WO2016178591A2 - Genetic markers and treatment of male obesity - Google Patents
Genetic markers and treatment of male obesity Download PDFInfo
- Publication number
- WO2016178591A2 WO2016178591A2 PCT/PT2016/050008 PT2016050008W WO2016178591A2 WO 2016178591 A2 WO2016178591 A2 WO 2016178591A2 PT 2016050008 W PT2016050008 W PT 2016050008W WO 2016178591 A2 WO2016178591 A2 WO 2016178591A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- subject
- assay
- solvate
- hydrate
- tautomer
- Prior art date
Links
- 0 CCC*C(C[C@](C)N)CNCC Chemical compound CCC*C(C[C@](C)N)CNCC 0.000 description 5
- XRQZLILAVBRIBQ-UHFFFAOYSA-N CC(C(C)=C1)N(C)C1C(O)=O Chemical compound CC(C(C)=C1)N(C)C1C(O)=O XRQZLILAVBRIBQ-UHFFFAOYSA-N 0.000 description 1
- CNKDOSNYWHDBNT-UHFFFAOYSA-N CC(C)(SC1C2)SC1N(C)C2C(O)=O Chemical compound CC(C)(SC1C2)SC1N(C)C2C(O)=O CNKDOSNYWHDBNT-UHFFFAOYSA-N 0.000 description 1
- BCMOCODTHZYPEI-UHFFFAOYSA-N CC(C)CN(C)CC(O)=O Chemical compound CC(C)CN(C)CC(O)=O BCMOCODTHZYPEI-UHFFFAOYSA-N 0.000 description 1
- DDGHFNSZKFJZDI-UHFFFAOYSA-N CC(C1)C(C)N(C)C1C(O)=O Chemical compound CC(C1)C(C)N(C)C1C(O)=O DDGHFNSZKFJZDI-UHFFFAOYSA-N 0.000 description 1
- MVTQBDMMPISLHW-UHFFFAOYSA-N CC(C=C1)N(C)C1C(O)=O Chemical compound CC(C=C1)N(C)C1C(O)=O MVTQBDMMPISLHW-UHFFFAOYSA-N 0.000 description 1
- CQGBVYPEUSQFCT-UHFFFAOYSA-N CC(CC1)CC(C2)C1N(C)C2C(O)=O Chemical compound CC(CC1)CC(C2)C1N(C)C2C(O)=O CQGBVYPEUSQFCT-UHFFFAOYSA-N 0.000 description 1
- FMLUIOBITQCLEE-UHFFFAOYSA-N CC(CCC1)CN(C)C1C(O)=O Chemical compound CC(CCC1)CN(C)C1C(O)=O FMLUIOBITQCLEE-UHFFFAOYSA-N 0.000 description 1
- ZITGPKZGTXAJSM-UHFFFAOYSA-N CC(CCC1C2)CC1N(C)C2C(O)=O Chemical compound CC(CCC1C2)CC1N(C)C2C(O)=O ZITGPKZGTXAJSM-UHFFFAOYSA-N 0.000 description 1
- UNIKQYIJSJGRRS-UHFFFAOYSA-N CCC(C(O)=O)N(C)C Chemical compound CCC(C(O)=O)N(C)C UNIKQYIJSJGRRS-UHFFFAOYSA-N 0.000 description 1
- PGYZIEVASKOIHO-UHFFFAOYSA-N CCC(C1)CN(C)C1C(O)=O Chemical compound CCC(C1)CN(C)C1C(O)=O PGYZIEVASKOIHO-UHFFFAOYSA-N 0.000 description 1
- MAWGMRQFCUEYCT-UHFFFAOYSA-N CCCC(C1)CN(C)C1C(O)=O Chemical compound CCCC(C1)CN(C)C1C(O)=O MAWGMRQFCUEYCT-UHFFFAOYSA-N 0.000 description 1
- IZYJZFQAHHGLEJ-UHFFFAOYSA-N CN(C(C1)C(NC#N)O)C2C1CCCC2 Chemical compound CN(C(C1)C(NC#N)O)C2C1CCCC2 IZYJZFQAHHGLEJ-UHFFFAOYSA-N 0.000 description 1
- UMKBBQSBRAEHPV-UHFFFAOYSA-N CN(C(C1)C(NO)=O)C2C1CCCC2 Chemical compound CN(C(C1)C(NO)=O)C2C1CCCC2 UMKBBQSBRAEHPV-UHFFFAOYSA-N 0.000 description 1
- UAWZDWSFCINKFK-UHFFFAOYSA-N CN(C(C1)C(O)=O)C2C1CCCC2 Chemical compound CN(C(C1)C(O)=O)C2C1CCCC2 UAWZDWSFCINKFK-UHFFFAOYSA-N 0.000 description 1
- LJFGHBPTHQDNDU-UHFFFAOYSA-N CN(C(C1)S(N)(=O)=O)C2C1CCCC2 Chemical compound CN(C(C1)S(N)(=O)=O)C2C1CCCC2 LJFGHBPTHQDNDU-UHFFFAOYSA-N 0.000 description 1
- STECRDROGVXUIL-UHFFFAOYSA-N CN(C(C1)c2nnn[nH]2)C2C1CCCC2 Chemical compound CN(C(C1)c2nnn[nH]2)C2C1CCCC2 STECRDROGVXUIL-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/403—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil condensed with carbocyclic rings, e.g. carbazole
- A61K31/404—Indoles, e.g. pindolol
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q1/00—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions
- C12Q1/68—Measuring or testing processes involving enzymes, nucleic acids or microorganisms; Compositions therefor; Processes of preparing such compositions involving nucleic acids
- C12Q1/6876—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes
- C12Q1/6883—Nucleic acid products used in the analysis of nucleic acids, e.g. primers or probes for diseases caused by alterations of genetic material
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/106—Pharmacogenomics, i.e. genetic variability in individual responses to drugs and drug metabolism
-
- C—CHEMISTRY; METALLURGY
- C12—BIOCHEMISTRY; BEER; SPIRITS; WINE; VINEGAR; MICROBIOLOGY; ENZYMOLOGY; MUTATION OR GENETIC ENGINEERING
- C12Q—MEASURING OR TESTING PROCESSES INVOLVING ENZYMES, NUCLEIC ACIDS OR MICROORGANISMS; COMPOSITIONS OR TEST PAPERS THEREFOR; PROCESSES OF PREPARING SUCH COMPOSITIONS; CONDITION-RESPONSIVE CONTROL IN MICROBIOLOGICAL OR ENZYMOLOGICAL PROCESSES
- C12Q2600/00—Oligonucleotides characterized by their use
- C12Q2600/156—Polymorphic or mutational markers
Landscapes
- Health & Medical Sciences (AREA)
- Chemical & Material Sciences (AREA)
- Life Sciences & Earth Sciences (AREA)
- Organic Chemistry (AREA)
- General Health & Medical Sciences (AREA)
- Engineering & Computer Science (AREA)
- Proteomics, Peptides & Aminoacids (AREA)
- Wood Science & Technology (AREA)
- Genetics & Genomics (AREA)
- Analytical Chemistry (AREA)
- Medicinal Chemistry (AREA)
- Veterinary Medicine (AREA)
- Public Health (AREA)
- Pharmacology & Pharmacy (AREA)
- Bioinformatics & Cheminformatics (AREA)
- Animal Behavior & Ethology (AREA)
- Zoology (AREA)
- General Chemical & Material Sciences (AREA)
- Biotechnology (AREA)
- Epidemiology (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Pathology (AREA)
- Obesity (AREA)
- Hematology (AREA)
- Physics & Mathematics (AREA)
- Biophysics (AREA)
- Nuclear Medicine, Radiotherapy & Molecular Imaging (AREA)
- Immunology (AREA)
- Microbiology (AREA)
- Molecular Biology (AREA)
- Diabetes (AREA)
- Child & Adolescent Psychology (AREA)
- Biochemistry (AREA)
- General Engineering & Computer Science (AREA)
- Medicines That Contain Protein Lipid Enzymes And Other Medicines (AREA)
- Pharmaceuticals Containing Other Organic And Inorganic Compounds (AREA)
Abstract
Some aspects of this disclosure provide methods for treating obesity in subjects carrying a variant allele of the carboxypeptidase D (CPD) gene with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; or a compound of Formulae III-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. Some aspects of this disclosure also provide methods for identifying a subject predisposed to obesity and/or sensitive to treatment with perindopril; a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; or a compound of Formulae III- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof based on the subject carrying a variant allele of the CPD gene. Pharmaceutical compositions for treating obesity and kits for detecting a variant allele of the CPD gene and/or for identifying a subject who is predisposed to obesity or who would benefit from treatment with perindopril; a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; or a compound of Formulae III- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof are also provided.
Description
GENETIC MARKERS AND TREATMENT OF MALE OBESITY
BACKGROUND
[0001] Obesity is a major risk factor for several disorders, particularly cardiovascular diseases, diabetes, cancer, sleep disorders and musculoskeletal disorders. Obesity, mostly male- type or waist-predominant obesity, is also a significant risk factor for metabolic syndrome. The latter is a combination of disorders (high blood pressure, high blood cholesterol and high triglyceride levels, together with an inflammatory state, leading to high prevalence of atherosclerosis and prothrombotic events) that strongly predispose to cardiovascular diseases and diabetes.
[0002] Obesity is considered a worldwide problem and with its prevalence steadily increasing not only in high income countries, obesity is becoming a serious global public health issue. According to the World Health Organization (WHO), it is the fifth leading risk factor for deaths globally and at least 2.8 million adults die each year as a consequence of being obese. In 2008, over 500 million people were obese, corresponding to more than 10% of the world's adult population. In 2011, more than 40 million children were overweight. Currently, the number of deaths linked to obesity is higher than the number of deaths linked to being underweight.
[0003] Diet and exercise are often insufficient to address obesity and drug therapies are helpful, but the outcomes have so far been modest. Nowadays, few therapeutic options exist and many drugs used for weight loss have been withdrawn from the market due to serious side effects. Thus, safer and more potent alternatives are needed (Glandt and Raz, 2011).
SUMMARY
[0004] The high prevalence and costs associated with obesity, together with the lack of markers for obesity risk assessment, diagnosis, and prognosis as well as the lack of effective therapies demonstrate an unmet need in this area. The work described herein describes the surprising discovery that certain nucleotide sequence variants found in the CPD gene, also referred to herein as variant alleles of the CPD gene, are associated with a predisposition to obesity. The testing of several chemical compounds in an animal model of obesity harboring a variant allele of the CPD gene and the subsequent identification of one particular group of compounds that can effectively treat obesity in such a model is also described herein. For example, it was surprisingly discovered that obesity in a subject carrying a variant allele of the
CPD gene can successfully be treated with perindopril or a stereoisomer, a tautomer, a polymorph, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof.
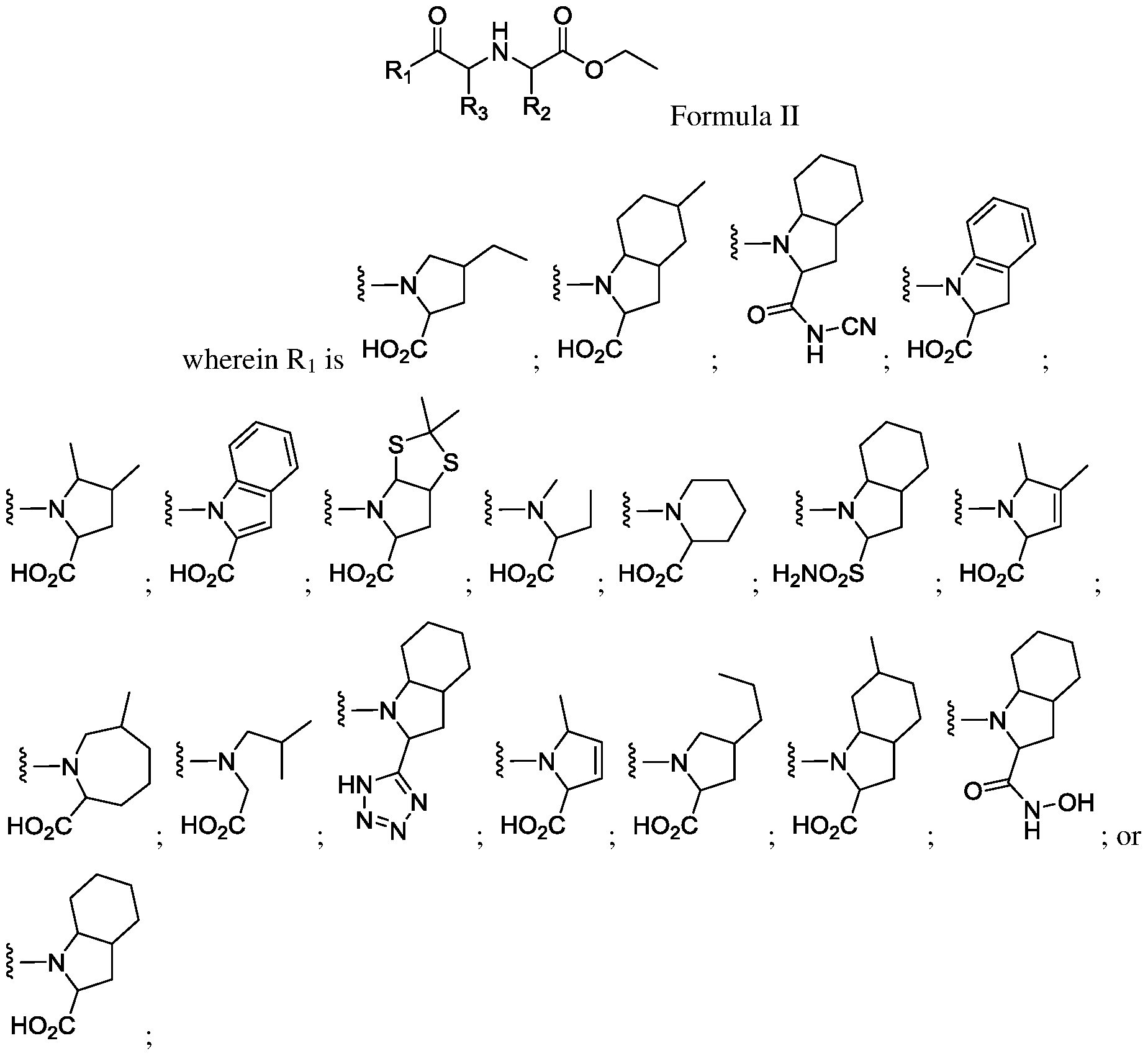
[0005] In one aspect, this disclosure provides a compound of Formula II, or a
harmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof;
[0009] Some aspects of this disclosure provide therapeutic methods for treating obesity in a subject carrying a variant allele of the CPD gene by administering perindopril or a stereoisomer, a tautomer, a polymorph, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof to the subject either alone or in combination with one or more anti-obesity agents. Some aspects of this disclosure provide biomarkers that are based on variant alleles of the CPD gene which are associated with obesity. Some aspects of this disclosure provide methods for detecting such variant alleles of the CPD gene using genotyping assays provided herein or otherwise known in the art.
[0010] Some aspects of this disclosure are based on the recognition that an individual's predisposition to obesity as well as the individual's responsiveness to treatment of obesity with perindopril, or a stereoisomer, a tautomer, a polymorph, a hydrate, a solvate, or a
pharmaceutically acceptable salt thereof, can be predicted based on detecting a variant allele of the CPD gene in the subject. Accordingly, some aspects of this disclosure provide diagnostic and prognostic methods for identifying a subject predisposed to obesity and/or responsive to treatment of obesity with perindopril, or a stereoisomer, a tautomer, a polymorph, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof. Some aspects of this disclosure provide methods of diagnosis and prognosis of a predisposition to develop obesity in a subject based on a detection of variant alleles of the CPD gene in the subject. Further, some aspects of this
disclosure provide methods of selecting a subject for treatment with perindopril or a stereoisomer, a tautomer, a polymorph, a hydrate, a solvate, or a pharmaceutically acceptable salt thereof based on a detection of variant alleles of the CPD gene in the subject.
[0011] Some aspects of this disclosure provide methods for treating obesity in a subject, comprising administering a therapeutically effective amount of perindopril, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the method comprises administering an effective amount of perindopril arginine to the subject. In some embodiments, the method comprises administering an effective amount of perindopril erbumine to the subject. In some embodiments, the subject carries a variant allele of the CPD gene. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the subject has a BMI (kg/m2) of > 30.00. In some embodiments, the subject has a BMI (kg/m ) of 30.00-34.99 (obesity class I). In some embodiments, the subject has a BMI of 35.00-39.99 (obesity class II). In some embodiments, the subject has a BMI of > 40.0 (obesity class III). In some embodiments, the method further comprises administering an effective amount of one or more agents selected from the group consisting of: an anti-obesity agent, an anti-hypertensive agent, a diuretic agent, and a antidiabetic agent. Some examples of antidiabetic agents may include biguanides (e.g., metformin), sulfonylureas (e.g., glimepiride), meglitinides (e.g., repaglinide), thiazolidinediones (e.g., pioglitazone), dipeptidyl peptidase IV inhibitors (e.g., sitagliptin), and a-glucosidase inhibitors (e.g., acarbose). In some embodiments, the antidiabetic agent is administered orally. In some embodiments, the perindopril, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered simultaneously. In some embodiments, the perindopril, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered separately. In some
embodiments, the method further comprises determining whether the subject carries a variant allele of the CPD gene. In some embodiments, the method comprises detecting one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406 in an allele of the CPD gene of the subject. In some embodiments, the method further comprises selecting the subject
for administration of perindopril based on the subject carrying a variant allele of the CPD gene. In some embodiments, the subject is male.
[0012] Some aspects of this disclosure provide methods for treating obesity in a subject, comprising administering a therapeutically effective amount of trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the subject carries a variant allele of the CPD gene. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the subject has a BMI (kg/m2) of > 30.00. In some
embodiments, the subject has a BMI (kg/m ) of 30.00-34.99 (obesity class I). In some embodiments, the subject has a BMI of 35.00-39.99 (obesity class II). In some embodiments, the subject has a BMI of > 40.0 (obesity class III). In some embodiments, the method further comprises administering an effective amount of one or more agents selected from the group consisting of: an anti-obesity agent, an anti-hypertensive agent, a diuretic agent, and a antidiabetic agent. Some examples of antidiabetic agents may include biguanides (e.g., metformin), sulfonylureas (e.g., glimepiride), meglitinides (e.g., repaglinide), thiazolidinediones (e.g., pioglitazone), dipeptidyl peptidase IV inhibitors (e.g., sitagliptin), and a-glucosidase inhibitors (e.g., acarbose). In some embodiments, the antidiabetic agent is administered orally. In some embodiments, the trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered simultaneously. In some embodiments, the trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered separately. In some embodiments, the method further comprises determining whether the subject carries a variant allele of the CPD gene. In some embodiments, the method comprises detecting one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406 in an allele of the CPD gene of the subject. In some embodiments, the method further comprises selecting the subject for administration of
perindopril based on the subject carrying a variant allele of the CPD gene. In some embodiments, the subject is male.
[0013] Some aspects of this disclosure provide methods for treating obesity in a subject, comprising administering a therapeutically effective amount of a compound of Formula II, or a harmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof;
8/190
[0016] In other aspec ϊ S . ¾
[0017] Some aspects of this disclosure provide methods for treating obesity in a subject, comprising administering a therapeutically effective amount of a compound of Formulae III-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof;
[0018] In some embodiments, the subject carries a variant allele of the CPD gene. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the subject has a BMI (kg/m2) of > 30.00. In some embodiments, the subject has a BMI (kg/m2) of 30.00- 34.99 (obesity class I). In some embodiments, the subject has a BMI of 35.00-39.99 (obesity class II). In some embodiments, the subject has a BMI of > 40.0 (obesity class III). In some embodiments, the method further comprises administering an effective amount of one or more agents selected from the group consisting of: an anti-obesity agent, an anti-hypertensive agent, a
diuretic agent, and a antidiabetic agent. Some examples of antidiabetic agents may include biguanides (e.g., metformin), sulfonylureas (e.g., glimepiride), meglitinides (e.g., repaglinide), fhiazolidinediones (e.g., pioglitazone), dipeptidyl peptidase IV inhibitors (e.g., sitagliptin), and a-glucosidase inhibitors (e.g., acarbose). In some embodiments, the antidiabetic agent is administered orally. In some embodiments, the trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered simultaneously. In some embodiments, the trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered separately. In some embodiments, the method further comprises determining whether the subject carries a variant allele of the CPD gene. In some embodiments, the method comprises detecting one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406 in an allele of the CPD gene of the subject. In some embodiments, the method further comprises selecting the subject for administration of perindopril based on the subject carrying a variant allele of the CPD gene. In some embodiments, the subject is male.
[0019] Some aspects of this disclosure provide methods for treating obesity in a subject, the method comprising (i) determining whether the subject carries a variant allele of the CPD gene; and (ii) if the subject is determined to carry a variant allele of the CPD gene, administering an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject. In some embodiments, step (i) comprises detecting the variant allele of the CPD gene in the genome of a cell of the subject. In some embodiments, the method further comprises obtaining a cell or tissue sample from the subject. In some embodiments, the method comprises isolating genomic DNA from a cell or tissue obtained from the subject. In some embodiments, step (i) comprises performing a genotyping assay on genomic DNA obtained from the subject. In some embodiments, the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and
rs9406. In some embodiments, the subject has a BMI (kg/m ) of > 30.00. In some embodiments, the subject has a BMI (kg/m ) of 30.00-34.99 (obesity class I). In some embodiments, the subject has a BMI of 35.00-39.99 (obesity class II). In some embodiments, the subject has a BMI of > 40.0 (obesity class III). In some embodiments, the method comprises administering an effective amount of perindopril arginine or of perindopril erbumine to the subject. In some embodiments, the method further comprises administering an effective amount of one or more agents selected from the group consisting of: an anti-obesity agent, an antihypertensive agent, a diuretic agent, and a antidiabetic agent. Some examples of antidiabetic agents may include biguanides (e.g., metformin), sulfonylureas (e.g., glimepiride), meglitinides (e.g., repaglinide), thiazolidinediones (e.g., pioglitazone), dipeptidyl peptidase IV inhibitors (e.g., sitagliptin), and a-glucosidase inhibitors (e.g., acarbose). In some embodiments, the antidiabetic agent is administered orally. In some embodiments, the perindopril, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered simultaneously. In some embodiments, the perindopril, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered separately. In some embodiments, the method further comprises selecting the subject for administration of perindopril based on the subject carrying a variant allele of the CPD gene. In some embodiments, the subject is male.
[0020] Some aspects of this disclosure provide methods for treating obesity in a subject, the method comprising (i) determining whether the subject carries a variant allele of the CPD gene; and (ii) if the subject is determined to carry a variant allele of the CPD gene, administering an effective amount of any compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) to the subject. In some embodiments, step (i) comprises detecting the variant allele of the CPD gene in the genome of a cell of the subject. In some embodiments, the method further comprises obtaining a cell or tissue sample from the subject. In some embodiments, the method comprises isolating genomic DNA from a cell or tissue obtained from the subject. In some embodiments, step (i) comprises performing a genotyping assay on genomic DNA obtained from the subject. In some embodiments, the genotyping assay is an allele-specific PCR assay, a
primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the subject has a BMI (kg/m2) of > 30.00. In some embodiments, the subject has a BMI (kg/m2) of 30.00-34.99 (obesity class I). In some embodiments, the subject has a BMI of 35.00-39.99 (obesity class II). In some embodiments, the subject has a BMI of > 40.0 (obesity class III). In some embodiments, the method further comprises administering an effective amount of one or more agents selected from the group consisting of: an anti-obesity agent, an anti -hypertensive agent, a diuretic agent, and a antidiabetic agent. Some examples of antidiabetic agents may include biguanides (e.g., metformin), sulfonylureas (e.g., glimepiride), meglitinides (e.g., repaglinide), thiazolidinediones (e.g., pioglitazone), dipeptidyl peptidase IV inhibitors (e.g., sitagliptin), and a-glucosidase inhibitors (e.g., acarbose). In some embodiments, the antidiabetic agent is administered orally. In some embodiments, the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and the one or more agents are administered
simultaneously. In some embodiments, the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) are administered separately. In some embodiments, the method further comprises selecting the subject for administration of the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) based on the subject carrying a variant allele of the CPD gene. In some embodiments, the subject is male.
[0021] Some aspects of this disclosure provide methods for identifying a subject who is predisposed to obesity, comprising (i) determining whether the subject carries a variant allele of
the CPD gene; and (ii) if the subject is determined to carry variant allele of the CPD gene, identifying the subject as predisposed to obesity. In some embodiments, step (i) comprises detecting the variant allele of the CPD gene in the genome of a cell of the subject. In some embodiments, the method further comprises obtaining a cell or tissue sample from the subject. In some embodiments, the method comprises isolating genomic DNA from a cell or tissue obtained from the subject. In some embodiments, step (i) comprises performing a genotyping assay on genomic DNA obtained from the subject. In some embodiments, the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay. In some embodiments, the allele-specific PCR assay is an intercalating dye assay, a FRET primer assay, or an ALPHASCREEN™ assay. In some embodiments, the primer extension assay is an ARMS (amplification refractory mutation system) assay, a kinetic or real-time PCR assay, a SNPSTREAM™ assay, a GENETIC BIT ANALYSIS™ (GBA) assay, a multiplex minisequencing assay, a SNAPSHOT™ assay, a PYROSEQUENCING™ assay, a MASSEXTEND™ assay, a MASSARRAY™ assay, a MALDI mass spectrometry-based assay, a microarray minisequencing assay, an APEX (arrayed primer extension) assay, a sequence specific priming (SSP) assay, a microarray primer extension assay, a tag array assay, a coded microsphere assay, a template-directed incorporation (TDI) assay, or a fluorescence polarization assay. In some embodiments, the oligonucleotide ligation assay is a colorimetric oligonucleotide ligation assay (OLA), a sequence-coded OLA, a microarray ligation assay, a ligase chain reaction assay, a padlock probe assay, or a rolling circle amplification assay. In some embodiments, the hybridization assay is a reverse dot blot assay, a line probe assay (LiPA), a microarray assay, a dynamic allele-specific hybridization (DASH) assay, a PNA or locked nucleic acid (LNA) probe assay, a TAQMAN™ assay, or a molecular beacon assay. In some embodiments, the endonuclease cleavage assay is a restriction site analysis (RFLP), or an INVADER™ assay. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the method further comprises determining whether the subject is obese. In some embodiments, the subject is not obese. In some embodiments, the method further comprises administering healthcare to the subject to prevent the subject from becoming obese. In some embodiments, the method further comprises monitoring the body weight of the subject
and, if the subject is determined to be obese during the monitoring, selecting the subject to treatment with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the method further comprises administering an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject. In some embodiments, the subject is male.
[0022] Some aspects of this disclosure provide methods for identifying a subject who is predisposed to obesity, comprising (i) determining whether the subject carries a variant allele of the CPD gene; and (ii) if the subject is determined to carry variant allele of the CPD gene, identifying the subject as predisposed to obesity. In some embodiments, step (i) comprises detecting the variant allele of the CPD gene in the genome of a cell of the subject. In some embodiments, the method further comprises obtaining a cell or tissue sample from the subject. In some embodiments, the method comprises isolating genomic DNA from a cell or tissue obtained from the subject. In some embodiments, step (i) comprises performing a genotyping assay on genomic DNA obtained from the subject. In some embodiments, the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay. In some embodiments, the allele-specific PCR assay is an intercalating dye assay, a FRET primer assay, or an ALPHASCREEN™ assay. In some embodiments, the primer extension assay is an ARMS (amplification refractory mutation system) assay, a kinetic or real-time PCR assay, a SNPSTREAM™ assay, a GENETIC BIT ANALYSIS™ (GBA) assay, a multiplex minisequencing assay, a SNAPSHOT™ assay, a PYROSEQUENCING™ assay, a MASSEXTEND™ assay, a MASSARRAY™ assay, a MALDI mass spectrometry-based assay, a microarray minisequencing assay, an APEX (arrayed primer extension) assay, a sequence specific priming (SSP) assay, a microarray primer extension assay, a tag array assay, a coded microsphere assay, a template-directed incorporation (TDI) assay, or a fluorescence polarization assay. In some embodiments, the oligonucleotide ligation assay is a colorimetric oligonucleotide ligation assay (OLA), a sequence-coded OLA, a microarray ligation assay, a ligase chain reaction assay, a padlock probe assay, or a rolling circle amplification assay. In some embodiments, the hybridization assay is a reverse dot blot assay, a line probe assay (LiPA), a microarray assay, a dynamic allele-specific hybridization (DASH) assay, a PNA or locked nucleic acid (LNA) probe assay, a TAQMAN™ assay, or a molecular
beacon assay. In some embodiments, the endonuclease cleavage assay is a restriction site analysis (RFLP), or an INVADER™ assay. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the method further comprises determining whether the subject is obese. In some embodiments, the subject is not obese. In some embodiments, the method further comprises administering healthcare to the subject to prevent the subject from becoming obese. In some embodiments, the method further comprises monitoring the body weight of the subject and, if the subject is determined to be obese during the monitoring, selecting the subject to treatment with the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). In some embodiments, the method further comprises administering an effective amount of the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) to the subject. In some embodiments, the subject is male.
[0023] Some aspects of this disclosure provide methods for selecting a subject for treatment of obesity with perindopril or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, the method comprising (i) determining whether an obese subject carries a variant allele of the CPD gene; and (ii) if the subject is determined to carry a CPD gene, selecting the subject for treatment of obesity with perindopril or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the method further comprises administering an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject. In some embodiments, step (i) comprises performing a genotyping assay. In some embodiments, the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay. In some embodiments, the allele-specific PCR assay is an intercalating dye
assay, a FRET primer assay, or an ALPHASCREEN assay. In some embodiments, the primer extension assay is an ARMS (amplification refractory mutation system) assay, a kinetic or realtime PCR assay, a SNPSTREAM™ assay, a GENETIC BIT ANALYSIS™ (GBA) assay, a multiplex minisequencing assay, a SNAPSHOT™ assay, a PYROSEQUENCING™ assay, a MASSEXTEND™ assay, a MASSARRAY™ assay, a MALDI mass spectrometry-based assay, a microarray minisequencing assay, an APEX (arrayed primer extension) assay, a sequence specific priming (SSP) assay, a microarray primer extension assay, a tag array assay, a coded microsphere assay, a template-directed incorporation (TDI) assay, or a fluorescence polarization assay. In some embodiments, the oligonucleotide ligation assay is a colorimetric oligonucleotide ligation assay (OLA), a sequence-coded OLA, a microarray ligation assay, a ligase chain reaction assay, a padlock probe assay, or a rolling circle amplification assay. In some embodiments, the hybridization assay is a reverse dot blot assay, a line probe assay (LiPA), a microarray assay, a dynamic allele-specific hybridization (DASH) assay, a PNA or locked nucleic acid (LNA) probe assay, a TAQMAN™ assay, or a molecular beacon assay. In some embodiments, the endonuclease cleavage assay is a restriction site analysis (RFLP), or an INVADER™ assay. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the subject is male.
[0024] Some aspects of this disclosure provide methods for selecting a subject for treatment of obesity with the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), the method comprising (i) determining whether an obese subject carries a variant allele of the CPD gene; and (ii) if the subject is determined to carry a CPD gene, selecting the subject for treatment of obesity with the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). In some embodiments, the method further comprises administering an effective amount
of the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) to the subject. In some embodiments, step (i) comprises performing a genotyping assay. In some embodiments, the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay. In some embodiments, the allele-specific PCR assay is an intercalating dye assay, a FRET primer assay, or an ALPHASCREEN™ assay. In some embodiments, the primer extension assay is an ARMS (amplification refractory mutation system) assay, a kinetic or real-time PCR assay, a SNPSTREAM™ assay, a GENETIC BIT ANALYSIS™ (GBA) assay, a multiplex
minisequencing assay, a SNAPSHOT™ assay, a PYROSEQUENCING™ assay, a
MASSEXTEND™ assay, a MASSARRAY™ assay, a MALDI mass spectrometry-based assay, a microarray minisequencing assay, an APEX (arrayed primer extension) assay, a sequence specific priming (SSP) assay, a microarray primer extension assay, a tag array assay, a coded microsphere assay, a template-directed incorporation (TDI) assay, or a fluorescence polarization assay. In some embodiments, the oligonucleotide ligation assay is a colorimetric oligonucleotide ligation assay (OLA), a sequence-coded OLA, a microarray ligation assay, a ligase chain reaction assay, a padlock probe assay, or a rolling circle amplification assay. In some embodiments, the hybridization assay is a reverse dot blot assay, a line probe assay (LiPA), a microarray assay, a dynamic allele-specific hybridization (DASH) assay, a PNA or locked nucleic acid (LNA) probe assay, a TAQMAN™ assay, or a molecular beacon assay. In some embodiments, the endonuclease cleavage assay is a restriction site analysis (RFLP), or an INVADER™ assay. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the subject is male.
[0025] In some embodiments of the therapeutic and diagnostic methods provided herein, a subject is treated or selected for treatment with perindopril, wherein the subject does not have hypertension, cardiovascular disease, and/or coronary artery disease (CAD).
[0026] In some embodiments of the therapeutic and diagnostic methods provided herein, a subject is treated or selected for treatment with the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), wherein the subject does not have hypertension, cardiovascular disease, and/or coronary artery disease (CAD).
[0027] Some aspects of this disclosure provide pharmaceutical compositions comprising an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for use in the treatment of obesity in a subject. Some aspects of this disclosure provide pharmaceutical compositions comprising an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for administration to an obese subject. In some embodiments, the pharmaceutical composition further comprises an effective amount of one or more agents selected from the group consisting of an anti-obesity agent, an anti-hypertensive agent, a diuretic agent, and an antidiabetic agent. In some embodiments, (i) the subject is male; (ii) the subject carries a variant allele of the CPD gene; and/or (iii) the subject has been selected for treatment with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof based on the subject carrying a variant allele of the CPD gene. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the composition comprises an effective amount of perindopril arginine or of perindopril erbumine. In some embodiments, the composition comprises about 0.01 mg, about 0.02 mg, about 0.03 mg, about 0.04 mg, about 0.05 mg, about 0.06 mg, about 0.07 mg, about 0.08 mg, about 0.09 mg, or about 0.1 mg of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof per kg body weight of the subject.
[0028] Some aspects of this disclosure provide pharmaceutical compositions comprising the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically
acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), for use in the treatment of obesity in a subject. Some aspects of this disclosure provide pharmaceutical compositions comprising an effective amount of the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), for administration to an obese subject. In some embodiments, the pharmaceutical composition further comprises an effective amount of one or more agents selected from the group consisting of an anti-obesity agent, an anti-hypertensive agent, a diuretic agent, and an antidiabetic agent. In some embodiments, (i) the subject is male; (ii) the subject carries a variant allele of the CPD gene; and/or (iii) the subject has been selected for treatment with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof based on the subject carrying a variant allele of the CPD gene. In some embodiments, the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the composition comprises about 0.01 mg, about 0.02 mg, about 0.03 mg, about 0.04 mg, about 0.05 mg, about 0.06 mg, about 0.07 mg, about 0.08 mg, about 0.09 mg, or about 0.1 mg of the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) per kg body weight of the subject.
[0029] Some aspects of this disclosure provide kits for detecting a variant allele of the
CPD gene and/or for identifying a subject who is predisposed to obesity, comprising reagents for performing a genotyping assay for detecting a variant allele of the CPD gene in genomic DNA obtained from the subject. Some aspects of this disclosure provide kits for identifying a subject who would benefit from treatment with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, the kit comprising reagents for performing a genotyping assay for detecting a variant allele of the CPD gene in genomic DNA obtained from the subject. In some embodiments, the kit comprises a primer, primer pair, or
probe for detecting the variant allele of the CPD gene. In some embodiments, the kit comprises a primer, primer pair, or probe for detecting one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
[0030] Some aspects of this disclosure provide kits for detecting a variant allele of the
CPD gene and/or for identifying a subject who is predisposed to obesity, comprising reagents for performing a genotyping assay for detecting a variant allele of the CPD gene in genomic DNA obtained from the subject. Some aspects of this disclosure provide kits for identifying a subject who would benefit from treatment with the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), the kit comprising reagents for performing a genotyping assay for detecting a variant allele of the CPD gene in genomic DNA obtained from the subject. In some embodiments, the kit comprises a primer, primer pair, or probe for detecting the variant allele of the CPD gene. In some embodiments, the kit comprises a primer, primer pair, or probe for detecting one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
[0031] The summary above is meant to illustrate, in a non-limiting manner, some of the embodiments, advantages, features, and uses of the technology disclosed herein. Other embodiments, advantages, features, and uses of the technology disclosed herein will be apparent from the Detailed Description, the Drawings, the Examples, and the Claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0032] Figure 1. Diagram of the exon structure of the CPD gene. The darker regions represent the coding frame.
[0033] Figure 2. Diagram of the location of the 10 identified SNPs with respect to the intron/exon structure and coding sequence of the CPD gene. The SNP locations shown in Fig. 2 are approximate.
[0034] Figure 3. Diagram illustrating the strategy for a gene association study that was used to discover associations between SNPs and the male obesity phenotype.
DEFINITIONS
[0035] As used herein and in the claims, the singular forms "a," "an," and "the" include the singular and the plural reference unless the context clearly indicates otherwise. Thus, for example, a reference to "an agent" includes a single agent and a plurality of such agents.
[0036] The term "effective amount," as used herein, refers to an amount of a biologically active agent that is sufficient to elicit a desired biological or clinical response. In some embodiments, a "therapeutically effective amount" of a composition provided herein (e.g., of a drug, pharmaceutical composition, combination, anti-obesity agent, or medicament) is the amount which, when administered to an obese subject, is sufficient to treat the obesity, or produce a desired clinical effect, e.g., a certain amount of weight loss within a certain time frame. For example, in some embodiments, an effective amount of perindopril, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, may refer to an amount that is sufficient to induce a measurable weight loss in an obese subject. For example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof refers to the amount sufficient or the amount required to induce the loss of at least 5%, at least 10%, at least 15%, at least 20%, at least 25, at least 30%, at least 35%, at least 40%, at least 45%, or at least 50%, of body weight within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced.
[0037] The term "mutation," as used herein, refers to a substitution of a residue within a sequence, e.g., a nucleic acid or amino acid sequence, with another residue, or a deletion or insertion of one or more residues within a sequence. Mutations are typically described herein by identifying the original residue followed by the position of the residue within the sequence and by the identity of the newly substituted residue. Various methods for making the amino acid substitutions (mutations) provided herein are well known in the art, and are provided by, for example, Green and Sambrook, Molecular Cloning: A Laboratory Manual (4th ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (2012)).
[0038] The term "nucleic acid" and "nucleic acid molecule," as used herein, refers to a compound comprising a nucleobase and an acidic moiety, e.g., a nucleoside, a nucleotide, or a
polymer of nucleotides. Typically, polymeric nucleic acids, e.g., nucleic acid molecules comprising three or more nucleotides are linear molecules, in which adjacent nucleotides are linked to each other via a phosphodiester linkage. In some embodiments, "nucleic acid" refers to individual nucleic acid residues (e.g. nucleotides and/or nucleosides). In some embodiments, "nucleic acid" refers to an oligonucleotide chain comprising three or more individual nucleotide residues. As used herein, the terms "oligonucleotide" and "polynucleotide" can be used interchangeably to refer to a polymer of nucleotides (e.g., a string of at least three nucleotides). In some embodiments, "nucleic acid" encompasses RNA as well as single and/or double- stranded DNA. Nucleic acids may be naturally occurring, for example, in the context of a genome, a transcript, an mRNA, tRNA, rRNA, siRNA, snRNA, a plasmid, cosmid, chromosome, chromatid, or other naturally occurring nucleic acid molecule. On the other hand, a nucleic acid molecule may be a non-naturally occurring molecule, e.g., a recombinant DNA or RNA, an artificial chromosome, an engineered genome, or fragment thereof, or a synthetic DNA, RNA, DNA/RNA hybrid, or including non-naturally occurring nucleotides or nucleosides.
Furthermore, the terms "nucleic acid," "DNA," "RNA," and/or similar terms include nucleic acid analogs, e.g., analogs having other than a phosphodiester backbone. Nucleic acids can be purified from natural sources, produced using recombinant expression systems and optionally purified, chemically synthesized, etc. Where appropriate, e.g., in the case of chemically synthesized molecules, nucleic acids can comprise nucleoside analogs such as analogs having chemically modified bases or sugars, and backbone modifications. A nucleic acid sequence is presented in the 5' to 3' direction unless otherwise indicated. In some embodiments, a nucleic acid is or comprises natural nucleosides (e.g. adenosine, thymidine, guanosine, cytidine, uridine, deoxyadenosine, deoxythymidine, deoxyguanosine, and deoxycytidine); nucleoside analogs (e.g., 2-aminoadenosine, 2-thiothymidine, inosine, pyrrolo-pyrimidine, 3-methyl adenosine, 5- methylcytidine, 2-aminoadenosine, C5-bromouridine, C5-fluorouridine, C5-iodouridine, C5- propynyl-uridine, C5-propynyl-cytidine, C5-methylcytidine, 2-aminoadenosine, 7- deazaadenosine, 7-deazaguanosine, 8-oxoadenosine, 8-oxoguanosine, 0(6)-methylguanine, and 2-thiocytidine); chemically modified bases; biologically modified bases (e.g., methylated bases); intercalated bases; modified sugars (e.g., 2'-fluororibose, ribose, 2'-deoxyribose, arabinose, and hexose); and/or modified phosphate groups (e.g., phosphorothioates and 5'-N-phosphoramidite linkages).
[0039] The term "protein," as used herein interchangeably with the terms "peptide," and
"polypeptide" refers to a polymer of amino acid residues linked together by peptide (amide) bonds. The terms refer to a protein, peptide, or polypeptide of any size, structure, or function. Typically, a protein, peptide, or polypeptide will be at least three amino acids long. A protein, peptide, or polypeptide may refer to an individual protein or a collection of proteins. One or more of the amino acids in a protein, peptide, or polypeptide may be modified, for example, by the addition of a chemical entity such as a carbohydrate group, a hydroxyl group, a phosphate group, a farnesyl group, an isofarnesyl group, a fatty acid group, a linker for conjugation, functionalization, or other modification, etc. A protein, peptide, or polypeptide may also be a single molecule or may be a multi-molecular complex. A protein, peptide, or polypeptide may be a fragment of a naturally occurring protein or peptide. A protein, peptide, or polypeptide may be naturally occurring, recombinant, or synthetic, or any combination thereof. Methods for the detection, sequence analysis, expression, and purification of proteins are well known, and include those described by Green and Sambrook, Molecular Cloning: A Laboratory Manual (4th ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. (2012)), the entire contents of which are incorporated herein by reference.
[0040] The term "subject," as used herein, refers to an individual organism, for example, an individual mammal. In some embodiments, the subject is a human. In some embodiments, the subject is a non-human mammal. In some embodiments, the subject is a non-human primate. In some embodiments, the subject is a rodent. In some embodiments, the subject is a sheep, a goat, a cattle, a cat, or a dog. In some embodiments, the subject is a vertebrate, an amphibian, a reptile, a fish, an insect, a fly, or a nematode. In some embodiments, the subject is a research animal. In some embodiments, the subject is genetically engineered, e.g., a genetically engineered non-human subject. The subject may be of either gender and at any stage of development. In some embodiments, the subject is a male subject. In some embodiments, the subject may be a vertebrate, mammal, or domestic animal. Accordingly, the agents,
compositions, and medicaments provided herein may be used in human and/or veterinary therapeutic applications. In some embodiments, the subject is a human being. In some embodiments, the subject may be a white Caucasian, or sharing other attributes of race, ethnicity, gender, age, weight, etc., with the subjects comprised in the population group in Example 3.
[0041] The term "obese" and "obesity" is used herein according to the WHO (WHO,
2000) definition represented in Table 1. One way to classify body weight status in humans is the Body Mass Index (BMI), which is calculated as the ratio of weight to height squared (kg/m2) and is commonly used to classify overweight and obesity in adults. The WHO classification of body weight is represented in Table 1.
Table 1. WHO classification of body weight status in humans
WHO obesity: preventing and managing the global epidemic. Report of a WHO consultation.
WHO Technical report series 894. Geneva: World Health Organization, 2000.
Based on the WHO classification, obesity can be defined by a Body Mass Index of 30.00 and higher, and obesity can be further classified into three classes (class I-III).
[0042] Thus, an obese individual can be defined as someone with a BMI (WHO, 2000) of about 30 kg/m or more; a morbidly obese individual can be defined as someone with a BMI of about 40 kg/m or more. In some embodiments, the obesity being referred to may be class I, class II, or class III obesity, as defined in Table 1 herein, or any combination thereof. In some embodiments, the obesity being referred to is at least class II obesity, and is preferably class III obesity.
[0043] The term "treatment," "treat," and "treating," refers to a clinical intervention aimed to reverse, alleviate, delay the onset of, or inhibit the progression of a disease or disorder, or one or more symptoms thereof, as described herein, for example, the onset, progression, or one or more symptoms of obesity. In some embodiments, treatment may be administered after one or more symptoms have developed, e.g., after a BMI of more than 30 has been reached, and/or after a disease has been diagnosed. In some embodiments, treatment may be administered in the absence of symptoms, e.g., to prevent or delay onset of a symptom or inhibit onset or
progression of a disease, e.g., in a pre-obese subject, or to inhibit progression from a lower class of obesity (e.g., class I or class II obesity) to a higher class of obesity (e.g., class III obesity). For example, treatment may be administered to a responsive individual prior to the onset of symptoms (e.g., in light of a history of symptoms and/or in light of genetic susceptibility factors, such as, for example, a variant allele of the CPD gene). Treatment may also be continued after symptoms have resolved, e.g., after the subject has lost weight and/or is not considered obese any more, for example, to prevent or delay weight gain back to an obese status. In some embodiments, a treatment as provided by some aspects of this invention is aimed to reduce the weight of the subject to a point that the subject is not considered obese anymore. In some such embodiments, treatment may continue until the subject reaches a BMI of less than 30 kg/m . In some embodiments, treatment may continue until the subject has reached a BMI associated with a lower class of obesity as compared to the class the subject was in when treatment started. For example, in some embodiments, treatment includes decreasing the BMI of the subject from a BMI associated with class III obesity to a BMI associated with class II or class I obesity, or reducing the BMI of the subject from a BMI associated with class II obesity to a BMI associated with class I obesity. In some embodiments, treatment may include a reduction of the BMI or of the weight of the subject by at least 5%, at least 10%, at least 20%, at least 25%, at least 30%, at least 40%, or at least 50% within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. In some embodiments, longer time frames are contemplated.
[0044] The term "probe" or "hybridization probe" refers to a nucleic acid molecule comprising a nucleotide sequence that is complementary to the nucleotide sequence of a target nucleic acid molecule, and is thus capable of binding in a sequence-specific manner to the complementary sequence of the target nucleic acid molecule via base-pairing. In some embodiments, such probes include, for example, nucleic acid molecules, peptide nucleic acids (PNAs) and locked nucleic acids (LNAs). In some embodiments, a probe is at least 5, at least 10, at least 15, at least 20, at least 25, at least 30, at least 35, at least 40, at least 45, at least 50, at least 60, at least 70, at least 80, at least 90, at least 100, at least 150, at least 200, at least 250, at least 300, at least 400, or at least 500 nucleotides long. In some embodiments, the probe comprises a sequence or perfect complementarity to a sequence of at least 5, at least 10, at least
15, at least 20, at least 25, at least 30, at least 35, at least 40, at least 45, at least 50, at least 60, at least 70, at least 80, at least 90, at least 100, at least 150, at least 200, at least 250, at least 300, at least 400, or at least 500 contiguous nucleotides of the target nucleic acid molecule, e.g., a target sequence of a CPD gene known to be characteristic for an allelic variant. Typically, a probe is used to detect a target nucleic acid via hybridization of the probe to the target nucleic acid. Suitable conditions for probe:target hybridization will be apparent to those of skill in the art based on the present disclosure and additional suitable conditions will be known to those of skill in the art. Hybridization may be performed under more or less stringent conditions, for example, in 3x sodium chloride/sodium citrate (SSC) at approximately 45 °C, approximately 50 °C, approximately 60 °C, approximately 65 °C, or approximately 70 °C, followed by at least one wash in 0.2x SSC/0.1% SDS at approximately 20-75 °C. As used herein, the term "probe" also includes primers. In some embodiments, probes and/or primers are sometimes referred to herein as "oligonucleotides."
[0045] The term "primer" refers to a nucleic acid molecule that can hybridize to a primer hybridization site of a nucleic acid template, e.g., a target nucleic acid molecule, via base pairing and that can be elongated by a polymerase, for example, Taq, Pfu, Pwo, Tfl, rTth, Tli, Tma, Bst, 9°Nm, Vent, or Phusion polymerase during a PCR. A primer, accordingly, includes a free 3'-OH group or other group amenable to the addition of nucleotide monomers by a polymerase. In some embodiments, only a 3' portion of the primer hybridizes to the primer hybridization site. In other embodiments, the whole primer hybridizes to the primer hybridization site. A primer includes a nucleotide sequence complementary to that of the primer hybridization site it hybridizes to. It should be noted, that primer hybridization may tolerate nucleotide -nucleotide mismatches, and, therefore, "complementary" does not require complete complementarity, but only a degree of complementarity sufficient for hybridization. Typically, a primer includes between 15 to 35 nucleotides. However, a primer may be longer or shorter than that, for example, ranging in length from 5-100 nucleotides. In a PCR assay, a primer hybridizes with a primer hybridization site of a nucleic acid template during the annealing step, is elongated by nucleotide addition in the elongation step, and the hybridization of elongated primer and template are broken during the denaturing step. In some embodiments, exemplary hybridization conditions for short probes and primers to target nucleic acid molecules may be about 5-12 °C below the calculated melting temperature™ of the complementary sequences of the primer and the target nucleic acid
molecule. Methods for calculating the Tm of a given probe or primer are known and include, without limitation, those utilizing the following formulae: Tm = 4 °C x (number of guanines (Gs) and cytosines (Cs) in the primer) + 2 °C x (number of adenines (As) and thymines (Ts) in the primer) for oligonucleotide of <14 bases in length and assuming a reaction is carried out in the presence of 50 mM monovalent cations. For longer oligonucleotides, the following formula can be used: Tm = 64.9 °C + 41 °C x (number of Gs and Cs in the primer - 16.4)/N, where N is the length of the primer. Another commonly used formula takes into account the salt concentration of the reaction (Rychlik, supra; Sambrook, supra; Mueller, supra.): Tm = 81.5 °C + 16.6 °C x (logio[Na+] + [K+]) + 0.41 °C x (%GC) - 675/N, where N is the number of nucleotides in the oligonucleotide. The aforementioned formulae provide a starting point for certain applications; however, the design of particular probes and primers may take into account additional or different factors. Methods for design of probes and primers for use in the methods provided herein, e.g., for the detection of variant alleles of the CPD gene, are well known in the art.
[0046] The term "primer extension," interchangeably used with the term "primer elongation", refers to the extension of a primer that hybridizes to a nucleic acid template by the addition of nucleotides complementary to the nucleic acid sequence of the template. In a PCR, this primer extension is usually performed by a thermophilic polymerase, for example, Taq, Pfu, Pwo, Tfl, rTth, Tli, Tma, Bst, 9°Nm, Vent, or Phusion polymerase. Additional polymerases suitable for primer extension will be apparent to those of skill in the art based on the present disclosure, which is not limited in this respect.
[0047] The term "primer hybridization site" refers to a nucleotide sequence that a primer can hybridize to. A primer hybridization sites may be part of a nucleic acid template, e.g., a target nucleic acid molecule. The primer hybridization site may be 100% homologous to the primer sequence, or may be less than 100% homologous (e.g., 99.9%, 99%, 98%, 97%, 96%, 95%, 90%, 85% homologous). The length and sequence of a primer hybridization site is dependent on the specific application. Length and nucleotide sequence can impact PCR parameters such as annealing temperature and cycle length. Usually, a primer hybridization site is between 10-40 bases long. In some embodiments, a primer hybridization site may be shorter or longer than that, depending on primer sequence and intended hybridization parameters. Methods to design primers for annealing and extension in view of hybridization and extension parameters
and methods of adapting hybridization and extension conditions in view of specific primer length and/or sequence are well known in the art.
[0048] The term "array" or "oligonucleotide array" refers to a plurality of
oligonucleotide primers or probes conjugated to a solid support, e.g., to a membrane or glass surface. In some embodiments, an array used for detecting a variant allele of the CPD gene may be used for detecting a CPD SNP disclosed herein. Accordingly, in some such embodiments, an array may be a SNP array, comprising, for example, a set of probes that can detect the presence of a variant allele of the CPD gene as disclosed herein.
[0049] The term "nucleic acid variant," "polymorphism," or "variant allele" refers to one of two or more alternative nucleotide sequences or alleles in a given population, e.g., to one of two or more alleles of a gene (e.g., the CPD gene) in a population of human subjects. A
"polymorphic site" is the locus at which sequence divergence occurs. A polymorphic site in an allele, e.g., of a gene or genomic region, results in the existence of at least two alleles of the respective gene or genomic region. A diallelic polymorphism correlates to two alleles. A triallelic polymorphism correlates to three alleles. Diploid organisms may be homozygous or heterozygous for allelic forms. A polymorphic site may be as small as one nucleotide. Such polymorphic sites that comprise a single nucleotide are also sometimes referred to as a single nucleotide polymorphism (SNP). Additional, non-limiting examples of polymorphic sites include: restriction fragment length polymorphisms (RFLPs), variable number of tandem repeats (VNTRs), hypervariable regions, minisatellites, dinucleotide repeats, trinucleotide repeats, tetranucleotide repeats and simple sequence repeats. In some embodiments, reference to a "polymorphism" can also encompass a set of polymorphisms (i.e., a haplotype).
[0050] The term "contacting," as used herein, refers to bringing a first molecule, for example, a nucleic acid molecule (e.g., a genomic nucleic acid molecule), and a second molecule, for example, a second nucleic acid molecule (e.g. a primer or probe), together in a manner that the molecules can bind, hybridize, or otherwise interact with each other. Contacting may be accomplished in a liquid medium, for example, in a reaction or hybridization buffer under suitable conditions. Suitable conditions will be apparent to those of skill in the art based on the present disclosure and knowledge in the art. The disclosure is not limited in this respect.
[0051] The term "melting temperature" (Tm) is an art-recognized term and refers to the temperature at which hybridization of two nucleotide strands is destabilized so that the two
nucleotide strands separate (or dissociate). In PCR, the melting temperature is the temperature at which a primer hybridized to a template dissociates from the template.
[0052] The term "polymerase chain reaction" (PCR) is an art recognized term and refers to a method of amplifying a nucleic acid molecule. PCR uses thermal cycling, consisting of cycles of repeated heating and cooling of a PCR sample including the nucleic acid molecule(s) to be amplified. A typical PCR cycle includes a denaturation (or melting) step, an annealing step, and an elongation (or extension) step. A typical PCR includes between 12 and 40 cycles. A PCR may further include an initialization step, for example, if each activation of a hot start polymerase is performed, a hold step, a final extension or hold step, and a final cooling step. PCR reagents include a buffer, for example, a buffer including Mg2+ ions, one or more primers, nucleotides, and a thermophilic polymerase, for example, Taq, Pfu, Pwo, Tfl, rTth, Tli, Tma, Bst, 9°Nm, Vent, or Phusion polymerase. A PCR product also sometimes referred to as an
"amplicon," is a nucleic acid generated as a result of a PCR. PCR protocols are well known in the art, for example, as described in Chapter 8 ("In vitro amplification of DNA by the
polymerase chain reaction") of Sambrook et al., Molecular Cloning: A laboratory Manual, Volumes 1-3, Cold Spring Harbor Laboratory Press, 2001. Reagents and reagent kits for PCR are available from numerous commercial suppliers. The term "quantitative PCR" (qPCR) refers to a method used to measure the quantity of a PCR product. If the quantity of a PCR product is measured in real time, the method is referred to as "quantitative, real-time PCR".
[0053] The term "suitable conditions," interchangeably used with the term "conditions suitable," refers to conditions that are suitable for a specific hybridization, reaction, interaction, or other event to take place. For example, conditions suitable for a primer or probe to hybridize to a target sequence may include a suitable medium allowing both primer or probe and target nucleic acid molecule to interact, for example, an aqueous and, if necessary, a buffering agent, a certain temperature, pH, or osmolarity. The suitable conditions for any given hybridization, reaction, or interaction will, of course, depend on the specific reaction or interaction. Suitable conditions for the reactions or interactions described herein are well known to those in the relevant chemical and molecular biological arts. For example, suitable conditions for nucleic acid hybridization, primer extension, restriction digestion, and linker ligation are described herein and in Sambrook et al., Molecular Cloning: A Laboratory Manual, Volumes 1-3, Cold Spring Harbor Laboratory Press, 2001, incorporated herein by reference. Further, suitable conditions for
various chemical reactions are described herein and, for example, in Smith and March, March 's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Wiley-Interscience, 6th edition, 2007, incorporated herein by reference.
[0054] The term "single nucleotide polymorphism (SNP)" refers to a polymorphic site occupied by a single nucleotide, i.e., a site of genetic variation within a population that affects a single nucleotide. The different nucleotides occurring at the site of polymorphism, e.g., A, T, G, or C, determine the allele of the polymorphic site (the different sequences with respect to the polymorphism are referred to as an allele). Many known SNPs do not have four alleles, but only two alleles. Frequently, the SNP site is preceded, and followed, by highly conserved sequences of the allele. SNPs are the most frequently observed type of genetic variation in a population. SNPs appear when a single nucleotide is altered, either by substitution, addition or deletion. A SNP usually arises due to substitution of one nucleotide for another at the polymorphic site. Replacement of one purine by another purine or one pyrimidine by another pyrimidine, is called a transition. Replacement of a purine by a pyrimidine or vice versa is called a transversion. A synonymous SNP refers to a substitution of one nucleotide for another in the coding region that does not change the amino acid sequence of the encoded polypeptide. A non-synonymous SNP refers to a substitution of one nucleotide for another in the coding region that changes the amino acid sequence of the encoded polypeptide. A SNP may also arise from a deletion or an insertion of a nucleotide or nucleotides relative to a reference allele. As used herein, the term "cluster" of SNPs refers to three or more SNPs that occur within 100 kilobases of each other in a particular polymorphic site, wherein all of the SNPs have a p-value e4 ( < 1 x 10"4).
[0055] The term "haplotype" refers to the designation of a set of polymorphisms or alleles of polymorphic sites within a gene of an individual. For example, a "112" haplotype might refer to a gene comprising allele 1 at each of the first two polymorphic sites and allele 2 at the third polymorphic site. A "diplotype" is a haplotype pair. A haplotype can be a set of SNPs on a single chromosome of a chromosome pair that are associated statistically. It is frequently observed that certain combinations of SNP alleles occur more frequently in a population than would be expected based on a mere statistical distribution. Such multi-SNP alleles thus include a number of associated SNP alleles. It is thought that such SNP associations, and the identification of a few alleles of a haplotype sequence, can unambiguously identify all other polymorphic sites in its region in most individuals in a population.
[0056] The term "isolated," as used herein, e.g., in the context of isolated nucleic acids, refers to a nucleic acid molecule that has been removed from its natural environment, such as, a genomic nucleic acid molecule that has been removed from its cellular or tissue environment, or has been purified in any way. A purified nucleic acid can comprise (on a molar basis) at least about 50, 80 or 90% of all macromolecular or nucleic acid species present.
[0057] The term "reference nucleotide sequence" used interchangeably with the term
"reference sequence" is herein defined to be for a nucleotide sequence of a particular gene, for example, in NCBI databases (www.ncbi.nlm.nih.gov). Alleles that differ from the reference are referred to as "variant" alleles. The polypeptide encoded by the reference nucleotide sequence is the "reference" polypeptide with a particular reference amino acid sequence, and polypeptides encoded by variant alleles are referred to as "variant" polypeptides with variant amino acid sequences. Nucleotide sequence variants can result in changes affecting properties of a polypeptide.
[0058] The term "susceptibility", "predisposition", "propensity" and "risk" refer to either an increased or decreased likelihood of an individual developing a disorder (e.g. a condition, illness, disorder or disease) relative to a reference likelihood observed or expected in a control population. In some embodiments, the control population may comprise individuals in the population (e.g., matched by age, gender, race, ethnicity, and/or body weight) that are healthy, that are not obese, or that do not have the genotype or phenotype assayed for. In some embodiments, the reference likelihood is an average likelihood of developing a disease (e.g., obesity) for the entire population (e.g., matched by age, gender, race, ethnicity, and/or body weight), without respect to the health or genetic status of the individuals comprised in the population.
[0059] The term "diagnosis" refers to the detection of a disease, syndrome, disorder or condition in a subject, or to distinguishing or identifying a person having or at risk for developing a particular disease, syndrome, disorder or condition. In some embodiments, the terms "diagnosing" and "screening" are used interchangeably (e.g. a person skilled in the art can diagnose a propensity to develop the disease).
[0060] The terms "prognosis" refers to a prediction of an outcome of a disease, syndrome, disorder or condition.
[0061] The term "screen" or "screening" as used herein includes processes intended for the identification of subjects having a specific genotype or phenotype as well as to diagnosing a disease in a subject, or determining the susceptibility, propensity, or risk of an asymptomatic subject for developing a disorder. Screening also includes the prognosis of a subject, i.e., when a subject has been diagnosed with a disorder, determining in advance the progress of the disorder as well as the assessment of efficacy of therapy options to treat a disorder.
[0062] The term "anti-obesity agent", as used herein, refers to a drug that is known to reduce the total body weight of a subject by at least 5% within a month after treatment with the drug has commenced. In some embodiments, an anti-obesity agent is thus an agent that decreases energy intake, and/or that increases energy expenditure or weight loss.
[0063] The term "portion", "fragment" and/or "truncated form", when used in reference to a gene or gene product, e.g., a CPD gene or a gene product encoded by the CPD gene, refers to a nucleic acid or polypeptide sequence that is less than the full-length sequence (e.g.,. less than the full-length CPD gene or the transcript or protein encoded by the full-length CPD gene). A portion or fragment or truncated form of a CPD gene or CPD gene product may be at least 25, at least 50, at least 75, at least 100, at least 150, at least 200, at least 250, or at least 300
nucleotides, codons, or amino acids in length.
[0064] The term "gene" refers to a linear sequence of nucleotides along a segment of
DNA that provides the coded instructions for expression of at least one gene product. In some embodiments, a gene is comprised within genomic DNA, for example, as part of a chromosome. In some embodiments, the term "gene" includes not only the sequence encoding the gene product (e.g., RNA or protein), but also proximate chromosomal sequences that control expression or are otherwise associated with the coding sequence, such as , for example, the introns and exons of the coding sequence, the regulatory regions such as the promoter region and possible other regulatory sequences, such as 5' UTR, 3' UTR. In some embodiments, sequences further up- or downstream of the gene, such as enhancers in cis are also included in the term..
[0065] The term "gene product" refers to a transcript, e.g., a primary transcript, a processed transcript (e.g. an mRNA), or a protein that is encoded by the gene. A "protein coding region" is a region of a DNA or RNA sequence that encodes a polypeptide or protein.
[0066] The term "assay" refers to a procedure for determining the presence, absence, or quantity of a target substance, e.g., of a target nucleic acid or gene product, by detecting or
measuring the quantity of the target substance in a sample, e.g., in a sample obtained from a subject or derived from a biological sample obtained from the subject.
[0067] The term "sample" or "biological sample" refers to a sample of tissue or fluid isolated from a subject, including, but not limited to, tissue biopsy, plasma, serum, whole blood, spinal fluid, lymph fluid, the external sections of the skin, respiratory, intestinal and
genitourinary tracts, tears, saliva, milk, urine, blood cells, tumors, organs. Also included are samples of in vitro cell culture constituents (including, but not limited to, conditioned medium resulting from the growth of cells in culture medium, putatively virally infected cells, recombinant cells, and cell components).
DETAILED DESCRIPTION
Introduction
[0068] Typically, obesity is caused by an energy imbalance between calories consumed and calories expended. Over the past years, advances in molecular biology techniques have allowed new developments relating to obesity etiology. While obesity was first thought to be caused by environmental and life style choices or by single gene mutations (monogenic obesity), it is now believed that the majority of cases are more complex and multifactorial, involving environmental and genetic factors mediated by numerous genes which could contribute to the phenotype of polygenic obesity (Mutch and Clement, 2006).
[0069] Identifying the genes involved in obesity could thus assist in furthering understanding of how the associated phenotype arises. The identification of obesity-associated genes would provide new therapeutic targets, and help to identify an optimal drug for a given individual harboring a specific genotype - the basis of personalized medicine.
[0070] To date, three genetic variants known as single nucleotide polymorphisms (SNPs) have been documented as associated with an overweight or obese phenotype in Asian populations: the SNP rs662799 in the APOA5 gene; the SNP rs6235, in PCSK1 gene; and the SNP rs2236518 in the of PRDM16 gene.
[0071] The effect of the SNP, rs662799, in APOA5 (the gene encoding Apolipoprotein
A-V in humans) on central obesity was evident only in obese males, and was not observed in obese females or non-obese participants. This study highlighted the gender-specific and weight- sensitive effects of APOA5 rs662799 on central obesity in Taiwanese individuals, and showed
that these effects are dyslipidemia- independent and weight-loss responsive. Though APOA5 rs662799 polymorphism is unfavorable in obese patients, this predicament is improved prominently by weight management (Hsu et al, 2013).
[0072] Another study reports that the SNP, rs6235, in PCSKl (the gene encoding proprotein convertase subtilisin/kexin type 1 in humans) exhibits a significant association with being overweight among male subjects, but not among female subjects. PCSKl rs6235 may contribute to the risk of being overweight in men and predict obesity-related metabolic traits such as waist circumference and diastolic blood pressure in Taiwanese individuals (Hsiao et al, 2013).
[0073] A third study investigated the contribution of genetic variants of PRDM16 (the gene encoding PR domain containing 16 in humans) to obesity. rs2236518 was the only SNP associated with BMI in males. No significant associations were detected in females, nor was a relationship found between any haplotype and obesity-related phenotypes (Yue et al, 2013).
[0074] The obese phenotype conferred by the SNP rs662799 of the APOA5 gene is manageable with a restricted diet, and the SNP rs6235 of the PCSKl gene is linked to the risk of being overweight only, but not to being obese. No SNPs have yet been reported to confer a risk for severe cases of obesity, e.g., for morbid obesity, or for cases of obesity where controlled diet and exercise may not have any substantive therapeutic effect.
[0075] The identification of genetic causes of severe obesity or obesity that cannot easily be treated by lifestyle changes, as provided in the present disclosure, is thus useful for identifying suitable drug targets and suitable drugs for treating such cases of obesity.
Identification of the CPD Gene as a Target in the Treatment of Obesity
[0076] Some aspects of this disclosure are based on the discovery that the CPD gene contributes strongly to obesity in males, as illustrated in the work described in the Examples section. In particular, a correlation between certain polymorphic sites in the CPD gene and the likelihood of developing obesity has been discovered in male subjects. This is surprising, given that CPD currently has an unknown role in lipid metabolism.
[0077] The carboxypeptidase D (CPD) gene encodes a metallocarboxypeptidase enzyme.
The CPD gene is located in the 17ql 1.2 chromosomal region and has been found to encompass approximately 88.3 kb of genomic sequence (see, e.g., Timblin et al., 2002, the entire contents of
which are incorporated herein by reference). The gene can express multiple transcript variants as a result of alternative polyadenylation signals (Timblin et al., 2002). The
metallocarboxypeptidase enzyme family can be divided into 2 subfamilies based on sequence similarities: the pancreatic carboxypeptidase-like and the regulatory B-type carboxypeptidase subfamilies. Carboxypeptidase D has been identified as a regulatory B-type carboxypeptidase. The CPD gene is a homolog of duck gpl80, a hepatitis B virus binding protein.
[0078] CPD structural data, including transcript, cDNA, and amino acid sequence data for human CPD, are well known to those of skill in the art and include, without limitation, the entries in the EMBL/GenBank Data Libraries under accession number U65090. In human, two protein isoforms have been reported: Isoform 1, as described, e.g., by mRNA accession number NM_001304.4 and protein accession number NP_001295.2, and Isoform 2, as described, e.g., by mRNA accession number NM_001199775.1 and protein accession number NP_001186704.1. An exemplary gene sequence for human CPD is found under GenBank Gene ID 1362, and is shown in SEQ ID NO: 1. A diagrammatic representation of the exon structure of the human CPD gene is provided in Figure 1.
[0079] Those of ordinary skill in the art will understand that the sequences provided herein, including those related to the GenBank database entries above, are representative of some wild-type CPD sequences occurring in humans, but are not meant to limit the term "CPD" or the scope of this disclosure. It will also be understood that additional wild-type CPD sequences exist, and such additional sequences will be apparent to those of skill in the art based on the present disclosure and the knowledge in the art. Such CPD sequences include, for example, those CPD sequences referred to in Garcia-Pardo J, et al. , Amyloid formation by human carboxypeptidase D transthyretin-like domain under physiological conditions. J Biol Chem, (2014); Thomas LN, et al., Carboxypeptidase-D is elevated in prostate cancer and its anti- apoptotic activity is abolished by combined androgen and prolactin receptor targeting. Prostate (2014); Koirala S, et al., Prolactin/Stat5 and androgen R1881 coactivate carboxypeptidase-D gene in breast cancer cells. Mol Endocrinol (2014); Jin T, et al., SiRNA-targeted
carboxypeptidase D inhibits hepatocellular carcinoma growth. Cell Biol Int (2013); Abdelmagid S A, et al. , and Prolactin and estrogen up-regulate carboxypeptidase-d to promote nitric oxide production and survival of mcf-7 breast cancer cells. Endocrinology (2008), the entire contents of each of which are incorporated herein by reference.
[0080] It will be understood that while some embodiments of the present disclosure relate to the CPD sequences provided herein, some embodiments embrace nucleic acid sequences that are homologous to the provided sequences, e.g., CPD sequences in species other than human, or to sequence variants (e.g., to variant alleles of the CPD gene), or sequences derived from or analogous to the sequences provided herein, e.g., to sequences comprising substantially identical to any of the CPD sequences provided herein. Such sequences include functional variants and functional fragments of the CPD sequences provided herein. In some embodiments, the term "substantially identical" to a CPD sequence provided herein refers to a sequence that is at least 70%, at least 80%, at least 90%, at least 92%, at least 95%, at least 97%, at least 98%, or at least 99% identical to any of the CPD sequences provided herein.
[0081] Little is known regarding the expression, localization, regulation or role of CPD.
It is widely distributed, suggesting a broad role for the enzyme. This 180-kDa membrane -bound metalloproteinase cleaves C-terminal arginine and lysine residues (Skidgel and Erdos, 1998). Intracellularly, CPD is found mostly in the trans-Golgi network, where it processes polypeptides or pro-hormones that transit the secretory pathway. It is also present in the plasma membrane (Varlamov et al. 1998) and cell nucleus (O'Malley et al., 2005). The enzymatic properties of the soluble and membrane forms are very similar.
[0082] Unlike carboxypeptidase E (CPE) and all other members of the
metallocarboxypeptidase gene family, CPD is unique in that it contains multiple
carboxypeptidase domains. Human, rat, mouse, and duck CPD contain three carboxypeptidase- like domains followed by a transmembrane domain and a 58-residue cytosolic tail. Of the three carboxypeptidase-like domains, only the first two have enzyme activity toward standard substrates. Several of the key catalytic residues are missing from the third carboxypeptidase-like domain of human, rat, and duck CPD, consistent with the observed lack of enzyme activity toward standard carboxypeptidase substrates (Sidyelyeva et al., 2002). The physiological function of the inactive domain 3 remains unclear, but it may function as a binding protein, as this domain of duck CPD was shown to bind the duck hepatitis B-virus pre-S protein (Eng et al., 1998). The enzymatic properties of the first and second domains of CPD differ in several aspects: optimum pH and substrate specificity. Although both domains are highly specific for C- terminal basic amino acids, the first domain prefers C-terminal Arg, whereas the second domain
prefers C-terminal Lys. The combination of the two domains therefore results in an enzyme with a broader range of activity than either domain alone (Sidyelyeva et al., 2002).
[0083] A 2011 study described a potential link between CPD and CPE on type 2 diabetes. Insulin is processed from its pro-hormone precursor by a series of enzymes. The final step in insulin processing involves the removal of C-terminal amino acid residues by CPE, which is known to be widely distributed in the brain, pituitary and neuroendocrine tissues. Mice lacking CPE are obese and glucose intolerant. It should be noted that mice lacking CPE do not die from diabetes and still produce a small amount of mature insulin, suggesting that alternative pro-insulin processing enzymes exist in the pancreatic β-cell. The most likely candidate for this compensation is the related enzyme, CPD. Nonetheless, it was demonstrated that the expression and sub-cellular localization of CPE and CPD are regulated by distinct extracellular signals; that CPD was significantly upregulated by elevated glucose, while CPE was not; and that low doses of insulin also increased CPD protein levels, consistent with a role for autocrine signaling. Surprisingly, the loss of CPE did not affect the levels of CPD. Knockdown of CPD exerted no effect on CPE protein levels. In conclusion, CPD is a poor surrogate for CPE in the pancreatic β- cell. The precise function of CPD in these cells remains uncovered (Chu et al., 2011). Furthermore, the role of CPD in trafficking, first identified in ducks as the Hepatitis B virus receptor, suggests that this protein may be more than a peptide -processing enzyme (Eng et al., 1998).
Variant alleles of the CPD Gene associated with Obesity
[0084] It has now surprisingly been discovered that mutations or nucleic acid variants in the CPD gene that are associated with an impaired or decreased expression level or function of the CPD protein, including SNPs such as those disclosed herein, can be used as nucleic acid biomarkers of obesity, e.g., of male obesity. This includes, for example, any mutations that lead to a frameshift in the reading frame of the CPD gene encoding the CPD protein, nonsense mutations that result in a premature stop codon or a nonsense codon in the transcribed mRNA, and thus in a truncated protein product, and missense mutations that result in an amino acid substitution which negatively affects protein function. In some embodiments, impairing mutations also include, without limitation, gene fusions, deletions, duplications, or inversions of nucleotide sequences, mutations resulting in alternative splice acceptor sites, resulting in aberrant
splicing patterns, and mutations in nucleotide sequences controlling the expression of the CPD gene, such as mutations in transcription factor binding sites, or in enhancer and promoter sequences. Additional mutations that impair or decrease the function of a protein will be known to those of skill in the art and the present disclosure is not limited in this respect.
[0085] In some embodiments, the mutations or nucleic acid variants used as biomarkers for obesity are SNPs. In general, the genomic distribution of SNPs is not homogenous, but occurring in non-coding regions (DNA between genes) more frequently than in coding ones. In that instance SNPs act as biological markers, helping locate genes associated with disease. When SNPs occur within a gene or in a regulatory region, it can lead to the expression of a different protein form, thereby playing a direct role in disease. Nevertheless, even when SNPs occur in non-coding regions, they could be responsible for differential or alternative splicing, or altered protein expression levels (Sachidanandam et al., 2001).
[0086] SNPs are identified herein according to their reference SNP identity number
("rs...") assigned by the dbSNP database of the National Center for Biotechnology Information (NCBI), either GRCh37.13 (annotation release 105) or GRCh 38 (annotation release 106), the entire contents of each of which are incorporated herein by reference.
[0087] Numerous SNPS in the CPD gene have been reported. Exemplary CPD SNPs, including synonymous SNPs (having no effect on the amino acid sequence of the expressed CPD protein), missense SNPs, and nonsense SNPs, are listed in Table 6.
[0088] Some aspects of this disclosure are based on the surprising discovery that intronic
SNPs, i.e., variant alleles that affect an intronic sequence of the CPD gene, can be used as biomarkers for obesity, e.g., for male obesity. Some exemplary intronic SNPs that are provided herein as biomarkers for obesity, e.g., for male obesity, include, but are not limited to, the SNPs shown in Table 2.
rs4795551 17: 28,750,942 T/A Intergenic/Unknown rs9913237 17: 28,760,939 G/C Intergenic/Unknown rs2253256 17: 28,778,720 C/G Intergenic/Unknown rs6505188 17: 28,784,382 G/A Intergenic/Unknown rs9911455 17: 28,788,586 G/A Intergenic/Unknown rs9406 17: 28,794,020 G/A Intergenic/Unknown
*according to NCBI Assembly Build Number 37 (GRCh37.13, Annotation Release 105, forward strand); ** ancestral allele on the left
Table 2. Exemplary SNPs within intronic regions of the CPD gene that are associated with obesity
[0089] The dbSNP database entry summaries (GRCh 38, annotation release 106) for each or the SNPs listed in Table 2 are provided below. The entire contents of the full dbSNP database entry for each of these SNPs are incorporated herein by reference.
[0090] s9913111 [Homo sapiens]
TTGTTTGTTTGTTTCGCTCACAGAT[A/G]TATCTATCCCTGGAATGTCCTTTGG
Chromosome: 17:30382868
Gene: CPD (GeneView)
Functional Consequence: intron variant
Validated: by 1000G,by 2hit 2allele, by cluster, by frequency, by hapmap
Global MAF: G=0.4347/2177 HGVS: NC_000017.10:g.28709886G>A,
NC_000017.11 :g.30382868G>A, NM_001199775. l :c.6-
2121 G>A, NM_001304.4: c.747-2121 G>A
[0091] rs719601 [Homo sapiens]
CAAAAATATTTGCCTTGTATATGCC[A/G]TTTTATAACCTTTTTTGATGCATGG
Chromosome: 17:30404397
Gene: CPD (GeneView)
Functional Consequence: intron variant
Validated: by 1000G, by 2hit 2allele, by cluster, by frequency
Global MAF: G=0.4065/2036 HGVS: NC_000017.10:g.28731415G>A,
NC_000017.11 :g.30 04397G>A, NM_001199775.1 :c.254- 16444G>A, NM_001304.4:c.995- 16444G>A
[0092] s2041374 [Homo sapiens]
GGTTGAAATTGGAGCAGAGAGCTGG[A/G]GTTGAGGGAGTTAGTCAAGTAGTAA Chromosome: 17:30413905
Gene: CPD (GeneView)
Functional Consequence: intron variant
Validated: by 1000G, by 2hit 2allele, by cluster, by frequency, by hapmap
Global MAF: G=0.4054/2030 HGVS: NC_000017.10:g.28740923G>A,
NC_000017.11 :g.30413905G>A, NC_000017.9:g.25765049G>A, NM_001 199775. l:c.254-6936G>A, NM_001304.4:c.995- 6936G>A
[0093] rs4343337 [Homo sapiens]
GGCTTTTTGCTTCCAATCCCCCACG[C/G]CTTTCCAGACCATACTGTCTTCTAT Chromosome: 17:30417754
Gene: CPD (GeneView)
Functional Consequence: intron variant
Validated: by 1000G, by 2hit 2allele, by cluster, by frequency, by hapmap
Global MAF: C=0.4303/2155 HGVS: NC_000017.10:g.28744772C>G,
NC_000017.11 :g.30417754C>G, NC_000017.5:g.28596642C>G, NM_001199775. l:c.254-3087C>G, NM_001304.4:c.995- 3087C>G
[0094] s4795551 [Homo sapiens]
GTGTCTTCCACTTGGTAATGGTTCT[A/T]ATTTACTAGAAATAGAGTATAAGAC Chromosome: 17:30423924
Gene: CPD (GeneView)
Functional Consequence: intron variant
Validated: by 1000G, by 2hit 2allele, by cluster, by frequency, by hapmap
Global MAF: A=0.3181/1593 HGVS: NC_000017.10:g.28750942A>T,
NC_000017.11 :g.30423924A>T,
ΝΜ_001 199775.1 : c.1108+227 A>T,
NM_001304.4:c.1849+227 A>T
[0095] s9913237 [Homo sapiens]
TTG ATCCC ACTGC ATTCTGCCTCCT [C/G] G A ATCTTGTGTCTTC ATATCTT ATT
Chromosome: 17:30433921
Gene: CPD (Gene View)
Functional Consequence: intron variant
Validated: by 1000G, by 2hit 2allele, by cluster, by frequency, by hapmap
Global MAF: C=0.4038/2022 HGVS: NC_000017.10:g.28760939C>G,
NC_000017.11 :g.30433921C>G,
NMJX) 1199775.1 : c.1386+2040C>G,
NM_001304.4:c.2127+2040C>G
[0096] rs2253256 [Homo sapiens]
GCTAGTAACTCGTTAATTTCTGTTT[C/G]TGCTTCAGTTGGTTGACAGGACTAG
Chromosome: 17:30451702
Gene: CPD (GeneView)
Functional Consequence: intron variant
Validated: by 1000G, by cluster, by frequency, by hapmap, by submitter
Global MAF: G=0.3187/1596 HGVS: NC_000017.10:g.28778720G>C,
NC_000017.11 :g.30451702G>C, NM_001 199775.1 :c.2329-9G>C,
NM_001304.4:c.3070-9G>C
[0097] rs6505188 [Homo sapiens]
CATTTTATTTGTTAATTCATGTGTC[A/G]ATGGGCACTTGGATTTCTACTTTTT
Chromosome: 17:30457364
Gene: CPD (GeneView)
Functional Consequence: intron variant
Validated: by 1000G, by 2hit 2allele, by cluster, by frequency Global
MAF: A=0.3181/1593 HGVS: NC_000017.10:g.28784382A>G,
NC_000017.11 :g.30457364A>G, NC_000017.5:g.28636252A>G,
NM_001199775. l:c.2757+838A>G,
NM 001304.4:c.3498+838A>G
[0098] s9911455 [Homo sapiens]
GAATATCACAGAGTTACTGGAGTCA[A/G]TATACCATGGTGGGAAGGAAATCAC Chromosome: 17:30461568
Gene: CPD (GeneView)
Functional Consequence: intron variant
Validated: by 1000G, by 2hit 2allele, by cluster, by frequency Global
MAF: G=0.4347/2177 HGVS: NC_000017.10:g.28788586G>A,
NC_000017.11 :g.30461568G>A,
NM_001199775. l:c.2889+257G>A,
NM_001304.4:c.3630+257G>A
[0099] rs9406 [Homo sapiens]
GGAAAACAAGACTTTCCCAGCTTGT[A/G]TTACCTAGAAGCGTGAATGTATAGG Chromosome: 17:30467002
Gene: CPD (GeneView)
Functional Consequence: utr variant 3 prime
Validated: by 1000G, by 2hit 2allele, by cluster, by frequency, by hapmap
Global MAF: A=0.3167/1586 HGVS: NC_000017.10:g.28794020A>G,
NC_000017.11 :g.30467002A>G, NM_001199775. l :c.*2188A>G,
NM_001304.4:c.*2188A>G
[00100] The association between the nucleic acid variants within the CPD gene provided herein and obesity, as demonstrated herein for the first time, e.g., the association between the intronic SNPs provided in Table 2 and male obesity, is unexpected. The work presented herein provides the first strong genetic risk factor for male obesity found on chromosome 17. The discovery that nucleic acid variants in the CPD gene are associated with male obesity has a number of specific applications, including the identification of subjects to ascertain risk of developing obesity and identification of new and optimal therapeutic approaches for individuals afflicted with, or at increased risk of developing, obesity. Applications and uses of the association are described herein.
Compositions, compounds, and agents
[00101] Some aspects of the present disclosure are based on the unexpected discovery that certain forms of obesity, e.g., of male obesity, that are characterized by the presence of one or more nucleic acid variants in the CPD gene, can be treated with an ACE inhibitor, for example, with a dicarboxylate-containing ACE inhibitor. Accordingly, the present disclosure provides compositions, methods, and strategies for the treatment of obesity in a specific patient population with a dicarboxylate-containing ACE inhibitor. In some embodiments, the patient population includes males harboring a variant allele of the CPD gene, e.g., a variant allele comprising a SNP listed in Table 2 and/or a SNP listed in Table 6. In some embodiments, the dicarboxylate- containing ACE inhibitor is perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for use in the treatment of male obesity.
[00102] Some aspects of the present disclosure are based on the unexpected discovery that certain forms of obesity, e.g., of male obesity, that are characterized by the presence of one or more nucleic acid variants in the CPD gene, can be treated with the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). Accordingly, the present disclosure provides compositions, methods, and strategies for the treatment of obesity in a specific patient population with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). In some embodiments, the patient population includes males harboring a variant allele of the CPD gene, e.g., a variant allele comprising a SNP listed in Table 2 and/or a SNP listed in Table 6.
[00103] As demonstrated in Example 4, it has surprisingly been found that the
dicarboxylate-containing ACE inhibitor perindopril can be used to treat obesity in male individuals. Some aspects of this disclosure, accordingly, provide perindopril, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, as well as compositions comprising perindopril, or a pharmaceutically acceptable salt,
stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for the treatment of obesity, e.g., male obesity.
[00104] ACE (angiotensin-converting-enzyme) inhibitors are widely known and used for the treatment of hypertension and congestive heart failure. In this regard, ACE is a component of the blood pressure -regulating renin-angiotensin system; the inhibition of ACE thus decreases the tension of blood vessels and the blood volume, thereby lowering blood pressure. ACE inhibitors have also been found useful in other cardiovascular and renal diseases, including acute myocardial infarction and renal failure.
[00105] These inhibitors can be divided into three groups based on their molecular structure. Dicarboxylate-containing ACE inhibitors make up the largest group, and these include, but are not limited to, enalapril, ramipril, quinapril, perindopril, lisinopril, benazepril, imidapril, trandolapril and cilazapril.
[00106] Perindopril is a compound as provided by Formula (I):
[00107] Perindopril is available, for example, under the brand names: Aceon, Acertil,
Actiprex, Armix, Coverene, Coverex, Coversum, Prenessa, Prestarium, Prexanil, Prexum, Procaptan, Provinace, Covinace, and Pericard. Some embodiments of this disclosure provide pharmaceutical preparations for the treatment of male obesity that comprise perindopril in a form resembling that or perindopril in the compositions provided under any of these brand names. Some embodiments of this disclosure provide methods using a composition available under any of these brand names for the treatment of obesity, e.g., male obesity.
[00108] Perindopril is the free acid form of perindopril erbumine or perindopril arginine, both of which are available for oral administration. Perindopril is a pro-drug, which is metabolized in the liver by hydrolysis of the ester group to form perindoprilat, the biologically active metabolite (i.e. the ACE inhibitor). In humans, hepatic esterases appear to be responsible for the hydrolysis of perindopril.
[00109] ACE inhibition, e.g., by perindoprilat, has two main effects: it inhibits angiotensin
II formation and potentiates bradykinin. Angiotensin II is a potent vasoconstrictor and a negative feedback mediator for renin activity. Thus, as a result of lower angiotensin II plasma levels, blood pressure decreases and plasma renin activity increases. Decreases in plasma angiotensin II levels reduce aldosterone secretion, with a subsequent decrease in sodium and water retention. It also dilates arterioles, thereby lowering total peripheral vascular resistance.
[00110] As with other ACE inhibitors, perindopril' s side effects are non-serious, including coughing, dizziness, back pain, drug intolerance, hypotension, fatigue, asthenia, headache, disturbances of mood and/or sleep and - very rarely - taste impairment, epigastric discomfort, nausea, abdominal pain, rash, increase in blood urea and creatinine, angioneurotic edema and decreases in hemoglobin, red cells and platelets.
[00111] Therefore, perindopril's suitability for oral administration and its low toxicity, as observed over several years as a consequence of perindopril being on the market, make it suitable for chronic use, and thus for treating obesity.
[00112] In some embodiments, the present disclosure provides compositions comprising a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for administration to an obese subject carrying a variant allele of the CPD gene as provided herein. In some embodiments the composition is a pharmaceutical composition for administration to a male subject having class I, class II, or class III obesity, and carrying a variant allele of the CPD gene as described herein, e.g., an allele characterized by one or more SNPs listed in Table 2.
[00113] In some embodiments, the present disclosure provides compositions comprising a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), for administration to an obese
subject carrying a variant allele of the CPD gene as provided herein. In some embodiments the composition is a pharmaceutical composition for administration to a male subject having class I, class II, or class III obesity, and carrying a variant allele of the CPD gene as described herein, e.g., an allele characterized by one or more SNPs listed in Table 2.
[00114] The compositions provided herein comprise an effective amount of the active ingredient (e.g., of a dicarboxylate-containing ACE inhibitor) for the treatment of obesity in a subject, e.g., a male subject exhibiting class I, class II, or class III obesity. In some embodiments, the compositions comprise an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for the treatment of obesity in a subject. In some embodiments, an effective amount is the amount which, when administered to an obese subject, is sufficient to treat the obesity, or to produce a certain amount of weight loss within a certain time frame.
[00115] The compositions provided herein comprise an effective amount of the active ingredient (e.g., of a dicarboxylate-containing ACE inhibitor) for the treatment of obesity in a subject, e.g., a male subject exhibiting class I, class II, or class III obesity. In some embodiments, the compositions comprise an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), for the treatment of obesity in a subject. In some embodiments, an effective amount is the amount which, when administered to an obese subject, is sufficient to treat the obesity, or to produce a certain amount of weight loss within a certain time frame.
[00116] For example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, may refer to an amount that is sufficient to induce a measurable weight loss in an obese subject. For example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof refers to the amount sufficient or the amount required to induce the loss of at least 5%, at least 10%, at least 15%, at least 20%, at least 25, at least 30%, at least 35%, at least 40%, at least 45%, or at least 50%, of body weight within a certain time frame after treatment has commenced, e.g., within 1
month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 1 1 months or 12 months, 18 months, or 24 months after treatment has commenced.
[00117] For example, in some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), or solvate thereof, may refer to an amount that is sufficient to induce a measurable weight loss in an obese subject. For example, in some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) refers to the amount sufficient or the amount required to induce the loss of at least 5%, at least 10%, at least 15%, at least 20%, at least 25, at least 30%, at least 35%, at least 40%, at least 45%, or at least 50%, of body weight within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 1 1 months or 12 months, 18 months, or 24 months after treatment has commenced.
[00118] In some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject, e.g., a reduction by at least 1 , at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least 11 , at least 12, at least 13, at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23, at least 24, at least 25, at least 26, at least 27, at least 28, at least 29, or at least 30 points within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced.
[00119] In some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof;
a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject, e.g., a reduction by at least 1 , at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least 11 , at least 12, at least 13, at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21, at least 22, at least 23, at least 24, at least 25, at least 26, at least 27, at least 28, at least 29, or at least 30 points within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 1 1 months or 12 months, 18 months, or 24 months after treatment has commenced.
[00120] In some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject resulting in the subject's BMI being classified in a WHO BMI class that is lower than the WHO BMI class the subject's BMI was in before treatment was commenced. The different WHO BMI classes are listed in Table 1. For example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as morbidly obese (class III) before treatment commenced that results in the subject being classified as obese (class I or II), overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 1 1 months or 12 months, 18 months, or 24 months after treatment has commenced.
[00121] In some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject resulting in the subject's BMI being classified in a WHO BMI class that is lower than the WHO BMI class the subject's BMI was in before treatment was commenced. The different WHO BMI classes are listed in Table 1. For example, in some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril,
spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as morbidly obese (class III) before treatment commenced that results in the subject being classified as obese (class I or II), overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 1 1 months or 12 months, 18 months, or 24 months after treatment has commenced.
[00122] For another example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as obese (class II) before treatment commenced that results in the subject being classified as obese (class I), overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 1 1 months or 12 months, 18 months, or 24 months after treatment has commenced. For yet another example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as obese (class I) before treatment commenced that results in the subject being classified as overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. As will be appreciated by the skilled artisan, the effective amount of an agent, e.g., of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, may vary depending on various factors as, for example, on the desired biological response, e.g., on the amount of weight loss desired and the target time frame for the desired weight loss, and on the specific agent being used.
[00123] For another example, in some embodiments, an effective amount of of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an
combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as obese (class II) before treatment commenced that results in the subject being classified as obese (class I), overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. For yet another example, in some embodiments, an effective amount of of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as obese (class I) before treatment commenced that results in the subject being classified as overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. As will be appreciated by the skilled artisan, the effective amount of an agent, e.g. , of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), may vary depending on various factors as, for example, on the desired biological response, e.g., on the amount of weight loss desired and the target time frame for the desired weight loss, and on the specific agent being used.
[00124] Some aspects of this disclosure provide compositions and methods for the treatment of obesity, e.g., male obesity, with a dicarboxylate-containing ACE inhibitor. In some embodiments, the dicarboxylate-containing ACE inhibitor is perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for use in the treatment of male obesity. Any suitable form of the dicarboxylate-containing ACE inhibitor perindopril can be used, including any pharmaceutically acceptable salt, polymorph, solvate,
hydrate, or clathrate thereof. Suitable forms of this active agent are well known in the art. It may be provided, for example, as the tert-butylamine salt (i.e. perindopril erbumine) or as perindopril arginine. In some embodiments, perindopril is provided as perindopril erbumine, as disclosed in Drug Bank DB00790 (APRD01178), for example.
[00125] Some aspects of this disclosure provide compositions and methods for the treatment of obesity, e.g., male obesity, with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), for use in the treatment of male obesity. Any suitable form of the compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) can be used, including any pharmaceutically acceptable salt, polymorph, solvate, hydrate, or clathrate thereof.
[00126] Perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, may be prepared as described in WO/2004/046172; WO/2004/075889; WO/2004/099138; WO/2005/100317; WO/2005/066198; WO/2005/054276; WO72005/023843; WO72005/012333; WO/2001/058868. Perindopril erbumine and perindopril arginine may be prepared as described in WO/2012/044189; WO/2008/114270;
WO/2007/020009; WO/2007/02012; WO/2006/101462; WO/2006/070276; WO/2005/037788 and WO/2013/102740; WO/2009/157018; WO/2007/099216, respectively.
[00127] In some embodiments, the dicarboxylate-containing ACE inhibitor perindopril is for use in the treatment of a subject for obesity as described herein. The obesity may be class I, II or III, is preferably at least class II and is most preferably class III. In some embodiments, the subject is male.
[00128] In some embodiments, a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) is for use in the treatment of a subject for obesity as described herein. The obesity may
be class I, II or III, is preferably at least class II and is most preferably class III. In some embodiments, the subject is male.
[00129] Some aspects of this disclosure provide an anti-obesity agent for use in the treatment of obesity in a male subject, characterized in that the subject has a nucleic acid variant within his CPD gene. In some embodiments, an anti-obesity agent for use in the treatment of obesity in a male subject is provided, characterized in that the subject has been selected as having a nucleic acid variant within his CPD gene. In some embodiments, an anti-obesity agent for use in a method of treating obesity in a male subject is provided, characterized in that the method comprises determining whether the subject has a nucleic acid variant within his CPD gene and treating the subject with the anti-obesity agent if so. In some embodiments, the anti- obesity agent comprises the dicarboxylate-containing ACE inhibitor perindopril. The anti- obesity agent may also comprise a known anti-obesity agent, such as orlistat
(tetrahydrolipstatin), lorcaserin, phentermine, phentermine-topiramate, rimonabant or sibutramine, in combination with an ACE inhibitor, e.g., with perindopril. Any suitable anti- obesity agent may be used in this manner according to some aspects of this disclosure.
[00130] Some aspects of this disclosure provide an anti-obesity agent for use in the treatment of obesity in a male subject, characterized in that the subject has a nucleic acid variant within his CPD gene. In some embodiments, an anti-obesity agent for use in the treatment of obesity in a male subject is provided, characterized in that the subject has been selected as having a nucleic acid variant within his CPD gene. In some embodiments, an anti-obesity agent for use in a method of treating obesity in a male subject is provided, characterized in that the method comprises determining whether the subject has a nucleic acid variant within his CPD gene and treating the subject with the anti-obesity agent if so. In some embodiments, the anti- obesity agent comprises a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). The anti-obesity agent may also comprise a known anti-obesity agent, such as orlistat (tetrahydrolipstatin), lorcaserin, phentermine, phentermine-topiramate, rimonabant or sibutramine, in combination with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable
salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). Any suitable anti-obesity agent may be used in this manner according to some aspects of this disclosure.
[00131] In some embodiments, the anti-obesity agents, including the dicarboxylate- containing ACE inhibitor perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, as described herein is formulated as known in the art. Perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof can be formulated as described in WO/2007/025695;
WO/2007/088035. Perindopril erbumine and perindopril arginine can be formulated as described in WO2007/092758; WO/2007/058634 and WO/2007/099216, respectively. Suitable
formulations are also described herein. The agents can be used in the treatment of male obesity according to any of the methods described herein. Thus, any and all of the relevant embodiments of the formulations and methods that are described below apply equally to the agents described herein.
[00132] In some embodiments, the anti-obesity agents, including a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), as described herein is formulated as known in the art. Suitable formulations are also described herein. The agents can be used in the treatment of male obesity according to any of the methods described herein. Thus, any and all of the relevant embodiments of the formulations and methods that are described below apply equally to the agents described herein.
[00133] The present disclosure provides compositions comprising a compound or combination of compounds provided herein, e.g., a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for administration to a subject having obesity and carrying a variant allele of the CPD gene as disclosed herein. Thus, the present invention provides pharmaceutical compositions comprising a compound or combination of compounds provided herein, e.g., a dicarboxylate-containing ACE inhibitor, such as, for example,
perindopril, or a a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. Pharmaceutical compositions may optionally comprise one or more additional therapeutically active substances, for example, an ACE inhibitor, an anti-obesity agent, an anti-diabetic agent, or an agent used to treat cardiovascular disease. In some embodiments, a method of administering pharmaceutical compositions comprising a compound or combination of compounds provided herein, e.g., a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, to a subject in need thereof is provided. In some embodiments, the compositions provided herein are administered to human subjects. In some embodiments, the subjects are male.
[00134] The present disclosure provides compositions comprising a compound or combination of compounds provided herein, e.g., including a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), for administration to a subject having obesity and carrying a variant allele of the CPD gene as disclosed herein. Thus, the present invention provides pharmaceutical compositions comprising a compound or combination of compounds provided herein, e.g., including a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). Pharmaceutical compositions may optionally comprise one or more additional therapeutically active substances, for example, an ACE inhibitor, an anti-obesity agent, an antidiabetic agent, or an agent used to treat cardiovascular disease. In some embodiments, a method of administering pharmaceutical compositions comprising a compound or combination of compounds provided herein, e.g., including a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), to a subject in need thereof is provided. In some embodiments, the
compositions provided herein are administered to human subjects. In some embodiments, the subjects are male.
[00135] For the purposes of the present disclosure, the phrase "active ingredient" generally refers to an active agent such as a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and may include additional active agents as provided herein. Although the descriptions of pharmaceutical compositions provided herein are principally directed to pharmaceutical compositions which are suitable for administration to humans, it will be understood by the skilled artisan that such compositions are generally also suitable for administration to animals. If required, modification of pharmaceutical compositions suitable for administration to humans in order to render the compositions suitable for
administration to various animals is well understood, and the ordinarily skilled person in the art will be able to design and perform such modification with merely ordinary, if any,
experimentation. Subjects to which administration of the pharmaceutical compositions is contemplated include, but are not limited to, humans and/or other primates; mammals, e.g., cattle, pigs, horses, sheep, cats, dogs, rodents, mice, hamsters, and/or rats; birds, e.g., chickens, ducks, geese, and turkeys.
[00136] For the purposes of the present disclosure, the phrase "active ingredient" generally refers to a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and may include additional active agents as provided herein. Although the descriptions of pharmaceutical compositions provided herein are principally directed to pharmaceutical compositions which are suitable for administration to humans, it will be understood by the skilled artisan that such compositions are generally also suitable for administration to animals. If required, modification of pharmaceutical compositions suitable for administration to humans in order to render the compositions suitable for administration to various animals is well understood, and the ordinarily skilled person in the art will be able to design and perform such modification with merely ordinary, if any, experimentation. Subjects to which administration of the pharmaceutical compositions is contemplated include, but are not limited to, humans and/or
other primates; mammals, e.g., cattle, pigs, horses, sheep, cats, dogs, rodents, mice, hamsters, and/or rats; birds, e.g., chickens, ducks, geese, and turkeys.
[00137] Formulations of the pharmaceutical compositions described herein may be prepared by any method known or hereafter developed in the art of pharmacology. In general, such preparatory methods include the step of bringing the active ingredient, e.g., a dicarboxylate- containing ACE inhibitor, such as, for example, perindopril, or a a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, into association with an excipient and/or one or more other accessory ingredients, and then, if necessary and/or desirable, shaping and/or packaging the product into a desired single- or multi-dose unit.
[00138] Formulations of the pharmaceutical compositions described herein may be prepared by any method known or hereafter developed in the art of pharmacology. In general, such preparatory methods include the step of bringing the active ingredient, e.g., a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), into association with an excipient and/or one or more other accessory ingredients, and then, if necessary and/or desirable, shaping and/or packaging the product into a desired single- or multi-dose unit.
[00139] A pharmaceutical composition comprising a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, may be prepared, packaged, and/or sold in bulk, as a single unit dose, and/or as a plurality of single unit doses. As used herein, a "unit dose" is discrete amount of the pharmaceutical composition comprising a predetermined amount of the active ingredient, e.g., a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. The amount of the active ingredient is generally equal to the dosage of the active ingredient which would be administered to a subject and/or a convenient fraction of such a dosage such as, for example, one -half or one-third of such a dosage.
[00140] A pharmaceutical composition comprising a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof;
a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), may be prepared, packaged, and/or sold in bulk, as a single unit dose, and/or as a plurality of single unit doses. As used herein, a "unit dose" is discrete amount of the pharmaceutical composition comprising a predetermined amount of the active ingredient, e.g., ., a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). The amount of the active ingredient is generally equal to the dosage of the active ingredient which would be administered to a subject and/or a convenient fraction of such a dosage such as, for example, one -half or one-third of such a dosage.
[00141] Relative amounts of the active ingredient, the pharmaceutically acceptable excipient, and/or any additional ingredients in a pharmaceutical composition in accordance with the invention will vary, depending upon the identity, size, and/or condition of the subject treated and further depending upon the route by which the composition is to be administered. By way of example, the composition may comprise between 0.1% and 100% (w/w) of active ingredient, e.g. a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and, optionally, any additional active ingredients, such as, for example, an anti-obesity agent. Pharmaceutical formulations as provided herein may additionally comprise a pharmaceutically acceptable excipient, which, as used herein, includes any and all solvents, dispersion media, diluents, or other liquid vehicles, dispersion or suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants and the like, as suited to the particular dosage form desired. Remington's The Science and Practice of
Pharmacy, 21st Edition, A. R. Gennaro (Lippincott, Williams & Wilkins, Baltimore, MD, 2006; incorporated herein by reference) discloses various excipients used in formulating
pharmaceutical compositions and known techniques for the preparation thereof. Except insofar as any conventional excipient medium is incompatible with a substance or its derivatives, such as by producing any undesirable biological effect or otherwise interacting in a deleterious manner with any other component(s) of the pharmaceutical composition, its use is contemplated to be within the scope of this invention.
[00142] Relative amounts of the active ingredient, the pharmaceutically acceptable excipient, and/or any additional ingredients in a pharmaceutical composition in accordance with the invention will vary, depending upon the identity, size, and/or condition of the subject treated and further depending upon the route by which the composition is to be administered. By way of example, the composition may comprise between 0.1% and 100% (w/w) of active ingredient, e.g. a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and, optionally, any additional active ingredients, such as, for example, an anti-obesity agent. Pharmaceutical formulations as provided herein may additionally comprise a pharmaceutically acceptable excipient, which, as used herein, includes any and all solvents, dispersion media, diluents, or other liquid vehicles, dispersion or suspension aids, surface active agents, isotonic agents, thickening or emulsifying agents, preservatives, solid binders, lubricants and the like, as suited to the particular dosage form desired. Remington's The Science and Practice of Pharmacy, 21st Edition, A. R. Gennaro (Lippincott, Williams & Wilkins, Baltimore, MD, 2006; incorporated herein by reference) discloses various excipients used in formulating pharmaceutical
compositions and known techniques for the preparation thereof. Except insofar as any conventional excipient medium is incompatible with a substance or its derivatives, such as by producing any undesirable biological effect or otherwise interacting in a deleterious manner with any other component(s) of the pharmaceutical composition, its use is contemplated to be within the scope of this invention.
[00143] In some embodiments, a pharmaceutically acceptable excipient is at least 95%, at least 96%, at least 97%, at least 98%, at least 99%, or 100% pure. In some embodiments, an excipient is approved for use in humans and for veterinary use. In some embodiments, an excipient is approved by United States Food and Drug Administration. In some embodiments, an excipient is pharmaceutical grade. In some embodiments, an excipient meets the standards of the United States Pharmacopoeia (USP), the European Pharmacopoeia (EP), the British
Pharmacopoeia, and/or the International Pharmacopoeia.
[00144] Pharmaceutically acceptable excipients used in the manufacture of pharmaceutical compositions include, but are not limited to, inert diluents, dispersing and/or granulating agents,
surface active agents and/or emulsifiers, disintegrating agents, binding agents, preservatives, buffering agents, lubricating agents, and/or oils. Such excipients may optionally be included in pharmaceutical formulations. Excipients such as cocoa butter and suppository waxes, coloring agents, coating agents, sweetening, flavoring, and/or perfuming agents can be present in the composition, according to the judgment of the formulator.
[00145] Exemplary diluents include, but are not limited to, calcium carbonate, sodium carbonate, calcium phosphate, dicalcium phosphate, calcium sulfate, calcium hydrogen phosphate, sodium phosphate lactose, sucrose, cellulose, microcrystalline cellulose, kaolin, mannitol, sorbitol, inositol, sodium chloride, dry starch, cornstarch, powdered sugar, etc., and/or combinations thereof.
[00146] Exemplary granulating and/or dispersing agents include, but are not limited to, potato starch, corn starch, tapioca starch, sodium starch glycolate, clays, alginic acid, guar gum, citrus pulp, agar, bentonite, cellulose and wood products, natural sponge, cation-exchange resins, calcium carbonate, silicates, sodium carbonate, cross-linked poly(vinyl-pyrrolidone)
(crospovidone), sodium carboxymethyl starch (sodium starch glycolate), carboxymethyl cellulose, cross-linked sodium carboxymethyl cellulose (croscarmellose), methylcellulose, pregelatinized starch (starch 1500), microcrystalline starch, water insoluble starch, calcium carboxymethyl cellulose, magnesium aluminum silicate (Veegum), sodium lauryl sulfate, quaternary ammonium compounds, etc., and/or combinations thereof.
[00147] Exemplary surface active agents and/or emulsifiers include, but are not limited to, natural emulsifiers (e.g. acacia, agar, alginic acid, sodium alginate, tragacanth, chondrux, cholesterol, xanthan, pectin, gelatin, egg yolk, casein, wool fat, cholesterol, wax, and lecithin),
®
colloidal clays (e.g. bentonite [aluminum silicate] and Veegum [magnesium aluminum silicate]), long chain amino acid derivatives, high molecular weight alcohols (e.g. stearyl alcohol, cetyl alcohol, oleyl alcohol, triacetin monostearate, ethylene glycol distearate, glyceryl monostearate, and propylene glycol monostearate, polyvinyl alcohol), carbomers (e.g. carboxy polymethylene, polyacrylic acid, acrylic acid polymer, and carboxyvinyl polymer), carrageenan, cellulosic derivatives (e.g. carboxymethylcellulose sodium, powdered cellulose, hydroxymethyl cellulose, hydroxypropyl cellulose, hydroxypropyl methylcellulose, methylcellulose), sorbitan
®
fatty acid esters (e.g. polyoxyethylene sorbitan monolaurate [Tween 20], polyoxyethylene
® ®
sorbitan [Tween 60], polyoxyethylene sorbitan monooleate [Tween 80], sorbitan
monopalmitate [Span 40], sorbitan monostearate [Span 60], sorbitan tristearate [Span 65], glyceryl monooleate, sorbitan monooleate [Span® 80]), polyoxyethylene esters (e.g.
polyoxyethylene monostearate [Myrj®45], polyoxyethylene hydrogenated castor oil,
polyethoxylated castor oil, polyoxymethylene stearate, and Solutol®), sucrose fatty acid esters, polyethylene glycol fatty acid esters (e.g. Cremophor®), polyoxyethylene ethers, (e.g.
polyoxyethylene lauryl ether [Brij 30]), poly(vinyl-pyrrolidone), diethylene glycol monolaurate, triethanolamine oleate, sodium oleate, potassium oleate, ethyl oleate, oleic acid, ethyl laurate, sodium lauryl sulfate, Pluronic F 68, Poloxamer 188, cetrimonium bromide, cetylpyridinium chloride, benzalkonium chloride, docusate sodium, etc. and/or combinations thereof.
[00148] Exemplary binding agents include, but are not limited to, starch (e.g. cornstarch and starch paste); gelatin; sugars (e.g. sucrose, glucose, dextrose, dextrin, molasses, lactose, lactitol, mannitol,); natural and synthetic gums (e.g. acacia, sodium alginate, extract of Irish moss, panwar gum, ghatti gum, mucilage of isapol husks, carboxymethylcellulose,
methylcellulose, ethylcellulose, hydroxyethylcellulose, hydroxypropyl cellulose, hydroxypropyl methylcellulose, microcrystalline cellulose, cellulose acetate, poly(vinyl-pyrrolidone), magnesium aluminum silicate (Veegum®), and larch arabogalactan); alginates; polyethylene oxide; polyethylene glycol; inorganic calcium salts; silicic acid; polymethacrylates; waxes; water; alcohol; etc. ; and combinations thereof.
[00149] Exemplary preservatives may include, but are not limited to, antioxidants, chelating agents, antimicrobial preservatives, antifungal preservatives, alcohol preservatives, acidic preservatives, and/or other preservatives. Exemplary antioxidants include, but are not limited to, alpha tocopherol, ascorbic acid, acorbyl palmitate, butylated hydroxyanisole, butylated hydroxytoluene, monothioglycerol, potassium metabisulfite, propionic acid, propyl gallate, sodium ascorbate, sodium bisulfite, sodium metabisulfite, and/or sodium sulfite.
Exemplary chelating agents include ethylenediaminetetraacetic acid (EDTA), citric acid monohydrate, disodium edetate, dipotassium edetate, edetic acid, fumaric acid, malic acid, phosphoric acid, sodium edetate, tartaric acid, and/or trisodium edetate. Exemplary
antimicrobial preservatives include, but are not limited to, benzalkonium chloride, benzethonium chloride, benzyl alcohol, bronopol, cetrimide, cetylpyridinium chloride, chlorhexidine, chlorobutanol, chlorocresol, chloroxylenol, cresol, ethyl alcohol, glycerin, hexetidine, imidurea, phenol, phenoxyethanol, phenylethyl alcohol, phenylmercuric nitrate, propylene glycol, and/or
thimerosal. Exemplary antifungal preservatives include, but are not limited to, butyl paraben, methyl paraben, ethyl paraben, propyl paraben, benzoic acid, hydroxybenzoic acid, potassium benzoate, potassium sorbate, sodium benzoate, sodium propionate, and/or sorbic acid.
Exemplary alcohol preservatives include, but are not limited to, ethanol, polyethylene glycol, phenol, phenolic compounds, bisphenol, chlorobutanol, hydroxybenzoate, and/or phenylethyl alcohol. Exemplary acidic preservatives include, but are not limited to, vitamin A, vitamin C, vitamin E, beta-carotene, citric acid, acetic acid, dehydroacetic acid, ascorbic acid, sorbic acid, and/or phytic acid. Other preservatives include, but are not limited to, tocopherol, tocopherol acetate, deteroxime mesylate, cetrimide, butylated hydroxyanisol (BHA), butylated
hydroxytoluened (BHT), ethylenediamine, sodium lauryl sulfate (SLS), sodium lauryl ether sulfate (SLES), sodium bisulfite, sodium metabisulfite, potassium sulfite, potassium
metabisulfite, Glydant Plus®, Phenonip®, methylparaben, Germall®115, Germaben®II,
Neolone™, Kathon™, and/or Euxyl®.
[00150] Exemplary buffering agents include, but are not limited to, citrate buffer solutions, acetate buffer solutions, phosphate buffer solutions, ammonium chloride, calcium carbonate, calcium chloride, calcium citrate, calcium glubionate, calcium gluceptate, calcium gluconate, D-gluconic acid, calcium glycerophosphate, calcium lactate, propanoic acid, calcium levulinate, pentanoic acid, dibasic calcium phosphate, phosphoric acid, tribasic calcium phosphate, calcium hydroxide phosphate, potassium acetate, potassium chloride, potassium gluconate, potassium mixtures, dibasic potassium phosphate, monobasic potassium phosphate, potassium phosphate mixtures, sodium acetate, sodium bicarbonate, sodium chloride, sodium citrate, sodium lactate, dibasic sodium phosphate, monobasic sodium phosphate, sodium phosphate mixtures, tromethamine, magnesium hydroxide, aluminum hydroxide, alginic acid, pyrogen-free water, isotonic saline, Ringer's solution, ethyl alcohol, etc., and/or combinations thereof.
[00151] Exemplary lubricating agents include, but are not limited to, magnesium stearate, calcium stearate, stearic acid, silica, talc, malt, glyceryl behanate, hydrogenated vegetable oils, polyethylene glycol, sodium benzoate, sodium acetate, sodium chloride, leucine, magnesium lauryl sulfate, sodium lauryl sulfate, etc. , and combinations thereof.
[00152] Exemplary oils include, but are not limited to, almond, apricot kernel, avocado, babassu, bergamot, black current seed, borage, cade, camomile, canola, caraway, carnauba,
castor, cinnamon, cocoa butter, coconut, cod liver, coffee, corn, cotton seed, emu, eucalyptus, evening primrose, fish, flaxseed, geraniol, gourd, grape seed, hazel nut, hyssop, isopropyl myristate, jojoba, kukui nut, lavandin, lavender, lemon, litsea cubeba, macademia nut, mallow, mango seed, meadowfoam seed, mink, nutmeg, olive, orange, orange roughy, palm, palm kernel, peach kernel, peanut, poppy seed, pumpkin seed, rapeseed, rice bran, rosemary, safflower, sandalwood, sasquana, savoury, sea buckthorn, sesame, shea butter, silicone, soybean, sunflower, tea tree, thistle, tsubaki, vetiver, walnut, and wheat germ oils. Exemplary oils include, but are not limited to, butyl stearate, caprylic triglyceride, capric triglyceride, cyclomethicone, diethyl sebacate, dimethicone 360, isopropyl myristate, mineral oil,
octyldodecanol, oleyl alcohol, silicone oil, and/or combinations thereof.
[00153] Solid dosage forms for oral administration include capsules, tablets, pills, powders, and granules. In such solid dosage forms, an active ingredient is mixed with at least one inert, pharmaceutically acceptable excipient such as sodium citrate or dicalcium phosphate and/or fillers or extenders (e.g. starches, lactose, sucrose, glucose, mannitol, and silicic acid), binders (e.g. carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose, and acacia), humectants (e.g. glycerol), disintegrating agents (e.g. agar, calcium carbonate, potato or tapioca starch, alginic acid, certain silicates, and sodium carbonate), solution retarding agents (e.g. paraffin), absorption accelerators (e.g. quaternary ammonium compounds), wetting agents (e.g. cetyl alcohol and glycerol monostearate), absorbents (e.g. kaolin and bentonite clay), and lubricants (e.g. talc, calcium stearate, magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate), and mixtures thereof. In the case of capsules, tablets and pills, the dosage form may comprise buffering agents.
[00154] Solid compositions of a similar type may be employed as fillers in soft and hard- filled gelatin capsules using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like. Solid dosage forms of tablets, dragees, capsules, pills, and granules can be prepared with coatings and shells such as enteric coatings and other coatings well known in the pharmaceutical formulating art. They may optionally comprise opacifying agents and can be of a composition that they release the active ingredient(s) only, or
preferentially, in a certain part of the intestinal tract, optionally, in a delayed manner. Examples of embedding compositions which can be used include polymeric substances and waxes. Solid compositions of a similar type may be employed as fillers in soft and hard-filled gelatin capsules
using such excipients as lactose or milk sugar as well as high molecular weight polyethylene glycols and the like.
[00155] Liquid dosage forms for oral and parenteral administration include, but are not limited to, pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions, syrups, and/or elixirs. In addition to active ingredients, liquid dosage forms may comprise inert diluents commonly used in the art such as, for example, water or other solvents, solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol, dimethylformamide, oils (in particular, cottonseed, groundnut, corn, germ, olive, castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof. Besides inert diluents, oral compositions can include adjuvants such as wetting agents, emulsifying and suspending agents, sweetening, flavoring, and/or perfuming agents. In certain embodiments for parenteral administration, compositions are mixed with solubilizing agents such as Cremophor , alcohols, oils, modified oils, glycols, polysorbates, cyclodextrins, polymers, and/or combinations thereof.
[00156] Injectable preparations, for example, sterile injectable aqueous or oleaginous suspensions may be formulated according to the known art using suitable dispersing agents, wetting agents, and/or suspending agents. Sterile injectable preparations may be sterile injectable solutions, suspensions, and/or emulsions in nontoxic parenterally acceptable diluents and/or solvents, for example, as a solution in 1,3-butanediol. Among the acceptable vehicles and solvents that may be employed are water, Ringer's solution, U.S.P., and isotonic sodium chloride solution. Sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose any bland fixed oil can be employed including synthetic mono- or diglycerides. Fatty acids such as oleic acid can be used in the preparation of injectables.
[00157] Injectable formulations can be sterilized, for example, by filtration through a bacterial-retaining filter, and/or by incorporating sterilizing agents in the form of sterile solid compositions which can be dissolved or dispersed in sterile water or other sterile injectable medium prior to use.
[0064] In order to prolong the effect of an active ingredient, it is often desirable to slow the absorption of the active ingredient from subcutaneous or intramuscular injection. This may be accomplished by the use of a liquid suspension of crystalline or amorphous material with poor
water solubility. The rate of absorption of the drug then depends upon its rate of dissolution which, in turn, may depend upon crystal size and crystalline form. Alternatively, delayed absorption of a parenterally administered drug form is accomplished by dissolving or suspending the drug in an oil vehicle. Injectable depot forms are made by forming microencapsule matrices of the drug in biodegradable polymers such as polylactide-polyglycolide. Depending upon the ratio of drug to polymer and the nature of the particular polymer employed, the rate of drug release can be controlled. Examples of other biodegradable polymers include poly(orthoesters) and poly( anhydrides). Depot injectable formulations are prepared by entrapping the drug in liposomes or microemulsions which are compatible with body tissues.
[00158] Dosage forms for topical and/or transdermal administration of a composition may include ointments, pastes, creams, lotions, gels, powders, solutions, sprays, inhalants and/or patches. Generally, an active ingredient is admixed under sterile conditions with a
pharmaceutically acceptable excipient and/or any needed preservatives and/or buffers as may be required. Additionally, the present invention contemplates the use of transdermal patches, which often have the added advantage of providing controlled delivery of a compound to the body. Such dosage forms may be prepared, for example, by dissolving and/or dispensing the compound in the proper medium. Alternatively or additionally, rate may be controlled by either providing a rate controlling membrane and/or by dispersing the compound in a polymer matrix and/or gel.
[00159] Suitable devices for use in delivering intradermal pharmaceutical compositions described herein include short needle devices such as those described in U.S. Patents 4,886,499; 5,190,521; 5,328,483; 5,527,288; 4,270,537; 5,015,235; 5,141,496; and 5,417,662. Intradermal compositions may be administered by devices which limit the effective penetration length of a needle into the skin, such as those described in PCT publication WO 99/34850 and functional equivalents thereof. Jet injection devices which deliver liquid compositions to the dermis via a liquid jet injector and/or via a needle which pierces the stratum corneum and produces a jet which reaches the dermis are suitable. Jet injection devices are described, for example, in U.S. Patents 5,480,381 ; 5,599,302; 5,334,144; 5,993,412; 5,649,912; 5,569,189; 5,704,911;
5,383,851; 5,893,397; 5,466,220; 5,339,163; 5,312,335; 5,503,627; 5,064,413; 5,520,639;
4,596,556; 4,790,824; 4,941,880; 4,940,460; and PCT publications WO 97/37705 and WO 97/13537. Ballistic powder/particle delivery devices which use compressed gas to accelerate vaccine in powder form through the outer layers of the skin to the dermis are suitable.
Alternatively or additionally, conventional syringes may be used in the classical mantoux method of intradermal administration.
[00160] Formulations suitable for topical administration include, but are not limited to, liquid and/or semi liquid preparations such as liniments, lotions, oil in water and/or water in oil emulsions such as creams, ointments and/or pastes, and/or solutions and/or suspensions.
Topically-administrable formulations may, for example, comprise from about 1% to about 10% (w/w) active ingredient, although the concentration of active ingredient may be as high as the solubility limit of the active ingredient in the solvent. Formulations for topical administration may further comprise one or more of the additional ingredients described herein.
[00161] A pharmaceutical composition may be prepared, packaged, and/or sold in a formulation suitable for pulmonary administration via the buccal cavity. Such a formulation may comprise dry particles which comprise the active ingredient and which have a diameter in the range from about 0.5 nm to about 7 nm or from about 1 nm to about 6 nm. Such compositions are conveniently in the form of dry powders for administration using a device comprising a dry powder reservoir to which a stream of propellant may be directed to disperse the powder and/or using a self-propelling solvent/powder dispensing container such as a device comprising the active ingredient dissolved and/or suspended in a low -boiling propellant in a sealed container. Such powders comprise particles wherein at least 98% of the particles by weight have a diameter greater than 0.5 nm and at least 95% of the particles by number have a diameter less than 7 nm. Alternatively, at least 95% of the particles by weight have a diameter greater than 1 nm and at least 90% of the particles by number have a diameter less than 6 nm. Dry powder compositions may include a solid fine powder diluent such as sugar and are conveniently provided in a unit dose form.
[00162] Low boiling propellants generally include liquid propellants having a boiling point of below 65 °F at atmospheric pressure. Generally the propellant may constitute 50% to 99.9% (w/w) of the composition, and active ingredient may constitute 0.1% to 20% (w/w) of the composition. A propellant may further comprise additional ingredients such as a liquid non- ionic and/or solid anionic surfactant and/or a solid diluent (which may have a particle size of the same order as particles comprising the active ingredient).
[00163] Pharmaceutical compositions formulated for pulmonary delivery may provide an active ingredient in the form of droplets of a solution and/or suspension. Such formulations may
be prepared, packaged, and/or sold as aqueous and/or dilute alcoholic solutions and/or suspensions, optionally sterile, comprising active ingredient, and may conveniently be administered using any nebulization and/or atomization device. Such formulations may further comprise one or more additional ingredients including, but not limited to, a flavoring agent such as saccharin sodium, a volatile oil, a buffering agent, a surface active agent, and/or a preservative such as methylhydroxybenzoate. Droplets provided by this route of administration may have an average diameter in the range from about 0.1 nm to about 200 nm.
[00164] Formulations described herein as being useful for pulmonary delivery are useful for intranasal delivery of a pharmaceutical composition. Another formulation suitable for intranasal administration is a coarse powder comprising the active ingredient and having an average particle from about 0.2 μπι to 500 μπι. Such a formulation is administered in the manner in which snuff is taken, i.e. by rapid inhalation through the nasal passage from a container of the powder held close to the nose.
[00165] Formulations suitable for nasal administration may, for example, comprise from about as little as 0.1% (w/w) and as much as 100% (w/w) of active ingredient, and may comprise one or more of the additional ingredients described herein. A pharmaceutical composition may be prepared, packaged, and/or sold in a formulation suitable for buccal administration. Such formulations may, for example, be in the form of tablets and/or lozenges made using
conventional methods, and may, for example, 0.1 % to 20% (w/w) active ingredient, the balance comprising an orally dissolvable and/or degradable composition and, optionally, one or more of the additional ingredients described herein. Alternately, formulations suitable for buccal administration may comprise a powder and/or an aerosolized and/or atomized solution and/or suspension comprising active ingredient. Such powdered, aerosolized, and/or atomized formulations, when dispersed, may have an average particle and/or droplet size in the range from about 0.1 nm to about 200 nm, and may further comprise one or more of any additional ingredients described herein.
[00166] A pharmaceutical composition may be prepared, packaged, and/or sold in a formulation suitable for ophthalmic administration. Such formulations may, for example, be in the form of eye drops including, for example, a 0.1/1.0% (w/w) solution and/or suspension of the active ingredient in an aqueous or oily liquid excipient. Such drops may further comprise buffering agents, salts, and/or one or more other of any additional ingredients described herein.
Other opthalmically-administrable formulations which are useful include those which comprise the active ingredient in microcrystalline form and/or in a liposomal preparation. Ear drops and/or eye drops are contemplated as being within the scope of this invention.
[00167] General considerations in the formulation and/or manufacture of pharmaceutical agents may be found, for example, in Remington: The Science and Practice of Pharmacy 21st ed., Lippincott Williams & Wilkins, 2005 (incorporated herein by reference).
[00168] Still further encompassed by the invention are pharmaceutical packs and/or kits.
Pharmaceutical packs and/or kits provided may comprise a provided composition and a container (e.g., a vial, ampoule, bottle, syringe, and/or dispenser package, or other suitable container). In some embodiments, provided kits may optionally further include a second container comprising a suitable aqueous carrier for dilution or suspension of the provided composition for preparation of administration to a subject. In some embodiments, contents of provided formulation container and solvent container combine to form at least one unit dosage form.
[00169] Optionally, a single container may comprise one or more compartments for containing a provided composition, and/or appropriate aqueous carrier for suspension or dilution. In some embodiments, a single container can be appropriate for modification such that the container may receive a physical modification so as to allow combination of compartments and/or components of individual compartments. For example, a foil or plastic bag may comprise two or more compartments separated by a perforated seal which can be broken so as to allow combination of contents of two individual compartments once the signal to break the seal is generated. A pharmaceutical pack or kit may thus comprise such multi-compartment containers including a provided composition and appropriate solvent and/or appropriate aqueous carrier for suspension.
[00170] Optionally, instructions for use are additionally provided in such kits of the invention. Such instructions may provide, generally, for example, instructions for dosage and administration. In other embodiments, instructions may further provide additional detail relating to specialized instructions for particular containers and/or systems for administration. Still further, instructions may provide specialized instructions for use in conjunction and/or in combination with additional therapy.
Methods for treating Obesity
[00171] Some aspects of this disclosure provide methods for treating obesity, e.g., in a male subject. Typically, the methods for treating obesity provided herein comprise
administering a composition provided herein to a subject in need thereof, e.g., a composition comprising a therapeutically effective amount of a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the subject is male. In some embodiments, the subject is morbidly obese. In some embodiments, the subject exhibits class I or class II obesity. In some embodiments, the method comprises administering the dicarboxylate- containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof as the only or the main active ingredient for treating obesity in the subject. In some embodiments, the method comprises administering a combination of active ingredients to the subject, e.g., a combination of a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and an anti- obesity agent as provided herein.
[00172] Some aspects of this disclosure provide methods for treating obesity, e.g., in a male subject. Typically, the methods for treating obesity provided herein comprise
administering a composition provided herein to a subject in need thereof, e.g., a composition comprising a therapeutically effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). In some embodiments, the subject is male. In some embodiments, the subject is morbidly obese. In some embodiments, the subject exhibits class I or class II obesity. In some embodiments, the method comprises administering a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) as the only or the main active ingredient for treating obesity in the subject. In some embodiments, the method comprises
administering a combination of active ingredients to the subject, e.g., a combination of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and an anti-obesity agent as provided herein.
[00173] In some embodiments, a "therapeutically effective amount" of a composition provided herein (e.g., of a drug, pharmaceutical composition, combination, anti-obesity agent, or medicament) is the amount which, when administered to an obese subject, is sufficient to treat the obesity, or produce a desired clinical effect, e.g., a certain amount of weight loss within a certain time frame. For example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, may refer to an amount that is sufficient to induce a measurable weight loss in an obese subject. For example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof refers to the amount sufficient or the amount required to induce the loss of at least 5%, at least 10%, at least 15%, at least 20%, at least 25, at least 30%, at least 35%, at least 40%, at least 45%, or at least 50%, of body weight within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 1 1 months or 12 months, 18 months, or 24 months after treatment has commenced. In some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject, e.g., a reduction by at least 1, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least 11, at least 12, at least 13, at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21 , at least 22, at least 23, at least 24, at least 25, at least 26, at least 27, at least 28, at least 29, or at least 30 points within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. In some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof
refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject resulting in the subject's BMI being classified in a WHO BMI class that is lower than the WHO BMI class the subject's BMI was in before treatment was commenced. The different WHO BMI classes are listed in Table 1. For example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as morbidly obese (class III) before treatment commenced that results in the subject being classified as obese (class I or II), overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 1 1 months or 12 months, 18 months, or 24 months after treatment has commenced. For another example, in some embodiments, an effective amount of perindopril, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as obese (class II) before treatment commenced that results in the subject being classified as obese (class I), overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. For yet another example, in some embodiments, an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as obese (class I) before treatment commenced that results in the subject being classified as overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. As will be appreciated by the skilled artisan, the effective amount of an agent, e.g., of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, may vary depending on various factors as, for example, on the desired biological response, e.g., on the amount of weight loss desired and the target time frame for the desired weight loss, and on the specific agent being used.
[00174] In some embodiments, a "therapeutically effective amount" of a composition provided herein (e.g., of a drug, pharmaceutical composition, combination, anti-obesity agent, or medicament) is the amount which, when administered to an obese subject, is sufficient to treat the obesity, or produce a desired clinical effect, e.g., a certain amount of weight loss within a certain time frame. For example, in some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), may refer to an amount that is sufficient to induce a measurable weight loss in an obese subject. For example, in some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) refers to the amount sufficient or the amount required to induce the loss of at least 5%, at least 10%, at least 15%, at least 20%, at least 25, at least 30%, at least 35%, at least 40%, at least 45%, or at least 50%, of body weight within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. In some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject, e.g., a reduction by at least 1, at least 2, at least 3, at least 4, at least 5, at least 6, at least 7, at least 8, at least 9, at least 10, at least 11, at least 12, at least 13, at least 14, at least 15, at least 16, at least 17, at least 18, at least 19, at least 20, at least 21 , at least 22, at least 23, at least 24, at least 25, at least 26, at least 27, at least 28, at least 29, or at least 30 points within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after
treatment has commenced. In some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject resulting in the subject's BMI being classified in a WHO BMI class that is lower than the WHO BMI class the subject's BMI was in before treatment was commenced. The different WHO BMI classes are listed in Table 1. For example, in some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as morbidly obese (class III) before treatment commenced that results in the subject being classified as obese (class I or II), overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. For another example, in some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as obese (class II) before treatment commenced that results in the subject being classified as obese (class I), overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. For yet another example, in some embodiments, an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of
Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), refers to the amount sufficient or the amount required to induce a reduction in the BMI of a subject that was classified as obese (class I) before treatment commenced that results in the subject being classified as overweight, or normal within a certain time frame after treatment has commenced, e.g., within 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months, 11 months or 12 months, 18 months, or 24 months after treatment has commenced. As will be appreciated by the skilled artisan, the effective amount of an agent, e.g., of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), may vary depending on various factors as, for example, on the desired biological response, e.g., on the amount of weight loss desired and the target time frame for the desired weight loss, and on the specific agent being used.
[00175] In some embodiments, methods for treating obesity in a subject are provided that further comprise determining whether the subject carries a variant allele of the CPD gene associated with obesity as provided herein, e.g., a mutation resulting in impairment or a reduction in CPD activity, and if the subject carries such a variant allele, administering an anti- obesity agent as provided herein, e.g., a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, to the subject. In some embodiments, the variant allele of the CPD gene associated with obesity is a SNP listed in Table 2. In some embodiments, the variant allele of the CPD gene associated with obesity is a SNP associated with a missense or nonsense mutation as listed in Table 6. In some embodiments, the variant allele of the CPD gene associated with obesity is a deletion, inversion, substitution, or gene fusion.
[00176] In some embodiments, methods for treating obesity in a subject are provided that further comprise determining whether the subject carries a variant allele of the CPD gene associated with obesity as provided herein, e.g., a mutation resulting in impairment or a reduction in CPD activity, and if the subject carries such a variant allele, administering an anti- obesity agent as provided herein, e.g., a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable
salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), to the subject. In some embodiments, the variant allele of the CPD gene associated with obesity is a SNP listed in Table 2. In some embodiments, the variant allele of the CPD gene associated with obesity is a SNP associated with a missense or nonsense mutation as listed in Table 6. In some embodiments, the variant allele of the CPD gene associated with obesity is a deletion, inversion, substitution, or gene fusion.
[00177] In some embodiments, the method further comprises determining whether the subject has a nucleic acid variant within his CPD gene are described further herein.
[00178] The obesity and subject to be treated are also as described herein. The treatment
(administration) step of either aspect may be carried out as follows.
[00179] It will be appreciated that anti-obesity agents, such as the dicarboxylate- containing ACE inhibitor perindopril, and medicaments according to the invention may be used in a monotherapy (i.e. the sole use of an agent or medicament capable of reducing the percentage change of total body weight by at least 5% or increasing energy expenditure or weight loss) for treating, ameliorating or preventing male obesity. Alternatively, anti-obesity agents, such as the dicarboxylate-containing ACE inhibitor perindopril, and medicaments according to the invention may be used as an adjunct to, or in combination with, known therapies for treating, ameliorating, or preventing obesity. For example, the dicarboxylate-containing ACE inhibitor perindopril may be used in combination with known active agents, compounds and medicaments for treating obesity and obesity-related conditions, such as orlistat, lorcaserin, phentermine or phentermine- topiramate. Or, the agent or medicament may be used in combination with an obesity management programme, such as a modified diet and exercise programme, gastroplasty or other suitable procedure.
[00180] It will be appreciated that anti-obesity agents, such as a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and medicaments according to the invention may be used in a monotherapy (i.e. the sole use of an agent or medicament capable of reducing the percentage change of total body weight by at least 5% or increasing energy expenditure or
weight loss) for treating, ameliorating or preventing male obesity. Alternatively, anti-obesity agents, such as a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and medicaments according to the invention may be used as an adjunct to, or in combination with, known therapies for treating, ameliorating, or preventing obesity. For example, a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) may be used in combination with known active agents, compounds and medicaments for treating obesity and obesity-related conditions, such as orlistat, lorcaserin, phentermine or phentermine- topiramate. Or, the agent or medicament may be used in combination with an obesity management programme, such as a modified diet and exercise programme, gastroplasty or other suitable procedure.
[00181] The term "combination" in the present invention is used as known to persons skilled in the art and may be present as a fixed combination, a non-fixed combination or kit-of- parts.
[00182] A "fixed combination" in the present invention is used as known to persons skilled in the art and is defined as a combination wherein a first active ingredient, such as the dicarboxylate-containing ACE inhibitor perindopril, and a second active ingredient are present together in one unit dosage or in a single entity. One example of a "fixed combination" is a pharmaceutical composition wherein the said first active ingredient and the said second active ingredient are present in a mixture for simultaneous administration, such as in a formulation. Another example of a "fixed combination" is a pharmaceutical combination wherein the said first active ingredient and the said second active ingredient are present in one unit without being in a mixture.
[00183] A "fixed combination" in the present invention is used as known to persons skilled in the art and is defined as a combination wherein a first active ingredient, such as a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an
combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and a second active ingredient are present together in one unit dosage or in a single entity. One example of a "fixed
combination" is a pharmaceutical composition wherein the said first active ingredient and the said second active ingredient are present in a mixture for simultaneous administration, such as in a formulation. Another example of a "fixed combination" is a pharmaceutical combination wherein the said first active ingredient and the said second active ingredient are present in one unit without being in a mixture.
[00184] A non-fixed combination or "kit-of-parts" in the present invention is used as known to persons skilled in the art and is defined as a combination wherein the said first active ingredient, such as the dicarboxylate-containing ACE inhibitor perindopril, and the said second active ingredient are present in more than one unit. One example of a non-fixed combination or kit-of-parts is a combination wherein the said first active ingredient and the said second active ingredient are present separately. The components of the non-fixed combination or kit-of-parts may be administered separately, sequentially, simultaneously (e.g. concomitantly), concurrently or chronologically staggered.
[00185] A non-fixed combination or "kit-of-parts" in the present invention is used as known to persons skilled in the art and is defined as a combination wherein the said first active ingredient, such as a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and the said second active ingredient are present in more than one unit. One example of a non-fixed combination or kit-of-parts is a combination wherein the said first active ingredient and the said second active ingredient are present separately. The components of the non-fixed combination or kit-of-parts may be administered separately, sequentially, simultaneously (e.g. concomitantly), concurrently or chronologically staggered.
[00186] The anti-obesity agents, such as the dicarboxylate-containing ACE inhibitor perindopril, and medicaments according to the invention may be combined in compositions having a number of different forms depending, in particular, on the manner in which the
composition is to be used. Thus, for example, the composition may be in the form of a powder, tablet, capsule, liquid, aerosol, spray, micellar solution, liposome suspension or any other suitable form that may be administered to an individual in need of treatment. It will be appreciated that the vehicle used in the compositions according to the invention should be one which is well-tolerated by the subject to whom it is given, and preferably enables delivery of the active ingredient to the cell and tissue types of interest, such as the brain, adrenal glands and liver.
[00187] The anti-obesity agents, such as a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), and medicaments according to the invention may be combined in compositions having a number of different forms depending, in particular, on the manner in which the composition is to be used. Thus, for example, the composition may be in the form of a powder, tablet, capsule, liquid, aerosol, spray, micellar solution, liposome suspension or any other suitable form that may be administered to an individual in need of treatment. It will be appreciated that the vehicle used in the compositions according to the invention should be one which is well-tolerated by the subject to whom it is given, and preferably enables delivery of the active ingredient to the cell and tissue types of interest, such as the brain, adrenal glands and liver.
[00188] Chronic oral use of the anti-obesity agents and medicaments according to the invention is preferred. Suitable formulations for such use are known in the art. Further examples are provided here.
[00189] A pharmaceutically acceptable vehicle is suitably used to formulate
pharmaceutical compositions. In one embodiment, the pharmaceutically acceptable vehicle may be a solid, and the composition may be in the form of a powder or tablet. A solid
pharmaceutically acceptable vehicle may include one or more substances which may also act as flavouring agents, lubricants, solubihzers, suspending agents, dyes, fillers, glidants, compression aids, inert binders, sweeteners, preservatives, dyes, coatings, or tablet-disintegrating agents. The vehicle may also be an encapsulating material. In powders, the vehicle is a finely divided solid that is in admixture with the finely divided active agents according to the invention. In tablets,
the active agent may be mixed with a vehicle having the necessary compression properties in suitable proportions and compacted in the shape and size desired. The powders and tablets preferably contain up to 99% of the active agents. Suitable solid vehicles include, for example, calcium phosphate, magnesium stearate, talc, sugars, lactose, dextrin, starch, gelatine, cellulose, polyvinylpyrrolidone, low melting waxes and ion exchange resins.
[00190] However, the pharmaceutical vehicle may be a liquid, and the pharmaceutical composition is in the form of a solution. Liquid vehicles are used in preparing solutions, suspensions, emulsions, syrups, elixirs and pressurized compositions. The active agent according to the invention may be dissolved or suspended in a pharmaceutically acceptable liquid vehicle such as water, an organic solvent, a mixture of both or pharmaceutically acceptable oils or fats. The liquid vehicle can contain other suitable pharmaceutical additives such as solubilizers, emulsifiers, buffers, preservatives, sweeteners, flavouring agents, suspending agents, thickening agents, colours, viscosity regulators, stabilisers or osmo- regulators. Suitable examples of liquid vehicles for oral and parenteral administration include water (partially containing additives as above, e.g. cellulose derivatives, preferably sodium carboxymethyl cellulose solution), alcohols (including monohydric alcohols and polyhydric alcohols, e.g. glycols) and their derivatives, and oils (e.g. fractionated coconut oil and arachis oil). For parenteral administration, the vehicle can also be an oily ester such as ethyl oleate and isopropyl myristate. Sterile liquid vehicles are useful in sterile liquid form compositions for parenteral administration. The liquid vehicle for pressurised compositions can be a halogenated hydrocarbon or other pharmaceutically acceptable propellant.
[00191] Liquid pharmaceutical compositions, which are sterile solutions or suspensions, can be utilised by, for example, intramuscular, intrathecal, epidural, intraperitoneal, intravenous and particularly subcutaneous injection. The agent may be prepared as a sterile solid
composition that may be dissolved or suspended at the time of administration using sterile water, saline, or other appropriate sterile injectable medium.
[00192] Medicaments comprising anti-obesity agents and compounds of the invention may be used in a number of ways. Oral administration is preferred, in which case the agents or compounds may be contained within a composition that may, for example, be ingested orally in the form of a tablet, capsule or liquid. Compositions comprising agents or compounds of the invention may be administered by inhalation (e.g. intranasally).
[00193] The agents and medicaments of the invention may be administered orally in the form of a sterile solution or suspension containing other solutes or suspending agents (for example, enough saline or glucose to make the solution isotonic), bile salts, acacia, gelatin, sorbitan monoleate, polysorbate 80 (oleate esters of sorbitol and its anhydrides copolymerised with ethylene oxide) and the like. The agents and medicaments used according to the invention can also be administered orally either in liquid or solid composition form. Compositions suitable for oral administration include solid forms, such as pills, capsules, granules, tablets, and powders, and liquid forms, such as solutions, syrups, elixirs, and suspensions. Forms useful for parenteral administration include sterile solutions, emulsions, and suspensions.
[00194] Agents and medicaments according to the invention may also be incorporated within a slow- or delayed-release device. Such devices may, for example, be inserted on or under the skin, and the agent or medicament may be released over weeks or even months. The device may be located at least adjacent the treatment site. Such devices may be particularly advantageous when long-term treatment with agents and medicaments used according to the invention is required and which would normally require frequent administration (e.g. at least daily injection).
[00195] In some embodiments, agents and medicaments according to the invention may be administered to a subject by injection into the blood stream or directly into a site requiring treatment. Injections may be intravenous (bolus or infusion) or subcutaneous (bolus or infusion), or intradermal (bolus or infusion).
[00196] It will be appreciated that the amount of the agent that is required is determined by its biological activity and bioavailability, which in turn depends on the mode of
administration, the physiochemical properties of the agent, and whether it is being used as a monotherapy or in a combined therapy. The frequency of administration will also be influenced by the half-life of the agent within the subject being treated. Optimal dosages to be administered may be determined by those skilled in the art, and will vary with the particular agent in use, the strength of the pharmaceutical composition or medicament, the mode of administration, and the advancement of the disease. Additional factors depending on the particular subject being treated will result in a need to adjust dosages, including the subject's age, weight and diet and the time of administration.
[00197] Generally, a daily dose of between 0.001 g kg of body weight and 10 mg/kg of body weight of agent or medicament according to the invention may be used for treating, ameliorating, or preventing male obesity, depending upon which agent or medicament is used. More preferably, the daily dose is between 0.01 g kg of body weight and 1 mg/kg of body weight, more preferably between 0.1 g kg and 100 g kg body weight, and most preferably between approximately 0.1 μg kg and 10 μg kg body weight.
[00198] A therapeutically effective amount of agent is suitably administered to the subject.
For example, the therapeutically effective amount of drug used may be from about 0.001 ng to about 1 mg, and preferably from about 0.01 ng to about 100 ng. It is preferred that the amount of drug is an amount from about 0.1 ng to about 10 ng, and most preferably from about 0.5 ng to about 5 ng.
[00199] The agent or medicament may be administered before, during or after onset of male obesity. Daily doses may be given as a single administration (e.g. a single daily injection, a single daily tablet intake, etc.). Alternatively, the agent or medicament may require
administration twice or more times during a day. As an example, agents and medicaments may be administered as two (or more, depending upon the severity of the disease being treated) daily doses of between 0.07 μg and 700 mg (i.e. assuming a body weight of 70 kg). A patient receiving treatment may take a first dose upon waking and then a second dose in the evening (if on a two dose regime) or at 3- or 4-hourly intervals thereafter. Alternatively, a slow release device may be used to provide optimal doses of agents and medicaments according to the invention to a patient without the need to administer repeated doses. Known procedures, such as those conventionally employed by the pharmaceutical industry (e.g. in vivo experimentation, clinical trials, etc.), may be used to form specific formulations of the agents and medicaments according to the invention and precise therapeutic regimes (such as daily doses of the agents and medicaments and the frequency of administration). As an example, patients may receive perindopril 8mg, once daily, for 12 weeks.
[00200] It will be appreciated that the combination therapy as described herein may comprise the administration of one or more additional therapeutics, e.g., that improve the combination therapy's bioavailability, reduce and/or modify their metabolism, inhibit their excretion, and/or modify their distribution within the body, and/or aid in the prophylaxis, diagnosis, or imaging of the condition. Exemplary additional therapeutics include, but are not
limited to, small organic molecules such as drug compounds (e.g. , compounds approved by the Food and Drugs Administration as provided in the Code of Federal Regulations (CFR)), peptides, proteins, carbohydrates, monosaccharides, oligosaccharides, polysaccharides, nucleoproteins, mucoproteins, lipoproteins, synthetic polypeptides or proteins, small molecules linked to proteins, glycoproteins, steroids, nucleic acids, DNAs, RNAs, nucleotides, nucleosides, oligonucleotides, antisense oligonucleotides, lipids, hormones, vitamins and cells.
[00201] For example, compositions as provided herein, e.g., compositions comprising a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, may be administered in combination with anti-obesity agents, anti-diabetic agents, and/or agents for treating cardiovascular disease in order to improve the treatment of obesity. The term "in combination with," as used in this context, is not intended to imply that the active agents must be administered at the same time and/or formulated for delivery together, although these methods of delivery are within the scope of the invention. Compositions can be administered in temporal proximity, e.g. , concurrently with, prior to, or subsequent to, one or more other desired therapeutics or medical procedures. In general, each active agent will be administered at a dose and/or on a time schedule determined for that active agent.
[00202] For example, compositions as provided herein, e.g., compositions comprising a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), may be administered in combination with anti-obesity agents, anti-diabetic agents, and/or agents for treating
cardiovascular disease in order to improve the treatment of obesity. The term "in combination with," as used in this context, is not intended to imply that the active agents must be
administered at the same time and/or formulated for delivery together, although these methods of delivery are within the scope of the invention. Compositions can be administered in temporal proximity, e.g. , concurrently with, prior to, or subsequent to, one or more other desired therapeutics or medical procedures. In general, each active agent will be administered at a dose and/or on a time schedule determined for that active agent.
[00203] Those of skill in the art will understand that the dosage of the active agents will have to be adjusted when used in combination. Such dose adjustments are routine in the art, and suitable strategies for finding appropriate dosages for combination therapies include, without limitation, those described in Mandrekar, Dose-finding trial designs for combination therapies in oncology. Clin OncoL 32(2):65-7 (2014), the entire contents of which are incorporated herein by reference. In general, it is expected that the active agents utilized in combination be utilized at levels that do not exceed the levels at which they are utilized individually. In some embodiments, the levels utilized in combination will be lower than those utilized individually.
[00204] Any of the active agents as described herein may be formulated in dosage unit form for ease of administration and uniformity of dosage. It will be understood, however, that the total daily usage of the active agents will be decided by the attending physician within the scope of sound medical judgment. The specific therapeutically effective dose level for any particular subject or organism will depend upon a variety of factors including the disease, disorder, or condition being treated and the severity of the disorder; the activity of the specific agent employed; the specific composition employed; the age, body weight, general health, sex and diet of the subject; the time of administration, route of administration, and rate of excretion of the specific agent employed; the duration of the treatment; and like factors well known in the medical arts.
[00205] The active agents can be administered by any route, including enteral {e.g., oral), parenteral, intravenous, intramuscular, intra-arterial, intramedullary, intrathecal, subcutaneous, intraventricular, transdermal, interdermal, rectal, intravaginal, intraperitoneal, topical (as by powders, ointments, creams, and/or drops), mucosal, nasal, buccal, sublingual; by intratracheal instillation, bronchial instillation, and/or inhalation; and/or as an oral spray, nasal spray, and/or aerosol. Specifically contemplated routes are oral administration, intravenous administration {e.g., systemic intravenous injection), regional administration via blood and/or lymph supply, and/or direct administration to an affected site. In general the most appropriate route of administration will depend upon a variety of factors including the nature of the agent {e.g., its stability in the environment of the gastrointestinal tract), the condition of the subject {e.g. , whether the subject is able to tolerate oral administration).
[00206] The exact amount of an active agent required to achieve an effective amount will vary from subject to subject, depending, for example, on species, age, and general condition of a
subject, severity of the side effects or disorder, identity of the particular compound(s), mode of administration, and the like. The desired dosage can be delivered three times a day, two times a day, once a day, every other day, every third day, every week, every two weeks, every three weeks, or every four weeks. In certain embodiments, the desired dosage can be delivered using multiple administrations (e.g., two, three, four, five, six, seven, eight, nine, ten, eleven, twelve, thirteen, fourteen, or more administrations).
[00207] In certain embodiments, an effective amount of an active agent for administration one or more times a day to an obese adult human may comprise about 0.0001 mg to about 3000 mg, about 0.0001 mg to about 2000 mg, about 0.0001 mg to about 1000 mg, about 0.001 mg to about 1000 mg, about 0.01 mg to about 1000 mg, about 0.1 mg to about 1000 mg, about 1 mg to about 1000 mg, about 1 mg to about 100 mg, about 0.1 mg to about 10 mg, or about 0.1 mg to about 15 mg, of a compound per unit dosage form.
[00208] In certain embodiments, the active agent may be administered orally or parenterally to an adult human at dosage levels sufficient to deliver from about 0.001 mg/kg to about 100 mg/kg, from about 0.01 mg/kg to about 50 mg/kg, preferably from about 0.1 mg/kg to about 40 mg/kg, preferably from about 0.5 mg/kg to about 30 mg/kg, from about 0.01 mg/kg to about 10 mg/kg, from about 0.1 mg/kg to about 10 mg/kg, and more preferably from about 0.01 mg/kg to about 1 mg/kg, of subject body weight per day, one or more times a day, to obtain the desired therapeutic effect.
[00209] It will be appreciated that dose ranges as described herein provide guidance for the administration of provided pharmaceutical compositions to an adult. The amount to be administered to, for example, a child or an adolescent can be determined by a medical practitioner or person skilled in the art and can be lower or the same as that administered to an adult.
Diagnostic and Prognostic Methods
[00210] Some aspects of the present disclosure provide methods for selecting a subject for treatment of obesity with a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the method comprises identifying a male subject carrying a variant allele within the CPD gene. In some embodiments, the method comprises
determining that the subject is obese, e.g., that the subject exhibits class I, II, or III obesity. In some embodiments, the method comprises determining that the subject is male.
[00211] Some aspects of the present disclosure provide methods for selecting a subject for treatment of obesity with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). In some embodiments, the method comprises identifying a male subject carrying a variant allele within the CPD gene. In some embodiments, the method comprises determining that the subject is obese, e.g., that the subject exhibits class I, II, or III obesity. In some embodiments, the method comprises determining that the subject is male.
[00212] Some aspects of this disclosure provide methods for identifying a subject who is predisposed to obesity, comprising (i) determining whether the subject carries a variant allele of the CPD gene; and (ii) if the subject is determined to carry variant allele of the CPD gene, identifying the subject as predisposed to obesity. In some embodiments, step (i) comprises detecting the variant allele of the CPD gene in the genome of a cell of the subject. In some embodiments, the method further comprises obtaining a cell or tissue sample from the subject. In some embodiments, the method comprises isolating genomic DNA from a cell or tissue obtained from the subject.
[00213] Some aspects of this disclosure provide methods for selecting a subject for treatment of obesity with perindopril or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. The methods generally comprise (i) determining whether an obese subject carries a variant allele of the CPD gene; and (ii) if the subject is determined to carry a CPD gene, selecting the subject for treatment of obesity with perindopril or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the method further comprises administering an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject.
[00214] Some aspects of this disclosure provide methods for selecting a subject for treatment of obesity with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt,
stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). The methods generally comprise (i) determining whether an obese subject carries a variant allele of the CPD gene; and (ii) if the subject is determined to carry a CPD gene, selecting the subject for treatment of obesity with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). In some embodiments, the method further comprises administering an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II- V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) to the subject.
[00215] In some embodiments of the methods for identifying a subject who is predisposed to obesity or the methods for selecting a subject for treatment of obesity with perindopril or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, step (i) comprises performing a genotyping assay on genomic DNA obtained from the subject. In some embodiments, the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay. In some embodiments, the allele-specific PCR assay is an intercalating dye assay, a FRET primer assay, or an ALPHASCREEN™ assay. In some embodiments, the primer extension assay is an ARMS (amplification refractory mutation system) assay, a kinetic or real-time PCR assay, a SNPSTREAM™ assay, a GENETIC BIT ANALYSIS™ (GBA) assay, a multiplex
minisequencing assay, a SNAPSHOT™ assay, a PYROSEQUENCING™ assay, a
MASSEXTEND™ assay, a MASSARRAY™ assay, a MALDI mass spectrometry-based assay, a microarray minisequencing assay, an APEX (arrayed primer extension) assay, a sequence specific priming (SSP) assay, a microarray primer extension assay, a tag array assay, a coded microsphere assay, a template-directed incorporation (TDI) assay, or a fluorescence polarization assay. In some embodiments, the oligonucleotide ligation assay is a colorimetric oligonucleotide ligation assay (OLA), a sequence-coded OLA, a microarray ligation assay, a ligase chain
reaction assay, a padlock probe assay, or a rolling circle amplification assay. In some embodiments, the hybridization assay is a reverse dot blot assay, a line probe assay (LiPA), a microarray assay, a dynamic allele-specific hybridization (DASH) assay, a PNA or locked nucleic acid (LNA) probe assay, a TAQMAN™ assay, or a molecular beacon assay. In some embodiments, the endonuclease cleavage assay is a restriction site analysis (RFLP), or an INVADER™ assay.
[00216] In some embodiments of the methods for identifying a subject who is predisposed to obesity or the methods for selecting a subject for treatment of obesity with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), step (i) comprises performing a genotyping assay on genomic DNA obtained from the subject. In some embodiments, the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay. In some embodiments, the allele- specific PCR assay is an intercalating dye assay, a FRET primer assay, or an
ALPHASCREEN™ assay. In some embodiments, the primer extension assay is an ARMS (amplification refractory mutation system) assay, a kinetic or real-time PCR assay, a
SNPSTREAM™ assay, a GENETIC BIT ANALYSIS™ (GBA) assay, a multiplex
minisequencing assay, a SNAPSHOT™ assay, a PYROSEQUENCING™ assay, a
MASSEXTEND™ assay, a MASSARRAY™ assay, a MALDI mass spectrometry-based assay, a microarray minisequencing assay, an APEX (arrayed primer extension) assay, a sequence specific priming (SSP) assay, a microarray primer extension assay, a tag array assay, a coded microsphere assay, a template-directed incorporation (TDI) assay, or a fluorescence polarization assay. In some embodiments, the oligonucleotide ligation assay is a colorimetric oligonucleotide ligation assay (OLA), a sequence-coded OLA, a microarray ligation assay, a ligase chain reaction assay, a padlock probe assay, or a rolling circle amplification assay. In some embodiments, the hybridization assay is a reverse dot blot assay, a line probe assay (LiPA), a microarray assay, a dynamic allele-specific hybridization (DASH) assay, a PNA or locked nucleic acid (LNA) probe assay, a TAQMAN™ assay, or a molecular beacon assay. In some
embodiments, the endonuclease cleavage assay is a restriction site analysis (RFLP), or an INVADER™ assay.
[00217] In some embodiments of the methods for identifying a subject who is predisposed to obesity or the methods for selecting a subject for treatment of obesity with perindopril or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, the variant allele of the CPD gene comprises one or more alleles provided herein, e.g., a variant allele characterized by a SNP associated with obesity as provided herein, e.g., with a SNP listed in Table 2 or in Table 6. In some embodiments, the SNP is selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the method further comprises determining whether the subject is obese. In some embodiments, the subject is not obese. In some
embodiments, the method further comprises administering healthcare to the subject to prevent the subject from becoming obese. In some embodiments, the method further comprises monitoring the body weight of the subject and, if the subject is determined to be obese during the monitoring, selecting the subject to treatment with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the method further comprises administering an effective amount of perindopril, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject. In some embodiments, the subject is male.
[00218] In some embodiments of the methods for identifying a subject who is predisposed to obesity or the methods for selecting a subject for treatment of obesity with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof), the variant allele of the CPD gene comprises one or more alleles provided herein, e.g., a variant allele characterized by a SNP associated with obesity as provided herein, e.g., with a SNP listed in Table 2 or in Table 6. In some embodiments, the SNP is selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. In some embodiments, the method further comprises determining whether the subject is obese. In some embodiments, the subject is not obese. In some embodiments, the method further
comprises administering healthcare to the subject to prevent the subject from becoming obese. In some embodiments, the method further comprises monitoring the body weight of the subject and, if the subject is determined to be obese during the monitoring, selecting the subject to treatment with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). In some embodiments, the method further comprises administering an effective amount of a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) to the subject. In some embodiments, the subject is male.
[00219] The step of determining or detecting the presence of a variant allele of the CPD gene according to various methods of the present disclosure may be carried out in vivo or in vitro. In one embodiment, detection of a variant allele of the CPD gene is performed in vitro on a biological sample obtained from the individual.
[00220] The biological sample may be a body fluid (e.g., blood, urine, sputum, saliva), or a cell or tissue sample, including, for example, and without limitation, a mucosal scraping sample). A nucleic acid comprising a sequence of interest, e.g., a genomic sequence of the CPD gene, may be obtained from a biological sample comprising DNA (e.g. gDNA or cDNA) or RNA (e.g. mRNA). If required, concentration and/or isolation of the nucleic acid from the sample can be done by any method known in the art or using commercial kits (such as the QIAamp DNA Blood Kit from Qiagen (Hilden, Germany) for the isolation of nucleic acids from blood samples, the 'High pure PCR Template Preparation Kit' (Roche Diagnostics, Basel, Switzerland) or the DNA purification kits (PureGene, Gentra, Minneapolis, US). Other well- known procedures for the isolation of DNA or RNA from a biological sample are also available (see for example Sambrook et al., Molecular Cloning: a Laboratory Manual, Cold Spring Harbor Laboratory Press, 1989, Cold Spring Harbor, US; and Ausubel et al., Current Protocols in Molecular Biology, 2003, John Wiley & Sons). When the quantity of nucleic acid is low or insufficient for the assessment, the nucleic acid of interest may be amplified. Amplification may
be accomplished by methods known in the art, including, for example, the PCR, ligase chain reaction (LCR), nucleic acid sequence-based amplification (NASBA), strand displacement amplification, rolling circle amplification, T7 -polymerase amplification and reverse transcription PCR (RT-PCR). The methods of the present disclosure may comprise a step of isolating nucleic acids from a biological sample and/or an amplification step.
[00221] Numerous methods suitable for detecting variant alleles or haploptypes of genes or genomic regions, including methods suitable for the detection of single nucleotide
polymorphisms in genomic sequences obtained from a subject are known in the art. Exemplary suitable genotyping assays for determining the presence of a variant allele of the CPD gene in a subject include, without limitation, allele-specific PCR methods such as intercalating dye, FRET primers, and Alphascreen(TM); primer extension methods such as ARMS (amplification refractory mutation system), kinetic or real-time PCR, SNPstream(TM), Genetic Bit
Analysis(TM) (GBA), multiplex minisequencing, SnaPshot(TM), Pyrosequencing(TM), MassEXTEND(TM), MassArray(TM), the MALDI mass spectrometry-based "GOOD" assay, microarray minisequencing, APEX (arrayed primer extension), sequence specific priming (SSP), microarray primer extension, Tag arrays, coded microspheres, template-directed incorporation (TDI), fluorescence polarization; oligonucleotide ligation methods such as colorimetric OLA (oligonucleotide ligation assay), sequence-coded OLA, microarray ligation, ligase chain reaction, padlock probes, and rolling circle amplification; hybridization methods such as reverse dot blot, line probe assay (LiPA), GeneChip(TM) microarrays, dynamic allele-specific hybridization (DASH), PNA and locked nucleic acid (LNA) probes, TaqMan(TM) (5' nuclease assay), and molecular beacons; and endonuclease cleavage methods such as restriction site analysis (RFLP) and Invader(TM) assay. The detection of the presence or absence of a variant allele may also, for example, be determined by DNA or RNA hybridization, sequencing, PCR, primer extension, multiplex ligation-dependent probe amplification (MLPA), oligonucleotide ligation assay (OLA) or restriction site analysis.
[00222] In some embodiments, a reference sequence for the CPD gene is be defined by the nucleotide sequence of SEQ ID NO: 1. Variants of the CPD gene may therefore be detected in a subject by comparing the sequence information obtained from the subject to the sequence of SEQ ID NO: 1.
[00223] Sequence identity between nucleotide sequences can be determined by comparing an alignment of the sequences. When an equivalent position in the compared sequences is occupied by the same base, then the molecules are identical at that position. Scoring an alignment as a percentage of identity is a function of the number of identical bases at positions shared by the compared sequences. When comparing sequences, optimal alignments may require gaps to be introduced into one or more of the sequences to take into consideration possible insertions and deletions in the sequences. Sequence comparison methods may employ gap penalties so that, for the same number of identical molecules in sequences being compared, a sequence alignment with as few gaps as possible, reflecting higher relatedness between the two compared sequences, will achieve a higher score than one with many gaps. Calculation of maximum percent identity involves the production of an optimal alignment, taking into consideration gap penalties.
[00224] Suitable computer programs for carrying out sequence comparisons are widely available in the commercial and public sector. Examples include MatGat (Campanella et al., 2003; program available from http://bitincka.com/ledion/matgat), Gap (Needleman & Wunsch, 1970, supra), FASTA (Altschul et al., 1990; program available from http://www.ebi.ac.uk/fasta), Clustal W 2.0 and X 2.0 (Larkin et al., 2007,; program available from
http://www.ebi.ac.uk/tools/clustalw2) and EMBOSS Pairwise Alignment Algorithms
(Needleman & Wunsch, 1970, supra; Kruskal, 1983; programs available from
http://www.ebi.ac.uk/tools/emboss/align). All programs may be run using default parameters.
[00225] The percentage identity for two sequences may take different values depending on:- (i) the method used to align the sequences or structural alignment from 3D comparison; and (ii) the parameters used by the alignment method, for example, local vs global alignment, the pair-score matrix used (e.g. BLOSUM62, PAM250, Gonnet etc.), and gap-penalty, e.g.
functional form and constants. Having made the alignment, there are many different ways of calculating percentage identity between the two sequences. For example, one may divide the number of identities by: (i) the length of shortest sequence; (ii) the length of alignment; (iii) the mean length of sequence; (iv) the number of non-gap positions; or (iv) the number of equivalenced positions excluding overhangs. Furthermore, it will be appreciated that percentage identity is also strongly length dependent. Therefore, the shorter a pair of sequences is, the higher the sequence identity one may expect to occur by chance. It is sometimes desirable to describe
sequence identity between two sequences in reference to a particular length or region (e.g. two sequences may be described as having at least 95% identity over a length of at least 50, 100, 250, 500, 750 or 1000 base pairs).
[00226] Hence, it will be appreciated that the accurate alignment of DNA sequences is a complex process. The popular multiple alignment program ClustalW (Larkin et al., 2007, supra) is a preferred way for generating multiple alignments of DNA in accordance with the invention. Suitable parameters for DNA alignments using ClustalW may be as follows: Gap Open Penalty = 15.0, Gap Extension Penalty = 6.66, Matrix = Identity, ENDGAP = -1 and GAPDIST = 4. Those skilled in the art will be aware that it may be necessary to vary these and other parameters for optimal sequence alignment.
[00227] Calculation of percentage identities between two polynucleotide sequences may then be calculated from such an alignment as (N/T)*100, where N is the number of positions at which the sequences share an identical residue, and T is the total number of positions compared including gaps but excluding overhangs. In some embodiments, calculating percentage identity between two sequences comprises (i) preparing a sequence alignment using the ClustalW program using a suitable set of parameters, for example, as set out above; and (ii) inserting the values of N and T into the following formula:- Sequence Identity = (N/T)* 100.
[00228] Alternative methods for identifying similar sequences will be known to those skilled in the art. For example, a substantially similar nucleotide sequence will be encoded by a sequence which hybridises to the sequence shown in SEQ ID NO: 1 or its complement under stringent conditions.
[00229] The nucleic acid variant may be within a non-coding region of the CPD gene.
The 10 SNPs identified herein in Table 2 are in intronic regions scattered throughout the gene. In some embodiments, the SNPs are located in exonic regions, e.g., the missense and nonsense mutations listed in Table 6. According to some embodiments, the variant allele comprises a SNP. In some embodiments, the SNP is selected from the group consisting of: rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406. These 10 SNPs, alone or in any combination, are strongly associated with obesity or an increased risk for having or developing the same, as demonstrated in Example 3.
[00230] Determining the presence of a variant allele of the CPD gene may thus comprise performing one or more assays from the group consisting of: nucleic acid amplification (for
example, polymerase chain reaction (PCR)), primer extension, restriction endonuclease digestion, sequencing, oligonucleotide hybridization (such as SNP-specific oligonucleotide hybridization), and a DNAse protection assay. Further methods suitable for determining a variant allele of the CPD gene are described herein and additional suitable methods will be apparent to the skilled artisan based on the present disclosure.
[00231] In some embodiments, the step of determining the presence or absence of a variant allele within the CPD gene in a subject comprises determining the presence or absence of a CPD haplotype of the subject, by detecting one or more or all SNPs as defined herein. The term "haplotype" refer to specific nucleic acid variants (such as SNP polymorphisms) within the CPD gene. For example, an individual may have a haplotype comprising one or more of the 10 SNPs identified herein.
[00232] In some embodiments, determining or detecting the presence or absence of a variant allele of the CPD gene may include determining or detecting the presence or absence of just one, or of at least two, three, four, five, six, seven, eight, nine or 10 nucleic variant alleles in the CPD gene, e.g., by determining or detecting the presence or absence of just one, or at least two, three, four, five, six, seven, eight, nine or all 10 of the SNPs identified in Table 2.
[00233] According to some aspects of this disclosure, determining or detecting the presence or absence of a variant allele of the CPD gene may be achieved with one or more CPD allele-specific primer or primer-pair, and/or with one or more CPD-specific probes (such as oligonucleotide probes, PNA probes and/or other artificial probes). Suitable primers, primer pairs, and probes for detecting variant alleles of genes, including variant alleles of the CPD gene, are well known to those of skill in the art.
[00234] In some embodiments, determining that the subject carries a variant allele of the
CPD gene comprises determining the expression level of a CPD gene product, e.g., a CPD mRNA or protein in a cell or tissue of the subject. In some embodiments, if the expression level of the CPD gene is lower than the expression level expected or observed in a healthy subject or population, then the subject is determined to carry a variant allele of the CPD gene. The term "expression level", as used herein, refers to information about the level of one or more gene products (e.g., an mRNA, a protein, or a combination thereof) in a cell or tissue. In some embodiments, the detection of one or more gene mutations, and/or a decrease in expression levels as described herein may be based on one or more measurements or assays, for example, a
quantitative or semi-quantitative value of expression of the CPD gene, reflective of the signal obtained from a quantitative or semi-quantitative assay detecting the abundance of a gene product (e.g., a protein or a nucleic acid transcript encoded by a CPD gene). Suitable assays for the detection of gene expression products are well known to those of skill in the art and include, for example, western blots, ELISA, RT-PCR (e.g., end-point RT-PCR, real-time PCR, or qPCR), protein or nucleic acid microarray, and massive parallel sequencing assays. However, any suitable assay may be used based on hybridization, specific binding (e.g., antibody binding), or any other technique, as aspects of the invention are not limited in this respect.
[00235] In some embodiments, the presence of one or more gene mutations, and/or a decrease in expression levels as described herein may involve a plurality of data points, for example, quantitative or semi-quantitative values of expression and/or one or sequence or mutation data points. In some embodiments, the presence of one or more gene mutations, and/or an increase or decrease in expression levels as described herein may be evaluated in a biopsy sample. Methods for the detection or for the generation of data for one or more gene mutations, and/or an increase or decrease in expression levels as described herein are well known to those in the art and include, for example, southern blot, western blot, ELISA, northern blot, reverse northern blot, RT-PCR (e.g. endpoint, real time, or qPCR), microarray (for either protein or transcript detection), SNP analysis, PCR, hybridization assays, sequencing assays, etc., or any combination thereof (for exemplary detection methods, see, e.g., Sambrook et al., Molecular Cloning: A Laboratory Manual, Third Edition (3 Volume Set), Cold Spring Harbor Laboratory Press; 3rd edition (January 15, 2001), ISBN-10: 0879695773; Robert Griitzmann (Editor), Christian Pilarsky (Editor), Cancer Gene Profiling: Methods and Protocols (Methods in
Molecular Biology), Humana Press; 1st edition (November 6, 2009), ISBN-10: 1934115762, both incorporated herein by reference for disclosure of gene product detection and expression profiling methods).
[00236] In some embodiments, a quantitative expression value is a value reflecting the abundance of a gene transcript in the starting sample, for example, a tumor cell or tissue sample. In some embodiments, a semi-quantitative expression value is a value reflecting the abundance of a gene transcript in the starting sample in relation to a control or reference quantity, e.g., a quantity measured or expected in a healthy cell or in a cell of the same type obtained from a healthy individual. Methods of calculating semi-quantitative expression values are well known
to those in the art. Appropriate control or reference quantities for the generation of semiquantitative expression values are well known to those in the art and include, for example, expression values of housekeeping genes (e.g., beta-actin or GAPDH), external controls (e.g., spiked in RNA or DNA controls not usually expressed in the cell to be analyzed), overall expression values (e.g., all expression values obtained from a cell added together), or historic or empiric values.
[00237] Some aspects of this disclosure provide methods of determining whether a subject is predisposed to develop obesity, wherein the method comprises determining whether the subject carries a variant allele within the CPD gene associated with obesity as provided herein. In some embodiments, the method further comprises identifying the subject as predisposed to obesity if the subject is determined to carry a variant allele of the CPD gene that is associated with obesity as provided herein. In some embodiments, the method further comprises administering healthcare to the subject. For example, in some embodiments, the subject is not obese, and the healthcare may include lifestyle and clinical measures to manage the subject's weight. In some embodiments, the healthcare may include administering a dicarboxylate- containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject.
[00238] Some aspects of this disclosure provide methods of determining whether a subject is predisposed to develop obesity, wherein the method comprises determining whether the subject carries a variant allele within the CPD gene associated with obesity as provided herein. In some embodiments, the method further comprises identifying the subject as predisposed to obesity if the subject is determined to carry a variant allele of the CPD gene that is associated with obesity as provided herein. In some embodiments, the method further comprises administering healthcare to the subject. For example, in some embodiments, the subject is not obese, and the healthcare may include lifestyle and clinical measures to manage the subject's weight. In some embodiments, the healthcare may include administering a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof) to the subject.
[00239] Some aspects of this disclosure provide methods for identifying a male subject who would benefit from treatment with an anti-obesity agent provide herein, e.g., with a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof. In some embodiments, the method comprises determining the presence or absence of a variant allele within the CPD gene. Again, it will be understood that the presence of the variant allele indicates that the subject would benefit from treatment with the agent.
[00240] Some aspects of this disclosure provide methods for identifying a male subject who would benefit from treatment with an anti-obesity agent provide herein, e.g., with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof). In some embodiments, the method comprises determining the presence or absence of a variant allele within the CPD gene. Again, it will be understood that the presence of the variant allele indicates that the subject would benefit from treatment with the agent.
[00241] In a preferred embodiment, the anti-obesity agent is the dicarboxylate-containing
ACE inhibitor perindopril.
[00242] In a preferred embodiment, the anti-obesity agent is with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof).
[00243] The methods provided herein may further comprise a step of treating the subject based on the results of the method (for example, with a dicarboxylate-containing ACE inhibitor, such as, for example, perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, as described herein). That is to say, if the methods result in the presence of a nucleic acid variant being detected in the subject's CPD gene, the subject could be said to be predisposed to obesity and likely to benefit from treatment with an anti- obesity agent. The method may thus comprise a treatment step. Details of suitable treatment steps are provided herein.
[00244] The methods provided herein may further comprise a step of treating the subject based on the results of the method (for example, with with a compound delineated herein (e.g., trandolapril, spirapril, moexipril, clizapril, or temocapril, or an combination thereof, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formulae II-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof)f, as described herein). That is to say, if the methods result in the presence of a nucleic acid variant being detected in the subject's CPD gene, the subject could be said to be predisposed to obesity and likely to benefit from treatment with an anti-obesity agent. The method may thus comprise a treatment step. Details of suitable treatment steps are provided herein.
Kits & Molecular Tools
[00245] Some aspects of this disclosure provide kits for carrying out the methods provided herein. For example, in some embodiments, a kit for detecting a variant allele of the CPD gene and/or for identifying a subject who is predisposed to obesity, the kit comprising reagents for performing a genotyping assay for detecting a variant allele of the CPD gene in genomic DNA obtained from the subject. In some embodiments, a kit for identifying a subject who would benefit from treatment with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, the kit comprising reagents for performing a genotyping assay for detecting a variant allele of the CPD gene in genomic DNA obtained from the subject. In some embodiments, the kit comprises a primer, primer pair, or probe for detecting the variant allele of the CPD gene. In some embodiments, the kit comprises a primer, primer pair, or probe for detecting one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
[00246] In some embodiments, a kit for identifying a male subject who is predisposed to obesity, comprising means for determining the presence or absence of a nucleic acid variant within the CPD gene in the subject's nucleic acids. Additionally provided, according to an eighth aspect of the invention, is a kit for identifying a male subject who would benefit from treatment with an anti-obesity agent, comprising means for detecting the presence or absence of a nucleic acid variant within the CPD gene in the subject's nucleic acids.
[00247] It will be understood that the positive determination or detection of a nucleic acid variant within the subject's CPD gene means that the subject is predisposed to obesity and likely to benefit from treatment with an anti-obesity agent. The subjects, obesity, anti-obesity agents and nucleic acid variants may be as already defined herein. The means for determining whether a male subject has a nucleic acid variant within his CPD gene are described further herein.
[00248] In some embodiments, the kit may further comprise instructions for identifying whether the individual is predisposed to obesity, or would benefit from treatment with an anti- obesity agent, based on the determination of the presence or absence of a nucleic acid variant. Again, it will be understood that the positive determination of the presence of a nucleic acid variant within the subject's CPD gene means that the subject is predisposed to obesity and likely to benefit from treatment with an anti-obesity agent.
[00249] Further provided is an obesity diagnosis kit which comprises means for determining the CPD haplotype of a male subject. The obesity diagnosis kit may be a kit for determining a predisposition to male obesity. The means for determining the haplotype may be as defined herein. The obesity and subject may be as defined herein. Additionally provided is the use of the obesity diagnosis kit as defined above to determine the CPD haplotype of a male individual and/or to determine a predisposition to male obesity.
[00250] Also provided is the use of one or more probes capable of binding specifically to a region of nucleic acid which includes a SNP distinctive of the CPD gene in a male individual for the diagnosis of obesity or a predisposition to obesity in the individual, or to identify whether the individual would benefit from treatment with an anti-obesity agent. The design of suitable probes will be within the remit of the skilled person. Additionally provided is the use of a primer capable of amplifying CPD nucleic acid which includes an SNP distinctive of a CPD allele for the diagnosis of male obesity or a predisposition to obesity, or to identify whether an individual would benefit from treatment with an anti-obesity agent. The design of suitable primers will be within the remit of the skilled person. The SNPs may be as defined herein.
[00251] Some aspects of this disclosure provide the use of kit as provided herein for identifying a male subject who is predisposed to obesity and/or for identifying a male subject who would benefit from treatment with an anti-obesity agent. The subject, obesity and anti- obesity agent may be as defined herein.
[00252] The kits provided herein may comprise a positive control and/or a negative control. Thus, the test sample may be compared to the positive and/or negative control, in order to determine whether or not the sample contains a nucleic acid variant of the invention. The positive control may comprise any nucleic acid variant, as described herein.
[00253] Some of the embodiments, advantages, features, and uses of the technology disclosed herein will be more fully understood from the Examples below. The Examples are intended to illustrate some of the benefits of the present disclosure and to describe particular embodiments, but are not intended to exemplify the full scope of the disclosure and, accordingly, do not limit the scope of the disclosure.
Compounds of the Invention
[00254] Compounds delineated herein (i.e., Formulae I-V) include salt, hydrate and solvates thereof. They include all compounds delineated in schemes herein, whether intermediate or final compounds in a process.
[00255] Compounds of the invention can be obtained from natural sources or made or modified made by means known in the art of organic synthesis. Methods for optimizing reaction conditions, if necessary minimizing competing by-products, are known in the art. Reaction optimization and scale-up may advantageously utilize high-speed parallel synthesis equipment and computer-controlled microreactors (e.g. Design And Optimization in Organic Synthesis, 2nd Edition, Carlson R, Ed, 2005; Elsevier Science Ltd.; Jahnisch, K et al, Angew. Chem. Int. Ed. Engl. 2004 43: 406; and references therein). Additional reaction schemes and protocols may be determined by the skilled artesian by use of commercially available structure-searchable database software, for instance, SciFinder® (CAS division of the American Chemical Society) and CrossFire Beilstein® (Elsevier MDL), or by appropriate keyword searching using an internet search engine such as Google® or keyword databases such as the US Patent and Trademark Office text database
[00256] The compounds herein may also contain linkages (e.g., carbon-carbon bonds) wherein bond rotation is restricted about that particular linkage, e.g. restriction resulting from the presence of a ring or double bond. Accordingly, all cis/trans and E/Z isomers are expressly included in the present invention. The compounds herein may also be represented in multiple tautomeric forms, in such instances, the invention expressly includes all tautomeric forms of the
compounds described herein, even though only a single tautomeric form may be represented. All such isomeric forms of such compounds herein are expressly included in the present invention. All crystal forms and polymorphs of the compounds described herein are expressly included in the present invention. All hydrate and solvate forms of the compounds described herein are expressly included in the present invention. Also embodied are extracts and fractions comprising compounds of the invention. The term isomers is intended to include diastereoisomers, enantiomers, regioisomers, structural isomers, rotational isomers, tautomers, and the like. For compounds which contain one or more stereogenic centers, e.g., chiral compounds, the methods of the invention may be carried out with an enantiomerically enriched compound, a racemate, or a mixture of diastereomers.
[00257] Preferred enantiomerically enriched compounds have an enantiomeric excess of
50% or more, more preferably the compound has an enantiomeric excess of 60%, 70%, 80%, 90%, 95%, 98%, or 99% or more. In preferred embodiments, only one enantiomer or diastereomer of a chiral compound of the invention is administered to cells or a subject.
EXAMPLES
Example 1: Gene screening
[00258] The study was designed to identify genes relevant for obesity in flies.
[00259] Gene screening was performed in Drosophila melanogaster, the fruit fly, as a model organism.
[00260] The fruit fly has one of the longest histories in terms of usage as a model organism and has been widely used in genetic and development studies. As a result, there is available a large collection of genetic tools and a vast amount of data about the biology of flies that is very well characterized and catalogued.
[00261] Moreover, the main signaling pathways and molecular mechanisms are conserved between Drosophila and humans. The use of Drosophila as a model system therefore represents a good compromise between relevance to humans, biological relevance, and cost and amount of time involved, when compared to other animal models.
[00262] In addition, a comparative analysis between the human genome and the genome of Drosophila melanogaster suggests that about 75% of the known human disease-related genes are conserved in flies. Therefore, it is possible to create models of certain human diseases in
flies, which can ultimately be used to screen large libraries of compounds as new therapeutic approaches.
Methods and Materials
a) Animal model
[00263] Drosophila melanogaster were used for the gene screening, and approximately
800 different genes were tested. These genes are selected based on the availability of fly stocks in Bloomington Stock Center, harboring a mutation in homologous genes.
One of the mutants tested was the svr mutant fly (Sidyelyeva et al. 2006). The svr gene is the homologue of the human CPD gene.
b) Gene Screening
[00264] For each genotype, male flies were collected after eclosion and then aged until day 7 in 10% yeast: 10% sugar food. Flies were food-deprived for 1 h to reduce recently ingested carbohydrates in the digestive tract, and homogenized in PBS buffer. Each gene was tested using three replicates and experiments were repeated at least twice.
[00265] Three parameters were measured in order to find obese and lean phenotypes: wet weight, protein content (performed using BCA Protein Assay from Pierce, according to manufacturer's protocol) and triglycerides content (performed using TRIGS kit from Randox Laboratories according to manufacturer's protocol).
[00266] The genotypes showing most extreme phenotypes in one or all of those parameters were validated with two secondary assays: fat content determination and starvation resistance.
[00267] For the fat content protocol, 200 males were collected and aged in 10% yeast: 10% sugar food until day 7. Flies were transferred to a microcentrifuge and weighed (wet weight). After killing flies at -80 °C, they were transferred to glass test tubes and dried at 65 °C for 32 hours until evaporation of all water in the tissues and weighed again (dry weight). To perform the extraction of neutral fats, ether was added to each tube and kept at room temperature for 24 hours. After removing all the ether, tubes were placed again at 65 °C for another 24 hours and then flies were finally weighed (fat-free dry weight). Fat percentage was determined by the ratio between the difference of dry weight - fat-free dry weight and wet weight.
[00268] For the starvation resistance protocol, 100 male flies were collected after eclosion and aged in 10% yeast: 10% sugar food until day 7. For each genotype, flies were transferred to vials with 0.5% agar and incubated at 25 °C. Mortality rate was determined by counting the number of dead flies every day. The average survival rate and the average starvation half-life were determined.
Results
[00269] Drosophila genes were identified in the genetic screen, harboring mutations that are responsible for decreasing or changing gene function causing a lean or obese phenotype. These genes are shown in Table 3.
Table 3. Genes identified in the screen, causing a lean or obese phenotype
[00270] The svr gene (the homologue of the human CPD gene) was responsible for an obese phenotype in flies. It was not previously known that flies with a mutation on the svr gene express an obese phenotype.
Example 2: Human homologues and SNPs identification
[00271] The study was designed to find human homologues to the fly genes identified in
Example 1 and, ultimately, the SNPs that occur on these human genes.
[00272] A suitable study of polygenic obesity is based on the analysis of SNPs located within or near a candidate gene, such as one having a phenotypic effect following genetic manipulation (e.g. a loss-of-function model). If a candidate gene appears promising after experiments in vitro and in vivo (animal models), its association with the obese phenotype can then be evaluated in case control and patients suffering from the disease.
Methods
[00273] Protein sequences from Drosophila melanogaster genes of interest were obtained from the Flybase website. Those sequences were used to perform a BLAST search
(blast.ncbi.nlm.nih.gov/Blast.cgi). The best hit for Homo sapiens was considered the human homologue of the fly gene. A separate search, using Life Technologies' website, was performed to find the SNPs available for a given human gene.
Results
[00274] 51 genes and over 700 SNPs were found in the search. These SNPs were selected for a subsequent gene association study. Being publically reported in the 1000 Genomes database, the SNPs are 'validated' as actual SNPs, and have frequency data for a reasonable population size.
Example 3: Gene association study
[00275] The study was designed to identify any associations between the SNPs identified in Example 2 and the obesity phenotype. The study was carried out by allelic discrimination of 741 SNPs in 266 human DNA samples from two groups of individuals with opposite clinical phenotypes: those of a normal weight and those classed as overweight. The study thus examined genetic variation in the CPD gene, and analyzed whether this variation was associated with obesity in males.
[00276] The overall strategy for the study is illustrated in Figure 3.
Methods
a) SNPs under study and TaqMan® OpenArray® genotyping
[00277] Genotyping analysis was performed using the TaqMan OpenArray Genotyping
System from Life Technologies. The 51 genes identified in Example 2 as homologues to the fly genes were studied; these genes are identified in Table 4. Selection of the 741 SNPs in 51 genes was performed, according to criteria suggested by Life Technologies to promote the success rate of the genotyping assays. Life Technologies was responsible for designing and manufacturing the required genotyping assays and also for their preloading into the OpenArray plates. Four projects of the format ' 192-assays per array' were set out: projects A, B, C and D, including 192, 189, 190 and 170 SNPs, respectively (Table 4). There was no difference between projects, except for the SNPs tested in each one. In this specific genotyping system, each DNA array (project) could contain 192 DNA hybridisation probes and, therefore, all 741 SNPs had to be distributed among four DNA arrays, according to the Life Technologies criteria to promote the success rate of the genotyping reactions. A total of 27 DNA spots were left blank, hence the different number of SNPs in each project.
[00278] All 266 human DNA samples were submitted to four DNA arrays (A, B, C and
D), in order to simultaneously genotype the 741 SNPs in each sample.
EHMT2 2 2 3 3 10
FTO 4 4 4 4 16
FXR1 5 5 5 4 19
GAK 4 3 2 4 13
GBE1 6 6 5 6 23
GGT1 3 4 3 1 11
GNAQ 7 6 7 7 27
HSPD1 3 2 2 1 8
HYOU1 2 2 1 1 6
ISL1 2 4 1 2 9
KIAA0664 2 2 3 2 9
LEP 0 0 1 0 1
LEPR 2 1 1 2 6
LIPC 1 1 0 0 2
LZTR1 3 2 2 1 8
MAN2B1 2 4 1 2 9
MAP2K7 2 0 1 2 5
MARK2 4 3 3 3 13
MC4R 1 2 2 2 7
MEGF10 6 7 7 6 26
MYCBP2 8 7 6 5 26
NT5C2 7 5 5 5 22
NUP210 6 5 5 5 21
PAPD5 4 5 5 3 17
PARG 2 3 2 2 9
PITPNMl 1 3 2 0 6
PLCB4 9 10 8 8 35
PRODH 1 2 2 1 6
PYY 0 0 1 0 1
RALGPS1 8 5 7 7 27
RICS 0 0 1 0 1
SBNOl 5 3 4 1 13
SLC5A8 2 2 3 3 10
SPNS1 1 1 3 2 7
TNIK 7 8 9 6 30
TRPC5 8 9 8 7 32
TUBB2B 2 1 1 0 4
51 192 189 190 170 741
Table 4. Total number of SNPs analyzed per gene in each project and also in the overall genotyping study. b) DNA extraction from saliva swabs
[00279] Saliva swabs were obtained from 266 individuals belonging to two different groups with the following clinical phenotypes:
i) Individuals that, at some point in their lives, had reached a BMI of <22 kg/m2, but never a BMI of >25 kg/m ; and
ii) Individuals having had gastroplasty, i.e. the morbidly obese, having a BMI of over 40 kg/m2.
[00280] Cell lysis was performed by adding 1 ml of buffer (10 mM Tris pH 8; 10 mM
EDTA; 0.1 M NaCl; 2% SDS), containing 40 mM dithiothreitol (DTT) and 0.2 ng proteinase K, to each saliva swab. The lysis mixture was incubated at 56 °C, for at least three hours. DNA was extracted using the standard phenol-chloroform method (Walsh et al, 1992), then washed with MilliQ water and concentrated using Amicon Ultra 30K centrifugal filter devices
(Millipore, Massachusetts), according to the manufacturer's instructions.
c) Preparation and quality control of the DNA samples
[00281] As is known in the art, DNA samples isolated from saliva swabs require purification prior to absorbance measurement and amplification in order to ensure accurate results. The samples should thus be "free" of PCR inhibitors and should have ratios of absorbance at 260 nm and 280 nm, and at 260 nm and 230 nm, (i.e. A260/230 and A260/280
ratios) greater than or equal to 1.7 according to NanoDrop manufacturer's instructions. The optical density (OD) ratios were measured on a NanoDrop® spectrophotometer.
[00282] All samples were quantified by fluorimetry using PicoGreen® dsDNA
Quantitation Reagent (Molecular Probes, Inc., Eugene, Oregon). The recommended starting concentration for human DNA samples is 50 ng/μΐ (in an unchangeable volume per plate).
However, the majority of samples were below this value. Upon preliminary tests using lower initial concentrations, it was observed that concentrations ranging from 15 to 25 ng/μΐ also yielded good call rates. Whenever possible, DNA samples at initial concentrations lower than 15 ng/μΐ were concentrated by precipitation with glycogen and isopropanol.
[00283] A total of 266 DNA samples from two groups of individuals with opposite clinical phenotypes were considered for genotyping analysis.
d) DNA loading, thermal cycling and imaging of plates
[00284] When possible, DNA samples were normalized to 25 ng/μΐ and a total of 200 ng was used for simultaneous genotyping of the 192 SNPs in each one of the four projects. One non-template control was used per plate to determine the genotyping clusters and check for contaminations.
® ®
[00285] Before plate loading, TaqMan OpenArray Master Mix was added to the normalized DNA samples (1: 1). Sample loading into the plates was done using the OpenArray® AccuFill™ Instrument (Applied Biosystems). The loaded plate was placed into a TaqMan®
® ® ®
OpenArray Case filled with TaqMan OpenArray Immersion Fluid and then sealed with TaqMan® OpenArray® Sealing Glue in the OpenArray® Case Sealing Station (Applied
Biosystems). Thermal cycling was performed in a Dual Flat Block GeneAmp ® PCR System 9700 (Applied Biosystems).
[00286] Plate imaging, that is the acquisition of genotypes, was done using the
OpenArray® NT Imager with the OpenArray® SNP Genotyping Analysis Software (Applied Biosystems).
e ) Data analysis
[00287] Data analysis was performed using the TaqMan ® Genotyper Software by autocalling as the call method. All calls or genotypes were manually reviewed and corrected if needed.
[00288] Hardy-Weinberg Equilibrium was performed for both genotype and allele frequencies. Data were then analyzed for genotypic and allelic association tests. To test the statistical significance of a given SNP, the effect of the polymorphism with the null model was compared using the likelihood ratio test: LRT = 2 (log Liknull - log Likother), where "other" refers to codominant, recessive, dominant and overdominant models. When these tests were not sensitive enough to discriminate between models, other criteria, like the Akaike information (AIC), gave a measure to choose the right model of inheritance. These tests were also performed considering sex traits. The odd ratio (ORs) with 95% confidence intervals and the p-value for the likelihood ratio test of association was calculated for each SNP and genetic model. Several genome-wide Manhattan plots were also constructed to improve the view over the statistically significant associations and a Bonferroni/False Discovery Rate (FDR) corrected level applied.
Results
a) Technical aspects and general results
[00289] All non-template controls gave the expected results, demonstrating no
contamination during the genotyping procedure. In addition, all genotyped males only yielded hemizygous genotypes for SNPs located on the X chromosome, indicating accuracy and low error rate.
[00290] Despite the use of less than the recommended concentration of 50 ng/μΐ DNA and also the non-normalisation of all samples due to the limitations in terms of starting
concentrations, 95.1% (253 out of 266) were genotyped with a call rate greater than 90%, meaning that 13 samples were filtered out from the study.
[00291] Concerning the genotyping assays, 3.1% (23 out of 741) were excluded from the study: 12 (1.6%) due to call rates below the 85% limit and another 11 assays (1.5%) because the respective genotypes clustered with low level of separation compromising the accuracy and reliability of these data.
[00292] Upon this first filtering, the genotypes obtained in a total of 253 DNA samples for
718 SNPs were statistically analysed for association tests.
b) Statistical analysis
[00293] SNP genotype frequencies were examined for Hardy-Weinberg equilibrium
(HWE) and 7.9% (57 out of 718) were found to be inconsistent with HWE. There are many
possible reasons for a significant deviation from HWE, such as natural selection, population admixture, inbreeding, structural variations like copy-number variations (CNVs), small population size, low quality of samples and experimental errors. Conventionally, SNPs significantly deviated from HWE are discarded before further analysis.
[00294] In addition, 21.8% (157 out of 718) were monomorphic assays, reporting just one genotyping cluster or one genotype. For that reason, these non-informative SNPs were also excluded before statistical association tests.
[00295] A total of 504 genotyping assays were then analyzed for genotypic and allelic risk association tests.
[00296] Significant differences (values of p<0.05) between the two groups of individuals with opposite clinical phenotypes were found for 50 SNPs referring to 22 different genes.
[00297] From these, CPD was the gene with more SNPs (10 out of 15 tested) presenting statistically significant differences between the two groups being compared. In this regard, the CPD gene appears to contribute strongly to male obesity, despite its currently unknown role in lipid metabolism.
[00298] The 10 SNPs that presented statistically significant differences between the two groups being compared are as follows: rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406 (identified in Table 2).
Example 4: Pharmacological Screening
[00299] The study was designed to identify any known compounds having a previously unknown efficacy in the treatment of obesity.
a) Drug library
[00300] For screening purposes a collection of 1040 known drugs that have reached clinical trials in the USA from MicroSource Discovery Systems, Inc (USA) was used. This library represents a wide variety of compounds of known therapeutic uses, with remarkable structural diversity, providing an opportunity to uncover additional and previously unknown activities in compounds that have already been extensively tested in toxicological assays and clinical trials. All compounds are already off -patent or research into the same has been discontinued after the compounds showed poor results in clinical trials for the original indication. Some compounds were excluded from the study (e.g. antibiotics), since their use is not
compatible with chronic administration. In all, more than 500 chemical compounds were ultimately tested in the model organism.
b) Animal model
[00301] The animal model used in the pharmacological screening was the same as that for the gene screening described in Example 1, i.e. Drosophila melanogaster harboring a loss-of- function mutation in the svr gene.
c) Drug administration
[00302] Flies (males) were collected after eclosion and transferred at day 1 to vials containing 10% Yeast and 10% Sugar food and different concentrations of drug (0.1, 1 and 10 μΜ) or solvent (dimethyl sulfoxide (DMSO)) as control. The flies were aged until day 7 on this food; vials were changed every two days. Each drug was tested using three replicates for each concentration and solvent control.
d) Biochemical assays
[00303] At day 7 after eclosion, flies were food-deprived for one hour to reduce recently ingested carbohydrates in the digestive tract, since the whole animal content was to be analyzed. Flies were weighed and homogenized for determination of protein content (using the bicinchoninic acid (BCA) assay, Pierce) and lipid (triglycerides) content (using the Triglicerides (Trigs) assay, Randox). Both tests are linear and concentrations were determined by
interpolation from a calibration curve with known standards.
e ) Data analysis
[00304] Data analysis was performed using GraphPad Software. For each drug, dose- response curves for three different parameters (Trigs, Trigs/BCA ratio, Weight and
Weight/BCA) were plotted.
Results
[00305] Of the over 500 chemical compounds tested in the svr loss-of-function flies, one compound, perindopril (Microsource Discovery Systems' ref. 01505212), showed a significant effect on triglyceride (TAG) levels, at a concentration of 10 μΜ, thus being a promising candidate for obesity treatment in male patients with mutations on the CPD gene.
[00306] The over 500 chemical compounds tested in the svr loss-of-function flies included, amongst other, Enalapril, Lisinopril, Benazepril, Captopril, Quinapril, and Ramipril,
but in contrast to perindopril, these compounds did not exhibit a significant effect on triglyceride (TAG) levels.
[00307] Results for this compound are summarized in Table 5. For each parameter, a mean percentage of reduction (compared to control) and standard deviation (St Dev) are presented. These results represent at least 25 different experiments, this being the number of experiments that were performed in order to obtain a result with strong statistical significance.
Table 5. Effect of perindopril (10 μΜ) in svr loss-of-function flies.
Example 5: Clinical trial
[00308] The study was designed to assess the efficacy and tolerability of perindopril in overweight or obese male subjects with genetic variations in the CPD gene.
[00309] Moreover, the study was designed to evaluate the CPD genotyping as a valid biomarker for prediction of clinical response to perindopril and the effect of perindopril in other weight control parameters, such as body composition, waist circumference and waist/hip ratio, and fasting circulating metabolic parameters in the different patient groups. Another goal is to evaluate the tolerability and safety of perindopril in the different patient groups.
[00310] A suitable study of efficacy and tolerability of perindopril is based on an interventional, phase II, multicenter, blinded and controlled clinical trial.
[00311] The primary endpoint is the proportion of patients who lose 5% body weight from baseline. The primary analysis compare the proportion of patients with 5% body weight loss between patient groups.
Methods
Investigational Product
[00312] Perindopril biconvex, film-coated tablets.
Sample size
[00313] Assuming that -10% of subjects in the different genotypic groups without the required phenotype will attain the target weight loss (5%) and that at least 20% in the genotypic
group of interest should achieve that target of weight reduction to be clinically meaningful, based on a two-sample test of equality of binomial proportions at a 0.05 level of significance and a
70% power, a sample size of 47 patients per genotypic group is required. As the penetration of each genotype is estimated to be of approximately 33% and assuming a dropout rate of approximately 10%, the study enrol approximately 165 patients.
Main inclusion criteria
[00314] Men with 18 years or more;
Body Mass Index (BMI) from 30.0 to 40.0 kg/m2;
Consumption of breakfast and dinner on a daily basis;
Willingness and ability to comply with the study requirements;
Ability to understand and sign informed consent.
Main exclusion criteria
[00315] History of obesity with a known cause (e.g., hypothyroidism, Cushing's disease);
Significant variation in weight (more 10%) in the past 3 months before screening visit;
History of anorexia nervosa, bulimia, or binge-eating disorder;
Systolic blood pressure <110 mmHg;
History of hypersensitivity to perindopril, or related compounds, or to any of the inactive ingredients;
History of weight -reduction diet or receiving any drugs to treat obesity within the 3 months prior to screening;
History of clinically significant gastro-intestinal disease;
History of major gastro-intestinal surgery other than appendectomy or uncomplicated cholecystectomy;
Previous gastric restrictive surgery or other surgical procedures to induce weight loss;
Liposuction within the last 3 months before screening;
Treatment with any investigational drug or device within 1 month before the start of the run-in period;
Hepatic or renal impairment;
Blood sampling
[00316] Blood sampling was performed at screening and end-of-study visits for clinical safety laboratory assessments and, at each three weeks of the treatment period. Genotyping was performed on entry to the treatment period.
Safety assessments
[00317] Safety was evaluated through the assessment of adverse events, vital signs, clinical laboratory tests and 12-lead ECG. Adverse events were monitored throughout the study. Vital signs were recorded at each scheduled visit. 12-lead ECG was recorded at screening and end-of-study visit. Haematology and biochemistry tests were performed at screening and end-of- study visit.
Study design
[00318] The study has consisted of 3 periods: a pre-treatment period that included a 7-day screening and a 4- week run-in, a 12-week open-label treatment period, and a post-treatment follow-up period.
[00319] In the pre-treatment period, and after giving written informed consent, patients underwent screening evaluations. Patients who successfully completed the screening have entered the 4-week run-in period where they have been given dietary and exercise counselling as standardized non-drug therapy for weight loss. After the run-in period, patients that fulfilled all the selection criteria have started the treatment period where they have received perindopril 8mg, once daily, for 12 weeks.
[00320] At the start of the treatment period, all patients have undergone a genetic testing to understand their CPD genotype, but the results have not been shared with the investigational team and data analysts and was not used as an exclusion criteria. During the 12 weeks of treatment, all patients have continued on the study diet and exercise (non-drug therapy) in addition to the study drug. They have visited the study site approximately every 6 weeks for safety and efficacy assessments. For efficacy assessments, patients have been weighed and BMI calculated at each visit; waist and hip circumference, body composition and fat mass analysis have also been calculated. Additional effectiveness assessments such as levels of fasting haemoglobin type Ale (HbAlc), glucose, cholesterol, triglycerides, insulin, C-peptide have been collected at predefined scheduled time points. At admission to and end of the treatment period, all patients have completed a questionnaire to record the effect of body weight on their daily lives.
[00321] In the post-treatment phase, approximately 4 weeks after receiving their last dose of study drug, patients have returned to the study site for a follow-up visit.
Conclusions
[00322] Genetic variation in the CPD gene is associated with obesity in the cohort of male patients tested. SNPs rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406, in particular, have been found to be associated with male obesity. These SNPs, as shown in Figure 2, span the non-coding regions in the CPD gene. It is suggested therefore that other SNPs within the CPD gene may also be associated with male obesity.
[00323] Thus, nucleic acid variants in the CPD gene, including SNPs such as those identified above, can be used as indicators (genetic markers) of obesity in males, particularly as diagnostic tools for identifying individuals with a predisposition for the condition and facilitating drug development. For this latter purpose, identifying the CPD gene as being involved in male obesity has provided not only a promising therapeutic target, but has also helped to identify a pharmacological agent having a therapeutic effect.
Example 4: Synthesis of Compounds of Formula II
Compounds of Formula II can be prepared according to synthetic methods known in the art and as delineated herein. For example, the following compounds of Formula II can be prepared in a similar fashion as used for the preparation of perindopril and other dicarboxylate ACE inhibitors- with certain changes (e.g., US6835843; US4914214; EP0049658A1; EP0116842A2;
EP0308341A1; EP0309324A1 ; US7279595; US7326794; US7358372; US7368580;
US7666896; US7674814; US20050119492; US20060183920; US20060189813;
US20070172524; US20070185335; US20070197821; US20080051584; US20090162420;
US20100172995; WO2008114270A1; US7534896; US7179833; US7183308; US7208607; US7223872; EP1319668A1 ; EP1321471A1; US8686161; US20110301357), each of which is expressly incorporated by reference herein in their entirety.
114/190
115/190
116/190
117/190
118/190
119/190
Altschul et al, 1990, J Mol Biol 215: 403-410.
Anderson M and Young K. (1985). Quantitative filter hybridization. Pages 73-111 in B Hames and S Higgins (eds) Nucleic acid hybridization, a practical approach. Oxford: IRI Press.
Berger SL and Kimmel AR. (1987) Methods in Enzymology. Vol. 152: Guide to molecular cloning techniques. San Diego: Academic Press, Inc.
Campanella et al, 2003, BMC Bioinformatics 4: 29.
Chu et al, 201 1, Islets 3(4): 155-65.
Eng et al, 1998, J Biol Chem 273(14): 8382 - 8.
Glandt et al, 201 1, J Obes 2011 : 636181.
Hsiao et al, 2013, Gene 533(1): 32-7.
Hsu et al, 2013, Nutr Diabetes 3: e61.
Kruskal (1983). In: Time warps, string edits and macromolecules: the theory and practice of sequence comparison, Sankoff & Kruskal (eds), pp 1 -44, Addison Wesley.
Larkin et al, 2007, Bioinformatics 23: 2947-2948.
Mueller PR et al ( 1993) In: Current Protocols in Molecular Biology. 15.5. New York: Greene
Publishing Associates, Inc. and John Wiley and Sons.
Mutch et al, 2006, PLoS Genet. 2(12): el 88.
Needleman et al, 1970, J Mol Biol 48: 443-453.
O'Malley et al, 2005, Biochem J 390 (3): 665-673.
Pearson et al, 1998, Proc Natl Acad Sci USA 85: 2444.
Rychlik et al, 1989, Nucl Acids Res 17: 8543.
Sachidanandam et al, 2001, Nature. 409 (6822): 928-33.
Sambrook et al (1989) Molecular Cloning: a Laboratory Manual. 2nd Ed., Vol. 1-3. Cold Spring
Harbor Laboratory.
Sidyelyeva et al, 2002, J Biol Chem 277(51): 49613-20.
Sidyelyeva et al, 2006, J Biol Chem 281 (19): 13844-13852.
Skidgel et al, 1998, Immunol Rev 161 : 129-141.
Smith et al, 1981, Adv Appl Math. 2: 482.
Song et al, 1995, J Biol Chem 270 (42): 25007-25013.
Song et al, 1996, J Biol Chem 271 (46): 28884-28889.
Timblin et al, 2002, Int Immunopharmacol 2: 1907-1917.
Varlamov et al, 1998, J Cell Science 1 11 (7): 877-885.
Walsh et al, 1992, J Forensic Sci 37(2): 387-95.
WHO obesity: preventing and managing the global epidemic. Report of a WHO consultation.
WHO Technical report series 894. Geneva: World Health Organization, 2000.
Yue et al, 2013, Acta Pharmacol Sin. 34(5): 710-6.
For World Health Organization see www.who.int.
[00324] All publications, patents, patent applications, publication, and database entries
(e.g., sequence database entries) mentioned herein, e.g., in the Background, Summary, Detailed Description, Examples, and/or References sections, are hereby incorporated by reference in their entirety as if each individual publication, patent, patent application, publication, and database entry was specifically and individually incorporated herein by reference. In case of conflict, the present application, including any definitions herein, will control.
EQUIVALENTS AND SCOPE
[00325] Those skilled in the art will recognize, or be able to ascertain using no more than routine experimentation, many equivalents of the embodiments described herein. The scope of the present disclosure is not intended to be limited to the above description, but rather is as set forth in the appended claims.
[00326] Articles such as "a," "an," and "the" may mean one or more than one unless indicated to the contrary or otherwise evident from the context. Claims or descriptions that include "or" between two or more members of a group are considered satisfied if one, more than one, or all of the group members are present, unless indicated to the contrary or otherwise evident from the context. The disclosure of a group that includes "or" between two or more group members provides embodiments in which exactly one member of the group is present, embodiments in which more than one members of the group are present, and embodiments in which all of the group members are present. For purposes of brevity those embodiments have not been individually spelled out herein, but it will be understood that each of these embodiments is provided herein and may be specifically claimed or disclaimed.
[00327] It is to be understood that the invention encompasses all variations, combinations, and permutations in which one or more limitation, element, clause, or descriptive term, from one or more of the claims or from one or more relevant portion of the description, is introduced into another claim. For example, a claim that is dependent on another claim can be modified to include one or more of the limitations found in any other claim that is dependent on the same base claim. Furthermore, where the claims recite a composition, it is to be understood that
methods of making or using the composition according to any of the methods of making or using disclosed herein or according to methods known in the art, if any, are included, unless otherwise indicated or unless it would be evident to one of ordinary skill in the art that a contradiction or inconsistency would arise.
[00328] Where elements are presented as lists, e.g. , in Markush group format, it is to be understood that every possible subgroup of the elements is also disclosed, and that any element or subgroup of elements can be removed from the group. It is also noted that the term
"comprising" is intended to be open and permits the inclusion of additional elements or steps. It should be understood that, in general, where an embodiment, product, or method is referred to as comprising particular elements, features, or steps, embodiments, products, or methods that consist, or consist essentially of, such elements, features, or steps, are provided as well. For purposes of brevity those embodiments have not been individually spelled out herein, but it will be understood that each of these embodiments is provided herein and may be specifically claimed or disclaimed.
[00329] Where ranges are given, endpoints are included. Furthermore, it is to be understood that unless otherwise indicated or otherwise evident from the context and/or the understanding of one of ordinary skill in the art, values that are expressed as ranges can assume any specific value within the stated ranges in some embodiments, to the tenth of the unit of the lower limit of the range, unless the context clearly dictates otherwise. For purposes of brevity, the values in each range have not been individually spelled out herein, but it will be understood that each of these values is provided herein and may be specifically claimed or disclaimed. It is also to be understood that unless otherwise indicated or otherwise evident from the context and/or the understanding of one of ordinary skill in the art, values expressed as ranges can assume any subrange within the given range, wherein the endpoints of the subrange are expressed to the same degree of accuracy as the tenth of the unit of the lower limit of the range.
[00330] In addition, it is to be understood that any particular embodiment of the present invention may be explicitly excluded from any one or more of the claims. Where ranges are given, any value within the range may explicitly be excluded from any one or more of the claims. Any embodiment, element, feature, application, or aspect of the compositions and/or methods of the invention, can be excluded from any one or more claims. For purposes of
brevity, all of the embodiments in which one or more elements, features, purposes, or aspects is excluded are not set forth explicitly herein.
SEQUENCES
[00331] The human CPD nucleotide and amino acid sequences provided herein are exemplary only and serve to illustrate some embodiments of the present disclosure. Additional CPD sequences, including CPD sequence variants, CPD sequences from species other than human, and CPD sequences comprising certain SNPs disclosed herein or otherwise known in the art will be apparent to the person of ordinary skill in the art based on the instant disclosure and the knowledge in the art.
[00332] >gil568815581:30378910-30469657 Homo sapiens chromosome 17, GRCh38.p2
Primary Assembly
TCCGGCGGGCCCCCGCCGCCCGGAGCGCTGAGCCGCGGGAGCGGAGCCGGGGTTAGCGGCGCTGCTGGAAGATGGCG AGCGGCCGGGACGAGCGGCCGCCTTGGCGGCTAGGGCGGCTCCTGTTGCTCATGTGCCTGCTGCTGCTGGGGAGCTC GGCCCGGGCGGCTCACATCAAGAAGGCGGAGGCGACTACCACAACTACGAGCGCGGGCGCCGAGGCGGCCGAGGGCC AGTTCGACCGCTACTACCACGAAGAGGAGTTGGAGTCGGCGCTGAGGGAGGCGGCGGCCGCGGGCCTCCCCGGCCTG GCCCGCCTCTTTAGCATCGGCCGCTCGGTGGAAGGCCGGCCGCTGTGGGTGCTTCGCCTCACCGCCGGCCTGGGGTC GCTAATCCCTGAGGGCGACGCGGGGCCTGACGCTGCCGGGCCCGACGCTGCGGGGCCGCTGCTGCCCGGCCGGCCCC AGGTGAAGCTGGTGGGCAACATGCATGGCGACGAGACCGTGTCGCGCCAGGTGTTGATCTACTTGGCCCGCGAGCTG GCGGCCGGCTACCGCCGCGGGGACCCGCGCCTGGTCCGCCTGCTCAACACCACCGACGTGTACCTGCTGCCCAGCCT CAACCCCGATGGCTTCGAGCGTGCCCGCGAGGGCGACTGTGGCTTCGGCGACGGCGGCCCGTCCGGGGCCAGCGGCC GCGACAATAGTCGCGGCCGCGACCTCAACCGAAGCTTTCCCGACCAGTTTAGCACCGGCGAACCCCCCGCCCTGGAC GAGGTGCCCGAGGTGCGCGCCCTCATCGAGTGGATCCGCAGGAACAAGTGAGTGTTGCCTGCCCCCTCCCCGTCCGT GTGAGCCTCCAAGGGCCGAGGCTGGTTCCGGCACCCAGTAGGCGCTCAGACAATGCTGGCATAAGGGGTGGCGGTGG TGAAGGTGAAGGGAGACACCCTGTAACGGGGACAGGGCCCAGGCCGCGTAGCCTCCCGTCCTGCTAATCATCAAAGA ATTGCCTCCTAGAGGCTGTCATTTGTTCAAGGGATGGCTGGAGGGGACACTCCTTTCTGACATTTCTGGTCCCTATT CTTACAGGGGCATAGAATATGAGCGAGAAGAGATCTTAAGGATAACCTAGGTCAGCTCACACATTTTATAGATAAGC TGTCAATGTTGTTATCTTATATCAAAAGTGCCTAATGTTATAGGCGCTGTGCTAATTTTTTCTGTGACTGCAGTATC TCACTTTATTTTGGCCACAACCTGATGTCATAGACACCATCAGTTTTGCATACGGGGAAATTGAGGGTCAGATTGGT TAACTGATTTACATAGGAGTGTGGCTGGGACTTAGAATTAGGCAATCTGATTCCCGATAGTCCTCTTAGCCACGGAG GGACACTGGAGCCCAAAAATGGCAGATGATATTCCCAGGATCTTCCCCTTGCCTATTTGACAGAAGCTGATTTTTCT CCAATTTTTACCTCTGACTCAGCTTAAGAAGATATGGACTTAGCTGGAGAACTTGCACAGATCTGTGAGCCGCTTCA TTTTAGACTCTGAAGGGTTTGTGATGTCATTTGTGCACCTTATTAGCCTGGGCAGAATGGCTAATGGTCACACTTCT AATACACACTTTAAGTGTTATTAAGCATTGCATGAGGTTTAAAAAATTACAAAGTAGAAGAAGGAGGACCTCAAGAA ATTAGTATAAAACAAGAGAATGTTATAAATATTTATGAGGTATGTTGGTGTACCCAGTTAAACTTTACAGGAATTGT TCCCACTGGCAAGATGTGTGTGGGCTGCAATAACACGGACCATCTCTGTGGAAGAGGTAGAGCTTGAGCTATCTCCA GAGATGGGCAGGATTTTCAGCAAAAAGGAGAGGAATAAAATAAGAGGGAACTCACTCCAGATGGCATAGTGTCTGAA GTGAGCAGGCATCTCTTCCCCTTTTCTAGCATCAGCTTTTCCCCATTTTTGTATCTTCATAAGGCTTTTGAAGCAAT AACTGATCATTCCATCTGGATTAAAAGAAGATTTCTGAGGGACTGCTAAGATAAAGAAGTTTCCCTGCGGGACTTGG AATCATTTCTTCTACAAGATTTCAGTCATATATAGAAGATCTAGAAAATTCTTGAGTGTTAGGACAGTTCCTGAGTC TTTTCGTCTTCTATCTTTTACTTTACTCCTCTTGATAAGCTCTCTTGTAGATATATAATTTCACATAGACTTTCTGG GAGGATTTTTTTTTCTTTTAAATACGATCGTGTCATCACCTCTGAACATCTGCGTAAGAAATATGACTTTGACATAC ACTTATTGACAGAGCTGTGATGGCCAGGGCTTTTTGGAGATGATACAACAAATGCACCGTTTGCATTGCTTAAATGT TTAAAATGCTCTGTCCTTGCACCTTTAAAATGCTGTGATCCTTGCAGGGATGTAAATTCTGTGCCTGGATTTTTCTT TTCAGTATTAGTTGTGCTGTGACCAAATATGAAAATAAGCTTTTTCTTGGTGCAAGTCGCCTTGAGGCTATACAGAG
TCATTTTGGTCTGTGGCTGTGGACTACCCTAATCATATCTGTTCTT CAAAT AT GTAACTCTCATTAATCAAAAGGGA AAGTAGGTGATCATATATGGTAAAGAGTACATCCATCCTAACTCAGTCTTCAAGAATTCACTCAAAATGCGTACTCT GT G CT AT T AAC CAAT AACAAAGGACACT GAT AT GT GAAT AAAAT T AAAAACAC AAG CAC ACAC ACAAT T T GAT AT T G ATGCCTAAATATAGTGTCAACTTTATGGATGGGATATTTTTAAATACTCAAACATTAGTCTGTATGGGAAAACTACC TTTTTCAGTGTATTTTGTGAGGTAAACCATTTGAGTAATTTCTTGATATCCTATTCACCCAGCTATATTCGAATTAG ATTTTTGTTGTTATTTCGAATATGTAAAATTTTAAGGCTTAATTTCCTCCAGCTTGGGAAAATGAAGTTTATTTTCT TTGTTTCACTTTATACTTCATCCAATGGATGTTGAGACAAATACTTAAGAAAATATATAAAAAAATTAACTTTATCA TT CAAAT GAA GAT TTT AT AGTTGACTCCTATTTTTTTATAGTTGACCGCTATGCTTCTTTAAGT GAAT AT ACCTGCT TAAAT TTT CAT AG GT AAT T AGCAT AT AAAT GAT AT T T GAAT GTGTTTGT GAAAAGC AT TAT T ACAGT GT T GAGAGT G GGTGCAGATGATT TTT TTGGGGGCTTATGTATACATATCTATGATTGTCT GAAT CTTTGAAATTTAGTTTGTCAGAT ATCAAGTTATCTTAATTTTTTTCAAGTTATCTACAGGGTTACCTGGATAGCGTAATAGAGTTAATTTAGAGATTAAC TGTTTAATATTTATAAACTCATTTGTAATATCTCTAATATATTTACCAGCTACTTTAAATCTTATGCAAGGAATGAT AT GT GAT ACT GAAGT C AAGT AT GAGAAACAT T T CAGAGAAT T GAT T T GAAT GGAAAC CAT AAAGT AC CT GT CAAAT A AGATTGGAGTTGATTTGCAAAGTTGATTTGACAAAAACTCAAAGCATAATCTTACAGCCATTGTAGTCACTTTCTCT TGAAATCTTCATTGTAAGAATGACACTGGTACTTTCAAAACTGTGTAATTACAGTAAGGAAAATCGAAGT GAAT TTT ATTACTCAAGTAGATTGCAAATATTTCATTGTTCAAAAGTATTTGATTAGGCCATTTTGACAATATTTCTGAGAAGT GTTGGAAGAAATGTTGAACAAAATGAGAAACAGCATTTTGTAGAGAATCTTGGATTTTAAAGTTTTACTTAACATGT GTAATTAAAGTAGCATGCTTTACTTATTTAGAATATTTAATTATCCATTTTATTTTACTAGAATATGAATGCCCAGA GGGTATTTGTTTGTTTGTTTCGCTCACAGATGTATCTATCCCTGGAATGTCCTTTGGGATGTATCTGTCCCTGAAAC GTCATTTGGCAAATAGTTGACACTCAAGAAATGTTTGTTGCCGAATGGATTACCTGACCTGCCCCAGCCTTCTTATA AC T T T C C C AG AGT C AG T G G G G GAAAAAT AC T T AAAAAT T G T T T TAG AAT T AC AAAC C AG AGAG TAAAT T GT T AAT AC CTTAGCTATAGGGGAGAGGTGGTTAGAGTGGAGTTAGAAAGTTAGAATATTTACTTAGTTTTTGTTTCTGTATCATT CTATTACCCAAGGACTGGCAGTGGAAGTGGGGAGTAGAATGATAGTTTGTAAGGCAGTGTTTCCTTAAACAGGATGC TAGATACCTCCAAAGAAATGAACATTTGTTGAGCAGGGGCTATAAAGTAGTCTTTATGCCAGATTATGTTTCATGTG TGTTATCTTAATTTTCATGACAACTCTGGGAAATGGGTAGCATTAATCCCCATTTTACAAATGAAAGGAAAACTGAG GTTCAGCGAATTTAAAGGCAGTAACTTCTCCTGATTATGTTATATTACTGGTGAGCAACAGATCTGGGAGACGGAAT GTTCTTACCCAAATTTGTCTCCAAAGCCTGTGCCATTGCCACTACACCAAATGGCATTCTTCTCTGGTGTCTGGAAG GGTGCTAATTATAATTATATTAGTTTTTAGCTAGTTTCTTTTCATGGTCTATGCTAGTGTTTAATCAGTGTCCTTAT TGGCAAAGATCTGTCTTCTGATGAACAGATTTTGTGTTACAAGTAATTTTCATTGTTTGATCTTCTCTGGGATAATA CTACTCATCATGCTTAAAATAGTCTTTCAAATTAGACATGATAGGTTTACTTTTTTTTCAAGCATATAGAAAAATCT TATATATCAACCCCCTTTAGAATTTCTGTAGGTCAGTTAATTTCAACAACAGAAAGGCAGAAGCAAGAAAATCCTAT TTAGAATGGAAAAAGAAAAGTATTTAATCTCTACTGATAGTAATGGTTTAAGCTGTTATATAAGGGAAAGATGCTTA AATAGGACTTTAGCATTTTTTTTGCCTTTAGGTTTTAGCTTTTACCATAAAAGGAATTCTTCAGTATTTCTATATAG ATAAGTTTGACCAAATTGTAAATTGGTTCACTTAAAAGTAGAAGCATTAAAAAAAATGTTTCTTTTACAGGAGCAGC CTTGCCTCCATGTCAATTTATTTTTTTAATGGCCCAATCAGAAACATTGGGCCTTGAAAGAGCTGCTTCTACAGTGA TGT ATT GGAGGACTAGTCTTTGA CAT GGTGTATTGTTTTTCTCTGTAAGCAGCTCACT GAAT CAGGTTGTTTTCTTT GTTTGTTTTTTTCCT GAAT CTTCTCAACTATGGCTTCTCTCTTGAAAATGTGAATT TAG TAT ATT GTGAAACTGGAG TGAAACACAAAATATCCTTTTAAAAATTAGACTTCCTATCTTAAAAATTCTCTTTGGACGGTAAATGTAAAAAGCAT AAAAGTTATTAACCTAACATATATGCAATGAATATTTATATATACCAGCCATATTTTCTGTGGAATTTACGTATTAT GAAATACAAGTCGTAGACTGAACTATATGAAATTGTCATTTTTTTAGGTCAAAAAAATGGGTGAGGGCCAGGCATAG TGGCTCATGCCTCTAATCCCAGCATTTTGGGAGGCCTGGCAACATGGCGAGACCCCATCACTACCAAAAAGAAAAGG GT GAAT ATT GGCAAGCTCAAAGCT CAT ATAGTTCAACCT AAT ACATGAATTTAGAATTCCCT AAAT AAGACATTTCC TGAAATATTAATTTATACTGTGT TAT CCAGCTATTT TTT CAAT GAAGGAAAACTCTTTAAAGTTAGTTTAGGTTAAC CT GAAT TGTTAAAGGTGTTTGAAATCTTAAATT GAT AATACTTTCTTTCCATTTTCTTTTTTTGTTGTTGTTAGTCT CCTTATAACAGATAACAGCTGATAGAGTTGTTTTCTAGGTCTCCAAGAGAGATATTGGAGTTTATTTCTAAAAGATT TTGTGGAATTTTTTTAATGCATGGTGTTTTGTGTCCTTTTTTAGTGATACGTGATTTGGTTGTATTTTCAAAGGTTT GTGCTTTCTGGAAATCTGCATGGTGGCTCAGTGGTAGCAAGCTATCCTTTTGATGATTCTCCAGAACATAAGGCCAC TGGAATCTATAGCAAAACCTCAGAT GAT GAAGT ATT TAAAT ACTTGGCAAAAGCTT AT GCTTCAAACCACCCCATAA TGAAAACTGGTGAGCCTCATTGTCCAGGAGATGAAGACGAGACTTTCAAAGATGGAATCACAAACGGCGCACATTGG TATGATGTGGAAGGTATGCAAAGCATTGAGTTTGCTACATTTTCCCCCTTGTTCGTTGAATTTTGTTATGTGTATTC T GAG C AAAAAAGAAGAAAC T C T G TAG AAAT C T AAC T T T AAAAG AT AT T TAT TTTTATTTT GAAAAT T T CAAAT C T AC AG C AAAAT T G AAG GAC T AC AT GAAGAC CTATCTGTCCTT CAT T TAAAT T CAC CAC TGT TAT C T T AT C C C AGAT G G C T TGCCTCTCCCTCTCTCTTTTTCACTCTCTACATGCACGTGTATGTGCATGCACACACACACACACACACACACACAC AATCACACAATTTTTTTGCCAAACCATCCAAAAGTAGGTTGCAGATTTCACAACACTTTACCTATAAGTTCCTCAGC ATGTATTTCCCAAGTACAGGGACATTTTCCTGTTTAACTATATACTATTACCATAACTGAGAAATCAATGATAATTT AATATTATCATACCACATGCCATCCGTAACTCAGATTTCCCCCATTATGTCCAAAGTAGCCTTGGTAGCCCCTTTTT ATCCAGATCTAGTATCCAATCAAAGTTCAAGCATTGCAGTTGGCTCTTGTAAAGAGACCGTGCTAATAGTCCTATGG
AAAAAT T C CAT AAT CT AGAT T GT CAC T T T C T T CAT G GT AAGAT TAG GT C AGGT T T C C CAGCAAGAC TAT TAT AT AGA TCATGTTGCGCATTATATCAGGAGACCCATAACGTCAAGCTGCAAGTGGCCAAGTTGGACAGCTTGGTTAACATGAT GACCACCAGCAACCAGGTCTCTCCATTTAAATTTAAAATGTATATATTTTTTAAGAGATAGGGTCTCACTCTGTTAC CCAGGCTGCAGTGCAATAGTACAATCATAGCTCACTGTAATCACAAGCTCCTGGGCGCAAGCAATACTCCTGCCTCA ATCTCCTGAGTAGCTGGAACTATAGATGTGTGCCACCATGCTAGCTAATTTTTAAATTTTTTGTAGAGATGGGGTAT CGCTGTGTTGCCGAGGCTGATCTCAAACTCCTGACCTCAAGGGATCCTCTTACCTCAAACTCCCAAAGTGCTGGGAT TAT AGAT GTGAGCCACTTCACCCAGCCCAGATCTCTCCATTTTACATAAAGGCACATTTTCCTTTTGTATTTT AAT C T GT GAG GT GAT CAT GGAAT GCTGTTTTTTT TAT T T T TAT T GT G GT AAAACAT AC AT AT AT AT AT ACAT AAC AT GAAA T T T GACAGT T T AACT GT T T AAAC AT T T T TAAGT GT AGAGC T CAGTG GT ACT AAT T T T CACAAT GT T GT G CAAC CAT C ACCACTATTTCTAAAACTTGTTCATCATCCCAAACAGAAACTCGGTAGGCATTAAATAATAATTCCCACTTCCATCT CTATCCCTTACCCCCAGCCATTGGTAACCTCTAATCTACTTTCTATCTCTGTGAATTTGCCCATTTTAGATATTTTA TAT AACT GGAGTTATACAAT ATT CTTCCTTTTGCATCAGGCTT ATT TTTCTTAACAT AAT ATT CTTAAGGGT GAT CC AT TTTGTTGCAGG TAT C AGAAC T T T AT T CAT TTTTATAGCT GAAT AAT AT T C C T T G AAT GT AT AT G T T AGT AAT AC A TTTTGTTTATCCATCTATTGATGAGCACTGGGTTGTTTCCACCTTGGCTACTAGGAATAATGCGTAATGAACGTTGG CGTTCAGGTATCTGTATGAGTCCTTATTTTCAATTCGTCTGGATATATACCCAGGAGTAGAATTGCTGGGTCAAATG GT AAT T CT AT GT T T AG CT T T GAC AGACT AT T T T C CACAGT AGAT AT AC CAT T T ACAT T C C CAC CAG CAAT AT AT AAG AAT TCCAATTTCT TAT GAAATGT TAT TTTTTAAAGAATTGTTTGTTGTTGTTGTTGTTGTTTTCAGAT GGAAT TTCA CTCTTGTTGCCCAGGCTGGAGTATAGTGGCATGATCTTGGCTCACTGTAACCTCCGCCTCCTGGCTTCAAGTGATTC TCCAGCCTCAGCCTCCCAAGTAGCTGGGATTACAGGTGCCTGCCACCAGGCCCAGCTAATTTTTTGTATTTTTAGTA GAGATAGGGTTCCATCATGTTGGCCAGGCTGGTCTCGAACTCCTGACCTCGAGTGATCTACCTGTCTCAGCCTCCCA AAGTGCTGGAATTATGTATGAGCCACCGTGCCTGGCAAGAATTGTTAATACAAGCTTGAACTGTTTACCTTTTCTCT TTAAATGCTATGAAAACAGCTAT TACT CTTTTTAAACTTTAGGTATGCTTT GAAT AAT AACGTTTTTGTTTTT AT GC ACA GAT GTAGATGTAGAGGGAACTTAGAAGGAATAAGT CAAC CAAT GGAAATGT CAT CAGTGTAAATTTTTTCTTTT CTAGGAGTCTGCCCATGATGAAATGCCTTAGGTCTTCCTTACCTTTTTATTGGCAGTCACATTATAAAGTGTCTTAT GACAT G T C T C T T AAT G TAT AT T T AAAC C T T T GAAT TACTCTTTTACTTTT TAT AT C AAG C CAT AT GT AC C C AAAAT G GGTCATAAGTTTGAGAATTTAATACTTTCGTTACTGGATCTTTGGGGTATTGCTTTTCTGGTTGGAAACCTCTGTGC CTGGTGGTGCCTTTGCCCAAGTTCTTGTCCTGCATCCAGGAAGAATGAGGTATGCAGACAAGTGGAGGGTGAGCAAG ACAAAGAGGAGCTTTATTGAGCTCAGAGGAGACCGGCAGTGGGCATCTCCTCTCTGTAGGCAGGTCACCCAGTCAAG TGTTCAGCCCTCAGCACAAAGGAACCCTGGAGTGGGTGGCCCCTCTCTACAGGCAGGTCATCCTGATGAGTGTTCAG CTTCAGCTTTCAGCAGAGAGGAGCTACCCTCTGGTTGTCCCATCATCTCTCCATCCTCTGCCTTGCTCTGACTGAGC CCAGGGCTTTTATGGACCTCAGAGGGGAGGAAGTGTATGCCGATTGGTTCATGGGCGCCACGGGCAGCCTGGAAAAG GCACAAGTCCCCACTCCAGTCTATGGGACTGGCAGCCTGGCCCCCAGCCTTCAGGACCTCCCTGGCCTGAAGGTGGG GCCTCACCAGGGACCCCCTCCCTTATACCCAGGAGCTAGTCTGCCTCTTGCTGCTTTCCATGGCACCCCGGCTGCTC GTGCTAAGGGGCACCTGCAAGCCAGTGCTGAGTCGCCCTCAGTCCCCAACCTTGGTTCCCTCTTCCATGCTTGTCAG TGCCCAGTCCGGTGGGGGCCCAGGCAACAGGGCCCAAGCATGTGCGTACCCCTCCGGGCAGT GACAGT GCGCCGCCT CAGCTC CAAC CCCAATCCCGGATAGGAGCA GAC GCAAGAATGGGGAGAGGCCAGGCAGCAGGAGCAGGCATCTCCAA GCCTGCAAGGGTAAGGGGGGCCTAGGAGGTCGGGGGACGCAGCTGCTGCCAGCTCCCAGCTCCCGCCGACTCAGTGG AGCATGCAGCCCCAGCCGTGCCCCCTGGGAGCCTGTGGCGGGTGGCTCCTAATCCTCGCTGGGCCTGGGCCAGTGTC TGGGCCAGGGGCGACATTGCCACAAGCTTCCCTGTTGCCCTGGGGCTAAGGAGTGGCCCGGAGCTGATTGCGGGCCT GGGGCCAGGCTGTCAGGAGTGTCAGGCTGGGAGGTCACCCCACGTGTGGCGGACACTGGGGACTTGGCCCCAAGTGG CCCGCGCAGAGCCTCCTCCCGAGCCCAGGAACCCGGGACCCTTAGTGGGGTGGGCGCAGTGGCTGCACTTCTGGCCA GATCCCCAAAGCTGGAGCAGCTCCTGCTTTCCGCCCCGCCCCCGAAGCACAGCCCCAGCTCCGCATAGGGGTTCCTC TCTGCCTGACTACATTACTCCCCCGCTGTGCTGCTCTGGGCCTGACCCCATTACGGCAGCCCCCAGGGCAGCGGGCT CTGGGGGGTGGCAGCGGGCTGTGGGGGGTGGCTGCAGAAGGGGTGGCTGTCCGCCTCCTTCCCGCACCTTCCGGCCT GCTGCTGCTATCACTTTCTAGAATTTTCTGATCCTCCTATAGTTTCTACCAGTGTTTCTTGTATATCAGAAGCATTC AAAAAGAGGT T T GACAT ACT AT T T AGAAGT GAAAC CTTCTCCC CAT AAC AAT T AGAGGT GAT T T GAAAAT CAT T AAA AATTTTTTTGTAGATAATGGACTTACTTTAATGTGTAATTTTTACATTTCAGTCCTTGGGAAATATATTTAATAAAA CCTAACTTTGAACTGGTGGTTTTGTAATCTAGTCAAGTTGCTTCATTAATTTGAGATGATGTTACTTAAAATAATGG GGTAAATACTTAACTTTGCCTTTTTGGAAAAAGTTGTAAAAGGTTAGAAAGTAATTTCTAACTGACAATTAATTGAG GAAGGTAAATGTATCTCATACTACTTAAAAAATAAATATCCGGCCTCTACAAAACGTAGGTGTACTTTGGTATTAAG GCCAGAGATTATTTGGTGAAGCTAGTTATCATCAGATTTTTCTGATTATTCAGACATCTGAAGAAGCTACATTATGC TGCTGTCATTTTTATTTTCATGAGCATCAACTTTTCTCTTGTTTCAGCTGAATGTCGGATATGTCCTATGTTATTTC TACAATAACTGGCTTATTTCTCTTCGTACCTCAGCAAATATTGAGCAGTTTCCCCTTGAAGAGGAGGCTGTGATGGT TTTAGTTTCAACAGGGTGAATTCATTGTGTTTAGTGAGTAAGATGTTGTGAAACCTTGTGACTAGGCATATATTCAA AAAACCCGGATGTCCTGGAGTAATTTTTTTTTTTTTTTTGAGACAGAGTCTTGCTGTGTTGCCCAGGCTGGAGTGCA GGCGTGATCTCAGCTCACTGCAACCTCTGCCTCCTGGGTTCAGATGATTCCCGTGCCTCAGCCTCTTGAGCAGCTGG GATTGCAGGTGTGTGCCACCATGCTGGCTAATTTTTTGTATTTTAAGTAGAGACAGGGTTTTACCATGTTGACCAGG
CTGGTCTTGAATTCCTGGCCTCAAGTGATCTGCCCACCTTGGCCTCCCAAAGTGCTGGGATTATAAACGGGAGCCAC ATGCTCAGCCGAGTAATTTTACTGATGTTCTATGTGATATATTTAGGAATTCTAGCTTAAAGAAGTATAGACCTTTA GAGCTGAAAGT GAAT AT ATT GAT TTAGTCCAACCCTTTTGTTTTAGAGAAAATCAAGGCCTGAGAGAGGAACT AACT TGTGCAATATAATGTATTAATATAGCAAATATTTACTGAGTTCCTGCCGTGCCACATATACTGTATTAGGTGATGTA CCGGTATACAGTGAGAGCAAAACAGAAACAACTCCTGCTCTGAGGAATCAGATAGTATATTAGTAGTGTGGAATCTG GGGTGATTTGGGCCCTAGTGCCTTTGCCTTCATTGCCAGGGCTGGCCCAGCATATCTAGCTGCCTATGTGGGTGAGC CTTGCTT CAT TCAACATGTCTCACAAACCTCAGGATCCATTTAGGAAATGGCTATCCTAATAAGGCT GAT GAAT AGC CACATCCCCTTCCAAAGCCTCGTTATTGTGAGACCTACTCTGGACTTGTTCATGGAGTAAGATGCCTTTTTTTTTTT TTGAGATGGAGTTTCGCTCTTGTTGCCCAGGGTGGAGTGCAATGGCACAATCTCAGCTCACTGCAACCTCTGCCTCT TGGGTTCAAGCGATTCTAGTGCCTCAGTCTCCTGAGTAGCTGAGGTTACGGGCATGTACCACCATGCCCGGCTAATT TTTGTATTTTTAGTAGAAACAGGGTTTTAC CAT GTTGGTCAAGCTGGTCTT GAAT TCCAGACCTCAAGT GAT CCACC TGCCTCGCCCTCCCAAAGTGCTGGGATTACAGGCCTGAGCTACCGTGCCTGGCCGCCTTTTTTTTTTTTTTTTTTTT TAATCACAGTGCGCTGATTTGTATGCCATTTGCCTACCGTTCTTTCTTCACAGTTTTGAAGACATTGTAAAAATCAT ATGATGTTTTGGTATGTTTATTCCAAAGCAGATTTAATAAAAACTGAACTGTAGTTTTAGGTCAACTTTTAAGCCAT GGCGTGTGCTTGTAGTCGCAGATACTTGGGAGGCCAAGGCGGGAGGATCTTGAGTTCAAGCCCAGCCTGGGCAAAAT AAT GAG AC CCTGTCTC C AAG AAAAC AAAC AGAC AAAAAAG AAT TGCCTTCTTT C AAAT TAT AC AAT T T AAG GAAG G G CAGGCAAC GT AT AGT T GAAAT TAT T T GAT C AAA GAT CAT AGT T T TAT GT AACT T T TAGAAGT T T T C T T G GGCAGAAC ATTGTTATATTTTATTTTCTTGCAGATAGAAGAACAGTAGAAGGCAGTGCAATGTAGTGGCAAAGAGAGAGCTTTGG AAT CCCGTTGCCTTAGGTTGGAATCACAACTCTGCCTTGACTAGCTGTGTGCGTGTGTGTGTGTGGTGTGGGT GAGA GACAAATTAATGAATTTCTCTAAGCCTCAATTCCCACTTAT AT AAAATT AAGAT AAATACTATCTACTCACAA GAT T AGGAGGCTCAAGTGAGAATGTGTGTAAAGCACTTTAGCCTAGCATCTGGTGCCATGGCTACTTATATCTACTAGAGT TTAAACTTACTCTTGAAGATAGGGACTTTGTTTTATTCCATTTTCTATTTCTAGCACACACAACCATGCCTGGTATA TAT AAG GT AAAT AAT AGT CT T T T T GGAAT AAAT GAAT T T T T GC CAT C C GAAAC AT AAAT ACAT AGT TAT T T GAT CT T TGGATAATCTTTTTTTTTTTTTTTTTTGGAGACAAAGTCTCACTCTGTCACCCAGGCCGGAGTGAAGTGGCGCAATC TCGGCTCACTGCAGCCTCTGCCTCCCAGGTTCCAGCGATTCTCCTGCCTCAGCCTCCCAGTTAGCTGGTGTTACAAG CATGCACCACTACGCCTGGCTAATTTTTGTATTTTTAGTAGAGACGGGGTTTCACCATGTTGACCAGGCTGCTCTCT AACT CCTGACCTCAGGT GAT CTGCTCGCCTCGGCCTCCCAAAGTGCTAGGATTACAGGCATGAGCCACTGCGCCCGG CCTAAAATCTTAGATCTTTAGTTAATCTTATAACAATTTATAAAAATATTCTTCCTAGTTTTGCTCCTACTCTGAGT AAATAAGCTTGCCTTACTGGTAGATCCTAGATCAGTATTATTTGAACTTTTTTTTCTTACTTTTAACCTATAATATG AAATACCTTTTAGTTGTGTATGTACATTTCATATATATGTGTGTACATTAAAAAATTTCATGGAACAATACTTTTAC TATACATTCTGGTATTATTTCTTTTAGTTTGTTCTATATCATTCTTTAAAAAATATGTCTGAACCCACTAATATATC T CACAAC C CACT AAT G CAT GAT GAC CTTCAGTTT GAAAAAACACT AT T C T T GAT CACCCTGTG GT AGAAT T CAT T T A TTTTCAAATTCTAAGTAGGAACAAAACACCTTACAAGTTGTGTGTGATAACTTATTATTTGGGTTTTTCCTCCCAAC TTGTTTATATGTTTCAGTTATTTGCAGTGAGGACTGGGCCACAGATATTTATCTCAGATGTTCTACTTTTAGATTGC TTTCCCTAAAATACGAATGAGTGTTGAGTTAGGAAATTGGTCAGAAATATAGTTGTCAGGCCTATGTATTGCTCATG TGAGTCTATGGATTCTATTATTAGTTAGGACAGCTGCCATAAAGCTTTATATTTGTCTTCTTAAGCCACTATAAACT TGAGGCTGAGCCTTTTCAAACTAGTAATTATGATTGTTTTCTCCTCTGGAAGAAGAATAAACTGTTCTTTGGATTTA TCAAATTTTCTTCCCTCTCCTTTTATACAATGGACAGAGTTAT GAAAT GTGGCCAGAAAGCAT AT AAT GTAACCAAT TCAGTTTTTTGTGTGAATATCAACTCCTTTTATCATGCAGACAACTATTCTTTCCCCAAACTGGAGGTCAGACTGAG CAGTAGCAGTGTGCTTTAAATATTGAAGTATGTTTGGCTGGAAGTAGATTGTTGATGGAAAAGGAAGAGCATGGGGA AAATAATTACCCACCTGTTTTCAACAATGCTGTTGAAAGTTTAGATTTGGTCCAGAAATGGACCAGAAAGCTTATTT T T GAGGAGT AT TTTCTGTGT CAT T AAAT AG GAT AT TAT GAAT T AAC C CAGT AACAC CAGAAAAT T GT AAT CT AAAAC AAAAAG GAAT AAGAT GAACAGT AT GT AACT AT AAAAAT AC AAT AT C T GAT AT T AAGAAACAGACAAAT C CAT AAAT A TAG TAGGAAGTTTAAAATAGGCCTCTTTCAGAACTAGTAGGTCATAGAGAT CAT AACT GTTAGATCACAAAAATGTG AGT AAGGATACAGTGGATTT GAAT AA CAT AAAT AAACCAATCT GAC CTAATGAACGCGTACAGAAT ACT GTCCTTAT ATCTAATTTTCCTGCAGAGTCTGTTTACTAATTTGAGGTGTGGATGACTAAGAAACCAGCTGTCACGCTTAGGGAAG ATGCATCTGGTTCTGTTAGCGCTTGTGTTACTTTGTTTTTAAGATTATATATTAAAAACTAAACAACAAAGATACAC AGGTAAAATAGGCCCAGGAGCAGTGGAGCACACTTGTAATCCCAGCACTTTGGGAGGCTGAAGTAGGAGGATTGCTT GAGGCCAGGAGTTTAAGACCTGGGCAACTGGTGAGACCTCATCTCTTAAAAAATACAAAAATTAGTGGGGCATGGTG ATGCACCCCTGTAGTCCCAGCTACTTGGGAGGCTGAGGGGCTGAGGCAGCAAGATAGTTTGAGCTCAGGAATGTGAG GCTGCAGTGAGCTATGATTGCACCACTGCACTCCAGCCTTTTTTTTTTTTTTTTTTTTTTTTTTTTTGACAGGGGAA GACCCGTGTCAAAAAAAAAAGAAAAAAAAAAGCTTATAATACAGGGTTCATAGGATCAAGTCTAATACTTCTTAGCA TGGGAGCGAAGATCCCACAGTCTGACTCAAGCCTATAAGTTTAGCCTCAGCTCCTACCTCTCCTCTGCCACCGGACT ACTTGTCCTTCCCAAATGTGAACCTTCACATGTTAGGCTATCCTCTGTCTGAAGTGCTTTTCACCAGTTTAGCTGTA ACCTCTTCTGAAACCTCTCCTATAATAACTGTCACTATCCTACCTTCGAGAGCTTTTTGTTTATACAGTACCATCAG TAT AAT ACT TAT C CAC T TAT GT T GT T AAT AGT TTTTTTGTTTCCTGTTAGTGTGT GAAT T CT T T GAT GAT AAG GAC A GTGTAGTGCTATTCGTTTTGGAGTTATCTTTGTGCAAAATGTATTGGCAGGAAAGAGTCCTAGAGGAGGGAAAACTA
GAGAGGTGTTTACAGTCATCTGGGATGAGGTCTCTTTGGAGATAAGGGCTTTACCTAGAGGGCATGTAGGGGAATGA AAAGGGAGTATAAGATACATATATAAGAAGTTATGCAAGAAGAACTGCAGAATTGACCATCGTTTAAATAGCAGAAC AGAAT G T C AAAGAGAG GAT G T C AAAG AT AT GAC T T T GAG GAT T T T GAG C T T G GAT T AGAAAGAAT G G GAGT G G GAAA AACTAATTGGATGGTGGGGATTGGGAGACAGCAAGCGAGTGAGCATGTGTGTATGTGTGTGTGTGTGTGTGTGTGTG TTCGTTCAGGTGAAAGAGGAGTGAAAACTGGAAATATTAATAGTACTTGATGTACTAGTCACAAGTACCCAATGGGC AGTTAGGGAATAGGTTCTAGAGCTTGGGAGAGAGGTCAAGATGAGAAAGAGATTTAGAAGTTTTGAGACATTTGGAA AGCATATGGCAGTGTAGATGATTTTGAAAATTGAATTAATAGTGTTTTCTGGCATAATTTAATTGCGTGGAGCTAGA AGT T CAAC CAGAACAT AT AT AT AAAT GAAC AGAT GG GT GC T T T T T T T T T T T T T T T T T T T T T AC CT C T AAAGT GAAT G C AT T T C GT AC T G G GAT GT T T T C C T T AAG GAAT G C C AAC AAT T C AGAT AT T AAT T C T C AG C C T G AT C AT AT T T T AAAG GTACAGTTGATAATCAAATGTGCATTAGTAAAGTGCTTTTGGTCTTACCTGATCTTGCACATTAAAGACCTGGTTGA GGCTTTTTCTTCCCTTTATTTTCTCTTTTAAAATCTGAGTGCTGCCTAATTTAAAAGGAAAGCAAAAAGGTAAAACA ATACTCCAAAACA GAAT ATT TGCTTGGAGTCACGAGTTGGCTT TAG TAAATTGAAGCATAACCTAG TAT AAAGACTG ACCTTTAGGAGCAGTGCAGTTGTGTACTTTGTAACGT GAT AAT AGGCCT AAAT GAAGAGAACCTAACACTCCTTTAG GATTTGGCCCCCAGTCTTTAGATCTAATGTGTATAAATTGGATGTACTTTTTTCTTTTCCTTTTTTCCTGTTTAACT CAGGTGGTAGAGAATGGGAGAGTACATTTAAAAAAAAAAAAAAGGAAAATACAGTTCAAAGAGCTATGTGGGAGACA GTGTTAACCAAGTTAGACAAAAATGAAAACAGATGTAGGACTGTGTAATTTAGTTTAACTCTGTAGAATCATATCAT TAGCAAATATATATTCAGTGGGAGTGGTGAAGGAAATAGATTTTCACAAGATAGGTTGCTCAGAATACAAAGACCAG GTTCAGTTAGTTAAGATTTGATCAATGTGAACTTGAATTTAAAAATTAGGAATTTGGGGAATTTTTTTAAGAGCAAC CTCTGATTTTTCCATTGATTTGGCCCAGATTTTCCACCTAAAATTTTTTTACAAACGTTATGCTCATTAGCTTTTAA AAATATCTCAGAAAGTACAAAATTATCACTTAATAACTCACTTATTGCCTATAAAAAATTGTTCAGGAAATTCAAAG TCGTGAGAGAATTTCTCACTATCCTTCAAAGTAGTTTTAATAAAGTAATATCATTAAGCTCCAAGTTGAAATATAGG GTAGAAATTGTCTGTCTATATTCCTAGACTTTAAAATGCTTTTCCTATTTAAAAAAACACATTGTAGTTTCCTTGTA AAAGATTAATAGGGTTCTTGTGTAATGCAGTTTGACACTTTATAGTTGGCCTGCAGAGGCAAGTAAAAAAAAAAACA AAAAAAACTTGCTATGTTTCTTAACACTGAAGCATTGGCTGTATTTTTTCTTTTGGCTTTCTGTTTGAGACAGAAGA GT GAAT CTTTAGTATCTTT GAAT GAAGGATTGAATTTATT AAT CTCCCCTCCCCTTAAAAGCT AGAT CTTTCTGTGG TGAAAA GAGT GAT AAGAGACTTGTTTCCTAATCAAGACGGATT GAG TAT CTTAAAACACATGAAGATTAGTTGTGCT ACAAATCGATTGAAAGAGTTTAATGGGTACGAGTAGATCACTTGATCAGAAGATTTAGCTATCCGTAACAATTAGCA TTGGTATT GAT CACAATTTGCAG TAT TACT AGACAGATTCCTAATGGTTACACTTT GAGT CTTGTTCTTT GAT ACT G GTAAATGAATGGTAGGTTTCTTTCTTTCCTTTCCTTTCCTTTCATTTCCTCTTCCTTTCTTTCCTTCTTTCCTTCCT CTCTCTCTCTCTCTCTTAATTTTTAGATCTCAGTTTTCATACGTATTCTTTCCTAAAATTAATCTGCCTGAGAAAGA TACTTGCTGTGGAGGTTCTATCTCATATTCTCTTCTCCCAATTGACTGAAGACAGGCATACTTTTCTACCCACCTGA ATCACATTATGGTGTATTGCCTTGGAGTTTTACAAACTTCCAGGGCACTAAAACAAAAGGAACAATTTGGTGATTAC TTTAATGGTTAAACAGTACCATAAGAGCATCACCCCCAATTTTTCTTTGAAAGATGTATTTTCAGTAATTATTTCTT ATATTTACTATTTTATTATTTACCAAAATTTGTAAAAAAAACTATGCCTTTTTGATAAATTGTGTGCTAGTGACTAT T GT AAT AAT T CAAT T T AGAG GAC ACAGAAT T AAT AC T T AGAGC AT TACAGT CAT T G GAAAT T AAGAAAAAT T T AAAT ACACT AACAT ACT AT AGAT T GAAAAAT TAT AGAAT GAT T G GT GAAA GAC T T T C AAG CAT GCAG GT AT AT CACAT T C A AAT AGCATT CT GT GGAGGAAGTAGAAT GGAAAAAAAGTT CAAAGAGAAAAAGAGAAGAT GT GGCT GGGTAT GGT GGC TCAGGCCTATGATCCTGGCACTTTGGGAGGCCGATGCAGAAGGATTAGTTGAGCTGAGGACTTCAAGACCAGCCTGA GCAACATAGT GAGATAT GT CT CT ACAAAAAGTAAAAATAAAAAAAT TAGCCAGGCAT GGT GGT GCACAT CT GCAGT C CAGTCTACTCAGGAGGCTAAGGTGGGAGGATCACTTGAGCCTGGAGGGCAAGGCTGCAGTGAGCTGTGACTACGCCA CT GCACT GCAGCT T GGGT GACAGAGCAAGACT C CT GT CT CAAAAAAAAAAAAAAAAAAAAAAAAAGAAGATAT AAAA TTCCTTAAAGAGGCATGTTCAGGCAACTTTTAAATAGATATTTCTAGAAATCAAATCTCTGGCATTTTTATTTGGAT AT AT T T AAAAT AAT AAT AT AAAAGT T T TAT T T AAAAAAAACT G GT CAT G GT GCAGT GGT T CAC ACAGT GT AAT T C C A GCACTTTAAGAGGCCAAGGTGGGAGGATTGCTTGTGCCCAGGAGTTCGAGACCAGCCTGGGCAAAATAGTGAGACAC TGTCTCTACAAAAAAAAAAAAAGAGAAAAATTTACATTATGCAGGATATTCCTTTGTTTACAACTATTTAAACTTAA GAT GAAAACT T GT GAAT AC CAAC T TAAAAAT T T GT GAAGC GT C GCAT AT TTTTTCAGTTATTT T AGT AT T AAC AAAC AAAT T GAAGAT CAT T G GT T TAT AT AAC C C C CT GAGAGACT AAT AGT AGAAT AGAACAGAAT AAT AGAAT AGAAT AGA ACAGAAT AGAAT AAT AGAAT AGAAT AAT AAT AGAAT AAT AGAAACT C GC T TAT T TAT GAT T GT T AAT AAGT AG GAT A AAT TGCAGAT CAT TTATACTATTAAAGTGCTTTATTGCTTTGAGGTGTGCCTGTAACT GAAT GTTTTACTTCCAAAA TTAAAATTTTCTTT GGCT GAAT AAAGAT AT TCTGAAACCTGACATTTCTCCTTTAATCTTTGTTGAGCCAAATGACA TAT CAACTTAAAAAGATAAGCAAAT AT ATT AACT GAAT CTGGCAGTAGCT GCAGCT GTTATTAATGTCAGGCCTATC TACAGATCAGT GGCT GCTCCCTGTTT GGGT GAACTCTAGCTTTGGGTAAGTAGGTTTTGTGTTGAGAAAAAAAAAAT TAA GAC AAC AAAC C T GAAC CAGCTACTTAGT TAG GAT TACTTATTATACAGTCTTT GAC AT T T G GT T T C AGAAAAAT AAAT GT T AAG T T T AAC AT AG AT G G C AAAT AAT G C C TAT C T GT GAC AT C T C AC AAAAAAT T C T T GAAT T AT T CAT AAC T T AAT GT C C C CAT T T G GT T C CAT GT T T T AAAAT AAT T AAAAAAAT AAAT T TAAAAAT AAT T T TAAAAAT AAAAAAAA TTTTTT T AAAT AAT T AAAAAAAAAT T C CT AAAC CAAAACT T GAAGT T GAT GAAT AAAGAC GT T T T GAT AT T CT AAT G CTAGGATAGTTTATCTTTTTGTCTTATGAAATAAAAATTAGTTTGCTACTCTTCAGACATT TAT GAGT TTGAAGAAG
ATAAAG GT AGACAT CACAAC T CAT TAAT TGCCTTCT GAAAACAGT T CAT TAT T AT AGAC T CAGAGGAAAC GT G CT T T ACT T T TAT GAGAT T GT AT CAAGACAGAGAT AAAT AT T T T AT AAT AT AT T T AT GT T AT T GAT T T CAAT AT AAT AACAT CGTGGTCAGAGAACACCCTTTGTATGTAAGTTTGACCTAGGTCAAACTTAAATCATACAAAGTTTGACCTAGGATAT GGTCAGACTTGGTGCATGTTCCATTTGTACTTGAAAAGCATGTGTATTCTGCCATTGTTGGGTGGGGTATAGTATAA ATATCATTTATAGATCTAGTTAGTTGATAGTATTATTACATTTTTCTCTATCCTTTGCTGATTGTCTTTTCTGCTGA TTACTGAGAGAAGAATGTTGAAATGTGCAACCAAAATTGTGGATTTACCCATTTTTCCTTTTAGTTCTATAAATTTT TGTTTTATATATTTCAAAATTCTGATTGTTGCATGCACATCTAGAATTATGTCGTTTTGGTGAATCTACACTTTTAT CGTTATGTAATGTCCCTCTTTATCTCTGGCATTTTTCCTTGCTCTGAAGTCTATTTTACCTGATAGTCATATAGCTA CTCCATCTTTTGATTTGTGTGTGTATGGTGTATTTTTTTCCTTTCTTTTAACTTACCTATACCATTATATTTAAAGT GAGTTTCTGACCTGGTGCGGTGGCTCATGCCTGTAATCCCAGCACTTTTGGAGGCTGAGGCAGGTGGATCACTTGAG GTCAGGAGTTCGAGACCAGCCTGGCCAAAATGGTGAAACCCCATCTCTAATAAAAATACAAAAATTAGCTGGGCATG GTGGCAGGCACCTGTAATCCCAGCTACTCGAGAGGCTGAGGCAAGAGAATGCTTAGGAAACCGGGAGGCAGAGGTTA CAGTGAGCCGAGATTGTACCACTGCACTCCAACCTGGGTAATACAGCGAGACTCTGTCTCAAAAAAT AAAT AAAT AA ATAATAAGTAAATAAATTGTGTTTCTTGTAGTTGCATAGAGTTGGGCCTTGTTTTTTAATGAGTTTTACAATTCCTG TCTTTTAGTTGGTGTGTTTGCACCATTTACACTGAATATAATTATTGTTATGTTTGGATTTAGGTCTACTTTCTCAT TTTTCAGTTTGCCTCTCTCATTTTAATCCCACTGCTTCCTTTTTCCTGCCTTCTATTGGATTGTGTTTTCAACGATA TCTTTTAGTTGCTCTAGTAAATACAAGTTACTACTTAACTTTTCACAGTTCTACCTAAAATTAATATTTTACTTCAA GAGGAATGTACGAACCTACCCACCATACCCTTTATCCTCCCCTTTTATGTTGTACTTAACTTATATATATATGCTGC ACTGAAAACCCTACTAGACAATGTTATAATTTTTACTTTCAACTGTCATACACAGTCTAAGGGACTTAAGGGGAGAA AAATAGCTGATTGTATTTACCATTTCTGTTGCTTTTCATTCATTCCTGAAGTTCTAAGTTTCTCTGTGGTATAACTT CTCTTCTGCCTGAAGAGCCTTCTTTAGCATTTCTTTTAAAGCAAGTATGCTTATAATGGATTATCTTAGTTTTCCAT CTCTGAGAATGTGTATTTTGCCATCATTCTTTTTTTTTTTTTTTTTTTTTTTT GAGAT GGAGTCTT ACT CTGTTGCC CAGGCTGGAGTGCATGGCGCGATCTCAGCTCACCGCAAGCTTTGCCTCCTGGGTTCACTCCATTCTCCTGCCTCAGC CTCCTGAGTAGCTGGGACTACAGGCGCCTACCACCACGCCCGGTTAATTTTTTTTTTTTTTTTTTTGTATTTTGTAT TTTTAGTGGAGATGGGGTTTCACCATGTTAGCCAAGATAGTCTTGATCTCCTGACCTCGTGATCCGCCCGTCTCGGC CTCCCAGAGTGCTGGGATTACAGGCGTGAGCCACTGCACCCGGCCTTGCCATCCTTCTTGAAGGACATTTTTATTGG ATATAAAACTCGGGGTTGTGGATTGTAGTTTGTTGTTTTTGCTGTTTGTTTTTTAGAGTGCTTTAATAATTTTGTGC CATTGCCTTCTGGCCTCCATGGCTTCTGCTGAGAAATTCAGTCATTTGAATTTTTCTTCCCCTTTATACGCACATCA TTTTTCTATGGCCACTTTCAAGACTATTTTATTGCTGCTGCTGCTGCTGCTTCTGCTTCTGCTTCTTCTGCTTCTGC TGCTGCTTCTTCTCCTTCTCCTTCTCCTCCTCCTTCTTCCTTCTCCTCCTCTTTCTCCTCTTACTCCTCTTCCTCTT CCTCCTCTTCTTCCTCTTCCTCTTCTTCCTCCTCTTCCTCTTCTTCCTCCTCTTCCTCTTCTTCCTCCTCTTCCTCT TCTTCCTCCTCCTCTTCTTCCTCTTCCTCCTCTTCCTCTTCTTCTTCCTCATCTTCTTCTTCTTCTTCCTCTTCTTC TTCTTCTTCCTCTTCCTGTTATTATTATTGTGAGACAGAGTTTGGCTGTGTTTCCCAGGCTGGAGTGCAGTGGTGCG GTCGTGGCTCACTGCAACCTCTGCCTCCCAGGCTCAAGCTATTTTCTTGCCTCAGCCTCCTAAGCAGCCGGGACTAC AGGCACGTGTCACAACACCTGGCTTATTTTTTTGTATTTTTTGTAGAGACAGGTTTTCAACATGTTGCCCCAGCTGG TCTCAAACTCCTGGGCTCAAGGGATCCGCCTGCCTCGGTCTCCCAAAATGTTGAGATTACAGGCATGAGCCATTGTG CCCAGCTGACTTTTTTTCTGTCTTTGGTTTTCAGCAGTTTGATTATAAAATACCCATGCATGGATTTTTTTGCACTT ATAGTGTTTGAGATTTGCTGAGCTTCTTGAAATGTAAGTTTTTGTCTTTTGCCAAATTTATGGTGTTTCTAGCCATT ATTTCACTTTTTTTTTTTTTTTAAATAGCATGGCGCCATTTTTTTTTCTCTCCTGAGACTCCAGTGATAACAATAGT AGGCCTTTTGCTATTGTTCTTAAAATTTTTTCTTCTTTTTTTTCAGATCTTTTTTTCTTCTTTCTCTGTTATTCATA AGGCAATTTCTACTTACCCATCTTCAAGATAACTCACTTTTTCCTATCATTTCCATTCTTCCATTAAACCCATTCAG TTTATTTTTTTAATTTAGTTACTGTATTTTTCTGAAATTCTGAAATTTCTACTTTTTTCTATTTTGCATGTTTGGTA TCTTTCTTTTACTTTAAGACTGTTTAATCTTACTTCCTTGGAAGGCTGGGCGTGGTGGTTCACACCTGTAATCTCAG CAATTCGGGAGGCCAAGGTGGGTGGATTACTTGAGGTCCAGAGTTTGAGACCAGCCTGGCCAACATGGTGAAACCCT GTCTCTACTAAAAATACAAAAATTAGCTGGACATGGTGGCACATGCCTGTAATCCCAGCTACTCAGAAGGCTGAGGG ACGAGAATTGCTTGAACCTGAGAGGCAGAGGTTGCAGTGAACT GAGAT CGTGCCACTGCACTCAAGCCTGGGTGACA GAGCGAGACTCTGTCTCAAAAAAACCAAACCAAAACAAAACGAAAACCTTAATTCCTTGTAACATGGTTATAATAAC TGCTTTAAGGTTAGTCTGGTAATTCCAGCATCTGTGTCACATTGGAGTTGGTGTCTGTTGACTTTTTTTCTTTATAA TATATTGAGCTTT TACT GGT TAT TTGAATGTCAAATAATTTTGGATTATATCCTGGACATTTTGAATCT TAT GCTAT GAGAT CTGGGTCTTGTTTAAATCCTATTAAAACAT AAT ACTTGTTGGCTGGGTGTGGTGGCTCACACCTAT AAT CCC AGCACTTTGAGAGGCCGAGGCGGGCAGATCACCTGAGGTCAAGAATTCAAGACCAGCCTAACCAACATGGAGAAACC CTGTCTCTACTAAAAATACAAAATTAGCTGGGCATGGTGGCACATGCTTGTAATCACAGCTACTCAGGAGGCTGAGG CAGGAGAATCGCTTTAACCTGGGAGGTGGAGGTTGCGGTGAGCAGAGATAGTGCCATTGCACTCCAGACTGGGCAAC AAGAAT GAAACTC CAT CT CAAAAAAATAAAATAAAATAAAAAT AAT AAT ACTT GT CT GT TT GT GCCAT CAGGGAAT G ACCCAGTTAGGTTCAGCTGCAAGTTTTGACTCGCCT TAT GTAAGCTGTAATTC CAAT GTCAGTTCAGTTTTCAAAGC CTTTATAGTGCTATTCAGATCTGTACTGTATGTGCACCACTCAGGGGCCAGTCTGGGTGCTGGGCAGTAACCTGTTT TGTAGTTCAGTTCTCAGTGCTTTTGGTGTGCTGTTTAAGGTCAGACCTGTACATGTGCAGTTTAGAGATGATCCCAG
GTGGTCACAGGCAATTTTATGAGATCCCTTTCTCACATTCCCTCATATCTGCCATCTCACTAAAGTCTCTGTCTTCC AGG GT C T C CAGT AT AGAAGAT AAAT AAAGACAT GAAAGAT CT C CTT T CAT GGGAAAGAAAGGAT GT GCAATAT CAT T T GT TAT CAGT GAAAT ACAAAT TAAAAC CAC AAT T AGAT AC CACTCGTCTTC C ACT AGAAT GC T TAAAAT T TAAGAGA CTTTCCAGTGGCATGAGCAACTTGTTAGGTGATGATGCAGAATAACTAAAACATGGATACATTGCTGGTGGTAATAT GAATATGGTACAACCACTTTAGAAAACAATTTCTTACAAAGTTAAACTTACTATAAGATCCCTCCATGCCACCTCTA GAT AT T T AC C CAAAAGAT AG GAAAAC AT AT GC C CAC AT T AAGAT CT AT G CACAGCT T T AAT CAC CAAAAAACAAGAA ACAACT GAAAT AAC CAT CAC AT GAT GAACAGAT AAG CAAAT T GAAC T AC T T T T AT T CAAT AGAAT ACT TAGT AAT GT AAT GGAC CAT T TAT AT AT GAAGAAT AT AGAT GAGTCT CAAAAG CAT TAT GCTAAGT GAAAGAAGC C T AAT ACAAAAG GCTATATACTATATGATTTTATTTATATGACATTCTAGAGAAGGCAGAAATATAGGGAAAGAAATTAGATTAATGGT AACCCAGGGTCTGGGAACCTTCTGGGATATAGAAATGTTCTGTATTTTTATTGTGGTGGTAGTTATGTGACTGTATA CATTGCACTGTACATGTAAATGGGATGAATTTTATAGCATATAAGTTATTATACCTCCATAAACTTGACTTTTAAAA AGGCTTTAAAGCACCGTGTTAACTAGATTGTCAAAATTCTTTTGAATCATTTACTTAATACTTAAGTGAAAGGAAAT TATTTAAGAACCTCTGTATGCTTCAAAGAAGAATAAAATTCAAAGTATCATGCAAAAATATTTGCCTTGTATATGCC GTTTTATAACCTTTTTTGATGCATGGTAAATATCGTGACAATAAATATATGCCTGCAGTAATTTAAATGGCTGCATA GTAGCCCATTTTATATCATAATTTACTAAACTGGTCTTTCATTTTTGAACATTTAGATTGCTTCCAGTTTTATGCTC TTTCAATTTGAATACTATAGCCCTCATCCTTATAGCTATTATCTTGGAATAAATTTCTAGAAAAGGAATTTCTGGTT GAGGTCATACCTATTTTAAAGATTTTATATTCATATTACTAAATTTTCACCCAGAAATGTTCTATAATCCTTTGTCA TCTCTTTTTTTTCTTTCTTTTTAATGAAAGACACTTCATCTTTCATTTTCTTTAAAATTTCTGATGGCAGCTTTTTG GCCAACAGATATTTTACTAGCTTTTTGGTAGTTGAATCTGTTAGGTAGTTAAATCTTTTAGGTAGTTAAATCTATTC ATCTATTATTCCTCTCATGGTTTTATACTACTTTGTTGTCATACTTAGAAGGGCTTTCCTCATTCTGAGAGAGTAAA AACATTTATGTTGGCTTCTGGTTTCATTAATGGCTTCATTTTTATGTTAAATTCCATATGGATTAAAGAGTTTACTT GGTATGATACTGTAAACCAAAAAGTATCTGTCTCTAAGATGGGTCT CAAT CAATTTAGAAGTT TAT TTTGCCAAGGT TAAGGA CAT GCCCGGAAGACAT AAAT ACAGTCACAGAAACCGTCTGTGGTCTTTCCCTTTCTCCAAAGATGACTTTT GAGGGCTTCAATATTTAAAGGGGAAAAGTGAGCTGGAGGGGAAAGAGTAAAGGTCATCCACATAGTGCAAGAGAAAA GGAGCAGTTGGGGGAATAGTCAATTATGTATTCATCTCATGCTCAGTAAATGGGCACTTTTCATGAGATAAGGTGAA CATGAAAGAGCTACCTGTGGAGATATTTACCCCTTTATCTGTAGCTATCTGCTTAGGAACAAAAGGAAAGGCAGCTT CTTGCATGACTCAGCTTTCAGCTTAATTTTTTTCTTTTGGCATAGTGAATTGAGTGAATTTGGCATACCTTTCACAA TAT GAT GT AAAAC T C CAC CAGT AT TTTTTGCCACCTAGT T AAC CT AGT C AAC C ACT T T T AAC C AT GT AAGAGT T AC T AATTTTTTCCCCCATTGGTTTTAAATACCTTGATCATATTCCAAATGCTTTTGTTTCAGTTACTGTGATTTCTATGT TGTTCCGTTGGTCTCTTTATTTTTATACCTGAAGTGAACTGCTTTAGTAAGTGTAGTTTTATTATATGTATTTTAGT AATATGGCAATGCAGGTCCCCTCTTAAACCACTCTTATTTTTTAATTTTTTGGGAGATATTCTCAGCCATTTCTCAG AAATTCTCACAAAGTTACTGGTACAGTTTAACTTCTAAATCATTATGTCCAGTTGCAACAAAAAAATCTATGTTTTC AT AAT AT T TAT AAAT T AAT T T G G G GAAAT T T GAT AT T T T TAT AAG CCTCTTAT C T AGAG G CAT AAT AC AC T T T AC T A AAAT C T C C T AC AT C C C T C AG TAG CAT T T TAT AAT AT T GAT T TAT AT TAT G CAT T T TAT TAT AC T T CAT C C TAT AC AT TTTGTGATTTCGTTGCTGGAAACTTTTTAAAAATTATATTTGCTGATTATTGCTGAAATACAGTAAAAAATATTGAT TTTTATATTGTTTTTAAGTAGTCATCTTTTTATTTCCAGTGTCATTTTACATGACTTTTTATTTAACTTAAATATGG TCTTATCAAAGAAAAATTTGGGGACTGTATGTCAGGATTCAACTAATATTTTCTATTTCCATTAGATTTTACACTGT GAAGCTAATATTAAAATCAGTCTATAAGACCTAAAATAAAAGAGGCTTGTCTCCATGAATCTGACTTTTAGTAATTG TTTGTAGAGATATATATTAAAAAGTCAATATTTATATTATATGCTCTGTCAAACTAAGGATGCTAAATGTATTTTCT TCT CTT GGAATTAAGTCTTATTT TAAAAT GATTTCTGTC CTT CCTCCCTACTTGCTACCTGGGGGAT AT ATTTGTAT GTGTCATTAGATGGAATTCACAAGTTATGGTCCAATTAAAGTTACCTTTAAAAACCTGATTAGGTAACACTCTAAAA TGT TACT GTAAACTT GAT CTTCTTGCTGGGTCTTAGCTAG TAT TTCTTAAATTGCTGCTAAAATAGGACAATTAAGG GAT AGACAAAAGAATGCTAGGACTCAAGCCTGGGGATTAT AGT GT TACT CTACGTCATTGCCTTCTAGCAT AAT GAA AGTGTGAGTTGGCCATGTTTGCAAATTAACACAGCTATTAAATTTTACATATGCAAAAAAACCCTTAGATTAGTTTA CATTTATATGGCCCTGAAATTGTTATTATTATTTTTTCTTTTTTATTATACTTTAAGTTCTAGGGTACATGTGCATA ATGTGCAGGTTTGTTACATAGGTATACATGTGTCATGTTGGTTTGCTGCACCCATCAGCTTATCATTTACATTAGGT ATTTCTCCTAATGCTATCCCTCCCCCAGTACCCCACCCGCCTACAGGTCCTGGTGTATGATGTTCCCTGCCCTGTGT CCATGTGTTCTCATTGTTCAATTCCCACCTATGAGTGAGAACATACGGTGTTTGGTTTTCTGTCCTTGTGATAGTTT GCTGAGAATGTGGTAGTTTCCAGCTTCATCTATGTCCCTGCAAAGGACATGAACTCATTCTTTTTTATGGCTGCATT GTATT C CAT GGTGTATATGTGCACATTTTCTTAATC CAGT CTGTCATT GAT GGACACTTGGGTTGGTTCCAAGT CTT TGCTATTGTGAATAGTGCCGCAATAAACATACATGTGCATGTGTCTTTATAGTAGCATGATTTATAATCCTTTGGGT ATATACCCAGTAATAGGATCGCTGGGTCAAATGTTATTTCTAGTTCTAGATCCTTGAGGAATCGCCACACTGACTTC CACAATGGTTGAACTAATTTACACTCCCACCAACAGTGTAAAAGCTTTCCAGTTTCTCTACATCGTCTCCAGTATCT ATT GTTTCCTGACTTTTTAAT GAT CGCCATTCTAACTGGCAT GAAAT GGTATCTCATTGTGGTTTT GAT TTGCGTTT CT CT GAT GAC CAGT GAT GAT GAGCATTTTTTCCTGTGTCTGTTGGCTGCATAAATGT CTT CTT TTGAGAAGTGTCTG TTCCTTTCCTTTGCCCACTTTTTGATGGGGTTGTTTGTTTTTTTCTTGTAAATTTGTTTAAGTTCTTTATAGATTCT GGATATTAGCCCTTTGTCAGATGGGCAGATTGCAAAAATTTTCTCCCATTCTGTAGGTTGCCTGTTCACTCTGATGG
TAGTTTCTTTTGCTGTGCAGAAGCTCTTTAGTTTAGTTAGATCCCATTTGTCTATTTTGGCTTTTGTTGCCATTGCT TTTGGTGTTTTAGTCATGAAGTCTTTGTCCATGCCTATGTCCTCAATGGTATTGCCTAGATTTTCTTCTAGGGTTTT TATGCTTTTAGGTCGTACGTTTAATTCTTTAAAACATCTTGAGTTAATTTTTGTATGAGGTGTAAGGAAGGGATCCA GTTTCAGCTTCCTACATATGGCTACCCACTTTTCCCAGCACCATTTATTGAATAGGGAATCCTTTGCCCATTTCTTG TTTTTGTCAGGTTTGTCAAATATCAGATGGTTGTAGATGTGTGGTGTTATTTCTAAGGCCTCTGTTCTGTTCTGTAT CTGTTTTGGTACCAGTACTATGCTATTTTGGTGACTGTAGCCTTGTAGTATAGTTTGAAGTCAGGTAGCATGATGCC TCCAGCTTTGTTCTTTTTGCTTAGGATTGTCTTGGCTATGCAGGCTCTTATTTGGTTCCATATGAAGTTTAAAGTAG TTTTTTCCAATTCTGTGAAGAAAGTAATTGGTAGCTTGATGGGGATGGCATTGAATCTATAAATGACCTTGGGCAGT ATGGCCATTTTCACGATATTGATTCTTCCTATCCATGAGCATGGAATGTTCTTCCATTTGTTTGTGTCTTCTTTTAT TTCATTGAGCAGTGGTTTGTAGTTCTCCTTGAAAAGGTCTTTCACATCCCTTGTAAGTTGTATTCCTAGGTATTTTA TTCTCTTTGTAGCAATTGTGAATGGGAGTTCACTCATGATTTGGCTCTCTGTATGTCTGTTATTGGTGTATAGGAAT GCTTGTGATTTTTGCACATTGATTTTGTATCCTGAGACTTTGCTGAAGTTGCTTATCAGCTTAAGGAGATTTGGGGC TGAGACCATGGAGTTTTCTAAATATACAGTCATGCCATCTGCAAACAGGGACAATTTGAGTTCCTCTTTTCCTAGTT GAATACCCTTTATTTCTTTCTCTTGCCTGATTGCCCTGGCCAGAACTTCCAACACTATGTTGAACAGGAGTGGTGAG AGAGGGCATCCTTGTGTTGTGCCAGTTTTCAAAGGGAATGCTTGCAGTATTCAAAAAAACTGGCACAGTATTCTTGT GCCAGTTTTCAAAGGGAATGCTTGCCCATTCAGTATGATACTGACTGTGGGTTTGTTATAAATAGCTCTTATTATTT TGAGATACATTCCATCAATACCTAGTTTATTGAGAGTTTTTAGCATGAAGGGCTGTTGAATTTTGTCTAAGGCCTTT TCAGTATCTATTGAGATAATCATGTGGTTTTTGTCATTTGGTTGTGTTTATGTGATGGATTACATTTATTGATTTGC ATATGTTGAAACAGCCTTGCATCCCAGGGATGAAGCTGACTTGATCGTGGTGGATAAGCTTTTTGATGTGCTTCTGG ATTCGGTTTTCCATTATTTTATTAAGGATTTTCGCATCGACATTCATCAGGGATATTGGTCTAAAATTCTCTTTTTT TGTTGTGTCTCTGCCAGGCTTTGGTTATCAGGATGATGCTGGCCTCATAAAATGAGTTAGGGAGGATTCCCTCCTTT TCTACTGATTGGAATAGTTTCAGAAGGAATGGTACCAGCTCCTCTTTGTACCTCTGGTAGAATTCAGCGGTGAATCC CTCTGCTCCTGGACTTTATTTGGTTGGTAGGCT ATT ATT GCCTCAATTTCAAAACCTGTTATTGGTCTATTAA GAGA TTCAACTTCCTCCTGGTTTAGTCTTGGGAGGGTGTATGTGTCCGGAAATTTATCCATTTCTTCTAGATTTTCTAGTT TATTTGTGTAGAGCGGTTTATAGTATTCTCTGATGGTAGTTTGTATTTCTGTGGGATCGGTGGTGATATCCCCTTTA TCATTTTTTATTGCATCTATTTGATTCTTCTCTCTTTTCTTCTTTATTAGTCTTGCTAGCAGTCTATCAATTCTGTT GATCTTTTCAAAAAACCAGCTCCTGGATTCATTGATTTTTTTTGAATGGTTTTTTATGTCTCTATCTCCTTCAGTTC TGCTCTGATTTTAGTTATTTCTTGCCTTCTGCTAGCTTTTGAATGTGTTTGCTCTTGCTTCTCTAGTTCTTTTAATT GTGATGTTAGGGTGTCAATTTTAGCTCTTTCCTGCTTTCTCTTGTGGGCATTTAGTGCTATAAATTTCCCTCTACAC ACTGCTTTAAATGTGTCCTAGAGATTCTGGTATGTTGTGTATTTGATCTCATTGGTTTCAAAGAACATCTTTATTTC TGCCTTCATTTCGTTATTTATCCAGTAGTCATTCAGGAGTAGGTTGTTCAGTTGCCATGTAGTTGTGTAGTTTTGAG TGAGTTTCTTAATCCTGAGTTCTAATTTGATTGTACTGTGGCCCGAGAGACAGTTTGTTGTGATTTCTGTTCTTTTA TATTTGTTGAGGAGTGTTTTACTTCCAATTATGAGGTCAATTTTCGCATAAGTGTGATATGATGCTGAGAAGAATGT ATATTCTGTTGATTTGGAGTGGAGAGTTCTGTAGATGTCTATTAGGTCCACTTGGTGCAGAGCTGAGTTCAATTCCT GGATATCCTTGTTAACCTTCTGTCTCATTGATCTGTCTAATGTTGATAGTGGGGTGTTAAAGTCTCCCATTATCATT GTGTGGGAGCCTAAGTCTCTTTGTAGGTCTCTAAGGACTTGCTTTATGAATCTGGGTGCTCCTGTATTGCATGCATA TGTATTTAGGATAGTTAGCTCTTCTTGTTGAATTGATCCCTTTACCATTATGTAATGGCCTTCTTTGTCTCTTTTGA TCTTTGTTGGTTTAAAGTCTGTTTTATTAGAGACTAGGATTGCAACCCATGCATTTTTTTGCTCTCCATTTGCTTGG TAGATCTTCCTCCATCCCTTTATTTTGAGCCTATGTGTGTCTCTGCACATGAGATGGGTTTCCTGAATACAGCACAC TGATGGGTCTTGACTCTTTATCCAGTTTGTCAGTCTGTGTCTTTTAATTGGAGCATTTAGCCCATTTACATTTAAGG TTAATGTTGTTATGTGTGAATTTGATCCTGTCATTATGATGTTAGCTGGTTATTTTGCCCGTTAATTGATGTAGTTT CTTCACAGCATGAATGGTCTTTGCAATTTGGCATGTTTTTGCGGTGGCTGGTACTGGTTGCTCTTTTCAATGTTTAG TGCTTCCTTTAGGAGCTCTTGTAAGGCAGGTCTGGTGGTGACAAAATCTCTCAGCATTTGCTTGTCTGTAAAGGATT TGATTTGCCCTTCACTTACGAAGCTTAGTTTGGCTGGATATGAAATTCTGGATTGAAAATTCTTTCTTTTAAGAATG TTGAATATTGGCCCCCACTCTCTTCTGGCTTGTAGGGTTTCTGACGAGAGATCTGCTGTTAGTCCGATGGGCTTCCC TTTGTGGGTAACCTGACCTTTCTCTCTGGCTGCCTTTAACATTTTTTCCTTCATTTCAACCTTGGTTAATCTGACAA TTATGTGTCTTGGAGTTGCTCTTCTTGAGGAATATCTTTGTGGTGGTCTCCATATTTCCTGAATTTGAATATTGGCC TGCCTTGCTAGGTTGGAGAAGTTCTCCTGGATAATATCCTGAAGAGTGTTTTCTAACTTGGTTCCATTCTCCCCGTC ACTTTCAGGTACACCAATCAAATGTAGATTTGGTTTTTTTACATAGTCCCATATTTCTTGGAGGCTTTTTTCGTTTC TTTTTACTCTTTTTTCTCTAATCTTGTCTTCTCGTTTTATTTCATTAATTTGATCTTCAACCACGATAGCCTTTCTT CCACTTAATCGAATTGGCTATTGAAGCTTGTGCATGCGTCATGAAGTTCTCGTACTGTGGTTTTCAGCTCCATCAGG TCATTTAAGGTCTTCTCTACACTGTTTATTCTAGTTAGCCATTCGTCTAACCTTTTTTCAAGGTTTTTAGCTTCCTT GCGATGGGTTAGAACATGTTTCTTTAGCTCGGAGAAGTTTGTTATTACCAACCTTCGGAAGCCTACTTCTGTCAGCT TGTGAAAGTCATTCTCCATCCAGTTTTGTTCCGTTGCTGGCAAGGAGCTGCGATCCTTTTCAGGAGAAGAGGCGCTC TGGTTTTTGGAATTTTCAGCTTTTCAGCCCTGGTTTCTCCCTATCTTTGTGGTTTTATCTACCTTTGGTCTTTGATG TTGGTGACCTACAGATGGGGTTTTGGTGTGGATGTCCTTTTTGCTGATGTTGATGCTATTCCTTCCTGTTTGTTAAT TTTCCTTCTAACAGGCCCCTCAGCTGCAGGTCTGTTGGAGTTTGCTGGAGGTCCACTCCGGACCCTGTTTGCCTAGG
TCTCACCAGTGGAGGCTGCAGAACAGCAAATATTGCAGAACAGCAAATATTGCTGCCTGATCCTTCCTCTGGAAGCA TCATCCCAGAGGGGCACCTGCCTGTATGAGGTGTCTGTCGGCCCCTACTGGGAGGTGTCTCCCAGTCAGTCTACATG GGATCAGGGACCCACTTGAAGAGGCAGTCTGTTCATTCTCAGAGCTTGAATGCCATGCTGGGAGAACCACTGCTCTC TTCAGAGCTGTCAGTCAGGGACGTTTAAGTTTGCAGAAGTTGTCTGCTGCCTTTTTTTCAGCTATGCCCTGCCCACA GAGGTGGAATCTATAGAGGCAGTAGGCCTTGCTGAGCTACGGTGGGCTCTGCCTAGTTCGAGCTTCCCAGCTGCTTT GTTAACCTACTCAAGCCTCAGCAATGGCAGACGCCCCTCCCGCCATGAGGCTGCAGCCTCGCAGGTTGATCTCAGAC TGCTGCAGTAGCAGTGAATAAGGCTTCGTGGGTGTGGGACCCACCGAGCCAGGCACAGGAGGGAATCTTCTGGTCTG CTGGTTGCTAAGACCATGGGAAAAGCACAGTATTTGGGCAAAAGAGTACTGTTTTTCCAGGTACAGTCTGTCATGGC TTCCCTTGGCTAGGAAAGGGAAATCTCCCCACCCCTTGCACTTCCCAGTTGAGGTGACGCCCTGCCCTGCTTCGACT TGCCCTCCGTGGGCTGCACCCACTGTCCAACCAGTCCCAATGAGATGAACCAGGTATCTCAGTTGGAAATGCAGAAA TCTACCCCTCTTCTGTGTCAATCTTGTCGATCTTGCTGGGAGCTGCAGACCGGAGCTGTTCCTATTCGGCCATCTTG GAAGTGACTGAAATTGTTATTTTTATGTGTTCTCTGTTTTGCTTCCTCACCAAGACTCTAAACTCTGGATGGAAGTA TATCTGTTGTTTATAATTCCCAATAGTCTTAGTATAATCCCTTACACACAACGACCATTGTATTGTGACCAATGTAG TAGTAAAAATTGGAAAAGATTTTCAATAAATATTATGACAATGAATGCACGTGTGTTTACGTGTATATGTACAAGTT AAAGTTACCAAAGATGATTATAGGTCAGCAATGACTAAAGGTGACTAATATCATAATGACTAAATATGGCTTGGTAG G GAT AG GAAAAT C AG CAT C T T AC C CAT T T C C T G C C AGAC T C AAG GT GAAT AAG AGAAAG C T AAAAG AT TGCCTTGCC CTAGGAGACAAGGTTAAGACCGAGTTACTAGGCTTATGGTGGTCATAAACCTGCAAATATTCTGAGAAGTCAAAGTC T GT T T G AC AT C C C CAT AT G GAT AT T C AAT G G G CAT T T C AAAC T T AAT G C T T T AAGAAC AAAC AT C T T AAT T T C C CAT TGCTCCAACCTCCTTTTATTCATTTAACATATATTTATTGAAGGGCTACTATGTGGCAGGTACTGTTTTAGTCTTGG AATGTCCATCAATGGGCATAACAAAGATCTCTGCCCTTGTGTTGTTTATAATCTGGCAGGGAGAAACAGGCAGTAGA TAGTAAACACAGTAGGTGAATTGTTAAGTGTTGTAGAAAAAGCAACAAGGTAGAAAAAGGGTAAGAGGAACTGGGAG TGCTGGTGGGATGGGCTTGGGGATAGTGATGGAGAGATGTAAGTAGCAACTTCAATTGGGGAGGTCAGGATGACCTG ACTCCATTGTCAGGTTGAAATTGGAGCAGAGAGCTGGGGTTGAGGGAGTTAGTCAAGTAGTAAGGTTATCCGGGGGA AGAGCATTCCAGCAAAGGGAACAAGTAGAGCAAAGTCAGGAGGTACTTAGAGTATCCCGTGAACAGCAAGGAGGCCA TGTGTCTGCTGCAGAGCCAGTGATGGAGAGAGTATAAAAGGAGGTTTAGGAAGGAAAGCTGGGTGATGGGGATCAAA TCATACAGGGCCTTAGGCCACTGAAAAGACTTTGGCTTTTAGTCTGAGTGAAATACGGGGAGTCTCTGAACTGTTTT GAACAGAGAAATAACATCTGACTTGCATTTTAACAGAATTACTCTGGCATCTCTGTTGAGTAAAGAATATAAAACGG CAAGGGCAGGAACAGGGGAACCTGTTAAGAGCAATTGCAGAGGCTGGGCGCAGTGGCTCATGCCTGTAATCCCAGCA CTTTGGGAGGCCAAGGTGGGTGGATCACGGGGTCAGGAGATCGAGACCATCCTGGCCAACATGGTGAAACCCCGTCT CTACTAAAAATACAAAAATACAAAAAATTAGCTGGGCACGGTGGCGGGCGCCTGTAGTCCCAGCTACTCGGGAAGCT GAG G C AG GAG AAT G G CAT GAAT C C G G GAG G C AG AG CTTGCAGT GAG C T G AGAT C AC AC C AC T G C AC TCCAGCCTGGG TAACAGAGCGAGACTGTCTCGGAAAAAAAAAAAAAAGAGTGATTGCAGAAATACATGCAGAGAAATGGCAGTGGCTT GTACAGGGCCACAGCAATGGACATGAGGATGAACATATTTTGGATTCTGAACATATTTTGAAGTTAGAGTCGGCATG GTTTTCTGACACTTTGGATATAAAATGT GAAT CCAAGGCTTTTTGCCTGAGCAACT ATT TAATAGAAGAATGAAAGT GCCATTAACTGCAGTGGGAAAGACTGTGGGTGGAGCAGGTTTGGAGAAGATAAGTAGTTCAGTTTTGGATATGTTAA GTTTGTGATATCCAAGTAAAGATTTTGAATAGGCAGTTGGATGTACAAGTCTGGAGTCTGGGAAGAAATCTAGGCTG GGGACAGAAATTTGAGGGTTGTCACCCTGTAGATCATGTTTAAAACCACGGGACTGTATGAGTTTCATTATACCAAG GAAATAAGTAGTAAGAGAAGAGGACCAAAGGCAGAACCTTAGGGATGCCACCTCTGTTGCCTTTGTTCTCTTTCATC TTTTCAGTAAATGGCACTACTATCTCCCTGATTTCTCAAGCCTAAAATCTAGAAGTCGTCCTTGATTTCTTCCTTCT ACATCCAGACCCGTCCTCTTTGTCTCCACTACCACCAACCTAGTCCAAGTCACCATTATCTTCTAATACATCTCCAT GCTTCCCCATTTGTCCCCCTACAGTCATTTTCCATCCAGCAGTCAGAAT GAT CTTGACA GAAT GAAGGTAATTCTAA TTGTATCACACCTCTGTGCAAAGCCTGCCAGTGTTCTGCACAGCAAACATTGT GAAT GAAT GTTCTGACTCACAAAT CAC T GT GAAAT AT T GT C CAAC GT GAAACAGAGT GAAAAGG CAG C CT ACAGAAAGGAAAAAAAT AT T T T T T T CT T AGA ATAAAATATTCTAATAAAATATTAACCCCCTAAAAAGGGGTTAATATCCAGAATATACAAAGAACTCCTACAACATA AC AAT AAAAAAC AAAT AAT C T AAT T TAAAAAT G G G CAAAG GACT TAAAT G GAC AT T T C T C T AAAGAAG AT AT AC AC A T G G C CAAC AAAC AT T G AAAAGAT G C T C T AC AT CAT GAAT CAT CAC AT AC C CAT GT T CAT T G CAG CAT T AT T CAC AAT TAC CAAGAGGT GGAAGCAAT TAAAT GT CAATT GACAGAT GAGT GGATAAAGAAAAGTTT GTAT ATACAAAAAAT GGA AT C T T AT T C AG C C T T AAAAAAGAAG G GAAT C C T AT CAT AT G C T AT AAC AT G G C T GAAC C T T AG G GAT AT TAT G C T AA GT GAAAT AAGCCAAAAAGACAAAATACTGCATGATTTCACTTAT AT GAAGTATCTGAAGTAGTCAAACTCTTAGAAA CAGAAAGTAGAAAGGTGGTTGCCAAGAGCCGGGGAGAGTGGCAAAAGGGGAGTTGTTTATAATGGATATGGAGCTTC ATT TTTTGCGAGAT GAAAAT GTTCTAGGGCTGGGTGCGGTGGGTCACGCCTGT AAT CCCAGCACTTTGGGAGGCCAA GGCGGGCGGATCACCTGAGGTCAGGAGTTCGAGACCAGCCTCAACATGGGGAAACCCTGTCTCTACTAAAAAAAATA CAAAATTAGCCGGGCGTAGTGCTGCATGCCTGTAATCTCAGCTACTCAGGAGGCTGAGGCAGGAGAATTGCTTGAAC CTGGGAGGCGGAGGTTGCGGTGAGCCGAGATTGAGCCATTGCACTCCAGCCTGGGCAACAAGAGCGAAAGTCCGTCT CAAAAAAAAGAAAAT GTT CT AGAGCT CTAT T GCACAGCAAT GT GGATAT AGTT AACACTAT T GT ACT GTACAT GTAA AAATGGTTAATATGGTGAAATTTGTATTATGTGTTCTTTAGCACAATAAAACAAAAACCAAATCCCTCCTGTGGTTC CTACTGCACTTAGAATAAAATCCCCAACTTCTTGCCCTAGCTGCCAAAGCTGTTTATGATGTTGTTTTGCCAGAATC
CCTCGACCTCAACTTGTGCCATTCTTCCTTTGTCTGTTATTGTCCAGCCACAGTAGCTTTCTTTCTATTCCTTACAC TTACCAAACTTTTCCCCCTGCCTTTGAAATGTTCTTCCCGCTGACTTTTACCAGGCTAGCTGCATCTTGTTTTTCAG ATCTTGACTTAATGTTACCTCCTCATGAAGGCTTTTTATAACTACCTAGTCTCTAGTTGCCACCAATCACTAGCTGT ATCACATCACTCTACTTAAATTTTCTAGGCCGGGCGCGGTGGCTCACGCCTGTAATCCCAGCACTTTGGGAGGCTGA GGCGGGTGGATCACGAGGTCAAGAGATGGAGACCAGCCTGGCCAACATGGTGAAACCCCGTCTCTACTAAAAATACA GAAAATTAGCTGGGTGTGGTGGCATGAGCCTGTAGTCCTAGGTACTCGGGAGGTTGAGGCAGGAGAATTGCTTGAAC CTGGGAGGTGGAGGTTGCAGTGAGCCGAGATCGCGCCACTGCACTCCAGCCTGGTGACAGAGTGAGACTCAGTCTCC AAAAAAAAAAAAAAATTCTGCATAGCACTTCTCACTATCTGATATTTTTCTTATTTATATTTGTTTACTGTCTGCTT C C C CT AT GTAAGAAT T CT AT AAGT T T T T TAT AT T T TAGAAT T TAAAGT T T T TAAAT T T T AAAAGAAT GT T AC GAGAA AGCAGGGACCTTGTCTGTTTTGTTCATCACCATATCCCCAAGCACCTAGAATAGTGCCTGGCATATACTAGGTGCTC AATAAATATTTGCCGAAAGAAAACTGAACTTCCTAGTCTTAAATGATCATTTCTTGAAAAGGACTACTCTTCTCCCC AAGCTCCAGACCTGTCTTTTTGAATTCCTTCTTGGGTATTTCTGTCTAAATATTCTACAAATACCTCAAATTCAACA TGCCCCTTTCTGCTCAATTACTCTTACAGACCTTATTTTTTCTGTTAATGGCATCATCATTAGCCCAATTGATCAGG CCAGAGGTCCTCTTTGATTCCTTTCTTTCTCTCAACTCCCACTATCTATCAGATCCTGTCAGTATCTTGTCTCACAT CTTTCCCTTCTCTTCCACAGAGTTGCTACCACATCATTTTAGGCCTAATTACTTTTTACTTGTATCATTGTAAGAAA CTGCCTAACCGGGCTTTTTGCTTCCAATCCCCCACGCCTTTCCAGACCATACTGTCTTCTATTCATCATACTACTTT TCTGTTCAAAACTAACAAATGAAATGCCATTTTTTTTTATGCTTTGGAAAGCCACGGAAAACTTTATCCTCAGCCTC CTTTAGACCCTACTTACCTTTCCAGCCTCACCATTCATGACATCCCTGCAGCTCTGTGCTGCTGCCCTCATTCTGTG GCCTTATCAGCCTCATAGCTGGTGGTCACATACACCAGCAGCCTCCACCTTTACAAAGCCATTTTCCCTGACTGCAG TGCTCCTGAACCCCCGCTCCTTTTGGCTGACTTACAGTTTTATCTTTAAGATATAACTAAAAAACTGCCTCCCCAAG AAGCCTTTATAGATCTTCCCTCAACTCCAAAATTGAAATTAGTGGCATAGACCCATAATATCTCTATTATTAATATC ATTTTTTTCTTTTTTTTTCCAAGATAGGTCTTACTTTGTTACCCAGACTGGAGTGCAGTGGCGCAATCTTGGCTCAC TGCAGCCTTGAACTCCTGAGCTTAAGCAATCCTCCCACCTCAACCTCCTGAGTAGCTATGATGACAGGCATGTGCCA CCATGCCCAGCTAATTTTTTTGTATTTTTTGTAGAGATGGGGTTTTGCTATGTTGCCTAGGCTGGTCTCAAACTCCT GAGCTCAAGCAGTCTGCCCAGGCTTCCAAAATATTTATTGAGCACCTACTATATGCCAGGCACTATTCTAGGTACTG CCCTCAGCCTCCCAAAGTGTTAGGATTACAGGCATGAGCCACCTCTCCCAGCCTATTTCATTTTTTAAATTGTGGTA AAGT AT ACAT AAT AT AAAAT T T AC CAT T GT AAC T GT T T T T AAG GGT ACAAT T C C CT T GC AT T AAGT ACAT T T ACAT T GCTGTACAACATTCACCACTGTCCTCTGCTATCATATTTAATTATTTATGGATGTAGTTGTCCCCTCCTCTGATTGG AAACAAAACGCTTGGCGTTATTTGTAAGTGCTGAATTAACATGGATGAACATGAATATAATCAGATCATCTCCTGAG ATGAGATGTCATGTCACAAGTATCCTTGTGATCCTGCTTGGGTAACTTCAGGATTGTGTTCAGTTTTCTTCCTTGTA CTTATTCAAAATGGCATTGGTTAGAGTTCACGGAGTCCTGGATTTTTAAAGGTTCTTTGCCTAATTTCTTCTTTACA AGTTTTTGGTGCGTACACTTAACCTAACTTTTTTGTCTTACCAATATCTATCCTATAAATATGCCAGTGAAATGGAG CATATTGCGTTAGGTAAGGTGTGCATATTTCAAGAACTAGATGTGTTTCAAAGCACATAATGAAACTTCAGCAAAAA TGAGATTAAGAGTTTAGGCTCCAATAATATTGCTGCGATTTTATTTCTGTCAAAGTTTTGTTATTTTTGCTGTATTG TTTGCTTTTCTTCAATGTTTTGTAAGCCCCGGTTCTTTGATGAACTCCTAATAAGCTGTGCTTAAAACTATGACATT GACCTTTCTCCAGAAACAGGTTTCAGAATTGTCTGTTTTTCATGTTCTCTCCCTGCCTTTTCTCTTGTAATCAAATC AGCATGTTTTTGTTTTTGTTTTGGAGACAGAGTCGCACTCTGTTGCCCAGGCTGGAGTGCAGTGGCACTATCTCTGC TAACTGCAACCTCTGCCTCCTGGGTTCAAGCGATTCTTCTGCCTCAGCCTCCCGAGTAGCTGGGACTACAGGCACGT GCCACCACACCCAGCTAATTTTTTGTATTTTTAGTAGAGACAGGGTTTCACCATGTTGCCCAGACTAGTCTCAAACT CCTGAGCTCAGGCAATCTGCCCGCCTCCACCTCACAAAGTGCTAGGATTACAGGCATGGGCCATTGCACCCAGCCTA AATCAGCATGTATTTTTAAACATCATAGAATGCGAGGGTTTGAAGTGTCTTTTGTGATCATCTAACATTCACTTTGC AT AT AAAT AAAT AAAT AAAG AC C CAT GT T AAT G C C AAAG G GAG T GAGT G G C AG AAG AAT C TAT GAC G GAAG CAT AT C TTTTGGTGCTGTCAATGAAAGGGAAACAAATGCTTGGCATTATTTGTAAGTGCTGAATTAACATGGATGAACATGAA AATAATCAGATCATCAGTTATTCTCCCTCCTGATCTGAAGGATCCTGTGTGACATCATCTGAGATGCACTGGGGGTT GAG GAAAGAT GAC C C T T AAAAT C C AC AT TTGTACTTTC C AAG AG C AAG G T GAGAT C AT AGAG AAG TTACACCCGCTC CTTTCTCCACTTTTTAGTCTTTATCCACTAAAAACAAGATGTGAGAAAATCAAGTGACAAGAATGATAGGGTAATAG GCTGTCAACAAAAT GAGT GTGTGGGCTTATATAGGTATAAACAT GGT GAAGGT GAT AGACCATGGATGCTTAGTCCC TTTTAAGTTCCATCTTCCGTTTAACACATTTTGAAAGAATTTATTGTGTCTTATTTTCCCCTGATTTGTGTAGCTGT GCCACCCTAGATAACAGTTTTTCTTTTTGCTCTTGCCACCTTCTT GAT AAAGGCAAGTACAGT TAGAAT CGTAGGT A CTTAATAATGCTTCGGCATGAATATGCTTCACAACTTT GAGAT GTGTACCCTT TAT TTTCTTTCTGTTAT AAAT CTT AATTTCTTTGTGCCCTACTTTTCATATCTGTAAAATGGATGAAATAGTAGTATCTGCCTCATATAATTGCTGTAAAG ATT AAAT GCT AAT ACA CAT AAAGCTCTTAGAACAGGTCT GGT GTATAGTAAACACACAAAGCTGCT ATT ATTATTGT TACTGGTATTAACTGATATATTCATACTTTTTATAACTGCTCATCCTATTAATTAAATGTATTTTTTCACTATATAT TCTTTTTCAATGTATATTCCTTTTAATCTTCCTTCTTAGCCTCCACTATAGTTCAGATGCCTTCTCCAATTTCAAAC AATTTAATGAAAAGAAAAGAAAATTTATACATCCAGAGATGGCTGATTTAATTTCTTCAGTCTTTTGGAGGGTAGAA TTATTTTCCTTCTGAGAGTAAACTTATTTTTTCTATGTTACAGGTGGTATGCAAGATTACAATTATGTGTGGGCCAA CTGTTTT GAGAT CACATTAGAACTGT CTT GTTGCAAGTACCCACCT GCT TCACAGCTTCGACAGGAATGGGAGAACA
ATCGTGAGTCTTTGATCACATTGATTGAAAAGGTAAAAGTAGATGACTGGAATGTTGGGGTATAGAAACAGGATTAA AT AAG G GAGAAAT T T G GAT G CAT GT GAAT G AT AAT C TAT AC AG TAT TAG AT GT CAT TTATCTCTTTTTATTACTTAA GATTATAAATTATCTTTAAAGATTATTAGATTATAATCTATTAAAACATATTCTAGCTCTGCTTCCCCTGCCCCTTA CCCCCAGCTCTCTGCTCCATAGGGCTTCCTTTTCCTATCTTGGAACACT TACT TAT ACAAACTGAAAT AAT TAGCCA AATTTGTACTCTCAGGTTCTTACAATGTCATGTGATCATATTTGCTAGTGAGCCTTTACCACTATATGAACCATAGA CAT CAGTTTAATTTAAACATCAC CCA GACT AAT ACAGCAATT GAAT ACACCAGCAGTTACACCTTGTTGAGAAAATT AGAAATCTGGCTTTGAGGAGAGGGAATTTTAAAAGCACCTTACTAGTGTCGAGTTCAGACATATGACTTAAGTTTAA GGATTTAATACTTCTGTCTGTATTTTATTTAAACTTCAAGGGATTGAAAATGTTTTGGGGGAGAGAGAAGAGTCACT TTATTTTTTAGAAATTCTAGTATCGGTGCAGAGAGAAATTATGCGCTCTTGCCTTCCAAATTCACCGTCTCTGAGCT TGGATTCTTGTCTTCTTAGGTTCACATTGGAGTGAAAGGATTTGTTAAAGATTCCATAACAGGATCTGGGTTAGAGA ATGCAACCATCTCAGTGGCTGGTATTAATCATAATATCACAACAGGCAGATTTGGTGATTTCTACCGATTACTTGTT CCTGGAACTTACAACCTTACAGTAGTTTTAACTGGGTAAGAATTTAAACTATGTAGACTCTTAGTTAAAATGTCAAG TCTCTGTTTTATATCTGAGACAGAAATATAGTCTAGTACATGTTGTTTATTTTTTGTGGCTTTTATTTTTCCTTATT TTCTGATTTTTCTCGTTTTATAATAAGAAAATACTATTGTGGAGTTTTTTGTCACATAGTCTAATAGTACAATGGGA TGTGAAGCATAGTTGTAGCATTAGAGGAAGGACAATTTTTAAAACTTGAAAAGATATTCAGCTGTGTAAAGCATTGC CTTAAAGCTAACCGAATACCAGAGTAGAAGAAACCCATTGCTCTGTAAATGACATTTTGAAGAACTGTCTTATCAGC TTTCTAATATATTAAAT GAAT GGTTTCCCACCTCCCCTGGAAAGCTTTCTGGAAGCTCACTTT GAT AAGGATTTAAT TGATAACCTTTAATCTTGAGCTTTCCTACTTGAAAAAGAAGTTGGAATGTCAGTTTGATAAGACACTGCAAAGAATT GTGTAATTTATCCCTTCTTT GAG TACT GTAGCAGTGGTTACAATGGAGTAACAACCACATCTTTCAACATGTTCTTT CCTTTGAGTGTAATTTTTTTGGTATACATTCTAGTGTGTGGTACTTGAGAAGATTTGGTGAGAGAAATAGTTGTTCA ATCTGGGGTTCTTTGTGAGATAATGTCCTAAAACTCTTAAATTTACACATCTCTCTTTATTTAGTTTGAGTTTTGAT AGATCGCTAAGTGTAAGAGGATGAAAAGGCTACTGTGCTTTTATAATATTAAAGCATTGTATGTCTTAACTATAAAC CAT T T ACT T T T T C AAAT T T T T T T T T C AGGT AT AT GC CAT T GAC T GT T AC T AAT GT AGT G GT GAAAGAAG GAC C AGC C ACAGAGGTGGATTTTTCTCTTAGGCCAACTGTAACTTCAGTAATCCCTGACACGACAGAGGCTGTATCAACTGCTAG CACAGTTGCTATACCTAATATTCTTTCTGGAACATCATCCTCCTACCAGCCAATTCAGCCAAAGGACTTTCACCACC ACCATTTCCCTGATATGGAAATCTTCTTGAGAAGGTTTGCCAATGAATATCCTAACATTACCCGGCTTTATTCCTTG GGAAAATCAGTAGAGTCAAGAGAACTTTATGTGATGGAGATATCTGATAATCCGGGTGTCCATGAACCAGGTAATTG GTATGGTCTTACACATAATTTCAGTAGTGCCCCTCAAAGCCATGTTCAATATTGCTATGTTTCATTCAGAAAGGTCG CCTACAGT T T GT AT AACT GG CAT T AAGAAAAC C T AACT AAAAT T AAAAGAAAG CAAAAAAGAAAAT GT AAC CAAT C T TTTCCCAGAAGAGAAGGAAATCCTTTTGCTAACCCATATTAGGCATGGTATTTAATAAGTGCATAGATTAGATTTAT TCTGCTAATAGTGTTTTATTTATTTATATTATATAGCATATATATGCTTTGAGCCTGCTCAGACATATGTATTAGTA AGGAAGCTGCTTTATAAGATGTTTCTGAAGGTTTTTTCTGCCTCCTTTGCCAAAATACATGTTTAAAAAATTTTTTA GTATAGGATTTATATATGTTGCGGAAAAAAACTGAGTCCATGATGATTAAGAAGGAAAAAAAGCCTTCCATGTAATT AT TTTGTTTT C AG GT G AAC C AGAAT T T AAG T AC AT T G GAAAT AT G CAT G GAAAT GAAGT G G T T G GAAGAGAAC T G C T GTTGAACCTCATAGAATACCTTTGTAAGAACTTTGGAACAGACCCTGAAGTCACAGATTTGGTTCATAACACTAGAA TTCACCTTATGCCATC CAT GAAT CCT GAT GGGT AT GAAAAGTCCCAGGAAGGT AAA GAAT AGCATTTAATTCTTAAT CAT T T GAT CAT CAT AT AGAAT GATTATTTT GAT G GAGAAG AGAAT T T GAAC TGGTTCTTTTG GAAT T T C T T C CAAT T TTTTTTCCTTTTAATTTATGATTGATTTTAGCTCTTGTATTCTTATATGATGATCTGGTCTGTTCAACAATTAGGTG ATCAAATTTATATATTTAGTGGTGTCTTCCACTTGGTAATGGTTCTAATTTACTAGAAATAGAGTATAAGACTTTTA TAAATTGTTTTATTTTAGCAATAGTTCTGTTGACAAAGAGTCAAACTCTGTAAAATATTTGAAGAGATTTATTCTGA GCCAAATATGAGTGACCATGGCCCATGACACAGCCCTCAGACCCAGAGGACATGTGTTCAGGGTAGTCAGGGTGCAG CTTGGTTTTAAACATTTTAAGAAGACATGAGACATCAGTCAGACACATTTAAGATATACATTGGCTTGGTCCAGAAA TCGGGCGAGGGTTGAAAGATTTATTAT CAAT CGAAAGGAATGTCT GGGT TAGGATAAGAGGTTGTAGTT AC CAAAGT TTGATCATGCAGATGAAGCCTCCAGGTAGCAGGCTTCACAGAGAATAGATTGTAAATGTTTCTTATCAGACTTAAGG TCTGTGTTGGTATTAATGCTGATTGACTTTTCCTGATTTCCAAAAGAGAGGAGGCATAATGAGGCATGTCTGACCCC CAT TTCCCAT CAT GGCCT GAAC CAGTCTTTCAGGTT AACT TTGGAGTGCCGTGGTCAAGAGGAGGGAATCTGTT GAG ATAGGCCCTGCCTATTGAGCGGGGCCTTAGAATTTTGTTTTGGGTTTACAGTTCTTATTAAATCATTATTTTGGGAA CCT TAT TT CAT AAGTGAAAAACCAAACCA GAT AT CTGTCTGAGTCAGT GACT CTGTTTT GACT CTCAGTTCTACTCC CTCTGCATATTCCAGACTTGCTAATTTATTGACTTCTCATTTCTTTGCCTTCCCTGGGCAAGCCCATCTCTCCTGGG GCGTTATAGTGTGGGATGGGACAGCAGACCCTTATGCACAACACTAGGGGAGTAAGAATTCATCTTTTCCCAGCCCA TGATTTCTTCCAGGCAATGATATCTATCAGGCACCATAGTATAATATGGAGCCTCATGACTTATTACTAGCACTGAC CTGACCATTGGGAGGATCAGTTGGTCATTAGTTGGTCATTAGTACCCAGAAGTTGGTCATTAGTACCCCAGAAGCTC ACTTGAACAAATAGGCACAAGATCTTACTTATATATGTATATATTTCAGATTTTTCCCCCTAAGGTTCTCATTCTTT GTCAGTGCCACAAGTCCCTTCTACATACTCCCCAGACCTTTCTGAAAGGTGGAACTAATTACACTAGTGAGCCCTCC AGTTTTCTTTAGTCTCTTGCAATTTTTCTCATTATAATGACAATAGTAAGGCTCCCACTCCCTATTAAAATGGAATA TGCACAGTTAAGCTTAAATTGAACTTTCTCCTCCATCAGGTTAAACTTCCAGCTGTGCTCTTGGCTAACTTTACCTT TTTGTGATCACTTTAATGTGAGCATATCACAATTTTCTTTTCATTTGCTCTATTTCTTACAATGTCTTAGTGAGCTT
TATACAAGTACAGAGAGGAGTATAGAGTTCCTGGCCAGGCGTGGTGGCTCATGCTTATAATCCCAGCACTTTGGGAG GTCGAGGTAGATGGATCATTTGAGACCAGGAGTTCAAGAGCAGCCTGGCCAACATGGTGAAACCCTGTTTCTACTAA AAAT AC AAAAAAT T AG C T G G AC GTGGTGGTG C AC AC C T GT AAT TCCAGCTACTCGG GAG AC T GAG G C AC AAGAAT T G CTTTAACCTGGGAGGTGGAGGTAGCAGTGAGCCGAGATGGCGCCACTGCACTACAGTCTAGGCCACAGAGTGAGACT CTGTCTCAAAAAAAAAAAAAAAAACAAATAGAGTTCCCCTCTGATCAGTAATTTCCACTCAACATTGTGATAATGTC ATTATATGTGGATTATTGACACTTAAACAGTGCTTTAATATAATTTGTCTAATTGGAATATTTTTCCTCCCAGATTA TGAGCAGAGGGCAAAAATAAAAAGATAATGGTTTTACCTCTCTGTTCAAAATATCTGGAAGAGATACAGTTTTTAAA CACGCAATGTGAAAAAGATGTGTTTTAACTGTAATGCCTGGCCAGGTGCTGTGGCTCACGCCTGTCATCCCAGCACT TTGGGAGGCCAAGGTGGGTGGATCACGAGGTCAGGAGTTCGAGACCAGCCTGGCCAACATGGTGAAACCTCATCTCT ACTAAAGATACAAAAAATTAGCTGGGCGTGGTGGCGCACACCTGTAATCCCAGCTACTCGGGAGGCTGAGGCAGGAG AATGGCGTGAACCCAGGAGGCAGAGGTTGCAGTAAGCCAAGATTGTGCCATTGCACTCCAGCCTGGGCGACAAGGCG AGGCTCCGTCTCAAAAAAAAAAAAAAAAAAAAGAGGTAATGCCTGACAGGTTCTTCCTGCCTGCTGCACAGGCAAAA CAGTTCATTGAGACCATGGTATCGCAGTAAAGAGTTTAATTAATGCAAGGCCAGCCACGCAGGAGAATTGGAGTTAT C AC T C AAAT CAGTCTCCCT G AAG G C T C AG AG GTTAGGGTTTTT C AAAGAT AG T T T GAT G GAC AAG G GAC T AG AGAAT GGGTTGATTGGTTGGGGATGAAATCATAGGGATGTGGAAAACAGTCCTTATGTGCTGAGTCAGCTTCTAGTTGGGAA CCACAGGACCAGCTGAGTTACAACTCACTCACTGGTCCAGGTGACGTCAGCTGGTTGTCAGAAATGCAAAAGTCAGA ATAACATCTCAGAAGGCCAATCTTAGGTTCTACAGTAGTGATGTTATCTACAGGAGTAGTTGAGGAAGTTACAAATC TTAACAACTTCCAAAACAATGACTGGTTATCCTTTAATTACACATACATGTTAGCCAAATTCAGGCCCCTCTTATAA TCCCAACCTTGTGACCTTTCATTAGTTTTACAAAGGTGGTTTAGTTTTGGGAAAGGTTGTTATCATCCTTGCTTTAA GAT TAAACTGTAAGCT AAAT TTCTCCCAAAGTTAGCTTGGCCGATGCCCAGGAAT GAC CAAGGACAGCTTGGAGGTT AGAAGTAAGGTGGAGTCTGGCTGGGCGTGGTGGCTCACTCCTGTAATCCTAGCACTTTGGGAGGCTGAGGGGGGTGG GTCACCTGATGTCAGGAGTTCAAGACCAGCCTGGCCAACATGGTGAAACCCCATCTCTACTAATAAATACAAAAATT AGCTGGGCATGATGGCAGGTGCCTGTAATTCCAGCTACTTGGGAGGCTGAGATGGGAGAATCGCTTGAACCCGGGAG ATGGTGGTTGCAGTGAGCTGAGATTGCGCCACTGCACTCCAGCCTGGGTGACTGAGTGAGACTCCATCTCAAAAAAA AAAAAAAAGGCGGAGTCAACCATGTCAGATTTCTTTTACTGTTATAATTTTGCAAAGACAGTTTCATAATCATAGGT GATTGATAATAGCGAAGCCTGATGTGTTCACATTTGCCTTGAGTTAAGGTATCTAAAGTAGTACGTGTTGGAATAAA AT AT AAGT GAAC AT G G C T TAT AT T C AAAT C C T T TAT CAT AT CAT AC AG GAG AT T C AAT AAG T GT AAT T G G C AGAAAC AACAGCAACAACTTTGACCTGAACCGAAATTTCCCAGACCAGTTTGTTCAGATCACAGATCCTACGCAACCAGAAAC TATTGCTGTAATGAGCTGGATGAAGTCCTATCCATTTGTACTTTCAGCAAACCTGCATGGAGGTATGGCAACTTTAT ATTCTACTAATCAGTTCTTGTTGAGAGCATTTGGAAATCCTGGTGGAATTTTATCTGTTTGTAGTGTTATGCTTTCT TAAAATGAGTATCCTGTTACTGCTCTTATGGCAGACAACAGTAAAGGTCTTTTCTCATTACTTTTTCAGCCAGTGTC CTGGCTGTTTCTTTCCTCCTTCTTTCTCTCTTTTTTTCCTCCTCTTCTCTTCTCCTTTTACCCCCATCTCTTCTCTC CACCCCTTTCTCCTCTCTTTTCTCACCTACTACTTCTCTTTCCCTTCTCTTACCTCCTCTCCCCTTTCTTCTCTGTC TTGTCTTTTTTCTCCTCTCCTCTCCTTCTCCCCCGCCTGTCCCCCTACTCTGTTCTTCCTCCTCCTCTCCTCTTCCT CTCATAGTTCTTCAGGTATTTTGTATTTATCTCTCACATTGGGAACTTGGTAACATATTCTTTGTCATTCATAAAAA TTGATCCAAATGTTTAGTTCTGTTTTCTCCCTGAAGTTTAATAAACTCTTTTATGTAAATTTAGATTTTGATATTCT CTTTTTTACTTGGTATATCCTTAACTGCAGCAGAGTGACCACATTCTCATGTAGTACCTGAGCTCATATACCTCCAT ATAATTTAGCAGGATGGATCGGTTGAATAGGATGAGTGTCAAGATTAAAAATCGCCACAATGTCCAACTAAGGTTGC GAAT AAGAGT GAAGAT AT CAGT AC C C AAGAGAAC CAAACT GT GT C C ACACAT AAAC T CACT T GAAC AAAAT GT T CAC TCATTTAATGTACAGTGCTCATGTATATGAATGTTCAATGTACATGAACGTTCATTCAGGATTATTCACAATAACAA AAAAGTTGAAACAACCCAAACAACCAT CAT CT GAT AAATAGAT AAAT AAAAT GTGGTCTGTCCTTACAAT GAAT ATT ATTTGGCTCGAAAAGGAATCAAGTGCTGATACATGCTACAACATAGATCCTTGAAACCTTACACTAAGTGACAAAAG C CAGT C AT AAT AGAC C ACAT AGT GCAT GAT TAT TAT TTTTTTCTTTAT C AAGT T GAT TAT AT T T T T AT GAAAT T T T T AGAATAGGCATATCCATAGAGACAGGAAGTAGATTAAAGATTGTATTGGGCTGGAGTGAATAGTGGTAAGAGGTGAT AGGGGAGTGACTGCTAATGGATTTTGGGGTTTCTTTTTGGAGTTATGAAAATGTTCTATAACTGGATAGTGGTAGTG GT T GT ACAAC C CT GT GAAT AT AAAC AACT T T AAAT GGGT GAAT T GC AT G GT AT T T GAAT T ACAT CAGT AAAAC T AC T AAAAAAAAAAAAG T GAC AAG TAT TAG CAC C AG G G G C AAT G G G G AAG AAAT T T AGT T T C AAC AT T T AC T G GAAT AT C C AGAAGTACCATAGTGGAGACTTCATGTCATATAGAGTAGCCTGCCATTTTAGAATGACAGTACATACTGATGGATAT TTAGATTTAGGAGGTTAATTGAGATTCTCCAAAAATTGCTTTGAAAGATTGCCAATAGTAGAAAAAAGTACTTCTAC AGTTGGTAATCATATATTGTTCTCAAAGAGTCAAATAAGAACTATGAAGTTATACCCTGGTTGGCAAAATAAATTTT TTGTTTTCTTTTATTATATTTTATGTGTTTAATGCTATGTTTGATAAAATTCACTTATTTAGAGCTTTAAAAATAGT CT CAT AAT AAAT CACA CAT CAT GTTGGCTAAATCTGGAAAGTT AAT AAT AGTTACATTC CAGT GAAAAT AAGT CACA GAT TTACAGATGTGGTGTCCAGACCTTGGCATGGTGAGAGGTAGGAATCTT CAGT CAT GCAGACATTTTTCTTT CCA TGACATATTTTGTTTTCT CAT CTTAAAATCCTTATTTAAATGGAGTTT GAAAT CTTGGCAAAGCAGTTTGAAGGAGA ATT TCTTTGTTGCTGTACAGGAGTAAAAAATCCTCATAGAGTCCGCAGCTGGAGCTTGT GAAT TAAGCT GAT AAAAG ACAGATTAAAAGAAAAAAGCATACAAATTTTATTTGATGTTTGTATGTGGCACAGGCAACAGGCAGTGGGGGAGTTC ATAGGAAAGACGTGAAACCCTCAAAAAAGCAGTGTTAAGGACTTAGTGCTCTTATAACAAAGGGTAATACCTTGTGG
AAAAGTGACTACACAAAGGAAGGGGTTTAGACTTCTAGGGGTAAAAAATTGTGGGAAAGTGACTAGGAAATATGCGG GGGAAACTAATGAAAGATGAGGGTTATTTTACTAAGGTTTGTTTGTGTCAACT CAT CTTGGTGTTGACTATCC CATC TCTGATGGTACCAGGAAGGTGCCTTTCTCACGGGAAATTTATGCCCTAATTTTAGGCAGAAAGAGGGAGGGTAGATA GCCTTTCCTGCATCTGCTGTTTCTCAGTTGCCTTCAGCTCTAAATAATTAACATGCTAAAGAGTTATATTCTGGGGC AGAATGTTCTGATTCCTTTCCCTGCCATCTTTATAATTTAGAAGGATCTTGAAAGAAGATAGGGGAAAGCAATATTC T T T T T C AT AAAAT T T C AAAAAT T T T GAT AC T AAT T T T T T T AT T GAG GT GAAGT T T ACAT AAT T C CAGT G GC GT T T AG TGCATTCATAGTGTTTTGCAACCACCCCCTCTATCTTGTTCCAAAACATTTTTATCTCCGCAAAAGGATACCCCACA CCCATTTAACAGTGACTCCCCTTTCCCTTCTCCCCTCAACCTCTGGTAACCACCAATCTTTGTTCTGTCTCTAGGGA TTTACCTATTCTGGATATTTCATAGAAATGGAATCCTATAACATGTGACCTTTCGTGTCTGGCTTCTTTCATTTAGC ATAGTGTTTTCAAGGTTCACTCATGTTGTGGCATATATCAGTACTTCATTCCTTTCTATGGTTGAATAATAGTACAT T GT AT AT ACAT AT GT AT AT AT CAAT AT CAC AT T T GC T T TAT C CAT T T AC GT AC T GAT GGACAT TTGGGTTGTTTTGA CTTTTTGACTACAATGAAAAATGCTTCTATGCCCATTGGCATTCAAATATCTATTTGAGTTCCTGTTTTCTTTTCCC CGTGCCCCCCTTGTTTTGAAACAGAGTCTCACTCTGTTACCCAGGCTGGAGTGTAAGTGGTGTGATCATGGCTCACT GCAGCCTCAAACTCCTGAGCTCAGGCAGTCCTCCCACCTCAGCCTCCCAAGTAGCTGGAACTGCAGGCATGCACCAC CACACCTGGCTTATTTTTAATTTTTTGTAGAGACAGGGTCTCC CAGT GTTGCCCAGGCT GAT CTTGAACTCTTGGGC TCAAGCAATCCACCTGCCTGCCTTGGCCTCCCAAAGTGCTGAGATTATAGGTGTGAACTACTGTACGCAGCCCCTGT ATTCAGTTCTTTTGGGTGTACACTTAGGAGTGAAATTGTCATATGATAATTCCATGTTAAGCTTTTTGAGGAATTGC CAAACTGTTTTCCACAGAGGCTGGACTATTTTACATTCCCGCCAGCAATGTATGAGGTTCCAGTTTCTTCATATCCT CACCACCACTTATTATTTTCTATTTTTATTATTATAGCTGTCCTAGTGGGTGAGAAGTAGGACCTCATTTTGGTTTT GGTGTGCATTTCCACAATAACTAATGATGCCGAGGATCTTTTCATGAGCTTGTTGGCCATTTATCTATCTTCTTTGG AGAAATATCTGTTCAAGTCCTTTGTCCAATTTTTAATTGAGTTTTTGTCTTTGTTGTTAAGTTGTAAGAGTTTTTAA ATACTGGACACTGGACCCTTATCAGGCTTGCAAAACATTTTCCCCCATTCTATAGGTTGTCTTTTCTCTTTCTTATG TTCTTGATGCATAAAAGTTTTTGATTTTGATGGAATCCCGTATATCTGTTTTTTTCTTTTGTTGCTAGTCCTTTCCG TATTGATGCTAATTATTTTTTTGAGGTAGACTGTTTGGTAGAAGTACTATAAATCTTTTTTTGTGGCTTTAGAAAAT TAGCCATTTGAAAGATATCTTCTCACTTATTCACTTTGAATTGAAGAAATTAGTGTAATTTTCATTTCTGTTGAGGA AATTCTGCTTGGCAAATTTTATTGTTTTCTCTTCTCTGGCAGTATTCTGAAAAGCTTTATGTTAATAATCCATCTTG AAACAGACAACATGATACATTTTCCTCTTTTCCCTTATAGGTTCTTTGGTGGTTAACTACCCTTTTGATGATGATGA ACAAGGACTTGCCACATATAGTAAATCACCAGATGATGCTGTGTTCCAACAAATAGCACTTTCTTATTCCAAGGTAG GCTTGTCTTTGAATATAAAATGTTACAAAATTAATTCTTTTATTTAAAAATATGCTTTAAAGTCCTGCAAGACTCAG GTCAAGTGCTTCCATAGTTAATAAAGCATGACTTTTTCAGCTGTTTCCGAAAAATAGCTTTTTCTCCTGTTTCCCAC CTCACTTTTGAATACACTACATGTCTTTCTCCATTGGCCTTTTATCTATGGTTTTAGCACAGTACCTGGAACATATA CATGTCAATTTATTTCATTTGACAAATAAGTTATAAGCCCAGTAAAGTAGGCCAAGATAAGGGCTTAATGAATTATA GCTCTGGAGCCAAATCTTGCCCACCACCTGTTTTTGTAAAT AAAAT TGT ATT GACA CACAGCCACAGCTAT ATT TTT ATATATTGTGTGTGGCTGCTTTCTCCTTCAACATTTCAAAGTAAGTCAGTATAATTTACTATATTAACAAACTAAAG AAGGAAAATC CAGT GAT CAAAGGGCAAAGTTGGGTAGTTTCAACAAAGACTTTTGCATCTAAAAAGTCTGAAAT ATT TGCTTTCTGGTTTTTTACAGGAAAAAATTGCCAAACTTTAGTCTAAGGTATTTAAGATCCTGTAGTTACATATAGCA AGGAAAAGCAGGCAGAAATTTCTGTATTTTCCAAACTTCCTATGATAAACATGGATTACTCTTACAATCGGGAATGT TAAAATGTGTGTGTGTGTGAAAATTTAATTTAAAAATCGTTCTTAGGACCAGGCTTGGTGGCTCATGCCTGAAATCG TAGCACTTTGGTAGGGCAAGGCAGGAGAATTGCTTGAGCTCTCAGGAGTTTGAGACCAGACTGGGCAACATAGCAAG AGCTTGCCTCTACTAAAAATAAAAAATAAAAAAAAAATTAGCCGGTTGTGGTGACATGCATTTGTTGTTCCAGCTAC TTAGGAGGCTGAGGTGGGAGGATTGCTCTATCCCAGGAGATTGAGGTT GAAGT GAGCTT GAT CACAATACTAT ACT C TAGCCTGGGTGACAAAGCTGAGACCCTGTCTCAAAAAAAT AAAAAT TGTCTTAGAAAACATGTTAAAAGTTGAGGCT TGTAAACTAAGCTTGTTAGTTTCAATGTTTGGTGTTACTACTGACTTAGTATTGTTATTGGTTATATTGGTACTATT ATTGGTTATAGGTAATGAATATTATTACTGTAACTCTGCACTCTCCAGTCACAGTCCTCCATCCTTCCACTTTTCCC ATTCCCTCATCCTCTGCAAATGAATCTTGTGAGATTGTTCAGAGTTTCTACTAGAGGAGTACAGCTGTTCATTTCAC CTTAGAGAATTATTGCTTTCTGTTTGGACATATATGTTTTTTTGAAGTACATACGTGGAGTAATAAGAGAATGTATA GTGTCCCAACCATCAAAATAAAAATTAAATGGCTGGATTTTGCATATTTTCTCATCACATTCTATGCTCATTCTCAT GATTTCTGCTTTTCGCCTTCATTTTGGCTTCTAGTTCCTTATCTTCTCTTTGTTTTCTTAGACCATTAACCCTTTTG ATT TGATTTACCTTTCTCTGTAGGGTAGACCCATGGACATACCTTTT CAAT GATTCTCCTAAGGGTCTTGTACACCC CTTTGACATAACTGCTTTGCCCTCCAGTCCTCAGTAATTACCATTATCTCTTTTCTCCATACCAATATTACAAGTAC AGCCTAGAGTGGTCACACTGTCACGGCAATTTGCTATAGTCCATATGTGTGCTCTCCAACTTCAGCTGAACCCTCAG TCCTACATGGCAACATTTCCTGTTTAGCTACTTGACTCCCTGGCTCAGTTCCCACTATGGTGATTCAGAGCCTTTAT CCTCTATCTGAGCCTCCTGTTCCAGCTCTATCCCTCTTATTCATAGTAGATCACTTTGCATTGAACTCAAGTAGAAC TTT GAGTAATCT GAT GTGAGTTCACTAAGATTCTGTCTTCTCAATTGTTTCT CAT GTGTGTCTTAA CAAT ATT CTCC TCCATTTCTTGATCCCACTGCATTCTGCCTCCTCGAATCTTGTGTCTTCATATCTTATTTTATAAATGAGGAAGGTG ATAATTAAACTCCCCAGTCTTCACTCACAGCCT TTT TTCTCTTGGT CAAT AAGCAGCTCCTCT AAAAAT ATT GTCTT CTGCTAACCT TAG TAAGCCCCCATGAAGCTACCATTCAAAGTTTCTTTATTTT TACT CTTCAAAACTTCTCAAAAGT
AGATAGTCTTGGGAGACATCTGAGCAGCCAAAGACAATGATGGCTATGTGATAACCTATCTCCCAGGGTTTTTCTTT AAACAATATAAAACGACTTAAAAACTACCAAATTTTACACAAAACCACACTGTTGGCATCACAAAAGATCCTGAAAC TGCAAAATAACTGTAAATTTTTTAAAAATCAAGAAGTCTCAGCATGATCTATCATGCACCTACCCCACCTTCGCCCC ACCACAAGGCTTTGTGGTGAGGTAAAACAGACCAAGAAAACTTACGGAGAAGACAGAAGTGGGTGCCAGTGAAGGGC AGCTGTGAAGGTAATTTGGAATGACCAC CAT ACAT CACAT AT GCACTAAATCCAAAAATACTAAAA GAG TAACTGGG C AG AT T AGAG C T C T G G AAT AC AG G GAAGAG AT T T C AAAAT G C AC AC AAC T T AAAG GAT C T G GT G T GAT T T AAT G G GA TAAGTACAATTTAAAAGGAGAGGATGGTTACAGGGAGATCTTCCAAACTCATAGCTTCTGGAGAAGGAAAAAAAGAG GCAAAGATAGAAATGAAGAAGTGCTCCTTGGCAATTAGATGGTGAATGGGAAAAGGAAAAAGTGGGGAGAAATGTAA GGTTT T ACAAGAC AGAAAAGAAC T T T AAAAT CAGAAGAT T CT C AGC T C C AC C C T T AC CAC CAAGAC AAAT AAAT AAC CACACTGAGGTACCTGGATTTTGCTAGACCGACAGAAAAGTGTACCATTAAGCTAGGAATAATATTAAATGCCCAAT AT CAT AAAAAT AAAGAGGAAAAT AAAGT C CAT G GAAAAT GAT T GCAGAAAAT T AGGAAAT GAAACT T CACAT AT AGA CACTAAGGAGACCCTACCATTAGCAAAAAATAATCGAGAAGCAGAAGAAAACTGTTCAGTTGGCATTCTAAACAGAT TAAGTATACCCAAATATACGTTGAGAATATGAGAAACTACCTCAACTCAGATAGTCAAAAAATAAAAACAGAGGCCA G GAAAC AAAAAAC AG G GAT AAAT AAAAT AAAGT T TAT AG G AC T C AG GAAAAAAAT G GAAGAAAAAG AC AAAAT TAT C TCTGAAATGAAGAAT AAAT GACAAAAGTTAGGGAACT CAT ACAT CCAATTTCAAACTTACTACAAAGCTACAGTAAT TAAGACAGTGTGGTACTGGCATAAAGACAGACATATAGATCAATGAAATAGAACTGAGAACCCAGGAATAAATTCTT ATGTTGATGGTCAGTTGATTTTTTTTTTTAGAAAAGGATGCCAAGACAATTCAATAGGCAAAGAATAGATTTTTTAA ATAAATGGTGCTGGGACAATTGGATATTTATATGCAAAAGAATAAACTAGATACCTTCCTCACACCACACCCCAAAA TTAGCTTAAAATAGGTCATAAACCTAAATATAGGAACTAAAACTATAAAACTAAGAAAAAATAGGAATACATTTTTG TGATTTGGTCCTCCTCTAGATATGACATCGGAAGCACAAGTGACAAAAAAAGATTGATACATTGGACATAATCCAGA TTTAAAACTTCTT TACT GCAAAAAAAAACTGTATTTGTTTCCTAGTTCTGCTGTAACAAAATT CCA CAAACTGGGTG GTCTAAGACAACAGGAATTTATTGTCTCATAGTTTTGGAGGCCAGAAGTCCAAAATCACAGTGTTGGCAGGGCCATT CTCCCTCT GAAACT GTGGAAGAATCCTTCCTTGTTTCTCCCTAGTTTCTGGTGCTTTGCTGGCAACCATTGGCATTC CTTCACTTCTAGGTGCATCACTCCAACCCTTCATCATCACACGGCATTCTCCCTCTGTCTTCGTATCCCAGTTTCCC CTTTGTACAAGGACACCGTCATATTGGATTAGGGCCCATGCTAATCACCTTATTTTAACTTGATTACCTCTGTAAGG AACTTATTTTTAAATAAGATCACATTTTGAGGTACTGAGGGTTAGGACTTCAATATATTTTTGGAGGACACAACTCA ATCCATAACATATAACATCCAGGAAGTAAAAAGACAACCCACAGAAGCTGGGCTTGGTGACACGTGCCTGTAGTCCC AGCTACTTGGGAGGCTGAGGCCGAAGGATTGCTTGAGCCCAAGAAGTAGAGGCTATCGTGAGCTATGATCATGCCAC T G C AC T C C AG C T T T G G T GAC AGAAG G AGAC CCCAGCCCC CAAAAAAT AAAT AAAT AGAT GAC C C AC AGAAT AAGAG A AAATTTTTGTAAATCATATATTTTGATAAAGGTGTTATATCTAAAATATATAAAGAACTCTTATGGCTAAATAATAA AAAGACAAAT CAT CCAAGT GAAAAAT AGGCAAAGGAT CT GAAT GGACAT TT CT CT GAAGAAGAGAAAAT GGCCAAT A AGC ACAT GAAAAGAT G CT CAAT AT TAT TAG C CAT T AGAGAAAT GCAAGT CAAT AC CACAAT GAGAT AC CTCTTGTTA C C C AC T AGAAT G G C T G T AAT T T T AAAAAAT G GAAAAT AT G CAT AG G C AAAG G T G GAAAAAT T GAAAC C T T AC AT AC A TTGCTGGTGGGGTGTAAAATGGTATAACTGCTTTGAAAAACAGTCTGGCAGTGCTTTAAAACATTAAATATAGAATT GTAATATGACCCAGCATTCCACTGCTAGGTATATACCCAAGAGAAATGGAAACATGTATTCACACAAAAACTTGTAC ATGAATATTTGTAGCAGCATTATTCATAATAGCCCCAAATGGAAGCAACCAAAATGTCCATCAACTGATGAATGGGT TAACAAACT GATAAGT AGACATACAAAAT CAT AC AG T G GAAT AT TATTCAGC C AT AAAAAGT AAT GAAGTAAT GAT A CACAGT ACAACTT GAAT GAACCT TAAAAAC CTACTAAGT GAAAGAAGT CACAAGAGACT ACAT ATT GTAT GAT T CAG TTTATATGTAATGTTCAGAGTAGGCAAACCCATAGAGACAGAAAGTAGATTGGTGGTTGCCTAGGGTTTGGAGTGGG GGTGGGGAGTAGGGGTTGGGGAAAAATGGAGAATGCTAATAGGTATAGAGTTTCTTTTTGGGGT GAT GAAAAT GTCC TAAAATTTAT TAT GGTGGGTGGTTATACAACTCTAT GAAT ACT AAAAACCATTGAATTGTACACTTTAAATGGAGTC AAT GTGTGGAATGTGAATTATATCT CAAT AAAGT GGATTTTGAAAACCTAAAGAAGACTGAGGGGTGGAGGATCCAT TCTGTATTCGTATATAAGTTAAAAAAATGAGATCTAAAAGTTATATACCAAGCCCGGTGTGATGGCTCACACCTGTA ATCTCAACACTTTGGGAGGCCGAGGTGGGCAGATTCGTTGAGCCCAGGAGTTCGAGACCAGCCTGGGCAACATAGTC AAACCCCATCCCTACAAAAAATACAAAAATTAGCCAGGTGTGTGGGTGCATGCCTATAGTCCCAGCTCCTCAGGAGG GTGAGGTGGAAGGATCACTTGAGCGTGGGGAGATTGAGGCTGCAGTGAGCCTGTGTTTCCACTACTGCACTCTGAGC T GG GT GACAAACAAGAC CCAGTTT CAAAAC CAAAAC AAAAC CAAAACAAAAC C AAAGCAAAAGAGC AT G CAT AT AAT TATTACAGTAATCTAAAATTATGGTTTTTATTTGATAAGAGAGTGATGGGATGATAAGCCTATAATTGGCATATTAA GTTTCTAGATGGGGATCGTTTATTTTATTTTGTTTTTGTTAGGACAGAGTCTTGCTTTGTCACCCAGGCTGTAGTGC AGTGGTGCAATCATGGTTTACTGCAGCCTCAACCTCCTGGGGTCAAGCTATTTTCCCATCTCAGCCTCCCAAGGAGC TGGTTCTACAGACATGTAGCACCATGTGTGGCTTTTTTTTTTTTTTTTTTTTTTTTGTAGAGATGAGGTCTCACTGT ATTGCCCAGGCTGGTCTTGAACTTCTGAGCTCAAGCAGTTCTCCAACCTTGGCCTCCCAAAGTGTTGGGATTACAGG TGTGATCTTACCTGTCCCTGTTTATATATTTTAAATGGTTAATTCTCTGTGGTAGAAAAATGGGAAATTATTCTTTT TCTCTTCACACTTTCAGTACTTTTTTTCTGTTTCAAAAAGAGATAAATGAAGTTTT GAC CAT CTTTCAAAGCT CAAT TTCTTCTAGGAAACTCCCCCTGCCACTCT CAT CAGATGTAATCTTTCTCTCCTCTTGCTTT CAAT GGCATGCATTTG TGTCTC CAT GGCAGTTTTTCTTACCCTTCATTGAGT TAT CAGTTGGACACCTACT GTAT GCTAGATGCTGAGGACAC AGAGACACAAACTGTTTCAAGGAATAGTGTATTGTCTTTAGGGAAGAGAAAGGTGCAACTAGTTGTAATAGGGTAAG
ATAATTGTACAGAGGGCAGTCAGAGCACAGAGGGGCCATCTTATTCCTGCTAGCAGTCAGGGAAAGCTGCTTGCTTT TATTGTGTGTGTATTATCTTTTTTGGACTTCAAGCTCCTTGAAAGCATGAAACGAAAGTATAAATATGAGCAGAAAT TTTTTTTAGAGAGGGGTTCAGCTAGGTTTTATATTACTTGTAATTGCGAGTAATAAATGAAGTCAATATTTTCTTAC GGAGCACATTGTATTTTGATTTTTAAAAATAGATAAGTTCAGATAGTGAAAAATCAAAATGGCATAAAAAGCTATAC ACTGAAAAGTCTCACTGCTCATAGCCCCACCCCACTCCCACCTCCACCCCTCACATACCCTGTTGGAAGCCACTTTT AT T AT T T T C C T GT AT C T T T C CAGT AT T T T T T T T T GAT GGAGAT AC AAAC AAAT AGAAGT AAAGAT T CT T T T T GT T T T TCCAGGAAAATTCCCAGATGTTTCAAGGTAGACCTTGCAAGAATATGTATCCTAATGAATATTTTCCTCATGGAATA ACAAATGGAGCTAGTTGGTATAATGTGCCAGGTAAAGATTCTTTTATATCAAGGCCTTACAACTTGATGGCCTTTCT GCTTTCCTAGATGGTGCTTCTTGTTATTATCTTTTAGTTTTGAGGAATAATAATTATTTTTAAAACATGGTTTAAAT AGTTTAAAATATTTAAATAATCTTTTCAACTACTTTTATGTTTGATGTACGTTGAGGGTTGAGTAAGAATAATGCAC TAAAACTTGATTAAATGGTTACATTTTACTTCTGGCTATTAGAAAGAATCTTACATGTGCTAATAGAAGAGTGTAAG CTATATTTGAATTCTTTTTTTTCTTTACAACGTTACTTTCTTTTTTTTTTTTTCTTTTCTTTTTTTATTATACTTTA GGTTTTAGGGTACATGTGCACATTGTGCAGGTTAGTTACATATGTATACATGTGCCATGCTGGTGCGCTGCACCCAC TAACTCGTCATCTAGCATTAGGTATATCTCCCAATGCTATCCCTCCCCCCTCCCCCCACCCCACCACAGTCCCCAGA GTGTGATATTCCCTTTCCTGTGTCCATGTGATCTCATTGTTCAATTCCCACCTATGAGTGAGAATATGCGGTGTTTG GTTTTTTGTTCTTGCGATAGTT TACT GAGAAT GAT GATTTCCAATTT CAT CCATGTCCCTGCAAAGGACATGAACTC ATCATTTTTTATGGCTGCATAGTATTCCATGGTGTATATGTGCCACATTTTCTTAATCCAGTCTATCATTGTTGGAC ATTTGGGTTGGTTCCAAGTCTTTGCTATTGTGAATAATGCCGCAATAAACATACGTGTGCATGTGTCTTTATAGCAG CATGATTTATAGTCATTTGGGTATATACCCAGTAATGGGATGGCTGGGTCAAATGGTATTTCTAGTTCTAGATCCCT GAGGAATCGCCACACTGACTTCCACAATGGTTGAACTAGTTTACAGTCCCACCAACAATGTAAAAGTGTTCCTATTT CTCCACATCCTCTCCTGCACCTGTTGTTTCCTGACTTTTTAATGATTGCCATTCTAACTGGTGTGAGATGGTATCTC ATT GT GGTTTTGATTTGCATTTCTCT GAT GGCCAGT GAT GAT GAGCATTTTTT CAT GTGTTTTTTGGCTGCAT AAAT GTCTTCTTTTGAGAAGTGTCTGTTCATGTCCTTCGCCCACTTTTTGATGGGGTTGTTTGTTTTTTTCTTGTAAATTT GTTGGAGTTCATTGTAGATTCTGGATATTAGCCCTTTGTCAGATGAGTAGGTTGCGAAAATTTTCTCCCATTTTGTA GGTTGCCTGTTCACTCTGATGGTAGTTTCTTTTGCTGTGCAGAAGCTCTTTAGTTTAATTAGATCCCATTTGTCAAT TTTGTCTTTTGTTGCCATTGCTTTTGGTGTTTTGGACATGAAGTCCTTGCCCATGCCTATGTCCTGAATGGTCATGC CTAGGTTTTCTTCTAGGGTTTTTATGGTTTTAGGTCTAACGTTTAAATCTTTAATCCATCTTGAATTGATTTTTGTA TAAGGTATAAGGAAGGGATCCAGTTTCAGCTTTCTACATATGGCTAGCCAGTTTTCCCAGCACCATTTATTAAATAG GGAATCCTTTCCCCATTTCTTGTTTTTCTCAGGTTTGTCAAAGATCAGACAGTTGTAGGTATGTGGTGTTATTTCTG AGGGCTCTGTTCTGTTCCATTGATCTATATCTCTGTTTTGGTACCAGTACCATGCTGTTTTGGTTACTGTAGCCTTG TAGTAAAGTTTGAAGTCAGGTAGTGTGATGCCTCCAGCTTTGTTCTTTTGGCTTAGGATTGACTTGGCGATGCGGGC TCTTTTTTGGTTCCATATGAACTTTAAAGTAGTTTTTTCCAATTCTGTGAAGAAAGTCATTGGTAGCTTGATGGGGA TGGCATTGAATCTGTAAATTACCTTGGGCAGTATGGCCATTTTCACAATATTGATTCTTCCTACCCATGAGCATGGA ATGTTCTTCCATTTGTTTGTATCCTCTTTTATTTCCTTGAGCAGTGGTTTGTAGTTCTCCTTGAAGAGGTCCTTCAC ATCCCTTGTAAGTTGGATTCCTAGGTATTTTATTCTCTTTGAAGCAATTGTGAATGGGAGTTCACTCATGATTTGGC TCTCTGTTTGTCTGTTGTTGGTGTATAAGAATGCTTGTGATTTTTGTACATTGATTTTGTATCCTGAGACTTTGCTG AAGTTGCTTATCAGCTTAAGGAGATTTTGGGCTGAGACAATGGGGTTTTCTAGATATACAATCATGTCATCTGCAAA CAGGGACAATTTGACTTCCTCTTTTCCTAATTGAATACCCTTTATTTCCTTCTCCTGCCTAATTGCCCTGGCCAGAA CTTCCAACAC TAT GTTGAATAGGAGTGGT GAGA GAGGGCATCCCTGTCTTGTGCCAGTTTTCAAAGGGAATGCTTCC AGT TTT T GCC CAT TCAGTATGATATTGGCTGTGGGTTTGTCATAGATAGCTCT TAT TAT TTTGAAATACGTCC CATC AATACCTAATTTATTGAGAGTTTTTAGCATGAAGAGTTGTTGAATTTTGTCAAAGGCTTTTTCTGCATCTATTGAGA TAATCATGTGGTTTTTGTCTTTGGTTCTGTTTATATGCTGGATTACATTTATTGATTTGCGTATATTGAACCAGCCT TGCATCCCAGGGATGAAGCCCACTTGATCATGGTGGATAAGCTTTTTGATGTGCTGCTGGATTCAGTTTGCCAGTAT TTTATTGAGGATTTTTGCATCAATGTTCATCAAGGATATTGGTCTAAAATTCTCTTTTTTTGTTGTGTCTCTGCCCG GCTTTGGTATCAGAATGATGCTGGCCTCATAAAATGAGTTAGGGAGGATTCCCTCTTTTTCTATTGATTGGAATAGT TTCAGAAGGAATGGTACCAGTTCCTCCTTGTACCTCTGGTAGAATTCGGCTGTAAATCCATCTGGTCCTGGACTCTT TTTGGTTGGTAAGCTATTGATTATTGCCACAATTTCAGCTCCTGTTATTGGTCTATTAAGAGATTCAACTTCTTCCA CATGAAGGTATAGGTGTTTTGCTTGTATCGCAAGCATCAAAGTGCCCTCACATGAACACGTTAGCTTATCAATGCTG ATAAGAGATAAATTTGGCTTTTTACTAGGAATGTTGCTTACCTTTTGGGATCTGAGCTGTTTAGAACTTCTTCAGAG TGACTAATTTTAATTTTTATCTTTTAGGAGGAATGCAGGACTGGAACTATTTACAAACAAATTGCTTTGAAGTGACT ATTGAACTAGGTTGTGTGAAATATCCACTTGAGAAAGAGCTGCCAAACTTTTGGGAACAGAATCGAAGATCACTAAT CCAGTTTATGAAACAGGTGACTATTCAGGAGTGAAGTATGAAATTTCTCCCGAGGGTGAAAAGATTTTTGTGCGTAC ACGTGGTTTTTGTGCAGTGATTTTGGAATACACTCTTTAAAATTTCAATTCATTTTAGTATATTTAACCAGTTTGTA TATTCCAAGGTGTTTAATATATTTATCTAGAAAATTCGTGAATGGAAATGAGTTTATTTGTATATGTGAGGAAAAGT TGAAGAAAATGATTGAACAAGGTAGTGATATTTTGCTCTTAAGGGTTAGTGGTCACTTTTTACCCGTGTTTAAAAAA GACCAGCATAAATTGGATACACACCTAATCATTGGCCAAGTGATAGAAAGATGGGCTGTGGAGTGAATGTTTTTATT CTTTTTCTTGTTGCCAGTGATTTACTGTGAAATGATTAATTTCCTTTTGTTTGGATATGTGTGGTGGCCAGTAGGTA
AAGATTATAAGAAATATACTAATGTTAATTAGGATAATCAGACTAATACTTTCTGCCAGTACATTTCTGCATTTTTC TTTTTCCTGAGTGGTGTGACATAAAGTCTGTGAGTTTTTTTTACTATATTTAAAGACATTTTATTCTGAAAAATTTT AAAT AT ACAGAAAAGT TAT GACT AGC CAAT AAT TAT T C CT AT AC CT AGAT T T AACAT T T T T AACAT T T T GT TAT AT T TGCTTTATTT T AAT GT AAAT T T T AT T T ACACAC T CACAT ACAC AT ACAT AT T T T T GACT GAAC TAT T GT AAT AT AAA TTGCAGGTATGGTATTTTACCCCTAAATATTTCAGCATCTATCTCCTAAAAATAAGGAGCTACTACATACTATCTCT AAGTGCTACTGTCCTACCCAAGAAAATTAACAGTAATTCCCTAATATTACCTAATACTTAGTTTATATTTAAATGTC CATATTTGTCCCCAAAATGTAATTTTACAGCTGTATTTTATGACCAGGATCTAGTCAAGTTTCATGCATTGCATTGG ATTATTATATCTCTTTACTATTCTTTGAACTATAAGAGTCCTTCTGTTTTTAAAAAATGCCATTTCCTTTTGAAAAG GCCAGGTCTGTTTTCTTGTAGAATGTCATATATTCTGAAATCATCAGAATGTGCTAAAGTTTAAACAGTGTTATTTA TATTCTTCATGATAAAGAGAAACAAAGCCTATGAAAAATTAGCCTTCAGAAATATTTTAATGAACATTTACATTGAA CTT TACT CTGAACTCCTAAATTCCTGTTTGTTC CAT TTATTTGTGTTGA CAAT CTGTTATTTTGAAT AT GACCTCCC AAGATAATTAGATGATGATGTTTATTATTGAAATCTGGTGTCCTTGTACTTAATCCAGGTTCATCAGGGCGTCAGAG GATTTGTTCTAGATGCCACAGATGGCAGGGGTATATTAAATGCCACCATTAGTGTTGCTGAGATTAATCACCCAGTG ACTACTTACAAAACTGGAGATTACTGGCGTCTCTTGGTTCCAGGAACTTATAAAATCACAGCATCTGCTCGAGGGTG AGT GACT GAATGCTTTGAAATAGAGTGCTCGGAGGACAAA CAT GGCTCCATTCTGGAGGAT TAT TACTAGAACGTTC TAGAGAGCCTGTTAAGCAACTTTGAATGGATTGCTGCGAAGTTTAGGGCTGGT TAT ACCTTT GACT CTCCTTTAGTG GCA GAT AGAGTACACGAAAAAAAAAAAAAGGAAAGATTCCTGGGGT AAAT ATT CTAGTT ATT GCTT AGT T GACT CAT TGTGACATGCTTCCAACCTGGACATTAAACAAGACATTTTAGAATACACATCAAATCCTGATCCTCATGGCCTTAGG TTTTTTATTTAAAAGGAGAGTTAGGCTGAAGTAATTCTACAGTCAGTTCCAATTCATAGTAGAATTTATTGGAAATG T GT T CAAGAT AGT TTTTCCTT TAAAG GAGT AAC AGAAGT GT T AGT T GAT GAAG CAC AAAGAAC AT AGAAAAT G CAT A GAAAGAACATTTCTCGTTCTTTT AAT TTGCTAAATACAGGTAAGCCCAAAGCAATGCAGTCTATTT AACAT GCTAGT AACTTTTTTTTTTTTTTTTTTTTTTTTTTGAGACAGAGCTTCTCTCTATCGCCCAGGCTGGAGTGCATGGCACTATC TCAGCTGCCTGCAACTTCTGCCTCCTGGGTTCAAGGGAT GCTT GTGCCTCAGCCTCCCAAGTAGTT GAT ATTACTGG TGTGTGCCACCACACCCGACTAATTTTTGTATTTTTAGTAGAGACAGGGTTTCTCTTGTAGTTTTCTTTTTTATCTT TTGATAAGTATAGATGTTACCACAGGATATTTTCCCTTCCTCCTGCTAGCTAACTTATTTCTGAAGAGTTAGTTTGA ATACCCATTGAAGTAAGGGTAAAATATGAAAGCATTTTAGTCGTTGCCTTTGTTTTTTAATGGTTTTCAGATATCTA GTGGTCCGTAAATGAATGGGTTAGAACCTCACACTAATGAGTAATGAGGTCTTTTCTGTGATTCTTCCATTTTTGGC AGAGTGTTAACTAAAGCAGCAGGGGATCCGAGAAACAGGGTGAGAAGGATGTGTTTGGCAGAGAATGTCATAACCTA GCACCTCTGCAACTTTCCCACCCGTATACCCTGATAGAAGACAAGTGAATTTAAGTTCCAGGAGACATGGGAGTATC CACTACAAGCACTAAATTTCTTTTGAAGCCATATATTTAATCTTAAAAACCTAGGCCAGGTGCGGTGGCTCAGGTCT GTAATCCCAGCACTTTGGGAGGCAGAGGCAGGTGGATCACTTGAGGTCAGAAGTTTGAGACCAGCCTGGCCAACATG GTGAAATCCTGTCTCTACTAAAAATACAAAAATTATCCAGGCATGGTGGTGGGCACCTGCAATCCCAGCTACTCAGG AGGCTGAGGCAGGAGAATCACTTGAACCCAGGAGGCGGAGGTTGCAGTGAACTGAGATGGTGCCACTGCACTCCAGC C T G G GT GAC AGAG C AAGAC T C CAT C T T AAAAAAAAT CAT G AAAAAAAAT C CAT T CAT AT T T T G T AAT AT AC C CAT G T CTATTCTTTATTAATGTATTAAAAAT AAT AAAT TACT CAACGTTAAAATTTTGTCTCTTTAGGAAACTGCACCA CAT TTATCCCAAGGATTCTTACGTTCTCTGCACACCTGCCTTCCCAGGGTTTGGGTACTCCAGCTGCAGGAGTGTGGTTC T GAT CTGTCTCCTTTATCATAGGTATAATCCAGTTACCAAGAATGT GACT GTCAAGAGTGAAGGCGCTATTCAGGTC AAC T T CAC AC T T G T T C GAT C C T C AAC AGAT T C AAAC AAT G AAT C AAAGAAAG G AAAAG GGGCTAGCAGCAG CAC C AA TGATGCCAGTGATCCAACTACTAAAGAGTTTGAAACTTTAATTAAAGACCTTTCAGCGGAGAATGGTTTGGAAAGCC T CAT GT TACGCT C CT C CT CAAAT CT GGCT CT GGCT CTTTAT CGATACCATT CCTACAAA GACT TAT CAGAGTTTCTG AGAGGACTTGTAATGAACTATCCACATATTACAAATCTTACCAAGTAAGTGTCACTTTCTATTGTCTTTTTTTTTTT T T C AAGAC AG T AAAAT CAGC AT T AGC CAT GT T GAAT AGT T GT AAT T T AT AT AGAAAT AT T T G G GAAC AG AAT GT ACA GAGAGTTGGAGTTTACTTATTTTGCAACATGTATTT GCTT GGA GAT CTT TTTTTTTTAATTATGCTTTAAGTTTTAG GGTACATGTGCACAACGTGCAGGTTTGTTACATATGTATACATGTGCCATGTTGGTGTGCTGCACCCATTAACTCGT CATTTACATTAGGTATATCTCCTAATGCTATCCCTCCCCCCTACCCCTACCCCACAACAGACCCCAGGGTGTGATGT TCCCCTTCCTGTGTCCAAGTGTTCTCATTGTTCAGTTCCCACCTATGAGTGAGAACATGCAGTGTTTGGTTTTTTGT CCTTGCGATAGTTTGCTGAGAATGATGGTTTCCAGCTTCATCCATGTCCCTACAAAGGACATGAACTCATAATTTTT TATGGTTGCATAGTATTCCATGGTGTATATGTGCCACATTTTCTTAATCCAGTCTATCATTGATGGACATTTGGGTT GGTTCCAAGTCTTTGCTATTGTGAATAGTGCCACAATAAACATACGTGTGCATGTGTCTTTATAGCACCATGATTTA TAGTCCTTTGGGTATATACCCAGTAATGGGAT GGCT GGGT CAAAT GGTATTTCCAGTTCTAGATCCCTGAGGAATTG CAACAC T GAC T T C CAC AAT G GT T GAACT AGT T TACAGT C C CAC CAACAAT GT AAAAGT GT T C C TAT T T C T C CACAT C CTCTCCAGCACCTGTTGTTTCCTGACTTTTTAATGATTGCTATTCTAACTGATGTGAGATGGTATCTCACTGTGGTT TTGATTTGCATTCTCT GAT GGCCAGT GAT GAT GAGCATTTTTTTGTGTGT CTT TT GGCT GCATAAATGT CTT CTTTT GAGAAGTGTCTGTTCATATCCTTCACCCACTTTTTGATGGGGTTGTTTGTATTTTTCTTGTAAATTTGTTTAAGTTC TTTGTAGATTCTGGATATTAGCCCTTTGTCAGATGAGTAGATTGCAAAAATTTTCTTCCATTCTGTAGGTTGCCTGT TCACTCTGATGGTAGTTTCTTTTGCTGTGCAGAAGCTCTTTAGTTTAATGAGATCCCATTTGTCAATTTTGGCTTTT GTTGCCATTGCTATTGGTGTTTTAGACATAAAGTT CTT GCCCATGCCTATGTCCT GAAT GGTATTGCCTAGGTTTTC
T T C T AG GGT T T T T AT G GT T T T AG GT C T AAC AT GT AAGT CT T T AAT C CAT C GT GAAT T AAT T T T T GT AT AAGGT GT AA GGAAGGGGTGCTTGGAGATCTTTTTAATAGTGAATCTTGGGAACTTTAAGGAGAATTACTTGTTAGATTAGAAGTAG ATAGCCTGTGATTCCAGGAAATGTAAAATTGCTCTTCCTGATAATTATTTTGACTGATTGTAAAACCCTTTCCATCA CTCAGCTACAGATCTATTAATATGTTCTGACTAAATATTGTTTAGTTTGTTTTATTTTGCTTGGGGCAAGGGAGGAA TTTAGAACTGTAAGCTCACCTGTTAGGGATTATGTGTAACTTTTTTTTTTTAATTAGAAAATGAAGCCTAGTTGCTG TCCTAATTCTAGTCTCTATTCACAGTCTCTTACTTGAAGCTTTAGGGACATGTGTGATCGTGGGTCCCAGGGAAAAC ATACAGTCCTGGCTTATGTAAAACTGATAGCTGAGATAGCCTTTACCTTCACTTTGTGGTGGTTGCTCTGCCTCACT TGCACTGCTTCATTTTAGATTTTGTTACAGTTACTCCAAAATTATTTCTATGATTTGTTGATTCCTATGGTTTGCCT GTTGTAGTTAAGGTCTTTACATTTTGCCTTGAGCATATAGTTCTCTGCTGCAAACCTCATGAACATTATTTAGCCTT TTACCTTTTTACTTTGTCTTTCTTCATGGGTCCTTTTAATTTCATCAGAAAACTTAGTTCCTCAAATTCTGATTTAT AATATTCATAGATGCAGAGTGATACTTAACTTTCTTTGGCAATACCAATTACTTTTCTTCCTTAACTGATTCTTGGA TATTTCTGGCTATTTCAGGTTTTAATATCATACAATCTTAGTGCCATAGAGTTCATATACTTTTTTATTTTAAAATA AAACCAGGCTGGGCACAGTGGCTCACACCTGTAATCCCAGCACTTTGGGAGGCCCAGGTGGGAGGATCGCTTGAGGC CAGGAGCTCCAGTTTACGTTGAACTATGATCACACCACTGTACTCCAGCCTAGGTGACAGTAAGATCCTGTCTCTAA AACAAACAAAAATTGGGCATTATTTTGCTGTCTGTTTACTTAGTTGATTTAATTTTTTTAACCTCCTAATTCAAGAC ACACTTTTTAGTTAAATATTTTCTCAAAATATGCCACACATGCCAGTGGTGGGGCACAAGAGAGTTTTAGGTGGTAC ACCAACAGGCACTAAGTACCATTGAATAGCGTCATGAGAAAGCAATAAGGTTTCTGTTAATTTTTCTCAATTTAATA TGATTATTTTTTTCTGTCCAATTCTTGTAAATCTCCCTTTATGCTAAAGAAAGCAGACCTTAAGCTCAAAGCTCTTG GGCAAGCAGAGTATTTGACCATTGTTTAATTTTCATTGAATTAGTATTTTTTTCCCCACTCAAATTACTTTTACTGT GAAACTGGTGTTGCATTTATAGTAAAATATAACGTGACTTATTTACAAATATTAATTTTGATTAAAAAGGGAGGGCC AGGGTGGTGGCGCATGCTGTAATCCCAGCACTTTGGGAGGCTGAGGTGGGAGGATTGCTTGAGGCCAGAAGCGCTTG AAACCAGCCTCAGCAATATAGCAAGACCCTGTCTCTACAAAATTTTTTTTAAAAAATTAGCTAGGCATGGTGGTGCA AACCTGTAGTACTACTCCCTTAGGAGACTGAGGCCAGAGGATCACTTGAGTCCTGGAATTCAAGGCTGCAGTGAGCT ATGATCAAGCCATTGCATTCCAGCTTGGGTGACATAGTGAGACCCTGTCTCTTAAAAATATAATGACAATAATATAA ATAAATAAATAAATAAATAAGGGAGGTTATCTATGTTCATACATACTTAAATATTTCTGTAATAAAAATTTAATTTG TGAGTTAGTACAAAATATATTAACTAAATAGTAGTGTAGATTAAAGAAGATAGGGCAAATATTGTGATGATTGTATG AGAAT TACT GAAT T T G GAAG GAT G GT AAAC T AAAT CAT AAT C T T T C C T AAAT C T T T C C T AAAT C T GAT AAT AT CAT C TATTTGATAAATGTTCTTTGGTCAAGGAAAACTAATTTTCTTAAGGTTTTCTGATGCAGTGATTCACTCTTTAAAGA CTTTTTAAAACTTTTTGTGTTTTGTTTTCATTTCTATTTTTAAAGTCCATAAGTCAACATTAACATAGTGCTTGATT ACTTGCTGATTTTTTTGTTTCTGGTTTTTGAAAGTTTGGGACAGAGCACTGAATATCGTCACATTTGGTCCCTTGAA ATCTCCAATAAGCCCAATGTATCTGAGCCTGAAGAACCAAAGATTCGTTTTGTTGCTGGTATCCATGGAAATGCGCC AGTTGGAACTGAACTGCTTTTGGCTCTGGCAGAATTTCTCTGCCTGAACTACAAAAAGAACCCAGCTGTTACCCAAG TAAGAGAATAGCCGAGGTTGACATGCTTTAAAAGGAAAAAGCTCAACATTAATATCAGGGCACCATTTTTAAAATTT TTAATTTCTTGCTAGATTTTACAGAGTCACTTGTAGTACATTATGAACTTTACTCTTTAAAAATGAACCATCTTTGT AGTATATACCCTTTGTTAATAGTGTCTTTCTATTATATTTCCTTGTATGTCTTAAGAGGCATGCAGGAAAGAATACT AAAAATTTACATTCAATTTCCTACTCCGTCATTTAATAGCCACACTACCCTTCCAGTCTTTATTCATAGTTCTTTAC TTTTTTTTTTTTTTTTTGAGACAGAGTCCTGCTCTATCACCCAGGCTTGAGTGCAATGGCATGATCTCGGCTCACTG CAACCTCCACCTCCTGGATTCAAGCAATTATCCCGCCTCAGCTTCCTGAGTAGCTGGGACTACAGATGCCCACCACC ATGCCCGGCTAATTTTTTTATATTTTTGGTAGAGACGGGGTTTTGCCATGTTGGCCACGCTGGTCTCGAACTCCTGA CCTCAGGTAATCCACCTCCTTTGGCCTCCCAAAAATGCTGTGATTACAGGCGTGAGCCATGGCGCCTGGCCAGTTTT TTACATTCTGACTTGAGGGACTTCCATTCTGAATTACTTATAACTAGGACACTATATTGAAAAATCTAGTGAAAGCC TAGTTTTTCAGTATAGTTTTCTTTTTCATGTAATACATAAAAAATTATTGGACATCTTGGCTGCTAAGAGATTTTAG AAAAAGCAATATGGTGTTATCTACTTTAGCAATCTGTGACTACAGAAAAGAACTTTGACTGGGGTGTGATGGCTCAT GCCTGTAATCACAACACTTCAGGGGGCTGAAGAGAGAGGACCACTTGAGGCCAGGCGTTTGAGACCAGCCAGAGCAG CAGAGTGATACCTTGTCTCTACAAAAATTTAAAAAATTAGCTGGGCCTTGTGGTGCACACCTGTAATCCTAGCTACT TGGGACGCTAAAGTGGGAGGATCACTTGAGCCTAGTAGGTCAAGACTGCAGTGAGCTGTGATTGCACCACTGCATTC CAGCCTGGGTAACAGGCTGGAATCTCAAAAACAAACAAAACAAAACAAAACTTTGACCCAGAGGCTTGGGCACGTAT GATTAAGCATCCTAGTTGTACGTTGCCTCAGAGAAGGGAAGGAAAGAGTGAAACACTCACTAATTGGCACTGTCCAT AGTGGACTTAGGAAAGGGAAAAAGAACTGATATTTATTGAATACATATTTTGTGCTGGGTAATATACCAGGTATTTT ATATACGTCATTATATCCTCAACCCTGTGATAATGATTATCTCCATTTTATAAATGTAGAAACTGAGGCAGTCCCAC AGCTAGAAACTGGTAAAACTAGAATTTAAACCCACGTATGTCTACTTTGAAGCCCAGTGCAGTTCTGCCTCCCCAAA ACAAATTGGGAAAGAATTGATGTCATGATTAGGATCAGAAGGGACCTGTGTAATTAGGTGACATGAACTTAAATCAG ACTGACCAAAAATTATGTTATCTTCCTTTGTGGGACAGGTGCTCTGTGTTGCGAACTTACTCAACTCTGCCATTAGA GCACAAAACAGT CAT AT GTAAAT GAATAGT CAT GCT TAT GT GT CAGTAAAACT TTATAT AC AAT AG C AG GC AC CAAC TGGATTTGGCCTGCTGTTAGTGGTTTCTCACCCCTGTTCTAAGTCACTGGAAGGTCTCTGGGAACATTAGCTGTACA GTAGTTTTTTGCTTATTTTTAATATATTTGAAGCAAAAGGAAATTTAATTTTTTATTTTTTCATTCATCTTAGTTCT GTCTTTTAAATTAACTATGTGTAGAATAAACAGACTTTCTGTTTGAGGCATGAGAGGGTACCCATTGATTCTACATG
CAGCTAGTAACTCGTTAATTTCTGTTTGTGCTTCAGTTGGTTGACAGGACTAGGATTGTGATTGTCCCTTCTCTAAA TCCAGATGGGCGAGAGAGAGCTCAAGAGAAAGACTGTACTTCAAAAATAGGACAAACAAATGCTCGTGGCAAAGATT TGGATACAGACTTCACAAGTAAGACTAATTTTTAGGCTACTAAAGTACTTAGGAGATAATTTTCTTTCTGCTAGAAG ATTGATTGATCCTATTTTGTCAGTGAATAATGGAGTACTTTCTTGTCTGGGTTATGAATGTGAGGTATAAATGAATT C AG AC AT C T G GAAAAG T T T T T GAAT T T T T C T C C AT T AT T AT T T G C AAAAAT AAT T T AAG T T T T T C AT AG AAAT T C AA TCCATTATATTGGGATGTTTGTTTCTTCACTAGTTGTGTAGAGATGTGTATTCTCTCTCCCCTCCTATGTATGTGTG TATGTGTATACACGCACAACACACACGTATGTATATTGATTTGGAGAGCCCTATTTCTTATTTACTTAAATGGGACC TTTTTCATTGAATATGTATCATTGACATGGTTCAATTTCAGTACTTATAGATCTCTAACATTCTTTTTCTTTTTTTT TTTTTTGAGACAGAGTCTCACTCTGTTGCCCAGGCTGGAGTGCAATGGTACAATCTTGGCTCGCCTCCGCCTCTTGG GTTAAGCAAT TAT CCTGCCTCAGCCTCCT GAAT AGCTGGGATTACAGGGACCCGCCAC CAT GCCTGGCCAATTTTTG TATTTTTAGTAGAGATGGGGTTTTATCATGTTGACCAGGCTGGTCTTGAACTCCCGACCTCGGGTGATCCGCCTGCC CAGCCTCCCAAAGTGCTGGGATTATAGGCATTAGCCACTGTGCCAGGCCCAACATTATTTTCTAATGGCATCTAGTA TGC CAT GAAT CTATGACAGT ACT CTTAATT TAT T CAAC CATTTTCT ACT GATAGAT ATT TACT TTATTTCTGATTTT TTGCTTTTGTAAACAGTGCTGCAGAGAACACCCGGCTTTCTGAAACTCTAATTTTCTTCCAAAGATAATGTATTTGT C CAT T T T C AC AC T G C T GAT AAAG G CAT AC C C AAGAC T G GAAAG AAAAG G AGT T T T AAT T T GAC T T AC AG C T C T AC AT GGCTGGGGAGGTTTCACAATCATGCTGCAGGATGAAAGCCACTTCTTACATGGTGGCAGCAAGAGAGTGTGAGAAGG AAG C AAAAG C AGAT C T GAT AAAC C CAT C AG AT C T CAT AAG AC T TAT T C AC TAT C AC AAG AAT AAT AC G G GAAAGAC C CGCCCC CAT GATTCAGTTACCTCCCACCGGCTCCCTCCCACAA CAAGT GGGAATTCTGGGAGATAAAATTCGAGTTG AGATTTGAGTGGGGACACAGCCAAACCATATCATTCTGCCACTGGCCCCTCCCAAATCTCATGTCCTCACATTTCAA AAC C AAT CAT G C C T T C C CAAC AG T C C C C C AAAG T C T TAT T T C AG CAT T AAC T C AAAAGT C C AC AGT C CAAC AT C T C A TCTAAGACAGGTCCCTTCTGCCTCTGAGGCTGTAAAATCAAAAGCAAGTTAGTTACTTCCTAGATAAAATAGGGGTA CAGGCATTGAGTAAATACAGCCATTCCAAATGGGAGAAATTGGCCAAAATGGAGGGGCTACAGGCCCCATGCAAGTT CAGAATCCAGCAGGGCAGTCAAATCTTAAAAGTTCAAAAATTTTCTCCTTTGACTCCATGTCTTGCATCTAGGTCAT GCTGATGCAAGAAGTGGGTTCCCATGGTCTTGGGCAGCTCCACCCCTGTGGCTTTACAGGGTACAGGCTGTCTCCCA GCTGCTTTCACCAGCTGGCATTGAGTGTCTGTGGCTTTTCCAGGGGCATGGTGCAAGCTGTCGTGGATCTACCATTC TGGAGTCTGGAGGATGGTGGCCCTCTTCTCACAGCTCCACTAGGCAGTACCTCAGTAGGGACTCTGTGTGGGGGCTC TGATCCCACATTTCCCTTCTGCACTGCCCTAGCAGAGGTTCTCCATGAGGGGCCTGCCCCTGCAGCAAACTTTTGCC TGGACATCCAAGCTTTTCCATACATCTTCTGAAATCTAGGTGGAGGTTCCCAAACATCAATTCTTGACTTTTTTGCA CCCGCGGGCTCAATGCCACATGGAAGCTGCCAGGTCTCGGGGCTTCCACCCATTGAAGCAACAGCCCGAGCTGTACC TTGGCCCCTTTTAGTCACAGCCGGCACAGCTGGGACACAGGGCAC CAAGT CCCTAGATTGCCCCCAGCACGGGGACC CTGGGCCCAGCCCACAAAACCAGTTTTTCCTCCTGGGCCTCCAGGCCTTTGATGGGAGGGGCTGCCGAGAAGGTCTC TGACATGGCCTGGAGACATTTTCCCCATGGTCTTGGGGATTAACATTAGGCTCCTGGCTACTTATGCAAATTTCTGC AGCTGGGTT GAAT TTCTCCCCAGAAAATGGGTTTTTCTTTTCT ATT GCAT AGT CAGGCTGCAAATTTTCCAAACTTT TAT GCTTTGCTTCCCTTATAAAACT GAAT GCCTTTAACAGCACCCATGTCACCTCTT GAAT GCTTTGCTGCTTAGAA ATTTCTTCCGCCAGATACCCTAAATCATCTCTCTCAGATTCAAAGTTCCAAAAATCTCTAGGGCAGGGGCAAAATGC TGCCAGTCTCTTTGCTAAAACATAACAAGAAT CAT CTTTGCTCCAGTTCCCAACAAGTTCATTATCTCCGTCT GAGA C C AC C T C AG C C T G GAC CTTATTGGT CAT AT C AC TAT C AG CAT T T T T GT C AAAG C CAT T CAAC AAGT C T C T AG G AAG T TCTATGCTTTCCCACATTTTCCCATCTTCTTCTGAGCCCTACAAACTATTCCAACCTCTGCCTGTTACCCAGCTCCA AAGCTGCTTCCACATTTTTGGGTATCTTTTCAGCAACACCTGGCTCTATTGGTACCAATTTACTGTATTAGTCTGTT TTCAAACTGCTAATAAAGACATACTCGAGACTGGGAAGAAAAGGAGATTTAATTGGACTTACAGTCCCACATGGCTG G G GAG G T T T C AC AAT CAT AG C G GAG GAT GAAAG G C AC T T C T T AC AT GGCAGTGG C AAGAGAG AAT GAGAAG G AAG T G AAAGTGGAATCCCCTGGTAAACC CAT CAGATCTTGTGAGACTT ATT CAC TAT CACAAGAAT AGCAT GGCAAAGACCC GCCCCCATGATTCAGTCACCTCCCACTGGGTACCTCCCACAACACGTGGGAATTCTGGGAGATACAATTCAAGTTGA GATTTGGGTGGGGGTACAGCCAAACCATATCAGATAGGTTATTTATATATTCCCTAAATTATTTTGTAAAGCTGGAT ATGCTTATTCTTTTTGTTTTTTTAATAAGATTTTTCTTTAACTTGCTTAGAGTCATTAGTATATACACATCCTGAAG TACATAATAAAACTCAGACTAACCTACCTTCTGTAATGTATCTTTCTTGCTTGAGATAGTGGCTTATGGAGAAATAA AAT AT T TAT C T AC AAAT T T T T AAGT T AGCAT T T T GAT T GT AT T CAAGT AGAGAAT AT T T T CT T AGAT AC T CAT ACT A AGATTTAAAATTT GAT ATTT TAT ATT TGACTTTTCTCTTTTTT AGAT AAT GCCTCCCAACCTGAGACCAAAGC CATC ATTGAAAATTTGATTCAAAAACAGGACTTTAGTCTTTCTGTTGCCTTAGATGGTGGTTCCATGCTGGTCACATATCC TTATGACAAGCCAGTACAGACAGGTATGTAGAATGTCATTTTATATATATACTGTTTAAGCTTAGGTAGCAAATCCC AAT T AAGT AAT GTCCCTTC C ACAT T T T TAT AAAT AGT GAGCAT T T GAGC ACACT C TAT GAGC AAAT T AC CAAC CAAA GAA GAT TGTACAT ACAT CAGCAGTTCAGTT ACT TGGAGAGCTCAGATCT AAT GGTAGCCCAGCTTT ATT GAGTCTAG CTATCTTGTTCCATCTGTTCCATTTAGTCCACAAGCATTTACTGAGCAATTATTATGTCTGTAAATATTGAGAATAT AAAAGT GAGTATGACACGGTTTCTGCCT CAT GAAACTCCT AT GTAACCAAATACTAACAGTACAGATTTGTCCTTCC CTGTTTTAAACTTTGACTAAGTGGTTGCACCTTATGGTGAGTTAAATAGTTGGTTGTTTAATCTCTGTGGGGTCGCA GTATATGGAGATAGTTTGGGTGGTTTTCAGTGCATCTTACAAAGAATATTGTTATTAGGCTACAGTTGTCCCAAAAT GCAGTAAGACATACCAAAACAAGTTTGTATTTTAGTGTGATAGTCTGTTGTGAAAACTCACCATTAGAACCTTTTCA
TGTAAAGAGGATGTTTGTCTTCTATTATCTTGGGCTAAATAAAACCTTTTAGGGTTAATTATAGTAACAGTATACTT AAG T G G AGT G C AAAAT T TAT TAG GAG AAAAAGAT GAC T T G TAT C CAT GAAG GT T AAC T T G G G G GAAAAAAAT CAT T T GGATTTCTCCACTGACTTCTAAATTTTTCTTTCTATAGTGGAAAATAAAGAGACTCTGAAGCATTTGGCATCTCTTT AT G CAAATAAT CAT C CAT C CAT G CAC AT GGGT CAG CCCAGTTGCC CAAAT AAAT C AGGT AGAT GT T T T C CAT AC C T T TTGTTATTGCTGTTGTTGTTGTTGCTTTTGTGGGGAAAGGAGTTTCACCTTAAGGTCTTCCTGATTCCACATCTCTT AAT G C T T T TAT AG AT G AGAAT AT T C C AG GAG GAGT AAT G C GT G GAG C AG AAT G G CAT AG T CAC CTGGGCAG CAT GAA GGTATGCTTTCTAGAACATGGTTAGAATGGAGTTATGGCCAGGCACAATGCCGTATGTGTAAACCCAGTGCTTTGGG AGGCCAAGGCAGGTGGATTTTCTGAGTTTGGGAGTTCAAGACCAGCCTGGCCAACATGGTGAAACCCCATTTCTACT AAAAATATAAAAATTAGCT GGGT GTGGTGGCAGACGCCCGTAATCCCAGCTACTTGGGAGGCT GAGT CAGGAGAATC GCTTGAACCTGGGAGGCGGAGGTTGCAGT GAGT CGAGCCGAGATCGCAC TACT GCACTCCAGCCTGGGCAACAGAGC AAGATT CCAT CT CAAAAAAAAAAAAAAAAAAAAAAAGAAT GGAGTT TAT GAAT AT CAGC CATT GAGTACAAGAATAG GTGACTTTTCTGGTATTGTAAAGTGAATGGCATCTGCCTCATGGCTTTCTTGACATGTTTAGCCCTTACATTAACCC GAC AGT ACT T T T T T GG C CAT T T T AAC CAT T T T T AAT T GT ACAGT T T AGT GACAT T AAT T AAAT GTTGTGCCC CAAAA ACCATATCTATTTCCAAAACTTCATTTGCCCCAGACAGAAACTCTGTACCCACTAAGCAATAACTCCCTATTCCCCT CTCCCACGGCTCCTGGAACCCCTGTCTACTTTCTGCCTCTAT GAAT TTGCCTATTCTAGGTATTT CAT GTAAGTAGA ATCATACAATATTTACCCTTTCACTTAGCAAAATGTTTTTAGGATTCATCCATTTTAGAGCATGTATCATTACTTCA TT CATT TTTATGGCTGAATAATATTCTATT TAT GTTTATACCACATTTTATTTGTTAATT CAT GTGTCAATGGGCAC TTGGATTTCTACTTTTTGGCTGTTGCAAATGATGTTGCCATGAAGATTGGTATTGCAGGTATCTGTTTGAACCCTGT GCTGCCATGAACATTGGTATTACAAGTATCTGTCACTTCTTTGGGGTAGATACCTAGGAGTGGAATTGCCTGGTTAT ATTGTAAGTCTATGTTTCACCTTTTGAGGAACCACCAAACTGTTTTCCACAGCAGCTGCACCATTTTACATTCCTTA CCAGCAATGTTTGAGGTTTCCAATTTTCCCATATCCTCTCCTCACCAACACTTATTTGCTGGTTTTTTTTTTTTTAA ACA GAGT CTCACTTTGTTGCTCAGGCTGGAGTGCAGTGGCTATGGCTCCTTCGCAGCTT ATT GCAGCCTGAACCTCC CAGGCCCAAGCAATCCCCCCACCTCAGCCTCCTGAGTAGCCAAGACCACCCGCATGCACCACCATGCTGAACCAATT ATTTTTAATTCTTTGTAGAGACAGGGTCTCCCTTTGTTGCCTAGGCTGGAGTTCTTTATATATTAATATAAAAGGCT GAGCGTGGTGGTGGCTCATGCCTATAATCCCAGCACTTTGGGAGGCCGAGGCGGGAGGATCACCTGAGGTCAGGAGT TTGAGACCAGCCTGGCCAACATGGTGAAACCCATCTCTACTAAAAATACAAAAAAATTAGCTGGGCGTGGTGGCAGG TGCCTGTAATCCCAGCTACTTGGGAGGCTGAGGCAGGAGAATCGCTTGAACTTGGGAAGCAGAGGTTTCAGTGAGCC GAGATTACACCATTGCACTCCAGCCTGGGCAACAAACACGAAACTCTGTCTCAAAAAAACGAATAAACAAACAAAAC CCATTCTGGATGCTGTGTATTCTAGATAGGAAATTCTTATCAGATACATGATTTGCAAGTATTTTCTCCTATTCTTT AGGTTACCTTTTAAATTTCTTGATTATGTCCTTTGATACATGAGTTTTTAATTGTAACGAAATCCAGTGTACCTGTT TTTGTTGTTGTTGCTTTTGCAGTTGTTATCCTATCTAAGGATCTGTTGCTAAATCCAAGGTCATGAAGATGCCCCGC CCCACC CCAT GTTTTCTTTTCTTTTTTTTGGAGGGCAAATGTGGGAT GAGT GGGAGGGGCGGGGTACATGGCCCCCC TGCCCACCTCCCTGCTCCCTGCCGCCTATCCTTGTGTTTTCTTTGAAGAGTTTTATGGGCTTGGCACGGTGGCTCAG GCCTGTAATCTGAGCACTTTGGGAGGCCAAGGT GGGT GGAT CATT T GAT GT CAGGAGTT CAA GAC CAGC CTGGCCAA CATGTTGAAACCCCATATACTAAAAATACAAACATTAGCCAGGCTGTAGTGCTGCGCACCTGTAATCCCAGCTACCC GGGAGGCTGTGGCAGGAGAATTGCTTGAGCCTGGGATGGGAAGATTGCGAAGAGCCGAGATCTTGCCACTTCACTTG AGT CTGGGCAACAGAGTGAGACCCTGTCTCAAAAAAAAAAAAAAGAAAAAAAAGAGTTTTAT GGAT CAAGCTGCCAT ATT TCAGT CATT GAT GCAGTTTATTTTTTATGGTAT GAAG TAGGAGTTCAACTTCGTTTGTTTGTATGTAGAAATTC AGT TGTGTCAGCACTATTTGTTGAAAGACTATTCTTTCCCCACT GAAT GGTTTTGGTATCCTTCCCAAAAAT GAGT T TACCATAGATGTGTGGATTTATTTCTGGACTCTCAATTCTATTCCATTGGTCTATATGTTTGCCTTTATGCCAGTAT CACTTTTTTTTTTTTTTTTTTGGTTGTTGTTTTCCTTTTTTTTAATTTATTTTTTATTTATTTTTATTTATTATTTA TTATTTATTATTTATTTTTTTATTATACTTTAAGTTTTAGGGTACATGTGCACAACGTGCAGGTTAGTTACATATGT ATACATGTGCCATGTTGGTGTGCTGCACTCATCAACTCATCATTTAACATTAGGTATATCTCCTAATGCTATCCCTC CCCCGACCCCACAACAGGCCCTGGTGTGTGATGTTCCCCTTCCTGTGTCCATGTGTTCTCATTGTTCAGTCCCCACC TAT GAGT GAGAACATGCGGTGTTTGGTTTTTTGTCCTTGCGATAGTTTGCTGAGAAT GAT GGTTTCCAGCTT CAT CA GTATCACTGTTTT GAT TAATGTAGGTTTGTTGTAGTAAGTTGTAAAATTGGAACGTGT GAGT CTTTCAGCTTT ATT G TTCTTAAAGATTGTTTTGGCTATTCAGGGCCCCTTGT CATT CCAT TTGAATTTGAGCATTGGCTTTTCTTTTTCTGA GAAAAGGGGTGTTGGAATTTTGTTAGATGTTGTGTTAAAACCGTATATCACTTTGTGTAGTATT GACAT CTCTACAA TAT T GTCCTCCTATCCATTATTTAGGTCTTCTTTAATTTCTGACAGCATTTTAATT CAT TGTC CAT CCACTT CAAAT TAGGCAGGAGTTGTTAGCAGAT GGGT GGTTGGCT GAT CAT TTT ATT CACATGTGCAACCAGAAAATTGACTTTATTC CCATTT CT GC CT GTCTTCCCTCCTTTATTT ACT AAGAACTTAC TACT GGGAGGCATTTTGCTAT AT TAGT GAT AAAT AAAAT AT ACT GCCTGGCCTATTGTAC GAAT TAACTGTGTTACATAATTAATTGATTT GAT CTGGAAGAGAGCAACTC TCAGCAGGCCGTGAGAAATTAAAGACACAAAAAATTATATCTAGTCAGAGATACAGCCTTTTCTCTTTAGAGGAGAT GGCATTTGGGCTGCAGAGAT AGT GAAGCA GAA CATT CCA GAAAGAGGAAGCAGTACATAGCAGAAGTCT AAT TTAGT TAGAATATGGGGTGTAATGCAGAGCCACAGAAGTTTAAGAATGGAAAGAGTGCTTGTTGCTTTGAATGCTTTGTTTT AAT AAGAATGGGAAACTGAGGTTTTGAGCAGGGGTAACAAT ATT GACAT GAT CTGAGCT AT GCTATGGGAAAACTAA TCTCTAAATAATCAGTTAAGATAGTTTGGAGGAAGGAGAGACTGGTACAGGGAGACCGGTTTGGAATTATCTAGACA
AAAAGTAATGAGGGCCTAGGTTATGCTAGTGGCAACGAGAGTAGAGTCAAAGGATGACATTTTAGATATTCTATAGA GGTAGAAT T GACAAGAT T CAGCAAAT TAT G GAT GAT AGT GAGAT AGAGAAGT C AAAGAC AAGAGGT T T CAT T G CT AT GTAAGAAAATGAGTTAATACCATTAACTGAGTTAGGAAAAAGAAGAGATTTTGGGAAAAGGTGGTAAGTTGGGTTTT TGAACCTGTCTGCACAGGCAGTTCCTGTACCTTGATAATATCAATGCCGTATTGCAGGGAGTGGATATTGTCACCAG CAGGGGTCCAGGTTGAGGGTATGAGAGAGCTGATGATCATTATCTCCATAGCTTCAAGTACAACTGTGACATTTCTG GGTTTTGAGGGAAGCTGACCCTCTTCTGCATGTACTCATTCATTCATTTCTTCAGCAAACCCGAATAAACCCCATCT ACCTGGCACTACCAGGCACTGTGGGAAGCACTGAAGATAGGAAAATGAATTCGACATAGCCCCTGTCCTCAAGGGCT CTCATAGAGTTTGTGATTGATTATAATACAGAGAGGTTTATTTATATTTGTCATAAAATGCTTTACAGCTTCTTTGT TTACGTCAGCTACGTTTTCTTTTCATTTACTCCATTTGTTGTATTACAAAGCCTTATTCTTTCTTTTACTTATTAAT TTTCCCACATTTGATTTTGAACTTTATATTCTAGGATTATAGTGTCACCTATGGCCATTGTCCGGAAATCACAGTAT ACACAAGCTGCTGTTACTTTCCTAGTGCTGCACGACTCCCTTCCTTGTGGGCAGACAATAAGAGATCTCTTCTTAGT ATGTTAGTGGAGGTGAGTCTTTTCCTTTTAACTAGAGGCAAACTCCCAGGAATATGTTCAGTGAAAACCTTTATCAA AAC AT TGTCTTTTAC C AAAAAT GT AT AT TAT T T AT T T TAAAT T T AT AT AGAAAGAAGAGT T T T G GT CAT T T TAT GT T TCCTAAATAGATGCCATTTATTCACATCTCTGGAATCTGCTATAATTTAGGTAATTTGACCTTGCAAGATGCTGCAA GTTCTTTAATTTGAATATCACAGAGTTACTGGAGTCAGTATACCATGGTGGGAAGGAAATCACTCAAGGCATTGGAA GATTGGGTTCTAATGCTTCAGGCTATGACAATATCAGAGTTCTCTTTTATTTCCAGCTCTGTGAGCCTGAATATTGA ATGAATAGCAAACAAGGTCAGAGTCAGTGGGGAGGGAAGAGAAGAAGCAGGCCATACAGAGTTTGATACTTGTTTGT GTGTGGTTTTTGTTTTGTTTGTTTGTTTGGTTGGTTTTGGTCATGCCTCAAGCTAGTGCCTCTACCATGTCTGGCAT TATGACAACATAAATTTATATTTTAAACATTTTTTCAGGTTCACAAGGGAGTTCATGGATTTGTTAAAGATAAGACT GGAAAGCCAATCTCTAAAGCAGTCATTGTACTTAATGAAGGAATAAAGGTACAAACAAAAGAGGGAGGTTATTTCCA TGTACTCTTAGCGCCAGGTGTCCATAACATTATTGCCATCGCTGATGGGTACCAGCAACAACATTCACAGGTAAGAA ACTCAAATTGAGTAGCATCATGTAAATTTTTATTCTTAATAATACTTCTGTTTTATTTTGAAACTCTTGTTAGAAAT CTTGAAGGGAATAAAGAAATTAAAAGTGTGACTGCCTCTTGATTATGATATCTTGTTATCCTTGTACCCCTTGCTAA CAAAAGGGAAAATTTTCTAGCATTTTGTATACTTTAGATGGCAATGCATCATCTCCTTAGTTTGCTATATGGGGCCT AATAATATAGCAGGGATACAGTACACCTTAAGCAGAATTTGTCATGATTCTTACACTTTCTCCTTCTAGGTCTTTGT GCATCATGATGCAGCTAGTTCTGTGGTGATAGTCTTTGACACAGATAACCGGATATTTGGTTTGCCAAGGGAGCTTG TGGTAACTGTATCAGGTAAAGACATTTTGATTTTTAGTAGTAAAAGTTAAAAACAATCTTGACATTTCAATATGAGA GTGGGTCACCTCTCTCATATTGATCCTGAAACTAATCACTTGTCTTCTAATGTCTCCTTATCTAAATGTGATTTTCC TGGACCTCTGTTCTCGCATATGTTCTCCTGGCTTGCCTTTCTCTGCCGAGACCAGCTCCATCAAGGAGACCCTAACC CAGCGGTGCTAGAGAAATTAAAGACACACACAGAAATATAGAGGTGTCAAGTGGGAAATCAGGGGTCTCATAGCCTT CAGCGCTGAGAACCTCCAACAGAGATTTACCCAGGTGTTTATTAACAGCAAGCCAGTCATTAGCATTGTTTCTATAG ATATTATATTAGCTGAAAGTATCCTTTATGGGAAACGAAGGGATGGGCCAAAATAAAGGGATGGGTTGGGCTAGTTA TCTGCAGCAGGAGCATGTCCTTAAGGCACAGATCACTCATGCTATTGTTTGTGGTTTAAGAACGCCTTTAAGCAGTT TTCCGCCCTGGGCGGGCCAGGTGTTCCTTGCCTTCATTCCGGTAAACCCACAACCTTCTAGTGTGGATGTCATGGCC ATCATGAACATGTCACAGTGCTGCAGAGATTTTGTTTATGGCCAGTTTTGGGGCCAGTTTATGGCCATATTTTGGGG GGCCTGTTCCCAACACTTCTCTTTCTTCAGGTCTATACTCAAACGTCACCTCCACGGAGAGGCTTTCCCTGATCACC CTAGCTACCCCTGTCACTATCTCTATCCTGCTTTTTTTCATTATAGCACTTTCATAACATTATATATTTGTTCATGT ATTTGTCTATATTCAATCTCTCCCACTAAAACATAAGTTCTGAGAGAGGGAGGAATCTACCTGTATCCTCCAAGCCT AGAACGATGGTTAGCACGCAGTGGGTCTTAATGAAGAGTTGTTGAATGAATGAATGATTTGCTTTATATTAAAGTCA TAAGTTCCTAGGACTTTGACTTATATTAGTTCCGCTTTTTCAAGATCCAGGAAAGAGGAAATGTTTGTTTACGCTGG AACTAATTCATGTCATTGCTTGGATTACTGAAAGGTTAGGAAGGAAAAAAATGTGGTAAAATGAATACTCTGCTATA CTTTATCTATTTGATACAAACAGCCTTAGTTTATTTCTAAGTGCAGCTTGGATGAGGCAGAATAACCAAGTTTTATC ACAGGGTTCCTGTGGGAGAGACCCTTAGTTGAGAGAAAGTAGTATATTGGAAACATGGCCCAATCATAGATCCTGTG TGGTGTGTTTAAAGGGTTCTTAGCTTGTCTCAGATAAAGTGTAAATAACTTAGTGGTTAAAAACACAGGAGGTAGAA TTCAGC CAT GGTT TAAAT CTGGCTCTGTCACCCATTAGCT ATT GTGAACTTAGTTTTTATTACTTTTAGTTTCTAAC ACTTTTATTTTGATAACCTTGTACAAATTTAAAATAAACAAGGAGATTTGGATGAAATGAAAGTTTACTGGAAAGGA AAAGTATAATAGAAGTCATAAAAAGCAGGTTGCAGAACAGTAGACACAGTATAATTTCATTATGGTAAAAGAAAAAA AATACATGTACGTGCATATGCATAAAGAAAGATCTGGAGCCAGGCATGGTGGCTCACGCCTGTAATACCAGCACTTT GGGAGGCCGAGGCAGGTGGATCACTTGAAGTCAGGAGTTCTAGACTAGCCTGGGCAACATGGCAAAACCCTATCTCT AGCAAAAATAAAAAAAAATTAGCCAGGTGTGGTGGCATGCACCTGTGGTACTACTTGGGAGGCTGAGGTGGGAGGAT TACACACACACACAAAAGATCTTATAACTAAAAATGATCTCTGGGTAATGGAAATGTAAATATAGGAGGTTTTTTGG TTTTGGCTTTTGTTAGTTTGCTTATCTGTATTTTTAAACTTTGTATATTGTACATATACTGCTTCTATAATAATAAA GGTTGTTCTTAAGTGCATGAAATAGAAATAAAATAAATGAGGGGTATATTATTAAAAGTTCCATTGTAAGCGGCTGC TTATTAATATCCTTAAAGTATAATATCACCCACATGATGGTTTTTGTTTGTCAGGTGCTACTATGTCGGCATTGATC CTAACAGCTTGCATTATTTGGTGCATCTGCTCAATCAAGTCTAATAGACACAAGGATGGCTTTCATCGGCTCAGGCA GCATCATGATGAGTATGAAGATGAAATTCGCATGATGTCTACCGGCTCCAAGAAGTCCCTCCTAAGCCATGAGTTCC AGGAT GAAAC AGACAC T GAAGAG GAAACAT TAT AT T CT AGCAAACAT T GAAAAACACAT T T T G CAT AT C T C C CAGCA
TAAGTACCAAGCAAAATTACAGTTCCTCTTGGGAGAACACTGCATTAAGAAGAGAGACTCTCTTGCTTCTTCAAAGA GCTTTGGGAAATTAAATTGCTAAATTTGTATTCTCTGTGAATTTCACTGGCAGTTTTGAACTTCCCTTCCTTAAAGT ACTCTAAACCTTTAAAAAAAAATCTGATTTATGCAGCAGAGATGGGACAGCCACTTTTTCTTTTTAATTTAAGATGA GCTATTTGGAGCTTATGTAATAATGGCATAAAGCCAACTAGAGGATGTTGTATTTTGCACATCAGATGTTTACTAGT GGCTTTAGTATTTTTCTTTGTTTTAAATGGCCAAAAGAATCCAGAAACATTAAGGCAGGGACAGCAGTCAGAATCGA CATAAAGCTTTAAAAACTCAAGGTTTTTTCAACCTACTGAGGAGTACTTTTCTCTAGTTGTTAAATAGCTGGAGTTT TTCTTATTCAGGTTTAATGGAGGTTGAATTGATTTTTAAACACATATAACAGTAGGAAATGAATAAATGGGCTTCTG CAT TTGGCTTTCTACCTGTTCCAAGGCTAGATCGGAACTGGTAGACTACGCTGTAAGCAGGATTT CACT ACCTCTCT TAAGGTTTAGCAAACTTCTAAATAGCCCATTTTAAGGGAGAACTTACTAACTTTATTGTGAAAGGTCTAAATGCCCA CTT GAAT GAAGCT GAGAGAGAGAT CT AGCAAAAGCT AAAACT CAT GTT GT CTAT CT TT GAACT T GGTAAAAAC CCAC AGGTGCTGCTGCTTATATCTGTGAAGCACTAGCTTATTCTAGGAATGCCTGATTCTTTAATATTGCCTAAATCGGAA CCTTTTT CTAT GTTGCACACATGGTTTTCAGATGACCCAGCCATCTACAAGATCT GAAT TCTACTGAAAAT AT CTAG AAATGTGGAAGAGACCTACTTGCACATTCTTAACCTGTATTTGAACACAAAATATCTATACTTCATGCTCCAGCCCA AGCCTATACCCTGTAATAGCATACTATTATT GAAAT CGCTTGACCGGT CTT GTTCACATAGGCCTCTGGGAGT GAT T TGGTTCTTTGCCCTAATGTTTCATTTGACGGTCTCTTTTTGATCAACCAATTTTTCTAAAAGTTCAGTCGAAAGCTT TTAAGT AT AGCTTCCTCCCTTGAAAAAAAATGTAAACTATGACTGCTGAGT GAT AAAA CACT GTGGTGT GAAAGT GT CATCTTCACTGCCAATCAGGCAAAGACCGGAAAGATTTGCATTTTATTATGTCTGTCTTATCATGCAATGGAAATGA TGCTTTTTGTAAGTATGCATCTTACCAATGATGTAACGGTTTAATACCTTTGAATGTTTTAATAACCAAGTTGCTGC T GAACT TATACTAAATCAGGGGACCAAAAAACTTGCTCTTAT CTT CTCAAATTGTATT CTAT AT CCATT AAT GTATC AGTTATCCCAAAGCCTTCAGGTGGAGGGGTTTACCACCTTCCTAGGTCGTTCAACCAGGTTTTGTGAGGAATGCATT CAAAGTGGCTTTATAAAAGAAGATTTTCTTTAGCAAGAATAATGAGGTCATGTCATTTGTTAATAAGTATCTGTGAT AAATCCGTGGTTCAAGGTTAAGC CAT TCTGGTATTCTGGTATTAGCAACTGTAAATTCTGCCACCT CAT ACAT GGAA CAGAGCTTGTGGGATGCTAATAGTTAGTGAAG TAT ACAT GAT TTAATTTCTAAT AAT CTTTATGTTTTCTTTAAGGA TGGTGGTGTATTGCTCTTTTTCAGCTTTATTTTTAAGAGTACAGTCAGGAAACCAACAAGGGGCCTAAGAGTGGCTG CCCCTGCTTGGGACATTACAGCAAGTGAAACAAAGTTAATGTGACAAGCTTTGCTTTGTTATCATTGGTCTTCACTA GAGGATACCTTTTACATGTACTTCTCTCTTGGATCAAATATGTCTTTAACTGTACATCTCAGTGGCTGGAGGCCATG C CT T T T AAGC AT GT GT AAAAT T T T T AAAGAAAT GAACAT ACAC AT AGT T AT T T T AG T AAT AT T T C C T GAAAGAAAAA CCAAATTCTGCTATAAGT CTT GAT CTTCAAT GAACT TTTAAAT AAT GCATTTAGCTGGAAAACAAGACTTTCCCAGC TTGTATTACCTAGAAGCGTGAATGTATAGGATACCTGACTACTAAGACTATATTCTCAGCCCTGCCCTGTCTTTTAT TTGCGGGTCTAATCTAATATTAGAATATATTAACCGCTTAAGGCATTGAAGCCATATGGGATGGGGAATGCATTTCT TCAGTGTTTCTCCGAGAGACTTTCCATTTCCTTGGAGTTATGGCGGCAAGTAAGTATCATAGTATTAAGAAATTTGC CTAAATCTGAGTTGTGCCTTTCTTTACTCACAAGGCATGGGCTTTGTCCTGGTGATCAGTTTGTAAGCCTTCTTCCT TCCCAGCTCCTTAATAAAAGCAAAGT GAT TGAGTAGGTAAT GTT CAAAGTGTCTGCCTGTGT ACAT GTACTTGTATT GATTATGTAGTTCAGTAAGATGTGCCCAAGTCATTTCAGAAAGAAAGACCCTTCAGTTTTGATGCATTTTGCTGAAC ACTTGGGTAGTGAGTGGGATCCTATCCAGTTGAGGAATGCTTGCAATGCTCATTGAAGGGATTTGCTTTGGGACTTT GTCATCTTCCAGAAAGGAAACATATTGTATATTTGGCCCAGTGTGATTGATTGCTTTATCTTTGGTAACTTTTACTT GAAT GGGATTTGCTGAATTAATGACT ATT GAAT TTAAAACTAATTATGAGTTGACAAATAAATAAAAGGTAGT GTT T AT GTCTGAGCTTATTGT GTT TGAGCTAACACCAGGT TACT CAGTAACCATGACCTGCTCCT CCATT TCCATTT ATT C TCAACATTAAATAGTTTTAT CTT GTT GTT GCCA GAAAT GCACTTGTGCCAGGT ATT GTCCCTGCTGTATGAAAAGCT TCTTGGCAATGAATTCTGTAATAGTGCCCTACATTATGGTTTTCTGGTGGAATTGTTTTAACAGTGACAACCCAGGA T T T C CAAT AT AT TTTTGTTT TAT T GT TAT T AC CAAAAAT T C CACT AT GAT T GAT GT T CAGT GAT T T T CTAT AG CAAC TTTTTTGGTAACTCTTTGGGTTTCTGATTTGTTTTAGCTAAAATTTTGGGGATATGATTTGGGTCTTTGATTAATGT CAGCT GAACT TGGATTTCTAGTT CAT GAAGAAATCTCTCCCAATACCCATTTATC CTAT TTTTAGCAAT AAT TCGTT AAT GAT T C CACT T GAT T T T C AGAAT AT TGTCCTGGTT GAT T T T GAT T T GACAG CAT ACAT TAT GAAAT T T GAAAGT A GGTTACCATTTTGAGGCAGTTGGATATAAATTATGTAAATATGTATGATTATGATTTTTATAAATGGCATAACATGA GTGTACTAACTACCTTCTATGCTGGCCATGCTACAGATTTTCTGGAGGTATGACAATAGTATTTTTTTATGCTCAGA TTAAAAAT CAGCT TTTCACCTCTCCAGTTTTTCCAAGT GAT ACT CCCAGTTCTAGAGCAATCTACAGCTGTTT AT GT GAGGTGCCCAACACCCATT CAT CTCAAGTGCTT CAGT CTT TGGTTTATTT CAT GCACTGTGCCTTCAAAAT GAAAT T TTTAAAAGGGACTTTAAATGAAGTTGAATAGTAGTTTTTAAAAGT CAAT TTGTAATTTATGT GAAAT CTAACTGTAA TGAGGTCCTTTCTGTTTTTTATATGTAAACAGATCTACTAATCCTGTATAAAAGTTATTTTACGATGTTTGTCTTTC T T T GT GT T T T GT C T CAT AAT CT T T T T T CAGAT G CAAT AT G C C G GAAAAAGT T AT AG GT C CAGT T T GAAAAT T AT T T A GTT TTTCTGC CTAT GCTAGTGGAAAAATAGTACCAGGATCAGAATACAGGGTATCACCTATGGAAT GTT TCTGTATT TAT GAATTGACTCAAAAGAAAGCTTT GTT TCT GAAAT CGCAT TAT GTAG TAGCCA CAGT TTTCTGTTTGTAGCTCAG CT AGAT T GT TAT AT AT GTT CAAT CAT T T CACAAT AACAAC ACAAAACT G GT CAT T GAAAGGT T T T T AT GT AC G CAT T TTAAACTTGTTCGTTAAAAATTTGGTCCTTTTTCCAGGTGAGGCCCAGTTAGAATAATGTGTCCCGGCACTTTTAGG CACAGCAAGGAT GAAT T CAAT AT CCCCTTTTCACTTAGCAACAATGTGTTACTTCTACCCTAATAGGAATTGGGAAA GCAAAGTTGT AT GAGAAACAGACTCTGCT GAT AAAG TACT CAT AGT CCA GACCAGA GAAAT AT AAATGGAAATAGGT
TATATTTCAATAGTGATTGGTTCATCTAAAAGTCTCTGCTGTAAAGGAAATAAAGCATAGAGGTTTGAGCATGGACT TTGGAGTTGGACCAATCTGTGACTGATTCTTCGTTCTGCTACTTGCTTCCAATGTGACCTTGTACAAGTTTCTTGAC ATTCTCTGAGCCTCAGTTTCTCTACTGGTTGAATAATCCTTGATAGGATTGCAGTGGAAAATTAAATGAAATAATGT TAGCAAAGGTCCCAACATAATATTTGACTTGGAATTGAATGCCCATGGTAACCAGCATCATTTTCCTTCATGTGATG TCTTCTTATGCCTTTGAAAGAAAGTTACTTTATCAAATGTATAAATAAAGATCTGTTTATAGGTGATCTTTTTAATT TAGAAGAAATTCTGAGACACAAATAAAAAAAGAAATTTTTTA (SEQ ID NO: 1)
[00333] >gil22004648lgblU65090.3IHSU65090 Homo sapiens carboxypeptidase D mRNA, complete cds
CGGCGCTGCTGGAAGATGGCGAGCGGCCGGGACGAGCGGCCGCATTGGCGGCTAGGGCGGCTCCTGTTGCTCATGTG CCTGCTGCTGCTGGGGAGCTCGGCCCGGGCGGCTCACATCAAGAAGGCGGAGGCGACTACCACAACTACGAGCGCGG GCGCCGAGGCGGCCGAGGGCCAGTTCGACCGCTACTACCACGAAGAGGAGTTGGAGTCGGCGCTGAGGGAGGCGGCG GCCGCGGGCCTCCCCGGCCTGGCCCGCCTCTTTAGCATCGGCCGCTCGGTGGAAGGCCGGCCGCTGTGGGTGCTTCG CCTCACCGCCGGCCTGGGGTCGCTAATCCCTGAGGGCGACGCGGGGCCTGACGCTGCCGGGCCCGACGCTGCGGGGC CGCTGCTGCCCGGCCGGCCCCAGGTGAAGCTGGTGGGCAACATGCATGGCGACGAGACCGTGTCGCGCCAGGTGTTG ATCTACTTGGCCCGCGAGCTGGCGGCCGGCTACCGCCGCGGGGACCCGCGCCTGGTCCGCCTGCTCAACACCACCGA CGTGTACCTGCTGCCCAGCCTCAACCCCGATGGCTTCGAGCGTGCCCGCGAGGGCGACTGTGGCTTCGGCGACGGCG GCCCGTCCGGGGCCAGCGGCCGCGACAATAGTCGCGGCCGCGACCTCAACCGAAGCTTTCCCGACCAGTTTAGCACC GGCGAACCCCCCGCCCTGGACGAGGTGCCCGAGGTGCGCGCCCTCATCGAGTGGATCCGCAGGAACAAGTTTGTGCT TTCTGGAAATCTGCATGGTGGCTCAGTGGTAGCAAGCTATCCTTTTGATGATTCTCCAGAACATAAGGCCACTGGAA TCTATAGCAAAACCTCAGATGATGAAGTATTTAAATACTTGGCAAAAGCTTATGCTTCAAACCACCCCATAATGAAA ACTGGTGAGCCTCATTGTCCAGGAGATGAAGACGAGACTTTCAAAGATGGAATCACAAACGGCGCACATTGGTATGA TGTGGAAGGTGGTATGCAAGATTACAATTATGTGTGGGCCAACTGTTTTGAGATCACATTAGAACTGTCTTGTTGCA AGTACCCACCTGCTTCACAGCTTCGACAGGAATGGGAGAACAATCGTGAGTCTTTGATCACATTGATTGAAAAGGTT CACATTGGAGTGAAAGGATTTGTTAAAGATTCCATAACAGGATCTGGGTTAGAGAATGCAACCATCTCAGTGGCTGG TATTAATCATAATATCACAACAGGCAGATTTGGTGATTTCTACCGATTACTTGTTCCTGGAACTTACAACCTTACAG TAGTTTTAACTGGGTATATGCCATTGACTGTTACTAATGTAGTGGTGAAAGAAGGACCAGCCACAGAGGTGGATTTT TCTCTTAGGCCAACTGTAACTTCAGTAATCCCTGACACGACAGAGGCTGTATCAACTGCTAGCACAGTTGCTATACC TAATATTCTTTCTGGAACATCATCCTCCTACCAGCCAATTCAGCCAAAGGACTTTCACCACCACCATTTCCCTGATA TGGAAATCTTCTTGAGAAGGTTTGCCAATGAATATCCTAACATTACCCGGCTTTATTCCTTGGGAAAATCAGTAGAG TCAAGAGAACTTTATGTGATGGAGATATCTGATAATCCGGGTGTCCATGAACCAGGTGAACCAGAATTTAAGTACAT TGGAAATATGCATGGAAATGAAGTGGTTGGAAGAGAACTGCTGTTGAACCTCATAGAATACCTTTGTAAGAACTTTG GAACAGACCCTGAAGTCACAGATTTGGTTCATAACACTAGAATTCACCTTATGCCATCCATGAATCCTGATGGGTAT GAAAAGTCCCAGGAAGGAGATTCAATAAGTGTAATTGGCAGAAACAACAGCAACAACTTTGACCTGAACCGAAATTT CCCAGACCAGTTTGTTCAGATCACAGATCCTACGCAACCAGAAACTATTGCTGTAATGAGCTGGATGAAGTCCTATC CATTTGTACTTTCAGCAAACCTGCATGGAGGTTCTTTGGTGGTTAACTACCCTTTTGATGATGATGAACAAGGACTT GCCACATATAGTAAATCACCAGATGATGCTGTGTTCCAACAAATAGCACTTTCTTATTCCAAGGAAAATTCCCAGAT GTTTCAAGGTAGACCTTGCAAGAATATGTATCCTAATGAATATTTTCCTCATGGAATAACAAATGGAGCTAGTTGGT ATAATGTGCCAGGAGGAATGCAGGACTGGAACTATTTACAAACAAATTGCTTTGAAGTGACTATTGAACTAGGTTGT GTGAAATATCCACTTGAGAAAGAGCTGCCAAACTTTTGGGAACAGAATCGAAGATCACTAATCCAGTTTATGAAACA GGTTCATCAGGGCGTCAGAGGATTTGTTCTAGATGCCACAGATGGCAGGGGTATATTAAATGCCACCATTAGTGTTG CTGAGATTAATCACCCAGTGACTACTTACAAAACTGGAGATTACTGGCGTCTCTTGGTTCCAGGAACTTATAAAATC ACAGCATCTGCTCGAGGGTATAATCCAGTTACCAAGAATGTGACTGTCAAGAGTGAAGGCGCTATTCAGGTCAACTT CACACTTGTTCGATCCTCAACAGATTCAAACAATGAATCAAAGAAAGGAAAAGGGGCTAGCAGCAGCACCAATGATG CCAGTGATCCAACTACTAAAGAGTTTGAAACTTTAATTAAAGACCTTTCAGCGGAGAATGGTTTGGAAAGCCTCATG TTACGCTCCTCCTCAAATCTGGCTCTGGCTCTTTATCGATACCATTCCTACAAAGACTTATCAGAGTTTCTGAGAGG ACTTGTAATGAACTATCCACATATTACAAATCTTACCAATTTGGGACAGAGCACTGAATATCGTCACATTTGGTCCC TTGAAATCTCCAATAAGCCCAATGTATCTGAGCCTGAAGAACCAAAGATTCGTTTTGTTGCTGGTATCCATGGAAAT GCGCCAGTTGGAACTGAACTGCTTTTGGCTCTGGCAGAATTTCTCTGCCTGAACTACAAAAAGAACCCAGCTGTTAC CCAATTGGTTGACAGGACTAGGATTGTGATTGTCCCTTCTCTAAATCCAGATGGGCGAGAGAGAGCTCAAGAGAAAG ACTGTACTTCAAAAATAGGACAAACAAATGCTCGTGGCAAAGATTTGGATACAGACTTCACAAATAATGCCTCCCAA CCTGAGACCAAAGCCATCATTGAAAATTTGATTCAAAAACAGAACTTTAGTCTTTCTGTTGCCTTAGATGGTGGTTC CATGCTGGTCACATATCCATATGACAAGCCAGTACAGACAGTGGAAAATAAAGAGACTCTGAAGCATTTGGCATCTC TTTATGCAAATAATCATCCATCCATGCACATGGGTCAGCCCAGTTGCCCAAATAAATCAGATGAGAATATTCCAGGA
GGAGTAATGCGTGGAGCAGAATGGCATAGTCACCTGGGCAGCATGAAGGATTATAGTGTCACCTATGGCCATTGTCC GGAAATCACAGTATACACAAGCTGCTGTTACTTTCCTAGTGCTGCACGACTCCCTTCCTTGTGGGCAGACAATAAGA GATCTCTTCTTAGTATGTTAGTGGAGGTTCACAAGGGAGTTCATGGATTTGTTAAAGATAAGACTGGAAAGCCAATC TCTAAAGCAGTCATTGTACTTAATGAAGGAATAAAGGTACAAACAAAAGAGGGAGGTTATTTCCATGTACTCTTAGC GCCAGGTGTCCATAACATTATTGCCATCGCTGATGGGTACCAGCAACAACATTCACAGGTCTTTGTGCATCATGATG CAGCTAGTTCTGTGGTGATAGTCTTTGACACAGATAACCGGATATTTGGTTTGCCAAGGGAGCTTGTGGTAACTGTA TCAGGTGCTACTATGTCGGCATTGATCCTAACAGCTTGCATTATTTGGTGCATCTGCTCAATCAAGTCTAATAGACA CAAGGATGGCTTTCATCGGCTCAGGCAGCATCATGATGAGTATGAAGATGAAATTCGCATGATGTCTACCGGCTCCA AGAAGTCCCTCCTAAGCCATGAGTTCCAGGATGAAACAGACACTGAAGAGGAAACATTATATTCTAGCAAACATTGA AAAACACATTTTGCATATCTCCCAGCATAAGTACCAAGCAAAATTACAGTTCCTCTTGGGAGAACACTGCATTAAGA AGAGAGACTCTCTTGCTTCTTCAAAGAGCTTTGGGAAATTAAATTGCTAAATTTGTATTCTCTGTGAATTTCACTGG CAGTTTTGAACTTCCCTTCCTTAAAGTACTCTAAACCTTTAAAAAAAAATCTGATTTATGCAGCAGAGATGGGACAG CCACTTTTTCTTTTTAATTTAAGATGAGCTATTTGGAGCTTATGTAATAATGGCATAAAGCCAACTAGAGGATGTTG TATTTTGCACATCAGATGTTTACTAGTGGCTTTAGTATTTTTCTTTGTTTTAAATGGCCAAAAGAATCCAGAAACAT TAAGGCAGGGACAGCAGTCAGAATCGACATAAAGCTTTAAAAACTCAAGGTTTTTTCAACCTACTGAGGAGTACTTT TCTCTAGTTGTTAAATAGCTGGAGTTTTTCTTATTCAGGTTTAATGGAGGTTGAATTGATTTTTAAACACATATAAC AGTAGGAAATGAATAAATGGGCTTCTGCATTTGGCTTTCTACCTGTTCCAAGGCTAGATCGGAACTGGTAGACTACG CTGTAAGCAGGATTTCACTACCTCTCTTAAGGTTTAGCAAACTTCTAAATAGCCCATTTTAAGGGAGAACTTACTAA CTTTATTGTGAAAGGTCTAAATGCCCACTTGAATGAAGCTGAGAGAGAGATCTAGCAAAAGCTAAAACTCATGTTGT CTATCTTTGAACTTGGTAAAAACCCACAGGTGCTGCTGCTTATATCTGTGAAGCACTAGCTTATTCTAGGAATGCCT GATTCTTTAATATTGCCTAAATCGGAACCTTTTTCTATGTTGCACACATGGTTTTCAGATGACCCAGCCATCTACAA GATCTGAATTCTACTGAAAATATCTAGAAATGTGGAAGAGACCTACTTGCACATTCTTAACCTGTATTTGAACACAA AATATCTATACTTCATGCTCCAGCCCAAGCCTATACCCTGTAATAGCATACTATTATTGAAATCGCTTGACCGGTCT TGTTCACATAGGCCTCTGGGAGTGATTTGGTTCTTTGCCCTAATGTTTCATTTGACGGTCTCTTTTTGATCAACCAA TTTTTCTAAAAGTTCAGTCGAAAGCTTTTAAGTATAGCTTCCTCCCTTGAAAAAAAATGTAAACTATGACTGCTGAG TGATAAAACACTGTGGTGTGAAAGTGTCATCTTCACTGCCAATCAGGCAAAGACCGGAAAGATTTGCATTTTATTAT GTCTGTCTTATCATGCAATGGAAATGATGCTTTTTGTAAGTATGCATCTTACCAATGATGTAACGGTTTAATACCTT TGAATGTTTTAATAACCAAGTTGCTGCTGAACTTATACTAAATCAGGGGACCAAAAAACTTGCTCTTATCTTCTCAA ATTGTATTCTATATCCATTAATGTATCAGTTATCCCAAAGCCTTCAGGTGGAGGGGTTTACCACCTTCCTAGGTCGT TCAACCAGGTTTTGTGAGGAATGCATTCAAAGTGGCTTTATAAAAGAAGATTTTCTTTAGCAAGAAAAAAAAAAAAA AAAAAAAAAAAAAAA (SEQ ID NO: 2)
[00334] >gil315138988lreflNM_001304.4l Homo sapiens carboxypeptidase D (CPD), transcript variant 1 , mRNA
GCCGCCCGGAGCGCTGAGCCGCGGGAGCGGAGCCGGGGTTAGCGGCGCTGCTGGAAGATGGCGAGCGGCCGGGACGA GCGGCCGCCTTGGCGGCTAGGGCGGCTCCTGTTGCTCATGTGCCTGCTGCTGCTGGGGAGCTCGGCCCGGGCGGCTC ACATCAAGAAGGCGGAGGCGACTACCACAACTACGAGCGCGGGCGCCGAGGCGGCCGAGGGCCAGTTCGACCGCTAC TACCACGAAGAGGAGTTGGAGTCGGCGCTGAGGGAGGCGGCGGCCGCGGGCCTCCCCGGCCTGGCCCGCCTCTTTAG CATCGGCCGCTCGGTGGAAGGCCGGCCGCTGTGGGTGCTTCGCCTCACCGCCGGCCTGGGGTCGCTAATCCCTGAGG GCGACGCGGGGCCTGACGCTGCCGGGCCCGACGCTGCGGGGCCGCTGCTGCCCGGCCGGCCCCAGGTGAAGCTGGTG GGCAACATGCATGGCGACGAGACCGTGTCGCGCCAGGTGTTGATCTACTTGGCCCGCGAGCTGGCGGCCGGCTACCG CCGCGGGGACCCGCGCCTGGTCCGCCTGCTCAACACCACCGACGTGTACCTGCTGCCCAGCCTCAACCCCGATGGCT TCGAGCGTGCCCGCGAGGGCGACTGTGGCTTCGGCGACGGCGGCCCGTCCGGGGCCAGCGGCCGCGACAATAGTCGC GGCCGCGACCTCAACCGAAGCTTTCCCGACCAGTTTAGCACCGGCGAACCCCCCGCCCTGGACGAGGTGCCCGAGGT GCGCGCCCTCATCGAGTGGATCCGCAGGAACAAGTTTGTGCTTTCTGGAAATCTGCATGGTGGCTCAGTGGTAGCAA GCTATCCTTTTGATGATTCTCCAGAACATAAGGCCACTGGAATCTATAGCAAAACCTCAGATGATGAAGTATTTAAA TACTTGGCAAAAGCTTATGCTTCAAACCACCCCATAATGAAAACTGGTGAGCCTCATTGTCCAGGAGATGAAGACGA GACTTTCAAAGATGGAATCACAAACGGCGCACATTGGTATGATGTGGAAGGTGGTATGCAAGATTACAATTATGTGT GGGCCAACTGTTTTGAGATCACATTAGAACTGTCTTGTTGCAAGTACCCACCTGCTTCACAGCTTCGACAGGAATGG GAGAACAATCGTGAGTCTTTGATCACATTGATTGAAAAGGTTCACATTGGAGTGAAAGGATTTGTTAAAGATTCCAT AACAGGATCTGGGTTAGAGAATGCAACCATCTCAGTGGCTGGTATTAATCATAATATCACAACAGGCAGATTTGGTG ATTTCTACCGATTACTTGTTCCTGGAACTTACAACCTTACAGTAGTTTTAACTGGGTATATGCCATTGACTGTTACT AATGTAGTGGTGAAAGAAGGACCAGCCACAGAGGTGGATTTTTCTCTTAGGCCAACTGTAACTTCAGTAATCCCTGA CACGACAGAGGCTGTATCAACTGCTAGCACAGTTGCTATACCTAATATTCTTTCTGGAACATCATCCTCCTACCAGC
CAATTCAGCCAAAGGACTTTCACCACCACCATTTCCCTGATATGGAAATCTTCTTGAGAAGGTTTGCCAATGAATAT CCTAACATTACCCGGCTTTATTCCTTGGGAAAATCAGTAGAGTCAAGAGAACTTTATGTGATGGAGATATCTGATAA TCCGGGTGTCCATGAACCAGGTGAACCAGAATTTAAGTACATTGGAAATATGCATGGAAATGAAGTGGTTGGAAGAG AACTGCTGTTGAACCTCATAGAATACCTTTGTAAGAACTTTGGAACAGACCCTGAAGTCACAGATTTGGTTCATAAC ACTAGAATTCACCTTATGCCATCCATGAATCCTGATGGGTATGAAAAGTCCCAGGAAGGAGATTCAATAAGTGTAAT T G G C AG AAAC AAC AG C AAC AAC T T T G AC C T GAAC C G AAAT T T C C C AGAC C AGT T T G T T C AGAT C AC AGAT C C T AC G C AACCAGAAACTATTGCTGTAATGAGCTGGATGAAGTCCTATCCATTTGTACTTTCAGCAAACCTGCATGGAGGTTCT TTGGTGGTTAACTACCCTTTTGATGATGATGAACAAGGACTTGCCACATATAGTAAATCACCAGATGATGCTGTGTT CCAACAAATAGCACTTTCTTATTCCAAGGAAAATTCCCAGATGTTTCAAGGTAGACCTTGCAAGAATATGTATCCTA ATGAATATTTTCCTCATGGAATAACAAATGGAGCTAGTTGGTATAATGTGCCAGGAGGAATGCAGGACTGGAACTAT TTACAAACAAATTGCTTTGAAGTGACTATTGAACTAGGTTGTGTGAAATATCCACTTGAGAAAGAGCTGCCAAACTT TTGGGAACAGAATCGAAGATCACTAATCCAGTTTATGAAACAGGTTCATCAGGGCGTCAGAGGATTTGTTCTAGATG CCACAGATGGCAGGGGTATATTAAATGCCACCATTAGTGTTGCTGAGATTAATCACCCAGTGACTACTTACAAAACT GGAGATTACTGGCGTCTCTTGGTTCCAGGAACTTATAAAATCACAGCATCTGCTCGAGGGTATAATCCAGTTACCAA GAATGTGACTGTCAAGAGTGAAGGCGCTATTCAGGTCAACTTCACACTTGTTCGATCCTCAACAGATTCAAACAATG AATCAAAGAAAGGAAAAGGGGCTAGCAGCAGCACCAATGATGCCAGTGATCCAACTACTAAAGAGTTTGAAACTTTA ATTAAAGACCTTTCAGCGGAGAATGGTTTGGAAAGCCTCATGTTACGCTCCTCCTCAAATCTGGCTCTGGCTCTTTA TCGATACCATTCCTACAAAGACTTATCAGAGTTTCTGAGAGGACTTGTAATGAACTATCCACATATTACAAATCTTA CCAATTTGGGACAGAGCACTGAATATCGTCACATTTGGTCCCTTGAAATCTCCAATAAGCCCAATGTATCTGAGCCT GAAGAACCAAAGATTCGTTTTGTTGCTGGTATCCATGGAAATGCGCCAGTTGGAACTGAACTGCTTTTGGCTCTGGC AGAATTTCTCTGCCTGAACTACAAAAAGAACCCAGCTGTTACCCAATTGGTTGACAGGACTAGGATTGTGATTGTCC C T T C T C T AAAT C C AGAT G G G C GAGAG AGAG C T C AAG AGAAAGAC T G T AC T T CAAAAAT AG GAC AAAC AAAT G C T C G T GGCAAAGATTTGGATACAGACTTCACAAATAATGCCTCCCAACCTGAGACCAAAGCCATCATTGAAAATTTGATTCA AAAACAGGACTTTAGTCTTTCTGTTGCCTTAGATGGTGGTTCCATGCTGGTCACATATCCTTATGACAAGCCAGTAC AGAC AG T G GAAAAT AAAGAG AC T C T G AAG CAT T T G G CAT CTCTTTATG C AAAT AAT CAT C CAT C CAT G C AC AT G G G T C AG CCCAGTTGCC C AAAT AAAT C AGAT GAG AAT AT T C C AG GAG GAG T AAT G C G T G GAG C AGAAT G G CAT AGT C AC C T GGGCAGCATGAAGGATTATAGTGTCACCTATGGCCATTGTCCGGAAATCACAGTATACACAAGCTGCTGTTACTTTC CTAGTGCTGCACGACTCCCTTCCTTGTGGGCAGACAATAAGAGATCTCTTCTTAGTATGTTAGTGGAGGTTCACAAG GGAGTTCATGGATTTGTTAAAGATAAGACTGGAAAGCCAATCTCTAAAGCAGTCATTGTACTTAATGAAGGAATAAA GGTACAAACAAAAGAGGGAGGTTATTTCCATGTACTCTTAGCGCCAGGTGTCCATAACATTATTGCCATCGCTGATG GGTACCAGCAACAACATTCACAGGTCTTTGTGCATCATGATGCAGCTAGTTCTGTGGTGATAGTCTTTGACACAGAT AACCGGATATTTGGTTTGCCAAGGGAGCTTGTGGTAACTGTATCAGGTGCTACTATGTCGGCATTGATCCTAACAGC TTGCATTATTTGGTGCATCTGCTCAATCAAGTCTAATAGACACAAGGATGGCTTTCATCGGCTCAGGCAGCATCATG ATGAGTATGAAGATGAAATTCGCATGATGTCTACCGGCTCCAAGAAGTCCCTCCTAAGCCATGAGTTCCAGGATGAA ACAGACACTGAAGAGGAAACATTATATTCTAGCAAACATTGAAAAACACATTTTGCATATCTCCCAGCATAAGTACC AAGCAAAATTACAGTTCCTCTTGGGAGAACACTGCATTAAGAAGAGAGACTCTCTTGCTTCTTCAAAGAGCTTTGGG AAATTAAATTGCTAAATTTGTATTCTCTGTGAATTTCACTGGCAGTTTTGAACTTCCCTTCCTTAAAGTACTCTAAA CCTTTAAAAAAAAATCTGATTTATGCAGCAGAGATGGGACAGCCACTTTTTCTTTTTAATTTAAGATGAGCTATTTG GAGCTTATGTAATAATGGCATAAAGCCAACTAGAGGATGTTGTATTTTGCACATCAGATGTTTACTAGTGGCTTTAG TAT TTTTCTTTGTTTT AAAT GGCCAAAAGAATCCAGAAACATTAAGGCAGGGACAGCAGTCAGAATCGACATAAAGC TTTAAAAACTCAAGGTTTTTTCAACCTACTGAGGAGTACTTTTCTCTAGTTGTTAAATAGCTGGAGTTTTTCTTATT CAGGTTTAATGGAGGTTGAATTGATTTTTAAACACATATAACAGTAGGAAATGAATAAATGGGCTTCTGCATTTGGC TTTCTACCTGTTCCAAGGCTAGATCGGAACTGGTAGACTACGCTGTAAGCAGGATTTCACTACCTCTCTTAAGGTTT AGCAAACTTCTAAATAGCCCATTTTAAGGGAGAACTTACTAACTTTATTGTGAAAGGTCTAAATGCCCACTTGAATG AAGCTGAGAGAGAGATCTAGCAAAAGCTAAAACTCATGTTGTCTATCTTTGAACTTGGTAAAAACCCACAGGTGCTG CTGCTTATATCTGTGAAGCACTAGCTTATTCTAGGAATGCCTGATTCTTTAATATTGCCTAAATCGGAACCTTTTTC TAT GTTGCACACATGGTTTTCAGATGACCCAGC CAT CTACAAGATCTGAATTCTACT GAAAAT AT CTAGAAATGTGG AAGAGACCTACTTGCACATTCTTAACCTGTATTTGAACACAAAATATCTATACTTCATGCTCCAGCCCAAGCCTATA CCCTGTAATAGCATACTATTATTGAAATCGCTTGACCGGTCTTGTTCACATAGGCCTCTGGGAGTGATTTGGTTCTT TGCCCTAATGTTTCATTTGACGGTCTCTTTTTGATCAACCAATTTTTCTAAAAGTTCAGTCGAAAGCTTTTAAGTAT AGCTTCCTCCCTTGAAAAAAAATGTAAACTATGACTGCTGAGTGATAAAACACTGTGGTGTGAAAGTGTCATCTTCA CTGCCAATCAGGCAAAGACCGGAAAGATTTGCATTTTATTATGTCTGTCTTATCATGCAATGGAAATGATGCTTTTT GT AAGT AT GC AT C T T AC CAAT GAT GT AAC G GT T T AAT AC C T T T GAAT GT T T T AAT AAC CAAGT T GC T GC T GAACTTA TACTAAATCAGGGGACCAAAAAACTTGCTCTTATCTTCTCAAATTGTATTCTATATCCATTAATGTATCAGTTATCC CAAAGCCTTCAGGTGGAGGGGTTTACCACCTTCCTAGGTCGTTCAACCAGGTTTTGTGAGGAATGCATTCAAAGTGG CTT TAT AAAAGAA GAT TTTCTTTAGCAAGAAT AAT GAGGT CAT GTCATTTGTT AAT AAG TAT CTGT GAT AAAT CCGT GGTTCAAGGTTAAGCCATTCTGGTATTCTGGTATTAGCAACTGTAAATTCTGCCACCTCATACATGGAACAGAGCTT
GTGGGATGCTAATAGTTAGTGAAGTATACATGATTTAATTTCTAATAATCTTTATGTTTTCTTTAAGGATGGTGGTG TATTGCTCTTTTTCAGCTTTATTTTTAAGAGTACAGTCAGGAAACCAACAAGGGGCCTAAGAGTGGCTGCCCCTGCT TGGGACATTACAGCAAGTGAAACAAAGTTAATGTGACAAGCTTTGCTTTGTTATCATTGGTCTTCACTAGAGGATAC CTTTTACATGTACTTCTCTCTTGGATCAAATATGTCTTTAACTGTACATCTCAGTGGCTGGAGGCCATGCCTTTTAA GCATGTGTAAAATTTTTAAAGAAATGAACATACACATAGTTATTTTAGTAATATTTCCTGAAAGAAAAACCAAATTC TGCTATAAGTCTTGATCTTCAATGAACTTTTAAATAATGCATTTAGCTGGAAAACAAGACTTTCCCAGCTTGTATTA CCTAGAAGCGTGAATGTATAGGATACCTGACTACTAAGACTATATTCTCAGCCCTGCCCTGTCTTTTATTTGCGGGT CTAATCTAATATTAGAATATATTAACCGCTTAAGGCATTGAAGCCATATGGGATGGGGAATGCATTTCTTCAGTGTT TCTCCGAGAGACTTTCCATTTCCTTGGAGTTATGGCGGCAAGTAAGTATCATAGTATTAAGAAATTTGCCTAAATCT GAGTTGTGCCTTTCTTTACTCACAAGGCATGGGCTTTGTCCTGGTGATCAGTTTGTAAGCCTTCTTCCTTCCCAGCT CCTTAATAAAAGCAAAGTGATTGAGTAGGTAATGTTCAAAGTGTCTGCCTGTGTACATGTACTTGTATTGATTATGT AGTTCAGTAAGATGTGCCCAAGTCATTTCAGAAAGAAAGACCCTTCAGTTTTGATGCATTTTGCTGAACACTTGGGT AGTGAGTGGGATCCTATCCAGTTGAGGAATGCTTGCAATGCTCATTGAAGGGATTTGCTTTGGGACTTTGTCATCTT CCAGAAAGGAAACATATTGTATATTTGGCCCAGTGTGATTGATTGCTTTATCTTTGGTAACTTTTACTTGAATGGGA TTTGCTGAATTAATGACTATTGAATTTAAAACTAATTATGAGTTGACAAATAAATAAAAGGTAGTGTTTATGTCTGA GCTTATTGTGTTTGAGCTAACACCAGGTTACTCAGTAACCATGACCTGCTCCTCCATTTCCATTTATTCTCAACATT AAATAGTTTTATCTTGTTGTTGCCAGAAATGCACTTGTGCCAGGTATTGTCCCTGCTGTATGAAAAGCTTCTTGGCA ATGAATTCTGTAATAGTGCCCTACATTATGGTTTTCTGGTGGAATTGTTTTAACAGTGACAACCCAGGATTTCCAAT ATATTTTTGTTTTATTGTTATTACCAAAAATTCCACTATGATTGATGTTCAGTGATTTTCTATAGCAACTTTTTTGG TAACTCTTTGGGTTTCTGATTTGTTTTAGCTAAAATTTTGGGGATATGATTTGGGTCTTTGATTAATGTCAGCTGAA CTTGGATTTCTAGTTCATGAAGAAATCTCTCCCAATACCCATTTATCCTATTTTTAGCAATAATTCGTTAATGATTC CACTTGATTTTCAGAATATTGTCCTGGTTGATTTTGATTTGACAGCATACATTATGAAATTTGAAAGTAGGTTACCA TTTTGAGGCAGTTGGATATAAATTATGTAAATATGTATGATTATGATTTTTATAAATGGCATAACATGAGTGTACTA ACTACCTTCTATGCTGGCCATGCTACAGATTTTCTGGAGGTATGACAATAGTATTTTTTTATGCTCAGATTAAAAAT CAGCTTTTCACCTCTCCAGTTTTTCCAAGTGATACTCCCAGTTCTAGAGCAATCTACAGCTGTTTATGTGAGGTGCC CAACACCCATTCATCTCAAGTGCTTCAGTCTTTGGTTTATTTCATGCACTGTGCCTTCAAAATGAAATTTTTAAAAG GGACTTTAAATGAAGTTGAATAGTAGTTTTTAAAAGTCAATTTGTAATTTATGTGAAATCTAACTGTAATGAGGTCC TTTCTGTTTTTTATATGTAAACAGATCTACTAATCCTGTATAAAAGTTATTTTACGATGTTTGTCTTTCTTTGTGTT TTGTCTCATAATCTTTTTTCAGATGCAATATGCCGGAAAAAGTTATAGGTCCAGTTTGAAAATTATTTAGTTTTTCT GCCTATGCTAGTGGAAAAATAGTACCAGGATCAGAATACAGGGTATCACCTATGGAATGTTTCTGTATTTATGAATT GACTCAAAAGAAAGCTTTGTTTCTGAAATCGCATTATGTAGTAGCCACAGTTTTCTGTTTGTAGCTCAGCTAGATTG TTATATATGTTCAATCATTTCACAATAACAACACAAAACTGGTCATTGAAAGGTTTTTATGTACGCATTTTAAACTT GTTCGTTAAAAATTTGGTCCTTTTTCCAGGTGAGGCCCAGTTAGAATAATGTGTCCCGGCACTTTTAGGCACAGCAA GGATGAATTCAATATCCCCTTTTCACTTAGCAACAATGTGTTACTTCTACCCTAATAGGAATTGGGAAAGCAAAGTT GTATGAGAAACAGACTCTGCTGATAAAGTACTCATAGTCCAGACCAGAGAAATATAAATGGAAATAGGTTATATTTC AATAGTGATTGGTTCATCTAAAAGTCTCTGCTGTAAAGGAAATAAAGCATAGAGGTTTGAGCATGGACTTTGGAGTT GGACCAATCTGTGACTGATTCTTCGTTCTGCTACTTGCTTCCAATGTGACCTTGTACAAGTTTCTTGACATTCTCTG AGCCTCAGTTTCTCTACTGGTTGAATAATCCTTGATAGGATTGCAGTGGAAAATTAAATGAAATAATGTTAGCAAAG GTCCCAACATAATATTTGACTTGGAATTGAATGCCCATGGTAACCAGCATCATTTTCCTTCATGTGATGTCTTCTTA TGCCTTTGAAAGAAAGTTACTTTATCAAATGTATAAATAAAGATCTGTTTATAGGTGATCTTTTTAATTTAGAAGAA ATTCTGAGACACAAATAAAAAAAGAAATTTTTTAAAAAAAAAAAAAAAAAA (SEQ ID NO: 3)
[00335] >gil22202611lreflNP_001295.21 carboxypeptidase D isoform 1 precursor [Homo sapiens]
MASGRDERPPWRLGRLLLLMCLLLLGSSARAAHIKKAEATTTTTSAGAEAAEGQFDRYYHEEELESALREAAAAGLP GLARLFSIGRSVEGRPLWVLRLTAGLGSLI PEGDAGPDAAGPDAAGPLLPGRPQVKLVGNMHGDETVSRQVLIYLAR ELAAGYRRGDPRLVRLLNTTDVYLLPSLNPDGFERAREGDCGFGDGGPSGASGRDNSRGRDLNRSFPDQFSTGEPPA LDEVPEVRALIEWIRRNKFVLSGNLHGGSWASYPFDDSPEHKATGIYSKTSDDEVFKYLAKAYASNHPIMKTGEPH CPGDEDETFKDGITNGAHWYDVEGGMQDYNYVWANCFEITLELSCCKYPPASQLRQEWENNRESLITLI EKVHIGVK GFVKDS ITGSGLENATI SVAGINHNITTGRFGDFYRLLVPGTYNLTVVLTGYMPLTVTNVVVKEGPATEVDFSLRPT VTSVI PDTTEAVSTASTVAI PNI LSGTSSSYQPIQPKDFHHHHFPDMEI FLRRFANEYPNITRLYSLGKSVESRELY VMEI SDNPGVHEPGEPEFKYIGNMHGNEVVGRELLLNLIEYLCKNFGTDPEVTDLVHNTRIHLMPSMNPDGYEKSQE GDSISVIGRNNSNNFDLNRNFPDQFVQITDPTQPETIAVMSWMKSYPFVLSANLHGGSLWNYPFDDDEQGLATYSK SPDDAVFQQIALSYSKENSQMFQGRPCKNMYPNEYFPHGITNGASWYNVPGGMQDWNYLQTNCFEVTIELGCVKYPL
EKELPNFWEQNRRSLIQFMKQVHQGVRGFVLDATDGRGILNATI SVAEINHPVTTYKTGDYWRLLVPGTYKITASAR GYNPVTKNVTVKSEGAIQVNFTLVRS STDSNNESKKGKGASSSTNDASDPTTKEFETLI KDLSAENGLESLMLRSS S NLALALYRYHSYKDLSEFLRGLVMNYPHITNLTNLGQSTEYRHIWSLEI SNKPNVSEPEEPKI RFVAGIHGNAPVGT ELLLALAEFLCLNYKKNPAVTQLVDRTRIVIVPSLNPDGRERAQEKDCTSKI GQTNARGKDLDTDFTNNASQPETKA IIENLIQKQDFSLSVALDGGSMLVTYPYDKPVQTVENKETLKHLASLYANNHPSMHMGQPSCPNKSDENIPGGVMRG AEWHSHLGSMKDYSVTYGHCPEITVYTSCCYFPSAARLPSLWADNKRSLLSMLVEVHKGVHGFVKDKTGKPI SKAVI VLNEGI KVQTKEGGYFHVLLAPGVHNI IAIADGYQQQHSQVFVHHDAASSVVIVFDTDNRI FGLPRELWTVSGATM SALILTACIIWCICSIKSNRHKDGFHRLRQHHDEYEDEIRMMSTGSKKSLLSHEFQDETDTEEETLYSSKH (SEQ ID NO: 4)
[00336] >gil315138989lreflNM_001199775.11 Homo sapiens carboxypeptidase D (CPD), transcript variant 2, mRNA
ATTTGTGCACCTTATTAGCCTGGGCAGAATGGCTAATGGTCACACTTCTAATACACACTTTAAGTGTTATTAAGCAT TGCATGAGGTTTAAAAAATTACAAAGTAGAAGAAGGAGGACCTCAAGAAATTAGTATAAAACAAGAGAATGTTATAA ATATTTATGAGGTTTGTGCTTTCTGGAAATCTGCATGGTGGCTCAGTGGTAGCAAGCTATCCTTTTGATGATTCTCC AGAACATAAGGCCACTGGAATCTATAGCAAAACCTCAGATGATGAAGTATTTAAATACTTGGCAAAAGCTTATGCTT CAAACCACCCCATAATGAAAACTGGTGAGCCTCATTGTCCAGGAGATGAAGACGAGACTTTCAAAGATGGAATCACA AACGGCGCACATTGGTATGATGTGGAAGGTGGTATGCAAGATTACAATTATGTGTGGGCCAACTGTTTTGAGATCAC ATTAGAACTGTCTTGTTGCAAGTACCCACCTGCTTCACAGCTTCGACAGGAATGGGAGAACAATCGTGAGTCTTTGA TCACATTGATTGAAAAGGTTCACATTGGAGTGAAAGGATTTGTTAAAGATTCCATAACAGGATCTGGGTTAGAGAAT GCAACCATCTCAGTGGCTGGTATTAATCATAATATCACAACAGGCAGATTTGGTGATTTCTACCGATTACTTGTTCC TGGAACTTACAACCTTACAGTAGTTTTAACTGGGTATATGCCATTGACTGTTACTAATGTAGTGGTGAAAGAAGGAC CAGCCACAGAGGTGGATTTTTCTCTTAGGCCAACTGTAACTTCAGTAATCCCTGACACGACAGAGGCTGTATCAACT GCTAGCACAGTTGCTATACCTAATATTCTTTCTGGAACATCATCCTCCTACCAGCCAATTCAGCCAAAGGACTTTCA CCACCACCATTTCCCTGATATGGAAATCTTCTTGAGAAGGTTTGCCAATGAATATCCTAACATTACCCGGCTTTATT CCTTGGGAAAATCAGTAGAGTCAAGAGAACTTTATGTGATGGAGATATCTGATAATCCGGGTGTCCATGAACCAGGT GAACCAGAATTTAAGTACATTGGAAATATGCATGGAAATGAAGTGGTTGGAAGAGAACTGCTGTTGAACCTCATAGA ATACCTTTGTAAGAACTTTGGAACAGACCCTGAAGTCACAGATTTGGTTCATAACACTAGAATTCACCTTATGCCAT CCATGAATCCTGATGGGTATGAAAAGTCCCAGGAAGGAGATTCAATAAGTGTAATTGGCAGAAACAACAGCAACAAC TTTGACCTGAACCGAAATTTCCCAGACCAGTTTGTTCAGATCACAGATCCTACGCAACCAGAAACTATTGCTGTAAT GAGCTGGATGAAGTCCTATCCATTTGTACTTTCAGCAAACCTGCATGGAGGTTCTTTGGTGGTTAACTACCCTTTTG ATGATGATGAACAAGGACTTGCCACATATAGTAAATCACCAGATGATGCTGTGTTCCAACAAATAGCACTTTCTTAT TCCAAGGAAAATTCCCAGATGTTTCAAGGTAGACCTTGCAAGAATATGTATCCTAATGAATATTTTCCTCATGGAAT AACAAATGGAGCTAGTTGGTATAATGTGCCAGGAGGAATGCAGGACTGGAACTATTTACAAACAAATTGCTTTGAAG TGACTATTGAACTAGGTTGTGTGAAATATCCACTTGAGAAAGAGCTGCCAAACTTTTGGGAACAGAATCGAAGATCA CTAATCCAGTTTATGAAACAGGTTCATCAGGGCGTCAGAGGATTTGTTCTAGATGCCACAGATGGCAGGGGTATATT AAATGCCACCATTAGTGTTGCTGAGATTAATCACCCAGTGACTACTTACAAAACTGGAGATTACTGGCGTCTCTTGG TTCCAGGAACTTATAAAATCACAGCATCTGCTCGAGGGTATAATCCAGTTACCAAGAATGTGACTGTCAAGAGTGAA GGCGCTATTCAGGTCAACTTCACACTTGTTCGATCCTCAACAGATTCAAACAATGAATCAAAGAAAGGAAAAGGGGC TAGCAGCAGCACCAATGATGCCAGTGATCCAACTACTAAAGAGTTTGAAACTTTAATTAAAGACCTTTCAGCGGAGA ATGGTTTGGAAAGCCTCATGTTACGCTCCTCCTCAAATCTGGCTCTGGCTCTTTATCGATACCATTCCTACAAAGAC TTATCAGAGTTTCTGAGAGGACTTGTAATGAACTATCCACATATTACAAATCTTACCAATTTGGGACAGAGCACTGA ATATCGTCACATTTGGTCCCTTGAAATCTCCAATAAGCCCAATGTATCTGAGCCTGAAGAACCAAAGATTCGTTTTG TTGCTGGTATCCATGGAAATGCGCCAGTTGGAACTGAACTGCTTTTGGCTCTGGCAGAATTTCTCTGCCTGAACTAC AAAAAGAACCCAGCTGTTACCCAATTGGTTGACAGGACTAGGATTGTGATTGTCCCTTCTCTAAATCCAGATGGGCG AGAGAGAGCTCAAGAGAAAGACTGTACTTCAAAAATAGGACAAACAAATGCTCGTGGCAAAGATTTGGATACAGACT TCACAAATAATGCCTCCCAACCTGAGACCAAAGCCATCATTGAAAATTTGATTCAAAAACAGGACTTTAGTCTTTCT GTTGCCTTAGATGGTGGTTCCATGCTGGTCACATATCCTTATGACAAGCCAGTACAGACAGTGGAAAATAAAGAGAC TCTGAAGCATTTGGCATCTCTTTATGCAAATAATCATCCATCCATGCACATGGGTCAGCCCAGTTGCCCAAATAAAT CAGATGAGAATATTCCAGGAGGAGTAATGCGTGGAGCAGAATGGCATAGTCACCTGGGCAGCATGAAGGATTATAGT GTCACCTATGGCCATTGTCCGGAAATCACAGTATACACAAGCTGCTGTTACTTTCCTAGTGCTGCACGACTCCCTTC CTTGTGGGCAGACAATAAGAGATCTCTTCTTAGTATGTTAGTGGAGGTTCACAAGGGAGTTCATGGATTTGTTAAAG ATAAGACTGGAAAGCCAATCTCTAAAGCAGTCATTGTACTTAATGAAGGAATAAAGGTACAAACAAAAGAGGGAGGT TATTTCCATGTACTCTTAGCGCCAGGTGTCCATAACATTATTGCCATCGCTGATGGGTACCAGCAACAACATTCACA
GGTCTTTGTGCATCATGATGCAGCTAGTTCTGTGGTGATAGTCTTTGACACAGATAACCGGATATTTGGTTTGCCAA GGGAGCTTGTGGTAACTGTATCAGGTGCTACTATGTCGGCATTGATCCTAACAGCTTGCATTATTTGGTGCATCTGC TCAATCAAGTCTAATAGACACAAGGATGGCTTTCATCGGCTCAGGCAGCATCATGATGAGTATGAAGATGAAATTCG CATGATGTCTACCGGCTCCAAGAAGTCCCTCCTAAGCCATGAGTTCCAGGATGAAACAGACACTGAAGAGGAAACAT TATATTCTAGCAAACATTGAAAAACACATTTTGCATATCTCCCAGCATAAGTACCAAGCAAAATTACAGTTCCTCTT GGGAGAACACTGCATTAAGAAGAGAGACTCTCTTGCTTCTTCAAAGAGCTTTGGGAAATTAAATTGCTAAATTTGTA TTCTCTGTGAATTTCACTGGCAGTTTTGAACTTCCCTTCCTTAAAGTACTCTAAACCTTTAAAAAAAAATCTGATTT ATGCAGCAGAGATGGGACAGCCACTTTTTCTTTTTAATTTAAGATGAGCTATTTGGAGCTTATGTAATAATGGCATA AAGCCAACTAGAGGATGTTGTATTTTGCACATCAGATGTTTACTAGTGGCTTTAGTATTTTTCTTTGTTTTAAATGG CCAAAAGAATCCAGAAACATTAAGGCAGGGACAGCAGTCAGAATCGACATAAAGCTTTAAAAACTCAAGGTTTTTTC AACCTACTGAGGAGTACTTTTCTCTAGTTGTTAAATAGCTGGAGTTTTTCTTATTCAGGTTTAATGGAGGTTGAATT GATTTTTAAACACATATAACAGTAGGAAATGAATAAATGGGCTTCTGCATTTGGCTTTCTACCTGTTCCAAGGCTAG ATCGGAACTGGTAGACTACGCTGTAAGCAGGATTTCACTACCTCTCTTAAGGTTTAGCAAACTTCTAAATAGCCCAT TTTAAGGGAGAACTTACTAACTTTATTGTGAAAGGTCTAAATGCCCACTTGAATGAAGCTGAGAGAGAGATCTAGCA AAAGCTAAAACTCATGTTGTCTATCTTTGAACTTGGTAAAAACCCACAGGTGCTGCTGCTTATATCTGTGAAGCACT AGCTTATTCTAGGAATGCCTGATTCTTTAATATTGCCTAAATCGGAACCTTTTTCTATGTTGCACACATGGTTTTCA GATGACCCAGCCATCTACAAGATCTGAATTCTACTGAAAATATCTAGAAATGTGGAAGAGACCTACTTGCACATTCT TAACCTGTATTTGAACACAAAATATCTATACTTCATGCTCCAGCCCAAGCCTATACCCTGTAATAGCATACTATTAT TGAAATCGCTTGACCGGTCTTGTTCACATAGGCCTCTGGGAGTGATTTGGTTCTTTGCCCTAATGTTTCATTTGACG GTCTCTTTTT GAT CAACCAATTTTTCTAAAAGTTCAGTCGAAAGCTTTT AAGT ATAGCTTCCTCCCTTGAAAAAAAA TGTAAACTATGACTGCTGAGTGATAAAACACTGTGGTGTGAAAGTGTCATCTTCACTGCCAATCAGGCAAAGACCGG AAAGAT T T GC AT T T T AT T AT GT C T GT CT T AT CAT GC AAT G GAAAT GAT G CT T T T T GT AAGT AT G CAT C T T AC CAAT G ATGTAACGGTTTAATACCTTTGAATGTTTTAATAACCAAGTTGCTGCTGAACTTATACTAAATCAGGGGACCAAAAA ACTTGCTCTTATCTTCTCAAATTGTATTCTATATCCATTAATGTATCAGTTATCCCAAAGCCTTCAGGTGGAGGGGT TTACCACCTTCCTAGGTCGTTCAACCAGGTTTTGTGAGGAATGCATTCAAAGTGGCTTTATAAAAGAAGATTTTCTT TAG CAAGAAT AAT GAG GT CAT GT CAT T T GT T AAT AAGT AT CT GT GAT AAAT CCGTGGTT CAAG GT TAAG C CAT T CT G GTATTCTGGTATTAGCAACTGTAAATTCTGCCACCTCATACATGGAACAGAGCTTGTGGGATGCTAATAGTTAGTGA AGTATACATGATTTAATTTCTAATAATCTTTATGTTTTCTTTAAGGATGGTGGTGTATTGCTCTTTTTCAGCTTTAT TTTTAAGAGTACAGTCAGGAAACCAACAAGGGGCCTAAGAGTGGCTGCCCCTGCTTGGGACATTACAGCAAGTGAAA CAAAGTTAATGTGACAAGCTTTGCTTTGTTATCATTGGTCTTCACTAGAGGATACCTTTTACATGTACTTCTCTCTT GGATCAAATATGTCTTTAACTGTACATCTCAGTGGCTGGAGGCCATGCCTTTTAAGCATGTGTAAAATTTTTAAAGA AAT GAACAT ACAC AT AGT TAT T T T AGT AAT AT T T C C T GAAAGAAAAAC C AAAT T C T GC TAT AAGT CT T GAT CT T C AA TGAACTTTTAAATAATGCATTTAGCTGGAAAACAAGACTTTCCCAGCTTGTATTACCTAGAAGCGTGAATGTATAGG ATACCTGACTACTAAGACTATATTCTCAGCCCTGCCCTGTCTTTTATTTGCGGGTCTAATCTAATATTAGAATATAT TAACCGCTTAAGGCATTGAAGCCATATGGGATGGGGAATGCATTTCTTCAGTGTTTCTCCGAGAGACTTTCCATTTC CTTGGAGTTATGGCGGCAAGTAAGTAT CAT AGT ATT AAGAAATTTGCCT AAAT CTGAGTTGTGCCTTTCTTTACTCA CAAGGCATGGGCTTTGTCCTGGTGATCAGTTTGTAAGCCTTCTTCCTTCCCAGCTCCTTAATAAAAGCAAAGTGATT GAGTAGGTAATGTTCAAAGTGTCTGCCTGTGTACATGTACTTGTATTGATTATGTAGTTCAGTAAGATGTGCCCAAG TCATTTCAGAAAGAAAGACCCTTCAGTTTTGATGCATTTTGCTGAACACTTGGGTAGTGAGTGGGATCCTATCCAGT TGAGGAATGCTTGCAATGCTCATTGAAGGGATTTGCTTTGGGACTTTGTCATCTTCCAGAAAGGAAACATATTGTAT ATTTGGCCCAGTGTGATTGATTGCTTTATCTTTGGTAACTTTTACTTGAATGGGATTTGCTGAATTAATGACTATTG AAT TTAAAACTAATTATGAGTTGACAAAT AAAT AAAAGGT AGT GTT TAT GTCTGAGCT TAT TGTGTTTGAGCTAACA CCAGGT TACT CAGTAACCAT GACCTGCTCCTCCATTTCCATTT ATT CTCAACATTAAATAGTTTTATCTTGTTGTTG CCA GAAAT GCACTTGTGCCAGGT ATT GTCCCTGCTGTATGAAAAGCTTCTTGGCAATGAATTCTGT AAT AGT GCCCT ACAT T AT GGT T T T CT G GT GGAAT T GT T T TAACAGT GACAAC C C AGGAT T T C CAAT AT AT TTTTGTTT TAT T GT T AT T ACCAAAAATTCCACTATGATTGATGTTCAGTGATTTTCTATAGCAACTTTTTTGGTAACTCTTTGGGTTTCTGATTT GTTTTAGCTAAAATTTTGGGGATATGATTTGGGTCTTTGATTAATGTCAGCTGAACTTGGATTTCTAGTTCATGAAG AAAT CTCTCCCAATACCCATTTATCCTATTTTTAGCAATAATTCGTTAAT GAT TCCACTT GAT TTTCAGAAT ATT GT CCTGGTTGATTTTGATTTGACAGCATACATTATGAAATTTGAAAGTAGGTTACCATTTTGAGGCAGTTGGATATAAA TTATGTAAATATGTATGATTATGATTTTTATAAATGGCATAACATGAGTGTACTAACTACCTTCTATGCTGGCCATG CTACAGATTTTCTGGAGGTATGA CAAT AGT ATT TTTTTATGCTCAGATTAAAAATCAGCTTTTCACCTCTCCAGTTT TTCCAAGTGATACTCCCAGTTCTAGAGCAATCTACAGCTGTTTATGTGAGGTGCCCAACACCCATTCATCTCAAGTG CTTCAGTCTTTGGTTTATTTCATGCACTGTGCCTTCAAAATGAAATTTTTAAAAGGGACTTTAAATGAAGTTGAATA GT AGT T T T TAAAAGT CAAT T T GT AAT T TAT GT GAAAT CTAACT GT AAT GAGGT CCTTTCTGTTTTT TAT AT GT AAAC AGATCTACTAATCCTGTATAAAAGTTATTTTACGATGTTTGTCTTTCTTTGTGTTTTGTCTCATAATCTTTTTTCAG ATGCAATATGCCGGAAAAAGTTATAGGTCCAGTTTGAAAATTATTTAGTTTTTCTGCCTATGCTAGTGGAAAAATAG TACCAGGATCAGAATACAGGGTATCACCTAT GGAAT GTTTCTGTATTTATGAATTGACTCAAAAGAAAGCTTT GTT T
CTGAAATCGCATTATGTAGTAGCCACAGTTTTCTGTTTGTAGCTCAGCTAGATTGTTATATATGTTCAATCATTTCA CAATAACAACACAAAACTGGTCATTGAAAGGTTTTTATGTACGCATTTTAAACTTGTTCGTTAAAAATTTGGTCCTT TTTCCAGGTGAGGCCCAGTTAGAATAATGTGTCCCGGCACTTTTAGGCACAGCAAGGATGAATTCAATATCCCCTTT TCACTTAGCAACAATGTGTTACTTCTACCCTAATAGGAATTGGGAAAGCAAAGTTGTATGAGAAACAGACTCTGCTG ATAAAGTACTCATAGTCCAGACCAGAGAAATATAAATGGAAATAGGTTATATTTCAATAGTGATTGGTTCATCTAAA AGTCTCTGCTGTAAAGGAAATAAAGCATAGAGGTTTGAGCATGGACTTTGGAGTTGGACCAATCTGTGACTGATTCT TCGTTCTGCTACTTGCTTCCAATGTGACCTTGTACAAGTTTCTTGACATTCTCTGAGCCTCAGTTTCTCTACTGGTT GAATAATCCTTGATAGGATTGCAGTGGAAAATTAAATGAAATAATGTTAGCAAAGGTCCCAACATAATATTTGACTT GGAATTGAATGCCCATGGTAACCAGCATCATTTTCCTTCATGTGATGTCTTCTTATGCCTTTGAAAGAAAGTTACTT TATCAAATGTATAAATAAAGATCTGTTTATAGGTGATCTTTTTAATTTAGAAGAAATTCTGAGACACAAATAAAAAA AGAAATTTTTTAAAAAAAAAAAAAAAAAA (SEQ ID NO: 5)
[00337] >gi 1315138990lreflNP_001186704.11 carboxypeptidase D isoform 2 [Homo sapiens]
MRFVLSGNLHGGSWASYPFDDSPEHKATGIYSKTSDDEVFKYLAKAYASNHPIMKTGEPHCPGDEDETFKDGITNG AHWYDVEGGMQDYNYVWANCFEITLELSCCKYPPASQLRQEWENNRESLITLI EKVHIGVKGFVKDSITGSGLENAT I SVAGINHNITTGRFGDFYRLLVPGTYNLTVVLTGYMPLTVTNVVVKEGPATEVDFSLRPTVTSVI PDTTEAVSTAS TVAI PNILSGTSS SYQPIQPKDFHHHHFPDMEI FLRRFANEYPNITRLYSLGKSVESRELYVMEI SDNPGVHEPGEP EFKYIGNMHGNEWGRELLLNLI EYLCKNFGTDPEVTDLVHNTRIHLMPSMNPDGYEKSQEGDSI SVIGRNNSNNFD LNRNFPDQFVQITDPTQPETIAVMSWMKSYPFVLSANLHGGSLWNYPFDDDEQGLATYSKSPDDAVFQQIALSYSK ENSQMFQGRPCKNMYPNEYFPHGITNGASWYNVPGGMQDWNYLQTNCFEVTI ELGCVKYPLEKELPNFWEQNRRSLI QFMKQVHQGVRGFVLDATDGRGI LNATI SVAEINHPVTTYKTGDYWRLLVPGTYKITASARGYNPVTKNVTVKSEGA IQVNFTLVRS STDSNNESKKGKGASS STNDASDPTTKEFETLI KDLSAENGLESLMLRS SSNLALALYRYHSYKDLS EFLRGLVMNYPHITNLTNLGQSTEYRHIWSLEI SNKPNVSEPEEPKIRFVAGIHGNAPVGTELLLALAEFLCLNYKK NPAVTQLVDRTRIVIVPSLNPDGRERAQEKDCTSKI GQTNARGKDLDTDFTNNASQPETKAI I ENLIQKQDFSLSVA LDGGSMLVTYPYDKPVQTVENKETLKHLASLYANNHPSMHMGQPSCPNKSDENI PGGVMRGAEWHSHLGSMKDYSVT YGHCPEITVYTSCCYFPSAARLPSLWADNKRSLLSMLVEVHKGVHGFVKDKTGKPI SKAVIVLNEGIKVQTKEGGYF HVLLAPGVHNI IAIADGYQQQHSQVFVHHDAAS SWIVFDTDNRI FGLPRELWTVSGATMSALILTACI IWCICS I KSNRHKDGFHRLRQHHDEYEDEI RMMSTGSKKSLLSHEFQDETDTEEETLYSSKH (SEQ ID NO: 6)
[00338] Numerous SNPs have been reported for the CPD gene. A list of exemplary known SNPs is provided below. For each SNP, the wild-type sequence is provided (contig ref.) and any changes in the amino acid sequence of the encoded protein is described. MAF: Minor allele frequency (if known); Codon pos: position of the SNP within the respective codon (nucleotide 1, 2, or 3); AA pos: position of the affected amino acid residue within the CPD protein. gene model Contig Label Contig mrna protein snp count
(contig mRNA GRCh38 NT_010783.16 NM_001304.4 NP_001295.2 140, coding transcript):
Chr. mRNA dbSNP rs# „ dbSNP Protein Codon AA
. ■ j MAF Function „ ,
position pos cluster id allele residue pos pos
30385019 196 rsl 17723754 0.0190 synonymous T Gly [G] 3 12
contig ref. C Gly [G] 3 12
30385084 261 rs372685833 missense G Arg [R] 2 34 contig ref. A Lys [K] 2 34
30385101 278 rs557108475 0.0002 missense A He [I] 1 40 contig ref. G Val [V] 1 40
30385104 281 rs369436295 missense G Val [V] 1 41 contig ref. T Phe [F] 1 41
30385116 293 rs 1804932 missense T Ser [S] 1 45 contig ref. G Ala [A] 1 45
30385143 320 rs 193233043 0.0002 missense G Val [V] 1 54 contig ref. A He [I] 1 54
30385152 329 rs372998203 0.0008 missense G Ala [A] 1 57 contig ref. A Thr [T] 1 57
30385185 362 rs376246870 missense A Lys [K] 1 68 contig ref. G Glu [E] 1 68
30385193 370 rs200206773 missense G Leu [L] 3 70 contig ref. C Phe [F] 3 70
30385197 374 rs377027055 missense C His [H] 1 72 contig ref. G Asp [D] 1 72
30385211 388 rs370362858 synonymous T Asn [N] 3 76 contig ref. C Asn [N] 3 76
30385212 389 rs530827592 missense A Ser [S] 1 77 contig ref. G Gly [G] 1 77
30385215 392 rs373819167 missense A Thr [T] 1 78 contig ref. G Ala [A] 1 78
30385220 397 rs561370635 0.0002 synonymous C His [H] 3 79 contig ref. T His [H] 3 79
30385225 402 rs367857380 missense G Cys [C] 2 81 contig ref. A Tyr [Y] 2 81
30420842 415 rs200035413 0.0002 synonymous C Gly [G] 3 85 contig ref. T Gly [G] 3 85
30420843 416 rs 181949706 0.0002 missense A Ser [S] 1 86 contig ref. G Gly [G] 1 86
30420846 419 rs 146972525 missense G Val [V] 1 87 contig ref. A Met [M] 1 87
30420859 432 rs573932634 0.0002 missense G Ser [S] 2 91 contig ref. A Asn [N] 2 91
30420864 437 rs 113671462 missense A Met [M] 1 93 contig ref. G Val [V] 1 93
30420918 491 rs540974519 0.0002 missense T Ser [S] 1 111 contig ref. C Pro [P] 1 111
30420919 492 rs371151229 0.0002 missense T Leu [L] 2 111
contig ref. C Pro [P] 2 111
30420934 507 rs377211802 missense A Gin [Q] 2 116 contig ref. G Arg [R] 2 116
30420949 522 rs368820920 missense G Ser [S] 2 121 contig ref. A Asn [N] 2 121
30420955 528 rsl47588955 missense A His [H] 2 123 contig ref. G Arg [R] 2 123
30421700 593 rs201345496 0.0002 missense G Val [V] 1 145 contig ref. A He [I] 1 145
30421720 613 rs369470432 missense C Asp [D] 3 151 contig ref. G Glu [E] 3 151
30421727 620 rsl90169686 0.0002 missense G Ala [A] 1 154 contig ref. A Thr [T] 1 154
30421748 641 rs565159852 missense G Asp [D] 1 161 contig ref. A Asn [N] 1 161
30421758 651 rs544645153 0.0002 missense C Thr [T] 2 164 contig ref. T Ile [I] 2 164
30421770 663 rs 148993764 0.0008 missense C Thr [T] 2 168 contig ref. G Arg [R] 2 168
30421773 666 rs201240868 missense G Cys [C] 2 169 contig ref. T Phe [F] 2 169
30421777 670 rs 145806162 synonymous A Gly [G] 3 170 contig ref. T Gly [G] 3 170
30421808 701 rs574948338 0.0002 missense C His [H] 1 181 contig ref. T Tyr [Y] 1 181
30421814 707 rs373237520 missense T Phe [F] 1 183 contig ref. C Leu [L] 1 183
30422727 780 rsl7857301 missense G Gly [G] 2 207 contig ref. A Glu [E] 2 207
30422746 799 rsl l7331885 0.0034 synonymous A Arg [R] 3 213 contig ref. G Arg [R] 3 213
30422775 828 rs 192520453 0.0012 missense G Arg [R] 2 223 missense T Met [M] 2 223 contig ref. C Thr [T] 2 223
30422787 840 rs77951927 missense G Gly [G] 2 227 contig ref. T Val [V] 2 227
30422788 841 rs 149444194 0.0004 synonymous T Val [V] 3 227 contig ref. A Val [V] 3 227
30422798 851 rs 143814240 missense C Arg [R] 1 231 contig ref. A Ser [S] 1 231
30422804 857 rsl48156095 missense A He [I] 1 233
contig ref. G Val [V] 1 233
30422809 862 rs200417693 0.0002 synonymous A Ala [A] 3 234 contig ref. T Ala [A] 3 234
30422830 883 rs71372220 synonymous G Gly [G] 3 241 contig ref. A Gly [G] 3 241
30422831 884 rs372959172 missense G Ala [A] 1 242 contig ref. A Thr [T] 1 242
30422836 889 rsl41947353 synonymous T Ser [S] 3 243 contig ref. A Ser [S] 3 243
30422862 915 rs61743601 0.0298 missense G Arg [R] 2 252 contig ref. A Lys [K] 2 252
30422879 932 rsl7854355 missense A Asn [N] 1 258 contig ref. C His [H] 1 258
30422884 937 rs 138876763 synonymous T Phe [F] 3 259 contig ref. C Phe [F] 3 259
30422919 972 rs541307210 0.0002 missense G Ser [S] 2 271 contig ref. A Asn [N] 2 271
30422940 993 rs61745982 missense A Gin [Q] 2 278 contig ref. G Arg [R] 2 278
30423526 1097 rs531960935 missense G Val [V] 1 313 contig ref. A He [I] 1 313
30423546 1117 rs370537953 synonymous C Asn [N] 3 319 contig ref. T Asn [N] 3 319
30423589 1160 rs375154790 missense A He [I] 1 334 contig ref. C Leu [L] 1 334
30423605 1176 rs568492127 0.0002 missense A Glu [E] 2 339 contig ref. G Gly [G] 2 339
30423634 1205 rs370944637 missense T Tyr [Y] 1 349 contig ref. C His [H] 1 349
30423641 1212 rs535880946 0.0002 missense T He [I] 2 351 contig ref. C Thr [T] 2 351
30427424 1302 rs372336205 missense C Thr [T] 2 381 contig ref. G Ser [S] 2 381
30427468 1346 rs375764127 missense G Glu [E] 1 396 contig ref. C Gin [Q] 1 396
30427484 1362 rsl88883639 0.0006 missense T Met [M] 2 401 contig ref. C Thr [T] 2 401
30427494 1372 rs556234135 0.0002 missense C Asp [D] 3 404 contig ref. A Glu [E] 3 404
30427503 1381 rs374917563 synonymous C Ala [A] 3 407 contig ref. T Ala [A] 3 407
30427546 1424 rs 148834529 0.0002 missense G Asp [D] 1 422 contig ref. A Asn [N] 1 422
30427551 1429 rs541966951 0.0002 synonymous A Leu [L] 3 423 contig ref. G Leu [L] 3 423
30431778 1443 rsl42023021 missense C Ser [S] 2 428 contig ref. T Leu [L] 2 428
30431785 1450 rs 16965763 0.0012 synonymous G Val [V] 3 430 contig ref. T Val [V] 3 430
30431791 1456 rs 139252097 synonymous T Tyr [Y] 3 432 contig ref. C Tyr [Y] 3 432
30431809 1474 rs201471087 0.0002 synonymous G Glu [E] 3 438 contig ref. A Glu [E] 3 438
30431815 1480 rsl49755386 0.0014 synonymous G Gly [G] 3 440 contig ref. A Gly [G] 3 440
30431822 1487 rs567752059 missense T Ser [S] 1 443 contig ref. A Thr [T] 1 443
30431824 1489 rs 186958459 0.0002 synonymous G Thr [T] 3 443 contig ref. A Thr [T] 3 443
30431871 1536 rs 199780759 missense G Cys [C] 2 459 contig ref. C Ser [S] 2 459
30439050 1622 rs377554415 missense G Asp [D] 1 488 contig ref. A Asn [N] 1 488
30439062 1634 rs370666752 missense C Arg [R] 1 492 contig ref. T Trp [W] 1 492
30442354 1696 rs535181733 0.0002 synonymous C Val [V] 3 512 contig ref. G Val [V] 3 512
30442381 1723 rs61746216 synonymous C Tyr [Y] 3 521 contig ref. T Tyr [Y] 3 521
30442402 1744 rsl44616874 synonymous C Pro [P] 3 528 contig ref. A Pro [P] 3 528
30443813 1804 rs536025849 synonymous T Gly [G] 3 548 contig ref. C Gly [G] 3 548
30443814 1805 rsl48468921 0.0004 missense A He [I] 1 549 contig ref. G Val [V] 1 549
30443844 1835 rsl45254310 missense A Ser [S] 1 559 contig ref. G Gly [G] 1 559
30443862 1853 rs371672835 missense A Thr [T] 1 565 contig ref. G Ala [A] 1 565
30443868 1859 rs 150162461 0.0002 missense G Val [V] 1 567 contig ref. A He [I] 1 567
30443894 1885 rs 142098304 synonymous G Pro [P] 3 575
contig ref. A Pro [P] 3 575
30443933 1924 rs558741330 0.0002 synonymous A Leu [L] 3 588 contig ref. G Leu [L] 3 588
30443967 1958 rs376371688 nonsense T 1 600 contig ref. C Arg [R] 1 600
30445737 2009 rs61733802 missense G Val [V] 1 617 contig ref. A He [I] 1 617
30445770 2042 rs 185549649 0.0002 missense G Ala [A] 1 628 contig ref. A Thr [T] 1 628
30445783 2055 rs527978533 0.0002 missense G Ser [S] 2 632 contig ref. A Asn [N] 2 632
30445822 2094 rs552326377 0.0002 missense G Ser [S] 2 645 contig ref. A Asn [N] 2 645
30445838 2110 rs9900046 synonymous C Pro [P] 3 650 contig ref. A Pro [P] 3 650
30445839 2111 rs28396255 missense C Pro [P] 1 651 contig ref. A Thr [T] 1 651
30445840 2112 rs373014036 0.0002 missense T Ile [I] 2 651 contig ref. C Thr [T] 2 651
30445842 2114 rs77379543 0.0004 missense T Ser [S] 1 652 contig ref. A Thr [T] 1 652
30445843 2115 rs 1860543 0.0010 missense T Ile [I] 2 652 contig ref. C Thr [T] 2 652
30445880 2152 rs 187666987 0.0004 synonymous A Ala [A] 3 664 contig ref. G Ala [A] 3 664
30445881 2153 rs535600272 0.0002 nonsense T 1 665 contig ref. G Glu [E] 1 665
30445908 2180 rs 192769344 0.0002 missense T Cys [C] 1 674 contig ref. C Arg [R] 1 674
30445909 2181 rs200096968 missense A His [H] 2 674 contig ref. G Arg [R] 2 674
30445932 2204 rs201829180 missense T Ser [S] 1 682 contig ref. G Ala [A] 1 682
30445968 2240 rs201995465 0.0002 missense C Gin [Q] 1 694 contig ref. G Glu [E] 1 694
30449570 2310 rs 185947591 missense C Ala [A] 2 717 contig ref. A Glu [E] 2 717
30449613 2353 rsl 11536427 synonymous C Asn [N] 3 731 contig ref. T Asn [N] 3 731
30449640 2380 rsl51154082 missense G Met [M] 3 740 contig ref. T He [I] 3 740
30449657 2397 rs 1043140 missense C Thr [T] 2 746 contig ref. T Ile [I] 2 746
30449714 2454 rs370754352 missense T Phe [F] 2 765 contig ref. G Cys [C] 2 765
30449735 2475 rs77713107 0.0002 missense A Gin [Q] 2 772 contig ref. C Pro [P] 2 772
30451763 2541 rsl40102865 missense T Leu [L] 2 794 contig ref. G Arg [R] 2 794
30451799 2577 rs72809814 0.0002 missense C Thr [T] 2 806 contig ref. T Ile [I] 2 806
30451817 2595 rsl50285239 missense A His [H] 2 812 contig ref. G Arg [R] 2 812
30455343 2629 rs 138340045 synonymous C Asn [N] 3 823 contig ref. T Asn [N] 3 823
30455344 2630 rs558201013 0.0002 missense A Thr [T] 1 824 contig ref. G Ala [A] 1 824
30455454 2740 rs76006071 missense A Glu [E] 3 860 contig ref. C Asp [D] 3 860
30456257 2758 rsl41390381 0.0002 synonymous A Val [V] 3 866 contig ref. G Val [V] 3 866
30456275 2776 rs376102015 synonymous A Leu [L] 3 872 contig ref. G Leu [L] 3 872
30456321 2822 rs373920031 missense G Val [V] 1 888 contig ref. A Met [M] 1 888
30456328 2829 rsl45126411 0.0010 missense T Leu [L] 2 890 contig ref. A Gin [Q] 2 890
30456466 2857 rs 138943830 missense C Asp [D] 3 899 contig ref. G Glu [E] 3 899
30456477 2868 rs201697680 0.0002 missense A Glu [E] 2 903 contig ref. G Gly [G] 2 903
30461252 2990 rs201346549 missense T Ser [S] 1 944 contig ref. G Ala [A] 1 944
30461272 3010 rs 145197774 0.0002 nonsense A 3 950 contig ref. G Trp ;w] 3 950
30461284 3022 rs562746499 0.0002 synonymous A Lys [K] 3 954 contig ref. G Lys [K] 3 954
30461285 3023 rs201106434 missense G Gly [G] 1 955 contig ref. A Arg [R] 1 955
30461302 3040 rs530054675 0.0002 missense C He [I] 3 960 contig ref. G Met [M] 3 960
30461955 3128 rs376251337 missense C Arg [R] 1 990
contig ref. G Gly [G] 1 990
30461995 3168 rs370902603 missense C Ala [A] 2 1003 contig ref. T Val [V] 2 1003
30462007 3180 rs 146827629 0.0002 missense T Leu [L] 2 1007 contig ref. C Pro [P] 2 1007
30462051 3224 rs 142087464 missense G Glu [E] 1 1022 contig ref. C Gin [Q] 1 1022
30462428 3294 rs568380104 0.0002 missense A Gin [Q] 2 1045 contig ref. G Arg [R] 2 1045
30462464 3330 rs 146329476 missense C Ala [A] 2 1057 contig ref. T Val [V] 2 1057
30464601 3349 rs75135188 0.0150 synonymous A Ser [S] 3 1063 contig ref. G Ser [S] 3 1063
30464644 3392 rs 148102054 0.0002 missense G Val [V] 1 1078 contig ref. A He [I] 1 1078
30464654 3402 rs 141840596 missense G Ser [S] 2 1081 contig ref. A Asn [N] 2 1081
30464656 3404 rs370170270 missense G Gly [G] 1 1082 contig ref. A Arg [R] 1 1082
30464716 3464 rs 199922587 missense T Cys [C] 1 1102 contig ref. C Arg [R] 1 1102
30464717 3465 rs367904758 missense A His [H] 2 1102 contig ref. G Arg [R] 2 1102
30464728 3476 rsl 15003383 0.0004 missense G Ala [A] 1 1106 contig ref. A Thr [T] 1 1106
30464730 3478 rs200857905 synonymous G Thr [T] 3 1106 contig ref. C Thr [T] 3 1106
30464731 3479 rs200892550 missense A Ser [S] 1 1107 contig ref. G Gly [G] 1 1107
30464811 3559 rs375525159 synonymous C His [H] 3 1133 contig ref. T His [H] 3 1133
30465049 3797 rs71902832 0.0615 -
Table 6. Exemplary SNPs and variant alleles of the CPD gene.
Claims
1. A method for treating obesity in a subject carrying a variant allele of the CPD gene, comprising administering a therapeutically effective amount of perindopril, or a
pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject.
2. A method for treating obesity in a subject carrying a variant allele of the CPD gene, comprising administering a therapeutically effective amount a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the sub ect;
3. A method for treating obesity in a subject carrying a variant allele of the CPD gene, comprising administering a therapeutically effective amount of a compound of Formulae III-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject;
ormula III (also referred to as RB 106);
4. The method of any one of claims 1-3, wherein the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
5. The method of any one of claims 1 and 4, wherein the method comprises administering an effective amount of perindopril arginine to the subject.
6. The method of any one of claims 1 and 4-5, wherein the method comprises administering an effective amount of perindopril erbumine to the subject.
7. The method of any one of claims 1-6, wherein the method comprises administering about 0.01 mg, about 0.02 mg, about 0.03 mg, about 0.04 mg, about 0.05 mg, about 0.06 mg, about 0.07 mg, about 0.08 mg, about 0.09 mg, or about 0.1 mg of the compound, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof per kg body weight of the subject.
8. The method of any one of claims 1-7, wherein the subject has a BMI (kg/m2) of > 30.00.
9. The method of any one of claims 1-7, wherein the subject has a BMI (kg/m ) of 30.00-34.99 (obesity class I).
10. The method of any one of claims 1-7, wherein the subject has a BMI of 35.00-39.99 (obesity class II).
11. The method of any one of claims 1-7, wherein the subject has a BMI of > 40.0 (obesity class III).
12. The method of any one of claims 1-11, wherein the method further comprises administering an effective amount of one or more agents selected from the group consisting of: an anti-obesity agent, an anti-hypertensive agent, a diuretic agent, and a antidiabetic agent.
13. The method of claim 12, wherein the antidiabetic agent is administered orally.
14. The method of claim 12, wherein the compound, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered simultaneously.
15. The method of claim 12, wherein the compound, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered separately.
16. The method of any one of claims 1-15, wherein the method further comprises determining whether the subject carries a variant allele of the CPD gene.
17. The method of claim 16, wherein the method comprises detecting one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406 in an allele of the CPD gene of the subject.
18. The method of any one of claims 1-16, wherein the method further comprises selecting the subject for administration of the compound based on the subject carrying a variant allele of the CPD gene.
19. The method of any one of claims 1-18, wherein the subject is male.
20. A method for treating obesity in a subject, the method comprising
(i) determining whether the subject carries a variant allele of the CPD gene; and
(ii) if the subject is determined to carry a variant allele of the CPD gene, administering effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject.
21. A method for treating obesity in a subject, the method comprising
(i) determining whether the subject carries a variant allele of the CPD gene; and
(ii) if the subject is determined to carry a variant allele of the CPD gene, administering effective amount of a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer tautomer, polymorph, hydrate, or solvate thereof to the subject;
22. A method for treating obesity in a subject, the method comprising
(i) determining whether the subject carries a variant allele of the CPD gene; and
(ii) if the subject is determined to carry a variant allele of the CPD gene, administering an effective amount of a compound of Formulae III-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject;
23. The method of any one of claims 20-22, wherein step (i) comprises detecting the variant allele of the CPD gene in the genome of a cell of the subject.
24. The method of any one of claims 20-23, wherein the method further comprises obtaining a cell or tissue sample from the subject.
25. The method of any one of claims 20-24, wherein the method comprises isolating genomic DNA from a cell or tissue obtained from the subject.
26. The method of any one of claims 20-25, wherein step (i) comprises performing a genotyping assay on genomic DNA obtained from the subject.
27. The method of claim 26, wherein the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay.
28. The method of any one of claims 20-27, wherein the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
29. The method of any one of claims 20-28, wherein the subject has a BMI (kg/m2) of > 30.00.
30. The method of any one of claims 20-28, wherein the subject has a BMI (kg/m ) of 30.00- 34.99 (obesity class I).
31. The method of any one of claims 20-28, wherein the subject has a BMI of 35.00-39.99 (obesity class II).
32. The method of any one of claims 20-28, wherein the subject has a BMI of > 40.0 (obesity class III).
33. The method of any one of claims 20 and 23-32, wherein the method comprises administering an effective amount of perindopril arginine or of perindopril erbumine to the subject.
34. The method of any one of claims 20-32, wherein the method further comprises administering an effective amount of one or more agents selected from the group consisting of: an anti-obesity agent, an anti-hypertensive agent, a diuretic agent, and a antidiabetic agent.
35. The method of claim 34, wherein the antidiabetic agent is administered orally.
36. The method of claim 34, wherein the compound, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered simultaneously.
37. The method of claim 34, wherein the compound, or the pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, and the one or more agents are administered separately.
38. The method of any one of claims 20-37, wherein the method further comprises selecting the subject for administration of the compound based on the subject carrying a variant allele of the CPD gene.
39. The method of any one of claims 20-38, wherein the subject is male.
40. A method for identifying a subject who is predisposed to obesity, comprising
(i) determining whether the subject carries a variant allele of the CPD gene; and
(ii) if the subject is determined to carry variant allele of the CPD gene, identifying the subject as predisposed to obesity.
41. The method of claim 40, wherein step (i) comprises detecting the variant allele of the CPD gene in the genome of a cell of the subject.
42. The method of claim 40 or 41, wherein the method further comprises obtaining a cell or tissue sample from the subject.
43. The method of any one of claims 40-42, wherein the method comprises isolating genomic DNA from a cell or tissue obtained from the subject.
44. The method of any one of claims 40-43, wherein step (i) comprises performing a genotyping assay on genomic DNA obtained from the subject.
45. The method of claim 44, wherein the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay.
46. The method of claim 45, wherein the allele-specific PCR assay is an intercalating dye assay, a FRET primer assay, or an ALPHASCREEN™ assay.
47. The method of claim 45, wherein the primer extension assay is an ARMS (amplification refractory mutation system) assay, a kinetic or real-time PCR assay, a SNPSTREAM™ assay, a GENETIC BIT ANALYSIS™ (GBA) assay, a multiplex minisequencing assay, a
SNAPSHOT™ assay, a PYROSEQUENCING™ assay, a MASSEXTEND™ assay, a
MASSARRAY™ assay, a MALDI mass spectrometry-based assay, a microarray
minisequencing assay, an APEX (arrayed primer extension) assay, a sequence specific priming (SSP) assay, a microarray primer extension assay, a tag array assay, a coded microsphere assay, a template-directed incorporation (TDI) assay, or a fluorescence polarization assay.
48. The method of claim 45, wherein the oligonucleotide ligation assay is a colorimetric oligonucleotide ligation assay (OLA), a sequence-coded OLA, a microarray ligation assay, a ligase chain reaction assay, a padlock probe assay, or a rolling circle amplification assay.
49. The method of claim 45, wherein the hybridization assay is a reverse dot blot assay, a line probe assay (LiPA), a microarray assay, a dynamic allele-specific hybridization (DASH) assay, a PNA or locked nucleic acid (LNA) probe assay, a TAQMAN™ assay, or a molecular beacon assay.
50. The method of claim 45, wherein the endonuclease cleavage assay is a restriction site analysis (RFLP), or an INVADER™ assay.
51. The method of any one of claims 40-50, wherein the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
52. The method of any one of claims 40-51 , wherein the method further comprises determining whether the subject is obese.
53. The method of any one of claims 40-52, wherein the subject is not obese.
54. The method of claim 53, wherein the method further comprises administering healthcare to the subject to prevent the subject from becoming obese.
55. The method of claim 53 or 54, wherein the method further comprises monitoring the body weight of the subject and, if the subject is determined to be obese during the monitoring, selecting the subject to treatment with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; or a compound of Formulae III-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof.
56. The method of any one of claims 40-55, wherein the method further comprises
administering an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; or a compound of Formulae III-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject.
57. The method of any one of claims 40-56, wherein the subject is male.
58. A method for selecting a subject for treatment of obesity with perindopril or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, the method comprising
(i) determining whether an obese subject carries a variant allele of the CPD gene; and
(ii) if the subject is determined to carry a CPD gene, selecting the subject for treatment of obesity with perindopril or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof.
59. A method for selecting a subject for treatment of obesity with a compound of Formula II or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, the method comprising
(i) determining whether an obese subject carries a variant allele of the CPD gene; and
(ii) if the subject is determined to carry a CPD gene, selecting the subject for treatment of obesity with the compound of Formula II or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof
Formula II
60. A method for selecting a subject for treatment of obesity with a compound of Formulae III- V or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, the method comprising
(i) determining whether an obese subject carries a variant allele of the CPD gene; and
(ii) if the subject is determined to carry a CPD gene, selecting the subject for treatment of obesity with the compound of Formulae III-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof;
61. The method of any one of claims 58-60, wherein the method further comprises
administering an effective amount of the compound, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof to the subject.
62. The method of any one of claims 58-61, wherein step (i) comprises performing a genotyping assay.
63. The method of claim 62, wherein the genotyping assay is an allele-specific PCR assay, a primer extension assay, an oligonucleotide ligation assay, a hybridization assay, or an endonuclease assay.
64. The method of claim 63, wherein the allele-specific PCR assay is an intercalating dye assay, a FRET primer assay, or an ALPHASCREEN™ assay.
65. The method of claim 64, wherein the primer extension assay is an ARMS (amplification refractory mutation system) assay, a kinetic or real-time PCR assay, a SNPSTREAM™ assay, a GENETIC BIT ANALYSIS™ (GBA) assay, a multiplex minisequencing assay, a
SNAPSHOT™ assay, a PYROSEQUENCING™ assay, a MASSEXTEND™ assay, a
MASSARRAY™ assay, a MALDI mass spectrometry-based assay, a microarray
minisequencing assay, an APEX (arrayed primer extension) assay, a sequence specific priming (SSP) assay, a microarray primer extension assay, a tag array assay, a coded microsphere assay, a template-directed incorporation (TDI) assay, or a fluorescence polarization assay.
66. The method of claim 64, wherein the oligonucleotide ligation assay is a colorimetric oligonucleotide ligation assay (OLA), a sequence-coded OLA, a microarray ligation assay, a ligase chain reaction assay, a padlock probe assay, or a rolling circle amplification assay.
67. The method of claim 64, wherein the hybridization assay is a reverse dot blot assay, a line probe assay (LiPA), a microarray assay, a dynamic allele-specific hybridization (DASH) assay, a PNA or locked nucleic acid (LNA) probe assay, a TAQMAN™ assay, or a molecular beacon assay.
68. The method of claim 64, wherein the endonuclease cleavage assay is a restriction site analysis (RFLP), or an INVADER™ assay.
69. The method of any one of claims 58-68, wherein the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
70. The method of any one of claims 58-69, wherein the subject is male.
71. A pharmaceutical composition comprising an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for use in the treatment of obesity in a subject.
72. A pharmaceutical composition comprising an effective amount of a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for use in the treatment of obesit in a subject;
Formula II
74. A pharmaceutical composition comprising an effective amount of perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for administration to an obese subject.
75. A pharmaceutical composition comprising an effective amount of a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, for administration to an obese sub ect;
Formula II
77. The pharmaceutical composition of any one of claims 71-76, wherein the pharmaceutical composition further comprises an effective amount of one or more agents selected from the group consisting of an anti-obesity agent, an anti-hypertensive agent, a diuretic agent, and an antidiabetic agent.
78. The pharmaceutical composition of any one of claims 71-77, wherein (i) the subject is male; (ii) the subject carries a variant allele of the CPD gene; and/or (iii) the subject has been selected for treatment with the compound, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof based on the subject carrying a variant allele of the CPD gene.
79. The pharmaceutical composition of claim 78, wherein the variant allele of the CPD gene comprises one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
80. The pharmaceutical composition of any one of claims 71, 74, and 77-79, wherein the composition comprises an effective amount of perindopril arginine or of perindopril erbumine.
81. The pharmaceutical composition of any one of claims 71-80, wherein the composition comprises about 0.01 mg, about 0.02 mg, about 0.03 mg, about 0.04 mg, about 0.05 mg, about 0.06 mg, about 0.07 mg, about 0.08 mg, about 0.09 mg, or about 0.1 mg of the compound, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof per kg body weight of the subject.
82. A kit for detecting a variant allele of the CPD gene and/or for identifying a subject who is predisposed to obesity, comprising reagents for performing a genotyping assay for detecting a variant allele of the CPD gene in genomic DNA obtained from the subject.
83. A kit for identifying a subject who would benefit from treatment with perindopril, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; a compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof; or a compound of Formulae III-V, or a pharmaceutically acceptable salt, stereoisomer, tautomer, polymorph, hydrate, or solvate thereof, the kit comprising reagents for performing a genotyping assay for detecting a variant allele of the CPD gene in genomic DNA obtained from the subject.
84. The kit of claim 82 or 83, wherein the kit comprises a primer, primer pair, or probe for detecting the variant allele of the CPD gene.
85. The kit of any one of claims 82-84, wherein the kit comprises a primer, primer pair, or probe for detecting one or more SNPs selected from the group consisting of rs9913111, rs719601, rs2041374, rs4343337, rs4795551, rs9913237, rs2253256, rs6505188, rs9911455 and rs9406.
86. The method of any one of claims 1-70, wherein the subject does not have hypertension.
87. The method of any one of claims 1-70, or 86 wherein the subject does not have
cardiovascular disease.
88. The method of any one of claims 1-70, 86, or 87, wherein the subject does not have coronary artery disease (CAD).
89. A compound of Formula II, or a pharmaceutically acceptable salt, stereoisomer, tautomer, olymorph, hydrate, or solvate thereof;
179/190
180/190
181/190
182/190
183/190
184/190
185/190
186/190
187/190
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201562157329P | 2015-05-05 | 2015-05-05 | |
| US62/157,329 | 2015-05-05 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2016178591A2 true WO2016178591A2 (en) | 2016-11-10 |
| WO2016178591A3 WO2016178591A3 (en) | 2017-11-23 |
Family
ID=56148631
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/PT2016/050008 WO2016178591A2 (en) | 2015-05-05 | 2016-05-05 | Genetic markers and treatment of male obesity |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2016178591A2 (en) |
Citations (66)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4270537A (en) | 1979-11-19 | 1981-06-02 | Romaine Richard A | Automatic hypodermic syringe |
| EP0049658A1 (en) | 1980-10-02 | 1982-04-14 | Adir | Substituted imino diacids, their preparation and pharmaceutical preparations containing them |
| EP0116842A2 (en) | 1983-01-22 | 1984-08-29 | Boehringer Ingelheim Kg | Amino-acids derivatives, process for their preparation and their use |
| US4596556A (en) | 1985-03-25 | 1986-06-24 | Bioject, Inc. | Hypodermic injection apparatus |
| US4790824A (en) | 1987-06-19 | 1988-12-13 | Bioject, Inc. | Non-invasive hypodermic injection device |
| EP0308341A1 (en) | 1987-09-17 | 1989-03-22 | Adir Et Compagnie | Process for the industrial synthesis of perindopril and for its principal synthesis intermediates |
| EP0309324A1 (en) | 1987-09-17 | 1989-03-29 | Adir Et Compagnie | Process for the preparation of N-alkylated amino acids and their esters, use in the synthesis of carboxyalkyl dipeptides |
| US4886499A (en) | 1986-12-18 | 1989-12-12 | Hoffmann-La Roche Inc. | Portable injection appliance |
| US4940460A (en) | 1987-06-19 | 1990-07-10 | Bioject, Inc. | Patient-fillable and non-invasive hypodermic injection device assembly |
| US4941880A (en) | 1987-06-19 | 1990-07-17 | Bioject, Inc. | Pre-filled ampule and non-invasive hypodermic injection device assembly |
| US5015235A (en) | 1987-02-20 | 1991-05-14 | National Carpet Equipment, Inc. | Syringe needle combination |
| US5064413A (en) | 1989-11-09 | 1991-11-12 | Bioject, Inc. | Needleless hypodermic injection device |
| US5141496A (en) | 1988-11-03 | 1992-08-25 | Tino Dalto | Spring impelled syringe guide with skin penetration depth adjustment |
| US5190521A (en) | 1990-08-22 | 1993-03-02 | Tecnol Medical Products, Inc. | Apparatus and method for raising a skin wheal and anesthetizing skin |
| US5312335A (en) | 1989-11-09 | 1994-05-17 | Bioject Inc. | Needleless hypodermic injection device |
| US5328483A (en) | 1992-02-27 | 1994-07-12 | Jacoby Richard M | Intradermal injection device with medication and needle guard |
| US5334144A (en) | 1992-10-30 | 1994-08-02 | Becton, Dickinson And Company | Single use disposable needleless injector |
| US5339163A (en) | 1988-03-16 | 1994-08-16 | Canon Kabushiki Kaisha | Automatic exposure control device using plural image plane detection areas |
| US5383851A (en) | 1992-07-24 | 1995-01-24 | Bioject Inc. | Needleless hypodermic injection device |
| US5417662A (en) | 1991-09-13 | 1995-05-23 | Pharmacia Ab | Injection needle arrangement |
| US5466220A (en) | 1994-03-08 | 1995-11-14 | Bioject, Inc. | Drug vial mixing and transfer device |
| US5480381A (en) | 1991-08-23 | 1996-01-02 | Weston Medical Limited | Needle-less injector |
| US5527288A (en) | 1990-12-13 | 1996-06-18 | Elan Medical Technologies Limited | Intradermal drug delivery device and method for intradermal delivery of drugs |
| US5569189A (en) | 1992-09-28 | 1996-10-29 | Equidyne Systems, Inc. | hypodermic jet injector |
| US5599302A (en) | 1995-01-09 | 1997-02-04 | Medi-Ject Corporation | Medical injection system and method, gas spring thereof and launching device using gas spring |
| WO1997013537A1 (en) | 1995-10-10 | 1997-04-17 | Visionary Medical Products Corporation | Gas pressured needle-less injection device |
| US5649912A (en) | 1994-03-07 | 1997-07-22 | Bioject, Inc. | Ampule filling device |
| WO1997037705A1 (en) | 1996-04-11 | 1997-10-16 | Weston Medical Limited | Spring-powered dispensing device for medical purposes |
| US5893397A (en) | 1996-01-12 | 1999-04-13 | Bioject Inc. | Medication vial/syringe liquid-transfer apparatus |
| WO1999034850A1 (en) | 1998-01-08 | 1999-07-15 | Fiderm S.R.L. | Device for controlling the penetration depth of a needle, for application to an injection syringe |
| US5993412A (en) | 1997-05-19 | 1999-11-30 | Bioject, Inc. | Injection apparatus |
| WO2001058868A1 (en) | 2000-04-06 | 2001-08-16 | Les Laboratoires Servier | Method for synthesis of perindopril and its pharmaceutically acceptable salts |
| EP1319668A1 (en) | 2003-03-12 | 2003-06-18 | Les Laboratoires Servier | Method for synthesis of (2S,3aS,7aS)-1-((S)-alanyl)-octahydro-1H-indole-2-carboxylic acid derivatives and use in the synthesis of perindopril |
| EP1321471A1 (en) | 2003-03-12 | 2003-06-25 | Les Laboratoires Servier | Method for synthesis of perindopril and its pharmaceutically acceptable salts |
| WO2004046172A1 (en) | 2002-11-18 | 2004-06-03 | Cipla Ltd. | Perindopril |
| WO2004075889A1 (en) | 2003-02-28 | 2004-09-10 | Lupin Limited | Process for preparation of perindopril and salts thereof |
| WO2004099138A2 (en) | 2003-05-12 | 2004-11-18 | Cipla Limited | Process for the preparation of perindopril |
| WO2005012333A2 (en) | 2003-07-31 | 2005-02-10 | Les Laboratoires Servier | Novel method for the synthesis of perindopril and the pharmaceutically acceptable salts thereof |
| WO2005023843A1 (en) | 2003-08-29 | 2005-03-17 | Les Laboratoires Servier | Novel method for the synthesis of perindopril and the pharmaceutically-acceptable salts thereof |
| WO2005037788A1 (en) | 2003-10-21 | 2005-04-28 | Lupin Ltd. | Novel method for preparation of crystalline perindopril erbumine |
| US20050119492A1 (en) | 2002-01-30 | 2005-06-02 | Guyla Simig | Process for the preparation of high purity perindopril and intermediates useful in the synthesis |
| WO2005054276A1 (en) | 2003-11-19 | 2005-06-16 | Les Laboratoires Servier | Method for synthesis of perindopril and the pharmaceutically-acceptable salts thereof |
| WO2005066198A1 (en) | 2003-12-10 | 2005-07-21 | Les Laboratoires Servier | Method for the synthesis of perindopril and the pharmaceutically-acceptable salts thereof |
| WO2005100317A1 (en) | 2004-04-13 | 2005-10-27 | Neopharma Limited | Process for the preparation of perindopril |
| WO2006070276A1 (en) | 2004-12-31 | 2006-07-06 | Quimica Sintetica, S.A. | Process for preparing perindopril erbumine |
| US20060189813A1 (en) | 2003-07-31 | 2006-08-24 | Claude Fugier | Novel method for the synthesis of perinidopril and the pharmaceutically acceptable salts thereof |
| WO2006101462A2 (en) | 2005-03-22 | 2006-09-28 | Vulm, A.S. | Pharmaceutical preparation comprising perindopril erbumine and method of preparation and stabilisation thereof |
| WO2007002012A1 (en) | 2005-06-21 | 2007-01-04 | Ams Research Corporation | Apparatus for securing a urethral sling to pubic bone |
| US7179833B2 (en) | 2003-06-30 | 2007-02-20 | Les Laboratoires Servier | Method of synthesising perindopril and the pharmaceutically acceptable salts thereof |
| WO2007020009A1 (en) | 2005-08-12 | 2007-02-22 | Sandoz Ag | New crystalline form of perindopril erbumine |
| US7183308B1 (en) | 2003-08-29 | 2007-02-27 | Les Laboratoires Servier | Metod for the synthesis of perindopril and the pharmaceutically acceptable salts thereof |
| WO2007025695A1 (en) | 2005-08-30 | 2007-03-08 | Lek Pharmaceuticals D.D. | Pharmaceutical composition comprising perindopril or its salts |
| WO2007058634A1 (en) | 2005-11-17 | 2007-05-24 | Diagen Smartno Pri Ljubljani, D.O.O. | Stable formulation of amorphous perindopril salts, a process for their preparation, especially industrial preparation, and their use in the therapy of hypertension |
| US20070172524A1 (en) | 2004-03-29 | 2007-07-26 | Krka, Tovarna Zdravil, D.D., Novo Mesto | Process for preparing a solid pharmaceutical composition |
| WO2007088035A1 (en) | 2006-02-02 | 2007-08-09 | Lek Pharmaceuticals D.D. | A pharmaceutical composition comprising perindopril |
| WO2007092758A2 (en) | 2006-02-03 | 2007-08-16 | Dr. Reddy's Laboratories Ltd. | Crystalline forms of perindopril erbumine |
| WO2007099216A2 (en) | 2006-02-28 | 2007-09-07 | Les Laboratoires Servier | β-CRYSTALLINE FORM OF PERINDOPRIL ARGININE SALT, METHOD FOR MAKING SAME, AND PHARMACEUTICAL COMPOSITIONS CONTAINING SAME |
| US20080051584A1 (en) | 2004-05-14 | 2008-02-28 | Les Laboratoires Servier | Process For The Preparation Of Perindopril And Salts Thereof |
| WO2008114270A1 (en) | 2007-03-22 | 2008-09-25 | Aarti Healthcare Limited | Process for the preparation of perindopril erbumine salt and novel polymorph (s) thereof |
| WO2008119838A1 (en) | 2007-04-03 | 2008-10-09 | Centre National De La Recherche Scientifique (Cnrs) | Fto gene polymorphisms associated to obesity and/or type ii diabetes |
| US7534896B2 (en) | 2003-08-29 | 2009-05-19 | Les Laboratories Servier | Process for the synthesis of perindopril and its pharmaceutically acceptable salts |
| US20090162420A1 (en) | 2005-12-05 | 2009-06-25 | Karin Klokkers | Matrix-Controlled Transdermal System Comprising Salts of ACE Inhibitor Dicarboxylic Acids |
| WO2009157018A2 (en) | 2008-06-24 | 2009-12-30 | Matrix Laboratories Ltd | Novel polymorphic forms of perindopril (l)-arginine and process for the preparation thereof |
| WO2011058232A1 (en) | 2009-11-16 | 2011-05-19 | Mas-Metabolic Analytical Services Oy | Nutrigenetic biomarkers for obesity and type 2 diabetes |
| WO2012044189A1 (en) | 2010-09-29 | 2012-04-05 | Instituto Superior Técnico | A new hydrated crystalline form of perindopril erbumine, methods for its preparation and its use in pharmaceutical preparations |
| WO2013102740A1 (en) | 2012-01-05 | 2013-07-11 | Les Laboratoires Servier | Method for the preparation of perindopril arginine salt |
Family Cites Families (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3177130D1 (en) * | 1980-08-30 | 1990-01-11 | Hoechst Ag | AMINO ACID DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF, MEANS CONTAINING THEM AND THE USE THEREOF. |
| US7985571B1 (en) * | 2002-06-07 | 2011-07-26 | Ryogen Llc | Isolated genomic polynucleotide fragments from chromosome 17 that encode human carboxypeptidase D |
| US20070038386A1 (en) * | 2003-08-05 | 2007-02-15 | Schadt Eric E | Computer systems and methods for inferring casuality from cellular constituent abundance data |
-
2016
- 2016-05-05 WO PCT/PT2016/050008 patent/WO2016178591A2/en active Application Filing
Patent Citations (85)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4270537A (en) | 1979-11-19 | 1981-06-02 | Romaine Richard A | Automatic hypodermic syringe |
| EP0049658A1 (en) | 1980-10-02 | 1982-04-14 | Adir | Substituted imino diacids, their preparation and pharmaceutical preparations containing them |
| EP0116842A2 (en) | 1983-01-22 | 1984-08-29 | Boehringer Ingelheim Kg | Amino-acids derivatives, process for their preparation and their use |
| US4596556A (en) | 1985-03-25 | 1986-06-24 | Bioject, Inc. | Hypodermic injection apparatus |
| US4886499A (en) | 1986-12-18 | 1989-12-12 | Hoffmann-La Roche Inc. | Portable injection appliance |
| US5015235A (en) | 1987-02-20 | 1991-05-14 | National Carpet Equipment, Inc. | Syringe needle combination |
| US4940460A (en) | 1987-06-19 | 1990-07-10 | Bioject, Inc. | Patient-fillable and non-invasive hypodermic injection device assembly |
| US4790824A (en) | 1987-06-19 | 1988-12-13 | Bioject, Inc. | Non-invasive hypodermic injection device |
| US4941880A (en) | 1987-06-19 | 1990-07-17 | Bioject, Inc. | Pre-filled ampule and non-invasive hypodermic injection device assembly |
| US4914214A (en) | 1987-09-17 | 1990-04-03 | Adir Et Cie | Process for the industrial synthesis of perindopril |
| EP0309324A1 (en) | 1987-09-17 | 1989-03-29 | Adir Et Compagnie | Process for the preparation of N-alkylated amino acids and their esters, use in the synthesis of carboxyalkyl dipeptides |
| EP0308341A1 (en) | 1987-09-17 | 1989-03-22 | Adir Et Compagnie | Process for the industrial synthesis of perindopril and for its principal synthesis intermediates |
| US5339163A (en) | 1988-03-16 | 1994-08-16 | Canon Kabushiki Kaisha | Automatic exposure control device using plural image plane detection areas |
| US5141496A (en) | 1988-11-03 | 1992-08-25 | Tino Dalto | Spring impelled syringe guide with skin penetration depth adjustment |
| US5503627A (en) | 1989-11-09 | 1996-04-02 | Bioject, Inc. | Ampule for needleless injection |
| US5064413A (en) | 1989-11-09 | 1991-11-12 | Bioject, Inc. | Needleless hypodermic injection device |
| US5312335A (en) | 1989-11-09 | 1994-05-17 | Bioject Inc. | Needleless hypodermic injection device |
| US5190521A (en) | 1990-08-22 | 1993-03-02 | Tecnol Medical Products, Inc. | Apparatus and method for raising a skin wheal and anesthetizing skin |
| US5527288A (en) | 1990-12-13 | 1996-06-18 | Elan Medical Technologies Limited | Intradermal drug delivery device and method for intradermal delivery of drugs |
| US5480381A (en) | 1991-08-23 | 1996-01-02 | Weston Medical Limited | Needle-less injector |
| US5417662A (en) | 1991-09-13 | 1995-05-23 | Pharmacia Ab | Injection needle arrangement |
| US5328483A (en) | 1992-02-27 | 1994-07-12 | Jacoby Richard M | Intradermal injection device with medication and needle guard |
| US5383851A (en) | 1992-07-24 | 1995-01-24 | Bioject Inc. | Needleless hypodermic injection device |
| US5520639A (en) | 1992-07-24 | 1996-05-28 | Bioject, Inc. | Needleless hypodermic injection methods and device |
| US5704911A (en) | 1992-09-28 | 1998-01-06 | Equidyne Systems, Inc. | Needleless hypodermic jet injector |
| US5569189A (en) | 1992-09-28 | 1996-10-29 | Equidyne Systems, Inc. | hypodermic jet injector |
| US5334144A (en) | 1992-10-30 | 1994-08-02 | Becton, Dickinson And Company | Single use disposable needleless injector |
| US5649912A (en) | 1994-03-07 | 1997-07-22 | Bioject, Inc. | Ampule filling device |
| US5466220A (en) | 1994-03-08 | 1995-11-14 | Bioject, Inc. | Drug vial mixing and transfer device |
| US5599302A (en) | 1995-01-09 | 1997-02-04 | Medi-Ject Corporation | Medical injection system and method, gas spring thereof and launching device using gas spring |
| WO1997013537A1 (en) | 1995-10-10 | 1997-04-17 | Visionary Medical Products Corporation | Gas pressured needle-less injection device |
| US5893397A (en) | 1996-01-12 | 1999-04-13 | Bioject Inc. | Medication vial/syringe liquid-transfer apparatus |
| WO1997037705A1 (en) | 1996-04-11 | 1997-10-16 | Weston Medical Limited | Spring-powered dispensing device for medical purposes |
| US5993412A (en) | 1997-05-19 | 1999-11-30 | Bioject, Inc. | Injection apparatus |
| WO1999034850A1 (en) | 1998-01-08 | 1999-07-15 | Fiderm S.R.L. | Device for controlling the penetration depth of a needle, for application to an injection syringe |
| US6835843B2 (en) | 2000-04-06 | 2004-12-28 | Les Laboratoires Servier | Method for synthesis of perindopril and its pharmaceutically acceptable salts |
| WO2001058868A1 (en) | 2000-04-06 | 2001-08-16 | Les Laboratoires Servier | Method for synthesis of perindopril and its pharmaceutically acceptable salts |
| US7326794B2 (en) | 2002-01-30 | 2008-02-05 | Les Laboratoires Servier | Process for the preparation of high purity perindopril and intermediates useful in the synthesis |
| US7279595B2 (en) | 2002-01-30 | 2007-10-09 | Les Laboratoires Servier | Process for the preparation of high purity perindopril |
| US20050119492A1 (en) | 2002-01-30 | 2005-06-02 | Guyla Simig | Process for the preparation of high purity perindopril and intermediates useful in the synthesis |
| US20070197821A1 (en) | 2002-01-30 | 2007-08-23 | Les Laboratoires Servier | Process for the preparation of high purity perindopril |
| WO2004046172A1 (en) | 2002-11-18 | 2004-06-03 | Cipla Ltd. | Perindopril |
| WO2004075889A1 (en) | 2003-02-28 | 2004-09-10 | Lupin Limited | Process for preparation of perindopril and salts thereof |
| EP1319668A1 (en) | 2003-03-12 | 2003-06-18 | Les Laboratoires Servier | Method for synthesis of (2S,3aS,7aS)-1-((S)-alanyl)-octahydro-1H-indole-2-carboxylic acid derivatives and use in the synthesis of perindopril |
| EP1321471A1 (en) | 2003-03-12 | 2003-06-25 | Les Laboratoires Servier | Method for synthesis of perindopril and its pharmaceutically acceptable salts |
| WO2004099138A2 (en) | 2003-05-12 | 2004-11-18 | Cipla Limited | Process for the preparation of perindopril |
| US7179833B2 (en) | 2003-06-30 | 2007-02-20 | Les Laboratoires Servier | Method of synthesising perindopril and the pharmaceutically acceptable salts thereof |
| WO2005012333A2 (en) | 2003-07-31 | 2005-02-10 | Les Laboratoires Servier | Novel method for the synthesis of perindopril and the pharmaceutically acceptable salts thereof |
| US7368580B2 (en) | 2003-07-31 | 2008-05-06 | Les Laboratories Servier | Method for the synthesis of perindopril and the pharmaceutically acceptable salts thereof |
| US20060183920A1 (en) | 2003-07-31 | 2006-08-17 | Claude Fugier | Novel method for the synthesis of perindopril and the pharmaceutically acceptable salts thereof |
| US20060189813A1 (en) | 2003-07-31 | 2006-08-24 | Claude Fugier | Novel method for the synthesis of perinidopril and the pharmaceutically acceptable salts thereof |
| US7358372B2 (en) | 2003-07-31 | 2008-04-15 | Les Laboratories Servier | Method for the synthesis of perindopril and the pharmaceutically acceptable salts thereof |
| US7223872B2 (en) | 2003-08-29 | 2007-05-29 | Les Laboratoires Servier | Process for the synthesis of perindopril and its pharmaceutically acceptable salts |
| WO2005023843A1 (en) | 2003-08-29 | 2005-03-17 | Les Laboratoires Servier | Novel method for the synthesis of perindopril and the pharmaceutically-acceptable salts thereof |
| US7534896B2 (en) | 2003-08-29 | 2009-05-19 | Les Laboratories Servier | Process for the synthesis of perindopril and its pharmaceutically acceptable salts |
| US7183308B1 (en) | 2003-08-29 | 2007-02-27 | Les Laboratoires Servier | Metod for the synthesis of perindopril and the pharmaceutically acceptable salts thereof |
| WO2005037788A1 (en) | 2003-10-21 | 2005-04-28 | Lupin Ltd. | Novel method for preparation of crystalline perindopril erbumine |
| WO2005054276A1 (en) | 2003-11-19 | 2005-06-16 | Les Laboratoires Servier | Method for synthesis of perindopril and the pharmaceutically-acceptable salts thereof |
| US7208607B1 (en) | 2003-11-19 | 2007-04-24 | Les Laboratoires Servier | Process for synthesis of perindopril and pharmaceutically acceptable salts thereof |
| WO2005066198A1 (en) | 2003-12-10 | 2005-07-21 | Les Laboratoires Servier | Method for the synthesis of perindopril and the pharmaceutically-acceptable salts thereof |
| US20100172995A1 (en) | 2004-03-29 | 2010-07-08 | Les Laboratoires Servier | Process For Preparing A Solid Pharmaceutical Composition |
| US20070172524A1 (en) | 2004-03-29 | 2007-07-26 | Krka, Tovarna Zdravil, D.D., Novo Mesto | Process for preparing a solid pharmaceutical composition |
| US7666896B2 (en) | 2004-04-13 | 2010-02-23 | Cipla Limited | Process for the preparation of perindopril |
| US20070185335A1 (en) | 2004-04-13 | 2007-08-09 | Kankan Rajendra N | Process for the preparation of perindopril |
| WO2005100317A1 (en) | 2004-04-13 | 2005-10-27 | Neopharma Limited | Process for the preparation of perindopril |
| US7674814B2 (en) | 2004-05-14 | 2010-03-09 | Les Laboratoires Servier | Process for the preparation of perindopril and salts thereof |
| US20080051584A1 (en) | 2004-05-14 | 2008-02-28 | Les Laboratoires Servier | Process For The Preparation Of Perindopril And Salts Thereof |
| WO2006070276A1 (en) | 2004-12-31 | 2006-07-06 | Quimica Sintetica, S.A. | Process for preparing perindopril erbumine |
| WO2006101462A2 (en) | 2005-03-22 | 2006-09-28 | Vulm, A.S. | Pharmaceutical preparation comprising perindopril erbumine and method of preparation and stabilisation thereof |
| WO2007002012A1 (en) | 2005-06-21 | 2007-01-04 | Ams Research Corporation | Apparatus for securing a urethral sling to pubic bone |
| WO2007020009A1 (en) | 2005-08-12 | 2007-02-22 | Sandoz Ag | New crystalline form of perindopril erbumine |
| WO2007025695A1 (en) | 2005-08-30 | 2007-03-08 | Lek Pharmaceuticals D.D. | Pharmaceutical composition comprising perindopril or its salts |
| WO2007058634A1 (en) | 2005-11-17 | 2007-05-24 | Diagen Smartno Pri Ljubljani, D.O.O. | Stable formulation of amorphous perindopril salts, a process for their preparation, especially industrial preparation, and their use in the therapy of hypertension |
| US20090162420A1 (en) | 2005-12-05 | 2009-06-25 | Karin Klokkers | Matrix-Controlled Transdermal System Comprising Salts of ACE Inhibitor Dicarboxylic Acids |
| WO2007088035A1 (en) | 2006-02-02 | 2007-08-09 | Lek Pharmaceuticals D.D. | A pharmaceutical composition comprising perindopril |
| WO2007092758A2 (en) | 2006-02-03 | 2007-08-16 | Dr. Reddy's Laboratories Ltd. | Crystalline forms of perindopril erbumine |
| WO2007099216A2 (en) | 2006-02-28 | 2007-09-07 | Les Laboratoires Servier | β-CRYSTALLINE FORM OF PERINDOPRIL ARGININE SALT, METHOD FOR MAKING SAME, AND PHARMACEUTICAL COMPOSITIONS CONTAINING SAME |
| WO2008114270A1 (en) | 2007-03-22 | 2008-09-25 | Aarti Healthcare Limited | Process for the preparation of perindopril erbumine salt and novel polymorph (s) thereof |
| WO2008119838A1 (en) | 2007-04-03 | 2008-10-09 | Centre National De La Recherche Scientifique (Cnrs) | Fto gene polymorphisms associated to obesity and/or type ii diabetes |
| WO2009157018A2 (en) | 2008-06-24 | 2009-12-30 | Matrix Laboratories Ltd | Novel polymorphic forms of perindopril (l)-arginine and process for the preparation thereof |
| US20110301357A1 (en) | 2008-06-24 | 2011-12-08 | Matrix Laboratories Ltd | Novel polymorphic forms of perindopril (l)-arginine and process for the preparation thereof |
| US8686161B2 (en) | 2008-06-24 | 2014-04-01 | Mylan Laboratories Limited | Polymorphic forms of perindopril (L)-arginine and process for the preparation thereof |
| WO2011058232A1 (en) | 2009-11-16 | 2011-05-19 | Mas-Metabolic Analytical Services Oy | Nutrigenetic biomarkers for obesity and type 2 diabetes |
| WO2012044189A1 (en) | 2010-09-29 | 2012-04-05 | Instituto Superior Técnico | A new hydrated crystalline form of perindopril erbumine, methods for its preparation and its use in pharmaceutical preparations |
| WO2013102740A1 (en) | 2012-01-05 | 2013-07-11 | Les Laboratoires Servier | Method for the preparation of perindopril arginine salt |
Non-Patent Citations (47)
| Title |
|---|
| A. R. GENNARO: "Remington's The Science and Practice of Pharmacy, 21" Edition,", 2006, LIPPINCOTT, WILLIAMS & WILKINS |
| ABDELMAGID SA ET AL.: "Prolactin and estrogen up-regulate carboxypeptidase-d to promote nitric oxide production and survival of mcf-7 breast cancer cells", ENDOCRINOLOGY, 2008 |
| ALTSCHUL ET AL., J MOL BIOL, vol. 215, 1990, pages 403 - 410 |
| ANDERSON M; YOUNG K.: "Nucleic acid hybridization, a practical approach", 1985, IRI PRESS, article "Quantitative filter hybridization", pages: 73 - 111 |
| AUSUBEL ET AL.: "Current Protocols in Molecular Biology", 2003, JOHN WILEY & SONS |
| BERGER SL; KIMMEL AR: "Methods in Enzymology. Vol. 152: Guide to molecular cloning techniques", 1987, ACADEMIC PRESS, INC |
| CAMPANELLA ET AL., BMC BIOINFONNATICS, vol. 4, 2003, pages 29 |
| CARLSON R,: "Design And Optimization in Organic Synthesis, 2nd Edition,", 2005, ELSEVIER SCIENCE LTD. |
| CHU ET AL., ISLETS, vol. 3, no. 4, 2011, pages 155 - 65 |
| ENG ET AL., J BIOL CHEM, vol. 273, no. 14, 1998, pages 8382 - 8 |
| GARCIA-PARDO J ET AL.: "Amyloid formation by human carboxypeptidase D transthyretin-like domain under physiological conditions", JBIOL CHEM, 2014 |
| GLANDT ET AL., J OBES, 2011, pages 636181 |
| GREEN; SAMBROOK: "Molecular Cloning: A Laboratory Manual, 4th ed.,", 2012, COLD SPRING HARBOR LABORATORY PRESS |
| HSIAO ET AL., GENE, vol. 533, no. 1, 2013, pages 32 - 7 |
| HSU ET AL., NUTR DIABETES, vol. 3, 2013, pages E61 |
| JAHNISCH, K ET AL., ANGEW. CHEM. INT. ED. ENGL., vol. 43, 2004, pages 406 |
| JIN T ET AL.: "SiRNA-targeted carboxypeptidase D inhibits hepatocellular carcinoma growth", CELL BIOL INT, 2013 |
| KOIRALA S ET AL.: "Prolactin/Stat5 and androgen R1881 coactivate carboxypeptidase-D gene in breast cancer cells", MOL ENDOCRINOL, 2014 |
| KRUSKAL: "Time warps, string edits and macromolecules: the theory and practice of sequence comparison", 1983, ADDISON WESLEY, pages: 1 - 44 |
| LARKIN ET AL., BIOINFONNATICS, vol. 23, 2007, pages 2947 - 2948 |
| MANDREKAR: "Dose-finding trial designs for combination therapies in oncology", JCLIN ONCOL, vol. 32, no. 2, 2014, pages 65 - 7 |
| MUELLER PR ET AL.: "Current Protocols in Molecular Biology", vol. 15.5, 1993, NEW YORK: GREENE PUBLISHING ASSOCIATES, INC. AND JOHN WILEY AND SONS |
| MUTCH ET AL., PLOS GENET., vol. 2, no. 12, 2006, pages E188 |
| NEEDLEMAN ET AL., J MOL BIOL, vol. 48, 1970, pages 443 - 453 |
| O'MALLEY ET AL., BIOCHEM J, vol. 390, no. 3, 2005, pages 665 - 673 |
| PEARSON ET AL., PROC NATL ACAD SCI USA, vol. 85, 1998, pages 2444 |
| ROBERT GRÜTZMANN , CHRISTIAN PILARSKY: "Cancer Gene Profiling: Methods and Protocols (Methods in Molecular Biology)", 6 November 2009, HUMANA PRESS |
| RYCHLIK ET AL., NUCL ACIDS RES, vol. 17, 1989, pages 8543 |
| SACHIDANANDAM ET AL., NATURE, vol. 409, no. 6822, 2001, pages 928 - 33 |
| SAMBROOK ET AL.: "Molecular Cloning: a Laboratory Manual", 1989, COLD SPRING HARBOR LABORATORY PRESS |
| SAMBROOK ET AL.: "Molecular Cloning: A Laboratory Manual", vol. 1-3, 2001, COLD SPRING HARBOR LABORATORY PRESS |
| SAMBROOK ET AL.: "Molecular Cloning: A laboratory Manual", vol. 1-3, 2001, COLD SPRING HARBOR LABORATORY PRESS, article "In vitro amplification of DNA by the polymerase chain reaction" |
| SAMBROOK ET AL.: "Molecular Cloning: A Laboratory Manual, Third Edition", 15 January 2001, COLD SPRING HARBOR LABORATORY PRESS, ISBN: 10: 087969577 |
| SAMBROOK ET AL.: "Molecular Cloning: a Laboratory Manual. 2nd Ed.,", vol. 1-3, 1989, COLD SPRING HARBOR LABORATORY |
| SIDYELYEVA ET AL., J BIOL CHEM, vol. 277, no. 51, 2002, pages 49613 - 20 |
| SIDYELYEVA ET AL., J BIOL CHEM, vol. 281, no. 19, 2006, pages 13844 - 13852 |
| SKIDGEL ET AL., IMMUNOL REV, vol. 161, 1998, pages 129 - 141 |
| SMITH ET AL., ADV APPL MATH., vol. 2, 1981, pages 482 |
| SMITH; MARCH: "Marc 's Advanced Organic Chemistry: Reactions, Mechanisms, and structure, 6th edition", 2007, WILEY-INTERSCIENCE |
| SONG ET AL., J BIOL CHEM, vol. 270, no. 42, 1995, pages 25007 - 25013 |
| SONG ET AL., J BIOL CHEM, vol. 271, no. 46, 1996, pages 28884 - 28889 |
| THOMAS LN ET AL.: "Carboxypeptidase-D is elevated in prostate cancer and its anti-apoptotic activity is abolished by combined androgen and prolactin receptor targeting", PROSTATE, 2014 |
| TIMBLIN ET AL., INT IMMUNOPHARMACOL, vol. 2, 2002, pages 1907 - 1917 |
| VARLAMOV ET AL., J CELL SCIENCE, vol. 111, no. 7, 1998, pages 877 - 885 |
| WALSH ET AL., J FORENSIC SCI, vol. 37, no. 2, 1992, pages 387 - 95 |
| WORLD HEALTH ORGANIZATION: "WHO obesity: preventing and managing the global epidemic", WHO TECHNICAL REPORT SERIES 894., 2000 |
| YUE ET AL., ACTA PHARMACOL SIN, vol. 34, no. 5, 2013, pages 710 - 6 |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2016178591A3 (en) | 2017-11-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| Hayashida et al. | Analgesic requirements after major abdominal surgery are associated with OPRM1 gene polymorphism genotype and haplotype | |
| Itokawa et al. | A microsatellite repeat in the promoter of the N-methyl-D-aspartate receptor 2A subunit (GRIN2A) gene suppresses transcriptional activity and correlates with chronic outcome in schizophrenia | |
| Gong et al. | Gene polymorphisms of OPRM1 A118G and ABCB1 C3435T may influence opioid requirements in Chinese patients with cancer pain | |
| CA2882487A1 (en) | Genetic polymorphisms associated with stroke, methods of detection and uses thereof | |
| EP1414477A2 (en) | Repeat sequences of the ca125 gene and their use for diagnostic and therapeutic interventions | |
| US10085979B2 (en) | Combinations for the treatment of neuroblastoma | |
| EP1470246A2 (en) | Methods to treat diabetes and related conditions based on polymorphisms in the tcf1 gene | |
| CA3159162A1 (en) | Antisense oligomers for treatment of conditions and diseases | |
| US20200246369A1 (en) | Antisense oligonucleotide and composition for prevention or treatment of glycogen storage disease type ia | |
| US20220226278A1 (en) | Optimized sigma-1 agonist method of responder selection and treatment | |
| Gaddam et al. | γ peptide nucleic acid-based miR-122 inhibition rescues vascular endothelial dysfunction in mice fed a high-fat diet | |
| US20190388391A1 (en) | Novel erythromelalgia treatment | |
| EP2291544B1 (en) | Genetic alterations on chromosomes 21q, 6q and 15q and methods of use thereof for the diagnosis and treatment of type i diabetes | |
| JP2015155422A (en) | Genotype specific methods for treating human subjects using 4-methylpyrazole | |
| US20090263368A1 (en) | Genetic variations associated with psychiatric disorders | |
| WO2016178591A2 (en) | Genetic markers and treatment of male obesity | |
| Kim et al. | Influence of ABCC2, SLCO1B1, and ABCG2 polymorphisms on the pharmacokinetics of olmesartan | |
| EP3200784B1 (en) | Small fibre neuropathy treatment | |
| US20150133309A1 (en) | Compositions and Methods for Diagnosing and Treating Neoplasia | |
| JP2021523139A (en) | Compositions and methods for slowing the progression of nephrolithiasis | |
| MX2013008068A (en) | Disease risk factors and methods of use. | |
| US10493059B2 (en) | Paroxysmal extreme pain disorder treatment | |
| WO2008000803A1 (en) | Identification and use of gprc variants in the treatment and diagnosis of parkinson's disease | |
| KR101514744B1 (en) | Single nucleotide polymophism of Human multidrug and toxin extrusion 2 gene and use thereof | |
| AU2011320568A1 (en) | Methods and compositions for assessing and treating cancer |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 16730920 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 16730920 Country of ref document: EP Kind code of ref document: A2 |