WO2016084972A1 - Oil-rich composition - Google Patents
Oil-rich composition Download PDFInfo
- Publication number
- WO2016084972A1 WO2016084972A1 PCT/JP2015/083516 JP2015083516W WO2016084972A1 WO 2016084972 A1 WO2016084972 A1 WO 2016084972A1 JP 2015083516 W JP2015083516 W JP 2015083516W WO 2016084972 A1 WO2016084972 A1 WO 2016084972A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- composition according
- composition
- oil
- acid
- Prior art date
Links
- 0 CC1=C*(*)=C(C)*1* Chemical compound CC1=C*(*)=C(C)*1* 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/06—Preparations for styling the hair, e.g. by temporary shaping or colouring
- A61Q5/065—Preparations for temporary colouring the hair, e.g. direct dyes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/40—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing nitrogen
- A61K8/45—Derivatives containing from 2 to 10 oxyalkylene groups
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/73—Polysaccharides
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/73—Polysaccharides
- A61K8/731—Cellulose; Quaternized cellulose derivatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/84—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions otherwise than those involving only carbon-carbon unsaturated bonds
- A61K8/86—Polyethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/84—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions otherwise than those involving only carbon-carbon unsaturated bonds
- A61K8/89—Polysiloxanes
- A61K8/891—Polysiloxanes saturated, e.g. dimethicone, phenyl trimethicone, C24-C28 methicone or stearyl dimethicone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/92—Oils, fats or waxes; Derivatives thereof, e.g. hydrogenation products thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/10—Preparations for permanently dyeing the hair
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K2800/00—Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects
- A61K2800/40—Chemical, physico-chemical or functional or structural properties of particular ingredients
- A61K2800/48—Thickener, Thickening system
Definitions
- the present invention relates to a composition comprising a relatively large amount of oil, which is preferably used for keratin fibers such as hair.
- compositions comprising a large amount of fatty materials have been proposed.
- Such compositions may comprise more than 20% by weight of fatty compounds, relative to the total weight of the composition, in combination with an alkaline agent, with or without an oxidative dye.
- Such compositions can provide high bleaching or coloring ability with a relatively small amount of alkaline agent and advantageously without ammonia.
- hair color treatment products using a direct dye are widely used there days, but the products have an issue of less color uptake as compared to permanent or semi-permanent hair color using an oxidative dye.
- compositions including a large amount of fatty materials tends to be unstable over time.
- An objective of the present invention is to provide a stable composition, in particular a stable cosmetic composition, which is preferably used for keratin fibers such as hair, with improved color uptake.
- composition in particular a cosmetic composition, which is preferably used for keratin fibers such as hair, comprising:
- the amount of the (a) oil is 25% by weight or more relative to the total weight of the composition
- the weight ratio of the (a) oil/the (c) thickener is from 25 to 100.
- the (a) oil may be selected from the group consisting of hydrocarbon oils and silicone oils.
- the amount of the (a) oil may range from 25 to 90% by weight, preferably from 30 to 80% by weight, and more preferably from 35 to 70% by weight, relative to the total weight of the composition.
- the polyoxyalkylenated fatty alcohols may be selected from saturated or unsaturated, linear or branched, oxyalkylenated C 8 -C 30 alcohols.
- the polyoxyalkylenated fatty alcohols may include from 1 to 100 ethyleneoxide units, preferably from 2 to 50 ethyleneoxide units, and more preferably from 2 to 30 ethyleneoxide units.
- the amount of the (b) nonionic surfactant may range from 0.01 to 15% by weight, preferably from 0.1 to 10% by weight, and more preferably from 0.5 to 5% by weight, relative to the total weight of the composition.
- the (c) thickener may be selected from polysaccharides.
- the amount of the (c) thickener may range from 0.1 to 15%) by weight, preferably from 0.2 to 10% by weight, and more preferably from 0.25 to 5% by weight, relative to the total weight of the composition.
- the weight ratio of the (a) oil/the (c) thickener may be from 25 to 90, preferably from 25 to 80, and more preferably from 25 to 70.
- the composition according to the present invention may further comprise (e) at least one dye selected from the group consisting of direct dyes and oxidative dyes.
- the direct dyes may be selected from synthetic direct dyes.
- the synthetic direct dyes may be selected from the group consisting of: hydrophilic synthetic direct dyes selected from the group consisting of cationic synthetic direct dyes, nonionic synthetic direct dyes, and anionic synthetic direct dyes; and hydrophobic synthetic direct dyes.
- the amount of the (e) dye may range from 0.001 to 10% by weight, preferably from 0.01 to 5% by weight, and more preferably from 0.1 to 3% by weight, relative to the total weight of the composition.
- composition according to the present invention be intended for dyeing keratin fibers, preferably hair.
- the present invention also relates to a cosmetic process for keratin fibers, preferably hair, comprising the steps of applying the composition according to the present invention to the keratin fibers.
- a stable composition in particular a stable cosmetic composition, which is preferably used for keratin fibers such as hair, with improved color uptake, if the composition includes a combination of selected specific ingredients under specific conditions.
- the present invention is a composition, in particular a cosmetic composition, which is preferably used for keratin fibers such as hair, comprising:
- the amount of the (a) oil is 25% by weight or more relative to the total weight of the composition
- the weight ratio of the (a) oil/the (c) thickener is from 25 to 100.
- composition according to the present invention can provide improved color uptake.
- the keratin fibers treated with the composition according to the present invention can be effectively colored.
- composition according to the present invention is stable over time.
- stable here means that the aspect of the composition does not change or substantially change for 30 minutes or more, preferably about a few hours or more, and more preferably about a few months or more.
- the composition according to the present invention can maintain its aspect for a long period of time.
- the aspect of the composition according to the present invention does not change or substantially change for a long period of time. Since the composition according to the present invention is stable over time, the composition can preferably be a cosmetic composition.
- the composition according to the present invention can provide enhanced coloring ability with a relatively small amount of alkaline agent even without ammonia.
- the composition according to the present invention can have an appropriate viscosity.
- composition according to the present invention will be explained in a more detailed manner.
- the composition according to the present invention comprises at least one (a) oil.
- oil means a fatty compound or substance which is in the form of a liquid at room temperature (25 °C) under atmospheric pressure (760 rnmHg).
- oils those generally used in cosmetics may be used alone or in combination thereof. These oils may be volatile or non- volatile, preferably non-volatile.
- the (a) oil may be a non-polar oil such as a hydrocarbon oil, a silicone oil, or the like; a polar oil such as a plant or animal oil and an ester oil or an ether oil; or a mixture thereof.
- the (a) oil be selected from the group consisting of oils of plant or animal origin, synthetic oils, silicone oils, and hydrocarbon oils.
- plant oils examples include, for example, linseed oil, camellia oil, macadamia nut oil, corn oil, mink oil, olive oil, avocado oil, sasanqua oil, castor oil, safflower oil, jojoba oil, sunflower oil, almond oil, rapeseed oil, sesame oil, soybean oil, peanut oil, and mixtures thereof.
- animal oils mention may be made of, for example, squalene and squalane.
- alkane oils such as isododecane and isohexadecane
- ester oils such as isododecane and isohexadecane
- ether oils such as triglycerides
- the ester oils are preferably liquid esters of saturated or unsaturated, linear or branched C 1 -C 26 aliphatic monoacids or polyacids and of saturated or unsaturated, linear or branched C 1 -C 26 aliphatic monoalcohols or polyalcohols, the total number of carbon atoms of the esters being greater than or equal to 10.
- the esters of monoalcohols at least one from among the alcohol and the acid from which the esters of the invention are derived is branched.
- ethyl palmitate ethyl hexyl palmitate
- isopropyl palmitate dicaprylyl carbonate
- alkyl myristates such as isopropyl myristate or ethyl myristate
- isocetyl stearate 2-ethylhexyl isononanoate
- isononyl isononanoate isodecyl neopentanoate
- isostearyl neopentanoate isostearyl neopentanoate.
- Esters of C 4 -C 22 dicarboxylic or tricarboxylic acids and of C 1 -C 22 alcohols and esters of monocarboxylic, dicarboxylic, or tricarboxylic acids and of non-sugar C 4 -C 26 dihydroxy, trihydroxy, tetrahydroxy, or pentahydroxy alcohols may also be used.
- sugar esters and diesters of C 6 -C 30 and preferably C 12 -C 22 fatty acids.
- sucrose means oxygen-bearing hydrocarbon-based compounds containing several alcohol functions, with or without aldehyde or ketone functions, and which comprise at least 4 carbon atoms. These sugars may be monosaccharides, oligosaccharides, or polysaccharides.
- sugars examples include sucrose (or saccharose), glucose, galactose, ribose, fucose, maltose, fructose, mannose, arabinose, xylose, and lactose, and derivatives thereof, especially alkyl derivatives, such as methyl derivatives, for instance methylglucose.
- the sugar esters of fatty acids may be chosen especially from the group comprising the esters or mixtures of esters of sugars described previously and of linear or branched, saturated or unsaturated C 6 -C 3 o and preferably C 12 -C 22 fatty acids. If they are unsaturated, these compounds may have one to three conjugated or non-conjugated carbon-carbon double bonds.
- the esters according to this variant may also be selected from monoesters, diesters, triesters, tetraesters, and polyesters, and mixtures thereof.
- esters may be, for example, oleates, laurates, palmitates, myristates, behenates, cocoates, stearates, linoleates, linolenates, caprates, and arachidonates, or mixtures thereof such as, especially, oleopalmitate, oleostearate, and palmitostearate mixed esters, as well as pentaerythrityl tetraethyl hexanoate.
- monoesters and diesters and especially sucrose, glucose or methylglucose monooleates or dioleates, stearates, behenates, oleopalmitates, linoleates, linolenates, and oleostearates.
- Glucate® DO is a methylglucose dioleate.
- ester oils mention may be made of, for example, diisopropyl adipate, dioctyl adipate, 2-ethylhexyl hexanoate, ethyl laurate, cetyl octanoate, octyldodecyl octanoate, isodecyl neopentanoate, myristyl propionate, 2-ethylhexyl 2-ethylhexanoate, 2-ethylhexyl octanoate, 2-ethylhexyl caprylate/caprate, methyl palmitate, ethyl palmitate, isopropyl palmitate, ethylhexyl palmitate, isohexyl laurate, hexyl laurate, isocetyl stearate, isopropyl isostearate, isopropyl myristate,
- artificial triglycerides mention may be made of, for example, glyceryl frimyristate, glyceryl tripalmitate, glyceryl trilinolenate, glyceryl trilaurate, glyceryl tricaprate, glyceryl tricaprylate, glyceryl tri(caprate/caprylate), and glyceryl tri(caprate/caprylate/linolenate).
- silicone oils mention may be made of, for example, linear organopolysiloxanes such as dimethylpolysiloxane, methylphenylpolysiloxane, methylhydrogenpolysiloxane, and the like; cyclic organopolysiloxanes such as octamethylcyclotetrasiloxane,
- decamethylcyclopentasiloxane dodecamethylcyclohexasiloxane, and the like; and mixtures thereof.
- the silicone oil is chosen from liquid polydialkylsiloxanes, especially liquid
- PDMS polydimethylsiloxanes
- liquid polyorganosiloxanes comprising at least one aryl group.
- silicone oils may also be organomodified.
- organomodified silicones that can be used in accordance with the present invention are silicone oils as defined above and comprising in their structure one or more organofunctional groups attached via a hydrocarbon-based group.
- Organopolysiloxanes are defined in greater detail in Walter Noll's Chemistry and Technology of Silicones ( 1968), Academic Press. They may be volatile or non- volatile.
- Volatile or non- volatile silicone oils such as volatile or non- volatile polydimethylsiloxanes (PDMS) containing a linear or cyclic silicone chain, that are liquid or pasty at ambient temperature, in particular cyclopolydimethylsiloxanes (cyclomethicones) such as eyclohexasiloxane;
- PDMS volatile or non- volatile polydimethylsiloxanes
- cyclomethicones such as eyclohexasiloxane
- dimethicones diphenylmethyldiphenyltrisiloxanes, 2-phenylemyltrimethyl siloxysilicates, and polymethylphenylsiloxanes, may be used.
- Hydrocarbon oils may be chosen from:
- linear or branched, optionally cyclic, C 6 -C 16 lower alkanes examples that may be mentioned include hexane, undecane, dodecane, tridecane, and isoparaffins, for instance isohexadecane, isododecane, and isodecane; and
- linear or branched hydrocarbons containing more than 16 carbon atoms such as liquid paraffins, liquid petroleum jelly, polydecenes, and hydrogenated polyisobutenes such as Parleam®, and squalane.
- hydrocarbon oils examples include linear or branched hydrocarbons such as mineral oil (e.g., liquid paraffin), paraffin, vaseline or petrolatum, naphthalenes, and the like; hydrogenated polyisobutene, isoeicosan, and decene/butene copolymer; and mixtures thereof.
- mineral oil e.g., liquid paraffin
- paraffin paraffin
- vaseline or petrolatum naphthalenes, and the like
- hydrogenated polyisobutene isoeicosan
- decene/butene copolymer and mixtures thereof.
- the (a) oil be chosen from hydrocarbon oils and silicone oils.
- the amount in the composition according to the present invention of the (a) oil is 25% by weight or more relative to the total weight of the composition, and it may range from 25 to 90% by weight, preferably from 30 to 80% by weight, and more preferably from 35 to 70% by weight, relative to the total weight of the composition.
- the amount of the (a) oil may range from 30 to 90% by weight, preferably from 30 to 80% by weight, and more preferably from 30 to 70% by weight, relative to the total weight of the composition.
- the amount of the (a) oil may range from 35 to 90% by weight, preferably from 35 to 80% by weight, and more preferably from 35 to 70% by weight, relative to the total weight of the composition.
- the amount of the (a) oil may range from 40 to 90% by weight, preferably from 40 to 80% by weight, and more preferably from 40 to 70% by weight, relative to the total weight of the composition. In a furthermore preferred embodiment, the amount of the (a) oil may range from 50 to 90% by weight, preferably from 50 to 80% by weight, and more preferably from 50 to 70% by weight, relative to the total weight of the composition.
- composition according to the present invention comprises at least one nonionic surfactant selected from the group consisting of polyoxyalkylenated fatty alcohols.
- a single type of the polyoxyalkylenated fatty alcohol may be used, but two or more different types of the
- polyoxyalkylenated fatty alcohol may be used in combination.
- the polyoxyalkylenated fatty alcohols are also called as polyalkylene glycol ether of fatty alcohols or polyoxyalkylene alkyl ether, and mean ethers of one or more fatty alcohols and of one or more oxyalkylene polymers.
- the polyoxyalkylenated fatty alcohols may be selected from saturated or unsaturated, linear or branched, polyoxyalkylenated C 8 -C 3 o alcohols.
- the oxyalkylene units are more particularly oxyethylene or oxypropylene units, or a combination thereof, preferably oxyethylene units. It is preferable that the polyoxyalkylenated fatty alcohols include from 1 to 100 ethyleneoxide units, more preferably from 2 to 50 ethyleneoxide units, and even more preferably from 2 to 30 ethyleneoxide units.
- the nonionic surfactants do not comprise any oxypropylene units.
- polyoxyethylenated fatty alcohol or C 8 -C 30 alcohols
- examples of polyoxyethylenated fatty alcohol (or C 8 -C 30 alcohols) that may be mentioned include the adducts of ethylene oxide with lauryl alcohol, especially those containing from 2 to 50 oxyethylene units and more particularly those containing from 2 to 20 oxyethylene units
- polyoxyalkylenated fatty alcohols may range from 0.01 to 15% by weight, preferably from 0.1 to 10% by weight, and more preferably from 0.5 to 5% by weight, relative to the total weight of the composition.
- composition according to the present invention comprises at least, one (c) thickener.
- a single type of thickener may be used, but two or more different types of thickener may be used in combination.
- the (c) thickener may be selected from hydrophilic polymers, preferably water-soluble polymers.
- the (c) thickener be selected from the group consisting of:
- association thickener means an amphiphilic thickener comprising both hydrophilic units and hydrophobic units, for example, at least one C 8 -C 30 fatty chain and at least one hydrophilic unit.
- associative thickeners that may be used are associative polymers chosen from: (aa) nonionic amphiphilic polymers comprising at least one fatty chain and at least one hydrophilic unit;
- anionic amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit;
- (cc) cationic amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit;
- amphoteric amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit
- fatty chain contains from 8 to 30 carbon atoms.
- the (aa) nonionic amphiphilic polymers comprising at least one fatty chain and at least one hydrophilic unit may, for example, be chosen from:
- celluloses modified with groups comprising at least one fatty chain examples that may be mentioned include:
- hydroxyethylcelluloses modified with groups comprising at least one fatty chain chosen from alkyl, arylalkyl, and alkylaryl groups, and in which the alkyl groups are, for example, C 8- c22, such as the product Natrosol Plus Grade 330 CS(C 1-6 alkyls) sold by the company Aqualon, and the product Bermocoll EHM 100 sold by the company Berol Nobel, and
- celluloses modified with polyalkylene glycol alkylphenyl ether groups such as the product Amercell Polymer HM-1500 (polyethylene glycol (15) nonylphenyl ether) sold by the company Amerchol.
- hydroxypropyl guars modified with groups comprising at least one fatty chain such as the product Esaflor HM 22 (C 22 alkyl chain) sold by the company Lamberti, and the products Miracare XC95-3 (C 1 alkyl chain) and RE205-1 (C 20 alkyl chain) sold by the company Rhodia Chimie.
- polyether urethanes comprising at least one fatty chain, such as C 10 -C 30 alkyl or alkenyl groups, for instance the products Elfacos T 210 and Elfacos T 212 sold by the company Akzo or the products Aculyn 44 and Aculyn 46 sold by the company Rohm & Haas.
- copolymers of hydrophilic acrylates or methacrylates and of hydrophobic monomers comprising at least one fatty chain such as a polyethylene glycol methacrylate/lauryl methacrylate copolymer.
- the (bb) anionic amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit may, for example, be chosen from those comprising at least one fatty-chain allyl ether unit and at least one hydrophilic unit comprising an ethylenic unsaturated anionic monomelic unit, for example, a vinylcarboxylic acid unit and further, for example, be chosen from units derived from acrylic acids, methacrylic acids, and mixtures thereof, wherein the fatty-chain allyl ether unit corresponds to the monomer of formula (I) below:
- CH 2 C(R 1 )CH 2 OB n R (I) in which Ri is chosen from H and CH 3 , B is an ethyleneoxy radical, n is chosen from zero and integers ranging from 1 to 100, R is chosen from hydrocarbon-based radicals chosen from alkyl, arylalkyl, aryl, alkylaryl, and cycloalkyl radicals, containing from 10 to 30 carbon atoms, and further, for example, from 10 to 24 carbon atoms and even further, for example, from 12 to 18 carbon atoms.
- a unit of formula (I) is, for example, a unit in which Ri can be H, n can be equal to 10, and R can be a stearyl (C 18 ) radical.
- anionic amphiphilic polymers are, for example, polymers formed from 20% to 60% by weight of acrylic acid and/or of methacrylic acid, from 5% to 60% by weight of lower alkyl (meth)acrylates, from 2% to 50% by weight of fatty-chain allyl ether of formula (I), and from 0% to 1%) by weight of a crosslinking agent which is a well-known copolymerizable unsaturated polyethylenic monomer, for example, diallyl phthalate, allyl(meth)acrylate, divinylbenzene, (poly)ethylene glycol dimethacrylate, and methylenebisacrylamide.
- a crosslinking agent which is a well-known copolymerizable unsaturated polyethylenic monomer, for example, diallyl phthalate, allyl(meth)acrylate, divinylbenzene, (poly)ethylene glycol dimethacrylate, and methylenebisacrylamide.
- polymers examples include crosslinked terpolymers of methacrylic acid, of ethyl acrylate, and of polyethylene glycol (10 EO) stearyl ether (Steareth-10), such as those sold by the company Ciba under the names Salcare SC 80 and Salcare SC 90, which are aqueous 30% emulsions of a crosslinked terpolymer of methacrylic acid, of ethyl acrylate, and of steareth-10 allyl ether (40/50/10).
- Salcare SC 80 and Salcare SC 90 are aqueous 30% emulsions of a crosslinked terpolymer of methacrylic acid, of ethyl acrylate, and of steareth-10 allyl ether (40/50/10).
- the anionic amphiphilic polymers may further be chosen, for example, from those comprising at least one hydrophilic unit of unsaturated olefinic carboxylic acid type, and at least one hydrophobic unit of a type such as a ( C 10 -C 30 ) alkyl ester of an unsaturated carboxylic acid.
- the hydrophilic unit of unsaturated olefinic carboxylic acid type corresponds to, for example, the monomer of formula (II) below:
- R 1 is chosen from H, CH 3 , and C 2 H , i.e., acrylic acid, methacrylic acid, and ethacrylic acid units.
- the hydrophobic unit of a type such as a (C 10 -C 30 ) alkyl ester of an unsaturated carboxylic acid corresponds to, for example, the monomer of formula (III) below:
- R 1 is chosen from H, CH 3 , and C 2 H 5 (i.e., acrylate, methacrylate, and ethacrylate units) and is, for example, chosen from, for example, H (acrylate units) and CH 3 (methacrylate units), and R is chosen from C 10 -C3 0 alkyl radicals, for example, C 12 -C 2 2 alkyl radicals.
- Examples of (C 10 -C 3 o)alkyl esters of unsaturated carboxylic acids include lauryl acrylate, stearyl acrylate, decyl acrylate, isodecyl acrylate, and dodecyl acrylate, and the corresponding methacrylates, lauryl methacrylate, stearyl methacrylate, decyl methacrylate, isodecyl methacrylate, and dodecyl methacrylate.
- Anionic amphiphilic polymers of this type are disclosed and prepared, for example, according to U.S. Pat. Nos. 3,915,921 and 4,509,949.
- anionic amphiphilic polymers that can be used may further be chosen from polymers formed from a mixture of monomers comprising:
- R 1 is chosen from H and CH 3
- R 2 is chosen from C 10 -C 30 alkyl radicals, such as alkyl radicals containing from 12 to 22 carbon atoms, and a crosslinking agent; such as polymers derived from 95% to 60% by weight of the acrylic acid (hydrophilic unit), 4% to 40% by weight of C 1 o-C 30 alkyl acrylate (hydrophobic unit), and 0%» to 6% by weight of crosslinking polymerizable monomer, or polymers derived from 98% to 96% by weight of the acrylic acid (hydrophilic unit), 1% to 4% by weight of C 10 -C 30 alkyl acrylate (hydrophobic unit), and 0.1% to 0.6% by weight of crosslinking polymerizable monomer; or
- acrylic acid and lauryl methacrylate such as the polymers formed from 66% by weight of acrylic acid and 34% by weight of lauryl methacrylate.
- the crosslinking agent can be a monomer comprising a group
- Pemulen TR 1 the products sold by the company Goodrich under the trade names Pemulen TR 1 , Pemulen TR2, and Carbopol 1382, and further, for example, Pemulen TRl, and the product sold by the company S.E.P.C. under the name Coatex SX.
- anionic amphiphilic fatty-chain polymers mention may also be made, for example, of the ethoxylated copolymer of methacrylic acid/methyl acrylate/alkyl
- Viscophobe DB 1000 dimethyl-meta-isopropenylbenzylisocyanate sold under the name Viscophobe DB 1000 by the company Amerchol.
- the (cc) cationic amphiphilic polymers used are, for example, chosen from quatemized cellulose derivatives and polyacrylates comprising amino side groups.
- the quatemized cellulose derivatives are, for example, chosen from
- quatemized celluloses modified with groups comprising at least one fatty chain, such as alkyl, arylalkyl, and alkylaryl groups comprising at least 8 carbon atoms, and mixtures thereof, and quatemized hydroxyethylcelluloses modified with groups comprising at least one fatty chain, such as alkyl, arylalkyl, and alkylaryl groups comprising at least 8 carbon atoms, and mixtures thereof.
- Quatemized and non-quaternized polyacrylates comprising amino side groups have, for example, hydrophobic groups, such as Steareth 20 (polyoxy-ethylenated (20) stearyl alcohol) and
- the alkyl radicals borne by the above quatemized celluloses and hydroxyethylcelluloses contain from 8 to 30 carbon atoms.
- the aryl radicals for example, are chosen from phenyl, benzyl, naphthyl, and anthryl groups.
- Examples of quatemized alkylhydroxyethyl-celluloses comprising C 8 -C 30 fatty chains are the products Quatrisoft LM 200, Quatrisoft LM-X 529-18-A, Quatrisoft LM-X 529-18B (C 12 alkyl), and Quatrisoft LM-X 529-8 (C 18 alkyl) sold by the company Amerchol, and the products Crodacel QM, Crodacel QL (C 12 alkyl), and Crodacel QS (C 18 alkyl) sold by the company Croda.
- polyacrylates comprising amino side chains are the polymers 8781-124B or
- amphoteric amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit
- alkyl radical is, for example, a stearyl radical.
- the associative thickeners in the compositions can have, for example, in solution or in dispersion at a concentration of 1% active material in water, a viscosity, measured using a Rheomat RM 180 rheometer at 25°C, of greater than 0.1 ps and further, for example, of greater than 0.2 cp, at a shear rate of 200 s -1 .
- crosslinked acrylic acid homopolymers that may be mentioned are those crosslinked with an allylic alcohol ether of the sugar series, such as the products sold under the names Carbopol 980, 981, 954, 2984, and 5984 by the company Goodrich or the products sold under the names Synthalen M and Synthalen K by the company 3 VSA.
- the crosslinked copolymers of (meth)acrylic acid and of C 1 -C 1 alkyl acrylate can be chosen from crosslinked copolymers of methacrylic acid and of ethyl acrylate as an aqueous dispersion comprising 38% active material sold, for example, under the name Viscoatex 538C by the company Coatex, and crosslinked copolymers of acrylic acid and of ethyl acrylate as an aqueous dispersion comprising 28% active material sold under the name Aculyn 33 by the company Rohm & Haas.
- Crosslinked copolymers of methacrylic acid and of ethyl acrylate include an aqueous dispersion comprising 30% active material sold under the name CARBOPOL AQUA SF-1 by the company NOVEON.
- nonionic homopolymers or copolymers comprising ethylenically unsaturated monomers of ester and/or amide type
- copolymer and BPA 500 by the company Kobo (polymethyl methacrylate).
- Ammonium acrylate homopolymers that may be mentioned include the product sold under the name Microsap PAS 5193 by the company Hoechst.
- Copolymers of ammonium acrylate and of acrylarnide include the product sold under the name Bozepol C Wunsch or the product PAS 5193 sold by the company Hoechst (which are described and prepared in documents FR-2 416 723, U.S. Pat. No. 2,798,053, and U.S. Pat. No. 2,923,692). It is preferable that the (c) thickener is selected from polysaccharides.
- the polysaccharides are, for example, chosen from glucans, modified and unmodified starches (such as those derived, for example, from cereals, for instance wheat, corn, or rice, from vegetables, for instance yellow peas, and tubers, for instance potatoes or cassava), amylose, amylopectin, glycogen, dextrans, celluloses, and derivatives thereof (e.g., methylcelluloses, hydroxyalkylcelluloses, ethyl hydroxyethylcelluloses, and carboxymethylcelluloses), mannans, xylans, lignins, arabans, galactans, galacturonans, chitin, chitosans, glucuronoxylans,
- arabinoxylans such as guar gums, and nonionic derivatives thereof (e.g., hydroxypropyl guar), and xanthan gums, and mixtures thereof.
- polysaccharides that may be used are chosen from those described, for example, in “Encyclopedia of Chemical Technology", Kirk-Othmer, Third Edition, 1982, volume 3, pp. 896-900, and volume 15, pp. 439-458, in "Polymers in Nature” by E. A. MacGregor and C. T. Greenwood, published by John Wiley & Sons, Chapter 6, pp. 240-328, 1980, and in "Industrial Gums-Polysaccharides and their Derivatives", edited by Roy L. Whistler, Second Edition, published by Academic Press Inc., the content of these three publications being entirely incorporated by reference.
- starches for example, starches, guar gums, celluloses, and derivatives thereof can be used.
- starches that may be used, mention may be made, for example, of macromolecules in the form of polymers comprising base units which are anhydroglucose units.
- base units which are anhydroglucose units.
- amylose linear polymer
- amylopectin branched polymer
- the relative proportions of amylose and amylopectin, as well as their degree of polymerization, can vary according to the botanical origin of the starches.
- the molecules of starches used may have cereals or tubers as their botanical origin.
- the starches can be, for example, chosen from maize, rice, cassava, tapioca, barley, potato, wheat, sorghum, and pea starches.
- Starches generally exist in the form of a white powder, insoluble in cold water, whose elementary particle size ranges from 3 to 100 microns.
- the starches may be optionally C 1 -C hydroxyalkylated or C 1 -C acylated (such as acetylated).
- the starches may have also undergone heat treatments.
- Distarch phosphates or compounds rich in distarch phosphate such as the product provided under the references PREJEL VA-70-T AGGL (gelatinized hydroxypropylated cassava distarch phosphate) or PREJEL TKl (gelatinized cassava distarch phosphate) or PREJEL 200 (gelatinized acetylated cassava distarch phosphate) by the company AVEBE, may also be used.
- the guar gums can be modified or unmodified.
- the unmodified guar gums are, for example, the products sold under the name Vidogum GH 175 by the company Unipectine and under the names Meypro-Guar 50 and Jaguar C by the company Meyhall.
- the modified nonionic guar gums are, for example, modified with C 1 -C 6 hydroxyalkyl groups.
- hydroxyalkyl groups that may be mentioned, for example, are hydroxymethyl, hydroxyethyl, hydroxypropyl, and hydroxybutyl groups.
- These guar gums are well known in the prior art and can be prepared, for example, by reacting the corresponding alkene oxides such as propylene oxides, with the guar gum so as to obtain a guar gum modified with hydroxypropyl groups.
- the degree of hydroxyalkylation which corresponds to the number of alkylene oxide molecules consumed by the number of free hydroxyl functions present on the guar gum, may, for example, range from 0.4 to 1.2.
- nonionic guar gums optionally modified with hydroxyalkyl groups are sold, for example, under the trade names Jaguar HP8, Jaguar HP60, Jaguar HP120, Jaguar DC 293, and Jaguar HP 105 by the company Rhodia Chimie (Meyhall) or under the name Galactasol 43 ⁇ 4FD2 by the company Aqualon.
- celluloses and cellulose derivatives such as cellulose modified with hydroxylalkyl groups
- cellulose modified with hydroxylalkyl groups are, for example, hydroxypropylmethylcellulose, hydroxyethylcellulose, and hydroxypropylcelluloses, as well as hydrophobicized hydroxypropylmethylcellulose.
- the fatty alcohols used as thickners do not contain polyoxyalkylenated groups and are, for example, chosen from myristyl alcohol, cetyl alcohol, stearyl alcohol, and behenyl alcohol.
- the (c) thickener may represent an amount ranging, for example, from 0.1% to 15% by weight, and further, for example, from 0.2% to 10% by weight, and even further, for example, from 0.25% to 5% by weight, relative to the total weight of the composition.
- weight ratio of the (a) oil/the (c) thickener be from 25 to 90, preferably from 25 to 80, and more preferably from 25 to 70.
- composition according to the present invention comprises (d) water.
- the amount of (d) water is not limited, and may be from 30 to 75%» by weight, preferably from 35 to 70%) by weight, and more preferably 40 to 60% by weight, relative to the total weight of the composition.
- composition according to the present invention may comprise at least one (e) dye.
- a single type of dye may be used, but two or more different types of dyes may be used in combination.
- the (e) dye may be selected from the group consisting of direct dyes and oxidative dyes.
- the direct dye means a colored substance which does not require the use of an oxidizing agent in order to develop its color.
- the direct dyes may be selected from natural direct dyes.
- natural dye is understood to mean any dye or dye precursor that is naturally occurring and is produced either by extraction (and optionally purification) from a plant matrix optionally in the presence of natural compounds such as ash or ammonia, or by chemical synthesis.
- quinone dyes such as lawsone and juglone
- alizarin purpurin
- laccaic acid carminic acid
- kermesic acid purpurogallin
- protocatechaldehyde indigoids (such as indigo), sorghum
- isatin betanin
- curcuminoids such as curcumin
- spinulosin various types of chlorophyll and chlorophyllin, hematoxylin, hematein, brazilein, brazilin, safflower dyes (such as carthamin)
- flavonoids such as rutin, quercetin, catechin, epicatechin, morin, apigenidin, and sandalwood
- anthocyans such as apigeninidin and apigenidin
- carotenoids tannins, orceins, santalins, and cochineal carmine.
- extracts or decoctions containing natural direct dye(s) in particular henna-based extracts, curcuma longa extract, sorghum leaf-sheath extract, haematoxylon campechianum extract, green tea extract, pine bark extract, cocoa extract, and logwood extract.
- the natural dye be chosen from the group consisting of curcuminoids, santalins, chlorophyllin, haematoxylin, haematein, brazilein, brazilin, sorghum, laccaic acid, lawsone, juglone, alizarin, purpurin, carminic acid, kermesic acid, purpurogallin, protocatechaldehyde, indigoids, isatin, spinulosin, apigenidin, orcein, betanin, flavonoids, anthocyans, and extracts or decoctions containing these compounds.
- the natural dyes may be preferably chosen, for example, from hydroxylated quinones, indigoids, hydroxyflavones, santalins A and B, isatin and its derivatives, and brasilin and its hydroxylated derivative.
- the hydroxylated quinones are preferably benzoquinones, naphthoquinones, and mono- or polyhydroxylated anthraquinones which are optionally substituted with groups such as alkyl, alkoxy, alkenyl, chloro, phenyl, hydroxyalkyl, and carboxyl.
- the naphthoquinones are preferably lawsone, juglone, flaviolin, naphthazarin, naphthopurpurin, lapachol, plumbagin, chloroplumbagin, droserone, shikonin,
- the benzoquinones are preferably spinulosin, atromentin, aurentioglyocladin,
- the anthraquinones are preferably alizarin, quinizarin, purpurin, carminic acid, chrysophanol, kermesic acid, rhein, aloe emodin, pseudopurpurin, quinizarincarboxylic acid, frangula emodin, 2-methylquinizarin, 1-hydroxyanthraquinone, and 2-hydroxyanthraquinone.
- the indigoids are preferably indigo, indirubin, isoindigo ,and Tyrian purple.
- the hydroxyflavones are preferably quercetin and morin.
- the direct dyes may be selected from synthetic direct dyes.
- synthetic direct dye is understood to mean any dye or dye precursor that is produced by chemical synthesis.
- the synthetic direct dye may be hydrophilic or hydrophobic.
- the synthetic direct dye may be a hydrophilic direct dye selected from the group consisting of acidic (anionic) direct dyes, basic (cationic) direct dyes and neutral (noninic) direct dyes, and a hydrophobic direct dye which cover all possible types of direct dyes, such as so-called nitro dyes, HC dyes, azo, methine, carbonyl, azine, nitro(hetero)aryl types or tri(hetero)arylmethane direct dyes, ⁇ orphyrins and
- Acidic direct dyes have an anionic moiety in their chemical structure.
- Basic direct dyes have a cationic moiety in their chemical structure.
- Neutral direct dyes are nonionic.
- the dyes of the family of the carbonyls mention may be made, for example, of synthetic dyes chosen from acridone, benzoquinone, anthraquinone, naphthoquinone,
- benzanthrone anthranthrone, pyranthrone, pyrazolanthrone, pyrimidinoanthrone, flavanthrone, indanthrone, flavone, (iso)violanthrone, isoindolinone, benzimidazolone, isoquinolinone, anthrapyridone, pyrazoloquinazolone, perinone, quinacridone, quinophthalone, naphthalimide, anthrapyrimidine, diketopyrrolopyrrole, or coumarin dyes.
- dyes of the family of the cyclic azines mention may in particular be made of azine, xanthene, thioxanthene, fluorindine, acridine, (di)oxazine, (di)thiazine, or pyronine dyes.
- nitro(hetero)aromatic dyes are more particularly nitrobenzene or nitropyridine direct dyes.
- cationic or noncationic compounds optionally comprising one or more metals or metal ions, such as, for example, alkali and alkaline earth metals, zinc, and silicon.
- azacarbocyanines such as tetraazacarbocyanines (tetraazapentamethines), quinone, and in particular anthraquinone, naphthoquinone, or benzoquinone direct dyes, or azine, xanthene, triarylmethane, indoamine, phthalocyanine, and ⁇ orphyrin direct dyes, alone or as mixtures.
- these synthetic direct dyes are chosen from nitrobenzene dyes, azo, azomethine, or methine direct dyes, and tetraazacarbocyanines (tetraazapentamethines); alone or as mixtures.
- D represents a nitrogen atom or the -CH group
- R 1 and R 2 which are identical or different, represent a hydrogen atom; a C 1 -C 4 alkyl radical which can be substituted by a -CN, -OH, or -NH 2 radical or can form, with a carbon atom of the benzene ring, an optionally oxygen-comprising or nitrogen-comprising heterocycle which can be substituted by one or more C 1 -C 4 alkyl radicals; or a 4'-aminophenyl radical,
- R 3 and R' 3 which are identical or different, represent a hydrogen atom, a halogen atom chosen from chlorine, bromine, iodine, and fluorine, a cyano radical, a C 1 -C 4 alkyl radical, a C 1 -C 4 alkoxy radical, or an acetyloxy radical
- X- represents an anion, preferably chosen from chloride, methyl sulphate, and acetate
- A represents a group chosen from the following structures:
- R 4 represents a C 1 -C 4 alkyl radical which can be sub tituted by a hydroxyl radical
- R 5 represents a hydrogen atom, a C 1 -C 4 alkoxy radical, or a halogen atom, such as bromine, chlorine, iodine, or fluorine,
- R 6 represents a hydrogen atom or a C 1 -C 4 alkyl radical or forms, with a carbon atom in the benzene ring, a heterocycle which optionally comprises oxygen and/or is optionally substituted by one or more C 1 -C 4 alkyl groups,
- X- represents a cosmetically acceptable anion preferably chosen from chloride, methyl sulphate, and acetate,
- E represents a group chosen from the following structures:
- R' represents a C 1 -C 4 alkyl radical
- E can also denote a group with the following structure:
- R' represents a C 1 -C 4 alkyl radical.
- cationic direct dyes are also particularly suitable to the invention:
- X- represents an anion preferably chosen from chloride, iodide, methyl sulphate, ethyl sulphate, or acetate.
- cationic direct dyes mention may be made of 4-nitro-o-phenylenediamine, 2-nitro-p-phenylenediamine, N,N'-bis-(2-hydroxyemyl)-2-nirro-p-phenylenediamine,
- the direct dye be selected from acidic direct dyes.
- the anionic direct dyes are commonly known as “acidic direct dyes” for their affinity with alkaline substances (see, for example, “Industrial Dyes, Chemistry, Properties, Application”, Klaus Hunger Ed. Wiley- VCH Verlag GmbH & Co KGaA, Weinheim 2003).
- Anionic or acid dyes are known in the literature (see, for example, “ Ullman s ' Encyclopedia of Industrial Chemistry”, Azo Dyes, 2005 Wiley- VCH Verlag GmbH & Co. KGaA, Weinheim 10.1002/14356007.a03 245, point 3.2; ibid, Textile Auxiliaries, 2002 Wiley- VCH Verlag GmbH & Co. KGaA, Weinheim 10.1002/14356007.a26227 and "Ashford's Dictionary of Industrial Chemicals", Second Edition, p. 14-p. 39, 2001). . .
- the preferred anionic dyes of formula of the invention are chosen from acidic nitro direct dyes, acidic azo dyes, acidic azine dyes, acidic triarylmethane dyes, acidic indoamine dyes, acidic anthraquinone dyes, anionic styryl dyes, and indigoids and acidic natural dyes; each of these dyes containing at least one sulfonate, phosphonate or carboxylate group bearing a cationic counterion X + , where X + represents an organic or mineral cationic counter ion preferably chosen from alkali and alkaline-earth metals, such as Na + and K + .
- Preferred acid dyes may be chosen from the dyes below.
- R 7 , Rs, R9, Rio, R'7, R's, R'9 and R'io which may be identical or different, represent a hydrogen atom or a group chosen from:
- X, X' and X which may be identical or different, representing an oxygen or sulfur atom, or NR with R representing a hydrogen atom or an alkyl group; (0) 2 S(0-)-, X + as defined previously;
- R"-S(0) 2 - with R" representing a hydrogen atom or an alkyl, aryl, (di)(alkyl)amino or aryl(alkyl)amino group; preferably a phenylarnino or phenyl group;
- aryl(alkyl)amino optionally substituted with one or more groups chosen from i) nitro; ii) nitroso; iii) (0) 2 S(0-)-, X + and iv) alkoxy with X*;
- heteroaryl preferably a benzothiazolyl group
- cycloalkyl especially cyclohexyl
- Ar-N N- with Ar representing an optionally substituted aryl group, preferably a phenyl optionally substituted with one or more alkyl, (0) 2 S(0 ⁇ )-, X + or phenylarnino groups; or alternatively two contiguous groups R 7 with Rg or Re with R9 or R9 with Rio together form a fused benzo group A' ; and R' 7 with R'g or R'g with R'9 or R'9 with R'io together form a fused benzo group B'; with A' and B' optionally substituted with one or more groups chosen from i) nitro; ii) nitroso; iii) (0) 2 S(0-)-, X 4 ; iv) hydroxyl; v) mercapto; vi) (di)(alkyl)amino; vii) R°-C(X)-X'-; viii) R°-X'-C(X)-; ix) RoX
- W represents a sigma bond ⁇ , an oxygen or sulfur atom, or a divalent radical i) -NR- with R as defined previously, or ii) methylene -C(Ra)(Rt,)- with R 3 ⁇ 4 and Rb, which may be identical or different, representing a hydrogen atom or an aryl group, or alternatively Ra and R b form, together with the carbon atom that bears them, a spiro cycloalkyl; preferably W represents a sulfur atom or Ra and Rb together form a cyclohexyl;
- formulae (II) and (IT) comprise at least one sulfonate (0)2S(0-)-, X + or phosphonate (0)P(0 2 -) 2X + or carboxylate (O)C(O-)-, X + radical on one of the rings A, A', B, B' or C with X + as defined previously.
- dyes of formula (II) mention may be made of Acid Red 1, Acid Red 4, Acid Red 13, Acid Red 14, Acid Red 18, Acid Red 27, Acid Red 32, Acid Red 33, Acid Red 35, Acid Red 37, Acid Red 40, Acid Red 41, Acid Red 42, Acid Red 44, Acid Red 68, Acid Red 73, Acid Red 135, Acid Red 138, Acid Red 184, Food Red 1, Food Red 13, Food Red 17, Orange 4, Acid Orange 6, Acid Orange 7, Acid Orange 10, Acid Orange 19, Acid Orange 20, Acid Orange 24, Acid Yellow 9, Acid Yellow 36, Acid Yellow 199, Food Yellow 3; Acid Violet 7, Acid Violet 14, Acid Blue 113, Acid Blue 117, Acid Black 1, Acid Brown 4, Acid Brown 20, Acid Black 26, Acid Black 52, Food Black 1 , Food Black 2, Pigment Red 57;
- dyes of formula (IT) mention may be made of Acid Red 111, Acid Red 134, Acid yellow 38;
- R 22 , R 23 , R 24 , R 25 , R 26 and R 27 which may be identical or different, represent a hydrogen or halogen atom or a group chosen from:
- aryloxy or arylthio optionally substituted, preferably substituted with one or more groups chosen from alkyl and (0)2S(0-)-, X + with X + as defined previously; aryl(alkyl)amino optionally substituted with one or more groups chosen from alkyl and
- Z' represents a hydrogen atom or a group NR 28 R 29 with R 28 and R 29 , which may be identical or different, representing a hydrogen atom or a group chosen from:
- polyhydroxyalkyl such as hydroxyethyl
- - aryl optionally substituted with one or more groups, particularly i) alkyl such as methyl,
- R° represents an alkyl group
- cycloakyl especially cyclohexyl
- Z represents a group chosen from hydroxyl and NR' 28 R' 29 with R' 28 and R' 29 , which may be identical or different, representing the same atoms or groups as R 28 and R 29 as defined previously; it being understood that formulae ( ⁇ ) and (III') comprise at least one sulfonate group (0) 2 S(0 " )-, X + with X + as defined previously.
- dyes of formula (III) mention may be made of Acid Blue 25, Acid Blue 43, Acid Blue 62, Acid Blue 78, Acid Blue 129, Acid Blue 138, Acid Blue 140, Acid Blue 251, Acid Green 25, Acid Green 41, Acid Violet 42, Acid Violet 43, Mordant Red 3; EXT Violet 2,
- R 61 represents a hydrogen or halogen atom or an alkyl group
- R 6 2, R 63 and R 64 which may be identical or different, represent a hydrogen atom or a group (0) 2 S(0 ⁇ )-, X ⁇ with X* " as defined previously;
- R 61 with R 62 , or R 63 and R 64 together form a benzo group optionally substituted with one or more groups (0) 2 S(0 " )-, X* " with X 4" as defined previously;
- G represents an oxygen or sulfur atom or a group NRe with R» representing a hydrogen atom or an alkyl group; particularly G represents an oxygen atom;
- formula (IV) comprises at least one sulfonate group (0) 2 S(0 " )-, X ⁇ with X ⁇ as defined previously.
- dyes of formula (IV) mention may be made of Acid Yellow 2, Acid Yellow 3 and Acid Yellow 5.
- nonionic direct dyes mention may be made of HC Red 13, HC Red 7, HC Blue 2, HC Yellow 4, HC Yellow 2, HC Red 3, 4-amino-3-nitrophenol,
- hydrophobic direct dyes mention may be made of Solvent Black 3, Solvent Blue 104, Solvent Blue 134, Solvent Blue 14, Disperse Blue 14, Solvent Red 2, Solvent Brown 5, Solvent Green 5, Solvent Orange 2, Solvent Orange 1 , Disperse Orange 24, Solvent Orange 63, Solvent Red 49, Solvent Red 1, Solvent Red 26, Solvent Red 27, Solvent Red 18, Solvent Red 23, Solvent Red 4, Disperse Orange 7, Disperse Blue 72, Disperse Violet 26, Disperse Yellow 16, Disperse Yellow 82, Disperse Yellow 54, Solvent Yellow 29, Solvent Yellow 163, Solvent Yellow 3, Solvent Yellow 56, Solvent Yellow 18, Solvent Yellow 98, Solvent Yellow 12, Solvent Yellow 14, Disperse Red 13, Disperse Green 9, Disperse Blue 148, Disperse Violet 63, Disperse Blue 60, and Solvent Orange 15.
- the synthetic direct dye may be selected from fluorescent dyes. Two or more types of the fluorescent dye may be used in combination. The use of some fluorescent dyes may make it possible to obtain, on dark hair, colors which are more visible than with conventional hydrophilic or hydrophobic direct dyes. Furthermore, these fluorescent dyes, when applied to dark hair, may also make it possible to lighten the hair without damaging it.
- fluorescent dyes is understood to mean fluorescent compounds and optical brighteners. In at least one embodiment, the fluorescent dye is soluble in the medium of the composition.
- Fluorescent dyes are fluorescent compounds which absorb visible radiation, for example, wavelengths ranging from 400 to 800 nm, and which are capable of re-emitting light in the visible region at a higher wavelength.
- the fluorescent dyes useful for the present invention re-emit orange-colored fluorescent light. They exhibit, for instance, a maximum re-emission wavelength ranging from 500 to 700 nm.
- Non-limiting examples of fluorescent dyes include compounds known in the art, for example, those described in Ullmann's Encyclopedia of Industrial Chemistry, Release 2004, 7th edition, “Fluorescent Dyes” chapter.
- the optical brighteners of the present disclosure also known under the name of "brighteners”, or “fluorescent brighteners”, or “fluorescent brightening agents” or “F WA”, or “fluorescent whitening agents”, or “whiteners”, or “fluorescent whiteners”, are colorless transparent compounds as they do not absorb radiation in visible light but only in ultraviolet light (wavelengths ranging from 200 to 400 nanometers) and convert the energy absorbed into fluorescent light of higher wavelength emitted in the visible part of the spectrum, generally in the blue and/or green, that is to say in wavelengths ranging from 400 to 550 nanometers.
- Optical brighteners are known in the art, for example, they are described in Ullmann's
- the fluorescent dyes which can be used in the composition of the present disclosure include compounds known from the art, for example, those described in French Patent No. 2 830 189.
- Soluble fluorescent compounds that may especially be mentioned include those belonging to the following families: naphthalimides, coumarins, xanthenes, and in particular
- xanthenodiquinolizines and azaxanthenes naphtholactams; azlactones; oxazines; thiazines;
- dioxazines dioxazines; azo compounds; azomethines; methines; pyrazines; stilbenes; ketopyrroles; and pyrenes.
- the fluorescent dyes are preferred, more particularly, those re-emitting orange-colored fluorescent light.
- the oxidative dyes may be selected from oxidation bases and couplers.
- the oxidation base can be selected from those conventionally known in oxidation dyeing, preferably from the group consisting of ortho- and para-phenylenediamines, double bases, ortho- and para-aminophenols, heterocyclic bases, and the acid addition salts thereof.
- Ri represents a hydrogen atom, a C 1 -C 4 alkyl radical, a monohydroxy(C 1 -C 4 alkyl) radical, a polyhydroxy-(C 2 -C 4 alkyl) radical, a (C 1 -C 4 )alkoxy(C 1 -C 4 )alkyl radical, a C 1 -C 4 alkylradical substituted with a nitrogen-containing group, a phenyl radical, or a 4'-aminophenyl radical;
- R 2 represents a hydrogen atom, a C 1 -C 4 alkyl radical, a monohydroxy(C 1 -C 4 alkyl) radical, a polyhydroxy(C 2 -C 4 alkyl) radical, a (CrC ⁇ alkoxyiC 1 -C ⁇ alkyl radical, or a C 1 -Q alkyl radical substituted with a nitrogen-containing group;
- Ri and R 2 may also form with the nitrogen atom carrying them a 5- or 6-membered
- nitrogen-containing heterocycle optionally substituted with one or more alkyl, hydroxyl, or ureido groups
- R 3 represents a hydrogen atom, a halogen atom such as a chlorine atom, a C 1 -C 4 alkyl radical, a sulpho radical, a carboxyl radical, a monohydroxy(C 1 -C 4 alkyl) radical, a hydroxy(C 1 -C 4 alkoxy) radical, an ace1ylamino( C 1 -C 4 alkoxy) radical, a mesylamino(C 1 -C 4 alkoxy) radical, or a carbamoylamino(C 1 -C 4 alkoxy) radical; and
- R4 represents a hydrogen or halogen atom or a C 1 -C 4 alkyl radical.
- nitrogen-containing groups of formula (I) above there may be mentioned in particular the amino, mono(C 1 -C 4 )alkylamino, (C 1 -C 4 )dialkylamino, (C 1 -C ⁇ trialkylamino,
- para-phenylenediamines of formula (I) above there may be mentioned more particularly para-phenylenediamine, para-tolylenediamine, 2-chloro-paraphenylenediamine, 2,3-dimethyl-para-phenylenediamine, 2,6-dimemyl-para-phenylenediamine,
- N-(P,Y-dmydroxypropyl)-para-phenylenediamine N-(4 ' -ammophenyl)-para-phenylenediamine
- N-phenyl-para-phenylenediamine 2- ⁇ -hydroxyethyloxy-para-phenylenediamine
- para-phenylenediamines of formula (I) above there are most particularly preferred para-phenylene&amine, para-tolylenediamine, 2-isopropyl-paraphenylenediamine,
- double bases are understood to mean compounds containing at least two aromatic rings on which amino and/or hydroxyl groups are carried.
- double bases which can be used as oxidation bases in the dyeing compositions in accordance with the invention, there may be mentioned in particular compounds corresponding to the following formula II), and their addition salts with an acid:
- - Z 1 and Z 2 which are identical or different, represent a hydroxyl or -NH 2 radical which may be substituted with a C 1 -C 4 alkyl radical or with a linking arm Y;
- the linking arm Y represents a linear or branched alkylene chain comprising from 1 to 14 carbon atoms, which may be interrupted by or which may end with one or more nitrogen-containing groups and/or one or more heteroatoms such as oxygen, sulphur, or nitrogen atoms, and optionally substituted with one or more hydroxyl or C 1 -C 6 alkoxy radicals;
- R 5 and 3 ⁇ 4 represent a hydrogen or halogen atom, a C 1 -C 4 alkyl radical, a monohydroxy(C 1 -C 4 alkyl) radical, a polyhydroxy(C 2 -C 4 alkyl) radical, an amino(C 1 -C 4 alkyl) radical, or a linking arm
- R 7 , Rs, R 9 , R 10 , Rn, and R 12 which are identical or different, represent a hydrogen atom, a linking arm Y, or a C 1 -C 4 alkyl radical; it being understood that the compounds of formula (II) contain only one linking arm Y per molecule.
- nitrogen-containing groups of formula (II) above there may be mentioned in particular the amino, mono(C 1 -C 4 )alkylamino, (C 1 -C 4 )dialkylamino, (C 1 -C 4 )trialkylarnino, monohydroxy(C 1 -C 4 )alkylamino, imidazolinium, and ammonium radicals.
- R 13 represents a hydrogen atom, or a halogen atom such as fluorine, a C 1 -C 4 alkyl,
- R 14 represents a hydrogen atom, or a halogen atom such as fluorine, a C 1 -C 4 alkyl,
- para-arninophenols of formula (III) above there may be mentioned more particularly para-aminophenol, 4-amino-3-methylphenol, 4-amino-3-fluorophenol,
- ormo-aminophenols which can be used as oxidation bases in the context of the present invention are chosen in particular from 2-aminophenol, 2-amino-l-hydroxy-5-methylbenzene, 2-amino-l-hydroxy-6-methylbenzene, 5-acetamido-2-aminophenol, and their addition salts with an acid.
- heterocyclic bases which can be used as oxidation bases in the dyeing compositions in accordance with the invention, there may be mentioned more particularly pyridine derivatives, pyrimidine derivatives, pyrazole derivatives, and their addition salts with an acid.
- pyridine derivatives there may be mentioned more particularly the compounds described for example in Patents GB 1,026,978 and GB 1,153,196, such as 2,5-diaminopyridine, 2-(4-memoxyphenyl)ancdno-3-aminopyridine, 2,3-diamino-6-methoxypyridine,
- pyrimidine derivatives there may be mentioned more particularly the compounds described, for example, in Patents DE 2 359 399; JP 88-169571; and JP 91-10659, or patent application WO 96/15765, such as 2,4,5, 6-tetraammopyrimidine,
- pyrazole derivatives there may be mentioned more particularly the compounds described in Patents DE 3 843 892 and DE 4 133 957 and patent applications WO 94/08969, WO 94/08970, FR-A-2 733 749, and DE 195 43 988 such as 4,5-diamino-l -methylpyrazole,
- heterocyclic bases which can be used as oxidation bases, there may be mentioned more particularly diarninopyrazolopyrazolones and especially
- the couplers may be an oxidation coupler which can be selected from those conventionally known in oxidation dyeing, preferably from the group consisting of meta-phenylenediamines, meta-aminophenols, meta-diphenols, naphthols, heterocyclic couplers, and the acid addition salts thereof.
- the heterocyclic couplers may be selected from the group consisting of indole derivatives, indoline derivatives, sesamol and its derivatives, pyridine derivatives, pyrazolotriazole derivatives, pyrazolones, indazoles, benzimidazoles, benzothiazoles, benzoxazoles, 1,3-benzodioxoles, quinolines, and their addition salts with an acid.
- couplers are more particularly chosen from 2,4-diamino-l-( ⁇ -hydroxyethyloxy)benzene,
- 2-cUoro-3-amino-6-methylphenol 1 ,3-dihydroxybenzene, 1 ,3-dihydroxy-2-methylbenzene, 4-chloro-l ,3-dihydroxybenzene, 2-amino-4-( ⁇ -hydroxyethylamino)-l -methoxybenzene, 1 ,3-diaminobenzene, 2-methyl-5-hydroxyethylaminophenol, 4-amino-2-hydroxytoluene, 1 ,3-bis(2,4-diaminophenoxy)-propane, sesamol,
- the addition acid salts of the oxidation bases and couplers are chosen in particular from hydrochlorides, hydrobromides, sulfates, citrates, succinates, tartrates, lactates, tosylates, benzenesulfonates, phosphates, and acetates.
- composition used for the present invention contains the (b) dye(s) in an amount of 0.001% by weight or more, and may contain, for example, 0.001 to 10% by weight, preferably 0.01 to 5% by weight, and more preferably 0.1 to 3% by weight, relative to the total weight of the composition.
- composition according to the present invention may comprise at least one additional surfactant other than the above ingredient (b).
- the additional surfactant used in the present invention may be selected from the group consisting of anionic surfactants, amphoteric surfactants, cationic surfactants, and nonionic surfactants.
- Two or more additional surfactants may be used in combination.
- a single type of additional surfactant or a combination of different types of additional surfactants may be used.
- the composition according to the present invention does not comprise PEG-40 hydrogenated castor oil in an amount of 1% by weight or more relative to the total weight of the composition. According to one preferred embodiment of the invention, the composition according to the present invention does not comprise PEG-40 hydrogenated castor oil.
- the composition according to the present invention does not comprise a nonionic surfactant other than polyoxyalkylenated fatty alcohol in an amount of 0.5% by weight or more relative to the total weight of the composition. According to one preferred embodiment of the invention, the composition according to the present invention does not comprise a nonionic surfactant other than polyoxyalkylenated fatty alcohol.
- the amount of the additional surfactant(s) may range from 0.1 to 30% by weight, preferably from 1 to 25% by weight, and more preferably from 3 to 20% by weight, relative to the total weight of the composition according to the present invention.
- composition according to the present invention may also comprise an effective amount of other ingredients, which are preferably common in cosmetic products, such as various common adjuvants, antiageing agents, whitening agents, anti-greasy skin agents, sequestering agents such as EDTA and etidronic acid, UV-screening agents, preserving agents such as chlorphenesin, vitamins, or provitamins, for instance, panthenol, opacifiers, fragrances, plant extracts, cationic polymers, and so on.
- other ingredients which are preferably common in cosmetic products, such as various common adjuvants, antiageing agents, whitening agents, anti-greasy skin agents, sequestering agents such as EDTA and etidronic acid, UV-screening agents, preserving agents such as chlorphenesin, vitamins, or provitamins, for instance, panthenol, opacifiers, fragrances, plant extracts, cationic polymers, and so on.
- the composition according to the present invention may further comprise at least one organic solvent.
- the organic solvent is preferably water miscible.
- the organic solvent there may be mentioned, for example, C 1 -C 4 alkanols, such as ethanol and isopropanol; aromatic alcohols such as benzyl alcohol and phenoxyethanol; analogous products; and mixtures thereof.
- the organic water-soluble solvents may be present in an amount ranging from less than 10% by weight, preferably from 5% by weight or less, and more preferably from 1% by weight or less, relative to the total weight of the composition.
- composition according to the present invention can be prepared by mixing the above essential and optional ingredients in accordance with a conventional process.
- the pH of the composition according to the present invention may be controlled.
- the pH may be, for example, from 3 to 7, preferably from 4 to 7, and more preferably from 5 to 7, if the (e) dye is selected from acidic direct dyes.
- the pH may be, for example, from 7 to 11, preferably from 7 to 10, and more preferably from 7 to 9, if the (e) dye is selected from basic direct dyes and oxidative dyes.
- the pH may be adjusted to the desired value using at least one acidifying agent and/or at least one basifying agent.
- the acidifying agents can be, for example, mineral or organic acids, for instance hydrochloric acid, orthophosphoric acid, carboxylic acids, for instance tartaric acid, citric acid, and lactic acid, or sulphonic acids.
- the basifying agent can be, for example, ammonium hydroxide, alkali metal hydroxide, alkali earth metal hydroxide, alkali metal carbonates, alkanolarnines such as mono-, di-, and
- triethanolamines and also their derivatives, preferably sodium or potassium hydroxide and compounds of the formula below.
- R denotes an alkylene such as propylene optionally substituted by a hydroxyl or a C 1 -C 4 alkyl radical
- R LS R 2 , R 3> and P M independently denote a hydrogen atom, an alkyl radical, or a C 1 -C 4 hydroxyalkyl radical, which may be exemplified by 1,3-propanediamine, and derivatives thereof.
- Arginine, urea, and monoethanolamine may be preferable.
- the acidifying or basifying agent may be present in an amount ranging from less than 10% by weight, preferably from 1% by weight or less, and more preferably from 0.5% by weight or less, relative to the total weight of the composition.
- composition according to the present invention be in the form of an emulsion, more preferably a fine emulsion, and even more preferably a nano- or micro-emulsion.
- the composition according to the present invention may be in the form of an O/W emulsion, preferably an O/W nano- or micro-emulsion.
- the dye is selected from direct dyes
- the composition according to the present invention may be a so-called one-part composition or a ready-to-use composition.
- the expression "ready-to-use composition" is defined herein as a composition to be applied immediately to keratin fibers such as hair.
- the so-called one-part composition or the ready-to-use composition may be in a form of hair color treatment composition.
- a so-called one-part composition does not need to mix ingredients in the composition prior to use. Therefore, it is easy for a consumer to use the composition according to the present invention for dyeing keratin fibers. Furthermore, stable coloring of keratin fibers is possible for the composition according to the present invention, because it is not possible to fail to mix ingredients in a precise mixing ratio which is required for two-part compositions for dyeing keratin fibers.
- composition according to the present invention can be used as a cosmetic composition.
- composition according to the present invention can be used for a non-therapeutic process, such as a cosmetic process, for treating the skin, hair, mucous membranes, nails, eyelashes, eyebrows, and/or scalp, by being applied to the skin, hair, mucous membranes, nails, eyelashes, eyebrows, and/or scalp.
- a non-therapeutic process such as a cosmetic process, for treating the skin, hair, mucous membranes, nails, eyelashes, eyebrows, and/or scalp, by being applied to the skin, hair, mucous membranes, nails, eyelashes, eyebrows, and/or scalp.
- the present invention also relates to a use of the composition according to the present invention, as it is or in care products and/or washing products and/or make-up products and/or
- the composition according to the present invention can be used, as it is, as the above product, or the composition according to the present invention can be used as an element of the above products.
- the composition according to the present invention can be added to or combined with any other elements to form the above products.
- the care product may be a lotion, a cream, a serum, a hair tonic, a hair conditioner, a sun screening agent, and the like.
- the washing product may be a shampoo, a face wash, a hand wash, and the like.
- the make-up product may be a foundation, a mascara, a lipstick, a lip gloss, a blusher, an eye shadow, a nail varnish, and the like.
- the make-up-removing product may be a make-up cleansing agent and the like.
- the composition according to the present invention may be intended for dyeing a keratin substance, in particular keratin fibers.
- the compostion according to the present invention can be used as a cosmetic composition, in particular for keratin fibers.
- composition according to the present invention can be used for a non-therapeutic process, such as a cosmetic process, for dyeing keratin fibers such as eyelashes, eyebrows, and hair, comprising the step of applying the composition according to the present invention onto the keratin fibers.
- a non-therapeutic process such as a cosmetic process, for dyeing keratin fibers such as eyelashes, eyebrows, and hair
- the keratin fibers to which the composition according to the present invention has been applied can be left for an appropriate time which is required to treat the keratin fibers.
- the time length for the treatment is not limited, but it may be from 1 minute to 1 hours, preferably 1 minute to 30 minutes, and more preferably 1 minute to 15 minutes.
- the time for dyeing the keratin fibers may be from 1 to 20 minutes, preferably 5 to 15 minutes.
- the keratin fibers may be treated at a room temperature.
- the keratin fibers can be heated at 25C to 65°C, preferably 30°C to 60 °C, more preferably35 °C to 55°C, more preferably 40 °C to 50 °C, during the step of applying the composition according to the present invention to the keratin fibers, and/or the step of leaving the keratin fibers to which the composition according to the present invention has been applied.
- the keratin fibers may be rinsed after the step of applying the composition according to the keratin fibers onto the keratin fibers and/or after the step of leaving the keratin fibers to which the composition according to the present invention has been applied.
- Examples 1-3 and Comparative Examples 1-3 The following compositions according to Examples 1-3 and Comparative Examples 1-3, shown in Table 1 , were prepared by mixing the components shown in Table 1. The numerical values for the amounts of the components shown in Table 1 are all based on "% by weight" as active raw materials.
- compositions according to Examples 4-11 and Comparative Examples 4-7 shown in Table 2, were prepared by mixing the components shown in Table 2.
- the numerical values for the amounts of the components shown in Table 2 are all based on "% by weight" as active raw materials.
- Comparative Examples 4-7 were evaluated by visual observations in accordance with the following criteria.
- compositions according to Example 12 and Comparative Example 8, shown in Table 3 were prepared by mixing the components shown in Table 3.
- the numerical values for the amounts of the components shown in Table 3 are all based on "% by weight" as active raw materials.
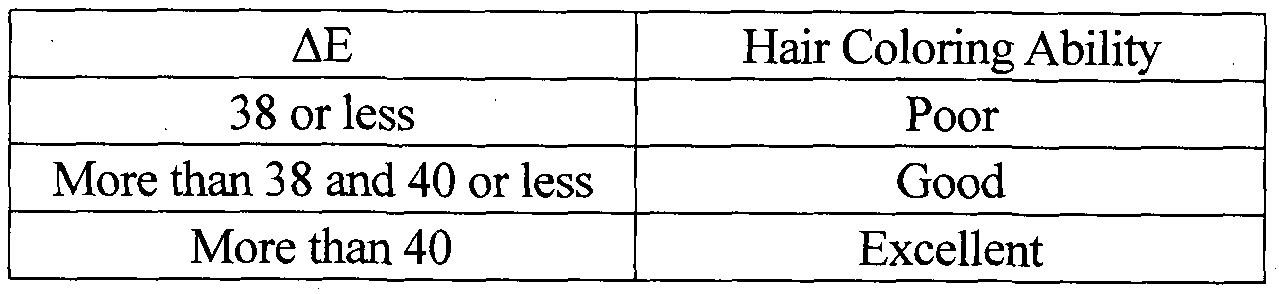
- compositions according to Example 12 and Comparative Example 8 were applied onto a swatch of white goat hair in a weight ratio of 5: 1 (the compositiomthe hair swatch). The applied hair swatch was left for 5 minutes at 27°C. It was then washed out with shampoo and dried. The color of the dyed hair swatch was then measured by Minolta CM-3600d. Based on the color (this was already measured before the application of the composition) of the undyed hair swatch and the measured color of the dyed hair swatch, ⁇ (between the color of the undyed original hair and the color of the dyed hair under the L a b system) was calculated. For the evaluation of hair coloring ability, following criteria was used.
Abstract
The present invention relates to a composition, in particular a cosmetic composition, which is preferably used for keratin fibers such as hair, comprising: (a) at least one oil; (b) at least one nonionic surfactant selected from the group consisting of polyoxyalkylenated fatty alcohols; (c) at least one thickener; and (d) water, wherein the amount of the (a) oil is 25% by weight or more relative to the total weight of the composition, and the weight ratio of the (a) oil/the (c) thickener is from 25 to 100. The composition according to the present invention can provide improved color uptake. Thus, the keratin fibers treated with the composition according to the present invention can be effectively colored. The composition according to the present invention is stable over time. Therefore, the composition according to the present invention can maintain its aspect for a long period of time. In other words, the aspect of the composition according to the present invention does not substantially change for a long period of time.
Description
DESCRIPTION
OIL-RICH COMPOSITION TECHNICAL FIELD
The present invention relates to a composition comprising a relatively large amount of oil, which is preferably used for keratin fibers such as hair. BACKGROUND ART
In addition to higher performance of products for bleaching or coloring hair, consumers of such products are more and more sensitive to the usage quality of the products. From the viewpoint of usage quality, the following, for example, can be regarded as major drawbacks: malodor from ammonia, which is typically contained as an alkaline agent in conventional hair bleaching or coloring products; difficulty in self-handling conventional hair bleaching or coloring products in the form of a liquid, gel or cream; risk of the product dripping off during application to the hair; and the like. In order to reduce the problems occurring with the pungent odor of ammonia, total or partial replacement of this alkaline agent with another one, such as monoemanolamine, has been proposed. However, the consequence of this modification often results in a decrease of the bleaching or coloring efficiency of the products. Recently, as an alternative to hair dyeing or bleaching products based on ammonia as an alkaline agent, compositions comprising a large amount of fatty materials have been proposed. Such compositions may comprise more than 20% by weight of fatty compounds, relative to the total weight of the composition, in combination with an alkaline agent, with or without an oxidative dye. Such compositions can provide high bleaching or coloring ability with a relatively small amount of alkaline agent and advantageously without ammonia.
Also, hair color treatment products using a direct dye are widely used there days, but the products have an issue of less color uptake as compared to permanent or semi-permanent hair color using an oxidative dye.
DISCLOSURE OF INVENTION
However, there is still a need to further improve the color uptake of the above compositions mcluding a large amount of fatty materials.
In addition, a composition including a large amount of fatty materials tends to be unstable over time.
An objective of the present invention is to provide a stable composition, in particular a stable cosmetic composition, which is preferably used for keratin fibers such as hair, with improved
color uptake.
The above objective of the present invention can be achieved by a composition, in particular a cosmetic composition, which is preferably used for keratin fibers such as hair, comprising:
(a) at least one oil;
(b) at least one nonionic surfactant selected from the group consisting of polyoxyalkylenated fatty alcohols;
(c) at least one thickener; and
(d) water,
wherein
the amount of the (a) oil is 25% by weight or more relative to the total weight of the composition, and
the weight ratio of the (a) oil/the (c) thickener is from 25 to 100. The (a) oil may be selected from the group consisting of hydrocarbon oils and silicone oils.
The amount of the (a) oil may range from 25 to 90% by weight, preferably from 30 to 80% by weight, and more preferably from 35 to 70% by weight, relative to the total weight of the composition.
The polyoxyalkylenated fatty alcohols may be selected from saturated or unsaturated, linear or branched, oxyalkylenated C8-C30 alcohols.
The polyoxyalkylenated fatty alcohols may include from 1 to 100 ethyleneoxide units, preferably from 2 to 50 ethyleneoxide units, and more preferably from 2 to 30 ethyleneoxide units.
The amount of the (b) nonionic surfactant may range from 0.01 to 15% by weight, preferably from 0.1 to 10% by weight, and more preferably from 0.5 to 5% by weight, relative to the total weight of the composition.
The (c) thickener may be selected from polysaccharides.
The amount of the (c) thickener may range from 0.1 to 15%) by weight, preferably from 0.2 to 10% by weight, and more preferably from 0.25 to 5% by weight, relative to the total weight of the composition.
The weight ratio of the (a) oil/the (c) thickener may be from 25 to 90, preferably from 25 to 80, and more preferably from 25 to 70. The composition according to the present invention may further comprise (e) at least one dye selected from the group consisting of direct dyes and oxidative dyes.
The direct dyes may be selected from synthetic direct dyes. The synthetic direct dyes may be selected from the group consisting of: hydrophilic synthetic
direct dyes selected from the group consisting of cationic synthetic direct dyes, nonionic synthetic direct dyes, and anionic synthetic direct dyes; and hydrophobic synthetic direct dyes.
The amount of the (e) dye may range from 0.001 to 10% by weight, preferably from 0.01 to 5% by weight, and more preferably from 0.1 to 3% by weight, relative to the total weight of the composition.
It is preferable that the composition according to the present invention be intended for dyeing keratin fibers, preferably hair.
Furthermore, the present invention also relates to a cosmetic process for keratin fibers, preferably hair, comprising the steps of applying the composition according to the present invention to the keratin fibers. BEST MODE FOR CARRYING OUT THE INVENTION
After diligent research, the inventors have discovered that it is possible to provide a stable composition, in particular a stable cosmetic composition, which is preferably used for keratin fibers such as hair, with improved color uptake, if the composition includes a combination of selected specific ingredients under specific conditions.
Thus, the present invention is a composition, in particular a cosmetic composition, which is preferably used for keratin fibers such as hair, comprising:
(a) at least one oil;
(b) at least one nonionic surfactant selected from the group consisting of polyoxyalkylenated fatty alcohols;
(c) at least one thickener; and
(d) water,
wherein
the amount of the (a) oil is 25% by weight or more relative to the total weight of the composition, and
the weight ratio of the (a) oil/the (c) thickener is from 25 to 100.
The composition according to the present invention can provide improved color uptake. Thus, the keratin fibers treated with the composition according to the present invention can be effectively colored.
The composition according to the present invention is stable over time. The term "stable" here means that the aspect of the composition does not change or substantially change for 30 minutes or more, preferably about a few hours or more, and more preferably about a few months or more.
The composition according to the present invention can maintain its aspect for a long period of time. In other words, the aspect of the composition according to the present invention does not change or substantially change for a long period of time.
Since the composition according to the present invention is stable over time, the composition can preferably be a cosmetic composition.
In addition, if the (e) dye is present and is selected from oxidative dyes, the composition according to the present invention can provide enhanced coloring ability with a relatively small amount of alkaline agent even without ammonia.
Furthermore, due to the presence of the thickener, the composition according to the present invention can have an appropriate viscosity. Thus, it is easy for the composition according to the present invention to be applied onto keratin fibers such as hair with hand or fingers, and the risk of the composition according to the present invention dripping off during the application to the keratin fibers can be reduced.
Hereinafter, the composition according to the present invention will be explained in a more detailed manner.
[Oil]
The composition according to the present invention comprises at least one (a) oil. Here, "oil" means a fatty compound or substance which is in the form of a liquid at room temperature (25 °C) under atmospheric pressure (760 rnmHg). As the oils, those generally used in cosmetics may be used alone or in combination thereof. These oils may be volatile or non- volatile, preferably non-volatile. The (a) oil may be a non-polar oil such as a hydrocarbon oil, a silicone oil, or the like; a polar oil such as a plant or animal oil and an ester oil or an ether oil; or a mixture thereof.
It is preferable that the (a) oil be selected from the group consisting of oils of plant or animal origin, synthetic oils, silicone oils, and hydrocarbon oils.
As examples of plant oils, mention may be made of, for example, linseed oil, camellia oil, macadamia nut oil, corn oil, mink oil, olive oil, avocado oil, sasanqua oil, castor oil, safflower oil, jojoba oil, sunflower oil, almond oil, rapeseed oil, sesame oil, soybean oil, peanut oil, and mixtures thereof.
As examples of animal oils, mention may be made of, for example, squalene and squalane.
As examples of synthetic oils, mention may be made of alkane oils such as isododecane and isohexadecane, ester oils, ether oils, and artificial triglycerides.
The ester oils are preferably liquid esters of saturated or unsaturated, linear or branched C1-C26 aliphatic monoacids or polyacids and of saturated or unsaturated, linear or branched C1-C26 aliphatic monoalcohols or polyalcohols, the total number of carbon atoms of the esters being greater than or equal to 10.
Preferably, for the esters of monoalcohols, at least one from among the alcohol and the acid from which the esters of the invention are derived is branched.
Among the monoesters of monoacids and of monoalcohols, mention may be made of ethyl palmitate, ethyl hexyl palmitate, isopropyl palmitate, dicaprylyl carbonate, alkyl myristates such as isopropyl myristate or ethyl myristate, isocetyl stearate, 2-ethylhexyl isononanoate, isononyl isononanoate, isodecyl neopentanoate, and isostearyl neopentanoate.
Esters of C4-C22 dicarboxylic or tricarboxylic acids and of C1-C22 alcohols and esters of monocarboxylic, dicarboxylic, or tricarboxylic acids and of non-sugar C4-C26 dihydroxy, trihydroxy, tetrahydroxy, or pentahydroxy alcohols may also be used.
Mention may especially be made of: diethyl sebacate; isopropyl lauroyl sarcosinate; diisopropyl sebacate; bis(2-ethylhexyl) sebacate; diisopropyl adipate; di-n-propyl adipate; dioctyl adipate; bis(2-ethylhexyl) adipate; diisostearyl adipate; bis(2-ethylhexyl) maleate; triisopropyl citrate; triisocetyl citrate; triisostearyl citrate; glyceryl trilactate; glyceryl trioctanoate; trioctyldodecyl citrate; trioleyl citrate; neopentyl glycol diheptanoate; and diethylene glycol diisononanoate.
As ester oils, one can use sugar esters and diesters of C6-C30 and preferably C12-C22 fatty acids. It is recalled that the term "sugar" means oxygen-bearing hydrocarbon-based compounds containing several alcohol functions, with or without aldehyde or ketone functions, and which comprise at least 4 carbon atoms. These sugars may be monosaccharides, oligosaccharides, or polysaccharides. Examples of suitable sugars that may be mentioned include sucrose (or saccharose), glucose, galactose, ribose, fucose, maltose, fructose, mannose, arabinose, xylose, and lactose, and derivatives thereof, especially alkyl derivatives, such as methyl derivatives, for instance methylglucose. The sugar esters of fatty acids may be chosen especially from the group comprising the esters or mixtures of esters of sugars described previously and of linear or branched, saturated or unsaturated C6-C3o and preferably C12-C22 fatty acids. If they are unsaturated, these compounds may have one to three conjugated or non-conjugated carbon-carbon double bonds. The esters according to this variant may also be selected from monoesters, diesters, triesters, tetraesters, and polyesters, and mixtures thereof.
These esters may be, for example, oleates, laurates, palmitates, myristates, behenates, cocoates, stearates, linoleates, linolenates, caprates, and arachidonates, or mixtures thereof such as, especially, oleopalmitate, oleostearate, and palmitostearate mixed esters, as well as pentaerythrityl tetraethyl hexanoate.
More particularly, use is made of monoesters and diesters and especially sucrose, glucose or methylglucose monooleates or dioleates, stearates, behenates, oleopalmitates, linoleates, linolenates, and oleostearates.
An example that may be mentioned is the product sold under the name Glucate® DO by the company Amerchol, which is a methylglucose dioleate. As examples of preferable ester oils, mention may be made of, for example, diisopropyl adipate, dioctyl adipate, 2-ethylhexyl hexanoate, ethyl laurate, cetyl octanoate, octyldodecyl octanoate, isodecyl neopentanoate, myristyl propionate, 2-ethylhexyl 2-ethylhexanoate, 2-ethylhexyl octanoate, 2-ethylhexyl caprylate/caprate, methyl palmitate, ethyl palmitate, isopropyl palmitate, ethylhexyl palmitate, isohexyl laurate, hexyl laurate, isocetyl stearate, isopropyl isostearate, isopropyl myristate, isodecyl oleate, glyceryl tri(2-ethylhexanoate), pentaerythrithyl
tetra(2-ethylhexanoate), 2-ethylhexyl succinate, diethyl sebacate, and mixtures thereof.
As examples of artificial triglycerides, mention may be made of, for example, glyceryl frimyristate, glyceryl tripalmitate, glyceryl trilinolenate, glyceryl trilaurate, glyceryl tricaprate, glyceryl tricaprylate, glyceryl tri(caprate/caprylate), and glyceryl tri(caprate/caprylate/linolenate).
As examples of silicone oils, mention may be made of, for example, linear organopolysiloxanes such as dimethylpolysiloxane, methylphenylpolysiloxane, methylhydrogenpolysiloxane, and the like; cyclic organopolysiloxanes such as octamethylcyclotetrasiloxane,
decamethylcyclopentasiloxane, dodecamethylcyclohexasiloxane, and the like; and mixtures thereof.
Preferably, the silicone oil is chosen from liquid polydialkylsiloxanes, especially liquid
polydimethylsiloxanes (PDMS) and liquid polyorganosiloxanes comprising at least one aryl group.
These silicone oils may also be organomodified. The organomodified silicones that can be used in accordance with the present invention are silicone oils as defined above and comprising in their structure one or more organofunctional groups attached via a hydrocarbon-based group.
Organopolysiloxanes are defined in greater detail in Walter Noll's Chemistry and Technology of Silicones ( 1968), Academic Press. They may be volatile or non- volatile.
Volatile or non- volatile silicone oils, such as volatile or non- volatile polydimethylsiloxanes (PDMS) containing a linear or cyclic silicone chain, that are liquid or pasty at ambient temperature, in particular cyclopolydimethylsiloxanes (cyclomethicones) such as eyclohexasiloxane;
polydimethylsiloxanes containing alkyl, alkoxy, or phenyl groups that are pendent or at the end of the silicone chain, which groups have from 2 to 24 carbon atoms; phenyl silicones such as phenyl tamethicones, phenyl dimethicones, phenyltrimethylsiloxydiphenylsiloxanes, diphenyl
dimethicones, diphenylmethyldiphenyltrisiloxanes, 2-phenylemyltrimethyl siloxysilicates, and polymethylphenylsiloxanes, may be used.
Hydrocarbon oils may be chosen from:
linear or branched, optionally cyclic, C6-C16 lower alkanes. Examples that may be mentioned include hexane, undecane, dodecane, tridecane, and isoparaffins, for instance
isohexadecane, isododecane, and isodecane; and
linear or branched hydrocarbons containing more than 16 carbon atoms, such as liquid paraffins, liquid petroleum jelly, polydecenes, and hydrogenated polyisobutenes such as Parleam®, and squalane.
As preferable examples of hydrocarbon oils, mention may be made of, for example, linear or branched hydrocarbons such as mineral oil (e.g., liquid paraffin), paraffin, vaseline or petrolatum, naphthalenes, and the like; hydrogenated polyisobutene, isoeicosan, and decene/butene copolymer; and mixtures thereof.
It is preferable that the (a) oil be chosen from hydrocarbon oils and silicone oils.
The amount in the composition according to the present invention of the (a) oil is 25% by weight or more relative to the total weight of the composition, and it may range from 25 to 90% by weight, preferably from 30 to 80% by weight, and more preferably from 35 to 70% by weight, relative to the total weight of the composition.
In one embodiment, the amount of the (a) oil may range from 30 to 90% by weight, preferably from 30 to 80% by weight, and more preferably from 30 to 70% by weight, relative to the total weight of the composition.
In a preferred embodiment, the amount of the (a) oil may range from 35 to 90% by weight, preferably from 35 to 80% by weight, and more preferably from 35 to 70% by weight, relative to the total weight of the composition.
In a more preferred embodiment, the amount of the (a) oil may range from 40 to 90% by weight, preferably from 40 to 80% by weight, and more preferably from 40 to 70% by weight, relative to the total weight of the composition. In a furthermore preferred embodiment, the amount of the (a) oil may range from 50 to 90% by weight, preferably from 50 to 80% by weight, and more preferably from 50 to 70% by weight, relative to the total weight of the composition.
[Nonionic Surfactant]
The composition according to the present invention comprises at least one nonionic surfactant selected from the group consisting of polyoxyalkylenated fatty alcohols. A single type of the polyoxyalkylenated fatty alcohol may be used, but two or more different types of the
polyoxyalkylenated fatty alcohol may be used in combination.
The polyoxyalkylenated fatty alcohols are also called as polyalkylene glycol ether of fatty alcohols or polyoxyalkylene alkyl ether, and mean ethers of one or more fatty alcohols and of one or more oxyalkylene polymers. The polyoxyalkylenated fatty alcohols may be selected from saturated or unsaturated, linear or
branched, polyoxyalkylenated C8-C3o alcohols.
The oxyalkylene units are more particularly oxyethylene or oxypropylene units, or a combination thereof, preferably oxyethylene units. It is preferable that the polyoxyalkylenated fatty alcohols include from 1 to 100 ethyleneoxide units, more preferably from 2 to 50 ethyleneoxide units, and even more preferably from 2 to 30 ethyleneoxide units. Advantageously, the nonionic surfactants do not comprise any oxypropylene units.
Examples of polyoxyethylenated fatty alcohol (or C8-C30 alcohols) that may be mentioned include the adducts of ethylene oxide with lauryl alcohol, especially those containing from 2 to 50 oxyethylene units and more particularly those containing from 2 to 20 oxyethylene units
(Laureth-2 to Laureth-20, as the CTFA names); the adducts of ethylene oxide with behenyl alcohol, especially those containing from 2 to 50 oxyethylene units and more particularly those containing from 2 to 20 oxyethylene units (Beheneth-2 to Beheneth-20, as the CTFA names); the adducts of ethylene oxide with cetearyl alcohol (mixture of cetyl alcohol and stearyl alcohol), especially those containing from 2 to 30 oxyethylene units (Ceteareth-2 to Ceteareth-30, as the CTFA names); the adducts of ethylene oxide with cetyl alcohol, especially those containing from 2 to 30 oxyethylene units (Ceteth-2 to Ceteth-30, as the CTFA names); the adducts of ethylene oxide with stearyl alcohol, especially those containing from 2 to 50 oxyethylene units and more particularly those containing from 2 to 20 oxyethylene units (Steareth-2 to Steareth-20, as the CTFA names); the adducts of ethylene oxide with isostearyl alcohol, especially those containing from 2 to 50 oxyethylene units (Isosteareth-2 to Isosteareth-50, as the CTFA names); and mixtures thereof. According to an embodiment, the composition according to the invention contains at least one fatty alcohol comprising from 2 to 9 ethyleneoxide units and at least one fatty alcohol comprising from 10 to 30 ethyleneoxide units.
The amount of the (b) nonionic surfactant selected from the group consisting of
polyoxyalkylenated fatty alcohols may range from 0.01 to 15% by weight, preferably from 0.1 to 10% by weight, and more preferably from 0.5 to 5% by weight, relative to the total weight of the composition.
[Thickener]
The composition according to the present invention comprises at least, one (c) thickener. A single type of thickener may be used, but two or more different types of thickener may be used in combination. The (c) thickener may be selected from hydrophilic polymers, preferably water-soluble polymers.
It is preferable that the (c) thickener be selected from the group consisting of:
(i) associative thickeners;
(ii) crosslinked acrylic acid homopolymers;
(iii) crosslinked copolymers of (meth)acrylic acid and of ( C1-C6)alkyl acrylate;
(iv) nonionic homopolymers and copolymers comprising ethylenically unsaturated monomers of ester and/or amide type;
(v) ammonium acrylate homopolymers and copolymers of ammonium acrylate and of acrylamide;
(vi) polysaccharides; and
(vii) Co-C3o fatty alcohols.
(i) As used herein, the expression "associative thickener" means an amphiphilic thickener comprising both hydrophilic units and hydrophobic units, for example, at least one C8-C30 fatty chain and at least one hydrophilic unit.
Representative associative thickeners that may be used are associative polymers chosen from: (aa) nonionic amphiphilic polymers comprising at least one fatty chain and at least one hydrophilic unit;
(bb) anionic amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit;
(cc) cationic amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit; and
(dd) amphoteric amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit,
wherein the fatty chain contains from 8 to 30 carbon atoms.
The (aa) nonionic amphiphilic polymers comprising at least one fatty chain and at least one hydrophilic unit may, for example, be chosen from:
(1) celluloses modified with groups comprising at least one fatty chain; examples that may be mentioned include:
hydroxyethylcelluloses modified with groups comprising at least one fatty chain chosen from alkyl, arylalkyl, and alkylaryl groups, and in which the alkyl groups are, for example, C8-c22, such as the product Natrosol Plus Grade 330 CS(C1-6 alkyls) sold by the company Aqualon, and the product Bermocoll EHM 100 sold by the company Berol Nobel, and
celluloses modified with polyalkylene glycol alkylphenyl ether groups, such as the product Amercell Polymer HM-1500 (polyethylene glycol (15) nonylphenyl ether) sold by the company Amerchol.
(2) hydroxypropyl guars modified with groups comprising at least one fatty chain, such as the product Esaflor HM 22 (C22 alkyl chain) sold by the company Lamberti, and the products Miracare XC95-3 (C1 alkyl chain) and RE205-1 (C20 alkyl chain) sold by the company Rhodia Chimie.
(3) polyether urethanes comprising at least one fatty chain, such as C10-C30 alkyl or alkenyl groups, for instance the products Elfacos T 210 and Elfacos T 212 sold by the company Akzo or the products Aculyn 44 and Aculyn 46 sold by the company Rohm & Haas.
(4) copolymers of vinylpyrrolidone and of hydrophobic fatty-chain monomers;
examples that may be mentioned include:
the products Antaron V216 and Ganex V216 (vinylpyrrolidone/hexadecene copolymer) sold by the company I.S.P., and
the products Antaron V220 and Ganex V220 (vinylpyrrolidone/eicosene copolymer) sold by the company I.S.P.
(5) copolymers of C1-C6 alkyl acrylates or methacrylates and of amphiphilic monomers comprising at least one fatty chain, such as the oxyethylenated methyl
methacrylate/stearyl acrylate copolymer sold by the company Goldschmidt under the nameAntil 208.
(6) copolymers of hydrophilic acrylates or methacrylates and of hydrophobic monomers comprising at least one fatty chain, such as a polyethylene glycol methacrylate/lauryl methacrylate copolymer.
The (bb) anionic amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit, may, for example, be chosen from those comprising at least one fatty-chain allyl ether unit and at least one hydrophilic unit comprising an ethylenic unsaturated anionic monomelic unit, for example, a vinylcarboxylic acid unit and further, for example, be chosen from units derived from acrylic acids, methacrylic acids, and mixtures thereof, wherein the fatty-chain allyl ether unit corresponds to the monomer of formula (I) below:
CH2=C(R1)CH2OBnR (I) in which Ri is chosen from H and CH3, B is an ethyleneoxy radical, n is chosen from zero and integers ranging from 1 to 100, R is chosen from hydrocarbon-based radicals chosen from alkyl, arylalkyl, aryl, alkylaryl, and cycloalkyl radicals, containing from 10 to 30 carbon atoms, and further, for example, from 10 to 24 carbon atoms and even further, for example, from 12 to 18 carbon atoms.
In one embodiment, a unit of formula (I) is, for example, a unit in which Ri can be H, n can be equal to 10, and R can be a stearyl (C18) radical.
Anionic amphiphilic polymers of this type are described and prepared, according to an emulsion polymerization process, in patent EP-0216 479 B2.
In one embodiment, anionic amphiphilic polymers are, for example, polymers formed from 20% to 60% by weight of acrylic acid and/or of methacrylic acid, from 5% to 60% by weight of lower alkyl (meth)acrylates, from 2% to 50% by weight of fatty-chain allyl ether of formula (I), and from 0% to 1%) by weight of a crosslinking agent which is a well-known copolymerizable unsaturated polyethylenic monomer, for example, diallyl phthalate, allyl(meth)acrylate, divinylbenzene, (poly)ethylene glycol dimethacrylate, and methylenebisacrylamide.
Examples of such polymers are crosslinked terpolymers of methacrylic acid, of ethyl acrylate, and of polyethylene glycol (10 EO) stearyl ether (Steareth-10), such as those sold by the company Ciba under the names Salcare SC 80 and Salcare SC 90, which are aqueous 30% emulsions of a crosslinked terpolymer of methacrylic acid, of ethyl acrylate, and of steareth-10 allyl ether (40/50/10). The anionic amphiphilic polymers may further be chosen, for example, from those comprising at
least one hydrophilic unit of unsaturated olefinic carboxylic acid type, and at least one hydrophobic unit of a type such as a ( C10-C30) alkyl ester of an unsaturated carboxylic acid. The hydrophilic unit of unsaturated olefinic carboxylic acid type corresponds to, for example, the monomer of formula (II) below:
in which R1 is chosen from H, CH3, and C2H , i.e., acrylic acid, methacrylic acid, and ethacrylic acid units. The hydrophobic unit of a type such as a (C10-C30) alkyl ester of an unsaturated carboxylic acid corresponds to, for example, the monomer of formula (III) below:
in which R1 is chosen from H, CH3, and C2H5 (i.e., acrylate, methacrylate, and ethacrylate units) and is, for example, chosen from, for example, H (acrylate units) and CH3 (methacrylate units), and R is chosen from C10-C30 alkyl radicals, for example, C12-C22 alkyl radicals.
Examples of (C10-C3o)alkyl esters of unsaturated carboxylic acids include lauryl acrylate, stearyl acrylate, decyl acrylate, isodecyl acrylate, and dodecyl acrylate, and the corresponding methacrylates, lauryl methacrylate, stearyl methacrylate, decyl methacrylate, isodecyl methacrylate, and dodecyl methacrylate.
Anionic amphiphilic polymers of this type are disclosed and prepared, for example, according to U.S. Pat. Nos. 3,915,921 and 4,509,949.
Representative anionic amphiphilic polymers that can be used may further be chosen from polymers formed from a mixture of monomers comprising:
(7) acrylic acid, an ester of formula (IV) below:
in which R1 is chosen from H and CH3, R2 is chosen from C10-C30 alkyl radicals, such as alkyl radicals containing from 12 to 22 carbon atoms, and a crosslinking agent; such as polymers derived from 95% to 60% by weight of the acrylic acid (hydrophilic unit), 4% to 40% by weight of C1o-C30 alkyl acrylate (hydrophobic unit), and 0%» to 6% by weight of crosslinking polymerizable monomer, or polymers derived from 98% to 96% by
weight of the acrylic acid (hydrophilic unit), 1% to 4% by weight of C10-C30 alkyl acrylate (hydrophobic unit), and 0.1% to 0.6% by weight of crosslinking polymerizable monomer; or
(8) acrylic acid and lauryl methacrylate, such as the polymers formed from 66% by weight of acrylic acid and 34% by weight of lauryl methacrylate.
with at least one other polymerizable group whose unsaturated bonds are not conjugated.
Mention may be made, for example, of polyallyl ethers such as polyallylsucrose and
polyallylpentaerythritol.
Among said polymers above, mention may be made, for example, of the products sold by the company Goodrich under the trade names Pemulen TR 1 , Pemulen TR2, and Carbopol 1382, and further, for example, Pemulen TRl, and the product sold by the company S.E.P.C. under the name Coatex SX.
Among anionic amphiphilic fatty-chain polymers, mention may also be made, for example, of the ethoxylated copolymer of methacrylic acid/methyl acrylate/alkyl
dimethyl-meta-isopropenylbenzylisocyanate sold under the name Viscophobe DB 1000 by the company Amerchol.
The (cc) cationic amphiphilic polymers used are, for example, chosen from quatemized cellulose derivatives and polyacrylates comprising amino side groups.
The quatemized cellulose derivatives are, for example, chosen from
quatemized celluloses modified with groups comprising at least one fatty chain, such as alkyl, arylalkyl, and alkylaryl groups comprising at least 8 carbon atoms, and mixtures thereof, and quatemized hydroxyethylcelluloses modified with groups comprising at least one fatty chain, such as alkyl, arylalkyl, and alkylaryl groups comprising at least 8 carbon atoms, and mixtures thereof.
Quatemized and non-quaternized polyacrylates comprising amino side groups have, for example, hydrophobic groups, such as Steareth 20 (polyoxy-ethylenated (20) stearyl alcohol) and
(C10-C30)alkyl PEG-20 itaconate.
The alkyl radicals borne by the above quatemized celluloses and hydroxyethylcelluloses, for example, contain from 8 to 30 carbon atoms. The aryl radicals, for example, are chosen from phenyl, benzyl, naphthyl, and anthryl groups.
Examples of quatemized alkylhydroxyethyl-celluloses comprising C8-C30 fatty chains are the products Quatrisoft LM 200, Quatrisoft LM-X 529-18-A, Quatrisoft LM-X 529-18B (C12 alkyl),
and Quatrisoft LM-X 529-8 (C18 alkyl) sold by the company Amerchol, and the products Crodacel QM, Crodacel QL (C12 alkyl), and Crodacel QS (C18 alkyl) sold by the company Croda.
Examples of polyacrylates comprising amino side chains are the polymers 8781-124B or
9492-103 and Structure Plus from the company National Starch.
Among the (dd) amphoteric amphiphilic polymers comprising at least one hydrophilic unit and at least one fatty-chain unit, mention may be made, for example, of copolymers of
memacrylarnidopropyltrimethylammonium chloride/acrylic acid/C10-C3o alkyl methacrylate, wherein the alkyl radical is, for example, a stearyl radical.
The associative thickeners in the compositions can have, for example, in solution or in dispersion at a concentration of 1% active material in water, a viscosity, measured using a Rheomat RM 180 rheometer at 25°C, of greater than 0.1 ps and further, for example, of greater than 0.2 cp, at a shear rate of 200 s-1.
(ii) Among the crosslinked acrylic acid homopolymers that may be mentioned are those crosslinked with an allylic alcohol ether of the sugar series, such as the products sold under the names Carbopol 980, 981, 954, 2984, and 5984 by the company Goodrich or the products sold under the names Synthalen M and Synthalen K by the company 3 VSA.
(iii) The crosslinked copolymers of (meth)acrylic acid and of C1-C1 alkyl acrylate can be chosen from crosslinked copolymers of methacrylic acid and of ethyl acrylate as an aqueous dispersion comprising 38% active material sold, for example, under the name Viscoatex 538C by the company Coatex, and crosslinked copolymers of acrylic acid and of ethyl acrylate as an aqueous dispersion comprising 28% active material sold under the name Aculyn 33 by the company Rohm & Haas. Crosslinked copolymers of methacrylic acid and of ethyl acrylate include an aqueous dispersion comprising 30% active material sold under the name CARBOPOL AQUA SF-1 by the company NOVEON.
(iv) Among the nonionic homopolymers or copolymers comprising ethylenically unsaturated monomers of ester and/or amide type, mention may be made of the products sold under the names: Cyanamer P250 by the company Cytec (polyacrylamide); PMMA MBX-8C by the company US Cosmetics (methyl methacrylate/ethylene glycol dimethacrylate copolymer);
Acryloid B66 by the company Rohm & Haas (butyl methacrylate/methyl methacrylate
copolymer); and BPA 500 by the company Kobo (polymethyl methacrylate).
(v) Ammonium acrylate homopolymers that may be mentioned include the product sold under the name Microsap PAS 5193 by the company Hoechst.
Copolymers of ammonium acrylate and of acrylarnide include the product sold under the name Bozepol C Nouveau or the product PAS 5193 sold by the company Hoechst (which are described and prepared in documents FR-2 416 723, U.S. Pat. No. 2,798,053, and U.S. Pat. No. 2,923,692). It is preferable that the (c) thickener is selected from polysaccharides.
(vi) The polysaccharides are, for example, chosen from glucans, modified and unmodified starches (such as those derived, for example, from cereals, for instance wheat, corn, or rice, from vegetables, for instance yellow peas, and tubers, for instance potatoes or cassava), amylose, amylopectin, glycogen, dextrans, celluloses, and derivatives thereof (e.g., methylcelluloses, hydroxyalkylcelluloses, ethyl hydroxyethylcelluloses, and carboxymethylcelluloses), mannans, xylans, lignins, arabans, galactans, galacturonans, chitin, chitosans, glucuronoxylans,
arabinoxylans, xyloglucans, glucomannans, pectic acids, and pectins, alginic acid and alginates, arabinogalactans, carrageenans, agars, glycosaminoglucans, gum arables, gum tragacanths, ghatti gums, karaya gums, carob gums, galactomannans, such as guar gums, and nonionic derivatives thereof (e.g., hydroxypropyl guar), and xanthan gums, and mixtures thereof.
For example, the polysaccharides that may be used are chosen from those described, for example, in "Encyclopedia of Chemical Technology", Kirk-Othmer, Third Edition, 1982, volume 3, pp. 896-900, and volume 15, pp. 439-458, in "Polymers in Nature" by E. A. MacGregor and C. T. Greenwood, published by John Wiley & Sons, Chapter 6, pp. 240-328, 1980, and in "Industrial Gums-Polysaccharides and their Derivatives", edited by Roy L. Whistler, Second Edition, published by Academic Press Inc., the content of these three publications being entirely incorporated by reference.
For example, starches, guar gums, celluloses, and derivatives thereof can be used.
Among the starches that may be used, mention may be made, for example, of macromolecules in the form of polymers comprising base units which are anhydroglucose units. The number of these units and their assembly make it possible to distinguish between amylose (linear polymer) and amylopectin (branched polymer). The relative proportions of amylose and amylopectin, as well as their degree of polymerization, can vary according to the botanical origin of the starches.
The molecules of starches used may have cereals or tubers as their botanical origin. Thus, the starches can be, for example, chosen from maize, rice, cassava, tapioca, barley, potato, wheat, sorghum, and pea starches.
Starches generally exist in the form of a white powder, insoluble in cold water, whose elementary particle size ranges from 3 to 100 microns.
The starches may be optionally C1-C hydroxyalkylated or C1-C acylated (such as acetylated). The starches may have also undergone heat treatments.
Distarch phosphates or compounds rich in distarch phosphate, such as the product provided under the references PREJEL VA-70-T AGGL (gelatinized hydroxypropylated cassava distarch phosphate) or PREJEL TKl (gelatinized cassava distarch phosphate) or PREJEL 200 (gelatinized acetylated cassava distarch phosphate) by the company AVEBE, may also be used.
The guar gums can be modified or unmodified.
The unmodified guar gums are, for example, the products sold under the name Vidogum GH 175 by the company Unipectine and under the names Meypro-Guar 50 and Jaguar C by the company Meyhall. The modified nonionic guar gums are, for example, modified with C1-C6 hydroxyalkyl groups.
Among the hydroxyalkyl groups that may be mentioned, for example, are hydroxymethyl, hydroxyethyl, hydroxypropyl, and hydroxybutyl groups. These guar gums are well known in the prior art and can be prepared, for example, by reacting the corresponding alkene oxides such as propylene oxides, with the guar gum so as to obtain a guar gum modified with hydroxypropyl groups.
The degree of hydroxyalkylation, which corresponds to the number of alkylene oxide molecules consumed by the number of free hydroxyl functions present on the guar gum, may, for example, range from 0.4 to 1.2.
Such nonionic guar gums optionally modified with hydroxyalkyl groups are sold, for example, under the trade names Jaguar HP8, Jaguar HP60, Jaguar HP120, Jaguar DC 293, and Jaguar HP 105 by the company Rhodia Chimie (Meyhall) or under the name Galactasol 4¾FD2 by the company Aqualon.
Among the celluloses and cellulose derivatives, such as cellulose modified with hydroxylalkyl groups, that are used are, for example, hydroxypropylmethylcellulose, hydroxyethylcellulose, and hydroxypropylcelluloses, as well as hydrophobicized hydroxypropylmethylcellulose. Mention may be made of the products sold under the names Klucel E F, Klucel H, Klucel L H F, Klucel M F, and Klucel G by the company Aqualon.
(vii) The fatty alcohols used as thickners do not contain polyoxyalkylenated groups and are, for example, chosen from myristyl alcohol, cetyl alcohol, stearyl alcohol, and behenyl alcohol.
The (c) thickener may represent an amount ranging, for example, from 0.1% to 15% by weight, and further, for example, from 0.2% to 10% by weight, and even further, for example, from 0.25% to 5% by weight, relative to the total weight of the composition.
It is preferable that weight ratio of the (a) oil/the (c) thickener be from 25 to 90, preferably from 25 to 80, and more preferably from 25 to 70.
[Water]
The composition according to the present invention comprises (d) water.
The amount of (d) water is not limited, and may be from 30 to 75%» by weight, preferably from 35 to 70%) by weight, and more preferably 40 to 60% by weight, relative to the total weight of the composition.
[Dye]
The composition according to the present invention may comprise at least one (e) dye. A single type of dye may be used, but two or more different types of dyes may be used in combination.
The (e) dye may be selected from the group consisting of direct dyes and oxidative dyes.
The direct dye means a colored substance which does not require the use of an oxidizing agent in order to develop its color.
The direct dyes may be selected from natural direct dyes.
The expression "natural dye" is understood to mean any dye or dye precursor that is naturally occurring and is produced either by extraction (and optionally purification) from a plant matrix optionally in the presence of natural compounds such as ash or ammonia, or by chemical synthesis.
As natural dyes, mention may be made of quinone dyes (such as lawsone and juglone), alizarin, purpurin, laccaic acid, carminic acid, kermesic acid, purpurogallin, protocatechaldehyde, indigoids (such as indigo), sorghum, isatin, betanin, curcuminoids (such as curcumin), spinulosin, various types of chlorophyll and chlorophyllin, hematoxylin, hematein, brazilein, brazilin, safflower dyes (such as carthamin), flavonoids (such as rutin, quercetin, catechin, epicatechin, morin, apigenidin, and sandalwood), anthocyans (such as apigeninidin and apigenidin), carotenoids, tannins, orceins, santalins, and cochineal carmine.
It is also possible to use extracts or decoctions containing natural direct dye(s), in particular henna-based extracts, curcuma longa extract, sorghum leaf-sheath extract, haematoxylon campechianum extract, green tea extract, pine bark extract, cocoa extract, and logwood extract.
It is preferable that the natural dye be chosen from the group consisting of curcuminoids, santalins, chlorophyllin, haematoxylin, haematein, brazilein, brazilin, sorghum, laccaic acid, lawsone, juglone, alizarin, purpurin, carminic acid, kermesic acid, purpurogallin, protocatechaldehyde, indigoids, isatin, spinulosin, apigenidin, orcein, betanin, flavonoids, anthocyans, and extracts or decoctions containing these compounds.
Alternatively, the natural dyes may be preferably chosen, for example, from hydroxylated quinones, indigoids, hydroxyflavones, santalins A and B, isatin and its derivatives, and brasilin and its hydroxylated derivative.
The hydroxylated quinones are preferably benzoquinones, naphthoquinones, and mono- or polyhydroxylated anthraquinones which are optionally substituted with groups such as alkyl, alkoxy, alkenyl, chloro, phenyl, hydroxyalkyl, and carboxyl. The naphthoquinones are preferably lawsone, juglone, flaviolin, naphthazarin, naphthopurpurin,
lapachol, plumbagin, chloroplumbagin, droserone, shikonin,
2- hydroxy-3 -methyl- 1 ,4-naphthoquinone, 3 ,5-dihydroxy- 1 ,4-naphthoquinone,
2,5-dihydroxy-l ,4-naphthoquinone, 2-methoxy-5-hydroxy-l ,4-naphthoquinone, and
3- memoxy-5-hydroxy-l,4-naphmoquinone.
The benzoquinones are preferably spinulosin, atromentin, aurentioglyocladin,
2,5-dihydroxy-6-methylbenzoquinone, 2-hydroxy-3-methyl-6-methoxybenzoquinone, 2, 5-dihydroxy-3,6-diphenylbenzoquinone, 2,3-dimethyl-5-hydroxy-6-methoxybenzoquinone, and 2,5-dihydroxy-6-isopropylbenzoquinone.
The anthraquinones are preferably alizarin, quinizarin, purpurin, carminic acid, chrysophanol, kermesic acid, rhein, aloe emodin, pseudopurpurin, quinizarincarboxylic acid, frangula emodin, 2-methylquinizarin, 1-hydroxyanthraquinone, and 2-hydroxyanthraquinone. The indigoids are preferably indigo, indirubin, isoindigo ,and Tyrian purple.
The hydroxyflavones are preferably quercetin and morin.
The direct dyes may be selected from synthetic direct dyes.
The expression "synthetic direct dye" is understood to mean any dye or dye precursor that is produced by chemical synthesis.
The synthetic direct dye may be hydrophilic or hydrophobic. The synthetic direct dye may be a hydrophilic direct dye selected from the group consisting of acidic (anionic) direct dyes, basic (cationic) direct dyes and neutral (noninic) direct dyes, and a hydrophobic direct dye which cover all possible types of direct dyes, such as so-called nitro dyes, HC dyes, azo, methine, carbonyl, azine, nitro(hetero)aryl types or tri(hetero)arylmethane direct dyes, ρorphyrins and
phthalocyanines, alone or as mixtures. Acidic direct dyes have an anionic moiety in their chemical structure. Basic direct dyes have a cationic moiety in their chemical structure.
Neutral direct dyes are nonionic.
More particularly, the azo dyes comprise an -N=N- functional group, the two nitrogen atoms of which are not simultaneously involved in a ring. However, it is not ruled out for one of the two nitrogen atoms of the -N=N- sequence to be involved in a ring.
The dyes of the family of the methines are more particularly compounds comprising at least one sequence chosen from >C=C< and -N=C<, the two atoms of which are not simultaneously involved in a ring. However, it is specified that one of the nitrogen or carbon atoms of the sequences can be involved in a ring. More particularly, the dyes of this family result from compounds of the following types: true methine (comprising one or more above-mentioned -C=C- sequences); azomethine (comprising at least one or more -C=N- sequences) with, for example, the azacarbocyanines and their isomers, the diazacarbocyanines and their isomers, the tefraazacarbocyanines; mono- and diarylmethane; indoamines (or diphenylamines); indophenols; and indoanilines.
As regards the dyes of the family of the carbonyls, mention may be made, for example, of synthetic dyes chosen from acridone, benzoquinone, anthraquinone, naphthoquinone,
benzanthrone, anthranthrone, pyranthrone, pyrazolanthrone, pyrimidinoanthrone, flavanthrone, indanthrone, flavone, (iso)violanthrone, isoindolinone, benzimidazolone, isoquinolinone, anthrapyridone, pyrazoloquinazolone, perinone, quinacridone, quinophthalone, naphthalimide, anthrapyrimidine, diketopyrrolopyrrole, or coumarin dyes.
As regards the dyes of the family of the cyclic azines, mention may in particular be made of azine, xanthene, thioxanthene, fluorindine, acridine, (di)oxazine, (di)thiazine, or pyronine dyes.
The nitro(hetero)aromatic dyes are more particularly nitrobenzene or nitropyridine direct dyes.
As regards the dyes of porphyrin or phthalocyanine type, use may be made of cationic or noncationic compounds optionally comprising one or more metals or metal ions, such as, for example, alkali and alkaline earth metals, zinc, and silicon.
Mention may be made, as examples of synthetic direct dyes which are particularly suitable, of nitrobenzene dyes, azo, azomethine, or methine direct dyes, azacarbocyanines, such as tetraazacarbocyanines (tetraazapentamethines), quinone, and in particular anthraquinone, naphthoquinone, or benzoquinone direct dyes, or azine, xanthene, triarylmethane, indoamine, phthalocyanine, and ρorphyrin direct dyes, alone or as mixtures. More preferably still, these synthetic direct dyes are chosen from nitrobenzene dyes, azo, azomethine, or methine direct dyes, and tetraazacarbocyanines (tetraazapentamethines); alone or as mixtures.
Mention may be made, among the azo, azomethine, methine, or tetraazapentamethine direct dyes which can be used according to the invention, of the cationic dyes described in Patent
Applications WO 95/15144, WO 95/01772, and EP 714 954; FR 2 189 006, FR 2 285 851, FR-2 140205, EP 1 378 544, and EP 1 674 073.
Thus, mention may be made of the cationic direct dyes corresponding to the following formulae:
in which:
D represents a nitrogen atom or the -CH group,
R1 and R2, which are identical or different, represent a hydrogen atom; a C1-C4 alkyl radical which can be substituted by a -CN, -OH, or -NH2 radical or can form, with a carbon atom of the benzene ring, an optionally oxygen-comprising or nitrogen-comprising heterocycle which can be substituted by one or more C1-C4 alkyl radicals; or a 4'-aminophenyl radical,
R3 and R'3, which are identical or different, represent a hydrogen atom, a halogen atom chosen from chlorine, bromine, iodine, and fluorine, a cyano radical, a C1-C4 alkyl radical, a C1-C4 alkoxy radical, or an acetyloxy radical,
X- represents an anion, preferably chosen from chloride, methyl sulphate, and acetate, A represents a group chosen from the following structures:
in which:
R5 represents a hydrogen atom, a C1-C4 alkoxy radical, or a halogen atom, such as bromine, chlorine, iodine, or fluorine,
R6 represents a hydrogen atom or a C1-C4 alkyl radical or forms, with a carbon atom in the benzene ring, a heterocycle which optionally comprises oxygen and/or is optionally substituted by one or more C1-C4 alkyl groups,
R7 represents a hydrogen atom or a halogen atom, such as bromine, chlorine, iodine, or fluorine, D\ and D2, which are identical or different, represent a nitrogen atom or the -CH group, m = 0 or l,
X- represents a cosmetically acceptable anion preferably chosen from chloride, methyl sulphate, and acetate,
in which R' represents a C1-C4 alkyl radical;
when m = 0 and when D\ represents a nitrogen atom, then E can also denote a group with the following structure:
in which R' represents a C1-C4 alkyl radical. The following cationic direct dyes are also particularly suitable to the invention:
X- represents an anion preferably chosen from chloride, iodide, methyl sulphate, ethyl sulphate, or acetate.
As examples of the cationic direct dyes, mention may be made of 4-nitro-o-phenylenediamine, 2-nitro-p-phenylenediamine, N,N'-bis-(2-hydroxyemyl)-2-nirro-p-phenylenediamine,
4-nitrophenylaminoethylurea, and 2-amino-6-chloro-4-nitrophenol,
2,6-diammo-3-((pyridine-3-yl)-azo)pyridine.
According to an embodiment, it is preferable that the direct dye be selected from acidic direct dyes. The anionic direct dyes are commonly known as "acidic direct dyes" for their affinity with alkaline substances (see, for example, "Industrial Dyes, Chemistry, Properties, Application", Klaus Hunger Ed. Wiley- VCH Verlag GmbH & Co KGaA, Weinheim 2003). Anionic or acid dyes are known in the literature (see, for example, " Ullman s ' Encyclopedia of Industrial Chemistry", Azo Dyes, 2005 Wiley- VCH Verlag GmbH & Co. KGaA, Weinheim 10.1002/14356007.a03 245, point 3.2; ibid, Textile Auxiliaries, 2002 Wiley- VCH Verlag GmbH & Co. KGaA, Weinheim 10.1002/14356007.a26227 and "Ashford's Dictionary of Industrial Chemicals", Second Edition, p. 14-p. 39, 2001). . .
The term "anionic direct dyes" means any direct dye comprising in its structure at least one sulfonate group S03- and/or at least one carboxylate group C(0)0- and/or at least one phosphonate group Ρ(=0)ΟΌ- and optionally one or more anionic groups G- with G-, which may be identical or different, representing an anionic group chosen from alkoxide O-, thioalkoxide S-, phosphonate, carboxylate and thiocarboxylate: C(Q)Q'- with Q and Q', which may be identical or different, representing an oxygen or sulfur atom; preferably, G- represents a carboxylate, i.e. Q and Q' represent an oxygen atom.
The preferred anionic dyes of formula of the invention are chosen from acidic nitro direct dyes, acidic azo dyes, acidic azine dyes, acidic triarylmethane dyes, acidic indoamine dyes, acidic anthraquinone dyes, anionic styryl dyes, and indigoids and acidic natural dyes; each of these dyes containing at least one sulfonate, phosphonate or carboxylate group bearing a cationic counterion X+, where X+ represents an organic or mineral cationic counter ion preferably chosen from alkali and alkaline-earth metals, such as Na+ and K+.
Preferred acid dyes may be chosen from the dyes below. The diary I anionic azo dyes of formula (II) or (ΙΓ):
in which formulae (II) and (IT):
R7, Rs, R9, Rio, R'7, R's, R'9 and R'io, which may be identical or different, represent a hydrogen atom or a group chosen from:
alkyl;
alkoxy, alkylthio;
hydroxyl, mercapto;
nitro;
R°-C(X)-X'-, R°-X'-C(X)-, R°-X'-C(X)-X"- with R° representing a hydrogen atom or an alkyl or aryl group; X, X' and X", which may be identical or different, representing an oxygen or sulfur atom, or NR with R representing a hydrogen atom or an alkyl group; (0)2S(0-)-, X+ as defined previously;
(O)CCT-, X+ as defined previously;
(0)P(02-)-, 2 X+ as defined previously;
R"-S(0)2-, with R" representing a hydrogen atom or an alkyl, aryl, (di)(alkyl)amino or aryl(alkyl)amino group; preferably a phenylarnino or phenyl group;
R'"-S(0)2-X'- with R'" representing an alkyl or optionally substituted aryl group, X' as defined previously;
(di)(alkyl)amino;
aryl(alkyl)amino optionally substituted with one or more groups chosen from i) nitro; ii) nitroso; iii) (0)2S(0-)-, X+ and iv) alkoxy with X*;
optionally substituted heteroaryl; preferably a benzothiazolyl group;
cycloalkyl; especially cyclohexyl,
Ar-N=N- with Ar representing an optionally substituted aryl group, preferably a phenyl optionally substituted with one or more alkyl, (0)2S(0~)-, X+ or phenylarnino groups; or alternatively two contiguous groups R7 with Rg or Re with R9 or R9 with Rio together form a fused benzo group A' ; and R'7 with R'g or R'g with R'9 or R'9 with R'io together form a fused benzo group B'; with A' and B' optionally substituted with one or more groups chosen from i) nitro; ii) nitroso; iii) (0)2S(0-)-, X4; iv) hydroxyl; v) mercapto; vi) (di)(alkyl)amino; vii) R°-C(X)-X'-; viii) R°-X'-C(X)-; ix) RºX'-C(X)-X"-; x) Ar-N=N-
and xi) optionally substituted aryl(alkyl)amino; with X+, R°, X, X', X" and Ar as defined previously;
W represents a sigma bond σ, an oxygen or sulfur atom, or a divalent radical i) -NR- with R as defined previously, or ii) methylene -C(Ra)(Rt,)- with R¾ and Rb, which may be identical or different, representing a hydrogen atom or an aryl group, or alternatively Ra and Rb form, together with the carbon atom that bears them, a spiro cycloalkyl; preferably W represents a sulfur atom or Ra and Rb together form a cyclohexyl;
it being understood that formulae (II) and (IT) comprise at least one sulfonate (0)2S(0-)-, X+ or phosphonate (0)P(02-) 2X+or carboxylate (O)C(O-)-, X+ radical on one of the rings A, A', B, B' or C with X+ as defined previously.
As examples of dyes of formula (II), mention may be made of Acid Red 1, Acid Red 4, Acid Red 13, Acid Red 14, Acid Red 18, Acid Red 27, Acid Red 32, Acid Red 33, Acid Red 35, Acid Red 37, Acid Red 40, Acid Red 41, Acid Red 42, Acid Red 44, Acid Red 68, Acid Red 73, Acid Red 135, Acid Red 138, Acid Red 184, Food Red 1, Food Red 13, Food Red 17, Orange 4, Acid Orange 6, Acid Orange 7, Acid Orange 10, Acid Orange 19, Acid Orange 20, Acid Orange 24, Acid Yellow 9, Acid Yellow 36, Acid Yellow 199, Food Yellow 3; Acid Violet 7, Acid Violet 14, Acid Blue 113, Acid Blue 117, Acid Black 1, Acid Brown 4, Acid Brown 20, Acid Black 26, Acid Black 52, Food Black 1 , Food Black 2, Pigment Red 57;
and as examples of dyes of formula (IT), mention may be made of Acid Red 111, Acid Red 134, Acid yellow 38;
The anthra uinone dyes of formulae (III) and (ΠΓ):
R22, R23, R24, R25, R26 and R27, which may be identical or different, represent a hydrogen or halogen atom or a group chosen from:
alkyl;
hydroxyl, mercapto;
- alkoxy, alkylthio;
aryloxy or arylthio optionally substituted, preferably substituted with one or more groups chosen from alkyl and (0)2S(0-)-, X+ with X+ as defined previously;
aryl(alkyl)amino optionally substituted with one or more groups chosen from alkyl and
(0)2S(0")-, X* with X* as defined previously;
(di)(alkyl)amino;
(di)(hydroxyalkyl)amino;
- (0)2S(0")-, X* with X* as defined previously;
Z' represents a hydrogen atom or a group NR28R29 with R28 and R29, which may be identical or different, representing a hydrogen atom or a group chosen from:
alkyl;
polyhydroxyalkyl such as hydroxyethyl;
- aryl optionally substituted with one or more groups, particularly i) alkyl such as methyl,
H-dodecyl, n-butyl; ii) (0)2S(0>, X" with X+ as defined previously; iii) R°-C(X)-X'-,
R°-X'-C(X)-, R°-X'-C(X)-X"- with R°, X, X' and X" as defined previously, preferably
R° represents an alkyl group;
cycloakyl; especially cyclohexyl;
Z represents a group chosen from hydroxyl and NR'28R'29 with R'28 and R'29, which may be identical or different, representing the same atoms or groups as R28 and R29 as defined previously; it being understood that formulae (ΙΠ) and (III') comprise at least one sulfonate group (0)2S(0")-, X+ with X+ as defined previously. As examples of dyes of formula (III), mention may be made of Acid Blue 25, Acid Blue 43, Acid Blue 62, Acid Blue 78, Acid Blue 129, Acid Blue 138, Acid Blue 140, Acid Blue 251, Acid Green 25, Acid Green 41, Acid Violet 42, Acid Violet 43, Mordant Red 3; EXT Violet 2,
and as examples of dyes of formula (ΠΓ), mention may be made of Acid Black 48; The quinoline-based dyes of formula (IV):
in which formula (TV):
R61 represents a hydrogen or halogen atom or an alkyl group;
R62, R63 and R64, which may be identical or different, represent a hydrogen atom or a group (0)2S(0~)-, X^ with X*" as defined previously;
or alternatively R61 with R62, or R63 and R64 , together form a benzo group optionally substituted with one or more groups (0)2S(0")-, X*" with X4" as defined previously;
G represents an oxygen or sulfur atom or a group NRe with R» representing a hydrogen atom or an alkyl group; particularly G represents an oxygen atom;
it being understood that formula (IV) comprises at least one sulfonate group (0)2S(0")-, X^ with X^ as defined previously.
As examples of dyes of formula (IV), mention may be made of Acid Yellow 2, Acid Yellow 3 and Acid Yellow 5.
As examples of the nonionic direct dyes, mention may be made of HC Red 13, HC Red 7, HC Blue 2, HC Yellow 4, HC Yellow 2, HC Red 3, 4-amino-3-nitrophenol,
1 -hydroxyethylarnino-5-nitroanisole, 3-nitro-p-(hydroxyemylamino)phenol,
3-methylamino-4-nitrophenoxyethanol, 2-nitro-5-(glyceryl)methylaniline, HC Violet 1, HC Orange 2, HC Yellow 9, HC Red 10, HC Red 11, 2-hydroxyethylpicramic acid, HC Blue 12, 3 -nitro-4-(N -(β-hydroxyemyl)amino)-toluene,
2-(N-(β-memoxyemyl)ammo)-5-(N,N-bis(hydroxyethyl)amino)-nitrobenzen, HC Yellow 10, HC
Violet 2, 4-hydroxypropylamino-3-nitrophenol, and HC Blue 14.
As examples of the hydrophobic direct dyes mention may be made of Solvent Black 3, Solvent Blue 104, Solvent Blue 134, Solvent Blue 14, Disperse Blue 14, Solvent Red 2, Solvent Brown 5, Solvent Green 5, Solvent Orange 2, Solvent Orange 1 , Disperse Orange 24, Solvent Orange 63, Solvent Red 49, Solvent Red 1, Solvent Red 26, Solvent Red 27, Solvent Red 18, Solvent Red 23, Solvent Red 4, Disperse Orange 7, Disperse Blue 72, Disperse Violet 26, Disperse Yellow 16, Disperse Yellow 82, Disperse Yellow 54, Solvent Yellow 29, Solvent Yellow 163, Solvent Yellow 3, Solvent Yellow 56, Solvent Yellow 18, Solvent Yellow 98, Solvent Yellow 12, Solvent Yellow 14, Disperse Red 13, Disperse Green 9, Disperse Blue 148, Disperse Violet 63, Disperse Blue 60, and Solvent Orange 15.
The synthetic direct dye may be selected from fluorescent dyes. Two or more types of the fluorescent dye may be used in combination. The use of some fluorescent dyes may make it possible to obtain, on dark hair, colors which are more visible than with conventional hydrophilic or hydrophobic direct dyes. Furthermore, these fluorescent dyes, when applied to dark hair, may also make it possible to lighten the hair without damaging it. As used herein, the term "fluorescent dyes" is understood to mean fluorescent compounds and optical brighteners. In at least one embodiment, the fluorescent dye is soluble in the medium of the composition.
Fluorescent dyes are fluorescent compounds which absorb visible radiation, for example, wavelengths ranging from 400 to 800 nm, and which are capable of re-emitting light in the visible region at a higher wavelength.
According to one embodiment, the fluorescent dyes useful for the present invention re-emit orange-colored fluorescent light. They exhibit, for instance, a maximum re-emission wavelength ranging from 500 to 700 nm.
Non-limiting examples of fluorescent dyes include compounds known in the art, for example, those described in Ullmann's Encyclopedia of Industrial Chemistry, Release 2004, 7th edition, "Fluorescent Dyes" chapter.
The optical brighteners of the present disclosure, also known under the name of "brighteners", or "fluorescent brighteners", or "fluorescent brightening agents" or "F WA", or "fluorescent whitening agents", or "whiteners", or "fluorescent whiteners", are colorless transparent compounds as they do not absorb radiation in visible light but only in ultraviolet light (wavelengths ranging from 200 to 400 nanometers) and convert the energy absorbed into fluorescent light of higher wavelength emitted in the visible part of the spectrum, generally in the blue and/or green, that is to say in wavelengths ranging from 400 to 550 nanometers.
Optical brighteners are known in the art, for example, they are described in Ullmann's
Encyclopedia of Industrial Chemistry (2002), "Optical Brighteners" and Kirk-Othmer
Encyclopedia of Chemical Technology (1995): "Fluorescent Whitening Agents".
The fluorescent dyes which can be used in the composition of the present disclosure include compounds known from the art, for example, those described in French Patent No. 2 830 189.
Soluble fluorescent compounds that may especially be mentioned include those belonging to the following families: naphthalimides, coumarins, xanthenes, and in particular
xanthenodiquinolizines and azaxanthenes; naphtholactams; azlactones; oxazines; thiazines;
dioxazines; azo compounds; azomethines; methines; pyrazines; stilbenes; ketopyrroles; and pyrenes.
If present, the fluorescent dyes are preferred, more particularly, those re-emitting orange-colored fluorescent light. The oxidative dyes may be selected from oxidation bases and couplers.
The oxidation base can be selected from those conventionally known in oxidation dyeing, preferably from the group consisting of ortho- and para-phenylenediamines, double bases, ortho- and para-aminophenols, heterocyclic bases, and the acid addition salts thereof.
There may be mentioned in particular:
- (I) the para-phenylenediamines of the following formula (I) and their addition salts with an acid:
in which:
Ri represents a hydrogen atom, a C1-C4 alkyl radical, a monohydroxy(C1-C4 alkyl) radical, a polyhydroxy-(C2-C4 alkyl) radical, a (C1-C4)alkoxy(C1-C4)alkyl radical, a C1-C4 alkylradical substituted with a nitrogen-containing group, a phenyl radical, or a 4'-aminophenyl radical;
R2 represents a hydrogen atom, a C1-C4 alkyl radical, a monohydroxy(C1-C4 alkyl) radical, a polyhydroxy(C2-C4 alkyl) radical, a (CrC^alkoxyiC1-C^alkyl radical, or a C1-Q alkyl radical
substituted with a nitrogen-containing group;
Ri and R2 may also form with the nitrogen atom carrying them a 5- or 6-membered
nitrogen-containing heterocycle optionally substituted with one or more alkyl, hydroxyl, or ureido groups;
R3 represents a hydrogen atom, a halogen atom such as a chlorine atom, a C1-C4 alkyl radical, a sulpho radical, a carboxyl radical, a monohydroxy(C1-C4 alkyl) radical, a hydroxy(C1-C4 alkoxy) radical, an ace1ylamino( C1-C4 alkoxy) radical, a mesylamino(C1-C4 alkoxy) radical, or a carbamoylamino(C1-C4 alkoxy) radical; and
R4 represents a hydrogen or halogen atom or a C1-C4 alkyl radical.
Among the nitrogen-containing groups of formula (I) above, there may be mentioned in particular the amino, mono(C1-C4)alkylamino, (C1-C4)dialkylamino, (C1-C^trialkylamino,
monohydroxy(C1-C4)alkylamino, di(monohydroxy(C1-C4)alkyl)amino, imidazolinium, and ammonium radicals.
Among the para-phenylenediamines of formula (I) above, there may be mentioned more particularly para-phenylenediamine, para-tolylenediamine, 2-chloro-paraphenylenediamine, 2,3-dimethyl-para-phenylenediamine, 2,6-dimemyl-para-phenylenediamine,
2,6-diemyl-para-phenylenediamine, 2,5-dimethyl-para-phenylenediamine,
Ν,Ν-dimemylpara-phenylenediamine, N,N-diethyl-para-phenylenediamine,
Ν,Ν-dipropyl-paraphenylenediamine, 4-amino-N,N-diethyl-3 -methylaniline,
N,N-bis(β-hydroxyemyl)-paraphenylenediamine,
4-N,N-bis(β-hydroxyemyl)arrimo-2-memylaniline,
4-N,N-bis(β-hydroxyemyl)ammo-2-chloroaniline, 2-β-hydroxyethyl-para-phenylenediamine, 2-fluoro-paraphenylenediamine, 2-isopropyl-para-phenylenediamine,
N-(β-hydroxypropyl)-paraphenylenediamine, 2-hydroxymemyl-para-phenylenediamine,
N,N-dimemyl-3-memylpara-phenylenediamine,
N,N-(emyl-β-hydroxyethyl)-para-phenylenediamine,
N-(P,Y-dmydroxypropyl)-para-phenylenediamine, N-(4 ' -ammophenyl)-para-phenylenediamine, N-phenyl-para-phenylenediamine, 2-β-hydroxyethyloxy-para-phenylenediamine,
2-β-acetylamino-emyloxy-para-phenylenediamine, N-(β-memoxyemyl)-para-phenylenediamine, 2-methyl- 1 -Ν-β-hydroxyemyl-para-phenylenediarnine, N-(4-aminophenyl)-3 -hydroxy-pyrrolidine, 2-[{2-[(4-Ammophenyl)ammo]emyl}(2-hydroxyethyl)amino]-ethanol, and their addition salts with an acid.
Among the para-phenylenediamines of formula (I) above, there are most particularly preferred para-phenylene&amine, para-tolylenediamine, 2-isopropyl-paraphenylenediamine,
2-β-hydroxyemyl-para-phenylenediamine, 2-β-hydroxyethyloxy-para-phenylenediarnine,
2,6-dimethyl-para-phenylenediamine, 2,6-diethyl-para-phenylenediamine,
2,3-dimethyl-para-phenylenediamine, N,N-bis(β-hydroxyemyl)-para-phenylenediarnine,
2-cWoro-para-phenylenediamine, and their addition salts with an acid.
- (II) According to the invention, "double bases" are understood to mean compounds containing at least two aromatic rings on which amino and/or hydroxyl groups are carried.
Among the double bases which can be used as oxidation bases in the dyeing compositions in accordance with the invention, there may be mentioned in particular compounds corresponding to the following formula II), and their addition salts with an acid:
in which:
- Z1 and Z2, which are identical or different, represent a hydroxyl or -NH2 radical which may be substituted with a C1-C4 alkyl radical or with a linking arm Y;
- the linking arm Y represents a linear or branched alkylene chain comprising from 1 to 14 carbon atoms, which may be interrupted by or which may end with one or more nitrogen-containing groups and/or one or more heteroatoms such as oxygen, sulphur, or nitrogen atoms, and optionally substituted with one or more hydroxyl or C1-C6 alkoxy radicals;
- R5 and ¾ represent a hydrogen or halogen atom, a C1-C4 alkyl radical, a monohydroxy(C1-C4 alkyl) radical, a polyhydroxy(C2-C4 alkyl) radical, an amino(C1-C4 alkyl) radical, or a linking arm
Y;
- R7, Rs, R9, R10, Rn, and R12, which are identical or different, represent a hydrogen atom, a linking arm Y, or a C1-C4 alkyl radical; it being understood that the compounds of formula (II) contain only one linking arm Y per molecule.
Among the nitrogen-containing groups of formula (II) above, there may be mentioned in particular the amino, mono(C1-C4)alkylamino, (C1-C4)dialkylamino, (C1-C4)trialkylarnino, monohydroxy(C1-C4)alkylamino, imidazolinium, and ammonium radicals.
Among the double bases of formulae (II) above, there may be mentioned more particularly N,N'-bis(β-hydroxyethyl)-N,N'-bis(4'-ammophenyl)-l,3-diaminopropanol,
N,N'-bis(β-hydroxyemyl)-N,N'-bis(4'-ammophenyl)emylenediamine,
N,N'-bis(4-animophenyl)-tetramemylenediamine,
Ν,Ν' -bis(β-hydroxyethyl)-N,N' -bis(4-ammophenyl)tetramethylenediamine,
N,N'-bis(4-memylammophenyl)tetramethylenediamine,
Ν,Ν' -bis(ethyl)-N,N' -bis(4 ' -amino-3 ' -memylphenyl)emylene-diamine,
l,8-bis(2,5-diaminophenoxy)-3,5-dioxaoctane, and their addition salts with an acid.
Among these double bases of formula (II),
Ν,Ν' -bis(β-hydroxyethyl)-N,N' -bis(4' -aminophenyl)- 1 ,3-diaminopropanol,
l,8-bis(2,5-diaminophenoxy)-3,5-dioxaoctane, or one of their addition salts with an acid are particularly preferred.
- (Ill) The para-aminophenols corresponding to the following formula (III), and their addition salts with an acid:
in which:
- R13 represents a hydrogen atom, or a halogen atom such as fluorine, a C1-C4 alkyl,
monohydroxy(C1-C4 alkyl), (C1-C4)alkoxy(C1-C4)-alkyl, amino(C1-C4 alkyl), or
hydroxy(C1 -C4)alkylamino-(C1-C4 alkyl) radical,
- R14 represents a hydrogen atom, or a halogen atom such as fluorine, a C1-C4 alkyl,
Among the para-arninophenols of formula (III) above, there may be mentioned more particularly para-aminophenol, 4-amino-3-methylphenol, 4-amino-3-fluorophenol,
4-amino-3 -hydroxymethylphenol, 4-amino-2-methylphenol, 4-amino-2-hydroxymethylphenol, 4-amino-2-methoxymethylphenol, 4-ammo-2-aminomethylphenol,
4-amino-2-(β-hydroxyethylaminomethyl)phenol, and their addition salts with an acid.
- (IV) The ormo-aminophenols which can be used as oxidation bases in the context of the present invention are chosen in particular from 2-aminophenol, 2-amino-l-hydroxy-5-methylbenzene, 2-amino-l-hydroxy-6-methylbenzene, 5-acetamido-2-aminophenol, and their addition salts with an acid.
- (V) Among the heterocyclic bases which can be used as oxidation bases in the dyeing compositions in accordance with the invention, there may be mentioned more particularly pyridine derivatives, pyrimidine derivatives, pyrazole derivatives, and their addition salts with an acid.
Among the pyridine derivatives, there may be mentioned more particularly the compounds described for example in Patents GB 1,026,978 and GB 1,153,196, such as 2,5-diaminopyridine, 2-(4-memoxyphenyl)ancdno-3-aminopyridine, 2,3-diamino-6-methoxypyridine,
2-(β-memoxyethyl)ammo-3-ammo-6-methoxypyridine, 3,4-diaminopyridine, and their addition salts with an acid.
Among the pyrimidine derivatives, there may be mentioned more particularly the compounds described, for example, in Patents DE 2 359 399; JP 88-169571; and JP 91-10659, or patent application WO 96/15765, such as 2,4,5, 6-tetraammopyrimidine,
4-hydroxy-2,5,6-triarninopyrimidine, 2-hydroxy-4,5,6-triarninopyrirnidine,
2.4- dmydroxy-5,6-diaminopyrirnidine, 2,5,6-triammo-pyrimidine, and the pyrazolopyrirnidine derivatives such as those mentioned in patent application FR-A-2 750 048 and among which there may be mentioned pyrazolo[l,5-a]-pyrimidme-3,7-diamine;
2.5- dimethyl-pyrazolo[l ,5-a]-pyrimidine-3,7-diamine; pyrazolo[l ,5-a]pyrimidine-3,5-diamine; 2,7-dimemylpyrazolo[l,5-a]pyrirm^me-3,5-dianiine; 3-ammopyrazolo[l,5-a]pyrimidin-7-ol;
3-amino-pyrazolo[l,5-a]pyrimidin-5-ol; 2-(3-amino-pyrazolo-[l,5-a]pyrimidin-7-ylamino)ethanol,
2-(7-aminopyrazolo [ 1 ,5 -a]pyrimidin-3 -ylamino)ethanol,
2- [(3 -amino-pyrazolo [1,5 -a]pyrimidin-7-yl)-(2-hydroxy-ethyl)amino] -ethanol,
2- [(7-aminopyrazolo[ 1 ,5-a]-pyrimidin-3-yl)-(2-hyckoxyethyl)amino]ethanol,
5,6-dimethylpyrazolo-[l,5-a]pyrimidine-3,7-diamine,
2,6-dimethylpyrazolo-[ 1 ,5-a]pyrimidine-3 ,7-diamine,
2,5,N7,N7-tetramethyl-pyrazolo[l ,5-a]pyrimidine-3,7-diamine,
3- amino-5-methyl-7-iniidazolylpropyl-aminopyrazolo[l,5-a]-pyrimidine their addition salts and their tautomeric forms, when a tautomeric equilibrium exists, and their addition salts with an acid.
Among the pyrazole derivatives, there may be mentioned more particularly the compounds described in Patents DE 3 843 892 and DE 4 133 957 and patent applications WO 94/08969, WO 94/08970, FR-A-2 733 749, and DE 195 43 988 such as 4,5-diamino-l -methylpyrazole,
3.4- diaminopyrazole, 4,5-diamino-l-(4'-chlorobenzyl)-pyrazole,
4,5-diamino- 1 ,3-dimethylpyrazole, 4,5-diamino-3-methyl- 1 -phenylpyrazole,
4.5- diammo-l-methyl-3-phenylpyrazole, 4-ammo-l,3-dimethyl-5-hydrazino-pyrazole,
1- benzyl-4,5-diamino-3-methyl-pyrazole, 4,5-diamino-3-tert-butyl-l-methylpyrazole,
4,5-diamino-l-tertbutyl-3-methylpyrazole, 4,5-dianiino-l-(β-hydroxyethyl)-3-methylpyrazole, 4,5-diamino- l-(β-hydroxyethyl)pyrazole, 4,5-diamino-l -ethyl-3 -methylpyrazole,
4,5-diamino-l-ethyl-3-(4'-methoxyphenyl)pyrazole,
4,5-diamino- l-ethyl-3-hydroxy-methylpyrazole, 4,5-diamino-3-hydroxymethyl-l-methylpyrazole, 4,5-diamino-3-hydroxymethyl-l-isopropyl-pyrazole, 4,5-diamino-3-methyl-l-isopropyl-pyrazole,
4- amino-5-(2'-animoemyl)ammo-l,3-dimethylpyrazole, 3,4,5-triaminopyrazole,
1 -memyl-3,4,5-triamino-pyrazole, 3,5-diarnino- 1 -methyl-4-methylaminopyrazole,
3,5-diammo-4-(β-hydroxy-emyl)amino- 1 -methylpyrazole, and their addition salts with an acid.
Among the heterocyclic bases which can be used as oxidation bases, there may be mentioned more particularly diarninopyrazolopyrazolones and especially
2,3-diammo-6,7-dihydro-lH5H-[pyrazolol,2,a]pyrazol-l-one and the addition salts of these diarninopyrazolopyrazolones with an acid.
The couplers may be an oxidation coupler which can be selected from those conventionally known in oxidation dyeing, preferably from the group consisting of meta-phenylenediamines, meta-aminophenols, meta-diphenols, naphthols, heterocyclic couplers, and the acid addition salts thereof.
The heterocyclic couplers may be selected from the group consisting of indole derivatives, indoline derivatives, sesamol and its derivatives, pyridine derivatives, pyrazolotriazole derivatives, pyrazolones, indazoles, benzimidazoles, benzothiazoles, benzoxazoles, 1,3-benzodioxoles, quinolines, and their addition salts with an acid.
These couplers are more particularly chosen from 2,4-diamino-l-(β-hydroxyethyloxy)benzene,
2- methyl-5-aminophenol, 5-N-(β-hydroxyethyl)amino-2-methylphenol, 3-aminophenol,
2-cUoro-3-amino-6-methylphenol, 1 ,3-dihydroxybenzene, 1 ,3-dihydroxy-2-methylbenzene, 4-chloro-l ,3-dihydroxybenzene, 2-amino-4-(β-hydroxyethylamino)-l -methoxybenzene,
1 ,3-diaminobenzene, 2-methyl-5-hydroxyethylaminophenol, 4-amino-2-hydroxytoluene, 1 ,3-bis(2,4-diaminophenoxy)-propane, sesamol,
l-amino-2-methoxy-4,5-niethylene-dioxybenzene, a-naphthol, 6-hydroxyindole, 4-hydroxyindole, 4-hydroxy-N-methylindole, 6-hydroxy-indoline, 2,6-dihydroxy-4-methylpyridine,
l-H-3-methylpyrazol-5-one, l-phenyl-3-methylpyrazol-5-one, 2-amino-3-hydroxypyridine,
3,6-dimethyl-pyrazolo[3,2-c]-l,2,4-triazole, 2,6-dimethylpyrazolo[l,5-b]-l,2,4-triazole, and their addition salts with an acid.
In general, the addition acid salts of the oxidation bases and couplers are chosen in particular from hydrochlorides, hydrobromides, sulfates, citrates, succinates, tartrates, lactates, tosylates, benzenesulfonates, phosphates, and acetates.
The composition used for the present invention contains the (b) dye(s) in an amount of 0.001% by weight or more, and may contain, for example, 0.001 to 10% by weight, preferably 0.01 to 5% by weight, and more preferably 0.1 to 3% by weight, relative to the total weight of the composition.
[Additional Surfactant]
The composition according to the present invention may comprise at least one additional surfactant other than the above ingredient (b).
The additional surfactant used in the present invention may be selected from the group consisting of anionic surfactants, amphoteric surfactants, cationic surfactants, and nonionic surfactants.
Two or more additional surfactants may be used in combination. Thus, a single type of additional surfactant or a combination of different types of additional surfactants may be used.
According to one preferred embodiment of the invention, the composition according to the present invention does not comprise PEG-40 hydrogenated castor oil in an amount of 1% by weight or more relative to the total weight of the composition. According to one preferred embodiment of the invention, the composition according to the present invention does not comprise PEG-40 hydrogenated castor oil.
According to one preferred embodiment of the invention, the composition according to the present invention does not comprise a nonionic surfactant other than polyoxyalkylenated fatty alcohol in an amount of 0.5% by weight or more relative to the total weight of the composition. According to one preferred embodiment of the invention, the composition according to the present invention does not comprise a nonionic surfactant other than polyoxyalkylenated fatty alcohol.
According to one embodiment of the present invention, the amount of the additional surfactant(s) may range from 0.1 to 30% by weight, preferably from 1 to 25% by weight, and more preferably from 3 to 20% by weight, relative to the total weight of the composition according to the present invention.
[Other Ingredients]
The composition according to the present invention may also comprise an effective amount of other ingredients, which are preferably common in cosmetic products, such as various common adjuvants, antiageing agents, whitening agents, anti-greasy skin agents, sequestering agents such as EDTA and etidronic acid, UV-screening agents, preserving agents such as chlorphenesin, vitamins, or provitamins, for instance, panthenol, opacifiers, fragrances, plant extracts, cationic polymers, and so on.
The composition according to the present invention may further comprise at least one organic solvent. Thus, the organic solvent is preferably water miscible. As the organic solvent, there may be mentioned, for example, C1-C4 alkanols, such as ethanol and isopropanol; aromatic alcohols such as benzyl alcohol and phenoxyethanol; analogous products; and mixtures thereof.
The organic water-soluble solvents may be present in an amount ranging from less than 10% by weight, preferably from 5% by weight or less, and more preferably from 1% by weight or less, relative to the total weight of the composition.
[Preparation and Properties]
The composition according to the present invention can be prepared by mixing the above essential and optional ingredients in accordance with a conventional process.
The pH of the composition according to the present invention may be controlled. The pH may be, for example, from 3 to 7, preferably from 4 to 7, and more preferably from 5 to 7, if the (e) dye is selected from acidic direct dyes. The pH may be, for example, from 7 to 11, preferably from 7 to 10, and more preferably from 7 to 9, if the (e) dye is selected from basic direct dyes and oxidative dyes.
The pH may be adjusted to the desired value using at least one acidifying agent and/or at least one basifying agent.
The acidifying agents can be, for example, mineral or organic acids, for instance hydrochloric acid, orthophosphoric acid, carboxylic acids, for instance tartaric acid, citric acid, and lactic acid, or sulphonic acids. The basifying agent can be, for example, ammonium hydroxide, alkali metal hydroxide, alkali earth metal hydroxide, alkali metal carbonates, alkanolarnines such as mono-, di-, and
triethanolamines, and also their derivatives, preferably sodium or potassium hydroxide and compounds of the formula below.
wherein
R denotes an alkylene such as propylene optionally substituted by a hydroxyl or a C1-C4 alkyl
radical, and RLS R2, R3> and PM independently denote a hydrogen atom, an alkyl radical, or a C1-C4 hydroxyalkyl radical, which may be exemplified by 1,3-propanediamine, and derivatives thereof. Arginine, urea, and monoethanolamine may be preferable. The acidifying or basifying agent may be present in an amount ranging from less than 10% by weight, preferably from 1% by weight or less, and more preferably from 0.5% by weight or less, relative to the total weight of the composition.
It is preferable that the composition according to the present invention be in the form of an emulsion, more preferably a fine emulsion, and even more preferably a nano- or micro-emulsion.
The composition according to the present invention may be in the form of an O/W emulsion, preferably an O/W nano- or micro-emulsion. If the dye is selected from direct dyes, the composition according to the present invention may be a so-called one-part composition or a ready-to-use composition. For the purposes of the present invention, the expression "ready-to-use composition" is defined herein as a composition to be applied immediately to keratin fibers such as hair. The so-called one-part composition or the ready-to-use composition may be in a form of hair color treatment composition.
As compared to a so-called two-part composition, a so-called one-part composition does not need to mix ingredients in the composition prior to use. Therefore, it is easy for a consumer to use the composition according to the present invention for dyeing keratin fibers. Furthermore, stable coloring of keratin fibers is possible for the composition according to the present invention, because it is not possible to fail to mix ingredients in a precise mixing ratio which is required for two-part compositions for dyeing keratin fibers.
[Process] The composition according to the present invention can be used as a cosmetic composition.
Thus, the composition according to the present invention can be used for a non-therapeutic process, such as a cosmetic process, for treating the skin, hair, mucous membranes, nails, eyelashes, eyebrows, and/or scalp, by being applied to the skin, hair, mucous membranes, nails, eyelashes, eyebrows, and/or scalp.
The present invention also relates to a use of the composition according to the present invention, as it is or in care products and/or washing products and/or make-up products and/or
make-up-removing products for the body and/or facial skin and/or mucous membranes and/or scalp and/or hair and/or nails and/or eyelashes and/or eyebrows.
In other words, the composition according to the present invention can be used, as it is, as the above product, or the composition according to the present invention can be used as an element of the above products. For example the composition according to the present invention can be added to or combined with any other elements to form the above products.
The care product may be a lotion, a cream, a serum, a hair tonic, a hair conditioner, a sun screening agent, and the like. The washing product may be a shampoo, a face wash, a hand wash, and the like. The make-up product may be a foundation, a mascara, a lipstick, a lip gloss, a blusher, an eye shadow, a nail varnish, and the like. The make-up-removing product may be a make-up cleansing agent and the like.
In a preferred embodiment, the composition according to the present invention may be intended for dyeing a keratin substance, in particular keratin fibers. Thus, the compostion according to the present invention can be used as a cosmetic composition, in particular for keratin fibers.
The composition according to the present invention can be used for a non-therapeutic process, such as a cosmetic process, for dyeing keratin fibers such as eyelashes, eyebrows, and hair, comprising the step of applying the composition according to the present invention onto the keratin fibers.
The keratin fibers to which the composition according to the present invention has been applied can be left for an appropriate time which is required to treat the keratin fibers. The time length for the treatment is not limited, but it may be from 1 minute to 1 hours, preferably 1 minute to 30 minutes, and more preferably 1 minute to 15 minutes. For example, the time for dyeing the keratin fibers may be from 1 to 20 minutes, preferably 5 to 15 minutes.
The keratin fibers may be treated at a room temperature. Alternatively, the keratin fibers can be heated at 25C to 65°C, preferably 30°C to 60 °C, more preferably35 °C to 55°C, more preferably 40 °C to 50 °C, during the step of applying the composition according to the present invention to the keratin fibers, and/or the step of leaving the keratin fibers to which the composition according to the present invention has been applied.
The keratin fibers may be rinsed after the step of applying the composition according to the keratin fibers onto the keratin fibers and/or after the step of leaving the keratin fibers to which the composition according to the present invention has been applied.
EXAMPLES The present invention will be described in more detail by way of examples, which however should not be construed as limiting the scope of the present invention.
[Examples 1-3 and Comparative Examples 1-3] The following compositions according to Examples 1-3 and Comparative Examples 1-3, shown in Table 1 , were prepared by mixing the components shown in Table 1. The numerical values for the amounts of the components shown in Table 1 are all based on "% by weight" as active raw materials.
Table 1
[Evaluation] Each of the compositions according to Examples 1-3 and Comparative Examples 1-3 was applied onto a swatch of white goat hair in a weight ratio of 5 : 1 (the compositiomthe hair swatch). The applied hair swatch was left for 5 minutes at 27°C. It was then washed out with shampoo and dried. The color of the dyed hair swatch was then measured by Minolta CM-3600d. Based on the color (this was already measured before the application of the composition) of the undyed hair swatch and the measured color of the dyed hair swatch, ΔΕ (between the color of the undyed original hair and the color of the dyed hair under the L a b system) was calculated. For the evaluation of hair coloring ability, the following criteria was used.
Hair Coloring Criteria
The evaluation results are shown in Table 1.
It is clear from the experimental data in Table 1 that the presence of oil in an amount of more than 25% by weight can provide better hair coloring ability.
[Examples 4-11 and Comparative Examples 4-7]
The following compositions according to Examples 4-11 and Comparative Examples 4-7, shown in Table 2, were prepared by mixing the components shown in Table 2. The numerical values for
the amounts of the components shown in Table 2 are all based on "% by weight" as active raw materials.
Table 2
(Abbreviations)
E: Excellent
F: Fair
P: Poor
N/A: Not Available (Cream could not be prepared)
[Evaluation]
The aspect and stability of each of the compositions according to Examples 4-11 and
Comparative Examples 4-7 were evaluated by visual observations in accordance with the following criteria.
(Aspect Criteria)
(Stability Criteria)
The evaluation results are shown in Table 2. It is clear from the experimental data in Table 2 that the presence of polyoxyalkylenated fatty alcohol(s) can provide a better aspect and stability if the weight ratio of oil/thickener is within from 25 to 100.
[Example 12 and Comparative Example 8]
The following compositions according to Example 12 and Comparative Example 8, shown in Table 3, were prepared by mixing the components shown in Table 3. The numerical values for the amounts of the components shown in Table 3 are all based on "% by weight" as active raw materials.
Table 3
[Evaluation]
Each of the compositions according to Example 12 and Comparative Example 8 was applied onto a swatch of white goat hair in a weight ratio of 5: 1 (the compositiomthe hair swatch). The applied hair swatch was left for 5 minutes at 27°C. It was then washed out with shampoo and dried. The color of the dyed hair swatch was then measured by Minolta CM-3600d. Based on the color (this was already measured before the application of the composition) of the undyed hair swatch and the measured color of the dyed hair swatch, ΔΕ (between the color of the undyed original hair and the color of the dyed hair under the L a b system) was calculated. For the evaluation of hair coloring ability, following criteria was used.
Hair Coloring Criteria
The evaluation results are shown in Table 3.
It is clear from the experimental data in Table 3 that polyoxyalkylenated fatty alcohols can provide better hair coloring ability than poloxyethylenated and hydrogenated castor oil.
Claims
CLAIMS 1. A composition, comprising:
(a) at least one oil;
(b) at least one nonionic surfactant selected from the group consisting of
polyoxyalkylenated fatty alcohols;
(c) at least one thickener; and
(d) water,
wherein
the amount of the (a) oil is 25% by weight or more relative to the total weight of the composition, and
the weight ratio of the (a) oil/the (c) thickener is from 25 to 100. 2. The composition according to Claim 1 , wherein the (a) oil is selected from the group consisting of hydrocarbon oils and silicone oils. 3. The composition according to Claim 1 or 2, wherein the amount of the (a) oil ranges from 25 to 90%» by weight, preferably from 30 to 80% by weight, and more preferably from 35 to 70% by weight, relative to the total weight of the composition. 4. The composition according to any one of Claims 1 to 3, wherein the
polyoxyalkylenated fatty alcohols are selected from saturated or unsaturated, linear or branched, oxyalkylenated C8-C3o alcohols. 5. The composition according to any one of Claims 1 to 4, wherein the
polyoxyalkylenated fatty alcohols include from 1 to 100 ethyleneoxide units, preferably from 2 to 50 ethyleneoxide units, and more preferably from 2 to 30 ethyleneoxide units. 6. The composition according to any one of Claims 1 to 5 wherein the amount of the
(b) nonionic surfactant ranges from 0.01 to 15% by weight, preferably from 0.1 to 10% by weight, and more preferably from 0.5 to 5% by weight, relative to the total weight of the composition. 7. The composition according to any one of Claims 1 to 6, wherein the (c) thickener is selected from polysaccharides. 8. The composition according to any one of Claims 1 to 7 wherein the amount of the
(c) thickener ranges from 0.1 to 15% by weight, preferably from 0.2 to 10% by weight, and more preferably from 0.25 to 5% by weight, relative to the total weight of the composition. 9. The composition according to any one of Claims 1 to 8, wherein the weight ratio of the (a) oil/the (c) thickener is from 25 to 90, preferably from 25 to 80, and more preferably from 25 to 70. 10. The composition according to any one of Claims 1 to 9, further comprising (e) at least one dye selected from the group consisting of direct dyes and oxidative dyes. 11. The composition according to Claim 10, wherein the direct dyes are selected from
synthetic direct dyes. 12. The composition according to Claim 11 , wherein the synthetic direct dyes are
selected from the group consisting of hydrophilic synthetic direct dyes selected from the group consisting of cationic synthetic direct dyes, nonionic synthetic direct dyes, and anionic synthetic direct dyes; and hydrophobic synthetic direct dyes. 13. The composition according to any one of Claims 10 to 12, wherein the amount of the (e) dye ranges from 0.001 to 10% by weight, preferably from 0.01 to 5% by weight, and more preferably from 0.1 to 3% by weight, relative to the total weight of the composition. 14. The composition according to any one of Claims 1 to 13, wherein the composition is intended for dyeing keratin fibers, preferably hair. 15. A cosmetic process for keratin fibers, preferably hair, comprising the steps of
applying the composition according to any one of Claims 1 to 14 to the keratin fibers.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP2014-238844 | 2014-11-26 | ||
| JP2014238844A JP2016098221A (en) | 2014-11-26 | 2014-11-26 | Oil-rich composition |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2016084972A1 true WO2016084972A1 (en) | 2016-06-02 |
Family
ID=54838395
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/JP2015/083516 WO2016084972A1 (en) | 2014-11-26 | 2015-11-20 | Oil-rich composition |
Country Status (2)
| Country | Link |
|---|---|
| JP (1) | JP2016098221A (en) |
| WO (1) | WO2016084972A1 (en) |
Citations (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2798053A (en) | 1952-09-03 | 1957-07-02 | Goodrich Co B F | Carboxylic polymers |
| US2923692A (en) | 1954-01-25 | 1960-02-02 | Goodrich Co B F | Mucilaginous composition comprising salt of crosslinked carboxylic polymer and method of preparing same |
| GB1026978A (en) | 1962-03-30 | 1966-04-20 | Schwarzkopf Verwaltung G M B H | Method of dyeing hair |
| GB1153196A (en) | 1965-07-07 | 1969-05-29 | Schwarzkopf Verwaltung G M B H | Method of Dyeing Hair |
| FR2140205A1 (en) | 1971-06-04 | 1973-01-12 | Oreal | |
| FR2189006A1 (en) | 1972-06-19 | 1974-01-25 | Oreal | |
| DE2359399A1 (en) | 1973-11-29 | 1975-06-12 | Henkel & Cie Gmbh | Tetraaminopyrimidines as developers in oxidation hair dyes - esp. used with meta aminophenol couplers for blue shading dyes |
| US3915921A (en) | 1974-07-02 | 1975-10-28 | Goodrich Co B F | Unsaturated carboxylic acid-long chain alkyl ester copolymers and tri-polymers water thickening agents and emulsifiers |
| FR2285851A1 (en) | 1974-09-27 | 1976-04-23 | Oreal | 3-AMINO PYRIDINE DERIVATIVES AND TINCTORIAL COMPOSITIONS CONTAINING THEM |
| FR2416723A1 (en) | 1978-02-14 | 1979-09-07 | Hoechst Ag | USE OF CROSS-LINKED POLYMERS TO RAISE THE VISCOSITY OF COSMETIC, PHARMACEUTICAL OR TECHNICAL PRODUCTS |
| US4509949A (en) | 1983-06-13 | 1985-04-09 | The B. F. Goodrich Company | Water thickening agents consisting of copolymers of crosslinked acrylic acids and esters |
| DE3843892A1 (en) | 1988-12-24 | 1990-06-28 | Wella Ag | OXIDATION HAIR AGENTS CONTAINING DIAMINOPYRAZOL DERIVATIVES AND NEW DIAMINOPYRAZOLE DERIVATIVES |
| JPH0310659A (en) | 1989-06-07 | 1991-01-18 | Ichimaru Pharcos Co Ltd | Iron-rich hemoferrum and production thereof |
| DE4133957A1 (en) | 1991-10-14 | 1993-04-15 | Wella Ag | HAIR DYE CONTAINING AMINOPYRAZOLE DERIVATIVES AND NEW PYRAZOLE DERIVATIVES |
| WO1994008969A1 (en) | 1992-10-16 | 1994-04-28 | Wella Aktiengesellschaft | Process for producing 4,5-diamino pyrazole derivatives, their use for colouring hair and novel pyrazole derivatives |
| WO1994008970A1 (en) | 1992-10-16 | 1994-04-28 | Wella Aktiengesellschaft | Oxidation hair dye containing 4,5-diaminopyrazole derivatives and novel 4,5-diaminopyrazole derivatives and process for their production |
| EP0216479B2 (en) | 1985-08-12 | 1994-08-24 | Ciba Specialty Chemicals Water Treatments Limited | Polymeric thickeners and their production |
| WO1995001772A1 (en) | 1993-07-05 | 1995-01-19 | Ciba-Geigy Ag | Process for dyeing keratin-containing fibres |
| WO1995015144A1 (en) | 1993-11-30 | 1995-06-08 | Ciba-Geigy Ag | Cationic dyes for keratin-containing fibres |
| WO1996015765A1 (en) | 1994-11-17 | 1996-05-30 | Henkel Kommanditgesellschaft Auf Aktien | Oxidation dyes |
| EP0714954A2 (en) | 1994-11-03 | 1996-06-05 | Ciba-Geigy Ag | Cationic iminazoleazodyestuffs |
| FR2733749A1 (en) | 1995-05-05 | 1996-11-08 | Oreal | COMPOSITIONS FOR DYEING KERATINIC FIBERS CONTAINING DIAMINO PYRAZOLES, DYEING PROCESS, NOVEL DIAMINO PYRAZOLES, AND PREPARATION METHOD THEREOF |
| DE19543988A1 (en) | 1995-11-25 | 1997-05-28 | Wella Ag | Oxidative hair dye composition |
| FR2750048A1 (en) | 1996-06-21 | 1997-12-26 | Oreal | KERATIN FIBER DYEING COMPOSITIONS CONTAINING PYRAZOLO- (1,5-A) -PYRIMIDINE DERIVATIVES, DYEING PROCESS, NOVEL PYRAZOLO- (1,5-A) -PYRIMIDINE DERIVATIVES AND PROCESS FOR PREPARING THE SAME |
| EP1106167A2 (en) * | 1999-12-03 | 2001-06-13 | L'oreal | Dye compositions for keratin fibers comprising a nonionic compound |
| FR2830189A1 (en) | 2001-09-28 | 2003-04-04 | Oreal | LIGHTENING DYE COMPOSITION FOR HUMAN KERATIN FIBERS |
| EP1378544A2 (en) | 2002-07-05 | 2004-01-07 | L'oreal | Tetraaza-pentamethine derivatives and their application for dyeing keratinous fibres |
| EP1674073A1 (en) | 2004-12-23 | 2006-06-28 | L'oreal | Use of specific porphyrins or phtalocyanins for colouring human keratinic material, compositions comprising them, colouring process and components |
| US20060242773A1 (en) * | 2005-04-29 | 2006-11-02 | L'oreal | Direct emulsion for bleaching hair |
| EP2198927A2 (en) * | 2008-12-19 | 2010-06-23 | L'oreal | Composition comprising a fatty substance and a particular oxyethylene surfactant, dyeing or lightening process using it, and devices therefor |
| WO2013004772A2 (en) * | 2011-07-05 | 2013-01-10 | L'oreal | Dye composition comprising an alkoxylated fatty alcohol ether and a fatty alcohol |
| WO2014020146A2 (en) * | 2012-08-02 | 2014-02-06 | L'oreal | Dyeing composition comprising at least one fatty substance, at least one oxidizing agent and at least one non-ionic, anionic and amphoteric surfactant |
Family Cites Families (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPH03135432A (en) * | 1989-10-23 | 1991-06-10 | Sanyo Chem Ind Ltd | Emulsion of silicone compound and emulsifying method |
| JPH06271433A (en) * | 1991-07-25 | 1994-09-27 | Sanyo Chem Ind Ltd | Hair dye |
| JPH06247833A (en) * | 1993-02-23 | 1994-09-06 | Kao Corp | Composition for dyeing keratinous fiber |
| DE19642622A1 (en) * | 1996-10-16 | 1998-04-23 | Wella Ag | Hair treatment agent with long-lasting setting properties |
| JPH10265354A (en) * | 1997-03-26 | 1998-10-06 | Shiseido Co Ltd | Hair dye |
| JP2000044445A (en) * | 1998-07-30 | 2000-02-15 | Shiseido Co Ltd | Oil-in-water type hair dye |
| FR2831802B1 (en) * | 2001-11-08 | 2004-10-15 | Oreal | COSMETIC COMPOSITIONS CONTAINING AN AMINO SILICONE AND A THICKENING AGENT AND THEIR USES |
| JP2004035493A (en) * | 2002-07-04 | 2004-02-05 | Shiseido Co Ltd | Hairdye |
| JP2005194206A (en) * | 2003-12-26 | 2005-07-21 | Kao Corp | Two-pack type hair cosmetic |
| JP2005336126A (en) * | 2004-05-28 | 2005-12-08 | Shiseido Co Ltd | Hair dye |
| JP4351970B2 (en) * | 2004-08-31 | 2009-10-28 | ホーユー株式会社 | Oily hair cosmetic composition |
| JP2006143663A (en) * | 2004-11-22 | 2006-06-08 | Kao Corp | Hair dye composition |
| FR2940079B1 (en) * | 2008-12-19 | 2011-02-18 | Oreal | COMPOSITION COMPRISING AT LEAST ONE SOLID FATTY ALCOHOL, METHOD FOR COLORING THE SAME AND DEVICES THEREOF |
| FR2940103B1 (en) * | 2008-12-19 | 2011-06-10 | Oreal | METHOD FOR LIGHTENING COLORING KERATINIC MATERIALS USING AN EMULSION COMPRISING A COLORANT AND AN ALKALI AGENT AND AN OXIDIZING COMPOSITION |
| CN103079539A (en) * | 2010-07-30 | 2013-05-01 | 道康宁东丽株式会社 | Cosmetic for hair containing sugar alcohol-modified silicone |
| FR2970173B1 (en) * | 2011-01-10 | 2013-07-05 | Oreal | COLORING OR LIGHTENING PROCESS USING A RICH BODY COMPOSITION COMPRISING A SOLID ALCOHOL AND ESTER, COMPOSITIONS AND DEVICE |
| RU2667000C2 (en) * | 2011-07-05 | 2018-09-13 | Л'Ореаль | Cosmetic composition enriched in fatty substances containing ester of polyoxyalkylated fatty alcohol and direct dye and/or oxidation dye, coloring method and device |
| FR2983407B1 (en) * | 2011-12-06 | 2014-01-03 | Oreal | AQUEOUS OIL-RICH COMPOSITION AND ITS USE IN AN OXIDATION OR DECOLORATION COLORING PROCESS |
-
2014
- 2014-11-26 JP JP2014238844A patent/JP2016098221A/en active Pending
-
2015
- 2015-11-20 WO PCT/JP2015/083516 patent/WO2016084972A1/en active Application Filing
Patent Citations (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US2798053A (en) | 1952-09-03 | 1957-07-02 | Goodrich Co B F | Carboxylic polymers |
| US2923692A (en) | 1954-01-25 | 1960-02-02 | Goodrich Co B F | Mucilaginous composition comprising salt of crosslinked carboxylic polymer and method of preparing same |
| GB1026978A (en) | 1962-03-30 | 1966-04-20 | Schwarzkopf Verwaltung G M B H | Method of dyeing hair |
| GB1153196A (en) | 1965-07-07 | 1969-05-29 | Schwarzkopf Verwaltung G M B H | Method of Dyeing Hair |
| FR2140205A1 (en) | 1971-06-04 | 1973-01-12 | Oreal | |
| FR2189006A1 (en) | 1972-06-19 | 1974-01-25 | Oreal | |
| DE2359399A1 (en) | 1973-11-29 | 1975-06-12 | Henkel & Cie Gmbh | Tetraaminopyrimidines as developers in oxidation hair dyes - esp. used with meta aminophenol couplers for blue shading dyes |
| US3915921A (en) | 1974-07-02 | 1975-10-28 | Goodrich Co B F | Unsaturated carboxylic acid-long chain alkyl ester copolymers and tri-polymers water thickening agents and emulsifiers |
| FR2285851A1 (en) | 1974-09-27 | 1976-04-23 | Oreal | 3-AMINO PYRIDINE DERIVATIVES AND TINCTORIAL COMPOSITIONS CONTAINING THEM |
| FR2416723A1 (en) | 1978-02-14 | 1979-09-07 | Hoechst Ag | USE OF CROSS-LINKED POLYMERS TO RAISE THE VISCOSITY OF COSMETIC, PHARMACEUTICAL OR TECHNICAL PRODUCTS |
| US4509949A (en) | 1983-06-13 | 1985-04-09 | The B. F. Goodrich Company | Water thickening agents consisting of copolymers of crosslinked acrylic acids and esters |
| EP0216479B2 (en) | 1985-08-12 | 1994-08-24 | Ciba Specialty Chemicals Water Treatments Limited | Polymeric thickeners and their production |
| DE3843892A1 (en) | 1988-12-24 | 1990-06-28 | Wella Ag | OXIDATION HAIR AGENTS CONTAINING DIAMINOPYRAZOL DERIVATIVES AND NEW DIAMINOPYRAZOLE DERIVATIVES |
| JPH0310659A (en) | 1989-06-07 | 1991-01-18 | Ichimaru Pharcos Co Ltd | Iron-rich hemoferrum and production thereof |
| DE4133957A1 (en) | 1991-10-14 | 1993-04-15 | Wella Ag | HAIR DYE CONTAINING AMINOPYRAZOLE DERIVATIVES AND NEW PYRAZOLE DERIVATIVES |
| WO1994008969A1 (en) | 1992-10-16 | 1994-04-28 | Wella Aktiengesellschaft | Process for producing 4,5-diamino pyrazole derivatives, their use for colouring hair and novel pyrazole derivatives |
| WO1994008970A1 (en) | 1992-10-16 | 1994-04-28 | Wella Aktiengesellschaft | Oxidation hair dye containing 4,5-diaminopyrazole derivatives and novel 4,5-diaminopyrazole derivatives and process for their production |
| WO1995001772A1 (en) | 1993-07-05 | 1995-01-19 | Ciba-Geigy Ag | Process for dyeing keratin-containing fibres |
| WO1995015144A1 (en) | 1993-11-30 | 1995-06-08 | Ciba-Geigy Ag | Cationic dyes for keratin-containing fibres |
| EP0714954A2 (en) | 1994-11-03 | 1996-06-05 | Ciba-Geigy Ag | Cationic iminazoleazodyestuffs |
| WO1996015765A1 (en) | 1994-11-17 | 1996-05-30 | Henkel Kommanditgesellschaft Auf Aktien | Oxidation dyes |
| FR2733749A1 (en) | 1995-05-05 | 1996-11-08 | Oreal | COMPOSITIONS FOR DYEING KERATINIC FIBERS CONTAINING DIAMINO PYRAZOLES, DYEING PROCESS, NOVEL DIAMINO PYRAZOLES, AND PREPARATION METHOD THEREOF |
| DE19543988A1 (en) | 1995-11-25 | 1997-05-28 | Wella Ag | Oxidative hair dye composition |
| FR2750048A1 (en) | 1996-06-21 | 1997-12-26 | Oreal | KERATIN FIBER DYEING COMPOSITIONS CONTAINING PYRAZOLO- (1,5-A) -PYRIMIDINE DERIVATIVES, DYEING PROCESS, NOVEL PYRAZOLO- (1,5-A) -PYRIMIDINE DERIVATIVES AND PROCESS FOR PREPARING THE SAME |
| EP1106167A2 (en) * | 1999-12-03 | 2001-06-13 | L'oreal | Dye compositions for keratin fibers comprising a nonionic compound |
| FR2830189A1 (en) | 2001-09-28 | 2003-04-04 | Oreal | LIGHTENING DYE COMPOSITION FOR HUMAN KERATIN FIBERS |
| EP1378544A2 (en) | 2002-07-05 | 2004-01-07 | L'oreal | Tetraaza-pentamethine derivatives and their application for dyeing keratinous fibres |
| EP1674073A1 (en) | 2004-12-23 | 2006-06-28 | L'oreal | Use of specific porphyrins or phtalocyanins for colouring human keratinic material, compositions comprising them, colouring process and components |
| US20060242773A1 (en) * | 2005-04-29 | 2006-11-02 | L'oreal | Direct emulsion for bleaching hair |
| EP2198927A2 (en) * | 2008-12-19 | 2010-06-23 | L'oreal | Composition comprising a fatty substance and a particular oxyethylene surfactant, dyeing or lightening process using it, and devices therefor |
| WO2013004772A2 (en) * | 2011-07-05 | 2013-01-10 | L'oreal | Dye composition comprising an alkoxylated fatty alcohol ether and a fatty alcohol |
| WO2014020146A2 (en) * | 2012-08-02 | 2014-02-06 | L'oreal | Dyeing composition comprising at least one fatty substance, at least one oxidizing agent and at least one non-ionic, anionic and amphoteric surfactant |
Non-Patent Citations (11)
Also Published As
| Publication number | Publication date |
|---|---|
| JP2016098221A (en) | 2016-05-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2016084971A1 (en) | Silicone oil-rich composition | |
| US10772818B2 (en) | Methods and kits comprising an antioxidant booster composition for improving color durability in artificially colored hair | |
| FR2954127A1 (en) | COLORING AND / OR DECOLOURING AGENT OF TWO-PART KERATINOUS FIBERS, COMPRISING A BODY AND A SEQUESTRING AGENT. | |
| US20110203605A1 (en) | Methods and kits for providing lift and then a permanent color to hair | |
| EP2642976A2 (en) | Process for stripping keratin fibres using a composition comprising a sulfinic acid derivative and an acidic aqueous composition | |
| FR2954121A1 (en) | COLORING AND / OR DECOLOURING AGENT OF TWO - PART KERATIN FIBERS, COMPRISING A PARTICULAR FOLDER AND A REDUCTONE. | |
| EP2473235B1 (en) | Composition comprising an acid dye of indigoid type and dyeing method | |
| WO2010097558A2 (en) | Composition containing a natural or synthetic dye and an aliphatic monohydroxylated alcohol and keratin fibre dyeing method using same | |
| EP3086856A1 (en) | Packaging article comprising an envelope and an anhydrous dye composition comprising a direct dye, use of the same and process for dyeing keratin fibres | |
| FR2954093A1 (en) | AGENT FOR COLORING AND / OR DECOLORIZING KERATINIC FIBERS IN TWO OR MORE PARTS IN THE FORM OF EMULSION AND DISPERSION | |
| US10111816B2 (en) | Composition for altering the color of keratin fibers | |
| US20090282624A1 (en) | Composition comprising a hydrophobic dye and an alkylene carbonate or a lactone and dyeing of keratinous fibres | |
| US10328009B1 (en) | Methods and compositions for improving the durability of color in artificially colored hair | |
| US20070033744A1 (en) | Composition for simultaneously bleaching and dyeing keratin fibers, comprising at least one anionic or nonionic direct dye and at least one associative polymer | |
| EP3886795B1 (en) | Composition for keratin fibers comprising a direct dye | |
| WO2016084972A1 (en) | Oil-rich composition | |
| JP7362237B2 (en) | Method and kit for dyeing keratin fibers | |
| FR2917974A1 (en) | Anhydrous composition, useful for dyeing human keratin fibers e.g. hair, comprises direct dyes e.g. azodyes, hydrogen peroxide complex and a polymer containing heterocyclic vinyl monomer, and alkaline agents e.g. metasilicate | |
| JP2016017031A (en) | Method of preparing cosmetic composition for keratin fiber | |
| WO2023228870A1 (en) | Composition for coloring keratin fibers | |
| FR2942594A1 (en) | Composition, useful for dyeing of human keratin fibers, preferably hair, comprises in a medium, one or more natural dyes e.g. lawsone, juglone, alizarin and purpurin, and 3-phenyl-1-propanol | |
| JP7237469B2 (en) | Method and kit for dyeing keratin fibers | |
| JP2023173161A (en) | Composition for coloring keratin fibers | |
| FR3137835A1 (en) | Composition for coloring keratinous fibers | |
| FR2917973A1 (en) | Anhydrous composition, useful for dyeing human keratin fibers e.g. hair, comprises oxidation dye precursors e.g. para-phenylene diamine, hydrogen peroxide complex and polymer containing heterocyclic vinyl monomer, and alkaline agent |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15807711 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15807711 Country of ref document: EP Kind code of ref document: A1 |