WO2015179772A1 - Deuterated phenylquinazolinone and phenylisoquinolinone compounds - Google Patents
Deuterated phenylquinazolinone and phenylisoquinolinone compounds Download PDFInfo
- Publication number
- WO2015179772A1 WO2015179772A1 PCT/US2015/032194 US2015032194W WO2015179772A1 WO 2015179772 A1 WO2015179772 A1 WO 2015179772A1 US 2015032194 W US2015032194 W US 2015032194W WO 2015179772 A1 WO2015179772 A1 WO 2015179772A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- deuterium
- compound
- formula
- atom
- optionally substituted
- Prior art date
Links
- 0 CC(C)(C)OC(NC(*)(*)C(ON(C(CC1)=O)C1=O)=O)=O Chemical compound CC(C)(C)OC(NC(*)(*)C(ON(C(CC1)=O)C1=O)=O)=O 0.000 description 2
- UZOTUSBWWZAVQD-UHFFFAOYSA-N Nc1c(C(Nc2ccccc2)=O)c(F)ccc1 Chemical compound Nc1c(C(Nc2ccccc2)=O)c(F)ccc1 UZOTUSBWWZAVQD-UHFFFAOYSA-N 0.000 description 1
- GQVRPSFDUVNMDA-UHFFFAOYSA-N [O-][N+](c1c(C(Nc2ccccc2)=O)c(F)ccc1)=O Chemical compound [O-][N+](c1c(C(Nc2ccccc2)=O)c(F)ccc1)=O GQVRPSFDUVNMDA-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D487/00—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00
- C07D487/02—Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, not provided for by groups C07D451/00 - C07D477/00 in which the condensed system contains two hetero rings
- C07D487/04—Ortho-condensed systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/185—Acids; Anhydrides, halides or salts thereof, e.g. sulfur acids, imidic, hydrazonic or hydroximic acids
- A61K31/19—Carboxylic acids, e.g. valproic acid
- A61K31/195—Carboxylic acids, e.g. valproic acid having an amino group
- A61K31/196—Carboxylic acids, e.g. valproic acid having an amino group the amino group being directly attached to a ring, e.g. anthranilic acid, mefenamic acid, diclofenac, chlorambucil
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/41—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with two or more ring hetero atoms, at least one of which being nitrogen, e.g. tetrazole
- A61K31/4164—1,3-Diazoles
- A61K31/4184—1,3-Diazoles condensed with carbocyclic rings, e.g. benzimidazoles
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/505—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim
- A61K31/519—Pyrimidines; Hydrogenated pyrimidines, e.g. trimethoprim ortho- or peri-condensed with heterocyclic rings
- A61K31/52—Purines, e.g. adenine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/69—Boron compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7052—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides
- A61K31/706—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom
- A61K31/7064—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines
- A61K31/7076—Compounds having saccharide radicals and heterocyclic rings having nitrogen as a ring hetero atom, e.g. nucleosides, nucleotides containing six-membered rings with nitrogen as a ring hetero atom containing condensed or non-condensed pyrimidines containing purines, e.g. adenosine, adenylic acid
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
- A61P35/02—Antineoplastic agents specific for leukemia
Definitions
- ADME absorption, distribution, metabolism and/or excretion
- ADME limitation that affects many medicines is the formation of toxic or biologically reactive metabolites.
- some patients receiving the drug may experience toxicities, or the safe dosing of such drugs may be limited such that patients receive a suboptimal amount of the active agent.
- modifying dosing intervals or formulation approaches can help to reduce clinical adverse effects, but often the formation of such undesirable metabolites is intrinsic to the metabolism of the compound.
- a metabolic inhibitor will be co-administered with a drug that is cleared too rapidly.
- a drug that is cleared too rapidly.
- the FDA recommends that these drugs be co-dosed with ritonavir, an inhibitor of cytochrome P450 enzyme 3A4 (CYP3A4), the enzyme typically responsible for their metabolism (see Kempf, D.J. et al., Antimicrobial agents and chemotherapy, 1997, 41(3): 654-60).
- CYP3A4 cytochrome P450 enzyme 3A4
- Ritonavir causes adverse effects and adds to the pill burden for HIV patients who must already take a combination of different drugs.
- the CYP2D6 inhibitor quinidine has been added to dextromethorphan for the purpose of reducing rapid CYP2D6 metabolism of dextromethorphan in a treatment of pseudobulbar affect.
- Quinidine has unwanted side effects that greatly limit its use in potential combination therapy (see Wang, L et al, Clinical Pharmacology and Therapeutics, 1994, 56(6 Pt 1): 659-67; and FDA label for quinidine at www.accessdata.fda.gov).
- a potentially attractive strategy for improving a drug's metabolic properties is deuterium modification.
- Deuterium is a safe, stable, nonradioactive isotope of hydrogen. Compared to hydrogen, deuterium forms stronger bonds with carbon. In select cases, the increased bond strength imparted by deuterium can positively impact the AD ME properties of a drug, creating the potential for improved drug efficacy, safety, and/or tolerability.
- the size and shape of deuterium are essentially identical to those of hydrogen, replacement of hydrogen by deuterium would not be expected to affect the biochemical potency and selectivity of the drug as compared to the original chemical entity that contains only hydrogen.
- This invention relates to novel 3-phenylquinazolin-4-one compounds, and pharmaceutically acceptable salts thereof.
- This invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions that are beneficially treated by administering an inhibitor of phosphoinositide 3-kinase, also referred to as phosphatidylinositol 3-kinase (PI3K), particularly the delta isoform.
- PI3K phosphatidylinositol 3-kinase
- Idelalisib also known as (5)-2-(l-((9H-purin-6-yl)amino)propyl)-5-fluoro-3- phenylquinazolin-4(3H)-one, GS 1101 , GS- 1101 or C AL- 101, inhibits PI3K.
- PI3K delta signaling in particular, plays a key role the activation, proliferation, survival and trafficking of B lymphocytes and is hyperactive in many B-cell malignancies.
- Idelalisib selectively inhibits PI3K activity over other kinases, and selectively inhibits PI3K delta over the alpha, beta, and gamma forms of PI3K.
- Idelalisib is approved for treatment of chronic lymphocytic leukemia (CLL) and follicular lymphoma, and is currently in clinical studies to treat small lymphocytic lymphoma (SLL), non-Hodgkin's lymphoma, Hodgkin's lymphoma, diffuse large B-cell lymphoma, lymphoplasmacytoid lymphoma, marginal zone lymphoma, and mantle cell lymphoma, either alone or in combination with one or more second therapeutic agents.
- CLL chronic lymphocytic leukemia
- SLL small lymphocytic lymphoma
- SLL small lymphocytic lymphoma
- Hodgkin's lymphoma Hodgkin's lymphoma
- diffuse large B-cell lymphoma lymphoplasmacytoid lymphoma
- marginal zone lymphoma marginal zone lymphoma
- mantle cell lymphoma either alone or in combination with one or more second therapeutic agents
- FIGURE 1 shows Metabolic Stability of Compound 120 versus Idelalisib in Human CYP3A4 SupersomesTM
- FIGURE 2 shows Metabolic Stability of Compound 120 versus Idelalisib in Human Liver Cytosol
- treat means decrease, suppress, attenuate, diminish, arrest, or stabilize the development or progression of a disease (e.g., a disease or disorder delineated herein), lessen the severity of the disease or improve the symptoms associated with the disease.
- a disease e.g., a disease or disorder delineated herein
- Disease means any condition or disorder that damages or interferes with the normal function of a cell, tissue, or organ.
- alkyl refers to a monovalent saturated hydrocarbon group. Ci-C 6 alkyl is an alkyl having from 1 to 6 carbon atoms. An alkyl may be linear or
- alkyl groups include methyl; ethyl; propyl, including n-propyl and isopropyl; butyl, including n-butyl, isobutyl, sec-butyl, and t-butyl; pentyl, including, for example, n-pentyl, isopentyl, and neopentyl; and hexyl, including, for example, n- hexyl and 2-methylpentyl.
- alkylene by itself or as part of another substituent refers to a saturated straight-chain or branched divalent group having the stated number of carbon atoms and derived from the removal of two hydrogen atoms from the corresponding alkane.
- straight chained and branched alkylene groups include -CH 2 - (methylene), -CH 2 -CH 2 - (ethylene), -CH 2 -CH 2 -CH 2 -
- alkenyl refers to a monovalent unsaturated hydrocarbon group where the unsaturation is represented by a double bond.
- C2-C6 alkenyl is an alkenyl having from 2 to 6 carbon atoms.
- alkynyl refers to a monovalent unsaturated hydrocarbon group where the unsaturation is represented by a triple bond.
- C2-C6 alkynyl is an alkenyl having from 2 to 6 carbon atoms.
- An alkynyl may be linear or branched. Examples of alkynyl groups include CH ⁇ C-, -C ⁇ C(CH 3 ), CH 3 -C ⁇ C-CH 2 -, CH 3 -C ⁇ C-CH 2 -CH 2 and CH 3 -C ⁇ C- CH(CH 3 )-CH 2 -.
- cycloalkyl refers to a monocyclic or bicyclic monovalent saturated or non-aromatic unsaturated hydrocarbon ring system.
- C 3 -Cio cycloalkyl refers to a cycloalkyl wherein the number of ring carbon atoms is from 3 to 10. Examples of C 3 -Cio cycloalkyl include C 3 -C 6 cycloalkyl.
- Bicyclic ring systems include fused, bridged, and spirocyclic ring systems.
- cycloalkyl groups include, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cis- and trans- decalinyl, norbornyl, and spiro[4.5]decanyl.
- Carbocyclyl refers to a monocyclic or bicyclic monovalent saturated or non-aromatic unsaturated hydrocarbon ring system.
- C 3 -Cio carbocyclyl refers to a carbocyclyl wherein the number of ring carbon atoms is from 3 to 10. Examples of C 3 -Cio carbocyclyl include C 3 -C 6 carbocyclyl.
- Bicyclic ring systems include fused, bridged, and spirocyclic ring systems.
- carbocyclyl groups include, cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cycloheptyl, cis- and trans-decalinyl, norbornyl, norbornenyl, and spiro[4.5]decanyl.
- heterocycloalkyl refers to a monocyclic or bicyclic monovalent saturated or non-aromatic unsaturated ring system wherein from 1 to 4 ring atoms are heteroatoms independently selected from the group consisting of O, N and S.
- the term "3 to 10-membered heterocycloalkyl” refers to a heterocycloalkyl wherein the number of ring atoms is from 3 to 10. Examples of 3 to 10-membered heterocycloalkyl include 3 to 6-membered heterocycloalkyl.
- Bicyclic ring systems include fused, bridged, and spirocyclic ring systems.
- heterocycloalkyl groups include azepanyl, azetidinyl, aziridinyl, imidazolidinyl, morpholinyl, oxazolidinyl, oxazolidinyl, piperazinyl, piperidinyl, pyrazolidinyl, pyrrolidinyl, quinuclidinyl, and thiomorpholinyl.
- the nitrogen, phosphorus, carbon or sulfur atoms can be optionally oxidized to various oxidation states.
- the group -S(O) 0-2 - refers to -S-(sulfide), -S(0)-(sulfoxide), and -SO2- (sulfone) respectively.
- nitrogens particularly but not exclusively, are meant to include their corresponding N-oxide form, although not explicitly defined as such in a particular example.
- annular nitrogen atoms can be optionally quatemized; and the ring substituent can be partially or fully saturated or aromatic.
- Aryl by itself or as part of another substituent refers to a monovalent aromatic hydrocarbon group having the stated number of carbon atoms (i.e., C5-C14 means from 5 to 14 carbon atoms).
- Typical aryl groups include, but are not limited to, groups derived from aceanthrylene, acenaphthylene, acephenanthrylene, anthracene, azulene, benzene, chrysene, coronene, fluoranthene, fluorene, hexacene, hexaphene, hexylene, as-indacene, 5-indacene, indane, indene, naphthalene, octacene, octophene, octalene, ovalene, penta- 2,4-diene, pentacene, pentalene, pentaphene, perylene, phenalene, phenanthren
- Arylalkyl by itself or as part of another substituent refers to an acyclic alkyl group in which one of the hydrogen atoms bonded to a carbon atom, typically a terminal or sp 3 carbon atom, is replaced with an aryl group.
- Typical arylalkyl groups include, but are not limited to, benzyl, 2-phenylethan-l-yl, 2-phenylethen-l-yl, naphthylmethyl, 2- naphthylethan-l-yl, 2-naphthylethen-l-yl, naphthobenzyl, 2-naphthophenylethan-l-yl and the like.
- the alkyl moiety of the arylalkyl group is (Ci-Ce) and the aryl moiety is (C5-C14).
- the alkyl group is (C1-C3) and the aryl moiety is (C5-C10), such as (C6-C10).
- heteroaryl refers to a monovalent aromatic monocyclic ring system wherein at least one ring atoms is a heteroatom independently selected from the group consisting of O, N and S.
- 5-membered heteroaryl refers to a heteroaryl wherein the number of ring atoms is 5. Examples of 5-membered heteroaryl groups include pyrrolyl, pyrazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, furazanyl, imidazolinyl, and triazolyl.
- Heteroarylalkyl by itself or as part of another substituent refers to an acyclic alkyl group in which one of the hydrogen atoms bonded to a carbon atom, typically a terminal or sp 3 carbon atom, is replaced with a heteroaryl group.
- the alkyl moiety of the heteroarylalkyl is (Ci-Ce) alkyl and the heteroaryl moiety is a 5-14- membered heteroaryl.
- the alkyl moiety is (C1-C3) alkyl and the heteroaryl moiety is a 5-10 membered heteroaryl.
- Halogen or "Halo” by themselves or as part of another substituent refers to fluorine, chlorine, bromine and iodine, or fluoro, chloro, bromo and iodo.
- any atom not specifically designated as a particular isotope is meant to represent any stable isotope of that atom.
- a position is designated specifically as “H” or “hydrogen”
- the position is understood to have hydrogen at its natural abundance isotopic composition.
- a position is designated specifically as “D” or “deuterium”
- the position is understood to have deuterium at an abundance that is at least 3340 times greater than the natural abundance of deuterium, which is 0.015% (i.e., at least 50.1% incorporation of deuterium).
- isotopic enrichment factor means the ratio between the isotopic abundance and the natural abundance of a specified isotope.
- a compound of this invention has an isotopic enrichment factor for each designated deuterium atom of at least 3500 (52.5% deuterium
- incorporation at each designated deuterium atom at least 4000 (60% deuterium incorporation), at least 4500 (67.5% deuterium incorporation), at least 5000 (75% deuterium), at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), at least 6333.3 (95% deuterium incorporation), at least 6466.7 (97% deuterium incorporation), at least 6600 (99% deuterium incorporation), or at least 6633.3 (99.5%) deuterium incorporation).
- isotopologue refers to a species in which the chemical structure differs from a specific compound of this invention only in the isotopic composition thereof.
- a compound represented by a particular chemical structure containing indicated deuterium atoms will also contain lesser amounts of isotopologues having hydrogen atoms at one or more of the designated deuterium positions in that structure.
- the relative amount of such isotopologues in a compound of this invention will depend upon a number of factors including the isotopic purity of deuterated reagents used to make the compound and the efficiency of incorporation of deuterium in the various synthesis steps used to prepare the compound.
- the relative amount of such isotopologues in toto will be less than 49.9% of the compound. In other embodiments, the relative amount of such isotopologues in toto will be less than 47.5%, less than 40%, less than 32.5%, less than 25%, less than 17.5%, less than 10%, less than 5%, less than 3%, less than 1%, or less than 0.5% of the compound.
- the invention also provides salts of the compounds of the invention.
- a salt of a compound of this invention is formed between an acid and a basic group of the compound, such as an amino functional group, or a base and an acidic group of the compound, such as a carboxyl functional group.
- the compound is a pharmaceutically acceptable acid addition salt.
- pharmaceutically acceptable refers to a component that is, within the scope of sound medical judgment, suitable for use in contact with the tissues of humans and other mammals without undue toxicity, irritation, allergic response and the like, and are commensurate with a reasonable benefit/risk ratio.
- pharmaceutically acceptable salt means any non-toxic salt that, upon administration to a recipient, is capable of providing, either directly or indirectly, a compound of this invention.
- pharmaceutically acceptable counterion is an ionic portion of a salt that is not toxic when released from the salt upon administration to a recipient.
- Acids commonly employed to form pharmaceutically acceptable salts include inorganic acids such as hydrogen bisulfide, hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid and phosphoric acid, as well as organic acids such as para- toluenesulfonic acid, salicylic acid, tartaric acid, bitartaric acid, ascorbic acid, maleic acid, besylic acid, fumaric acid, gluconic acid, glucuronic acid, formic acid, glutamic acid, methanesulfonic acid, ethanesulfonic acid, benzenesulfonic acid, lactic acid, oxalic acid, para-bromophenylsulfonic acid, carbonic acid, succinic acid, citric acid, benzoic acid and acetic acid, as well as related inorganic and organic acids.
- organic acids such as para- toluenesulfonic acid, salicylic acid, tartaric acid, bitartaric acid, ascorbic acid
- salts thus include sulfate, pyrosulfate, bisulfate, sulfite, bisulfite, phosphate, monohydrogenphosphate, dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide, acetate, propionate, decanoate, caprylate, acrylate, formate, isobutyrate, caprate, heptanoate, propiolate, oxalate, malonate, succinate, suberate, sebacate, fumarate, maleate, butyne-l,4-dioate, hexyne-l,6-dioate, benzoate, chlorobenzoate, methylbenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, phthalate, terephthalate, sulfonate, xylene sulfonate, phenylacetate, phenylpropionate
- the compounds of the present invention may contain an asymmetric carbon atom, for example, as the result of deuterium substitution or otherwise.
- compounds of this invention can exist as either individual enantiomers, or mixtures of the two enantiomers. Accordingly, a compound of the present invention may exist as either a racemic mixture or a scalemic mixture, or as individual respective stereoisomers that are substantially free from another possible stereoisomer.
- substantially free of other stereoisomers means less than 25% of other stereoisomers, preferably less than 10% of other stereoisomers, more preferably less than 5% of other stereoisomers and most preferably less than 2% of other stereoisomers are present.
- stable compounds refers to compounds which possess stability sufficient to allow for their manufacture and which maintain the integrity of the compound for a sufficient period of time to be useful for the purposes detailed herein (e.g., formulation into therapeutic products, intermediates for use in production of therapeutic compounds, isolatable or storable intermediate compounds, treating a disease or condition responsive to therapeutic agents).
- Substituted with deuterium refers to the replacement of one or more hydrogen atoms with a corresponding number of deuterium atoms.
- variable may be referred to generally (e.g., "each R") or may be referred to specifically (e.g., R 1 , R 2 , R 3 , etc.). Unless otherwise indicated, when a variable is referred to generally, it is meant to include all specific embodiments of that particular variable.
- the present invention provides a compound of Formula (AB):
- X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
- Z is N or CY 4d ;
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or deuterium;
- R is methyl optionally substituted with 1 -3 deuterium or ethyl optionally
- R, X, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 comprises a deuterium atom
- Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each deuterium, then at least one of Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , and Y 4c is deuterium.
- X is CI, F, -CF 3 or -CD 3 .
- R is -CH 3 , -CD 3 , -CH 2 CH 3 , -CD 2 CH 3 ,
- the compound of Formula (AB) has the structure of Formula (A):
- X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or
- R is methyl optionally substituted with 1 -3 deuterium or ethyl optionally
- R, X, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 comprises a deuterium atom.
- X is CI, F, -CF 3 or -CD 3 .
- R is -CH 3 , -CD 3 , -CH 2 CH 3 , -CD 2 CH 3 , -CH 2 CD 3 , or -CD 2 CD 3 .
- the compound of Formula (A) has the structure of Formula (A-I):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or
- R is selected from the group consisting of: -CH2CH3, -CH2CD3, -CD2CH3
- R at least one of R, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 comprises a deuterium atom.
- the compound of Formula (A) has the structure of Formula ( ⁇ - ⁇ ):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or
- R is -CH3 or -CD 3 ;
- R at least one of R, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y* and Y 7 comprises a deuterium atom.
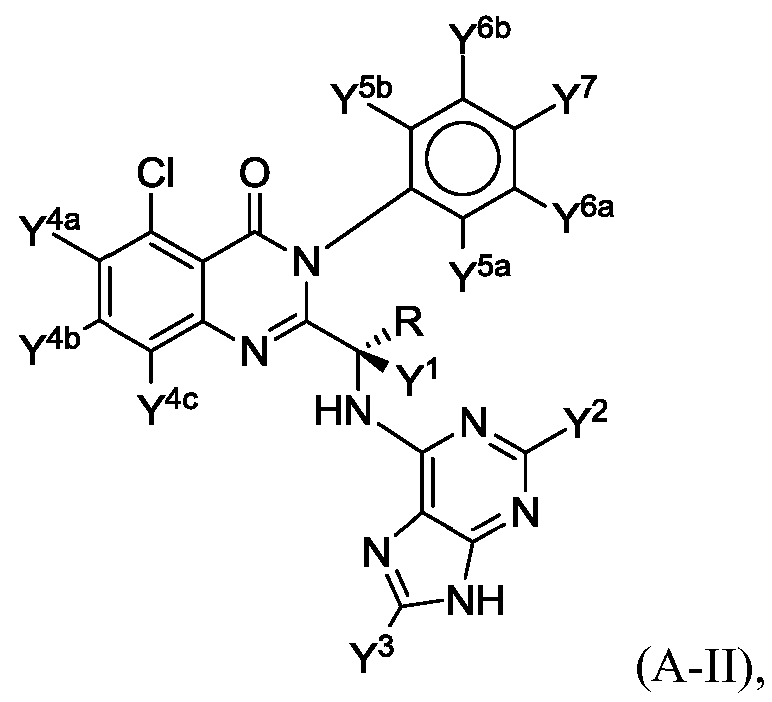
- the compound of Formula (A) has the structure of Formula (A-II):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH2CH3, -CH2CD3,
- R at least one of R, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 comprises a deuterium atom.
- the compound of Formula (A) has the structure of Formula
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or
- R is selected from the group consisting of: -CH3, -CD 3 , -CH2CH3, -CH2CD3, -CD 2 CH 3 and -CD 2 CD 3 .
- the compound of Formula (A) has the structure of Formula (A-IV):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH 2 CH 3 , -CH 2 CD 3 , -CD 2 CH 3 and -CD 2 CD 3 ;
- R at least one of R, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y* and Y 7 comprises a deuterium atom.
- the compound of Formula (AB) has the structure of Formula (B):
- X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or deuterium;
- R is methyl optionally substituted with 1 -3 deuterium or ethyl optionally
- R, X, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 comprises a deuterium atom
- Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each deuterium, then at least one of Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , and Y 4c is deuterium.
- X is CI, F, -CF 3 or -CD 3 .
- R is -CH 3 , -CD 3 , -CH 2 CH 3 , -CD 2 CH 3 ,
- the compound of Formula (B) has the structure of Formula (B-I):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or deuterium; R is selected from the group consisting
- Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y* and Y 7 comprises a deuterium atom.
- the compound of Formula (B) has the structure of Formula
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or deuterium;
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH 2 -CH 3 , -CH 2 -CD 3 ,
- R at least one of R, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y* and Y 7 comprises a deuterium atom;
- the compound of Formula (B) has the structure of Formula (B-III):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydrogen or deuterium;
- R is selected from the group consisting of: -CH3, -CD3, -CH2-CH3, -CH2-CD3, -CD2-CH3 and -CD2-CD3.
- the compound of Formula (B) has the structure of Formula (B-IV):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each independently hydi
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH2-CH3, -CH2-CD3, -CD2-CH3 and -CD2-CD3;
- R at least one of R, Y 1 , Y 2 , Y 3 , Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y* and Y 7 comprises a deuterium atom.
- Y 1 is deuterium.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 2 is deuterium.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 3 is deuterium.
- Y 2 and Y 3 are the same.
- Y 2 and Y 3 can be deuterium.
- Y 2 and Y 3 can be hydrogen.
- Y 1 , Y 2 and Y 3 are the same.
- Y 1 , Y 2 and Y 3 can be deuterium.
- Y 1 , Y 2 and Y 3 can be hydrogen.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 4a is deuterium.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 4b is deuterium.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 4c is deuterium.
- Y 4a , Y 4b and Y 4c are the same.
- Y 4a , Y 4b and Y 4c can be deuterium.
- Y 4a , Y 4b and Y 4c can be hydrogen.
- Y 4a , Y 4b , Y 4c and Y 4d are the same.
- Y 4a , Y 4b , Y 4c and Y 4d can be deuterium.
- Y 4a , Y 4b , Y 4c and Y 4d can be hydrogen.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 5a is deuterium.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 5b is deuterium.
- Y 5a and Y 5b are the same.
- Y 5a and Y 5b can be deuterium.
- Y 5a and Y 5b can be hydrogen.
- A, A-I, A- ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 6a is deuterium.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 6b is deuterium.
- Y 6a and Y 6b are the same.
- Y 6a and Y 6b can be deuterium.
- Y 6a and Y 6b can be hydrogen.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 7 is deuterium.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV,Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are the same.
- Y 5a , Y 5b , Y 6a , Y* and Y 7 can be deuterium.
- Y 5a , Y 5b , Y 6a , Y 6b and Y 7 can be hydrogen.
- A, A-I, ⁇ - ⁇ , A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are the same.
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 can be deuterium.
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 can be hydrogen.
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y* and Y 7 are the same.
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y 6b and Y 7 can be deuterium.
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5a , Y 5b , Y 6a , Y* and Y 7 can be hydrogen.
- R is selected from the group consisting of: -CH2CD3 and -CD2CD3.
- R can be -CH2CD3.
- R is -CD2CD3.
- Y , Y and Y are each hydrogen, Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each deuterium, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 1 (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- Y 4a , Y 4b and Y 4c are each hydrogen, Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each deuterium, R is -CH2CH3, and the compound is selected from any one of the compounds (Cmpd) set forth in Table la (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- Y 4a , Y 4b , Y 4c and Y 4d are each hydrogen, Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each deuterium, and the compound is selected from any one of the compounds (Cmpd) set forth in Table lb (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- Y 4a , Y 4b and Y 4c are each deuterium, Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each hydrogen, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 2:
- any atom not designated as deuterium is present at its natural isotopic abundance.
- Y 4a , Y 4b and Y 4c are each deuterium, Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each hydrogen, R is -CH2CH3, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 2a:
- Table 2a Exemplary Embodiments of Formula (A-I) Compound Y 1 Y 2 Y 3
- Y 4a , Y 4b , Y 4c and Y 4d are each deuterium, Y 5a , Y 5b , Y 6a , Y 6b and Y 7 are each hydrogen, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 2b:
- any atom not designated as deuterium is present at its natural isotopic abundance.
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b , and Y 7 are each deuterium and the compound is selected from any one of the compounds (Cmpd) set forth in Table 3 (below):
- Y 4a , Y 4b , Y 4c , Y 5a , Y 5b , Y 6a , Y 6b , and Y 7 are each deuterium, R is -CH2CH3, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 3a (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- Y 4a , Y 4b , Y 4c , Y 4d , Y 5 Y 5b , Y 6a , Y 6b , and Y 7 are each deuterium, and the compound is selected from any one the compounds (Cmpd) set forth in Table 3b (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the compound of Formula (A-I) has the structure of Formula (A-Ia):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is selected from the group consisting of: -C bC ⁇ -C bCDs, -CD2CH3, and - CD2CD3;
- At least one of R, Y 1 , Y 2 , and Y 3 comprises a deuterium atom.
- the compound of Formula ( ⁇ - ⁇ ) has the structure of Formula (A-Ia'):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is -CH 3 or -CD 3 ;
- At least one of R, Y 1 , Y 2 , and Y 3 comprises a deuterium atom.
- the compound of Formula (A-II) has the structure of Formula (A-IIa):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH 2 CH 3 ,
- At least one of R, Y 1 , Y 2 and Y 3 comprises a deuterium atom.
- the compound of Formula (A-III) has the structure of Formula (A-IIIa):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH2CH3, -CH2CD3, -CD 2 CH 3 and -CD 2 CD 3 .
- the compound of Formula (A-IV) has the structure of
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH 2 CH 3 ,
- the compound of Formula (B-I) has the structure of Formula (B-Ia):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH2CH3,
- At least one of R, Y 1 , Y 2 , and Y 3 comprises a deuterium atom.
- the compound of Formula (B-II) has the structure of Formula (B-IIa):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is selected from the group consisting of: -CH3, -CD 3 , -CH2CH3,
- At least one of R, Y 1 , Y 2 and Y 3 comprises a deuterium atom
- the compound of Formula (B-III) has the structure of Formula (B-IIIa):
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH2CH3, -CH2CD3, -CD 2 CH 3 and -CD 2 CD 3 .
- the compound of Formula (B-IV) has the structure of
- Y 1 , Y 2 and Y 3 are each independently hydrogen or deuterium
- R is selected from the group consisting of: -CH 3 , -CD 3 , -CH 2 CH 3 , -CH 2 CD 3 , -CD 2 CH 3 and -CD 2 CD 3 ;
- At least one of R, Y 1 , Y 2 and Y 3 comprises a deuterium atom.
- Y 1 is deuterium.
- Y 2 is deuterium.
- Y 3 is deuterium.
- Y 2 and Y 3 are the same.
- Y 2 and Y 3 can be deuterium.
- Y 1 , Y 2 and Y 3 are the same.
- Y 1 , Y 2 , and Y 3 can be deuterium.
- R is selected from the group consisting of: -CH2CD3 and -CD2CD3.
- R can be -CH2CD3.
- R is -CD2CD3.
- R is selected from the group consisting of: -CH2CH3 and -CD2CD3.
- R can be -CH2CH3.
- R is selected from the group consisting of: -CH3 and -CD3.
- R can be -CH3.
- R is -CD3.
- the compound of Formula (A-Ia) is selected from any one of the compounds (Cmpd) set forth in Table 4 (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the compound of Formula (B-Ia) is selected from any the compounds (Cmpd) set forth in Table 4c (below):
- the compound of Formula (A-IIa) is selected from any one of the compounds (Cmpd) set forth in Table 5a (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the compound of Formula (B-IIa) is selected from any the compounds (Cmpd) set forth in Table 5b (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the compound of Formula (A-IIIa) is selected from any one of the compounds (Cmpd) set forth in Table 6a (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the compound of Formula (B-IIIa) is selected from any one of the compounds (Cmpd) set forth in Table 6b (below): Table 6b: Exemplary Embodiments of Formula (B-IIIa)
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the compound of Formula (A-IVa) is selected from any one of the compounds (Cmpd) set forth in Table 7a (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- the compound of Formula (B-IVa) is selected from any one of the compounds (Cmpd) set forth in Table 7b (below):
- any atom not designated as deuterium is present at its natural isotopic abundance.
- any atom not designated as deuterium in any of the embodiments set forth above is present at its natural isotopic abundance.
- Such methods can be carried out utilizing corresponding deuterated and optionally, other isotope-containing reagents and/or intermediates to synthesize the compounds delineated herein, or invoking standard synthetic protocols known in the art for introducing isotopic atoms to a chemical structure.
- Reagents and conditions (a) (i) (COCl) 2 , DMF (cat); (ii) aniline, base such as NaHC0 3 ; (b) (i) SOCl 2 , DMF (cat); (ii) (12), base such as Et 3 N; (c) Zn, AcOH; (d) (i) TFA; (ii) K 2 C0 ; (e) (16), DIPEA
- compound (13) can be readily prepared with %-atom d incorporation values of 95% or greater under these conditions.
- Amine 15 is then allowed to react at elevated temperature in a solvent such as tert-butyl alcohol with an optionally deuterium- substituted 6-bromopurine (16) in the presence of a base such as di-isopropylethylamine to afford compounds of Formula A-Ia or A-Ia'.
- Reagents and conditions (a) Zn, AcOH; (b) DIEA, DMAP
- Boc-protected 2-aminobutyric acid analogs (12) are prepared from the corresponding amino acid (19) using methods known to one of ordinary skill in the art. Many of the corresponding amino acids (19) are commercially available (examples 19a, 19c, 19e and 19h) or may be prepared using known methods from commercially available optionally deuterium-substituted intermediates.
- 2-Aminobutyric acid analog 19b is prepared according to the procedure described by O'Reily, E. et. al. in Amino Acids (2010), 39(3), 849-858.
- Analogs 19d and 19g are prepared according to the procedure of Stirling, I. et. al. described in J. Chem. Soc. Perkin I: Org.
- deuterium-substituted 6-bromopurine analogs (16) used in the preparation of compounds of Formula AB and all sub-formula of AB are prepared from hypoxanthine (20) in high yield with %-atom d values of 95% using the hydrogen- deuterium exchange method of Sajiki, H. described in WO 2004/046066 to afford (21). Subsequent treatment of (21) with phosphorous tribromide at elevated temperature affords compound (16).
- Compounds of Formula B, B-I, B-Ia, B-II, B-IIa, B-III, B-IIIa, B-IV and B- IVa can be prepared using methods analogous to those disclosed in US 2009312319 and to that shown in the above schemes using appropriately deuterated starting materials which are commercially available or prepared according to routine methods known to one of skill in the art.
- the invention also provides pharmaceutical compositions comprising an effective amount of a compound of Formula AB and/or any sub-formula of AB (e.g., including any of the formulae herein), or a pharmaceutically acceptable salt of said compound; and a pharmaceutically acceptable carrier.
- the carrier(s) are "acceptable" in the sense of being compatible with the other ingredients of the formulation and, in the case of a
- Pharmaceutically acceptable carriers, adjuvants and vehicles that may be used in the pharmaceutical compositions of this invention include, but are not limited to, ion exchangers, alumina, aluminum stearate, lecithin, serum proteins, such as human serum albumin, buffer substances such as phosphates, glycine, sorbic acid, potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts or electrolytes, such as protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone, cellulose-based substances, polyethylene glycol, sodium carboxymethylcellulose, polyacrylates, waxes, polyethylene -polyoxypropylene-block polymers, polyethylene glycol and wool fat.
- the solubility and bioavailability of the compounds of the present invention in pharmaceutical compositions may be enhanced by methods well-known in the art.
- One method includes the use of lipid excipients in the formulation. See “Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs (Drugs and the Pharmaceutical Sciences),” David J. Hauss, ed. Informa Healthcare, 2007; and “Role of Lipid Excipients in Modifying Oral and Parenteral Drug Delivery: Basic Principles and Biological Examples," Kishor M. Wasan, ed. Wiley-Interscience, 2006.
- Another known method of enhancing bioavailability is the use of an amorphous form of a compound of this invention optionally formulated with a poloxamer, such as LUTPvOLTM and PLURONICTM (BASF Corporation), or block copolymers of ethylene oxide and propylene oxide. See United States patent 7,014,866; and United States patent publications 20060094744 and 20060079502.
- compositions of the invention include those suitable for oral, rectal, nasal, topical (including buccal and sublingual), vaginal or parenteral (including subcutaneous, intramuscular, intravenous and intradermal) administration.
- the compound of the formulae herein is administered transdermally (e.g., using a transdermal patch or iontophoretic techniques).
- Other formulations may conveniently be presented in unit dosage form, e.g., tablets, sustained release capsules, and in liposomes, and may be prepared by any methods well known in the art of pharmacy. See, for example, Remington: The Science and Practice of Pharmacy, Lippincott Williams & Wilkins, Baltimore, MD (20th ed. 2000).
- Such preparative methods include the step of bringing into association with the molecule to be administered ingredients such as the carrier that constitutes one or more accessory ingredients.
- ingredients such as the carrier that constitutes one or more accessory ingredients.
- the compositions are prepared by uniformly and intimately bringing into association the active ingredients with liquid carriers, liposomes or finely divided solid carriers, or both, and then, if necessary, shaping the product.
- compositions of the present invention suitable for oral administration may be presented as discrete units such as capsules, sachets, or tablets each containing a predetermined amount of the active ingredient; a powder or granules; a solution or a suspension in an aqueous liquid or a non-aqueous liquid; an oil -in- water liquid emulsion; a water-in-oil liquid emulsion; packed in liposomes; or as a bolus, etc.
- Soft gelatin capsules can be useful for containing such suspensions, which may beneficially increase the rate of compound absorption.
- carriers that are commonly used include lactose and corn starch.
- Lubricating agents such as magnesium stearate, are also typically added.
- useful diluents include lactose and dried cornstarch.
- aqueous suspensions are administered orally, the active ingredient is combined with emulsifying and suspending agents. If desired, certain sweetening and/or flavoring and/or coloring agents may be added.
- compositions suitable for oral administration include lozenges comprising the ingredients in a flavored basis, usually sucrose and acacia or tragacanth; and pastilles comprising the active ingredient in an inert basis such as gelatin and glycerin, or sucrose and acacia.
- compositions suitable for parenteral administration include aqueous and nonaqueous sterile injection solutions which may contain anti -oxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient; and aqueous and non-aqueous sterile suspensions which may include suspending agents and thickening agents.
- the formulations may be presented in unit- dose or multi-dose containers, for example, sealed ampules and vials, and may be stored in a freeze dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example water for injections, immediately prior to use.
- Extemporaneous injection solutions and suspensions may be prepared from sterile powders, granules and tablets.
- Such injection solutions may be in the form, for example, of a sterile injectable aqueous or oleaginous suspension.
- This suspension may be formulated according to techniques known in the art using suitable dispersing or wetting agents (such as, for example, Tween 80) and suspending agents.
- the sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic parenterally-acceptable diluent or solvent, for example, as a solution in 1,3-butanediol.
- the acceptable vehicles and solvents that may be employed are mannitol, water, Ringer's solution and isotonic sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed oil may be employed including synthetic mono- or diglycerides.
- Fatty acids, such as oleic acid and its glyceride derivatives are useful in the preparation of injectables, as are natural pharmaceutically-acceptable oils, such as olive oil or castor oil, especially in their polyoxyethylated versions.
- These oil solutions or suspensions may also contain a long- chain alcohol diluent or dispersant.
- compositions of this invention may be administered in the form of suppositories for rectal administration.
- These compositions can be prepared by mixing a compound of this invention with a suitable non-irritating excipient which is solid at room temperature but liquid at the rectal temperature and therefore will melt in the rectum to release the active components.
- suitable non-irritating excipient include, but are not limited to, cocoa butter, beeswax and polyethylene glycols.
- compositions of this invention may be administered by nasal aerosol or inhalation.
- Such compositions are prepared according to techniques well- known in the art of pharmaceutical formulation and may be prepared as solutions in saline, employing benzyl alcohol or other suitable preservatives, absorption promoters to enhance bioavailability, fluorocarbons, and/or other solubilizing or dispersing agents known in the art. See, e.g.: Rabinowitz JD and Zaffaroni AC, US Patent 6,803,031, assigned to Alexza Molecular Delivery Corporation.
- Topical administration of the pharmaceutical compositions of this invention is especially useful when the desired treatment involves areas or organs readily accessible by topical application.
- the pharmaceutical composition should be formulated with a suitable ointment containing the active components suspended or dissolved in a carrier.
- Carriers for topical administration of the compounds of this invention include, but are not limited to, mineral oil, liquid petroleum, white petroleum, propylene glycol, polyoxyethylene polyoxypropylene compound, emulsifying wax, and water.
- the pharmaceutical composition can be formulated with a suitable lotion or cream containing the active compound suspended or dissolved in a carrier.
- Suitable carriers include, but are not limited to, mineral oil, sorbitan monostearate, polysorbate 60, cetyl esters wax, cetearyl alcohol, 2- octyldodecanol, benzyl alcohol, and water.
- the pharmaceutical compositions of this invention may also be topically applied to the lower intestinal tract by rectal suppository formulation or in a suitable enema formulation. Topically-transdermal patches and iontophoretic administration are also included in this invention.
- Application of the subject therapeutics may be local, so as to be administered at the site of interest.
- Various techniques can be used for providing the subject
- compositions at the site of interest such as injection, use of catheters, trocars, projectiles, pluronic gel, stents, sustained drug release polymers or other device which provides for internal access.
- the compounds of this invention may be incorporated into compositions for coating an implantable medical device, such as prostheses, artificial valves, vascular grafts, stents, or catheters.
- an implantable medical device such as prostheses, artificial valves, vascular grafts, stents, or catheters.
- Suitable coatings and the general preparation of coated implantable devices are known in the art and are exemplified in US Patents 6,099,562; 5,886,026; and 5,304,121.
- the coatings are typically biocompatible polymeric materials such as a hydrogel polymer,
- the invention provides a method of coating an implantable medical device comprising the step of contacting said device with the coating composition described above. It will be obvious to those skilled in the art that the coating of the device will occur prior to implantation into a mammal.
- the invention provides a method of impregnating an implantable drug release device comprising the step of contacting said drug release device with a compound or composition of this invention.
- Implantable drug release devices include, but are not limited to, biodegradable polymer capsules or bullets, non-degradable, diffusible polymer capsules and biodegradable polymer wafers.
- the invention provides an implantable medical device coated with a compound or a composition comprising a compound of this invention, such that said compound is therapeutically active.
- the invention provides an implantable drug release device impregnated with or containing a compound or a composition comprising a compound of this invention, such that said compound is released from said device and is therapeutically active.
- composition of this invention may be painted onto the organ, or a composition of this invention may be applied in any other convenient way.
- a composition of this invention further comprises a second therapeutic agent.
- the second therapeutic agent may be selected from any compound or therapeutic agent known to have or that demonstrates advantageous properties when administered with a compound having the same mechanism of action as idelalisib.
- Such agents include those indicated as being useful in combination with idelalisib, including but not limited to, those described in US 7,932,260; US 8,138,195; US 8,207,153; US 8,546,409; US 8,586,597; US RE44599; WO 2005/113554.
- the second therapeutic agent is an agent useful in the treatment of a disease or condition selected from rheumatoid arthritis, monoarticular arthritis, osteoarthritis, gouty arthritis, spondylitis; Behcet disease; sepsis, septic shock, endotoxic shock, gram negative sepsis, gram positive sepsis, and toxic shock syndrome; multiple organ injury syndrome secondary to septicemia, trauma, or hemorrhage; ophthalmic disorders, such as allergic conjunctivitis, vernal conjunctivitis, uveitis, and thyroid- associated opthalmopathy; eosinophilic granuloma; pulmonary or respiratory disorders, such as chronic bronchitis, allergic rhinitis, silicosis, pulmonary sarcoidosis, pleurisy, alveolitis, vasculitis, pneumonia, bronchiectasis, and pulmonary oxygen toxicity;
- a disease or condition selected from rheumatoi
- fibrosis such as cystic fibrosis
- keloid formation or scar tissue formation atherosclerosis
- autoimmune diseases such as systemic lupus erythematosus (SLE), autoimmune thyroiditis, multiple sclerosis, some forms of diabetes, and Reynaud's syndrome
- transplant rejection disorders such as GVHD and allograft rejection
- chronic glomerulonephritis inflammatory bowel diseases, such as chronic inflammatory bowel disease (CIBD), Crohn's disease, ulcerative colitis, and necrotizing enterocolitis
- inflammatory dermatoses such as contact dermatitis, atopic dermatitis, psoriasis, or urticaria
- fever and myalgias due to infection central or peripheral nervous system inflammatory disorders, such as meningitis, encephalitis, and brain or spinal cord injury due to minor trauma; Sjogren's syndrome; diseases involving leukocyte diapede
- cancers related to or derived from B lymphocytes or B lymphocyte progenitors including, without limitation, lymphomas, e.g., malignant neoplasms of lymphoid and reticuloendothelial tissues, such as Burkitt's lymphoma, Hodgkin's lymphoma, non-Hodgkin's lymphomas, lymphocytic lymphomas and the like; multiple myelomas; leukemias, such as lymphocytic leukemias, chronic myeloid (myelogenous) leukemias, and the like; and diseases characterized by histamine release, i.e., allergic disorders, including disorders such as chronic obstructive pulmonary disease (COPD), asthma, ARDS, emphysema, and related disorders.
- lymphomas e.g., malignant neoplasms of lymphoid and reticuloendothelial tissues, such as Burkitt's lymphoma,
- the second therapeutic agent is selected from rituximab, bendamustine, ofatumumab, fludarabine, everolimus, bortezomib, chlorambucil, lenalidomide, GS-9973, or combinations thereof.
- the invention provides separate dosage forms of a compound of this invention and one or more of any of the above-described second therapeutic agents, wherein the compound and second therapeutic agent are associated with one another.
- association with one another means that the separate dosage forms are packaged together or otherwise attached to one another such that it is readily apparent that the separate dosage forms are intended to be sold and administered together (within less than 24 hours of one another, consecutively or simultaneously).
- the compound of the present invention is present in an effective amount.
- effective amount refers to an amount which, when administered in a proper dosing regimen, is sufficient to treat the target disorder.
- an effective amount of a compound of this invention can range from about 100 mg to about 300 mg per day, from about 20 mg to about 600 mg per day, from about 10 mg to about 1500 mg per day, or from about 1 mg to about 3000 mg per day.
- the compound may be administered three times a day, twice a day, once a day, or once every other day, over the course of treatment.
- Effective doses will also vary, as recognized by those skilled in the art, depending on the diseases treated, the severity of the disease, the route of administration, the sex, age and general health condition of the subject, excipient usage, the possibility of co- usage with other therapeutic treatments such as use of other agents and the judgment of the treating physician. For example, guidance for selecting an effective dose can be determined by reference to the prescribing information for idelalisib.
- an effective amount of the second therapeutic agent is between about 20% and 100% of the dosage normally utilized in a monotherapy regime using just that agent.
- an effective amount is between about 70% and 100% of the normal monotherapeutic dose.
- the normal monotherapeutic dosages of these second therapeutic agents are well known in the art. See, e.g., Wells et al, eds., Pharmacotherapy Handbook, 2nd Edition,
- the invention provides a method of modulating the activity of PI3K (also known as phosphatidylinositol 3-kinase, PI 3-kinase, PI3K, PI(3)K, or PI-3K) in a cell, comprising contacting a cell with one or more compounds of Formula AB herein, or any sub-formula of AB (e.g., including any of the formulae herein), or a pharmaceutically acceptable salt thereof.
- the modulating is inhibiting.
- the inhibiting is selective for PI3K delta over PI3K alpha, PI3K beta, and PI3K gamma.
- the invention provides a method of treating a disease that is beneficially treated by idelalisib in a subject in need thereof, comprising the step of administering to the subject an effective amount of a compound or a composition of this invention.
- the subject is a patient in need of such treatment.
- diseases are well known in the art and are disclosed in, but not limited to the following patents and published applications: US 7,932,260; US 8,138,195; US 8,207,153; US 8,546,409; US 8,586,597; US RE44599; PCT WO 2005/113554.
- Such diseases include, but are not limited to, rheumatoid arthritis, monoarticular arthritis, osteoarthritis, gouty arthritis, spondylitis; Behcet disease; sepsis, septic shock, endotoxic shock, gram negative sepsis, gram positive sepsis, and toxic shock syndrome; multiple organ injury syndrome secondary to septicemia, trauma, or hemorrhage; ophthalmic disorders, such as allergic conjunctivitis, vernal conjunctivitis, uveitis, and thyroid- associated opthalmopathy; eosinophilic granuloma; pulmonary or respiratory disorders, such as asthma, chronic bronchitis, allergic rhinitis, ARDS, chronic pulmonary inflammatory disease (e.g., chronic obstructive pulmonary disease), silicosis, pulmonary sarcoidosis, pleurisy, alveolitis, vasculitis, emphysema, pneumonia, bronchiec
- transplant rejection disorders such as GVHD and allograft rejection; chronic
- inflammatory bowel diseases such as chronic inflammatory bowel disease (CIBD), Crohn's disease, ulcerative colitis, and necrotizing enterocolitis;
- inflammatory dermatoses such as contact dermatitis, atopic dermatitis, psoriasis, or urticaria; fever and myalgias due to infection; central or peripheral nervous system inflammatory disorders, such as meningitis, encephalitis, and brain or spinal cord injury due to minor trauma; Sjogren's syndrome; diseases involving leukocyte diapedesis;
- alcoholic hepatitis bacterial pneumonia; antigen-antibody complex mediated diseases; hypovolemic shock; Type I diabetes mellitus; acute and delayed hypersensitivity; disease states due to leukocyte dyscrasia and metastasis; thermal injury; granulocyte transfusion- associated syndromes; cytokine-induced toxicity; reperfusion injury such as vascular stroke (including global and focal ischemia), hemorrhagic shock, myocardial ischemia or infarction, organ transplantation, and cerebral vasospasm; ischemia resulting from thromboembolytic occlusion of a cerebral vessel, traumatic head injury,
- lymphomas e.g., malignant neoplasms of lymphoid and reticuloendothelial tissues, such as Burkitt's lymphoma, Hodgkin's lymphoma, non-Hodgkin's lymphomas, lymphocytic lymphomas and the like; multiple myelomas; leukemias, such as lymphocytic leukemias, chronic myeloid (myelogenous) leukemias, and the like; and diseases characterized by histamine release, i.e., allergic disorders and related disorders.
- lymphomas e.g., malignant neoplasms of lymphoid and reticuloendothelial tissues, such as Burkitt's lymphoma, Hodgkin's lymphoma, non-Hodgkin's lymphomas, lymphocytic lymphomas and the like
- leukemias such as lymphocytic leukemias, chronic myeloid (mye
- the method of this invention is used to treat a disease or condition selected from chronic lymphocytic leukemia (including those with a 17p deletion, relapsed, refractory, and previously untreated conditions), small lymphocytic lymphoma (including relapsed or refractory), non-Hodgkin's lymphoma (including relapsed or refractory indolent B-cell non-Hodgkin's lymphoma), Hodgkin lymphoma (including relapsed or refractory), diffuse large B-cell lymphoma, follicular lymphoma (including recurrent follicular lymphoma), lymphoplasmacytoid lymphoma, marginal zone lymphoma, and mantle cell lymphoma (including relapsed or refractory) in a subject in need thereof.
- chronic lymphocytic leukemia including those with a 17p deletion, relapsed, refractory, and previously untreated conditions
- Identifying a subject in need of such treatment can be in the judgment of a subject or a health care professional and can be subjective (e.g. opinion) or objective (e.g.
- any of the above methods of treatment comprises the further step of co-administering to the subject in need thereof one or more second therapeutic agents.
- the choice of second therapeutic agent may be made from any second therapeutic agent known to be useful for co -administration with idelalisib.
- the choice of second therapeutic agent is also dependent upon the particular disease or condition to be treated. Examples of second therapeutic agents that may be employed in the methods of this invention are those set forth above for use in combination
- compositions comprising a compound of this invention and a second therapeutic agent.
- the combination therapies of this invention include co-administering a compound of Formula AB or any sub-formula of AB (e.g., including any of the formulae herein) and a second therapeutic agent to a subject in need thereof for treatment of the following conditions (with the particular second therapeutic agent indicated in parentheses following the indication): relapsed or refractory chronic lymphocytic leukemia (rituximab, bendamustine, ofatumumab, fludarabine, everolimus, bortezomib, chlorambucil, lenalidomide, GS-9973, or combinations thereof), previously treated chronic lymphocytic leukemia (bendamustine, rituximab, ofatumumab, or combinations thereof), previously untreated chronic lymphocytic leukemia (bendamustine, rituximab, and combinations thereof), previously untreated B-cell chronic lymphocytic leukemia with 17p deletion (rituximab,
- GS-9973 has been reported in Burke, R. T. et al. Oncotarget 2014, 5(4), page 908-915, and is also known as 6-(lH-indazol-6-yl)-N-(4-morpholinophenyl)imidazo[l,2- a]pyrazin-8-amine.
- GS-9973 has the structure:
- co-administered means that the second therapeutic agent may be administered together with a compound of this invention as part of a single dosage form (such as a composition of this invention comprising a compound of the invention and an second therapeutic agent as described above) or as separate, multiple dosage forms.
- the additional agent may be administered prior to, consecutively with, or following the administration of a compound of this invention.
- both the compounds of this invention and the second therapeutic agent(s) are administered by conventional methods.
- composition of this invention comprising both a compound of the invention and a second therapeutic agent, to a subject does not preclude the separate administration of that same therapeutic agent, any other second therapeutic agent or any compound of this invention to said subject at another time during a course of treatment.
- the effective amount of the compound of this invention is less than its effective amount would be where the second therapeutic agent is not
- the effective amount of the second therapeutic agent is less than its effective amount would be where the compound of this invention is not administered. In this way, undesired side effects associated with high doses of either agent may be minimized.
- Other potential advantages including without limitation improved dosing regimens and/or reduced drug cost) will be apparent to those of skill in the art.
- the invention provides the use of a compound of Formula AB and/or any sub-formula of AB (e.g., including any of the formulae herein) alone or together with one or more of the above-described second therapeutic agents in the manufacture of a medicament, either as a single composition or as separate dosage forms, for treatment in a subject of a disease, disorder or symptom set forth above.
- a compound of Formula AB and/or any sub-formula of AB e.g., including any of the formulae herein for use in the treatment in a subject of a disease, disorder or symptom thereof delineated herein.
- Example 1 (S -2-(l -((9H-purin-6-yl-2,8-d2 amino propyn-5-fiuoro-3- phenylquinazolin-4(3H)-one (Compound 120).
- Step 2 6-Chloro-7H-purine-2,8-d? (22).
- the resulting solid was suspended in N,N-dimethylaniline (2.4 mL, 18.7 mmol), cooled to 0 °C and phosphoryl trichloride (6.5 mL, 71.5 mmol) was added.
- the mixture was heated at 110 °C for 1 hour to afford a clear, red-brown solution. After cooling, the mixture was concentrated under reduced pressure, and ice-water (30 mL) was added.
- the mixture was stirred for 30 minutes and extracted with MTBE (10 mL x 3).
- the aqueous layer was rendered basic (pH 9-10) with 2N NaOH at 0 °C, and further extracted with MTBE (10 mL x 3) and subsequently acidified to pH 6 with IN HCl.
- the resulting aqueous layer was concentrated under reduced pressure to a volume of 15 mL, cooled to 10 °C, and a solid that separated was filtered, and the filtrate was concentrated to dryness.
- the residual material was suspended in water (5 mL), stirred at room temperature for 30 minutes, cooled to 10 °C, filtered and dried under vacuum to afford 21a (260 mg, 25% yield) as an off-white solid.
- Step 3 S -2-(l-((9H-Purin-6-yl-2,8-d7 amino propyn-5-fiuoro-3- phenylquinazolin-4(3H)-one (120). To a solution of 22 (260 mg, 1.67 mmol) and 15a (WO2005113556) (550 mg, 1.84 mmol), in DMSO-de (10 mL) was added
- reactions were initiated by addition of pre-warmed NADPH solution.
- the final reaction volume was 0.5 mL and contained 200 pmol/mL CYP3A4 supersomesTM, 1.0 ⁇ test compound, and 2 mM NADPH in 0.1 M potassium phosphate buffer, pH 7.4, and 3 mM MgCb.
- the reaction mixtures were incubated at 37 °C, and 50 ⁇ , aliquots were removed at 0, 5, 10, 20, 30 and 45 minutes and added to 96-well plates which contained 50 ⁇ , of ice-cold ACN with internal standard to stop the reactions.
- the plates were stored at 4 °C for 20 minutes after which 100 of water was added to the wells of the plate before centrifugation to pellet precipitated proteins.
- Supernatants were transferred to another 96-well plate and analyzed for amounts of parent remaining by LC -MS/MS using an Applied Bio-systems API 4000 mass spectrometer.
- DMSO fetal sulfate
- acetonitrile ACN
- reaction mixtures were incubated at 37 °C, and 50 ⁇ , aliquots were removed at 0, 10, 20, 30, 45, 60, 90 and 120 minutes and added to 96-well plates which contained 100 ⁇ , of ice-cold ACN with internal standard to stop the reactions.
- the plates were stored at 4 °C for 20 minutes after which 50 ⁇ , of water/ACN (1 : 1), was added to the wells of the plate before
- Microsomal Assay Human liver microsomes (20 mg/mL) are obtained from Xenotech, LLC (Lenexa, KS). ⁇ -nicotinamide adenine dinucleotide phosphate, reduced form (NADPH), magnesium chloride (MgCb), and dimethyl sulfoxide (DMSO) are purchased from Sigma-Aldrich.
- 7.5 mM stock solutions of test compounds are prepared in DMSO.
- the 7.5 mM stock solutions are diluted to 12.5-50 ⁇ in acetonitrile (ACN).
- ACN acetonitrile
- the 20 mg/mL human liver microsomes are diluted to 0.625 mg/mL in 0.1 M potassium phosphate buffer, pH 7.4, containing 3 mM MgCl 2 .
- the diluted microsomes are added to wells of a 96-well deep-well polypropylene plate in triplicate.
- a 10 ⁇ , aliquot of the 12.5-50 ⁇ test compound is added to the microsomes and the mixture is pre-warmed for 10 minutes. Reactions are initiated by addition of pre-warmed NADPH solution.
- the final reaction volume is 0.5 mL and contains 0.5 mg/mL human liver microsomes, 0.25-1.0 ⁇ test compound, and 2 mM NADPH in 0.1 M potassium phosphate buffer, pH 7.4, and 3 mM MgCl 2 .
- the reaction mixtures are incubated at 37 °C, and 50 ⁇ , aliquots are removed at 0, 5, 10, 20, and 30 minutes and added to shallow- well 96-well plates which contain 50 ⁇ ⁇ of ice-cold ACN with internal standard to stop the reactions.
- the plates are stored at 4 °C for 20 minutes after which 100 ⁇ ⁇ of water is added to the wells of the plate before centrifugation to pellet precipitated proteins.
Abstract
This invention relates to novel 3-phenylquinazolin-4-one compounds, and pharmaceutically acceptable salts thereof. This invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions that are beneficially treated by administering an inhibitor of phosphoinositide 3-kinase (PI3K).
Description
DEUTERATED PHENYLQUINAZOLINONE AND PHENYLISOQUINOLINONE
COMPOUNDS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Serial No. 62/002,399 filed May 23, 2014 and U.S. Provisional Serial No. 62/066,236 filed October 20, 2014. This disclosure of the prior applications are considered part of (and are incorporated by reference in) the disclosure of this application.
BACKGROUND OF THE INVENTION
[0002] Many current medicines suffer from poor absorption, distribution, metabolism and/or excretion (ADME) properties that prevent their wider use or limit their use in certain indications. Poor ADME properties are also a major reason for the failure of drug candidates in clinical trials. While formulation technologies and prodrug strategies can be employed in some cases to improve certain ADME properties, these approaches often fail to address the underlying ADME problems that exist for many drugs and drug candidates. One such problem is rapid metabolism that causes a number of drugs, which otherwise would be highly effective in treating a disease, to be cleared too rapidly from the body. A possible solution to rapid drug clearance is frequent or high dosing to attain a sufficiently high plasma level of drug. This, however, introduces a number of potential treatment problems such as poor patient compliance with the dosing regimen, side effects that become more acute with higher doses, and increased cost of treatment. A rapidly metabolized drug may also expose patients to undesirable toxic or reactive metabolites.
[0003] Another ADME limitation that affects many medicines is the formation of toxic or biologically reactive metabolites. As a result, some patients receiving the drug may experience toxicities, or the safe dosing of such drugs may be limited such that patients receive a suboptimal amount of the active agent. In certain cases, modifying dosing intervals or formulation approaches can help to reduce clinical adverse effects, but often the formation of such undesirable metabolites is intrinsic to the metabolism of the compound.
[0004] In some select cases, a metabolic inhibitor will be co-administered with a drug that is cleared too rapidly. Such is the case with the protease inhibitor class of drugs that
are used to treat HIV infection. The FDA recommends that these drugs be co-dosed with ritonavir, an inhibitor of cytochrome P450 enzyme 3A4 (CYP3A4), the enzyme typically responsible for their metabolism (see Kempf, D.J. et al., Antimicrobial agents and chemotherapy, 1997, 41(3): 654-60). Ritonavir, however, causes adverse effects and adds to the pill burden for HIV patients who must already take a combination of different drugs. Similarly, the CYP2D6 inhibitor quinidine has been added to dextromethorphan for the purpose of reducing rapid CYP2D6 metabolism of dextromethorphan in a treatment of pseudobulbar affect. Quinidine, however, has unwanted side effects that greatly limit its use in potential combination therapy (see Wang, L et al, Clinical Pharmacology and Therapeutics, 1994, 56(6 Pt 1): 659-67; and FDA label for quinidine at www.accessdata.fda.gov).
[0005] In general, combining drugs with cytochrome P450 inhibitors is not a satisfactory strategy for decreasing drug clearance. The inhibition of a CYP enzyme's activity can affect the metabolism and clearance of other drugs metabolized by that same enzyme. CYP inhibition can cause other drugs to accumulate in the body to toxic levels.
[0006] A potentially attractive strategy for improving a drug's metabolic properties is deuterium modification. In this approach, one attempts to slow the CYP -mediated metabolism of a drug or to reduce the formation of undesirable metabolites by replacing one or more hydrogen atoms with deuterium atoms. Deuterium is a safe, stable, nonradioactive isotope of hydrogen. Compared to hydrogen, deuterium forms stronger bonds with carbon. In select cases, the increased bond strength imparted by deuterium can positively impact the AD ME properties of a drug, creating the potential for improved drug efficacy, safety, and/or tolerability. At the same time, because the size and shape of deuterium are essentially identical to those of hydrogen, replacement of hydrogen by deuterium would not be expected to affect the biochemical potency and selectivity of the drug as compared to the original chemical entity that contains only hydrogen.
[0007] Over the past 35 years, the effects of deuterium substitution on the rate of metabolism have been reported for a very small percentage of approved drugs (see, e.g., Blake, MI et al, J Pharm Sci, 1975, 64:367-91; Foster, AB, Adv Drug Res 1985, 14:1-40 ("Foster"); Kushner, DJ et al, Can J Physiol Pharmacol 1999, 79-88; Fisher, MB et al, Curr Opin Drug Discov Devel, 2006, 9: 101-09 ("Fisher")). The results have been
variable and unpredictable. For some compounds deuteration caused decreased metabolic clearance in vivo. For others, there was no change in metabolism. Still others demonstrated increased metabolic clearance. The variability in deuterium effects has also led experts to question or dismiss deuterium modification as a viable drug design strategy for inhibiting adverse metabolism (see Foster at p. 35 and Fisher at p. 101).
[0008] The effects of deuterium modification on a drug's metabolic properties are not predictable even when deuterium atoms are incorporated at known sites of metabolism. Only by actually preparing and testing a deuterated drug can one determine if and how the rate of metabolism will differ from that of its non-deuterated counterpart. See, for example, Fukuto et al. (J. Med. Chem. 1991, 34, 2871-76). Many drugs have multiple sites where metabolism is possible. The site(s) where deuterium substitution is required and the extent of deuteration necessary to see an effect on metabolism, if any, will be different for each drug.
SUMMARY OF THE INVENTION
[0009] This invention relates to novel 3-phenylquinazolin-4-one compounds, and pharmaceutically acceptable salts thereof. This invention also provides compositions comprising a compound of this invention and the use of such compositions in methods of treating diseases and conditions that are beneficially treated by administering an inhibitor of phosphoinositide 3-kinase, also referred to as phosphatidylinositol 3-kinase (PI3K), particularly the delta isoform.
[0010] Idelalisib, also known as (5)-2-(l-((9H-purin-6-yl)amino)propyl)-5-fluoro-3- phenylquinazolin-4(3H)-one, GS 1101 , GS- 1101 or C AL- 101, inhibits PI3K. PI3K delta signaling, in particular, plays a key role the activation, proliferation, survival and trafficking of B lymphocytes and is hyperactive in many B-cell malignancies. Idelalisib selectively inhibits PI3K activity over other kinases, and selectively inhibits PI3K delta over the alpha, beta, and gamma forms of PI3K.
[0011] Idelalisib is approved for treatment of chronic lymphocytic leukemia (CLL) and follicular lymphoma, and is currently in clinical studies to treat small lymphocytic lymphoma (SLL), non-Hodgkin's lymphoma, Hodgkin's lymphoma, diffuse large B-cell lymphoma, lymphoplasmacytoid lymphoma, marginal zone lymphoma, and mantle cell
lymphoma, either alone or in combination with one or more second therapeutic agents.
[0012] Despite the beneficial activities of idelalisib, there is a continuing need for new compounds to treat the aforementioned diseases and conditions.
DESCRIPTION OF DRAWINGS
[0013] FIGURE 1 shows Metabolic Stability of Compound 120 versus Idelalisib in Human CYP3A4 Supersomes™
[0014] FIGURE 2 shows Metabolic Stability of Compound 120 versus Idelalisib in Human Liver Cytosol
DETAILED DESCRIPTION OF THE INVENTION
Definitions
[0015] The term "treat" means decrease, suppress, attenuate, diminish, arrest, or stabilize the development or progression of a disease (e.g., a disease or disorder delineated herein), lessen the severity of the disease or improve the symptoms associated with the disease.
[0016] "Disease" means any condition or disorder that damages or interferes with the normal function of a cell, tissue, or organ.
[0017] "The term "alkyl" refers to a monovalent saturated hydrocarbon group. Ci-C 6 alkyl is an alkyl having from 1 to 6 carbon atoms. An alkyl may be linear or
branched. Examples of alkyl groups include methyl; ethyl; propyl, including n-propyl and isopropyl; butyl, including n-butyl, isobutyl, sec-butyl, and t-butyl; pentyl, including, for example, n-pentyl, isopentyl, and neopentyl; and hexyl, including, for example, n- hexyl and 2-methylpentyl.
[0018] Unless otherwise specified, "alkylene" by itself or as part of another substituent refers to a saturated straight-chain or branched divalent group having the stated number of carbon atoms and derived from the removal of two hydrogen atoms from the corresponding alkane. Examples of straight chained and branched alkylene groups include -CH2- (methylene), -CH2-CH2- (ethylene), -CH2-CH2-CH2-
(propylene), -C(CH3)2-, -CH2-CH(CH3)-, -CH2-CH2-CH2-CH2-, -CH2-CH2-CH2-CH2-
CH2- (pentylene), -CH2-CH(CH3)-CH2-, and -CH2-C(CH3)2-CH2-.
[0019] The term "alkenyl" refers to a monovalent unsaturated hydrocarbon group where
the unsaturation is represented by a double bond. C2-C6 alkenyl is an alkenyl having from 2 to 6 carbon atoms. An alkenyl may be linear or branched. Examples of alkenyl groups include CH2=CH-, CH2=C(CH3)-, CH2=CH-CH2-, CH3-CH=CH-CH2-, CH3- CH=C(CH3)- and CH3-CH=CH-CH(CH3)-CH2-. Where double bond stereoisomerism is possible, the stereochemistry of an alkenyl may be (E), (Z), or a mixture thereof.
[0020] The term "alkynyl" refers to a monovalent unsaturated hydrocarbon group where the unsaturation is represented by a triple bond. C2-C6 alkynyl is an alkenyl having from 2 to 6 carbon atoms. An alkynyl may be linear or branched. Examples of alkynyl groups include CH≡C-, -C≡C(CH3), CH3-C≡C-CH2-, CH3-C≡C-CH2-CH2 and CH3-C≡C- CH(CH3)-CH2-.
[0021] The term "cycloalkyl" refers to a monocyclic or bicyclic monovalent saturated or non-aromatic unsaturated hydrocarbon ring system. The term "C3-Cio cycloalkyl" refers to a cycloalkyl wherein the number of ring carbon atoms is from 3 to 10. Examples of C3-Cio cycloalkyl include C3-C6 cycloalkyl. Bicyclic ring systems include fused, bridged, and spirocyclic ring systems. More particular examples of cycloalkyl groups include, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, cis- and trans- decalinyl, norbornyl, and spiro[4.5]decanyl.
[0022] The term "carbocyclyl" refers to a monocyclic or bicyclic monovalent saturated or non-aromatic unsaturated hydrocarbon ring system. The term "C3-Cio carbocyclyl" refers to a carbocyclyl wherein the number of ring carbon atoms is from 3 to 10. Examples of C3-Cio carbocyclyl include C3-C6 carbocyclyl. Bicyclic ring systems include fused, bridged, and spirocyclic ring systems. More particular examples of carbocyclyl groups include, cyclopropyl, cyclobutyl, cyclopentyl, cyclopentenyl, cyclohexyl, cyclohexenyl, cycloheptyl, cis- and trans-decalinyl, norbornyl, norbornenyl, and spiro[4.5]decanyl.
[0023] The term "heterocycloalkyl" refers to a monocyclic or bicyclic monovalent saturated or non-aromatic unsaturated ring system wherein from 1 to 4 ring atoms are heteroatoms independently selected from the group consisting of O, N and S. The term "3 to 10-membered heterocycloalkyl" refers to a heterocycloalkyl wherein the number of ring atoms is from 3 to 10. Examples of 3 to 10-membered heterocycloalkyl include 3 to 6-membered heterocycloalkyl. Bicyclic ring systems include fused, bridged, and spirocyclic ring systems. More particular examples of heterocycloalkyl groups include
azepanyl, azetidinyl, aziridinyl, imidazolidinyl, morpholinyl, oxazolidinyl, oxazolidinyl, piperazinyl, piperidinyl, pyrazolidinyl, pyrrolidinyl, quinuclidinyl, and thiomorpholinyl.
[0024] In the above heterocycloalkyl substituents, the nitrogen, phosphorus, carbon or sulfur atoms can be optionally oxidized to various oxidation states. In a specific example, the group -S(O)0-2-, refers to -S-(sulfide), -S(0)-(sulfoxide), and -SO2- (sulfone) respectively. For convenience, nitrogens, particularly but not exclusively, are meant to include their corresponding N-oxide form, although not explicitly defined as such in a particular example. Thus, for a compound of the invention having, for example, a pyridyl ring; the corresponding pyridyl-N-oxide is meant to be included as another compound of the invention. In addition, annular nitrogen atoms can be optionally quatemized; and the ring substituent can be partially or fully saturated or aromatic.
[0025] "Aryl" by itself or as part of another substituent refers to a monovalent aromatic hydrocarbon group having the stated number of carbon atoms (i.e., C5-C14 means from 5 to 14 carbon atoms). Typical aryl groups include, but are not limited to, groups derived from aceanthrylene, acenaphthylene, acephenanthrylene, anthracene, azulene, benzene, chrysene, coronene, fluoranthene, fluorene, hexacene, hexaphene, hexylene, as-indacene, 5-indacene, indane, indene, naphthalene, octacene, octophene, octalene, ovalene, penta- 2,4-diene, pentacene, pentalene, pentaphene, perylene, phenalene, phenanthrene, picene, pleiadene, pyrene, pyranthrene, rubicene, triphenylene, trinaphthylene, and the like. In a specific embodiment, the aryl group is cyclopentadienyl, phenyl or naphthyl. In a more specific embodiment, the aryl group is phenyl or naphthyl.
[0026] "Arylalkyl" by itself or as part of another substituent refers to an acyclic alkyl group in which one of the hydrogen atoms bonded to a carbon atom, typically a terminal or sp3 carbon atom, is replaced with an aryl group. Typical arylalkyl groups include, but are not limited to, benzyl, 2-phenylethan-l-yl, 2-phenylethen-l-yl, naphthylmethyl, 2- naphthylethan-l-yl, 2-naphthylethen-l-yl, naphthobenzyl, 2-naphthophenylethan-l-yl and the like. In one embodiment, the alkyl moiety of the arylalkyl group is (Ci-Ce) and the aryl moiety is (C5-C14). In a more specific embodiment the alkyl group is (C1-C3) and the aryl moiety is (C5-C10), such as (C6-C10).
[0027] The term "heteroaryl" refers to a monovalent aromatic monocyclic ring system wherein at least one ring atoms is a heteroatom independently selected from the group
consisting of O, N and S. The term 5-membered heteroaryl refers to a heteroaryl wherein the number of ring atoms is 5. Examples of 5-membered heteroaryl groups include pyrrolyl, pyrazolyl, oxazolyl, isoxazolyl, thiazolyl, isothiazolyl, oxadiazolyl, thiadiazolyl, furazanyl, imidazolinyl, and triazolyl.
[0028] "Heteroarylalkyl" by itself or as part of another substituent refers to an acyclic alkyl group in which one of the hydrogen atoms bonded to a carbon atom, typically a terminal or sp3 carbon atom, is replaced with a heteroaryl group. In one embodiment, the alkyl moiety of the heteroarylalkyl is (Ci-Ce) alkyl and the heteroaryl moiety is a 5-14- membered heteroaryl. In a more specific embodiment, the alkyl moiety is (C1-C3) alkyl and the heteroaryl moiety is a 5-10 membered heteroaryl.
[0029] "Halogen" or "Halo" by themselves or as part of another substituent refers to fluorine, chlorine, bromine and iodine, or fluoro, chloro, bromo and iodo.
[0030] It will be recognized that some variation of natural isotopic abundance occurs in a synthesized compound depending upon the origin of chemical materials used in the synthesis. Thus, a preparation of idelalisib will inherently contain small amounts of deuterated isotopologues. The concentration of naturally abundant stable hydrogen and carbon isotopes, notwithstanding this variation, is small and immaterial as compared to the degree of stable isotopic substitution of compounds of this invention. See, for instance, Wada, E et al, Seikagaku, 1994, 66: 15; Gannes, LZ et al, Comp Biochem Physiol Mol Integr Physiol, 1998, 1 19:725.
[0031] In the compounds of this invention any atom not specifically designated as a particular isotope is meant to represent any stable isotope of that atom. Unless otherwise stated, when a position is designated specifically as "H" or "hydrogen", the position is understood to have hydrogen at its natural abundance isotopic composition. Also unless otherwise stated, when a position is designated specifically as "D" or "deuterium", the position is understood to have deuterium at an abundance that is at least 3340 times greater than the natural abundance of deuterium, which is 0.015% (i.e., at least 50.1% incorporation of deuterium).
[0032] The term "isotopic enrichment factor" as used herein means the ratio between the isotopic abundance and the natural abundance of a specified isotope.
[0033] In other embodiments, a compound of this invention has an isotopic enrichment factor for each designated deuterium atom of at least 3500 (52.5% deuterium
incorporation at each designated deuterium atom), at least 4000 (60% deuterium incorporation), at least 4500 (67.5% deuterium incorporation), at least 5000 (75% deuterium), at least 5500 (82.5% deuterium incorporation), at least 6000 (90% deuterium incorporation), at least 6333.3 (95% deuterium incorporation), at least 6466.7 (97% deuterium incorporation), at least 6600 (99% deuterium incorporation), or at least 6633.3 (99.5%) deuterium incorporation).
[0034] The term "isotopologue" refers to a species in which the chemical structure differs from a specific compound of this invention only in the isotopic composition thereof.
[0035] The term "compound," when referring to a compound of this invention, refers to a collection of molecules having an identical chemical structure, except that there may be isotopic variation among the constituent atoms of the molecules. Thus, it will be clear to those of skill in the art that a compound represented by a particular chemical structure containing indicated deuterium atoms, will also contain lesser amounts of isotopologues having hydrogen atoms at one or more of the designated deuterium positions in that structure. The relative amount of such isotopologues in a compound of this invention will depend upon a number of factors including the isotopic purity of deuterated reagents used to make the compound and the efficiency of incorporation of deuterium in the various synthesis steps used to prepare the compound. However, as set forth above the relative amount of such isotopologues in toto will be less than 49.9% of the compound. In other embodiments, the relative amount of such isotopologues in toto will be less than 47.5%, less than 40%, less than 32.5%, less than 25%, less than 17.5%, less than 10%, less than 5%, less than 3%, less than 1%, or less than 0.5% of the compound.
[0036] The invention also provides salts of the compounds of the invention.
[0037] A salt of a compound of this invention is formed between an acid and a basic group of the compound, such as an amino functional group, or a base and an acidic group of the compound, such as a carboxyl functional group. According to another
embodiment, the compound is a pharmaceutically acceptable acid addition salt.
[0038] The term "pharmaceutically acceptable," as used herein, refers to a component that is, within the scope of sound medical judgment, suitable for use in contact with the
tissues of humans and other mammals without undue toxicity, irritation, allergic response and the like, and are commensurate with a reasonable benefit/risk ratio. A
"pharmaceutically acceptable salt" means any non-toxic salt that, upon administration to a recipient, is capable of providing, either directly or indirectly, a compound of this invention. A "pharmaceutically acceptable counterion" is an ionic portion of a salt that is not toxic when released from the salt upon administration to a recipient.
[0039] Acids commonly employed to form pharmaceutically acceptable salts include inorganic acids such as hydrogen bisulfide, hydrochloric acid, hydrobromic acid, hydroiodic acid, sulfuric acid and phosphoric acid, as well as organic acids such as para- toluenesulfonic acid, salicylic acid, tartaric acid, bitartaric acid, ascorbic acid, maleic acid, besylic acid, fumaric acid, gluconic acid, glucuronic acid, formic acid, glutamic acid, methanesulfonic acid, ethanesulfonic acid, benzenesulfonic acid, lactic acid, oxalic acid, para-bromophenylsulfonic acid, carbonic acid, succinic acid, citric acid, benzoic acid and acetic acid, as well as related inorganic and organic acids. Such
pharmaceutically acceptable salts thus include sulfate, pyrosulfate, bisulfate, sulfite, bisulfite, phosphate, monohydrogenphosphate, dihydrogenphosphate, metaphosphate, pyrophosphate, chloride, bromide, iodide, acetate, propionate, decanoate, caprylate, acrylate, formate, isobutyrate, caprate, heptanoate, propiolate, oxalate, malonate, succinate, suberate, sebacate, fumarate, maleate, butyne-l,4-dioate, hexyne-l,6-dioate, benzoate, chlorobenzoate, methylbenzoate, dinitrobenzoate, hydroxybenzoate, methoxybenzoate, phthalate, terephthalate, sulfonate, xylene sulfonate, phenylacetate, phenylpropionate, phenylbutyrate, citrate, lactate, β-hydroxybutyrate, glycolate, maleate, tartrate, methanesulfonate, propanesulfonate, naphthalene- 1 -sulfonate, naphthalene-2- sulfonate, mandelate and other salts. In one embodiment, pharmaceutically acceptable acid addition salts include those formed with mineral acids such as hydrochloric acid and hydrobromic acid, and especially those formed with organic acids such as maleic acid.
[0040] The compounds of the present invention (e.g., compounds of Formula (I) and/or Formula (la)), may contain an asymmetric carbon atom, for example, as the result of deuterium substitution or otherwise. As such, compounds of this invention can exist as either individual enantiomers, or mixtures of the two enantiomers. Accordingly, a compound of the present invention may exist as either a racemic mixture or a scalemic
mixture, or as individual respective stereoisomers that are substantially free from another possible stereoisomer. The term "substantially free of other stereoisomers" as used herein means less than 25% of other stereoisomers, preferably less than 10% of other stereoisomers, more preferably less than 5% of other stereoisomers and most preferably less than 2% of other stereoisomers are present. Methods of obtaining or synthesizing an individual enantiomer for a given compound are known in the art and may be applied as practicable to final compounds or to starting material or intermediates.
[0041] Unless otherwise indicated, when a disclosed compound is named or depicted by a structure without specifying the stereochemistry and has one or more chiral centers, it is understood to represent all possible stereoisomers of the compound.
[0042] The term "stable compounds," as used herein, refers to compounds which possess stability sufficient to allow for their manufacture and which maintain the integrity of the compound for a sufficient period of time to be useful for the purposes detailed herein (e.g., formulation into therapeutic products, intermediates for use in production of therapeutic compounds, isolatable or storable intermediate compounds, treating a disease or condition responsive to therapeutic agents).
[0043] "D" and "d" both refer to deuterium. "Stereoisomer" refers to both enantiomers and diastereomers. "Tert" and "t-" each refer to tertiary. "US" refers to the United States of America.
[0044] "Substituted with deuterium" refers to the replacement of one or more hydrogen atoms with a corresponding number of deuterium atoms.
[0045] Throughout this specification, a variable may be referred to generally (e.g., "each R") or may be referred to specifically (e.g., R1, R2, R3, etc.). Unless otherwise indicated, when a variable is referred to generally, it is meant to include all specific embodiments of that particular variable.
Therapeutic Compounds
wherein:
X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
Z is N or CY4d;
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or deuterium;
R is methyl optionally substituted with 1 -3 deuterium or ethyl optionally
substituted with 1-5 deuterium; and
at least one of R, X, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 comprises a deuterium atom;
provided that if Z is CY4d, Y4d is hydrogen, X is chloro, and R is -CH3, then a) when at least one of Y2 and Y3 is deuterium, then at least one of Y1, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y* and Y7 is deuterium; and
b) when Y5a, Y5b, Y6a, Y6b and Y7 are each deuterium, then at least one of Y1, Y2, Y3, Y4a, Y4b, and Y4c is deuterium.
[0047] In some embodiments of Formula AB, X is CI, F, -CF3 or -CD3.
[0048] In some embodiments of Formula AB, R is -CH3, -CD3, -CH2CH3, -CD2CH3,
[0049] -CH2CD3, or -CD2CD3.
wherein:
X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or
deuterium;
R is methyl optionally substituted with 1 -3 deuterium or ethyl optionally
substituted with 1-5 deuterium; and
at least one of R, X, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 comprises a deuterium atom.
[0051] In some embodiments of Formula A, X is CI, F, -CF3 or -CD3.
[0052] In some embodiments of Formula A, R is -CH3, -CD3, -CH2CH3, -CD2CH3, -CH2CD3, or -CD2CD3.
[0053] In some embodiments, the compound of Formula (A) has the structure of Formula (A-I):
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or
deuterium;
R is selected from the group consisting of: -CH2CH3, -CH2CD3, -CD2CH3
and -CD2CD3; and
at least one of R, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 comprises a deuterium atom.
[0054] In some embodiments, the compound of Formula (A) has the structure of Formula (Α-Γ):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or
deuterium;
R is -CH3 or -CD3; and
at least one of R, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y* and Y7 comprises a deuterium atom.
[0055] In some embodiments, the compound of Formula (A) has the structure of Formula (A-II):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or
deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3, -CH2CD3,
-CD2CH3 and -CD2CD3; and
at least one of R, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 comprises a deuterium atom.
[0056] In some embodiments, the compound of Formula (A) has the structure of Formula
(A-III):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or
deuterium; and
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3, -CH2CD3,
-CD2CH3 and -CD2CD3.
[0057] In some embodiments, the compound of Formula (A) has the structure of Formula (A-IV):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or
deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3, -CH2CD3, -CD2CH3 and -CD2CD3; and
at least one of R, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y* and Y7 comprises a deuterium atom.
[0058] In some embodiments, the compound of Formula (AB) has the structure of Formula (B):
wherein:
X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or deuterium;
R is methyl optionally substituted with 1 -3 deuterium or ethyl optionally
substituted with 1-5 deuterium; and
at least one of R, X, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 comprises a deuterium atom;
provided that if Y4d is hydrogen, X is chloro, and R is -CH3, then
a) when at least one of Y2 and Y3 is deuterium, then at least one of Y1, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y* and Y7 is deuterium; and
b) when Y5a, Y5b, Y6a, Y6b and Y7 are each deuterium, then at least one of Y1, Y2, Y3, Y4a, Y4b, and Y4c is deuterium.
[0059] In some embodiments of Formula B, X is CI, F, -CF3 or -CD3.
[0060] In some embodiments of Formula B, R is -CH3, -CD3, -CH2CH3, -CD2CH3,
-CH2CD3, or -CD2CD3.
[0061] In some embodiments, the compound of Formula (B) has the structure of Formula (B-I):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or deuterium;
R is selected from the group consisting
of: -CH3, -CD3, -CH2CH3, -CH2CD3, -CD2CH3 and -CD2CD3; and at least one of R, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y* and Y7 comprises a deuterium atom.
[0062] In some embodiments, the compound of Formula (B) has the structure of Formula
(Β-Π):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2-CH3, -CH2-CD3,
-CD2-CH3 and -CD2-CD3; and
at least one of R, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y* and Y7 comprises a deuterium atom;
provided that if Y4d is hydrogen, and R is -CH3 then
a) when at least one of Y2 and Y3 is deuterium, then at least one of Y1, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y* and Y7 is deuterium; and
b) when Y5a, Y5b, Y6a, Y6b and Y7 are each deuterium, then at least one of Y1, Y2, Y3, Y4a, Y4b, and Y4c is deuterium.
[0063] In some embodiments, the compound of Formula (B) has the structure of Formula (B-III):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or deuterium; and
R is selected from the group consisting of: -CH3, -CD3, -CH2-CH3, -CH2-CD3, -CD2-CH3 and -CD2-CD3.
[0064] In some embodiments, the compound of Formula (B) has the structure of Formula (B-IV):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydi
deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2-CH3, -CH2-CD3, -CD2-CH3 and -CD2-CD3; and
at least one of R, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y* and Y7 comprises a deuterium atom.
[0065] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y1 is deuterium.
[0066] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y2 is deuterium.
[0067] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y3 is deuterium.
[0068] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y2 and Y3 are the same. For example, Y2 and Y3 can be deuterium. As another example, Y2 and Y3 can be hydrogen.
[0069] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y1, Y2 and Y3 are the same. For example, Y1, Y2 and Y3 can be deuterium. As another example, Y1, Y2 and Y3 can be hydrogen.
[0070] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y4a is deuterium.
[0071] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y4b is deuterium.
[0072] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y4c is deuterium.
[0073] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y4a, Y4b and Y4c are the same. For example Y4a, Y4b and Y4c can be deuterium. As another example Y4a, Y4b and Y4c can be hydrogen.
[0074] In some embodiments of Formula AB, B, B-I, B-II, B-III or B-IV, Y4a, Y4b, Y4c and Y4d are the same. For example Y4a, Y4b, Y4c and Y4d can be deuterium. As another example Y4a, Y4b, Y4c and Y4d can be hydrogen.
[0075] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y5a is deuterium.
[0076] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y5b is deuterium.
[0077] In some embodiments of Formula AB, A, A-I, A- Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y5a and Y5b are the same. For example, Y5a and Y5b can be deuterium. As another example, Y5a and Y5b can be hydrogen.
[0078] In some embodiments of Formula AB, A, A-I, A- Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y6a is deuterium.
[0079] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y6b is deuterium.
[0080] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y6a and Y6b are the same. For example, Y6a and Y6b can be deuterium. As another example, Y6a and Y6b can be hydrogen.
[0081] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y7 is deuterium.
[0082] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV,Y5a, Y5b, Y6a, Y6b and Y7 are the same. For example, Y5a, Y5b, Y6a, Y* and Y7 can be deuterium. As another example, Y5a, Y5b, Y6a, Y6b and Y7 can be hydrogen.
[0083] In some embodiments of Formula AB, A, A-I, Α-Γ, A-II, A-III, A-IV, B, B-I, fill, B-III or B-IV, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 are the same. For example, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 can be deuterium. As another example, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 can be hydrogen.
[0084] In some embodiments of Formula AB, B, B-I, B-II, B-III or B-IV, Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y* and Y7 are the same. For example, Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 can be deuterium. As another example, Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y* and Y7 can be hydrogen.
[0085] In some embodiments of Formula AB, A, A-I, A-II, A-III, A-IV, B, B-I, B-II, B- III or B-IV, R is selected from the group consisting of: -CH2CD3 and -CD2CD3. For example, R can be -CH2CD3. In some embodiments, R is -CD2CD3.
[0086] In one embodiment of a compound of Formula (A-I), Y , Y and Y are each hydrogen, Y5a, Y5b, Y6a, Y6b and Y7 are each deuterium, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 1 (below):
Table 1 : Exemplary Embodiments of Formula (A-I)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0087] In one embodiment of a compound of Formula (A-I), Y4a, Y4b and Y4c are each hydrogen, Y5a, Y5b, Y6a, Y6b and Y7 are each deuterium, R is -CH2CH3, and the compound is selected from any one of the compounds (Cmpd) set forth in Table la (below):
118 D D H
119 D H D
120 H D D
121 D D D
122 H D H
123 H H D
124 H H H
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0088] In one embodiment of a compound of Formula (B-II), Y4a, Y4b, Y4c and Y4d are each hydrogen, Y5a, Y5b, Y6a, Y6b and Y7 are each deuterium, and the compound is selected from any one of the compounds (Cmpd) set forth in Table lb (below):
Table lb: Exemplary Embodiments of Formula (B-II)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0089] In one embodiment of the compound of Formula (A-I), Y4a, Y4b and Y4c are each deuterium, Y5a, Y5b, Y6a, Y6b and Y7 are each hydrogen, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 2:
Table 2: Exemplary Embodiments of Formula (A-I)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0090] In one embodiment of the compound of Formula (A-I), Y4a, Y4b and Y4c are each deuterium, Y5a, Y5b, Y6a, Y6b and Y7 are each hydrogen, R is -CH2CH3, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 2a:
Table 2a: Exemplary Embodiments of Formula (A-I)
Compound Y1 Y2 Y3
217 D H H
218 D D H
219 D H D
220 H D D
221 D D D
222 H D H
223 H H D
224 H H H or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0091] In one embodiment of the compound of Formula (B-II), Y4a, Y4b, Y4c and Y4d are each deuterium, Y5a, Y5b, Y6a, Y6b and Y7 are each hydrogen, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 2b:
Table 2b: Exemplary Embodiments of Formula (B-II)
239 H H D -CDs
240 H H H -CDs
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0092] In one embodiment of the compound of Formula (A-I), Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b, and Y7 are each deuterium and the compound is selected from any one of the compounds (Cmpd) set forth in Table 3 (below):
Table 3: Exemplary Embodiments of Formula (A-I)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0093] In one embodiment of the compound of Formula (A-I), Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b, and Y7 are each deuterium, R is -CH2CH3, and the compound is selected from any one of the compounds (Cmpd) set forth in Table 3a (below):
Table 3a: Exemplary Embodiments of Formula (A-I)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0094] In one embodiment of the compound of Formula (B-II), Y4a, Y4b, Y4c, Y4d, Y5 Y5b, Y6a, Y6b, and Y7 are each deuterium, and the compound is selected from any one the compounds (Cmpd) set forth in Table 3b (below):
Table 3b: Exemplary Embodiments of Formula (B-II)
335 D H D -CDs
336 H D D -CDs
337 D D D -CDs
338 H D H -CDs
339 H H D -CDs
340 H H H -CDs
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0095] In some embodiments, the compound of Formula (A-I) has the structure of Formula (A-Ia):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -C bC ^-C bCDs, -CD2CH3, and - CD2CD3; and
at least one of R, Y1, Y2, and Y3 comprises a deuterium atom.
[0096] In some embodiments, the compound of Formula (Α-Γ) has the structure of Formula (A-Ia'):
Y3 (A-Ia')
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is -CH3 or -CD3; and
at least one of R, Y1, Y2, and Y3 comprises a deuterium atom.
[0097] In some embodiments, the compound of Formula (A-II) has the structure of Formula (A-IIa):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3,
-CD2CH3 and -CD2CD3; and
at least one of R, Y1, Y2 and Y3 comprises a deuterium atom.
[0098] In some embodiments, the compound of Formula (A-III) has the structure of Formula (A-IIIa):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium; and
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3, -CH2CD3, -CD2CH3 and -CD2CD3.
[0099] In some embodiments, the compound of Formula (A-IV) has the structure of
Formula (A-IVa):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3,
-CD2CH3 and -CD2CD3; and
least one of R, Y1, Y2 and Y3 comprises a deuterium atom.
[0100] In some embodiments, the compound of Formula (B-I) has the structure of Formula (B-Ia):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3,
-CH2CD3, -CD2CH3, and -CD2CD3; and
at least one of R, Y1, Y2, and Y3 comprises a deuterium atom.
[0101] In some embodiments, the compound of Formula (B-II) has the structure of Formula (B-IIa):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3,
-CD2CH3 and -CD2CD3; and
at least one of R, Y1, Y2 and Y3 comprises a deuterium atom;
provided that when R is -CH3, then Y1 is deuterium.
[0102] In some embodiments, the compound of Formula (B-III) has the structure of Formula (B-IIIa):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium; and
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3, -CH2CD3, -CD2CH3 and -CD2CD3.
[0103] In some embodiments, the compound of Formula (B-IV) has the structure of
Formula (B-IVa):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2CH3, -CH2CD3, -CD2CH3 and -CD2CD3; and
at least one of R, Y1, Y2 and Y3 comprises a deuterium atom.
[0104] In some embodiments of Formula A-Ia, A-Ia', A-IIa, A- Ilia, A-IVa, B-Ia, B-IIa, B-IIIa or B-IVa, Y1 is deuterium.
[0105] In some embodiments of Formula A-Ia, A-Ia', A-IIa, A-IIIa, A-IVa, B-Ia, B-IIa, B-IIIa or B-IVa, Y2 is deuterium.
[0106] In some embodiments of Formula A-Ia, A-Ia', A-IIa, A-IIIa, A-IVa, B-Ia, B-IIa, B-IIIa or B-IVa, Y3 is deuterium.
[0107] In some embodiments of Formula A-Ia, A-Ia' ,A-IIa, A-IIIa, A-IVa, B-Ia, B-IIa, B-IIIa or B-IVa, Y2 and Y3 are the same. For example, Y2 and Y3 can be deuterium.
[0108] In some embodiments of Formula A-Ia, A-Ia', A-IIa, A-IIIa, A-IVa, B-Ia, B-IIa, B-IIIa or B-IVa, Y1, Y2 and Y3 are the same. For example, Y1, Y2, and Y3 can be deuterium.
[0109] In some embodiments of Formula A-Ia, A-IIa, A-IIIa, A-IVa, B-Ia, B-IIa, B-IIIa or B-IVa, R is selected from the group consisting of: -CH2CD3 and -CD2CD3. For example, R can be -CH2CD3. In some embodiments, R is -CD2CD3.
[0110] In some embodiments of Formula A-Ia, A-IIa, A-IIIa, A-IVa, B-Ia, B-IIa, B-IIIa or B-IVa, R is selected from the group consisting of: -CH2CH3 and -CD2CD3. For example, R can be -CH2CH3.
[0111] In some embodiments of Formula A-Ia', A-IIa, A-IIIa, A-IVa, B-Ia, B-IIa, B-IIIa or B-IVa, R is selected from the group consisting of: -CH3 and -CD3. For example, R can be -CH3. In some embodiments, R is -CD3.
[0112] In one embodiment, the compound of Formula (A-Ia) is selected from any one of the compounds (Cmpd) set forth in Table 4 (below):
Table 4: Exemplary Embodiments of Formula (A-Ia)
409 D D H -CH2-CD3
410 H D H -CH2-CD3
411 H D H -CD2-CD3
412 H H D -CD2-CD3
413 H H H -CD2-CD3
414 H H H -CH2-CD3
415 D H D -CH2-CD3
416 H H D -CH2-CD3 or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0113] In one embodiment, the compound of Formula (A-Ia), wherein R = CH2CH3, is selected from any one of the compounds (Cmpd) set forth in Table 4a (below):
Table 4a: Exemplary Embodiments of Formula (A-Ia)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0114] In one embodiment, the compound of Formula (A-Ia'), wherein R = CH3, is selected from any one of the compounds (Cmpd) set forth in Table 4b (below):
Table 4b: Exemplary Embodiments of Formula (A-Ia)
426 D H D
427 H D D
428 D D D
429 H D H
430 H H D
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0115] In one embodiment, the compound of Formula (B-Ia) is selected from any the compounds (Cmpd) set forth in Table 4c (below):
Table 4c: Exemplary Embodiments of Formula (B-Ia)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0116] In one embodiment, the compound of Formula (A-IIa) is selected from any one of the compounds (Cmpd) set forth in Table 5a (below):
Table 5a: Exemplary Embodiments of Formula (Ila)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0117] In one embodiment, the compound of Formula (B-IIa) is selected from any the compounds (Cmpd) set forth in Table 5b (below):
Table 5b: Exemplary Embodiments of Formula (B-IIa)
521 D H D -CDs
522 H D D -CDs
523 D D D -CDs
524 H D H -CDs
525 H H D -CDs
526 H H H -CDs
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0118] In one embodiment, the compound of Formula (A-IIIa) is selected from any one of the compounds (Cmpd) set forth in Table 6a (below):
Table 6a: Exemplary Embodiments of Formula (A-IIIa)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0119] In one embodiment, the compound of Formula (B-IIIa) is selected from any one of the compounds (Cmpd) set forth in Table 6b (below):
Table 6b: Exemplary Embodiments of Formula (B-IIIa)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0120] In one embodiment, the compound of Formula (A-IVa) is selected from any one of the compounds (Cmpd) set forth in Table 7a (below):
Table 7a: Exemplary Embodiments of Formula (A-IVa)
708 D H H CH3
709 D D H CH3
710 D H D CH3
711 H D D CH3
712 D D D CH3
713 H D H CH3
714 H H D CH3
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0121] In one embodiment, the compound of Formula (B-IVa) is selected from any one of the compounds (Cmpd) set forth in Table 7b (below):
Table 7b: Exemplary Embodiments of Formula (B-IVa)
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
[0122] In another set of embodiments, any atom not designated as deuterium in any of the embodiments set forth above is present at its natural isotopic abundance.
[0123] The synthesis of compounds of the invention may be readily achieved by synthetic chemists of ordinary skill by reference to the Exemplary Synthesis and Examples disclosed herein. Relevant procedures analogous to those of use for the preparation of compounds of Formula AB, A, A-I, A-Ia, Α-Γ, A-Ia', A-II, A-IIa, A-III, A-IIIa, A-IV, A-IVa, B, B-I, B-Ia, B-II, B-IIa, B-III, B-IIIa, B-IV and B-IVa and intermediates thereof are disclosed, for instance in the following patents and patent applications: US 7,932,260; US 8,138,195; US 8,207,153; US 8,546,409; US 8,586,597; US 2009312319; US 2013/231356 US RE44599; and WO 2005/113554.
[0124] Such methods can be carried out utilizing corresponding deuterated and optionally, other isotope-containing reagents and/or intermediates to synthesize the compounds delineated herein, or invoking standard synthetic protocols known in the art for introducing isotopic atoms to a chemical structure.
Exemplary Synthesis
[0125] A convenient method for synthesizing compounds of Formula (A-I), (Α-Γ), (A- Ia) and/or (A-Ia') is depicted in Scheme 1, below.
[0126] Scheme 1 : General Synthesis of Compounds of Formula A-Ia and A-Ia'
10 11 (12) 13
14 15
Reagents and conditions: (a) (i) (COCl)2, DMF (cat); (ii) aniline, base such as NaHC03; (b) (i) SOCl2, DMF (cat); (ii) (12), base such as Et3N; (c) Zn, AcOH; (d) (i) TFA; (ii) K2C0 ; (e) (16), DIPEA
[0127] In a manner analogous to procedures described by Fowler (US 8207153), commercially available 2-fluoro-6-nitrobenzoic acid (10) is treated with an activating agent such as oxalyl chloride using a catalyst such as DMF, then allowed to react with aniline in the presence of a base such as NaHC03, to afford benzamide (11). Reaction of (11) at elevated temperature with thionyl chloride as solvent in the presence of a catalyst such as DMF, followed by treatment with an optionally deuterium-substituted Boc- protected 2-amino butyric acid (12), or Boc-protected 2-amino propanoic acid (12), and a base such as triethylamine, affords the bis-amide (13). It is expected that compound (13) can be readily prepared with %-atom d incorporation values of 95% or greater under these conditions. Treatment of an acetic acid solution containing compound (13) with powdered elemental zinc affords dihydroquinazolinone (14). Subsequent cleavage of the Boc-group with trifluoro acetic acid followed by treatment with a base such as potassium carbonate affords the free amine (15). Amine 15 is then allowed to react at elevated temperature in a solvent such as tert-butyl alcohol with an optionally deuterium- substituted 6-bromopurine (16) in the presence of a base such as di-isopropylethylamine to afford compounds of Formula A-Ia or A-Ia'. Using commercially available reagents and deuterated reagents that can be readily prepared by known methods, compounds of Formula A-Ia or A-Ia' can be prepared with greater than 90% or greater than 95% deuterium incorporation at each position designated as D.
[0128] An alternative method to prepare compound (14) used in the preparation of compounds of Formula A-Ia or A-Ia' is outlined in Scheme 2. 0129] Scheme 2: Alternative Synthesis of Compound (14)
11 17
Reagents and conditions: (a) Zn, AcOH; (b) DIEA, DMAP
[0130] An acetic acid solution containing compound (11) is treated with powdered elemental zinc to afford aniline (17). Subsequent coupling of (17) in a thermal reaction with an optionally deuterium-substituted Boc-protected 2-amino butyric acid activated ester (18), or an optionally deuterium-substituted Boc-protected 2-amino propanoic acid activated ester (18), affords compound (14).
[0131] Synthesis of compounds (12) used in the preparation of compounds of Formula A-Ia are outlined below in Scheme 3.
[0132] Scheme 3: Synthesis of 2-Aminobutyric Acid Compounds (12)
(12a) Y1 = H, R = CH2CH3
[0133] Boc-protected 2-aminobutyric acid analogs (12) are prepared from the corresponding amino acid (19) using methods known to one of ordinary skill in the art. Many of the corresponding amino acids (19) are commercially available (examples 19a, 19c, 19e and 19h) or may be prepared using known methods from commercially available optionally deuterium-substituted intermediates. 2-Aminobutyric acid analog
19b is prepared according to the procedure described by O'Reily, E. et. al. in Amino Acids (2010), 39(3), 849-858. Analogs 19d and 19g are prepared according to the procedure of Stirling, I. et. al. described in J. Chem. Soc. Perkin I: Org. and Bioorg. (1997)(5), 667-680. Compound 19f is prepared according to the procedure of Esaki, N. et. al. described in Anal. Biochem. (1982), 119(2) 281-5. Boc-protected 2- aminopropanoic acid analogs may be prepared in a similar manner. It is expected that compounds (12) can be readily prepared with %-atom d incorporation values of 95% or greater under these conditions.
[0134] Synthesis of compounds (16) used in the preparation of compounds of Formula AB and all sub-formula of AB are outlined below in Scheme 4.
[0135] Scheme 4: Preparation of Compounds (16)
16
20 21
Y2, Y3 = H or D
Reagents and conditions: (a) 10% Pd/C, D20, H2; (b) PBr3
[0136] Optionally deuterium-substituted 6-bromopurine analogs (16) used in the preparation of compounds of Formula AB and all sub-formula of AB are prepared from hypoxanthine (20) in high yield with %-atom d values of 95% using the hydrogen- deuterium exchange method of Sajiki, H. described in WO 2004/046066 to afford (21). Subsequent treatment of (21) with phosphorous tribromide at elevated temperature affords compound (16).
[0137] Compounds of Formula A, A-II, A-IIa, A-III, A- Ilia, A-IV and A- IVa can be prepared in a manner analogous to that shown in the above schemes using appropriately deuterated starting materials which are commercially available or prepared according to routine methods known to one of skill in the art.
[0138] Compounds of Formula B, B-I, B-Ia, B-II, B-IIa, B-III, B-IIIa, B-IV and B- IVa can be prepared using methods analogous to those disclosed in US 2009312319 and to that shown in the above schemes using appropriately deuterated starting materials which
are commercially available or prepared according to routine methods known to one of skill in the art.
[0139] The specific approaches and compounds shown above are not intended to be limiting. The chemical structures in the schemes herein depict variables that are hereby defined commensurately with chemical group definitions (moieties, atoms, etc.) of the corresponding position in the compound formulae herein, whether identified by the same variable name (i.e., R1, R2, R3, etc.) or not. The suitability of a chemical group in a compound structure for use in the synthesis of another compound is within
the knowledge of one of ordinary skill in the art.
[0140] Additional methods of synthesizing compounds of the invention and their synthetic precursors, including those within routes not explicitly shown in schemes herein, are within the means of chemists of ordinary skill in the art. Synthetic chemistry transformations and protecting group methodologies (protection and deprotection) useful in synthesizing the applicable compounds are known in the art and include, for example, those described in Larock R, Comprehensive Organic Transformations, VCH Publishers (1989); Greene, TW et al, Protective Groups in Organic Synthesis, 3rd Ed., John Wiley and Sons (1999); Fieser, L et al., Fieser and Fieser 's Reagents for Organic Synthesis, John Wiley and Sons (1994); and Paquette, L, ed., Encyclopedia of Reagents for Organic Synthesis, John Wiley and Sons (1995) and subsequent editions thereof.
[0141] Combinations of substituents and variables envisioned by this invention are only those that result in the formation of stable compounds.
Compositions
[0142] The invention also provides pharmaceutical compositions comprising an effective amount of a compound of Formula AB and/or any sub-formula of AB (e.g., including any of the formulae herein), or a pharmaceutically acceptable salt of said compound; and a pharmaceutically acceptable carrier. The carrier(s) are "acceptable" in the sense of being compatible with the other ingredients of the formulation and, in the case of a
pharmaceutically acceptable carrier, not deleterious to the recipient thereof in an amount used in the medicament.
[0143] Pharmaceutically acceptable carriers, adjuvants and vehicles that may be used in the pharmaceutical compositions of this invention include, but are not limited to, ion exchangers, alumina, aluminum stearate, lecithin, serum proteins, such as human serum albumin, buffer substances such as phosphates, glycine, sorbic acid, potassium sorbate, partial glyceride mixtures of saturated vegetable fatty acids, water, salts or electrolytes, such as protamine sulfate, disodium hydrogen phosphate, potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica, magnesium trisilicate, polyvinyl pyrrolidone, cellulose-based substances, polyethylene glycol, sodium carboxymethylcellulose, polyacrylates, waxes, polyethylene -polyoxypropylene-block polymers, polyethylene glycol and wool fat.
[0144] If required, the solubility and bioavailability of the compounds of the present invention in pharmaceutical compositions may be enhanced by methods well-known in the art. One method includes the use of lipid excipients in the formulation. See "Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs (Drugs and the Pharmaceutical Sciences)," David J. Hauss, ed. Informa Healthcare, 2007; and "Role of Lipid Excipients in Modifying Oral and Parenteral Drug Delivery: Basic Principles and Biological Examples," Kishor M. Wasan, ed. Wiley-Interscience, 2006.
[0145] Another known method of enhancing bioavailability is the use of an amorphous form of a compound of this invention optionally formulated with a poloxamer, such as LUTPvOL™ and PLURONIC™ (BASF Corporation), or block copolymers of ethylene oxide and propylene oxide. See United States patent 7,014,866; and United States patent publications 20060094744 and 20060079502.
[0146] The pharmaceutical compositions of the invention include those suitable for oral, rectal, nasal, topical (including buccal and sublingual), vaginal or parenteral (including subcutaneous, intramuscular, intravenous and intradermal) administration. In certain embodiments, the compound of the formulae herein is administered transdermally (e.g., using a transdermal patch or iontophoretic techniques). Other formulations may conveniently be presented in unit dosage form, e.g., tablets, sustained release capsules, and in liposomes, and may be prepared by any methods well known in the art of pharmacy. See, for example, Remington: The Science and Practice of Pharmacy, Lippincott Williams & Wilkins, Baltimore, MD (20th ed. 2000).
[0147] Such preparative methods include the step of bringing into association with the molecule to be administered ingredients such as the carrier that constitutes one or more accessory ingredients. In general, the compositions are prepared by uniformly and intimately bringing into association the active ingredients with liquid carriers, liposomes or finely divided solid carriers, or both, and then, if necessary, shaping the product.
[0148] In certain embodiments, the compound is administered orally. Compositions of the present invention suitable for oral administration may be presented as discrete units such as capsules, sachets, or tablets each containing a predetermined amount of the active ingredient; a powder or granules; a solution or a suspension in an aqueous liquid or a non-aqueous liquid; an oil -in- water liquid emulsion; a water-in-oil liquid emulsion; packed in liposomes; or as a bolus, etc. Soft gelatin capsules can be useful for containing such suspensions, which may beneficially increase the rate of compound absorption.
[0149] In the case of tablets for oral use, carriers that are commonly used include lactose and corn starch. Lubricating agents, such as magnesium stearate, are also typically added. For oral administration in a capsule form, useful diluents include lactose and dried cornstarch. When aqueous suspensions are administered orally, the active ingredient is combined with emulsifying and suspending agents. If desired, certain sweetening and/or flavoring and/or coloring agents may be added.
[0150] Compositions suitable for oral administration include lozenges comprising the ingredients in a flavored basis, usually sucrose and acacia or tragacanth; and pastilles comprising the active ingredient in an inert basis such as gelatin and glycerin, or sucrose and acacia.
[0151] Compositions suitable for parenteral administration include aqueous and nonaqueous sterile injection solutions which may contain anti -oxidants, buffers, bacteriostats and solutes which render the formulation isotonic with the blood of the intended recipient; and aqueous and non-aqueous sterile suspensions which may include suspending agents and thickening agents. The formulations may be presented in unit- dose or multi-dose containers, for example, sealed ampules and vials, and may be stored in a freeze dried (lyophilized) condition requiring only the addition of the sterile liquid carrier, for example water for injections, immediately prior to use. Extemporaneous
injection solutions and suspensions may be prepared from sterile powders, granules and tablets.
[0152] Such injection solutions may be in the form, for example, of a sterile injectable aqueous or oleaginous suspension. This suspension may be formulated according to techniques known in the art using suitable dispersing or wetting agents (such as, for example, Tween 80) and suspending agents. The sterile injectable preparation may also be a sterile injectable solution or suspension in a non-toxic parenterally-acceptable diluent or solvent, for example, as a solution in 1,3-butanediol. Among the acceptable vehicles and solvents that may be employed are mannitol, water, Ringer's solution and isotonic sodium chloride solution. In addition, sterile, fixed oils are conventionally employed as a solvent or suspending medium. For this purpose, any bland fixed oil may be employed including synthetic mono- or diglycerides. Fatty acids, such as oleic acid and its glyceride derivatives are useful in the preparation of injectables, as are natural pharmaceutically-acceptable oils, such as olive oil or castor oil, especially in their polyoxyethylated versions. These oil solutions or suspensions may also contain a long- chain alcohol diluent or dispersant.
[0153] The pharmaceutical compositions of this invention may be administered in the form of suppositories for rectal administration. These compositions can be prepared by mixing a compound of this invention with a suitable non-irritating excipient which is solid at room temperature but liquid at the rectal temperature and therefore will melt in the rectum to release the active components. Such materials include, but are not limited to, cocoa butter, beeswax and polyethylene glycols.
[0154] The pharmaceutical compositions of this invention may be administered by nasal aerosol or inhalation. Such compositions are prepared according to techniques well- known in the art of pharmaceutical formulation and may be prepared as solutions in saline, employing benzyl alcohol or other suitable preservatives, absorption promoters to enhance bioavailability, fluorocarbons, and/or other solubilizing or dispersing agents known in the art. See, e.g.: Rabinowitz JD and Zaffaroni AC, US Patent 6,803,031, assigned to Alexza Molecular Delivery Corporation.
[0155] Topical administration of the pharmaceutical compositions of this invention is especially useful when the desired treatment involves areas or organs readily accessible
by topical application. For topical application topically to the skin, the pharmaceutical composition should be formulated with a suitable ointment containing the active components suspended or dissolved in a carrier. Carriers for topical administration of the compounds of this invention include, but are not limited to, mineral oil, liquid petroleum, white petroleum, propylene glycol, polyoxyethylene polyoxypropylene compound, emulsifying wax, and water. Alternatively, the pharmaceutical composition can be formulated with a suitable lotion or cream containing the active compound suspended or dissolved in a carrier. Suitable carriers include, but are not limited to, mineral oil, sorbitan monostearate, polysorbate 60, cetyl esters wax, cetearyl alcohol, 2- octyldodecanol, benzyl alcohol, and water. The pharmaceutical compositions of this invention may also be topically applied to the lower intestinal tract by rectal suppository formulation or in a suitable enema formulation. Topically-transdermal patches and iontophoretic administration are also included in this invention.
[0156] Application of the subject therapeutics may be local, so as to be administered at the site of interest. Various techniques can be used for providing the subject
compositions at the site of interest, such as injection, use of catheters, trocars, projectiles, pluronic gel, stents, sustained drug release polymers or other device which provides for internal access.
[0157] Thus, according to yet another embodiment, the compounds of this invention may be incorporated into compositions for coating an implantable medical device, such as prostheses, artificial valves, vascular grafts, stents, or catheters. Suitable coatings and the general preparation of coated implantable devices are known in the art and are exemplified in US Patents 6,099,562; 5,886,026; and 5,304,121. The coatings are typically biocompatible polymeric materials such as a hydrogel polymer,
polymethyldisiloxane, polycaprolactone, polyethylene glycol, polylactic acid, ethylene vinyl acetate, and mixtures thereof. The coatings may optionally be further covered by a suitable topcoat of fluorosilicone, polysaccharides, polyethylene glycol, phospholipids or combinations thereof to impart controlled release characteristics in the composition. Coatings for invasive devices are to be included within the definition of pharmaceutically acceptable carrier, adjuvant or vehicle, as those terms are used herein.
[0158] According to another embodiment, the invention provides a method of coating an implantable medical device comprising the step of contacting said device with the coating composition described above. It will be obvious to those skilled in the art that the coating of the device will occur prior to implantation into a mammal.
[0159] According to another embodiment, the invention provides a method of impregnating an implantable drug release device comprising the step of contacting said drug release device with a compound or composition of this invention. Implantable drug release devices include, but are not limited to, biodegradable polymer capsules or bullets, non-degradable, diffusible polymer capsules and biodegradable polymer wafers.
[0160] According to another embodiment, the invention provides an implantable medical device coated with a compound or a composition comprising a compound of this invention, such that said compound is therapeutically active.
[0161] According to another embodiment, the invention provides an implantable drug release device impregnated with or containing a compound or a composition comprising a compound of this invention, such that said compound is released from said device and is therapeutically active.
[0162] Where an organ or tissue is accessible because of removal from the subject, such organ or tissue may be bathed in a medium containing a composition of this invention, a composition of this invention may be painted onto the organ, or a composition of this invention may be applied in any other convenient way.
[0163] In another embodiment, a composition of this invention further comprises a second therapeutic agent. The second therapeutic agent may be selected from any compound or therapeutic agent known to have or that demonstrates advantageous properties when administered with a compound having the same mechanism of action as idelalisib. Such agents include those indicated as being useful in combination with idelalisib, including but not limited to, those described in US 7,932,260; US 8,138,195; US 8,207,153; US 8,546,409; US 8,586,597; US RE44599; WO 2005/113554.
[0164] Preferably, the second therapeutic agent is an agent useful in the treatment of a disease or condition selected from rheumatoid arthritis, monoarticular arthritis, osteoarthritis, gouty arthritis, spondylitis; Behcet disease; sepsis, septic shock, endotoxic shock, gram negative sepsis, gram positive sepsis, and toxic shock syndrome; multiple
organ injury syndrome secondary to septicemia, trauma, or hemorrhage; ophthalmic disorders, such as allergic conjunctivitis, vernal conjunctivitis, uveitis, and thyroid- associated opthalmopathy; eosinophilic granuloma; pulmonary or respiratory disorders, such as chronic bronchitis, allergic rhinitis, silicosis, pulmonary sarcoidosis, pleurisy, alveolitis, vasculitis, pneumonia, bronchiectasis, and pulmonary oxygen toxicity;
reperfusion injury of the myocardium, brain, or extremities; fibrosis, such as cystic fibrosis; keloid formation or scar tissue formation; atherosclerosis; autoimmune diseases, such as systemic lupus erythematosus (SLE), autoimmune thyroiditis, multiple sclerosis, some forms of diabetes, and Reynaud's syndrome; transplant rejection disorders such as GVHD and allograft rejection; chronic glomerulonephritis; inflammatory bowel diseases, such as chronic inflammatory bowel disease (CIBD), Crohn's disease, ulcerative colitis, and necrotizing enterocolitis; inflammatory dermatoses, such as contact dermatitis, atopic dermatitis, psoriasis, or urticaria; fever and myalgias due to infection; central or peripheral nervous system inflammatory disorders, such as meningitis, encephalitis, and brain or spinal cord injury due to minor trauma; Sjogren's syndrome; diseases involving leukocyte diapedesis; alcoholic hepatitis; bacterial pneumonia; antigen-antibody complex mediated diseases; hypovolemic shock; Type I diabetes mellitus; acute and delayed hypersensitivity; disease states due to leukocyte dyscrasia and metastasis; thermal injury; granulocyte transfusion-associated syndromes; cytokine -induced toxicity; reperfusion injury such as vascular stroke (including global and focal ischemia), hemorrhagic shock, myocardial ischemia or infarction, organ transplantation, and cerebral vasospasm;
ischemia resulting from thromboembolytic occlusion of a cerebral vessel, traumatic head injury, edema, or brain tumor; cardiac ischemia; diseases of the bone such as
osteoporosis, Paget's disease, and related bone resorption disorders; cancers related to or derived from B lymphocytes or B lymphocyte progenitors including, without limitation, lymphomas, e.g., malignant neoplasms of lymphoid and reticuloendothelial tissues, such as Burkitt's lymphoma, Hodgkin's lymphoma, non-Hodgkin's lymphomas, lymphocytic lymphomas and the like; multiple myelomas; leukemias, such as lymphocytic leukemias, chronic myeloid (myelogenous) leukemias, and the like; and diseases characterized by histamine release, i.e., allergic disorders, including disorders such as chronic obstructive pulmonary disease (COPD), asthma, ARDS, emphysema, and related disorders.
[0165] In one embodiment, the second therapeutic agent is selected from rituximab, bendamustine, ofatumumab, fludarabine, everolimus, bortezomib, chlorambucil, lenalidomide, GS-9973, or combinations thereof.
[0166] In another embodiment, the invention provides separate dosage forms of a compound of this invention and one or more of any of the above-described second therapeutic agents, wherein the compound and second therapeutic agent are associated with one another. The term "associated with one another" as used herein means that the separate dosage forms are packaged together or otherwise attached to one another such that it is readily apparent that the separate dosage forms are intended to be sold and administered together (within less than 24 hours of one another, consecutively or simultaneously).
[0167] In the pharmaceutical compositions of the invention, the compound of the present invention is present in an effective amount. As used herein, the term "effective amount" refers to an amount which, when administered in a proper dosing regimen, is sufficient to treat the target disorder.
[0168] The interrelationship of dosages for animals and humans (based on milligrams per meter squared of body surface) is described in Freireich et al, Cancer Chemother. Rep, 1966, 50: 219. Body surface area may be approximately determined from height and weight of the subject. See, e.g., Scientific Tables, Geigy Pharmaceuticals, Ardsley, N.Y., 1970, 537.
In one embodiment, an effective amount of a compound of this invention can range from about 100 mg to about 300 mg per day, from about 20 mg to about 600 mg per day, from about 10 mg to about 1500 mg per day, or from about 1 mg to about 3000 mg per day. In certain aspects of this embodiment, the compound may be administered three times a day, twice a day, once a day, or once every other day, over the course of treatment.
[0169] Effective doses will also vary, as recognized by those skilled in the art, depending on the diseases treated, the severity of the disease, the route of administration, the sex, age and general health condition of the subject, excipient usage, the possibility of co- usage with other therapeutic treatments such as use of other agents and the judgment of the treating physician. For example, guidance for selecting an effective dose can be determined by reference to the prescribing information for idelalisib.
[0170] For pharmaceutical compositions that comprise a second therapeutic agent, an effective amount of the second therapeutic agent is between about 20% and 100% of the dosage normally utilized in a monotherapy regime using just that agent. Preferably, an effective amount is between about 70% and 100% of the normal monotherapeutic dose. The normal monotherapeutic dosages of these second therapeutic agents are well known in the art. See, e.g., Wells et al, eds., Pharmacotherapy Handbook, 2nd Edition,
Appleton and Lange, Stamford, Conn. (2000); PDR Pharmacopoeia, Tarascon Pocket Pharmacopoeia 2000, Deluxe Edition, Tarascon Publishing, Loma Linda, Calif. (2000), each of which references are incorporated herein by reference in their entirety.
[0171] It is expected that some of the second therapeutic agents referenced above will act synergistically with the compounds of this invention. When this occurs, it will allow the effective dosage of the second therapeutic agent and/or the compound of this invention to be reduced from that required in a monotherapy. This has the advantage of minimizing toxic side effects of either the second therapeutic agent of a compound of this invention, synergistic improvements in efficacy, improved ease of administration or use and/or reduced overall expense of compound preparation or formulation.
Methods of Treatment
[0172] In another embodiment, the invention provides a method of modulating the activity of PI3K (also known as phosphatidylinositol 3-kinase, PI 3-kinase, PI3K, PI(3)K, or PI-3K) in a cell, comprising contacting a cell with one or more compounds of Formula AB herein, or any sub-formula of AB (e.g., including any of the formulae herein), or a pharmaceutically acceptable salt thereof. In some embodiments, the modulating is inhibiting. In some embodiments, the inhibiting is selective for PI3K delta over PI3K alpha, PI3K beta, and PI3K gamma.
[0173] According to another embodiment, the invention provides a method of treating a disease that is beneficially treated by idelalisib in a subject in need thereof, comprising the step of administering to the subject an effective amount of a compound or a composition of this invention. In one embodiment the subject is a patient in need of such treatment. Such diseases are well known in the art and are disclosed in, but not limited to the following patents and published applications: US 7,932,260; US 8,138,195; US
8,207,153; US 8,546,409; US 8,586,597; US RE44599; PCT WO 2005/113554. Such diseases include, but are not limited to, rheumatoid arthritis, monoarticular arthritis, osteoarthritis, gouty arthritis, spondylitis; Behcet disease; sepsis, septic shock, endotoxic shock, gram negative sepsis, gram positive sepsis, and toxic shock syndrome; multiple organ injury syndrome secondary to septicemia, trauma, or hemorrhage; ophthalmic disorders, such as allergic conjunctivitis, vernal conjunctivitis, uveitis, and thyroid- associated opthalmopathy; eosinophilic granuloma; pulmonary or respiratory disorders, such as asthma, chronic bronchitis, allergic rhinitis, ARDS, chronic pulmonary inflammatory disease (e.g., chronic obstructive pulmonary disease), silicosis, pulmonary sarcoidosis, pleurisy, alveolitis, vasculitis, emphysema, pneumonia, bronchiectasis, and pulmonary oxygen toxicity; reperfusion injury of the myocardium, brain, or extremities; fibrosis, such as cystic fibrosis; keloid formation or scar tissue formation; atherosclerosis; autoimmune diseases, such as systemic lupus erythematosus (SLE), autoimmune thyroiditis, multiple sclerosis, some forms of diabetes, and Reynaud's syndrome;
transplant rejection disorders such as GVHD and allograft rejection; chronic
glomerulonephritis; inflammatory bowel diseases, such as chronic inflammatory bowel disease (CIBD), Crohn's disease, ulcerative colitis, and necrotizing enterocolitis;
inflammatory dermatoses, such as contact dermatitis, atopic dermatitis, psoriasis, or urticaria; fever and myalgias due to infection; central or peripheral nervous system inflammatory disorders, such as meningitis, encephalitis, and brain or spinal cord injury due to minor trauma; Sjogren's syndrome; diseases involving leukocyte diapedesis;
alcoholic hepatitis; bacterial pneumonia; antigen-antibody complex mediated diseases; hypovolemic shock; Type I diabetes mellitus; acute and delayed hypersensitivity; disease states due to leukocyte dyscrasia and metastasis; thermal injury; granulocyte transfusion- associated syndromes; cytokine-induced toxicity; reperfusion injury such as vascular stroke (including global and focal ischemia), hemorrhagic shock, myocardial ischemia or infarction, organ transplantation, and cerebral vasospasm; ischemia resulting from thromboembolytic occlusion of a cerebral vessel, traumatic head injury,
edema, or brain tumor; cardiac ischemia; diseases of the bone such as osteoporosis, Paget's disease, and related bone resorption disorders; cancers related to or derived from B lymphocytes or B lymphocyte progenitors including, without limitation, lymphomas,
e.g., malignant neoplasms of lymphoid and reticuloendothelial tissues, such as Burkitt's lymphoma, Hodgkin's lymphoma, non-Hodgkin's lymphomas, lymphocytic lymphomas and the like; multiple myelomas; leukemias, such as lymphocytic leukemias, chronic myeloid (myelogenous) leukemias, and the like; and diseases characterized by histamine release, i.e., allergic disorders and related disorders.
[0174] In one particular embodiment, the method of this invention is used to treat a disease or condition selected from chronic lymphocytic leukemia (including those with a 17p deletion, relapsed, refractory, and previously untreated conditions), small lymphocytic lymphoma (including relapsed or refractory), non-Hodgkin's lymphoma (including relapsed or refractory indolent B-cell non-Hodgkin's lymphoma), Hodgkin lymphoma (including relapsed or refractory), diffuse large B-cell lymphoma, follicular lymphoma (including recurrent follicular lymphoma), lymphoplasmacytoid lymphoma, marginal zone lymphoma, and mantle cell lymphoma (including relapsed or refractory) in a subject in need thereof.
[0175] Identifying a subject in need of such treatment can be in the judgment of a subject or a health care professional and can be subjective (e.g. opinion) or objective (e.g.
measurable by a test or diagnostic method).
[0176] In another embodiment, any of the above methods of treatment comprises the further step of co-administering to the subject in need thereof one or more second therapeutic agents. The choice of second therapeutic agent may be made from any second therapeutic agent known to be useful for co -administration with idelalisib. The choice of second therapeutic agent is also dependent upon the particular disease or condition to be treated. Examples of second therapeutic agents that may be employed in the methods of this invention are those set forth above for use in combination
compositions comprising a compound of this invention and a second therapeutic agent.
[0177] In particular, the combination therapies of this invention include co-administering a compound of Formula AB or any sub-formula of AB (e.g., including any of the formulae herein) and a second therapeutic agent to a subject in need thereof for treatment of the following conditions (with the particular second therapeutic agent indicated in parentheses following the indication): relapsed or refractory chronic lymphocytic leukemia (rituximab, bendamustine, ofatumumab, fludarabine, everolimus, bortezomib,
chlorambucil, lenalidomide, GS-9973, or combinations thereof), previously treated chronic lymphocytic leukemia (bendamustine, rituximab, ofatumumab, or combinations thereof), previously untreated chronic lymphocytic leukemia (bendamustine, rituximab, and combinations thereof), previously untreated B-cell chronic lymphocytic leukemia with 17p deletion (rituximab), relapsed or refractory mantle cell lymphoma (rituximab, bendamustine, ofatumumab, fludarabine, everolimus, bortezomib, chlorambucil, lenalidomide, GS-9973, and combinations thereof), relapsed or refractory indolent B-cell non-Hodgkin's lymphoma (rituximab, bendamustine, ofatumumab, fludarabine, everolimus, bortezomib, chlorambucil, lenalidomide, GS-9973, and combinations thereof), previously treated non-Hodgkin's lymphoma (bendamustine, rituximab, and combinations thereof), diffuse large B-cell lymphoma (GS-9973), small lymphocytic lymphoma (rituximab), and recurrent follicular lymphoma (rituximab, lenalidomide, and combinations thereof).
[0178] GS-9973 has been reported in Burke, R. T. et al. Oncotarget 2014, 5(4), page 908-915, and is also known as 6-(lH-indazol-6-yl)-N-(4-morpholinophenyl)imidazo[l,2- a]pyrazin-8-amine. GS-9973 has the structure:
[0179] The term "co-administered" as used herein means that the second therapeutic agent may be administered together with a compound of this invention as part of a single dosage form (such as a composition of this invention comprising a compound of the invention and an second therapeutic agent as described above) or as separate, multiple dosage forms. Alternatively, the additional agent may be administered prior to, consecutively with, or following the administration of a compound of this invention. In such combination therapy treatment, both the compounds of this invention and the second therapeutic agent(s) are administered by conventional methods. The administration of a composition of this invention, comprising both a compound of the invention and a second
therapeutic agent, to a subject does not preclude the separate administration of that same therapeutic agent, any other second therapeutic agent or any compound of this invention to said subject at another time during a course of treatment.
[0180] Effective amounts of these second therapeutic agents are well known to those skilled in the art and guidance for dosing may be found in patents and published patent applications referenced herein, as well as in Wells et al., eds., Pharmacotherapy
Handbook, 2nd Edition, Appleton and Lange, Stamford, Conn. (2000); PDR
Pharmacopoeia, Tarascon Pocket Pharmacopoeia 2000, Deluxe Edition, Tarascon Publishing, Loma Linda, Calif. (2000), and other medical texts. However, it is well within the skilled artisan's purview to determine the second therapeutic agent's optimal effective-amount range.
[0181] In one embodiment of the invention, where a second therapeutic agent is administered to a subject, the effective amount of the compound of this invention is less than its effective amount would be where the second therapeutic agent is not
administered. In another embodiment, the effective amount of the second therapeutic agent is less than its effective amount would be where the compound of this invention is not administered. In this way, undesired side effects associated with high doses of either agent may be minimized. Other potential advantages (including without limitation improved dosing regimens and/or reduced drug cost) will be apparent to those of skill in the art.
[0182] In yet another aspect, the invention provides the use of a compound of Formula AB and/or any sub-formula of AB (e.g., including any of the formulae herein) alone or together with one or more of the above-described second therapeutic agents in the manufacture of a medicament, either as a single composition or as separate dosage forms, for treatment in a subject of a disease, disorder or symptom set forth above. Another aspect of the invention is a compound of Formula AB and/or any sub-formula of AB (e.g., including any of the formulae herein) for use in the treatment in a subject of a disease, disorder or symptom thereof delineated herein.
Examples
[0183] Example 1. (S -2-(l -((9H-purin-6-yl-2,8-d2 amino propyn-5-fiuoro-3-
phenylquinazolin-4(3H)-one (Compound 120).
Scheme 5. Pre aration of (Compound 120)
Compound 120
Step 1. 3,9-Dihydro-6H-purin-6-one-2,8-d2 (21a). To a solution of 3,9-dihydro- 6H-purin-6-one (20) (8 g, 59 mmol ) in D20 (350 mL, 99 atom% D, Sigma-Aldrich) in a 2 L Parr bomb was added 10% Pd/C (800 mg). The mixture was stirred under hydrogen (15 psi) at room temperature for 20 minutes. Hydrogen was evacuated and replaced with nitrogen, and the resulting mixture was heated to 130 °C at 100 psi with stirring for 48 hours. The mixture was cooled to 80 °C and filtered through a pad of celite. The resulting solid was cooled to 10 °C, filtered and washed with D20 during isolation. The above procedure was repeated on the resulting solid (5 g) and was dried under vacuum to afford 21a (3.2 g, 24%) as a white solid.
Step 2. 6-Chloro-7H-purine-2,8-d? (22). A solution of 21a (900 mg, 6.5 mmol) in D20 (50 mL, 99 atom%> D, Sigma-Aldrich) at 70 °C was stirred for 30 minutes and concentrated to dryness. The resulting solid was suspended in N,N-dimethylaniline (2.4 mL, 18.7 mmol), cooled to 0 °C and phosphoryl trichloride (6.5 mL, 71.5 mmol) was added. The mixture was heated at 110 °C for 1 hour to afford a clear, red-brown solution. After cooling, the mixture was concentrated under reduced pressure, and ice-water (30 mL) was added. The mixture was stirred for 30 minutes and extracted with MTBE (10
mL x 3). The aqueous layer was rendered basic (pH 9-10) with 2N NaOH at 0 °C, and further extracted with MTBE (10 mL x 3) and subsequently acidified to pH 6 with IN HCl. The resulting aqueous layer was concentrated under reduced pressure to a volume of 15 mL, cooled to 10 °C, and a solid that separated was filtered, and the filtrate was concentrated to dryness. The residual material was suspended in water (5 mL), stirred at room temperature for 30 minutes, cooled to 10 °C, filtered and dried under vacuum to afford 21a (260 mg, 25% yield) as an off-white solid.
[0184] Step 3. (S -2-(l-((9H-Purin-6-yl-2,8-d7 amino propyn-5-fiuoro-3- phenylquinazolin-4(3H)-one (120). To a solution of 22 (260 mg, 1.67 mmol) and 15a (WO2005113556) (550 mg, 1.84 mmol), in DMSO-de (10 mL) was added
diisopropylethylamine (0.58 mL, 3.4 mmol). The mixture was stirred at 105 °C for 16 hours, cooled to room temperature, poured onto ice-water (100 mL) and stirred for 15 minutes. The resulting mixture was extracted with dichloromethane (100 mL x 3), and the combined organic layers were dried over sodium sulfate, filtered and concentrated. The residual material was purified using Analogix automated chromatography system eluting with 0-4% methanol in dichloromethane. Product fractions were pooled and evaporated to afford compound 120 (150 mg, 21% yield) as an off-white solid. The chiral purity was found to be 93.5%> ee. (Chiralcel OD-H 25cm x 4.6mm, 5 μιη, 85%> hexane + 15% EtOH, 0.75 mL/min, 210 nm, retention time = 13.056 min). 1H NMR (DMSO-de, 300 MHz) δ 12.73 (br s, 1H), 7.74-7.81 (m, 1H), 7.44-7.58 (m, 5H), 7.26-7.28 (m, 1H), 7.19-7.23 (m, 1H), 4.81-4.93 (br m, 1H), 1.92-2.0 (m, 1H), 1.81-1.89 (m, 2H), 0.75-0.79 (m, 3H); MS (ESI) 418.2 [(M + H)+].
[0185] Example 2. Evaluation of Metabolic Stability of Compound 120 in Human CYP3A4 Supersomes™
[0186] SUPERSOMES™ Assay. 7.5 mM stock solutions of Compound 120 and idelalisib were prepared in DMSO. The 7.5 mM stock solutions were diluted to 50 μΜ in acetonitrile (ACN). Human CYP3A4 supersomes™ (2000 pmol/mL, purchased from BD Biosciences™) were diluted to 250 pmol/mL in 0.1 M potassium phosphate buffer, pH 7.4, containing 3 mM MgCh. The diluted supersomes were added to wells of a 96-well polypropylene plate in triplicate. A 10 aliquot of the 50 μΜ test compound was
added to the supersomes and the mixture was pre-warmed for 7 minutes. Reactions were initiated by addition of pre-warmed NADPH solution. The final reaction volume was 0.5 mL and contained 200 pmol/mL CYP3A4 supersomes™, 1.0 μΜ test compound, and 2 mM NADPH in 0.1 M potassium phosphate buffer, pH 7.4, and 3 mM MgCb. The reaction mixtures were incubated at 37 °C, and 50 μΐ, aliquots were removed at 0, 5, 10, 20, 30 and 45 minutes and added to 96-well plates which contained 50 μΐ, of ice-cold ACN with internal standard to stop the reactions. The plates were stored at 4 °C for 20 minutes after which 100 of water was added to the wells of the plate before centrifugation to pellet precipitated proteins. Supernatants were transferred to another 96-well plate and analyzed for amounts of parent remaining by LC -MS/MS using an Applied Bio-systems API 4000 mass spectrometer.
[0187] Data analysis: The in vitro half-lives (ti/2 values) for test compounds were calculated from the slopes of the linear regression of LN(% parent remaining) vs incubation time relationship:
[0188] in vitro t ½ = 0.693/k, where k = -[slope of linear regression of % parent remaining (In) vs incubation time] .
[0189] The results of this experiment are shown in Table 7 and Figure 1. As shown in Table 7, the average half-life of idelalisib was calculated to be 65.9 minutes. In contrast, Compound 120 was more stable in the supersomes with a calculated average half-life of 91.6 minutes. This represents an average 39% increase in ti/2 for compound 120.
Table 7: Metabolic Stability of Compound 120 versus Idelalisib in Human CYP3A4 Supersomes™
*% Δ = [(deuterated species)-(nondeuterated species)](100)/(nondeuterated species)
[0190] Example 3. Evaluation of Metabolic Stability of Compound 120 in Human Liver Cytosol.
[0191] 7.5 mM stock solutions of Compound 120 and idelalisib were prepared in
DMSO. The 7.5 mM stock solutions were diluted to 50 μΜ in acetonitrile (ACN). 500 aliquots of human liver cytosol (10 mg/mL, obtained from Xenotech, LLC) were added to wells of a 96-well polypropylene plate in triplicate and pre -warmed for 7 minutes. Reactions were initiated by addition of 10 aliquot of the 50 μΜ test compound to the pre-warmed cytosol. The final reaction volume was 0.5 mL and contained 10 mg/mL cytosol and 1.0 μΜ test compound. The reaction mixtures were incubated at 37 °C, and 50 μΐ, aliquots were removed at 0, 10, 20, 30, 45, 60, 90 and 120 minutes and added to 96-well plates which contained 100 μΐ, of ice-cold ACN with internal standard to stop the reactions. The plates were stored at 4 °C for 20 minutes after which 50 μΐ, of water/ACN (1 : 1), was added to the wells of the plate before
centrifugation to pellet precipitated proteins. Supernatants were transferred to another 96-well plate and analyzed for amounts of parent remaining by LC -MS/MS using an Applied Bio-systems API 4000 mass spectrometer.
[0192] Data analysis: The % parent remaining was calculated based on area ratio at 0 and 120 minutes.
[0193] The results of this experiment are shown in Table 8 and Figure 2. As shown in Table 8, the average % parent remaining after 120 minutes for idelalisib was calculated to be 67%. The average % parent remaining after 120 minutes for Compound 120 was calculated to be 91%. This represents an average 36% increase in the amount of parent remaining for compound 120.
Table 8: Metabolic Stability of Compound 120 versus Idelalisib in Human Liver Cytosol
Compound Experiment 1 Experiment Experiment Ave ± SD
2 3 (% Δ)
Compound 120 91 82 99 91±5.4
(36%*)
*% Δ = [(deuterated species)-(nonc euterated species)](100)/(nondeuterated species)
Example 4. Evaluation of Metabolic Stability
[0194] Microsomal Assay: Human liver microsomes (20 mg/mL) are obtained from Xenotech, LLC (Lenexa, KS). β -nicotinamide adenine dinucleotide phosphate, reduced form (NADPH), magnesium chloride (MgCb), and dimethyl sulfoxide (DMSO) are purchased from Sigma-Aldrich.
[0195] Determination of Metabolic Stability: 7.5 mM stock solutions of test compounds are prepared in DMSO. The 7.5 mM stock solutions are diluted to 12.5-50 μΜ in acetonitrile (ACN). The 20 mg/mL human liver microsomes are diluted to 0.625 mg/mL in 0.1 M potassium phosphate buffer, pH 7.4, containing 3 mM MgCl2. The diluted microsomes are added to wells of a 96-well deep-well polypropylene plate in triplicate. A 10 μΐ, aliquot of the 12.5-50 μΜ test compound is added to the microsomes and the mixture is pre-warmed for 10 minutes. Reactions are initiated by addition of pre-warmed NADPH solution. The final reaction volume is 0.5 mL and contains 0.5 mg/mL human liver microsomes, 0.25-1.0 μΜ test compound, and 2 mM NADPH in 0.1 M potassium phosphate buffer, pH 7.4, and 3 mM MgCl2. The reaction mixtures are incubated at 37 °C, and 50 μΐ, aliquots are removed at 0, 5, 10, 20, and 30 minutes and added to shallow- well 96-well plates which contain 50 μΐ^ of ice-cold ACN with internal standard to stop the reactions. The plates are stored at 4 °C for 20 minutes after which 100 μΐ^ of water is added to the wells of the plate before centrifugation to pellet precipitated proteins. Supernatants are transferred to another 96-well plate and analyzed for amounts of parent remaining by LC-MS/MS using an Applied Bio-systems API 4000 mass spectrometer. The same procedure is followed for the non-deuterated counterpart of the compound of Formula AB and/or any sub-formula of AB (e.g., including any of the formulae herein) and the positive control, 7-ethoxycoumarin (1 μΜ). Testing is done in
triplicate.
[0196] Data analysis: The in vitro ti/2s for test compounds are calculated from the slopes of the linear regression of % parent remaining (In) vs incubation time relationship,
in vitro t ½ = 0.693/k
k = -[slope of linear regression of % parent remaining (In) vs incubation time]
[0197] Data analysis is performed using Microsoft Excel Software.
[0198] Without further description, it is believed that one of ordinary skill in the art can, using the preceding description and the illustrative examples, make and utilize the compounds of the present invention and practice the claimed methods. It should be understood that the foregoing discussion and examples merely present a detailed description of certain preferred embodiments. It will be apparent to those of ordinary skill in the art that various modifications and equivalents can be made without departing from the spirit and scope of the invention.
Claims
A compound, or p thereof, of Formula (AB)
wherein:
X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
Z is N or CY4d;
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or deuterium;
R is methyl optionally substituted with 1-3 deuterium or ethyl optionally
substituted with 1-5 deuterium; and
at least one of R, X, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 comprises a deuterium atom;
provided that if Z is CY4d, Y4d is hydrogen, X is chloro, and R is -CH3, then a. when at least one of Y2 and Y3 is deuterium, then at least one of Y1, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 is deuterium; and
b. when Y5a, Y5b, Y6a, Y6b and Y7 are each deuterium, then at least one of Y1, Y2, Y3, Y4a, Y4b, and Y4c is deuterium.
2. The compound of claim 1 , or pharmaceutically acceptable salt thereof, wherein the compound is a compound of Formula (A):
wherein:
X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or
deuterium;
R is methyl optionally substituted with 1 -3 deuterium or ethyl optionally
substituted with 1-5 deuterium; and
at least one of R, X, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 comprises a deuterium atom.
3. The compound of claim 2, or pharmaceutically acceptable salt thereof, wherein the compound is a compound of Formula, of Formula (A-Ia):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -CH2-CH3 -CH2-CD3, -CD2-CH3, and -CD2-CD3;
at least one of R, Y1, Y2, and Y3 comprises a deuterium atom.
4. The compound of claim 1 , or pharmaceutically acceptable salt thereof, wherein the compound is a compound of Formula (B):
wherein:
X is selected from halogen and methyl optionally substituted with 1-3 deuterium or optionally substituted with 1-3 fluorine;
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
Y4a, Y4b, Y4c, Y4d, Y5a, Y5b, Y6a, Y6b and Y7 are each independently hydrogen or deuterium;
R is methyl optionally substituted with 1-3 deuterium or ethyl optionally
substituted with 1-5 deuterium; and
at least one of R, X, Y1, Y2, Y3, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 comprises a deuterium atom;
provided that if Y4d is hydrogen, X is chloro, and R is -CH3, then
a. when at least one of Y2 and Y3 is deuterium, then at least one of Y1, Y4a, Y4b, Y4c, Y5a, Y5b, Y6a, Y6b and Y7 is deuterium; and
b. when Y5a, Y5b, Y6a, Y6b and Y7 are each deuterium, then at least one of Y1, Y2, Y3, Y4a, Y4b, and Y4c is deuterium.
5. The compound of claim 4, or pharmaceutically acceptable salt thereof, wherein the compound is a compound of Formula (B-IIa):
wherein:
Y1, Y2 and Y3 are each independently hydrogen or deuterium;
R is selected from the group consisting of: -CH3, -CD3, -CH2CFi3, -CH2CD3,
-CD2CH3 and -CD2CD3; and
at least one of R, Y1, Y2 and Y3 comprises a deuterium atom;
provided that when R is -CH3, then Y1 is deuterium.
6. The compound of any one of claims 1-5, wherein Y1 is deuterium.
7. The compound of any one of claims 1-5, wherein Y2 is deuterium.
8. The compound of any one of claims 1-5, wherein Y3 is deuterium.
9. The compound of any one of claims 1-5, wherein Y2 and Y3 are the same.
10. The compound of claim 9, wherein Y2 and Y3 are deuterium.
11. The compound of any one of claims 1-5, wherein Y1, Y2 and Y3 are the same.
12. The compound of claim 11, wherein Y1, Y2, and Y3 are deuterium.
13. The compound of any one of claims 1-5, wherein R is -CH2-CH3 or -CD2-CD3.
14. The compound of claim 13, wherein R is -CH2-CH3.
15. The compound of claim 13, wherein R is -CD2-CD3.
16. The compound of any one of claims 1, 2, 4 or 5, wherein R is -CH3 or -CD3.
17. The compound of claim 16, wherein R is -CH3.
18. The compound of claim 16, wherein R is -CD3.
19. The compound of any of claims 1-18, wherein any atom not designated as
deuterium is present at its natural isotopic abundance.
The compound of claim 3, wherein the compound is selected from any one of the compounds in the table below:
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
The compound of claim 3, wherein R is -CH2CH3 and the compound is selected from any one of the compounds in the table below:
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
The compound of claim 5, wherein the compound is selected from any one of the compounds in the table below:
526 H H H -CDs
or a pharmaceutically acceptable salt thereof, wherein any atom not designated as deuterium is present at its natural isotopic abundance.
23. A pharmaceutical composition comprising a compound of claim 1 or a
pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier.
24. A method of modulating the activity of PI3K in a cell, the method comprising contacting a cell with a pharmaceutical composition of claim 23.
25. The method of claim 24, wherein the modulating is inhibiting.
26. The method of claim 25, wherein the inhibiting selectively inhibits PI3K delta over PI3K alpha, PI3K beta, and PI3K gamma.
27. A method of treating a disease or condition selected from the group consisting of: chronic lymphocytic leukemia, small lymphocytic lymphoma, non-Hodgkin's lymphoma, Hodgkin's lymphoma, diffuse large B-cell lymphoma, follicular lymphoma, lymphoplasmacytoid lymphoma, marginal zone lymphoma, and mantle cell lymphoma, comprising the step of administering to the subject in need thereof a composition of claim 23.
28. The method of claim 27, wherein the composition of claim 23 is co-administered with a second therapeutic agent.
29. The method of claim 28, wherein the second therapeutic agent is selected from the group consisting of: rituximab, bendamustine, ofatumumab, fludarabine, everolimus, bortezomib, chlorambucil, lenalidomide, GS-9973, and combinations thereof.
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US201462002399P | 2014-05-23 | 2014-05-23 | |
| US62/002,399 | 2014-05-23 | ||
| US201462066236P | 2014-10-20 | 2014-10-20 | |
| US62/066,236 | 2014-10-20 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2015179772A1 true WO2015179772A1 (en) | 2015-11-26 |
Family
ID=53284628
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2015/032194 WO2015179772A1 (en) | 2014-05-23 | 2015-05-22 | Deuterated phenylquinazolinone and phenylisoquinolinone compounds |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2015179772A1 (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9522146B2 (en) | 2009-07-15 | 2016-12-20 | Intellikine Llc | Substituted Isoquinolin-1(2H)-one compounds, compositions, and methods thereof |
| US9527847B2 (en) | 2012-06-25 | 2016-12-27 | Infinity Pharmaceuticals, Inc. | Treatment of lupus, fibrotic conditions, and inflammatory myopathies and other disorders using PI3 kinase inhibitors |
| WO2017087207A1 (en) * | 2015-11-16 | 2017-05-26 | NeuForm Pharmaceuticals, Inc. | Deuterated compounds for treating hematologic malignant, inflammatory and autoimmune diseases |
| EP3101020A4 (en) * | 2014-01-30 | 2017-06-14 | Suzhou Zelgen Biopharmaceutical Co., Ltd. | Deuterated quinazolinone compound and pharmaceutical composition comprising same |
| US9822131B2 (en) | 2008-01-04 | 2017-11-21 | Intellikine Llc | Certain chemical entities, compositions and methods |
| USRE46621E1 (en) | 2011-01-10 | 2017-12-05 | Infinity Pharmaceuticals, Inc. | Processes for preparing isoquinolinones and solid forms of isoquinolinones |
| CN108137592A (en) * | 2015-11-03 | 2018-06-08 | 纽弗姆制药有限公司 | For treat the deuterated compound of leukemia with and combinations thereof and method |
| WO2021046315A3 (en) * | 2019-09-05 | 2021-04-22 | Wisconsin Alumni Research Foundation | Inhibitors of encephalitic alphaviruses |
| US11110096B2 (en) | 2014-04-16 | 2021-09-07 | Infinity Pharmaceuticals, Inc. | Combination therapies |
| US11147818B2 (en) | 2016-06-24 | 2021-10-19 | Infinity Pharmaceuticals, Inc. | Combination therapies |
Citations (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5304121A (en) | 1990-12-28 | 1994-04-19 | Boston Scientific Corporation | Drug delivery system making use of a hydrogel polymer coating |
| US5886026A (en) | 1993-07-19 | 1999-03-23 | Angiotech Pharmaceuticals Inc. | Anti-angiogenic compositions and methods of use |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| WO2004046066A1 (en) | 2002-11-15 | 2004-06-03 | Wako Pure Chemical Industries, Ltd. | Method for deuteration or tritiation of heterocyclic ring |
| US6803031B2 (en) | 2001-05-24 | 2004-10-12 | Alexza Molecular Delivery Corporation | Delivery of erectile dysfunction drugs through an inhalation route |
| WO2005113554A2 (en) | 2004-05-13 | 2005-12-01 | Icos Corporation | Method of preparing 3-phenyl-2-[9h-purin-6-ylamino)-methyl]-3h-quinazolin-4-one and substituted and related compounds |
| US7014866B2 (en) | 2001-05-03 | 2006-03-21 | Hoffmann-La Roche Inc. | High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same |
| US20060079502A1 (en) | 1999-11-02 | 2006-04-13 | Steffen Lang | Pharmaceutical compositions |
| US20060094744A1 (en) | 2004-09-29 | 2006-05-04 | Maryanoff Cynthia A | Pharmaceutical dosage forms of stable amorphous rapamycin like compounds |
| US20090312319A1 (en) | 2008-01-04 | 2009-12-17 | Intellikine | Certain chemical entities, compositions and methods |
| US8138195B2 (en) | 2000-04-25 | 2012-03-20 | Icos Corporation | Inhibitors of human phosphatidylinositol 3-kinase delta |
| US8546409B2 (en) | 2009-04-20 | 2013-10-01 | Gilead Calistoga Llc | Methods of treatment for solid tumors |
-
2015
- 2015-05-22 WO PCT/US2015/032194 patent/WO2015179772A1/en active Application Filing
Patent Citations (19)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5304121A (en) | 1990-12-28 | 1994-04-19 | Boston Scientific Corporation | Drug delivery system making use of a hydrogel polymer coating |
| US5886026A (en) | 1993-07-19 | 1999-03-23 | Angiotech Pharmaceuticals Inc. | Anti-angiogenic compositions and methods of use |
| US6099562A (en) | 1996-06-13 | 2000-08-08 | Schneider (Usa) Inc. | Drug coating with topcoat |
| US20060079502A1 (en) | 1999-11-02 | 2006-04-13 | Steffen Lang | Pharmaceutical compositions |
| US8138195B2 (en) | 2000-04-25 | 2012-03-20 | Icos Corporation | Inhibitors of human phosphatidylinositol 3-kinase delta |
| US7014866B2 (en) | 2001-05-03 | 2006-03-21 | Hoffmann-La Roche Inc. | High dose solid unit oral pharmaceutical dosage form of amorphous nelfinavir mesylate and process for making same |
| US6803031B2 (en) | 2001-05-24 | 2004-10-12 | Alexza Molecular Delivery Corporation | Delivery of erectile dysfunction drugs through an inhalation route |
| WO2004046066A1 (en) | 2002-11-15 | 2004-06-03 | Wako Pure Chemical Industries, Ltd. | Method for deuteration or tritiation of heterocyclic ring |
| WO2005113556A1 (en) | 2004-05-13 | 2005-12-01 | Icos Corporation | Quinazolinones as inhibitors of human phosphatidylinositol 3-kinase delta |
| WO2005113554A2 (en) | 2004-05-13 | 2005-12-01 | Icos Corporation | Method of preparing 3-phenyl-2-[9h-purin-6-ylamino)-methyl]-3h-quinazolin-4-one and substituted and related compounds |
| US7932260B2 (en) | 2004-05-13 | 2011-04-26 | Icos Corporation | Quinazolinones as inhibitors of human phosphatidylinositol 3-kinase delta |
| US8207153B2 (en) | 2004-05-13 | 2012-06-26 | Icos Corporation | Quinazolinones as inhibitors of human phosphatidylinositol 3-kinase delta |
| US20130231356A1 (en) | 2004-05-13 | 2013-09-05 | Icos Corporation | Quinazolinones as inhibitors of human phosphatidylinositol 3-kinase delta |
| USRE44599E1 (en) | 2004-05-13 | 2013-11-12 | Icos Corporation | Quinazolinones as inhibitors of human phosphatidylinositol 3-kinase delta |
| US8586597B2 (en) | 2004-05-13 | 2013-11-19 | Icos Corporation | 6-fluoro-3-phenyl-2-[1-(9H-purin-6-ylamino)ethyl]-3H-quinazolin-4-one as an inhibitor of human phosphatidylinositol 3-kinase delta |
| US20060094744A1 (en) | 2004-09-29 | 2006-05-04 | Maryanoff Cynthia A | Pharmaceutical dosage forms of stable amorphous rapamycin like compounds |
| US20090312319A1 (en) | 2008-01-04 | 2009-12-17 | Intellikine | Certain chemical entities, compositions and methods |
| US8546409B2 (en) | 2009-04-20 | 2013-10-01 | Gilead Calistoga Llc | Methods of treatment for solid tumors |
| WO2011008302A1 (en) * | 2009-07-15 | 2011-01-20 | Intellikine, Inc. | Certain chemical entities, compositions and methods |
Non-Patent Citations (26)
| Title |
|---|
| "Encyclopedia ofreagentsfor Organic Synthesis", 1995, JOHN WILEY AND SONS |
| "Geigy Pharmaceuticals", 1970, ARDSLEY, article "Scientific Tables", pages: 537 |
| "Oral Lipid-Based Formulations: Enhancing the Bioavailability of Poorly Water-Soluble Drugs (Drugs and the Pharmaceutical Sciences", 2007, INFORMA HEALTHCARE |
| "Pharmacotherapy Handbook", 2000, APPLETON AND LANGE |
| "Remington: The Science and Practice of Pharmacy", 2000, LIPPINCOTT WILLIAMS & WILKINS |
| "Role of Lipid Excipients in Modifying Oral and Parenteral Drug Delivery: Basic Principles and Biological Examples", 2006, WILEY-INTERSCIENCE |
| "Tarascon Pocket Pharmacopoeia 2000", 2000, TARASCON PUBLISHING |
| BLAKE, MI ET AL., J PHARM SCI, vol. 64, 1975, pages 367 - 91 |
| BURKE, R. T. ET AL., ONCOTARGET, vol. 5, no. 4, 2014, pages 908 - 915 |
| ESAKI, N., ANAL. BIOCHEM., vol. 119, no. 2, 1982, pages 281 - 5 |
| FIESER, L ET AL.: "Fieser and Fieser 's Reagentsfor Organic Synthesis", 1994, JOHN WILEY AND SONS |
| FISHER, MB ET AL., CURR OPIN DRUG DISCOV DEVEL, vol. 9, 2006, pages 101 - 09 |
| FOSTER A B: "Deuterium isotope effects in the metabolism of drugs and xenobiotics: implications for drug design", ADVANCES IN DRUG RESEARCH, ACADEMIC PRESS, LONDON, GB, vol. 14, 1 January 1985 (1985-01-01), pages 1 - 40, XP009086953, ISSN: 0065-2490 * |
| FOSTER, AB, ADV DRUG RES, vol. 14, 1985, pages 1 - 40 |
| FREIREICH ET AL., CANCER CHEMOTHER. REP, vol. 50, 1966, pages 219 |
| FUKUTO ET AL., J. MED. CHEM., vol. 34, 1991, pages 2871 - 76 |
| GANNES, LZ ET AL., COMP BIOCHEM PHYSIOL MOL INTEGR PHYSIOL, vol. 119, 1998, pages 725 |
| GREENE, TW ET AL.: "Protective Groups in Organic Synthesis", 1999, JOHN WILEY AND SONS |
| HAO CHEN ET AL: "Biotransformation of GS-1101 (CAL-101), a Potent and Selective Inhibitor of PI3K delta for the Treatment of Patients with Hematologic Malignancies -- Chen et al. 26 (1): 850.10 -- The FASEB Journal", THE FASEB JOURNAL, vol. 26, 1 January 2012 (2012-01-01), pages 850.10, XP055199787, ISSN: 1530-6860 * |
| KEMPF, D.J. ET AL., ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, vol. 41, no. 3, 1997, pages 654 - 60 |
| KUSHNER, DJ ET AL., CAN J PHYSIOL PHARMACOL, 1999, pages 79 - 88 |
| LAROCK R: "Comprehensive Organic Transformations", 1989, VCH PUBLISHERS |
| O'REILY, E., AMINO ACIDS, vol. 39, no. 3, 2010, pages 849 - 858 |
| STIRLING, I., J. CHEM. SOC. PERKIN I: ORG. AND BIOORG., 1997, pages 667 - 680 |
| WADA, E ET AL., SEIKAGAKU, vol. 66, 1994, pages 15 |
| WANG, L ET AL., CLINICAL PHARMACOLOGY AND THERAPEUTICS, vol. 56, 1994, pages 659 - 67 |
Cited By (20)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9655892B2 (en) | 2008-01-04 | 2017-05-23 | Intellikine Llc | Certain chemical entities, compositions and methods |
| US11433065B2 (en) | 2008-01-04 | 2022-09-06 | Intellikine Llc | Certain chemical entities, compositions and methods |
| US9822131B2 (en) | 2008-01-04 | 2017-11-21 | Intellikine Llc | Certain chemical entities, compositions and methods |
| US9522146B2 (en) | 2009-07-15 | 2016-12-20 | Intellikine Llc | Substituted Isoquinolin-1(2H)-one compounds, compositions, and methods thereof |
| US11312718B2 (en) | 2011-01-10 | 2022-04-26 | Infinity Pharmaceuticals, Inc. | Formulations of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one |
| USRE46621E1 (en) | 2011-01-10 | 2017-12-05 | Infinity Pharmaceuticals, Inc. | Processes for preparing isoquinolinones and solid forms of isoquinolinones |
| US9840505B2 (en) | 2011-01-10 | 2017-12-12 | Infinity Pharmaceuticals, Inc. | Solid forms of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1 (2H)-one and methods of use thereof |
| US10550122B2 (en) | 2011-01-10 | 2020-02-04 | Infinity Pharmaceuticals, Inc. | Solid forms of (S)-3-(1-(9H-purin-6-ylamino)ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one and methods of use thereof |
| US9527847B2 (en) | 2012-06-25 | 2016-12-27 | Infinity Pharmaceuticals, Inc. | Treatment of lupus, fibrotic conditions, and inflammatory myopathies and other disorders using PI3 kinase inhibitors |
| US10414767B2 (en) | 2014-01-30 | 2019-09-17 | Suzhou Zelgen Biopharmaceuticals Co., Ltd. | Deuterated quinazolinone compound and pharmaceutical composition comprising same |
| EP3101020A4 (en) * | 2014-01-30 | 2017-06-14 | Suzhou Zelgen Biopharmaceutical Co., Ltd. | Deuterated quinazolinone compound and pharmaceutical composition comprising same |
| US11110096B2 (en) | 2014-04-16 | 2021-09-07 | Infinity Pharmaceuticals, Inc. | Combination therapies |
| US11944631B2 (en) | 2014-04-16 | 2024-04-02 | Infinity Pharmaceuticals, Inc. | Combination therapies |
| CN108137592A (en) * | 2015-11-03 | 2018-06-08 | 纽弗姆制药有限公司 | For treat the deuterated compound of leukemia with and combinations thereof and method |
| CN108137592B (en) * | 2015-11-03 | 2021-03-05 | 纽弗姆制药有限公司 | Deuterated compounds and compositions and methods thereof for treating leukemia |
| CN108699061A (en) * | 2015-11-16 | 2018-10-23 | 纽弗姆制药有限公司 | For treating hematologic malignancies, inflammation and the deuterated compound of autoimmune disease |
| CN108699061B (en) * | 2015-11-16 | 2022-07-05 | 纽弗姆制药有限公司 | Deuterated compounds for the treatment of hematological malignancies, inflammation, and autoimmune diseases |
| WO2017087207A1 (en) * | 2015-11-16 | 2017-05-26 | NeuForm Pharmaceuticals, Inc. | Deuterated compounds for treating hematologic malignant, inflammatory and autoimmune diseases |
| US11147818B2 (en) | 2016-06-24 | 2021-10-19 | Infinity Pharmaceuticals, Inc. | Combination therapies |
| WO2021046315A3 (en) * | 2019-09-05 | 2021-04-22 | Wisconsin Alumni Research Foundation | Inhibitors of encephalitic alphaviruses |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2015179772A1 (en) | Deuterated phenylquinazolinone and phenylisoquinolinone compounds | |
| AU2013296627B2 (en) | Deuterated ibrutinib | |
| AU2014235462C1 (en) | Deuterated palbociclib | |
| AU2018271227A1 (en) | Deuterated derivatives of ruxolitinib | |
| WO2011063001A1 (en) | Niacin prodrugs and deuterated versions thereof | |
| EP2872159A2 (en) | Deuterated carfilzomib | |
| WO2011116066A1 (en) | Derivatives of dimethylcurcumin | |
| US8993570B2 (en) | Substituted triazolo-pyridazine derivatives | |
| WO2014110189A1 (en) | Deuterated momelotinib | |
| WO2011017612A1 (en) | Substituted diphenylpyrazine derivatives | |
| AU2011223895A1 (en) | Fluorouracil derivatives | |
| EP2836492B1 (en) | Substituted xanthine derivatives | |
| US8557815B2 (en) | Substituted triazolophthalazine derivatives | |
| WO2015031741A1 (en) | Deuterated derivatives of a thienotriazolodiazapine bromodomain-containing protein inhibitor | |
| WO2012154728A1 (en) | Deuterated n-butyl bumetanide | |
| WO2016089814A1 (en) | Deuterated analogues of daclatasvir | |
| WO2014159511A1 (en) | Deuterated pacritinib | |
| EP3105232A1 (en) | Substituted triazolobenzodiazepines | |
| WO2010068480A1 (en) | Deuterated derivatives of dimeboline | |
| WO2012027579A1 (en) | Synthetic triterpenoid derivatives | |
| WO2018013686A1 (en) | Deuterated idalopirdine | |
| WO2014008417A1 (en) | Deuterated vercirnon |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 15727226 Country of ref document: EP Kind code of ref document: A1 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 15727226 Country of ref document: EP Kind code of ref document: A1 |