WO2010088450A2 - Methods for treating diseases associated with the modulation of serca - Google Patents
Methods for treating diseases associated with the modulation of serca Download PDFInfo
- Publication number
- WO2010088450A2 WO2010088450A2 PCT/US2010/022486 US2010022486W WO2010088450A2 WO 2010088450 A2 WO2010088450 A2 WO 2010088450A2 US 2010022486 W US2010022486 W US 2010022486W WO 2010088450 A2 WO2010088450 A2 WO 2010088450A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- formula
- pharmaceutically acceptable
- stereoisomer
- acceptable derivative
- Prior art date
Links
- 0 CC(C)C1C*CC1 Chemical compound CC(C)C1C*CC1 0.000 description 27
- UHOVQNZJYSORNB-UHFFFAOYSA-N c1ccccc1 Chemical compound c1ccccc1 UHOVQNZJYSORNB-UHFFFAOYSA-N 0.000 description 4
- VGXJOGSDKWVMIE-UHFFFAOYSA-N CC(CCc1ccccc1)NC(c(cc1)ccc1F)=O Chemical compound CC(CCc1ccccc1)NC(c(cc1)ccc1F)=O VGXJOGSDKWVMIE-UHFFFAOYSA-N 0.000 description 3
- TTZUSADFLLMFBP-UHFFFAOYSA-N COc1cc(OC)cc(CNC(CCc2ccccc2)=O)c1 Chemical compound COc1cc(OC)cc(CNC(CCc2ccccc2)=O)c1 TTZUSADFLLMFBP-UHFFFAOYSA-N 0.000 description 3
- KHFNIWQUUZMMEJ-UHFFFAOYSA-N CC(CCc1ccc[o]1)NC(c1cc(C(F)(F)F)ccc1)=O Chemical compound CC(CCc1ccc[o]1)NC(c1cc(C(F)(F)F)ccc1)=O KHFNIWQUUZMMEJ-UHFFFAOYSA-N 0.000 description 2
- YFNSBYHZYCMCNZ-UHFFFAOYSA-N CC(CCc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O Chemical compound CC(CCc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O YFNSBYHZYCMCNZ-UHFFFAOYSA-N 0.000 description 2
- WYHKDYANIUUHGH-UHFFFAOYSA-N CC(CNCc(cccc1)c1O)NC(c1cc(OC)cc(OC)c1)=O Chemical compound CC(CNCc(cccc1)c1O)NC(c1cc(OC)cc(OC)c1)=O WYHKDYANIUUHGH-UHFFFAOYSA-N 0.000 description 2
- NZKOAOQHCXLKNX-UHFFFAOYSA-N CC(CNCc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O Chemical compound CC(CNCc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O NZKOAOQHCXLKNX-UHFFFAOYSA-N 0.000 description 2
- WZPXPCMFTCCGQB-UHFFFAOYSA-N CC(CNc1ccccc1)NC(c1cc(O)cc(O)c1)=O Chemical compound CC(CNc1ccccc1)NC(c1cc(O)cc(O)c1)=O WZPXPCMFTCCGQB-UHFFFAOYSA-N 0.000 description 2
- FTHPLWDYWAKYCY-UHFFFAOYSA-N COc1cc(C(Cl)=O)cc(OC)c1 Chemical compound COc1cc(C(Cl)=O)cc(OC)c1 FTHPLWDYWAKYCY-UHFFFAOYSA-N 0.000 description 2
- NVKQYRYUTSGRIY-UHFFFAOYSA-N COc1cc(C(N(CC2)CCN2c2ccccc2)=O)ccc1 Chemical compound COc1cc(C(N(CC2)CCN2c2ccccc2)=O)ccc1 NVKQYRYUTSGRIY-UHFFFAOYSA-N 0.000 description 2
- VQCIKIMYDJDDCI-GFCCVEGCSA-N C[C@H](CCc1ccccc1)NC(c1cc(C(F)(F)F)cc(C(F)(F)F)c1)=O Chemical compound C[C@H](CCc1ccccc1)NC(c1cc(C(F)(F)F)cc(C(F)(F)F)c1)=O VQCIKIMYDJDDCI-GFCCVEGCSA-N 0.000 description 2
- RNVCVTLRINQCPJ-UHFFFAOYSA-N Cc1ccccc1N Chemical compound Cc1ccccc1N RNVCVTLRINQCPJ-UHFFFAOYSA-N 0.000 description 2
- YZTJYBJCZXZGCT-UHFFFAOYSA-N C(C1)NCCN1c1ccccc1 Chemical compound C(C1)NCCN1c1ccccc1 YZTJYBJCZXZGCT-UHFFFAOYSA-N 0.000 description 1
- WPIMBZGVPVKFDT-UHFFFAOYSA-N CC(C)Oc1cccc(C(Cl)=O)c1 Chemical compound CC(C)Oc1cccc(C(Cl)=O)c1 WPIMBZGVPVKFDT-UHFFFAOYSA-N 0.000 description 1
- GDTHHMBQQCODIZ-MRXNPFEDSA-N CC(C)Oc1cccc(C(N[C@H](C)CCc2ccccc2)=O)c1 Chemical compound CC(C)Oc1cccc(C(N[C@H](C)CCc2ccccc2)=O)c1 GDTHHMBQQCODIZ-MRXNPFEDSA-N 0.000 description 1
- WQAHPYIDHFCXGY-UHFFFAOYSA-N CC(CCCc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O Chemical compound CC(CCCc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O WQAHPYIDHFCXGY-UHFFFAOYSA-N 0.000 description 1
- DLVADZCWXZUTQC-UHFFFAOYSA-N CC(CCc(cc1)ccc1OC)NC(c1cc(C#N)ccc1)=O Chemical compound CC(CCc(cc1)ccc1OC)NC(c1cc(C#N)ccc1)=O DLVADZCWXZUTQC-UHFFFAOYSA-N 0.000 description 1
- LDGPVJINECUAHS-UHFFFAOYSA-N CC(CCc(cc1)ccc1OC)NC(c1cccc(OC)c1)=O Chemical compound CC(CCc(cc1)ccc1OC)NC(c1cccc(OC)c1)=O LDGPVJINECUAHS-UHFFFAOYSA-N 0.000 description 1
- LQRYXCGWXPKDDY-UHFFFAOYSA-N CC(CCc1ccc[o]1)NC(c1cc(C#N)ccc1)=O Chemical compound CC(CCc1ccc[o]1)NC(c1cc(C#N)ccc1)=O LQRYXCGWXPKDDY-UHFFFAOYSA-N 0.000 description 1
- YQBVZRASNZMRMC-UHFFFAOYSA-N CC(CCc1ccc[o]1)NC(c1cc(OC)cc(OC)c1)=O Chemical compound CC(CCc1ccc[o]1)NC(c1cc(OC)cc(OC)c1)=O YQBVZRASNZMRMC-UHFFFAOYSA-N 0.000 description 1
- XZASUCFWNAKTQV-UHFFFAOYSA-N CC(CCc1ccc[o]1)NC(c1cccc(OC)c1)=O Chemical compound CC(CCc1ccc[o]1)NC(c1cccc(OC)c1)=O XZASUCFWNAKTQV-UHFFFAOYSA-N 0.000 description 1
- XVJAPHLNCYZISZ-UHFFFAOYSA-N CC(CCc1ccccc1)C(Nc1cc(OC)cc(OC)c1)=O Chemical compound CC(CCc1ccccc1)C(Nc1cc(OC)cc(OC)c1)=O XVJAPHLNCYZISZ-UHFFFAOYSA-N 0.000 description 1
- UENDBSQAOQJPRW-UHFFFAOYSA-N CC(CCc1ccccc1)NC(c(cc1OC)cc(OC)c1OC)=O Chemical compound CC(CCc1ccccc1)NC(c(cc1OC)cc(OC)c1OC)=O UENDBSQAOQJPRW-UHFFFAOYSA-N 0.000 description 1
- VQCIKIMYDJDDCI-UHFFFAOYSA-N CC(CCc1ccccc1)NC(c1cc(C(F)(F)F)cc(C(F)(F)F)c1)=O Chemical compound CC(CCc1ccccc1)NC(c1cc(C(F)(F)F)cc(C(F)(F)F)c1)=O VQCIKIMYDJDDCI-UHFFFAOYSA-N 0.000 description 1
- BPPKVHUDDDOLIQ-UHFFFAOYSA-N CC(CCc1ccccc1)NC(c1cccc(C(F)(F)F)c1)=O Chemical compound CC(CCc1ccccc1)NC(c1cccc(C(F)(F)F)c1)=O BPPKVHUDDDOLIQ-UHFFFAOYSA-N 0.000 description 1
- VCXOFTBPXAKNEC-UHFFFAOYSA-N CC(CCc1ccccc1)NC(c1cccc(OC)c1)=O Chemical compound CC(CCc1ccccc1)NC(c1cccc(OC)c1)=O VCXOFTBPXAKNEC-UHFFFAOYSA-N 0.000 description 1
- DOSJHTBJZDKKDU-UHFFFAOYSA-N CC(CN(CCC1)c2c1cccc2)NC(c1cc(OC)cc(OC)c1)=O Chemical compound CC(CN(CCC1)c2c1cccc2)NC(c1cc(OC)cc(OC)c1)=O DOSJHTBJZDKKDU-UHFFFAOYSA-N 0.000 description 1
- WGTPROAWBAOPNU-UHFFFAOYSA-N CC(CNc1ccccc1)NC Chemical compound CC(CNc1ccccc1)NC WGTPROAWBAOPNU-UHFFFAOYSA-N 0.000 description 1
- MESBMALJOIAUKQ-UHFFFAOYSA-N CC(CNc1ccccc1)NC(OC(C)(C)C)=O Chemical compound CC(CNc1ccccc1)NC(OC(C)(C)C)=O MESBMALJOIAUKQ-UHFFFAOYSA-N 0.000 description 1
- KADUUQXOHQEXAP-UHFFFAOYSA-N CC(CNc1ccccc1)NC(c1cc(F)ccc1)=O Chemical compound CC(CNc1ccccc1)NC(c1cc(F)ccc1)=O KADUUQXOHQEXAP-UHFFFAOYSA-N 0.000 description 1
- JDVDVFZRRBWBLZ-UHFFFAOYSA-N CC(CNc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O Chemical compound CC(CNc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O JDVDVFZRRBWBLZ-UHFFFAOYSA-N 0.000 description 1
- UYHTUQHYGKAYJM-UHFFFAOYSA-N CC(c1cc(OC(F)(F)F)ccc1)=O Chemical compound CC(c1cc(OC(F)(F)F)ccc1)=O UYHTUQHYGKAYJM-UHFFFAOYSA-N 0.000 description 1
- VOQGDJPVMANQLY-UHFFFAOYSA-N CCOc1cc(OCC)cc(C(NC(C)CCc2ccc[o]2)=O)c1 Chemical compound CCOc1cc(OCC)cc(C(NC(C)CCc2ccc[o]2)=O)c1 VOQGDJPVMANQLY-UHFFFAOYSA-N 0.000 description 1
- REEFZZBWLTTYAR-UHFFFAOYSA-N CCOc1cc(OCC)cc(C(NC(C)CNCc2ccccc2)=O)c1 Chemical compound CCOc1cc(OCC)cc(C(NC(C)CNCc2ccccc2)=O)c1 REEFZZBWLTTYAR-UHFFFAOYSA-N 0.000 description 1
- VCYSUBMQCHARPH-UHFFFAOYSA-N COc1cc(OC)cc(C(N(CC2)CCN2c2ccccc2)=O)c1 Chemical compound COc1cc(OC)cc(C(N(CC2)CCN2c2ccccc2)=O)c1 VCYSUBMQCHARPH-UHFFFAOYSA-N 0.000 description 1
- WECUIGDEWBNQJJ-SECBINFHSA-N C[C@H](CCc1ccccc1)N Chemical compound C[C@H](CCc1ccccc1)N WECUIGDEWBNQJJ-SECBINFHSA-N 0.000 description 1
- YFNSBYHZYCMCNZ-CQSZACIVSA-N C[C@H](CCc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O Chemical compound C[C@H](CCc1ccccc1)NC(c1cc(OC)cc(OC)c1)=O YFNSBYHZYCMCNZ-CQSZACIVSA-N 0.000 description 1
- VVETYJRPGACGCD-CYBMUJFWSA-N C[C@H](CCc1ccccc1)NC(c1cccc(F)c1)=O Chemical compound C[C@H](CCc1ccccc1)NC(c1cccc(F)c1)=O VVETYJRPGACGCD-CYBMUJFWSA-N 0.000 description 1
- WZPXPCMFTCCGQB-LLVKDONJSA-N C[C@H](CNc1ccccc1)NC(c1cc(O)cc(O)c1)=O Chemical compound C[C@H](CNc1ccccc1)NC(c1cc(O)cc(O)c1)=O WZPXPCMFTCCGQB-LLVKDONJSA-N 0.000 description 1
- MWRIYYGLQNVVOD-CYBMUJFWSA-N C[C@H](CNc1ccccc1)NC(c1cc(OC)ccc1)=O Chemical compound C[C@H](CNc1ccccc1)NC(c1cc(OC)ccc1)=O MWRIYYGLQNVVOD-CYBMUJFWSA-N 0.000 description 1
- SXPRXEYTYNZGDA-UHFFFAOYSA-N I[n]1ncc2c1cccc2 Chemical compound I[n]1ncc2c1cccc2 SXPRXEYTYNZGDA-UHFFFAOYSA-N 0.000 description 1
- LYUQWQRTDLVQGA-UHFFFAOYSA-N NCCCc1ccccc1 Chemical compound NCCCc1ccccc1 LYUQWQRTDLVQGA-UHFFFAOYSA-N 0.000 description 1
- RNDFPMCITGXZKB-UHFFFAOYSA-N O=C(c1cccc(OC(F)(F)F)c1)NCCCc1ccccc1 Chemical compound O=C(c1cccc(OC(F)(F)F)c1)NCCCc1ccccc1 RNDFPMCITGXZKB-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/42—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/44—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring
- C07C235/46—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring having the nitrogen atoms of the carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P11/00—Drugs for disorders of the respiratory system
- A61P11/06—Antiasthmatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/48—Drugs for disorders of the endocrine system of the pancreatic hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/01—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C233/16—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
- C07C233/17—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom
- C07C233/22—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom having the carbon atom of the carboxamide group bound to an acyclic carbon atom of a carbon skeleton containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/01—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms
- C07C233/16—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
- C07C233/24—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by a carbon atom of a six-membered aromatic ring
- C07C233/29—Carboxylic acid amides having carbon atoms of carboxamide groups bound to hydrogen atoms or to acyclic carbon atoms having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by a carbon atom of a six-membered aromatic ring having the carbon atom of the carboxamide group bound to an acyclic carbon atom of a carbon skeleton containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/64—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings
- C07C233/65—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atoms of the carboxamide groups bound to hydrogen atoms or to carbon atoms of unsubstituted hydrocarbon radicals
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/64—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings
- C07C233/67—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
- C07C233/68—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom
- C07C233/73—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom of a carbon skeleton containing six-membered aromatic rings
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C233/00—Carboxylic acid amides
- C07C233/64—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings
- C07C233/77—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by amino groups
- C07C233/78—Carboxylic acid amides having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings having the nitrogen atom of at least one of the carboxamide groups bound to a carbon atom of a hydrocarbon radical substituted by amino groups with the substituted hydrocarbon radical bound to the nitrogen atom of the carboxamide group by an acyclic carbon atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/42—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/44—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring
- C07C235/48—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring having the nitrogen atom of at least one of the carboxamide groups bound to an acyclic carbon atom of a hydrocarbon radical substituted by singly-bound oxygen atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C235/00—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms
- C07C235/42—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton
- C07C235/44—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring
- C07C235/50—Carboxylic acid amides, the carbon skeleton of the acid part being further substituted by oxygen atoms having carbon atoms of carboxamide groups bound to carbon atoms of six-membered aromatic rings and singly-bound oxygen atoms bound to the same carbon skeleton with carbon atoms of carboxamide groups and singly-bound oxygen atoms bound to carbon atoms of the same non-condensed six-membered aromatic ring having the nitrogen atom of at least one of the carboxamide groups bound to an acyclic carbon atom of a hydrocarbon radical substituted by nitrogen atoms not being part of nitro or nitroso groups
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07C—ACYCLIC OR CARBOCYCLIC COMPOUNDS
- C07C255/00—Carboxylic acid nitriles
- C07C255/49—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton
- C07C255/57—Carboxylic acid nitriles having cyano groups bound to carbon atoms of six-membered aromatic rings of a carbon skeleton containing cyano groups and carboxyl groups, other than cyano groups, bound to the carbon skeleton
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/08—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon radicals, substituted by hetero atoms, attached to ring carbon atoms
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D211/00—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings
- C07D211/04—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D211/06—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D211/08—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms
- C07D211/10—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with radicals containing only carbon and hydrogen atoms attached to ring carbon atoms
- C07D211/16—Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hydrocarbon or substituted hydrocarbon radicals directly attached to ring carbon atoms with radicals containing only carbon and hydrogen atoms attached to ring carbon atoms with acylated ring nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D215/00—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems
- C07D215/02—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom
- C07D215/04—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, directly attached to the ring carbon atoms
- C07D215/06—Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom with only hydrogen atoms or radicals containing only hydrogen and carbon atoms, directly attached to the ring carbon atoms having only hydrogen atoms, hydrocarbon or substituted hydrocarbon radicals, attached to the ring nitrogen atom
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D241/00—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings
- C07D241/02—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings
- C07D241/04—Heterocyclic compounds containing 1,4-diazine or hydrogenated 1,4-diazine rings not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D307/00—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom
- C07D307/02—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings
- C07D307/04—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members
- C07D307/10—Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom not condensed with other rings having no double bonds between ring members or between ring members and non-ring members with substituted hydrocarbon radicals attached to ring carbon atoms
- C07D307/14—Radicals substituted by nitrogen atoms not forming part of a nitro radical
Definitions
- SERCA Sarcoplasmic/endoplasmic reticulum calcium ATP-ase
- the disease is heart failure, stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, a disease associated with a cardiac disease, urinary incontinence, cancer, or asthma.
- kits for treating heart failure comprising administering a compound of formula I:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
- R x is, independently at each occurrence, H, alkyl, or aryl;
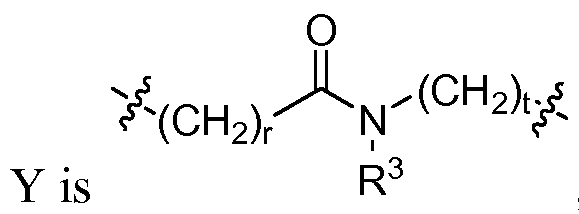
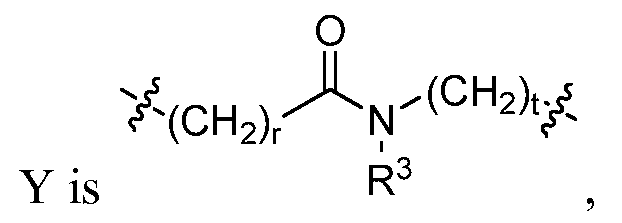
- R 1 , n, Q, Y, and J are selected from (i) and (ii) as follows:
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; and wherein R 3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R >x is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4; Q is H or alkyl; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R .3 i •s H, or alkyl; or R > 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- provided herein are methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula I
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
- R x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
- R 1 , n, Q, Y, and J are selected from (i) and (ii) as follows:
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; with the proviso that R 1 is not indolyl; and
- r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula Ia:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy;
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and R 1 , n, Q, J, and Y are selected from (i) and (ii) as follows: (i) n is an integer from
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0011] In some embodiments, provided herein are methods for treating cancer comprising administering a compound of formula I:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
- R 1 , n, J, Q, and Y are selected from (i) and (ii) as follows:
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
- ⁇ is, independently at each occurrence, H, alkyl, or aryl; and wherein R 3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and R 1 , n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl
- r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R >x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and n is an integer from 1-4; Q is H or alkyl; R 1 is aryl, heteroaryl, substituted aryl, substituted hetero
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl; and R 1 , n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl
- R 3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
- kits for treating diabetes comprising administering a compound of formula Ia:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R >x is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4; Q is H or alkyl; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- a compound of formula I comprising administering a compound of formula I:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl; and R 1 , n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R 3 wherein R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; with the proviso that R 1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
- a compound of formula Ia comprising administering a compound of formula Ia:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof. 4.
- FIG. 1 shows a graph depicting pCA versus ATP ase activity.
- FIG. 2 shows Ca-ATPase dose-response of compound A.
- FIG. 3 shows Ca-ATPase dose-response of compound B.
- FIG. 4 shows representative traces of sarcomere shortening as a reproducible index of contractility.
- FIG. 5 shows the effects of a compound described herein on normal myocytes.
- FIG. 6 shows the effects of a compound described herein on failing myocytes.
- FIG. 7 shows the rate dependence of a compound described herein.
- the left bar represents the control.
- the right bar represents the compound.
- FIG. 8 shows the change in sarcomere length for myocytes treated with thapsigargin, a compound described herein, and both thapsigargin and the compound.
- FIG. 9 shows the effects of a compound described herein on cellular action potential.
- FIG. 10 shows ECG traces before (top) and after(bottom) perfusion of a compound described herein.
- FIG. 11 shows a graph depicting percent contractility versus concentration for a compound described herein.
- FIG. 12 shows a table summarizing Rat PK Data for two exemplary compounds.
- FIG. 13 shows the Pmax for a compound described herein in a mouse in vivo hemodynamic study.
- FIG. 14 shows the acute increase in dP/dtmax for a compound described herein in a mouse in vivo hemodynamic study.
- FIG. 15 shows the effects of a compound described herein on cardiac output in a mouse in vivo hemodynamic study.
- FIG. 16 shows the effects of a compound described herein on ejection fraction in a mouse in vivo hemodynamic study.
- alkoxy refers to * s wherein R is alkyl.
- alkyl refers to a uni-valent hydrocarbon chain or group of about 1 to about 20 carbons. In some embodiments, the alkyl contains about 1 to about 15 carbons. In some embodiments, the alkyl contains about 1 to about 10 carbons. In some embodiments, the alkyl contains about 1 to about 8 carbons. In some embodiments, the alkyl contains about 1 to about 6 carbons. In some embodiments, the alkyl contains about 1 to about 3 carbons. In some embodiments, the alkyl contains 1 to 2 carbons. In some embodiments, the alkyl is primary. In some embodiments, the alkyl is secondary. In some embodiments, the alkyl is tertiary.
- the alkyl is methyl, ethyl, n-propyl, isopropyl, isobutyl, n-butyl, sec-butyl, tert- butyl, isopentyl, neopentyl, tert-pentyl, or isohexyl.
- the alkyl is methyl, ethyl, n-propyl, or isopropyl.
- the alkyl is methyl.
- the alkyl is tert-butyl.
- the alkyl is a straight hydrocarbon chain. In some embodiments, the alkyl is a branched hydrocarbon chain.
- aralkyl refers to * ( ⁇ H2)n ⁇ ? wherein R aryl and n is an integer from 1 to 20. In some embodiments, n is an 1 to 10. In some embodiments, n is 1-5. In some embodiments, n is 1-3. In some embodiments, n is 1. In some embodiments, n is 2. In some embodiments, n is 3.
- aryl refers to a uni-valent monocyclic or multicyclic aromatic group containing from 6 to about 30 carbons. In some embodiments, the aryl contains about 6 to about 15 carbons. In some embodiments, the aryl contains about 6 to about 10 carbons. In some embodiments, the aryl is fluorenyl, phenyl, or naphthyl. In some embodiments, the aryl is phenyl. In some embodiments, the aryl in monocyclic.
- cycloalkyl refers to a univalent monocyclic or multicyclic alkyl group containing from 3 to about 30 carbons. In some embodiments, the cycloalkyl contains 5 to about 15 carbons. In some embodiments, the cycloalkyl contains about 5 to about 10 carbons.
- the cycloalkyl contains about 5 to about 8 carbons. In some embodiments, the cycloalkyl is cyclopentyl or cyclohexyl. In some embodiments, the cycloalkyl is monocyclic.
- halo refers to halogen. In some embodiments, halo is F, Cl, Br, or
- halo is F. In some embodiments, halo is Cl. In some embodiments, halo is Br. In some embodiments, halo is I.
- haloalkyl refers to a uni-valent alkyl group substituted with one or more halo group or groups. In some embodiments, the haloalkyl is substituted with 1-3 halo
- the haloalkyl is [0044]
- heteroaryl refers to a uni-valent monocyclic or multicyclic aromatic ring system containing about 5 to about 15 ring atomsm, wherein at least one ring atom is a heteroatom. In some embodiments, the heteroaryl contains 5 to about 10 ring atoms. In some embodiments, the heteroaryl contains 5 or 6 ring atoms. In some embodiments, the heteroaryl is monocyclic. In some embodiments, the heteroatom is N, O, or S. In some embodiments, the heteroaryl contains one heteroatom. In some embodiments, the heteroaryl contains 1 to 3 N atoms.
- the heteroaryl contains one O or S atom and one or two N atoms.
- the heteroaryl is furyl, imidazolyl, pyrimidinyl, tetrazolyl, thienyl, pyridyl, pyrrolyl, thiazolyl, isothiazolyl, oxazolyl, isoxazolyl, thiazolyl, quinolinyl, or isoquinolinyl.
- the heteroaryl is furyl.
- the aryl is monocyclic.
- heterocyclyl refers to a uni-valent monocyclic or multicyclic non- aromatic ring system containing about 3-30 ring atoms, wherein at least one ring atom is a heteroatom. In some embodiments, the heterocyclyl contains 5 to about 10 ring atoms. In some embodiments, the heterocyclyl contains 5 or 6 ring atoms. In some embodiments, the heteroatom is N, O, or S. In some embodiments, the heterocyclyl is monocyclic.
- substituted aryl and “substituted heteroaryl” refers to an aryl or a
- heteroaryl substituted with one or more alkyl, alkoxy, aryloxy, hydroxyl, -NR Z R Z , ,
- the compounds provided herein and the compounds used in the compositions and methods provided herein may contain chiral centers. Such chiral centers may be of either the (R) or (S) configuration, or may be a mixture thereof. Thus, the compounds provided herein may be enantiomerically pure, or be stereoisomeric or diastereomeric mixtures.
- a given stereoisomer of a compound descried herein or given stereoisomer of a compound used in the compositions and methods described herein is substantially free of other stereoisomers.
- substantially free means that a given compound is at least 80% by weight of a given stereoisomer.
- the compound is at least 85% by weight of a given stereoisomer. In some embodiments, the compound is at least 90% by weight of a given stereoisomer. In some embodiments, the compound is at least 95% by weight of a given stereoisomer. In some embodiments, the compound is at least 98% by weight of a given stereoisomer.
- the compounds described herein may be a pharmaceutically acceptable derivative.
- Pharmaceutically acceptable derivatives of a compound include, but are not limited to, salts, esters, enol ethers, enol esters, acetals, ketals, orthoesters, hemiacetals, hemiketals, acids, bases, solvates or hydrates thereof. Such derivatives may be readily prepared by those of skill in this art using known methods for such derivatization.
- salts include, but are not limited to, amine salts, such as but not limited to N 5 N'- dibenzylethylenediamine, chloroprocaine, choline, ammonia, diethanolamine and other hydroxyalkylamines, ethylenediamine, N-methylglucamine, procaine, N-benzylphenethylamine, l-para-chlorobenzyl-2-pyrrolidin-r-ylmethylbenzimidazole, diethylamine and other alkylamines, piperazine and tris(hydroxymethyl)aminomethane; alkali metal salts, such as but not limited to lithium, potassium and sodium; alkali earth metal salts, such as but not limited to barium, calcium and magnesium; transition metal salts, such as but not limited to zinc; and inorganic salts, such as but not limited to, sodium hydrogen phosphate and disodium phosphate; and also including, but not limited to, salts of mineral acids, such as but not limited to hydroch
- esters include, but are not limited to, alkyl, alkenyl, alkynyl, aryl, aralkyl, and cycloalkyl esters of acidic groups, including, but not limited to, carboxylic acids, phosphoric acids, phosphinic acids, sulfonic acids, sulf ⁇ nic acids and boronic acids.
- Pharmaceutically acceptable solvates and hydrates are complexes of a compound with one or more solvent or water molecules, or 1 to about 100, or 1 to about 10, or one to about 2, 3 or 4, solvent or water molecules. It is to be understood that the pharmaceutically acceptable derivates described herein include, but are not limited to, produgs.
- Prodrugs include, but are not limited to, a derivative of a compound that reacts under biological conditions (in vitro or in vivo) to provide that compound.
- such derivative hydrolyzes or oxidizes under biological conditions to provide the compound.
- prodrugs include, but are not limited to, derivatives that comprise biohydrolyzable moieties such as biohydrolyzable amides, biohydrolyzable esters, biohydrolyzable carbamates, biohydrolyzable carbonates, biohydrolyzable ureides, and biohydrolyzable phosphate analogs.
- Prodrugs can typically be prepared using well-known methods, such as those described in 1 Burger's Medicinal Chemistry and Drug Discovery, 172-178, 949-982 (Manfred E. Wolff ed., 5th ed. 1995), and Design of Prodrugs (H. Bundgaard ed., Elselvier, New York 1985, incorporated herein by reference in its entirety).
- the Ca-ATP ase (SERCA) of the sarcoplasmic reticulum (SR) functions to remove calcium ions from the cytoplasm.
- SERCA re-sequesters the calcium back into the internal sacroplasmic reticulum pool, thus priming the next quantal release of calcium.
- provided herein are methods of treating a disease associated with the modulation of SERCA comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods of treating a disease associated with the modulation of SERC A2 comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods of treating a disease associated with the modulation of SERCA2a comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
- provided herein are methods of treating a disease associated with the modulation of SERCA2b comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
- methods of treating a disease associated with decreased SERCA2a activity comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
- methods of treating a disease associated with the modulation of SERC A2a via increasing the activity of SERCA2a comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
- SERCA2 regulates cardiac function by promoting both cardiac relaxation and contractility. Furthermore, in cardiac muscle, SERCA2a is regulated by phospholamban (PLB), which inhibits SERCA at submicromolar calcium. The regulation of PLB influences cardiac relaxation and contractility. Phosphorylation of PLB by protein kinase A causes PLB to release its inhibitory grip on SERC A2. Abnormal PLB/SERC A2 ratios and associated defective calcium cycling is a feature of some forms of human and experimental heart failure. Therefore, without being bound by any theory, compounds that have the capacity to increase SERC A2 activity or release inhibition of SERCA2 by interacting with the SERC A2 -PLB interface may play a role in improving myocyte contractility and cardiac function.
- PLB phospholamban
- provided herein are methods for treating diseases associated with phospholamban activity or phospholamban levels by administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
- methods for treating a disease associated with the modulation of SERCA and/or PLB comprising administering a compound described herein.
- the disease is heart failure, stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, a disease associated with a cardiac disease, urinary incontinence, cancer, asthma, diabetes, or Alzheimer's disease.
- methods of treating heart failure comprising administering a compound of formula I: i; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl;
- R 1 , n, Q, Y, and J are selected from (i) and (ii) as follows:
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; and
- R 3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
- R x is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
- R x is, independently at each occurrence, H, alkyl, or aryl; and n is 0;
- J is CH or N
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; and
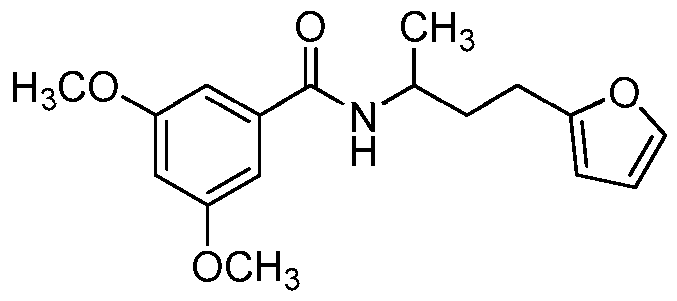
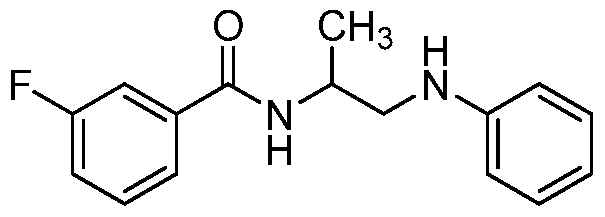
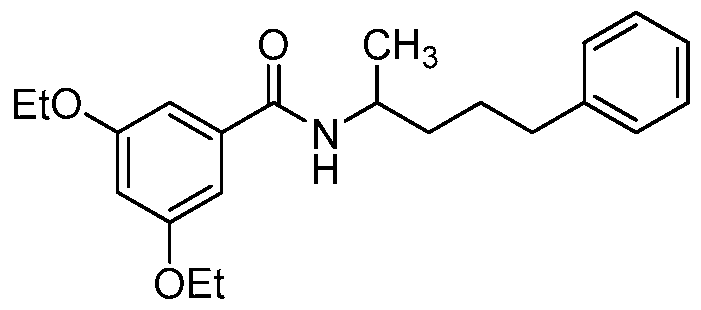
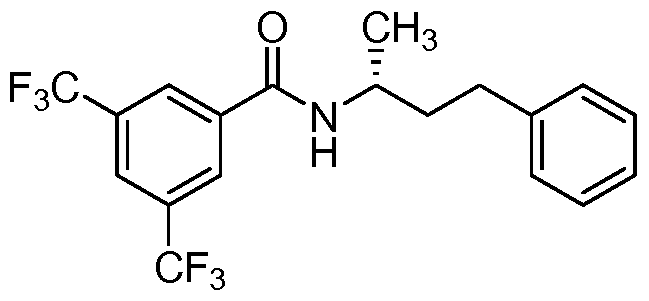
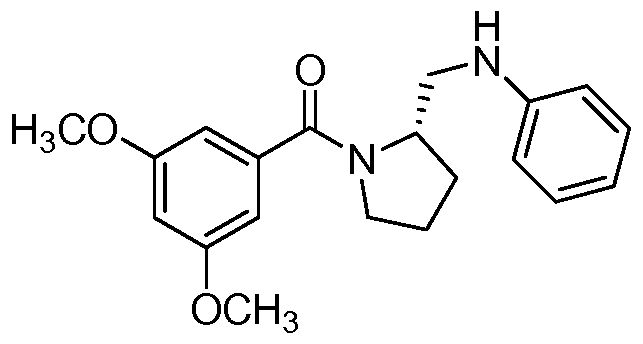
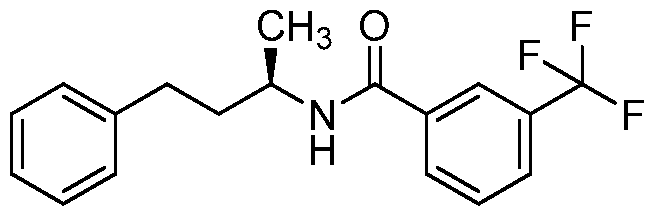
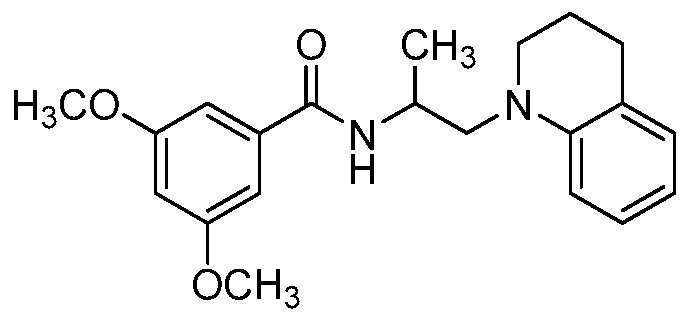
- the compound of formula I is: or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is:
- the compound of formula I is
- the compound of formula I is
- the compound of formula I is:
- the compound of formula I is wherein R 1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is
- the compound of formula I is
- the compound of formula I is:
- the compound of formula I is:
- A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro. [0077] In some embodiments, A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
- A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
- A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
- B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
- B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
- B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
- At least one of A, B, and C is not hydrogen. [0085] In some embodiments, at least two of A, B, and C is not hydrogen. [0086] In some embodiments, B is hydrogen, and A and C are not hydrogen. [0087] In some embodiments, B is hydrogen, A and C are identical. [0088] In some embodiments, when A is para to Y and is not hydrogen, then A is not isopropoxy, when B is para to Y and is not hydrogen, then B is not isopropoxy, and when C is para to Y and is not hydrogen, then C is not isopropoxy.
- n is 1-2.
- n 1
- n is 2-4.
- n is 2-3.
- n is 2.
- Q is H or alkyl of 1-3 carbons.
- Q is H or alkyl of 1-2 carbons.
- Q is H or methyl
- Q is alkyl of 1-3 carbons.
- Q is alkyl of 1-2 carbons.
- Q is methyl
- R 1 is monocyclic.
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR 2 -X.
- R 1 is aryl, heteroaryl, substituted aryl, or NR 2 -X.
- R 1 is aryl, heteroaryl or substituted aryl.
- R 1 is aryl or heteroaryl.
- R 1 is aryl
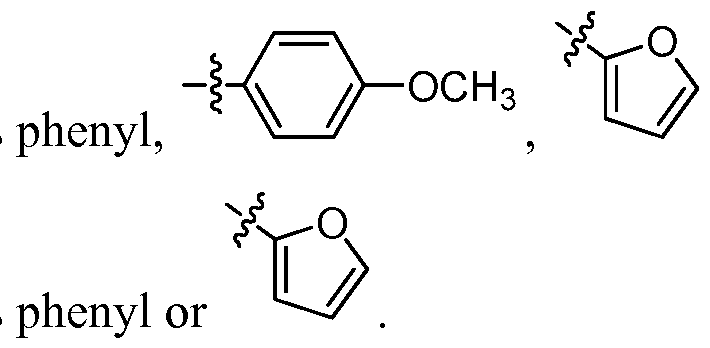
- R 1 is phenyl
- R 1 is phenyl; , or
- R 1 is phenyl, X) , or
- R 1 is phenyl or [00113] In some embodiments, R 1 is not indolyl. [00114] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
- X is aryl or aralkyl.
- X is aryl
- X is phenyl
- R 2 is H or alkyl or 1-3 carbons.
- R 2 is H.
- R 3 is H or alkyl.
- R 3 is H or alkyl or 1-3 carbons.
- R 3 is H.
- R 3 and Q are joined together to form ring.
- R and Q are joined together to form a 5-6 membered ring.
- t is 0, and R 3 and Q are joined together to form a 5-6 membered rin g-
- t is 0 and R 3 and Q are joined together to form a 5 membered ring.
- r is 0-2.
- r is 0-1.
- r is 0.
- t is 0-2.
- t is 0-1.
- t is 0.
- r is 0-1 and t is 0.
- r is 0 and t is 0.
- the compound of formula I is N-(00135]
- a and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or thereof.
- the compound of fomula I is N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoe
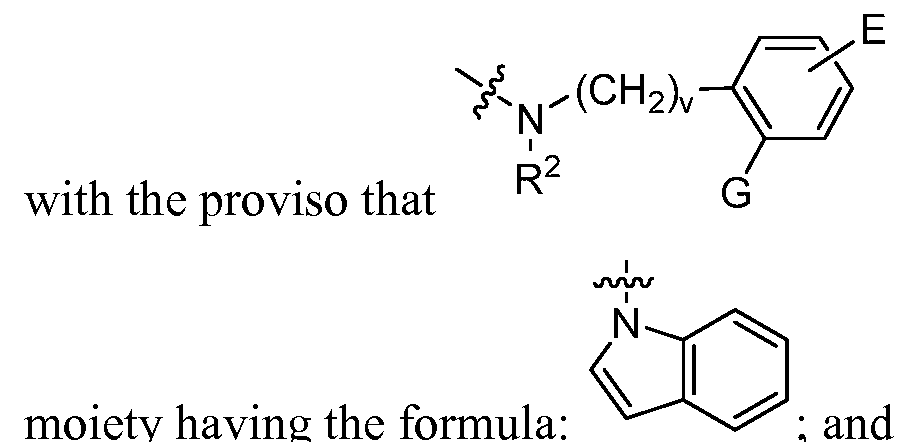
- v is an integer from 0 to 3;
- A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO 2 ; wherein at least one of A and B is not H;
- E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
- R 3 is H or alkyl of 1-3 carbons, Q is methyl, or R 3 and Q are joined together to form a 5- 6 membered ring; and R 2 and G are selected from (i) and (ii) as follows: (i) v is 0,
- R 2 is H or alkyl of 1-3 carbons
- G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO 2 , orG is joined together with R 2 to form a 5-6 membered ring; with the proviso that R 2 G ' i s not a substituted or unsubstituted
- the compound of fomula I is H or alkyl of 1-3 carbons; and G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 ; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- R 2 is H or alkyl of 1-3 carbons; and G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 ; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- v is an integer from 0 to 3;
- a and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1- 3 carbons, -CN, azido, or NO 2 ; wherein at least one of A and B is not H;
- R 3 is H or alkyl of 1-3 carbons, Q is methyl, or R 3 and Q are joined together to form a 5- 6 membered ring; and R 2 and G are selected from (i) and (ii) as follows: (i) v is 0,
- R 2 is H or alkyl of 1-3 carbons
- G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 , or G is joined together with R 2 to form a 5-6 membered ring;
- R 2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 ; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- / G — is not a substuted or unsubstituted moiety
- R 2 is not a substuted or unsubstituted moiety
- a and B are each, independently, H, hydroxyl, or alkoxy of 1-3 carbons; R 3 is H or alkyl of 1-3 carbons, Q is methyl, R 2 is H, alkyl of 1-3 carbons, and G is H, or hydroxyl.
- the compound of formula I has the following formula:
- the compound of formula I has the following formula:
- the compound of formula I is a compound of formula II:
- the compound of formula I is a compound of formula III:
- the compound of formula I is a compound of formula IV:
- the compound of formula I is a compound of formula V:
- the compound of formula I is a compound of formula VI:
- the compound of formula I is a compound of formula VII:
- the compound of formula I is a compound of formula VIII:
- the compound of formula I is a compound of formula IX:
- the compound of formula I is a compound of formula X:
- the compound of formula I is a compound of formula XI:
- the compound of formula I is a compound of formula XII:
- the compound of formula I is a compound of formula XIII:
- the compound of formula I is a compound of formula XIV:
- the compound of formula I is a compound of formula XV:
- the compound of formula I is a compound of formula XVI:
- the compound of formula I is a compound of formula XVII:
- the compound of formula I is a compound of formula XVIII:
- the compound of formula I is a compound of formula XIX:
- the compound of formula I is a compound of formula XX:
- the compound of formula I is a compound of formula XXI:
- the compound of formula I is a compound of formula XXII:
- the compound of formula I is a compound of formula XXIII:
- the compound of formula I is a compound of formula XXIV:
- the compound of formula I is a compound of formula XXV:
- the compound of formula I is a compound of formula XXVI:
- the compound of formula I is a compound of formula XXVII:
- the compound of formula I is a compound of formula XXVIII:
- the compound of formula I is a compound of formula XXIX: XXIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is a compound of formula XXX:
- the compound of formula I is a compound of formula XXXI:
- the compound of formula I is a compound of formula XXXII:
- the compound of formula I is a compound of formula XXXIII:
- the compound of formula I is a compound of formula XXXIV:
- the compound of formula I is a compound of formula XXXV:
- the compound of formula I is a compound of formula XXXVI:
- the compound of formula I is a compound of formula XXXVII:
- the compound of formula I is a compound of formula
- XXXVIII XXXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is a compound of formula XXXIX:
- the compound of formula I is a compound of formula XL:
- the compound of formula I is a compound of formula XLI:
- the compound of formula I is a compound of formula XLII:
- the compound of formula I is a compound of formula XLIII:
- the compound of formula I is a compound of formula XLIV
- the compound of formula I is a compound of formula XLV
- the compound of formula I is a compound of formula XLVI
- the compound of formula I is a compound of formula XLVII XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof. [00189] In some embodiments, the compound of formula I is a compound of formula XLVIII
- the compound of formula I is a compound of formula XLIX
- the compound of formula I is a compound of formula L
- the compound of formula I is a compound of formula LI
- the compound of formula I is a compound of formula LII
- the compound of formula I is a compound of formula LII
- the heart failure is acute heart failure, chronic heart failure, end-stage heart failure, congestive heart failure, right heart failure, left heart failure, forward heart failure, backward heart failure, Class I, II, III, or IV heart failure as defined by New York Heart Association Functional Classification, systolic heart failure, diastolic heart failure, low- output heart failure, or high-output heart failure.
- inotropic drugs i.e., drugs that increase contractility
- methods for treating heart failure comprising administering a compound of formula I, or a pharmaceutically acceptable derivative or stereoisomer, wherein the administration of the compound results in improved survival.
- methods for treating heart failure comprising administering a compound of formula I, or a pharmaceutically acceptable derivative or stereoisomer, wherein the administration of the compound reduces the oxygen cost of contractility.
- provided herein are methods for treating stenosis or restenosis comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
- vascular smooth muscle cells VSMC
- SERCA2a inhibits proliferation through inactivation of calcineurin (PP2B) and its target transcription factor NFAT (nuclear factor of activated T-cells), resulting in lowering of cyclin Dl and pRb levels.
- PP2B calcineurin
- NFAT target transcription factor of activated T-cells
- provided herein are methods for treating a disease associated with neointima formation comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- methods for treating a disease associated with calcineurin (PP2B) comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- methods for treating a disease associated with NFAT comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- Hemodialysis vascular access dysfunction is a cause of hospitalization in the hemodialysis population and is associated with very significant morbidity.
- Roy-Chaudhury et al. American Journal of Kidney Diseases, Vol. 50, No. 5, 2007: pp 782-790, incorporated herein by reference by its entirety.
- arteriovenous fistulas AVFs
- Roy-Chaudhury et al. reported the presence of aggressive neointimal hyperplasia in venous segment specimens from patients with early AVF failure.
- provided herein are methods for treating arteriovenous fistula failure comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
- methods for inhibiting stenosis in AVF comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- a cardiac disease or a disease associated with cardiac disease comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- cardiac diseases include, but are not limited to, ischemia, arrhythmia, myocardial infarction, pulmonary hypertension, transplant rejection, abnormal heart contractility, non-ischemic cardiomyopathy, mitral valve regurgitation, aortic stenosis or regurgitation, or abnormal Ca 2+ metabolism.
- the pulmonary hypertension is primary or secondary.
- the pulmonary hypertension is group 1, 2, 3, 4, or 5 pulonary hypertension, as classified by the Third WHO World Symposium on PAH, Venice 2003.
- the pulmonary hypertension is pulmonary arterial hypertension; pulmonary hypertension with left heart disease pulmonary hypertension associated with lung disorders, hypoxemia, or both; or pulmonary hypertension due to chronic thrombotic or embolic disorders.
- provided herein are methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula I
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
- R x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
- R 1 , n, Q, Y, and J are selected from (i) and (ii) as follows:
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; with the proviso that R 1 is not indolyl; and
- R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
- kits for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula Ia:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
- R x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and not hydrogen, then A is not isopropoxy, if B is para to Y and not hydrogen, then B is not isopropoxy, and if C is para to Y and not hydrogen, then C is not isopropoxy;
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; and
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- provided herein are methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula I
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
- R x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and n is 0;
- J is CH or N
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; and
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is
- the compound of formula I is
- the compound of formula I is:
- the compound of formula I is
- R 1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is: or a pharmaceutically acceptable derivative or stereoisomer thereof [00213] In some embodiments, the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is
- the compound of formula I is or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is
- the compound of formula I is:
- the compound of formula I is:
- A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
- A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
- A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF 3 , or cyano.
- B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
- B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
- B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF 3 , or cyano.
- A when A is para to Y and is not hydrogen, then A is not alkoxy, when B is para to Y and is not hydrogen, then B is not alkoxy, and when C is para to Y and is not hydrogen, then C is not alkoxy.
- At least one of A, B, and C is not hydrogen.
- At least two of A, B, and C is not hydrogen.
- B is hydrogen
- a and C are not hydrogen
- B is hydrogen
- a and C are identical.
- R x is H.
- n is 1-3.
- n is 1-2.
- n 1
- n is 2-4.
- n is 2-3.
- n is 2.
- Q is H or alkyl of 1-3 carbons.
- Q is H or alkyl of 1-2 carbons. [00245] In some embodiments, Q is H or methyl.
- Q is alkyl of 1-3 carbons.
- Q is alkyl of 1-2 carbons.
- Q is methyl
- R 1 is monocyclic.
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR 2 -X.
- R 1 is aryl, heteroaryl, substituted aryl, or NR 2 -X.
- R 1 is aryl, heteroaryl or substituted aryl.
- R 1 is aryl or heteroaryl.
- R 1 is aryl
- R 1 is phenyl
- R 1 is phenyl
- R 1 is phenyl, *o , or
- R 1 is phenyl or
- X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
- X is aryl or aralkyl.
- X is aryl
- X is phenyl
- R 2 is H or alkyl or 1-3 carbons.
- R 2 is H.
- R J is H or alkyl.
- R J is H or alkyl or 1-3 carbons.
- R J is H.
- R 3 and Q are joined together to form ring.
- R 3 and Q are joined together to form a 5-6 membered ring.
- t is 0, and R 3 and Q are joined together to form a 5-6 membered rin g-
- t is 0 and R 3 and Q are joined together to form a 5 membered ring.
- r is 0-2.
- r is 0-1.
- r is 0.
- t is 0-2.
- t is 0-1.
- t is 0.
- r is 0-1 and t is 0.
- r is 0 and t is 0.
- the compound of formula I is N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl
- a and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or
- R 1 is phenyl or y I> ;. or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of fomula I is N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl
- v is an integer from 0 to 3;
- A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO 2 ; wherein at least one of A and B is not H; E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
- R 3 is H or alkyl of 1-3 carbons, Q is methyl, or R 3 and Q are joined together to form a 5-
- R 2 and G are selected from (i) and (ii) as follows:
- R 2 is H or alkyl of 1-3 carbons
- G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 , orG is joined together with R 2 to form a 5-6 membered ring;
- R 2 is H or alkyl of 1-3 carbons
- G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 ; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of fomula I is
- v is an integer from 0 to 3;
- a and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-
- R 3 is H or alkyl of 1-3 carbons, Q is methyl, or R 3 and Q are joined together to form a 5- 6 membered ring; and R 2 and G are selected from (i) and (ii) as follows: (i) v is 0,
- R 2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or
- R 2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 ; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- R >22 CH2)( /— _/
- G is not a substuted or unsubstituted moiety
- a and B are each, independently, H, hydroxyl, or alkoxy of
- R 3 is H or alkyl of 1-3 carbons, Q is methyl, R 2 is H, alkyl of 1-3 carbons, and G is
- the compound of formula I has the following formula: or a pharmaceutically acceptable derivative thereof. [00287] In further embodiments, the compound of formula I has the following formula:
- the compound of formula I is a compound of formula II:
- the compound of formula I is a compound of formula III:
- the compound of formula I is a compound of formula IV:
- the compound of formula I is a compound of formula V:
- the compound of formula I is a compound of formula VI:
- the compound of formula I is a compound of formula VII:
- the compound of formula I is a compound of formula VIII:
- the compound of formula I is a compound of formula IX:
- the compound of formula I is a compound of formula X:
- the compound of formula I is a compound of formula XI:
- the compound of formula I is a compound of formula XII:
- the compound of formula I is a compound of formula XIII:
- the compound of formula I is a compound of formula XIV:
- the compound of formula I is a compound of formula XV:
- the compound of formula I is a compound of formula XVI:
- the compound of formula I is a compound of formula XVII:
- the compound of formula I is a compound of formula XVIII:
- the compound of formula I is a compound of formula XIX:
- the compound of formula I is a compound of formula XX:
- the compound of formula I is a compound of formula XXI:
- the compound of formula I is a compound of formula XXII:
- the compound of formula I is a compound of formula XXIII:
- the compound of formula I is a compound of formula XXIV:
- the compound of formula I is a compound of formula XXV:
- the compound of formula I is a compound of formula XXVI:
- the compound of formula I is a compound of formula XXVII:
- the compound of formula I is a compound of formula XXVIII:
- the compound of formula I is a compound of formula XXIX:
- the compound of formula I is a compound of formula XXX:
- the compound of formula I is a compound of formula XXXI: XXXI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is a compound of formula XXXII:
- the compound of formula I is a compound of formula XXXIII:
- the compound of formula I is a compound of formula XXXIV:
- the compound of formula I is a compound of formula XXXV:
- the compound of formula I is a compound of formula XXXVI:
- the compound of formula I is a compound of formula XXXVII:
- the compound of formula I is a compound of formula
- the compound of formula I is a compound of formula XXXIX:
- the compound of formula I is a compound of formula XL: or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is a compound of formula XLI:
- the compound of formula I is a compound of formula XLII:
- the compound of formula I is a compound of formula XLIII:
- the compound of formula I is a compound of formula XLIV
- the compound of formula I is a compound of formula XLV
- the compound of formula I is a compound of formula XLVI
- the compound of formula I is a compound of formula XLVII
- the compound of formula I is a compound of formula XLVIII
- the compound of formula I is a compound of formula XLIX XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is a compound of formula L
- the compound of formula I is a compound of formula LI
- the compound of formula I is a compound of formula LII
- the compound of formula I is a compound of formula LII
- SERC A2a modulation may also be invoked in the treatment of urinary incontinence diseases.
- SERCA2a modulation may be effected in, inter alia, urethral sphincter muscle cells, urinary bladder muscle cells, pelvic floor muscle cells, detrusor muscle cells, or abdominal muscle cells.
- kits for treating urinary incontinence comprising administering a compound of formula I:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R >x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and R 1 , n, Q, J, and Y are selected from (i) and (ii) as follows: (i) n is an integer from
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R 3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- kits for treating urinary incontinence comprising administering a compound of formula Ia:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; n is an integer from 1-4; Q is H or alkyl; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or
- R 2 is H or alkyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- kits for treating urinary incontinence comprising administering a compound of formula I:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
- R x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and n is 0;
- J is CH or N
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is
- the compound of formula I is or a pharmaceutically acceptable derivative thereof.
- the compound of formula I is:
- the compound of formula I is
- R 1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is: or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is: or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is
- the compound of formula I is
- the compound of formula I is
- the compound of formula I is or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is:
- the compound of formula I is:
- A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
- A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
- A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
- B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
- B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
- B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF 3 , or cyano.
- At least one of A, B, and C is not hydrogen.
- At least two of A, B, and C is not hydrogen.
- B is hydrogen
- a and C are not hydrogen
- B is hydrogen
- a and C are identical.
- R is H.
- n is 1-3.
- n is 1-2.
- n 1
- n is 2-4.
- n is 2-3.
- n is 2.
- Q is H or alkyl of 1-3 carbons.
- Q is H or alkyl of 1-2 carbons.
- Q is H or methyl
- Q is alkyl of 1-3 carbons.
- Q is alkyl of 1-2 carbons.
- Q is methyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR 2 -X.
- R 1 is aryl, heteroaryl, substituted aryl, or NR 2 -X.
- R 1 is aryl, heteroaryl or substituted aryl.
- R 1 is aryl or heteroaryl.
- R 1 is aryl
- R 1 is phenyl
- R 1 is phenyl; , or
- R 1 is phenyl, y I> or
- R 1 is phenyl or y I>
- X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
- X is aryl or aralkyl.
- X is aryl
- X is phenyl
- R 2 is H or alkyl or 1-3 carbons.
- R 2 is H.
- R 3 is H or alkyl.
- R 3 is H or alkyl or 1-3 carbons.
- R is H.
- R and Q are joined together to form ring.
- R and Q are joined together to form a 5-6 membered ring.
- t is 0, and R 3 and Q are joined together to form a 5-6 membered ring.
- t is 0 and R 3 and Q are joined together to form a 5 membered ring.
- r is 0-2.
- r is 0-1.
- r is 0.
- t is 0-2.
- t is 0-1.
- t is 0.
- r is 0-1 and t is 0.
- r is 0 and t is 0.
- the compound of formula I is wherein A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or thereof.
- the compound of fomula I is N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoe
- v is an integer from 0 to 3;
- A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO 2 ; wherein at least one of A and B is not H;
- E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
- R 3 is H or alkyl of 1-3 carbons, Q is methyl, or R 3 and Q are joined together to form a 5- 6 membered ring; and R 2 and G are selected from (i) and (ii) as follows: (i) v is 0,
- R 2 is H or alkyl of 1-3 carbons
- G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 , orG is joined together with R 2 to form a 5-6 membered ring; with the proviso that is not a substituted or unsubstituted
- R 2 is H or alkyl of 1-3 carbons
- G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 ; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of fomula I is
- v is an integer from 0 to 3;
- a and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1- 3 carbons, -CN, azido, or NO 2 ; wherein at least one of A and B is not H;
- R 3 is H or alkyl of 1-3 carbons, Q is methyl, or R 3 and Q are joined together to form a 5- 6 membered ring; and R 2 and G are selected from (i) and (ii) as follows: (i) v is 0,
- R 2 is H or alkyl of 1-3 carbons
- G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO 2 , or G is joined together with R 2 to form a 5-6 membered ring;
- R 2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or
- R >2 CH2)v
- [0 2 J G is not a substuted or unsubstituted moiety
- R2 /— _/ G is not a substuted or unsubstituted moiety
- a and B are each, independently, H, hydroxyl, or alkoxy of 1-3 carbons; R 3 is H or alkyl of 1-3 carbons, Q is methyl, R 2 is H, alkyl of 1-3 carbons, and G is H, or hydroxyl.
- the compound of formula I has the following formula:
- the compound of formula I has the following formula:
- the compound of formula I is a compound of formula II: ii; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is a compound of formula III:
- the compound of formula I is a compound of formula IV:
- the compound of formula I is a compound of formula V:
- the compound of formula I is a compound of formula VI:
- the compound of formula I is a compound of formula VII:
- the compound of formula I is a compound of formula VIII:
- the compound of formula I is a compound of formula IX:
- the compound of formula I is a compound of formula X:
- the compound of formula I is a compound of formula XI:
- the compound of formula I is a compound of formula XII:
- the compound of formula I is a compound of formula XIII:
- the compound of formula I is a compound of formula XIV:
- the compound of formula I is a compound of formula XV:
- the compound of formula I is a compound of formula XVI:
- the compound of formula I is a compound of formula XVII:
- the compound of formula I is a compound of formula XVIII:
- the compound of formula I is a compound of formula XIX:
- the compound of formula I is a compound of formula XX:
- the compound of formula I is a compound of formula XXI:
- the compound of formula I is a compound of formula XXII:
- the compound of formula I is a compound of formula XXIII:
- the compound of formula I is a compound of formula XXIV:
- the compound of formula I is a compound of formula XXV:
- the compound of formula I is a compound of formula XXVI:
- the compound of formula I is a compound of formula XXVII:
- the compound of formula I is a compound of formula XXVIII:
- the compound of formula I is a compound of formula XXIX:
- the compound of formula I is a compound of formula XXX:
- the compound of formula I is a compound of formula XXXI:
- the compound of formula I is a compound of formula XXXII:
- the compound of formula I is a compound of formula XXXIII:
- the compound of formula I is a compound of formula XXXIV:
- the compound of formula I is a compound of formula XXXV:
- the compound of formula I is a compound of formula XXXVI:
- the compound of formula I is a compound of formula XXXVII:
- the compound of formula I is a compound of formula
- the compound of formula I is a compound of formula XXXIX:
- the compound of formula I is a compound of formula XL:
- the compound of formula I is a compound of formula XLI: or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is a compound of formula XLII:
- the compound of formula I is a compound of formula XLIII:
- the compound of formula I is a compound of formula XLIV
- the compound of formula I is a compound of formula XLV
- the compound of formula I is a compound of formula XLVI
- the compound of formula I is a compound of formula XLVII
- the compound of formula I is a compound of formula XLVIII
- the compound of formula I is a compound of formula XLIX
- the compound of formula I is a compound of formula L
- the compound of formula I is a compound of formula LI
- the compound of formula I is a compound of formula LII
- the compound of formula I is a compound of formula LII
- the urinary incontinence is urge incontinence, stress incontinence, urinary retention with overflow incontinence, ectopic ureter, partial or total incompetence of the urinary sphincter, or neurogenic bladder dysfunction.
- the modulation of cytosolic calcium levels has been reported to be associated with apoptosis. See, e.g., Zobos, WO 2004/108083, the entirety of which is incorporated herein by reference. In several different cell types, excessive cytosolic calcium levels has been shown to induce apotosis.
- cyclooxygenase 2 (COX-2) inhibitors such as celecoxib
- COX-2 cyclooxygenase 2
- Sch ⁇ nthal Cancer Lett. (2008), doi: 10.1016/j.canlet.2008.07.005, the entirety of which is incorporated herein by reference. It is thought that the inhibition of COX-2 is not involved in the anticancer effect of celecoxib; rather, celcoxib inhibits tumor growth via inhibition of SERCA. Id. at 4-5. Sch ⁇ nthal is now taking advantage of this discovery by designing and synthesizing new compounds that even more effectively target SERCA, which may lead to the discovery of anticancer agents. Id.
- kits for inducing apoptosis comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound is a SERCA or SERCA2a inhibitor.
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
- R 1 , n, J, Q, and Y are selected from (i) and (ii) as follows:
- n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R 3 is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
- R 2 is H or alkyl; with the proviso that R 1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R 3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- kits for treating cancer comprising administering a compound of formula Ia:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- -NR X R X -COOR X , -CONR X R X , aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SR x , wherein R >x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and not hydrogen, then A is not isopropoxy, if B is para to Y and not hydrogen, then B is not isopropoxy, and if C is para to Y and not hydrogen, then C is not isopropoxy; n is an integer from 1-4;
- Q is H or alkyl
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R 1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- R is H, or alkyl; or R 3 and Q are joined together to form a 5-7 membered ring; or, if R 1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R 3 may be linked to R 1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
- kits for treating cancer comprising administering a compound of formula I:
- A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
- R x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and n is 0;
- J is CH or N
- R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR 2 -X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R 2 is H or alkyl; with the proviso that R 1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O O
- R ⁇ is, independently at each occurrence, H, alkyl, or aryl;
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is or a pharmaceutically acceptable derivative or stereoisomer thereof. [00485] In some embodiments, the compound of formula I is
- the compound of formula I is:
- the compound of formula I is
- R 1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is:
- the compound of formula I is: or a pharmaceutically acceptable derivative or stereoisomer thereof.
- the compound of formula I is:
- the compound of formula I is:
- the compound of formula I is
- the compound of formula I is
- the compound of formula I is or a pharmaceutically acceptable derivative or stereoisomer thereof. [00495] In some embodiments, the compound of formula I is
- the compound of formula I is:
- the compound of formula I is:
- A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
- A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
- A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF 3 , or cyano.
- B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
- B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
- B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
- B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
- At least one of A, B, and C is not hydrogen.
- At least two of A, B, and C is not hydrogen.
- B is hydrogen
- a and C are not hydrogen
- B is hydrogen
- a and C are identical.
- R x is H.
- n is 1-3.
- n is 1-2.
- n 1
- n is 2-4.
- n is 2-3.
- n is 2.
- Q is H or alkyl of 1-3 carbons.
- Q is H or alkyl of 1-2 carbons.
- Q is H or methyl
- Q is alkyl of 1-3 carbons.
- Q is alkyl of 1-2 carbons.
- Q is methyl
- R 1 is monocyclic. [00526] In some embodiments, R 1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR 2 -X.
- R 1 is aryl, heteroaryl, substituted aryl, or NR 2 -X.
- R 1 is aryl, heteroaryl or substituted aryl.
- R 1 is aryl or heteroaryl.
- R 1 is aryl
- R 1 is phenyl
- R 1 is phenyl; . , wherein R is alkyl; , or
- R 1 is phenyl, , or
- R 1 is phenyl or [00535] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl. [00536] In some embodiments, X is aryl or aralkyl. [00537] In some embodiments, X is aryl. [00538] In some embodiments, X is phenyl. [00539] In some embodiments, R 2 is H or alkyl or 1-3 carbons. [00540] In some embodiments, R 2 is H. [00541] In some embodiments, R 3 is H or alkyl.
- R J is H or alkyl or 1-3 carbons.
- R 3 is H.
- R J and Q are joined together to form ring.
- R J and Q are joined together to form a 5-6 membered ring.
- t is 0, and R 3 and Q are joined together to form a 5-6 membered ring.
- t is 0 and R 3 and Q are joined together to form a 5 membered ring.
- r is 0-2.
- r is 0-1.
- r is 0.
- t is 0-2.
- t is 0-1.
- t is 0.
- r is 0-1 and t is 0.
- r is 0 and t is 0.
- a and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or thereof.
- the compound of fomula I is N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoethyl-N-(2-aminoethyl)-2-aminoe
- v is an integer from 0 to 3;
Abstract
Provided herein are compounds, compositions, and methods for treating or ameliorating diseases associated with the modulation of SERCA.
Description
METHODS FOR TREATING DISEASES ASSOCIATED WITH THE MODULATION
OF SERCA
[0001] This application claims priority to U.S. Provisional Application No. 61/148,893, filed January 30, 2009, U.S. Provisional Application No. 61/225,897, filed July 15, 2009, and U.S. Provisional Application No. 61/259,587, filed November 9, 2009, the entireties of which are incorporated herein by reference.
1. FIELD
[0002] Provided herein are compounds, compositions, and methods for treating, preventing, or ameliorating diseases associated with SERCA activity. Further, provided herein are compounds, compositions, and methods for treating or ameliorating heart failure.
2. BACKGROUND
[0003] Sarcoplasmic/endoplasmic reticulum calcium ATP-ase (SERCA) functions to remove calcium ions from the cytoplasm. The modulation of SERCA is associated with various disorders, including but not limited to heart failure, urinary incontinence, cancer, asthma, diabetes, or Alzheimer's disease. Thus, there is a continuing need for compounds, compositions, and methods the modulation of SERCA.
3. SUMMARY
[0004] Provided herein are methods for treating a disease associated with the modulation of SERCA and/or PLB comprising administering a compound described herein. In some embodiments, the disease is heart failure, stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, a disease associated with a cardiac disease, urinary incontinence, cancer, or asthma.
[0005] In some embodiments, provided herein are methods of treating heart failure comprising administering a compound of formula I:
^N^NRXRX ^N^ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and
R1, n, Q, Y, and J are selected from (i) and (ii) as follows:
(i) n is an integer from 1-4;
J is CH;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0006] In some embodiments, provided herein are methods of treating heart failure comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SR x , wherein R >x is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R .3 i •s H, or alkyl; or R > 3 and Q are joined together to form a 5-7 membered ring;
or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0007] In some embodiments, provided herein are methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula I
^N NRXRX ^N ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
R1, n, Q, Y, and J are selected from (i) and (ii) as follows:
(i) n is an integer from 1-4;
J is CH;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl;
or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; with the proviso that R1 is not indolyl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0008] In some embodiments, provided herein are methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof.
[0009] In some embodiments, provided herein are methods of treating urinary incontinence comprising administering a compound of formula I:
I; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
, -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and R1, n, Q, J, and Y are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, Rγ , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY;
wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N-^NRYRY ^N^ORY
-NRYRY, RY , amido, RY , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0010] In some embodiments, provided herein are methods of treating urinary incontinence comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, RY , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0011] In some embodiments, provided herein are methods for treating cancer comprising administering a compound of formula I:
I; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or
-SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
R1, n, J, Q, and Y are selected from (i) and (ii) as follows:
(i) n is an integer from 1-4;
J is CH;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl;
or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
-NRYRY,
-COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N-^NRYRY >s!N^ORY
-NRYRY, RY , amido, RY , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0012] In some embodiments, provided herein are methods for treating cancer comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SR x , wherein R >x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system;
wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, Rγ , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0013] In some embodiments, provided herein are methods of treating asthma or chronic obstructive pulmonary disease comprising administering a compound of formula I:
-NRXRX,
, -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -
SR x wherein Rx is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is
not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and R1, n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3
or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0014] In some embodiments, provided herein are methods of treating asthma or chronic obstructive pulmonary disease comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SR x , wherein R >x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and n is an integer from 1-4; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring;
or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [0015] In some embodiments, provided herein are methods of treating diabetes comprising administering a compound of formula I:
-NRXRX,
, -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or - SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and R1, n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R3 is H, or alkyl;
or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
[0016] In some embodiments, provided herein are methods of treating diabetes comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SR x , wherein R >x is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X,
wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[0017] In some embodiments, provided herein are methods of treating Alzheimer's disease comprising administering a compound of formula I:
I; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
, -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or - SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and R1, n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X,
wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
-NRYRY,
, -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
^(CH2)r ΛN-(CH2)t^ Y is R3 wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; with the proviso that R1 is not indolyl;
wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, Rγ , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
[0018] In some embodiments, provided herein are methods of treating Alzheimer's disease comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
, -COORX, -CONRXRX, aryl, monocylic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl;
or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
-NRYRY,
, -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof. 4. BRIEF DESCRIPTION OF THE FIGURES
[0019] FIG. 1 shows a graph depicting pCA versus ATP ase activity.
[0020] FIG. 2 shows Ca-ATPase dose-response of compound A.
[0021] FIG. 3 shows Ca-ATPase dose-response of compound B.
[0022] FIG. 4 shows representative traces of sarcomere shortening as a reproducible index of contractility.
[0023] FIG. 5 shows the effects of a compound described herein on normal myocytes.
[0024] FIG. 6 shows the effects of a compound described herein on failing myocytes.
[0025] FIG. 7 shows the rate dependence of a compound described herein. The left bar represents the control. The right bar represents the compound.
[0026] FIG. 8 shows the change in sarcomere length for myocytes treated with thapsigargin, a compound described herein, and both thapsigargin and the compound.
[0027] FIG. 9 shows the effects of a compound described herein on cellular action potential.
[0028] FIG. 10 shows ECG traces before (top) and after(bottom) perfusion of a compound described herein.
[0029] FIG. 11 shows a graph depicting percent contractility versus concentration for a compound described herein.
[0030] FIG. 12 shows a table summarizing Rat PK Data for two exemplary compounds.
[0031] FIG. 13 shows the Pmax for a compound described herein in a mouse in vivo hemodynamic study.
[0032] FIG. 14 shows the acute increase in dP/dtmax for a compound described herein in a mouse in vivo hemodynamic study.
[0033] FIG. 15 shows the effects of a compound described herein on cardiac output in a mouse in vivo hemodynamic study.
[0034] FIG. 16 shows the effects of a compound described herein on ejection fraction in a mouse in vivo hemodynamic study.
5. DETAILED DESCRIPTION
5.1. DEFINITIONS
[0035] Unless defined otherwise, all technical and scientific terms used herein have the same meaning as is commonly understood by one of ordinary skill in the art. Where chemical groups, moieties, or substitutents are defined, a dash, a dash bisected with a jagged dash, or a dash dissected with a swiggly line represents a possible point of attachment. Where a chemical moiety or substituent is attached to a ring and where the point of attachment is not on a particular ring atom, the chemical moiety or substituent may be attached to any ring atom. In the event that there are a plurality of definitions for a term used herein, the definitions provided in this section prevail unless stated otherwise.
~I"OR [0036] As used herein, "alkoxy" refers to * s wherein R is alkyl.
[0037] As used herein, "alkyl" refers to a uni-valent hydrocarbon chain or group of about 1 to about 20 carbons. In some embodiments, the alkyl contains about 1 to about 15 carbons. In some embodiments, the alkyl contains about 1 to about 10 carbons. In some embodiments, the alkyl contains about 1 to about 8 carbons. In some embodiments, the alkyl contains about 1 to about 6 carbons. In some embodiments, the alkyl contains about 1 to about 3 carbons. In some embodiments, the alkyl contains 1 to 2 carbons. In some embodiments, the alkyl is primary. In
some embodiments, the alkyl is secondary. In some embodiments, the alkyl is tertiary. In some embodiments, the alkyl is methyl, ethyl, n-propyl, isopropyl, isobutyl, n-butyl, sec-butyl, tert- butyl, isopentyl, neopentyl, tert-pentyl, or isohexyl. In some embodiments, the alkyl is methyl, ethyl, n-propyl, or isopropyl. In some embodiments, the alkyl is methyl. In some embodiments, the alkyl is tert-butyl. In some embodiments, the alkyl is a straight hydrocarbon chain. In some embodiments, the alkyl is a branched hydrocarbon chain.
O
[0038] As used herein, "amido" refers to R' , wherein R' is H or alkyl and R' ' is alkyl.
[0039] As used herein, "aralkyl" refers to * (^H2)n κ ? wherein R aryl and n is an integer from 1 to 20. In some embodiments, n is an 1 to 10. In some embodiments, n is 1-5. In some embodiments, n is 1-3. In some embodiments, n is 1. In some embodiments, n is 2. In some embodiments, n is 3.
[0040] As used herein, "aryl" refers to a uni-valent monocyclic or multicyclic aromatic group containing from 6 to about 30 carbons. In some embodiments, the aryl contains about 6 to about 15 carbons. In some embodiments, the aryl contains about 6 to about 10 carbons. In some embodiments, the aryl is fluorenyl, phenyl, or naphthyl. In some embodiments, the aryl is phenyl. In some embodiments, the aryl in monocyclic.
[0041] As used herein, "cycloalkyl" refers to a univalent monocyclic or multicyclic alkyl group containing from 3 to about 30 carbons. In some embodiments, the cycloalkyl contains 5 to about 15 carbons. In some embodiments, the cycloalkyl contains about 5 to about 10 carbons.
In some embodiments, the cycloalkyl contains about 5 to about 8 carbons. In some embodiments, the cycloalkyl is cyclopentyl or cyclohexyl. In some embodiments, the cycloalkyl is monocyclic.
[0042] As used herein, "halo" refers to halogen. In some embodiments, halo is F, Cl, Br, or
I. In some embodiments, halo is F. In some embodiments, halo is Cl. In some embodiments, halo is Br. In some embodiments, halo is I.
[0043] As used herein, "haloalkyl" refers to a uni-valent alkyl group substituted with one or more halo group or groups. In some embodiments, the haloalkyl is substituted with 1-3 halo
- -CF, group or groups. In some embodiments, the haloalkyl is
[0044] As used herein, "heteroaryl" refers to a uni-valent monocyclic or multicyclic aromatic ring system containing about 5 to about 15 ring atomsm, wherein at least one ring atom is a heteroatom. In some embodiments, the heteroaryl contains 5 to about 10 ring atoms. In some embodiments, the heteroaryl contains 5 or 6 ring atoms. In some embodiments, the heteroaryl is monocyclic. In some embodiments, the heteroatom is N, O, or S. In some embodiments, the heteroaryl contains one heteroatom. In some embodiments, the heteroaryl contains 1 to 3 N atoms. In some embodiments, the heteroaryl contains one O or S atom and one or two N atoms. In some embodiments, the heteroaryl is furyl, imidazolyl, pyrimidinyl, tetrazolyl, thienyl, pyridyl, pyrrolyl, thiazolyl, isothiazolyl, oxazolyl, isoxazolyl, thiazolyl, quinolinyl, or isoquinolinyl. In some embodiments, the heteroaryl is furyl. In some embodiments, the aryl is monocyclic.
[0045] As used herein, "heterocyclyl" refers to a uni-valent monocyclic or multicyclic non- aromatic ring system containing about 3-30 ring atoms, wherein at least one ring atom is a heteroatom. In some embodiments, the heterocyclyl contains 5 to about 10 ring atoms. In some embodiments, the heterocyclyl contains 5 or 6 ring atoms. In some embodiments, the heteroatom is N, O, or S. In some embodiments, the heterocyclyl is monocyclic.
[0046] As used herein, "substituted aryl" and "substituted heteroaryl" refers to an aryl or a
amido,
, -COORZ, -CONRZRZ, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRZ, wherein Rz is, independently at each occurrence, H, alkyl, or aryl, unless otherwise indicated.
[0047] It is to be understood that the compounds provided herein and the compounds used in the compositions and methods provided herein may contain chiral centers. Such chiral centers may be of either the (R) or (S) configuration, or may be a mixture thereof. Thus, the compounds provided herein may be enantiomerically pure, or be stereoisomeric or diastereomeric mixtures. In some embodiments, a given stereoisomer of a compound descried herein or given stereoisomer of a compound used in the compositions and methods described herein is substantially free of other stereoisomers. The term "substantially free" means that a given
compound is at least 80% by weight of a given stereoisomer. In some embodiments, the compound is at least 85% by weight of a given stereoisomer. In some embodiments, the compound is at least 90% by weight of a given stereoisomer. In some embodiments, the compound is at least 95% by weight of a given stereoisomer. In some embodiments, the compound is at least 98% by weight of a given stereoisomer.
[0048] One of skill in the art will recognize that administration of a compound in its (R) form is equivalent, for compounds that undergo epimerization in vivo, to administration of the compound in its (S) form.
[0049] It is to be understood that the compounds described herein may be a pharmaceutically acceptable derivative. Pharmaceutically acceptable derivatives of a compound include, but are not limited to, salts, esters, enol ethers, enol esters, acetals, ketals, orthoesters, hemiacetals, hemiketals, acids, bases, solvates or hydrates thereof. Such derivatives may be readily prepared by those of skill in this art using known methods for such derivatization. Pharmaceutically acceptable salts include, but are not limited to, amine salts, such as but not limited to N5N'- dibenzylethylenediamine, chloroprocaine, choline, ammonia, diethanolamine and other hydroxyalkylamines, ethylenediamine, N-methylglucamine, procaine, N-benzylphenethylamine, l-para-chlorobenzyl-2-pyrrolidin-r-ylmethylbenzimidazole, diethylamine and other alkylamines, piperazine and tris(hydroxymethyl)aminomethane; alkali metal salts, such as but not limited to lithium, potassium and sodium; alkali earth metal salts, such as but not limited to barium, calcium and magnesium; transition metal salts, such as but not limited to zinc; and inorganic salts, such as but not limited to, sodium hydrogen phosphate and disodium phosphate; and also including, but not limited to, salts of mineral acids, such as but not limited to hydrochlorides and sulfates; and salts of organic acids, such as but not limited to acetates, lactates, malates, tartrates, citrates, ascorbates, succinates, butyrates, valerates, mesylates, and fumarates. Pharmaceutically acceptable esters include, but are not limited to, alkyl, alkenyl, alkynyl, aryl, aralkyl, and cycloalkyl esters of acidic groups, including, but not limited to, carboxylic acids, phosphoric acids, phosphinic acids, sulfonic acids, sulfϊnic acids and boronic acids. Pharmaceutically acceptable enol ethers include, but are not limited to, derivatives of formula C=C(OR) where R is alkyl, alkenyl, alkynyl, aryl, aralkyl and cycloalkyl. Pharmaceutically acceptable enol esters include, but are not limited to, derivatives of formula C=C(OC(O)R) where R is hydrogen, alkyl, alkenyl, alkynyl, aryl, aralkyl and cycloalkyl.
Pharmaceutically acceptable solvates and hydrates are complexes of a compound with one or more solvent or water molecules, or 1 to about 100, or 1 to about 10, or one to about 2, 3 or 4, solvent or water molecules. It is to be understood that the pharmaceutically acceptable derivates described herein include, but are not limited to, produgs. Prodrugs include, but are not limited to, a derivative of a compound that reacts under biological conditions (in vitro or in vivo) to provide that compound. In some embodiments, such derivative hydrolyzes or oxidizes under biological conditions to provide the compound. Examples of prodrugs include, but are not limited to, derivatives that comprise biohydrolyzable moieties such as biohydrolyzable amides, biohydrolyzable esters, biohydrolyzable carbamates, biohydrolyzable carbonates, biohydrolyzable ureides, and biohydrolyzable phosphate analogs. Prodrugs can typically be prepared using well-known methods, such as those described in 1 Burger's Medicinal Chemistry and Drug Discovery, 172-178, 949-982 (Manfred E. Wolff ed., 5th ed. 1995), and Design of Prodrugs (H. Bundgaard ed., Elselvier, New York 1985, incorporated herein by reference in its entirety). 5.2. METHODS OF TREATMENT
[0050] Without being bound by any theory, the Ca-ATP ase (SERCA) of the sarcoplasmic reticulum (SR) functions to remove calcium ions from the cytoplasm. Without being bound by any theory, SERCA re-sequesters the calcium back into the internal sacroplasmic reticulum pool, thus priming the next quantal release of calcium.
[0051] In some embodiments, provided herein are methods of treating a disease associated with the modulation of SERCA comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods of treating a disease associated with the modulation of SERC A2 comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods of treating a disease associated with the modulation of SERCA2a comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods of treating a disease associated with the modulation of SERCA2b comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
[0052] In some embodiments, provided herein are methods of treating a disease associated with decreased SERCA2a activity comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods of treating a disease associated with the modulation of SERC A2a via increasing the activity of SERCA2a comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
[0053] Without being bound by any theory, in cardiac muscle, SERCA2 regulates cardiac function by promoting both cardiac relaxation and contractility. Furthermore, in cardiac muscle, SERCA2a is regulated by phospholamban (PLB), which inhibits SERCA at submicromolar calcium. The regulation of PLB influences cardiac relaxation and contractility. Phosphorylation of PLB by protein kinase A causes PLB to release its inhibitory grip on SERC A2. Abnormal PLB/SERC A2 ratios and associated defective calcium cycling is a feature of some forms of human and experimental heart failure. Therefore, without being bound by any theory, compounds that have the capacity to increase SERC A2 activity or release inhibition of SERCA2 by interacting with the SERC A2 -PLB interface may play a role in improving myocyte contractility and cardiac function.
[0054] In some embodiments, provided herein are methods for treating diseases associated with phospholamban activity or phospholamban levels by administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. [0055] In some embodiments, provided herein are methods for treating a disease associated with the modulation of SERCA and/or PLB comprising administering a compound described herein. In some embodiments, the disease is heart failure, stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, a disease associated with a cardiac disease, urinary incontinence, cancer, asthma, diabetes, or Alzheimer's disease. [0056] In some embodiments, provided herein are methods of treating heart failure comprising administering a compound of formula I:
i; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and
R1, n, Q, Y, and J are selected from (i) and (ii) as follows:
(i) n is an integer from 1-4;
J is CH;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X,
wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof. [0057] In some embodiments, provided herein are methods of treating heart failure comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRXR* >*N^ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof. [0058] In some embodiments, provided herein are methods of treating heart failure comprising administering a compound of formula I:
I; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
^N NRXRX *'H ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and n is 0;
J is CH or N;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof. [0059] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0060] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0061] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [0062] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [0063] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0064] In some embodiments, the compound of formula I is
wherein R1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[0065] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof [0066] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0067] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0068] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0069] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0070] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0071] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0072] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [0073] In some embodiments, the compound of formula I is:
[0074] In some embodiments, the compound of formula I is:
[0075] In some embodiments, A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[0076] In some embodiments, A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro. [0077] In some embodiments, A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[0078] In some embodiments, A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[0079] In some embodiments, A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[0080] In some embodiments, B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[0081] In some embodiments, B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[0082] In some embodiments, B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[0083] In some embodiments, B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[0084] In some embodiments, at least one of A, B, and C is not hydrogen. [0085] In some embodiments, at least two of A, B, and C is not hydrogen. [0086] In some embodiments, B is hydrogen, and A and C are not hydrogen. [0087] In some embodiments, B is hydrogen, A and C are identical. [0088] In some embodiments, when A is para to Y and is not hydrogen, then A is not isopropoxy, when B is para to Y and is not hydrogen, then B is not isopropoxy, and when C is para to Y and is not hydrogen, then C is not isopropoxy.
[0089] In some embodiments, when A is para to Y and is not hydrogen, then A is not alkoxy, when B is para to Y and is not hydrogen, then B is not alkoxy, and when C is para to Y and is not hydrogen, then C is not alkoxy. [0090] In some embodiments, Rx is H.
[0091] In some embodiments, n is 1-3.
[0092] In some embodiments, n is 1-2.
[0093] In some embodiments, n is 1.
[0094] In some embodiments, n is 2-4.
[0095] In some embodiments, n is 2-3.
[0096] In some embodiments, n is 2.
[0097] In some embodiments, Q is H or alkyl of 1-3 carbons.
[0098] In some embodiments, Q is H or alkyl of 1-2 carbons.
[0099] In some embodiments, Q is H or methyl.
[00100] In some embodiments, Q is alkyl of 1-3 carbons.
[00101] In some embodiments, Q is alkyl of 1-2 carbons.
[00102] In some embodiments, Q is methyl.
[00103] In some embodiments, R1 is monocyclic.
[00104] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR2-X.
[00105] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, or NR2-X.
[00106] In some embodiments, R1 is aryl, heteroaryl or substituted aryl.
[00107] In some embodiments, R1 is aryl or heteroaryl.
[00108] In some embodiments, R1 is aryl.
[00109] In some embodiments, R1 is phenyl.
[00112] In some embodiments, R1 is
phenyl or [00113] In some embodiments, R1 is not indolyl.
[00114] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
[00115] In some embodiments, X is aryl or aralkyl.
[00116] In some embodiments, X is aryl.
[00117] In some embodiments, X is phenyl.
[00118] In some embodiments, R2 is H or alkyl or 1-3 carbons.
[00119] In some embodiments, R2 is H.
[00120] In some embodiments, R3 is H or alkyl.
[00121] In some embodiments, R3 is H or alkyl or 1-3 carbons.
[00122] In some embodiments, R3 is H.
[00123] In some embodiments, R3 and Q are joined together to form ring.
[00124] In some embodiments, R and Q are joined together to form a 5-6 membered ring.
[00125] In some embodiments, t is 0, and R3 and Q are joined together to form a 5-6 membered rin g-
[00126] In some embodiments, t is 0 and R3 and Q are joined together to form a 5 membered ring.
[00127] In some embodiments, r is 0-2.
[00128] In some embodiments, r is 0-1.
[00129] In some embodiments, r is 0.
[00130] In some embodiments, t is 0-2.
[00131] In some embodiments, t is 0-1.
[00132] In some embodiments, t is 0.
[00133] In some embodiments, r is 0-1 and t is 0.
[00134] In some embodiments, r is 0 and t is 0.
[00135] In some embodiments, the compound of formula I is
wherein A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or
thereof.
[00136] In some embodiments, the compound of fomula I is
A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2, orG is joined together with R2 to form a 5-6 membered ring;
with the proviso that R2 G ' is not a substituted or unsubstituted
moiety having the formula:
; and (ii) v is 1 to 3, R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00137] In some embodiments, the compound of fomula I is
A and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1- 3 carbons, -CN, azido, or NO2; wherein at least one of A and B is not H;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, or G is joined together with R2 to form a 5-6 membered ring;
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
Λ R2 _/
[00138] In some embodiments, / G— is not a substuted or unsubstituted moiety
[00139] In some embodiments, R2 is not a substuted or unsubstituted moiety
[00140] In further embodiments, A and B are each, independently, H, hydroxyl, or alkoxy of 1-3 carbons; R3 is H or alkyl of 1-3 carbons, Q is methyl, R2 is H, alkyl of 1-3 carbons, and G is H, or hydroxyl.
[00141] In further embodiments, the compound of formula I has the following formula:
[00142] In further embodiments, the compound of formula I has the following formula:
[00143] In some embodiments, the compound of formula I is a compound of formula II:
[00144] In some embodiments, the compound of formula I is a compound of formula III:
III; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00145] In some embodiments, the compound of formula I is a compound of formula IV:
IV; a pharmaceutically acceptable derivative or stereoisomer thereof.
[00146] In some embodiments, the compound of formula I is a compound of formula V:
V; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00147] In some embodiments, the compound of formula I is a compound of formula VI:
VI; or a pharmaceutically acceptable derivative thereof.
VII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00149] In some embodiments, the compound of formula I is a compound of formula VIII:
VIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00150] In some embodiments, the compound of formula I is a compound of formula IX:
IX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00151] In some embodiments, the compound of formula I is a compound of formula X:
X; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00152] In some embodiments, the compound of formula I is a compound of formula XI:
[00153] In some embodiments, the compound of formula I is a compound of formula XII:
XII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00154] In some embodiments, the compound of formula I is a compound of formula XIII:
[00155] In some embodiments, the compound of formula I is a compound of formula XIV:
XIV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00156] In some embodiments, the compound of formula I is a compound of formula XV:
XV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00158] In some embodiments, the compound of formula I is a compound of formula XVII:
XVII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00159] In some embodiments, the compound of formula I is a compound of formula XVIII:
XVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00160] In some embodiments, the compound of formula I is a compound of formula XIX:
XIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00161] In some embodiments, the compound of formula I is a compound of formula XX:
XX;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00162] In some embodiments, the compound of formula I is a compound of formula XXI:
XXI; or a pharmaceutically acceptable derivative thereof. [00163] In some embodiments, the compound of formula I is a compound of formula XXII:
XXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00164] In some embodiments, the compound of formula I is a compound of formula XXIII:
XXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00165] Is some embodiments, the compound of formula I is a compound of formula XXIV:
XXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00166] In some embodiments, the compound of formula I is a compound of formula XXV:
XXV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00167] In some embodiments, the compound of formula I is a compound of formula XXVI:
XXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00168] In some embodiments, the compound of formula I is a compound of formula XXVII:
XXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00169] In some embodiments, the compound of formula I is a compound of formula XXVIII:
XXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00170] In some embodiments, the compound of formula I is a compound of formula XXIX:
XXIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00171] In some embodiments, the compound of formula I is a compound of formula XXX:
XXX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00172] In some embodiments, the compound of formula I is a compound of formula XXXI:
[00173] In some embodiments, the compound of formula I is a compound of formula XXXII:
XXXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XXXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00175] In some embodiments, the compound of formula I is a compound of formula XXXIV:
XXXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00176] In some embodiments, the compound of formula I is a compound of formula XXXV:
XXXV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00177] In some embodiments, the compound of formula I is a compound of formula XXXVI:
XXXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00178] In some embodiments, the compound of formula I is a compound of formula XXXVII:
XXXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00179] In some embodiments, the compound of formula I is a compound of formula
[00180] In some embodiments, the compound of formula I is a compound of formula XXXIX:
[00181] In some embodiments, the compound of formula I is a compound of formula XL:
[00182] In some embodiments, the compound of formula I is a compound of formula XLI:
XLII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00184] In some embodiments, the compound of formula I is a compound of formula XLIII:
XLIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00185] In some embodiments, the compound of formula I is a compound of formula XLIV
XLIV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00186] In some embodiments, the compound of formula I is a compound of formula XLV
XLV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00187] In some embodiments, the compound of formula I is a compound of formula XLVI
XLVI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00188] In some embodiments, the compound of formula I is a compound of formula XLVII
XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof. [00189] In some embodiments, the compound of formula I is a compound of formula XLVIII
XLVIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00190] In some embodiments, the compound of formula I is a compound of formula XLIX
XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof. [00191] In some embodiments, the compound of formula I is a compound of formula L
L or a pharmaceutically acceptable derivative or stereoisomer thereof. [00192] In some embodiments, the compound of formula I is a compound of formula LI
LI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00193] In some embodiments, the compound of formula I is a compound of formula LII
LII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00194] In some embodiments, the compound of formula I is a compound of formula LII
LIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00195] In some embodiments, the heart failure is acute heart failure, chronic heart failure, end-stage heart failure, congestive heart failure, right heart failure, left heart failure, forward heart failure, backward heart failure, Class I, II, III, or IV heart failure as defined by New York Heart Association Functional Classification, systolic heart failure, diastolic heart failure, low- output heart failure, or high-output heart failure.
[00196] The search for positive inotropic drugs, i.e., drugs that increase contractility, that can be used to treat heart failure has been extensive However, all current inotropic drugs that increase contractility also have a negative effect on long term survival. In some embodiments, provided herein are methods for treating heart failure comprising administering a compound of formula I, or a pharmaceutically acceptable derivative or stereoisomer, wherein the administration of the compound results in improved survival. In some embodiments, provided herein are methods for treating heart failure comprising administering a compound of formula I, or a pharmaceutically acceptable derivative or stereoisomer, wherein the administration of the compound reduces the oxygen cost of contractility.
[00197] In some embodiments, provided herein are methods for treating stenosis or restenosis comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00198] Lipskaia et al. reported that increasing SERC A2a activity inhibits vascular smooth muscle cells (VSMC) proliferation and neointima formation. Circulation Research. 2005; 97(5): 488-495, incorporated herein by reference by its entirety. It was reported that SERCA2a inhibits
proliferation through inactivation of calcineurin (PP2B) and its target transcription factor NFAT (nuclear factor of activated T-cells), resulting in lowering of cyclin Dl and pRb levels. [00199] In some embodiments, provided herein are methods for treating a disease associated with vascular smooth muscle cell proliferation comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods for treating a disease associated with neointima formation comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods for treating a disease associated with calcineurin (PP2B) comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods for treating a disease associated with NFAT comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00200] Hemodialysis vascular access dysfunction is a cause of hospitalization in the hemodialysis population and is associated with very significant morbidity. Roy-Chaudhury et al., American Journal of Kidney Diseases, Vol. 50, No. 5, 2007: pp 782-790, incorporated herein by reference by its entirety. While arteriovenous fistulas (AVFs) are used as a permanent form of dialysis access, they have significant problems with early AVF failure. Roy-Chaudhury et al. reported the presence of aggressive neointimal hyperplasia in venous segment specimens from patients with early AVF failure.
[00201] In some embodiments, provided herein are methods for treating arteriovenous fistula failure comprising administering a compound described herein or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, provided herein are methods for inhibiting stenosis in AVF comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00202] In some embodiments, provided herein are methods for treating a cardiac disease or a disease associated with cardiac disease comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof. Such cardiac diseases include, but are not limited to, ischemia, arrhythmia, myocardial infarction, pulmonary hypertension, transplant rejection, abnormal heart contractility, non-ischemic cardiomyopathy, mitral valve regurgitation, aortic stenosis or regurgitation, or abnormal Ca2+ metabolism. In
some embodiments, the pulmonary hypertension is primary or secondary. In some embodiments, the pulmonary hypertension is group 1, 2, 3, 4, or 5 pulonary hypertension, as classified by the Third WHO World Symposium on PAH, Venice 2003. In some embodiments, the pulmonary hypertension is pulmonary arterial hypertension; pulmonary hypertension with left heart disease pulmonary hypertension associated with lung disorders, hypoxemia, or both; or pulmonary hypertension due to chronic thrombotic or embolic disorders.
[00203] In some embodiments, provided herein are methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula I
^N^NRXRX >^N^ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
R1, n, Q, Y, and J are selected from (i) and (ii) as follows:
(i) n is an integer from 1-4;
J is CH;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl;
with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; with the proviso that R1 is not indolyl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00204] In some embodiments, provided herein are methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula Ia:
^N^NRXRX ^N^ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and not hydrogen, then A is not isopropoxy, if B is para to Y and not hydrogen, then B is not isopropoxy, and if C is para to Y and not hydrogen, then C is not isopropoxy; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00205] In some embodiments, provided herein are methods for treating stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with
NFAT, arteriovenous fistula failure, a cardiac disease, or a disease associated with a cardiac disease comprising administering a compound of formula I
^N NRXRX ^N ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and n is 0;
J is CH or N;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof. [00206] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00207] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00208] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [00209] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [00210] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00211] In some embodiments, the compound of formula I is
wherein R1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00212] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof [00213] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00214] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00215] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00216] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00217] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00218] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00219] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00220] In some embodiments, the compound of formula I is:
[00221] In some embodiments, the compound of formula I is:
[00222] In some embodiments, A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00223] In some embodiments, A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00224] In some embodiments, A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00225] In some embodiments, A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00226] In some embodiments, A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00227] In some embodiments, B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00228] In some embodiments, B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00229] In some embodiments, B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00230] In some embodiments, B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00231] In some embodiments, when A is para to Y and is not hydrogen, then A is not alkoxy, when B is para to Y and is not hydrogen, then B is not alkoxy, and when C is para to Y and is not hydrogen, then C is not alkoxy.
[00232] In some embodiments, at least one of A, B, and C is not hydrogen.
[00233] In some embodiments, at least two of A, B, and C is not hydrogen.
[00234] In some embodiments, B is hydrogen, and A and C are not hydrogen.
[00235] In some embodiments, B is hydrogen, A and C are identical.
[00236] In some embodiments, Rx is H.
[00237] In some embodiments, n is 1-3.
[00238] In some embodiments, n is 1-2.
[00239] In some embodiments, n is 1.
[00240] In some embodiments, n is 2-4.
[00241] In some embodiments, n is 2-3.
[00242] In some embodiments, n is 2.
[00243] In some embodiments, Q is H or alkyl of 1-3 carbons.
[00244] In some embodiments, Q is H or alkyl of 1-2 carbons.
[00245] In some embodiments, Q is H or methyl.
[00246] In some embodiments, Q is alkyl of 1-3 carbons.
[00247] In some embodiments, Q is alkyl of 1-2 carbons.
[00248] In some embodiments, Q is methyl.
[00249] In some embodiments, R1 is monocyclic.
[00250] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR2-X.
[00251] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, or NR2-X.
[00252] In some embodiments, R1 is aryl, heteroaryl or substituted aryl.
[00253] In some embodiments, R1 is aryl or heteroaryl.
[00254] In some embodiments, R1 is aryl.
[00255] In some embodiments, R1 is phenyl.
[00259] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
[00260] In some embodiments, X is aryl or aralkyl.
[00261] In some embodiments, X is aryl.
[00262] In some embodiments, X is phenyl.
[00263] In some embodiments, R2 is H or alkyl or 1-3 carbons.
[00264] In some embodiments, R2 is H.
[00265] In some embodiments, RJ is H or alkyl.
[00266] In some embodiments, RJ is H or alkyl or 1-3 carbons.
[00267] In some embodiments, RJ is H.
[00268] In some embodiments, R3 and Q are joined together to form ring.
[00269] In some embodiments, R3 and Q are joined together to form a 5-6 membered ring.
[00270] In some embodiments, t is 0, and R3 and Q are joined together to form a 5-6 membered rin g-
[00271] In some embodiments, t is 0 and R3 and Q are joined together to form a 5 membered ring.
[00272] In some embodiments, r is 0-2.
[00273] In some embodiments, r is 0-1.
[00274] In some embodiments, r is 0.
[00275] In some embodiments, t is 0-2.
[00276] In some embodiments, t is 0-1.
[00277] In some embodiments, t is 0.
[00278] In some embodiments, r is 0-1 and t is 0.
[00279] In some embodiments, r is 0 and t is 0.
[00280] In some embodiments, the compound of formula I is
-CF3; and R1 is phenyl or yI> ;. or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00281] In some embodiments, the compound of fomula I is
A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5-
6 membered ring; and R2 and G are selected from (i) and (ii) as follows:
(i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, orG is joined together with R2 to form a 5-6 membered ring;
(ii) v is 1 to 3,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00282] In some embodiments, the compound of fomula I is
A and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-
3 carbons, -CN, azido, or NO2; wherein at least one of A and B is not H;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows:
(i) v is 0,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or
NO2, or G is joined together with R2 to form a 5-6 membered ring;
with the proviso that is not a substituted or unsubstituted
(ii) v is 1-3,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
0 me embodiments, R >22 CH2)( /— _/
[00283] In so G is not a substuted or unsubstituted moiety
having the formula
[00285] In further embodiments, A and B are each, independently, H, hydroxyl, or alkoxy of
1-3 carbons; R3 is H or alkyl of 1-3 carbons, Q is methyl, R2 is H, alkyl of 1-3 carbons, and G is
H, or hydroxyl.
[00286] In further embodiments, the compound of formula I has the following formula:
or a pharmaceutically acceptable derivative thereof. [00287] In further embodiments, the compound of formula I has the following formula:
[00288] In some embodiments, the compound of formula I is a compound of formula II:
[00289] In some embodiments, the compound of formula I is a compound of formula III:
HI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00290] In some embodiments, the compound of formula I is a compound of formula IV:
IV; a pharmaceutically acceptable derivative or stereoisomer thereof.
[00291] In some embodiments, the compound of formula I is a compound of formula V:
V; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00292] In some embodiments, the compound of formula I is a compound of formula VI:
VI; or a pharmaceutically acceptable derivative thereof.
[00293] In some embodiments, the compound of formula I is a compound of formula VII:
VII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00294] In some embodiments, the compound of formula I is a compound of formula VIII:
VIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
IX; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00296] In some embodiments, the compound of formula I is a compound of formula X:
X; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00297] In some embodiments, the compound of formula I is a compound of formula XI:
XI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00298] In some embodiments, the compound of formula I is a compound of formula XII:
XII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00299] In some embodiments, the compound of formula I is a compound of formula XIII:
[00300] In some embodiments, the compound of formula I is a compound of formula XIV:
XIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00301] In some embodiments, the compound of formula I is a compound of formula XV:
XV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00302] In some embodiments, the compound of formula I is a compound of formula XVI:
XVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00303] In some embodiments, the compound of formula I is a compound of formula XVII:
XVII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00304] In some embodiments, the compound of formula I is a compound of formula XVIII:
XVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00305] In some embodiments, the compound of formula I is a compound of formula XIX:
XIX; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00306] In some embodiments, the compound of formula I is a compound of formula XX:
XX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00307] In some embodiments, the compound of formula I is a compound of formula XXI:
XXI; or a pharmaceutically acceptable derivative thereof. [00308] In some embodiments, the compound of formula I is a compound of formula XXII:
XXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00309] In some embodiments, the compound of formula I is a compound of formula XXIII:
XXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00310] Is some embodiments, the compound of formula I is a compound of formula XXIV:
XXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00311] In some embodiments, the compound of formula I is a compound of formula XXV:
XXV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00312] In some embodiments, the compound of formula I is a compound of formula XXVI:
XXVI;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00313] In some embodiments, the compound of formula I is a compound of formula XXVII:
XXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00314] In some embodiments, the compound of formula I is a compound of formula XXVIII:
XXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00315] In some embodiments, the compound of formula I is a compound of formula XXIX:
[00316] In some embodiments, the compound of formula I is a compound of formula XXX:
XXX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00317] In some embodiments, the compound of formula I is a compound of formula XXXI:
XXXI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00318] In some embodiments, the compound of formula I is a compound of formula XXXII:
XXXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00319] In some embodiments, the compound of formula I is a compound of formula XXXIII:
XXXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00320] In some embodiments, the compound of formula I is a compound of formula XXXIV:
XXXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00321] In some embodiments, the compound of formula I is a compound of formula XXXV:
XXXV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XXXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00323] In some embodiments, the compound of formula I is a compound of formula XXXVII:
XXXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00324] In some embodiments, the compound of formula I is a compound of formula
XXXVIII:
[00325] In some embodiments, the compound of formula I is a compound of formula XXXIX:
[00326] In some embodiments, the compound of formula I is a compound of formula XL:
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00327] In some embodiments, the compound of formula I is a compound of formula XLI:
[00328] In some embodiments, the compound of formula I is a compound of formula XLII:
XLII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00329] In some embodiments, the compound of formula I is a compound of formula XLIII:
XLIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
XLIV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00331] In some embodiments, the compound of formula I is a compound of formula XLV
XLV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00332] In some embodiments, the compound of formula I is a compound of formula XLVI
XLVI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00333] In some embodiments, the compound of formula I is a compound of formula XLVII
XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof. [00334] In some embodiments, the compound of formula I is a compound of formula XLVIII
XLVIII or a pharmaceutically acceptable derivative or stereoisomer thereof. [00335] In some embodiments, the compound of formula I is a compound of formula XLIX
XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof. [00336] In some embodiments, the compound of formula I is a compound of formula L
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00337] In some embodiments, the compound of formula I is a compound of formula LI
LI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00338] In some embodiments, the compound of formula I is a compound of formula LII
LII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00339] In some embodiments, the compound of formula I is a compound of formula LII
LIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00340] SERC A2a modulation may also be invoked in the treatment of urinary incontinence diseases. SERCA2a modulation may be effected in, inter alia, urethral sphincter muscle cells,
urinary bladder muscle cells, pelvic floor muscle cells, detrusor muscle cells, or abdominal muscle cells.
[00341] In some embodiments, provided herein are methods of treating urinary incontinence comprising administering a compound of formula I:
-NRXRX,
, -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SR x , wherein R >x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and R1, n, Q, J, and Y are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system;
wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, Rγ , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY >*N^ORY
-NRYRY, RY , amido, RY , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 and Q are joined together to form a 5-7 membered ring;
and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00342] In some embodiments, provided herein are methods of treating urinary incontinence comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
, -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; n is an integer from 1-4; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
O O
, Jl s X
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, Rγ , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY;
wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00343] In some embodiments, provided herein are methods of treating urinary incontinence comprising administering a compound of formula I:
I; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
^N NRXRX *'H ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, heteroaryl, heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and n is 0;
J is CH or N;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
O O
, Jl , JJ
-NRYRY, RY , amido, Rγ , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00344] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00345] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00346] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [00347] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof.. [00348] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00349] In some embodiments, the compound of formula I is
wherein R1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00350] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof [00351] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00352] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00353] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00354] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00355] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00356] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00357] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00358] In some embodiments, the compound of formula I is:
[00359] In some embodiments, the compound of formula I is:
[00360] In some embodiments, A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00361] In some embodiments, A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro. [00362] In some embodiments, A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00363] In some embodiments, A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00364] In some embodiments, A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00365] In some embodiments, B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00366] In some embodiments, B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00367] In some embodiments, B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00368] In some embodiments, B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00369] In some embodiments, at least one of A, B, and C is not hydrogen.
[00370] In some embodiments, at least two of A, B, and C is not hydrogen.
[00371] In some embodiments, B is hydrogen, and A and C are not hydrogen.
[00372] In some embodiments, B is hydrogen, A and C are identical.
[00373] In some embodiments, R is H.
[00374] In some embodiments, n is 1-3.
[00375] In some embodiments, n is 1-2.
[00376] In some embodiments, n is 1.
[00377] In some embodiments, n is 2-4.
[00378] In some embodiments, n is 2-3.
[00379] In some embodiments, n is 2.
[00380] In some embodiments, Q is H or alkyl of 1-3 carbons.
[00381] In some embodiments, Q is H or alkyl of 1-2 carbons.
[00382] In some embodiments, Q is H or methyl.
[00383] In some embodiments, Q is alkyl of 1-3 carbons.
[00384] In some embodiments, Q is alkyl of 1-2 carbons.
[00385] In some embodiments, Q is methyl.
[00386] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR2-X.
[00387] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, or NR2-X.
[00388] In some embodiments, R1 is aryl, heteroaryl or substituted aryl.
[00389] In some embodiments, R1 is aryl or heteroaryl.
[00390] In some embodiments, R1 is aryl.
[00391] In some embodiments, R1 is phenyl.
[00394] In some embodiments, R1 is phenyl or yI>
[00395] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
[00396] In some embodiments, X is aryl or aralkyl.
[00397] In some embodiments, X is aryl.
[00398] In some embodiments, X is phenyl.
[00399] In some embodiments, R2 is H or alkyl or 1-3 carbons.
[00400] In some embodiments, R2 is H.
[00401] In some embodiments, R3 is H or alkyl.
[00402] In some embodiments, R3 is H or alkyl or 1-3 carbons.
[00403] In some embodiments, R is H.
[00404] In some embodiments, R and Q are joined together to form ring.
[00405] In some embodiments, R and Q are joined together to form a 5-6 membered ring.
[00406] In some embodiments, t is 0, and R3 and Q are joined together to form a 5-6 membered ring.
[00407] In some embodiments, t is 0 and R3 and Q are joined together to form a 5 membered ring.
[00408] In some embodiments, r is 0-2.
[00409] In some embodiments, r is 0-1.
[00410] In some embodiments, r is 0.
[00411] In some embodiments, t is 0-2.
[00412] In some embodiments, t is 0-1.
[00413] In some embodiments, t is 0.
[00414] In some embodiments, r is 0-1 and t is 0.
[00415] In some embodiments, r is 0 and t is 0.
[00416] In some embodiments, the compound of formula I is
wherein A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or
thereof.
[00417] In some embodiments, the compound of fomula I is
A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, orG is joined together with R2 to form a 5-6 membered ring;
with the proviso that is not a substituted or unsubstituted
(ii) v is 1 to 3,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, the compound of fomula I is
A and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1- 3 carbons, -CN, azido, or NO2; wherein at least one of A and B is not H;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, or G is joined together with R2 to form a 5-6 membered ring;
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or
NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
0419] In some embodiments, R >2 CH2)v
[0 2 J G is not a substuted or unsubstituted moiety
Λ
[00420] In some embodiments, R2 /— _/ G is not a substuted or unsubstituted moiety
[00421] In further embodiments, A and B are each, independently, H, hydroxyl, or alkoxy of 1-3 carbons; R3 is H or alkyl of 1-3 carbons, Q is methyl, R2 is H, alkyl of 1-3 carbons, and G is H, or hydroxyl.
[00422] In further embodiments, the compound of formula I has the following formula:
[00423] In further embodiments, the compound of formula I has the following formula:
B
O Q R2 Q . or a pharmaceutically acceptable derivative thereof.
[00424] In some embodiments, the compound of formula I is a compound of formula II:
ii; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00425] In some embodiments, the compound of formula I is a compound of formula III:
HI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00426] In some embodiments, the compound of formula I is a compound of formula IV:
IV; a pharmaceutically acceptable derivative or stereoisomer thereof.
[00427] In some embodiments, the compound of formula I is a compound of formula V:
V; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00428] In some embodiments, the compound of formula I is a compound of formula VI:
VI; or a pharmaceutically acceptable derivative thereof.
[00429] In some embodiments, the compound of formula I is a compound of formula VII:
VII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00430] In some embodiments, the compound of formula I is a compound of formula VIII:
VIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00431] In some embodiments, the compound of formula I is a compound of formula IX:
IX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00432] In some embodiments, the compound of formula I is a compound of formula X:
[00433] In some embodiments, the compound of formula I is a compound of formula XI:
[00434] In some embodiments, the compound of formula I is a compound of formula XII:
XII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00435] In some embodiments, the compound of formula I is a compound of formula XIII:
XIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00436] In some embodiments, the compound of formula I is a compound of formula XIV:
XIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00437] In some embodiments, the compound of formula I is a compound of formula XV:
XV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00438] In some embodiments, the compound of formula I is a compound of formula XVI:
XVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00439] In some embodiments, the compound of formula I is a compound of formula XVII:
XVII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00440] In some embodiments, the compound of formula I is a compound of formula XVIII:
XVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XIX; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00442] In some embodiments, the compound of formula I is a compound of formula XX:
XX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00443] In some embodiments, the compound of formula I is a compound of formula XXI:
XXI; or a pharmaceutically acceptable derivative thereof. [00444] In some embodiments, the compound of formula I is a compound of formula XXII:
XXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00445] In some embodiments, the compound of formula I is a compound of formula XXIII:
[00446] Is some embodiments, the compound of formula I is a compound of formula XXIV:
XXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00447] In some embodiments, the compound of formula I is a compound of formula XXV:
XXV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00448] In some embodiments, the compound of formula I is a compound of formula XXVI:
XXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00449] In some embodiments, the compound of formula I is a compound of formula XXVII:
XXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00451] In some embodiments, the compound of formula I is a compound of formula XXIX:
[00452] In some embodiments, the compound of formula I is a compound of formula XXX:
XXX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00453] In some embodiments, the compound of formula I is a compound of formula XXXI:
XXXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00455] In some embodiments, the compound of formula I is a compound of formula XXXIII:
XXXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00456] In some embodiments, the compound of formula I is a compound of formula XXXIV:
XXXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00457] In some embodiments, the compound of formula I is a compound of formula XXXV:
XXXV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00458] In some embodiments, the compound of formula I is a compound of formula XXXVI:
XXXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00459] In some embodiments, the compound of formula I is a compound of formula XXXVII:
XXXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00460] In some embodiments, the compound of formula I is a compound of formula
XXXVIII:
XXXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00461] In some embodiments, the compound of formula I is a compound of formula XXXIX:
[00462] In some embodiments, the compound of formula I is a compound of formula XL:
[00463] In some embodiments, the compound of formula I is a compound of formula XLI:
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00464] In some embodiments, the compound of formula I is a compound of formula XLII:
XLII; or a pharmaceutically acceptable derivative or stereoisomer thereof
[00465] In some embodiments, the compound of formula I is a compound of formula XLIII:
XLIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00466] In some embodiments, the compound of formula I is a compound of formula XLIV
XLIV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00467] In some embodiments, the compound of formula I is a compound of formula XLV
XLV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00468] In some embodiments, the compound of formula I is a compound of formula XLVI
XLVI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00469] In some embodiments, the compound of formula I is a compound of formula XLVII
XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof. [00470] In some embodiments, the compound of formula I is a compound of formula XLVIII
[00471] In some embodiments, the compound of formula I is a compound of formula XLIX
XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof. [00472] In some embodiments, the compound of formula I is a compound of formula L
or a pharmaceutically acceptable derivative or stereoisomer thereof.
LI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00474] In some embodiments, the compound of formula I is a compound of formula LII
LII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00475] In some embodiments, the compound of formula I is a compound of formula LII
LIII or a pharmaceutically acceptable derivative or stereoisomer thereof. [00476] In some embodiments, the urinary incontinence is urge incontinence, stress incontinence, urinary retention with overflow incontinence, ectopic ureter, partial or total incompetence of the urinary sphincter, or neurogenic bladder dysfunction. [00477] The modulation of cytosolic calcium levels has been reported to be associated with apoptosis. See, e.g., Zeilig, WO 2004/108083, the entirety of which is incorporated herein by reference. In several different cell types, excessive cytosolic calcium levels has been shown to induce apotosis. Id.; see also Nicotera et al., Cell Calcium (1998) 23: 173-180. For example, Christensen et al. reported that the growth of LNCaP prostate cancer cells in mice was inhibited by administration of thapsigargin, which is a SERCA inhibitor. Bioorg. Med. Chem. 14 (2006) 2810-2815, incorporated herein by reference in its entirety. The authors attributed this observed inhibition to the potency of thapsigargin as an inhibitor of SERCA. Id. at 2810. Specifically, the authors explained that SERCA inhibition induces an increase in cytosolic calcium concentration, which eventually causes apoptosis. Id. In another example, cyclooxygenase 2 (COX-2) inhibitors, such as celecoxib, have emerged as potential anticancer agents. Schόnthal, Cancer
Lett. (2008), doi: 10.1016/j.canlet.2008.07.005, the entirety of which is incorporated herein by reference. It is thought that the inhibition of COX-2 is not involved in the anticancer effect of celecoxib; rather, celcoxib inhibits tumor growth via inhibition of SERCA. Id. at 4-5. Schόnthal is now taking advantage of this discovery by designing and synthesizing new compounds that even more effectively target SERCA, which may lead to the discovery of anticancer agents. Id. [00478] In some embodiments, provided herein are methods for inducing apoptosis comprising administering a compound described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof. In further embodiments, the compound is a SERCA or SERCA2a inhibitor.
[00479] In some embodiments, provided herein are methods for treating cancer comprising administering a compound of formula I:
-NRXRX,
, -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or
-SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
R1, n, J, Q, and Y are selected from (i) and (ii) as follows:
(i) n is an integer from 1-4;
J is CH;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, RY , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; with the proviso that R1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
-NRYRY,
, -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY;
wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00480] In some embodiments, provided herein are methods for treating cancer comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SR x , wherein R >x is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and not hydrogen, then A is not isopropoxy, if B is para to Y and not hydrogen, then B is not isopropoxy, and if C is para to Y and not hydrogen, then C is not isopropoxy; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system;
wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, Rγ , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00481] In some embodiments, provided herein are methods for treating cancer comprising administering a compound of formula I:
-NRXRX,
, -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or
-SR^ wherein Rx is, independently at each occurrence, H, alkyl, or aryl, with the proviso that if A is para to Y and is not hydrogen, then A is not isopropoxy, if B is para to Y and is not hydrogen, then B is not isopropoxy, and if C is para to Y and is not hydrogen, then C is not isopropoxy; and
n is 0;
J is CH or N;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N-^NRYRY ^N^ORY
-NRYRY, RY , amido, RY , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00482] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00483] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00484] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00485] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [00486] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00487] In some embodiments, the compound of formula I is
wherein R1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00488] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof [00489] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00490] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00491] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00492] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00493] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00494] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00495] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00496] In some embodiments, the compound of formula I is:
[00497] In some embodiments, the compound of formula I is:
[00498] In some embodiments, A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00499] In some embodiments, A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro. [00500] In some embodiments, A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00501] In some embodiments, A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00502] In some embodiments, A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00503] In some embodiments, B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00504] In some embodiments, B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00505] In some embodiments, B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00506] In some embodiments, B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00507] In some embodiments, when A is para to Y and is not hydrogen, then A is not alkoxy, when B is para to Y and is not hydrogen, then B is not alkoxy, and when C is para to Y and is not hydrogen, then C is not alkoxy.
[00508] In some embodiments, at least one of A, B, and C is not hydrogen.
[00509] In some embodiments, at least two of A, B, and C is not hydrogen.
[00510] In some embodiments, B is hydrogen, and A and C are not hydrogen.
[00511] In some embodiments, B is hydrogen, A and C are identical.
[00512] In some embodiments, Rx is H.
[00513] In some embodiments, n is 1-3.
[00514] In some embodiments, n is 1-2.
[00515] In some embodiments, n is 1.
[00516] In some embodiments, n is 2-4.
[00517] In some embodiments, n is 2-3.
[00518] In some embodiments, n is 2.
[00519] In some embodiments, Q is H or alkyl of 1-3 carbons.
[00520] In some embodiments, Q is H or alkyl of 1-2 carbons.
[00521] In some embodiments, Q is H or methyl.
[00522] In some embodiments, Q is alkyl of 1-3 carbons.
[00523] In some embodiments, Q is alkyl of 1-2 carbons.
[00524] In some embodiments, Q is methyl.
[00525] In some embodiments, R1 is monocyclic.
[00526] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR2-X.
[00527] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, or NR2-X.
[00528] In some embodiments, R1 is aryl, heteroaryl or substituted aryl.
[00529] In some embodiments, R1 is aryl or heteroaryl.
[00530] In some embodiments, R1 is aryl.
[00531] In some embodiments, R1 is phenyl.
[00533] In some embodiments, R1 is phenyl, , or
[00534] In some embodiments, R1 is
phenyl or [00535] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl. [00536] In some embodiments, X is aryl or aralkyl. [00537] In some embodiments, X is aryl. [00538] In some embodiments, X is phenyl. [00539] In some embodiments, R2 is H or alkyl or 1-3 carbons. [00540] In some embodiments, R2 is H. [00541] In some embodiments, R3 is H or alkyl. [00542] In some embodiments, RJ is H or alkyl or 1-3 carbons. [00543] In some embodiments, R3 is H. [00544] In some embodiments, RJ and Q are joined together to form ring. [00545] In some embodiments, RJ and Q are joined together to form a 5-6 membered ring. [00546] In some embodiments, t is 0, and R3 and Q are joined together to form a 5-6 membered ring.
[00547] In some embodiments, t is 0 and R3 and Q are joined together to form a 5 membered ring.
[00548] In some embodiments, r is 0-2.
[00549] In some embodiments, r is 0-1.
[00550] In some embodiments, r is 0.
[00551] In some embodiments, t is 0-2.
[00552] In some embodiments, t is 0-1.
[00553] In some embodiments, t is 0.
[00554] In some embodiments, r is 0-1 and t is 0.
[00555] In some embodiments, r is 0 and t is 0.
[00556] In some embodiments, the compound of formula I is
wherein A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or
thereof.
[00557] In some embodiments, the compound of fomula I is
A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R is H or alkyl of 1-3 carbons, Q is methyl, or R and Q are joined together to form a 5- 6 membered ring;
and R2 and G are selected from (i) and (ii) as follows:
(i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido,
Or NO2, orG is joined together with R2 to form a 5-6 membered ring;
is not a substituted or unsubstituted
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, the compound of fomula I is
A and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1- 3 carbons, -CN, azido, or NO2; wherein at least one of A and B is not H;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2,
or G is joined together with R to form a 5-6 membered ring;
with the proviso that is not a substituted or unsubstituted
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptalble derivative or stereoisomer thereof.
[00559] In some embodiments, R2 G is not a substuted or unsubstituted moiety
Vt CHΛiJ
[00560] In some embodiments, R2 J G is not a substuted or unsubstituted moiety
[00561] In further embodiments, A and B are each, independently, H, hydroxyl, or alkoxy of 1-3 carbons; R3 is H or alkyl of 1-3 carbons, Q is methyl, R2 is H, alkyl of 1-3 carbons, and G is H, or hydroxyl.
[00562] In further embodiments, the compound of formula I has the following formula:
[00563] In further embodiments, the compound of formula I has the following formula:
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00564] In some embodiments, the compound of formula I is a compound of formula II:
[00565] In some embodiments, the compound of formula I is a compound of formula III:
III; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00566] In some embodiments, the compound of formula I is a compound of formula IV:
IV; a pharmaceutically acceptable derivative or stereoisomer thereof.
[00567] In some embodiments, the compound of formula I is a compound of formula V:
V;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00568] In some embodiments, the compound of formula I is a compound of formula VI:
VI; or a pharmaceutically acceptable derivative thereof.
[00569] In some embodiments, the compound of formula I is a compound of formula VII:
VII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00570] In some embodiments, the compound of formula I is a compound of formula VIII:
VIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00571] In some embodiments, the compound of formula I is a compound of formula IX:
IX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
X; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00573] In some embodiments, the compound of formula I is a compound of formula XI:
[00574] In some embodiments, the compound of formula I is a compound of formula XII:
XII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00575] In some embodiments, the compound of formula I is a compound of formula XIII:
XIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00576] In some embodiments, the compound of formula I is a compound of formula XIV:
XIV;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00577] In some embodiments, the compound of formula I is a compound of formula XV:
XV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00578] In some embodiments, the compound of formula I is a compound of formula XVI:
XVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00579] In some embodiments, the compound of formula I is a compound of formula XVII:
XVII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00580] In some embodiments, the compound of formula I is a compound of formula XVIII:
XVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00582] In some embodiments, the compound of formula I is a compound of formula XX:
XX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00583] In some embodiments, the compound of formula I is a compound of formula XXI:
XXI; or a pharmaceutically acceptable derivative thereof. [00584] In some embodiments, the compound of formula I is a compound of formula XXII:
XXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00586] Is some embodiments, the compound of formula I is a compound of formula XXIV:
XXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00587] In some embodiments, the compound of formula I is a compound of formula XXV:
XXV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00588] In some embodiments, the compound of formula I is a compound of formula XXVI:
XXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00589] In some embodiments, the compound of formula I is a compound of formula XXVII:
XXVII;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00590] In some embodiments, the compound of formula I is a compound of formula XXVIII:
XXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00591] In some embodiments, the compound of formula I is a compound of formula XXIX:
[00592] In some embodiments, the compound of formula I is a compound of formula XXX:
XXX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00593] In some embodiments, the compound of formula I is a compound of formula XXXI:
XXXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00595] In some embodiments, the compound of formula I is a compound of formula XXXIII:
XXXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00596] In some embodiments, the compound of formula I is a compound of formula XXXIV:
XXXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00597] In some embodiments, the compound of formula I is a compound of formula XXXV:
XXXV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00598] In some embodiments, the compound of formula I is a compound of formula XXXVI:
XXXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00599] In some embodiments, the compound of formula I is a compound of formula XXXVII:
XXXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00600] In some embodiments, the compound of formula I is a compound of formula
XXXVIII:
[00601] In some embodiments, the compound of formula I is a compound of formula XXXIX:
[00602] In some embodiments, the compound of formula I is a compound of formula XL:
[00603] In some embodiments, the compound of formula I is a compound of formula XLI:
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00604] In some embodiments, the compound of formula I is a compound of formula XLII:
XLII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00605] In some embodiments, the compound of formula I is a compound of formula XLIII:
XLIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00606] In some embodiments, the compound of formula I is a compound of formula XLIV
XLIV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00607] In some embodiments, the compound of formula I is a compound of formula XLV
XLV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00608] In some embodiments, the compound of formula I is a compound of formula XLVI
XLVI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00609] In some embodiments, the compound of formula I is a compound of formula XLVII
XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof. [00610] In some embodiments, the compound of formula I is a compound of formula XLVIII
[00611] In some embodiments, the compound of formula I is a compound of formula XLIX
XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof. [00612] In some embodiments, the compound of formula I is a compound of formula L
or a pharmaceutically acceptable derivative or stereoisomer thereof.
LI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00614] In some embodiments, the compound of formula I is a compound of formula LII
LII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00615] In some embodiments, the compound of formula I is a compound of formula LII
LIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00616] Specific examples of cancer include, but are not limited to, cancers of the skin, such as melanoma; lymph node; breast; cervix; uterus; gastrointestinal tract; lung; ovary; prostate; colon; rectum; mouth; brain; head and neck; throat; testes; kidney; pancreas; bone; spleen; liver; bladder; larynx; nasal passages; and AIDS-related cancers. The compounds of formula I are also useful for treating cancers of the blood and bone marrow, such as multiple myeloma and acute and chronic leukemias, for example, lymphoblastic, myelogenous, lymphocytic, and myelocytic leukemias. Further, the compounds of formula I can be used for treating, preventing or managing either primary or metastatic tumors.
[00617] Other specific cancers include, but are not limited to, advanced malignancy, amyloidosis, neuroblastoma, meningioma, hemangiopericytoma, multiple brain metastase, glioblastoma multiforms, glioblastoma, brain stem glioma, poor prognosis malignant brain tumor, malignant glioma, recurrent malignant glioma, anaplastic astrocytoma, anaplastic oligodendroglioma, neuroendocrine tumor, rectal adenocarcinoma, Dukes C & D colorectal cancer, unresectable colorectal carcinoma, metastatic hepatocellular carcinoma, Kaposi's
sarcoma, karotype acute myeloblastic leukemia, chronic lymphocytic leukemia (CLL), Hodgkin's lymphoma, non-Hodgkin's lymphoma, cutaneous T-CeIl lymphoma, cutaneous B- CeIl lymphoma, diffuse large B-CeIl lymphoma, low grade follicular lymphoma, metastatic melanoma (localized melanoma, including, but not limited to, ocular melanoma), malignant mesothelioma, malignant pleural effusion mesothelioma syndrome, peritoneal carcinoma, papillary serous carcinoma, gynecologic sarcoma, soft tissue sarcoma, scleroderma, cutaneous vasculitis, Langerhans cell histiocytosis, leiomyosarcoma, fϊbrodysplasia ossificans progressive, hormone refractory prostate cancer, resected high-risk soft tissue sarcoma, unrescectable hepatocellular carcinoma, Waldenstrom's macroglobulinemia, smoldering myeloma, indolent myeloma, fallopian tube cancer, androgen independent prostate cancer, androgen dependent stage IV non-metastatic prostate cancer, hormone-insensitive prostate cancer, chemotherapy- insensitive prostate cancer, papillary thyroid carcinoma, follicular thyroid carcinoma, medullary thyroid carcinoma, and leiomyoma. In a specific embodiment, the cancer is metastatic. In another embodiment, the cancer is refractory or resistance to chemotherapy or radiation. [00618] In one embodiment, provided herein are methods of treating various forms of leukemias such as chronic lymphocytic leukemia, chronic myelocytic leukemia, acute lymphoblastic leukemia, acute myelogenous leukemia and acute myeloblastic leukemia, including leukemias that are relapsed, refractory or resistant. The term "leukemia" refers to malignant neoplasms of the blood-forming tissues. The leukemia includes, but is not limited to, chronic lymphocytic leukemia, chronic myelocytic leukemia, acute lymphoblastic leukemia, acute myelogenous leukemia and acute myeloblastic leukemia. The leukemia can be relapsed, refractory or resistant to conventional therapy. The term "relapsed" refers to a situation where patients who have had a remission of leukemia after therapy have a return of leukemia cells in the marrow and a decrease in normal blood cells. The term "refractory or resistant" refers to a circumstance where patients, even after intensive treatment, have residual leukemia cells in their marrow.
[00619] In another embodiment, provided herein are methods of treating, preventing or managing various types of lymphomas, including Non-Hodgkin's lymphoma (NHL). The term "lymphoma" refers a heterogenous group of neoplasms arising in the reticuloendothelial and lymphatic systems. "NHL" refers to malignant monoclonal proliferation of lymphoid cells in sites of the immune system, including lymph nodes, bone marrow, spleen, liver and
gastrointestinal tract. Examples of NHL include, but are not limited to, mantle cell lymphoma (MCL), lymphocytic lymphoma of intermediate differentiation, intermediate lymphocytic lymphoma (ILL), diffuse poorly differentiated lymphocytic lymphoma (PDL), centrocytic lymphoma, diffuse small-cleaved cell lymphoma (DSCCL), follicular lymphoma, and any type of the mantle cell lymphomas that can be seen under the microscope (nodular, diffuse, blastic and mentle zone lymphoma).
[00620] Asthma is a chronic inflammatory disease. In the United States, more than 22 million people are known to have asthma. Asthma is accompanied by extensive changes in normal airway tissue architecture, also known as remodeling. A component of remodeling involves an increased amount of smooth muschle in the airways. Phenotypic modulation of airway smooth muscle (ASM) is an aspect of airway remodeling in asthma that is characterized by enhanced proliferation and secretion of pro-inflammatory chemokines. See Mahn et al., PNAS, 106(26): 10775-10780 (2009), which is incorporated herein by reference in its entirety. These activities are regulated by the concentration of free Ca2+ in the cytosol ([Ca2+J1). A rise in [(Ca2+), is normalized by rapid reuptake of Ca2+ into sarcoplasmic reticulum stores by the sarco/endoplasmic reticulum Ca2+ pump. Mahn et al report that SERC A2 protein expression is reduced in ASM from asthmatics. That is, they report that diminished SERCA expression contributes to airway remodeling in asthma. Depleted SERC A- function leading to abnormal calcium homeostatis in asthmatic ASM was reported by Mahn et al. to be associated with a sustained increase in [Ca2+J1 and enhanced proliferative and secretory function. [00621] It has also been reported that in patients with chronic obstructive pulmonary disease (COPD), SERCA2 concentration is reduced. Moria et al. Arch Bronconeumol, 43(l):4-8 (2007), incorporated herein by referenced in its entirety. Moria et al. report that the protein is tyrosine-nitrated in skeletal muscle from patients with low body mass index compared to those with normal body mass indiex. Moria et al. note that these results indicate the presence of a previously unrecognized cellular alteration in skeletal nuscle from patients with COPD and low muscle weight.
[00622] In some embodiments, provided herein are methods of treating asthma or chronic obstructive pulmonary disease comprising administering a compound of formula I:
i; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or - SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and R1, n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system;
and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [00623] In some embodiments, provided herein are methods of treating asthma or chronic obstructive pulmonary disease comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
, -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SR x , wherein R >x is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [00624] In some embodiments, provided herein are methods of treating asthma or chronic obstructive pulmonary disease comprising administering a compound of formula I:
^N^NRXR* >*N^ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or -
SR x wherein Rx is, independently at each occurrence, H, alkyl, or aryl; with the proviso that if A is meta to Y and is not hydrogen, then A is not tetrazolyl or substituted tetrazolyl, if B is meta to Y and is not hydrogen, then B is
not tetrazolyl or substituted tetrazolyl, and if C is meta to Y and is not hydrogen, then C is not tetrazolyl or substituted tetrazolyl; and n is 0;
J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof; or a pharmaceutical composition containing such compound or derivative or stereoisomer thereof. [00625] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00626] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00627] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00628] In some embodiments, the compound of formula I is
[00629] In some embodiments, the compound of formula I is:
[00630] In some embodiments, the compound of formula I is
wherein R1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00631] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof [00632] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00633] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00634] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00635] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00636] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00637] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00638] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00639] In some embodiments, the compound of formula I is:
[00640] In some embodiments, the compound of formula I is:
[00641] In some embodiments, A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00642] In some embodiments, A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro. [00643] In some embodiments, A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00644] In some embodiments, A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00645] In some embodiments, A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00646] In some embodiments, B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00647] In some embodiments, B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00648] In some embodiments, B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00649] In some embodiments, B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00650] In some embodiments, at least one of A, B, and C is not hydrogen.
[00651] In some embodiments, at least two of A, B, and C is not hydrogen.
[00652] In some embodiments, B is hydrogen, and A and C are not hydrogen.
[00653] In some embodiments, B is hydrogen, A and C are identical.
[00654] In some embodiments, when A is para to Y and is not hydrogen, then A is not isopropoxy, when B is para to Y and is not hydrogen, then B is not isopropoxy, and when C is para to Y and is not hydrogen, then C is not isopropoxy.
[00655] In some embodiments, when A is para to Y and is not hydrogen, then A is not alkoxy, when B is para to Y and is not hydrogen, then B is not alkoxy, and when C is para to Y and is not hydrogen, then C is not alkoxy.
[00656] In some embodiments, Rx is H.
[00657] In some embodiments, n is 1-3.
[00658] In some embodiments, n is 1-2.
[00659] In some embodiments, n is 1.
[00660] In some embodiments, n is 2-4.
[00661] In some embodiments, n is 2-3.
[00662] In some embodiments, n is 2.
[00663] In some embodiments, Q is H or alkyl of 1-3 carbons.
[00664] In some embodiments, Q is H or alkyl of 1-2 carbons.
[00665] In some embodiments, Q is H or methyl.
[00666] In some embodiments, Q is alkyl of 1-3 carbons.
[00667] In some embodiments, Q is alkyl of 1-2 carbons.
[00668] In some embodiments, Q is methyl.
[00669] In some embodiments, R1 is monocyclic.
[00670] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR2-X.
[00671] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, or NR2-X.
[00672] In some embodiments, R1 is aryl, heteroaryl or substituted aryl.
[00673] In some embodiments, R1 is aryl or heteroaryl.
[00674] In some embodiments, R1 is aryl. [00675] In some embodiments, R1 is phenyl.
[00678] In some embodiments, R1 is phenyl or yI>
[00679] In some embodiments, R1 is not indolyl.
[00680] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
[00681] In some embodiments, X is aryl or aralkyl.
[00682] In some embodiments, X is aryl.
[00683] In some embodiments, X is phenyl.
[00684] In some embodiments, R2 is H or alkyl or 1-3 carbons.
[00685] In some embodiments, R2 is H.
[00686] In some embodiments, R3 is H or alkyl.
[00687] In some embodiments, R3 is H or alkyl or 1-3 carbons.
[00688] In some embodiments, R is H.
[00689] In some embodiments, R and Q are joined together to form ring.
[00690] In some embodiments, R and Q are joined together to form a 5-6 membered ring.
[00691] In some embodiments, t is 0, and R3 and Q are joined together to form a 5-6 membered ring.
[00692] In some embodiments, t is 0 and R3 and Q are joined together to form a 5 membered ring.
[00693] In some embodiments, r is 0-2.
[00694] In some embodiments, r is 0-1.
[00695] In some embodiments, r is 0.
[00696] In some embodiments, t is 0-2.
[00697] In some embodiments, t is 0-1.
[00698] In some embodiments, t is 0.
[00699] In some embodiments, r is 0-1 and t is 0.
[00700] In some embodiments, r is 0 and t is 0.
[00701] In some embodiments, the compound of formula I is
wherein A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or
thereof.
[00702] In some embodiments, the compound of fomula I is
A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2,
orG is joined together with R2 to form a 5-6 membered ring;
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof. In some embodiments, the compound of fomula I is
A and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1- 3 carbons, -CN, azido, or NO2; wherein at least one of A and B is not H;
R is H or alkyl of 1-3 carbons, Q is methyl, or R and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, or G is joined together with R2 to form a 5-6 membered ring;
with the proviso that R2 J G is not a substituted or unsubstituted
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
having the formula
[00706] In further embodiments, A and B are each, independently, H, hydroxyl, or alkoxy of 1-3 carbons; R3 is H or alkyl of 1-3 carbons, Q is methyl, R2 is H, alkyl of 1-3 carbons, and G is H, or hydroxyl.
[00707] In further embodiments, the compound of formula I has the following formula:
B
O Q R2 G . or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00708] In further embodiments, the compound of formula I has the following formula:
or a pharmaceutically acceptable derivative or stereisomer thereof..
[00709] In some embodiments, the compound of formula I is a compound of formula II:
[00710] In some embodiments, the compound of formula I is a compound of formula III:
III; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00711] In some embodiments, the compound of formula I is a compound of formula IV:
IV; a pharmaceutically acceptable derivative or stereoisomer thereof.
[00712] In some embodiments, the compound of formula I is a compound of formula V:
V;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00713] In some embodiments, the compound of formula I is a compound of formula VI:
VI; or a pharmaceutically acceptable derivative thereof.
[00714] In some embodiments, the compound of formula I is a compound of formula VII:
VII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00715] In some embodiments, the compound of formula I is a compound of formula VIII:
VIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00716] In some embodiments, the compound of formula I is a compound of formula IX:
IX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
X; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00718] In some embodiments, the compound of formula I is a compound of formula XI:
[00719] In some embodiments, the compound of formula I is a compound of formula XII:
XII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00720] In some embodiments, the compound of formula I is a compound of formula XIII:
XIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00721] In some embodiments, the compound of formula I is a compound of formula XIV:
XIV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00722] In some embodiments, the compound of formula I is a compound of formula XV:
XV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00723] In some embodiments, the compound of formula I is a compound of formula XVI:
XVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00724] In some embodiments, the compound of formula I is a compound of formula XVII:
XVII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00725] In some embodiments, the compound of formula I is a compound of formula XVIII:
XVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00726] In some embodiments, the compound of formula I is a compound of formula XIX:
XIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00727] In some embodiments, the compound of formula I is a compound of formula XX:
XX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00728] In some embodiments, the compound of formula I is a compound of formula XXI:
XXI; or a pharmaceutically acceptable derivative thereof. [00729] In some embodiments, the compound of formula I is a compound of formula XXII:
XXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00730] In some embodiments, the compound of formula I is a compound of formula XXIII:
XXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00731] Is some embodiments, the compound of formula I is a compound of formula XXIV:
XXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00732] In some embodiments, the compound of formula I is a compound of formula XXV:
XXV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00733] In some embodiments, the compound of formula I is a compound of formula XXVI:
XXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00734] In some embodiments, the compound of formula I is a compound of formula XXVII:
XXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00735] In some embodiments, the compound of formula I is a compound of formula XXVIII:
XXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00736] In some embodiments, the compound of formula I is a compound of formula XXIX:
XXX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00738] In some embodiments, the compound of formula I is a compound of formula XXXI:
[00739] In some embodiments, the compound of formula I is a compound of formula XXXII:
XXXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00740] In some embodiments, the compound of formula I is a compound of formula XXXIII:
XXXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00741] In some embodiments, the compound of formula I is a compound of formula XXXIV:
XXXIV;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00742] In some embodiments, the compound of formula I is a compound of formula XXXV:
XXXV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00743] In some embodiments, the compound of formula I is a compound of formula XXXVI:
XXXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00744] In some embodiments, the compound of formula I is a compound of formula XXXVII:
XXXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00745] In some embodiments, the compound of formula I is a compound of formula
XXXVIII:
[00746] In some embodiments, the compound of formula I is a compound of formula XXXIX:
XXXIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00747] In some embodiments, the compound of formula I is a compound of formula XL:
[00748] In some embodiments, the compound of formula I is a compound of formula XLI:
[00749] In some embodiments, the compound of formula I is a compound of formula XLII:
XLII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00750] In some embodiments, the compound of formula I is a compound of formula XLIII:
XLIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00751] In some embodiments, the compound of formula I is a compound of formula XLIV
XLIV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00752] In some embodiments, the compound of formula I is a compound of formula XLV
XLV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00753] In some embodiments, the compound of formula I is a compound of formula XLVI
XLVI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00754] In some embodiments, the compound of formula I is a compound of formula XLVII
XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00755] In some embodiments, the compound of formula I is a compound of formula XLVIII
XLVIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00756] In some embodiments, the compound of formula I is a compound of formula XLIX
XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof. [00757] In some embodiments, the compound of formula I is a compound of formula L
L or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00758] In some embodiments, the compound of formula I is a compound of formula LI
LI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00759] In some embodiments, the compound of formula I is a compound of formula LII
LII or a pharmaceutically acceptable derivative or stereoisomer thereof.
LIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00761] In some embodiments, the asthma is mild intermittent asthma, which is characterized by a symptom frequency of once per week, nighttime symptoms of less than or equal to twice per month, or an FEV or PEF greater than or equal to 80% predicted. In some embodiments, the asthma is mild persistent asthma, which is characterized by a symptom frequency of greater than 2 times a weeks but less than 1 time a day, nighttime symptoms of greater than two times a month, or an FEV or PEF greater than or equal to 80% predicted. In some embodiments, the asthma is moderate persistent asthma, which is characterized by daily symptoms, nighttime symptoms of greater than one time a week, or an FEV or PEF of greater thant 60% to less than 80% predicted. . In some embodiments, the asthma is severe persistent asthma, which is characterized by continual symptoms, frequent nighttime symptoms, or an FEV or PEF of less than or equal to 60% predicted.
[00762] In some embodiments, the asthma is instinsic asthma, extrinsic asthma, bronchitic asthma, exercise-induced asthma, occupational asthma, asthma induced following bacterial infection, asthma in children, wheezy infant syndrome, atopic asthma, non-atopic asthma, atopic bronchial IgE -mediated asthma, essential asthma, true asthma, instrinsic asthma casued by pathophysiologic disturbances, extrinsic asthma casued by environmental factors, bronchial asthma, essential asthma of unknown or inapprent cause, emphysematous asthma, allergen induced asthma, cold air induced asthma, incipient asthma, bronchilytis, morning dipping, or spontaneous idiopathic asthma.
[00763] Studies based on patients with diabetes and animal models of diabetes show that intracellular calcium levels are increased in most tissues. See, .e.g., Levy et al., The American Journal of Medicine, 96: 260-273 (1994). Indeed, diabetes is associated with abnormal intracellular calcium homeostaisis. This defect is found in both diabetes type I and type II and widespread among tissues. The activities of SERCA are altered in a tissue specific manner. [00764] In some embodiments, provided herein are methods of treating diabetes comprising administering a compound of formula I:
i; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or - SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and R1, n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X,
wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; and
wherein R3 and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00765] In some embodiments, provided herein are methods of treating diabetes comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRXR* >*N^ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00766] In some embodiments, provided herein are methods of treating diabetes comprising administering a compound of formula I:
I; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl, O O
^N NRXRX *'H ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or - SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and n is 0;
J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof. [00767] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00768] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00769] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [00770] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [00771] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00772] In some embodiments, the compound of formula I is
wherein R1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00773] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof [00774] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00775] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00776] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00777] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00778] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00779] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00780] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00781] In some embodiments, the compound of formula I is:
[00782] In some embodiments, the compound of formula I is:
[00783] In some embodiments, A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00784] In some embodiments, A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro. [00785] In some embodiments, A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00786] In some embodiments, A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00787] In some embodiments, A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00788] In some embodiments, B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00789] In some embodiments, B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00790] In some embodiments, B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00791] In some embodiments, B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00792] In some embodiments, at least one of A, B, and C is not hydrogen. [00793] In some embodiments, at least two of A, B, and C is not hydrogen. [00794] In some embodiments, B is hydrogen, and A and C are not hydrogen. [00795] In some embodiments, B is hydrogen, A and C are identical. [00796] In some embodiments, when A is para to Y and is not hydrogen, then A is not isopropoxy, when B is para to Y and is not hydrogen, then B is not isopropoxy, and when C is para to Y and is not hydrogen, then C is not isopropoxy.
[00797] In some embodiments, when A is para to Y and is not hydrogen, then A is not alkoxy, when B is para to Y and is not hydrogen, then B is not alkoxy, and when C is para to Y and is not hydrogen, then C is not alkoxy. [00798] In some embodiments, Rx is H.
[00799] In some embodiments, n is 1-3.
[00800] In some embodiments, n is 1-2.
[00801] In some embodiments, n is 1.
[00802] In some embodiments, n is 2-4.
[00803] In some embodiments, n is 2-3.
[00804] In some embodiments, n is 2.
[00805] In some embodiments, Q is H or alkyl of 1-3 carbons.
[00806] In some embodiments, Q is H or alkyl of 1-2 carbons.
[00807] In some embodiments, Q is H or methyl.
[00808] In some embodiments, Q is alkyl of 1-3 carbons.
[00809] In some embodiments, Q is alkyl of 1-2 carbons.
[00810] In some embodiments, Q is methyl.
[00811] In some embodiments, R1 is monocyclic.
[00812] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR2-X.
[00813] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, or NR2-X.
[00814] In some embodiments, R1 is aryl, heteroaryl or substituted aryl.
[00815] In some embodiments, R1 is aryl or heteroaryl.
[00816] In some embodiments, R1 is aryl.
[00817] In some embodiments, R1 is phenyl.
[00820] In some embodiments, R1 is
phenyl or [00821] In some embodiments, R1 is not indolyl.
[00822] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
[00823] In some embodiments, X is aryl or aralkyl.
[00824] In some embodiments, X is aryl.
[00825] In some embodiments, X is phenyl.
[00826] In some embodiments, R2 is H or alkyl or 1-3 carbons.
[00827] In some embodiments, R2 is H.
[00828] In some embodiments, R3 is H or alkyl.
[00829] In some embodiments, R3 is H or alkyl or 1-3 carbons.
[00830] In some embodiments, R3 is H.
[00831] In some embodiments, R3 and Q are joined together to form ring.
[00832] In some embodiments, R and Q are joined together to form a 5-6 membered ring.
[00833] In some embodiments, t is 0, and R3 and Q are joined together to form a 5-6 membered rin g-
[00834] In some embodiments, t is 0 and R3 and Q are joined together to form a 5 membered ring.
[00835] In some embodiments, r is 0-2.
[00836] In some embodiments, r is 0-1.
[00837] In some embodiments, r is 0.
[00838] In some embodiments, t is 0-2.
[00839] In some embodiments, t is 0-1.
[00840] In some embodiments, t is 0.
[00841] In some embodiments, r is 0-1 and t is 0.
[00842] In some embodiments, r is 0 and t is 0.
[00843] In some embodiments, the compound of formula I is
wherein A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, cyano, or
thereof.
[00844] In some embodiments, the compound of fomula I is
A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2, orG is joined together with R2 to form a 5-6 membered ring;
with the proviso that R2 G ' is not a substituted or unsubstituted
moiety having the formula:
; and (ii) v is 1 to 3, R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00845] In some embodiments, the compound of fomula I is
A and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1- 3 carbons, -CN, azido, or NO2; wherein at least one of A and B is not H;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, or G is joined together with R2 to form a 5-6 membered ring;
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
Λ R2 _/
[00846] In some embodiments, / G— is not a substuted or unsubstituted moiety
[00848] In further embodiments, A and B are each, independently, H, hydroxyl, or alkoxy of 1-3 carbons; R3 is H or alkyl of 1-3 carbons, Q is methyl, R2 is H, alkyl of 1-3 carbons, and G is H, or hydroxyl.
[00849] In further embodiments, the compound of formula I has the following formula:
[00850] In further embodiments, the compound of formula I has the following formula:
[00851] In some embodiments, the compound of formula I is a compound of formula II:
[00852] In some embodiments, the compound of formula I is a compound of formula III:
III; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00853] In some embodiments, the compound of formula I is a compound of formula IV:
IV; a pharmaceutically acceptable derivative or stereoisomer thereof.
[00854] In some embodiments, the compound of formula I is a compound of formula V:
V; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00855] In some embodiments, the compound of formula I is a compound of formula VI:
VI; or a pharmaceutically acceptable derivative thereof.
VII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00857] In some embodiments, the compound of formula I is a compound of formula VIII:
VIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00858] In some embodiments, the compound of formula I is a compound of formula IX:
IX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00859] In some embodiments, the compound of formula I is a compound of formula X:
X; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00860] In some embodiments, the compound of formula I is a compound of formula XI:
[00861] In some embodiments, the compound of formula I is a compound of formula XII:
XII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00862] In some embodiments, the compound of formula I is a compound of formula XIII:
[00863] In some embodiments, the compound of formula I is a compound of formula XIV:
XIV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00864] In some embodiments, the compound of formula I is a compound of formula XV:
XV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00866] In some embodiments, the compound of formula I is a compound of formula XVII:
XVII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00867] In some embodiments, the compound of formula I is a compound of formula XVIII:
XVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00868] In some embodiments, the compound of formula I is a compound of formula XIX:
XIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00869] In some embodiments, the compound of formula I is a compound of formula XX:
XX;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00870] In some embodiments, the compound of formula I is a compound of formula XXI:
XXI; or a pharmaceutically acceptable derivative thereof.
[00871] In some embodiments, the compound of formula I is a compound of formula XXII:
XXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00872] In some embodiments, the compound of formula I is a compound of formula XXIII:
XXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00873] Is some embodiments, the compound of formula I is a compound of formula XXIV:
XXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00874] In some embodiments, the compound of formula I is a compound of formula XXV:
XXV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00875] In some embodiments, the compound of formula I is a compound of formula XXVI:
XXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00876] In some embodiments, the compound of formula I is a compound of formula XXVII:
XXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00877] In some embodiments, the compound of formula I is a compound of formula XXVIII:
XXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00878] In some embodiments, the compound of formula I is a compound of formula XXIX:
XXIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00879] In some embodiments, the compound of formula I is a compound of formula XXX:
XXX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00880] In some embodiments, the compound of formula I is a compound of formula XXXI:
[00881] In some embodiments, the compound of formula I is a compound of formula XXXII:
XXXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XXXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00883] In some embodiments, the compound of formula I is a compound of formula XXXIV:
XXXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00884] In some embodiments, the compound of formula I is a compound of formula XXXV:
XXXV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00885] In some embodiments, the compound of formula I is a compound of formula XXXVI:
XXXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00886] In some embodiments, the compound of formula I is a compound of formula XXXVII:
XXXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00887] In some embodiments, the compound of formula I is a compound of formula
[00888] In some embodiments, the compound of formula I is a compound of formula XXXIX:
[00889] In some embodiments, the compound of formula I is a compound of formula XL:
[00890] In some embodiments, the compound of formula I is a compound of formula XLI:
XLII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00892] In some embodiments, the compound of formula I is a compound of formula XLIII:
XLIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00893] In some embodiments, the compound of formula I is a compound of formula XLIV
XLIV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00894] In some embodiments, the compound of formula I is a compound of formula XLV
XLV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00895] In some embodiments, the compound of formula I is a compound of formula XLVI
XLVI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00896] In some embodiments, the compound of formula I is a compound of formula XLVII
XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof. [00897] In some embodiments, the compound of formula I is a compound of formula XLVIII
XLVIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00898] In some embodiments, the compound of formula I is a compound of formula XLIX
XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof. [00899] In some embodiments, the compound of formula I is a compound of formula L
L or a pharmaceutically acceptable derivative or stereoisomer thereof. [00900] In some embodiments, the compound of formula I is a compound of formula LI
LI or a pharmaceutically acceptable derivative or stereoisomer thereof.
LII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00902] In some embodiments, the compound of formula I is a compound of formula LII
LIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00903] In some embodiments, the diabetes is type 1 diabetes or type 2, diabetes. In some embodiments, the diabetes is gestational diabetes, juvenile diabetes, congenital diabetes, systic fibrosis-related diabetes, monogenic diabetes, or maturity onsent diabetes of the young.
[00904] Calcium dysregulation is involved in Alzheimer's disease pathogenesis. See, e.g., Yu et al., Progress in Neurobiology, 89: 240-255 (2009). Almost the whole brain pathology process that is observed in Alzheimer's disease is accompanied by systemic calcium changes, including synaptic dysfunction, mitochondrial dysfunction, presenilins mutation, Aβ production, and Tau phosphorylation.
[00905] In some embodiments, provided herein are methods of treating Alzheimer's disease comprising administering a compound of formula I:
I; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
, -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or - SRX,
wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and R1, n, Q, Y, and J are selected from (i) and (ii) as follows: (i) n is an integer from 1-4; J is CH; Q is H or alkyl; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, RY , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; and (ii) n is 0; J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; with the proviso that R1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl,
-NRYRY,
-COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00906] In some embodiments, provided herein are methods of treating Alzheimer's disease comprising administering a compound of formula Ia:
Ia; wherein A, B, and C are each, independently, H, alkyl, alkoxy, aryloxy, hydroxyl,
-NRXRX,
-COORX, -CONRXRX, aryl, monocylic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRX, wherein Rx is, independently at each occurrence, H, alkyl, or aryl; n is an integer from 1-4;
Q is H or alkyl;
R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and
R2 is H or alkyl; with the proviso that R1 is not indolyl; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, Q may be linked with R1 to form a bicyclic ring system; wherein the substituent, if present, is alkyl, alkoxy, aryloxy, hydroxyl, O O
^N^NRYRY ^N^ORY
-NRYRY, RY , amido, RY , -COORY, -CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R3 is H, or alkyl; or R3 and Q are joined together to form a 5-7 membered ring; or, if R1 is aryl, heteroaryl, substituted aryl, or substituted heteroaryl, R3 may be linked to R1 to form a bicyclic ring system; and r and t are each, independently, an integer from 0-3; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00907] In some embodiments, provided herein are methods of treating Alzheimer's disease comprising administering a compound of formula I:
^N^NRXR* >*N^ORX
-NRXRX, Rx , amido, Rx , -COORX, -CONRXRX, aryl, monocyclic heteroaryl, monocyclic heterocyclyl, cycloalkyl, halo, haloalkyl, cyano, nitro, azido, or - SRX,
wherein Rx is, independently at each occurrence, H, alkyl, or aryl; and n is 0;
J is CH or N; R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, methyl, or NR2-X, wherein X is aryl, aralkyl, heteroaryl, heteroaralkyl, substituted aryl, substituted heteroaryl, substituted aralkyl, or substituted heteroaralkyl; and R2 is H or alkyl; with the proviso that R1 is not indolyl; wherein the substituent, if present, is alkyl, alkoxy, aryloxy,
-CONRYRY, aryl, heteroaryl, cycloalkyl, halo, haloalkyl, cyano, nitro, or -SRY; wherein Rγ is, independently at each occurrence, H, alkyl, or aryl; and
wherein R and Q are joined together to form a 5-7 membered ring; and r and t are each, independently, an integer from 0-3 or a pharmaceutically acceptable derivative or stereoisomer thereof. [00908] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00909] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00910] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [00911] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative thereof. [00912] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00913] In some embodiments, the compound of formula I is
wherein R1 is phenyl or substituted phenyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00914] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof [00915] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00916] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00917] In some embodiments, the compound of formula I is:
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00918] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00919] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00920] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00921] In some embodiments, the compound of formula I is
or a pharmaceutically acceptable derivative or stereoisomer thereof. [00922] In some embodiments, the compound of formula I is:
[00923] In some embodiments, the compound of formula I is:
[00924] In some embodiments, A, B, and C are each, independently, H, alkyl, alkoxy, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00925] In some embodiments, A, B, and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro. [00926] In some embodiments, A, B, and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00927] In some embodiments, A, B, and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00928] In some embodiments, A, B, and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00929] In some embodiments, B is H; and A and C are each, independently, H, alkyl of 1-3 carbons, alkoxy of 1-3 carbons, hydroxyl, amino, amido, halo, haloalkyl of 1-3 carbons, cyano, or nitro.
[00930] In some embodiments, B is H; and A and C are each, independently, H, alkoxy, hydroxyl, halo, haloalkyl, or cyano.
[00931] In some embodiments, B is H; and A and C are each, independently, H, alkoxy of 1-3 carbons, hydroxyl, halo, haloalkyl of 1-3 carbons, or cyano.
[00932] In some embodiments, B is H; and A and C are each, independently, H, methoxy, ethoxy, hydroxyl, -F, -CF3, or cyano.
[00933] In some embodiments, at least one of A, B, and C is not hydrogen.
[00934] In some embodiments, at least two of A, B, and C is not hydrogen.
[00935] In some embodiments, B is hydrogen, and A and C are not hydrogen.
[00936] In some embodiments, B is hydrogen, A and C are identical.
[00937] In some embodiments, when A is para to Y and is not hydrogen, then A is not isopropoxy, when B is para to Y and is not hydrogen, then B is not isopropoxy, and when C is para to Y and is not hydrogen, then C is not isopropoxy.
[00938] In some embodiments, when A is para to Y and is not hydrogen, then A is not alkoxy, when B is para to Y and is not hydrogen, then B is not alkoxy, and when C is para to Y and is not hydrogen, then C is not alkoxy.
[00939] In some embodiments, Rx is H.
[00940] In some embodiments, n is 1-3.
[00941] In some embodiments, n is 1-2.
[00942] In some embodiments, n is 1.
[00943] In some embodiments, n is 2-4.
[00944] In some embodiments, n is 2-3.
[00945] In some embodiments, n is 2.
[00946] In some embodiments, Q is H or alkyl of 1-3 carbons.
[00947] In some embodiments, Q is H or alkyl of 1-2 carbons.
[00948] In some embodiments, Q is H or methyl.
[00949] In some embodiments, Q is alkyl of 1-3 carbons.
[00950] In some embodiments, Q is alkyl of 1-2 carbons.
[00951] In some embodiments, Q is methyl.
[00952] In some embodiments, R1 is monocyclic.
[00953] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, substituted heteroaryl, or NR2-X.
[00954] In some embodiments, R1 is aryl, heteroaryl, substituted aryl, or NR2-X.
[00955] In some embodiments, R1 is aryl, heteroaryl or substituted aryl.
[00956] In some embodiments, R1 is aryl or heteroaryl.
[00957] In some embodiments, R1 is aryl.
[00958] In some embodiments, R1 is phenyl.
S=>?RΛ
[00959] In some embodiments, R1 is phenyl; x l ^0N
, wherein R4 is alkyl; , or
[00960] In some embodiments, R1 is phenyl, or
[00962] In some embodiments, R1 is not indolyl.
[00963] In some embodiments, X is aryl, aralkyl, substituted aryl, or substituted aralkyl.
[00964] In some embodiments, X is aryl or aralkyl.
[00965] In some embodiments, X is aryl.
[00966] In some embodiments, X is phenyl.
[00967] In some embodiments, R2 is H or alkyl or 1-3 carbons.
[00968] In some embodiments, R2 is H.
[00969] In some embodiments, RJ is H or alkyl.
[00970] In some embodiments, RJ is H or alkyl or 1-3 carbons.
[00971] In some embodiments, RJ is H.
[00972] In some embodiments, RJ and Q are joined together to form ring.
[00973] In some embodiments, RJ and Q are joined together to form a 5-6 membered ring
[00974] In some embodiments, t is 0, and R3 and Q are joined together to form a 5-6 membered ring.
[00975] In some embodiments, t is 0 and R3 and Q are joined together to form a 5 membered ring.
[00976] In some embodiments, r is 0-2.
[00977] In some embodiments, r is 0-1.
[00978] In some embodiments, r is 0.
[00979] In some embodiments, t is 0-2.
[00980] In some embodiments, t is 0-1.
[00981] In some embodiments, t is 0.
[00982] In some embodiments, r is 0-1 and t is 0.
[00983] In some embodiments, r is 0 and t is 0.
[00984] In some embodiments, the compound of formula I is
-CF3; and R1 is phenyl or yi> ;. or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00985] In some embodiments, the compound of fomula I is
A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5-
6 membered ring; and R2 and G are selected from (i) and (ii) as follows:
(i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, orG is joined together with R2 to form a 5-6 membered ring;
is not a substituted or unsubstituted
(ii) v is 1 to 3,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00986] In some embodiments, the compound of fomula I is
A and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1- 3 carbons, -CN, azido, or NO2; wherein at least one of A and B is not H;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or
NO2, or G is joined together with R2 to form a 5-6 membered ring;
with the proviso that G is not a substituted or unsubstituted
(ii) v is 1-3,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
00987] In some embodiments, R >2
[ 2 J G is not a substuted or unsubstituted moiety
having the formula
[00989] In further embodiments, A and B are each, independently, H, hydroxyl, or alkoxy of
1-3 carbons; R3 is H or alkyl of 1-3 carbons, Q is methyl, R2 is H, alkyl of 1-3 carbons, and G is
H, or hydroxyl.
[00990] In further embodiments, the compound of formula I has the following formula:
or a pharmaceutically acceptable derivative thereof. [00991] In further embodiments, the compound of formula I has the following formula:
[00992] In some embodiments, the compound of formula I is a compound of formula II:
[00993] In some embodiments, the compound of formula I is a compound of formula III:
HI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00994] In some embodiments, the compound of formula I is a compound of formula IV:
IV; a pharmaceutically acceptable derivative or stereoisomer thereof.
[00995] In some embodiments, the compound of formula I is a compound of formula V:
V; or a pharmaceutically acceptable derivative or stereoisomer thereof. [00996] In some embodiments, the compound of formula I is a compound of formula VI:
VI; or a pharmaceutically acceptable derivative thereof.
[00997] In some embodiments, the compound of formula I is a compound of formula VII:
VII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[00998] In some embodiments, the compound of formula I is a compound of formula VIII:
VIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
IX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001000] In some embodiments, the compound of formula I is a compound of formula X:
X; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001001] In some embodiments, the compound of formula I is a compound of formula XI:
XI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001002] In some embodiments, the compound of formula I is a compound of formula XII:
XII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001003] In some embodiments, the compound of formula I is a compound of formula XIII:
XIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001004] In some embodiments, the compound of formula I is a compound of formula XIV:
XIV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001005] In some embodiments, the compound of formula I is a compound of formula XV:
XV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001006] In some embodiments, the compound of formula I is a compound of formula XVI:
XVI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001007] In some embodiments, the compound of formula I is a compound of formula XVII:
XVII;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001008] In some embodiments, the compound of formula I is a compound of formula XVIII:
XVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001009] In some embodiments, the compound of formula I is a compound of formula XIX:
XIX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001010] In some embodiments, the compound of formula I is a compound of formula XX:
XX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001011] In some embodiments, the compound of formula I is a compound of formula XXI:
XXI; or a pharmaceutically acceptable derivative thereof. [001012] In some embodiments, the compound of formula I is a compound of formula XXII:
XXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001013] In some embodiments, the compound of formula I is a compound of formula XXIII:
XXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001014] Is some embodiments, the compound of formula I is a compound of formula XXIV:
XXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001015] In some embodiments, the compound of formula I is a compound of formula XXV:
XXV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001016] In some embodiments, the compound of formula I is a compound of formula XXVI:
XXVI;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001017] In some embodiments, the compound of formula I is a compound of formula XXVII:
XXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001018] In some embodiments, the compound of formula I is a compound of formula XXVIII:
XXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001019] In some embodiments, the compound of formula I is a compound of formula XXIX:
[001020] In some embodiments, the compound of formula I is a compound of formula XXX:
XXX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001021] In some embodiments, the compound of formula I is a compound of formula XXXI:
XXXI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001022] In some embodiments, the compound of formula I is a compound of formula XXXII:
XXXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001023] In some embodiments, the compound of formula I is a compound of formula XXXIII:
XXXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001024] In some embodiments, the compound of formula I is a compound of formula XXXIV:
XXXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001025] In some embodiments, the compound of formula I is a compound of formula XXXV:
XXXV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XXXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001027] In some embodiments, the compound of formula I is a compound of formula XXXVII:
XXXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001028] In some embodiments, the compound of formula I is a compound of formula
XXXVIII:
[001029] In some embodiments, the compound of formula I is a compound of formula XXXIX:
[001030] In some embodiments, the compound of formula I is a compound of formula XL:
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001031] In some embodiments, the compound of formula I is a compound of formula XLI:
[001032] In some embodiments, the compound of formula I is a compound of formula XLII:
XLII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001033] In some embodiments, the compound of formula I is a compound of formula XLIII:
XLIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
XLIV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001035] In some embodiments, the compound of formula I is a compound of formula XLV
XLV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001036] In some embodiments, the compound of formula I is a compound of formula XLVI
XLVI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001037] In some embodiments, the compound of formula I is a compound of formula XLVII
XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof. [001038] In some embodiments, the compound of formula I is a compound of formula XLVIII
XLVIII or a pharmaceutically acceptable derivative or stereoisomer thereof. [001039] In some embodiments, the compound of formula I is a compound of formula XLIX
XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof. [001040] In some embodiments, the compound of formula I is a compound of formula L
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001041] In some embodiments, the compound of formula I is a compound of formula LI
LI or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001042] In some embodiments, the compound of formula I is a compound of formula LII
LII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001043] In some embodiments, the compound of formula I is a compound of formula LII
LIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
5.3. FORMULATION OF PHARMACEUTICAL COMPOSITIONS
[001044] In some embodiments, provided herein are pharmaceutical compositions comprising one or more compounds described herein, or a pharmaceutically acceptable derivative or stereoisomer thereof and a pharmaceutically acceptable vehicle, carrier, diluent, or excipient, or a mixture thereof.
[001045] In one embodiment, provided herein are pharmaceutical compositions in modified release dosage forms, which comprise a compound described herein, or pharmaceutically acceptable derivative thereof, and one or more release controlling excipients as described herein. Suitable modified release dosage vehicles include, but are not limited to, hydrophilic or hydrophobic matrix devices, water-soluble separating layer coatings, enteric coatings, osmotic devices, multiparticulate devices, and combinations thereof. The pharmaceutical compositions may also comprise non-release controlling excipients.
[001046] Further provided herein are pharmaceutical compositions in enteric coated dosage forms, which comprise a compound described herein, or a pharmaceutically acceptable derivative thereof, and one or more release controlling excipients for use in an enteric coated dosage form. The pharmaceutical compositions may also comprise non-release controlling excipients.
[001047] Additionally provided are pharmaceutical compositions in a dosage form that has an instant releasing component and at least one delayed releasing component, and is capable of giving a discontinuous release of the compound in the form of at least two consecutive pulses separated in time from 0.1 up to 24 hours. The pharmaceutical compositions comprise a compound described herein, or a pharmaceutically acceptable derivative thereof, and one or more release controlling and non-release controlling excipients, such as those excipients suitable for a disruptable semi-permeable membrane and as swellable substances.
[001048] In certain embodiments, provided herein are pharmaceutical compositions in a dosage form for oral administration to a subject, which comprise a compound described herein, or a pharmaceutically acceptable derivative thereof, and one or more pharmaceutically acceptable excipients or carriers, enclosed in an intermediate reactive layer comprising a gastric juice- resistant polymeric layered material partially neutralized with alkali and having cation exchange capacity and a gastric juice-resistant outer layer.
[001049] In one embodiment, the pharmaceutical compositions herein may be provided in unit- dosage forms or multiple-dosage forms. Unit-dosage forms, as used herein, refer to physically discrete units suitable for administration to human and animal subjects and packaged individually as is known in the art. Each unit-dose contains a predetermined quantity of the active ingredient(s) sufficient to produce the desired therapeutic effect, in association with the required pharmaceutical carriers or excipients. Examples of unit-dosage forms include ampoules, syringes, and individually packaged tablets and capsules. Unit-dosage forms may be administered in fractions or multiples thereof. A multiple-dosage form is a plurality of identical unit-dosage forms packaged in a single container to be administered in segregated unit-dosage form. Examples of multiple-dosage forms include vials, bottles of tablets or capsules, or bottles of pints or gallons.
[001050] The compounds described herein may be administered alone, or in combination with one or more other compounds provided herein, one or more other active ingredients. The pharmaceutical compositions that comprise a compound provided herein may be formulated in various dosage forms for oral, parenteral, and topical administration. The pharmaceutical compositions may also be formulated as modified release dosage forms, including delayed-, extended-, prolonged-, sustained-, pulsatile-, controlled-, accelerated- and fast-, targeted-, programmed-release, and gastric retention dosage forms. These dosage forms can be prepared according to conventional methods and techniques known to those skilled in the art (see, Remington: The Science and Practice of Pharmacy, supra; Modified-Release Drug Deliver Technology, Rathbone et al., Eds., Drugs and the Pharmaceutical Science, Marcel Dekker, Inc.: New York, NY, 2002; Vol. 126).
[001051] The pharmaceutical compositions provided herein may be administered at once, or multiple times at intervals of time. It is understood that the precise dosage and duration of treatment may vary with the age, weight, and condition of the patient being treated, and may be determined empirically using known testing protocols or by extrapolation from in vivo or in vitro test or diagnostic data. It is further understood that for any particular individual, specific dosage regimens should be adjusted over time according to the individual need and the professional judgment of the person administering or supervising the administration of the formulations.
5.3.1. Oral Administration
[001052] The pharmaceutical compositions provided herein may be provided in solid, semisolid, or liquid dosage forms for oral administration. As used herein, oral administration also includes buccal, lingual, and sublingual administration. Suitable oral dosage forms include, but are not limited to, tablets, capsules, pills, troches, lozenges, pastilles, cachets, pellets, medicated chewing gum, granules, bulk powders, effervescent or non-effervescent powders or granules, solutions, emulsions, suspensions, solutions, wafers, sprinkles, elixirs, and syrups. In addition to the active ingredient(s), the pharmaceutical compositions may contain one or more pharmaceutically acceptable carriers or excipients, including, but not limited to, binders, fillers, diluents, disintegrants, wetting agents, lubricants, glidants, coloring agents, dye-migration inhibitors, sweetening agents, and flavoring agents.
[001053] Binders or granulators impart cohesiveness to a tablet to ensure that the tablet remains intact after compression. Suitable binders or granulators include, but are not limited to, starches, such as corn starch, potato starch, and pre-gelatinized starch (e.g., STARCH 1500); gelatin; sugars, such as sucrose, glucose, dextrose, molasses, and lactose; natural and synthetic gums, such as acacia, alginic acid, alginates, extract of Irish moss, Panwar gum, ghatti gum, mucilage of isabgol husks, carboxymethylcellulose, methylcellulose, polyvinylpyrrolidone (PVP), Veegum, larch arabogalactan, powdered tragacanth, and guar gum; celluloses, such as ethyl cellulose, cellulose acetate, carboxymethyl cellulose calcium, sodium carboxymethyl cellulose, methyl cellulose, hydroxyethylcellulose (HEC), hydroxypropylcellulose (HPC), hydroxypropyl methyl cellulose (HPMC); micro crystalline celluloses, such as AVICEL-PH-101, AVICEL-PH- 103, AVICEL PvC-581, AVICEL-PH-105 (FMC Corp., Marcus Hook, PA); and mixtures thereof. Suitable fillers include, but are not limited to, talc, calcium carbonate, microcrystalline cellulose, powdered cellulose, dextrates, kaolin, mannitol, silicic acid, sorbitol, starch, pre- gelatinized starch, and mixtures thereof. The binder or filler may be present from about 50 to about 99% by weight in the pharmaceutical compositions provided herein. [001054] Suitable diluents include, but are not limited to, dicalcium phosphate, calcium sulfate, lactose, sorbitol, sucrose, inositol, cellulose, kaolin, mannitol, sodium chloride, dry starch, and powdered sugar. Certain diluents, such as mannitol, lactose, sorbitol, sucrose, and inositol, when present in sufficient quantity, can impart properties to some compressed tablets that permit
disintegration in the mouth by chewing. Such compressed tablets can be used as chewable tablets.
[001055] Suitable disintegrants include, but are not limited to, agar; bentonite; celluloses, such as methylcellulose and carboxymethylcellulose; wood products; natural sponge; cation-exchange resins; alginic acid; gums, such as guar gum and Veegum HV; citrus pulp; cross-linked celluloses, such as croscarmellose; cross-linked polymers, such as crospovidone; cross-linked starches; calcium carbonate; microcrystalline cellulose, such as sodium starch glycolate; polacrilin potassium; starches, such as corn starch, potato starch, tapioca starch, and pre- gelatinized starch; clays; aligns; and mixtures thereof. The amount of disintegrant in the pharmaceutical compositions provided herein varies upon the type of formulation, and is readily discernible to those of ordinary skill in the art. The pharmaceutical compositions provided herein may contain from about 0.5 to about 15% or from about 1 to about 5% by weight of a disintegrant.
[001056] Suitable lubricants include, but are not limited to, calcium stearate; magnesium stearate; mineral oil; light mineral oil; glycerin; sorbitol; mannitol; glycols, such as glycerol behenate and polyethylene glycol (PEG); stearic acid; sodium lauryl sulfate; talc; hydrogenated vegetable oil, including peanut oil, cottonseed oil, sunflower oil, sesame oil, olive oil, corn oil, and soybean oil; zinc stearate; ethyl oleate; ethyl laureate; agar; starch; lycopodium; silica or silica gels, such as AEROSIL® 200 (W.R. Grace Co., Baltimore, MD) and CAB-O-SIL® (Cabot Co. of Boston, MA); and mixtures thereof. The pharmaceutical compositions provided herein may contain about 0.1 to about 5% by weight of a lubricant.
[001057] Suitable glidants include colloidal silicon dioxide, CAB-O-SIL® (Cabot Co. of Boston, MA), and asbestos-free talc. Coloring agents include any of the approved, certified, water soluble FD&C dyes, and water insoluble FD&C dyes suspended on alumina hydrate, and color lakes and mixtures thereof. A color lake is the combination by adsorption of a water- soluble dye to a hydrous oxide of a heavy metal, resulting in an insoluble form of the dye. Flavoring agents include natural flavors extracted from plants, such as fruits, and synthetic blends of compounds which produce a pleasant taste sensation, such as peppermint and methyl salicylate. Sweetening agents include sucrose, lactose, mannitol, syrups, glycerin, and artificial sweeteners, such as saccharin and aspartame. Suitable emulsifying agents include gelatin, acacia, tragacanth, bentonite, and surfactants, such as polyoxyethylene sorbitan monooleate
(TWEEN 20), polyoxyethylene sorbitan monooleate 80 (TWEEN 80), and triethanolamine oleate. Suspending and dispersing agents include sodium carboxymethylcellulose, pectin, tragacanth, Veegum, acacia, sodium carbomethylcellulose, hydroxypropyl methylcellulose, and polyvinylpyrolidone. Preservatives include glycerin, methyl and propylparaben, benzoic add, sodium benzoate and alcohol. Wetting agents include propylene glycol monostearate, sorbitan monooleate, diethylene glycol monolaurate, and polyoxyethylene lauryl ether. Solvents include glycerin, sorbitol, ethyl alcohol, and syrup. Examples of non-aqueous liquids utilized in emulsions include mineral oil and cottonseed oil. Organic acids include citric and tartaric acid. Sources of carbon dioxide include sodium bicarbonate and sodium carbonate. [001058] It should be understood that many carriers and excipients may serve several functions, even within the same formulation.
[001059] The pharmaceutical compositions provided herein may be provided as compressed tablets, tablet triturates, chewable lozenges, rapidly dissolving tablets, multiple compressed tablets, or enteric-coating tablets, sugar-coated, or film-coated tablets. Enteric-coated tablets are compressed tablets coated with substances that resist the action of stomach acid but dissolve or disintegrate in the intestine, thus protecting the active ingredients from the acidic environment of the stomach. Enteric-coatings include, but are not limited to, fatty acids, fats, phenylsalicylate, waxes, shellac, ammoniated shellac, and cellulose acetate phthalates. Sugar-coated tablets are compressed tablets surrounded by a sugar coating, which may be beneficial in covering up objectionable tastes or odors and in protecting the tablets from oxidation. Film-coated tablets are compressed tablets that are covered with a thin layer or film of a water-soluble material. Film coatings include, but are not limited to, hydroxyethylcellulose, sodium carboxymethylcellulose, polyethylene glycol 4000, and cellulose acetate phthalate. Film coating imparts the same general characteristics as sugar coating. Multiple compressed tablets are compressed tablets made by more than one compression cycle, including layered tablets, and press-coated or dry-coated tablets.
[001060] The tablet dosage forms may be prepared from the active ingredient in powdered, crystalline, or granular forms, alone or in combination with one or more carriers or excipients described herein, including binders, disintegrants, controlled-release polymers, lubricants, diluents, and/or colorants. Flavoring and sweetening agents are especially useful in the formation of chewable tablets and lozenges.
[001061] The pharmaceutical compositions provided herein may be provided as soft or hard capsules, which can be made from gelatin, methylcellulose, starch, or calcium alginate. The hard gelatin capsule, also known as the dry-filled capsule (DFC), consists of two sections, one slipping over the other, thus completely enclosing the active ingredient. The soft elastic capsule (SEC) is a soft, globular shell, such as a gelatin shell, which is plasticized by the addition of glycerin, sorbitol, or a similar polyol. The soft gelatin shells may contain a preservative to prevent the growth of microorganisms. Suitable preservatives are those as described herein, including methyl- and propyl-parabens, and sorbic acid. The liquid, semisolid, and solid dosage forms provided herein may be encapsulated in a capsule. Suitable liquid and semisolid dosage forms include solutions and suspensions in propylene carbonate, vegetable oils, or triglycerides. Capsules containing such solutions can be prepared as described in U.S. Pat. Nos. 4,328,245; 4,409,239; and 4,410,545. The capsules may also be coated as known by those of skill in the art in order to modify or sustain dissolution of the active ingredient.
[001062] The pharmaceutical compositions provided herein may be provided in liquid and semisolid dosage forms, including emulsions, solutions, suspensions, elixirs, and syrups. An emulsion is a two-phase system, in which one liquid is dispersed in the form of small globules throughout another liquid, which can be oil-in-water or water-in-oil. Emulsions may include a pharmaceutically acceptable non-aqueous liquids or solvent, emulsifying agent, and preservative. Suspensions may include a pharmaceutically acceptable suspending agent and preservative. Aqueous alcoholic solutions may include a pharmaceutically acceptable acetal, such as a di(lower alkyl) acetal of a lower alkyl aldehyde, e.g., acetaldehyde diethyl acetal; and a water- miscible solvent having one or more hydroxyl groups, such as propylene glycol and ethanol. Elixirs are clear, sweetened, and hydroalcoholic solutions. Syrups are concentrated aqueous solutions of a sugar, for example, sucrose, and may also contain a preservative. For a liquid dosage form, for example, a solution in a polyethylene glycol may be diluted with a sufficient quantity of a pharmaceutically acceptable liquid carrier, e.g. , water, to be measured conveniently for administration.
[001063] Other useful liquid and semisolid dosage forms include, but are not limited to, those containing the active ingredient(s) provided herein, and a dialkylated mono- or poly-alkylene glycol, including, 1 ,2-dimethoxymethane, diglyme, triglyme, tetraglyme, polyethylene glycol- 350-dimethyl ether, polyethylene glycol-550-dimethyl ether, polyethylene glycol-750-dimethyl
ether, wherein 350, 550, and 750 refer to the approximate average molecular weight of the polyethylene glycol. These formulations may further comprise one or more antioxidants, such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), propyl gallate, vitamin E, hydroquinone, hydroxycoumarins, ethanolamine, lecithin, cephalin, ascorbic acid, malic acid, sorbitol, phosphoric acid, bisulfite, sodium metabisulfite, thiodipropionic acid and its esters, and dithiocarbamates .
[001064] The pharmaceutical compositions provided herein for oral administration may be also provided in the forms of liposomes, micelles, microspheres, or nanosystems. Micellar dosage forms can be prepared as described in U.S. Pat. No. 6,350,458.
[001065] The pharmaceutical compositions provided herein may be provided as non- effervescent or effervescent, granules and powders, to be reconstituted into a liquid dosage form. Pharmaceutically acceptable carriers and excipients used in the non-effervescent granules or powders may include diluents, sweeteners, and wetting agents. Pharmaceutically acceptable carriers and excipients used in the effervescent granules or powders may include organic acids and a source of carbon dioxide.
[001066] Coloring and flavoring agents can be used in all of the above dosage forms. [001067] The pharmaceutical compositions provided herein may be formulated as immediate or modified release dosage forms, including delayed-, sustained, pulsed-, controlled, targeted-, and programmed-release forms.
[001068] The pharmaceutical compositions provided herein may be co-formulated with other active ingredients which do not impair the desired therapeutic action, or with substances that supplement the desired action, such as antacids, proton pump inhibitors, and H2-receptor antagonists. 5.3.2. Parenteral Administration
[001069] The pharmaceutical compositions provided herein may be administered parenterally by injection, infusion, or implantation, for local or systemic administration. Parenteral administration, as used herein, include intravenous, intraarterial, intraperitoneal, intrathecal, intraventricular, intraurethral, intrasternal, intracranial, intramuscular, intrasynovial, and subcutaneous administration.
[001070] The pharmaceutical compositions provided herein may be formulated in any dosage forms that are suitable for parenteral administration, including solutions, suspensions, emulsions,
micelles, liposomes, microspheres, nanosystems, and solid forms suitable for solutions or suspensions in liquid prior to injection. Such dosage forms can be prepared according to conventional methods known to those skilled in the art of pharmaceutical science (see, Remington: The Science and Practice of Pharmacy, supra).
[001071] The pharmaceutical compositions intended for parenteral administration may include one or more pharmaceutically acceptable carriers and excipients, including, but not limited to, aqueous vehicles, water-miscible vehicles, non-aqueous vehicles, antimicrobial agents or preservatives against the growth of microorganisms, stabilizers, solubility enhancers, isotonic agents, buffering agents, antioxidants, local anesthetics, suspending and dispersing agents, wetting or emulsifying agents, complexing agents, sequestering or chelating agents, cryoprotectants, lyoprotectants, thickening agents, pH adjusting agents, and inert gases. [001072] Suitable aqueous vehicles include, but are not limited to, water, saline, physiological saline or phosphate buffered saline (PBS), sodium chloride injection, Ringers injection, isotonic dextrose injection, sterile water injection, dextrose and lactated Ringers injection. Non-aqueous vehicles include, but are not limited to, fixed oils of vegetable origin, castor oil, corn oil, cottonseed oil, olive oil, peanut oil, peppermint oil, safflower oil, sesame oil, soybean oil, hydrogenated vegetable oils, hydrogenated soybean oil, and medium-chain triglycerides of coconut oil, and palm seed oil. Water-miscible vehicles include, but are not limited to, ethanol, 1,3-butanediol, liquid polyethylene glycol (e.g., polyethylene glycol 300 and polyethylene glycol 400), propylene glycol, glycerin, JV-methyl-2-pyrrolidone, dimethylacetamide, and dimethy lsulfoxide .
[001073] Suitable antimicrobial agents or preservatives include, but are not limited to, phenols, cresols, mercurials, benzyl alcohol, chlorobutanol, methyl and propyl p-hydroxybenzates, thimerosal, benzalkonium chloride, benzethonium chloride, methyl- and propylparabens, and sorbic acid. Suitable isotonic agents include, but are not limited to, sodium chloride, glycerin, and dextrose. Suitable buffering agents include, but are not limited to, phosphate and citrate. Suitable antioxidants are those as described herein, including bisulfite and sodium metabisulfite. Suitable local anesthetics include, but are not limited to, procaine hydrochloride. Suitable suspending and dispersing agents are those as described herein, including sodium carboxymethylcelluose, hydroxypropyl methylcellulose, and polyvinylpyrrolidone. Suitable emulsifying agents include those described herein, including polyoxyethylene sorbitan
monolaurate, polyoxyethylene sorbitan monooleate 80, and triethanolamine oleate. Suitable sequestering or chelating agents include, but are not limited to EDTA. Suitable pH adjusting agents include, but are not limited to, sodium hydroxide, hydrochloric acid, citric acid, and lactic acid. Suitable complexing agents include, but are not limited to, cyclodextrins, including alpha- cyclodextrin, beta-cyclodextrin, hydroxypropyl-beta-cyclodextrin, sulfobutylether-beta- cyclodextrin, and sulfobutylether 7-beta-cyclodextrin (CAPTISOL®, CyDex, Lenexa, KS). [001074] The pharmaceutical compositions provided herein may be formulated for single or multiple dosage administration. The single dosage formulations are packaged in an ampule, a vial, or a syringe. The multiple dosage parenteral formulations must contain an antimicrobial agent at bacteriostatic or fungistatic concentrations. All parenteral formulations must be sterile, as known and practiced in the art.
[001075] In one embodiment, the pharmaceutical compositions are provided as ready-to-use sterile solutions. In another embodiment, the pharmaceutical compositions are provided as sterile dry soluble products, including lyophilized powders and hypodermic tablets, to be reconstituted with a vehicle prior to use. In yet another embodiment, the pharmaceutical compositions are provided as ready-to-use sterile suspensions. In yet another embodiment, the pharmaceutical compositions are provided as sterile dry insoluble products to be reconstituted with a vehicle prior to use. In still another embodiment, the pharmaceutical compositions are provided as ready-to-use sterile emulsions.
[001076] The pharmaceutical compositions provided herein may be formulated as immediate or modified release dosage forms, including delayed-, sustained, pulsed-, controlled, targeted-, and programmed-release forms.
[001077] The pharmaceutical compositions may be formulated as a suspension, solid, semisolid, or thixotropic liquid, for administration as an implanted depot. In one embodiment, the pharmaceutical compositions provided herein are dispersed in a solid inner matrix, which is surrounded by an outer polymeric membrane that is insoluble in body fluids but allows the active ingredient in the pharmaceutical compositions diffuse through.
[001078] Suitable inner matrixes include polymethylmethacrylate, polybutylmethacrylate, plasticized or unplasticized polyvinylchloride, plasticized nylon, plasticized polyethyleneterephthalate, natural rubber, polyisoprene, polyisobutylene, polybutadiene, polyethylene, ethylene-vinylacetate copolymers, silicone rubbers, polydimethylsiloxanes,
silicone carbonate copolymers, hydrophilic polymers, such as hydrogels of esters of acrylic and methacrylic acid, collagen, cross-linked polyvinylalcohol, and cross-linked partially hydrolyzed polyvinyl acetate.
[001079] Suitable outer polymeric membranes include polyethylene, polypropylene, ethylene/propylene copolymers, ethylene/ethyl acrylate copolymers, ethylene/vinylacetate copolymers, silicone rubbers, polydimethyl siloxanes, neoprene rubber, chlorinated polyethylene, polyvinylchloride, vinylchloride copolymers with vinyl acetate, vinylidene chloride, ethylene and propylene, ionomer polyethylene terephthalate, butyl rubber epichlorohydrin rubbers, ethylene/vinyl alcohol copolymer, ethylene/vinyl acetate/vinyl alcohol terpolymer, and ethylene/vinyloxyethanol copolymer.
5.3.3. Topical Administration
[001080] The pharmaceutical compositions provided herein may be administered topically to the skin, orifices, or mucosa. The topical administration, as used herein, include (intra)dermal, conjuctival, intracorneal, intraocular, ophthalmic, auricular, transdermal, nasal, vaginal, uretheral, respiratory, and rectal administration.
[001081] The pharmaceutical compositions provided herein may be formulated in any dosage forms that are suitable for topical administration for local or systemic effect, including emulsions, solutions, suspensions, creams, gels, hydrogels, ointments, dusting powders, dressings, elixirs, lotions, suspensions, tinctures, pastes, foams, films, aerosols, irrigations, sprays, suppositories, bandages, dermal patches. The topical formulation of the pharmaceutical compositions provided herein may also comprise liposomes, micelles, microspheres, nanosystems, and mixtures thereof.
[001082] Pharmaceutically acceptable carriers and excipients suitable for use in the topical formulations provided herein include, but are not limited to, aqueous vehicles, water-miscible vehicles, non-aqueous vehicles, antimicrobial agents or preservatives against the growth of microorganisms, stabilizers, solubility enhancers, isotonic agents, buffering agents, antioxidants, local anesthetics, suspending and dispersing agents, wetting or emulsifying agents, complexing agents, sequestering or chelating agents, penetration enhancers, cryopretectants, lyoprotectants, thickening agents, and inert gases.
[001083] The pharmaceutical compositions may also be administered topically by electroporation, iontophoresis, phonophoresis, sonophoresis and microneedle or needle-free injection, such as POWDERJECT™ (Chiron Corp., Emeryville, CA), and BIOJECT™ (Bioject Medical Technologies Inc., Tualatin, OR).
[001084] The pharmaceutical compositions provided herein may be provided in the forms of ointments, creams, and gels. Suitable ointment vehicles include oleaginous or hydrocarbon bases, including such as lard, benzoinated lard, olive oil, cottonseed oil, and other oils, white petrolatum; emulsifiable or absorption bases, such as hydrophilic petrolatum, hydroxystearin sulfate, and anhydrous lanolin; water-removable bases, such as hydrophilic ointment; water- soluble ointment bases, including polyethylene glycols of varying molecular weight; emulsion bases, either water-in-oil (W/O) emulsions or oil-in-water (O/W) emulsions, including cetyl alcohol, glyceryl monostearate, lanolin, and stearic acid (see, Remington: The Science and Practice of Pharmacy, supra). These vehicles are emollient but generally require addition of antioxidants and preservatives.
[001085] Suitable cream base can be oil-in-water or water-in-oil. Cream vehicles may be water-washable, and contain an oil phase, an emulsifier, and an aqueous phase. The oil phase is also called the "internal" phase, which is generally comprised of petrolatum and a fatty alcohol such as cetyl or stearyl alcohol. The aqueous phase usually, although not necessarily, exceeds the oil phase in volume, and generally contains a humectant. The emulsifier in a cream formulation may be a nonionic, anionic, cationic, or amphoteric surfactant. [001086] Gels are semisolid, suspension-type systems. Single -phase gels contain organic macromolecules distributed substantially uniformly throughout the liquid carrier. Suitable gelling agents include crosslinked acrylic acid polymers, such as carbomers, carboxypolyalkylenes, Carbopol®; hydrophilic polymers, such as polyethylene oxides, polyoxyethylene-polyoxypropylene copolymers, and polyvinylalcohol; cellulosic polymers, such as hydroxypropyl cellulose, hydroxyethyl cellulose, hydroxypropyl methylcellulose, hydroxypropyl methylcellulose phthalate, and methylcellulose; gums, such as tragacanth and xanthan gum; sodium alginate; and gelatin. In order to prepare a uniform gel, dispersing agents such as alcohol or glycerin can be added, or the gelling agent can be dispersed by trituration, mechanical mixing, and/or stirring.
[001087] The pharmaceutical compositions provided herein may be administered rectally, urethrally, vaginally, or perivaginally in the forms of suppositories, pessaries, bougies, poultices or cataplasm, pastes, powders, dressings, creams, plasters, contraceptives, ointments, solutions, emulsions, suspensions, tampons, gels, foams, sprays, or enemas. These dosage forms can be manufactured using conventional processes as described in Remington: The Science and Practice of Pharmacy, supra.
[001088] Rectal, urethral, and vaginal suppositories are solid bodies for insertion into body orifices, which are solid at ordinary temperatures but melt or soften at body temperature to release the active ingredient(s) inside the orifices. Pharmaceutically acceptable carriers utilized in rectal and vaginal suppositories include vehicles, such as stiffening agents, which produce a melting point in the proximity of body temperature, when formulated with the pharmaceutical compositions provided herein; and antioxidants as described herein, including bisulfite and sodium metabisulfite. Suitable vehicles include, but are not limited to, cocoa butter (theobroma oil), glycerin-gelatin, carbowax (polyoxyethylene glycol), spermaceti, paraffin, white and yellow wax, and appropriate mixtures of mono-, di- and triglycerides of fatty acids, hydrogels, such as polyvinyl alcohol, hydroxyethyl methacrylate, polyacrylic acid; glycerinated gelatin. Combinations of the various vehicles may be used. Rectal and vaginal suppositories may be prepared by the compressed method or molding. The typical weight of a rectal and vaginal suppository is about 2 to 3 g.
[001089] The pharmaceutical compositions provided herein may be administered ophthalmically in the forms of solutions, suspensions, ointments, emulsions, gel-forming solutions, powders for solutions, gels, ocular inserts, and implants.
[001090] The pharmaceutical compositions provided herein may be administered intranasally or by inhalation to the respiratory tract. The pharmaceutical compositions may be provided in the form of an aerosol or solution for delivery using a pressurized container, pump, spray, atomizer, such as an atomizer using electrohydrodynamics to produce a fine mist, or nebulizer, alone or in combination with a suitable propellant, such as 1,1,1,2-tetrafluoroethane or 1,1, 1,2,3, 3,3-heptafluoropropane. The pharmaceutical compositions may also be provided as a dry powder for insufflation, alone or in combination with an inert carrier such as lactose or phospholipids; and nasal drops. For intranasal use, the powder may comprise a bioadhesive agent, including chitosan or cyclodextrin.
[001091] Solutions or suspensions for use in a pressurized container, pump, spray, atomizer, or nebulizer may be formulated to contain ethanol, aqueous ethanol, or a suitable alternative agent for dispersing, solubilizing, or extending release of the active ingredient provided herein, a propellant as solvent; and/or a surfactant, such as sorbitan trioleate, oleic acid, or an oligolactic acid.
[001092] The pharmaceutical compositions provided herein may be micronized to a size suitable for delivery by inhalation, such as 50 micrometers or less, or 10 micrometers or less. Particles of such sizes may be prepared using a comminuting method known to those skilled in the art, such as spiral jet milling, fluid bed jet milling, supercritical fluid processing to form nanoparticles, high pressure homogenization, or spray drying. The pharmaceutical compositions provided herein may also be micronized to a size suitable for injection.
[001093] Capsules, blisters and cartridges for use in an inhaler or insufflator may be formulated to contain a powder mix of the pharmaceutical compositions provided herein; a suitable powder base, such as lactose or starch; and a performance modifier, such as /-leucine, mannitol, or magnesium stearate. The lactose may be anhydrous or in the form of the monohydrate. Other suitable excipients include dextran, glucose, maltose, sorbitol, xylitol, fructose, sucrose, and trehalose. The pharmaceutical compositions provided herein for inhaled/intranasal administration may further comprise a suitable flavor, such as menthol and levomenthol, or sweeteners, such as saccharin or saccharin sodium.
[001094] The pharmaceutical compositions provided herein for topical administration may be formulated to be immediate release or modified release, including delayed-, sustained-, pulsed-, controlled-, targeted, and programmed release. 5.3.4. Nanoparticulate Formulation
[001095] The pharmaceutical compositions provided herein can also be formulated as nanoparticulates, which comprises particles, granules, or capsules, with diameters of about 1 nm, about 10 nm, about 50 nm, about 100 nm, about 150 nm, about 200 nm, about 250 nm, about 300 nm, about 350 nm, about 400 nm, about 450 nm, about 500 nm, about 600 nm, about 700 nm, about 800 nm, about 900 nm, about 1000 nm, or about 2000 nm. In some embodiments, the pharmaceutical compositions provided herein comprises a multiplicity of particles, granules or capsules having diameters ranging from about 1 nm to about 2000 nm, from about 10 nm to about 1000 nm, from about 10 nm to about 500 nm, from about 10 nm to about 400 nm, from
about 10 nm to about 300 nm, from about 10 nm to about 250 nm, from about 10 nm to about 200 nm, from about 10 nm to about 150 nm, from about 10 nm to about 100 nm, from about 25 nm to about 250 nm, or from about 50 nm to about 300 nm. In one embodiments, the nanoparticulates are nanocrystals, or nanosuspensions. The nanoparticulates comprising the active drug ingredient(s) optionally further comprise other therapeutic agents and/or one or more other ingredient(s). In one embodiment, the other therapeutic agents and/or one or more other ingredient is/are excipients as described herein elsewhere. In one embodiment, the other therapeutic agents and/or one or more other ingredient is/are carriers, micelles, dendrimers, metals, polymers, antibodies, proteins, liposomes, lipids, lipoproteins, glycoproteins, cyclodextrins, or viral vectors. The surface of the nanoparticulates is optionally coated with material known to one skilled in the art. In one embodiment, the material is a proteins, polymer, ligand, film coating, hydrophilic coatings, enteric coating, delayed-release coating, enteric polymer, water-swellable polymer, or water-soluble polymer. The polymers in the nanoparticulate formulation provided herein may be biodegradable. The nanoparticulates may, in one embodiment, be stabilized by proteins or polymers. The shape of the nanoparticulates may be spherical or non-spherical. The nanoparticulates provided herein can be made by processes known to those skilled in the art. In one embodiment, the process is milling, wet or dry granulation, homogenization, sprayed-dry dispersion, freeze-drying, hot-melting matrices, pelletization, direct compress, precipitation, supercritical-fluid dispersion, or emulsifϊcation- diffusion. The nanoparticulates can be further formulated as a solid, a semi-solid, or a liquid. The nanoparticulates can be further processed. In one embodiment, the nanoparticulates are further processed as a tablet, a capsule, a suspension, or a lyophilized powder. See generally, Nanoparticle Technology for Drug Delivery (Ram B. Gupta & Uday B. Kompella ed., Informa HealthCare 2006); Nanoparticulates As Drug Carrier (Vladimir P. Torchilin ed., World Scientific Publishing Co. 2006); Nanoparticulate Drug Delivery Systems (Deepak Thassu, Michel Deleers, & Yashwant Pathak ed., Informa HealthCare 2007); see also, BaIa et al., PLGA Nanoparticles in Drug Delivery: the State of the Art, Crit. Rev. Ther. Drug Carrier Syst. 21(5):387-422 (2004); Agnihotri et al., Recent Advances on Chitosan-Based Micro- and Nanoparticles in Drug Delivery, J. Controlled Release 100(l):5-28 (2004); Vauthier et al., Drug Delivery to Resistant Tumors: the Potential ofPoly(Alkyl Cyanoacrylate) Nanoparticles, J. Controlled Release 93(2): 151-60 (2003); Panyam et al., Biodegradable Nanoparticles for Drug
and Gene Delivery to Cells and Tissue, Adv. Drug Deliv. Rev. 55(3):329-47 (2003); Hans et al, Biodegradable Nanoparticles for Drug Delivery and Targeting, Cur. Opin. Solid State & Mat. Sci. 6(4):319-27 (2002); Lockman et al., Nanoparticle Technology for Drug Delivery Across the Blood-Brain Barrier, Drug Dev. Ind. Pharm. 28(1): 1-13 (2002); Takeuchi et al., Mucoadhesive Nanoparticulate Systems for Peptide Drug Delivery, Adv. Drug Deliv. Rev. 47(l):39-54 (2001); Soppimath et al., Biodegradable Polymeric Nanoparticles as Drug Delivery Devices, J. Controlled Release 70(1 -2): 1-20 (2001); Muller et al., Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery — A Review of the State of the Art, Eur. J. Pharm. Biopharm. 50(1): 161-77 (2000); Labhasetwar et al., Nanoparticle Drug Delivery System for Restenosis, Adv. Drug Deliv. Rev. 24(l):63-85 (1997).
[001096] The pharmaceutical composition comprising nanoparticulates may be administered by any means or route of administration known in the art. In one embodiment, the compositions are administered orally, parenthetically, ocularly, nasally, or topically. In one embodiment, the compositions are administered as an inhaler, an aerosol, a lozenge, a suppository, or an implant. The pharmaceutical composition comprising nanoparticulates can be fabricated to effect immediate release, controlled release, delayed release, sustained release, slow release, and/or targeted release of the active drug ingredient in the body of the subject being treated. The pharmaceutical composition comprising nanoparticulates may be formulated to target particular tissues, cells, receptors, proteins, genes, DNA sequences, or areas of the body of the subject to be treated.
5.3.5. Stents
[001097] In some embodiments, a stent is coated with, or contains, the compound of formula I. The stent may, in some embodiments, be implanted into a blood vessel in a patient.
5.3.6. Modified Release
[001098] The pharmaceutical compositions provided herein may be formulated as a modified release dosage form. As used herein, the term "modified release" refers to a dosage form in which the rate or place of release of the active ingredient(s) is different from that of an immediate dosage form when administered by the same route. Modified release dosage forms include delayed-, extended-, prolonged-, sustained-, pulsatile- or pulsed-, controlled-, accelerated- and fast-, targeted-, programmed-release, and gastric retention dosage forms. The pharmaceutical compositions in modified release dosage forms can be prepared using a variety
of modified release devices and methods known to those skilled in the art, including, but not limited to, matrix controlled release devices, osmotic controlled release devices, multiparticulate controlled release devices, ion-exchange resins, enteric coatings, multilayered coatings, microspheres, liposomes, and combinations thereof. The release rate of the active ingredient(s) can also be modified by varying the particle sizes and polymorphorism of the active ingredient(s).
[001099] Examples of modified release include, but are not limited to, those described in U.S.
Pat. Nos.: 3,845,770; 3,916,899; 3,536,809; 3,598,123; 4,008,719; 5,674,533; 5,059,595;
5,591,767; 5,120,548; 5,073,543; 5,639,476; 5,354,556; 5,639,480; 5,733,566; 5,739,108;
5,891,474; 5,922,356; 5,972,891; 5,980,945; 5,993,855; 6,045,830; 6,087,324; 6,113,943;
6,197,350; 6,248,363; 6,264,970; 6,267,981; 6,376,461; 6,419,961; 6,589,548; 6,613,358; and
6,699,500.
5.3.6.1. Matrix Controlled Release Devices
[001100] The pharmaceutical compositions provided herein in a modified release dosage form may be fabricated using a matrix controlled release device known to those skilled in the art (see, Takada et al in "Encyclopedia of Controlled Drug Delivery," Vol. 2, Mathiowitz ed., Wiley, 1999).
[001101] In one embodiment, the pharmaceutical compositions provided herein in a modified release dosage form is formulated using an erodible matrix device, which is water-swellable, erodible, or soluble polymers, including synthetic polymers, and naturally occurring polymers and derivatives, such as polysaccharides and proteins.
[001102] Materials useful in forming an erodible matrix include, but are not limited to, chitin, chitosan, dextran, and pullulan; gum agar, gum arabic, gum karaya, locust bean gum, gum tragacanth, carrageenans, gum ghatti, guar gum, xanthan gum, and scleroglucan; starches, such as dextrin and maltodextrin; hydrophilic colloids, such as pectin; phosphatides, such as lecithin; alginates; propylene glycol alginate; gelatin; collagen; and cellulosics, such as ethyl cellulose (EC), methylethyl cellulose (MEC), carboxymethyl cellulose (CMC), CMEC, hydroxyethyl cellulose (HEC), hydroxypropyl cellulose (HPC), cellulose acetate (CA), cellulose propionate (CP), cellulose butyrate (CB), cellulose acetate butyrate (CAB), CAP, CAT, hydroxypropyl methyl cellulose (HPMC), HPMCP, HPMCAS, hydroxypropyl methyl cellulose acetate trimellitate (HPMCAT), and ethylhydroxy ethylcellulose (EHEC); polyvinyl pyrrolidone;
polyvinyl alcohol; polyvinyl acetate; glycerol fatty acid esters; polyacrylamide; polyacrylic acid; copolymers of ethacrylic acid or methacrylic acid (EUDRAGIT®, Rohm America, Inc., Piscataway, NJ); poly(2-hydroxyethyl-methacrylate); polylactides; copolymers of L-glutamic acid and ethyl-L-glutamate; degradable lactic acid-glycolic acid copolymers; poly-D-(-)-3- hydroxybutyric acid; and other acrylic acid derivatives, such as homopolymers and copolymers of butylmethacrylate, methylmethacrylate, ethylmethacrylate, ethylacrylate, (2- dimethylaminoethyl)methacrylate, and (trimethylaminoethyl)methacrylate chloride. [001103] In another embodiment, the pharmaceutical compositions are formulated with a non- erodible matrix device. The active ingredient(s) is dissolved or dispersed in an inert matrix and is released primarily by diffusion through the inert matrix once administered. Materials suitable for use as a non-erodible matrix device include, but are not limited to, insoluble plastics, such as polyethylene, polypropylene, polyisoprene, polyisobutylene, polybutadiene, polymethylmethacrylate, polybutylmethacrylate, chlorinated polyethylene, polyvinylchloride, methyl acrylate -methyl methacrylate copolymers, ethylene-vinylacetate copolymers, ethylene/propylene copolymers, ethylene/ethyl acrylate copolymers, vinylchloride copolymers with vinyl acetate, vinylidene chloride, ethylene and propylene, ionomer polyethylene terephthalate, butyl rubber epichlorohydrin rubbers, ethylene/vinyl alcohol copolymer, ethylene/vinyl acetate/vinyl alcohol terpolymer, and ethylene/vinyloxyethanol copolymer, polyvinyl chloride, plasticized nylon, plasticized polyethyleneterephthalate, natural rubber, silicone rubbers, polydimethylsiloxanes, silicone carbonate copolymers, hydrophilic polymers, such as ethyl cellulose, cellulose acetate, crospovidone, and cross-linked partially hydrolyzed polyvinyl acetate,; and fatty compounds, such as carnauba wax, microcrystalline wax, and triglycerides.
[001104] In a matrix controlled release system, the desired release kinetics can be controlled, for example, via the polymer type employed, the polymer viscosity, the particle sizes of the polymer and/or the active ingredient(s), the ratio of the active ingredient(s) versus the polymer, and other excipients in the compositions.
[001105] The pharmaceutical compositions provided herein in a modified release dosage form may be prepared by methods known to those skilled in the art, including direct compression, dry or wet granulation followed by compression, melt-granulation followed by compression.
5.3.6.2. Osmotic Controlled Release Devices
[001106] The pharmaceutical compositions provided herein in a modified release dosage form may be fabricated using an osmotic controlled release device, including one-chamber system, two-chamber system, asymmetric membrane technology (AMT), and extruding core system (ECS). In general, such devices have at least two components: (a) the core which contains the active ingredient(s); and (b) a semipermeable membrane with at least one delivery port, which encapsulates the core. The semipermeable membrane controls the influx of water to the core from an aqueous environment of use so as to cause drug release by extrusion through the delivery port(s).
[001107] In addition to the active ingredient(s), the core of the osmotic device optionally includes an osmotic agent, which creates a driving force for transport of water from the environment of use into the core of the device. One class of osmotic agents water- swellable hydrophilic polymers, which are also referred to as "osmopolymers" and "hydrogels," include, but not limited to, hydrophilic vinyl and acrylic polymers, polysaccharides such as calcium alginate, polyethylene oxide (PEO), polyethylene glycol (PEG), polypropylene glycol (PPG), poly(2-hydroxyethyl methacrylate), poly(acrylic) acid, poly(methacrylic) acid, polyvinylpyrrolidone (PVP), crosslinked PVP, polyvinyl alcohol (PVA), PVA/PVP copolymers, PVA/PVP copolymers with hydrophobic monomers such as methyl methacrylate and vinyl acetate, hydrophilic polyurethanes containing large PEO blocks, sodium croscarmellose, carrageenan, hydroxyethyl cellulose (HEC), hydroxypropyl cellulose (HPC), hydroxypropyl methyl cellulose (HPMC), carboxymethyl cellulose (CMC) and carboxyethyl, cellulose (CEC), sodium alginate, polycarbophil, gelatin, xanthan gum, and sodium starch glycolate. [001108] The other class of osmotic agents is osmogens, which are capable of imbibing water to affect an osmotic pressure gradient across the barrier of the surrounding coating. Suitable osmogens include, but are not limited to, inorganic salts, such as magnesium sulfate, magnesium chloride, calcium chloride, sodium chloride, lithium chloride, potassium sulfate, potassium phosphate, sodium carbonate, sodium sulfite, lithium sulfate, potassium chloride, and sodium sulfate; sugars, such as dextrose, fructose, glucose, inositol, lactose, maltose, mannitol, raffϊnose, sorbitol, sucrose, trehalose, and xylitol; organic acids, such as ascorbic acid, benzoic acid, fumaric acid, citric acid, maleic acid, sebacic acid, sorbic acid, adipic acid, edetic acid, glutamic acid, p-tolunesulfonic acid, succinic acid, and tartaric acid; urea; and mixtures thereof.
[001109] Osmotic agents of different dissolution rates may be employed to influence how rapidly the active ingredient(s) is initially delivered from the dosage form. For example, amorphous sugars, such as Mannogeme EZ (SPI Pharma, Lewes, DE) can be used to provide faster delivery during the first couple of hours to promptly produce the desired therapeutic effect, and gradually and continually release of the remaining amount to maintain the desired level of therapeutic or prophylactic effect over an extended period of time. In this case, the active ingredient(s) is released at such a rate to replace the amount of the active ingredient metabolized and excreted.
[001110] The core may also include a wide variety of other excipients and carriers as described herein to enhance the performance of the dosage form or to promote stability or processing. [001111] Materials useful in forming the semipermeable membrane include various grades of acrylics, vinyls, ethers, polyamides, polyesters, and cellulosic derivatives that are water- permeable and water-insoluble at physiologically relevant pHs, or are susceptible to being rendered water-insoluble by chemical alteration, such as crosslinking. Examples of suitable polymers useful in forming the coating, include plasticized, unplasticized, and reinforced cellulose acetate (CA), cellulose diacetate, cellulose triacetate, CA propionate, cellulose nitrate, cellulose acetate butyrate (CAB), CA ethyl carbamate, CAP, CA methyl carbamate, CA succinate, cellulose acetate trimellitate (CAT), CA dimethylaminoacetate, CA ethyl carbonate, CA chloroacetate, CA ethyl oxalate, CA methyl sulfonate, CA butyl sulfonate, CA p-toluene sulfonate, agar acetate, amylose triacetate, beta glucan acetate, beta glucan triacetate, acetaldehyde dimethyl acetate, triacetate of locust bean gum, hydroxlated ethylene -vinylacetate, EC, PEG, PPG, PEG/PPG copolymers, PVP, HEC, HPC, CMC, CMEC, HPMC, HPMCP, HPMCAS, HPMCAT, poly(acrylic) acids and esters and poly-(methacrylic) acids and esters and copolymers thereof, starch, dextran, dextrin, chitosan, collagen, gelatin, polyalkenes, polyethers, polysulfones, polyethersulfones, polystyrenes, polyvinyl halides, polyvinyl esters and ethers, natural waxes, and synthetic waxes.
[001112] Semipermeable membrane may also be a hydrophobic microporous membrane, wherein the pores are substantially filled with a gas and are not wetted by the aqueous medium but are permeable to water vapor, as disclosed in U.S. Pat. No. 5,798,119. Such hydrophobic but water- vapor permeable membrane are typically composed of hydrophobic polymers such as polyalkenes, polyethylene, polypropylene, polytetrafluoroethylene, polyacrylic acid derivatives,
polyethers, polysulfones, polyethersulfones, polystyrenes, polyvinyl halides, polyvinylidene fluoride, polyvinyl esters and ethers, natural waxes, and synthetic waxes.
[001113] The delivery port(s) on the semipermeable membrane may be formed post-coating by mechanical or laser drilling. Delivery port(s) may also be formed in situ by erosion of a plug of water-soluble material or by rupture of a thinner portion of the membrane over an indentation in the core. In addition, delivery ports may be formed during coating process, as in the case of asymmetric membrane coatings of the type disclosed in U.S. Pat. Nos. 5,612,059 and 5,698,220. [001114] The total amount of the active ingredient(s) released and the release rate can substantially by modulated via the thickness and porosity of the semipermeable membrane, the composition of the core, and the number, size, and position of the delivery ports. [001115] The pharmaceutical compositions in an osmotic controlled-release dosage form may further comprise additional conventional excipients as described herein to promote performance or processing of the formulation.
[001116] The osmotic controlled-release dosage forms can be prepared according to conventional methods and techniques known to those skilled in the art (see, Remington: The Science and Practice of Pharmacy, supra; Santus and Baker, J. Controlled Release 1995, 55, 1- 21; Verma et al., Drug Development and Industrial Pharmacy 2000, 26, 695-708; Verma et al., J. Controlled Release 2002, 79, 7-27).
[001117] In certain embodiments, the pharmaceutical compositions provided herein are formulated as AMT controlled-release dosage form, which comprises an asymmetric osmotic membrane that coats a core comprising the active ingredient(s) and other pharmaceutically acceptable excipients. See, U.S. Pat. No. 5,612,059 and WO 2002/17918. The AMT controlled- release dosage forms can be prepared according to conventional methods and techniques known to those skilled in the art, including direct compression, dry granulation, wet granulation, and a dip-coating method.
[001118] In certain embodiment, the pharmaceutical compositions provided herein are formulated as ESC controlled-release dosage form, which comprises an osmotic membrane that coats a core comprising the active ingredient(s), hydroxylethyl cellulose, and other pharmaceutically acceptable excipients.
5.3.6.3. Multiparticulate Controlled Release Devices
[001119] The pharmaceutical compositions provided herein in a modified release dosage form may be fabricated as a multiparticulate controlled release device, which comprises a multiplicity of particles, granules, or pellets, ranging from about 10 μm to about 3 mm, about 50 μm to about 2.5 mm, or from about 100 μm to 1 mm in diameter. Such multiparticulates may be made by the processes know to those skilled in the art, including wet-and dry-granulation, extrusion/spheronization, roller-compaction, melt-congealing, and by spray-coating seed cores. See, for example, Multiparticulate Oral Drug Delivery; Marcel Dekker: 1994; and Pharmaceutical P elletization Technology; Marcel Dekker: 1989.
[001120] Other excipients as described herein may be blended with the pharmaceutical compositions to aid in processing and forming the multiparticulates. The resulting particles may themselves constitute the multiparticulate device or may be coated by various film-forming materials, such as enteric polymers, water-swellable, and water-soluble polymers. The multiparticulates can be further processed as a capsule or a tablet.
5.3.6.4. Targeted Delivery
[001121] The pharmaceutical compositions provided herein may also be formulated to be targeted to a particular tissue, receptor, or other area of the body of the subject to be treated, including liposome-, resealed erythrocyte-, and antibody-based delivery systems. Examples include, but are not limited to, U.S. Pat. Nos. 6,316,652; 6,274,552; 6,271,359; 6,253,872; 6,139,865; 6,131,570; 6,120,751; 6,071,495; 6,060,082; 6,048,736; 6,039,975; 6,004,534; 5,985,307; 5,972,366; 5,900,252; 5,840,674; 5,759,542; and 5,709,874. 5.3.7. Inhalers
5.3.7.1. Metered dose inhalers
[001122] The pharmaceutical compositions herein may be formulated to be administered via a metered dose inhaler. Metered dose inhalers are medicament dispensers suitable for dispensing medicament in aerosol form, wherein the medicament is comprised in an aerosol container suitable for containing a propellant-based aerosol medicament formulation. [001123] Suitable propelleants include chlorofluorocarbon propellants and chlorofluorocarbon propellants, including but not limited to CFC-11, CFC- 12, CFC-113, hyrdofluoroalkanes, including but not limited to HFA- 134a or HFA 227), or dichlorodifluoromethane.
5.3.7.2. Dry powder inhalers
[001124] The pharmaceutical compositions herein may be formulated to be administered via a dry powder inhaler. Dry power inhalers (DPI) use a mechanism such as a burst of gas to create a cloud of dry powder inside a container, which can then be inhaled by the patient. DPIs are also well known in the art and can be purchased from a number of vendors which include, for example, Fisons, Glaxo-Wellcome, Inhale Therapeutic Systems, ML Laboratories, Qdose and Vectura. DPIs include multiple dose DPI ("MDDPI") system, which allows for the delivery of more than one therapeutic dose. MDDPIs are available from companies such as AstraZeneca, Glaxo Wellcome, IVAX, Schering Plough, SkyePharma and Vectura. For example, capsules and cartridges of gelatin for use in an inhaler or insufflator can be formulated containing a powder mix of the compound and a suitable powder base such as lactose or starch for these systems. 5.3.8. Nebulizers
[001125] The pharmaceutical compositions herein may be formulated to be administered via a nebulizer. A nebulizer is an instrument that is capable of generating very fine liquid droplets for inhalation into the lung. Within this instrument, the nebulizing liquid or solution is atomized into a mist of droplets with a broad size distribution by methods known to those of skill in the art, including, but not limited to, compressed air, ultrasonic waves, or a vibrating orifice. Nebulizers may further contain, e.g., a baffle which, along with the housing of the instrument, selectively removes large droplets from the mist by impaction. Thus, the mist inhaled into the lung contains fine aerosol droplets.
[001126] The nebilizer and can be unit dose or multidose. Nebulizers are available from, e.g., Pari GmbH (Stamberg, Germany), DeVilbiss Healthcare (Heston, Middlesex, UK), Healthdyne, Vital Signs, Baxter, Allied Health Care, Invacare, Hudson, Omron, Bremed, AirSep, Luminscope, Medisana, Siemens, Aerogen, Mountain Medical, Aerosol Medical Ltd. (Colchester, Essex, UK), AFP Medical (Rugby, Warwickshire, UK), Bard Ltd. (Sunderland, UK), Carri-Med Ltd (Dorking, UK), Plaem Nuiva (Brescia, Italy), Henleys Medical Supplies (London, UK), Intersurgical (Berkshire, UK), Lifecare Hospital Supplies (Leies, UK), Medic- Aid Ltd. (West Sussex, UK), Medix Ltd. (Essex, UK), Sinclair Medical Ltd. (Surrey, UK), and many others.
[001127] Nebulizers for use herein include, but are not limited to, jet nebulizers (optionally sold with compressors), ultrasonic nebulizers, and others. Exemplary jet nebulizers for use herein
include Pari LC plus/ProNeb, Pari LC plus/ProNeb Turbo, Pari LC plus/Dura Neb 1000 & 2000, Pari LC plus/Walkhaler, Pari LC plus/Pari Master, Pari LC star, Omron CompAir XL Portable Nebulizer System (NE-C 18 and JetAir Disposable nebulizer), Omron CompAir Elite Compressor Nebulizer System (NE-C21 and Elite Air Reusable Nebilizer), Pari LC Plus or Pari LC Star nebulizer with Proneb Ultra compressor, Pulmo-aide, Pulmo-aide LT, Pulmo-aide traveler, Invacare Passport, Inspiration Healthdyne 626, Pulmo-Neb Traveler, DeVilbiss 646, Whisper Jet, Acorn II, Misty-Neb, Allied aerosol, Schuco Home Care, Lexan Plasic Pocet Neb, SideStream Hand Held Neb, Mobil Mist, Up-Draft, Up-Draft II, T Up-Draft, ISO-NEB, AVA- NEB, Micro Mist, and PulmoMate. Exemplary ultrasonic nebulizers for use herein include MicroAir, Ultai, Siemens Ultra Nebulizer 145, CompAir, Puinosonic, Scout, 5003 Ultrasonic Neb, 5110 Ultrasonic Neb, 5004 Desk Ultrasonic Nebulizer, Mystique Ultrasonic, Luminscope's Ultrasonic Nebulizer, Medisana Ultrasonic Nebulizer, Microstat Ultrasonic Nebulizer, and MABISMist Hand Held Ultrasonic Nebulizer. Other nebulizers for use herein include 5000 Electromagnetic Neb, 5001 Electromagnetic Neb 5002 Rotary Piston Neb, Lumineb I Piston Nebulizer 5500, AERONEB.TM. Portable Nebuizer System, AERODOSE.TM. Inhaler, AeroEclipse Breath Actuated Nebulizer, HALOLITE.TM. system (Profile Therapeutics), AKITA.RTM. systems (InaMed, Germany), Mystic system (BattellePharma), RESPIMAT.RTM. (Boehringer Ingelheim), AERX.RTM. (Aradigm), and E-FLOW.TM. (Pari). 5.4. ARTICLES OF MANUFACTURE
[001128] The compounds or pharmaceutically acceptable derivatives can be packaged as articles of manufacture containing packaging material, a compound or pharmaceutically acceptable derivative thereof provided herein, which is used for treatment, prevention or amelioration of one or more symptoms associated with SERC A2 activity, and a label that indicates that the compound or pharmaceutically acceptable derivative thereof is used for treatment, prevention or amelioration of one or more symptoms diseases associated with SERCA2.
[001129] The articles of manufacture provided herein contain packaging materials. Packaging materials for use in packaging pharmaceutical products are well known to those of skill in the art. See, e.g., U.S. Patent Nos. 5,323,907, 5,052,558 and 5,033,252. Examples of pharmaceutical packaging materials include, but are not limited to, blister packs, bottles, tubes, inhalers, pumps, bags, vials, containers, syringes, bottles, and any packaging material suitable for
a selected formulation and intended mode of administration and treatment. A wide array of formulations of the compounds and compositions provided herein are contemplated. 5.5. COMPOUNDS
[001130] In some embodiments, provided herein are compounds having the following formula:
A, and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, Or NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, orG is joined together with R2 to form a 5-6 membered ring;
with the proviso that R2 J G is not a substituted or unsubstituted
(ii) v is 1 to 3,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001131] In some embodiments, provided herein are compounds having the following formula:
A and B are each, independently, H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-
3 carbons, -CN, azido, or NO2; wherein at least one of A and B is not H;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2, or G is joined together with R2 to form a 5-6 membered ring;
with the proviso that is not a substituted or unsubstituted
R2 is H or alkyl of 1-3 carbons; and G is H, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001134] In further embodiments, A and B are each, independently, H, hydroxyl, or alkoxy of
1-3 carbons; R3 is H or alkyl of 1-3 carbons, Q is methyl, R2 is H, alkyl of 1-3 carbons, and G is
H, or hydroxyl.
[001135] In further embodiments, the compound has the following formula:
[001136] In further embodiments, the compound has the following formula:
[001137] In some embodiments, provided herein is a compound of formula III, IV, V, VI, VII, X, XI, XII, XIII, XIV, XV, XVI, XVII, XVIII, XX, or XXI:
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001138] In some embodiments, provided herein is a compound of formula III, VI, VII, X, XI,
XV, XVII, XVIII, or XX:
HI; VI;
VII; X;
XX;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001139] In some embodiments, provided herein is a compound of formula III:
III; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001140] In some embodiments, provided herein is a compound of formula IV:
IV; a pharmaceutically acceptable derivative or stereoisomer thereof.
[001141] In some embodiments, provided herein is a compound of formula V:
V; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001142] In some embodiments, provided herein is a compound of formula VI:
VI; or a pharmaceutically acceptable derivative thereof.
[001143] In some embodiments, provided herein is a compound of formula VII:
VII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001144] In some embodiments, provided herein is a compound of formula X:
X; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001145] In some embodiments, provided herein is a compound of formula XI:
[001146] In some embodiments, provided herein is a compound of formula XII:
XII;
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001147] In some embodiments, provided herein is a compound of formula XIII:
XIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001148] In some embodiments, provided herein is a compound of formula XIV:
XIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001149] In some embodiments, provided herein is a compound of formula XV:
XV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001150] In some embodiments, provided herein is a compound of formula XVI:
XVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001151] In some embodiments, the compound of formula I is a compound of formula XVII:
XVII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001152] In some embodiments, provided herein is a compound of formula XVIII:
XVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001153] In some embodiments, provided herein is a compound of formula XX:
XX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001154] In some embodiments, provided herein is a compound of formula XXI:
XXI; or a pharmaceutically acceptable derivative thereof. [001155] In some embodiments, provided herein is a compound of formula XXIII:
XXIII; or a pharmaceutically acceptable derivative thereof. [001156] In some embodiments, provided herein is a compound of formula XXIV:
XXIV or a pharmaceutically acceptable derivative thereof. [001157] In some embodiments, provided herein is a compound of formula XXV:
XXV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001158] In some embodiments, provided herein is a compound of formula XXVI:
XXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001159] In some embodiments, provided herein is a compound of formula XXVII:
XXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001160] In some embodiments, provided herein is a compound of formula XXVIII:
XXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001161] In some embodiments, provided herein is a compound of formula XXIX:
XXIX; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001162] In some embodiments, provided herein is a compound of formula XXX:
XXX; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001163] In some embodiments, provided herein is a compound of formula XXXI:
XXXI; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001164] In some embodiments, provided herein is a compound of formula XXXII:
XXXII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001165] In some embodiments, provided herein is a compound of formula XXXIII:
XXXIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001166] In some embodiments, provided herein is a compound of formula XXXIV:
XXXIV; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001167] In some embodiments, provided herein is a compound of formula XXXV:
XXXV; or a pharmaceutically acceptable derivative or stereoisomer thereof.
XXXVI; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001169] In some embodiments, provided herein is a compound of formula XXXVII:
XXXVII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001170] In some embodiments, provided herein is a compound of formula XXXVIII:
XXXVIII; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001171] In some embodiments, provided herein is a compound of formula XXXIX:
XXXIX; or a pharmaceutically acceptable derivative or stereoisomer thereof. [001172] In some embodiments, provided herein is a compound of f formula XL:
or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001173] In some embodiments, provided herein is a compound of formula XLI:
[001174] In some embodiments, provided herein is a compound of formula XLII:
XLII; or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001175] In some embodiments, provided herein is a compound of formula XLIII:
XLIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001176] In some embodiments, provided herein is a compound of formula XLIV
XLIV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001177] In some embodiments, provided herein is a compound of formula XLV
XLV or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001178] In some embodiments, provided herein is a compound of formula XLVI
XLVI or a pharmaceutically acceptable derivative or stereoisomer thereof. [001179] In some embodiments, provided herein is a compound of formula XLVII
XLVII or a pharmaceutically acceptable derivative or stereoisomer thereof. [001180] In some embodiments, provided herein is a compound of formula XLVIII
XLVIII or a pharmaceutically acceptable derivative or stereoisomer thereof. [001181] In some embodiments, provided herein is a compound of formula XLIX
XLIX or a pharmaceutically acceptable derivative or stereoisomer thereof. [001182] In some embodiments, provided herein is a compound of formula LI
LI or a pharmaceutically acceptable derivative or stereoisomer thereof.
LII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001184] In some embodiments, the compound of formula I is a compound of formula LII
LIII or a pharmaceutically acceptable derivative or stereoisomer thereof.
[001185] It is understood that the detailed description and accompanying examples are merely illustrative, and are not to be taken as limitations upon the scope of the subject matter. Various changes and modifications to the disclosed embodiments will be apparent to those skilled in the art. Such changes and modifications, including without limitation those relating to the chemical structures, substituents, derivatives, intermediates, syntheses, formulations and/or methods of use provided herein, may be made without departing from the spirit and scope thereof. Included within the scope of the subject matter described herein are all combinations of the embodiments described herein.U.S. patents and publications referenced herein are incorporated by reference. 6. EXAMPLES
[001186] The subject matter described herein is further illustrated by the following examples, which are not to be considered as limitative of its scope. 6.1. SYNTHESIS OF COMPOUNDS
6.1.1. Synthesis of Compounds II, VII, VIII, IX, X, XI, XIV, XV, XVI, XVII, and
XXII
[001187] The synthesis of compounds II, VII, VIII, IX, X, XI, XIV, XV, XVI, XVII, and XXII were performed following the general scheme above. To a stirred solution of 4-phenylbutan-2-
amine (0.5 mmol) in dichloromethane (2 niL) at room temperature, was added diisopropylethylamine (0.75 mmol) and the appropriate benzoyl chloride (0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and/or 1H NMR. Compound II: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 8.66 min; ESI-MS m/z 314.2 [M+H]+. Compound VII: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 7.89 min; ESI-MS m/z 286.2 [M+H]+. Compound VIII: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 8.70 min; ESI-MS m/z 277.2 [M+H]+. Compound IX: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 8.88 min; ESI-MS m/z 288.2 [M+H]+. Compound X: RP- HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 9.06 min; ESI-MS m/z 381.2 [M+H]+. Compound XI: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 9.43 min; ESI-MS m/z 431.2 [M+H]+. Compound XIV: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.23 min; ESI-MS m/z 272 [M+H]+. Compound XV: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.20 min; ESI-MS m/z 284 [M+H]+. Compound XVI: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.67 min; ESI-MS m/z 343 [M+H]+. Compound XVII: RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret Time = 2.51 min; ESI-MS m/z 342 [M+H]+. Compound XXII: RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.87 min; ESI-MS m/z 321 [M+H]+. 6.1.2. Synthesis of Compounds III and XVIII
[001188] The synthesis of compounds III and XVIII were performed following the general scheme above. To a stirred solution of 5-phenylpentan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and the appropriate benzoyl chloride (0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and/or 1H NMR. Compound III: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 8.74
min; ESI-MS m/z 328.3 [M+H]+. Compound XVIII: RP-HPLC (H2O/ACN) 1 ml/min, 4.5 min, 254 nM): Ret. Time: 2.25 min; ESI-MS m/z 356 [M+H]+. 6.1.3. Synthesis of Compound IV
[001189] The synthesis of compound IV was performed as shown above. To a stirred solution of 4-(4-methoxyphenyl)butan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3,5-dimethoxybenzoyl chloride (0.5 mmol). The reaction mixture was stirred for 15 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 8.64 min; ESI-MS m/z 344.3 [M+H]+. 6.1.4. Synthesis of Compound V
[001190] The synthesis of compound V was performed as shown above. To a stirred solution of pentan-2-amine (0.5 mmol) in dimethylformamide (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3,5-dimethoxybenzoyl chloride (0.5 mmol). The reaction mixture was stirred for 15 h at room temperature and then quenched with MeOH. The mixture was purified by reverse-phase LC and analyzed for purity by LCMS and 1H NMR. RP- HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 8.37 min; ESI-MS m/z 252.3 [M+H]+.
6.1.5. Synthesis of Compound VI
[001191] The synthesis of compound VI was performed as shown above. To a stirred solution of (R)-4-phenylbutan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3,5-dimethoxybenzoyl chloride (0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.21 min; ESI-MS m/z 314 [M+H]+. [001192] Alternative Synthesis of Compound VI
R, R (major) S, R
4-phenylbutan-2-one (1.5 g, 10 mmole) and (i?)-l-phenylethanamine (1.2 g, 10 mmol) were dissolved in anhydrous ethanol and stirred for 20 min at rt. Raney Nickel (0.2 g) was added and the solution placed under hydrogen (1 atm) at rt and stirred for 5 h. After removal of the catalyst, the product mixture was concentrated and (i?)-4-phenyl-JV-((i?)-l-phenylethyl)butan-2- amine was isolated via silica gel column chromatography (EtOAc/hexanes), concentrated and hydrogenated with 20% Pd(OH)2 in ethanol at 55 psi for 2Oh to provide (i?)-4-phenylbutan-2- amine after filtration and solvent removal. To a stirred solution of (i?)-4-phenylbutan-2-amine (75 mg, 0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (DIEA, 0.75 mmol) and 3,5-dimethoxybenzoyl chloride (100 mg, 0.5
mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2θ/ACN(10- 95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.21 min; ESI-MS m/z 314 [M+H]+. Chiral Analysis. Column: Daicel CHIRALCEL OD-RH (0.46 cm x 15 cm), eluent: H2O/ACN (20-95%), run time: 10.5 min, flow rate: 1.0 mL/min, detection: UV 254 nm, tR = 8.5 min for R- isomer, 9.0 min for S-isomer. Optical rotation value: [α]D = -25.09 (>98% ee). 6.1.6. Synthesis of Compound XIX and XX
[001193] The synthesis of compounds XIX and XX were performed following the scheme above. To a stirred solution of 4-(furan-2-yl)butan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and the appropriate benzoyl chloride (0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and/or 1H NMR. Compound XIX: RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.92 min; ESI-MS m/z 304.1 [M+H]+. Compound XX: RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.33 min; ESI-MS m/z 332 [M+H]+. 6.1.7. Synthesis of Compound XXI
[001194] The synthesis of compound XXI was performed as shown above. To a stirred solution of (S)-Λ/-(pyrrolidin-2-ylmethyl)aniline (0.5 mmol) in dimethylformamide (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3,5-dimethoxybenzoyl chloride (0.5 mmol). The reaction mixture was stirred for 15 h at room temperature and then quenched with MeOH. The mixture was purified by reverse-phase LC and analyzed for purity
by LCMS and 1H NMR. RP-HPLC (H2OZACN(S-QS0Zo) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.95 min; ESI-MS m/z 341 [M+H]+. 6.1.8. Synthesis of Compound XII
[001195] The synthesis of compound XII was performed as shown above. To a stirred solution of 3,5-dimethoxyaniline (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 2-methyl-4-phenylbutanoyl chloride (0.5 mmol). The reaction mixture was stirred for 2 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 8.60 min; ESI-MS m/z 314.2 [M+H]+.
6.1.9. Synthesis of Compound XIII
[001196] The synthesis of compound XIII was performed as shown above. To a stirred solution of 3,5-dimethoxybenzylamine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-phenylpropanoyl chloride (0.5 mmol). The reaction mixture was stirred for 2 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1U NMR. RP-HPLC (H2O/ACN(5-95%) 1 ml/min, 12 min, 254 nM): Ret. Time = 8.03 min; ESI-MS m/z 300.3 [M+H]+.
6.1.10. Synthesis of Compound XXIII
[001197] The synthesis of compound XXIII was performed as shown above. To a suspension of Boc-Ala-OH (300 mg, 1.6 mmol) in THF (5 mL) was added CDI (280 mg, 1.7 mmol). The reaction mixture refluxed for 1 h, cooled to rt, followed by drop wise addition of aniline (160 mg, 1.7 mmol) in THF (3 ml). After stirring for 2 h at rt, the solvent was evaporated, water was added and the reaction was extracted with EtOAc. The organic layer was separated and washed with aqueous NaOH, aqueous HCl, and brine, dried over Na2SO4, and concentrated. This residue (320 mg, 1.2 mmol) was dissolved in dry THF (5 mL) and added drop wise to a suspension of LiAlH4 (180 mg, 4.8 mmol) in dry THF (2 mL) at 00C. This reaction mixture was stirred at 45°C for 2 h, quenched with water, and extracted with EtOAc. The organic layer was combined and washed with water and dried over anhydrous Na2SO4 followed by concentration. The residue was purified by flash chromatography on silica gel and the product was treated with excess EtOAc/ HCl (3.5 mol/L, 1.5 mL) and stirred at rt for 2 h to afford the bis-HCl salt. This amine (223 mg, 1 mmol) was treated with 1 equivalent of 3,5-dimethoxybenzoyl chloride (200 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diisopropylethylamine. After stirring at rt for 3 h the reaction mixture was concentrated and purified via reverse-phase HPLC (C- 18 column, ACN/H20) to give compound XXIII as a white solid. The compound was analyzed for purity by LCMS. RP-HPLC (H20/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.91 min; ESI-MS m/z 315 [M+H]+.
6.1.11. Synthesis of Compound XXIV
[001198] Benzylamine (214 mg, 2 mmol) and Boc-alanal (346 mg, 2 mmol) were dissolved in dichloromethane containing 1% v/v acetic acid (5 mL) and treated with sodium cyanoborohydride (157 mg, 2.5 mmol). After stirring 5 h at rt, the reaction was quenched with aqueous NaHCO3 , extracted with ethyl acetate, concentrated, and purified via flash chromatography. The product (264 mg, 1 mmol) was treated with 1.5 equivalents of 9- fluorenylmethyloxycarbonyl chloride (388 mg, 1.5 mmol) in THF (2 mL) containing diisopropylethylamine (387 mg, 3 mmol). After extraction and evaporation, the Fmoc intermediate (400 mg, 0.8 mmol) was stirred for 1 h in 20% TFA v/v in DCM to afford Boc removal, evaporated, and treated with 1 equivalent of 3,5-dimethoxybenzoyl chloride (200 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diispropylethylamine. After stirring at rt for 3 h the reaction mixture was concentrated followed by suspension and stirring (2 h) in 20% piperidine v/v in DCM to afford Fmoc removal. After evaporation, the residue was purified via reverse-phase HPLC (C- 18 column, ACN/H2O) to give compound XXIV as a yellow solid. The compound was analyzed for purity by LCMS. RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.15 min; ESI-MS m/z 329 [M+H]+.
6.1.12. Synthesis of Compound XXV
[001199] To a suspension of Boc-Ala-OH (300 mg, 1.6 mmol) in THF (5 niL) was added CDI (280 mg, 1.7 mmol). The reaction mixture was refluxed for 1 h, cooled to rt, and 1,2,3,4- tetrahydroquinoline (226 mg, 1.7 mmol) was added. After stirring for 2 h at rt, the solvent was evaporated, water was added and the reaction was extracted with EtOAc. The organic layer was separated and washed with aqueous NaOH, aqueous HCl, and brine, dried over Na2SO4, and concentrated. This residue (1.2 mmol) was dissolved in dry THF (5 mL) and added drop wise to a suspension OfLiAlH4 (180 mg, 4.8 mmol) in dry THF (2 mL) at 00C. This reaction mixture was stirred at 45°C for 2 h, quenched with water, and extracted with EtOAc. The organic layer was combined and washed with water and dried over anhydrous Na2SO4 followed by concentration. The residue was purified by flash chromatography on silica gel and the product was treated with excess EtOAc/ HCl (3.5 mol/L, 1.5 mL) to afford the bis-HCl salt. This amine (1 mmol) was treated with 1 equivalent of 3,5-dimethoxybenzoyl chloride (200 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diisopropylethylamine. After stirring at rt for 3 h the reaction mixture was concentrated and purified via reverse-phase HPLC (C-18 column, ACN/H2O) to give Compound XXV. The compound was analyzed for purity by LCMS. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.34 min; ESI-MS m/z 355 [M+H]+
6.1.13. Synthesis of Compound XXVI
[001200] 2-Hydroxybenzylamine (246 mg, 2 mmol) and Boc-alanal (346 mg, 2 mmol) were dissolved in anhydrous methanol (5 rnL). After stirring at rt for 2 h, sodium triacetoxyborohydride (530 mg, 2.5 mmol) was added and the solution was stirred for 25 h at rt. The reaction was quenched with aqueous NaHCO3 , extracted with ethyl acetate, concentrated, and purified via flash chromatography. The product (1 mmol) was treated with 1.5 equivalents of 9-fluorenylmethyloxycarbonyl chloride (388 mg, 1.5 mmol) in THF (2 mL) containing diisopropylethylamine (387 mg, 3 mmol). After extraction and evaporation, the Fmoc intermediate (0.8 mmol) was stirred for 1 h in 20% TFA v/v in DCM to afford Boc removal, evaporated, and treated with 1 equivalent of 3,5-dimethoxybenzoyl chloride (200 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diispropylethylamine. After stirring at rt for 3 h the reaction mixture was concentrated then suspended and stirred for 2 h in 20% piperidine v/v in DCM to afford Fmoc removal. After evaporation, the residue was purified via reverse-phase HPLC (C- 18 column, ACN/H2O) to give Compound XXVI. The compound was analyzed for purity by LCMS. RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.00 min; ESI-MS m/z 345 [M+H]+
6.1.14. Synthesis of Compound XXVII
[001201] To a suspension of Boc-Ala-OH (300 mg, 1.6 mmol) in THF (5 niL) was added CDI (280 mg, 1.7 mmol). The reaction mixture was refluxed for 1 h, cooled to rt, and aniline (160 mg, 1.7 mmol) in THF (3 ml) was added. After stirring for an additional 2 h at rt, the solvent was evaporated, water was added and the reaction was extracted with EtOAc. The organic layer was separated and washed with aqueous NaOH, aqueous HCl, and brine, dried over Na2SO4, and concentrated. This residue (320 mg, 1.2 mmol) was dissolved in dry THF (5 mL) and added drop wise to a suspension OfLiAlH4 (180 mg, 4.8 mmol) in dry THF (2 mL) at 00C. This reaction mixture was stirred at 45°C for 2 h, quenched with water, and extracted with EtOAc. The organic layer was combined and washed with water and dried over anhydrous Na2SO4 followed by concentration. The residue was purified by flash chromatography on silica gel and the product was treated with excess EtOAc/ HCl (3.5 mol/L, 1.5 mL) to afford the bis-HCl salt. This amine (1 mmol) was treated with 1 equivalent of 3-methoxybenzoyl chloride (170 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diisopropylethylamine. After stirring at rt for 3 h, the reaction mixture was concentrated and purified via reverse-phase HPLC (C-18 column, ACN/H2O) to give Compound XXVII. The compound was analyzed for purity by LCMS. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 0.97 min; ESI-MS m/z 285 [M+H]+
6.1.15. Synthesis of Compound XXVIII
[001202] To a suspension of Boc-Ala-OH (300 mg, 1.6 mmol) in THF (5 niL) was added CDI (280 mg, 1.7 mmol). The reaction mixture was refluxed for 1 h, cooled to rt, and aniline (160 mg, 1.7 mmol) in THF (3 ml) was added. After stirring for an additional 2 h at rt, the solvent was evaporated, water was added and the reaction was extracted with EtOAc. The organic layer was separated and washed with aqueous NaOH, aqueous HCl, and brine, dried over Na2SO4, and concentrated. This residue (320 mg, 1.2 mmol) was dissolved in dry THF (5 mL) and added drop wise to a suspension OfLiAlH4 (180 mg, 4.8 mmol) in dry THF (2 mL) at 00C. This reaction mixture was stirred at 45°C for 2 h, quenched with water, and extracted with EtOAc. The organic layer was combined and washed with water and dried over anhydrous Na2SO4 followed by concentration. The residue was purified by flash chromatography on silica gel and the product was treated with excess EtOAc/ HCl (3.5 mol/L, 1.5 mL) to afford the bis-HCl salt. This amine (1 mmol) was treated with 1 equivalent of 3,5-dihydroxybenzoic acid (154 mg, 1 mmol), 1.2 equivalents of benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP, 624 mg, 1.2 mmol) in DMF (2 mL) containing 2 equivalents of diisopropylethylamine. After stirring at rt for 20 h, the reaction mixture was concentrated and purified via reverse-phase HPLC (C-18 column, ACN/H2O) to give Compound XXVIII. RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.15 min; ESI-MS m/z 287 [M+H]+
[001203] Benzylamine (214 mg, 2 mmol) and Boc-alanal (346 mg, 2 mmol) were dissolved in dichloromethane containing 1% v/v acetic acid (5 mL) and treated with sodium cyanoborohydride (157 mg, 2.5 mmol). After stirring 5 h at rt, the reaction was quenched with aqueous NaHCO3 , extracted with ethyl acetate, concentrated, and purified via flash chromatography. The product (264 mg, 1 mmol) was treated with 1.5 equivalents of 9- fluorenylmethyloxycarbonyl chloride (388 mg, 1.5 mmol) in THF (2 mL) containing diisopropylethylamine (387 mg, 3 mmol). After extraction and evaporation, the Fmoc intermediate (400 mg, 0.8 mmol) was stirred for 1 h in 20% TFA v/v in DCM to afford Boc removal, evaporated, and treated with 1 equivalent of 3,5-diethoxybenzoyl chloride (228 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diispropylethylamine. After stirring at rt for 3 h the reaction mixture was concentrated followed by suspension and stirring (2 h) in 20% piperidine v/v in DCM to afford Fmoc removal. After evaporation, the residue was purified via reverse-phase HPLC (C- 18 column, ACN/H2O) to give Compound XXIX. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.35 min; ESI-MS m/z 357 [M+H]+
[001204] Benzylamine (214 mg, 2 mmol) and Boc-alanal (346 mg, 2 mmol) were dissolved in dichloromethane containing 1% v/v acetic acid (5 mL) and treated with sodium cyanoborohydride (157 mg, 2.5 mmol). After stirring 5 h at rt, the reaction was quenched with aqueous NaHCO3 , extracted with ethyl acetate, concentrated, and purified via flash chromatography. The product (264 mg, 1 mmol) was treated with 1.5 equivalents of 9- fluorenylmethyloxycarbonyl chloride (388 mg, 1.5 mmol) in THF (2 mL) containing diisopropylethylamine (387 mg, 3 mmol). After extraction and evaporation, the Fmoc intermediate (400 mg, 0.8 mmol) was stirred for 1 h in 20% TFA v/v in DCM to afford Boc removal, evaporated, and treated with 1 equivalent of 3-methoxybenzoyl chloride (170 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diispropylethylamine. After stirring at rt for 3 h the reaction mixture was concentrated followed by suspension and stirring (2 h) in 20% piperidine v/v in DCM to afford Fmoc removal. After evaporation, the residue was purified via reverse-phase HPLC (C- 18 column, ACN/H2O) to give Compound XXX. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.02 min; ESI-MS m/z 299 [M+H]+ 6.1.18. Synthesis of Compound XXXI
[001205] To a stirred solution of Λ/-(pyrrolidin-2-ylmethyl)aniline in dimethylformamide (at room temperature diisopropylethylamine is added. The reaction mixture is stirred for 15 h at
room temperature and then quenched with MeOH. The mixture iss purified by reverse-phase LC and analyzed for purity by LCMS and 1H NMR. 6.1.19. Synthesis of Compound XXXII
[001206] To a stirred solution of (7?)-4-phenylbutan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-methoxybenzoyl chloride (0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo and the final product was purified by reverse-phase HPLC (C- 18 column, ACN/H2O) to give Compound XXXII. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.17 min; ESI-MS m/z 284 [M+H]+ 6.1.20. Synthesis of Compound XXXIII
[001207] Aniline (186 mg, 2 mmol) and boc-D-alanal (346 mg, 2 mmol) were dissolved in anhydrous methanol (5 mL). After stirring for 2 h at rt, sodium triacetoxyborohydride (530 mg, 2.5 mmol) was added and the solution was stirred for 25 h at rt. The reaction was quenched with aqueous NaHCO3, extracted with ethyl acetate, concentrated, and purified via flash chromatography. A portion of this intermediate (0.8 mmol) was stirred for 1 h in 20% TFA v/v in DCM to afford Boc removal, evaporated, and treated with 1 equivalent of 3-methoxybenzoyl chloride (170 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diispropylethylamine (DIEA). After stirring at rt for 3 h the reaction mixture was concentrated and purified via reverse-phase HPLC (C- 18 column, ACN/H2O) to give Compound XXXIII. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 0.98 min; ESI-MS m/z 285 [M+H]+
6.1.21. Synthesis of Compound XXXIV
[001208] To a stirred solution of (7?)-4-phenylbutan-2-amine (0.5 mmol) in dichloromethane (2 rnL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-fluorobenzoyl chloride (0.5 mmol). The reaction mixture was stirred for 2 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo and the final product was purified by reverse-phase HPLC (C- 18 column, ACN/H2O) to give Compound XXXIV. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.20 min; ESI-MS m/z 272 [M+H]+ 6.1.22. Synthesis of Compound XXXV
[001209] To a stirred solution of (i?)-4-phenylbutan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3- (trifluoromethyl)benzoyl chloride (0.5 mmol). The reaction mixture was stirred for 3 h at room temperature and then quenched with MeOH. The solvent was evaporated in vacuo and the final product was purified by reverse-phase HPLC (C- 18 column, ACN/H2O) to give Compound XXXV: RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.46 min; ESI-MS m/z 322 [M+H]+
[001210] To a suspension of Boc-Ala-OH (300 mg, 1.6 mmol) in THF (5 niL) was added CDI (280 mg, 1.7 mmol). The reaction mixture was refluxed for 1 h, cooled to rt, and aniline (160 mg, 1.7 mmol) in THF (3 ml) was added. After stirring for an additional 2 h at rt, the solvent was evaporated, water was added and the reaction was extracted with EtOAc. The organic layer was separated and washed with aqueous NaOH, aqueous HCl, and brine, dried over Na2SO4, and concentrated. This residue (320 mg, 1.2 mmol) was dissolved in dry THF (5 mL) and added drop wise to a suspension OfLiAlH4 (180 mg, 4.8 mmol) in dry THF (2 mL) at 00C. This reaction mixture was stirred at 45°C for 2 h, quenched with water, and extracted with EtOAc. The organic layer was combined and washed with water and dried over anhydrous Na2SO4 followed by concentration. The residue was purified by flash chromatography on silica gel and the product was treated with excess EtOAc/ HCl (3.5 mol/L, 1.5 mL) to afford the bis-HCl salt. This amine (1 mmol) was treated with 1 equivalent of 3-fluorobenzoyl chloride (159 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diisopropylethylamine. After stirring at rt for 3 h, the reaction mixture was concentrated and purified via reverse-phase HPLC (C-18 column, ACN/H2O) to give Compound XXXVI. RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.87 min; ESI-MS m/z 273 [M+H]+
[001211] To a suspension of Boc-Ala-OH (300 mg, 1.6 mmol) in THF (5 niL) was added CDI (280 mg, 1.7 mmol). The reaction mixture was refluxed for 1 h, cooled to rt, and aniline (160 mg, 1.7 mmol) in THF (3 ml) was added. After stirring for an additional 2 h at rt, the solvent was evaporated, water was added and the reaction was extracted with EtOAc. The organic layer was separated and washed with aqueous NaOH, aqueous HCl, and brine, dried over Na2SO4, and concentrated. This residue (320 mg, 1.2 mmol) was dissolved in dry THF (5 mL) and added drop wise to a suspension OfLiAlH4 (180 mg, 4.8 mmol) in dry THF (2 mL) at 00C. This reaction mixture was stirred at 45°C for 2 h, quenched with water, and extracted with EtOAc. The organic layer was combined and washed with water and dried over anhydrous Na2SO4 followed by concentration. The residue was purified by flash chromatography on silica gel and the product was treated with excess EtOAc/ HCl (3.5 mol/L, 1.5 mL) to afford the bis-HCl salt. This amine (1 mmol) was treated with 1 equivalent of 3-(trifluoromethoxy)benzoyl chloride (209 mg, 1 mmol) in DCM (2 mL) containing 2 equivalents of diisopropylethylamine. After stirring at rt for 3 h, the reaction mixture was concentrated and purified via reverse-phase HPLC (C- 18 column, ACN/H2O) to give Compound XXXVII. RP-HPLC (H2O/ACN( 10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 2.20 min; ESI-MS m/z 323 [M+H]+ 6.1.25. Synthesis of Compound XXXVIII
[001212] To a stirred solution 4-phenylpiperidine (81 mg, 0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (97 mg, 0.75 mmol) and 3- methoxybenzoyl chloride (85 mg, 0.5 mmol). The reaction mixture was stirred for 5 h at room
temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2OZACN(SO-QS0ZO) 1 ml/min, 3 min, 254 nM): Ret. Time = 1.48 min; ESI-MS m/z 296 [M+H]+. 6.1.26. Synthesis of Compound XXXIX
[001213] To a stirred solution 1-phenylpiperazine (81 mg, 0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (97 mg, 0.75 mmol) and 3- methoxybenzoyl chloride (85 mg, 0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1U NMR. RP-HPLC (H2O/ACN(30-95%) 1 ml/min, 3 min, 254 nM): Ret. Time = 1.25 min; ESI-MS m/z 297 [M+H]+. 6.1.27. Synthesis of Compound XL
[001214] To a stirred solution 4-phenylpiperidine (81 mg, 0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (97 mg, 0.75 mmol) and 3,5- dimethoxybenzoyl chloride (100 mg, 0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(30-95%) 1 ml/min, 3 min, 254 nM): Ret. Time = 1.52 min; ESI-MS m/z 326 [M+H]+.
6.1.28. Synthesis of Compound XLI
[001215] To a stirred solution 1-phenylpiperazine (81 mg, 0.5 mmol) in dichloromethane (2 rnL) at room temperature, was added diisopropylethylamine (97 mg, 0.75 mmol) and 3,5- dimethoxybenzoyl chloride (100 mg, 0.5 mmol). The reaction mixture was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by silica gel flash chromatography and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(30-95%) 1 ml/min, 3 min, 254 nM): Ret. Time = 1.32 min; ESI-MS m/z 327 [M+H]+. 6.1.29. Synthesis of Compound XLII
Aniline (186 mg, 2 mmol) and boc-D-alanal (346 mg, 2 mmol) were dissolved in anhydrous methanol (5 mL). After stirring for 2 h at rt, sodium triacetoxyborohydride (530 mg, 2.5 mmol) was added and the solution was stirred for 25 h at rt. The reaction was quenched with aqueous NaHCO3, extracted with ethyl acetate, concentrated, and purified via flash chromatography. A portion of this intermediate (1.1 mmol) was stirred for 1 h in 20% TFA v/v in DCM to afford Boc removal, and this amine (1 mmol) was treated with 1 equivalent of 3,5-dihydroxybenzoic acid (154 mg, 1 mmol), 1.2 equivalents of benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP, 624 mg, 1.2 mmol) in DMF (2 mL) containing 2 equivalents of diisopropylethylamine. After stirring at rt for 20 h, the reaction mixture was concentrated and purified via reverse-phase HPLC (C- 18 column, ACNZH2O) to give the title compound.
RP-HPLC (H2OZACN(IO-QS0ZO) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.14 min; ESI-MS m/z
287 [M+H]+
CHIRAL ANALYSIS [Daicel Chiralcel OD-RH column (150 x 4.6 mm), UV detection at 254 nm, flow rate of 1.0 mL/min, mobile phase: H2O/ACN(10-95%), Run time = 10 min]: Ret. Time
= 4.16 min; ESI-MS m/z 287 [M+H]+
6.1.30. Synthesis of Compound XLIII
[001216] To a stirred solution (i?)-4-phenylbutan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3,5- bis(trifluoromethyl)benzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.81 min; ESI-MS m/z 390 [M+H]+. 6.1.31. Synthesis of Compound XLIV
[001217] To a stirred solution (i?)-4-phenylbutan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3- isopropoxybenzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.68 min; ESI-MS m/z 312 [M+H]+.
6.1.32. Synthesis of Compound XLV
[001218] To a stirred solution (7?)-4-phenylbutan-2-amine (0.5 mmol) in dichloromethane (2 rnL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3- (trifluoromethoxy)benzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.75 min; ESI-MS m/z 338 [M+H]+. 6.1.33. Synthesis of Compound XLVI
[001219] To a stirred solution (i?)-4-phenylbutan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-cyanobenzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(10- 95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.34 min; ESI-MS m/z 279 [M+H]+.
[001220] To a stirred solution 2-amino-4-(4-methoxyphenyl)butan-l-ylium (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-methoxybenzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.32 min; ESI-MS m/z 314 [M+H]+. 6.1.35. Synthesis of Compound XLVIII
[001221] To a stirred solution 2-amino-4-(4-methoxyphenyl)butan-l-ylium (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-(trifluoromethyl)benzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.32 min; ESI-MS m/z 314 [M+H]+. 6.1.36. Synthesis of Compound XLIX
[001222] To a stirred solution 2-amino-4-(4-methoxyphenyl)butan-l-ylium (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-cyanobenzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.26 min; ESI-MS m/z 309 [M+H]+. 6.1.37. Synthesis of Compound L
[001223] To a stirred solution 4-(furan-2-yl)butan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-methoxybenzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1U NMR. RP-HPLC (H2O/ACN(10- 95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.14 min; ESI-MS m/z 274 [M+H]+. 6.1.38. Synthesis of Compound LI
[001224] To a stirred solution 4-(furan-2-yl)butan-2-amine (0.5 mmol) in dichloromethane (2 mL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3- (trifluoromethyl)benzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR.
RP-HPLC (H2OZACN(IO-QS0ZO) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.34 min; ESI-MS m/z 312 [M+H]+.
6.1.39. Synthesis of Compound LII
[001225] To a stirred solution 4-(furan-2-yl)butan-2-amine (0.5 mmol) in dichloromethane (2 rnL) at room temperature, was added diisopropylethylamine (0.75 mmol) and 3-cyanobenzoyl chloride (0.5 mmol). The reaction was stirred for 5 h at room temperature and then quenched with MeOH. The reaction mixture was extracted into DCM and the organic layer was washed with water and brine. The solvent was evaporated in vacuo, and the residue was purified by reverse-phase HPLC and analyzed for purity by LCMS and 1H NMR. RP-HPLC (H2O/ACN(10- 95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 3.06 min; ESI-MS m/z 269 [M+H]+. 6.1.40. Synthesis of Compound LIII
Aniline (186 mg, 2 mmol), boc-L-alanine (378 mg, 2 mmol) and l-ethyl-3-(3- dimethylaminopropyl)carbodiimide (EDC, 420 mg, 2.2 mmol) were dissolved in DCM (5 mL) to which JV,jV-diisopropylethylamine (DIEA, 322 mg, 2.5 mmol) and aniline (186 mg, 2 mmol) were added. The reaction mixture was stirred for 24 hrs at rt. Water (20 mL) was added and the organic layer was separated and washed with aqueous NaOH, aqueous HCl, and brine, dried over Na2SO4, and concentrated to give intermediate A. To a mixture of A (396 mg, 1.5 mmol) in dry THF (10 mL) was added LiAlH4 (IM in THF, 3 ml) drop wise at rt. The reaction mixture was stirred at rt for 2 h, quenched by addition of water, and extracted with EtOAc. The organic layer
was combined and washed with water and dried over anhydrous Na2SO4 followed by concentration. The residue was purified by flash chromatography on silica gel and treated with anhydrous HCl (4M in dioxane, 1 mL) and concentrated to afford intermediate B which was treated with 3,5-dihydroxybenzoic acid (230 mg, 1.5 mmol) and l-ethyl-3-(3- dimethylaminopropyl)carbodiimide (EDC, 382 mg, 2.0 mmol) in DCM containing 2 equivalents of Λ/,Λ/-diisopropylethylamine (3 mmol). After stirring at rt for 20 h water (20 mL) was added and the organic layer was separated and washed with aqueous NaOH, aqueous HCl, and brine, dried over Na2SO4, and concentrated to give crude (iS)-3,5-dihydroxy-JV-(l- (phenylamino)propan-2-yl)benzamide which was concentrated and purified via reverse-phase HPLC (C- 18 column, ACN/H2O).
RP-HPLC (H2O/ACN(10-95%) 1 ml/min, 4.5 min, 254 nM): Ret. Time = 1.14 min; ESI-MS m/z 287 [M+H]+
CHIRAL ANALYSIS [Daicel Chiralcel OD-RH column (150 x 4.6 mm), UV detection at 254 nm, flow rate of 1.0 mL/min, mobile phase: H2O/ACN(10-95%), Run time = 10 min]: Ret. Time = 4.22 min; ESI-MS m/z 287 [M+H]+ 6.2. DETERMINATION OF ACTIVITY
6.2.1. ATPase Assay in Membranes - General Procedure
[001226] Without being bound to any theory, the Ca-ATPase (SERCA) of the sarcoplasmic reticulum (SR) functions to remove calcium ions from the cytoplasm. ATP-ase 2 (SERC A2) re- sequesters the calcium back into the internal sarcoplasmic reticulum pool, thus priming the next quantal release of calcium. SERC A2 thereby regulates cardiac function by promoting both cardiac relaxation and contractility. In cardiac muscle, SERC A2a is regulated by phospholamban (PLB) which inhibits SERCA at submicromolar calcium. The regulation of PLB is a pivotal event in cardiac relaxation and contractility and phosphorylation of PLB by protein kinase A causes PLB to release its inhibitory grip on SERC A2. Abnormal PLN/SERCA2 ratios and associated defective calcium cycling is a feature of some forms of human and experimental heart failure.
[001227] This procedure evaluates small molecules for their potential to reverse the inhibitory effect of phospholamban (PLB) on SERCA by measuring sarcoplasmic reticulum calcium- dependent ATPase activity (Ca-ATPase).
[001228] The procedure uses solid-phase peptide synthesis of PLB and functional membrane co-reconstitution with purified SERCA, or heterologous co-expression of PLB and SERCA in HEK cells. FRET between labeled SERCA and PLB is measured to assess compound effects on the SERCA-PLB complex, followed by Ca-ATPase measurements in an NADH-linked, enzyme- coupled microtiter plate assay. The procedure characterizes the binding of compounds to the SERCA-PLB complex.
[001229] The procedure is designed, inter alia, to establish whether small molecule compounds have the potential to be effective in reversing SERCA inhibition and restoring myocyte function (contractility and relaxation) in the failing human myocardium following acute decompensated heart failure.
6.2.1.1. Materials, Reagents, and Equipment
6.2.1.1.1. Materials and Equipment
[001230] Blender; Centrifuge; Cheesecloth; Cold Room (approximately 4°C); Computer and custom software program for calculating Ca++; Freezer at -6O0C or below; Microfuge Tubes
Microtiter Plates - 96 wells; Microtiter Reader (SpectraMAX Plus by Molecular Devices or equivalent); Softmax Software; and Tissue Homogenizer.
6.2.1.1.2. Cells, Reagents, Standards, and Media
[001231] A23187 Ionophore ; Adenosine triphosphate (ATP); Aprotinin ; Benzamidine; Reagents for Biuret protein determination; Dimethyl Sulfoxide (DMSO); EGTA; Lactate Dehydrogenase (LDH); Leupeptin ; Magnesium chloride (MgCl2); MOPS; NADH ; PEP; Pefabloc SC; Pepstatin A; Potassium Chloride (KCl); 2-propanol; Pyruvate Kinsase (PK); Reagents for Biuret Method; Sodium Bicarbonate; Sucrose; and TRIS (tris(hydroxymethyl)aminomethane).
6.2.1.2. Procedures
6.2.1.2.1. Solution Preparations
[001232] Prepare reagents, filter, and store at ambient conditions for no more than two weeks unless indicated otherwise below.
[001233] 10 mM Sodium Bicarbonate Solution/10 mM TRIS Solution, pH 7.0, with Protease Inhibitors (10 mL/L Aprotinin (0.1 mg/mL), Leupeptin (0.1 mg/mL), Benzamidine (80 mM), Pefabloc SC (100 mM)and Pepstatin A (0.1 mg/mL)).
[001234] 10 mM Sodium Bicarbonate Solution/10 mM TRIS Solution, pH 7.0, with Protease Inhibitors (10 mL/L Aprotinin (0.1 mg/mL), Leupeptin (0.1 mg/mL), Benzamidine (80 mM), Pefabloc SC (100 mM)and Pepstatin A (0.1 mg/mL)).
6.2.1.2.2. Test Compounds
[001235] The long term storage temperature for test compounds is -700C or below.
[001236] Powdered, unreconstituted compounds can be stored at -200C or below for up to two weeks.
[001237] Each compound is weighed out (1-2 mg) and dissolved in DMSO at 10 mM to prepare a long-term storage stock solution. This solution is further diluted to 1.0 mM, which is used in the assays.
6.2.1.2.3. Animal Procedures
A. Species
[001238] Porcine: Purebred Yorkshire
B. Euthanasia
[001239] Euthanize animals according to standard procedures recommended by the American Veterinary Medical Association and approved by the Institution's Animal Care and Use Committee.
C. Preparation of Porcine Cardiac SR
[001240] Purify sarcoplasmic reticulum vesicles from porcine hearts using the procedure described below.
1. Place the fresh porcine heart into 10 mM Sodium Bicarbonate Solution, pH 7.0, containing protease inhibitors and transport to the lab.
2. In a cold room, snip the atria and fat etc. off of the ventricles.
3. Blend each ventricle in the Sodium Bicarbonate Solution (~5 mL/g of tissue) and centrifuge at 11,000 x g for 20 min, 40C.
4. Filter the supernatants through four layers of cheesecloth and store on ice.
5. Measure the supernatant volume and add enough KCl to make a 0.6 M solution. Stir on ice for 1 hour.
6. Centrifuge the solution at 100,000 x g for 30 min, 40C and resuspend the resulting pellet in 10% Sucrose Solution with Protease Inhibitors.
7. Centrifuge the solution at 100,000 x g for 30 min, 40C and resuspend the resulting pellet in ~2 rnL of 10% Sucrose Solution with Protease Inhibitors.
8. Aliquot the cardiac SR prep into microfuge tubes (500 μL/tube) and flash-freeze. Store at less than -6O0C.
6.2.1.2.4. Protein Content
[001241] Determine the protein concentration using the Biuret method. Gornall et al. J. Biol. Chem. Ill (1949), pp. 751-766.
6.2.1.2.5. ATPase Assay
[001242] Perform ATPase activity measurements using an NADH-linked assay, microtiter plates (96 wells, 8x12) on a SpectraMAX Plus.
1. Plate different samples in each row and different calcium concentrations in each column. Ensure that each plate contain two rows of controls (1% DMSO) and two different compounds in triplicate.
2. Test each compound at 10 μM twice per day and on two different days for a total n=4.
3. The total well volume is 200 μL, and each well should contain 6.67 μg porcine cardiac SR (Balog et al., Am. J. Physiol. Heart Ore. Physiol., (2005) 290: H794-9, incorporated herein by reference in its entirety) or rabbit skeletal tissue prepared using Celladon procedure TM002 (Birmachu et al. Biochemistry, (1989) 28, pp. 3940-3947, incorporated herein by reference in its entirety) 50 mM MOPS, 100 mM KCl, 5 mM MgC12, 1 mM EGTA, 0.7 μg A23187 Ionophore, 0.2 mM NADH, 0.5 mM PEP, 2.5 mM ATP, 2 IU PK, 2 IU LDH and 90 mM sucrose.
4. The volume of CaCl2 necessary for a set ionized Ca2+ was calculated according to the method of Fabiato and Fabiato (J. Physiol (Paris) 1979, 75:463-505), using the RECIPC (computer program developed by Steven Robertson and revised by Neil Rrandall and Carl Johnson) to calculate the volume of 20 mM Ca2+ needed.
5. Monitor the assays at 340 nm, and the decreasing absorbance of NADH converted into ATPase activity [(μM substrate hydro lyzed)(mg SR)-I (min)- 1].
6.2.2. Sarcomere Shortening Assay - General Procedure
[001243] Without being bound by any theory, in mammalian cardiac muscle cells, calcium regulation by sarcoplasmic reticulum calcium ATPase (SERC A2) and phospholamban (PLB) is an essential pre-requisite for myocyte contraction and relaxation. In hearts undergoing acute decompensated heart failure, decreased SERCA2 activity results in abnormal cellular calcium handling and decreased cardiac contractility. Therefore, compounds that have the capacity to directly increase SERC A2 activity or release the inhibition of SERC A2 by interacting at the SERCA2-PLB interface, may play a role in improving myocyte contractility and cardiac function. In response to myocyte stimulation, physical changes in some of the cellular contractility proteins such as sarcomere length can be assessed as a direct measure of cellular contractility. Compounds that have the ability to decrease sarcomere length and increase fractional shortening may be developed as potential therapeutics for the treatment of acute decompensated heart failure. 6.2.2.1. Materials, Reagents, and Equipment
6.2.2.1.1. Materials and Equipment
[001244] Animal restrainer; Cell strainer filter, sterile (BD Falcon REF 352360 or equivalent); CCD camera (Ionoptix Myocam, Ionoptix, Milton, MA or equivalent); Data acquisition software package (custom, Ion Wizard version 4.4 or equivalent); Digital (Field) simulator; Digital roller perfusion pump (Masterflex or equivalent); Filters, 0.2 μm; Imaging chamber with a perfusion inflow route (custom-made, Ionoptix or equivalent); Langendorff apparatus; Microscope, inverted epi- fluorescent (Nikon TSlOO or equivalent); Microscope slides; Myopacer (Ion Optix Myopacer, Ionoptix, Milton, MA); Petri dishes ;Platinum electrodes; Surgical scissors, fine, sterile; Syringes; Temperature -regulated perfusion apparatus; Tube, plastic, sterile; and Water bath, 370C water bath (Cole-Palmer or equivalent).
6.2.2.1.2. Reagents, Standards, and Media
[001245] 2,3, Butanedione Monoxine (BDM); Calcium Chloride (CaCl2); Calf Serum (Not heat-inactivated); Collagenase II; Distilled Water; Fura2-AM; Glucose; Heparin; HEPES Sodium Salt (NaO4S); Heptahydrate (MgSO4-7H2); Ketamine; Magnesium Chloride (MgCl2); Magnesium Sulphate; MEM (Sigma 4780); Penicillin; Potassium Chloride (KCl); Potassium
Phosphate (KH2PO4); Sodium Chloride (NaCl); Sodium Phosphate (Na2HPO4); Sodium Bicarbonate (NaHCOs); and Taurine. 6.2.2.2. Procedures
6.2.2.2.1. Solution Preparations
A. Perfusion Buffer
[001246] 1. Prepare Ix Perfusion Buffer from a 1OX stock solution, which is stored at -2O0C. Perfusion buffer is prepared from 1OX stock solution by diluting in distilled water at the time of use.
2. Adjust pH to 7.0 with HCl and filter sterilize.
3. Alternatively use SIGMA KH Buffer k3735-lL.
Materials Mol.Wt Final Cone. For Ix For 1Ox
(g/Mol.) (mM) g/L g/L
Sodium Chloride (NaCl) 58.4 120.4 7.03 70.3
Potassium Chloride (KCl) 74.6 14.7 1.1 11.0
Potassium Phosphate (KH2PO4) 136.1 0.6 0.082 0.82
Sodium Phosphate (Na2HPO4) 142.0 0.6 0.085 0.85
Magnesium sulphate 246.5 246.5 1.2 0.3 3.0
Heptahydrate(MgSO4-7H2))
HEPES sodium salt (NaO4S) IM 10 2.6 26
Sodium Bicarbonate (NaHCO3) 84 4.6 0.39 3.9
Taurine 125.1 30 3.75 37.5
2,3,Butanedione Monoxine (BDM) 101.1 10 1.0 10
Glucose 180.2 5.5 1.0 10
B. Myocyte Digestion Buffer
[001247] Prepare Myocyte Digestion Buffer using the materials and quantities listed below; store at 40C until use.
Material Quantity Per Heart
Perfusion Buffer 50 rnL
Collagenase II 40 rnL
10OmM CaCl2 15 μL
C. Myocyte Stopping Buffer
[001248] Prepare Myocyte Stopping Buffer using the materials and quantities listed below; store at 40C until use.
Material Final Concentration Quantity Per Heart
1 X Perfusion Buffer 18 rnL
Calf serum (not heat inactivated) 10% 2 mL
100 mM CaCl2 12.5 μL 2.5 μL
D. Myocyte Plating Medium
[001249] Prepare Myocyte Plating Buffer using the materials and quantities listed below; store at 40C until use.
Material Final Concentration Volume
MEM (Sigma 4780) 42.5 mL
Calf serum 10% 5 mL
2,3,Butanedione Monoxine 1O mM I mL
(BDM)
Penicillin lOO units/mL 0.5 mL
E. HEPES Buffer
[001250] Prepare HEPES Buffer using the materials and quantities listed below; glucose and calcium chloride are not added until the day of use; store at 40C until use.
Material Grams/Liter
NaCl 7.66
KCl 0.298
HEPES 2.38
MgCl2 0.203
CaCl2 (M.W. 147.02) 0.1471
Glucose 1.801
I M CaCl2 10O mL 1 items are not added until the day of isolation
F. Tyrode's Solution
[001251] Prepare Tyrode's Solution in 1 Liter of distilled water using the materials and quantities listed below; store at 40C until use. Material Grams/Liter
NaCl 8
KCl 0.2
CaCl2 0.2
MgCl2 0.1
NaH2PO4 0.05
NaHCO3 1
D-Glucose 1
6.2.2.2.2. Test Compounds
[001252] The long term storage temperature for test compounds is -700C or below. Powdered, unreconstituted compounds can be stored at -200C or below for up to two weeks. Each compound is diluted to 10 mM using DMSO as a solvent.
6.2.2.2.3. Animal Procedures
A. Animals
[001253] Use one adult male Sprague Dawley rat (220 - 270 gram), at least 6 weeks of age at the time of sacrifice, for each cardiac myocyte isolation procedure. The total number of animals used will be dependent on the number of compounds to be tested.
B. Anesthesia
[001254] Place rats into a restrainer and administer intramuscularly (IM) a 1 :2 mixture of ketamine and heparin (0.5 ml ketamine, 1 mL heparin) to induce anesthesia.
C. Euthanasia
[001255] Euthanize rats following anesthesia by cervical dislocation.
D. Isolation of Rat Left Ventricular Cardiomyocytes
[001256] The isolation of rat cardiomyocytes is the most critical component of the fractional shortening assay. Care must be taken to ensure that myocyte integrity and viability is maintained for functional characterization. Individual cell integrity is more crucial than % of total cell viability.
[001257] Ideal conditions include 25-40% confluency and response to stimulus. Do not use myocytes isolated from cells with greater than 90% confluency or that have no spontaneous contraction without stimuli.
[001258] The myocyte isolation procedure takes place in calcium-free media to prevent heart contraction.
1. Perform a thoracotomy at the interstitium of the 4th rib and rapidly excise the heart.
2. Immerse the heart, dissected free of fat, onto a Petri dish containing ice cold IX Perfusion Buffer.
3. Cannulate the aorta for retrograde perfusion with a sequence of the following solutions: Perfusion Buffer, Collagenase Buffer, Myocyte Stopping Buffer, HEPES Buffer, Myocyte Plating Medium, Tyrodes Solution. a. Once the heart is carefully cannulated at the aorta as a Langendorff perfused preparation, excise the left and right atria and perfuse the ventricles with Perfusion Buffer at 370C for 4 minutes to remove all blood. A digital roller perfusion pump and 370C water bath are required for the perfusion procedure. Deliver tissue perfusion at a constant flow rate (8 mL/min) with media pre-
warmed to 420C and oxygenated (95% O2, 5% CO2) before passage through a temperature -regulated perfusion apparatus which sets the final fluid temperature at 370C before reaching the heart preparation. b. Switch the perfusate to Collagenase Buffer (yellow color) for a total of 18 minutes. The Collagenase Buffer takes approximately 2 minutes to perfuse through the perfusion equipment and equilibrate at 370C. c. Set the timer to perfuse the suspended ventricles for 16 minutes. To ensure the perfusion equipment is functioning, check that the color of the buffer that is collected in a sterile vial is pale yellow. d. Monitor the digestion process by gently squeezing the ventricles to ensure that they are softened. e. At the end of the digestion period, switch the solution to 35 mL of the Myocyte Stopping Buffer with 1.5 μL (ImM) CaCl2. Perfuse the heart and rinse free of collagenase for 5 minutes at constant pressure and flow. f. Following collagenase digestion and rinsing, remove the heart from the Langendorff apparatus and carefully detached from the aortic cannula.
4. Transfer the heart to a Petri-dish and separate the ventricles using an incision that runs along the left anterior descending coronary artery. Using fine sterile surgical scissors, section and mince the LV free wall.
5. Add 20 mL of Myocyte Stopping Buffer to a sterile plastic tube and decant the chopped ventricular myocyte 'soup' into it the buffer. Allow the myocytes to settle and separate from the non-myocytes, keeping the tube upright for 15 minutes.
6. Decant the supernatant and add 20 mL of new Myocyte Stopping Buffer to the cell suspension. Filter the 20 mL 'soup' through a sterile cell strainer filter to remove all remaining non-myocytes.
7. Gradually replace the remaining 4 mL of Perfusate Buffer with HEPES Buffer, 1 mL at a time. Allow the 4 mL suspension to settle upright for 15 minutes. Remove 1 mL of the supernatant and replace with HEPES Buffer. Repeat this process 3 more times over a period of 60 minutes until the myocytes are suspended in 4 mL HEPES Buffer. This process helps to minimize cell death due to an abrupt rise in extracellular calcium and allows the myocytes to recover.
6.2.2.2.4. Myocyte Preparation, Staining and Application of Compound
[001259] 1. Transfer a sample of the solution from the step above to a microscope slide to check for preparation purity, cell viability, muscle striations and z-bands. Typical preparations contain 80% rod-shaped, quiescent, Ca2+-tolerant myocytes, which have well-defined cross striations and sarcomere patterns.
2. Allow the myocytes to recover from the isolation procedure, incubating in Tyrodes Solution containing ImM CaCl2 for a total of 1 hour. Store remaining cells in Myocyte Plating Medium.
3. Transfer 100 μL of the myocyte suspension to 1 mL vials. Add 1 μL of Fura2-AM to each vial and incubate for 10 minutes while cells are maintained in darkened room.
4. Sediment the cells, suction the Fura2-AM containing supernatant, and replace with Tyrodes Solution.
5. Add 10 μL of each test compound (lOμM) to each vial. All experiments are first conducted in the presence of ImM CaCl2. Incubate each compound in the presence of myocytes for 30 minutes.
6.2.2.2.5. Myocyte Pacing
[001260] Use a custom-designed myopacer and digital (Field) simulator that allows fine control of voltage strength, frequency and duration for pacing rat cardiomyocytes. Pulse, voltage, frequency and duration are readily adjusted to minimize the amount of output power used to field stimulate myocytes which helps to maintain myocyte stability and viability. Mount a custom- made (Ionoptix) imaging chamber with a perfusion inflow route on an adjustable stage of an inverted epi- fluorescent Nikon microscope, filled with 500 μL oxygenated Tyrodes Solution.
1. Add 100 μL of normal cell suspension to the chamber and allow the suspension to settle for 5 minutes before turning steady-state pacing to the lowest output voltage at a pulse width of 2 ms and a frequency of IHz. Use platinum electrodes to field-stimulate the myocytes.
2. Gradually raise the output voltage by increments of 1 volt until the majority of cells in the imaging window start contract in exact synchrony with the delivered pacing stimuli, thereby defining the diastolic threshold for field stimulation.
3. Set the pacing voltage to 1.2 x diastolic threshold for the remainder of the recordings in a given group of cells.
4. Mix 890 μL HEPES buffer, 100 μL cell suspension and 10 μL of test compound at 10 rnM concentration. 500 μL of mixture are subsequently added to the imaging chamber.
6.2.2.2.6. Sarcomere Shortening Assay
[001261] Sarcomere length is recorded from single myocytes using an epi-fluorescence inverted microscope with an attached CCD camera. Only rod-shaped, striated cells that exhibit a clear and stable contraction by visual inspection using the 4Ox objective of the microscope are included in the study.
1. Move the stage of the microscope using a fine, adjustable micromanipulator that translates the chamber-holding stage in the x-y direction until a target contracting cell appears in the middle of the microscope viewing field.
2. Using a built-in shutter, redirect the optical path towards a high resolution CCD camera that is mounted onto the main arm of the Nikon microscope.
3. Display digital images in real time using a custom- written data acquisition software package, which interferes with the CCD camera.
4. Rotate the CCD camera using a stabilizing rotating arm that provides 360 degree rotational flexibility to orient the myocyte along the longitudinal dimension of the measurement field, which is continuously displayed to the investigator on the computer monitor.
5. Minimize background noise and enhance the myocyte image contrast using the digital imaging software (Ionoptix Ltd, Ion Wizard version 4.4).
6. Using digital calipers, track and register a region of interest within the center of the field of the cardiac myocyte in both time and space during each myocyte contraction.
7. Measure the average sarcomere length within the user-determined window using the Ionoptix software that determines the average periodicity of the Z-line density based on the fast Fourier transform algorithm.
8. Calculate unloaded sarcomere shortening as the difference between peak systolic length and maximum diastolic length. Determine kinetic parameters for the shortening transient using a curve-fitting algorithm (Ionoptix).
6.2.2.2.7. Cell Sampling
[001262] 1. Use only freshly isolated, striated, rod-shaped viable cells for the fractional shortening assay.
2. In order to maintain accuracy during experimentation and ensure sampling of healthy cells, assess fractional shortening in up to 5 cells from each imaging chamber.
3. Randomly sample sham control cells and cells incubated with test compounds.
4. To account for inter-rat variation, test each compound in different batches of cardiomyocyes isolated on different days.
5. Assessment of fractional shortening on a minimum of 20 cells/compound, 30 second per cell is essential.
6. Compare results against a compound of known fractional shortening parameters. 6.2.3. Compound Evaluation - Functional Effects on Serca
[001263] Two exemplary compounds, "compound A" and "compound B" were evaluated in this study. Ca-ATPase assays were performed at a series of [Ca2+] corresponding to the physiological range defined by systole and diastole (pCa 5.5 and 7.5, respectively). The ATP hydrolysis rate was measured by an NADH-enzyme linked assay, with Vmax and pKca determined by fitting the ATPase calcium-dependence to the Hill function (Figure 1, inset). 10 μM compound A increases Vmax by about 15% with no effect on pKca (Figure 1 and Table 1). [001264] Ca-ATPase measurements were conducted in the presence of a range of test- compound concentrations, relative to controls conducted in the presence of vector (DMSO). [001265] Dose-response studies show that compound A is a significant Vmax activator in both CSR and SSR, slightly more potent in SSR (Figure 2 and Tables 2a-2b). Increased potency in SSR relative to CSR correlates with increased contractility in excised atrial muscle, compared with vertricular (not shown). Compound B was found to be more potent (Figure 3, Table 3a-3b).
Table 1. SSR Ca-Curves illustrating compound A effect on Vmax (Corresponds to Figure 1).
DMSO Compound pCa CTRL SEM A SEM
5.4 3.88725 0.19799 4.4655 0.0735
5.6 3.93825 0.13195 4.675 0.038
5.8 3.7925 0.15378 4.399 0.021
6 3.40325 0.2203 3.9065 0.2605
6.2 2.67425 0.11687 3.1025 0.0555
6.4 1.928 0.08906 2.181 0.077
6.6 1.17825 0.04216 1.3055 0.0235
6.8 0.75625 0.05254 0.8365 0.0305
7 0.50275 0.00776 0.535 0.03
7.5 0.2705 0.01 188 0.2785 0.0105
8 0.19433 0.01625 0.1945 0.0085
Table 2A. CSR Ca-ATPase V max and pKca dose response to Compound A
(Corresponds to Figure 2, left panel).
[compound ΔVmax ΔpKca
A](μM) %change SEM p (% of PLB shift) SEM p n
0.03 -1.8 1.0 3.3 1.6 7
0.1 -2.0 1.7 0.5 2.0 7
0.3 -2.1 1.0 3.0 2.8 7
1 -0.5 1.3 1.7 1.2 7
3 3.4 1.7 1.2 2.6 7
10 6.8 1.9 0.002 -2.8 3.3 0.002 12
Table 2B. SSR Ca-ATPase Vmax and pKca dose response to compound A (Corresponds to Figure 2, right panel). [compound i ΔVmax ΔpKca
A](μM) %%cchhaannggee SSEEMM p ((%% ooff PPLLBB sshhiifftt)) SSEEMM p n
.03 -1.8 1.2 0.5 2.7 7
0.1 4.4 1.1 -3.5 2.2 7
0.3 4.0 2.5 -5.2 0.9 7
1 5.3 1.9 -2.0 1.8 7
3 9.1 2.1 -2.6 3.7 7
10 18.8 2.0 0.003 --99..00 44..00 0.0001 10
Table 3A. CSR Ca-ATPase Vmax and pKca dose response to Compound B (Corresponds to Figure 3, left panel).
[Compound ΔVmax ΔpKca
B](μM) %change SEM P (% of PLB shift) SEM p n
0.02 -4.6 1.1 3.4 2.0 2
0.1 -1.4 2.2 -8.4 10.1 2
0.5 -2.4 0.5 4.5 3.5 2
1 1.4 1.8 1.1 4.5 2
4 6.5 0.2 -3.9 0.5 2
10 15.8 2.1 0.0001 -13.9 2.2 0.0003 10
Table 3B. CSR Ca-ATPase Vmax and pKca dose response to Compound B (Corresponds to Figure 3, right panel). [Compound ΔVmax ΔpKca
B](μM) "/ochange SEM p (% of PLB shift) SEM P n
0.02 1.3 0.6 -2.1 4.1 2
0.1 1.7 1.1 -0.5 3.8 2
0.5 5.0 2.0 -7.9 2.2 2
1 10.4 0.6 -6.5 2.0 2
4 18.0 3.7 -15.6 6.5 2
10 33.3 3.1 0.001 -31.4 3.1 0.00004 6
6.2.4. Evaluation of Exemplary Compounds in Sarcomere Shortening Assay
[001266] Certain compounds were evaluated for their ability to increase sarcomere shortening Unless otherwise inidicated, sarcomere shortening (SS) was evaluated at 10 μM. + means a statistically significant positive change in SS as follows:
+: <10%
++: 10%-20%
+++: 20%-30%
Table 2
[001267] An exemplary compound described herein was evaluated as set forth in this section.
6.2.5.1. A compound described herein increases myocyte contractility in vitro
[001268] The effects of an exemplary compound described herein at a concentration of lOμM on contractility in ventricular myocytes that were isolated from normal and failing rat hearts were investigated. Contractile performance was carefully assessed by measuring the extent of sarcomere shortening during field stimulation of myocytes at a constant rate of IHz. In a subset of experiments, myocytes were field stimulated at a wide range of rates (0.5-3Hz) in order to determine the rate dependent effects of the compound on contractility. Shown in Figure 4 are representative traces illustrating the periodic and reproducible degree of sarcomere shortening during the course of myocyte contraction. These representative traces highlight the robust nature of this metric as a reliable index of cellular contractility. On average, myocytes that were pre- incubated with lOμM of the exemplary compound of exhibited a highly significant (p<0.05) increase in sarcomere shortening compared to untreated cells, indicating marked enhancement (by >25%) in contractility, Figure 5.
[001269] To further determine the efficacy of the compound in producing positive inotropic effects in diseased myocytes, a reproducible model of heart failure produced by ascending aortic banding in the rat was used. As shown in Figure 6, pretreatment of failing myocytes with lOμM of the compound also significantly increased sarcomere shortening in a comparable manner to its effects in normal myocytes. Furthermore, rate dependence of the compound was tested by measuring the extent of sarcomere shortening during field stimulation at 0.5, 1, 2, and 3Hz in untreated compared to treated myocytes. As shown in Figure 7, lOμM the compound enhanced contractility at all tested frequencies.
6.2.5.2. Effects of the compound are related to SERCA2a function
[001270] To determine if the positive inotropic effect of the compound was related to a potential change in SERCA2a activity, Thapsigargin was used to pharmacologically inhibit SERCA2a function. Thapsigargin markedly suppressed contractility in untreated myocytes. Remarkably, Thapsigargin also completely reversed the positive inotropic effects that were afforded by the compound (Figure 8). These data suggest that intracellular calcium cycling, in general, and SERC A2a specifically are likely targets of the compound.
6.2.5.3. Effects of the compound on cellular electrophysiological properties
[001271] Several pharmacological agents known to increase contractility and/or perturb intracellular calcium cycling produce major electrophysiological changes that could promote arrhythmias, in part, by prolonging the cellular action potential. To determine whether the positive inotropic effects produced by the compound in myocytes were associated with major cellular electrophysiological abnormalities, action potentials were recorded from myocytes using the whole cell patch clamp technique in current clamp mode. As shown in Figure 9, the compound did not alter the depolarization phase of the action potential, but rather hastened repolarization. These cellular electrophysiological findings are consistent with potential SERCA2a activation by the compound. Finally, an investigation as to whether perfusion with the compound enhanced the propensity for arrhythmias in retrogradely perfused rat hearts was performed. As shown in Figure 10, perfusion with the compound did not alter major electrocardiographic features and did not predispose to arrhythmias, ex vivo as volume conducted electrocardiograms were comparable before and after treatment with the compound.
6.2.5.4. Ex vivo Contractility Assay Using Normal Rat Cardiac Tissues
[001272] This study was designed to evaluate the potential inotropic effects of the compound on the fresh isolated cardiac tissues from the normal male Sprague Dawley rats. Either the atrial tissues (A) and ventricular tissues (V) were paced into a organ bath with an electrical field stimulator at IHz with square wave pulses of 0.35 msec duration. After reaching steady state, the test compounds were added cumulatively (0.1-10 μM). Isoproterenol (ISO) and the vehicle buffers were used as positive and negative controls respectively. The compound was tested at 0.1, 0.3, 1, 3 and 10 μM. Data in Figure 11 is presented as a percent variation in the force of contraction with respect to control (% Contractility): Contractile force % = ( Fa -
Fb ) /Fbχ 100%. Fa was the contractile force of the isolated heart tissues after drug administration; whereas Fb represented the contractile force of isolated heart tissues before drug administration.
[001273] The results shown that the compound provides a statistically significant increase in contractile force in a dose-dependent manner. Its effects on rat atria and ventricular tissues were similar. The vehicle buffer had no effect on force of contraction of isolated rat tissues.
6.2.5.5. Rat Pharmacokinetic Study
[001274] The objective of this study was to evaluate the oral uptake and pharmacokinetic profiles of the compound in rats. After the compound was administered orally (gavage) at 10 mg/ kg or intravenous (IV) at 2 mg / kg to male Sprague-Dawley rats, the blood samples were collected from retro-orbital puncture at 0, 0.017, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 24 hours post- dosing. All plasma samples were being analyzed using an API3000 LC-MS/MS (liquid chromatography/mass spectrometry/mass spectrometry). The PK parameters were generated using a non-compartment model. Mean plasma concentration data for the compound in rats following intravenous and oral administration are tabulated below:
[001275] Following an IV bolus injection of the compound at 2 mg / kg, the systemic clearance was 3.21 L/hr/kg, which corresponded to 96.92% of rat hepatic blood flow (3.31 L/hr/kg). The terminal elimination half-life (Ty2Z) was 2.06 hr, while Vz was 9.44 L/kg. The Maximum plasma concentration Cmax (at 1 minutes after dosing) was 8.75 nmol/mL, AUQo-∞) was 630.3645 ± 76.6174 μg/L*hr. The volume of distribution at terminal phase was 9.47 L/kg, which was corresponded tol4.14 fold of the total body water (0.67 L/kg) in the rats. Following oral administration of the compound at a dose of 10 mg/kg, the Cmax and Tmax for the compound
were 0.24 nmol/mL and 0.26 hr, respectively; the AUCo-∞ and half-life (Ty2) were 98.33 μg/L*hr and 1.61 hr, respectively. The bioavailability of the compound was 2.94%. No abnormalities such as convulsion, salivation, vomit, watery feces, etc. were observed during the course of study.
[001276] Following a similar procedure, a second exemplary compound described herein was tested. Following an IV bolus injection of the compound at 2 mg / kg, the systemic clearance was 5.14 L/hr/kg. The terminal elimination half-life (Ty2Z) was 4.06 hr, while Vz was 30.53 L/kg. The Maximum plasma concentration Cmax (at 1 minutes after dosing) was 3.40 nmol/mL, AUC(O-O0) was 390.35 μg/L*hr. Following oral administration of the compound at a dose of 10 mg/kg, the Cmax and Tmax for the compound were 0.29 nmol/mL and 0.42 hr, respectively; the AUCo-∞ and half-life (Ty2) were 282.66 μg/L*hr and 4.78 hr, respectively. Figure 12 summarizes the results for the evaluated compounds.
[001277] The bioavailability of the tested compounds was calculated from the dose-corrected area under curve (AUC) non-intravenous divded by AUC intravenous. The formula for calculating F for a drug administered by the oral route (po) is given below.
F = rAUCy*doseτv [AUC]iv * dosepo
6.2.5.6. Dog Pharmacokinetic Study
[001278] The objective of this study was to evaluate the oral uptake and pharmacokinetic profiles of the compound in male Beagle dogs. After the compound was administered orally (gavage) at 10 mg/ kg into 3 dogs, the blood samples were collected at 0, 0.017, 0.083, 0.25, 0.5, 1, 2, 4, 6, 8, and 24 hours post-dosing and were stored at -2O0C. After 7-days of wash-out period of time, these animals were intravenously administered (IV) of the compound at 1 mg / kg, the blood samples were collected at the same time points listed above. The concentrations of the compound in plasma were determined using a high performance liquid chromatography /mass spectrometry (HPLC/MS/MS) method. The PK parameters were generated using a non- compartment model. Mean plasma concentration data for the compound in dogs following intravenous and oral administration are tabulated below.
[001279] Following an IV bolus injection of the compound at 1 mg / kg, the systemic clearance was 3.12 L/hr/kg, which corresponded to 173.93% of dog hepatic blood flow (1.85 L/hr/kg). The value of terminal elimination half-life (Tm) was 0.7198 hr; the Cmax (at 1 minutes after dosing) was 2.93 nmol/mL, while the value of AUQo-∞) was 324.91 μg/L*hr. The volume of distribution at terminal phase was 3.23 L/kg, which was corresponded to 5.39 fold of the total body water (0.60 L/kg) in the dogs. Following oral administration of the compound at a dose of 10 mg/kg, Cmax and Tmax for the compound were 2.34 nmol/mL and 0.42 hr, respectively; AUC(o-∞) and half-life (Ty2) were 1107.17 μg/L*hr and 1.48 hr, respectively. The bioavailability of the compound was 33.78 %. No abnormalities were observed during the course of study. 6.2.5.7. The compound increases contractility in vivo
[001280] An investigation as to whether the increase in myocyte contractility observed in vitro translates into increased global cardiac function, in vivo, was performed. Using invasive hemodynamic measurements and pressure-volume loop recordings, various indices of load independent cardiac function were compared at baseline, and during infusion with 1) control vehicle, 2) the compound, and 3) isoproterenol.
[001281] An adult, male C57 Black mouse was cage-anesthetized and maintained throughout the experimental procedure with isofluorane. A closed-chest procedure was utilized (Lips et al,
Basic Res. Cardiol., 2004 99(5): 351-9) and a longitudinal incision of carotid artery was performed. The catheter/transducer was inserted into the carotid artery and gently advanced into the aorta where pressure recordings were obtained prior to further advancement and calibration of the device in the left ventricle.
[001282] A second incision was made and the jugular vein was cannulated to receive a delivery catheter for the intravenous administration of vehicle, the compound and isoprotenerol, a beta-2 receptor agonist with known cardio-hemodynamic effects.
[001283] The recording system was set up to obtain PV loop recordings (1,000 loops/second) to establish baseline LV function and measure trends in heart rate, stroke volume and
+DP/DTmax at 5 second intervals.
[001284] Following calibration of the device in the left ventricle and determination of consistent PV loops, a 50 μl bolus of so luto I/DMA (vehicle) was delivered IV into the jugular vein. The vena cava was exposed by incision and a Q-tip was used to depress the vena cava and obtain the ESPVR. This process was repeated three times for vehicle and three times for the compound (50 uL bolus at 4ug/g and by infusion lml/min for 1 min) and isoprotenerol (5OuL bolus at 0. lng/g). A 5 -minute washout period was provided between doses to allow the LV to return to baseline.
[001285] The average effects of vehicle, the compound, and isoprotenerol on cardiac function are shown in Figures 13-16.
[001286] The compound significantly increased cardiac function in vivo by enhancing both the cardiac output and ejection fraction compared to baseline and vehicle injection. Interestingly, the positive inotropic effects of the compound were comparable to beta adrenergic stimulation with isoproterenol.
[001287] All references cited are incorporated herein by reference in their entireties.
Claims
1. A compound of formula:
wherein A and B are each, independently, H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or -NO2; wherein at least one of A and B is not H;
E is H, F, Br, I, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, or azido;
R3 is H or alkyl of 1-3 carbons, Q is methyl, or R3 and Q are joined together to form a 5- 6 membered ring; and v, R2 and G are selected from (i) and (ii) as follows: (i) v is 0,
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons,
-CN, azido, or -NO2,
Or G is joined together with R2 to form a 5-6 membered ring;
R2 is H or alkyl of 1-3 carbons; and
G is H, halo, hydroxyl, alkoxy of 1-3 carbons, haloalkyl of 1-3 carbons, -CN, azido, or -NO2; or a pharmaceutically acceptable derivative or stereoisomer thereof.
2. The compound of claim 1, wherein A and B are each, independently, H, hydroxyl, or alkoxy of 1-3 carbons, or a pharmaceutically acceptable derivative or stereoisomer thereof.
3. The compound of claim 1 or 2, wherein A and B are each, independently, hydroxyl or methoxy, or a pharmaceutically acceptable derivative or stereoisomer thereof.
4. The compound of any of claims 1-3, wherein A and B are both hydroxyl, or a pharmaceutically acceptable derivative or stereoisomer thereof.
5. The compound of any of claim 1-3, wherein A and B are both methoxy, or a pharmaceutically acceptable derivative or stereoisomer thereof.
6. The compound of any of claims 1-5, wherein E is H, or a pharmaceutically acceptable derivative or stereoisomer thereof.
7. The compound of any of claims 1-6, wherein R2 is H, or a pharmaceutically acceptable derivative or stereoisomer thereof.
8. The compound of any of claims 1-7, wherein R is H, or a pharmaceutically acceptable derivative or stereoisomer thereof.
9. The compound of any of claims 1-8, wherein v is 0, or a pharmaceutically acceptable derivative or stereoisomer thereof.
10. The compound of any of claims 1-9, wherein G is H, or a pharmaceutically acceptable derivative or stereoisomer thereof.
11. A compound selected from:
12. The compound of any of claims 1-11, wherein the compound has the following formula:
13. The compound or pharmaceutically acceptable derivative or stereoisomer thereof of any of claims 1-12, wherein the compound or pharmaceutically acceptable derivative or stereoisomer thereof is a pharmaceutically acceptable salt.
14. The compound or pharmaceutically acceptable derivative or stereoisomer thereof of any of claims 1-13, wherein the compound or pharmaceutically acceptable derivative or stereoisomer thereof is a hydrochloride salt.
15. The compound of any of claims 1-14, where the compound has the following formula:
16. A pharmaceutical composition comprising the compound or pharmaceutically acceptable derivative or stereoisomer thereof of any of claims 1-15 and a pharmaceutically acceptable carrier.
17. A method for treating or preventing heart failure, or ameliorating one ore more symptoms thereof, comprising administering the compound or pharmaceutically acceptable derivative or stereoisomer thereof of any of claims 1-15 or the pharmaceutical composition of claim 16.
18. The method of claim 17, wherein the heart failure is acute heart failure, chronic heart failure, end-stage heart failure, congestive heart failure, right heart failure, left heart failure, forward heart failure, backward heart failure, class I heart failure, class II heart failure III, class IV heart failure, systolic heart failure, diastolic heart failure, low-output heart failure, or high- output heart failure.
19. A method for treating or preventing, stenosis, restenosis, a disease associated with vascular smooth muscle cell proliferation, a disease associated with neointima formation, a disease associated with calcineurin PP2B, a disease associated with NFAT, a disease associated with SERCA, arteriovenous fistula failure, a cardiac disease, a disease associated with a cardiac disease, urinary incontinence, cancer, asthma, diabetes, Alzheimer's disease, or pulmonary hypertension; or ameliorating one ore more symptoms thereof, comprising administering the compound or pharmaceutically acceptable derivative or stereoisomer thereof of any of claims 1- 15 or the pharmaceutical composition of claim 16.
Applications Claiming Priority (6)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US14889309P | 2009-01-30 | 2009-01-30 | |
| US61/148,893 | 2009-01-30 | ||
| US22589709P | 2009-07-15 | 2009-07-15 | |
| US61/225,897 | 2009-07-15 | ||
| US25958709P | 2009-11-09 | 2009-11-09 | |
| US61/259,587 | 2009-11-09 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2010088450A2 true WO2010088450A2 (en) | 2010-08-05 |
| WO2010088450A3 WO2010088450A3 (en) | 2010-12-23 |
Family
ID=42244474
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2010/022486 WO2010088450A2 (en) | 2009-01-30 | 2010-01-29 | Methods for treating diseases associated with the modulation of serca |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2010088450A2 (en) |
Cited By (12)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2014524482A (en) * | 2011-08-25 | 2014-09-22 | コグニション セラピューティクス,インコーポレイテッド | Compositions and methods for treating neurodegenerative diseases |
| WO2014173868A1 (en) | 2013-04-22 | 2014-10-30 | Universitaet Des Saarlandes | Serca inhibitor and calmodulin antagonist combination |
| JP2014529613A (en) * | 2011-08-25 | 2014-11-13 | コグニションセラピューティクス,インコーポレイテッド | Compositions and methods for treating neurodegenerative diseases |
| CN105237406A (en) * | 2015-10-14 | 2016-01-13 | 湖南华腾制药有限公司 | Synthesis method of (R)-(-)-1-methyl-3-amphetamine |
| WO2016032569A1 (en) * | 2014-08-29 | 2016-03-03 | Celladon Corporation | Quinolines and their use for treating endoplasmic reticulum stress-caused diseases |
| US9796672B2 (en) | 2014-01-31 | 2017-10-24 | Cognition Therapeutics, Inc. | Isoindoline compositions and methods for treating neurodegenerative disease |
| US9815770B2 (en) | 2009-07-31 | 2017-11-14 | Cognition Therapeutics, Inc. | Inhibitors of cognitive decline |
| WO2021046034A1 (en) * | 2019-09-05 | 2021-03-11 | University Of Houston System | Small molecule liver x receptor modulators and uses thereof |
| US11013717B1 (en) | 2018-01-31 | 2021-05-25 | Flagship Pioneering Innovations V, Inc. | Methods and compositions for treating cancer using SERCA pump inhibitors |
| US11214540B2 (en) | 2017-05-15 | 2022-01-04 | Cognition Therapeutics, Inc. | Compositions for treating neurodegenerative diseases |
| US11730729B2 (en) | 2020-07-20 | 2023-08-22 | Neurodon Corporation | Quinolines that modulate SERCA and their use for treating disease |
| US11827626B2 (en) | 2014-08-29 | 2023-11-28 | Neurodon Corporation | Quinolines that modulate SERCA and their use for treating disease |
Citations (59)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3536809A (en) | 1969-02-17 | 1970-10-27 | Alza Corp | Medication method |
| US3598123A (en) | 1969-04-01 | 1971-08-10 | Alza Corp | Bandage for administering drugs |
| US3845770A (en) | 1972-06-05 | 1974-11-05 | Alza Corp | Osmatic dispensing device for releasing beneficial agent |
| US3916899A (en) | 1973-04-25 | 1975-11-04 | Alza Corp | Osmotic dispensing device with maximum and minimum sizes for the passageway |
| US4008719A (en) | 1976-02-02 | 1977-02-22 | Alza Corporation | Osmotic system having laminar arrangement for programming delivery of active agent |
| US4328245A (en) | 1981-02-13 | 1982-05-04 | Syntex (U.S.A.) Inc. | Carbonate diester solutions of PGE-type compounds |
| US4409239A (en) | 1982-01-21 | 1983-10-11 | Syntex (U.S.A.) Inc. | Propylene glycol diester solutions of PGE-type compounds |
| US4410545A (en) | 1981-02-13 | 1983-10-18 | Syntex (U.S.A.) Inc. | Carbonate diester solutions of PGE-type compounds |
| US5033252A (en) | 1987-12-23 | 1991-07-23 | Entravision, Inc. | Method of packaging and sterilizing a pharmaceutical product |
| US5052558A (en) | 1987-12-23 | 1991-10-01 | Entravision, Inc. | Packaged pharmaceutical product |
| US5059595A (en) | 1989-03-22 | 1991-10-22 | Bioresearch, S.P.A. | Pharmaceutical compositions containing 5-methyltetrahydrofolic acid, 5-formyltetrahydrofolic acid and their pharmaceutically acceptable salts in controlled-release form active in the therapy of organic mental disturbances |
| US5073543A (en) | 1988-07-21 | 1991-12-17 | G. D. Searle & Co. | Controlled release formulations of trophic factors in ganglioside-lipsome vehicle |
| US5120548A (en) | 1989-11-07 | 1992-06-09 | Merck & Co., Inc. | Swelling modulated polymeric drug delivery device |
| US5323907A (en) | 1992-06-23 | 1994-06-28 | Multi-Comp, Inc. | Child resistant package assembly for dispensing pharmaceutical medications |
| US5354556A (en) | 1984-10-30 | 1994-10-11 | Elan Corporation, Plc | Controlled release powder and process for its preparation |
| US5591767A (en) | 1993-01-25 | 1997-01-07 | Pharmetrix Corporation | Liquid reservoir transdermal patch for the administration of ketorolac |
| US5612059A (en) | 1988-08-30 | 1997-03-18 | Pfizer Inc. | Use of asymmetric membranes in delivery devices |
| US5639476A (en) | 1992-01-27 | 1997-06-17 | Euro-Celtique, S.A. | Controlled release formulations coated with aqueous dispersions of acrylic polymers |
| US5639480A (en) | 1989-07-07 | 1997-06-17 | Sandoz Ltd. | Sustained release formulations of water soluble peptides |
| US5674533A (en) | 1994-07-07 | 1997-10-07 | Recordati, S.A., Chemical And Pharmaceutical Company | Pharmaceutical composition for the controlled release of moguisteine in a liquid suspension |
| US5709874A (en) | 1993-04-14 | 1998-01-20 | Emory University | Device for local drug delivery and methods for using the same |
| US5733566A (en) | 1990-05-15 | 1998-03-31 | Alkermes Controlled Therapeutics Inc. Ii | Controlled release of antiparasitic agents in animals |
| US5739108A (en) | 1984-10-04 | 1998-04-14 | Monsanto Company | Prolonged release of biologically active polypeptides |
| US5759542A (en) | 1994-08-05 | 1998-06-02 | New England Deaconess Hospital Corporation | Compositions and methods for the delivery of drugs by platelets for the treatment of cardiovascular and other diseases |
| US5798119A (en) | 1995-06-13 | 1998-08-25 | S. C. Johnson & Son, Inc. | Osmotic-delivery devices having vapor-permeable coatings |
| US5840674A (en) | 1990-11-01 | 1998-11-24 | Oregon Health Sciences University | Covalent microparticle-drug conjugates for biological targeting |
| US5891474A (en) | 1997-01-29 | 1999-04-06 | Poli Industria Chimica, S.P.A. | Time-specific controlled release dosage formulations and method of preparing same |
| US5900252A (en) | 1990-04-17 | 1999-05-04 | Eurand International S.P.A. | Method for targeted and controlled release of drugs in the intestinal tract and more particularly in the colon |
| US5922356A (en) | 1996-10-09 | 1999-07-13 | Sumitomo Pharmaceuticals Company, Limited | Sustained release formulation |
| US5972366A (en) | 1994-11-28 | 1999-10-26 | The Unites States Of America As Represented By The Secretary Of The Army | Drug releasing surgical implant or dressing material |
| US5972891A (en) | 1992-12-07 | 1999-10-26 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| US5980945A (en) | 1996-01-16 | 1999-11-09 | Societe De Conseils De Recherches Et D'applications Scientifique S.A. | Sustained release drug formulations |
| US5985307A (en) | 1993-04-14 | 1999-11-16 | Emory University | Device and method for non-occlusive localized drug delivery |
| US5993855A (en) | 1995-09-18 | 1999-11-30 | Shiseido Company, Ltd. | Delayed drug-releasing microspheres |
| US6004534A (en) | 1993-07-23 | 1999-12-21 | Massachusetts Institute Of Technology | Targeted polymerized liposomes for improved drug delivery |
| US6039975A (en) | 1995-10-17 | 2000-03-21 | Hoffman-La Roche Inc. | Colon targeted delivery system |
| US6045830A (en) | 1995-09-04 | 2000-04-04 | Takeda Chemical Industries, Ltd. | Method of production of sustained-release preparation |
| US6048736A (en) | 1998-04-29 | 2000-04-11 | Kosak; Kenneth M. | Cyclodextrin polymers for carrying and releasing drugs |
| US6060082A (en) | 1997-04-18 | 2000-05-09 | Massachusetts Institute Of Technology | Polymerized liposomes targeted to M cells and useful for oral or mucosal drug delivery |
| US6071495A (en) | 1989-12-22 | 2000-06-06 | Imarx Pharmaceutical Corp. | Targeted gas and gaseous precursor-filled liposomes |
| US6087324A (en) | 1993-06-24 | 2000-07-11 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| US6113943A (en) | 1996-10-31 | 2000-09-05 | Takeda Chemical Industries, Ltd. | Sustained-release preparation capable of releasing a physiologically active substance |
| US6120751A (en) | 1997-03-21 | 2000-09-19 | Imarx Pharmaceutical Corp. | Charged lipids and uses for the same |
| US6131570A (en) | 1998-06-30 | 2000-10-17 | Aradigm Corporation | Temperature controlling device for aerosol drug delivery |
| US6139865A (en) | 1996-10-01 | 2000-10-31 | Eurand America, Inc. | Taste-masked microcapsule compositions and methods of manufacture |
| US6197350B1 (en) | 1996-12-20 | 2001-03-06 | Takeda Chemical Industries, Ltd. | Method of producing a sustained-release preparation |
| US6248363B1 (en) | 1999-11-23 | 2001-06-19 | Lipocine, Inc. | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| US6253872B1 (en) | 1996-05-29 | 2001-07-03 | Gmundner Fertigteile Gesellschaft M.B.H & Co., Kg | Track soundproofing arrangement |
| US6264970B1 (en) | 1996-06-26 | 2001-07-24 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| US6267981B1 (en) | 1995-06-27 | 2001-07-31 | Takeda Chemical Industries, Ltd. | Method of producing sustained-release preparation |
| US6271359B1 (en) | 1999-04-14 | 2001-08-07 | Musc Foundation For Research Development | Tissue-specific and pathogen-specific toxic agents and ribozymes |
| US6274552B1 (en) | 1993-03-18 | 2001-08-14 | Cytimmune Sciences, Inc. | Composition and method for delivery of biologically-active factors |
| US6316652B1 (en) | 1995-06-06 | 2001-11-13 | Kosta Steliou | Drug mitochondrial targeting agents |
| US6350458B1 (en) | 1998-02-10 | 2002-02-26 | Generex Pharmaceuticals Incorporated | Mixed micellar drug deliver system and method of preparation |
| WO2002017918A2 (en) | 2000-08-30 | 2002-03-07 | Pfizer Products Inc. | Sustained release formulations for growth hormone secretagogues |
| US6419961B1 (en) | 1996-08-29 | 2002-07-16 | Takeda Chemical Industries, Ltd. | Sustained release microcapsules of a bioactive substance and a biodegradable polymer |
| US6589548B1 (en) | 1998-05-16 | 2003-07-08 | Mogam Biotechnology Research Institute | Controlled drug delivery system using the conjugation of drug to biodegradable polyester |
| US6613358B2 (en) | 1998-03-18 | 2003-09-02 | Theodore W. Randolph | Sustained-release composition including amorphous polymer |
| WO2004108083A2 (en) | 2003-05-30 | 2004-12-16 | Cyclegen, Inc. | Methods for the selective treatment of tumors by calcium-mediated induction of apoptosis |
-
2010
- 2010-01-29 WO PCT/US2010/022486 patent/WO2010088450A2/en active Application Filing
Patent Citations (62)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3536809A (en) | 1969-02-17 | 1970-10-27 | Alza Corp | Medication method |
| US3598123A (en) | 1969-04-01 | 1971-08-10 | Alza Corp | Bandage for administering drugs |
| US3845770A (en) | 1972-06-05 | 1974-11-05 | Alza Corp | Osmatic dispensing device for releasing beneficial agent |
| US3916899A (en) | 1973-04-25 | 1975-11-04 | Alza Corp | Osmotic dispensing device with maximum and minimum sizes for the passageway |
| US4008719A (en) | 1976-02-02 | 1977-02-22 | Alza Corporation | Osmotic system having laminar arrangement for programming delivery of active agent |
| US4410545A (en) | 1981-02-13 | 1983-10-18 | Syntex (U.S.A.) Inc. | Carbonate diester solutions of PGE-type compounds |
| US4328245A (en) | 1981-02-13 | 1982-05-04 | Syntex (U.S.A.) Inc. | Carbonate diester solutions of PGE-type compounds |
| US4409239A (en) | 1982-01-21 | 1983-10-11 | Syntex (U.S.A.) Inc. | Propylene glycol diester solutions of PGE-type compounds |
| US5739108A (en) | 1984-10-04 | 1998-04-14 | Monsanto Company | Prolonged release of biologically active polypeptides |
| US5354556A (en) | 1984-10-30 | 1994-10-11 | Elan Corporation, Plc | Controlled release powder and process for its preparation |
| US5033252A (en) | 1987-12-23 | 1991-07-23 | Entravision, Inc. | Method of packaging and sterilizing a pharmaceutical product |
| US5052558A (en) | 1987-12-23 | 1991-10-01 | Entravision, Inc. | Packaged pharmaceutical product |
| US5073543A (en) | 1988-07-21 | 1991-12-17 | G. D. Searle & Co. | Controlled release formulations of trophic factors in ganglioside-lipsome vehicle |
| US5612059A (en) | 1988-08-30 | 1997-03-18 | Pfizer Inc. | Use of asymmetric membranes in delivery devices |
| US5698220A (en) | 1988-08-30 | 1997-12-16 | Pfizer Inc. | Asymmetric membranes in delivery devices |
| US5059595A (en) | 1989-03-22 | 1991-10-22 | Bioresearch, S.P.A. | Pharmaceutical compositions containing 5-methyltetrahydrofolic acid, 5-formyltetrahydrofolic acid and their pharmaceutically acceptable salts in controlled-release form active in the therapy of organic mental disturbances |
| US5639480A (en) | 1989-07-07 | 1997-06-17 | Sandoz Ltd. | Sustained release formulations of water soluble peptides |
| US5120548A (en) | 1989-11-07 | 1992-06-09 | Merck & Co., Inc. | Swelling modulated polymeric drug delivery device |
| US6071495A (en) | 1989-12-22 | 2000-06-06 | Imarx Pharmaceutical Corp. | Targeted gas and gaseous precursor-filled liposomes |
| US5900252A (en) | 1990-04-17 | 1999-05-04 | Eurand International S.P.A. | Method for targeted and controlled release of drugs in the intestinal tract and more particularly in the colon |
| US5733566A (en) | 1990-05-15 | 1998-03-31 | Alkermes Controlled Therapeutics Inc. Ii | Controlled release of antiparasitic agents in animals |
| US5840674A (en) | 1990-11-01 | 1998-11-24 | Oregon Health Sciences University | Covalent microparticle-drug conjugates for biological targeting |
| US5639476A (en) | 1992-01-27 | 1997-06-17 | Euro-Celtique, S.A. | Controlled release formulations coated with aqueous dispersions of acrylic polymers |
| US5323907A (en) | 1992-06-23 | 1994-06-28 | Multi-Comp, Inc. | Child resistant package assembly for dispensing pharmaceutical medications |
| US5972891A (en) | 1992-12-07 | 1999-10-26 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| US5591767A (en) | 1993-01-25 | 1997-01-07 | Pharmetrix Corporation | Liquid reservoir transdermal patch for the administration of ketorolac |
| US6274552B1 (en) | 1993-03-18 | 2001-08-14 | Cytimmune Sciences, Inc. | Composition and method for delivery of biologically-active factors |
| US5709874A (en) | 1993-04-14 | 1998-01-20 | Emory University | Device for local drug delivery and methods for using the same |
| US5985307A (en) | 1993-04-14 | 1999-11-16 | Emory University | Device and method for non-occlusive localized drug delivery |
| US6376461B1 (en) | 1993-06-24 | 2002-04-23 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| US6087324A (en) | 1993-06-24 | 2000-07-11 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| US6004534A (en) | 1993-07-23 | 1999-12-21 | Massachusetts Institute Of Technology | Targeted polymerized liposomes for improved drug delivery |
| US5674533A (en) | 1994-07-07 | 1997-10-07 | Recordati, S.A., Chemical And Pharmaceutical Company | Pharmaceutical composition for the controlled release of moguisteine in a liquid suspension |
| US5759542A (en) | 1994-08-05 | 1998-06-02 | New England Deaconess Hospital Corporation | Compositions and methods for the delivery of drugs by platelets for the treatment of cardiovascular and other diseases |
| US5972366A (en) | 1994-11-28 | 1999-10-26 | The Unites States Of America As Represented By The Secretary Of The Army | Drug releasing surgical implant or dressing material |
| US6316652B1 (en) | 1995-06-06 | 2001-11-13 | Kosta Steliou | Drug mitochondrial targeting agents |
| US5798119A (en) | 1995-06-13 | 1998-08-25 | S. C. Johnson & Son, Inc. | Osmotic-delivery devices having vapor-permeable coatings |
| US6267981B1 (en) | 1995-06-27 | 2001-07-31 | Takeda Chemical Industries, Ltd. | Method of producing sustained-release preparation |
| US6045830A (en) | 1995-09-04 | 2000-04-04 | Takeda Chemical Industries, Ltd. | Method of production of sustained-release preparation |
| US5993855A (en) | 1995-09-18 | 1999-11-30 | Shiseido Company, Ltd. | Delayed drug-releasing microspheres |
| US6039975A (en) | 1995-10-17 | 2000-03-21 | Hoffman-La Roche Inc. | Colon targeted delivery system |
| US5980945A (en) | 1996-01-16 | 1999-11-09 | Societe De Conseils De Recherches Et D'applications Scientifique S.A. | Sustained release drug formulations |
| US6253872B1 (en) | 1996-05-29 | 2001-07-03 | Gmundner Fertigteile Gesellschaft M.B.H & Co., Kg | Track soundproofing arrangement |
| US6264970B1 (en) | 1996-06-26 | 2001-07-24 | Takeda Chemical Industries, Ltd. | Sustained-release preparation |
| US6419961B1 (en) | 1996-08-29 | 2002-07-16 | Takeda Chemical Industries, Ltd. | Sustained release microcapsules of a bioactive substance and a biodegradable polymer |
| US6139865A (en) | 1996-10-01 | 2000-10-31 | Eurand America, Inc. | Taste-masked microcapsule compositions and methods of manufacture |
| US5922356A (en) | 1996-10-09 | 1999-07-13 | Sumitomo Pharmaceuticals Company, Limited | Sustained release formulation |
| US6699500B2 (en) | 1996-10-31 | 2004-03-02 | Takeda Chemical Industries, Ltd. | Sustained-release preparation capable of releasing a physiologically active substance |
| US6113943A (en) | 1996-10-31 | 2000-09-05 | Takeda Chemical Industries, Ltd. | Sustained-release preparation capable of releasing a physiologically active substance |
| US6197350B1 (en) | 1996-12-20 | 2001-03-06 | Takeda Chemical Industries, Ltd. | Method of producing a sustained-release preparation |
| US5891474A (en) | 1997-01-29 | 1999-04-06 | Poli Industria Chimica, S.P.A. | Time-specific controlled release dosage formulations and method of preparing same |
| US6120751A (en) | 1997-03-21 | 2000-09-19 | Imarx Pharmaceutical Corp. | Charged lipids and uses for the same |
| US6060082A (en) | 1997-04-18 | 2000-05-09 | Massachusetts Institute Of Technology | Polymerized liposomes targeted to M cells and useful for oral or mucosal drug delivery |
| US6350458B1 (en) | 1998-02-10 | 2002-02-26 | Generex Pharmaceuticals Incorporated | Mixed micellar drug deliver system and method of preparation |
| US6613358B2 (en) | 1998-03-18 | 2003-09-02 | Theodore W. Randolph | Sustained-release composition including amorphous polymer |
| US6048736A (en) | 1998-04-29 | 2000-04-11 | Kosak; Kenneth M. | Cyclodextrin polymers for carrying and releasing drugs |
| US6589548B1 (en) | 1998-05-16 | 2003-07-08 | Mogam Biotechnology Research Institute | Controlled drug delivery system using the conjugation of drug to biodegradable polyester |
| US6131570A (en) | 1998-06-30 | 2000-10-17 | Aradigm Corporation | Temperature controlling device for aerosol drug delivery |
| US6271359B1 (en) | 1999-04-14 | 2001-08-07 | Musc Foundation For Research Development | Tissue-specific and pathogen-specific toxic agents and ribozymes |
| US6248363B1 (en) | 1999-11-23 | 2001-06-19 | Lipocine, Inc. | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| WO2002017918A2 (en) | 2000-08-30 | 2002-03-07 | Pfizer Products Inc. | Sustained release formulations for growth hormone secretagogues |
| WO2004108083A2 (en) | 2003-05-30 | 2004-12-16 | Cyclegen, Inc. | Methods for the selective treatment of tumors by calcium-mediated induction of apoptosis |
Non-Patent Citations (35)
| Title |
|---|
| "Multiparticulate Oral Drug Delivery", 1994, MARCEL DEKKER |
| "Pharmaceutical Pelletization Technology", 1989, MARCEL DEKKER |
| AGNIHOTRI ET AL.: "Recent Advances on Chitosan-Based Micro- and Nanoparticles in Drug Delivery", J. CONTROLLED RELEASE, vol. 100, no. 1, 2004, pages 5 - 28, XP004604081, DOI: doi:10.1016/j.jconrel.2004.08.010 |
| BALA ET AL.: "PLGA Nanoparticles in Drug Delivery: the State of the Art", CRIT. REV. THER. DRUG CARRIER SYST., vol. 21, no. 5, 2004, pages 387 - 422, XP009167778, DOI: doi:10.1615/CritRevTherDrugCarrierSyst.v21.i5.20 |
| BALOG ET AL., AM. J PHYSIOL. HEART CIRC. PHYSIOL., vol. 290, 2005, pages H794 - 9 |
| BIOORG. MED. CHEM., vol. 14, 2006, pages 2810 - 2815 |
| BIRMACHU ET AL., BIOCHEMISTRY, vol. 28, 1989, pages 3940 - 3947 |
| CIRCULATION RESEARCH., vol. 97, no. 5, 2005, pages 488 - 495 |
| DEEPAK THASSU, MICHEL DELEERS, & YASHWANT PATHAK: "Nanoparticulate Drug Delivery Systems", 2007, INFORMA HEALTHCARE |
| FABIATO; FABIATO, J. PHYSIOL (PARIS), vol. 75, 1979, pages 463 - 505 |
| GORNALL ET AL., J BIOL. CHEM., vol. 177, 1949, pages 751 - 766 |
| H. BUNDGAARD: "Design of Prodrugs", 1985, ELSELVIER |
| HANS ET AL.: "Biodegradable Nanoparticles for Drug Delivery and Targeting", CUR. OPIN. SOLID STATE & MAT. SCI., vol. 6, no. 4, 2002, pages 319 - 27 |
| LABHASETWAR ET AL.: "Nanoparticle Drug Delivery System for Restenosis", ADV. DRUG DELIV. REV., vol. 24, no. 1, 1997, pages 63 - 85, XP055179211 |
| LIPS ET AL., BASIC RES. CARDIOL., vol. 99, no. 5, 2004, pages 351 - 9 |
| LOCKMAN ET AL.: "Nanoparticle Technology for Drug Delivery Across the Blood-Brain Barrier", DRUG DEV. IND. PHARM., vol. 28, no. 1, 2002, pages 1 - 13, XP008026571, DOI: doi:10.1081/DDC-120001481 |
| MAHN ET AL., PNAS, vol. 106, no. 26, 2009, pages 10775 - 10780 |
| MANFRED E. WOLFF: "Burger's Medicinal Chemistry and Drug Discovery, 5th ed.", 1995, pages: 172-78 - 949-82 |
| MORIA ET AL., ARCH BRONCONEUMOL, vol. 43, no. 1, 2007, pages 4 - 8 |
| MULLER ET AL.: "Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery A Review of the State of the Art", EUR. J. PHARM. BIOPHARM., vol. 50, no. 1, 2000, pages 161 - 77, XP004257186, DOI: doi:10.1016/S0939-6411(00)00087-4 |
| NICOTERA ET AL., CELL CALCIUM, vol. 23, 1998, pages 173 - 180 |
| PANYAM ET AL.: "Biodegradable Nanoparticlesfor Drug and Gene Delivery to Cells and Tissue", ADV. DRUG DELIV. REV., vol. 55, no. 3, 2003, pages 329 - 47, XP008096954, DOI: doi:10.1016/S0169-409X(02)00228-4 |
| RAM B. GUPTA & UDAY B. KOMPELLA: "Nanoparticle Technology for Drug Delivery", 2006, INFORMA HEALTHCARE |
| RATHBONE ET AL.,: "Drugs and the Pharmaceutical Science", vol. 126, 2002, MARCEL DEKKER, INC., article "Modified-Release Drug Deliver Technology" |
| ROY-CHAUDHURY ET AL., AMERICAN JOURNAL OF KIDNEY DISEASES, vol. 50, no. 5, 2007, pages 782 - 790 |
| SANTUS; BAKER, J CONTROLLED RELEASE, vol. 35, 1995, pages 1 - 21 |
| SCH6NTHAL, CANCER LETT., 2008 |
| SOPPIMATH ET AL.: "Biodegradable Polymeric Nanoparticles as Drug Delivery Devices", J. CONTROLLED RELEASE, vol. 70, no. 1-2, 2001, pages 1 - 20, XP002520453, DOI: doi:10.1016/S0168-3659(00)00339-4 |
| TAKADA ET AL.: "Encyclopedia of Controlled Drug Delivery", vol. 2, 1999, WILEY |
| TAKEUCHI ET AL.: "Mucoadhesive Nanoparticulate Systems for Peptide Drug Delivery", ADV. DRUG DELIV. REV., vol. 47, no. 1, 2001, pages 39 - 54, XP008117909, DOI: doi:10.1016/S0169-409X(00)00120-4 |
| VAUTHIER ET AL.: "Drug Delivery to Resistant Tumors: the Potential of Poly(Alkyl Cyanoacrylate) Nanoparticles", J. CONTROLLED RELEASE, vol. 93, no. 2, 2003, pages 151 - 60, XP004473635, DOI: doi:10.1016/j.jconrel.2003.08.005 |
| VERMA ET AL., DRUG DEVELOPMENT AND INDUSTRIAL PHARMACY, vol. 26, 2000, pages 695 - 708 |
| VERMA ET AL., J CONTROLLED RELEASE, vol. 79, 2002, pages 7 - 27 |
| VLADIMIR P. TORCHILIN: "Nanoparticulates As Drug Carrier", 2006, WORLD SCIENTIFIC PUBLISHING CO. |
| YU ET AL., PROGRESS IN NEUROBIOLOGY, vol. 89, 2009, pages 240 - 255 |
Cited By (18)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9815770B2 (en) | 2009-07-31 | 2017-11-14 | Cognition Therapeutics, Inc. | Inhibitors of cognitive decline |
| JP2014529613A (en) * | 2011-08-25 | 2014-11-13 | コグニションセラピューティクス,インコーポレイテッド | Compositions and methods for treating neurodegenerative diseases |
| JP2014524482A (en) * | 2011-08-25 | 2014-09-22 | コグニション セラピューティクス,インコーポレイテッド | Compositions and methods for treating neurodegenerative diseases |
| JP2017206545A (en) * | 2011-08-25 | 2017-11-24 | コグニション セラピューティクス,インコーポレイテッド | Compositions and methods for treating neurodegenerative disease |
| WO2014173868A1 (en) | 2013-04-22 | 2014-10-30 | Universitaet Des Saarlandes | Serca inhibitor and calmodulin antagonist combination |
| US10611728B2 (en) | 2014-01-31 | 2020-04-07 | Cognition Therapeutics, Inc. | Isoindoline compositions and methods for treating neurodegenerative disease |
| US11691947B2 (en) | 2014-01-31 | 2023-07-04 | Cognition Therapeutics, Inc. | Isoindoline compositions and methods for treating neurodegenerative disease |
| US9796672B2 (en) | 2014-01-31 | 2017-10-24 | Cognition Therapeutics, Inc. | Isoindoline compositions and methods for treating neurodegenerative disease |
| US10207991B2 (en) | 2014-01-31 | 2019-02-19 | Cognition Therapeutics, Inc. | Isoindoline compositions and methods for treating neurodegenerative disease |
| WO2016032569A1 (en) * | 2014-08-29 | 2016-03-03 | Celladon Corporation | Quinolines and their use for treating endoplasmic reticulum stress-caused diseases |
| US11827626B2 (en) | 2014-08-29 | 2023-11-28 | Neurodon Corporation | Quinolines that modulate SERCA and their use for treating disease |
| CN105237406A (en) * | 2015-10-14 | 2016-01-13 | 湖南华腾制药有限公司 | Synthesis method of (R)-(-)-1-methyl-3-amphetamine |
| US11214540B2 (en) | 2017-05-15 | 2022-01-04 | Cognition Therapeutics, Inc. | Compositions for treating neurodegenerative diseases |
| US11013717B1 (en) | 2018-01-31 | 2021-05-25 | Flagship Pioneering Innovations V, Inc. | Methods and compositions for treating cancer using SERCA pump inhibitors |
| WO2021046034A1 (en) * | 2019-09-05 | 2021-03-11 | University Of Houston System | Small molecule liver x receptor modulators and uses thereof |
| CN114599638A (en) * | 2019-09-05 | 2022-06-07 | 休斯敦大学系统 | Small molecule liver X receptor modulators and uses thereof |
| US11730729B2 (en) | 2020-07-20 | 2023-08-22 | Neurodon Corporation | Quinolines that modulate SERCA and their use for treating disease |
| US11890279B2 (en) | 2020-07-20 | 2024-02-06 | Neurodon Corporation | Quinolines that modulate SERCA and their use for treating disease |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2010088450A3 (en) | 2010-12-23 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2010088450A2 (en) | Methods for treating diseases associated with the modulation of serca | |
| US10919865B2 (en) | Substituted diaminocarboxamide and diaminocarbonitrile pyrimidines, compositions thereof, and methods of treatment therewith | |
| EP1249450B1 (en) | Benzimidazole derivatives as human chymase inhibitors | |
| CN102341385B (en) | Piperazine compound capable of inhibiting prostaglandin d synthase | |
| WO2010057833A1 (en) | Compounds for treatment of duchenne muscular dystrophy | |
| JP2011516442A (en) | Compounds for treating muscular dystrophy | |
| CN110522731A (en) | Aromatic heterocycle compounds as anti-inflammatory compound | |
| JP4413135B2 (en) | Heteroaryl compounds that inhibit leukocyte adhesion mediated by α4 integrin | |
| TW200811142A (en) | Pyrimidinyl sulfonamide compounds which inhibit leukocyte adhesion mediated by VLA-4 | |
| JP2005089334A (en) | 8-hydroxyadenine compound | |
| CN101641327A (en) | 5-[3-(3-hydroxyphenoxy) azetidine-1-yl]-5-methyl-2, the hydrochloride of 2-phenylbenzene hexanamide | |
| BR112016010515B1 (en) | USE OF A GLUTARIMIDE DERIVATIVE COMPOUND IN THE TREATMENT OF EOSINOPHILIC DISEASES | |
| JP2021512922A (en) | Heteroaryl compounds, their pharmaceutical compositions, and their therapeutic use | |
| WO2016070697A1 (en) | Crystalline form of jak kinase inhibitor bisulfate and a preparation method thereof | |
| BRPI0912171B1 (en) | glucocorticoid receptor activating compound, and, composition | |
| CN109071531A (en) | For treating or preventing the hydrochloride Form and preparation method thereof of JAK related disease drug | |
| WO2022183963A1 (en) | 8-(picolinamide) substituted coumarin compound, and preparation method therefor and use thereof | |
| CN102112446A (en) | Phenanthrenone compounds, compositions and methods | |
| CN102688250A (en) | Synthesis and application of azo derivatives as inhibitor of RSK2 | |
| WO2023155757A1 (en) | Use of sarsasapogenin structure-based derivative and pharmaceutical composition thereof | |
| WO2024061172A1 (en) | Prolyl hydroxylase inhibitor and use thereof | |
| AU2015201030B2 (en) | Substituted diaminocarboxamide and diaminocarbonitrile pyrimidines, compositions thereof, and methods of treatment therewith | |
| CN117562915A (en) | Application of adenosine derivative in preparing medicament for preventing, relieving or treating fibrosis diseases | |
| CN103910718B (en) | Double cyclosubstituted pyrazolone azo compounds, preparation method and use | |
| CN117430562A (en) | 4-methyl-2-oxo-3, 6-dihydropyrimidine compound and preparation method and application thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 10703386 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 10703386 Country of ref document: EP Kind code of ref document: A2 |