WO2008152444A2 - Foamable waterless compositions with modulating agents - Google Patents
Foamable waterless compositions with modulating agents Download PDFInfo
- Publication number
- WO2008152444A2 WO2008152444A2 PCT/IB2007/004628 IB2007004628W WO2008152444A2 WO 2008152444 A2 WO2008152444 A2 WO 2008152444A2 IB 2007004628 W IB2007004628 W IB 2007004628W WO 2008152444 A2 WO2008152444 A2 WO 2008152444A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- acid
- agent
- composition
- agents
- group
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0012—Galenical forms characterised by the site of application
- A61K9/0014—Skin, i.e. galenical aspects of topical compositions
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/10—Dispersions; Emulsions
- A61K9/12—Aerosols; Foams
- A61K9/122—Foams; Dry foams
Definitions
- This invention relates to pharmaceutical and cosmetic compositions, particularly waterless compositions.
- External topical administration is an important route for the administration of drugs in disease treatment.
- Many groups of drugs including, for example, antibiotic, anti-fungal, anti-inflammatory, anesthetic, analgesic, antiallergic, corticosteroid, anti-psoriasis, retinoid, vitamins and antiproliferative medications are preferably administered in hydrophobic media, namely ointment.
- ointments often form an impermeable barrier, so that metabolic products and excreta from the wounds to which they are applied are not easily removed or drained away.
- Some active agents are known to be generally unstable or susceptible to isomerisation or to breakdown, resulting in loss of activity and the use of stabilizers, anti oxidants antimicrobials and buffers and the like in aqueous compositions to protect active or cosmetic agents is known.
- the problems of protecting active pharmaceutical and cosmetic agents in waterless environments, such as polar compositions are multifold and can vary according to the type of waterless environment and the nature of the agent being used. It has been surprisingly found that factors like small levels of acid residues in the raw materials can be significant in influencing agent stability. Similarly, the presence of low levels of metal ions can act to catalyze reactions or breakdown. Likewise, the presence of agents in a waterless environment that results in ionization or leads to oxidation can act to cause reactions or breakdown. There is therefore a need for simple and elegant solutions to stabilize active ingredients in a waterless or substantially environment.
- Foams and, in particular, foam emulsions are complicated systems which do not form under all circumstances. Changes in foam emulsion composition, such as by the addition of active ingredients may destabilize the foam. There is, therefore, a need for a foam composition, which provides desirable properties to the skin and can remain stable whilst accommodating a variety of active ingredients.
- Formulations based on oil or ointment, emollients have a number of useful attributes making them suitable candidates for topical pharmaceutical and cosmetic compositions including foamable compositions. They are inherently stable and inert which are clearly desirable characteristics. They are able to moisturize and soften the skin and in appropriate amounts can act as a protective or barrier layer and can form a barrier to water. By appropriate formulation they can act to improve drug delivery to the skin and yet remain resistant to being washed off. On the other hand they are by their nature greasy materials and can be difficult to formulate particlarly into a topical foamable composition that can deliver substantially uniform and stable composition or foam that ameliorates or overcomes the look and feel of a greasy material, especially where that composition is waterless or substantially so. It is further a problem to incorporate into such a vehicle pharmaceutically effective amounts of one or more active pharmaceutical ingredients such that they are uniformly present throughout the formulation and are effectively delivered without the use of an alcohol in the formulation.
- the present invention relates to a waterless composition as a vehicle in which an active pharmaceutical or cosmetic agent, when added is stable or stabilized.
- the present invention also relates to a foamable waterless composition as a vehicle in which an active pharmaceutical or cosmetic agent, when added is stable or stabilized by the presence of a modulating agent.
- the present invention more particularly relates to modulating agents that are able to be effective in relatively low concentrations and which have little or no significant effect on the compositions and on the foam compositions of the present invention.
- a waterless composition as a vehicle in which a modulating agent is incorporated and which is capable of stabilizing an active pharmaceutical or cosmetic agent, when added.
- a waterless vehicle an active agent that is susceptible, to one or more of reaction, breakdown, ionization or oxidation and a modulating agent.
- the waterless vehicle comprises a hydrophobic solvent. In another embodiment it comprises a hydrophilic solvent.
- the carrier includes: a waterless solvent, a stabilizing surfactant, and or a polymeric agent, and in which is incorporated a modulating agent.
- the carrier is a foamable carrier, and includes: a waterless solvent, a stabilizing surfactant, and or a polymeric agent, and a propellant and in which is incorporated a modulating agent.
- the carrier and modulating agent formulation is capable of stabilizing an active pharmaceutical or cosmetic agent in a substantially waterless environment.
- a waterless composition comprising one or more active agents and a carrier for topical delivery of the one or more active agents suitable for stabilizing at least one of said one or more active agents, said carrier comprising a waterless solvent and an effective amount of a modulating agent; wherein the at least one active agent is chemically unstable in the carrier in the absence of a modulating agent; wherein the modulating agent is capable of and is selected to modulate or adjust the artificial pH of the carrier to an artificial pH or to provide an artificial pH buffering effect such that the chemical stability of the at least one active agent is increased as compared to its stability in the carrier without the modulating agent.
- pharmaceutical or cosmetic composition is foamable and, includes: a waterless solvent, a stabilizing surfactant, and or a polymeric agent, a modulating agent, a propellant and one or more active pharmaceutical or cosmetic agents.
- the active agent itself can have characteristics of a modifying agent.
- the carrier includes: a. a waterless solvent comprising about 25% to about 98% of at least polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol or combinations thereof; b. a modulating agent; c. a stabilizing agent selected from the group consisting of a surface-active agent; and about 0.01 % to about 5% by weight of at least one polymeric agent or mixtures thereof; wherein the carrier is shakable and wherein if the composition is stored in an aerosol container and comprises a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition it will upon release expand to form a breakable foam.
- the active agent is susceptible to reaction, breakdown, ionization, or oxidization.
- the foamable pharmaceutical composition includes:
- a waterless solvent comprising about 25% to about 98% of at least polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol or combinations thereof;
- a stabilizing agent selected from the group consisting of a surface- active agent and about 0.01% to about 5% by weight of at least one polymeric agent;
- an effective amount of an active pharmaceutical or cosmetic agent wherein the carrier is shakable and wherein the composition is stored in an aerosol container and upon release expands to form a breakable foam.
- a foamabie waterless composition comprising: a. a therapeutically effective concentration of one or more active agents; b. a carrier for topical delivery of the one or more active agents suitable for stabilizing at least one of said one or more active agents, comprising i. about 25% to about 98% of at least polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol; ii. 0% to about 75% of a secondary polar solvent; iii. an effective amount of a modulating agent iv.
- a stabilizing agent selected from the group consisting of a surface-active agent; about 0,01 % to about 5% by weight of at least one polymeric agent and mixtures thereof.
- a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition wherein the at least one active agent is chemically unstable in the carrier in the absence of a modulating agent; wherein the modulating agent is capable of and is selected to modulate or adjust the artificial pH of the carrier to an artificial pH or to provide an artificial pH buffering effect such that the chemical stability of the at least one active agent is increased as compared to its stability in the carrier without the modulating agent.
- the carrier is shakable or flowable; and wherein the composition is stored in an aerosol container and upon release expands to form a breakable foam.
- the present invention further relates to said composition comprising one or more additional active agents.
- the present invention further relates to said composition comprising one or more additional or secondary polar solvents.
- the foamable cosmetic or pharmaceutical composition is non-flammable, wherein said gas propellant contains hydrofluorocarbon.
- a method of treating a disorder of mammalian subject comprising: administering a composition to a target site, the composition comprising: one or more active agents and a carrier for topical delivery of the one or more active agents suitable for stabilizing at least one of said one or more active agents, said carrier comprising a waterless solvent and an effective amount of a modulating agent; wherein the at least one active agent is chemically unstable in the carrier in the absence of a modulating agent; wherein the modulating agent is capable of and is selected to modulate or adjust the artificial pH of the carrier to an artificial pH or to provide an artificial pH buffering effect such that the chemical stability of the at least one active agent is increased as compared to its stability in the carrier without the modulating agent.
- a method of treating a disorder of mammalian subject comprising: administering a foamable composition to a target site, the composition comprising: a foamable waterless composition comprising: a. a therapeutically effective concentration of one or more active agents; b. a carrier for topical delivery of the one or more active agents suitable for stabilizing at least one of said one or more active agents, comprising i. about 25% to about 98% of at least polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol; ii. 0% to about 75% of a secondary polar solvent; iii. an effective amount of a modulating agent iv.
- a stabilizing agent selected from the group consisting of a surface-active agent; about 0.01 % to about 5% by weight of at least one polymeric agent and mixtures thereof.
- a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition wherein the at least one active agent is chemically unstable in the carrier in the absence of a modulating agent; wherein the modulating agent is capable of and is selected to modulate or adjust the artificial pH of the carrier to an artificial pH or to provide an artificial pH buffering effect such that the chemical stability of the at least one active agent is increased as compared to its stability in the carrier without the modulating agent.
- the carrier is shakable or flowable; and wherein the composition is stored in an aerosol container and upon release expands to form a breakable foam.
- foamable waterless composition comprising: a. a therapeutically effective concentration of one or more active agents; b. a carrier delivery of the one or more active agents suitable for stabilizing at least one of said one or more active agents, comprising i. about 25% to about 98% of at least polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol; ii. 0% to about 75% of a secondary polar solvent; iii. an effective amount of an modulating agent iv. a stabilizing agent selected from the group consisting of a surface-active agent; about 0.01 % to about 5% by weight of at least one polymeric agent and mixtures thereof.
- a therapeutically effective concentration of one or more active agents comprising i. about 25% to about 98% of at least polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol; ii. 0% to about 75% of a secondary polar solvent; iii. an effective amount
- a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition
- the at least one active agent is chemically unstable in the carrier in the absence of a modulating agent
- the modulating agent comprises an effective amount of one or more agents that are capable of impeding said destabilization and or are capable of stabilizing the active agent by chelating a metal ion such that the modulating agent is capable of reducing in the waterless carrier the availability of metal ions so the stability of at least one active agent is improved when compared to its stability in the carrier without the modulating agent
- the carrier is shakable or flowable
- the composition is stored in an aerosol container and upon release expands to form a breakable foam
- a carrier where the modulating agent acts by impeding ionization and or by impeding oxidation.
- a foamable waterless carrier for use with a therapeutically effective concentration of one or more active agents comprising:
- polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol;0% to about 75% of a secondary polar solvent;
- a stabilizing agent selected from the group consisting of a surface-active agent; about 0.01 % to about 5% by weight of at least one polymeric agent and mixtures thereof.
- a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition wherein at least one active agent if present is susceptible, to one or more of reaction, breakdown, ionization or oxidation; wherein the modulating agent comprises an effective amount of one or more agents that are capable of impeding said destabilization and or are capable of stabilizing the active agent primarily by modulating or adjusting the artificial pH of the carrier such that the modulating agent is capable of producing in the waterless carrier an artificial pH or an artificial pH buffering effect at about a pH or pH range in which the stability of at least one active agent is improved when compared to its stability in the carrier without the modulating agent and or by chelating a metal ion such that the modulating agent is capable of reducing in the waterless carrier the availability of metal ions so the group consisting of a
- the present invention further provides a method of treating, alleviating or preventing a disorder of mammalian subject, comprising administering a therapeutically effective amount of the above-mentioned compositions to an afflicted target site.
- the present invention further provides use of a therapeutically effective amount of the above-mentioned compositions as a medicament or in the manufacture of a medicament. [0037] The present invention further provides a therapeutically effective amount of the above-mentioned compositions for use as a medicament or in the manufacture of a medicament.
- a waterless composition for use as a vehicle in which an active pharmaceutical or cosmetic agent, when added is stable or stabilized.
- Active pharmaceutical and cosmetic agents are more generally referred to as a therapeutic agent.
- a waterless composition as a vehicle in which a modulating agent is incorporated and which is capable of stabilizing an active pharmaceutical or cosmetic agent, when added.
- the carrier and modulating agent formulation is capable of stabilizing an active pharmaceutical or cosmetic agent in a substantially waterless or mon aqueous environment.
- the carrier and modulating agent formulation is capable of stabilizing an active pharmaceutical or cosmetic agent in a substantially waterless environment, where the active agent has low or minimal susceptibility to water and can withstand up to about 10% water and more preferably upto about 5% water.
- a waterless composition for use as a vehicle in which an active pharmaceutical or cosmetic agent, when added is stable or stabilized by the presence of a modulating agent.
- a foamable waterless composition for use as a vehicle in which an active pharmaceutical or cosmetic agent, when added is stable or stabilized by the presence of a modulating agent.
- compositions containing a modulating agent as described herein are ideal carriers for active pharmaceutical ingredients that are soluble in polar solvents and which may be potentially unstable in an aqueous environment, for example, following a change in pH, or the introduction a metal catalyst or in the presence of an ionization or oxidation agent.
- the modifying or modulating affect of the modulating agent on the waterless environment prevents or minimizes a so-called 'change in the artificial pH range' and/or locks up available metal ions which could otherwise act as a catalyst in the waterless environment and/or impedes ionization or oxidation of the active agent .
- modulating agents are identified, which by their presence are able to stabilize active pharmaceutical or cosmetic agents in a waterless or substantially waterless environment. More particularly, they can be effective at relatively low concentrations that have little or no significant effect on the foam compositions of the present invention.
- a waterless composition as a vehicle in which a modulating agent is incorporated and which is capable of stabilizing an active pharmaceutical or cosmetic agent, when added.
- a waterless vehicle an active agent that is susceptible, to one or more of reaction, breakdown, ionization or oxidation and a modulating agent.
- the waterless vehicle comprises a hydrophobic solvent. In another embodiment it comprises a hydrophilic solvent.
- the carrier includes: a waterless solvent, a stabilizing surfactant, and or a polymeric agent, and in which is incorporated a modulating agent.
- the carrier is a foamable carrier, and includes: a waterless solvent, a stabilizing surfactant, and or a polymeric agent, and a propellant and in which is incorporated a modulating agent.
- the carrier comprises a stabilizing agent comprising a surfactant and a polymeric agent. This is particularly helpful for foamable compositions where the combination can be synergistic or complimentary in providing a robust stable foam of quality with a reasonable collase time.
- the carrier and modulating agent formulation is capable of stabilizing an active pharmaceutical or cosmetic agent in a substantially waterless environment.
- pharmaceutical or cosmetic composition is foamable and, includes: a waterless solvent, a stabilizing surfactant, and or a polymeric agent, a modulating agent, a propellant and one or more active pharmaceutical or cosmetic agents.
- the active agent itself can have characteristics of a modifying agent.
- the carrier includes:
- a waterless solvent comprising about 25% to about 98% of at least polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol or combinations thereof;
- a stabilizing agent selected from the group consisting of a surface- active agent and about 0.01 % to about 5% by weight of at least one polymeric agent and mixtures thereof; wherein the carrier is shakable or flowable and wherein if the composition is stored in an aerosol container and further comprises a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition it will upon release expand to form a breakable foam.
- the active agent is susceptible to reaction, breakdown, ionization, or oxidization.
- the active agent itself can have characteristics of a modifying agent.
- the foamable carrier includes:
- a waterless solvent comprising about 25% to about 98% of at least polar solvent selected from the group consisting of (1) a polyol; and (2) a polyethylene glycol or combinations thereof;
- a stabilizing agents selectedfrom from the group consisting of a surface-active agent;and about 0.01 % to about 5% by weight of at least one polymeric agent and mixtures thereof; and
- a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition; wherein the composition is shakable or flowable; and wherein the composition is stored in an aerosol container and upon release expands to form a breakable foam.
- the foamable pharmaceutical composition includes:
- a waterless solvent comprising about 25% to about 98% of at least polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol or combinations thereof;
- an effective amount of an active pharmaceutical or cosmetic agent wherein the carrier is shakable or flowable; and wherein the composition is stored in an aerosol container and upon release expands to form a breakable foam.
- the polymeric agent is selected from a bioadhesive agent, a gelling agent, a film forming agent and a phase change agent and can be from about 0.01 % to about 5% by weight, preferably 0,2 to 3%.
- the ratio of polymeric agent to surfactant is about 1 :10 to about 10:1 ; about 1 :5 to about 5:1 ; about 3:7 to about 7:3; and about 2:1 to about 1 :2.
- polymeric agent is however not straightforward.
- the polymers should be miscible or swell in the waterless solvent. It has been found that in the case of modified cellulose that lower molecular weight cellulose polymer derivatives are preferable.
- the polymeric agent is hydroxypropyl cellulose.
- the polymeric agent is or Carbomer such as Carbopol 934®.
- the pre-foamable carrier; the pre-foamable pharmaceutical or cosmetic composition; the foamable carrier, or the foamable pharmaceutical or cosmetic composition further includes 0.1% to about 75% of a secondary polar solvent.
- a vehicle additive preferably a foamable vehicle additive that is suitable for use with not merely one type of active pharmaceutical ingredient (“API") but is adaptable for use with one or more API's from a wide range of different types of API's with appropriate and usually relatively minimal or minor adjustment.
- API active pharmaceutical ingredient
- a buffer, stabilizer, anti ionization agent or an antioxidant as would be appreciated by a person skilled in the art with the benefit of the teachings herein.
- one or more pharmaceutical or cosmetic base compositions that are suitable for use with modulating agents.
- a vehicle additive preferably a foamable one that is suitable for use as a vehicle base for delivery for API's, which are by their nature unstable or reactive or isomerizes or oxidize.
- the modulating agent is preferably between about 0.05% to about 5% by weight of the composition.
- the surfactant and polymeric agent and their amounts are selected so that the composition is sufficiently shakable or in certain limited cases so that it is flowable albeit not shakable so that extrusion and substantially uniform composition formation, particularly foam extrusion and formation is not hampered.
- the maximum effective amount of surfactant and polymeric agent that may be used may be limited by the need for shakability and as a minimum by the need for flowability.
- the propellant is preferably between about 5% to about 12% by weight of the composition.
- the pharmaceutical or cosmetic foamable product is non-flammable.
- waterless is meant that the composition contains no or substantially no, free or unassociated or absorbed water. It will be understood by a person of the art that the waterless solvents and substances miscible with them of the present invention can be hydrophilic and can contain water in an associated or unfree or absorbed form and may absorb water from the atmosphere and the ability to do so is its hygroscopic water capacity.
- the carrier comprises an active pharmaceutical or cosmetic agent, which degrades in the presence of water, and in such cases the present of water in the composition is clearly not desirable.
- the composition is waterless.
- the active agent may tolerate the presence of a small amount of water and the waterless composition is substantially non-aqueous.
- substantially non-aqueous is intended to indicate that the waterless composition has water content below about 5%, preferably below about 2%, such as below about 1.5%.
- the active agent has low or minimal sensitivity and can tolerate water to about 10% or less.
- the foamable carrier Upon release from an aerosol container, the foamable carrier forms an expanded foam suitable for the treatment of an infected surface and for topical administration to the skin, a body surface, a body cavity or a mucosal surface.
- the waterless solvent comprises about 25% to about 98% of at least a polar solvent selected from the group consisting of (1 ) a polyol; and (2) a polyethylene glycol or combinations thereof.
- the waterless solvent comprises about 0.1 % to about 75% of a secondary polar solvent.
- Polar solvent
- polar solvent as used herein, is not intended to characterize the solubilization capabilities of the solvent for any specific active agent or any other component of the foamable composition. Rather, such information is provided to aid in the identification of materials suitable for use as a part in the foamable compositions described herein.
- the polar solvent is a polyol.
- a polyol is an organic substance that contains at least two hydroxy groups in its molecular structure.
- the foamable carrier contains at least one diol (a compound that contains two hydroxy groups in its molecular structure).
- diols include propylene glycol (e.g., 1 ,2-propylene glycol and 1 ,3-propylene glycol), butanediol (e.g., 1 ,2-butanediol, 1 ,3-butanediol, 2,3- butanediol and 1 ,4-butanediol), butanediol (e.g., 1 ,3-butanediol and 1 ,4- butenediol), butynediol, pentanediol (e.g., pentane-1 ,2-diol, pentane-1 ,3-diol, pentane-1 ,4-diol, pentane-1 ,5-diol, pen
- the foamable carrier contains at least one triol (a compound that contains three hydroxy groups in its molecular structure), such as glycerin, butane-1 ,2,3-triol, butane-1 ,2,4-triol and hexane- 1 ,2,6-triol.
- triol a compound that contains three hydroxy groups in its molecular structure
- the polyol is a mixture of polyols.
- the mixture of polyols contains at least one diol and at least one triol. According to certain embodiments the ratio between the diol and triol is between 9:1 and 1 :1.
- part of mixture of polyols is a saccharide. Exemplary saccharides include, but are not limited to monosaccharide, disaccharides, oligosaccharides and sugar alcohols.
- a monosaccharide is a simple sugar that cannot be hydrolyzed to smaller units.
- Exemplary monosaccharide compounds are ribose, glucose, fructose and galactose.
- Disaccharides are made up of two monosaccharides joined together, such as sucrose, maltose and lactose.
- a sugar alcohol also known as a polyol, polyhydric alcohol, or polyalcohol
- saccharide whose carbonyl group (aldehyde or ketone, reducing sugar) has been reduced to a primary or secondary hydroxyl group. They are commonly used for replacing sucrose in foodstuffs, often in combination with high intensity artificial sweeteners to counter the low sweetness.
- Some exemplary sugar alcohols, which are suitable for use according to the present invention are mannitol, sorbitol, xylitol, maltitol, lactitol. (Maltitol and lactitol are not completely hydrogenated compounds - they are a monosaccharide combined with a polyhydric alcohol).
- Mixtures of polyols, including (1 ) at least one polyol selected from a diol and a triol; and (2) a saccharide are contemplated within the scope of the present invention.
- the polar solvent consists of a polymerized ethylene glycol, namely polyethylene glycol, which is also termed "PEG".
- PEG polyethylene glycol
- the PEG is selected from the group consisting of PEG 200, PEG 300, PEG 400, PEG 600, PEG 1000, PEG 4000, PEG 6000 and PEG 8000.
- the foamable carrier according to the present invention can contain a single PEG or a mixture of two or more PEGs.
- the concentration of the PEG should be in a level that results in viscosity, prior to filling of the composition into aerosol canisters, of less than 12,000 CPs, and more preferably, less than 10,000 CPs.
- a secondary polar solvent is added to the foamable composition of the present invention.
- the secondary polar solvent is selected from a variety of organic solvents that are typically miscible on both water and oil.
- Examples of polar solvent that can be contained in the foamable carrier of the present invention include dimethyl isosorbide, tetrahydrofurfuryl alcohol polyethyleneglycol ether (glycofurol), DMSO, pyrrolidones, (such as N-Methyl-2- pyrrolidone and 1-Methyl-2-pyrrolidinone), ethyl proxitol, dimethylacetamide (DMAc), PEG-type surfactants and alpha hydroxy acids, such as lactic acid and glycolic acid.
- DMAc dimethylacetamide
- a secondary solvent in a waterless foam composition can help improve delivery of active agents to a target area.
- Foam compositions of the present invention, for which the solvent includes a secondary solvent can increase the levels of the active agent in the waterless composition and thus, provide high delivery and improved therapy.
- polyols, PEGs and polar solvents possess a high solubilizing power and thus, they can enable increased concentrations of a pharmaceutical active agent.
- Polyols, PEGs and polar solvents are also known for their skin penetration enhancement properties. These properties enable high drug bioavailability in the target area of treatment, resulting in an enhanced therapeutic effect.
- combinations of a polyol, PEGs and a secondary polar solvent exhibit an increased permeability across the skin, as suggested, for example, in Eur J Pharm Biopharm. 1998 Nov; 46(3):265-71.
- the foamable carrier contains (1 ) at least one polar solvent, selected from a polyol (selected from a diol and a triol) and PEG; and (2) at least one secondary polar solvent.
- the foamable carrier contains (1 ) a mixture of at least two polyols; and (2) at least one secondary polar solvent.
- the foamable carrier contains a mixture of at least one polyol and at least one PEG; yet in other embodiments the foamable carrier contains (1 ) a mixture of at least one polyol and at least one PEG and (2) at least one secondary polar solvent.
- the ratio between the polyol and/or PEG and the secondary polar solvent is between 9:1 and 1 :1.
- the polyol is selected from the group consisting of propylene glycol, hexylene glycol and glycerin (and mixtures thereof); and the secondary polar solvent is selected from the group consisting of dimethyl isosorbide, diethylene glycol monoethyl ether, a liquid polyethylene glycol and glycofurol.
- the foamable carrier contains (1 ) at least one polyol; and (2) dimethyl isosorbide.
- Short chain alcohols such as ethanol and propanol are known as polar solvents, however, according to one or more embodiments, the composition of the present invention is substantially alcohol-free, i.e., free of short chain alcohols.
- Short chain alcohols having up to 5 carbon atoms in their carbon chain skeleton and one hydroxyl group, such as ethanol, propanol, isopropanol, butanol, iso-butanol, t-butanol and pentanol, are considered less desirable polar solvents due to their skin-irritating effect.
- the composition is substantially alcohol-free and includes less than about 5% final concentration of lower alcohols, preferably less than about 2%, more preferably less than about 1%.
- a short chain alcohol can be included in the composition, as long as the ratio between the short chain alcohol and the polyol is less than 1 :4 by weight.
- modulating agent is used to describe an agent which can improve the stability of or stabilize a carrier or a foamable composition and or an active agent by modulating the effect of a substance or residue present in the carrier or composition.
- the substance or residue may for example be acidic or basic and potentially alter an artificial pH in a waterless or substantially non aqueous environment or it may be one or more metal ions which may act as a potential catalyst in a waterless or substantially non aqueous environment or it may be an ionisation agent or it may be an oxidizing agent.
- the modulating agent is used in a waterless composition. In one or more embodiments the modulating agent is used in a substantially non aqueous composition [0105] In one or more embodiments the modulating agent is used to describe an agent which can affect pH in an aqueous solution.
- the agent can be any of the known buffering systems used in pharmaceutical or cosmetic formulations as would be appreciated by a man of the art. It can also be an organic acid, a carboxylic acid, a fatty acid an amino acid, an aromatic acid, an alpha or beta hydroxyl acid an organic base or a nitrogen containing compound.
- the modulating agent is used to describe an agent, which is a chelating or sequestering or complexing agent that is sufficiently soluble or functional in the waterless solvent to enable it to "mop up” or “lock” metal ions.
- modulating agent is used to describe an agent which can effect pH in an aqueous solution
- modulating agent more particularly means an acid or base or buffer system or combinations thereof, which is introduced into or is present in and acts to modulate the ionic or polar characteristics and any acidity or basesity balance of a waterless or substantially non aqueous carrier, composition, foamable carrier or foamable composition or resultant foam of the present invention.
- the substance or residue can be introduced into the formulation from any one or more of the ingredients, some of which themselves may have acidic or basic properties.
- the polymer or solvent may contain basic residues in which case it may be desirable or beneficial to add an acid.
- the surfactant may contain some acid residues in which case the addition of a base may be desirable and beneficial.
- more than one ingredient may contain residues which may ameliorate or compound their significance. For example if one ingredient provided weak acid residues and another stronger acid residues the artificial pH in a waterless environment should be lower. In contrast, if one residue was acid and the other basic the net effect in the formulation maybe significantly reduced.
- the active ingredient may favor an acidic pH or more significantly may need to be maintained at a certain acidic pH otherwise it may readily isomerize, chemically react or breakdown, in which case introducing acidic components might be of help.
- the active ingredient may favor a basic pH or more significantly may need to be maintained at a certain basic pH otherwise it may readily hydrolyse, undergo rearrangement, isomerize, chemically react or breakdown, in which case introducing basic components might be of help.
- sufficient modulating agent is added to achieve an artificial pH in which the active agent is preferably stable.
- Such artificial pH may be acidic, maybe basic or may be neutral.
- pH, pKa, and pKb, buffers and the like are used in classical measurements of an aqueous solution. Such measurements are artificial in a waterless environment. Nevertheless, reference to and description below of such terms are made for convenience and clarity, since such terms are well defined and understood with reference to aqueous solutions and further due to the lack of an appropriate uniform way of describing and identifying the artificial or virtual pH. pK etc in a waterless environment in relation to the present invention. Although predictions of artificial pH can be made using dilution techniques of measurements of waterless formulations diluted in water they are formulation sensitive and specific and have to be carefully calibrated with complex formulas.
- Waterless medium can be polar and protic yet it does not conform to classical ionic behavior.
- a buffer as defined by Van Slyke [Van Slyke, J. Biol. Chem. 52, 525 (1922)], is "a substance which by its presence in solution increases the amount of acid or alkali that must be added to cause unit change in pH".
- a buffer solution is a solution of a definite pH made up in such a way that this pH alters only gradually with the addition of alkali or acid.
- Such a solution consists of a solution of a salt of the week acid in the presence of the three acid itself. The pH of the solution is determined by the dissociation equilibrium of the free acid.
- An acid can be a strong acid or a weak acid.
- a strong acid is an acid, which is a virtually 100% ionized in solution.
- a week acid is one which does not ionize fully when it is dissolved in water. The lower the value for pKa, the stronger is the acid and likewise, the higher the value for pKa the weaker is the acid.
- a base can be a strong base or a weak base.
- a strong base is something, which is fully ionic with 100% hydroxide ions.
- a weak base is one which does not convert fully into hydroxide ions in solution. The lower the value for pKb, the stronger is the base and likewise, the higher the value for pKb the weaker is the base.
- composition's pH selected from the group consisting of:
- R a and R b are independently selected from the group consisting of H, F, Cl, Br, and saturated or unsaturated, isomeric or non-isomeric, straight, branched, or cyclic C1-C2 5 alkyl, aralkyl, or aryl groups, wherein each of R 3 and R b may be optionally substituted with an OH, SH, CHO, COOH group;
- R a and R b are independently selected from the group consisting of H, F, Cl, Br, and saturated or unsaturated, isomeric or non-isomeric, straight, branched, or cyclic C1-C25 alkyl, aralkyl, or aryl groups, wherein each of R a and R b may be optionally substituted with an OH, SH, CHO, COOH group.
- alpha hydroxyl acids and beta hydroxyl acids include, but are not limited to alpha-hydroxybutyric acid, alpha-hydroxyisobutyhc acid, alpha- hydroxyisocaproic acid, alpha-hydroxyisovaleric acid, atrolactic acid, beta- hydroxybutyric acid, beta-phenyl lactic acid, beta-phenylpyruvic acid, citric acid, pyruvic acid, galacturonic acid, glucoheptonic acid, glucoheptono 1 ,4-lactone, gluconic acid, gluconolactone, glucuronic acid, glucuronolactone, glycolic acid, lactic acid, mandelic acid, mucic acid, pyruvic acid, saccharic acid, saccharic acid 1 ,4-lactone, tartaric acid and tartronic acid, 3-Hydroxybutanoic acid, quinic acid, isocitric acid, tropic acid, trethocanic acid, chlorolactic acid, quinic acid,
- Exemplary aromatic acids include, but are not limited to benzoic acid, toluic acid, dimethyl benzoic acid, phthalic acid and nicotinic acid (heterocyclic).
- the aromatic ring can contain solely carbon atoms or a combination of carbon atoms and other atoms (heterocyclic), wherein R-i, R 2 , R 3 and R 4 are independently selected from the group consisting of H, F, Cl, Br, and saturated or unsaturated, isomeric or non-isomeric, straight, branched, or cyclic C 1 -C 25 alkyl, aralkyl, or aryl groups.
- Formula Ilia wherein R is H, is identified as salicylic acid, yet another example of an aromatic hydroxyl acid is acetylsalicylic acid.
- R a and R b are independently selected from the group consisting of H and saturated or unsaturated, isomeric or non-isomeric, straight, branched, or cyclic C 1 -C 25 alkyl, aralkyl, or aryl groups, wherein R 3 may be optionally substituted with an F, Cl, Br 1 I, OH, CHO, COOH, or alkoxy group having 1 to 9 carbon atoms;
- Short chain carboxylic acids include formic acid, acetic acid and propionic acid.
- a fatty acid is a carboxylic acid often with a long unbranched aliphatic tail (chain), which is either saturated or unsaturated.
- Carboxylic acids as short as butyric acid (4 carbon atoms) are considered to be fatty acids, while fatty acids derived from natural fats and oils may be assumed to have at least 8 carbon atoms, as exemplified in the following list:.
- the carbon atom chain of the fatty acid may have at least one double bond, as exemplifies in the following list:
- Alpha-linolenic, docosahexaenoic, and eicosapentaenoic acids are examples of omega-3 fatty acids.
- Linoleic acid and arachidonic acid are omega-6 fatty acids.
- Oleic and erucic acid are omega-9 fatty acids.
- a further class of carboxylic acids according to the present invention comprises a carboxylic acid, wherein the carbon atom chain is branched, such as valproic acid.
- the carbon chain of the fatty acid or fatty alcohol can be substituted with a hydroxyl group, such as 12-hydroxy stearic acid.
- Short chain carboxylic acids such as formic acid and acetic acid are reasonably strong acids (pKa 3.77 and 4.76, respectively). Longer chain fatty acids have higher pKa values.
- Nonanoic acid for example, has a pKa of 4.96.
- a dicarboxylic acid is an organic compound, having two carboxylic acid moieties on its carbon atom skeleton. They have the general molecular formula HOOC-(CH 2 ) ⁇ -COOH.
- the dicarboxylic acid is a short-chain dicarboxylic acid.
- dicarboxylic acid group is derived from natural products or from synthesis, having "n" value from 4 up to 21.
- the carbon atom skeleton of the dicarboxylic acid can be saturated or unsaturated, such as in the case of undecylenic acid, maleic acid and fumaric acid.
- the dicarboxylic acid is branched, i.e., the carbon atom skeleton of the dicarboxylic acid can be substituted with at least one group selected from the group consisting of R, F, Cl, Br, OH, OR, SH, CHO, COOH, and NR 3 R b , wherein R, R 3 and R b are independently selected from the group consisting of H, saturated or unsaturated, isomeric or non-isomeric, straight, branched, or cyclic C1-C2 5 alkyl, aralkyl, or aryl groups.
- Exemplary branched dicarboxylic acids include aspartic acid, alpha-ketoglutaric acid, adipic acid 2-amino, aconitic acid, benzene dicarboxylic acid, citramalic acid, citric acid, cystathionine, glucuronic acid, glutamic acid, itaconic acid, malic acid, mucic acid, oxalacetic acid, diamino pimelic acid, saccharic acid, dimethyl succinic acid, tartaric acid, tartronic acid,
- the organic acid is selected from the group consisting of ascorbic acid, isoascorbic acid, ethanesulfonic acid, glycerophosphoric acid, acetohydroxamic acid, aconitic acid, alpha-ketocaproic acid, aminomalonic acid, hippuric acid, hydrochloric acid, methanesulfonic acid, oxalic acid, phosphoric acid, sorbic acid, iminodiacetic acid, carnitine, nicotinic acid and retinoids, such as retinoic acid and isotretinoin.
- the acidic strength of the carboxyl, amino and ionizable R-groups in amino acids can be defined by the association constant, Ka or more commonly the negative logarithm of Ka, the pKa.
- Ka the association constant
- pKa the negative logarithm of Ka
- nonprotein amino acids are also suitable in accordance to the present invention, including but not limited to: B-alanine (3-alanine), 4- aminobutyrate (GABA), 3-cyanoalanine (B-cyanoalanine), 2-aminobutyric acid, 2- methylene-4-aminobutyric acid, 3-methylene-4-aminobutyric acid, 2- aminoisobutyric acid, 5-aminolevulinic acid, 2-amino-4-methylhexanoic acid (homoisoleucine), 2-amino-4-methylhex-4-enoic acid, 2-amino-4-methylhex-5- ynoic acid, 2-amino-3-methylpentanoic acid, 2-aminoadipic acid, 4- ethylideneglutamic acid, 3-aminoglutaric acid, 2-aminopimelic acid, N4- ethylasparagine, N4-methylasparagine, erythro-4-methylg
- erythro-3-hydroxyaspartic acid 4-hydroxyarginine, 4-hydroxycitrulline, threo-4- hydroxyglutamic acid, 3,4-dihydroxyglutamic acid, 3-hydroxy-4-methylglutamic acid, 3-hydroxy-4-methyleneglutamic acid, 4-hydroxy-4-methylglutamic acid, 4- hydroxy-glutamine, N5-(2-hydroxyethyl)glutamine, 5-hydroxynorleucine, threo-4- hydroxyhomoarginine, homoserine, O-acetylhomoserine, O-oxalylhomoserine, O- phosphohomoserine, 4-hydroxyisoleucine, 5-hydroxymethylhomocysteine, threo- 3-hydroxyleucine, 5-hydroxyleucine, 2-hydroxylysine, 4-hydroxylysine, 5- hydroxylysine, N6-acetyl-5-hydroxylysine, N6-trimethyl-5-hydroxylysine, 4- hydroxyomithine, mimosine
- the organic modulating agent is a dimer or oligomer of amino acids.
- the organic modulating agent is an organic base.
- the organic base is a nitrogen-containing organic compound.
- the nitrogen-containing organic modulating compound is selected from, primary, secondary, tertiary and quaternary amines.
- the organic primary amine may include alkylamines such as methylamine and ethylamine; ethanolamine such as monoethanolamine and monoisopropanolamines; diamines such as ethylenediamine and 1 ,2- diaminopropane.
- the organic secondary amines include dialkylamines such as dimethylamine and diethylamine, diethanolamine and diisopropanolamine; N- methylethanolamine and N-ethylethanolamine.
- the organic tertiary amines include trialkylamines such as triethylamine and triethylamine; triethanolamine; N- methyldiethanolamine and tri-isopropanolamine.
- the quaternary compounds preferably include basic choline.
- the nitrogen-containing organic modulating compound is an amide.
- Amides are derivatives of oxoacids in which an acidic hydroxy group has been replaced by an amino or substituted amino group.
- Compounds having one, two, or threeacyl groups on a given nitrogen are generically included and may be designated as primary, secondary and tertiary amides, respectively.
- the nitrogen-containing organic modulating compound is an amine oxide, amine oxide are compounds derived from tertiary amines by the attachment of one oxygen atom to the nitrogen atom: RaN + -O " .
- the nitrogen-containing organic modulating compound is a lactam.
- Lactams are cyclic amides amino carboxylic acids, having a 1-azacycloalkan-2-one structure, or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring, as exemplified in the following structures:
- Urea is another example of a nitrogen-containing modulating compound.
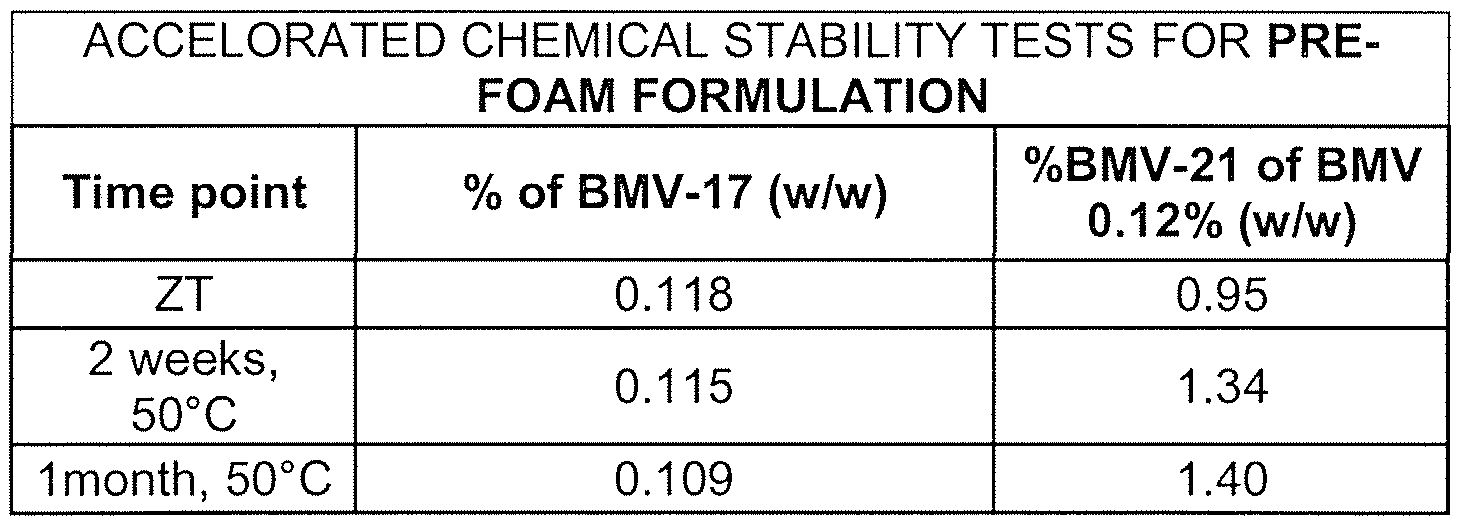
- the modulating agent to the foamable composition of the present invention is useful for stabilizing pharmaceutical and cosmetic active agents which are unstable in certain pH conditions. It is known, for example, that active agents, which contain ester bond in their structure tend to undergo hydrolysis of the ester bond at basic pH levels. Therefore, the addition of an agent which avoids the formation of basic pH condition and thus, prevents degradation of such active agents. Many steroid compounds are known to undergo rearrangement at high pH, and again, adding an acidic modulating agent helps prevent such degradation. This is clearly exemplified by the stark difference in stability results for BMV in the absence and presence of a modulating agent as demonstrated in Examples 1 and 2 below. Another example of a pH-sensitive active agent is vitamin D, which degrades at low pH levels. In such a case, the addition of a basic modulating agent, such as triethanol amine is useful to maintain acceptable stability of this active agents as can be seen an exemplified in Example 5 below.
- the stability of an active agent may be influenced by small quantities of, for example, metal ions in the waterless compositions.
- metal ions may act as a catalyst in facilitating the reaction or breakdown of the agent.
- the modulating agent can be a chelating or sequestering or complexing agent on its own.
- an effective amount of chelating agent which is sufficiently soluble or functional in the waterless solvent to enable it to "mop up" or "lock” metal ions.
- the chelating agent is selected from the group consisting of acetyl trihexyl citrate, aminotrimethylene phosphonic acid, beta-alanine diacetic acid, bismuth citrate, calcium disodium edta, citric acid, cyclohexanediamine tetraacetic acid, diammonium citrate, dibutyl oxalate, diethyl oxalate, diisobutyl oxalate, diisopropyl oxalate, dilithium oxalate, dimethyl oxalate, dipotassium edta, dipotassium oxalate, dipropyl oxalate, disodium edta, disodium edta-copper, disodium pyrophosphate, edta, etidronic acid, hedta, methyl cyclodextrin, o
- the chelating agent is selected from the group consisting of ethylenediaminetetraacetic acid ("EDTA”) and salts thereof such as disodium EDTA, tetrasodium EDTA and calsium disodium EDTA; diethylenetriaminepentaacetic acid (“DTPA”) and salts thereof; hydroxyethlethylenediaminetri acetic acid (“HEDTA”) and salts thereof and nitrilotriacetic acid (“NTA”); more preferably EDTA, HEDTA and their salts; most preferably EDTA and its salts.
- EDTA ethylenediaminetetraacetic acid
- DTPA diethylenetriaminepentaacetic acid
- HEDTA hydroxyethlethylenediaminetri acetic acid
- NTA nitrilotriacetic acid
- a preferred non limiting example of the chelating agent is EDTA.
- the chelating and sequestering agent is present in the composition at a level of up to about 5.0%, preferably 1.0 percent, by weight, of the composition.
- Combinations of Modulating Agents may be a useful aspect of the present invention.
- combinations of a strong acid and a weak base; a weak acid and a strong base: a weak acid and a strong acid; a weak base and a strong base; a weak base and a second weak base ; and a weak acid and a second weak acid may prove more effective in protecting or stabilizing an active agent in the waterless solvents of the present invention than a base or acid on its own.
- each of the active agents may have an artificial pH at which it can be more stable in a waterless composition.
- ascorbic acid in aqueous solution is known to be more stable at an acidic pH of about 5.4. It is therefore desirable to add a combination of modulating agents in a waterless composition that will generate an artificial pH approximately of the order of or equivalent to that at which ascorbic acid is more stable in a waterless medium, In order to do so it will be appreciated a man of the art that that some adjustment will have to be made as ascorbic acid is itself a strong acid and displays the characteristics of a modulating agent. Similarly chelating agents may be usefully used in combination with another modulating agent such as an acid, a base or a buffer system or with various combinations of modulating agents.
- the modulating agent to the foamable composition of the present invention is further useful for adjusting the pH of the target area of application.
- Skin is the first line of defense against all elements, such as microorganisms, wind, and pollutants, and it's the acid mantle, a fine film with a slightly acidic pH on the surface of the skin that provides protection for the skin. It plays a very important role as an integral part of the barrier function of the stratum corneum. Recent studies have demonstrated that increased enzyme activity of phospholipase A2 is related to the formation of the acid mantle in the stratum corneum. This combination makes the skin less permeable to water and other polar compounds. Normal skin surface pH is between 4 and 6.5 in healthy people, though it varies among the different areas of the skin.
- Newborn infants do have a higher skin surface pH compared to adults, but this normalizes within three days. Therefore, it is important to maintain skin surface pH in order to prevent susceptibility to bacterial skin infections or skin damage and disease.
- adding a modulating agent which contributes to the stabilization of skin pH at the desirable level, is advantageous.
- the organic modulating agent can serve to stabilize an active agent in the foam composition, it often provides additional therapeutic properties to the composition.
- the following table exemplifies, in a non-limiting fashion, the therapeutic benefits expected from an organic modulating agent. It is to be understood that this table provides a non-exhaustive list of modulating agents that possess therapeutic effects, however, many other compounds listed in the present specification also possess therapeutic benefits.
- the modulating agent may also be a preservative or an antioxidant or an ionization agent. Any preservative, antioxidant or ionization agents suitable for pharmaceutical or cosmetic application may be used. Non limiting examples of antioxidants are tocopherol succinate, propyl galate, butylated hydroxy toluene and butyl hydroxy anisol. Ionization agents may be positive or may be negative depending on the environment and the active agent or composition that is to be protected. Ionization agents may for example act to protect or reduce sensitivity of active agents.
- Non limiting examples of positive ionization agents are benzyl conium chloride, and cetyl pyridium chloride.
- Non limiting examples of negative ionization agents are sodium lauryl sulphate, sodium lauryl lactylate and phospholipids.
- the modulating agent is a flavonoid.
- Flavonoids are a large group of polyphenols antioxidant compounds, which often occur as glycosides and are ubiquitously present in foods of plant origin. Some flavonoids (e.g. quercetin, rutin) are available as dietary supplements. Flavonoids can be further subdivided into:
- flavonols e.g. kaempferol, quercetin and myricetin
- flavones e.g. apigenin and luteolin
- flavonones e.g. hesperetin, naringenin, eriodictyol
- flavan-3-ols e.g. (+)-catechin, (+)-gallocatechin, (-)-epicatechin, (-)- epigallocatechin
- anthocyanins e.g. cyanidin, delphinidin, malvidin, pelargonidin, peonidin, petunidin
- Flavonoids are distinguished from the carotenoids.
- flavonoids The activities of flavonoids are dependent on their chemical structure.Estimates of dietary flavonoid intake vary from 10 to 100 mg daily, but may be several hundreds of milligrams a day. Dietary supplements of quercetin and rutin provide around 500 mg in a single dose.
- Flavonoids may have a potential role in the prevention of CVD, cancer and cataracts and possibly other diseases, for anti-viral activity., and they may be useful in treating ulcers, include hemorrhoids, allergy, asthma, menopausal symptoms and the prevention of habitual abortion.
- quercetin As a dietary supplement, quercetin is promoted for prevention and treatment of atherosclerosis and hyperlipidaemia, diabetes, cataracts, hay fever, peptic ulcer, inflammation, prevention of cancer and for treating prostatitis.
- a preliminary, double-blind, placebo-controlled trial in chronic non-bacterial prostatitis showed that quercetin reduced pain and improved quality of life, but had no effect on voiding dysfunction.
- rutin is used to reduce capillary permeability and treat symptoms of varicose veins. In combination with bromelain and trypsin, rutin is used to treat osteoarthritis.
- a non limiting list of flavonoid compounds is: benzquercin, diosmin, ethoxazorutoside, flavodate, sodium hesperidin, leucocianido, monoxerutin, oxerutin, quercetin, rutoside, rosmarinic acid. The above information was noted from Dietary Supplements, Electronic Version, Pharmaceutical Press 2007.
- a single flavonoid is provided and in a further embodiment a combination of two or more flavonoid are provided.
- the flavonoids act synergistically.
- water soluble flavonoids are combined with water insoluble flavonoids. It is known for example that whole polyphenols extracts have greater antioxidant effect than their known individual components.
- flavonoids are used in combination with other phenolics.
- one or more flavonoids are provided in combination with one or more vitamins.
- flavonoids are provided that are more reactive than the vitamins.
- the flavonoids act as a conservational agent.
- the Microsponges are rigid, porous and spongelike round microscopic particles of cross-linked polymer beads (e.g., polystyrene or copolymers thereof), each defining a substantially noncollapsible pore network.
- the Microsponges can be loaded with an active ingredient and can provide a controlled time release of the active ingredient to skin or to a mucosal membrane upon application of the formulation. The slow release is intended to reduce irritation by the active.
- Microsponge® delivery technology was developed by Advanced Polymer Systems.
- the composition comprisises one or more active agents loaded into Microponges with a waterless carrier comprising a modulating agent.
- the composition of the present invention contains a polymeric agent. It has been documented that the presence of a polymeric agent is necessary for the creation of foam, having fine bubble structure, which does not readily collapse upon release from the pressurized aerosol can.
- the polymeric agent serves to stabilize the foam composition and to control drug residence in the target organ.
- the polymeric agent is soluble or readily dispersible in the polyol; or in the mixture of a polyol and an additional polar solvent.
- Non-limiting examples of polymeric agents that are soluble or readily dispersible in propylene glycol are Hydroxypropylcellulose and carbomer (homopolymer of acrylic acid is crosslinked with an allyl ether pentaerythritol, an ally! ether of sucrose, or an allyl ether of propylene, such as Carbopol® 934, Carbopol® 940, Carbopo® 941 , Carbopol® 980 and Carbopol® 981.
- polymeric agents are suitable for use according to the present invention provided that they are soluble or readily dispersible in the polyol; or in the mixture of a polyol and an additional polar solvent, on a case by case basis.
- Exemplary polymeric agents include, in a non-limiting manner, naturally-occurring polymeric materials, such as locust bean gum, sodium alginate, sodium caseinate, egg albumin, gelatin agar, carrageenin gum, sodium alginate, xanthan gum, quince seed extract, tragacanth gum, guar gum, cationic guars, hydroxypropyl guar gum, starch, amine-bearing polymers such as chitosan; acidic polymers obtainable from natural sources, such as alginic acid and hyaluronic acid; chemically modified starches and the like, carboxyvinyl polymers, polyvinylpyrrolidone, polyvinyl alcohol, polyacrylic acid polymers. polymethacrylic acid polymers, polyvinyl acetate polymers, polyvinyl chloride polymers, polyvinylidene chloride polymers and the like.
- naturally-occurring polymeric materials such as locust bean gum, sodium alginate, sodium
- Additional exemplary polymeric agents include semi-synthetic polymeric materials such as cellulose ethers, such as, hydroxypropyl cellulose, Polyethylene glycol, having molecular weight of 1000 or more (e.g., PEG 1 ,000, PEG 4,000, PEG 6,000 and PEG 10,000) also have gelling capacity and while they are considered herein as "secondary polar solvents", as detailed herein, they are also considered polymeric agents.
- semi-synthetic polymeric materials such as cellulose ethers, such as, hydroxypropyl cellulose, Polyethylene glycol, having molecular weight of 1000 or more (e.g., PEG 1 ,000, PEG 4,000, PEG 6,000 and PEG 10,000) also have gelling capacity and while they are considered herein as "secondary polar solvents", as detailed herein, they are also considered polymeric agents.
- the concentration of the polymeric agent should be selected so that the composition, after filling into aerosol canisters, is flowable, and can be shaken in the canister.
- the concentration of the polymeric agent is selected such that the viscosity of the composition, prior to filling of the composition into aerosol canisters, is less than 12,000 CPs, and more preferably, less than 10,000 CPs.
- the composition further contains a surface-active agent.
- Surface- active agents include any agent linking oil and water in the composition, in the form of emulsion.
- a surfactant's hydrophilic/lipophilic balance (HLB) describes the emulsifier's affinity toward water or oil. HLB is defined for non-ionic surfactants. The HLB scale ranges from 1 (totally lipophilic) to 20 (totally hydrophilic), with 10 representing an equal balance of both characteristics.
- Lipophilic emulsifiers form water-in-oil (w/o) emulsions; hydrophilic surfactants form oil-in-water (o/w) emulsions.
- the HLB of a blend of two emulsifiers equals the weight fraction of emulsifier A times its HLB value plus the weight fraction of emulsifier B times its HLB value (weighted average).
- a single surfactant may suffice.
- a combination of two or more surfactants is desired.
- Reference to a surfactant in the specification can also apply to a combination of surfactants or a surfactant system. As will be appreciated by a person skilled in the art which surfactant or surfactant system is more appropriate is related to the vehicle and intended purpose. In general terms a combination of surfactants can be significant in producing breakable forms of good quality.
- the composition contains a single surface active agent having an HLB value between about 2 and 9, or more than one surface active agent and the weighted average of their HLB values is between about 2 and about 9.
- the composition contains a single surface active agent having an HLB value between about 7 and 14, or more than one surface active agent and the weighted average of their HLB values is between about 7 and about 14.
- the composition contains a single surface active agent having an HLB value between about 9 and about 19, or more than one surface active agent and the weighted average of their HLB values is between about 9 and about 19.
- HLB values In a waterless or substantially waterless environment a wide range of HLB values may be suitable.
- the composition contains a non-ionic surfactant.
- non-ionic surfactants include a polysorbate, polyoxyethylene (20) sorbitan monostearate, polyoxyethylene (20) sorbitan monooleate, a polyoxyethylene fatty acid ester, Myrj 45, Myrj 49, Myrj 52 and Myrj 59; a polyoxyethylene alkyl ether, polyoxyethylene cetyl ether, polyoxyethylene palmityl ether, polyethylene oxide hexadecyl ether, polyethylene glycol cetyl ether, steareths such as steareth 2, brij 21 , brij 721 , brij 38, brij 52, brij 56 and brij W1 , a sucrose ester, a partial ester of sorbitol and its anhydrides, sorbitan monolaurate, sorbitan monolaurate, a monoglyceride, a diglycer
- the surfactant is an ether for example polyoxyethylene (26) glycerol ether.
- surfactants are selected which can provide a close packed surfactant layer.
- combinations of at least two surfactants are selected.
- they should be complex emulgators and more preferably they should both be of a similar molecular type;for example, a pair of ethers, like steareth 2 and steareth 21 , or a pair of esters, for example, PEG-40 stearate and polysorbate 80.
- the surfactants can be ethers.
- POE esters cannot be used and a combination of sorbitan laurate and sorbitan stearate or a combination of sucrose stearic acid ester mixtures and sodium laurate may be used. All these combinations due to their versatility and strength may also be used satisfactorily and effectively with ether frormulations, although the amounts and proportion may be varied according to the formulation and its objectives as will be appreciated by a man of the art.
- a series of dextrin derivative surfactants prepared by the reaction of the propylene glycol polyglucosides with a hydrophobic oxirane-containing material of the glycidyl ether are highly biodegradable. [Hong-Rong Wang and Keng-Ming Chen, Colloids and Surfaces A: Physicochemical and Engineering Aspects Volume 281 , Issues 1-3, 15 June 2006, Pages 190-193] .
- Non-limiting examples of non-ionic surfactants that have HLB of about 7 to about 12 include steareth 2 (HLB-4.9); glyceryl monostearate/PEG 100 stearate ( Av HLB-11.2); stearate Laureth 4 (HLB-9.7) and cetomacrogol ether (e.g., polyethylene glycol 1000 monocetyl ether).
- Non-limiting examples of preferred surfactants which have a HLB of 4-19 are set out in the Table below:
- Polyglycerized Fatty Acids such as:
- the surface active agent is a complex emulgator in which the combination of two or more surface active agents can be more effective than a single surfactant and provides a more stable formulation or improved foam quality than a single surfactant.
- the complex emulgator comprises a combination of surfactants wherein there is a difference of about 4 or more units between the HLB values of the two surfactants or there is a significant difference in the chemical nature or structure of the two or more surfactants.
- surfactant systems are, combinations of polyoxyethylene alkyl ethers, such as Brij 59 / BrijiO; Brij 52 / Brij 10; Steareth 2 / Steareth 20; Steareth 2 / Steareth 21 (Brij 72 / Brij 721); combinations of polyoxyethylene stearates such as Myrj 52 / Myrj 59; combinations of sucrose esters, such as Surphope 1816 / Surphope 1807; combinations of sorbitan esters, such as Span 20 / Span 80; Span 20 / Span 60; combinations of sucrose esters and sorbitan esters, such as Surphope 1811 and Span 60; combinations of liquid polysorbate detergents and PEG compounds, such as Tween 80 / PEG- 40 stearate; methyl glucaso sequistearate; polymeric emulsifiers, such as Permulen (TRI or TR2); liquid crystal systems, such as Arlatone (2121
- the surfactant is preferably one or more of the following: a combination of steareth-2 and steareth-21 on their own or in combination with glyceryl monostearate (GMS); in certain other embodiments the surfactant is a combination of polysorbate 80 and PEG-40 stearate. In certain other embodiments the surfactant is a combination of glyceryl monostearate/PEG 100 stearate. In certain other embodiments the surfactant is a combination of two or more of stearate 21 , PEG 40 stearate, and polysorbate 80. In certain orher embodiments the surfactant is a combination of two or more of laureth 4, span ⁇ O, and polysorbate 80.
- the surfactant is a combination of two or more of GMS and ceteareth. In certain other embodiments the surfactant is a combination of two or more of steareth 21 , ceteareth 20, ceteth 2 and laureth 4 In certain other embodiments the surfactant is a combination of ceteareth 20 and polysorbate 40 stearate. In certain orther embodiments the surfactant is a combination of span 60 and GMS. In certain other embodiments the surfactant is a combination of two or all of PEG 40 stearate, sorbitan stearate and polysorbate 60
- the surfactant is one or more of sucrose stearic acid esters, sorbitan laureth, and sorbitan stearate.
- non-ionic surfactants with significant hydrophobic and hydrophilic components, increase the emulsifier or foam stabilization characteristics of the composition.
- using combinations of surfactants with high and low HLB's to provide a relatively close packed surfactant layer may strengthen the formulation.
- the stability of the composition can be improved when a combination of at least one non-ionic surfactant having HLB of less than 9 and at least one non-ionic surfactant having HLB of equal or more than 9 is employed.
- the ratio between the at least one non-ionic surfactant having HLB of less than 9 and the at least one non-ionic surfactant having HLB of equal or more than 9, is between 1 :8 and 8:1 , or at a ratio of 4:1 to 1 :4.
- the resultant HLB of such a blend of at least two emulsifiers is preferably between about 9 and about 14.
- a combination of at least one non- ionic surfactant having HLB of less than 9 and at least one non-ionic surfactant having HLB of equal or more than 9 is employed, at a ratio of between 1 :8 and 8:1 , or at a ratio of 4:1 to 1 :4, wherein the HLB of the combination of emulsifiers is preferably between about 5 and about 18.
- the surface active agent is selected from the group of cationic, zwitterionic, amphoteric and ampholytic surfactants, such as sodium methyl cocoyl taurate, sodium methyl oleoyl taurate, sodium lauryl sulfate, triethanolamine lauryl sulfate and betaines.
- amphiphilic molecules can show lyotropic liquid-crystalline phase sequences depending on the volume balances between the hydrophilic part and hydrophobic part. These structures are formed through the micro-phase segregation of two Many amphiphilic molecules can show lyotropic liquid- crystalline phase sequences depending on the volume balances between the hydrophilic part and hydrophobic part. These structures are formed through the micro-phase segregation of two incompatible components on a nanometer scale. Soap is an everyday example of a lyotropic liquid crystal.
- surfactants tend to form lyotropic liquid crystals in emulsions interface (oil-in- water) and exert a stabilizing effect [0200]
- the surfactant is a surfactant or surfactant combination is capable of or which tends to form liquid crystals.
- Surfactants which tend to form liquid crystals may improve the quality of foams.
- Non limiting examples of surfactants with postulated tendency to form interfacial liquid crystals are; phospholipids, alkyl glucosides, sucrose esters, sorbitan esters.
- the at least one surface active agent is liquid. Moreover for the purposes of formulating with liquid ethers a liqiid surfactant is preferred
- the liquid surfactant is a polysorbate, preferably polysorbate 80 or 60.
- the at least one surface active agent is solid, semi solid or waxy. In a further embodiment they are soluble in oil and in another embodiment have a HLB of less than about 12.
- HLB values may not be so applicable to non ionic surfactants, for example, with liquid crystals or with silicones. Also HLB values may be of lesser significance in a waterless or substantially non-aqueous environment.
- the surfactant can be, a surfactant system comprising of a surfactant and a co surfactant, a waxy emulsifier, a liquid crystal emulsifier, an emulsifier which is solid or semi solid at room temperature and pressure, or combinations of two or more agents in an appropriate proportion as will be appreciated a person skilled in the art.
- a solid or semi solid emulsifier combination it can also comprise a solid or semi solid emulsifier and a liquid emulsifier.
- at least one surfactant is a liquid.
- the surface-active agent includes at least one non-ionic surfactant.
- Ionic surfactants are known to be irritants. Therefore, non-ionic surfactants are preferred in applications including sensitive tissue such as found in most mucosal tissues, especially when they are infected or inflamed. Non-ionic surfactants alone can provide formulations and foams of good or excellent quality in the carriers and compositions of the present invention.
- the composition contains a non-ionic surfactant.
- the composition includes a mixture of non-ionic surfactants as the sole surface active agent.
- the foamable composition includes a mixture of at least one non-ionic surfactant and at least one ionic surfactant in a ratio in the range of about 100:1 to 6:1.
- the non- ionic to ionic surfactant ratio is greater than about 6:1 , or greater than about 8:1 ; or greater than about 14: 1 , or greater than about 16: 1 , or greater than about 20: 1.
- surface active agent comprises a combination of a non- ionic surfactant and an ionic surfactant, at a ratio of between 1 :1 and 20:1
- a combination of a non-ionic surfactant and an ionic surfactant is employed, at a ratio of between 1 :1 and 20:1 , or at a ratio of 4:1 to 10:1 ; for example, about 1 :1 , about 4:1 , about 8:1 , about 12:1 , about 16: 1 and about 20: 1 or at a ratio of 4: 1 to 10: 1 , for example, about 4: 1 , about 6:1 , about 8:1 and about 10:1.
- the upper amount of surfactant that may be used may be limited by the shakability of the composition. If the surfactant is non liquid, it can make the formulation to viscous or solid. Subject to its miscibily solid surfactants may be added first, and may require gentle warming and then cooling before being combined with the other ingredients. In general terms, as the amount of non-liquid surfactant is increased the shakability of the formulation reduces until a limitation point is reached where the formulation can become non shakable and unsuitable. Thus in one embodiment, any effective amount of surfactant may be used provided the formulation remains shakable.
- the upper limit for foamable formulations may be determined by flowability such that any effective amount can be used provided the formulation is sufficiently flowable to be able to flow through an actuator valve and be released and still expand to form a good quality foam. This may be due without being bound by any theory to one or more of a number of factors such as the viscosity, the softness, the lack of crystals, the pseudoplastic or semi pseudo plastic nature of the composition and the dissolution of the propellant into the composition.
- the amount of surfactant or combination of surfactants is between about 0.05% to about 20%; between about 0.05% to about 15%. or between about 0.05% to about 10%.
- the concentration of surface active agent is between about 0.2% and about 8%. In a more preferred embodiments the concentration of surface active agent is between about 1% and about 6% or between about 1% and about 4%.
- the surface active agent does not contain a polyoxyethylene (POE) moiety, such as polysorbate surfactants, POE fatty acid esters, and POE alkyl ethers, because the active agent is incompatible with such surface active agents.
- POE polyoxyethylene
- the active agent pimecrolimus is not stable the presence of POE moieties, yet benefits greatly from the use of dicarboxylic esters as penetration enhancers. In such cases, alternative surface active agents are employed.
- POE - free surfactants include non-ethoxylated sorbitan esters, such as sorbitan monopalmitate, sorbitan monostearate, sorbitan tristearate, sorbitan monooleate, sorbitan trioleate, sorbitan monolaurate and sorbitan sesquioleate; glycerol fatty acid esters, such as glycerol monostearate and glycerol monooleate; mono-, di- and tri-esters of sucrose with fatty acids (sucrose esters), sucrose stearate, sucrose distearate sucrose palmitate and sucrose laurate; and alkyl polyglycosides, such as lauryl diglucoside.
- sorbitan esters such as sorbitan monopalmitate, sorbitan monostearate, sorbitan tristearate, sorbitan monooleate, sorbitan trioleate, sorbitan monolaurate and sorb
- the surface-active agent includes mono- , di- and tri-esters of sucrose with fatty acids (sucrose esters), prepared from sucrose and esters of fatty acids or by extraction from sucro-glycerides.
- sucrose esters include those having high monoester content, which have higher HLB values.
- the surfactant includes at least one surfactant selected from a polyoxyethylene fatty ether, a polyoxyethylene fatty ester.a carbohydrate ester and a sucrose ester.
- non-ionic surfactants include steareth-2, steareth-20, steareth-21 , ceteareth 2, PEG-100 stearyl ether, cetearyl glucoside, methyl glucose sesquistearate, sorbitan monostearate, GMS NE and span 20.
- non limiting other examples of surfactant combinations are glyceryl stearate and PEG 100 stearate and Iaureth4; steareth 2, PEG 100 searate and Iaureth4; and cetearyl glucoside and cetearyl alcohol.
- the surfactant containing formulations are further boosted by a foam adjuvant for example stearyl alcohol.
- a non limiting example of a combination of surfactants having a weighted average of their HLB values of 11 is Glyceryl Stearate and PEG-100 Stearate (for example trade name "simulsol 165" from Sepic).
- the foamable carrier further contains at least one hydrophobic solvent.
- hydrophobic solvent as used herein, is not intended to characterize the solubilization capabilities of the solvent for any specific active agent or any other component of the foamable composition. Rather, such information is provided to aid in the identification of materials suitable for use as a part in the foamable compositions described herein.
- a "hydrophobic solvent” as used herein refers to a material having solubility in distilled water at ambient temperature of less than about 1 gm per 100 ml_, more preferable less than about 0.5 gm per 100 ml_, and most preferably less than about 0.1 gm per 100 ml_.
- the hydrophobic organic carrier is an oil, such as mineral oil, isopropyl palmitate, isopropyl isostearate, diisopropyl adipate, diisopropyl dimerate, maleated soybean oil, octyl palmitate, cetyl lactate, cetyl ricinoleate, tocopheryl acetate, acetylated lanolin alcohol, cetyl acetate, phenyl trimethicone, glyceryl oleate, tocopheryl linoleate, wheat germ glycerides, arachidyl propionate, myristyl lactate, decyl oleate, propylene glycol ricinoleate, isopropyl lanolate, pentaerythrityl tetrastearate, neopentylglycol dicaprylate/dicaprate, isononyl isononanoate,
- oil such as mineral oil, iso
- a humectant is a substance that helps retain moisture and also prevents rapid evaporation.
- suitable heumectants are propylene glycol, propylene glycol derivatives, and glycerin.
- Other examples of humectants and moisturizers may be found in the Handbook of Pharmaceutical Additives published by Gower. Suitable ones for use with and soluble in the waterless compositions of the present invention may be selected as will be appreciated by a person skilled in the art.
- a moisturizer is a substance that helps retain moisture or add back moisture to the skin.
- examples are allantoin, petrolatum, urea, lactic acid, sodium PCV, glycerin, shea butter, caprylic/capric/stearic triglyceride, candelilla wax, propylene glycol, lanolin, hydrogenated oils, squalene, sodium hyaluronate and lysine PCA.
- Glycerine and sodium pCA work in combination. Other examples may be found in the Handbook of Pharmaceutical Additives published by Gower.
- compositions of the present invention may in one or more embodiments usefully comprise in addition a humectant or a moisturizer or combinations thereof.
- a foam adjuvant is included in the foamable carriers of the present invention to increase the foaming capacity of surfactants and/or to stabilize the foam.
- the foam adjuvant agent includes fatty alcohols having 15 or more carbons in their carbon chain, such as cetyl alcohol and stearyl alcohol (or mixtures thereof).
- fatty alcohols are arachidyl alcohol (C20), behenyl alcohol (C22), 1-triacontanol (C30), as well as alcohols with longer carbon chains (up to C50).
- Fatty alcohols derived from beeswax and including a mixture of alcohols, a majority of which has at least 20 carbon atoms in their carbon chain, are especially well suited as foam adjuvant agents.
- the amount of the fatty alcohol required to support the foam system is inversely related to the length of its carbon chains.
- Foam adjuvants, as defined herein are also useful in facilitating improved spreadability and absorption of the composition.
- the foam adjuvant agent includes fatty acids having 16 or more carbons in their carbon chain, such as hexadecanoic acid (C16) stearic acid (C18), arachidic acid (C20), behenic acid (C22), octacosanoic acid (C28), as well as fatty acids with longer carbon chains (up to C50), or mixtures thereof.
- fatty acids having 16 or more carbons in their carbon chain, such as hexadecanoic acid (C16) stearic acid (C18), arachidic acid (C20), behenic acid (C22), octacosanoic acid (C28), as well as fatty acids with longer carbon chains (up to C50), or mixtures thereof.
- fatty acids having 16 or more carbons in their carbon chain such as hexadecanoic acid (C16) stearic acid (C18), arachidic acid (C20), behenic acid (C22), octacosanoic
- the carbon atom chain of the fatty alcohol or the fatty acid may have at least one double bond.
- a further class of foam adjuvant agent includes a branched fatty alcohol or fatty acid.
- the carbon chain of the fatty acid or fatty alcohol also can be substituted with a hydroxy! group, such as 12-hydroxy stearic acid.
- a composition of the present invention includes one or more additional components.
- additional components include but are not limited to anti perspirants, anti-static agents, buffering agents, bulking agents, chelating agents, cleansers, colorants, conditioners, deodorants, diluents, dyes, emollients, fragrances, hair conditioners, humectants, pearlescent aids, perfuming agents, permeation enhancers, pH-adjusting agents, preservatives, protectants, skin penetration enhancers, softeners, solubilizers, sunscreens, sun blocking agents, sunless tanning agents, viscosity modifiers and vitamins and flavonoids.
- a specific additional component may have more than one activity, function or effect.
- the additional component is a pH adjusting agent or a buffering agent.
- Suitable buffering agents include but are not limited to acetic acid, adipic acid, calcium hydroxide, citric acid, glycine, hydrochloric acid, lactic acid, magnesium aluminometasilicates, phosphoric acid, sodium carbonate, sodium citrate, sodium hydroxide, sorbic acid, succinic acid, tartaric acid, and derivatives, salts and mixtures thereof.
- the additional component is an emollient.
- Suitable emollients include but are not limited to mineral oil, lanolin oil, coconut oil, cocoa butter, olive oil, aloe vera extract, jojoba oil, castor oil, fatty acids, fatty alcohols, diisopropyl adipate, hydroxybenzoate esters, benzoic acid esters of C9 to C15 alcohols, isononyl iso-nonanoate, silicone oils, polyethers, C12 to C15 alkyl benzoates, oleic acid, stearic fatty acid, cetyl alcohols, hexadecyl alcohol, dimethyl polysiloxane, polyoxypropylene cetyl ether, polyoxypropylene butyl ether, and derivatives, esters, salts and mixtures thereof.
- the additional component is a humectant.
- Suitable humectants include but are not limited to guanidine, urea, glycolic acid, glycolate salts, ammonium glycolate, quaternary alkyl ammonium glycolate, lactic acid, lactate salts, ammonium lactate, quaternary alkyl ammonium lactate, aloe vera, aloe vera gel, allantoin, urazole, alkoxylated glucose, hyaluronic acid, lactamide monoethanolamine, acetamide monoethanolamine and derivatives, esters, salts and mixtures thereof.
- the additional component is a preservative.
- Suitable preservatives include but are not limited to C12 to C15 alkyl benzoates, alkyl p-hydroxybenzoates, aloe vera extract, ascorbic acid, benzalkonium chloride, benzoic acid, benzoic acid esters of C9 to C15 alcohols, butylated hydroxytoluene, castor oil, cetyl alcohols, chlorocresol, citric acid, cocoa butter, coconut oil, diazolidinyl urea, diisopropyl adipate, dimethyl polysiloxane, DMDM hydantoin, ethanol, fatty acids, fatty alcohols, hexadecyl alcohol, hydroxybenzoate esters, iodopropynyl butylcarbamate, isononyl iso- nonanoate, jojoba oil, lanolin oil, methylparaben, mineral oil,
- the additional component is a skin penetration enhancer.
- Suitable skin penetration enhancers include but are not limited to acetone, acyl lactylates, acyl peptides, acylsarcosinates, alkanolamine salts of fatty acids, alkyl benzene sulphonates, alkyl ether sulphates, alkyl sulphates, anionic surface-active agents, benzyl benzoate, benzyl salicylate, butan-1 ,4-diol, butyl benzoate, butyl laurate, butyl myristate, butyl stearate, cationic surface-active agents, citric acid, cocoamidopropylbetaine, decyl methyl sulfoxide, decyl oleate, dibutyl azelate, dibutyl phthalate, dibenzyl sebacate, dibutyl sebacate, dibutyl dibutyl
- composition with modulating agent may be used as is without addition of a propellant.
- propellant is added to form a foamable compostion that produces a foam when the formulation is expelled from a pressurized canister.
- the foam may be of various qualities as indicated herein. In a preferred embodiment the foam is of about good quality. In a more preferred embodiment it is of about excellent quality.
- suitable propellants include volatile hydrocarbons such as butane, propane, isobutane and fluorocarbon gases, or mixtures thereof.
- the propellant is AP 70 which is a mixture of propane, isobutene and butane.
- the propellant makes up about 5-25 wt% of the foamable composition. In some circumstances the propellant may be upto 35%.
- the propellants are used to generate and administer the foamable composition as a foam.
- the total composition including propellant, foamable compositions and optional ingredients is referred to as the foamable composition.
- Such propellants include, but are not limited to, hydrofluorocarbon (HFC) propellants, which contain no chlorine atoms, and as such, fall completely outside concerns about stratospheric ozone destruction by chlorofluorocarbons or other chlorinated hydrocarbons.
- HFC hydrofluorocarbon
- Exemplary non-flammable propellants include propellants made by DuPont under the registered trademark Dymel, such as 1 ,1 ,1 ,2 tetrafluorethane (Dymel 134), and 1 ,1 ,1 ,2,3,3,3 heptafluoropropane (Dymel 227) 1 ,1 , difluoro ethane (Dymel 152) and 1 ,1 ,1 ,3,3,3 hexafluoropropane HFCs possess Ozone Depletion Potential of 0.00 and thus, they are allowed for use as propellant in aerosol products.
- Dymel such as 1 ,1 ,1 ,2 tetrafluorethane (Dymel 134), and 1 ,1 ,1 ,2,3,3,3 heptafluoropropane (Dymel 227)
- foamable emulsions including HFC as the propellant can be improved in comparison with the same composition made with a hydrocarbon propellant.
- foamable compositions comprise a combination of a HFC and a hydrocarbon propellant such as n-butane or mixtures of hydrocarbom propellants such as propane, isobutane and butane.
- a hydrocarbon propellant such as n-butane or mixtures of hydrocarbom propellants such as propane, isobutane and butane.
- a pharmaceutical or cosmetic composition manufactured using the foamable carrier of the present invention is very easy to use. When applied onto the afflicted body surface of mammals, i.e., humans or animals, it is in a foam state, allowing free application without spillage. Upon further application of a mechanical force, e.g., by rubbing the composition onto the body surface, it freely spreads on the surface and is rapidly absorbed.
- the foamable composition of the present invention is stable, having an acceptable shelf-life of months or preferably at least one year, or more preferably, at least two years at ambient temperature, as revealed in accelerated stability tests.
- Organic carriers and propellants tend to impair the stability of emulsions and to interfere with the formation of stable foam upon release from a pressurized container. It has been observed, however, that the foamable compositions according to the present invention are surprisingly stable. Following accelerated stability studies, they demonstrate desirable texture; they form fine bubble structures that do not break immediately upon contact with a surface, spread easily on the treated area and absorb quickly.
- composition should also be free flowing, to allow it to flow through the aperture of the container, e.g., and aerosol container, and create an acceptable foam.
- Foam quality can be graded as follows:
- Grade E excellent: very rich and creamy in appearance, does not show any bubble structure or shows a very fine (small) bubble structure; does not rapidly become dull; upon spreading on the skin, the foam retains the creaminess property and does not appear watery.
- Grade G (good): rich and creamy in appearance, very small bubble size, "dulls” more rapidly than an excellent foam, retains creaminess upon spreading on the skin, and does not become watery.
- Grade FG (fairly good): a moderate amount of creaminess noticeable, bubble structure is noticeable; upon spreading on the skin the product dulls rapidly and becomes somewhat lower in apparent viscosity.
- Grade F very little creaminess noticeable, larger bubble structure than a "fairly good” foam, upon spreading on the skin it becomes thin in appearance and watery.
- Grade P no creaminess noticeable, large bubble structure, and when spread on the skin it becomes very thin and watery in appearance.
- Grade VP dry foam, large very dull bubbles, difficult to spread on the skin.
- Topically administrable foams are typically of quality grade E or G, when released from the aerosol container. Smaller bubbles are indicative of more stable foam, which does not collapse spontaneously immediately upon discharge from the container. The finer foam structure looks and feels smoother, thus increasing its usability and appeal.
- a further aspect of the foam is breakability. Thermally sensitive foams immediately collapse upon exposure to skin temperature and, therefore, cannot be applied on the hand and afterwards delivered to the afflicted area.
- the foam of the present invention has several advantages, when compared with hydroalcoholic foam compositions, such as (1) Breakability.
- the foam of the present invention is thermally stable and breakable under sheer force but is not "quick breaking which allows comfortable application and well directed administration to the target area;
- foams Another property of the foam is specific gravity, as measured upon release from the aerosol can.
- foams typically have specific gravity of less than 0.12 g/mL; or less than 0.10 g/ml_; or less than 0.08 g/mL, depending on their composition and on the propellant concentration.
- compositions, the foamable compositions and the foams of the present invention are ideal vehicles for active pharmaceutical ingredients and active cosmetic ingredients.
- active pharmaceutical ingredients and active cosmetic ingredients are collectively termed “active agent” or “active agents”.
- a foamable composition, comprising an active agent has the following advantages:
- the foamable composition provides a preferred solvent for active agents, particularly water-insoluble agents.
- a polyol and/or a PEG and a secondary polar solvent in the foamable composition facilitates a co-solvent effect, resulting increased concentrations of soluble active agent in the dosage form, thus facilitating enhanced skin penetration of the active agent.
- increased penetration is positively correlated with improved clinical outcome.
- attaining an increased drug penetration into the target site of action enables a decrease of treatment frequency, for example, from twice or three times daily to once daily.
- Polyols and PEGs; and combinations of a polyol and/or PEG with a secondary polar solvent are known as skin penetration enhancers, thus, increasing drug residence in the target area and increasing clinical efficacy, as detailed above.
- compositions contains no water, or merely up to 5% and water minimizes the probability of degradation of water- sensitive active agents.
- a foam containing a polyol and/or PEG with no water at all can be formed in accordance with the composition and process of the present invention. Such compositions ensure high stability of water sensitive active agents.
- the foamable polyol composition in contained in an impermeable pressurized packaging presentation is impermeable and thus, the active agent is not exposed to environmental degradation factors, such as light and oxidating agent during storage.
- the composition includes at least one active agent. a. a therapeutically effective concentration of an active agent; b. a modulating agent; c. about 50% to about 98% of a polar solvent, selected from the group consisting of a polyol and a polyethylene glycol; d. 0% to about 48% of a secondary polar solvent; e. about 0.2% to about 5% by weight of a surface-active agent; f. about 0.01% to about 5% by weight of at least one polymeric agent; and for a foamable composition additionally g. a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition. wherein the composition is stored in an aerosol container and upon release expands to form a breakable foam.
- Suitable active agents include but are not limited to active herbal extracts, acaricides, age spot and keratose removing agents, allergen, analgesics, local anesthetics, antiacne agents, antiallergic agents, antiaging agents, antibacterials, antibiotics, antibum agents, anticancer agents, antidandruff agents, antidepressants, antidermatitis agents, antiedemics, antihistamines, antihelminths, antihyperkeratolyte agents, antiinflammatory agents, antiirritants, antilipemics, antimicrobials, antimycotics, antiproliferative agents, antioxidants, anti-wrinkle agents, antipruritics, antipsoriatic agents, antirosacea agents antiseborrheic agents, antiseptic, antiswelling agents, antiviral agents, antiyeast agents, astringents, topical cardiovascular agents, chemotherapeutic agents, corticosteroids, dicarboxylic acids, disinfectidi
- the active agent is an active herbal extract.
- Suitable active herbal extracts include but are not limited to angelica, anise oil, astragali radix, azalea, benzyl acetate, birch tar oil, bomyl acetate, cacumen biotae, camphor, cantharidin, capsicum, cineole, cinnamon bark, cinnamon leaf, citronella, citroneliol, citronellyl acetate, citronellyl formate, eucalyptus, eugenyl acetate, flos carthami, fructus mori, garlic, geraniol, geranium, geranyl acetate, habanera, isobutyl angelicate, lavender, ledum latifolium, ledum palustre, lemongrass, limonene, linalool, linalyl acetate, methyl anthranilate, methyl cinnamate, mezer
- the active agent is an acaricide.
- Suitable acaricides include but are not limited to amitraz, flumethrin, fluvalinate and derivatives, esters, salts and mixtures thereof.
- the active agent is an age spot and keratoses removing agent.
- Suitable age spot and keratoses removing agent include but are not limited to hydroxy acids, azelaic acid and other related dicarboxylic acids, retinoids, kojic acid, arbutin, nicotinic, ascorbic acid, hydroquinone and derivatives, esters, salts and mixtures thereof.
- Certain nonsteroidal anti-inflammatory agents, such as diclofenac are also useful for the treatment of keratoses.
- the active agent is an analgesic.
- Suitable analgesics include but are not limited to benzocaine, butamben picrate, dibucaine, dimethisoquin, dyclonine, lidocaine, pramoxine, tetracaine, salicylates and derivatives, esters, salts and mixtures thereof.
- the active agent is a local anesthetic.
- suitable local anesthetics include but are not limited to benzocaine, benzyl alcohol, bupivacaine, butamben picrate, chlorprocaine, cocaine, dibucaine, dimethisoquin, dyclonine, etidocaine, hexylcaine, ketamine, lidocaine, mepivacaine, phenol, pramoxine, procaine, tetracaine, salicylates and derivatives, esters, salts and mixtures thereof.
- the active agent is an antiacne agent.
- Suitable antiacne agents include but are not limited to N- acetylcysteine, adapalene, azelaic acid, benzoyl peroxide, cholate, clindamycin, deoxycholate, erythromycin, flavinoids, glycolic acid, meclocycline, metronidazol, mupirocin, octopirox, phenoxy ethanol, phenoxy proponol, pyruvic acid, resorcinol, retinoic acid, salicylic acid, scymnol sulfate, sulfacetamide-sulfur, sulfur, tazarotene, tetracycline, tretinoin triclosan and derivatives, esters, salts and mixtures thereof.
- the active agent is an antiaging agent.
- Suitable antiaging agents include but are not limited to sulfur- containing D and L amino acids, alpha-hydroxy acids s, beta-hydroxy acids (e.g. salicylic acid), urea, hyaluronic acid, phytic acid, lipoic acid; lysophosphatidic acid, skin peel agents (e.g., phenol, resorcinol and the like), vitamin B3 compounds (e.g., niacinamide, nicotinic acid and nicotinic acid salts and esters, including non-vasodilating esters of nicotinic acid (such as tocopheryl nicotinate), nicotinyl amino acids, nicotinyl alcohol esters of carboxylic acids, nicotinic acid N- oxide and niacinamide N-oxide), vitamin B5 and retinoids (e.g., retinol, retina
- the active agent is an antibiotic.
- antibiotic as used herein shall include, but is not limited to, any substance being destructive to or inhibiting the growth of bacteria or any substance having the capacity to inhibit the growth of or to destroy bacteria.
- the antibiotic agent is selected from the group consisting of a beta-lactam antibiotic, an aminoglycoside, an ansa-type antibiotic, an anthraquinone, an azole, an antibiotic glycopeptide, a macrolide, an antibiotic nucleoside, an antibiotic peptide, an antibiotic polyene, an antibiotic polyether, an antibiotic quinolone, an antibiotic steroid, a sulfonamide, an antibiotic metal, an oxidizing agent, a periodate, a hypochlorite, a permanganate, a substance that release free radicals and/or active oxygen, a cationic antimicrobial agent, a quaternary ammonium compound, a biguanide, a triguanide, a bisbiguanide, a polymeric biguanide, and analogs, derivatives, salts, ions and complexes thereof.
- a beta-lactam antibiotic an aminoglycoside, an ansa-type antibiotic, an anthraquinone
- Suitable antibiotics include but are not limited to amanfadine hydrochloride, amanfadine sulfate, amikacin, amikacin sulfate, aminoglycosides, amoxicillin, ampicillin, ansamycins, bacitracin, beta-lactams, candicidin, capreomycin, carbenicillin, cephalexin, cephaloridine, cephalothin, cefazolin, cephapirin, cephradine, cephaloglycin, chloramphenicols, chlorhexidine, chlorhexidine gluconate, chlorhexidine hydrochloride, chloroxine, chlorquinaldol, chlortetracycline, chlortetracycline hydrochloride, ciprofloxacin, circulin, clindamycin, clindamycin hydrochloride, clotrimazole, cloxacillin, demeclocycline, diclosxacillin, di
- the active agent is an antidandruff agent.
- Suitable antidandruff agents include but are not limited to aminexil, benzalkonium chloride, benzethonium chloride, 3-bromo-1-chloro-5,5- dimethyl-hydantoin, chloramine B, chloramine T, chlorhexidine, N- chlorosuccinimide.climbazole- , 1 ,3-dibromo-5,5-dimethylhydantoin, 1 ,3-dichloro- 5,5-dimethyl-hydantoin, betulinic acid, betulonic acid, celastrol, crataegolic acid, cromakalin, cyproterone acetate, dutasteride, finesteride, ibuprofen, ketoconozole, oleanolic acid, phenytoin, picrotone olamine, salicylic acid, selenium sulphides,
- the active agent is an antihistamine.
- Suitable antihistamines include but are not limited to chlorcyclizine, diphenhydramine, mepyramine, methapyrilene, tripelennamine and derivatives, esters, salts and mixtures thereof.
- the active agent is an antimycotic Also termed antifungal agent.
- antimycotic and “antifungal” as used herein include, but is not limited to, any substance being destructive to or inhibiting the growth of fungi and yeast or any substance having the capacity to inhibit the growth of or to destroy fungi and/or yeast.
- the antifungal agent is an agent that is useful in the treatment of a superficial fungal infection of the skin, dermatophytosis, microsporum, trichophyton and epidermophyton infections, candidiasis, oral candidiasis (thrush), candidiasis of the skin and genital mucous membrane, Candida paronychia, which inflicts the nail and nail bed and genital and vaginal Candida, which inflict genitalia and the vagina.
- Suitable antimycotics include but are not limited to allylamines, amorolfine, amphotericin B, azole compounds, bifonazole, butoconazole, chloroxine, clotrimazole, ciclopirox olamine, clotrimazole, econazole, elubiol, fenticonazole, fluconazole, flucytosine (5FC), griseofulvin, itraconazole, ketoconazole, mafenide acetate, miconazole, naftifine, natamycin, tolnaftate, nystatin, polyenes, oxiconazole, sulbentine, sulconazole, terbinafine, terconazole, tioconazole, undecylenic acid and derivatives, esters, salts and mixtures thereof.
- the active agent is an antipruritic.
- Suitable antipruritics include but are not limited to menthol, methdilazine, trimeprazine, urea and derivatives, esters, salts and mixtures thereof.
- the active agent is an additional antipsoriatic agent.
- additional antipsoriatic agents include but are not limited to 6-aminonicotinamide, 6-aminonicotinic acid, 2- aminopyrazinamide, anthralin, 6-carbamoylnicotinamide, 6-chloronicotinamide, 2- carbamoylpyrazinamide, corticosteroids, 6-dimethylaminonicotinamide, dithranol, 6-formylaminonicotinamide, 6-hydroxy nicotinic acid, 6-substituted nicotinamides, 6-substituted nicotinic acid, 2-substituted pyrazinamide, tazarotene, thionicotinamide, trichothecene mycotoxins and derivatives, esters, salts and mixtures thereof.
- the active agent is an antirosacea agent.
- Suitable antirosacea agents include but are not limited to azelaic acid, metronidazole, sulfacetamide and derivatives, esters, salts and mixtures thereof.
- Certain nonsteroidal anti-inflammatory agents, such as salicylic acid, salycilates, piroxicam and diclofenac are also useful for the treatment of Rosacea.
- the active agent is an antiseborrheic agent.
- Suitable antiseborrheic agents include but are not limited to glycolic acid, salicylic acid, selenium sulfide, zinc pyrithione, a dicarboxylic acid, such as azelaic acid and derivatives, esters, salts and mixtures thereof.
- the active agent is an antiviral agent.
- Suitable antiviral agents include but are not limited to acyclovir, gancyclovir, ribavirin, amantadine, rimantadine nucleoside-analog reverse transcriptase inhibitors, such as zidovudine, didanosine, zalcitabine, tavudine, lamivudine and vidarabine, non-nucleoside reverse transcriptase inhibitors, such as nevirapine and delavirdine, protease inhibitors, such as saquinavir, ritonavir, indinavir and nelfinavir, and interferons and derivatives, esters, salts and mixtures thereof.
- the active agent is a chemotherapeutic agent.
- chemotherapeutic agents include but are not limited to daunorubicin, doxorubicin, idarubicin, amrubicin, pirarubicin, epirubicin, mitoxantrone, etoposide, teniposide, vinblastine, vincristine, mitomycin C, 5-FU, paclitaxel, docetaxel, actinomycin D, colchicine, topotecan, irinotecan, gemcitabine cyclosporin, verapamil, valspodor, probenecid, MK571 , GF120918, LY335979, biricodar, terfenadine, quinidine, pervilleine A, XR9576 and derivatives, esters, salts and mixtures thereof.
- the active agent is a corticosteroid.
- Suitable corticosteroids include but are not limited to alclometasone dipropionate, amcinafel, amcinafide, amcinonide, beclomethasone, beclomethasone dipropionate, betamethsone, betamethasone benzoate, betamethasone dexamethasone-phosphate, dipropionate, betamethasone valerate, budesonide, chloroprednisone, chlorprednisone acetate, clescinolone, clobetasol, clobetasol propionate, clobetasol valerate, clobetasone, clobetasone butyrate, clocortelone, cortisone, cortodoxone, craposone butyrate, desonide, desoxymethasone, dexamethasone, desoxycorticosterone acetate, dichlorisone, dif
- -methyl dexamethasone methylprednisoione, methylprednisolone acetate, mometasone furoate, paramethasone, prednisolone, prednisone, pregnenolone, progesterone, spironolactone, triamcinolone, triamcinolone acetonide and derivatives, esters, salts and mixtures thereof.
- the active agent is a hair growth regulator.
- Suitable hair growth regulators include but are not limited to N- acetylgalactosamine, N-acetylglucosamine, N-acetylmannosamine, acitretin, aminexil, ascomycin, asiatic acid, azelaic acid, benzalkonium chloride, benzethonium chloride, benzydamine, benzyl nicotinate, benzoyl peroxide, benzyl peroxide, betulinic acid, betulonic acid, calcium pantothenate, celastrol, cepharanthine, chlorpheniramine maleate, clinacycin hydrochloride, crataegolic acid, cromakalin, cyproterone acetate, diazoxide, diphenhydramine hydrochloride, dutasteride, estradiol, ethyl-2-hydroxypropanoate, fina
- the active agent is a hormone.
- suitable hormones include but are not limited to methyltestosterone, androsterone, androsterone acetate, androsterone propionate, androsterone benzoate, androsteronediol, androsteronediol-3-acetate, androsteronediol-17- acetate, androsteronediol 3-17-diacetate, androsteronediol-17-benzoate, androsteronedione, androstenedione, androstenediol, dehydroepiandrosterone, sodium dehydroepiandrosterone sulfate, dromostanolone, dromostanolone propionate, ethylestrenol, fluoxymesterone, nandrolone phenpropionate, nandrolone decanoate, nandrolone furylpropionate, nandrolone
- the active agent is a hydroxyacid.
- Suitable hydroxy acids include but are not limited to agaricic acid, aleuritic acid, allaric acid, altraric acid, arabiraric acid, ascorbic acid, atrolactic acid, benzilic acid, citramalic acid, citric acid, dihydroxytartaric acid, erythraric acid, galactaric acid, galacturonic acid, glucaric acid, glucuronic acid, glyceric acid, glycolic acid, gularic acid, gulonic acid, hydroxypyruvic acid, idaric acid, isocitric acid, lactic acid, lyxaric acid, malic acid, mandelic acid, mannaric acid, methyllactic acid, mucic acid, phenyllactic acid, pyruvic acid, quinic acid, ribaric acid, ribonic acid, saccharic acid, talaric acid, tartaric acid, tartronic acid, threa
- the active agent is a keratolytic agent.
- keratolytic agent is used herein to mean a compound which loosens and removes the stratum corneum of the skin, or alters the structure of the keratin layers of skin. Keratolytic agents are used in the treatment of many dermatological disorders, which involve dry skin, hyperkeratiinization (such as prsoriasis), skin itching (such as xerosis), acne and rosacea.
- Suitable keratolytic agents include but are not limited to N- acetylcysteine, azelaic acid, cresols, dihydroxy benzene compounds, such as resorcinol and hydroquinone, alpha-hydroxy acids, such as lactic acid and glycolic acid, phenol, pyruvic acid, resorcinol, sulfur, salicylic acid, retinoic acid, isoretinoic acid, retinol, retinal, urea and derivatives, esters, salts and mixtures thereof.
- the active agent is a lactam.
- lactams include but are not limited to L-galactono-1 ,4-lactam, L- arabino-1 ,5-lactam, D-fucono-1 ,5-lactam, D-glucaro-1 ,4-lactam, D-glucurono-6,3- lactam, 2,5-tri-O-acetyl-D-glucurono-6,3-lactam, 2-acetamido-2-deoxyglucono- 1 ,5-1- actam, 2-acetamido-2-deoxygalactono-1 ,5-lactam, D-glucaro-1 , 4:6,3- dilactam- , L-idaro-1 ,5-lactam, 2,3,5,tri-O-acetyl-D-glucaro-1 ,4-lactam, 2,5-di-O- acetyl-D-
- the active agent is a nonsteroidal anti-inflammatory agent.
- Suitable non-steroidal anti-inflammatory agent include but are not limited to azelaic acid, oxicams, piroxicam, isoxicam, tenoxicam, sudoxicam, CP-14,304, salicylates, aspirin, disalcid, benorylate, trilisate, safapryn, solprin, diflunisal, fendosal, acetic acid derivatives, diclofenac, fenclofenac, indomethacin, sulindac, tolmetin, isoxepac, furofenac, tiopinac, zidometacin, acematacin, fentiazac, zomepirac, clindanac, oxepinac, felbinac, ketorolac, fenamates, mefenamic, meclofenamic, flufen
- the active agent is insecticide.
- insecticide is used herein to mean a compound which kills, inhibits the growth of, impeded the proliferation of or repels insects.
- Insecticides include, for example, agents that can kill lice, flees, ticks, mites, scabies and mousquitos, as well as agents that repel such insects.
- Suitable insecticides include but are not limited to DDT, lindane, malathion, permethrin, allethrin, biopermethrin, transpermethrin, phenothrin, diethyl-m-toluamide, dimethyl phthalate, piperonyl butoxide, pyrethroids and derivatives, esters, salts and mixtures thereof.
- the active agent is a vasodilator.
- Suitable vasodilators include but are not limited to agents that modulate the activity of the enzyme nitric oxide synthase, nicotinic acid, ethyl nicotinate, amyl nitrite, amyl nitrate, ethyl nitrite, butyl nitrite, isobutyl nitrite, glyceryl trinitrate, octyl nitrite, sodium nitrite, sodium nitroprusside, clonitrate, erythrityl tetranitrate, isosorbide mononitrate, isosorbide dinitrate, mannitol hexanitrate, pentaerythritol tetranitrate, penetrinitol, triethanolamine trinitrate, trolnitrate phosphate (triethanolamine trinitrate diphosphate
- the active agent is a vasoconstrictor.
- Suitable vasodilators include but are not limited to ephedrine, epinephrine, phenylephrine, angiotensin, vasopressin; an extract ephedra sinica (ma huang), polygonum bistorta (bistort root), hamamelis virginiana (witch hazel), hydrastis canadensis (goldenseal), lycopus virginicus (bugleweed), aspidosperma quebracho (quebracho bianco), cytisus scoparius (scotch broom) and cypressand and derivatives, esters, salts and mixtures thereof.
- the active agent is a retinoid.
- Suitable retinoids include but are not limited to retinol, retinal, retinoic acid, all-trans retinoic acid, isotretinoin, tazarotene, adapalene, 13-c/s-retinoic acid, acitretin all-trans beta carotene, alpha carotene, lycopene, 9-cis-beta- carotene, lutein and zeaxanthin.
- the active agent is a vitamin D analog.
- Suitable retinoids include but are not limited to calcipotriene, cholecalciferol, 25-hydroxycholecalciferol, 1 ⁇ -dihydroxycholecalciferol, ergocalciferol, 1 ⁇ -dihydroxyergocalciferol, 22,23-dihydroergocalciferol, 1 ,24,25-trihydroxycholecalciferol, previtamin D 3 , tachysterob (also termed tacalciol), isovitamin D 3 , dihydrotachysterob, (I S)-hydroxycalciol, (24R)- hydroxycalcidiol, 25-fluorocalciol, ercalcidiol, ertacalciol, (5E)-isocalcioI, 22,23- dihydroercalciol, (24S)-methylcalciol, (5£)-(10S
- the active agent is selected from the group consisting of an immunosuppressants and immunoregulating agents.
- Suitable immunosuppressants and immunoregulating agents include but are not limited to cyclic peptides, such as cyclosporine, tacrolimus, tresperimus, pimecrolimus, sirolimus (rapamycin), verolimus, laflunimus, laquinimod, imiquimod derivatives, esters, salts and mixtures thereof.
- the immunomodulator is a calcineurin Inhibitor.
- the active agent is a wart remover.
- wart removers include but are not limited to imiquimod, podophyllotoxin and derivatives, esters, salts and mixtures thereof.
- the active agent is a photodynamic therapy (PDT) agent.
- PDT agents include but are not limited to modified porphyrins, chlorins, bacteriochlorins, phthalocyanines, naphthalocyanines, pheophorbides, purpurins, m-THPC, mono-L-aspartyl chlorin e6, bacteriochlorins, phthalocyanines, benzoporphyrin derivatives, as well as photosensitiser precursors, such as aminolevulinic acid and derivatives, esters, salts and mixtures thereof.
- the active agent is an antioxidant or a radical scavenger.
- Suitable antioxidants and radical scavengers agents include but are not limited to ascorbic acid, ascorbyl esters of fatty acids, magnesium ascorbyl phosphate, sodium ascorbyl phosphate, ascorbyl sorbate, tocopherol, tocopheryl sorbate, tocopheryl acetate, butylated hydroxy benzoic acid, ⁇ -hydroxy ⁇ . ⁇ j. ⁇ -tetramethylchroman ⁇ -carboxylic acid, gallic acid, propyl gallate, uric acid, sorbic acid, lipoic acid, diethylhydroxylamine, amino-guanidine, glutathione, dihydroxy fumaric acid, lycine pidolate, arginine pilolate, nordihydroguaiaretic acid, bioflavonoids, curcumin, lysine, methionine, proline, superoxide dismutas
- the active agent is a self- tanning agent, such as dihydroxyacetone.
- the active agent is an agent, capable of treating hyperhidrosis.
- Suitable hyperhidrosis agents include but are not limited to anticholinergic drugs, boric acid, tannic acid, resorcinol, potassium permanganate, formaldehyde, glutaraldehyde, methenamine, a Lewis acid, aluminum chloride, aluminum chlorohydrates, zirconium chlorohydrates, aluminum-zirconium-Glycine (AZG) complex, aluminum hydroxybromide, a glycopyrrolate compound, a 5-alpha-reductase inhibitor, finasteride, epristeride, flutamide, spironolactone, saw palmetto extract, cholestan-3-one, a mono- and dicarboxylic acid having 4 to 18 carbon atoms, botulinum toxin, a 5-HT2C receptor antagonist, a 5-HT2C receptor antagonist, ketanserin, ritanserin, mianserin,
- the active agent is a sunscreen agent.
- Suitable sunscreen agents include but are not limited to titanium dioxide, zinc oxide, zirconium oxide, iron oxide, p-aminobenzoic acid and its derivatives (ethyl, isobutyl, glyceryl esters; p-dimethylaminobenzoic acid); anthranilic acid derivatives (i.e., o-amino-benzoates, methyl, menthyl, phenyl, benzyl, phenylethyl, linalyl, terpinyl, and cyclohexenyl esters); salicylates (amyl, phenyl, octyl, benzyl, menthyl, glyceryl, and di-pro-pyleneglycol esters); cinnamic acid derivatives (menthyl and benzyl esters, a-phenyl cinnamonitrile; butyl
- the active agent is a figure- forming agent and an agent, capable of treating cellulite.
- Suitable such agents include but are not limited to baldderwack extract, butcher's, broom, cayenne, dandelion, red clover, ginkgo biloba, horse chestnut, witch hazel and borage oil, caffeic acid, nicotinic acid, theophiline and pentoxyphilline and salts and derivatives thereof.
- ⁇ disorders of the skin, body cavity or mucosal surface involve a combination of etiological factors.
- etiological factors e.g., fungal and bacterial infections and that are inflamed and have symptoms of redness and/or itching warrant therapy that combines an anti-infective agent and an anti-inflammatory agent.
- combining at least two active agents that treat different etiological factors results in a synergistic effect and consequently higher success rate of the treatment.
- the composition contains two active agents, where each of the active agents require a different pH environment in order to remain stable.
- the active agents require a different pH environment in order to remain stable.
- corticosteroids are typically stable at acidic pH values (they have a maximum stability at a pH of about 4-6) and of vitamin D analogues are typically stable at basic pH values (they have a maximum stability at pH values above about 8).
- the composition is substantially non-aqueous or preferably waterless.
- substantially non-aqueous is intended to indicate that the composition has a water content below about 5%, preferably below about 2%, such as below about 1.5%.
- the formulation can then be modulated by a modulating agent addative that provides, for example, an artificial pH closer to the more sensitive of the two sensitive actives or alternatively closer to the more pH sensitive of the two actives.
- a modulating agent addative that provides, for example, an artificial pH closer to the more sensitive of the two sensitive actives or alternatively closer to the more pH sensitive of the two actives.
- the actve pharmaceutical or cosmetic agent is an agent which is sensitive to pH such that it is more stable at a certain pH or pH range and tends to react or break down or isomerise at a different pH or pH range.
- the active agent includes the following non limiting examples: steroids (most are stable at acidic ph of about 4.5 ); antibiotics, such as tetracycline's which are stable at acidic ph (e.g.
- antibiotics such as, ampicillin, benzylpenicillin, carbenicillin, cephadroxil, cefotaxime, cephalotin, and cephradine
- triamcinolone nystatin (stable at about ph 7.0); sulfacetamide; and amphotericin
- vitamins are generally ph sensitive and examples are ascorbic acid which is stable in slightly acidic conditions and vitamin D analogues that are stable at basic pH, and nicotine amide
- NSAID's such as aspirin and indomethacin; atropine; piloarpine; procaine amide; aztrenam; barbiturates
- captopril nucletide derivatives such as cytarabineepinephrine
- parabens such as ethylparaben methlparaben
- anticancer agents such as fluorouracil and iodoxur
- foamable carrier of the present invention is suitable for treating any infected surface.
- foamable carrier is suitable for administration to the skin, a body surface, a body cavity or mucosal surface, e.g., the cavity and/or the mucosa of the nose, mouth, eye, ear, respiratory system, vagina or rectum (severally and interchangeably termed herein "target site").
- the foamable composition of the present invention is useful in treating an animal or a human patient having any one of a variety of dermatological disorders, including dermatological pain, dermatological inflammation, acne, acne vulgaris, inflammatory acne, non-inflammatory acne, acne fulminans, nodular papulopustular acne, acne conglobata, dermatitis, bacterial skin infections, fungal skin infections, viral skin infections, parasitic skin infections, skin neoplasia, skin neoplasms, pruritis, cellulitis, acute lymphangitis, lymphadenitis, erysipelas, cutaneous abscesses, necrotizing subcutaneous infections, scalded skin syndrome, folliculitis, furuncles, hidradenitis suppurativa, carbuncles, paronychial infections, rashes, erythrasma, impetigo, ecthyma,
- the foamable composition of the present invention is suitable for treating a disorder of a body cavity or mucosal surface, e.g., the mucosa of the nose, mouth, eye, ear, respiratory system, vagina or rectum.
- Non limiting examples of such conditions include chlamydia infection, gonorrhea infection, hepatitis B, herpes, HIV/AIDS, human papillomavirus (HPV), genital warts, bacterial vaginosis, candidiasis, chancroid, granuloma Inguinale, lymphogranloma venereum, mucopurulent cervicitis (MPC), molluscum contagiosum, nongonococcal urethritis (NGU), trichomoniasis, vulvar disorders, vulvodynia, vulvar pain, yeast infection, vulvar dystrophy, vulvar intraepithelial neoplasia (VIN), contact dermatitis, pelvic inflammation, endometritis, salpingitis, oophoritis, genital cancer, cancer of the cervix, cancer of the vagina, vaginal dryness, dyspareuni
- the composition is useful for the treatment of an infection.
- the composition is suitable for the treatment of an infectiion, selected from the group of a bacterial infection, a fungal infection, a yeast infection, a viral infection and a parasitic infection.
- the composition is useful for the treatment of wound, ulcer and bum. This use is particularly important since the composition of the present invention creates a thin, semi-occlusive layer, which coats the damaged tissue, while allowing exudates to be released from the tissue.
- composition of the present invention is also suitable for administering a hormone to the skin or to a mucosal membrane or to a body cavity, in order to deliver the hormone into the tissue of the target organ, in any disorder that responds to treatment with a hormone.
- the water-free composition In light of the hygroscopic nature of the water-free composition, it is further suitable for the treatment and prevention of post-surgical adhesions. Adhesions are scars that form abnormal connections between tissue surfaces. Post-surgical adhesion formation is a natural consequence of surgery, resulting when tissue repairs itself following incision, cauterization, suturing, or other means of trauma.
- the foam When comprising appropriate protective agents, the foam is suitable for the treatment or prevention of post surgical adhesions.
- the use of foam is particularly advantageous because foam can expand in the body cavity and penetrate into hidden areas that cannot be reached by any other alternative means of administration.
- the foamable composition is substantially alcohol-free, i.e., free of short chain alcohols.
- Short chain alcohols having up to 5 carbon atoms in their carbon chain skeleton and one hydroxyl group, such as ethanol, propanol, isopropanol, butaneol, iso-butaneol, t-butaneol and pentanol, are considered less desirable solvents or polar solvents due to their skin-irritating effect.
- the composition is substantially alcohol-free and includes less than about 5% final concentration of lower alcohols, preferably less than about 2%, more preferably less than about 1 %.
- 'Shakability means that the composition contains some or sufficient flow to allow the composition to be mixed or remixed on shaking. That is, it has fluid or semi fluid properties. In some very limited cases it may still be possible to have a foamable composition which is flowable but not apparently shakable.
- a breakable foam is thermally stable or substantially so, yet breaks under sheer force.
- the breakable foam of the present invention is not "quick breaking", i.e., it does not readily collapse upon exposure to body temperature environment. Sheer-force breakability of the foam is clearly advantageous over thermally induced breakability, (due to, for example, the presence of alcohol) since it allows comfortable application and well directed administration to the target area.
- chemical instability of one or more active agents is meant that at least one of the one or more active agents is susceptible to one or more of inter alia reaction, breakdown, ionization or oxidation or the rate thereof is increased when incorporated into a pharmaceutical or cosmetic carrier that is non aqueous or substantially non aqueous and not comprising a modulating agent .
- the formulas of the present invention may be made in the following general way with appropriate adjustments for each formulation as will be appreciated by someone skilled in the art.
- Polymers if any, are mixed, swelled and solubilized in the waterless medium, when necessary, with appropriate heat until it forms a clear solution.
- Stabilizing surfactants added usually with heat, until a homogeneous mixture is obtained, the mixture is then allowed to cool.
- the remainder of the ingredients are then added with mixing until they have dissolved in the medium.
- the active agent is usually added at the end once the modulating agent has been incorporated.
- the canisters are then filled with the above waterless formula, sealed and crimped with a valve and pressurized with the propellant.
- the foamable formulation may be prorduced under nitrogen and under vacuum. Whilst the whole process can be carried out under an oxygen free environment, it can be sufficient to apply a vacuum after heating and mixing all the ingredients to obtain an emulsion or homogenous liquid.
- the production chamber is equipped to apply a vacuum but if not the formulation can be for example placed in a dessicator to remove oxygen prior to filing and crimping.
- Each aerosol canister is filled with PFF and crimped with valve using vacuum crimping machine.
- the process of applying a vacuum will cause most of the oxygen present to be eliminated.
- Addition of hydrocarbon propellant may without being bound by any theory further help to reduce the likelihood of any remaining oxygen reacting with the active ingredient.
- Pressurizing is carried out using a hydrocarbon gas or gas mixture.
- Canisters are filled and then warmed for 30 sec in a warm bath at 5O 0 C and well shaken immediately thereafter.
- Each pressurized canister is subjected to bubble and crimping integrity testing by immersing the canister in a 60°C water bath for 2 minutes. Canisters are observed for leakage as determined by the generation of bubbles. Canisters releasing bubbles are rejected.
- LFRA100 instrument is used to characterize hardness.
- a probe is inserted into the test material.
- the resistance of the material to compression is measured by a calibrated load cell and reported in units of grams on the texture analyzer instrument display.
- Preferably at least three repeat tests are made.
- the textural characteristics of a dispensed foam can affect the degree of dermal penetration, efficacy, spreadability and acceptability to the user. The results can also be looked at as an indicator of softness. Note: the foam sample is dispensed into an aluminum sample holder and filled to the top of the holder.
- Collapse time is examined by dispensing a given quantity of foam and photographing sequentially its appearance with time during incubation at 36 0 C. It is useful for evaluating foam products, which maintain structural stability at skin temperature for at least 1 min.
- Viscosity is measured with Brookfield LVDV-II + PRO with spindle SC4- 25 at ambient temperature and 10, 5 and 1 RPM. Viscosity is usually measured at 10RPM. However, at about the apparent upper limit for the spindle of ⁇ >50,000CP, the viscosity at 1 RPM may be measured, although the figures are of a higher magnitude.
- the conductance meter is calibrated.
- the electrode is inserted into a sufficient volume of composition and the conductance reading is recorded. Mesurements with foamable compositions are made prior to addition of propellant.
- the amount of active agent present is analysed in foam expelled from various presurized canisters containing foam formulations using HPLC. Analysis is carried out at zero time and at appropriate time intervals thereafter.
- the canisters are stored in controlled temperature incubators at 5C, at 25C, at, 4OC and at 5OC. At appropriate time intervals canisters are removed and the amount of active agent in the foam sample is measured.
- BMV, BMV-21 and IS are extracted by heating, cooling and centrifugation of the sample solution.
- the clear layer is analyzed by UPLC using a C-18 column; elution is performed with a mobile phase containing acetonitrile:water 55:45.
- the content of BMV and BMV-21 is calculated using the respective peak area ratio against average peak area ratio of BMV standard (0.2 mg/mL) to the IS present in the standard solution at the same concentration as in samples.
- the column type is Acquity BEH C-18, 1.7 ⁇ m, 50 x 2.1 mm in 40 0 C, the flow is 0.5 mL/min, the detector: UV, at 254 nm injection volume is 4 ⁇ L.; the diluents is 0.1% acetic acid in water : acetonitrile, 45:55 (v/v) and the mobile phase content is water : acetonitrile, 45:55 (v/v). The run time is 4 min.
- the method can be carried out by HPLC using a different but appropriate column of the same type.
- Bubble Size Foams are made of gas bubbles entrapped in liquid.
- the bubble size and distribution reflects in the visual texture and smoothness of the foam.
- Foam bubbles size is determined by dispensing a foam sample on a glass slide, taking a picture of the foam surface with a digital camera equipped with a macro lens. The diameter of about 30 bubbles is measured manually relatively to calibration standard template. Statistical parameters such as mean bubble diameter, standard deviation and quartiles are then determined. Measuring diameter may also be undertaken with image analysis software.
- the camera used was a Nikon D40X Camera (resolution 10MP) equipped with Sigma Macro Lens (ref: APO MACRO 150mm F2.8 EX DG HSM). Pictures obtained are cropped to keep a squared region of 400 pixels x 400 pixels.
- Example 1 A Waterless Steroid Foamable Composition with out a Modulating Agent
- Example 3 A Waterless Steroid Foamable Composition where the Modulating Agent is Stearic Acid
- Example-4 A Waterless Steroid Foamable Composition With A Penetration Enhancer DMI in the absence of an added Modulating Agent.
- Example 5 A Waterless Vitamin D3 Derivative Foamable Composition where the Modulating Agent is Triethoanolamine:
- waterless solvent is a combination of two polyethylene glycols:
- waterless solvent is a combination of two polyethylene glycols and propylene glycol:
- lidocaine and prilocaine may act as its own modulating agent.
- Another modulating agent can be used to modify the artificial pH to be of the order of the pH at which lidocaine and prilocaine are respectively most stable.
- Example 9 Demonstration of Excellent Minimal Foams Produced with two Different Surfactants Steareth 2 and Montanov 68 (cetearyl alcohol and cetearyl glucoside)
- Example 10 Examples of foams containing a steroid active agent with Carbomer (synthetic high molecular weight crosslinked polymers of acrylic acid) as the polymeric agent
- Stearic acid a waxy solid acts as a foam adjuvant, thickener and Modulating Agent to produce good quality foam.
- the selection of Modulating Agent is not per se obvious with a waterless formulation.
- isostearic acid results in poor foam presumably but without being bound by any theory suggested due to it being a liquid wax and not having the thickening effect of its closely related counterpart stearic acid plus its isostearic acids asymmetrical shape even though they both are CI8.
Abstract
Description
Claims
Priority Applications (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP07874548A EP2097065A2 (en) | 2006-11-29 | 2007-11-29 | Foamable waterless compositions with modulating agents |
| AU2007355106A AU2007355106A1 (en) | 2006-11-29 | 2007-11-29 | Foamable waterless compositions with modulating agents |
| IL198688A IL198688A (en) | 2006-11-29 | 2009-05-11 | Composition with modulating agents |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US86162006P | 2006-11-29 | 2006-11-29 | |
| US60/861,620 | 2006-11-29 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2008152444A2 true WO2008152444A2 (en) | 2008-12-18 |
| WO2008152444A3 WO2008152444A3 (en) | 2009-06-18 |
Family
ID=40130252
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2007/004628 WO2008152444A2 (en) | 2006-11-29 | 2007-11-29 | Foamable waterless compositions with modulating agents |
Country Status (4)
| Country | Link |
|---|---|
| EP (1) | EP2097065A2 (en) |
| AU (1) | AU2007355106A1 (en) |
| IL (1) | IL198688A (en) |
| WO (1) | WO2008152444A2 (en) |
Cited By (83)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010125470A3 (en) * | 2009-04-28 | 2011-06-16 | Foamix Ltd. | Foamable vehicle and pharmaceutical compositions comprising aprotic polar solvents and uses thereof |
| WO2011121062A1 (en) | 2010-03-31 | 2011-10-06 | Helmut Vockner | Composition for the treatment of periodontitis and other inflammatory infectious diseases |
| WO2011154004A1 (en) * | 2010-06-11 | 2011-12-15 | Leo Pharma A/S | A pharmaceutical spray composition comprising a vitamin d analogue and a corticosteroid |
| EP2429517A1 (en) * | 2009-05-15 | 2012-03-21 | Biocepta Corporation | Compositions suitable for the topical treatment of fungal infections of the skin and nails |
| US20120070390A1 (en) * | 2010-04-21 | 2012-03-22 | Phillips D Howard | Topical drug delivery system with dual carriers |
| WO2012096839A1 (en) * | 2011-01-11 | 2012-07-19 | Jr Chem, Llc | Compositions for anorectal use and methods for treating anorectal disorders |
| US8343945B2 (en) | 2007-12-07 | 2013-01-01 | Foamix Ltd. | Carriers, formulations, methods for formulating unstable active agents for external application and uses thereof |
| US8357422B2 (en) | 2010-07-02 | 2013-01-22 | Rbc Life Sciences, Inc. | Dietary supplement |
| US8486376B2 (en) | 2002-10-25 | 2013-07-16 | Foamix Ltd. | Moisturizing foam containing lanolin |
| US8486374B2 (en) | 2003-08-04 | 2013-07-16 | Foamix Ltd. | Hydrophilic, non-aqueous pharmaceutical carriers and compositions and uses |
| US8507561B2 (en) | 2009-01-22 | 2013-08-13 | Absorption Pharmaceuticals, LLC | Desensitizing drug product |
| US8518376B2 (en) | 2007-12-07 | 2013-08-27 | Foamix Ltd. | Oil-based foamable carriers and formulations |
| US8586008B2 (en) | 2003-01-24 | 2013-11-19 | Stiefel West Coast, Llc | Pharmaceutical foam |
| US8633178B2 (en) | 2011-11-23 | 2014-01-21 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8636982B2 (en) | 2007-08-07 | 2014-01-28 | Foamix Ltd. | Wax foamable vehicle and pharmaceutical compositions thereof |
| US20140031366A1 (en) * | 2010-12-16 | 2014-01-30 | Board Of Regents, The University Of Texas System | Azole pharmaceutical formulations for parenteral administration and methods for preparing and using the same as treatment of diseases sensitive to azole compounds |
| US8709385B2 (en) | 2008-01-14 | 2014-04-29 | Foamix Ltd. | Poloxamer foamable pharmaceutical compositions with active agents and/or therapeutic cells and uses |
| US8722021B2 (en) | 2002-10-25 | 2014-05-13 | Foamix Ltd. | Foamable carriers |
| US8741265B2 (en) | 2002-10-25 | 2014-06-03 | Foamix Ltd. | Penetrating pharmaceutical foam |
| US8795693B2 (en) | 2003-08-04 | 2014-08-05 | Foamix Ltd. | Compositions with modulating agents |
| US8795635B2 (en) | 2006-11-14 | 2014-08-05 | Foamix Ltd. | Substantially non-aqueous foamable petrolatum based pharmaceutical and cosmetic compositions and their uses |
| US8808716B2 (en) | 2009-02-25 | 2014-08-19 | Stiefel Research Australia Pty Ltd | Topical foam composition |
| US8871184B2 (en) | 2009-10-02 | 2014-10-28 | Foamix Ltd. | Topical tetracycline compositions |
| US8933059B2 (en) | 2012-06-18 | 2015-01-13 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| WO2014028397A3 (en) * | 2012-08-13 | 2015-07-16 | Teva Pharmaceutical Industries Ltd. | Laquinimod for treatment of gaba mediated disorders |
| US9167813B2 (en) | 2009-07-29 | 2015-10-27 | Foamix Pharmaceuticals Ltd. | Non surfactant hydro-alcoholic foamable compositions, breakable foams and their uses |
| US9180091B2 (en) | 2012-12-21 | 2015-11-10 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| EP2954886A1 (en) * | 2014-06-12 | 2015-12-16 | Ceva Sante Animale | Pheromonal composite foam compositions |
| US9289382B2 (en) | 2012-06-18 | 2016-03-22 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US9427605B2 (en) | 2005-03-24 | 2016-08-30 | Novan, Inc. | Cosmetic treatment with nitric oxide, device for performing said treatment and manufacturing method therefor |
| US9526738B2 (en) | 2009-08-21 | 2016-12-27 | Novan, Inc. | Topical gels and methods of using the same |
| US9622947B2 (en) | 2002-10-25 | 2017-04-18 | Foamix Pharmaceuticals Ltd. | Foamable composition combining a polar solvent and a hydrophobic carrier |
| US9636405B2 (en) | 2003-08-04 | 2017-05-02 | Foamix Pharmaceuticals Ltd. | Foamable vehicle and pharmaceutical compositions thereof |
| US9668972B2 (en) | 2002-10-25 | 2017-06-06 | Foamix Pharmaceuticals Ltd. | Nonsteroidal immunomodulating kit and composition and uses thereof |
| US9757397B2 (en) | 2011-07-05 | 2017-09-12 | Novan, Inc. | Methods of manufacturing topical compositions and apparatus for the same |
| US9849142B2 (en) | 2009-10-02 | 2017-12-26 | Foamix Pharmaceuticals Ltd. | Methods for accelerated return of skin integrity and for the treatment of impetigo |
| US9855211B2 (en) | 2013-02-28 | 2018-01-02 | Novan, Inc. | Topical compositions and methods of using the same |
| US9919072B2 (en) | 2009-08-21 | 2018-03-20 | Novan, Inc. | Wound dressings, methods of using the same and methods of forming the same |
| US9931349B2 (en) | 2016-04-01 | 2018-04-03 | Therapeuticsmd, Inc. | Steroid hormone pharmaceutical composition |
| US10052386B2 (en) | 2012-06-18 | 2018-08-21 | Therapeuticsmd, Inc. | Progesterone formulations |
| US10064870B2 (en) | 2015-11-03 | 2018-09-04 | Zoetis Services Llc | Sol-gel polymer composites and uses thereof |
| US10098894B2 (en) | 2014-07-29 | 2018-10-16 | Therapeuticsmd, Inc. | Transdermal cream |
| CN108949296A (en) * | 2018-08-15 | 2018-12-07 | 江苏四新界面剂科技有限公司 | Monocrystalline silicon cutting surfaces activating agent |
| US10206932B2 (en) | 2014-05-22 | 2019-02-19 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10206947B2 (en) | 2013-08-08 | 2019-02-19 | Novan, Inc. | Topical compositions and methods of using the same |
| US10226483B2 (en) | 2013-08-08 | 2019-03-12 | Novan, Inc. | Topical compositions and methods of using the same |
| CN109453150A (en) * | 2011-02-11 | 2019-03-12 | 莫贝里制药公司 | New antifungal composition |
| US10258630B2 (en) | 2014-10-22 | 2019-04-16 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10265334B2 (en) | 2011-07-05 | 2019-04-23 | Novan, Inc. | Anhydrous compositions |
| US10286077B2 (en) | 2016-04-01 | 2019-05-14 | Therapeuticsmd, Inc. | Steroid hormone compositions in medium chain oils |
| US10322082B2 (en) | 2014-07-11 | 2019-06-18 | Novan, Inc. | Topical antiviral compositions and methods of using the same |
| US10322085B2 (en) | 2002-10-25 | 2019-06-18 | Foamix Pharmaceuticals Ltd. | Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof |
| US10322081B2 (en) | 2014-07-11 | 2019-06-18 | Novan, Inc. | Topical antiviral compositions and methods of using the same |
| US10328087B2 (en) | 2015-07-23 | 2019-06-25 | Therapeuticsmd, Inc. | Formulations for solubilizing hormones |
| US10350166B2 (en) | 2009-07-29 | 2019-07-16 | Foamix Pharmaceuticals Ltd. | Non surface active agent non polymeric agent hydro-alcoholic foamable compositions, breakable foams and their uses |
| US10398641B2 (en) | 2016-09-08 | 2019-09-03 | Foamix Pharmaceuticals Ltd. | Compositions and methods for treating rosacea and acne |
| WO2019175290A1 (en) | 2018-03-13 | 2019-09-19 | Beckley Canopy Therapeutics Limited | Cannabis or cannabis derived compositions for promoting cessation of chemical dependence |
| US10471148B2 (en) | 2012-06-18 | 2019-11-12 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable PK profile |
| US10471072B2 (en) | 2012-12-21 | 2019-11-12 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10537581B2 (en) | 2012-12-21 | 2020-01-21 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10548890B2 (en) | 2011-04-28 | 2020-02-04 | Platform Brightworks Two, Ltd. | Parenteral formulations of lipophilic pharmaceutical agents and methods for preparing and using the same |
| WO2020039073A1 (en) | 2018-08-24 | 2020-02-27 | UNION therapeutics A/S | Halogenated salicylanilides for the treatment of dermatitis |
| WO2020089470A1 (en) | 2018-11-02 | 2020-05-07 | UNION therapeutics A/S | Halogenated salicylanilides for treating the symptoms of dermatitis |
| WO2020089467A1 (en) | 2018-11-02 | 2020-05-07 | UNION therapeutics A/S | Dosage regimen |
| US10716305B2 (en) | 2015-01-23 | 2020-07-21 | Biocidium Biopharmaceuticals Inc. | Anti-bacterial compositions |
| US10806740B2 (en) | 2012-06-18 | 2020-10-20 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10821077B2 (en) | 2002-10-25 | 2020-11-03 | Foamix Pharmaceuticals Ltd. | Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof |
| US10849864B2 (en) | 2015-07-28 | 2020-12-01 | Novan, Inc. | Combinations and methods for the treatment and/or prevention of fungal infections |
| US10874108B2 (en) | 2016-05-04 | 2020-12-29 | 5d Health Protection Group Ltd. | Anti-microbial compositions |
| US10912743B2 (en) | 2016-03-02 | 2021-02-09 | Novan, Inc. | Compositions for treating inflammation and methods of treating the same |
| US10925689B2 (en) | 2014-07-14 | 2021-02-23 | Novan, Inc. | Nitric oxide releasing nail coating compositions, nitric oxide releasing nail coatings, and methods of using the same |
| US11077194B2 (en) | 2012-03-14 | 2021-08-03 | Novan, Inc. | Nitric oxide releasing pharmaceutical compositions |
| US11166980B2 (en) | 2016-04-13 | 2021-11-09 | Novan, Inc. | Compositions, systems, kits, and methods for treating an infection |
| RU2759587C2 (en) * | 2016-10-18 | 2021-11-15 | Филип Моррис Продактс С.А. | Ready-made composition for evaporation of electronic vaping device and electronic vaping device |
| RU2762342C2 (en) * | 2016-10-18 | 2021-12-20 | Филип Моррис Продактс С.А. | Ready-made composition for evaporation of electronic vaping device and electronic vaping device with such a composition |
| US11213587B2 (en) | 2010-11-22 | 2022-01-04 | Bausch Health Ireland Limited | Pharmaceutical formulations containing corticosteroids for topical administration |
| US11246875B2 (en) | 2012-12-21 | 2022-02-15 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11266661B2 (en) | 2012-12-21 | 2022-03-08 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11285171B2 (en) | 2018-03-01 | 2022-03-29 | Novan, Inc. | Nitric oxide releasing suppositories and methods of use thereof |
| US11648256B2 (en) | 2015-06-18 | 2023-05-16 | Bausch Health Ireland Limited | Topical compositions and methods for treating psoriasis |
| US11839656B2 (en) | 2010-11-22 | 2023-12-12 | Bausch Health Ireland Limited | Pharmaceutical formulations containing corticosteroids for topical administration |
| DE102022116721A1 (en) | 2022-07-05 | 2024-01-11 | Paul Hartmann Ag | Absorbent incontinence article as a disposable disposable product and method for producing the incontinence article |
| US11957753B2 (en) | 2018-04-30 | 2024-04-16 | Bausch Health Ireland Limited | Pharmaceutical formulations containing corticosteroids for topical administration |
Families Citing this family (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8512718B2 (en) | 2000-07-03 | 2013-08-20 | Foamix Ltd. | Pharmaceutical composition for topical application |
| US20080138296A1 (en) | 2002-10-25 | 2008-06-12 | Foamix Ltd. | Foam prepared from nanoemulsions and uses |
| US9211259B2 (en) | 2002-11-29 | 2015-12-15 | Foamix Pharmaceuticals Ltd. | Antibiotic kit and composition and uses thereof |
| US8900554B2 (en) | 2002-10-25 | 2014-12-02 | Foamix Pharmaceuticals Ltd. | Foamable composition and uses thereof |
| US7820145B2 (en) | 2003-08-04 | 2010-10-26 | Foamix Ltd. | Oleaginous pharmaceutical and cosmetic foam |
| US7575739B2 (en) | 2003-04-28 | 2009-08-18 | Foamix Ltd. | Foamable iodine composition |
| US9439857B2 (en) | 2007-11-30 | 2016-09-13 | Foamix Pharmaceuticals Ltd. | Foam containing benzoyl peroxide |
| CN105424884B (en) * | 2015-11-18 | 2017-04-05 | 中国海洋石油总公司 | A kind of detection method of poly- table binary composite oil-displacing system concentration of component |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB933486A (en) * | 1960-10-26 | 1963-08-08 | Vantorex Ltd | Improvements in or relating to aerosol foams |
| WO1991011991A1 (en) * | 1990-02-09 | 1991-08-22 | Kabi Pharmacia Ab | Foamable composition for pharmaceutical use, use thereof and method of treatment |
| US5536743A (en) * | 1988-01-15 | 1996-07-16 | Curatek Pharmaceuticals Limited Partnership | Intravaginal treatment of vaginal infections with buffered metronidazole compositions |
| WO2008075207A2 (en) * | 2006-04-04 | 2008-06-26 | Foamix Ltd. | Anti-infection augmentation foamable compositions and kit and uses thereof |
-
2007
- 2007-11-29 EP EP07874548A patent/EP2097065A2/en not_active Withdrawn
- 2007-11-29 AU AU2007355106A patent/AU2007355106A1/en not_active Abandoned
- 2007-11-29 WO PCT/IB2007/004628 patent/WO2008152444A2/en active Application Filing
-
2009
- 2009-05-11 IL IL198688A patent/IL198688A/en not_active IP Right Cessation
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB933486A (en) * | 1960-10-26 | 1963-08-08 | Vantorex Ltd | Improvements in or relating to aerosol foams |
| US5536743A (en) * | 1988-01-15 | 1996-07-16 | Curatek Pharmaceuticals Limited Partnership | Intravaginal treatment of vaginal infections with buffered metronidazole compositions |
| WO1991011991A1 (en) * | 1990-02-09 | 1991-08-22 | Kabi Pharmacia Ab | Foamable composition for pharmaceutical use, use thereof and method of treatment |
| WO2008075207A2 (en) * | 2006-04-04 | 2008-06-26 | Foamix Ltd. | Anti-infection augmentation foamable compositions and kit and uses thereof |
Cited By (189)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8486376B2 (en) | 2002-10-25 | 2013-07-16 | Foamix Ltd. | Moisturizing foam containing lanolin |
| US10322085B2 (en) | 2002-10-25 | 2019-06-18 | Foamix Pharmaceuticals Ltd. | Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof |
| US10117812B2 (en) | 2002-10-25 | 2018-11-06 | Foamix Pharmaceuticals Ltd. | Foamable composition combining a polar solvent and a hydrophobic carrier |
| US9622947B2 (en) | 2002-10-25 | 2017-04-18 | Foamix Pharmaceuticals Ltd. | Foamable composition combining a polar solvent and a hydrophobic carrier |
| US8741265B2 (en) | 2002-10-25 | 2014-06-03 | Foamix Ltd. | Penetrating pharmaceutical foam |
| US8722021B2 (en) | 2002-10-25 | 2014-05-13 | Foamix Ltd. | Foamable carriers |
| US9713643B2 (en) | 2002-10-25 | 2017-07-25 | Foamix Pharmaceuticals Ltd. | Foamable carriers |
| US11033491B2 (en) | 2002-10-25 | 2021-06-15 | Vyne Therapeutics Inc. | Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof |
| US9668972B2 (en) | 2002-10-25 | 2017-06-06 | Foamix Pharmaceuticals Ltd. | Nonsteroidal immunomodulating kit and composition and uses thereof |
| US10821077B2 (en) | 2002-10-25 | 2020-11-03 | Foamix Pharmaceuticals Ltd. | Dicarboxylic acid foamable vehicle and pharmaceutical compositions thereof |
| US8586008B2 (en) | 2003-01-24 | 2013-11-19 | Stiefel West Coast, Llc | Pharmaceutical foam |
| US9486394B2 (en) | 2003-01-24 | 2016-11-08 | Stiefel West Coast, Llc | Pharmaceutical foam |
| US8486374B2 (en) | 2003-08-04 | 2013-07-16 | Foamix Ltd. | Hydrophilic, non-aqueous pharmaceutical carriers and compositions and uses |
| US9636405B2 (en) | 2003-08-04 | 2017-05-02 | Foamix Pharmaceuticals Ltd. | Foamable vehicle and pharmaceutical compositions thereof |
| US8795693B2 (en) | 2003-08-04 | 2014-08-05 | Foamix Ltd. | Compositions with modulating agents |
| US9427605B2 (en) | 2005-03-24 | 2016-08-30 | Novan, Inc. | Cosmetic treatment with nitric oxide, device for performing said treatment and manufacturing method therefor |
| US9682021B2 (en) | 2006-11-14 | 2017-06-20 | Foamix Pharmaceuticals Ltd. | Substantially non-aqueous foamable petrolatum based pharmaceutical and cosmetic compositions and their uses |
| US8795635B2 (en) | 2006-11-14 | 2014-08-05 | Foamix Ltd. | Substantially non-aqueous foamable petrolatum based pharmaceutical and cosmetic compositions and their uses |
| US11103454B2 (en) | 2007-08-07 | 2021-08-31 | Vyne Therapeutics Inc. | Wax foamable vehicle and pharmaceutical compositions thereof |
| US10369102B2 (en) | 2007-08-07 | 2019-08-06 | Foamix Pharmaceuticals Ltd. | Wax foamable vehicle and pharmaceutical compositions thereof |
| US8636982B2 (en) | 2007-08-07 | 2014-01-28 | Foamix Ltd. | Wax foamable vehicle and pharmaceutical compositions thereof |
| US9662298B2 (en) | 2007-08-07 | 2017-05-30 | Foamix Pharmaceuticals Ltd. | Wax foamable vehicle and pharmaceutical compositions thereof |
| US9549898B2 (en) | 2007-12-07 | 2017-01-24 | Foamix Pharmaceuticals Ltd. | Oil and liquid silicone foamable carriers and formulations |
| US8343945B2 (en) | 2007-12-07 | 2013-01-01 | Foamix Ltd. | Carriers, formulations, methods for formulating unstable active agents for external application and uses thereof |
| US9795564B2 (en) | 2007-12-07 | 2017-10-24 | Foamix Pharmaceuticals Ltd. | Oil-based foamable carriers and formulations |
| US11433025B2 (en) | 2007-12-07 | 2022-09-06 | Vyne Therapeutics Inc. | Oil foamable carriers and formulations |
| US8518376B2 (en) | 2007-12-07 | 2013-08-27 | Foamix Ltd. | Oil-based foamable carriers and formulations |
| US8900553B2 (en) | 2007-12-07 | 2014-12-02 | Foamix Pharmaceuticals Ltd. | Oil and liquid silicone foamable carriers and formulations |
| US8709385B2 (en) | 2008-01-14 | 2014-04-29 | Foamix Ltd. | Poloxamer foamable pharmaceutical compositions with active agents and/or therapeutic cells and uses |
| US8637577B2 (en) | 2009-01-22 | 2014-01-28 | Absorption Pharmaceuticals, LLC | Desensitizing drug product |
| US8563616B2 (en) | 2009-01-22 | 2013-10-22 | Absorption Pharmaceuticals, LLC | Desensitizing drug product |
| US8507561B2 (en) | 2009-01-22 | 2013-08-13 | Absorption Pharmaceuticals, LLC | Desensitizing drug product |
| US8808716B2 (en) | 2009-02-25 | 2014-08-19 | Stiefel Research Australia Pty Ltd | Topical foam composition |
| US10688071B2 (en) | 2009-02-25 | 2020-06-23 | Mayne Pharma Llc | Topical foam composition |
| US10568859B2 (en) | 2009-02-25 | 2020-02-25 | Mayne Pharma Llc | Topical foam composition |
| US10363216B2 (en) | 2009-04-28 | 2019-07-30 | Foamix Pharmaceuticals Ltd. | Foamable vehicles and pharmaceutical compositions comprising aprotic polar solvents and uses thereof |
| US10213384B2 (en) | 2009-04-28 | 2019-02-26 | Foamix Pharmaceuticals Ltd. | Foamable vehicles and pharmaceutical compositions comprising aprotic polar solvents and uses thereof |
| US9884017B2 (en) | 2009-04-28 | 2018-02-06 | Foamix Pharmaceuticals Ltd. | Foamable vehicles and pharmaceutical compositions comprising aprotic polar solvents and uses thereof |
| WO2010125470A3 (en) * | 2009-04-28 | 2011-06-16 | Foamix Ltd. | Foamable vehicle and pharmaceutical compositions comprising aprotic polar solvents and uses thereof |
| US10588858B2 (en) | 2009-04-28 | 2020-03-17 | Foamix Pharmaceuticals Ltd. | Foamable vehicles and pharmaceutical compositions comprising aprotic polar solvents and uses thereof |
| EP2429517A1 (en) * | 2009-05-15 | 2012-03-21 | Biocepta Corporation | Compositions suitable for the topical treatment of fungal infections of the skin and nails |
| CN102427811A (en) * | 2009-05-15 | 2012-04-25 | 碧奥塞普达公司 | Compositions suitable for the topical treatment of fungal infections of the skin and nails |
| EP2429517A4 (en) * | 2009-05-15 | 2013-03-27 | Biocepta Corp | Compositions suitable for the topical treatment of fungal infections of the skin and nails |
| US9167813B2 (en) | 2009-07-29 | 2015-10-27 | Foamix Pharmaceuticals Ltd. | Non surfactant hydro-alcoholic foamable compositions, breakable foams and their uses |
| US10092588B2 (en) | 2009-07-29 | 2018-10-09 | Foamix Pharmaceuticals Ltd. | Foamable compositions, breakable foams and their uses |
| US11219631B2 (en) | 2009-07-29 | 2022-01-11 | Vyne Pharmaceuticals Inc. | Foamable compositions, breakable foams and their uses |
| US10350166B2 (en) | 2009-07-29 | 2019-07-16 | Foamix Pharmaceuticals Ltd. | Non surface active agent non polymeric agent hydro-alcoholic foamable compositions, breakable foams and their uses |
| US9919072B2 (en) | 2009-08-21 | 2018-03-20 | Novan, Inc. | Wound dressings, methods of using the same and methods of forming the same |
| US11583608B2 (en) | 2009-08-21 | 2023-02-21 | Novan, Inc. | Wound dressings, methods of using the same and methods of forming the same |
| US9526738B2 (en) | 2009-08-21 | 2016-12-27 | Novan, Inc. | Topical gels and methods of using the same |
| US9737561B2 (en) | 2009-08-21 | 2017-08-22 | Novan, Inc. | Topical gels and methods of using the same |
| US10376538B2 (en) | 2009-08-21 | 2019-08-13 | Novan, Inc. | Topical gels and methods of using the same |
| US10029013B2 (en) | 2009-10-02 | 2018-07-24 | Foamix Pharmaceuticals Ltd. | Surfactant-free, water-free formable composition and breakable foams and their uses |
| US10517882B2 (en) | 2009-10-02 | 2019-12-31 | Foamix Pharmaceuticals Ltd. | Method for healing of an infected acne lesion without scarring |
| US10265404B2 (en) | 2009-10-02 | 2019-04-23 | Foamix Pharmaceuticals Ltd. | Compositions, gels and foams with rheology modulators and uses thereof |
| EP2482788B1 (en) * | 2009-10-02 | 2022-12-14 | Journey Medical Corporation | Topical tetracycline compositions |
| US10238746B2 (en) | 2009-10-02 | 2019-03-26 | Foamix Pharmaceuticals Ltd | Surfactant-free water-free foamable compositions, breakable foams and gels and their uses |
| US9675700B2 (en) | 2009-10-02 | 2017-06-13 | Foamix Pharmaceuticals Ltd. | Topical tetracycline compositions |
| US10213512B2 (en) | 2009-10-02 | 2019-02-26 | Foamix Pharmaceuticals Ltd. | Topical tetracycline compositions |
| US10967063B2 (en) | 2009-10-02 | 2021-04-06 | Vyne Therapeutics Inc. | Surfactant-free, water-free formable composition and breakable foams and their uses |
| US10946101B2 (en) | 2009-10-02 | 2021-03-16 | Vyne Therapeutics Inc. | Surfactant-free water-free foamable compositions, breakable foams and gels and their uses |
| US10463742B2 (en) | 2009-10-02 | 2019-11-05 | Foamix Pharmaceuticals Ltd. | Topical tetracycline compositions |
| US10137200B2 (en) | 2009-10-02 | 2018-11-27 | Foamix Pharmaceuticals Ltd. | Surfactant-free water-free foamable compositions, breakable foams and gels and their uses |
| US9849142B2 (en) | 2009-10-02 | 2017-12-26 | Foamix Pharmaceuticals Ltd. | Methods for accelerated return of skin integrity and for the treatment of impetigo |
| US10610599B2 (en) | 2009-10-02 | 2020-04-07 | Foamix Pharmaceuticals Ltd. | Topical tetracycline compositions |
| US8871184B2 (en) | 2009-10-02 | 2014-10-28 | Foamix Ltd. | Topical tetracycline compositions |
| US10086080B2 (en) | 2009-10-02 | 2018-10-02 | Foamix Pharmaceuticals Ltd. | Topical tetracycline compositions |
| US10821187B2 (en) | 2009-10-02 | 2020-11-03 | Foamix Pharmaceuticals Ltd. | Compositions, gels and foams with rheology modulators and uses thereof |
| US10322186B2 (en) | 2009-10-02 | 2019-06-18 | Foamix Pharmaceuticals Ltd. | Topical tetracycline compositions |
| US10835613B2 (en) | 2009-10-02 | 2020-11-17 | Foamix Pharmaceuticals Ltd. | Compositions, gels and foams with rheology modulators and uses thereof |
| WO2011121062A1 (en) | 2010-03-31 | 2011-10-06 | Helmut Vockner | Composition for the treatment of periodontitis and other inflammatory infectious diseases |
| US20120070390A1 (en) * | 2010-04-21 | 2012-03-22 | Phillips D Howard | Topical drug delivery system with dual carriers |
| US10716799B2 (en) | 2010-06-11 | 2020-07-21 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamin D analogue and a corticosteroid |
| US20170065618A1 (en) * | 2010-06-11 | 2017-03-09 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamin d analogue and a corticosteroid |
| WO2011154004A1 (en) * | 2010-06-11 | 2011-12-15 | Leo Pharma A/S | A pharmaceutical spray composition comprising a vitamin d analogue and a corticosteroid |
| US10130640B2 (en) | 2010-06-11 | 2018-11-20 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamin D analogue and a corticosteroid |
| US10660908B2 (en) | 2010-06-11 | 2020-05-26 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamin D analogue and a corticosteroid |
| US10617698B2 (en) | 2010-06-11 | 2020-04-14 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamind D analogue and a corticosteroid |
| US9119781B2 (en) | 2010-06-11 | 2015-09-01 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamin D analogue and a corticosteroid |
| US10688108B2 (en) | 2010-06-11 | 2020-06-23 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamin D analogue and a corticosteroid |
| EP2859886A1 (en) * | 2010-06-11 | 2015-04-15 | Leo Pharma A/S | A pharmaceutical spray composition |
| RU2576607C2 (en) * | 2010-06-11 | 2016-03-10 | Лео Фарма А/С | Pharmaceutical aerosol formulation containing vitamin d analogue and corticosteroid |
| US20130123720A1 (en) * | 2010-06-11 | 2013-05-16 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamin d analogue and a corticosteroid |
| US9566286B2 (en) | 2010-06-11 | 2017-02-14 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamin D analogue and a corticosteroid |
| US10682364B2 (en) | 2010-06-11 | 2020-06-16 | Leo Pharma A/S | Pharmaceutical spray composition comprising a vitamind D analogue and a corticosteroid |
| US8357422B2 (en) | 2010-07-02 | 2013-01-22 | Rbc Life Sciences, Inc. | Dietary supplement |
| US11839656B2 (en) | 2010-11-22 | 2023-12-12 | Bausch Health Ireland Limited | Pharmaceutical formulations containing corticosteroids for topical administration |
| US11213587B2 (en) | 2010-11-22 | 2022-01-04 | Bausch Health Ireland Limited | Pharmaceutical formulations containing corticosteroids for topical administration |
| US20140031366A1 (en) * | 2010-12-16 | 2014-01-30 | Board Of Regents, The University Of Texas System | Azole pharmaceutical formulations for parenteral administration and methods for preparing and using the same as treatment of diseases sensitive to azole compounds |
| US10307418B2 (en) * | 2010-12-16 | 2019-06-04 | Platform Brightworks Two, Ltd. | Azole pharmaceutical formulations for parenteral administration and methods for preparing and using the same as treatment of diseases sensitive to azole compounds |
| WO2012096839A1 (en) * | 2011-01-11 | 2012-07-19 | Jr Chem, Llc | Compositions for anorectal use and methods for treating anorectal disorders |
| US8952057B2 (en) | 2011-01-11 | 2015-02-10 | Jr Chem, Llc | Compositions for anorectal use and methods for treating anorectal disorders |
| CN109453150A (en) * | 2011-02-11 | 2019-03-12 | 莫贝里制药公司 | New antifungal composition |
| US10548890B2 (en) | 2011-04-28 | 2020-02-04 | Platform Brightworks Two, Ltd. | Parenteral formulations of lipophilic pharmaceutical agents and methods for preparing and using the same |
| US11045466B2 (en) | 2011-04-28 | 2021-06-29 | Platform Brightworks Two, Ltd. | Parenteral formulations of lipophilic pharmaceutical agents and methods for preparing and using the same |
| US10500220B2 (en) | 2011-07-05 | 2019-12-10 | Novan, Inc. | Topical compositions |
| US10265334B2 (en) | 2011-07-05 | 2019-04-23 | Novan, Inc. | Anhydrous compositions |
| US9757397B2 (en) | 2011-07-05 | 2017-09-12 | Novan, Inc. | Methods of manufacturing topical compositions and apparatus for the same |
| US8987237B2 (en) | 2011-11-23 | 2015-03-24 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8633178B2 (en) | 2011-11-23 | 2014-01-21 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8846648B2 (en) | 2011-11-23 | 2014-09-30 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8846649B2 (en) | 2011-11-23 | 2014-09-30 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10675288B2 (en) | 2011-11-23 | 2020-06-09 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11793819B2 (en) | 2011-11-23 | 2023-10-24 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US9248136B2 (en) | 2011-11-23 | 2016-02-02 | Therapeuticsmd, Inc. | Transdermal hormone replacement therapies |
| US11103516B2 (en) | 2011-11-23 | 2021-08-31 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11077194B2 (en) | 2012-03-14 | 2021-08-03 | Novan, Inc. | Nitric oxide releasing pharmaceutical compositions |
| US10052386B2 (en) | 2012-06-18 | 2018-08-21 | Therapeuticsmd, Inc. | Progesterone formulations |
| US11865179B2 (en) | 2012-06-18 | 2024-01-09 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable PK profile |
| US11529360B2 (en) | 2012-06-18 | 2022-12-20 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8987238B2 (en) | 2012-06-18 | 2015-03-24 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11110099B2 (en) | 2012-06-18 | 2021-09-07 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US9006222B2 (en) | 2012-06-18 | 2015-04-14 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10806740B2 (en) | 2012-06-18 | 2020-10-20 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US9289382B2 (en) | 2012-06-18 | 2016-03-22 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11166963B2 (en) | 2012-06-18 | 2021-11-09 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11033626B2 (en) | 2012-06-18 | 2021-06-15 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable pk profile |
| US10639375B2 (en) | 2012-06-18 | 2020-05-05 | Therapeuticsmd, Inc. | Progesterone formulations |
| US9012434B2 (en) | 2012-06-18 | 2015-04-21 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8933059B2 (en) | 2012-06-18 | 2015-01-13 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US9301920B2 (en) | 2012-06-18 | 2016-04-05 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10471148B2 (en) | 2012-06-18 | 2019-11-12 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable PK profile |
| WO2014028397A3 (en) * | 2012-08-13 | 2015-07-16 | Teva Pharmaceutical Industries Ltd. | Laquinimod for treatment of gaba mediated disorders |
| US10537581B2 (en) | 2012-12-21 | 2020-01-21 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11304959B2 (en) | 2012-12-21 | 2022-04-19 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11622933B2 (en) | 2012-12-21 | 2023-04-11 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US9180091B2 (en) | 2012-12-21 | 2015-11-10 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US11246875B2 (en) | 2012-12-21 | 2022-02-15 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11266661B2 (en) | 2012-12-21 | 2022-03-08 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11497709B2 (en) | 2012-12-21 | 2022-11-15 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10806697B2 (en) | 2012-12-21 | 2020-10-20 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11351182B2 (en) | 2012-12-21 | 2022-06-07 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10568891B2 (en) | 2012-12-21 | 2020-02-25 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11065197B2 (en) | 2012-12-21 | 2021-07-20 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US10471072B2 (en) | 2012-12-21 | 2019-11-12 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10835487B2 (en) | 2012-12-21 | 2020-11-17 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11241445B2 (en) | 2012-12-21 | 2022-02-08 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11123283B2 (en) | 2012-12-21 | 2021-09-21 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US11116717B2 (en) | 2012-12-21 | 2021-09-14 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US10888516B2 (en) | 2012-12-21 | 2021-01-12 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US11285098B2 (en) | 2013-02-28 | 2022-03-29 | Novan, Inc. | Topical compositions and methods of using the same |
| US9855211B2 (en) | 2013-02-28 | 2018-01-02 | Novan, Inc. | Topical compositions and methods of using the same |
| US10258564B2 (en) | 2013-02-28 | 2019-04-16 | Novan, Inc. | Topical compositions and methods of using the same |
| US11813284B2 (en) | 2013-08-08 | 2023-11-14 | Novan, Inc. | Topical compositions and methods of using the same |
| US10828323B2 (en) | 2013-08-08 | 2020-11-10 | Novan, Inc. | Topical compositions and methods of using the same |
| US10206947B2 (en) | 2013-08-08 | 2019-02-19 | Novan, Inc. | Topical compositions and methods of using the same |
| US10226483B2 (en) | 2013-08-08 | 2019-03-12 | Novan, Inc. | Topical compositions and methods of using the same |
| US11103513B2 (en) | 2014-05-22 | 2021-08-31 | TherapeuticsMD | Natural combination hormone replacement formulations and therapies |
| US10206932B2 (en) | 2014-05-22 | 2019-02-19 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| EP2954886A1 (en) * | 2014-06-12 | 2015-12-16 | Ceva Sante Animale | Pheromonal composite foam compositions |
| US10322081B2 (en) | 2014-07-11 | 2019-06-18 | Novan, Inc. | Topical antiviral compositions and methods of using the same |
| US11040006B2 (en) | 2014-07-11 | 2021-06-22 | Novan, Inc. | Topical antiviral compositions, delivery systems, and methods of using the same |
| US10322082B2 (en) | 2014-07-11 | 2019-06-18 | Novan, Inc. | Topical antiviral compositions and methods of using the same |
| US10736839B2 (en) | 2014-07-11 | 2020-08-11 | Novan, Inc. | Topical antiviral compositions, delivery systems, and methods of using the same |
| US11723858B2 (en) | 2014-07-11 | 2023-08-15 | Novan, Inc. | Topical antiviral compositions, delivery systems, and methods of using the same |
| US10925689B2 (en) | 2014-07-14 | 2021-02-23 | Novan, Inc. | Nitric oxide releasing nail coating compositions, nitric oxide releasing nail coatings, and methods of using the same |
| US10098894B2 (en) | 2014-07-29 | 2018-10-16 | Therapeuticsmd, Inc. | Transdermal cream |
| US10398708B2 (en) | 2014-10-22 | 2019-09-03 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10258630B2 (en) | 2014-10-22 | 2019-04-16 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10668082B2 (en) | 2014-10-22 | 2020-06-02 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10716305B2 (en) | 2015-01-23 | 2020-07-21 | Biocidium Biopharmaceuticals Inc. | Anti-bacterial compositions |
| US11363814B2 (en) | 2015-01-23 | 2022-06-21 | Biocidium Ip Holdco, Co. | Anti-bacterial compositions |
| US11679115B2 (en) | 2015-06-18 | 2023-06-20 | Bausch Health Ireland Limited | Topical compositions and methods for treating psoriasis |
| US11679116B2 (en) | 2015-06-18 | 2023-06-20 | Bausch Health Ireland Limited | Topical compositions and methods for treating psoriasis |
| US11648256B2 (en) | 2015-06-18 | 2023-05-16 | Bausch Health Ireland Limited | Topical compositions and methods for treating psoriasis |
| US10328087B2 (en) | 2015-07-23 | 2019-06-25 | Therapeuticsmd, Inc. | Formulations for solubilizing hormones |
| US10912783B2 (en) | 2015-07-23 | 2021-02-09 | Therapeuticsmd, Inc. | Formulations for solubilizing hormones |
| US10849864B2 (en) | 2015-07-28 | 2020-12-01 | Novan, Inc. | Combinations and methods for the treatment and/or prevention of fungal infections |
| US10729698B2 (en) | 2015-11-03 | 2020-08-04 | Zoetis Services Llc | Sol-gel polymer composites and uses thereof |
| US10064870B2 (en) | 2015-11-03 | 2018-09-04 | Zoetis Services Llc | Sol-gel polymer composites and uses thereof |
| US10912743B2 (en) | 2016-03-02 | 2021-02-09 | Novan, Inc. | Compositions for treating inflammation and methods of treating the same |
| US10532059B2 (en) | 2016-04-01 | 2020-01-14 | Therapeuticsmd, Inc. | Steroid hormone pharmaceutical composition |
| US9931349B2 (en) | 2016-04-01 | 2018-04-03 | Therapeuticsmd, Inc. | Steroid hormone pharmaceutical composition |
| US10286077B2 (en) | 2016-04-01 | 2019-05-14 | Therapeuticsmd, Inc. | Steroid hormone compositions in medium chain oils |
| US11166980B2 (en) | 2016-04-13 | 2021-11-09 | Novan, Inc. | Compositions, systems, kits, and methods for treating an infection |
| US10874108B2 (en) | 2016-05-04 | 2020-12-29 | 5d Health Protection Group Ltd. | Anti-microbial compositions |
| US10398641B2 (en) | 2016-09-08 | 2019-09-03 | Foamix Pharmaceuticals Ltd. | Compositions and methods for treating rosacea and acne |
| US11324691B2 (en) | 2016-09-08 | 2022-05-10 | Journey Medical Corporation | Compositions and methods for treating rosacea and acne |
| US10849847B2 (en) | 2016-09-08 | 2020-12-01 | Foamix Pharamaceuticals Ltd. | Compositions and methods for treating rosacea and acne |
| RU2762342C2 (en) * | 2016-10-18 | 2021-12-20 | Филип Моррис Продактс С.А. | Ready-made composition for evaporation of electronic vaping device and electronic vaping device with such a composition |
| RU2759587C2 (en) * | 2016-10-18 | 2021-11-15 | Филип Моррис Продактс С.А. | Ready-made composition for evaporation of electronic vaping device and electronic vaping device |
| US11285171B2 (en) | 2018-03-01 | 2022-03-29 | Novan, Inc. | Nitric oxide releasing suppositories and methods of use thereof |
| WO2019175290A1 (en) | 2018-03-13 | 2019-09-19 | Beckley Canopy Therapeutics Limited | Cannabis or cannabis derived compositions for promoting cessation of chemical dependence |
| US11957753B2 (en) | 2018-04-30 | 2024-04-16 | Bausch Health Ireland Limited | Pharmaceutical formulations containing corticosteroids for topical administration |
| CN108949296A (en) * | 2018-08-15 | 2018-12-07 | 江苏四新界面剂科技有限公司 | Monocrystalline silicon cutting surfaces activating agent |
| WO2020039073A1 (en) | 2018-08-24 | 2020-02-27 | UNION therapeutics A/S | Halogenated salicylanilides for the treatment of dermatitis |
| WO2020089467A1 (en) | 2018-11-02 | 2020-05-07 | UNION therapeutics A/S | Dosage regimen |
| WO2020089470A1 (en) | 2018-11-02 | 2020-05-07 | UNION therapeutics A/S | Halogenated salicylanilides for treating the symptoms of dermatitis |
| DE102022116721A1 (en) | 2022-07-05 | 2024-01-11 | Paul Hartmann Ag | Absorbent incontinence article as a disposable disposable product and method for producing the incontinence article |
Also Published As
| Publication number | Publication date |
|---|---|
| IL198688A0 (en) | 2010-02-17 |
| AU2007355106A1 (en) | 2008-12-18 |
| WO2008152444A3 (en) | 2009-06-18 |
| EP2097065A2 (en) | 2009-09-09 |
| IL198688A (en) | 2015-06-30 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US9101662B2 (en) | Compositions with modulating agents | |
| US11433025B2 (en) | Oil foamable carriers and formulations | |
| EP2097065A2 (en) | Foamable waterless compositions with modulating agents | |
| US9636405B2 (en) | Foamable vehicle and pharmaceutical compositions thereof | |
| US10588858B2 (en) | Foamable vehicles and pharmaceutical compositions comprising aprotic polar solvents and uses thereof | |
| US20080069779A1 (en) | Foamable vehicle and vitamin and flavonoid pharmaceutical compositions thereof | |
| AU2006313443A1 (en) | Foamable vehicle and pharmaceutical compositions thereof | |
| DK2494959T3 (en) | Foam Bart dicarboxylsyrebærestof and pharmaceutical compositions thereof | |
| WO2008038140A2 (en) | Foamable vehicle comprising polypropylene glycol alkyl ether and pharmaceutical compositions thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007874548 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 198688 Country of ref document: IL |
|
| NENP | Non-entry into the national phase |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2007355106 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2403/KOLNP/2009 Country of ref document: IN |
|
| ENP | Entry into the national phase |
Ref document number: 2007355106 Country of ref document: AU Date of ref document: 20071129 Kind code of ref document: A |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07874548 Country of ref document: EP Kind code of ref document: A2 |