WO2007141635A2 - Detergents formulations with low impact on the environment - Google Patents
Detergents formulations with low impact on the environment Download PDFInfo
- Publication number
- WO2007141635A2 WO2007141635A2 PCT/IB2007/001505 IB2007001505W WO2007141635A2 WO 2007141635 A2 WO2007141635 A2 WO 2007141635A2 IB 2007001505 W IB2007001505 W IB 2007001505W WO 2007141635 A2 WO2007141635 A2 WO 2007141635A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sodium
- formulation
- dry
- acid
- components
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/26—Organic compounds containing nitrogen
- C11D3/33—Amino carboxylic acids
-
- C—CHEMISTRY; METALLURGY
- C11—ANIMAL OR VEGETABLE OILS, FATS, FATTY SUBSTANCES OR WAXES; FATTY ACIDS THEREFROM; DETERGENTS; CANDLES
- C11D—DETERGENT COMPOSITIONS; USE OF SINGLE SUBSTANCES AS DETERGENTS; SOAP OR SOAP-MAKING; RESIN SOAPS; RECOVERY OF GLYCEROL
- C11D3/00—Other compounding ingredients of detergent compositions covered in group C11D1/00
- C11D3/16—Organic compounds
- C11D3/20—Organic compounds containing oxygen
- C11D3/2075—Carboxylic acids-salts thereof
- C11D3/2086—Hydroxy carboxylic acids-salts thereof
Definitions
- the present invention relates to formulations of detergents for laundry, dishwashers, washing-up, hard surfaces cleaning, containing one or more surfactants, if necessary one or more electrolytes and one or more sequestering agents.
- the main features of these formulations concern toxicology and environmental toxicology, as well as cleaning efficacy.
- a detergent formulation is based on a surfactant, if necessary an electrolyte and a sequestering agent, or a set of them.
- a surfactant if necessary an electrolyte and a sequestering agent, or a set of them.
- EP1137752 (Unilever) which considers, among other components, either IDS or HIDS (0,05 - 1 ,5 % by weight) for laundry detergents without bleaching agents, aimed to preserve fabrics colours.
- IDS or HIDS (0,05 - 1 ,5 % by weight) for laundry detergents without bleaching agents, aimed to preserve fabrics colours.
- Two very important matters associated with household detergents are environmental impact and toxicological hazard.
- nonbiodegradable complexing agents are: EDTA and sodium salt (C.A.S. 60-00-4 and 64-02-8), phosphonates (ATMP - C.A.S. 6419-19- 8; EDTMP - C.A.S. 1429-50-1 ; HDTMP - C.A.S. 23605-74-5; DTPMP - C.A.S. 15827- 60-8; HEDP, etidronic acid - C.A.S. 2809-21-4) and polycarboxylated (acrylic acid omopolymers - C.A.S.
- EDTA and its salts are very little biodegradable: recently it was experienced a slow biodegradation at alkaline pH and without heavy metals, but these particular conditions are likely neither in surface waters nor in activated sludge plants (European Union - Risk Assessment Report Vol.49: ⁇ detic acid (EDTA)", 2004).
- Polycarboxylated are very little biodegradable aerobically and aren't at all anaerobically; in sewage treatment plants, they are adsorbed upon sludges (Danish EPA: “Environmental and Health Assessment of Substances in Household and Cosmetic Detergent products", 100-103, 615-2001).
- Phosphonates are very little biodegradable aerobically (HERA - Human & Environmental Risk Assessment on ingredients of European household cleaning products: “Phosphonates", 2004) and almost nonbiodegradable anaerobically (Danish EPA: “Environmental and Health Assessment of Substances in Household and Cosmetic Detergent products", 96-100, 615-2001); in particular conditions (catalysis made by some transition metals ions, at specific pH ranges) they're photodegradable; nevertheless, in real environments photodegradation is prevented by other physico- chemical mechanisms, such as low exposition to sun light (for example: muddy or deep waters) and, above all, absorbance in soil and sediments, which is reversible only after treatment either with strong acids or with more concentrated salted solutions. These are all conditions very far from real conditions (FISCHER K: "Distribution and elimination of HEDP in aquatic test systems", WATER RES; 27 (3). 1993. 485-493).
- zeolites C AS. 1318-02-1
- Zeolites are water insoluble aluminosilicates which act as ionic exchangers.
- Zeolites precipitate onto rivers bottom and in some cases cover it at all; they create problems in sewage and wastewater plants too, because they raise sludges to be wasted at the end of the cycle.
- Antiredeposition agents generally contained in detergents behave in various ways: some of them (acrylic polymers and acrylic-maleic copolymers) have low acute toxicity, but are very slowly biodegradable; instead, carboxymethylcellulose (CMC, C.A.S. 9004- 32-4) is readily biodegradable, but last studies (RTECS database) testify carcinogenicity (subcutaneous route) and mutagenicity in reproductive system (oral route) in rats at relatively low dosages (140 mg/Kg).
- CMC carboxymethylcellulose
- Preservatives and perfumes have heavy outcomes both on the environment and human health : generally both kind of chemicals are very slowly biodegradable, very toxic on aquatic organisms and sensitizing.
- 5-chloro-2-methyl-2H- isothiazolin-3-one and 2-methyl-2H-isothiazolin-3-one are mutagens according to the Ames test;
- 2-Bromo-2-nitropropane-1 ,3-diol (Bronopol) is quite biodegradable, but byproducts are carcinogens (nitrosamines).
- Fragrances may contain sensitizing or allergizing chemicals, even if in small concentrations; such chemicals are often toxic or very toxic for aquatic organisms.
- sensitizing or allergizing chemicals such chemicals are often toxic or very toxic for aquatic organisms.
- limonene [(r)-p-mentha-1 ,8-diene, C.A.S. 5989-27-5; labelling: Xi, N R10-38-
- dishwasher “ecologic” detergents contain polyacrylates or polyacrylates- sulphonates and phosphonates, sometimes CMC too; laundry “ecologic” detergents contain phosphonates, polyacrylates, zeolite, acrylic-maleic copolymers, silycone- paraffine mixture, optical brightener, CMC; in multipurpose “ecologic” detergents are present phosphonates, polycarboxylates, non biodegradable preservatives (methylisothiazolinone) and allergizing fragrances (limonene). Such components are present in relatively low concentrations, but these low values must be parametrized to the large quantities poured in water every day and their tendency to accumulate.
- the present invention is aimed to detergents formulations with low impact on environment and human health, together with good cleaning efficacy.
- Formulations here described are preferably water based and can be liquid or gel/paste shaped. They include at least one surfactant, or a collection of surfactants, if needed an electrolyte and at least a sequestering agent, chosen into the following group: iminodisuccinic acid tetrasodium salt (IDS), N,N-diacetic glutamic acid tetrasodium salt (GLDA), sodium glucoheptonate, sodium gluconate.
- IDS iminodisuccinic acid tetrasodium salt
- GLDA N,N-diacetic glutamic acid tetrasodium salt
- sodium glucoheptonate sodium gluconate.
- the surfactants used may belong to 3 categories: anionic, non-ionic and amphoteric/zwitterionic.
- Sequestering agents / Builders In detergency, a sequestering agent acts mainly as a calcium ad magnesium lime controller, in order to avoid lime to precipitate upon clothes and hard surfaces both as carbonates and as insoluble fatty acids esters. Moreover, they complex heavy metals ions (Fe 3+ , Cu 2+ , etc.), which may create coloured stains upon clothes and surfaces.
- formulations contain all rapidly biodegradable sequestering agents with low toxicological impact, chosen among the following:
- IDS iminodisuccinic acid tetrasodium salt
- N,N-diacetic glutamic acid tetrasodium salt having the following molecular formula (II):
- sequestering agents could be accompanied by the use of a builder chosen among alkali metal orthophosphate, diphosphate and triphosphate.
- Phosphates if used in very small quantities, give a little contribute to eutrophication, with regard to detergents used in the past or to current other uses (such as agricultural ones). Instead, using few quantities may allow to avoid to use more pollutant chemicals.
- the described above sequestering agents may be matched with an additional sequestering/antiredeposition agent selected from the group consisting of citric acid, citric acid tri alkali metals salt, polyaspartate, alkali metals orthophosphate, diphosphate and/or triphosphate and mixture thereof.
- formulations may be present other chemicals with the same toxycological and eco-toxycological features described above, for example the following: solvents (short chain alcohols, esters, etc.), thickening agents, antiredeposition agents, perfumes, colouring agents, preservatives, antibacterial agents, bleaching agents with or without bleach activators, scrubbing agents, bittering agents, etc..
- solvents short chain alcohols, esters, etc.
- thickening agents for example the following: thickening agents, antiredeposition agents, perfumes, colouring agents, preservatives, antibacterial agents, bleaching agents with or without bleach activators, scrubbing agents, bittering agents, etc.
- other components is generally used referring to those components, but sometimes the formulations report some specific chemicals selected from this group.
- EXAMPLE 1 detergent for laundry machine or washing clothes by hand; particularly, formulation E is preferably used for delicate laundry wash.
- formulation E it is particularly preferred either the formulation with 2 wt% or the formulation with 5 wt% Sodium lactate.
- EXAMPLE 2 Washing-up and/or hard surfaces degreasing detergents.
- EXAMPLE 3 alkaline machine dishwasher / glasswasher / potwasher, also suitable for certain hard surfaces other than cooking tools (ex. car spareparts, etc.).
- formulation A it is particularly preferred either the formulation with 7 wt% N,N-diacetic glutamic acid sodium salt and 4 wt% Polyaspartate, or the formulation with 8 wt% N, N- diacetic glutamic acid sodium salt and 3 wt% Polyaspartate.
- formulation E it is particularly preferred either the formulation with 3 wt% N,N-diacetic glutamic acid sodium salt and 3 wt% lminodisuccinic acid sodium salt, or the formulation with 5 wt% N 1 N- diacetic glutamic acid sodium salt and 5 wt% lminodisuccinic acid sodium salt; both formulations E are suitable to contain preferably either 6 wt% or 12 wt% alkali hydroxide.
- EXAMPLE 4 rinse-aid for dishwasher / glasswasher / potwasher and/or anti-lime for hard surfaces.
- EXAMPLE 5 multipurpose detergents for hard surfaces.
- EXAMPLE 6 pretreating detergents for laundry.
Abstract
Detergents formulations with low impact on environment and human health, together with good cleaning efficacy, particularly for laundry, crockery or hard surfaces, containing at least one surfactant and at least one sequestering agent, in which at least one sequestering agent is selected from the group consisting of: - iminodisuccinic acid tetrasodium salt (IDS); - N,N-diacetic glutamic acid tetrasodium salt (GLDA); - sodium glucoheptonate; sodium gluconate; and mixtures thereof.
Description
"Detergents formulations with low impact on the environment"
DESCRIPTION INVENTION OBJECT
The present invention relates to formulations of detergents for laundry, dishwashers, washing-up, hard surfaces cleaning, containing one or more surfactants, if necessary one or more electrolytes and one or more sequestering agents. The main features of these formulations concern toxicology and environmental toxicology, as well as cleaning efficacy.
BACKGROUND ART Generally, a detergent formulation is based on a surfactant, if necessary an electrolyte and a sequestering agent, or a set of them. An example is
EP1137752 (Unilever) which considers, among other components, either IDS or HIDS (0,05 - 1 ,5 % by weight) for laundry detergents without bleaching agents, aimed to preserve fabrics colours. Two very important matters associated with household detergents are environmental impact and toxicological hazard.
Surfactants are sometimes toxic, as it was recently demonstrated for ethoxylated nonylphenols, almost ubiquitous until they were recognized as likely endocrine disruptors and fast taken off the market. Tensides can also create various problems in aquatic environments: they act on water surface tension and some of them are foam boosters, so they promote the formation of persistent foams upon rivers and lakes surfaces either preventing oxygen-carbon dioxide exchanging or permeating natural membranes.
Further concern for both toxicity and environmental impact is represented by sequestering agents. Generally used nonbiodegradable complexing agents are: EDTA and sodium salt (C.A.S. 60-00-4 and 64-02-8), phosphonates (ATMP - C.A.S. 6419-19- 8; EDTMP - C.A.S. 1429-50-1 ; HDTMP - C.A.S. 23605-74-5; DTPMP - C.A.S. 15827- 60-8; HEDP, etidronic acid - C.A.S. 2809-21-4) and polycarboxylated (acrylic acid omopolymers - C.A.S. 9003-04-7 and 9003-01-4; acrylic acid and maleic anhydride copolymers - C.A.S. 52255-49-9).
All of them can easily transfer and concentrate heavy metals (among them lead, cadmium and mercury) into various organisms of the food chain.
EDTA and its salts are very little biodegradable: recently it was experienced a slow biodegradation at alkaline pH and without heavy metals, but these particular conditions are likely neither in surface waters nor in activated sludge plants (European Union - Risk Assessment Report Vol.49: Εdetic acid (EDTA)", 2004).
Polycarboxylated are very little biodegradable aerobically and aren't at all anaerobically; in sewage treatment plants, they are adsorbed upon sludges (Danish EPA: "Environmental and Health Assessment of Substances in Household and Cosmetic Detergent products", 100-103, 615-2001).
Phosphonates are very little biodegradable aerobically (HERA - Human & Environmental Risk Assessment on ingredients of European household cleaning products: "Phosphonates", 2004) and almost nonbiodegradable anaerobically (Danish EPA: "Environmental and Health Assessment of Substances in Household and Cosmetic Detergent products", 96-100, 615-2001); in particular conditions (catalysis made by some transition metals ions, at specific pH ranges) they're photodegradable; nevertheless, in real environments photodegradation is prevented by other physico- chemical mechanisms, such as low exposition to sun light (for example: muddy or deep waters) and, above all, absorbance in soil and sediments, which is reversible only after treatment either with strong acids or with more concentrated salted solutions. These are all conditions very far from real conditions (FISCHER K: "Distribution and elimination of HEDP in aquatic test systems", WATER RES; 27 (3). 1993. 485-493).
Usually powder laundry detergents contain zeolites (C AS. 1318-02-1) too; they are water insoluble aluminosilicates which act as ionic exchangers. Zeolites precipitate onto rivers bottom and in some cases cover it at all; they create problems in sewage and wastewater plants too, because they raise sludges to be wasted at the end of the cycle.
There are many other chemicals with low or no biodegradability at all, commonly used as detergents ingredients. Among them, sylicone based antifoaming agents. Although they're relatively chemically inert, their action may damage aquifers and natural
membranes.
To improve fabrics look after washing, laundry detergents usually contain optical brighteners. Although they're not a concern toxicologically, (HERA: "Fluorescent
Brightener FWA-T, 2004; "Fluorescent Brightener FWA-5", 2003), they are completely useless from the washing power point of view; their temporary action has only commercial profit.
Antiredeposition agents generally contained in detergents behave in various ways: some of them (acrylic polymers and acrylic-maleic copolymers) have low acute toxicity, but are very slowly biodegradable; instead, carboxymethylcellulose (CMC, C.A.S. 9004- 32-4) is readily biodegradable, but last studies (RTECS database) testify carcinogenicity (subcutaneous route) and mutagenicity in reproductive system (oral route) in rats at relatively low dosages (140 mg/Kg).
Preservatives and perfumes have heavy outcomes both on the environment and human health : generally both kind of chemicals are very slowly biodegradable, very toxic on aquatic organisms and sensitizing. For example: 5-chloro-2-methyl-2H- isothiazolin-3-one and 2-methyl-2H-isothiazolin-3-one are mutagens according to the Ames test; 2-Bromo-2-nitropropane-1 ,3-diol (Bronopol) is quite biodegradable, but byproducts are carcinogens (nitrosamines).
Fragrances may contain sensitizing or allergizing chemicals, even if in small concentrations; such chemicals are often toxic or very toxic for aquatic organisms. For example: limonene [(r)-p-mentha-1 ,8-diene, C.A.S. 5989-27-5; labelling: Xi, N R10-38-
43-50/53], 2,4,6-trinitro-1,3-dimethyl-5-terz-butylbenzene (C.A.S. 81-15-2, labelling: N
R44, R51/53), hydroxycitronellal (C.A.S. 107-75-5; labelling: Xi R36, R43), cinnamyl alcohol (C.A.S. 104-54-1 , labelling: Xi R43), diphenil etere (C.A.S. 101-84-8, labelling: Xi, N, R38, 51/53).
Taking into consideration all of these features, we can see what an impact may have large quantities of detergents put every year into rivers or lakes water. In addition, for some kinds of detergents there's also a direct or indirect toxicological impact because they get in touch with users, for example washing-up detergents (direct contact with the
skin), or dishwasher and laundry detergents (indirect contact due to machines incomplete rinsing of dishes surface or clothes).
A lot of toxic or polluting raw materials are not written upon detergents labels or safety data sheets, because their concentrations are lower than law limits (Dir 1999/45/CE relating to the classification, packaging and labelling of dangerous preparations); in fact such chemicals are present and those with low biodegradability tend to accumulate in the environment and may get into the food chain.
This situation is getting a little better thank to the recent Reg 2004/648/CE, which obliges detergents producers (with the exception of dispensations) to use only rapidly (aerobically) biodegradable surfactants; it is restricted to surfactants without considering all other chemicals, in many cases more harmful to the environment and to human health. It seems to be more and more important to search for low environmental impact and low toxicity formulations. Recently, the most careful consumers are looking for ecologic products. So, household detergents market offers many products defined "environmentally friendly"; in our opinion, this definition is seldom supported by facts.
Most of these products contain chemicals with very low biodegradability.
For example: dishwasher "ecologic" detergents contain polyacrylates or polyacrylates- sulphonates and phosphonates, sometimes CMC too; laundry "ecologic" detergents contain phosphonates, polyacrylates, zeolite, acrylic-maleic copolymers, silycone- paraffine mixture, optical brightener, CMC; in multipurpose "ecologic" detergents are present phosphonates, polycarboxylates, non biodegradable preservatives (methylisothiazolinone) and allergizing fragrances (limonene). Such components are present in relatively low concentrations, but these low values must be parametrized to the large quantities poured in water every day and their tendency to accumulate. SUMMARY OF THE INVENTION
The present invention is aimed to detergents formulations with low impact on environment and human health, together with good cleaning efficacy.
In order to reach these features, only rapidly biodegradable chemicals are used, according to OECD Tests A-F and equivalent tests (see also Reg CE/2004/648),
together with low tendency to bioaccumulate (Log Kow < 3 and other toxicological data (low acute and chronic human toxicity, low toxicity for aquatic organisms).
Those features don't concern mineral electrolytes, such as alkali metals hydroxides, carbonates, hydrogencarbonates, silicates and chlorides, which are made with chemicals naturally present in the environment; in addition, their pH tends to neutralize simply for diluition into wastewater.
Since all chosen organic chemicals are rapidly biodegradable, microorganisms naturally present in the environment are able to degrade the detergents residuals in a reasonable time, even if wastewaters don't flow to a sewage treatment plant. This feature is added to the low toxicity of molecules and to the good cleaning performance.
Formulations here described are preferably water based and can be liquid or gel/paste shaped. They include at least one surfactant, or a collection of surfactants, if needed an electrolyte and at least a sequestering agent, chosen into the following group: iminodisuccinic acid tetrasodium salt (IDS), N,N-diacetic glutamic acid tetrasodium salt (GLDA), sodium glucoheptonate, sodium gluconate.
DETAILED DESCRIPTION OF THE INVENTION
In the following paper, the invention is thoroughly described, with specific reference to the surfactants and sequestering agents used, together with some examples of formulations. Surfactants
The surfactants used may belong to 3 categories: anionic, non-ionic and amphoteric/zwitterionic.
The first selection we made was about both the impact on the environment and the toxicity: in addition to be rapidly biodegradable (according to complete biodegradability standard required by Reg CE/2004/648), they had to be anaerobically biodegradable and to have a low toxicity on aquatic organisms (LC50 > 1mg/l), considering that any surfactant - soap too - is quite toxic for fishes. This is one of the reasons why it is important for a tenside to be rapidly biodegradable. Preferably, in this invention are used:
- alkali metals alkyl ether sulfates (<= 4 EO);
- alkali metals alkyl isethyonates;
- alkali metals soaps;
- ethoxylated fatty alcohol (<= 6 EO); - cocodiethanolamide;
- alkylpolyglucosides (preferably: alkyl = C4 - C11), as hydrotrope;
- alkyliminodipropionate, as hydrotrope;
- alkylamidopropyl betaine.
In the following table are listed average acute toxicity values [from Danish EPA: "Environmental and Health Assessment of Substances in Household and Cosmetic Detergent products", 17-93, 615-2001 , and from US National Library of Medicine - SIS (Specialized Information Services)]:
Among the group of surfactants, some are non persistently foam boosters. This feature allows to see very well where the detergent is acting (for ex.: during washing-up),
anyhow losing foam consistency when reaching the wastewater. In this way, even if wastewater don't flow up to a sewage treatment plant, the danger to damage natural ecosystems with floating foams is minimized. Sequestering agents / Builders In detergency, a sequestering agent acts mainly as a calcium ad magnesium lime controller, in order to avoid lime to precipitate upon clothes and hard surfaces both as carbonates and as insoluble fatty acids esters. Moreover, they complex heavy metals ions (Fe3+, Cu2+, etc.), which may create coloured stains upon clothes and surfaces.
In this invention, formulations contain all rapidly biodegradable sequestering agents with low toxicological impact, chosen among the following:
• iminodisuccinic acid tetrasodium salt (IDS), also called N-(1 ,2-dicarboxyethyl)-aspartic acid tetrasodium salt, having the following molecular formula (I):
• sodium glucoheptonate, having the following molecular formula (III):
sodium gluconate, having the following molecular formula (IV):
In the following table are listed average acute toxicity values, taken from Safety Data Sheets provided from the products suppliers:
The use of one or more than one sequestering agents could be accompanied by the use of a builder chosen among alkali metal orthophosphate, diphosphate and triphosphate.
Phosphates, if used in very small quantities, give a little contribute to eutrophication, with regard to detergents used in the past or to current other uses (such as agricultural ones). Instead, using few quantities may allow to avoid to use more pollutant chemicals. The described above sequestering agents may be matched with an additional
sequestering/antiredeposition agent selected from the group consisting of citric acid, citric acid tri alkali metals salt, polyaspartate, alkali metals orthophosphate, diphosphate and/or triphosphate and mixture thereof. Other components In these formulations may be present other chemicals with the same toxycological and eco-toxycological features described above, for example the following: solvents (short chain alcohols, esters, etc.), thickening agents, antiredeposition agents, perfumes, colouring agents, preservatives, antibacterial agents, bleaching agents with or without bleach activators, scrubbing agents, bittering agents, etc.. The phrase "other components" is generally used referring to those components, but sometimes the formulations report some specific chemicals selected from this group. EXAMPLES
The following paper reports some examples of preferred formulations, used as laundry detergent, dishwasher, multipurpose, etc. For each use, two or more formulations are written, called A, B, C, ... For each component, a preferred concentration range and a single value (between brackets) are written; the single value is related to an even more preferred formulation. All concentrations are expressed as %, that is the dry chemical weight (in grams) in comparison to 100 grams of the aqueous formulations.
EXAMPLE 1: detergent for laundry machine or washing clothes by hand; particularly, formulation E is preferably used for delicate laundry wash.
In formulation E, it is particularly preferred either the formulation with 2 wt% or the formulation with 5 wt% Sodium lactate.
EXAMPLE 2: Washing-up and/or hard surfaces degreasing detergents.
EXAMPLE 3: alkaline machine dishwasher / glasswasher / potwasher, also suitable for certain hard surfaces other than cooking tools (ex. car spareparts, etc.).
In formulation A, it is particularly preferred either the formulation with 7 wt% N,N-diacetic glutamic acid sodium salt and 4 wt% Polyaspartate, or the formulation with 8 wt% N, N- diacetic glutamic acid sodium salt and 3 wt% Polyaspartate. In formulation E, it is particularly preferred either the formulation with 3 wt% N,N-diacetic glutamic acid sodium salt and 3 wt% lminodisuccinic acid sodium salt, or the formulation with 5 wt% N1N- diacetic glutamic acid sodium salt and 5 wt% lminodisuccinic acid sodium salt; both formulations E are suitable to contain preferably either 6 wt% or 12 wt% alkali hydroxide.
EXAMPLE 4: rinse-aid for dishwasher / glasswasher / potwasher and/or anti-lime for hard surfaces.
EXAMPLE 5: multipurpose detergents for hard surfaces.
Claims
1. Detergent formulation, particularly for laundry, crockery or hard surfaces, containing at least one surfactant and at least one sequestering agent, characterized in that at least one sequestering agent is selected from the group consisting of: - iminodisuccinic acid tetrasodium salt (IDS), having the following molecular formula (I):
- N,N-diacetic glutamic acid tetrasodium salt (GLDA), having the following molecular formula (II):
(II) sodium glucoheptonate, having the following molecular formula (III):
(IV) and mixtures thereof.
2. Formulations according to Claim 1, characterized in that at least one surfactant selected from the group consisting of alkali metals alkylsulfates, alkali metals alkyl ether sulfates, alkali metals alkyl isethyonates, alkali metals soaps, ethoxylated fatty alcohol, cocodiethanolamide, alkylpolyglucosides, alkyliminodipropionate, alkylamidopropyl betaine and mixtures thereof.
3. Formulation according to Claim 1 or Claim 2, characterized in that it contains also solvents, thickening agents, antifoam agents, antiredeposition agents, perfumes, colouring agents, preservatives, antimicrobial agents, bleaching agents with or without bleach activators, scrubbing agents, bittering agents, alone or in mixture.
4. Formulation as claimed in any of the preceding claims, particularly suitable for laundry machine or washing clothes by hand, characterized in that it comprises (dry wt%):
Sodium lauryl sulfate 0,05 - 3,5 Potassium soap 5,5 - 9,5 Ethoxylated fatty alcohol 5,0 - 9,0 Amphoteric surfactant 0,05 - 3,5 Sodium gluconate 0,5 - 4,5 Sodium carbonate 2,0 - 6,0 Sodium chloride 0,05 - 3,5 Other components, water to 100%
5. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for laundry machine or washing clothes by hand, characterized in that it comprises (dry wt%):
Sodium lauryl sulfate 0,01 - 3,0 Sodium alkyl ether sulfate 5,0 - 9,0 Potassium soap 5,5-9,5 ethoxylated fatty alcohol 5,0-9,0 Amphoteric surfactant 0,05-3,5 - Sodium gluconate 0,5-4,5
Sodium carbonate 2,0-6,0
Sodium chloride 0,05 - 3,5
Other components, water to 100%
6. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for laundry machine or washing clothes by hand, characterized in that it comprises (dry wt%):
Potassium soap 7,0-12,0 ethoxylated fatty alcohol 5,5-10,5 Hydrotrope surfactant 1,0-4,0
Sodium gluconate 0,5-5,5 - Sodium carbonate 2,0-6,0
Sodium chloride 0,05 - 3,5
Other components, water to 100%
7. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for laundry machine or washing clothes by hand, characterized in that it comprises (dry wt%): - ethoxylated fatty alcohol 2,5-7,5
Hydrotrope surfactant 0,5 - 4,5
Sodium gluconate 2,5 - 7,5
N, N-diacetic glutamic acid sodium salt 0,05-4,0 lminodisuccinic acid sodium salt 1,6- 4,0 - sodium citrate dihydrated 2,5 - 7,5
Sodium lactate 0,5 - 7,0
Sodium carbonate 3,0-7,0
Sodium chloride 0,05 - 3,5
Other components, water to 100%
8. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for laundry machine or washing clothes by hand, preferably used for delicate laundry wash, characterized in that it comprises (dry wt%):
Sodium alkyl ether sulfate 0,5 - 5,5 - ethoxylated fatty alcohol 2,5 - 7,5
Hydrotrope surfactant 0,5 - 4,5
Sodium gluconate 0,5 - 5,5 lminodisuccinic acid sodium salt 1 ,6 - 4,0 Sodium lactate 0,5 - 7,0 - Sodium chloride 0,05 - 3,5
Citric acid or lactic acid 0,01 - 1,5
Other components, water to 100%
9. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for washing- up /hard surfaces degreasing detergents, characterized in that it comprises (dry wt%):
Sodium lauryl sulfate 3,5 - 7,5 ethoxylated fatty alcohol 1 ,5 - 5,5 alkylamidopropyl betaine 0,05 - 4,0 lminodisuccinic acid sodium salt 0,05 - 2,5 - Citric acid 0,01 - 1 ,5
Sodium chloride 0,05 - 4,0
Other components, water to 100%
10. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for washing- up /hard surfaces degreasing detergents, characterized in that it comprises (dry wt%):
Sodium lauryl sulfate 7,5 - 11 ,5 ethoxylated fatty alcohol 7,0 - 11 ,0 alkylamidopropyl betaine 0,05 - 4,0
Potassium stearic soap 0,05 - 3,0 Citric acid 0,01 - 1 ,5
Sodium gluconate 0,5 - 4,5
Sodium chloride 0,05 - 4,0
Other components, water to 100%
11. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for washing- up /hard surfaces degreasing detergents, characterized in that it comprises (dry
Ό : ethoxylated fatty alcohol 0,5 - 5,5
Sodium alkyl ether sulfate 2,5 - 7,5 - alkylamidopropyl betaine 0,05 - 3,0
Hydrotrope surfactant 0,05 - 3,0
Sodium lactate 1 ,5 - 6,5 lminodisuccinic acid sodium salt 0,05 - 3,0
Citric acid or lactic acid 0,01 - 1 ,5 - Sodium chloride 0,05 - 3,5
Other components, water to 100%
12. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for washing- up /hard surfaces degreasing detergents, characterized in that it comprises (dry wt%): - ethoxylated fatty alcohol 3,5 - 8,5
Sodium alkyl ether sulfate 2,5 - 7,5 alkylamidopropyl betaine 0,05 - 3,0
Hydrotrope surfactant 2,0 - 6,0
Sodium lactate 1 ,5 - 6,5 - lminodisuccinic acid sodium salt 0,05 - 3,5
Citric acid or lactic acid 0,01 - 1 ,5
Sodium chloride 0,05 - 3,5
Other components, water to 100%
13. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for washing- up /hard surfaces degreasing detergents, characterized in that it comprises (dry
%,: ethoxylated fatty alcohol 0,5 - 5,5
Hydrotrope surfactant 2,0 - 7,0 - Sodium lactate 1 ,5 - 6,5 lminodisuccinic acid sodium salt 0,05 - 4,0 Citric acid or lactic acid 0,01 - 3,0
Sodium gluconate 2,5 - 7,5
Sodium chloride 0,05 - 3,5 - Other components, water to 100%
14. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for machine dishwasher / glasswasher / potwasher, also suitable for certain hard surfaces other than cooking tools (ex. car spareparts, etc.). characterized in that it comprises (dry wt%): - N,N-diacetic glutamic acid sodium salt 5,0 - 10,0
Sodium citrate dihydrated 5,0 - 10,0
Hydrotrope surfactant 0,05 - 3,0
Ethoxylated fatty alcohols 0,05 - 3,0
Sodium hydroxide 0,05 - 3,5 - Sodium metasilicate 3,0 - 7,0
Polyaspartate 2,0 - 7,0
Other components, water to 100%
15. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for machine dishwasher / glasswasher / potwasher, also suitable for certain hard surfaces other than cooking tools (ex. car spareparts, etc.). characterized in that it comprises (dry wt%):
N, N-diacetic glutamic acid sodium salt 8,0 - 13,0 Sodium citrate dihydrated 5,0 - 10,0
Hydrotrope surfactant 0,01 - 2,5 Ethoxylated fatty alcohols 0,01 - 2,5
Sodium hydroxide 9,0 - 13,0
Polyaspartate 2,0 - 7,0
Other components, water to 100%
16. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for machine dishwasher / glasswasher / potwasher, also suitable for certain hard surfaces other than cooking tools (ex. car spareparts, etc.). characterized in that it comprises (dry wt%):
N,N-diacetic glutamic acid sodium salt 0,5 - 5,5 - lminodisuccinic acid sodium salt 0,5 - 5,5
Sodium citrate dihydrated 5,0 - 11 ,0
Sodium gluconate 2,5 - 7,5
Sodium carbonate 0,01 - 3,0
Hydrotrope surfactant 0,05 - 3,0 - Ethoxylated fatty alcohols 0,01 - 2,5
Sodium hydroxide 0,05 - 3,5
Sodium metasilicate 3,0 - 7,0
Other components, water to 100%
17. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for machine dishwasher / glasswasher / potwasher, also suitable for certain hard surfaces other than cooking tools (ex. car spareparts, etc.). characterized in that it comprises (dry wt%):
N,N-diacetic glutamic acid sodium salt 0,1 - 8,5 lminodisuccinic acid sodium salt 0,1 - 8,5 - Sodium citrate dihydrated 5,0 - 11 ,0
Sodium gluconate 2,5 - 7,5
Sodium carbonate 0,01 - 3,0
Hydrotrope surfactant 0,1 - 3,5
Ethoxylated fatty alcohols 0,1 - 3,5 Sodium hydroxide 0,05 - 3,5
Sodium metasilicate 3,0 - 7,0
Other components, water to 100%
18. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for machine dishwasher / glasswasher / potwasher, also suitable for certain hard surfaces other than cooking tools (ex. car spareparts, etc.). characterized in that it comprises (dry wt%):
N,N-diacetic glutamic acid sodium salt 0,1 - 8,5 lminodisuccinic acid sodium salt 0,1 - 8,5 - Sodium citrate dihydrated 5,0 - 11 ,0
Sodium gluconate 2,5 - 7,5
Sodium carbonate 0,01 - 3,0
Hydrotrope surfactant 0,05 - 3,0
Ethoxylated fatty alcohols 0,01 - 2,5 - Sodium hydroxide 5,0 - 15,0
Sodium metasilicate 3,0 - 7,0
Other components, water to 100%
19. Formulation as claimed in any of the Claims 1 to 3, particularly used as rinse-aid for dishwasher / glasswasher / potwasher and/or anti-lime for hard surfaces, characterized in that it comprises (dry wt%): lminodisuccinic acid sodium salt 0,05 - 2,5
Citric acid monohydrated 3,0 - 7,0
Hydrotrope surfactant 9,0 - 13,0
Ethoxylated fatty alcohols 9,0 - 13,0 - Alcohols 5,0 - 9,0
Ethyl lactate 0,05 - 3,0
Other components, water to 100%
20. Formulation as claimed in any of the Claims 1 to 3, particularly used as rinse-aid for dishwasher / glasswasher / potwasher and/or anti-lime for hard surfaces, characterized in that it comprises (dry wt%): lminodisuccinic acid sodium salt 0,05 - 2,5 Citric acid monohydrated 10,0- 14,0
Ethoxylated fatty alcohols 16,0 - 21 ,0 - Alcohols 11 ,0 - 16,0
Other components, water to 100%
21. Formulation as claimed in any of the Claims 1 to 3, particularly used as rinse-aid for dishwasher / glasswasher / potwasher and/or anti-lime for hard surfaces, characterized in that it comprises (dry wt%): - lminodisuccinic acid sodium salt 0,05 - 3,0
Citric acid monohydrated 10,0 - 14,0
Hydrotrope surfactant 0,05 - 3,0
Alcohols 3,0 - 7,0
Other components, water to 100%
22. Formulation as claimed in any of the Claims 1 to 3, particularly used as rinse-aid for dishwasher / glasswasher / potwasher and/or anti-lime for hard surfaces, characterized in that it comprises (dry wt%): lminodisuccinic acid sodium salt 0,05 - 2,5 Citric acid monohydrated 10,0 - 14,0 - Lactic acid 0,1 - 4,0
Ethoxylated fatty alcohols 16,0 - 21 ,0
- Alcohols 11 ,0 - 16,0
Other components, water to 100%
23. Formulation as claimed in any of the Claims 1 to 3, particularly used as anti-lime for hard surfaces, characterized in that it comprises (dry wt%): lminodisuccinic acid sodium salt 0,1 - 4,0 Citric acid monohydrated 5,0 - 9,0
Lactic acid 5,0 - 9,0
Sodium alkyl ether sulfate 0,01 - 5,0 Alkylamidopropyl betaine 0,01 - 5,0
Alcohols 5,0 - 9,0
Other components, water to 100%
24. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for multipurpose detergents for hard surfaces, characterized in that it comprises (dry wt%): lminodisuccinic acid sodium salt 0,05 - 2,0 Hydrotrope surfactant 4,0 - 8,0
Ethoxylated fatty alcohols 0,05 - 2,5 - Alcohols 5,0 - 10,0
Ethyl lactate 0,05 - 3,0
Other components, water to 100%
25. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for multipurpose detergents for hard surfaces, characterized in that it comprises (dry wt%): lminodisuccinic acid sodium salt 0,05 - 2,0
Hydrotrope surfactant 0,05 - 3,0
Ethoxylated fatty alcohols 0,05 - 2,5
Alcohols 5,0 - 10,0 - Other components, water to 100%
26. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for multipurpose detergents for hard surfaces, characterized in that it comprises (dry
%): lminodisuccinic acid sodium salt 0,1 - 4,0
Sodium alkyl ether sulfate 0,01 - 3,5
Alcohols 5,0 - 10,0
Alkyl lactate 0,1 - 4,0
Sodium lactate 0,01 - 3,0
Citric acid or lactic acid 0,05 - 2,5 Other components, water to 100%
27. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for multipurpose detergents for hard surfaces, characterized in that it comprises (dry
%.: - lminodisuccinic acid sodium salt 0,1 - 4,0
Hydrotrope surfactant 0,05 - 3,0
Alcohols 5,0 - 10,0
- Alkyl lactate 0,1 - 4,0
Sodium lactate 0,01 - 3,0 - Citric acid or lactic acid 0,05 - 2,5
Other components, water to 100%
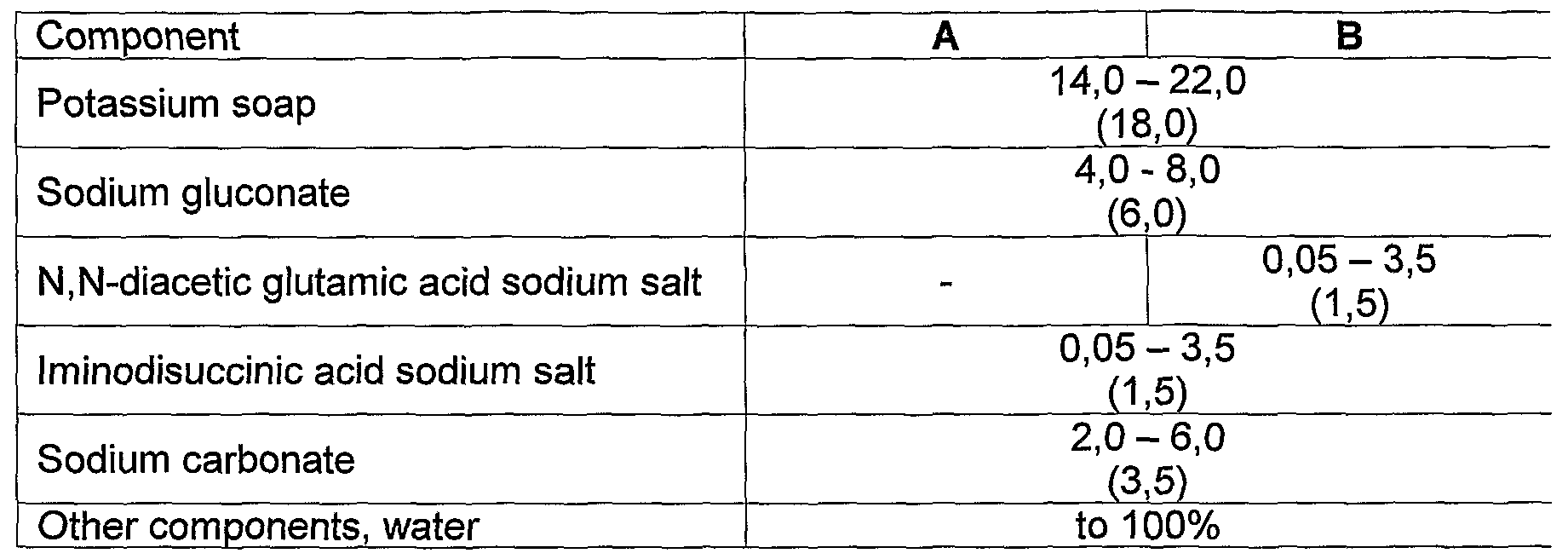
28. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for pretreating additive detergents for laundry, characterized in that it comprises (dry
%y. - Potassium soap 14,0 - 22,0
Sodium gluconate 4,0 - 8,0 lminodisuccinic acid sodium salt 0,05 - 3,5
Sodium carbonate 3,0 - 7,0
Other components, water to 100%
29. Formulation as claimed in any of the Claims 1 to 3, particularly suitable for pretreating detergents for laundry, characterized in that it comprises (dry wt%):
Potassium soap 14,0 - 22,0
Sodium gluconate 4,0 - 8,0
N,N-diacetic glutamic acid sodium salt 0,05 - 3,5 - lminodisuccinic acid sodium salt 0,05 - 3,5
Sodium carbonate 3,0 - 7,0
Other components, water to 100%
30. Formulation as claimed in any of the preceding claims, characterized in that it is an aqueous solution, or an aqueous gel/ paste.
31. Formulation as claimed in Claims 14, 15, 16, 17 and 18, characterized in that it is a powder, containing if necessary an additional concentration of sodium carbonate (5 - 50 wt%).
32. Detergent for laundry machine and/or clothes by hand, dishwashers, washing-up, hard surfaces, characterized in that its formulation follows at least one of the preceding claims.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| ITCR2006A000016 | 2006-06-07 | ||
| ITCR20060016 ITCR20060016A1 (en) | 2006-06-07 | 2006-06-07 | DETERGENT FORMULATIONS AT LOW ENVIRONMENTAL IMPACT |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007141635A2 true WO2007141635A2 (en) | 2007-12-13 |
| WO2007141635A3 WO2007141635A3 (en) | 2008-09-04 |
Family
ID=38780313
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2007/001505 WO2007141635A2 (en) | 2006-06-07 | 2007-05-30 | Detergents formulations with low impact on the environment |
Country Status (2)
| Country | Link |
|---|---|
| IT (1) | ITCR20060016A1 (en) |
| WO (1) | WO2007141635A2 (en) |
Cited By (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2255776A1 (en) * | 2009-05-26 | 2010-12-01 | PURAC Biochem BV | Cleansing composition |
| WO2012038755A1 (en) * | 2010-09-23 | 2012-03-29 | Innospec Limited | Automatic dishwashing composition |
| JP2013518983A (en) * | 2010-02-09 | 2013-05-23 | ビーエーエスエフ ソシエタス・ヨーロピア | Detergent composition |
| ES2428799A1 (en) * | 2012-05-04 | 2013-11-11 | Javier DÍAZ RODRÍGUEZ | Cleaning product degreaser for multiple surfaces (Machine-translation by Google Translate, not legally binding) |
| JP2014196514A (en) * | 2009-06-19 | 2014-10-16 | ザ プロクター アンド ギャンブルカンパニー | Liquid hand dishwashing detergent composition |
| US9115014B1 (en) * | 2014-09-18 | 2015-08-25 | Silk Water Solutions Inc. | Composition and method for water conditioning |
| WO2018007298A1 (en) * | 2016-07-05 | 2018-01-11 | Henkel Ag & Co. Kgaa | Dishwasher agent containing saccharic acid and amino carboxylic acid |
| DE102018206661A1 (en) * | 2018-04-30 | 2019-10-31 | Henkel Ag & Co. Kgaa | Detergent composition for automatic dishwashing filter cleaning |
| EP3666871A1 (en) * | 2018-12-12 | 2020-06-17 | Henkel AG & Co. KGaA | Washing or cleaning agents containing iminodisuccinate and / or iminotrisuccinate |
| CN111394207A (en) * | 2020-04-22 | 2020-07-10 | 周栋 | Water-soluble film dish washing tablet and preparation method thereof |
| CN111910191A (en) * | 2020-07-24 | 2020-11-10 | 苏州波菲特新材料科技有限公司 | Powder degreasing agent, preparation method thereof and drying device |
| EP3593896A4 (en) * | 2017-03-07 | 2020-12-23 | Kurita Water Industries Ltd. | Water treatment chemical, method for preparing same, and method for washing polyamide-based reverse osmosis membrane |
| WO2023275269A1 (en) | 2021-06-30 | 2023-01-05 | Nouryon Chemicals International B.V. | Chelate-amphoteric surfactant liquid concentrates and use thereof in cleaning applications |
| US11891588B2 (en) | 2019-07-31 | 2024-02-06 | Ecolab Usa Inc. | Personal protective equipment free delimer compositions o |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN110003998A (en) * | 2019-04-25 | 2019-07-12 | 湖南日用化学科学研究所有限公司 | A kind of long-acting fluorescent brightener added detergent and preparation method thereof with highly effective chelating ability |
Citations (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3807668A1 (en) * | 1988-03-09 | 1989-09-21 | Henkel Kgaa | Additive containing dimethyl sulphoxide for alkaline cleaning solutions and its use |
| WO1992011346A1 (en) * | 1990-12-21 | 1992-07-09 | Kommentus Ecogreen Aktiebolag | A detergent and its manufacture |
| EP0512371A2 (en) * | 1991-05-02 | 1992-11-11 | DISPO-Kommerz AG | Granular phosphate-free agents for automatic dishwashing |
| WO1993004152A1 (en) * | 1991-08-16 | 1993-03-04 | Kommentus Ecogreen Aktiebolag | A powder detergent for dishwashing machines, and its manufacture |
| DE4228043A1 (en) * | 1992-08-24 | 1994-03-03 | Henkel Kgaa | Builder for detergents |
| DE4300658A1 (en) * | 1993-01-13 | 1994-07-14 | Henkel Kgaa | Liq or pasty washing and cleaning compsn contg anionic and nonionic tenside |
| US5364551A (en) * | 1993-09-17 | 1994-11-15 | Ecolab Inc. | Reduced misting oven cleaner |
| EP0626470A1 (en) * | 1993-05-28 | 1994-11-30 | VAW Aluminium AG | Process and device for cleaning aluminium chips |
| GB2281567A (en) * | 1993-07-24 | 1995-03-08 | Jolly Sailor Marine Products L | Cleaning compositions |
| DE4408502A1 (en) * | 1994-02-18 | 1995-08-24 | Henkel Ecolab Gmbh & Co Ohg | Spray-dried granules with high bulk density |
| EP0758678A2 (en) * | 1995-08-11 | 1997-02-19 | HENKEL-ECOLAB GmbH & CO. OHG | Cleaning agent for protective working garment |
| US5622569A (en) * | 1995-06-02 | 1997-04-22 | Aluminum Company Of America | Aluminum rigid container sheet cleaner and cleaning method |
| EP0783034A2 (en) * | 1995-12-22 | 1997-07-09 | Nitto Chemical Industry Co., Ltd. | Chelating agent and detergent comprising the same |
| WO1998013467A1 (en) * | 1996-09-27 | 1998-04-02 | Unilever N.V. | Aqueous structured liquid detergent composition comprising aminocarboxylate sequestrant |
| US5849095A (en) * | 1996-04-09 | 1998-12-15 | Rouillard; Carol | Anti-etch bottle washing solution |
| EP0969080A1 (en) * | 1998-07-03 | 2000-01-05 | Showa Denko Kabushiki Kaisha | Liquid detergent composition |
| US6013612A (en) * | 1997-10-22 | 2000-01-11 | Showa Denko K.K. | Cleaning agent composition |
| US6034046A (en) * | 1999-03-26 | 2000-03-07 | Colgate Palmolive Company | All purpose liquid bathroom cleaning compositions |
| EP1067172A2 (en) * | 1997-03-12 | 2001-01-10 | Showa Denko Kabushiki Kaisha | Detergent composition |
| US6240935B1 (en) * | 2000-03-30 | 2001-06-05 | The Boeing Company | Boelube R dissolving alkaline cleaning solution |
| WO2001046371A1 (en) * | 1999-12-21 | 2001-06-28 | Unilever Plc | Detergent compositions |
| EP1138756A2 (en) * | 2000-03-29 | 2001-10-04 | Henkel Kommanditgesellschaft auf Aktien | Detergent Tablets comprising special surfactant granulates |
| US20020004475A1 (en) * | 2000-06-02 | 2002-01-10 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Detergent compositions |
| DE10100720A1 (en) * | 2001-01-10 | 2002-07-11 | Beiersdorf Ag | Cosmetic and dermatological detergent compositions containing an effective amount of iminodisuccinic acid and / or its salts |
| US20020112294A1 (en) * | 1999-12-17 | 2002-08-22 | Kuzmenka Daniel Joseph | Dye fixing composition |
| US6440924B1 (en) * | 1998-03-16 | 2002-08-27 | Henkel Kommanditgesellschaft Auf Aktien | Aqueous multiphase detergents with immiscible phases |
| US6455487B1 (en) * | 2002-03-21 | 2002-09-24 | Colgate Palmolive Company | Liquid cleaning composition containing a preservative and an effective chelating agent |
| WO2003018735A1 (en) * | 2001-08-27 | 2003-03-06 | Henkel Kommanditgesellschaft Auf Aktien | Screen cleaning fluid and use thereof for vehicle screens |
| WO2003083024A1 (en) * | 2002-03-27 | 2003-10-09 | Colgate-Palmolive Company | Liquid dish cleaning compositions having improved preservative system |
| US20030195129A1 (en) * | 2001-07-24 | 2003-10-16 | Akira Ishikawa | Laundering pretreatment composition for clothing |
| WO2004015047A2 (en) * | 2002-08-13 | 2004-02-19 | Mcintyre Group, Ltd. | High concentration surfactant compositions and methods |
| US20040058839A1 (en) * | 2002-09-23 | 2004-03-25 | Tadrowski Tami J. | Cleaning solutions for carbon removal |
| US20060019854A1 (en) * | 2004-07-21 | 2006-01-26 | Johnsondiversey. Inc. | Paper mill cleaner with taed |
| CA2578868A1 (en) * | 2004-09-06 | 2006-03-16 | Furukawa Techno Material Co., Ltd. | Surfactant-based composition |
| WO2007025944A1 (en) * | 2005-08-31 | 2007-03-08 | Basf Aktiengesellschaft | Cleaning formulations for machine dishwashing comprising hydrophilically modified polycarboxylates |
| WO2007052064A1 (en) * | 2005-11-07 | 2007-05-10 | Reckitt Benckiser N.V. | Composition |
-
2006
- 2006-06-07 IT ITCR20060016 patent/ITCR20060016A1/en unknown
-
2007
- 2007-05-30 WO PCT/IB2007/001505 patent/WO2007141635A2/en active Application Filing
Patent Citations (36)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE3807668A1 (en) * | 1988-03-09 | 1989-09-21 | Henkel Kgaa | Additive containing dimethyl sulphoxide for alkaline cleaning solutions and its use |
| WO1992011346A1 (en) * | 1990-12-21 | 1992-07-09 | Kommentus Ecogreen Aktiebolag | A detergent and its manufacture |
| EP0512371A2 (en) * | 1991-05-02 | 1992-11-11 | DISPO-Kommerz AG | Granular phosphate-free agents for automatic dishwashing |
| WO1993004152A1 (en) * | 1991-08-16 | 1993-03-04 | Kommentus Ecogreen Aktiebolag | A powder detergent for dishwashing machines, and its manufacture |
| DE4228043A1 (en) * | 1992-08-24 | 1994-03-03 | Henkel Kgaa | Builder for detergents |
| DE4300658A1 (en) * | 1993-01-13 | 1994-07-14 | Henkel Kgaa | Liq or pasty washing and cleaning compsn contg anionic and nonionic tenside |
| EP0626470A1 (en) * | 1993-05-28 | 1994-11-30 | VAW Aluminium AG | Process and device for cleaning aluminium chips |
| GB2281567A (en) * | 1993-07-24 | 1995-03-08 | Jolly Sailor Marine Products L | Cleaning compositions |
| US5364551A (en) * | 1993-09-17 | 1994-11-15 | Ecolab Inc. | Reduced misting oven cleaner |
| DE4408502A1 (en) * | 1994-02-18 | 1995-08-24 | Henkel Ecolab Gmbh & Co Ohg | Spray-dried granules with high bulk density |
| US5622569A (en) * | 1995-06-02 | 1997-04-22 | Aluminum Company Of America | Aluminum rigid container sheet cleaner and cleaning method |
| EP0758678A2 (en) * | 1995-08-11 | 1997-02-19 | HENKEL-ECOLAB GmbH & CO. OHG | Cleaning agent for protective working garment |
| EP0783034A2 (en) * | 1995-12-22 | 1997-07-09 | Nitto Chemical Industry Co., Ltd. | Chelating agent and detergent comprising the same |
| US5849095A (en) * | 1996-04-09 | 1998-12-15 | Rouillard; Carol | Anti-etch bottle washing solution |
| WO1998013467A1 (en) * | 1996-09-27 | 1998-04-02 | Unilever N.V. | Aqueous structured liquid detergent composition comprising aminocarboxylate sequestrant |
| EP1067172A2 (en) * | 1997-03-12 | 2001-01-10 | Showa Denko Kabushiki Kaisha | Detergent composition |
| US6013612A (en) * | 1997-10-22 | 2000-01-11 | Showa Denko K.K. | Cleaning agent composition |
| US6440924B1 (en) * | 1998-03-16 | 2002-08-27 | Henkel Kommanditgesellschaft Auf Aktien | Aqueous multiphase detergents with immiscible phases |
| EP0969080A1 (en) * | 1998-07-03 | 2000-01-05 | Showa Denko Kabushiki Kaisha | Liquid detergent composition |
| US6034046A (en) * | 1999-03-26 | 2000-03-07 | Colgate Palmolive Company | All purpose liquid bathroom cleaning compositions |
| US20020112294A1 (en) * | 1999-12-17 | 2002-08-22 | Kuzmenka Daniel Joseph | Dye fixing composition |
| WO2001046371A1 (en) * | 1999-12-21 | 2001-06-28 | Unilever Plc | Detergent compositions |
| EP1138756A2 (en) * | 2000-03-29 | 2001-10-04 | Henkel Kommanditgesellschaft auf Aktien | Detergent Tablets comprising special surfactant granulates |
| US6240935B1 (en) * | 2000-03-30 | 2001-06-05 | The Boeing Company | Boelube R dissolving alkaline cleaning solution |
| US20020004475A1 (en) * | 2000-06-02 | 2002-01-10 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Detergent compositions |
| DE10100720A1 (en) * | 2001-01-10 | 2002-07-11 | Beiersdorf Ag | Cosmetic and dermatological detergent compositions containing an effective amount of iminodisuccinic acid and / or its salts |
| US20030195129A1 (en) * | 2001-07-24 | 2003-10-16 | Akira Ishikawa | Laundering pretreatment composition for clothing |
| WO2003018735A1 (en) * | 2001-08-27 | 2003-03-06 | Henkel Kommanditgesellschaft Auf Aktien | Screen cleaning fluid and use thereof for vehicle screens |
| US6455487B1 (en) * | 2002-03-21 | 2002-09-24 | Colgate Palmolive Company | Liquid cleaning composition containing a preservative and an effective chelating agent |
| WO2003083024A1 (en) * | 2002-03-27 | 2003-10-09 | Colgate-Palmolive Company | Liquid dish cleaning compositions having improved preservative system |
| WO2004015047A2 (en) * | 2002-08-13 | 2004-02-19 | Mcintyre Group, Ltd. | High concentration surfactant compositions and methods |
| US20040058839A1 (en) * | 2002-09-23 | 2004-03-25 | Tadrowski Tami J. | Cleaning solutions for carbon removal |
| US20060019854A1 (en) * | 2004-07-21 | 2006-01-26 | Johnsondiversey. Inc. | Paper mill cleaner with taed |
| CA2578868A1 (en) * | 2004-09-06 | 2006-03-16 | Furukawa Techno Material Co., Ltd. | Surfactant-based composition |
| WO2007025944A1 (en) * | 2005-08-31 | 2007-03-08 | Basf Aktiengesellschaft | Cleaning formulations for machine dishwashing comprising hydrophilically modified polycarboxylates |
| WO2007052064A1 (en) * | 2005-11-07 | 2007-05-10 | Reckitt Benckiser N.V. | Composition |
Cited By (28)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2010136478A2 (en) * | 2009-05-26 | 2010-12-02 | Purac Biochem Bv | Method for improving the mildness of cleansing compositions |
| WO2010136478A3 (en) * | 2009-05-26 | 2011-12-01 | Purac Biochem Bv | Method for improving the mildness of cleansing compositions |
| CN102711716A (en) * | 2009-05-26 | 2012-10-03 | 普拉克生化公司 | Method for improving the mildness of cleansing compositions |
| JP2012528109A (en) * | 2009-05-26 | 2012-11-12 | ピュラック バイオケム ビー.ブイ. | Methods for improving the calmness of cleansing compositions |
| EP2255776A1 (en) * | 2009-05-26 | 2010-12-01 | PURAC Biochem BV | Cleansing composition |
| JP2014196514A (en) * | 2009-06-19 | 2014-10-16 | ザ プロクター アンド ギャンブルカンパニー | Liquid hand dishwashing detergent composition |
| JP2013518983A (en) * | 2010-02-09 | 2013-05-23 | ビーエーエスエフ ソシエタス・ヨーロピア | Detergent composition |
| US9506020B2 (en) | 2010-09-23 | 2016-11-29 | Innospec Limited | Automatic dishwashing composition |
| WO2012038755A1 (en) * | 2010-09-23 | 2012-03-29 | Innospec Limited | Automatic dishwashing composition |
| ES2428799A1 (en) * | 2012-05-04 | 2013-11-11 | Javier DÍAZ RODRÍGUEZ | Cleaning product degreaser for multiple surfaces (Machine-translation by Google Translate, not legally binding) |
| US10081957B2 (en) * | 2014-09-18 | 2018-09-25 | Silk Water Solutions Inc. | Composition and method for water conditioning |
| US10829951B2 (en) | 2014-09-18 | 2020-11-10 | Silk Water Solutions Inc. | Composition and method for water conditioning |
| AU2015207864B1 (en) * | 2014-09-18 | 2015-11-12 | Silk Water Solutions Inc. | A composition and method for water conditioning |
| US9115014B1 (en) * | 2014-09-18 | 2015-08-25 | Silk Water Solutions Inc. | Composition and method for water conditioning |
| US10253514B2 (en) | 2014-09-18 | 2019-04-09 | Silk Water Solutions Inc. | Composition and method for water conditioning |
| WO2016042425A1 (en) * | 2014-09-18 | 2016-03-24 | Silk Water Solutions Inc. | A composition and method for water conditioning |
| AU2015316535B2 (en) * | 2014-09-18 | 2020-02-06 | Silk Water Solutions Inc. | A composition and method for water conditioning |
| WO2018007298A1 (en) * | 2016-07-05 | 2018-01-11 | Henkel Ag & Co. Kgaa | Dishwasher agent containing saccharic acid and amino carboxylic acid |
| EP3593896A4 (en) * | 2017-03-07 | 2020-12-23 | Kurita Water Industries Ltd. | Water treatment chemical, method for preparing same, and method for washing polyamide-based reverse osmosis membrane |
| US11400420B2 (en) | 2017-03-07 | 2022-08-02 | Kurita Water Industries Ltd. | Water treatment chemical, method for preparing same, and method for washing polyamide reverse osmosis membrane |
| DE102018206661A1 (en) * | 2018-04-30 | 2019-10-31 | Henkel Ag & Co. Kgaa | Detergent composition for automatic dishwashing filter cleaning |
| DE102018131883A1 (en) * | 2018-12-12 | 2020-06-18 | Henkel Ag & Co. Kgaa | Detergent or cleaning agent containing iminodisuccinate and / or iminotrisuccinate |
| EP3666871A1 (en) * | 2018-12-12 | 2020-06-17 | Henkel AG & Co. KGaA | Washing or cleaning agents containing iminodisuccinate and / or iminotrisuccinate |
| US11891588B2 (en) | 2019-07-31 | 2024-02-06 | Ecolab Usa Inc. | Personal protective equipment free delimer compositions o |
| CN111394207A (en) * | 2020-04-22 | 2020-07-10 | 周栋 | Water-soluble film dish washing tablet and preparation method thereof |
| CN111910191A (en) * | 2020-07-24 | 2020-11-10 | 苏州波菲特新材料科技有限公司 | Powder degreasing agent, preparation method thereof and drying device |
| CN111910191B (en) * | 2020-07-24 | 2024-03-08 | 苏州波菲特新材料科技有限公司 | Preparation facilities of powder degreaser |
| WO2023275269A1 (en) | 2021-06-30 | 2023-01-05 | Nouryon Chemicals International B.V. | Chelate-amphoteric surfactant liquid concentrates and use thereof in cleaning applications |
Also Published As
| Publication number | Publication date |
|---|---|
| ITCR20060016A1 (en) | 2007-12-08 |
| WO2007141635A3 (en) | 2008-09-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007141635A2 (en) | Detergents formulations with low impact on the environment | |
| CA2500920C (en) | Non-polymer thickening agent and cleaning composition | |
| EP2297290B1 (en) | Biodegradable scale control composition for use in highly concentrated alkaline detergents | |
| Yangxin et al. | Development of surfactants and builders in detergent formulations | |
| CA2512207C (en) | Cleaning concentrate | |
| CA2767310C (en) | Method of removing/preventing redeposition of protein soils | |
| CN102292428B (en) | Development of an aluminum hydroxycarboxylate builder | |
| ES2638446T3 (en) | Cleaning composition | |
| WO2009024747A2 (en) | Environmentally acceptable hard surface treatment compositions | |
| CN103649291A (en) | Calcium sequestering composition | |
| CN103215144A (en) | Acidic lasting wall built-up type toilet bowl cleaner and preparation method thereof | |
| US20090082245A1 (en) | Method for formulating a branded cleaning product | |
| CN111534388A (en) | Low-foam easy-to-bleach sterilization laundry detergent and preparation method thereof | |
| JP3927623B2 (en) | Cleaning composition | |
| EP1067172B1 (en) | Detergent composition | |
| JP2002530517A (en) | Encapsulated detergent | |
| CA2649239C (en) | Formulations with unexpected cleaning performance incorporating a biodegradable chelant | |
| KR101140217B1 (en) | Eco-friendly detergent composition for cleaning water reservoir | |
| JP2015196778A (en) | Method for washing tablewares | |
| WO2015171091A1 (en) | Use of oxidized humic acid its salts and derivatives in laundry compositions | |
| WO2015171090A1 (en) | Use of oxidized humic acid its salts and derivatives in cleaning compositions | |
| WO2010061239A1 (en) | Method for formulating a branded cleaning product | |
| WO2015171089A1 (en) | Use of oxidized humic acid its salts and derivatives in dishwashing compositions | |
| Gambogi et al. | Dishwashing with detergents | |
| Mehta et al. | Scaling Problems in Home Care Applications |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 07734783 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: RU |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 07734783 Country of ref document: EP Kind code of ref document: A2 |