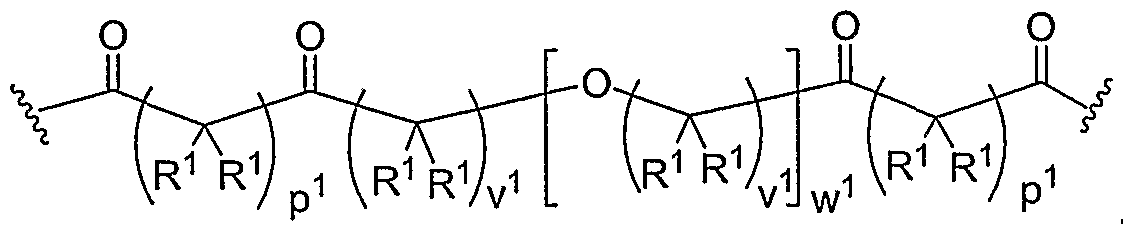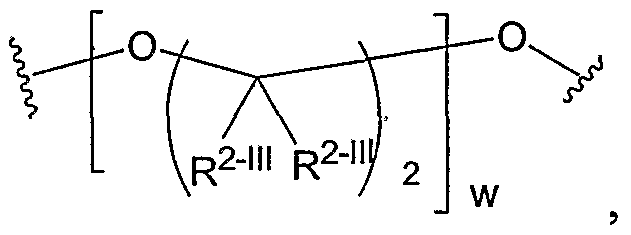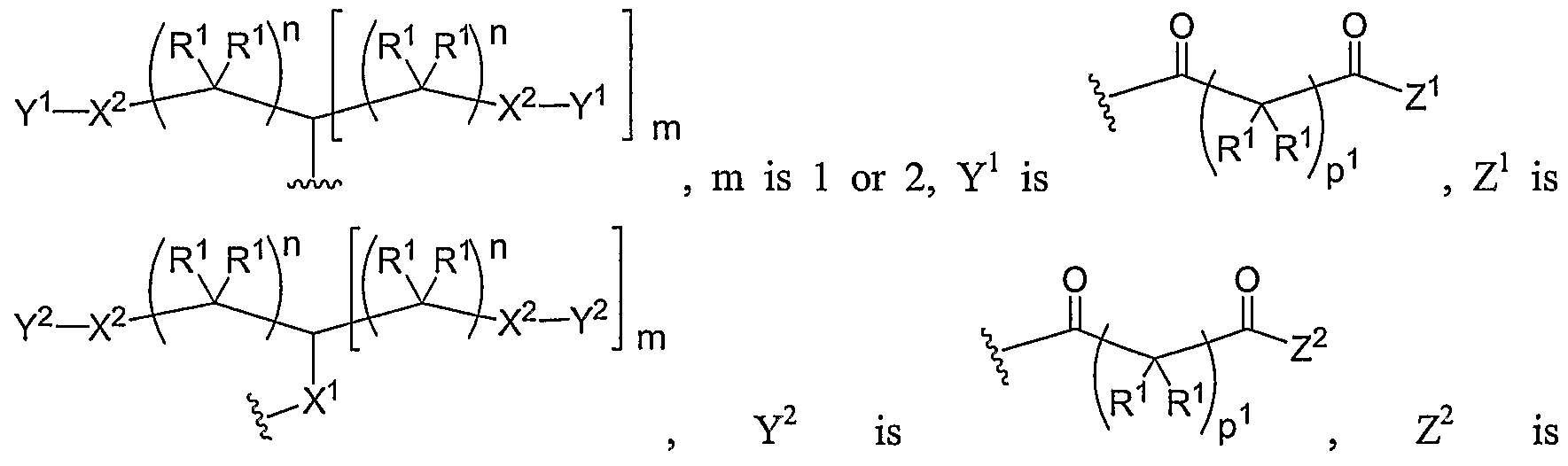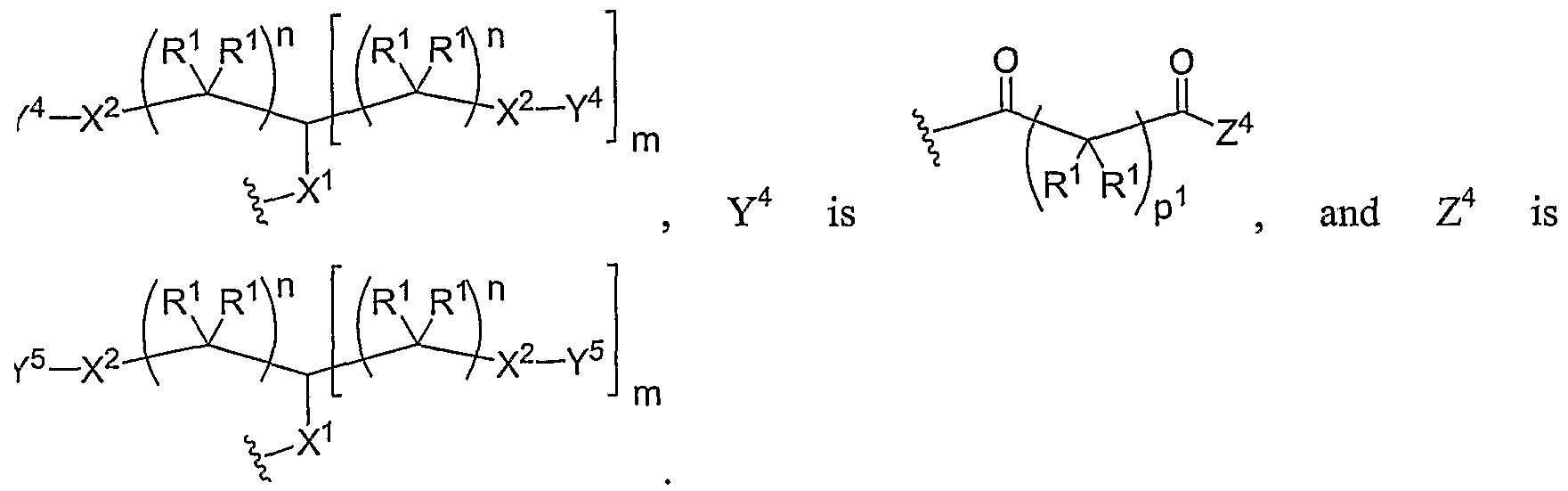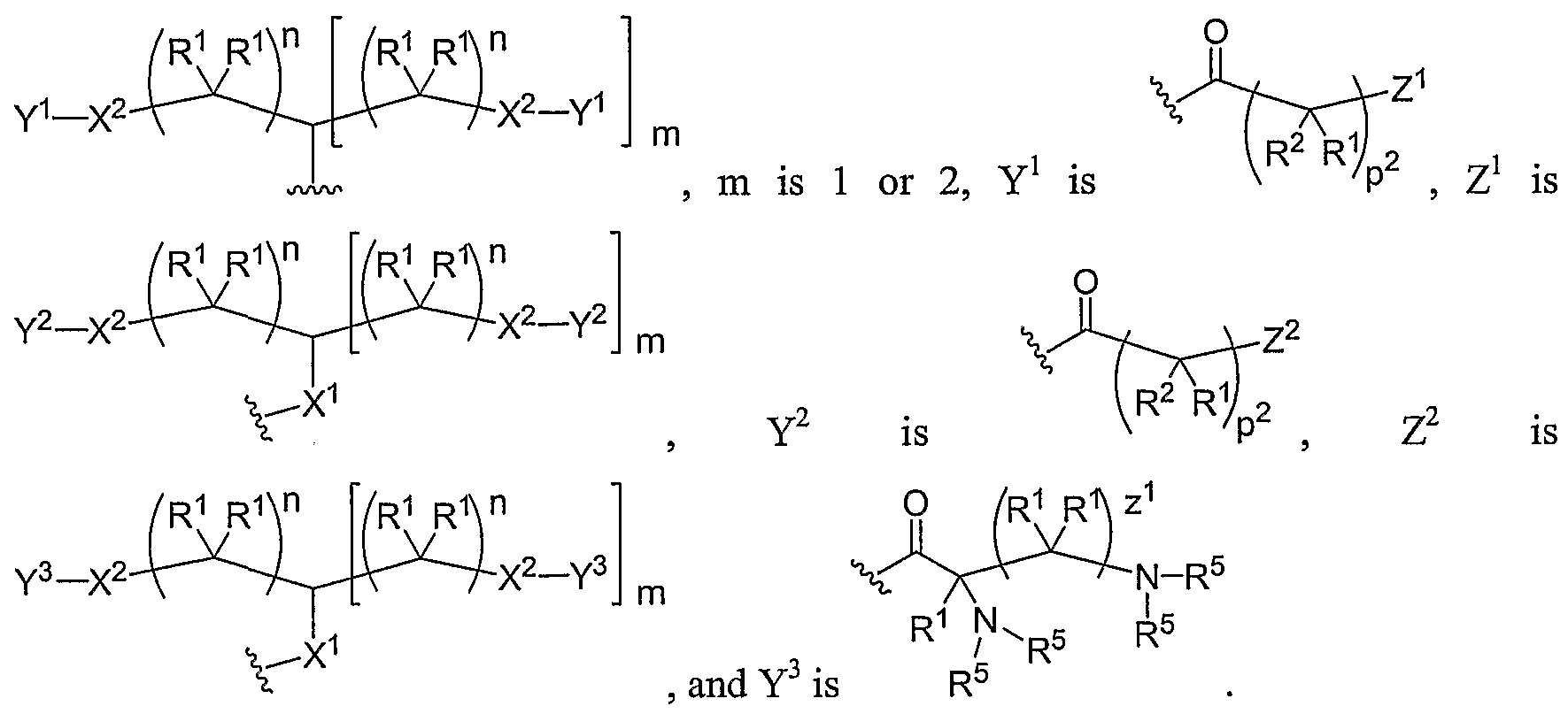WO2007001926A2 - Low-swelling hydrogel sealants for wound repair - Google Patents
Low-swelling hydrogel sealants for wound repair Download PDFInfo
- Publication number
- WO2007001926A2 WO2007001926A2 PCT/US2006/023639 US2006023639W WO2007001926A2 WO 2007001926 A2 WO2007001926 A2 WO 2007001926A2 US 2006023639 W US2006023639 W US 2006023639W WO 2007001926 A2 WO2007001926 A2 WO 2007001926A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- occurrence
- represents independently
- compound
- formula
- alkyl
- Prior art date
Links
- 0 C*P(C)(N(*)O*)OC Chemical compound C*P(C)(N(*)O*)OC 0.000 description 74
- JDFXJJLFADUZIY-UHFFFAOYSA-N CON(C(CC1)=O)C1=O Chemical compound CON(C(CC1)=O)C1=O JDFXJJLFADUZIY-UHFFFAOYSA-N 0.000 description 2
- MYMDPTPDEXPFMS-FNORWQNLSA-N CC(C=C)/N=C/C Chemical compound CC(C=C)/N=C/C MYMDPTPDEXPFMS-FNORWQNLSA-N 0.000 description 1
- MFGZEHUHXJBUOR-UHFFFAOYSA-N CC(CCNC(C(CCCCNC(CCC([N+](C(CC1)=O)(C1=O)[O-])=O)=O)NC(CCC([N+](C(CC1)=O)(C1=O)[O-])=O)=O)=O)OCCOCCNC(C(CCCCNC(CCC([N+](C(CC1)=O)(C1=O)[O-])=O)=O)NC(CCC([OH+][NH2+]C(CCC=O)=O)=O)=O)=O Chemical compound CC(CCNC(C(CCCCNC(CCC([N+](C(CC1)=O)(C1=O)[O-])=O)=O)NC(CCC([N+](C(CC1)=O)(C1=O)[O-])=O)=O)=O)OCCOCCNC(C(CCCCNC(CCC([N+](C(CC1)=O)(C1=O)[O-])=O)=O)NC(CCC([OH+][NH2+]C(CCC=O)=O)=O)=O)=O MFGZEHUHXJBUOR-UHFFFAOYSA-N 0.000 description 1
- KFYKZKISJBGVMR-UHFFFAOYSA-N CCC(C)NCC Chemical compound CCC(C)NCC KFYKZKISJBGVMR-UHFFFAOYSA-N 0.000 description 1
- WQZPUWMOQOEQHK-UHFFFAOYSA-N CON(C(CC1S(O)(=O)=O)=O)C1=O Chemical compound CON(C(CC1S(O)(=O)=O)=O)C1=O WQZPUWMOQOEQHK-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/30—Macromolecular organic or inorganic compounds, e.g. inorganic polyphosphates
- A61K47/34—Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyesters, polyamino acids, polysiloxanes, polyphosphazines, copolymers of polyalkylene glycol or poloxamers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/06—Ointments; Bases therefor; Other semi-solid forms, e.g. creams, sticks, gels
Definitions
- Sealants and adhesives play an important role in helping patients recover from surgery or trauma. Sealants and adhesives are useful in treating patients suffering from a variety of in vivo or topical conditions, including lacerations, tears, wounds, ulcers, and surgical procedures. Sealants or adhesives can generally be used in any indication or application that a suture is presently used, and the sealant or adhesive often provides a better outcome than when a suture is used. Sealants or adhesives can also be applied more quickly to the injury site and often provide a better seal over the wound. Various medicinal applications for sealants and adhesives are described below.
- Skin lacerations are tears in the skin produced by accidents, trauma, or as a result of a surgical procedure. Lacerations often require treatment in order to close the hole in the skin, stop bleeding, and prevent infection. Minor lacerations in the skin may be treated using an adhesive tissue to cover the wound. However, larger laceractions often require sutures or a glue to help seal the wound. For example, it is generally recommended that sutures or a glue be used to treat lacerations deeper than 0.25 inches having a jagged edge or loose flap of tissue. The location of the laceration may also affect the form of treatment. For example, it is advantageous to treat a skin laceration on a joint using a glue because adhesive tissue tends to limit mobility of the joint. The use of sutures or glues to treat skin lacerations can also reduce the chance of scar formation.
- Lacerations of the liver can occur from trauma or as a result of a surgical procedure.
- the liver is a highly vascularized organ and bleeds profusely when lacerated or traumatized.
- Liver lacerations are difficult to repair owing to the nature of liver tissue.
- Liver tissue has very weak cohesive strength, and, consequently, sutures and staples are not satisfactory because they may pull through the liver tissue.
- the lack of satisfactory wound treatment methods for liver lacerations combined with the fact that it is difficult to reach the veins that feed the liver renders liver lacerations particularly serious. In fact, severe lacerations of the liver often result in the patient's death due to bleeding. Thus, new materials to treat liver lacerations are needed. Cornea - Corneal Lacerations/Perforations
- Corneal perforations/lacerations are produced by a variety of medical conditions (e.g., infection, inflammation, xerosis, neurotrophication, and degeneration) and traumas (e.g., chemical, thermal, surgical, and penetrating).
- medical conditions e.g., infection, inflammation, xerosis, neurotrophication, and degeneration
- traumas e.g., chemical, thermal, surgical, and penetrating.
- corneal perforations often lead to loss of vision and a decrease in an individual's quality of life.
- different treatments are currently available ranging from suturing the wound to a cornea graft.
- the surgical procedures are difficult given the delicate composition of the cornea and the severity of the wound which increases the likelihood for leakage and severe astigmatism after surgery.
- tissue adhesives glues
- This type of treatment is advantageous because the method is simple, quick, safe, and provides quick restoration of globe integrity, thereby avoiding further complications.
- an adhesive should: 1) bind to the tissue (necrosed or not, very often wet) with an adequate adhesion force; 2) be non-toxic; 3) be biodegradable or resorbable; 4) be sterilizable; and 5) not interfere with the healing process.
- alkyl-cyanoacrylates are available for the repair of small perforations.
- these "super glues” have several disadvantages.
- the monomers used to form the alkyl-cyanoacrylate polymer adhesive can be toxic with formation of formaldehyde.
- Alkyl-cyanoacrylates can also polymerize too quickly, which complicates administering the adhesive to the wound.
- the alkyl-cyanoacrylate polymer also has a hard, rough surface causing patient discomfort and the need to wear a contact lens.
- Adhesive hemostats based on fibrin, are usually constituted of fibrinogen, thrombin and factor XIII. Systems with fibrinogen and photosensitizers activated with light are also being tested. If adhesive hemostats have intrinsic properties which meet the requirements for a tissue adhesive, autologous products (time consuming in an emergency) or severe treatments before clinical use are needed to avoid any contamination to the patient.
- Sealants for corneal perforations generally should 1) not impair normal vision, 2) quickly restore the intraocular pressure '1OP), 3) maintain the structural integrity of the eye, 4) promote healing, 5) adhere to moist issue surfaces, 6) possess solute diffusion properties which are molecular weight dependent md favorable for normal cornea function, 7) possess rheological properties that allow for iontroUed placement of the polymer on the wound, and 8) polymerize under mild ;onditions.
- sutures for wound repair has several limitations and disadvantages.
- sutures such as 10-0 nylon (which s often the preferred suture for corneal repair as well as in other in vivo areas) can act as a iidus for infection and incite corneal inflammation and vascularization.
- the Dropensity for corneal scarring increases with persistent inflammation and vascularization.
- corneal suturing often yields uneven healing and resultant regular and irregular astigmatism.
- sutures are prone to becoming loose and/or broken and may require additional attention for prompt removal.
- effective suturing requires technical skills that vary widely from surgeon to surgeon and can also involve prolonged operative time.
- a trephine a special round cutting tool called a trephine.
- the donor cornea is cut to a matching size. Then, the donor cornea is placed upon the eye and secured in place with approximately 16 sutures around the transplant.
- a sutureless procedure would be highly desirable and would offer the following advantages: 1) sutures provide a site for infection, 2) the sutured cornea takes about 3 months to heal before the sutures need to be removed, and 3) the strain applied to the new cornea tissue from the sutures can distort the cornea.
- an ocular adhesive could serve as an adjuvant to sutures and/or reduce the necessary number of sutures.
- phacoemulsification Clear corneal incisions in the temporal cornea offer several advantages with phacoemulsification.
- the major advantage associated with phacoemulsification is the reduction in size of the entrance wound. Smaller wounds require fewer sutures or even no sutures at all, minimizing induction of astigmatis ⁇ i j decreasing bleeding and subconjunctival hemorrhage, and speeding the recovery of visual acuity. See Agapitos, P. J. Curr. Opin. Ophthalmol. 1993, 4, 39-43 and LyIe, W. A.; Jin, G. J. J. Cataract Refract. Surg. 1996, 22, 1456-1460.
- OCT optical coherence tomography
- Laser-assisted in situ keratomileusis is the popular refractive surgical procedure where a thin, hinged corneal flap is created by a microkeratome blade. This flap is then moved aside to allow an excimer laser beam to ablate the corneal stromal tissue with extreme precision for the correction of myopia and astigmatism. At the conclusion of the procedure, the flap is then repositioned and allowed to heal. However, with trauma, this flap can become dislocated prior to healing, resulting in flap striae (folds) and severe visual loss. When this complication occurs, treatment involves prompt replacement of the flap and flap suturing. The use of sutures has limitations and drawbacks as discussed above.
- Cataracts or other diseases or injuires that lead to a poorly functioning or damaged lens require the natural lens to be replaced.
- the optical properties of the normal eye lens are the consequence of a high concentration of proteins called "crystallins" forming a natural hydrogel.
- crystallins proteins that form a natural hydrogel.
- the anatomical basis of accommodation includes the lens substance, lens capsule, zonular fibers, ciliary muscle and the elastic part of the choroid. Accommodation occurs through accurately controlled adjustments in the shape and thickness of the lens.
- the capsular bag is essential in transmitting the various extralenticular forces to the lens substance.
- Modern cataract surgery can be done through a small incision (usually 2.5-3.5 mm). Once the incision is made, the anterior chamber is filled with a viscoelastic and the capsular bag is pricked with a needle. From this incision, a small continuous circular capsulorhexis (CCC) approximately 1.5 mm in diameter is performed using capsulorhexis forceps. Next endocapsular phacoemulsification is performed and the lens epithelial cells are removed by aspiration.
- CCC circular capsulorhexis
- the polymerization can be slowed by adding iophendylate to the monomers, but the reaction still occurs in two to three seconds. Risks of retinal tear at the edge of the treated hole can also be observed because of the hardness of the polymerized cyanoacrylate.
- the vitreous is normally a clear, gel-like substance that fills the center of the eye. It makes up approximately 2/3 of the eye's volume, giving it form and shape before birth. Certain problems affecting the back of the eye may require a vitrectomy, or surgical removal of the vitreous.
- a vitrectomy the surgeon creates small incisions/punctures in the eye (sclerotomies) for separate instruments. These incisions are placed in the pars plana of the eye, which is located just behind the iris but in front of the retina.
- the instruments which pass through these incisions include a light pipe, an infusion port, and the vitrectomy cutting device.
- each sclerotomy site is closed with a single interrupted suture of 8-0 silk or 7-0 polyglycolic acid suture.
- the eye is filled with fluid until the vitreous is replaced as the eye secretes aqueous and nutritive fluids.
- Some of the most common eye conditions that require vitrectomy include 1) complications from diabetic retinopathy, such as retinal detachment or bleeding, 2) macular hole, 3) retinal detachment, 4) pre-retinal membrane fibrosis, 5) bleeding inside the eye (vitreous hemorrhage), 6) injury or infection, and 7) certain problems related to previous eye surgery.
- Leaking filtering blebs after glaucoma surgery are difficult to manage and can lead to serious, vision-threatening complications. Leaking blebs can result in hypotony and shallowing of the anterior chamber, choroidal effusion, maculopathy, retinal, and choroidal folds, suprachoroidal hemorrhage, corneal decompensation, peripheral anterior synechiae, and cataract formation. A leaking bleb can also lead to the loss of bleb function and to the severe complications of endophthalmaitis. The incidence of bleb leaks increases with the use of antimetabolites. Bleb leaks in eyes treated with 5-fluorouracil or mitomycin C may occur in as many as 20% to 40% of patients.
- Bleb leaks in eyes treated with antimetabolities may be difficult to heal because of thin avascular tissue and because of abnormal fibrovascular response. If the leak persists despite the use of conservative management, a 9-0 to 10-0 nylon or absorbable suture on a tapered vascular needle can be used to close the conjunctival wound. In a thin-walled or avascular bleb, a suture may not be advisable because it could tear the tissue and cause a larger leak.
- Fibrin adhesives have been used to close bleb leaks.
- the adhesive is applied to conjunctival wound simultaneously with thrombin to form a fibrin clot at the application site.
- the operative field must be dry during the application because fibrin will not adhere to wet tissue.
- Cyanoacrylate glue may be used to close a conjuctival opening.
- To apply the glue the surrounding tissue must be dried and a single drop of the cyanoacrylate is placed.
- the operative must be careful not to seal the applicator to the tissue or to seal surrounding tissue with glue given its quick reaction.
- a soft contact lens is then applied over the glue to decrease patient discomfort. However, this procedure can actually worsen the problem if the cyanoacrylate tears from the bleb and causes a larger wound.
- Blepharoplasty is an operation to remove excess skin, fat and muscle from around the eyes to correct droopy eyelids and bagginess under the eyes. It can be performed on the upper lids and lower lids, at the same time or separately. The operation may be done using either conventional or laser techniques. For surgery on the upper eyelids, cuts are made into the natural lines and creases in the lid, and into the laughter lines at the corner of the eye. For surgery on the lower eyelids, a cut is usually made just below the eyelashes. This means the scars run along the eye's natural folds, concealing them as much as possible. Excess fat, muscle and loose skin are removed, and the cut is closed using sutures. If only fat is being removed, sometimes the cut is made on the inside of the lower eyelid, leaving no visible scar. A tissue adhesive could provide a more effective means to secure the cuts made during these procedures. Summary of the Invention
- the present invention broadly relates to methods and compositions for sealing a wound on a patient using a hydrogel sealant/adhesive.
- a sealant comprising dendrimeric macromolecules that form a hydrogel.
- the sealants of the present invention further comprise a polymer, such as polyvinylpyrrolidone, poly(N-isopropylacrylamide), or a copolymer of poly(ethylene glycol) and poly(propylene glycol).
- the sealants of the present invention further comprise a pharmaceutical agent, such as an antibiotic, antimicrobial agent, or antiinflammatory agent.
- the sealants of the invention comprise a hydrogel that swells less than about 400 wt% upon hydration.
- the sealants of the present invention may be used to treat a wound on a patient that is topical or in vivo.
- the sealants of the present invention can act as a barrier to bacteria and other organisms.
- the sealants of the invention comprise a dendrimeric macromolecule and an alkyl diacid compound, wherein the alkyl diacid compound has a molecular weight of less than about 1000 g/mol.
- the compositions used to seal a wound comprise at least one dendrimeric macromolecule and at least one polyethylene glycol polymer.
- the dendrimeric compounds used to form the hydrogel sealant have an acrylate group attached at the periphery of the dendrimer. Treatment of acrylate-capped dendrimeric compounds with ultraviolet radiation or a chemical initiator causes the dendrimeric compounds to polymerize, thereby forming a seal.
- the dendrimeric compounds have a lysine, cysteine, isocysteine residue or other nucleophilic group attached to the periphery of the dendrimer.
- a dendrimeric compound containing two or more electrophilic groups such as an aldehyde, activated ester, or acrylate
- a linear or dendrimeric polyethyelene glycol containing two or more electrophilic groups such as an aldehyde, activated ester, or acrylate
- compositions used to seal the wound comprise a compound that has a polylysine core to which cysteine, isocysteine, or other nucleophilic groups are attached.
- Another aspect of the present invention relates to a method of sealing a wound on a patient comprising the steps of applying an effective amount of a dendrimeric compound to a wound on a patient and treating the dendrimeric compound with a polymerization agent.
- the dendrimeric compound is administered in the form of an aqueous solution.
- the polymerization agent is ultraviolet light or visible light.
- the dendrimeric compound is administered in the form of a gel.
- the dendrimeric compound is administered in the form of a patch.
- Another aspect of the present invention relates to a method of sealing a wound on a patient comprising the steps of treating a dendrimeric compound with a polymerization agent to form a repair agent, and applying the repair agent to a wound on a patient.
- the method further comprises the step of sterilizing the dendrimeric compound or repair agent.
- kits for sealing a wound comprising a polymerizable dendrimeric compound that forms a hydrogel and a system for delivering the polymerizable dendrimeric compound to a wound on a patient.
- the kit further comprises a polymerization agent.
- the system is a syringe.
- Figure 1 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
- Figure 2 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
- Figure 3 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
- Figure 4 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
- Figure 5 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
- Figure 6 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
- Figure 7 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
- Figure 8 depicts a dendrimer terminated with nucleoside groups amenable to the invention.
- Figure 9 depicts dendrimers and compounds useful for making dendrimers amenable to the present invention.
- Figure 10 depicts a dendrimer amenable to the present invention.
- Figure 11 depicts photocrosslinkable PEG 34 oo-(PGLSA-MA 4 ) 2 macromer 1 for hydrogel formation.
- Figure 13 depicts the complex modulus ⁇ G* ⁇ , storage modulus G', loss modulus G" and loss angle ⁇ of crosslinked hydrogel samples at 4 different concentrations of macromer 1 in PBS.
- Figure 14 depicts a determination of the compressive modulus E, as a function of concentration of macromer 1 in PBS.
- the compression-relaxation experiment for 20 % 1 is shown on the left, the linear curve fits for all concentrations and the resulting compressive modulus E is shown on the right.
- Figure 15 depicts histological sections of 7.5 % and 15 % macromer 1 concentration hydrogels after 2 and 4 weeks incubation. Top: Red indicates proteoglycans in the Safranin-0 stained sections, green indicates collagen in the Masson's Trichrome stained sections. Bottom: Red indicates type I or II collagen in the immunostained sections, no significant type I collagen was detected at either concentration. The length of the inserted bar is 100 ⁇ m.
- Figure 16 depicts a plot of swelling versus time for occular sealants in Example 120.
- Figure 17 depicts a plot of average swelling results versus time for occular sealants in Example 120.
- Figure 18 depicts a response surface plot of pH and buffer ratio effects for sealants in Example 121.
- Figure 19 depicts a contour plot of pH and buffer ratio effects for sealants in Example 121.
- Figure 20 depicts a response surface plot for swelling at 48 hours for sealants in Example 121.
- Figure 21 depicts a contour plot for swelling at 48 hours for sealants in Example 121.
- Figure 22 depicts a response surface plot for swelling at 72 hours for sealants in Example 121.
- Figure 23 depicts a contour plot for swelling at 72 hours for sealants in Example 121.
- Figure 24 depicts the effect of dendron buffer pH on swelling after 48 hours for sealants in Example 122.
- Figure 25 depicts the effect of dendron buffer pH on cure time for sealants in Example 122.
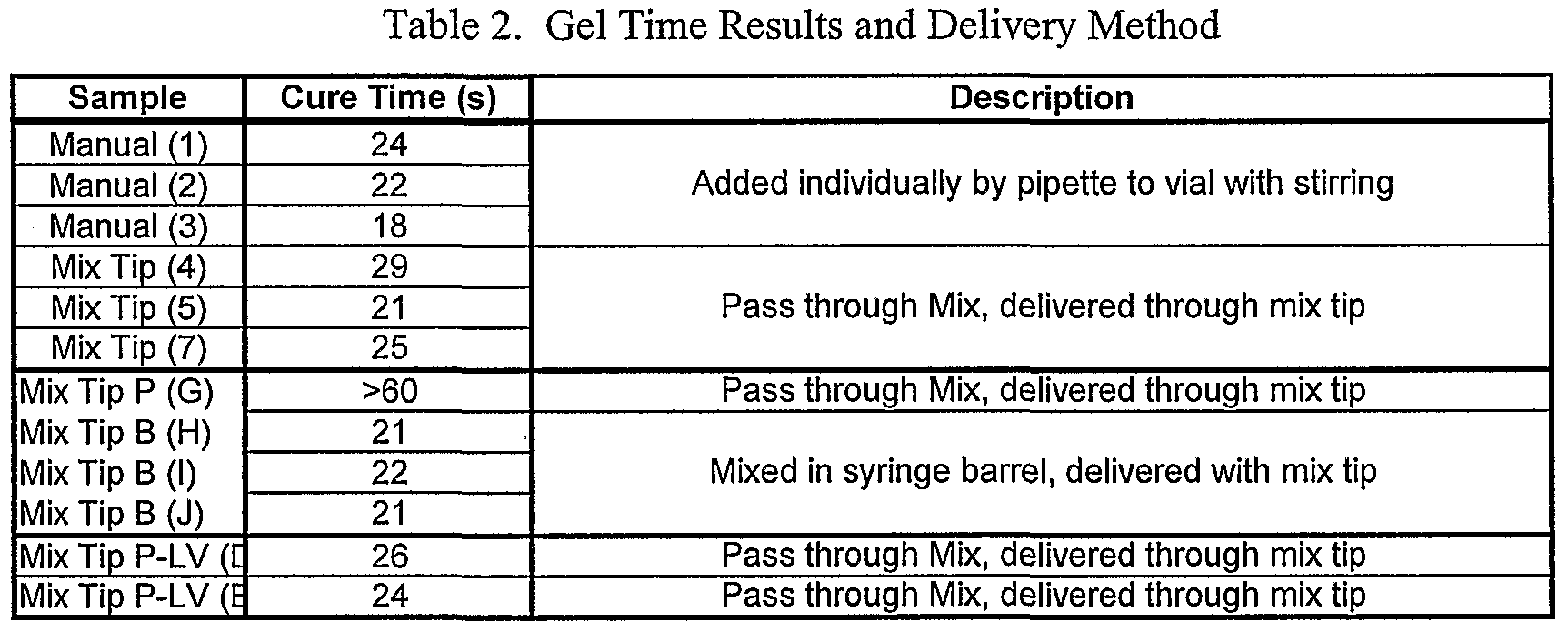
- Figure 26 depicts a swelling response surface plot for manually mixed samples for sealants in Example 123.
- Figure 27 depicts a swelling response surface plot for mix-tip samples for sealants in Example 123.
- Figure 28 depicts a cure time response surface plot for manually mixed samples (with contour plot) for sealants in Example 123.
- Figure 29 depicts a cure time response surface plot for mix-tip samples for sealants in Example 123.
- Figure 30 depicts a double-acting, single-barrel syringe.
- Figure 31 depicts a double-barrel syringe.
- the present invention broadly relates to methods and compositions for sealing a wound on a patient using a hydrogel sealant/adhesive.
- the sealant compositions of the present invention may be used in clinical treatments, such as sealing or repairing traumatic or surgical wounds.
- the sealants may be used for topical wounds or in vivo wounds, and the sealants may be formed in situ.
- the sealants of the invention may be used in situations where the site of the wound is not easily accessible and when sutureless surgery is desirable.
- the sealants of the present invention comprise dendrimeric macromolecules. In certain instances, the dendrimeric macromolecules form a hydrogel that swells less than about 400 wt% upon hydration.
- the dendrimeric macromolecular sealants of the present invention optionally comprise a polymer, such as polyvinylpyrrolidone, polyethylene glycol, poly(N-isopropylacrylamide), or a copolymer of poly(ethylene glycol) and poly(propylene glycol).
- the sealants of the invention comprise a dendrimeric macromolecule and an alkyl diacid compound, wherein the alkyl diacid compound has a molecular weight of less than about 1000 g/mol.
- the formulations used to prepare the sealant may be mixed with a pharmaceutical agent to aid in the repair of damaged tissue or organs.
- the pharmaceutical agent is a growth factor, antibiotic, antimicrobial agent, or antiinflammatory agent.
- the pharmaceutical agent may be encapsulated by the sealant or it may be bonded to the polymeric compounds forming the sealant.
- the dendrimeric macromolecules can be seeded with cells, and then used to repair the damaged tissue or organs.
- the polymers and cells can be mixed and then injected into the in vivo site and crosslinked in situ for tissue repair or replacement.
- the crosslinked polymers provide a three dimensional template for new cell growth.
- Crosslinking such as with a methacrylated functionalized denditic polymer, can be achieved using light or a chemical reaction.
- the crosslinkable biodendritic macromolecules can undergo a covalent or non- covalent crosslinking reaction to form a three-deminsional crosslinked gel or network.
- the crosslinking reaction is performed using light.
- the crosslinking reaction does not require light.
- the formulations used to prepare the hydrogel sealants/adhesives of the invention may be aqueous mixtures.
- the pH of the aqueous mixture may be adjusted to give hydrogel sealants with different physical properties. In certain instances, the pH of the mixture ranges from about 6.0 to about 10.0.
- the pH of the mixture ranges from about 7.5 to about 8.5.
- the aqueous mixture may further comprise a carbonate, phosphate, 1-piperazineethane sulfonic acid, 4-(2-hydroxyethyl)-monosodium salt (HEPES), or a citrate to modulate the pH of the mixture.
- the aqueous mixture further comprises a carbonate or phosphate.
- Dendritic polymers are globular monodispersed polymers composed of repeated branching units emitting from a central core. See US5714166; US4289872; US4435548; US5041516; US5362843; US5154853; US05739256; US5602226; US5514764; Bosman, A. W.; Janssen, H. M.; Meijer, E. W. Chem. Rev. 1999, 99, 1665-1688; Fischer, M.; Vogtle, F. Angew. Chem. Int. Ed. 1999, 38, 884-905; Zeng, F.; Zimmerman, S. C. Chem. Rev. 1997, 97, 1681-1712; Tomalia, D.
- dendrimers are highly ordered, possess high surface area to volume ratios, and exhibit numerous end groups for functionalization. Consequently, dendrimers display several favorable physical properties for both industrial and biomedical applications including: small polydispersity indexes (PDI), low viscosities, high solubility and miscibility, and excellent adhesive properties.
- PDI polydispersity indexes
- the majority of dendrimers investigated for biomedical/biotechnology applications e.g., MRI, gene delivery, and cancer treatment
- are derivatives of aromatic polyether or aliphatic amides are not ideal for in vivo uses. See Service, R. F.
- Biodendrimers are a novel class of dendritic macromolecules composed entirely of building blocks known to be biocompatible or degradable to natural metabolites in vivo.
- the synthesis, characterization, and use of biodendrimers composed of biocompatible or natural metabolite monomers such as glycerol, lactic acid, glycolic acid, succinic acid, ribose, adipic acid, malic acid, glucose, citric acid, glycine, lysine, cysteine, alanine, etc. are described herein.
- the dendritic structures of the invention contain glycerol and one or more of lactic acid, glycolic acid, succinic acid, ribose, adipic acid, malic acid, glucose, citric acid, glycine, lysine, cysteine, alanine, and the like as a building block.
- the dendrimer is terminated with a photoreactive group or nucleophilic group.
- the terminus of the dendrimer contains a nucleoside.
- An additional embodiment of the invention is a dendritic structure that is composed of all lysine resides, such that it is a generation one or higher, or it is a lysine dendritic macromolecule terminated with cystene residues such that it is a generation one or higher.
- One aspect of the present invention relates to the synthesis and fabrication of dendritic polymers and copolymers of polyesters, polyethers, polyether-esters, and polyamino acids or combinations thereof.
- linear poly(glycolic acid), ⁇ oly(lactic acid), and their copolymers are synthetic polyesters that have been approved by the FDA for certain uses, and have been used successfully as sutures, drug delivery carriers, and tissue engineering scaffold for organ failure or tissue loss (Gilding and Reed, Polymer, 20:1459 (1979); Mooney et al., Cell Transpl., 2:203 (1994); and Lewis, D. H.
- a further embodiment of this invention is to attach biological recognition units for cell recognition to the end groups or within the dendrimer structure.
- the tripeptide arginine-glycine-aspartic (RGD) can be added to the structure for cell binding.
- RGD tripeptide arginine-glycine-aspartic
- Barrera et al. described the synthesis of a poly(lactic acid) (pLAL) containing a low concentration of N-epsilon-carbobenzoxy-L-lysine units. The polymers were chemically modified through reaction of the lysine units to introduce arginine-glycine-aspartic acid peptide sequences or other growth factors to improve polymer-cell interactions (Barrera et al., J. Am. Chem.
- a dendritic polymer includes multiple end-groups for functionalization, crosslinked gels with high crosslinking-densities at low polymer concentration, globular structure, low viscosities, and a well-defined composition.
- Conventional linear polymers for medical applications cannot be controlled easily or modified through changes in the polymer's structure because these polymers (e.g., PLA) do not possess functional groups, other than end groups, that permit chemical modification to change their properties.
- these polymers do not adopt a well-defined structure in solution, thereby limiting the applications of these polymers. Consequently, the novel polymers described herein are substantially different.
- Gels are 3D polymeric materials which exhibit the ability to swell in water and to retain a fraction of water within the structure without dissolving.
- the physical properties exhibited by gels such as water content, sensitivity to environmental conditions ⁇ e.g., pH, temperature, solvent, and stress), softness, adhesivity, and rubbery consistency are favorable for biomedical and biotechnological applications.
- gels may be used as coatings ⁇ e.g. biosensors, catheters, and sutures), as "homogeneous" materials ⁇ e.g.
- one aspect of the present invention relates to a gel comprising cells to create an artificial cornea to replace or aid in the repair of a damaged cornea.
- the gel is formed from a dendrimeric compound.
- Another aspect of the present invention relates to a method and means for designing, constructing, and utilizing artificial dendritic matrices as temporary scaffolding for cellular growth and implantation.
- a further embodiment of the invention to provide biodegradable, non-toxic matrices which can be utilized for cell growth, both in vitro, in vivo, and in situ.
- the cell scaffold/matrix/gel can be formed in vitro or in situ by crosslinking.
- the present invention relates to a method for configuring and constructing biodegradable artificial matrices such that they not only provide a support for cell growth, but also allow and enhance vascularization and differentiation of the growing cell mass following implantation.
- the present invention relates to matrices in different configurations so that cell behavior and interaction with other cells, cell substrates, and molecular signals can be studied in vitro.
- the dendritic macromolecules of the present invention are useful as a tissue sealant or adhesive.
- the polymers of the invention may be used as an ophthalmic sealant or adhesive for corneal lacerations, retinal tears, corneal transplants, cataract procedures, and the like.
- the macromolecules of the invention may be used to seal a large variety of different types of wounds, and one of skill in the art will recognize that the sealant/adhesive has wide-spread application in ophthalmic and general surgeries.
- the sealants of the invention are used to seal a wound that is less than about 10 cm 2 , 5 cm 2 , or 1 cm 2 in size.
- the sealants of the invention are used to seal a wound that is less than about 5 cm, 2 cm, or 1 cm in length. In certain instances, the sealants of the invention are used to seal a wound that is less than about 0.5 cm, 0.2 cm, 0.1, or 0.05 cm in length.
- a further embodiment of this invention is to use biodendritic crosslinkable polymers for sealing corneal perforations.
- a further embodiment of this invention is to use biodendritic crosslinkable polymers for sealing retinal holes.
- a further embodiment of this invention is to use biodendritic crosslinkable polymers for sealing leaking blebs.
- a further embodiment of this invention is to use biodendritic crosslinkable polymers for sealing a corneal transplant.
- the crosslinkable polymers of the invention may be used when the site of the wound is not easily accessible or when sutureless surgery is desired.
- the crosslinkable sealants may also be useful in cardiovascular surgery (aortic dissection or anastomotic bleeding), urinary tract surgery (nephrotomy closure, urethral repair, or hypospadia repair), pulmonary surgery (sealing parenchymal & bronchial leaks, bronchopleural fistula repair, or persistent air leak repairs), G.I.
- Low-swelling hydrogels are advantagous because hydrogel sealants/adhesives that swell significantly can become dislodged from the application site and lose their adhesive strength.
- a hydrogel that swells about 700 wt% in aqueous solution can have poor performance in an ocular laceration.
- the low-swelling hydrogel is a hydrogel that swells less than about 400 wt% in an aqueous solution.
- the low-swelling hydrogel swells less than about 300 wt% or 200 wt% in an aqueous solution.
- the hydrogel sealants of the invention can form in less than about 30 minutes. In certain instances, the hydrogel sealants of the invention form in less than about 15 minutes, 5 minutes, or 1 minute.
- Biologically active agents may be incorporated in the dendritic gel.
- Biologically active agents amenable to the present invention include, without limitation, medicaments; vitamins; mineral supplements; substances used for the treatment, prevention, diagnosis, cure or mitigation of disease or illness; or substances which affect the structure or function of the body; or pro-drugs, which become biologically active or more active after they have been placed in a predetermined physiological environment.
- Preferred active agents amenable for use in the compositions of the present invention include growth factors, such as transforming growth factors (TGFs), fibroblast growth factors (FGFs), platelet derived growth factors (PDGFs), epidermal growth factors (EGFs), connective tissue activated peptides (CTAPs), osteogenic factors, and biologically active analogs, fragments, and derivatives of such growth factors.
- TGFs transforming growth factors
- FGFs fibroblast growth factors
- PDGFs platelet derived growth factors
- EGFs epidermal growth factors
- CTAPs connective tissue activated peptides
- osteogenic factors and biologically active analogs, fragments, and derivatives of such growth factors.
- TGF transforming growth factor
- TGF- ⁇ l transforming growth factor
- TGF- ⁇ 2 transforming growth factor- ⁇ 3
- bone morphogenetic proteins for example, BMP-I, BMP-2, BMP-3, BMP-4, BMP-5, BMP-6, BMP-7, BMP-8, and BMP-9
- heparin-binding growth factors for example, fibroblast growth factor (FGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and insulin-like growth factor (IGF)
- inhibins for example, Inhibin A, Inhibin B
- growth differentiating factors for example, GDF- 1
- activins for example, Activin A, Activin B, and Activin AB
- Representative categories of biologically active compounds amenable to the present invention also include anti-infective agents, anti-inflammatory agents, anti-neoplastic agents, analgesic agents, anti-thrombotic agents, diagnostic agents, mineral supplements, vitamins, and prodrugs.
- Representative analgesics include nonsteroidal anti-inflammatory drugs, opiate agonists and salicylates.
- anti-infective agents include anthelmintics, antianaerobics, antibiotics, aminoglycoside antibiotics, antifungal antibiotics, cephalosporin antibiotics, macrolide antibiotics, various beta-lactam antibiotics, penicillin antibiotics, quinolone antibiotics, sulfonamide antibiotics, tetracycline antibiotics, antimycobacterials, antituberculosis antimycobacterials, antiprotozoals, antimalarial antiprotozoals, antiviral agents, anti-retroviral agents, scabicides, and urinary anti- infectives.
- Representative antineoplastic agents include alkylating agents, nitrogen mustard alkylating agents, nitrosourea alkylating agents, antimetabolites, purine analog antimetabolites, pyrimidine analog antimetabolites, hormonal antineoplastics, natural antineoplastics, antibiotic natural antineoplastics, and vinca alkaloid natural antineoplastics.
- Specific compounds for the aforementioned categories are listed in U.S. 6,600,010 and Merck Index, 12 th Edition; S. Budavari, M. J. O'Neil, A. Smith, P. E. Heckelman, J. F. Kinneary, Ed.; Merck Research Laboratories: Whitehouse Station, NJ, 1996, both of which are hereby incorporated by reference.
- the biomaterials of the present invention are also useful as a wound dressing.
- tissue adhesives e.g., glues, sealants, patches, films and the like
- an ideal adhesive should 1) bind to the tissue (necrosed or not, sometimes wet) with an adequate adhesion force, 2) be non-toxic, 3) biodegradable or resorbable, 4) sterilizable, 5) selectively permeable to gases, 6) impermeable to bacteria, and 7) able to control evaporative water loss.
- the main function of the adhesive is to protect the wound and to enhance the healing process, or at least not prevent it.
- Adhesive hemostats based on fibrin
- fibrinogen/photosensitizers systems If their intrinsic properties meet the requirements for a tissue adhesive, autologous products (which are time consuming in emergency) or severe treatments before clinical use are needed to avoid any contamination to the patient.
- Synthetic materials mainly polymers and hydrogels, have also been developed for wound closure.
- Alkyl-cyanoacrylates are available for the repair of cornea perforations.
- One investigator observed no difference in healed skin incisions that were treated by suture or by ethyl-2-cyanoacrylate-"Mediglue” application.
- these "super glues” have disadvantages.
- Their monomers, in particular those with short alkyl chains, can be toxic, and they can polymerize too quickly leading to difficulty in treating the wound. Once polymerized, the surface of the glue is rough and hard. This can cause discomfort to the patient, and in case of cornea perforation treatment, a contact lens is often required.
- the low-swelling, polymeric dendritic sealants of the present invention can be prepared by crosslinking dendrimers or dendritic polymers using either light or a chemical crosslinking reaction.
- the dendritic polymers are cross-linked with linear polymers to form a crosslinked gel or network.
- the gels can be highly hydrated and hydrophilic.
- the adhesive/sealant is mixed with a linear or dendritic polymer, such as polyvinylpyrrolidone, to form an adhesive/sealant that is an interpentrating network adhesive/sealant.
- Such adhesives/sealants often have a higher viscosity, and thus can be applied to the injury site in a more controlled fashion.
- the polymer may be polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose or other cellulose derivatives, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n- isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
- the polymer may also be a copolymer of polyvinylpyrrolidone and one or more of the aforementioned polymers.
- the polymers are functionalized to contain groups that will react with each other to form the gel.
- the dendritic polymers can be chemically modified to have more than two functional groups.
- the dendritic polymers have nucleophilic groups, such as primary amino (-NH 2 ) groups or thiol (-SH) groups, which can react with electrophilic groups such as an acrylate, succinimidyl ester, maleimide, or aldehyde.
- Each functional group on a multifunctionally dendritic polymer is capable of covalently binding with another polymer, thereby effecting formation of the network by crosslmking between the polymers.
- covalently crosslinked networks can be formed by reacting an activated ester, such as a succinimidyl ester, with an amine or thiol, e.g., a terminal primary or secondary amine, lys, cys, and the like.
- an activated ester such as a succinimidyl ester
- an amine or thiol e.g., a terminal primary or secondary amine, lys, cys, and the like.
- Thiol- or cysteine-terminated dendritic structures that form a disulfide crosslinked network with another thiol- or cysteine- terminated dendritic or linear polymer will also form a gel.
- a gel is formed during the reaction of an aldehyde-functionalized small molecule or polymer and an amine- or cysteine-functionalized polymer.
- An additional method is to have a maleimide- or vinylsulfone-functionalized dendritic polymer react with a thiol-functionalized dendritic, linear, comb, or other polymer to form the gel.
- a functionalized succinimidyl glutarate dendritic polymer reacts with an acid-terminated dendritic, linear, comb, or other polymer to form the gel.
- An acrylate-functionalized polymer reacts with an amine- or thiol- functionalized polymer to form the crosslinked gel.
- a further embodiment of this invention is the use of a chemical peptide ligation reaction to create a crosslinked gel involving a dendritic polymer.
- the dendritic polymers have nucleophilic groups, such as primary amino groups or thiol groups, which can react with electrophilic groups such as an acrylate, succinimidyl ester, maleimide, or aldehyde on a small molecule.
- the dendritic polymer has nucleophilic groups capable of reacting with an activated diester of sebabic acid.
- Biodendrimers are often based on a core unit and contain branches.
- the core and branches may be composed of glycerol and lactic acid, glycerol and glycolic acid, glycerol and succinic acid, glycerol and adipic acid, and glycerol, succinic acid, and PEG.
- polymers such as PEG and PLA can be attached to the core unit or to a branch to make large starburst or dendritic polymers.
- the biodendrimeric macromolecular sealants of the present invention optionally comprise a polymer, such as polyvinylpyrrolidone, polyethylene glycol, poly(N-isopro ⁇ ylacrylamide), or a copolymer of polyethylene glycol) and poly(propylene glycol).
- the sealants of the invention comprise a dendrimeric macromolecule and an alkyl diacid compound, wherein the alkyl diacid compound has a molecular weight of less than about 1000 g/mol.
- One aspect of the present invention relates to a method for preparing and administrating in situ a biocompatible gel ex vivo, in vitro, or in vivo, comprising:
- a reactive composition by admixing a biocompatible crosslinking polymer having two different nucleophilic groups such as sulfhydryl and amine groups where there is at least one amine or sulfhydryl group on the polymer with a biocompatible crosslinking polymer B having amine and sulfhydryl-reactive groups, and further wherein the amine and sulfhydryl-reactive groups are capable of covalent reaction with the amine and sulfhydryl groups upon admixture of polymers A and B under effective crosslinking conditions to form a gel in less than one day; and
- Another aspect of the present invention relates to dendritic or branched polymers or copolymers composed of monomers synthesized by combining branching compounds with other linear or branched building blocks.
- Both components are known to be biocompatible or are natural metabolites in vivo including but not limited to glycerol, citric acid, lactic acid, glycolic acid, adipic acid, caproic acid, ribose, glucose, succinic acid, malic acid, amino acids, peptides, synthetic peptide analogs, poly(ethylene glycol), poly(hydroxyacids) [e.g., PGA. PLA], including where one of the monomers is a branched structure such as glycerol combined with one of the other components.
- the present invention relates to the aforementioned polymers derivatized with peripheral compounds possessing an olefin, including, but not limited to, acrylate and methacrylate.
- the present invention relates to the aforementioned polymers derivatized with peripheral compounds including, but not limited to, cysteine, lysine, other amino acids, or any other compounds that would provide terminal nucleophiles (including, but not limited to, amines, thiols, and hydroxyl groups) or electrophiles (including, but not limited to, NHS esters, maleimides, aldehydes, and ketones).
- peripheral compounds including, but not limited to, cysteine, lysine, other amino acids, or any other compounds that would provide terminal nucleophiles (including, but not limited to, amines, thiols, and hydroxyl groups) or electrophiles (including, but not limited to, NHS esters, maleimides, aldehydes, and ketones).
- the present invention relates to the aforementioned polymers for subsequent polymerization/crosslinking/reaction with another linear or branched structure with either olefmic, electrophilic or nucleophilic groups, respectively to form a gel.
- the present invention relates to the aforementioned polymers for subsequent polymerization/crosslinking/reaction with another linear or branched structure via a photopolymerization process (single or multi-photon process) to form a gel.
- Another aspect of the present invention relates to a branching structure with at least three functional groups composed of, but not limited to, glycerol, citric acid, malic acid, amino acids, peptides, synthetic peptide analogs, or other dendritic structures synthesized to produce terminal olefins (including but not limited to acrylate or methacrylate groups), nucleophiles (including but not limited to amines, thiols, and hydroxyl groups) or electrophiles (including but not limited to NHS esters, maleimides, aldehydes, and ketones) for subsequent polymerization/crosslinking with another linear or branched structure with either olefmic, electrophilic or nucleophilic groups, respectively.
- Another aspect of the present invention relates to a branching structure with at least three functional groups composed of, but not limited to, glycerol, citric acid, malic acid, amino acids, peptides, synthetic peptide analogs, or other dendritic structures derivatized with peripheral compounds including, but not limited to, cysteine, lysine, other amino acids, or any other compounds that would provide terminal olefins (including but not limited to acrylate or methacrylate groups), nucleophiles (including but not limited to amines, thiols, and hydroxyl groups) or electrophiles (including but not limited to NHS esters, maleimides, aldehydes, and ketones) for subsequent polymerization/crosslinking with another linear or branched structure with either olefmic, electrophilic or nucleophilic groups, respectively.
- functional groups composed of, but not limited to, glycerol, citric acid, malic acid, amino acids, peptides, synthetic peptide analogs
- Another aspect of the present invention relates to a branching structure composed of three lysine amino acids with four cysteine amino acids on the periphery with the structure CysLys(Cys)Lys(CysLys(Cys))OMe*4HCl as described in the examples.
- Another aspect of the present invention relates to a branching structure composed of three lysine amino acids with amines on the periphery with the structure (Lys)Lys(Lys)OMe»4HCl as described in the examples.
- the present invention relates to the aforementioned polymers for subsequent polymerization/crosslinking/reaction with another linear or branched structure with olefmic, electrophilic or nucleophilic groups to form a gel.
- the present invention relates to the aforementioned polymers for subsequent polymerization/crosslinking/reaction with another linear or branched structure through thiazolidine linkages to form a gel.
- the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a poly(ethylene glycol) having a molecular weight of about 200 to about 200,000 g/mol with at least two electrophilic groups.
- the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a poly(ethylene glycol) having a molecular weight of about 200 to about 200,000 g/mol with at least two nucleophilic groups
- the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a poly(ethylene glycol) having a molecular weight of about 200 to about 200,000 g/mol with functional groups including but not limited to olefins, aldehydes, maleimides, or NHS esters.
- the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a poly(ethylene glycol) having a molecular weight of about 200 to about 200,000 g/mol with aldehyde functional groups to form hydrogels through the formation of thiazolidine linkages.
- the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a copolymer comprising poly(ethylene glycol) and polypropylene glycol, wherein the copolymer has a molecular weight of about 200 to about 200,000 g/mol with functional groups including, but not limited to, olefins, aldehydes, maleimides, and NHS esters.
- the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with an alkyl diacid, wherein the alkyl diacid has a molecular weight of about 50 to about 1,000 g/mol and the alkyl diacid contains functional groups comprising NHS-esters.
- the present invention relates to the aforementioned formulations in which each of the components are dissolved or suspended in an aqueous solution wherein the said aqueous solution is selected from water, buffered aqueous media, saline, buffered saline, solutions of amino acids, solutions of sugars, solutions of vitamins, solutions of carbohydrates or combinations of any two or more thereof.
- the present invention relates to the application of the aforementioned formulation through a delivery device which physically separates the components until the components are physically mixed by the end user, including but not limited to a dual barrel syringe with a mixing device.
- Another aspect of the present invention relates to packaging of the aforementioned branching compounds in an aqueous solution at a preselected pH and molarity selected from the aqueous solutions described above and the packaging of the second compound in an aqueous solution at another preselected pH and molarity selected from the aqueous solutions described above.
- the pH and molarities of the two solutions produce a final desired solution with a different pH.
- the aqueous solution comprising the branching compounds further comprises a polymer, such as polyvinylpyrrolidone .
- Another aspect of the present invention relates to packaging of the aforementioned branching compounds in an aqueous solution at a preselected pH and molarity selected from the aqueous solutions described above and the packaging of the second compound in an aqueous solution at another preselected pH and molarity selected from the aqueous solutions described above.
- the contents are packaged free of oxygen and shielded from light.
- the pH and molarities of the two solutions produce a final desired solution with a different pH.
- Another aspect of the present invention relates to packaging of the aforementioned branching compounds as a powder and adding an aqueous solution at a preselected pH and molarity selected from the aqueous solutions described above before use.
- the second component may either be packaged by dissolving the second compound in an aqueous solution at another preselected pH and molarity selected from the aqueous solutions described above or packaged similar to the first compound in which the compound stored as a powder and an aqueous solution at a preselected pH and molarity selected from the aqueous solutions described above is added before use.
- the contents are packaged free of oxygen and shielded from light.
- the pH and molarities of the two solutions produce a final desired solution with a different pH.
- Another aspect of the present invention relates to the storage of the aforementioned cysteine-terminated polymers in an acidic, oxygen-free solution to minimize the formation of disulfide bonds.
- Another aspect of the present invention relates to the storage of the aforementioned aldehyde-terminated polymers in an acidic, oxygen-free solution to maximize the percent reactivity of the polymer and minimize aldol condensation and reverse Michael additions.
- Another aspect of the present invention relates to the addition of various additives that might be incorporated into the polymer formulations including, but not limited to, antioxidants, colorants, viscosity modifiers, plasticizers, small molecule carbohydrates, large molecule carbohydrates, amino acids, peptides, or other water soluble polymers (linear or branched).
- additives may be added to increase the shelf life, increase the polymerization rate, modify the pH or molarity of the solution, change the refractive index, modify the mechanical properties, change crosslinking density, decrease swelling, or aid in visualization.
- Another aspect of the present invention relates to the addition of various additives or anti-microbial agents such has polyhexamethylene biguanide (PHMB) that might be incorporated into the polymer formulations.
- PHMB polyhexamethylene biguanide
- Another aspect of the present invention relates to the resulting hydrogels formed by mixing the aforementioned compounds as described and prepared herein.
- the present invention relates to hydrogels formed by photopolymerization of the aforementioned compounds.
- Another aspect of the present invention relates to a method of using crosslinkable/polymerizable/reactionary dendritic polymers, branching structures, and their hydrogels for delivery of therapeutics.
- Another aspect of the present invention relates to a method of using a crosslinlcable/polymerizable/reactionary dendritic polymer or monomer for seeding with cells and subsequent in situ polymerization in vivo.
- Another aspect of the present invention relates to a crosslinkable/polymerizable/reactionary dendritic polymer or monomer wherein the crosslinking is of covalent, ionic, electrostatic, and/or hydrophobic nature.
- Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction involves a nucleophile and electrophile.
- Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction is a peptide ligation reaction.
- Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction is a Diels-Alder reaction.
- Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction is a Michael Addition reaction.
- Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction is a photochemical reaction using a UV or visible photoinitator chromophore.
- Another aspect of the present invention relates to a crosslinkable branched or dendritic polymer in combination with a crosslinkable linear, comb, multi-block, star, or dendritic polymer(s) for a medical or tissue engineering application.
- Another aspect of the present invention relates to a crosslinkable branched or dendritic polymer in combination with a crosslinkable monomer(s) for a medical or tissue engineering application.
- Another aspect of the present invention relates to a method of using a crosslinkable branched or dendritic polymer combined with a crosslinkable small molecule(s) (molecule weight less than about 1000 Daltons) for a medical or tissue engineering application.
- Another aspect of the present invention relates to a crosslinkable branched or dendritic polymer or monomer wherein the said crosslinking dendritic polymer is combined with one or more linear, comb, multi-block, star polymers or crosslinkable comb, multi- block, star polymers.
- Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the final polymeric form is a gel, film, fiber, or woven sheet.
- Another aspect of the present invention relates to the aforementioned polymers, branching structures, and their resulting hydrogels wherein the final polymeric form is a gel, film, fiber, or woven sheet.
- Another aspect of the present invention relates to the aforementioned polymers, branching structures, and their resulting hydrogels wherein the polymer or crosslinkable monomer is D or L configuration or a mixture.
- Another aspect of the present invention relates to the aforementioned polymers, branching structures, and their hydrogels wherein the dendritic structure is asymmetric at the surface such as a surface block structure where a carboxylate acid(s) and alkyl chains, or acrylate(s) and PEG(s) are present, for example, or within the core and inner layers of the dendrimer such as amide and ester linkages in the structure.
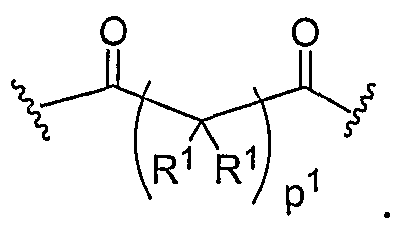
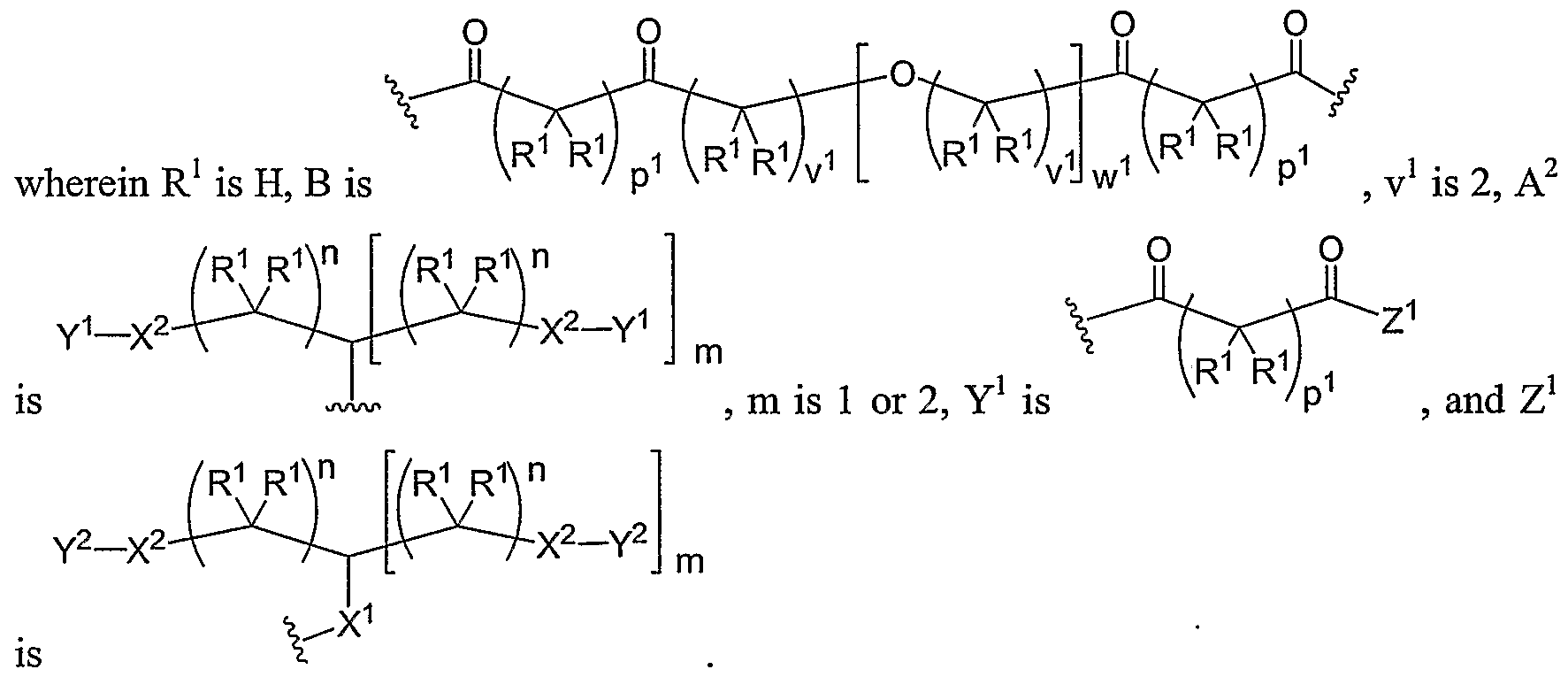
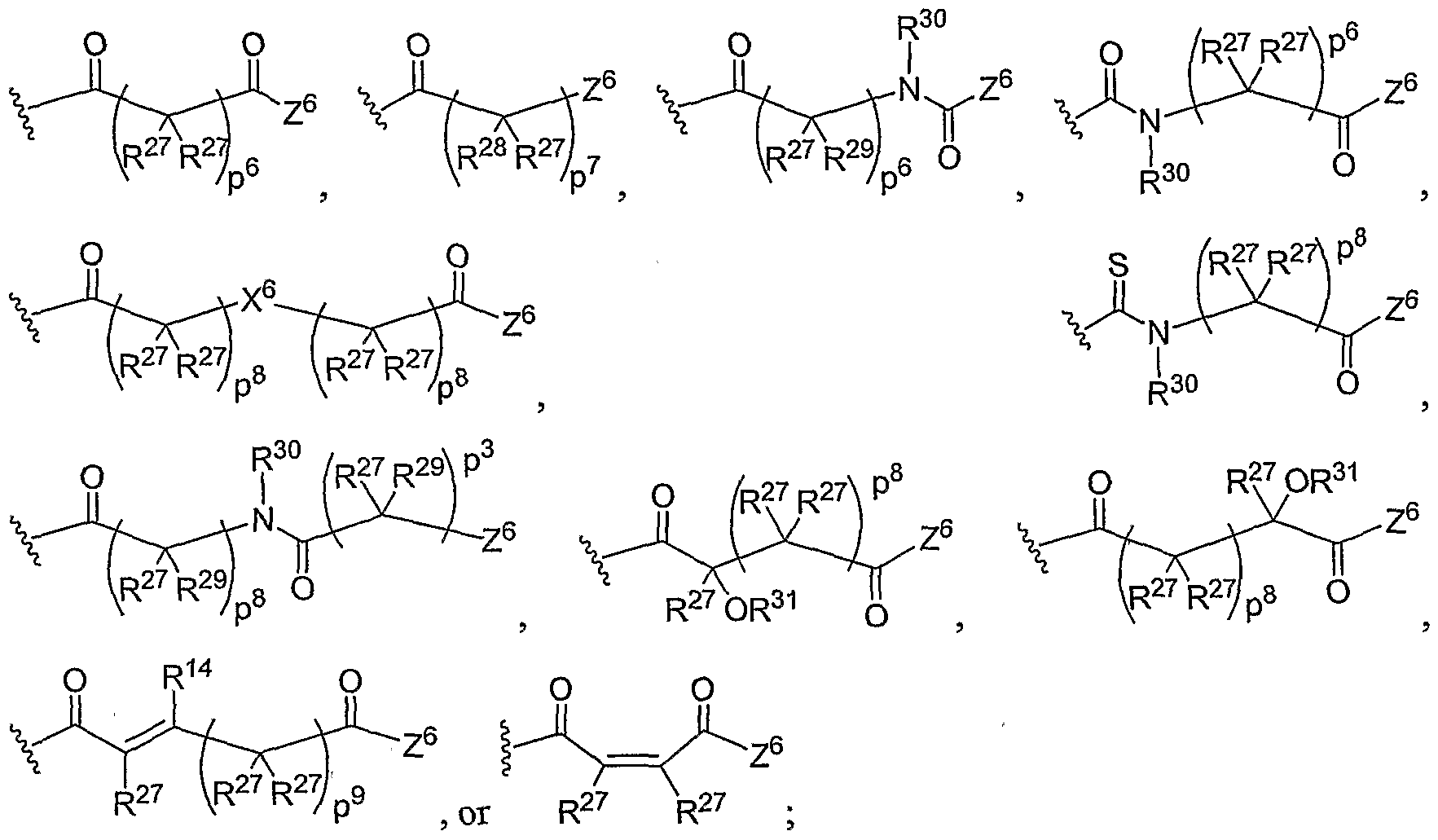
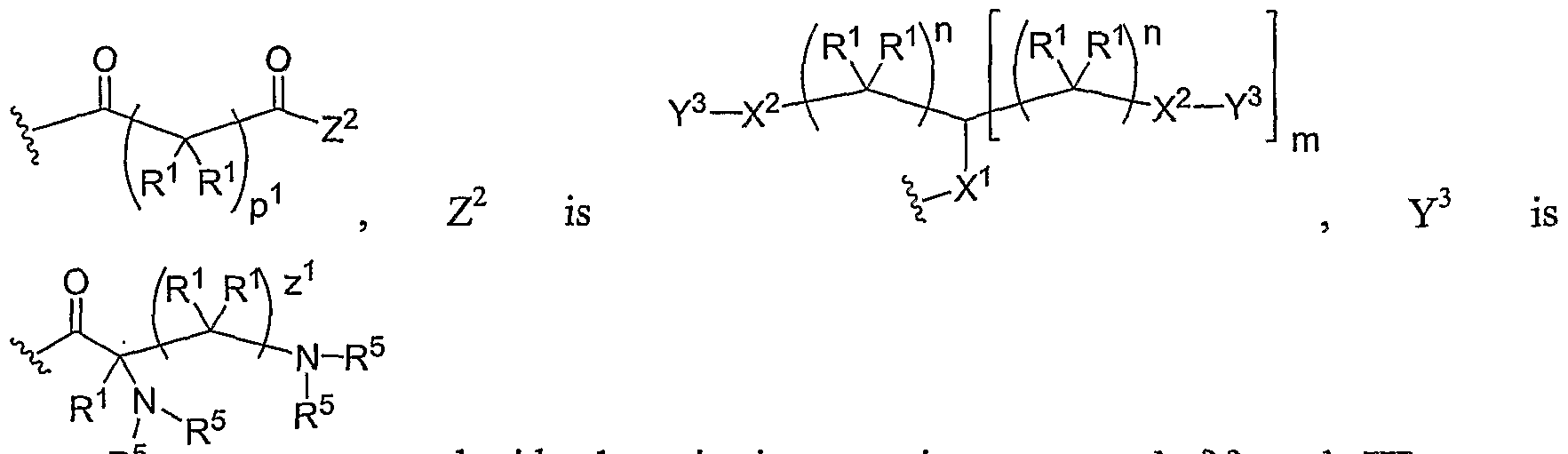
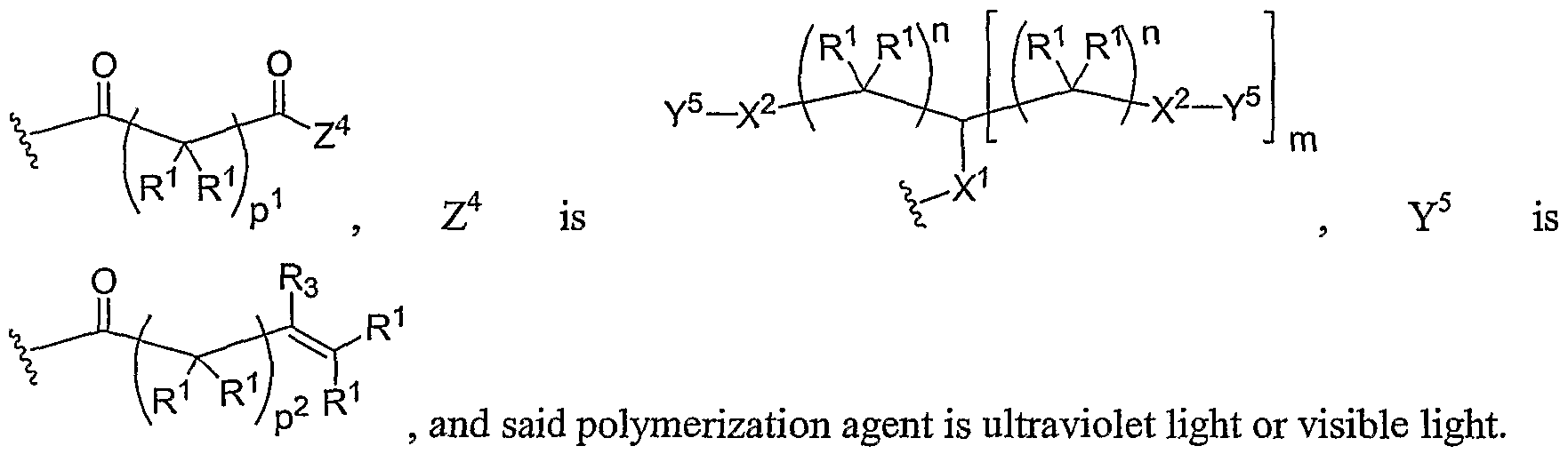
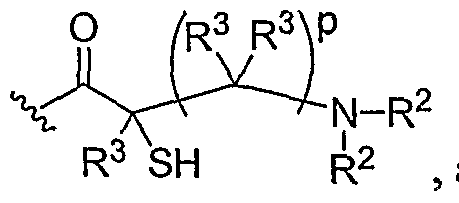
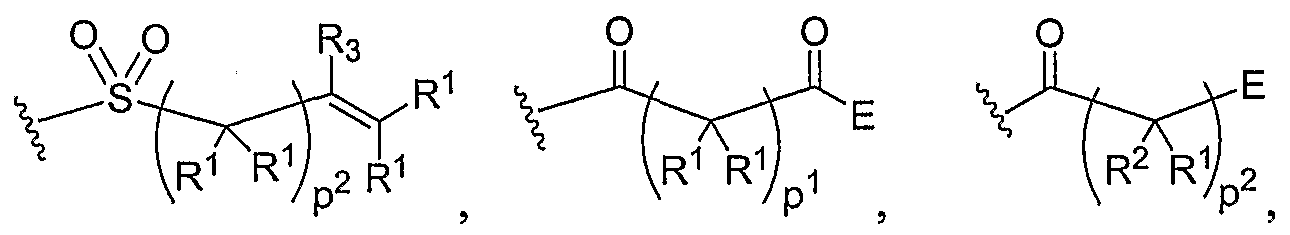
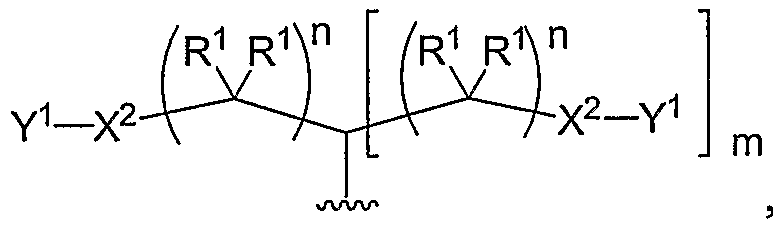
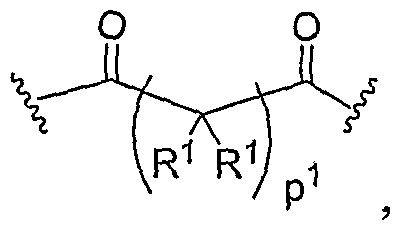
- Another aspect of the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer wherein the polymer is a star biodendritic polymer or copolymer as shown in at least one of the formulas below: where Y and X are the same or different at each occurrence and are O, S, Se, N(H), or P(H) and where R 1 , R 2 , R 3 , R 4 , R 5 , R ⁇ , R 7 , R 8 , A or Z are the same or different and include -H, -CH 3 , -OH, carboxylic acid, sulfate, phosphate, aldehyde, methoxy, amine, amide, thiol, disulfide, straight or branched chain alkane, straight or branched chain alkene, straight or branched chain ester, straight or branched chain ether, straight or branched chain silane, straight or branched chain urethane, straight or branched chain, carbonate,
- Another aspect of the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer where the straight or branched chain is of about 1-50 carbon atoms wherein the chain is fully saturated, fully unsaturated or any combination therein
- the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer where the straight or branched chain is of about 1-50 carbon atoms wherein the chain is fully saturated, fully unsaturated or any combination therein.
- the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer wherein straight or branched chains are the same number of carbons or different wherein R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , Rs, A or Z are any combination of the linkers including ester, silane, urea, amide, amine, carbamate, urethane, thiol-urethane, carbonate, thio-ether, thio-ester, sulfate, phosphate and ether.
- the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer which includes at least one chain selected from the group consisting of hydrocarbons, fluorocarbons, halocarbons, alkenes, and alkynes.
- the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer which includes at least one chain selected from the group consisting of linear and dendritic polymers.
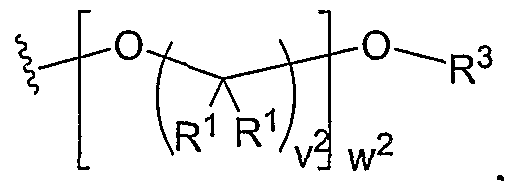
- the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer wherein said linear and dendritic polymers include at least one selected from the group consisting of polyethers, polyesters, polyamines, polyacrylic acids, polycarbonates, polyamino acids, polynucleic acids and polysaccharides of molecular weight ranging from about 200-1,000,000 g/mol, and wherein said chain contains 0, 1 or more than 1 photopolymerizable group.
- Another aspect of the present invention relates to a crosslinkable or noncrosslinkable polymer, wherein the polyether is PEG, and wherein the polyester is PLA, PGA or PLGA.
- Another aspect of the present invention relates to a linear polymer wherein the chain is a polymer or copolymer of a polyester, polyamide, polyether, or polycarbonate of or the aforementioned polymer in combination with a polyester, polyamide, polyether, or polycarbonate of:
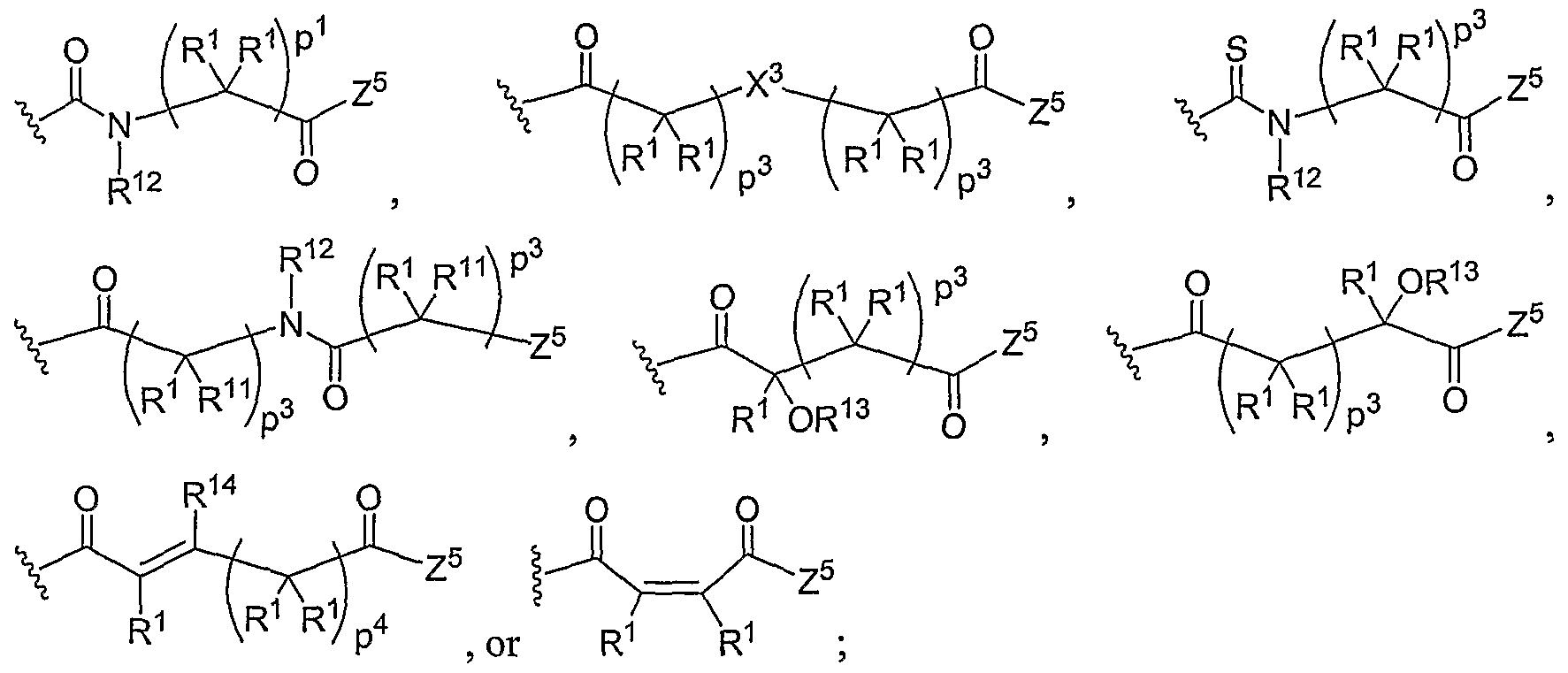
- the present invention relates to the aforementioned polymer comprised of repeating units of General Structure I, where A is O, S, Se, or N-R 7 .
- the present invention relates to the aforementioned polymer, where W, X, and Z are the same or different at each occurrence and are O, S, Se, N(H), or P(H).
- the present invention relates to the aforementioned polymer, where R 1 is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group.
- the present invention relates to the aforementioned polymer, where Ri is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group substituted internally or terminally by one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
- Ri is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group substituted internally or terminally by one or more hydroxyl, hydroxyether, carb
- the present invention relates to the aforementioned polymer, where Ri is a polymer (such as poly(ethylene glycol), poly(ethylene oxide), or a poly(hydroxyacid)), a carbohydrate, a protein, a polypeptide, an amino acid, a nucleic acid, a nucleotide, a polynucleotide, any DNA or RNA segment, a lipid, a polysaccharide, an antibody, a pharmaceutical agent, or any epitope for a biological receptor.
- Ri is a polymer (such as poly(ethylene glycol), poly(ethylene oxide), or a poly(hydroxyacid)), a carbohydrate, a protein, a polypeptide, an amino acid, a nucleic acid, a nucleotide, a polynucleotide, any DNA or RNA segment, a lipid, a polysaccharide, an antibody, a pharmaceutical agent, or any epitope for a biological receptor.
- Ri is a polymer (such
- the present invention relates to the aforementioned polymer, where Ri is a photocrosslinkable, chemically, or ionically crosslinkable group.
- the present invention relates to the aforementioned polymer, in which D is a straight or branched alkyl chain of about 1-5 carbons, m is 0 or 1, and R 2 , R 3 , R 4 , R 5 , R 6 , and R 7 are the same or different at each occurrence and are hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, alkoxy, aryloxy, olefin, alkylamine, dialkylamine, arylamine, diarylamine, alkylamide, dialkylamide, arylamide, diarylamide, alkylaryl, or arylalkyl group.
- the present invention relates to the aforementioned polymer comprised of repeating units of General Structure II, where L, N, and J are the same or different at each occurrence and are O, S, Se, N(H), or P(H).
- the present invention relates to the aforementioned polymer where Rj is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group.
- the present invention relates to the aforementioned polymer where Ri is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group substituted internally or terminally by one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
- the present invention relates to the aforementioned polymer where Ri is a polymer selected from the group consisting of poly(ethylene glycols), poly(ethylene oxides), and poly(hydroxyacids, or is a carbohydrate, a protein, a polypeptide, an amino acid, a nucleic acid, a nucleotide, a polynucleotide, a DNA or RNA segment, a lipid, a polysaccharide, an antibody, a pharmaceutical agent, or an epitope for a biological receptor.
- Ri is a polymer selected from the group consisting of poly(ethylene glycols), poly(ethylene oxides), and poly(hydroxyacids, or is a carbohydrate, a protein, a polypeptide, an amino acid, a nucleic acid, a nucleotide, a polynucleotide, a DNA or RNA segment, a lipid, a polysaccharide, an antibody, a pharmaceutical agent, or an epitope for a
- the present invention relates to the aforementioned polymer where R] is a photocrosslinkable, chemically, or ionically crosslinkable group.
- the present invention relates to the aforementioned polymer, where D is a straight or branched alkyl chain of about 1-5 carbons, q and r are the same or different at each occurrence and are 0 or 1, and R 7 , R 8 , R9, Rio, Rn, Ri 2 , Ri 3 , and R] 4 are the same or different at each occurrence and are hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, alkoxy, aryloxy, olefin, alkylamine, dialkylamine, arylamine, diarylamine, alkylamide, dialkylamide, arylamide, diarylamide, alkylaryl, or arylalkyl group.
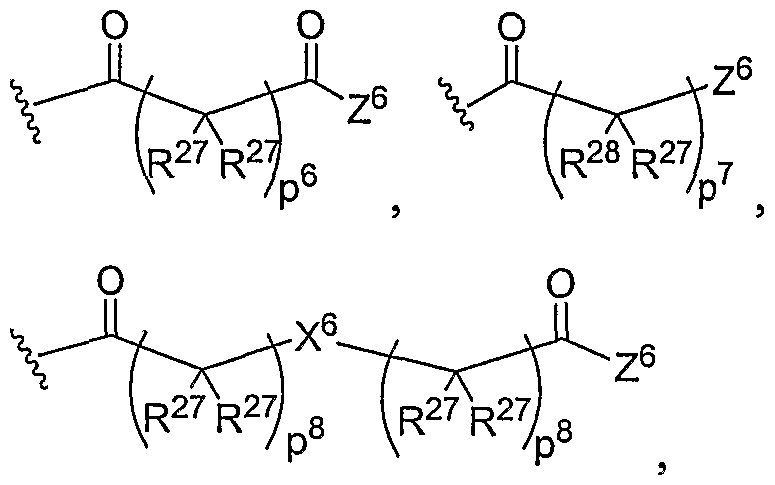
- the present invention relates to the aforementioned block or random copolymer comprised of repeating units of General Structure III, where M, T, and Q are the same or different at each occurrence and are O, S, Se, N(H), or P(H), e is 0 or 1-9, and Ri5 is a straight or branched alkyl chain of about 1-5 carbons, unsubstituted or substituted with one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
- M, T, and Q are the same or different at each occurrence and are O, S, Se, N(H), or P(H)
- e is 0 or 1-9
- Ri5 is a straight or branched alkyl chain of about 1-5 carbons, unsubstituted or substituted with one or more
- the present invention relates to the aforementioned block or random copolymer comprised of repeating units of General Structure III, where M, T, and Q are the same or different at each occurrence and are O, S, Se, N(H), or P(H), and Rj 5 is a straight or branched alkyl chain of about 1-5 carbons, unsubstituted or substituted with one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
- the present invention relates to the aforementioned block or random copolymer comprised of repeating units of General Structure III, where M, T, and Q are the same or different at each occurrence and are O, S, Se, N(H), or P(H), and Rl 5 is a straight or branched alkyl chain of about 1-5 carbons, unsubstituted or substituted with one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
- Another aspect of the present invention relates to a higher order block or random copolymer comprised of three or more different repeating units, and having one or more repeating units described above, such as a polyglyerol glycine carbonate-polyglycerol succinic acid copolymer.
- Another aspect of the present invention relates to a block or random copolymer as described above, which includes at least one terminal crosslinkable group selected from the group consisting of amines, thiols, amides, phosphates, sulfates, hydroxides, alkenes, and alkynes.
- the present invention relates to the aforementioned block or random copolymer where X, Y, M is O, S, N-H, N-R, and wherein R is -H, CH 2 , CR 2 , Se or an isoelectronic species of oxygen.
- the present invention relates to the aforementioned block or random copolymer wherein an amino acid(s) is attached to Ri, R 2 , R 3 , R 4 , R 5 , A, and/or Z.
- the present invention relates to the aforementioned block or random copolymer wherein a polypeptide(s) is attached to Ri, R 2 , R 3 , R 4 , R 5 , A, and/or Z.
- the present invention relates to the aforementioned block or random copolymer wherein an antibody(ies) is attached to Ri, R 2 , R 3 , R 4 , R5, A, and/or Z. In certain instances, the present invention relates to the aforementioned block or random copolymer wherein a nucleotide(s) is attached to R 1 , R 2 , R 3 , R 4 , R 5 A, and/or Z.
- the present invention relates to the aforementioned block or random copolymer wherein a nucleoside(s) is attached to R 1 , R 2 , R 3 , R 4 , R 5> A, and/or Z.
- the present invention relates to the aforementioned block or random copolymer wherein an oligonucleotide(s) is attached to R 1 , R 2 , R 3 , R 4 , R 5, A, and/or Z.
- the present invention relates to the aforementioned block or random copolymer wherein a ligand(s) is attached to R 1 , R 2 , R 3 , R 4 , R 5 , A, and/or Z that binds to a biological receptor.
- the present invention relates to the aforementioned block or random copolymer wherein a pharmaceutical agent(s) is attached to R 1 , R 2 , R 3 , R 4 , R 5, A, and/or Z.
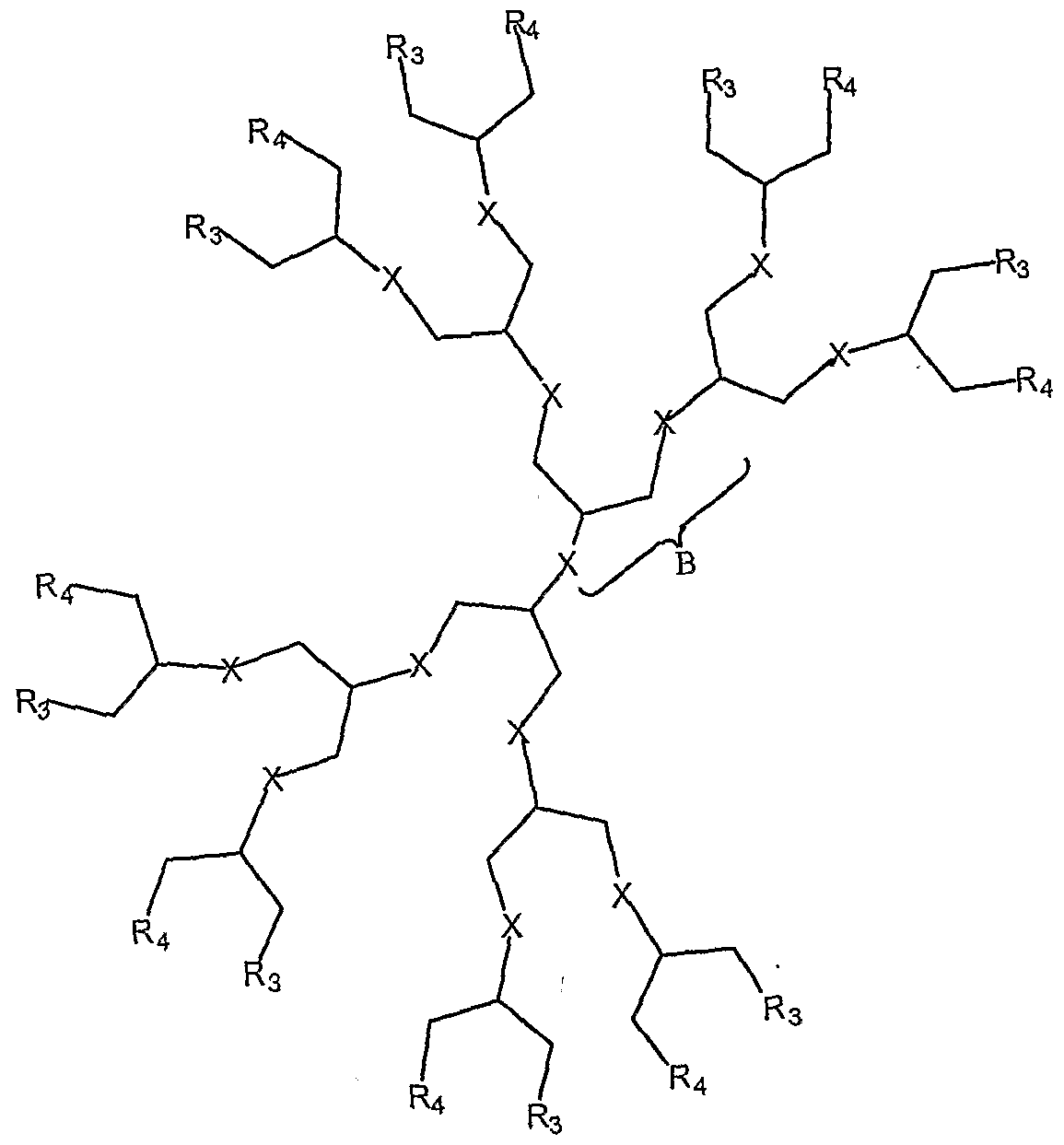
- the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer or copolymer wherein the polymer is a dendritic macromolecule including at least one polymer selected from the group consisting of dendrimers, hybrid linear-dendrimers, dendrons, or hyperbranched polymers according to one of the general formulas or such similar structures below: where R 3 , R 4 , which may be the same or different, are a repeat pattern of B, and n is about 0 to 50.
- the present invention relates to the aforementioned polymer, wherein X, Y, M is O, S, N-H, N-R, wherein R is -H, CH 2 , CR 2 or a chain as defined above, Se or any isoelectronic species of oxygen
- the present invention relates to the aforementioned polymer, wherein X, Y, M is O, S, N-H, N-R, wherein R is -H, CH 2 , CR 2 or a chain as defined above, Se or any isoelectronic species of oxygen.
- the present invention relates to the aforementioned polymer where R 3 and R 4 are carboxylic acid with a protecting group such as but not limited to a phthalimidomethyl ester, a t-butyldimethylsilyl ester, or a t-butyldiphenylsilyl ester.
- a protecting group such as but not limited to a phthalimidomethyl ester, a t-butyldimethylsilyl ester, or a t-butyldiphenylsilyl ester.
- the present invention relates to the aforementioned polymer where R 3 , R 4 , A, and Z are the same or different, R 3 and R 4 are repeated a certain number of times, and terminate in -H, -OH, -CH 3 , carboxylic acid, sulfate, phosphate, aldehyde, activated ester, methoxy, amine, amide, thiol, disulfide, straight or branched chain allcane, straight or branched chain alkene, straight or branched chain ester, straight or branched chain ether, straight or branched chain silane, straight or branched chain urethane, straight or branched chain, carbonate, straight or branched chain sulfate, straight or branched chain phosphate, straight or branched chain thiol urethane, straight or branched chain amine, straight or branched chain thiol urea, straight or branched chain thiol ether, straight or
- the present invention relates to the aforementioned polymer having a straight or branched chain of 1-50 carbon atoms and wherein the chain is fully saturated, fully unsaturated or any combination therein.
- the present invention relates to the aforementioned polymer wherein straight or branched chains are the same number of carbons or different and wherein R 3 , R 4 , A, Z are any combination of linkers selected from the group consisting of esters, silanes, ureas, amides, amines, urethanes, thiol-urethanes, carbonates, carbamates, thio-ethers, thio-esters, sulfates, phosphates and ethers.
- the present invention relates to the aforementioned polymer wherein chains include at least one selected from hydrocarbons, fluorocarbons, halocarbons, alkenes, and alkynes.
- the present invention relates to the aforementioned polymer wherein said chains include polyethers, polyesters, polyamines, polyacrylic acids, polyamino acids, polynucleic acids and polysaccharides of molecular weight ranging from 200-1,000,000, and wherein said chain contains 1 or more crosslinkable or photopolymerizable group.
- the present invention relates to the aforementioned polymer wherein the chains include at least one of PEG, PLA, PGA, PGLA, and PMMA.
- the present invention relates to the aforementioned block or random copolymer, which includes at least one terminal crosslinkable or photopolymerizable group selected from the group consisting of amines, thiols, amides, phosphates, sulfates, hydroxides, alkenes, activated esters, malemides, aldehydes, and alkynes.
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with amino acid(s), such as cysteine, attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with polypeptide(s) attached to Z, A, R 3 , and/or R 4 . In certain instances, the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with an antibody(ies) or single chain antibody(ies) attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with a nucleotide(s) attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with a nucleoside(s) attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with oligonucleotide(s) attached to Z 5 A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with ligand(s) attached to Z, A, R 3 , and/or R 4 that binds to a biological receptor.
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with a pharmaceutical agent(s) attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with a pharmaceutical agent attached to Z, A, R 3 , and/or R 4 and is at least one selected from the group consisting of antibacterial, anticancer, anti-inflammatory, and antiviral.
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times to produce a polymer in which a pharmaceutical agent(s) is encapsulated or chemically bound to the polymer.
- the present invention relates to the aforementioned polymer wherein camptothecin or a derivative of campothethcm is encapsulated
- the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with a carbohydrate(s) attached to Z, A, R 3 , and/or R 4 . In certain instances, the present invention relates to the aforementioned polymer wherein R 3 and R 4 are repeated a certain number of times and terminates with the carbohydrate mannose or sialic acid attached to the polymer.
- the present invention relates to the aforementioned polymer which includes a polymer or copolymer of a polyester, polyamide, polyether, or polycarbonate at the center or periphery of the polymers above taken from the structures below.
- the present invention relates to the aforementioned polymer block or random copolymer which includes at least one terminal or internal crosslinkable group selected from the group consisting of amines, thiols, amides, phosphates, sulfates, hydroxides, alkenes, and alkynes.
- the present invention relates to the aforementioned polymer wherein X, Y, M is O, S, N-H, N-R, wherein R is -H, CH 2 , CR 2 or a chain as defined above, Se or any isoelectronic species of oxygen.
- the present invention relates to the aforementioned polymer wherein an amino acid(s) is attached to Z, A, R 3 , and/or R 4 . In certain instances, the present invention relates to the aforementioned polymer wherein a polypeptide(s) is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein an antibody(ies) or single chain antibody(ies) is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein a nucleotide(s) is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein a nucleoside(s) is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein an oligonucleotide(s) is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein a ligand(s) is attached to Z, A, R 3 , and/or R 4 that binds to a biological receptor.
- the present invention relates to the aforementioned polymer wherein a pharmaceutical agent(s) is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein a carbohydrate(s) is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein a PET or MRI contrast agent(s) is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein the contrast agent is Gd(DPTA).
- the present invention relates to the aforementioned polymer wherein an iodated compound(s) for X-ray imaging is attached to Z, A, R 3 , and/or R 4 .
- the present invention relates to the aforementioned polymer wherein a pharmaceutical agent(s) is attached to Z, A, R 3 , and/or R 4 and is at least one selected from the group consisting of antibacterial, anticancer, anti-inflammatory, and antiviral.
- the present invention relates to the aforementioned polymer wherein the carbohydrate is mannose or sialic acid is covalently attached to the polymer.
- Another aspect of the present invention relates to a surgical procedure which comprises using a photopolymerizable, or chemically crosslmkable, or non-covalently crosslinkable dendritic polymer or copolymer.
- Another aspect of the present invention relates to a surgical procedure wherein said dendritic polymer or copolymer is dissolved or suspended in an non-aqueous liquid such as soybean oil, mineral oil, corn oil, rapeseed oil, coconut oil, olive oil, safflower oil, cottonseed oil, aliphatic, cycloaliphatic or aromatic hydrocarbons having 4-30 carbon atoms, aliphatic or aromatic alcohols having 1-30 carbon atoms, aliphatic or aromatic esters having 2-30 carbon atoms, alkyl, aryl or cyclic ethers having 2-30 carbon atoms, alkyl or aryl halides having 1-30 carbon atoms and optionally having more than one halogen substituent, ketones having 3-30 carbon atoms, polyalkylene glycol or combinations of any two or more thereof.
- an non-aqueous liquid such as soybean oil, mineral oil, corn oil, rapeseed oil, coconut oil, olive oil, safflower oil, cottonseed oil,
- the present invention relates to the surgical procedure wherein the supramolecular structure of the dendrimer is an emulsion.
- the present invention relates to the dendritic polymer or copolymer which optionally contains at least one stereochemical center.
- the present invention relates to the dendritic polymer or copolymer which is of D or L configuration.
- the present invention relates to the dendritic polymer or copolymer wherein the final dendritic polymer or monomer is chiral or is achiral.
- the present invention relates to the dendritic polymer or copolymer which contains at least one site where the branching is incomplete.
- the present invention relates to a crosslinkable/photocrosslinkable/reactionary dendritic polymer or copolymer which contains at least one site where the branching is incomplete.
- the present invention relates to a crosslinkable/photocrosslinkable/reactionary dendritic polymer or copolymer which contains at least one site where the branching is incomplete which forms a hydrogel.
- the present invention relates to a crosslinlcable/photocrosslinkable/reactionary dendritic polymer or copolymer which contains at least one site where the branching is incomplete and used for drug delivery.
- the present invention relates to a dendritic polymer or copolymer made by a convergent or divergent synthesis.
- the dendritic polymer of the invention relates to
- Another aspect of the invention relates to a hydrogel sealant formed by mixing two synthetic polymers or compounds that form a gel which swells less than 150 w/w or w/v or v/v %, wherein the synthetic polymer is not albumin or gelatin.
- Another aspect of the invention relates to a hydrogel sealant formed by mixing two synthetic polymers or compounds that forms a gel via nucleophilic/electrophilic reactions which swells less than 150 w/w or w/v or v/v %, wherein the synthetic polymer is not albumin or gelatin.
- Another aspect of the invention relates to a sealant formed with two separate electrophilic or nucleophilic synthetic polymers/compounds in solution mixed with the corresponding synthetic electrophilic or nucleophilic polymer.
- Another aspect of the invention relates to a crosslinkable composition comprised of a first crosslinkable component having two or greater sets of two nucleophilic groups (an amine and a sulhydryl) directly reacting with one electrophilic group of a second crosslinkable component having two or greater electrophilic groups, each capable of reacting with the said two nucleophilic groups, to form a covalent five membered ring structure (thiazolidine ring) wherein each of the first and second crosslinkable components is synthetic, dissolved in aqueous solution, and crosslinking of the composition results in a biocompatible crosslinked hydrogel in less than 10 minutes.
- Another aspect of the invention relates to a stable crosslinkable composition
- a stable crosslinkable composition comprised of a first crosslinkable component having two or greater sets of 1,2-aminothiol groups directly reacting with one electrophilic (or aldehyde) group of a second crosslinkable component having two or greater electrophilic groups, each capable of reacting with the 1,2-aminothiol, to form a covalent five membered ring structure (thiazolidine ring) wherein each of the first and second crosslinkable components is synthetic, dissolved in aqueous solution , the crosslinking reaction is highly polymer specific, (ie it does not bind to tissue) and crosslinking of the composition results in a biocompatible crosslinked hydrogel on a tissue surface in less than 10 minutes.
- kits comprising at least one of the aforementioned stable crosslinkable compositions; and instructions for its use.
- Another aspect of the invention relates to a method of forming a three dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of:
- step (c) simultaneous with or subsequent to step (b), applying the first synthetic polymer and the synthetic small molecule to the first tissue surface; and (d) allowing the first synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
- the present invention relates to the aforementioned method, further comprising contacting the first tissue surface with a second surface after step (c) but before step (d) to effect adhesion between the first tissue surface and the second surface.
- Another aspect of the invention relates to a method of forming a three dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of:
- step (c) simultaneous with or subsequent to step (b), applying the first synthetic polymer and the synthetic small molecule to the first tissue surface;
- the present invention relates to the aforementioned method, further comprising contacting the first tissue surface with a second surface after step (c) but before step (d) to effect adhesion between the first tissue surface and the second surface.
- the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 400%.
- the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 300%.
- the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 200%.
- the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 100%.
- the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 50%.
- the present invention relates to any one of the aforementioned methods, wherein the synthetic polymer does not include collagen, collagen derivatives, or chemically modified collagens such as gelatin, hyaluronic acid or chemically modified derivatives of hyaluronic acid, albumin from any source or chemically modified derivatives of albumin fo ⁇ n any source, thrombin or chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
- the present invention relates to the aforementioned method, further comprising the step of sterilizing the three-dimensional synthetic polymer matrix and/or the materials used to prepare, the matrix.
- the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
- the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10 "
- the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10 "
- a variety of procedures are known in the art for sterilizing a chemical composition. Sterilization may be accomplished by chemical, physical, or irradiation techniques. Examples of chemical methods include exposure to ethylene oxide or hydrogen peroxide vapor. Examples of physical methods include sterilization by heat (dry or moist), retort camiing, and filtration. The British Pharmacopoeia recommends heating at a minimum of 160 0 C for not less than 2 hours, a minimum of 170 0 C for not less than 1 hour and a minimum of 180 0 C for not less than 30 minutes for effective sterilization. For examples of heat sterilization, see U.S. Patent 6,136,326, which is hereby incorporated by reference. Passing the chemical composition through a membrane can be used to sterilize a composition.
- the composition is filtered through a small pore filter such as a 0.22 micron filter which comprises material inert to the composition being filtered.
- a small pore filter such as a 0.22 micron filter which comprises material inert to the composition being filtered.
- the filtration is conducted in a Class 100,000 or better clean room.
- irradiation methods include gamma irradiation, electron beam irradiation, microwave irradiation, and irradiation using visible light.
- One preferred method is electron beam irradiation, as described in U.S. Patents 6,743,858; 6,248,800; and 6,143,805, each of which is hereby incorporated by reference.
- the two main groups of electron beam accelerators are: (1) a Dynamitron, which uses an insulated core transformer, and (2) radio frequency (RP) linear accelerators (linacs).
- the Dynamitron is a particle accelerator (4.5 MeV) designed to impart energy to electrons.
- the high energy electrons are generated and accelerated by the electrostatic fields of the accelerator electrodes arranged within the length of the glass-insulated beam tube (acceleration tube).
- These electrons traveling through an extension of the evacuation beam tube and beam transport (drift pipe) are subjected to a magnet deflection system in order to produce a "scanned" beam, prior to leaving the vacuum enclosure through a beam window.
- the dose can be adjusted with the control of the percent scan, the beam current, and the conveyor speed.
- the electron-beam radiation employed may be maintained at an initial fluence of at least about 2 ⁇ Curie/cm 2 , at least about 5 ⁇ Curie/cm 2 , at least about 8 ⁇ Curie/cm 2 , or at least about 10 ⁇ Curie/cm 2 .
- the electron-beam radiation employed has an initial fluence of from about 2 to about 25 ⁇ Curie/cm 2 .
- the electron- beam dosage is from about 5 to 50 kGray, or from about 15 to about 20 kGray with the specific dosage being selected relative to the density of material being subjected to electron-beam radiation as well as the amount of bioburden estimated to be therein. Such factors are well within the skill of the art.
- the composition to be sterilized may be in any type of at least partially electron beam permeable container such as glass or plastic.
- the container may be sealed or have an opening.
- glass containers include ampules, vials, syringes, pipettes, applicators, and the like.
- the penetration of electron beam irradiation is a function of the packaging. If there is not enough penetration from the side of a stationary electron beam, the container may be flipped or rotated to achieve adequate penetration. Alternatively, the electron beam source can be moved about a stationary package. In order to determine the dose distribution and dose penetration in product load, a dose map can be performed. This will identify the minimum and maximum dose zone within a product.
- the visible light for sterilization can be generated using any conventional generator of sufficient power and breadth of wavelength to effect sterilization. Generators are commercially available under the trade name PureBright® in-line sterilization systems from PurePulse Technologies, Inc. 4241 Ponderosa Ave, San Diego, Calif. 92123, USA.
- PureBright® in-line sterilization system employs visible light to sterilize clear liquids at an intensity approximately 90000 times greater than surface sunlight. If the amount of UV light penetration is of concern, conventional UV absorbing materials can be used to filter out the UV light.
- the composition is sterilized to provide a Sterility Assurance Level (SAL) of at least about 10 "3 .
- SAL Sterility Assurance Level
- the Sterility Assurance Level measurement standard is described, for example, in ISO/CD 14937, the entire disclosure of which is incorporated herein by reference.
- the Sterility Assurance Level may be at least about 10 "4 , at least about 10 "5 , or at least about 10 "6 .
- the materials used to form the seal on a wound may be delivered to the wound on a patient before the hydrogel forms.
- a large number of delivery systems are known in the art and are amenable to the present invention.
- a polymerizable dendrimeric compound is delivered to a wound on a patient.
- a polymerizable dendrimeric compound is combined with a polyermization agent to form a repair mixture, and the repair mixture is delivered to the wound on a patient.
- the materials delivered to the wound on a patient have been sterilized.
- the delivery system may be a single-barrel syringe system.
- the single-barrel syringe is a double acting, single-barrel syringe system as displayed in Figure
- a delivery device that flows two or more streams of liquid in a mixing chamber may be preferable.
- a delivery device that mixes two solids and two liquids and then separately flows these streams of liquid to a mixing chamber may be advantageous.
- a delivery system is used to deliver the sealant-forming materials to the wound, wherein at least two dry, reactive components are stored together in a dry state and introduced into a liquid component(s) at the time of use to form a mixture that forms a hydrogel.
- generation refers to the number of branched repeat units which emanate from the central core.
- G3 PGLSA dendrimer has three branching layers not including the core.
- polymerize refers to the process of converting a monomer to a chain of momomers, wherein the chain of momomers comprises at least about 5 monomers. In certain instances, the chain of monomers comprises at least about 10 or 15 momomers. In certain instances, the chain of monomers comprises at least about 25 or 40 momomers. In certain instances, the chain of monomers comprises at least about 50 or 75 momomers. In certain instances, the chain of monomers comprises at least about 100 or 150 momomers.
- the term "polymerize" indicates that at least one of the functional groups capable of forming a bond in the polymerization reaction forms a bond with another compound, generally speaking, the other compound is another monomer.
- at least about 10% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer.
- at least about 25% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer.
- at least about 50% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer.
- At least about 75% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer. In certain instances, about 20% to about 50% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer.
- the term "polymerize" only requires that at least some of the monomer units in a given solution react to form a chain of monomers. In certain instances, about 10% to about 30% of the monomers react to form a chain of monomers. In certain instances, about 30% to about 50% of the monomers react to form a chain of monomers. In certain instances, about 50% to about 75% of the monomers react to form a chain of monomers.
- the term “seal” or “sealing” as used herein indicates that a protective barrier is formed over the wound.
- the protective barrier is a continuous layer.
- the protective barrier is a discontinous layer, i.e., a layer that has holes or pores in the layer.
- the discontinous layer comprises less than about 25% holes.
- the discontinous layer comprises about less than 15% holes.
- the discontinous layer comprises about less than 5% holes.
- certain fluids or gases can penetrate through the layer.
- the fluid is a liquid located in the eye.
- the fluid is water.
- the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 40 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 60 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 80 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 100 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 120 or about 150 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 180 or about 200 mm Hg.
- PEG-ALD 2 refers to poly(ethylene glycol) dialdehyde.
- the aldehyde is the represented by -(CR 2 ) n C(H)O, wherein R is hydrogen or Ci-C 6 alkyl, and n is an integer from 0 to about 15. In certain instances, the aldehyde is the radical of acetaldehyde, butyraldehyde, or propionaldehyde.
- amino acid refers to the radical of N-hydroxy succinimide.
- sulfoNHS refers to O or a salt thereof.
- Mw weight average molecular weight in g/mol.
- alkali metal refer to those elements listed in Group 1 of the periodic table. The following elements are alkali metals: Li, Na, K, Rb, Cs, and Fr.
- PLA refers to poly(lactic acid).
- the PLA has a weight average molecular weight in the range of about 300 g/mol to about 800,000 g/mol. In certain instances, the PLA has a weight average molecular weight in the range of about 1000 g/mol to about 100,000 g/mol.
- PGA refers to poly(glycolic acid).
- the PGA has a weight average molecular weight in the range of about 300 g/mol to about 800,000 g/mol. In certain instances, the PGA has a weight average molecular weight in the range of about 1000 g/mol to about 100,000 g/mol.
- PLGA refers to a copolymer of lactic acid and glycolic acid. In certain instances, the PLGA has a weight average molecular weight in the range of about 300 g/mol to about 800,000 g/mol. In certain instances, the PLGA has a weight average molecular weight in the range of about 1000 g/mol to about 100,000 g/mol.
- heteroatom is art-recognized and refers to an atom of any element other than carbon or hydrogen.
- Illustrative heteroatoms include boron, nitrogen, oxygen, phosphorus, sulfur and selenium.
- alkyl is art-recognized, and includes saturated aliphatic groups, including straight-chain alkyl groups, branched-chain alkyl groups, cycloalkyl (alicyclic) groups, alkyl substituted cycloalkyl groups, and cycloalkyl substituted alkyl groups.
- a straight chain or branched chain alkyl has about 30 or fewer carbon atoms in its backbone (e.g., C]-C 3O for straight chain, C 3 -C 3O for branched chain), and alternatively, about 20 or fewer.
- cycloalkyls have from about 3 to about 10 carbon atoms in their ring structure, and alternatively about 5, 6 or 7 carbons in the ring structure.
- lower alkyl refers to an alkyl group, as defined above, but having from one to about ten carbons, alternatively from one to about six carbon atoms in its backbone structure.
- lower alkenyl and “lower alkynyl” have similar chain lengths.
- aralkyl is art-recognized and refers to an alkyl group substituted with an aryl group (e.g., an aromatic or heteroaromatic group).
- alkenyl and alkynyl are art-recognized and refer to unsaturated aliphatic groups analogous in length and possible substitution to the alkyls described above, but that contain at least one double or triple bond respectively.
- aryl is art-recognized and refers to 5-, 6- and 7-membered single-ring aromatic groups that may include from zero to four heteroatoms, for example, benzene, naphthalene, anthracene, pyrene, pyrrole, furan, thiophene, imidazole, oxazole, thiazole, triazole, pyrazole, pyridine, pyrazine, pyridazine and pyrimidine, and the like.
- aryl groups having heteroatoms in the ring structure may also be referred to as "aryl heterocycles" or “heteroaromatics.”
- the aromatic ring may be substituted at one or more ring positions with such substituents as described above, for example, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, alkoxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio, sulfonyl, sulfonamide, ketone, aldehyde, ester, heterocyclyl, aromatic or heteroaromatic moieties, - CF 3 , -CN, or the like.
- aryl also includes polycyclic ring systems having two or more cyclic rings in which two or more carbons are common to two adjoining rings (the rings are "fused rings") wherein at least one of the rings is aromatic, e.g., the other cyclic rings may be cycloalkyls, cycloalkenyls, cycloalkynyls, aryls and/or heterocyclyls.
- ortho, meta and para are art-recognized and refer to 1,2-, 1,3- and 1,4- disubstituted benzenes, respectively.
- 1,2-dimethylbenzene and ortho-dimethvlbenzene are synonymous.
- heterocyclyl refers to 3- to about 10-membered ring structures, alternatively 3- to about 7-membered rings, whose ring structures include one to four heteroatoms.
- Heterocycles may also be polycycles.
- Heterocyclyl groups include, for example, thiophene, thianthrene, furan, pyran, isobenzofuran, chromene, xanthene, phenoxanthene, pyrrole, imidazole, pyrazole, isothiazole, isoxazole, pyridine, pyrazine, pyrimidine, pyridazine, indolizine, isoindole, indole, indazole, purine, quinolizine, isoquinoline, quinoline, phthalazine, naphthyridine, quinoxaline, quinazoline, cinnoline, pteridine, carbazole, carboline, phenanthridine, acridine, pyrimidine, phenanthroline, phenazine, phenarsazine, phenothiazine, furazan, phenoxazine, pyrrolidine, o
- the heterocyclic ring may be substituted at one or more positions with such substituents as ' described above, as for example, halogen, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio, sulfonyl, ketone, aldehyde, ester, a heterocyclyl, an aromatic or heteroaromatic moiety, -CF 3 , -CN, or the like.
- substituents as ' described above, as for example, halogen, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate, carbony
- polycyclyl or “polycyclic group” are art-recognized and refer to two or • more rings (e.g., cycloalkyls, cycloalkenyls, cycloalkynyls, aryls and/or heterocyclyls) in which two or more carbons are common to two adjoining rings, e.g., the rings are "fused rings". Rings that are joined through non-adjacent atoms are termed "bridged" rings.
- Each of the rings of the polycycle may be substituted with such substituents as described above, as for example, halogen, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio, sulfonyl, ketone, aldehyde, ester, a heterocyclyl, an aromatic or heteroaromatic moiety, -CF 3 , -CN, or the like.
- substituents as described above, as for example, halogen, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate, carbonyl, carboxyl, si
- carrier is art-recognized and refers to an aromatic or non-aromatic ring in which each atom of the ring is carbon.
- nitro is art-recognized and refers to -NO 2 ;
- halogen is art- recognized and refers to -F, -Cl, -Br or -I;
- sulfhydryl is art-recognized and refers to -SH;
- hydroxyl means -OH;
- sulfonyl is art-recognized and refers to -SO 2 " .
- Halide designates the corresponding anion of the halogens, and "pseudohalide” has the definition set forth on 560 of "Advanced Inorganic Chemistry" by Cotton and Wilkinson.
- amine and “amino” are art-recognized and refer to both unsubstituted and substituted amines, e.g., a moiety that may be represented by the general formulas:
- R50, R51 and R52 each independently represent a hydrogen, an alkyl, an alkenyl, - (CH 2 ) m -R61, or R50 and R51, taken together with the N atom to which they are attached complete a heterocycle having from 4 to 8 atoms in the ring structure;
- R61 represents an aryl, a cycloalkyl, a cycloalkenyl, a heterocycle or a polycycle; and m is zero or an integer in the range of 1 to 8.
- R50 and R51 (and optionally R52) each independently represent a hydrogen, an alkyl, an alkenyl, or -(CH 2 ) m -R61.
- alkylamine includes an amine group, as defined above, having a substituted or unsubstituted alkyl attached thereto, i.e., at least one of R50 and R51 is an alkyl group.
- acylamino is art-recognized and refers to a moiety that may be represented by the general formula:
- R50 is as defined above
- R54 represents a hydrogen, an alkyl, an alkenyl or - (CH 2 ) m -R61, where m and R61 are as defined above.
- amino is art recognized as an amino-substituted carbonyl and includes a moiety that may be represented by the general formula:
- alkylthio refers to an alkyl group, as defined above, having a sulfur radical attached thereto.
- the "alkylthio" moiety is represented by one of -S-alkyl, -S-alkenyl, -S-alkynyl, and -S-(CH 2 ) m -R61, wherein m and R61 are defined above.
- Representative alkylthio groups include methylthio, ethyl thio, and the like.
- te ⁇ n "carboxyl” is art recognized and includes such moieties as may be represented by the general formulas: wherein X50 is a bond or represents an oxygen or a sulfur, and R55 and R56 represents a hydrogen, an alkyl, an alkenyl, -(CH 2 ) m -R61or a pharmaceutically acceptable salt, R56 represents a hydrogen, an alkyl, an alkenyl or -(CH2) m -R61, where m and R61 are defined above. Where X50 is an oxygen and R55 or R56 is not hydrogen, the formula represents an "ester".
- oxime and "oxime ether” are art-recognized and refer to moieties that may be represented by the general formula:
- R75 is hydrogen, alkyl, cycloalkyl, alkenyl, a ⁇ kyny ⁇ , aryl, aralkyl, or -(CH 2 ) m -R61.
- the moiety is an "oxime” when R is H; and it is an "oxime ether” when R is alkyl, cycloalkyl, alkenyl, alkynyl, aryl, aralkyl, or -(CH 2 ) m -R61.
- alkoxyl or "alkoxy” are art-recognized and refer to an alkyl group, as defined above, having an oxygen radical attached thereto.
- Representative alkoxyl groups include methoxy, ethoxy, propyloxy, tert-butoxy and the like.
- An "ether” is two hydrocarbons covalently linked by an oxygen. Accordingly, the substituent of an alkyl that renders that alkyl an ether is or resembles an alkoxyl, such as may be represented by one of -O-alkyl, -O-alkenyl, -O-alkynyl, -O--(CH 2 ) m -R61, where m and R61 are described above.
- R57 is an electron pair, hydrogen, alkyl, cycloalkyl, or aryl.
- R57 is as defined above.
- sulfamoyl is art-recognized and refers to a moiety that may be represented by the general formula:
- sulfonyl is art-recognized and refers to a moiety that may be represented by the general formula:
- R58 is one of the following: hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl or heteroaryl.
- sulfoxido is art-recognized and refers to a moiety that may be represented by the general formula:
- phosphoryl is art-recognized and may in general be represented by the formula:
- Q50 represents S or O
- R59 represents hydrogen, a lower alkyl or an aryl.
- the phosphoryl group of the phosphorylalkyl may be represented by the general formulas:
- R60 represents a lower alkyl or an aryl.
- Analogous substitutions may be made to alkenyl and alkynyl groups to produce, for example, aminoalkenyls, aminoalkynyls, amidoalkenyls, amidoalkynyls, iminoalkenyls, iminoalkynyls, thioalkenyls, thioalkynyls, carbonyl-substituted alkenyls or alkynyls.
- each expression e.g. alkyl, m, n, and the like, when it occurs more than once in any structure, is intended to be independent of its definition elsewhere in the same structure.
- selenoalkyl is art-recognized and refers to an alkyl group having a substituted seleno group attached thereto.
- exemplary "selenoethers" which may be substituted on the alkyl are selected from one of -Se-alkyl, -Se-alkenyl, -Se-alkynyl, and - Se-(CH 2 ) m -R61, m and R61 being defined above.
- triflyl, tosyl, mesyl, and nonaflyl are art-recognized and refer to trifluoromethanesulfonyl, p-toluenesulfonyl, methanesulfonyl, and nonafluorobutanesulfonyl groups, respectively.
- triflate, tosylate, mesylate, and nonaflate are art-recognized and refer to trifluoromethanesulfonate ester, p-toluenesulfonate ester, methanesulfonate ester, and nonafluorobutanesulfonate ester functional groups and molecules that contain said groups, respectively.
- Me, Et, Ph, Tf, Nf, Ts, and Ms represent methyl, ethyl, phenyl, trifluoromethanesulfonyl, nonafluorobutanesulfonyl, />-toluenesulfonyl and methanesulfonyl, respectively.
- a more comprehensive list of the abbreviations utilized by organic chemists of ordinary skill in the art appears in the first issue of each volume of the Journal of Organic Chemistry: this list is typically presented in a table entitled Standard List of Abbreviations.
- compositions of the present invention may exist in particular geometric or stereoisomeric forms.
- polymers of the present invention may also be optically active.
- the present invention contemplates all such compounds, including cis- and trans-isomers, R- and S-enantiomers, diastereomers, (D)-isomers, (L)- isomers, the racemic mixtures thereof, and other mixtures thereof, as falling within the scope of the invention.
- Additional asymmetric carbon atoms may be present in a substituent such as an alkyl group. All such isomers, as well as mixtures thereof, are intended to be included in this invention.
- a particular enantiomer of compound of the present invention may be prepared by asymmetric synthesis, or by derivation with a chiral auxiliary, where the resulting diastereomeric mixture is separated and the auxiliary group cleaved to provide the pure desired enantiomers.
- the molecule contains a basic functional group, such as amino, or an acidic functional group, such as carboxyl, diastereomeric salts are formed with an appropriate optically-active acid or base, followed by resolution of the diastereomers thus formed by fractional crystallization or chromatographic means well known in the art, and subsequent recovery of the pure enantiomers.
- substitution or “substituted with” includes the implicit proviso that such substitution is in accordance with permitted valence of the substituted atom and the substituent, and that the substitution results in a stable compound, e.g., which does not spontaneously undergo transformation such as by rearrangement, cyclization, elimination, or other reaction.
- substituted is also contemplated to include all permissible substituents of organic compounds.
- the permissible substituents include acyclic and cyclic, branched and unbranched, carbocyclic and heterocyclic, aromatic and nonaromatic substituents of organic compounds.
- Illustrative substituents include, for example, those described herein above.
- the permissible substituents may be one or more and the same or different for appropriate organic compounds.
- the heteroatoms such as nitrogen may have hydrogen substituents and/or any permissible substituents of organic compounds described herein which satisfy the valences of the heteroatoms. This invention is not intended to be limited in any manner by the permissible substituents of organic compounds.
- protecting group means temporary substituents which protect a potentially reactive functional group from undesired chemical transformations.
- protecting groups include esters of carboxylic acids, silyl ethers of alcohols, and acetals and ketals of aldehydes and ketones, respectively.
- the field of protecting group chemistry has been reviewed (Greene, T. W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 2 nd ed.; Wiley: New York, 1991). Protected forms of the inventive compounds are included within the scope of this invention.
- One aspect of the present invention relates to a method of sealing a wound on a patient, comprising the steps of: applying an effective amount of a dendrimeric compound of formula Ia or formula Ib to a wound on a patient and treating said dendrimeric compound with a polymerization agent sufficient to polymerize said dendrimeric compound, wherein said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, or an oxidizing agent, wherein formula Ia is represented by:
- a 2 is alkyl, aryl, aralkyl, -Si(R 3 ) 3 , or
- A represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
- Y represents independently for each occurrence R 4 , A A 4 ,
- Z 1 represents independently for each occurrence -X 1 -R 4 , E, or
- Z ⁇ -2 z represents independently for each occurrence -X 1 -R 5 , E, or
- Y 3 represents independently for each occurrence R 6 , A 4 ,
- Z represents independently for each occurrence -X 1 - n R6 , E, or
- Y 4 represents independently for each occurrence R 7 , A 4 ,
- Z represents independently for each occurrence -X 1 -R r.7 , E, or
- Y 5 represents independently for each occurrence R 8 , A 4 ,
- Z 5 represents independently for each occurrence -X 1 -R 8 , E, or
- Y represents independently for each occurrence R 5 A ,
- R 1 represents independently for each occurrence H, alkyl, or halogen
- R 2 represents independently for each occurrence H, alkyl, -OH, -N(R 1 °) 2 , -SH, hydroxyalkyl, or -[C(R ⁇ 2 ] C1 R 16 ;
- R 3 represents independently for each occurrence alkyl, aryl, or aralkyl;
- R 4 , R 5 , R 6 , R 7 , R 8 , and R 9 are H;
- R 10 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R u represents independently for each occurrence H, -OH, -N(R 10 ) 2 , -SH, alkyl, hydroxyalkyl, or -[C(R') 2 ] d R 16 ;
- R 12 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 13 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 14 represents independently for each occurrence H, alkyl, or -CO 2 R 10
- R 15 represents independently for each occurrence H, alkyl, or -OR 10 ;
- R 16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R 10 ) 2 , -SH, -S-alkyl, -CO 2 R 10 , -C(O)N(R 10 ) 2 , or - C(NH 2 )N(R 10 ) 2 ;
- v 1 and v 2 each represent independently for each occurrence 2, 3, or 4; w 1 and w 2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is O, 1, 2, 3, 4, or 5; z represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z 2 and z 3 each represent independently for each occurrence 1, 2, 3, 4, or 5; X 1 and X 2 each represent independently for each occurrence O or -N(R 10 )-; X 3 represents independently for each occurrence O, N(R 10 ), or C(R 15 )(CO 2 R 10 );
- A represents independently for each occurrence
- X 5 represents independently for each occurrence O or -N(R 22 )-;
- R 17 represents independently for each occurrence H, -(C(R 19 ) 2 ) h SH,
- R represents independently for each occurrence H or alkyl
- R 19 represents independently for each occurrence H, halogen, or alkyl
- R 20 represents independently for each occurrence H or alkyl
- R 21 represents independently for each occurrence H, -(C(R 19 ) 2 ) h SH, -
- R ,22 represents independently for each occurrence H, alkyl, aryl, or aralkyl; n 1 and h each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p 5 represents independently for each occurrence 1, 2, 3, 4, or 5; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive; said formula II is represented by:
- R represents independently for each occurrence H or R 5' "
- R " ! represents independently for each occurrence H or alkyl
- R 3" ⁇ represents independently for each occurrence H, halogen, or alkyl
- R 4"11 represents independently for each occurrence alkyl, aryl, or aralkyl
- R 5'11 represents independently for each occurrence H or R 2" " and z represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; said formula HI is represented by:
- R ' represents independently for each occurrence H, alkyl, or halogen
- R ,3'- " i m ⁇ is fluoroalkyl, chloroalkyl, -CH 2 NO 2 , or
- x represents independently for each occurrence 0, 1, 2, 3, 4, 5, 6, 7, or 8;
- y represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8;
- z represents independently for each occurrence 0, 1, or 2;
- v represents independently for each occurrence 2, 3, or 4;
- w is an integer in the range of about 5 to about 700, inclusive;
- a 2 is alkyl, aryl, aralkyl,r -Si(R 3 ) 3 , or
- a 3 represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
- Y represents independently for each occurrence R ,
- Z represents independently for each occurrence -X 1 - ⁇ R.4 , E, or
- Y represents independently for each occurrence R ,
- Z 2 represents independently for each occurrence -X'-R 5 , E, or
- Y represents independently for each occurrence R ,
- Z 3 represents independently for each occurrence -X -R , E, or
- Y 4 represents independently for each occurrence R 7 ,
- Z 4 represents independently for each occurrence -X 1 -R 7 , E, or
- Y represents independently for each
- Z 5 represents independently for each occurrence -X 1 -R 8 , E, or
- Y represents independently for each occurrence R ,
- R 1 represents independently for each occurrence H, allcyl, or halogen
- R represents independently for each occurrence H, alkyl, -OH, -N(R 10 ) ⁇ 2 , -SH, hydroxyalkyl, or -[C(R 1 ) 2 ] d R 16 ;
- R 3 represents independently for each occurrence alkyl, aryl, or aralkyl;
- R 4 , R 5 , R 6 , R 7 , R 8 , and R 9 are H;
- R 10 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 11 represents independently for each occurrence H, -OH, -N(R 1 °) 2 , -SH, alkyl, hydroxyalkyl, or -[C(R ] ) 2 ] d R 16 ;
- R 12 represents independently for each occurrence H, allcyl, aryl, or aralkyl
- R 13 represents independently for each occurrence H, allcyl, aryl, or aralkyl
- R 14 represents independently for each occurrence H, alkyl, or -CO 2 R 10
- R 15 represents independently for each occurrence H, allcyl, or -OR 10 ;
- R 16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R 10 ) 2 , -SH, -S-allcyl, -CO 2 R 10 , -C(O)N(R 10 ) 2 , or - C(NH 2 )N(R 10 ) 2 ;
- n represents independently for each occurrence 1, 2, 3, 4, 5, or 6;
- p 1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8;
- p 2 represents independently for each occurrence 0, 1, 2, 3, or 4;
- p 3 represents independently for each occurrence 1, 2, or 3;
- p 4 represents independently for each occurrence 0, 1, 2, or 3;
- d represents independently for each occurrence 1, 2, 3, 4, 5, or 6;
- t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
- v 1 and v 2 each represent independently for each occurrence 2, 3, or 4; w 1 and w 2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is 0, 1, 2, 3, 4, or 5; z 1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z 2 and z 3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
- X 1 and X 2 each represent independently for each occurrence O or -N(R 10 )-; X 3 represents independently for each occurrence O, N(R 10 ), or C(R 15 XCO 2 R 1 °); and
- E represents independently for each occurrence H, - [C(R 1 ⁇ ] n C(O)H, , or
- X 6 represents independently for each occurrence O or -N(R 30 )-;
- R 23 represents independently for each occurrence
- R 24 represents independently for each occurrence H or alkyl
- R i25 represents independently for each occurrence H, halogen, or alkyl
- R 26 represents independently for each occurrence H or alkyl
- R 27 represents independently for each occurrence H, alkyl, or halogen
- R 28 represents independently for each occurrence H, alkyl, -OH, -N(R 30 ) 2 , -SH, or hydroxyalkyl;
- R 29 represents independently for each occurrence H, -OH, -N(R 30 ) 2 , -SH, alkyl, or hydroxyalkyl;
- R 30 and R 31 represent independently for each occurrence H, alkyl, aryl, or aralkyl; represents independently for each occurrence E 1 or
- R , 32 represents independently for each
- Z 7 represents independently for each occurrence E 1 or
- R >33 represents independently for each
- R 34 represents independently for each occurrence H, alkyl, or -CO 2 R 30 ;
- E 1 represents independently for each occurrence H, -[C(R 24 ) 2 ] j C(O)H,
- the present invention relates to the aforementioned method, wherein said dendrimeric compound is a compound of formula Ia, and said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, or an oxidizing agent. In certain instances, the present invention relates to the aforementioned method,
- A is , and m is 1 or 2.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method, wherein Z 1 represents independently for each occurrence -X -R or
- the present invention relates to the aforementioned method, wherein Z 2 represents independently for each occurrence -X 1 -R 5 or
- the present invention relates to the aforementioned method, wherein Z 3 represents independently for each occurrence -X 1 -R 6 or
- the present invention relates to the aforementioned method, wherein Z 4 represents independently for each occurrence -X 1 -R 7 or
- the present invention relates to the aforementioned method, wherein Z 5 represents independently for each occurrence -X 1 -R 8 or
- the present invention relates to the aforementioned method, wherein X 1 is O.
- the present invention relates to the aforementioned method, wherein X 1 and X 2 are O.
- the present invention relates to the aforementioned method, wherein n is 1.
- the present invention relates to the aforementioned method, wherein p 1 is 2, 3, or 4.
- the present invention relates to the aforementioned method, wherein p 2 is 1.
- the present invention relates to the aforementioned method, wherein R 1 is H.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is H
- B is m
- said polymerization agent is ultraviolet light or visible light.
- the present invention relates to the aforementioned method
- R 1 is H
- B is m
- the present invention relates to the aforementioned method
- said polymerization agent is a compound of formula III.
- the present invention relates to the aforementioned method
- R is H
- B is m
- the present invention relates to the aforementioned method
- R 1 is H
- B is , m
- the present invention relates to the aforementioned method
- R 1 is H
- B is
- the present invention relates to the aforementioned method,
- R 1 is H
- B is m
- the present invention relates to the aforementioned method, wherein p 1 is 1, 2, 3, or 4.
- the present invention relates to the aforementioned method, wherein p 1 is 2.
- the present invention relates to the aforementioned method, wherein p 1 is 4.
- the present invention relates to the aforementioned method, wherein m is 1.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- said polymerization agent is ultraviolet light or visible light.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is H, B is , m is 1
- said polymerization agent is a compound of formula III.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is H, B is , m is 1
- the present invention relates to the aforementioned method, wherein p 1 is 1, 2, 3, or 4.
- the present invention relates to the aforementioned method, wherein p is 2.
- the present invention relates to the aforementioned method, wherein p 1 is 4.
- the present invention relates to the aforementioned method, wherein m is 1.
- the present invention relates to the aforementioned method, wherein R 2 is (Ci-C 3 )alkyl.
- the present invention relates to the aforementioned method
- R 1 is H, B is and v is 2.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is H
- B is polymerization agent is ultraviolet light or visible light.
- the present invention relates to the aforementioned method
- R 1 is H
- B is
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is H
- B is
- polymerization agent is ultraviolet light or visible light.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- polymerization agent is ultraviolet light or visible light.
- the present invention relates to the aforementioned method
- R 1 is H
- B is v 1 is 2
- the present invention relates to the aforementioned method, wherein w 1 is an integer in the range of about 50 to about 250.
- the present invention relates to the aforementioned method, wherein w 1 is an integer in the range of about 60 to about 90.
- the present invention relates to the aforementioned method, wherein p 1 is 2.
- the present invention relates to the aforementioned method, wherein m is 1.
- the present invention relates to the aforementioned method, wherein p 1 is 2, p 2 is 0, and R 3 is (Ci-Cs)alkyl.
- the present invention relates to the aforementioned method, wherein p 1 is 2, p 2 is 0, R 3 is (Ci-C5)alkyl, and w 1 is an integer in the range of about 60 to about 90.
- the present invention relates to the aforementioned method
- R 1 is H
- B is A 2 is
- R 3 is alkyl
- v 2 is
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- said polymerization agent is ultraviolet light or visible light.
- the present invention relates to the aforementioned method
- R 1 is H
- B R 3 is alkyl
- v 2 is
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is H
- B is A 2 IS
- R 3 is alkyl
- v 2 i is
- the present invention relates to the aforementioned method, wherein p 1 is 2.
- the present invention relates to the aforementioned method, wherein m is 1. In certain instances, the present invention relates to the aforementioned method, wherein p 1 is 2, p 2 is 0, and R 3 is (Ci-C 5 )alkyl.
- the present invention relates to the aforementioned method, wherein p 1 is 2, p 2 is 0, and R 3 is (Ci-Cs)alkyl, and w 2 is an integer in the range of about 60 to about 90.
- the present invention relates to the aforementioned method
- R 1 is H
- B is , m
- the present invention relates to the aforementioned method
- R 1 is H
- B is , m
- the present invention relates to the aforementioned method
- R 1 is H
- B is
- the present invention relates to the aforementioned method
- R 1 is H
- B is m
- the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula II.
- the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula III.
- the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula III, R 1"111 is -C(O)H, and R 2"111 is H. In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula in, R 1"111 is -C(O)H, R 2'111 is
- the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula III, R 2"111 is -C(O)H, R 2"111 is
- B 1"111 is w ⁇ an( j w is an integer in the range of about 60-90.
- the present invention relates to the aforementioned method, said compound of formula IK is
- the present invention relates to the aforementioned method, wherein said compound of formula Ia is
- n is an integer in the range of about 70 to about 80, and said polymerization agent is UV light.
- the present invention relates to the aforementioned method, wherein said dendrimeric compound is a compound of formula Ib. In certain embodiments, the present invention relates to the aforementioned method, wherein v is 2.
- the present invention relates to the aforementioned method, wherein X 5 is -N(H)-.
- the present invention relates to the aforementioned method, wherein R 18 is H.
- the present invention relates to the aforementioned method, wherein R 19 is H.
- the present invention relates to the aforementioned method, wherein R 20 is H.
- the present invention relates to the aforementioned method, wherein w is an integer in the range of about 20-500.
- the present invention relates to the aforementioned method, wherein w is an integer in the range of about 40-250.
- the present invention relates to the aforementioned method, wherein w is an integer in the range of about 60-90.
- the present invention relates to the aforementioned method, said compound of formula Ib is
- said polymerization agent is a compound of formula V.
- the present invention relates to the aforementioned method, wherein v is 2.
- the present invention relates to the aforementioned method, wherein X 6 is -N(H)-.
- the present invention relates to the aforementioned method, wherein R 24 is H. In certain embodiments, the present invention relates to the aforementioned method, wherein R 25 is H.
- the present invention relates to the aforementioned method, wherein R 26 is H.
- the present invention relates to the aforementioned method, wherein w is an integer in the range of about 20-500.
- the present invention relates to the aforementioned method, wherein w is an integer in the range of about 40-250.
- the present invention relates to the aforementioned method, wherein w is an integer in the range of about 60-90.
- the present invention relates to the aforementioned method, wherein R 23 represents independently for each occurrence
- the present invention relates to the aforementioned method, wherein R 23 represents independently for each occurrence
- the present invention relates to the aforementioned method, said compound of formula V is
- the present invention relates to the aforementioned method, said compound of formula V is
- the present invention relates to the aforementioned method, wherein said polymerization agent is an oxidizing agent.
- the present invention relates to the aforementioned method, wherein said polymerization agent is O 2 .
- the present invention relates to the aforementioned method, wherein said polymerization agent is ultraviolet light or visible light.
- the present invention relates to the aforementioned method, wherein said polymerization agent is ultraviolet light.
- the present invention relates to the aforementioned method, wherein said polymerization agent is light with a ⁇ of 400-600 nm.
- the present invention relates to the aforementioned method, wherein said polymerization agent is light with a ⁇ of 450-550 nm.
- the present invention relates to the aforementioned method, wherein said polymerization agent is light with a ⁇ of 488-514 nm.
- the present invention relates to the aforementioned method, wherein said patient is a primate, equine, feline, or canine.
- the present invention relates to the aforementioned method, wherein said patient is a human.
- the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
- the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
- the present invention relates to the aforementioned method, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
- the present invention relates to the aforementioned method, wherein said wound is a corneal laceration or corneal perforation.
- the present invention relates to the aforementioned method, wherein said wound is less than about 10 cm in size.
- the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm 2 in size.
- the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm 2 in size.
- the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm in length.
- the present invention relates to the aforementioned method, wherein said wound is less than about 2 cm in length.
- the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm in length.
- the present invention relates to the aforementioned method, wherein said wound is less than about 0.5 cm in length.
- the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 2% w/w to about 40% w/w. In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 5% w/w to about 20% w/w.
- the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 6% w/w to about 10% w/w.
- the present invention relates to the aforementioned method, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
- the present invention relates to the aforementioned method, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
- the present invention relates to the aforementioned method, further comprising the step of admixing a photoinitiator with said compound of formula Ia prior to treating said compound of formula Ia with said polymerization agent.
- the present invention relates to the aforementioned method, wherein said photoinitiator is eosin-Y.
- the present invention relates to the aforementioned method, further comprising the step of admixing a natural polymer with said dendrimeric compound.
- the present invention relates to the aforementioned method, wherein said natural polymer is HA, collagen, or a GAG fragment.
- the present invention relates to the aforementioned method, further comprising the step of admixing at least one cell with said dendrimeric compound.
- the present invention relates to the aforementioned method, wherein said cell is a stem cell.
- the present invention relates to the aforementioned method, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
- the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2- hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), polyvinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
- the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone.
- the present invention relates to the aforementioned method, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
- the present invention relates to the aforementioned method, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
- the present invention relates to the aforementioned method, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or a biologically active analog, fragment, or derivative thereof.
- the present invention relates to the aforementioned method, wherein said pharmaceutical agent is polyhexamethylene biguanide.
- the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 400 wt%.
- the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 200 wt%.
- the present invention relates to the aforementioned method, further comprising the step of sterilizing said dendrimeric compound.
- the present invention relates to the aforementioned method, further comprising the step of sterilizing said dendrimeric compound and said polymerization agent, wherein said polymerization agent is selected from the group consisting of a compound of fo ⁇ nula II, a compound of formula III, a compound of formula IV, and a compound of formula V.
- the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
- the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about lo- 3 .
- the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10- 5 .
- Another aspect of the present invention relates to a method of sealing a wound on a patient, comprising the steps of: applying an effective amount of a compound of formula VI to a wound on a patient and treating said compound of formula VI with a polymerization agent sufficient to polymerize said compound of formula VI, wherein said polymerization agent is an oxidizing agent or a compound of formula VII, wherein formula VI is represented by:
- R represents independently for each occurrence H or alkyl
- R represents independently for each occurrence H, halogen, or alkyl
- R 4 represents independently for each occurrence alkyl, aryl, or aralkyl
- R 5 represents independently for each occurrence -(C(R 3 ) 2 ) m SH, -C(O)(C(R 3 ) 2 ) m SH,
- R 1 ⁇ i represents independently -(C(R 2 - v ⁇ ) 2 ) x C(O)H, -C(O)(C(R 2 - VII ) 2 ) y C(O)H, -
- R 2'v ⁇ represents independently for each occurrence H, alkyl, or halogen
- R 3 - v ⁇ is fluoroalkyl, chloroalkyl, -CH 2 NO 2 , B is alkyl diradical, heteroalkyl diradical, or v 2 ⁇ 11 represents independently for each occurrence 2, 3, or 4; and w 2"v ⁇ is an integer in the range of about 5 to 700, inclusive.
- the present invention relates to the aforementioned method, wherein said polymerization agent is an oxidizing agent.
- the present invention relates to the aforementioned method, wherein said polymerization agent is O 2 .
- the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII.
- the present invention relates to the aforementioned method, wherein B is an alkyl diradical. ' '
- the present invention relates to the aforementioned method, said compound of formula VII is
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method, wherein w 2"vu is an integer in the range of about 50 to about 250.
- the present invention relates to the aforementioned method, wherein w 2"v ⁇ is an integer in the range of about 60 to about 90.
- the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R 2'vu is -C(O)H, and R 2"
- the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R 2"v ⁇ is -C(O)H, R 2 ⁇ v ⁇ is
- the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R 2 ⁇ v ⁇ is -C(O)H, R 2'v ⁇ is
- H, B is v 2"vu is 2, and w 2"v ⁇ is an integer in the range of about 60-90.
- the present invention relates to the aforementioned method, wherein n is 3, 4, or 5.
- the present invention relates to the aforementioned method, wherein n is 4.
- the present invention relates to the aforementioned method, wherein R 2 is H.
- the present invention relates to the aforementioned method, wherein R 3 is H.
- the present invention relates to the aforementioned method, wherein R 4 is alkyl.
- the present invention relates to the aforementioned method, wherein R is methyl or ethyl.
- the present invention relates to the aforementioned method, wherein n is 4, R 2 and R 3 are H, and R 4 is alkyl.
- the present invention relates to the aforementioned method
- R 1 is In certain instances, the present invention relates to the aforementioned method
- R 1 is and p is 1.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is is l.
- the present invention relates to the aforementioned method
- n 4, R 2 and R 3 are H, R 4 is methyl, R 1 is R 2 , and p is 1.
- the present invention relates to the aforementioned method
- n 4, R 2 and R 3 are H, R 4 is methyl, R 1 is R 3 SH R 2 , and p is 1.
- the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and a Bronsted acid.
- the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HA, wherein A is halogen or -O 2 CR 6 , and R 6 is alkyl, fluoroalkyl, aryl, or aralkyl.
- the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and an acid selected from group consisting of HCl and HBr. In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HO 2 CR 6 , wherein R 6 is fluoroalkyl.
- the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and CF 3 CO 2 H.
- the present invention relates to the aforementioned method, wherein said patient is a primate, equine, feline, or canine.
- the present invention relates to the aforementioned method, wherein said patient is a human.
- the present invention relates to the aforementioned method, further comprising the step of admixing a natural polymer with said compound of formula VI.
- the present invention relates to the aforementioned method, wherein said natural polymer is HA, collagen, or a GAG fragment.
- the present invention relates to the aforementioned method, further comprising the step of admixing at least one cell with said compound of formula VI.
- the present invention relates to the aforementioned method, wherein said cell is a stem cell.
- the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
- said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
- the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
- the present invention relates to the aforementioned method, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound. In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration or corneal perforation.
- the present invention relates to the aforementioned method, wherein said wound is less than about 10 cm 2 in size.
- the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm 2 in size.
- the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm 2 in size.
- the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm in length.
- the present invention relates to the aforementioned method, wherein said wound is less than about 2 cm in length.
- the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm in length.
- the present invention relates to the aforementioned method, wherein said wound is less than about 0.5 cm in length.
- the present invention relates to the aforementioned method, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
- the present invention relates to the aforementioned method, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
- the present invention relates to the aforementioned method, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
- the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2- hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), polyvinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
- said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2- hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), polyvinyl alcohol), poly(lactic acid), poly(glycolic acid),
- the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone.
- the present invention relates to the aforementioned method, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
- the present invention relates to the aforementioned method, wherein said pharmaceutical agent is an antibiotic, antimicrobial agent, antiinflammatory agent, or growth factor.
- the present invention relates to the aforementioned method, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
- the present invention relates to the aforementioned method, wherein said pharmaceutical agent is polyhexamethylene biguanide.
- the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 400 wt%.
- the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 200 wt%.
- the present invention relates to the aforementioned method, further comprising the step of sterilizing said compound of formula VI.
- the present invention relates to the aforementioned method, further comprising the step of sterilizing said compound of formula VI and said polymerization agent, wherein said polymerization agent is a compound of formula VII.
- the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation. In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10 "
- the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10 "
- Another aspect of the present invention relates to a method of sealing a wound on a patient, comprising the steps of: treating a dendrimeric compound of formula Ia or formula Ib with a polymerization agent to form a repair agent and applying said repair agent to a wound on a patient, wherein said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, or an oxidizing agent, wherein formula Ia is represented by:
- a 2 is alkyl, aryl, aralkyl, -Si(R 3 ) 3 , or
- A represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
- Z 1 represents independently for each occurrence -X 1 -R 4 , E, or
- Y 2 represents independently for each occurrence R 5 , A 4 ,
- Z represents independently for each occurrence -X 1 - ⁇ R-,5 , E, or
- Z 3 represents independently for each occurrence -X 1 -R 6 , E, or
- Y 4 represents independently for each occurrence R 7 , A 4 ,
- Z 4 represents independently for each occurrence -X'-R/, E, or
- Y 5 represents independently for each occurrence R 8 , A 4 ,
- Z 5 represents independently for each occurrence -X 1 -R 8 , E, or
- R 1 represents independently for each occurrence H, alkyl, or halogen
- R 2 represents independently for each occurrence H, alkyl, -OH, -N(R 1 °) 2 , -SH, hydroxyalkyl, or -[C(R') 2 ] d R 16 ;
- R 3 represents independently for each occurrence alkyl, aryl, or aralkyl;
- R 4 , R 5 , R 6 , R 7 , R 8 , and R 9 are H;
- R 10 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 11 represents independently for each occurrence H, -OH, -N(R 10 ) 2 , -SH, alkyl, hydroxyalkyl, or -[C(R 1 ⁇ dR 16 ;
- R 12 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 13 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 14 represents independently for each occurrence H, alkyl, or -CO 2 R 10
- R 15 represents independently for each occurrence H, alkyl, or -OR 10 ;
- R 16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R 10 ) 2 , -SH, -S-alkyl, -CO 2 R 10 , -C(O)N(R 10 ) 2 , or - C(NH 2 )N(R 10 ),;
- v 1 and v 2 each represent independently for each occurrence 2, 3, or 4; w ! and w 2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is 0, 1, 2, 3, 4, or 5; z 1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z 2 and z 3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
- X 1 and X 2 each represent independently for each occurrence O or -N(R 10 )-;
- X 3 represents independently for each occurrence O, N(R 10 ), or C(R 15 )(CO 2 R 10 ); -
- a 4 represents independently for each occurrence
- X 5 represents independently for each occurrence O or -N(R 22 )-;
- R 17 represents independently for each occurrence H, -(C(R 19 ) 2 ) h SH, -
- R 18 represents independently for each occurrence H or alkyl
- R 19 represents independently for each occurrence H, halogen, or alkyl
- R 20 represents independently for each occurrence H or alkyl
- R 21 represents independently for each occurrence H, -(C(R 19 ) 2 )hSH, -
- R 22 represents independently for each occurrence H, alkyl, aryl, or aralkyl; n 1 and h each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p 5 represents independently for each occurrence 1, 2, 3, 4, or 5; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive; said formula II is represented by: wherein
- R 1"11 represents independently for each occurrence H or R 5" "
- R 2"11 represents independently for each occurrence H or alkyl
- R 3"11 represents independently for each occurrence H, halogen, or alkyl
- R 4"11 represents independently for each occurrence alkyl, aryl, or aralkyl
- R 5'11 represents independently for each occurrence H or R 2 "" ; and z represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; said formula III is represented by:
- R 1 - 111 is -(C(R 2 - m ) 2 ) x C(O)H, -C(O)(C(R 2 - m ) 2 ) y C(O)H, -(C(R 2 - i ⁇ ) 2 ) x C(O)R 3 - ⁇ i , or - C(O)(C(R 2" ⁇ i ) 2 ) y C(O)R 3 - ⁇ i ;
- R 2'111 represents independently for each occurrence H, alkyl, or halogen; IT ,3- ⁇ 1 "i is fluoroalkyl, chloroalkyl, -CH 2 NO 2 , or
- x represents independently for each occurrence 0, 1, 2, 3, 4, 5, 6, 7, or 8;
- y represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8;
- z represents independently for each occurrence 0, 1, or 2;
- v represents independently for each occurrence 2, 3, or 4;
- w is an integer in the range of about 5 to about 700, inclusive;
- a 3 represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
- Y 1 represents independently for each occurrence R 4 ,
- Z 1 represents independently for each occurrence -X'-R 4 , E, or
- Y represents independently for each occurrence R ,
- Y 3 represents independently for each occurrence R 6 .
- Z represents independently for each occurrence -X 1 -R r> 6 , E, or
- Y 4 represents independently for each occurrence R 7 ,
- Z 4 represents independently for each occurrence -X 1 -R 7 , E, or
- Y represents independently for each occurrence R ,
- Z 5 represents independently for each occurrence -X 1 -R 8 , E, or
- Y 6 represents independently for each occurrence R 9 ,
- R 1 represents independently for each occurrence H, alkyl, or halogen
- R 2 represents independently for each occurrence H, alkyl, -OH, -N(R I0 ) 2 , -SH, hydroxyalkyl, or -[C(R 1 ) 2 ] d R 16 ;
- R 3 represents independently for each occurrence alkyl, aryl, or aralkyl;
- R 4 , R 5 , R 6 , R 7 , R 8 , and R 9 are H;
- R 10 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 11 represents independently for each occurrence H, -OH, -N(R 10 ) 2 , -SH, alkyl, hydroxyalkyl, or
- R 12 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 13 represents independently for each occurrence H, alkyl, aryl, or aralkyl
- R 14 represents independently for each occurrence H, alkyl, or -CO 2 R 10
- R 15 represents independently for each occurrence H, alkyl, or -OR 10 ;
- R 16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R 10 ) 2 , -SH, -S-alkyl, -CO 2 R 10 , -C(O)N(R 10 ) 2 , or - C(NH 2 )N(R 10 ) 2 ;
- v 1 and v 2 each represent independently for each occurrence 2, 3, or 4; w 1 and w 2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is O, 1, 2, 3, 4, or 5; z 1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z 2 and z 3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
- X 1 and X 2 each represent independently for each occurrence O or -N(R 10 )-; X 3 represents independently for each occurrence O, N(R 10 ), or C(R I5 )(CO 2 R 10 ); and
- E represents independently for each occurrence H, -[C(R ) 2 ] n C(O)H, , or
- X 6 represents independently for each occurrence O or -N(R 30 )-;
- R 23 represents independently for each occurrence
- R represents independently for each occurrence H or alkyl
- R 25 represents independently for each occurrence H, halogen, or alkyl
- R 26 represents independently for each occurrence H or alkyl
- R 27 represents independently for each occurrence H, alkyl, or halogen
- R 28 represents independently for each occurrence H, alkyl, -OH, -N(R 30 ) 2 , -SH, or hydroxyalkyl;
- R 29 represents independently for each occurrence H, -OH, -N(R 30 ) 2 , -SH, alkyl, or hydroxyalkyl;
- R 30 and R 31 represent independently for each occurrence H, alkyl, aryl, or aralkyl;
- Z 6 represents independently for each occurrence E 1 or
- R represents independently for each occurrence
- Z 7 represents independently for each occurrence E 1 or
- R >33 represents independently for each occurrence
- R >34 represents independently for each occurrence H, alkyl, or -CO 2 R 30.
- E 1 represents independently for each occurrence H, -[C(R 24 ) 2 ] j C(O)H,
- p 6 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8
- p 7 represents independently for each occurrence 0, 1, 2, 3, or 4
- p 8 represents independently for each occurrence 1, 2, or 3
- p 9 represents independently for each occurrence 0, 1, 2, or 3
- n 2 and j each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8
- m 1 represents independently for each occurrence 1 or 2
- v represents independently for each occurrence 2, 3, or 4
- w is an integer in the range of about 5 to about 700, inclusive.
- the present invention relates to the aforementioned method, wherein said dendrimeric compound is a compound of formula Ia, and said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, or an oxidizing agent.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method, wherein Z 1 represents independently for each occurrence -X 1 -R 4 or
- the present invention relates to the aforementioned method, wherein Z 2 represents independently for each occurrence -X 1 -R 5 or
- the present invention relates to the aforementioned method, wherein Z 3 represents independently for each occurrence -X ⁇ R 5 or
- the present invention relates to the aforementioned method, wherein Z 4 represents independently for each occurrence -X 1 -R 7 or
- the present invention relates to the aforementioned method, wherein Z 5 represents independently for each occurrence -X 1 -R 8 or
- the present invention relates to the aforementioned method, wherein X 1 is O.
- the present invention relates to the aforementioned method, wherein X 1 and X 2 are O.
- the present invention relates to the aforementioned method, wherein n is 1.
- the present invention relates to the aforementioned method, wherein p 1 is 2, 3, or 4.
- the present invention relates to the aforementioned method, wherein p 2 is 1.
- the present invention relates to the aforementioned method, wherein R 1 is H.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is H
- B is , m
- the present invention relates to the aforementioned method
- R 1 is H
- B is , A ⁇ 2M.-s_ , m
- Y 1 IS is or visible light.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 5 and said polymerization agent is a compound of formula in.
- the present invention relates to the aforementioned method
- R 1 is H
- B is , m
- the present invention relates to the aforementioned method
- R 1 is H
- B is m
- the present invention relates to the aforementioned method
- R is H
- B is , m
- Y 4 groups are H, about 1/2 of the Y 4 groups are polymerization agent is ultraviolet light or visible light.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method, wherein p 1 is 1, 2, 3, or 4.
- the present invention relates to the aforementioned method, wherein p 1 is 2.
- the present invention relates to the aforementioned method, wherein p is 4.
- the present invention relates to the aforementioned method, wherein m is 1.
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- the present invention relates to the aforementioned method
- R 1 is H, B is , m is 1
- the present invention relates to the aforementioned method
Abstract
One aspect of the invention relates to a sealant comprising dendrimeric macromolecules that form a hydrogel. In certain instances, the sealants comprise a hydrogel that swells less than 400 wt% upon hydration. In certain instances, the sealants further comprise pharmaceutical agent such as antibiotic, antimicrobial agent, or anti-inflammatory agent. Effective amounts of the sealants of the invention can be used topically or in vivo to treat wounds, and can also used as barrier against bacteria and other organisms. The invention also comprises kit for sealing a wound comprising polymerizable dendrimeric compound that forms a hydrogel and a system for delivering the polymerizable dendrimeric compound to a wound on a patient.
Description
Low-Swelling Hydrogel Sealants for Wound Repair Background of the Invention
Sealants and adhesives play an important role in helping patients recover from surgery or trauma. Sealants and adhesives are useful in treating patients suffering from a variety of in vivo or topical conditions, including lacerations, tears, wounds, ulcers, and surgical procedures. Sealants or adhesives can generally be used in any indication or application that a suture is presently used, and the sealant or adhesive often provides a better outcome than when a suture is used. Sealants or adhesives can also be applied more quickly to the injury site and often provide a better seal over the wound. Various medicinal applications for sealants and adhesives are described below.
Skin Lacerations
Skin lacerations are tears in the skin produced by accidents, trauma, or as a result of a surgical procedure. Lacerations often require treatment in order to close the hole in the skin, stop bleeding, and prevent infection. Minor lacerations in the skin may be treated using an adhesive tissue to cover the wound. However, larger laceractions often require sutures or a glue to help seal the wound. For example, it is generally recommended that sutures or a glue be used to treat lacerations deeper than 0.25 inches having a jagged edge or loose flap of tissue. The location of the laceration may also affect the form of treatment. For example, it is advantageous to treat a skin laceration on a joint using a glue because adhesive tissue tends to limit mobility of the joint. The use of sutures or glues to treat skin lacerations can also reduce the chance of scar formation.
Liver Lacerations
Lacerations of the liver can occur from trauma or as a result of a surgical procedure. The liver is a highly vascularized organ and bleeds profusely when lacerated or traumatized. Liver lacerations are difficult to repair owing to the nature of liver tissue. Liver tissue has very weak cohesive strength, and, consequently, sutures and staples are not satisfactory because they may pull through the liver tissue. The lack of satisfactory wound treatment methods for liver lacerations combined with the fact that it is difficult to reach the veins that feed the liver renders liver lacerations particularly serious. In fact, severe lacerations of the liver often result in the patient's death due to bleeding. Thus, new materials to treat liver lacerations are needed.
Cornea - Corneal Lacerations/Perforations
Corneal perforations/lacerations are produced by a variety of medical conditions (e.g., infection, inflammation, xerosis, neurotrophication, and degeneration) and traumas (e.g., chemical, thermal, surgical, and penetrating). Unfortunately, corneal perforations often lead to loss of vision and a decrease in an individual's quality of life. Depending on the type and the origin of the perforation, different treatments are currently available ranging from suturing the wound to a cornea graft. However, the surgical procedures are difficult given the delicate composition of the cornea and the severity of the wound which increases the likelihood for leakage and severe astigmatism after surgery. In certain cases, perforations that cannot be treated by standard suture procedures are treated with tissue adhesives (glues) to repair the wound. This type of treatment is advantageous because the method is simple, quick, safe, and provides quick restoration of globe integrity, thereby avoiding further complications. In addition to easy and fast application to the wound, an adhesive should: 1) bind to the tissue (necrosed or not, very often wet) with an adequate adhesion force; 2) be non-toxic; 3) be biodegradable or resorbable; 4) be sterilizable; and 5) not interfere with the healing process.
Various alkyl-cyanoacrylates are available for the repair of small perforations. However, these "super glues" have several disadvantages. For example, the monomers used to form the alkyl-cyanoacrylate polymer adhesive, particularly those with short alkyl chains, can be toxic with formation of formaldehyde. Alkyl-cyanoacrylates can also polymerize too quickly, which complicates administering the adhesive to the wound. The alkyl-cyanoacrylate polymer also has a hard, rough surface causing patient discomfort and the need to wear a contact lens. In addition, a number of complications have been reported including cataract formation, corneal infiltration, glaucoma, giant papillary conjunctivitis, and symblepharon formation when cyanoacrylates are used as a corneal sealant. Finally, additional surgical intervention has been required in more than 60% of patients.
Other glues for wound repair have been developed. Adhesive hemostats, based on fibrin, are usually constituted of fibrinogen, thrombin and factor XIII. Systems with fibrinogen and photosensitizers activated with light are also being tested. If adhesive hemostats have intrinsic properties which meet the requirements for a tissue adhesive, autologous products (time consuming in an emergency) or severe treatments before clinical use are needed to avoid any contamination to the patient. Sealants for corneal perforations
generally should 1) not impair normal vision, 2) quickly restore the intraocular pressure '1OP), 3) maintain the structural integrity of the eye, 4) promote healing, 5) adhere to moist issue surfaces, 6) possess solute diffusion properties which are molecular weight dependent md favorable for normal cornea function, 7) possess rheological properties that allow for iontroUed placement of the polymer on the wound, and 8) polymerize under mild ;onditions.
The use of sutures for wound repair has several limitations and disadvantages. First, iuture placement inflicts trauma to corneal tissues, especially when multiple passes are leeded. Second, although suture material has improved, sutures such as 10-0 nylon (which s often the preferred suture for corneal repair as well as in other in vivo areas) can act as a iidus for infection and incite corneal inflammation and vascularization. Notably, the Dropensity for corneal scarring increases with persistent inflammation and vascularization. ■Third, corneal suturing often yields uneven healing and resultant regular and irregular astigmatism. Fourth, sutures are prone to becoming loose and/or broken and may require additional attention for prompt removal. Finally, effective suturing requires technical skills that vary widely from surgeon to surgeon and can also involve prolonged operative time.
Cornea - Corneal Transplants
During a corneal transplant or penetrating keratoplasty surgery, the diseased cornea is removed with a special round cutting tool called a trephine. The donor cornea is cut to a matching size. Then, the donor cornea is placed upon the eye and secured in place with approximately 16 sutures around the transplant. A sutureless procedure would be highly desirable and would offer the following advantages: 1) sutures provide a site for infection, 2) the sutured cornea takes about 3 months to heal before the sutures need to be removed, and 3) the strain applied to the new cornea tissue from the sutures can distort the cornea. Notably, an ocular adhesive could serve as an adjuvant to sutures and/or reduce the necessary number of sutures.
Cornea - Clear Corneal Incision
Clear corneal incisions in the temporal cornea offer several advantages with phacoemulsification. The major advantage associated with phacoemulsification is the reduction in size of the entrance wound. Smaller wounds require fewer sutures or even no sutures at all, minimizing induction of astigmatisπij decreasing bleeding and subconjunctival hemorrhage, and speeding the recovery of visual acuity. See Agapitos, P.
J. Curr. Opin. Ophthalmol. 1993, 4, 39-43 and LyIe, W. A.; Jin, G. J. J. Cataract Refract. Surg. 1996, 22, 1456-1460. Surgeons typically examine the clear corneal incisions at the completion of the procedure by inflating the anterior chamber with balanced salt solution and applying pressure to the anterior cornea to check for leakage from the wound. If there is some leakage, the wound may be hydrated with balanced saline solution to seal fully the wound. This is done by injecting balanced saline solution into the open stromal edges. Hydration forces the two edges of the wound together, creating a tight seal. The endothelial cell pump can then remove the fluid from both the anterior and posterior portions of the wound, further sealing the wound together. See Fine, I. H. J. Cataract Refract. Surg. 1991, 17 (Suppl), 672-676. These tests for fluid flow, however, make several assumptions, including that the eye will remain well pressurized during the early postoperative period and that the hydrated wound will not be rapidly deturgesced by the corneal endothelium. One additional assumption is that the absence of aqueous outflow from the wound correlates with the inability of surface fluid from the tear film to flow into the wound, possibly contaminating the aqueous humor and predisposing to infection. However, intraocular pressure is known to vary in the postoperative period, frequently dropping to less than 5 mm Hg, and telemetric intraocular pressure monitoring devices suggest that large fluctuations in intraocular pressure occur in individual eyes in response to blinking. See Shingleton, B. J.; Wadhwani, R. A.; O'Donoghue, M. W.; Baylus, S.; Hoey, H. J. Cataract Refract. Surg. 2001, 27, 524-527 and Percicot, C. L.; Schnell, C. R.; Debon, C; Hariton, C. J. Pharmacol Toxicol. Methods 1996, 36, 223-228.
In a recent study, optical coherence tomography (OCT) confirmed that the morphology of clear corneal incision wounds was not constant but varied in response to changes in the intraocular pressure. See McDonnell, P. J.; Taban, M.; Sarayba, M.; Rao, B.; Zhang, J.; Schiffman, R.; Chen, Z. P. Ophthalmology 2003, 110, 2342-2348. When the eyes were well pressurized (20 mm Hg or higher), the chambers were deeply formed, and the wound edges were well apposed. Elevation of intraocular pressure up to 40 to 50 mm Hg did not result in any separation of the wound edges. As the intraocular pressure was reduced to 10 mm Hg and below, the wound edges progressively separated. The separation began at the internal aspect of the wound, with posterior migration of the posterior and peripheral wound leaflet. This separation resulted in a wedge-shaped gaping in the internal aspect of the incision. Coincident with this wound margin separation, the spontaneous flow of aqueous through the wound was observed, and the chamber became shallower. Elevating
the intraocular pressure resulted in prompt closure of the corneal wound at its superficial margin, termination of fluid leakage from the wound, and deepening of the anterior chamber. India ink was also applied to the surface of the cornea and quickly became visible through the operating microscope within the clear corneal incisions. Histologic examination of the wounds confirmed partial penetration of India ink particles along the edges of the incisions in every cornea. These studies demonstrated that a transient reduction of intraocular pressure might result in poor wound apposition in clear corneal incisions, with the potential for fluid flow across the cornea and into the anterior chamber along with the attendant risk of endophthalmitis. See McDonnell, P. J.; Taban, M.; Sarayba, M.; Rao, B.; Zhang, J.; Schiffman, R.; Chen, Z. P. Ophthalmology 2003, 110, 2342-2348.
Nonetheless, a progressive increase in the percentage of surgeons preferring self- sealing clear corneal incisions over scleral tunnel incisions in the United States and Europe has occurred over the past decade. See Learning, D. V. J. Cataract Refract. Surg. 1995, 21, 378-385 and Learning, D. V. J. Cataract Refract. Surg. 2001, 27, 948-955. Some studies, however, reveal an increased incidence of postoperative endophthalmitis after clear corneal cataract incisions and a recent, retrospective, case-controlled study, reported that clear corneal incisions were a statistically significant risk factor for acute post-cataract surgery endophthalmitis when compared with scleral tunnel incisions. See John, M. E.; Noblitt, R. Endophthalmitis. Scleral tunnel vs. clear corneal incision; Slack, Inc.: Thorofare, NJ, 2001; Colleaux, K. M.; Hamilton, W. K. Can. J. Ophthalmol. 2000, JJ, 373-378; Nagaki, Y.; Hayasaka, S.; Kadoi, C; Matsumoto, M.; Yanagisawa, S.; Watanabe, K.; Watanabe, K.; Hayasaka, Y.; Ikeda, N.; Sato, S.; Kataoka, Y.; Togashi, M.; Abe, T. J. Cataract. Refract. Surg. 2003, 29, 20-26; Stonecipher, K. G.; Parmley, V. C; Jensen, H.; Rowsey, J. J. Arch. Ophthalmol. 1991, 109, 1562-1563; Lertsumitkul, S.; Myers, P. C; O'Rourke, M. T.; Chandra, J. Clin. Exp. Ophthalmol. 2001, 29, 400-405; and Blake, A. C; Holekamp, N. M.; Bohigian, G.; Thompson, P. A. Am. J. Ophthalmol. 2003, 136, 300-305. The visual outcome following severe endophthalmitis is always guarded. In a Western Australian Endophthalmitis Study more than half of the subjects suffered visual impairment, with 41% poorer than 20/200, 53% poorer than 20/125, and 58% poorer than 20/40. See Semmens, J. B.; Li5 J.; Morlet, N.; Ng, J. CHn. Exp. Ophthalmol. 2003, 31, 213-219. Post-cataract endophthalmitis remains a potentially blinding complication of a sight-restoring procedure.
Refractive Surgery - Laser-assisted in situ Keratomileusis (LASIK)
Laser-assisted in situ keratomileusis is the popular refractive surgical procedure where a thin, hinged corneal flap is created by a microkeratome blade. This flap is then moved aside to allow an excimer laser beam to ablate the corneal stromal tissue with extreme precision for the correction of myopia and astigmatism. At the conclusion of the procedure, the flap is then repositioned and allowed to heal. However, with trauma, this flap can become dislocated prior to healing, resulting in flap striae (folds) and severe visual loss. When this complication occurs, treatment involves prompt replacement of the flap and flap suturing. The use of sutures has limitations and drawbacks as discussed above. These novel adhesives could also play a useful role in the treatment of LASIK flap dislocations and striae (folds). These visually debilitating flap complications are not uncommon following LASIK, and are currently treated by flap repositioning and suturing. Notably, the flap repositioning and suturing procedure requires considerable operative time and technical skill. Hence, a tissue adhesive could provide a more effective means to secure the flap.
Refractive surgery — Lens Replacement
Cataracts or other diseases or injuires that lead to a poorly functioning or damaged lens require the natural lens to be replaced. The optical properties of the normal eye lens are the consequence of a high concentration of proteins called "crystallins" forming a natural hydrogel. In vertebrate lenses, a range of differently sized protein assemblies, the alpha -, beta -, and gαmzwα-crystallins, are found creating a medium of high refractive index. The anatomical basis of accommodation includes the lens substance, lens capsule, zonular fibers, ciliary muscle and the elastic part of the choroid. Accommodation occurs through accurately controlled adjustments in the shape and thickness of the lens. The capsular bag is essential in transmitting the various extralenticular forces to the lens substance.
Modern cataract surgery can be done through a small incision (usually 2.5-3.5 mm). Once the incision is made, the anterior chamber is filled with a viscoelastic and the capsular bag is pricked with a needle. From this incision, a small continuous circular capsulorhexis (CCC) approximately 1.5 mm in diameter is performed using capsulorhexis forceps. Next endocapsular phacoemulsification is performed and the lens epithelial cells are removed by aspiration.
Retina - Retinal Holes
Techniques commonly used for the treatment of retinal holes, such as cryotherapy, diathermy and photocoagulation, are unsuccessful in the case of complicated retinal detachment, mainly because of the delay in the application and the weak strength of the chorioretinal adhesion. Cyanoacrylate retinopexy has been used in special cases. It has also been demonstrated that the chorioretinal adhesion is stronger and lasts longer than the earlier techniques. As noted previously with regard to corneal perforation treatment, the extremely rapid polymerization of cyanoacrylate glues, difficulty in using them in aqueous conditions, and the toxicity are disadvantages associated with this method. The polymerization can be slowed by adding iophendylate to the monomers, but the reaction still occurs in two to three seconds. Risks of retinal tear at the edge of the treated hole can also be observed because of the hardness of the polymerized cyanoacrylate.
Retina — Vitrectomy/Sclerotomy Incisions
The vitreous is normally a clear, gel-like substance that fills the center of the eye. It makes up approximately 2/3 of the eye's volume, giving it form and shape before birth. Certain problems affecting the back of the eye may require a vitrectomy, or surgical removal of the vitreous. During a vitrectomy, the surgeon creates small incisions/punctures in the eye (sclerotomies) for separate instruments. These incisions are placed in the pars plana of the eye, which is located just behind the iris but in front of the retina. The instruments which pass through these incisions include a light pipe, an infusion port, and the vitrectomy cutting device. Upon completion of pars plana vitrectomy, each sclerotomy site is closed with a single interrupted suture of 8-0 silk or 7-0 polyglycolic acid suture. After a vitrectomy, the eye is filled with fluid until the vitreous is replaced as the eye secretes aqueous and nutritive fluids.
Some of the most common eye conditions that require vitrectomy include 1) complications from diabetic retinopathy, such as retinal detachment or bleeding, 2) macular hole, 3) retinal detachment, 4) pre-retinal membrane fibrosis, 5) bleeding inside the eye (vitreous hemorrhage), 6) injury or infection, and 7) certain problems related to previous eye surgery.
Glaucoma - Leaking Bleb
Leaking filtering blebs after glaucoma surgery are difficult to manage and can lead to serious, vision-threatening complications. Leaking blebs can result in hypotony and shallowing of the anterior chamber, choroidal effusion, maculopathy, retinal, and choroidal
folds, suprachoroidal hemorrhage, corneal decompensation, peripheral anterior synechiae, and cataract formation. A leaking bleb can also lead to the loss of bleb function and to the severe complications of endophthalmaitis. The incidence of bleb leaks increases with the use of antimetabolites. Bleb leaks in eyes treated with 5-fluorouracil or mitomycin C may occur in as many as 20% to 40% of patients. Bleb leaks in eyes treated with antimetabolities may be difficult to heal because of thin avascular tissue and because of abnormal fibrovascular response. If the leak persists despite the use of conservative management, a 9-0 to 10-0 nylon or absorbable suture on a tapered vascular needle can be used to close the conjunctival wound. In a thin-walled or avascular bleb, a suture may not be advisable because it could tear the tissue and cause a larger leak.
Fibrin adhesives have been used to close bleb leaks. The adhesive is applied to conjunctival wound simultaneously with thrombin to form a fibrin clot at the application site. The operative field must be dry during the application because fibrin will not adhere to wet tissue. Cyanoacrylate glue may be used to close a conjuctival opening. To apply the glue, the surrounding tissue must be dried and a single drop of the cyanoacrylate is placed. The operative must be careful not to seal the applicator to the tissue or to seal surrounding tissue with glue given its quick reaction. A soft contact lens is then applied over the glue to decrease patient discomfort. However, this procedure can actually worsen the problem if the cyanoacrylate tears from the bleb and causes a larger wound.
Oculoplastics - Blepharoplasty Incisions
Blepharoplasty is an operation to remove excess skin, fat and muscle from around the eyes to correct droopy eyelids and bagginess under the eyes. It can be performed on the upper lids and lower lids, at the same time or separately. The operation may be done using either conventional or laser techniques. For surgery on the upper eyelids, cuts are made into the natural lines and creases in the lid, and into the laughter lines at the corner of the eye. For surgery on the lower eyelids, a cut is usually made just below the eyelashes. This means the scars run along the eye's natural folds, concealing them as much as possible. Excess fat, muscle and loose skin are removed, and the cut is closed using sutures. If only fat is being removed, sometimes the cut is made on the inside of the lower eyelid, leaving no visible scar. A tissue adhesive could provide a more effective means to secure the cuts made during these procedures.
Summary of the Invention
The present invention broadly relates to methods and compositions for sealing a wound on a patient using a hydrogel sealant/adhesive. One aspect of the present invention relates to a sealant comprising dendrimeric macromolecules that form a hydrogel. In certain instances, the sealants of the present invention further comprise a polymer, such as polyvinylpyrrolidone, poly(N-isopropylacrylamide), or a copolymer of poly(ethylene glycol) and poly(propylene glycol). In certain instances, the sealants of the present invention further comprise a pharmaceutical agent, such as an antibiotic, antimicrobial agent, or antiinflammatory agent. In certain instances, the sealants of the invention comprise a hydrogel that swells less than about 400 wt% upon hydration. The sealants of the present invention may be used to treat a wound on a patient that is topical or in vivo. In addition, the sealants of the present invention can act as a barrier to bacteria and other organisms. In certain instances, the sealants of the invention comprise a dendrimeric macromolecule and an alkyl diacid compound, wherein the alkyl diacid compound has a molecular weight of less than about 1000 g/mol.
In certain instances, the compositions used to seal a wound comprise at least one dendrimeric macromolecule and at least one polyethylene glycol polymer. In certain instances, the dendrimeric compounds used to form the hydrogel sealant have an acrylate group attached at the periphery of the dendrimer. Treatment of acrylate-capped dendrimeric compounds with ultraviolet radiation or a chemical initiator causes the dendrimeric compounds to polymerize, thereby forming a seal. In certain instances, the dendrimeric compounds have a lysine, cysteine, isocysteine residue or other nucleophilic group attached to the periphery of the dendrimer. Addition of a dendrimeric compound containing two or more electrophilic groups, such as an aldehyde, activated ester, or acrylate, to the lysine-, cysteine-, or isocysteine-capped dendrimers produces a polymeric compound that can form a seal. Alternatively, addition of a linear or dendrimeric polyethyelene glycol containing two or more electrophilic groups, such as an aldehyde, activated ester, or acrylate, to the lysine-, cysteine-, or isocysteine-capped dendrimers produces a polymeric compound that can form a seal. In a further embodiment, addition of an alkyl-chain compound containing two or more electrophilic groups, such as an aldehyde, activated ester, or acrylate, to the lysine-, cysteine-, or isocysteine-capped dendrimers produces a polymeric compound that can form a seal. In certain instances, the compositions used to seal the wound comprise a compound that has a polylysine core to
which cysteine, isocysteine, or other nucleophilic groups are attached. Addition of a compound containing two or more electrophilic groups, such as aldehydes, activated esters, or acrylates, to the cysteine- or isocysteine-capped polylysine compounds produces a polymeric compound that can form a seal.
Another aspect of the present invention relates to a method of sealing a wound on a patient comprising the steps of applying an effective amount of a dendrimeric compound to a wound on a patient and treating the dendrimeric compound with a polymerization agent. In certain instances, the dendrimeric compound is administered in the form of an aqueous solution. In certain instances, the polymerization agent is ultraviolet light or visible light. In certain instances, the dendrimeric compound is administered in the form of a gel. In certain instances, the dendrimeric compound is administered in the form of a patch. Another aspect of the present invention relates to a method of sealing a wound on a patient comprising the steps of treating a dendrimeric compound with a polymerization agent to form a repair agent, and applying the repair agent to a wound on a patient. In certain instances, the method further comprises the step of sterilizing the dendrimeric compound or repair agent.
Another aspect of the present invention relates to a kit for sealing a wound comprising a polymerizable dendrimeric compound that forms a hydrogel and a system for delivering the polymerizable dendrimeric compound to a wound on a patient. In certain instances, the kit further comprises a polymerization agent. In certain instances, the system is a syringe.
Brief Description of Figures
Figure 1 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
Figure 2 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
Figure 3 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
Figure 4 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
Figure 5 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
Figure 6 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
Figure 7 depicts various monomers that can be used to prepare dendrimers used in methods of the invention.
Figure 8 depicts a dendrimer terminated with nucleoside groups amenable to the invention.
Figure 9 depicts dendrimers and compounds useful for making dendrimers amenable to the present invention.
Figure 10 depicts a dendrimer amenable to the present invention.
Figure 11 depicts photocrosslinkable PEG34oo-(PGLSA-MA4)2macromer 1 for hydrogel formation.
Figure 12 depicts the normalized weight of hydrogel samples at 7.5, 10, and 15 % macromer concentration (n = 3), stored in PBS (left, PBS = Dulbecco's Phosphate Buffer Saline) or chondrocyte culture medium (right), at 37 0C, as a function of time.
Figure 13 depicts the complex modulus \G*\, storage modulus G', loss modulus G" and loss angle δ of crosslinked hydrogel samples at 4 different concentrations of macromer 1 in PBS.
Figure 14 depicts a determination of the compressive modulus E, as a function of concentration of macromer 1 in PBS. The compression-relaxation experiment for 20 % 1 is shown on the left, the linear curve fits for all concentrations and the resulting compressive modulus E is shown on the right.
Figure 15 depicts histological sections of 7.5 % and 15 % macromer 1 concentration hydrogels after 2 and 4 weeks incubation. Top: Red indicates proteoglycans in the Safranin-0 stained sections, green indicates collagen in the Masson's Trichrome stained sections. Bottom: Red indicates type I or II collagen in the immunostained sections, no significant type I collagen was detected at either concentration. The length of the inserted bar is 100 μm.
Figure 16 depicts a plot of swelling versus time for occular sealants in Example 120.
Figure 17 depicts a plot of average swelling results versus time for occular sealants in Example 120.
Figure 18 depicts a response surface plot of pH and buffer ratio effects for sealants in Example 121.
Figure 19 depicts a contour plot of pH and buffer ratio effects for sealants in Example 121.
Figure 20 depicts a response surface plot for swelling at 48 hours for sealants in Example 121.
Figure 21 depicts a contour plot for swelling at 48 hours for sealants in Example 121.
Figure 22 depicts a response surface plot for swelling at 72 hours for sealants in Example 121.
Figure 23 depicts a contour plot for swelling at 72 hours for sealants in Example 121.
Figure 24 depicts the effect of dendron buffer pH on swelling after 48 hours for sealants in Example 122.
Figure 25 depicts the effect of dendron buffer pH on cure time for sealants in Example 122.
Figure 26 depicts a swelling response surface plot for manually mixed samples for sealants in Example 123.
Figure 27 depicts a swelling response surface plot for mix-tip samples for sealants in Example 123.
Figure 28 depicts a cure time response surface plot for manually mixed samples (with contour plot) for sealants in Example 123.
Figure 29 depicts a cure time response surface plot for mix-tip samples for sealants in Example 123.
Figure 30 depicts a double-acting, single-barrel syringe.
Figure 31 depicts a double-barrel syringe.
Detailed Description of the Invention
The present invention broadly relates to methods and compositions for sealing a wound on a patient using a hydrogel sealant/adhesive. The sealant compositions of the present invention may be used in clinical treatments, such as sealing or repairing traumatic or surgical wounds. The sealants may be used for topical wounds or in vivo wounds, and the sealants may be formed in situ. The sealants of the invention may be used in situations where the site of the wound is not easily accessible and when sutureless surgery is desirable. The sealants of the present invention comprise dendrimeric macromolecules. In certain instances, the dendrimeric macromolecules form a hydrogel that swells less than about 400 wt% upon hydration. The dendrimeric macromolecular sealants of the present invention optionally comprise a polymer, such as polyvinylpyrrolidone, polyethylene glycol, poly(N-isopropylacrylamide), or a copolymer of poly(ethylene glycol) and poly(propylene glycol). In certain instances, the sealants of the invention comprise a dendrimeric macromolecule and an alkyl diacid compound, wherein the alkyl diacid compound has a molecular weight of less than about 1000 g/mol.
The formulations used to prepare the sealant may be mixed with a pharmaceutical agent to aid in the repair of damaged tissue or organs. In certain instances, the pharmaceutical agent is a growth factor, antibiotic, antimicrobial agent, or antiinflammatory agent. The pharmaceutical agent may be encapsulated by the sealant or it may be bonded to the polymeric compounds forming the sealant. In certain instances, the dendrimeric macromolecules can be seeded with cells, and then used to repair the damaged tissue or organs. Alternatively, the polymers and cells can be mixed and then injected into the in vivo site and crosslinked in situ for tissue repair or replacement. The crosslinked polymers provide a three dimensional template for new cell growth. Crosslinking, such as with a methacrylated functionalized denditic polymer, can be achieved using light or a chemical reaction. The crosslinkable biodendritic macromolecules can undergo a covalent or non- covalent crosslinking reaction to form a three-deminsional crosslinked gel or network. In certain instances, the crosslinking reaction is performed using light. In certain instances, the crosslinking reaction does not require light.
The formulations used to prepare the hydrogel sealants/adhesives of the invention may be aqueous mixtures. The pH of the aqueous mixture may be adjusted to give hydrogel sealants with different physical properties. In certain instances, the pH of the mixture ranges from about 6.0 to about 10.0. In certain instances, the pH of the mixture ranges from about 7.5 to about 8.5. The aqueous mixture may further comprise a carbonate, phosphate, 1-piperazineethane sulfonic acid, 4-(2-hydroxyethyl)-monosodium salt (HEPES), or a citrate to modulate the pH of the mixture. In certain instances, the aqueous mixture further comprises a carbonate or phosphate.
Dendritic Macromolecules
Dendritic polymers are globular monodispersed polymers composed of repeated branching units emitting from a central core. See US5714166; US4289872; US4435548; US5041516; US5362843; US5154853; US05739256; US5602226; US5514764; Bosman, A. W.; Janssen, H. M.; Meijer, E. W. Chem. Rev. 1999, 99, 1665-1688; Fischer, M.; Vogtle, F. Angew. Chem. Int. Ed. 1999, 38, 884-905; Zeng, F.; Zimmerman, S. C. Chem. Rev. 1997, 97, 1681-1712; Tomalia, D. A.; Naylor, A. M.; Goddard, W. A. Angew. Chem. Int. Ed. Engl. 1990, 29, 138. These macromolecules are synthesized using either a divergent (from core to surface) (Buhleier, W.; Wehner, F. V.; Vogtle, F. Synthesis 1987, 155-158. Tomalia, D. A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Polymer Journal 1985, 17, 117-132. Tomalia, D. A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. Macromolecules 1986, 19, 2466. Newkome, G. R.; Yao, Z.; Baker, G. R.; Gupta, V. K. J. Org. Chem. 1985, 50, 2003) or a convergent approach (from surface to core). See Hawker, C. J.; Frechet, J. M. J. J. Am. Chem. Soc. 1990, 112, 163%-lβAl.
This research area has undergone tremendous growth in the last decade since the early work of Tomalia and Newkome. Compared to linear polymers, dendrimers are highly ordered, possess high surface area to volume ratios, and exhibit numerous end groups for functionalization. Consequently, dendrimers display several favorable physical properties for both industrial and biomedical applications including: small polydispersity indexes (PDI), low viscosities, high solubility and miscibility, and excellent adhesive properties. The majority of dendrimers investigated for biomedical/biotechnology applications (e.g., MRI, gene delivery, and cancer treatment) are derivatives of aromatic polyether or aliphatic
amides, and thus are not ideal for in vivo uses. See Service, R. F. Science 1995, 267, 458- 459; Lindhorst, T. K.; Kieburg, C. Angew. Chem. Int. Ed. 1996, 35, 1953-1956; Ashton, P. R.; Boyd, S. E.; Brown, C. L.; Yayaraman, N.; Stoddart, J. F. Angew. Chem. Int. Ed. 1997, 1997, 732-735; Wiener, E. C; Brechbeil, M. W.; Brothers, H.; Magin, R. L.; Gansow, O. A.; Tomalia, D. A.; Lauterbur, P. C. Magn. Reson. Med. 1994, 31, 1-8; Wiener, E. C; Auteri, F. P.; Chen, J. W.; Brechbeil, M. W.; Gansow, O. A.; Schneider, D. S.; Beldford, R. L.; Clarkson, R. B.; Lauterbur, P. C. J. Am. Chem. Soc. 1996, 118, IHA-IIZI; Toth, E.; Pubanz, D.; Vauthey, S.; Helm, L.; Merbach, A. E. Chem. Eur. J. 1996, 2, 1607-1615; Adam, G. A.; Neuerburg, J.; Spuntrup, E.; Muhl;er, A.; Scherer, K.; Gunther, R. W. J. Magn. Reson. Imag. 1994, 4, 462-466; Bourne, M. W.; Margerun, L.; Hylton, N.; Campion, B.; Lai, J. J.; Dereugin, N.; Higgins, C. B. J. Magn. Reson. Imag. 1996, 6, 305-310; Miller, A. D. Angew. Chem. Int. Ed. 1998, 37, 1768-1785; Kukowska-Latallo, J. F.; Bielinska, A. U.; Johnson, J.; Spinder, R.; Tomalia, D. A.; Baker, J. R. Proc. Natl. Acad. ScL 1996, 93, 4897-4902; Hawthorne, M. F. Angew. Chem. Int. Ed. 1993, 32, 950-984; and Qualmann, B.; Kessels M.M.; Musiol H.; Sierralta W.D.; Jungblut PW.; L., M. Angew. Chem. Int. Ed. 1996, 35, 909-911.
Biodendrimers are a novel class of dendritic macromolecules composed entirely of building blocks known to be biocompatible or degradable to natural metabolites in vivo. The synthesis, characterization, and use of biodendrimers composed of biocompatible or natural metabolite monomers such as glycerol, lactic acid, glycolic acid, succinic acid, ribose, adipic acid, malic acid, glucose, citric acid, glycine, lysine, cysteine, alanine, etc. are described herein. In certain instances, the dendritic structures of the invention contain glycerol and one or more of lactic acid, glycolic acid, succinic acid, ribose, adipic acid, malic acid, glucose, citric acid, glycine, lysine, cysteine, alanine, and the like as a building block. In certain instances, the dendrimer is terminated with a photoreactive group or nucleophilic group. In certain instances, the terminus of the dendrimer contains a nucleoside. An additional embodiment of the invention is a dendritic structure that is composed of all lysine resides, such that it is a generation one or higher, or it is a lysine dendritic macromolecule terminated with cystene residues such that it is a generation one or higher.
One aspect of the present invention relates to the synthesis and fabrication of dendritic polymers and copolymers of polyesters, polyethers, polyether-esters, and polyamino acids or combinations thereof. For example, linear poly(glycolic acid),
ρoly(lactic acid), and their copolymers are synthetic polyesters that have been approved by the FDA for certain uses, and have been used successfully as sutures, drug delivery carriers, and tissue engineering scaffold for organ failure or tissue loss (Gilding and Reed, Polymer, 20:1459 (1979); Mooney et al., Cell Transpl., 2:203 (1994); and Lewis, D. H. in Biodegradable Polymers as Drug Delivery Systems, Chasin, M., and Langer, R., Eds., Marcel Dekker, New York, 1990). In tissue engineering applications, isolated cells or cell clusters are attached onto or embedded in a synthetic biodegradable polymer scaffold and this polymer-cell scaffold is next implanted into recipients (Langer and Vacant", Science, 260:920 (1993). A large number of cell types have been used including cartilage cells (Freed et al., Bio/Technology, 12:689 (1994)). Like the novel biodendrimers described in this invention, the advantages include their degradability in the physiological environment to yield naturally occurring metabolic products and the ability to control their rate of degradation by varying the ratio of lactic acid. In the dendritic structures the degradation can be controlled by both the type of monomer used and the generation number.
A further embodiment of this invention is to attach biological recognition units for cell recognition to the end groups or within the dendrimer structure. For example, the tripeptide arginine-glycine-aspartic (RGD), can be added to the structure for cell binding. Barrera et al. described the synthesis of a poly(lactic acid) (pLAL) containing a low concentration of N-epsilon-carbobenzoxy-L-lysine units. The polymers were chemically modified through reaction of the lysine units to introduce arginine-glycine-aspartic acid peptide sequences or other growth factors to improve polymer-cell interactions (Barrera et al., J. Am. Chem. Soc, 115:11010 (1993); U.S. Pat. No. 5,399,665 to Bartera et al.). One limitation of the copolymers developed by Barrera et al. is that only a limited number of lysine units can be incorporated into the backbone. In many tissue engineering applications, the concentration of biologically active molecules attached to the linear polymer is too low to produce the desired interactions between the polymer and the body. Consequently, there is a need for the development of optimal materials for use as sealants, adhesives, or temporary scaffolds to support cell growth and tissue development in tissue engineering and wound repair applications. In addition, there is a need for methods for introducing functionalities such as polyamino acids, peptides, and carbohydrates into polyesters, polyether-esters, polycarbonates, and the like in order to improve the biocompatibility, biochemical, mechanical, and other properties of the polymers. Furthermore, the need exists for polyester-, polyether ester-, and polyester-amine materials
which include a sufficient concentration of derivatizable groups to permit the chemical modification of the polymer for different biomedical applications.
It is therefore an object of the invention to provide dendritic polymers and copolymers of polyesters and polyamino acids, polyethers, polyurethanes, polycarbonates, polycarbamates, polyamino alcohols or combinations of these polymer classess which can be chemically modified for different biomedical applications such as tissue engineering applications, wound management, contrast agents vehicles, drug delivery vechiles, etc. It is a further object of the invention to provide dendritic polymers and copolymers of polyesters and polyamino acids with improved properties such as biodegradability, biocompatibility, and mechanical strength. It is still another object of the invention to provide dendritic polymers that can be derivatized to include functionalities, such as peptide sequences or growth factors, to improve the interaction of the polymer with cells, tissues, or bone.
The advantages of a dendritic polymer include multiple end-groups for functionalization, crosslinked gels with high crosslinking-densities at low polymer concentration, globular structure, low viscosities, and a well-defined composition. Conventional linear polymers for medical applications cannot be controlled easily or modified through changes in the polymer's structure because these polymers (e.g., PLA) do not possess functional groups, other than end groups, that permit chemical modification to change their properties. Furthermore, these polymers do not adopt a well-defined structure in solution, thereby limiting the applications of these polymers. Consequently, the novel polymers described herein are substantially different.
Gels
Another aspect of the present invention relates to using dendritic polymeric gels, gel-cell, gel-drug compositions for surgeries, drug delivery, and tissue engineering. Gels are 3D polymeric materials which exhibit the ability to swell in water and to retain a fraction of water within the structure without dissolving. The physical properties exhibited by gels such as water content, sensitivity to environmental conditions {e.g., pH, temperature, solvent, and stress), softness, adhesivity, and rubbery consistency are favorable for biomedical and biotechnological applications. Indeed, gels may be used as coatings {e.g. biosensors, catheters, and sutures), as "homogeneous" materials {e.g. contact lenses, burn dressings, and dentures), and as devices {e.g. artificial organs and drug delivery
systems). See Peppas, N. A. Hydrogel in Medicine and Pharmacy, VoI I and II 1987; Wichterle, O.; Lira, D. Nature 1960, 185, 117-118; and Ottenbrite, R. M.; Huang, S. J.; Park, K. Hydrogels and Biodegradable polymers for Bioapplications 1994; Vol. 627, pp 268.
Gel matrices for the entrapment of cells, including stem cells, as artificial organs/tissues have been explored. These investigations indicate that encapsulation is a promising approach for a number of disease states including Parkinson's disease (L- dopamine cells), liver disease (hepatocyte cells), and diabetes (islets of Langerhans). In the past, islets of Langerhans (the insulin producing cells of the pancreas) have been embedded in an ionically crosslinked alginate (a natural hydrogel) microcapsule with a poly-L-lysine coating. This composition reduced blood sugar levels in diabetic mice following transplantation.
Consequently, one aspect of the present invention relates to a gel comprising cells to create an artificial cornea to replace or aid in the repair of a damaged cornea. In a preferred embodiment, the gel is formed from a dendrimeric compound. Another aspect of the present invention relates to a method and means for designing, constructing, and utilizing artificial dendritic matrices as temporary scaffolding for cellular growth and implantation. A further embodiment of the invention to provide biodegradable, non-toxic matrices which can be utilized for cell growth, both in vitro, in vivo, and in situ. The cell scaffold/matrix/gel can be formed in vitro or in situ by crosslinking. In certain instances, the present invention relates to a method for configuring and constructing biodegradable artificial matrices such that they not only provide a support for cell growth, but also allow and enhance vascularization and differentiation of the growing cell mass following implantation. In certain instances, the present invention relates to matrices in different configurations so that cell behavior and interaction with other cells, cell substrates, and molecular signals can be studied in vitro.
Sealants/ Adhesives
The dendritic macromolecules of the present invention are useful as a tissue sealant or adhesive. For example, the polymers of the invention may be used as an ophthalmic sealant or adhesive for corneal lacerations, retinal tears, corneal transplants, cataract procedures, and the like. Notably, the macromolecules of the invention may be used to seal
a large variety of different types of wounds, and one of skill in the art will recognize that the sealant/adhesive has wide-spread application in ophthalmic and general surgeries. In certain instances, the sealants of the invention are used to seal a wound that is less than about 10 cm2, 5 cm2, or 1 cm2 in size. In certain instances, the sealants of the invention are used to seal a wound that is less than about 5 cm, 2 cm, or 1 cm in length. In certain instances, the sealants of the invention are used to seal a wound that is less than about 0.5 cm, 0.2 cm, 0.1, or 0.05 cm in length. A further embodiment of this invention is to use biodendritic crosslinkable polymers for sealing corneal perforations. A further embodiment of this invention is to use biodendritic crosslinkable polymers for sealing retinal holes. A further embodiment of this invention is to use biodendritic crosslinkable polymers for sealing leaking blebs. A further embodiment of this invention is to use biodendritic crosslinkable polymers for sealing a corneal transplant.
In addition to ophthalmological applications, the crosslinkable polymers of the invention may be used when the site of the wound is not easily accessible or when sutureless surgery is desired. The crosslinkable sealants may also be useful in cardiovascular surgery (aortic dissection or anastomotic bleeding), urinary tract surgery (nephrotomy closure, urethral repair, or hypospadia repair), pulmonary surgery (sealing parenchymal & bronchial leaks, bronchopleural fistula repair, or persistent air leak repairs), G.I. tract and stomach surgery (parotid cutaneous fistula, tracheo-oesophageal fistula, or peptic ulcer repair), joint surgery (cartilage repair or meniscal repair), heart surgery (cardiac ventricular rupture repair), brain surgery (dural defect repairs), ear surgery (ear drum perforation), and post-surgical drainage reduction (mastectomy or axillary dissection). The ease of application coupled with the ability to quickly and precisely seal a wet or dry wound renders the sealants/adhesives of the present invention superior to sealants/adhesives described previously.
Another aspect of the invention relates to the use of low-swelling adhesives to treat wounds or injuries. Low-swelling hydrogels are advantagous because hydrogel sealants/adhesives that swell significantly can become dislodged from the application site and lose their adhesive strength. For example, a hydrogel that swells about 700 wt% in aqueous solution can have poor performance in an ocular laceration. In a preferred embodiment, the low-swelling hydrogel is a hydrogel that swells less than about 400 wt% in an aqueous solution. In certain embodiments, the low-swelling hydrogel swells less than about 300 wt% or 200 wt% in an aqueous solution. The hydrogel sealants of the invention
can form in less than about 30 minutes. In certain instances, the hydrogel sealants of the invention form in less than about 15 minutes, 5 minutes, or 1 minute.
Biologically Active Agents Within the Dendritic Gel/Network
Biologically active agents may be incorporated in the dendritic gel. Biologically active agents amenable to the present invention include, without limitation, medicaments; vitamins; mineral supplements; substances used for the treatment, prevention, diagnosis, cure or mitigation of disease or illness; or substances which affect the structure or function of the body; or pro-drugs, which become biologically active or more active after they have been placed in a predetermined physiological environment. Preferred active agents amenable for use in the compositions of the present invention include growth factors, such as transforming growth factors (TGFs), fibroblast growth factors (FGFs), platelet derived growth factors (PDGFs), epidermal growth factors (EGFs), connective tissue activated peptides (CTAPs), osteogenic factors, and biologically active analogs, fragments, and derivatives of such growth factors.
Members of the transforming growth factor (TGF) supergene family, which are multifunctional regulatory proteins, are preferred. Members of the TGF supergene family include the beta-transforming growth factors (for example, TGF-βl, TGF-β2, and TGF-β3); bone morphogenetic proteins (for example, BMP-I, BMP-2, BMP-3, BMP-4, BMP-5, BMP-6, BMP-7, BMP-8, and BMP-9); heparin-binding growth factors (for example, fibroblast growth factor (FGF), epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and insulin-like growth factor (IGF)); inhibins (for example, Inhibin A, Inhibin B); growth differentiating factors (for example, GDF- 1); and activins (for example, Activin A, Activin B, and Activin AB).
Representative categories of biologically active compounds amenable to the present invention also include anti-infective agents, anti-inflammatory agents, anti-neoplastic agents, analgesic agents, anti-thrombotic agents, diagnostic agents, mineral supplements, vitamins, and prodrugs. Representative analgesics include nonsteroidal anti-inflammatory drugs, opiate agonists and salicylates. Representative anti-infective agents include anthelmintics, antianaerobics, antibiotics, aminoglycoside antibiotics, antifungal antibiotics, cephalosporin antibiotics, macrolide antibiotics, various beta-lactam antibiotics, penicillin antibiotics, quinolone antibiotics, sulfonamide antibiotics, tetracycline antibiotics, antimycobacterials, antituberculosis antimycobacterials, antiprotozoals, antimalarial
antiprotozoals, antiviral agents, anti-retroviral agents, scabicides, and urinary anti- infectives. Representative antineoplastic agents include alkylating agents, nitrogen mustard alkylating agents, nitrosourea alkylating agents, antimetabolites, purine analog antimetabolites, pyrimidine analog antimetabolites, hormonal antineoplastics, natural antineoplastics, antibiotic natural antineoplastics, and vinca alkaloid natural antineoplastics. Specific compounds for the aforementioned categories are listed in U.S. 6,600,010 and Merck Index, 12th Edition; S. Budavari, M. J. O'Neil, A. Smith, P. E. Heckelman, J. F. Kinneary, Ed.; Merck Research Laboratories: Whitehouse Station, NJ, 1996, both of which are hereby incorporated by reference.
Wound dressings
The biomaterials of the present invention are also useful as a wound dressing. Although the suture technique is used to treat the majority of wounds, the use of tissue adhesives (e.g., glues, sealants, patches, films and the like) is an attractive alternative to the use of sutures. In addition to easy and rapid application to the wound, an ideal adhesive should 1) bind to the tissue (necrosed or not, sometimes wet) with an adequate adhesion force, 2) be non-toxic, 3) biodegradable or resorbable, 4) sterilizable, 5) selectively permeable to gases, 6) impermeable to bacteria, and 7) able to control evaporative water loss. Notably, the main function of the adhesive is to protect the wound and to enhance the healing process, or at least not prevent it.
As described above, numerous sealants have been investigated and used for different clinical applications. Adhesive hemostats, based on fibrin, are the most common products of biological origin. These sealants are usually constituted of fibrinogen, thrombin and factor XIII, as well as fibrinogen/photosensitizers systems. If their intrinsic properties meet the requirements for a tissue adhesive, autologous products (which are time consuming in emergency) or severe treatments before clinical use are needed to avoid any contamination to the patient.
Synthetic materials, mainly polymers and hydrogels, have also been developed for wound closure. Alkyl-cyanoacrylates are available for the repair of cornea perforations. One investigator observed no difference in healed skin incisions that were treated by suture or by ethyl-2-cyanoacrylate-"Mediglue" application. However, these "super glues" have disadvantages. Their monomers, in particular those with short alkyl chains, can be toxic,
and they can polymerize too quickly leading to difficulty in treating the wound. Once polymerized, the surface of the glue is rough and hard. This can cause discomfort to the patient, and in case of cornea perforation treatment, a contact lens is often required. Other materials have been commercialized such as "Biobrane II" (composite of polydimethylsiloxane on nylon fabric) and "Opsite" (polyurethane layer with vinyl ether coating on one side). A new polymeric hemostat (poly-N-aceryl glucosamine) has been studied for biomedical applications such as treatment of gastric varices in order to replace cyanoacrylate (vournakis). Adhesives based on modified gelatin are also found to treat skin wounds. Photopolymerizable poly(ethylene glycol) substituted with lactate and acrylate groups has been used to seal air leaks in lung surgery.
Crosslinked Gels or Networks
The low-swelling, polymeric dendritic sealants of the present invention can be prepared by crosslinking dendrimers or dendritic polymers using either light or a chemical crosslinking reaction. In certain instances, the dendritic polymers are cross-linked with linear polymers to form a crosslinked gel or network. The gels can be highly hydrated and hydrophilic. In certain instances, the adhesive/sealant is mixed with a linear or dendritic polymer, such as polyvinylpyrrolidone, to form an adhesive/sealant that is an interpentrating network adhesive/sealant. Such adhesives/sealants often have a higher viscosity, and thus can be applied to the injury site in a more controlled fashion. Importantly, such sealants adhere to the wound. In addition to polyvinylpyrrolidone, a large number of polymers known in the art are amenable to the present invention. For example, the polymer may be polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose or other cellulose derivatives, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n- isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers. The polymer may also be a copolymer of polyvinylpyrrolidone and one or more of the aforementioned polymers.
For the chemical crosslinking reaction that is not activited by light, the polymers are functionalized to contain groups that will react with each other to form the gel. For example, the dendritic polymers can be chemically modified to have more than two
functional groups. In certain instances, the dendritic polymers have nucleophilic groups, such as primary amino (-NH2) groups or thiol (-SH) groups, which can react with electrophilic groups such as an acrylate, succinimidyl ester, maleimide, or aldehyde. Each functional group on a multifunctionally dendritic polymer is capable of covalently binding with another polymer, thereby effecting formation of the network by crosslmking between the polymers.
Examples of covalently crosslinked networks can be formed by reacting an activated ester, such as a succinimidyl ester, with an amine or thiol, e.g., a terminal primary or secondary amine, lys, cys, and the like. Thiol- or cysteine-terminated dendritic structures that form a disulfide crosslinked network with another thiol- or cysteine- terminated dendritic or linear polymer will also form a gel. Alternatively, a gel is formed during the reaction of an aldehyde-functionalized small molecule or polymer and an amine- or cysteine-functionalized polymer. An additional method is to have a maleimide- or vinylsulfone-functionalized dendritic polymer react with a thiol-functionalized dendritic, linear, comb, or other polymer to form the gel. A functionalized succinimidyl glutarate dendritic polymer reacts with an acid-terminated dendritic, linear, comb, or other polymer to form the gel. An acrylate-functionalized polymer reacts with an amine- or thiol- functionalized polymer to form the crosslinked gel. A further embodiment of this invention is the use of a chemical peptide ligation reaction to create a crosslinked gel involving a dendritic polymer. In this reaction an aldehyde or aldehyde-acid reacts with a cysteine- functionalized polymer to form a gel or crosslinked network. In certain instances, the dendritic polymers have nucleophilic groups, such as primary amino groups or thiol groups, which can react with electrophilic groups such as an acrylate, succinimidyl ester, maleimide, or aldehyde on a small molecule. In certain instances, the dendritic polymer has nucleophilic groups capable of reacting with an activated diester of sebabic acid.
Biodendrimers are often based on a core unit and contain branches. The core and branches may be composed of glycerol and lactic acid, glycerol and glycolic acid, glycerol and succinic acid, glycerol and adipic acid, and glycerol, succinic acid, and PEG. After the core is synthesized, polymers such as PEG and PLA can be attached to the core unit or to a branch to make large starburst or dendritic polymers. The biodendrimeric macromolecular sealants of the present invention optionally comprise a polymer, such as polyvinylpyrrolidone, polyethylene glycol, poly(N-isoproρylacrylamide), or a copolymer of polyethylene glycol) and poly(propylene glycol). In certain instances, the sealants of the
invention comprise a dendrimeric macromolecule and an alkyl diacid compound, wherein the alkyl diacid compound has a molecular weight of less than about 1000 g/mol.
Below the present invention is described by reference to specific embodiments. This description is not meant to limit the scope of the invention, but to convey the essence of the invention. Additional embodiments may be readily envisioned by one of ordinary skill in the art, and such embodiments fall within the scope of the invention.
One aspect of the present invention relates to a method for preparing and administrating in situ a biocompatible gel ex vivo, in vitro, or in vivo, comprising:
(a) forming a reactive composition by admixing a biocompatible crosslinking polymer having two different nucleophilic groups such as sulfhydryl and amine groups where there is at least one amine or sulfhydryl group on the polymer with a biocompatible crosslinking polymer B having amine and sulfhydryl-reactive groups, and further wherein the amine and sulfhydryl-reactive groups are capable of covalent reaction with the amine and sulfhydryl groups upon admixture of polymers A and B under effective crosslinking conditions to form a gel in less than one day; and
(b) allowing the components of the reactive composition to crosslink and thereby form a gel.
Another aspect of the present invention relates to dendritic or branched polymers or copolymers composed of monomers synthesized by combining branching compounds with other linear or branched building blocks. Both components are known to be biocompatible or are natural metabolites in vivo including but not limited to glycerol, citric acid, lactic acid, glycolic acid, adipic acid, caproic acid, ribose, glucose, succinic acid, malic acid, amino acids, peptides, synthetic peptide analogs, poly(ethylene glycol), poly(hydroxyacids) [e.g., PGA. PLA], including where one of the monomers is a branched structure such as glycerol combined with one of the other components.
In certain instances, the present invention relates to the aforementioned polymers derivatized with peripheral compounds possessing an olefin, including, but not limited to, acrylate and methacrylate.
In certain instances, the present invention relates to the aforementioned polymers derivatized with peripheral compounds including, but not limited to, cysteine, lysine, other amino acids, or any other compounds that would provide terminal nucleophiles (including,
but not limited to, amines, thiols, and hydroxyl groups) or electrophiles (including, but not limited to, NHS esters, maleimides, aldehydes, and ketones).
In certain instances, the present invention relates to the aforementioned polymers for subsequent polymerization/crosslinking/reaction with another linear or branched structure with either olefmic, electrophilic or nucleophilic groups, respectively to form a gel.
In certain instances, the present invention relates to the aforementioned polymers for subsequent polymerization/crosslinking/reaction with another linear or branched structure via a photopolymerization process (single or multi-photon process) to form a gel.
Another aspect of the present invention relates to a branching structure with at least three functional groups composed of, but not limited to, glycerol, citric acid, malic acid, amino acids, peptides, synthetic peptide analogs, or other dendritic structures synthesized to produce terminal olefins (including but not limited to acrylate or methacrylate groups), nucleophiles (including but not limited to amines, thiols, and hydroxyl groups) or electrophiles (including but not limited to NHS esters, maleimides, aldehydes, and ketones) for subsequent polymerization/crosslinking with another linear or branched structure with either olefmic, electrophilic or nucleophilic groups, respectively.
Another aspect of the present invention relates to a branching structure with at least three functional groups composed of, but not limited to, glycerol, citric acid, malic acid, amino acids, peptides, synthetic peptide analogs, or other dendritic structures derivatized with peripheral compounds including, but not limited to, cysteine, lysine, other amino acids, or any other compounds that would provide terminal olefins (including but not limited to acrylate or methacrylate groups), nucleophiles (including but not limited to amines, thiols, and hydroxyl groups) or electrophiles (including but not limited to NHS esters, maleimides, aldehydes, and ketones) for subsequent polymerization/crosslinking with another linear or branched structure with either olefmic, electrophilic or nucleophilic groups, respectively.
Another aspect of the present invention relates to a branching structure composed of three lysine amino acids with four cysteine amino acids on the periphery with the structure CysLys(Cys)Lys(CysLys(Cys))OMe*4HCl as described in the examples.
Another aspect of the present invention relates to a branching structure composed of three lysine amino acids with amines on the periphery with the structure (Lys)Lys(Lys)OMe»4HCl as described in the examples.
In certain instances, the present invention relates to the aforementioned polymers for subsequent polymerization/crosslinking/reaction with another linear or branched structure with olefmic, electrophilic or nucleophilic groups to form a gel.
In certain instances, the present invention relates to the aforementioned polymers for subsequent polymerization/crosslinking/reaction with another linear or branched structure through thiazolidine linkages to form a gel.
In certain instances, the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a poly(ethylene glycol) having a molecular weight of about 200 to about 200,000 g/mol with at least two electrophilic groups.
In certain instances, the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a poly(ethylene glycol) having a molecular weight of about 200 to about 200,000 g/mol with at least two nucleophilic groups
In certain instances, the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a poly(ethylene glycol) having a molecular weight of about 200 to about 200,000 g/mol with functional groups including but not limited to olefins, aldehydes, maleimides, or NHS esters.
In certain instances, the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a poly(ethylene glycol) having a molecular weight of about 200 to about 200,000 g/mol with aldehyde functional groups to form hydrogels through the formation of thiazolidine linkages.
In certain instances, the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with a copolymer comprising poly(ethylene glycol) and polypropylene glycol, wherein the copolymer has a molecular weight of about 200 to about 200,000 g/mol with functional groups including, but not limited to, olefins, aldehydes, maleimides, and NHS esters.
In certain instances, the present invention relates to the aforementioned polymers undergoing polymerization/crosslinking with an alkyl diacid, wherein the alkyl diacid has a
molecular weight of about 50 to about 1,000 g/mol and the alkyl diacid contains functional groups comprising NHS-esters.
In certain instances, the present invention relates to the aforementioned formulations in which each of the components are dissolved or suspended in an aqueous solution wherein the said aqueous solution is selected from water, buffered aqueous media, saline, buffered saline, solutions of amino acids, solutions of sugars, solutions of vitamins, solutions of carbohydrates or combinations of any two or more thereof.
In certain instances, the present invention relates to the application of the aforementioned formulation through a delivery device which physically separates the components until the components are physically mixed by the end user, including but not limited to a dual barrel syringe with a mixing device.
Another aspect of the present invention relates to packaging of the aforementioned branching compounds in an aqueous solution at a preselected pH and molarity selected from the aqueous solutions described above and the packaging of the second compound in an aqueous solution at another preselected pH and molarity selected from the aqueous solutions described above. When combined, the pH and molarities of the two solutions produce a final desired solution with a different pH. In certain instances, the aqueous solution comprising the branching compounds further comprises a polymer, such as polyvinylpyrrolidone .
Another aspect of the present invention relates to packaging of the aforementioned branching compounds in an aqueous solution at a preselected pH and molarity selected from the aqueous solutions described above and the packaging of the second compound in an aqueous solution at another preselected pH and molarity selected from the aqueous solutions described above. The contents are packaged free of oxygen and shielded from light. When combined, the pH and molarities of the two solutions produce a final desired solution with a different pH.
Another aspect of the present invention relates to packaging of the aforementioned branching compounds as a powder and adding an aqueous solution at a preselected pH and molarity selected from the aqueous solutions described above before use. The second component may either be packaged by dissolving the second compound in an aqueous solution at another preselected pH and molarity selected from the aqueous solutions described above or packaged similar to the first compound in which the compound stored as
a powder and an aqueous solution at a preselected pH and molarity selected from the aqueous solutions described above is added before use. The contents are packaged free of oxygen and shielded from light. When combined, the pH and molarities of the two solutions produce a final desired solution with a different pH.
Another aspect of the present invention relates to the storage of the aforementioned cysteine-terminated polymers in an acidic, oxygen-free solution to minimize the formation of disulfide bonds.
Another aspect of the present invention relates to the storage of the aforementioned aldehyde-terminated polymers in an acidic, oxygen-free solution to maximize the percent reactivity of the polymer and minimize aldol condensation and reverse Michael additions.
Another aspect of the present invention relates to the addition of various additives that might be incorporated into the polymer formulations including, but not limited to, antioxidants, colorants, viscosity modifiers, plasticizers, small molecule carbohydrates, large molecule carbohydrates, amino acids, peptides, or other water soluble polymers (linear or branched). Such additives may be added to increase the shelf life, increase the polymerization rate, modify the pH or molarity of the solution, change the refractive index, modify the mechanical properties, change crosslinking density, decrease swelling, or aid in visualization.
Another aspect of the present invention relates to the addition of various additives or anti-microbial agents such has polyhexamethylene biguanide (PHMB) that might be incorporated into the polymer formulations.
Another aspect of the present invention relates to the resulting hydrogels formed by mixing the aforementioned compounds as described and prepared herein.
In certain instances, the present invention relates to hydrogels formed by photopolymerization of the aforementioned compounds.
Another aspect of the present invention relates to a method of using crosslinkable/polymerizable/reactionary dendritic polymers, branching structures, and their hydrogels for delivery of therapeutics.
Another aspect of the present invention relates to a method of using a crosslinlcable/polymerizable/reactionary dendritic polymer or monomer for seeding with cells and subsequent in situ polymerization in vivo.
Another aspect of the present invention relates to a crosslinkable/polymerizable/reactionary dendritic polymer or monomer wherein the crosslinking is of covalent, ionic, electrostatic, and/or hydrophobic nature.
Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction involves a nucleophile and electrophile.
Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction is a peptide ligation reaction.
Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction is a Diels-Alder reaction.
Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction is a Michael Addition reaction.
Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the crosslinking reaction is a photochemical reaction using a UV or visible photoinitator chromophore.
Another aspect of the present invention relates to a crosslinkable branched or dendritic polymer in combination with a crosslinkable linear, comb, multi-block, star, or dendritic polymer(s) for a medical or tissue engineering application.
Another aspect of the present invention relates to a crosslinkable branched or dendritic polymer in combination with a crosslinkable monomer(s) for a medical or tissue engineering application.
Another aspect of the present invention relates to a method of using a crosslinkable branched or dendritic polymer combined with a crosslinkable small molecule(s) (molecule weight less than about 1000 Daltons) for a medical or tissue engineering application.
Another aspect of the present invention relates to a crosslinkable branched or dendritic polymer or monomer wherein the said crosslinking dendritic polymer is combined with one or more linear, comb, multi-block, star polymers or crosslinkable comb, multi- block, star polymers.
Another aspect of the present invention relates to a crosslinkable dendritic polymer or monomer wherein the final polymeric form is a gel, film, fiber, or woven sheet.
Another aspect of the present invention relates to the aforementioned polymers, branching structures, and their resulting hydrogels wherein the final polymeric form is a gel, film, fiber, or woven sheet.
Another aspect of the present invention relates to the aforementioned polymers, branching structures, and their resulting hydrogels wherein the polymer or crosslinkable monomer is D or L configuration or a mixture.
Another aspect of the present invention relates to the aforementioned polymers, branching structures, and their hydrogels wherein the dendritic structure is asymmetric at the surface such as a surface block structure where a carboxylate acid(s) and alkyl chains, or acrylate(s) and PEG(s) are present, for example, or within the core and inner layers of the dendrimer such as amide and ester linkages in the structure.
Another aspect of the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer wherein the polymer is a star biodendritic polymer or copolymer as shown in at least one of the formulas below: where Y and X are the same or different at each occurrence and are O, S, Se, N(H), or P(H) and where R1, R2, R3, R4, R5, Rβ, R7, R8, A or Z are the same or different and include -H, -CH3, -OH, carboxylic acid, sulfate, phosphate, aldehyde, methoxy, amine, amide, thiol, disulfide, straight or branched chain alkane, straight or branched chain alkene, straight or branched chain ester, straight or branched chain ether, straight or branched chain silane, straight or branched chain urethane, straight or branched chain, carbonate, straight or branched chain sulfate, straight or branched chain phosphate, straight or branched chain thiol urethane, straight or branched chain amine, straight or branched chain thiol urea, straight or branched chain thiol ether, straight or branched chain thiol ester, or any combination thereof.
Another aspect of the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer where the straight or branched chain is of about 1-50 carbon atoms wherein the chain is fully saturated, fully unsaturated or any combination therein
In certain instances, the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer where the straight or branched chain is of about 1-50 carbon atoms wherein the chain is fully saturated, fully unsaturated or any combination therein.
In certain instances, the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer wherein straight or branched chains are the same number of carbons or different wherein R1, R2, R3, R4, R5, R6, R7, Rs, A or Z are any combination of the linkers including ester, silane, urea, amide, amine, carbamate, urethane, thiol-urethane, carbonate, thio-ether, thio-ester, sulfate, phosphate and ether.
In certain instances, the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer which includes at least one chain selected from the group consisting of hydrocarbons, fluorocarbons, halocarbons, alkenes, and alkynes.
In certain instances, the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer which includes at least one chain selected from the group consisting of linear and dendritic polymers.
In certain instances, the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer wherein said linear and dendritic polymers include at least one selected from the group consisting of polyethers, polyesters, polyamines, polyacrylic acids, polycarbonates, polyamino acids, polynucleic acids and polysaccharides of molecular weight ranging from about 200-1,000,000 g/mol, and wherein said chain contains 0, 1 or more than 1 photopolymerizable group.
Another aspect of the present invention relates to a crosslinkable or noncrosslinkable polymer, wherein the polyether is PEG, and wherein the polyester is PLA, PGA or PLGA.
Another aspect of the present invention relates to a linear polymer wherein the chain is a polymer or copolymer of a polyester, polyamide, polyether, or polycarbonate of or the aforementioned polymer in combination with a polyester, polyamide, polyether, or polycarbonate of:
General Structure I
General Structure II
In certain instances, the present invention relates to the aforementioned polymer comprised of repeating units of General Structure I, where A is O, S, Se, or N-R7.
In certain instances, the present invention relates to the aforementioned polymer, where W, X, and Z are the same or different at each occurrence and are O, S, Se, N(H), or P(H).
In certain instances, the present invention relates to the aforementioned polymer, where R1 is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group.
In certain instances, the present invention relates to the aforementioned polymer, where Ri is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group substituted internally or terminally by one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
In certain instances, the present invention relates to the aforementioned polymer, where Ri is a polymer (such as poly(ethylene glycol), poly(ethylene oxide), or a poly(hydroxyacid)), a carbohydrate, a protein, a polypeptide, an amino acid, a nucleic acid, a nucleotide, a polynucleotide, any DNA or RNA segment, a lipid, a polysaccharide, an antibody, a pharmaceutical agent, or any epitope for a biological receptor.
In certain instances, the present invention relates to the aforementioned polymer, where Ri is a photocrosslinkable, chemically, or ionically crosslinkable group.
In certain instances, the present invention relates to the aforementioned polymer, in which D is a straight or branched alkyl chain of about 1-5 carbons, m is 0 or 1, and R2, R3, R4, R5, R6, and R7 are the same or different at each occurrence and are hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, alkoxy, aryloxy, olefin, alkylamine, dialkylamine, arylamine, diarylamine, alkylamide, dialkylamide, arylamide, diarylamide, alkylaryl, or arylalkyl group.
In certain instances, the present invention relates to the aforementioned polymer comprised of repeating units of General Structure II, where L, N, and J are the same or different at each occurrence and are O, S, Se, N(H), or P(H).
In certain instances, the present invention relates to the aforementioned polymer where Rj is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group.
In certain instances, the present invention relates to the aforementioned polymer where Ri is hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, olefin, silyl, alkylsilyl, arylsilyl, alkylaryl, or arylalkyl group substituted internally or terminally by one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
In certain instances, the present invention relates to the aforementioned polymer where Ri is a polymer selected from the group consisting of poly(ethylene glycols), poly(ethylene oxides), and poly(hydroxyacids, or is a carbohydrate, a protein, a polypeptide, an amino acid, a nucleic acid, a nucleotide, a polynucleotide, a DNA or RNA segment, a lipid, a polysaccharide, an antibody, a pharmaceutical agent, or an epitope for a biological receptor.
In certain instances, the present invention relates to the aforementioned polymer where R] is a photocrosslinkable, chemically, or ionically crosslinkable group.
In certain instances, the present invention relates to the aforementioned polymer, where D is a straight or branched alkyl chain of about 1-5 carbons, q and r are the same or different at each occurrence and are 0 or 1, and R7, R8, R9, Rio, Rn, Ri2, Ri3, and R]4 are the same or different at each occurrence and are hydrogen, a straight or branched alkyl chain of about 1-20 carbons, cycloalkyl, aryl, alkoxy, aryloxy, olefin, alkylamine, dialkylamine, arylamine, diarylamine, alkylamide, dialkylamide, arylamide, diarylamide, alkylaryl, or arylalkyl group.
In certain instances, the present invention relates to the aforementioned block or random copolymer comprised of repeating units of General Structure III, where M, T, and Q are the same or different at each occurrence and are O, S, Se, N(H), or P(H), e is 0 or 1-9, and Ri5 is a straight or branched alkyl chain of about 1-5 carbons, unsubstituted or substituted with one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
In certain instances, the present invention relates to the aforementioned block or random copolymer comprised of repeating units of General Structure III, where M, T, and Q are the same or different at each occurrence and are O, S, Se, N(H), or P(H), and Rj5 is a straight or branched alkyl chain of about 1-5 carbons, unsubstituted or substituted with one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
In certain instances, the present invention relates to the aforementioned block or random copolymer comprised of repeating units of General Structure III, where M, T, and Q are the same or different at each occurrence and are O, S, Se, N(H), or P(H), and Rl 5 is a straight or branched alkyl chain of about 1-5 carbons, unsubstituted or substituted with one or more hydroxyl, hydroxyether, carboxyl, carboxyester, carboxyamide, amino, mono- or di-substituted amino, thiol, thioester, sulfate, phosphate, phosphonate, or halogen substituents.
Another aspect of the present invention relates to a higher order block or random copolymer comprised of three or more different repeating units, and having one or more repeating units described above, such as a polyglyerol glycine carbonate-polyglycerol succinic acid copolymer.
Another aspect of the present invention relates to a block or random copolymer as described above, which includes at least one terminal crosslinkable group selected from the group consisting of amines, thiols, amides, phosphates, sulfates, hydroxides, alkenes, and alkynes.
In certain instances, the present invention relates to the aforementioned block or random copolymer where X, Y, M is O, S, N-H, N-R, and wherein R is -H, CH2, CR2, Se or an isoelectronic species of oxygen.
In certain instances, the present invention relates to the aforementioned block or random copolymer wherein an amino acid(s) is attached to Ri, R2, R3, R4, R5, A, and/or Z.
In certain instances, the present invention relates to the aforementioned block or random copolymer wherein a polypeptide(s) is attached to Ri, R2, R3, R4, R5, A, and/or Z.
In certain instances, the present invention relates to the aforementioned block or random copolymer wherein an antibody(ies) is attached to Ri, R2, R3, R4, R5, A, and/or Z.
In certain instances, the present invention relates to the aforementioned block or random copolymer wherein a nucleotide(s) is attached to R1, R2, R3, R4, R5 A, and/or Z.
In certain instances, the present invention relates to the aforementioned block or random copolymer wherein a nucleoside(s) is attached to R1, R2, R3, R4, R5> A, and/or Z.
In certain instances, the present invention relates to the aforementioned block or random copolymer wherein an oligonucleotide(s) is attached to R1, R2, R3, R4, R5, A, and/or Z.
In certain instances, the present invention relates to the aforementioned block or random copolymer wherein a ligand(s) is attached to R1, R2, R3, R4, R5, A, and/or Z that binds to a biological receptor.
In certain instances, the present invention relates to the aforementioned block or random copolymer wherein a pharmaceutical agent(s) is attached to R1, R2, R3, R4, R5, A, and/or Z.
In certain instances, the present invention relates to the aforementioned crosslinkable or noncrosslinkable polymer or copolymer wherein the polymer is a dendritic macromolecule including at least one polymer selected from the group consisting of dendrimers, hybrid linear-dendrimers, dendrons, or hyperbranched polymers according to one of the general formulas or such similar structures below: where R3, R4, which may be the same or different, are a repeat pattern of B, and n is about 0 to 50.
In certain instances, the present invention relates to the aforementioned polymer, wherein X, Y, M is O, S, N-H, N-R, wherein R is -H, CH2, CR2 or a chain as defined above, Se or any isoelectronic species of oxygen
In certain instances, the present invention relates to the aforementioned polymer, wherein X, Y, M is O, S, N-H, N-R, wherein R is -H, CH2, CR2 or a chain as defined above, Se or any isoelectronic species of oxygen.
In certain instances, the present invention relates to the aforementioned polymer where R3 and R4 are carboxylic acid with a protecting group such as but not limited to a phthalimidomethyl ester, a t-butyldimethylsilyl ester, or a t-butyldiphenylsilyl ester.
In certain instances, the present invention relates to the aforementioned polymer where R3, R4, A, and Z are the same or different, R3 and R4 are repeated a certain number of times, and terminate in -H, -OH, -CH3, carboxylic acid, sulfate, phosphate, aldehyde, activated ester, methoxy, amine, amide, thiol, disulfide, straight or branched chain allcane, straight or branched chain alkene, straight or branched chain ester, straight or branched chain ether, straight or branched chain silane, straight or branched chain urethane, straight or branched chain, carbonate, straight or branched chain sulfate, straight or branched chain phosphate, straight or branched chain thiol urethane, straight or branched chain amine, straight or branched chain thiol urea, straight or branched chain thiol ether, straight or
branched chain thiol ester, or any combination thereof, and wherein c is a natural or unnatural amino acid.
In certain instances, the present invention relates to the aforementioned polymer having a straight or branched chain of 1-50 carbon atoms and wherein the chain is fully saturated, fully unsaturated or any combination therein.
In certain instances, the present invention relates to the aforementioned polymer wherein straight or branched chains are the same number of carbons or different and wherein R3, R4, A, Z are any combination of linkers selected from the group consisting of esters, silanes, ureas, amides, amines, urethanes, thiol-urethanes, carbonates, carbamates, thio-ethers, thio-esters, sulfates, phosphates and ethers.
In certain instances, the present invention relates to the aforementioned polymer wherein chains include at least one selected from hydrocarbons, fluorocarbons, halocarbons, alkenes, and alkynes.
In certain instances, the present invention relates to the aforementioned polymer wherein said chains include polyethers, polyesters, polyamines, polyacrylic acids, polyamino acids, polynucleic acids and polysaccharides of molecular weight ranging from 200-1,000,000, and wherein said chain contains 1 or more crosslinkable or photopolymerizable group.
In certain instances, the present invention relates to the aforementioned polymer wherein the chains include at least one of PEG, PLA, PGA, PGLA, and PMMA.
In certain instances, the present invention relates to the aforementioned block or random copolymer, which includes at least one terminal crosslinkable or photopolymerizable group selected from the group consisting of amines, thiols, amides, phosphates, sulfates, hydroxides, alkenes, activated esters, malemides, aldehydes, and alkynes.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with amino acid(s), such as cysteine, attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with polypeptide(s) attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with an antibody(ies) or single chain antibody(ies) attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with a nucleotide(s) attached to Z, A, R3, and/or R4.,
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with a nucleoside(s) attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with oligonucleotide(s) attached to Z5 A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with ligand(s) attached to Z, A, R3, and/or R4 that binds to a biological receptor.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with a pharmaceutical agent(s) attached to Z, A, R3, and/or R4.
1 In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with a pharmaceutical agent attached to Z, A, R3, and/or R4 and is at least one selected from the group consisting of antibacterial, anticancer, anti-inflammatory, and antiviral.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times to produce a polymer in which a pharmaceutical agent(s) is encapsulated or chemically bound to the polymer.
In certain instances, the present invention relates to the aforementioned polymer wherein camptothecin or a derivative of campothethcm is encapsulated
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with a carbohydrate(s) attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein R3 and R4 are repeated a certain number of times and terminates with the carbohydrate mannose or sialic acid attached to the polymer.
In certain instances, the present invention relates to the aforementioned polymer which includes a polymer or copolymer of a polyester, polyamide, polyether, or polycarbonate at the center or periphery of the polymers above taken from the structures below.
General Structure I
General Structure II
General Structure III
In certain instances, the present invention relates to the aforementioned polymer block or random copolymer which includes at least one terminal or internal crosslinkable group selected from the group consisting of amines, thiols, amides, phosphates, sulfates, hydroxides, alkenes, and alkynes.
In certain instances, the present invention relates to the aforementioned polymer wherein X, Y, M is O, S, N-H, N-R, wherein R is -H, CH2, CR2 or a chain as defined above, Se or any isoelectronic species of oxygen.
In certain instances, the present invention relates to the aforementioned polymer wherein an amino acid(s) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein a polypeptide(s) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein an antibody(ies) or single chain antibody(ies) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein a nucleotide(s) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein a nucleoside(s) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein an oligonucleotide(s) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein a ligand(s) is attached to Z, A, R3, and/or R4 that binds to a biological receptor.
In certain instances, the present invention relates to the aforementioned polymer wherein a pharmaceutical agent(s) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein a carbohydrate(s) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein a PET or MRI contrast agent(s) is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein the contrast agent is Gd(DPTA).
In certain instances, the present invention relates to the aforementioned polymer wherein an iodated compound(s) for X-ray imaging is attached to Z, A, R3, and/or R4.
In certain instances, the present invention relates to the aforementioned polymer wherein a pharmaceutical agent(s) is attached to Z, A, R3, and/or R4 and is at least one selected from the group consisting of antibacterial, anticancer, anti-inflammatory, and antiviral.
In certain instances, the present invention relates to the aforementioned polymer wherein the carbohydrate is mannose or sialic acid is covalently attached to the polymer.
Another aspect of the present invention relates to a surgical procedure which comprises using a photopolymerizable, or chemically crosslmkable, or non-covalently crosslinkable dendritic polymer or copolymer.
Another aspect of the present invention relates to a surgical procedure wherein said dendritic polymer or copolymer is dissolved or suspended in an non-aqueous liquid such as soybean oil, mineral oil, corn oil, rapeseed oil, coconut oil, olive oil, safflower oil, cottonseed oil, aliphatic, cycloaliphatic or aromatic hydrocarbons having 4-30 carbon atoms, aliphatic or aromatic alcohols having 1-30 carbon atoms, aliphatic or aromatic esters having 2-30 carbon atoms, alkyl, aryl or cyclic ethers having 2-30 carbon atoms, alkyl or aryl halides having 1-30 carbon atoms and optionally having more than one halogen substituent, ketones having 3-30 carbon atoms, polyalkylene glycol or combinations of any two or more thereof.
In certain instances, the present invention relates to the surgical procedure wherein the supramolecular structure of the dendrimer is an emulsion.
In certain instances, the present invention relates to the dendritic polymer or copolymer which optionally contains at least one stereochemical center.
In certain instances, the present invention relates to the dendritic polymer or copolymer which is of D or L configuration.
In certain instances, the present invention relates to the dendritic polymer or copolymer wherein the final dendritic polymer or monomer is chiral or is achiral.
In certain instances, the present invention relates to the dendritic polymer or copolymer which contains at least one site where the branching is incomplete.
In certain instances, the present invention relates to a crosslinkable/photocrosslinkable/reactionary dendritic polymer or copolymer which contains at least one site where the branching is incomplete.
In certain instances, the present invention relates to a crosslinkable/photocrosslinkable/reactionary dendritic polymer or copolymer which contains at least one site where the branching is incomplete which forms a hydrogel.
In certain instances, the present invention relates to a crosslinlcable/photocrosslinkable/reactionary dendritic polymer or copolymer which contains at least one site where the branching is incomplete and used for drug delivery.
In certain instances, the present invention relates to a dendritic polymer or copolymer made by a convergent or divergent synthesis.
In certain instances, the dendritic polymer of the invention relates to
Another aspect of the invention relates to a hydrogel sealant formed by mixing two synthetic polymers or compounds that form a gel which swells less than 150 w/w or w/v or v/v %, wherein the synthetic polymer is not albumin or gelatin.
Another aspect of the invention relates to a hydrogel sealant formed by mixing two synthetic polymers or compounds that forms a gel via nucleophilic/electrophilic reactions which swells less than 150 w/w or w/v or v/v %, wherein the synthetic polymer is not albumin or gelatin.
Another aspect of the invention relates to a sealant formed with two separate electrophilic or nucleophilic synthetic polymers/compounds in solution mixed with the corresponding synthetic electrophilic or nucleophilic polymer.
Another aspect of the invention relates to a crosslinkable composition comprised of a first crosslinkable component having two or greater sets of two nucleophilic groups (an amine and a sulhydryl) directly reacting with one electrophilic group of a second crosslinkable component having two or greater electrophilic groups, each capable of reacting with the said two nucleophilic groups, to form a covalent five membered ring structure (thiazolidine ring) wherein each of the first and second crosslinkable components is synthetic, dissolved in aqueous solution, and crosslinking of the composition results in a biocompatible crosslinked hydrogel in less than 10 minutes.
Another aspect of the invention relates to a stable crosslinkable composition comprised of a first crosslinkable component having two or greater sets of 1,2-aminothiol groups directly reacting with one electrophilic (or aldehyde) group of a second crosslinkable component having two or greater electrophilic groups, each capable of reacting with the 1,2-aminothiol, to form a covalent five membered ring structure (thiazolidine ring) wherein each of the first and second crosslinkable components is synthetic, dissolved in aqueous solution , the crosslinking reaction is highly polymer specific, (ie it does not bind to tissue) and crosslinking of the composition results in a biocompatible crosslinked hydrogel on a tissue surface in less than 10 minutes.
Another aspect of the present invention relates to a kit, comprising at least one of the aforementioned stable crosslinkable compositions; and instructions for its use.
Another aspect of the invention relates to a method of forming a three dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of:
(a) providing an aqueous solution of a synthetic polymer containing m nucleophilic groups and an aqueous solution of a second synthetic small molecule containing n electrophilic groups, wherein the electrophilic groups react with the nucleophilic groups to form covalent bonds therewith, wherein m and n are each greater than or equal to two, and wherein m+n is greater than or equal to five;
(b) contacting the synthetic polymer and the synthetic small molecule to initiate crosslinking; and
(c) simultaneous with or subsequent to step (b), applying the first synthetic polymer and the synthetic small molecule to the first tissue surface; and
(d) allowing the first synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
In certain instances, the present invention relates to the aforementioned method, further comprising contacting the first tissue surface with a second surface after step (c) but before step (d) to effect adhesion between the first tissue surface and the second surface.
Another aspect of the invention relates to a method of forming a three dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of:
(a) providing an aqueous solution of a synthetic polymer containing two nucleophilic groups and an aqueous solution of a second synthetic small molecule containing three or four electrophilic groups, wherein the electrophilic groups react with the nucleophilic groups to form covalent bonds;
(b) contacting the synthetic polymer and the synthetic small molecule to initiate crosslinking; and
(c) simultaneous with or subsequent to step (b), applying the first synthetic polymer and the synthetic small molecule to the first tissue surface; and
(d) allowing the first synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
In certain instances, the present invention relates to the aforementioned method, further comprising contacting the first tissue surface with a second surface after step (c) but before step (d) to effect adhesion between the first tissue surface and the second surface.
In certain instances, the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 400%.
In certain instances, the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 300%.
In certain instances, the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 200%.
In certain instances, the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 100%.
In certain instances, the present invention relates to any one of the aforementioned methods, wherein the formed three-dimensional matrix swells less than 50%.
In certain instances, the present invention relates to any one of the aforementioned methods, wherein the synthetic polymer does not include collagen, collagen derivatives, or chemically modified collagens such as gelatin, hyaluronic acid or chemically modified derivatives of hyaluronic acid, albumin from any source or chemically modified derivatives of albumin foπn any source, thrombin or chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing the three-dimensional synthetic polymer matrix and/or the materials used to prepare, the matrix.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"
3
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"
Sterilization Procedures
A variety of procedures are known in the art for sterilizing a chemical composition. Sterilization may be accomplished by chemical, physical, or irradiation techniques. Examples of chemical methods include exposure to ethylene oxide or hydrogen peroxide vapor. Examples of physical methods include sterilization by heat (dry or moist), retort camiing, and filtration. The British Pharmacopoeia recommends heating at a minimum of 160 0C for not less than 2 hours, a minimum of 170 0C for not less than 1 hour and a minimum of 180 0C for not less than 30 minutes for effective sterilization. For examples of heat sterilization, see U.S. Patent 6,136,326, which is hereby incorporated by reference. Passing the chemical composition through a membrane can be used to sterilize a
composition. For example, the composition is filtered through a small pore filter such as a 0.22 micron filter which comprises material inert to the composition being filtered. In certain instances, the filtration is conducted in a Class 100,000 or better clean room. Examples of irradiation methods include gamma irradiation, electron beam irradiation, microwave irradiation, and irradiation using visible light. One preferred method is electron beam irradiation, as described in U.S. Patents 6,743,858; 6,248,800; and 6,143,805, each of which is hereby incorporated by reference.
There are several sources for electron beam irradiation. The two main groups of electron beam accelerators are: (1) a Dynamitron, which uses an insulated core transformer, and (2) radio frequency (RP) linear accelerators (linacs). The Dynamitron is a particle accelerator (4.5 MeV) designed to impart energy to electrons. The high energy electrons are generated and accelerated by the electrostatic fields of the accelerator electrodes arranged within the length of the glass-insulated beam tube (acceleration tube). These electrons, traveling through an extension of the evacuation beam tube and beam transport (drift pipe) are subjected to a magnet deflection system in order to produce a "scanned" beam, prior to leaving the vacuum enclosure through a beam window. The dose can be adjusted with the control of the percent scan, the beam current, and the conveyor speed. In certain instances, the electron-beam radiation employed may be maintained at an initial fluence of at least about 2 μCurie/cm2, at least about 5 μCurie/cm2, at least about 8 μCurie/cm2, or at least about 10 μCurie/cm2. In certain instances, the electron-beam radiation employed has an initial fluence of from about 2 to about 25 μCurie/cm2. In certain instances, the electron- beam dosage is from about 5 to 50 kGray, or from about 15 to about 20 kGray with the specific dosage being selected relative to the density of material being subjected to electron-beam radiation as well as the amount of bioburden estimated to be therein. Such factors are well within the skill of the art.
The composition to be sterilized may be in any type of at least partially electron beam permeable container such as glass or plastic. In embodiments of the present invention, the container may be sealed or have an opening. Examples of glass containers include ampules, vials, syringes, pipettes, applicators, and the like. The penetration of electron beam irradiation is a function of the packaging. If there is not enough penetration from the side of a stationary electron beam, the container may be flipped or rotated to achieve adequate penetration. Alternatively, the electron beam source can be moved about a stationary package. In order to determine the dose distribution and dose penetration in
product load, a dose map can be performed. This will identify the minimum and maximum dose zone within a product.
Procedures for sterilization using visible light are described in U.S. Patent 6,579,916, which is hereby incorporated by reference. The visible light for sterilization can be generated using any conventional generator of sufficient power and breadth of wavelength to effect sterilization. Generators are commercially available under the trade name PureBright® in-line sterilization systems from PurePulse Technologies, Inc. 4241 Ponderosa Ave, San Diego, Calif. 92123, USA. The PureBright® in-line sterilization system employs visible light to sterilize clear liquids at an intensity approximately 90000 times greater than surface sunlight. If the amount of UV light penetration is of concern, conventional UV absorbing materials can be used to filter out the UV light.
In a preferred embodiment, the composition is sterilized to provide a Sterility Assurance Level (SAL) of at least about 10"3. The Sterility Assurance Level measurement standard is described, for example, in ISO/CD 14937, the entire disclosure of which is incorporated herein by reference. In certain embodiments, the Sterility Assurance Level may be at least about 10"4, at least about 10"5, or at least about 10"6.
Delivery Systems
The materials used to form the seal on a wound may be delivered to the wound on a patient before the hydrogel forms. A large number of delivery systems are known in the art and are amenable to the present invention. In certain instances, a polymerizable dendrimeric compound is delivered to a wound on a patient. Alternatively, a polymerizable dendrimeric compound is combined with a polyermization agent to form a repair mixture, and the repair mixture is delivered to the wound on a patient. In certain instances, the materials delivered to the wound on a patient have been sterilized.
The delivery system may be a single-barrel syringe system. In certain instances, the single-barrel syringe is a double acting, single-barrel syringe system as displayed in Figure
30. In certain situations, a double- or multi-barrel syringe system, as displayed in Figure
31, may be preferable. In instances where the polymerizable dendrimer is mixed with a polymerization agent prior to delivering the mixture to the wound on a patient, a delivery device that flows two or more streams of liquid in a mixing chamber may be preferable.
Alternatively, a delivery device that mixes two solids and two liquids and then separately flows these streams of liquid to a mixing chamber may be advantageous. In certain instances, a delivery system is used to deliver the sealant-forming materials to the wound, wherein at least two dry, reactive components are stored together in a dry state and introduced into a liquid component(s) at the time of use to form a mixture that forms a hydrogel.
Definitions
For convenience, certain terms employed in the specification, examples, and appended claims are collected here.
The term "generation" refers to the number of branched repeat units which emanate from the central core. For example a third generation (or G3) PGLSA dendrimer has three branching layers not including the core.
The term "polymerize" as used herein refers to the process of converting a monomer to a chain of momomers, wherein the chain of momomers comprises at least about 5 monomers. In certain instances, the chain of monomers comprises at least about 10 or 15 momomers. In certain instances, the chain of monomers comprises at least about 25 or 40 momomers. In certain instances, the chain of monomers comprises at least about 50 or 75 momomers. In certain instances, the chain of monomers comprises at least about 100 or 150 momomers. In instances wherein the monomeric unit has more than one functional group capable of forming a bond in the polymerization reaction, the term "polymerize" indicates that at least one of the functional groups capable of forming a bond in the polymerization reaction forms a bond with another compound, generally speaking, the other compound is another monomer. In certain instances, at least about 10% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer. In certain instances, at least about 25% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer. In certain instances, at least about 50% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer. In certain instances, at least about 75% of the functional groups capable of forming a bond in a polymerization reaction form a bond to another monomer. In certain instances, about 20% to about 50% of the functional groups capable of forming a bond in a polymerization reaction form a bond to
another monomer. Furthermore, the term "polymerize" only requires that at least some of the monomer units in a given solution react to form a chain of monomers. In certain instances, about 10% to about 30% of the monomers react to form a chain of monomers. In certain instances, about 30% to about 50% of the monomers react to form a chain of monomers. In certain instances, about 50% to about 75% of the monomers react to form a chain of monomers. In certain instances, about 75% to about 85% of the monomers react to form a chain of monomers. In certain instances, about 85% to about 95% of the monomers react to form a chain of monomers. In certain instances, greater than about 95% of the monomers react to form a chain of monomers.
The term "seal" or "sealing" as used herein indicates that a protective barrier is formed over the wound. In certain instances, the protective barrier is a continuous layer. In certain instances, the protective barrier is a discontinous layer, i.e., a layer that has holes or pores in the layer. In certain instances, the discontinous layer comprises less than about 25% holes. In certain instances, the discontinous layer comprises about less than 15% holes. In certain instances, the discontinous layer comprises about less than 5% holes. In the instance where the protective barrier is a continuous layer, certain fluids or gases can penetrate through the layer. In certain instances, the fluid is a liquid located in the eye. In certain instances, the fluid is water. In instances when the wound is an ophthalmic wound, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 40 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 60 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 80 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 100 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 120 or about 150 mm Hg. In certain instances, the seal prevents fluid from exiting the wound when the pressure in the eye is less than about 180 or about 200 mm Hg.
The term "PEG-ALD2" as used herein refers to poly(ethylene glycol) dialdehyde. The aldehyde is the represented by -(CR2)nC(H)O, wherein R is hydrogen or Ci-C6 alkyl, and n is an integer from 0 to about 15. In certain instances, the aldehyde is the radical of acetaldehyde, butyraldehyde, or propionaldehyde.
The term "NHS" as used herein refers to the radical of N-hydroxy succinimide.
The term "sulfoNHS" as used herein refers to
O or a salt thereof.
The term "Mw" as used herein refers to weight average molecular weight in g/mol.
The term "alkali metal" refer to those elements listed in Group 1 of the periodic table. The following elements are alkali metals: Li, Na, K, Rb, Cs, and Fr.
The term "PLA" refers to poly(lactic acid). In certain instances, the PLA has a weight average molecular weight in the range of about 300 g/mol to about 800,000 g/mol. In certain instances, the PLA has a weight average molecular weight in the range of about 1000 g/mol to about 100,000 g/mol.
The term "PGA" refers to poly(glycolic acid). In certain instances, the PGA has a weight average molecular weight in the range of about 300 g/mol to about 800,000 g/mol. In certain instances, the PGA has a weight average molecular weight in the range of about 1000 g/mol to about 100,000 g/mol.
The term "PLGA" refers to a copolymer of lactic acid and glycolic acid. In certain instances, the PLGA has a weight average molecular weight in the range of about 300 g/mol to about 800,000 g/mol. In certain instances, the PLGA has a weight average molecular weight in the range of about 1000 g/mol to about 100,000 g/mol.
The term "heteroatom" is art-recognized and refers to an atom of any element other than carbon or hydrogen. Illustrative heteroatoms include boron, nitrogen, oxygen, phosphorus, sulfur and selenium.
The term "alkyl" is art-recognized, and includes saturated aliphatic groups, including straight-chain alkyl groups, branched-chain alkyl groups, cycloalkyl (alicyclic) groups, alkyl substituted cycloalkyl groups, and cycloalkyl substituted alkyl groups. In certain embodiments, a straight chain or branched chain alkyl has about 30 or fewer carbon atoms in its backbone (e.g., C]-C3O for straight chain, C3-C3O for branched chain), and alternatively, about 20 or fewer. Likewise, cycloalkyls have from about 3 to about 10 carbon atoms in their ring structure, and alternatively about 5, 6 or 7 carbons in the ring structure.
Unless the number of carbons is otherwise specified, "lower alkyl" refers to an alkyl group, as defined above, but having from one to about ten carbons, alternatively from one
to about six carbon atoms in its backbone structure. Likewise, "lower alkenyl" and "lower alkynyl" have similar chain lengths.
The term "aralkyl" is art-recognized and refers to an alkyl group substituted with an aryl group (e.g., an aromatic or heteroaromatic group).
The terms "alkenyl" and "alkynyl" are art-recognized and refer to unsaturated aliphatic groups analogous in length and possible substitution to the alkyls described above, but that contain at least one double or triple bond respectively.
The term "aryl" is art-recognized and refers to 5-, 6- and 7-membered single-ring aromatic groups that may include from zero to four heteroatoms, for example, benzene, naphthalene, anthracene, pyrene, pyrrole, furan, thiophene, imidazole, oxazole, thiazole, triazole, pyrazole, pyridine, pyrazine, pyridazine and pyrimidine, and the like. Those aryl groups having heteroatoms in the ring structure may also be referred to as "aryl heterocycles" or "heteroaromatics." The aromatic ring may be substituted at one or more ring positions with such substituents as described above, for example, halogen, azide, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, alkoxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio, sulfonyl, sulfonamide, ketone, aldehyde, ester, heterocyclyl, aromatic or heteroaromatic moieties, - CF3, -CN, or the like. The term "aryl" also includes polycyclic ring systems having two or more cyclic rings in which two or more carbons are common to two adjoining rings (the rings are "fused rings") wherein at least one of the rings is aromatic, e.g., the other cyclic rings may be cycloalkyls, cycloalkenyls, cycloalkynyls, aryls and/or heterocyclyls.
The terms ortho, meta and para are art-recognized and refer to 1,2-, 1,3- and 1,4- disubstituted benzenes, respectively. For example, the names 1,2-dimethylbenzene and ortho-dimethvlbenzene are synonymous.
The terms "heterocyclyl", "heteroaryl", or "heterocyclic group" are art-recognized and refer to 3- to about 10-membered ring structures, alternatively 3- to about 7-membered rings, whose ring structures include one to four heteroatoms. Heterocycles may also be polycycles. Heterocyclyl groups include, for example, thiophene, thianthrene, furan, pyran, isobenzofuran, chromene, xanthene, phenoxanthene, pyrrole, imidazole, pyrazole, isothiazole, isoxazole, pyridine, pyrazine, pyrimidine, pyridazine, indolizine, isoindole, indole, indazole, purine, quinolizine, isoquinoline, quinoline, phthalazine, naphthyridine, quinoxaline, quinazoline, cinnoline, pteridine, carbazole, carboline, phenanthridine,
acridine, pyrimidine, phenanthroline, phenazine, phenarsazine, phenothiazine, furazan, phenoxazine, pyrrolidine, oxolane, thiolane, oxazole, piperidine, piperazine, morpholine, lactones, lactams such as azetidinones and pyrrolidinones, sultams, sultones, and the like. The heterocyclic ring may be substituted at one or more positions with such substituents as ' described above, as for example, halogen, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio, sulfonyl, ketone, aldehyde, ester, a heterocyclyl, an aromatic or heteroaromatic moiety, -CF3, -CN, or the like.
The terms "polycyclyl" or "polycyclic group" are art-recognized and refer to two or • more rings (e.g., cycloalkyls, cycloalkenyls, cycloalkynyls, aryls and/or heterocyclyls) in which two or more carbons are common to two adjoining rings, e.g., the rings are "fused rings". Rings that are joined through non-adjacent atoms are termed "bridged" rings. Each of the rings of the polycycle may be substituted with such substituents as described above, as for example, halogen, alkyl, aralkyl, alkenyl, alkynyl, cycloalkyl, hydroxyl, amino, nitro, sulfhydryl, imino, amido, phosphonate, phosphinate, carbonyl, carboxyl, silyl, ether, alkylthio, sulfonyl, ketone, aldehyde, ester, a heterocyclyl, an aromatic or heteroaromatic moiety, -CF3, -CN, or the like.
The term "carbocycle" is art-recognized and refers to an aromatic or non-aromatic ring in which each atom of the ring is carbon.
1 The term "nitro" is art-recognized and refers to -NO2; the term "halogen" is art- recognized and refers to -F, -Cl, -Br or -I; the term "sulfhydryl" is art-recognized and refers to -SH; the term "hydroxyl" means -OH; and the term "sulfonyl" is art-recognized and refers to -SO2 ". "Halide" designates the corresponding anion of the halogens, and "pseudohalide" has the definition set forth on 560 of "Advanced Inorganic Chemistry" by Cotton and Wilkinson.
The terms "amine" and "amino" are art-recognized and refer to both unsubstituted and substituted amines, e.g., a moiety that may be represented by the general formulas:
wherein R50, R51 and R52 each independently represent a hydrogen, an alkyl, an alkenyl, - (CH2)m-R61, or R50 and R51, taken together with the N atom to which they are attached complete a heterocycle having from 4 to 8 atoms in the ring structure; R61 represents an aryl, a cycloalkyl, a cycloalkenyl, a heterocycle or a polycycle; and m is zero or an integer in the range of 1 to 8. In other embodiments, R50 and R51 (and optionally R52) each independently represent a hydrogen, an alkyl, an alkenyl, or -(CH2)m-R61. Thus, the term "alkylamine" includes an amine group, as defined above, having a substituted or unsubstituted alkyl attached thereto, i.e., at least one of R50 and R51 is an alkyl group.
The term "acylamino" is art-recognized and refers to a moiety that may be represented by the general formula:
wherein R50 is as defined above, and R54 represents a hydrogen, an alkyl, an alkenyl or - (CH2)m-R61, where m and R61 are as defined above.
The term "amido" is art recognized as an amino-substituted carbonyl and includes a moiety that may be represented by the general formula:
wherein R50 and R51 are as defined above. Certain embodiments of the amide in the present invention will not include imides which may be unstable.
The term "alkylthio" refers to an alkyl group, as defined above, having a sulfur radical attached thereto. In certain embodiments, the "alkylthio" moiety is represented by one of -S-alkyl, -S-alkenyl, -S-alkynyl, and -S-(CH2)m-R61, wherein m and R61 are defined above. Representative alkylthio groups include methylthio, ethyl thio, and the like.
The teπn "carboxyl" is art recognized and includes such moieties as may be represented by the general formulas:
wherein X50 is a bond or represents an oxygen or a sulfur, and R55 and R56 represents a hydrogen, an alkyl, an alkenyl, -(CH2)m-R61or a pharmaceutically acceptable salt, R56 represents a hydrogen, an alkyl, an alkenyl or -(CH2)m-R61, where m and R61 are defined above. Where X50 is an oxygen and R55 or R56 is not hydrogen, the formula represents an "ester". Where X50 is an oxygen, and R55 is as defined above, the moiety is referred to herein as a carboxyl group, and particularly when R55 is a hydrogen, the formula represents a "carboxylic acid". Where X50 is an oxygen, and R56 is hydrogen, the formula represents a "formate". In general, where the oxygen atom of the above formula is replaced by sulfur, the formula represents a "thiolcarbonyl" group. Where X50 is a sulfur and R55 or R56 is not hydrogen, the formula represents a "thiolester." Where X50 is a sulfur and R55 is hydrogen, the formula represents a "thiolcarboxylic acid." Where X50 is a sulfur and R56 is hydrogen, the formula represents a "thiolformate." On the other hand, where X50 is a bond, and R55 is not hydrogen, the above formula represents a "ketone" group. Where X50 is a bond, and R55 is hydrogen, the above formula represents an "aldehyde" group.
The term "carbamoyl" refers to -0(C=O)NRR1, where R and R' are independently H, aliphatic groups, aryl groups or heteroaryl groups.
The term "oxo" refers to a carbonyl oxygen (=0).
The terms "oxime" and "oxime ether" are art-recognized and refer to moieties that may be represented by the general formula:
wherein R75 is hydrogen, alkyl, cycloalkyl, alkenyl, aϊkynyϊ, aryl, aralkyl, or -(CH2)m-R61. The moiety is an "oxime" when R is H; and it is an "oxime ether" when R is alkyl, cycloalkyl, alkenyl, alkynyl, aryl, aralkyl, or -(CH2)m-R61.
The terms "alkoxyl" or "alkoxy" are art-recognized and refer to an alkyl group, as defined above, having an oxygen radical attached thereto. Representative alkoxyl groups
include methoxy, ethoxy, propyloxy, tert-butoxy and the like. An "ether" is two hydrocarbons covalently linked by an oxygen. Accordingly, the substituent of an alkyl that renders that alkyl an ether is or resembles an alkoxyl, such as may be represented by one of -O-alkyl, -O-alkenyl, -O-alkynyl, -O--(CH2)m-R61, where m and R61 are described above.
The term "sulfonate" is art recognized and refers to a moiety that may be represented by the general formula:
The term "sulfate" is art recognized and includes a moiety that may be represented by the general formula:
The term "sulfonamido" is art recognized and includes a moiety that may be represented by the general formula:
The teπn "sulfamoyl" is art-recognized and refers to a moiety that may be represented by the general formula:
The term "sulfonyl" is art-recognized and refers to a moiety that may be represented by the general formula:
O
-R58
O in which R58 is one of the following: hydrogen, alkyl, alkenyl, alkynyl, cycloalkyl, heterocyclyl, aryl or heteroaryl.
The term "sulfoxido" is art-recognized and refers to a moiety that may be represented by the general formula:
-/
R58 in which R58 is defined above.
The term "phosphoryl" is art-recognized and may in general be represented by the formula:
wherein Q50 represents S or O, and R59 represents hydrogen, a lower alkyl or an aryl. When used to substitute, e.g., an alkyl, the phosphoryl group of the phosphorylalkyl may be represented by the general formulas:
wherein Q50 and R59, each independently, are defined above, and Q51 represents O, S or N. When Q50 is S, the phosphoryl moiety is a "phosphorothioate".
The term "phosphoramidite" is art-recognized and may be represented in the general formulas:
The term "phosphonamidite" is art-recognized and may be represented in the general formulas:
Analogous substitutions may be made to alkenyl and alkynyl groups to produce, for example, aminoalkenyls, aminoalkynyls, amidoalkenyls, amidoalkynyls, iminoalkenyls, iminoalkynyls, thioalkenyls, thioalkynyls, carbonyl-substituted alkenyls or alkynyls.
The definition of each expression, e.g. alkyl, m, n, and the like, when it occurs more than once in any structure, is intended to be independent of its definition elsewhere in the same structure.
The term "selenoalkyl" is art-recognized and refers to an alkyl group having a substituted seleno group attached thereto. Exemplary "selenoethers" which may be substituted on the alkyl are selected from one of -Se-alkyl, -Se-alkenyl, -Se-alkynyl, and - Se-(CH2)m-R61, m and R61 being defined above.
The terms triflyl, tosyl, mesyl, and nonaflyl are art-recognized and refer to trifluoromethanesulfonyl, p-toluenesulfonyl, methanesulfonyl, and nonafluorobutanesulfonyl groups, respectively. The terms triflate, tosylate, mesylate, and nonaflate are art-recognized and refer to trifluoromethanesulfonate ester, p-toluenesulfonate
ester, methanesulfonate ester, and nonafluorobutanesulfonate ester functional groups and molecules that contain said groups, respectively.
The abbreviations Me, Et, Ph, Tf, Nf, Ts, and Ms represent methyl, ethyl, phenyl, trifluoromethanesulfonyl, nonafluorobutanesulfonyl, />-toluenesulfonyl and methanesulfonyl, respectively. A more comprehensive list of the abbreviations utilized by organic chemists of ordinary skill in the art appears in the first issue of each volume of the Journal of Organic Chemistry: this list is typically presented in a table entitled Standard List of Abbreviations.
Certain compounds contained in compositions of the present invention may exist in particular geometric or stereoisomeric forms. In addition, polymers of the present invention may also be optically active. The present invention contemplates all such compounds, including cis- and trans-isomers, R- and S-enantiomers, diastereomers, (D)-isomers, (L)- isomers, the racemic mixtures thereof, and other mixtures thereof, as falling within the scope of the invention. Additional asymmetric carbon atoms may be present in a substituent such as an alkyl group. All such isomers, as well as mixtures thereof, are intended to be included in this invention.
If, for instance, a particular enantiomer of compound of the present invention is desired, it may be prepared by asymmetric synthesis, or by derivation with a chiral auxiliary, where the resulting diastereomeric mixture is separated and the auxiliary group cleaved to provide the pure desired enantiomers. Alternatively, where the molecule contains a basic functional group, such as amino, or an acidic functional group, such as carboxyl, diastereomeric salts are formed with an appropriate optically-active acid or base, followed by resolution of the diastereomers thus formed by fractional crystallization or chromatographic means well known in the art, and subsequent recovery of the pure enantiomers.
It will be understood that "substitution" or "substituted with" includes the implicit proviso that such substitution is in accordance with permitted valence of the substituted atom and the substituent, and that the substitution results in a stable compound, e.g., which does not spontaneously undergo transformation such as by rearrangement, cyclization, elimination, or other reaction.
The term "substituted" is also contemplated to include all permissible substituents of organic compounds. In a broad aspect, the permissible substituents include acyclic and
cyclic, branched and unbranched, carbocyclic and heterocyclic, aromatic and nonaromatic substituents of organic compounds. Illustrative substituents include, for example, those described herein above. The permissible substituents may be one or more and the same or different for appropriate organic compounds. For purposes of this invention, the heteroatoms such as nitrogen may have hydrogen substituents and/or any permissible substituents of organic compounds described herein which satisfy the valences of the heteroatoms. This invention is not intended to be limited in any manner by the permissible substituents of organic compounds.
The phrase "protecting group" as used herein means temporary substituents which protect a potentially reactive functional group from undesired chemical transformations. Examples of such protecting groups include esters of carboxylic acids, silyl ethers of alcohols, and acetals and ketals of aldehydes and ketones, respectively. The field of protecting group chemistry has been reviewed (Greene, T. W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 2nd ed.; Wiley: New York, 1991). Protected forms of the inventive compounds are included within the scope of this invention.
For purposes of this invention, the chemical elements are identified in accordance with the Periodic Table of the Elements, CAS version, Handbook of Chemistry and Physics, 67th Ed., 1986-87, inside cover.
Methods of the Invention
One aspect of the present invention relates to a method of sealing a wound on a patient, comprising the steps of: applying an effective amount of a dendrimeric compound of formula Ia or formula Ib to a wound on a patient and treating said dendrimeric compound with a polymerization agent sufficient to polymerize said dendrimeric compound, wherein said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, or an oxidizing agent, wherein formula Ia is represented by:
A represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
Y represents independently for each occurrence R 4 , A A 4 ,
Z represents independently for each occurrence -X 1 - nR6 , E, or
Z represents independently for each occurrence -X 1 -R r.7 , E, or
R1 represents independently for each occurrence H, alkyl, or halogen;
R2 represents independently for each occurrence H, alkyl, -OH, -N(R1 °)2, -SH, hydroxyalkyl, or -[C(R^2]C1R16;
R3 represents independently for each occurrence alkyl, aryl, or aralkyl; R4, R5, R6, R7, R8, and R9 are H;
R10 represents independently for each occurrence H, alkyl, aryl, or aralkyl; Ru represents independently for each occurrence H, -OH, -N(R10)2, -SH, alkyl, hydroxyalkyl, or -[C(R')2]dR16;
R12 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R13 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R14 represents independently for each occurrence H, alkyl, or -CO2R10; R15 represents independently for each occurrence H, alkyl, or -OR10;
R16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R10)2, -SH, -S-alkyl, -CO2R10, -C(O)N(R10)2, or - C(NH2)N(R10)2;
d represents independently for each occurrence 1, 2, 3, 4, 5, or 6; n represents independently for each occurrence 1, 2, 3, 4, 5, or 6; p1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p2 represents independently for each occurrence O, 1, 2, 3, or 4; p3 represents independently for each occurrence 1, 2, or 3; p represents independently for each occurrence 0, 1, 2, or 3; t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
v1 and v2 each represent independently for each occurrence 2, 3, or 4; w1 and w2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is O, 1, 2, 3, 4, or 5;
z represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z2 and z3 each represent independently for each occurrence 1, 2, 3, 4, or 5; X1 and X2 each represent independently for each occurrence O or -N(R10)-; X3 represents independently for each occurrence O, N(R10), or C(R15)(CO2R10);
E represents independently for each occurrence
provided that R only occurs once, R5 only occurs once, R6 only occurs once, R7 only occurs once, R8 only occurs once, and R9 only occurs once; said formula Ib is represented by:
Ib or a pharmaceutically acceptable salt, solvate, or hydrate thereof, wherein
X5 represents independently for each occurrence O or -N(R22)-;
R17 represents independently for each occurrence H, -(C(R19)2)hSH,
R represents independently for each occurrence H or alkyl;
R19 represents independently for each occurrence H, halogen, or alkyl;
R20 represents independently for each occurrence H or alkyl;
R21 represents independently for each occurrence H, -(C(R19)2)hSH, -
R ,22 represents independently for each occurrence H, alkyl, aryl, or aralkyl;
n1 and h each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p5 represents independently for each occurrence 1, 2, 3, 4, or 5; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive; said formula II is represented by:
II wherein
R " ! represents independently for each occurrence H or alkyl;
R3"π represents independently for each occurrence H, halogen, or alkyl;
R4"11 represents independently for each occurrence alkyl, aryl, or aralkyl; and
R5'11 represents independently for each occurrence H or
R2"" and z represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; said formula HI is represented by:
R1-lll B1-lll R1-lll in wherein
Ruπi is -(C(R2-m)2)xC(O)H, -C(O)(C(R2-πi)2)yC(O)H, -(C(R2-iπ)2)xC(O)R3-m, or C(O)(C(R2-πi)2)yC(O)R3-iπ;
R ' represents independently for each occurrence H, alkyl, or halogen;
R ,3'-"imπ is fluoroalkyl, chloroalkyl, -CH2NO2, or
x represents independently for each occurrence 0, 1, 2, 3, 4, 5, 6, 7, or 8;
y represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8;
z represents independently for each occurrence 0, 1, or 2;
v represents independently for each occurrence 2, 3, or 4; and
w is an integer in the range of about 5 to about 700, inclusive;
said formula IV is represented by:
A1— X1-B— X1— A2 rv wherein
A3 represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
Z represents independently for each occurrence -X 1 - τR.4 , E, or
Z2 represents independently for each occurrence -X'-R5, E, or
Y4 represents independently for each occurrence R7,
Z4 represents independently for each occurrence -X1 -R7, E, or
Y represents independently for each
Z5 represents independently for each occurrence -X1 -R8, E, or
R represents independently for each occurrence H, alkyl, -OH, -N(R 10 )\2, -SH, hydroxyalkyl, or -[C(R1)2]dR16;
R3 represents independently for each occurrence alkyl, aryl, or aralkyl; R4, R5, R6, R7, R8, and R9 are H;
R10 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R11 represents independently for each occurrence H, -OH, -N(R1 °)2, -SH, alkyl, hydroxyalkyl, or -[C(R])2]dR16;
R12 represents independently for each occurrence H, allcyl, aryl, or aralkyl; R13 represents independently for each occurrence H, allcyl, aryl, or aralkyl; R14 represents independently for each occurrence H, alkyl, or -CO2R10; R15 represents independently for each occurrence H, allcyl, or -OR10;
R16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R10)2, -SH, -S-allcyl, -CO2R10, -C(O)N(R10)2, or - C(NH2)N(R10)2;
n represents independently for each occurrence 1, 2, 3, 4, 5, or 6; p1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p2 represents independently for each occurrence 0, 1, 2, 3, or 4; p3 represents independently for each occurrence 1, 2, or 3; p4 represents independently for each occurrence 0, 1, 2, or 3; d represents independently for each occurrence 1, 2, 3, 4, 5, or 6; t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
v1 and v2 each represent independently for each occurrence 2, 3, or 4; w1 and w2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is 0, 1, 2, 3, 4, or 5; z1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z2 and z3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
X1 and X2 each represent independently for each occurrence O or -N(R10)-; X3 represents independently for each occurrence O, N(R10), or C(R15XCO2R1 °); and
said formula V is represented by:
X6 represents independently for each occurrence O or -N(R30)-;
R23 represents independently for each occurrence
R24 represents independently for each occurrence H or alkyl;
R i25 represents independently for each occurrence H, halogen, or alkyl;
R26 represents independently for each occurrence H or alkyl;
R27 represents independently for each occurrence H, alkyl, or halogen;
R28 represents independently for each occurrence H, alkyl, -OH, -N(R30)2, -SH, or hydroxyalkyl;
R29 represents independently for each occurrence H, -OH, -N(R30)2, -SH, alkyl, or hydroxyalkyl;
R30 and R31 represent independently for each occurrence H, alkyl, aryl, or aralkyl;
represents independently for each occurrence E1 or
R , 32 represents independently for each
Z7 represents independently for each occurrence E1 or
R >33 represents independently for each
p represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p7 represents independently for each occurrence 0, 1, 2, 3, or 4; p8 represents independently for each occurrence 1, 2, or 3; p9 represents independently for each occurrence 0, 1, 2, or 3; n2 and j each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; m1 represents independently for each occurrence 1 or 2; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive.
In certain instances, the present invention relates to the aforementioned method, wherein said dendrimeric compound is a compound of formula Ia, and said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, or an oxidizing agent.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein Z1 represents independently for each occurrence -X -R or
In certain instances, the present invention relates to the aforementioned method, wherein Z2 represents independently for each occurrence -X1 -R5 or
In certain instances, the present invention relates to the aforementioned method, wherein Z3 represents independently for each occurrence -X1 -R6 or
2.
In certain instances, the present invention relates to the aforementioned method, wherein Z4 represents independently for each occurrence -X1 -R7 or
In certain instances, the present invention relates to the aforementioned method, wherein Z5 represents independently for each occurrence -X1 -R8 or
In certain instances, the present invention relates to the aforementioned method, wherein X1 is O.
In certain instances, the present invention relates to the aforementioned method, wherein X1 and X2 are O.
In certain instances, the present invention relates to the aforementioned method, wherein n is 1.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, 3, or 4.
In certain instances, the present invention relates to the aforementioned method, wherein p2 is 1.
In certain instances, the present invention relates to the aforementioned method, wherein R1 is H.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
groups are H, about 1/2 of the
and said polymerization agent is ultraviolet light or visible light.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 1, 2, 3, or 4.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 4.
In certain instances, the present invention relates to the aforementioned method, wherein m is 1.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 1, 2, 3, or 4.
In certain instances, the present invention relates to the aforementioned method, wherein p is 2.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 4.
In certain instances, the present invention relates to the aforementioned method, wherein m is 1.
In certain instances, the present invention relates to the aforementioned method, wherein R2 is (Ci-C3)alkyl.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein w1 is an integer in the range of about 50 to about 250.
In certain instances, the present invention relates to the aforementioned method, wherein w1 is an integer in the range of about 60 to about 90.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2.
In certain instances, the present invention relates to the aforementioned method, wherein m is 1.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, p2 is 0, and R3 is (Ci-Cs)alkyl.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, p2 is 0, R3 is (Ci-C5)alkyl, and w1 is an integer in the range of about 60 to about 90.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
is
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2.
In certain instances, the present invention relates to the aforementioned method, wherein m is 1.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, p2 is 0, and R3 is (Ci-C5)alkyl.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, p2 is 0, and R3 is (Ci-Cs)alkyl, and w2 is an integer in the range of about 60 to about 90.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula II.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula III.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula III, R1"111 is -C(O)H, and R2"111 is H.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula in, R1"111 is -C(O)H, R2'111 is
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula III, R2"111 is -C(O)H, R2"111 is
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula IK is
In certain instances, the present invention relates to the aforementioned method, wherein said compound of formula Ia is
In certain embodiments, the present invention relates to the aforementioned method, wherein said dendrimeric compound is a compound of formula Ib.
In certain embodiments, the present invention relates to the aforementioned method, wherein v is 2.
In certain embodiments, the present invention relates to the aforementioned method, wherein X5 is -N(H)-.
In certain embodiments, the present invention relates to the aforementioned method, wherein R18 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein R19 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein R20 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 20-500.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 40-250.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 60-90.
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula Ib is
In certain embodiments, the present invention relates to the aforementioned method, said polymerization agent is a compound of formula V.
In certain embodiments, the present invention relates to the aforementioned method, wherein v is 2.
In certain embodiments, the present invention relates to the aforementioned method, wherein X6 is -N(H)-.
In certain embodiments, the present invention relates to the aforementioned method, wherein R24 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein R25 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein R26 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 20-500.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 40-250.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 60-90.
In certain embodiments, the present invention relates to the aforementioned method, wherein R23 represents independently for each occurrence
In certain embodiments, the present invention relates to the aforementioned method, wherein R23 represents independently for each occurrence
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula V is
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula V is
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is an oxidizing agent.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is O2.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is ultraviolet light or visible light.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is ultraviolet light.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is light with a λ of 400-600 nm.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is light with a λ of 450-550 nm.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is light with a λ of 488-514 nm.
In certain embodiments, the present invention relates to the aforementioned method, wherein said patient is a primate, equine, feline, or canine.
In certain embodiments, the present invention relates to the aforementioned method, wherein said patient is a human.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration or corneal perforation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 10 cm in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 2 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 0.5 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 2% w/w to about 40% w/w.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 5% w/w to about 20% w/w.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 6% w/w to about 10% w/w.
In certain embodiments, the present invention relates to the aforementioned method, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
In certain embodiments, the present invention relates to the aforementioned method, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing a photoinitiator with said compound of formula Ia prior to treating said compound of formula Ia with said polymerization agent.
In certain embodiments, the present invention relates to the aforementioned method, wherein said photoinitiator is eosin-Y.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing a natural polymer with said dendrimeric compound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said natural polymer is HA, collagen, or a GAG fragment.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing at least one cell with said dendrimeric compound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said cell is a stem cell.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2- hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), polyvinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or a biologically active analog, fragment, or derivative thereof.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is polyhexamethylene biguanide.
In certain embodiments, the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 400 wt%.
In certain embodiments, the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 200 wt%.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing said dendrimeric compound.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing said dendrimeric compound and said
polymerization agent, wherein said polymerization agent is selected from the group consisting of a compound of foπnula II, a compound of formula III, a compound of formula IV, and a compound of formula V.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about lo-3.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10-5.
Another aspect of the present invention relates to a method of sealing a wound on a patient, comprising the steps of: applying an effective amount of a compound of formula VI to a wound on a patient and treating said compound of formula VI with a polymerization agent sufficient to polymerize said compound of formula VI, wherein said polymerization agent is an oxidizing agent or a compound of formula VII, wherein formula VI is represented by:
VI or a pharmaceutically acceptable salt, solvate, or hydrate thereof, wherein
R1 represents independently for each occurrence
R represents independently for each occurrence H or alkyl;
R represents independently for each occurrence H, halogen, or alkyl;
R4 represents independently for each occurrence alkyl, aryl, or aralkyl;
R5 represents independently for each occurrence -(C(R3)2)mSH, -C(O)(C(R3)2)mSH,
-CO2(C(R3)2)mSH, -C(O)N(R2)(C(R3)2)mSH,
n and m each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p is 1, 2, 3, 4, or 5; and said formula VII is represented by:
R1Λαi represents independently -(C(R2-vπ)2)xC(O)H, -C(O)(C(R2-VII)2)yC(O)H, -
R2'vπ represents independently for each occurrence H, alkyl, or halogen;
R3-vπ is fluoroalkyl, chloroalkyl, -CH2NO2,
B is alkyl diradical, heteroalkyl diradical, or
v2^11 represents independently for each occurrence 2, 3, or 4; and w2"vπ is an integer in the range of about 5 to 700, inclusive.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is an oxidizing agent.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is O2.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII.
In certain embodiments, the present invention relates to the aforementioned method, wherein B is an alkyl diradical. ' '
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula VII is
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein w2"vu is an integer in the range of about 50 to about 250.
In certain instances, the present invention relates to the aforementioned method, wherein w2"vπ is an integer in the range of about 60 to about 90.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R2'vu is -C(O)H, and R2"
VII is H.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R2"vπ is -C(O)H, R2~vπ is
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R2~vπ is -C(O)H, R2'vπ is
In certain instances, the present invention relates to the aforementioned method, wherein n is 3, 4, or 5.
In certain instances, the present invention relates to the aforementioned method, wherein n is 4.
In certain instances, the present invention relates to the aforementioned method, wherein R2 is H.
In certain instances, the present invention relates to the aforementioned method, wherein R3 is H.
In certain instances, the present invention relates to the aforementioned method, wherein R4 is alkyl.
In certain instances, the present invention relates to the aforementioned method, wherein R is methyl or ethyl.
In certain instances, the present invention relates to the aforementioned method, wherein n is 4, R2 and R3 are H, and R4 is alkyl.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and a Bronsted acid.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HA, wherein A is halogen or -O2CR6, and R6 is alkyl, fluoroalkyl, aryl, or aralkyl.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and an acid selected from group consisting of HCl and HBr.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HO2CR6, wherein R6 is fluoroalkyl.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and CF3CO2H.
In certain embodiments, the present invention relates to the aforementioned method, wherein said patient is a primate, equine, feline, or canine.
In certain embodiments, the present invention relates to the aforementioned method, wherein said patient is a human.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing a natural polymer with said compound of formula VI.
In certain embodiments, the present invention relates to the aforementioned method, wherein said natural polymer is HA, collagen, or a GAG fragment.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing at least one cell with said compound of formula VI.
In certain embodiments, the present invention relates to the aforementioned method, wherein said cell is a stem cell.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration or corneal perforation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 10 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 2 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 0.5 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2- hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), polyvinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate,
latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is an antibiotic, antimicrobial agent, antiinflammatory agent, or growth factor.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is polyhexamethylene biguanide.
In certain embodiments, the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 400 wt%.
In certain embodiments, the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 200 wt%.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing said compound of formula VI.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing said compound of formula VI and said polymerization agent, wherein said polymerization agent is a compound of formula VII.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"
3
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"
Another aspect of the present invention relates to a method of sealing a wound on a patient, comprising the steps of: treating a dendrimeric compound of formula Ia or formula Ib with a polymerization agent to form a repair agent and applying said repair agent to a wound on a patient, wherein said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, or an oxidizing agent, wherein formula Ia is represented by:
A1— X1-B— X1— A2 Ia wherein
A represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
Z1 represents independently for each occurrence -X1 -R4 , E, or
Y2 represents independently for each occurrence R5, A4,
Z represents independently for each occurrence -X 1 - τR-,5 , E, or
Z3 represents independently for each occurrence -X1 -R6 , E, or
Y4 represents independently for each occurrence R7, A4,
Z4 represents independently for each occurrence -X'-R/, E, or
Y5 represents independently for each occurrence R8, A4,
R1 represents independently for each occurrence H, alkyl, or halogen;
R2 represents independently for each occurrence H, alkyl, -OH, -N(R1 °)2, -SH, hydroxyalkyl, or -[C(R')2]dR16;
R3 represents independently for each occurrence alkyl, aryl, or aralkyl; R4, R5, R6, R7, R8, and R9 are H;
R10 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R11 represents independently for each occurrence H, -OH, -N(R10)2, -SH, alkyl, hydroxyalkyl, or -[C(R1 ^dR16;
R12 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R13 represents independently for each occurrence H, alkyl, aryl, or aralkyl;
R14 represents independently for each occurrence H, alkyl, or -CO2R10; R15 represents independently for each occurrence H, alkyl, or -OR10;
R16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R10)2, -SH, -S-alkyl, -CO2R10, -C(O)N(R10)2, or - C(NH2)N(R10),;
d represents independently for each occurrence 1, 2, 3, 4, 5, or 6; n represents independently for each occurrence 1, 2, 3, 4, 5, or 6; p represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p2 represents independently for each occurrence 0, 1, 2, 3, or 4; p3 represents independently for each occurrence 1, 2, or 3; p4 represents independently for each occurrence 0, 1, 2, or 3; t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
v1 and v2 each represent independently for each occurrence 2, 3, or 4; w! and w2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is 0, 1, 2, 3, 4, or 5; z1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z2 and z3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
X1 and X2 each represent independently for each occurrence O or -N(R10)-;
X3 represents independently for each occurrence O, N(R10), or C(R15)(CO2R10);
-
E represents independently
provided that R4 only occurs once, R5 only occurs once, R6 only occurs once, R7 only occurs once, R8 only occurs once, and R9 only occurs once; said formula Ib is represented by:
Ib or a pharmaceutically acceptable salt, solvate, or hydrate thereof,
wherein
X5 represents independently for each occurrence O or -N(R22)-;
R17 represents independently for each occurrence H, -(C(R19)2)hSH, -
R18 represents independently for each occurrence H or alkyl;
R19 represents independently for each occurrence H, halogen, or alkyl;
R20 represents independently for each occurrence H or alkyl;
R21 represents independently for each occurrence H, -(C(R19)2)hSH, -
R22 represents independently for each occurrence H, alkyl, aryl, or aralkyl; n1 and h each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p5 represents independently for each occurrence 1, 2, 3, 4, or 5; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive; said formula II is represented by:
wherein
R2"11 represents independently for each occurrence H or alkyl;
R3"11 represents independently for each occurrence H, halogen, or alkyl;
R4"11 represents independently for each occurrence alkyl, aryl, or aralkyl; and
R5'11 represents independently for each occurrence H or
R2"" ; and z represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; said formula III is represented by:
R-I-IlI g1-lll R-I-IIl in wherein
R1-111 is -(C(R2-m)2)xC(O)H, -C(O)(C(R2-m)2)yC(O)H, -(C(R2-iπ)2)xC(O)R3-πi, or - C(O)(C(R2"πi)2)yC(O)R3-πi;
R2'111 represents independently for each occurrence H, alkyl, or halogen;
IT ,3-π1"i is fluoroalkyl, chloroalkyl, -CH2NO2, or
x represents independently for each occurrence 0, 1, 2, 3, 4, 5, 6, 7, or 8;
y represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8;
z represents independently for each occurrence 0, 1, or 2;
v represents independently for each occurrence 2, 3, or 4; and
w is an integer in the range of about 5 to about 700, inclusive;
said formula IV is represented by:
A1— X1-B— X1— A2 IV wherein
or
A3 represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
Y represents independently for each occurrence R ,
Z represents independently for each occurrence -X 1 -R r> 6 , E, or
Z4 represents independently for each occurrence -X1 -R7, E, or
Z5 represents independently for each occurrence -X1 -R8, E, or
Y6 represents independently for each occurrence R9,
R2 represents independently for each occurrence H, alkyl, -OH, -N(RI0)2, -SH, hydroxyalkyl, or -[C(R1)2]dR16;
R3 represents independently for each occurrence alkyl, aryl, or aralkyl; R4, R5, R6, R7, R8, and R9 are H;
R10 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R11 represents independently for each occurrence H, -OH, -N(R10)2, -SH, alkyl, hydroxyalkyl, or
R12 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R13 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R14 represents independently for each occurrence H, alkyl, or -CO2R10; R15 represents independently for each occurrence H, alkyl, or -OR10;
R16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R10)2, -SH, -S-alkyl, -CO2R10, -C(O)N(R10)2, or - C(NH2)N(R10)2;
n represents independently for each occurrence 1, 2, 3, 4, 5, or 6; p1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p2 represents independently for each occurrence 0, 1, 2, 3, or 4; p3 represents independently for each occurrence 1, 2, or 3; p4 represents independently for each occurrence 0, 1, 2, or 3; d represents independently for each occurrence 1, 2, 3, 4, 5, or 6;
t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
v1 and v2 each represent independently for each occurrence 2, 3, or 4; w1 and w2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is O, 1, 2, 3, 4, or 5; z1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z2 and z3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
X1 and X2 each represent independently for each occurrence O or -N(R10)-; X3 represents independently for each occurrence O, N(R10), or C(RI5)(CO2R10); and
said formula V is represented by:
or a pharmaceutically acceptable salt, solvate, or hydrate thereof, wherein
X6 represents independently for each occurrence O or -N(R30)-;
R represents independently for each occurrence H or alkyl;
R25 represents independently for each occurrence H, halogen, or alkyl;
R26 represents independently for each occurrence H or alkyl;
R27 represents independently for each occurrence H, alkyl, or halogen;
R28 represents independently for each occurrence H, alkyl, -OH, -N(R30)2, -SH, or hydroxyalkyl;
R29 represents independently for each occurrence H, -OH, -N(R30)2, -SH, alkyl, or hydroxyalkyl;
R30 and R31 represent independently for each occurrence H, alkyl, aryl, or aralkyl; Z6 represents independently for each occurrence E1 or
R >33 represents independently for each occurrence
R >34 represents independently for each occurrence H, alkyl, or -CO2R 30.
p6 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p7 represents independently for each occurrence 0, 1, 2, 3, or 4; p8 represents independently for each occurrence 1, 2, or 3; p9 represents independently for each occurrence 0, 1, 2, or 3; n2 and j each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; m1 represents independently for each occurrence 1 or 2; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive.
In certain instances, the present invention relates to the aforementioned method, wherein said dendrimeric compound is a compound of formula Ia, and said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, or an oxidizing agent.
In certain instances, the present invention relates to the aforementioned method,
wherein A1
, and m is 1 or 2.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein Z1 represents independently for each occurrence -X1 -R4 or
In certain instances, the present invention relates to the aforementioned method, wherein Z2 represents independently for each occurrence -X1 -R5 or
In certain instances, the present invention relates to the aforementioned method, wherein Z3 represents independently for each occurrence -X^R5 or
In certain instances, the present invention relates to the aforementioned method, wherein Z4 represents independently for each occurrence -X1 -R7 or
2.
In certain instances, the present invention relates to the aforementioned method, wherein Z5 represents independently for each occurrence -X1 -R8 or
In certain instances, the present invention relates to the aforementioned method, wherein X1 is O.
In certain instances, the present invention relates to the aforementioned method, wherein X1 and X2 are O.
In certain instances, the present invention relates to the aforementioned method, wherein n is 1.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, 3, or 4.
In certain instances, the present invention relates to the aforementioned method, wherein p2 is 1.
In certain instances, the present invention relates to the aforementioned method, wherein R1 is H.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
groups are H, about 1/2 of the Y4 groups are
polymerization agent is ultraviolet light or visible light.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 1, 2, 3, or 4.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2.
In certain instances, the present invention relates to the aforementioned method, wherein p is 4.
In certain instances, the present invention relates to the aforementioned method, wherein m is 1.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
, m is 1
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 1, 2, 3, or 4.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 4.
In certain instances, the present invention relates to the aforementioned method, wherein m is 1.
In certain instances, the present invention relates to the aforementioned method, wherein R2 is (C1-C3)alkyl.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein w1 is an integer in the range of about 50 to about 250.
In certain instances, the present invention relates to the aforementioned method, wherein w1 is an integer in the range of about 60 to about 90.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2.
In certain instances, the present invention relates to the aforementioned method, wherein m is 1.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, p2 is 0, and R3 is (Ci-C5)alkyl.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, p2 is 0, R3 is (d-CsJalkyl, and w1 is an integer in the range of about 60 to about 90.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2.
In certain instances, the present invention relates to the aforementioned method, wherein m is 1.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, p2 is 0, and R3 is (Cι-C5)alkyl.
In certain instances, the present invention relates to the aforementioned method, wherein p1 is 2, p2 is 0, and R3 is (Ci-C5)alkyl, and w2 is an integer in the range of about 60 to about 90.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula II.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula III.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula IK, R1"111 is -C(O)H, and R2"m is H.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization, agent is a compound of formula III, Rum is -C(O)H, R2"111 is
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula III, R2"111 is -C(O)H, R2"111 is
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula III is
In certain instances, the present invention relates to the aforementioned method, wherein said compound of formula Ia is
In certain embodiments, the present invention relates to the aforementioned method, wherein said dendrimeric compound is a compound of formula Ib.
In certain embodiments, the present invention relates to the aforementioned method, wherein v is 2.
In certain embodiments, the present invention relates to the aforementioned method, wherein X5 is -N(H)-.
In certain embodiments, the present invention relates to the aforementioned method, wherein R18 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein R19 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein R20 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 20-500.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 40-250.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 60-90.
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula Ib is
In certain embodiments, the present invention relates to the aforementioned method, said polymerization agent is a compound of formula V.
In certain embodiments, the present invention relates to the aforementioned method, wherein v is 2.
In certain embodiments, the present invention relates to the aforementioned method, wherein X6 is -N(H)-.
In certain embodiments, the present invention relates to the aforementioned method, wherein R24 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein R25 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein R26 is H.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 20-500.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 40-250.
In certain embodiments, the present invention relates to the aforementioned method, wherein w is an integer in the range of about 60-90.
In certain embodiments, the present invention relates to the aforementioned method, wherein R23 represents independently for each occurrence
In certain embodiments, the present invention relates to the aforementioned method, wherein R23 represents independently for each occurrence
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula V is
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula V is
ertain embodiments, the present invention relates to the aforementioned method, wherein aid polymerization agent is an oxidizing agent.
In certain embodiments, the present invention relates to the aforementioned method, /herein said polymerization agent is O2.
In certain embodiments, the present invention relates to the aforementioned method, /herein said polymerization agent is ultraviolet light or visible light.
In certain embodiments, the present invention relates to the aforementioned method, /herein said polymerization agent is ultraviolet light.
In certain embodiments, the present invention relates to the aforementioned method, therein said polymerization agent is light with a λ of 400-600 nm.
In certain embodiments, the present invention relates to the aforementioned method, therein said polymerization agent is light with a λ of 450-550 nm.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymerization agent is light with a λ of 488-514 nm.
In certain embodiments, the present invention relates to the aforementioned method, wherein said patient is a primate, equine, feline, or canine.
In certain embodiments, the present invention relates to the aforementioned method, wherein said patient is a human.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration or corneal perforation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 10 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 2 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 0.5 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 2% w/w to about 40% w/w.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 5% w/w to about 20% w/w.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 6% w/w to about 10% w/w.
In certain embodiments, the present invention relates to the aforementioned method, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
In certain embodiments, the present invention relates to the aforementioned method, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
In certain embodiments, the present invention relates to the aforementioned method, wherein said repair agent is an aqueous mixture that has a pH in the range of about 5.5 to about 9.5.
In certain embodiments, the present invention relates to the aforementioned method, wherein said repair agent is an aqueous mixture that has a pH in the range of about 6.5 to about 7.5.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing a photoinitiator with said compound of formula Ia prior to treating said compound of formula Ia with said polymerization agent.
In certain embodiments, the present invention relates to the aforementioned method, wherein said photoinitiator is eosin-Y.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing a natural polymer with said dendrimeric compound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said natural polymer is HA, collagen, or a GAG fragment.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing at least one cell with said dendrimeric compound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said cell is a stem cell.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2- hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is polyhexamethylene biguanide.
In certain embodiments, the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 400 wt%.
In certain embodiments, the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 200 wt%.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing said dendrimeric compound.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing said dendrimeric compound and said polymerization agent, wherein said polymerization agent is selected from the group consisting of a compound of formula II, a compound of formula III, a compound of formula IV, and a compound of formula V.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation., microwave irradiation, or visible light irradiation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"
3
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"
Another aspect of the present invention relates to a method of sealing a wound on a patient, comprising the steps of: treating a compound of formula VI with a polymerization agent to form a repair agent and applying said repair agent to a wound on a patient, wherein said polymerization
agent is an oxidizing agent or a compound of formula VII, wherein formula VI is represented by:
VI or a pharmaceutically acceptable salt, solvate, or hydrate thereof, wherein
R1 represents independently for each occurrence H, -(C(R3)2)mSH,
R2 represents independently for each occurrence H or alkyl;
R3 represents independently for each occurrence H, halogen, or alkyl;
R4 represents independently for each occurrence alkyl, aryl, or aralkyl;
R5 represents independently for each occurrence -(C(R3)2)mSH, -C(O)(C(R3)2)mSH,
-CO2(C(R3)2)mSH, -C(O)N(R2)(C(R3)2)mSH,
n and m each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p is 1, 2, 3, 4, or 5; and said formula VII is represented by:
R1-VII_B_RI-VII VII
wherein
, l-VII
R'"vu represents independently -(C(R )T2-V VIUIλ)2)XC(O)H, -C(O)(C(R ,2/-"VvI"K)2)yC(O)H,
R >2-"VII represents independently for each occurrence H, alkyl, or halogen;
B is alkyl diradical, heteroalkyl diradical, or
v2"vπ represents independently for each occurrence 2, 3, or 4; and w2"vπ is an integer in the range of about 5 to 700, inclusive.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is an oxidizing agent.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is O2.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII.
In certain embodiments, the present invention relates to the aforementioned method, wherein B is an alkyl diradical.
In certain embodiments, the present invention relates to the aforementioned method, said compound of formula VII is
In certain instances, the present invention relates to the aforementioned method, wherein w2^11 is an integer in the range of about 50 to about 250.
In certain instances, the present invention relates to the aforementioned method, wherein w2"vπ is an integer in the range of about 60 to about 90.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R2"vπ is -C(O)H, and R2" VII is H.
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R2"vπ is -C(O)H, R2~vπ is
In certain instances, the present invention relates to the aforementioned method, wherein said polymerization agent is a compound of formula VII, R2"vπ is -C(O)H, R2~VI1 is
In certain instances, the present invention relates to the aforementioned method, wherein n is 3, 4, or 5.
In certain instances, the present invention relates to the aforementioned method, wherein n is 4.
In certain instances, the present invention relates to the aforementioned method, wherein R2 is H.
In certain instances, the present invention relates to the aforementioned method, wherein R3 is H.
In certain instances, the present invention relates to the aforementioned method, wherein R4 is alkyl.
In certain instances, the present invention relates to the aforementioned method, wherein R4 is methyl or ethyl.
In certain instances, the present invention relates to the aforementioned method, wherein n is 4, R2 and R3 are H, and R4 is alkyl.
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
In certain instances, the present invention relates to the aforementioned method,
wherein n is 4, R2 and R3 are H, R4 is methyl, R1 is
R2 , and p is 1.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and a Brδnsted acid.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HA, wherein A is halogen or -O2CR6, and R6 is alkyl, fluoroalkyl, aryl, or aralkyl.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and an acid selected from group consisting of HCl and HBr.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HO2CR6, wherein R6 is fluoroalkyl.
In certain instances, the present invention relates to the aforementioned method, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and CF3CO2H.
In certain embodiments, the present invention relates to the aforementioned method, wherein said patient is a primate, equine, feline, or canine.
In certain embodiments, the present invention relates to the aforementioned method, wherein said patient is a human.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing a natural polymer with said compound of formula VI.
In certain embodiments, the present invention relates to the aforementioned method, wherein said natural polymer is HA, collagen, or a GAG fragment.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of admixing at least one cell with said compound of formula VI.
In certain embodiments, the present invention relates to the aforementioned method, wherein said cell is a stem cell.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is a corneal laceration or corneal perforation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 10 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm2 in size.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 5 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 2 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 1 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said wound is less than about 0.5 cm in length.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
In certain embodiments, the present invention relates to the aforementioned method, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2- hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
In certain embodiments, the present invention relates to the aforementioned method, wherein said polymer is polyvinylpyrrolidone.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is an antibiotic, antimicrobial agent, antiinflammatory agent, or growth factor.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is polyhexamethylene biguanide.
In certain embodiments, the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 400 wt%.
In certain embodiments, the present invention relates to the aforementioned method, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 200 wt%.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing said compound of formula VI.
In certain embodiments, the present invention relates to the aforementioned method, further comprising the step of sterilizing said compound of formula VI and said polymerization agent, wherein said polymerization agent is a compound of formula VII.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about
10-3.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about
10"5.
Another aspect of the invention relates to a method for preparing a sealant, comprising the step of: admixing a first biocompatible crosslinking polymer comprising at least two different nucleophilic groups with a second biocompatible crosslinking polymer comprising at least one amine-reactive group and at least one sulfhydryl-reactive group to form a gel, wherein said amine- and sulfhydryl-reactive groups are capable of a covalent reaction with said nucleophilic groups of said first crosslinking polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed in less than about one-half hour following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method,
first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed in less than about 5 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed in less than about 1 minute following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said nucleophilic groups on said first biocompatible polymer are sulfhydryl and amine.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed ex vivo.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed in vitro.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed in vivo.
In certain embodiments, the present invention relates to the aforementioned method further comprising the step of administering an effective amount of said sealant to a patient.
Another aspect of the invention relates to a method for preparing a sealant, comprising the step of: admixing a first biocompatible crosslinlcing polymer comprising at least two different nucleophilic groups with a second biocompatible crosslinking polymer comprising at least one sulfhydryl-reactive group to form a sealant, wherein said sulfhydryl-reactive group is capable of a covalent reaction with said nucleophilic groups of said first crosslinking polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed in less than about one-half hour following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method,
wherein said sealant is formed in less than about 15 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed in less than about 5 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed in less than about 1 minute following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said nucleophilic groups on said first biocompatible polymer are sulfhydryl and amine.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed ex vivo.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed in vitro.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed in vivo.
In certain embodiments, the present invention relates to the aforementioned method further comprising the step of administering an effective amount of said sealant to a patient.
Another aspect of the invention relates to a method for preparing a biocompatible gel, comprising the step of: admixing a first biocompatible crosslinking polymer comprising at least one amine group and at least one sulfhydryl group with a second biocompatible crosslinking polymer comprising at least one aldehyde to form a sealant, wherein said amine group and said sulfhydryl group are capable of covalent reaction with the aldehyde group to form a thiazolidine linkage.
In certain embodiments, the present invention relates to the aforementioned method, wherein said gel is formed in less than about one-half hour following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said gel is formed in less than about 15 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said gel is formed in less than about 5 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said gel is formed in less than about 1 minute following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed ex vivo.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed in vitro.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed in vivo.
In certain embodiments, the present invention relates to the aforementioned method further comprising the step of administering an effective amount of said gel to a patient.
Another aspect of the invention relates to a method for preparing a biocompatible sealant, comprising the step of: admixing a first biocompatible crosslinking polymer comprising a histidine amino acid group with a second biocompatible crosslinking polymer comprising an electrophilic group to form a sealant, wherein said histidine group of said first polymer and said electrophilic group of said second polymer are capable of reaction to an amide linkage.
In certain embodiments, the present invention relates to the aforementioned method, wherein said electrophilic group is a thiocarboxylic acid or acyl disulfide.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed in less than about one-half hour following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method,
wherein said sealant is formed in less than about 15 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed. in less than about 5 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said sealant is formed in less than about 1 minute following admixing of said first biocompatible polymer with said second biocompatible polymer.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed ex vivo.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed in vitro.
In certain embodiments, the present invention relates to the aforementioned method, wherein said method is performed in vivo.
In certain embodiments, the present invention relates to the aforementioned method further comprising the step of administering an effective amount of said sealant to a patient.
Compositions of the Itivention
One aspect of the present invention relates to a sealant comprising a dendrimeric macromolecule that forms a hydrogel which swells less than about 400 wt% in an aqueous solution.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said dendrimeric macromolecule is the compound formed by treating a dendrimeric compound of formula Ia or formula Ib with a polymerization agent selected from the group consisting of ultraviolet light, visible light, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, or an oxidizing agent, wherein formula Ia, formula Ib, formula II, formula in, formula IV, and formula V are as defined above.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said dendrimeric macromolecule is the compound formed by treating a
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said dendrimeric macromolecule is the compound formed by treating a dendrimeric compound of formula Ia or formula Ib with a polymerization agent selected from the group consisting of ultraviolet light and visible light.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said dendrimeric macromolecule is the compound formed by treating a dendrimeric compound of formula VI with a polymerization agent selected from the group consisting of oxidizing agent or a compound of formula VII, wherein formula VI and formula VI are as defined above.
In certain embodiments, the present invention relates to the aforementioned sealant, further comprising a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2- hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said polymer is polyvinylpyrrolidone.
In certain embodiments, the present invention relates to the aforementioned sealant, further comprising a pharmaceutical agent.
In certain embodiments, the present invention relates to the aforementioned method, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said pharmaceutical agent is polyhexamethylene biguanide.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said hydrogel swells less than about 250 wt%.
In certain embodiments, the present invention relates to the aforementioned sealant, wherein said hydrogel swells less than about 150 wt%.
Another aspect of the present invention relates to a formulation for foπning a sealant, comprising water and a dendrimeric compound that forms a hydrogel which swells less than about 400 wt% in an aqueous solution, wherein said dendrimeric compound is a compound of formula Ia, a compound of formula Ib, or a compound of formula VI; and said formulation forms a hydrogel in less than about 5 minutes when treated with a polymerization agent selected from the group consisting of ultraviolet light, visible light, an oxidizing agent, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, and a compound of formula VII; and said formulae Ia, Ib, II, III, IV, V, VI, and VII are as defined above.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said hydrogel swells less than about 250 wt%.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said hydrogel swells less than about 150 wt%.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said formulation forms a hydrogel in less than about 2 minutes when treated with a polymerization agent.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said formulation forms a hydrogel in less than about 1 minute when treated with a polymerization agent.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said formulation forms a hydrogel in less than about 30 seconds when treated with a polymerization agent.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said formulation has a pH in the range of about 5.5 to about 9.5.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said formulation has a pH in the range of about 6.5 to about 7.5.
In certain embodiments, the present invention relates to the aforementioned formulation, further comprising a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
In certain embodiments, the present invention relates to the aforementioned formulation, wherein said polymer is polyvinylpyrrolidone.
Kits of the Invention
One aspect of the present invention relates to a kit for sealing a wound comprising: a polymerizable dendrimeric compound that forms a hydrogel which swells less than about 400 wt%; and a system for delivering said polymerizable dendrimeric compound to a wound on a patient.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said system is a syringe.
In certain embodiments, the present invention relates to the aforementioned kit, further comprising a polymerization agent.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said polymerization agent is a compound of formula II, a compound of formula III, a compound of formula IV,. a compound of formula V, or a compound of formula VII; wherein formula II, formula III, formula IV, formula V, and formula VII are as defined above.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said polymerization agent is a compound of formula III or a compound of formula IV, and said formula III and formula IV are as defined above.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said dendrimeric is a compound of formula Ia, formula Ib, or formula VI; wherein formula Ia, formula Ib, and formula VI are as defined above.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said dendrimeric compound is represented by formula Ia or formula Ib, wherein formula Ia and formula Ib are as defined above.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said kit has a sterility assurance level of at least about 10'3.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said kit has a sterility assurance level of at least about 10"5.
In certain embodiments, the present invention relates to the aforementioned kit, further comprising a pharmaceutical agent.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
In certain embodiments, the present invention relates to the aforementioned kit, wherein said pharmaceutical agent is polyhexamethylene biguanide.
In certain embodiments, the present invention relates to the aforementioned kit, further comprising a desiccant.
Another aspect of the present invention relates to a hydrogel sealant formed by mixing two synthetic polymers or compounds that form a gel which swells less than about 150 w/w %, wherein said synthetic polymer is not albumin or gelatin.
In certain instances, the present invention relates to the aforementioned sealant, wherein said gel swells less than about 150 w/v %.
In certain instances, the present invention relates to the aforementioned sealant, wherein said gel swells less than about 150 v/v %.
In certain instances, the present invention relates to the aforementioned sealant, wherein said gel forms via a nucleophilic/electrophilic reaction.
Another aspect of the invention relates to a sealant formed by mixing at least two nucleophilic synthetic polymers or compounds in solution with at least one synthetic electrophilic polymer.
Another aspect of the invention relates to a sealant formed by mixing at least two electrophilic synthetic polymers or compounds in solution with at least one synthetic nucleophilic polymer.
In certain instances, the present invention relates to the aforementioned sealant, wherein the synthetic polymer does not include collagen, collagen derivatives, chemically modified collagens, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
Another aspect of the present invention relates to a crosslinkable composition comprising a first crosslinkable component having two or more sets of two nucleophilic groups that can react with a second crosslinkable component having two or more electrophilic groups, each capable of reacting with the two nucleophilic groups, to form a covalent five-membered ring structure.
In certain instances, the present invention relates to the aforementioned composition, wherein each of the first and second crosslinkable components is synthetic and dissolved in an aqueous solution.
In certain instances, the present invention relates to the aforementioned composition, wherein crosslinking of the composition results in a biocompatible crosslinked hydrogel in less than about 10 minutes.
In certain instances, the present invention relates to the aforementioned composition, wherein the five-membered ring structure is a thiazolidine ring.
Another aspect of the present invention relates to a stable crosslinkable composition comprising a first crosslinkable component having at least two sets of 1,2-aminothiol groups that can react with an electrophilic group of a second crosslinkable component having at least two electrophilic groups, each capable of reacting with the 1,2-aminothiol, to form a covalent five-membered ring structure, wherein each of the first and second crosslinkable components is synthetic and dissolved in an aqueous solution.
In certain instances, the present invention relates to the aforementioned composition, wherein the first and second crosslinkable components do not form a covalant bond with tissue.
In certain instances, the present invention relates to the aforementioned composition, wherein the composition results in a biocompatible crosslinked hydrogel on a tissue surface in less than about 10 minutes.
In certain instances, the present invention relates to the aforementioned composition, wherein the electrophilic group is an aldehyde.
In certain instances, the present invention relates to the aforementioned composition, wherein the five-membered ring structure is a thiazolidine ring
In certain instances, the present invention relates to the aforementioned composition, wherein the crosslinkable components do not include collagen, collagen derivatives, chemically modified collagens, gelatin, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
In certain instances, the present invention relates to the aforementioned composition, wherein the crosslinkable composition forms a three-dimensional matrix that swells less than about 400%.
In certain instances, the present invention relates to the aforementioned composition, wherein the crosslinkable composition forms a three-dimensional matrix that swells less than about 300%.
In certain instances, the present invention relates to the aforementioned composition, wherein the crosslinkable composition forms a three-dimensional matrix that swells less than about 200%.
In certain instances, the present invention relates to the aforementioned composition, wherein the crosslinkable composition forms a three-dimensional matrix that swells less than about 100%.
In certain instances, the present invention relates to the aforementioned composition, wherein the crosslinkable composition forms a three-dimensional matrix that swells less than about 50%.
In certain instances, the present invention relates to the aforementioned composition, wherein the first component has a molecular weight of less than about 1000 g/mol.
In certain instances, the present invention relates to the aforementioned composition, wherein the first component has a molecular weight of less than about 500 g/mol.
In certain instances, the present invention relates to the aforementioned composition, wherein the weight ratio of the first component to the second component is less than about 10:1.
In certain instances, the present invention relates to the aforementioned composition, wherein the weight ratio of the first component to the second component is less than about 5:1.
Another aspect of the present invention relates to a method for forming a three- dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of:
(a) providing an aqueous solution of a synthetic polymer comprising at least two nucleophilic groups and an aqueous solution of a synthetic small molecule comprising at least two electrophilic groups, wherein the electrophilic groups react with the nucleophilic groups to form covalent bonds, wherein the number of nucleophilic groups plus electrophilic groups is at least five;
(b) applying the synthetic polymer and the synthetic small molecule to the first tissue surface; and
(c) allowing the synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
In certain instances, the present invention relates to the aforementioned method, further comprising the step of contacting the first tissue surface with a second surface after step (b) but before step (c) to effect adhesion between the first tissue surface and the second surface.
Another aspect of the present invention relates to a method for forming a three- dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of:
(a) providing an aqueous solution of a synthetic polymer comprising at least two nucleophilic groups and an aqueous solution of a synthetic small molecule comprising at least two electrophilic groups, wherein the electrophilic groups react with the nucleophilic groups to form covalent bonds, wherein the number of nucleophilic groups plus electrophilic groups is at least five;
(b) contacting the synthetic polymer and the synthetic small molecule to initiate crosslinking to form a repair agent; and
(c) applying the repair agent to the first tissue surface; and
(d) allowing the synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
In certain instances, the present invention relates to the aforementioned method, further comprising contacting the first tissue surface with a second surface after step (c) but before step (d) to effect adhesion between the first tissue surface and the second surface.
Another aspect of the present invention relates to a method for forming a three- dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of: a) providing an aqueous solution of a synthetic polymer comprising at least two nucleophilic groups and providing an aqueous solution of a synthetic small molecule comprising at least three electrophilic groups, wherein the electrophilic groups can react with the nucleophilic groups to form covalent bonds;
b) applying the synthetic polymer and the synthetic small molecule to the first tissue surface to initiate crosslinking; and c) allowing the synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
In certain instances, the present invention relates to the aforementioned method, further comprising the step of contacting the first tissue surface with a second surface after step (b) but before step (c) to effect adhesion between the first tissue surface and the second surface.
Another aspect of the present invention relates to a method for forming a three- dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of: a) providing an aqueous solution of a synthetic polymer comprising at least two nucleophilic groups and providing an aqueous solution of a synthetic small molecule comprising at least three electrophilic groups, wherein the electrophilic groups can react with the nucleophilic groups to form covalent bonds; b) contacting the synthetic polymer and the synthetic small molecule to initiate crosslinking to form a repair agent; c) applying the repair agent to the first tissue surface; and d) allowing the first synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three-dimensional matrix.
In certain instances, the present invention relates to the aforementioned method, further comprising the step of contacting the first tissue surface with a second surface after step (c) but before step (d) to effect adhesion between the first tissue surface and the second surface.
In certain instances, the present invention relates to the aforementioned method, wherein the three-dimensional matrix swells less than about 400%.
In certain instances, the present invention relates to the aforementioned method, wherein the three-dimensional matrix swells less than about 300%.
In certain instances, the present invention relates to the aforementioned method, wherein the three-dimensional matrix swells less than about 200%.
In certain instances, the present invention relates to the aforementioned method, wherein the three-dimensional matrix swells less than about 100%.
In certain instances, the present invention relates to the aforementioned method, wherein the three-dimensional matrix swells less than about 50%.
In certain instances, the present invention relates to the aforementioned method, wherein the synthetic polymer does not include collagen, collagen derivatives, chemically modified collagens, gelatin, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
In certain instances, the present invention relates to the aforementioned method, further comprising the step of sterilizing the synthetic polymer and synthetic small molecule.
In certain instances, the present invention relates to the aforementioned method, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
In certain instances, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about lo-3.
In certain instances, the present invention relates to the aforementioned method, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10-5.
Exemplification
The invention now being generally described, it will be more readily understood by reference to the following examples, which are included merely for purposes of illustration of certain aspects and embodiments of the present invention, and are not intended to limit the invention.
Example 1
Synthesis of 2-[(m-l,3-benzylidene glycerol)-2-propionic acid] - cώ-l,3-O-Benzylidene glycerol (10.9 g, 60.4 mmol) was dissolved in 1,4-dioxane (250 niL) followed by the addition of NaH (7.0 g, 0.30 mol). The reaction mixture was stirred at room temperature for one hour before cooling to 0 0C. 2-Bromopropionic acid (8.64 mL, 96 mmol) was then added over a 15 minute period of time. The reaction mixture was allowed to return to room temperature and then stirred at 50 0C for 12 hours before it was cooled to 0 0C and quenched with ethanol followed by the addition of water (250 mL). The solution was adjusted to 4.0 pH using IN HCl and extracted with CH2Cl2 (200 mL). This procedure was repeated once again after re-adjusting the pH to 4.0. The combined organic phase was dried with Na2SO4, gravity filtered, and evaporated. The solid was stirred in ethyl ether (50 mL) for 45 minutes and cooled to -25 0C for 3 hours before collecting 11.7 g of the white powder (77.3 % yield). 1H NMR (400 MHz, CDCl3): δ 1.51 (d, 3, CH-CH3, J=7.00 Hz), 3.46 (m, 1, -CH2-CH-CH2-, J=I.71 Hz), 4.04 (m, 2, -CH2-CH-CH11, J=I.71 Hz), 4.22 (q, 1, CH-CH3, J= 7.00 Hz), 4.29 (m, 2, -CH2-CH-CH21, J=1.71 Hz), 5.54 (s, 1, CH), 7.34 (m, 3, arom. CHX 7.46 (m, 2, arom. CH). 13C NMR (400 MHz, CDCl3): δ 176.05 (COOH), 137.82 (CH), 129.34 (CH), 128.52 (CH), 126.26 (CH), 101.79 (CH), 72.83 (CH), 70.70 (CH), 69.28 (CH2), 69.09 (CH2), 18.79 (CH3). FTIR: v (cm4) 1714 (C=O), 1455 (CH2 bend), 1401 (CH3 bend). GC-MS 253 m/z (MH+) (Theory: 252 m/z (M+)). GC-MS 253 m/z (MH+) (Theory: 252 m/z (M+)) Elemental Analysis C: 61.75 %; H 6.37 % (Theoiy: C: 61.90 %; H 6.39 %).
Example 2
Synthesis of benzylidene protected [G0]-PGLLA-bzld - 2-[(ew-l,3-benzylidene glycerol)-2-propionic acid] (4.02 g, 15.9 mmol), cw-l,3-C>-benzylideneglycerol (2.62 g, 14.5 mmol), and DPTS (1.21 g, 4.10 mmol) were dissolved in CH2Cl2 (40 mL). The reaction flask was flushed with nitrogen and then DCC (3.61 g, 17.5 mmol) was added. Stirring at room temperature was continued for 14 hours under a nitrogen atmosphere. Upon reaction completion, the DCC-urea was filtered and washed with a small amount of CH2Cl2 (10 mL) and the filtrate was evaporated. The crude product was purified by silica gel chromatography, eluting with 3:97 MeOH:CH2Cl2. The product was dissolved in minimal CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. Ethyl ether was decanted and the precipitate was exposed to reduced pressure to yield 5.63 g of a white powder (94.0 % yield). 1H NMR (400 MHz,
CDCl3): δ 1.56 (d, 6, CH-CH3, J=6.84 Hz), 3.47 (m, 2, -CH2-CH-CH2-, J=I .71 Hz), 3.99 (m, 2, -CH2-CH-CH2=, J=1.71 Hz), 4.14 (m, 2, -CH2-CH-CH2=, J=I .71 Hz), 4.25 (m, 2, - CH2-CH-CH2=), 4.31 (m, 1, -CH2-CH-CH2=), 4.37 (q, 1, CH-CH3, J= 6.84 Hz), 4.42 (m, 1, - CH2-CH-CH2=), 4.72 (m, 1, -CH2-CH-CH2-, J=I.71 Hz), 5.49 (s, 1, CH), 5.53 (s, 1, CH), 7.34 (m, 6, arom. CH)1 7.47 (m, 4, arom. CH). 13C NMR (400 MHz, CDCl3): δ 173.53 (COOR), 138.32 (CH), 137.97 (CH), 129.36 (CH), 129.10 (CH), 128.54 (CH), 128.40 (CH), 126.42 (CH), 126.20 (CH), 101.51 (CH), 101.46 (CH), 72.88 (CH), 70.80 (CH2), 70.23 (CH), 69.08 (CH2), 69.02 (CH2), 68.19 (CH2), 66.83 (CH), 19.34 (CH3). FTIR: v (cm"1) 1743 (C=O), 1452 (CH2 bend), 1389 (CH3 bend). GC-MS 415 nVz (MH+) (Theory: 414 m/z (M+)) Elemental Analysis C: 66.63 %; H 6.33 % (Theory C: 66.65 %; H 6.32 %).
Example 3
Synthesis of [GO]-PGLLA-OH - Pd/C (10%) (10 % w/w) was added to a solution of benzylidene protected [GO]-PGLLA (5.49 g, 13.2 mmol) in EtOAc/MeOH (3:1, 40 mL). The flask was evacuated and filled with 50 psi of H2 before shaking for 20 minutes. The catalyst was filtered and washed with EtOAc (10 mL). The filtrate was then evaporated to give 2.94 g of a colorless, viscous oil (94.0 % yield). 1H NMR (400 MHz, (CD3)2CO): δ 1.08 (m, 1, CH3), 1.36 (m, 2, CH3), 3.65 (broad m, 9, -CH2-CH-CH2-), 4.20 (broad m, 3, - CH2-CH-CH2-). 13C NMR (400 MHz, (CD3)2SO): δ 174.03 (COOR), 81.53 (CH), 76.66 (CH), 74.30 (CH), 61.82 (CH2), 61.69 (CH2), 60.37 (CH2), 19.62 (CH3). FTIR: v (cm"l) 3383 (OH), 1737 (C=O). GC MS 239 m/z (MH+) (Theory: 238 m/z (M+)) Elemental Analysis C: 45.52 %; H 7.65 % (Theory C: 45.37 %; H 7.62%).
Example 4
Synthesis of benzylidene protected [Gl]-PGLLA-bzld - 2-[(αs-l,3-benzylidene glycerol)-2-propionic acid] (4.41 g, 17.50 mmol), [GO]-PGLLA (0.791 g, 3.32 mmol), and DPTS (2.46 g, 8.36 mmol), were dissolved in DMF (80 mL). The reaction flask was flushed with nitrogen and then DCC (5.31 g, 25.74 mmol) was added. The contents were stirred at room temperature for 14 hours under nitrogen atmosphere. The DMF was removed under high vacuum and the remaining residue was dissolved in CH2Cl2. The DCC-urea was filtered and washed with a small amount of CH2Cl2 (20 mL) and the filtrate was concentrated. The crude product was purified by silica gel chromatography, eluting with 3:97 MeOH:CH2Cl2. The product was dissolved in minimal CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC.
Ethyl ether was decanted and the precipitate was exposed to reduced pressure to yield 3.45 g of a white powder (88.3 % yield). 1H NMR (400 MHz, CDCl3): δ 1.33 (m, 3, CH3), 1.47 (m, 12, CH3), 3.41 (m, 4, CH,), 3.76 (m, 2, -CH2-CH-CH2-), 3.97 (m, 4, -CH2-CH-CH2-), 4.10 (m, 4, -CH2-CH-CH2-), 4.28 (m, 20, -CH2-CH-CH2-), 5.30 (m, 1, CH), 5.49 (m, 4, CH), 7.30 (m, 12, arom. CH), 7.46 (m, 8, arom. CH). 13C NMR (400 MHz, CDCl3): δ 173.16 (COOR), 138.24 (CH), 129.14 (CH), 128.40 (CH), 126.36 (CH), 101.47 (CH), 72.68 (CH), 70.54 (CH2), 70.12 (CH), 68.13 (CH2), 19.27 (CH3), 18.99 (CH3). FTIR: y cm" ') 1745 (C=O), 1451 (CH2 bend), 1386 (CH3 bend). FAB MS 1175.6 m/z (MH+) (Theory: 1175.2 m/z (M+)) Elemental Analysis C: 62.11 %; H 6.46 % (Theory C: 62.34 %; H 6.35%). SEC Mw: 1280, Mn: 1260, PDI: 1.01.
Example 5
Synthesis of [Gl]-PGLLA-OH - Pd/C (10%) (10 % w/w) was added to a solution of benzylidene protected [Gl]-PGLLA (0.270 g, 0.230 mmol) in THF (15 mL). The flask was evacuated and filled with 50 psi of H2 before shaking for 15 minutes. The catalyst was filtered and washed with THF (10 mL). The filtrate was then evaporated to give 0.178 g of a colorless, viscous oil (94.0 % yield). 1H NMR (400 MHz, (CD3)2CO): δ 1.41 (m, 5, CH3), 1.49 (m, 10, CH3), 3.53 (m, 2, -CH2-CH-CH2-), 3.63 (m, 11, -CH2-CH-CH2-), 3.74 (m, 4, -CH2-CH-CH2-), 3.93 (m, 3, -CH2-CH-CH2-), 4.23 (m, 5, -CH2-CH-CH2-), 4.39 (m, 10, -CH2-CH-CH2-). 13C NMR (400 MHz, CD3Cl): δ 169.64 (COOR), 74.53 (CH), 72.97 (CH), 72.74 (CH), 69.95 (CH2), 68.97 (CH), 62.73 (CH2), 61.76 (CH2), 19.42 (CH3), 18.13 (CH3), 17.56 (CH3). FTIR: v (cm"1) 3409 (OH), 1733 (C=O), 1453 (CH2 bend), 1374 (CH3 bend). FAB MS 823.3 m/z (MH+) (Theory: 822.8 m/z (M+)) Elemental Analysis C: 47.72 %; H 7.41 % (Theory C: 48.17 %; H 7.11 %). SEC Mw: 1100, Mn: 1090, PDI: 1.01.
Example 6
Synthesis of benzylidene protected [G2]-PGLLA-bzld - 2-[(cώ-l,3-benzylidene glycerol)-2-propionic acid] (8.029 g, 31.83 mmol), DCC (9.140 g, 44.30 mmol), and DPTS (4.629 g, 15.74 mmol) were dissolved in THF (80 mL). The reaction flask was flushed with nitrogen and stirred for 30 minutes before [Gl]-PGLLA (0.825 g, 1.00 mmol) was added by dissolving in a minimal amount of THF. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. The DCC-urea was filtered and washed with a small amount of THF (20 mL). The THF filtrate was evaporated and the crude product was purified by silica gel chromatography, eluting with 3:97 MeOH:CH2Cl2.
Tie product was dissolved in minimal CH2Cl2, filtered (to remove any DCU), and irecipitated in ethyl ether at -20 0C to remove remaining DCC. Ethyl ether was decanted md the precipitate was exposed to reduced pressure to yield 2.09 g of a white powder (77 K yield). 1H NMR (400 MHz, CDCl3): δ 1.33 (m, 15, CH3), 1.46 (m, 24, CH3), 3.40 (m, 3, CH5), 3.77 (m, 5, -CH2-CH-CH2-), 3.95 (m, 10, -CH2-CH-CH2-), 4.06 (m, 12, -CH2-CH- CH2-), 4.28 (m, 47, -CH2-CH-CH2-), 5.49 (m, 8, CH), 7.30 (m, 24, arom. CH), 7.47 (m, 16, arom. CH). 13C NMR (400 MHz, CDCl3): δ 173.15 (COOR), 138.28 (CH), 129.12 (CH), 128.40 (CH), 126.36 (CH), 101.44 (CH), 72.69 (CH), 70.54 (CH2), 70.12 (CH), 68.13 (CH2), 19.23 (CH3). FTIR: y cm"') 1746 (C=O), 1452 (CH2 bend), 1386 (CH3 bend). FAB MS 2697.0 m/z (MH+) (Theory: 2696.8 m/z (M+)) Elemental Analysis C: 60.86 %; H 6.37% (Theory C: 61.02 %; H 6.35 %). SEC Mw: 2350, Mn: 2310, PDI: 1.01.
Example 7
Synthesis of [G2]-PGLLA-OH - Pd/C (10%) (10 % w/w) was added to a solution of benzylidene protected [G2]-PGLLA (0.095 g, 0.035 mmol) in THF (10 mL). The flask was evacuated and filled with 50 psi of H2 before shaking for 15 minutes. The catalyst was filtered and washed with THF (10 mL). The filtrate was evaporated to give 0.061 g of a colorless viscous oil (88.0 % yield). 1H NMR (400 MHz, (CD3)2CO): δ 1.36 (m, 39, CH3), 3.61 (m, 48, -CH2-CH-CH2-), 3.94 (m, 10, -CH2-CH-CH2-), 4.16 (m, 6, -CH2-CH- CH2-), 4.35 (m, 29, -CH2-CH-CH2-). 13C NMR (400 MHz, (CD3)2CO): δ 174.37 (COOR), 81.98 (CH), 74.16 (CH), 70.46 (CH), 62.32 (CH2), 62.09 (CH2), 18.76 (CH3). FTIR: v (cm" ') 3431 (OH), 1741 (C=O), 1453 (CH2 bend), 1376 (CH3 bend). MALDI-TOF MS 1991.8 m/z (MH+) (Theory: 1991.9m/z (M+)). SEC Mw: 2170, Mn: 2130, PDI: 1.01.
Example 8
Synthesis of [G2]-PGLLA-Ac - [G2J-PGLLA (0.098 g, 0.049 mmol) was dissolved in 5 mL of pyridine. Acetic anhydride (6.0 mL, 64 mmol) was then added via syringe and the reaction mixture was stirred at 40 0C for 8 hours. Pyridine and acetic anhydride were removed under high vacuum. The product was isolated on a prep TLC eluting with 4:96 MeOH: CH3Cl. 1H NMR (400 MHz, CD3Cl): δ 1.22 (m, 15, CH3), 1.39 (m, 24, CH3), 2.05 (m, 48, CH3), 3.62 - 4.21 (broad multiplets, 83, -CH2-CH-CH2-). 13C NMR (400 MHz, CD3Cl): δ 172.69 (COOR), 170.87 (COOR), 75.15 (CH), 74.60 (CH), 70.46 (CH), 63.68 (CH2), 63.17 (CH2), 29.88 (CH3), 21.02 (CH3), 19.01 (CH3). FAB MS 2665.0 m/z (MH+)
(Theory: 2664.5 m/z (M+)) Elemental Analysis C: 50.70 %; H 6.71 % (Theory C: 50.94 %; H 6.43 %).
Example 9
Synthesis of benzylidene protected [G3]-PGLLA-bzld - 2-[(cw-l,3-benzylidene glycerol)-2-propionic acid] (0.376 g, 1.49 mmol), DCC (0.463 g, 2.24 mmol), and DPTS (0.200 g, 0.680 mmol) were dissolved in THF (15 mL). The reaction flask was flushed with nitrogen and stirred for 1.5 hours before [G2]-PGLLA (0.070 g, 0.035 mmol) was added by dissolving in a minimal amount of THF. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. The DCC-urea was filtered and washed with a small amount of THF (20 mL). The THF filtrate was evaporated and the crude product was purified by silica gel chromatography, eluting with 3:97 MeOH:CH2Cl2. The product was dissolved in minimal CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. Ethyl ether was decanted and the precipitate was exposed to reduced pressure to yield 0.164 g of a white powder (89.1 % yield). 1H NMR (400 MHz, CDCl3): δ 1.32 (m, 39, CH3), 1.45 (m, 48, CH3), 3.38 (m, 16, CH,), 3.77 (m, 14, -CH2-CH-CH2-), 3.97 (m, 20, -CH2-CH-CH2-), 4.07 (m, 24, - CH2-CH-CH2-), 4.24 (m, 97, -CH2-CH-CH2-), 4.39 (m, 8, -CH2-CH-CH2-), 5.47 (m, 16, CH), 7.31 (m, 48, arom. CH), 7.44 (m, 32, arom. CH). 13C NMR (400 MHz, CDCl3): δ 173.14 (COOR), 138.28 (CH), 129.12 (CH), 128.40 (CH), 126.36 (CH), 101.41 (CH), 72.68 (CH), 70.56 (CH2), 70.13 (CH), 68.11 (CH2), 19.25 (CH3), 19.02 (CH3). FTIR: y cm" x) 1744 (C=O), 1451 (CH2 bend), 1385 (CH3 bend). MALDI MS 5743.3 m/z (MH+) (Theory: 5739.9 m/z (M+)) Elemental Analysis C: 60.32 %; H 6.34% (Theory C: 60.47 %; H 6.36 %). SEC Mw: 4370, Mn: 4310, PDI: 1.01.
Example 10
Synthesis of [G3]-PGLLA-OH - Pd/C (10%) (10 % w/w) was added to a solution of benzylidene protected [G3]-PGLLA (0.095 g, 0.035 mmol) in THF (15 mL). The flask was evacuated and filled with 50 psi of H2 before shaking for 15 minutes. The catalyst was filtered and washed with THF (10 mL). The filtrate was evaporated to give 0.128 g of a colorless viscous oil (95.4 % yield). 1H NMR (400 MHz, (CD3)2CO): δ 1.37 (m, 87, CH3), 3.56 (m, 83, -CH2-CH-CH2- or -CH-CH3), 3.78 (m, 13, -CH2-CH-CH2- or -CH-CH3), 4.01 (m, 14, -CH2-CH-CH2- or -CH-CH3), 4.18 (m, 13, -CH2-CH-CH2- or -CH-CH3), 4.39 (m, 56, -CH2-CH-CH2- or -CH-CH3). 13C NMR (400 MHz, (CD3)2CO): δ 174.37 (COOR),
82.01 (CH), 74.16 (CH), 62.35 (CH2), 62.15 (CH2), 18.80 (CH3). FTIR: v (cm-1) 3434 (OH), 1738 (C=O), 1452 (CH2 bend), 1376 (CH3 bend). MALDI MS 4332.5 m/z (MH+) (Theory. 4330.2 m/z (M+)) Elemental Analysis C: 49.56 %; H 7.21 % (Theory C: 49.09 %; H 6.94%). SEC Mw: 4110, Mn: 4060, PDI: 1.01.
Example 11
Synthesis of [G0]-PGLSA-bzId - Succinic acid (1.57 g, 13.3 mmol), cώ-1,3-0- benzylideneglycerol (5.05 g, 28.0 mmol), and DPTS (4.07 g, 13.8 mmol) were dissolved in CH2Cl2 (120 mL). The reaction flask was flushed with nitrogen and then DCC (8.19 g, 39.7 mmol) was added. Stirring at room temperature was continued for 14 hours under a nitrogen atmosphere. Upon reaction completion, the DCC-urea was filtered and washed with a small amount of CH2Cl2 (20 mL). The crude product was purified by silica gel chromatography, eluting with 3:97 methanol: CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. Following vacuum filtration, 5.28 g of a white solid was collected (90 % yield). 1H NMR (CDCl3): δ 2.78 (s, 4, -CH2-CH2-), 4.08 (m, 4, -CH2-CH-CH2-), 4.23 (m, 4, -CH2-CH-CH2-), 4.69 (m, 2, -CH2-CH-CH2-, J=I.54 Hz, 1.71 Hz), 5.50 (s, 2, CH), 7.34 (m, 6, arom. CH), 7.48 (m, 4, arom. CH). 13C NMR (CDCl3): δ 172.32 (COOR), 138.03 (CH), 129.23 (CH), 128.48 (CH), 126.24 (CH), 101.33 (CH), 69.16 (CH2), 66.50 (CH), 29.57 (CH2). FTIR: v (cm4) 2992 (aliph. C-H stretch), 1727 (C=O). GC-MS 443 m/z (MH+) (Theory: 442 m/z (M+)). HR FAB 442.1635 m/z (M+) (Theory: 442.1628 m/z (M+)). Elemental Analysis C: 65.25 %; H 5.85 % (Theory C: 65.15 %; H 5.92 %).
Example 12
Synthesis of [GO]-PGLSA-OH - Pd/C (10 % w/w) was added to a solution of benzylidene protected [GO]-PGLSA (2.04 g, 4.61 mmol) in THF (30 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 50 psi of H2 before shaking for 10 hours. The catalyst was filtered and washed with THF (20 mL). The filtrate was evaporated to give 1.18 g of a clear viscous oil (97 % yield). 1H NMR (CD3OD): δ 2.67 (s, 4, -CH4-CH2- ), 3.64 (m, 8, -CH2-CH-CH2-), 4.87 (m, 2, -CH2-CH-CH2-). 13C NMR (CD3OD): δ 172.77 (COOR), 75.84 (CH2), 60.41 (CH), 28.96 (CH2). 13C NMR ((CD3)2CO): δ 171.99 (COOR), 76.15 (CH2), 60.89 (CH). FTIR: v (cm4) 3299 (OH), 1728 (C=O). GC-MS 284 m/z (M+NH4 +) (Theory: 266 m/z (M+)). Elemental Analysis C: 44.94 %; H 6.87 % (Theory C: 45.11 %; H 6.81%).
Example 13
Synthesis of 2-(cώ-l,3-0-benzylidene glycerol)succinic acid mono ester - cis-1,3-0- Ben2ylideneglycerol (9.90 g, 54.9 mmol) was dissolved in pyridine (100 mL) followed by the addition of succinic anhydride (8.35 g, 83.4 mmol). The reaction mixture was stirred at room temperature for 18 hours before the pyridine was removed under vacuum at 40 0C. The remaining solid was dissolved in CH2Cl2 (100 mL) and washed three times with cold 0.2 N HCl (100 mL), or until the aqueous phase remained at pH 1. The organic phase was evaporated and the solid was dissolved in deionized water (300 mL). 1 N NaOH was added until pH 7 was obtained and the product was dissolved in solution. The aqueous phase was extracted with CH2Cl2 (200 mL) and then readjusted to pH 4. The aqueous phase was subsequently extracted twice with CH2Cl2 (200 mL), dried with Na2SO4, filtered, and evaporated. The solid was stirred in ethyl ether (50 mL) and cooled to -25 0C for 3 hours before collecting 14.6 g of a white powder (95 % yield). 1H NMR (CDCl3): δ 2.68 (m, 4, - CH2-CH2-), 4.13 (m, 2, -CH2-CH-CH2-), 4.33 (m, 2, -CH2-CH-CH2-), 4.70 (m, 1, -CH2-CH- CH2-), 5.51 (s, 1, CH), 7.34 (m, 3, arom. CH), 7.47 (m, 2, arom. CH). 13C NMR (CDCl3): δ 178.07 (COOH), 172.38 (COOR), 137.95 (CH), 129.33 (CH), 128.51 (CH), 126.26 (CH), 101.43 (CH), 69.15 (CH2), 66.57 (CH), 29.24 (CH2), 29.05 (CH2). FTIR: v (cm4) 2931 (aliph. C-H stretch), 1713 (C=O). GC-MS 281 m/z (MH+) (Theory: 280 m/z (M+)). Elemental Analysis C: 60.07 %; H 5.80 % (Theory: C: 59.99 %; H 5.75 %).
Example 14
Synthesis of [Gl]-PGLSA-bzld - 2-(cώ-l,3-<9-Benzylidene glycerol)succinic acid mono ester (6.33 g, 22.6 mmol), [GO]-PGLSA (1.07 g, 4.02 mmol), and DPTS (2.51 g, 8.53 mmol) were dissolved in THF (60 mL). The reaction flask was flushed with nitrogen and then DCC (7.04 g, 34.1 mmol) was added. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of THF (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 3:97 to 5:95 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. The ethyl ether was decanted and the precipitate was isolated to yield 5.11 g of a white powder (97 % yield). 1H NMR (CDCl3): δ 2.58 (m, 4, -CH2-CH2-), 2.63 (m, 8, -CH2-CH2-), 2.71 (m, 8, -CH2- CH2-), 4.12 (m, 12, -CH2-CH-CH2-), 4.23 (m, 12, -CH2-CH-CH2-), 4.69 (m, 4, -CH2-CH- CH2-), 5.20 (m, 2, -CH2-CH-CH2-), 5.51 (m, 4, CH), 7.33 (m, 12, arom. CH), 7.46 (m, 8,
arom. CH). 13C NMR (CDCl3): δ 172.28 (COOR), 171.91 (COOR), 171.53 (COOR), 138.03 (CH), 129.26 (CH), 128.48 (CH), 126.22 (CH), 101.32 (CH), 69.50 (CH), 69.16 (CH2), 66.54 (CH), 62.49 (CH2), 29.36 (CH2), 29.03 (CH2). FTIR: v (cm-1) 2858 (aliph. C- H stretch), 1731 (C=O). FAB MS 1315.6 m/z (MH+) (Theory: 1315.3 m/z (M+)). Elemental Analysis C: 60.13 %; H 5.82 % (Theory C: 60.27 %; H 5.67%). SEC Mw: 1460, Mn: 1450, PDI: 1.01.
Example 15
Synthesis of [Gl]-PGLSA-OH - Pd/C (10 % w/w) was added to a solution of benzylidene protected [Gl]-PGLSA (0.270 g, 0.230 mmol) in THF (20 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 50 psi of H2 before shaking for 10 hours. The catalyst was filtered and washed with THF (20 mL). The filtrate was evaporated to give 0.178 g of a colorless, viscous oil (94 % yield). 1H NMR (CD3OD): δ 2.63 (m, 20, - CH2-CH2-), 3.52 (m, 4, -CH2-CH-CH2-), 3.64 (m, 8, -CH2-CH-CH2-), 3.80 (m, 2, -CH2-CH- CH2-), 4.05 (m, 2, -CH2-CH-CH2-), 4.14 (m, 2, -CH2-CH-CH2-), 4.21 (m, 4, -CH2-CH-CH2- ), 4.30 (m, 4, -CH2-CH-CH2-), 4.85 (m, 2, -CH2-CH-CH2-), 5.25 (m, 2, -CH2-CH-CH2-). 13C NMR (CD3OD): δ 172.82 (COOR), 172.58 (COOR), 172.48 (COOR), 172.08 (COOR),
75.82 (CH), 69.90 (CH), 69.68 (CH), 65.66 (CH2), 62.85 (CH2), 62.30 (CH2), 60.43 (CH2),
28.83 (CH2), 28.61 (CH2). FTIR: v (cm"1) 3405 (OH), 2943 (aliph. C-H stretch), 1726 (C=O). FAB MS 963.2 m/z (MH+) (Theory: 962.9 m/z (M+)). Elemental Analysis C: 47.13 %; H 6.11 % (Theory C: 47.40 %; H 6.07 %). SEC Mw: 1510, Mn: 1500, PDI: 1.01.
Example 16
Synthesis of [G2]-PGLSA-bzld - 2-(cώ-l,3-O-Benzylidene glycerol)succinic acid mono ester (4.72 g, 16.84 mmol), [Gl]-PGLSA (1.34 g, 1.39 mmol), and DPTS (1.77 g, 6.02 mmol) were dissolved in THF (100 mL). The reaction flask was flushed with nitrogen and then DCC (4.62 g, 22.4 mmol) was added. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of THF (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 3:97 to 5:95 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. The ethyl ether was decanted and the precipitate was isolated to yield 4.00 g of a white powder (94 % yield). 1H NMR (CDCl3): δ 2.59 (broad m, 26, -CH2-CH2-), 2.69 (broad m, 52, -CH2-CH2-), 4.13
(m, 28, -CH2-CH-CH2-), 4.13 (m, 28, -CH2-CH-CH2-), 4.69 (m, 8, -CH2-CH-CH2-), 5.22 (m, 6, -CH2-CH-CH2-), 5.50 (s, 8, CH), 7.32 (m, 24, arom. CH), 7.47 (m, 16, arom. CH). 13C NMR (CDCl3): δ 172.27 (COOR), 171.88 (COOR), 171.60 (COOR), 138.04 (CH), 129.25 (CH), 128.47 (CH), 126.21 (CH), 101.30 (CH), 69.48 (CH), 69.15 (CH2), 66.54 (CH), 62.57 (CH2), 29.35 (CH2), 29.18 (CH2) 29.03 (CH2), 28.84 (CH2). FTIR: ycm"1) 2969 (aliph. C-H stretch), 1733 (C=O). FAB MS 3060.7 m/z (MH+) (Theory: 3060.9 m/z (M+)). Elemental Analysis C: 59.20 %; H 5.64 % (Theory C: 58.86 %; H 5.60 %). SEC Mw: 3030, Mn: 2990, PDI: 1.01.
Example 17
Synthesis of [G2]-PGLSA-OH - Pd/C (10 % w/w) was added to a solution of benzylidene protected [G2]-PGLSA (2.04 g, 0.667 mmol) in THF (20 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 50 psi of H2 before shaking for 10 hours. The catalyst was filtered and washed with THF (20 mL). The filtrate was evaporated to give 1.49 g of a colorless, viscous oil (95 % yield). 1H NMR (CD3OD): δ 2.64 (m, 52, - CH2-CH2-), 3.53 (m, 16, -CH2-CH-CH2-), 3.64 (m, 4, -CH2-CH-CH2-), 3.80 (m, 8, -CH2- CH-CH2-), 4.06 (m, 8, -CH2-CH-CH2-), 4.14 (m, 6, -CH2-CH-CH2-), 4.21 (m, 11, -CH2- CH-CH2-), 4.30 (m, 11, -CH2-CH-CH2-), 5.25 (m, 6, -CH2-CH-CH2-). 13C NMR (CD3OD): δ 172.83 (COOR), 172.59 (COOR), 172.49 (COOR), 69.91 (CH), 69.69 (CH), 65.68 (CH2), 62.88 (CH2), 62.37 (CH2), 28.61 (CH2). FTIR: v (cm-1) 3429 (OH), 2952 (aliph. C-H stretch), 1728 (C=O). MALDI MS 2357.3 m/z (MH+) (Theory: 2356.1 m/z (M+)). Elemental Analysis C: 48.32 %; H 5.97 % (Theory C: 47.92 %; H 5.90%). SEC Mw: 3060, Mn: 3000, PDI: 1.02.
Example 18
Synthesis of succinic acid monomethallyl ester (SAME) - 2-Methyl-2-propen-l-ol (4.90 mL, 58.2 mmol) was dissolved in pyridine (20 mL) followed by the addition of succinic anhydride (7.15 g, 71.4 mmol). The reaction mixture was stirred at room temperature for 15 hours before the pyridine was removed under vacuum at 30 0C. The remaining liquid was dissolved in CH2Cl2 (100 mL) and washed two times with cold 0.2 N HCl (100 mL). The organic phase was dried with Na2SO4, gravity filtered, and evaporated to give 9.25 g of a clear liquid (92 % yield). 1H NMR (CDCl3): δ 1.70 (s, 3, CH3), 2.64 (m, 4, -CH2-CH2-), 4.48 (s, 2, -CH2-), 4.88 (m, 1, vinyl CH2), 4.93 (m, 1, vinyl CH2). 13C NMR (CDCl3): δ 178.58 (COOH), 172.05 (COOR), 139.88 (CH), 113.31 (CH2), 68.31 (CH2), 29.11 (CH2),
28.99 (CH2), 19.59 (CH3). FTIR: v (cm-1) 2939 (aliph. C-H stretch), 1711 (C=O). GC-MS 173 m/z (MH+) (Theory: 172 m/z (M+)). Elemental Analysis C: 55.51 %; H 7.09 % (Theory: C: 55.81 %; H 7.02 %).
Example 19
Synthesis of [G2] -PGLSA-SAME - Succinic acid monomethallyl ester (0.826 g, 4.80 mmol), [G2]-PGLSA (0.401 g, 0.170 mmol), and DPTS (0.712 g, 2.42 mmol) were dissolved in THF (50 niL). The reaction flask was flushed with nitrogen and then DCC (1.52 g, 7.37 mmol) was added. Stirring at room temperature was continued for 14 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of CH2Cl2 (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 3:97 to 5:95 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. The ethyl ether was decanted and the precipitate was isolated to yield 0.558 g of a clear colorless oil (68.2 % yield). 1H NMR (CDCl3): δl.72 (s, 48, CH3), 2.63 (m, 116, -CH2-CH2-), 4.16 (m, 23, -CH2-CH-CH2-), 4.27 (m, 23, -CH2-CH-CH2-), 4.48 (s, 32, -CH2-), 4.89 (s, 16, vinyl CH2), 4.94 (s, 16, vinyl CH2), 5.24 (m, 14, -CH2-CH-CH2-). 13C NMR (CDCl3): δ 171.91 (COOR), 171.67 (COOR), 139.98 (CH), 113.22 (CH2), 69.43 (CH), 68.31 (CH2), 62.56 (CH2), 29.10 (CH2), 29.02 (CH2) 28.83 (CH2), 19.66 (CH3). FTIR: yOm"1) 2969 (aliph. C-H stretch), 1734 (C=O). MALDI MS 4840.9 m/z (MH+) (Theory: 4838.7 m/z (M+)). Elemental Analysis C: 55.37 %; H 6.22 % (Theory C: 55.35%; H 6.29%). SEC Mw: 5310, Mn: 5230, PDI: 1.02.
Example 20
Synthesis of [G3]-PGLSA-bzId - 2-(cώ-l,3-O-Ben2ylidene glycerol)succinic acid mono ester (2.77 g, 9.89 mmol), [G2J-PGLSA (1.00 g, 0.425 mmol), and DPTS (1.30 g, 4.42 mmol) were dissolved in THF (40 mL). The reaction flask was flushed with nitrogen and then DCC (2.67 g, 12.9 mmol) was added. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of THF (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 3:97 to 5:95 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU)3 and precipitated in ethyl ether at -20 0C to remove remaining DCC. The ethyl ether was decanted and the precipitate was isolated to yield 3.51 g of a white powder (90 % yield).
1H NMR (CDCl3): δ 2.57 - 2.72 (broad m, 116, -CH2-CH2-), 4.12 (m, 60, -CH2-CH-CH2-), 4.23 (m, 60, -CH2-CH-CH2-), 4.68 (m, 16, -CH2-CH-CH2-), 5.22 (m, 14, -CH2-CH-CH2-), 5.49 (s, 16, CH), 7.33 (m, 48, arom. CH), 7.46 (m, 32, arom. CH). 13C NMR (CDCl3): δ 172.31 (COOR), 171.97 (COOR), 171.65 (COOR), 138.01 (CH), 129.28 (CH), 128.49 (CH), 126.21 (CH), 101.28 (CH), 69.45 (CH), 69.16 (CH2), 66.53 (CH), 62.59 (CH2), 29.32 (CH2), 29.16 (CH2) 29.01 (CH2), 28.81 (CH2). FTIR: ycnf1) 2984 (aliph. C-H stretch), 1733 (C=O). MALDI MS 6553.4 m/z (MH+) (Theory: 6552.2 m/z (M+)). Elemental Analysis C: 58.50 %; H 5.66 % (Theory C: 58.29 %; H 5.57 %). SEC Mw: 5550, Mn: 5480, PDI: 1.01.
Example 21
Synthesis of [G3]-PGLSA-OH - Pd/C (10 % w/w) was added to a solution of benzylidene protected [G3]-PGLSA (1.23 g, 0.188 mmol) in 9: 1 THF/MeOH (20 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 50 psi of H2 before shaking for 10 hours. The catalyst was filtered and washed with 9:1 THF/MeOH (20 mL). The filtrate was evaporated to give 0.923 g of a colorless, viscous oil (95 % yield). 1H NMR (CD3OD): δ 2.64 (m, 116, -CH2-CH2-), 3.51 (m, 26, -CH2-CH-CH2-), 3.67 (m, 28, -CH2-CH-CH2-), 3.80 (m, 12, -CH2-CH-CH2-), 4.05 (m, 14, -CH2-CH-CH2-), 4.14 (m, 14, -CH2-CH-CH2-), 4.22 (m, 22, -CH2-CH-CH2-), 4.30 (m, 22, -CH2-CH-CH2-), 5.26 (m, 14, -CH2-CH-CH2). 13C NMR (CD3OD): δ 172.86 (COOR), 69.91 (CH), 67.64 (CH), 65.67 (CH2), 62.87 (CH2), 62.41 (CH2), 28.61 (CH2). FTIR: v (cm4) 3442 (OH)5 2959 (aliph. C-H stretch), 1731 (C=O). MALDI MS 5144.8 m/z (MH+) (Theory: 5142.5 m/z (M+)). Elemental Analysis C: 48.07 %; H 5.84 % (Theory C: 48.11 %; H 5.84 %). SEC Mw: 5440, Mn: 5370, PDI: 1.01.
Example 22
Synthesis of [G4]-PGLSA-bzld - 2-(cz5-l,3-C>-Benzylidene glycerol)succinic acid mono ester (2.43 g, 8.67 mmol), [G3J-PGLSA (0.787 g, 0.153 mmol), and DPTS (1.30 g, 4.42 mmol) were dissolved in 10:1 THF/DMF (40 mL). The reaction flask was flushed with nitrogen and then DCC (2.63 g, 12.7 mmol) was added. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon completion, solvents were removed under vacuum and the remaining solids were redissolved CH2Cl2. The DCC-urea was filtered and washed with a small amount of CH2Cl2 (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with
3:97 to 5:95 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. The ethyl ether was decanted and the precipitate was exposed to reduced pressure to yield 1.50 g of a white powder (73 % yield). 1H NMR (CDCl3): δ 2.63 (m, 70, -CH2-CH2-), 2.72 (m, 146, - CH2-CH2-), 2.90 (m, 32, -CH2-CH2-), 4.14 (m, 100, -CH2-CH-CH2-), 4.25 (m, 100, -CH2- CH-CH2-), 4.70 (m, 32, -CH2-CH-CH2-), 5.25 (m, 16, -CH2-CH-CH2-), 5.52 (s, 32, CIJ), 7.33 (m, 96, arom. CH), 7.47 (m, 64, arom. CH). 13C NMR (CDCl3): δ 172.27 (COOR), 171.90 (COOR), 171.57 (COOR), 138.08 (CH), 129.25 (CH), 128.47 (CH), 126.23 (CH), 101.27 (CH), 69.49 (CH), 69.13 (CH2), 66.54 (CH), 62.45 (CH2), 29.34 (CH2), 29.02 (CH2), 28.83 (CH2). FTIR: VDnT1) 2978 (aliph. C-H stretch), 1733 (C=O). MALDI MS 13536.8 m/z (MH+) (Theory: 13534.7 m/z (M+)). Elemental Analysis C: 58.20 %; H 5.56 % (Theory C: 58.04 %; H 5.56 %). SEC Mw: 9000, Mn: 8900, PDI: 1.01.
Example 23
Synthesis of [G4]-PGLSA-OH - Pd/C (10 % w/w) was added to a solution of benzylidene protected [G4]-PGLSA (0.477 g, 0.0352 mmol) in 9:1 THF/MeOH (20 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 50 psi of H2 before shaking for 10 hours. The catalyst was filtered and washed with 9:1 THF/MeOH (20 mL). The filtrate was evaporated to give 0.351 g of a colorless, viscous oil (93 % yield). 1H NMR (CD3OD): δ 2.65 (m, 244, -CH2-CH2-), 3.53 (m, 50, -CH2-CH-CH2), 3.65 (m, 22, -CH2-CH-CH2-), 3.81 (m, 28, -CH2-CH-CH2-), 4.05 (m, 32, -CH2-CH-CH2-), 4.14 (m, 32, -CH2-CH-CH2-), 4.24 (m, 60, -CH2-CH-CH2-), 4.30 (m, 60, -CH2-CH-CH2-), 5.26 (m, 32, -CH2-CH-CH2-). 13C NMR (CD3OD): δl72.94 (COOR), 69.92 (CH), 65.72 (CH2), 62.91 (CH2), 28.67 (CH2). FTIR: v (cm-1) 3444 (OH), 2931 (aliph. C-H stretch), 1729 (C=O). MALDI MS 10715.6 m/z (MH+) (Theory: 10715.3 m/z (M+)). Elemental Analysis C: 48.50 %; H 5.83 % (Theory C: 48.20 %; H 5.81 %). SEC Mw: 8800, Mn: 8720, PDI: 1.01.
Example 24
The PGLSA dendrimers or other dendrimers described herein can also be synthesized through Accelerated Syntheses for example:
Example 24.1 Synthesis of 2-(cis-l,3-O-benzyIidene glycerol)succinic acid mono ester anhydride - 2-(cw-l,3-O-Benzylidene glycerol)succinic acid mono ester (50.00 g, 178.4 mmol) ) and DCC (22.09 g, 107.0 mmol) were dissolved in DCM (300 mL) and stirred for 14 hours. The DCU precipitate was collected by filtration and washed with DCM (50 mL).
The organic phase was directly added to 900 mL of hexanes. The hexanes and precipitate were cooled to -20 0C for 3 hours before 46.11 g of precipitate was collected after filtration (95 % yield). 1H NMR (CDCl3): δ 2.75 (m, 4, -CH2-CH2-), 4.12 (m, 4, -CH2-CH-CH2-), 4.25 (m, 4, -CH2-CH-CH2-), 4.71 (m, 2, -CH2-CH-CH2-), 5.52 (s, 2, CH), 7.34 (m, 6, arom. CH), 7.47 (m, 4, arom. CH). 13C NMR (CDCl3): δ 171.77 (COOR), 167.99 (-COOCO-), 137.96 (CH), 129.29 (CH), 128.51 (CH), 126.20 (CH), 101.36 (CH), 69.13 (CH2), 66.76 (CH), 30.37 (CH2), 28.94 (CH2). FTIR: v (cm-1) 2938 (aliph. C-H stretch), 1815 (C=O), 1730 (C=O). FAB-MS 543.2 m/z [M-H]+ (Theory: 542.53 m/z [M]+). Elemental Analysis C: 61.83 %; H 5.70 % (Theory: C: 61.99 %; H 5.57 %).
Example 24.2 Synthesis of [Gl]-PGLSA-bzld - Pd(OH)2/C (10% w/w) and activated carbon were added to a solution of [GO]-PGLSA-bzld (3.571 g, 8.071 mmol) in THF (25 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The catalyst and activated carbon were filtered off and washed with THF (50 mL). 2-(cώ-l,3-0-benzylidene glycerol)succinic acid mono ester anhydride (21.990 g, 40.532 mmol) and then DMAP (0.514 g, 4.207 mmol) were directly added to the deprotected core in the THF ( more THF was added to give a total volume of 100 mL). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Any remaining anhydride was quenched by the addition of n-propanol (4.0 mL, 44 mmol), which was allowed to stir for another 5 hours. The THF was removed under vacuum and the remaining contents were dissolved in DCM (250 mL) and washed once with 0.1 N HCl (200 mL) and three times with saturated sodium bicarbonate (200 mL). The organic phase was dried with Na2SO4, filtered, and concentrated before the dendrimer was precipitated in hexanes (450 mL) and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 10.29 g of a white solid (96.9 % yield). 1H NMR, 13C NMR, FTIR, MALDI-TOF MS, Elemental Analysis, and SEC have been previously reported. Tg (0C): 36.7 to 42.4, 39.5 at half-height.
Example 24.3 Synthesis of [G2]-PGLSA-bzld (186) - Pd(OH)2/C (10% w/w) and activated carbon were added to a solution of [Gl] -PGLS A-bzld (4.40 g, 3.43 mmol) in THF (50 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The catalyst and activated carbon were filtered off and washed with THF (50 mL). 2-(cw-l,3-Obenzylidene glycerol)succinic acid mono ester anhydride (18.459 g, 34.024 mmol) and then DMAP (0.831 g, 6.802 mmol) were directly added to the deprotected dendrimer in the THF. The reaction was stirred at room temperature for 14
hours under nitrogen atmosphere. Any remaining anhydride was quenched by the addition of n-propanol (3.0 mL, 33 mmol), which was allowed to stir for another 5 hours. The THF was removed under vacuum and the remaining contents were dissolved in DCM (400 mL) and washed once with 0.1 N HCl (300 mL) and three times with saturated sodium bicarbonate (300 mL). The organic phase was dried with Na2SO4, filtered, and concentrated before the dendrimer was precipitated in hexanes (900 mL) and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 9.85 g of a white solid (96.2 % yield). 1H NMR, 13C NMR, FTIR, MALDI-TOF MS, Elemental Analysis, and SEC have been previously reported. Tg (0C): 39.3 to 45.4, 42.3 at half-height. Example 24.4 Synthesis of [G3]-PGLSA-bzld - Pd(OH)2/C (10% w/w) and activated carbon were added to a solution of [G2]-PGLSA-bzld (12.81 g, 4.218 mmol) in THF (100 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The catalyst and activated carbon were filtered off and washed with THF (100 mL). From this solution, 1.822 g of [G2]-PGLSA-OH in THF was removed form the mixture. Next, 2-(cw-l,3-O-benzylidene glycerol)succinic acid mono ester anhydride (45.9154 g, 84.632 mmol) and then DMAP (1.5592 g, 12.763 mmol) were directly added to the deprotected core in the THF. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Any remaining anhydride was quenched by the addition of n-propanol (8.0 mL, 88 mmol), which was allowed to stir for another 5 hours. The THF was removed under vacuum and the remaining contents were dissolved in DCM (500 mL) and washed once with 0.1 N HCl (400 mL) and three times with saturated sodium bicarbonate (400 mL). The organic phase was dried with Na2SO4, filtered, and concentrated before the dendrimer was precipitated in hexanes (800 mL) and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 20.37 g of a white solid (91.4 % yield). 1H NMR, 13C NMR, FTIR, MALDI-TOF MS, Elemental Analysis, and SEC have been previously reported. Tg (0C): 43.1 to 48.3, 45.7 at half-height.
Example 24.5 Synthesis of [G3]-PGLSA-OH - Pd(OH)2/C (10% w/w) and activated carbon were added to a solution of [G3]-PGLSA-bzld (3.571 g, 8.071 mmol) in THF/MeOH (9:1) (25 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The catalyst and activated carbon were filtered off and washed with more of the THF/MeOH solution (50 mL) before the solvents were evaporated. The product was used directly in next reaction
Example 24.6 Synthesis of [G4]-PGLSA-bzld - The deprotected core was dissolved in the THF/dimethyl acetimide (10:1) (200 mL) and 2-(cw-l,3-O-benzylidene glycerol)succinic acid mono ester anhydride (60.83 g, 0.11212 mmol) and then DMAP (1.63 g, 13.342 mmol) were directly added to the reaction flask. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Any remaining anhydride was quenched by the addition of n-propanol (4.0 mL, 44 mmol), which was allowed to stir for another 5 hours. The solvents were removed under vacuum and the remaining contents were dissolved in DCM (250 mL) and washed once with 0.1 N HCl (200 mL) and three times with saturated sodium bicarbonate (200 mL). The organic phase was dried with Na2SO4, filtered, and concentrated before the dendrimer was precipitated in hexanes (450 mL) and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 33.25 g of a white solid (88.15 % yield). 1H NMR, 13C NMR, FTIR, MALDI-TOF MS, Elemental Analysis, and SEC have been previously reported. Tg (0C): 43.6 to 49.6, 47.0 at half-height.
Example 24.7 Synthesis of [G5]-PGLSA-bzId - [G4] -PGLSA-OFf (0.2052 g, 0.0192 mmol) and 2-(cώ-l,3-O-Benzylidene glycerol)succinic acid mono ester anhydride (0.067 g, 0.548 mmol), were dissolved in 1 :1 THF/DMF (15 mL). DMAP (1.152 g, 2.123 mmol) was added and the reaction flask was flushed with nitrogen. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Any remaining anhydride was quenched by the addition of water (4.0 mL) which was allowed to stir for another 5 hours. The solvents were removed under vacuum and the remaining contents were dissolved in DCM (150 mL) and washed once with 0.1 N HCl (100 mL) and three times with saturated sodium bicarbonate (100 mL). The organic phase was dried with Na2SO4, filtered, and concentrated before the dendrimer was precipitated in hexanes (450 mL) and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 0.414 g of a white solid (78.6 % yield). 1H NMR (CDCl3): δ 2.57-2.69 (broad m, 488, -CH2-CH2-), 4.07-4.21 (m, 507, -CH2-CH-CH2-), 4.66 (m, 64, -CH2-CH-CH2-), 5.19 (m, 63, -CH2-CH- CH2-), 5.48 (s, 64, CH), 7.31 (m, 194, arom. CH), 7.44 (m, 128, arom. CH). 13C NMR (CDCl3): δ 172.28 (COOR), 171.91 (COOR), 171.61 (COOR), 138.08 (CH), 129.25 (CH), 128.47 (CH), 126.23 (CH), 101.24 (CH), 69.47 (CH), 69.12 (CH2), 66.54 (CH), 62.45 (CH2), 29.33 (CH2), 29.17 (CH2), 29.02 (CH2), 28.83 (CH2). MALDI MS 27059 m/z [M- H]+ (Theory: 27500 m/z [M]+). SEC Mw: 16150, Mn: 15870, PDI: 1.02.
Example 25
Syntheses of [Gn]-PGLSA Dendrons with Focal NHS Activated Ester Example 25.1 Synthesis of [2-(cis-l,3-O-benzyIidene glyceroty-N-succinimidyl] succinate (bzld-[Gl]-PGLSA-NHS dendron) - 2-(czs-l,3-0-benzylidene glycerol)succinic acid mono ester (11.47 g, 40.92 mmol), N-hydroxy succinimide (4.85 g, 42.18 mmol), and DPTS (4.26 g, 14.50 mmol), were dissolved in CH2Cl2 (100 mL). The reaction flask was flushed with nitrogen and then DCC (13.44 g, 65.14 mmol) was added. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. The DCC-urea was filtered and washed with a small amount OfCH2Cl2 (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 3:97 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. Following vacuum filtration, 13.0 g of a white solid was collected (84 % yield). 1H NMR (400 MHz, CDCl3): δ 2.76 (broad s, 4, -CH2-CH2-), 2.85 (m, 2, -CHa-CH2-), 2.96 (m, 2, -CH2-CH2-), 4.13 (m, 2, -CH2-CH-CH2-), 4.27 (m, 2, -CH2-CH-CHa-), 4.72 (m, 1, -CH2-CH-CH2-), 5.52 (s, 1, CH), 7.34 (m, 3, arom. CH), 7.47 (m, 2, arom. CH). 13C NMR (400 MHz, CDCl3): δ 171.32 (COOR), 169.12 (COOR), 167.82 (COOR), 137.96 (CH), 129.30 (CH), 128.51 (CH), 126.23 (CH), 101.38 (CH), 69.11 (CH2), 66.94 (CH), 29.08 (CH2), 26.51 (CH2), 25.74 (CH2). FTIR: v (cm"1) 29318 (aliph. C-H stretch), 1820.09 and 1727 (C=O). GC- MS 378 m/z [M-H]+ (Theory: 377 m/z [M]+). Elemental Analysis C: 57.22 %; H 5.07 % (Theory: C: 57.29 %; H 5.08 %).
Example 25.2 Synthesis of bzld-[G2]-PGLSA-NHS dendron - Pd/C (10% w/w) was added to a solution of bzld-[Gl]-PGLSA-NHS dendron (0.514 g, 1.36 mmol) in THF (20 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 20 min. The catalyst and activated carbon were filtered off and washed with THF (50 mL). 2-(cw-l,3-(9-benzylidene glycerol)succinic acid mono ester (0.975 g, 3.48 mmol) and DPTS (0.475 g, 1.61 mmol) were directly added to this solution. The reaction flask was flushed with nitrogen and then DCC (1.08 g, 5.24 mmol) was added. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. The DCC-urea was filtered and washed with a small amount of THF (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 3:97 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. Following
vacuum filtration, 0.991 g of a white solid was collected (70 % yield). 1H NMR (400 MHz, CDCl3): δ 2.63 (broad s, 4, -CH2-CH2-), 2.72 (m, 10, -CH2-CH2-), 2.90 (t, 2, -CH2-CH2-), 4.14 (m, 6, -CH2-CH-CH2-), 4.25 (m, 6, -CH2-CH-CH2-), 4.70 (m, 2, -CH2-CH-CH2-), 5.25 (m, 1, -CH2-CH-CH2-), 5.52 (s, 2, CH), 7.33 (m, 6, arom. CH), 7.47 (m, 4, arom. CH). 13C NMR (400 MHz, CDCl3): δ 172.31 (COOR), 171.92 (COOR), 170.35 (COOR), 169.12 (COOR), 167.80 (COOR), 138.02 (CH), 129.27 (CH), 128.49 (CH), 126.21 (CH), 101.33 (CH), 69.97 (CH2), 69.17 (CH2), 66.53 (CH), 62.49 (CH2), 29.38 (CH2), 29.05 (CH2), 26.35 (CH2), 25.74 (CH2). FAB MS 814.3 m/z [M-H]+ (Theory: 813.8 m/z [M]+). Elemental Analysis C: 57.42 %; H 5.40 % (Theory: C: 57.56 %; H 5.33 %).
Example 25.3 Synthesis of bz!d-[G3]-PGLSA-NHS dendron - Pd/C (10% w/w) was added to a solution of bzld-[G2] -PGLSA-NHS dendron (0.687 g, 0.844 mmol) in THF (20 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 20 min. The catalyst and activated carbon were filtered off and washed with THF (50 mL). 2-(cώ-l,3-O-benzylidene glycerol)succinic acid mono ester (1.269 g, 4.53 mmol) and DPTS (0.657 g, 2.23 mmol) were directly added to this solution. The reaction flask was flushed with nitrogen and then DCC (1.08 g, 5.24 mmol) was added. The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. The DCC-urea was filtered and washed with a small amount of THF (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 3:97 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. Following vacuum filtration, 0.796 g of a white solid was collected (72 % yield). 1H NMR (400 MHz, CDCl3): δ 2.59 (m, 9, -CH2-CH2-), 2.63 (m, 9, -CH2-CH2-), 2.74 (m, 12, -CH2-CH2-), 2.89 (t, 2, -CH2-CH2-), 4.14 (m, 14, -CH2-CH-CH2-), 4.24 (m, 14, -CH2-CH-CH2-), 4.70 (m, 4, - CH2-CH-CH2-), 5.20 (m, 2, -CH2-CH-CH2-), 5.26 (m, 1, -CH2-CH-CH2-), 5.51 (s, 4, CH), 7.33 (m, 12, arom. CH), 7.47 (m, 8, arom. CH). 13C NMR (400 MHz, CDCl3): δ 172.29 (COOR), 171.92 (COOR), 171.59 (COOR), 169.23 (COOR), 167.87 (COOR), 138.03 (CH), 129.27 (CH), 128.48 (CH), 126.21 (CH), 101.33 (CH), 69.51 (CH2), 69.17 (CH2), 66.54 (CH), 62.51 (CH2), 29.37 (CH2), 29.04 (CH2), 28.86 (CH2), 25.73 (CH2). FAB MS 1686.7 m/z [M-H]+ (Theory: 1686.6 m/z [M]+). Elemental Analysis C: 57.52 %; H 5.53 % (Theory: C: 57.68 %; H 5.44 %).
Example 26
Synthesis of [G2]-PGLSA-(Z)Lys(Z) - Z-Lys(Z)-OH (1.88 g, 4.53 mmol), [G2J-PGLSA (0.401 g, 0.170 mmol), and DPTS (0.66 g, 2.24 mmol) were dissolved in THF (20 mL). The reaction flask was flushed with nitrogen and then DCC (1.43 g, 6.93 mmol) was added. Stirring at room temperature was continued for 24 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of THF (20 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 2:98 to 4:96 methanol:CH2Cl2. The product was dissolved in CH2Cl2, filtered (to remove any DCU), and precipitated in ethyl ether at -20 0C to remove remaining DCC. The ethyl ether was decanted and the precipitate was isolated to yield 1.69 g of a white solid (95.1 % yield). 1H NMR (CDCl3): δ 1.28 (broad s, 32, -CH2-CH2-CH2- CH2-NH-), 1.43 (broad s, 32, -CH2-CH2-CH2-CH2-NH-), 1.59 (broad s, 16, -CH2-CH2-CH2- CH2-NH-), 1.72 (broad s, 16, -CH2-CH2-CH2-CH2-NH-), 1.59 (broad s, 32, -CH2-CH2-CH2- CH2-NH-), 2.54 (broad s, 52, -CH2-CH2-), 4.09-4.28 (broad m, 23, -CH2-CH-CH2- and - CH2-CHCO-NH-), 5.00 (s, 32, -CH2-Ph), 5.03 (s, 32, -CH2-Ph), 5.18 (m, 14, -CH2-CH- CH2-), 7.25 (m, 165, arom. CH). 13C NMR (CDCl3): δ 171.98 (COOR), 171.51 (COOR), 156.80 (COOR), 156.34 (COOR), 136.84 (CH), 136.44 (CH), 128.67 (CH), 128.29 (CH), 67.19 (CH), 66.76 (CH), 62.58 (CH2), 53.96 (CH), 40.62 (CH2), 31.80 (CH2) 29.49 (CH2), 28.89 (CH2), 28.73 (CH2), 22.56 (CH2). MALDI MS 8708.0 m/z [M-H]+ (Theory: 8699.0 m/z [M]+). SEC Mw: 7330, Mn: 7220, PDI: 1.01.
Example 27
Synthesis of [G2]-PGLSA-Lys - [G2]-PGLSA-Z-Lys(Z) (59.0 mg, 0.00678 mmol), was dissolved in DMF (3 mL). The reaction flask was flushed with nitrogen and then 10% Pd/C (400 mg) was added and stirred vigorously. To this stirring solution, formic acid was slowly added via syringe. The solution began to bubble and give off heat. Stirring at room temperature was continued for 14 hours under nitrogen atmosphere. Upon completion, Pd/C was filtered and washed with a small amount of 1 N HCl (10 mL), which was added to the DMF solution containing the dendrimer. The resulting solution was added drip wise into a large excess of acetone. The contents were cooled to -20 0C over night. The acetone was decanted and the precipitate was isolated to yield 29.0 mg of product (96.3 % yield). 1H NMR (CDCl3): δ 1.39 (broad m, 32, -CH2-CH2-CH2-CH2-NH-), 1.60 (broad m, 32, -CH2- CH2-CH2-CH2-NH-), 1.83 (broad m, 16, -CH2-Ca-CH2-CH2-NH-), 1.92 (broad m, 16, -
CH2-CH2-CH2-CH2-NH-), 2.53-2.60 (broad m, 52, -CH2-CH2-), 2.87 (broad m, 32, -CH2- CH2-CH2-CH2-NH-), 4.08 (broad m, 20, -CH2-CH-CH2- and -CH2-CHCO-NH-), 4.09 (broad m, 23, -CH2-CH-CH2- and -CH2-CHCO-NH-), 4.21 (broad m, 25, -CH2-CH-CH2- and -CH2-CHCO-NH-), 4.35 (broad m, 16, -CH2-CH-CH2- and -CH2-CHC0-NH-), 4.43 (broad m, 16, -CH2-CH-CH2- and -CH2-CHCO-NH-), 5.19 (m, 5, -CH2-CH-CH2-), 5.30 (m, 8, -CH2-CH-CH2-). 13C NMR (CDCl3): δ 174.35 (COOR), 173.74 (COOR), 169.67 (COOR), 70.04 (CH), 64.21 (CH2), 63.01 (CH2), 52.72 (CH2), 39.17 (CH2), 37.07 (CH2), 29.38 (CH2) 28.81 (CH2), 26.44 (CH2), 21.78 (CH2), 21.71 (CH2). MALDI MS 4404 m/z [M-H]+ (Theory: 4407 m/z [M]+). SEC Mw: 7730, Mn: 7580, PDI: 1.02.
Example 28
Synthesis of [G2]-PGLSA-COOH - [G2]-PGLSA-OH (0.636 g, 0.270 mmol) was dissolved in pyridine (20 mL) and stirred while succinic anhydride (0.649 g, 6.485 mmol) was added. The reaction mixture was stirred for 16 hours at 35 oC before the pyridine was removed under reduced pressure. The contents were partially dissolved in DCM (15 mL), and 0.1 N HCl (15 mL) was then added and the mixture was stirred for an additional 15 minutes. After stirring, the organic and aqueous phases separated and a layer was formed between the two phases. While avoiding the interface, most of the aqueous and organic phases were removed. This washing procedure with 15 mL of DCM and 0.1 N HCl was repeated two more times. Any remaining organic or aqueous phase was removed first by rotoevaporation followed by lyopholization to yield 0.990 g of a highly viscous liquid (92.7 % yield). MALDI MS 3958.4 m/z [M+H]+, (Theory: 3957.2 m/z [M]+).
Example 29
Synthesis of [G4] -PGLSA-COOH and [G4]-PGLSA-COO-Na+ - [G4]-PGLSA-OH (0.140 g, 0.0131 mmol) was dissolved in pyridine (10 mL) and stirred while succinic anhydride (0.167 g, 1.68 mmol) was added. The reaction mixture was stirred for 16 hours before the pyridine was removed under reduced pressure. The contents were partially dissolved in DCM (15 mL), and 0.1 N HCl (15 mL) was then added and the mixture was stirred for an additional 15 minutes. After stirring, the organic and aqueous phases separated and a layer was formed between the two phases. While avoiding the interface, most of the aqueous and organic phases were removed. This washing procedure with 15 mL of DCM and 0.1 N HCl was repeated two more times. Any remaining organic or aqueous
phase was removed first by rotoevaporation followed by lyopholization to yield 0.191 g of a highly viscous liquid (85 % yield). To dissolve the polymer in water, deionized water (10 mL) and brine (0.5 mL) were added to the solution and 0.05 N NaOH was added drop-wise to the stirring solution until the pH remained at 7.0, The dendrimer was purified via dialysis with 7,000 MW cutoff dialysis tubing for 24 hours in DI water. The water was then removed via lyopholization to obtain a white solid. 1H NMR (D2O): δ 2.32 (m, 130, -CH2- CH2-), 2.46 (m, 133, -CH2-CH2-), 2.58 (m, 228, -CH2-CH2-) 4.13-4.21 (m, 240, -CH2-CH- CH2-), 5.18 (m, 62, -CH2-CH-CH2-). 13C NMR (D2O): δ 180.72 (COOH), 175.37 (COOH), 173.52 (COOR), 70.14 (CH), 69.76 (CH), 62.80 (CH2), 34.31 (CH2), 32.10 (CH2), 30.72 (CH2), 29.01 (CH2). FTIR: v (cm-1) 3368 (OH), 2964 (aliph. C-H stretch), 1732 (C=O), 1567 (asym COO- stretch), 1409 (sym COO" stretch), 1149 (C-O stretch). MALDI MS 17168 m/z [M + Na]+, 8602 m/z [M + Na]2+, (Theory: 17120.0 m/z [M]+). SEC Mw: 8330, Mn: 7780, PDI: 1.11.
Example 30
Synthesis of 2-(tert-Butyldiphenylsilanyloxy)-succinic acid 4-(2-phenyl-[l,3]dioxan-5- yl) ester - L-Malic acid (2.00 g, 15.0 mmol) was dissolved in pyridine (25 mL) and tert- butylchlorodiphenylsilane (3.9 mL, 15.0 mmol) was added via syringe. The reaction was stirred for 14 hours before the pyridine was removed by vacuum. The remaining residue was dissolved in DCM (100 mL) and washed with 0.2 N HCl (2x 100 mL). The organic layer was dried with Na2SO4, filtered, and evaporated. Crude 2-{tert- butyldiphenylsilanyloxy) succinic acid was subsequently dissolved in a 2: 1 mixture of trifluoroacetic anhydride and THF (50 mL) respectively and heated to 50 0C for 2 hours. The solvents were removed by vacuum and the crude mixture was azeotroped with toluene. The crude anhydride was dissolved in pyridine and cώ-l,3-O-benzylideneglycerol (2.7 g, 54.9 mmol) was added before the solution was stirred another 14 hours. The pyridine was removed by vacuum. The remaining residue was dissolved in DCM (100 mL) and washed with 0.2 N HCl (2x 100 mL). The organic layer was dried with Na2SO4, filtered, and evaporated. The crude product was purified by silica gel chromatography, eluting with 79:20:1 to 59:40:1 hexane: ethyl acetate: acetic acid. 0.99 g of a viscous clear liquid were isolated following evaporation of solvents (90 % yield) evaporated to give 1.18 g of a clear viscous oil (12.3% yield). 1H NMR (400 MHz, CDCl3): δ 2.67 (s, 9, -CH3), 2.78 (broad m, 2, -CH2-CH-), 3.64 (broad m, 4, -CH2-CH-CH2-), 4.87 (m, 1, -CH2-CH-CH2-) 2.78 (t, 1, -
The crude anhydride was dissolved in pyridine and α,y~l,3-6>-benzylideneglycerol (2.7 g, 54.9 mmol) was added before the solution was stirred another 14 hours. The pyridine was removed by vacuum. The remaining residue was dissolved in DCM (100 niL) and washed with 0.2 N HCl (2x 100 mL). The organic layer was dried with Na2SO4, filtered, and evaporated. The crude product was purified by silica gel chromatography, eluting with 79:20:1 to 59:40:1 hexane: ethyl acetate: acetic acid. 0.99 g of a viscous clear liquid were isolated following evaporation of solvents (90 % yield) evaporated to give 1.18 g of a clear viscous oil (12.3% yield). 1H NMR (400 MHz, CDCl3): δ 2.67 (s, 9, -CH3), 2.78 (broad m, 2, -CH2-CH-), 3.64 (broad m, 4, -CH2-CH-CH2-), 4.87 (m, 1, -CH2-CH-CH2-) 2.78 (t, 1, - CH2-CH-), 5.50 (s, 1, CH), 7.34 (broad ra, 4, arom. CH), 7.48 (broad m, 11, arσm. CH). 13C NMR (100.6 MHz, CDCl3): δ 177.54 (COOH), 175.91 (COOH), 171.63 (COOR), 138.00 (CH)5 136.20 (CH), 136.14 (CH), 132.94 (CH), 130.20 (CH), 129.25 (CH), 128.41 (CH), 127.95 (CH), 127.81 (CH), 126.35 (CH), 101.41 (CH), 69.43 (CH2), 68.78 (CH), 66.91 (CH2), 39.92 (CH), 26.99 (CH3), 20.95 (CH), 19.53 (CH2). FAB-MS 535.2 m/z [M+Hf (Theory: 534.67 m/z [M]+).
Example 31
Synthesis of [GO]-PGLAA-bzld - Adipic acid (6.474 g, 44.300 mmol), cis-l,3-O- benzylideneglycerol (17.571 g, 97.508 mmol), and DPTS (10.01 g, 34.03 mmol) were dissolved in DCM (120 mL) followed by the addition of DCC (28.260 g, 136.96 mmol). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon reaction completion, the DCC-urea was filtered and washed with a small amount of DCM (50 mL). The crude product was purified by silica gel chromatography, eluting with 2% MeOH in DCM. The appropriate isolated fractions were concentrated, filtered (to remove any DCU), and directly precipitated in hexanes and cooled to -20 0C overnight. Following vacuum filtration, 12.694 g of a white solid was collected (60.8 % yield). 1H NMR (400 MHz, CDCl3): δ 1.72 (s, 4, -CH2-CH2-CH2-CH2-), 2.45 (s, 4, -CH2-CH2-CH2-CH2-), 4.12 (m, 4, -CH2-CH-CH2-), 4.25 (m, 4, -CH2-CH-CH2-), 4.68 (m, 2, -CH2-CH-CH2-), 5.52 (s, 2, CH), 7.34 (m, 6, arom. CH), 7.48 (m, 4, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 173.47 (COOR), 138.01 (CH), 129.27 (CH), 128.50 (CH), 126.22 (CH), 101.43 (CH)5 69.30 (CH2), 66.08 (CH), 34.15 (CH2), 24.49 (CH2). FAB 471.2 m/z [M+H]+ (Theory: 470.5I mZz [M]+).
Example 32
Synthesis of [GO]-PGLAA-OH - Pd(OH)2/C (10% w/w) was added to a solution of [GO]- PGLAA-bzld (2.161 g, 4.593 mmol) in THF (30 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi OfH2 before shaking for 10 hours. The catalyst was filtered and washed with THF solution (50 mL). The filtrate was evaporated to give 1.303 g of a clear viscous oil (96.4 % yield). 1H NMR (400 MHz, CD3OD): δ 1.64 (m, 4, -CH2-CH2-CH2-CH2-), 2.36 (m, 4, -CH2-CH2-CH2-CH2-), 3.51 (m, 1, -CH2-CH-CH2-), 3.64 (m, 5, -CH2-CH-CH2-), 3.78 (m, 1, -CH2-CH-CH2-), 4.03 (m, 1, -CH2-CH-CH2-), 4.12 (m, 1, -CH2-CH-CH2-). 13C NMR (100.6 MHz, CD3OD): δ 173.76 (COOR), 75.43 (CH), 69.91 (CH), 65.33 (CH2), 62.83 (CH2), 60.49 (CH2), 33.52 (CH2), 33.31 (CH2), 24.12 (CH2). FAB MS 295.30 m/z [M+H]+ (Theory: 294.30 m/z [M]+).
Example 33
Synthesis of adipic anhydride - Adipic acid (96.28 g, 0.6588 mol) and acetic anhydride (400 mL) were combined and refluxed at 160 0C for four hours. Afterwards, the acetic acid/anhydride was removed under vacuum. Next the depolymerization catalyst, zinc acetate monohydrate, was added along with a distillation apparatus and the heat was slowly increased. After 100 0C, nothing was collected until 200 0C when 68.79 g of a clear colorless liquid was collected (82.5 % yield). 1H NMR (400 MHz, CDCl3): δ 1.91 (m, 4, - CH2-CH2-CH2-CH2-), 2.67 (m, 4, -CH2-CH2-CH2-CH2-). 13C NMR (100.6 MHz, CDCl3): δ 168.38 (-COOCO-), 34.60 (CH2), 22.37 (CH2). GC-MS 128 m/z [M]+ (Theory: 128.12 m/z [M]+).
Example 34 Synthesis of 2-(«s-l,3-0-benzyIidene glycerol)adipic acid mono ester cώ-l,3-O-benzylideneglycerol (68.74 g, 0.5365 mol) was dissolved in pyridine (150 mL) followed by the addition of adipic anhydride (82.50 g, 0.4578 mol). The reaction mixture was stirred at room temperature for 18 hours before the pyridine was removed under vacuum at 35 0C. The remaining solid was dissolved in DCM (400 mL) and washed two times with 0.2 N HCl (400 mL), or until the aqueous phase remained at pH 1. The organic phase was evaporated and the solid was added to deionized water (300 mL). 1 N NaOH was added until pH 7 was obtained and the product was in the aqueous solution. The aqueous phase was washed with DCM (400 mL), to extract any remaining adipic
anhydride, and then readjusted to pH 4. The aqueous phase was subsequently extracted twice with DCM (400 mL), dried with Na2SO4, filtered, and evaporated to afford 67.53 g of a white powder (47.80 % yield). 1H NMR (400 MHz, CDCl3): δ 1.70 (m, 4, -CH2-CH2- CH2-CH2-), 2.35 (m, 2, -CH2-CH2-CH2-CH2-), 2.44 (m, 2, -CH2-CH2-CH2-CH2-), 4.13 (m, 2, -CH2-CH-CH2-), 4.25 (m, 2, -CH2-CH-CH2-), 4.67 (m, 1, -CH2-CH-CH2-), 5.53 (s, 1, CH), 7.33 (m, 3, arom. CH), 7.47 (m, 2, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 178.98 (COOH), 173.48 (COOR), 137.97 (CH), 129.30 (CH), 128.51 (CH), 126.22 (CH), 101.45 (CH), 69.28 (CH2), 66.13 (CH), 34.13 (CH2), 33.71 (CH2), 24.43 (CH2), 24.21 (CH2). FAB MS 309.1 m/z (MH+) (Theory: 308.33 m/z (M+)).
Example 35
Synthesis of [Gl]-PGLAA-bzld - First, 2-(cώ-l,3-0-benzylidene glycerol)adipic acid mono ester (7.226 g, 23.434 mmol), [GO]-PGLAA-OH (1.222 g, 4.152 mmol), and DPTS (2.830 g, 9.621 mmol) were dissolved in THF (100 mL) followed by the addition of DCC (4.32 g, 21.0 mmol). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon reaction completion, the DCC-urea was filtered and washed with a small amount of THF (50 mL). The crude product was purified by silica gel chromatography, eluting with 1/1 to 4/1 EtOAc :hexanes. The appropriate isolated fractions were concentrated, filtered (to remove any DCU), and directly precipitated in hexanes and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 5.99 g of a sticky solid (99.1 % yield). 1H NMR (400 MHz, CDCl3): δ 1.63 (m, 20, - CH2-CH2-CH2-CH2-), 2.32 (m, 12, -CH2-CH2-CH2-CH2-), 2.43 (m, 8, -CH2-CH2-CH2-CH2- ), 4.10 (m, 12, -CH2-CH-CH2-), 4.25 (m, 12, -CH2-CH-CH2-), 4.68 (m, 4, -CH2-CH-CH2-), 5.21 (m, 2, -CH2-CH-CH2-), 5.51 (s, 4, CH), 7.32 (m, 12, arom. CH), 7.47 (m, 8, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 173.40 (COOR), 172.87 (COOR), 172.55 (COOR), 138.02 (CH), 129.28 (CH), 128.49 (CH), 126.21 (CH), 101.39 (CH), 69.28 (CH2), 66.11 (CH), 62.39 (CH2), 34.08 (CH2), 33.90 (CH2), 33.75 (CH2), 24.37 (CH2). FAB MS 1455.6 m/z [M+H]+ (Theory: 1455.54 m/z [M]+).
Example 36
Synthesis of [Gl]-PGLAA-OH - Pd(OH)2/C (10% w/w) was added to a solution of [Gl]- PGLAA-bzld (4.870 g, 3.346 mmol) in THF (50 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The
catalyst was filtered and washed with THF solution (50 mL). The filtrate was evaporated to give 3.669 g of a clear viscous oil (99.5 % yield). 1H NMR (400 MHz, CD3OD): δ 1.63 (m, 20, -CH2-CH2-CH2-CH2-), 2.36 (m, 20, -CH2-CH2-CH2-CH2-), 3.52 (m, 2, -CH2-CH-CH2-), 3.59-3.69 (broad m, 12, -CH2-CH-CH2-), 3.79 (m, 1, -CH2-CH-CH2-), 4.03 (m, 1, -CH2- CH-CH2-), 4.14 (m, 5, -CH2-CH-CH2-), 4.32 (m, 4, -CH2-CH-CH2-), 5.24 (m, 2, -CH2-CH- CH2-). 13C NMR (100.6 MHz, CD3OD): δ 173.64 (COOR), 173.36 (COOR), 172.93 (COOR), 75.42 (CH), 69.93 (CH), 69.47 (CH), 65.36 (CH2), 62.87 (CH2), 62.15 (CH2), 60.50 (CH2), 33.49 (CH2), 33.35 (CH2), 33.20 (CH2), 24.11 (CH2). MALDI-TOF MS 1125.8 m/z [MR-Na]+ (Theory: 1103.11 m/z [M]+).
Example 37
Synthesis of [G2]-PGLAA-bzld - 2-(cώ-l,3-O-benzylidene glycerol)adipic acid mono ester (10.012 g, 32.472 mmol), [Gl]-PGLAA-OH (3.397 g, 3.079 mmol), and DPTS (2.508 g, 8.527 mmol) were dissolved in THF (100 mL) followed by the addition of DCC (4.62 g, 22.4 mmol). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon reaction completion, the DCC-urea was filtered and washed with a small amount of THF (50 mL). The crude product was purified by silica gel chromatography, eluting with 2% MeOH in DCM. The appropriate isolated fractions were concentrated, filtered (to remove any DCU), and directly precipitated in hexanes and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 9.39 g of a sticky wax (89.0 % yield). 1H NMR (400 MHz, CDCl3): δ 1.63 (m, 52, -CH2-CH2-CH2- CH2-), 2.31 (m, 36, -CH2-CH2-CH2-CH2-), 2.41 (m, 16, -CH2-CH2-CH2-CH2-), 4.05 (m, 28, -CH2-CH-CH2-), 4.25 (m, 28, -CH2-CH-CH2-), 4.67 (m, 8, -CH2-CH-CH2-), 5.21 (m, 6, - CH2-CH-CH2-), 5.51 (s, 8, CH), 7.33 (m, 24, arom. CH), 7.46 (m, 16, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 173.39 (COOR), 172.87 (COOR), 172.54 (COOR), 138.02 (CH), 129.27 (CH), 128.49 (CH), 126.21 (CH), 101.38 (CH), 69.27 (CH2), 66.11 (CH), 62.39 (CH2), 34.08 (CH2), 33.74 (CH2), 33.67 (CH2), 24.37 (CH2). MALDI MS 3449.2 m/z [M+Naf (Theory: 3425.61 m/z [M]+).
Example 38
Synthesis of [G2]-PGLAA-OH - Pd(OH)2/C (10% w/w) was added to a solution of [G2]- PGLAA-bzld (8.02 g, 2.34 mmol) in THF (100 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The catalyst was
filtered and washed with THF solution (50 mL). The filtrate was evaporated to give 6.360 g of a clear viscous oil (99.4 % yield). 1H NMR (400 MHz, CD3OD): δ 1.62 (m, 52, -CH2- CH2-CH2-CH2-), 2.35 (m, 52, -CH2-CH2-CH2-CH2-), 3.52 (m, 5, -CH2-CH-CH2-), 3.59- 3.71 (broad m, 25, -CH2-CH-CH2-), 3.79 (m, 3, -CH2-CH-CH2-), 4.03 (m, 3, -CH2-CH- CH2-), 4.14 (m, 15, -CH2-CH-CH2-), 4.33 (m, 12, -CH2-CH-CH2-), 5.25 (m, 6, -CH2-CH- CH2-). 13C NMR (100.6 MHz, CD3OD): δ 173.63 (COOR), 173.27 (COOR), 172.92 (COOR), 75.42 (CH), 69.94 (CH), 69.47 (CH), 65.38 (CH2), 62.89 (CH2), 62.17 (CH2), 60.52 (CH2), 33.51 (CH2), 33.39 (CH2), 33.22 (CH2), 24.12 (CH2). MALDI-TOF MS 2744.3 m/z [M+Naf (Theory: 2720.75 m/z [M]+).
Example 39
Synthesis of [G3]-PGLAA-bzld - 2-(cw-l,3-O-benzylidene glycerol)adipic acid mono ester (12.626 g, 40.950 mmol), [G2]-PGLAA-OH (5.263 g, 1.934 mmol), and DPTS (3.232 g, 10.989 mmol) were dissolved in THF (100 mL) followed by the addition of DCC (12.581 g, 60.975 mmol). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon reaction completion, the DCC-urea was filtered and washed with a small amount of THF (60 mL). The crude product was purified by silica gel chromatography, eluting with 1.5 to 3.0 % MeOH in DCM. The appropriate isolated fractions were concentrated, filtered (to remove any DCU), and directly precipitated in hexanes and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 12.22 g of a sticky wax (85.8 % yield). 1H NMR (400 MHz, CDCl3): δ 1.63 (broad m, 130, -CH2-CH2-CH2-CH2-), 2.31 (m, 90, -CH2-CH2-CH2-CH2-), 2.41 (m, 32, -CH2-CH2- CH2-CH2-), 4.10 (m, 62, -CH2-CH-CH2-), 4.24 (m, 62, -CH2-CH-CH2-), 4.67 (m, 16, -CH2- CH-CH2-), 5.19 (m, 14, -CH2-CH-CH2-), 5.51 (s, 16, CH), 7.32 (m, 48, arom. CH), 7.46 (m, 32, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 173.38 (COOR), 172.89 (COOR), 172.48 (COOR), 138.03 (CH), 129.27 (CH), 128.49 (CH), 126.21 (CH), 101.36 (CH), 69.26 (CH2), 66.11 (CH), 62.29 (CH2), 34.08 (CH2), 33.83 (CH2), 33.74 (CH2), 33.67 (CH2), 24.43 (CH2), 24.36 (CH2). MALDI-TOF MS 7390 m/z [M+Naf (Theory: 7365.73 m/z [M]+).
Example 40
Synthesis of IG3]-PGLAA-OH - Pd(OH)2/C (10% w/w) was added to a solution of [G3]- PGLAA-bzld (11.03 g, 1.497 mmol) in THF (125 mL). The flask for catalytic
hydrogenolysis was evacuated and filled with 60 psi Of H2 before shaking for 10 hours. The catalyst was filtered and washed with THF solution (75 mL). The filtrate was evaporated to give 8.69 g of a clear viscous oil (97.5 % yield). 1H NMR (400 MHz, CD3OD): δ 1.63 (m, 124, -CH2-CH2-CH2-CH2-), 2.35 (m, 127, -CEb-CH2-CH2-CH^-), 3.52 (m, 7, -CH2-CH- CH2-), 3.60-3.71 (broad m, 55, -CH2-CH-CH2-), 3.79 (m, 4, -CH2-CH-CH2-), 4.04 (m, 5, - CH2-CH-CH2-), 4.14 (m, 34, -CH2-CH-CH2-), 4.32 (m, 29, -CH2-CH-CH2-), 5.25 (m, 14, - CH2-CH-CH2-). 13C NMR (100.6 MHz, CD3OD): δ 173.82 (COOR)5 173.63 (COOR), 173.36 (COOR), 173.27 (COOR), 172.92 (COOR), 75.45 (CH), 75.40 (CH), 69.96 (CH), 69.48 (CH), 65.40 (CH2), 62.92 (CH2), 62.23 (CH2), 60.54 (CH2), 33.53 (CH2), 33.25 (CH2), 24.15 (CH2). MALDI-TOF MS 5975.0 ni/z [M+Na]+ (Theory: 5956.02 m/z [M]+).
Example 41
Synthesis of [GO]-PGLSA-[Gl]-PGLAA-bzld - 2-(cw-l,3-O-benzylidene glycerol)adipic acid mono ester (11.793 g, 38.248 mmol), [GO]-PGLSA-OH (1.185 g, 4.449 mmol), and DPTS (2.853 g, 9.700 mmol) were dissolved in THF (50 mL) followed by the addition of DCC (7.216 g, 34.973 mmol). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of THF (50 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 1/1 to 4/1 EtOAc:hexanes. The appropriate isolated fractions were concentrated, filtered (to remove any remaining DCU), and directly precipitated in hexanes and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 7.173 g of a sticky solid (97 % yield). 1H NMR (400 MHz, CDCl3): δ 1.65 (m, 16, -CH2-CH2-CH2-CH2-), 2.33 (m, 8, -CH2-CH2- CH2-CH2-), 2.42 (m, 8, -CH2-CH2-CH2-CH2-), 2.59 (m, 4, -CH2-CH2-), 4.11 (m, 12, -CH2- CH-CH2-), 4.24 (m, 12, -CH2-CH-CH2-), 4.67 (m, 4, -CH2-CH-CH2-), 5.20 (m, 2, -CH2- CH-CH2-), 5.51 (s, 4, CH), 7.33 (m, 12, arom. CH), 7.47 (m, 8, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 173.41 (COOR), 172.92 (COOR), 171.48 (COOR), 138.02 (CH), 129.28 (CH), 128.49 (CH), 126.21 (CH), 101.38 (CH), 69.65 (CH), 69.27 (CH2), 66.11 (CH), 62.19 (CH2), 34.09 (CH2), 33.73 (CH2), 28.97 (CH2), 24.44 (CH2), 24.36 (CH2). FAB MS 1425.5 m/z [M+H]+ (Theory: 1427.49 m/z [M]+). SEC Mw: 1670, Mn: 1650, PDI: 1.01.
Example 42
Synthesis of [G0]-PGLSA-[G1]-PGLAA-OH - Pd(OH)2/C (10% w/w) was added to a solution of [GO]-PGLSA-[Gl ]-PGLAA-bzld (5.900 g, 4.133 mmol) in THF (50 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The catalyst was filtered and washed with THF (50 mL). The filtrate was evaporated to give 4.407 g of a colorless, viscous oil (99 % yield). 1H NMR (400 MHz, CD3OD): δ 1.63 (m, 16, -CH2-CH2-CH2-CH2-), 2.36 (m, 16, -CH2-CH2-CH2-CH2-), 2.61 (m, 4, -CH2-CH2-), 3.52 (m, 3, -CH2-CH-CH2-), 3.59-3.65 (broad m, 9, -CH2-CH-CH2-), 3.69 (m, 2, -CH2-CH-CH2-), 3.79 (m, 2, -CH2-CH-CH2-), 4.03 (m, 2, -CH2-CH-CH2-), 4.15 (m, 5, -CH2-CH-CH2-), 4.30 (m, 4, -CH2-CH-CH2-), 5.25 (m, 2, -CH2-CH-CH2-). 13C NMR (100.6 MHz, CD3OD): δ 173.85 (COOR), 173.67 (COOR), 173.41 (COOR), 171.95 (COOR), 75.42 (CH), 69.93 (CH), 69.78 (CH), 65.36 (CH2), 62.87 (CH2), 62.04 (CH2), 60.50 (CH2), 33.50 (CH2), 33.29 (CH2), 33.19 (CH2), 28.61 (CH2), 24.12 (CH2). MALDI- TOF MS 1097.5 m/z [M+Na]+ (Theory: 1075.06 m/z [M]+). SEC Mw: 1680, Mn: 1660, PDI: 1.01.
Example 43
Synthesis of [G0]-PGLSA-[Gl]-PGLAA~[G2]-PGLSA-bzId - 2-(cώ-l,3-0-benzylidene glycerol)succinic acid mono ester (12.758 g, 45.520 mmol), [GO]-PGLSA-[G1]-PGLAA- OH (4.284 g, 3.984 mmol), and DPTS (5.112 g, 17.381 mmol) were dissolved in THF (100 mL) followed by the addition of DCC (13.912 g, 67.436 mmol). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of THF (50 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 2% MeOH in DCM. The appropriate isolated fractions were concentrated, filtered (to remove any remaining DCU), and directly precipitated in hexanes and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 10.84 g of a white solid (85.7 % yield). 1H NMR (400 MHz, CDCl3): δ 1.60 (m, 17, -CH2-CH2-CH2-CH2-), 2.30 (m, 17, -CH2-CH2-CH2-CH2-), 2.63 (m, 20, -CH2-CH2-), 2.72 (m, 16, -CH2-CH2-), 4.11 (m, 29, -CH2-CH-CH2-), 4.23 (m, 29, -CH2-CH-CH2-), 4.70 (m, 8, -CH2-CH-CH2-), 5.20 (m, 6, - CH2-CH-CH2-), 5.51 (s, 8, CH), 7.34 (m, 12, arom. CH), 7.46 (m, 8, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 173.41 (COOR), 172.92 (COOR), 171.48 (COOR), 138.02 (CH), 129.28 (CH), 128.49 (CH), 126.21 (CH), 101.38 (CH), 69.65 (CH), 69.27 (CH2), 66.11
(CH), 62.19 (CH2), 34.09 (CH2), 33.73 (CH2), 28.97 (CH2), 24.44 (CH2), 24.36 (CH2). MALDI-TOF MS 3172.7 m/z [M+Naf (Theory: 3173.13 m/z [M]+). SEC Mw: 360O5 Mn: 3540, PDI: 1.02.
Example 44
Synthesis of [GO]-PGLSA-[GI]-PGLAA-[GI]-PGLSA-OH - Pd(OH)2/C (10% w/w) was added to a solution of [GO]-PGLSA-[G l]-PGLAA-[G2]-PGLSA-bzld (5.251 g, 1.655 mmol) in THF (100 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The catalyst was filtered and washed with THF (50 mL). The filtrate was evaporated to give 4.011 g of a colorless, viscous oil (98.2 % yield). 1H NMR (400 MHz, CD3OD): δ 1.62 (m, 17, -CH2-CH2-CH2-CH2-), 2.36 (m, 17, -CH2-CH2-CH2-CH2-), 2.64 (m, 36, -CH2-CH2-), 3.52 (m, 2, -CH2-CH-CH2-), 3.60-3.66 (broad m, 26, -CH2-CH-CH2-), 3.69 (m, 9, -CH2-CH-CH2-), 3.80 (m, 1, -CH2-CH-CH2-), 4.18 (m, 14, -CH2-CH-CH2-), 4.32 (m, 12, -CH2-CH-CH2-), 5.25 (m, 6, -CH2-CH-CH2-). 13C NMR (100.6 MHz, CD3OD): δ 173.38 (COOR), 173.05 (COOR), 172.56 (COOR), 172.24 (COOR), 172.00 (COOR), 75.81 (CH), 69.80 (CH), 69.35 (CH), 67.65 (CH2), 65.68 (CH2), 62.87 (CH2), 62.42 (CH2), 62.11 (CH2), 60.43 (CH2), 33.49 (CH2), 33.20 (CH2), 28.83 (CH2), 28.64 (CH2), 25.28 (CH2), 24.09 (CH2). MALDI-TOF MS 2492.0 m/z [M+Na]+ (Theory: 2468.27 m/z [M]+). SEC Mw: 3390, Mn: 3340, PDI: 1.02.
Example 45
Synthesis of [GO]-PGLSA-[Gl]-PGLAA-[Gl]-PGLSA-[GS]-PGLAA^zId - 2-(αs-l,3- O-benzylidene glycerol)adipic acid mono ester (10.751 g, 34.869 mmol), [GO]-PGLSA- [G1]-PGLAA-[G2]-PGLSA-OH (3.771 g, 1.528 mmol), and DPTS (1.463 g, 4.975 mmol) were dissolved in THF (120 mL) followed by the addition of DCC (10.598 g, 51.365 mmol). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of THF (50 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 1.5% MeOH in DCM. The appropriate isolated fractions were concentrated, filtered (to remove any remaining DCU), and directly precipitated in hexanes and cooled to -20 0C overnight. The hexanes were decanted and the precipitate was isolated to yield 9.88 g of a sticky solid (90.9 % yield). 1H NMR (400 MHz, CDCl3): δ 1.65 (m, 81, -CH2-CH2-CH2-CH2-), 2.31 (m, 52, -CH2-CH2-CH2-CH2-), 2.42 (m, 32, -CH2- CH2-CH2-CH2-), 2.58 (m, 36 -CH2-CH2-), 4.10 (m, 62, -CH2-CH-CH2-), 4.23 (m, 62, -CH2-
CH-CH2-), 4.66 (m, 16, -CH2-CH-CH2-), 5.19 (m, 14, -CH2-CH-CH2-), 5.51 (s, 16, CH), 7.33 (m, 47, arom. CH), 7.46 (m, 32, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 173.39 (COOR), 172.90 (COOR)3 171.82 (COOR), 171.53 (COOR), 138.04 (CH), 129.26 (CH), 128.49 (CH), 126.22 (CH)5 101.36 (CH), 69.65 (CH), 69.26 (CH2), 66.11 (CH), 62.64 (CH2), 62.15 (CH2), 34.07 (CH2), 33.73 (CH2), 28.96 (CH2), 28.80 (CH2), 24.43 (CH2), 24.35 (CH2). MALDI-TOF MS 7137.3 m/z [M+Na]+ (Theory: 7113.25 m/z [M]+). SEC Mw: 7160, Mn: 7060, PDI: 1.01.
Example 46
Synthesis of [GO]-PGLSA-[GI]-PGLAA-[GI]-PGLSA-[GS]-PGLAA-OH - Pd(OH)2/C (10% w/w) was added to a solution of [GO]-PGLSA-[G1]-PGLAA-[G2]-PGLSA-[G3]- PGLAA-bzld (9.175 g, 1.290 mmol) in THF (100 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi OfH2 before shaking for 10 hours. The catalyst was filtered and washed with THF (50 mL). The filtrate was evaporated to give 7.218 g of a colorless, viscous oil (98.1 % yield). 1H NMR (400 MHz, CD3OD): δ 1.63 (m, 83, -CH2-CH2-CH2-CH2-), 2.37 (m, 83, -CH2-CH2-CH2-CH2-), 2.61 (m, 36, -CH2-CH2-), 3.52 (m, 8, -CH2-CH-CH2-), 3.60-3.71 (broad m, 57, -CH2-CH-CH2-), 3.80 (m, 4, -CH2- CH-CH2-), 4.03 (m, 5, -CH2-CH-CH2-), 4.11-4.23 (m, 34, -CH2-CH-CH2-), 4.30 (m, 29, - CH2-CH-CH2-), 5.25 (m, 14, -CH2-CH-CH2-). 13C NMR (100.6 MHz, CD3OD): δ 173.85 (COOR), 173.67 (COOR), 173.41 (COOR), 171.95 (COOR), 75.42 (CH), 69.93 (CH), 69.78 (CH), 65.36 (CH2), 62.87 (CH2), 62.04 (CH2), 60.50 (CH2), 33.50 (CH2), 33.29 (CH2), 33.19 (CH2), 28.61 (CH2), 24.12 (CH2). MALDI-TOF MS 5730.3 m/z [M+Naf (Theory: 5703.54 m/z [M]+). SEC Mw: 6570, Mn: 6490, PDI: 1.01.
Example 47
Synthesis of [GO]-PGLSA-[G1]-PGLAA-[G2]-PGLSA-[G3]-PGLAA-[G4]-PGLSA- bzld - 2~(cz5-l,3-O-benzylidene glycerol)succinic acid mono ester (11.572 g, 41.286 mmol), [GO]-PGLSA-[GI]-PGLAA-^]-PGLSA-[GS]-PGLAA-OH (5.593 g, 0.981 mmol), and DPTS (4.094 g, 13.919 mmol) were dissolved in THF (80 mL) followed by the addition of DCC (12.596 g, 61.048 mmol). The reaction was stirred at room temperature for 14 hours under nitrogen atmosphere. Upon completion, the DCC-urea was filtered and washed with a small amount of THF (50 mL) and the solvent was evaporated. The crude product was purified by silica gel chromatography, eluting with 1.5% to 5.0% MeOH in
DCM. The appropriate isolated fractions were concentrated, filtered (to remove any remaining DCU), and directly precipitated in hexanes and cooled to -20 0C over 48 hours. The hexanes were decanted and the precipitate was isolated to yield 11.5O g of a white solid (83.2 % yield). 1H NMR (400 MHz, CDCl3): δ 1.59 (m, 83, -CH2-CH2-CH2-CH2-), 2.30 (m, 83, -CH2-CH2-CH2-CH2-), 2.62 (m, 104, -CH2-CH2-), 2.70 (m, 63, -CH2-CH2-), 4.12 (m, 130, -CH2-CH-CH2-), 4.22 (m, 130, -CH2-CH-CH2-), 4.68 (m, 32, -CH2-CH-CH2-), 5.18 (m, 30, -CH2-CH-CH2-), 5.50 (s, 32, CH), 7.33 (m, 97, arom. CH), 7.46 (m, 66, arom. CH). 13C NMR (100.6 MHz, CDCl3): δ 172.88 (COOR), 172.53 (COOR), 172.25 (COOR), 171.89 (COOR), 138.04 (CH), 129.26 (CH), 128.48 (CH), 126.22 (CH), 101.28 (CH), 69.14 (CH2), 66.54 (CH), 62.60 (CH2), 33.81 (CH2), 33.66 (CH2), 29.35 (CH2), 29.03 (CH2), 24.30 (CH2). SEC Mw: 10440, Mn: 10290, PDI: 1.02.
Example 48 Synthesis of [GO]-PGLSA-[G1]-PGLAA-[G2]-PGLSA-[G3]-PGLAA-[G4]-PGLSA-
OH - Pd(OH)2/C (10% w/w) was added to a solution of [GO]-PGLSA-[G1]-PGLAA-[G2]- PGLSA-[G3]-PGLAA-[G4]-PGLSA-bzld (2.084 g, 0.1478 mmol) in THF (80 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 10 hours. The catalyst was filtered and washed with THF (75 mL). The filtrate was evaporated to give 1.652 g of a colorless, viscous oil (99.1 % yield). 1H NMR (400 MHz, CD3OD): δ 1.62 (m, 80, -CH2-CH2-CH2-CH2-), 2.37 (m, 80, -CH2-CH2-CH2-CH2-), 2.64 (m, 164, -CH2-CH2-), 3.52 (m, 12, -CH2-CH-CH2-), 3.63-3.71 (broad m, 160, -CH2-CH- CH2-), 3.80 (m, 6, -CH2-CH-CH2-), 4.06 (m, 14, -CH2-CH-CH2-), 4.20 (m, 62, -CH2-CH- CH2-), 4.30 (m, 60, -CH2-CH-CH2-), 5.25 (m, 30, -CH2-CH-CH2-). 13C NMR (100.6 MHz, CD3OD): δ 173.40 (COOR), 173.06 (COOR), 172.58 (COOR), 75.82 (CH), 69.90 (CH), 69.34 (CH), 67.64 (CH2), 62.45 (CH2), 62.15 (CH2), 60.46 (CH2), 33.25 (CH2), 28.87 (CH2), 28.67 (CH2), 25.27 (CH2), 24.12 (CH2). MALDI-TOF MS 11299.1 m/z [M+Na]+ (Theory: 11276.39 m/z [M]+). SEC Mw: 9150, Mn: 9000, PDI: 1.02.
Example 49
Synthesis of PEG-([G0]-PGLSA-bzId)2 - This example is shown for PEG of 3400 Mw5 but we have also used PEG of 10,000 and 20,000 Mw. PEG, Mn=3400, (10.0 g, 2.94 mmol), which was dried under vacuum at 120 0C for three hours, and [2-(cis-l,3-O-
benzylidene glycero^-N-succinimidyl] succinate (4.03 g, 10.7 mmol) were dissolved in CH2Cl2 (100 mL) and stirred under nitrogen. TEA (2.0 mL, 14 mmol) was added by syringe and stirring was continued for 14 hours. Any remaining activated ester was quenched by the addition of fresh TEA (1.0 mL, 7.2 mmol) and n-propanol (1.0 mL, 11 mmol), which was allowed to stir for another 10 hours. After removing most of the solvent, the product was precipitated in cold ethyl ether (700 mL) and collected to yield 11.1 g of a white solid (97 % yield). 1H NMR obtained. Elemental Analysis C: 55.31 %; H 8.58 % (Theory C: 55.56 %; H 8.66 %). MALDI MS Mw: 4020, Mn: 3940, PDI: 1.02. SEC Mw: 3980, Mn: 3950, PDI: 1.03.
Example 50
Synthesis of PEG-([G0]-PGLSA-OH)2 - Pd/C (10 % w/w) was added to a solution of PEG-([G0]-PGLSA-bzld)2 (5.07 g, 1.29 mmol) in 80 mL of 9:1 ethyl acetate/methanol. The apparatus for catalytic hydrogenolysis was evacuated and filled with 50 psi of H2 before shaking for 8 hours. The catalyst was filtered off and washed with ethyl acetate (20 mL). The filtrate was evaporated and the remaining white solid was redissolved in a minimal amount of CH2Cl2 (15 mL)and precipitated in cold ethyl ether (600 mL) to give 4.52 g of a white solid (93 % yield). 1H NMR obtained. Elemental Analysis C: 53.49 %; H 8.78 % (Theory C: 53.69 %; H 8.85 %). MALDI MS Mw: 3780, Mn: 3730, PDI: 1.01. SEC Mw: 3860, Mn: 3710, PDI: 1.021.
Example 51
Synthesis of PEG-([Gl]-PGLSA-bzld)2 - PEG-([G0]-PGLSA-OH)2 (5.81 g, 1.55 mmol), which was dried under vacuum at 80 0C for three hours, and [2-(czs-l,3-0-benzylidene glycerol)-N-succinimidyl] succinate (4.35 g, 11.5 mmol) were dissolved in CH2Cl2 (70 mL) and stirred under nitrogen. TEA (1.75 mL, 13.0 mmol) was added by syringe and stirring was continued for 14 hours. Any remaining activated ester was quenched by the addition of fresh TEA (1.0 mL, 7.2 mmol) and n-propanol (1.0 mL, 11 mmol), which was allowed to stir for another 10 hours. After removing most of the solvent, the product was precipitated in cold ethyl ether (700 mL) and collected to yield 7.15 g (96 % yield). 1H NMR obtained. MALDI MS Mw: 4520, Mn: 4480, PDI: 1.01. SEC Mw: 4420, Mn: 4240, PDI: 1.04.
Example 52
Synthesis of PEG-([G1]-PGLSA-OH)2 - Pd/C (10 % w/w) was added to a solution of PEG-([Gl]-PGLSA-bzld)2 (5.53 g, 1.15 mmol) in 80 niL of 9:1 ethyl acetate/methanol. The apparatus for catalytic hydrogenolysis was evacuated and filled with 50 psi of H2 before shaking for 8 hours. The catalyst was filtered off and washed with ethyl acetate (20 mL). The filtrate was evaporated and the remaining white solid was redissolved in a minimal amount of CH2Cl2 (15 mL) and precipitated in cold ethyl ether (700 mL) to give 4.71 g of a white solid (92 % yield). 1H NMR obtained. MALDI MS Mw: 4320, Mn: 4280, PDI: 1.01. SEC Mw: 4390, Mn: 4230, PDI: 1.04.
Example 53
Synthesis of PEG-([G1]-PGLSA-MA)2 - PEG-([G1]-PGLS A-OH)2 (1.03 g, 0.232 mmol), which was dried under vacuum at 80 0C for three hours, was dissolved in CH2Cl2 (40 mL) and stirred under nitrogen before the addition of methacryloyl chloride (1.93 g, 5.12 mmol). TEA (0.80 mL, 5.74 mmol) was added by syringe and stirring was continued for 14 hours. The mixture was diluted with more CH2Cl2 (60 mL) and washed twice with 0.1 N HCl (100 mL). After drying with Na2SO4, filtering, and removing most of the solvent, the product was precipitated in cold ethyl ether and collected to yield 1.08 g (94 % yield). 1H NMR obtained. SEC Mw: 4610, Mn: 4420, PDI: 1.04.
Example 54
Synthesis of PEG-([G2]-PGLSA-bzld)2 - PEG-([G1]-PGLSA-OH)2 (0.697 g, 0.150 mmol), which was dried under vacuum at 80 0C for three hours, and [2-(cώ-l,3-<9- benzylidene glyceroty-N-succinimidyl] succinate (1.01 g, 2.68 mmol) were dissolved in CH2Cl2 (30 mL) and stirred under nitrogen. TEA (0.50 mL, 3.59 mmol) was added by syringe and stirring was continued for 14 hours. Any remaining activated ester was quenched by the addition of fresh TEA (1.0 mL, 7.2 mmol) and n-propanol (1.0 mL, 11 mmol), which was allowed to stir for another 10 hours. After removing most of the solvent, the product was precipitated in cold ethyl ether (400 mL) and collected to yield 0.940 g (93 % yield). 1H NMR obtained.
Example 55
Synthesis of ([GI]-PGLSA-MA)2-PEG - ([G1]-PGLSA-OH)2-PEG (0.500 g, 0.113 mmol) was dissolved in DCM (15 mL) and stirred under nitrogen before methacrylic anhydride (0.56 mL, 3.76 mmol) was added by syringe. DMAP (86.0 mg, 0.704 mmol) was added and stirring was continued for 14 hours. Any remaining anhydride was quenched by the addition of methanol (0.1 mL, 3.95 mmol), which was allowed to stir for another 5 hours. The reaction was diluted with DCM (35 mL) and washed with 0.1 N HCl (50 mL) and brine (50 mL). The organic phase was dried with Na2SO4 and filtered before the PEG-based dendrimer was precipitated in cold (-20 0C) ethyl ether (300 mL) and collected to yield 0.519 g of a white solid (93 % yield). 1H NMR (CDCl3): δ 1.90 (m, 19, -CH3), 2.61 (m, 21, -CH2-CH2-), 3.42 (t, 2, -CH2-CH2-), 3.55-3.65 (broad m, 285, -CH2-CH2-), 3.77 (t, 2, -CH2- CH2-), 4.09-4.37 (broad m, 29, -CH2-CH-CH2-), 5.22 (m, 2, -CH2-CH-CH2-), 5.35 (m, 2, - CH2-CH-CH2-), 5.57 (m, 6, CH), 6.07 (m, 6, CH). 13C NMR (CDCl3): δ 171.89 (COOR)5 135.84 (CH), 126.64 (CH), 70.75 (CH2), 69.45 (CH), 62.61 (CH2), 28.87 (CH2), 18.43 (CH3). FTIR: v (cm"1) 2873 (aliph. C-H stretch), 1736 (C=O). MALDI MS Mw: 5012, Mn: 4897, PDI: 1.02. SEC Mw: 3910, Mn: 3740, PDI: 1.04. Tm = 40.8.
Example 56
Synthesis of ([G2]-PGLSA-bzld)2-PEG - ([G1]-PGLSA-OH)2-PEG (3.25 g, 0.737 mmol), and 2-(ezs-l,3-O-benzylidene glycerol)succinic acid mono ester anhydride (12.68 g, 23.37 mmol) were dissolved in DCM (50 mL) and stirred under nitrogen. DMAP (0.588 g, 4.81 mmol) was added and stirring was continued for 14 hours. Any remaining anhydride was quenched by the addition of n-propanol (2.5 mL, 28 mmol), which was allowed to stir for another 5 hours. The reaction was diluted with DCM (50 mL) and washed with 0.1 N HCl (100 mL), saturated sodium bicarbonate (100 mL 3x), and brine (100 mL). The organic phase was dried with Na2SO4, filtered, and concentrated before the PEG-based dendrimer was precipitated in cold (-20 0C) ethyl ether (400 mL) and collected to yield 4.57 g of a white solid (91 % yield). 1H NMR (CDCl3): δ 2.61 (broad m, 4O5 -CH2-CH2-), 2.72 (broad m, 16, -CH2-CH2-), 3.43 (t, 2, -CH2-CH2-), 3.55-3.65 (broad m, 280, -CH2-CH2-), 3.77 (t, 2, -CH2-CH2-), 4.13 (broad m, 28, -CH2-CH-CH2-), 4.22 (broad m5 28, -CH2-CH- CH2-), 4.69 (m, 8, -CH2-CH-CH2-), 5.20 (m, 6, -CH2-CH-CH2-), 5.50 (s, S5 CH)5 7.32 (m, 24, arom. CH), 7.46 (m, 16, arom. CH). 13C NMR (CDCl3): δ 172.28 (COOR)5 171.91 (COOR), 171.57 (COOR), 138.01 (CH)5 129.26 (CH), 128.48 (CH), 126.21 (CH), 101.33
23S
(CH), 70.56 (CH2), 69.50 (CH), 69.16 (CH2), 66.53 (CH), 64.08 (CH2), 29.49 (CH2), 29.21 (CH2). FTIR: v (cm'1) 2879(aliph. C-H stretch), 1736 (C=O). MALDI MS Mw: 6642, Mn: 6492, PDI: 1.02. SEC Mw: 4860, Mn: 4680, PDI: 1.04. Tm = 31.4.
Example 57
Synthesis of ([G2]-PGLSA-OH)2-PEG - Pd(OH)2/C (10 % w/w) was added to a solution of ([G2]-PGLSA-bzld)2-PEG (3.26 g, 0.500 mmol) in 25 mL of 2:1 DCM/methanol. The apparatus for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 8 hours. The catalyst was filtered off and washed with DCM (20 mL). The PEG-based dendrimer was isolated after evaporation of solvents to give 2.86 g of a white solid (98 % yield). 1H NMR (CDCl3): δ 2.63 (broad m, 56, -CH2-CH2-), 3.42 (s, 4, -CH2- CH2-), 3.50-3.67 (broad m, 285, -CH2-CH2-), 3.72 (broad m, 27, -CH2-CH-CH2-), 4.14-4.29 (broad m, 32, -CH2-CH-CH2-), 4.88 (m, 8, -CH2-CH-CH2-), 5.22 (m, 6, -CH2-CH-CH2-). 13C NMR (CDCl3): δ 172.56 (COOR), 172.32 (COOR), 76.01 (CH), 70.78 (CH2), 69.56 (CH), 69.22 (CH2), 64.14 (CH2), 63.52 (CH2), 62.60 (CH2), 61.93 (CH2), 29.44 (CH2), 29.21 (CH2), 28.98 (CH2). FTIR: v (cm-1) 3452 (OH), 288. (aliph. C-H stretch), 1735 (C=O). MALDI MS Mw: 5910, Mn: 5788, PDI: 1.02. SEC Mw: 5340, Mn: 5210, PDI: 1.03. Tm = 36.5.
Example 58
Synthesis of ([G2]-PGLSA-MA)2-PEG - ([G2] -PGLSA-OH)2-PEG (0.501 g, 0.0863 mmol) was dissolved in DCM (15 mL) and stirred under nitrogen before methacrylic anhydride (0.50 mL, 3.36 mmol) was added by syringe. DMAP (72.1 mg, 0.990 mmol) was added and stirring was continued for 14 hours. Any remaining anhydride was quenched by the addition of methanol (0.1 mL, 3.95 mmol), which was allowed to stir for another 5 hours. The reaction was diluted with DCM (35 mL) and washed with 0.1 N HCl (50 mL) and brine (50 mL). The organic phase was dried with Na2SO4 and filtered before the PEG-based dendrimer was precipitated in cold (-20 0C) ethyl ether (300 mL) and collected to yield 0.534 g of a white solid (90 % yield). 1H NMR (CDCl3): δ 1.89 (m, 47, - CH3), 2.60 (m, 65, -CH2-CH2-), 3.56-3.67 (broad m, 387, -CH2-CH2-), 3.77 (t, 2, -CH2- CH2-), 4.12-4.37 (broad m, 81, -CH2-CH-CH2-), 5.21 (m, 13, -CH2-CH-CH2-), 5.33 (m, 7, - CH2-CH-CH2-), 5.56 (m, 16, CH), 6.06 (m, 16, CH). 13C NMR (CDCl3): δ 171.89 (COOR), 135.84 (CH), 126.64 (CH), 70.75 (CH2), 69.45 (CH), 62.61 (CH2), 28.87 (CH2),
18.43 (CH3). Fim: v (cm"1) 2873 (aliph. C-H stretch), 1736 (C=O). %). MALDI MS Mw: 6956, Mn: 6792, PDI: 1.02. SEC Mw: 4580, Mn: 4390, PDI: 1.04. Tm = 27.0.
Example 59
Synthesis of ([G3]-PGLSA-bzld)2-PEG - ([G2] -PGLSA-OH)2-PEG (2.13 g, 0.367 mmol), and 2-(c/s-l,3-O-ben2ylidene glycerol)succinic acid mono ester anhydride (12.71 g, 23.43 mmol) were dissolved in DCM (45 niL) and stirred under nitrogen. DMAP (0.608 g, 4.98 mmol) was added and stirring was continued for 14 hours. Any remaining anhydride was quenched by the addition of n-proρanol (2.0 mL, 22 mmol), which was allowed to stir for another 5 hours. The reaction was diluted with DCM (55 mL) and washed with 0.1 N HCl (100 mL), saturated sodium bicarbonate (100 mL 3x), and brine (100 mL). The organic phase was dried with Na2SO4, filtered, and concentrated before the PEG-based dendrimer was precipitated in cold (-20 0C) ethyl ether (400 mL) overnight and collected to yield 3.35 g of a white solid (92 % yield). 1H NMR (CDCl3): δ 2.61 (broad m, 84, -CH2-CH2-), 2.74 (broad m, 36, -CH2-CH2-), 3.43 (t, 2, -CH2-CH2-), 3.56-3.65 (broad m, 278, -CH2-CH2-), 3.78 (t, 2, -CH2-CH2-), 4.13 (broad m, 60, -CH2-CH-CH2-), 4.21 (broad m, 60, -CH2-CH- CH2-), 4.69 (m, 16, -CH2-CH-CH2-), 5.19 (m, 14, -CH2-CH-CH2-), 5.50 (s, 16, CH), 7.32 (m, 46, arom. CH), 7.46 (m, 30, arom. CH). 13C NMR (CDCl3): δ 172.28 (COOR), 171.91 (COOR), 138.03 (CH), 129.26 (CH), 128.48 (CH), 126.21 (CH), 101.31 (CH), 70.76 (CH2), 69.49 (CH), 69.16 (CH2), 66.53 (CH), 62.47 (CH2), 29.35 (CH2), 29.02 (CH2), 28.83 (CH2). FTIR: v (cm-1) 2868 (aliph. C-H stretch), 1735 (C=O). MALDI MS Mw: 10215, Mn: 9985, PDI: 1.02. SEC Mw: 7020, Mn: 6900, PDI: 1.02. Tg = -13.6.
Example 60
Synthesis of ([G31-PGLSA-OH)2-PEG - Pd(OH)2/C (10 % w/w) was added to a solution of ([G3]-PGLSA-bzld)2-PEG (2.88 g, 0.288 mmol) in 30 mL of 2: 1 DCM/methanol. The apparatus for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 8 hours. The catalyst was filtered off and washed with DCM (20 mL). The PEG-based dendrimer was isolated after evaporation of solvents to give 2.86 g of a white solid (98 % yield). 1H NMR ((CD3)2CO):δ 2.64 (broad m, 120, -CH2-CH2-), 3.49-3.60 (broad m, 286, -CH2-CH2-), 3.64-3.75 (broad m, 33, -CH2-CH-CH2-), 4.00-4.12 (broad m, 42, -CH2-CH-CH2-), 4.13-4.29 (broad m, 68, -CH2-CH-CH2-), 4.64 (t, 2, -CH2-CH-CH2-), 4.85 (t, 2, -CH2-CH-CH2-), 5.26 (m, 14, -CH2-CH-CH2-). 13C NMR ((CD3)2CO): δ 171.85 (COOR), 171.64 (COOR), 76.09 (CH), 73.70 (CH2), 70.56 (CH), 69.52 (CH2), 66.19 (CH),
63.87 (CH2), 62.31 (CH2), 61.65 (CH2), 60.69 (CH2). FTIR: v (cm"1) 3432 (OH), 2925 (aliph. C-H stretch), 1734 (C=O). MALDI MS Mw: 8765, Mn: 8575, PDI: 1.02. SEC Mw: 8090, Mn: 7820, PDI: 1.03. Tg = -38.2.
Example 61
Synthesis of ([G3] -PGLSA-MA)2-PEG - ([G3]-PGLSA-OH)2-PEG (0.223 g, 0.0260 mmol) was dissolved in THF (15 niL) and stirred under nitrogen before methacrylic anhydride (1.10 niL, 7.38 mmol) was added by syringe. DMAP (90.0 mg, 0.737 mmol) was added and stirring was continued for 14 hours. Any remaining anhydride was quenched by the addition of methanol (0.2 mL, 7.89 mmol), which was allowed to stir for i another 5 hours. The reaction was diluted with DCM (35 mL) and washed with 0.1 N HCl (50 mL) and brine (50 mL). The organic phase was dried with Na2SO4 and filtered before the PEG-based dendrimer was precipitated in cold (-20 0C) ethyl ether (300 mL) and collected to yield 0.248 g of a white solid (89 % yield). 1H NMR (CDCl3): δ 1.90 (m, 76, - CH3), 2.62 (m, 111, -CH2-CH2-), 3.56-3.67 (broad m, 285, -CH2-CH2-), 4.14-4.38 (broad m, 114, -CH2-CH-CH2-), 5.23 (m, 13, -CH2-CH-CH2-), 5.35 (m, 10, -CH2-CH-CH2-), 5.56 (m, 25, CH), 6.07 (m, 25, CH). 13C NMR (CDCl3): δ 171.87 (COOR), 135.91 (CH), 126.71 (CH), 70.76 (CH2), 69.47 (CH), 62.62 (CH2), 28.88 (CH2), 18.43 (CH3). FTIR: v (cnT1) 2874 (aliph. C-H stretch), 1734 (C=O). MALDI MS Mw: 10722, Mn: 10498, PDI: 1.02. SEC Mw: 7000, Mn: 6820, PDI: 1.03. Tg = -37.9.
Example 62
Synthesis of ([G4]-PGLSA-bzId)2-PEG - ([G3]-PGLSA-OH)2-PEG (1.82 g, 0.212 mmol), and 2-(cw-l,3-O-benzylidene glycerol)succinic acid mono ester anhydride (15.93 g, 29.36 mmol) were dissolved in THF (50 mL) and stirred under nitrogen. DMAP (0.537 g, 4.40 mmol) was added and stirring was continued for 14 hours. Any remaining anhydride was quenched by the addition of n-propanol (2.5 mL, 28 mmol), which was allowed to stir for another 5 hours. The reaction was diluted with DCM (50 mL) and washed with 0.1 N HCl (100 mL), saturated sodium bicarbonate (100 mL 3x), and brine (100 mL). The organic phase was dried with Na2SO4, filtered, and concentrated before the PEG-based dendrimer was precipitated in ethyl ether (400 mL) and collected to yield 3.11 g of a white solid (87 % yield). 1H NMR (CDCl3): δ 2.61 (broad m, 180, -CH2-CH2-), 2.70 (broad m, 64, -CH2-CH2-), 3.43 (t, 2, -CH2-CH2-), 3.56-3.65 (broad m, 286, -CH2-CH2-), 3.78 (t, 2, - CH2-CH2-), 4.11 (broad m, 125, -CH2-CH-CH2-), 4.23 (broad m, 125, -CH2-CH-CH2-),
4.68 (m, 32, -CH2-CH-CH2-), 5.20 (m, 30, -CH2-CH-CH2-), 5.49 (s, 32, CH), 7.32 (m, 93, arom. CH), 7,46 (m, 62, arom. CH). 13C NMR (CDCl3): δ 172.28 (COOR), 171.90 (COOR), 171.60 (COOR), 138.04 (CH), 129.26 (CH), 128.48 (CH), 126.21 (CH), 101.29 (CH), 70.76 (CH2), 69.46 (CH), 69.15 (CH2), 66.53 (CH), 62.57 (CH2), 29.34 (CH2), 29.18 (CH2), 29.02 (CH2), 28.83 (CH2). FTIR: v (cm'1) 2865 (aliph. C-H stretch), 1734 (C=O). MALDI MS Mw: 17289, Mn: 16968, PDI: 1.02. SEC Mw: 8110, Mn: 7950, PDI: 1.02. Tg = 5.3.
Example 63
Synthesis of ([G4]-PGLSA-OH)2-PEG - Pd(OH)2/C (10 % w/w) was added to a solution of ([G4]-PGLSA-bzld)2-PEG (2.88 g, 0.170 mmol) in 30 mL of 2:1 DCM/methanol. The apparatus for catalytic hydrogenolysis was evacuated and filled with 60 psi of H2 before shaking for 8 hours. The catalyst was filtered off and washed with DCM (20 mL). The PEG-based dendrimer was isolated after evaporation of solvents to give 2.86 g of a white solid (98 % yield). 1H NMR ((CD3)2CO):δ 2.64 (broad m, 248, -CH2-CH2-), 3.49-3.60 (broad m, 296, -CH2-CH2-), 3.66 (broad m, 50, -CH2-CH-CH2-), 3.82 (broad m, 42, -CH2- CH-CH2-), 4.04-4.16 (broad m, 66, -CH2-CH-CH2-), 4.28 (broad m, 124, -CH2-CH-CH2-), 4.86 (m, 10, -CH2-CH-CH2-), 5.27 (m, 30, -CH2-CH-CH2-). 13C NMR ((CD3)2CO): δ 172.20 (COOR), 70.45 (CH2), 70.10 (CH), 69.92 (CH2), 65.96 (CH), 62.31 (CH2). FTIR: v (cm"1) 3445 (OH), 2931 (aliph. C-H stretch), 1713 (C-O). MALDI MS Mw: 14402, Mn: 14146, PDI: 1.02. SEC Mw: 9130, Mn: 8980, PDI: 1.02. Tg = -18.0.
Example 64 Synthesis of bzld-[Gl]-PGLSA-TBDPS
4.00 g (0.014 mol) of bzld-[G I]-PGLSA-CO2H and 3.24 g (0.048 mol) of imidazole were stirred in 15 mL of DMF. Next, 6.4 mL (0.024 mol) of diphenyl-t-butyl silyl chloride were added and the reaction was stirred at 25 0C for 48 hours. The DMF was removed, the product was dissolved in CH2Cl2, washed with sat. NaHCO3 and water, dried over Na2SO4, filtered, rotoevaporated, and dried on the vacuum line. The product was purified by column chromatography (4:1 hexanes:EtOAc) affording 6.38 g of product as a viscous opaque oil (86% yield). Rf = 0.13 in 4:1 hexanes:EtOAc. 1H NMR (CDCI3): δ 1.09 (s, 9H, t-butyl), 2.78-2.84 (m, 4H, -CH2-CH2), 4.11-4.15 (m, 2Η, -CH2-CH-CH2-), 4.23-4.26 (m, 2Η, -CH2- CH-CH2-), 4.70-4.71 (m, 1Η, -CH2-CH-CH2-), 5.54 (s, IH, CH), 7.33-7.42, 7.48-7.50,
7.67-7.68 (m, 15H, arom. bzld and phenyl CH) ppm. 13C NMR (CDCl3): δ 19.34 (-C- (CΗ3)3), 27.07 (-C-(CH3)S), 29.72, 30.96 (succ. -CH2-), 66.46, 69.18 (glycerol, 2C, -CH2-), 101.39 (O-CH-O), 126.23, 127.94, 128.50, 129.28, 130.29, 131.93, 135.51 (arom. CH), 137.99 (arom. bzld -C-), 171.53, 172.52 (succ. ~C(=O)-) ppm. GC-MS: 519.2 m/z (MH+) (theory: 518.2 m/z (M+)). HR-FAB: 517.2028 m/z (M-H+) (theory: 518.2125 m/z (M+)). Elemental analysis: C, 69.18%; H, 6.69% (theory: C, 69.47%; H, 6.61%).
Example 65 Synthesis Of HO-[Gl]-PGLSA-TBDPS
2.41 g (4.65 mmol) of bzld-[Gl]-PGLSA-TBDPS was dissolved in 45 mL of THF, and 1.0 g of 20% Pd(OH)2ZC was added. The solution was then placed in a Parr tube on a hydrogenator, evacuated, flushed with hydrogen, and shaken under 50 psi H2 for 3 hours. The solution was then filtered over wet celite. The product was purified by column chromatography (1:1 Hex:EtOAc increasing to 1:4 Hex:EtOAc) to yield 1.9 g of a clear oil (95% yield). 1H NMR (CDCl3): δ 1.08 (s, 9H, t-butyl), 2.02 (b s, 2H, -OH), 2.64-2.85 (m, 4Η, -CH2-CH2), 3.70-3.72, 4.07-4.14 (m, 4Η, -CH2-CH-CH2-), 4.83-4.86 (m, 1Η, -CH2- CH-CH2-), 7.33-7.44, 7.62-7.65 (m, 1OH, arom. phenyl CH) ppm. 13C NMR (CDCl3): δ 19.30 (-C-CCHs)3), 27.03 (-C-(CH3)S), 29.77, 31.37 (succ. -CH2-), 62.45 (glycerol, -CH2-), 75.86 (CH2-CH-CH2), 127.97, 130.36, 132.67, 135.49 (phenyl CH), 172.65, 178.24 (succ. - C(O)-) ppm. FAB-MS: 431 m/z (M-H+) (theory: 430.57 m/z (M+)). Acetyl derivative of compound HO-[G1]-PGLSA-TBDPS:
Compound HO-[G1]-PGLSA-TBDPS was a hydroscopic oil and repeated attempts to obtain satisfactory EA failed. Thus, we decided to prepare the acetyl analog for elemental analysis. 0.44 g (1.02 mmol) of HO-[G1]-PGLSA-TBDPS was stirred in 30 mL of CH2Cl2 with 0.30 g (1.02 mmol) of DPTS, 0.15 mL (2.66 mmol) of freshly distilled acetic acid, and 0.63 g (3.07 mmol) of DCC. The solution was stirred at room temperature for 18 hours. The DCU precipitate was filtered and the solution was evaporated. A solution of 1:1 ethyl acetate :hexane was added and impurities precipitated. The solution was filtered, concentrated and further purified by column chromatography (3:1 hexanes:EtOAc), to afford 0.44 g of product (83% yield). Rf = 0.19 (4:1 hexanes:EtOAc) 1H NMR (CDCl3): δ 1.08 (s, 9H, t-butyl), 1.87-1.93 (m, 6H, -CH3), 2.50-2.71 (m, 4Η, - CH2-CH2), 3.96-4.19 (m, 4Η, -CH2-CH-CH2-), 5.06-5.18 (m, 1Η, -CH2-CH-CH2-), 7.22- 7.33, 7.51-7.56 (m, 1OH, phenyl CH) ppm. 13C NMR (CDCl3): δ 19.10 (-C-(CΗ3)3), 20.61 (OC-CH3), 26.82 (-C-(CHa)3), 29.14, 30.62 (succ. -CH2-), 62.12, 69.28 (glycerol, -CH2-),
127.71, 130.09, 131.65, 135.27 (arom. CH), 170.52, 171.19, 171.58 ( -C(O)-) ppm. FAB- MS: 515.4 m/z (MH+) (theory: 514.6 m/z (M+)). Elemental analysis: C, 62.76%; H, 6.69% (theory: C, 63.01%; H, 6.66%). SEC: Mw = 547, Mn = 528, PDI = 1.04.
Example 66 Synthesis of bzld-[G2]-PGLSA-TBDPS
1.90 g (4.41 mmol) Of HO-[Gl]-PGLSA-TBDPS was stirred in 100 mL Of CH2Cl2 with 1.30 g (1 equiv; 4.41 mmol) of DPTS, 2.72 g (9.70 mmol; 2.2 equiv) of 2(cis-l,3-0- ben2ylidene glycerol)succinic acid monoester, and 2.00 g (9.70 mmol; 2.2 equiv) of DCC. The solution was stirred at RT for 18 hours. The DCU precipitate was filtered off and the solution was evaporated. A solution of 1 : 1 ethyl acetate-.hexanes was added and impurities precipitated. The solution was filtered, concentrated and further purified by column chromatography (1 :1 hexanes:EtOAc) to afford 3.70 g of product (88% yield). Rf = 0.216 (1:1 hexanes.ΕtOAc). 1H NMR (CDCl3): δ 1.08 (s, 9H, t-butyl), 2.57-2.79 (m, 12H, -CH2- CH2), 4.08-4.14, 4.16-4.22 (m, 12Η, -CH2-CH-CH2-), 4.70-4.71 (m, 2Η, -CH2-CH-CH2-), 5.21 (m, IH, CH), 5.49-5.54 (m, 1Η, CH), 7.32-7.41, 7.47-7.49, 7.64-7.58 (m, 20Η, arom. bzld and phenyl CH) ppm. 13C NMR (CDCl3): δ 19.31 (-C-(CΗ3)3), 27.04 (-C-(CH3)S), 28.98, 29.33, 30.81 (succ. -CH2-), 62.48, 66.50, 69.16, 69.43 (glycerol, -CH2-), 101.33 (O- CH-O), 126.22, 127.95, 128.49, 129.26, 130.32, 131.92, 135.49 (arom. CH), 138.02 (arom. bzld -C-), 171.93, 172.28 (succ. -C(O)-) ppm. GC-MS: 955.3 m/z (MH+) (theory: 954.4 m/z (M+)). Elemental analysis: C, 64.35%; H, 6.29% (theory: C, 64.14%; H, 6.12%). SEC: Mw = 940, Mn = 930, PDI = 1.01.
Example 67 Synthesis of bzld-[G2]-PGLSA-acid
1.00 g (1.04 mmol) of bzld-[G2]-PGLSA-TBDPS was dissolved in 75 mL of THF. Next, 1.25 g (3.96 mmol) of tetrabutylammonium fluoride trihydrate was added to the solution and it was stirred at RT for 1 hour. After one hour the reaction was complete as indicated by TLC. The solution was diluted with 25 mL OfH2O and acidified with IN HCl to a pH of 3. The product was extracted into CH2Cl2, dried over Na2SO4, concentrated and dried on the vacuum line. The product was purified by column chromatography (0-5% MeOH in CH2Cl2; Rf = 0.24) for 0.65 g of product (87% yield). 1H NMR (CDCl3): δ 2.55- 2.77 (m, 12H, -CH2-CH2), 4.10-4.17, 4.24-4.31 (m, 12H5 -CH2-CH-CH2-), 4.74-4.75 (m, 2Η, -CH2-CH-CH2-), 5.28-5.31 (m, IH, CH), 5.52-5.54 (m, 2Η, CH)5 7.33-7.38, 7.47-7.49 (m, 10Η, arom. bzld CH) ppm. 13C NMR (CDCl3): δ 28.72, 29.03, 29.38 (succ. -CH2-),
62.68, 66.56, 69.16 (glycerol, -CH2-), 101.44 (O-CH-O), 126.23, 128.50, 129.33 (arom. CH), 137.75 (arom. bzld -C-), 172.67, 175.16 (succ. -C(=O)-) ppm. GC-MS: 715.2 m/z (M-H') (theory: 716.2 m/z (M+)). Elemental analysis: C, 58.71%; H, 5.82% (theory: C, 58.66%; H, 5.63%). SEC: Mw = 810, Mn = 800, PDI = 1.01.
Example 68 Synthesis of HO-[G2]-PGLSA-TBDPS
1.55 g (1.62 mmol) of bzld-[G2]-PGLSA-TBDPS was dissolved in 40 mL of THF and 1.0 g of 20% Pd(OH)2/C was added. The solution was then placed in a Parr tube on a hydrogenator and shaken under 50 psi H2 for 4 hours. The solution was then filtered over wet celite, rotoevaporated, and purified by column chromatography (0-25% acetone in EtOAc) to yield 1.12 g of product (95% yield). Rf = 0.25 (1:3 acetone:EtOAc). 1H NMR (CDCl3): δ 1.07 (s, 9H, t-butyl), 2.25 (b s, 4H, -OH), 2.58-2.82 (m, 12Η, -CH2-CH2), 3.71- 3.74, 4.09-4.26 (m, 12Η, -CH2-CH-CH2-), 4.87-4.99, 5.24-5.25 (m, 3Η, -CH2-CH-CH2-), 7.34-7.43, 7.63-7.48 (m, 1OH, phenyl CH) ppm. 13C NMR (CDCl3): δ 14.52 (-C-(CΗ3)3), 25.78 (-C-(CHs)3), 26.99, 29.30, 30.51, 30.81 (succ. -CH2-), 62.08, 63.44, 68.17, 70.23 (glycerol, -CH2-), 125.71, 127.96, 130.35, 135.45 (phenyl), 171.94, 172.40 (succ. -C(=O)-) ppm. GC-MS: 779.5 m/z (MH+) (theory: 778.3 m/z (M+)). SEC: Mw = 800, Mn = 792, PDI =1.01 Acetyl derivative of HO-[G2]-PGLSA-TBDPS:
Compound HO-[G2]-PGLSA-TBDPS was a hydroscopic oil and repeated attempts to obtain satisfactory EA failed. Thus, we decided to prepare the acetyl analog for elemental analysis. 0.55 g (0.70 mmol) of HO-[G2]-PGLSA-TBDPS was stirred in 40 mL of CH2Cl2 with 0.39 g (1.34 mmol) of DPTS, 0.19 mL (3.36 mmol) of freshly distilled acetic acid, and 0.87 g (4.20 mmol) of DCC. The solution was stirred at RT for 18 hours. The DCU precipitate was filtered and the solution was evaporated. The residue was resuspended in a minimum of CH2Cl2, cooled to 10 0C and filtered. The resulting solution was concentrated and further purified by column chromatography (0-5% acetone in CH2Cl2) to afford 0.49g of product (66% yield). Rf = 0.17 (5% acetone in CH2Cl2) 1H NMR (CDCl3): δ 1.07 (s, 9H, t-butyl), 2.04 (s, 12H, -CH3), 2.55-2.83 (m, 12Η, -CH2-CH2), 4.09-4.32 (m, 12Η, -CH2-CH-CH2-), 5.20-5.29 (m, 3H5 -CH2-CH-CH2-), 7.32-7.44, 7.61- 7.67 (m, 1OH, phenyl CH) ppm. 13C NMR (CDCl3): δ 19.10 (-C-(CΗ3)3), 20.67 (OC-CH3), 26.82 (-C-(CH3)3), 28.60, 28.80, 29.10, 30.59 (succ. -CH2-), 62.11, 62.31, 69.39 (glycerol, -CH2-), 127.72, 130.09, 131.67, 135.27 (arom. CH), 170.50, 171.33, 171.61 ( -C(O)-)
ppm. FAB-MS: 947.9 m/z (MH+) (theory: 947.0 m/z (M+)). Elemental analysis: C, 57.15%; H, 6.26% (theory: C, 57.07%; H, 6.17%). SEC: Mw = 1075, Mn = 1041, PDI = 1.03.
Example 69 Synthesis of bzld-[G3] -PGLS A-TBDPS
The bzld-[G3]-PGLSA-TBDPS dendron was synthesized by two methods, first by coupling of a bzld-[G2]-PGLSA-acid dendron to a HO-[G1]-PGLSA-TBDPS dendron convergently, and second by coupling compound to a HO-[G2]-PGLSA-TBDPS dendron (7) divergently.
Convergently: 1.05 g (1.47 mmol) of bzld-[G2]-PGLSA-acid was stirred in 75 mL of CH2Cl2, and 0.29 g (0.67 mmol) of HO-[G1]-PGLSA-TBDPS, 0.20 g (0.67 mmol) DPTS, and 0.41 g (2.00 mmol) DCC were added. The solution was stirred at RT for 48 hours. The DCU precipitate was filtered off and the solution was evaporated. The product was purified by column chromatography (3:7 hexanes: EtOAc, Rf = 0.08) with a yield of 0.99 g (82% yield).
Divergently: 0.55 g (0.71 mmol) of a HO-[G2]-PGLSA-TBDPS was stirred in 50 mL Of CH2Cl2, and 0.42 g (1.41 mmol) of DPTS, 0.871 g (3.11 mmol) of 2(cis-l,3-0- Benzylidene Glycerol)Succinic Acid Monoester, and 0.64 g (3.12 mmol) of DCC were added. The solution was stirred under nitrogen at RT for 18 hours. The DCU precipitate was filtered and the solution was evaporated. The product was purified by column chromatography (3:7 hexanes: EtOAc) to afford 0.71 g of product (54% yield). Rf = 0.08 (3:7 hexanes:EtOAc). 1H NMR (CDCl3): δ 1.08 (s, 9H, t-butyl), 2.54-2.92 (m, 28H, -CH2- CH2), 4.08-4.15, 4.22-4.27 (m, 28Η, -CH2-CH-CH2-), 4.71 (s, 4Η, -CH2-CH-CH2-), 5.21- 5.24 (m, 3H, CH), 5.52 (s, 4Η, CH), 7.31-7.42, 7.42-7.49, 7.65-7.67 (m, 30Η, arom. bzld and phenyl CH) ppm. 13C NMR (CDCl3): δ 19.31 (-C-(CΗ3)3), 27.04 (-C-(CH3)3), 29.35, 30.81 (succ. -CH2-), 62.49, 66.53, 69.16, 69.47 (glycerol, -CH2-), 101.33 (0-CH-O), 126.21, 127.94, 128.48, 129.26, 130.32, 135.47 (arom. CH), 138.02 (arom. bzld -C-), 171.90, 172.28 (succ. -C(O)-) ppm. GC-MS: 1825.6 m/z (M-H+) (theory: 1827.9 m/z (M+)). HR-FAB: 1825.6124 m/z (M-H+) (theory: 1826.6233 m/z (M+)). Elemental analysis: C, 60.66%; H, 5.85% (theory: C, 61.11%; H, 5.85%). SEC: Mw = 1830, Mn = 1810, PDI = LOl.
Example 70 Synthesis of bzld-[G3]-PGLSA-acid
2.00 g (1.09 mmol) of bzld-[G3]-PGLSA-TBDPS was dissolved in 125 niL of THF. Next, 1.3 g (4.1 mmol) of tetrabutylammonium fluoride trihydrate was added to the solution. The mixture was stirred at RT for 1 hour. After one hour the reaction was complete as indicated by TLC. The solution was diluted with 25 mL of H2O and acidified with IN HCl to a pH of 3. The product was extracted into CH2Cl2, dried over Na2SO4, rotoevaporated and dried on the vacuum line. The product was purified by column chromatography (0-5% MeOH in CH2Cl2) to afford 1.44 g of product (83% yield). Rf = 0.21 (5% MeOH in CH2Cl2). 1H NMR (CDCl3): δ 2.58-2.75 (m, 28H, -CH2-CH2), 4.11- 4.16, 4.19-4.27 (m, 28Η, -CH2-CH-CH2-), 4.71-4.72 (m, 4Η, -CH2-CH-CH2-), 5.21-5.28 (m, 3H, CH), 5.52-5.53 (m, 4Η, CH), 7.32-7.37, 7.46-7.49 (m, 20Η, arom. bzld CH) ppm. 13C NMR (CDCl3): δ 29.05, 29.36 (succ. -CH2-), 62.51, 66.58, 69.16 (glycerol, -CH2-), 101.36 (0-CH-O), 126.21, 128.49, 129.29 (arom. CH), 137.95 (arom. bzld -C-), 171.83, 173.01 (succ. -C(O)-) ppm. GC-MS: 1587.5 m/z (M-H+) (theory. 1588.5 m/z (M+)). Elemental analysis: C, 58.02%; H, 5.60% (theory. C, 58.18%; H, 5.58%). SEC: Mw = 1650, Mn = 1620, PDI = 1.02.
Example 71 Synthesis of HO-[G3]-PGLSA-TBDPS
0.53 g (0.29 mmol) of bzld-[G3]-PGLSA-TBDPS was dissolved in 50 mL of THF in a Parr tube. 0.4 g of 20% Pd(OH)2/C was added and the flask was evacuated and filled with 50 psi of H2. The mixture was shaken for 8 hours, then filtered over wet celite. The filtrate was dried to produce a clear oil which was purified by column chromatography (0- 50% acetone in EtOAc) to afford 0.38 g of product (88% yield). Rf = 0.23 (1: 1 acetone:EtOAc). 1H NMR (CDCl3): δl.3 (s, 9H, t-butyl), 2.52-2.86 (m, 28H, -CH2-CH2), 3.44-3.94 (m, 24, -CH2-CH-CH2- and -OH), 4.10-4.38, (m, 12Η, -CH2-CH-CH2-), 4.82- 4.92 (m, 4Η, CH), 5.18-5.30 (m, 3Η, CH), 7.28-7.43, 7.50-7.54, 7.60-7.66 (m, 10Η, phenyl CH) ppm. 13C NMR (CDCl3): δ 19.04 (-C-(CΗ3)3), 24.44 (-C-(CH3)3), 26.76, 27.12, 28.82, 28.97, 29.10, 30.57 (succ. -CH2-), 61.17, 62.33, 63.21, 69.30, 75.52 (glycerol, -CH2-), 127.72, 130.11, 131.57, 134.36, 135.20 (arom. CH), 171.66, 171.72/ 171.99, 172.27, 172.38, 172.46 (succ. -C(O)-) ppm. MALDI-MS: 1475.56 m/z (MH+) (theory: 1475.5 111/Z (M+)). SEC: Mw = 2101, Mn = 1994, PDI = 1.05.
Acetyl derivative of compound of HO-[G3]-PGLSA-TBDPS:
Compound HO-[G3]-PGLSA-TBDPS was a hydroscopic oil and repeated attempts to obtain satisfactory EA failed. Thus, we decided to prepare the acetyl analog for elemental analysis. 0.24 g (0.16 mmol) of HO-[G3]-PGLS A-TBDPS was stirred in 40 mL of CH2Cl2 with 0.19 g (0.65 mmol) of DPTS, 0.09 mL (1.55 mmol) of freshly distilled acetic acid, and 0.40 g (1.94 mmol) of DCC. The solution was stirred at RT for 18 hours. The DCU precipitate was filtered and the solution was evaporated. The residue was resuspended in a minimum Of CH2Cl2, cooled to 10 0C and filtered. The resulting solution was concentrated and further purified by column chromatography (8:2 hexanes:EtOAc to 3:7 hexanes:EtOAc) to afford 0.18 g of product (63% yield). Rf = 0.15 (3:7 hexanes:EtOAc) 1H NMR (CDCl3): δ 1.10 (s, 9H, t-butyl), 1.99 (s, 24H, -CH3), 2.48-2.78 (m, 28Η, -CH2-CH2), 4.02-4.30 (m, 28Η, -CH2-CH-CH2-), 5.12-5.26 (m, 7Η, -CH2-CH- CH2-), 7.25-7.38, 7.55-7.61 (m, 1OH, phenyl CH) ppm. 13C NMR (CDCl3): δ 18.87 (-C- (CΗ3)3), 20.46 (OC-CH3), 26.61 (-C-(CH3)3), 26.95, 28.47, 28.55, 28.64, 28.90, 30.39 (succ. -CH2-), 61.90, 62.10, 69.02, 69.22 (glycerol, -CH2-), 127.52, 129.90, 131.48, 135.05 (arom. CH), 170.26, 171.14, 171.40, 171.46 ( -C(=O)-) ppm. FAB-MS: 1812.2 m/z (MH+) (theory: 1811.8 m/z (M+)). Elemental analysis: C, 53.95%; H, 6.12% (theory: C, 53.70%; H, 5.90%). SEC: Mw = 1943, Mn = 1882, PDI = 1.03.
Example 72 Synthesis of bzld-[G4]-PGLSA-TBDPS
The bzld-[G4]-PGLSA-TBDPS dendron was synthesized by two methods, first by coupling of bzld-[G2]-PGLSA-acid dendron to a HO-[G2]-PGLSA-TBDPS dendron convergently, and secondly by coupling the monoester 2(cis-l,3-O-Benzylidene Glycerol)Succinic Acid Monoester to a HO-[G3]-PGLSA-TBDPS dendron divergently.
Convergently: 0.14 g (0.18 mmol) of HO-[G2]-PGLSA-TBDPS was dissolved in 30 mL Of CH2Cl2. Next, 0.05 g (0.18 mmol) of DPTS, 0.82 g (1.10 mmol) of bzld-[G2]- PGLSA-acid and 0.22 g (1.10 mmol) of DCC were added. The solution was stirred at RT under nitrogen for 72 hours. The DCU was filtered, the filtrate was concentrated to dryness and the residue was resuspended in a minimum of cold THF. The solution was filtered, concentrated and purified by column chromatography (1:1 hexanes:EtOAc to 1 :4 hexanes:EtOAc, Rf = 0.14) to afford 0.48 g of product (75% yield).
Divergently: 0.38 g (0.26 mmol) of HO-[G3]-PGLSA-TBDPS was dissolved in 50 mL Of CH2Cl2. Next, 1.00 g (3.57 mmol) of 2(cis-l,3-O-Benzylidene Glycerol)Succinic
Acid Monoester, 0.10 g (0.34 mmol) of DPTS, and 0.656 g (3.57 mmol) of DCC were added to the mixture. The solution was stirred for 48 hours under nitrogen at RT. The DCU precipitate was filtered, concentrated and purified by column chromatography (1:1 hexanes:EtOAc to 1:4 hexanes:EtOAc, Rf = 0.14) to afford 0.572 g of product (60% yield). 1H NMR (CDCl3): δ 1.07 (s, 9H, t-butyl), 2.55-2.77 (m, 6OH, -CH2-CH2), 4.07-4.15, 4.22- 4.25 (m, 60Η, -CH2-CH-CH2-), 4.70 (s, 8Η, -CH2-CH-CH2-), 5.19-5.21 (m, 7H, CH), 5.51 (s, 8Η, CH), 7.30-7.40, 7.46-7.48, 7.63-7.65 (m, 50Η, arom. bzld and phenyl CH) ppm. 13C NMR (CDCl3): δ 14.40 (-C-(CΗ3)3), 27.03 (-C-(CH3)3), 29.02, 29.35 (succ. -CH2-), 62.47, 66.53, 69.16, 69.49 (glycerol, -CH2-), 101.31 (0-CH-O), 126.21, 127.94, 128.48, 129.26, 135.47 (arom. CH), 138.03 (arom. bzld -C-), 171.50, 171.90, 172.27 (succ. -C(O)-) ppm. MALDI-MS: 3574.54 m/z (MH+) (theory: 3573.54 m/z (M+)). Elemental analysis: C, 59.49%; H, 5.70% (theory: C, 59.19%; H, 5.74%). SEC: Mw = 3420, Mn = 3350, PDI = 1.02.
Example 73 Synthesis of [G3]-PGLSA-bzld Dendrimer
0.019 g (0.084 mmol) of [GO]-PGLSA-OH, 12 was dissolved in 50 mL Of CH2Cl2. Next, 0.64 g (0.40 mmol) of compound bzld-[G3]-PGLSA-acid, 0.074 g (0.25 mmol) of DPTS, and 0.10 g of DCC (0.50 mmol) were added. The solution was stirred for 72 hours at RT under nitrogen. The DCU was filtered off and the filtrate was concentrated. The additional DCU was precipitated in cold THF and filtered. The product was purified by column chromatography (0-5% MeOH in CH2Cl2) to yield 0.40 g of product (73% yield). 1H NMR (CDCl3): δ 2.60-2.74 (m, 116H, -CH2-CH2), 4.08-4.17 (m, 60Η, -CH2-CH-CH2-), 4.22-4.26 (m, 60Η, -CH2-CH-CH2-), 4.70 (s, 16Η, -CH2-CH-CH2-), 5.20-5.23 (m, 14H, CH), 5.51 (s, 16Η, CH), 7.32-7.36, 7.46-7.48 (m, 80Η, arom. bzld CH) ppm. 13C NMR (CDCl3): δ 29.02, 29.35 (succ. -CH2-), 62.47, 66.54, 69.16 (glycerol, -CH2-), 101.31 (O- CH-O), 126.21, 128.48, 129.26 (arom. CH), 138.01 (arom. bzld -C-), 171.83, 172.29 (succ. -C(O)-) ppm. MALDI: 6553.4 m/z (MH+) (theory: 6552.2 m/z (M+). Elemental analysis: C, 58.50%; H, 5.48% (theory: C, 58.29%; H, 5.57%). SEC: Mw = 4740, Mn = 4590, PDI = LOl.
Example 74 Synthesis of [G3J-PGLSA-OH Dendrimer, 14
0.33 g (0.051 mmol) of [G3 J-PGLS A-bzld was dissolved in 50 mL of a 9:1 solution of THF and MeOH in a Pan- tube. Next, 0.50 g of 20% Pd(OH)2/C was added and the flask
was evacuated and filled with 50 psi of H2. The mixture was shaken for 7 hours, then filtered over wet celite. The filtrate was dried to produce 0.25 g of a clear oil (0.049 mmol, 97% yield). 1H NMR (CD3OD): 52.64 (m, 116, -CH2-CH2-), 3.51 (m, 26, -CH2-CH- CH2-), 3.67 (m, 28, -CH2-CH-CH2-), 3.80 (m, 12, -CH2-CH-CH2-), 4.05 (m, 14, -CH2- CH-CH2-), 4.14 (m, 14, -CH2-CH-CH2-), 4.22 (m, 22; -CH2-CH-CH2-), 4.30 (m, 22, - CH2-CH-CH2-), 5.26 (m, 14, -CH2-CH-CH2) ppm. 13C NMR (CD3OD): δ. 28.61 (CH2), 62.41 (CH2), 62.87 (CH2), 65.67 (CH2), 67.64 (CH), 69.91 (CH), 172.86 (COOR) ppm. MALDI-MS: 5144.8 m/z (MH+) (theory: 5142.5 rn/z (M+)). Elemental analysis: C, 48.07%; H, 5.84% (theory: C, 48.11%; H, 5.84%). SEC Mw: 5440; Mn: 5370; PDI: 1.01.
Example 75 Synthesis of [G3] -PGLSA-MA Dendrimer (50% derivatized)
0.22 g (0.041 mmol) of [G3J-PGLSA-OH was dissolved in 5 mL of DMF. Next, 0.20 g (1.66 mmol) of DMAP was then added followed by 0.10 mL (0.67 mmol, 0.5 equiv. to the peripheral hydroxyl groups on [G3 J-PGLSA-OH) of freshly distilled methacrylic anhydride. After 4.5 hours the reaction was complete as indicated by TLC. 0.03 mL (0.67 mmol) of MeOH was added to the reaction and allowed to stir for an additional 20 minutes. The solution was precipitated into 300 mL of cold ethyl ether. The ether was decanted off and the remaining oily reside was diluted with 20 mL of CH2Cl2. The organic phase was washed with 1 N HCl and brine. The organic phase was dried over Na2SO4, filtered, and concentrated to approximately 2 mL. This concentrated solution was precipitated in 300 mL of cold ethyl ether. The ether was decanted off and the resulting oily residue was dried under reduced pressure to yield 0.20 g of product (78% yield). 1H NMR (CDCl3): δ 1.90 (s, 42H, -CH3), 2.55-2.77 (m, 116Η, -CH2-CH2), 3.61-3.78 (m, 30Η, -CH2-CH-CH2-), 4.07- 4.30 (m, 120H, -CH2-CH-CH2-), 5.58-5.62 (m, 16Η, =CH), 6.03-6.16 (m, 16Η, =CH) ppm. 13C NMR (CDCl3): δ 18.24 (-CH3), 29.56, 29.75 (succ. -CH2-), 61.52, 62.09, 62.14, 65.17, 65.83, 69.39, 69.56, 70.04, 73.23, 75.89 (glycerol -CH2-), 171.04, 171.25, 171.37, 171.58, 171.79, 172.14, 172.51 ppm. MALDI-MS: 6224.6 m/z (MH+) (theory: 6231.6 m/z (M+)). SEC: Mw = 3525, Mn = 2708, PDI = 1.30.
Example 76 Synthesis of bzϊd-[G3]-PGLSA-PEG-OMe
0.29 g (0.18 mmol) of bzId-[G3]-PGLSA-acid was dissolved in 75 mL Of CH2Cl2. Next 0.45 g (0.09 mmol) of 5000 MW polyethylene glycol) mono-methyl ether (PEG- OMe; MALDI-MS: Mw = 5147, Mn = 5074, PDI = 1.01), 0.037 g (0.18 mmol) of DCC, and 0.026 g (0.09 mmol) of DPTS were added to the solution. The solution was stirred under nitrogen at RT for 168 hours. The DCU was filtered and the filtrate was concentrated to dryness. The resulting residue was resuspended in THF, cooled, and the DCU was filtered. The resulting solution was precipitated in ethyl ether. The solid was dissolved in THF, stirred with Amberlyst A-21 ion-exchange resin (Aldrich) (weakly basic resin) to eliminate the excess 9. The solution was filtered and the filtrate was dried over Na2SO4, dissolved in CH2Cl2, washed with 0.1 N HCl, and dried over Na2SO4 to yield 0.53 g of a solid white product (89% yield). 1H NMR (CDCl3): δ 2.60-2.73 (m, 28H, -CH2-CH2), 3.36 (s, MME CH3) 3.57-3.64 (m, 406Η, PEG CH2), 4.11-4.26 (m, 28Η, -CH2-CH-CH2-), 4.71 (m, 4Η, -CH2-CH-CH2-), 5.21-5.23 (m, 3H, CH), 5.52-5.54 (m, 4Η, CH), 7.32-7.37, 7.46- 7.49 (m, 20Η, arom. bzld CH) ppm. 13C NMR (CDCl3): δ 29.36, 29.90 (succ. -CH2-), 62.48, 66.53, 69.17 (glycerol, -CH2-), 70.77 (PEG, -CH2-), 101.33 (0-CH-O), 126.21, 128.48, 129.26 (arom. CH), 137.80 (arom. bzld -C-), 171.90 (succ. -C(O)-) ppm. MALDI-MS: Mw = 6671, Mn = 6628 PDI = 1.01 (theoretical MW = 6588). SEC: Mw = 6990, Mn = 6670, PDI = 1.04.
Example 77 Synthesis of HO-[G3]-PGLSA-PEG~OMe
0.52 g of bzld-[G3]-PGLSA-PEG-OMe was dissolved in 40 mL of THF. Next, 0.10 g of 20% Pd(OH)2/C was added. The reaction vessel was evacuated and flushed with hydrogen. The solution was shaken for 3 hours under 50 psi H2 at RT. The Pd(OH)2ZC was removed by filtering over wet celite. The filtrate was dried and precipitated in ethyl ether to yield 0.40 g of an opaque hydroscopic solid (83% yield). 1H NMR (CDCl3): δ 2.60-2.70 (m, 28H, -CH2-CH2), 3.36 (s, MME CH3) 3.53-3.78 (b m, 422Η, PEG CH2 and -CH2-CH- CH2-), 4.17-4.27 (m, 12Η, -CH2-CH-CH2-), 4.92 (m, 4Η, -CH2-CH-CH2-), 5.21-5.23 (m, 3H, CH) ppm. 13C NMR (DMSO): δ 29.14, 29.36 (succ. -CH2-), 60.25 (-CH3 OMe), 63.22, 66.54, 69.87 (glycerol, -CH2-), 70.43 (PEG, -CH2-), 172.35, 172.57 (succ. -C(O)-)
ppm. MALDI-MS: Mw = 6302, Mn = 6260, PDI = 1.01 (theoretical MW = 6136). SEC: Mw = 6660, Mn = 6460, PDI = 1.03.
Example 78 Synthesis of MA- [G3] -PGLSA-PEG-OMe
0.39 g (0.064 mmol) of HO-[G3]-PGLSA-PEG-OMe was dissolved in 30 mL of CH2Cl2. Next, 10 mg (0.08 mmol) of DMAP and 0.15 mL methacrylic anhydride (1.0 mmol) were added and the solution was stirred at RT under nitrogen overnight. The solution was then washed with 0.1 N HCl, dried over Na2SO4, condensed, and precipitated in ether to afford 0.41 g of product (96% yield). 1H NMR (CDCl3): δ 1.92 (s, 24 H, -CH3- methacrylate), 2.63 (m, 28H, -CH2-CH2), 3.36 (s, MME CH3) 3.59-3.67 (m, 406Η, PEG CH2), 4.19-4.39 (m, 28Η, -CH2-CH-CH2-), 5.24 (m, 4Η, -CH2-CH-CH2), 5.35 (m, 3H, CH), 5.59 (s, 8Η, -CH2- methacrylate), 6.10 (s, 8Η, -CH2- methacrylate) ppm. MALDI-MS: Mw = 7080, Mn = 7008, PDI = 1.01 (theoretical MW = 6780). SEC: Mw = 6918, Mn = 6465, PDI = 1.07.
Example 79 Synthesis of Myr-[G2]-PGLSA-TBDPS
0.45 g (0.58 mmol) of compound OΗ-[G2]-PGLSA-TBDPS was dissolved in 75 mL of CH2Cl2 with 0.63 g (2.77 mmol) of myristic acid(Myr), 0.34 g (1.16 mmol) of DPTS, and 0.72 g (3.47 mmol) of DCC. The reaction was stirred at RT for 16 hours. The DCU precipitate was filtered and the solution was evaporated. The residue was resuspended in 50 mL of ethanol, cooled to 0 0C for 6 hours and filtered. The precipitate was resuspended in 75 mL of CH2Cl2, washed with 75 mL of H2O, dried over Na2SO4, and the solvent evaporated to yield 0.84 g of product (89% yield). 1H NMR (CDCl3): δ 0.80- 0.89 (t, 12H, -CH3), 1.08 (s, 9Η, t-butyl), 1.14-1.34 (m, 8OH, myristic -CH2-), 1.50-1.64 (m, 8Η, C(=O)-CH2-CH2-CH2-), 2.22-2.33 (t, 8H, C(=O)-CH2-CH2-), 2.53-2.83 (m, 12H, succinic -CH2-CH2), 4.08-4.34 (m, 12Η, -CH2-CH-CH2-), 5.18-5.30 (m, 3Η, -CH2-CH- CH2-), 7.32-7.44, 7.61-7.67 (m, 1OH, phenyl CH) ppm. 13C NMR (CDCl3): δ 14.25, 22.67, 24.81, 26.85, 28.81, 28.79, 29.12, 29.24, 29.36, 29.53, 29.64, 31.97, 34.05, 61.88, 62.34, 69.17, 127.66, 130.13, 135.28, 138.77, 171.34, 171.69, 173.32 ppm. FAB-MS: 1620.1 m/z (MH+) (theory: 1620.29 m/z (M+)). Elemental analysis: C, 68.84%; H, 9.69% (theory: C, 68.94%; H, 9.58%). SEC: Mw = 2168, Mn = 2135, PDI = 1.02.
Example 80 Synthesis of Myr-[G2]-PGLSA-acid
0.81 g (0.50 mmol) of Myr-[G2]-PGLSA-TBPDS was dissolved in 100 mL of THF. Next, 0.55 g (1.75 mmol) of tefrabutylarnmonium fluoride trihydrate was added to the solution. The mixture was stirred at RT for 1 hour. After one hour the reaction was complete as indicated by TLC. The solution was diluted with 25 mL OfH2O and acidified with IN HCl to a pH of 3. The product was extracted into EtOAc, dried over Na2SO4, rotoevaporated and dried on the vacuum line. The product was purified by column chromatography (0-3% MeOH in CH2Cl2) to afford 0.60 g of product (87% yield). Rf = 0.23 (3% MeOH in CH2Cl2). 1H NMR (CDCl3): δ 0.82-0.88 (t, 12H, -CH3), 1.20-1.31 (m, 80Η, myristic -CH2-), 1.53-1.64 (m, 8Η, -C(O)-CH2-CH2-CH2-), 2.26-2.33 (t, 8H, -C(O)- CH2-CH2-), 2.60-2.68 (m, 12H, -CH2-CH2-), 4.11-4.34 (m, 12Η, -CH2-CH-CH2-), 5.19-5.35 (m, 3Η, -CH2-CH-CH2-) ppm. 13C NMR (CDCl3): δ 14.16, 22.78, 24.98, 28.56, 28.87, 29.07, 29.24, 29.47, 29.63, 29.87, 32.01, 34.04, 62.02, 62.64, 69.16, 69.93, 171.47, 171.68, 173.51 ppm. FAB-MS: 1382.9 TnZz (M-H+) (theory: 1381.9 m/z (M+)). Elemental analysis: C, 66.72%; H, 9.91% (theory: C, 66.92%; H, 9.92%). SEC: Mw = 2074, Mn = 2040, PDI = 1.02.
Example 81 Synthesis of 2-benzyl-l,3-di(Myr-[G2]-PGLSA)2-gIycerol
0.85 g (0.62 mmol) of compound Myr-[G2]-PGLSA-acid was dissolved in 75 mL Of CH2Cl2 with 0.05 g (0.26 mmol) of 2-benzyl-glycerol, 0.08 g (0.26 mmol) of DPTS, and 0.16 g (0.77 mmol) of DCC. The reaction was stirred at RT for 16 hours. The DCU precipitate was filtered and the solution was evaporated. The residue was resuspended in 50 mL of ethanol, cooled to 0 0C for 6 hours and filtered. The precipitate was purified by column chromatography (20-50% EtOAc in hexanes) to yield 0.63 g of product (85% yield). Rf = 0.17 (30% EtOAc in hexanes). 1H NMR (CDCl3): δ 0.81-0.88 (t, 24H, -CH3), 1.17-1.34 (m, 160Η, myristic -CH2-), 1.52-1.63 (m, 16Η, C(=O)-CH2-CH2-CH2-), 2.24-2.32 (t, 16H, C(O)-CH2-CH2-), 2.58-2.66 (m, 24H, succinic -CH2-CH2), 3.77-3.85 (m, 1Η, - CH2-CH-CH2-), 4.04-4.38 (m, 28H, -CH2-CH-CH2-), 4.59-4.65 (s, 2Η, benzyl -CH2-), 5.17-5.34 (m, 6Η, -CH2-CH-CH2-), 7.25-7.34 (m, 5H, aromatic CH) ppm. MALDI-MS:
2933.4 m/z (M+Na+) (theory: 2933.0 m/z (M-HKa+)). Elemental analysis: C, 67.92%; H, 9.79% (theory: C, 67.69%; H, 9.77%). SEC: Mw = 4388, Mn = 4258, PDI = 1.03.
Example 82 Synthesis of l,3-di(Myr-[G2]-PGLSA)2-glycerol
0.47 g (0.16 mmol) of 2-benzyl-l,3-di(Myr-[G2]-PGLSA)2-glycerol was dissolved in 20 mL of THF and 0.5 g of 10% Pd/C was added. The solution was then placed in a Parr tube on a hydrogenator and shaken under 50 psi H2 for 10 hours. The solution was then filtered over wet celite, rotoevaporated, to yield the product.
Example 83 Synthesis of bz-SA-[G2]-PGLSA-TBDPS
0.77 g (0.99 mmol) of compound HO-[G2]-PGLSA-TBDPS was dissolved in 75 mL of CH2Cl2 with 0.99 g (4.76 mmol) of benzylated succinic acid (bz-sa), 0.58 g (1.98 mmol) of DPTS, and 1.23 g (5.91 mmol) of DCC. The reaction was stirred at RT for 16 hours. The DCU precipitate was filtered and the solution was evaporated. The residue was resuspended in a minimum Of CH2Cl2, cooled to 10 0C for 1 hour and filtered. The solution was concentrated under reduced pressure and purified by column chromatography (30-50% EtOAc in hexanes) to afford 1.21 g of product (79% yield). Rf - 0.18 (40% EtOAc in hexanes). 1H NMR (CDCl3): δ 1.08 (s, 9H, t-butyl), 2.55-2.81 (m, 28H, succinic -CH2- CH2), 4.06-4.37 (m, 12Η, -CH2-CH-CH2-), 5.11 (s, 8Η, benzyl -CH2-), 5.18-5.29 (m, 3Η, - CH2-CH-CH2-), 7.22-7.44, 7.61-7.67 (m, 3OH, aromatic CH) ppm. 13C NMR (CDCl3): δ 19.13, 26.81, 28.42, 28.64, 28.70, 28.9I5 29.07, 30.56, 62.68, 66.72, 69.07, 73.69, 127.68, 128.23, 128.54, 130.06, 131.73, 135.21, 135.77, 171.64, 171.73, 171.90 ppm. FAB-MS: 1539.6 m/z (MH+) (theory: 1539.7 m/z (M+)). Elemental analysis: C, 63.35%; H, 6.02% (theory: C, 63.19%; H, 5.89%).
Example 84 Synthesis of bz-SA-[G2]-PGLSA-acid
1.12 g (0.73 mmol) of bz-SA-[G2]-PGLSA-TBDPS was dissolved in 100 mL of THF. Next, 0.89 g (2.76 mmol) of tetrabutylammonium fluoride trihydrate was added to the solution. The mixture was stirred at RT for 1 hour. After one hour the reaction was complete as indicated by TLC. The solution was diluted with 25 mL of H2O and acidified
with IN HCl to a pH of 3. The product was extracted into EtOAc, dried over Na2SO4, rotoevaporated and dried on the vacuum line. The product was purified by column chromatography (0-3% MeOH in CH2Cl2) to afford 0.71 g of product (75% yield). Rf = 0.18 (3% MeOH in CH2Cl2). 1H NMR (CDCl3): δ 2.54-2.69 (m, 28H, -CH2-CH2), 4.11- 4.31 (m, 12Η, -CH2-CH-CH2-), 5.09 (s, 8Η, benzyl -CH2-), 5.18-5.25 (m, 3Η, -CH2-CH- CH2-), 7.25-7.36 (m, 2OH, aromatic CH) ppm. 13C NMR (CDCl3): δ 28.57, 28.78, 28.94, 62.28, 62.43, 66.60, 69.16, 69.37, 128.24, 128.29, 128.61, 128.57, 171.33, 171.79, 171.95 ppm. FAB-MS: 1301.5 m/z (M-H+) (theory: 1301.3 m/z (M+)). Elemental analysis: C, 60.23%; H, 5.81% (theory: C, 60.00%; H, 5.58%). SEC: Mw = 1415, Mn = 1379, PDI = 1.03.
Example 85 Synthesis of bz-SA-[G4]-PGLSA-TBDPS
0.07 g (0.08 mmol) of compound HO-[G2]-PGLSA-TBDPS was dissolved in 40 mL Of CH2Cl2 with 0.53 g (0.41 mmol) of bz-SA-[G2]-PGLSA-acid, 0.05 g (0.17 mmol) of DPTS, and 0.11 g (0.51 mmol) of DCC. The reaction was stirred at RT for 48 hours. The DCU precipitate was filtered and the solution was evaporated. The residue was resuspended in a minimum Of CH2Cl2, cooled to 10 0C for 1 hour and filtered. The solution was concentrated under reduced pressure and purified by column chromatography (30-80% EtOAc in hexanes) to afford 0.40 g of product (80% yield). Rf = 0.18 (65% EtOAc in hexanes). 1H NMR (CDCl3): δ 1.07 (s, 9H, t-butyl), 2.53-2.81 (m, 124H, succinic -CH2- CH2), 4.10-4.31 (m, 60Η, -CH2-CH-CH2-), 5.09 (s, 32Η, benzyl -CH2-), 5.18-5.28 (m, 15Η, -CH2-CH-CH2-), 7.25-7.41, 7.45-7.49, 7.61-7.66 (m, 9OH, aromatic CH) ppm. 13C NMR (CDCl3): δ 26.72, 28.52, 28.73, 28.87, 62.15, 66.43, 68.84, 69.16, 125.91, 127.64, 128.11, 128.33, 128.46, 130.01, 135.16, 135.66, 171.25, 171.54, 171.64, 171.81 ppm. MALDI-MS: XXX m/z (MH+) (theory: XXX m/z (M+)). Elemental analysis: C, 60.70%; H, 5.74% (theory: C, 60.34%; H, 5.63%). SEC: Mw = 5142, Mn = 5064, PDI = 1.02.
Example 86 Synthesis of bz-SA-[G4]-PGLSA-acid
0.22 g (0.04 mmol) of bz-SA-[G4]-PGLSA-TBDPS was dissolved in 12 mL of THF. Next, 0.04 g (0.13 mmol) of tetrabutylammonium fluoride trihydrate was added to
the solution. The mixture was stirred at RT for 4 hours. The solution was diluted with 5 mL Of H2O and acidified with IN HCl to a pH of 3. Additional THF was added drop wise to keep product in solution. The product was extracted into EtOAc, dried over Na2SO4, rotoevaporated and dried on the vacuum line. The product was purified by column chromatography (20-100% EtOAc in hexanes) to afford the product. 1H NMR (CDCl3): δ 2.46-2.84 (m, 124H, -CH2-CH2), 4.12-4.49 (m, 60Η, -CH2-CH-CH2-), 5.02-5.36 (m, 57Η, benzyl -CH2- and -CH2-CH-CH2-), 7.25-7.48 (m, 8OH, aromatic CH) ppm. 13C NMR (CDCl3): δ 28.79, 28.93, 62.21, 66.51, 69.24, 127.64, 128.17, 128.52, 135.69, 171.34, 171.73, 171.91 ppm.
Example 87 Synthesis of ZLys(Z)-OPFP
DCC (5.45 g, 26 mmol) was added in five portions over 10 minutes to a solution of ZLys(Z)OΗ (10 g, 24 mmol) and 1.1 equiv of pentafiuorophenol in freshly distilled CH2Cl2 (40 mL). The reaction mixture was stirred under N2 at 25 0C for 2 h, filtered to remove the insoluble urea, concentrated to ~ 20 mL under reduced pressure, and then stored at 4 0C for 2 h. An additional filtration removed further urea, and the filtrate was diluted with hexane (25 mL) and stored at 4 0C for 4h. The resultant white precipitate was collected by filtration, washed with DCM/hexane (1:2, 3x5 mL), and dried in vacuum; yield 13.37 g (980Zo)-1H NMR (CDCl3): δ 1.46 (m, 2, CH2-CH2); 1.54 (m, 2, CH2-CH2); 1.84 (m, 1, CH2- CH); 2.00 (m, 1, CH2-CH); 3.19 (m, 2, CH2-NH); 4.67 (m, 1, CH2-CH); 4.8 (m, 1, NH); 5.03 (m, 2, CH2-O); 5.11 (s, 2, CH2-O); 5.54 (m, 1, NH); 7.3 (m, 10, arom CH). 19F NMR (CDC13): δ -162.26 (t, 2, CF); -157.60 (t, 1, CF); -152.72 (d, 2, CF). Elemental analysis: (theory: C, 57.93; Η, 4.34) found C, 58.12; Η, 4.40
Example 88 Synthesis of ZLys(Z)Lys(ZLys(Z))OMe
LysOMe. 2ΗC1 (1.43 g, 6 mmol) was dissolved in DMF (45 mL) with the DIEA (2.35 g, 18 mmol), and then the HOBT (2.25 g, 14 mmol) was added. After 5 minutes ZLys(Z)OPFP (12.5 g, 21 mmol) in DCM (30 mL) was added at 0 0C for 10 minutes. The mixture was stirred for 24 h at RT under N2. After concentration under vacuum the mixture was dissolved in DCM (50 mL) washed with NaHCO3 (2x150 mL), water (2x150 mL) and then dried over NaSO4. The solvent was removed, and the mixture was precipitated in ether
to lead a pure white compound 5.72 g (98%).1H NMR (CDCl3): δ 1.35-1.79 (m, 18, CH2- CH2); 2.87 (m, 1, CH2-NH); 3.13 (m, 4, CH2-NH); 3.40 (m, 1, CH2-NH); 3.63 (s, 3, CH3); 4.16 (m, 1, CH-NΗ); 4.34 (m, 1, CH-NΗ); 4.38 (m, 1, CH-NΗ); 4.88-5.02 (4 x s, 8, CH2- O); 5.13 (m, 1, CH2-NH); 5.28 (m, 1, CH2-NH); 5.94 (d, I5 CΗ-NH); 6.25 (d, 1, CΗ-NH); 6.88 (m, 1, CΗ2-NH);7.19-7.27 (m, 20, arom CH). 7.43 (d, 1, CΗ-NH). FAB MS: 953.4 m/z (MH+) (theory. 952.4 m/z (M+)). Elemental analysis: (theory: C, 64.27; H, 6.77; Ν, 8.82; O, 20.14) found C, 63.98; H, 6.79; Ν, 8.81; O, 20.39.
Example 89 Synthesis of LysLys(Lys)OMe» 4HCl
Pd/C (10% w/w) was added to a solution of ZLys(Z)Lys(ZLys(Z))OMe (1 g, 1 mmol) in MeOH (50 mL). The flask for catalytic hydrogenolysis was evacuated and filled with 50 psi ofH2 before shaking for 1O h. The catalyst was filtered and washed with MeOH (20 mL). The filtered was acidified with HCl gas. The acid solution was evaporated to give 578 mg of the white compound (98%).1H ΝMR (DMSO-d6): δ 1.36-1.81 (m, 18, CH2- CH2); 2.75 (m, 4, CH2-NH3 +); 3.12 (m, 2, CH2-NH); 3.65 (s, 3, CH3); 3.82 (m, 1, CH-NΗ); 3.98 (m, 1, CH-NΗ); 4.25 (m, 1, CH-NΗ); 8.20-8.45 (m, 12, NH3 +); 8.88 (t, 1, CH2-NH); 9.18 (d, 1, CΗ-NH). FAB MS: 417.4 m/z (MH+- 4HC1) (theory: 416.3 m/z (M+)). Elemental analysis: (theory: C, 40.65; H, 7.72; Cl, 25.26; N, 14.97) found C540.31; H57.87; Cl5 25.10; N, 14.97.
Example 90 Synthesis of IsoCysOH
L-cysteine hydrochloride monohydrate (100 g, 0.569 mol) was refluxed in dry acetone (1.5 L) under dry nitrogen for 1.5 hours. The white precipitate was collected by filtration and refluxed a second time in dry acetone. Again the white solid was collected to yield 103.6 g of pure product (92 % yield).
Example 91 Synthesis of IsoCys(Boc)OH
To a suspension of IsoCysOH (144 g, 0.727 mol) and άi-tert-hutyl dicarbonate (206 g, 0.943 mol) in dry acetonitrile was added DIEA (140 mL, 0.803 mol). The suspension
was allowed to stir for two days. Afterward, the acetonitrile was removed in vacuo, and the remaining oil was redissolved in ethyl ether and concentrated once more to an oily solid. The oily solid was again dissolved in ethyl ether and the amine salts were removed by vacuum filtration through Celite. The ethereal filtrate was washed with 0.1 N HCl (2x), water (2x), and brine (Ix), dried with sodium sulfate, and concentrated to a clear oil which was dissolved in hexanes and concentrated to a white solid in vacuo. Crystallization from hexanes yielded 142 g of a white solid (75 % yield). FAB MS: 260.1 m/z (MH") (theory: 261.1 m/z (M+)). Elemental analysis: (theory: 50.55; H, 7.33; N, 5.36; O, 24.49; S, 12.27) found C, 50.26; H, 7.30; N, 5.20; S, 12.11.
Example 92 Synthesis of IsoCys(Boc)OPFP
DCC (4.11 g, 20 mmol) was added in five portions over 10 min to a solution of IsoCys(Boc)OH (4.8 g, 18 mmol) and 1.1 equiv of pentafluorophenol (3.42, 20 mmol)in freshly distilled CH2Cl2 (25 mL). The reaction mixture was stirred under N2 at 25 0C for 2 h, filtered to remove the insoluble urea, concentrated to ~ 20 mL under reduced pressure, and then stored at 4 0C for 2 h. An additional filtration removed further urea, and the product was crystallized from hot hexane. The resultant white precipitate was collected by filtration and dried in vacuum; yield 5.8 g (950Zo)-1H NMR (CDCl3): δ 1.43 (s, 6, Boc CH3); 1.49 (s, 3, Boc CH3); 1.81 (s, 3, Isopr CH3); 1.87 (s, 3, Isopr CH3); 3.24 (d-d, 1, CH2); 3.43 (d-d, 1, CH2); 5.14 (d, 1, CH). 19F NMR (CDC13): δ -162.24 (t, 2, CF); -157.70 (t, 1, CF); - 152.94 (d, 2, CF). GC MS: 445.0 m/z (M + NH4 +) (theory: 427.0 m/z (M+)). Elemental analysis: (theory: C, 47.77; H, 4.25; N, 3.28; S, 7.50) found C, 47.74; H, 4.19; N, 3.35; S, 7.48
Example 93 Synthesis of isoCys(Boc)Lys(isoCys(Boc))Lys(isoCys(Boc)Lys(isoCys(Boc)))OMe
LysLys(Lys)OMe (500 mg, 0.8 mmol) was dissolved in DMF (25 mL) with DIEA (550 mg, 4 mmol, and then HOBT (695 mg, 4 mmol) was added. After 5 minutes the IsoCys(Boc)OPFP, (2.78 g, 5.6 mmol) in DCM (21 mL) was added at 0 0C for 10 minutes. The mixture was stirred for 24 h at RT under N2. After concentration under vacuum the
mixture was dissolved in DCM (40 mL) washed by NaHCO3 (2x100 mL), water (2x100 mL) and dried over NaSO4. Evaporation of organic solvent gave an oil that was purified by silica gel chromatography (DCM-MeOH = 96/4): yield 951 mg (740Zo)-1H NMR (CDCl3): δ 1.19-1.68 (m, 18, CH2-CH2); 1.43 (s, 36, Boc CH3); 1.74 and 1.83 (2 x s, 24, Isopr CH3); 3.22 (m, 14, CH2-NH and CH2-S); 3.68 (s, 3, CH3-O); 4.29 (m, 1, CH-NΗ); 4.40 (m, 1, CH- NH); 4.49 (m, 1, CH-NH); 4.69 (m, 4, CH-N); 6.40-7.00 (m, 6, NH)-13C NMR (CDC13): δ 22.69-25.47 (CH2); 28.96-30.27 (CH3); 31.43 (CH2-S); 34.32; 37.11; 39.98; 52.74-53.32; 67.90; 72.00-74-10 (isopr Q; 82.05 (Boc Q; 152.32-154.23 (OCO-NH); 163.17 (CO- OCH3); 171.58-173.07 (CO). FAB MS: 1389.6 m/z (MH+) (theory: 1388.6 m/z (M+)). HR MS: 1390.8784 m/z (MH+) (theory: 1390.8799 m/z (MH+)). Elemental analysis: (theory: C, 54.44; H, 7.83; N, 10.08; S, 9.23) found C, 53.93; H, 7.70; N, 9.92; S, 9.15.
Example 94 Synthesis of isoCysLys(isoCys)Lys(isoCysLys(isoCys))OMe«4TFA
TFA (5 mL) was added in 10 portions over 10 min to a solution of isoCys(Boc)Lys(isoCys(Boc))Lys(isoCys(Boc)Lys(isoCys(Boc)))OMe, (600 mg, 0.4 mmol) in freshly distilled CH2Cl2 (30 mL) at 0 0C. The reaction mixture was stirred under N2 for 25 0C for 2 h. The solvent was removed by vacuum, and the mixture was precipitated in ether to afford a pure white compound 417 mg (97 0Zo)-1H NMR (CD3OD): δ 1.39-1.81 (m, 18, CH2-CH2); 1.73 (s, 24, Isopr CH3); 3.13-3.31 (m, 10, CH2-NH); 3.56 (m, 4, CH2-S); 3.68 (s, 3, CH3-O); 4.27 (m, 1, CH-NΗ); 4.36 (m, 2, CH-NΗ); 4.56 (m, 4, CH- NH-C)-13C NMR (CD3OD): δ 24.83-24.96 (CH2); 29.43-30.71 (CH3); 32.87-33.74 (CH2- S); 36.71-36.95; 40.94-41.38; 53.68-56.18; 65.78; 75.55-75.78 (isopr Q; 165.31 (CO- OCH3); 170.17-174.88 (CO). FAB MS: 990.4 m/z (MH+) (theory: 989.4 m/z (M+)). Elemental analysis: (theory: C, 42.38; H, 5.58; N, 9.69; S, 8.87) found C, 42.10; H, 5.77; N, 9.92; S, 9.01.
Example 95 Synthesis of CysLys(Cys)Lys(CysLys(Cys))OMe»4HCI isoCysLys(isoCys)Lys(isoCysLys(isoCys))OMe,(400 mg, 0.4 mmol) was dissolved in HCl IN-MeOH 50/50 (60 mL), and stirred under N2 at 25 0C for 4 h. The solvent was removed by vacuum, and the mixture was precipitated in ether to lead a pure white compound 350 mg (90 %). 1H NMR (DMSO d-6): δ 1.30-1.76 (m, 18, CH2-CH2); 2.76-
3.19 (m, 18, CH2-NH, CH2-SH and CH2-SH); 3.63 (s, 3, CH3-O); 4.02 (m, 2, CH-NH3 +); 4.13 (m, 2, CH-NH3 +); 4.18 (m, 2, CH-NH); 4.32 (m, 1, NH-CH-CO2CH3); 8.18, 8.47 and 8.81 (m, 18, NH and NH3 +). 13C NMR (DMSO d-6): δ 23.34-25.87 (CH2); 28.91; 31.99; 49.23; 52.49; 54.56; 167.36 (CO-OCH3); 172.26-178.39 (CO). FAB MS: 829.6 m/z (MH+) (theory: 828.3 m/z (M+)). HR MS: 829.3581 m/z (MH+) (theory: 828.3478 m/z (M+)). Elemental analysis: (theory: C, 38.19; H, 6.62; N, 14.37; S, 13.16) found: C, 37.99; H, 6.67; N, 14.21.
Example 96 Synthesis of the (succinic acid)2-PEG
(OH)2-PEG (10 g, 3 mmol) was dissolved in pyridine (30 mL) with succinic anhydride (5.88 g, 60 mmol), and stirred under N2 at 25 0C for 4 h. The solvent was removed by vacuum, and the mixture was precipitated in ether to afford a product 10.48 g (99 %).
Example 97 Synthesis of (succinic acid NHS)2-PEG
(succinic acid)2-PEG (Ig, 0.3 mmol) was dissolved in DCM with EDCI and DMAP and N-hydroxysuccinimide was added. The reaction was stirred at RT for 24 hours and the product isolated by precipitation. NMR obtained.
Example 98 Synthesis of (succinic acid cesium SaIt)2-PEG
(succinic acid)2-PEG (Ig, 0.3 mmol) was dissolved in water and the pH was adjusted to 7.5 with CsCO3. The solvent was removed to obtain the pure compound (99%).
Example 99 Synthesis of (dimethyl acetal succinic ester)2-PEG
(dimethyl acetal succinic ester)2-PEG was prepared by reaction of (succinic acid cesium SaIt)2-PEG3(Ig, 0.3 mmol), with bromoacetaldehyde dimethyl acetal (133 μl, 1.2
mmol) in DMF (5 mL) at 60 0C for 3 days. The solvent was removed by vacuum, and the mixture was precipitated in ether.
Example 100 Synthesis of (dialdehyde succinic estertø-PEG
(dialdehyde succinic ester)2-PEG was obtain by treatment of (dimethyl acetal succinic ester)2-PEG, with TFA (5% H2O) in CH2Cl2 (1:3) at room temperature for 20 minutes. The solvent was removed by vacuum, and the product was precipitated in ethyl ether.
Example 101 Synthesis of PEG-([G1]-PGLSA-NHS)2
PEG-([G1 J-PGLSA-OH)2 (1.03 g, 0.232 mmol), which was dried under vacuum at 80 0C for three hours, was then dissolved in CH2Cl2 (40 mL). EDCI, DMAP, and N- hydrosuccinimide were added and the reaction was stirred for 24 hours. The product was isolated by precipitation in cold ethyl ether.
Example 102 Synthesis of PEG-(lys)2
(NH2)-PEG (1.0 g), which was dried under vacuum at 80 0C for three hours, was then dissolved in DMF (45 mL) with the DIEA (2.35 g, 18 mmol). HOBT was then added. After 5 minutes ZLys(Z)OPFP in DCM (30 mL) was added at 0 0C for 10 minutes. The mixture was stirred for 24 h at RT under N2. The reaction was then stopped and the product precipitated in ethyl ether. The Z groups were removed using Pd/C (10% w/w) and hydrogen gas. A solution of the intermediate was dissolved in MeOH (50 mL) and pour into the hydrogenation flask. The flask for catalytic hydrogenolysis was evacuated and filled with 50 psi of H2 before shaking for 1O h. The catalyst was filtered and washed with MeOH (20 mL). The product was isolated by precipitation in ethyl ether.
Example 103 Synthesis of PEG-(lys-succinate-NHS2)2
PEG-(lys)2 (1.0 g), which was dried under vacuum at 80 0C for three hours, was dissolved in CH2Cl2 (40 mL) and then succinic anhydride was added. The reaction was stirred for 24 hours and the succinic acid derivatized product was isolated by precipitation in ethyl ether. Next, this intermediate was dissolved in CH2Cl2 (40 mL) EDCI, DMAP, and N-hydrosuccinimide were added and the reaction was stirred for 24 hours. The product was isolated by precipitation in cold ethyl ether.
Example 104
Preparation of a non-covalently crosslinked gel/network using (didodecane methyl amine)2-PEG
The (didodecane methyl amine)2-PEG was prepared in two steps by first treating (NH2)-PEG with 8 equivalents of bromododecane, 15 equivalents of NaCO3 in reflux ethanol to obtain (didodecane amine)2-PEG. The (didodecane amine)2-PEG, 1, was then treated with methyl iodine to afford (didodecane methyl amine)2-PEG after precipitation in ether.
This cationic-hydrophobic linear polymer is likely to form a gel with the carboxylated terminated dendritic polymers.
Example 105
General Procedure for the Preparation of a Hydrogel Through Photocrosslinking ([GI]-PGLSA-MA)2-PEG
Five μL of solution containing 0.5% EY in HEPES buffer (0.1 M, 7.4 pH), 100 μL of 5.0 M TEA, and 1 μL of VP were mixed with 2 mL of a 55 % w/v solution of the dendritic polymer in HEPES buffer. Upon laser exposure (argon ion laser, λmax = 488 and 514 nm, 200 mW) for 60 s, the pink viscous liquid crosslinked into a clear, soft, flexible hydrogel. This reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties. The crosslinked process can be with a UV or visible light system.
Example 106
General Procedure for the Preparation of a Hydrogel Through Photocrosslinking [G3]-PGLSA-MA
Gels were prepared by dissolving [G3J-PGLSA-MA, DMPA, and VP (1,000:10:1 respectively) in CH2Cl2. The polymer solution was exposed to UV light from a UVP BLAK-RAY long wave ultraviolet lamp for 5 minutes. This reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties. The polymer may be crosslinked with a UV or visible light absorbing system.
Example 107
General Procedure for the Preparation of a Hydrogel Through Photocrosslinking MA-[G3]-PGLSA-PEG-OMe
Five μL of a solution of 0.5% EY in HEPES buffer (0.1 M, 7.4 pH), 100 μL of 5.0 M TEA, and 1 μL of VP were mixed with 2 mL of a 55 % w/v solution of the dendritic polymer in HEPES buffer. Upon laser exposure (argon ion laser, λmax = 488 and 514 nm, 200 mW) for 60 s, the pink viscous liquid crosslinked into a clear, soft, flexible hydrogel. This reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties. The polymer may be crosslinked with a UV or visible light absorbing system.
Example 108
General Procedure for the Preparation of a Hydrogel Through Treatment of LysLys(Lys)OMe with ([GI]-PGLSA-MA)2-PEG
The gel was prepared by mixing an aqueous solution of the LysLys(Lys)OMe dendron with the ([G1]-PGLSA-MA)2-PEG. For example, the dendron dissolved at 33% w/w in phosphate buffer pH=8.2 (10 mg dendron in 20 μl) and the ([Gl]-PGLSA-MA)2- PEG was dissolved at 50% w/w (50 mg ([G1]-PGLSA-MA)2-PEG in 50 μL) in the same buffer. These two solutions were mixed together to lead a gel. This reaction can be
performed under a variety of concentrations of polymer to prepare gels with much different physical and mechanical properties.
Example 109
General Procedure for the Preparation of a Hydrogel Through Treatment of LysLys(Lys)OMe with PEG n-hydroxysuccinimide ((NHS)2-PEG)
The gel was prepared by mixing an aqueous solution of the LysLys(Lys)OMe dendron with the PEG NHS. For example, the dendron dissolved at 33% w/w in phosphate buffer pH=8.2 (10 mg dendron in 20 μl) and the PEG diNHS (commercially available, Mw = 3400) was dissolved at 55% w/w (50 mg PEG diNHS in 40 μL) in the same buffer. These two solutions were mixed together to lead a gel. Gelation was over in a few minutes. This reaction can be performed under a variety of concentrations of polymer to prepare gels with much different physical and mechanical properties.
Example 110
General Procedure for the Preparation of a Hydrogel Through Treatment of LysLys(Lys)OMe with PEG dimaleimide ((MAL)2-PEG)
The gel was prepared by mixing an aqueous solution of the LysLys(Lys)OMe dendron with the PEG-MAL. For example, the dendron dissolved at 33% w/w in phosphate buffer pH=8.2 (10 mg dendron in 20 μl) and the PEG dimaleimide (commercially available, Mw = 3400) was dissolved at 55% w/w (50 mg PEG dimaleimide in 40 μL) in the same buffer. These two solutions were mixed together to lead a gel. Gelation occurs over 15 minutes. This reaction can be performed under a variety of concentrations of polymer to prepare gels with much different physical and mechanical properties.
Example 111
General Procedure for the Preparation of a Hydrogel Through Treatment of LysLys(Cys)Lys(CysLys(Cys))OMe»HCl with PEG dialdehdye ((CHO)2-PEG) or (dialdehyde succinic ester)2-PEG
The gel was prepared by mixing an aqueous solution of the CysLys(Cys)Lys(CysLys(Cys))OMe*4HCl dendrons with the peg-dialdehyde or (dialdehyde succinic ester)2-PEG. For example, the dendron dissolved at 33% w/w in
phosphate buffer pH=7 (10 mg dendron in 20 μl) and the PEG compound was dissolved at 55% w/w (50 mg PEG in 40 μl) in the same buffer. These two solutions were mixed together to lead a gel. Gelation occurs almost immediately. This reaction can be performed under a variety of concentrations of polymer to prepare gels with much different physical and mechanical properties.
Example 112
General Procedure for the Preparation of a Hydrogel Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl with PEG NHS ((NHS)2-PEG)
The gel was prepared by mixing an aqueous solution of the CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl dendrons with the PEG(NHS)2. For example, the dendron dissolved at 33% w/w in phosphate buffer pH=7 (10 mg dendron in 20 μl) and the PEG compound was dissolved at 55% w/w (50 mg PEG in 40 μl) in the same buffer. These two solutions were mixed together to lead a gel. Gelation occurs almost immediately. This reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties.
Example 113
General Procedure for the Preparation of a Hydrogel Through the Formation of Disulfide Bonds
The gel was prepared by allowing a solution of 22 mg of CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl in 40 μL in phosphate buffered solution to rest for one week. The solution forms a weak hydrogel.
Example 114
The mechanical properties of photocrosslinked gels composed of a [G3]-PGLSA dendrimer, a ([Gl]-PGLSA)2-PEG dendritic-linear hybrid macromolecule, a [G3]- PGLSA-PEG-OMe dendritic-linear hybrid macromolecule, a PEG (3400 Mw), and a PEG (8000 Mw) were determined. These three biodendritic polymers were selected since they span a range of biodendrimer compositions and structures. Cylindrical constructs of the photocrosslinked hydrogels were prepared as previously described, and evaluated for their compressive and shear properties on a dynamic mechanical spectrometer. Results showed a minimal sensitivity of the shear properties to concentration for the ([Gl]-PGLSA)2-PEG
biodendrimer, with a much stronger sensitivity to the compressive moduli. The biodendrimer-based hydrogel showed highly elastic properties (δ = 7°) at 7.5% crosslinkable biodendrimer concentration and more visco-elastic behavior at 15 and 20% crosslinkable biodendrimer concentrations (δ = 61°). The dynamic shear stiffness, however, varied little over the range of concentrations from 10-20%, with values less than 2 kPa. In contrast, the compressive modulus E showed a stronger concentration dependence ranging from 5 kPa at 7.5% to 21 kPa at 20% macromer concentration. There was a strong sensitivity of the shear stiffness to concentration, from G* < 1 kPa for the 20% crosslinkable ([G3]-PGLSA)-PEG biodendrimer concentration, to nearly 1000 kPa for the 50% concentration of the [G3]-PGLSA crosslinkable biodendrimer. All hydrogel networks formed from the [G3] biodendrimers were highly elastic. This illustrates a unique feature of the biodendrimer scaffolds, that the physical properties are a nonlinear function of the dendrimer "generation" and macromer concentration, reflecting an interaction between higher numbers of available crosslinking sites (i.e., higher generation), structure (dendrimer vs. hybrid dendritic-linear polymer), size, and flexibility prior to crosslinking (e.g., ([G3]- PGLSA)-PEG vs. ([Gl]-PGLSA)2-PEG vs. [G3]-PGLSA)).
Table 1. Mechanical Properties of photocrosslinked gels.
■t^eq |G*|
% w/v Gel δ / °
/kPa (KPa)
20 % ([GI]-PGLSA-MA)2-PEG 34 1.7 61
15 % ([GI]-PGLSA-MA)2-PEG 20 1.7 61
10 % ([GI]-PGLSA-MA)2-PEG 6.4 1.3 14
7.5 % ([GI]-PGLSA-MA)2-PEG 3.7 1.2 7
50% [G3]-PGLSA-MA 940 7
20 % ([G3]-PGLSA-MA)-PEG 0.21 —
* Data collected at 10 rad/sec.
Example 115
General. All chemicals and culture media were used as received and were stored at room temperature, in the dark, or 4 0C where appropriate. All errors are reported as data ± one
standard deviation. AU macromer concentrations were reported as percentage weight macromer per weight solvent; macromer solutions showed densities of 1.04 ± 0.02, 1.03 ± 0.01, and 1.04 ± 0.03 for solutions at 7.5, 10, and 15 % macromer 1 in PBS, respectively. Chondrocyte growth medium consisted of DMEM supplemented with 10 % FBS, 2.5 mg/mL phosphate-C, and 5 mL penicillin/streptomycin (Invitrogen, Carlsbad CA). Washing medium consisted of DMEM high glucose with L-glutamine, 110 mg/mL sodium pyruvate with pyridoxine HCl, 3.3 mL/L 300x stock gentamycin, 10 mL/L 10Ox stock kanamycin, and 5 mL/L 20Ox fungizone (Invitrogen, Carlsbad CA).
Part I: Hydrogel Synthesis. The PEG34Oo-(PGLSA-MA4)2 macromer 1 (Figure 11) was synthesized from poly(ethylene glycol) with an weight average molecular weight of 3400 g/mol as a core and biodendrimers based on glycerol (GL) and succinic acid (SA), as described previously by Carnahan et al. See Carnahan, M. A.; Middleton, C; Kim, J.; Kim, T.; Grinstaff, M. W., J. Am. Chem. Soc. 2002, 124, 5291-5293. In order to evaluate the mechanical properties of the crosslinked biodendrimer macromer 1 solutions at 7.5, 10, 15, and 20 % w/w in PBS (Dulbecco's Phosphate Buffered Saline, Invitrogen) were mixed with 5 % photoinitiator solution (0.1 % eosin-Y, 4 % N-vinyl-2-pyrrolidinone, and 40 % triethanolamine in PBS, Invitrogen). The resulting solution was subsequently crosslinked in cylindrical molds (ø S mm, h = 2 mm) with long-wave UV (30 minutes), a filtered Xenon arc lamp (2 minutes with a Spectra-Physics/Oriel, 300W Xe lamp with filter #59070, yielding -100 mW at 510 nm), or an Argon laser (60 seconds at 514 run, 200 mW, Ultima SE 120V, Lumenis), to form a three-dimensional turgid hydrogel. The resulting pellets were stored in PBS at room temperature or 37 0C. Due to the absence of significant swelling, all concentrations reported here are initial macromer concentrations, uncorrected for swelling after crosslinking.
Part II: Hydrogel Swelling and Degradation. Cylindrical hydrogel samples for swelling and degradation testing were prepared by crosslinking biodendrimer solutions at 7.5, 10, and 15 % w/w (n = 3) with the eosin-based initiator mentioned in the previous section in cylindrical molds (ø 8 mm, h = 2 mm) with a filtered Xenon arc lamp (2 minutes with a Spectra-Physics/Oriel 300W Xe lamp with filter #59070, yielding -100 mW at 510 nm). The crosslinked hydrogel pellets were subsequently stored at 37 0C in phosphate buffered saline (PBS, Invitrogen) or chondrocyte cell culture medium, both supplemented with 0.1 % NaN3 to prevent bacterial or fungal infection. The weight of the samples was measured over a period of 35 days, and was normalized by the weight of the samples immediately
after crosslinking. The weight change over time used as an estimate of swelling and hydrogel degradation in cell culture medium or PBS, in the absence of chondrocytes. The results are presented in Figure 12.
Part HI: Mechanical Testing. Cylindrical hydrogel samples for dynamic mechanical testing were prepared by crosslinking biodendrimer solutions at 7.5, 10, 15, and 20 % w/w with the eosin-based initiator mentioned above in cylindrical molds (ø 8 mm, h = 2 mm) with long- wave UV (30 minutes). The samples were removed from the molds and were subsequently allowed to equilibrate in PBS solution for 3 days at room temperature. After equilibration, the samples were investigated in a strain-controlled rheometer (ARES, Rheometrics Scientific) at 0.1 to 100 Hz (maximum strain amplitude of 0.05) with a parallel-plate geometry immersed in a PBS bath. No significant frequency dependence was detected in the observed frequency range. The frequency independent dynamic shear modulus I G* I , storage modulus G', loss modulus G", and the loss angle δ are consequently shown as averaged from experiments at 5 to 10 rad/s, allowing estimation of the errors from the standard deviation. Compressive stress-relaxation experiments were performed on similar samples up to 20 % compressive strain in increments of 5 % to determine the equilibrium compressive modulus. The compressive moduli were determined by linear regression of the equilibrium stress versus strain data; errors were estimated from the standard deviation. The results are presented in Figures 13 and 14. Part IV: Chondrocyte Encapsulation. Chondrocytes were isolated from the femoral condyles of skeletally immature porcine knees (3-5 months) using an enzymatic digestion protocol described previously. See Kuettner, K.; Pauli, B.; Gall, G.; Memoli, V.; Schenk, R., J. Cell. Biol. 1982, 93 (3), 743-50. Cells were washed with washing medium and were subsequently re-suspended at 2x107 cells/mL in either a 7.5 % w/w or 15 % w/w biodendrimer solution with 5 % of the photoinitiator system. Samples (100 μL) of the cell suspensions were placed in cylindrical molds (ø 8 mm, h = 2 mm) and crosslinked with an argon-ion laser (514 nm, 200 mW, 60 sec, Ultima SE 120V, Lumenis, Santa Clara, CA) to create cell-gel constructs. These constructs were placed in individual wells and cultured in chondrocyte culture medium in a humidified atmosphere at 37 0C with 5 % CO2. The culture medium was replaced every 3 days. Unseeded constructs incubated under the same conditions served as controls. Constructs containing cells were harvested at 2, 4, and 12 weeks (n = 3) and processed for histology. Control constructs without chondrocytes were harvested at 12 weeks (n = 2) and studied similarly.
Part V: Histology and Immunohistochemistry. Cell-gel constructs were placed in paraformaldehyde for 30 minutes, followed by dehydration in a graded series of ethanol prior to embedding in paraffin. Paraffin-embedded sections were stained with H&E, Safranin-O, or Masson's Trichrome for histological evaluation. Sections were also immunolabelled for the presence of types I (Sigma C2456) and II (DSHB II-II6B3) collagen, with visualization via a horseradish-conjugated secondary antibody. The results are presented in Figure 15.
Example 116
General Procedure for the Preparation of a Hydrogel Through Photocrosslinking ([GI]-PGLSA-MA)2-PEG in the presence of Polyvinylpyrrolidone
Five μL of solution containing 0.5% EY in HEPES buffer (0.1 M, 7.4 pH)> 100 μL of 5.0 M TEA, and 1 μL of VP were mixed with 2 mL of a 55 % w/v solution of the dendritic polymer in HEPES buffer. To this solution was added polyvinylpyrrolidone (weight average molecular weight of 55,000 g/mol) such that the final concentration was 2.5 wt%. Upon laser exposure (argon ion laser, λmax = 488 and 514 nm, 200 mW) for 60 s, the pink viscous liquid crosslinked into a clear, soft, flexible hydrogel. This reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties. The crosslinking process can be performed with UV or visible light.
Example 117
General Procedure for the Preparation of a Hydrogel Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe»4HCI with PEG(NHS)2 in the presence of Polyvinylpyrrolidone
The gel was prepared by mixing an aqueous solution of the CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl dendrons with the PEG(NHS)2. For example, the dendron dissolved at 33% w/w in phosphate buffer pH=7 (10 mg dendron in 20 μl) and the PEG compound was dissolved at 55% w/w (50 mg PEG in 40 μl) in the same buffer. To the PEG solution was added polyvinylpyrrolidone (weight average molecular weight of 55,000 g/mol) such that the final concentration was 2.5 wt% in the sealant formulation. These two solutions were mixed together to afford a gel. Gelation occurs within 30
seconds. This reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties.
Example 118
General Procedure for the Preparation of an Antimicrobial Hydrogel Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl with PEG(NHS)2 in the presence of PHMB
The gel was prepared by mixing an aqueous solution of the CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl dendrons with the PEG(NHS)2. For example, the dendron dissolved at 33% w/w in phosphate buffer pH=7 (10 mg dendron in 20 μl) and the PEG compound was dissolved at 55% w/w (50 mg PEG in 40 μl) in the same buffer. To the PEG solution was added polyhexamethylenei biguanide (PHMB), a well known antimicrobial such that the final concentration was 0.05 wt% or lower in the sealant formulation. These two solutions were mixed together to afford a gel. Gelation occurs within 30 seconds. PHMB did not affect hydrogel sealant formation. This reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties.
Example 119
Set Time for CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl with PEG(NHS)2 to Form a Sealant/Adhesive
A tunable and controlled set time of the adhesive is a desired characteristic since it can ensure a good seal with the tissue but also because it can be optimized with the delivery device. A gel-time test, or time to cure test, was developed for the sealant/adhesive. The test required a magnetic stir plate that was set to 1050-1100 rpm using a calibrated tachometer. A 1.5 mL autosampler vial containing a micro stir bar was then placed on the stir plate. The dendron (4.7 mg) and the PEG (32.8 mg) were dissolved in their respective buffers and added to the vial by: 1) manually using a pipetter, 2) pass through mixing using two connected syringes and the mix tip, 3) materials added to the individual barrels of the double barreled syringe, dissolved, and expressed into the through the mix tip, or 4) double volume with pass through mixing. Timekeeping began as soon as the materials were mixing in the vial. The cure time occurred when the material gelled enough to stop rotation of the stir bar. The weight of the polymer express into the vials was determined.
The results were analyzed by comparing the individual and average cure times for the different dispensing methods. Hydrogel sealants/adhesives were formed generally between 11 and 30 seconds, and there was no significant difference in the set times between the different delivery methods. However, dissolving the powders in the syringe rather than using the two syringe pass-through method provided a better result since the adapters tended to leak. If leakage occurred, the concentration/stoichiometry of the reactants was mismatched; thus the sealant could take more than a minute to gel.
Example 120
Swelling Observed for Hydrogel formed from
CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl and PEG(NHS)2 to form a Sealant/Adhesive
Swelling was performed by soaking the polymerized material in buffer and monitoring the weight gain over time. After waiting at least 15 minutes for the materials to fully cure, 1.0 mL of phosphate-buffered saline was added to the vial and the swelling time started. The weight gain due to swelling was measured at multiple time points by decanting
off the buffer, drying the residual liquid off the polymer using a cotton swab and nitrogen, and weighing the vial. The buffer was then replaced and swelling continued.
The degree of swelling was determined by dividing the weight of the polymerized material at t=0 into the weight of the swollen polymer. The target time was the point at which swelling reached (or nearly reached) a plateau, and any additional swelling would be insignificant when comparing different materials/methods. Figure 16 is a plot of the swelling results. The target point seems to appear at 48 hours. The average swelling results (Figure 17) also indicate that use of the mix tip did not provide as good a network when compared to the manual mix in the vial. The results are more consistent and show less swelling when mixed manually or directly. Importantly, these hydrogel sealants/adhesives swell to less than 150 wt%. Thus, one aspect of the invention relates to hydrogel systems that have tunable swelling, wherein the swelling can range from more than about 500% to less than about 150%.
Effect of pH on Set Time and Swelling for Hydrogel Formed from CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl and PEG(NHS)2 to Form Sealants/Adhesives
The optimal buffer system was determined to optimize the gel time and swelling of the materials. Generally, the rate of polymerization increased with increasing dendron buffer pH, when the PEG buffer remained at a constant pH. A formulation was found that possessed the appropriate setting time (< 30 seconds) while yielding minimal swelling (<150 wt%). The poly(ethylene glycol) of PEG(NHS)2 has a number average molecular weight of 3400 g/mol.
Three different pHs for the dendron buffer (a carbonate buffer) and a pH 7.0 phosphate buffer solution for the PEG-SPA mixture were investigated (PEG refers to poly(ethylene glycol), SPA refers to succinimidyl propionic acid). The buffers were prepared in 5 different millimolar concentration ratios of phosphate to carbonate ranging from 1 to 300 millimolar. The respective phosphates and carbonates at different specified molarities were used to dissolve the PEG-SPA and dendron respectively before combining the dissolved solutions to form a hydrogel. The five buffer combinations containing different ratios of phosphates to carbonates were prepared from individual stock solutions of the monobasic and dibasic components; each final solution was made from blending and dilution. The different pH buffers for the dendron were further prepared by adjustment with a NaOH solution. The manual mix method was used to determine the gel time. Swelling experiments were performed with the hydrogel in phosphate buffered saline (PBS) at pH 7.4 using time points of 48 and 72 h. The experiment was repeated in triplicate to reduce error.
Table 4. Design for Buffer Study
* Buffer Ratio refers to the ratio of carbonate concentration in the dendron buffer to the concentration of phosphate in the PEG-SPA buffer.
Table 5. Average Gel Time and Swelling Results for All Groups
The three individual groups were averaged and the data is shown in Figures 18 and 19. As expected, the cure time decreased (approximately 5 seconds) when the buffer pH was increased from 8.5 to 9.5. Changing the buffer ratio while holding pH of the dendron constant had a similar effect. Increasing pH and maximizing the concentration of carbonate in the dendron buffer rendered these effects additive, reducing the gel time by approximately 12 seconds. These effects are clearly seen in the response surface (Figure 18) and contour (Figure 19) plots.
These factors affected the degree of hydrogel swelling differently. The swelling at low buffer ratio changed little across the range of dendron buffer pH. However, the effect was magnified slightly at higher buffer ratios. Similarly, swelling changed little at low pH across the range of buffer ratios, which magnified the differences at the higher pH. The response surface plot (Figure 20) shows a minimum in the swelling curve at a buffer ratio of 150. The effect of pH on the curve at that buffer ratio is relatively insignificant. Therefore, the 150 buffer ratio appears to be an appropriate choice for minimizing swelling. Similar conclusions can be made using the contour plot in Figure 21. These results appear to hold in the pH range of 8.5 to approximately 9.1, at the 150 buffer ratio.
The 48-hour results are consistent with those at 72 hours; however, the effect of the minimum at the 150 buffer ratio reduced (Figure 22). The contour plot in Figure 23 shows an additional area of reduced swelling in the range of pH 8.7 to 8.9 at the 150 buffer ratio. This further defines the area in which the buffers should be formulated.
The conclusions from this study are: 1) a buffer ratio of 150 provided the least swelling, and 2) a pH of 9.0 would be the most appropriate for the dendron buffer. These parameters optimize the balance between minimizing the gel time and swelling.
Example 122
Effect of pH and Buffer Concentration on Set Time and Swelling for CysLys(Cys)Lys(CysLys(Cys))OMe»4HCI with PEG(NHS)2 to form Sealants/ Adhesives
After determining the appropriate buffer ratio and pH for the PEG and the dendron, respectively, it was determined that lowering the overall concentration of buffer in the two part system may further reduce swelling. Moreover, an equal ratio of reactive functional groups would provide a tighter network. The volumes of the buffers were also adjusted to
provide equivalent weight percentages of components and volumes of solution, once the powders dissolved.
Buffers were prepared for the dendron and the PEG at the concentrations listed in Table 6 below. The buffers were adjusted to pH 7.0 and 9.0 for the PEG and the dendron, respectively. Total Buffer, as listed in Table 6, refers to the total buffer concentration in the experiment after combining the pH 9 carbonate buffer and pH 7 phosphate buffers, for example. The weights of the individual units used and the volumes of buffer are shown in Table 7. The dendron was weighed as a 4x sample into a single container in order to minimize weighing errors that may be associated with a 4.7 mg sample. The buffer was added to the dendron at 4x scale also. However, a Ix scale of each solution was used for the actual testing.
The standard procedures were followed for determining the gel time and degree of swelling.
Table 6. Buffer Concentrations
The results for the gel time of the various systems (Table 8) indicated that there was no significant difference in set time. The 150-ratio sample, determined as optimal from the previous study, provided the lowest gel time, showing a minimum at that buffer level. The relationship of the overall buffer concentration to the degree of swelling was not very significant. However, there may be a concentration of buffer (approximately 240 mM) that may cause a significant increase in swelling when exceeded. The can be seen in the difference between the samples with overall buffer concentrations of 240 mM and 280 mM. The 280 mM sample also had the longest average gel time with a large standard deviation.
An extension of the study was performed to further investigate the effect of the dendron pH on the system. Materials were tested with buffers of varying concentrations, but with constant pH's of 7.0 for the PEG and 9.5 for the dendron. Previous studies have indicated that this may reduce the average gel time of the samples.
Table 9
Table 10
The effect of dendron buffer pH on cure time and swelling was conducted using Lys3-Cys4-OMe dendron and PEG-SPA. The PEG-SPA buffer pH was 7.0 for all samples. The dendron buffer pH was varied from 9.0 to 10.5 at a constant concentration of 200 mM (150:1 ratio). The dendron was measured out in bulk, while the PEG-SPA was measured out as single units. The curing test was conducted using direct mixing and the swelling was
measured after 48 hours. The average results for the tests are shown in Figures 24 and 25. Note: The dendron sample at pH=10.0 did not dissolve completely, since it is difficult to dissolve it in the higher pH buffers, which affected the results.
Example 123
Effect of Stoichiometery on Set Time and Swelling for CysLys(Cys)Lys(CysLys(Cys))OMe-4HCl with PEG(NHS)2 to form Sealants/Adhesives
After determining the buffer concentration and buffer pH, a design of experiments (DOE) was performed to determine the sensitivity of cure time and swelling to the stoichiometry of the dendron and PEG. The design involved setting the balanced equivalents weights of the dendron (4.7 mg) and the PEG (36.0 mg) to equal one equivalent (1.0 equiv) in the design (Table 11). The range of equivalents for each reactant was set from 0.9 to 1.1, using 1.0 as the center point. The chosen design was a central composite response surface that was rotatable, with points outside the range at 0.86 and 1.14 equivalents. The design and material weights are shown in the table below. The same design was also applied to use of the solutions in the syringe/mix-tip combination device. The responses monitored for the samples were cure time and swelling after 48 h.
Table 11. Stoichiometry Experimental Design
The samples for the dendron were measured at 5x weight into a centrifuge tube, while the PEG was measured at Ix. Appropriate amounts of pH 7.0 and pH 9.5 were added to the tubes, and the samples allowed to mix until dissolved. The samples were then transferred to the curing vial, 135 μL each, adding the PEG first. Set time was then
measured. Phosphate-buffered saline was added to the samples, which were allowed to swell for 48 h. The samples were subsequently drained, dried and weighed.
Results for all manually mixed samples are shown in Table 12. The cure times ranged from 26-99 sec and the swelling ranged from 41.8-66.7%. Their averages, along with the results for the mix-tip samples, are shown in Table 13. There is no significant difference in the Mix-Tip results versus the manual mixed averages for cure time and swelling, considering only one sample was tested for each group using the tip.
Table 12. Cure time and Swelling Results for Manually Mixed Samples
The results were analyzed using a statistical DOE software package. The model was the rotatable central composite design with two center points and two replicates. The results indicate that the low equivalents of dendron combined with the high for the PEG produces the lowest level of swelling in the manually mixed samples. This is seen in the response surface plot (Figure 26). There is an apparent swelling plateau along the range of dendron equivalents when the PEG level is at its lowest value. The opposite is true at the highest level of PEG, where the swelling runs from minimum to near maximum value with increasing dendron equivalents. These trends appear to reverse when looking at across the PEG range at the high and low values of the dendron. Therefore, to achieve the lowest swelling in the manually mixed samples, the balance should generally be 0.9 equiv dendron and 1.1 equiv PEG. The degree of swelling is low and the range is very tight at all combinations.
The same trends appear to apply to the mix-tip samples. However, the degree of swelling is higher, and therefore the trends are more pronounced (Figure 27). The minimum value for swelling in the mix-tip samples is almost equivalent to that of the manually mixed samples. The mix-tip appears to have a greater sensitivity to the stoichiometry of the two reactants.
The cure time results indicate that the trends are the inverse of the swelling results (Figure 28). The longer cure times occur at the combination that gave the lowest degree of swelling: 0.9 equiv dendron and 1.1 equiv PEG. The set time is more sensitive to changes in the equivalents of the dendron than that of the PEG. High equivalents of dendron and the nominal quantity of PEG give the lowest cure time. The same trends and almost identical cure time values can be seen for the mix-tip samples (Figure 29).
Example 124
Buffer for CysLys(Cys)Lys(CysLys(Cys))OMe-4HCl with PEG(NHS)2 to form a Sealant/Adhesive
The components, pH, and ionic strength of the buffer solution can be important for sealant/adhesive use. The following buffer was selected for this adhesive formulation after examination of different buffers. One acceptable range for the ionic strength in this formulation for a sealant application is ± 25%.
118 mM NaCl
5.I mM KCl
100-15O mM Na2HPO4
20-3O mM NaHCO3
2.I mM CaCl2
2.0 mM MgCl2
Example 125
Antimicrobial Properties of a Hydrogel Formed from
CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl and PEG(NHS)2
The antimicrobial properties of the hydrogel were tested by incubating the crosslinked adhesive/sealant with the organism Bacillus Atrophaeus (ATCC 9372) at a concentration of 10,000 cfu. The test employed a non-sterile adhesive, a sterile adhesive, and a positive control (N=5/group). After 24 hours, only the positive control supports the bacteria. Thus, the hydrogel adhesive acts as a barrier to bacteria.
Example 126
Antimicrobial Properties of a Hydrogel Formed from
CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl and PEG(NHS)2 Containing 0.005 wt% Polyhexamethylene biguanide (PHMB)
The antimicrobial properties of a hydrogel containing polyhexamethylene biguanide (PHMB) were tested by incubating the crosslinked adhesive/sealant with the organism
Bacillus Atrophaeus (ATCC 9372) at a concentration of 10,000 cfu. The test employed a non-sterile adhesive, a sterile adhesive, and a positive control (N=5/group). After 24 hours, only the positive control supports the bacteria. Thus, the hydrogel with PHMB adhesive acts as a barrier to bacteria, and the hydrogel functions as an antibacterial formulation.
Example 127
E-Beam Sterilization of a Hydrogel Sealant Formed from CysLys(Cys)Lys(CysLys(Cys))OMe-4HCl and PEG(NHS)2
The sealant formulation was sterilized using E-beam. After E-beam sterilization, the dendron and PEG(NHS)2 solutions foπned a hydrogel sealant with 30 seconds. Thus, E- beam sterilization is an acceptable sterilization method for the hydrogels of the invention.
Example 128
Securing a Corneal Incision with a Hydrogel Sealant Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl with PEG(NHS)2
The rabbits (N=3) were anesthetized by intramuscular injection of a combination of ketamine hydrochloride and xylazine (34 mg/kg + 5 mg/kg) at a dose of 0.6 mL/kg. Each rabbit was injected subcutaneously with 0.2 mg/kg buprenorphine. Following general anesthesia, a peri-ocular surgical prep was performed by flushing the eyes with a 1:10 dilution of povidone iodine in saline and swabbing the eyelids and surrounding skin. The eyes were flushed with balanced salt solution (BSS), making sure any hair or debris is removed. Following the preparation, an antibiotic-steroid solution, such as Maxitrol® (neomycin/polymyxin/dexamethasone) and 0.5% proparacaine hydrochloride topical anesthetic was instilled into each eye. The rabbit was positioned under the operating microscope and draped. A wire lid speculum was placed. IOP measurement was made prior to the creation of the incision. The conjunctiva was opened and reflected back on the dorsomedial side of the eye. A 2.7-3.0 mm linear incision at the limbus (single plane incision) will be made with a Becton-Dickenson 3 mm slit knife (or equivalent from an alternate manufacturer). At a spot opposite the incision, ~180° limbal, slight pressure (Seidel Test) was applied to the eye with a sterile swab to demonstrate leakage of aqueous humor. Before applying the test material, the incision was dried with a sterile surgical
swab. Approximately 0.03 mL of the adhesive was applied and the adhesive was gelled within 30s. Upon application of slight pressure, no leakage was observed and the wound was sealed for all eyes.
Example 129 General Procedure for the Eye Surgeries Involving a Corneal Transplant
An enucleated human eye (NC Eye Bank) or pig eye was placed under a surgical microscope with the cornea facing upwards. A 5.5 mm central corneal trephination was made in an enucleated eye, and then this newly-formed button will then be autografted back to the original eye. For the biodendrimer sealant formulations, 20 μL sealant was applied to the wound edges to secure the autograft after 8 or 16 sutures were put into place. Leaking/bursting pressures for all eyes was determined as done for the corneal laceration studies. Evidence of major wound leakage or wound dehiscence was used as endpoints for our bursting pressure studies. Fluorescein dye will be applied to the wound and the surrounding area using a Fluorets strip (Chavvin) to look for wound leakage. Specifically, the crosslinkable polymer sealant system containing the
CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl dendron and PEG(NHS)2, (3400 Mw) was used. The crosslinkable polymer system was applied to the wound and it sealed the wound in less than 20 seconds. Similar results were obtained when using CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl dendron and PEG-dialdehyde (PEG-ALD2) or poly(ethylene glycol) dimaleimide (PEG-MAL2) to form the hydrogel sealant, wherein the PEG had a weight average molecular weight of 3400 g/mol.
Example 130
General Procedure for the Securing a Skin Laceration Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl with PEG(NHS)2 to Form a
Sealant/Adhesive
A 3 cm by 5 mm incision was made in pigskin through the dermis using a scalpel ex vivo (N=3). The wound was dried with a tissue and the crosslinkable polymer sealant system containing the CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl dendron and PEG(NHS)2, (3400 Mw) was applied as the two edges of the tissue were squeezed. The sealant filled the skin defect and was gelled within 30 seconds.
Example 131
General Procedure for Securing a Liver Laceration Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl with PEG(NHS)2 to Form a Sealant/Adhesive
A wound 3 cm long and 5 mm deep was made in chicken liver using a scalpel ex vivo (N=3). The wound was dried with a tissue, and the crosslinkable polymer sealant system containing the CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl dendron and PEG(NHS)2, (3400 Mw) was applied as the two edges of the tissue were squeezed. The sealant filled the liver injury and was gelled within 30 seconds.
Example 132
Securing a Corneal Incision with a Hydrogel Sealant Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe-4HCl with PEG(NHS)2 in the presence of Polyvinylpyrrolidone
Rabbits (N=3) were anesthetized by intramuscular injection of a combination of ketamine hydrochloride and xylazine (34 mg/kg + 5 mg/kg) at a dose of 0.6 mL/kg. Each rabbit was injected subcutaneously with 0.2 mg/kg buprenorphine. Following general anesthesia, a peri-ocular surgical preparation was performed by flushing the eyes with a 1:10 dilution of povidone iodine in saline and swabbing the eyelids and surrounding skin. The eyes were flushed with balanced salt solution (BSS), making sure any hair or debris is removed. Following the prep, an antibiotic-steroid solution, such as Maxitrol (neomycin/polymyxin/dexamethasone) and 0.5% proparacaine hydrochloride topical anesthetic was instilled into each eye. The rabbit was positioned under the operating microscope and draped. A wire lid speculum was placed. IOP measurement was made prior to the creation of the incision. The conjunctiva was opened and reflected back on the dorsomedial side of the eye. A 2.7-3.0 mm linear incision near the conjutiva (single plane incision) will be made with a Becton-Dickenson 3 mm slit knife (or equivalent from an alternate manufacturer). At a spot opposite the incision, ~180° limbal, slight pressure (Seidel Test) was applied to the eye with a sterile swab to demonstrate leakage of aqueous humor. Before applying the test material, the incision will be dried with a sterile surgical swab. Approximately 0.03 mL of the adhesive containing the PVP was applied and the adhesive was gelled within 30s. Upon application of slight pressure no leakage was observed and the wound was sealed for all eyes. With the PVP present in the formulation,
the adhesive remained on the eye for approximately 5 days and longer than when PVP was not used in the formulation.
Example 133
General Procedure for the Preparation of a Hydrogel Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl with Sebacic di-sulfoNHS ((sulfoNHS)2-SA)
A gel was prepared by mixing an aqueous solution of CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl dendrons with (sulfoNHS)2-SA. For example, the dendron was dissolved in phosphate buffer pH=7 and the (sulfoNHS)2-SA compound was dissolved in the same buffer. These two solutions were mixed together to give a gel. Gelation occurs almost immediately. The reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties.
Example 134
General Procedure for the Preparation of a Hydrogel Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe»4HCl with Sebacic di-sulfoNHS ((sulfoNHS)2-SA) and PEG(NHS)2
A gel was prepared by mixing an aqueous solution of CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl dendrons with (sulfoNHS)2-SA. For example, the dendron was dissolved in phosphate buffer pH=7 and (sulfoNHS)2-SA and PEG(NHS)2 were dissolved in the same buffer at a ratio of 1 : 1. These two solutions were then mixed together to lead a gel. Gelation occurs almost immediately. The reaction can be performed under a variety of concentrations of reagents to prepare gels with different physical and mechanical properties.
Example 135
General Procedure for the Preparation of a Hydrogel Through Treatment of CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl with a Low Molecular Weight ((NHS)2- PEG) (PEG molecular weight = 400 g/mol)
A gel was prepared by mixing an aqueous solution of CysLys(Cys)Lys(CysLys(Cys))OMe«4HCl dendrons with PEG(NHS)2. For
example, the dendron was dissolved in phosphate buffer pH=7 and the PEG compound was dissolved in the same buffer. These two solutions were mixed together to give a gel. Gelation occurs almost immediately. This reaction can be performed under a variety of concentrations of polymer to prepare gels with different physical and mechanical properties.
Incorporation by Reference
All of the patents and publications cited herein are hereby incorporated by reference.
Equivalents
Those skilled in the art will recognize, or be able to ascertain using no more than routine experimentation, many equivalents to the specific embodiments of the invention described herein. Such equivalents are intended to be encompassed by the following claims.
Claims
We claim:
1. A method of sealing a wound on a patient, comprising the steps of: applying an effective amount of a dendrimeric compound of formula Ia or formula Ib to a wound on a patient and treating said dendrimeric compound with a polymerization agent sufficient to polymerize said dendrimeric compound, wherein said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, or an oxidizing agent, wherein formula Ia is represented by:
A1— X1-B— X1— A2 Ia wherein
A represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
Y1 represents independently for each occurrence R4, A4,
Z1 represents independently for each occurrence -X1 -R4 , E, or
Y5 represents independently for each occurrence R8, A4,
Z5 represents independently for each occurrence -X1 -R8, E, or
R1 represents independently for each occurrence H, alkyl, or halogen;
R2 represents independently for each occurrence H, alkyl, -OH, -N(R10)2, -SH, hydroxyalkyl, or -[C(R])2]dR16;
R3 represents independently for each occurrence alkyl, aryl, or aralkyl; R4, R5, R6, R7, R8, and R9 are H;
R10 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R11 represents independently for each occurrence H, -OH, -N(R10)2, -SH, alkyl, hydroxyalkyl, or
R12 represents independently for each occurrence H, alkyl, aryl, or aralkyl;
R13 represents independently for each occurrence H, alkyl, aryl, or aralkyl;
R14 represents independently for each occurrence H, alkyl, or -CO2R10;
R15 represents independently for each occurrence H, alkyl, or -OR10;
R16 represents independently for each occurrence phenyl, hydroxyphenyl, rrolidyl, imidazolyl, indolyl, -N(R10)2, -SH, -S-alkyl, -CO2R . 1'0 , 1ON py ", -C(O)N(R1U)2, or - C(NH2)N(R10)2;
d represents independently for each occurrence 1, 2, 3, 4, 5, or 6; n represents independently for each occurrence 1, 2, 3, 4, 5, or 6;
p1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p2 represents independently for each occurrence 0, 1, 2, 3, or 4; p3 represents independently for each occurrence 1, 2, or 3; p4 represents independently for each occurrence 0, 1, 2, or 3; t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
v1 and v2 each represent independently for each occurrence 2, 3, or 4; w1 and w2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is O, 1, 2, 3, 4, or 5; z1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z2 and z3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
X1 and X2 each represent independently for each occurrence O or -N(R10)-;
X3 represents independently for each occurrence O, N(R10), or C(R15)(CO2R10);
provided that R4 only occurs once, R5 only occurs once, R6 only occurs once, R7 only occurs once, R8 only occurs once, and R9 only occurs once; said formula Ib is represented by:
Ib or a pharmaceutically acceptable salt, solvate, or hydrate thereof, wherein
X5 represents independently for each occurrence O or -N(R22)-;
R17 represents independently for each occurrence H, -(C(R19)2)hSH, -
R , 18 represents independently for each occurrence H or alkyl;
R19 represents independently for each occurrence H, halogen, or alkyl;
R20 represents independently for each occurrence H or alkyl;
R21 represents independently for each occurrence H, -(C(R19)2)hSH,
R22 represents independently for each occurrence H, alkyl, aryl, or aralkyl; n1 and h each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p5 represents independently for each occurrence 1, 2, 3, 4, or 5; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive; said formula II is represented by:
π wherein
R2"11 represents independently for each occurrence H or alkyl;
R3"11 represents independently for each occurrence H, halogen, or alkyl;
R4"11 represents independently for each occurrence alkyl, aryl, or aralkyl; and
R5"11 represents independently for each occurrence H or
R2'" and z represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; said formula IQ is represented by:
R-I-IIl β1-lll R-I-IIl
III wherein
R2"111 represents independently for each occurrence H, alkyl, or halogen;
x represents independently for each occurrence 0, 1, 2, 3, 4, 5, 6, 7, or 8;
y represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8;
z represents independently for each occurrence 0, 1, or 2;
v represents independently for each occurrence 2, 3, or 4; and
w is an integer in the range of about 5 to about 700, inclusive;
said formula IV is represented by:
A1— X1-B— X1— A2
IV wherein
A \. 3 represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
Z1 represents independently for each occurrence -X^R4, E, or
Y2 represents independently for each occurrence R ,
Z represents independently for each occurrence -X 1 - rR.5 , E, or
Z represents independently for each occurrence -X -R , E, or
Z5 represents independently for each occurrence -X1 -R8, E, or
Y represents independently for each occurrence R ,
R1 represents independently for each occurrence H, alkyl, or halogen;
R represents independently for each occurrence H, alkyl, -OH, -N(R 10 )\2, -SH, hydroxyalkyl, or -[C(R^2]CiR16;
R3 represents independently for each occurrence alkyl, aryl, or aralkyl;
R4, R5, R6, R7, R8, and R9 are H;
R10 represents independently for each occurrence H, alkyl, aryl, or aralkyl;
R11 represents independently for each occurrence H, -OH, -N(R1 °)2, -SH, alley 1, hydroxyalkyl, or
R12 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R13 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R14 represents independently for each occurrence H, alkyl, or -CO2R10; R15 represents independently for each occurrence H, alkyl, or -OR10;
R16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R10)2, -SH, -S-alkyl, -CO2R10, -C(O)N(R10)2, or - C(NH2)N(R10)2;
n represents independently for each occurrence 1, 2, 3, 4, 5, or 6; p1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p2 represents independently for each occurrence 0, 1, 2, 3, or 4; p3 represents independently for each occurrence 1, 2, or 3; p4 represents independently for each occurrence 0, 1, 2, or 3; d represents independently for each occurrence 1, 2, 3, 4, 5, or 6; t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
v1 and v2 each represent independently for each occurrence 2, 3, or 4; w1 and w2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is O, 1, 2, 3, 4, or 5; z1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z2 and z3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
X1 and X2 each represent independently for each occurrence O or -N(R10)-;
X3 represents independently for each occurrence O, N(R10), or C(R^)(CO2R10); and
E represents independently for each occurrence H,
or
or a pharmaceutically acceptable salt, solvate, or hydrate thereof,
wherein
X6 represents independently for each occurrence O or -N(R30)-;
R23 represents independently for each occurrence
R24 represents independently for each occurrence H or alkyl;
R25 represents independently for each occurrence H, halogen, or alkyl;
R26 represents independently for each occurrence H or alkyl;
R27 represents independently for each occurrence H, alkyl, or halogen;
R28 represents independently for each occurrence H, alkyl, -OH, -N(R30)2, -SH, or hydroxyalkyl;
R29 represents independently for each occurrence H, -OH, -N(R30)2, -SH, alkyl, or hydroxyalkyl;
R30 and R31 represent independently for each occurrence H, alkyl, aryl, or aralkyl;
Z6 represents independently for each occurrence E or
R >32 represents independently for each occurre
R ,34 represents independently for each occurrence H, alkyl, or -CO2R 30.
p ,6 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p7 represents independently for each occurrence 0, 1, 2, 3, or 4; p8 represents independently for each occurrence 1, 2, or 3;
p9 represents independently for each occurrence 0, 1, 2, or 3; n2 and j each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; m represents independently for each occurrence 1 or 2; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive.
2. The method of claim 1, wherein said dendrimeric compound is a compound of formula Ia, and said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, or an oxidizing agent.
5. The method of claim 1, wherein Z1 represents independently for each occurrence -X1 -R'
6. The method of claim 1, wherein Z2 represents independently for each occurrence -X1 -R5
7. The method of claim 1, wherein Z represents independently for each occurrence -X -R
8. The method of claim 1, wherein Z4 represents independently for each occurrence -X'-R7
9. The method of claim 1, wherein Z represents independently for each occurrence -X -R
10. The method of claim 1, wherein X1 is O.
11. The method of claim 1 , wherein X1 and X2 are O.
12. The method of claim 1, wherein n is 1.
13. The method of claim 1, wherein p1 is 2, 3, or 4.
14. The method of claim 1, wherein p is 1.
15. The method of claim 1, wherein R1 is H.
O O
19. The method of claim 1, wherein R1 is H, B is R1 RV y P 1 , A Λ 22 i •s
27. The method of any one of claims 16-26, whereinp is 1, 2, 3, or 4.
28. The method of any one of claims 16-26, whereinp1 is 2.
29. The method of any one of claims 16-26, wherein p1 is 4.
30. The method of any one of claims 16-26, wherein m is 1.
31. The method of claim 1, wherein B is ≠
is
35. The method of claim 1, wherein R1 is H, B is is
40. The method of any one of claims 31-39, wherein p1 is 1 , 2, 3, or 4.
41. The method of any one of claims 31-39, wherein p1 is 2.
42. The method of any one of claims 31-39, wherein p1 is 4.
43. The method of any one of claims 31-39, wherein m is 1.
44. The method of any one of claims 31-39, wherein R2 is (Ci-C3)alkyl.
46. The method of claim 1, wherein R1 is H, B is
47. The method of claim 1, wherein R1 is H, B is
49. The method of claim 1, wherein R1 is H, B is
50. The method of claim 1, wherein R is H, B is
51. The method of claim 1, wherein R1 is H, B is
52. The method of claim 1, wherein R1 is H, B is
53. The method of claim 1, wherein R1 is H, B IS
54. The method of claim 1, wherein R1 is H, B is
55. The method of claim 1, wherein R1 is H, B is
56. The method of claim 1, wherein R is H, B is
57. The method of any one of claims 45-56, wherein w1 is an integer in the range of about 50 to about 250.
58. The method of any one of claims 45-56, wherein w! is an integer in the range of about 60 to about 90.
59. The method of any one of claims 45-56, wherein p1 is 2.
60. The method of any one of claims 45-56, wherein m is 1.
61. The method of any one of claims 45-56, wherein p1 is 2, p2 is 0, and R3 is (Ci-Cs)alkyl.
62. The method of any one of claims 45-56, wherein p1 is 2, p2 is 0, R3 is (Ci-Csjalkyl, and w! is an integer in the range of about 60 to about 90.
65. The method of claim 1, wherein R1 is H, B is
71. The method of any one of claims 63-70, wherein p1 is 2.
72. The method of any one of claims 63-70, wherein m is 1.
73. The method of any one of claims 63-70, wherein p1 is 2, p2 is 0, and R3 is (Ci-C5)alkyl.
74. The method of any one of claims 63-70, wherein p1 is 2, p2 is 0, and R3 is (Ci-C5)alkyl, and w is an integer in the range of about 60 to about 90.
79. The method of claim 1, wherein said polymerization agent is a compound of formula II.
80. The method of claim 1, wherein said polymerization agent is a compound of formula in.
81. The method of claim 1, wherein said polymerization agent is a compound of formula III, R1-111 is -C(O)H, and R2-111 is H.
82. The method of claim 1, wherein said polymerization agent is a compound of formula
83. The method of claim 1, wherein said polymerization agent is a compound of formula
84. The method of claim 1, wherein said compound of formula HI is
85. The method of claim 1, wherein said compound of formula Ia is
86. The method of claim 1, wherein said dendrimeric compound is a compound of formula Ib.
87. The method of claim 86, wherein v is 2.
88. The method of claim 86, wherein X5 is -N(H)-.
89. The method of claim 86, wherein R18 is H.
90. The method of claim 86, wherein R19 is H.
91. The method of claim 86, wherein R20 is H.
92. The method of any one of claims 86-91, wherein w is an integer in the range of about 20-500.
93. The method of any one of claims 86-91, wherein w is an integer in the range of about 40-250.
94. The method of any one of claims 86-91, wherein w is an integer in the range of about 60-90.
95. The method of claim 86, wherein said compound of formula Ib is
96. The method of claim 1, wherein said polymerization agent is a compound of formula V.
97. The method of claim 96, wherein v is 2.
98. The method of claim 96, wherein X6 is -N(H)-.
99. The method of claim 96, wherein R24 is H.
100. The method of claim 96, wherein R25 is H.
101. The method of claim 96, wherein R26 is H.
102. The method of one of claims 96-101, wherein w is an integer in the range of about 20- 500.
103. The method of one of claims 96-101, wherein w is an integer in the range of about 40- 250.
104. The method of one of claims 96-101, wherein w is an integer in the range of about 60- 90.
105. The method of one of claims 96-101, wherein R23 represents independently for each occurrence
106. The method of claim 96 wherein R23 represents independently for each occurrence
108. The method of claim 96, said compound of formula V is
109. The method of claim 1, wherein said polymerization agent is an oxidizing agent.
110. The method of claim 1, wherein said polymerization agent is O2.
111. The method of claim 1 , wherein said polymerization agent is ultraviolet light or visible light.
112. The method of claim 1, wherein said polymerization agent is ultraviolet light.
113. The method of claim 1, wherein said polymerization agent is light with a λ of 400-600 nm.
114. The method of claim 1, wherein said polymerization agent is light with a λ of 450-550 nm.
115. The method of claim 1, wherein said polymerization agent is light with a λ of 488-514 nm.
116. The method of claim 1, wherein said patient is a primate, equine, feline, or canine.
117. The method of claim 1, wherein said patient is a human.
118. The method of claim 1, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
119. The method of claim 1, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
120. The method of claim 1, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
121. The method of claim 1, wherein said wound is a corneal laceration or corneal perforation.
122. The method of claim 1, wherein said wound is less than about 10 cm2 in size.
123. The method of claim 1, wherein said wound is less than about 5 cm2 in size.
124. The method of claim 1, wherein said wound is less than about 1 cm2 in size.
125. The method of claim 1, wherein said wound is less than about 5 cm in length.
126. The method of claim 1, wherein said wound is less than about 2 cm in length.
127. The method of claim 1, wherein said wound is less than about 1 cm in length.
128. The method of claim 1, wherein said wound is less than about 0.5 cm in length.
129. The method of claim 1, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 2% w/w to about 40% w/w.
130. The method of claim 1, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 5% w/w to about 20% w/w.
131. The method of claim 1, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 6% w/w to about 10% w/w.
132. The method of claim 1, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
133. The method of claim 1, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
134. The method of claim 1, further comprising the step of admixing a photoinitiator with said compound of formula Ia prior to treating said compound of formula Ia with said polymerization agent.
135. The method of claim 134, wherein said photoinitiator is eosin-Y.
136. The method of claim 1, further comprising the step of admixing a natural polymer with said dendrimeric compound.
137. The method of claim 136, wherein said natural polymer is HA, collagen, or a GAG fragment.
138. The method of claim 1, further comprising the step of admixing at least one cell with said dendrimeric compound.
139. The method of claim 138, wherein said cell is a stem cell.
140. The method of claim 1, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
141. The method of claim 140, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
142. The method of claim 140, wherein said polymer is polyvinylpyrrolidone.
143. The method of claim 1, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
144. The method of claim 143, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
145. The method of claim 143, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or a biologically active analog, fragment, or derivative thereof.
146. The method of claim 143, wherein said pharmaceutical agent is polyhexamethylene biguanide.
147. The method of claim 1, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 400 wt%.
148. The method of claim 1, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 200 wt%.
149. The method of claim 1, further comprising the step of sterilizing said dendrimeric compound.
150. The method of claim 1, further comprising the step of sterilizing said dendrimeric compound and said polymerization agent, wherein said polymerization agent is selected from the group consisting of a compound of formula II, a compound of formula III, a compound of formula IV, and a compound of formula V.
151. The method of claim 149 or 150, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
152. The method of claim 151, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"3.
153. The method of claim 151, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"5.
154. A method of sealing a wound on a patient, comprising the steps of:
applying an effective amount of a compound of formula VI to a wound on a patient and treating said compound of formula VI with a polymerization agent sufficient to polymerize said compound of formula VI, wherein said polymerization agent is an oxidizing agent or a compound of formula VII, wherein formula VI is represented by:
VI or a pharmaceutically acceptable salt, solvate, or hydrate thereof, wherein
R1 represents independently for each occurrence
R2 represents independently for each occurrence H or alkyl;
R3 represents independently for each occurrence H, halogen, or alkyl;
R4 represents independently for each occurrence alkyl, aryl, or aralkyl;
R5 represents independently for each occurrence -(C(R3)2)mSH, -C(O)(C(R3)2)mSH,
-CO2(C(R3)2)mSH, -C(O)N(R2)(C(R3)2)mSH,
n and m each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p is 1, 2, 3, 4, or 5; and said formula VII is represented by:
R1-VI1_B_R1-VII vπ vherein
RNVU represents independently -(C(irvl')2)xC(O)H,
R2"vu represents independently for each occurrence H, alkyl, or halogen;
B is alkyl diradical, heteroalkyl diradical, or
v2"VI1 represents independently for each occurrence 2, 3, or 4; and w2"^11 is an integer in the range of about 5 to 700, inclusive.
155. The method of claim 154, wherein said polymerization agent is an oxidizing agent.
156. The method of claim 154, wherein said polymerization agent is O2.
157. The method of claim 154, wherein said polymerization agent is a compound of formula VII.
158. The method of claim 154, wherein B is an alkyl diradical.
159. The method of claim 154, said compound of formula VII is
161. The method of claim 154, wherein w ..2-"VII . is an integer in the range of about 50 to about 250.
162. The method of claim 154, wherein w2"vπ is an integer in the range of about 60 to about 90.
163. The method of claim 154, wherein said polymerization agent is a compound of formula VII, R2-VI1 is -C(O)H, and R2Λαi is H.
164. The method of claim 154, wherein said polymerization agent is a compound of
VII is 2.
165. The method of claim 154, wherein said polymerization agent is a compound of
2, and w2"vπ is an integer in the range of about 60-90.
166. The method of claim 154, wherein n is 3, 4, or 5.
167. The method of claim 154, wherein n is 4.
168. The method of claim 154, wherein R2 is H.
169. The method of claim 154, wherein R3 is H.
170. The method of claim 154, wherein R4 is alkyl.
171. The method of claim 154, wherein R4 is methyl or ethyl.
172. The method of claim 154, wherein n is 4, R2 and R3 are H, and R4 is alkyl.
177. The method of claim 154, wherein n is 4, R2 and R3 are H, R4 is methyl, R1 is
178. The method of claim 154, wherein n is 4, R2 and R3 are H, R4 is methyl, R1 is
179. The method of claim 154, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and a Brδnsted acid.
180. The method of claim 154, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HA, wherein A is halogen or -O2CR6, and R6 is alkyl, fluoroalkyl, aryl, or aralkyl.
181. The method of claim 154, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and an acid selected from group consisting of HCl and HBr.
182. The method of claim 154, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HO2CR6, wherein R6 is fluoroalkyl.
183. The method of claim 154, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and CF3CO2H.
184. The method of claim 154 wherein said patient is a primate, equine, feline, or canine.
185. The method of claim 154, wherein said patient is a human.
186. The method of claim 154, further comprising the step of admixing a natural polymer with said compound of formula VI.
187. The method of claim 154, further comprising the step of admixing at least one cell with said compound of formula VI.
188. The method of claim 154, wherein said cell is a stem cell.
189. The method of claim 154, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
190. The method of claim 154, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
191. The method of claim 154, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
192. The method of claim 154, wherein said wound is a corneal laceration or corneal perforation.
193. The method of claim 154, wherein said wound is less than about 10 cm2 in size.
194. The method of claim 154, wherein said wound is less than about 5 cm in size.
195. The method of claim 154, wherein said wound is less than about 1 cm2 in size.
196. The method of claim 154, wherein said wound is less than about 5 cm in length.
197. The method of claim 154, wherein said wound is less than about 2 cm in length.
198. The method of claim 154, wherein said wound is less than about 1 cm in length.
199. The method of claim 154, wherein said wound is less than about 0.5 cm in length.
200. The method of claim 154, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
201. The method of claim 154, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
202. The method of claim 154, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
203. The method of claim 202, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
204. The method of claim 202, wherein said polymer is polyvinylpyrrolidone.
205. The method of claim 154, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
206. The method of claim 205, wherein said pharmaceutical agent is an antibiotic, antimicrobial agent, antiinflammatory agent, or growth factor.
207. The method of claim 205, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
208. The method of claim 205, wherein said pharmaceutical agent is polyhexamethylene biguanide.
209. The method of claim 154, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 400 wt%.
210. The method of claim 154, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 200 wt%.
211. The method of claim 154, further comprising the step of sterilizing said compound of foπnula VI.
212. The method of claim 154, further comprising the step of sterilizing said compound of formula VI and said polymerization agent, wherein said polymerization agent is a compound of foπnula VII.
213. The method of claim 211 or 212, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
214. The method of claim 213, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"3.
215. The method of claim 213, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"5.
216. A method of sealing a wound on a patient, comprising the steps of: treating a dendrimeric compound of formula Ia or formula Ib with a polymerization agent to form a repair agent and applying said repair agent to a wound on a patient, wherein said polymerization agent is ultraviolet light, visible light, a compound of formula π, a compound of formula III, a compound of formula IV, a compound of formula V, or an oxidizing agent, wherein formula Ia is represented by:
A1— X1-B— X1— A2 Ia wherein
A2 is alkyl, aryl, aralkyl, -Si(R3)3,
or
A represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
Z1 represents independently for each occurrence -X1 -R4 , E, or
Z represents independently for each occurrence -X 1 - rR>5 , E, or
Z* represents independently for each occurrence -X'-R0 , E, or
Y4 represents independently for each occurrence R7, A4,
Z represents independently for each occurrence -X 1 -R π7 , E, or
Y5 represents independently for each occurrence R8, A4,
R1 represents independently for each occurrence H, alkyl, or halogen;
R3 represents independently for each occurrence alkyl, aryl, or aralkyl; R4, R5, R6, R7, R8, and R9 are H;
R10 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R11 represents independently for each occurrence H, -OH, -N(R10)2, -SH5 alkyl, hydroxyalkyl, or -[C(R1)2]dR16;
R12 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R13 represents independently for each occurrence H, alkyl, aryl, or aralkyl;
R14 represents independently for each occurrence H, alkyl, or -CO2R10; R15 represents independently for each occurrence H, alkyl, or -OR10;
R16 represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R10)2, -SH, -S-alkyl, -CO2R10, -C(O)N(R10)2, or - C(NH2)N(R10)2;
d represents independently for each occurrence 1, 2, 3, 4, 5, or 6; n represents independently for each occurrence 1, 2, 3, 4, 5, or 6; p1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p2 represents independently for each occurrence 0, 1, 2, 3, or 4; p3 represents independently for each occurrence 1, 2, or 3; p4 represents independently for each occurrence 0, 1, 2, or 3; t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
v and v each represent independently for each occurrence 2, 3, or 4; w1 and w2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is O, 1, 2, 3, 4, or 5; z1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z2 and z3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
X1 and X2 each represent independently for each occurrence O or -N(R10)-;
X3 represents independently for each occurrence O, N(R10), or C(R15)(CO2R10);
provided that R4 only occurs once, R5 only occurs once, R6 only occurs once, R7 only occurs once, R8 only occurs once, and R9 only occurs once; said formula Ib is represented by:
Ib or a pharmaceutically acceptable salt, solvate, or hydrate thereof,
wherein
X represents independently for each occurrence O or -N(R 22χ )-;
R represents independently for each occurrence H, -(C(R 19 )\2)hSH,
R represents independently for each occurrence H or alkyl;
R1 represents independently for each occurrence H, halogen, or alkyl;
R20 represents independently for each occurrence H or alkyl;
R21 represents independently for each occurrence H, -(C(R19)2)hSH, -
R22 represents independently for each occurrence H, alkyl, aryl, or aralkyl; n1 and h each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p5 represents independently for each occurrence 1, 2, 3, 4, or 5; v represents independently for each occurrence 2, 3, or 4; and w is an integer in the range of about 5 to about 700, inclusive; said formula II is represented by:
wherein
R2'11 represents independently for each occurrence H or alkyl;
R3"11 represents independently for each occurrence H, halogen, or alkyl;
R4"11 represents independently for each occurrence alkyl, aryl, or aralkyl; and
R5'11 represents independently for each occurrence H or
R2"" and z represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; said formula III is represented by:
R-I-IIl g1-lll R1-III
III wherein
R1-111 is -(C(R2-πi)2)xC(O)H, -C(O)(C(R2-iπ)2)yC(O)H, -(C(R2-m)2)xC(O)R3-m, or - C(0)(C(R2-πi)2)yC(O)R3"iπ;
x represents independently for each occurrence 0, 1, 2, 3, 4, 5, 6, 7, or 8;
y represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8;
z represents independently for each occurrence 0, 1, or 2;
v represents independently for each occurrence 2, 3, or 4; and
w is an integer in the range of about 5 to about 700, inclusive;
said formula IV is represented by:
A1— X1-B~X1— A2 rv wherein
A3 represents independently for each occurrence alkyl, cycloalkyl, heteroalkyl, heterocycloalkyl, aryl, heteroaryl, or aralkyl;
Z represents independently for each occurrence -X 1 - rR,5 , E, or
Y3 represents independently for each occurrence R ,
Z3 represents independently for each occurrence -X1 -R0 , E, or
Y represents independently for each occurrence R7,
Z4 represents independently for each occurrence -X^R7, E, or
Z5 represents independently for each occurrence -X1 -R8, E, or
R1 represents independently for each occurrence H, alkyl, or halogen;
R2 represents independently for each occurrence H, alkyl, -OH, -N(R1 °)2, -SH, hydroxyalkyl, or -[C(R1^dR16;
R3 represents independently for each occurrence alkyl, aryl, or aralkyl; R4, R5, R6, R7, R8, and R9 are H;
R , 10 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R11 represents independently for each occurrence H, -OH, -N(R10)2, -SH, alkyl, hydroxyalkyl, or -[CCR^jdR16;
R represents independently for each occurrence H, alkyl, aryl, or aralkyl; R13 represents independently for each occurrence H, alkyl, aryl, or aralkyl; R14 represents independently for each occurrence H, alkyl, or -CO2R10;
R . 15 represents independently for each occurrence H, alkyl, or -OR io. ;
R represents independently for each occurrence phenyl, hydroxyphenyl, pyrrolidyl, imidazolyl, indolyl, -N(R10)2, -SH, -S-alkyl, -CO2R10, -C(O)N(R10)2, or - C(NH2)N(R10)2;
n represents independently for each occurrence 1, 2, 3, 4, 5, or 6; p1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p2 represents independently for each occurrence 0, 1, 2, 3, or 4; p3 represents independently for each occurrence 1, 2, or 3; p4 represents independently for each occurrence 0, 1, 2, or 3; d represents independently for each occurrence 1, 2, 3, 4, 5, or 6;
t represents independently for each occurrence 2, 3, 4, or 5 in accord with the rules of valence;
v1 and v2 each represent independently for each occurrence 2, 3, or 4; w1 and w2 each represent independently for each occurrence an integer from about 5 to about 700, inclusive; x is 1, 2, or 3; y is 0, 1, 2, 3, 4, or 5; z1 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; z2 and z3 each represent independently for each occurrence 1, 2, 3, 4, or 5;
X1 and X2 each represent independently for each occurrence O or -N(R10)-; X3 represents independently for each occurrence O, N(R10), or C(R15)(CO2R10); and
said formula V is represented by:
or a pharmaceutically acceptable salt, solvate, or hydrate thereof, wherein
X represents independently for each occurrence O or -N(R 30N )-;
R ,24 represents independently for each occurrence H or alkyl;
R ,25 represents independently for each occurrence H, halogen, or alkyl;
R ,26 represents independently for each occurrence H or alkyl;
R27 represents independently for each occurrence H, alkyl, or halogen;
R28 represents independently for each occurrence H, alkyl, -OH, -N(R30)2, -SH, or hydroxyalkyl;
R29 represents independently for each occurrence H, -OH, -N(R30)2, -SH, alkyl, or hydroxyalkyl;
R30 and R31 represent independently for each occurrence H, alkyl, aryl, or aralkyl; Z6 represents independently for each occurrence E1 or
Z7 represents independently for each occurrence E1 or
R ,33 represents independently for each
R34 represents independently for each occurrence H, alkyl, or -CO2R 30.
p6 represents independently for each occurrence 1, 2, 3, 4, 5, 6, 7; or 8; p7 represents independently for each occurrence O, 1, 2, 3, or 4; p8 represents independently for each occurrence 1, 2, or 3; p9 represents independently for each occurrence 0, 1, 2, or 3; n2 and j each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; m1 represents independently for each occurrence 1 or 2; v represents independently for each occurrence 2, 3, or 4; and w is an integer in. the range of about 5 to about 700, inclusive.
217. The method of claim 216, wherein said dendrimeric compound is a compound of formula Ia, and said polymerization agent is ultraviolet light, visible light, a compound of formula II, a compound of formula III, or an oxidizing agent.
218. The method of claim 216, wherein A1 is
, and m is 1 or 2.
219. The method of claim 216, wherein A 2 i •s_
220. The method of claim 216, wherein Z represents independently for each occurrence -
222. The method of claim 216, wherein Z3 represents independently for each occurrence -
223. The method of claim 216, wherein Z4 represents independently for each occurrence -
224. The method of claim 216, wherein Z5 represents independently for each occurrence -
225. The method of claim 216, wherein X1 is O.
226. The method of claim 216, wherein X1 and X2 are O.
227. The method of claim 216, wherein n is 1.
228. The method of claim 216, wherein p1 is 2, 3, or 4.
229. The method of claim 216, wherein p2 is 1.
230. The method of claim 216, wherein R1 is H.
and Z1 is
the Y groups
242. The method of any one of claims 231-241, wherein p1 is 1, 2, 3, or 4.
243. The method of any one of claims 231-241, wherein p1 is 2.
244. The method of any one of claims 231-241, wherein p1 is 4.
245. The method of any one of claims 231-241, wherein m is 1.
247. The method of claim 216, wherein R1 is H, B
255. The method of any one of claims 246-254, wherein p1 is 1, 2, 3, or 4.
256. The method of any one of claims 246-254, wherein p1 is 2.
257. The method of any one of claims 246-254, wherein p1 is 4.
258. The method of any one of claims 246-254, wherein m is 1.
259. The method of any one of claims 246-254, wherein R2 is (Q-C^alkyl.
260. The method of claim 216, wherein B is
261. The method of claim 216, wherein R1 is H, B is
262. The method of claim 216, wherein R1 is H, B is
263. The method of claim 216, wherein R is H, B is
264. The method of claim 216, wherein R is H, B is
265. The method of claim 216, wherein R1 is H, B is
266. The method of claim 216, wherein R1 is H, B is
267. The method of claim 216, wherein R is H, B is
268. The method of claim 216, wherein R is H, B is
269. The method of claim 216, wherein R1 is H, B is
270. The method of claim 216, wherein R1 is H, B is
271. The method of claim 216, wherein R1 is H, B is
272. The method of any one of claims 260-271, wherein w1 is an integer in the range of about 50 to about 250.
273. The method of any one of claims 260-271, wherein w1 is an integer in the range of about 60 to about 90.
274. The method of any one of claims 260-271, wherein p1 is 2.
275. The method of any one of claims 260-271, wherein m is 1.
276. The method of any one of claims 260-271, wherein p1 is 2, p2 is 0, and R3 is (Ci- C5)alkyl.
277. The method of any one of claims 260-271, wherein p1 is 2, p2 is 0, R3 is (Ci-C5)alkyl, and w1 is an integer in the range of about 60 to about 90.
278. The method of claim 216, wherein R1 is H, B is A2 is
280. The method of claim 216, wherein R1 is H, B is
283. The method of claim 216, wherein R1 is H, B is
.84. The method of claim 216, wherein R1 is H, B
s
286. The method of any one of claims 278-285, wherein p1 is 2.
287. The method of any one of claims 278-285, wherein m is 1.
288. The method of any one of claims 278-285, wherein p1 is 2, p2 is 0, and R3 is (Ci- C5)alkyl.
289. The method of any one of claims 278-285, wherein p1 is 2, p2 is 0, and R3 is (Ci- Cs)alkyl, and w2 is an integer in the range of about 60 to about 90.
290. The method of claim 216, wherein R1 is H, B is
91 The method of claim 216, wherein R1 is H, B is , A is
294. The method of claim 216, wherein said polymerization agent is a compound of formula II.
295. The method of claim 216, wherein said polymerization agent is a compound of formula III.
296. The method of claim 216, wherein said polymerization agent is a compound of formula III, R1"111 is -C(O)H, and R2-111 is H.
297. The method of claim 216, wherein said polymerization agent is a compound of
298. The method of claim 216, wherein said polymerization agent is a compound of
formula III, R2411 is -C(O)H, R2"111 is H, B1"111 is
and w is an integer in the range of about 60-90.
299. The method of claim 216, said compound of formula HI is
300. The method of claim 216, wherein said compound of formula Ia is
301. The method of claim 216, wherein said dendrimeric compound is a compound of formula Ib.
302. The method of claim 301, wherein v is 2.
303. The method of claim 301, wherein X5 is -N(H)-.
304. The method of claim 301, wherein R18 is H.
305. The method of claim 301, wherein R19 is H.
306. The method of claim 301, wherein R20 is H.
307. The method of any one of claims 301-306, wherein w is an integer in the range of about 20-500.
308. The method of any one of claims 301-306, wherein w is an integer in the range of about 40-250.
309. The method of any one of claims 301-306, wherein w is an integer in the range of about 60-90.
310. The method of claim 216, said compound of formula Ib is
311. The method of claim 311, said polymerization agent is a compound of formula V.
312. The method of claim 311, wherein v is 2.
313. The method of claim 311, wherein X6 is -N(H)-.
314. The method of claim 311, wherein R24 is H.
315. The method of claim 311, wherein R25 is H.
316. The method of claim 311, wherein R26 is H.
317. The method of any one of claims 311-316, wherein w is an integer in the range of about 20-500.
318. The method of any one of claims 311-316, wherein w is an integer in the range of about 40-250.
319. The method of any one of claims 311-316, wherein w is an integer in the range of about 60-90.
320. The method of claim 311, wherein R23 represents independently for each occurrence
321. The method of claim 311, wherein R "-represents independently for each occurrence
322. The method of claim 311, said compound of formula V is
323. The method of claim 311, said compound of formula V is
324. The method of claim 216, wherein said polymerization agent is an oxidizing agent.
325. The method of claim 324, wherein said polymerization agent is O2.
326. The method of claim 216, wherein said polymerization agent is ultraviolet light or visible light.
327. The method of claim 216, wherein said polymerization agent is ultraviolet light.
328. The method of claim 216, wherein said polymerization agent is light with a λ of 400- 600 nm.
329. The method of claim 216, wherein said polymerization agent is light with a λ of 450- 550 nm.
330. The method of claim 216, wherein said polymerization agent is light with a λ of 488- 514 nm.
331. The method of claim 216, wherein said patient is a primate, equine, feline, or canine.
332. The method of claim 216, wherein said patient is a human.
333. The method of claim 216, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
334. The method of claim 216, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
335. The method of claim 216, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
336. The method of claim 216, wherein said wound is a corneal laceration or corneal perforation.
337. The method of claim 216, wherein said wound is less than about 10 cm2 in size.
338. The method of claim 216, wherein said wound is less than about 5 cm2 in size.
339. The method of claim 216, wherein said wound is less than about 1 cm2 in size.
340. The method of claim 216, wherein said wound is less than about 5 cm in length.
341. The method of claim 216, wherein said wound is less than about 2 cm in length.
342. The method of claim 216, wherein said wound is less than about 1 cm in length.
343. The method of claim 216, wherein said wound is less than about 0.5 cm in length.
344. The method of claim 216, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 2% w/w to about 40% w/w.
345. The method of claim 216, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 5% w/w to about 20% w/w.
346. The method of claim 216, wherein said compound of formula Ia is dissolved in at least one solvent, and said compound of formula Ia has a concentration in the range of about 6% w/w to about 10% w/w.
347. The method of claim 216, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
348. The method of claim 216, wherein said dendrimeric compound is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
349. The method of claim 216, wherein said repair agent is an aqueous mixture that has a pH in the range of about 5.5 to about 9.5.
350. The method of claim 216, wherein said repair agent is an aqueous mixture that has a pH in the range of about 6.5 to about 7.5.
351. The method of claim 216, further comprising the step of admixing a photoinitiator with said compound of formula Ia prior to treating said compound of formula Ia with said polymerization agent.
352. The method of claim 351, wherein said photoinitiator is eosin-Y.
353. The method of claim 216, further comprising the step of admixing a natural polymer with said dendrimeric compound.
354. The method of claim 353, wherein said natural polymer is HA, collagen, or a GAG fragment.
355. The method of claim 216, further comprising the step of admixing at least one cell with said dendrimeric compound.
356. The method of claim 355, wherein said cell is a stem cell.
357. The method of claim 216, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
358. The method of claim 357, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose, a cellulose derivative,
polypropylene glycol), poly(ethylene glycol), polyvinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
359. The method of claim 357, wherein said polymer is polyvinylpyrrolidone.
360. The method of claim 216, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
361. The method of claim 360, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
362. The method of claim 360, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
363. The method of claim 360, wherein said pharmaceutical agent is polyhexamethylene biguanide.
364. The method of claim 216, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 400 wt%.
365. The method of claim 216, wherein the hydrogel formed from treating said dendrimeric compound with a polymerization agent swells less than about 200 wt%.
366. The method of claim 216, further comprising the step of sterilizing said dendrimeric compound.
367. The method of claim 216, further comprising the step of sterilizing said dendrimeric compound and said polymerization agent, wherein said polymerization agent is selected from the group consisting of a compound of formula II, a compound of formula in, a compound of formula IV, and a compound of formula V.
368. The method of claim 367, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
369. The method of claim 366 or 367, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"3.
370. The method of claim 366 or 367, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"5.
371. A method of sealing a wound on a patient, comprising the steps of: treating a compound of formula VI with a polymerization agent to form a repair agent and applying said repair agent to a wound on a patient, wherein said polymerization agent is an oxidizing agent or a compound of formula VII, wherein formula VI is represented by:
VI or a pharmaceutically acceptable salt, solvate, or hydrate thereof, wherein
R1 represents independently for each occurrence H, -(C(R3)2)mSH,
Rz represents independently for each occurrence H or alkyl; R3 represents independently for each occurrence H, halogen, or alkyl; R4 represents independently for each occurrence alkyl, aryl, or aralkyl;
R5 represents independently for each occurrence -(C(R3)2)mSH, -C(O)(C(R3)2)mSH,
n and m each represent independently for each occurrence 1, 2, 3, 4, 5, 6, 7, or 8; p is 1, 2, 3, 4, or 5; and said formula VII is represented by: p1-VII_g_p1-VII
VII wherein
R]-vπ represents independently -(C(R2-VII)2)XC(O)H, -C(O)(C(R2-VII)2)yC(O)H, -
R represents independently for each occurrence H, alkyl, or halogen;
B is alkyl diradical, heteroalkyl diradical, or
; v " π represents independently for each occurrence 2, 3, or 4; and w2"vπ is an integer in the range of about 5 to 700, inclusive.
372. The method of claim 371, wherein said polymerization agent is an oxidizing agent.
373. The method of claim 371, wherein said polymerization agent is O2.
374. The method of claim 371, wherein said polymerization agent is a compound of formula VII.
375. The method of claim 374, wherein B is an alkyl diradical.
378. The method of claim 377, wherein w2'vπ is an integer in the range of about 50 to about 250.
379. The method of claim 377, wherein w2"vπ is an integer in the range of about 60 to about 90.
380. The method of claim 371, wherein said polymerization agent is a compound of formula VII, R2'vπ is -C(O)H, and R2Λαi is H.
381. The method of claim 371, wherein said polymerization agent is a compound of
VII is 2.
382. The method of claim 371, wherein said polymerization agent is a compound of
2, and w2"vπ is an integer in the range of about 60-90.
383. The method of claim 371, wherein n is 3, 4, or 5.
384. The method of claim 371, wherein n is 4.
385. The method of claim 371, wherein R2 is H.
386. The method of claim 371, wherein R3 is H.
387. The method of claim 371, wherein R4 is alkyl.
388. The method of claim 371, wherein R4 is methyl or ethyl.
389. The method of claim 371, wherein n is 4, R2 and R3 are H, and R4 is alkyl.
390. The method of claim 371, wherein R1
394. The method of claim 371, wherein n is 4, R2 and R3 are H, R4 is methyl, R1 is
395. The method of claim 371, wherein n is 4, R2 and R3 are H, R4 is methyl, R1 is
396. The method of claim 371, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and a Brδnsted acid.
397. The method of claim 371, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HA, wherein A is halogen or -O2CR6, and R6 is alkyl, fluoroalkyl, aryl, or aralkyl.
398. The method of claim 371, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and an acid selected from group consisting of HCl and HBr.
399. The method of claim 371, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and HO2CR6, wherein R6 is fluoroalkyl.
400. The method of claim 371, wherein said pharmaceutically acceptable salt is a complex formed by said compound of formula VI and CF3CO2H.
401. The method of claim 371, wherein said patient is a primate, equine, feline, or canine.
402. The method of claim 371, wherein said patient is a human.
403. The method of claim 371, further comprising the step of admixing a natural polymer with said compound of formula VI.
404. The method of claim 403, wherein said natural polymer is HA, collagen, or a GAG fragment.
405. The method of claim 371, further comprising the step of admixing at least one cell with said compound of formula VI.
406. The method of claim 405, wherein said cell is a stem cell.
407. The method of claim 371, wherein said wound is a skin laceration, liver laceration, ophthalmic wound, arterial laceration, lung laceration, laceration of tissue in the gastrointestinal tract, cartilage wound, heart laceration, laceration of tissue in the urinary track, brain laceration, ear laceration, kidney laceration, or pancreatic laceration.
408. The method of claim 371, wherein said wound is a skin laceration, liver laceration, or ophthalmic wound.
409. The method of claim 371, wherein said wound is a corneal laceration, corneal perforation, retinal tear, retinal hole, leaking bleb, corneal incision, or corneal transplant wound.
410. The method of claim 371, wherein said wound is a corneal laceration or corneal perforation.
411. The method of claim 371, wherein said wound is less than about 10 cm in size.
412. The method of claim 371, wherein said wound is less than about 5 cm2 in size.
413. The method of claim 371, wherein said wound is less than about 1 cm2 in size.
414. The method of claim 371, wherein said wound is less than about 5 cm in length.
415. The method of claim 371, wherein said wound is less than about 2 cm in length.
416. The method of claim 371, wherein said wound is less than about 1 cm in length.
417. The method of claim 371, wherein said wound is less than about 0.5 cm in length.
418. The method of claim 371, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 5.5 to about 9.5.
419. The method of claim 371, wherein said compound of formula VI is dissolved in an aqueous solution that has a pH in the range of about 6.5 to about 7.5.
420. The method of claim 371, further comprising the step of applying a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol to said wound of said patient.
421. The method of claim 420, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
422. The method of claim 420, wherein said polymer is polyvinylpyrrolidone.
423. The method of claim 371, further comprising the step of applying a pharmaceutical agent to said wound of said patient.
424. The method of claim 423, wherein said pharmaceutical agent is an antibiotic, antimicrobial agent, antiinflammatory agent, or growth factor.
425. The method of claim 423, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
426. The method of claim 423, wherein said pharmaceutical agent is polyhexamethylene biguanide.
427. The method of claim 371, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 400 wt%.
428. The method of claim 371, wherein the hydrogel formed from treating said compound of formula VI with a polymerization agent swells less than about 200 wt%.
429. The method of claim 371, further comprising the step of sterilizing said compound of formula VI.
430. The method of claim 371, further comprising the step of sterilizing said compound of formula VI and said polymerization agent, wherein said polymerization agent is a compound of formula VH.
431. The method of claim 430, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
432. The method of claim 430 or 431, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"3.
433. The method of claim 430 or 431, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"5.
434. A method for preparing a sealant, comprising the step of: admixing a first biocompatible crosslinking polymer comprising at least two different nucleophilic groups with a second biocompatible crosslinking polymer comprising at least one amine-reactive group and at least one sulfhydryl-reactive group to form a gel, wherein said amine- and sulfhydryl-reactive groups are capable of a covalent reaction with said nucleophilic groups of said first crosslinking polymer.
435. The method of claim 434, wherein said sealant is formed in less than about one-half hour following admixing of said first biocompatible polymer with said second biocompatible polymer.
436. The method of claim 434, wherein said sealant is formed in less than about 15 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
437. The method of claim 434, wherein said sealant is formed in less than about 5 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
438. The method of claim 434, wherein said sealant is formed in less than about 1 minute following admixing of said first biocompatible polymer with said second biocompatible polymer.
439. The method of claim 434, wherein said nucleophilic groups on said first biocompatible polymer are sulfhydryl and amine.
440. The method of claim 434, wherein said method is performed ex vivo.
441. The method of claim 434, wherein said method is performed in vitro.
442. The method of claim 434, wherein said method is performed in vivo.
443. The method of claim 434, further comprising the step of administering an effective amount of said sealant to a patient.
444. A method for preparing a sealant, comprising the step of: admixing a first biocompatible crosslinking polymer comprising at least two different nucleophilic groups with a second biocompatible crosslinking polymer comprising at least one sulfhydryl-reactive group to form a sealant, wherein said sulfhydryl-reactive group is capable of a covalent reaction with said nucleophilic groups of said first crosslinking polymer.
445. The method of claim 444, wherein said sealant is formed in less than about one-half hour following admixing of said first biocompatible polymer with said second biocompatible polymer.
446. The method of claim 444, wherein said sealant is formed in less than about 15 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
447. The method of claim 444, wherein said sealant is formed in less than about 5 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
448. The method of claim 444, wherein said sealant is formed in less than about 1 minute following admixing of said first biocompatible polymer with said second biocompatible polymer.
449. The method of claim 444, wherein said nucleophilic groups on said first biocompatible polymer are sulfhydryl and amine.
450. The method of claim 444, wherein said method is performed ex vivo.
451. The method of claim 444, wherein said method is performed in vitro.
452. The method of claim 444, wherein said method is performed in vivo.
453. The method of claim 444, further comprising the step of administering an effective amount of said sealant to a patient.
454. A method for preparing a biocompatible gel, comprising the step of: admixing a first biocompatible crosslinking polymer comprising at least one amine group and at least one sulfhydryl group with a second biocompatible crosslinking polymer comprising at least one aldehyde to form a sealant, wherein said amine group and said sulfhydryl group are capable of covalent reaction with the aldehyde group to form a thiazolidine linkage.
455. The method of claim 454, wherein said gel is formed in less than about one-half hour following admixing of said first biocompatible polymer with said second biocompatible polymer.
456. The method of claim 454, wherein said gel is formed in less than about 15 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
457. The method of claim 454, wherein said gel is formed in less than about 5 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
458. The method of claim 454, wherein said gel is foπned in less than about 1 minute following admixing of said first biocompatible polymer with said second biocompatible polymer.
459. The method of claim 454, wherein said method is performed ex vivo.
460. The method of claim 454, wherein said method is performed in vitro.
461. The method of claim 454, wherein said method is performed in vivo.
462. The method of claim 454, further comprising the step of administering an effective amount of said gel to a patient.
463. A method for preparing a biocompatible sealant, comprising the step of: admixing a first biocompatible crosslinking polymer comprising a histidine amino acid group with a second biocompatible crosslinking polymer comprising an electrophilic group to form a sealant, wherein said histidine group of said first polymer and said
electrophilic group of said second polymer are capable of reaction to an amide linkage.
464. The method of claim 463, wherein said electrophilic group is a thiocarboxylic acid or acyl disulfide.
465. The method of claim 463, wherein said sealant is formed in less than about one-half hour following admixing of said first biocompatible polymer with said second biocompatible polymer.
466. The method of claim 463, wherein said sealant is formed in less than about 15 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
467. The method of claim 463, wherein said sealant is formed in less than about 5 minutes following admixing of said first biocompatible polymer with said second biocompatible polymer.
468. The method of claim 463, wherein said sealant is formed in less than about 1 minute following admixing of said first biocompatible polymer with said second biocompatible polymer.
469. The method of claim 463, wherein said method is performed ex vivo.
470. The method of claim 463, wherein said method is performed in vitro.
471. The method of claim 463, wherein said method is performed in vivo.
All. The method of claim 463, further comprising the step of administering an effective amount of said sealant to a patient.
473. A sealant comprising a dendrimeric macromolecule that forms a hydrogel which swells less than about 400 wt% in an aqueous solution.
474. The sealant of claim 473, wherein said dendrimeric macromolecule is the compound formed by treating a dendrimeric compound of formula Ia or formula Ib with a polymerization agent selected from the group consisting of ultraviolet light, visible light, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, or an oxidizing agent, wherein formula Ia, formula Ib, formula II, formula III, formula IV, and formula V are as defined in the specification.
475. The sealant of claim 473, wherein said dendrimeric macromolecule is the compound formed by treating a dendrimeric compound of formula Ia or formula Ib with a
polymerization agent selected from the group consisting of ultraviolet light and visible light.
476. The sealant of claim 473, wherein said dendrimeric macromolecule is the compound formed by treating a dendrimeric compound of formula VI with a polymerization agent selected from the group consisting of oxidizing agent or a compound of formula VII, wherein formula VI and formula VI are as defined in the specification.
477. The sealant of claim 473, further comprising a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol.
478. The sealant of claim 477, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
479. The sealant of claim 477, wherein said polymer is polyvinylpyrrolidone.
480. The sealant of claim 473, further comprising a pharmaceutical agent.
481. The sealant of claim 480, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
482. The sealant of claim 480, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
483. The sealant of claim 480, wherein said pharmaceutical agent is polyhexamethylene biguanide.
484. The sealant of claim 473, wherein said hydrogel swells less than about 250 wt%.
485. The sealant of claim 473, wherein said hydrogel swells less than about 150 wt%.
486. A formulation for forming a sealant, comprising water and a dendrimeric compound that forms a hydrogel which swells less than about 400 wt% in an aqueous solution, wherein said dendrimeric compound is a compound of formula Ia, a compound of formula Ib, or a compound of formula VI; and said formulation forms a hydrogel in less than about
5 minutes when treated with a polymerization agent selected from the group consisting of ultraviolet light, visible light, an oxidizing agent, a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, and a compound of formula VII; and said formulae Ia, Ib, II, IH, IV, V, VI, and VII are as defined in the specification.
487. The formulation of claim 486, wherein said hydrogel swells less than about 250 wt%.
488. The formulation of claim 486, wherein said hydrogel swells less than about 150 wt%.
489. The formulation of claim 486, wherein said formulation forms a hydrogel in less than about 2 minutes when treated with a polymerization agent.
490. The formulation of claim 486, wherein said formulation forms a hydrogel in less than about 1 minute when treated with a polymerization agent.
491. The formulation of claim 486, wherein said formulation forms a hydrogel in less than about 30 seconds when treated with a polymerization agent.
492. The formulation of claim 486, wherein said formulation has a pH in the range of about 5.5 to about 9.5.
493. The formulation of claim 486, wherein said formulation has a pH in the range of about 6.5 to about 7.5.
494. The formulation of claim 486, further comprising a polymer having a weight average molecular weight of about 500 g/mol to about 800,000 g/mol.
495. The formulation of claim 494, wherein said polymer is polyvinylpyrrolidone, polyvinylpyrrolidone iodide, starch, 2-hydroxyethyl cellulose, a cellulose derivative, poly(propylene glycol), poly(ethylene glycol), poly(vinyl alcohol), poly(lactic acid), poly(glycolic acid), polycaprolactone, poly(n-isopropylacrylamide), polyacrylamide, polyacrylic acid, a polymethylmethacrylate, latex, hyaluronic acid, an alginate, a gelatin, or a copolymer of one or more of the aforementioned polymers.
496. The formulation of claim 494, wherein said polymer is polyvinylpyrrolidone.
497. A kit for sealing a wound comprising: a polymerizable dendrimeric compound that forms a hydrogel which swells less than about 400 wt%; and
a system for delivering said polymerizable dendrimeric compound to a wound on a patient.
498. The kit of claim 497, wherein said system is a syringe.
499. The kit of claim 497, further comprising a polymerization agent.
500. The kit of claim 499, wherein said polymerization agent is a compound of formula II, a compound of formula III, a compound of formula IV, a compound of formula V, or a compound of formula VII; wherein formula II, formula HI, formula IV, foπnula V, and formula VII are as defined in the specification.
501. The kit of claim 499, wherein said polymerization agent is a compound of formula III or a compound of formula IV, and said foπnula III and formula IV are as defined in the specification.
502. The kit of claim 497, wherein said dendrimeric is a compound of formula Ia, formula Ib, or formula VI; wherein formula Ia, formula Ib, and formula VI are as defined in the specification.
503. The kit of claim 497, wherein said dendrimeric compound is represented by formula Ia or formula Ib, wherein formula Ia and formula Ib are as defined in the specification.
504. The kit of any one of claims 497-503, wherein said kit has a sterility assurance level of at least about 10"3.
505. The kit one of claims 497-503, wherein said kit has a sterility assurance level of at least about 10"5.
506. The kit of claim 497, further comprising a pharmaceutical agent.
507. The kit of claim 506, wherein said pharmaceutical agent is an antibiotic, antimicrobial compound, antiinflammatory compound, or growth factor.
508. The kit of claim 506, wherein said pharmaceutical agent is a transforming growth factor, fibroblast growth factor, platelet derived growth factor, epidermal growth factor, connective tissue activated peptide, osteogenic factor, or biologically active analog, fragment, or derivative thereof.
509. The kit of claim 506, wherein said pharmaceutical agent is polyhexamethylene biguanide.
510. The kit of claim 497, further comprising a desiccant.
511. A hydrogel sealant formed by mixing two synthetic polymers or compounds that foπn a gel which swells less than about 150 w/w %, wherein said synthetic polymer is not albumin or gelatin.
512. The sealant of claim 511, wherein said gel swells less than about 150 w/v %.
513. The sealant of claim 511, wherein said gel swells less than about 150 v/v %.
514. The sealant of claim 511, wherein said gel forms via a nucleophilic/electrophilic reaction.
515. A sealant formed by mixing at least two nucleophilic synthetic polymers or compounds in solution with at least one synthetic electrophilic polymer.
516. A sealant formed by mixing at least two electrophilic synthetic polymers or compounds in solution with at least one synthetic nucleophilic polymer.
517. The sealant of any one of claims 511-516, wherein the synthetic polymer does not include collagen, collagen derivatives, chemically modified collagens, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
518. A crosslinkable composition comprising a first crosslinkable component having two or more sets of two nucleophilic groups that can react with a second crosslinkable component having two or more electrophilic groups, each capable of reacting with the two nucleophilic groups, to form a covalent five-membered ring structure.
519. The composition of claim 518, wherein each of the first and second crosslinkable components is synthetic and dissolved in an aqueous solution.
520. The composition of claim 518, wherein crosslinking of the composition results in a biocompatible crosslinked hydrogel in less than about 10 minutes.
521. The composition of claim 518, wherein the nucleophilic groups are an amine or sulhydryl.
522. The composition of claim 518, wherein the five-membered ring structure is a thiazolidine ring.
523. A stable crosslinkable composition comprising a first crosslinkable component having at least two sets of 1,2-aminothiol groups that can react with an electrophilic group of a
second crosslinkable component having at least two electrophilic groups, each capable of reacting with the 1,2-aminothiol, to form a covalent fϊve-membered ring structure, wherein each of the first and second crosslinkable components is synthetic and dissolved in an aqueous solution.
524. The composition of claim 523, wherein the first and second crosslinkable components do not form a covalant bond with tissue.
525. The composition of claim 523, wherein the composition results in a biocompatible crosslinked hydrogel on a tissue surface in less than about 10 minutes.
526. The composition of claim 523, wherein the electrophilic group is an aldehyde.
527. The composition of claim 523, wherein the five-membered ring structure is a thiazolidine ring.
528. The composition of any one of claims 523-527, wherein the crosslinkable components do not include collagen, collagen derivatives, chemically modified collagens, gelatin, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
529. The composition of claim 523, wherein the crosslinkable composition forms a three- dimensional matrix that swells less than about 400%.
530. The composition of claim 523, wherein the crosslinkable composition forms a three- dimensional matrix that swells less than about 300%.
531. The composition of claim 523, wherein the crosslinkable composition forms a three- dimensional matrix that swells less than about 200%.
532. The composition of claim 523, wherein the crosslinkable composition forms a three- dimensional matrix that swells less than about 100%.
533. The composition of claim 523, wherein the crosslinkable composition forms a three- dimensional matrix that swells less than about 50%.
534. The composition of claim 523, wherein the first component has a molecular weight of less than about 1000 g/mol.
535. The composition of claim 523, wherein the first component has a molecular weight of less than about 500 g/mol.
536. The composition of claim 523, wherein the weight ratio of the first component to the second component is less- than about 10:1.
537. The composition of claim 523, wherein the weight ratio of the first component to the second component is less than about 5:1.
538. A method for forming a three-dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of:
(a) providing an aqueous solution of a synthetic polymer comprising at least two nucleophilic groups and an aqueous solution of a synthetic small molecule comprising at least two electrophilic groups, wherein the electrophilic groups react with the nucleophilic groups to form covalent bonds, wherein the number of nucleophilic groups plus electrophilic groups is at least five;
(b) applying the synthetic polymer and the synthetic small molecule to the first tissue surface; and
(c) allowing the synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
539. The method of claim 538, further comprising the step of contacting the first tissue surface with a second surface after step (b) but before step (c) to effect adhesion between the first tissue surface and the second surface.
540. A method for forming a three-dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of:
(a) providing an aqueous solution of a synthetic polymer comprising at least two nucleophilic groups and an aqueous solution of a synthetic small molecule comprising at least two electrophilic groups, wherein the electrophilic groups react with the nucleophilic groups to form covalent bonds, wherein the number of nucleophilic groups plus electrophilic groups is at least five;
(b) contacting the synthetic polymer and the synthetic small molecule to initiate crosslinking to form a repair agent; and
(c) applying the repair agent to the first tissue surface; and
(d) allowing the synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
541. The method of claim 540, further comprising contacting the first tissue surface with a second surface after step (c) but before step (d) to effect adhesion between the first tissue surface and the second surface.
542. A method for forming a three-dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of: a) providing an aqueous solution of a synthetic polymer comprising at least two nucleophilic groups and providing an aqueous solution of a synthetic small molecule comprising at least three electrophilic groups, wherein the electrophilic groups can react with the nucleophilic groups to form covalent bonds; b) applying the synthetic polymer and the synthetic small molecule to the first tissue surface to initiate crosslinking; and c) allowing the synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three dimensional matrix.
543. The method of claim 542, further comprising the step of contacting the first tissue surface with a second surface after step (b) but before step (c) to effect adhesion between the first tissue surface and the second surface.
544. A method for forming a three-dimensional synthetic polymer matrix on a first tissue surface, comprising the steps of: a) providing an aqueous solution of a synthetic polymer comprising at least two nucleophilic groups and providing an aqueous solution of a synthetic small molecule comprising at least three electrophilic groups, wherein the electrophilic groups can react with the nucleophilic groups to form covalent bonds; b) contacting the synthetic polymer and the synthetic small molecule to initiate crosslinking to form a repair agent; c) applying the repair agent to the first tissue surface; and d) allowing the first synthetic polymer and the synthetic small molecule to become crosslinked to one another to form a three-dimensional matrix.
545. The method of claim 544, further comprising the step of contacting the first tissue surface with a second surface after step (c) but before step (d) to effect adhesion between the first tissue surface and the second surface.
546. The method of any one of claims 538-544, wherein the three-dimensional matrix swells less than about 400%.
547. The method of any one of claims 538-544, wherein the three-dimensional matrix swells less than about 300%.
548. The method of any one of claims 538-544, wherein the three-dimensional matrix swells less than about 200%.
549. The method of any one of claims 538-544, wherein the three-dimensional matrix swells less than about 100%.
550. The method of any one of claims 538-544, wherein the three-dimensional matrix swells less than about 50%.
551. The method of claim 546, wherein the synthetic polymer does not include collagen, collagen derivatives, chemically modified collagens, gelatin, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
552. The method of claim 547, wherein the synthetic polymer does not include collagen, collagen derivatives, chemically modified collagens, gelatin, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
553. The method of claim 548, wherein the synthetic polymer does not include collagen, collagen derivatives, chemically modified collagens, gelatin, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
554. The method of claim 549, wherein the synthetic polymer does not include collagen, collagen derivatives, chemically modified collagens, gelatin, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified
derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
555. The method of claim 550, wherein the synthetic polymer does not include collagen, collagen derivatives, chemically modified collagens, gelatin, hyaluronic acid, chemically modified derivatives of hyaluronic acid, albumin from any source, chemically modified derivatives of albumin form any source, thrombin, chemically modified derivatives of thrombin, fibrinogen, or chemically modified derivatives of fibrinogen.
556. The method of any one of claims 538-544, further comprising the step of sterilizing the synthetic polymer and synthetic small molecule.
557. The method of claim 556, wherein said sterilizing is performed by treatment with ethylene oxide, hydrogen peroxide, heat, gamma irradiation, electron beam irradiation, microwave irradiation, or visible light irradiation.
558. The method of claim 556, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"3.
559. The method of claim 556, wherein said sterilizing is effective to achieve a sterility assurance level of at least about 10"5.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US69388505P | 2005-06-24 | 2005-06-24 | |
| US60/693,885 | 2005-06-24 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2007001926A2 true WO2007001926A2 (en) | 2007-01-04 |
| WO2007001926A3 WO2007001926A3 (en) | 2007-12-06 |
Family
ID=37595693
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2006/023639 WO2007001926A2 (en) | 2005-06-24 | 2006-06-19 | Low-swelling hydrogel sealants for wound repair |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2007001926A2 (en) |
Cited By (63)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2008016983A2 (en) * | 2006-08-02 | 2008-02-07 | Baxter International Inc. | Rapidly acting dry sealant and methods for use and manufacture |
| US7597882B2 (en) | 2006-04-24 | 2009-10-06 | Incept Llc | Protein crosslinkers, crosslinking methods and applications thereof |
| WO2010093873A2 (en) | 2009-02-12 | 2010-08-19 | Incept, Llc | Drug delivery through hydrogel plugs |
| US7862538B2 (en) | 2008-02-04 | 2011-01-04 | Incept Llc | Surgical delivery system for medical sealant |
| WO2011084465A2 (en) | 2009-12-15 | 2011-07-14 | Incept, Llc | Implants and biodegradable fiducial markers |
| US8092820B2 (en) | 2001-07-17 | 2012-01-10 | Baxter International Inc. | Dry hemostatic compositions and methods for their preparation |
| US8262608B2 (en) | 2007-01-25 | 2012-09-11 | Hyperbranch Medical Technology, Inc. | Applicators for multiple component formulations and the like, and methods of use thereof |
| US8303981B2 (en) | 1996-08-27 | 2012-11-06 | Baxter International Inc. | Fragmented polymeric compositions and methods for their use |
| US8357378B2 (en) | 1996-08-27 | 2013-01-22 | Baxter International Inc. | Fragmented polymeric compositions and methods for their use |
| US8366689B2 (en) | 2008-09-30 | 2013-02-05 | Avedro, Inc. | Method for making structural changes in corneal fibrils |
| US8414911B2 (en) | 2006-10-24 | 2013-04-09 | The Regents Of The University Of California | Photochemical therapy to affect mechanical and/or chemical properties of body tissue |
| WO2013086015A1 (en) | 2011-12-05 | 2013-06-13 | Incept, Llc | Medical organogel processes and compositions |
| US8545487B2 (en) | 2007-12-05 | 2013-10-01 | Avedro Inc. | Eye therapy system |
| US8574277B2 (en) | 2009-10-21 | 2013-11-05 | Avedro Inc. | Eye therapy |
| US8603511B2 (en) | 1996-08-27 | 2013-12-10 | Baxter International, Inc. | Fragmented polymeric compositions and methods for their use |
| US8664463B2 (en) | 2010-10-06 | 2014-03-04 | Alfred E. Mann Institute For Biomedical Engineering At The University Of Southern California | Reversible adhesives |
| US8703122B2 (en) | 2006-05-31 | 2014-04-22 | Baxter International Inc. | Method for directed cell in-growth and controlled tissue regeneration in spinal surgery |
| US8703170B2 (en) | 2010-04-07 | 2014-04-22 | Baxter International Inc. | Hemostatic sponge |
| US8771258B2 (en) | 2009-12-16 | 2014-07-08 | Baxter International Inc. | Hemostatic sponge |
| US8790698B2 (en) | 2007-10-30 | 2014-07-29 | Baxter International Inc. | Use of a regenerative biofunctional collagen biomatrix for treating visceral or parietal defects |
| US8834864B2 (en) | 2003-06-05 | 2014-09-16 | Baxter International Inc. | Methods for repairing and regenerating human dura mater |
| US8940335B2 (en) | 2010-06-01 | 2015-01-27 | Baxter International Inc. | Process for making dry and stable hemostatic compositions |
| US8961501B2 (en) | 2010-09-17 | 2015-02-24 | Incept, Llc | Method for applying flowable hydrogels to a cornea |
| US9005609B2 (en) | 2003-08-07 | 2015-04-14 | Ethicon, Inc. | Hemostatic compositions containing sterile thrombin |
| US9020580B2 (en) | 2011-06-02 | 2015-04-28 | Avedro, Inc. | Systems and methods for monitoring time based photo active agent delivery or photo active marker presence |
| US9039783B2 (en) | 2009-05-18 | 2015-05-26 | Baxter International, Inc. | Method for the improvement of mesh implant biocompatibility |
| US9044308B2 (en) | 2011-05-24 | 2015-06-02 | Avedro, Inc. | Systems and methods for reshaping an eye feature |
| US9084728B2 (en) | 2010-06-01 | 2015-07-21 | Baxter International Inc. | Process for making dry and stable hemostatic compositions |
| US9125807B2 (en) | 2007-07-09 | 2015-09-08 | Incept Llc | Adhesive hydrogels for ophthalmic drug delivery |
| US9162006B2 (en) | 2009-06-16 | 2015-10-20 | Baxter International Inc. | Hemostatic sponge |
| WO2016094646A1 (en) | 2014-12-10 | 2016-06-16 | Incept, Llc | Hydrogel drug delivery implants |
| US9393344B2 (en) | 2006-01-11 | 2016-07-19 | Hyperbranch Medical Technology, Inc. | Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices |
| US9408945B2 (en) | 2010-06-01 | 2016-08-09 | Baxter International Inc. | Process for making dry and stable hemostatic compositions |
| US9463004B2 (en) | 2009-05-04 | 2016-10-11 | Incept, Llc. | Biomaterials for track and puncture closure |
| WO2016183296A1 (en) | 2015-05-12 | 2016-11-17 | Incept, Llc | Drug delivery from hydrogels |
| WO2017091749A1 (en) | 2015-11-25 | 2017-06-01 | Incept, Llc | Shape changing drug delivery devices and methods |
| US9707126B2 (en) | 2009-10-21 | 2017-07-18 | Avedro, Inc. | Systems and methods for corneal cross-linking with pulsed light |
| US9724078B2 (en) | 2013-06-21 | 2017-08-08 | Ferrosan Medical Devices A/S | Vacuum expanded dry composition and syringe for retaining same |
| US9821025B2 (en) | 2011-10-11 | 2017-11-21 | Baxter International Inc. | Hemostatic compositions |
| US9833541B2 (en) | 2011-10-27 | 2017-12-05 | Baxter International Inc. | Hemostatic compositions |
| WO2018058048A1 (en) | 2016-09-23 | 2018-03-29 | Incept, Llc | Intracameral drug delivery depots |
| EA029534B1 (en) * | 2008-11-07 | 2018-04-30 | Клокс Текнолоджиз Инк. | Combination of an oxidant and a photoactivator for the healing of wounds |
| US9999703B2 (en) | 2012-06-12 | 2018-06-19 | Ferrosan Medical Devices A/S | Dry haemostatic composition |
| US10028657B2 (en) | 2015-05-22 | 2018-07-24 | Avedro, Inc. | Systems and methods for monitoring cross-linking activity for corneal treatments |
| US10114205B2 (en) | 2014-11-13 | 2018-10-30 | Avedro, Inc. | Multipass virtually imaged phased array etalon |
| US10111980B2 (en) | 2013-12-11 | 2018-10-30 | Ferrosan Medical Devices A/S | Dry composition comprising an extrusion enhancer |
| US10226417B2 (en) | 2011-09-16 | 2019-03-12 | Peter Jarrett | Drug delivery systems and applications |
| CN109529025A (en) * | 2018-11-26 | 2019-03-29 | 浙江舒是生物科技有限公司 | A kind of medical cosmetology Special sterilizing liquid |
| US10258809B2 (en) | 2015-04-24 | 2019-04-16 | Avedro, Inc. | Systems and methods for photoactivating a photosensitizer applied to an eye |
| US10322170B2 (en) | 2011-10-11 | 2019-06-18 | Baxter International Inc. | Hemostatic compositions |
| US10350111B2 (en) | 2014-10-27 | 2019-07-16 | Avedro, Inc. | Systems and methods for cross-linking treatments of an eye |
| WO2020006255A1 (en) * | 2018-06-27 | 2020-01-02 | The Board Of Trustees Of The Leland Stanford Junior University | Compositions and methods for in situ-forming tissue constructs |
| US10550187B2 (en) | 2014-10-24 | 2020-02-04 | Incept, Llc | Extra luminal scaffold |
| US10653837B2 (en) | 2014-12-24 | 2020-05-19 | Ferrosan Medical Devices A/S | Syringe for retaining and mixing first and second substances |
| US10918796B2 (en) | 2015-07-03 | 2021-02-16 | Ferrosan Medical Devices A/S | Syringe for mixing two components and for retaining a vacuum in a storage condition |
| US11046818B2 (en) | 2014-10-13 | 2021-06-29 | Ferrosan Medical Devices A/S | Dry composition for use in haemostasis and wound healing |
| US11109849B2 (en) | 2012-03-06 | 2021-09-07 | Ferrosan Medical Devices A/S | Pressurized container containing haemostatic paste |
| US11179576B2 (en) | 2010-03-19 | 2021-11-23 | Avedro, Inc. | Systems and methods for applying and monitoring eye therapy |
| US11207410B2 (en) | 2015-07-21 | 2021-12-28 | Avedro, Inc. | Systems and methods for treatments of an eye with a photosensitizer |
| CN113929792A (en) * | 2020-07-13 | 2022-01-14 | 孛朗孚(杭州)生物科技有限公司 | Aldehyde modified hyaluronic acid (sodium) and synthesis method and application thereof |
| US11642244B2 (en) | 2019-08-06 | 2023-05-09 | Avedro, Inc. | Photoactivation systems and methods for corneal cross-linking treatments |
| US11766356B2 (en) | 2018-03-08 | 2023-09-26 | Avedro, Inc. | Micro-devices for treatment of an eye |
| US11801324B2 (en) | 2018-05-09 | 2023-10-31 | Ferrosan Medical Devices A/S | Method for preparing a haemostatic composition |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2009109194A2 (en) | 2008-02-29 | 2009-09-11 | Ferrosan A/S | Device for promotion of hemostasis and/or wound healing |
| US9498114B2 (en) | 2013-06-18 | 2016-11-22 | Avedro, Inc. | Systems and methods for determining biomechanical properties of the eye for applying treatment |
| US9498122B2 (en) | 2013-06-18 | 2016-11-22 | Avedro, Inc. | Systems and methods for determining biomechanical properties of the eye for applying treatment |
Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6261687B1 (en) * | 1997-02-14 | 2001-07-17 | Reliance Electric Technologies, Llc | Oxygen plasma resistant polymer for electrical devices |
| US6719988B2 (en) * | 1997-02-21 | 2004-04-13 | The Regents Of The University Of California | Antiseptic compositions for inactivating prions |
-
2006
- 2006-06-19 WO PCT/US2006/023639 patent/WO2007001926A2/en active Application Filing
Patent Citations (2)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6261687B1 (en) * | 1997-02-14 | 2001-07-17 | Reliance Electric Technologies, Llc | Oxygen plasma resistant polymer for electrical devices |
| US6719988B2 (en) * | 1997-02-21 | 2004-04-13 | The Regents Of The University Of California | Antiseptic compositions for inactivating prions |
Non-Patent Citations (1)
| Title |
|---|
| VELAZQUEZ A.J. ET AL.: 'New Dendritic Adhesives for Sutureless Ophthalmic Surgical Procedures' VITRO STUDIES OF CORNEAL LACERATION REPAIR, ARCH OPHTHALMOLO vol. 122, June 2004, pages 867 - 870 * |
Cited By (117)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US8512729B2 (en) | 1996-08-27 | 2013-08-20 | Baxter International Inc. | Fragmented polymeric compositions and methods for their use |
| US8603511B2 (en) | 1996-08-27 | 2013-12-10 | Baxter International, Inc. | Fragmented polymeric compositions and methods for their use |
| US8357378B2 (en) | 1996-08-27 | 2013-01-22 | Baxter International Inc. | Fragmented polymeric compositions and methods for their use |
| US8303981B2 (en) | 1996-08-27 | 2012-11-06 | Baxter International Inc. | Fragmented polymeric compositions and methods for their use |
| US8092820B2 (en) | 2001-07-17 | 2012-01-10 | Baxter International Inc. | Dry hemostatic compositions and methods for their preparation |
| US8383141B2 (en) | 2001-07-17 | 2013-02-26 | Baxter International Inc. | Dry hemostatic compositions and methods for their preparation |
| US8834864B2 (en) | 2003-06-05 | 2014-09-16 | Baxter International Inc. | Methods for repairing and regenerating human dura mater |
| US9005609B2 (en) | 2003-08-07 | 2015-04-14 | Ethicon, Inc. | Hemostatic compositions containing sterile thrombin |
| US11027043B1 (en) | 2006-01-11 | 2021-06-08 | Hyperbranch Medical Technology, Inc. | Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices |
| US11826485B2 (en) | 2006-01-11 | 2023-11-28 | Hyperbranch Medical Technology, Inc. | Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices |
| US9878066B2 (en) | 2006-01-11 | 2018-01-30 | Hyperbranch Medical Technology, Inc. | Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices |
| US10688216B2 (en) | 2006-01-11 | 2020-06-23 | Hyperbranch Medical Technology, Inc. | Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices |
| US9393344B2 (en) | 2006-01-11 | 2016-07-19 | Hyperbranch Medical Technology, Inc. | Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices |
| US9498557B2 (en) | 2006-04-24 | 2016-11-22 | Incept, Llc | Crosslinking methods and applications thereof |
| US7597882B2 (en) | 2006-04-24 | 2009-10-06 | Incept Llc | Protein crosslinkers, crosslinking methods and applications thereof |
| US8703122B2 (en) | 2006-05-31 | 2014-04-22 | Baxter International Inc. | Method for directed cell in-growth and controlled tissue regeneration in spinal surgery |
| WO2008016983A3 (en) * | 2006-08-02 | 2009-03-26 | Baxter Int | Rapidly acting dry sealant and methods for use and manufacture |
| AU2007281138B2 (en) * | 2006-08-02 | 2013-05-02 | Baxter Healthcare S.A. | Rapidly acting dry sealant and methods for use and manufacture |
| KR101401873B1 (en) * | 2006-08-02 | 2014-05-29 | 박스터 헬쓰케어 에스에이 | Rapidly acting dry sealant and methods for use and manufacture |
| WO2008016983A2 (en) * | 2006-08-02 | 2008-02-07 | Baxter International Inc. | Rapidly acting dry sealant and methods for use and manufacture |
| EP2468310A1 (en) * | 2006-08-02 | 2012-06-27 | Baxter International Inc | Rapidly acting dry sealant and methods for use and manufacture |
| US8414911B2 (en) | 2006-10-24 | 2013-04-09 | The Regents Of The University Of California | Photochemical therapy to affect mechanical and/or chemical properties of body tissue |
| US8262608B2 (en) | 2007-01-25 | 2012-09-11 | Hyperbranch Medical Technology, Inc. | Applicators for multiple component formulations and the like, and methods of use thereof |
| US9125807B2 (en) | 2007-07-09 | 2015-09-08 | Incept Llc | Adhesive hydrogels for ophthalmic drug delivery |
| US10251954B2 (en) | 2007-07-09 | 2019-04-09 | Incept, Llc | Hydrogel polymeric compositions and methods |
| US11324828B2 (en) | 2007-07-09 | 2022-05-10 | Incept, Llc | Hydrogel polymeric compositions and methods |
| US9775906B2 (en) | 2007-07-09 | 2017-10-03 | Incept Llc | Hydrogel polymeric compositions and methods |
| US8790698B2 (en) | 2007-10-30 | 2014-07-29 | Baxter International Inc. | Use of a regenerative biofunctional collagen biomatrix for treating visceral or parietal defects |
| US8545487B2 (en) | 2007-12-05 | 2013-10-01 | Avedro Inc. | Eye therapy system |
| US7862538B2 (en) | 2008-02-04 | 2011-01-04 | Incept Llc | Surgical delivery system for medical sealant |
| US8366689B2 (en) | 2008-09-30 | 2013-02-05 | Avedro, Inc. | Method for making structural changes in corneal fibrils |
| EA029534B1 (en) * | 2008-11-07 | 2018-04-30 | Клокс Текнолоджиз Инк. | Combination of an oxidant and a photoactivator for the healing of wounds |
| WO2010093873A2 (en) | 2009-02-12 | 2010-08-19 | Incept, Llc | Drug delivery through hydrogel plugs |
| US8409606B2 (en) | 2009-02-12 | 2013-04-02 | Incept, Llc | Drug delivery through hydrogel plugs |
| US9463004B2 (en) | 2009-05-04 | 2016-10-11 | Incept, Llc. | Biomaterials for track and puncture closure |
| US9993298B2 (en) | 2009-05-18 | 2018-06-12 | Baxter International Inc. | Method for the improvement of mesh implant biocompatibility |
| US9039783B2 (en) | 2009-05-18 | 2015-05-26 | Baxter International, Inc. | Method for the improvement of mesh implant biocompatibility |
| US9162006B2 (en) | 2009-06-16 | 2015-10-20 | Baxter International Inc. | Hemostatic sponge |
| US8870934B2 (en) | 2009-10-21 | 2014-10-28 | Avedro, Inc. | Eye therapy system |
| US8574277B2 (en) | 2009-10-21 | 2013-11-05 | Avedro Inc. | Eye therapy |
| US9707126B2 (en) | 2009-10-21 | 2017-07-18 | Avedro, Inc. | Systems and methods for corneal cross-linking with pulsed light |
| US11160883B2 (en) | 2009-12-15 | 2021-11-02 | Incept, Llc | Echolucent implant composition and methods |
| US8383161B2 (en) | 2009-12-15 | 2013-02-26 | Incept, Llc | Radioopaque covalently crosslinked hydrogel particle implants |
| EP3960215A1 (en) | 2009-12-15 | 2022-03-02 | Incept, LLC | Implants and biodegradable fiducial markers |
| US9308283B2 (en) | 2009-12-15 | 2016-04-12 | Incept, Llc | Implants and biodegradable fiducial markers |
| US11786612B2 (en) | 2009-12-15 | 2023-10-17 | Incept, Llc | Implant and biodegradable tissue marker compositions and methods |
| US11154624B2 (en) | 2009-12-15 | 2021-10-26 | Incept, Llc | Echolucent implant compositions and methods |
| US10272164B2 (en) | 2009-12-15 | 2019-04-30 | Incept, Llc | Implants and biodegradable tissue markers |
| US11083802B2 (en) | 2009-12-15 | 2021-08-10 | Incept, Llc | Echolucent implant compositions and methods |
| US9669117B2 (en) | 2009-12-15 | 2017-06-06 | Incept, Llc | Implants and biodegradable tissue markers |
| WO2011084465A2 (en) | 2009-12-15 | 2011-07-14 | Incept, Llc | Implants and biodegradable fiducial markers |
| EP3583960A1 (en) | 2009-12-15 | 2019-12-25 | Incept, LLC | Implants and biodegradable fiducial markers |
| US10786581B2 (en) | 2009-12-15 | 2020-09-29 | Incept, Llc | Implants and biodegradable tissue markers |
| US9517287B2 (en) | 2009-12-16 | 2016-12-13 | Baxter International, Inc. | Hemostatic sponge |
| US11071804B2 (en) | 2009-12-16 | 2021-07-27 | Baxter International Inc. | Hemostatic sponge |
| US9872934B2 (en) | 2009-12-16 | 2018-01-23 | Baxter International Inc. | Hemostatic sponge |
| US8771258B2 (en) | 2009-12-16 | 2014-07-08 | Baxter International Inc. | Hemostatic sponge |
| US11179576B2 (en) | 2010-03-19 | 2021-11-23 | Avedro, Inc. | Systems and methods for applying and monitoring eye therapy |
| US8703170B2 (en) | 2010-04-07 | 2014-04-22 | Baxter International Inc. | Hemostatic sponge |
| US11478566B2 (en) | 2010-04-07 | 2022-10-25 | Baxter International Inc. | Hemostatic sponge |
| US10441674B2 (en) | 2010-04-07 | 2019-10-15 | Baxter International Inc. | Hemostatic sponge |
| US9375505B2 (en) | 2010-04-07 | 2016-06-28 | Baxter International Inc. | Hemostatic sponge |
| US10245348B2 (en) | 2010-06-01 | 2019-04-02 | Baxter International Inc. | Process for making dry and stable hemostatic compositions |
| US9408945B2 (en) | 2010-06-01 | 2016-08-09 | Baxter International Inc. | Process for making dry and stable hemostatic compositions |
| US10994045B2 (en) | 2010-06-01 | 2021-05-04 | Baxter International Inc. | Process for making dry and stable hemostatic compositions |
| US8940335B2 (en) | 2010-06-01 | 2015-01-27 | Baxter International Inc. | Process for making dry and stable hemostatic compositions |
| US9084728B2 (en) | 2010-06-01 | 2015-07-21 | Baxter International Inc. | Process for making dry and stable hemostatic compositions |
| US8961501B2 (en) | 2010-09-17 | 2015-02-24 | Incept, Llc | Method for applying flowable hydrogels to a cornea |
| US8664463B2 (en) | 2010-10-06 | 2014-03-04 | Alfred E. Mann Institute For Biomedical Engineering At The University Of Southern California | Reversible adhesives |
| US9044308B2 (en) | 2011-05-24 | 2015-06-02 | Avedro, Inc. | Systems and methods for reshaping an eye feature |
| US10137239B2 (en) | 2011-06-02 | 2018-11-27 | Avedro, Inc. | Systems and methods for monitoring time based photo active agent delivery or photo active marker presence |
| US9020580B2 (en) | 2011-06-02 | 2015-04-28 | Avedro, Inc. | Systems and methods for monitoring time based photo active agent delivery or photo active marker presence |
| US10226417B2 (en) | 2011-09-16 | 2019-03-12 | Peter Jarrett | Drug delivery systems and applications |
| US10322170B2 (en) | 2011-10-11 | 2019-06-18 | Baxter International Inc. | Hemostatic compositions |
| US9821025B2 (en) | 2011-10-11 | 2017-11-21 | Baxter International Inc. | Hemostatic compositions |
| US9833541B2 (en) | 2011-10-27 | 2017-12-05 | Baxter International Inc. | Hemostatic compositions |
| EP3613413A1 (en) | 2011-12-05 | 2020-02-26 | Incept, LLC | Medical organogel processes and compositions |
| WO2013086015A1 (en) | 2011-12-05 | 2013-06-13 | Incept, Llc | Medical organogel processes and compositions |
| US11109849B2 (en) | 2012-03-06 | 2021-09-07 | Ferrosan Medical Devices A/S | Pressurized container containing haemostatic paste |
| US10799611B2 (en) | 2012-06-12 | 2020-10-13 | Ferrosan Medical Devices A/S | Dry haemostatic composition |
| US9999703B2 (en) | 2012-06-12 | 2018-06-19 | Ferrosan Medical Devices A/S | Dry haemostatic composition |
| US10595837B2 (en) | 2013-06-21 | 2020-03-24 | Ferrosan Medical Devices A/S | Vacuum expanded dry composition and syringe for retaining same |
| US9724078B2 (en) | 2013-06-21 | 2017-08-08 | Ferrosan Medical Devices A/S | Vacuum expanded dry composition and syringe for retaining same |
| US11103616B2 (en) | 2013-12-11 | 2021-08-31 | Ferrosan Medical Devices A/S | Dry composition comprising an extrusion enhancer |
| US10111980B2 (en) | 2013-12-11 | 2018-10-30 | Ferrosan Medical Devices A/S | Dry composition comprising an extrusion enhancer |
| US11046818B2 (en) | 2014-10-13 | 2021-06-29 | Ferrosan Medical Devices A/S | Dry composition for use in haemostasis and wound healing |
| US10550187B2 (en) | 2014-10-24 | 2020-02-04 | Incept, Llc | Extra luminal scaffold |
| US11377498B2 (en) | 2014-10-24 | 2022-07-05 | Incept, Llc | Extra luminal scaffold |
| US10350111B2 (en) | 2014-10-27 | 2019-07-16 | Avedro, Inc. | Systems and methods for cross-linking treatments of an eye |
| US11219553B2 (en) | 2014-10-27 | 2022-01-11 | Avedro, Inc. | Systems and methods for cross-linking treatments of an eye |
| US10114205B2 (en) | 2014-11-13 | 2018-10-30 | Avedro, Inc. | Multipass virtually imaged phased array etalon |
| EP3858329A1 (en) | 2014-12-10 | 2021-08-04 | Incept, LLC | Hydrogel drug delivery implants |
| WO2016094646A1 (en) | 2014-12-10 | 2016-06-16 | Incept, Llc | Hydrogel drug delivery implants |
| US10653837B2 (en) | 2014-12-24 | 2020-05-19 | Ferrosan Medical Devices A/S | Syringe for retaining and mixing first and second substances |
| US11167149B2 (en) | 2015-04-24 | 2021-11-09 | Avedro, Inc. | Systems and methods for photoactivating a photosensitizer applied to an eye |
| US10258809B2 (en) | 2015-04-24 | 2019-04-16 | Avedro, Inc. | Systems and methods for photoactivating a photosensitizer applied to an eye |
| WO2016183296A1 (en) | 2015-05-12 | 2016-11-17 | Incept, Llc | Drug delivery from hydrogels |
| US11369591B2 (en) | 2015-05-12 | 2022-06-28 | Incept, Llc | Drug delivery from hydrogels |
| EP4279064A2 (en) | 2015-05-12 | 2023-11-22 | Incept, LLC | Drug delivery from hydrogels |
| US10028657B2 (en) | 2015-05-22 | 2018-07-24 | Avedro, Inc. | Systems and methods for monitoring cross-linking activity for corneal treatments |
| US10918796B2 (en) | 2015-07-03 | 2021-02-16 | Ferrosan Medical Devices A/S | Syringe for mixing two components and for retaining a vacuum in a storage condition |
| US11207410B2 (en) | 2015-07-21 | 2021-12-28 | Avedro, Inc. | Systems and methods for treatments of an eye with a photosensitizer |
| US10420724B2 (en) | 2015-11-25 | 2019-09-24 | Incept, Llc | Shape changing drug delivery devices and methods |
| US11938223B2 (en) | 2015-11-25 | 2024-03-26 | Incept, Llc | Shape changing drug delivery devices and methods |
| WO2017091749A1 (en) | 2015-11-25 | 2017-06-01 | Incept, Llc | Shape changing drug delivery devices and methods |
| US10786462B2 (en) | 2015-11-25 | 2020-09-29 | Incept, Llc | Shape changing drug delivery devices and methods |
| US11413250B2 (en) | 2015-11-25 | 2022-08-16 | Incept, Llc | Shape changing drug delivery devices and methods |
| WO2018058048A1 (en) | 2016-09-23 | 2018-03-29 | Incept, Llc | Intracameral drug delivery depots |
| EP4197527A1 (en) | 2016-09-23 | 2023-06-21 | Incept, LLC | Intracameral drug delivery depots |
| US11766356B2 (en) | 2018-03-08 | 2023-09-26 | Avedro, Inc. | Micro-devices for treatment of an eye |
| US11801324B2 (en) | 2018-05-09 | 2023-10-31 | Ferrosan Medical Devices A/S | Method for preparing a haemostatic composition |
| US20210244659A1 (en) * | 2018-06-27 | 2021-08-12 | The Board Of Trustees Of The Leland Stanford Junior University | Compositions and methods for in situ-forming tissue constructs |
| WO2020006255A1 (en) * | 2018-06-27 | 2020-01-02 | The Board Of Trustees Of The Leland Stanford Junior University | Compositions and methods for in situ-forming tissue constructs |
| CN109529025A (en) * | 2018-11-26 | 2019-03-29 | 浙江舒是生物科技有限公司 | A kind of medical cosmetology Special sterilizing liquid |
| US11642244B2 (en) | 2019-08-06 | 2023-05-09 | Avedro, Inc. | Photoactivation systems and methods for corneal cross-linking treatments |
| CN113929792B (en) * | 2020-07-13 | 2023-03-10 | 孛朗孚(杭州)生物科技有限公司 | Aldehyde modified hyaluronic acid (sodium) and synthesis method and application thereof |
| CN113929792A (en) * | 2020-07-13 | 2022-01-14 | 孛朗孚(杭州)生物科技有限公司 | Aldehyde modified hyaluronic acid (sodium) and synthesis method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2007001926A3 (en) | 2007-12-06 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2007001926A2 (en) | Low-swelling hydrogel sealants for wound repair | |
| US11027043B1 (en) | Crosslinked gels comprising polyalkyleneimines, and their uses as medical devices | |
| JP5739668B2 (en) | Crosslinked polyalkyleneimine hydrogels with adjustable degradation rate | |
| WO2006031358A2 (en) | Dendritic polymers, crosslinked gels, and their uses as ophthalmic sealants and lenses | |
| US10646614B2 (en) | Dissolvable hydrogel compositions for wound management and methods of use | |
| AU2017217397B2 (en) | Dendrimer-bioadhesive polymer hydrogel nanoglue and use thereof | |
| US20040086479A1 (en) | Novel dendritic polymers, crosslinked gels, and their biomedical uses | |
| RU2486907C2 (en) | Imidised biopolymer adhesive and hydrogel | |
| WO2006031388A2 (en) | Dentritic polymers, crosslinked gels, and their uses in orthopedic applications | |
| WO2007005249A2 (en) | Nanoparticles and dendritic-polymer-based hydrogels comprising them | |
| JP2004530452A (en) | Non-attractable transition viscoelastic materials for use in surgery |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 06773438 Country of ref document: EP Kind code of ref document: A2 |