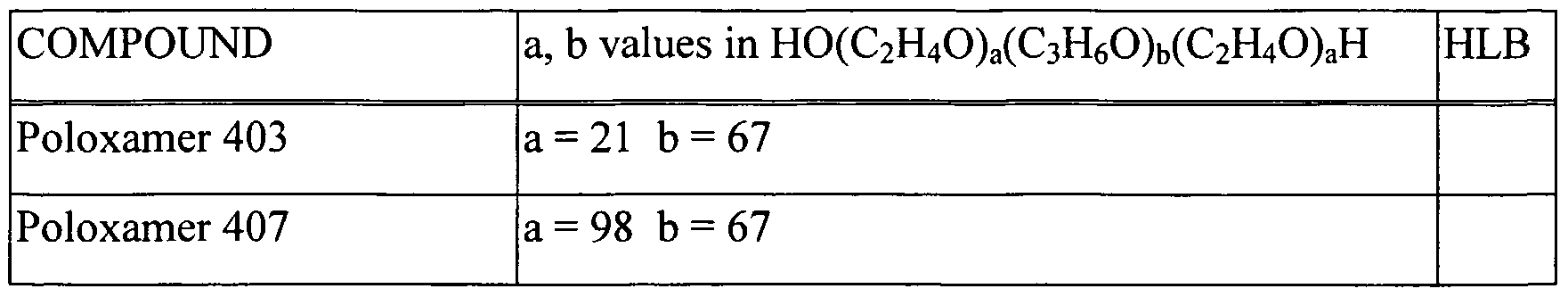WO2001037808A1 - Solid carriers for improved delivery of active ingredients in pharmaceutical compositions - Google Patents
Solid carriers for improved delivery of active ingredients in pharmaceutical compositions Download PDFInfo
- Publication number
- WO2001037808A1 WO2001037808A1 PCT/US2000/032255 US0032255W WO0137808A1 WO 2001037808 A1 WO2001037808 A1 WO 2001037808A1 US 0032255 W US0032255 W US 0032255W WO 0137808 A1 WO0137808 A1 WO 0137808A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- agents
- pharmaceutical composition
- active ingredient
- vaccine
- sodium
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/14—Esters of carboxylic acids, e.g. fatty acid monoglycerides, medium-chain triglycerides, parabens or PEG fatty acid esters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/335—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin
- A61K31/35—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom
- A61K31/352—Heterocyclic compounds having oxygen as the only ring hetero atom, e.g. fungichromin having six-membered rings with one oxygen as the only ring hetero atom condensed with carbocyclic rings, e.g. methantheline
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/435—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with one nitrogen as the only ring hetero atom
- A61K31/47—Quinolines; Isoquinolines
- A61K31/4709—Non-condensed quinolines and containing further heterocyclic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/495—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with two or more nitrogen atoms as the only ring heteroatoms, e.g. piperazine or tetrazines
- A61K31/496—Non-condensed piperazines containing further heterocyclic rings, e.g. rifampin, thiothixene
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/535—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having six-membered rings with at least one nitrogen and one oxygen as the ring hetero atoms, e.g. 1,2-oxazines
- A61K31/5375—1,4-Oxazines, e.g. morpholine
- A61K31/5383—1,4-Oxazines, e.g. morpholine ortho- or peri-condensed with heterocyclic ring systems
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/55—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole
- A61K31/551—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having seven-membered rings, e.g. azelastine, pentylenetetrazole having two nitrogen atoms, e.g. dilazep
- A61K31/5513—1,4-Benzodiazepines, e.g. diazepam or clozapine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/56—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids
- A61K31/565—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol
- A61K31/568—Compounds containing cyclopenta[a]hydrophenanthrene ring systems; Derivatives thereof, e.g. steroids not substituted in position 17 beta by a carbon atom, e.g. estrane, estradiol substituted in positions 10 and 13 by a chain having at least one carbon atom, e.g. androstanes, e.g. testosterone
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/70—Carbohydrates; Sugars; Derivatives thereof
- A61K31/7042—Compounds having saccharide radicals and heterocyclic rings
- A61K31/7048—Compounds having saccharide radicals and heterocyclic rings having oxygen as a ring hetero atom, e.g. leucoglucosan, hesperidin, erythromycin, nystatin, digitoxin or digoxin
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K47/00—Medicinal preparations characterised by the non-active ingredients used, e.g. carriers or inert additives; Targeting or modifying agents chemically bound to the active ingredient
- A61K47/06—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite
- A61K47/08—Organic compounds, e.g. natural or synthetic hydrocarbons, polyolefins, mineral oil, petrolatum or ozokerite containing oxygen, e.g. ethers, acetals, ketones, quinones, aldehydes, peroxides
- A61K47/10—Alcohols; Phenols; Salts thereof, e.g. glycerol; Polyethylene glycols [PEG]; Poloxamers; PEG/POE alkyl ethers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/1605—Excipients; Inactive ingredients
- A61K9/1617—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/14—Particulate form, e.g. powders, Processes for size reducing of pure drugs or the resulting products, Pure drug nanoparticles
- A61K9/16—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction
- A61K9/167—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction with an outer layer or coating comprising drug; with chemically bound drugs or non-active substances on their surface
- A61K9/1676—Agglomerates; Granulates; Microbeadlets ; Microspheres; Pellets; Solid products obtained by spray drying, spray freeze drying, spray congealing,(multiple) emulsion solvent evaporation or extraction with an outer layer or coating comprising drug; with chemically bound drugs or non-active substances on their surface having a drug-free core with discrete complete coating layer containing drug
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/4841—Filling excipients; Inactive ingredients
- A61K9/4858—Organic compounds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5073—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings
- A61K9/5078—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings with drug-free core
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
- A61P1/04—Drugs for disorders of the alimentary tract or the digestive system for ulcers, gastritis or reflux esophagitis, e.g. antacids, inhibitors of acid secretion, mucosal protectants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P13/00—Drugs for disorders of the urinary system
- A61P13/08—Drugs for disorders of the urinary system of the prostate
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P15/00—Drugs for genital or sexual disorders; Contraceptives
- A61P15/10—Drugs for genital or sexual disorders; Contraceptives for impotence
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/12—Keratolytics, e.g. wart or anti-corn preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/06—Antigout agents, e.g. antihyperuricemic or uricosuric agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/08—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease
- A61P19/10—Drugs for skeletal disorders for bone diseases, e.g. rachitism, Paget's disease for osteoporosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P21/00—Drugs for disorders of the muscular or neuromuscular system
- A61P21/02—Muscle relaxants, e.g. for tetanus or cramps
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/04—Centrally acting analgesics, e.g. opioids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/06—Antimigraine agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/20—Hypnotics; Sedatives
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/26—Psychostimulants, e.g. nicotine, cocaine
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/04—Anorexiants; Antiobesity agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/06—Antihyperlipidemics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P3/00—Drugs for disorders of the metabolism
- A61P3/08—Drugs for disorders of the metabolism for glucose homeostasis
- A61P3/10—Drugs for disorders of the metabolism for glucose homeostasis for hyperglycaemia, e.g. antidiabetics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/04—Antibacterial agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/10—Antimycotics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/02—Antiprotozoals, e.g. for leishmaniasis, trichomoniasis, toxoplasmosis
- A61P33/06—Antimalarials
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P33/00—Antiparasitic agents
- A61P33/10—Anthelmintics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/14—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4
- A61P5/16—Drugs for disorders of the endocrine system of the thyroid hormones, e.g. T3, T4 for decreasing, blocking or antagonising the activity of the thyroid hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/24—Drugs for disorders of the endocrine system of the sex hormones
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P5/00—Drugs for disorders of the endocrine system
- A61P5/38—Drugs for disorders of the endocrine system of the suprarenal hormones
- A61P5/40—Mineralocorticosteroids, e.g. aldosterone; Drugs increasing or potentiating the activity of mineralocorticosteroids
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/10—Antioedematous agents; Diuretics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/04—Inotropic agents, i.e. stimulants of cardiac contraction; Drugs for heart failure
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/06—Antiarrhythmics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/10—Drugs for disorders of the cardiovascular system for treating ischaemic or atherosclerotic diseases, e.g. antianginal drugs, coronary vasodilators, drugs for myocardial infarction, retinopathy, cerebrovascula insufficiency, renal arteriosclerosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
- A61P9/12—Antihypertensives
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B82—NANOTECHNOLOGY
- B82Y—SPECIFIC USES OR APPLICATIONS OF NANOSTRUCTURES; MEASUREMENT OR ANALYSIS OF NANOSTRUCTURES; MANUFACTURE OR TREATMENT OF NANOSTRUCTURES
- B82Y5/00—Nanobiotechnology or nanomedicine, e.g. protein engineering or drug delivery
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5015—Organic compounds, e.g. fats, sugars
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5005—Wall or coating material
- A61K9/5021—Organic macromolecular compounds
- A61K9/5026—Organic macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyvinyl pyrrolidone, poly(meth)acrylates
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5084—Mixtures of one or more drugs in different galenical forms, at least one of which being granules, microcapsules or (coated) microparticles according to A61K9/16 or A61K9/50, e.g. for obtaining a specific release pattern or for combining different drugs
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y02—TECHNOLOGIES OR APPLICATIONS FOR MITIGATION OR ADAPTATION AGAINST CLIMATE CHANGE
- Y02A—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE
- Y02A50/00—TECHNOLOGIES FOR ADAPTATION TO CLIMATE CHANGE in human health protection, e.g. against extreme weather
- Y02A50/30—Against vector-borne diseases, e.g. mosquito-borne, fly-borne, tick-borne or waterborne diseases whose impact is exacerbated by climate change
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/902—Specified use of nanostructure
- Y10S977/904—Specified use of nanostructure for medical, immunological, body treatment, or diagnosis
- Y10S977/906—Drug delivery
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S977/00—Nanotechnology
- Y10S977/902—Specified use of nanostructure
- Y10S977/904—Specified use of nanostructure for medical, immunological, body treatment, or diagnosis
- Y10S977/927—Diagnostic contrast agent
Definitions
- the present invention relates to pharmaceutical delivery systems for pharmaceutical active ingredients, such as drugs, nutritionals, cosmeceuticals, and diagnostic agents.
- pharmaceutical active ingredients such as drugs, nutritionals, cosmeceuticals, and diagnostic agents.
- the present invention provides compositions and dosage forms including solid carriers for improved delivery of pharmaceutical active ingredients.
- Hydrophobic active ingredients such as progesterone, cyclosporine, itraconazole and glyburide present delivery challenges due to their poor aqueous solubility and slow dissolution rate.
- hydrophobic drugs Several commercial products of these hydrophobic drugs are available, the various products using different methods to try to enhance in vivo performance.
- One approach is size reduction by micronization, such as in Prometrium (micronized progesterone) and Micronase (micronized glyburide).
- Other approaches include size reduction in emulsion formulations, such as in Sandimmune (cyclosporine emulsion) and NeOral (cyclosporine microemulsion). These approaches suffer from several disadvantages.
- Micronization/nanonization presents processing and stability challenges, as well as dissolution limitations, since the micronized/nanosized drug still possesses a high degree of crystallinity.
- Liquid formulations present drug precipitation and packaging challenges, due to solvent evaporation.
- non-solid formulations are more prone to chemical instability and capsule-shell incompatibility, leading to the possibility of leakage upon storage.
- hydrophilic active ingredients For hydrophilic active ingredients, the formulation challenges are different. Although these compounds are readily soluble in the aqueous gastrointestinal environment, they are poorly absorbed, due to poor membrane permeability and/or enzymatic degradation. Surfactants and lipophilic additives have been reported to improve membrane permeability; see, e.g., LeCluyse and Sutton, "In vitro models for selection of development candidates. Permeability studies to define mechanisms of absorption enhancement", Advanced Drug Delivery Reviews, 23, 163-183 (1997). However, these compositions fail to maintain effective levels and type of enhancers for bioacceptable absorption enhancement. Most solid dosage forms of hydrophilic active ingredients exhibit poor or no absorption of the active. Moreover, these non-solid formulations suffer from the disadvantages of chemical instability, leakage and capsule shell incompatibility as discussed above.
- Solid carriers for pharmaceutical active ingredients offer potential advantages over micronized drugs, emulsions or solubilized formulations.
- Solid carriers typically of size less than about 2 mm, can easily pass through the stomach, thus making the performance less prone to gastric emptying variability. Further, the problems of leakage and other disadvantages of liquid formulations are not present in solid carrier formulations. To date, however, such solid carrier formulations generally have been limited to a few specific drugs, due to difficulties in formulating appropriate drug/excipient compositions to effectively coat the active ingredient onto a carrier particle.
- the present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered.
- the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate.
- the encapsulation coat includes at least one ionic or non-ionic hydrophilic surfactant.
- the encapsulation coat can include a pharmaceutical active ingredient, a lipophilic component such as a lipophilic surfactant or a triglyceride, or both a pharmaceutical active ingredient and a lipophilic component.
- the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate.
- the encapsulation coat includes a lipophilic component, such as a lipophilic surfactant or a triglyceride.
- the encapsulation coat can include a pharmaceutical active ingredient, an ionic or non-ionic hydrophilic surfactant, or both a pharmaceutical active ingredient and a hydrophilic surfactant.
- the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate.
- the encapsulation coat includes a pharmaceutical active ingredient and an ionic or non- ionic hydrophilic surfactant; a pharmaceutical active ingredient and a lipophilic component such as a lipophilic surfactant or a triglyceride; or a pharmaceutical active ingredient and both a hydrophilic surfactant and a lipophilic component.
- the solid pharmaceutical composition includes a solid carrier, wherein the solid carrier is formed of at least two components selected from the group consisting of pharmaceutical active ingredients; ionic or non-ionic hydrophilic surfactants; and lipophilic components such as lipophilic surfactants and triglycerides.
- the present invention also provides dosage forms of any of the solid pharmaceutical compositions, and methods of using the solid pharmaceutical compositions.
- Figure 1 is a graph showing the extent of dissolution/release of glyburide as a function of time for a composition according to the present invention and two prior art compositions.
- Figure 2 A is a graph showing the extent of dissolution/release of progesterone as a function of time for two compositions according to the present invention and the pure bulk drug.
- Figure 2B is a graph showing the extent of dissolution/release of progesterone as a function of time for two compositions of the present invention, a conventional commercial formulation of progesterone, and the pure bulk drug.
- Figure 3 is a graph showing the extent of dissolution/release of omeprazole as a function of time for two compositions according to the present invention and a prior art composition.
- the present invention provides solid pharmaceutical compositions for improved delivery of a wide variety of pharmaceutical active ingredients contained therein or separately administered.
- the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate.
- the encapsulation coat can include different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides.
- the solid pharmaceutical composition includes a solid carrier, the solid carrier being formed of different combinations of pharmaceutical active ingredients, hydrophilic surfactant, lipophilic surfactants and triglycerides.
- any of the components of the compositions of the present invention can be used as supplied commercially, or can be preprocessed by agglomeration, air suspension chilling, air suspension drying, balling, coacervation, comminution, compression, pelletization, cryopelletization, extrusion, granulation, homogenization, inclusion complexation, lyophilization, melting, mixing, molding, pan coating, solvent dehydration, sonication, spheronization, spray chilling, spray congealing, spray drying, or other processes known in the art.
- the various components can also be pre-coated or encapsulated. These various processes and coatings are described in more detail below.
- the active ingredients suitable for use in the pharmaceutical compositions and methods of the present invention are not particularly limited, as the compositions are surprisingly capable of effectively delivering a wide variety of active ingredients.
- the active ingredient can be hydrophilic, lipophilic, amphiphilic or hydrophobic, and can be solubilized, dispersed, or partially solubilized and dispersed, in the encapsulation coat.
- the active ingredient can be provided separately from the solid pharmaceutical composition, such as for co-administration.
- Such active ingredients can be any compound or mixture of compounds having therapeutic or other value when administered to an animal, particularly to a mammal, such as drugs, nutrients, cosmeceuticals, diagnostic agents, nutritional agents, and the like.
- the categorization of an active ingredient as hydrophilic or hydrophobic may change, depending upon the particular salts, isomers, analogs and derivatives used.
- the active ingredient agent is hydrophobic.
- Hydrophobic active ingredients are compounds with little or no water solubility. Intrinsic water solubilities (i.e., water solubility of the unionized form) for hydrophobic active ingredients are less than about 1% by weight, and typically less than about 0.1% or 0.01% by weight.
- the active ingredient is a hydrophobic drug.
- the active ingredient is a nutrient, a cosmeceutical, a diagnostic agent or a nutritional agent.
- Suitable hydrophobic active ingredients are not limited by therapeutic category, and can be, for example, analgesics, anti-inflammatory agents, anthelmintics, anti- arrhythmic agents, anti-bacterial agents, anti-viral agents, anti-coagulants, anti- depressants, anti-diabetics, anti-epileptics, anti-fungal agents, anti-gout agents, anti- hypertensive agents, anti-malarials, anti-migraine agents, anti-muscarinic agents, anti- neoplastic agents, erectile dysfunction improvement agents, immunosuppressants, anti- protozoal agents, anti-thyroid agents, anxiolytic agents, sedatives, hypnotics, neuroleptics, ⁇ -Blockers, cardiac inotropic agents, corticosteroids, diuretics, anti- parkinsonian agents, gastro-intestinal agents, histamine receptor antagonists, keratolytics, lipid regulating agents, anti-anginal agents, cox-2 inhibitors,
- hydrophobic active ingredients are: acutretin, albendazole, albuterol, aminogluthemide, amiodarone, amlodipine, amphetamine, amphotericin B, atorvastatin, atovaquone, azithromycin, baclofen, beclomethsone, benezepril, benzonatate, betamethasone, bicalutanide, budesonide, bupropion, busulphan, butenafine, calcifediol, calciprotiene, calcitriol, camptothecan, candesartan, capsaicin, carbamezepine, carotenes, celecoxib, cerivistatin, cetrizine, chlorpheniramine, cholecalciferol, cilostazol, cimetidine, cinnarizine, ciprofloxacin, cisapride, clarithromycin, clemastine, clomiphene
- preferred active ingredients include: acutretin, albendazole, albuterol, aminogluthemide, amiodarone, amlodipine, amphetamine, amphotericin B, atorvastatin, atovaquone, azithromycin, baclofen, benzonatate, bicalutanide, busulphan, butenafine, calcifediol, calciprotiene, calcitriol, camptothecan, capsaicin, carbamezepine, carotenes, celecoxib, cerivistatin, chlorpheniramine, cholecaliferol, cimetidine, cinnarizine, ciprofloxacin, cisapride, citrizine, clarithromycin, clemastine, clomiphene, codeine, coenzyme Q10, cyclosporine, danazol, dantrolene, dexchlopheniramine, di
- hydrophobic active ingredients include: acutretin, albuterol, aminogluthemide, amiodarone, amlodipine, amprenavir, atorvastatin, atovaquone, baclofen, benzonatate, bicalutanide, busulphan, calcifediol, calciprotiene, calcitriol, camptothecan, capsaicin, carbamezepine, carotenes, celecoxib, chlo ⁇ heniramine, cholecaliferol, cimetidine, cinnarizine, cisapride, citrizine, clemastine, coenzyme Q10, cyclosporine, danazol, dantrolene, dexchlopheniramine, diclofenac, dihydro epiandrosterone, dihydroergotamine, dihyrotachysterol, efavirenz, ergocalciferol, ergotamine, essential fatty acids
- hydrophobic active ingredients include: amlodipine, amprenavir, atorvastatin, atovaquone, celecoxib, cisapride, coenzyme Q10, cyclosporine, famotidine, fenofibrate, fexofenadine, finasteride, ibuprofen, itraconazole, lanosprazole, loratadine, lovastatin, megesterol acetate, montelukast, nabumetone, nizatidine, omeprazole, oxaprozin, paclitaxel, paricalcitol, pioglitazone, pranlukast, progesterone, pseudo- ephedrine, rabeprazole, rapamycin, refocoxib, repaglinide, rimexolone, ritanovir, rosiglitazone, saquinavir, sildena
- the active ingredient is hydrophilic.
- Amphiphilic compounds are also included within the class of hydrophilic active ingredients. Apparent water solubilities for hydrophilic active ingredients are greater than about 0.1% by weight, and typically greater than about 1% by weight.
- the hydrophilic active ingredient is a hydrophilic drug.
- the hydrophilic active ingredient is a cosmeceutical, a diagnostic agent, or a nutritional agent.
- Suitable hydrophilic active ingredients are not limited by therapeutic category, and can be, for example, analgesics, anti-inflammatory agents, anthelmintics, anti- arrhythmic agents, anti-bacterial agents, anti-viral agents, anti-coagulants, anti- depressants, anti-diabetics, anti-epileptics, anti-fungal agents, anti-gout agents, anti- hypertensive agents, anti-malarials, anti-migraine agents, anti-muscarinic agents, anti- neoplastic agents, erectile dysfunction improvement agents, immunosuppressants, anti- protozoal agents, anti-thyroid agents, anxiolytic agents, sedatives, hypnotics, neuroleptics, Q-Blockers, cardiac inotropic agents, corticosteroids, diuretics, anti- parkinsonian agents, gastro-intestinal agents, histamine receptor antagonists, keratolytics, lipid regulating agents, anti-anginal agents, cox-2 inhibitors, le
- the hydrophilic active ingredient can be a cytokine, a peptidomimetic, a peptide, a protein, a toxoid, a serum, an antibody, a vaccine, a nucleoside, a nucleotide, a portion of genetic material, a nucleic acid, or a mixture thereof.
- hydrophilic active ingredients include: acarbose; acyclovir; acetyl cysteine; acetylcholine chloride; alatrofloxacin; alendronate; alglucerase; amantadine hydrochloride; ambenomium; amifostine; amiloride hydrochloride; aminocaproic acid; amphotericin B; antihemophilic factor (human); antihemophilic factor (porcine); antihemophilic factor (recombinant); aprotinin; asparaginase; atenolol; atracurium besylate; atropine; azithromycin; aztreonam; BCG vaccine; bacitracin; becalermin; belladona; bepridil hydrochloride; bleomycin sulfate; calcitonin human; calcitonin salmon; carboplatin; capecitabine; capreomycin sulfate; cef
- TNK-tPA trandolapril; trimetrexate gluconate; trospectinomycin; trovafloxacin; tubocurarine chloride; tumor necrosis factor; typhoid vaccine live; urea; urokinase; vancomycin; valaciclovir; valsartan; varicella virus vaccine live; vasopressin and vasopressin derivatives; vecoronium bromide; vinblastin; vincristine; vinorelbine; vitamin B12 ; warfarin sodium; yellow fever vaccine; zalcitabine; zanamavir; zolandronate; zidovudine; pharmaceutically acceptable salts, isomers and derivatives thereof; and mixtures thereof.
- preferred active ingredients include acarbose; acyclovir; atracurium besylate; alendronate; alglucerase; amantadine hydrochloride; amphotericin B; antihemophilic factor (human); antihemophilic factor (porcine); antihemophilic factor (recombinant; azithromycin; calcitonin human; calcitonin salmon; capecitabine; cefazolin sodium; cefonicid sodium; cefoperazone; cefoxitin sodium; ceftizoxime; ceftriaxone; cefuroxime axetil; cephalexin; chrionic gonadotropin; cidofovir; cladribine ; clindamycin and clindamycin derivatives; cortocotropin; cosyntropin; cromalyn sodium; cytarabine; daltaperin sodium; danaproid; desmopressin; didanos
- hydrophilic active ingredients include acarbose; alendronate; amantadine hydrochloride; azithromycin; calcitonin human; calcitonin salmon; ceftriaxone; cefuroxime axetil; chrionic gonadotropin; cromalyn sodium; daltaperin sodium; danaproid; desmopressin; didanosine; editronate disodium; enoxaprin sodium; epoetin alpha; factor IX; famiciclovir; foscarnet sodium; ganciclovir; granulocyte colony stimulating factor; granulocyte-macrophage stimulating factor; growth hormones- recombinant human; growth hormone- Bovine; glucagon; gonadotropin releasing hormone and synthetic analogs thereof; GnRH; gonadorelin; heparin sodium; indinavir sulfate; influenza virus vaccine; inter leukin-2; interleukin-3; insulin-
- hydrophilic surfactants can be used to provide any of several advantageous characteristics to the compositions, including: increased solubility of the active ingredient in the solid carrier; improved dissolution of the active ingredient; improved solubilization of the active ingredient upon dissolution; enhanced abso ⁇ tion and/or bioavailability of the active ingredient, particularly a hydrophilic active ingredient; and improved stability, both physical and chemical, of the active ingredient.
- the hydrophilic surfactant can be a single hydrophilic surfactant or a mixture of hydrophilic surfactants, and can be ionic or non-ionic.
- various embodiments of the invention include a lipophilic component, which can be a lipophilic surfactant, including a mixture of lipophilic surfactants, a triglyceride, or a mixture thereof.
- the lipophilic surfactant can provide any of the advantageous characteristics listed above for hydrophilic surfactants, as well as further enhancing the function of the surfactants.
- a compound To function as a surfactant, a compound must necessarily include polar or charged hydrophilic moieties as well as non-polar hydrophobic (lipophilic) moieties; i.e., a surfactant compound must be amphiphilic.
- An empirical parameter commonly used to characterize the relative hydrophilicity and lipophilicity of non-ionic amphiphilic compounds is the hydrophilic-lipophilic balance (the "HLB" value).
- HLB hydrophilic-lipophilic balance
- hydrophilic surfactants are generally considered to be those compounds having an HLB value greater than about 10, as well as anionic, cationic, or zwitterionic compounds for which the HLB scale is not generally applicable.
- lipophilic surfactants are compounds having an HLB value less than about 10.
- HLB value of a surfactant is merely a rough guide generally used to enable formulation of industrial, pharmaceutical and cosmetic emulsions.
- HLB values can differ by as much as about 8 HLB units, depending upon the empirical method chosen to determine the HLB value (Schott, J. Pharm. Sciences, 79(1), 87-88 (1990)).
- polypropylene oxide containing block copolymers polypropylene oxide containing block copolymers, available commercially as PLURONIC® surfactants, BASF Co ⁇ .
- the HLB values may not accurately reflect the true physical chemical nature of the compounds.
- Suitable surfactants can be anionic, cationic, zwitterionic or non-ionic. Such surfactants can be grouped into the following general chemical classes detailed in the Tables herein.
- the HLB values given in the Tables below generally represent the HLB value as reported by the manufacturer of the corresponding commercial product. In cases where more than one commercial product is listed, the HLB value in the Tables is the value as reported for one of the commercial products, a rough average of the reported values, or a value that, in the judgment of the present inventors, is more reliable.
- the invention is not limited to the surfactants in the Tables, which show representative, but not exclusive, lists of available surfactants.
- refined, distilled or fractionated surfactants, purified fractions thereof, or re- esterified fractions are also within the scope of the invention, although not specifically listed in the Tables.
- PEG polyethylene glycol
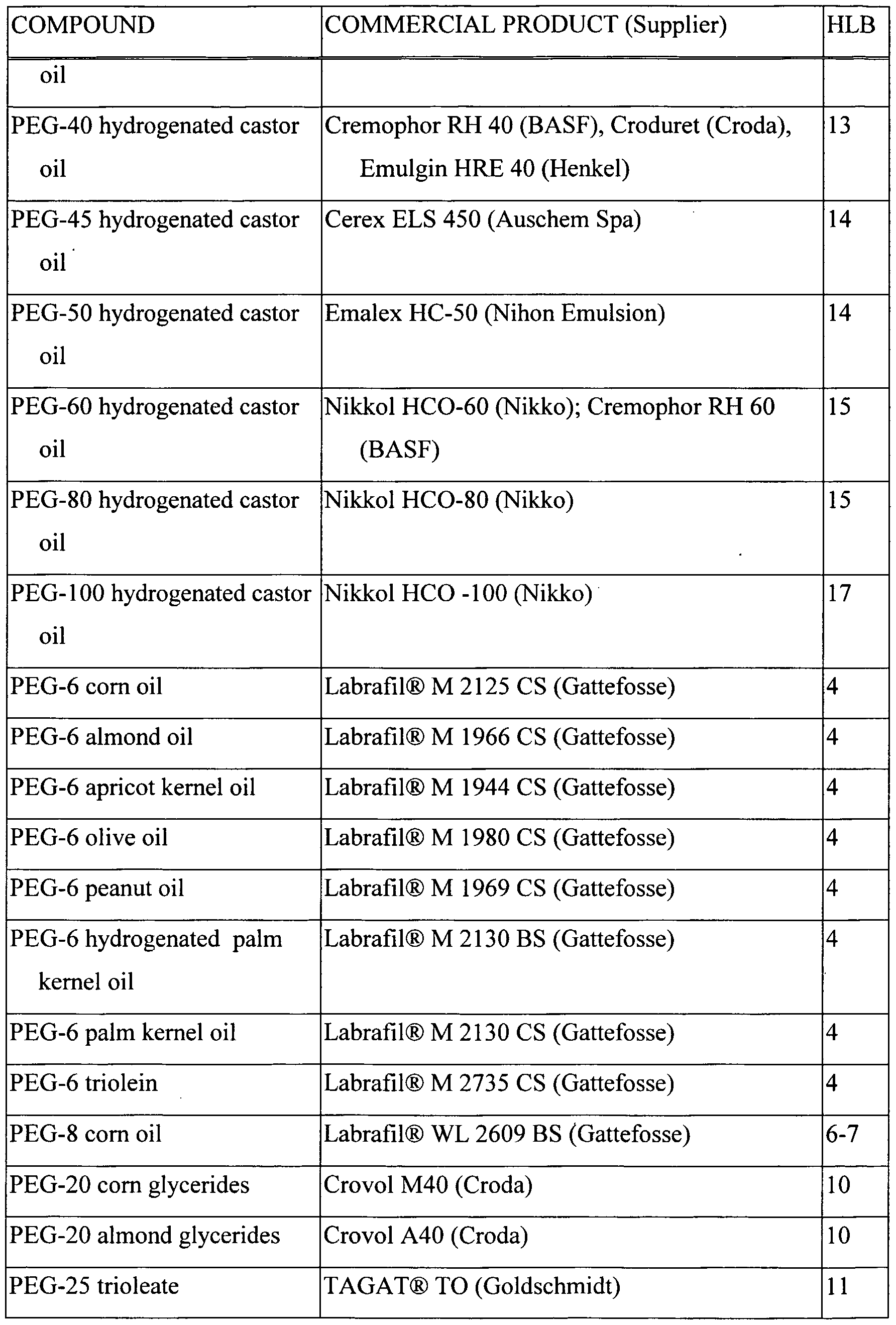
- Table 1 PEG-Fatty Acid Monoester Surfactants
- Polyethylene glycol (PEG) fatty acid diesters are also suitable for use as surfactants in the compositions of the present invention.
- Representative PEG-fatty acid diesters are shown in Table 2.
- mixtures of surfactants are also useful in the present invention, including mixtures of two or more commercial surfactant products.
- PEG-fatty acid esters are marketed commercially as mixtures or mono- and diesters.
- Representative surfactant mixtures are shown in Table 3.
- a large number of surfactants of different degrees of lipophilicity or hydrophilicity can be prepared by reaction of alcohols or polyalcohols with a variety of natural and/or hydrogenated oils.
- the oils used are castor oil or hydrogenated castor oil, or an edible vegetable oil such as corn oil, olive oil, peanut oil, palm kernel oil, apricot kernel oil, or almond oil.
- Preferred alcohols include glycerol, propylene glycol, ethylene glycol, polyethylene glycol, sorbitol, and pentaerythritol.
- Representative surfactants of this class suitable for use in the present invention are shown in Table 5.
- Polyglycerol esters of fatty acids are also suitable surfactants for the present invention.
- suitable polyglyceryl esters are shown in Table 6. Table 6: Polyglycerized Fatty Acids
- esters of propylene glycol and fatty acids are suitable surfactants for use in the present invention.
- Examples of surfactants of this class are given in Table 7.
- mixtures of surfactants are also suitable for use in the present invention.
- mixtures of propylene glycol fatty acid esters and glycerol fatty acid esters are suitable and are commercially available. Examples of these surfactants are shown in Table 8. Table 8: Glycerol/Propylene Glycol Fatty Acid Esters
- a particularly important class of surfactants is the class of mono- and diglycerides. These surfactants are generally lipophilic. Examples of these surfactants are given in Table 9.
- Sterols and derivatives of sterols are suitable surfactants for use in the present invention. These surfactants can be hydrophilic or lipophilic. Examples of surfactants of this class are shown in Table 10. Table 10: Sterol and Sterol Derivative Surfactants
- PEG-sorbitan fatty acid esters are available and are suitable for use as surfactants in the present invention.
- these surfactants are hydrophilic, although several lipophilic surfactants of this class can be used. Examples of these surfactants are shown in Table 11.
- Ethers of polyethylene glycol and alkyl alcohols are suitable surfactants for use in the present invention. Examples of these surfactants are shown in Table 12.
- Esters of sugars are suitable surfactants for use in the present invention. Examples of such surfactants are shown in Table 13.
- hydrophilic PEG-alkyl phenol surfactants are available, and are suitable for use in the present invention. Examples of these surfactants are shown in Table 14. Table 14: Polyethylene Glycol Alkyl Phenol Surfactants
- the POE-POP block copolymers are a unique class of polymeric surfactants.
- the unique structure of the surfactants, with hydrophilic POE and lipophilic POP moieties in well-defined ratios and positions, provides a wide variety of surfactants suitable for use in the present invention.
- These surfactants are available under various trade names, including Synperonic PE series (ICI); Pluronic® series (BASF), Emkalyx, Lutrol (BASF), Supronic, Monolan, Pluracare, and Plurodac.
- the generic term for these polymers is "poloxamer” (CAS 9003-11-6). These polymers have the formula:
- Sorbitan esters of fatty acids are suitable surfactants for use in the present invention. Examples of these surfactants are shown in Table 16.
- Esters of lower alcohols (C 2 to C 4 ) and fatty acids (C 8 to C] 8 ) are suitable surfactants for use in the present invention. Examples of these surfactants are shown in Table 17.
- Ionic surfactants including cationic, anionic and zwitterionic surfactants, are suitable hydrophilic surfactants for use in the present invention.
- Preferred anionic surfactants include fatty acid salts and bile salts.
- Preferred cationic surfactants include carnitines.
- preferred ionic surfactants include sodium oleate, sodium lauryl sulfate, sodium lauryl sarcosinate, sodium dioctyl sulfosuccinate, sodium cholate, sodium taurocholate; lauroyl carnitine; palmitoyl carnitine; and myristoyl carnitine. Examples of such surfactants are shown in Table 18. For simplicity, typical counterions are shown in the entries in the Table.
- any bioacceptable counterion may be used.
- the fatty acids are shown as sodium salts, other cation counterions can also be used, such as alkali metal cations or ammonium.
- these ionic surfactants are generally available as pure compounds, rather than commercial
- Ionizable surfactants when present in their unionized (neutral, non-salt) form, are lipophilic surfactants suitable for use in the compositions of the present invention.
- Particular examples of such surfactants include free fatty acids, particularly C 6 -C 22 fatty acids, and bile acids.
- suitable unionized ionizable surfactants include the free fatty acid and bile acid forms of any of the fatty acid salts and bile salts shown in Table 18. 2.20 Derivatives of Fat-Soluble Vitamins
- Derivatives of oil-soluble vitamins such as vitamins A, D, E, K, etc. are also useful surfactants for the compositions of the present invention.
- An example of such a derivative is tocopheryl PEG- 1000 succinate (TPGS, available from Eastman).
- surfactants are preferred.
- surfactants or mixtures of surfactants that solidify at ambient room temperature are most preferred.
- Preferred non-ionic hydrophilic surfactants include alkylglucosides; alkylmaltosides; alkylthioglucosides; lauryl macrogolglycerides; polyoxyethylene alkyl ethers; polyoxyethylene alkylphenols; polyethylene glycol fatty acids esters; polyethylene glycol glycerol fatty acid esters; polyoxyethylene sorbitan fatty acid esters; polyoxyethylene-polyoxypropylene block copolymers; polyglycerol fatty acid esters; polyoxyethylene glycerides; polyoxyethylene sterols, derivatives, and analogues thereof; polyoxyethylene vegetable oils; polyoxyethylene hydrogenated vegetable oils; reaction mixtures of polyols with fatty acids, glycerides, vegetable oils, hydrogenated vegetable oils, and sterols; sugar esters, sugar ethers; sucroglycerides; polyethoxylated fat-soluble vitamins or derivatives; and mixtures thereof.
- the non-ionic hydrophilic surfactant is selected from the group consisting of polyoxyethylene alkylethers; polyethylene glycol fatty acids esters; polyethylene glycol glycerol fatty acid esters; polyoxyethylene sorbitan fatty acid esters; polyoxyethylene-polyoxypropylene block copolymers; polyglyceryl fatty acid esters; polyoxyethylene glycerides; polyoxyethylene vegetable oils; and polyoxyethylene hydrogenated vegetable oils.
- the glyceride can be a monoglyceride, diglyceride, triglyceride, or a mixture.
- non-ionic hydrophilic surfactants that are reaction mixtures of polyols and fatty acids, glycerides, vegetable oils, hydrogenated vegetable oils or sterols. These reaction mixtures are largely composed of the transesterification products of the reaction, along with often complex mixtures of other reaction products.
- the polyol is preferably glycerol, ethylene glycol, polyethylene glycol, sorbitol, propylene glycol, pentaerythritol, or a saccharide.
- the hydrophilic surfactant can also be, or include as a component, an ionic surfactant.
- Preferred ionic surfactants include alkyl ammonium salts; bile acids and salts, analogues, and derivatives thereof; fusidic acid and derivatives thereof; fatty acid derivatives of amino acids, oligopeptides, and polypeptides; glyceride derivatives of amino acids, oligopeptides, and polypeptides; acyl lactylates; mono-,diacetylated tartaric acid esters of mono-,diglycerides; succinylated monoglycerides; citric acid esters of mono-,diglycerides; alginate salts; propylene glycol alginate; lecithins and hydrogenated lecithins; lysolecithin and hydrogenated lysolecithins; lysophospholipids and derivatives thereof; phospholipids and derivatives thereof; salts of alkylsul
- More preferable ionic surfactants include bile acids and salts, analogues, and derivatives thereof; lecithins, lysolecithin, phospholipids, lysophospholipids and derivatives thereof; salts of alkylsulfates; salts of fatty acids; sodium docusate; acyl lactylates; mono-,diacetylated tartaric acid esters of mono-,diglycerides; succinylated monoglycerides; citric acid esters of mono-,diglycerides; carnitines; and mixtures thereof.
- preferred ionic surfactants are lecithin, lysolecithin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, phosphatidic acid, phosphatidylserine, lysophosphatidylcholine, lysophosphatidylethanolamine, lysophosphatidylglycerol, lysophosphatidic acid, lysophosphatidylserine, PEG- phosphatidylethanolamine, PVP-phosphatidylethanolamine, lactylic esters of fatty acids, stearoyl-2-lactylate, stearoyl lactylate, succinylated monoglycerides, mono/diacetylated tartaric acid esters of mono/diglycerides, citric acid esters of mono/diglycerides, cholate, taurocholate, glycocholate, deoxycholate, taurodeoxycholate
- Particularly preferred ionic surfactants are lecithin, lysolecithin, phosphatidylcholine, phosphatidylethanolamine, phosphatidylglycerol, lysophosphatidylcholine, PEG-phosphatidylethanolamine, lactylic esters of fatty acids, stearoyl-2-lactylate, stearoyl lactylate, succinylated monoglycerides, mono/diacetylated tartaric acid esters of mono/diglycerides, citric acid esters of mono/diglycerides, cholate, taurocholate, glycocholate, deoxycholate, taurodeoxycholate, glycodeoxycholate, cholylsarcosine, caproate, caprylate, caprate, laurate, oleate, lauryl sulfate, docusate, and salts and mixtures thereof, with the most preferred ionic surfactants being lecithin, lac
- Preferred lipophilic surfactants are alcohols; polyoxyethylene alkylethers; fatty acids; glycerol fatty acid esters; acetylated glycerol fatty acid esters; lower alcohol fatty acids esters; polyethylene glycol fatty acids esters; polyethylene glycol glycerol fatty acid esters; polypropylene glycol fatty acid esters; polyoxyethylene glycerides; lactic acid derivatives of mono/diglycerides; propylene glycol diglycerides; sorbitan fatty acid esters; polyoxyethylene sorbitan fatty acid esters; polyoxyethylene-polyoxypropylene block copolymers; transesterified vegetable oils; sterols; sterol derivatives; sugar esters; sugar ethers; sucroglycerides; polyoxyethylene vegetable oils; and polyoxyethylene hydrogenated vegetable oils.
- lipophilic surfactants can be reaction mixtures of polyols and fatty acids, glycerides, vegetable oils, hydrogenated vegetable oils, and sterols.
- the lipophilic surfactant is selected from the group consisting of fatty acids; lower alcohol fatty acid esters; polyethylene glycol glycerol fatty acid esters; polypropylene glycol fatty acid esters; polyoxyethylene glycerides; glycerol fatty acid esters; acetylated glycerol fatty acid esters; lactic acid derivatives of mono/diglycerides; sorbitan fatty acid esters; polyoxyethylene sorbitan fatty acid esters; polyoxyethylene- polyoxypropylene block copolymers; polyoxyethylene vegetable oils; polyoxyethylene hydrogenated vegetable oils; and reaction mixtures of polyols and fatty acids, glycerides, vegetable oils, hydrogenated vegetable oils, and sterols.
- lower alcohol fatty acids esters More preferred are lower alcohol fatty acids esters; polypropylene glycol fatty acid esters; propylene glycol fatty acid esters; glycerol fatty acid esters; acetylated glycerol fatty acid esters; lactic acid derivatives of mono/diglycerides; sorbitan fatty acid esters; polyoxyethylene vegetable oils; and mixtures thereof, with glycerol fatty acid esters and acetylated glycerol fatty acid esters being most preferred.
- the esters are preferably mono- or diglycerides, or mixtures of mono- and diglycerides, where the fatty acid moiety is a C 6 to C 22 fatty acid.
- lipophilic surfactants which are the reaction mixture of polyols and fatty acids, glycerides, vegetable oils, hydrogenated vegetable oils, and sterols.
- Preferred polyols are polyethylene glycol, sorbitol, propylene glycol, and pentaerythritol.
- the lipophilic component can be a lipophilic surfactant or a triglyceride.
- Preferred triglycerides are those which solidify at ambient room temperature, with or without addition of appropriate additives, or those which in combination with particular surfactants and/or active ingredients solidify at room temperature. Examples of triglycerides suitable for use in the present invention are shown in Table 19. In general, these triglycerides are readily available from commercial sources. For several triglycerides, representative commercial products and/or commercial suppliers are listed. Table 19: Triglycerides
- Fractionated triglycerides modified triglycerides, synthetic triglycerides, and mixtures of triglycerides are also within the scope of the invention.
- Preferred triglycerides include vegetable oils, fish oils, animal fats, hydrogenated vegetable oils, partially hydrogenated vegetable oils, medium and long-chain triglycerides, and structured triglycerides. It should be appreciated that several commercial surfactant compositions contain small to moderate amounts of triglycerides, typically as a result of incomplete reaction of a triglyceride starting material in, for example, a transesterification reaction. Such commercial surfactant compositions, while nominally referred to as "surfactants”, may be suitable to provide all or part of the triglyceride component for the compositions of the present invention.
- compositions containing triglycerides include some members of the surfactant families Gelucires (Gattefosse), Maisines (Gattefosse), and Imwitors (H ⁇ ls). Specific examples of these compositions are: Gelucire 44/14 (saturated polyglycolized glycerides)
- Gelucire 33/01 (semi-synthetic triglycerides of C 8 -C ⁇ 8 saturated fatty acids)
- Gelucire 39/01 (semi-synthetic glycerides) other Gelucires, such as 37/06, 43/01 , 35/10, 37/02, 46/07, 48/09, 50/02, 62/05, etc.
- Imwitor 742 (caprylic/capric glycerides)
- compositions having significant triglyceride content are known to those skilled in the art. It should be appreciated that such compositions, which contain triglycerides as well as surfactants, may be suitable to provide all or part of the triglyceride component of the compositions of the present invention, as well as all or part of the surfactant component.
- the substrate of the compositions of the present invention can be a powder or a multiparticulate, such as a granule, a pellet, a bead, a spherule, a beadlet, a microcapsule, a millisphere, a nanocapsule, a nanosphere, a microsphere, a platelet, a minitablet, a tablet or a capsule.
- a powder constitutes a finely divided (milled, micronized, nanosized, precipitated) form of an active ingredient or additive molecular aggregates or a compound aggregate of multiple components or a physical mixture of aggregates of an active ingredient and/or additives.
- Such substrates can be formed of various materials known in the art, such as, for example:
- Sugars such as lactose, sucrose or dextrose; Polysaccharides, such as maltodextrin or dextrates;
- Cellulosics such as microcrystalline cellulose or microcrystalline cellulose/sodium carboxymethyl cellulose; Inorganics, such as dicalcium phosphate, hydroxyapitite, tricalcium phosphate, talc, or titania; and
- Polyols such as mannitol, xylitol, sorbitol or cyclodextrin.
- the substrate can also be formed of any of the active ingredients, surfactants, triglycerides or additives described herein.
- the substrate is a solid form of an additive, an active ingredient, a surfactant, or a triglyceride; a complex of an additive, surfactant or triglyceride and an active ingredient; a coprecipitate of an additive, surfactant or triglyceride and an active ingredient, or a mixture thereof.
- the substrate need not be a solid material, although o often it will be a solid.
- the encapsulation coat on the substrate may act as a solid "shell" surrounding and encapsulating a liquid or semi-liquid substrate material.
- Such substrates are also within the scope of the present invention, as it is ultimately the carrier, of which the substrate is a part, which must be a solid.
- the solid pharmaceutical compositions of the present invention can optionally include one or more additives, sometimes referred to as excipients.
- the additives can be contained in an encapsulation coat in compositions which include an encapsulation coat, or can be part of the solid carrier, such as coated to an encapsulation coat, or contained within the components forming the solid carrier.
- the additives can be contained in the pharmaceutical composition but not part of the solid carrier itself. Specific, non-limiting examples of additives are described below.
- Suitable additives are those commonly utilized to facilitate the processes involving the preparation of the solid carrier, the encapsulation coating, or the pharmaceutical dosage form. These processes include agglomeration, air suspension chilling, air suspension drying, balling, coacervation, comminution, compression, pelletization, cryopelletization, extrusion, granulation, homogenization, inclusion complexation, lyophilization, nanoencapsulation, melting, mixing, molding, pan coating, solvent dehydration, sonication, spheronization, spray chilling, spray congealing, spray drying, or other processes known in the art.
- the additive can also be pre-coated or encapsulated. Appropriate coatings are well known in the art, and are further described in the sections below. Based on the functionality of the additives, examples of the additives are as follows:
- the pharmaceutical compositions of the present invention can optionally include one or more solubilizers, i.e., additives to increase the solubility of the pharmaceutical active ingredient or other composition components in the solid carrier.
- Suitable solubilizers for use in the compositions of the present invention include: alcohols and polyols, such as ethanol, isopropanol, butanol, benzyl alcohol, ethylene glycol, propylene glycol, butanediols and isomers thereof, glycerol, pentaerythritol, sorbitol, mannitol, transcutol, dimethyl isosorbide, polyethylene glycol, polypropylene glycol, polyvinylalcohol, hydroxypropyl methylcellulose and other cellulose derivatives, cyclodextrins and cyclodextrin derivatives; ethers of polyethylene glycols having an average molecular weight of about 200 to about 6000, such as tetra
- Preferred solubilizers include triacetin, triethylcitrate, ethyl oleate, ethyl caprylate, dimethylacetamide, N-methylpyrrolidone, N-hydroxyethylpyrrolidone, polyvinylpyrroli-done, hydroxypropyl methylcellulose, hydroxypropyl cyclodextrins, ethanol, polyethylene glycol 200-600, glycofurol, transcutol, propylene glycol, and dimethyl isosorbide.
- Particularly preferred solubilizers include sorbitol, glycerol, triacetin, ethyl alcohol, PEG-400, glycofurol and propylene glycol.
- the amount of solubilizer that can be included in compositions of the present invention is not particularly limited.
- the amount of a given solubilizer is limited to a bioacceptable amount, which is readily determined by one of skill in the art.
- the compositions can include an enzyme inhibiting agent.
- Enzyme inhibiting agents are shown for example, in Bernskop-Schnurch, A., "The use of inhibitory agents to overcome enzymatic barrier to perorally administered therapeutic peptides and proteins", J. Controlled Release 52, 1-16 (1998), the disclosure of which is inco ⁇ orated herein by reference.
- inhibitory agents can be divided into the following classes: Inhibitors that are not based on amino acids, such as P-aminobenzamidine, FK- 448, camostat mesylate, sodium glycocholate;
- Amino acids and modified amino acids such as aminoboronic acid derivatives and n-acetylcysteine
- Peptides and modified peptides such as bacitracin, phosphinic acid dipeptide derivatives, pepstatin, antipain, leupeptin, chymostatin, elastatin, bestatin, hosphoramindon, puromycin, cytochalasin potatocarboxy peptidase inhibitor, and amastatin;
- Polypeptide protese inhibitors such as aprotinin (bovine pancreatic trypsin inhibitor), Bowman-Birk inhibitor and soybean trypsin inhibitor, chicken egg white trypsin inhibitor, chicken ovoinhibitor, and human pancreatic trypsin inhibitor.
- Complexing agents such as EDTA, EGTA, 1,10- phenanthroline and hydroxychinoline; and
- Mucoadhesive polymers and polymer-inhibitor conjugates such as polyacrylate derivatives, chitosan, cellulosics, chitosan-EDTA, chitosan-EDTA-antipain, polyacrylic acid-bacitracin, carboxymethyl cellulose-pepstatin, polyacrylic acid-B woman-Birk inhibitor.
- the choice and levels of the enzyme inhibitor are based on toxicity, specificity of the proteases and the potency of the inhibition.
- the inhibitor can be suspended or solubilized in the composition preconcentrate, or added to the aqueous diluent or as a beverage.
- an inhibitor can function solely or in combination as: a competitive inhibitor, by binding at the substrate binding site of the enzyme, thereby preventing the access to the substrate; examples of inhibitors believed to operate by this mechanism are antipain, elastatinal and the Bowman Birk inhibitor; a non-competitive inhibitor which can be simultaneously bound to the enzyme site along with the substrate, as their binding sites are not identical; and/or a complexing agent due to loss in enzymatic activity caused by deprivation of essential metal ions out of the enzyme structure.
- additives conventionally used in pharmaceutical compositions can be included, and these additives are well known in the art.
- additives include: anti-adherents (anti-sticking agents, glidants, flow promoters, lubricants) such as talc, magnesium stearate, fumed silica (Carbosil, Aerosil), micronized silica (Syloid No.
- FP 244, Grace U.S.A. polyethylene glycols, surfactants, waxes, stearic acid, stearic acid salts, stearic acid derivatives, starch, hydrogenated vegetable oils, sodium benzoate, sodium acetate, leucine, PEG-4000 and magnesium lauryl sulfate; anticoagulants, such as acetylated monoglycerides; antifoaming agents, such as long-chain alcohols and silicone derivatives; antioxidants.
- binders such as BHT, BHA, gallic acid, propyl gallate, ascorbic acid, ascorbyl palmitate, 4-hydroxymethyl-2,6-di-tert-butyl phenol, and tocopherol; binders (adhesives), i.e., agents that impart cohesive properties to powdered materials through particle-particle bonding, such as matrix binders (dry starch, dry sugars), film binders (PVP, starch paste, celluloses, bentonite, sucrose), and chemical binders (polymeric cellulose derivatives, such as carboxy methyl cellulose, HPC and HPMC; sugar syrups; corn syrup; water soluble polysaccharides such as acacia, tragacanth, guar and alginates; gelatin; gelatin hydrolysate; agar; sucrose; dextrose; and non-cellulosic binders, such as PVP, PEG, vinyl pyrrolidone copolymers, pregelatinized
- the acid is a pharmaceutically acceptable acid, such as hydrochloric acid, hydrobromic acid, hydriodic acid, sulfuric acid, nitric acid, boric acid, phosphoric acid, acetic acid, acrylic acid, adipic acid, alginic acid, alkanesulfonic acid, amino acids, ascorbic acid, benzoic acid, boric acid, butyric acid, carbonic acid, citric acid, fatty acids, formic acid, fumaric acid, gluconic acid, hydroquinosulfonic acid, isoascorbic acid, lactic acid, maleic acid, methanesulfonic acid, oxalic acid, para- bromophenylsulfonic acid, propionic acid, p-toluenesulfonic acid, salicylic acid, stearic acid, succinic acid, tannic acid, tartaric acid, thioglycolic acid, toluenesulfonic acid and uric acid, and where
- acetylated monoglycerides glycerin, triacetin, propylene glycol, phthalate esters (e.g., diethyl phthalate, dibutyl phthalate), castor oil, sorbitol and dibutyl seccate; preservatives, such as ascorbic acid, boric acid, sorbic acid, benzoic acid, and salts thereof, parabens, phenols, benzyl alcohol, and quaternary ammonium compounds; solvents, such as alcohols, ketones, esters, chlorinated hydrocarbons and water; sweeteners, including natural sweeteners such as maltose, sucrose, glucose, sorbitol, glycerin and dextrins, and artificial sweeteners, such as aspartame, saccharine and saccharine salts; and thickeners (vis
- Additives can also be materials such as proteins (e.g., collagen, gelatin, Zein, gluten, mussel protein, lipoprotein); carbohydrates (e.g., alginates, carrageenan, cellulose derivatives, pectin, starch, chitosan); gums (e.g., xanthan gum, gum arabic); spermaceti; natural or synthetic waxes; camuaba wax; fatty acids (e.g., stearic acid, hydroxystearic acid); fatty alcohols; sugars; shellacs, such as those based on sugars (e.g., lactose, sucrose, dextrose) or starches; polysaccharide-based shellacs (e.g., maltodextrin and maltodextrin derivatives, dextrates, cyclodextrin and cyclodextrin derivatives); cellulosic-based shellacs (e.g., microcrystalline cellulose, sodium carboxy
- compositions of the present invention can be processed by agglomeration, air suspension chilling, air suspension drying, balling, coacervation, coating, comminution, compression, cryopelletization, encapsulation, extrusion, wet granulation, dry granulation, homogenization, inclusion complexation, lyophilization, melting, microencapsulation, mixing, molding, pan coating, solvent dehydration, sonication, spheronization, spray chilling, spray congealing, spray drying, or other processes known in the art.
- compositions can be provided in the form of a minicapsule, a capsule, a tablet, an implant, a troche, a lozenge (minitablet), a temporary or permanent suspension, an ovule, a suppository, a wafer, a chewable tablet, a quick or fast dissolving tablet, an effervescent tablet, a buccal or sublingual solid, a granule, a film, a sprinkle, a pellet, a bead, a pill, a powder, a triturate, a platelet, a strip or a sachet.
- compositions can also be administered as a "dry syrup", where the finished dosage form is placed directly on the tongue and swallowed or followed with a drink or beverage. These forms are well known in the art and are packaged appropriately.
- the compositions can be formulated for oral, nasal, buccal, ocular, urethral, transmucosal, vaginal, topical or rectal delivery, although oral delivery is presently preferred.
- the pharmaceutical composition and/or the solid carrier particles can be coated with one or more enteric coatings, seal coatings, film coatings, barrier coatings, compress coatings, fast disintegrating coatings, or enzyme degradable coatings. Multiple coatings can be applied for desired performance.
- the dosage form can be designed for immediate release, pulsatile release, controlled release, extended release, delayed release, targeted release, synchronized release, or targeted delayed release.
- solid carriers can be made of various component types and levels or thicknesses of coats, with or without an active ingredient. Such diverse solid carriers can be blended in a dosage form to achieve a desired performance. The definitions of these terms are known to those skilled in the art.
- the dosage form release profile can be effected by a polymeric matrix composition, a coated matrix composition, a multiparticulate composition, a coated multiparticulate composition, an ion-exchange resin-based composition, an osmosis-based composition, or a biodegradable polymeric composition.
- a polymeric matrix composition effected by a polymeric matrix composition, a coated matrix composition, a multiparticulate composition, a coated multiparticulate composition, an ion-exchange resin-based composition, an osmosis-based composition, or a biodegradable polymeric composition.
- the release may be effected through favorable diffusion, dissolution, erosion, ion-exchange, osmosis or combinations thereof.
- the capsule When formulated as a capsule, the capsule can be a hard or soft gelatin capsule, a starch capsule, or a cellulosic capsule. Although not limited to capsules, such dosage forms can further be coated with, for example, a seal coating, an enteric coating, an extended release coating, or a targeted delayed release coating. These various coatings are known in the art, but for clarity, the following brief descriptions are provided:
- Seal coating, or coating with isolation layers Thin layers of up to 20 microns in thickness can be applied for variety of reasons, including for particle porosity reduction, to reduce dust, for chemical protection, to mask taste, to reduce odor, to minimize gastrointestinal irritation, etc.
- the isolating effect is proportional to the thickness of the coating.
- Water soluble cellulose ethers are preferred for this application.
- HPMC and ethyl cellulose in combination, or Eudragit El 00 may be particularly suitable for taste masking applications.
- Traditional enteric coating materials listed elsewhere can also be applied to form an isolating layer.
- Extended release coating means a coating designed to effect delivery over an extended period of time.
- the extended release coating is a pH-independent coating formed of, for example, ethyl cellulose, hydroxypropyl cellulose, methylcellulose, hydroxymethyl cellulose, hydroxyethyl cellulose, acrylic esters, or sodium carboxymethyl cellulose.
- Various extended release dosage forms can be readily designed by one skilled in art to achieve delivery to both the small and large intestines, to only the small intestine, or to only the large intestine, depending upon the choice of coating materials and/or coating thickness.
- Enteric coating relates to a mixture of pharmaceutically acceptable excipients which is applied to, combined with, mixed with or otherwise added to the carrier or composition.
- the coating may be applied to a compressed or molded or extruded tablet, a gelatin capsule, and/or pellets, beads, granules or particles of the carrier or composition.
- the coating may be applied through an aqueous dispersion or after dissolving in appropriate solvent. Additional additives and their levels, and selection of a primary coating material or materials will depend on the following properties:
- Dosage forms of the compositions of the present invention can also be formulated as enteric coated delayed release oral dosage forms, i.e., as an oral dosage form of a pharmaceutical composition as described herein which utilizes an enteric coating to effect release in the lower gastrointestinal tract.
- the enteric coated dosage form may be a compressed or molded or extruded tablet/mold (coated or uncoated) containing granules, pellets, beads or particles of the active ingredient and/or other composition components, which are themselves coated or uncoated.
- the enteric coated oral dosage form may also be a capsule (coated or uncoated) containing pellets, beads or granules of the solid carrier or the composition, which are themselves coated or uncoated.
- delayed release refers to the delivery so that the release can be accomplished at some generally predictable location in the lower intestinal tract more distal to that which would have been accomplished if there had been no delayed release alterations.
- the preferred method for delay of release is coating. Any coatings should be applied to a sufficient thickness such that the entire coating does not dissolve in the gastrointestinal fluids at pH below about 5, but does dissolve at pH about 5 and above. It is expected that any anionic polymer exhibiting a pH-dependent solubility profile can be used as an enteric coating in the practice of the present invention to achieve delivery to the lower gastrointestinal tract.
- the preferred polymers for use in the present invention are anionic carboxylic polymers. The more preferred polymers and compatible mixtures thereof, and some of their properties, include, but are not limited to:
- Shellac also called purified lac, a refined product obtained from the resinous secretion of an insect. This coating dissolves in media of pH >7.
- Acrylic polymers (preferred).
- the performance of acrylic polymers can vary based on the degree and type of substitution.
- suitable acrylic polymers include methacrylic acid copolymers and ammonio methacrylate copolymers.
- the Eudragit series E, L, S, RL, RS and NE (Rohm Pharma) are available as solubilized in organic solvent, aqueous dispersion, or dry powders.
- the Eudragit series RL ,NE, and RS are insoluble in the gastrointestinal tract but are permeable and are used primarily for extended release.
- the Eudragit series E dissolve in the stomach.
- the Eudragit series L, L-30D and S are insoluble in stomach and dissolve in the intestine.
- Cellulose Derivatives also preferred.
- suitable cellulose derivatives are: ethyl cellulose; reaction mixtures of partial acetate esters of cellulose with phthalic anhydride. The performance can vary based on the degree and type of substitution.
- Cellulose acetate phthalate (CAP) dissolves in pH > 6. Aquateric
- FMC is an aqueous based system and is a spray dried CAP psuedolatex with particles ⁇ l ⁇ m.
- Other components in Aquateric can include pluronics, Tweens, and acetylated monoglycerides; cellulose acetate trimellitate (Eastman); methylcellulose (Pharmacoat, Methocel); hydroxypropyl methyl cellulose phthalate (HPMCP).
- HP-50, HP-55, HP-55S, HP-55F grades are suitable; hydroxypropyl methyl cellulose succinate (HPMCS; AQOAT (Shin Etsu)).
- HPMCS hydroxypropyl methyl cellulose succinate
- AQOAT Shin Etsu
- the performance can vary based on the degree and type of substitution. Suitable grades include AS-LG (LF), which dissolves at pH 5, AS-MG (MF), which dissolves at pH 5.5, and AS-HG (HF), which dissolves at higher pH.
- AS-LG LF
- AS-MG MF
- AS-HG AS-HG
- PVAP Poly Vinyl Acetate Phthalate
- the coating can, and usually does, contain a plasticizer and possibly other coating excipients such as colorants, talc, and/or magnesium stearate, which are well known in the art.
- Suitable plasticizers include: triethyl citrate (Citroflex 2), triacetin (glyceryl triacetate), acetyl triethyl citrate (Citroflec A2), Carbowax 400 (polyethylene glycol 400), diethyl phthalate, tributyl citrate, acetylated monoglycerides, glycerol, fatty acid esters, propylene glycol, and dibutyl phthalate.
- anionic carboxylic acrylic polymers usually will contain 10-25% by weight of a plasticizer, especially dibutyl phthalate, polyethylene glycol, triethyl citrate and triacetin.
- a plasticizer especially dibutyl phthalate, polyethylene glycol, triethyl citrate and triacetin.
- Conventional coating techniques such as spray or pan coating are employed to apply coatings. The coating thickness must be sufficient to ensure that the oral dosage form remains intact until the desired site of topical delivery in the lower intestinal tract is reached.
- Colorants, detackifiers, surfactants, antifoaming agents, lubricants, stabilizers such as hydroxy propyl cellulose, acid/base may be added to the coatings besides plasticizers to solubilize or disperse the coating material, and to improve coating performance and the coated product.
- a particularly suitable methacrylic copolymer is Eudragit L.RTM, particularly L- 30D.RTM and Eudragit 100-55.RTM, manufactured by Rohm Pharma, Germany.
- Eudragit L-30 D.RTM the ratio of free carboxyl groups to ester groups is approximately 1 :1.
- the copolymer is known to be insoluble in gastrointestinal fluids having pH below 5.5, generally 1.5-5.5, i.e., the pH generally present in the fluid of the upper gastrointestinal tract, but readily soluble or partially soluble at pH above 5.5, i.e., the pH generally present in the fluid of lower gastrointestinal tract.
- Eudragit S.RTM Another methacrylic acid polymer which is suitable for use in coating the composition or solid carrier which can be employed in the compositions and methods described herein, either alone or in combination with other coatings, is Eudragit S.RTM, manufactured by Rohm Pharma, Germany. Eudragit S.RTM. differs from Eudragit L-30- D.RTM only insofar as the ratio of free carboxyl groups to ester groups is approximately 1 :2. Eudragit S.RTM is insoluble at pH below 5.5, but unlike Eudragit L-30-D.RTM, is poorly soluble in gastrointestinal fluids having pH of 5.5-7.0, such as is present in the small intestine media.
- This copolymer is soluble at pH 7.0 and above, i.e., the pH generally found in the colon.
- Eudragit S.RTM can be used alone as a coating to provide delivery of beginning at the large intestine via a delayed release mechanism.
- Eudragit S.RTM being poorly soluble in intestinal fluids below pH 7, can be used in combination with Eudragit L-30-D.RTM, soluble in intestinal fluids above pH 5.5, in order to effect a delayed release composition.
- Preferred materials include shellac, acrylic polymers, cellulosic derivatives, polyvinyl acetate phthalate, and mixtures thereof. More preferred materials include Eudragit series E, L, S, RL, RS, NE, L.RTM, L300.RTM, S.RTM, 100-55RTM, cellulose acetate phthalate, Aquateric, cellulose acetate trimellitate, ethyl cellulose, hydroxypropyl methyl cellulose phthalate, hydroxypropyl methyl cellulose succinate, poly vinyl acetate phthalate, and Cotteric.
- Most preferred materials include Eudragit series L.RTM, L300.RTM, S.RTM, L100-55RTM, cellulose acetate phthalate, Aquateric, ethyl cellulose, hydroxypropyl methyl cellulose phthalate, hydroxypropyl methyl cellulose succinate, poly vinyl acetate phthalate, and Cotteric. Extended release, and targeted delayed release coatings for dosage forms of the compositions of the present invention are described more completely in U.S. Patent Nos. 5,622,721 and 5,686,105, the disclosures of which are inco ⁇ orated herein by reference in their entirety.
- Immediate release coating of solid carriers is commonly used to improve product elegance as well as for a moisture barrier, and taste and odor masking. Rapid breakdown of the film in gastric media is important, leading to effective disintegration and dissolution.
- Eudragit RD100 Rostm
- It is a combination of a water insoluble cationic methacrylate copolymer with a water soluble cellulose ether. In powder form, it is readily dispensible into an easily sprayable suspension that dries to leave a smooth film. Such films rapidly disintegrate in aqueous media at a rate that is independent of pH and film thickness.
- compositions of the present invention can be prepared by a variety of processes to apply an encapsulation coat onto a substrate or to forma substrate-free solid carrier such as a multiparticulate or a powder.
- the commonly utilized coating and pelletization processes include balling, spheronization, extrusion, spray congealing, spray drying, pan coating, fluidized bed coating, melt extrusion, crystallization, cryopelletization, nanoencapsulation, coacervation, etc. It is also clear to one skilled in the art that appropriate additives can also be introduced to the composition or during the processes to facilitate the preparation of the solid carrier or the dosage forms, depending on the need of the individual process.
- a coating process frequently involves spraying a coating solution onto a substrate.
- the coating solution can be a molten solution of the encapsulation coat composition free of a dispersing medium.
- the coating solution can also be prepared by solubilizing or suspending the composition of the encapsulation coat in an aqueous medium, an organic solvent, a supercritical fluid, or a mixture thereof.
- the residual dispersing medium can be further removed to a desirable level utilizing appropriate drying processes, such as vacuum evaporation, heating, freeze drying, etc.
- a pelletization process typically involves preparing a molten solution of the composition of the solid carrier or a dispersion of the composition of the solid carrier solubilized or suspended in an aqueous medium, an organic solvent, a supercritical fluid, or a mixture thereof. Such solution or dispersion is then passed through a certain opening to achieve the desired shape, size, and other properties. Similarly, appropriate drying processes can be adopted to control the level of the residual dispersing medium, if necessary.
- the processes described above, the combination of the processes, or the modification of the processes are well know in the art. Some of the processes are briefly described herein for reference.
- pellets are very much like granules and bead; the techniques for producing pellets can also produce granules, beads, etc.
- Pellets, granules or beads are formed with the aid of a pelletizer, spheronizer or extruder.
- the pelletizer, spheronizer or extruder is able to form approximately spherical bodies from a mass of finely divided particles continuously, by a rolling or tumbling action on a flat or curved surface with the addition of a liquid.
- Pelletizers can be classified based on the angle of their axis as horizontal dram or inclined dish pelletizers.
- Rotary fluidized granulators can also be used for pelletization.
- a standard fluidized drier bowl can be replaced with a rotating plate as an air distributor.
- a binder liquid is sprayed from via one or two binary nozzles located axially to the rotational movement of the powder bed. This operation results in rounding of the granules to approximately spherical pellets.
- Such balling or agitation techniques can be influenced by operating conditions, such as bridging/binding liquid requirements, residence time of the material in the pelletizer, speed and angle of inclination of the pelletizer, amount of material fed to the pelletizer, choice and levels of binder, etc.
- the components of the invention can also be self binding. Liquid components can be pelletized with an the aid of suitable solidifying, binding or thickening agents.
- binder for a given application is readily determined by one skilled in the art.
- the binder must be capable of wetting the surfaces of the particle being pelletized or granulated. Binders must have sufficient wet strength to allow agglomerates to be handled, and sufficient dry strength to make them suitable for their intended pu ⁇ oses. Each process, however, makes use of a different system of forces and may require a different agglomerate strength.
- the final selection of the binder should be made on the basis of the type of equipment that is used.
- the size and size distribution of pellets, bulk density, strength and flow properties also affect the performance of the pellets, and these properties can be adjusted by one skilled in the art by the inclusion of additives, choice of equipment, and processing conditions.
- Extrusion Extmsion is a well-known method of applying pressure to a composition (damp or melted) until it flows through an orifice or a defined opening.
- the extrudable length varies with the physical characteristics of the material to be extruded, the method of extmsion, and the process of manipulation of the particles after extmsion.
- Various types of extmsion devices can be employed, such as screw, sieve and basket, roll, and ram extruders.
- Encapsulation by Extmsion In this method, the lipid composition in the form of an emulsion is added to a low moisture melt of low maltodextrin, or sugar, or modified edible starch, mixed and extmded into a cold bath. The solidified composition can be further ground down. Optionally, centrifugal extmsion can be utilized for efficiency.
- Melt Extmsion Components of the invention can be melted and extruded with a continuous, solvent free extmsion process, with or without inclusion of additives. Such a process is well-established and well-known to skilled practitioners in the art. Spheronization
- Spheronization is the process of converting material into spheres, the shape with the lowest surface area to volume ratio. Spheronization typically begins with damp extmded particles. The extmded particles are broken into uniform lengths instantaneously and gradually transformed into spherical shapes. In addition, powdered raw materials, which require addition of either liquid or material from a mixer, can be processed in an air-assisted spheronizer.
- Spray congealing is method that is generally used in changing the stmcture of the materials, to obtain free flowing powders from liquids and to provide pellets ranging in size from about 0.25 to 2.0 mm.
- Spray congealing is process in which a substance of interest is allowed to melt, disperse, or dissolve in a hot melt of other additives, and is then sprayed into an air chamber wherein the temperature is below the melting point of the formulation components, to provide spherical congealed pellets.
- the air removes the latent heat of fusion.
- the temperature of the cooled air used depends on the freezing point of the product.
- the particles are held together by solid bonds formed from the congealed melts.
- the particles Due to the absence of solvent evaporation in most spray congealing processes, the particles are generally non porous and strong, and remain intact upon agitation.
- the characteristics of the final congealed product depend in part on the properties of the additives used.
- the rate of feeding and inlet/outlet temperatures are adjusted to ensure congealing of the atomized liquid droplet.
- the feed should have adequate viscosity to ensure homogeneity.
- the conversion of molten feed into powder is a single, continuous step. Proper atomization and a controlled cooling rate are critical to obtain high surface area, uniform and homogeneous congealed pellets. Adjustment of these parameters is readily achieved by one skilled in the art.
- the spray congealing method is particularly suitable for heat labile substances, since ambient temperature is used to dry, and for moisture sensitive substances, since non-aqueous compositions can be utilized.
- Spray congealing is similar to spray drying, except that no solvent is utilized. Spray congealing is a uniform and rapid process, and is completed before the product comes in contact with any equipment surface. Most additives that are solid at room temperature and melt without decomposition are suitable for this method.
- spray dryers operating with cool inlet air have been used for spray congealing.
- atomization of molten mass can be employed, such as pressure, or pneumatic or centrifugal atomization.
- pressure or pneumatic or centrifugal atomization.
- formulation aspects such as matrix materials, viscosity, and processing factors, such as temperature, atomization and cooling rate affect the quality (mo ⁇ hology, particle size distribution, polymophism and dissolution characteristics) of spray congealed pellets.
- the spray congealed particles may be used in tablet granulation form, encapsulation form, or can be inco ⁇ orated into a liquid suspension form.
- Solvent Dehydration (Spray Drying)
- the oily material is commonly mixed with a polymeric material, such as gelatin, vegetable gum, modified starch, dextrin, or other appropriate additives.
- An emulsifier is added, if needed, to form an oil-in- water emulsion.
- the emulsion is atomized into a column of heated air in a drying chamber, resulting in rapid evaporation of water.
- the emulsion is atomized directly into a polar solvent, such as isopropanol, ethanol, glycerol or polyglycols, to dehydrate the aerosolized particle.
- compositions containing lipophilic actives or additives that result in lipophilic cores are particularly suitable for compositions containing lipophilic actives or additives that result in lipophilic cores.
- Spray drying/solvent dehydration can also be applied to hydrophilic active ingredients or additives to form an oil in water emulsion which is spray dried. This results in a homogenous solid composition.
- water or organic solvent based formulations can be spray dried by using inert process gas, such as nitrogen, argon and the like. Crystallization
- Components of the present invention can be dissolved in appropriate solvents and subjected to spherical crystallization techniques well-known in the art.
- Nanoencapsulation involves solubilizing an aqueous solution of an active ingredient and other components in a weakly polar vehicle. Micelles are formed with the active in an organic outer phase. Then, an amphiphilic monomer is added to the lipophilic external phase. The mixed micelles thus formed are then polymerized with the aid of a suitable procedure, such as UV or gamma radiation, heat, or chemical agents, the hardened solidified micelles are made to undergo phase exchange by replacing an outer lipophilic vehicle by water. By selecting appropriate monomers, networking agents and auxiliary materials, nanoncapsules as small as 80 to 250 nm can be prepared.
- Components of the present invention can be dispersed in a supercritical fluid and crystallized as needed.
- Current techniques involving supercritical fluids include precipitation by rapid expansion of supercritical solutions, gas anti-solvent processes, and precipitation from gas saturated solutions.
- Coacervation is a transfer of macromolecules with film properties from a solvated state in a coacervation phase into a phase in which there is a film around each particle.
- the coacervation method involves dispersing the composition in a dispersion of a polymeric colloid, such as gelatin alginate, and shock treating the mixture with temperature or pH, etc., to generate a two-phase system.
- the desired phase is then hardened with a cross-linking agent, such as glutaraldehyde.
- the cryopelletization procedure allows conversion of a molten mass, aqueous solution or suspension into solid, bead-like particles.
- the molten mass solutions or suspensions are dripped by means of an appropriately designed device into liquid nitrogen.
- the production of small drops and liquid nitrogen cooling permit very rapid and uniform freezing of the material processed.
- the pellets are further dried in conventional freeze dryers.
- Cryopelletization can also be carried out under aseptic conditions for sterile processing.
- the most critical step producing spherical particles by globulization is the droplet formation. Droplet formation is influenced by formulation related variables, such as the nature of the active ingredient and additives, viscosity, total solid content, surface tension, etc. Extra care must be undertaken with processing of suspensions to ensure homogeneity.
- Enteric matrix pellets can be formed that include polyacrylic acid (e.g. Carbopol) with a high molecular weight polyethylene (such as PEG-20,000).
- polyacrylic acid e.g. Carbopol
- a high molecular weight polyethylene such as PEG-20,000
- microencapsulation applies to enclosure or encasement in microcapsules. Microencapsulation is a means of applying coatings to small particles of solids or droplets of liquids and dispersions.
- coating covers the surfaces of the microcapsules.
- protected covers the surfaces of the microcapsules.
- layered are commonly used interchangeably with the term “encapsulated”. All of these terms can be used to refer to practically any core material that is encased or enclosed in an outer shell.
- Typical equipment used to apply coating includes a conventional pan (Pellegrini; Italy), a modified perforated pan (multicoater, Thomas Eng., IL) or a Wurster coater in a Glatt powder doater/granulator (Glatt Airtechniques). Solvent Based Solution Coating
- Solvent-based coating is when the components of the invention are solubilized and/or dispersed in a solvent.
- the solvent can be aqueous.
- the components can be emulsified with an appropriate emulsifier, organic solvent, or a supercritical fluid. Solvents with a lower melting point than water and higher evaporation numbers are preferred. Solvent mixtures with other organic solvents or water are often employed to get appropriate viscosity and component solubilization.
- Typical solvents include ethanol, methanol, isopropanol, acetone, dichloromethane, trichloromethane and ethyl acetate. Appropriate polymers can also be added as needed.
- Cellulosic derivatives and polymethacrylates are particularly suitable additives for organic solvent coating. Dissolution and solubilization of the components is facilitated by rigorous stirring or heating. Plasticizers may be also be added to stimulate dissolution. Colorants and antisticking agents can be employed as needed.
- Substrate surface area, shape, porosity and stability are important determinants of good coating.
- Spherical particles are preferred, and these may be produced through spheronization or a spherical crystallization process. Crystals or compact granules from dry compaction or extrusion processes, often available commercially, serve as good substrates.
- Encapsulation can be conducted by traditional pan coating or fluidized bed techniques. Several process (air supply, temperature, spray rate, spray system, powder feed, attrition) and formulation factors determine the quality of the end product, and one skilled in the art can readily adjust such parameters as needed.
- Air suspension in a rotary fluidized bed granulator can be used to deposit the encapsulation coat on to a substrate, thus allowing a high rate of dmg application with low dmg loss. Furthermore, both aqueous and organic solvents can be used.
- the Wurster process an air suspension technique, is more suitable for encapsulations involving very fine powders.
- This process entails using coating materials that can be applied in a molten state.
- the selection of proper coating materials depends on melting point, melting point range and the viscosity in the liquid state.
- a fluidized bed is ideal for molten coatings of substrates that range from about 100 microns to about 2000 microns in size. Fluidized bed coating, spraying molten materials, involves achieving a proper balance of process parameters that allow proper encapsulation to occur.
- Substrate particles that are suspended and separated from each other by the fluidization air enter a zone of finely atomized coating liquid. Coating occurs as the liquid droplets, which are substantially smaller in size than substrate, impact the particles, spread, and solidify.
- Some critical success parameters include bed temperature, atomization, atomization fluid temperature, or droplet size, spray type, spray rate, rate of coating droplet solidification on particle surfaces, particle size, shape, etc '
- Inert materials such as sodium chloride, citric acid, potassium chloride can serve as substrates.
- One skilled in the art can readily adjust such parameters to achieve a satisfactory product.
- compositions of the present invention do not include a seed particle, such as a conventional drug or other additive aggregate starch or sugar bead. Instead, the compositions are processed, and the components are chosen, such that a solid composition is formed without the need to coat the composition onto a substrate bead.
- Such compositions can be in the form of beadlets, beads, granules, pellets, etc., that have an approximately homogenous distribution of active ingredient, surfactant, triglyceride and/or additives.
- compositions can be produced by means of balling in pelletizers or fluid bed granulators, and compaction or extrusion/spheronization.
- these compositions can be produced using solvent-free spray congealing processes or dropping (globulization) methods.
- Dropping procedures involve conversion of aqueous solutions or suspensions to a solid form. Congealing of the liquid droplets in cooling baths can aided by a chemical reaction (e.g., insoluble salt or complex formation), a sol/gel transition, or by freezing in a coolant bath of liquid nitrogen or halogenated hydrocarbons.
- the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate.
- the encapsulation coat includes at least one ionic or non-ionic hydrophilic surfactant.
- the encapsulation coat can include a pharmaceutical active ingredient, a lipophilic component such as a lipophilic surfactant or a triglyceride, or both a pharmaceutical active ingredient and a lipophilic component.
- Prior art has used surfactants in formulating coated bead compositions to provide a wetting function, to enable hydrophobic drugs to properly adhere to beads and/or water-soluble binders.
- U.S. Patent No. 4,717,569 to Harrison et al. discloses coated bead compositions of hydrophobic steroid compounds wetted by a hydrophilic surfactant and adhered to the beads by a water-soluble binder.
- the steroid compound is present as finely divided particles, held to the beads by the binder.
- the present inventors have su ⁇ risingly found that proper choice of surfactants and other components allows compositions to be prepared with a wide variety of hydrophilic or hydrophobic active ingredients.
- the present inventors have found that surfactants at higher levels, i.e., in amounts far in excess of the amounts necessary or appropriate for a wetting function, enable a pharmaceutical active ingredient to be fully or at least partially solubilized in the encapsulation coating material itself, rather than merely physically bound in a binder matrix.
- binders can optionally be used in the compositions of the present invention
- the higher surfactant concentrations of the present invention i.e., solubilizing amounts, obviate the need for binders and render them optional instead of necessary.
- the amount of hydrophilic surfactant used in this embodiment can be adjusted so as to at least partially solubilize the pharmaceutical active ingredient, with the optional lipophilic surfactants and triglycerides chosen to further increase the pharmaceutical active ingredient's solubility.
- a further advantage believed to accme from the pharmaceutical compositions of the present invention is that upon administration of the composition to a patient, the high levels of surfactants and other components present in the composition facilitate the rapid solubilization of the pharmaceutical active ingredient.
- compositions of the present invention contains a dmg in a form which requires further solubilization in vivo, such as by emulsification and micellization in the gastrointestinal tract
- the active ingredient in compositions of the present invention is at least partially solubilized in the composition itself, and is further provided with surfactants and other components in the composition to facilitate rapid dispersion (emulsification/micellization) and sustained solubilization of the active ingredient upon administration.
- the encapsulation coat can alternatively be formulated without an active ingredient.
- an active ingredient can be provided in the composition itself but not in the encapsulation coat, if desired. While not presently preferred, such a formulation delivers the active ingredient to the patient along with the surfactants and other components to facilitate dispersion (emulsification/micellization), thus still providing more rapid active ingredient presentation to the abso ⁇ tion site.
- the active ingredient can be administered in a separate dosage form, including a conventional dosage form, prior to, concurrently with, or subsequent to administration of the present compositions, to achieve similar advantages.
- the optional lipophilic surfactant and triglycerides can be used as desired to further enhance solubilization of the active ingredient, or to promote dispersion (emulsification/micellization) in vivo, or to promote in vivo abso ⁇ tion at the abso ⁇ tion site.
- the materials of the encapsulation coat provides components to promote efficient transport of the active ingredient across the barrier membrane to promote more effective abso ⁇ tion.
- the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate.
- the encapsulation coat includes a lipophilic component, such as a lipophilic surfactant or a triglyceride.
- the encapsulation coat can include a pharmaceutical active ingredient, an ionic or non-ionic hydrophilic surfactant, or both a pharmaceutical active ingredient and a hydrophilic surfactant.
- the lipophilic surfactant or triglyceride can be present in amounts to enable at least partial solubilization of an active ingredient in the encapsulation coat, in the composition, or separately administered.
- the solid pharmaceutical composition effectively presents a lipophilic component with or without an active ingredient to help promote abso ⁇ tion of a hydrophilic active.
- the solid pharmaceutical composition includes a solid carrier, the solid carrier including a substrate and an encapsulation coat on the substrate.
- the encapsulation coat includes a pharmaceutical active ingredient and an ionic or non- ionic hydrophilic surfactant; a pharmaceutical active ingredient and a lipophilic component such as a lipophilic surfactant or a triglyceride; or a pharmaceutical active ingredient and both a hydrophilic surfactant and a lipophilic component.
- the solid pharmaceutical composition includes a solid carrier, wherein the solid carrier is formed of at least two components selected from the group consisting of pharmaceutical active ingredients; ionic or non-ionic hydrophilic surfactants; and lipophilic components such as lipophilic surfactants and triglycerides.
- the solid pharmaceutical composition is formulated without the need for a substrate seed particle.
- the active ingredient, surfactants and triglycerides in the chosen combination are processed, with appropriate excipients if necessary, to form solid carriers in the absence of a seed substrate.
- the components are chosen to at least partially solubilize the active ingredient, as described above.
- the present invention also provides methods of using the above-described pharmaceutical composition.
- the present invention provides a method of treating a patient with a pharmaceutical active ingredient, the method including the steps of providing a dosage form of a pharmaceutical composition as described above, including an active ingredient, and administering the dosage form to the patient.
- the patient can be an animal, preferably a mammal, and more preferably a human.
- the present invention provides a method including the steps of providing a dosage form of a pharmaceutical composition as described above, providing a dosage form of a pharmaceutical active ingredient, and administering the dosage forms to the patient.
- This method is advantageous when all or part of the active ingredient or an additional active ingredient is to be administered to the patient in a separate dosage form prior to, concurrently with, or subsequent to administration of the pharmaceutical composition.
- the present invention provides a method of improving the palatability and/or masking the taste of a pharmaceutical active ingredient, by providing the active ingredient in a pharmaceutical composition as described above. Since the active ingredient is encapsulated in a lipid coat, it will not instantaneously dissolve in the mouth, but will instead dissolve in a region past the oral cavity, thereby substantially avoiding or at least reducing undesirable contact between the active ingredient and the mouth.
- compositions enable gastric resistance or acid degradation reduction of the active ingredient.
- the solid carrier improves the chemical stability of the active ingredient.
- the solid carrier protects the upper gastrointestinal tract from the adverse effects of the active ingredient.
- the present invention provides a method of improving the dissolution and/or abso ⁇ tion of a pharmaceutical active ingredient, by administering the active ingredient in a composition as described above, or co-administering the active ingredient with a composition as described above.
- compositions according to the present invention were prepared as follows. The specific components used are detailed in Examples 2-5.
- a spraying solution of the coating materials was prepared by dissolving the desired amount of the active ingredient and mixing with the hydrophilic and/or lipophilic surfactants in an organic solvent or a mixture of organic solvents.
- the organic solvent used for the coating solution was a mixture of methylene chloride and isopropyl alcohol in a 3:1 to 1 :1 weight ratio.
- Commercially available sugar beads (30/35 mesh size) were coated in a conventional coating pan having a spray gun (Campbell Hausfield, DH 7500) with a nozzle diameter of 1.2 mm and an air pressure of 25 psi. The bed temperature was maintained at approximately 32 °C during the spraying process.
- talc talc
- a pharmaceutical composition was prepared according to the method of Example 1, having a substrate particle, an active ingredient (glyburide), and a mixture of a hydrophilic surfactant (PEG-40 stearate) and a lipophilic surfactant (glycerol monolaurate).
- the components and their amounts were as follows:
- a pharmaceutical composition was prepared according to the method of Example 1 , having a substrate particle, an active ingredient (progesterone), a mixture of a hydrophilic surfactant (Solulan C-24) and two lipophilic components (deoxycholic acid and distilled monoglycerides).
- the components and their amounts were as follows:
- a pharmaceutical composition was prepared according to the method of Example 1, having a substrate particle, an active ingredient (itraconazole), a mixture of non-ionic hydrophilic surfactants (Cremophor RH-40 and PEG- 150 monostearate), an ionic hydrophilic surfactant (sodium taurocholate) and a lipophilic surfactant (glycerol monolaurate).
- active ingredient itraconazole
- non-ionic hydrophilic surfactants Cosmetic RH-40 and PEG- 150 monostearate
- an ionic hydrophilic surfactant sodium taurocholate
- glycerol monolaurate a lipophilic surfactant
- Example 5 Composition IV
- a pharmaceutical composition was prepared according to the method of Example 1 , having a substrate particle, an active ingredient (omeprazole), a mixture of a two hydrophilic surfactants (PEG- 150 monostearate and PEG-40 monostearate), and a triglyceride-containing lipophilic component (Maisine 35-1).
- the components and their amounts were as follows:
- the dried, coated beads of Example 3 were further seal coated by a polymer layer.
- the seal coating polymer layer was applied to the progesterone-coated beads in a Uni-Glatt fluid bed coater.
- Polyvinylpyrrolidone (PVP-K30) was dissolved in isopropyl alcohol to form a 5% w/w solution.
- This seal coating solution was sprayed onto the coated beads with a Wurster bottom spray insert.
- the inlet and outlet air temperature were maintained at 30 and 40 °C, respectively.
- the beads were further dried by supplying dry air at 50-55°C for 5-15 minutes.
- the seal coated beads were then allowed to cool in the apparatus by supplying dry air at 20-25°C for 5-15 minutes.
- the dried, seal coated beads were collected and stored in a container.
- Example 7 Protective Coating The dried, coated beads of Example 5 were further coated with a protective polymer layer.
- the protective coating was applied to the omeprazole coated beads by spraying with a hydroxypropyl methylcellulose (HPMC) solution.
- HPMC hydroxypropyl methylcellulose
- the protective coating HPMC solution was prepared by dissolving 6 grams of HPMC in ethanol. To this solution, methyl ene chloride was added followed by 2 mL of triethylcitrate as a plasticizer and 1 g of talc, the HPMC solution was sprayed on the beads as described in Example 6, and the protective coated beads were then dried and collected.
- Example 5 The dried, coated beads of Example 5 were further coated with an enteric coating layer.
- the enteric layer was applied to the omeprazole coated beads by spraying a
- Eudragit L100 solution Eudragit L100 is an acrylate polymer commercially available from Rohm Pharma.
- the spraying solution was prepared by dispersing 15 g of Eudragit LI 00 in 200 mL of ethanol to give a clear solution. To this solution, 4 g of triethyl citrate was then added as a plasticizer. 2 grams of purified talc was also added to the solution. The solution was then sprayed onto the beads, and the beads were dried, as described in Example 6.
- a comparative dissolution study was performed on three forms of an active ingredient: the glyburide coated beads of Example 2, a commercially available glyburide product (Micronase®, available from Pharmacia & Upjohn), and the pure glyburide bulk dmg.
- the dissolution study was performed using a USP dissolution type 2 apparatus. For each of the three forms, material equivalent to 5 mg of glyburide was used for each triplicated dissolution mn in 500 mL of isotonic pH 7.4 phosphate buffer. The dissolution medium was maintained at 37 °C and constantly stirred at a speed of 100 ⁇ rn. The dissolution media were sampled at 15, 30, 45, 60, 120 and 180 minutes.
- the HPLC assay was performed on a Varian 9010 system by injecting 50 ⁇ L of the sample.
- the sample was separated on a Phenominex Cl 8 column by running a mobile phase of 75:25 v/v methanol/phosphate buffer (0.1 M potassium dihydrogen phosphate, pH adjusted to 3.5 using phosphoric acid), at a flow rate of 1 mL/min, at ambient temperature.
- Glyburide was detected by its UV abso ⁇ tion at 229 nm.
- the results of the comparative dissolution measurement were expressed as the percent of glyburide dissolved/released in the dissolution medium at a given time, relative to the initial total glyburide content present in the dissolution medium (5 mg/500 mL). The results are shown in Figure 1 , with the error bars representing the standard deviation. As the Figure shows, the glyburide coated beads of the present invention showed a superior dissolution profile in the rate, the extent, and the variability of glyburide dissolved/released into the dissolution medium, compared to the commercial Micronase® and the pure bulk dmg.
- a comparative dissolution study was performed on three forms of an active ingredient: the progesterone coated beads of Example 3, the seal coated, progesterone coated beads of Example 6, and the pure progesterone bulk dmg.
- the dissolution study was performed using a USP dissolution type 2 apparatus.
- material equivalent to 100 mg of progesterone was used for each duplicated dissolution mn in 900 mL of isotonic pH 7.4 phosphate buffer containing 0.5% w/v of Tween 80.
- the dissolution medium was maintained at 37 °C and constantly stirred at a speed of 100 ⁇ m.
- the dissolution media were sampled at 30, 60, 120 and 180 minutes.
- the HPLC assay was performed on a Varian 9010 system by injecting 50 ⁇ L of the sample.
- the sample was separated on a Phenominex C 18 column by running a mobile phase of 75:25 v/v methanol/phosphate buffer (0.1 M potassium dihydrogen phosphate, pH adjusted to 3.5 using phosphoric acid), at a flow rate of 1 mL/min, at ambient temperature.
- Glyburide was detected by its UV abso ⁇ tion at 229 nm.
- the results of the comparative dissolution measurement were expressed as the percent of progesterone dissolved/released in the dissolution medium at a given time, relative to the initial total progesterone content present in the dissolution medium (100 mg/900 mL).
- the results are shown in Figure 2A.
- the progesterone coated beads of the present invention, with or without a seal coating showed superior dissolution profiles in both the rate and the extent of progesterone dissolved/released into the dissolution medium, compared to the pure bulk dmg.
- Prometrium® is a capsule dosage form in which micronized progesterone is suspended in a blend of vegetable oils.
- the dissolution of the Prometrium® capsule was performed using a USP dissolution type 1 apparatus, and the dissolution of the other samples was performed using a USP dissolution type 2 apparatus.
- material equivalent to 100 mg of progesterone was used for each dissolution mn in 900 mL of isotonic pH 7.4 phosphate buffer.
- the dissolution medium was maintained at 37 °C and constantly stirred at a speed of 100 ⁇ m.
- the dissolution media were sampled at 15, 30, 45, 60 and 180 minutes. At each time point, 3 mL of the medium was sampled, and the medium was replenished with 3 mL of fresh buffer/Tween solution.
- the samples were filtered through a 0.45 ⁇ filter immediately after the sampling. The filtrates were then diluted in methanol to an appropriate concentration for a progesterone-specific HPLC assay.
- the HPLC assay was performed on a Varian 9010 system by injecting 50 ⁇ L of the sample.
- the sample was separated on a Phenominex C18 column by running a mobile phase of 75:25 v/v methanol/phosphate buffer (0.1 M potassium dihydrogen phosphate, pH adjusted to 3.5 using phosphoric acid), at a flow rate of 1 mL/min, at ambient temperature. Glyburide was detected by its UV abso ⁇ tion at 229 nm.
- the results of the comparative dissolution measurement were expressed as the percent of progesterone dissolved/released in the dissolution medium at a given time, relative to the initial total progesterone content present in the dissolution medium (100 mg/900 mL). The results are shown in Figure 2B.
- the progesterone coated beads of the present invention showed superior dissolution profiles in both the rate and the extent of progesterone dissolved/released into the dissolution medium, compared to the commercial Prometrium® and the pure bulk dmg.
- Example 12 Comparative Dissolution Study IV A comparative dissolution study was performed comparing the rate and extent of dissolution of the protective coated, omeprazole coated beads of Example 7, the enteric coated, omeprazole coated beads of Example 8 and a commercially available omeprazole product (Prilosec®, available from Astra Zeneca). The dissolution study was performed using a USP dissolution type 2 apparatus. For each of the three dosage forms, material equivalent to 5 mg of omeprazole was used for each dissolution mn in 500 mL of isotonic pH 7.4 phosphate buffer. The dissolution medium was maintained at 37 °C and constantly stirred at a speed of 100 ⁇ m. The dissolution media were sampled at 15, 30, 45 and 60 minutes.
- the results of the comparative dissolution measurement were expressed as the percent of omeprazole dissolved in the dissolution medium at a given time, relative to the initial total omeprazole content present in the dissolution medium (5 mg/500 mL).
- the results are shown in Figure 3.
- the omeprazole coated beads of the present invention showed superior dissolution profiles in both the rate and the extent of omeprazole dissolved/released into the dissolution medium, compared to the commercial Prilose® product.
- compositions that can be prepared according to the present invention. It should be appreciated that the compositions can be prepared in the absence of the active ingredients and appropriate, amounts of the active ingredients in any given dosage form then can be administered together or separately with the composition. It should also be appreciated that the compositions can further include additional additives, excipients, and other components for the pu ⁇ ose of facilitating the processes involving the preparation of the composition or the pharmaceutical dosage form, as described herein, as is well-known to those skilled in the art.
Abstract
Description
Claims
Priority Applications (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU17981/01A AU1798101A (en) | 1999-11-23 | 2000-11-22 | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| JP2001539423A JP2003517470A (en) | 1999-11-23 | 2000-11-22 | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| CA2391923A CA2391923C (en) | 1999-11-23 | 2000-11-22 | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| EP00980761A EP1233756A4 (en) | 1999-11-23 | 2000-11-22 | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/447,690 | 1999-11-23 | ||
| US09/447,690 US6248363B1 (en) | 1999-11-23 | 1999-11-23 | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2001037808A1 true WO2001037808A1 (en) | 2001-05-31 |
Family
ID=23777347
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US2000/032255 WO2001037808A1 (en) | 1999-11-23 | 2000-11-22 | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
Country Status (6)
| Country | Link |
|---|---|
| US (9) | US6248363B1 (en) |
| EP (1) | EP1233756A4 (en) |
| JP (2) | JP2003517470A (en) |
| AU (1) | AU1798101A (en) |
| CA (2) | CA2706113A1 (en) |
| WO (1) | WO2001037808A1 (en) |
Cited By (151)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001082905A1 (en) * | 2000-04-28 | 2001-11-08 | Glaxosmithkline Kabushiki Kaisha | Pharmaceutical formulation comprising carrier beads coated with a layer of valaciclovir |
| WO2001087355A1 (en) * | 2000-05-18 | 2001-11-22 | Australian Rural Group Limited | Lipophilic medicament |
| WO2002092062A2 (en) * | 2001-05-15 | 2002-11-21 | Leo Pharma A/S | Combination of vitamin d analogue and pyrimidine nucleoside analogue |
| WO2002094258A1 (en) * | 2001-05-21 | 2002-11-28 | Noveon Ip Holdings Corp. | Improved dosage form of n-acetyl cysteine |
| WO2003003999A2 (en) * | 2001-07-02 | 2003-01-16 | Elan Corporation, Plc. | Delivery of a bioactive material |
| EP1287821A1 (en) * | 2001-09-04 | 2003-03-05 | Pfizer Limited | Multiparticulate formulations of atorvastatin calcium having a modulated rate of drug release |
| WO2003075895A1 (en) * | 2002-03-11 | 2003-09-18 | Novartis Ag | Tasted masked veterinary solid compositions |
| WO2003105607A1 (en) * | 2002-06-12 | 2003-12-24 | Nutralease Ltd. | Nano-sized self-assembled structured liquids |
| WO2004047811A1 (en) * | 2002-11-22 | 2004-06-10 | Centurion, Inc. | Controlled release formulation of tamsulosin or pharmaceutically acceptable salts thereof for treatment of the signs and symptoms of benign prostatic hyperplasia |
| WO2004047798A2 (en) * | 2002-11-22 | 2004-06-10 | Omega Farma Ehf | Formulations of finasteride |
| EP1429732A1 (en) * | 2001-07-10 | 2004-06-23 | Kos Life Sciences, Inc. | A core formulation |
| EP1453536A1 (en) * | 2001-12-12 | 2004-09-08 | F H Faulding & Co. Limited | Composition for the preservation of viruses |
| EP1461044A1 (en) * | 2001-12-03 | 2004-09-29 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin d compounds |
| JP2004534074A (en) * | 2001-06-14 | 2004-11-11 | ヤゴテック アーゲー | New composition |
| WO2005018626A1 (en) | 2003-08-22 | 2005-03-03 | Fournier Laboratories Ireland Limited | Pharmaceutical composition comprising a combination of metformin and a statin |
| WO2005020993A1 (en) * | 2003-08-29 | 2005-03-10 | Lifecycle Pharma A/S | Modified release compositions comprising tacrolimus |
| WO2005042623A1 (en) * | 2003-10-23 | 2005-05-12 | University Of Nottingham | Preparing active polymer extrudates |
| WO2005041958A1 (en) * | 2003-10-21 | 2005-05-12 | Smithkline Beecham Pharmco Puerto Rico Incorporated | Novel compositions |
| JPWO2003059389A1 (en) * | 2002-01-16 | 2005-05-19 | 山之内製薬株式会社 | Pharmaceutical composition for improving oral absorption |
| WO2005065645A2 (en) * | 2003-12-31 | 2005-07-21 | Actavis Group Hf | Donepezil formulations |
| JP2005523293A (en) * | 2002-02-26 | 2005-08-04 | アストラゼネカ アクチボラグ | Iressa pharmaceutical formulation containing water-soluble cellulose derivative |
| WO2005094791A1 (en) * | 2004-03-19 | 2005-10-13 | Eczacibasi Ozgun Kimyasal Urunler San. Tic. A.S. | Preparation of lipid coated cefuroxime axetil |
| EP1626695A2 (en) * | 2003-05-07 | 2006-02-22 | Afmedica, Inc. | Compositions and methods for reducing scar tissue formation |
| EP1631239A2 (en) * | 2003-06-11 | 2006-03-08 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin d compounds |
| EP1641437A1 (en) * | 2003-07-09 | 2006-04-05 | Chong Kun Dang Pharmaceutical Corp. | The solid dispersion of tacrolimus |
| WO2006036967A1 (en) * | 2004-09-28 | 2006-04-06 | Atrium Medical Corporation | Solubilizing a drug for use in a coating |
| JP2006518380A (en) * | 2003-01-31 | 2006-08-10 | スミスクライン・ビーチャム・コーポレイション | Solid dispersion composition |
| EP1759692A2 (en) * | 2003-03-10 | 2007-03-07 | Novartis AG | Taste-masked solid veterinary compositions |
| WO2007077581A2 (en) * | 2006-01-02 | 2007-07-12 | Rubicon Research Private Limited | Pharmaceutical compositions |
| EP1809295A2 (en) * | 2004-11-10 | 2007-07-25 | Derray Technology, Inc. | Novel formulations of eprosartan with enhanced bioavailability |
| EP1811967A2 (en) * | 2004-11-08 | 2007-08-01 | Biokey, Inc. | Methods and formulations for making pharmaceutical compositions containing bupropion |
| EP1874271A1 (en) * | 2005-04-12 | 2008-01-09 | Elan Pharma International Limited | Modified release compositions comprising a fluorocytidine derivative for the treatment of cancer |
| WO2008030161A1 (en) * | 2006-09-05 | 2008-03-13 | Astrazeneca Ab | Pharmaceutical composition comprising candesartan cilexetil |
| WO2008033024A2 (en) * | 2006-09-15 | 2008-03-20 | Echo Pharmaceuticals B.V. | Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances |
| US7348027B2 (en) * | 2005-04-08 | 2008-03-25 | Bayer Healthcare Llc | Taste masked veterinary formulation |
| EP1905432A1 (en) * | 2006-06-30 | 2008-04-02 | ELAN CORPORATION, Plc | Nanoparticulate and controlled release compositions comprising a cephalosporin |
| WO2008083192A2 (en) * | 2006-12-28 | 2008-07-10 | Repros Therapeutics Inc. | Methods and formulations for improved bioavailability of antiprogestins |
| WO2008065097A3 (en) * | 2006-11-28 | 2008-07-17 | Liconsa Laboratorios Sa | Stabilized solid pharmaceutical composition of candesartan cilexetil |
| WO2008061226A3 (en) * | 2006-11-17 | 2008-07-31 | Supernus Pharmaceuticals Inc | Sustained-release formulations of topiramate |
| WO2009068458A3 (en) * | 2007-11-27 | 2009-08-20 | Univ Friedrich Alexander Er | Encapsulated microparticles with virus-containing core and production method for microparticles |
| WO2009131537A1 (en) * | 2008-04-25 | 2009-10-29 | Karolinska Institutet Innovations Ab | New therapy of treatment of the irritable bowel syndrome. |
| WO2010005480A2 (en) * | 2008-06-16 | 2010-01-14 | Biovascular, Inc. | Controlled release compositions of agents that reduce circulating levels of platelets and methods therefor |
| US7696162B2 (en) | 2001-03-23 | 2010-04-13 | Sanofi-Aventis Deutschland Gmbh | Zinc-free and low-zinc insulin preparations having improved stability |
| EP2206518A1 (en) * | 2007-10-25 | 2010-07-14 | Astellas Pharma Inc. | Pharmaceutical composition containing lipophilic il-2 production inhibitor |
| US7771751B2 (en) | 2005-08-31 | 2010-08-10 | Abraxis Bioscience, Llc | Compositions comprising poorly water soluble pharmaceutical agents and antimicrobial agents |
| EP1411921B2 (en) † | 2001-07-20 | 2010-08-18 | Novartis AG | Pharmaceutical compositions containing terbinafine and use thereof |
| EP2217211A1 (en) * | 2007-10-29 | 2010-08-18 | SRI International | An orally-absorbed solid dose formulation for vancomycin |
| CN101167697B (en) * | 2006-10-26 | 2011-03-30 | 中国科学院上海药物研究所 | Donepezils compound long-acting slow-releasing and controlled-releasing composition and preparation method thereof |
| CN1859909B (en) * | 2003-08-29 | 2011-04-06 | 生命周期药物公司 | Solid dispersions comprising tacrolimus |
| US7932263B2 (en) | 2003-09-26 | 2011-04-26 | Astrazeneca Ab | Therapeutic treatment |
| EP2323627A1 (en) * | 2008-06-23 | 2011-05-25 | Bionetwork | Novel rapid-effect pharmaceutical forms and uses of resulting pharmaceutical compositions |
| US7976853B2 (en) | 2003-01-29 | 2011-07-12 | Takeda Pharmaceutical Company Limited | Process for producing coated preparation |
| US8003690B2 (en) | 2002-12-13 | 2011-08-23 | Jagotec Ag | Topical nanoparticulate spironolactone formulation |
| US8007830B2 (en) | 2001-10-26 | 2011-08-30 | Merck Frosst Canada & Co. | Granule formation |
| ITMI20101512A1 (en) * | 2010-08-06 | 2012-02-07 | Sofar Spa | COMPOSITIONS OF BECLOMETASONE DIPROPIONIONATO IN GASTRORESISTANT MICROSPHERES WITH MODIFIED RELEASE AND PROCESS FOR THEIR ACHIEVEMENT |
| CN102423299A (en) * | 2011-12-20 | 2012-04-25 | 中国热带农业科学院农产品加工研究所 | Preparation method for novel drug-loaded chitosan nano-microspheres |
| CN102657626A (en) * | 2012-05-23 | 2012-09-12 | 重庆康刻尔制药有限公司 | Medicinal composite tablet of pioglitazone medicine |
| US8324192B2 (en) | 2005-11-12 | 2012-12-04 | The Regents Of The University Of California | Viscous budesonide for the treatment of inflammatory diseases of the gastrointestinal tract |
| US8497258B2 (en) | 2005-11-12 | 2013-07-30 | The Regents Of The University Of California | Viscous budesonide for the treatment of inflammatory diseases of the gastrointestinal tract |
| US8633178B2 (en) | 2011-11-23 | 2014-01-21 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8637512B2 (en) | 2002-07-29 | 2014-01-28 | Glaxo Group Limited | Formulations and method of treatment |
| US8652523B2 (en) | 2002-07-26 | 2014-02-18 | Flamel Technologies | Oral pharmaceutical formulation in the form of a plurality of microcapsules for prolonged release of active principle(s) with slow solubility |
| US8664239B2 (en) | 2007-05-30 | 2014-03-04 | Veloxis Pharmaceuticals A/S | Tacrolimus for improved treatment of transplant patients |
| US8679545B2 (en) | 2005-11-12 | 2014-03-25 | The Regents Of The University Of California | Topical corticosteroids for the treatment of inflammatory diseases of the gastrointestinal tract |
| EP2712867A1 (en) * | 2005-12-14 | 2014-04-02 | Abbott Laboratories | Compositions of tetrazole derivatives of sirolimus and anti-oxidants |
| US8802087B2 (en) | 2004-03-22 | 2014-08-12 | Abbott Products Gmbh | Pharmaceutical compositions of lipase-containing products, in particular of pancreation |
| US8858978B2 (en) | 2004-09-28 | 2014-10-14 | Atrium Medical Corporation | Heat cured gel and method of making |
| US8865695B2 (en) | 2009-01-08 | 2014-10-21 | Lipocine Inc. | Steroidal compositions |
| US8865692B2 (en) | 2007-11-13 | 2014-10-21 | Meritage Pharma, Inc | Compositions for the treatment of gastrointestinal inflammation |
| US8906419B2 (en) | 2003-08-06 | 2014-12-09 | Nostrum Pharmaceuticals, Inc. | Pharmaceutical composition containing water soluble drug |
| US8911777B2 (en) | 2007-04-04 | 2014-12-16 | Sigmoid Pharma Limited | Pharmaceutical composition of tacrolimus |
| US8933059B2 (en) | 2012-06-18 | 2015-01-13 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8951570B2 (en) | 2007-04-26 | 2015-02-10 | Sigmoid Pharma Limited | Manufacture of multiple minicapsules |
| US8980870B2 (en) | 2002-09-24 | 2015-03-17 | Boehringer Ingelheim International Gmbh | Solid telmisartan pharmaceutical formulations |
| US9000040B2 (en) | 2004-09-28 | 2015-04-07 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US9012506B2 (en) | 2004-09-28 | 2015-04-21 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US9034858B2 (en) | 2010-11-30 | 2015-05-19 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| EP2314277A4 (en) * | 2008-07-28 | 2015-06-03 | Kao Corp | External preparation for skin, and wrinkle-repairing agent |
| US9180091B2 (en) | 2012-12-21 | 2015-11-10 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US9198871B2 (en) | 2005-08-15 | 2015-12-01 | Abbott Products Gmbh | Delayed release pancreatin compositions |
| US9220681B2 (en) | 2012-07-05 | 2015-12-29 | Sigmoid Pharma Limited | Formulations |
| US9220820B2 (en) | 2005-10-15 | 2015-12-29 | Atrium Medical Corporation | Hydrophobic cross-linked gels for bioabsorbable drug carrier coatings |
| US9226900B2 (en) | 2008-07-11 | 2016-01-05 | Critical Pharmaceuticals Limited | Process for preparing microparticles |
| US9238029B2 (en) | 2004-06-16 | 2016-01-19 | Takeda Pharmaceuticals U.S.A., Inc. | Multiple PPI dosage form |
| US9241910B2 (en) | 2008-03-11 | 2016-01-26 | Takeda Pharmaceutical Company Limited | Orally-disintegrating solid preparation |
| US9278070B2 (en) | 2009-05-18 | 2016-03-08 | Sigmoid Pharma Limited | Composition comprising oil drops |
| US9278161B2 (en) | 2005-09-28 | 2016-03-08 | Atrium Medical Corporation | Tissue-separating fatty acid adhesion barrier |
| US9289382B2 (en) | 2012-06-18 | 2016-03-22 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US9314474B2 (en) | 2010-07-15 | 2016-04-19 | Hybrigenics, Sa | Formulations of 14-epi-analogues of vitamin D |
| US9320746B2 (en) | 2013-02-21 | 2016-04-26 | Sigmoid Pharma Limited | Method for treating intestinal fibrosis |
| US9358241B2 (en) | 2010-11-30 | 2016-06-07 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US9364519B2 (en) | 2011-09-01 | 2016-06-14 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition for use in the treatment of a neurodegenerative disease |
| US9408893B2 (en) | 2011-08-29 | 2016-08-09 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical combination for use in glycemic control in diabetes type 2 patients |
| US9408858B2 (en) | 2007-04-25 | 2016-08-09 | Opko Renal, Llc | Method for treating secondary hyperparathyroidism in CKD |
| US9427423B2 (en) | 2009-03-10 | 2016-08-30 | Atrium Medical Corporation | Fatty-acid based particles |
| US9492596B2 (en) | 2006-11-06 | 2016-11-15 | Atrium Medical Corporation | Barrier layer with underlying medical device and one or more reinforcing support structures |
| US9498485B2 (en) | 2014-08-28 | 2016-11-22 | Lipocine Inc. | Bioavailable solid state (17-β)-hydroxy-4-androsten-3-one esters |
| US9526764B2 (en) | 2008-10-17 | 2016-12-27 | Sanofi-Aventis Deutschland Gmbh | Combination of an insulin and a GLP-1-agonist |
| US9539302B2 (en) | 2009-06-18 | 2017-01-10 | Allergan, Inc. | Safe desmopressin administration |
| US9549918B2 (en) | 2008-05-30 | 2017-01-24 | Veloxis Pharmaceuticals A/S | Stabilized tacrolimus composition |
| US9592324B2 (en) | 2006-11-06 | 2017-03-14 | Atrium Medical Corporation | Tissue separating device with reinforced support for anchoring mechanisms |
| US9687445B2 (en) | 2012-04-12 | 2017-06-27 | Lts Lohmann Therapie-Systeme Ag | Oral film containing opiate enteric-release beads |
| US9707176B2 (en) | 2009-11-13 | 2017-07-18 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition comprising a GLP-1 agonist and methionine |
| US9744137B2 (en) | 2006-08-31 | 2017-08-29 | Supernus Pharmaceuticals, Inc. | Topiramate compositions and methods of enhancing its bioavailability |
| US9801982B2 (en) | 2004-09-28 | 2017-10-31 | Atrium Medical Corporation | Implantable barrier device |
| US9821032B2 (en) | 2011-05-13 | 2017-11-21 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical combination for improving glycemic control as add-on therapy to basal insulin |
| US9821024B2 (en) | 2010-11-25 | 2017-11-21 | Sigmoid Pharma Limited | Immunomodulatory compositions |
| US9861644B2 (en) | 2013-03-15 | 2018-01-09 | Opko Ireland Global Holdings, Ltd. | Stabilized modified release vitamin D formulation and method of administering same |
| US9867880B2 (en) | 2012-06-13 | 2018-01-16 | Atrium Medical Corporation | Cured oil-hydrogel biomaterial compositions for controlled drug delivery |
| US9878036B2 (en) | 2009-08-12 | 2018-01-30 | Sigmoid Pharma Limited | Immunomodulatory compositions comprising a polymer matrix and an oil phase |
| US9931349B2 (en) | 2016-04-01 | 2018-04-03 | Therapeuticsmd, Inc. | Steroid hormone pharmaceutical composition |
| US9943530B2 (en) | 2006-02-03 | 2018-04-17 | Opko Renal, Llc | Treating vitamin D insufficiency and deficiency with 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 |
| US9950039B2 (en) | 2014-12-12 | 2018-04-24 | Sanofi-Aventis Deutschland Gmbh | Insulin glargine/lixisenatide fixed ratio formulation |
| US9981013B2 (en) | 2010-08-30 | 2018-05-29 | Sanofi-Aventis Deutschland Gmbh | Use of AVE0010 for the treatment of diabetes mellitus type 2 |
| US10029011B2 (en) | 2009-11-13 | 2018-07-24 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition comprising a GLP-1 agonist, an insulin and methionine |
| US10052386B2 (en) | 2012-06-18 | 2018-08-21 | Therapeuticsmd, Inc. | Progesterone formulations |
| US10072256B2 (en) | 2006-05-22 | 2018-09-11 | Abbott Products Gmbh | Process for separating and determining the viral load in a pancreatin sample |
| US10076494B2 (en) | 2016-06-16 | 2018-09-18 | Dexcel Pharma Technologies Ltd. | Stable orally disintegrating pharmaceutical compositions |
| US10098894B2 (en) | 2014-07-29 | 2018-10-16 | Therapeuticsmd, Inc. | Transdermal cream |
| US10143706B2 (en) | 2016-06-29 | 2018-12-04 | Cannscience Innovations, Inc. | Decarboxylated cannabis resins, uses thereof and methods of making same |
| US10159713B2 (en) | 2015-03-18 | 2018-12-25 | Sanofi-Aventis Deutschland Gmbh | Treatment of type 2 diabetes mellitus patients |
| US10206932B2 (en) | 2014-05-22 | 2019-02-19 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10220047B2 (en) | 2014-08-07 | 2019-03-05 | Opko Ireland Global Holdings, Ltd. | Adjunctive therapy with 25-hydroxyvitamin D and articles therefor |
| US10258630B2 (en) | 2014-10-22 | 2019-04-16 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| EP3395340B1 (en) | 2003-09-12 | 2019-04-17 | Amgen, Inc | Rapid dissolution formulation of cinacalcet hcl |
| US10286077B2 (en) | 2016-04-01 | 2019-05-14 | Therapeuticsmd, Inc. | Steroid hormone compositions in medium chain oils |
| US10293052B2 (en) | 2007-11-13 | 2019-05-21 | Meritage Pharma, Inc. | Compositions for the treatment of gastrointestinal inflammation |
| US10302660B2 (en) | 2008-04-02 | 2019-05-28 | Opko Renal, Llc | Methods useful for vitamin D deficiency and related disorders |
| US10322213B2 (en) | 2010-07-16 | 2019-06-18 | Atrium Medical Corporation | Compositions and methods for altering the rate of hydrolysis of cured oil-based materials |
| US10328087B2 (en) | 2015-07-23 | 2019-06-25 | Therapeuticsmd, Inc. | Formulations for solubilizing hormones |
| US10434147B2 (en) | 2015-03-13 | 2019-10-08 | Sanofi-Aventis Deutschland Gmbh | Treatment type 2 diabetes mellitus patients |
| US10434138B2 (en) | 2013-11-08 | 2019-10-08 | Sublimity Therapeutics Limited | Formulations |
| US10471148B2 (en) | 2012-06-18 | 2019-11-12 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable PK profile |
| US10471072B2 (en) | 2012-12-21 | 2019-11-12 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10537581B2 (en) | 2012-12-21 | 2020-01-21 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10561615B2 (en) | 2010-12-10 | 2020-02-18 | Lipocine Inc. | Testosterone undecanoate compositions |
| US10668089B2 (en) | 2006-06-21 | 2020-06-02 | Opko Ireland Global Holdings, Ltd. | Method of treating and preventing secondary hyperparathyroidism |
| US10704037B2 (en) | 2005-07-29 | 2020-07-07 | Abbott Products Gmbh | Processes for the manufacture and use of pancreatin |
| WO2020187455A1 (en) | 2019-03-20 | 2020-09-24 | Pharmathen S.A. | Prolonged release formulation comprising tacrolimus |
| US10806740B2 (en) | 2012-06-18 | 2020-10-20 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10864304B2 (en) | 2009-08-11 | 2020-12-15 | Atrium Medical Corporation | Anti-infective antimicrobial-containing biomaterials |
| US10993987B2 (en) | 2014-11-07 | 2021-05-04 | Sublimity Therapeutics Limited | Compositions comprising Cyclosporin |
| US11173168B2 (en) | 2016-03-28 | 2021-11-16 | Eirgen Pharma Ltd. | Methods of treating vitamin D insufficiency in chronic kidney disease |
| US11246875B2 (en) | 2012-12-21 | 2022-02-15 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11266607B2 (en) | 2005-08-15 | 2022-03-08 | AbbVie Pharmaceuticals GmbH | Process for the manufacture and use of pancreatin micropellet cores |
| US11266661B2 (en) | 2012-12-21 | 2022-03-08 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11559530B2 (en) | 2016-11-28 | 2023-01-24 | Lipocine Inc. | Oral testosterone undecanoate therapy |
| US11672809B2 (en) | 2010-03-29 | 2023-06-13 | Eirgen Pharma Ltd. | Methods and compositions for reducing parathyroid levels |
| US11707467B2 (en) | 2014-08-28 | 2023-07-25 | Lipocine Inc. | (17-ß)-3-oxoandrost-4-en-17YL tridecanoate compositions and methods of their preparation and use |
| US11752158B2 (en) | 2007-04-25 | 2023-09-12 | Eirgen Pharma Ltd. | Method of treating vitamin D insufficiency and deficiency |
| US11801253B2 (en) | 2007-04-25 | 2023-10-31 | Opko Renal, Llc | Method of safely and effectively treating and preventing secondary hyperparathyroidism in chronic kidney disease |
Families Citing this family (1393)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5744500A (en) * | 1990-01-03 | 1998-04-28 | Teva Pharmaceutical Industries, Ltd. | Use of R-enantiomer of N-propargyl-1-aminoindan, salts, and compositions thereof |
| US6428771B1 (en) * | 1995-05-15 | 2002-08-06 | Pharmaceutical Discovery Corporation | Method for drug delivery to the pulmonary system |
| US6645988B2 (en) * | 1996-01-04 | 2003-11-11 | Curators Of The University Of Missouri | Substituted benzimidazole dosage forms and method of using same |
| US6489346B1 (en) * | 1996-01-04 | 2002-12-03 | The Curators Of The University Of Missouri | Substituted benzimidazole dosage forms and method of using same |
| US6699885B2 (en) * | 1996-01-04 | 2004-03-02 | The Curators Of The University Of Missouri | Substituted benzimidazole dosage forms and methods of using same |
| US5840737A (en) | 1996-01-04 | 1998-11-24 | The Curators Of The University Of Missouri | Omeprazole solution and method for using same |
| US20030215507A1 (en) * | 1996-03-25 | 2003-11-20 | Wyeth | Extended release formulation |
| FR2754177B1 (en) * | 1996-10-07 | 1999-08-06 | Sanofi Sa | PHARMACEUTICAL MICROSPHERES OF VALPROIC ACID FOR ORAL ADMINISTRATION |
| US20050004049A1 (en) * | 1997-03-11 | 2005-01-06 | Elan Pharma International Limited | Novel griseofulvin compositions |
| GB9800936D0 (en) * | 1997-05-10 | 1998-03-11 | Univ Nottingham | Biofunctional polymers |
| US20030077229A1 (en) * | 1997-10-01 | 2003-04-24 | Dugger Harry A. | Buccal, polar and non-polar spray or capsule containing cardiovascular or renal drugs |
| KR100627614B1 (en) * | 1998-04-20 | 2006-09-25 | 에자이 가부시키가이샤 | Medicament comprising stabilized compositions containing benzimidazole-type compounds |
| US20040087512A1 (en) * | 1998-05-12 | 2004-05-06 | Teter Beverly B. | In-situ formation of CLA |
| US7022683B1 (en) * | 1998-05-13 | 2006-04-04 | Carrington Laboratories, Inc. | Pharmacological compositions comprising pectins having high molecular weights and low degrees of methoxylation |
| KR100314496B1 (en) | 1998-05-28 | 2001-11-22 | 윤동진 | Non-thrombogenic heparin derivatives, process for preparation and use thereof |
| NZ330726A (en) * | 1998-06-18 | 2000-10-27 | Dec Res | Intra-vaginal delivery unit or composition containing a cyclodextrin which improves absorbtion of 17-beta oestradiol or oestradiol benzoate |
| US6413713B1 (en) * | 1998-10-30 | 2002-07-02 | Hyperbaric Systems | Method for preserving blood platelets |
| US20070122481A1 (en) * | 1998-11-02 | 2007-05-31 | Elan Corporation Plc | Modified Release Compositions Comprising a Fluorocytidine Derivative for the Treatment of Cancer |
| US20080113025A1 (en) * | 1998-11-02 | 2008-05-15 | Elan Pharma International Limited | Compositions comprising nanoparticulate naproxen and controlled release hydrocodone |
| US20090297597A1 (en) * | 1998-11-02 | 2009-12-03 | Gary Liversidge | Modified Release Ticlopidine Compositions |
| JP4809533B2 (en) * | 1998-11-20 | 2011-11-09 | オバン・エナジー・リミテッド | Dispersible phospholipid stabilized microparticles |
| GB9903547D0 (en) | 1999-02-16 | 1999-04-07 | Novartis Ag | Organic compounds |
| US7658938B2 (en) * | 1999-02-22 | 2010-02-09 | Merrion Reasearch III Limited | Solid oral dosage form containing an enhancer |
| US6248363B1 (en) * | 1999-11-23 | 2001-06-19 | Lipocine, Inc. | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| HUP0200483A3 (en) * | 1999-03-11 | 2004-07-28 | Fujisawa Pharmaceutical Co | Liposome preparations |
| US6616942B1 (en) * | 1999-03-29 | 2003-09-09 | Soft Gel Technologies, Inc. | Coenzyme Q10 formulation and process methodology for soft gel capsules manufacturing |
| US7164034B2 (en) | 1999-06-10 | 2007-01-16 | Pfizer Inc. | Alpha2delta ligands for fibromyalgia and other disorders |
| US20080207755A1 (en) * | 2000-05-31 | 2008-08-28 | Pfizer Inc | Alpha 2 Delta Ligands For Fibromyalgia and Other Disorders |
| CA2374760A1 (en) * | 1999-06-18 | 2000-12-28 | Takeda Chemical Industries, Ltd. | Quickly disintegrating solid preparations |
| US7169889B1 (en) * | 1999-06-19 | 2007-01-30 | Biocon Limited | Insulin prodrugs hydrolyzable in vivo to yield peglylated insulin |
| US9006175B2 (en) * | 1999-06-29 | 2015-04-14 | Mannkind Corporation | Potentiation of glucose elimination |
| EP2280004B1 (en) * | 1999-06-29 | 2016-04-20 | MannKind Corporation | Pharmaceutical formulations comprising insulin complexed with a diketopiperazine |
| US6982281B1 (en) * | 2000-11-17 | 2006-01-03 | Lipocine Inc | Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs |
| US20150374826A1 (en) * | 1999-06-30 | 2015-12-31 | Lipocine Inc. | Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs |
| US20030236236A1 (en) * | 1999-06-30 | 2003-12-25 | Feng-Jing Chen | Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs |
| ES2231275T3 (en) | 1999-10-08 | 2005-05-16 | Affinium Pharmaceuticals, Inc. | FAB INHIBITORS I. |
| JP4081273B2 (en) | 1999-10-20 | 2008-04-23 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | Method for stabilizing benzimidazole compounds |
| US7364752B1 (en) | 1999-11-12 | 2008-04-29 | Abbott Laboratories | Solid dispersion pharamaceutical formulations |
| EP1175205B1 (en) * | 1999-11-12 | 2006-06-14 | Abbott Laboratories | Solid dispersion comprising ritonavir, fenofibrate or griseofulvin |
| US20030180352A1 (en) * | 1999-11-23 | 2003-09-25 | Patel Mahesh V. | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| US20060034937A1 (en) * | 1999-11-23 | 2006-02-16 | Mahesh Patel | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| CZ300615B6 (en) * | 1999-11-30 | 2009-07-01 | Panacea Biotec Limited | Fast dissolving pharmaceutical composition in solid dosage form with prolonged sweet taste and process for preparing thereof |
| FR2803538B1 (en) * | 1999-12-15 | 2002-06-07 | Separex Sa | METHOD AND DEVICE FOR CAPTURING FINE PARTICLES BY PERCOLATION IN A BED OF GRANULES |
| CA2397601A1 (en) * | 1999-12-16 | 2001-06-21 | Teva Pharmaceutical Industries, Ltd. | Novel processes for making- and a new crystalline form of- leflunomide |
| US6632429B1 (en) | 1999-12-17 | 2003-10-14 | Joan M. Fallon | Methods for treating pervasive development disorders |
| US20040062803A1 (en) * | 1999-12-22 | 2004-04-01 | Hedden David B. | Sustained-release formulation of a cyclooxygenase-2 inhibitor |
| HUP0204372A3 (en) | 1999-12-23 | 2004-06-28 | Pfizer Prod Inc | Pharmaceutical compositions providing enhanced drug concentrations |
| SK285128B6 (en) * | 1999-12-28 | 2006-07-07 | Zentiva, A. S. | A remedy with controlled release comprising tramadol hydrochloride and method for preparation thereof |
| US20040009229A1 (en) * | 2000-01-05 | 2004-01-15 | Unger Evan Charles | Stabilized nanoparticle formulations of camptotheca derivatives |
| EP1255565A1 (en) * | 2000-01-13 | 2002-11-13 | MERCK PATENT GmbH | Pharmaceutical preparations containing 2-pyrrolidone as the dissolving intermediary |
| SE0000090D0 (en) * | 2000-01-13 | 2000-01-13 | Astrazeneca Ab | Method and apparatus for monitoring |
| ATE340563T1 (en) * | 2000-02-04 | 2006-10-15 | Depomed Inc | SHELL AND CORE TYPE DOSAGE FORM WITH A RELEASE OF ACTIVE INGREDIENTS APPROACHING TO THE ZERO ORDER |
| DE10007771A1 (en) * | 2000-02-14 | 2001-08-23 | Kleine & Steube Entoxin Gmbh | Immunomodulatory compositions, processes for their preparation and their use |
| US6544555B2 (en) | 2000-02-24 | 2003-04-08 | Advancis Pharmaceutical Corp. | Antibiotic product, use and formulation thereof |
| FR2805761B1 (en) * | 2000-03-02 | 2002-08-30 | Mainelab | LIPID NANOCAPSULES, METHOD OF PREPARATION AND USE AS A MEDICAMENT |
| KR20010100194A (en) * | 2000-03-13 | 2001-11-14 | 박호군 | Composition and formulation for solubilization of various compounds and preparation method thereof |
| AU2001244584B2 (en) * | 2000-03-31 | 2006-01-19 | Kirin Pharma Kabushiki Kaisha | Powdery preparation for transmucosal administration containing a polymeric form of drug and exhibiting improved storage stability |
| DK1145717T3 (en) * | 2000-04-13 | 2004-08-02 | Pfizer Prod Inc | Synergistic effect of glyburide and milrinone |
| WO2001078725A2 (en) * | 2000-04-13 | 2001-10-25 | Synthon B.V. | Modified release formulations containing a hypnotic agent |
| US6884778B2 (en) * | 2000-04-14 | 2005-04-26 | William Marsh Rice University | Biocompatible macromers |
| KR100381834B1 (en) * | 2000-05-20 | 2003-04-26 | 이상득 | Solid dispersion system of pranlukast with improved dissolution, and the method thereof |
| FR2809309B1 (en) * | 2000-05-23 | 2004-06-11 | Mainelab | EXTENDED RELEASE MICROSPHERES FOR INJECTION DELIVERY |
| FR2809310B1 (en) * | 2000-05-26 | 2004-02-13 | Centre Nat Rech Scient | USE OF BIGUANIDE DERIVATIVES FOR MANUFACTURING A MEDICINAL PRODUCT HAVING A HEALING EFFECT |
| DE10026698A1 (en) | 2000-05-30 | 2001-12-06 | Basf Ag | Self-emulsifying active ingredient formulation and use of this formulation |
| GB0015617D0 (en) * | 2000-06-26 | 2000-08-16 | Vectura Ltd | Improved preparations for dermal delivery of active substances |
| US20100010101A1 (en) * | 2000-07-05 | 2010-01-14 | Capricorn Pharma, Inc. | Rapid-Melt Compositions and Methods of Making Same |
| AU2001279284A1 (en) * | 2000-07-05 | 2002-01-14 | Capricorn Pharma, Inc | Rapid-melt semi-solid compositions, methods of making same and methods of using same |
| US6375982B1 (en) * | 2000-07-05 | 2002-04-23 | Capricorn Pharma, Inc. | Rapid-melt semi-solid compositions, methods of making same and method of using same |
| USRE44145E1 (en) | 2000-07-07 | 2013-04-09 | A.V. Topchiev Institute Of Petrochemical Synthesis | Preparation of hydrophilic pressure sensitive adhesives having optimized adhesive properties |
| US6967028B2 (en) * | 2000-07-31 | 2005-11-22 | Mainelab | Prolonged release microspheres for injectable administration |
| US6555543B2 (en) | 2000-08-04 | 2003-04-29 | Dmi Biosciences, Inc. | Method of using diketopiperazines and composition containing them |
| DK2116257T3 (en) * | 2000-08-09 | 2013-02-04 | Alk Abello As | Parenteral vaccine formulations and applications thereof |
| US20070053895A1 (en) | 2000-08-14 | 2007-03-08 | Fallon Joan M | Method of treating and diagnosing parkinsons disease and related dysautonomic disorders |
| US6503894B1 (en) | 2000-08-30 | 2003-01-07 | Unimed Pharmaceuticals, Inc. | Pharmaceutical composition and method for treating hypogonadism |
| US20040043061A1 (en) * | 2000-09-15 | 2004-03-04 | Leon Daniel S. | Dissolvable films comprising suspended, non-soluble pharmaceutically active ingredients, apparatus and methods for their manufacture and use |
| ATE270544T1 (en) | 2000-09-22 | 2004-07-15 | Galephar M F | SEMI-SOLID MEDICINAL PREPARATION CONTAINING ISOTRETINOIN |
| US20020119192A1 (en) * | 2000-09-22 | 2002-08-29 | Vishwanathan Narayanan Badri | Controlled release formulations for oral administration |
| CA2423431A1 (en) * | 2000-10-06 | 2002-04-11 | Durect Corporation | Devices and methods for management of inflammation |
| AU2007202061B2 (en) * | 2000-10-06 | 2009-03-05 | Durect Corporation | Devices and methods for management of inflammation |
| US20020068078A1 (en) | 2000-10-13 | 2002-06-06 | Rudnic Edward M. | Antifungal product, use and formulation thereof |
| AU2001211213A1 (en) * | 2000-10-20 | 2002-04-29 | Galephar M/F | Stable oral formulation containing benzimidazole derivative |
| US8030002B2 (en) | 2000-11-16 | 2011-10-04 | Curemark Llc | Methods for diagnosing pervasive development disorders, dysautonomia and other neurological conditions |
| DE10058119A1 (en) * | 2000-11-22 | 2002-05-23 | Bayer Ag | Pharmaceutical kit containing repinotan, for use in acute treatment of neurological disorders such as stroke, including assay composition for determining body repinotan levels to optimize dosage |
| MY137726A (en) * | 2000-11-22 | 2009-03-31 | Nycomed Gmbh | Freeze-dried pantoprazole preparation and pantoprazole injection |
| US20030162733A1 (en) * | 2000-11-27 | 2003-08-28 | Haynes Joel R. | Nucleic acid adjuvants |
| EP1337273A2 (en) * | 2000-11-28 | 2003-08-27 | Transform Pharmaceuticals, Inc. | Pharmaceutical formulations comprising paclitaxel, derivatives, and pharmaceutically acceptable salts thereof |
| US6599522B2 (en) * | 2000-12-01 | 2003-07-29 | Lakshminarayan Rao V. Mokshagundam | Triglyceride reducing agent |
| US20030072807A1 (en) * | 2000-12-22 | 2003-04-17 | Wong Joseph Chung-Tak | Solid particulate antifungal compositions for pharmaceutical use |
| US6884436B2 (en) * | 2000-12-22 | 2005-04-26 | Baxter International Inc. | Method for preparing submicron particle suspensions |
| US6607784B2 (en) * | 2000-12-22 | 2003-08-19 | Baxter International Inc. | Microprecipitation method for preparing submicron suspensions |
| US20040256749A1 (en) * | 2000-12-22 | 2004-12-23 | Mahesh Chaubal | Process for production of essentially solvent-free small particles |
| US7193084B2 (en) * | 2000-12-22 | 2007-03-20 | Baxter International Inc. | Polymorphic form of itraconazole |
| US8067032B2 (en) | 2000-12-22 | 2011-11-29 | Baxter International Inc. | Method for preparing submicron particles of antineoplastic agents |
| US9700866B2 (en) * | 2000-12-22 | 2017-07-11 | Baxter International Inc. | Surfactant systems for delivery of organic compounds |
| US20030096013A1 (en) * | 2000-12-22 | 2003-05-22 | Jane Werling | Preparation of submicron sized particles with polymorph control |
| US20050048126A1 (en) * | 2000-12-22 | 2005-03-03 | Barrett Rabinow | Formulation to render an antimicrobial drug potent against organisms normally considered to be resistant to the drug |
| US20030054015A1 (en) * | 2000-12-25 | 2003-03-20 | Shinichiro Haze | Sympathetic-activating perfume composition |
| US7060675B2 (en) * | 2001-02-15 | 2006-06-13 | Nobex Corporation | Methods of treating diabetes mellitus |
| US6867183B2 (en) * | 2001-02-15 | 2005-03-15 | Nobex Corporation | Pharmaceutical compositions of insulin drug-oligomer conjugates and methods of treating diseases therewith |
| CA2994779C (en) | 2001-02-19 | 2020-08-25 | Novartis Ag | Use of 40-o-(2-hydroxyethyl)-rapamycin for inhibiting growth of a solid tumour of the brain other than lymphatic cancer |
| US6524615B2 (en) * | 2001-02-21 | 2003-02-25 | Kos Pharmaceuticals, Incorporated | Controlled release pharmaceutical composition |
| US6534088B2 (en) * | 2001-02-22 | 2003-03-18 | Skyepharma Canada Inc. | Fibrate-statin combinations with reduced fed-fasted effects |
| KR100510356B1 (en) * | 2001-02-27 | 2005-08-24 | 룀 게엠베하 운트 콤파니 카게 | Pharmaceutical formulations comprising a coating and binding agent with improved storage stability and process for the preparation thereof |
| US6777000B2 (en) * | 2001-02-28 | 2004-08-17 | Carrington Laboratories, Inc. | In-situ gel formation of pectin |
| US7494669B2 (en) * | 2001-02-28 | 2009-02-24 | Carrington Laboratories, Inc. | Delivery of physiological agents with in-situ gels comprising anionic polysaccharides |
| FR2821747B1 (en) * | 2001-03-09 | 2004-07-02 | Ethypharm Lab Prod Ethiques | SUSPENSION OF TELITHROMYCIN WITH A MASK TASTE |
| KR200249057Y1 (en) * | 2001-03-22 | 2001-10-19 | 김진환 | Sewage backflow integrated into the lid and base. Odor Prevention Device |
| DE60230934D1 (en) * | 2001-04-06 | 2009-03-05 | Affinium Pharm Inc | FAB I INHIBITORS |
| US7431710B2 (en) | 2002-04-08 | 2008-10-07 | Glaukos Corporation | Ocular implants with anchors and methods thereof |
| US6503532B1 (en) * | 2001-04-13 | 2003-01-07 | Murty Pharmaceuticals, Inc. | Pharmaceutical composition containing tetrahydrocannabinol and a transdermal/transcutaneous delivery method thereof |
| US8728445B2 (en) | 2001-05-01 | 2014-05-20 | A.V. Topchiev Institute Of Petrochemical Synthesis, Russian Academy Of Sciences | Hydrogel Compositions |
| US20050215727A1 (en) | 2001-05-01 | 2005-09-29 | Corium | Water-absorbent adhesive compositions and associated methods of manufacture and use |
| DE60239528D1 (en) | 2001-05-01 | 2011-05-05 | Corium International Redwood City | TWO-PHASE, WATER-ABSORBING BIOADHESIVE COMPOSITION |
| WO2002087543A1 (en) * | 2001-05-01 | 2002-11-07 | Biozone Laboratories, Inc. | Sustained release formulations for nifedipine, dextromethorphan, and danazol |
| US8840918B2 (en) | 2001-05-01 | 2014-09-23 | A. V. Topchiev Institute of Petrochemical Synthesis, Russian Academy of Sciences | Hydrogel compositions for tooth whitening |
| US8541021B2 (en) | 2001-05-01 | 2013-09-24 | A.V. Topchiev Institute Of Petrochemical Synthesis | Hydrogel compositions demonstrating phase separation on contact with aqueous media |
| US8206738B2 (en) | 2001-05-01 | 2012-06-26 | Corium International, Inc. | Hydrogel compositions with an erodible backing member |
| US20050113510A1 (en) | 2001-05-01 | 2005-05-26 | Feldstein Mikhail M. | Method of preparing polymeric adhesive compositions utilizing the mechanism of interaction between the polymer components |
| US20030070584A1 (en) * | 2001-05-15 | 2003-04-17 | Cynthia Gulian | Dip coating compositions containing cellulose ethers |
| US20030072731A1 (en) * | 2001-05-15 | 2003-04-17 | Cynthia Gulian | Dip coating compositions containing starch or dextrin |
| AU4061702A (en) * | 2001-05-15 | 2003-04-03 | Mcneil-Ppc, Inc. | Dip coating compositions containing starch or dextrin |
| JP2002348474A (en) * | 2001-05-23 | 2002-12-04 | Dow Corning Toray Silicone Co Ltd | Polyorganosiloxane microemulsion composition and cosmetic raw material |
| JP4865958B2 (en) | 2001-05-23 | 2012-02-01 | 株式会社トクホン | Analgesic anti-inflammatory patch with local action |
| US20080058424A1 (en) * | 2002-05-23 | 2008-03-06 | Cephalon, Inc. | Novel pharmaceutical formulations of modafinil |
| MXPA03010705A (en) * | 2001-05-25 | 2004-05-27 | Cephalon Inc | Solid pharmaceutical formulations comprising modafinil. |
| US20030070679A1 (en) * | 2001-06-01 | 2003-04-17 | Boehringer Ingelheim Pharma Kg | Capsules containing inhalable tiotropium |
| US6828297B2 (en) * | 2001-06-04 | 2004-12-07 | Nobex Corporation | Mixtures of insulin drug-oligomer conjugates comprising polyalkylene glycol, uses thereof, and methods of making same |
| US6828305B2 (en) * | 2001-06-04 | 2004-12-07 | Nobex Corporation | Mixtures of growth hormone drug-oligomer conjugates comprising polyalkylene glycol, uses thereof, and methods of making same |
| US6713452B2 (en) | 2001-06-04 | 2004-03-30 | Nobex Corporation | Mixtures of calcitonin drug-oligomer conjugates comprising polyalkylene glycol, uses thereof, and methods of making same |
| US7713932B2 (en) | 2001-06-04 | 2010-05-11 | Biocon Limited | Calcitonin drug-oligomer conjugates, and uses thereof |
| US6835802B2 (en) | 2001-06-04 | 2004-12-28 | Nobex Corporation | Methods of synthesizing substantially monodispersed mixtures of polymers having polyethylene glycol moieties |
| US7244703B2 (en) * | 2001-06-22 | 2007-07-17 | Bentley Pharmaceuticals, Inc. | Pharmaceutical compositions and methods for peptide treatment |
| ITMI20011337A1 (en) * | 2001-06-26 | 2002-12-26 | Farmatron Ltd | ORAL PHARMACEUTICAL COMPOSITIONS WITH MODIFIED RELEASE OF THE ACTIVE SUBSTANCE |
| ITMI20011338A1 (en) * | 2001-06-26 | 2002-12-26 | Farmatron Ltd | ORAL PHARMACEUTICAL COMPOSITIONS WITH IMMEDIATE RELEASE OF THE ACTIVE INGREDIENT |
| US7014867B2 (en) * | 2001-06-28 | 2006-03-21 | Ucb Farchim Sa | Tablet comprising cetirizine and pseudoephedrine |
| FR2826549A1 (en) * | 2001-06-28 | 2003-01-03 | Roquette Freres | PROCESS FOR THE PREPARATION OF A COMPRESSED EDULCORANT TABLET AND A COMPRESSED EDULCORANT THUS OBTAINED |
| US7173064B2 (en) * | 2001-07-09 | 2007-02-06 | Repros Therapeutics Inc. | Methods and compositions with trans-clomiphene for treating wasting and lipodystrophy |
| CN1954807A (en) * | 2001-07-09 | 2007-05-02 | 佐纳根有限公司 | Clomiphene compositions rich in trans-clomiphene |
| US7737185B2 (en) * | 2001-07-09 | 2010-06-15 | Repros Therapeutics Inc. | Methods and compositions with trans-clomiphene |
| CN100335045C (en) * | 2001-07-17 | 2007-09-05 | 出光兴产株式会社 | Preventive agent for ascites in poultry |
| US6720002B2 (en) * | 2001-07-20 | 2004-04-13 | R.P. Scherer Technologies, Inc. | Antihistamine formulations for soft capsule dosage forms |
| GB0118300D0 (en) * | 2001-07-26 | 2001-09-19 | Cortendo Ab | Formulations |
| FR2827770B1 (en) * | 2001-07-27 | 2005-08-19 | Gattefosse Ets Sa | ORAL PHARMACEUTICAL COMPOSITION COMPRISING AN ACTIVE INGREDIENT LIKELY TO BE SUBSTANTIALLY EFFECT OF FIRST INTESTINAL PASSAGE |
| US20080305173A1 (en) * | 2001-07-31 | 2008-12-11 | Beuford Arlie Bogue | Amorphous drug beads |
| US20030068375A1 (en) | 2001-08-06 | 2003-04-10 | Curtis Wright | Pharmaceutical formulation containing gelling agent |
| US20030153590A1 (en) * | 2001-08-14 | 2003-08-14 | Oy Contral Pharma Ltd | Method of treating alcoholism or alcohol abuse |
| US7097868B2 (en) * | 2001-08-23 | 2006-08-29 | Bio-Dar Ltd. | Stable coated microcapsules |
| US6488952B1 (en) * | 2001-08-28 | 2002-12-03 | John P. Kennedy | Semisolid therapeutic delivery system and combination semisolid, multiparticulate, therapeutic delivery system |
| EP1429728A1 (en) * | 2001-08-29 | 2004-06-23 | SRL Technologies, Inc. | Sustained release preparations |
| WO2003020210A2 (en) * | 2001-08-29 | 2003-03-13 | Umd, Inc. | Vaginal delivery of chemotherapeutic agents and inhibitors of membrane efflux systems for cancer therapy |
| US7338971B2 (en) * | 2001-08-30 | 2008-03-04 | El-Naggar Mawaheb M | Treatment of inflammatory, cancer, and thrombosis disorders |
| US7166571B2 (en) * | 2001-09-07 | 2007-01-23 | Biocon Limited | Insulin polypeptide-oligomer conjugates, proinsulin polypeptide-oligomer conjugates and methods of synthesizing same |
| US6913903B2 (en) * | 2001-09-07 | 2005-07-05 | Nobex Corporation | Methods of synthesizing insulin polypeptide-oligomer conjugates, and proinsulin polypeptide-oligomer conjugates and methods of synthesizing same |
| US7196059B2 (en) * | 2001-09-07 | 2007-03-27 | Biocon Limited | Pharmaceutical compositions of insulin drug-oligomer conjugates and methods of treating diseases therewith |
| US7030082B2 (en) * | 2001-09-07 | 2006-04-18 | Nobex Corporation | Pharmaceutical compositions of drug-oligomer conjugates and methods of treating disease therewith |
| US7312192B2 (en) * | 2001-09-07 | 2007-12-25 | Biocon Limited | Insulin polypeptide-oligomer conjugates, proinsulin polypeptide-oligomer conjugates and methods of synthesizing same |
| US6770625B2 (en) | 2001-09-07 | 2004-08-03 | Nobex Corporation | Pharmaceutical compositions of calcitonin drug-oligomer conjugates and methods of treating diseases therewith |
| US20030091634A1 (en) * | 2001-09-14 | 2003-05-15 | Pawan Seth | Delayed release tablet of venlafaxin |
| US20060003012A9 (en) | 2001-09-26 | 2006-01-05 | Sean Brynjelsen | Preparation of submicron solid particle suspensions by sonication of multiphase systems |
| MXPA04002446A (en) | 2001-09-26 | 2004-07-23 | Baxter Int | Preparation of submicron sized nanoparticles via dispersion and solvent or liquid phase removal. |
| US8309118B2 (en) | 2001-09-28 | 2012-11-13 | Mcneil-Ppc, Inc. | Film forming compositions containing sucralose |
| ES2261782T3 (en) * | 2001-10-10 | 2006-11-16 | Boehringer Ingelheim Pharmaceuticals Inc. | DUST TREATMENT WITH PRESSURIZED GASEOUS FLUIDS. |
| US7112340B2 (en) * | 2001-10-19 | 2006-09-26 | Baxter International Inc. | Compositions of and method for preparing stable particles in a frozen aqueous matrix |
| US20030091630A1 (en) * | 2001-10-25 | 2003-05-15 | Jenny Louie-Helm | Formulation of an erodible, gastric retentive oral dosage form using in vitro disintegration test data |
| US20030152622A1 (en) * | 2001-10-25 | 2003-08-14 | Jenny Louie-Helm | Formulation of an erodible, gastric retentive oral diuretic |
| CA2409552A1 (en) | 2001-10-25 | 2003-04-25 | Depomed, Inc. | Gastric retentive oral dosage form with restricted drug release in the lower gastrointestinal tract |
| RU2311903C2 (en) * | 2001-11-07 | 2007-12-10 | Синтон Б.В. | Tamzulosin tablets |
| US20040142902A1 (en) * | 2001-11-08 | 2004-07-22 | Struijker- Boudier Harry A.J. | Implant dosage form and use thereof for the delivery of a cholosterol lowering agent |
| JP4031232B2 (en) * | 2001-11-09 | 2008-01-09 | カプスゲル・ジャパン株式会社 | New capsule |
| ITMI20012366A1 (en) * | 2001-11-09 | 2003-05-09 | Farmatron Ltd | THERAPEUTIC SYSTEMS STABILIZED WITH IMMEDIATE RELEASE AND / OR MODIFIED FOR THE ORAL ADMINISTRATION OF ACTIVE AND / OR EXCIPIENT PRINCIPLES AND / OR WINGS |
| WO2003045357A1 (en) * | 2001-11-27 | 2003-06-05 | Transform Pharmaceuticals, Inc. | Oral pharmaceutical formulations comprising paclitaxel, derivatives and methods of administration thereof |
| US6455557B1 (en) | 2001-11-28 | 2002-09-24 | Elan Pharmaceuticals, Inc. | Method of reducing somnolence in patients treated with tizanidine |
| US20040220240A1 (en) * | 2001-11-28 | 2004-11-04 | Pellegrini Cara A. | Method of increasing the extent of absorption of tizanidine |
| US6652891B2 (en) | 2001-12-12 | 2003-11-25 | Herbasway Laboratories, Llc | Co-enzyme Q10 dietary supplement |
| US7183321B2 (en) * | 2001-12-17 | 2007-02-27 | Bristol-Myers Squibb Company | Antidiabetic formulation and method |
| AU2002353659A1 (en) * | 2001-12-18 | 2003-07-15 | Synthon B.V. | Simvastatin dosage forms |
| US20050220869A1 (en) * | 2001-12-20 | 2005-10-06 | Federico Stroppolo | Particulate compositions |
| EP1455594B2 (en) * | 2001-12-20 | 2015-07-22 | Société des Produits Nestlé S.A. | A food product containing gel capsules or tablets |
| US6992191B2 (en) * | 2001-12-20 | 2006-01-31 | Teva Pharmaceutical Industries, Ltd. | Hydrogenation of precursors to thiazolidinedione antihyperglycemics |
| US20030139386A1 (en) * | 2001-12-21 | 2003-07-24 | Sophie Cote | Pharmaceutical compositions based on azetidine derivatives |
| FR2834212B1 (en) * | 2001-12-27 | 2004-07-09 | Besins Int Belgique | USE OF IMMEDIATE RELEASE POWDER IN PHARMACEUTICAL AND NUTRACEUTICAL COMPOSITIONS |
| US20030165566A1 (en) * | 2002-01-10 | 2003-09-04 | O'toole Edel | Sedative non-benzodiazepine formulations |
| SE0200154D0 (en) * | 2002-01-21 | 2002-01-21 | Galenica Ab | New process |
| US20030162827A1 (en) * | 2002-01-30 | 2003-08-28 | Suresh Venkataram | HMG CoA reductase inhibiting composition, method of preparation thereof and method for competitively inhibiting HMG CoA reductase using such composition |
| US20040116514A1 (en) * | 2002-01-31 | 2004-06-17 | Hoyoku Nishino | Compositions for preventing human cancer and method of preventing human cancer |
| GB0203296D0 (en) * | 2002-02-12 | 2002-03-27 | Glaxo Group Ltd | Novel composition |
| ES2302913T3 (en) * | 2002-02-14 | 2008-08-01 | Dsm Ip Assets B.V. | FORMULATIONS BASED ON COENZYME Q-10. |
| GB2398005B (en) * | 2002-02-20 | 2005-09-14 | Strides Arcolab Ltd | Orally administrable pharmaceutical formulation |
| US8128957B1 (en) | 2002-02-21 | 2012-03-06 | Valeant International (Barbados) Srl | Modified release compositions of at least one form of tramadol |
| US20050182056A9 (en) * | 2002-02-21 | 2005-08-18 | Seth Pawan | Modified release formulations of at least one form of tramadol |
| US20030161875A1 (en) * | 2002-02-27 | 2003-08-28 | Deepak Murpani | Fast dissolving tablets of cyclooxygenase-2 enzyme inhibitors |
| GB0205868D0 (en) * | 2002-03-13 | 2002-04-24 | Univ Nottingham | Polymer composite with internally distributed deposition matter |
| WO2003080149A2 (en) | 2002-03-20 | 2003-10-02 | Mannkind Corporation | Inhalation apparatus |
| US20030219482A1 (en) * | 2002-03-21 | 2003-11-27 | Chaudhari Sunil Sudhakar | Multiparticulate compositions for once-a-day administration |
| KR101061351B1 (en) | 2002-04-09 | 2011-08-31 | 플라멜 테크놀로지스 | Oral Suspension of Active Ingredient Microcapsules |
| US8100844B2 (en) * | 2002-04-25 | 2012-01-24 | Ultraflex Systems, Inc. | Ambulating ankle and knee joints with bidirectional dampening and assistance using elastomeric restraint |
| GB0210397D0 (en) | 2002-05-07 | 2002-06-12 | Ferring Bv | Pharmaceutical formulations |
| US20040043070A1 (en) * | 2002-05-14 | 2004-03-04 | Ayres James W. | Hot melt coating by direct blending and coated substrates |
| US20050215552A1 (en) * | 2002-05-17 | 2005-09-29 | Gadde Kishore M | Method for treating obesity |
| KR101072885B1 (en) | 2002-05-17 | 2011-10-17 | 듀크 유니버시티 | Method for treating obesity |
| US7229644B2 (en) * | 2002-05-23 | 2007-06-12 | Cephalon, Inc. | Pharmaceutical formulations of modafinil |
| US20040033257A1 (en) * | 2002-05-30 | 2004-02-19 | Strides Inc. | Pharmaceutical formulation in a drug delivery system and process for preparing the same |
| US6824763B2 (en) | 2002-05-30 | 2004-11-30 | Kimberly-Clark Worldwide, Inc. | Anti-fungal powder having enhanced excipient properties |
| US20030224046A1 (en) * | 2002-06-03 | 2003-12-04 | Vinay Rao | Unit-dose combination composition for the simultaneous delivery of a short-acting and a long-acting oral hypoglycemic agent |
| DE10227232A1 (en) * | 2002-06-18 | 2004-01-15 | Aventis Pharma Deutschland Gmbh | Sour insulin preparations with improved stability |
| AR039744A1 (en) * | 2002-06-26 | 2005-03-09 | Alza Corp | METHODS AND DOSAGE FORMS TO INCREASE THE SOLUBILITY OF PHARMACOS COMPOSITIONS FOR CONTROLLED ADMINISTRATION |
| KR20050054483A (en) * | 2002-06-28 | 2005-06-10 | 알자 코포레이션 | Transdermal drug delivery devices having coated microprotrusions |
| US20040005339A1 (en) * | 2002-06-28 | 2004-01-08 | Shojaei Amir H. | Formulations of fenofibrate and/or fenofibrate derivatives with improved oral bioavailability |
| WO2004002458A1 (en) * | 2002-06-28 | 2004-01-08 | Shire Laboratories Inc. | Formulations of fenofibrate and/or fenofibrate derivatives with improved oral bioavailability |
| US7771707B2 (en) | 2004-06-12 | 2010-08-10 | Collegium Pharmaceutical, Inc. | Abuse-deterrent drug formulations |
| US10004729B2 (en) | 2002-07-05 | 2018-06-26 | Collegium Pharmaceutical, Inc. | Tamper-resistant pharmaceutical compositions of opioids and other drugs |
| KR20040010306A (en) * | 2002-07-22 | 2004-01-31 | (주)나노하이브리드 | A Hybrid Of Itraconazole, Cyclosporine Or Carvedilol With A Layered Silicate And A Process For Preparing The Same |
| DE10234260A1 (en) * | 2002-07-27 | 2004-02-05 | Beiersdorf Ag | Soap-containing cleaning substrate |
| US20050208132A1 (en) * | 2002-07-29 | 2005-09-22 | Gayatri Sathyan | Methods and dosage forms for reducing side effects of benzisozazole derivatives |
| AU2003256848A1 (en) * | 2002-07-29 | 2004-02-16 | Alza Corporation | Formulations and dosage forms for controlled delivery of topiramate |
| US20050232995A1 (en) * | 2002-07-29 | 2005-10-20 | Yam Nyomi V | Methods and dosage forms for controlled delivery of paliperidone and risperidone |
| GB2399084B (en) * | 2002-07-30 | 2007-01-31 | Univ Liverpool | Porous beads and method of production thereof |
| US7429619B2 (en) * | 2002-08-02 | 2008-09-30 | Mcneil Consumer Healthcare | Polyacrylic film forming compositions |
| GB2391472B (en) * | 2002-08-02 | 2004-12-08 | Satishchandra Punambhai Patel | Pharmaceutical compositions |
| US20040033262A1 (en) * | 2002-08-19 | 2004-02-19 | Orchid Health Care | Sustained release pharmaceutical composition of a cephalosporin antibiotic |
| US6986884B2 (en) * | 2002-09-04 | 2006-01-17 | Rosenberg E William | Composition and method for treating soft nails |
| JP4279254B2 (en) * | 2002-09-05 | 2009-06-17 | ニューロジェシックス, インコーポレイテッド | Compositions and kits for removal of irritating compounds from body surfaces |
| US8524200B2 (en) | 2002-09-11 | 2013-09-03 | The Procter & Gamble Company | Tooth whitening products |
| US20040116532A1 (en) * | 2002-09-13 | 2004-06-17 | Craig Heacock | Pharmaceutical formulations of modafinil |
| US7785627B2 (en) | 2002-09-20 | 2010-08-31 | Watson Pharmaceuticals, Inc. | Pharmaceutical formulation containing a biguanide and a thiazolidinedione derivative |
| US8084058B2 (en) | 2002-09-20 | 2011-12-27 | Watson Pharmaceuticals, Inc. | Pharmaceutical formulation containing a biguanide and a thiazolidinedione derivative |
| US7959946B2 (en) | 2002-09-20 | 2011-06-14 | Watson Pharmaceuticals, Inc. | Pharmaceutical formulation containing a biguanide and a thiazolidinedione derivative |
| US9060941B2 (en) * | 2002-09-20 | 2015-06-23 | Actavis, Inc. | Pharmaceutical formulation containing a biguanide and a thiazolidinedione derivative |
| WO2004026256A2 (en) * | 2002-09-20 | 2004-04-01 | Alpharma, Inc. | Sustained-release opioid formulations and methods of use |
| US20040220153A1 (en) * | 2002-09-24 | 2004-11-04 | Jost-Price Edward Roydon | Methods and reagents for the treatment of diseases and disorders associated with increased levels of proinflammatory cytokines |
| US20040062778A1 (en) * | 2002-09-26 | 2004-04-01 | Adi Shefer | Surface dissolution and/or bulk erosion controlled release compositions and devices |
| US6702850B1 (en) * | 2002-09-30 | 2004-03-09 | Mediplex Corporation Korea | Multi-coated drug-eluting stent for antithrombosis and antirestenosis |
| DK1571970T3 (en) * | 2002-10-02 | 2011-11-28 | Dmi Biosciences Inc | Diagnosis and monitoring of diseases |
| US6966990B2 (en) | 2002-10-11 | 2005-11-22 | Ferro Corporation | Composite particles and method for preparing |
| US8487002B2 (en) * | 2002-10-25 | 2013-07-16 | Paladin Labs Inc. | Controlled-release compositions |
| TWI319713B (en) * | 2002-10-25 | 2010-01-21 | Sustained-release tramadol formulations with 24-hour efficacy | |
| CA2503150A1 (en) * | 2002-10-31 | 2004-05-21 | Supergen, Inc. | Pharmaceutical formulations targeting specific regions of the gastrointestinal tract |
| DE10251963A1 (en) * | 2002-11-08 | 2004-05-19 | Lts Lohmann Therapie-Systeme Ag | Wafer-form transmucosal dosage form, comprising solution of active agent, e.g. for combating drug abuse, in phosphatidyl choline fraction, providing both rapid and constant release via the oral cavity |
| MXPA05005038A (en) * | 2002-11-12 | 2005-07-01 | Teva Pharma | Pharmaceutical compositions and dosage forms for buccal and sublingual delivery of tizanidine and methods of administering tizanidine sublingually or bucally. |
| KR100508518B1 (en) * | 2002-11-13 | 2005-08-17 | 한미약품 주식회사 | Method for the preparation of paclitaxel solid dispersion by using the supercritical fluid process and paclitaxel solid dispersion prepared thereby |
| US9561182B2 (en) * | 2003-08-22 | 2017-02-07 | Cure Pharmaceutical Corporation | Edible films for administration of medicaments to animals, methods for their manufacture and methods for their use for the treatment of animals |
| US8999372B2 (en) * | 2002-11-14 | 2015-04-07 | Cure Pharmaceutical Corporation | Methods for modulating dissolution, bioavailability, bioequivalence and drug delivery profile of thin film drug delivery systems, controlled-release thin film dosage formats, and methods for their manufacture and use |
| US20040131662A1 (en) | 2003-11-12 | 2004-07-08 | Davidson Robert S. | Method and apparatus for minimizing heat, moisture, and shear damage to medicants and other compositions during incorporation of same with edible films |
| US20040191302A1 (en) | 2003-03-28 | 2004-09-30 | Davidson Robert S. | Method and apparatus for minimizing heat, moisture, and shear damage to medicants and other compositions during incorporation of same with edible films |
| US20040109927A1 (en) * | 2002-11-27 | 2004-06-10 | Ang Jit Fu | Carbonate-based anti-caking agent with reduced gas release properties |
| CA2507685A1 (en) * | 2002-11-28 | 2004-06-10 | Themis Laboratories Private Limited | Process for manufacturing sustained release microbeads containing venlafaxine hci |
| ES2518316T3 (en) | 2002-12-06 | 2014-11-05 | Debiopharm International Sa | Heterocyclic compounds, their manufacturing methods and their use in therapy |
| US7670627B2 (en) * | 2002-12-09 | 2010-03-02 | Salvona Ip Llc | pH triggered targeted controlled release systems for the delivery of pharmaceutical active ingredients |
| US7166671B2 (en) * | 2002-12-10 | 2007-01-23 | Cellresin Technologies, Llc | Grafted cyclodextrin |
| US7385004B2 (en) * | 2002-12-10 | 2008-06-10 | Cellresin Technologies, Llc | Enhanced lubrication in polyolefin closure with polyolefin grafted cyclodextrin |
| US8129450B2 (en) | 2002-12-10 | 2012-03-06 | Cellresin Technologies, Llc | Articles having a polymer grafted cyclodextrin |
| US20040115226A1 (en) * | 2002-12-12 | 2004-06-17 | Wenji Li | Free-flowing solid formulations with improved bio-availability of poorly water soluble drugs and process for making the same |
| NZ523128A (en) * | 2002-12-12 | 2006-01-27 | Ashmont Holdings Ltd | Anthelmintic formulations containing avermectins and or milbemycins |
| PL377520A1 (en) * | 2002-12-13 | 2006-02-06 | Warner-Lambert Company Llc | Pregabalin derivatives for the treatment of fibromyalgia and other disorders |
| US20040115287A1 (en) * | 2002-12-17 | 2004-06-17 | Lipocine, Inc. | Hydrophobic active agent compositions and methods |
| US7160830B2 (en) * | 2002-12-18 | 2007-01-09 | Albemarle Netherlands, B.V. | Process for the preparation of catalyst microspheres |
| IN192381B (en) * | 2002-12-20 | 2004-04-10 | Ranbaxy Lab | |
| EP1578193A4 (en) * | 2002-12-23 | 2011-06-15 | Vical Inc | Method for freeze-drying nucleic acid/block copolymer/cationic surfactant complexes |
| US7381422B2 (en) * | 2002-12-23 | 2008-06-03 | Vical Incorporated | Method for producing sterile polynucleotide based medicaments |
| US20040127551A1 (en) * | 2002-12-27 | 2004-07-01 | Kai Zhang | Taxane-based compositions and methods of use |
| US20040197408A1 (en) * | 2002-12-30 | 2004-10-07 | Angiotech International Ag | Amino acids in micelle preparation |
| US20040142903A1 (en) * | 2003-01-16 | 2004-07-22 | Par Pharmaceutical Inc. | Bioavailable fenofibrate compositions, methods for treating hyperlipidemia and hypercholesterolemia and processes for the preparation of such compositions |
| US20080213363A1 (en) * | 2003-01-23 | 2008-09-04 | Singh Nikhilesh N | Methods and compositions for delivering 5-HT3 antagonists across the oral mucosa |
| US20040162273A1 (en) * | 2003-01-23 | 2004-08-19 | The Procter & Gamble Company | Powder pharmaceutical compositions |
| US20040147564A1 (en) * | 2003-01-29 | 2004-07-29 | Rao Vinay U. | Combinations of glimepiride and the thiazolidinedione for treatment of diabetes |
| US20040186155A1 (en) * | 2003-01-30 | 2004-09-23 | Dayno Jeffrey Marc | Combination therapy for the treatment or prevention of migraine |
| US20040156894A1 (en) * | 2003-02-07 | 2004-08-12 | Grother Leon Paul | Use of edible acids in fast-dispersing pharmaceutical solid dosage forms |
| US7083748B2 (en) * | 2003-02-07 | 2006-08-01 | Ferro Corporation | Method and apparatus for continuous particle production using supercritical fluid |
| US6931888B2 (en) * | 2003-02-07 | 2005-08-23 | Ferro Corporation | Lyophilization method and apparatus for producing particles |
| SI21402A (en) * | 2003-02-12 | 2004-08-31 | LEK farmacevtska dru�ba d.d. | Lined particles and pharmaceutical forms |
| JP2006518751A (en) * | 2003-02-20 | 2006-08-17 | サンタラス インコーポレイティッド | Novel formulation for rapid and sustained suppression of gastric acid, omeprazole antacid complex-immediate release |
| US20050220870A1 (en) * | 2003-02-20 | 2005-10-06 | Bonnie Hepburn | Novel formulation, omeprazole antacid complex-immediate release for rapid and sustained suppression of gastric acid |
| WO2005005010A2 (en) * | 2003-02-21 | 2005-01-20 | Ferro Corporation | Methods and apparatus for producing composite particles using supercritical fluid as plasticizing and extracting agent |
| US7638138B2 (en) * | 2003-02-21 | 2009-12-29 | Translational Research, Ltd. | Compositions for nasal administration of pharmaceuticals |
| US20040185119A1 (en) * | 2003-02-26 | 2004-09-23 | Theuer Richard C. | Method and compositions for treating gastric hyperacidity while diminishing the likelihood of producing vitamin deficiency |
| FR2853831A1 (en) * | 2003-03-05 | 2004-10-22 | Usv Ltd | SOLID DOSAGE FOR ORAL USE OF METFORMIN AND GLYBURIDE AND PROCESS FOR PREPARING THE SAME |
| BRPI0408323A (en) * | 2003-03-14 | 2006-03-07 | Nirmal Mulye | process for the preparation of prolonged release tablets |
| US7858607B2 (en) * | 2003-03-14 | 2010-12-28 | Mamchur Stephen A | System for use by compounding pharmacists to produce hormone replacement medicine customized for each consumer |
| DE602004016831D1 (en) | 2003-03-17 | 2008-11-13 | Affinium Pharm Inc | PHARMACEUTICAL COMPOSITIONS INHIBITORS OF FAB I AND OTHER ANTIBIOTICS CONTAINED |
| US20040185009A1 (en) * | 2003-03-19 | 2004-09-23 | Dexcel Pharma Technologies Ltd. | Composition and device for treating periodontal diseases |
| EP1622603A4 (en) * | 2003-03-25 | 2010-03-24 | Kiel Lab Inc | Phenolic acid salts of gabapentin in solid dosage forms and methods of use |
| WO2004093866A1 (en) * | 2003-03-25 | 2004-11-04 | Kiel Laboratories, Inc. | Process for preparing phenolic acid salts of gabapentin |
| US20040191298A1 (en) * | 2003-03-26 | 2004-09-30 | Fredrik Nicklasson | New formulations and use thereof |
| KR100974482B1 (en) | 2003-03-27 | 2010-08-10 | 가부시키가이샤 바이오악티스 | Powder medicine applicator for nasal cavity |
| US20050152967A1 (en) * | 2003-03-28 | 2005-07-14 | Pfab, Lp | Dynamic variable release |
| WO2004084870A1 (en) * | 2003-03-28 | 2004-10-07 | Sigmoid Biotechnologies Limited | Solid oral dosage form containing seamless microcapsules |
| CA2520321A1 (en) * | 2003-04-04 | 2004-10-14 | Pharmacia Corporation | Oral extended release compressed tablets of multiparticulates |
| CA2521925A1 (en) * | 2003-04-08 | 2004-10-28 | Algorx Pharmaceuticals, Inc. | Preparation and purification of synthetic capsaicin |
| WO2004091506A2 (en) * | 2003-04-10 | 2004-10-28 | Ivax Research, Inc. | Taxane-based compositions and methods of use |
| US20040208932A1 (en) * | 2003-04-17 | 2004-10-21 | Ramachandran Thembalath | Stabilized paroxetine hydrochloride formulation |
| TR200300510A2 (en) * | 2003-04-18 | 2004-11-22 | Sanovel �La� Sanay� Ve T�Caret A.�. | Dispersing alendronate microparticle formulation |
| JP2006524260A (en) * | 2003-04-23 | 2006-10-26 | ヒューマン バイオシステムズ | Improved methods and solutions for donor organ storage |
| MXPA05011557A (en) * | 2003-04-29 | 2006-03-09 | Orexigen Therapeutics Inc | Compositions for affecting weight loss. |
| ES2277030T3 (en) * | 2003-05-02 | 2007-07-01 | Dexcel Ltd. | FORMULATION IN TABLETS OF VENLAFAXINE OF PROLONGED RELEASE. |
| US7030102B1 (en) * | 2003-05-06 | 2006-04-18 | Bioactives, Llc | Highly bioavailable coenzyme Q-10 cyclodextrin complex |
| BRPI0410098A (en) * | 2003-05-06 | 2006-05-16 | Nirmal Mulye | controlled release formulation of erythromycin derivatives |
| PE20050150A1 (en) * | 2003-05-08 | 2005-03-22 | Altana Pharma Ag | A DOSAGE FORM CONTAINING (S) -PANTOPRAZOLE AS AN ACTIVE INGREDIENT |
| CL2004000983A1 (en) * | 2003-05-08 | 2005-03-04 | Altana Pharma Ag | ORAL PHARMACEUTICAL COMPOSITION IN THE FORM OF A TABLET THAT INCLUDES DIHYDRATED MAGNETIC PANTOPRAZOL, WHERE THE TABLET FORM IS COMPOSED BY A NUCLEUS, A MIDDLE COAT AND AN OUTER LAYER; AND USE OF PHARMACEUTICAL COMPOSITION IN ULCERAS AND |
| US20060008531A1 (en) * | 2003-05-08 | 2006-01-12 | Ferro Corporation | Method for producing solid-lipid composite drug particles |
| GB0310919D0 (en) * | 2003-05-13 | 2003-06-18 | West Pharm Serv Drug Res Ltd | Pharmaceutical compositions |
| US20070141071A1 (en) * | 2003-05-14 | 2007-06-21 | Oregon State University | Hot melt coating by direct blending and coated substrates |
| ES2575563T3 (en) * | 2003-05-15 | 2016-06-29 | Ampio Pharmaceuticals, Inc. | Treatment of diseases mediated by T lymphocytes |
| US20040234579A1 (en) * | 2003-05-22 | 2004-11-25 | Mark D. Finke, Inc. | Dietary supplements and methods of preparing and administering dietary supplements |
| IN2003MU00504A (en) * | 2003-06-05 | 2005-05-13 | Alembic Ltd | |
| WO2004110492A2 (en) * | 2003-06-06 | 2004-12-23 | Glaxo Group Limited | Composition comprising triptans and nsaids |
| US20050020546A1 (en) * | 2003-06-11 | 2005-01-27 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin D compounds |
| US7655692B2 (en) * | 2003-06-12 | 2010-02-02 | Pfizer Inc. | Process for forming amorphous atorvastatin |
| SE0301880D0 (en) * | 2003-06-25 | 2003-06-25 | Astrazeneca Uk Ltd | New drug delivery composition |
| US7314640B2 (en) * | 2003-07-11 | 2008-01-01 | Mongkol Sriwongjanya | Formulation and process for drug loaded cores |
| EP1498122A1 (en) * | 2003-07-18 | 2005-01-19 | Aventis Pharma S.A. | Semi-solid systems containing azetidine derivatives |
| MXPA06000529A (en) * | 2003-07-18 | 2006-08-11 | Santarus Inc | Pharmaceutical composition for inhibiting acid secretion. |
| US20060246116A1 (en) * | 2003-07-18 | 2006-11-02 | Petworks, Llc | Formula and method for the delivery of medications to animals |
| EP1648417A4 (en) * | 2003-07-18 | 2010-01-20 | Santarus Inc | Pharmaceutical formulation and method for treating acid-caused gastrointestinal disorders |
| WO2005016253A2 (en) * | 2003-07-18 | 2005-02-24 | Petworks, Llc | Formula nd method for the delivery of oral medications to animals |
| US8993599B2 (en) * | 2003-07-18 | 2015-03-31 | Santarus, Inc. | Pharmaceutical formulations useful for inhibiting acid secretion and methods for making and using them |
| US8313775B2 (en) | 2003-07-21 | 2012-11-20 | Shionogi Inc. | Antibiotic product, use and formulation thereof |
| JP2006528185A (en) | 2003-07-21 | 2006-12-14 | アドバンシス ファーマスーティカル コーポレイション | Antibiotic preparations, their use and preparation |
| US8313776B2 (en) | 2003-07-21 | 2012-11-20 | Shionogi Inc. | Antibiotic product, use and formulation thereof |
| US20050058707A1 (en) * | 2003-08-06 | 2005-03-17 | Iran Reyes | Uniform delivery of topiramate over prolonged period of time with enhanced dispersion formulation |
| NZ545747A (en) | 2003-08-06 | 2010-06-25 | Senomyx Inc | T1R hetero-oligomeric taste receptors, cell lines that express said receptors, and taste compounds |
| US20060286037A1 (en) * | 2003-08-08 | 2006-12-21 | Ono Pharmaceutical Co., Ltd. | Heart-slowing drug containing short-acting ß blocker as the active ingredient |
| CA2524300C (en) * | 2003-08-08 | 2008-10-28 | Biovail Laboratories International Srl | Modified-release tablet of bupropion hydrochloride |
| EP1653925A1 (en) | 2003-08-11 | 2006-05-10 | Advancis Pharmaceutical Corporation | Robust pellet |
| WO2005013960A1 (en) * | 2003-08-12 | 2005-02-17 | Kyungdong Pharm. Co., Ltd. | Preparing method for controlled released type tablet tamsulosin hcl and the tablet thereof |
| JP2007502294A (en) | 2003-08-12 | 2007-02-08 | アドバンシス ファーマスーティカル コーポレイション | Antibiotic preparations, their use and preparation |
| US20050089502A1 (en) * | 2003-08-21 | 2005-04-28 | Todd Schansberg | Effervescent delivery system |
| AU2004268549A1 (en) * | 2003-08-22 | 2005-03-10 | Alza Corporation | Stepwise delivery of topiramate over prolonged period of time |
| US20050107350A1 (en) * | 2003-08-22 | 2005-05-19 | Pharmacia Corporation | Method for the treatment or prevention of bone disorders with a cyclooxygenase-2 inhibitor alone and in combination with a bone disorder treatment agent and compositions therewith |
| SI2792363T1 (en) * | 2003-08-26 | 2016-11-30 | Shire Biopharmaceuticals Holdings Ireland Limited | Pharmaceutical formulation comprising lanthanum compounds |
| US20050085479A1 (en) * | 2003-08-27 | 2005-04-21 | Pharmacia Corporation | Mediated central nervous system compositions of a cyclooxygenase-2 selective inhibitor and a corticotropin releasing factor antagonist for the treatment of ischemic disorders or injury |
| US8377952B2 (en) * | 2003-08-28 | 2013-02-19 | Abbott Laboratories | Solid pharmaceutical dosage formulation |
| US8025899B2 (en) * | 2003-08-28 | 2011-09-27 | Abbott Laboratories | Solid pharmaceutical dosage form |
| US20050187278A1 (en) * | 2003-08-28 | 2005-08-25 | Pharmacia Corporation | Treatment or prevention of vascular disorders with Cox-2 inhibitors in combination with cyclic AMP-specific phosphodiesterase inhibitors |
| CA2535780A1 (en) | 2003-08-29 | 2005-03-17 | Advancis Pharmaceuticals Corporation | Antibiotic product, use and formulation thereof |
| GB0503942D0 (en) * | 2005-02-25 | 2005-04-06 | Novartis Ag | Purification process |
| GB0320312D0 (en) * | 2003-08-29 | 2003-10-01 | Novartis Ag | Purification process |
| US20050197512A1 (en) * | 2003-08-29 | 2005-09-08 | Ulrich Beutler | Purification process |
| JP2005075804A (en) * | 2003-09-03 | 2005-03-24 | Toyo Capsule Kk | Medicinal composition including menatetrenone |
| WO2005023225A1 (en) * | 2003-09-05 | 2005-03-17 | Ranbaxy Laboratories Limited | Cilostazol adsorbate |
| US20050058670A1 (en) * | 2003-09-09 | 2005-03-17 | Jong-Soo Woo | Oral itraconazole composition which is not affected by ingested food and process for preparing same |
| GB0321256D0 (en) * | 2003-09-11 | 2003-10-08 | Generics Uk Ltd | Novel crystalline compounds |
| JP2007513869A (en) | 2003-09-15 | 2007-05-31 | アドバンシス ファーマスーティカル コーポレイション | Antibiotic preparations, their use and preparation |
| US20050059583A1 (en) | 2003-09-15 | 2005-03-17 | Allergan, Inc. | Methods of providing therapeutic effects using cyclosporin components |
| EP1670325A1 (en) | 2003-09-29 | 2006-06-21 | Soft Gel Technologies, Inc. | SOLUBILIZED CoQ-10 |
| US8124072B2 (en) | 2003-09-29 | 2012-02-28 | Soft Gel Technologies, Inc. | Solubilized CoQ-10 |
| NZ546349A (en) * | 2003-09-29 | 2010-03-26 | Cipla Ltd | Pharmaceutical formulation with improved stability |
| US20050069590A1 (en) * | 2003-09-30 | 2005-03-31 | Buehler Gail K. | Stable suspensions for medicinal dosages |
| BR0318535A (en) * | 2003-09-30 | 2006-09-12 | Lupin Ltd | pharmaceutical composition for controlled drug delivery, process for preparing a pharmaceutical composition and controlled release composition |
| TWI372066B (en) * | 2003-10-01 | 2012-09-11 | Wyeth Corp | Pantoprazole multiparticulate formulations |
| AR045957A1 (en) * | 2003-10-03 | 2005-11-16 | Novartis Ag | PHARMACEUTICAL COMPOSITION AND COMBINATION |
| US9173847B2 (en) * | 2003-10-10 | 2015-11-03 | Veloxis Pharmaceuticals A/S | Tablet comprising a fibrate |
| EP1680086A2 (en) * | 2003-10-10 | 2006-07-19 | LifeCycle Pharma A/S | A solid dosage form comprising a fibrate and a statin |
| US20050220881A1 (en) * | 2003-10-10 | 2005-10-06 | Bvm Holding Co. | Pharmaceutical composition |
| US20050096390A1 (en) * | 2003-10-10 | 2005-05-05 | Per Holm | Compositions comprising fenofibrate and pravastatin |
| US20070014846A1 (en) * | 2003-10-10 | 2007-01-18 | Lifecycle Pharma A/S | Pharmaceutical compositions comprising fenofibrate and atorvastatin |
| CA2540984C (en) | 2003-10-10 | 2011-02-08 | Lifecycle Pharma A/S | A solid dosage form comprising a fibrate |
| US20060172006A1 (en) * | 2003-10-10 | 2006-08-03 | Vincent Lenaerts | Sustained-release tramadol formulations with 24-hour clinical efficacy |
| US20050096391A1 (en) * | 2003-10-10 | 2005-05-05 | Per Holm | Compositions comprising fenofibrate and rosuvastatin |
| US20050084531A1 (en) * | 2003-10-16 | 2005-04-21 | Jatin Desai | Tablet with aqueous-based sustained release coating |
| US7338171B2 (en) * | 2003-10-27 | 2008-03-04 | Jen-Chuen Hsieh | Method and apparatus for visual drive control |
| US20070092562A1 (en) * | 2003-10-28 | 2007-04-26 | Spi Pharma, Inc. | Product and process for increasing compactibility of carbohydrates |
| WO2005044193A2 (en) * | 2003-10-28 | 2005-05-19 | Spi Pharma, Inc. | Product and process for increasing compactibility of carbohydrates |
| US20050096365A1 (en) * | 2003-11-03 | 2005-05-05 | David Fikstad | Pharmaceutical compositions with synchronized solubilizer release |
| US20060003002A1 (en) * | 2003-11-03 | 2006-01-05 | Lipocine, Inc. | Pharmaceutical compositions with synchronized solubilizer release |
| US8987322B2 (en) * | 2003-11-04 | 2015-03-24 | Circ Pharma Research And Development Limited | Pharmaceutical formulations for carrier-mediated transport statins and uses thereof |
| EP2210605B1 (en) * | 2003-11-04 | 2017-03-01 | TCD Royalty Sub, LLC | Once daily dosage forms of trospium |
| EP1680100A4 (en) | 2003-11-04 | 2012-08-08 | Supernus Pharmaceuticals Inc | Compositions of quaternary ammonium containing bioavailability enhancers |
| SE0302924D0 (en) * | 2003-11-05 | 2003-11-05 | Camurus Ab | Pharmaceutical composition having a cationic excipient |
| US20070292498A1 (en) * | 2003-11-05 | 2007-12-20 | Warren Hall | Combinations of proton pump inhibitors, sleep aids, buffers and pain relievers |
| WO2005046662A2 (en) * | 2003-11-07 | 2005-05-26 | Jj Pharma, Inc. | Hdl-boosting combination therapy complexes |
| US20050101605A1 (en) * | 2003-11-07 | 2005-05-12 | Ahmed Salah U. | Oral liquid formulations of methotrexate |
| DK1530965T3 (en) * | 2003-11-11 | 2006-07-17 | Mattern Udo | Controlled release delivery system for nasal application |
| US8784869B2 (en) | 2003-11-11 | 2014-07-22 | Mattern Pharma Ag | Controlled release delivery system for nasal applications and methods of treatment |
| DE60305043T2 (en) * | 2003-11-13 | 2006-11-30 | Ferring B.V. | Blister packaging and solid dosage form containing desmopressin |
| US7387793B2 (en) | 2003-11-14 | 2008-06-17 | Eurand, Inc. | Modified release dosage forms of skeletal muscle relaxants |
| US7470435B2 (en) * | 2003-11-17 | 2008-12-30 | Andrx Pharmaceuticals, Llc | Extended release venlafaxine formulation |
| EP1614412A1 (en) † | 2003-11-18 | 2006-01-11 | Helm AG | Process for the manufacture of free-flowing, powdery atorvastatin adsorbates |
| WO2005062722A2 (en) * | 2003-11-21 | 2005-07-14 | Sun Pharmaceutical Industries Limited | Fexofenadine containing pharmaceutical formulation |
| CZ300438B6 (en) * | 2003-11-25 | 2009-05-20 | Pliva Hrvatska D.O.O. | Process for preparing solid medicament form for oral administration with instantaneous release of active substance and containing as the active substance finasteride polymorphous form |
| US7201920B2 (en) | 2003-11-26 | 2007-04-10 | Acura Pharmaceuticals, Inc. | Methods and compositions for deterring abuse of opioid containing dosage forms |
| WO2005060943A1 (en) * | 2003-11-28 | 2005-07-07 | Glenmark Pharmaceuticals Ltd. | Antifungal oral dosage forms and the methods preparation |
| US20050118265A1 (en) * | 2003-11-28 | 2005-06-02 | Anandi Krishnan | Antifungal oral dosage forms and the methods for preparation |
| WO2005053727A2 (en) * | 2003-11-29 | 2005-06-16 | Sangstat Medical Corporation | Pharmaceutical compositions for bioactive peptide agents |
| CA2547774A1 (en) * | 2003-12-04 | 2005-06-16 | Pfizer Products Inc. | Method for making pharmaceutical multiparticulates |
| BRPI0416534A (en) * | 2003-12-04 | 2007-01-09 | Pfizer Prod Inc | multiparticulate compositions with improved stability |
| US6984403B2 (en) * | 2003-12-04 | 2006-01-10 | Pfizer Inc. | Azithromycin dosage forms with reduced side effects |
| WO2005053640A2 (en) * | 2003-12-04 | 2005-06-16 | Pfizer Products Inc. | Azithromycin multiparticulate dosage forms by liquid-based processes |
| WO2005053639A2 (en) * | 2003-12-04 | 2005-06-16 | Pfizer Products Inc. | Controlled release multiparticulates formed with dissolution enhancers |
| WO2005053652A1 (en) | 2003-12-04 | 2005-06-16 | Pfizer Products Inc. | Multiparticulate crystalline drug compositions containing a poloxamer and a glyceride |
| CN1889931A (en) * | 2003-12-04 | 2007-01-03 | 辉瑞产品公司 | Spray-congeal process using an extruder for preparing multiparticulate azithromycin compositions containing preferably a poloxamer and a glyceride |
| ES2600577T3 (en) * | 2003-12-04 | 2017-02-09 | Bend Research, Inc. | Spray-solidification process using an extruder to prepare multiparticulate compositions of crystalline drugs |
| AU2004296784B2 (en) | 2003-12-08 | 2010-12-02 | Cpex Pharmaceuticals, Inc. | Pharmaceutical compositions and methods for insulin treatment |
| US9359585B2 (en) * | 2003-12-08 | 2016-06-07 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Stable nonaqueous reactive skin care and cleansing compositions having a continuous and a discontinuous phase |
| ITMI20032399A1 (en) * | 2003-12-09 | 2005-06-10 | Zambon Spa | PHARMACEUTICAL COMPOSITION CONTAINING GABAPENTIN. |
| ATE301455T1 (en) * | 2003-12-09 | 2005-08-15 | Helm Ag | PHARMACEUTICAL PREPARATION CONTAINING VALACICLOVIR |
| US7446101B1 (en) * | 2003-12-12 | 2008-11-04 | Bioactives, Llc | Bioavailable carotenoid-cyclodextrin formulations for soft-gels and other encapsulation systems |
| US6783772B1 (en) * | 2003-12-12 | 2004-08-31 | Sanjeev Khandelwal | Pharmaceutical preparations containing alendronate sodium |
| JP5183068B2 (en) * | 2003-12-22 | 2013-04-17 | フィンレイ,ウォーレン,エイチ | Powder formation by atmospheric spray freeze drying |
| US7846462B2 (en) * | 2003-12-22 | 2010-12-07 | Unilever Home & Personal Care Usa, Division Of Conopco, Inc. | Personal care implement containing a stable reactive skin care and cleansing composition |
| US20050136121A1 (en) * | 2003-12-22 | 2005-06-23 | Shear/Kershman Laboratories, Inc. | Oral peptide delivery system with improved bioavailability |
| AR046773A1 (en) * | 2003-12-23 | 2005-12-21 | Novartis Ag | PHARMACEUTICAL FORMULATIONS OF BISPHOSPHONATES |
| EP1701706A2 (en) * | 2003-12-29 | 2006-09-20 | Alza Corporation | Novel drug compositions and dosage forms |
| JP2007517063A (en) * | 2003-12-29 | 2007-06-28 | アルザ・コーポレーシヨン | Novel pharmaceutical compositions and dosage forms of topiramate |
| US20050175696A1 (en) * | 2003-12-29 | 2005-08-11 | David Edgren | Drug granule coatings that impart smear resistance during mechanical compression |
| BRPI0418330A (en) * | 2003-12-31 | 2007-05-02 | Pfizer Prod Inc | solid compositions of low solubility drugs and poloxamers |
| CA2551254A1 (en) * | 2003-12-31 | 2005-07-21 | Pfizer Products Inc. | Stabilized pharmaceutical solid compositions of low-solubility drugs, poloxamers, and stabilizing polymers |
| AU2005204378B2 (en) * | 2004-01-12 | 2009-01-22 | Mannkind Corporation | A method of reducing serum proinsulin levels in type 2 diabetics |
| US7713959B2 (en) * | 2004-01-13 | 2010-05-11 | Duke University | Compositions of an anticonvulsant and mirtazapine to prevent weight gain |
| KR20060128995A (en) * | 2004-01-13 | 2006-12-14 | 듀크 유니버시티 | Compositions of an anticonvulsant and an antipsychotic drug for affecting weight loss |
| US20050152887A1 (en) * | 2004-01-14 | 2005-07-14 | Doctor's Signature Sales And Marketing International Corp. [Dba Life Force International | Protonic formulation |
| US7658721B2 (en) * | 2004-01-16 | 2010-02-09 | Biodel Inc. | Sublingual drug delivery device |
| KR20160106194A (en) | 2004-01-22 | 2016-09-09 | 유니버시티 오브 마이애미 | Topical co-enzyme q10 formulations and methodns of use |
| US20050164952A1 (en) * | 2004-01-23 | 2005-07-28 | Vital Pharmaceuticals, Inc. | Delivery system for growth hormone releasing peptides |
| CN1929819B (en) | 2004-01-30 | 2012-02-29 | 考里安国际公司 | Rapidly dissolving film for delivery of an active agent |
| JP2007520555A (en) * | 2004-02-05 | 2007-07-26 | バクスター・インターナショナル・インコーポレイテッド | Dispersants prepared by use of self-stabilizing agents |
| AU2005213472A1 (en) * | 2004-02-10 | 2005-08-25 | Santarus, Inc. | Combination of proton pump inhibitor, buffering agent, and nonsteroidal anti-inflammatory agent |
| KR100582350B1 (en) * | 2004-02-17 | 2006-05-22 | 한미약품 주식회사 | Tamsulosin hydrochloride composition for oral administration and controlled-release granule formulation thereof |
| JP4874651B2 (en) * | 2004-02-17 | 2012-02-15 | エーザイ・アール・アンド・ディー・マネジメント株式会社 | Soft capsule |
| KR20150038745A (en) * | 2004-02-17 | 2015-04-08 | 트랜스셉트 파마슈티칼스, 인코포레이티드 | Compositions for delivering hypnotic agents across the oral mucosa and methods of use thereof |
| US8642079B2 (en) * | 2004-02-23 | 2014-02-04 | Hormos Medical Corporation | Solid formulations of ospemifene |
| US20050196355A1 (en) * | 2004-03-03 | 2005-09-08 | Constantine Georgiades | Film products having controlled disintegration properties |
| US20070269519A1 (en) * | 2004-03-03 | 2007-11-22 | Constantine Georgiades | Whitening products |
| US20080003248A1 (en) * | 2004-03-03 | 2008-01-03 | Constantine Georgiades | Whitening products |
| WO2005087198A1 (en) * | 2004-03-10 | 2005-09-22 | Ranbaxy Laboratories Limited | Processes for the preparation of solid dosage forms of amorphous valganciclovir hydrochloride |
| US20080248999A1 (en) * | 2007-04-04 | 2008-10-09 | Biodel Inc. | Amylin formulations |
| US20080085298A1 (en) * | 2004-03-12 | 2008-04-10 | Biodel, Inc. | Rapid Mucosal Gel or Film Insulin Compositions |
| US20080090753A1 (en) | 2004-03-12 | 2008-04-17 | Biodel, Inc. | Rapid Acting Injectable Insulin Compositions |
| US20080096800A1 (en) * | 2004-03-12 | 2008-04-24 | Biodel, Inc. | Rapid mucosal gel or film insulin compositions |
| EP1591114A1 (en) * | 2004-03-12 | 2005-11-02 | Fournier Laboratories Ireland Limited | Use of metformin and orlistat for the treatment or prevention of obesity |
| ES2398838T3 (en) * | 2004-03-12 | 2013-03-22 | Biodel, Inc. | Fast-acting drug delivery compositions |
| US20050202079A1 (en) * | 2004-03-15 | 2005-09-15 | Mylan Pharmaceuticals Inc. | Novel orally administrable formulation of nitrofurantoin and a method for preparing said formulation |
| US7468428B2 (en) | 2004-03-17 | 2008-12-23 | App Pharmaceuticals, Llc | Lyophilized azithromycin formulation |
| US20050208145A1 (en) * | 2004-03-19 | 2005-09-22 | Thava Vasanthan | Grain fiber compositions and methods of use |
| WO2005094842A1 (en) * | 2004-03-30 | 2005-10-13 | Transition Therapeutics Inc. | Vitamin b12-containing compositions and methods of use |
| US8003122B2 (en) * | 2004-03-31 | 2011-08-23 | Cordis Corporation | Device for local and/or regional delivery employing liquid formulations of therapeutic agents |
| US7989490B2 (en) * | 2004-06-02 | 2011-08-02 | Cordis Corporation | Injectable formulations of taxanes for cad treatment |
| US20070225337A1 (en) * | 2004-03-31 | 2007-09-27 | Sandoz Ag | Novel Co-Precipitate of Amorphous Rosiglitazone |
| WO2005097061A1 (en) * | 2004-04-01 | 2005-10-20 | Procyte Corporation | Encapsulated peptide copper complexes and compositions and methods related thereto |
| US20050226927A1 (en) * | 2004-04-02 | 2005-10-13 | Impax Laboratories, Inc. | Pharmaceutical dosage forms having immediate release and/or controlled release properties that contain a GABAB receptor agonist |
| US8007827B2 (en) * | 2004-04-02 | 2011-08-30 | Impax Laboratories, Inc. | Pharmaceutical dosage forms having immediate release and/or controlled release properties |
| US20050220873A1 (en) * | 2004-04-02 | 2005-10-06 | Chien-Hsuan Han | Pharmaceutical dosage forms having immediate and controlled release properties that contain a GABAB receptor agonist |
| US7850987B2 (en) * | 2004-04-08 | 2010-12-14 | Micronutrient, Llc | Nutrient composition(s) and system(s) for individualized, responsive dosing regimens |
| US7785619B2 (en) * | 2004-04-08 | 2010-08-31 | Micro Nutrient, Llc | Pharmanutrient composition(s) and system(s) for individualized, responsive dosing regimens |
| US20050226906A1 (en) * | 2004-04-08 | 2005-10-13 | Micro Nutrient, Llc | Nutrient system for individualized responsive dosing regimens |
| WO2005117870A2 (en) * | 2004-04-16 | 2005-12-15 | Santarus, Inc. | Combination of proton pump inhibitor, buffering agent, and prokinetic agent |
| WO2005105040A2 (en) * | 2004-04-26 | 2005-11-10 | Micelle Products, Inc. | Water-soluble formulations of fat soluble vitamins and pharmaceutical agents and their applications |
| CN1968692A (en) * | 2004-05-03 | 2007-05-23 | 杜克大学 | Compositions for affecting weight loss |
| EP1750669A1 (en) * | 2004-05-04 | 2007-02-14 | Boehringer Ingelheim International Gmbh | Solid pharmaceutical form comprising an ltb4 antagonist |
| US9149482B2 (en) * | 2004-05-10 | 2015-10-06 | Lupin Ltd. | Pharmaceutical formulation of cefixime for enhanced bioavailability |
| US20060127468A1 (en) * | 2004-05-19 | 2006-06-15 | Kolodney Michael S | Methods and related compositions for reduction of fat and skin tightening |
| US7754230B2 (en) * | 2004-05-19 | 2010-07-13 | The Regents Of The University Of California | Methods and related compositions for reduction of fat |
| ES2372499T3 (en) * | 2004-05-19 | 2012-01-20 | Los Angeles Biomedical Research Institute At Harbor-Ucla Medical Center | USE OF A DETERGENT FOR NON-SURGICAL FAT DISPOSAL. |
| US20080286359A1 (en) * | 2004-05-24 | 2008-11-20 | Richard John Dansereau | Low Dosage Forms Of Risedronate Or Its Salts |
| US7645460B2 (en) * | 2004-05-24 | 2010-01-12 | The Procter & Gamble Company | Dosage forms of risedronate |
| US7645459B2 (en) | 2004-05-24 | 2010-01-12 | The Procter & Gamble Company | Dosage forms of bisphosphonates |
| ATE480592T1 (en) * | 2004-05-24 | 2010-09-15 | Cellresin Tech Llc | AMPHOTERE GRAFTED BARRIER MATERIALS |
| US20080287400A1 (en) * | 2004-05-24 | 2008-11-20 | Richard John Dansereau | Low Dosage Forms Of Risedronate Or Its Salts |
| US8815916B2 (en) * | 2004-05-25 | 2014-08-26 | Santarus, Inc. | Pharmaceutical formulations useful for inhibiting acid secretion and methods for making and using them |
| US8906940B2 (en) * | 2004-05-25 | 2014-12-09 | Santarus, Inc. | Pharmaceutical formulations useful for inhibiting acid secretion and methods for making and using them |
| US20050265955A1 (en) * | 2004-05-28 | 2005-12-01 | Mallinckrodt Inc. | Sustained release preparations |
| PL1828167T3 (en) | 2004-06-04 | 2015-02-27 | Debiopharm Int Sa | Acrylamide derivatives as antibiotic agents |
| EP1750862B1 (en) | 2004-06-04 | 2011-01-05 | Teva Pharmaceutical Industries Ltd. | Pharmaceutical composition containing irbesartan |
| US20060002986A1 (en) * | 2004-06-09 | 2006-01-05 | Smithkline Beecham Corporation | Pharmaceutical product |
| EP1753398B1 (en) * | 2004-06-10 | 2018-09-19 | Glatt Air Techniques, Inc. | Controlled release matrix pharmaceutical dosage formulation |
| US20060002999A1 (en) * | 2004-06-17 | 2006-01-05 | Forest Laboratories, Inc. | Immediate release formulations of 1-aminocyclohexane compounds, memantine and neramexane |
| ITMI20041295A1 (en) | 2004-06-25 | 2004-09-25 | Cosmo Spa | ORAL ANTI-MICROBIAL PHARMACEUTICAL COMPOSITIONS |
| WO2006005017A2 (en) * | 2004-06-30 | 2006-01-12 | Valeant Research & Development | Oral composition comprising carbamylating agent |
| WO2006014427A1 (en) | 2004-07-02 | 2006-02-09 | Advancis Pharmaceutical Corporation | Tablet for pulsed delivery |
| KR100553160B1 (en) * | 2004-07-09 | 2006-02-22 | 한국콜마 주식회사 | Nano sized phospholipid liposome composition comprising coenzym Q10 and manufacturing method thereof |
| TWI302944B (en) * | 2004-07-12 | 2008-11-11 | Method for removing virus activity from biological materials | |
| WO2006019916A1 (en) * | 2004-07-14 | 2006-02-23 | Repros Therapeutics Inc. | Trans-clomiphene for the treatment of benign prostate hypertrophy, prostate cancer, hypogonadism, elevated triglycerides and high cholesterol |
| US7875700B2 (en) | 2004-07-19 | 2011-01-25 | Biocon Limited | Cation complexes of insulin compound conjugates, formulation and uses thereof |
| DE102004035938A1 (en) * | 2004-07-23 | 2006-02-16 | Röhm GmbH & Co. KG | Process for the preparation of coated drug forms with stable drug release profile |
| CA2574925A1 (en) * | 2004-07-26 | 2006-02-09 | Teva Pharmaceutical Industries Ltd. | Pharmaceutical dosage forms including rasagiline |
| WO2006078320A2 (en) | 2004-08-04 | 2006-07-27 | Brookwood Pharmaceuticals, Inc. | Methods for manufacturing delivery devices and devices thereof |
| RU2393877C2 (en) | 2004-08-05 | 2010-07-10 | Кориум Интернэшнл, Инк. | Adhesive composition |
| WO2006016530A1 (en) * | 2004-08-10 | 2006-02-16 | Translational Research, Ltd. | Transnasal composition having immediate action and high absorbability |
| US20060039966A1 (en) * | 2004-08-12 | 2006-02-23 | Miller Bruce A Jr | Dosage delivery apparatus for improving user acceptance of oral supplements and medicaments and methods for manufacturing same |
| DE102004039270A1 (en) * | 2004-08-13 | 2006-02-23 | J. S. Staedtler Gmbh & Co. Kg | Moldable mass e.g. in the model building and/or prototype building, comprises dried (by supplying energy source) and hardened (by using microwave equipment) mass |
| EP1791531A1 (en) * | 2004-08-20 | 2007-06-06 | Alpharma, Inc. | Paroxetine formulations |
| JP5078014B2 (en) | 2004-08-20 | 2012-11-21 | マンカインド コーポレイション | Catalytic reaction of diketopiperazine synthesis. |
| MX2007002189A (en) | 2004-08-23 | 2008-01-11 | Mannkind Corp | Diketopiperazine salts, diketomorpholine salts or diketodioxane salts for drug delivery. |
| US8119153B2 (en) * | 2004-08-26 | 2012-02-21 | Boston Scientific Scimed, Inc. | Stents with drug eluting coatings |
| WO2006024138A1 (en) * | 2004-08-30 | 2006-03-09 | Taro Pharmaceutical Industries Ltd. | A thermoreversible pharmaceutical formulation for anti-microbial agents comprising poloxamer polymers and hydroxy fatty acid ester of polyethylene glycol |
| US20060134212A1 (en) * | 2004-09-02 | 2006-06-22 | Forest Laboratories, Inc. | Lercanidipine immediate release compositions |
| US20060165789A1 (en) * | 2004-09-09 | 2006-07-27 | Forest Laboratories, Inc. | Lercanidipine modified release compositions |
| US20060165788A1 (en) * | 2004-09-09 | 2006-07-27 | Wattanaporn Abramowitz | Lercanidipine pH dependent pulsatile release compositions |
| US8750983B2 (en) * | 2004-09-20 | 2014-06-10 | P Tech, Llc | Therapeutic system |
| PL1811979T3 (en) * | 2004-09-27 | 2009-04-30 | Sigmoid Pharma Ltd | Microcapsules comprising a methylxanthine and a corticosteroid |
| US20060115499A1 (en) * | 2004-09-27 | 2006-06-01 | Alk-Abello A/S | Liquid allergy vaccine formulation for oromucosal administration |
| US20060198838A1 (en) * | 2004-09-28 | 2006-09-07 | Fallon Joan M | Combination enzyme for cystic fibrosis |
| US7740875B2 (en) * | 2004-10-08 | 2010-06-22 | Mediquest Therapeutics, Inc. | Organo-gel formulations for therapeutic applications |
| US20060078580A1 (en) | 2004-10-08 | 2006-04-13 | Mediquest Therapeutics, Inc. | Organo-gel formulations for therapeutic applications |
| US20060079514A1 (en) * | 2004-10-13 | 2006-04-13 | Victory Pharma Incorporated | Methods and compositions including methscopolamine bromide |
| US20060079513A1 (en) * | 2004-10-13 | 2006-04-13 | Preston David M | Methods and compositions including methscopolamine nitrate |
| KR100591786B1 (en) * | 2004-10-19 | 2006-06-26 | 휴먼팜 주식회사 | Pharmaceutical composition containing pranlukast and preparation process thereof |
| SG10201404796QA (en) | 2004-10-20 | 2014-10-30 | Endorech Inc | Sex steroid precursors alone or in combination with a selective estrogen receptor modulator and/or with estrogens and/or a type 5 cgmp phosphodiesterase inhibitor for the prevention and treatment of vaginal dryness and sexual dysfunction in postmenopausal women |
| US20060088591A1 (en) * | 2004-10-22 | 2006-04-27 | Jinghua Yuan | Tablets from a poorly compressible substance |
| WO2006047022A1 (en) | 2004-10-25 | 2006-05-04 | Virginia Commonwealth University | Nuclear sulfated oxysterol, potent regulator of cholesterol homeostasis, for therapy of hypercholesterolemia, hyperlipidemia, and atherosclerosis |
| KR101086254B1 (en) * | 2004-11-04 | 2011-11-24 | 에스케이케미칼주식회사 | Soild dispersion composition of pranlukast with improved bioavailability and the method of preparing the solid dispersion |
| US20060193908A1 (en) * | 2004-11-09 | 2006-08-31 | Burnside Beth A | Extended release formulations of poorly soluble antibiotics |
| WO2006054175A2 (en) * | 2004-11-18 | 2006-05-26 | Aurobindo Pharma Limited | Stable dosage forms of acid labile drug |
| FR2878161B1 (en) * | 2004-11-23 | 2008-10-31 | Flamel Technologies Sa | ORAL MEDICINE FORM, SOLID AND DESIGNED TO AVOID MEASUREMENT |
| FR2878158B1 (en) * | 2004-11-24 | 2009-01-16 | Flamel Technologies Sa | ORAL PHARMACEUTICAL FORM, SOLID MICROPARTICULAR DESIGNED TO PREVENT MEASUREMENT |
| US20060148903A1 (en) * | 2004-11-24 | 2006-07-06 | Algorx Pharmaceuticals, Inc. | Capsaicinoid gel formulation and uses thereof |
| CA2588296A1 (en) | 2004-11-24 | 2006-06-01 | Neuromolecular Pharmaceuticals, Inc. | Composition comprising an nmda receptor antagonist and levodopa and use thereof for treating neurological disease |
| US9149433B2 (en) * | 2004-11-30 | 2015-10-06 | Basf Corporation | Method for formation of micro-prilled polymers |
| CN101132781A (en) * | 2004-12-09 | 2008-02-27 | 茵西斯医疗公司 | Room-temperature stable dronabinol formulations |
| KR101118199B1 (en) | 2004-12-20 | 2012-03-15 | 주식회사 삼양홀딩스 | Polymeric micelle composition for solubilizing tacrolimus |
| EP1842557B1 (en) | 2004-12-22 | 2013-10-23 | Nitto Denko Corporation | Drug carrier and drug carrier kit for inhibiting fibrosis |
| US20120269886A1 (en) | 2004-12-22 | 2012-10-25 | Nitto Denko Corporation | Therapeutic agent for pulmonary fibrosis |
| DE102005042875A1 (en) | 2004-12-23 | 2006-09-21 | Grünenthal GmbH | Fast-release dosage forms for antibiotics |
| US20060246003A1 (en) * | 2004-12-27 | 2006-11-02 | Eisai Co. Ltd. | Composition containing anti-dementia drug |
| US20060280789A1 (en) * | 2004-12-27 | 2006-12-14 | Eisai Research Institute | Sustained release formulations |
| US20070129402A1 (en) * | 2004-12-27 | 2007-06-07 | Eisai Research Institute | Sustained release formulations |
| AU2005320547B2 (en) * | 2004-12-27 | 2009-02-05 | Eisai R & D Management Co., Ltd. | Method for stabilizing anti-dementia drug |
| US20090208579A1 (en) * | 2004-12-27 | 2009-08-20 | Eisai R & D Management Co., Ltd. | Matrix Type Sustained-Release Preparation Containing Basic Drug or Salt Thereof, and Method for Manufacturing the Same |
| US20060140820A1 (en) | 2004-12-28 | 2006-06-29 | Udo Mattern | Use of a container of an inorganic additive containing plastic material |
| CN100434068C (en) * | 2004-12-30 | 2008-11-19 | 天津药物研究院 | Bexarotene gel and its preparation method |
| US20060147518A1 (en) * | 2004-12-30 | 2006-07-06 | Pierre Fabre Medicament | Stable solid dispersion of a derivative of vinca alkaloid and process for manufacturing it |
| US7758884B2 (en) * | 2005-01-28 | 2010-07-20 | Kemin Industries, Inc. | Formulation for increasing the deposition of dietary carotenoids in eggs |
| BRPI0606528A8 (en) | 2005-02-04 | 2018-03-13 | Repros Therapeutics Inc | composition for use in the manufacture of male infertility treatment medicines |
| US20060198886A1 (en) * | 2005-03-01 | 2006-09-07 | Jenkins Richard B | Medicament having coated methenamine combined with acidifier |
| WO2006092449A2 (en) * | 2005-03-02 | 2006-09-08 | Metanomics Gmbh | Process for the production of fine chemicals |
| FR2883179B1 (en) * | 2005-03-18 | 2009-04-17 | Ethypharm Sa | COATED TABLET |
| RU2413508C2 (en) * | 2005-03-22 | 2011-03-10 | Репрос Терапьютикс Инк. | Trans-clomiphene dosing regimen |
| AU2006230557A1 (en) * | 2005-03-31 | 2006-10-05 | King Pharmaceuticals Research & Development, Inc. | Controlled release pharmaceutical compositions of liothyronine and methods of making and using the same |
| EP1865941B8 (en) * | 2005-04-01 | 2010-12-22 | Neurogesx, Inc. | Oils of capsaicinoids and methods of making and using the same |
| US20090156545A1 (en) * | 2005-04-01 | 2009-06-18 | Hostetler Karl Y | Substituted Phosphate Esters of Nucleoside Phosphonates |
| WO2006137953A1 (en) | 2005-04-01 | 2006-12-28 | The Regents Of The Univerisity Of California | Phosphono-pent-2-en-1-yl nucleosides and analogs |
| JP5002904B2 (en) * | 2005-04-04 | 2012-08-15 | ブラザー工業株式会社 | Radio tag communication apparatus, communication method thereof, and communication program thereof |
| DK1874282T3 (en) * | 2005-04-06 | 2010-10-25 | Adamas Pharmaceuticals Inc | Methods and Preparations for the Treatment of CNS Disorders |
| ES2326354B1 (en) * | 2005-04-13 | 2010-07-08 | Elan Pharma International Limited | COMPOSITIONS OF CONTROLLED RELEASE AND NANOPARTICULATES THAT INCLUDE DERIVATIVES OF PROSTAGLANDINA. |
| US8492369B2 (en) | 2010-04-12 | 2013-07-23 | Clarus Therapeutics Inc | Oral testosterone ester formulations and methods of treating testosterone deficiency comprising same |
| KR20080009201A (en) * | 2005-04-15 | 2008-01-25 | 클라루스 쎄러퓨틱스, 아이엔씨. | Pharmaceutical delivery systems for hydrophobic drugs and compositions comprising same |
| US20100119607A1 (en) * | 2005-04-18 | 2010-05-13 | Rubicon Research Pvt. Ltd. | Bioenhanced compositions |
| JP2008536929A (en) * | 2005-04-18 | 2008-09-11 | ルビコン・リサーチ・ピーヴィーティー・エルティーディー | Bio-enhancing composition |
| BRPI0610780A2 (en) * | 2005-04-22 | 2016-09-06 | Teva Pharma | disintegrating tablet pharmaceutical formulation, and methods for treating a patient in need of olanzapine treatment, and for producing the tablet pharmaceutical formulation |
| US20060240108A1 (en) * | 2005-04-26 | 2006-10-26 | Bernard Bobby L | Cellulosic films incorporating a pharmaceutically acceptable plasticizer with enhanced wettability |
| US20060240051A1 (en) * | 2005-04-26 | 2006-10-26 | Singleton Andy H | Eutectic blends containing a water soluble vitamin derivative |
| WO2006118265A1 (en) * | 2005-04-28 | 2006-11-09 | Eisai R & D Management Co., Ltd. | Composition containing antidementia agent |
| WO2006119280A2 (en) * | 2005-05-03 | 2006-11-09 | Handylab, Inc. | Lyophilized pellets |
| CA2609330A1 (en) * | 2005-05-25 | 2006-11-30 | Transcept Pharmaceuticals, Inc. | Solid compositions and methods for treating middle-of-the night insomnia |
| US20070225322A1 (en) * | 2005-05-25 | 2007-09-27 | Transoral Pharmaceuticals, Inc. | Compositions and methods for treating middle-of-the night insomnia |
| US20070287740A1 (en) * | 2005-05-25 | 2007-12-13 | Transcept Pharmaceuticals, Inc. | Compositions and methods of treating middle-of-the night insomnia |
| ATE543489T1 (en) * | 2005-05-26 | 2012-02-15 | Teva Womens Health Inc | ORAL DOSAGE FORMS CONTAINING PROGESTERONE AND METHODS OF USE AND MANUFACTURING THEREOF |
| WO2006131806A2 (en) * | 2005-06-07 | 2006-12-14 | Pfizer Products Inc. | Multiparticulates comprising low-solubility drugs and carriers that result in rapid drug release |
| EP1901718A4 (en) * | 2005-06-13 | 2012-07-18 | Elan Pharma Int Ltd | Modified release ticlopidine compositions |
| US20060280787A1 (en) * | 2005-06-14 | 2006-12-14 | Baxter International Inc. | Pharmaceutical formulation of the tubulin inhibitor indibulin for oral administration with improved pharmacokinetic properties, and process for the manufacture thereof |
| JP2008543842A (en) * | 2005-06-14 | 2008-12-04 | バクスター インターナショナル インコーポレイテッド | Pharmaceutical formulations for minimizing drug-drug interactions |
| WO2006134610A1 (en) * | 2005-06-16 | 2006-12-21 | Hetero Drugs Limited | Efavirenz pharmaceutical composition having enhanced dissolution profile |
| JP2008546786A (en) * | 2005-06-23 | 2008-12-25 | シェーリング コーポレイション | Rapidly absorbable oral formulation of PDE5 inhibitor |
| US8435558B1 (en) | 2005-06-28 | 2013-05-07 | University Of South Florida | Ultrasound enhancement of drug release across non-ionic surfactant membranes |
| US7981442B2 (en) * | 2005-06-28 | 2011-07-19 | University Of South Florida | Ultrasound enhancement of drug release across non-ionic surfactant membranes |
| AR054805A1 (en) * | 2005-06-29 | 2007-07-18 | Stiefel Laboratories | TOPICAL COMPOSITIONS FOR SKIN TREATMENT |
| US20070014847A1 (en) * | 2005-07-05 | 2007-01-18 | Ahmed Salah U | Coated capsules and methods of making and using the same |
| CN100402035C (en) * | 2005-07-07 | 2008-07-16 | 石药集团中奇制药技术(石家庄)有限公司 | A pharmaceutical composition of microencapsulated cefuroxime axetil |
| US20110045065A1 (en) * | 2005-07-11 | 2011-02-24 | Ashok Vasantray Vyas | Substance having antioxidant, geroprotective and anti-ischemic activity and method for the preparation thereof |
| US7276476B2 (en) * | 2005-07-13 | 2007-10-02 | Allergan, Inc. | Cyclosporin compositions |
| US20070015693A1 (en) * | 2005-07-13 | 2007-01-18 | Allergan, Inc. | Cyclosporin compositions |
| US7288520B2 (en) * | 2005-07-13 | 2007-10-30 | Allergan, Inc. | Cyclosporin compositions |
| US7297679B2 (en) * | 2005-07-13 | 2007-11-20 | Allergan, Inc. | Cyclosporin compositions |
| US7202209B2 (en) | 2005-07-13 | 2007-04-10 | Allergan, Inc. | Cyclosporin compositions |
| US20070015691A1 (en) | 2005-07-13 | 2007-01-18 | Allergan, Inc. | Cyclosporin compositions |
| NZ565846A (en) * | 2005-07-18 | 2011-12-22 | Horizon Therapeutics Inc | Medicaments containing famotidine and ibuprofen and administration of same |
| US8067451B2 (en) * | 2006-07-18 | 2011-11-29 | Horizon Pharma Usa, Inc. | Methods and medicaments for administration of ibuprofen |
| US20080020040A1 (en) * | 2006-07-18 | 2008-01-24 | Horizon Therapeutics, Inc. | Unit dose form for administration of ibuprofen |
| US20080021078A1 (en) * | 2006-07-18 | 2008-01-24 | Horizon Therapeutics, Inc. | Methods and medicaments for administration of ibuprofen |
| US20070020333A1 (en) * | 2005-07-20 | 2007-01-25 | Chin-Chih Chiang | Controlled release of hypnotic agents |
| US7501393B2 (en) * | 2005-07-27 | 2009-03-10 | Allergan, Inc. | Pharmaceutical compositions comprising cyclosporins |
| US10532028B2 (en) * | 2005-07-28 | 2020-01-14 | Isp Investments Llc | Method to improve characteristics of spray dried powders and granulated materials, and the products thereby produced |
| KR100693461B1 (en) * | 2005-07-29 | 2007-03-12 | 동국제약 주식회사 | Pharmaceutical Composition Comprising a Macrolide Antibiotic As an Active Ingredient, and Preparation Method thereof, and Sustained Release Compositions Comprising the same |
| BRPI0614907A2 (en) * | 2005-08-01 | 2011-04-19 | Teva Pharma | processes for the preparation of tizanidine compositions and uses in neurological treatments of medicaments based thereon |
| EP1749528A1 (en) | 2005-08-05 | 2007-02-07 | Pharma C S.A. | Pharmaceutical combinations containing a mu opioid agonist and an inhibitor of NO production |
| US20070036859A1 (en) * | 2005-08-11 | 2007-02-15 | Perry Ronald L | Sustained release antihistamine and decongestant composition |
| WO2007022345A2 (en) * | 2005-08-17 | 2007-02-22 | Fleming And Company, Pharmaceuticals | Vitamin b12 nasal spray and method of use |
| US8394812B2 (en) | 2005-08-24 | 2013-03-12 | Penwest Pharmaceuticals Co. | Sustained release formulations of nalbuphine |
| WO2007025005A2 (en) | 2005-08-24 | 2007-03-01 | Penwest Pharmaceuticals Co. | Sustained release formulations of nalbuphine |
| KR101233235B1 (en) * | 2005-08-26 | 2013-02-15 | 에스케이케미칼주식회사 | Pharmaceutical composition of pranlukast solid-dispersion with improved early dissolution rate and the method of preparing the composition |
| US20080058282A1 (en) | 2005-08-30 | 2008-03-06 | Fallon Joan M | Use of lactulose in the treatment of autism |
| WO2007026271A1 (en) * | 2005-09-02 | 2007-03-08 | Firmenich Sa | Clear flavor microemulsions comprising sugar esters of fatty acids |
| RU2005128993A (en) * | 2005-09-08 | 2007-03-20 | Общество С Ограниченной Ответственностью Исследовательский Центр "Комкон" (Ru) | MEANS FOR CORRECTION OF STRESS-INDUCED NEUROMEDIATOR, NEURO-ENDOCRINE AND METABOLIC DISORDERS, AND ALSO THE METHOD FOR PREVENTION AND TREATMENT OF THEIR PATHOLOGICALLY SIMILAR TO THEM |
| ES2620293T3 (en) * | 2005-09-09 | 2017-06-28 | Paladin Labs Inc. | Sustained drug release composition |
| KR100754953B1 (en) | 2005-09-14 | 2007-09-04 | 주식회사 대웅 | A solublized material comprising ubidecarenone, aqueous solution and process for preparation thereof |
| US7803404B2 (en) | 2005-09-14 | 2010-09-28 | Mannkind Corporation | Method of drug formulation based on increasing the affinity of active agents for crystalline microparticle surfaces |
| US7473684B2 (en) | 2005-09-16 | 2009-01-06 | Selamine Limited | Bisphosphonate formulation |
| US20070116695A1 (en) * | 2005-09-21 | 2007-05-24 | Fallon Joan M | Pharmaceutical preparations for attention deficit disorder, attention deficit hyperactivity disorder and other associated disorders |
| US20070086952A1 (en) * | 2005-09-29 | 2007-04-19 | Biodel, Inc. | Rapid Acting and Prolonged Acting Inhalable Insulin Preparations |
| US7713929B2 (en) * | 2006-04-12 | 2010-05-11 | Biodel Inc. | Rapid acting and long acting insulin combination formulations |
| US8084420B2 (en) * | 2005-09-29 | 2011-12-27 | Biodel Inc. | Rapid acting and long acting insulin combination formulations |
| CA2521272A1 (en) * | 2005-10-04 | 2007-04-04 | Bernard Charles Sherman | Capsules comprising topiramate |
| EP1945185B1 (en) * | 2005-10-12 | 2016-02-03 | Proventiv Therapeutics, LLC | Methods and articles for treating 25-hydroxyvitamin d insufficiency and deficiency |
| JP5584415B2 (en) | 2005-10-12 | 2014-09-03 | ユニメッド・ファーマシューティカルズ・エルエルシー | Improved testosterone gel formulation and method of use |
| US9839667B2 (en) | 2005-10-14 | 2017-12-12 | Allergan, Inc. | Prevention and treatment of ocular side effects with a cyclosporin |
| US7745400B2 (en) * | 2005-10-14 | 2010-06-29 | Gregg Feinerman | Prevention and treatment of ocular side effects with a cyclosporin |
| US20070141106A1 (en) * | 2005-10-19 | 2007-06-21 | Bonutti Peter M | Drug eluting implant |
| EP1954262A4 (en) * | 2005-11-07 | 2012-05-02 | Russell M Jaffe | Compositions for regulating metabolic disorders and methods of use thereof |
| US8652529B2 (en) * | 2005-11-10 | 2014-02-18 | Flamel Technologies | Anti-misuse microparticulate oral pharmaceutical form |
| ATE438381T1 (en) * | 2005-11-18 | 2009-08-15 | Synhton B V | ZOLPIDEME TABLETS |
| BRPI0520669A2 (en) * | 2005-11-21 | 2009-06-02 | Teva Pharma | pharmaceutical dosage that reduces the effect of food found for atorvastatin administration |
| ES2761812T3 (en) | 2005-11-22 | 2020-05-21 | Nalpropion Pharmaceuticals Inc | Composition and methods of increasing insulin sensitivity |
| TWI425944B (en) * | 2005-11-28 | 2014-02-11 | Orexigen Therapeutics Inc | Sustained-release formulation of zonisamide |
| US7858609B2 (en) | 2005-11-28 | 2010-12-28 | Marinus Pharmaceuticals | Solid ganaxolone formulations and methods for the making and use thereof |
| KR20080075027A (en) * | 2005-12-05 | 2008-08-13 | 아피늄 파마슈티컬스, 인크. | Heterocyclylacrylamide compounds as fabi inhibitors and antibacterial agents |
| US8778924B2 (en) | 2006-12-04 | 2014-07-15 | Shionogi Inc. | Modified release amoxicillin products |
| US8357394B2 (en) | 2005-12-08 | 2013-01-22 | Shionogi Inc. | Compositions and methods for improved efficacy of penicillin-type antibiotics |
| US9572886B2 (en) | 2005-12-22 | 2017-02-21 | Nitto Denko Corporation | Agent for treating myelofibrosis |
| ES2664086T3 (en) | 2005-12-30 | 2018-04-18 | Zensun (Shanghai) Science & Technology, Co., Ltd. | Extended release of neurregulin to improve cardiac function |
| AR054215A1 (en) | 2006-01-20 | 2007-06-13 | Eriochem Sa | A PHARMACEUTICAL FORMULATION OF A TAXANE, A SOLID COMPOSITION OF A LIOFILIZED TAXAN FROM AN ACETIC ACID SOLUTION, A PROCEDURE FOR THE PREPARATION OF A SOLID COMPOSITION OF A TAXANE, A SOLUBILIZING COMPOSITION OF A LIOFILIZED TAXANE AND AN ELEMENTARY KIT |
| WO2007092312A2 (en) * | 2006-02-03 | 2007-08-16 | Stiefel Laboratories, Inc. | Topical skin treating compositions |
| ES2459018T3 (en) | 2006-02-09 | 2014-05-07 | Alba Therapeutics Corporation | Close junction effector formulations |
| US20070190141A1 (en) * | 2006-02-16 | 2007-08-16 | Aaron Dely | Extended release opiate composition |
| FR2897267A1 (en) * | 2006-02-16 | 2007-08-17 | Flamel Technologies Sa | MULTIMICROPARTICULAR PHARMACEUTICAL FORMS FOR PER OS ADMINISTRATION |
| IN2015DN00888A (en) | 2006-02-22 | 2015-07-10 | Mannkind Corp | |
| WO2007100822A2 (en) * | 2006-02-24 | 2007-09-07 | Teva Pharmaceutical Industries Ltd. | Fluvastatin sodium pharmaceutical compositions |
| SE0600482L (en) * | 2006-03-02 | 2006-11-14 | Ferring Int Ct Sa | Pharmaceutical composition comprising desmopressin, silica and starch |
| EP2383271B1 (en) | 2006-03-13 | 2013-07-10 | Kyorin Pharmaceutical Co., Ltd. | Aminoquinolones as GSK-3 Inhibitors |
| JP2009530280A (en) * | 2006-03-13 | 2009-08-27 | エンサイシブ・ファーマシューティカルズ・インコーポレイテッド | Formulation of sitaxsentan sodium |
| WO2007106494A2 (en) * | 2006-03-13 | 2007-09-20 | Encysive Pharmaceuticals, Inc. | Methods and compositions for treatment of diastolic heart failure |
| ES2378573T3 (en) | 2006-03-16 | 2012-04-16 | Tris Pharma, Inc. | Modified release formulations containing ion exchange drug-resin complexes |
| CN101410012A (en) * | 2006-03-28 | 2009-04-15 | 杰佛林制药公司 | Formulations of low dose non-steroidal anti-inflammatory drugs and beta-cyclodextrin |
| EP2522343A1 (en) * | 2006-03-28 | 2012-11-14 | Javelin Pharmaceuticals, Inc. | Formulations of Low Dose Diclofenac and Beta-Cyclodextrin |
| EP2206494B1 (en) | 2006-03-31 | 2015-12-02 | Stiefel Research Australia Pty Ltd | Foamable suspension gel |
| CA2648594C (en) * | 2006-04-07 | 2012-10-16 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| MX2008013165A (en) * | 2006-04-12 | 2009-01-29 | Biodel Inc | Rapid acting and long acting insulin combination formulations. |
| JP2007308480A (en) * | 2006-04-20 | 2007-11-29 | Shin Etsu Chem Co Ltd | Solid preparation containing enteric solid dispersion |
| KR101387563B1 (en) | 2006-04-21 | 2014-04-25 | 세노믹스, 인코포레이티드 | Comestible compositions comprising high potency savory flavorants, and processes for producing them |
| US20080096819A1 (en) * | 2006-05-02 | 2008-04-24 | Allozyne, Inc. | Amino acid substituted molecules |
| CA2653748A1 (en) * | 2006-05-02 | 2007-11-15 | Allozyne, Inc. | Non-natural amino acid substituted polypeptides |
| CA2894933C (en) * | 2006-05-02 | 2017-10-03 | University Of Miami | Topical co-enzyme q10 formulations and treatment of pain, fatigue and wounds |
| US8299052B2 (en) | 2006-05-05 | 2012-10-30 | Shionogi Inc. | Pharmaceutical compositions and methods for improved bacterial eradication |
| EP2019679B1 (en) | 2006-05-23 | 2018-06-20 | Theracos, Inc. | Glucose transport inhibitors and methods of use |
| US20070281014A1 (en) * | 2006-06-01 | 2007-12-06 | Cima Labs, Inc. | Prednisolone salt formulations |
| US8916195B2 (en) | 2006-06-05 | 2014-12-23 | Orexigen Therapeutics, Inc. | Sustained release formulation of naltrexone |
| US20070286900A1 (en) * | 2006-06-09 | 2007-12-13 | Catherine Herry | Low dose tablets of opioid analgesics and preparation process |
| US7893053B2 (en) | 2006-06-16 | 2011-02-22 | Theracos, Inc. | Treating psychological conditions using muscarinic receptor M1 antagonists |
| US8748419B2 (en) | 2006-06-16 | 2014-06-10 | Theracos, Inc. | Treating obesity with muscarinic receptor M1 antagonists |
| US20080026061A1 (en) * | 2006-06-22 | 2008-01-31 | Reichwein John F | Crystalline N-(4-chloro-3-methyl-5-isoxazolyl)-2-[2-methyl-4.5-(methylenedioxy)phenylacetyl]-thiophene-3-sulfonamide |
| US20080014257A1 (en) * | 2006-07-14 | 2008-01-17 | Par Pharmaceutical, Inc. | Oral dosage forms |
| US8067033B2 (en) | 2007-11-30 | 2011-11-29 | Horizon Pharma Usa, Inc. | Stable compositions of famotidine and ibuprofen |
| EP2687533B1 (en) | 2006-07-20 | 2017-07-19 | Debiopharm International SA | Acrylamide derivatives as FAB I inhibitors |
| EA015304B1 (en) | 2006-08-03 | 2011-06-30 | Нитек Фарма Аг | Delayed-release glucocorticoid treatment of rheumatoid disease |
| WO2008019146A2 (en) * | 2006-08-04 | 2008-02-14 | Insys Therapeutics Inc. | Aqueous dronabinol formulations |
| EP2061509A2 (en) * | 2006-08-14 | 2009-05-27 | Wayne State University | Polymer-surfactant nanoparticles for sustained release of compounds |
| EP2051696A2 (en) * | 2006-08-18 | 2009-04-29 | Morton Grove Pharmaceuticals, Inc. | Stable liquid levetiracetam compositions and methods |
| US20080057123A1 (en) | 2006-08-30 | 2008-03-06 | Jagotec Ag | Controlled Release Formulations |
| WO2008027963A2 (en) * | 2006-08-31 | 2008-03-06 | Horizon Therapeutics, Inc. | Nsaid dose unit formulations with h2-receptor antagonists and methods of use |
| WO2008028193A2 (en) * | 2006-09-01 | 2008-03-06 | Pharmion Corporation | Colon-targeted oral formulations of cytidine analogs |
| BRPI0716897A2 (en) | 2006-09-21 | 2013-10-22 | Activx Biosciences Inc | COMPOUND OR PHARMACEUTICALLY ACCEPTABLE DERIVATIVE OF THE SAME, PHARMACEUTICAL COMPOSITION, METHODS FOR INHIBITING AN ACTION OF A SERINE HYDROLASE AND FOR TREATMENT OF A DISEASE MEDIATED BY SERINA HYDROLASE, AND ARTICLE OF MANUFACTURING |
| US20080075785A1 (en) * | 2006-09-22 | 2008-03-27 | San-Laung Chow | Controlled release hydrogel formulation |
| US20110165236A1 (en) * | 2006-09-22 | 2011-07-07 | Biokey, Inc. | Controlled release hydrogel formulation |
| CN101600417B (en) | 2006-10-04 | 2012-05-30 | M&P专利股份公司 | Controlled release delivery system for nasal application of neurotransmitters |
| US20090092658A1 (en) * | 2007-10-05 | 2009-04-09 | Santarus, Inc. | Novel formulations of proton pump inhibitors and methods of using these formulations |
| US20080145411A1 (en) * | 2006-10-06 | 2008-06-19 | Kaneka Corporation | Composition of high absorbability for oral administration comprising oxidized coenzyme q10 |
| US20080085310A1 (en) * | 2006-10-06 | 2008-04-10 | Maria Oksana Bachynsky | Capecitabine rapidly disintegrating tablets |
| EP2124556B1 (en) | 2006-10-09 | 2014-09-03 | Charleston Laboratories, Inc. | Pharmaceutical compositions |
| CA2666149A1 (en) | 2006-10-19 | 2008-04-24 | Auspex Pharmaceuticals, Inc. | Substituted indoles |
| AU2007309281B2 (en) * | 2006-10-20 | 2013-10-31 | Johnson & Johnson Consumer Inc. | Acetaminophen / ibuprofen combinations |
| CA2667890C (en) * | 2006-10-31 | 2015-01-27 | Surmodics Pharmaceuticals, Inc. | Spheronized polymer particles |
| WO2008057604A2 (en) * | 2006-11-08 | 2008-05-15 | The Regents Of The University Of California | Small molecule therapeutics, syntheses of analogues and derivatives and methods of use |
| WO2008060964A2 (en) | 2006-11-09 | 2008-05-22 | Orexigen Therapeutics, Inc. | Unit dosage package and methods for administering weight loss medications |
| US8088786B2 (en) | 2006-11-09 | 2012-01-03 | Orexigen Therapeutics, Inc. | Layered pharmaceutical formulations |
| DE102006053385B4 (en) | 2006-11-13 | 2014-05-08 | Universitätsklinikum Freiburg | Enterococcus faecalis antigen |
| US8998846B2 (en) | 2006-11-20 | 2015-04-07 | Lutonix, Inc. | Drug releasing coatings for balloon catheters |
| US8425459B2 (en) * | 2006-11-20 | 2013-04-23 | Lutonix, Inc. | Medical device rapid drug releasing coatings comprising a therapeutic agent and a contrast agent |
| US8430055B2 (en) | 2008-08-29 | 2013-04-30 | Lutonix, Inc. | Methods and apparatuses for coating balloon catheters |
| US9700704B2 (en) | 2006-11-20 | 2017-07-11 | Lutonix, Inc. | Drug releasing coatings for balloon catheters |
| US8414910B2 (en) | 2006-11-20 | 2013-04-09 | Lutonix, Inc. | Drug releasing coatings for medical devices |
| US9737640B2 (en) | 2006-11-20 | 2017-08-22 | Lutonix, Inc. | Drug releasing coatings for medical devices |
| US8414526B2 (en) | 2006-11-20 | 2013-04-09 | Lutonix, Inc. | Medical device rapid drug releasing coatings comprising oils, fatty acids, and/or lipids |
| US8414909B2 (en) | 2006-11-20 | 2013-04-09 | Lutonix, Inc. | Drug releasing coatings for medical devices |
| US8414525B2 (en) * | 2006-11-20 | 2013-04-09 | Lutonix, Inc. | Drug releasing coatings for medical devices |
| US20080276935A1 (en) | 2006-11-20 | 2008-11-13 | Lixiao Wang | Treatment of asthma and chronic obstructive pulmonary disease with anti-proliferate and anti-inflammatory drugs |
| BRPI0719280A2 (en) | 2006-11-27 | 2014-03-11 | Lundbeck & Co As H | COMPOUND, PHARMACEUTICAL COMPOSITION, METHODS FOR MODULATING THE ACTIVITY OF A P2X7 IN VITRO RECEIVER AND IN A PATIENT, TO TREAT A CONDITION RESPONSIBLE FOR P2X7 RECEIVER MODULATION IN A PATIENT, TO INHIBIT THE DEVELOPMENT OF A GALLULLE CELL IN A PITCH TO DETERMINE THE PRESENCE OR ABSENCE OF P2X7 RECEIVER IN A SAMPLE, PACKAGED PHARMACEUTICAL PREPARATION, METHOD FOR TREATING OR PREVENTING CIRROSIS IN A PATIENT, AND USING A COMPOUND. |
| CA2669815A1 (en) | 2006-11-28 | 2008-06-05 | Marinus Pharmaceuticals | Nanoparticulate formulations and methods for the making and use thereof |
| GB0624084D0 (en) * | 2006-12-01 | 2007-01-10 | Selamine Ltd | Ramipril amino acid salts |
| GB0624090D0 (en) * | 2006-12-01 | 2007-01-10 | Selamine Ltd | Ramipril amine salts |
| GB0624087D0 (en) * | 2006-12-01 | 2007-01-10 | Selamine Ltd | Ramipril combination salt |
| AU2007329373B2 (en) * | 2006-12-04 | 2013-06-20 | Supernus Pharmaceuticals, Inc. | Enhanced immediate release formulations of topiramate |
| MX2009005947A (en) * | 2006-12-05 | 2009-06-17 | Novartis Ag | Microemulsion dosage forms of valsartan and methods of making the same. |
| US9555167B2 (en) * | 2006-12-11 | 2017-01-31 | 3M Innovative Properties Company | Biocompatible antimicrobial compositions |
| US20080200890A1 (en) * | 2006-12-11 | 2008-08-21 | 3M Innovative Properties Company | Antimicrobial disposable absorbent articles |
| WO2008076780A2 (en) * | 2006-12-14 | 2008-06-26 | Isp Investments Inc. | Amorphous valsartan and the production thereof |
| WO2008080037A2 (en) * | 2006-12-21 | 2008-07-03 | Isp Investments Inc. | Carotenoids of enhanced bioavailability |
| EP2125739A1 (en) * | 2006-12-22 | 2009-12-02 | Encysive Pharmaceuticals, Inc. | Modulators of c3a receptor and methods of use thereof |
| US8337817B2 (en) * | 2006-12-26 | 2012-12-25 | Shin Nippon Biomedical Laboratories, Ltd. | Preparation for transnasal application |
| US8814930B2 (en) | 2007-01-19 | 2014-08-26 | Elixir Medical Corporation | Biodegradable endoprosthesis and methods for their fabrication |
| US20080177373A1 (en) * | 2007-01-19 | 2008-07-24 | Elixir Medical Corporation | Endoprosthesis structures having supporting features |
| US20130150943A1 (en) | 2007-01-19 | 2013-06-13 | Elixir Medical Corporation | Biodegradable endoprostheses and methods for their fabrication |
| WO2008092057A2 (en) * | 2007-01-26 | 2008-07-31 | Isp Investments Inc. | Formulation process method to produce spray dried products |
| WO2008092046A2 (en) * | 2007-01-26 | 2008-07-31 | Isp Investments Inc. | Amorphous oxcarbazepine and the production thereof |
| PT1958617E (en) * | 2007-02-14 | 2010-10-28 | Lesvi Laboratorios Sl | Pharmaceutical compositions containing quetiapine fumarate |
| KR100868295B1 (en) | 2007-02-15 | 2008-11-11 | 풍림무약주식회사 | Solid dispersion containing leflunomide and preparation method thereof |
| EP3255045A1 (en) | 2007-02-16 | 2017-12-13 | Debiopharm International SA | Salts, prodrugs and polymorphs of fab i inhibitors |
| PL2144604T3 (en) * | 2007-02-28 | 2012-02-29 | Conatus Pharmaceuticals Inc | Methods for the treatment of chronic viral hepatitis C using RO 113-0830 |
| WO2008106167A1 (en) * | 2007-02-28 | 2008-09-04 | Conatus Pharmaceuticals, Inc. | Combination therapy comprising matrix metalloproteinase inhibitors and caspase inhibitors for the treatment of liver diseases |
| ES2605371T3 (en) | 2007-03-15 | 2017-03-14 | Auspex Pharmaceuticals, Inc. | D9-Deuterated Venlafaxine |
| US8343541B2 (en) | 2007-03-15 | 2013-01-01 | Soft Gel Technologies, Inc. | Ubiquinol and alpha lipoic acid compositions |
| US20100143471A1 (en) * | 2007-03-21 | 2010-06-10 | Lupin Limited | Novel reduced dose pharmaceutical compositions of fexofenadine and pseudoephedrine |
| UA93148C2 (en) | 2007-03-29 | 2011-01-10 | Панасэа Биотэк Лимитэд | modified release dosage form of tacrolimus |
| TWI407971B (en) | 2007-03-30 | 2013-09-11 | Nitto Denko Corp | Cancer cells and tumor-related fibroblasts |
| DK2064222T3 (en) | 2007-04-02 | 2014-07-21 | Theracos Inc | Benzylic glycoside derivatives and methods for their use |
| WO2008134518A2 (en) * | 2007-04-25 | 2008-11-06 | Cytochroma Inc. | Methods and compounds for vitamin d therapy |
| EP2527336A1 (en) | 2007-04-25 | 2012-11-28 | Concert Pharmaceuticals Inc. | Deuterated analogues of cilostazol |
| NZ581362A (en) * | 2007-04-27 | 2011-07-29 | Cydex Pharmaceuticals Inc | Formulations containing clopidogrel and sulfoalkyl ether cyclodextrin and methods of use |
| JP2010526054A (en) * | 2007-05-01 | 2010-07-29 | シグモイド・ファーマ・リミテッド | Combined pharmaceutical composition |
| US7892776B2 (en) | 2007-05-04 | 2011-02-22 | The Regents Of The University Of California | Screening assay to identify modulators of protein kinase A |
| US7537532B2 (en) * | 2007-05-16 | 2009-05-26 | Young Carl D | Handle for implement and method |
| US20080286357A1 (en) * | 2007-05-17 | 2008-11-20 | Balchem Corporation | Multi-functional particulate delivery system for pharmacologically active ingredients |
| US8426467B2 (en) | 2007-05-22 | 2013-04-23 | Baxter International Inc. | Colored esmolol concentrate |
| US8722736B2 (en) | 2007-05-22 | 2014-05-13 | Baxter International Inc. | Multi-dose concentrate esmolol with benzyl alcohol |
| WO2008144888A1 (en) * | 2007-05-25 | 2008-12-04 | The University Of British Columbia | Formulations for the oral administration of therapeutic agents and related methods |
| US20090004284A1 (en) * | 2007-06-26 | 2009-01-01 | Watson Pharmaceuticals, Inc. | Controlled release tamsulosin hydrochloride formulation |
| GB0712316D0 (en) * | 2007-06-26 | 2007-08-01 | Entripneur Ltd | A novel powder and its method of manufacture |
| US20090004285A1 (en) * | 2007-06-29 | 2009-01-01 | Liangping Yu | Stable non-disintegrating dosage forms and method of making same |
| FR2918277B1 (en) | 2007-07-06 | 2012-10-05 | Coretecholding | NOVEL PROCESS FOR THE PRODUCTION OF HYDRODISPERSIBLE DRY PHARMACEUTICAL FORMS AND THE HYDRODISPERSIBLE COMPOSITIONS THUS OBTAINED |
| EA201000016A1 (en) * | 2007-07-12 | 2010-10-29 | Трагара Фармасьютикалс, Инк. | METHODS AND COMPOSITIONS FOR THE TREATMENT OF CANCER, TUMORS AND DISTURBANCES ASSOCIATED WITH TUMORS |
| US8110226B2 (en) * | 2007-07-20 | 2012-02-07 | Mylan Pharmaceuticals Inc. | Drug formulations having inert sealed cores |
| CN101091890A (en) * | 2007-07-26 | 2007-12-26 | 沈阳药科大学 | Composite type emulsifier, and emulsion prepared by using the emulsifier, and preparation method |
| US20090036414A1 (en) * | 2007-08-02 | 2009-02-05 | Mutual Pharmaceutical Company, Inc. | Mesalamine Formulations |
| US8399410B2 (en) | 2007-08-06 | 2013-03-19 | Allergan, Inc. | Methods and devices for desmopressin drug delivery |
| JP2010535774A (en) * | 2007-08-06 | 2010-11-25 | インシス セラピューティクス インコーポレイテッド | Oral cannabinoid liquid formulations and methods of treatment |
| US8268806B2 (en) | 2007-08-10 | 2012-09-18 | Endorecherche, Inc. | Pharmaceutical compositions |
| CA2707840A1 (en) | 2007-08-20 | 2009-02-26 | Allozyne, Inc. | Amino acid substituted molecules |
| WO2009026537A1 (en) | 2007-08-23 | 2009-02-26 | Theracos, Inc. | Benzylbenzene derivatives and methods of use |
| US20090060983A1 (en) * | 2007-08-30 | 2009-03-05 | Bunick Frank J | Method And Composition For Making An Orally Disintegrating Dosage Form |
| ATE531721T1 (en) * | 2007-09-11 | 2011-11-15 | Kyorin Seiyaku Kk | CYANOAMINOQUINOLONES AS GSK-3 INHIBITORS |
| US8476261B2 (en) | 2007-09-12 | 2013-07-02 | Kyorin Pharmaceutical Co., Ltd. | Spirocyclic aminoquinolones as GSK-3 inhibitors |
| KR100790954B1 (en) * | 2007-09-13 | 2008-01-04 | 영남대학교 산학협력단 | Novel composite of gelatin ultra-micro capsule containing itraconazole |
| US8003621B2 (en) | 2007-09-14 | 2011-08-23 | Nitto Denko Corporation | Drug carriers |
| EP2200613B1 (en) | 2007-09-21 | 2018-09-05 | The Johns Hopkins University | Phenazine derivatives and uses thereof |
| AR063111A1 (en) | 2007-10-03 | 2008-12-30 | Eriochem Sa | A PHARMACEUTICAL FORMULATION OF TAXANO |
| CN101918019B (en) | 2007-10-08 | 2014-11-26 | 奥里尼亚制药有限公司 | Ophthalmic compositions comprising calcineurin inhibitors or mTOR inhibitors |
| EP2826475B1 (en) | 2007-10-16 | 2019-03-20 | Repros Therapeutics Inc. | Trans-clomiphene for treating diabetes in hypogonadal men |
| MX2010003979A (en) | 2007-10-16 | 2010-06-02 | Biocon Ltd | An orally administerable solid pharmaceutical composition and a process thereof. |
| GB0720967D0 (en) * | 2007-10-25 | 2007-12-05 | Protophama Ltd | Anti-material pharmaceutical composition |
| KR101560176B1 (en) * | 2007-10-31 | 2015-10-14 | 맥네일-피피씨, 인코포레이티드 | Orally disintegrated dosage form |
| US20100216754A1 (en) * | 2007-11-13 | 2010-08-26 | Meritage Pharma, Inc. | Compositions for the treatment of inflammation of the gastrointestinal tract |
| CN101969956B (en) * | 2007-11-13 | 2014-03-05 | 梅里蒂奇制药公司 | Corticosteroid compositions |
| US20090123551A1 (en) * | 2007-11-13 | 2009-05-14 | Meritage Pharma, Inc. | Gastrointestinal delivery systems |
| US8802156B2 (en) | 2007-11-14 | 2014-08-12 | Laboratorios Farmacéuticos Rovi, S.A. | Pharmaceutical forms for the release of active compounds |
| KR100920106B1 (en) * | 2007-11-14 | 2009-10-01 | 경북대학교 산학협력단 | Controlled drug carrier for deliverying sildenafil citrate transdermally and patch containing the same |
| TWI468167B (en) * | 2007-11-16 | 2015-01-11 | 威佛(國際)股份有限公司 | Pharmaceutical compositions |
| WO2009066299A2 (en) * | 2007-11-23 | 2009-05-28 | Rappaport Family Institute For Research | Use of haptoglobin genotyping in diagnosis and treatment of cardiovascular disease |
| JP2011506318A (en) * | 2007-12-06 | 2011-03-03 | デュレクト コーポレーション | Oral pharmaceutical formulation |
| WO2009085952A1 (en) | 2007-12-20 | 2009-07-09 | Brookwood Pharmaceuticals, Inc. | Process for preparing microparticles having a low residual solvent volume |
| WO2009089117A1 (en) * | 2008-01-04 | 2009-07-16 | Hormel Foods Corporation | Encapsulation of oxidatively unstable compounds |
| ES2590604T3 (en) * | 2008-01-04 | 2016-11-22 | Schabar Research Associates Llc | Composition comprising an analgesic and an antihistamine |
| US20110020519A1 (en) * | 2008-01-04 | 2011-01-27 | Aveka, Inc. | Encapsulation of oxidatively unstable compounds |
| US8193182B2 (en) | 2008-01-04 | 2012-06-05 | Intellikine, Inc. | Substituted isoquinolin-1(2H)-ones, and methods of use thereof |
| WO2009089181A1 (en) * | 2008-01-04 | 2009-07-16 | Blodel, Inc. | Insulin formulations for insulin release as a function of tissue glucose levels |
| CA3066426A1 (en) | 2008-01-09 | 2009-07-16 | Charleston Laboratories, Inc. | Pharmaceutical compositions comprising an antiemetic and an opioid analgesic |
| JP2011511674A (en) | 2008-02-07 | 2011-04-14 | ユニヴァーシティ オブ ワシントン | Circumferential aerosol device |
| US20100021538A1 (en) * | 2008-02-29 | 2010-01-28 | Youngro Byun | Pharmaceutical compositions containing heparin derivatives |
| WO2009113061A1 (en) * | 2008-03-10 | 2009-09-17 | Dexcel Pharma Technologies Ltd. | Humidity-resistant drug formulations and methods of preparation thereof |
| CA2718416C (en) * | 2008-03-13 | 2018-01-02 | Mallinckrodt Inc. | Multi-function, foot-activated controller for imaging system |
| US8658163B2 (en) * | 2008-03-13 | 2014-02-25 | Curemark Llc | Compositions and use thereof for treating symptoms of preeclampsia |
| HUE032873T2 (en) | 2008-03-17 | 2017-11-28 | Ambit Biosciences Corp | 1-(3-(6,7-dimethoxyquinazolin-4-yloxy)phenyl)-3-(5-(1,1,1-trifluoro-2-methylpropan-2-yl)isoxazol-3-yl)urea as raf kinase modulator in the treatment of cancer diseases |
| US8282977B2 (en) | 2008-03-20 | 2012-10-09 | Virun, Inc. | Compositions containing non-polar compounds |
| US8409601B2 (en) | 2008-03-31 | 2013-04-02 | Cordis Corporation | Rapamycin coated expandable devices |
| US8420110B2 (en) * | 2008-03-31 | 2013-04-16 | Cordis Corporation | Drug coated expandable devices |
| SG189687A1 (en) | 2008-04-11 | 2013-05-31 | Cytotech Labs Llc | Methods and use of inducing apoptosis in cancer cells |
| US8222272B2 (en) * | 2008-04-11 | 2012-07-17 | Roxane Laboratories, Inc. | Pharmaceutical formulation and process comprising a solid dispersion of macrolide (tacrolimus) |
| US8084025B2 (en) | 2008-04-18 | 2011-12-27 | Curemark Llc | Method for the treatment of the symptoms of drug and alcohol addiction |
| US20090264392A1 (en) * | 2008-04-21 | 2009-10-22 | Meritage Pharma, Inc. | Treating eosinophilic esophagitis |
| US20090280169A1 (en) * | 2008-05-07 | 2009-11-12 | Merrion Research Iii Limited | Compositions of peptides and processes of preparation thereof |
| EP2116618A1 (en) | 2008-05-09 | 2009-11-11 | Agency for Science, Technology And Research | Diagnosis and treatment of Kawasaki disease |
| US20090298882A1 (en) * | 2008-05-13 | 2009-12-03 | Muller George W | Thioxoisoindoline compounds and compositions comprising and methods of using the same |
| MX2010012514A (en) * | 2008-05-20 | 2011-05-30 | Cerenis Therapeutics S A | Niacin and nsaid for combination therapy. |
| ES2677548T3 (en) * | 2008-05-21 | 2018-08-03 | Ferring B.V. | Orodispersible desmopressin to increase the initial period of uninterrupted sleep due to nocturia |
| US20100286045A1 (en) | 2008-05-21 | 2010-11-11 | Bjarke Mirner Klein | Methods comprising desmopressin |
| WO2009146320A1 (en) | 2008-05-27 | 2009-12-03 | Dmi Life Sciences, Inc. | Therapeutic methods and compounds |
| US20090306025A1 (en) * | 2008-05-30 | 2009-12-10 | Fairfield Clinical Trials, Llc | Method and composition for skin inflammation and discoloration |
| JP2011521973A (en) | 2008-05-30 | 2011-07-28 | オレキシジェン・セラピューティクス・インコーポレーテッド | Methods for treating visceral fat conditions |
| US8485378B2 (en) * | 2009-04-08 | 2013-07-16 | General Mills, Inc. | Multi-container packages for dispensing liquid and dry food |
| US9045262B2 (en) * | 2008-06-10 | 2015-06-02 | General Mills, Inc. | Packages for dispensing liquid and dry food |
| US9199779B2 (en) * | 2008-06-10 | 2015-12-01 | General Mills, Inc. | Packages for dispensing liquid and dry food |
| US8815318B2 (en) * | 2008-06-10 | 2014-08-26 | General Mills, Inc. | Packages for dispensing liquid and dry food |
| US8173621B2 (en) | 2008-06-11 | 2012-05-08 | Gilead Pharmasset Llc | Nucleoside cyclicphosphates |
| KR101933816B1 (en) | 2008-06-13 | 2019-03-29 | 맨카인드 코포레이션 | A dry powder inhaler and system for drug delivery |
| US8485180B2 (en) | 2008-06-13 | 2013-07-16 | Mannkind Corporation | Dry powder drug delivery system |
| FR2932387B1 (en) * | 2008-06-16 | 2010-09-17 | Cll Pharma | ORAL COMPOSITION CONTAINING AN ANTIPLATELET AGENT OF THE FAMILY OF THIENOPYRIDINES IN THE BASIC FORM. |
| WO2009155612A2 (en) * | 2008-06-20 | 2009-12-23 | Genvault Corporation | Sample collection and storage devices and methods of use thereof |
| WO2009155581A1 (en) | 2008-06-20 | 2009-12-23 | Mannkind Corporation | An interactive apparatus and method for real-time profiling of inhalation efforts |
| US8337931B2 (en) * | 2008-06-23 | 2012-12-25 | Virun, Inc. | Compositions containing non-polar compounds |
| US9320780B2 (en) * | 2008-06-26 | 2016-04-26 | Curemark Llc | Methods and compositions for the treatment of symptoms of Williams Syndrome |
| US20090324730A1 (en) * | 2008-06-26 | 2009-12-31 | Fallon Joan M | Methods and compositions for the treatment of symptoms of complex regional pain syndrome |
| EP2318035B1 (en) * | 2008-07-01 | 2019-06-12 | Curemark, Llc | Methods and compositions for the treatment of symptoms of neurological and mental health disorders |
| EP2307434B1 (en) | 2008-07-02 | 2014-02-12 | IDENIX Pharmaceuticals, Inc. | Compounds and pharmaceutical compositions for the treatment of viral infections |
| US20100003319A1 (en) * | 2008-07-02 | 2010-01-07 | Glenmark Generics Ltd. | Raloxifene immediate release tablets |
| BRPI0916769A2 (en) | 2008-07-15 | 2017-09-26 | Theracos Inc | deuterated benzylbenzene derivatives and methods of use |
| JP5713897B2 (en) * | 2008-07-16 | 2015-05-07 | エボニック コーポレイションEvonik Corporation | Process for preparing microparticles containing bioactive peptides |
| RU2018129725A (en) * | 2008-07-18 | 2019-03-20 | Байомод Консептс Инк. | PRODUCTS RELEASING THE ACTIVE INGREDIENT |
| US10776453B2 (en) * | 2008-08-04 | 2020-09-15 | Galenagen, Llc | Systems and methods employing remote data gathering and monitoring for diagnosing, staging, and treatment of Parkinsons disease, movement and neurological disorders, and chronic pain |
| WO2010017056A1 (en) * | 2008-08-07 | 2010-02-11 | Merck & Co., Inc. | Orally administered solid formulations containing a hydrophobic drug and tpgs |
| TWI494123B (en) | 2008-08-11 | 2015-08-01 | Mannkind Corp | Use of ultrarapid acting insulin |
| ES2599330T3 (en) | 2008-08-22 | 2017-02-01 | Theracos Sub, Llc | Process for the preparation of SGLT2 inhibitors |
| CA2638240C (en) * | 2008-08-29 | 2010-02-02 | Alexander Macgregor | Method of treating dysglycemia and glucose excursions |
| JP2012501681A (en) * | 2008-09-12 | 2012-01-26 | ジェンボールト コーポレイション | Matrix and media for storage and stabilization of biomolecules |
| WO2010033812A1 (en) * | 2008-09-18 | 2010-03-25 | Variation Biotechnologies, Inc. | Compositions and methods for treating hepatitis a. |
| US20100092447A1 (en) * | 2008-10-03 | 2010-04-15 | Fallon Joan M | Methods and compositions for the treatment of symptoms of prion diseases |
| CA2740509A1 (en) * | 2008-10-30 | 2010-05-06 | Medlite A/S | Formulation for treatment of vaginal dryness |
| WO2010053487A1 (en) | 2008-11-07 | 2010-05-14 | Cydex Pharmaceuticals, Inc. | Composition containing sulfoalkyl ether cyclodextrin and latanoprost |
| ES2645692T3 (en) * | 2008-11-11 | 2017-12-07 | The Board Of Regents,The University Of Texas System | Rapamycin microcapsules and their use for cancer treatment |
| EP2379561B1 (en) | 2008-11-25 | 2015-11-04 | University Of Rochester | Mlk inhibitors and methods of use |
| IT1392214B1 (en) * | 2008-11-28 | 2012-02-22 | Sila S R L | PROCESS FOR THE PRODUCTION OF A COMPOUND OF N-BUTIRRIC ACID IN MICROCAPSULATED FORM, INTENDED FOR ANIMAL OR HUMAN POWER |
| WO2010065492A1 (en) * | 2008-12-02 | 2010-06-10 | Sciele Pharma, Inc. | Alpha2-adrenergic agonist a calcium channel blocker composition |
| NZ617066A (en) | 2008-12-23 | 2015-02-27 | Gilead Pharmasset Llc | Nucleoside analogs |
| EA019295B1 (en) | 2008-12-23 | 2014-02-28 | Джилид Фармассет, Ллс. | Synthesis of purine nucleosides and process for preparing them |
| AU2009329867B2 (en) | 2008-12-23 | 2015-01-29 | Gilead Pharmasset Llc | Nucleoside phosphoramidates |
| US8314106B2 (en) | 2008-12-29 | 2012-11-20 | Mannkind Corporation | Substituted diketopiperazine analogs for use as drug delivery agents |
| CN102307892A (en) | 2008-12-31 | 2012-01-04 | 西尼克斯公司 | Derivatives of cyclosporin A |
| US20100221328A1 (en) * | 2008-12-31 | 2010-09-02 | Wertz Christian F | Sustained-release formulations |
| EP2947100B1 (en) | 2009-01-06 | 2019-05-08 | Galenagen, LLC | Oral compositions for the treatment or the prevention of infections by E. Coli |
| US9107419B2 (en) | 2009-01-06 | 2015-08-18 | Curelon Llc | Compositions and methods for treatment or prevention of Staphylococcus aureus infections and for the eradication or reduction of Staphylococcus aureus on surfaces |
| US8784879B2 (en) | 2009-01-14 | 2014-07-22 | Corium International, Inc. | Transdermal administration of tamsulosin |
| WO2010084188A1 (en) * | 2009-01-26 | 2010-07-29 | Nitec Pharma Ag | Delayed-release glucocorticoid treatment of asthma |
| MA33060B1 (en) * | 2009-01-28 | 2012-02-01 | Novartis Ag | Pharmacy capsules of organic compounds |
| WO2010088450A2 (en) | 2009-01-30 | 2010-08-05 | Celladon Corporation | Methods for treating diseases associated with the modulation of serca |
| NZ582836A (en) * | 2009-01-30 | 2011-06-30 | Nitec Pharma Ag | Delayed-release glucocorticoid treatment of rheumatoid arthritis by improving signs and symptoms, showing major or complete clinical response and by preventing from joint damage |
| US8568793B2 (en) | 2009-02-11 | 2013-10-29 | Hope Medical Enterprises, Inc. | Sodium nitrite-containing pharmaceutical compositions |
| JP5890182B2 (en) | 2009-02-12 | 2016-03-22 | インセプト エルエルシー | Drug delivery with hydrogel plugs |
| US20100209475A1 (en) * | 2009-02-19 | 2010-08-19 | Biomet Manufacturing Corp. | Medical implants having a drug delivery coating |
| CA2751854A1 (en) * | 2009-02-25 | 2010-09-02 | Merrion Research Iii Limited | Composition and drug delivery of bisphosphonates |
| JP5746981B2 (en) | 2009-02-27 | 2015-07-08 | アムビト ビオスシエンセス コルポラチオン | JAK kinase-regulated quinazoline derivatives and methods of use thereof |
| US9060927B2 (en) * | 2009-03-03 | 2015-06-23 | Biodel Inc. | Insulin formulations for rapid uptake |
| US8101593B2 (en) | 2009-03-03 | 2012-01-24 | Kythera Biopharmaceuticals, Inc. | Formulations of deoxycholic acid and salts thereof |
| US8193372B2 (en) | 2009-03-04 | 2012-06-05 | Idenix Pharmaceuticals, Inc. | Phosphothiophene and phosphothiazole HCV polymerase inhibitors |
| JP2012520314A (en) | 2009-03-11 | 2012-09-06 | アムビト ビオスシエンセス コルポラチオン | Combination of indazolylaminopyrrolotriazine and taxane for cancer treatment |
| CN102421784B (en) * | 2009-03-11 | 2015-09-30 | 杏林制药株式会社 | As the 7-cycloalkyl amino quinolone of GSK-3 inhibitor |
| PL2405963T3 (en) | 2009-03-11 | 2014-04-30 | Mannkind Corp | Apparatus, system and method for measuring resistance of an inhaler |
| WO2010107507A1 (en) * | 2009-03-20 | 2010-09-23 | Incube Labs, Llc | Solid drug delivery apparatus, formulations and methods of use |
| WO2010110686A1 (en) | 2009-03-27 | 2010-09-30 | Pathway Therapeutics Limited | Pyrimidinyl and 1,3,5 triazinyl benzimidazoles and their use in cancer therapy |
| KR20110136880A (en) | 2009-03-27 | 2011-12-21 | 패스웨이 테라퓨틱스 인코포레이티드 | Pyrimidinyl and 1,3,5-triazinyl benzimidazole sulfonamides and their use in cancer therapy |
| US9056050B2 (en) | 2009-04-13 | 2015-06-16 | Curemark Llc | Enzyme delivery systems and methods of preparation and use |
| EP2419085A4 (en) * | 2009-04-14 | 2013-04-24 | Univ California | Improved oral drug devices and drug formulations |
| US20130274352A1 (en) * | 2009-04-14 | 2013-10-17 | The Regents Of The University Of California | Oral Drug Devices and Drug Formulations |
| MY162440A (en) | 2009-04-22 | 2017-06-15 | Axikin Pharmaceuticals Inc | 2,5-disubstituted arylsulfonamide ccr3 antagonists |
| MX2011011139A (en) | 2009-04-22 | 2012-01-27 | Axikin Pharmaceuticals Inc | 2,5-disubstituted arylsulfonamide ccr3 antagonists. |
| KR20120034627A (en) | 2009-04-22 | 2012-04-12 | 액시킨 파마수티컬스 인코포레이티드 | Arylsulfonamide ccr3 antagonists |
| MX2011011958A (en) | 2009-05-11 | 2012-02-13 | Berg Biosystems Llc | Methods for treatment of metabolic disorders using epimetabolic shifters, multidimensional intracellular molecules, or environmental influencers. |
| ES2350075B1 (en) * | 2009-05-11 | 2011-11-10 | Maria Pilar Mateo Herrero | MICROENCAPSULATED COMPOSITION BASED ON SAPONIFIED EUROPEAN OLEA, SUUSO AND ITS OBTAINING PROCEDURE. |
| US20100291160A1 (en) * | 2009-05-13 | 2010-11-18 | Carver David R | Pharmaceutical system for trans-membrane delivery |
| HUE047755T2 (en) | 2009-05-13 | 2020-05-28 | Cydex Pharmaceuticals Inc | Pharmaceutical compositions comprising prasugrel and cyclodextrin derivatives and methods of making and using the same |
| US9101539B2 (en) * | 2009-05-15 | 2015-08-11 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal pharmaceutical compositions with improved pharmacokinetics |
| US10206813B2 (en) | 2009-05-18 | 2019-02-19 | Dose Medical Corporation | Implants with controlled drug delivery features and methods of using same |
| TWI576352B (en) | 2009-05-20 | 2017-04-01 | 基利法瑪席特有限責任公司 | Nucleoside phosphoramidates |
| WO2010133961A1 (en) | 2009-05-22 | 2010-11-25 | Inventia Healthcare Private Limited | Extended release compositions of cyclobenzaprine |
| US9968564B2 (en) | 2009-06-05 | 2018-05-15 | Intercontinental Great Brands Llc | Delivery of functional compounds |
| US8859003B2 (en) * | 2009-06-05 | 2014-10-14 | Intercontinental Great Brands Llc | Preparation of an enteric release system |
| US20100310726A1 (en) * | 2009-06-05 | 2010-12-09 | Kraft Foods Global Brands Llc | Novel Preparation of an Enteric Release System |
| US20100307542A1 (en) * | 2009-06-05 | 2010-12-09 | Kraft Foods Global Brands Llc | Method of Reducing Surface Oil on Encapsulated Material |
| EP2266546A1 (en) * | 2009-06-08 | 2010-12-29 | Advancell Advanced in Vitro Cell Technologies,S.A. | Process for the preparation of colloidal systems for the delivery of active compounds |
| AU2010259184B2 (en) | 2009-06-09 | 2015-08-13 | Aurinia Pharmaceuticals Inc. | Topical drug delivery systems for ophthalmic use |
| US8937150B2 (en) | 2009-06-11 | 2015-01-20 | Abbvie Inc. | Anti-viral compounds |
| RS53856B1 (en) | 2009-06-11 | 2015-08-31 | Abbvie Bahamas Ltd. | Heterocyclic compounds as inhibitors of hepatitis c virus (hcv) |
| BRPI1013154B1 (en) | 2009-06-12 | 2020-04-07 | Mannkind Corp | MICROPARTICLES OF DICETOPIPERAZINE WITH SPECIFIC SURFACE AREAS DEFINED, DRY POWDER UNDERSTANDING THE REFERRED MICROPARTICLES, METHOD FOR FORMATION OF THE REFERENCESMICROPARTICLES AND THE FORMATION OF MICROPARTYSTEMS |
| WO2010144865A2 (en) | 2009-06-12 | 2010-12-16 | Meritage Pharma, Inc. | Methods for treating gastrointestinal disorders |
| EP2451950B9 (en) | 2009-07-06 | 2016-11-23 | Variation Biotechnologies Inc. | Methods for preparing vesicles and formulations produced therefrom |
| US9907746B2 (en) | 2009-07-06 | 2018-03-06 | Variation Biotechnologies, Inc. | Methods for preparing vesicles and formulations produced therefrom |
| WO2011003870A2 (en) | 2009-07-06 | 2011-01-13 | Creabilis S.A. | Mini-pegylated corticosteroids, compositions including same, and methods of making and using same |
| CA2767008C (en) | 2009-07-07 | 2018-01-30 | Pathway Therapeutics, Inc. | Pyrimidinyl and 1,3,5-triazinyl benzimidazoles and their use in cancer therapy |
| PT2451435T (en) | 2009-07-08 | 2018-01-15 | Hope Medical Entpr Inc D B A Hope Pharmaceuticals | Sodium thiosulfate-containing pharmaceutical compositions |
| CA2767576C (en) | 2009-07-08 | 2020-03-10 | Charleston Laboratories Inc. | Pharmaceutical compositions comprising an antiemetic and an opioid analgesic |
| CA2804265C (en) | 2009-07-10 | 2019-02-19 | Linzy O. Scott, Iii | Methods and compositions for treating thyroid-related medical conditions with reduced folates |
| CA2768024A1 (en) * | 2009-07-20 | 2011-01-27 | Vetegen, Llc | A stable pharmaceutical omeprazole formulation for oral administration |
| WO2011009961A1 (en) | 2009-07-24 | 2011-01-27 | Virologik Gmbh | Combination of proteasome inhibitors and anti-hepatitis medication for treating hepatitis |
| US8951558B2 (en) * | 2009-07-30 | 2015-02-10 | Evonik Röhm Gmbh | Aqueous carbonated medium containing an amino(meth)acrylate polymer or copolymer |
| GB2472327B (en) * | 2009-07-31 | 2013-03-13 | Shin Nippon Biomedical Lab Ltd | Intranasal granisetron and nasal applicator |
| US8735374B2 (en) * | 2009-07-31 | 2014-05-27 | Intelgenx Corp. | Oral mucoadhesive dosage form |
| AR077712A1 (en) | 2009-08-05 | 2011-09-14 | Idenix Pharmaceuticals Inc | SERINA PROTEASA MACROCICLICA INHIBITORS |
| KR20120059558A (en) | 2009-08-19 | 2012-06-08 | 암비트 바이오사이언시즈 코포레이션 | Biaryl compounds and methods of use thereof |
| PT2473475T (en) | 2009-08-31 | 2017-08-02 | Zynerba Pharmaceuticals Inc | Use of cannabidiol prodrugs in topical and transdermal administration with microneedles |
| KR101636958B1 (en) * | 2009-09-11 | 2016-07-06 | 주식회사 대웅제약 | Ursodesoxycholic Acid-Synthetic Hydrotalcite-Eudragit Nanohybrid, Pharmaceutical Composition Containing the Same and Method for Preparing the Same |
| EP2477612A2 (en) * | 2009-09-17 | 2012-07-25 | Evonik Degussa Corporation | Implant devices that differ by release profile and methods of making and using same |
| NZ617025A (en) * | 2009-09-18 | 2014-03-28 | Sanofi Sa | (z)-2-cyano-3-hydroxy-but-2-enoic acid-(4’-trifluormethylphenyl)-amide tablet formulations with improved stability |
| US20110318411A1 (en) | 2010-06-24 | 2011-12-29 | Luber Joseph R | Multi-layered orally disintegrating tablet and the manufacture thereof |
| US8313768B2 (en) | 2009-09-24 | 2012-11-20 | Mcneil-Ppc, Inc. | Manufacture of tablet having immediate release region and sustained release region |
| US20110070286A1 (en) * | 2009-09-24 | 2011-03-24 | Andreas Hugerth | Process for the manufacture of nicotine-comprising chewing gum and nicotine-comprising chewing gum manufactured according to said process |
| US8807979B2 (en) | 2009-09-24 | 2014-08-19 | Mcneil-Ppc, Inc. | Machine for the manufacture of dosage forms utilizing radiofrequency energy |
| WO2011041414A1 (en) | 2009-09-30 | 2011-04-07 | Acura Pharmaceuticals, Inc. | Methods and compositions for deterring abuse |
| CN102548544B (en) * | 2009-10-09 | 2015-01-21 | 永进药品工业株式会社 | Pharmaceutical composition with both immediate and extended release characteristics |
| US8357398B2 (en) * | 2009-10-21 | 2013-01-22 | Alitair Pharmaceuticals Inc. | Benzonatate compositions and methods of use |
| US9511125B2 (en) | 2009-10-21 | 2016-12-06 | Curemark Llc | Methods and compositions for the treatment of influenza |
| WO2011056566A2 (en) | 2009-10-26 | 2011-05-12 | Sunesis Pharmaceuticals, Inc. | Compounds and methods for treatment of cancer |
| WO2011050457A1 (en) | 2009-10-26 | 2011-05-05 | The University Of British Columbia | Stabilized formulation for oral administration of therapeutic agents and related methods |
| CA2778698A1 (en) | 2009-11-03 | 2011-05-12 | Mannkind Corporation | An apparatus and method for simulating inhalation efforts |
| WO2011056764A1 (en) | 2009-11-05 | 2011-05-12 | Ambit Biosciences Corp. | Isotopically enriched or fluorinated imidazo[2,1-b][1,3]benzothiazoles |
| US8663671B2 (en) | 2009-11-05 | 2014-03-04 | Philip Morris Usa Inc. | Methods and compositions for producing hydrogel capsules coated for low permeability and physical integrity |
| US20110112125A1 (en) * | 2009-11-09 | 2011-05-12 | Tritech Biopharmaceuticals Co., Ltd. | Novel hair growth composition |
| US10391059B2 (en) | 2009-11-11 | 2019-08-27 | Rapamycin Holdings, Inc. | Oral rapamycin nanoparticle preparations and use |
| US9283211B1 (en) | 2009-11-11 | 2016-03-15 | Rapamycin Holdings, Llc | Oral rapamycin preparation and use for stomatitis |
| WO2011062614A1 (en) * | 2009-11-20 | 2011-05-26 | Tonix Pharmaceuticals, Inc. | Methods and compositions for treating symptoms associated with post-traumatic stress disorder using cyclobenzaprine |
| WO2011064769A1 (en) | 2009-11-24 | 2011-06-03 | Yissum Research Development Company Of The Hebrew University Of Jerusalem Ltd. | Methods and pharmaceutical compositions for the treatment of hot flashes |
| AU2010325746B2 (en) * | 2009-12-02 | 2016-02-25 | Adare Pharmaceuticals S.R.L. | Fexofenadine microcapsules and compositions containing them |
| US8741343B2 (en) | 2009-12-02 | 2014-06-03 | Adamas Pharmaceuticals, Inc. | Method of administering amantadine prior to a sleep period |
| WO2011069002A1 (en) | 2009-12-02 | 2011-06-09 | Alquest Therapeutics, Inc. | Organoselenium compounds and uses thereof |
| US10610528B2 (en) | 2009-12-08 | 2020-04-07 | Intelgenx Corp. | Solid oral film dosage forms and methods for making same |
| US20110136815A1 (en) | 2009-12-08 | 2011-06-09 | Horst Zerbe | Solid oral film dosage forms and methods for making same |
| US10668060B2 (en) | 2009-12-10 | 2020-06-02 | Collegium Pharmaceutical, Inc. | Tamper-resistant pharmaceutical compositions of opioids and other drugs |
| CN102822175A (en) | 2009-12-18 | 2012-12-12 | 埃迪尼克斯医药公司 | 5,5-fused arylene or heteroarylene hepatitis C virus inhibitors |
| SG181896A1 (en) * | 2009-12-23 | 2012-07-30 | Map Pharmaceuticals Inc | Novel ergoline analogs |
| AU2010339460A1 (en) | 2009-12-30 | 2012-07-19 | Scynexis Inc. | Cyclosporine analogues |
| WO2011082384A2 (en) | 2009-12-31 | 2011-07-07 | Differential Drug Development Associates, Llc | Modulation of solubility, stability, absorption, metabolism, and pharmacokinetic profile of lipophilic drugs by sterols |
| EP2521537A2 (en) | 2010-01-04 | 2012-11-14 | Wockhardt Limited | Pharmaceutical composition for modified delivery of actives |
| JP6196041B2 (en) | 2010-01-11 | 2017-09-13 | オレキシジェン・セラピューティクス・インコーポレーテッド | Methods for providing weight loss therapy in patients with major depression |
| US8603568B2 (en) * | 2010-01-15 | 2013-12-10 | Kemin Industries, Inc. | Hydrolyzed lecithin product to improve digestibility |
| EP2359812A1 (en) * | 2010-01-18 | 2011-08-24 | Cephalon France | Oral lyophilised compositions |
| WO2011089166A1 (en) | 2010-01-19 | 2011-07-28 | Virologik Gmbh | Semicarbazone proteasome inhibitors for treating hiv and hepatitis infection |
| US20110182985A1 (en) * | 2010-01-28 | 2011-07-28 | Coughlan David C | Solid Pharmaceutical Composition with Enhancers and Methods of Preparing thereof |
| WO2011094890A1 (en) | 2010-02-02 | 2011-08-11 | Argusina Inc. | Phenylalanine derivatives and their use as non-peptide glp-1 receptor modulators |
| WO2011097525A1 (en) | 2010-02-05 | 2011-08-11 | Tragara Pharmaceuticals, Inc. | Solid state forms of macrocyclic kinase inhibitors |
| MX337169B (en) | 2010-02-11 | 2016-02-16 | Celgene Corp | Arylmethoxy isoindoline derivatives and compositions comprising and methods of using the same. |
| US20110200669A1 (en) * | 2010-02-15 | 2011-08-18 | Winston Laboratories, Inc. | Method and compositions of civamide to treat disease of the intestines |
| CA2791964A1 (en) | 2010-03-02 | 2011-09-09 | Axikin Pharmaceuticals, Inc. | Isotopically enriched arylsulfonamide ccr3 antagonists |
| US20140294951A1 (en) * | 2011-10-26 | 2014-10-02 | Joseph M. Fayad | Oral formulations mimetic of Roux-en-Y gastric bypass actions on the ileal brake; Compositions, methods of treatment, diagnostics and systems for treatment of metabolic syndrome manifestations including insulin resistance, fatty liver disease, hyperlipidemia, and T2D |
| US20130273154A1 (en) * | 2011-03-02 | 2013-10-17 | Joseph M. Fayad | Oral formulations Mimetic of Roux-en-Y gastric bypass actions on the ileal brake; Compositions, Methods of Treatment, Diagnostics and Systems for treatment of metabolic syndrome manifestations including insulin resistance, fatty liver disease, hpperlipidemia, and type 2 diabetes |
| WO2012118712A2 (en) | 2011-03-02 | 2012-09-07 | Jerome Schentag | Compositions, methods of treatment and diagnostics for treatment of hepatic steatosis alone or in combination with a hepatitis c virus infection |
| WO2011112747A1 (en) * | 2010-03-09 | 2011-09-15 | Catholic Healthcare West | Methods for inhibiting preterm labor and uterine contractility disorders and preventing cervical ripening |
| WO2011112689A2 (en) | 2010-03-11 | 2011-09-15 | Ambit Biosciences Corp. | Saltz of an indazolylpyrrolotriazine |
| ES2664793T3 (en) | 2010-03-12 | 2018-04-23 | Berg Llc | Intravenous formulations of coenzyme Q10 (CoQ10) and methods of use thereof |
| EP2547655B1 (en) | 2010-03-17 | 2016-03-09 | Axikin Pharmaceuticals, Inc. | Arylsulfonamide ccr3 antagonists |
| KR101622441B1 (en) | 2010-03-23 | 2016-05-18 | 버런, 아이엔씨. | Nanoemulsion including sucrose fatty acid ester |
| WO2011120033A1 (en) * | 2010-03-26 | 2011-09-29 | Merrion Research Iii Limited | Pharmaceutical compositions of selective factor xa inhibitors for oral administration |
| TWI540319B (en) | 2010-03-29 | 2016-07-01 | 中央研究院 | Quantitative measurement of nano/micro particle endocytosis with cell mass spectrometry |
| AU2011235044A1 (en) | 2010-03-31 | 2012-11-22 | Gilead Pharmasset Llc | Stereoselective synthesis of phosphorus containing actives |
| US8563530B2 (en) | 2010-03-31 | 2013-10-22 | Gilead Pharmassel LLC | Purine nucleoside phosphoramidate |
| EP2552933A1 (en) | 2010-03-31 | 2013-02-06 | Gilead Pharmasset LLC | Purine nucleoside phosphoramidate |
| US8581001B2 (en) | 2010-04-16 | 2013-11-12 | Codman & Shurtleff | Metformin-cysteine prodrug |
| US20110268809A1 (en) | 2010-04-28 | 2011-11-03 | Paul Andrew Brinkley | Nicotine-Containing Pharmaceutical Compositions |
| US20110274628A1 (en) | 2010-05-07 | 2011-11-10 | Borschke August J | Nicotine-containing pharmaceutical compositions |
| CN103153994B (en) | 2010-05-24 | 2016-02-10 | 罗切斯特大学 | Bicyclic heteroaryl kinase inhibitor and using method |
| CN107029223A (en) | 2010-05-26 | 2017-08-11 | 西莱克塔生物科技公司 | Synthesize nano-carrier combined vaccine |
| WO2011150201A2 (en) | 2010-05-27 | 2011-12-01 | Ambit Biosciences Corporation | Azolyl amide compounds and methods of use thereof |
| WO2011150198A1 (en) | 2010-05-27 | 2011-12-01 | Ambit Biosciences Corporation | Azolyl urea compounds and methods of use thereof |
| CN104945318A (en) | 2010-06-01 | 2015-09-30 | 拜欧赛里克斯公司 | Hydroxypyridone derivatives, pharmaceutical compositions thereof, and their therapeutic use for treating proliferative diseases |
| CN103153309A (en) | 2010-06-01 | 2013-06-12 | 拜欧赛里克斯公司 | Methods of treating hematologic malignancies using 6-cyclohexyl-1-hydroxy-4-methyl-2(1h)-pyridone |
| CN104860907A (en) | 2010-06-07 | 2015-08-26 | 诺沃梅迪科斯有限公司 | Furanyl compounds and the use thereof |
| NZ605440A (en) * | 2010-06-10 | 2014-05-30 | Abbvie Bahamas Ltd | Solid compositions comprising an hcv inhibitor |
| CN102277748A (en) * | 2010-06-12 | 2011-12-14 | 罗莱家纺股份有限公司 | Using method of nanometer vitamin microcapsule finishing agent |
| WO2011153712A1 (en) | 2010-06-12 | 2011-12-15 | Theracos, Inc. | Crystalline form of benzylbenzene sglt2 inhibitor |
| US9375437B2 (en) | 2010-06-18 | 2016-06-28 | Lipocine Inc. | Progesterone containing oral dosage forms and kits |
| MX359281B (en) | 2010-06-21 | 2018-09-21 | Mannkind Corp | Dry powder drug delivery system and methods. |
| US8741373B2 (en) | 2010-06-21 | 2014-06-03 | Virun, Inc. | Compositions containing non-polar compounds |
| WO2011161666A2 (en) * | 2010-06-21 | 2011-12-29 | White Innovation Ltd. | Enclosed liquid capsules |
| US9757331B2 (en) * | 2010-06-24 | 2017-09-12 | Prayon Sa | Stabilized active compound |
| US20110319389A1 (en) | 2010-06-24 | 2011-12-29 | Tonix Pharmaceuticals, Inc. | Methods and compositions for treating fatigue associated with disordered sleep using very low dose cyclobenzaprine |
| GB201010954D0 (en) * | 2010-06-29 | 2010-08-11 | Edko Pazarlama Tanitim Ticaret | Compositions |
| AU2011276223C1 (en) | 2010-07-06 | 2016-05-12 | Variation Biotechnologies, Inc. | Compositions and methods for treating influenza |
| CN102309461B (en) * | 2010-07-09 | 2013-08-14 | 重庆医科大学 | Pyridostigmine bromide odor masking dispersible tablets and preparation method thereof |
| CN103096877B (en) * | 2010-07-15 | 2017-09-22 | 海布里詹尼克斯股份公司 | The new formulation of 14 epimerism analogs of vitamin D |
| US20130178522A1 (en) | 2010-07-19 | 2013-07-11 | James M. Jamison | Vitamin c and chromium-free vitamin k, and compositions thereof for treating an nfkb-mediated condition or disease |
| CN101953822B (en) * | 2010-07-30 | 2012-05-30 | 合肥立方制药股份有限公司 | Venlafaxine hydrochloride controlled release tablets and preparation method thereof |
| EP2611795B1 (en) | 2010-09-01 | 2016-05-04 | Ambit Biosciences Corporation | An optically active pyrazolylaminoquinazoline, and pharmaceutical compositions and methods of use thereof |
| EP2663553B1 (en) | 2010-09-01 | 2015-08-26 | Ambit Biosciences Corporation | Quinoline and isoquinoline derivatives for use as jak modulators |
| EP2611789A1 (en) | 2010-09-01 | 2013-07-10 | Ambit Biosciences Corporation | Quinazoline compounds and methods of use thereof |
| US20130225614A1 (en) | 2010-09-01 | 2013-08-29 | Ambit Biosciences Corporation | 4-azolylaminoquinazoline derivatives and methods of use thereof |
| WO2012030924A1 (en) | 2010-09-01 | 2012-03-08 | Ambit Biosciences Corporation | Azolopyridine and azolopyrimidine compounds and methods of use thereof |
| US20120053176A1 (en) | 2010-09-01 | 2012-03-01 | Ambit Biosciences Corp. | Adenosine a3 receptor modulating compounds and methods of use thereof |
| EP2611793A1 (en) | 2010-09-01 | 2013-07-10 | Ambit Biosciences Corporation | 2-cycloquinazoline derivatives and methods of use thereof |
| US20130225578A1 (en) | 2010-09-01 | 2013-08-29 | Ambit Biosciences Corporation | 7-cyclylquinazoline derivatives and methods of use thereof |
| WO2012030894A1 (en) | 2010-09-01 | 2012-03-08 | Ambit Biosciences Corporation | Thienopyridine and thienopyrimidine compounds and methods of use thereof |
| CA2809983A1 (en) | 2010-09-01 | 2012-03-08 | Ambit Biosciences Corporation | Hydrobromide salts of a pyrazolylaminoquinazoline |
| US8507496B2 (en) | 2010-09-07 | 2013-08-13 | Dmi Acquisition Corp. | Treatment of diseases |
| US8933055B2 (en) | 2010-09-22 | 2015-01-13 | Ecolab Usa Inc. | Antimicrobial compositions containing cationic active ingredients and quaternary sugar derived surfactants |
| WO2012044641A1 (en) | 2010-09-29 | 2012-04-05 | Pathway Therapeutics Inc. | 1,3,5-triazinyl benzimidazole sulfonamides and their use in cancer therapy |
| MX2013003954A (en) | 2010-10-11 | 2013-08-01 | Axikin Pharmaceuticals Inc | Salts of arylsulfonamide ccr3 antagonists. |
| US8808743B2 (en) * | 2010-10-20 | 2014-08-19 | William Wayne Howard | Benzonatate compositions and methods of use |
| US8865198B2 (en) | 2010-10-25 | 2014-10-21 | Dexcel Pharma Technologies Ltd. | Method for treating a periodontal disease |
| EP2632471B1 (en) | 2010-10-27 | 2019-05-01 | Dignity Health | Trimegestone (tmg) for treatment of preterm birth |
| US10695431B2 (en) | 2010-10-29 | 2020-06-30 | Infirst Healthcare Limited | Solid solution compositions and use in cardiovascular disease |
| US8895536B2 (en) | 2010-10-29 | 2014-11-25 | Infirst Healthcare Ltd. | Compositions and methods for treating chronic inflammation and inflammatory diseases |
| US11730709B2 (en) | 2010-10-29 | 2023-08-22 | Infirst Healthcare Limited | Compositions and methods for treating severe pain |
| US9504664B2 (en) | 2010-10-29 | 2016-11-29 | Infirst Healthcare Limited | Compositions and methods for treating severe pain |
| US9737500B2 (en) | 2010-10-29 | 2017-08-22 | Infirst Healthcare Limited | Compositions and methods for treating severe pain |
| US10695432B2 (en) | 2010-10-29 | 2020-06-30 | Infirst Healthcare Limited | Solid solution compositions and use in severe pain |
| US9744132B2 (en) | 2010-10-29 | 2017-08-29 | Infirst Healthcare Limited | Solid solution compositions and use in chronic inflammation |
| US11224659B2 (en) | 2010-10-29 | 2022-01-18 | Infirst Healthcare Limited | Solid solution compositions and use in severe pain |
| US9308213B2 (en) | 2010-10-29 | 2016-04-12 | Infirst Healthcare Limited | Solid solution compositions and use in chronic inflammation |
| US11202831B2 (en) | 2010-10-29 | 2021-12-21 | Infirst Healthcare Limited | Solid solution compositions and use in cardiovascular disease |
| US9271950B2 (en) | 2010-10-29 | 2016-03-01 | Infirst Healthcare Limited | Compositions for treating chronic inflammation and inflammatory diseases |
| US9994443B2 (en) | 2010-11-05 | 2018-06-12 | Selecta Biosciences, Inc. | Modified nicotinic compounds and related methods |
| CA2817577A1 (en) | 2010-11-10 | 2012-05-18 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US10238684B2 (en) | 2010-11-18 | 2019-03-26 | Foundational Biosystems, Llc | Micro- and nano-quantity sleep enhancing nutrient composition and method of enhancing central nervous system protein clearance using same |
| US20180153904A1 (en) | 2010-11-30 | 2018-06-07 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| WO2012075140A1 (en) | 2010-11-30 | 2012-06-07 | Pharmasset, Inc. | Compounds |
| US20140079686A1 (en) | 2010-12-06 | 2014-03-20 | Shikha P. Barman | Methods For Treating Baldness And Promoting Hair Growth |
| WO2012080050A1 (en) | 2010-12-14 | 2012-06-21 | F. Hoffmann-La Roche Ag | Solid forms of a phenoxybenzenesulfonyl compound |
| DK2663309T3 (en) | 2011-01-10 | 2017-06-19 | Infinity Pharmaceuticals Inc | METHODS FOR PRODUCING ISOQUINOLINONES AND SOLID FORMS OF ISOQUINOLINONES |
| MX359103B (en) | 2011-01-13 | 2018-09-14 | Variation Biotechnologies Inc | Compositions and methods for treating viral infections. |
| CA2825152A1 (en) | 2011-01-31 | 2012-08-09 | Celgene Corporation | Pharmaceutical compositions of cytidine analogs and methods of use thereof |
| TW201309690A (en) | 2011-02-10 | 2013-03-01 | Idenix Pharmaceuticals Inc | Macrocyclic serine protease inhibitors, pharmaceutical compositions thereof, and their use for treating HCV infections |
| ES2716865T3 (en) | 2011-02-18 | 2019-06-17 | Scripps Research Inst | Directed differentiation of oligodendrocyte precursor cells to a myelinating cell target |
| WO2012112940A1 (en) | 2011-02-18 | 2012-08-23 | Kythera Biopharmaceuticals, Inc. | Treatment of submental fat |
| EP2678007A4 (en) | 2011-02-25 | 2015-08-12 | Univ South Dakota | Protein nanocarriers for topical delivery |
| RU2612506C2 (en) | 2011-03-03 | 2017-03-09 | Импел Ньюрофарма Инк. | Nasal drug delivery device |
| JP5809296B2 (en) | 2011-03-03 | 2015-11-10 | シーチェイド ファーマシューティカルズ,インコーポレーテッド | Antifungal drugs and their use |
| US20120232159A1 (en) | 2011-03-07 | 2012-09-13 | Tonix Pharmaceuticals, Inc. | Methods and Compositions for Treating Depression using Cyclobenzaprine |
| RU2013145556A (en) | 2011-03-11 | 2015-04-20 | Селджин Корпорейшн | APPLICATION OF 3- (5-AMINO-2-METHYL-4-OXOCHINAZOLIN-3 (4H) -YL) PIPERIDINE-2,6-DION IN THE TREATMENT OF IMMUNE AND INFLAMMATORY DISEASES |
| EP2691388A1 (en) | 2011-03-28 | 2014-02-05 | MEI Pharma, Inc. | (fused ring arylamino and heterocyclylamino) pyrimidynyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| AU2012236722A1 (en) | 2011-03-28 | 2013-10-17 | Mei Pharma, Inc. | (alpha-substituted cycloalkylamino and heterocyclylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| TWI572599B (en) | 2011-03-28 | 2017-03-01 | Mei製藥公司 | (alpha-substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5-triazinyl benzimidazoles, pharmaceutical compositions thereof, and their use in treating proliferative diseases |
| US20120252721A1 (en) | 2011-03-31 | 2012-10-04 | Idenix Pharmaceuticals, Inc. | Methods for treating drug-resistant hepatitis c virus infection with a 5,5-fused arylene or heteroarylene hepatitis c virus inhibitor |
| WO2012154321A1 (en) | 2011-03-31 | 2012-11-15 | Idenix Pharmaceuticals, Inc. | Compounds and pharmaceutical compositions for the treatment of viral infections |
| MX353285B (en) | 2011-04-01 | 2018-01-05 | Mannkind Corp | Blister package for pharmaceutical cartridges. |
| ES2762451T3 (en) | 2011-04-04 | 2020-05-25 | Berg Llc | Treatment of tumors of the central nervous system with coenzyme Q10 |
| US8653058B2 (en) | 2011-04-05 | 2014-02-18 | Kythera Biopharmaceuticals, Inc. | Compositions comprising deoxycholic acid and salts thereof suitable for use in treating fat deposits |
| MX362974B (en) | 2011-04-21 | 2019-02-28 | Curemark Llc | Compounds for the treatment of neuropsychiatric disorders. |
| CA2835208C (en) | 2011-05-09 | 2019-08-20 | Impel Neuropharma, Inc. | Nozzles for nasal drug delivery |
| US9757388B2 (en) | 2011-05-13 | 2017-09-12 | Acerus Pharmaceuticals Srl | Intranasal methods of treating women for anorgasmia with 0.6% and 0.72% testosterone gels |
| US20130045958A1 (en) | 2011-05-13 | 2013-02-21 | Trimel Pharmaceuticals Corporation | Intranasal 0.15% and 0.24% testosterone gel formulations and use thereof for treating anorgasmia or hypoactive sexual desire disorder |
| US20130040923A1 (en) | 2011-05-13 | 2013-02-14 | Trimel Pharmaceuticals Corporation | Intranasal lower dosage strength testosterone gel formulations and use thereof for treating anorgasmia or hypoactive sexual desire disorder |
| US10201584B1 (en) | 2011-05-17 | 2019-02-12 | Abbvie Inc. | Compositions and methods for treating HCV |
| EP2526971A1 (en) * | 2011-05-25 | 2012-11-28 | ArisGen SA | Mucosal delivery of drugs |
| US8524664B2 (en) | 2011-06-02 | 2013-09-03 | Colorado Seminary, Which owns and Operates The Univeristy of Denver | Methods of treating overproduction of cortisol using ACTH antagonist peptides |
| CA2839270C (en) | 2011-06-17 | 2018-09-18 | Berg Llc | Inhalable liposomal pharmaceutical compositions |
| WO2012174472A1 (en) | 2011-06-17 | 2012-12-20 | Mannkind Corporation | High capacity diketopiperazine microparticles |
| JP2014517076A (en) | 2011-06-23 | 2014-07-17 | マップ・ファーマシューティカルズ・インコーポレイテッド | Novel fluoroergoline analogues |
| MX2014000648A (en) | 2011-07-19 | 2014-09-25 | Infinity Pharmaceuticals Inc | Heterocyclic compounds and uses thereof. |
| AU2012284088B2 (en) | 2011-07-19 | 2015-10-08 | Infinity Pharmaceuticals Inc. | Heterocyclic compounds and uses thereof |
| US8951996B2 (en) | 2011-07-28 | 2015-02-10 | Lipocine Inc. | 17-hydroxyprogesterone ester-containing oral compositions and related methods |
| UA113291C2 (en) | 2011-08-04 | 2017-01-10 | TRANSCLOMYPHENE METABOLITES AND THEIR APPLICATIONS | |
| JP6016343B2 (en) * | 2011-09-08 | 2016-10-26 | 株式会社ロッテ | Oral composition |
| KR20140075693A (en) | 2011-08-29 | 2014-06-19 | 인피니티 파마슈티칼스, 인코포레이티드 | Heterocyclic compounds and uses thereof |
| AR088441A1 (en) | 2011-09-12 | 2014-06-11 | Idenix Pharmaceuticals Inc | SUBSTITUTED CARBONYLOXYMETHYLPHOSPHORAMIDATE COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS FOR THE TREATMENT OF VIRAL INFECTIONS |
| JP2014526474A (en) | 2011-09-12 | 2014-10-06 | アイディニックス ファーマシューティカルズ インコーポレイテッド | Compounds and pharmaceutical compositions for the treatment of viral infections |
| US10226417B2 (en) | 2011-09-16 | 2019-03-12 | Peter Jarrett | Drug delivery systems and applications |
| US9474303B2 (en) | 2011-09-22 | 2016-10-25 | R.J. Reynolds Tobacco Company | Translucent smokeless tobacco product |
| US20130078307A1 (en) | 2011-09-22 | 2013-03-28 | Niconovum Usa, Inc. | Nicotine-containing pharmaceutical composition |
| US9629392B2 (en) | 2011-09-22 | 2017-04-25 | R.J. Reynolds Tobacco Company | Translucent smokeless tobacco product |
| US9084439B2 (en) | 2011-09-22 | 2015-07-21 | R.J. Reynolds Tobacco Company | Translucent smokeless tobacco product |
| WO2013049749A2 (en) | 2011-09-29 | 2013-04-04 | Plx Pharma Inc. | pH DEPENDENT CARRIERS FOR TARGETED RELEASE OF PHARMACEUTICALS ALONG THE GASTROINTESTINAL TRACT, COMPOSITIONS THEREFROM, AND MAKING AND USING SAME |
| US9630979B2 (en) | 2011-09-29 | 2017-04-25 | Infinity Pharmaceuticals, Inc. | Inhibitors of monoacylglycerol lipase and methods of their use |
| US8492368B2 (en) * | 2011-10-07 | 2013-07-23 | Florida State University Research Foundation | Nasal delivery mechanism for prophylactic and post-acute use of progesterone and/or its enantiomer for use in treatment of mild traumatic brain injuries |
| PL2766029T3 (en) | 2011-10-10 | 2020-08-24 | Ampio Pharmaceuticals, Inc. | Treatment of degenerative joint disease |
| KR20140075772A (en) | 2011-10-10 | 2014-06-19 | 앰피오 파마슈티컬스 인코퍼레이티드 | Implantable medical devices with increased immune tolerance, and methods for making and implanting |
| KR102066297B1 (en) | 2011-10-14 | 2020-01-14 | 암비트 바이오사이언시즈 코포레이션 | Heterocyclic compounds and use thereof as modulators of type iii receptor tyrosine kinases |
| US8507460B2 (en) | 2011-10-14 | 2013-08-13 | Idenix Pharmaceuticals, Inc. | Substituted 3′,5′-cyclic phosphates of purine nucleotide compounds and pharmaceutical compositions for the treatment of viral infections |
| US9308263B2 (en) | 2011-10-21 | 2016-04-12 | Seachaid Pharmaceuticals, Inc. | Pharmaceutical compositions and uses thereof |
| US9907748B2 (en) | 2011-10-21 | 2018-03-06 | Niconovum Usa, Inc. | Excipients for nicotine-containing therapeutic compositions |
| IN2014DN03093A (en) | 2011-10-24 | 2015-05-15 | Mannkind Corp | |
| SG10201605205VA (en) | 2011-10-28 | 2016-08-30 | Ampio Pharmaceuticals Inc | Treatment of rhinitis |
| KR20190090048A (en) | 2011-12-05 | 2019-07-31 | 인셉트, 엘엘씨 | Medical organogel processes and compositions |
| US8912215B2 (en) | 2011-12-13 | 2014-12-16 | Everon Biosciences, Inc. | Rapamycin composition |
| CA2859173A1 (en) | 2011-12-19 | 2013-06-27 | Map Pharmaceuticals, Inc. | Novel iso-ergoline derivatives |
| SG10201506202RA (en) | 2011-12-21 | 2015-09-29 | Map Pharmaceuticals Inc | Novel neuromodulatory compounds |
| US9034832B2 (en) | 2011-12-29 | 2015-05-19 | Abbvie Inc. | Solid compositions |
| US9724230B2 (en) | 2012-01-04 | 2017-08-08 | Sight Sciences, Inc. | Dry eye treatment apparatus and methods |
| US11285040B2 (en) | 2012-01-04 | 2022-03-29 | Sight Sciences, Inc. | Combination treatment systems |
| US9510972B2 (en) | 2012-01-04 | 2016-12-06 | Sight Sciences, Inc. | Dry eye treatment systems |
| US10973680B2 (en) | 2012-01-04 | 2021-04-13 | Sight Sciences, Inc. | Controller for dry eye treatment systems |
| CL2012000047A1 (en) * | 2012-01-06 | 2012-07-20 | Cultivos Hidrobiologicos Y Biotecnologia Aguamarina S A | Method for reducing particulate matter suspended in air or water, which comprises agglomerating the particulate matter suspended with negatively charged exopolysaccharides (eps). |
| US20140356399A1 (en) | 2012-01-12 | 2014-12-04 | Variation Biotechnologies, Inc. | Compositions and methods for treating viral infections |
| RU2698906C2 (en) | 2012-01-27 | 2019-09-02 | Вэриэйшн Биотекнолоджиз, Инк. | Methods and compositions for therapeutic agents |
| KR20190102084A (en) * | 2012-02-06 | 2019-09-02 | 메리얼 인코포레이티드 | Parasiticidal oral veterinary compositions comprising systemically-acting active agents, methods and uses thereof |
| US9763928B2 (en) | 2012-02-10 | 2017-09-19 | Niconovum Usa, Inc. | Multi-layer nicotine-containing pharmaceutical composition |
| WO2013130600A1 (en) | 2012-02-29 | 2013-09-06 | Ambit Biosciences Corporation | Solid forms comprising optically active pyrazolylaminoquinazoline, compositions thereof, and uses therewith |
| WO2013138617A1 (en) | 2012-03-16 | 2013-09-19 | Axikin Pharmaceuticals, Inc. | 3,5-diaminopyrazole kinase inhibitors |
| KR102236462B1 (en) | 2012-03-19 | 2021-04-08 | 시다라 세라퓨틱스, 인코포레이티드 | Dosing regimens for echinocandin class compounds |
| US8940742B2 (en) | 2012-04-10 | 2015-01-27 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| BR112014026351B1 (en) | 2012-04-25 | 2022-09-27 | Spi Pharma Inc | COMPOSITIONS COMPRISING MICROSPHERES AND PHARMACEUTICAL FORMULATION CONTAINING SUCH COMPOSITIONS |
| US9511028B2 (en) | 2012-05-01 | 2016-12-06 | Johnson & Johnson Consumer Inc. | Orally disintegrating tablet |
| US9233491B2 (en) | 2012-05-01 | 2016-01-12 | Johnson & Johnson Consumer Inc. | Machine for production of solid dosage forms |
| US9445971B2 (en) | 2012-05-01 | 2016-09-20 | Johnson & Johnson Consumer Inc. | Method of manufacturing solid dosage form |
| CA2871820C (en) | 2012-05-10 | 2020-11-03 | Painreform Ltd. | Depot formulations of a hydrophobic active ingredient and methods for preparation thereof |
| WO2013177195A1 (en) | 2012-05-22 | 2013-11-28 | Idenix Pharmaceuticals, Inc. | 3',5'-cyclic phosphate prodrugs for hcv infection |
| WO2013177219A1 (en) | 2012-05-22 | 2013-11-28 | Idenix Pharmaceuticals, Inc. | D-amino acid compounds for liver disease |
| US9109001B2 (en) | 2012-05-22 | 2015-08-18 | Idenix Pharmaceuticals, Inc. | 3′,5′-cyclic phosphoramidate prodrugs for HCV infection |
| US10350278B2 (en) | 2012-05-30 | 2019-07-16 | Curemark, Llc | Methods of treating Celiac disease |
| ES2924024T3 (en) | 2012-06-06 | 2022-10-04 | Nalpropion Pharmaceuticals Llc | Composition for use in a method for the treatment of overweight and obesity in patients with high cardiovascular risk |
| US9078925B2 (en) | 2012-06-18 | 2015-07-14 | Galephar Pharmaceutical Research, Inc. | Pharmaceutical semi-solid composition of isotretinoin |
| WO2013190384A1 (en) | 2012-06-19 | 2013-12-27 | Affinium Pharmaceuticals, Inc. | Prodrug derivatives of (e)-n-methyl-n-((3-methylbenzofuran-2-yl)methyl)-3-(7-oxo-5,6,7,8-tetrahydro-1,8-naphthyridin-3-yl)acrylamide |
| US9012640B2 (en) | 2012-06-22 | 2015-04-21 | Map Pharmaceuticals, Inc. | Cabergoline derivatives |
| SG10201605800UA (en) | 2012-07-12 | 2016-09-29 | Mannkind Corp | Dry powder drug delivery system and methods |
| PL2872121T3 (en) | 2012-07-12 | 2019-02-28 | SpecGx LLC | Extended release, abuse deterrent pharmaceutical compositions |
| KR20150041123A (en) | 2012-08-09 | 2015-04-15 | 셀진 코포레이션 | Salts and solid forms of (S)-3-(4-((4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione and compositions comprising and methods of using the same |
| CN114939119A (en) | 2012-08-09 | 2022-08-26 | 细胞基因公司 | Treatment of immune-related and inflammatory diseases |
| WO2014028610A1 (en) | 2012-08-15 | 2014-02-20 | Tris Pharma, Inc. | Methylphenidate extended release chewable tablet |
| US9029320B2 (en) * | 2012-08-27 | 2015-05-12 | Red Mountain Med Spa, LLC | Formulations and methods for weight loss and body contouring |
| CA2922849A1 (en) | 2012-08-31 | 2014-03-06 | Ixchel Pharma, Llc | Agents useful for treating obesity, diabetes and related disorders |
| EP2892884A1 (en) | 2012-09-07 | 2015-07-15 | Axikin Pharmaceuticals, Inc. | Isotopically enriched arylsulfonamide ccr3 antagonists |
| US9789063B2 (en) * | 2012-09-27 | 2017-10-17 | Basf Se | Storage-stable dust-free homogeneous particulate formulation |
| WO2014055647A1 (en) | 2012-10-03 | 2014-04-10 | Mei Pharma, Inc. | (sulfinyl and sulfonyl benzimidazolyl) pyrimidines and triazines, pharmaceutical compositions thereof, and their use for treating proliferative diseases |
| US10513534B2 (en) | 2012-10-08 | 2019-12-24 | Idenix Pharmaceuticals Llc | 2′-chloro nucleoside analogs for HCV infection |
| EP2909223B1 (en) | 2012-10-19 | 2017-03-22 | Idenix Pharmaceuticals LLC | Dinucleotide compounds for hcv infection |
| WO2014066239A1 (en) | 2012-10-22 | 2014-05-01 | Idenix Pharmaceuticals, Inc. | 2',4'-bridged nucleosides for hcv infection |
| WO2014066856A1 (en) | 2012-10-26 | 2014-05-01 | Mannkind Corporation | Inhalable influenza vaccine compositions and methods |
| DK2914296T4 (en) | 2012-11-01 | 2022-01-03 | Infinity Pharmaceuticals Inc | Treatment of cancers using PI3 kinase isoform modulators |
| EP2914294A1 (en) | 2012-11-02 | 2015-09-09 | Repros Therapeutics Inc. | Trans-clomiphene for use in cancer therapy |
| US20150272924A1 (en) | 2012-11-08 | 2015-10-01 | Summa Health System | Vitamin c, vitamin k, a polyphenol, and combinations thereof for wound healing |
| WO2014078436A1 (en) | 2012-11-14 | 2014-05-22 | Idenix Pharmaceuticals, Inc. | D-alanine ester of sp-nucleoside analog |
| US20140140951A1 (en) | 2012-11-14 | 2014-05-22 | Idenix Pharmaceuticals, Inc. | D-Alanine Ester of Rp-Nucleoside Analog |
| JP2015537029A (en) | 2012-11-14 | 2015-12-24 | ダブリュー・アール・グレース・アンド・カンパニー−コーンW R Grace & Co−Conn | Composition containing biologically active substance and irregular inorganic oxide |
| US9156781B2 (en) | 2012-11-30 | 2015-10-13 | Novomedix, Llc | Substituted biaryl sulfonamides and the use thereof |
| WO2014085795A1 (en) | 2012-11-30 | 2014-06-05 | University Of Rochester | Mixed lineage kinase inhibitors for hiv/aids therapies |
| RU2018141241A (en) | 2012-11-30 | 2019-01-24 | Экьюра Фармасьютикалз, Инк. | SELF-ADJUSTABLE RELEASE OF PHARMACEUTICAL INGREDIENT |
| US8859005B2 (en) | 2012-12-03 | 2014-10-14 | Intercontinental Great Brands Llc | Enteric delivery of functional ingredients suitable for hot comestible applications |
| JP2014114247A (en) * | 2012-12-11 | 2014-06-26 | Capsugel Belgium Nv | Oil-in-water type emulsion and method for producing the same |
| FR2999432B1 (en) * | 2012-12-17 | 2014-12-12 | Ethypharm Sa | ORODISPERSIBLE COMPRESSES OBTAINED BY COMPRESSION MOLDING |
| RU2015128609A (en) | 2012-12-19 | 2017-01-25 | КАШИВ ФАРМА, ЭлЭлСи | Oversaturated Stabilized Nanoparticles for Weakly Soluble Drugs |
| EP2935304A1 (en) | 2012-12-19 | 2015-10-28 | IDENIX Pharmaceuticals, Inc. | 4'-fluoro nucleosides for the treatment of hcv |
| SG11201504931SA (en) | 2012-12-21 | 2015-07-30 | Map Pharmaceuticals Inc | Novel methysergide derivatives |
| US9271951B2 (en) | 2012-12-21 | 2016-03-01 | Mylan Inc. | Levothyroxine formulation with acacia |
| US20140179784A1 (en) * | 2012-12-21 | 2014-06-26 | Mylan Inc. | Levothyroxine formulation with carrageenan |
| WO2014110305A1 (en) | 2013-01-11 | 2014-07-17 | Mayo Foundation For Medical Education And Research | Vitamins c and k for treating polycystic diseases |
| MX2015009045A (en) | 2013-01-14 | 2015-12-17 | Infirst Healthcare Ltd | Compositions and methods for treating severe pain. |
| SG11201505241WA (en) * | 2013-01-14 | 2015-08-28 | Infirst Healthcare Ltd | Solid solution compositions and use in severe pain |
| CN103040788A (en) * | 2013-01-19 | 2013-04-17 | 南京正宽医药科技有限公司 | Cefuroxime axetil capsule and preparation method thereof |
| HUE045625T2 (en) * | 2013-01-30 | 2019-12-30 | Daewoong Co Ltd | Pharmaceutical composition for protecting wounds, providing hemostasis, or preventing adhesion in the gastrointestinal tract |
| AU2014211715B2 (en) | 2013-02-04 | 2016-08-25 | Infirst Healthcare Limited | Compositions and methods for treating chronic inflammation and inflammatory diseases |
| US9309275B2 (en) | 2013-03-04 | 2016-04-12 | Idenix Pharmaceuticals Llc | 3′-deoxy nucleosides for the treatment of HCV |
| WO2014137930A1 (en) | 2013-03-04 | 2014-09-12 | Idenix Pharmaceuticals, Inc. | Thiophosphate nucleosides for the treatment of hcv |
| US20140255452A1 (en) | 2013-03-11 | 2014-09-11 | Niconovum Usa, Inc. | Method and apparatus for differentiating oral pouch products |
| US20160030401A1 (en) | 2013-03-13 | 2016-02-04 | The Board Of Regents Of The University Of Texas System | Use of mtor inhibitors for prevention of intestinal polyp growth and cancer |
| RU2719579C2 (en) * | 2013-03-14 | 2020-04-21 | Сидара Терапьютикс, Инк. | Dosing regimens for echinocandin class compounds |
| US11484534B2 (en) | 2013-03-14 | 2022-11-01 | Abbvie Inc. | Methods for treating HCV |
| US11744838B2 (en) | 2013-03-15 | 2023-09-05 | Acerus Biopharma Inc. | Methods of treating hypogonadism with transnasal testosterone bio-adhesive gel formulations in male with allergic rhinitis, and methods for preventing an allergic rhinitis event |
| EP2970194A1 (en) | 2013-03-15 | 2016-01-20 | Infinity Pharmaceuticals, Inc. | Salts and solid forms of isoquinolinones and composition comprising and methods of using the same |
| KR20150132508A (en) | 2013-03-15 | 2015-11-25 | 앰피오 파마슈티컬스 인코퍼레이티드 | Compositions for the mobilization, homing, expansion and differentiation of stem cells and methods of using the same |
| KR102499439B1 (en) | 2013-03-15 | 2023-02-13 | 맨카인드 코포레이션 | Microcrystalline diketopiperazine compositions and methods |
| CA3119755A1 (en) | 2013-03-15 | 2014-09-18 | Tonix Pharma Holdings Limited | Eutectic formulations of amitriptyline hydrochloride and mannitol |
| US9351517B2 (en) | 2013-03-15 | 2016-05-31 | Virun, Inc. | Formulations of water-soluble derivatives of vitamin E and compositions containing same |
| CN105188670B (en) | 2013-03-15 | 2018-11-02 | 马留斯医药有限责任公司 | Emulsion formulations |
| WO2014167439A1 (en) | 2013-03-26 | 2014-10-16 | Wockhardt Limited | Modified release pharmaceutical compositions of topiramate or salts thereof |
| US9187515B2 (en) | 2013-04-01 | 2015-11-17 | Idenix Pharmaceuticals Llc | 2′,4′-fluoro nucleosides for the treatment of HCV |
| CN113797343A (en) | 2013-04-08 | 2021-12-17 | 博格有限责任公司 | Treatment of cancer using coenzyme Q10 combination therapy |
| EA201591954A1 (en) * | 2013-04-12 | 2016-04-29 | Вайом Байосайнсиз Пвт. Лтд. | COMPOSITION AND COMPOSITION OF ANTI-MICROBAL MEANS, METHODS OF THEIR RECEPTION AND METHODS OF TREATING MICROBIAL INFECTIONS |
| WO2014172572A1 (en) * | 2013-04-18 | 2014-10-23 | Board Of Regents, The University Of Texas System | Antimicrobial wraps for medical implants |
| US20160074322A1 (en) * | 2013-04-25 | 2016-03-17 | Seachaid Pharmaceuticals, Inc. | Oral cefepime compositions and uses thereof |
| CA2909954C (en) | 2013-04-28 | 2021-03-23 | Impel Neuropharma, Inc. | Medical unit dose container |
| US20140335153A1 (en) * | 2013-05-09 | 2014-11-13 | Cure Pharmaceutical Corporation | Thin film with high load of active ingredient |
| DK3003309T3 (en) | 2013-05-30 | 2020-12-14 | Infinity Pharmaceuticals Inc | Treatment of cancer with PI3 kinase isoform modulators |
| EP3004130B1 (en) | 2013-06-05 | 2019-08-07 | Idenix Pharmaceuticals LLC. | 1',4'-thio nucleosides for the treatment of hcv |
| US10154971B2 (en) | 2013-06-17 | 2018-12-18 | Adamas Pharma, Llc | Methods of administering amantadine |
| CA2918369C (en) | 2013-07-18 | 2021-06-29 | Mannkind Corporation | Heat-stable dry powder pharmaceutical compositions and methods |
| EP3027636B1 (en) | 2013-08-01 | 2022-01-05 | Idenix Pharmaceuticals LLC | D-amino acid phosphoramidate pronucleotides of halogeno pyrimidine compounds for liver disease |
| WO2015021064A1 (en) | 2013-08-05 | 2015-02-12 | Mannkind Corporation | Insufflation apparatus and methods |
| JP6539274B2 (en) | 2013-08-12 | 2019-07-03 | ファーマシューティカル マニュファクチュアリング リサーチ サービシズ,インコーポレーテッド | Extruded immediate release abuse deterrent pills |
| RU2016111675A (en) | 2013-08-30 | 2017-10-04 | Эмбит Байосайенсиз Корпорейшн | COMPOUNDS OF BIARILACETAMIDE AND METHODS OF USE |
| WO2015035094A1 (en) | 2013-09-04 | 2015-03-12 | Berg Llc | Methods of treatment of cancer by continuous infusion of coenzyme q10 |
| NZ631142A (en) | 2013-09-18 | 2016-03-31 | Axikin Pharmaceuticals Inc | Pharmaceutically acceptable salts of 3,5-diaminopyrazole kinase inhibitors |
| CA2923753C (en) | 2013-09-19 | 2021-10-12 | Terumo Corporation | Polymer particles |
| AU2014321278B2 (en) | 2013-09-19 | 2016-11-10 | Microvention, Inc. | Polymer films |
| WO2015042375A1 (en) | 2013-09-20 | 2015-03-26 | Idenix Pharmaceuticals, Inc. | Hepatitis c virus inhibitors |
| WO2015051336A1 (en) | 2013-10-03 | 2015-04-09 | David Wise | Compositions and methods for treating pelvic pain and other conditions |
| WO2015051241A1 (en) | 2013-10-04 | 2015-04-09 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| NZ718430A (en) | 2013-10-04 | 2021-12-24 | Infinity Pharmaceuticals Inc | Heterocyclic compounds and uses thereof |
| US9757330B2 (en) | 2013-10-18 | 2017-09-12 | Industrial Technology Research Institute | Recipe for in-situ gel, and implant, drug delivery system formed thereby |
| US20160244452A1 (en) | 2013-10-21 | 2016-08-25 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| WO2015061683A1 (en) | 2013-10-25 | 2015-04-30 | Idenix Pharmaceuticals, Inc. | D-amino acid phosphoramidate and d-alanine thiophosphoramidate pronucleotides of nucleoside compounds useful for the treatment of hcv |
| EP3063165A1 (en) | 2013-11-01 | 2016-09-07 | Idenix Pharmaceuticals LLC | D-alanine phosphoramidate pronucleotides of 2'-methyl 2'-fluoro guanosine nucleoside compounds for the treatment of hcv |
| AU2014346486B2 (en) | 2013-11-08 | 2016-12-08 | Terumo Corporation | Polymer particles |
| GB201319792D0 (en) * | 2013-11-08 | 2013-12-25 | Sigmoid Pharma Ltd | Formulations |
| US10064905B1 (en) * | 2013-11-11 | 2018-09-04 | Ilysm, LLC | Pharmaceutical preparation |
| BR112016010942B1 (en) * | 2013-11-15 | 2020-11-24 | Dsm Ip Assets B.V | FORMULATION OF COMPOUNDS LITTLE SOLUBLE BY HOT FUSION EXTRUSION AND HOT FUSION EXTRUSION PROCESS FOR THE SAME PRODUCTION |
| CA2931458A1 (en) | 2013-11-27 | 2015-06-04 | Idenix Pharmaceuticals Llc | Nucleotides for the treatment of liver cancer |
| EP3074399A1 (en) | 2013-11-27 | 2016-10-05 | Idenix Pharmaceuticals LLC | 2'-dichloro and 2'-fluoro-2'-chloro nucleoside analogues for hcv infection |
| WO2015095391A1 (en) | 2013-12-17 | 2015-06-25 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded extended release abuse deterrent pill |
| US9492444B2 (en) | 2013-12-17 | 2016-11-15 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded extended release abuse deterrent pill |
| EP3083654A1 (en) | 2013-12-18 | 2016-10-26 | Idenix Pharmaceuticals LLC | 4'-or nucleosides for the treatment of hcv |
| PL3639828T3 (en) | 2013-12-24 | 2022-05-02 | Virginia Commonwealth University | Use of oxygenated cholesterol sulfates (ocs) for treating acute liver failure |
| US9700544B2 (en) | 2013-12-31 | 2017-07-11 | Neal K Vail | Oral rapamycin nanoparticle preparations |
| WO2015103490A1 (en) | 2014-01-03 | 2015-07-09 | Abbvie, Inc. | Solid antiviral dosage forms |
| MX368159B (en) | 2014-01-10 | 2019-09-20 | Johnson & Johnson Consumer Inc | Process for making tablet using radiofrequency and lossy coated particles. |
| JP6541687B2 (en) * | 2014-01-21 | 2019-07-10 | ビーピーエスアイ ホールディングス, エルエルシー | Immediate release film coating containing medium chain glycerides, and substrate coated thereby |
| EP3114122A1 (en) | 2014-03-05 | 2017-01-11 | Idenix Pharmaceuticals LLC | Solid forms of a flaviviridae virus inhibitor compound and salts thereof |
| EA201691872A1 (en) | 2014-03-19 | 2017-04-28 | Инфинити Фармасьютикалз, Инк. | HETEROCYCLIC COMPOUNDS FOR APPLICATION IN THE TREATMENT OF PI3K-GAMMA-MEDIATED DISORDERS |
| NO2723977T3 (en) | 2014-03-19 | 2018-03-10 | ||
| CA2943231C (en) | 2014-03-20 | 2023-10-24 | Capella Therapeutics, Inc. | Benzimidazole derivatives as erbb tyrosine kinase inhibitors for the treatment of cancer |
| ES2891734T3 (en) | 2014-03-20 | 2022-01-31 | Capella Therapeutics Inc | Benzimidazole derivatives as ERBB tyrosine kinase inhibitors for cancer treatment |
| AU2015237723B2 (en) | 2014-03-26 | 2018-04-26 | Sun Pharma Advanced Research Company Ltd. | Abuse deterrent immediate release biphasic matrix solid dosage form |
| AU2015237243A1 (en) * | 2014-03-28 | 2016-09-29 | Therapeuticsmd, Inc. | Progesterone formulations |
| US10307464B2 (en) | 2014-03-28 | 2019-06-04 | Mannkind Corporation | Use of ultrarapid acting insulin |
| WO2015157559A2 (en) | 2014-04-09 | 2015-10-15 | Siteone Therapeutics, Inc. | 10',11'-modified saxitoxins for the treatment of pain |
| US10202411B2 (en) | 2014-04-16 | 2019-02-12 | Idenix Pharmaceuticals Llc | 3′-substituted methyl or alkynyl nucleosides nucleotides for the treatment of HCV |
| WO2015168079A1 (en) | 2014-04-29 | 2015-11-05 | Infinity Pharmaceuticals, Inc. | Pyrimidine or pyridine derivatives useful as pi3k inhibitors |
| CA2948540A1 (en) | 2014-05-12 | 2015-11-19 | Conatus Pharmaceuticals Inc. | Treatment of the complications of chronic liver disease with caspase inhibitors |
| JP2017516779A (en) | 2014-05-28 | 2017-06-22 | アイデニクス・ファーマシューティカルズ・エルエルシー | Nucleoside derivatives for cancer treatment |
| WO2015184173A1 (en) | 2014-05-29 | 2015-12-03 | Dose Medical Corporation | Implants with controlled drug delivery features and methods of using same |
| US9527815B2 (en) | 2014-06-18 | 2016-12-27 | Biotheryx, Inc. | Hydroxypyridone derivatives, pharmaceutical compositions thereof, and their therapeutic use for treating inflammatory, neurodegenerative, or immune-mediated diseases |
| CN106559991B (en) | 2014-06-19 | 2019-09-20 | 阿里亚德医药股份有限公司 | Heteroaryl compound for kinase inhibition |
| WO2016005994A2 (en) * | 2014-07-06 | 2016-01-14 | Gattefosse India Pvt. Ltd. | Pharmaceutical composition comprising solid dispersion of bcs class ii drugs with gelucires |
| US9855234B2 (en) | 2014-07-08 | 2018-01-02 | Insys Development Company, Inc. | Diclofenac sublingual spray |
| US9499514B2 (en) | 2014-07-11 | 2016-11-22 | Celgene Corporation | Antiproliferative compounds and methods of use thereof |
| JP6371463B2 (en) | 2014-07-17 | 2018-08-08 | ファーマシューティカル マニュファクチュアリング リサーチ サービシズ,インコーポレーテッド | Immediate release abuse deterrent liquid filler form |
| US9956153B2 (en) | 2014-08-01 | 2018-05-01 | Ecolab Usa Inc. | Antimicrobial foaming compositions containing cationic active ingredients |
| US9850521B2 (en) * | 2014-08-01 | 2017-12-26 | Agilent Technologies, Inc. | In vitro assay buffer for Cas9 |
| US9730819B2 (en) | 2014-08-15 | 2017-08-15 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9855156B2 (en) * | 2014-08-15 | 2018-01-02 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9480588B2 (en) | 2014-08-15 | 2016-11-01 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| US9259339B1 (en) | 2014-08-15 | 2016-02-16 | Elixir Medical Corporation | Biodegradable endoprostheses and methods of their fabrication |
| RU2736513C2 (en) | 2014-08-18 | 2020-11-17 | Ампио Фармасьютикалз, Инк. | Treating pathological conditions of joints |
| BR112017004708A2 (en) | 2014-09-12 | 2017-12-05 | Tobira Therapeutics Inc | cenicriviroc combination therapy for fibrosis treatment |
| US10016363B2 (en) | 2014-09-18 | 2018-07-10 | Virun, Inc. | Pre-spray emulsions and powders containing non-polar compounds |
| AU2015317336B2 (en) | 2014-09-18 | 2021-01-21 | Tonix Pharma Holdings Limited | Eutectic formulations of Cyclobenzaprine hydrochloride |
| US9861611B2 (en) | 2014-09-18 | 2018-01-09 | Virun, Inc. | Formulations of water-soluble derivatives of vitamin E and soft gel compositions, concentrates and powders containing same |
| US20180250224A1 (en) * | 2014-09-19 | 2018-09-06 | Oxular Limited | Ophthalmic Drug Compositions |
| WO2016054365A1 (en) * | 2014-10-01 | 2016-04-07 | Als Mountaiin Llc | Pharmaceutical composition comprising aspirin, metformin, and serotonin with non-ionic surfactant |
| US10561806B2 (en) | 2014-10-02 | 2020-02-18 | Mannkind Corporation | Mouthpiece cover for an inhaler |
| US9708348B2 (en) | 2014-10-03 | 2017-07-18 | Infinity Pharmaceuticals, Inc. | Trisubstituted bicyclic heterocyclic compounds with kinase activities and uses thereof |
| AU2015336065A1 (en) | 2014-10-20 | 2017-05-04 | Pharmaceutical Manufacturing Research Services, Inc. | Extended release abuse deterrent liquid fill dosage form |
| AU2015335950B2 (en) | 2014-10-21 | 2018-01-25 | Takeda Pharmaceutical Company Limited | Crystalline forms of 5-chloro-N4-[-2-(dimethylphosphoryl) phenyl]-N2-{2-methoxy-4-[4-(4-methylpiperazin-1-yl) piperidin-1-yl] phenyl}pyrimidine-2,4-diamine |
| EP3209658A1 (en) | 2014-10-24 | 2017-08-30 | Biogen MA Inc. | Diterpenoid derivatives and methods of use thereof |
| IL296080B2 (en) * | 2014-11-17 | 2023-12-01 | Eisai R&D Man Co Ltd | Method for treating cancer |
| WO2016106309A1 (en) | 2014-12-23 | 2016-06-30 | Axikin Pharmaceuticals, Inc. | 3,5-diaminopyrazole kinase inhibitors |
| MX2017009406A (en) | 2015-01-20 | 2018-01-18 | Xoc Pharmaceuticals Inc | Isoergoline compounds and uses thereof. |
| CA2974117A1 (en) | 2015-01-20 | 2016-07-28 | Xoc Pharmaceuticals, Inc. | Ergoline compounds and uses thereof |
| CN107205948B (en) | 2015-01-29 | 2021-12-14 | 诺和诺德股份有限公司 | Tablets comprising a GLP-1 agonist and an enteric coating |
| US10172800B2 (en) | 2015-02-20 | 2019-01-08 | Osmotica Kereskedelmi Es Szolgaltato Kft | Controlled release dosage form with enhanced pharmacokinetics |
| US10300032B2 (en) | 2015-02-20 | 2019-05-28 | Osmotica Kereskedelmi Es Szolgaltato Kft | Controlled release dosage form |
| WO2016134200A1 (en) * | 2015-02-20 | 2016-08-25 | Enspire Group LLC | Soft gelatin capsules containing fexofenadine |
| BR112017017729A2 (en) | 2015-02-20 | 2018-04-10 | Osmotica Kereskedelmi Es Szolgaltato Kft | gaba receptor agonist controlled release oral dosage form |
| US10987328B2 (en) | 2015-02-20 | 2021-04-27 | Osmotica Kereskedelmi Es Szolgaltato Kft | Controlled release dosage form |
| GB201505938D0 (en) | 2015-04-08 | 2015-05-20 | Reckitt Benckiser Llc | Compositions comprising C0-Q10, krill oil and vitamin D |
| WO2016154592A1 (en) | 2015-03-26 | 2016-09-29 | Microvention, Inc. | Embiolic particles |
| US20170042806A1 (en) | 2015-04-29 | 2017-02-16 | Dexcel Pharma Technologies Ltd. | Orally disintegrating compositions |
| WO2016187355A1 (en) * | 2015-05-20 | 2016-11-24 | Glaukos Corporation | Therapeutic drug compositions and implants for delivery of same |
| US10815264B2 (en) | 2015-05-27 | 2020-10-27 | Southern Research Institute | Nucleotides for the treatment of cancer |
| RU2708254C2 (en) | 2015-05-28 | 2019-12-05 | Др. Редди'С Лабораториз Лтд. | Oral celecoxib composition for treating pain |
| US9763892B2 (en) | 2015-06-01 | 2017-09-19 | Autotelic Llc | Immediate release phospholipid-coated therapeutic agent nanoparticles and related methods |
| US10631564B2 (en) | 2015-06-19 | 2020-04-28 | University Of Southern California | Enterically coated microparticle compositions and methods for modified nutrient delivery |
| WO2016205701A1 (en) | 2015-06-19 | 2016-12-22 | University Of Southern California | Enteral fast access tract platform system |
| EP3310359A4 (en) | 2015-06-22 | 2019-04-03 | Lipocine Inc. | 17-hydroxyprogesterone ester-containing oral compositions and related methods |
| US11389512B2 (en) | 2015-06-22 | 2022-07-19 | Ampio Pharmaceuticals, Inc. | Use of low molecular weight fractions of human serum albumin in treating diseases |
| EP3313402B1 (en) | 2015-06-23 | 2023-12-27 | Neurocrine Biosciences, Inc. | Vmat2 inhibitors for treating neurological diseases or disorders |
| CA2991108A1 (en) | 2015-07-02 | 2017-01-05 | Civitas Therapeutics, Inc. | Triptan powders for pulmonary delivery |
| US9585867B2 (en) | 2015-08-06 | 2017-03-07 | Charles Everett Ankner | Cannabinod formulation for the sedation of a human or animal |
| KR20210048600A (en) | 2015-08-17 | 2021-05-03 | 쿠라 온콜로지, 인크. | Methods of treating cancer patients with farnesyl transferase inhibitors |
| US11103581B2 (en) | 2015-08-31 | 2021-08-31 | Acura Pharmaceuticals, Inc. | Methods and compositions for self-regulated release of active pharmaceutical ingredient |
| US11925578B2 (en) | 2015-09-02 | 2024-03-12 | Glaukos Corporation | Drug delivery implants with bi-directional delivery capacity |
| US11590228B1 (en) | 2015-09-08 | 2023-02-28 | Tris Pharma, Inc | Extended release amphetamine compositions |
| CA2998182A1 (en) | 2015-09-10 | 2017-03-16 | Impel Neuropharma, Inc. | In-line nasal delivery device |
| US11564833B2 (en) | 2015-09-25 | 2023-01-31 | Glaukos Corporation | Punctal implants with controlled drug delivery features and methods of using same |
| CN108473503A (en) | 2015-09-30 | 2018-08-31 | 赛特温治疗公司 | The saxitoxin class compound that 11,13- for treating pain is modified |
| CN108473489B (en) | 2015-10-30 | 2022-09-02 | 纽罗克里生物科学有限公司 | VALBENAZINE salts and polymorphs thereof |
| WO2017079566A1 (en) | 2015-11-05 | 2017-05-11 | Conatus Pharmaceuticals, Inc. | Caspase inhibitors for use in the treatment of liver cancer |
| US10112924B2 (en) | 2015-12-02 | 2018-10-30 | Astraea Therapeutics, Inc. | Piperdinyl nociceptin receptor compounds |
| US20170165252A1 (en) | 2015-12-10 | 2017-06-15 | Niconovum Usa Inc. | Protein-enriched therapeutic composition |
| LT3394057T (en) | 2015-12-23 | 2022-06-27 | Neurocrine Biosciences, Inc. | Synthetic method for preparation of (s)-(2r,3r,11br)-3-isobutyl-9,10-dimethoxy-2,3,4,6,7,11b-hexahydro-1h-pyrido[2,1,-a]lsoquinolin-2-yl 2-amino-3-methylbutanoate di(4-methylbenzenesulfonate) |
| WO2017117478A1 (en) | 2015-12-31 | 2017-07-06 | Conatus Pharmaceuticals Inc. | Methods of using caspase inhibitors in treatment of liver disease |
| SG10202003099XA (en) | 2016-01-08 | 2020-05-28 | Celgene Corp | Antiproliferative compounds, and their pharmaceutical compositions and uses |
| US10369188B2 (en) | 2016-01-08 | 2019-08-06 | Cidara Therapeutics, Inc. | Methods for preventing and treating pneumocystis infections |
| AR107320A1 (en) | 2016-01-08 | 2018-04-18 | Celgene Corp | SOLID FORMS OF 2- (4-CHLOROPHENYL) -N - ((2- (2,6-DIOXOPIPERIDIN-3-IL) -1-OXOINDOLIN-5-IL) METHYL) -2,2-DIFLUOROACETAMIDE AND ITS PHARMACEUTICAL COMPOSITIONS AND APPLICATIONS |
| US10648983B2 (en) | 2016-01-08 | 2020-05-12 | Celgene Corporation | Methods for treating cancer and the use of biomarkers as a predictor of clinical sensitivity to therapies |
| PL3419628T3 (en) | 2016-02-26 | 2021-05-31 | Debiopharm International Sa | Medicament for treatment of diabetic foot infections |
| WO2017161016A1 (en) | 2016-03-16 | 2017-09-21 | Cidara Therapeutics, Inc. | Dosing regimens for treatment of fungal infections |
| WO2017161116A1 (en) | 2016-03-17 | 2017-09-21 | Infinity Pharmaceuticals, Inc. | Isotopologues of isoquinolinone and quinazolinone compounds and uses thereof as pi3k kinase inhibitors |
| WO2017168174A1 (en) | 2016-04-02 | 2017-10-05 | N4 Pharma Uk Limited | New pharmaceutical forms of sildenafil |
| CN108884019A (en) | 2016-04-11 | 2018-11-23 | 克雷西奥生物科技有限公司 | Deuterated chloramines ketone derivatives |
| WO2017180794A1 (en) | 2016-04-13 | 2017-10-19 | Skyline Antiinfectives, Inc. | Deuterated o-sulfated beta-lactam hydroxamic acids and deuterated n-sulfated beta-lactams |
| EP3442479A1 (en) | 2016-04-20 | 2019-02-20 | Harold Alexander Heitzmann | Bioresorbable ocular drug delivery device |
| WO2017184968A1 (en) | 2016-04-22 | 2017-10-26 | Kura Oncology, Inc. | Methods of selecting cancer patients for treatment with farnesyltransferase inhibitors |
| KR102498741B1 (en) | 2016-04-29 | 2023-02-10 | 에프지에이치 바이오테크 인코포레이티드 | Di-substituted pyrazole compounds for the treatment of disease |
| EP3861961A1 (en) | 2016-05-16 | 2021-08-11 | Elixir Medical Corporation | Uncaging stent |
| US11622872B2 (en) | 2016-05-16 | 2023-04-11 | Elixir Medical Corporation | Uncaging stent |
| TWI753910B (en) | 2016-05-16 | 2022-02-01 | 美商拜歐斯瑞克斯公司 | Pyridinethiones, pharmaceutical compositions thereof, and their therapeutic use for treating a proliferative, inflammatory, neurodegenerative, or immune-mediated disease |
| US10517846B2 (en) * | 2016-05-26 | 2019-12-31 | Dr. Reddy's Laboratories Ltd. | Pharmaceutical compositions for treating acne |
| WO2017214269A1 (en) | 2016-06-08 | 2017-12-14 | Infinity Pharmaceuticals, Inc. | Heterocyclic compounds and uses thereof |
| US11541018B2 (en) | 2016-06-23 | 2023-01-03 | Corium, Llc | Adhesive matrix with hydrophilic and hydrophobic domains and a therapeutic agent |
| US9737530B1 (en) | 2016-06-23 | 2017-08-22 | Collegium Pharmaceutical, Inc. | Process of making stable abuse-deterrent oral formulations |
| WO2018002673A1 (en) | 2016-07-01 | 2018-01-04 | N4 Pharma Uk Limited | Novel formulations of angiotensin ii receptor antagonists |
| SG11201900712SA (en) | 2016-07-27 | 2019-02-27 | Corium Int Inc | Memantine transdermal delivery systems |
| US10016372B2 (en) | 2016-07-27 | 2018-07-10 | Corium International, Inc. | Transdermal delivery systems with pharmacokinetics bioequivalent to oral delivery |
| EP3490540A1 (en) | 2016-07-27 | 2019-06-05 | Corium International, Inc. | Sodium bicarbonatein situ |
| JP7048576B2 (en) * | 2016-08-02 | 2022-04-05 | デュレクト コーポレーション | A composition comprising oxygenated cholesterol sulfate and at least one of polyalkylene glycol, carboxymethyl cellulose, and polyoxyl glyceride. |
| ES2922933T3 (en) * | 2016-08-02 | 2022-09-21 | Univ Virginia Commonwealth | Compositions comprising 5-cholesten-3,25-diol 3-sulphate (25HC3S) or a pharmaceutically acceptable salt thereof and at least one cyclic oligosaccharide |
| WO2018027001A1 (en) * | 2016-08-03 | 2018-02-08 | Temple University-Of The Commonwealth System Of Higher Education | Microencapsulation of active agents |
| AU2017311412B2 (en) | 2016-08-11 | 2023-05-18 | Ovid Therapeutics Inc. | Methods and compositions for treatment of epileptic disorders |
| US11339142B2 (en) | 2016-09-07 | 2022-05-24 | Fgh Biotech, Inc. | Di-substituted pyrazole compounds for the treatment of diseases |
| US10391105B2 (en) | 2016-09-09 | 2019-08-27 | Marinus Pharmaceuticals Inc. | Methods of treating certain depressive disorders and delirium tremens |
| CN109982687A (en) | 2016-09-19 | 2019-07-05 | 梅制药公司 | Conjoint therapy |
| US10201632B2 (en) | 2016-09-28 | 2019-02-12 | Terumo Corporation | Polymer particles |
| PL3300724T3 (en) * | 2016-09-30 | 2020-04-30 | Erber Aktiengesellschaft | Particle containing at least a volatile substance and process for its preparation |
| RU2753864C2 (en) * | 2016-10-13 | 2021-08-24 | Каталент Ю.Кей. Суиндон Зайдис Лимитед | Lyophilized pharmaceutical compositions for vaginal delivery |
| AU2017353838A1 (en) | 2016-11-03 | 2019-05-23 | Kura Oncology, Inc. | Farnesyltransferase inhibitors for use in methods of treating cancer |
| US10106521B2 (en) | 2016-11-09 | 2018-10-23 | Phloronol, Inc. | Eckol derivatives, methods of synthesis and uses thereof |
| US10988442B2 (en) | 2016-11-09 | 2021-04-27 | Novomedix, Llc | Nitrite salts of 1,1-dimethylbiguanide, pharmaceutical compositions, and methods of use |
| EP3548007A4 (en) | 2016-12-01 | 2020-08-12 | Ignyta, Inc. | Methods for the treatment of cancer |
| JP2020500875A (en) | 2016-12-02 | 2020-01-16 | ニューロクライン バイオサイエンシーズ,インコーポレイテッド | Use of barbenazine for treating schizophrenia or schizophrenic affective disorder |
| ES2924903T3 (en) * | 2017-01-20 | 2022-10-11 | Univ Kansas | Macrocyclic peptides for use in the treatment of prostate or breast cancer |
| SG11201906883SA (en) | 2017-01-27 | 2019-08-27 | Neurocrine Biosciences Inc | Methods for the administration of certain vmat2 inhibitors |
| RU2729079C1 (en) | 2017-01-31 | 2020-08-04 | Кимберли-Кларк Ворлдвайд, Инк. | Antibacterial composition containing ester of benzoic acid, and methods for inhibiting bacterial growth by using thereof |
| US9956215B1 (en) | 2017-02-21 | 2018-05-01 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| MX2019009821A (en) | 2017-02-21 | 2019-12-02 | Kura Oncology Inc | Methods of treating cancer with farnesyltransferase inhibitors. |
| WO2018156585A1 (en) * | 2017-02-21 | 2018-08-30 | Ico Therapeutics Inc. | Solid oral formulations of amphotericin b |
| WO2018164996A1 (en) | 2017-03-06 | 2018-09-13 | Neurocrine Biosciences, Inc. | Dosing regimen for valbenazine |
| EP3595638A4 (en) * | 2017-03-15 | 2020-09-16 | Cerecin Inc. | Pharmaceutical compositions having high drug loadings of medium chain triglycerides and methods related thereto |
| US11555031B2 (en) | 2017-03-20 | 2023-01-17 | The Broad Institute, Inc. | Compounds and methods for regulating insulin secretion |
| US10493026B2 (en) | 2017-03-20 | 2019-12-03 | Johnson & Johnson Consumer Inc. | Process for making tablet using radiofrequency and lossy coated particles |
| US11236097B2 (en) | 2017-03-29 | 2022-02-01 | Siteone Therapeutics, Inc. | 11,13-modified saxitoxins for the treatment of pain |
| EP3601290B1 (en) | 2017-03-29 | 2021-11-24 | Siteone Therapeutics, Inc. | 11,13-modified saxitoxins for the treatment of pain |
| US20200179352A1 (en) | 2017-04-26 | 2020-06-11 | Neurocrine Biosciences, Inc. | Use of valbenazine for treating levodopa-induced dyskinesia |
| JOP20190219A1 (en) | 2017-05-09 | 2019-09-22 | Cardix Therapeutics LLC | Pharmaceutical compositions and methods of treating cardiovascular diseases |
| US10085999B1 (en) | 2017-05-10 | 2018-10-02 | Arixa Pharmaceuticals, Inc. | Beta-lactamase inhibitors and uses thereof |
| US20190224275A1 (en) | 2017-05-12 | 2019-07-25 | Aurinia Pharmaceuticals Inc. | Protocol for treatment of lupus nephritis |
| JP7173992B2 (en) | 2017-05-17 | 2022-11-17 | バーグ エルエルシー | Use of coenzyme Q10 preparations in the treatment and prevention of epidermolysis bullosa |
| CA3063535A1 (en) | 2017-05-19 | 2018-11-22 | Nflection Therapeutics, Inc. | Fused heteroaromatic-aniline compounds for treatment of dermal disorders |
| MA49141A (en) | 2017-05-19 | 2020-03-25 | Nflection Therapeutics Inc | PYRROLOPYRIDINE-ANILINE COMPOUNDS FOR THE TREATMENT OF SKIN CONDITIONS |
| EP3630758A1 (en) | 2017-06-01 | 2020-04-08 | Xoc Pharmaceuticals, Inc | Ergoline derivatives for use in medicine |
| US11197909B2 (en) | 2017-07-12 | 2021-12-14 | Cidara Therapeutics, Inc. | Compositions and methods for the treatment of fungal infections |
| US11517557B2 (en) | 2017-07-13 | 2022-12-06 | Tonix Pharmaceuticals Holding Corp. | Analogs of cyclobenzaprine and amitriptyline |
| US10806730B2 (en) | 2017-08-07 | 2020-10-20 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| TW201919628A (en) | 2017-08-07 | 2019-06-01 | 美商庫拉腫瘤技術股份有限公司 | Methods of treating cancer with FARNESYLTRANSFERASE inhibitors |
| WO2019040748A1 (en) | 2017-08-24 | 2019-02-28 | Adamas Pharma, Llc | Amantadine compositions, preparations thereof, and methods of use |
| CN111712229A (en) | 2017-09-15 | 2020-09-25 | 奥叙拉尔有限公司 | Ophthalmic delivery device |
| AU2018335259A1 (en) | 2017-09-21 | 2020-04-09 | Neurocrine Biosciences, Inc. | High dosage valbenazine formulation and compositions, methods, and kits related thereto |
| US11744967B2 (en) | 2017-09-26 | 2023-09-05 | Shin Nippon Biomedical Laboratories, Ltd. | Intranasal delivery devices |
| US10993941B2 (en) | 2017-10-10 | 2021-05-04 | Neurocrine Biosciences, Inc. | Methods for the administration of certain VMAT2 inhibitors |
| CA3077149A1 (en) | 2017-10-10 | 2019-04-18 | Neurocrine Biosciences, Inc. | Methods for the administration of (s)-2-amino-3-methyl-butyric acid (2r,3r,11br)-3-isobutyl-9,10-dimethoxy-1,3,4,6,7,11b-hexahydro-2h-pyrido[2,1-a]isoquinolin-2yl ester and salts thereof |
| JP2018043990A (en) * | 2017-10-11 | 2018-03-22 | カプスゲル・ベルギウム・ナムローゼ・フェンノートシャップCapsugel Belgium NV | Oil-in-water emulsion and method for producing the same |
| JP7191099B2 (en) | 2017-11-21 | 2022-12-16 | インペル ファーマシューティカルズ インコーポレイテッド | intranasal device with dip tube |
| US11395887B2 (en) | 2017-11-21 | 2022-07-26 | Impel Pharmaceuticals Inc. | Intranasal device with inlet interface |
| WO2019113269A1 (en) | 2017-12-08 | 2019-06-13 | Kura Oncology, Inc. | Methods of treating cancer patients with farnesyltransferase inhibitors |
| CN111447971A (en) | 2017-12-11 | 2020-07-24 | 通尼克斯制药控股有限公司 | Cyclobenzaprine treatment for agitation, psychosis and cognitive decline in dementia and neurodegenerative conditions |
| US11173132B2 (en) | 2017-12-20 | 2021-11-16 | Corium, Inc. | Transdermal adhesive composition comprising a volatile liquid therapeutic agent having low melting point |
| BR112020013750A8 (en) | 2018-01-05 | 2022-10-18 | Impel Neuropharma Inc | INTRANASAL DISPENSE OF OLANZAPINE BY PRECISION OLFATIVE DEVICE |
| JP7317020B2 (en) | 2018-01-05 | 2023-07-28 | インペル ファーマシューティカルズ インコーポレイテッド | Intranasal delivery of dihydroergotamine by a precision olfactory device |
| WO2019139871A1 (en) | 2018-01-10 | 2019-07-18 | Cura Therapeutics Llc | Pharmaceutical compositions comprising dicarboxylic acids and their therapeutic applications |
| JP7395480B2 (en) | 2018-01-10 | 2023-12-11 | クラ セラピューティクス, エルエルシー | Pharmaceutical compositions containing phenylsulfonamides and their therapeutic applications |
| MX2018000963A (en) | 2018-01-22 | 2018-12-13 | Federico Amezcua Amezcua | Synergistic pharmaceutical composition originated from the active enantiomer s-ketorolac tromethamine and tramadol hydrochloride. |
| US10231931B1 (en) | 2018-03-23 | 2019-03-19 | Genus Lifesciences Inc. | Thyroid hormone oral dosage forms and methods of using the same |
| SG11202011544UA (en) | 2018-06-14 | 2020-12-30 | Neurocrine Biosciences Inc | Vmat2 inhibitor compounds, compositions, and methods relating thereto |
| EP3807260A1 (en) | 2018-06-15 | 2021-04-21 | R. J. Reynolds Tobacco Company | Purification of nicotine |
| KR102145853B1 (en) * | 2018-06-19 | 2020-08-19 | 한국유나이티드제약 주식회사 | Pharma ceutical Composition Comprising Cilostazol and Statin derivatives |
| WO2020006341A1 (en) | 2018-06-29 | 2020-01-02 | Conatus Pharmaceuticals, Inc. | (s)-3-(2-(4-(benzyl)-3-oxopiperazin-1-yl)acetamido)-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic acid derivatives and related compounds as caspase inhibitors for treating cardiovascular diseases |
| WO2020009560A1 (en) | 2018-07-04 | 2020-01-09 | AMÉZCUA AMÉZCUA, Federico | Synergic pharmaceutical composition of the active enantiomer (s)-ketorolac and gabapentin for the treatment of neuropathic pain |
| KR20210034047A (en) | 2018-07-19 | 2021-03-29 | 임펠 뉴로파마 인코포레이티드 | Airway delivery of levodopa and dopa decarboxylase inhibitors for the treatment of Parkinson's disease |
| US11350657B2 (en) * | 2018-08-06 | 2022-06-07 | Pharmavite, Llc | Protein gummy composition |
| KR20210044817A (en) | 2018-08-15 | 2021-04-23 | 뉴로크린 바이오사이언시즈 인코퍼레이티드 | Methods of Administration of Specific VMAT2 Inhibitors |
| AU2019334202A1 (en) | 2018-09-06 | 2021-03-25 | Innopharmascreen, Inc. | Methods and compositions for treatment of asthma or parkinson's disease |
| US20220009938A1 (en) | 2018-10-03 | 2022-01-13 | Siteone Therapeutics, Inc. | 11,13-modified saxitoxins for the treatment of pain |
| US20220002396A1 (en) | 2018-11-01 | 2022-01-06 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| US11572344B2 (en) | 2018-11-20 | 2023-02-07 | Nflection Therapeutics, Inc. | Cyanoaryl-aniline compounds for treatment of dermal disorders |
| WO2020106307A1 (en) | 2018-11-20 | 2020-05-28 | Nflection Therapeutics, Inc. | Aryl-aniline and heteroaryl-aniline compounds for treatment of birthmarks |
| MA55143A (en) | 2018-11-20 | 2021-09-29 | H Lee Moffitt Cancer Center & Res Institute Inc | ARYL-ANILINE AND HETEROARYL-ANILINE COMPOUNDS FOR THE TREATMENT OF SKIN CANCERS |
| CA3120337A1 (en) | 2018-11-20 | 2020-05-28 | Nflection Therapeutics, Inc. | Naphthyridinone-aniline compounds for treatment of dermal disorders |
| WO2020118142A1 (en) | 2018-12-07 | 2020-06-11 | Marinus Pharmaceuticals, Inc. | Ganaxolone for use in prophylaxis and treatment of pospartum depression |
| JP7407461B2 (en) | 2018-12-19 | 2024-01-04 | シャイ・セラピューティクス・エルエルシー | Compounds that interact with the RAS superfamily for the treatment of cancer, inflammatory diseases, RAS diseases, and fibrotic diseases |
| WO2020132700A1 (en) | 2018-12-21 | 2020-06-25 | Fgh Biotech Inc. | Methods of using inhibitors of srebp in combination with niclosamide and analogs thereof |
| EP3897638A1 (en) | 2018-12-21 | 2021-10-27 | Kura Oncology, Inc. | Therapies for squamous cell carcinomas |
| EP3906017A4 (en) | 2019-01-03 | 2022-12-28 | Impel Pharmaceuticals Inc. | Nasal drug delivery device |
| EP3921038A1 (en) | 2019-02-06 | 2021-12-15 | Dice Alpha, Inc. | Il-17a modulators and uses thereof |
| US20220142983A1 (en) | 2019-03-01 | 2022-05-12 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| WO2020181165A1 (en) | 2019-03-07 | 2020-09-10 | Conatus Pharmaceuticals Inc. | Caspase inhibitors and methods of use thereof |
| US20220143006A1 (en) | 2019-03-15 | 2022-05-12 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| AU2020254492A1 (en) | 2019-03-29 | 2021-11-11 | Kura Oncology, Inc. | Methods of treating Squamous Cell Carcinomas with farnesyltransferase inhibitors |
| US20220168296A1 (en) | 2019-04-01 | 2022-06-02 | Kura Oncology, Inc. | Methods of treating cancer with farnesyltransferase inhibitors |
| WO2020223583A1 (en) | 2019-05-02 | 2020-11-05 | Kura Oncology, Inc. | Methods of treating acute myeloid leukemia with farnesyltransferase inhibitors |
| KR20220010011A (en) | 2019-05-17 | 2022-01-25 | 임펠 뉴로파마 인코포레이티드 | Disposable nasal delivery device |
| US11298336B2 (en) | 2019-05-30 | 2022-04-12 | Soluble Technologies, Inc. | Water soluble formulation |
| WO2020243538A1 (en) * | 2019-05-31 | 2020-12-03 | Primo Pharmatech Llc | Unit dosage form for transmucosal drug delivery of an active pharmaceutical ingredient |
| AU2020310190A1 (en) | 2019-07-11 | 2022-02-24 | Cura Therapeutics, Llc | Phenyl compounds and pharmaceutical compositions thereof, and their therapeutic applications |
| AU2020311404A1 (en) | 2019-07-11 | 2022-03-03 | Cura Therapeutics, Llc | Sulfone compounds and pharmaceutical compositions thereof, and their therapeutic applications for the treatment of neurodegenerative diseases |
| MX2022000570A (en) | 2019-07-26 | 2022-03-11 | Espervita Therapeutics Inc | Functionalized long-chain hydrocarbon mono- and di-carboxylic acids useful for the prevention or treatment of disease. |
| WO2021026124A1 (en) | 2019-08-05 | 2021-02-11 | Marinus Pharmaceuticals, Inc. | Ganaxolone for use in treatment of status epilepticus |
| US10940141B1 (en) | 2019-08-23 | 2021-03-09 | Neurocrine Biosciences, Inc. | Methods for the administration of certain VMAT2 inhibitors |
| AU2020348685A1 (en) | 2019-09-16 | 2022-04-14 | Dice Alpha, Inc. | IL-17A modulators and uses thereof |
| EP4034236A1 (en) | 2019-09-26 | 2022-08-03 | Abionyx Pharma SA | Compounds useful for treating liver diseases |
| JP2022551583A (en) | 2019-10-01 | 2022-12-12 | モレキュラー スキン セラピューティクス,インコーポレイティド | Benzoxazinone compounds as KLK5/7 dual inhibitors |
| CN114828889A (en) | 2019-12-06 | 2022-07-29 | 马瑞纳斯制药公司 | Ganaxolone for the treatment of tuberous sclerosis |
| US11633405B2 (en) | 2020-02-07 | 2023-04-25 | Therapeuticsmd, Inc. | Steroid hormone pharmaceutical formulations |
| US20210251947A1 (en) * | 2020-02-10 | 2021-08-19 | TRYAGx Labs Inc. | Stable formulations of dronabinol |
| EP4125820A4 (en) | 2020-03-26 | 2024-04-10 | Plx Opco Inc | Pharmaceutical carriers capable of ph dependent reconstitution and methods for making and using same |
| WO2021242970A1 (en) | 2020-05-29 | 2021-12-02 | Boulder Bioscience Llc | Methods for improved endovascular thrombectomy using 3,3'-diindolylmethane |
| US20230227466A1 (en) | 2020-06-18 | 2023-07-20 | Shy Therapeutics, Llc | Substituted thienopyrimidines that interact with the ras superfamily for the treatment of cancers, inflammatory diseases, rasopathies, and fibrotic disease |
| CA3124579A1 (en) | 2020-07-15 | 2022-01-15 | Schabar Research Associates Llc | Unit oral dose compositions composed of naproxen sodium and famotidine for the treatment of acute pain and the reduction of the severity of heartburn and/or the risk of heartburn |
| KR20230051164A (en) | 2020-07-15 | 2023-04-17 | 샤바르 리서치 어소시에이츠 엘엘씨 | Oral unit dosage composition consisting of ibuprofen and famotidine for treatment of acute pain and reduction of severity and/or risk of heartburn |
| US11786475B2 (en) | 2020-07-22 | 2023-10-17 | Soluble Technologies Inc. | Film-based dosage form |
| AU2021326546A1 (en) | 2020-08-14 | 2023-04-13 | Siteone Therapeutics, Inc. | Non-hydrated ketone inhibitors of NaV1.7 for the treatment of pain |
| WO2022040584A1 (en) * | 2020-08-21 | 2022-02-24 | Neuronasal, Inc. | Methods of administering glutathione precursors |
| US11541009B2 (en) | 2020-09-10 | 2023-01-03 | Curemark, Llc | Methods of prophylaxis of coronavirus infection and treatment of coronaviruses |
| WO2022165000A1 (en) | 2021-01-27 | 2022-08-04 | Shy Therapeutics, Llc | Methods for the treatment of fibrotic disease |
| WO2022164997A1 (en) | 2021-01-27 | 2022-08-04 | Shy Therapeutics, Llc | Methods for the treatment of fibrotic disease |
| US11452690B1 (en) | 2021-01-27 | 2022-09-27 | ECI Pharmaceuticals, LLC | Oral liquid compositions comprising amlodipine besylate and methods of using the same |
| WO2022189856A1 (en) | 2021-03-08 | 2022-09-15 | Abionyx Pharma Sa | Compounds useful for treating liver diseases |
| KR20230169979A (en) | 2021-03-10 | 2023-12-18 | 다이스 몰레큘스 에스브이, 인크. | Alpha V Beta 6 and Alpha V Beta 1 Integrin Inhibitors and Uses Thereof |
| EP4062907A1 (en) * | 2021-03-23 | 2022-09-28 | Substipharm | Formulation for oral administration of ivermectin and uses thereof |
| US11197819B1 (en) * | 2021-04-09 | 2021-12-14 | Drug Delivery Company, Llc | Extended release bioabsorbable subcutaneous medicinal dosage delivery implant system |
| WO2022226166A1 (en) | 2021-04-22 | 2022-10-27 | Protego Biopharma, Inc. | Spirocyclic imidazolidinones and imidazolidinediones for treatment of light chain amyloidosis |
| US11866639B2 (en) | 2021-04-29 | 2024-01-09 | Saudi Arabian Oil Company | Method and material to reduce acid-carbonate reaction rate by endothermic reaction |
| WO2022251533A1 (en) | 2021-05-27 | 2022-12-01 | Protego Biopharma, Inc. | Heteroaryl diamide ire1/xbp1s activators |
| CA3234276A1 (en) | 2021-11-30 | 2023-06-08 | Kura Oncology, Inc. | Macrocyclic compounds having farnesyltransferase inhibitory activity |
| WO2023129577A1 (en) | 2022-01-03 | 2023-07-06 | Lilac Therapeutics, Inc. | Cyclic thiol prodrugs |
| WO2023150345A1 (en) * | 2022-02-04 | 2023-08-10 | Intact Therapeutics, Inc. | Mesalamine pharmaceutical formulations and methods of use thereof |
| WO2023192817A1 (en) | 2022-03-28 | 2023-10-05 | Isosterix, Inc. | Inhibitors of the myst family of lysine acetyl transferases |
| US20230348909A1 (en) | 2022-03-30 | 2023-11-02 | Biomarin Pharmaceutical Inc. | Dystrophin exon skipping oligonucleotides |
| GB2619907A (en) | 2022-04-01 | 2023-12-27 | Kanna Health Ltd | Novel crystalline salt forms of mesembrine |
| US20230331693A1 (en) | 2022-04-14 | 2023-10-19 | Bristol-Myers Squibb Company | Gspt1 compounds and methods of use of the novel compounds |
| WO2023201348A1 (en) | 2022-04-15 | 2023-10-19 | Celgene Corporation | Methods for predicting responsiveness of lymphoma to drug and methods for treating lymphoma |
| WO2023211990A1 (en) | 2022-04-25 | 2023-11-02 | Siteone Therapeutics, Inc. | Bicyclic heterocyclic amide inhibitors of na v1.8 for the treatment of pain |
| WO2023215781A1 (en) | 2022-05-05 | 2023-11-09 | Biomarin Pharmaceutical Inc. | Method of treating duchenne muscular dystrophy |
| WO2024054832A1 (en) | 2022-09-09 | 2024-03-14 | Innovo Therapeutics, Inc. | CK1α AND DUAL CK1α / GSPT1 DEGRADING COMPOUNDS |
| WO2024073473A1 (en) | 2022-09-30 | 2024-04-04 | Boulder Bioscience Llc | Compositions comprising 3,3'-diindolylmethane for treating non-hemorrhagic closed head injury |
Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4717569A (en) * | 1984-06-04 | 1988-01-05 | Sterling Drug Inc. | Unit dosage form of sparingly soluble medicaments |
| US4849227A (en) * | 1986-03-21 | 1989-07-18 | Eurasiam Laboratories, Inc. | Pharmaceutical compositions |
| US5340589A (en) * | 1991-05-16 | 1994-08-23 | Sterling Winthrop Inc. | Low solubility drug-coated bead compositions |
| US5573783A (en) * | 1995-02-13 | 1996-11-12 | Nano Systems L.L.C. | Redispersible nanoparticulate film matrices with protective overcoats |
Family Cites Families (118)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3164520A (en) * | 1962-10-29 | 1965-01-05 | Olin Mathieson | Injectable steroid compositions containing at least 75% benzyl benzoate |
| US3510561A (en) * | 1965-05-20 | 1970-05-05 | Canada Packers Ltd | Sulfone-enhanced heparin absorption through mucous membranes |
| US4147783A (en) * | 1974-02-28 | 1979-04-03 | Akzona Incorporated | Oral pharmaceutical preparation |
| JPS5266616A (en) * | 1975-11-29 | 1977-06-02 | Sawai Seiyaku Kk | Manufacturing of solidified oily liquid substance |
| FR2408345A1 (en) * | 1976-11-30 | 1979-06-08 | Besins Jean Louis | NEW COMPOSITION WITH ANTI-CONCEPTIONAL ACTION |
| JPS53107408A (en) * | 1977-02-28 | 1978-09-19 | Yamanouchi Pharmaceut Co Ltd | Micellar preparation for rectal infusion |
| JPS5770824A (en) * | 1980-10-20 | 1982-05-01 | Nippon Saafuakutanto Kogyo Kk | Vehicle for medicine |
| US4439432A (en) * | 1982-03-22 | 1984-03-27 | Peat Raymond F | Treatment of progesterone deficiency and related conditions with a stable composition of progesterone and tocopherols |
| US4654327A (en) * | 1982-04-21 | 1987-03-31 | Research Corp. | Quaternary ammonium complexes of heparin |
| IL68769A (en) * | 1983-05-23 | 1986-02-28 | Hadassah Med Org | Pharmaceutical compositions containing insulin for oral administration |
| US4731384A (en) * | 1983-07-01 | 1988-03-15 | Troponwerke Gmbh & Co, Kg | Etofenamate formulation |
| US4832952A (en) * | 1983-07-07 | 1989-05-23 | American Home Products Corporation | Pharmaceutical composition containing a liquid lubricant |
| DE3331009A1 (en) * | 1983-08-27 | 1985-03-14 | Basf Ag, 6700 Ludwigshafen | METHOD FOR INCREASING THE ENTERAL RESORBABILITY OF HEPARIN OR. HEPARINOIDS AND THE SO AVAILABLE HEPARIN OR HEPARINOID PREPARATION |
| DE3406497A1 (en) * | 1984-02-23 | 1985-09-05 | Mueller Bernhard Willi Werner | HIGHLY DISPERSAL PHARMACEUTICAL MULTI-COMPONENT SYSTEMS AND METHOD FOR THEIR PRODUCTION |
| US4795327A (en) * | 1984-03-26 | 1989-01-03 | Forest Laboratories, Inc. | Controlled release solid drug dosage forms based on mixtures of water soluble nonionic cellulose ethers and anionic surfactants |
| US4572915A (en) * | 1984-05-01 | 1986-02-25 | Bioglan Laboratories | Clear micellized solutions of fat soluble essential nutrients |
| DE3421468A1 (en) * | 1984-06-08 | 1985-12-19 | Dr. Rentschler Arzneimittel Gmbh & Co, 7958 Laupheim | LIPID NANOPELLETS AS A CARRIER SYSTEM FOR MEDICINAL PRODUCTS FOR PERORAL USE |
| GB8903804D0 (en) * | 1989-02-20 | 1989-04-05 | Sandoz Ltd | Improvements in or relating to organic compounds |
| US4897269A (en) * | 1984-09-24 | 1990-01-30 | Mezei Associates Limited | Administration of drugs with multiphase liposomal delivery system |
| US4867984A (en) | 1984-11-06 | 1989-09-19 | Nagin K. Patel | Drug in bead form and process for preparing same |
| DE3500103A1 (en) * | 1985-01-04 | 1986-07-10 | R.P. Scherer GmbH, 6930 Eberbach | PHARMACEUTICAL PREPARATION WITH AN INTENSIVE SOLUTION IN WATER AND DIGESTIVE JUICES |
| FR2585246A1 (en) * | 1985-07-26 | 1987-01-30 | Cortial | PROCESS FOR OBTAINING SOLID PHARMACEUTICAL FORMS WITH PROLONGED RELEASE |
| US4717596A (en) * | 1985-10-30 | 1988-01-05 | International Business Machines Corporation | Method for vacuum vapor deposition with improved mass flow control |
| US5023108A (en) | 1986-01-13 | 1991-06-11 | Research Corporation | Aqueous dispersions of waxes and lipids for pharmaceutical coating |
| US5433959A (en) * | 1986-02-13 | 1995-07-18 | Takeda Chemical Industries, Ltd. | Stabilized pharmaceutical composition |
| CA1327010C (en) * | 1986-02-13 | 1994-02-15 | Tadashi Makino | Stabilized solid pharmaceutical composition containing antiulcer benzimidazole compound and its production |
| NZ221411A (en) * | 1986-08-11 | 1989-10-27 | Innovata Biomed Ltd | Pharmaceutical compositions containing microcapsules and a surfactant |
| HU205861B (en) * | 1986-12-19 | 1992-07-28 | Sandoz Ag | Process for producing hydrosole of pharmaceutically effective material |
| US4900734A (en) * | 1987-08-27 | 1990-02-13 | Maxson Wayne S | Novel pharmaceutical composition containing estradiol and progesterone for oral administration |
| GB8730011D0 (en) * | 1987-12-23 | 1988-02-03 | Smithkline Dauelsberg | Pharmaceutical compositions |
| FR2627696B1 (en) * | 1988-02-26 | 1991-09-13 | Fournier Innovation Synergie | NEW GALENIC FORM OF FENOFIBRATE |
| DE3807895A1 (en) * | 1988-03-10 | 1989-09-21 | Knoll Ag | PRODUCTS CONTAINING A CALCIUM ANTAGONIST AND A LIPID DOWNER |
| KR0148748B1 (en) * | 1988-09-16 | 1998-08-17 | 장 크라메르, 한스 루돌프 하우스 | A multiphase cyclosporin composition |
| US4994439A (en) * | 1989-01-19 | 1991-02-19 | California Biotechnology Inc. | Transmembrane formulations for drug administration |
| US5014656A (en) * | 1990-04-25 | 1991-05-14 | General Motors Corporation | Internal combustion engine having a permanent ground electrode and replaceable center electrode element |
| US5091188A (en) * | 1990-04-26 | 1992-02-25 | Haynes Duncan H | Phospholipid-coated microcrystals: injectable formulations of water-insoluble drugs |
| US5091187A (en) * | 1990-04-26 | 1992-02-25 | Haynes Duncan H | Phospholipid-coated microcrystals: injectable formulations of water-insoluble drugs |
| US5298497A (en) * | 1990-05-15 | 1994-03-29 | E. R. Squibb & Sons, Inc. | Method for preventing onset of hypertension employing a cholesterol lowering drug |
| ATE121618T1 (en) * | 1990-08-13 | 1995-05-15 | David W Yesair | MIXED LIPID-BICARBONATE COLLOIDAL PARTICLES FOR DELIVERING MEDICINAL AND CALORIES. |
| US5300529A (en) * | 1991-02-12 | 1994-04-05 | Isp Investments Inc. | Stable, clear, efficacious aqueous microemulsion compositions containing a high loading of a water-insoluble, agriculturally active chemical |
| US5403593A (en) * | 1991-03-04 | 1995-04-04 | Sandoz Ltd. | Melt granulated compositions for preparing sustained release dosage forms |
| TW212139B (en) | 1991-04-15 | 1993-09-01 | Yamanouchi Pharma Co Ltd | |
| US5380535A (en) | 1991-05-28 | 1995-01-10 | Geyer; Robert P. | Chewable drug-delivery compositions and methods for preparing the same |
| JPH0597672A (en) * | 1991-10-08 | 1993-04-20 | Terumo Corp | Amide derivative-containing solid preparation and its production |
| RU2120798C1 (en) * | 1991-11-22 | 1998-10-27 | Проктер энд Гэмбл Фармасьютикалз, Инк. | Solid pharmaceutical composition for oral administration |
| US5206219A (en) * | 1991-11-25 | 1993-04-27 | Applied Analytical Industries, Inc. | Oral compositions of proteinaceous medicaments |
| US5571533A (en) * | 1992-02-07 | 1996-11-05 | Recordati, S.A., Chemical And Pharmaceutical Company | Controlled-release mucoadhesive pharmaceutical composition for the oral administration of furosemide |
| SE9200951D0 (en) * | 1992-03-27 | 1992-03-27 | Kabi Pharmacia Ab | PHARMACEUTICAL COMPOSITION CONTAINING A DEFINED LIPID SYSTEM |
| GB9212511D0 (en) * | 1992-06-12 | 1992-07-22 | Cortecs Ltd | Pharmaceutical compositions |
| PH30929A (en) | 1992-09-03 | 1997-12-23 | Janssen Pharmaceutica Nv | Beads having a core coated with an antifungal and a polymer. |
| GB9300875D0 (en) * | 1993-01-18 | 1993-03-10 | Ucb Sa | Nanocapsule containing pharmaceutical compositions |
| BE1006990A5 (en) * | 1993-04-22 | 1995-02-07 | Univ Gent | METHOD AND COMPOSITION TO MAKE AN ACTIVE INGREDIENT IN A solid dosage form. |
| SE9302135D0 (en) * | 1993-06-18 | 1993-06-18 | Kabi Pharmacia Ab | NEW PHARMACEUTICAL COMPOSITION |
| ES2068762B1 (en) * | 1993-07-21 | 1995-12-01 | Lipotec Sa | A NEW PHARMACEUTICAL PREPARATION TO IMPROVE THE BIOAVAILABILITY OF DRUGS OF DIFFICULT ABSORPTION AND PROCEDURE FOR THEIR OBTAINING. |
| US6022852A (en) * | 1993-10-22 | 2000-02-08 | Hexal Ag | Pharmaceutical composition containing cyclosporin A |
| WO1995014037A1 (en) * | 1993-11-17 | 1995-05-26 | Ibah, Inc. | Transparent liquid for encapsulated drug delivery |
| DE4340781C3 (en) * | 1993-11-30 | 2000-01-27 | Novartis Ag | Liquid preparations containing cyclosporin and process for their preparation |
| KR0146671B1 (en) * | 1994-02-25 | 1998-08-17 | 김충환 | Cyclosporin-containing powder composition |
| US5811120A (en) | 1994-03-02 | 1998-09-22 | Eli Lilly And Company | Solid orally administerable raloxifene hydrochloride pharmaceutical formulation |
| GB9405304D0 (en) * | 1994-03-16 | 1994-04-27 | Scherer Ltd R P | Delivery systems for hydrophobic drugs |
| US5731355A (en) * | 1994-03-22 | 1998-03-24 | Zeneca Limited | Pharmaceutical compositions of propofol and edetate |
| GB9409778D0 (en) * | 1994-05-16 | 1994-07-06 | Dumex Ltd As | Compositions |
| US6692766B1 (en) * | 1994-06-15 | 2004-02-17 | Yissum Research Development Company Of The Hebrew University Of Jerusalem | Controlled release oral drug delivery system |
| US5616330A (en) * | 1994-07-19 | 1997-04-01 | Hemagen/Pfc | Stable oil-in-water emulsions incorporating a taxine (taxol) and method of making same |
| US5858398A (en) * | 1994-11-03 | 1999-01-12 | Isomed Inc. | Microparticular pharmaceutical compositions |
| US5629021A (en) * | 1995-01-31 | 1997-05-13 | Novavax, Inc. | Micellar nanoparticles |
| FR2730231B1 (en) * | 1995-02-02 | 1997-04-04 | Fournier Sca Lab | COMBINATION OF FENOFIBRATE AND VITAMIN E, USE IN THERAPEUTICS |
| SI9500173B (en) * | 1995-05-19 | 2002-02-28 | Lek, | Three-phase pharmaceutical form with constant and controlled release of amorphous active ingredient for single daily application |
| US5726181A (en) * | 1995-06-05 | 1998-03-10 | Bionumerik Pharmaceuticals, Inc. | Formulations and compositions of poorly water soluble camptothecin derivatives |
| DE19527661C2 (en) * | 1995-07-28 | 1998-02-19 | Optrex Europ Gmbh | Carrier comprising electrical conductors with an electronic component and method for contacting conductors of a substrate with contact warts of an electronic component |
| US6645988B2 (en) * | 1996-01-04 | 2003-11-11 | Curators Of The University Of Missouri | Substituted benzimidazole dosage forms and method of using same |
| US5858401A (en) * | 1996-01-22 | 1999-01-12 | Sidmak Laboratories, Inc. | Pharmaceutical composition for cyclosporines |
| DE19619045C1 (en) * | 1996-05-02 | 1997-11-13 | Jenapharm Gmbh | Use of combination products for the treatment of hypogonadal men and men with pituitary disorders |
| US5846971A (en) | 1996-06-28 | 1998-12-08 | Schering Corporation | Oral antifungal composition |
| EP0914100A1 (en) * | 1996-06-28 | 1999-05-12 | Schering Corporation | Oral composition comprising a triazole antifungal compound |
| US5883109A (en) * | 1996-07-24 | 1999-03-16 | Bristol-Myers Squibb Company | Method for lowering serum lipid levels employing an MTP inhibitor in combination with another cholesterol lowering drug |
| SE9603077D0 (en) * | 1996-08-29 | 1996-08-29 | Tetra Laval Holdings & Finance | An apparatus for and method of performing an animal-related action regarding at least a portion of the body of an animal |
| US5891469A (en) | 1997-04-02 | 1999-04-06 | Pharmos Corporation | Solid Coprecipitates for enhanced bioavailability of lipophilic substances |
| US6361796B1 (en) * | 1996-10-25 | 2002-03-26 | Shire Laboratories, Inc. | Soluble form osmotic dose delivery system |
| FR2758459B1 (en) * | 1997-01-17 | 1999-05-07 | Pharma Pass | FENOFIBRATE PHARMACEUTICAL COMPOSITION HAVING HIGH BIODAVAILABILITY AND PROCESS FOR PREPARING THE SAME |
| GB9700878D0 (en) * | 1997-01-17 | 1997-03-05 | Scherer Ltd R P | Dosage forms and method for ameliorating male erectile dysfunction |
| ES2384551T3 (en) * | 1997-03-05 | 2012-07-06 | Sugen, Inc. | Formulations for hydrophobic pharmaceutical agents |
| US5874418A (en) * | 1997-05-05 | 1999-02-23 | Cydex, Inc. | Sulfoalkyl ether cyclodextrin based solid pharmaceutical formulations and their use |
| US6046177A (en) * | 1997-05-05 | 2000-04-04 | Cydex, Inc. | Sulfoalkyl ether cyclodextrin based controlled release solid pharmaceutical formulations |
| KR100509130B1 (en) * | 1997-07-29 | 2005-08-18 | 파마시아 앤드 업존 캄파니 엘엘씨 | Self-Emulsifying Formulation for Lipophilic Compounds |
| IT1294760B1 (en) * | 1997-09-03 | 1999-04-12 | Jagotec Ag | PROCEDURE FOR THE PREPARATION OF PHARMACEUTICAL TABLETS ABLE TO RELEASE, ACCORDING TO PREDETERMINABLE SCHEMES, LITTLE ACTIVE INGREDIENTS |
| US20020013304A1 (en) * | 1997-10-28 | 2002-01-31 | Wilson Leland F. | As-needed administration of an androgenic agent to enhance female sexual desire and responsiveness |
| US6027747A (en) * | 1997-11-11 | 2000-02-22 | Terracol; Didier | Process for the production of dry pharmaceutical forms and the thus obtained pharmaceutical compositions |
| US5891845A (en) * | 1997-11-21 | 1999-04-06 | Fuisz Technologies Ltd. | Drug delivery systems utilizing liquid crystal structures |
| US6013665A (en) * | 1997-12-16 | 2000-01-11 | Abbott Laboratories | Method for enhancing the absorption and transport of lipid soluble compounds using structured glycerides |
| ID25908A (en) * | 1998-03-06 | 2000-11-09 | Novartis Ag | EMULSION PRACTONCENTRATES CONTAINING CYCLOSPORINE OR MACROLIDES |
| DK173431B1 (en) * | 1998-03-20 | 2000-10-23 | Gea Farmaceutisk Fabrik As | Pharmaceutical formulation comprising a 2 - [[(2-pyridinyl) methyl] sulfinyl] benzimidazole with anti-ulcer activity and progress |
| ES2157731B1 (en) * | 1998-07-21 | 2002-05-01 | Liconsa Liberacion Controlada | ORAL PHARMACEUTICAL PREPARATION OF AN ANTIFUNGIC ACTIVITY COMPOUND AND PROCEDURE FOR PREPARATION. |
| US6174547B1 (en) * | 1999-07-14 | 2001-01-16 | Alza Corporation | Dosage form comprising liquid formulation |
| US5993880A (en) * | 1998-10-01 | 1999-11-30 | Kraft Foods Inc. | Non-staining, acid-stable, cold-water-soluble, edible green color and compositions for preparing acidic foods and beverages |
| US6180138B1 (en) * | 1999-01-29 | 2001-01-30 | Abbott Laboratories | Process for preparing solid formulations of lipid-regulating agents with enhanced dissolution and absorption |
| US6383517B1 (en) * | 1999-01-29 | 2002-05-07 | Abbott Laboratories | Process for preparing solid formulations of lipid-regulating agents with enhanced dissolution and absorption |
| US6248363B1 (en) * | 1999-11-23 | 2001-06-19 | Lipocine, Inc. | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| US7374779B2 (en) * | 1999-02-26 | 2008-05-20 | Lipocine, Inc. | Pharmaceutical formulations and systems for improved absorption and multistage release of active agents |
| US6982281B1 (en) * | 2000-11-17 | 2006-01-03 | Lipocine Inc | Pharmaceutical compositions and dosage forms for administration of hydrophobic drugs |
| US6720001B2 (en) * | 1999-10-18 | 2004-04-13 | Lipocine, Inc. | Emulsion compositions for polyfunctional active ingredients |
| US20060034937A1 (en) * | 1999-11-23 | 2006-02-16 | Mahesh Patel | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions |
| PT1108425E (en) * | 1999-12-16 | 2005-10-31 | Medinfar Produtos Farmaceutico | NEW MULTI-MUNICIPAL PHARMACEUTICAL PREPARATIONS CONTAINING SUBSTITUTED BENZIMIDAZOES |
| HUP0204372A3 (en) * | 1999-12-23 | 2004-06-28 | Pfizer Prod Inc | Pharmaceutical compositions providing enhanced drug concentrations |
| EP2415462A1 (en) * | 1999-12-23 | 2012-02-08 | Mayne Pharma International Pty Ltd. | Improved pharmaceutical compositions for poorly soluble drugs |
| US6340471B1 (en) * | 1999-12-30 | 2002-01-22 | Alvin Kershman | Method for preparing solid delivery system for encapsulated and non-encapsulated pharmaceuticals |
| US20020102301A1 (en) * | 2000-01-13 | 2002-08-01 | Joseph Schwarz | Pharmaceutical solid self-emulsifying composition for sustained delivery of biologically active compounds and the process for preparation thereof |
| US7025979B2 (en) * | 2000-02-15 | 2006-04-11 | Schering Ag | Male contraceptive formulation comprising norethisterone |
| US6503894B1 (en) * | 2000-08-30 | 2003-01-07 | Unimed Pharmaceuticals, Inc. | Pharmaceutical composition and method for treating hypogonadism |
| US20030022875A1 (en) * | 2001-07-27 | 2003-01-30 | Wilson Leland F. | As-needed administration of orally active androgenic agents to enhance female sexual desire and responsiveness |
| US20040002445A1 (en) * | 2002-03-28 | 2004-01-01 | Rajneesh Taneja | Enhancement of endogenous gonadotropin production |
| WO2005011635A2 (en) * | 2003-08-04 | 2005-02-10 | Pfizer Products Inc. | Pharmaceutical compositions of adsorbates of amorphous drugs and lipophilic microphase-forming materials |
| AR047938A1 (en) * | 2003-08-25 | 2006-03-15 | Combinatorx Inc | FORMULATIONS, CONJUGATES AND COMBINATIONS OF PHARMACOS FOR THE TREATMENT OF NEOPLASMS |
| CA2540984C (en) * | 2003-10-10 | 2011-02-08 | Lifecycle Pharma A/S | A solid dosage form comprising a fibrate |
| US20060003002A1 (en) * | 2003-11-03 | 2006-01-05 | Lipocine, Inc. | Pharmaceutical compositions with synchronized solubilizer release |
| WO2006012502A2 (en) * | 2004-07-23 | 2006-02-02 | Rigel Pharmaceuticals, Inc. | Formulation of insoluble small molecule therapeutics in lipid-based carriers |
| US20060134210A1 (en) * | 2004-12-22 | 2006-06-22 | Astrazeneca Ab | Solid dosage form comprising proton pump inhibitor and suspension made thereof |
| GB0807605D0 (en) * | 2008-04-28 | 2008-06-04 | Diurnal Ltd | Lipid composition |
-
1999
- 1999-11-23 US US09/447,690 patent/US6248363B1/en not_active Expired - Lifetime
-
2000
- 2000-11-22 CA CA2706113A patent/CA2706113A1/en not_active Abandoned
- 2000-11-22 WO PCT/US2000/032255 patent/WO2001037808A1/en active Application Filing
- 2000-11-22 AU AU17981/01A patent/AU1798101A/en not_active Abandoned
- 2000-11-22 JP JP2001539423A patent/JP2003517470A/en active Pending
- 2000-11-22 EP EP00980761A patent/EP1233756A4/en not_active Ceased
- 2000-11-22 CA CA2391923A patent/CA2391923C/en not_active Expired - Lifetime
-
2001
- 2001-03-06 US US09/800,593 patent/US6569463B2/en not_active Expired - Lifetime
-
2003
- 2003-05-01 US US10/428,341 patent/US6923988B2/en not_active Expired - Lifetime
-
2008
- 2008-12-02 US US12/326,711 patent/US20090074859A1/en not_active Abandoned
-
2012
- 2012-02-14 JP JP2012029635A patent/JP2012116863A/en active Pending
-
2014
- 2014-08-14 US US14/460,188 patent/US20150224130A9/en not_active Abandoned
-
2015
- 2015-05-15 US US14/713,692 patent/US20150273067A1/en not_active Abandoned
-
2017
- 2017-06-16 US US15/625,764 patent/US20180125978A1/en not_active Abandoned
-
2019
- 2019-02-28 US US16/289,565 patent/US20200061191A1/en not_active Abandoned
-
2020
- 2020-06-30 US US16/917,731 patent/US20210008212A1/en not_active Abandoned
Patent Citations (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4717569A (en) * | 1984-06-04 | 1988-01-05 | Sterling Drug Inc. | Unit dosage form of sparingly soluble medicaments |
| US4849227A (en) * | 1986-03-21 | 1989-07-18 | Eurasiam Laboratories, Inc. | Pharmaceutical compositions |
| US5340589A (en) * | 1991-05-16 | 1994-08-23 | Sterling Winthrop Inc. | Low solubility drug-coated bead compositions |
| US5573783A (en) * | 1995-02-13 | 1996-11-12 | Nano Systems L.L.C. | Redispersible nanoparticulate film matrices with protective overcoats |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP1233756A4 * |
Cited By (331)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2001082905A1 (en) * | 2000-04-28 | 2001-11-08 | Glaxosmithkline Kabushiki Kaisha | Pharmaceutical formulation comprising carrier beads coated with a layer of valaciclovir |
| WO2001087355A1 (en) * | 2000-05-18 | 2001-11-22 | Australian Rural Group Limited | Lipophilic medicament |
| US7696162B2 (en) | 2001-03-23 | 2010-04-13 | Sanofi-Aventis Deutschland Gmbh | Zinc-free and low-zinc insulin preparations having improved stability |
| WO2002092062A2 (en) * | 2001-05-15 | 2002-11-21 | Leo Pharma A/S | Combination of vitamin d analogue and pyrimidine nucleoside analogue |
| WO2002092062A3 (en) * | 2001-05-15 | 2003-02-13 | Leo Pharma As | Combination of vitamin d analogue and pyrimidine nucleoside analogue |
| US6623754B2 (en) | 2001-05-21 | 2003-09-23 | Noveon Ip Holdings Corp. | Dosage form of N-acetyl cysteine |
| WO2002094258A1 (en) * | 2001-05-21 | 2002-11-28 | Noveon Ip Holdings Corp. | Improved dosage form of n-acetyl cysteine |
| JP2004534074A (en) * | 2001-06-14 | 2004-11-11 | ヤゴテック アーゲー | New composition |
| US8753683B2 (en) | 2001-07-02 | 2014-06-17 | Merrion Research Iii Limited | Delivery of a bioactive material |
| WO2003003999A3 (en) * | 2001-07-02 | 2003-06-19 | Elan Corp Plc | Delivery of a bioactive material |
| US7670626B2 (en) | 2001-07-02 | 2010-03-02 | Merrion Research Iii Limited | Delivery of a bioactive material |
| WO2003003999A2 (en) * | 2001-07-02 | 2003-01-16 | Elan Corporation, Plc. | Delivery of a bioactive material |
| EP1429732A1 (en) * | 2001-07-10 | 2004-06-23 | Kos Life Sciences, Inc. | A core formulation |
| EP1429732A4 (en) * | 2001-07-10 | 2005-01-26 | Kos Life Sciences Inc | A core formulation |
| EP1411921B2 (en) † | 2001-07-20 | 2010-08-18 | Novartis AG | Pharmaceutical compositions containing terbinafine and use thereof |
| EP1287821A1 (en) * | 2001-09-04 | 2003-03-05 | Pfizer Limited | Multiparticulate formulations of atorvastatin calcium having a modulated rate of drug release |
| US8007830B2 (en) | 2001-10-26 | 2011-08-30 | Merck Frosst Canada & Co. | Granule formation |
| EP1461044A4 (en) * | 2001-12-03 | 2007-06-13 | Novacea Inc | Pharmaceutical compositions comprising active vitamin d compounds |
| EP1461044A1 (en) * | 2001-12-03 | 2004-09-29 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin d compounds |
| EP1453536A1 (en) * | 2001-12-12 | 2004-09-08 | F H Faulding & Co. Limited | Composition for the preservation of viruses |
| EP1453536A4 (en) * | 2001-12-12 | 2009-08-26 | Mayne Pharma Int Pty Ltd | Composition for the preservation of viruses |
| JPWO2003059389A1 (en) * | 2002-01-16 | 2005-05-19 | 山之内製薬株式会社 | Pharmaceutical composition for improving oral absorption |
| JP5462429B2 (en) * | 2002-01-16 | 2014-04-02 | アステラス製薬株式会社 | Pharmaceutical composition for improving oral absorption |
| JP2005523293A (en) * | 2002-02-26 | 2005-08-04 | アストラゼネカ アクチボラグ | Iressa pharmaceutical formulation containing water-soluble cellulose derivative |
| EP1803448A3 (en) * | 2002-03-11 | 2007-09-12 | Novartis AG | Tasted masked veterinary solid compositions |
| US8617587B2 (en) | 2002-03-11 | 2013-12-31 | Novartis Ag | Tasted masked veterinary solid compositions |
| EP1803448A2 (en) * | 2002-03-11 | 2007-07-04 | Novartis AG | Tasted masked veterinary solid compositions |
| WO2003075895A1 (en) * | 2002-03-11 | 2003-09-18 | Novartis Ag | Tasted masked veterinary solid compositions |
| US7182950B2 (en) | 2002-06-12 | 2007-02-27 | Nutralease Ltd. | Nano-sized self-assembled liquid dilutable vehicles |
| WO2003105607A1 (en) * | 2002-06-12 | 2003-12-24 | Nutralease Ltd. | Nano-sized self-assembled structured liquids |
| US8652523B2 (en) | 2002-07-26 | 2014-02-18 | Flamel Technologies | Oral pharmaceutical formulation in the form of a plurality of microcapsules for prolonged release of active principle(s) with slow solubility |
| US8637512B2 (en) | 2002-07-29 | 2014-01-28 | Glaxo Group Limited | Formulations and method of treatment |
| US8980870B2 (en) | 2002-09-24 | 2015-03-17 | Boehringer Ingelheim International Gmbh | Solid telmisartan pharmaceutical formulations |
| WO2004047798A2 (en) * | 2002-11-22 | 2004-06-10 | Omega Farma Ehf | Formulations of finasteride |
| KR100592512B1 (en) * | 2002-11-22 | 2006-07-03 | 서울약품공업(주) | Extended Release Formulation of Tamsulosin or Pharmaceutically Acceptable Salt for Treating Evacuatory Insufficiency |
| WO2004047811A1 (en) * | 2002-11-22 | 2004-06-10 | Centurion, Inc. | Controlled release formulation of tamsulosin or pharmaceutically acceptable salts thereof for treatment of the signs and symptoms of benign prostatic hyperplasia |
| WO2004047798A3 (en) * | 2002-11-22 | 2004-07-29 | Omega Farma Ehf | Formulations of finasteride |
| US8003690B2 (en) | 2002-12-13 | 2011-08-23 | Jagotec Ag | Topical nanoparticulate spironolactone formulation |
| US7976853B2 (en) | 2003-01-29 | 2011-07-12 | Takeda Pharmaceutical Company Limited | Process for producing coated preparation |
| JP2006518380A (en) * | 2003-01-31 | 2006-08-10 | スミスクライン・ビーチャム・コーポレイション | Solid dispersion composition |
| EP1759692A2 (en) * | 2003-03-10 | 2007-03-07 | Novartis AG | Taste-masked solid veterinary compositions |
| EP1759692A3 (en) * | 2003-03-10 | 2007-09-12 | Novartis AG | Taste-masked solid veterinary compositions |
| EP1626695A4 (en) * | 2003-05-07 | 2009-12-30 | Afmedica Inc | Compositions and methods for reducing scar tissue formation |
| EP1626695A2 (en) * | 2003-05-07 | 2006-02-22 | Afmedica, Inc. | Compositions and methods for reducing scar tissue formation |
| EP1631239A2 (en) * | 2003-06-11 | 2006-03-08 | Novacea, Inc. | Pharmaceutical compositions comprising active vitamin d compounds |
| EP1631239A4 (en) * | 2003-06-11 | 2008-03-05 | Novacea Inc | Pharmaceutical compositions comprising active vitamin d compounds |
| EP1641437A4 (en) * | 2003-07-09 | 2009-06-03 | Chong Kun Dang Pharm Corp | The solid dispersion of tacrolimus |
| EP1641437A1 (en) * | 2003-07-09 | 2006-04-05 | Chong Kun Dang Pharmaceutical Corp. | The solid dispersion of tacrolimus |
| US8906419B2 (en) | 2003-08-06 | 2014-12-09 | Nostrum Pharmaceuticals, Inc. | Pharmaceutical composition containing water soluble drug |
| WO2005018626A1 (en) | 2003-08-22 | 2005-03-03 | Fournier Laboratories Ireland Limited | Pharmaceutical composition comprising a combination of metformin and a statin |
| US10548880B2 (en) | 2003-08-29 | 2020-02-04 | Veloxis Pharmaceuticals A/S | Solid dispersions comprising tacrolimus |
| US8586084B2 (en) | 2003-08-29 | 2013-11-19 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| US11077096B2 (en) | 2003-08-29 | 2021-08-03 | Veloxis Pharmaceuticals Inc. | Modified release compositions comprising tacrolimus |
| US8889186B2 (en) | 2003-08-29 | 2014-11-18 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| US8486993B2 (en) | 2003-08-29 | 2013-07-16 | Veloxis Pharmaceuticals A/S | Solid dispersions comprising tacrolimus |
| US8617599B2 (en) | 2003-08-29 | 2013-12-31 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| US8623411B2 (en) | 2003-08-29 | 2014-01-07 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| NO337869B1 (en) * | 2003-08-29 | 2016-07-04 | Veloxis Pharmaceuticals As | Solid dispersions comprising tacrolimus |
| AU2004267909B2 (en) * | 2003-08-29 | 2008-12-18 | Veloxis Pharmaceuticals, Inc. | Modified release compositions comprising tacrolimus |
| US8623410B2 (en) | 2003-08-29 | 2014-01-07 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| US9763920B2 (en) | 2003-08-29 | 2017-09-19 | Veloxis Pharmaceuticals A/S | Solid dispersions comprising tacrolimus |
| AU2004267910B2 (en) * | 2003-08-29 | 2011-01-06 | Veloxis Pharmaceuticals, Inc. | Solid dispersions comprising tacrolimus |
| CN1859909B (en) * | 2003-08-29 | 2011-04-06 | 生命周期药物公司 | Solid dispersions comprising tacrolimus |
| US9757362B2 (en) | 2003-08-29 | 2017-09-12 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| US8889185B2 (en) | 2003-08-29 | 2014-11-18 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| US9161907B2 (en) | 2003-08-29 | 2015-10-20 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| US11129815B2 (en) | 2003-08-29 | 2021-09-28 | Veloxis Pharmaceuticals Inc. | Solid dispersions comprising tacrolimus |
| US7994214B2 (en) | 2003-08-29 | 2011-08-09 | Lifecycle Pharma A/S | Solid dispersions comprising tacrolimus |
| US8591946B2 (en) | 2003-08-29 | 2013-11-26 | Veloxis Pharmaceuticals A/S | Modified release compositions comprising tacrolimus |
| WO2005020993A1 (en) * | 2003-08-29 | 2005-03-10 | Lifecycle Pharma A/S | Modified release compositions comprising tacrolimus |
| WO2005020994A1 (en) * | 2003-08-29 | 2005-03-10 | Lifecycle Pharma A/S | Solid dispersions comprising tacrolimus |
| EP3395340B1 (en) | 2003-09-12 | 2019-04-17 | Amgen, Inc | Rapid dissolution formulation of cinacalcet hcl |
| US7932263B2 (en) | 2003-09-26 | 2011-04-26 | Astrazeneca Ab | Therapeutic treatment |
| WO2005041958A1 (en) * | 2003-10-21 | 2005-05-12 | Smithkline Beecham Pharmco Puerto Rico Incorporated | Novel compositions |
| JP2007509120A (en) * | 2003-10-21 | 2007-04-12 | エスビー・ファルムコ・プエルト・リコ・インコーポレイテッド | New composition |
| WO2005042623A1 (en) * | 2003-10-23 | 2005-05-12 | University Of Nottingham | Preparing active polymer extrudates |
| WO2005065645A3 (en) * | 2003-12-31 | 2005-10-27 | Alpharma Inc | Donepezil formulations |
| WO2005065645A2 (en) * | 2003-12-31 | 2005-07-21 | Actavis Group Hf | Donepezil formulations |
| WO2005094791A1 (en) * | 2004-03-19 | 2005-10-13 | Eczacibasi Ozgun Kimyasal Urunler San. Tic. A.S. | Preparation of lipid coated cefuroxime axetil |
| US8802087B2 (en) | 2004-03-22 | 2014-08-12 | Abbott Products Gmbh | Pharmaceutical compositions of lipase-containing products, in particular of pancreation |
| US9238029B2 (en) | 2004-06-16 | 2016-01-19 | Takeda Pharmaceuticals U.S.A., Inc. | Multiple PPI dosage form |
| US9889152B2 (en) | 2004-06-16 | 2018-02-13 | Takeda Pharmaceuticals U.S.A., Inc. | Multiple PPI dosage form |
| US9012506B2 (en) | 2004-09-28 | 2015-04-21 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US10772995B2 (en) | 2004-09-28 | 2020-09-15 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| WO2006036967A1 (en) * | 2004-09-28 | 2006-04-06 | Atrium Medical Corporation | Solubilizing a drug for use in a coating |
| US10792312B2 (en) | 2004-09-28 | 2020-10-06 | Atrium Medical Corporation | Barrier layer |
| US9801913B2 (en) | 2004-09-28 | 2017-10-31 | Atrium Medical Corporation | Barrier layer |
| US10814043B2 (en) | 2004-09-28 | 2020-10-27 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US10869902B2 (en) | 2004-09-28 | 2020-12-22 | Atrium Medical Corporation | Cured gel and method of making |
| US9801982B2 (en) | 2004-09-28 | 2017-10-31 | Atrium Medical Corporation | Implantable barrier device |
| US9827352B2 (en) | 2004-09-28 | 2017-11-28 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US9000040B2 (en) | 2004-09-28 | 2015-04-07 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US11793912B2 (en) | 2004-09-28 | 2023-10-24 | Atrium Medical Corporation | Cross-linked fatty acid-based biomaterials |
| US8858978B2 (en) | 2004-09-28 | 2014-10-14 | Atrium Medical Corporation | Heat cured gel and method of making |
| US8962023B2 (en) | 2004-09-28 | 2015-02-24 | Atrium Medical Corporation | UV cured gel and method of making |
| US9682175B2 (en) | 2004-09-28 | 2017-06-20 | Atrium Medical Corporation | Coating material and medical device system including same |
| US10016465B2 (en) | 2004-09-28 | 2018-07-10 | Atrium Medical Corporation | Cured gel and method of making |
| EP1811967A4 (en) * | 2004-11-08 | 2008-08-20 | Biokey Inc | Methods and formulations for making pharmaceutical compositions containing bupropion |
| US8586085B2 (en) | 2004-11-08 | 2013-11-19 | Biokey, Inc. | Methods and formulations for making pharmaceutical compositions containing bupropion |
| EP1811967A2 (en) * | 2004-11-08 | 2007-08-01 | Biokey, Inc. | Methods and formulations for making pharmaceutical compositions containing bupropion |
| EP1809295A4 (en) * | 2004-11-10 | 2009-07-01 | Derray Technology Inc | Novel formulations of eprosartan with enhanced bioavailability |
| EP1809295A2 (en) * | 2004-11-10 | 2007-07-25 | Derray Technology, Inc. | Novel formulations of eprosartan with enhanced bioavailability |
| US7348027B2 (en) * | 2005-04-08 | 2008-03-25 | Bayer Healthcare Llc | Taste masked veterinary formulation |
| EP1874271A1 (en) * | 2005-04-12 | 2008-01-09 | Elan Pharma International Limited | Modified release compositions comprising a fluorocytidine derivative for the treatment of cancer |
| EP1874271A4 (en) * | 2005-04-12 | 2012-01-25 | Elan Pharma Int Ltd | Modified release compositions comprising a fluorocytidine derivative for the treatment of cancer |
| US10704037B2 (en) | 2005-07-29 | 2020-07-07 | Abbott Products Gmbh | Processes for the manufacture and use of pancreatin |
| US11266607B2 (en) | 2005-08-15 | 2022-03-08 | AbbVie Pharmaceuticals GmbH | Process for the manufacture and use of pancreatin micropellet cores |
| US9198871B2 (en) | 2005-08-15 | 2015-12-01 | Abbott Products Gmbh | Delayed release pancreatin compositions |
| US9308180B2 (en) | 2005-08-31 | 2016-04-12 | Abraxis Bioscience, Llc | Compositions and methods for preparation of poorly water soluble drugs with increased stability |
| US7771751B2 (en) | 2005-08-31 | 2010-08-10 | Abraxis Bioscience, Llc | Compositions comprising poorly water soluble pharmaceutical agents and antimicrobial agents |
| US11083823B2 (en) | 2005-09-28 | 2021-08-10 | Atrium Medical Corporation | Tissue-separating fatty acid adhesion barrier |
| US9278161B2 (en) | 2005-09-28 | 2016-03-08 | Atrium Medical Corporation | Tissue-separating fatty acid adhesion barrier |
| US9220820B2 (en) | 2005-10-15 | 2015-12-29 | Atrium Medical Corporation | Hydrophobic cross-linked gels for bioabsorbable drug carrier coatings |
| US8497258B2 (en) | 2005-11-12 | 2013-07-30 | The Regents Of The University Of California | Viscous budesonide for the treatment of inflammatory diseases of the gastrointestinal tract |
| US11413296B2 (en) | 2005-11-12 | 2022-08-16 | The Regents Of The University Of California | Viscous budesonide for the treatment of inflammatory diseases of the gastrointestinal tract |
| US11197822B2 (en) | 2005-11-12 | 2021-12-14 | The Regents Of The University Of California | Topical corticosteroids for the treatment of inflammatory diseases of the gastrointestinal tract |
| US8679545B2 (en) | 2005-11-12 | 2014-03-25 | The Regents Of The University Of California | Topical corticosteroids for the treatment of inflammatory diseases of the gastrointestinal tract |
| US8324192B2 (en) | 2005-11-12 | 2012-12-04 | The Regents Of The University Of California | Viscous budesonide for the treatment of inflammatory diseases of the gastrointestinal tract |
| US9119863B2 (en) | 2005-11-12 | 2015-09-01 | The Regents Of The University Of California | Viscous budesonide for the treatment of inflammatory diseases of the gastrointestinal tract |
| US10272037B2 (en) | 2005-11-12 | 2019-04-30 | The Regents Of The University Of California | Topical corticosteroids for the treatment of inflammatory diseases of the gastrointestinal tract |
| US9782347B2 (en) | 2005-11-12 | 2017-10-10 | The Regents Of The University Of California | Topical corticosteroids for the treatment of inflammatory diseases of the gastrointestinal tract |
| EP2712867A1 (en) * | 2005-12-14 | 2014-04-02 | Abbott Laboratories | Compositions of tetrazole derivatives of sirolimus and anti-oxidants |
| WO2007077581A2 (en) * | 2006-01-02 | 2007-07-12 | Rubicon Research Private Limited | Pharmaceutical compositions |
| WO2007077581A3 (en) * | 2006-01-02 | 2008-01-24 | Rubicon Res Private Ltd | Pharmaceutical compositions |
| US11911398B2 (en) | 2006-02-03 | 2024-02-27 | Opko Renal, Llc | Treating Vitamin D insufficiency and deficiency with 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 |
| US9943530B2 (en) | 2006-02-03 | 2018-04-17 | Opko Renal, Llc | Treating vitamin D insufficiency and deficiency with 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 |
| US11007204B2 (en) | 2006-02-03 | 2021-05-18 | Opko Renal, Llc | Treating vitamin D insufficiency and deficiency with 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 |
| US10213442B2 (en) | 2006-02-03 | 2019-02-26 | Opko Renal, Llc | Treating vitamin D insufficiency and deficiency with 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 |
| US10072256B2 (en) | 2006-05-22 | 2018-09-11 | Abbott Products Gmbh | Process for separating and determining the viral load in a pancreatin sample |
| US10668089B2 (en) | 2006-06-21 | 2020-06-02 | Opko Ireland Global Holdings, Ltd. | Method of treating and preventing secondary hyperparathyroidism |
| EP1905432A1 (en) * | 2006-06-30 | 2008-04-02 | ELAN CORPORATION, Plc | Nanoparticulate and controlled release compositions comprising a cephalosporin |
| US9744137B2 (en) | 2006-08-31 | 2017-08-29 | Supernus Pharmaceuticals, Inc. | Topiramate compositions and methods of enhancing its bioavailability |
| WO2008030161A1 (en) * | 2006-09-05 | 2008-03-13 | Astrazeneca Ab | Pharmaceutical composition comprising candesartan cilexetil |
| WO2008033024A2 (en) * | 2006-09-15 | 2008-03-20 | Echo Pharmaceuticals B.V. | Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances |
| CN101541299B (en) * | 2006-09-15 | 2013-04-17 | 艾可制药有限公司 | Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances |
| US9308175B2 (en) | 2006-09-15 | 2016-04-12 | Echo Pharmaceuticals B.V. | Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances |
| WO2008033024A3 (en) * | 2006-09-15 | 2008-05-22 | Echo Pharmaceuticals Bv | Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances |
| AU2007295179B2 (en) * | 2006-09-15 | 2013-05-30 | Echo Pharmaceuticals B.V. | Dosage unit for sublingual, buccal or oral administration of water-insoluble pharmaceutically active substances |
| CN101167697B (en) * | 2006-10-26 | 2011-03-30 | 中国科学院上海药物研究所 | Donepezils compound long-acting slow-releasing and controlled-releasing composition and preparation method thereof |
| US9492596B2 (en) | 2006-11-06 | 2016-11-15 | Atrium Medical Corporation | Barrier layer with underlying medical device and one or more reinforcing support structures |
| US9592324B2 (en) | 2006-11-06 | 2017-03-14 | Atrium Medical Corporation | Tissue separating device with reinforced support for anchoring mechanisms |
| US8298580B2 (en) | 2006-11-17 | 2012-10-30 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| US9622983B2 (en) | 2006-11-17 | 2017-04-18 | Supernus Pharmaceutcals, Inc. | Sustained-release formulations of topiramate |
| US8877248B1 (en) | 2006-11-17 | 2014-11-04 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| US8663683B2 (en) | 2006-11-17 | 2014-03-04 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| EP2394643A1 (en) * | 2006-11-17 | 2011-12-14 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| US8889191B2 (en) | 2006-11-17 | 2014-11-18 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| US10314790B2 (en) | 2006-11-17 | 2019-06-11 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| US8298576B2 (en) | 2006-11-17 | 2012-10-30 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| US9555004B2 (en) | 2006-11-17 | 2017-01-31 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| US9549940B2 (en) | 2006-11-17 | 2017-01-24 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| AU2007319141B2 (en) * | 2006-11-17 | 2013-01-10 | Supernus Pharmaceuticals Inc. | Sustained-release formulations of topiramate |
| US8992989B2 (en) | 2006-11-17 | 2015-03-31 | Supernus Pharmaceuticals, Inc. | Sustained-release formulations of topiramate |
| WO2008061226A3 (en) * | 2006-11-17 | 2008-07-31 | Supernus Pharmaceuticals Inc | Sustained-release formulations of topiramate |
| WO2008065097A3 (en) * | 2006-11-28 | 2008-07-17 | Liconsa Laboratorios Sa | Stabilized solid pharmaceutical composition of candesartan cilexetil |
| WO2008083192A3 (en) * | 2006-12-28 | 2008-11-27 | Repros Therapeutics Inc | Methods and formulations for improved bioavailability of antiprogestins |
| WO2008083192A2 (en) * | 2006-12-28 | 2008-07-10 | Repros Therapeutics Inc. | Methods and formulations for improved bioavailability of antiprogestins |
| US10434139B2 (en) | 2007-04-04 | 2019-10-08 | Sublimity Therapeutics Limited | Oral pharmaceutical composition |
| US10434140B2 (en) | 2007-04-04 | 2019-10-08 | Sublimity Therapeutics Limited | Pharmaceutical cyclosporin compositions |
| US9844513B2 (en) | 2007-04-04 | 2017-12-19 | Sigmoid Pharma Limited | Oral pharmaceutical composition |
| US8911777B2 (en) | 2007-04-04 | 2014-12-16 | Sigmoid Pharma Limited | Pharmaceutical composition of tacrolimus |
| US9675558B2 (en) | 2007-04-04 | 2017-06-13 | Sigmoid Pharma Limited | Pharmaceutical cyclosporin compositions |
| US9585844B2 (en) | 2007-04-04 | 2017-03-07 | Sigmoid Pharma Limited | Oral pharmaceutical composition |
| US9114071B2 (en) | 2007-04-04 | 2015-08-25 | Sigmoid Pharma Limited | Oral pharmaceutical composition |
| US9387179B2 (en) | 2007-04-04 | 2016-07-12 | Sigmoid Pharma Limited | Pharmaceutical cyclosporin compositions |
| US9498486B1 (en) | 2007-04-25 | 2016-11-22 | Opko Renal, Llc | Method for controlled release oral dosage of a vitamin D compound |
| US9925147B2 (en) | 2007-04-25 | 2018-03-27 | Opko Renal, Llc | Method for treating secondary hyperparathyroidism in CKD |
| US11154509B2 (en) | 2007-04-25 | 2021-10-26 | Eirgen Pharma Ltd. | Methods for controlled release oral dosage of a vitamin D compound |
| US9408858B2 (en) | 2007-04-25 | 2016-08-09 | Opko Renal, Llc | Method for treating secondary hyperparathyroidism in CKD |
| US11752158B2 (en) | 2007-04-25 | 2023-09-12 | Eirgen Pharma Ltd. | Method of treating vitamin D insufficiency and deficiency |
| US11801253B2 (en) | 2007-04-25 | 2023-10-31 | Opko Renal, Llc | Method of safely and effectively treating and preventing secondary hyperparathyroidism in chronic kidney disease |
| US9918940B2 (en) | 2007-04-25 | 2018-03-20 | Opko Renal, Llc | Methods for controlled release oral dosage of a vitamin D compound |
| US9402788B2 (en) | 2007-04-26 | 2016-08-02 | Sigmoid Pharma Limited | Manufacture of multiple minicapsules |
| US8951570B2 (en) | 2007-04-26 | 2015-02-10 | Sigmoid Pharma Limited | Manufacture of multiple minicapsules |
| US11123331B2 (en) | 2007-05-30 | 2021-09-21 | Veloxis Pharmaceuticals, Inc. | Tacrolimus for improved treatment of transplant patients |
| US8664239B2 (en) | 2007-05-30 | 2014-03-04 | Veloxis Pharmaceuticals A/S | Tacrolimus for improved treatment of transplant patients |
| US10864199B2 (en) | 2007-05-30 | 2020-12-15 | Veloxis Pharmaceuticals A/S | Tacrolimus for improved treatment of transplant patients |
| US11110081B2 (en) | 2007-05-30 | 2021-09-07 | Veloxis Pharmaceuticals, Inc. | Tacrolimus for improved treatment of transplant patients |
| EP2206518A4 (en) * | 2007-10-25 | 2012-05-09 | Astellas Pharma Inc | Pharmaceutical composition containing lipophilic il-2 production inhibitor |
| EP2206518A1 (en) * | 2007-10-25 | 2010-07-14 | Astellas Pharma Inc. | Pharmaceutical composition containing lipophilic il-2 production inhibitor |
| EP2217211A4 (en) * | 2007-10-29 | 2011-06-08 | Stanford Res Inst Int | An orally-absorbed solid dose formulation for vancomycin |
| EP2217211A1 (en) * | 2007-10-29 | 2010-08-18 | SRI International | An orally-absorbed solid dose formulation for vancomycin |
| US11357859B2 (en) | 2007-11-13 | 2022-06-14 | Viropharma Biologics Llc | Compositions for the treatment of gastrointestinal inflammation |
| US8865692B2 (en) | 2007-11-13 | 2014-10-21 | Meritage Pharma, Inc | Compositions for the treatment of gastrointestinal inflammation |
| US9050368B2 (en) | 2007-11-13 | 2015-06-09 | Meritage Pharma, Inc. | Corticosteroid compositions |
| US10293052B2 (en) | 2007-11-13 | 2019-05-21 | Meritage Pharma, Inc. | Compositions for the treatment of gastrointestinal inflammation |
| WO2009068458A3 (en) * | 2007-11-27 | 2009-08-20 | Univ Friedrich Alexander Er | Encapsulated microparticles with virus-containing core and production method for microparticles |
| US9241910B2 (en) | 2008-03-11 | 2016-01-26 | Takeda Pharmaceutical Company Limited | Orally-disintegrating solid preparation |
| US10302660B2 (en) | 2008-04-02 | 2019-05-28 | Opko Renal, Llc | Methods useful for vitamin D deficiency and related disorders |
| WO2009131537A1 (en) * | 2008-04-25 | 2009-10-29 | Karolinska Institutet Innovations Ab | New therapy of treatment of the irritable bowel syndrome. |
| US11419823B2 (en) | 2008-05-30 | 2022-08-23 | Veloxis Pharmaceuticals, Inc. | Stabilized tacrolimus composition |
| US9549918B2 (en) | 2008-05-30 | 2017-01-24 | Veloxis Pharmaceuticals A/S | Stabilized tacrolimus composition |
| US10166190B2 (en) | 2008-05-30 | 2019-01-01 | Veloxis Pharmaceuticals A/S | Stabilized tacrolimus composition |
| US9381198B2 (en) | 2008-06-16 | 2016-07-05 | Biovascular, Inc. | Controlled release compositions of agents that reduce circulating levels of platelets and methods therefor |
| WO2010005480A3 (en) * | 2008-06-16 | 2010-08-05 | Biovascular, Inc. | Controlled release compositions of agents that reduce circulating levels of platelets and methods therefor |
| WO2010005480A2 (en) * | 2008-06-16 | 2010-01-14 | Biovascular, Inc. | Controlled release compositions of agents that reduce circulating levels of platelets and methods therefor |
| US9040483B2 (en) | 2008-06-16 | 2015-05-26 | Biovascular, Inc. | Controlled release compositions of agents that reduce circulating levels of platelets and methods therefor |
| EP2323627A1 (en) * | 2008-06-23 | 2011-05-25 | Bionetwork | Novel rapid-effect pharmaceutical forms and uses of resulting pharmaceutical compositions |
| US9226900B2 (en) | 2008-07-11 | 2016-01-05 | Critical Pharmaceuticals Limited | Process for preparing microparticles |
| EP2314277A4 (en) * | 2008-07-28 | 2015-06-03 | Kao Corp | External preparation for skin, and wrinkle-repairing agent |
| US10117909B2 (en) | 2008-10-17 | 2018-11-06 | Sanofi-Aventis Deutschland Gmbh | Combination of an insulin and a GLP-1 agonist |
| US9526764B2 (en) | 2008-10-17 | 2016-12-27 | Sanofi-Aventis Deutschland Gmbh | Combination of an insulin and a GLP-1-agonist |
| US11304960B2 (en) | 2009-01-08 | 2022-04-19 | Chandrashekar Giliyar | Steroidal compositions |
| US11052096B2 (en) | 2009-01-08 | 2021-07-06 | Lipocine Inc. | Steroidal compositions |
| US8865695B2 (en) | 2009-01-08 | 2014-10-21 | Lipocine Inc. | Steroidal compositions |
| US11166929B2 (en) | 2009-03-10 | 2021-11-09 | Atrium Medical Corporation | Fatty-acid based particles |
| US10285964B2 (en) | 2009-03-10 | 2019-05-14 | Atrium Medical Corporation | Fatty-acid based particles |
| US9427423B2 (en) | 2009-03-10 | 2016-08-30 | Atrium Medical Corporation | Fatty-acid based particles |
| US9278070B2 (en) | 2009-05-18 | 2016-03-08 | Sigmoid Pharma Limited | Composition comprising oil drops |
| US9999651B2 (en) | 2009-05-18 | 2018-06-19 | Sigmoid Pharma Limited | Composition comprising oil drops |
| US9539302B2 (en) | 2009-06-18 | 2017-01-10 | Allergan, Inc. | Safe desmopressin administration |
| US11419914B2 (en) | 2009-06-18 | 2022-08-23 | Serenity Pharmaceuticals Llc | Safe desmopressin administration |
| US10864304B2 (en) | 2009-08-11 | 2020-12-15 | Atrium Medical Corporation | Anti-infective antimicrobial-containing biomaterials |
| US9878036B2 (en) | 2009-08-12 | 2018-01-30 | Sigmoid Pharma Limited | Immunomodulatory compositions comprising a polymer matrix and an oil phase |
| US10029011B2 (en) | 2009-11-13 | 2018-07-24 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition comprising a GLP-1 agonist, an insulin and methionine |
| US10028910B2 (en) | 2009-11-13 | 2018-07-24 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition comprising a GLP-1-agonist and methionine |
| US9707176B2 (en) | 2009-11-13 | 2017-07-18 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition comprising a GLP-1 agonist and methionine |
| US11672809B2 (en) | 2010-03-29 | 2023-06-13 | Eirgen Pharma Ltd. | Methods and compositions for reducing parathyroid levels |
| US9314474B2 (en) | 2010-07-15 | 2016-04-19 | Hybrigenics, Sa | Formulations of 14-epi-analogues of vitamin D |
| US10322213B2 (en) | 2010-07-16 | 2019-06-18 | Atrium Medical Corporation | Compositions and methods for altering the rate of hydrolysis of cured oil-based materials |
| US11097035B2 (en) | 2010-07-16 | 2021-08-24 | Atrium Medical Corporation | Compositions and methods for altering the rate of hydrolysis of cured oil-based materials |
| ITMI20101512A1 (en) * | 2010-08-06 | 2012-02-07 | Sofar Spa | COMPOSITIONS OF BECLOMETASONE DIPROPIONIONATO IN GASTRORESISTANT MICROSPHERES WITH MODIFIED RELEASE AND PROCESS FOR THEIR ACHIEVEMENT |
| US10028917B2 (en) | 2010-08-06 | 2018-07-24 | Sofar Spa | Beclomethasone dipropionate compositions in modified-release gastro-resistant microspheres and process for obtaining them |
| WO2012017385A3 (en) * | 2010-08-06 | 2012-04-19 | Sofar Spa | Beclomethasone dipropionate compositions in modified-release gastro-resistant microspheres and process for obtaining them |
| EP3009130A1 (en) * | 2010-08-06 | 2016-04-20 | SOFAR S.p.A. | Beclomethasone dipropionate compositions in modified-release gastro-resistant microspheres |
| US9981013B2 (en) | 2010-08-30 | 2018-05-29 | Sanofi-Aventis Deutschland Gmbh | Use of AVE0010 for the treatment of diabetes mellitus type 2 |
| US9821024B2 (en) | 2010-11-25 | 2017-11-21 | Sigmoid Pharma Limited | Immunomodulatory compositions |
| US9205057B2 (en) | 2010-11-30 | 2015-12-08 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US10799513B2 (en) | 2010-11-30 | 2020-10-13 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US9480690B2 (en) | 2010-11-30 | 2016-11-01 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US9034858B2 (en) | 2010-11-30 | 2015-05-19 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US10226473B2 (en) | 2010-11-30 | 2019-03-12 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US9757390B2 (en) | 2010-11-30 | 2017-09-12 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US9358241B2 (en) | 2010-11-30 | 2016-06-07 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US10716794B2 (en) | 2010-11-30 | 2020-07-21 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US10973833B2 (en) | 2010-11-30 | 2021-04-13 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US9949985B2 (en) | 2010-11-30 | 2018-04-24 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US9943527B2 (en) | 2010-11-30 | 2018-04-17 | Lipocine Inc. | High-strength testosterone undecanoate compositions |
| US10561615B2 (en) | 2010-12-10 | 2020-02-18 | Lipocine Inc. | Testosterone undecanoate compositions |
| US9821032B2 (en) | 2011-05-13 | 2017-11-21 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical combination for improving glycemic control as add-on therapy to basal insulin |
| US9408893B2 (en) | 2011-08-29 | 2016-08-09 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical combination for use in glycemic control in diabetes type 2 patients |
| US9987332B2 (en) | 2011-09-01 | 2018-06-05 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition for use in the treatment of a neurodegenerative disease |
| US9364519B2 (en) | 2011-09-01 | 2016-06-14 | Sanofi-Aventis Deutschland Gmbh | Pharmaceutical composition for use in the treatment of a neurodegenerative disease |
| US10675288B2 (en) | 2011-11-23 | 2020-06-09 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8846648B2 (en) | 2011-11-23 | 2014-09-30 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11103516B2 (en) | 2011-11-23 | 2021-08-31 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11793819B2 (en) | 2011-11-23 | 2023-10-24 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8987237B2 (en) | 2011-11-23 | 2015-03-24 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8846649B2 (en) | 2011-11-23 | 2014-09-30 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8633178B2 (en) | 2011-11-23 | 2014-01-21 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US9248136B2 (en) | 2011-11-23 | 2016-02-02 | Therapeuticsmd, Inc. | Transdermal hormone replacement therapies |
| CN102423299A (en) * | 2011-12-20 | 2012-04-25 | 中国热带农业科学院农产品加工研究所 | Preparation method for novel drug-loaded chitosan nano-microspheres |
| US9763879B2 (en) | 2012-04-12 | 2017-09-19 | Lts Lohmann Therapie-Systeme Ag | Oral film containing opiate enteric-release beads |
| US9687445B2 (en) | 2012-04-12 | 2017-06-27 | Lts Lohmann Therapie-Systeme Ag | Oral film containing opiate enteric-release beads |
| CN102657626A (en) * | 2012-05-23 | 2012-09-12 | 重庆康刻尔制药有限公司 | Medicinal composite tablet of pioglitazone medicine |
| US10888617B2 (en) | 2012-06-13 | 2021-01-12 | Atrium Medical Corporation | Cured oil-hydrogel biomaterial compositions for controlled drug delivery |
| US9867880B2 (en) | 2012-06-13 | 2018-01-16 | Atrium Medical Corporation | Cured oil-hydrogel biomaterial compositions for controlled drug delivery |
| US11166963B2 (en) | 2012-06-18 | 2021-11-09 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11110099B2 (en) | 2012-06-18 | 2021-09-07 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10471148B2 (en) | 2012-06-18 | 2019-11-12 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable PK profile |
| US10639375B2 (en) | 2012-06-18 | 2020-05-05 | Therapeuticsmd, Inc. | Progesterone formulations |
| US10052386B2 (en) | 2012-06-18 | 2018-08-21 | Therapeuticsmd, Inc. | Progesterone formulations |
| US9301920B2 (en) | 2012-06-18 | 2016-04-05 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11529360B2 (en) | 2012-06-18 | 2022-12-20 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US9012434B2 (en) | 2012-06-18 | 2015-04-21 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US8933059B2 (en) | 2012-06-18 | 2015-01-13 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US11033626B2 (en) | 2012-06-18 | 2021-06-15 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable pk profile |
| US9289382B2 (en) | 2012-06-18 | 2016-03-22 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11865179B2 (en) | 2012-06-18 | 2024-01-09 | Therapeuticsmd, Inc. | Progesterone formulations having a desirable PK profile |
| US8987238B2 (en) | 2012-06-18 | 2015-03-24 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US9006222B2 (en) | 2012-06-18 | 2015-04-14 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10806740B2 (en) | 2012-06-18 | 2020-10-20 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US9950051B2 (en) | 2012-07-05 | 2018-04-24 | Sigmoid Pharma Limited | Formulations |
| US9220681B2 (en) | 2012-07-05 | 2015-12-29 | Sigmoid Pharma Limited | Formulations |
| US11266661B2 (en) | 2012-12-21 | 2022-03-08 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10537581B2 (en) | 2012-12-21 | 2020-01-21 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11622933B2 (en) | 2012-12-21 | 2023-04-11 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US11497709B2 (en) | 2012-12-21 | 2022-11-15 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10835487B2 (en) | 2012-12-21 | 2020-11-17 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10888516B2 (en) | 2012-12-21 | 2021-01-12 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US10471072B2 (en) | 2012-12-21 | 2019-11-12 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11351182B2 (en) | 2012-12-21 | 2022-06-07 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11304959B2 (en) | 2012-12-21 | 2022-04-19 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11116717B2 (en) | 2012-12-21 | 2021-09-14 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US11246875B2 (en) | 2012-12-21 | 2022-02-15 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11241445B2 (en) | 2012-12-21 | 2022-02-08 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US9180091B2 (en) | 2012-12-21 | 2015-11-10 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US11065197B2 (en) | 2012-12-21 | 2021-07-20 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US10806697B2 (en) | 2012-12-21 | 2020-10-20 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US11123283B2 (en) | 2012-12-21 | 2021-09-21 | Therapeuticsmd, Inc. | Soluble estradiol capsule for vaginal insertion |
| US10568891B2 (en) | 2012-12-21 | 2020-02-25 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US9320746B2 (en) | 2013-02-21 | 2016-04-26 | Sigmoid Pharma Limited | Method for treating intestinal fibrosis |
| US9980902B2 (en) | 2013-02-21 | 2018-05-29 | Sigmoid Pharma Limited | Method for treating intestinal fibrosis |
| US10350224B2 (en) | 2013-03-15 | 2019-07-16 | Opko Ireland Global Holdings, Ltd. | Stabilized modified release vitamin D formulation and method of administering same |
| US11253528B2 (en) | 2013-03-15 | 2022-02-22 | Eirgen Pharma Ltd. | Stabilized modified release Vitamin D formulation and method of administering same |
| US10300078B2 (en) | 2013-03-15 | 2019-05-28 | Opko Ireland Global Holdings, Ltd. | Stabilized modified release vitamin D formulation and method of administering same |
| US10357502B2 (en) | 2013-03-15 | 2019-07-23 | Opko Ireland Global Holdings, Ltd. | Stabilized modified release vitamin D formulation and method of administering same |
| US9861644B2 (en) | 2013-03-15 | 2018-01-09 | Opko Ireland Global Holdings, Ltd. | Stabilized modified release vitamin D formulation and method of administering same |
| US10434138B2 (en) | 2013-11-08 | 2019-10-08 | Sublimity Therapeutics Limited | Formulations |
| US11103513B2 (en) | 2014-05-22 | 2021-08-31 | TherapeuticsMD | Natural combination hormone replacement formulations and therapies |
| US10206932B2 (en) | 2014-05-22 | 2019-02-19 | Therapeuticsmd, Inc. | Natural combination hormone replacement formulations and therapies |
| US10098894B2 (en) | 2014-07-29 | 2018-10-16 | Therapeuticsmd, Inc. | Transdermal cream |
| US10493084B2 (en) | 2014-08-07 | 2019-12-03 | Opko Ireland Global Holdings, Ltd. | Adjunctive therapy with 25-hydroxyvitamin D and articles therefor |
| US10220047B2 (en) | 2014-08-07 | 2019-03-05 | Opko Ireland Global Holdings, Ltd. | Adjunctive therapy with 25-hydroxyvitamin D and articles therefor |
| US11738033B2 (en) | 2014-08-07 | 2023-08-29 | Eirgen Pharma Ltd. | Adjunctive therapy with 25-hydroxyvitamin D and articles therefor |
| US11007205B2 (en) | 2014-08-07 | 2021-05-18 | Eirgen Pharma Ltd. | Adjunctive therapy with 25-hydroxyvitamin D and articles therefor |
| US11298365B2 (en) | 2014-08-28 | 2022-04-12 | Lipocine Inc. | Bioavailable solid state (17-β)-hydroxy-4-androsten-3-one esters |
| US11707467B2 (en) | 2014-08-28 | 2023-07-25 | Lipocine Inc. | (17-ß)-3-oxoandrost-4-en-17YL tridecanoate compositions and methods of their preparation and use |
| US9498485B2 (en) | 2014-08-28 | 2016-11-22 | Lipocine Inc. | Bioavailable solid state (17-β)-hydroxy-4-androsten-3-one esters |
| US11872235B1 (en) | 2014-08-28 | 2024-01-16 | Lipocine Inc. | Bioavailable solid state (17-β)-Hydroxy-4-Androsten-3-one esters |
| US9757389B2 (en) | 2014-08-28 | 2017-09-12 | Lipocine Inc. | Bioavailable solid state (17-β)-hydroxy-4-androsten-3-one esters |
| US10258630B2 (en) | 2014-10-22 | 2019-04-16 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10398708B2 (en) | 2014-10-22 | 2019-09-03 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10668082B2 (en) | 2014-10-22 | 2020-06-02 | Therapeuticsmd, Inc. | Vaginal inserted estradiol pharmaceutical compositions and methods |
| US10993987B2 (en) | 2014-11-07 | 2021-05-04 | Sublimity Therapeutics Limited | Compositions comprising Cyclosporin |
| US9950039B2 (en) | 2014-12-12 | 2018-04-24 | Sanofi-Aventis Deutschland Gmbh | Insulin glargine/lixisenatide fixed ratio formulation |
| US10434147B2 (en) | 2015-03-13 | 2019-10-08 | Sanofi-Aventis Deutschland Gmbh | Treatment type 2 diabetes mellitus patients |
| US10159713B2 (en) | 2015-03-18 | 2018-12-25 | Sanofi-Aventis Deutschland Gmbh | Treatment of type 2 diabetes mellitus patients |
| US10912783B2 (en) | 2015-07-23 | 2021-02-09 | Therapeuticsmd, Inc. | Formulations for solubilizing hormones |
| US10328087B2 (en) | 2015-07-23 | 2019-06-25 | Therapeuticsmd, Inc. | Formulations for solubilizing hormones |
| US11173168B2 (en) | 2016-03-28 | 2021-11-16 | Eirgen Pharma Ltd. | Methods of treating vitamin D insufficiency in chronic kidney disease |
| US10532059B2 (en) | 2016-04-01 | 2020-01-14 | Therapeuticsmd, Inc. | Steroid hormone pharmaceutical composition |
| US10286077B2 (en) | 2016-04-01 | 2019-05-14 | Therapeuticsmd, Inc. | Steroid hormone compositions in medium chain oils |
| US9931349B2 (en) | 2016-04-01 | 2018-04-03 | Therapeuticsmd, Inc. | Steroid hormone pharmaceutical composition |
| US10835488B2 (en) | 2016-06-16 | 2020-11-17 | Dexcel Pharma Technologies Ltd. | Stable orally disintegrating pharmaceutical compositions |
| US10076494B2 (en) | 2016-06-16 | 2018-09-18 | Dexcel Pharma Technologies Ltd. | Stable orally disintegrating pharmaceutical compositions |
| US10537592B2 (en) | 2016-06-29 | 2020-01-21 | CannScience Innovations Inc. | Decarboxylated cannabis resins, uses thereof and methods of making same |
| US10383892B2 (en) | 2016-06-29 | 2019-08-20 | CannScience Innovations Inc. | Decarboxylated cannabis resins, uses thereof and methods of making same |
| US10143706B2 (en) | 2016-06-29 | 2018-12-04 | Cannscience Innovations, Inc. | Decarboxylated cannabis resins, uses thereof and methods of making same |
| US11559530B2 (en) | 2016-11-28 | 2023-01-24 | Lipocine Inc. | Oral testosterone undecanoate therapy |
| WO2020187455A1 (en) | 2019-03-20 | 2020-09-24 | Pharmathen S.A. | Prolonged release formulation comprising tacrolimus |
Also Published As
| Publication number | Publication date |
|---|---|
| US20150224130A9 (en) | 2015-08-13 |
| JP2003517470A (en) | 2003-05-27 |
| CA2706113A1 (en) | 2001-05-31 |
| EP1233756A4 (en) | 2007-12-26 |
| US20140357586A1 (en) | 2014-12-04 |
| US20030064097A1 (en) | 2003-04-03 |
| EP1233756A1 (en) | 2002-08-28 |
| CA2391923C (en) | 2011-05-17 |
| US20200061191A1 (en) | 2020-02-27 |
| US20090074859A1 (en) | 2009-03-19 |
| CA2391923A1 (en) | 2001-05-31 |
| US20210008212A1 (en) | 2021-01-14 |
| US20150273067A1 (en) | 2015-10-01 |
| US6923988B2 (en) | 2005-08-02 |
| US20180125978A1 (en) | 2018-05-10 |
| US20030215496A1 (en) | 2003-11-20 |
| AU1798101A (en) | 2001-06-04 |
| US6569463B2 (en) | 2003-05-27 |
| JP2012116863A (en) | 2012-06-21 |
| US6248363B1 (en) | 2001-06-19 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20210008212A1 (en) | Solid Carriers for Improved Delivery of Active Ingredients in Pharmaceutical Compositions | |
| WO2007018943A2 (en) | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions | |
| TWI435718B (en) | The method for producing a wax-containing matrix particle containing a drug is carried out by using an extruder in the method and a sustained-release preparation containing Cilostazol | |
| US20030180352A1 (en) | Solid carriers for improved delivery of active ingredients in pharmaceutical compositions | |
| US7374779B2 (en) | Pharmaceutical formulations and systems for improved absorption and multistage release of active agents | |
| KR101563390B1 (en) | Novel composition based on gamma-hydroxybutyric acid | |
| US20080014257A1 (en) | Oral dosage forms | |
| CA2895534C (en) | Orally disintegrating tablet formulation for enhanced bioavailability | |
| US20120225118A1 (en) | Compositions for delivery of insoluble agents | |
| KR20010012402A (en) | Stable oral pharmaceutical dosage forms | |
| EP2153834A2 (en) | Extended release pharmaceutical compositions comprising quetiapine salts | |
| WO2012001705A2 (en) | Pharmaceutical compositions of (r)-lansoprazole | |
| CN101340882B (en) | Method of producing drug-containing wax matrix particles, extruder to be used in the method and sustained-release preparation containing cilostazol | |
| CN1713896B (en) | Spheroids, preparation method thereof and pharmaceutical compositions | |
| WO2006117803A2 (en) | Transmucosal drug delivery systems | |
| WO2008157228A1 (en) | New methods for taste-masking | |
| KR101506626B1 (en) | Sustained-release pharmaceutical composition comprising acebrophylline and a hydrophobic material | |
| WO2009006299A2 (en) | Multi-particulate systems | |
| KR20000018902A (en) | Antibacterial composition containing intraconazole | |
| CS217143B1 (en) | Medicinal preparation containing the pentoxyfylin |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AG AL AM AT AU AZ BA BB BG BR BY BZ CA CH CN CR CU CZ DE DK DM DZ EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MA MD MG MK MN MW MX MZ NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW MZ SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE TR BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 2391923 Country of ref document: CA |
|
| ENP | Entry into the national phase |
Ref country code: JP Ref document number: 2001 539423 Kind code of ref document: A Format of ref document f/p: F |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2000980761 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 2000980761 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |