WO2000017161A1 - Cyclic ester or amide derivatives - Google Patents
Cyclic ester or amide derivatives Download PDFInfo
- Publication number
- WO2000017161A1 WO2000017161A1 PCT/US1999/021290 US9921290W WO0017161A1 WO 2000017161 A1 WO2000017161 A1 WO 2000017161A1 US 9921290 W US9921290 W US 9921290W WO 0017161 A1 WO0017161 A1 WO 0017161A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound
- independently
- substituted
- cycloalkenyl
- heteroatom
- Prior art date
Links
- LJXDUBIKEZJTEJ-UHFFFAOYSA-N CCC(C)(C)C(C(N(CCC1)C1C(OC(CC1)CCC1(c1ccccc1)c1ccccc1)=O)=O)=O Chemical compound CCC(C)(C)C(C(N(CCC1)C1C(OC(CC1)CCC1(c1ccccc1)c1ccccc1)=O)=O)=O LJXDUBIKEZJTEJ-UHFFFAOYSA-N 0.000 description 2
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/10—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/16—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/02—Drugs for disorders of the nervous system for peripheral neuropathies
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
- A61P25/16—Anti-Parkinson drugs
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/28—Drugs for disorders of the nervous system for treating neurodegenerative disorders of the central nervous system, e.g. nootropic agents, cognition enhancers, drugs for treating Alzheimer's disease or other forms of dementia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/04—Immunostimulants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P37/00—Drugs for immunological or allergic disorders
- A61P37/02—Immunomodulators
- A61P37/06—Immunosuppressants, e.g. drugs for graft rejection
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P43/00—Drugs for specific purposes, not provided for in groups A61P1/00-A61P41/00
Definitions
- This invention relates to neurotrophic low molecular weight, small molecule cyclic ester or amide derivatives having an affinity for FKBP-type immunophilins, pharmaceutical compositions comprising the same, and methods of using the same to effect a neuronal activity.
- immunophilin refers to a number of proteins that serve as receptors for the principal immunosuppressant drugs, cyclosporin A (CsA) , FK506, and rapamycin.
- CsA cyclosporin A
- FKBPs FKBPs .
- Cyclosporin A binds to cyclophilin A, while FK506 and rapamycin bind to FKBP12.
- Immunophilins are known to have peptidyl-prolyl isomerase (PPIase) , or rota ase, enzyme activity. It has been determined that rotamase enzyme activity plays a role in the catalyzation of the interconvers on of the cis and zrans isomers of peptide and protein substrates for the immunophilin proteins .
- PPIase peptidyl-prolyl isomerase
- rotamase enzyme activity plays a role in the catalyzation of the interconvers on of the cis and zrans isomers of peptide and protein substrates for the immunophilin proteins .
- Immunophilins were originally discovered and studied in the immune tissue. It was initially postulated by those skilled in the art that inhibition of the immunophilins ' rotamase activity leads to inhibition of T-cell proliferation, thereby causing the immunosuppressive activity exhibited by immunosuppressant drugs, such as cyclosporin A, FK506, and rapamycin. Further study has shown that the inhibition of rotamase activity, in and of itself, does not result in immunosuppressive activity. Schreiber et al., Science, 1990, vol. 250, pp. 556- 559. Instead, immunosuppression appears to stem from the formation of a complex of immunosuppressant drugs with immunophilins.
- immunosuppressant drugs such as cyclosporin A, FK506, and rapamycin.
- the immunophilin-drug complexes interact with ternary protein targets as their mode of action. Schreiber et al., Cell , 1991, vol. 66, pp. 807-815.
- the immunophilin-drug complex binds to the enzyme calcineurin and inhibits the T-cell receptor signaling necessary to T-cell proliferation.
- the immunophilin-drug complex of FKBP-rapamycin interacts with the RAFT1/FRAP protein and inhibits the IL-2 receptor signaling also necessary to T-cell proliferation. In either case, T-cell proliferation is inhibited.
- immunophilins have also been found in the central nervous system. Immunophilin concentrations are 10-50 times greater in the central nervous system than in the immune system. Within neural tissues, immunophilins appear to influence nitric oxide synthesis, neurotransmitter release, and neuronal process extension. It has been found that picomolar concentrations of immunosuppressants such as FK506 and rapamycin stimulate neurite outgrowth in PC12 cells and sensory neurons such as dorsal root ganglion cells (DRGs) . Lyons et al., Proc. of Na tl . Acad. Sci . , 1994, vol. 91, pp. 3191-3195. In whole animal experiments, FK506 has been shown to stimulate nerve regeneration following facial nerve injury.
- immunosuppressants such as FK506 and rapamycin stimulate neurite outgrowth in PC12 cells and sensory neurons such as dorsal root ganglion cells (DRGs) . Lyons et al., Proc. of Na tl . Ac
- immunosuppressant drugs when administered chronically, immunosuppressant drugs exhibit a number of potentially serious side effects including nephrotoxicity, such as impairment of glomerular filtration and irreversible interstitial fibrosis (Kopp et al., J. Am . Soc . Nephrol . , 1991, 1:162); neurological deficits, such as involuntary tremors, or non-specific cerebral angina, such as non-localized headaches (De Groen et al., N. Engl . J. Med. , 1987, 317:861) ; and vascular hypertension with complications resulting therefrom (Kahan et al., N. Engl . J. Med. , 1989, 321:1725) .
- nephrotoxicity such as impairment of glomerular filtration and irreversible interstitial fibrosis (Kopp et al., J. Am . Soc . Nephrol . , 1991, 1:162)
- neurological deficits such as in
- neurodegenerative disorders such as Alzheimer's disease, Parkinson's disease and amyotrophic lateral sclerosis (ALS) , may occur due to the loss, or decreased availability, of a neurotrophic substance specific for a particular population of neurons affected in the disorder.
- ALS amyotrophic lateral sclerosis
- neurotrophic factors affecting specific neuronal populations in the central nervous system have been identified. For example, it has been hypothesized that Alzheimer's disease results from a decrease or loss of nerve growth factor (NGF) . It has thus been proposed to treat SDAT patients with exogenous nerve growth factor or other neurotrophic proteins, such as brain derived growth factor, glial derived growth factor, ciliary neurotrophic factor and neurotropin-3, to increase the survival of degenerating neuronal populations.
- NGF nerve growth factor
- the present invention provides compounds containing small molecule FKBP rotamase inhibitors for enhancing neurite outgrowth, and promoting neuronal growth and regeneration in various neuropathological situations where neuronal repair can be facilitated, including: peripheral nerve damage caused by physical injury or disease state such as diabetes; physical damage to the central nervous system (spinal cord and brain) ; brain damage associated with stroke; and neurological disorders relating to neurodegeneration, such as Parkinson's disease, SDAT (Alzheimer's disease) and amyotrophic lateral sclerosis.
- the inventive compounds are also useful for treating alopecia, promoting hair growth, treating vision disorder, improving vision, treating memory impairment and enhancing memory performance in an animal.
- the present invention relates to neurotrophic low molecular weight, small molecule cyclic ester and amide derivatives having an affinity for FKBP-type immunophilins.
- the compounds are potent inhibitors of the enzyme activity associated with immunophilin proteins, particularly peptidyl-prolyl isomerase, or rotamase, enzyme " activity.
- the compounds may or may not exert immunosuppressive activity.
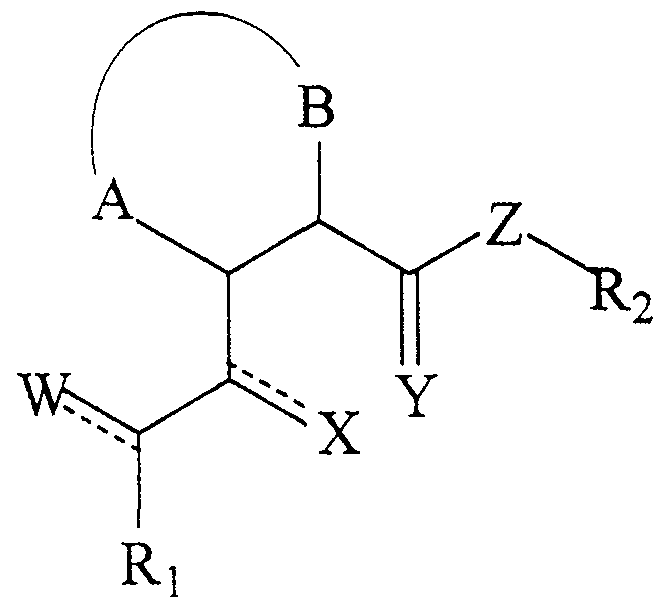
- the present invention relates to a compound of formula I
- a and B taken together with the carbon atoms to which they are respectively attached, form a 5-7 membered saturated or unsaturated carbocyclic or heterocyclic ring, said hererocyclic ring containing one or more heteroatom(s) independently selected from the group consisting of 0, S, SO, S0 2 , N, NH, and NR;
- R, R l r and R 2 are independently Cj-C, straight or branched chain alkyl, C 2 -C 9 straight or branched chain alkenyl, C 3 -C 9 cycloalkyl, C 3 -C 9 cycloalkenyl, or Ar, wherein said R, R,, and R 2 are independently unsubstituted or substituted with one or substituent (s) ;
- Ar is an aromatic, mono-, bi- or tricyclic, carbo- or heterocyclic ring having an individual ring size of 5-9 members, said heterocyclic ring containing one or more heteroatom(s) independently selected from the group consisting of 0, S, SO, S0 2 , N, NH and NR;
- W and X are independently 0, S, CH 2 or H 2 ;
- Y is 0 or S
- Z is 0, NH or NR.
- a and B taken together with the nitrogen and carbon atoms to which they are respectively attached, form a 5-7 membered saturated or unsaturated heterocyclic ring containing one or more heteroatom(s) independently selected from the group consisting of 0, S, SO, S0 2 , N, NH and NR;
- R and R are independently Ci-Cg straight or branched chain alkyl, C 2 -C 9 straight or branched chain alkenyl, C 3 -C 9 cycloalkyl, C 3 -C 9 cycloalkenyl, or Ar, wherein said R and R : are independently unsubstituted or substituted with one or more substituent (s) ;
- R 2 is C 3 -C 9 cycloalkyl, C 3 -C 9 cycloalkenyl or Ar, wherein said cycloalkyl or cycloalkenyl is unsubstituted or substituted with one or more substituent (s) , or said Ar is substituted with one or more substituent (s) ;
- Ar is an aromatic, mono-, bi- or tricyclic, carbo- or heterocyclic ring having an individual ring size of 5-9 members, said heterocyclic ring containing one or more heteroatom(s) independently selected from the group consisting of 0, S, SO, S0 2 , N, NH and NR;
- W and X are independently 0, S, CH 2 or H 2 ;
- Y is O or S
- Z is 0, NH or NR.
- the present invention also relates to a " " pharmaceutical composition comprising an effective amount of the compound of formula I, II or III, and a pharmaceutically acceptable carrier.
- the present invention further relates to a method of effecting a neuronal activity in an animal, comprising administering to said animal an effective amount of the compound of formula I, II or III.
- Alkyl means a branched or unbranched saturated hydrocarbon chain containing 1 to 6 carbon atoms, such as methyl, ethyl, propyl, iso-propyl, butyl, iso- butyl, tert-butyl, n-pentyl, n-hexyl, and the like, unless otherwise indicated.
- Alkoxy means the group -OR wherein R is alkyl as herein defined.
- R is a branched or unbranched saturated hydrocarbon chain containing 1 to 3 carbon atoms.
- Alopecia refers to deficient hair growth and partial or complete loss of hair, including without limitation androgenic alopecia (male pattern baldness) , toxic alopecia, alopecia senilis, alopecia areata, alopecia pelada and trichotillomania.
- Alopecia results when the pilar cycle is disturbed. The most frequent phenomenon is a shortening of the hair growth or anagen phase due to cessation of cell proliferation. This results in an early onset of the catagen phase, and consequently a large number of hairs in the telogen phase during which the follicles are detached from the dermal papillae, and the hairs fall out.
- Alopecia has a number of etiologies, including genetic factors, aging, local and systemic diseases, febrile conditions, mental stresses, hormonal problems, and secondary effects of drugs.
- “Enhancing memory performance” refers to improving or increasing the mental faculty by which to register, retain or recall past experiences, knowledge, ideas, sensations, thoughts or impressions. "Eye” refers to the anatomical structure responsible for vision in humans and other animals, and encompasses the following anatomical structures, without limitation: lens, vitreous body, ciliary body, posterior chamber, anterior chamber, pupil, cornea, iris, canal of Schlemm, zonules of Zinn, limbus, conjunctiva, choroid, retina, central vessels of the retina, optic nerve, fovea centralis, macula lutea, and sclera.
- Halo means fluoro, chloro, bromo, or iodo, unless otherwise indicated.
- “Isomers” refer to different compounds that have the same molecular formula.
- “Stereoisomers” are isomers that differ only in the way the atoms are arranged in space.
- Enantiomers are a pair of stereoisomers that are non-superimposable mirror images of each other.
- “Diastereoisomers” are stereoisomers which are not mirror images of each other.
- Racemic mixture means a mixture containing equal parts of individual enantiomers.
- Non-racemic mixture is a mixture containing unequal parts of individual enantiomers or stereoisomers.
- Memory impairment refers to a diminished mental registration, retention or recall of past experiences, knowledge, ideas, sensations, thoughts or impressions. Memory impairment may affect short and long-term information retention, facility with spatial relationships, memory (rehearsal) strategies, and verbal retrieval and production. Common causes of memory impairment are age, severe head trauma, brain anoxia or ischemia, alcoholic-nutritional diseases, and drug intoxications. Examples of memory impairment include, without limitation, benign forgetfulness, amnesia and any disorder in which memory deficiency is present, such as Korsakoff's amnesic psychosis, dementia and learning disorders. "Ophthalmological” refers to anything about or concerning the eye, without limitation, and is used interchangeably with “ocular,” “ophthalmic,”
- “Pharmaceutically acceptable salt, ester, or solvate” refers to a salt, ester, or solvate of a subject compound which possesses the desired pharmacological activity and which is neither biologically nor otherwise undesirable.
- a salt, ester, or solvate can be formed with inorganic acids such as acetate, adipate, alginate, aspartate, benzoate, benzenesulfonate, bisulfate, butyrate, citrate, camphorate, camphor sul fonate , cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate, fumarate, glucoheptanoate, gluconate, glycerophosphate, hemisulfate, heptanoate, hexanoate, hydrochloride, hydrobromide, hydroiodide, 2- hydroxyethanesulfonate , lactate, maleate, me
- base salts, esters, or solvates include ammonium salts; alkali metal salts, such as sodium and potassium salts; alkaline earth metal salts, such as calcium and magnesium salts; salts " with organic bases, such as dicyclohexylamine salts; N-methyl-D-glucamine; and salts with amino acids, such as arginine, lysine, and so forth.
- the basic nitrogen-containing groups can be quarternized with such agents as lower alkyl halides, such as methyl, ethyl, propyl, and butyl chlorides, bromides, and iodides; dialkyl sulfates, such as dimethyl, diethyl, dibutyl, and diamyl sulfates; long chain halides, such as decyl, lauryl, myristyl, and stearyl chlorides, bromides, and iodides; aralkyl halides, such as benzyl and phenethyl bromides; and others. Water or oil-soluble or dispersible products are thereby obtained.
- lower alkyl halides such as methyl, ethyl, propyl, and butyl chlorides, bromides, and iodides
- dialkyl sulfates such as dimethyl, diethyl, dibutyl, and diamyl sulfates
- Phenyl includes all possible isomeric phenyl radicals, optionally monosubstituted or multi-substi- tuted with substituents selected from the group consisting of alkyl, alkoxy, hydroxy, halo, and haloalkyl .
- Palm cycle refers to the life cycle of hair follicles, and includes three phases: (1) the anagen phase, the period of active hair growth which, insofar as scalp hair is concerned, lasts about three to five years;
- the catagen phase the period when growth stops and the follicle atrophies which, insofar as scalp hair is concerned, lasts about one to two weeks
- the telogen phase the rest period when hair progressively separates and finally falls out which, insofar as scalp hair is concerned, lasts about three to four months.
- 80 to 90 percent of the follicles are in the anagen phase, less than 1 percent being in the catagen phase, and the rest being in the telogen phase.
- the telogen phase hair is uniform in diameter with a slightly bulbous, non-pigmented root.
- hair has a large colored bulb at its root .
- Preventing vision degeneration includes the ability to prevent degeneration of vision in patients newly diagnosed as having a degenerative disease affecting vision, or at risk of developing a new degenerative disease affecting vision, and for preventing further degeneration of vision in patients who are already suffering from or have symptoms of a degenerative disease affecting vision.
- Promoter hair growth refers to maintaining, inducing, stimulating, accelerating, or revitalizing the germination of hair.
- “Promoting vision regeneration” refers to maintaining, improving, stimulating or accelerating recovery of, or revitalizing one or more components of the visual system in a manner which improves or enhances vision, either in the presence or absence of " any ophthalmologic disorder, disease, or injury. "Treating” refers to:
- Treating alopecia refers to:
- Terminal hair is coarse, pigmented, long hair in which the bulb of the hair follicle is seated deep in the dermis.
- Vellus hair is fine, thin, non-pigmented short hair in which the hair bulb is located superficially in the dermis. As alopecia progresses, the hairs change from the terminal to the vellus type.
- Vision refers to the ability of humans and other animals to process images, and is used interchangeably with “sight”, “seeing”, and other such terms, without limitation.
- Vision disorder refers to any disorder that affects or involves vision, including without limitation visual impairment, orbital disorders, disorders of the lacrimal apparatus, disorders of the eyelids, disorders of the conjunctiva, disorders of the cornea, cataracts, disorders of the uveal tract, disorders of the retina, disorders of the optic nerve or visual pathways, free radical induced eye disorders and diseases, immunologically-mediated eye disorders and diseases, eye injuries, and symptoms and complications of eye disease, eye disorder, or eye injury.
- Visual impairment refers to any dysfunction in vision including without limitation disturbances or diminution in vision (e.g., binocular, central, peripheral, scotopic) , visual acuity for objects near and for, visual field, ocular motility, color perception, adaptation to light and dark, accommodation, refraction, and lacrimation.
- disturbances or diminution in vision e.g., binocular, central, peripheral, scotopic
- visual acuity for objects near and for, visual field e.g., ocular motility, color perception, adaptation to light and dark, accommodation, refraction, and lacrimation.
- PDR Physicians ' Desk Reference
- the low molecular weight, small molecule FKBP inhibitor compounds of this invention have an affinity for FKBP-type immunophilins, such as FKBP12.
- FKBP-type immunophilins such as FKBP12.
- the compounds of this invention are bound to an FKBP-type immunophilin, they have been found to inhibit the prolyl-peptidyl cis-trans isomerase activity, or rotamase, activity of the binding protein.
- the compounds are effective in stimulating neurite growth, as well as treating alopecia, promoting hair growth, treating vision disorders, improving vision, treating memory impairment, and enhancing memory performance in an animal.
- the compounds may or may not be immunosuppressive .
- the cyclic ester or amide derivative may be a compound of formula I
- a and B taken together with the carbon atoms to which they are attached, form a 5-7 membered saturated or unsaturated carbocyclic or heterocyclic ring, said heterocyclic ring containing one or more heteroatom(s) independently selected from the group consisting of 0, S, SO, S0 2 , N, NH, and NR;
- R, R L and R 2 are independently ⁇ C g straight or branched chain alkyl, C 2 -C 9 straight or branched chain alkenyl, C 3 -C 9 cycloalkyl, C 3 -C 9 cycloalkenyl, or Ar, wherein said R, R x and R 2 are independently unsubstituted or substituted with one or more substituent (s) and the carbon atoms of said alkyl, alkenyl, cycloalkyl, and cycloalkenyl are independently unsubstituted or substituted with one or more heteroatom(s) ;
- Ar is an aromatic, mono-, bi- or tricyclic, carbo- or heterocyclic ring having an individual ring size " of 5-9 members, said heterocyclic ring containing one or more heteroatom(s) independently selected from the -group consisting of 0, S, SO, S0 2 , N, NH and NR;
- W and X are independently 0, S, CH 2 or H 2 ; ⁇ is 0 or S; and
- Z is 0, NH, or NR.
- the cyclic ester or amide derivative is a compound of formula II
- a and B taken together with the nitrogen and carbon atoms to which they are respectively attached, form a 5-7 membered saturated or unsaturated heterocyclic ring containing one or more heteroatom(s) independently selected from the group consisting of 0, S, SO, S0 2 , N, NH and NR;
- R and R are independently ⁇ g straight or branched chain alkyl, C 2 -C 9 straight or branched chain alkenylr C 3 -C 9 cycloalkyl, C 3 -C 9 cycloalkenyl, or Ar, wherein said R and R x are independently unsubstituted or substituted with one or more substituent (s) , and the carbon atoms of said alkyl, alkenyl, cycloalkyl, and cycloalkenyl are independently unsubstituted or substituted with one or more heteroatom(s) ;
- R 2 is C 3 -C 9 cycloalkyl, C 3 -C 9 cycloalkenyl or Ar, wherein said R 2 is unsubstituted or substituted with one or more substituent (s) , and the carbon atoms of said cycloalkyl and cycloalkenyl are independently substituted with one or more heteroatom(s) ;
- Ar is an aromatic, mono-, bi- or tricyclic, carbo- or heterocyclic ring having an individual ring size of 5-9 members, said heterocyclic ring containing one or more heteroatom(s) independently selected from the group consisting of 0, S, SO, S0 2 , N, NH and NR; W and X are independently 0, S, CH 2 or H 2 ; Y is 0 or S; and Z is 0, NH or NR.
- R 2 is substituted with (Ar) n , and n is 1-2.
- the cyclic ester or amide derivative is 4 , 4-diphenylcyclohexl (2S) -1- (3, 3-dimethyl-2-oxopentanoyl) -pyrrolidine-2- carboxylate, a compound of formula III
- R, R, and R 2 are C ⁇ -C 9 straight or branched chain alkyl, C 2 -C 9 straight or branched chain alkenyl, C,-C 9 alkoxy, C-C 9 alkenyloxy, phenoxy, benzyloxy, C 3 -C 8 cycloalkyl, C 5 -C 7 cycloalkenyl, hydroxy, carboxy, carbonyl, amino, amido, cyano, isocyano, nitro, nitroso, nitrilo, isonitrilo, imino, azo, diazo, sulfonyl, sulfoxy, thio, thiocarbonyl, thiocyano, formanilido, thioformamido, sulfhydryl, halo, haloalkyl, trifluoromethyl, and carbocyclic and heterocyclic moieties.
- Carbocyclic C 2 -C 9 straight or branched chain
- carbocyclic and heterocyclic moieties include, without limitation, phenyl, benzyl, naphthyl, indenyl, azulenyl, fluorenyl, anthracenyl, indolyl, isoindolyl, indolinyl, benzofuranyl, benzothiophenyl , indazolyl, benzimidazolyl , benzthiazolyl, tetrahydrofuranyl, tetrahyctropyranyl, pyridyl, pyrrolyl, pyrro idmyl, pyridmyl, pyrimidinyl, pu ⁇ nyl, quinolmyl, isoquinolinyl, tetrahydroquinolmyl, quinolizmyl, furyl, thiophenyl, lmidazolyl, oxazolyl, benzoxazolyl, thiazolyl, isoxxazo
- All the compounds of formulas I-III possess asymmetric centers and thus can be produced as mixtures of stereoisomers or as individual R- and S- stereoisomers .
- the individual stereoisomers may be obtained by using an optically active starting material, by resolving a racemic or non-racemic mixture of an intermediate at some appropriate stage of the synthesis, or by resolving the compounds of formulas I-III. It is understood that the compounds of formulas I-III encompass individual stereoisomers as well as mixtures (racemic and non-racemic) of stereoisomers. S-stereoisomers are most preferred.
- the present: invention also relates to a pharmaceutical composition
- a pharmaceutical composition comprising:
- the amount of the compound of formula I, II or III is effective for binding to an FKBP-type immunophilin.
- the amount of the compound of formula I, II or III is effective for effecting a neuronal activity in an animal.
- the compounds of the present invention have an affinity for the FK506 binding protein, particularly
- FKBP12 which is present in the brain.
- inventive compounds bind to FKBP in the brain, they exhibit excellent neurotrophic activity. This activity is useful in the stimulation of damaged neurons, the promotion of neuronal regeneration, the prevention of neurodegeneration, and the treatment of several neurological disorders known to be associated with neuronal degeneration and peripneral neuropatnies .
- the present invention further relates to a metho ⁇ of effecting a neuronal activity in an animal, comprising administering to sai ⁇ animal an effective amount of a compound of formula I, II or III.
- the neuronal activity is selected from the group consisting of stimulation of damaged neurons, promotion of neuronal regeneration, prevention of neurodegeneration and treatment of neurological disorder.
- the neurological disorders that may oe treated include but are not limited to: trigemmal neuralgia; glossopharyngeal neuralgia; Bell's Palsy; myasthenia gravis; muscular dystrophy; amyotrophic lateral sclerosis; progressive muscular atrophy; progressive bulbar inherited muscular atrophy; herniated, ruptured or prolapsed invertebrate disk syndromes; cervical spondylosis; plexus disorders; thoracic outlet destruction syndromes; peripheral neuropathies such as those caused by lead, dapsone, ticks, porphyria or Guillam-Barre syndrome; Alzheimer's disease; and Parkinson's ⁇ isease.
- the compounds of the present invention are particularly useful for treating a neurological disorder selected from the group consisting of: peripheral neuropathy caused by physical injury or disease state, traumatic injury to the brain, physical damage to the spinal cord, stroke associated with brain damage, and neurological disorder relating to neurodegeneration.
- a neurological disorder selected from the group consisting of: peripheral neuropathy caused by physical injury or disease state, traumatic injury to the brain, physical damage to the spinal cord, stroke associated with brain damage, and neurological disorder relating to neurodegeneration.
- Examples of neurological disorders relating to neurodegeneration are Alzheimer's Disease, Parkinson's Disease and amyotrophic lateral sclerosis.
- the compounds of the present invention may be administered orally, parenterally, by inhalation spray, topically, rectally, nasally, buccally, vaginally or via an implanted reservoir in dosage formulations containing conventional non-toxic pharmaceutically-acceptable carriers, adjuvants and vehicles.
- parenteral as used herein includes subcutaneous, intravenous, intramuscular, intraperitoneally, intrathecally, intraventricularly, intrasternal and intracranial injection or infusion techniques .
- the immunophilin-drug complex should readily penetrate the blood-brain barrier when peripherally administered.
- Compounds of this invention which cannot penetrate the blood-brain barrier can oe effectively administered by an mtraventricular route.
- the compoun ⁇ s may be m the form of a sterile mjectaole preparation, for example as a sterile injectable aqueous or oleaginous suspension.
- This suspension may oe formulated according to techniques know m the art using suitable dispersing or wetting agents and suspending agents.
- the sterile mjectable preparation may also oe a sterile mjectaole solution or suspension m a non-toxic parenterally-acceptable diluent or solvent, for example as a solution in 1,3- butanediol.
- the acceptable vehicles and solvents that may be employed are water, Ringer's solution and lsotomc sodium chloride solution.
- sterile, fixed oils are conventionally employed as a solvent or suspending medium.
- any bland fixed o l may be employed including synthetic mono- or diglycerides .
- Fatty acids such as oleic acid and its glyceride derivatives find use in the preparation of miectables, olive oil or castor oil, especially in their polyoxyethylated versions.
- These oil solutions or suspensions may also contain a long-chain alconol diluent or dispersant.
- the compounds may be administered orally in the form of capsules or tablets, for example, or as an aqueous suspension or solution.
- carriers which are commonly used include lactose and corn starch.
- Lubricating agents such as magnesium stearate, are also typically added.
- useful diluents include lactose and dried corn starch.
- aqueous suspensions are required for oral use, the active ingredient is combined with emulsifying and suspending agents. If desired, certain sweetening and/or flavoring and/or coloring agents may be added.
- the compounds of this invention may also be administered in the form of suppositories for rectal administration of the drug.
- These compositions can be prepared by mixing the drug with a suitable non- irritating excipient which is solid at room temperature but liquid at rectal temperature and therefore will melt in the rectum to release the drug.
- suitable non- irritating excipient include cocoa butter, beeswax and polyethylene glycols.
- the compounds of this invention may also be administered optically, especially when the conditions addressed for treatment involve areas or organs readily accessible by topical application, including neurological disorders of the eye, the skin, or the lower intestinal tract. Suitable topical formulations are readily prepared for each of these areas.
- the compounds can be formulated as micronized suspensions in isotonic, pH adjusted sterile saline, or, preferably, as solutions is isotonic, pH adjusted sterile saline, either with or without a preservative such as benzylalkonium chloride.
- the compounds may be formulated in an ointment sucn as petrolatum.
- the compounds can be formulated in a suitable ointment containing the compound suspended or dissolved in, for example, a mixture with one or more of the following: mineral oil, liquid petrolatum, white petrolatum, propylene glycol, polyoxyethylene polyoxypropylene compound, emulsifying wax and water.
- the compounds can be formulated in a suitable lotion or cream containing the active compound suspended or dissolved m, for example, a mixture of one or more of the following: mineral oil, sorbitan monostearate, polysorbate 60, cetyl esters wax, cetearyl alcohol, 2- octyldodecanol, benzyl alcohol and water.
- Topical application for the lower intestinal tract an be effected in a rectal suppository formulation (see above) or in a suitable enema formulation.
- Dosage levels on the order of about . i g to about 10,000 mg. of the active ingredient compound are useful in the treatment of the above conditions, with preferred levels of about 0. Img to about 1,000 mg.
- the amount of active ingredient that may be combined with the carrier materials to produce a single dosage form will vary depending upon the host treated and the particular mode of administration.
- a specific dose level for any particular patient will depend upon a variety of factors including the activity of the specific compound employed, the age, body weight, general health, sex, diet, time of administration, rate of excretion, drug combination, and the severity of the particular disease being treated and form of administration.
- the compounds can be administered with other neurotrophic agents such as neurotrophic growth factor (NGF) , glial derived growth factor, brain derived growth factor, ciliary neurotrophic factor, and neurotropin-3.
- NGF neurotrophic growth factor
- the dosage level of other neurotrophic drugs will depend upon the factors previously stated and the neurotrophic effectiveness of the drug combination.
- the cis- trans isomerization of an alanine- proline bond in a model substrate, N-succinyl-Ala-Ala- Pro-Phe-p-nitroanilide is monitored spectro- photometrically in a chymotrypsin-coupled assay, which releases para-nitroanilide from the trans form of the substrate.
- the inhibition of this reaction caused by the addition of different concentrations of inhibitor is determined, and the data is analyzed as a change in first-order rate constant as a function of inhibitor concentration to yield the apparent K 1 values.
- a plastic cuvette In a plastic cuvette are added 950 mL of ice cold assay buffer (25 mM HEPES, pH 7.8, 100 mM NaCl) , 10 mL of FKBP (2.5 mM in 10 mM Tris-Cl pH 7.5, 100 mM NaCl, 1 mM dithiothreitol) , 25 mL of chymotrypsin (50 mg/mL in 1 mM HC1) and 10 mL of test compound at various concentrations in dimethyl sulfoxide.
- ice cold assay buffer 25 mM HEPES, pH 7.8, 100 mM NaCl
- FKBP 2.5 mM in 10 mM Tris-Cl pH 7.5, 100 mM NaCl, 1 mM dithiothreitol
- 25 mL of chymotrypsin 50 mg/mL in 1 mM HC1
- 10 mL of test compound at various concentrations in
- the reaction is initiated by the addition of 5 mL of substrate (succinyl-Ala-Phe-Pro-Phe-para-nitroanilide, 5 mg/mL in 2.35 mM LiCl in trifluoroethanol) .
- substrate succinyl-Ala-Phe-Pro-Phe-para-nitroanilide, 5 mg/mL in 2.35 mM LiCl in trifluoroethanol
- the absorbance at 390 nM versus time is monitored for 90 sec using a spectrophotometer and the rate constants are determined from the absorbance versus time data files.
- FKBP-12 complexes with the inositol triphosphate receptor (IP 3 R) and the ryanodine receptor (RyR) . It is believed that the neurotrophic compounds of this invention disassociates FKBP-12 from these complexes causing the calcium channel to become "leaky” (Cameron et al., 1995). Calcium fluxes are involved in neurite extensions so that the IP 3 R receptor and the ryanodine receptor might be involved in the neurotrophic effects of drugs. Since the drugs bind to the same site as FKBP-12 as the IP 3 R receptor, one could assume that the drugs displace the channels from FKBP-12. Chick Dorsal Root Ganglion Cultures and Neurite Outgrowth
- Dorsal root ganglia are dissected from chick embryos of ten day gestation.
- Whole ganglion explants are cultured on thin layer Matrigel-coated 12 well plates with Liebovitz L15 plus high glucose media supplemented with 2 mM glutamine and 10% fetal calf serum, and also containing 10 ⁇ M cytosine ⁇ -D arabinofuranoside (Ara C) at 37°C in an environment containing 5% C0 2 . Twenty-four hours later, the DRGs are treated with various concentrations of nerve growth factor (NGF) , immunophilin ligands, or combinations of NFG plus drugs.
- NFG nerve growth factor
- the ganglia are visualized under phase contrast or Hoffman Modulation contrast with a Zeiss Axiovert inverted microscope. Photomicrographs of the explants are made, and neurite outgrowth is quantitated. Neurites longer than the DRG diameter are counted as positive, with total number of neurites quantitated per each experimental condition. Three to four DRGs are cultured per well, and each treatment is performed in duplicate. The compounds of the present invention promote neurite outgrowth in sensory neurons . Sciatic Nerve Axotomy
- neuroimmunophilin FKBP ligands which are compounds related to those of the present invention, just prior to the lesion and daily for 18 days following the lesion, results in significant regeneration of both axon number and the degree of myelination as compared to vehicle treated animals.
- the significant efficacy of neuroimmunophilin FKBP ligands is consistent with their potent activity in inhibiting rotamase activity and stimulating neurite outgrowth in chick DRGs.
- the neurotrophic effects of the compounds of the present invention are further demonstrated in an animal model of neurodegenerative disease: MPTP lesioning of dopaminergic neurons in mice is used as an animal model of Parkinson's Disease.
- Four week old male CD1 white mice are dosed i.p. with 30 mg/kg of MPTP for 5 days.
- a cyclic ester or amide derivative (10-40 mg/kg), or vehicle is administered s.c. along with the MPTP for 5 days, as well as for an additional 5 days following cessation of MPTP treatment.
- the animals are sacrificed and the striata are dissected and homogenized.
- Binding of [3H]CFT, a radioligand for the dopamine transporter, to the stiatal membranes is done to quantitate the level of the dopamine transporter (DAT) following lesion and drug treatment.
- DAT dopamine transporter
- Immunostaining is performed on saggital and coronal brain sections using anti-tyrosine hydoxylase Ig to quantitate survival and recovery of dopaminergic neurons.
- a substantial loss of functional dopaminergic terminals is observed as compared to non-lesioned animals.
- Lesioned animals receiving cyclic ester or amide derivatives show a nearly quantitative recovery of TH- stai ⁇ ed dopaminergic neurons.
- a patient is suffering from peripheral nerve damage caused by physical injury or disease state such as diabetes.
- a cyclic ester or amide derivative as identified above, or a pharmaceutical composition comprising the same, may be administered to the patient.
- Enhanced neurite outgrowth, and neuronal growth and regeneration are expected to occur following treatment.
- a patient is suffering from physical damage to the central nervous system (spinal cord and brain) .
- a cyclic ester or amide derivative as identified above, or a pharmaceutical composition comprising the same, may be administered to the patient.
- Enhanced neurite outgrowth, and neuronal growth and regeneration are expected to occur following treatment .
- Example 4 A patient is suffering from brain damage associated with stroke.
- a cyclic ester or amide derivative as identified above, or a pharmaceutical composition comprising the same, may be administered to tne patient.
- Enhanced neurite outgrowth, and neuronal growth and regeneration are expected to occur following treatment.
- a patient is suffering from neurodegeneration resulting from Parkinson's disease.
- a cyclic ester or amide derivative as identified above, or a pharmaceutical composition comprising tne same, may be administered to the patient.
- Enhanced neurite outgrowth, and neuronal growth and regeneration are expected to occur following treatment.
- a patient is suffering from neurodegeneration resulting from amyotrophic lateral sclerosis.
- a cyclic ester or amide derivative as identified above, or a pharmaceutical composition comprising the same, may be administered to the patient.
- Enhanced neurite outgrowth, and neuronal growth and regeneration are expected to occur following treatment.
- a patient is suffering from neurodegeneration resulting from SDAT (Alzheimer's disease).
- a cyclic ester or amide derivative as identified above, or a pharmaceutical composition comprising the same, may be administered to the patient.
- Enhanced neurite outgrowth, and neuronal growth and regeneration are expected to occur following treatment.
Abstract
Description
Claims
Priority Applications (7)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| EP99951470A EP1115703A1 (en) | 1998-09-21 | 1999-09-17 | Cyclic ester or amide derivatives |
| KR1020017003246A KR20010089253A (en) | 1998-09-21 | 1999-09-17 | Cyclic ester or amide derivatives |
| CA002341036A CA2341036A1 (en) | 1998-09-21 | 1999-09-17 | Cyclic ester or amide derivatives |
| AU63905/99A AU6390599A (en) | 1998-09-21 | 1999-09-17 | Cyclic ester or amide derivatives |
| JP2000574071A JP2002526474A (en) | 1998-09-21 | 1999-09-17 | Cyclic ester or amide derivative |
| IL14155299A IL141552A0 (en) | 1998-09-21 | 1999-09-17 | Cyclic ester or amide derivatives |
| HU0103565A HUP0103565A3 (en) | 1998-09-21 | 1999-09-17 | Cyclic ester or amide derivatives and pharmaceutical compositions containing them |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/157,566 US6462072B1 (en) | 1998-09-21 | 1998-09-21 | Cyclic ester or amide derivatives |
| US09/157,566 | 1998-09-21 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2000017161A1 true WO2000017161A1 (en) | 2000-03-30 |
Family
ID=22564303
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1999/021290 WO2000017161A1 (en) | 1998-09-21 | 1999-09-17 | Cyclic ester or amide derivatives |
Country Status (11)
| Country | Link |
|---|---|
| US (1) | US6462072B1 (en) |
| EP (1) | EP1115703A1 (en) |
| JP (1) | JP2002526474A (en) |
| KR (1) | KR20010089253A (en) |
| CN (1) | CN1323294A (en) |
| AU (1) | AU6390599A (en) |
| CA (1) | CA2341036A1 (en) |
| HU (1) | HUP0103565A3 (en) |
| IL (1) | IL141552A0 (en) |
| WO (1) | WO2000017161A1 (en) |
| ZA (1) | ZA200101934B (en) |
Families Citing this family (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| ES2290117T3 (en) * | 2000-02-15 | 2008-02-16 | Sugen, Inc. | PROTEIN QUINASE 2-INDOLIN INHIBITORS REPLACED WITH PIRROL. |
| AR042586A1 (en) | 2001-02-15 | 2005-06-29 | Sugen Inc | 3- (4-AMIDOPIRROL-2-ILMETILIDEN) -2-INDOLINONE AS INHIBITORS OF PROTEIN KINASE; YOUR PHARMACEUTICAL COMPOSITIONS; A METHOD FOR THE MODULATION OF THE CATALYTIC ACTIVITY OF PROTEINQUINASE; A METHOD TO TREAT OR PREVENT AN AFFECTION RELATED TO PROTEINQUINASE |
| BR0213185A (en) | 2001-10-10 | 2004-09-14 | Sugen Inc | 3- [4- (Substituted Heterocyclyl) -pyrrol-2-ylmethylidene] 2-indolinone derivatives as kinase inhibitors |
| WO2006004903A2 (en) * | 2004-06-28 | 2006-01-12 | Atherogenics, Inc. | 1,2-bis-(substituted-phenyl)-2-propen-1-ones and pharmaceutical compositions thereof |
Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992000278A1 (en) * | 1990-07-02 | 1992-01-09 | Vertex Pharmaceuticals Incorporated | Novel immunosuppressive compounds |
| WO1996040633A1 (en) * | 1995-06-07 | 1996-12-19 | Guilford Pharmaceuticals Inc. | Small molecule inhibitors of rotamase enzyme activity |
| WO1996041609A2 (en) * | 1995-06-08 | 1996-12-27 | Vertex Pharmaceuticals Incorporated | Methods and compositions for stimulating neurite growth |
| WO1998013355A1 (en) * | 1996-09-25 | 1998-04-02 | Guilford Pharmaceuticals Inc. | Heterocyclic esters and amides |
| WO1998055090A1 (en) * | 1997-06-04 | 1998-12-10 | Guilford Pharmaceuticals Inc. | Hair growth compositions and uses |
Family Cites Families (81)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4070361A (en) | 1977-04-21 | 1978-01-24 | E. R. Squibb & Sons, Inc. | Mercaptoalkylsulfonyl proline and pipecolic acid and esters thereof |
| US4206137A (en) | 1978-03-27 | 1980-06-03 | E. R. Squibb & Sons, Inc. | Thioalkanoylalkanoic acid compounds |
| IL58849A (en) | 1978-12-11 | 1983-03-31 | Merck & Co Inc | Carboxyalkyl dipeptides and derivatives thereof,their preparation and pharmaceutical compositions containing them |
| JPS55160757A (en) | 1979-05-31 | 1980-12-13 | Sumitomo Chem Co Ltd | Novel cyclopentanecarboxylic acid derivative and its preparation |
| US4310461A (en) | 1980-06-23 | 1982-01-12 | E. R. Squibb & Sons, Inc. | Imido, amido and amino derivatives of mercaptoacyl prolines and pipecolic acids |
| US4390695A (en) | 1980-06-23 | 1983-06-28 | E. R. Squibb & Sons, Inc. | Imido, amido and amino derivatives of mercaptoacyl prolines and pipecolic acids |
| US4578474A (en) | 1980-06-23 | 1986-03-25 | E. R. Squibb & Sons, Inc. | Imido, amido and amino derivatives of mercaptoacyl prolines and pipecolic acids |
| US4950649A (en) | 1980-09-12 | 1990-08-21 | University Of Illinois | Didemnins and nordidemnins |
| GR75019B (en) | 1980-09-17 | 1984-07-12 | Univ Miami | |
| DE3174844D1 (en) | 1980-10-23 | 1986-07-24 | Schering Corp | Carboxyalkyl dipeptides, processes for their production and pharmaceutical compositions containing them |
| ZA817261B (en) | 1980-10-23 | 1982-09-29 | Schering Corp | Carboxyalkyl dipeptides,processes for their production and pharmaceutical compositions containing them |
| ZA826022B (en) | 1981-08-21 | 1983-08-31 | Univ Miami | Novel complex amido and imido derivatives of carboxyalkyl peptides and thioethers and ethers of peptides |
| EP0088350B1 (en) | 1982-03-08 | 1985-02-20 | Schering Corporation | Carboxyalkyl dipeptides, processes for their production and pharmaceutical compositions containing them |
| US4531964A (en) | 1982-09-13 | 1985-07-30 | Nippon Kayaku Kabushiki Kaisha | Heterocyclic compound and a herbicidal composition containing said compound |
| US4574079A (en) | 1983-05-27 | 1986-03-04 | Gavras Haralambos P | Radiolabeled angiotensin converting enzyme inhibitors for radiolabeling mammalian organ sites |
| US4593102A (en) | 1984-04-10 | 1986-06-03 | A. H. Robins Company, Inc. | N-[(amino)alkyl]-1-pyrrolidine, 1-piperidine and 1-homopiperidinecarboxamides (and thiocarboxamides) with sulfur linked substitution in the 2, 3 or 4-position |
| DE3508251A1 (en) | 1985-03-08 | 1986-09-11 | Merck Patent Gmbh, 6100 Darmstadt | Dipeptides |
| CN86101850A (en) | 1985-03-22 | 1987-02-04 | 森得克斯(美国)有限公司 | N, the manufacture method and the purposes of N '-dialkyl group guanidine radicals dipeptides |
| KR960004900B1 (en) | 1986-09-10 | 1996-04-17 | 신텍스(유.에스.에이.) 인코포레이티드 | Selective amidination of diamines |
| IT1206078B (en) | 1987-06-03 | 1989-04-14 | Polifarma Spa | PROCEDURE FOR THE PRODUCTION OF 3-INDOLPIRUVIC ACID AND ITS DERIVATIVES THEIR PHARMACEUTICAL USE |
| US5187156A (en) | 1988-03-16 | 1993-02-16 | Fujisawa Pharmaceutical Co., Ltd. | Peptide compounds, processes for preparation thereof and pharmaceutical composition comprising the same |
| IL90872A0 (en) | 1988-07-08 | 1990-02-09 | Smithkline Beckman Corp | Retroviral protease binding peptides |
| DE3931051A1 (en) | 1988-09-22 | 1990-03-29 | Hoechst Ag | New herbicidal amine salts of herbicidal acids |
| EP0378318A1 (en) | 1989-01-11 | 1990-07-18 | Merck & Co. Inc. | Process for synthesis of FK-506 and tricarbonyl intermediates |
| EP0672648B1 (en) | 1989-04-15 | 1998-09-23 | Zaidan Hojin Biseibutsu Kagaku Kenkyu Kai | Threo (2R,3S)-3-amino-2-hydroxypentanoic acid and threo (2R,3S)-3-(p-methoxy-benzyloxycarbonyl/FMOC) amino-2-hydroxy-pentanoic acid |
| US5164525A (en) | 1989-06-30 | 1992-11-17 | Merck & Co., Inc. | Synthetic process for fk-506 type macrolide intermediates |
| US5703088A (en) | 1989-08-21 | 1997-12-30 | Beth Israel Deaconess Medical Center, Inc. | Topical application of spiperone or derivatives thereof for treatment of pathological conditions associated with immune responses |
| NZ234883A (en) | 1989-08-22 | 1995-01-27 | Merck Frosst Canada Inc | Quinolin-2-ylmethoxy indole derivatives, preparation and pharmaceutical compositions thereof |
| GB8922026D0 (en) | 1989-09-29 | 1989-11-15 | Pharma Mar Sa | Novel anti-viral and cytotoxic agent |
| US5115098A (en) | 1990-02-28 | 1992-05-19 | President And Fellows Of Harvard College | End-blocked peptides inhibiting binding capacity of gp120 |
| JPH04211648A (en) | 1990-07-27 | 1992-08-03 | Nippon Kayaku Co Ltd | Keto-acid amide derivative |
| DE4015255A1 (en) | 1990-05-12 | 1991-11-14 | Hoechst Ag | OXALYL-AMINOSA-E-LEED DERIVATIVES, METHOD FOR THE PRODUCTION THEREOF AND THEIR USE AS A MEDICAMENT FOR INHIBITING THE PROLYL HYDROXYLASE |
| JPH06500561A (en) | 1990-08-24 | 1994-01-20 | ジ・アップジョン・カンパニー | Aminopolyol-containing peptides as transition state mimetics |
| WO1992004370A1 (en) | 1990-08-29 | 1992-03-19 | Vertex Pharmaceuticals Incorporated | Modified di- and tripeptidyl immunosuppressive compounds |
| GB2247456A (en) | 1990-09-03 | 1992-03-04 | Fujisawa Pharmaceutical Co | Tetrahydropyrane compounds, a process for their production and a pharmaceutical composition containing the same |
| JPH04149166A (en) | 1990-10-12 | 1992-05-22 | Nippon Kayaku Co Ltd | Novel keto acid amide derivative |
| WO1992016501A1 (en) | 1991-03-20 | 1992-10-01 | Vertex Pharmaceuticals Incorporated | Tetrahydroxyalkane derivatives as inhibitors of hiv aspartyl protease |
| IT1245712B (en) | 1991-04-09 | 1994-10-14 | Boehringer Mannheim Italia | USEFUL HETEROCYCLIC AMINES THERAPY OF ASTHMA AND AIRWAY INFLAMMATION |
| US5147877A (en) | 1991-04-18 | 1992-09-15 | Merck & Co. Inc. | Semi-synthetic immunosuppressive macrolides |
| WO1992019745A1 (en) | 1991-05-08 | 1992-11-12 | Vertex Pharmaceuticals Incorporated | Rfkbp: a novel prolyl isomerase and rapamycin/fk506 binding protein |
| KR100244372B1 (en) | 1991-05-09 | 2000-03-02 | 조슈아 에스.보저 | Novel immunosuppressive compounds |
| MX9202466A (en) | 1991-05-24 | 1994-06-30 | Vertex Pharma | NOVELTY IMMUNOSUPPRESSIVE COMPOUNDS. |
| JPH05178824A (en) | 1991-08-05 | 1993-07-20 | Takeda Chem Ind Ltd | Asparagine derivative and its use |
| AU2803692A (en) | 1991-10-11 | 1993-05-03 | Vertex Pharmaceuticals Incorporated | Isolation of an mr 52,000 fk506 binding protein and molecular cloning of a corresponding human cdna |
| IL103394A0 (en) | 1991-10-11 | 1993-03-15 | Ciba Geigy | Pyrimidinyl and triazinyl ethers and thioethers,their preparation and their use as herbicides |
| AU3278293A (en) | 1991-12-20 | 1993-07-28 | Syntex (U.S.A.) Inc. | Cyclic amides of 3-amino-2-hydroxy-carboxylic acids as hiv-protease inhibitors |
| CA2091194A1 (en) * | 1992-04-08 | 1993-10-09 | Richard D. Connell | 2-oxo-ethyl derivatives as immunosuppressants |
| WO1993023548A2 (en) | 1992-05-20 | 1993-11-25 | Vertex Pharmaceuticals Incorporated | METHOD OF DETECTING TISSUE-SPECIFIC FK506 BINDING PROTEIN MESSENGER RNAs AND USES THEREOF |
| IT1254373B (en) | 1992-05-29 | 1995-09-14 | HETEROPROSTANOIDS, PROCEDURE FOR THEIR PREPARATION AND THEIR EMPLOYE THERAPEUTIC. | |
| US5334719A (en) | 1992-06-17 | 1994-08-02 | Merck Frosst Canada, Inc. | Bicyclic(azaaromatic)indoles as inhibitors of leukotriene bisynthesis |
| IS2334B (en) | 1992-09-08 | 2008-02-15 | Vertex Pharmaceuticals Inc., (A Massachusetts Corporation) | Aspartyl protease inhibitor of a new class of sulfonamides |
| US5723490A (en) | 1992-09-08 | 1998-03-03 | Vertex Pharmaceuticals Incorporated | THF-containing sulfonamide inhibitors of aspartyl protease |
| NZ314207A (en) | 1992-09-28 | 2000-12-22 | Vertex Pharma | 1-(2-Oxoacetyl)-piperidine-2-carboxylic acid derivatives as multi drug resistant cancer cell sensitizers |
| AU5748194A (en) | 1992-12-11 | 1994-07-04 | Vertex Pharmaceuticals Incorporated | Mannitol derivatives and their use as inhibitors of aspartyl protease |
| US5252579A (en) | 1993-02-16 | 1993-10-12 | American Home Products Corporation | Macrocyclic immunomodulators |
| US5631017A (en) | 1993-03-26 | 1997-05-20 | Beth Israel Deaconess Medical Center, Inc. | Topical application of buspirone for treatment of pathological conditions associated with immune responses |
| US5319098A (en) | 1993-05-18 | 1994-06-07 | Celgene Corporation | Process for the stereoselective preparation of L-alanyl-L-proline |
| US5798355A (en) | 1995-06-07 | 1998-08-25 | Gpi Nil Holdings, Inc. | Inhibitors of rotamase enzyme activity |
| IT1270882B (en) | 1993-10-05 | 1997-05-13 | Isagro Srl | FUNGICIDE-BASED OLIGOPEPTIDES |
| ES2130452T3 (en) | 1993-11-04 | 1999-07-01 | Abbott Lab | CYCLOBUTANE DERIVATIVES USED AS INHIBITORS OF SQUALENE-SYNTHESASE AND PROTEIN FARNESYL TRANSFERASE. |
| CN1146201A (en) | 1994-03-07 | 1997-03-26 | 沃泰克斯药物股份有限公司 | Sulphonamide derivatives as aspartyl protease inhibitors |
| US5744485A (en) | 1994-03-25 | 1998-04-28 | Vertex Pharmaceuticals Incorporated | Carbamates and ureas as modifiers of multi-drug resistance |
| US5716929A (en) | 1994-06-17 | 1998-02-10 | Vertex Pharmaceuticals, Inc. | Inhibitors of interleukin-1β converting enzyme |
| US5856116A (en) | 1994-06-17 | 1999-01-05 | Vertex Pharmaceuticals, Incorporated | Crystal structure and mutants of interleukin-1 beta converting enzyme |
| US5488816A (en) | 1994-07-21 | 1996-02-06 | Boehringer Mannheim Corporation | Method and apparatus for manufacturing a coagulation assay device in a continuous manner |
| WO1996006097A1 (en) | 1994-08-18 | 1996-02-29 | Ariad Gene Therapeutics, Inc. | New multimerizing agents |
| IL115685A (en) | 1994-11-16 | 2000-08-31 | Vertex Pharma | Amino acid derivatives pharmaceutical compositions containing the same and processes for the preparation thereof |
| US5543423A (en) | 1994-11-16 | 1996-08-06 | Vertex Pharmaceuticals, Incorporated | Amino acid derivatives with improved multi-drug resistance activity |
| US5621108A (en) | 1994-12-05 | 1997-04-15 | Trustees Of The University Of Pennsylvania | Processes and intermediates for preparing macrocycles |
| US5691372A (en) | 1995-04-19 | 1997-11-25 | Vertex Pharmaceuticals Incorporated | Oxygenated-Heterocycle containing sulfonamide inhibitors of aspartyl protease |
| US5726184A (en) | 1995-05-19 | 1998-03-10 | Vertex Pharmaceuticals Incorporated | Tetralin compounds with improved MDR activity |
| US5696135A (en) | 1995-06-07 | 1997-12-09 | Gpi Nil Holdings, Inc. | Inhibitors of rotamase enzyme activity effective at stimulating neuronal growth |
| US5614547A (en) | 1995-06-07 | 1997-03-25 | Guilford Pharmaceuticals Inc. | Small molecule inhibitors of rotamase enzyme |
| US5801197A (en) | 1995-10-31 | 1998-09-01 | Gpi Nil Holdings, Inc. | Rotamase enzyme activity inhibitors |
| US5786378A (en) | 1996-09-25 | 1998-07-28 | Gpi Nil Holdings, Inc. | Heterocyclic thioesters |
| US5811434A (en) | 1996-11-13 | 1998-09-22 | Vertex Pharmacueticals Incorporated | Methods and compositions for stimulating neurite growth |
| US5840736A (en) | 1996-11-13 | 1998-11-24 | Vertex Pharmaceuticals Incorporated | Methods and compositions for stimulating neurite growth |
| US5780484A (en) | 1996-11-13 | 1998-07-14 | Vertex Pharmaceuticals Incorporated | Methods for stimulating neurite growth with piperidine compounds |
| PT944645E (en) | 1996-12-06 | 2005-06-30 | Vertex Pharma | INHIBITORS OF INTERLEUCINA CONVERTER ENZYME 1BETA |
| US5721256A (en) | 1997-02-12 | 1998-02-24 | Gpi Nil Holdings, Inc. | Method of using neurotrophic sulfonamide compounds |
| BR9813373A (en) | 1997-12-05 | 2000-10-03 | Astrazeneca Uk Ltd | Compound, process for preparing it, pharmaceutical composition, process for preparing it, use of a compound, and, processes for effecting immunosuppression and for treating or reducing the risk of reversibly obstructive airway disease in a patient |
-
1998
- 1998-09-21 US US09/157,566 patent/US6462072B1/en not_active Expired - Fee Related
-
1999
- 1999-09-17 JP JP2000574071A patent/JP2002526474A/en active Pending
- 1999-09-17 AU AU63905/99A patent/AU6390599A/en not_active Abandoned
- 1999-09-17 IL IL14155299A patent/IL141552A0/en unknown
- 1999-09-17 CA CA002341036A patent/CA2341036A1/en not_active Abandoned
- 1999-09-17 KR KR1020017003246A patent/KR20010089253A/en not_active Application Discontinuation
- 1999-09-17 WO PCT/US1999/021290 patent/WO2000017161A1/en not_active Application Discontinuation
- 1999-09-17 HU HU0103565A patent/HUP0103565A3/en unknown
- 1999-09-17 CN CN99810925A patent/CN1323294A/en active Pending
- 1999-09-17 EP EP99951470A patent/EP1115703A1/en not_active Withdrawn
-
2001
- 2001-03-08 ZA ZA200101934A patent/ZA200101934B/en unknown
Patent Citations (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1992000278A1 (en) * | 1990-07-02 | 1992-01-09 | Vertex Pharmaceuticals Incorporated | Novel immunosuppressive compounds |
| WO1996040633A1 (en) * | 1995-06-07 | 1996-12-19 | Guilford Pharmaceuticals Inc. | Small molecule inhibitors of rotamase enzyme activity |
| WO1996041609A2 (en) * | 1995-06-08 | 1996-12-27 | Vertex Pharmaceuticals Incorporated | Methods and compositions for stimulating neurite growth |
| WO1998013355A1 (en) * | 1996-09-25 | 1998-04-02 | Guilford Pharmaceuticals Inc. | Heterocyclic esters and amides |
| WO1998055090A1 (en) * | 1997-06-04 | 1998-12-10 | Guilford Pharmaceuticals Inc. | Hair growth compositions and uses |
Non-Patent Citations (2)
| Title |
|---|
| HOLT D A ET AL: "DESIGN, SYNTHESIS, AND KINETIC EVALUATION OF HIGH-AFFINITY FKBP LIGANDS AND THE X-RAY CRYSTAL STRUCTURES OF THEIR COMPLEXES WITH FKBP12", JOURNAL OF THE AMERICAN CHEMICAL SOCIETY,US,AMERICAN CHEMICAL SOCIETY, WASHINGTON, DC, vol. 115, no. 22, pages 9925-9938, XP000676758, ISSN: 0002-7863 * |
| HOLT D A ET AL: "STRUCTURE-ACTIVITY STUDIES OF SYNTHETIC FKBP LIGANDS AS PEPTIDYL-PROLYL ISOMERASE INHIBITORS", BIOORGANIC & MEDICINAL CHEMISTRY LETTERS,GB,OXFORD, vol. 4, no. 2, pages 315-320, XP000645938, ISSN: 0960-894X * |
Also Published As
| Publication number | Publication date |
|---|---|
| HUP0103565A2 (en) | 2002-01-28 |
| CN1323294A (en) | 2001-11-21 |
| EP1115703A1 (en) | 2001-07-18 |
| CA2341036A1 (en) | 2000-03-30 |
| HUP0103565A3 (en) | 2002-02-28 |
| JP2002526474A (en) | 2002-08-20 |
| ZA200101934B (en) | 2002-09-18 |
| IL141552A0 (en) | 2002-03-10 |
| US6462072B1 (en) | 2002-10-08 |
| KR20010089253A (en) | 2001-09-29 |
| AU6390599A (en) | 2000-04-10 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US5786378A (en) | Heterocyclic thioesters | |
| AU732194C (en) | Heterocyclic esters and amides | |
| US5721256A (en) | Method of using neurotrophic sulfonamide compounds | |
| US6486151B2 (en) | N-oxides of heterocyclic esters, amides, thioesters, and ketones | |
| US5874449A (en) | N-linked sulfonamides of heterocyclic thioesters | |
| US5935989A (en) | N-linked ureas and carbamates of heterocyclic thioesters | |
| US6462072B1 (en) | Cyclic ester or amide derivatives | |
| WO2000010553A2 (en) | Carbamate and urea compositions and neurotrophic uses | |
| US6121273A (en) | N-linked sulfonamides of heterocyclic thioesters | |
| US6417189B1 (en) | AZA compounds, pharmaceutical compositions and methods of use | |
| MXPA01002919A (en) | Cyclic ester or amide derivatives | |
| CA2602791A1 (en) | Heterocyclic thioesters and ketones |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| WWE | Wipo information: entry into national phase |
Ref document number: 99810925.8 Country of ref document: CN |
|
| ENP | Entry into the national phase |
Ref document number: 1999 63905 Country of ref document: AU Kind code of ref document: A |
|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AL AM AT AU AZ BA BB BG BR BY CA CH CN CR CU CZ DE DK DM EE ES FI GB GD GE GH GM HR HU ID IL IN IS JP KE KG KP KR KZ LC LK LR LS LT LU LV MD MG MK MN MW MX NO NZ PL PT RO RU SD SE SG SI SK SL TJ TM TR TT TZ UA UG UZ VN YU ZA ZW |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SL SZ TZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| ENP | Entry into the national phase |
Ref document number: 2341036 Country of ref document: CA Ref document number: 2341036 Country of ref document: CA Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 141552 Country of ref document: IL |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 63905/99 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2001/01934 Country of ref document: ZA Ref document number: 200101934 Country of ref document: ZA |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020017003246 Country of ref document: KR |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/2001/002919 Country of ref document: MX |
|
| ENP | Entry into the national phase |
Ref document number: 2000 574071 Country of ref document: JP Kind code of ref document: A |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1999951470 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1999951470 Country of ref document: EP |
|
| REG | Reference to national code |
Ref country code: DE Ref legal event code: 8642 |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020017003246 Country of ref document: KR |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1999951470 Country of ref document: EP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1020017003246 Country of ref document: KR |