WO2000015611A1 - Branched alkyl pyrrolidine-3-carboxylic acids - Google Patents
Branched alkyl pyrrolidine-3-carboxylic acids Download PDFInfo
- Publication number
- WO2000015611A1 WO2000015611A1 PCT/US1999/018258 US9918258W WO0015611A1 WO 2000015611 A1 WO2000015611 A1 WO 2000015611A1 US 9918258 W US9918258 W US 9918258W WO 0015611 A1 WO0015611 A1 WO 0015611A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- compound according
- carboxylic acid
- pyrrolidine
- effective amount
- therapeutically effective
- Prior art date
Links
Classifications
-
- C—CHEMISTRY; METALLURGY
- C07—ORGANIC CHEMISTRY
- C07D—HETEROCYCLIC COMPOUNDS
- C07D207/00—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom
- C07D207/02—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom
- C07D207/04—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members
- C07D207/10—Heterocyclic compounds containing five-membered rings not condensed with other rings, with one nitrogen atom as the only ring hetero atom with only hydrogen or carbon atoms directly attached to the ring nitrogen atom having no double bonds between ring members or between ring members and non-ring members with hetero atoms or with carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals, directly attached to ring carbon atoms
- C07D207/16—Carbon atoms having three bonds to hetero atoms with at the most one bond to halogen, e.g. ester or nitrile radicals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P1/00—Drugs for disorders of the alimentary tract or the digestive system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P19/00—Drugs for skeletal disorders
- A61P19/02—Drugs for skeletal disorders for joint disorders, e.g. arthritis, arthrosis
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/08—Antiepileptics; Anticonvulsants
- A61P25/10—Antiepileptics; Anticonvulsants for petit-mal
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/14—Drugs for disorders of the nervous system for treating abnormal movements, e.g. chorea, dyskinesia
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/22—Anxiolytics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P25/00—Drugs for disorders of the nervous system
- A61P25/24—Antidepressants
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P29/00—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID]
- A61P29/02—Non-central analgesic, antipyretic or antiinflammatory agents, e.g. antirheumatic agents; Non-steroidal antiinflammatory drugs [NSAID] without antiinflammatory effect
Definitions
- Ri is hydrogen or a lower alkyl radical and n is 4, 5, or 6 are known in
- the uses disclosed are: protective effect against cramp induced by thiosemicarbazide; protective action against cardiazole cramp; the cerebral diseases, epilepsy, faintness attacks, hypokinesia, and cranial traumas; and improvement in cerebral functions.
- the compounds are useful in geriatric patients.
- the patents are hereby incorporated by reference.
- the compounds, prodrugs, and pharmaceutically acceptable salts are useful in a variety of disorders.
- the disorders include: convulsions such as in epilepsy, faintness attacks, hypokinesia, cranial disorders, neurodegenerative disorders, depression, anxiety, panic, pain, inflammatory disorders such as arthritis, irritable bowel syndrome, and neuropathological disorders.
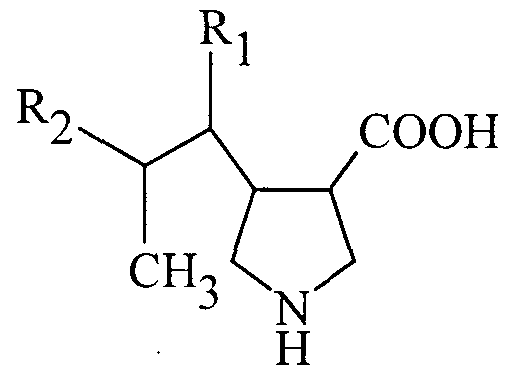
- the compounds are those of formula
- R ⁇ is hydrogen or a straight or branched alkyl of from 1 to 5 carbons
- R2 is a straight or branched alkyl of from 1 to 5 carbons; and Ri and R2 when taken together form a carbocyclic ring of from 3 to 7 atoms.
- Preferred compounds are those wherein R ⁇ is H, methyl, or ethyl
- R2 is methyl or ethyl.
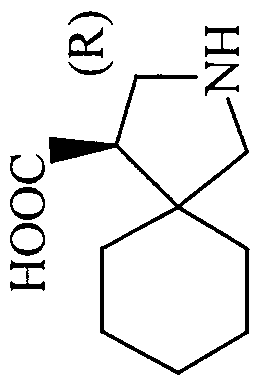
- the most preferred compounds are those wherein (cis)-4-isobutyl- pyrrolidine-3-carboxylic acid and (trans)-4-isobutyl-pyrrolidine-3-carboxylic acid.
- Other preferred compounds are those wherein R] and R2 are taken to form a carbocylic ring of from 3 to 7 atoms.
- More preferred compounds are those wherein Rj and R2 form a five or six membered ring.
- Novel intermediates useful in the preparation of the final compounds are also encompassed by the invention.
- R4 is alkyl of 3 or
- Such compounds are selected from: trans-4-isopropylpyrrolidine-3-carboxylic acid; trans-4-propyl-pyrrolidine-3-carboxylic acid; and trans-4-butyl-pyrrolidine-3-carboxylic acid.
- alkyl is a straight or branched group of from 1 to 5 carbon atoms including but not limited to methyl, ethyl, propyl, n-propyl, isopropyl, butyl, 2-butyl, tert-butyl, and pentyl.
- Preferred groups are methyl and tert-butyl.
- the stereocenters in Formula I can have independently be of either an R or S configuration.
- salts when R is hydrogen can be salts of appropriate inorganic or organic acids, for example, hydrochloric, sulphuric, phosphoric, acetic, oxalic, lactic, citric, malic, salicylic, malonic, maleic, succinic, and ascorbic.
- inorganic or organic acids for example, hydrochloric, sulphuric, phosphoric, acetic, oxalic, lactic, citric, malic, salicylic, malonic, maleic, succinic, and ascorbic.
- salts with alkali metals or alkaline earth metals for example, sodium, potassium, magnesium, or calcium are formed.
- Salts with quaternary ammonium ions can also be prepared with, for example, the tetramethyl-ammonium ion.
- Prodrugs of compounds I-VIII are included in the scope of the instant invention.
- Aminoacyl-glycolic and -lactic esters are known as prodrugs of amino acids (Wermuth C.G., Chemistry and Industry, 1980:433-435).
- the carbonyl group of the amino acids can be esterified by known means.
- Prodrugs and soft drugs are known in the art (Palomino E., Drugs of the Future, 1990;15(4):361- 368). The last two citations are hereby incorporated by reference.
- the effectiveness of an orally administered drug is dependent upon the drug's efficient transport across the mucosal epithelium and its stability in entero- hepatic circulation.
- a prodrug is a drug which has been chemically modified and may be biologically inactive at its site of action, but which may be degraded or modified by one or more enzymatic or other in vivo processes to the parent bioactive form.
- This chemically modified drug, or prodrug should have a different pharmacokinetic profile to the parent, enabling easier absorption across the mucosal epithelium, better salt formulation and/or solubility, improved systemic stability (for an increase in plasma half-life, for example).
- ester or amide derivatives which may be cleaved by, for example, esterases or Upases.
- ester derivatives the ester is derived from the carboxylic acid moiety of the drug molecule by known means.
- amide derivatives the amide may be derived from the carboxylic acid moiety or the amine moiety of the drug molecule by known means.
- derivatives that accumulate at a site of action through membrane selection of a prodrug form or modified prodrug form any combination of 1 to 3.
- the quaternary salt is termed a "soft" quaternary salt since, unlike normal quaternary salts, e.g., R-N + (CH 3 ) 3 , it can release the active drug on hydrolysis.
- Soft quaternary salts have useful physical properties compared with the basic drug or its salts. Water solubility may be increased compared with other salts, such as the hydrochloride, but more important there may be an increased abso ⁇ tion of the drug from the intestine. Increased abso ⁇ tion is probably due to the fact that the "soft" quaternary salt has surfactant properties and is capable of forming micelles and unionized ion pairs with bile acids, etc., which are able to penetrate the intestinal epithelium more effectively. The prodrug, after abso ⁇ tion, is rapidly hydrolyzed with release of the active parent drug. Certain of the compounds of the present invention can exist in unsolvated forms as well as solvated forms, including hydrated forms. In general, the solvated forms, including hydrated forms, are equivalent to unsolvated forms and are intended to be encompassed within the scope of the present invention.
- Certain of the compounds of the present invention possess one or more chiral centers and each center may exist in the R(D) or S(L) configuration.
- the present invention includes all enantiomeric and epimeric forms as well as the appropriate mixtures thereof.
- the compound of Example 1 is a mixture of all four possible stereoisomers.
- the compound of Example 6 is one of the isomers.
- the configuration of the cyclohexane ring carbon centers may be R or S in these compounds where a configuration can be defined.
- Compounds can also be assayed for biological activity using a [3 H] gabapentin binding assay as described in Suman Chauhan N., et al., Eur. J. Pharmacol., 1993;244:293-301.
- Table 2 above shows the binding affinity of the compounds of the invention to the ⁇ 2 ⁇ subunit.
- Neurontin® a marketed drug effective in the treatment of such disorders as epilepsy.
- Neurontin® is l-(aminomethyl)-cyclohexaneacetic acid of structural formula
- Gabapentin (Neurontin®) is about 0.10 to 0.12 ⁇ M in this assay.
- the compounds of the instant invention are expected, therefore, to exhibit pharmacologic properties comparable to gabapentin. For example, as agents for convulsions, anxiety, and pain.
- the present invention also relates to therapeutic use of the compounds of the mimetic as agents for neurodegenerative disorders.
- neurodegenerative disorders are, for example, Alzheimer's disease, Huntington's disease, Parkinson's disease, and Amyotrophic Lateral Sclerosis.
- the present invention also covers treating neurodegenerative disorders termed acute brain injury. These include but are not limited to: stroke, head trauma, and asphyxia.
- Stroke refers to a cerebral vascular disease and may also be referred to as a cerebral vascular incident (CVA) and includes acute thromboembolic stroke. Stroke includes both focal and global ischemia. Also, included are transient cerebral ischemic attacks and other cerebral vascular problems accompanied by cerebral ischemia. A patient undergoing carotid endarterectomy specifically or other cerebrovascular or vascular surgical procedures in general, or diagnostic vascular procedures including cerebral angiography and the like. Other incidents are head trauma, spinal cord trauma, or injury from general anoxia, hypoxia, hypoglycemia, hypotension as well as similar injuries seen during procedures from embole, hyperfusion, and hypoxia.
- CVA cerebral vascular incident
- the instant invention would be useful in a range of incidents, for example, during cardiac bypass surgery, in incidents of intracranial hemorrhage, in perinatal asphyxia, in cardiac arrest, and status epilepticus.
- Pain refers to acute as well as chronic pain.
- Acute pain is usually short-lived and is associated with hyperactivity of the sympathetic nervous system. Examples are postoperative pain and allodynia.
- Chronic pain is usually defined as pain persisting from 3 to 6 months and includes somatogenic pains and psychogenic pains. Other pain is nociceptive.
- Still other pain is caused by injury or infection of peripheral sensory nerves. It includes, but is not limited to pain from peripheral nerve trauma, he ⁇ es virus infection, diabetes mellitus, causalgia, plexus avulsion, neuroma, limb amputation, and vasculitis.
- Neuropathic pain is also caused by nerve damage from chronic alcoholism, human immunodeficiency virus infection, hypothyroidism, uremia, or vitamin deficiencies.
- Neuropathic pain includes, but is not limited to pain caused by nerve injury such as, for example, the pain diabetics suffer from.
- Psychogenic pain is that which occurs without an organic origin such as low back pain, atypical facial pain, and chronic headache.
- inflammatory pain osteoarthritic pain
- trigeminal neuralgia cancer pain
- diabetic neuropathy restless leg syndrome
- acute he ⁇ etic and posthe ⁇ etic neuralgia causalgia
- brachial plexus avulsion occipital neuralgia
- gout phantom limb
- burn and other forms of neuralgia, neuropathic and idiopathic pain syndrome.
- a skilled physician will be able to determine the appropriate situation in which subjects are susceptible to or at risk of, for example, stroke as well as suffering from stroke for administration by methods of the present invention.
- the compounds of the invention are also expected to be useful in the treatment of depression.
- Depression can be the result of organic disease, secondary to stress associated with personal loss, or idiopathic in origin. There is a strong tendency for familial occurrence of some forms of depression suggesting a mechanistic cause for at least some forms of depression.
- the diagnosis of depression is made primarily by quantification of alterations in patients' mood. These evaluations of mood are generally performed by a physician or quantified by a neuropsychologist using validated rating scales, such as the Hamilton Depression Rating Scale or the Brief Psychiatric Rating Scale. Numerous other scales have been developed to quantify and measure the degree of mood alterations in patients with depression, such as insomnia, difficulty with concentration, lack of energy, feelings of worthlessness, and guilt.
- the standards for diagnosis of depression as well as all psychiatric diagnoses are collected in the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) referred to as the DSM-IV-R manual published by the American Psychiatric Association,
- GABA is an inhibitory neurotransmitter with the central nervous system. Within the general context of inhibition, it seems likely that GAB A-mimetics might decrease or inhibit cerebral function and might therefore slow function and decrease mood leading to depression.
- the compounds of the instant invention may produce an anticonvulsant effect through the increase of newly created GABA at the synaptic junction. If gabapentin does indeed increase GABA levels or the effectiveness of GABA at the synaptic junction, then it could be classified as a GABA-mimetic and might decrease or inhibit cerebral function and might, therefore, slow function and decrease mood leading to depression.
- GABA agonist or GABA-mimetic might work just the opposite way by increasing mood and thus, be an antidepressant, is a new concept, different from the prevailing opinion of GABA activity heretofore.
- the compounds of the instant invention are also expected to be useful in the treatment of anxiety and of panic as demonstrated by means of standard pharmacological procedures.
- Nociceptive pressure thresholds were measured in the rat paw pressure test using an analgesymeter (Randall-Selitto method: Randall L.O. and Selitto J.J., "A method for measurement of analgesic activity on inflamed tissue," Arch. Int. Pharmacodyn., 1957;4:409-419).
- Male Sprague-Dawley rats (70-90 g) were trained on this apparatus before the test day. Pressure was gradually applied to the hind paw of each rat and nociceptive thresholds were determined as the pressure (g) required to elicit paw withdrawal. A cutoff point of 250 g was used to prevent any tissue damage to the paw.
- the apparatus is an open-topped box, 45 cm long, 27 cm wide, and 27 cm high, divided into a small (2/5) and a large (3/5) area by a partition that extended
- the total number of body postures exhibited by the animal towards the threat stimulus (a human standing approximately 0.5 m away from the marmoset cage and staring into the eyes of the marmoset) is recorded during the 2-minute test period.
- the body postures scored are slit stares, tail postures, scent marking of the cage/perches, piloerection, retreats, and arching of the back.
- Each animal is exposed to the threat stimulus twice on the test day before and after drug treatment.
- the difference between the two scores is analyzed using one-way analysis of variance followed by Dunnett's t-test. All drug treatments are carried out SC at least 2 hours after the first (control) threat.
- the pretreatment time for each compound is 40 minutes.
- Rat conflict Test Rats are trained to press levers for food reward in operant chambers.
- the schedule consists of alternations of four 4-minute unpunished periods on variable interval of 30 seconds signaled by chamber lights on and three 3-minute punished periods on fixed ratio 5 (by footshock concomitant to food delivery) signaled by chamber lights off.
- the degree of footshock is adjusted for each rat to obtain approximately 80% to 90% suppression of responding in comparison with unpunished responding.
- Rats receive saline vehicle on training days.
- mice Male DBA/2 mice, 3 to 4 weeks old were obtained from Jackson Laboratories Bar Harbour, Maine. Immediately before anticonvulsant testing, mice were placed upon a wire mesh, 4 inches square, suspended from a steel rod. The square was slowly inverted through 180° and mice observed for 30 seconds. Any mouse falling from the wire mesh was scored as ataxic (Coughenour L.L., McLean J.R., Parker R.B., "A new device for the rapid measurement of impaired motor function in mice,” Pharm. Biochem. Behav., 1977;6(3):351-3).
- Mice were placed into an enclosed acrylic plastic chamber (21 cm height, approximately 30 cm diameter) with a high-frequency speaker (4 cm diameter) in the center of the top lid.
- An audio signal generator (Protek model B-810) was used to produce a continuous sinusoidal tone that was swept linearly in frequency between 8 kHz and 16 kHz once each 10 msec.
- the average sound pressure level (SPL) during stimulation was approximately 100 dB at the floor of the chamber. Mice were placed within the chamber and allowed to acclimatize for one minute.
- mice in the vehicle-treated group responded to the sound stimulus (applied until tonic extension occurred, or for a maximum of 60 sec) with a characteristic seizure sequence consisting of wild running followed by clonic seizures, and later by tonic extension, and finally by respiratory arrest and death in 80% or more of the mice.
- vehicle-treated mice the entire sequence of seizures to respiratory arrest lasts approximately 15 to 20 seconds.
- the incidence of all the seizure phases in the drug-treated and vehicle-treated mice was recorded, and the occurrence of tonic seizures were used for calculating anticonvulsant ED50 values by probit analysis (Litchfield J.T., Wilcoxon F.
- the compounds of the instant invention are also expected to be useful in the treatment of pain and phobic disorders (Am. J. Pain Manag., 1995;5:7-9).
- the compounds of the instant invention are also expected to be useful in treating the symptoms of manic, acute or chronic, single upside, or recurring depression. They are also expected to be useful in treating and/or preventing bipolar disorder (United States Patent Number 5,510,381).
- TNBS trinitrobenzene sulfonic
- mice Male Sprague-Dawley rats (Janvier, Le Genest-St-Ilse, France) weighing 340-400 g are used. The animals are housed 3 per cage in a regulated environment (20 ⁇ 1°C, 50 + 5% humidity, with light 8:00 am to 8:00 pm). Under anesthesia (ketamine 80 mg/kg i.p; acepromazin 12 mg/kg ip), the injection of TNBS (50 mg/kg) or saline (1.5 mL/kg) is performed into the proximal colon (1 cm from the cecum). After the surgery, animals are individually housed in polypropylene cages and kept in a regulated environment (20 ⁇ 1°C, 50 + 5% humidity, with light 8:00 am to 8:00 pm) during 7 days.
- a regulated environment (20 ⁇ 1°C, 50 + 5% humidity, with light 8:00 am to 8:00 pm
- a balloon (5-6 cm length) is inserted by anus and kept in position (tip of balloon 5 cm from the anus) by taping the catheter to the base of the tail.
- the balloon is progressively inflated by step of 5 mm Hg, from 0 to 75 mm Hg, each step of inflation lasting 30 seconds.
- Each cycle of colonic distension is controlled by a standard barostat (ABS, St-Die, France).
- the threshold corresponds to the pressure which produced the first abdominal contraction and the cycle of distension is then discontinued.
- the colonic threshold (pressure expressed in mm Hg) is determined after performance of four cycles of distension on the same animal.
- Group C mean of the colonic threshold in the control group
- Group T mean of the colonic threshold in the TNBS-treated group
- Group A mean of the colonic threshold in the test compound-treated group
- TNBS is dissolved in EtOH 30% and injected under a volume of 0.5 mL/rat.
- TNBS is purchased from Fluka.
- Oral administration of the test compound or its vehicle is performed 1 hour before the colonic distension cycle.
- Sub-cutaneous administration of the test compound or its vehicle is performed 30 minutes before the colonic distension cycle.
- the compounds of the present invention can be prepared and administered in a wide variety of oral and parenteral dosage forms.
- the compounds of the present invention can be administered by injection, that is, intravenously, intramuscularly, intracutaneously, subcutaneously, intraduodenally, or intraperitoneally.
- the compounds of the present invention can be administered by inhalation, for example, intranasally.
- the compounds of the present invention can be administered transdermally.
- the following dosage forms may comprise as the active component, either a compound of Formula I or a corresponding pharmaceutically acceptable salt of a compound of Formula I.
- pharmaceutically acceptable carriers can be either solid or liquid.
- Solid form preparations include powders, tablets, pills, capsules, cachets, suppositories, and dispersible granules.
- a solid carrier can be one or more substances which may also act as diluents, flavoring agents, binders, preservatives, tablet disintegrating agents, or an encapsulating material.
- the carrier is a finely divided solid which is in a mixture with the finely divided active component.
- the active component is mixed with the carrier having the necessary binding properties in suitable proportions and compacted in the shape and size desired.
- the powders and tablets preferably contain from five or ten to about seventy percent of the active compound.
- Suitable carriers are magnesium carbonate, magnesium stearate, talc, sugar, lactose, pectin, dextrin, starch, gelatin, tragacanth, methylcellulose, sodium carboxymethylcellulose, a low melting wax, cocoa butter, and the like.
- the term "preparation" is intended to include the formulation of the active compound with encapsulating material as a carrier providing a capsule in which the active component with or without other carriers, is surrounded by a carrier, which is thus in association with it.
- cachets and lozenges are included. Tablets, powders, capsules, pills, cachets, and lozenges can be used as solid dosage forms suitable for oral administration.
- a low melting wax such as a mixture of fatty acid glycerides or cocoa butter
- the active component is dispersed homogeneously therein, as by stirring.
- the molten homogenous mixture is then poured into convenient sized molds, allowed to cool, and thereby to solidify.
- Liquid form preparations include solutions, suspensions, and emulsions, for example, water or water propylene glycol solutions.
- liquid preparations can be formulated in solution in aqueous polyethylene glycol solution.
- Aqueous solutions suitable for oral use can be prepared by dissolving the active component in water and adding suitable colorants, flavors, stabilizing and thickening agents as desired.
- Aqueous suspensions suitable for oral use can be made by dispersing the finely divided active component in water with viscous material, such as natural or synthetic gums, resins, methylcellulose, sodium carboxymethylcellulose, and other well-known suspending agents.
- viscous material such as natural or synthetic gums, resins, methylcellulose, sodium carboxymethylcellulose, and other well-known suspending agents.
- solid form preparations which are intended to be converted, shortly before use, to liquid form preparations for oral administration.
- liquid forms include solutions, suspensions, and emulsions.
- These preparations may contain, in addition to the active component, colorants, flavors, stabilizers, buffers, artificial and natural sweeteners, dispersants, thickeners, solubilizing agents, and the like.
- the pharmaceutical preparation is preferably in unit dosage form. In such form the preparation is subdivided into unit doses containing appropriate quantities of the active component.
- the unit dosage form can be a packaged preparation, the package containing discrete quantities of preparation, such as packeted tablets, capsules, and powders in vials or ampoules.
- the unit dosage form can be a capsules, tablet, cachet, or lozenge itself, or it can be the appropriate number of any of these in packaged form.
- the quantity of active component in a unit dose preparation may be varied or adjusted from 0.1 mg to 1 g according to the particular application and the potency of the active component.
- the drug may be administered three times daily as, for example, capsules of 100 or 300 mg.
- the composition can, if desired, also contain other compatible therapeutic agents.
- the compounds utilized in the pharmaceutical method of this invention are administered at the initial dosage of about 0.01 mg to about 100 mg/kg daily.
- a daily dose range of about 0.01 mg to about 100 mg/kg is preferred.
- the dosages may be varied depending upon the requirements of the patient, the severity of the condition being treated, and the compound being employed. Determination of the proper dosage for a particular situation is within the skill of the art. Generally, treatment is initiated with smaller dosages which are less than the optimum dose of the compound. Thereafter, the dosage is increased by small increments until the optimum effect under the circumstances is reached.
- the total daily dosage may be divided and administered in portions during the day, if desired.
- Step 1 Synthesis of 1,1 -Dibromo-4-methyl-pent-l-ene
- Step 2 Synthesis of 5-Methyl-hex-2-ynoic acid ethyl ester l,l-Dibromo-4-methyl-pent-l-ene 6 (40 g, 165.9 mmol) was dissolved in dry THF (120 mL) and cooled to -78°C. While stirring, «-butyllithium (1.6 M solution in hexane, 190.8 mL, 305 mmol) was added dropwise in a few minutes.
- Step 4 ( " cis)-l-Benzyl-4-isobutyl-pyrrolidine-3 -carboxylic acid ethyl ester N-Benzyl-N-(methoxymethyl)trimethylsilylmethylamine (4.0 g,
- N-Benzyl-N-(methoxymethyl)trimethylsilylmethylamine (2.84 g, 12 mmol), followed by TFA (1.0 M solution in CH2CI2, 1.0 mL, 1 mmol) were added to a solution of (E)-5-methyl-hex-2-enoic acid ethyl ester (1.56 g, 10.0 mmol) in methylene chloride (30 mL) maintained at -5°C under nitrogen atmosphere. After 15 minutes, the bath was removed and stirring was continued overnight. Saturated sodium bicarbonate was added, and the organic portion was separated, washed with brine, and dried.
- reaction mixture was stirred at 0°C for 1 hour, then diluted with water (40 mL). Sodium sulfite (0.85 g, 6.75 mmol) was added, and the mixture was extracted with ethyl acetate. The aqueous phase was adjusted to pH 5.0 with KH2PO4 (1.51 g, 11.1 mmol) and 10% HCl. This solution was extracted with isopropyl alcohol :methylene chloride (1 :3), which was dried over
Abstract
Description
Claims
Priority Applications (10)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AT99941063T ATE280154T1 (en) | 1998-09-14 | 1999-08-11 | BRANCHED ALKYLPYRROLIDINE-3-CARBONIC ACIDS |
| DE69921340T DE69921340T2 (en) | 1998-09-14 | 1999-08-11 | BRANCHED ALKYLPYRROLIDIN-3-CARBOXYLIC ACIDS |
| AU54787/99A AU5478799A (en) | 1998-09-14 | 1999-08-11 | Branched alkyl pyrrolidine-3-carboxylic acids |
| NZ510145A NZ510145A (en) | 1998-09-14 | 1999-08-11 | Branched alkyl pyrrolidine-3-carboxylic acids useful in protective action against cardiazole cramp and cerebral conditions such as epilepsy, hypokinesia and cranial traumas |
| JP2000570151A JP2002524551A (en) | 1998-09-14 | 1999-08-11 | Branched-chain alkylpyrrolidine-3-carboxylic acid |
| EP99941063A EP1112253B1 (en) | 1998-09-14 | 1999-08-11 | Branched alkyl pyrrolidine-3-carboxylic acids |
| US09/673,277 US6245801B1 (en) | 1998-09-14 | 1999-08-11 | Branched alkyl pyrrolidine-3-carboxylic acids |
| CA002339273A CA2339273C (en) | 1998-09-14 | 1999-08-11 | Branched alkyl pyrrolidine-3-carboxylic acids |
| KR1020017003209A KR20010075064A (en) | 1998-09-14 | 1999-08-11 | Branched alkyl pyrrolidine-3-carboxylic acids |
| BR9913701-1A BR9913701A (en) | 1998-09-14 | 1999-08-11 | branched alkyl pyrrolidine-3-carboxylic acids |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10015698P | 1998-09-14 | 1998-09-14 | |
| US60/100,156 | 1998-09-14 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO2000015611A1 true WO2000015611A1 (en) | 2000-03-23 |
Family
ID=22278367
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1999/018258 WO2000015611A1 (en) | 1998-09-14 | 1999-08-11 | Branched alkyl pyrrolidine-3-carboxylic acids |
Country Status (13)
| Country | Link |
|---|---|
| US (1) | US6245801B1 (en) |
| EP (1) | EP1112253B1 (en) |
| JP (1) | JP2002524551A (en) |
| KR (1) | KR20010075064A (en) |
| AT (1) | ATE280154T1 (en) |
| AU (1) | AU5478799A (en) |
| BR (1) | BR9913701A (en) |
| CA (1) | CA2339273C (en) |
| DE (1) | DE69921340T2 (en) |
| ES (1) | ES2228087T3 (en) |
| NZ (1) | NZ510145A (en) |
| WO (1) | WO2000015611A1 (en) |
| ZA (1) | ZA200100837B (en) |
Cited By (33)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2002022568A1 (en) * | 2000-09-14 | 2002-03-21 | Grünenthal GmbH | ss-THIO-AMINO ACIDS |
| WO2002028816A1 (en) * | 2000-09-30 | 2002-04-11 | Buschmann, Helmut | Substituted 5-amino-1-pentene-3-ol derivatives |
| WO2002030881A1 (en) * | 2000-09-30 | 2002-04-18 | Grünenthal GmbH | Sulfonylguanidine |
| WO2003104184A1 (en) | 2002-06-11 | 2003-12-18 | Xenoport, Inc. | Methods for synthesis of acyloxyalkyl derivatives of gaba analogs |
| WO2004054559A1 (en) * | 2002-12-13 | 2004-07-01 | Warner-Lambert Company Llc | Alpha2delta ligands for different pharmaceutical uses |
| US6833140B2 (en) | 2001-06-11 | 2004-12-21 | Xenoport, Inc. | Orally administered dosage forms of GABA analog prodrugs having reduced toxicity |
| US6900192B2 (en) | 2000-10-06 | 2005-05-31 | Xenoport, Inc. | Bile-acid conjugates for providing sustained systemic concentrations of drugs |
| US6992076B2 (en) | 2000-10-06 | 2006-01-31 | Xenoport, Inc. | Bile-acid derived compounds for providing sustained systemic concentrations of drugs after oral administration |
| US7026351B2 (en) | 2002-03-20 | 2006-04-11 | Xenoport, Inc. | Cyclic 1-(acyloxy)-alkyl prodrugs of GABA analogs, compositions and uses thereof |
| US7060727B2 (en) | 2002-12-11 | 2006-06-13 | Xenoport, Inc. | Prodrugs of fused GABA analogs, pharmaceutical compositions and uses thereof |
| US7250433B2 (en) | 2003-03-07 | 2007-07-31 | Warner Lambert Company Llc | Tetrazole and oxadiazolone substituted β-amino acid derivatives |
| US7420002B2 (en) | 2001-06-11 | 2008-09-02 | Xenoport | Amino acid conjugates providing for sustained systemic concentrations of GABA analogues |
| DE102007036068A1 (en) | 2007-08-01 | 2009-02-05 | Wacker Chemie Ag | Process for the preparation of alkylmethoxymethyltrimethylsilanylmethylamines |
| WO2010042759A2 (en) | 2008-10-08 | 2010-04-15 | Kyphia Pharmaceuticals Inc | Gaba conjugates and methods of use thereof |
| US7700652B2 (en) | 2003-09-11 | 2010-04-20 | Xenoport, Inc. | Treating urinary incontinence using prodrugs of GABA analogs |
| US7790708B2 (en) | 2001-06-11 | 2010-09-07 | Xenoport, Inc. | Prodrugs of GABA analogs, compositions and uses thereof |
| US7868043B2 (en) | 2008-01-25 | 2011-01-11 | Xenoport, Inc. | Mesophasic forms of (3S)-aminomethyl-5-methyl-hexanoic acid prodrugs and methods of use |
| US7872046B2 (en) | 2008-01-25 | 2011-01-18 | Xenoport, Inc. | Crystalline form of a (3S)-aminomethyl-5-methyl-hexanoic acid prodrug and methods of use |
| EP2343073A2 (en) | 2003-12-11 | 2011-07-13 | Sepracor Inc. | Combination of a sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression |
| US8048917B2 (en) | 2005-04-06 | 2011-11-01 | Xenoport, Inc. | Prodrugs of GABA analogs, compositions and uses thereof |
| US8062870B2 (en) | 2008-01-25 | 2011-11-22 | Xenoport, Inc. | Enantiomerically resolving acyloxyalkyl thiocarbonates used in synthesizing acyloxyalkyl carbamate prodrugs |
| US8114909B2 (en) | 2003-09-17 | 2012-02-14 | Xenoport, Inc. | Treating or preventing restless legs syndrome using prodrugs of GABA analogs |
| WO2014113299A1 (en) | 2013-01-15 | 2014-07-24 | Warsaw Orthopedic, Inc. | Clonidine compounds in a biodegradable fiber |
| WO2014117176A1 (en) | 2013-01-28 | 2014-07-31 | Lopez Hector L | Methods of improving tolerability, pharmacodynamics, and efficacy of b-alanine and use therefor |
| US8795725B2 (en) | 2004-11-04 | 2014-08-05 | Xenoport, Inc. | GABA analog prodrug sustained release oral dosage forms |
| KR20170127148A (en) * | 2016-05-11 | 2017-11-21 | 제이에스아이실리콘주식회사 | A Novel Silicone Coupling Agent Having a Tertiary Amine Substituted with a Ether Group and A method for Producing the Same |
| US9879019B2 (en) | 2015-10-16 | 2018-01-30 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-α]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| CN110038576A (en) * | 2019-05-05 | 2019-07-23 | 中国科学院兰州化学物理研究所 | A kind of load type metal catalyst and its preparation method and application |
| US10550126B2 (en) | 2015-10-16 | 2020-02-04 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-A]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11365198B2 (en) | 2015-10-16 | 2022-06-21 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11512092B2 (en) | 2015-10-16 | 2022-11-29 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11524964B2 (en) | 2015-10-16 | 2022-12-13 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11773106B2 (en) | 2015-10-16 | 2023-10-03 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
Families Citing this family (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6455571B1 (en) * | 1998-04-23 | 2002-09-24 | Abbott Laboratories | Inhibitors of neuraminidases |
| US20050209319A1 (en) * | 2004-03-18 | 2005-09-22 | Xenoport, Inc. | Treatment of local pain |
| CN111072543B (en) * | 2019-11-13 | 2021-06-04 | 北京海美桐医药科技有限公司 | Preparation method and application of (3R,4S) -4-ethylpyrrolidine-3-carboxylic acid compound |
Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4087544A (en) * | 1974-12-21 | 1978-05-02 | Warner-Lambert Company | Treatment of cranial dysfunctions using novel cyclic amino acids |
| WO1996006095A1 (en) * | 1994-08-19 | 1996-02-29 | Abbott Laboratories | Endothelin antagonists |
| WO1996015108A1 (en) * | 1994-11-14 | 1996-05-23 | Eli Lilly And Company | Pyrrolidinyl di-carboxylic acid derivatives as metabotropic glutamate receptor agonists |
-
1999
- 1999-08-11 KR KR1020017003209A patent/KR20010075064A/en not_active Application Discontinuation
- 1999-08-11 NZ NZ510145A patent/NZ510145A/en unknown
- 1999-08-11 WO PCT/US1999/018258 patent/WO2000015611A1/en active IP Right Grant
- 1999-08-11 EP EP99941063A patent/EP1112253B1/en not_active Expired - Lifetime
- 1999-08-11 US US09/673,277 patent/US6245801B1/en not_active Expired - Fee Related
- 1999-08-11 CA CA002339273A patent/CA2339273C/en not_active Expired - Fee Related
- 1999-08-11 JP JP2000570151A patent/JP2002524551A/en active Pending
- 1999-08-11 AU AU54787/99A patent/AU5478799A/en not_active Abandoned
- 1999-08-11 BR BR9913701-1A patent/BR9913701A/en not_active IP Right Cessation
- 1999-08-11 DE DE69921340T patent/DE69921340T2/en not_active Expired - Fee Related
- 1999-08-11 ES ES99941063T patent/ES2228087T3/en not_active Expired - Lifetime
- 1999-08-11 AT AT99941063T patent/ATE280154T1/en not_active IP Right Cessation
-
2001
- 2001-01-30 ZA ZA200100837A patent/ZA200100837B/en unknown
Patent Citations (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4087544A (en) * | 1974-12-21 | 1978-05-02 | Warner-Lambert Company | Treatment of cranial dysfunctions using novel cyclic amino acids |
| WO1996006095A1 (en) * | 1994-08-19 | 1996-02-29 | Abbott Laboratories | Endothelin antagonists |
| WO1996015108A1 (en) * | 1994-11-14 | 1996-05-23 | Eli Lilly And Company | Pyrrolidinyl di-carboxylic acid derivatives as metabotropic glutamate receptor agonists |
Cited By (83)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6846843B2 (en) | 2000-09-14 | 2005-01-25 | Gruenenthal Gmbh | β-thioamino acids |
| WO2002022568A1 (en) * | 2000-09-14 | 2002-03-21 | Grünenthal GmbH | ss-THIO-AMINO ACIDS |
| WO2002028816A1 (en) * | 2000-09-30 | 2002-04-11 | Buschmann, Helmut | Substituted 5-amino-1-pentene-3-ol derivatives |
| WO2002030881A1 (en) * | 2000-09-30 | 2002-04-18 | Grünenthal GmbH | Sulfonylguanidine |
| WO2002030869A1 (en) * | 2000-09-30 | 2002-04-18 | Grünenthal GmbH | 5-amino-1-pentene-3-ol substituted derivatives |
| US7671074B2 (en) | 2000-09-30 | 2010-03-02 | Gruenenthal Gmbh | Sulfonylguanidine compounds and pharmaceutical uses thereof |
| US6815443B2 (en) | 2000-09-30 | 2004-11-09 | Gruenenthal Gmbh | 5-Amino-1-pentene-3-ol substituted derivatives |
| US7601706B2 (en) | 2000-10-06 | 2009-10-13 | Xenoport, Inc. | Bile-acid conjugates providing for sustained systemic concentration of drugs |
| US7049305B2 (en) | 2000-10-06 | 2006-05-23 | Xenoport, Inc. | Bile-acid conjugates providing for sustained systemic concentration of drugs |
| US7601708B2 (en) | 2000-10-06 | 2009-10-13 | Xenoport, Inc. | Bile-acid derived compounds for providing sustained systemic concentrations of drugs after oral administration |
| US6900192B2 (en) | 2000-10-06 | 2005-05-31 | Xenoport, Inc. | Bile-acid conjugates for providing sustained systemic concentrations of drugs |
| US6992076B2 (en) | 2000-10-06 | 2006-01-31 | Xenoport, Inc. | Bile-acid derived compounds for providing sustained systemic concentrations of drugs after oral administration |
| EP2085087A1 (en) | 2001-06-11 | 2009-08-05 | Xenoport, Inc. | Prodrugs of gaba analogs, compositions and uses thereof |
| US8168623B2 (en) | 2001-06-11 | 2012-05-01 | Xenoport, Inc. | Prodrugs of GABA analogs, compositions and uses thereof |
| US7420002B2 (en) | 2001-06-11 | 2008-09-02 | Xenoport | Amino acid conjugates providing for sustained systemic concentrations of GABA analogues |
| US6833140B2 (en) | 2001-06-11 | 2004-12-21 | Xenoport, Inc. | Orally administered dosage forms of GABA analog prodrugs having reduced toxicity |
| US7790708B2 (en) | 2001-06-11 | 2010-09-07 | Xenoport, Inc. | Prodrugs of GABA analogs, compositions and uses thereof |
| US9238616B2 (en) | 2001-06-11 | 2016-01-19 | Xenoport, Inc. | Prodrugs of gaba analogs, compositions and uses thereof |
| US6818787B2 (en) | 2001-06-11 | 2004-11-16 | Xenoport, Inc. | Prodrugs of GABA analogs, compositions and uses thereof |
| US7645797B2 (en) | 2001-06-11 | 2010-01-12 | Xenoport, Inc. | Amino acid conjugates providing for sustained systemic concentrations of GABA analogues |
| US7026351B2 (en) | 2002-03-20 | 2006-04-11 | Xenoport, Inc. | Cyclic 1-(acyloxy)-alkyl prodrugs of GABA analogs, compositions and uses thereof |
| US7868041B2 (en) | 2002-03-20 | 2011-01-11 | Xenoport, Inc. | Cyclic 1-(acyloxy)-alkyl prodrugs of GABA analogs, compositions and uses thereof |
| US7569576B2 (en) | 2002-03-20 | 2009-08-04 | Xenoport, Inc. | Cyclic 1-(acyloxy)-alkyl prodrugs of GABA analogs, compositions and uses thereof |
| EP2275401A1 (en) | 2002-06-11 | 2011-01-19 | XenoPort, Inc. | Crystalline 1-{[(a-isobutanoyloxyethoxy)carbonyl]aminomethyl}-1-cyclohexane acetic acid |
| WO2003104184A1 (en) | 2002-06-11 | 2003-12-18 | Xenoport, Inc. | Methods for synthesis of acyloxyalkyl derivatives of gaba analogs |
| US7060727B2 (en) | 2002-12-11 | 2006-06-13 | Xenoport, Inc. | Prodrugs of fused GABA analogs, pharmaceutical compositions and uses thereof |
| WO2004054559A1 (en) * | 2002-12-13 | 2004-07-01 | Warner-Lambert Company Llc | Alpha2delta ligands for different pharmaceutical uses |
| AU2003303038B2 (en) * | 2002-12-13 | 2009-08-06 | Warner-Lambert Company Llc | Alpha2delta ligands for different pharmaceutical uses |
| US7250433B2 (en) | 2003-03-07 | 2007-07-31 | Warner Lambert Company Llc | Tetrazole and oxadiazolone substituted β-amino acid derivatives |
| US7700652B2 (en) | 2003-09-11 | 2010-04-20 | Xenoport, Inc. | Treating urinary incontinence using prodrugs of GABA analogs |
| US8114909B2 (en) | 2003-09-17 | 2012-02-14 | Xenoport, Inc. | Treating or preventing restless legs syndrome using prodrugs of GABA analogs |
| EP2343073A2 (en) | 2003-12-11 | 2011-07-13 | Sepracor Inc. | Combination of a sedative and a neurotransmitter modulator, and methods for improving sleep quality and treating depression |
| US8795725B2 (en) | 2004-11-04 | 2014-08-05 | Xenoport, Inc. | GABA analog prodrug sustained release oral dosage forms |
| US8906412B2 (en) | 2004-11-04 | 2014-12-09 | Xenoport, Inc. | GABA analog prodrug sustained release oral dosage forms |
| US8048917B2 (en) | 2005-04-06 | 2011-11-01 | Xenoport, Inc. | Prodrugs of GABA analogs, compositions and uses thereof |
| DE102007036068A1 (en) | 2007-08-01 | 2009-02-05 | Wacker Chemie Ag | Process for the preparation of alkylmethoxymethyltrimethylsilanylmethylamines |
| US7847117B2 (en) | 2007-08-01 | 2010-12-07 | Wacker Chemie Ag | Process for preparing alkyl(methoxymethyl)trimethylsilanylmethylamines |
| EP2050754A1 (en) | 2007-08-01 | 2009-04-22 | Wacker Chemie AG | Method for manufacturing alkyl-methoxymethyl-trimethylsilanylmethyl amines |
| US8062870B2 (en) | 2008-01-25 | 2011-11-22 | Xenoport, Inc. | Enantiomerically resolving acyloxyalkyl thiocarbonates used in synthesizing acyloxyalkyl carbamate prodrugs |
| US7872046B2 (en) | 2008-01-25 | 2011-01-18 | Xenoport, Inc. | Crystalline form of a (3S)-aminomethyl-5-methyl-hexanoic acid prodrug and methods of use |
| US7868043B2 (en) | 2008-01-25 | 2011-01-11 | Xenoport, Inc. | Mesophasic forms of (3S)-aminomethyl-5-methyl-hexanoic acid prodrugs and methods of use |
| US8258179B2 (en) | 2008-01-25 | 2012-09-04 | Xenoport, Inc. | Crystalline form of a (3S)-aminomethyl-5-methyl-hexanoic acid prodrug and methods of use |
| EP3075722A1 (en) | 2008-10-08 | 2016-10-05 | Xgene Pharmaceutical Inc | Gaba conjugates and methods of use thereof |
| WO2010042759A2 (en) | 2008-10-08 | 2010-04-15 | Kyphia Pharmaceuticals Inc | Gaba conjugates and methods of use thereof |
| WO2014113299A1 (en) | 2013-01-15 | 2014-07-24 | Warsaw Orthopedic, Inc. | Clonidine compounds in a biodegradable fiber |
| WO2014117176A1 (en) | 2013-01-28 | 2014-07-31 | Lopez Hector L | Methods of improving tolerability, pharmacodynamics, and efficacy of b-alanine and use therefor |
| US10995095B2 (en) | 2015-10-16 | 2021-05-04 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carb oxamide and solid state forms thereof |
| US11680069B2 (en) | 2015-10-16 | 2023-06-20 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US9879018B2 (en) | 2015-10-16 | 2018-01-30 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-α]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl and solid state forms thereof |
| US11795175B2 (en) | 2015-10-16 | 2023-10-24 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US9963459B1 (en) | 2015-10-16 | 2018-05-08 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-alpla]pyrrolo[2,3-e]-pyrazin-8-YL)-N-(2,2,2-Trifluoroethyl)pyrrol and solid state forms thereof |
| US10017517B2 (en) | 2015-10-16 | 2018-07-10 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-α]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluorethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11186584B2 (en) | 2015-10-16 | 2021-11-30 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US10202393B2 (en) | 2015-10-16 | 2019-02-12 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-α]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US10202394B2 (en) | 2015-10-16 | 2019-02-12 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US10344036B2 (en) | 2015-10-16 | 2019-07-09 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-#a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-#carboxamide and solid state forms thereof |
| US11780847B1 (en) | 2015-10-16 | 2023-10-10 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1- carboxamide and solid state forms thereof |
| US10519164B2 (en) | 2015-10-16 | 2019-12-31 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3,ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US10550126B2 (en) | 2015-10-16 | 2020-02-04 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-A]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US10597400B2 (en) | 2015-10-16 | 2020-03-24 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carb oxamide and solid state forms thereof |
| US10730883B2 (en) | 2015-10-16 | 2020-08-04 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US10981924B2 (en) | 2015-10-16 | 2021-04-20 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US10981923B2 (en) | 2015-10-16 | 2021-04-20 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[l,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11780848B2 (en) | 2015-10-16 | 2023-10-10 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1- carboxamide and solid state forms thereof |
| US9951080B2 (en) | 2015-10-16 | 2018-04-24 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-alpha]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11787815B1 (en) | 2015-10-16 | 2023-10-17 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11365198B2 (en) | 2015-10-16 | 2022-06-21 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11198697B1 (en) | 2015-10-16 | 2021-12-14 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11512092B2 (en) | 2015-10-16 | 2022-11-29 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11524964B2 (en) | 2015-10-16 | 2022-12-13 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11535624B2 (en) | 2015-10-16 | 2022-12-27 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-α]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11535626B2 (en) | 2015-10-16 | 2022-12-27 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1 carboxamide and solid state forms thereof |
| US11535625B2 (en) | 2015-10-16 | 2022-12-27 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11661425B2 (en) | 2015-10-16 | 2023-05-30 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US9879019B2 (en) | 2015-10-16 | 2018-01-30 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-α]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11718627B2 (en) | 2015-10-16 | 2023-08-08 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11767326B2 (en) | 2015-10-16 | 2023-09-26 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-n-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11773106B2 (en) | 2015-10-16 | 2023-10-03 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]-pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| US11773105B2 (en) | 2015-10-16 | 2023-10-03 | Abbvie Inc. | Processes for the preparation of (3S,4R)-3-ethyl-4-(3H-imidazo[1,2-a]pyrrolo[2,3-e]- pyrazin-8-yl)-N-(2,2,2-trifluoroethyl)pyrrolidine-1-carboxamide and solid state forms thereof |
| KR20170127148A (en) * | 2016-05-11 | 2017-11-21 | 제이에스아이실리콘주식회사 | A Novel Silicone Coupling Agent Having a Tertiary Amine Substituted with a Ether Group and A method for Producing the Same |
| KR101874792B1 (en) | 2016-05-11 | 2018-08-02 | 제이에스아이실리콘주식회사 | A Novel Silicone Coupling Agent Having a Tertiary Amine Substituted with a Ether Group and A method for Producing the Same |
| CN110038576A (en) * | 2019-05-05 | 2019-07-23 | 中国科学院兰州化学物理研究所 | A kind of load type metal catalyst and its preparation method and application |
| CN110038576B (en) * | 2019-05-05 | 2021-09-14 | 中国科学院兰州化学物理研究所 | Supported metal catalyst and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1112253B1 (en) | 2004-10-20 |
| DE69921340T2 (en) | 2005-03-17 |
| JP2002524551A (en) | 2002-08-06 |
| DE69921340D1 (en) | 2004-11-25 |
| ZA200100837B (en) | 2002-01-30 |
| CA2339273C (en) | 2005-10-18 |
| US6245801B1 (en) | 2001-06-12 |
| EP1112253A1 (en) | 2001-07-04 |
| CA2339273A1 (en) | 2000-03-23 |
| NZ510145A (en) | 2003-02-28 |
| ATE280154T1 (en) | 2004-11-15 |
| BR9913701A (en) | 2001-06-05 |
| AU5478799A (en) | 2000-04-03 |
| ES2228087T3 (en) | 2005-04-01 |
| KR20010075064A (en) | 2001-08-09 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| EP1112253B1 (en) | Branched alkyl pyrrolidine-3-carboxylic acids | |
| US6489352B2 (en) | Conformationally constrained compounds as pharmaceutical agents | |
| EP1226110B1 (en) | Bicyclic amino acids as pharmaceutical agents | |
| US7122678B2 (en) | Cyclic amino acids and derivatives thereof useful as pharmaceutical agents | |
| HRP970560A2 (en) | Substituted gamma aminobutyric acids as pharmaceutical agents | |
| EP1180094B1 (en) | Fused polycyclic amino acids as pharmaceutical agents | |
| EP1185524B1 (en) | 3-heteroarylalkyl substituted gaba analogs | |
| US6710190B1 (en) | 3-heteroarylalkyl substituted gaba analogs | |
| MXPA01001044A (en) | Branched alkyl pyrrolidine-3-carboxylic acids | |
| US20050250800A1 (en) | Conformationally constrained compounds as pharmaceutical agents | |
| MXPA00009494A (en) | Conformationally constrained amino acid compounds having affinity for the alpha2delta subunit of a calcium channel |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A1 Designated state(s): AE AL AU BA BB BG BR CA CN CR CU CZ DM EE GD GE HR HU ID IL IN IS JP KP KR LC LK LR LT LV MG MK MN MX NO NZ PL RO SG SI SK SL TR TT UA US UZ VN YU ZA |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A1 Designated state(s): GH GM KE LS MW SD SL SZ UG ZW AM AZ BY KG KZ MD RU TJ TM AT BE CH CY DE DK ES FI FR GB GR IE IT LU MC NL PT SE BF BJ CF CG CI CM GA GN GW ML MR NE SN TD TG |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 09673277 Country of ref document: US |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 54787/99 Country of ref document: AU |
|
| WWE | Wipo information: entry into national phase |
Ref document number: PA/a/2001/001044 Country of ref document: MX |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2001/00837 Country of ref document: ZA Ref document number: 200100837 Country of ref document: ZA |
|
| ENP | Entry into the national phase |
Ref document number: 2339273 Country of ref document: CA Ref country code: CA Ref document number: 2339273 Kind code of ref document: A Format of ref document f/p: F |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 510145 Country of ref document: NZ |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1999941063 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref country code: JP Ref document number: 2000 570151 Kind code of ref document: A Format of ref document f/p: F |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1020017003209 Country of ref document: KR |
|
| WWP | Wipo information: published in national office |
Ref document number: 1999941063 Country of ref document: EP |
|
| WWP | Wipo information: published in national office |
Ref document number: 1020017003209 Country of ref document: KR |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1020017003209 Country of ref document: KR |
|
| WWG | Wipo information: grant in national office |
Ref document number: 1999941063 Country of ref document: EP |