RANDOM PEPTIDES THAT BIND TO GASTRO-INTESTINAL TRACT (GIT) TRANSPORT RECEPTORS AND RELATED METHODS
This application claims priority to U.S. provisional application Serial No. 60/046,595 filed May 15, 1997, which is incorporated by reference herein in its entirety.
1. INTRODUCTION
The present invention relates generally to random peptides capable of specific binding to gastro-intestinal tract (GIT) transport receptors. In particular, this invention relates to peptide sequences and motifs, as well as derivatives thereof, which enhance drug delivery and transport through tissue, such as epithelial cells lining the lumenal side of the gastro-intestinal tract (GIT) . Production of peptides, derivatives and antibodies is also provided. The invention further relates to pharmaceutical compositions, formulations and related methods.
2. BACKGROUND OF THE INVENTION 2.1. Peptide Libraries There have been two different approaches to the construction of random peptide libraries. According to one approach, peptides have been chemically synthesized in vi tro in several formats. Examples of chemically synthesized libraries can be found in Fodor, S., et al . , 1991, Science 251: 767-773; Houghten, R., et al . , 1991, Nature 35 : 84-86; and Lam, K. , et al . , 1991, Nature 354 : 82-84.
A second approach to the construction of random peptide libraries has been to use the M13 phage, and, in particular, protein pIII of M13. The viral capsid protein of M13, protein III (pill) , is responsible for infection of bacteria. Several investigators have determined from mutational analysis that the 406 amino acid long pill capsid
protein has two domains. The C-terminus anchors the protein to the viral coat, while portions of the N-terminus of pill are essential for interaction with the E. coli pillin protein (Crissman, J.W. and Smith, G.P., 1984, Virology 132 : 445- 455) . Although the N-terminus of the pill protein has shown to be necessary for viral infection, the extreme N-terminus of the mature protein does tolerate alterations. In 1985, George Smith published experiments reporting the use of the pill protein of bacteriophage M13 as an experimental system for expressing a heterologous protein on the viral coat surface (Smith, G.P., 1985, Science 228 : 1315-1317). It was later recognized, independently by two groups, that the M13 phage piII gene display system could be a useful one for mapping antibody epitopes (De la Cruz, V., et al . , 1988, J. Biol. Chem. 263 : 4318-4322; Parmley, S.F. and Smith, G.P., 1988, Gene T2 - 305-318).
Parmley, S.F. and Smith, G.P., 1989, Adv. Exp. Med. Biol. 251: 215-218 suggested that short, synthetic DNA segments cloned into the pIII gene might represent a library of epitopes. These authors reasoned that since linear epitopes were often ~6 amino acids in length, it should be possible to use a random recombinant DNA library to express all possible hexapeptides to isolate epitopes that bind to antibodies. Scott, J.K. and Smith, G.P., 1990, Science 249 : 386-390 describe construction and expression of an "epitope library" of hexapeptides on the surface of M13. Cwirla, S.E., et al., 1990, Proc. Natl. Acad. Sci. USA 87: 6378-6382 also described a somewhat similar library of hexapeptides expressed as gene pill fusions of M13 fd phage. PCT Application WO 91/19818 published December 26, 1991 by Dower and Cwirla describes a similar library of pentameric to octameric random amino acid sequences. Devlin et al . , 1990, Science, 249 : 404-406, describes a peptide library of about 15 residues generated using an (NNS) coding scheme for oligonucleotide synthesis in which S is G or C. Christian and colleagues have described a phage display library,
expressing decapeptides (Christian, R.B., et al . , 1992, J. Mol. Biol. 227: 711-718).
Other investigators have used other viral capsid proteins for expression of non-viral DNA on the surface of phage particles. For example, the major capsid protein pVIII was so used by Cesareni, G., 1992, FEBS Lett. 307 : 66-70. Other bacteriophage than M13 have been used to construct peptide libraries. Four and six amino acid sequences corresponding to different segments of the Plasmodium falciparum major surface antigen have been cloned and expressed in the filamentous bacteriophage fd (Greenwood, J., et al., 1991, J. Mol. Biol. 120: 821-827).
Kay et al . , 1993, Gene 128 : 59-65 (Kay) discloses a method of constructing peptide libraries that encode peptides of totally random sequence that are longer than those of any prior conventional libraries. The libraries disclosed in Kay encode totally synthetic random peptides of greater than about 20 amino acids in length. Such libraries can be advantageously screened to identify peptides, polypeptides and/or other proteins having binding specificity for a variety of ligands. (See also U.S. Patent No. 5,498,538 dated March 12, 1996; and PCT Publication No. WO 94/18318 dated August 18, 1994.)
A comprehensive review of various types of peptide libraries can be found in Gallop et al . , 1994, J. Med. Chem. 37:1233-1251.
Screening of peptide libraries has often been done using an antibody as ligand (Parmley and Smith, 1989, Adv. Exp. Med. Biol. 251:215-218; Scott and Smith, 1990, Science 249:386-390) . In many cases, the aim of the screening is to identify peptides from the library that mimic the epitopes to which the antibodies are directed. Thus, given an available antibody, peptide libraries are excellent sources for identifying epitopes or epitope-like molecules of that antibody (Yayon et al . , 1993, Proc. Natl. Acad. Sci. USA 90:10643-10647) .
McCafferty et al . , 1990, Nature 348:552-554 used PCR to amplify immunoglobulin variable (V) region genes and cloned those genes into phage expression vectors. The authors suggested that phage libraries of V, diversity (D) , and joining (J) regions could be screened with antigen. The phage that bound to antigen could then be mutated in the antigen-binding loops of the antibody genes and rescreened. The process could be repeated several times, ultimately giving rise to phage which bind the antigen strongly. Marks et al . , 1991, J. Mol. Biol. 222:581-597 also used PCR to amplify immunoglobulin variable (V) region genes and cloned those genes into phage expression vectors .
Kang et al . , 1991, Proc. Natl. Acad. Sci. USA 88:4363-4366 created a phagemid vector that could be used to express the V and constant (C) regions of the heavy and light chains of an antibody specific for an antigen. The heavy and light chain V-C regions were engineered to combine in the periplasm to produce an antibody-like molecule with a functional antigen binding site. Infection of cells harboring this phagemid with helper phage resulted in the incorporation of the antibody-like molecule on the surface of phage that carried the phagemid DNA. This allowed for identification and enrichment of these phage by screening with the antigen. It was suggested that the enriched phage could be subject to mutation and further rounds of screening, leading to the isolation of antibody-like molecules that were capable of even stronger binding to the antigen.
Hoogenboom et al . , 1991, Nucleic Acids Res. 19:4133-4137 suggested that naive antibody genes might be cloned into phage display libraries. This would be followed by random mutation of the cloned antibody genes to generate high affinity variants.
Bass et al . , 1990, Proteins: Struct. Func . Genet. 8:309-314 fused human growth hormone (hGH) to the carboxy terminus of the gene III protein of phage fd. This fusion protein was built into a phagemid vector. When cells carrying the phagemid were infected with a helper phage,
about 10% of the phage particles produced displayed the fusion protein on their surfaces. These phage particles were enriched by screening with hGH receptor-coated beads. It was suggested that this system could be used to develop mutants of hGH with altered receptor binding characteristics.
Lowman et al . , 1991, Biochemistry 30:10832-10838 used an improved version of the system of Bass et al . described above to select for mutant hGH proteins with exceptionally high affinity for the hGH receptor. The authors randomly mutagenized the hGH-pIII fusion proteins at sites near the vicinity of 12 amino acids of hGH that had previously been identified as being important in receptor binding.
Balass et al . , 1993, Proc. Natl. Acad. Sci. USA 90:10638-10642 used a phage display library to isolate linear peptides that mimicked a conformationally dependent epitope of the nicotinic acetylcholine receptor. This was done by screening the library with a monoclonal antibody specific for the conformationally dependent epitope. The monoclonal antibody used was thought to be specific to the acetylcholine receptor's binding site for its natural ligand, acetylcholine .
2.2. Drug Delivery Systems The common routes of therapeutic drug administration are oral ingestion or parenteral (intravenous, subcutaneous and intramuscular) routes of administration. Intravenous drug administration suffers from numerous limitations, including (i) the risk of adverse effects resulting from rapid accumulation of high concentrations of drug, (ii) repeated injections which can cause patient discomfort; and (iii) the risk of infection at the site of repeated injections. Subcutaneous injection is not generally suitable for delivering large volumes or for irritating substances. Whereas oral administration is generally more convenient, it is limited where the therapeutic agent is not efficiently absorbed by the gastrointestinal tract. To date,
the development of oral formulations for the effective delivery of peptides, proteins and macromolecules has been an elusive target. Poor membrane permeability, enzymatic instability, large molecular size, and hydrophilic properties are four factors that have remained major hurdles for peptide and protein formulations (reviewed by Fix, J.A., 1996, J. Pharmac . Sci. 85:1282-1285). In order to develop an efficacious oral formulation, the peptide must be protected from the enzymatic environment of the gastrointestinal tract (GIT) , presented to the absorptive epithelial barrier in a sufficient concentration to effect transcellular flux (Fix, J.A., 1996, J. Pharmac. Sci. 85:1282-1285), and if possible "smuggled" across the epithelial barrier in an apical to basolateral direction. Site specific drug delivery or drug targeting can be achieved at different levels, including (i) primary targeting to a specific organ, (ii) secondary targeting to a specific cell type within that organ and (iii) tertiary targeting where the drug is delivered to specific intracellular structures { e . g. , the nucleus for genes)
(reviewed in Davis and Jllum, 1994, In: Targeting of Drugs 4, (Eds), Gregoriadis, McCormack and Poste, 183-194). At present there is a considerable amount of ongoing research work in the Drug Delivery Systems (DDS) area, and much of it addresses (i) targeting delivery and (ii) the development of non- invasive ways of getting macromolecules, peptides, proteins, products of the biotechnology industry, etc. into the body (Evers, P., 1995, Developments in Drug Delivery: Technology and Markets, Financial Times Management Report) . It is generally accepted that targeted drug delivery is crucial to the improved treatment of certain diseases, especially cancer, and not surprisingly many of the approaches to targeted drug delivery are focused in the cancer area. Many anticancer drugs are toxic to the body as well as to malignant cells. If a drug, or a delivery system, can be modified so that it "homes in" on the tumor, then by maximizing the drug concentration at the disease site, the
anti-cancer effect can be exploited to the full, while toxicity is greatly reduced. Tumors contain antigens which provoke the body to respond by producing antibodies designed to attach to the antigens and destroy them. Monoclonal antibodies are being used as both delivery vehicles targeted to tumor cells (reviewed by Pietersz, G.A., 1990, Bioconjugate Chem. 1:89-95) and as imaging agents to carry molecules of drug or imaging agent to the tumor surface.
2.3. Transport Pathways
The epithelial cells lining the lumenal side of the GIT are a major barrier to drug delivery following oral administration. However, there are four recognized transport pathways which can be exploited to facilitate drug delivery and transport: the transcellular, paracellular , carrier- mediated, and transcytotic pathways. The ability of a conventional drug, peptide, protein, macromolecule or nano- or microparticulate system to "interact" with one of these transport pathways may result in increased delivery of that drug or particle from the GIT to the underlying circulation. In the case of the receptor-mediated, carrier- mediated or transcytotic transport pathways, some of the uptake signals have been identified. These signals include, inter alia , folic acid, which interacts with the folate receptor, and cobalamin, which interacts with Intrinsic Factor. In addition, leucine- and tyrosine-based peptide sorting motifs or internalization sequences exist, such as YSKV, FPHL, YRGV, YQTI , TEQF, TEVM, TSAF, and YTRF (SEQ ID NOS:203, 204, 205, 206, 207, 208, 209, and 210, respectively) , which facilitate uptake or targeting of proteins using specific membrane receptors or binding sites to identify peptides that bind specifically to the receptor or binding site.
Non-receptor based assays to discover particular ligands have also been used. For instance, a strategy for identifying peptides that alter cellular function by scanning whole cells with phage display libraries is disclosed in Fong
et al . , Drug Development Research 33:64-70 (1994) . However, because whole cells, rather than intact tissue or polarized cell cultures, are used for screening phage display libraries, this procedure does not provide information regarding sequences whose primary function includes affecting transport across polarized cell layers.
Additionally, Stevenson et al . , Pharmaceutical Res. 12(9), S94 (1995) discloses the use of Caco-2 monolayers to screen a synthetic tripeptide combinatorial library for information relating to the permeability of di- and tri- peptides .
A method of identifying a peptide which permits or facilitates the transport of an active agent through human or animal tissues has been developed (see U.S. patent application Serial No. 08/746,411 filed November 8, 1996, which is incorporated by reference herein in its entirety) . Phage from a random phage library is plated onto or brought into contact with a first side, preferably the apical side, of a tissue sample, either in vitro, in vivo or in si tu, or polarized tissue cell culture. The phage which is transported to a second side of the tissue opposite the first side, preferably the basolateral side, is harvested to select transported phages . The transported phages are amplified in a host and this cycle is repeated (using the transported phage from the most recent cycle) to obtain a selected phage library containing phage which can be transported from the first side to the second side.
Discussion or citation of a reference hereinabove shall not be construed as meaning that such reference is prior art to the present invention.
3. SUMMARY OF THE INVENTION
The present invention relates generally to random peptides and peptide motifs capable of specific binding to GIT transport receptors. Such proteins can be identified using any random peptide library, e . g. , a chemically synthesized peptide library or a biologically expressed
peptide library. If a biological peptide expression library is used, the nucleic acid which encodes the peptide which binds to the ligand of choice can be recovered, and then sequenced to determine its nucleotide sequence and hence deduce the amino acid sequence that mediates binding. Alternatively, the amino acid sequence of an appropriate binding domain can be determined by direct determination of the amino acid sequence of a peptide selected from a peptide library containing chemically synthesized peptides. In a less preferred aspect, direct amino acid sequencing of a binding peptide selected from a biological peptide expression library can also be performed.
In particular, this invention relates to proteins ( e . g. , peptides) that are capable of facilitating transport of an active agent through a human or animal gastrointestinal tissue, and derivatives (e.g., fragments) and analogs thereof, and nucleotide sequences coding for said proteins and derivatives.
Preferably, the tissue through which transport is facilitated is of the duodenum, jejunum, ileum, ascending colon, transverse colon, descending colon, or pelvic colon. The tissue is most preferably epithelial cells lining the lumenal side of the GIT.
The proteins of the invention have use in facilitating transport of active agents from the lumenal side of the GIT into the systemic blood system, and/or in targeting active agents to the GIT. Thus, for example, by binding (covalently or noncovalently) a protein of the invention to an orally administered drug, the drug can be targeted to specific receptor sites or transport pathways which are known to operate in the human gastrointestinal tract, thus facilitating its absorption into the systemic system.
The invention also relates to derivatives and analogs of the invention which are functionally active, i.e., they are capable of displaying one or more known functional activities associated with a full-length peptide. Such
functional activities include but are not limited to antigenicity (ability to bind or to compete with GIT transport receptor-binding peptides for binding to an anti- GIT transport receptor antibody) and ability to bind or compete with full-length peptide for binding to a GIT transport receptor.
The invention further relates to fragments of (and derivatives and analogs thereof) GIT transport receptor- binding peptides which comprise one or more motifs of a GIT transport receptor-binding peptide.
Antibodies to GIT transport receptor-binding peptides and GIT transport receptor-binding peptide derivatives and analogs are additionally provided.
Methods of production of the GIT transport receptor-binding peptides, derivatives, fragments and analogs, e . g. , by recombinant means, are also provided.
The present invention also relates to therapeutic methods, pharmaceutical compositions and formulations based on GIT transport receptor-binding peptides. Formulations of the invention include but are not limited to GIT transport receptor-binding peptides or motifs and derivatives (including fragments) thereof; antibodies thereto; and nucleic acids encoding the GIT transport receptor-binding peptides or derivatives associated with an active agent. Preferably, the active agent is a drug or drug-containing nano- or microparticle .
The GIT transport-receptor binding proteins of the invention can also be used to determine levels of the GIT transport receptors in a sample by binding thereto. The GIT transport-receptor binding proteins can also be used to identify molecules that bind thereto, by contacting candidate test molecules under conditions conducive to binding, and detecting any binding that occurs.
4. DESCRIPTION OF THE FIGURES
Figure 1. Figure 1 shows the human PEPT1 predicted amino acid sequence determined from the sequence of the cDNA clone
coding for human PEPT1 (SEQ ID NO: 176) (Liang R. et al. J. Biol. Chem. 270 (12) : 6456-6463 (1995)), including the extracellular domain from amino acid 391 to 573 (Fei et al . , Nature 368:563 (1994)). Figures 2A-2C. Figures 2A-2C show the DNA sequence of the cDNA coding for the human intestinal peptide-associated transporter HPT1 and the corresponding putative amino acid sequence (bases 1 to 3345; Medline : 94204643 ) (SEQ ID NOS: 177 and 178, respectively) . Figures 3A-3B. Figures 3A-3B show the putative Human Sucrase-isomaltase complex (hSI) amino acid sequence determined from the sequence of the cDNA clone coding for human sucrase-isomaltase complex (SEQ ID NO: 179) (Chantret I., et al . , Biochem. J. 2JL5(Pt 3) :915-923 (1992). Figures 4A-4B. Figures 4A-4B show the D2H nucleotide and deduced amino acid sequence for the human D2H transporter (SEQ ID NOS:180 and 181, respectively) (Wells, R.G. et al.,J. Clin. Invest. 9J3: 1959-1963 (1993) . Figures 5A-5C. Figure 5A is a schematic summary of the cloning of the DNA insert present in gene III of the phages selected from the phage display libraries into the expression vector pGex-4T-2. The gene insert in gene III of the phages was amplified by PCR using DNA primers which flank the gene insert and which contained recognition sequences for specific restriction endonucleases at their extreme 5' sides. Alternatively, specific primers which amplify specific regions of the DNA inserts in gene III of the phages, and which contained recognition sequences for specific restriction endonucleases at their extreme 5' sides, were used in PCR amplification experiments. Following amplification of the gene inserts, the amplified PCR fragments were digested with the restriction endonucleases Xhol and Notl. Similarly the plasmid pGex-4T-2, which codes for the reporter protein glutathione S-transferase (GST) , was digested with the restriction endonucleases Sail and Notl. The digested PCR fragments were ligated into the digested plasmid pGex-4T-2 using T4 DNA Ligase and the ligated
products were transformed into competent Escherichia coli , with selection of transformants on agar plates containing selection antibiotic. The selected clones were cultured, the plasmids were recovered and the in-frame sequence of the DNA insert in the plasmids was confirmed by DNA sequencing. The correct clones were subsequently used for expression of the GST-fusion proteins (SEQ ID NO:182); Figure 5B shows the series of full-length P31 (designated P31) (SEQ ID NO: 43) and truncated peptides derived from P31 (clones # 101, 102, 103 and 119), (SEQ ID NOS:183, 184, 185, and 186, respectively) full-length PAX2 (designated PAX2) (SEQ ID NO: 55) and truncated peptides derived from PAX2 (clones # 104, 105, 106) (SEQ ID NOS:170, 187, and 188, respectively) and full-length DCX8 (DCX8) (SEQ ID NO: 23) and series of truncated peptides derived from DCX8 (clones # 107, 108, 109) (SEQ ID NOS: 189, 190, and 191, respectively) that were expressed as fusion proteins to GST. The construction of these GST- fusion proteins is shown in Figure 5A. Figure 5C shows the series of full-length P31 (designated P31) (SEQ ID NO:43) and truncated peptides derived from P31 (clones # 103, 110, 119, 111, and 112) (SEQ ID NOS:185, 192, 193, 194, and 195, respectively) , full-length PAX2 (designated PAX2) (SEQ ID NO: 55) and truncated peptides derived from PAX2 (clones # 106, 113, 114, 115) (SEQ ID NOS:188, 196, 197, and 198, respectively) and full-length SNilO (designated SNilO) (SEQ ID NO: 4) and series of truncated peptides derived from SNilO (clones # 116, 117, 118) (SEQ ID NOS:199, 200, and 201, respectively) that were expressed as fusion proteins to GST. The construction of these GST- fusion proteins is shown in Figure 5A. (Underlining and bold in Figs. 5A-5C are for orientation of the sequences.)
Figures 6A-6B. Figures 6A-6B show the binding of GST and GST- fusion proteins to recombinant hSI and to fixed C2BBel fixed cells as detected by ELISA assays. Figure 6A shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST-fusion proteins from SNilO (designated GST-SNilO) and SNi34 (designated GST-SNi34) to
recombinant hSI . Figure 6B shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST-fusion proteins from SNilO (designated GST-SNilO) and SNi34 (designated GST-SNi34) to fixed C2BBel cells. Figures 7A-7M. Figures 7A-7M show the binding of GST peptide and truncated fusion proteins to fixed Caco-2 cells, fixed C2BBel cells, and fixed A431 cells or to recombinant GIT transport receptors D2H, HPT1 , hPEPTl or to BSA using increasing concentrations (expressed as μg/ml on the X-axis) of the control GST protein and the GST- fusion proteins, as detected by ELISA assays. Figure 7A shows the binding of the control protein GST, which does not contain a fusion peptide, and the series of GST-fusion proteins from P31 including the fusion to full-length P31 peptide (designated P31) (SEQ ID NO:43) and clone # 101 (designated P31,101), clone # 102 (designated P31, 102) and clone # 103 (designated P31,103). Figure 7B shows the binding of the control protein GST, which does not contain a fusion peptide, and the series of GST- fusion proteins from PAX2 including the fusion to full-length PAX2 peptide (designated PAX2) and clone # 104 (designated PAX2,104), clone # 105 (designated PAX2 , 105) and clone # 106 (designated PAX2,106) (SEQ ID NOS:55, 170, 187, and 188, respectively) . Figure 7C shows the binding of the control protein GST, which does not contain a fusion peptide, and the series of GST- fusion proteins from DCX8 including the fusion to full-length DCX8 peptide (designated DCX8) and clone # 107 (designated DCX8,107), clone # 108 (designated DCX8 , 108) and clone # 109 (designated DCX8,109) (SEQ ID NOS: 23, 189, 190, and 191, respectively) . Figure 7D shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST- fusion proteins from DCX8 (designated GST-DCX8) and DCX11 (designated GST-DCX11) to recombinant D2H. Figure 7E shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST-fusion proteins from DCX8 (designated GST-DCX8) and DCX11 (designated GST-
DCX11) to fixed C2BBel cells. Figure 7F shows the binding of the control protein GST, which does not contain a fusion
peptide, and the GST-fusion proteins from P31 (designated GST-P31) and 5PAX5 (designated GST-5PAX5) to recombinant hPEPTl . Figure 7G shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST- fusion proteins from P31 (designated GST-P31) and 5PAX5 (designated GST-5PAX5) to fixed C2BBel cells. Figure 7H shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST- fusion proteins from HAX42 (designated GST-HAX42) and PAX2 (designated GST-PAX2) to recombinant HPT1. Figure 71 shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST- fusion proteins from HAX42 (designated GST-HAX42) and PAX2 (designated GST-PAX2) to fixed C2BBel cells. Figure 7J shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST- fusion proteins from P31 (designated GST-P31) and truncated derivatives clone # 101 (designated GST-P31-101) , clone # 102 (designated GST- P31-102) , clone # 103 (designated GST-P31-103) to either recombinant hPEPTl or to BSA.' Figure 7K shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST-fusion proteins from P31 (designated GST-P31) and truncated derivatives clone # 101 (designated GST-P31-101) , clone # 102 (designated GST-P31-102) , clone # 103 (designated GST-P31-103) to either fixed C2BBel cells or to fixed A431 cells. Figure 7L shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST-fusion proteins from PAX2 (designated GST-PAX2) and truncated derivatives clone # 104 (designated GST-PAX2- 104) , clone # 105 (designated GST-PAX2-105) , clone # 106 (designated GST-PAX2-106) to either recombinant hPEPTl or to BSA. Figure 7M shows the binding of the control protein GST, which does not contain a fusion peptide, and the GST- fusion proteins from PAX2 (designated GST-PAX2) and truncated derivatives clone # 106 (designated GST-PAX2-106) to either fixed Caco-2 cells or to fixed A431 cells.
Figures 8A-8D. Figure 8 shows the transport of GST or GST- peptide fusion derivatives across polarized Caco-2 cells in
an apical to basolateral direction as a function of time (1-4 hours) as detected by ELISA assays. Figure 8A shows the transport of either GST, the GST fusion to full-length P31 peptide (designated P31) (SEQ ID NO: 43) and the GST clone derivative clone # 103 (designated P31.103) across polarized Caco-2 cells in an apical to basolateral as a function of time (in hours) following initial administration of the proteins to the apical medium of polarized Caco-2 cells. The line designated No Protein corresponds to control assays in which buffer control was applied to the apical medium of polarized Caco-2 cells followed by sampling of the basolateral medium as a function of time (hours) and assay for GST by the ELISA assay. Figure 8B shows the transport of either GST, the GST fusion to full-length PAX2 peptide (designated PAX2) and the GST clone derivative clone # 106 (designated PAX2.106) across polarized Caco-2 cells in an apical to basolateral as a function of time (in hours) following initial administration of the proteins to the apical medium of polarized Caco-2 cells. The line designated No Protein corresponds to control assays in which buffer control was applied to the apical medium of polarized Caco-2 cells followed by sampling of the basolateral medium as a function of time (hours) and assay for GST by the ELISA assay. Figure 8C shows the transport of either GST, the GST fusion to full-length DCX8 peptide (designated DCX8), and the GST clone derivatives clone # 107 (designated DCX8.107) and clone # 109 (designated DCX8.109) across polarized Caco-2 cells in an apical to basolateral as a function of time (in hours) following initial administration of the proteins to the apical medium of polarized Caco-2 cells. The line designated No Protein corresponds to control assays in which buffer control was applied to the apical medium of polarized Caco-2 cells followed by sampling of the basolateral medium as a function of time (hours) and assay for GST by the ELISA assay. Figure 8D shows the amount of the GST and GST- fusion proteins (GST fusions to P31, P31-103, PAX2 , PAX2.106, DCX8 , DCX8-107, DCX8-109) , used in the experiments shown in panels
A-C above, in the apical medium of the polarized Caco-2 cells as detected by ELISA assay.
Figures 9A-9B. Figures 9A-9B show the inhibition of GST-P31 binding to C2BBel fixed cells with varying concentration of competitors while holding the concentration of GST-P31 constant at 0.015 μM; the peptide competitors are ZElan024 which is the dansylated peptide version of P31 (SEQ ID NO: 43) and ZElan044, ZElan049 and ZElan050 which are truncated, dansylated pieces of P31 (SEQ ID NO:43) . Data is presented as O.D. versus peptide concentration (Figure 9A) and as percent inhibition of GST-P31 binding versus peptide concentration (Figure 9B) .
Figures 10A-10C. Figures 10A-10C present a compilation of the results of competition ELISA studies of GST-P31, GST- PAX2, GST-SNilO and GST-HAX42 versus listed dansylated peptides on fixed C2BBel cells ("Z" denotes e -amino dansyl lysine) . The pi of the dansylated peptides is also included. Estimated IC50 values are in μM and where present, IC50 ranges refer to results from multiple assays. If the IC50 value could not be determined, a ">" or "<" symbol is used. The GST/C2BBel column shows GST protein binding to fixed C2BBel cells .
Figures 11A-11B. Figure 11A shows the transport of GST or GST-peptide fusion derivatives across polarized Caco-2 cells in an apical to basolateral direction at 0, 0.5, 2 and 4 hours as detected by ELISA assays and described elsewhere in the text in full detail. The proteins used in the assay included GST, GST-P31 fusion, GST-5PAX5 fusion, GST-DCX8 fusion, GST-DCX11 fusion, GST-PAX2 fusion, GST-HAX42 fusion, GST-SNi34 fusion and GST-SNilO fusion. The column designated No protein refers to control experiments in which buffer was applied to the apical medium of the cells and ELISA assay was performed on the corresponding basolateral medium of these cells at 0, 0.5, 2 and 4 hours post buffer addition. Figure 11B shows the internalization of GST or GST-peptide fusion derivatives within polarized Caco-2 cells following administration of the GST or GST-fusion protein derivatives
to the apical medium of polarized Caco-2 cells and subsequent recovery of the cells from the transwells and detection of the GST or GST fusions within the recovered cell lysates as detected by ELISA assays and as described elsewhere in the text in full detail . The proteins used in the assay included GST, GST-P31 fusion, GST-5PAX5 fusion, GST-DCX8 fusion, GST- DCX11 fusion, GST-PAX2 fusion, GST-HAX42 fusion, GST-SNi34 fusion and GST-SNilO fusion. The column designated No protein refers to control experiments in which buffer was applied to the apical medium of the cells and ELISA assay was performed on the corresponding cell lysates of these cells at the end of the experiment .
Figure 12. Figure 12 shows the binding of GST and GST- fusion proteins to fixed Caco-2 cells, and the corresponding proteins following digestion with the protease Thrombin which cleaves at a recognition site between the GST portion and the fused peptide portion of the GST- fusion protein. The symbol "-" refers to proteins which were not digested with thrombin and the symbol "+" refers to proteins which were digested with thrombin prior to use in the binding assay. The binding of the proteins to the fixed Caco-2 cells was detected by ELISA assays.
Figures 13A-13B. Figures 13A-13B show binding of peptide- coated nanoparticles to fixed Caco-2 cells. Figures 14A-14B. Figures 14A-14B show the binding of (A) dansylated peptide SNilO to the purified hSI receptor and BSA and (B) dansylated peptides and peptide-loaded insulin- containing PLGA particles to fixed C2BBel cells. Figure 14B depicts binding of dansylated peptides corresponding to P31 (SEQ ID NO:43), PAX2 , HAX42, and SNilO to fixed C2BBel cells, as well as the insulin-containing PLGA particles adsorbed with each of these peptides. Data is presented with background subtracted. Figures 15A-15B. Figure 15 shows the binding of peptide- coated particles to A) S100 and B) P100 fractions harvested from Caco-2 cells. The dilution series 1:2 - 1:64 represents particle concentrations in the range 0.0325-0.5 μg/well .
Data is presented with background subtracted. The particles are identified as follows: 939, no peptide; 1635, scrambled PAX2; 1726, P31 D-Arg 16-mer (ZElan053); 1756, HAX42; 1757, PAX2; 1758, HAX42/PAX2. Figures 16A-16B. Figure 16 shows the binding of dansylated peptides to P100 fractions harvested from Caco-2 cells. Peptides were assayed in the range 0.0032-2.5 μg/well . Data is presented with background subtracted. A) HAX42, P31 D-form (ZElan 053) and scrambled PAX2 ; B) PAX2 , HAX42 and scrambled PAX2.
Figures 17A-17B. Figures 17A and 17B show (A) the systemic blood glucose and (B) insulin levels following intestinal administration of control (PBS) ; insulin solution; insulin particles; all 8 peptides mix particles and study group peptide-particles according to this invention (lOOiu insulin loading) .
Figures 18A-18B. Figures 18A and 18B show the (A) systemic blood glucose and (B) insulin levels following intestinal administration of control (PBS) ; insulin solution; insulin particles and study group peptide-particles according to this invention (300iu insulin loading) .
Figure 19. Figure 19 shows the enhanced plasma levels of leuprolide upon administration of P31 (SEQ ID NO: 43) and PAX2 coated nanoparticles loaded with leuprolide relative to subcutaneous injection. Group 1 was administered leuprolide acetate (12.5 μg) subcutaneously. Group 2 was administered intraduodenally uncoated leuprolide acetate particles (600 μg, 1.5 ml) . Group 3 was intraduodenally administered leuprolide acetate particles coated with PAX2 (600 μg; 1.5 ml) . Group 4 was administered intraduodenally leuprolide acetate particles coated with P31 (SEQ ID NO:43) (600 μg, 1.5 ml) .
Figure 20. Figure 20 lists P31 (SEQ ID NO: 43) known protein homologies . Figures 21A-21C. Figures 21A-21C list DCX8 known protein homologies . Figure 22. Figure 22 lists DAB10 known protein homologies.
Figure 23. Figure 23 shows the DNA sequence (SEQ ID NO-211) and the corresponding amino acid sequence (SEQ ID NO: 212) for glutathione S-transferase (Smith and Johnson, 1988, Gene 7:31-40) .
5. DETAILED DESCRIPTION OF THE INVENTION
The present invention relates to proteins ( e . g. , peptides) that bind to GIT transport receptors and nucleic acids that encode such proteins. The invention further relates to fragments and other derivatives of such proteins. Nucleic acids encoding such fragments or derivatives are also within the scope of the invention. The invention further relates to fragments (and derivatives and analogs thereof) of GIT transport receptor-binding peptides which comprise one or more domains of the GIT transport receptor-binding peptides. The invention also relates to derivatives of GIT transport receptor-binding proteins and analogs of the invention which are functionally active, i . e . , they are capable of displaying one or more known functional activities associated with a full-length GIT transport receptor-binding peptide. Such functional activities include but are not limited to ability to bind to a GIT transport receptor, antigenicity [ability to bind (or compete with peptides for binding) to an anti-GIT transport receptor-binding peptide antibody] , immunogenicity (ability to generate antibody which binds to GIT transport receptor-binding peptide), etc.
Production of the foregoing proteins and derivatives, by, e.gr., recombinant methods, is also provided. Antibodies to GIT transport receptor-binding proteins, derivatives and analogs, are additionally provided. The present invention also relates to therapeutic and diagnostic methods and compositions based on GIT transport receptor-binding proteins and nucleic acids.
The invention is illustrated by way of examples infra .
For clarity of disclosure, and not by way of limitation, the detailed description of the invention is divided into the subsections which follow.
5.1. GIT Transport Receptor-Binding Peptides, Derivatives and Analogs
The invention relates to peptides that bind GIT transport receptors and derivatives (including but not limited to fragments) and analogs thereof. In specific embodiments, of the present invention, such peptides that 0 bind to GIT transport receptor include but are not limited to those containing as primary amino acid sequences, all or part of the amino acid sequences substantially as depicted in
Table 7 (SEQ ID NOS: 1-55). Nucleic acids encoding such peptides, derivatives and peptide analogs are also provided. 5
In one embodiment, the GIT transport receptor-binding peptides are encoded by the nucleic acids having the nucleotide sequences set forth in Table 8 infra (SEQ ID
NOS: 56-109) . Proteins whose amino acid sequence comprise, or alternatively, consist of SEQ ID NOS: 1-55 or a portion thereof that mediates binding to a GIT transport receptor are provided.
The production and use of derivatives and analogs related to GIT transport receptor-binding peptides are within the scope of the present invention. In a specific 25 embodiment, the derivative or analog is functionally active, i.e., capable of exhibiting one or more functional activities associated with a full-length GIT transport receptor-binding peptide. For example, such derivatives or analogs which have the desired immunogenicity or antigenicity can be used, in immunoassays, for immunization, etc. A specific embodiment relates to a GIT transport receptor-binding peptide fragment that can be bound by an anti-GIT transport receptor-binding peptide antibody. In a preferred aspect, the derivatives or ,_ analogs have the ability to bind to a GIT transport receptor. Derivatives or analogs of GIT transport receptor-binding peptides can be tested for the desired activity by procedures
known in the art, including binding to a GIT transport receptor domain or to Caco-2 cells, in vi tro, or to intestinal tissue, in vivo . (See the Examples infra . )
In particular, derivatives can be made by altering GIT transport receptor-binding peptide sequences by substitutions, additions or deletions that provide for functionally equivalent molecules. Due to the degeneracy of nucleotide coding sequences, other nucleotide sequences which encode substantially the same amino acid sequence may be used in the practice of the present invention. These include but are not limited to nucleotide sequences which are altered by the substitution of different codons that encode a functionally equivalent amino acid residue within the sequence, thus producing a silent change. Likewise, the GIT transport receptor-binding peptide derivatives of the invention include, but are not limited to, those containing, as a primary amino acid sequence, all or part of the amino acid sequence of a GIT transport receptor-binding peptide including altered sequences in which functionally equivalent amino acid residues are substituted for residues within the sequence resulting in a silent change. For example, one or more amino acid residues within the sequence can be substituted by another amino acid of a similar polarity which acts as a functional equivalent, resulting in a silent alteration. Substitutes for an amino acid within the sequence may be selected from other members of the class to which the amino acid belongs. For example, the nonpolar (hydrophobic) amino acids include alanine, leucine, isoleucine, valine, proline, phenylalanine, tryptophan and methionine . The polar neutral amino acids include glycine, serine, threonine, cysteine, tyrosine, asparagine, and glutamine. The positively charged (basic) amino acids include arginine, lysine and histidine. The negatively charged (acidic) amino acids include aspartic acid and glutamic acid.
In a specific embodiment of the invention, proteins consisting of or, alternatively, comprising all or a fragment
of a GIT transport receptor-binding peptide consisting of at least 5, 10, 15, 20, 25, 30 or 35 (contiguous) amino acids of the full-length GIT transport receptor-binding peptide are provided. In a specific embodiment, such proteins are not more than 20, 30, 40, 50, or 75 amino acids in length. Derivatives or analogs of GIT transport receptor-binding peptides include but are not limited to those molecules comprising regions that are substantially homologous to GIT transport receptor-binding peptides or fragments thereof ( e . g. , at least 50%, 60%, 70%, 80% or 90% identity) (e.g., over an identical size sequence or when compared to an aligned sequence in which the alignment is done by a computer homology program known in the art) or whose encoding nucleic acid is capable of hybridizing to a coding GIT transport receptor-binding peptide sequence, under stringent, moderately stringent, or nonstringent conditions.
In a specific embodiment, the GIT transport receptor-binding derivatives of the invention are not known proteins with homology to the GIT transport receptor-binding peptides of the invention or portions thereof.
The GIT transport receptor-binding peptide derivatives and analogs of the invention can be produced by various methods known in the art. The manipulations which result in their production can occur at the gene or protein level. For example, the cloned GIT transport receptor- binding peptide gene sequence can be modified by any of numerous strategies known in the art (Maniatis, T., 1990, Molecular Cloning, A Laboratory Manual, 2d ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, New York) . The sequence can be cleaved at appropriate sites with restriction endonuclease (s) , followed by further enzymatic modification if desired, isolated, and ligated in vi tro . In the production of the gene encoding a derivative or analog of GIT transport receptor-binding peptides, care should be taken to ensure that the modified gene remains within the same translational reading frame uninterrupted by translational
stop signals, in the gene region where the desired GIT transport receptor-binding peptides activity is encoded.
Additionally, nucleic acid sequences encoding the GIT transport receptor-binding peptides can be mutated in vitro or in vivo, to create and/or destroy translation, initiation, and/or termination sequences, or to create variations in coding regions and/or form new restriction endonuclease sites or destroy preexisting ones, to facilitate further in vi tro modification. Any technique for mutagenesis known in the art can be used, including but not limited to, chemical mutagenesis, in vi tro site-directed mutagenesis (Hutchinson, C, et al . , 1978, J. Biol. Chem 253:6551), use of TAB® linkers (Pharmacia) , use of PCR primers containing mutation (s) for use in amplification, etc. Manipulations of GIT transport receptor-binding peptide sequences may also be made at the protein level . Included within the scope of the invention are GIT transport receptor-binding peptide fragments or other derivatives or analogs which are differentially modified during or after translation or chemical synthesis, e.g., by glycosylation, acetylation, phosphorylation, amidation, derivatization by known protecting/blocking groups, proteolytic cleavage, linkage to an antibody molecule or other cellular ligand, etc. Any of numerous chemical modifications may be carried out by known techniques, including but not limited to specific chemical cleavage by cyanogen bromide, trypsin, chyτnotrypsin, papain, V8 protease, NaBH4; acetylation, formylation, oxidation, reduction; metabolic synthesis in the presence of tunicamycin; etc. In a specific embodiment, the amino- and/or carboxy-termini are modified.
In addition, GIT transport receptor-binding peptides and analogs and derivatives thereof can be chemically synthesized. For example, a peptide corresponding to all or a portion of a GIT transport receptor-binding peptide which comprises the desired domain or which mediates the desired activity in vi tro, can be synthesized by use of a peptide synthesizer. Furthermore, if desired, nonclassical
amino acids or chemical amino acid analogs can be introduced as a substitution or addition into the GIT transport receptor-binding peptide sequence. Non-classical amino acids include but are not limited to the D-isomers of the common amino acids, α-amino isobutyric acid, 4-aminobutyric acid, Abu, 2 -amino butyric acid, γ-Abu, e-Ahx, 6 -amino hexanoic acid, Aib, 2-amino isobutyric acid, 3-amino propionic acid, ornithine, norleucine, norvaline, hydroxyproline, sarcosine, citrulline, cysteic acid, t-butylglycine, t-butylalanine, phenylglycine, cyclohexylalanine, -alanine, fluoro-amino acids, designer amino acids such as -methyl amino acids, Cc.- methyl amino acids, Nα-methyl amino acids, and amino acid analogs in general. Furthermore, the amino acid can be D (dextrorotary) or L (levorotary) . In a specific embodiment, the GIT transport receptor-binding peptide derivative is a chimeric, or fusion, peptide comprising a GIT transport receptor-binding peptide or fragment thereof (preferably consisting of at least a domain or motif of the GIT transport receptor-binding peptide, or at least 6, 10, 15, 20, 25, 30 or all amino acids of the GIT transport receptor-binding peptides or a binding portion thereof) joined at its amino- or carboxy-terminus via a peptide bond to an amino acid sequence of a different peptide. In one embodiment, such a chimeric peptide is produced by recombinant expression of a nucleic acid encoding the protein (comprising a transport receptor-coding sequence joined in-frame to a coding sequence for a different protein) . Such a chimeric product can be made by ligating the appropriate nucleic acid sequences encoding the desired amino acid sequences to each other by methods known in the art, in the proper coding frame, and expressing the chimeric product by methods commonly known in the art. Alternatively, such a chimeric product may be made by protein synthetic techniques, e.g., by use of a peptide synthesizer. Chimeric genes comprising portions of GIT transport receptor fused to any heterologous protein-encoding sequences may be constructed. A specific embodiment relates to a chimeric
protein comprising a fragment of GIT transport receptor- binding peptides of at least six amino acids.
In another specific embodiment, the GIT transport receptor-binding peptide derivative is a molecule comprising 5 a region of homology with a GIT transport receptor-binding peptide. By way of example, in various embodiments, a first protein region can be considered "homologous" to a second protein region when the amino acid sequence of the first region is at least 30%, 40%, 50%, 60%, 70%, 75%, 80%, 90%, or
10 95% identical, when compared to any sequence in the second region of an equal number of amino acids as the number contained in the first region or when compared to an aligned sequence of the second region that has been aligned by a computer homology program known in the art. For example, a
15 molecule can comprise one or more regions homologous to a GIT transport receptor-binding peptide domain (see infra) or a portion thereof.
The GIT transport receptor-binding proteins and derivatives thereof of the invention can be assayed for
20 binding activity by suitable in vivo or in vi tro assays, e.g., as described in the examples infra and/or as will be known to the skilled artisan.
Other specific embodiments of derivatives and analogs are described in the subsection below and examples
25 sections infra .
5.2. Motifs/Derivatives of GIT Transport Receptor-Binding Peptides Containing One or More Domains of The Protein
In a specific embodiment, the invention relates to
30 GIT transport receptor-binding peptide derivatives and analogs, in particular GIT transport receptor-binding peptide fragments and derivatives of such fragments, that comprise, or alternatively consist of, one or more domains of a GIT transport receptor-binding peptide. In particular, examples
35 of such domains are identified in the examples infra .
5.3. Synthesis of Peptides
The peptides and derivatives of the present invention may be chemically synthesized or synthesized using recombinant DNA techniques .
5.3.1. Procedure For Solid Phase Synthesis Peptides may be prepared chemically by methods that are known in the art. For example, in brief, solid phase peptide synthesis consists of coupling the carboxyl group of the C-terminal amino acid to a resin and successively adding N-alpha protected amino acids. The protecting groups may be any known in the art . Before each new amino acid is added to the growing chain, the protecting group of the previous amino acid added to the chain is removed. The coupling of amino acids to appropriate resins is described by Rivier et al . , U.S. Patent No. 4,244,946. Such solid phase syntheses have been described, for example, by Merrifield, 1964, J. Am. Chem. Soc. 85:2149; Vale et al . , 1981, Science 213:1394-1397; Marki et al . , 1981, J. Am. Chem. Soc. 103:3178 and in U.S. Patent Nos . 4,305,872 and 4,316,891. In a preferred aspect, an automated peptide synthesizer is employed.
By way of example but not limitation, peptides can be synthesized on an Applied Biosystems Inc. ("ABI") model 431A automated peptide synthesizer using the "Fastmoc" synthesis protocol supplied by ABI, which uses
2- (lH-Benzotriazol-1-yl) -1,1,3,3, -tetramethyluronium hexafluorophosphate ("HBTU") (R. Knorr et al . , 1989, Tet . Lett., 30:1927) as coupling agent. Syntheses can be carried out on 0.25 mmol of commercially available 4- (2' , 4 ' -dimethoxyphenyl- (9-fluorenyl- methoxycarbonyl) -aminomethyl) -phenoxy polystyrene resin ("Rink resin" from Advanced ChemTech) (H. Rink, 1987, Tet. Lett. 28:3787). Fmoc amino acids (1 mmol) are coupled according to the Fastmoc protocol. The following side chain protected Fmoc amino acid derivatives are used: FmocArg(Pmc)OH; FmocAsn (Mbh) OH; FmocAsp ^Bu) OH; FmocCys (Acm) OH; FmocGlu ^Bu) OH; FmocGln (Mbh) OH; FmocHis (Tr) OH,•
FmocLys (Boc)OH; FmocSer (fcBu) OH; FmocThr (fcBu) OH; FmocTyr (fcBu) OH. [Abbreviations: Acm, acetamidomethyl ; Boc, tert-butoxycarbonyl ; "Bu, tert-butyl; Fmoc,
9-fluorenylmethoxycarbonyl ; Mbh, 4 , 4 ' -dimethoxybenzhydryl ; 5 Pmc, 2 , 2 , 5 , 7 , 8-pentamethylchroman-6-sulfonyl ; Tr, trityl] .
Synthesis is carried out using N-methylpyrrolidone (NMP) as solvent, with HBTU dissolved in
N, N-dimethylformamide (DMF) . Deprotection of the Fmoc group is effected using approximately 20% piperidine in NMP. At 0 the end of each synthesis the amount of peptide present is assayed by ultraviolet spectroscopy . A sample of dry peptide resin (about 3-10 mg) is weighed, then 20% piperidine in DMA (10 ml) is added. After 30 min sonication, the UV (ultraviolet) absorbance of the dibenzofulvene-piperidine 5 adduct (formed by cleavage of the N-terminal Fmoc group) is recorded at 301 nm. Peptide substitution (in mmol g"1) can be calculated according to the equation:
A x v substitution = x 1000
7800 x w 0 where A is the absorbance at 301 nm, v is the volume of 20% piperidine in DMA (in ml) , 7800 is the extinction coefficient
(in mol"1dm3cm"1) of the dibenzofulvene-piperidine adduct, and w is the weight of the peptide-resin sample (in mg) .
Finally, the N-terminal Fmoc group is cleaved using
25
20% piperidine in DMA, then acetylated using acetic anhydride and pyridine in DMA. The peptide resin is thoroughly washed with DMA, CH2C12 and finally diethyl ether.
5.3.2. Cleavage And Deprotection
30
By way of example but not limitation, cleavage and deprotection can be carried out as follows: The air-dried peptide resin is treated with ethylmethyl-sulfide (EtSMe) , ethanedithiol (EDT) , and thioanisole (PhSMe) for __ approximately 20 min. prior to addition of 95% aqueous trifluoracetic acid (TFA) . A total volume of approximately 50 ml of these reagents per gram of peptide-resin is used.
The following ratio is used: TFA:EtSMe :EDT : PhSMe (10:0.5:0.5:0.5) . The mixture is stirred for 3 h at room temperature under an atmosphere of N2. The mixture is filtered and the resin washed with TFA (2 x 3 ml) . The 5 combined filtrate is evaporated in vacuo, and anhydrous diethyl ether added to the yellow/orange residue. The resulting white precipitate is isolated by filtration. See King et al . , 1990, Int. J. Peptide Protein Res. 36:255-266 regarding various cleavage methods. 10
5.3.3. Purification of the Peptides Purification of the synthesized peptides can be carried out by standard methods including chromatography (e.g., ion exchange, affinity, and sizing column 15 chromatography, high performance liquid chromatography
(HPLC) ) , centrifugation, differential solubility, or by any other standard technique.
5.3.4. Biological Peptide Libraries
20 Biological peptide libraries can be used to express and identify peptides that bind to GIT transport receptors. According to this second approach, involving recombinant DNA techniques, peptides can, by way of example, be expressed in biological systems as either soluble fusion proteins or viral
25 capsid proteins.
5.3.4.1. Methods To Identify Binders: Construction Of Libraries
In a specific embodiment, the peptides of the
_n invention that specifically bind to GIT transport receptors are identified by screening a random peptide library by contacting the library with a ligand selected from among
HPT1, hPEPTl, D2H, or hSI (or a molecule consisting essentially of an extracellular domain thereof or fragment of
__ the domain) to identify members of the library that specifically bind to the ligand.
In a particular embodiment, a process to identify the peptides of the present method utilizes a library of recombinant vectors constructed by methods well known in the art and comprises screening a library of recombinant vectors 5 expressing inserted synthetic oligonucleotide sequences encoding extracellular GIT transport receptor domains, for example, attached to an accessible surface structural protein of a vector to isolate those members producing peptides that bind to HPT1, hPEPTl, D2H, or hSI . The nucleic acid sequence 0 of the inserted synthetic oligonucleotides of the isolated vector is determined and the amino acid sequence encoded can be deduced to identify a binding domain that binds the ligand of choice (e.g., HPT1 , hPEPTl, D2H, or hSI) .
The present invention encompasses a method for
15 identifying a peptide which binds to a ligand selected from among HPT1, hPEPTl, D2H, or hSI comprising: screening a library of random peptides with the ligand (or an extracellular domain or fragment thereof) under conditions conducive to ligand binding and isolating the peptide which
20 binds to the ligand. Additionally, the methods of the invention further comprise determining the nucleotide sequence encoding the binding domain of the peptide identified to deduce the amino acid sequence of the binding domain.
25
5.3.4.2. Preparation of Extracellular Domain Ligand
In a specific embodiment, molecules consisting essentially of an extracellular domain of the desired GIT
_n transport receptor or a fragment of an extracellular domain are used to screen a random peptide library for binding thereto. Preferably, a nucleic acid encoding the extracellular domain is cloned and recombinantly expressed, and the domain is then purified for use. The GIT transport
„ receptor is preferably selected from among HPT1, hPEPTl, D2H, or hSI.
5.3.4.3. Methods to Identify Binders: Screening Libraries
Once a suitable random peptide library has been constructed (or otherwise obtained) , the library is screened to identify peptides having binding affinity for the GIT transport receptor, e.g., HPTl, hPEPTl, D2H, or hSI . In a preferred aspect, the library is a TSAR library (see U.S. Patent No. 5,498,538 dated March 12, 1996 and PCT Publication WO 94/18318 dated August 18, 1994, both of which are incorporated by reference herein in their entireties) . 0 Screening the libraries can be accomplished by any of a variety of methods known to those of skill in the art. See, e.g., the following references, which disclose screening of peptide libraries: Parmley and Smith, 1989, Adv. Exp. Med. Biol. 251: 215-218; Scott and Smith, 1990, Science 249 : 386- 5 390; Fowlkes et al . , 1992; BioTechniques J : 422-427; Oldenburg et al . , 1992, Proc. Natl. Acad. Sci. USA J39.: 5393- 5397; Yu et al . , 1994, Cell 76.: 933-945; Staudt et al . , 1988, Science 241: 577-580; Bock et al . , 1992, Nature 355 : 564-566; Tuerk et al . , 1992, Proc. Natl. Acad. Sci. USA 89 : 6988-6992; 0 Ellington et al . , 1992, Nature 355 : 850-852; U.S. Patent No. 5,096,815, U.S. Patent No. 5,223,409, and U.S. Patent No. 5,198,346, all to Ladner et al . ; and Rebar and Pabo, 1993, Science 263 : 671-673. See also PCT publication WO 94/18318, dated August 18, 1994.
25
One of ordinary skill in the art will recognize that, with suitable modifications, the screening methods described below would be suitable for a wide variety of biological expression libraries.
Once a library has been constructed or otherwise
30 obtained, the library is screened to identify binding molecules having specific binding affinity for a ligand for a GIT transport receptor preferably selected from among HPTl, hPEPTl, D2H, or hSI . -j. Screening the libraries can be accomplished by any of a variety of methods known to those of skill in the art. Exemplary screening methods are described in Fowlkes et al . ,
1992, BioTechniques, 11:422-427 and include contacting the vectors with an immobilized target ligand and harvesting those vectors that bind to said ligand. Such useful screening methods, are designated "panning" methods. In panning methods useful to screen the present libraries, the target ligand can be immobilized on plates, beads (such as magnetic beads), sepharose, beads used in columns, etc. If desired, the immobilized target ligand can be "tagged", e.g., using labels such as biotin, fluoroscein isothiocyanate, rhodamine, etc. e.g. for FACS sorting. Panning is also disclosed in Parmley, S.F. and Smith, G.P., 1988, Gene 73 : 305-318.
In a particular embodiment of the invention, the library can be screened with a recombinant receptor domain. In another embodiment, the library can be screened successively with receptor domains and then on CaCO-2 cells.
For screening of the peptide libraries in vi tro, the solvent requirements involved in screening are not limited to aqueous solvents; thus, nonphysiological binding interactions and conditions different from those found in vivo can be exploited.
Screening a library can be achieved using a method comprising a first "enrichment" step and a second filter lift as follows. The following description is given by way of example, not limitation.
Binders from an expressed library (e.g., in phage) capable of binding to a given ligand ("positives") are initially enriched by one or two cycles of panning or affinity chromatography. A microtiter well is passively coated with the ligand (e.g., about 10 μg in 100 μl) . The well is then blocked with a solution of BSA to prevent nonspecific adherence of the phage of the library to the plastic surface. For example, about 1011 phage particles expressing peptides are then added to the well and incubated for several hours. Unbound phage are removed by repeated washing of the plate, and specifically bound phage are eluted using an acidic glycine-HCl solution or other elution buffer. The
eluted phage solution is neutralized with alkali, and amplified, e . g. , by infection of E. coli and plating on large petri dishes containing Luria broth (LB) in agar. Amplified cultures expressing the binding peptides are then titered and the process repeated. Alternatively, the ligand can be covalently coupled to agarose or acrylamide beads using commercially available activated bead reagents. The phage solution is then simply passed over a small column containing the coupled bead matrix which is then washed extensively and eluted with acid or other eluant . In either case, the goal is to enrich the positives to a frequency of about > 1/105.
Following enrichment, a filter lift assay is conducted. For example, when specific binders are expressed in phage, approximately 1-2 x 105 phage are added to 500 μl of log phase E. coli and plated on a large Luria Broth-agarose plate with 0.7% agarose in broth. The agarose is allowed to solidify, and a nitrocellulose filter (e.g., 0.45 μ) is placed on the agarose surface. A series of registration marks is made with a sterile needle to allow re-alignment of the filter and plate following development as described below. Phage plaques are allowed to develop by overnight incubation at 37 °C (the presence of the filter does not inhibit this process) . The filter is then removed from the plate with phage from each individual plaque adhered in si tu . The filter is then exposed to a solution of BSA or other blocking agent for 1-2 hours to prevent non-specific binding of the ligand (or "probe") .
The probe itself is labeled, for example, either by biotinylation (using commercial NHS-biotin) or direct enzyme labeling, e.g., with horse radish peroxidase or alkaline phosphatase. Probes labeled in this manner are indefinitely stable and can be re-used several times. The blocked filter is exposed to a solution of probe for several hours to allow the probe to bind in si tu to any phage on the filter displaying a peptide with significant affinity to the probe. The filter is then washed to remove unbound probe, and then developed by exposure to enzyme substrate solution (in the
case of directly labeled probe) or further exposed to a solution of enzyme-labeled avidin (in the case of biotinylated probe) . Positive phage plaques are identified by localized deposition of colored enzymatic cleavage product on the filter which corresponds to plaques on the original plate. The developed filter is simply realigned with the plate using the registration marks, and the "positive" plaques are cored from the agarose to recover the phage . Because of the high density of plaques on the original plate, it may be difficult to isolate a single plaque from the plate on the first pass. Accordingly, phage recovered from the initial core can be re-plated at low density and the process can be repeated to allow isolation of individual plaques and hence single clones of phage. Successful screening experiments are optimally conducted using 3 rounds of serial screening. The recovered cells are then plated at a low density to yield isolated colonies for individual analysis. The individual colonies are selected and used to inoculate LB culture medium containing ampicillin. After overnight culture at 37°C, the cultures are then spun down by centrifugation. Individual cell aliquots are then retested for binding to the target ligand attached to the beads. Binding to other beads having attached thereto a non-relevant ligand, can be used as a negative control.
One aspect of screening the libraries is that of elution. The following discussion is applicable to any system where the random peptide is expressed on a surface fusion molecule. It is conceivable that the conditions that disrupt the peptide-target interactions during recovery of the phage are specific for every given peptide sequence from a plurality of proteins expressed on phage. For example, certain interactions may be disrupted by acid pH but not by basic pH, and vice versa . Thus, it may be desirable to test a variety of elution conditions (including but not limited to pH 2-3, pH 12-13, excess target in competition, detergents, mild protein denaturants, urea, varying temperature, light,
presence or absence of metal ions, chelators, etc.) and compare the primary structures of the binding proteins expressed on the phage recovered for each set of conditions to determine the appropriate elution conditions for each ligand/binding protein combination. Some of these elution conditions may be incompatible with phage infection because they are bactericidal and will need to be removed by dialysis (i.e., dialysis bag, Centricon/Amicon microconcentrators) .
In a preferred embodiment, a phage display library of random peptides is screened to select phage expressing peptides that bind to a GIT transport receptor. Preferably, a first step is to isolate a preselected phage library. The "preselected phage library" is a library consisting of a subpopulation of a phage display library. This subpopulation can be formed by initially screening against either a target GIT transport receptor (or domain thereof) so as to permit the selection of a subpopulation of phages which specifically bind to the receptor. Alternatively, the subpopulation can be formed by screening against a target cell or cell type or tissue type or tissue barrier of the gastro-intestinal tract, so as to permit the selection of a subpopulation of phages which either bind specifically to the target cell or target cell type or target tissue or target tissue barrier, or which binds to and/or is transported across (or between) the target cell or target cell type or target tissue or target tissue barrier either in si tu or in vivo . This preselected phage library or subpopulation of selected phages can also be rescreened against the target GIT transport receptor, permitting the further selection of a subpopulation of phages which bind to the GIT transport receptor or target cell or target cell type or target tissue or target tissue barrier or which bind to and/or is transported across the target cell, target tissue or target tissue barrier either in si tu or in vivo . Such rescreening can be repeated from zero to 30 times with each successive "pre-selected phage library" generating additional pre-selected phage libraries.
In a preferred embodiment, a preselected phage library binding a ligand that is a GIT transport receptor preferably selected from among HPTl, hPEPTl, D2H, or hSI is obtained by an in vi tro screening step as described above, 5 and then the phage are optionally further characterized using in vi tro assays consisting of binding phage directly to the receptor domain of interest or, alternatively, to Caco-2 cells or using in vivo assays. In another preferred embodiment, in vivo assays are used that measure uptake of 0 phage by intestinal tissue or, alternatively, through the
GIT. In alternative embodiments, such further in vi tro or in vivo assays can be used as the initial screening step.
In vivo assays that may be used are described in the examples infra . 5
5.4. Generation of Antibodies to GIT Transport
Receptor-Binding Peptides and Derivatives Thereof
According to the invention, a GIT transport receptor-binding peptide, fragments or other derivatives, or analogs thereof, may be used as an immunogen to generate antibodies which immunospecifically bind such an immunogen.
Such antibodies include but are not limited to polyclonal , monoclonal, chimeric, single chain, Fab fragments, and an Fab expression library.
Various procedures known in the art may be used for the production of polyclonal antibodies to a GIT transport receptor-binding peptide or derivative or analog. For the production of antibody, various host animals can be immunized by injection with the native GIT transport receptor-binding peptides, or a synthetic version, or derivative (e.g., fragment) thereof, including but not limited to rabbits, mice, rats, fowl, etc. Various adjuvants may be used to increase the immunological response, depending on the host species, including but not limited to Freund's (complete and
_ 35_ incomplete) , mineral gels such as aluminum hydroxide, surface active substances such as lysolecithin, pluronic polyols, polyanions, peptides, oil emulsions, keyhole limpet
hemocyanins, dinitrophenol , and potentially useful human adjuvants such as BCG (bacille Calmette-Guerin) and corynebacterium parvum.
For preparation of monoclonal antibodies directed toward a GIT transport receptor-binding peptide or analog thereof, any technique which provides for the production of antibody molecules by continuous cell lines in culture may be used. For example, the hybridoma technique originally developed by Kohler and Milstein (1975, Nature 256:495-497), as well as the trioma technique, the human B-cell hybridoma technique (Kozbor et al . , 1983, Immunology Today 4:72), and the EBV-hybridoma technique to produce human monoclonal antibodies (Cole et al . , 1985, in Monoclonal Antibodies and Cancer Therapy, Alan R. Liss, Inc., pp. 77-96) . In an additional embodiment of the invention, monoclonal antibodies can be produced in germ- free animals utilizing recent technology (PCT/US90/02545) . According to the invention, human antibodies may be used and can be obtained by using human hybridomas (Cote et al . , 1983, Proc. Natl. Acad. Sci. U.S.A. 80:2026-2030) or by transforming human B cells with EBV virus in vi tro (Cole et al . , 1985, in Monoclonal Antibodies and Cancer Therapy, Alan R. Liss, pp. 77-96) . According to the invention, techniques developed for the production of "chimeric antibodies" (Morrison et al . , 1984, Proc. Natl. Acad. Sci. U.S.A. 81:6851-6855; Neuberger et al . , 1984, Nature 312:604-608; Takeda et al . , 1985, Nature 314:452-454) by splicing the genes from a mouse antibody molecule specific for GIT transport receptor-binding peptides together with genes from a human antibody molecule of appropriate biological activity can be used.
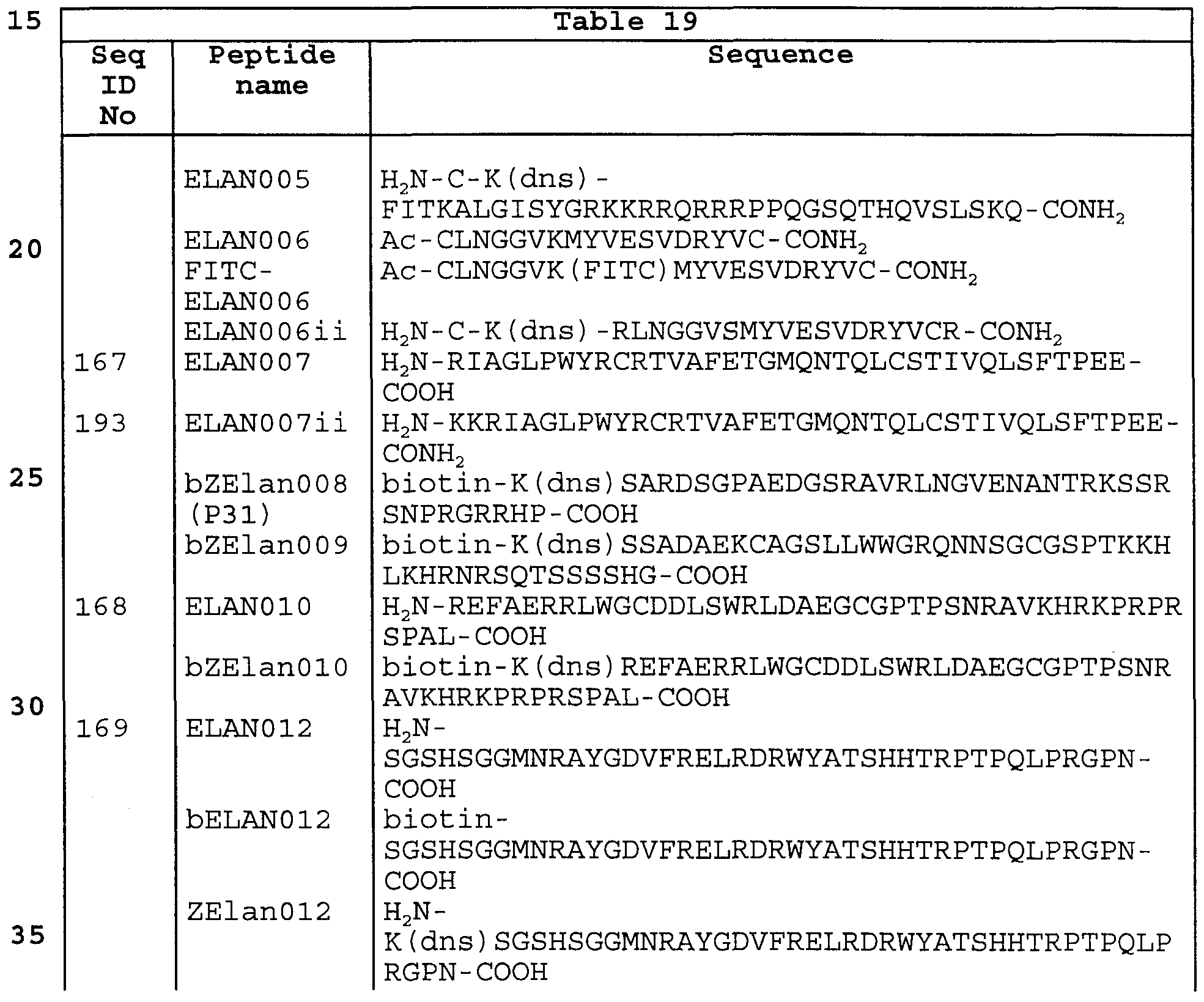
According to the invention, techniques described for the production of single chain antibodies (U.S. Patent 4,946,778) can be adapted to produce GIT transport receptor- binding peptide-specific single chain antibodies. An additional embodiment of the invention utilizes the techniques described for the construction of Fab expression libraries (Huse et al . , 1989, Science 246:1275-1281) to allow
rapid and easy identification of monoclonal Fab fragments with the desired specificity for GIT transport receptor- binding peptides, derivatives, or analogs.
Antibody fragments which contain the idiotype of the molecule can be generated by known techniques. For example, such fragments include but are not limited to: the F(ab')2 fragment which can be produced by pepsin digestion of the antibody molecule; the Fab' fragments which can be generated by reducing the disulfide bridges of the F(ab')2 fragment, the Fab fragments which can be generated by treating the antibody molecule with papain and a reducing agent, and Fv fragments.
In the production of antibodies, screening for the desired antibody can be accomplished by techniques known in the art, e.g. ELISA (enzyme-linked immunosorbent assay). For example, to select antibodies which recognize a specific domain of a GIT transport receptor-binding peptide, one may assay generated hybridomas for a product which binds to a GIT transport receptor-binding peptide fragment containing such a domain.
Antibodies specific to a domain of a GIT transport receptor-binding peptide are also provided.
The foregoing antibodies can be used in methods known in the art relating to the localization and activity of the GIT transport receptor-binding peptide sequences of the invention, e.g., for imaging these peptides after in vivo administration ( e . g. , to monitor treatment efficacy), measuring levels thereof in appropriate physiological samples, in diagnostic methods, etc. For instance, antibodies or antibody fragments specific to a domain of a GIT transport receptor-binding peptide or to a derivative of a peptide, such as a dansyl group or some other epitope introduced into the peptide, can be used to 1) identify the presence of the peptide on a nanoparticle or other substrate; 2) quantify the amount of peptide on the nanoparticle; 3) measure the level of the peptide in appropriate physiological samples; 4) perform im unohistology on tissue
samples; 5) image the peptide after in vivo administration; 6) purify the peptide from a mixture using an immunoaffinity column or 7) bind or fix the peptide to the surface of nanoparticle. This last use envisions attaching the antibody 5 (or fragment of the antibody) to the surface of drug-loaded nanoparticles or other substrate and then incubating this conjugate with the peptide. This procedure results in binding of the peptide in a certain fixed orientation, resulting in a particle that contains the peptide bound to
10 the antibody in such a way that the peptide is fully active. Abtides (or Antigen binding peptides) specific to a domain of a GIT transport receptor-binding peptide or to a derivative of a peptide, such as a dansyl group or some other epitope introduced into the peptide, can be used for the same
15 seven purposes identified above for antibodies.
5.5. Assays of GIT Transport Receptor-Binding Peptides, Derivatives and Analogs
The functional activity of GIT transport receptor-
_n binding peptides, derivatives and analogs can be assayed by various methods .
In a preferred embodiment, in which binding to a
GIT transport receptor is being assayed, the binding can be assayed by in vivo or in vi tro assays such as described in the examples infra, or by other means that are known in the art .
In another embodiment, where one is assaying for the ability to bind or compete with full-length GIT transport receptor-binding peptide for binding to anti-GIT transport
_- receptor-binding peptide antibody, various immunoassays known in the art can be used, including but not limited to competitive and non-competitive assay systems using techniques such as radioimmunoassays, ELISA (enzyme linked immunosorbent assay), "sandwich" immunoassays,
_.. immunoradiometric assays, gel diffusion precipitin reactions, immunodiffusion assays, in si tu immunoassays (using colloidal gold, enzyme or radioisotope labels, for example), western
blots, precipitation reactions, agglutination assays (e.g., gel agglutination assays, hemagglutination assays), complement fixation assays, immunofluorescence assays, protein A assays, and immunoelectrophoresis assays, etc. In one embodiment, antibody binding is detected by detecting a label on the primary antibody. In another embodiment, the primary antibody is detected by detecting binding of a secondary antibody or reagent to the primary antibody. In a further embodiment, the secondary antibody is labelled. Many means are known in the art for detecting binding in an immunoassay and are within the scope of the present invention.
Other methods will be known to the skilled artisan and are within the scope of the invention.
5.6. Uses
The invention provides compositions comprising the GIT transport receptor-binding proteins of the invention bound to a material comprising an active agent. Such compositions have use in targeting the active agent to the GIT and/or in facilitating transfer through the lumen of the GIT into the systemic circulation. Where the active agent is an imaging agent, such compositions can be administered in vivo to image the GIT (or particular transport receptors thereof) . Other active agents include but are not limited to: any drug or antigen or any drug- or antigen-loaded or drug- or antigen-encapsulated nanoparticle, microparticle, liposome, or micellar formulation capable of eliciting a biological response in a human or animal . Examples of drug- or antigen-loaded or drug- or antigen-encapsulated formulations include those in which the active agent is encapsulated or loaded into nano- or microparticles, such as biodegradable nano- or microparticles, and which have the GIT transport receptor-binding protein or derivative or analog adsorbed, coated or covalently bound, such as directly linked or linked via a linking moiety, onto the surface of the nano- or microparticle. Additionally, the protein, derivative or
analog can form the nano- or microparticle itself or the protein, derivative or analog can be covalently attached to the polymer or polymers used in the production of the biodegradable nano- or microparticles or drug-loaded or drug- encapsulated nano- or microparticles or the peptide can be directly conjugated to the active agent. Such conjugations to active agents include fusion proteins in which a DNA sequence coding for the peptide is fused in- frame to the gene or cDNA coding for a therapeutic peptide or protein such that the modified gene codes for a recombinant fusion protein.
In a preferred embodiment, the invention provides for treatment of various diseases and disorders by administration of a therapeutic compound (termed herein "Therapeutic"). Such "Therapeutics" include but are not limited to: GIT transport receptor-binding proteins, and analogs and derivatives (including fragments) thereof (e.g., as described hereinabove) that bind to GIT transport receptors, bound to an active agent of value in the treatment or prevention of a disease or disorder (preferably a mammalian, most preferably human, disease or disorder) . Therapeutics also include but are not limited to nucleic acids encoding the GIT transport receptor-binding proteins, analogs, or derivatives bound to such a therapeutic or prophylactic active agent. The active agent is preferably a drug.
Any drug known in the art may be used, depending upon the disease or disorder to be treated or prevented, and the type of subject to which it is to be administered. As used herein, the term "drug" includes, without limitation, any pharmaceutically active agent. Representative drugs include, but are not limited to, peptides or proteins, hormones, analgesics, anti-migraine agents, anti-coagulant agents, anti-emetic agents, cardiovascular agents, anti- hypertensive agents, narcotic antagonists, chelating agents, anti-anginal agents, chemotherapy agents, sedatives, anti- neoplasties, prostaglandins, and antidiuretic agents. Typical drugs include peptides, proteins or hormones such as
insulin, calcitonin, calcitonin gene regulating protein, atrial natriuretic protein, colony stimulating factor, betaseron, erythropoietin (EPO) , interferons such as a. , β or γ interferon, somatropin, somatotropin, somatostatin, insulin-like growth factor (somatomedins) , luteinizing hormone releasing hormone (LHRH) , tissue plasminogen activator (TPA) , growth hormone releasing hormone (GHRH) , oxytocin, estradiol, growth hormones, leuprolide acetate, factor VIII, interleukins such as interleukin-2 , and analogs thereof; analgesics such as fentanyl, sufentanil, butorphanol, buprenorphine, levorphanol, morphine, hydromorphone , hydocodone, oxymorphone, methadone, lidocaine, bupivacaine, diclofenac, naproxen, paverin, and analogs thereof; anti-migraine agents such as heparin, hirudin, and analogs thereof; anti -coagulant agents such as scopolamine, ondansetron, domperidone, etoclopramide, and analogs thereof; cardiovascular agents, anti-hypertensive agents and vasodilators such as diltiazem, clonidine, nifedipine, verapamil, isosorbide-5-mononitrate, organic nitrates, agents used in treatment of heart disorders and analogs thereof; sedatives such as benzodiazeines, phenothiozines and analogs thereof; narcotic antagonists such as naltrexone, naloxone and analogs thereof; chelating agents such as deferoxamine and analogs thereof; anti-diuretic agents such as desmopressin, vasopressin and analogs thereof; anti -anginal agents such as nitroglycerine and analogs thereof; anti- neoplasties such as 5-fluorouracil , bleomycin and analogs thereof; prostaglandins and analogs thereof; and chemotherapy agents such as vincristine and analogs thereof. Representative drugs also include but are not limited to antisense oligonucleotides, genes, gene correcting hybrid oligonucleotides, ribozymes, aptameric oligonucleotides, triple-helix forming oligonucleotides, inhibitors of signal transduction pathways, tyrosine kinase inhibitors and DNA modifying agents. Drugs that can be used also include, without limitation, systems containing gene therapeutics, including viral systems for therapeutic gene delivery such as
adenovirus, adeno-associated virus, retroviruses, herpes simplex virus, sindbus virus, liposomes, cationic lipids, dendrimers, and enzymes. For instance, gene delivery viruses can be modified such that they express the targeting peptide 5 on the surface so as to permit targeted gene delivery.
In a preferred embodiment, a Therapeutic is therapeutically or prophylactically administered to a human patient .
Additional descriptions and sources of Therapeutics 10 that can be used according to the invention are found in various Sections herein.
5.7. Therapeutic/Prophylactic Administration, Compositions and Formulations
The invention provides methods of treatment (and 15 prophylaxis) by administration to a subject of an effective amount of a Therapeutic of the invention. In a preferred aspect, the Therapeutic is substantially purified. The subject is preferably an animal, including but not limited to animals such as cows, pigs, horses, chickens, cats, dogs, etc., and is preferably a mammal, and most preferably a human .
As will be clear, any disease or disorder of interest amenable to therapy or prophylaxis by providing a drug in vivo systemically or by targeting a drug in vivo to 25 the GIT (by linkage to a GIT transport -receptor binding protein, derivative or analog of the invention) can be treated or prevented by administration of a Therapeutic of the invention. Such diseases may include but are not limited
_n to hypertension, diabetes, osteoporosis, hemophilia, anemia, cancer, migraine, and angina pectoris, to name but a few.
Any route of administration known in the art may be used, including but not limited to oral, nasal, topical, intravenous, intraperitoneal , intradermal, mucosal, ό - 5c intrathecal, intramuscular, etc. Preferably, administration is oral; in such an embodiment the GIT-transport binding protein, derivative or analog of the invention acts
advantageously to facilitate transport of the therapeutic active agent through the lumen of the GIT into the systemic circulation.
The present invention also provides therapeutic compositions/formulations. In a specific embodiment of the invention, a GIT transport receptor-binding peptide or motif of interest is associated with a therapeutically or prophylactically active agent, preferably a drug or drug- containing nano- or microparticle. More preferably, the active agent is a drug encapsulating or drug loaded nano- or microparticle, such as a biodegradable nano- or microparticle, in which the peptide is physically adsorbed or coated or covalently bonded, such as directly linked or linked via a linking moiety, onto the surface of the nano- or microparticle. Alternatively, the peptide can form the nano- or microparticle itself or can be directly conjugated to the active agent. Such conjugations include fusion proteins in which a DNA sequence coding for the peptide is fused in-frame to the gene or cDNA coding for a therapeutic peptide or protein, such that the modified gene codes for a recombinant fusion protein in which the "targeting" peptide is fused to the therapeutic peptide or protein and where the "targeting" peptide increases the absorption of the fusion protein from the GIT. Preferably the particles range in size from 200-600 nm.
Thus, in a specific embodiment, a GIT transport- binding protein is bound to a slow-release (controlled release) device containing a drug. In a specific embodiment, polymeric materials can be used ( see Medical Applications of Controlled Release, Langer and Wise (eds.), CRC Pres . , Boca Raton, Florida (1974) ; Controlled Drug Bioavailability, Drug Product Design and Performance, Smolen and Ball (eds.), Wiley, New York (1984); Ranger and Peppas, J. Macromol . Sci. Rev. Macromol. Chem. 23:61 (1983); see also Levy et al . , Science 228:190 (1985); During et al . , Ann. Neurol . 25:351 (1989); Howard et al . , J. Neurosurg. 71:105 (1989)).
The present invention also provides pharmaceutical compositions. Such compositions comprise a therapeutically effective amount of a Therapeutic, and a pharmaceutically acceptable carrier. In a specific embodiment, the term "pharmaceutically acceptable" means approved by a regulatory agency of the Federal or a state government or listed in the U.S. Pharmacopeia or other generally recognized pharmacopeia for use in animals, and more particularly in humans. The term "carrier" refers to a diluent, adjuvant, excipient, or vehicle with which the therapeutic is administered. Such pharmaceutical carriers can be sterile liquids, such as water and oils, including those of petroleum, animal, vegetable or synthetic origin, such as peanut oil, soybean oil, mineral oil, sesame oil and the like. Water is a preferred carrier when the pharmaceutical composition is administered intravenously. Saline solutions and aqueous dextrose and glycerol solutions can also be employed as liquid carriers, particularly for injectable solutions. Suitable pharmaceutical excipients include starch, glucose, lactose, sucrose, gelatin, malt, rice, flour, chalk, silica gel, sodium stearate, glycerol monostearate, talc, sodium chloride, dried skim milk, glycerol, propylene, glycol, water, ethanol and the like. The composition, if desired, can also contain minor amounts of wetting or emulsifying agents, or pH buffering agents. These compositions can take the form of solutions, suspensions, emulsion, tablets, pills, capsules, powders, sustained-release formulations and the like. The composition can be formulated as a suppository, with traditional binders and carriers such as triglycerides . Oral formulations can include standard carriers such as pharmaceutical grades of mannitol, lactose, starch, magnesium stearate, sodium saccharine, cellulose, magnesium carbonate, etc. Examples of suitable pharmaceutical carriers are described in "Remington's Pharmaceutical Sciences" by E.W. Martin. Such compositions will contain a therapeutically effective amount of the Therapeutic, preferably in purified
form, together with a suitable amount of carrier so as to provide the form for proper administration to the patient.
The Therapeutics of the invention can be formulated as neutral or salt forms. Pharmaceutically acceptable salts include those formed with free amino groups such as those derived from hydrochloric, phosphoric, acetic, oxalic, tartaric acids, etc., and those formed with free carboxyl groups such as those derived from sodium, potassium, ammonium, calcium, ferric hydroxides, isopropylamine, triethylamine, 2-ethylamino ethanol, histidine, procaine, etc .
The amount of the Therapeutic of the invention which will be effective in the treatment of a particular disorder or condition will depend on the nature of the disorder or condition, and can be determined by standard clinical techniques. In addition, in vi tro assays may optionally be employed to help identify optimal dosage ranges. The precise dose to be employed in the formulation will also depend on the route of administration, and the seriousness of the disease or disorder, and should be decided according to the judgment of the practitioner and each patient's circumstances.
6. EXAMPLES 6.1. Selection of GIT Receptor Targets
The HPTl, hPEPTl, D2H, and hSI receptors were selected for cloning as GIT receptor targets based on several criteria, including: (1) expression on surface of epithelial cells in gastro-intestinal tract (GIT) ; (2) expression along the length of small intestine (HPTl, hPEPTl, D2H) ;
(3) expression locally at high concentration (hSI) ; (4) large putative extracellular domains facing into the lumen of the GIT; and (5) extracellular domains that permit easy access and bioadhesion by targeting particles. The four recombinant receptor sites screened with the peptide libraries additionally have the following characteristics :
Receptor Characteristics
D2H Transport of neutral/basic amino acids; a transport activating protein for a range of amino acid translocases hSI Metabolism of sucrose and other sugars; represents 9% of brush border membrane protein in Jejunum
HPTl di/tri peptide transporter or facilitator of peptide transport hPEPTl di/tri peptide transporter
Figures 1-4 (SEQ ID NOS: 176, 178, 179, and 181, respectively) show the predicted amino acid sequences for hPEPTl, HPTl, hSI and D2H, respectively.
6.2. Cloning of Extracellular Domain of Selected Receptor Site
The following receptor domains were cloned and expressed as His-tag fusion proteins by standard techniques:
Domain (amino
Receptor acid residues) hPEPTla 391-571
HPTlb 29-273 hSIc 272-667
D2Hd 387-685
a Liang et al . , 1995, J. Biol. Chem. 270:6456-6463 b Dantzig et al . , 1994, Association of Intestinal
Peptide Transport with a Protein Related to the
Cadherin Superfamily c Chantret et al . , Biochem. J. 285:915-923 d Bertran et al . , J. Biol. Chem. 268:14842-14949
The receptor proteins were expressed as His-tag fusion proteins and affinity purified under denaturing conditions, using urea or guanidine HCI, utilizing the pET His-tag metal chelate affinity for Ni-NTA Agarose (Hochuli, E., Purification of recombinant proteins with metal chelate adsorbent, Genetic Engineering, Principals and Methods (J.K. Setlow, ed.), Plenum Press, NY, Vol. 12 (1990), pp. 87-98).
6.3. Phage Libraries
Three phage DC8, D38, and DC43 libraries expressing N-terminal pill fusions in M13 were used to identify peptides that bind to the GIT receptors. The D38 and DC43 libraries which are composed of 37 and 43 random amino acid domains, respectively, have been described previously (McConnell et al., 1995, Molecular Diversity, 1:165-176). The DC8 library is similar to the other two except that the random insert is 8 amino acids long flanked on each side by a cysteine residue (i.e. , CX8C) .
6.4. Biopanning
Three rounds of biopanning on the GIT receptors were performed generally by standard methods (McConnell et al . , 1995, Molecular Diversity, 1:165-176), using a mixture of the DC8 (1 x 1010 pfu) , D38 and DC43 (1 x 1011 pfu) phage libraries. After each round of panning the percentage of phage recovered was determined. Following the first two rounds of panning, the eluted phage were amplified overnight. Phage from the third pan were plated out and 100 plaques were picked, amplified overnight and screened in an ELISA assay for binding to the relevant receptor and BSA. After data analysis, phage clones were identified which had high absorbance in the ELISA assay and/or a good ratio of binding to target compared to binding to BSA. The Insulin Degrading Enzyme (IDE) and recombinant human tissue factor (hTF) were used as irrelevant controls. Several variations of the standard panning technique, discussed below, were used. Selection or panning methods followed one of two strategies. The first strategy involved panning the mixed libraries on the specific GIT receptor adsorbed to a solid surface. The second strategy panned the libraries twice against the GIT receptor and then against Caco-2 cells (Peterson and Mooseker, 1992, J. Cell Science 102:581-600), Selection methods are reflected in the clone nomenclature as described below:
S designates the clone was identified by binding to the hSI receptor domain.
D designates the clone was identified by binding to the D2H receptor domain. P designates the clone was identified by binding to the PEPT1 receptor domain.
H designates the clone was identified by binding to the HPT-1 receptor domain.
Phage designated Ni are from a solid phase band GIT receptor pan that used the standard procedure with the addition of Ni-NTA Agarose (Qiagen, Chatsworth, CA) . Receptor coated plates were blocked with 0.5% BSA/PBS containing 160μl Ni-NTA agarose and libraries were panned in the presence of 50μl Ni-NTA agarose. The receptor proteins were expressed as His-tag fusions. The His-tag has a high affinity for Ni-NTA Agarose. Blocking the plate and panning in the presence of Ni-NTA agarose minimized phage binding to the His-tag portion of the recombinant receptor.
Phage with the designation AX were eluted with acid and Factor Xa. Phage were first eluted by standard acid elution then Factor Xa (New England Biolabs, Beverly, MA: lμg protease in 300μl of 20mM Tris-HCL, lOOmM NaCI, 2mM CaCl2) was added to the panning plate and incubated 2 hours . Phage from both elution methods were pooled together then plated. Phage with the designation AB were eluted with acid and base. Phage were eluted first by standard acid elution then lOOmM triethylamine pH 12.1 was added to the panning plate for 10 minutes. Phage from both elution methods were pooled together then plated. C designates panning on receptor followed by Caco-2 cells. First and second round pans were performed on the receptor and the third round pan was on snapwells of Caco-2 cells. DCX11, DCX8 and DCX33 were identified by two pans on D2H receptor, third pan on Caco-2 cells. The third round Factor Xa eluate from the Caco-2 cells was screened by ELISA on D2H, BSA and fixed Caco-2 cells. For HCA3 the first two rounds of panning were performed on the HPT-1 receptor and
the third pan was on monolayers cultured on snapwells of Caco-2 cells.
Phage designated 5PAX were carried through five rounds of panning after which a number of phage were sequenced prior to screening by ELISA.
6.5. Sequencing of Selected Phage
The amino acid sequence of phage inserts demonstrating a good ratio of binding to receptor domains and/or Caco-2 cells over background BSA binding were deduced from the nucleotide sequence obtained by sequencing (Sequenase®, U.S. Biochemical Corp., Cleveland, OH) both DNA strands of the appropriate region in the viral genome . The third round acid eluate was screened by ELISA on HPT-1, BSA and Caco-2 fixed cells. Phage designated 5PAX were carried through five rounds of panning after which a number of phages were sequenced prior to screening by ELISA.
One well of a 24 well plate was coated with 10 μg/ml of GIT receptor and the plate was incubated overnight at 4°C. The plate was blocked with 0.5 BSA-PBS for one hour. A mixture of the DC8, D38 and DC43 phage libraries was added to the plate and the plate was incubated for 2 to 3 hours at room temperature on a rotator. After washing the well 10 times with 1% BSA plus 0.05% Tween 20 in PBS, the well was eluted with 0.05m glycine, pH2. The phage was then eluted with 0.2M NaP04. The eluted phage was titered on agar plates; the remaining phage was amplified overnight. The next day the amplified phage was added to a second coated plate and the panning procedure was repeated as described above . The eluted phage from the second pan as well as the amplified phage from the first pan was titered on agar plates. Following amplification overnight of the phage from the second pan, the panning procedure was repeated as described above. The phage eluted from the third pan and the amplified phage from the second pan were then titered overnight on agar plates. Isolated phage colonies were amplified overnight prior to use in an ELISA assay.
6.6. Receptor ELISA Procedure
96 well plates were coated overnight with GIT receptor, BSA and, optionally, IDE (insulin degrading enzyme, an irrelevant His-fusion protein) or hTF . The plates were 5 blocked for one hour with 0.5% BSA-PBS. After clarification, the amplified phage were diluted 1:100 in 1% BSA plus 0.05% Tween 20 in PBS and added to the plates. Following incubation of the plates on a rotator for 1 to 2 hours, the plates were washed 5 times with 1% BSA plus 0.05% Tween 20 in
10 PBS. Dilute anti-M13-HRP conjugate (anti-M13 antibody linked to horse radish peroxidase (HRP) ) was added to all the wells and the plate was incubated for one hour on a rotator. After the plates were washed 5 times, as described above, TMB substrate was added to the wells. The plates were read at
15 650nm absorbance.
RECEPTOR ELISA RESULTS:
Below are the results of ELISA assays which assessed the binding of phage panned on the hSI receptor to 20 microtiter plates coated with hSI and BSA. Table 1 shows the OD results as well as the ratio of hSI to BSA binding.
25
30
35
Table 1
Below are the results of an ELISA which assessed the binding of phage panned on the D2H receptor to microtiter plates coated with D2H and BSA. Table 2 shows the OD results as well as the ratio of D2H to BSA binding.
Below are the results of an ELISA which assessed the binding of phage panned for two rounds on the D2H receptor followed by a third round pan on Caco-2 snapwells. Binding to fixed Caco-2 cells, D2H and BSA was examined.
Table 3 shows the OD results as well as the ratio of D2H to BSA binding.
Table 3
Below are the results of an ELISA which assessed the binding of phage panned on the hPEPTl receptor to hPEPTl and BSA. Table 4 shows the OD results as well as the ratio of hPEPTl to BSA binding.
Table 4
Table 5 shows the results of an ELISA which assessed the binding of phage panned on the HPT-1 receptor to HPT-1 and BSA. The table shows the OD results as well as the ratio of HPT-1 to BSA binding.
Table 5
Table 6 shows the results of an ELISA which assessed the binding of phage panned for two rounds on the HPT-1 receptor followed by a third round pan on Caco-2 snapwells. Binding to fixed Caco-2 cells, HPT-1 and BSA was examined. The table shows the OD results as well as the ratio of HPT-1 to BSA binding.
Table 6
CELL ELISA PROCEDURE
Phage ELISA was used as described above with the following changes. Diluent and wash buffer was PBS containing 1%BSA and 0.05% Tween 20 and plates were washed five times at each wash step. Supernatant of infected bacterial cultures was diluted 1:100 and incubated with protein coated plates for 2-3 hours with mild agitation. Anti-M13 Horseradish peroxidase (HRP) conjugate (Pharmacia, Piscataway, NJ) was diluted 1:8000.
Fixed Caco-2, C2BBel, and A431 cell plates were prepared by growing cells on tissue culture treated microtiter plates. When cells were confluent, plates were fixed with 10% formaldehyde, washed twice with PBS and stored with 0.5%BSA-PBS at -20 °C. On the day of the assay, thawed
plates were treated with PBS containing 0.1% phenylhydrazine for one hour at 37°C followed by two PBS washes and blocking for One hour with 0.5%BSA-PBS. The standard ELISA procedure was followed at this point. Phage which showed specificity to a GIT receptor was further characterized by ELISA on a variety of recombinant proteins. Phage which continued to exhibit GIT receptor specificity was sequenced.
Table 7
TARGET BINDING PHAGE INSERT SEQUENCES: tιι-V . hSI ID. NO.
S15 1 RSGAYESPDGRGGRSYVGGGGGCGNIGRKHNLWGLRTASPACWD
S21 2 SPRSFWPWSRHESFGISNYLGCGYRTCISGTMTKSSPIYPRHS
S22 3 SSSSDWGGVPGKWRERFKGRGCGISITSVLTGKPNPCPEPKAA
SNilO 4 RVGQCTDSDVRRPWARSCAHQGCGAGTRNSHGCITRPLRQASAH
SNi28 5 SHSGGMNRAYGDVFRELRDRWNATSHHTRPTPQLPRGPN
SNi34 6 SPCGGSWGRFMQGGLFGGRTDGCGAHRNRTSASLEPPSSDY
SNi38 7 RGAADQRRGWSENLGLPRVGWDAIAHNSYTFTSRRPRPP
SNi45 8 SGGEVSSWGRVNDLCARVSWTGCGTARSARTDNKGFLPKHSSLR
SNiAX2 9 SDSDGDHYGLRGGVRCSLRDRGCGLALSTVHAGPPSFYPKLSSP
SNiAX4 10 RSLGNYGVTGTVDVTVLPMPGHANHLGVSSASSSDPPRR
SNiAX6 11 RTTTAKGCLLGSFGVLSGCSFTPTSPPPHLGYPPHSVN
SNiAXδ 12 SPKLSSVGVMTKVTELPTEGPNAISIPISATLGPRNPLR
D2H
DAB3 13 RWCGAELCNSVTKKFRPGWRDHANPSTHHRTPPPSQSSP
DAB7 14 RWCGADDPCGASRWRGGNSLFGCGLRCSAAQSTPSGRIHSTSTS
DAB10 15 SKSGEGGDSSRGETGWARVRSHAMTAGRFRWYNQLPSDR
DAB18 16 RSSANNCEWKSDWMRRACIARYANSSGPARAVDTKAAP
DAB24 17 SKWSWSSRWGSPQDKVEKTRAGCGGSPSSTNCHPYTFAPPPQAG
DAB30 18 SGFWEFSRGLWDGENRKSVRSGCGFRGSSAQGPCPVTPATIDKH
DAX15 19 SESGRCRSVSRWMTTWQTQKGGCGSNVSRGSPLDPSHQTGHATT
DAX23 20 REWRFAGPPLDLWAGPSLPSFNASSHPRALRTYWSQRPR
DAX24 21 RMEDIKNSGWRDSCRWGDLRPGCGSRQWYPSNMRSSRDYPAGGH
DAX27 22 SHPWYRHWNHGDFSGSGQSRHTPPESPHPGRPNATI
DCX8 23 RYKHDIGCDAGVDKKSSSVRGGCGAHSSPPRAGRGPRGTMVSRL
DCX11 24 SQGSKQCMQYRTGRLTVGSEYGCGMNPARHATPAYPARLLPRYR
DCX26 25 SGRTTSEISGLWGWGDDRSGYGWGNTLRPNYIPYRQATNRHRYT
DCX33 26 RWNWTVLPATGGHYWTRSTDYHAINNHRPSIPHQHPTPI DCX36 27 SWSSWNWSSKTTRLGDRATREGCGPSQSDGCPYNGRLTTVKPRT
DCX39 28 SGSLNAWQPRSWVGGAFRSHANNNLNPKPTMVTRHPT
DCX42 29 RYSGLSPRDNGPACSQEATLEGCGAQRLMSTRRKGRNSRPGWTL
DCX45 30 SVGNDKTSRPVSFYGRVSDLWNASLMPKRTPSSKRHDDG
hPEPTl
PAX9 31 RWPSVGYKGNGSDTIDVHSNDASTKRSLIYNHRRPLFP
PAX14 32 RTFENDGLGVGRSIQKKSDRWYASHNIRSHFASMSPAGK
PAX15 33 SYCRVKGGGEGGHTDSNLARSGCGKVARTSRLQHINPRATPPSR
PAX16 34 SWTRWGKHTHGGFVNKSPPGKNATSPYTDAQLPSDQGPP PAX17 35 SQVDSFRNSFRWYEPSRALCHGCGKRDTSTTRIHNSPSDSYPTR
PAX18 36 SFLRFQSPRFEDYSRTISRLRNATNPSNVSDAHNNRALA
PAX35 37 RSITDGGINEVDLSSVSNVLENANSHRAYRKHRPTLKRP
PAX38 38 SSKVSSPRDPTVPRKGGNVDYGCGHRSSARMPTSALSSITKCYT
PAX40 39 RASTQGGRGVAPEFGASVLGRGCGSATYYTNSTSCKDAMGHNYS PAX43 40 RWCEKHKFTAARCSAGAGFERDASRPPQPAHRDNTNRNA
PAX45 41 SFQVYPDHGLERHALDGTGPLYAMPGRWIRARPQNRDRQ
PAX46 42 SRCTDNEQCPDTGTRSRSVSNARYFSSRLLKTHAPHRP
P31 43 SARDSGPAEDGSRAVRLNGVENANTRKSSRSNPRGRRHP
P90 44 SSADAEKCAGSLLWWGRQNNSGCGSPTKKHLKHRNRSQTSSSSH 5PAX3 45 RPKNVADAYSSQDGAAAEETSHASNAARKSPKHKPLRRP
5PAX5 46 RGSTGTAGGERSGVLNLHTRDNASGSGFKPWYPSNRGHK
5PAX7 47 RWGWERSPSDYDSDMDLGARRYATRTHRAPPRVLKAPLP
5PAX12 48 RGWKCEGSQAAYGDKDIGRSRGCGSITKNNTNHAHPSHGAVAKI
HPT-1
HAX9 49 SREEANWDGYKREMSHRSRFWDATHLSRPRRPANSGDPN
HAX35 50 EWYSWKRSSKSTGLGDTATREGCGPSQSDGCPYNGRLTTVKPRK
HAX40 51 REFAERRLWGCDDLSWRLDAEGCGPTPSNRAVKHRKPRPRSPAL
HAX42 52 SDHALGTNLRSDNAKEPGDYNCCGNGNSTGRKVFNRRRPSAIPT HCA3 53 RHISEYSFANSHLMGGESKRKGCGINGSFSPTCPRSPTPAFRRT
H40 54 SRESGMWGSWWRGHRLNSTGGNANMNASLPPDPPVSTP
PAX2 55 STPPSREAYSRPYSVDSDSDTNAKHSSHNRRLRTRSRPN
Table 8
DNA Sequences for Clones used in in vivo Pan
S15 (SEQ ID NO: 56) TCTCACTCCTCGAGATCCGGCGCTTATGAGAGTCCGGATGGTCGGGGGGGTCGGAGCTATG TGGGGGGCGGGGGTGGNTGTGGTAACATTGGTCGGAAGCATAACCTGTGGGGGCTGCGTAC CGCGTCGCCGGCCTGCTGGGACTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
521 (SEQ ID NO: 57)
TCTCACTCCTCGAGTCCTCGCTCTTTCTGGCCCGTTGTGTCCCGGCATGAGTCGTTTGGGA TCTCTAACTATTTGGGNTGTGGTTATCGTACATGTATCTCCGGCACGATGACTAAGTCTAG CCCGATTTACCCTCGGCATTCGTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
522 (SEQ ID NO: 58)
TCTCACTCCTCGAGTAGTAGCTCCGATTGGGGTGGTGTGCCTGGGAAGGTGGTTAGGGAGC GCTTTAAGGGGCGCGGTTGTGGTATTTCCATCACCTCCGTGCTCACTGGGAAGCCCAATCC GTGTCCGGAGCCTAAGGCGGCCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
SNi 10 (SEQ ID NO: 59)
TCTCACTCCTCGAGAGTTGGCCAGTGCACGGATTCTGATGTGCGGCGTCCTTGGGCCAGGT CTTGCGCTCATCAGGGTTGTGGTGCGGGCACTCGCAACTCGCACGGCTGCATCACCCGTCC TCTCCGCCAGGCTAGCGCTCATTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
SNi 28 (SEQ ID NO: 60)
TCTCACTCCTCGAGCCACTCCGGTGGTATGAATAGGGCCTACGGGGATGTGTTTAGGGAGC TTCGTGATCGGTGGAACGCCACTTCCCACCACACTCGCCCCACCCCTCAGCTCCCCCGTGG GCCTAATTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
SNi 34 (SEQ ID NO: 61) TCTCACTCCTCGAGTCCGTGCGGGGGGTCGTGGGGGCGTTTTATGCAGGGTGGCCTTTTCG GCGGTAGGACTGATGGTTGTGGTGCCCATAGAAACCGCACTTCTGCGTCGTTAGAGCCCCC GAGCAGCGACTACTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
SNi 38 (SEQ ID NO: 62)
TCTCACTCCTCGAGGGGCGCCGCCGATCAGCGGCGGGGGTGGTCCGAGAACTTGGGGTTGC CTAGGGTGGGGTGGGACGCCATCGCTCACAATAGCTATACGTTCACCTCGCGCCGCCCGCG CCCCCCCTCTAGA
SNi 45 (SEQ ID NO: 63)
TCTCACTCCTCGAGCGGTGGGGAGGTCAGCTCCTGGGGCCGCGTGAATGACCTCTGCGCTA GGGTGAGTTGGACTGGTTGTGGTACTGCTCGTTCCGCGCGTACCGACAACAAAGGCTTTCT TCCTAAGCACTCGTCACTCCGCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
SNi AX2 (SEQ ID NO: 64)
TCTCACTCCTCGAGTGATAGTGACGGGGATCATTATGGGCTTCGGGGGGGGGTGCGTTGTT CGCTTCGTGATAGGGGTTGTGGTCTGGCCCTGTCCACCGTCCATGCTGGTCCCCCCTCTTT TTACCCCAAGCTCTCCAGCCCCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
SNi AX4 (SEQ ID NO: 65) TCTCACTCCTCGAGGAGCTTGGGTAATTATGGCGTCACCGGGACTGTGGACGTGACGGTTT TGCCCATGCCTGGCCACGCCAACCACCTTGGTGTCTCCTCCGCCTCTAGCTCTGATCCTCC GCGGCGCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
SNi AX6 (SEQ ID NO: 66)
TCTCACTCCTCGAGAACTACGACGGCTAAGGGGTGTCTTCTCGGAAGCTTCGGCGTTCTTA GTGGGTGCTCATTTACGCCAACCTCTCCACCGCCCCACCTAGGATACCCCCCCCACTCCGT CAATTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
SNi AX8 (SEQ ID NO: 67)
TCTCACTCCTCGAGCCCGAAGTTGTCCAGCGTGGGTGTTATGACTAAGGTCACGGAGCTGC CCACGGAGGGGCCTAACGCCATTAGTATTCCGATCTCCGCGACCCTCGGCCCGCGCAACCC GCTCCGCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAB3 (SEQ ID NO: 68)
TCTCACTCCTCGAGGTGGTGCGGCGCTGAGCTGTGCAACTCGGTGACTAAGAAGTTTCGCC CGGGCTGGCGGGATCACGCCAATCCCTCCACCCATCATCGTACTCCCCCGCCCAGCCAGTC CAGCCCTTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAB7 (SEQ ID NO: 69)
TCTCACTCCTCGAGGTGGTGCGGCGCTGATGACCCGTGTGGTGCCAGTCGTTGGCGGGGGG GCAACAGCTTGTTTGGTTGTGGTCTTCGTTGTAGTGCGGCGCAGAGCACCCCGAGTGGCAG GATCCATTCCACTTCGACCAGCTCTAGAATCGAAGGTGCGCTAGACCTTCGAGA
DAB10 (SEQ ID NO: 70) TCTCACTCCTCGAGTAAGTCCGGGGAGGGGGGTGACAGTAGCAGGGGCGAGACGGGCTGGG CGAGGGTTCGGTCTCACGCCATGACTGCTGGCCGCTTTCGGTGGTACAACCAGTTGCCCTC TGATCGGTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAB18 (SEQ ID NO: 71)
TCTCACTCCTCGAGGTCGAGCGCCAATAATTGCGAGTGGAAGTCTGATTGGATGCGCAGGG CCTGTATTGCTCGTTACGCCAACAGTTCGGGCCCCGCCCGCGCCGTCGACACTAAGGCCGC GCCCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAB24 (SEQ ID NO: 72)
TCTCACTCCTCGAGTAAGTGGTCGTGGAGTTCGAGGTGGGGCTCCCCGCAGGATAAGGTTG AGAAGACCAGGGCGGGTTGTGGTGGTAGTCCCAGCAGCACCAATTGTCACCCCTACACCTT TGCCCCCCCCCCGCAAGCCGGCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAB30 ( SEQ ID NO : 73 )
TCTCACTCCTCGAGTGGGTTCTGGGAGTTTAGCAGGGGGCTTTGGGATGGGGAGAACCGTA AGAGTGTCCGGTCGGGTTGTGGTTTTCGTGGCTCCTCTGCTCAGGGCCCGTGTCCGGTCAC GCCTGCCACCATTGACAAACACTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAX15 (SEQ ID NO: 74)
TCTCACTCCTCGAGTGAGAGCGGGCGGTGCCGTAGCGTGAGCCGGTGGATGACGACGTGGC AGACGCAGAAGGGCGGTTGTGGTTCCAATGTTTCCCGCGGTTCGCCCCTCGACCCCTCTCA CCAGACCGGGCATGCCACTACTTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAX23 (SEQ ID NO: 75)
TCTCACTCCTCGAGGGAGTGGAGGTTTGCCGGGCCGCCGTTGGACCTGTGGGCGGGTCCGA GCTTGCCCTCTTTTAACGCCAGTTCCCACCCTCGCGCCCTGCGCACCTATTGGTCCCAGCG GCCCCGCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAX24 (SEQ ID NO: 76) TCTCACTCCTCGAGGATGGAGGACATCAAGAACTCGGGGTGGAGGGACTCTTGTAGGTGGG GTGACCTGAGGCCTGGTTGTGGTAGCCGCCAGTGGTACCCCTCGAATATGCGTTCTAGCAG AGATTACCCCGCGGGGGGCCACTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DAX27 (SEQ ID NO: 77)
TCTCACTCCTCGAGTCATCCGTGGTACAGGCATTGGAACCATGGTGACTTCTCTGGTTCGG GCCAGTCACGCCACACCCCGCCGGAGAGCCCCCACCCCGGCCGCCCTAATGCCACCATTTC TAGAATCGAAGGTCGCGCTAGACCTTCGAG
DCX8 (SEQ ID NO: 78)
TCTCACTCCTCGAGATATAAGCACGATATCGGTTGCGATGCTGGGGTTGACAAGAAGTCGT CGTCTGTGCGTGGTGGTTGTGGTGCTCATTNGTCGCCACCCCGCGCCGGCCGTGGTCCTCG CGGCACGATGGTTAGCAGGCTTTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DCX11 (SEQ ID NO: 79)
TCTCACTCCTCGAGTCAGGGCTCCAAGCAGTGTATGCAGTACCGCACCGGTCGTTTGACGG TGGGGTCTGAGTATGGTTGTGGTATGAACCCCGCCCGCCATGCCACGCCCGCTTATCCGGC GCGCCTGCTGCCACGCTATCGCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DCX26 (SEQ ID NO: 80)
TCTCACTCCTCGAGTGGGCGGACTACTAGTGAGATTTCTGGGCTCTGGGGTTGGGGTGACG ACCGGAGCGGTTATGGTTGGGGTAACACGCTCCGCCCCAACTACATCCCTTATAGGCAGGC GACGAACAGGCATCGTTATACGTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DCX33 (SEQ ID NO: 81) TCTCACTCCTCGAGGTGGAATTGGACTGTCTTGCCCGCCACTGGCGGCCATTACTGGACGC GTTCGACGGACTATCACGCCATTAACAATCACAGGCCGAGCATCCCCCACCAGCATCCGAC CCCTATCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DCX36 ( SEQ ID NO : 82 )
TCTCACTCCTCGAGTTGGTCGTCGTGGAATTGGAGCTCTAAGACTACTCGTCTGGGCGACA GGGCGACTCGGGAGGGTTGTGGTCCCAGCCAGTCTGATGGCTGTCCTTATAACGGCCGCCT TACGACCGTCAAGCCTCGCACGTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DCX39 (SEQ ID NO: 83)
TCTCACTCCTCGAGTGGTAGTTTGAACGCATGGCAACCGCGGTCATGGGTGGGGGGCGCGT TCCGGTCACACGCCAACAATAACTTGAACCCCAAGCCCACCATGGTTACTNGTCACCCTAC CTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
DCX42 (SEQ ID NO: 84)
TCTCACTCCTCGAGGTATTCGGGTTTGTCCCCGCGGGACAACGGTCCCGCTTGTAGTCAGG AGGCTACCTTGGAGGGTTGTGGTGCGCAGAGGCTGATGTCCACCCGTCGCAAGGGCCGCAA CTCCCGCCCCGGGTGGACGCTCTCTAGAATCGAAGGTCGCGCTAGACCCTTCGAGA
DCX45 (SEQ ID NO: 85) TCTCACTCCTCGAGCGTGGGGAATGATAAGACTAGCAGGCCGGTTTCCTTCTACGGGCGCG TTAGTGATCTGTGGAACGCCAGCTTGATGCCGAAGCGTACTCCCAGCTCGAAGCGCCACGA TGATGGCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX2 (SEQ ID NO: 86)
TCTCACTCCTCGAGTACTCCCCCCAGTAGGGAGGCGTATAGTAGGCCCTATAGTGTCGATA GCGATTCGGATACGAACGCCAAGCACAGCTCCCACAACCGCCGTNTGCGGACGCGCAGCCG CCCGAACTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX9 (SEQ ID NO: 87)
TCTCACTCCTCGAGATGGCCTAGTGTGGGTTACAAGGGTAATGGCAGTGACACTATTGATG TTCACAGCAATGACGCCAGTACTAAGAGGTCCCTCATCTATAACCACCGCCGCCCCNTCTT TCCCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX14 (SEQ ID NO: 88)
TCTCACTCCTCGAGAACGTTTGAGAACGACGGGCTGGGCGTCGGCCGGTCTATTCAGAAGA AGTCGGATAGGTGGTACGCCAGCCACAACATTCGTAGCCATTTCGCGTCCATGTCTCCCGC TGGTAAGTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX15 (SEQ ID NO: 89)
TCTCACTCCTCGAGCTATTGTCGGGTTAAGGGTGGTGGGGAGGGGGGGCATACGGATTCCA ATCTGGCTAGGTCGGGTTGTGGTAAGGTGGCCAGGACCAGCAGGCTTCAGCATATCAACCC GCGCGCTACCCCCCCCTCCCGGTCTAGAATCGAAGGTC
PAX16 (SEQ ID NO: 90) TCTCACTCCTCGAGTTGGACTCGGTGGGGCAAGCACANTCATGGGGGGTTTGTGAACAAGT CTCCCCCTGGGAAGAACGCCACGAGCCCCTACACCGACGCCCAGCTGCCCAGTGATCAGGG TCCTCCCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX17 ( SEQ ID NO : 91 )
TCTCACTCCTCGAGTCAGGTTGATTCGTTTCGTAATAGCTTTCGGTGGTATGAGCCGAGCA GGGCTCTGTGCCATGGTTGTGGTAAGCGCGACACCTCCACCACTCGTATCCACAATAGCCC CAGCGACTCCTATCCTACACGCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX18 (SEQ ID NO: 92)
TCTCACTCCTCGAGCTTTTTGCGGTTCCAGAGTCCGAGGTTCGAGGATTACAGTAGGACGA TCTNTCGGTTGCGCAACGCCACGAACCCGAGTAATGTCTCCGATGCGCACAATAACCGGGC CTTGGCCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX35 (SEQ ID NO: 93)
TCTCACTCCTCGAGGAGCATCACCGACGGGGGCATCAATGAGGTGGACCTGAGTAGTGTGT CGAACGTTCTTGAGAACGCCAACTCGCATAGGGCCTACAGGAAGCATCGCCCGACCTTGAA GCGTCCTTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX38 (SEQ ID NO: 94) TCTCACTCCTCGAGTTCGAAGGTGAGCAGCCCGAGGGATCCGACGGTCCCGCGGAAGGGCG GCAATGTTGATTATGGTTGTGGTCACAGGTCTTCCGCCCGGATGCCTACCTCCGCTCTGTC GTCGATCACGAAGTGCTACACTTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX40 (SEQ ID NO: 95)
TCTCACTCCTCGAGAGCCAGTANGCAGGGCGGCCGGGGTGTTGCCCCTGAGTTTGGGGCGA GCGTTTTGGGTNGTGGTTGTGGTAGCGCCACTTATTACACGAACTCCACCAGCTGCAAGGA TGCTATGGGCCACAACTACTCGTCTAGAATCGAAGGTCGCGNTAGACCTTCGAGA
PAX43 (SEQ ID NO: 96)
TCTCACTCCTCGAGATGGTGCGAGAAGCACAAGTTTACGGCTGCGCGTTGCAGCGCGGGGG CGGGTTTTGAGAGGGANGCCAGCCGTCCGCCCCAGCCTGCCCACCGGGATAATACCAACCG TAATGCNTNTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX45 (SEQ ID NO: 97)
TCTCACTCCTCGAGTTTTCAGGTGTACCCGGACCATGGTCTGGAGAGGCATGCTTTGGACG GGACGGGTCCGCTTTACGCCATGCCCGGCCGCTGGATTAGGGCGCGTCCGCAGAACAGGGA CCGCCAGTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
PAX46 (SEQ ID NO: 98)
TCTCACTCCTCGAGCAGGTGTACGGACAACGAGCAGTGCCCCGATACCGGGANTAGGTCTC GTTCCGTTAGTAACGCCAGGTACTTTTCGAGCAGGTTGCTCAAGACTCACGCCCCCCATCG CCCTTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
P31 (SEQ ID NO: 99) TCTCACTCCTCGAGTGCCAGGGATAGCGGGCCTGCGGAGGATGGGTCCCGCGCCGTCCGGT TGAACGGGGTTGAGAACGCCAACACTAGGAAGTCCTCCCGCAGTAACCCGCGGGGTAGGCG CCATCCCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
P90 ( SEQ ID NO : 100 )
TCTCACTCCTCGAGTTCCGCCGATGCGGAGAAGTGTGCGGGCAGTCTGTTGTGGTGGGGTA GGCAGAACAACTCCGGTTGTGGTTCGCCCACGAAGAAGCATCTGAAGCACCGCAATCGCAG TCAGACCTCCTCTTCGTCCCACTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA 5PAX3 (SEQ ID NO: 101)
TCTCACTCCTCGAGACCGAAGAACGTGGCCGATGCTTATTCGTCTCAGGACGGGGCGGCGG CCGAGGAGACGTCTCACGCCAGTAATGCCGCGCGGAAGTCCCCTAAGCACAAGCCCTTGAG GCGGCCTTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
5PAX5 (SEQ ID NO: 102) TCTCACTCCTCGAGAGGCAGTACGGGGACGGCCGGCGGCGAGCGT
TGCACACCAGGGATAACGCCAGCGGCAGCGGTTTCAAACCGTGGTACCCTTCGAATCGGGG TCACAAGTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
5PAX7 (SEQ ID NO: 103)
TCTCACTCCTCGAGGTGGGGGTGGGAGAGGAGTCCGTCCGACTACGATTCTGATATGGACT TGGGGGCGAGGAGGTACGCCACCCGCACCCACCGCGCGCCCCCTCGCGTCTTGAAGGCTCC CCTGCCCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
5PAX12 (SEQ ID NO: 104)
TCTCACTCCTCGAGGCACTGGAAGTGCGAGGGCTCTCAGGCTGCCTACGGGGACAAGGATA TCGGGAGGTCCAGGGGTTGTGGTTCCATTACAAAGAATAACACTAATCACGCCCATCCTAG CCACGGCGCCGTTGCTAAGATCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
HAX9 (SEQ ID NO: 105)
TCTCACTCCTCGAGCCGCGAGGAGGCGAACTGGGACGGCTATAAGAGGGAGATGAGCCACC GGAGTCGCTTTTGGGACGCCACCCACCTGTCCCGCCCTCGCCGCCCCGCTAACTCTGGTGA CCCTAACTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA HAX40 (SEQ ID NO: 106)
TCTCACTCNTCGAGAGAGTTCGCGGAGAGGAGGTTGTGGGGGTGTGATGACCTGAGTTGGC GTCTCGACGCGGAGGGTTGTGGTCCCACTCCGAGCAATCGGGCCGTCAAGCATCGCAAGCC CCGCCCACGCTCCCCCGCACTCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
HAX42 (SEQ ID NO: 107) TCTCACTCNTNGAGTGATCACGCGTTGGGGACGAATCTGAGGTCTGACAATGCCAAGGAGC CGGGTGATTACAACTGTTGTGGTAACGGGAACTCTACCGGGCGAAAGGTTTTTAACCGTAG GCGCCCCTCCGCCATCCCCANTTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
HCA3 (SEQ ID NO: 108)
TCTCACTCCTCGAGGCATATTTCTGAGTATAGCTTTGCGAATTCCCACTTGATGGGTGGCG AGTCCAAGCGGAAGGGTTGTGGTATTAACGGCTCCTTTTCTCCCACTTGTCCCCGCTCCCC CACCCCAGCCTTCCGCCGCACCTCTAGAATCGAAGGTCGCGCTAGACCTTCGAGA
H40 ( SEQ ID NO : 109 )
TCTCACTCCTCGAGCCGGGAGAGCGGGATGTGGGGTAGTTGGTGGCGTGGTCACAGGTTGA ATTCCACGGGGGGTAACGCCAACATGAATGCTAGTCTGCCCCCCGACCCCCCTGTTTCCAC TCCGTCTAGAATCGAAGGTCGCGCTAGACCTTCGAG
Peptide Motifs
By comparison of the amino acid sequences of the clones binding GIT receptors, certain sequence similarities or "motifs" were recognized. These motifs can often represent the part of the sequence that is important for binding to the target. Table 9 identifies regions of sequence similarity or sequence motifs (in boldface) that were identified among GIT binding peptides (corresponding SEQ ID NOS. are shown in Table 7) .
Table 9
PEPT-
HPT1
P31 SARDSGPAEDGSRAVRLNGVENANTRKSSRSNPRGRRHP
PAX9 RWPSVGYKGNGSDTIDVHSNDASTKRSLIYNHRRPLFP
HAX42 SDHALGTNLRSDNAKEPGDYNCCGNGNSTGRK-VFNRRRPSAIPT
PAX2 STPPSREAYSRPYSVDSDSDTNAKHSSHNRRLRTRSRPN hSI
SNilO RVGQCTDSDVRRP ARSCAHQGCGAGTRNSHGCITRPLRQASAH
SNi38 RGAADQRRG SENLGLPRVGWDAIAHNSYTFTSRRPRPP
S15 RSGAYESPDGRGGRSYVGGGGGCGNIGRKHNLWGLRTASPACWD
SNi34 SPCGGSWGRFMQGGLFGGRTDGCGAHRNRTSASLEPPSSDY
D2H
DAB10 SKSGEGGDSSRGETGWARVRSHAMTAGRFRWYNQLPSDR
DAB30 SGFWEFSRGLWDGENRKSVRSGCGFRGSSAQGPCPVTPATIDKH
DCX8 RYKHDIGCDAGVDKKSSSVRGGCG-AHSSPPRAGRGPRGTMVSRL
Phage Binding to Caco-2 Cells
Phage expressing presumed GIT binding peptide inserts were also assayed by ELISA on fixed Caco-2 or C2BBel cells as follows. Cells were plated at 1 x 105 cells/well on 100 μl culture media and incubated at 30°C in 5% C02 overnight. 100 μl 25% formaldehyde was added to each well for 15 minutes. Contents of the wells were removed by inverting the plate. The plate was then washed 3 times with
DPBS . 0.1% phenylhydrazine DPBS solution was added to each well and incubated for 1 hr at 37°C. The plate was inverted and washed 3 times. The plate was blocked with 0.5% BSA-DPBS for 1 hr at room temperature. The plate was inverted and washed 3 times with 1% BPT (PBS containing 1% BSA and 0.05% Tween20) . Phage diluted with 1% BPT was added to wells containing fixed cells. Wells without phage added were used to determine background binding of the HRP conjugate. The plates were incubated 2-3 hours on a rotor at room temperature. Plates were washed as before. Plates were incubated with dilute anti-M13-HRP antibody in 1% BPT for 1 hour at room temperature. Following washing, TMB substrate was added and absorbance of the plates were read at 650 nm. Table 10 shows the relative binding of phage encoding peptides to fixed Caco-2 cells.
Table 10.
Relative binding of phage encoding peptides to fixed Caco-2 cells
Fixed Caco-2
Phage cell binding
SNilO ++
SNi34 +
P31 ++
5PAX5 ++
PAX2 +
HAX42 +
DCX8 +++
DCX11 +
HI +
M13mpl 18 -
In vivo phage selection: Further selection of phage expressing peptides capable of binding to the GIT or transporting the GIT was done as follows. The purified library was resuspended in a
buffer, such as TBS or PBS, and introduced onto one side of a tissue barrier, e.g., injected into the duodenum, jejunum, ileum, colon or other in vivo animal site using, for instance, a closed loop model or open loop model. Following injection, samples of bodily fluids located across the tissue barrier, e.g., samples of the portal circulation and/or systemic circulation, were withdrawn at predetermined time points, such as 0 to 90 minutes and/or 2 to 6 hours or more. An aliquot of the withdrawn sample (e.g., blood) was used to directly infect a host, e.g., E. coli , in order to confirm the presence of phage. The remaining sample was incubated, e.g., overnight incubation with E. coli at 37°C with shaking. The amplified phage present in the culture can be sequenced individually to determine the identity of peptides coded by the phage or, if further enrichment is desired, can be precipitated using PEG, and resuspended in PBS. The phage can then be further precipitated using PEG or used directly for administration to another animal using a closed or open GIT loop model system. Portal or systemic blood samples are collected and the phage transported into such circulation systems is subsequently amplified. In this manner, administration of the phage display library with, if desired, repeat administration of the amplified phage to the GIT of the animal, permitted the selection of phage which was transported from the GIT to the portal and/or systemic circulation of the animal .
If desired, following administration of the phage display library to the tissue barrier (e.g., GIT) of the animal model, the corresponding region of the tissue barrier can be recovered at the end of the procedures given above. This recovered tissue can be washed repeatedly in suitable buffers, e.g., PBS containing protease inhibitors and homogenized in, for example, PBS containing protease inhibitors. The homogenate can be used to infect a host, such as E. coli , thus permitting amplification of phages which bind tightly to the tissue barrier (e.g., intestinal tissue) . Alternatively, the recovered tissue can be
homogenized in suitable PBS buffers, washed repeatedly and the phage present in the final tissue homogenate can be amplified in E. coli . This approach permits amplification
(and subsequent identification of the associated peptides) of phages which either bind tightly to the tissue barrier (e.g., intestinal tissue) or which are internalized by the cells of the tissue barrier (e.g., epithelial cells of the intestinal tissue) . This selection approach of phage which bind to tissues or which are internalized by tissues can be repeated.
Treatment of animal tissue barriers in vivo with phage display populations
The purified phage display library (random or preselected) was diluted to 500 μl in PBS buffer and injected into the closed (or open) intestinal loop model (e.g., rat, rabbit or other species) . At time 0 and at successive time points after injection, a sample of either the portal circulation or systemic circulation was withdrawn. An aliquot of the withdrawn blood was incubated with E. coli , followed by plating for phage plaques or for transduction units or for colonies where the phage codes for resistance to antibiotics such as tetracycline. The remainder of the withdrawn blood sample (up to 150 μl) was incubated with 250 μl of E. coli and 5 ml of LB medium or other suitable growth medium. The E. coli cultures were incubated overnight by incubation at 37°C on a shaking platform. Blood samples taken at other time points (such as 15 min, 30 min, 45 min, 60 min, up to 6 hours) were processed in a similar manner, permitting amplification of phages present in the portal or systemic circulation in E. coli at these times. Following amplification, the amplified phage was recovered by PEG precipitation and resuspended in PBS buffer or TBS buffer. The titer of the amplified phage, before and after PEG precipitation, was determined. The amplified, PEG precipitated phage was diluted to a known phage titer
(generally between 108 and 1010 phage or plaque forming units (p.f.u.) per ml) and was injected into the GIT of the animal
closed (or open) loop model. Blood samples were collected from portal and/or systemic circulation at various time points and the phage transported into the blood samples were amplified in E. coli as given above for the first cycle. Subsequently, the phage was PEG-precipitated, resuspended, titered, diluted and injected into the GIT of the animal closed (or open) loop model. This procedure of phage injection followed by collection of portal and/or systemic blood samples and amplification of phage transported into these blood samples can be repeated, for example, up to 10 times, to permit the selection of phages which are preferentially transported from the GIT into the portal and/or systemic circulation.
6.7. Transport of Phage From Rat Lumen Into the Portal and Systemic Circulation
Phage from random phage display libraries as well as control phage were injected into the lumen of the rat gastro-intestinal tract ( in si tu rat closed loop model) . Blood was collected over time from either the systemic circulation or portal circulation and the number of phage which were transported to the circulation was determined by titering blood samples in E. coli .
The phage display libraries used in this study were D38 and DC43 in which gene III codes for random 38-mer and 43-mer peptides, respectively. As a negative control, the identical phage M13mpl8, in which gene III does not code for a "random" peptide sequence, was used. Both the library phages D38 and DC43 were prepared from E. coli , mixed together, dialyzed against PBS, precipitated using PEG/NaCl and were resuspended in PBS buffer. The M13mpl8 control was processed in a similar manner. The titer of each phage sample was determined and the phage samples were diluted in PBS to approximately the same titers prior to injection into the rat closed loop model .
For sampling from the systemic circulation, approximately 15 cm of the duodenum of Wistar rats was tied
off (closed loop model), approximately 0.5ml of phage solution was injected into the closed loop and blood (0.4ml) was sampled from the tail vein at various times. The time points used (in min) were: 0, 15, 30, 45, 60, 90, 120, 180, 240 and 300 minutes. For sampling from the portal circulation, the portal vein was catheterized, approximately 15 cm of the duodenum was tied off (closed loop model), 0.5ml of phage solution was injected into the closed loop and blood was sampled from the portal vein catheter at various times. As the portal sampling is delicate, sampling times were restricted to 15, 30, 45 and 60 minutes, where possible. The volume of phage injected into each animal was as follows:
ANIMALS ( 15 ) VOLUME OF PHAGE INJECTED R1 -R3 0 . 50 ml
R4 0 . 43 ml
R5 - R15 0 . 45 ml
The estimated number of transported phage has been adjusted to account for differences in volume injected into each animal (using 0.5 ml as the standard volume) .
To investigate transport into the systemic circulation, animals Rl , R2 and R3 received the control phage M13mpl8 and animals R4 , R5 , R6 and R7 received the test phage D38/DC43 mix. To investigate transport into the portal circulation, animals R8 , R9 and R10 received the control phage M13mpl8 and animals Rll, R12, R13 and R14 received the test phage D38/DC43 mix. Animal R15* received the combined phage samples from animals R4-R7 (see Table 11) which were sampled from the systemic circulation on day one, followed by amplification in E. coli , PEG precipitation and resuspension in PBS. On subsequent analysis, the titer of this phage was found to be 100 times greater than the other phage samples used for animals R8-R14. Thus, the data presented for animal R15* is adjusted down.
Approximately 0.4 ml of the blood was collected at each time point in each model system. 30 μl of the collected blood (systemic) was mixed with 100 μl of the prepared E. coli strain K91Kan, incubated at 37°C for 30 min, and plated out for plaque formation using Top Agarose on LB plates. Various negative controls were included in the titering experiments. The following day, the number of plaque forming units was determined. Similarly, 30 μl of the collected blood (portal) and serial dilutions (1:100, 1:1000) thereof was mixed with 100 μl of the prepared E. coli strain K91Kan, incubated at 37°C for 30 min, and plated out for plaque formation using Top Agarose on LB plates. The following day, the number of plaque forming units was determined. In addition, approximately 300 μl of the collected blood from each time point (systemic and portal) was incubated with 5ml of prepared E. coli strain K91Kan in modified growth media containing 5mM MgCl2/MgS04 at 37°C overnight with shaking (to permit phage amplification) . The samples were centrifuged and the cell pellet was discarded. Samples of the phage supernatant were collected, serially diluted (10~2, 10-4, 10"6, 10"8) in TBS buffer, and plated for plaques in order to determine the number of plaque forming units present in the amplified phage samples. Furthermore, an aliquot of phage was removed from the "amplified" supernatants obtained from test animals R4-R7 (samples from each time point were used) , combined, and precipitated using PEG for two hours. The precipitated phage was resuspended in PBS buffer and was injected into closed loop model of animal R15*, followed by portal sampling.
The number of phage transported from the closed loop model into the systemic circulation is presented in Table 11 hereafter. The number of phage transported from the closed loop model into the portal circulation is presented in Table 12 hereafter. These numbers are corrected for phage input difference and for volume input differences. Clearly, more phage are present in the portal samples than in the
systemic samples, indicative of either hepatic or RES clearance and/or phage instability in the systemic circulation. In addition, the uptake of phage from the GIT into the portal circulation is quite rapid, with substantial 5 number of phages detected within 15 minutes. The results from the portal sampling experiments would also indicate that the kinetics of uptake of phage from the D38/DC43 libraries is quicker than that of the control phage. Thus, there may be preferential uptake of phage coding for random peptide 0 sequences from the GIT into the portal circulation. In the case of animals R13, R14 and R15*, the % of the phage transported into the titered blood sample within the limited time frame (30, 45 and 15 mins, respectively) was estimated as 0.13%, 1.1% and 0.013%, respectively. 5
TABLE 11
NUMBER OF PHAGE TRANSPORTED FROM THE CLOSED LOOP MODEL INTO THE SYSTEMIC CIRCULATION
Animals Rl , R2 and R3 received the control phage M13mpl8.
Animals R4 , R5 , R6 and R7 received the test phage 5 D38/DC43 mix.
Table 12
NUMBER OF PHAGE TRANSPORTED FROM THE CLOSED LOOP MODEL INTO THE PORTAL CIRCULATION
Animals R8 , R9 and R10 received the control phage
M13mpl8.
Animals Rll, R12, R13 and R14 received the test phage D38/DC43 mix.
Animal R15* received the combined phage samples from animals R4-R7 (see Table 11) which were sampled from the systemic circulation on day one, followed by PEG precipitation and resuspension in PBS. On subsequent analysis, the titer of this phage was found to be 100 times greater than the other phage samples used for animals R8-R14. Thus, the data measuring phage transport into the portal circulation for animal R15* is adjusted down.
These studies demonstrated that both the control phage and the D38/DC43 phages are transported over time from the lumen of the GIT into the portal and systemic circulation, as demonstrated by titering the phage transported to the blood in E. coli . More phage were transported from the test phage samples into the portal circulation than the corresponding control phage sample . In addition, the kinetics of transport of the test phage into the portal circulation appeared to exceed that of the control phage. Phage from the D38/DC43 libraries which appeared in the systemic circulation of different animals (R4-R7) were pooled, amplified in E. coli , precipitated, and re-applied to the lumen of the GIT, followed by collection in the portal circulation and titering in E. coli . These selected phage were also transported from the lumen of the GIT into the portal circulation. This in si tu loop model may represent an
attractive screening model in which to identify peptide sequences which facilitate transport of phage and particles from the GIT into the circulation.
Using this screening model system, a number of preselected phage libraries now exist, including a one pass systemic phage library from animals R4-R7, a one-pass portal library from animals R11-R14, and a two pass, rapid transport, systemic-portal phage library SP-2 from animal R15*.
6.8. Transport of Phage From Preselected Phage Libraries From the Rat Lumen Into the Portal and Systemic Circulation
Four preselected phage libraries, GI-D2H, Gl-hSI, GI-
HPT1 and GI -hPEPTl, were constructed by pooling phage previously selected by screening random phage display libraries D38 and DC43 using the HPTl, HPEPTl, D2H and hSI receptor or binding sites located in the GIT. The phage pools, preselected phage libraries are shown in Table 13.
Note that the sequences for PAX2 , HAX1 , HAX5 , HAX6 , HAX10,
H10 and HAX44 are the same. Also, the sequence for HAX40 is the same as that for H44. The corresponding SEQ ID NOS. are shown in Table 7.
Table 13
PRESELECTED PHAGE LIBRARIES
D2H HSI HPTl hPEPTl
DAB3 S15 HAX9 PAX2 (H10)
DAB7 S21 HAX35 PAX9
DAB10 S22 HAX40 (H44) PAX14
DAB18 SNilO HAX42 PAX15
DAB24 SNi28 HCA3 PAX16
DAB30 SNi34 HAX1 PAX17
DAX15 SNi38 HAX5 PAX18
DAX23 SNi45 HAX6 PAX35
DAX24 SNiAX2 HAX10 PAX38
DAX27 SNiAX6 H40 PAX40
DCX8 SNiAX8 M13mpl8 PAX43
DCX11 M13mpl8 PAX45
DCX26 PAX46
DCX33 P31
DCX36 P90
DCX39 5PAX3
DCX42 5PAX5
DCX45 5PAX7
M13mpl8 5PAX12
H40 M13mpl8
Similar to methods described herein above, these preselected phage libraries together with the negative control phage M13mpl8 were injected into the rat closed loop model (6 animals per preselected phage library) , blood was collected over time from the portal circulation via the portal vein and, at the termination of the experiment, a systemic blood sample was collected from the tail vein and the intestinal tissue region from the closed loop was collected.
In particular, phages selected in vi tro to each receptor or binding site located in the GIT were amplified in E. coli , PEG-precipitated, resuspended in TBS and the titer of each phage sample was determined by plaquing in E. coli as described above. Subsequently, an equal number of each phage (8 x 108 phage) for each receptor site was pooled into a preselected phage library together with the negative control phage M13mpl8 and each preselected phage library was administered to 6 Wistar rats per library (rats 1-6; GI-D2H, rats 7-12; Gl-hSI, rats 13-18; GI -hPEPTl, and rats 19-24; GI- HPT1) . Using the in si tu loop model described above, 0.5 ml of preselected phage library solution was injected into the tied-off portion of the duodenum/jejunum. Blood was collected into heparinized tubes from the portal vein at 0, 15, 30, 45 and 60 minutes. A blood sample was taken from the systemic circulation at the end of the experiment. Similarly, the portion of the duodenum/jejunum used for phage injection was taken at the end of the experiment.
Thirty microliters of the collected portal blood (neat and 10~2, 10"4, 10"6 dilutions) was added to 30 μl E. coli K91Kan cells (overnight culture) and incubated at 37°C for 10 min. Subsequently, 3 ml of top agarose was added and the samples were plated for plaques. One hundred microliters of
the collected portal blood was added to lOOμl of E. coli K91Kan. Five milliliters of LB medium was then added and the samples were incubated at 37°C overnight in a rotating microbial incubator. The E. coli was removed by centrifugation and the amplified phage supernatant samples were either titered directly or were PEG-precipitated, resuspended in TBS and titered. Following titration of the amplified phage, samples containing phage from each set of animals were combined, adjusting the titer of each sample to the same titer, and were plated for plaques on LB agar plates (22cm2 square plates). Either 12,000 or 24,000 phage were plated for plaques .
Thirty microliters of the collected systemic blood (neat and 10"2, 10"4, 10"6 dilutions) was added to E. coli K91Kan cells, incubated at 37°C for 10 min. Three ml of top agarose was then added and the samples were plated for plaques. One hundred microliters of the collected systemic blood was added to lOOμl of E. coli K91Kan, incubated at 37°C for 10 min. Five milliliters of LB medium was then added and the samples were incubated at 37°C overnight in a rotating microbial incubator. The E. coli was removed by centrifugation and the amplified phage supernatant samples were either titered directly or were PEG-precipitated, resuspended in TBS and titered. Following titration of the amplified phage, samples containing phage from each set of animals were combined, adjusting the titer of each sample to the same titer, and were plated for plaques on LB agar plates (22cm2 square plates) . Either 12,000 or 24,000 phage were plated for plaques . The intestinal tissue portion used in each closed loop was excised. The tissue was cut into small segments, followed by 3 washings in sterile PBS containing protease inhibitors, and homogenized in an Ultra thorex homogeniser (Int-D samples) . Alternatively, the tissue (in PBS supplemented with protease inhibitors) was homogenized in an
Ultra Thorex homogenizer, washed 3 times in PBS containing protease inhibitors and resuspended in PBS containing
protease inhibitors (Int-G samples) . In each case, serial dilutions (neat and 10"2, 10"4, 10"6 dilutions) of the tissue homogenate was titered in E . coli . In addition, an aliquot (lOOμl) of the tissue homogenate was added to lOOμl of E . coli K91Kan, incubated at 37°C for 10 min, followed by addition of 5ml of LB medium and incubation overnight at 37°C in a rotating microbial incubator.
The phage amplified from the portal blood, systemic blood and intestinal tissue was plated for plaques. The plaques were transferred to Hybond-N Nylon filters, followed by denaturation (1.5M NaCI, 0.5M NaOH), neutralization (0.5M TRIS-HC1, pH7.4, 1.5M NaCI), and washing in 2X SSC buffer. The filters were air-dried, and the DNA was cross-linked to the filter (UV crosslinking: 2min, high setting) . The filters were incubated in pre-hybridization buffer (6X SSC, 5X Denhardt's solution, 0.1% SDS, 20μg/ml yeast tRNA) at 40°C- 45°C for at least 60 min.
Synthetic oligonucleotides, (22-mers), complimentary to regions coding for the receptor or binding sites used to create the preselected phage library, were synthesized (see Table 14 below) .
Table 14
OLIGONUCLEOTIDES USED IN IN VIVO SCREEN
CLONE NAME OLIGO SEQ.
ID. NO,
S15 MCCGGACTCTCATAAGCGCCGG3' 111
S21 5'ACAACGGGCCAGAAAGAGCGAG3 ' 112 S22 5 'ACACCACCCCAATCGGAGCTAC3 ' 113 SNilO 5'TCAGAATCCGTGCACTGGCCAA3' 114 SNi28 5'GCCCTATTCATACCACCGGAGT3 ' 115 SNi34 5' CATCAGTCCTACCGCCGAAAAG3 ' 116 SNi38 5' CGTATAGCTATTGTGAGCGATG3 ' 117 SNi45 5'ACGCGCGGAACGAGCAGTACCA3 ' 118 SNiAX2 5' CCATAATGATCCCCGTCACTAT3' 119 SNiAX6 5 'AGACACCCCTTAGCCGTCGTAG3 ' 120 SNiAXδ 5 'AGCTCCGTGACCTTAGTCATAA3 ' 121
CLONE NAME OLIGO SEQ. ID. NO.
DAB3 5 ' TGCACAGCTCAGCGCCGCACCA 3 122
DAB7 5'ACGGGTCATCAGCGCCGCACCA 3 123
DAB10 MGTCACCCCCCTCCCCGGACTT 3 124
DAB18 5'ACTCGCAATTATTGGCGCTCGA 3 125
DAB24 5'GTCTTCTCAACCTTATCCTGCG 3 126
DAB30 5 'AAAGCCCCCTGCTAAACTCCCA 3 127
DAX15 5'CTGCGTCTGCCACGTCGTCATC 3 128
DAX23 5'GTTAAAAGAGGGCAAGCTCGGA 3 129
DAX24 MCGAGTTCTTGATGTCCTCCAT 3 130
DAX27 B'TCCAATGCCTGTACCACGGATG 3 131
DCX8 5'TCGCAACCGATATCGTGCTTAT3 ' 132
DCX11 5'TGCATACACTGCTTGGAGCCCT3 ' 133
DCX26 5 'GAAATCTCACTAGTAGTCCGCC3' 134
DCX33 5'GCGGGCAAGACAGTCCAATTCC3 ' 135
DCX36 5'GAGCTCCAATTCCACGACGACC3 ' 136
DCX39 5 'GGTTGCCATGCGTTCAAACTAC3 ' 137
DCX42 5 'TCCCGCGGGGACAAACCCGAAT3 ' 138
DCX45 5' CTGCTAGTCTTATCATTCCCCA3 ' 139
PAX2 5 ' CTATCGACACTATAGGGCCTAC3 ' 140
PAX9 5 'TACCCTTGTAACCCACACTAGG3 ' 141
PAX14 5.TTCTTCTGAATAGACCGGCCGA3 ' 142
PAX15 5 ' CCACCACCCTTAACCCGACAAT3 ' 143
PAX16 5 'AGGGGGAGACTTGTTCACAAAC3 ' 144
PAX17 5' CGGCTCATACCACCGAAAGCTA3 ' 145
PAX18 5 'ATCGTCCTACTGTAATCCTCGA3 ' 146
PAX35 5 'GACACACTACTCAGGTCCACCT3' 147
PAX38 5' CCATAATCAACATTGCCGCCCT3 ' 148
PAX40 5' CAAAACGCTCGCCCCAAACTCA3 ' 149
PAX43 5'GTAAACTTGTGCTTCTCGCACC3 ' 150
PAX45 5' CCATGGTCCGGGTACACCTGAA3' 151
PAX46 5'GTTACTAACGGAACGAGACCTA3 ' 152
P31 5 'TGTTGGCGTTCTCAACCCCGTT3 ' 153
P90 5 'ACAACCGGAGTTGTTCTGCCTA3 ' 154
5PAX3 5'TAAGCATCGGCCACGTTCTTCG3 ' 155
5PAX5 5'TTATCCCTGGTGTGCAGGTTGA3 ' 156
CLONE NAME OLIGO SEQ.
ID. NO.
5PAX7 MATCAGAATCGTAGTCGGACGG3' 157
5PAX12 MTTTGTAATGGAACCACAACCC3' 158
HAX9 MGGTGGCTCATCTCCCTCTTAT3' 159 HAX35 5'ATCAGACTGGCTGGGACCACAA3' 160
HAX40 5'CACAACCTCCTCTCCGCGAACT3' 161
HAX42 5'AGATTCGTCCCCAACGCGTGAT3' 162
HCA3 5'GGGAATTCGCAAAGCTATACTC3' 163
H40 5'CCCCGTGGAATTCAACCTGTGA3' 164 M13 (positive) 5'GTCGTCTTTCCAGACGT3' 165
Ml3 (negative) 5'CTTGCATGCCTGCAGGTCGAC3' 166
The oligonucleotides (5pmol) were 5 ' end labelled with 32P-ATP and T4 polynucleotide kinase and approximately 2.5pmol of labelled oligonucleotide was used in hybridization studies. Hybridizations were performed at 40-45°C overnight in buffer containing 6X SSC, 5X Denhardt's solution, 0.1% SDS, 20μg/ml yeast tRNA and the radiolabeled synthetic oligonucleotide, followed by washings (20-30 min at 40-45°C) in the following buffers: (i) 2X SSC / 0.1% SDS, (ii) IX SSC / 0.1% SDS, (iii) 0. IX SSC / 0.1% SDS. The filters were air-dried and exposed for autoradiography for 15 hours, 24 hours or 72 hours.
Hybridization data indicated that all the oligonucleotide probes bound specifically to their phage target except for the HAX9 probe which apparently was not labeled. A negative control probe that hybridized only to M13mpl8 DNA showed a weak to negative signal in all samples tested (data not shown) .
Hybridization data for pools from each receptor group of rats was compiled. Tables 15, 16, 17 and 18 show a representative compilation of autoradiograph signals of the HSI, D2H, HPTl and hPEPTl receptor groups. These Tables show the phage absorption and uptake from the closed loop GIT model to portal and systemic circulation and phage absorption/internalization to intestinal tissue. In these Tables, Int-G refers to intestinal tissue homogenized prior
to washing and recovery while Int-D refers to intestinal tissue washed prior to homogenization and phage recovery. In all cases, leading phage candidates were present in more than one animal .
Table 15 SUMMARY OF AUTORADIOGRAPH SIGNALS OF HSI ANIMAL STUDY
*not detected
Table 16 SUMMARY OF AUTORADIOGRAPH SIGNALS OF D2H ANIMAL STUDY
Table 17 SUMMARY OF AUTORADIOGRAPH SIGNALS OF HPTl ANIMAL STUDY
Table 18 SUMMARY OF AUTORADIOGRAPH SIGNALS OF hPEPTl ANIMAL STUDY
Apart from the synthetic oligonucleotide to HAX9 , all oligonucleotides were initially confirmed to be radiolabeled, as determined by hybridization to the corresponding phage target (eg., phage S15 hybridized to the oligonucleotide S15) . In addition, under the experimental conditions used, the oligonucleotides essentially did not hybridize to the negative control phage template M13mpl8. Two oligonucleotides were synthesized to the phage M13mpl8 : (1) a positive oligonucleotide which hybridizes to a conserved sequence in both M13mpl8 and each of the GIT receptor or GIT binding site selected phages [designated M13 (positive) ] ; and (2) a negative oligonucleotide which only hybridizes to a sequence unique to the multiple cloning site of phage M13mpl8 and which does not hybridize to any of the GIT receptor or GIT binding site selected phages.
In the case of the hSI pool of phages, only four phages were transported from the closed loop model into the portal circulation: phages S15, SNi-10, SNi-34 and SNi-38. The other phages, S21, S22, SNi-28, SNi-45, SNiAX-2, SNiAX-6 and SNiAX-8, were not transported from the GIT into the portal circulation. In addition, phages SNi-10 and to a lesser extent phages S15 and S22 were found in the intestine samples or fractions, whereas the other phages were not. There was a very low presence (<0.1%) of the phage M13mpl8 in the Int-G samples. These results show that phages can be further selected from pre-selected libraries, permitting the identification of phages which are transported from the GIT closed loop into the portal circulation or phages which bind to or are internalized by intestinal tissue. In the case of the D2H pool of phages, there was a rank order by which phages were transported from the GIT closed loop model into the portal circulation, with phages DCXll and DAB10 preferably transported, followed by phages DCX8 , DAB30, DAB3 and DAB7. A number of phages from this pool were not transported into the portal circulation, including phages DAB18, DAB24, DAX15, DAX24, DAX27, DCX26, DCX36, DCX39, DCX42, DCX45. There is a very low level of transport of phage DAX23 from the GIT into the portal circulation. Similarly, only some of the phages were found in the intestinal samples fractions, including phages DAB30, DCX33, DAB7, DCXll, DCX45 and to a much lesser extent phages DAB3 , DAB10, DCX8 , DCX39, DCX42. Some phages were not found in the intestinal samples, including phages DAB18, DAB24, DAX15, DAX24, DCX26, and DCX36. There was a very low presence (<0.1%) of the phage M13mpl8 in the Int-G samples. These results showed that phages can be further selected from pre-selected libraries, permitting the identification of phages which are transported from the GIT closed loop into the portal circulation or phages which bind to or are internalized by intestinal tissue.
In the case of the HPTl pool of phages, there was a rank order by which phages were transported from the GIT closed
loop model into the portal or systemic circulation. Phage PAX2 (which was used at a 4X concentration relative to the other phages in this pool) followed by phage HAX42 was found in the portal and systemic circulation; phage H40 was found in the systemic circulation only. None of the phages in this pool were found in the intestine samples or fractions. Phage M13mpl8 was not found in the intestine fractions or systemic circulation, with very low incidence (<0.001%) in the portal circulation. These results show that phages can be further selected from pre-selected libraries, permitting the identification of phages which are transported from the GIT closed loop into the portal and/or systemic circulation or phages which bind to or are internalized by intestinal tissue . In the case of the hPEPTl pool of phages, the phages PAX2 and H40 were also included in this pool. A number of phages from this pool were found in the portal circulation, including phages P31 (SEQ ID NO:43), PAX46, PAX9, H40, PAX17, PAX40, PAX2, PAX14, 5PAX3 and 5PAX12. A number of phages were not found in the portal blood including the negative control phage M13mpl8, PAX15, PAX16, PAX18, PAX35, PAX38, PAX43, PAX45, P90, 5PAX5 and 5PAX7. The only phage found in the systemic circulation were phages 5PAX5 and P31 (SEQ ID NO: 43) . In addition, there was preferential binding of some phages to the intestine, including phages 5PAX12 , 5PAX7,
5PAX3, H40, P31 (SEQ ID NO:43), PAX9, and to a lesser extent phages PAX38 and PAX15. Some phages were not found in the intestine samples, including the negative control phage M13mpl8 and the phages PAX2 , PAX14 , PAX16, PAX18, PAX35, PAX45, PAX46, P90 and 5PAX5. These results show that phages can be further selected from pre-selected libraries, permitting the identification of phages which are transported from the GIT closed loop into the portal and/or systemic circulation or phages which bind to or are internalized by intestinal tissue.
Further Characterization of Select Sequences
Following initial screening of the four recombinant receptor sites (hPEPTl, HPTl, D2H, hSI) of the gastrointestinal tissue, with the phage display libraries, a series of phage were isolated which showed preferential binding to the respective target receptor sites in comparison to negative control protein BSA protein and the recombinant protein recombinant human tissue factor (hTF) (which, like the recombinant receptors of the gastrointestinal tissue, contained a poly-histidine tag at its NH2-terminal end) . In subsequent experiments same titers of the selected phage which bound to each target receptor site were combined into a single pool ( i . e . , one pool of HPTl binding phage, one pool of hPEPTl binding phage, one pool of D2H binding phage, and one pool of hSI binding phage) . Each pool was supplemented with an equivalent titer of the negative control phage M13mpl8. These phage pools were injected into a closed duodenal loop region of rat intestinal tissue and subsequently phage was harvested and recovered which was bound to and retained by the intestinal tissue and/or was absorbed from the intestinal loop into the portal and/or systemic circulation. In addition, a selection of the initial phages which bound to the target recombinant receptor site were analyzed for binding to either fixed Caco-2 cells and/or to fixed C2BBel cells. The selection of the final lead peptide sequences was based on the ability of the phage, coding for that peptide sequence (1) to bind to the target recombinant receptor site in vi tro in preference to its binding to the negative control proteins BSA and/or hTFs, (2) to bind to rat intestinal tissue following injection into a closed duodenal loop of rat intestinal tissue in preference to the negative control phage M13mpl8, (3) to be absorbed from rat intestinal tissue into either the portal and/or systemic circulation following injection into a closed duodenal loop of rat intestinal tissue in preference to the negative control phage M13mpl8, and (4) to bind to either fixed Caco-2 cells or fixed C2BBel cells in phage binding
studies in preference to the negative control phage M13mpl8. Peptides were also selected with consideration to the ease of chemical synthesis.
6.9. GST Fusion Proteins of GIT Targeting Peptides Construction of GST Fusion Proteins of GI Targeting Peptides
Glutathione S-transferase (GST) vectors encoding fusion proteins of GI targeting peptides were constructed in the vector pGEX4T-2 (source, Pharmacia Biotech, Piscataway, NJ) . Briefly, single-strand DNA from the clones of interest were amplified by the polymerase chain reaction. The amplified DNA was then cleaved with the restriction enzymes Xhol and Notl and then ligated into Sall/Notl cleaved pGEX4T-2. Following transformation, the DNA sequence for each construct was verified by sequencing.
For construction of the truncated versions of the GST fusion proteins, where the inserted sequence was less than 45 base pairs, overlapping oligonucleotides containing cohesive Sail and Notl termini, and encoding the sequence of interest, were annealed and then ligated directly into Sall/Notl cleaved pGEX4T-2. Following transformation, the DNA sequence for each construct was verified.
A diagrammatic representation of the various GST fusion protein constructs that have been synthesized is indicated in Figures 5A-5C.
Expression and Purification of GST Fusion Proteins
Escherichia coli BL21 cells containing GST fusion protein constructs were grown overnight in 2X YT media containing 100 μg/ml ampicillin (2X YT/amp) . Overnight cultures were diluted 1:100 in 2X YT broth (100 ml), and cells were grown to an A600 of 0.5 at 30°C, induced with ImM isopropyl-1-thio-B-D-galactopyranoside, and grown for an additional 3 h. Cells were harvested by centrifugation and resuspended in 5 ml of PBS containing a mixture of the proteinase inhibitors (Boehringer/Mannheim) . Cells were
sonicated on ice, and the cell lysates were centrifuged at 12,000 x g for 10 minutes at 4°C. Supernatant fractions were reacted for 30 minutes at room temperature with 2 ml of a 50% slurry of glutathione-Sepharose® 4B, washed 3 times with 1.5 ml of PBS (at room temperature) , and the bound GST fusion proteins were eluted by reaction for 10 minutes at room temperature with 3 X 1ml of 10 mM reduced glutathionein 50 mM Tris HCI pH 8.0. Protein was quantified by the Bio-Rad protein assay followed by characterization by SDS- polyacrylamide gel electrophoresis.
ELISA of GST fusion peptides
The standard ELISA procedure was modified as follows. GST proteins were diluted to an appropriate concentration in PBS containing 1%BSA and 0.05% Tween20
(1%BPT) , titered and incubated one hour at room temperature. Following five washes an anti-GST monoclonal antibody was added (Sigma, St. Louis Clone GST-2 diluted 1:10,000 in 1%BPT) and incubated one hour. After five more washes goat anti-mouse IgG2b-HRP was added (Southern Biotechnology
Associates Inc., Birmingham, AL, diluted 1:4000 in 1%BPT) and incubated one hour. After five washes plates were developed with TMB peroxidase substrate (Kirkegard and Perry, Gaithersburg, MD) . All data is presented with background binding subtracted.
Figure 6 shows the binding of GST-SNilO, GST-SNi34 and GST alone to the hSI receptor and to fixed C2BBel cells.
GST Fusion Proteins of Selected GIT Targeting Peptides Results show that GST-DXB8, GST-PAX2, GST-P31, GST-
SNilO and GST-SNi34 bound fixed Caco-2 or C2BBel cells (Figures 7 and 8) relative to GST control binding. GST-HAX42, GST-5PAX5, all showed weak to moderate binding relative to GST control. Interestingly, P31 truncation 103-GST fusion protein bound almost as well as full-length P31 (SEQ ID NO: 43) to fixed Caco-2 cells (A) . This suggests the portion
of the P31 sequence (SEQ ID NO: 43) responsible for binding resides in this portion. PAX2.107 bound similarly to full- length PAX2 ; therefore, this portion most likely contains the amino acid sequence responsible for binding (B) . In preliminary assays, none of the DCX8 truncations bound similarly to full-length DCX8 to Caco-2 cells suggesting the binding region spans more than one of these pieces .
Inhibition of Binding by Synthetic Peptides Binding of GST-P31 to fixed C2BBel Cells
The standard ELISA procedure was modified as follows. GST fusion proteins and peptides were diluted to an appropriate concentration in PBS containing 1% BSA and 0.05% Tween 20. Peptides were titered, a constant concentration of diluted GST protein was added to titered peptides and the mixture was incubated one hour at room temperature . Following five washes, an anti-GST monoclonal antibody was added (Sigma, St. Louis Clone GST-2 diluted 1:10,000 in 1% BPT) and incubated one hour. After five more washes goat anti-mouse IgG2b-HRP was added (Southern Biotechnology
Associates Inc., Birmingham, AL, diluted 1:4000 in 1% BPT) and incubated one hour. After five washes plates were developed with TMB peroxidase substrate (Kirkegard and Perry, Gaithersburg, MD) . All data is presented with background binding subtracted.
Figures 9A and 9B show the inhibition of GST-P31 binding to C2BBel fixed cells. The peptide competitors are ZElan024 which is the dansylated peptide version of P31 (SEQ ID NO: 43) and ZElan044, ZElan049 and ZElan050 which are truncated, dansylated pieces of P31 (SEQ ID NO: 43) . Data is presented as O.D. vs. peptide concentration and as percent inhibition of GST-P31 binding vs. peptide concentration. Uncompeted GST-P31 binding was considered as 100% binding. IC50 values are estimates using the 50% line on the percent inhibition graph.
GST-P31 and GST-PAX2 exhibited no crossreactive binding to ZElan024 (P31) (SEQ ID NO:43) and ZElanOlδ (PAX2)
at the 0.5 μg/ml concentration used in competition assays. GST-HAX42 exhibited crossreactivity to ZElan018 (PAX2) and ZElan021 (HAX42) at the 5 μg/ml concentration used in competition assays. Figures 10A-10C present a compilation of data generated by competition ELISA of GST-P31, GST-PAX2, GST- SNilO and GST-HAX42 versus various dansylated peptides on fixed C2BBel cells. IC50 values are in μM and include ranges determined from multiple assays. The GST/C2BBel column is a summary of GST protein binding to fixed C2BBel cells.
Binding to fixed Caco-2 Cells
Caco-2 cells were fixed, treated with phenylhydrazine and blocked as described above. Synthetic peptides (lOOμg/ml) were applied in duplicate to Caco-2 cells and serially diluted down the 96 -well plate. The corresponding GST-peptide fusion protein (lOμg) was added to each well and the plates were incubated for 2h at room temperature with agitation. Binding of the GST-peptide fusion proteins to the cells was assayed using the ELISA technique described above. GST-P31 binding was inhibited by ZElan024, ZElan028 and ZElan031 as well as the two D forms ZElan053 and ZElan054. GST-PAX2 binding was inhibited by ZElan032, ZElan033, and ZElan035. GST-HAX42 binding was not inhibited by ZElan021 (full length HAX42) but it was inhibited by ZElanOlδ (PAX2) and ZElan026 and ZElan038 (scrambled PAX2 peptides) .
Transport and Uptake of GST-Peptide Fusions into Live Caco-2 Cells
Transport and uptake of GST-peptide fusions and deletion derivatives across cultured polarized Caco-2 monolayers over 4 hours in HBSS buffer was examined using an anti-GST ELISA assay. In another experiment, transport and uptake of GST-peptide fusions and deletion derivatives across
cultured polarized Caco-2 monolayers over 24 hours in serum- free medium (SFM) was examined using an anti-GST ELISA assay.
Materials Buffered Hank's balanced salt solution (bHBSS) = lx
HBSS (Gibco CN.14065-031) supplemented with 0.011M glucose (lg/1) , 25 mM Hepes (15 mM acid (3.575g/l; Sigma CN.H3375); lOmM base (2.603g/l; Sigma CN.H1016)].
Chloroquine : Made up as lOmM solution in water [Sigma CN C6628]
Lysate buffer: 30 mM Tris-HCl pH8.0 ; ImM EDTA Serum- free medium (SFM) is normal medium without serum.
Method a) 4h HBSS study: Transepithelial electrical flux (TER) across the Caco-2 monolayers grown on snapwells (passage 33; 23 days old) was measured to confirm monolayer integrity before beginning the experiment . The medium was removed and the cells were washed once with bHBSS. bHBSS containing lOOμM chloroquine was added and the cells were incubated for 2h at 37°C. The bHBSS+chloroquine was replaced with 0.5ml bHBSS containing GST-peptide fusions (lOOμg/ml) and the cells were incubated as before. Basolateral samples were removed at the following times: 0, 0.5h, 2h, and 4h. At 4h, TER was measured, the apical medium was sampled and the apical reservoir was washed 6 times with HBSS. The cells were allowed to lyse for lh on ice in lysate buffer, after which, lysate sample was collected. All samples were stored at -70°C until assay by anti-GST ELISA. Before analysis, samples were normalized for protein content relative to each other using a BioRad protein assay. b) 24h SFM study: Transepithelial electrical flux (TER) across the Caco-2 monolayers grown on snapwells (passage 33; 23 days old) was measured to confirm monolayer integrity before beginning the experiment . The medium was removed and the cells were washed once with SFM. SFM
containing GST-peptide fusions (lOOμg/ml) was added to the cells which were incubated at 37°C for 24h at 5% C02. After 24 hours, TER readings were taken, and samples from the basolateral and apical reservoirs were removed. The apical reservoir was washed 6 times with PBS. The cells were allowed to lyse for lh on ice in lysate buffer, after which lysate sample was collected. All samples were stored at -70° until assay by anti-GST ELISA. Before analysis, samples were normalized for protein content relative to each other using a BioRad protein assay.
Results
All of the GST-peptide fusions and controls examined were transported across live Caco-2 monolayers. Full-length GST-P31 and GST-DCX8, but not truncations of these molecules had a higher flux than GST alone.
Internalization of GST-peptide fusions into polarized Caco-2 cells was investigated in two experiments. In experiment 1, 15μg of GST-peptide fusion was applied in bHBSS and internalized GST-peptide was recovered by lysing the cells after 4h. In experiment 2, lOμg of GST-peptide was applied in either a) bHBSS (lysate recovered after 4h) , or b) serum-free medium (lysate recovered after 24h) .
Figure 11A describes complete transport of GST- peptide across a polarized Caco-2 monolayer and does not necessarily refer to internalization, i.e., the GST-peptide was recovered from the basolateral reservoir of a snapwell but the proteins could have crossed the barrier by the paracellular route.
Effect of Thrombin Cleavage on Binding of GST-Peptide Fusions to Fixed Caco-2 Cells
Binding of intact and thrombin-cleaved GST-peptide fusions to fixed Caco-2 cells was compared. Reduced binding of the thrombin-cleaved GST-peptide fusions relative to intact fusions indicates that the peptide component of the fusion, and not the GST domain, mediates binding.
Method
Confluent Caco-2 monolayers grown in 96-well plates (p38) were fixed and treated with 0.1% phenylhydrazine before blocking with 0.1% BSA in PBS. Thirty micrograms of each GST-peptide was treated with bovine thrombin (lμ/ml; 0.4 NIH units; Sigma CN.T9681) for 18h at room temperature in 20mM Tris-HCl pH8.0, 150mM NaCI, 2.5mM CaCl2. Controls were similarly treated without addition of thrombin. Ten micrograms of each GST-peptide fusion was removed for PAGE analysis, and lOμg of fusions were added in duplicate to the fixed Caco-2 cells before 5-fold serial dilutions (1% BPT diluent) . The fusions were allowed to bind for lh at room temperature. Following 6 washes with 1% BPT, binding was assayed by ELISA.
Results
Results are shown in Figure 12.
Conclusions: PAGE analysis confirmed that the GST-peptide fusions were effectively cleaved with thrombin. Cleavage with thrombin significantly reduced detection of binding of GST-P31.103, GST-PAX2.106, GST-DCX8, GST-SNilO to fixed Caco- 2 cells, indicating that the peptide component, and not the GST domain, mediates binding.
6.10. Synthesis of Peptides
6.10.1. Procedure For Solid Phase Synthesis
Peptides may be prepared by methods that are known in the art. For example, in brief, solid phase peptide synthesis consists of coupling the carboxyl group of the C- terminal amino acid to a resin and successively adding N- alpha protected amino acids. The protecting groups may be any known in the art . Before each new amino acid is added to the growing chain, the protecting group of the previous amino acid added to the chain is removed. The coupling of amino acids to appropriate resins is described by Rivier et al . ,
U.S. Patent No. 4,244,946. Such solid phase syntheses have been described, for example, by Merrifield, 1964, J. Am.
Chem. Soc. 85:2149; Vale et al . , 1981, Science 213:1394-1397;
Marki et al . , 1981, J. Am. Chem. Soc. 103:3178 and in U.S. Patent Nos . 4,305,872 and 4,316,891. In a preferred aspect, an automated peptide synthesizer is employed.
By way of example but not limitation, peptides can be synthesized on an Applied Biosystems Inc. ("ABI") model
431A automated peptide synthesizer using the "Fastmoc" synthesis protocol supplied by ABI, which uses
2- (lH-Benzotriazol-1-yl) -1,1,3,3, -tetramethyluronium hexafluorophosphate ("HBTU") (R. Knorr et al . , 1989, Tet.
Lett., 30:1927) as coupling agent. Syntheses can be carried out on 0.25 mmol of commercially available 4- (2 ' , 4 ' -dimethoxyphenyl- (9-fluorenyl- methoxycarbonyl) -aminomethyl) -phenoxy polystyrene resin ("Rink resin" from Advanced ChemTech) (H. Rink, 1987, Tet.
Lett. 28:3787). Fmoc amino acids (1 mmol) are coupled according to the Fastmoc protocol. The following side chain protected Fmoc amino acid derivatives are used:
FmocArg ( mc) OH; FmocAsn (Mbh) OH; FmocAsp (fcBu) OH;
FmocCys (Acm) OH; FmocGlu (fcBu) OH; FmocGln (Mbh) OH; FmocHis (Tr) OH,•
FmocLys (Boc)OH; FmocSer ^Bu) OH; FmocThr ^Bu) OH;
FmocTyr (tBu) OH. [Abbreviations: Acm, acetamidomethyl ; Boc, tert-butoxycarbonyl ; fcBu, tert-butyl; Fmoc,
9-fluorenylmethoxycarbonyl ; Mbh, 4 , 4 ' -dimethoxybenzhydryl ;
Pmc, 2 , 2 , 5, 7, 8-pentamethylchroman-6-sulfonyl ; Tr, trityl] .
Synthesis is carried out using N-methylpyrrolidone (NMP) as solvent, with HBTU dissolved in N,N-dimethylformamide (DMF) . Deprotection of the Fmoc group is effected using approximately 20% piperidine in NMP. At the end of each synthesis the amount of peptide present is assayed by ultraviolet spectroscopy. A sample of dry peptide resin (about 3-10 mg) is weighed, then 20% piperidine in DMA (10 ml) is added. After 30 min sonication, the UV
(ultraviolet) absorbance of the dibenzofulvene-piperidine adduct (formed by cleavage of the N-terminal Fmoc group) is
recorded at 301 nm. Peptide substitution (in mmol g"1) can be calculated according to the equation:
A x v substitution = x 1000
7800 x w where A is the absorbance at 301 nm, v is the volume of 20% piperidine in DMA (in ml) , 7800 is the extinction coefficient (in molMm3cm"1) of the dibenzofulvene-piperidine adduct, and w is the weight of the peptide-resin sample (in mg) .
Finally, the N-terminal Fmoc group is cleaved using 20% piperidine in DMA, then acetylated using acetic anhydride and pyridine in DMA. The peptide resin is thoroughly washed with DMA, CH2C12 and finally diethyl ether.
6.10.2. Cleavage and Deprotection
By way of example but not limitation, cleavage and deprotection can be carried out as follows: The air-dried peptide resin is treated with ethylmethyl-sulfide (EtSMe) , ethanedithiol (EDT) , and thioanisole (PhSMe) for approximately 20 min. prior to addition of 95% aqueous trifluoracetic acid (TFA) . A total volume of approximately 50 ml of these reagents are used per gram of peptide-resin. The following ratio is used: TFA: EtSMe : EDT: PhSme (10:0.5:0.5:0.5). The mixture is stirred for 3 h at room temperature under an atmosphere of N2. The mixture is filtered and the resin washed with TFA (2 x 3 ml) . The combined filtrate is evaporated in vacuo, and anhydrous diethyl ether added to the yellow/orange residue. The resulting white precipitate is isolated by filtration. See King et al . , 1990, Int. J. Peptide Protein Res. 36:255-266 regarding various cleavage methods.
6.10.3. Purification of the Peptides
Purification of the synthesized peptides can be carried out by standard methods including chromatography (e.g., ion exchange, affinity, and sizing column chromatography, high performance liquid chromatography
(HPLC) ) , centrifugation, differential solubility, or by any other standard technique.
6.10.4. Conjugation of Peptides to Other Molecules
5
The peptides of the present invention may be linked to other molecules (e.g., a detectable label, a molecule facilitating adsorption to a solid substratum, or a toxin, according to various embodiments of the invention) by methods that are well known in the art . Such methods include the use
10 of homobifunctional and heterobifunctional cross-linking molecules .
The homobifunctional molecules have at least two reactive functional groups, which are the same. The reactive functional groups on a homobifunctional molecule include, for
15 example, aldehyde groups and active ester groups. Homobifunctional molecules having aldehyde groups include, for example, glutaraldehyde and subaraldehyde . The use of glutaraldehyde as a cross-linking agent was disclosed by Poznansky et al . , 1984, Science 223:1304-1306.
20
Homobifunctional molecules having at least two active ester units include esters of dicarboxylic acids and
N-hydroxysuccinimide . Some examples of such N-succinimidyl esters include disuccinimidyl suberate and dithio-bis-
(succinimidyl propionate) , and their soluble bis-sulfonic 25 acid and bis-sulfonate salts such as their sodium and potassium salts. These homobifunctional reagents are available from Pierce, Rockford, Illinois.
The heterobifunctional molecules have at least two
_n different reactive groups. Some examples of heterobifunctional reagents containing reactive disulfide bonds include N-succinimidyl 3- (2-pyridyl-dithio) propionate (Carlsson et al . , 1978, Biochem J. 173:723-737), sodium S-4- succinimidyloxycarbonyl-alpha-methylbenzylthiosulfate, and
„ 4-succinimidyloxycarbonyl-alpha-methyl- (2- pyridyldithio) toluene . N-succinimidyl 3- (2- pyridyldithio) propionate is preferred. Some examples of
heterobifunctional reagents comprising reactive groups having a double bond that reacts with a thiol group include succinimidyl 4- (N-maleimidomethyl) cyclohexahe-1-carboxylate and succinimidyl m-maleimidobenzoate . Other heterobifunctional molecules include succinimidyl 3 - (maleimido) propionate, sulfosuccinimidyl 4-(p- maleimido-phenyl) butyrate, sulfosuccinimidyl 4- (N- maleimidomethyl-cyclohexane) -1-carboxylate, maleimidobenzoyl-
N-hydroxy-succinimide ester. The sodium sulfonate salt of succinimidyl m-maleimidobenzoate is preferred. Many of the above-mentioned heterobifunctional reagents and their sulfonate salts are available from Pierce.
Additional information regarding how to make and use these as well as other polyfunctional reagents may be obtained from the following publications or others available in the art: Carlsson et al . , 1978, Biochem. J. 173:723-737;
Cumber et al . , 1985, Methods in Enzymology 112:207-224; Jue et al., 1978, Biochem 17:5399-5405; Sun et al . , 1974,
Biochem. 13:2334-2340; Blattler et al . , 1985, Biochem. 24:1517-152; Liu et al . , 1979, Biochem. 18:690-697; Youle and
Neville, 1980, Proc. Natl. Acad. Sci. USA 77:5483-5486;
Lerner et al . , 1981, Proc. Natl. Acad. Sci. USA 78:3403-3407;
Jung and Moroi, 1983, Biochem. Biophys . Acta 761:162;
Caulfield et al . , 1984, Biochem. 81:7772-7776; Staros, 1982, Biochem. 21:3950-3955; Yoshitake et al . , 1979, Eur. J.
Biochem. 101:395-399; Yoshitake et al . , 1982, J. Biochem.
92:1413-1424; Pilch and Czech, 1979, J. Biol. Chem. 254:3375-
3381; Novick et al . , 1987, J. Biol. Chem. 262:8483-8487;
Lomant and Fairbanks, 1976, J. Mol. Biol. 104:243-261; Hamada and Tsuruo, 1987, Anal. Biochem. 160:483-488; Hashida et al . ,
1984, J. Applied Biochem. 6:56-63.
Additionally, methods of cross-linking are reviewed by Means and Feeney, 1990, Bioconjugate Chem. 1:2-12.
6.10.4.1. Biotinylation of Peptides
Methods of biotinylating peptides are well known in the art . Any convenient method may be employed in the
practice of the invention. For example, the following procedure was used. Ten micrograms of peptide was dissolved in 100 μl of 0.1 % acetic acid. PBS (900μl) and 3.3 mg of biotin-LC-NHS (Pierce, Rockford, IL) was added. Following incubation for 30 minutes at room temperature the biotinylated peptides were purified over a Superose 12 column (Pharmacia, Piscataway, NJ) .
6.10.5. Synthetic Peptides Tables 19, 20 and 21 provide the primary structure for various synthetic peptides manufactured in the practice of the present invention.
SGSPPCGGSWGRFMQGGLFGGRTDGCGAHRNRTSASLEPPSSD
Y-CONH2
250 ELAN014 H2N-
SHSGGMNRAYGDVFRELRDRWNATSHHTRPTPQLPRGPNS-
CONH2 bZElan014 biotin-
K (dns) SHSGGMNRAYGDVFRELRDRWNATSHHTRPTPQLPRG
PNS-CONH2
ZElan014 H2N-
K (dns) SHSGGMNRAYGDVFRELRDRWNATSHHTRPTPQLPRG
PNS-CONH2
ZElan015 H2N- (DCXll) K (dns) SQGSKQCMQYRTGRLTVGSEYGCGMNPARHATPAYPA
RLLPRYR- CONH2
ZElan016 H2N- (SNilO) K (dns) RVGQCTDSDVRRPWARSCAHQGCGAGTRNSHGCITRP
LRQASAH- CONH2 bZElan017 biotin-K (dns) SGSGRVGQCTDSDVRRPWARSCA-CONH2 ZElan017 H2N-K (dns) RVGQCTDSDVRRPWARSCA-CONH2 ZElanOlδ H2N- (PAX2) K (dns) STPPSREAYSRPYSVDSDSDTNAKHSSHNRRLRTRSR
PNG-CONH2
ZElan019 H2N- (5PAX5) K (dns) RGSTGTAGGERSGVLNLHTRDNASGSGFKPWYPSNRG
HK-CONH2
ZElan020 H2N- K ( dns ) SGSGLYANPGMYSRLHSPA- CONH2
(CY09) bZElan020 biotin-K (dns) SGSGLYANPGMYSRLHSPA-CONH2
(CY09) ZElan021 H2N-
(HAX42) K (dns) SDHALGTNLRSDNAKEPGDYNCCGNGNSTGRKVFNRR
RPSAIPT - CONH2
ZElan022 H2N- (SNi34) K (dns) SPCGGSWGRFMQGGLFGGRTDGCGAHRNRTSASLEPP
SSDY-CONH2
ZElan023 H2N- (DCX8) K (dns) RYKHDIGCDAGVDKKSSSVRGGCGAHSSPPRAGRGPR
GTMVSRL- CONH2
ZElan024 H2N- (P31) K (dns) SARDSGPAEDGSRAVRLNGVENANTRKSSRSNPRGRR
HPGG-CONH2
ZElan025 H2N- (DAB10) K (dns) SKSGEGGDSSRGETGWARVRSHAMTAGRFRWYNQLPS
DR-CONH2
ZElan026 H2N- (PAX2/con K (dns) SEANLDGRKSRYSSPRRNSSTRPRTSPNSVHARYPST trol) DHD- CONH2 bELAN027 biot in- (PAX2) SGSGSTPPSREAYSRPYSVDSDSDTNAKHSSHNRRLRTRSRPN
G-CONH2
251 18C21 H2N-DTNAKHSSHNRRLRTRSRPNG-CONH2 Fmoc- Fmoc-K (dns) RVGQCTDSDVRRPWARSCAHQG-COOH Z16N23
252 16C23 H2N-CGAGTRNSHGCITRPLRQASAHG-CONH2
PST
H
2N- K dns) SSRSNPG-CONH
2 )
H2N- K dns) RSNPRG-CONH2 )
H2N- K dns)SNPRG-CONH2 )
H2N- K dns) PRGRRH-CONH2 )
H2N-K dns)RRHPG-CONH2 )
H2N- K dns) KSSRGN-CONH2
H2N- K dns) KTSERSQPRGRRQPG-CONH2
H2N-K dns) TrKSSrSNPrGrrHPG-CONH2
H2N- K dns) TRKSSrSNPRGrRHPG-CONH2
H,N-K dns) TNAKHSSHN-CONH2 )
H2N-K dns) RRLRTRSRPN-CONH2 )
H2N- K dns) RRLRTRSR-CONH2 )
H2N-K dns) RRLRTR-CONH2 )
H2N-K dns) rrLrTrSrPN-CONH2
H2N-K dns) SDHALGTNLRSDNAKEPGDYNCCGNG-CONH2 )
H,N-K dns) GDYNCCGNGNSTGRKVFNRRRPSAIPT-CONH2 )
H2N-K dns) SDHALGTNLRSDNAKEPG-CONH2 )
H2N-K dns) GDYNCCGNGNSTG-CONH2 )
H
2N-K dns) RKVFNRRRPSAIPT-CONH,
)
PS
,
GST fusion proteins of GIT peptides are shown in Table 21.
Table 21
ι→ O ts5
O Co
6.10.6. Peptide Stability
The relative stability for ZElan031, ZElan053 and ZElan054 was determined in simulated intestinal fluid (SIF) SIF was made by dissolving lOOmg of pancreatin (Sigma cat#P- 1625, lot# 122H0812)in 8.4ml of phosphate stock solution, adjusting the pH to 7.5 with 0.2N NaOH and adjusting the volume to 10ml with water.
Peptide (3.25mg) was dissolved in 3.25 ml of 10,000 fold diluted SIF solution at 37°C. Aliquots (0.7ml) of the digestion solution were then withdrawn at <lmin, lh, 3h, and 21h or 24h. The samples were quickly passed through a syringe filter (Millipore Millex-GV 0.22μm, part# SLGV025LS, lot# H2BM95250) and 300μL of the filtered solution was immediately injected onto a Hewlett-Packard HPLC system equipped with a C-δ column (Applied Biosystems column and guard column: column- p/n 0711-0023 Spheri-5 ODS 5μm, 220x4.6mm). The products were eluted at 1.5ml/min using an acetonitrile-water gradient. The major fluorescent peaks were collected, lyopholized and identified by MS analysis. The HPLC gradient used was:
Time
(min) Solvent Mixture
0 95% H20- 5% acetonitrile ( 0 . 1%TFA)
5 95% H20- 5%acetonitrile ( 0 . 1%TFA)
35 δ5% H20- 15% acetonitrile ( 0 . 1%TFA) linear solvent change
40 0% H2O- 100% acetonitrile ( 0 . 1%TFA)
45 95% H20- 5% acetonitrile ( 0 . 1%TFA)
52 95% H20- 5%acetonitrile ( 0 . 1%TFA)
As shown in Table 22, the relative stability (to SIF) for the three peptides was found to be
ZElan053>ZElanO54>ZElan031. Enzymatic cleavage of the peptide was found to occur at arginine and/or lysine as expected. The replacement of 1 -amino acids with their D-amino acid analogs significantly reduced the rate of proteolysis at these residues .
TABLE 22
Peptide Percent Remaining at: Rel. Stab.
1 m 1 h 3 h 24 h
ZElan031 100 38.7 0 0 3
ZElan054 97.4 58.2 11.6 2.7 2
ZElan053 100 98.3 98.1 94.0 1
7. CHARACTERIZATION OF PEPTIDE -COATED PARTICLES
Binding of Peptide-Coated PLGA Nanoparticles to Fixed Caco-2 Cells
Binding of nanoparticles coated with targeting peptides to fixed Caco-2 cells was investigated using an ELISA assay based on reaction of antibody with the dansyl moiety present on the peptides. Isoelectric points of selected synthetic peptides are shown in Table 23 (corresponding SEQ ID NOS. are shown in Table 7) . Corresponding dansylated synthetic GIT binding peptides are given in Table 24.
TABLE 23 Peptide Sequence pi
P31 SARDSGPAEDGSRAVRLNGVENANTRKSSRSNPRGRRHP 12.26
5PAX5 RGSTGTAGGERSGV N HTRDNASGSGFKP YPSNRGHK 11.49
SNilO RVGQCTDSDVRRPWARSCAHQGCGAGTRNSHGCITRPLRQASAH 10.45
SNi34 SPCGGSWGRFMQGGLFGGRTDGCGAHRNRTSAS EPPSSDY 8.25
DCXll SQGSKQCMQYRTGRLTVGSEYGCGMNPARHATPAYPARLLPRYR 10.44
DCX8 RYKHDIGCDAGVDKKSSSVRGGCGAHSSPPRAGRGPRGTMVSR 11.03
HAX42 SDHALGTN RSDNAKEPGDYNCCGNGNSTGRKVFNRRRPSAIPT 9.62
PAX2 STPPSREAYSRPYSVDSDSDTNAKHSSHNRRLRTRSRPN 11.26
TABLE 24
Peptide Sequence
P31 H2N-K(dns) SARDSGPAEDGSRAVRLNGVENANTRKSSRSNPRGRRHPGG-CONH2
5PAX5 H2N-K (dns) RGSTGTAGGERSGV NLHTRDNASGSGFKPWYPSNRGHK-CONH2
SNilO H2N-K (dns) RVGQCTDSDVRRPWARSCAHQGCGAGTRNSHGCITRPLRQASAH-CONH2
SNi34 H-N-K(dns) SPCGGSWGRFMQGGLFGGRTDGCGAHRNRTSASLEPPSSDY-CONH2
DCXll H2N-K(dns) SQGSKQCMQYRTGRLTVGSEYGCGMNPARHATPAYPARLLPRYR-CONH2
DCX8 H2N-K (dns) RYKHDIGCDAGVDKKSSSVRGGCGAHSSPPRAGRGPRGTMVSRL-CONH2
HAX42 H2N-K(dns) SDHALGTNLRSDNAKEPGDYNCCGNGNSTGRKVFNRRRPSAIPT-CONH2
PAX2 H2N-K(dns) STPPSREAYSRPYSVDSDSDTNAKHSSHNRR RTRSRPNG-CONH2
DAB10 H2N-K (dns) SKSGEGGDSSRGETG ARVRSHAMTAGRFR YNQ PSDR-CONH2
Method;
Confluent Caco-2 monolayers grown in 96-well plates (p38) were fixed and treated with 0.1% phenylhydrazine before blocking with 0.1% BSA in PBS. Control and dansyl peptide-
15 coated nanoparticles were resuspended in sterile water at lOmg/ml and stirred with a magnet for lh at room temperature. Samples consisted of: (1) blank nanoparticle control, (2) scrambled PAX2-coated nanoparticles, (3) PAX2-coated nanoparticles, (4) HAX42 -coated nanoparticles, 0 (5) PAX2/HAX42 -coated nanoparticles, and (6) 8 peptide-coated nanoparticles .
Nanoparticles were added to the cells at lOmg/ml in lOOμl 1%BSA-PBS (no TweenδO is used in this assay) and 2-fold serially-diluted. The 96-well plates were incubated for lh
? 5 at room temperature. The plates were washed 5 times with 1%BSA-PBS and lOOμl of anti-dansyl antibody (Cytogen DB3- 226.3; 0.5 μg/ml; batch May 1997) was added per well and the plates incubated lh at room temperature. The wells were washed 5 times with 1%BSA-PBS; lOOμl of goat anti-mouse λ:HRP 30 antibody (Southern Biotechnology CN. 1060-05; 1:10,000) was added per well, and the plates incubated lh at room temperature. After washing 5 times with 1%BSA-PBS, lOOμl of TMB peroxidase substrate (KPL CN. 50-76-00) was added to the wells and the optical density at 650nm was measured after 15
35 minutes.
As shown in Figures 13A-B, a decreasing anti-dansyl ELISA response was observed for nanoparticles coated with PAX2, HAX2, PAX2+HAX2, and a mixture of δ targeting peptides, when decreasing amounts of the nanoparticles were applied to fixed Caco-2 cells. No concentration effect was observed for blank nanoparticles or nanoparticles coated with a scrambled version of PAX2 peptide. Nanoparticles coated with PAX2 , HAX2, PAX2+HAX2, and the 8 peptide mix, showed increased response relative to blank nanoparticles or nanoparticles coated with a scrambled version of PAX2 peptide. The OD values were low relative to those normally observed for GST- peptide fusion binding to fixed Caco-2 cells.
Table 25 below shows the insulin potency and level of peptides coated onto the particles (measured by fluorescense) for formulation 1 particles (formulation by the coacervation method given below) .
Table 25
Peptide Blend L
Insulin Peptide mg/g μl/mg
PAX2 60.7 3.51
HAX42 55.9 2.93
PAX2 SCRAMBLED 57.7 1.26
P31 67.0 1.22
5PAX5 52.7 2.83
SNilO 59.5 1.75
SNi34 61.5 4.03
DCX8 59.1 1.87
DAB10 55.9 1.99
ELISA of dansylated peptides and insulin coated PLGA particles
The standard ELISA procedure was modified as follows. Peptides and particles were diluted to an appropriate concentration in PBS containing 1%BSA (particles were sonicated to achieve a homogeneous solution) , titered
and incubated one hour at room temperature. Following five washes with PBS containing 1%BSA, an in-house IgGlλ anti- dansyl monoclonal antibody was added (diluted to lμg/ml in 1%BSA-PBS) and the plates were incubated for one hour. After five more washes goat anti-mouse λ-HRP was added (Southern Biotechnology Associates Inc., Birmingham, AL, diluted 1:10,000 in 1%BSA-PBS) and the plates were incubated one hour. After five washes, plates were developed with TMB peroxidase substrate (Kirkegard and Perry, Gaithersburg, MD) . All data is presented with background binding subtracted. Tween 20 was not added to the diluent or the washes when insulin coated PLGA particles were included in the assay.
Figures 14A-14B show the binding of the dansylated peptide SNilO to hSI and BSA.
BINDING OF SYNTHETIC PEPTIDES AND PEPTIDE-COATED PARTICLES TO SI00 AND PI00 FRACTIONS DERIVED FROM CACO-2 CELLS 0
8.1. Detection of Binding to Membrane (P100) and Cytosolic (S100) fractions
Caco-2 cell membrane (P100) and cytosolic (S100) fractions were prepared using a modification of the method described in Kinsella, B. T., O'Mahony, D. J. and G. A. 5
FitzGerald, 1994, J. Biol. Chem. 26.9(47) : 29914-29919.
Confluent Caco-2 cell monolayers (grown in 75 cm2 flasks for up to 1 week at 37°C and 5% C02) were washed twice in
Dulbecco's PBS (DPBS) and the cells were harvested by 0 centrifugation at 1000 rpm after treatment with 10 mM EDTA-
DPBS . The cells were washed 3 times in DPBS and the final cell pellet was resuspended in 3 volumes of ice cold HED buffer (20 mM HEPES (pH 7.67), 1 mM EGTA, 0.5 mM dithiothreitol, 1 mM phenylmethylsulphonyl fluoride (PMSF) ) . _ The cells were allowed to swell for 5 min on ice prior to homogenization for 30 sec. The homogenates were centrifuged at 40,000 rpm for 45 min at 4°C. The supernatant (S100) was
removed and the pellet (P100) was resuspended in HEDG buffer (20 mM HEPES (pH 7.67), 1 mM EGTA, 0.5 mM dithiothreitol , 100 mM NaCI, 10% glycerol, 1 mM PMSF) . Protein concentrations were determined using the Bradford assay (Bradford, M. M., 5 1976, Anal. Biochem. 72 : 248-254).
Binding of peptide and/or peptide-coated PLGA particles to membrane (P100) and cytosolic (S100) fractions was assessed by detection of the dansyl moiety incorporated in the peptide. Costar ninety six well ELISA plates were 0 coated with S100 and P100 fractions (100 μg/ml in 0.05 M NaHC03) overnight at 4°C. The plates were blocked with 0.5% bovine serum albumin in DPBS for 1 h at room temperature and washed 3 times in 1% BSA-DPBS. Peptide-coated particles or peptides were dispersed in the same buffer and added to the 5 plates at concentrations in the range 0.0325 - 0.5 mg/well. After 1 h at room temperature the plates were washed 5 times in 1% BSA-DPBS and 100 μl of anti-dansyl antibody (Cytogen DB3-226.3; 0.5 μg/ml) was added per well. The plates were incubated for 1 h at room temperature. The wells were washed 0 3 times in 1% BSA-DPBS and 100 μl of goat anti-mouse IgGλ:HRP antibody (Southern Biotechnology 1060-05; 1:10,000) was added per well . The plates were incubated for 1 h at room temperature. After washing 3 times in 1% BSA-DPBS 100 μl of TMB substrate (3 , 3 ' , 5 ' , 5-tetramethylbenzidine; Microwell 5 Peroxidase Substrate System (Kirkegaard and Perry
Laboratories 50-76-00) ) was added and the optical density was measured at 650 nm at various time intervals.
8.2. Binding of Peptide-Coated PLGA particles 0 A novel assay system is provided by the instant invention for detection of binding of peptide-coated PLGA particles to membrane (P100) and cytosolic (S100) fractions derived from live Caco-2 cells. The absorbance readings obtained using this assay system were substantially higher 5 than those obtained using similar peptide-coated PLGA particle concentrations on fixed Caco-2 cells. This greater sensitivity together with the derivation of the S100 and P100
fractions from live Caco-2 cells suggests that this assay may be the assay system of choice for detection of peptide-coated PLGA particle binding. The assay was concentration dependent and peptide/particle correlation permitted differentiation between specific and non-specific binding interactions.
Binding of peptide-coated PLGA particles was assessed using S100 and P100 fractions derived from live Caco-2 cells as described above. The fractions were coated onto 96-well plates at lOμg/well in 0.05 M NaHC03 and peptide-coated PLGA particles were assayed by ELISA at concentrations in the range 0.0325 - 0.5 mg/well.
Figures 15A and 15B illustrate the data obtained on S100 and P100 fractions respectively for particles coated with no peptide, scrambled PAX2 (control) , P31 D-Arg 16 -mer (ZElan053), HAX42, PAX2 and HAX42/PAX2. Using particle concentrations of 0.0325 - 0.5 mg/well all test peptide- coated PLGA particles exhibited greater binding to both the S100 and P100 fractions than the scrambled PAX2 coated control particles. All particles except P31 D-Arg 16-mer (ZElan053) exhibited greater binding to the P100 fraction than the S100 fraction. Greater binding of the P31 D-Arg 16- mer (ZElan053) coated particles to the S100 fraction may be indicative of non-specific binding due to the D-Arg modification of the P31 peptide (SEQ ID NO: 43) . Binding of PLGA particles coated with varying concentrations of PAX2 peptide ranging from 0.05 - 5.0 mg/g was assessed using a) fixed Caco-2 cells (P35) and b) S100 and P100 fractions (Caco-2 P33) . The particles were assayed at concentrations in the range 0.03125 - 0.0625 mg/well. " Using a particle concentration of 0.0625 mg/well, all PAX2 coated particles except those coated at 0.05 mg/g exhibited greater binding to fixed Caco-2 cells than the scrambled PAX2 coated control particles. There appeared to be a concentration effect with increasing PAX2 peptide concentration resulting in improved Caco-2 cell binding (in the range 0.05 - 1.0 mg/g) . However all absorbance readings
were low and binding of the PAX2 (5 mg/g) was not consistent with this pattern.
Using particle concentrations of 0.03125 - 0.0625 mg/well all test peptide coated particles except PAX2 (0.05 mg/g) exhibited comparable or greater binding to both the S100 and P100 fractions than the scrambled PAX2 coated control particles. All particles exhibited greater binding to the P100 fraction than the S100 fraction. Binding to both the S100 and P100 fractions was directly proportional to the concentration of the PAX2 peptide on the particle. The absorbance readings obtained using this assay system were substantially higher than those obtained on the fixed Caco-2 cells .
The effect of blocking solution on binding of peptide- coated PLGA particles to P100 fractions (Caco-2 P35) was assessed using 1% bovine serum albumin (BSA) and 1% milk powder blocking solutions to assess background binding. The following particles were assayed at concentrations in the range 0.03125 - 0.0625 mg/well: no peptide; scrambled PAX2 ; and a range of PAX2 coated particles having peptide concentrations from 5-0.05 mg/g. As previously observed using 1% BSA, all test peptide coated particles except PAX2 coated at 0.05 mg/g exhibited comparable or greater binding to the P100 fractions than the scrambled PAX2 coated control particles. Binding to P100 fractions was directly proportional to the concentration of the PAX2 peptide on the particle (although in this instance PAX2 (5 mg/g) exhibited slightly lower binding than PAX2 (1 mg/g)) . A similar trend was observed using 1% milk powder and a particle concentration of 0.0625 mg/well. However all absorbance readings were low when 1% milk powder was used and the binding pattern was not detectable using particles at a concentration of 0.0625 mg/well.
Non-specific binding of peptide-coated PLGA particles to plastic was also assessed using 1% BSA and 1% milk powder blocking solutions. The binding pattern observed above could be detected when BSA was used; however, absorbance readings
- Ill -
were substantially lower and binding of particles PAX2 (0.1 and 0.05 mg/g respectively) was not detectable. When 1% milk powder was used, all absorbance readings were low and no binding pattern was detectable. BSA was chosen for blocking in subsequent assays .
8.3. Comparison of Peptide-Coated Particle and
Synthetic Peptide Binding to P100 fractions
Binding of dansylated peptides to P100 fractions was assessed to determine if peptide binding was predictive of peptide-coated particle binding. Figure 16 illustrates the data obtained for the dansylated peptides A) HAX42, P31
D-form and scrambled PAX2 and B) PA 2 , HAX42 and scrambled
PAX2.
Two consecutive assays produced substantial variations in absorbance readings. Initially, the HAX42 peptide exhibited strong binding when compared to the scrambled PAX2 control. The P31 D-form peptide (ZElan053) exhibited binding at the highest dilution only. In the repeat assay, HAX42 also exhibited significant binding compared to the scrambled
PAX2 control. However, the scrambled PAX2 control and HAX42 produced relatively high absorbance values compared to those obtained in the previous assay. The PAX2 peptide was indistinguishable from the scrambled PAX2 control.
Peptide/particle binding correlation is summarized as follows in Table 26 :
TABLE 26
Peptide/particle_assay correlation
Assay
Peptide correlation
HAX42 +
PAX2 +/-
P31 D-form -
Scrambled +/-
PAX2
+ positive; + /- equivocal ; - negative
Peptide/particle binding correlated well for the HAX42 peptide. In contrast, no correlation could be detected
for the P31 D-form (ZElan053) peptide. Since the P31 D-form peptide-coated particles exhibited greater binding to the S100 fraction than the P100 fraction (unlike the other test peptides) it appears that the particle binding interaction was non-specific or that some other molecule was competing for binding to the P100 fraction but not to the S100 fraction. Thus the peptide/particle assay correlation may be useful for distinguishing between specific and non-specific binding interactions. The scrambled PAX2 control produced variable results so that it was difficult to assess the PAX2 binding correlation.
8.4. Determination of HAX42 and PAX2 Binding Motif Sequences Peptides and GST fusion proteins of HAX42, PAX2 and various derivatives were assayed using peptide ELISA to P100 membrane fractions derived from Caco-2 cells. The GST-PAX2 protein and PAX2 peptide data indicate that a core binding motif lies in the amino acid sequence TNAKHSSHNRRLRTR (SEQ ID NO: ) otherwise named GST-106 and ZElan033. Similarly, the HAX42 peptide data suggest that a core binding motif for HAX42 lies in the amino acid sequence PGDYNCCGNCNSTG (SEQ ID NO: ), otherwise named ZElan091.
The peptides and proteins were analyzed by a dansylated peptide ELISA method in which 96 well plates were coated overnight at 4°C with lOOμl/well coating protein (normally lOOμg/ml P100 membrane fraction) in 0.05M carbonate buffer pH9.6. Nonspecific binding was blocked using 200μl/well, 2% Marvel/PBS for 2 hours at 37°C prior to incubation with dansylated peptides. The plates were washed three times with PBS/0.05% Tween 20 and after each subsequent incubation step. The peptides were diluted in blocking solution at a starting concentration of lOOμg/ml and diluted 1:2 downwards, lOOμl/well, followed by incubation at room temperature for 1 hour, exactly. A buffer blank control was included to ensure that background binding to plastic was not due to the antibodies used in the assay system. To detect the
dansylated peptides, a mouse anti-dansyl antibody (DB3, Cytogen Corp.) at 1:1340 dilution in blocking buffer and lOOμl/well was added followed by incubation at room temperature for 1 hour. The plates were then incubated with an anti -mouse λ-HRP conjugated antibody (Southern Biotech 1060-05) at a 1:10,000 dilution in blocking solution, lOOμl/well for 1 hour at room temperature. Plates were developed using 75μl/well Bionostics TMB substrate and incubated for approximately 10 minutes. The developing reaction was stopped using Bionostics Red Stop solution
(25μl/well) , and the optical density of the plates was read at 650nm.
GST-PAX2 Peptides - Relative Binding to P100 Fractions After subtraction of the GST-peptide binding to plastic from P100 binding values, the binding of GST-PAX2 peptides were represented as a ratio of GST-HAX42 binding to P100, which was given the arbitrary value of 1.00. The following ratios were determined from binding to P100 of GST-peptides at a peptide concentration of 20μg/ml. Bold denotes positive binding to the P100 membrane fraction.
Table 27
;ST-peptide Value
GST-HAX42 1.00
GST-PAX2 1.79
GST-104 0.01
GST-105 -0.08
GST-106 2.71
GST-113 0.26
GST-114 0.17
GST-115 0.36
GST 0.46
Table 28
GST-peptide Amino Acid Sequence
GST-PAX2 STPPSREAYSRPYSVDSDSDTNAKHSSHNRRLRTRSRPN
GST- 104 STPPSREAYSRPYSVDSDSD
GST- 105 STPPSREAYSRPYSVDSDSDTNAKHSSHN
GST-106 TNAKHSSHNRRLRTRSRPN
GST-113 TNAKHSSHN
GST- 114 SSHNRRLRTRSRPN
GST-115 RRLRTRSRPN
PAX2 Peptides - Relative Binding to P100 Fractions
ZElan021, full length HAX42, was given the arbitrary value of 1.00 for binding to P100 at a given peptide concentration determined from the signal-to-noise ratio data. PAX2 and its derivatives are given as a ratio of HAX42 value to reflect their binding abilities to P100 membrane fractions derived from a Caco-2 cell line as shown in Table 29. Table 30 provides a line-up of the PAX2 peptides showing the positive binding peptides in boldface. The GST-PAX2 peptide and PAX2 peptide data agree, demonstrating that a binding motif is in the amino acid sequence TNAKHSSHNRRLRTR (GST-106 and ZElan033) .
TABLE 29
Binding Binding value value
Binding Binding Binding at at value Binding value value 50μg/ml 50μg/ml
PAX2 at value at at (Jackson (Southern peptide 20μg/ml at 20μg/ml 50/ig/ml 50μg/ml Ab) Ab)
ZElan018 -0.33 1.07 0.95 1.01 ZElan032 1.43 2.87 0.95 1.06 ZElan033 0.35 1.57 0.80 0.66 ZElan035 0.12 0.43 0.81 0.77 ZElan055 0.99 0.73 1.10 0.59 ZElan056 0.00 0.16 0.21 0.21 ZElan057 0.08 0.56 0.25 ZElan058 0.05 0.47 0.16 ZElan073 0.07 -0.11 0.49 0.66 0.49 ZElan074 0.06 0.82 0.52 0.71 0.48 ZElan075 0.13 0.52 0.38 0.47 0.32 ZElan076 0.08 1.00 0.41 0.60 0.42 ZElan077 0.20 0.76 0.54 0.73 0.52 ZElan078 0.11 0.87 0.69 0.68 0.47 ZElan079 0.31 0.97 0.68 0.83 0.53 ZElan080 0.23 0.84 0.45 0.67 0.38 ZElan081 0.01 0.89 0.47 ZElan082 0.00 0.92 0.40 ZElan083 0.43 0.63 1.03 0.88 ZElan084 1.06 0.93 1.16 0.77
Table 30
PAX2 SEQ ID Peptide Amino acid sequence NO: ZElanOlδ H_N-K (dns) STPPSREAYSRPYSVDSDSDTNAKHSSHNRRLRTRSRPNG -CONE ZElan032 H-N-K (dns) TNAKHSSHNRRLRTRSRPN-CONH2 ZElan033 H2N-K (dns) TNAKHSSHNRRLRTR-CONH2 ZElan034 H2N-K (dns) SSHNRRLRTRSRPN-CONH2 ZElan035 H2N-K (dns) SSHNRRLRTR-CONHj ZElan055 H2N-K (dns) TNAKHSSHN-CONH2 ZElan056 H2N-K (dns) RR RTRSRPN-CONH2 ZElan057 H-N-K(dns) RRLRTRSR-CONH2 ZElan058 H2N-K (dns) RLRTR-CONH2 ZElan059 H2N-K (dns) rrLrTrSrPN-CONH2 ZElan073 H2N-K (dns) ASH RRLRTR-CONH2 ZElan074 H2N-K ( ns) SAHNRRLRTR-CONH2 ZElan075 H2N-K (dns) SSA RRLRTR-CONH2 ZElan076 H2N-K (dns) SSHARRLRTR-CONH2 ZElan077 H2N-K(dns) SSHNARLRTR-CONH2 ZElan078 H2N-K(dns) SSHNRALRTR-CONH2 ZElan079 H2N-K (dns) SSHNRRARTR-CONH2 ZElan080 H2N-K(dns) SSHNRRLATR-CONH2 ZElanOδl H2N-K (dns) SSHNRRLRAR-CONH2 ZElan082 H2N-K(dns) SSHNRRLRTA-CONH2 SCRAMBLED PAX2 PEPTIDES: ZElan083 H2N-K (dns) GRNHDWSSNTHKSYRSPRSASYPRLSNDRTDRTEPAPSS-CONH2 ZElan084 H2N-K (dns) RNTRNKTSRLSANPHRSHR-CONH2
HAX42 Peptides - Relative Binding to P100 Fractions
ZElan021, full length HAX42, was given the arbitrary value of 1.00 for binding to P100 at a given peptide concentration determined from the signal-to-noise ratio data. HAX42 and its derivatives are given as a ratio of HAX42 value to reflect their binding abilities to P100 membrane fractions derived from a Caco-2 cell line as shown in Table 31. Table 32 provides a line-up of the HAX42 peptides showing the positive binding peptides in boldface. A core binding motif appears to lie in the amino acid sequence PGDYNCCGNCNSTG (ZElan091) .
TABLE 31
HAX42 Binding Binding Binding Binding Binding Binding peptide value at value at value at value at value at value at
20μg/ml 50μg/ml 50μg/ml 25μg/ml 25μg/ml 25μg/ml
ZElan021 1. .00 1. .00 1.00 1.00 1.00 1.00
ZElan060 0. .44 0. .56 0.43
ZElan061 0. .20 0. .60 0.38
ZElan062 0. .11 0. .42 0.34
ZElan065 0, .00 0. .54 0.30
ZElan067 0, .08 0. .52 0.40
ZElan070 0. .59 0. .97 0.39
ZElan071 1. .22 0, .89 0.75
ZElan072 0, .83 0. .61 0.88
ZElan087 0.46 0.44
ZElan088 2.21 1.41 1.63
ZElan089 0.55 0.44 0.49
ZElan090 2.06 1.54 2.16
ZElan091 2.02 1.37 1.20
ZElan092 1.41 1.90 0.91
ZElan093 1.88 1.37 1.33
Table 32
HAX42 Amino acid seguence
Peptide
ZElan021 HjN-K (dns) SDHALGTNLRSDNAKEPGDYNCCGNGNSTGRKVFNRRRPSAIPT-CONH2 ZElanOβO H-N-K (dns) SDHALGTNLRSDNAKEPGDYNCCGNG-CONHj ZElan061 H2N-K (dns) GNGNSTGRKVFNRRRPSAIPT-CONH2 ZElan062 H-N-K (dns) SDHALGTNLRSDNAKEPG-CONH2 ZElan065 H2N-K (dns) RKVFNRRRPS-CONH2 ZElan067 H2N-K ( ns) NRRRPS-CONH2 ZElan070 H2N-K (dns) SDHALGTNLRSDNAKEPGDYNCCGNGNST-CONH2 ZElan071 H2N-K (dns) NLRSDNAKEPGDYNCCGNGNSTGRKVFNR-CONH2 ZElan072 H2N-K (dns) PGDYNCCGNGNSTGRKVFNRRRPSAIPT-CONHj ZElan087 H2N-K (dns) SDHALGTNLRSDNAKEPGDY-CONH2 ZElan088 H2N-K (dns) SDNAKEPGDYNCCGNGNSTG-CONHj ZElan089 H2N-K (dns) SDHALGTNLRSDNAK-CONH2 -CONH2 ZElan090 H2N-K (dns) EPGDYNCCGNGNSTG ZΞlan091 H2N-K (dns) PGDYNCCGNGNSTG-CONHj ZElan092 H2N-K (dns) PGDYNCCGNG-CONHj ZΞlan093 H2N-K (dns) NCCGNGNSTG-CONHj
9. FORMULATIONS
General Method for Preparation of Coacervated Particles.
Solid particles containing a Therapeutic as defined herein are prepared using a coacervation method. The are particles are formed from a polymer and have a particle size of between about lOnm and 500 μm, most preferably 50 to 800 nm. In addition the particles contain targeting ligands which are incorporated into the particles using a number of methods .
The organic phase (B) polymer of the general method given above may be soluble, permeable, impermeable,
biodegradable or gastroretentive . The polymer may consist of a mixture of polymer or copolymers and may be a natural or synthetic polymer. Representative biodegradable polymers include without limitation polyglycolides; polylactides ; poly (lactide-co-glycolides) , including DL, L and D forms; copolyoxalates ; polycaprolactone; polyesteramides; polyorthoesters; polyanhydrides ; polyalkylcyanoacrylates ; polyhydroxybutyrates; polyurethanes; albumin; casein; citosan derivatives; gelatin; acacia; celluloses; polysaccharides ; alginic acid; polypeptides; and the like, copolymers thereof, mixtures thereof and stereoisomers thereof. Representative synthetic polymers include alkyl celluloses; hydroxalkyl celluloses; cellulose ethers; cellulose esters; nitrocelluloses ; polymers of acrylic and methacrylic acids and esters thereof; dextrans; polyamides; polycarbonates; polyalkylenes; polyalkylene glycols; polyalkylene oxides; polyalkylene terephthalates ; polyvinyl alcohols; polyvinyl ethers; polyvinyl esters; polyvinyl halides; poyvinylpyrrolidone; polysiloxanes and polyurethanes and co- polymers thereof.
Typically, particles are formed using the following general method:
An aqueous solution (A) of a polymer, surface active agent, surface stabilising or modifying agent or salt, or surfactant preferably a polyvinyl alcohol (PVA) or derivative with a % hydrolysis 50 - 100% and a molecular weight range 500 - 500,000, most preferably 80-100% hydrolysis and 10,000-150,000 molecular weight, is introduced into a vessel . The mixture (A) is stirred under low shear conditions at 10- 2000 rpm, preferably 100-600 rpm. The pH and/or ionic strength of this solution may be modified using salts, buffers or other modifying agents. The viscosity of this solution may be modified using polymers, salts, or other viscosity enhancing or modifying agents. A polymer, preferably poly (lacide-co-glycolide) , polylactide, polyglycolide or a combination thereof or in any enantiomeric form or a covalent conjugate of the these
polymers with a targeting ligand is dissolved in water miscible organic solvents to form organic phase (B) . Most preferably, a combination of acetone and ethanol is used in a range of ratios from 0:100 acetone: ethanol to 100: 0 acetone: ethanol depending upon the polymer used.
Additional polymer(s), peptide(s) sugars, salts, natural/biological polymers or other agents may also be added to the organic phase (B) to modify the physical and chemical properties of the resultant particle product. A drug or bioactive substance may be introduced into either the aqueous phase (A) or the organic phase (B) . A targeting ligand may also be introduced into either the aqueous phase (A) or the organic phase (B) at this point. The organic phase (B) is added into the stirred aqueous phase (A) at a continuous rate. The solvent is evaporated, preferably by a rise in temperature over ambient and/or the use of a vacuum pump. The particles are now present as a suspension (C) . A targeting ligand may be introduced into the stirred suspension at this point. A secondary layer of polymer(s), peptide(s) sugars, salts, natural/biological polymers or other agents may be deposited on to the pre- formed particulate core by any suitable method at this stage.
The particles (D) are then separated from the suspension (C) using standard colloidal separation techniques, preferably by centrifugation at high xg' force, filtration, gel permeation chromatography, affinity chromatography or charge separation techniques . The supernatant is discarded and the particles (D) re-suspended in a washing solution (E) preferably water, salt solution, buffer or organic solvent (s) . The particles (D) are separated from the washing liquid in a similar manner as previously described and re-washed, commonly twice. A targeting ligand may be dissolved in washing solution (E) at the final washing stage and may be used to wash the particles (D) .
The particles may then be dried. Particles may then be further processed for example, tabletted, encapsulated or spray dried.
The release profile of the particles formed above may be varied from immediate to controlled or delayed release dependent upon the formulation used and/or desired.
Drug loading may be in the range 0-90% w/w. Targeting ligand loading may be in the range 0-90% w/w.
Specific examples include the following examples:
EXAMPLE 1; Peptide added at the final washing stage Product: Bovine Insulin loaded nanoparticles Aim: To prepare a 2g batch of insulin loaded nanoparticles at a theoretical loading of 50mg/g and with the peptide ZElan018 added. Formulation Details
RG504H (Lot no. 250583) 2. Og
Acetone 45ml
Ethanol : 5ml PVA (aq. 5%w/v) 400ml
Bovine Insulin (Lot no. 86H0674) lOOmg Peptide: PAX2 (ZElanOlδ) 10mg/50ml dH20
Experimental details: The 5% w/v PVA solution was prepared by heating water until near boiling point, adding PVA and stirring until cool. The organic phase was prepared by adding acetone, 45ml, and ethanol, 5ml, together. The polymer solution was prepared by adding RG504H, 2g, to the organic phase and stirring until dissolved. The IKA™ reactor vessel was set up, all seals greased and the temperature was set at 25°C. The PVA solution, 400ml, was added into the reactor vessel and stirred at 400 rpm.
Bovine insulin, lOOmg, was added into the stirring PVA solution. Using clean tubing and a green needle, the polymer solution was slowly dripped in the stirring PVA solution with the peristaltic pump set at 40. The solvent was allowed to
evaporate by opening the ports and allowing the dispersion to stir overnight at 400 rpm.
The suspension was centrifuged in a Beckman
Ultracentrifuge™ with swing-out rotor at 12,500 rpm, 4°C. The supernatant was decanted and discarded. The "cake" of particles was broken up and dH20 (200mls) was added to wash the particles. The centrifugation and washing steps were repeated twice .
The peptide solution, (ZElan018, lOmg in 50ml dH20) was prepared and added to the particles for a final washing stage. The suspended particles were centrifuged as before.
The supernatant liquid was decanted, the λ cake' broken up, and the particles were dried in the vacuum oven.
The particles were ground, placed in a securitainer and sent for analysis. The weight of particles recovered was
1.45g. A SEM showed discrete, reasonably spherical particles in the 300-500nm size range. The potency was 49.2mg/g (98.0% of label claim) . Peptide loading was 2.42 μg/mg (48.4% of label claim) .
EXAMPLE 2 : Peptide added at the beginning of manufacture
Product: Bovine Insulin loaded nanoparticles
Aim: To prepare a 2g batch of insulin loaded nanoparticles at a theoretical loading of 50mg/g and with the peptide ZElan018 added at the beginning of manufacture.
Formulation Details
RG504H (Lot no. 250583) 2. Og
Acetone 45ml
Ethanol : 5ml PVA(aq. 5%w/v) 400ml
Bovine Insulin (Lot no. 65H0640) lOOmg
Peptide: PAX2 (ZElanOlδii) lOmg
Experimental details: The 5% w/v PVA solution was prepared by heating water until near boiling point, adding PVA and stirring until cool. The organic phase was prepared by adding acetone,
45ml, and ethanol, 5ml, together. The polymer solution was prepared by adding RG504H (polyactide-co-glycolide, Boehringer Ingelheim) , 2g, to the organic phase prepared in step above and stirring until dissolved. The IKA™ reactor vessel was set up, all seals greased and the temperature was set at 25°C. The PVA solution, 400ml, was added into the reactor vessel and stirred at 400 rpm.
Bovine insulin, lOOmg, was added into the stirring PVA solution. PAX2 (ZElan018ii, lOmg) was added to the stirring PVA solution. Using clean tubing and a green needle, the polymer solution was slowly dripped into the stirring PVA solution with the peristaltic pump set at 40. The solvent was allowed to evaporate by opening the ports and allowing the dispersion to stir overnight at 400 rpm. The suspension was centrifuged in a Beckman Ultracentrifuge™ with swing-out rotor at 12,500 rpm, 4°C. The supernatant was decanted and discarded.
The "cake" of particles was broken up and dH20 (200ml) was added to wash the particles. The centrifugation and washing steps were repeated twice. The 'cake' was broken up and the particles were dried in the vacuum oven.
The particles were ground, placed in a securitainer and sent for analysis. The weight of the particles recovered was 1.6g. The potency was 47.3mg/g (94.6% of label claim) . Peptide loading was 1.689μg/mg (33.8% of label claim).
EXAMPLE 3 Peptide added 1 hour before centrifugation
Product: Bovine Insulin loaded nanoparticles
Aim: To prepare a lg batch of insulin loaded nanoparticles at a theoretical loading of 50mg/g and with the peptide ZElanOlδ added 1 hour before centrifugation.
Formulation Details
RG504H (Lot no. 250583) 1. Og
Acetone 22.5ml Ethanol: 2.5ml
PVA(aq. 5%w/v) 200ml
Bovine Insulin (Lot no. 65H0640) 50mg
Peptide: PAX2 (ZElanOlδ) 5mg
Experimental details:
The 5% w/v PVA solution was prepared by heating water until near boiling point, adding PVA and stirring until cool. The organic phase was prepared by adding acetone, 22.5ml, and ethanol, 2.5ml, together. The polymer solution was prepared by adding RG504H, lg, to the organic phase prepared above and stirring until dissolved. The IKA™ reactor vessel was set up, all seals greased and the temperature was set at 25°C. The PVA solution, 200ml, was added into the reactor vessel and stirred at 400 rpm.
Bovine insulin, 50mg, was added into the stirring PVA solution. Using clean tubing and a green needle, the polymer solution was slowly dripped in the stirring PVA solution with the peristaltic pump set at 40. The solvent was allowed to evaporate by opening the ports and allowing the dispersion to stir overnight at 400 rpm.
PAX2 (ZElanOlδ 5mg) was added to the stirring particle suspension. After 1 hr, the suspension was centrifuged in a Beckman Ultracentrifuge™ with swing-out rotor at 12,500 rpm, 4°C. The supernatant was decanted and discarded. The "cake" of particles was broken up and dH20 (200ml) was added to wash the particles. The centrifugation and washing steps were repeated twice.
The 'cake' was broken up and the particles were dried in the vacuum oven. The particles were ground, placed in a securitainer and sent for analysis. Potency was 20.75mg/g (41.5% of label claim). Peptide loading was 1.256μg/mg (25.12 % of label claim).
EXAMPLE 4: Leuprolide acetate loaded nanoparticles Aim: To prepare a 3g batch of leuprolide-acetate loaded nanoparticles at a theoretical loading of 20mg/g and with the peptide ZElan024 added. Formulation Details RG504H (Lot no. 271077) 3. Og
Acetone 67.5ml
Ethanol : 7.5ml
PVA(aq. 5%w/v) 600ml
Leuprolide acetate (Lot no. V14094) 60mg Peptide: P31 (ZElan024) 15mg/50ml dH20
Experimental details:
The PVA solution was prepared and the organic phase was prepared by adding acetone, 67.5ml, and ethanol, 7.5ml, together. The polymer solution was prepared by adding
RG504H, 3g, to the organic phase prepared above and stirring until dissolved. The IKA™ reactor vessel was set up, all seals greased and the temperature was set at 25°C. The PVA solution, 600ml, was added into the reactor vessel and stirred at 400 rpm.
Leuprolide acetate, 60mg, was added into the stirring PVA solution. Using clean tubing and a green needle, the polymer solution, was slowly dripped in the stirring PVA solution with the peristaltic pump set at 40. The solvent was allowed to evaporate by opening the ports and allowing the dispersion to stir overnight at 400 rpm. The suspension was centrifuged in a Beckman Ultracentrifuge™ with swing-out rotor at 15,000 rpm, 4°C. The supernatant was decanted and retained for analysis. The "cake" of particles was broken up and dH20
200ml) was added to wash the particles. The centrifugation and washing steps were repeated twice.
The peptide solution (P31 (SEQ ID NO: 43) , 15mg in 50ml dH20) was prepared and added to the particles for a final washing stage. The suspended particles were centrifuged as before. The supernatant liquid was decanted, and the particles were dried in the vacuum oven.
The particles were ground, placed in a securitainer and sent for analysis. The weight of particles recovered was 1.87g. SEM showed discrete, reasonably spherical particles in the 300-500nm size range. The potency was 4.7mg/g (23.4% of label claim) . Peptide loading was 1.76μg/mg.
EXAMPLE 5: Peptide added by spiking' polymer phase with polymer-peptide conjugate
Product: Bovine Insulin loaded nanoparticles
Aim: To prepare a 3g batch of insulin loaded 5 nanoparticles at a theoretical loading of 50mg/g and with the polymer-peptide conjugate PLGA-ZElan019 added.
Formulation Details
RG504H (Lot no. 2 077) 2.δ5g
RG504H-ZElan019 conjugattee 0.15g 10 ( 5PAX5-conjugate)
Acetone 67.5ml
Ethanol : 7.5ml
PVA(aq. 5%w/v) 600ml
Bovine Insulin (Lot no. 86H0674) 150mg 15
Experimental details :
The 5% w/v PVA solution was prepared by heating water until near boiling point, adding PVA and stirring until cool. The organic phase was prepared by adding acetone, 20 67.5ml, and ethanol, 7.5ml, together. The polymer solution was prepared by adding RG504H and the polymer-peptide conjugate to the organic phase and stirring until dissolved. The IKA™ reactor vessel was set up, all seals greased and the temperature was set at 25°C. The PVA 25 solution, 400ml, was added into the reactor vessel and stirred at 400 rpm.
Bovine insulin, lOOmg, was added into the stirring
PVA solution. Using clean tubing and a green needle, the polymer solution, was slowly dripped in the stirring PVA 30 solution with the peristaltic pump set at 40. The solvent was allowed to evaporate by opening the ports and allowing the dispersion to stir overnight at 400 rpm.
The suspension was centrifuged in a Beckman
Ultracentrifuge™ with swing-out rotor at 12,500 rpm, 4°C. 35 The supernatant was decanted and discarded. The "cake" of particles was broken up and dH20 (200ml) was added to wash the
particles . The centrifugation washing step was repeated twice .
The λ cake' was broken up and the particles were dried in the vacuum oven. The particles were ground, placed 5 in a securitainer and sent for analysis. The weight of particles recovered was 2.δg. The potency was 53.1mg/g 106.2% of label claim). Peptide loading was 4.02 μg/mg (80.4% of label claim).
10 10. ANIMAL STUDIES
Study 1
An open-loop study in which the test solution was injected directly into the ileum was done. Wistar rats (300- 350g) were fasted for 4 hours and anaesthetized by
15 intramuscular administration 15 to 20 minutes prior to administration of the test solution with a solution of ketamine [0.525 ml of ketamine (100 mg/ml) and 0.875 ml of acepromazine maleate-BP ACP (2mg/ml) ] . The rats were then injected with a test solution (injection volume: 1.5ml PBS)
20 intra-duodenally at 2-3 cm below the pyloris . The test solution contained either PLGA particles manufactured according to the coacervation procedure given above with or without targeting peptides or by the "spiked" method given above. Insulin (fast-acting bovine; 28.1 iu/mg) was
25 incorporated in the particles at 5% drug loading for a total of lOOiu insulin (70 mg particles) or 300iu insulin (210 mg particles) . Blood glucose values for the rats were measured using a Glucometer™ (Bayer; 0.1 to 33.3 m/mol/L) ; plasma insulin values were measured using a Phadeseph RIA Kit™
30 (Upjohn Pharmacia; 3 to 240 μU/ml-assayed in duplicate) .
Systemic and portal blood was sampled.
Study groups included animals receiving test solutions containing particles coated with the following peptides shown in Table 33. 35
Table 33
Study Group Receptor Peptide
I hSI SNilO
SNi34
II hPEPTl P31
5PAX5
III HPTl PAX2
HAX42
IV D2H DCX8
DCXll V ("spiked") hPEPTl P31-PLGA conjugate
5PAX5-PLGA conjugate
Control groups included: 1) PBS control (1.5ml) Open-Loop; 2) Insulin solution (liu/0.2ml) subcutaneous; 3) Insulin particles - no peptide (liu/0.2ml) subcutaneous; 4) Insulin particles/all 8 peptides mix (liu/0.2ml) subcutaneous; 5) Insulin loaded particles/peptide control (scrambled 5PAX5) (100iu/l .5ml) Open-Loop; 6) Insulin loaded particles/peptide control (scrambled 5PAX5) (300iu/l .5ml) Open-Loop; 7) Control particles (insulin-free) /all δ peptide mix (equivalent 100iu/l.5ml) Open-Loop; and 8) Control particles (insulin- free) /all 8 peptide mix (equivalent 300iu/l.5ml) Open-Loop. The following describes the pharmacokinetics for 300iu-loading:
Target Receptor F%* Fold- increase ** Stat. Sig.**
HPTl 10.37 17.0 <0.001
Spiked hPEPTl 4.94 7.5 0.005
PAX2 scrambled 3.50 3.6 NS
Mix-8 2.00 2.0 NS hPEPTl 1.60 1.5 NS
D2H 1.57 1.4 NS hSI 0.54 0.9 NS
* based on area under the curve (AUC) (l-4h) , base-line adjusted, relative to subcutaneous insulin solution liu ** Fold increase in AUC compared to insulin particles: 300iu Figures 17A and 17B show the systemic blood glucose and insulin levels following intestinal administration of control (PBS); insulin solution; insulin particles; all 8
peptides mix particles and study group peptide-particles (lOOiu) . Figures 18A and 18B show the systemic blood glucose and insulin levels following intestinal administration of control (PBS) ; insulin solution; insulin particles and study group peptide-particles (300iu) .
HPTl targeted peptide coated particles provided the most potent enhancement of the delivery of insulin over subcutaneous injection of insulin followed by hPEPTl spiked > PAX2 scrambled > mix-δ > hPEPTl > D2H > uncoated particles > hSI > solution. In a repeat study, the uncoated particles containing insulin gave similar profiles but the HPTl-peptide targeted particles gave a reduced profile (3 -fold) . The insulin-free PLGA particles and the all-8 mix particles did not show an effect on the basal insulin or glucose levels. The HPTl targeting particles, the PEPT1 spiked, targeting particles, and the PEPT1 targeting particles also reduced blood glucose levels indicative that the insulin delivered was bioactive. The other targeting particles were also shown to reduce blood glucose levels although not to the same extent as the HPTl and PEPT1 spiked particles. No histological differences were observed in the small intestine for any of the formulations evaluated.
Study 2 A second open-loop study, similar to study 1 above, was undertaken with the following treatment groups as shown in Table 34.
Table 34
Group Dose Description
Number Insulin (iu)
1 PBS control
2a 1 subcutaneous, bovine insulin
2b 2 subcutaneous, bovine insulin
2c 3 subcutaneous, bovine insulin
2d 4 subcutaneous, bovine insulin
2e 10 subcutaneous, bovine insulin
2f 20 subcutaneous, bovine insulin
2g 4 subcutaneous, human insulin
3 300 uncoated insulin particles
4 100 HAX42/PAX2 with 300 iu particle loading
5 300 HAX42/PAX2 (40mer) particles
6 300 HAX42 (40mer) particles
7 300 HAX42 particles + 10-fold excess free HAX42 (40mer)
8 300 PAX2 (40mer) particles
9 300 PAX2 freeze-dried (40mer) particles
10 300 PAX2 scrambled particles III (40mer)
11 300 PAX2 scrambled particles IV (19mer)
12 300 5PAX5/P31 (40mer) particles
13 300 P31 (40mer) particles
14 300 5PAX5 (40mer) particles
15 300 HAX42 (27mer) particles
16 300 PAX2 (20mer) particles
17 300 P31 (20mer) particles
18 300 PAX2 (15mer) particles
19 300 P31 (15mer) particles
20 300 P31 D-form 1(5 D-arginine) (16mer) particles
21 300 P31 D-form 11(2 D-arginine) (16mer) particles
22 300 HAX42 (lOmer)
Availability of insulin following administration was assessed relative to a 1 and 20iu subcutaneous dose because the response to increasing subcutaneous doses of bovine insulin does not increase linearly over the range of 1 to 20iu. Data up to three hours post-dosing was available for most animals. Therefore, availability was first assessed using individual AUC(0-3h) data estimated from baseline- subtracted data for which data up to 3 hours was available. This approach may lead to an underestimation of the availability as some animals that gave a high response often did not survive for 3 hours and, therefore, were excluded from the analyses . In an attempt to capture as much of these high responses observed at the earlier timepoints as possible, the mean baseline-subtracted plasma concentration
data was used to estimate an AUC for each group. Table 35 shows the results based on this second approach (AUC(0-3h) calculated from the mean plasma concentration data) .
Table 35
Group Dose iu Mean AUC(0.3h) F vs . 1 iu F vs. 20 iu
1 0 2.14
2a 1 875.27 100.00 28.86
2b 2 2439.36 139.35 40.22
2c 3 3671.44 139.82 40.36
2d 4 6912.18 197.43 56.98
2e 10 27224.41 311.04 89.77
2f 20 60651.28 346.47 100.00
2g 4 14255.49 407.17 117.52
3 300 10677.78 4.07 1.17
3 -Rat43 300 4645.06 1.77 0.51
4 100 3527.18 4.03 1.16
5 300 27112.26 10.33 2.98
6 300 33091.68 12.60 3.64
7 300 9303.09 3.54 1.02
8 300 34241.83 13.04 3.76
9 300 10968.83 4.18 1.21
10 300 27692.78 10.55 3.04
11 300 3004.29 1.14 0.33
12 300 18852.61 7.18 2.07
13 300 20278.43 7.72 2.23
14 300 17400.38 6.63 1.91
15 300 16775.69 6.39 1.84
16 300 14217.47 5.41 1.56
17 300 8197.97 3.12 0.90
18 300 25050.59 9.54 2.75
19 300 7927.96 3.02 0.87
20 300 21519.57 8.20 2.37
21 300 6322.41 2.41 0.69
22 300 12553.01 4.78 1.38
The data for group 3 (uncoated insulin particles) are expressed with and without Rat 43. This animal had an atypically high response to these uncoated particles and, therefore, may have biased the data for this group.
This data shows that a combination of peptide- coated particles (HAX42/PAX2 or 5PAX5/P31) shows no greater availability than particles coated with the individual peptides. Further, peptide-coated particles have a greater availability than uncoated peptides. Scrambling the 40mer
PAX2 peptide did not result in a loss of bioavailability . Scrambling the PAX2 peptide and reducing the size to 19mer resulted in a loss of bioavailability although this loss may be attributed in part to the reduction in peptide size. Reducing peptide size resulted in loss of bioavailability. The D-form of P31 (ZElan053) had increased bioavailability possibly due to greater resistance to peptide breakdown. A competitive excess of peptide resulted in a loss of bioavailability, and freeze drying caused a loss in bioavailability. By way of example, measurement of blood glucose levels showed that the HPTl and hPEPTl targeting particles incorporating HAX42, PA 2 , P31 (SEQ ID NO:43), and P31 D-form (ZElan053) reduced blood glucose levels indicating that the insulin delivered was bioactive. In further studies, insulin was recovered from the targeting particles following particle formation by dissolution and analyzed by electrophoresis in non-denaturing sodum dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) . The analysis of the insulin by non- denaturing SDS-PAGE and also by western blot transferred to membranes and subsequent screening with an antibody to insulin, indicated that the insulin was intact, with no evidence of degradation, dimerization, or aggregation during the process of particle formation.
Study 3
An intraduodenal open loop model study was carried out on Wistar rats (300-350g) . Group 1 was administered leuprolide acetate (12.5 μg) subcutaneously. Group 2 was administered intraduodenally uncoated leuprolide acetate particles (600 μg, 1.5 ml) . Group 3 was intraduodenally administered leuprolide acetate particles coated with PAX2 (600 μg; 1.5 ml). Group 4 was administered intraduodenally leuprolide acetate particles coated with P31 (SEQ ID NO: 43) (600 μg, 1.5 ml) . Figure 19 shows the leuprolide plasma concentration following administration to these four groups. Both the P31 (SEQ ID NO: 43) and the PAX2 coated leuprolide
particles administered intraduodenally provided enhanced plasma levels of leuprolide relative to subcutaneous injection.
Homologies of GIT transport-binding peptides to known proteins are shown in Figures 20, 21A-F, and 22 A-D.
The present invention is not to be limited in scope by the specific embodiments described herein. Indeed, various modifications of the invention in addition to those described herein will become apparent to those skilled in the art from the foregoing description and accompanying figures.
Such modifications are intended to fall within the scope of the appended claims. Various publications are cited herein, the disclosures of which are incorporated by reference in their entireties .
SEQUENCE LISTING
(1) GENERAL INFORMATION
(i) APPLICANTS: CYTOGEN CORPORATION and ELAN CORPORATION, pic
(ii) TITLE OF THE INVENTION: RANDOM PEPTIDES THAT BIND TO GASTROINTESTINAL TRACT (GIT) TRANSPORT RECEPTORS AND RELATED METHODS
(iii) NUMBER OF SEQUENCES: 265
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Pennie & Edmonds LLP (B) STREET: 1155 Avenue of the Americas
(C) CITY: New York
(D) STATE: New York
(E) COUNTRY: USA
(F) ZIP: 10036
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Diskette
(B) COMPUTER: IBM Compatible (C) OPERATING SYSTEM: DOS
(D) SOFTWARE: FastSEQ Version 2.0
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER:
(B) FILING DATE:
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION: (A) NAME: Misrock, S. Leslie
(B) REGISTRATION NUMBER: 18,872
(C) REFERENCE/DOCKET NUMBER: 1101-209-228
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: 212-790-9090
(B) TELEFAX: 212-869-9741
(C) TELEX: 66141 PENNIE
(2) INFORMATION FOR SEQ ID NO : 1 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO : 1 :
Arg Ser Gly Ala Tyr Glu Ser Pro Asp Gly Arg Gly Gly Arg Ser Tyr
1 5 10 15
Val Gly Gly Gly Gly Gly Cys Gly Asn He Gly Arg Lys His Asn Leu
20 25 30
Trp Gly Leu Arg Thr Ala Ser Pro Ala Cys Trp Asp 35 40
(2) INFORMATION FOR SEQ ID NO : 2 : (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (xi) SEQUENCE DESCRIPTION: SEQ ID NO : 2 :
Ser Pro Arg Ser Phe Trp Pro Val Val Ser Arg His Glu Ser Phe Gly
1 5 10 15
He Ser Asn Tyr Leu Gly Cys Gly Tyr Arg Thr Cys He Ser Gly Thr
20 25 30
Met Thr Lys Ser Ser Pro He Tyr Pro Arg His Ser 35 40 (2) INFORMATION FOR SEQ ID NO : 3 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS :
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:3:
Ser Ser Ser Ser Asp Trp Gly Gly Val Pro Gly Lys Val Val Arg Glu
1 5 10 15
Arg Phe Lys Gly Arg Gly Cys Gly He Ser He Thr Ser Val Leu Thr
20 25 30
Gly Lys Pro Asn Pro Cys Pro Glu Pro Lys Ala Ala 35 40
(2) INFORMATION FOR SEQ ID NO : 4 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO : 4 :
Arg Val Gly Gin Cys Thr Asp Ser Asp Val Arg Arg Pro Trp Ala Arg
1 5 10 15
Ser Cys Ala His Gin Gly Cys Gly Ala Gly Thr Arg Asn Ser His Gly
20 25 30
Cys He Thr Arg Pro Leu Arg Gin Ala Ser Ala His 35 40
(2) INFORMATION FOR SEQ ID NO : 5 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 5:
Ser His Ser Gly Gly Met Asn Arg Ala Tyr Gly Asp Val Phe Arg Glu
1 5 10 15
Leu Arg Asp Arg Trp Asn Ala Thr Ser His His Thr Arg Pro Thr Pro
20 25 30
Gin Leu Pro Arg Gly Pro Asn 35
(2) INFORMATION FOR SEQ ID NO : 6 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 41 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO : 6 :
Ser Pro Cys Gly Gly Ser Trp Gly Arg Phe Met Gin Gly Gly Leu Phe
1 5 10 15
Gly Gly Arg Thr Asp Gly Cys Gly Ala His Arg Asn Arg Thr Ser Ala
20 25 30
Ser Leu Glu Pro Pro Ser Ser Asp Tyr 35 40
(2) INFORMATION FOR SEQ ID NO : 7 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO : 7 :
Arg Gly Ala Ala Asp Gin Arg Arg Gly Trp Ser Glu Asn Leu Gly Leu
1 5 10 15
Pro Arg Val Gly Trp Asp Ala He Ala His Asn Ser Tyr Thr Phe Thr
20 25 30
Ser Arg Arg Pro Arg Pro Pro 35
(2) INFORMATION FOR SEQ ID NO: 8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO : 8 :
Ser Gly Gly Glu Val Ser Ser Trp Gly Arg Val Asn Asp Leu Cys Ala
1 5 10 15
Arg Val Ser Trp Thr Gly Cys Gly Thr Ala Arg Ser Ala Arg Thr Asp 20 25 30 Asn Lys Gly Phe Leu Pro Lys His Ser Ser Leu Arg 35 40
(2) INFORMATION FOR SEQ ID NO : 9 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY : unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO : 9 :
Ser Asp Ser Asp Gly Asp His Tyr Gly Leu Arg Gly Gly Val Arg Cys
1 5 10 15
Ser Leu Arg Asp Arg Gly Cys Gly Leu Ala Leu Ser Thr Val His Ala
20 25 30
Gly Pro Pro Ser Phe Tyr Pro Lys Leu Ser Ser Pro 35 40
(2) INFORMATION FOR SEQ ID NO: 10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 10:
Arg Ser Leu Gly Asn Tyr Gly Val Thr Gly Thr Val Asp Val Thr Val
1 5 10 15
Leu Pro Met Pro Gly His Ala Asn His Leu Gly Val Ser Ser Ala Ser
20 25 30
Ser Ser Asp Pro Pro Arg Arg 35
(2) INFORMATION FOR SEQ ID NO: 11:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 38 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 11:
Arg Thr Thr Thr Ala Lys Gly Cys Leu Leu Gly Ser Phe Gly Val Leu
1 5 10 15
Ser Gly Cys Ser Phe Thr Pro Thr Ser Pro Pro Pro His Leu Gly Tyr 20 25 30 Pro Pro His Ser Val Asn 35
(2) INFORMATION FOR SEQ ID NO: 12:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 12:
Ser Pro Lys Leu Ser Ser Val Gly Val Met Thr Lys Val Thr Glu Leu
1 5 10 15
Pro Thr Glu Gly Pro Asn Ala He Ser He Pro He Ser Ala Thr Leu
20 25 30
Gly Pro Arg Asn Pro Leu Arg 35
(2) INFORMATION FOR SEQ ID NO: 13:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:13:
Arg Trp Cys Gly Ala Glu Leu Cys Asn Ser Val Thr Lys Lys Phe Arg
1 5 10 15
Pro Gly Trp Arg Asp His Ala Asn Pro Ser Thr His His Arg Thr Pro
20 25 30
Pro Pro Ser Gin Ser Ser Pro 35
(2) INFORMATION FOR SEQ ID NO : 14 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (U) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 14:
Arg Trp Cys Gly Ala Asp Asp Pro Cys Gly Ala Ser Arg Trp Arg Gly
1 5 10 15
Gly Asn Ser Leu Phe Gly Cys Gly Leu Arg Cys Ser Ala Ala Gin Ser
20 25 30
Thr Pro Ser Gly Arg He His Ser Thr Ser Thr Ser 35 40
(2) INFORMATION FOR SEQ ID NO: 15:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 15
Ser Lys Ser Gly Glu Gly Gly Asp Ser Ser Arg Gly Glu Thr Gly Trp
1 5 10 15
Ala Arg Val Arg Ser His Ala Met Thr Ala Gly Arg Phe Arg Trp Tyr 20 25 30 Asn Gin Leu Pro Ser Asp Arg 35
(2) INFORMATION FOR SEQ ID NO: 16:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 38 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:16:
Arg Ser Ser Ala Asn Asn Cys Glu Trp Lys Ser Asp Trp Met Arg Arg
1 5 10 15
Ala Cys He Ala Arg Tyr Ala Asn Ser Ser Gly Pro Ala Arg Ala Val
20 25 30
Asp Thr Lys Ala Ala Pro 35
(2) INFORMATION FOR SEQ ID NO: 17:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 17:
Ser Lys Trp Ser Trp Ser Ser Arg Trp Gly Ser Pro Gin Asp Lys Val
1 5 10 15
Glu Lys Thr Arg Ala Gly Cys Gly Gly Ser Pro Ser Ser Thr Asn Cys
20 25 30
His Pro Tyr Thr Phe Ala Pro Pro Pro Gin Ala Gly 35 40
(2) INFORMATION FOR SEQ ID NO:18:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 18:
Ser Gly Phe Trp Glu Phe Ser Arg Gly Leu Trp Asp Gly Glu Asn Arg
1 5 10 15
Lys Ser Val Arg Ser Gly Cys Gly Phe Arg Gly Ser Ser Ala Gin Gly 20 25 30 Pro Cys Pro Val Thr Pro Ala Thr He Asp Lys His 35 40
(2) INFORMATION FOR SEQ ID NO: 19:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 19:
Ser Glu Ser Gly Arg Cys Arg Ser Val Ser Arg Trp Met Thr Thr Trp
1 5 10 15
Gin Thr Gin Lys Gly Gly Cys Gly Ser Asn Val Ser Arg Gly Ser Pro
20 25 30
Leu Asp Pro Ser His Gin Thr Gly His Ala Thr Thr
35 40
(2) INFORMATION FOR SEQ ID NO: 20:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 20:
Arg Glu Trp Arg Phe Ala Gly Pro Pro Leu Asp Leu Trp Ala Gly Pro
1 5 10 15
Ser Leu Pro Ser Phe Asn Ala Ser Ser His Pro Arg Ala Leu Arg Thr
20 25 30
Tyr Trp Ser Gin Arg Pro Arg 35
(2) INFORMATION FOR SEQ ID NO: 21:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 21:
Arg Met Glu Asp He Lys Asn Ser Gly Trp Arg Asp Ser Cys Arg Trp
1 5 10 15
Gly Asp Leu Arg Pro Gly Cys Gly Ser Arg Gin Trp Tyr Pro Ser Asn
20 25 30
Met Arg Ser Ser Arg Asp Tyr Pro Ala Gly Gly His 35 40
(2) INFORMATION FOR SEQ ID NO:22:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 36 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 22:
Ser His Pro Trp Tyr Arg His Trp Asn His Gly Asp Phe Ser Gly Ser
1 5 10 15
Gly Gin Ser Arg His Thr Pro Pro Glu Ser Pro His Pro Gly Arg Pro 20 25 30 Asn Ala Thr He 35
( 2 ) INFORMATION FOR SEQ ID NO : 23 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:23:
Arg Tyr Lys His Asp He Gly Cys Asp Ala Gly Val Asp Lys Lys Ser
1 5 10 15
Ser Ser Val Arg Gly Gly Cys Gly Ala His Ser Ser Pro Pro Arg Ala
20 25 30
Gly Arg Gly Pro Arg Gly Thr Met Val Ser Arg Leu 35 40
(2) INFORMATION FOR SEQ ID NO: 24:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:24:
Ser Gin Gly Ser Lys Gin Cys Met Gin Tyr Arg Thr Gly Arg Leu Thr
1 5 10 15
Val Gly Ser Glu Tyr Gly Cys Gly Met Asn Pro Ala Arg His Ala Thr
20 25 30
Pro Ala Tyr Pro Ala Arg Leu Leu Pro Arg Tyr Arg 35 40
(2) INFORMATION FOR SEQ ID NO: 25:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 25:
Ser Gly Arg Thr Thr Ser Glu He Ser Gly Leu Trp Gly Trp Gly Asp
1 5 10 15
Asp Arg Ser Gly Tyr Gly Trp Gly Asn Thr Leu Arg Pro Asn Tyr He 20 25 30 Pro Tyr Arg Gin Ala Thr Asn Arg His Arg Tyr Thr 35 40
(2) INFORMATION FOR SEQ ID NO: 26:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:26:
Arg Trp Asn Trp Thr Val Leu Pro Ala Thr Gly Gly His Tyr Trp Thr
1 5 10 15
Arg Ser Thr Asp Tyr His Ala He Asn Asn His Arg Pro Ser He Pro
20 25 30
His Gin His Pro Thr Pro He 35
(2) INFORMATION FOR SEQ ID NO: 27:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 27:
Ser Trp Ser Ser Trp Asn Trp Ser Ser Lys Thr Thr Arg Leu Gly Asp
1 5 10 15
Arg Ala Thr Arg Glu Gly Cys Gly Pro Ser Gin Ser Asp Gly Cys Pro
20 25 30
Tyr Asn Gly Arg Leu Thr Thr Val Lys Pro Arg Thr 35 40
(2) INFORMATION FOR SEQ ID NO: 28:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 37 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:28:
Ser Gly Ser Leu Asn Ala Trp Gin Pro Arg Ser Trp Val Gly Gly Ala
1 5 10 15
Phe Arg Ser His Ala Asn Asn Asn Leu Asn Pro Lys Pro Thr Met Val
20 25 30
Thr Arg His Pro Thr 35
(2) INFORMATION FOR SEQ ID NO: 29:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 29:
Arg Tyr Ser Gly Leu Ser Pro Arg Asp Asn Gly Pro Ala Cys Ser Gin
1 5 10 15
Glu Ala Thr Leu Glu Gly Cys Gly Ala Gin Arg Leu Met Ser Thr Arg 20 25 30 Arg Lys Gly Arg Asn Ser Arg Pro Gly Trp Thr Leu 35 40
(2) INFORMATION FOR SEQ ID NO: 30:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 30:
Ser Val Gly Asn Asp Lys Thr Ser Arg Pro Val Ser Phe Tyr Gly Arg
1 5 10 15
Val Ser Asp Leu Trp Asn Ala Ser Leu Met Pro Lys Arg Thr Pro Ser
20 25 30
Ser Lys Arg His Asp Asp Gly 35
(2) INFORMATION FOR SEQ ID NO: 31:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 38 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 31:
Arg Trp Pro Ser Val Gly Tyr Lys Gly Asn Gly Ser Asp Thr He Asp
1 5 10 15
Val His Ser Asn Asp Ala Ser Thr Lys Arg Ser Leu He Tyr Asn His
20 25 30
Arg Arg Pro Leu Phe Pro 35
(2) INFORMATION FOR SEQ ID NO: 32:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 32:
Arg Thr Phe Glu Asn Asp Gly Leu Gly Val Gly Arg Ser He Gin Lys
1 5 10 15
Lys Ser Asp Arg Trp Tyr Ala Ser His Asn He Arg Ser His Phe Ala 20 25 30 Ser Met Ser Pro Ala Gly Lys 35
(2) INFORMATION FOR SEQ ID NO: 33:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:33:
Ser Tyr Cys Arg Val Lys Gly Gly Gly Glu Gly Gly His Thr Asp Ser
1 5 10 15
Asn Leu Ala Arg Ser Gly Cys Gly Lys Val Ala Arg Thr Ser Arg Leu
20 25 30
Gin His He Asn Pro Arg Ala Thr Pro Pro Ser Arg 35 40
(2) INFORMATION FOR SEQ ID NO: 34:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 34:
Ser Trp Thr Arg Trp Gly Lys His Thr His Gly Gly Phe Val Asn Lys
1 5 10 15
Ser Pro Pro Gly Lys Asn Ala Thr Ser Pro Tyr Thr Asp Ala Gin Leu
20 25 30
Pro Ser Asp Gin Gly Pro Pro 35
(2) INFORMATION FOR SEQ ID NO: 35:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 35:
Ser Gin Val Asp Ser Phe Arg Asn Ser Phe Arg Trp Tyr Glu Pro Ser
1 5 10 15
Arg Ala Leu Cys His Gly Cys Gly Lys Arg Asp Thr Ser Thr Thr Arg
20 25 30
He His Asn Ser Pro Ser Asp Ser Tyr Pro Thr Arg 35 40
(2) INFORMATION FOR SEQ ID NO: 36:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 36
Ser Phe Leu Arg Phe Gin Ser Pro Arg Phe Glu Asp Tyr Ser Arg Thr
1 5 10 15
He Ser Arg Leu Arg Asn Ala Thr Asn Pro Ser Asn Val Ser Asp Ala 20 25 30 His Asn Asn Arg Ala Leu Ala 35
( 2 ) INFORMATION FOR SEQ ID NO : 37 :
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 37:
Arg Ser He Thr Asp Gly Gly He Asn Glu Val Asp Leu Ser Ser Val
1 5 10 15
Ser Asn Val Leu Glu Asn Ala Asn Ser His Arg Ala Tyr Arg Lys His
20 25 30
Arg Pro Thr Leu Lys Arg Pro 35
(2) INFORMATION FOR SEQ ID NO: 38:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 38:
Ser Ser Lys Val Ser Ser Pro Arg Asp Pro Thr Val Pro Arg Lys Gly
1 5 10 15
Gly Asn Val Asp Tyr Gly Cys Gly His Arg Ser Ser Ala Arg Met Pro
20 25 30
Thr Ser Ala Leu Ser Ser He Thr Lys Cys Tyr Thr 35 40
(2) INFORMATION FOR SEQ ID NO: 39:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS :
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 39:
Arg Ala Ser Thr Gin Gly Gly Arg Gly Val Ala Pro Glu Phe Gly Ala
1 5 10 15
Ser Val Leu Gly Arg Gly Cys Gly Ser Ala Thr Tyr Tyr Thr Asn Ser 20 25 30 Thr Ser Cys Lys Asp Ala Met Gly His Asn Tyr Ser 35 40
(2) INFORMATION FOR SEQ ID NO: 40:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 40:
Arg Trp Cys Glu Lys His Lys Phe Thr Ala Ala Arg Cys Ser Ala Gly
1 5 10 15
Ala Gly Phe Glu Arg Asp Ala Ser Arg Pro Pro Gin Pro Ala His Arg
20 25 30
Asp Asn Thr Asn Arg Asn Ala 35
(2) INFORMATION FOR SEQ ID NO: 41:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 41:
Ser Phe Gin Val Tyr Pro Asp His Gly Leu Glu Arg His Ala Leu Asp
1 5 10 15
Gly Thr Gly Pro Leu Tyr Ala Met Pro Gly Arg Trp He Arg Ala Arg
20 25 30
Pro Gin Asn Arg Asp Arg Gin 35
(2) INFORMATION FOR SEQ ID NO:42:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 38 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 42:
Ser Arg Cys Thr Asp Asn Glu Gin Cys Pro Asp Thr Gly Thr Arg Ser
1 5 10 15
Arg Ser Val Ser Asn Ala Arg Tyr Phe Ser Ser Arg Leu Leu Lys Thr
20 25 30
His Ala Pro His Arg Pro 5
(2) INFORMATION FOR SEQ ID NO:43:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 43:
Ser Ala Arg Asp Ser Gly Pro Ala Glu Asp Gly Ser Arg Ala Val Arg
1 5 10 15
Leu Asn Gly Val Glu Asn Ala Asn Thr Arg Lys Ser Ser Arg Ser Asn 20 25 30 Pro Arg Gly Arg Arg His Pro 35
(2) INFORMATION FOR SEQ ID NO: 44:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:44:
Ser Ser Ala Asp Ala Glu Lys Cys Ala Gly Ser Leu Leu Trp Trp Gly
1 5 10 15
Arg Gin Asn Asn Ser Gly Cys Gly Ser Pro Thr Lys Lys His Leu Lys
20 25 30
His Arg Asn Arg Ser Gin Thr Ser Ser Ser Ser His 35 40
(2) INFORMATION FOR SEQ ID NO: 45:
(i) SEQUENCE CHARACTERISTICS :
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 45:
Arg Pro Lys Asn Val Ala Asp Ala Tyr Ser Ser Gin Asp Gly Ala Ala
1 5 10 15
Ala Glu Glu Thr Ser His Ala Ser Asn Ala Ala Arg Lys Ser Pro Lys
20 25 30
His Lys Pro Leu Arg Arg Pro 35
(2) INFORMATION FOR SEQ ID NO: 46:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:46:
Arg Gly Ser Thr Gly Thr Ala Gly Gly Glu Arg Ser Gly Val Leu Asn
1 5 10 15
Leu His Thr Arg Asp Asn Ala Ser Gly Ser Gly Phe Lys Pro Trp Tyr 20 25 30 Pro Ser Asn Arg Gly His Lys 35
(2) INFORMATION FOR SEQ ID NO: 47:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 47:
Arg Trp Gly Trp Glu Arg Ser Pro Ser Asp Tyr Asp Ser Asp Met Asp
1 5 10 15
Leu Gly Ala Arg Arg Tyr Ala Thr Arg Thr His Arg Ala Pro Pro Arg
20 25 30
Val Leu Lys Ala Pro Leu Pro 35
(2) INFORMATION FOR SEQ ID NO:48:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 48:
Arg Gly Trp Lys Cys Glu Gly Ser Gin Ala Ala Tyr Gly Asp Lys Asp
1 5 10 15
He Gly Arg Ser Arg Gly Cys Gly Ser He Thr Lys Asn Asn Thr Asn
20 25 30
His Ala His Pro Ser His Gly Ala Val Ala Lys He 35 40
(2) INFORMATION FOR SEQ ID NO: 49:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 49:
Ser Arg Glu Glu Ala Asn Trp Asp Gly Tyr Lys Arg Glu Met Ser His
1 5 10 15
Arg Ser Arg Phe Trp Asp Ala Thr His Leu Ser Arg Pro Arg Arg Pro
20 25 30
Ala Asn Ser Gly Asp Pro Asn 35
(2) INFORMATION FOR SEQ ID NO: 50:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 50:
Glu Trp Tyr Ser Trp Lys Arg Ser Ser Lys Ser Thr Gly Leu Gly Asp
1 5 10 15
Thr Ala Thr Arg Glu Gly Cys Gly Pro Ser Gin Ser Asp Gly Cys Pro 20 25 30 Tyr Asn Gly Arg Leu Thr Thr Val Lys Pro Arg Lys 35 40
(2) INFORMATION FOR SEQ ID NO: 51:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 51:
Arg Glu Phe Ala Glu Arg Arg Leu Trp Gly Cys Asp Asp Leu Ser Trp
1 5 10 15
Arg Leu Asp Ala Glu Gly Cys Gly Pro Thr Pro Ser Asn Arg Ala Val
20 25 30
Lys His Arg Lys Pro Arg Pro Arg Ser Pro Ala Leu 35 40
(2) INFORMATION FOR SEQ ID NO: 52:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 52:
Ser Asp His Ala Leu Gly Thr Asn Leu Arg Ser Asp Asn Ala Lys Glu
1 5 10 15
Pro Gly Asp Tyr Asn Cys Cys Gly Asn Gly Asn Ser Thr Gly Arg Lys
20 25 30
Val Phe Asn Arg Arg Arg Pro Ser Ala He Pro Thr 35 40
(2) INFORMATION FOR SEQ ID NO:53:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:53:
Arg His He Ser Glu Tyr Ser Phe Ala Asn Ser His Leu Met Gly Gly
1 5 10 15
Glu Ser Lys Arg Lys Gly Cys Gly He Asn Gly Ser Phe Ser Pro Thr 20 25 30 Cys Pro Arg Ser Pro Thr Pro Ala Phe Arg Arg Thr 35 40
(2) INFORMATION FOR SEQ ID NO:54:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 38 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 54:
Ser Arg Glu Ser Gly Met Trp Gly Ser Trp Trp Arg Gly His Arg Leu
1 5 10 15
Asn Ser Thr Gly Gly Asn Ala Asn Met Asn Ala Ser Leu Pro Pro Asp
20 25 30
Pro Pro Val Ser Thr Pro 35
(2) INFORMATION FOR SEQ ID NO: 55:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 55:
Ser Thr Pro Pro Ser Arg Glu Ala Tyr Ser Arg Pro Tyr Ser Val Asp
1 5 10 15
Ser Asp Ser Asp Thr Asn Ala Lys His Ser Ser His Asn Arg Arg Leu
20 25 30
Arg Thr Arg Ser Arg Pro Asn 35
(2) INFORMATION FOR SEQ ID NO: 56:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:56:
TCTCACTCCT CGAGATCCGG CGCTTATGAG AGTCCGGATG GTCGGGGGGG TCGGAGCTAT 60 GTGGGGGGCG GGGGTGGNTG TGGTAACATT GGTCGGAAGC ATAACCTGTG GGGGCTGCGT 120 ACCGCGTCGC CGGCCTGCTG GGACTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 57;
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 57:
TCTCACTCCT CGAGTCCTCG CTCTTTCTGG CCCGTTGTGT CCCGGCATGA GTCGTTTGGG 60
ATCTCTAACT ATTTGGGNTG TGGTTATCGT ACATGTATCT CCGGCACGAT GACTAAGTCT 120
AGCCCGATTT ACCCTCGGCA TTCGTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 58:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 58:
TCTCACTCCT CGAGTAGTAG CTCCGATTGG GGTGGTGTGC CTGGGAAGGT GGTTAGGGAG 60 CGCTTTAAGG GGCGCGGTTG TGGTATTTCC ATCACCTCCG TGCTCACTGG GAAGCCCAAT 120 CCGTGTCCGG AGCCTAAGGC GGCCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 59:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 59:
TCTCACTCCT CGAGAGTTGG CCAGTGCACG GATTCTGATG TGCGGCGTCC TTGGGCCAGG 60 TCTTGCGCTC ATCAGGGTTG TGGTGCGGGC ACTCGCAACT CGCACGGCTG CATCACCCGT 120 CCTCTCCGCC AGGCTAGCGC TCATTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 60:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO:60:
TCTCACTCCT CGAGCCACTC CGGTGGTATG AATAGGGCCT ACGGGGATGT GTTTAGGGAG 60 CTTCGTGATC GGTGGAACGC CACTTCCCAC CACACTCGCC CCACCCCTCA GCTCCCCCGT 120 GGGCCTAATT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 61:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 168 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 61: TCTCACTCCT CGAGTCCGTG CGGGGGGTCG TGGGGGCGTT TTATGCAGGG TGGCCTTTTC 60 GGCGGTAGGA CTGATGGTTG TGGTGCCCAT AGAAACCGCA CTTCTGCGTC GTTAGAGCCC 120 CCGAGCAGCG ACTACTCTAG AATCGAAGGT CGCGCTAGAC CTTCGAGA 168
(2) INFORMATION FOR SEQ ID NO: 62:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 135 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:62:
TCTCACTCCT CGAGGGGCGC CGCCGATCAG CGGCGGGGGT GGTCCGAGAA CTTGGGGTTG 60 CCTAGGGTGG GGTGGGACGC CATCGCTCAC AATAGCTATA CGTTCACCTC GCGCCGCCCG 120 CGCCCCCCCT CTAGA 135
(2) INFORMATION FOR SEQ ID NO: 63:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO:63:
TCTCACTCCT CGAGCGGTGG GGAGGTCAGC TCCTGGGGCC GCGTGAATGA CCTCTGCGCT 60 AGGGTGAGTT GGACTGGTTG TGGTACTGCT CGTTCCGCGC GTACCGACAA CAAAGGCTTT 120 CTTCCTAAGC ACTCGTCACT CCGCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 64:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:64: TCTCACTCCT CGAGTGATAG TGACGGGGAT CATTATGGGC TTCGGGGGGG GGTGCGTTGT 60 TCGCTTCGTG ATAGGGGTTG TGGTCTGGCC CTGTCCACCG TCCATGCTGG TCCCCCCTCT 120 TTTTACCCCA AGCTCTCCAG CCCCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 65:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 65:
TCTCACTCCT CGAGGAGCTT GGGTAATTAT GGCGTCACCG GGACTGTGGA CGTGACGGTT 60
TTGCCCATGC CTGGCCACGC CAACCACCTT GGTGTCTCCT CCGCCTCTAG CTCTGATCCT 120 CCGCGGCGCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 66:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 159 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:66
TCTCACTCCT CGAGAACTAC GACGGCTAAG GGGTGTCTTC TCGGAAGCTT CGGCGTTCTT 60 AGTGGGTGCT CATTTACGCC AACCTCTCCA CCGCCCCACC TAGGATACCC CCCCCACTCC 120 GTCAATTCTA GAATCGAAGG TCGCGCTAGA CCTTCGAGA 159
(2) INFORMATION FOR SEQ ID NO: 67:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:67: TCTCACTCCT CGAGCCCGAA GTTGTCCAGC GTGGGTGTTA TGACTAAGGT CACGGAGCTG 60 CCCACGGAGG GGCCTAACGC CATTAGTATT CCGATCTCCG CGACCCTCGG CCCGCGCAAC 120 CCGCTCCGCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 68:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:68:
TCTCACTCCT CGAGGTGGTG CGGCGCTGAG CTGTGCAACT CGGTGACTAA GAAGTTTCGC 60
CCGGGCTGGC GGGATCACGC CAATCCCTCC ACCCATCATC GTACTCCCCC GCCCAGCCAG 120 TCCAGCCCTT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 69:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 176 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:69:
TCTCACTCCT CGAGGTGGTG CGGCGCTGAT GACCCGTGTG GTGCCAGTCG TTGGCGGGGG 60 GGCAACAGCT TGTTTGGTTG TGGTCTTCGT TGTAGTGCGG CGCAGAGCAC CCCGAGTGGC 120 AGGATCCATT CCACTTCGAC CAGCTCTAGA ATCGAAGGTG CGCTAGACCT TCGAGA 176 (2) INFORMATION FOR SEQ ID NO: 70:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 70:
TCTCACTCCT CGAGTAAGTC CGGGGAGGGG GGTGACAGTA GCAGGGGCGA GACGGGCTGG 60 GCGAGGGTTC GGTCTCACGC CATGACTGCT GGCCGCTTTC GGTGGTACAA CCAGTTGCCC 120
TCTGATCGGT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 71:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 159 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 71:
TCTCACTCCT CGAGGTCGAG CGCCAATAAT TGCGAGTGGA AGTCTGATTG GATGCGCAGG 60
GCCTGTATTG CTCGTTACGC CAACAGTTCG GGCCCCGCCC GCGCCGTCGA CACTAAGGCC 120 GCGCCCTCTA GAATCGAAGG TCGCGCTAGA CCTTCGAGA 159
(2) INFORMATION FOR SEQ ID NO: 72:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 72:
TCTCACTCCT CGAGTAAGTG GTCGTGGAGT TCGAGGTGGG GCTCCCCGCA GGATAAGGTT 60 GAGAAGACCA GGGCGGGTTG TGGTGGTAGT CCCAGCAGCA CCAATTGTCA CCCCTACACC 120 TTTGCCCCCC CCCCGCAAGC CGGCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177 (2) INFORMATION FOR SEQ ID NO: 73:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:73:
TCTCACTCCT CGAGTGGGTT CTGGGAGTTT AGCAGGGGGC TTTGGGATGG GGAGAACCGT 60 AAGAGTGTCC GGTCGGGTTG TGGTTTTCGT GGCTCCTCTG CTCAGGGCCC GTGTCCGGTC 120 ACGCCTGCCA CCATTGACAA ACACTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 74: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 74:
TCTCACTCCT CGAGTGAGAG CGGGCGGTGC CGTAGCGTGA GCCGGTGGAT GACGACGTGG 60 CAGACGCAGA AGGGCGGTTG TGGTTCCAAT GTTTCCCGCG GTTCGCCCCT CGACCCCTCT 120 CACCAGACCG GGCATGCCAC TACTTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 75:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 75:
TCTCACTCCT CGAGGGAGTG GAGGTTTGCC GGGCCGCCGT TGGACCTGTG GGCGGGTCCG 60 AGCTTGCCCT CTTTTAACGC CAGTTCCCAC CCTCGCGCCC TGCGCACCTA TTGGTCCCAG 120 CGGCCCCGCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 76:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 76:
TCTCACTCCT CGAGGATGGA GGACATCAAG AACTCGGGGT GGAGGGACTC TTGTAGGTGG 60 GGTGACCTGA GGCCTGGTTG TGGTAGCCGC CAGTGGTACC CCTCGAATAT GCGTTCTAGC 120 AGAGATTACC CCGCGGGGGG CCACTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 77: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 152 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 77:
TCTCACTCCT CGAGTCATCC GTGGTACAGG CATTGGAACC ATGGTGACTT CTCTGGTTCG 60
GGCCAGTCAC GCCACACCCC GCCGGAGAGC CCCCACCCCG GCCGCCCTAA TGCCACCATT 120 TCTAGAATCG AAGGTCGCGC TAGACCTTCG AG 152
(2) INFORMATION FOR SEQ ID NO: 78:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 177 base pairs (B) TYPE: nucleic acid
(C) STRANDEDNESS : single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 78:
TCTCACTCCT CGAGATATAA GCACGATATC GGTTGCGATG CTGGGGTTGA CAAGAAGTCG 60 TCGTCTGTGC GTGGTGGTTG TGGTGCTCAT TNGTCGCCAC CCCGCGCCGG CCGTGGTCCT 120
CGCGGCACGA TGGTTAGCAG GCTTTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 79:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 79:
TCTCACTCCT CGAGTCAGGG CTCCAAGCAG TGTATGCAGT ACCGCACCGG TCGTTTGACG 60 GTGGGGTCTG AGTATGGTTG TGGTATGAAC CCCGCCCGCC ATGCCACGCC CGCTTATCCG 120 GCGCGCCTGC TGCCACGCTA TCGCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 80: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 80:
TCTCACTCCT CGAGTGGGCG GACTACTAGT GAGATTTCTG GGCTCTGGGG TTGGGGTGAC 60
GACCGGAGCG GTTATGGTTG GGGTAACACG CTCCGCCCCA ACTACATCCC TTATAGGCAG 120 GCGACGAACA GGCATCGTTA TACGTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 81:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 162 base pairs (B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 81:
TCTCACTCCT CGAGGTGGAA TTGGACTGTC TTGCCCGCCA CTGGCGGCCA TTACTGGACG 60 CGTTCGACGG ACTATCACGC CATTAACAAT CACAGGCCGA GCATCCCCCA CCAGCATCCG 120
ACCCCTATCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 82:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single (D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:82:
TCTCACTCCT CGAGTTGGTC GTCGTGGAAT TGGAGCTCTA AGACTACTCG TCTGGGCGAC 60 AGGGCGACTC GGGAGGGTTG TGGTCCCAGC CAGTCTGATG GCTGTCCTTA TAACGGCCGC 120 CTTACGACCG TCAAGCCTCG CACGTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 83:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 156 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:83:
TCTCACTCCT CGAGTGGTAG TTTGAACGCA TGGCAACCGC GGTCATGGGT GGGGGGCGCG 60
TTCCGGTCAC ACGCCAACAA TAACTTGAAC CCCAAGCCCA CCATGGTTAC TNGTCACCCT 120 ACCTCTAGAA TCGAAGGTCG CGCTAGACCT TCGAGA 156
(2) INFORMATION FOR SEQ ID NO: 84:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 178 base pairs (B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 84:
TCTCACTCCT CGAGGTATTC GGGTTTGTCC CCGCGGGACA ACGGTCCCGC TTGTAGTCAG 60 GAGGCTACCT TGGAGGGTTG TGGTGCGCAG AGGCTGATGT CCACCCGTCG CAAGGGCCGC 120
AACTCCCGCC CCGGGTGGAC GCTCTCTAGA ATCGAAGGTC GCGCTAGACC CTTCGAGA 178
(2) INFORMATION FOR SEQ ID NO: 85:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single (D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 85:
TCTCACTCCT CGAGCGTGGG GAATGATAAG ACTAGCAGGC CGGTTTCCTT CTACGGGCGC 60 GTTAGTGATC TGTGGAACGC CAGCTTGATG CCGAAGCGTA CTCCCAGCTC GAAGCGCCAC 120 GATGATGGCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 86:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 86:
TCTCACTCCT CGAGTACTCC CCCCAGTAGG GAGGCGTATA GTAGGCCCTA TAGTGTCGAT 60 AGCGATTCGG ATACGAACGC CAAGCACAGC TCCCACAACC GCCGTNTGCG GACGCGCAGC 120 CGCCCGAACT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 87:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 159 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 87:
TCTCACTCCT CGAGATGGCC TAGTGTGGGT TACAAGGGTA ATGGCAGTGA CACTATTGAT 60 GTTCACAGCA ATGACGCCAG TACTAAGAGG TCCCTCATCT ATAACCACCG CCGCCCCNTC 120
TTTCCCTCTA GAATCGAAGG TCGCGCTAGA CCTTCGAGA 159
(2) INFORMATION FOR SEQ ID NO: 88:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single (D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 88:
TCTCACTCCT CGAGAACGTT TGAGAACGAC GGGCTGGGCG TCGGCCGGTC TATTCAGAAG 60 AAGTCGGATA GGTGGTACGC CAGCCACAAC ATTCGTAGCC ATTTCGCGTC CATGTCTCCC 120 GCTGGTAAGT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 89:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 160 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 89:
TCTCACTCCT CGAGCTATTG TCGGGTTAAG GGTGGTGGGG AGGGGGGGCA TACGGATTCC 60 AATCTGGCTA GGTCGGGTTG TGGTAAGGTG GCCAGGACCA GCAGGCTTCA GCATATCAAC 120 CCGCGCGCTA CCCCCCCCTC CCGGTCTAGA ATCGAAGGTC 160
(2) INFORMATION FOR SEQ ID NO: 90:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 90:
TCTCACTCCT CGAGTTGGAC TCGGTGGGGC AAGCACANTC ATGGGGGGTT TGTGAACAAG 60 TCTCCCCCTG GGAAGAACGC CACGAGCCCC TACACCGACG CCCAGCTGCC CAGTGATCAG 120 GGTCCTCCCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 91:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 91:
TCTCACTCCT CGAGTCAGGT TGATTCGTTT CGTAATAGCT TTCGGTGGTA TGAGCCGAGC 60 AGGGCTCTGT GCCATGGTTG TGGTAAGCGC GACACCTCCA CCACTCGTAT CCACAATAGC 120 CCCAGCGACT CCTATCCTAC ACGCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 92:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:92:
TCTCACTCCT CGAGCTTTTT GCGGTTCCAG AGTCCGAGGT TCGAGGATTA CAGTAGGACG 60 ATCTNTCGGT TGCGCAACGC CACGAACCCG AGTAATGTCT CCGATGCGCA CAATAACCGG 120 GCCTTGGCCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 93:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (x ) SEQUENCE DESCRIPTION: SEQ ID NO: 93:
TCTCACTCCT CGAGGAGCAT CACCGACGGG GGCATCAATG AGGTGGACCT GAGTAGTGTG 60 TCGAACGTTC TTGAGAACGC CAACTCGCAT AGGGCCTACA GGAAGCATCG CCCGACCTTG 120 AAGCGTCCTT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO : 94 :
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 94: TCTCACTCCT CGAGTTCGAA GGTGAGCAGC CCGAGGGATC CGACGGTCCC GCGGAAGGGC 60
GGCAATGTTG ATTATGGTTG TGGTCACAGG TCTTCCGCCC GGATGCCTAC CTCCGCTCTG 120 TCGTCGATCA CGAAGTGCTA CACTTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 95:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 95:
TCTCACTCCT CGAGAGCCAG TANGCAGGGC GGCCGGGGTG TTGCCCCTGA GTTTGGGGCG 60 AGCGTTTTGG GTNGTGGTTG TGGTAGCGCC ACTTATTACA CGAACTCCAC CAGCTGCAAG 120 GATGCTATGG GCCACAACTA CTCGTCTAGA ATCGAAGGTC GCGNTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 96:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO:96:
TCTCACTCCT CGAGATGGTG CGAGAAGCAC AAGTTTACGG CTGCGCGTTG CAGCGCGGGG 60 GCGGGTTTTG AGAGGGANGC CAGCCGTCCG CCCCAGCCTG CCCACCGGGA TAATACCAAC 120 CGTAATGCNT NTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 97:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 97: TCTCACTCCT CGAGTTTTCA GGTGTACCCG GACCATGGTC TGGAGAGGCA TGCTTTGGAC 60 GGGACGGGTC CGCTTTACGC CATGCCCGGC CGCTGGATTA GGGCGCGTCC GCAGAACAGG 120 GACCGCCAGT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 98:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 159 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 98:
TCTCACTCCT CGAGCAGGTG TACGGACAAC GAGCAGTGCC CCGATACCGG GANTAGGTCT 60
CGTTCCGTTA GTAACGCCAG GTACTTTTCG AGCAGGTTGC TCAAGACTCA CGCCCCCCAT 120 CGCCCTTCTA GAATCGAAGG TCGCGCTAGA CCTTCGAGA 159
(2) INFORMATION FOR SEQ ID NO: 99:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 99;
TCTCACTCCT CGAGTGCCAG GGATAGCGGG CCTGCGGAGG ATGGGTCCCG CGCCGTCCGG 60 TTGAACGGGG TTGAGAACGC CAACACTAGG AAGTCCTCCC GCAGTAACCC GCGGGGTAGG 120 CGCCATCCCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO: 100:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 100: TCTCACTCCT CGAGTTCCGC CGATGCGGAG AAGTGTGCGG GCAGTCTGTT GTGGTGGGGT 60 AGGCAGAACA ACTCCGGTTG TGGTTCGCCC ACGAAGAAGC ATCTGAAGCA CCGCAATCGC 120 AGTCAGACCT CCTCTTCGTC CCACTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 101:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 101:
TCTCACTCCT CGAGACCGAA GAACGTGGCC GATGCTTATT CGTCTCAGGA CGGGGCGGCG 60
GCCGAGGAGA CGTCTCACGC CAGTAATGCC GCGCGGAAGT CCCCTAAGCA CAAGCCCTTG 120 AGGCGGCCTT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO:102:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 102:
TCTCACTCCT CGAGAGGCAG TACGGGGACG GCCGGCGGCG AGCGTTCCGG GGTGCTCAAC 60 CTGCACACCA GGGATAACGC CAGCGGCAGC GGTTTCAAAC CGTGGTACCC TTCGAATCGG 120 GGTCACAAGT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162 (2) INFORMATION FOR SEQ ID NO:103:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 103:
TCTCACTCCT CGAGGTGGGG GTGGGAGAGG AGTCCGTCCG ACTACGATTC TGATATGGAC 60 TTGGGGGCGA GGAGGTACGC CACCCGCACC CACCGCGCGC CCCCTCGCGT CTTGAAGGCT 120
CCCCTGCCCT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162
(2) INFORMATION FOR SEQ ID NO:104:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 104:
TCTCACTCCT CGAGGCACTG GAAGTGCGAG GGCTCTCAGG CTGCCTACGG GGACAAGGAT 60
ATCGGGAGGT CCAGGGGTTG TGGTTCCATT ACAAAGAATA ACACTAATCA CGCCCATCCT 120 AGCCACGGCG CCGTTGCTAA GATCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 105:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 162 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 105:
TCTCACTCCT CGAGCCGCGA GGAGGCGAAC TGGGACGGCT ATAAGAGGGA GATGAGCCAC 60 CGGAGTCGCT TTTGGGACGC CACCCACCTG TCCCGCCCTC GCCGCCCCGC TAACTCTGGT 120 GACCCTAACT CTAGAATCGA AGGTCGCGCT AGACCTTCGA GA 162 (2) INFORMATION FOR SEQ ID NO: 106:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 106:
TCTCACTCNT CGAGAGAGTT CGCGGAGAGG AGGTTGTGGG GGTGTGATGA CCTGAGTTGG 60 CGTCTCGACG CGGAGGGTTG TGGTCCCACT CCGAGCAATC GGGCCGTCAA GCATCGCAAG 120 CCCCGCCCAC GCTCCCCCGC ACTCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO:107: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 107:
TCTCACTCNT NGAGTGATCA CGCGTTGGGG ACGAATCTGA GGTCTGACAA TGCCAAGGAG 60 CCGGGTGATT ACAACTGTTG TGGTAACGGG AACTCTACCG GGCGAAAGGT TTTTAACCGT 120 AGGCGCCCCT CCGCCATCCC CANTTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO:108:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 177 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 108:
TCTCACTCCT CGAGGCATAT TTCTGAGTAT AGCTTTGCGA ATTCCCACTT GATGGGTGGC 60 GAGTCCAAGC GGAAGGGTTG TGGTATTAAC GGCTCCTTTT CTCCCACTTG TCCCCGCTCC 120 CCCACCCCAG CCTTCCGCCG CACCTCTAGA ATCGAAGGTC GCGCTAGACC TTCGAGA 177
(2) INFORMATION FOR SEQ ID NO: 109:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 158 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 109:
TCTCACTCCT CGAGCCGGGA GAGCGGGATG TGGGGTAGTT GGTGGCGTGG TCACAGGTTG 60 AATTCCACGG GGGGTAACGC CAACATGAAT GCTAGTCTGC CCCCCGACCC CCCTGTTTCC 120 ACTCCGTCTA GAATCGAAGG TCGCGCTAGA CCTTCGAG 158
(2) INFORMATION FOR SEQ ID NO: 110: ( ) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 708 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 110:
Met Gly Met Ser Lys Ser His Ser Phe Phe Gly Tyr Pro Leu Ser He
1 5 10 15
Phe Phe He Val Val Asn Glu Phe Cys Glu Arg Phe Ser Tyr Tyr Gly
20 25 30
Met Arg Ala He Leu He Leu Tyr Phe Thr Asn Phe He Ser Trp Asp
35 40 45
Asp Asn Leu Ser Thr Ala He Tyr His Thr Phe Val Ala Leu Cys Tyr 50 55 60 Leu Thr Pro He Leu Gly Ala Leu He Ala Asp Ser Trp Leu Gly Lys 65 70 75 80
Phe Lys Thr He Val Ser Leu Ser He Val Tyr Thr He Gly Gin Ala
85 90 95
Val Thr Ser Val Ser Ser He Asn Asp Leu Thr Asp His Asn His Asp
100 105 110
Gly Thr Pro Asp Ser Leu Pro Val His Val Val Leu Ser Leu He Gly
115 120 125
Leu Ala Leu He Ala Leu Gly Thr Gly Gly He Lys Pro Cys Val Ser 130 135 140
Ala Phe Gly Gly Asp Gin Phe Glu Glu Gly Gin Glu Lys Gin Arg Asn 145 150 155 160
Arg Phe Phe Ser He Phe Tyr Leu Ala He Asn Ala Gly Ser Leu Leu 165 170 175
Ser Thr He He Thr Pro Met Leu Arg Val Gin Gin Cys Gly He His
180 185 190
Ser Lys Gin Ala Cys Tyr Pro Leu Ala Phe Gly Val Pro Ala Ala Leu
195 200 205
Met Ala Val Ala Leu He Val Phe Val Leu Gly Ser Gly Met Tyr Lys
210 215 220
Lys Phe Lys Pro Gin Gly Asn He Met Gly Lys Val Ala Lys Cys He 225 230 235 240
Gly Phe Ala He Lys Asn Arg Phe Arg His Arg Ser Lys Ala Phe Pro
245 250 255
Lys Arg Glu His Trp Leu Asp Trp Ala Lys Glu Lys Tyr Asp Glu Arg
260 265 270
Leu He Ser Gin He Lys Met Val Thr Arg Val Met Phe Leu Tyr He
275 280 285
Pro Leu Pro Met Phe Trp Ala Leu Phe Asp Gin Gin Gly Ser Arg Trp
290 295 300 Thr Leu Gin Ala Thr Thr Met Ser Gly Lys He Gly Ala Leu Glu He
305 310 315 320
Gin Pro Asp Gin Met Gin Thr Val Asn Ala He Leu He Val He Met
325 330 335
Val Pro He Phe Asp Ala Val Leu Tyr Pro Leu He Ala Lys Cys Gly
340 345 350
Phe Asn Phe Thr Ser Leu Lys Lys Met Ala Val Gly Met Val Leu Ala
355 360 365
Ser Met Ala Phe Val Val Ala Ala He Val Gin Val Glu He Asp Lys
370 375 380
Thr Leu Pro Val Phe Pro Lys Gly Asn Glu Val Gin He Lys Val Leu 385 390 395 400
Asn He Gly Asn Asn Thr Met Asn He Ser Leu Pro Gly Glu Met Val
405 410 415
Thr Leu Gly Pro Met Ser Gin Thr Asn Ala Phe Met Thr Phe Asp Val
420 425 430
Asn Lys Leu Thr Arg He Asn He Ser Ser Pro Gly Ser Pro Val Thr 435 440 445 A]-a al Tnr AsP AsP pl e LYS Gln G1Y Gln Ar9 Hi-S Tnr Leu Leu Val
450 455 460
Trp Ala Pro Asn His Tyr Gin Val Val Lys Asp Gly Leu Asn Gin Lys
465 470 475 480
Pro Glu Lys Gly Glu Asn Gly He Arg Phe Val Asn Thr Phe Asn Glu
485 490 495
Leu He Thr He Thr Met Ser Gly Lys Val Tyr Ala Asn He Ser Ser
500 505 510
Tyr Asn Ala Ser Thr Tyr Gin Phe Phe Pro Ser Gly He Lys Gly Phe 515 520 525
Thr He Ser Ser Thr Glu He Pro Pro Gin Cys Gin Pro Asn Phe Asn
530 535 540
Thr Phe Tyr Leu Glu Phe Gly Ser Ala Tyr Thr Tyr He Val Gin Arg 545 550 555 560
Lys Asn Asp Ser Cys Pro Glu Val Lys Val Phe Glu Asp He Ser Ala
565 570 575
Asn Thr Val Asn Met Ala Leu Gin He Pro Gin Tyr Phe Leu Leu Thr 580 585 590 Cys Gly Glu Val Val Phe Ser Val Thr Gly Leu Glu Phe Ser Tyr Ser 595 600 605
Gin Ala Pro Ser Asn Met Lys Ser Val Leu Gin Ala Gly Trp Leu Leu
610 615 620
Thr Val Ala Val Gly Asn He He Val Leu He Val Ala Gly Ala Gly 625 630 635 640
Gin Phe Ser Lys Gin Trp Ala Glu Tyr He Leu Phe Ala Ala Leu Leu
645 650 655
Leu Val Val Cys Val Val Phe Ala He Met Ala Arg Phe Tyr Thr Tyr 660 665 670
He Asn Pro Ala Glu He Glu Ala Gin Phe Asp Glu Asp Glu Lys Lys
675 680 685
Asn Arg Leu Glu Lys Ser Asn Pro Tyr Phe Met Ser Gly Ala Asn Ser 690 695 700
Gin Lys Gin Met 705
(2) INFORMATION FOR SEQ ID NO: 111:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 111: TCCGGACTCT CATAAGCGCC GG 22
(2) INFORMATION FOR SEQ ID NO: 112:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO : 112 :
ACAACGGGCC AGAAAGAGCG AG 22
(2) INFORMATION FOR SEQ ID NO: 113:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 113: ACACCACCCC AATCGGAGCT AC 22
(2) INFORMATION FOR SEQ ID NO: 114:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 114: TCAGAATCCG TGCACTGGCC AA 22
(2) INFORMATION FOR SEQ ID NO: 115: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 115: GCCCTATTCA TACCACCGGA GT 22
(2) INFORMATION FOR SEQ ID NO: 116:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 116:
CATCAGTCCT ACCGCCGAAA AG 22
(2) INFORMATION FOR SEQ ID NO: 117:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 117: CGTATAGCTA TTGTGAGCGA TG 22 (2) INFORMATION FOR SEQ ID NO: 118:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 118:
ACGCGCGGAA CGAGCAGTAC CA 22
(2) INFORMATION FOR SEQ ID NO: 119:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs (B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 119:
CCATAATGAT CCCCGTCACT AT 22
(2) INFORMATION FOR SEQ ID NO: 120:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 120: AGACACCCCT TAGCCGTCGT AG 22
(2) INFORMATION FOR SEQ ID NO: 121:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single (D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 121:
AGCTCCGTGA CCTTAGTCAT AA 22
(2) INFORMATION FOR SEQ ID NO: 122:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 122:
TGCACAGCTC AGCGCCGCAC CA 22
(2) INFORMATION FOR SEQ ID NO: 123:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:123: ACGGGTCATC AGCGCCGCAC CA 22 (2) INFORMATION FOR SEQ ID NO: 124:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 124: TGTCACCCCC CTCCCCGGAC TT 22
(2) INFORMATION FOR SEQ ID NO: 125:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 125: ACTCGCAATT ATTGGCGCTC GA 22
(2) INFORMATION FOR SEQ ID NO: 126: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 126: GTCTTCTCAA CCTTATCCTG CG 22
(2) INFORMATION FOR SEQ ID NO: 127:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single (D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 127:
AAAGCCCCCT GCTAAACTCC CA 22
(2) INFORMATION FOR SEQ ID NO: 128:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO:128:
CTGCGTCTGC CACGTCGTCA TC 22
(2) INFORMATION FOR SEQ ID NO: 129:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 129:
GTTAAAAGAG GGCAAGCTCG GA 22
(2) INFORMATION FOR SEQ ID NO: 130:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 130: CCGAGTTCTT GATGTCCTCC AT 22
(2) INFORMATION FOR SEQ ID NO: 131:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 131: TCCAATGCCT GTACCACGGA TG 22
(2) INFORMATION FOR SEQ ID NO: 132:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:132: TCGCAACCGA TATCGTGCTT AT 22
(2) INFORMATION FOR SEQ ID NO: 133:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 133; TGCATACACT GCTTGGAGCC CT 22
(2) INFORMATION FOR SEQ ID NO: 134:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 134:
GAAATCTCAC TAGTAGTCCG CC 22
(2) INFORMATION FOR SEQ ID NO: 135:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 135:
GCGGGCAAGA CAGTCCAATT CC 22
(2) INFORMATION FOR SEQ ID NO: 136:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 136: GAGCTCCAAT TCCACGACGA CC 22
(2) INFORMATION FOR SEQ ID NO: 137:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 137: GGTTGCCATG CGTTCAAACT AC 22
(2) INFORMATION FOR SEQ ID NO:138: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 138: TCCCGCGGGG ACAAACCCGA AT 22
(2) INFORMATION FOR SEQ ID NO: 139:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(,xi.,) SEQUENCE DESCRIPTION: SEQ ID NO: 139:
CTGCTAGTCT TATCATTCCC CA 22
(2) INFORMATION FOR SEQ ID NO: 140:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs (B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 140: CTATCGACAC TATAGGGCCT AC 22
(2) INFORMATION FOR SEQ ID NO: 141:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 141:
TACCCTTGTA ACCCACACTA GG 22
(2) INFORMATION FOR SEQ ID NO: 142:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:142: TTCTTCTGAA TAGACCGGCC GA 22
(2) INFORMATION FOR SEQ ID NO:143:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:143;
CCACCACCCT TAACCCGACA AT 22
(2) INFORMATION FOR SEQ ID NO: 144:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 144:
AGGGGGAGAC TTGTTCACAA AC 22 (2) INFORMATION FOR SEQ ID NO: 145:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 145:
CGGCTCATAC CACCGAAAGC TA 22
(2) INFORMATION FOR SEQ ID NO: 146:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs (B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 146: ATCGTCCTAC TGTAATCCTC GA 22
(2) INFORMATION FOR SEQ ID NO: 147:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:147:
GACACACTAC TCAGGTCCAC CT 22
(2) INFORMATION FOR SEQ ID NO: 148:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 148:
CCATAATCAA CATTGCCGCC CT 22
(2) INFORMATION FOR SEQ ID NO: 149:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 149: CAAAACGCTC GCCCCAAACT CA 22
(2) INFORMATION FOR SEQ ID NO: 150:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 150: GTAAACTTGT GCTTCTCGCA CC 22
(2) INFORMATION FOR SEQ ID NO: 151: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 151: CCATGGTCCG GGTACACCTG AA 22
(2) INFORMATION FOR SEQ ID NO:152:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single (D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 152:
GTTACTAACG GAACGAGACC TA 22
(2) INFORMATION FOR SEQ ID NO: 153:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ii) MOLECULE TYPE: DNA (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 153:
TGTTGGCGTT CTCAACCCCG TT 22
(2) INFORMATION FOR SEQ ID NO: 154:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 154:
ACAACCGGAG TTGTTCTGCC TA 22
(2) INFORMATION FOR SEQ ID NO: 155:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 155: TAAGCATCGG CCACGTTCTT CG 22
(2) INFORMATION FOR SEQ ID NO: 156:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 156: TTATCCCTGG TGTGCAGGTT GA 22
(2) INFORMATION FOR SEQ ID NO: 157: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 157:
TATCAGAATC GTAGTCGGAC GG 22
(2) INFORMATION FOR SEQ ID NO:158:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 158:
CTTTGTAATG GAACCACAAC CC 22
(2) INFORMATION FOR SEQ ID NO: 159:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs (B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 159: CGGTGGCTCA TCTCCCTCTT AT 22
(2) INFORMATION FOR SEQ ID NO: 160:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 160:
ATCAGACTGG CTGGGACCAC AA 22
(2) INFORMATION FOR SEQ ID NO: 161:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 161: CACAACCTCC TCTCCGCGAA CT 22
(2) INFORMATION FOR SEQ ID NO:162:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 162
AGATTCGTCC CCAACGCGTG AT 22
(2) INFORMATION FOR SEQ ID NO: 163:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid (C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:163:
GGGAATTCGC AAAGCTATAC TC 22 (2) INFORMATION FOR SEQ ID NO:164:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 22 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 164:
CCCCGTGGAA TTCAACCTGT GA 22
(2) INFORMATION FOR SEQ ID NO: 165:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 17 base pairs (B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 165: GTCGTCTTTC CAGACGT 17
(2) INFORMATION FOR SEQ ID NO: 166:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ϋ) MOLECULE TYPE: DNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 166:
CTTGCATGCC TGCAGGTCGA C 21
(2) INFORMATION FOR SEQ ID NO: 167:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 37 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 167:
Arg He Ala Gly Leu Pro Trp Tyr Arg Cys Arg Thr Val Ala Phe Glu
1 5 10 15
Thr Gly Met Gin Asn Thr Gin Leu Cys Ser Thr He Val Gin Leu Ser 20 25 30
Phe Thr Pro Glu Glu 35
(2) INFORMATION FOR SEQ ID NO: 168:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 168:
Arg Glu Phe Ala Glu Arg Arg Leu Trp Gly Cys Asp Asp Leu Ser Trp 1 5 10 15 Arg Leu Asp Ala Glu Gly Cys Gly Pro Thr Pro Ser Asn Arg Ala Val 20 25 30
Lys His Arg Lys Pro Arg Pro Arg Ser Pro Ala Leu 35 40
(2) INFORMATION FOR SEQ ID NO: 169:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 41 amino acids (B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 169:
Ser Gly Ser His Ser Gly Gly Met Asn Arg Ala Tyr Gly Asp Val Phe 1 5 10 15
Arg Glu Leu Arg Asp Arg Trp Tyr Ala Thr Ser His His Thr Arg Pro
20 25 30
Thr Pro Gin Leu Pro Arg Gly Pro Asn 35 40
(2) INFORMATION FOR SEQ ID NO: 170:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 170: Ser Thr Pro Pro Ser Arg Glu Ala Tyr Ser Arg Pro Tyr Ser Val Asp 1 5 10 15
Ser Asp Ser Asp 20
(2) INFORMATION FOR SEQ ID NO: 171:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 29 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 171:
Ser Thr Pro Pro Ser Arg Glu Ala Tyr Ser Arg Pro Tyr Ser Val Asp
1 5 10 15
Ser Asp Ser Asp Thr Asn Ala Lys His Ser Ser His Asn
20 25
(2) INFORMATION FOR SEQ ID NO: 172:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 172:
Thr Asn Ala Lys His Ser Ser His Asn Arg Arg Leu Arg Thr Arg Ser
1 5 10 15
Arg Pro Asn
(2) INFORMATION FOR SEQ ID NO: 173:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 9 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 173:
Thr Asn Ala Lys His Ser Ser His Asn 1 5
(2) INFORMATION FOR SEQ ID NO: 174:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 14 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 174: Ser Ser His Asn Arg Arg Leu Arg Thr Arg Ser Arg Pro Asn 1 5 10
(2) INFORMATION FOR SEQ ID NO: 175:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 175:
Arg Arg Leu Arg Thr Arg Ser Arg Pro Asn 1 5 10
(2) INFORMATION FOR SEQ ID NO:176:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 708 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 176: Met Gly Met Ser Lys Ser His Ser Phe Phe Gly Tyr Pro Leu Ser He 1 5 10 15
Phe Phe He Val Val Asn Glu Phe Cys Glu Arg Phe Ser Tyr Tyr Gly
20 25 30
Met Arg Ala He Leu He Leu Tyr Phe Thr Asn Phe He Ser Trp Asp
35 40 45
Asp Asn Leu Ser Thr Ala He Tyr His Thr Phe Val Ala Leu Cys Tyr
50 55 60
Leu Thr Pro He Leu Gly Ala Leu He Ala Asp Ser Trp Leu Gly Lys 65 70 75 80
Phe Lys Thr He Val Ser Leu Ser He Val Tyr Thr He Gly Gin Ala
85 90 95
Val Thr Ser Val Ser Ser He Asn Asp Leu Thr Asp His Asn His Asp
100 105 110
Gly Thr Pro Asp Ser Leu Pro Val His Val Val Leu Ser Leu He Gly
115 120 125
Leu Ala Leu He Ala Leu Gly Thr Gly Gly He Lys Pro Cys Val Ser
130 135 140 Ala Phe Gly Gly Asp Gin Phe Glu Glu Gly Gin Glu Lys Gin Arg Asn
145 150 155 160
Arg Phe Phe Ser He Phe Tyr Leu Ala He Asn Ala Gly Ser Leu Leu
165 170 175
Ser Thr He He Thr Pro Met Leu Arg Val Gin Gin Cys Gly He His
180 185 190
Ser Lys Gin Ala Cys Tyr Pro Leu Ala Phe Gly Val Pro Ala Ala Leu
195 200 205
Met Ala Val Ala Leu He Val Phe Val Leu Gly Ser Gly Met Tyr Lys 210 215 220
Lys Phe Lys Pro Gin Gly Asn He Met Gly Lys Val Ala Lys Cys He 225 230 235 240
Gly Phe Ala He Lys Asn Arg Phe Arg His Arg Ser Lys Ala Phe Pro
245 250 255
Lys Arg Glu His Trp Leu Asp Trp Ala Lys Glu Lys Tyr Asp Glu Arg
260 265 270
Leu He Ser Gin He Lys Met Val Thr Arg Val Met Phe Leu Tyr He 275 280 285 Pro Leu Pro Met Phe Trp Ala Leu Phe Asp Gin Gin Gly Ser Arg Trp 290 295 300
Thr Leu Gin Ala Thr Thr Met Ser Gly Lys He Gly Ala Leu Glu He 305 310 315 320
Gin Pro Asp Gin Met Gin Thr Val Asn Ala He Leu He Val He Met
325 330 335
Val Pro He Phe Asp Ala Val Leu Tyr Pro Leu He Ala Lys Cys Gly
340 345 350
Phe Asn Phe Thr Ser Leu Lys Lys Met Ala Val Gly Met Val Leu Ala
355 360 365
Ser Met Ala Phe Val Val Ala Ala He Val Gin Val Glu He Asp Lys
370 375 380 Thr Leu Pro Val Phe Pro Lys Gly Asn Glu Val Gin He Lys Val Leu
385 390 395 400
Asn He Gly Asn Asn Thr Met Asn He Ser Leu Pro Gly Glu Met Val
405 410 415
Thr Leu Gly Pro Met Ser Gin Thr Asn Ala Phe Met Thr Phe Asp Val
420 425 430
Asn Lys Leu Thr Arg He Asn He Ser Ser Pro Gly Ser Pro Val Thr
435 440 445
Ala Val Thr Asp Asp Phe Lys Gin Gly Gin Arg His Thr Leu Leu Val 450 455 460
Trp Ala Pro Asn His Tyr Gin Val Val Lys Asp Gly Leu Asn Gin Lys 465 470 475 480
Pro Glu Lys Gly Glu Asn Gly He Arg Phe Val Asn Thr Phe Asn Glu
485 490 495
Leu He Thr He Thr Met Ser Gly Lys Val Tyr Ala Asn He Ser Ser
500 505 510
Tyr Asn Ala Ser Thr Tyr Gin Phe Phe Pro Ser Gly He Lys Gly Phe 515 520 525 Thr He Ser Ser Thr Glu He Pro Pro Gin Cys Gin Pro Asn Phe Asn 530 535 540
Thr Phe Tyr Leu Glu Phe Gly Ser Ala Tyr Thr Tyr He Val Gin Arg 545 550 555 560
Lys Asn Asp Ser Cys Pro Glu Val Lys Val Phe Glu Asp He Ser Ala
565 570 575
Asn Thr Val Asn Met Ala Leu Gin He Pro Gin Tyr Phe Leu Leu Thr
580 585 590
Cys Gly Glu Val Val Phe Ser Val Thr Gly Leu Glu Phe Ser Tyr Ser 595 600 605
Gin Ala Pro Ser Asn Met Lys Ser Val Leu Gin Ala Gly Trp Leu Leu
610 615 620
Thr Val Ala Val Gly Asn He He Val Leu He Val Ala Gly Ala Gly 625 630 635 640
Gin Phe Ser Lys Gin Trp Ala Glu Tyr He Leu Phe Ala Ala Leu Leu
645 650 655
Leu Val Val Cys Val Val Phe Ala He Met Ala Arg Phe Tyr Thr Tyr 660 665 670 He Asn Pro Ala Glu He Glu Ala Gin Phe Asp Glu Asp Glu Lys Lys 675 680 685
Asn Arg Leu Glu Lys Ser Asn Pro Tyr Phe Met Ser Gly Ala Asn Ser
690 695 700
Gin Lys Gin Met 705
(2) INFORMATION FOR SEQ ID NO: 177: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 3345 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (ix) FEATURE: (A) NAME/KEY: Coding Sequence
(B) LOCATION: 88...2583 (D) OTHER INFORMATION:
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 177:
GAATTCCGTC TCGACCACTG AATGGAAGAA AAGGACTTTT AACCACCATT TTGTGACTTA 60 CAGAAAGGAA TTTGAATAAA GAAAACT ATG ATA CTT CAG GCC CAT CTT CAC TCC 114
Met He Leu Gin Ala His Leu His Ser 1 5 CTG TGT CTT CTT ATG CTT TAT TTG GCA ACT GGA TAT GGC CAA GAG GGG 162 Leu Cys Leu Leu Met Leu Tyr Leu Ala Thr Gly Tyr Gly Gin Glu Gly 10 15 20 25
AAG TTT AGT GGA CCC CTG AAA CCC ATG ACA TTT TCT ATT TAT GAA GGC 210 Lys Phe Ser Gly Pro Leu Lys Pro Met Thr Phe Ser He Tyr Glu Gly 30 35 40
CAA GAA CCG AGT CAA ATT ATA TTC CAG TTT AAG GCC AAT CCT CCT GCT 258 Gin Glu Pro Ser Gin He He Phe Gin Phe Lys Ala Asn Pro Pro Ala 45 50 55
GTG ACT TTT GAA CTA ACT GGG GAG ACA GAC AAC ATA TTT GTG ATA GAA 306 Val Thr Phe Glu Leu Thr Gly Glu Thr Asp Asn He Phe Val He Glu 60 65 70
CGG GAG GGA CTT CTG TAT TAC AAC AGA GCC TTG GAC AGG GAA ACA AGA 354 Arg Glu Gly Leu Leu Tyr Tyr Asn Arg Ala Leu Asp Arg Glu Thr Arg 75 80 85
TCT ACT CAC AAT CTC CAG GTT GCA GCC CTG GAC GCT AAT GGA ATT ATA 402 Ser Thr His Asn Leu Gin Val Ala Ala Leu Asp Ala Asn Gly He He 90 95 100 105
GTG GAG GGT CCA GTC CCT ATC ACC ATA GAA GTG AAG GAC ATC AAC GAC 450 Val Glu Gly Pro Val Pro He Thr He Glu Val Lys Asp He Asn Asp 110 115 120
AAT CGA CCC ACG TTT CTC CAG TCA AAG TAC GAA GGC TCA GTA AGG CAG 498
Asn Arg Pro Thr Phe Leu Gin Ser Lys Tyr Glu Gly Ser Val Arg Gin 125 130 135
AAC TCT CGC CCA GGA AAG CCC TTC TTG TAT GTC AAT GCC ACA GAC CTG 546 Asn Ser Arg Pro Gly Lys Pro Phe Leu Tyr Val Asn Ala Thr Asp Leu 140 145 150 GAT GAT CCG GCC ACT CCC AAT GGC CAG CTT TAT TAC CAG ATT GTC ATC 594 Asp Asp Pro Ala Thr Pro Asn Gly Gin Leu Tyr Tyr Gin He Val He 155 160 165
CAG CTT CCC ATG ATC AAC AAT GTC ATG TAC TTT CAG ATC AAC AAC AAA 642 Gin Leu Pro Met He Asn Asn Val Met Tyr Phe Gin He Asn Asn Lys 170 175 180 185
ACG GGA GCC ATC TCT CTT ACC CGA GAG GGA TCT CAG GAA TTG AAT CCT 690 Thr Gly Ala He Ser Leu Thr Arg Glu Gly Ser Gin Glu Leu Asn Pro
190 195 200
GCT AAG AAT CCT TCC TAT AAT CTG GTG ATC TCA GTG AAG GAC ATG GGA 738 Ala Lys Asn Pro Ser Tyr Asn Leu Val He Ser Val Lys Asp Met Gly 205 210 215
GGC CAG AGT GAG AAT TCC TTC AGT GAT ACC ACA TCT GTG GAT ATC ATA 786 Gly Gin Ser Glu Asn Ser Phe Ser Asp Thr Thr Ser Val Asp He He 220 225 230
GTG ACA GAG AAT ATT TGG AAA GCA CCA AAA CCT GTG GAG ATG GTG GAA 834 Val Thr Glu Asn He Trp Lys Ala Pro Lys Pro Val Glu Met Val Glu 235 240 245
AAC TCA ACT GAT CCT CAC CCC ATC AAA ATC ACT CAG GTG CGG TGG AAT 882 Asn Ser Thr Asp Pro His Pro He Lys He Thr Gin Val Arg Trp Asn 250 255 260 265
GAT CCC GGT GCA CAA TAT TCC TTA GTT GAC AAA GAG AAG CTG CCA AGA 930 Asp Pro Gly Ala Gin Tyr Ser Leu Val Asp Lys Glu Lys Leu Pro Arg 270 275 280
TTC CCA TTT TCA ATT GAC CAG GAA GGA GAT ATT TAC GTG ACT CAG CCC 978
Phe Pro Phe Ser He Asp Gin Glu Gly Asp He Tyr Val Thr Gin Pro 285 290 295
TTG GAC CGA GAA GAA AAG GAT GCA TAT GTT TTT TAT GCA GTT GCA AAG 1026 Leu Asp Arg Glu Glu Lys Asp Ala Tyr Val Phe Tyr Ala Val Ala Lys 300 305 310 GAT GAG TAC GGA AAA CCA CTT TCA TAT CCG CTG GAA ATT CAT GTA AAA 1074 Asp Glu Tyr Gly Lys Pro Leu Ser Tyr Pro Leu Glu He His Val Lys 315 320 325
GTT AAA GAT ATT AAT GAT AAT CCA CCT ACA TGT CCG TCA CCA GTA ACC 1122 Val Lys Asp He Asn Asp Asn Pro Pro Thr Cys Pro Ser Pro Val Thr 330 335 340 345
GTA TTT GAG GTC CAG GAG AAT GAA CGA CTG GGT AAC AGT ATC GGG ACC 1170 Val Phe Glu Val Gin Glu Asn Glu Arg Leu Gly Asn Ser He Gly Thr
350 355 360
CTT ACT GCA CAT GAC AGG GAT GAA GAA AAT ACT GCC AAC AGT TTT CTA 1218 Leu Thr Ala His Asp Arg Asp Glu Glu Asn Thr Ala Asn Ser Phe Leu 365 370 375
AAC TAC AGG ATT GTG GAG CAA ACT CCC AAA CTT CCC ATG GAT GGA CTC 1266 Asn Tyr Arg He Val Glu Gin Thr Pro Lys Leu Pro Met Asp Gly Leu 380 385 390
TTC CTA ATC CAA ACC TAT GCT GGA ATG TTA CAG TTA GCT AAA CAG TCC 1314 Phe Leu He Gin Thr Tyr Ala Gly Met Leu Gin Leu Ala Lys Gin Ser 395 400 405
TTG AAG AAG CAA GAT ACT CCT CAG TAC AAC TTA ACG ATA GAG GTG TCT 1362 Leu Lys Lys Gin Asp Thr Pro Gin Tyr Asn Leu Thr He Glu Val Ser 410 415 420 425
GAC AAA GAT TTC AAG ACC CTT TGT TTT GTG CAA ATC AAC GTT ATT GAT 1410
Asp Lys Asp Phe Lys Thr Leu Cys Phe Val Gin He Asn Val He Asp 430 435 440
ATC AAT GAT CAG ATC CCC ATC TTT GAA AAA TCA GAT TAT GGA AAC CTG 1458 He Asn Asp Gin He Pro He Phe Glu Lys Ser Asp Tyr Gly Asn Leu 445 450 455 ACT CTT GCT GAA GAC ACA AAC ATT GGG TCC ACC ATC TTA ACC ATC CAG 1506 Thr Leu Ala Glu Asp Thr Asn He Gly Ser Thr He Leu Thr He Gin 460 465 470
GCC ACT GAT GCT GAT GAG CCA TTT ACT GGG AGT TCT AAA ATT CTG TAT 1554 Ala Thr Asp Ala Asp Glu Pro Phe Thr Gly Ser Ser Lys He Leu Tyr 475 480 485
CAT ATC ATA AAG GGA GAC AGT GAG GGA CGC CTG GGG GTT GAC ACA GAT 1602 His He He Lys Gly Asp Ser Glu Gly Arg Leu Gly Val Asp Thr Asp 490 495 500 505
CCC CAT ACC AAC ACC GGA TAT GTC ATA ATT AAA AAG CCT CTT GAT TTT 1650 Pro His Thr Asn Thr Gly Tyr Val He He Lys Lys Pro Leu Asp Phe
510 515 520
GAA ACA GCA GCT GTT TCC AAC ATT GTG TTC AAA GCA GAA AAT CCT GAG 1698
Glu Thr Ala Ala Val Ser Asn He Val Phe Lys Ala Glu Asn Pro Glu 525 530 535
CCT CTA GTG TTT GGT GTG AAG TAC AAT GCA AGT TCT TTT GCC AAG TTC 1746
Pro Leu Val Phe Gly Val Lys Tyr Asn Ala Ser Ser Phe Ala Lys Phe 540 545 550
ACG CTT ATT GTG ACA GAT GTG AAT GAA GCA CCT CAA TTT TCC CAA CAC 1794
Thr Leu He Val Thr Asp Val Asn Glu Ala Pro Gin Phe Ser Gin His
555 560 565
GTA TTC CAA GCG AAA GTC AGT GAG GAT GTA GCT ATA GGC ACT AAA GTG 1842
Val Phe Gin Ala Lys Val Ser Glu Asp Val Ala He Gly Thr Lys Val 570 575 580 585
GGC AAT GTG ACT GCC AAG GAT CCA GAA GGT CTG GAC ATA AGC TAT TCA 1890 Gly Asn Val Thr Ala Lys Asp Pro Glu Gly Leu Asp He Ser Tyr Ser 590 595 600
CTG AGG GGA GAC ACA AGA GGT TGG CTT AAA ATT GAC CAC GTG ACT GGT 1938 Leu Arg Gly Asp Thr Arg Gly Trp Leu Lys He Asp His Val Thr Gly 605 610 615
GAG ATC TTT AGT GTG GCT CCA TTG GAC AGA GAA GCC GGA AGT CCA TAT 1986 Glu He Phe Ser Val Ala Pro Leu Asp Arg Glu Ala Gly Ser Pro Tyr 620 625 630
CGG GTA CAA GTG GTG GCC ACA GAA GTA GGG GGG TCT TCC TTA AGC TCT 2034 Arg Val Gin Val Val Ala Thr Glu Val Gly Gly Ser Ser Leu Ser Ser 635 640 645 GTG CA GAG TTC CAC CTG ATC CTT ATG GAT GTG AAT GAC AAC CCT CCC 2082 Val Ser Glu Phe His Leu He Leu Met Asp Val Asn Asp Asn Pro Pro 650 655 660 665
AGG CTA GCC AAG GAC TAC ACG GGC TTG TTC TTC TGC CAT CCC CTC AGT 2130 Arg Leu Ala Lys Asp Tyr Thr Gly Leu Phe Phe Cys His Pro Leu Ser 670 675 680
GCA CCT GGA AGT CTC ATT TTC GAG GCT ACT GAT GAT GAT CAG CAC TTA 2178 Ala Pro Gly Ser Leu He Phe Glu Ala Thr Asp Asp Asp Gin His Leu 685 690 695
TTT CGG GGT CCC CAT TTT ACA TTT TCC CTC GGC AGT GGA AGC TTA CAA 2226 Phe Arg Gly Pro His Phe Thr Phe Ser Leu Gly Ser Gly Ser Leu Gin 700 705 710
AAC GAC TGG GAA GTT TCC AAA ATC AAT GGT ACT CAT GCC CGA CTG TCT 2274 Asn Asp Trp Glu Val Ser Lys He Asn Gly Thr His Ala Arg Leu Ser 715 720 725
ACC AGG CAC ACA GAC TTT GAG GAG AGG GCG TAT GTC GTC TTG ATC CGC 2322 Thr Arg His Thr Asp Phe Glu Glu Arg Ala Tyr Val Val Leu He Arg 730 735 740 745
ATC AAT GAT GGG GGT CGG CCA CCC TTG GAA GGC ATT GTT TCT TTA CCA 2370 He Asn Asp Gly Gly Arg Pro Pro Leu Glu Gly He Val Ser Leu Pro 750 755 760
GTT ACA TTC TGC AGT TGT GTG GAA GGA AGT TGT TTC CGG CCA GCA GGT 2418 Val Thr Phe Cys Ser Cys Val Glu Gly Ser Cys Phe Arg Pro Ala Gly 765 770 775
CAC CAG ACT GGG ATA CCC ACT GTG GGC ATG GCA GTT GGT ATA CTG CTG 2466 His Gin Thr Gly He Pro Thr Val Gly Met Ala Val Gly He Leu Leu 780 785 790
ACC ACC CTT CTG GTG ATT GGT ATA ATT TTA GCA GTT GTG TTT ATC CGC 2514 Thr Thr Leu Leu Val He Gly He He Leu Ala Val Val Phe He Arg 795 800 805
ATA AAG AAG GAT AAA GGC AAA GAT AAT GTT GAA AGT GCT CAA GCA TCT 2562
He Lys Lys Asp Lys Gly Lys Asp Asn Val Glu Ser Ala Gin Ala Ser 810 815 820 825
GAA GTC AAA CCT CTG AGA AGC TGAATTTGAA AAGGAATGTT TGAATTTATA TAGC 2617 Glu Val Lys Pro Leu Arg Ser 830 AAGTGCTATT TCAGCAACAA CCATCTCATC CTATTACTTT TCATCTAACG TGCATTATAA 2677
TTTTTTAAAC AGATATTCCC TCTTGTCCTT TAATATTTGC TAAATATTTC TTTTTTGAGG 2737
TGGAGTCTTG CTCTGTCGCC CAGGCTGGAG TACAGTGGTG TGATCCCAGC TCACTGCAAC 2797
CTCCGCCTCC TGGGTTCACA TGATTCTCCT GCCTCAGCTT CCTAAGTAGC TGGGTTTACA 2857
GGCACCCACC ACCATGCCCA GCTAATTTTT GTATTTTTAA TAGAGACGGG GTTTCGCCAT 2917
TTGGCCAGGC TGGTCTTGAA CTCCTGACGT CAAGTGATCT GCCTGCCTTG GTCTCCCAAT 2977
ACAGGCATGA ACCACTGCAC CCACCTACTT AGATATTTCA TGTGCTATAG ACATTAGAGA 3037
GATTTTTCAT TTTTCCATGA CATTTTTCCT CTCTGCAAAT GGCTTAGCTA CTTGTGTTTT 3097
TCCCTTTTGG GGCAAGACAG ACTCATTAAA TATTCTGTAC ATTTTTTCTT TATCAAGGAG 3157 ATATATCAGT GTTGTCTCAT AGAACTGCCT GGATTCCATT TATGTTTTTT CTGATTCCAT 3217
CCTGTGTCCC CTTCATCCTT GACTCCTTTG GTATTTCACT GAATTTCAAA CATTTGTCAG 3277
AGAAGAAAAA AGTGAGGACT CAGGAAAAAT AAATAAATAA AAGAACAGCC TTTTGCGGCC 3337
GCGAATTC 3345
(2) INFORMATION FOR SEQ ID NO:178:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 832 amino acids (B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 178:
Met He Leu Gin Ala His Leu His Ser Leu Cys Leu Leu Met Leu Tyr 1 5 10 15
Leu Ala Thr Gly Tyr Gly Gin Glu Gly Lys Phe Ser Gly Pro Leu Lys
20 25 30
Pro Met Thr Phe Ser He Tyr Glu Gly Gin Glu Pro Ser Gin He He
35 40 45
Phe Gin Phe Lys Ala Asn Pro Pro Ala Val Thr Phe Glu Leu Thr Gly
50 55 60
Glu Thr Asp Asn He Phe Val He Glu Arg Glu Gly Leu Leu Tyr Tyr 65 70 75 80 Asn Arg Ala Leu Asp Arg Glu Thr Arg Ser Thr His Asn Leu Gin Val
85 90 95
Ala Ala Leu Asp Ala Asn Gly He He Val Glu Gly Pro Val Pro He
100 105 110
Thr He Glu Val Lys Asp He Asn Asp Asn Arg Pro Thr Phe Leu Gin
115 120 125
Ser Lys Tyr Glu Gly Ser Val Arg Gin Asn Ser Arg Pro Gly Lys Pro
130 135 140
Phe Leu Tyr Val Asn Ala Thr Asp Leu Asp Asp Pro Ala Thr Pro Asn 145 150 155 160
Gly Gin Leu Tyr Tyr Gin He Val He Gin Leu Pro Met He Asn Asn
165 170 175
Val Met Tyr Phe Gin He Asn Asn Lys Thr Gly Ala He Ser Leu Thr 180 185 190
Arg Glu Gly Ser Gin Glu Leu Asn Pro Ala Lys Asn Pro Ser Tyr Asn
195 200 205
Leu Val He Ser Val Lys Asp Met Gly Gly Gin Ser Glu Asn Ser Phe
210 215 220
Ser Asp Thr Thr Ser Val Asp He He Val Thr Glu Asn He Trp Lys 225 230 235 240
Ala Pro Lys Pro Val Glu Met Val Glu Asn Ser Thr Asp Pro His Pro 245 250 255
He Lys He Thr Gin Val Arg Trp Asn Asp Pro Gly Ala Gin Tyr Ser
260 265 270
Leu Val Asp Lys Glu Lys Leu Pro Arg Phe Pro Phe Ser He Asp Gin
275 280 285
Glu Gly Asp He Tyr Val Thr Gin Pro Leu Asp Arg Glu Glu Lys Asp
290 295 300
Ala Tyr Val Phe Tyr Ala Val Ala Lys Asp Glu Tyr Gly Lys Pro Leu 305 310 315 320 Ser Tyr Pro Leu Glu He His Val Lys Val Lys Asp He Asn Asp Asn
325 330 335
Pro Pro Thr Cys Pro Ser Pro Val Thr Val Phe Glu Val Gin Glu Asn
340 345 350
Glu Arg Leu Gly Asn Ser He Gly Thr Leu Thr Ala His Asp Arg Asp
355 360 365
Glu Glu Asn Thr Ala Asn Ser Phe Leu Asn Tyr Arg He Val Glu Gin
370 375 380
Thr Pro Lys Leu Pro Met Asp Gly Leu Phe Leu He Gin Thr Tyr Ala 385 390 395 400
Gly Met Leu Gin Leu Ala Lys Gin Ser Leu Lys Lys Gin Asp Thr Pro
405 410 415
Gin Tyr Asn Leu Thr He Glu Val Ser Asp Lys Asp Phe Lys Thr Leu
420 425 430
Cys Phe Val Gin He Asn Val He Asp He Asn Asp Gin He Pro He
435 440 445
Phe Glu Lys Ser Asp Tyr Gly Asn Leu Thr Leu Ala Glu Asp Thr Asn
450 455 460 He Gly Ser Thr He Leu Thr He Gin Ala Thr Asp Ala Asp Glu Pro
465 470 475 480
Phe Thr Gly Ser Ser Lys He Leu Tyr His He He Lys Gly Asp Ser
485 490 495
Glu Gly Arg Leu Gly Val Asp Thr Asp Pro His Thr Asn Thr Gly Tyr
500 505 510
Val He He Lys Lys Pro Leu Asp Phe Glu Thr Ala Ala Val Ser Asn
515 520 525
He Val Phe Lys Ala Glu Asn Pro Glu Pro Leu Val Phe Gly Val Lys 530 535 540
Tyr Asn Ala Ser Ser Phe Ala Lys Phe Thr Leu He Val Thr Asp Val 545 550 555 560
Asn Glu Ala Pro Gin Phe Ser Gin His Val Phe Gin Ala Lys Val Ser
565 570 575
Glu Asp Val Ala He Gly Thr Lys Val Gly Asn Val Thr Ala Lys Asp
580 585 590
Pro Glu Gly Leu Asp He Ser Tyr Ser Leu Arg Gly Asp Thr Arg Gly 595 600 605 Trp Leu Lys He Asp His Val Thr Gly Glu He Phe Ser Val Ala Pro 610 615 620
Leu Asp Arg Glu Ala Gly Ser Pro Tyr Arg Val Gin Val Val Ala Thr 625 630 635 640
Glu Val Gly Gly Ser Ser Leu Ser Ser Val Ser Glu Phe His Leu He
645 650 655
Leu Met Asp Val Asn Asp Asn Pro Pro Arg Leu Ala Lys Asp Tyr Thr
660 665 670
Gly Leu Phe Phe Cys His Pro Leu Ser Ala Pro Gly Ser Leu He Phe 675 680 685
Glu Ala Thr Asp Asp Asp Gin His Leu Phe Arg Gly Pro His Phe Thr
690 695 700
Phe Ser Leu Gly Ser Gly Ser Leu Gin Asn Asp Trp Glu Val Ser Lys 705 710 715 720
He Asn Gly Thr His Ala Arg Leu Ser Thr Arg His Thr Asp Phe Glu
725 730 735
Glu Arg Ala Tyr Val Val Leu He Arg He Asn Asp Gly Gly Arg Pro
740 745 750
Pro Leu Glu Gly He Val Ser Leu Pro Val Thr Phe Cys Ser Cys Val
755 760 765
Glu Gly Ser Cys Phe Arg Pro Ala Gly His Gin Thr Gly He Pro Thr
770 775 780
Val Gly Met Ala Val Gly He Leu Leu Thr Thr Leu Leu Val He Gly 785 790 795 800
He He Leu Ala Val Val Phe He Arg He Lys Lys Asp Lys Gly Lys
805 810 815
Asp Asn Val Glu Ser Ala Gin Ala Ser Glu Val Lys Pro Leu Arg Ser 820 825 830
(2) INFORMATION FOR SEQ ID NO: 179:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 1827 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 179:
Met Ala Arg Lys Lys Phe Ser Gly Leu Glu He Ser Leu He Val Leu
1 5 10 15
Phe Val He Val Thr He He Ala He Ala Leu He Val Val Leu Ala
20 25 30
Thr Lys Thr Pro Ala Val Asp Glu He Ser Asp Ser Thr Ser Thr Pro
35 40 45
Ala Thr Thr Arg Val Thr Thr Asn Pro Ser Asp Ser Gly Lys Cys Pro 50 55 60
Asn Val Leu Asn Asp Pro Val Asn Val Arg He Asn Cys He Pro Glu 65 70 75 80
Gin Phe Pro Thr Glu Gly He Cys Ala Gin Arg Gly Cys Cys Trp Arg
85 90 95
Pro Trp Asn Asp Ser Leu He Pro Trp Cys Phe Phe Val Asp Asn His
100 105 110
Gly Tyr Asn Val Gin Asp Met Thr Thr Thr Ser He Gly Val Glu Ala 115 120 125 Lys Leu Asn Arg He Pro Ser Pro Thr Leu Phe Gly Asn Asp He Asn 130 135 140
Ser Val Leu Phe Thr Thr Gin Asn Gin Thr Pro Asn Arg Phe Arg Phe 145 150 155 160
Lys He Thr Asp Pro Asn Asn Arg Arg Tyr Glu Val Pro His Gin Tyr
165 170 175
Val Lys Glu Phe Thr Gly Pro Thr Val Ser Asp Thr Leu Tyr Asp Val
180 185 190
Lys Val Ala Gin Asn Pro Phe Ser He Gin Val He Arg Lys Ser Asn 195 200 205
Gly Lys Thr Leu Phe Asp Thr Ser He Gly Pro Leu Val Tyr Ser Asp
210 215 220
Gin Tyr Leu Gin He Ser Ala Arg Leu Pro Ser Asp Tyr He Tyr Gly 225 230 235 240
He Gly Glu Gin Val His Lys Arg Phe Arg His Asp Leu Ser Trp Lys
245 250 255
Thr Trp Pro He Phe Thr Arg Asp Gin Leu Pro Gly Asp Asn Asn Asn 260 265 270 Asn Leu Tyr Gly His Gin Thr Phe Phe Met Cys He Glu Asp Thr Ser 275 280 285
Gly Lys Ser Phe Gly Val Phe Leu Met Asn Ser Asn Ala Met Glu He
290 295 300
Phe He Gin Pro Thr Pro He Val Thr Tyr Arg Val Thr Gly Gly He
305 310 315 320
Leu Asp Phe Tyr He Leu Leu Gly Asp Thr Pro Glu Gin Val Val Gin
325 330 335
Gin Tyr Gin Gin Leu Val Gly Leu Pro Ala Met Pro Ala Tyr Trp Asn
340 345 350
Leu Gly Phe Gin Leu Ser Arg Trp Asn Tyr Lys Ser Leu Asp Val Val
355 360 365
Lys Glu Val Val Arg Arg Asn Arg Glu Ala Gly He Pro Phe Asp Thr
370 375 380
Gin Val Thr Asp He Asp Tyr Met Glu Asp Lys Lys Asp Phe Thr Tyr 385 390 395 400
Asp Gin Val Ala Phe Asn Gly Leu Pro Gin Phe Val Gin Asp Leu His
405 410 415
Asp His Gly Gin Lys Tyr Val He He Leu Asp Pro Ala He Ser He
420 425 430
Gly Arg Arg Ala Asn Gly Thr Thr Tyr Ala Thr Tyr Glu Arg Gly Asn
435 440 445
Thr Gin His Val Trp He Asn Glu Ser Asp Gly Ser Thr Pro He He
450 455 460
Gly Glu Val Trp Pro Gly Leu Thr Val Tyr Pro Asp Phe Thr Asn Pro 465 470 475 480
Asn Cys He Asp Trp Trp Ala Asn Glu Cys Ser He Phe His Gin Glu
485 490 495
Val Gin Tyr Asp Gly Leu Trp He Asp Met Asn Glu Val Ser Ser Phe 500 505 510 He Gin Gly Ser Thr Lys Gly Cys Asn Val Asn Lys Leu Asn Tyr Pro 515 520 525
Pro Phe Thr Pro Asp He Leu Asp Lys Leu Met Tyr Ser Lys Thr He
530 535 540
Cys Met Asp Ala Val Gin Asn Trp Gly Lys Gin Tyr Asp Val His Ser 545 550 555 560
Leu Tyr Gly Tyr Ser Met Ala He Ala Thr Glu Gin Ala Val Gin Lys
565 570 575
Val Phe Pro Asn Lys Arg Ser Phe He Leu Thr Arg Ser Thr Phe Ala 580 585 590
Gly Ser Gly Arg His Ala Ala His Trp Leu Gly Asp Asn Thr Ala Ser
595 600 605
Trp Glu Gin Met Glu Trp Ser He Thr Gly Met Leu Glu Phe Ser Leu
610 615 620
Phe Gly He Pro Leu Val Gly Ala Asp He Cys Gly Phe Val Ala Glu 625 630 635 640
Thr Thr Glu Glu Leu Cys Arg Arg Trp Met Gin Leu Gly Ala Phe Tyr 645 650 655 Pro Phe Ser Arg Asn His Asn Ser Asp Gly Tyr Glu His Gin Asp Pro 660 665 670
Ala Phe Phe Gly Gin Asn Ser Leu Leu Val Lys Ser Ser Arg Gin Tyr
675 680 685
Leu Thr He Arg Tyr Thr Leu Leu Pro Phe Leu Tyr Thr Leu Phe Tyr
690 695 700
Lys Ala His Val Phe Gly Glu Thr Val Ala Arg Pro Val Leu His Glu 705 710 715 720
Phe Tyr Glu Asp Thr Asn Ser Trp He Glu Asp Thr Glu Phe Leu Trp 725 730 735
Gly Pro Ala Leu Leu He Thr Pro Val Leu Lys Gin Gly Ala Asp Thr
740 745 750
Val Ser Ala Tyr He Pro Asp Ala He Trp Tyr Asp Tyr Glu Ser Gly
755 760 765
Ala Lys Arg Pro Trp Arg Lys Gin Arg Val Asp Met Tyr Leu Pro Ala
770 775 780
Asp Lys He Gly Leu His Leu Arg Gly Gly Tyr He He Pro He Gin 785 790 795 800 Glu Pro Asp Val Thr Thr Thr Ala Ser Arg Lys Asn Pro Leu Gly Leu
805 810 815
He Val Ala Leu Gly Glu Asn Asn Thr Ala Lys Gly Asp Phe Phe Trp
820 825 830
Asp Asp Gly Glu Thr Lys Asp Thr He Gin Asn Gly Asn Tyr He Leu
835 840 845
Tyr Thr Phe Ser Val Ser Asn Asn Thr Leu Asp He Val Cys Thr His
850 855 860
Ser Ser Tyr Gin Glu Gly Thr Thr Leu Ala Phe Gin Thr Val Lys He 865 870 875 880
Leu Gly Leu Thr Asp Ser Val Thr Glu Val Arg Val Ala Glu Asn Asn
885 890 895
Gin Pro Met Asn Ala His Ser Asn Phe Thr Tyr Asp Ala Ser Asn Gin
900 905 910
Val Leu Leu He Ala Asp Leu Lys Leu Asn Leu Gly Arg Asn Phe Ser
915 920 925
Val Gin Trp Asn Gin He Phe Ser Glu Asn Glu Arg Phe Asn Cys Tyr
930 935 940
Pro Asp Ala Asp Leu Ala Thr Glu Gin Lys Cys Thr Gin Arg Gly Cys 945 950 955 960
Val Trp Arg Thr Gly Ser Ser Leu Ser Lys Ala Pro Glu Cys Tyr Phe
965 970 975
Pro Arg Gin Asp Asn Ser Tyr Ser Val Asn Ser Ala Arg Tyr Ser Ser
980 985 990
Met Gly He Thr Ala Asp Leu Gin Leu Asn Thr Ala Asn Ala Arg He
995 1000 1005
Lys Leu Pro Ser Asp Pro He Ser Thr Leu Arg Val Glu Val Lys Tyr
1010 1015 1020
His Lys Asn Asp Met Leu Gin Phe Lys He Tyr Asp Pro Gin Lys Lys 025 1030 1035 1040 Arg Tyr Glu Val Pro Val Pro Leu Asn He Pro Thr Thr Pro He Ser
1045 1050 1055
Thr Tyr Glu Asp Arg Leu Tyr Asp Val Glu He Lys Glu Asn Pro Phe
1060 1065 1070
Gly He Gin He Arg Arg Arg Ser Ser Gly Arg Val He Trp Asp Ser
1075 1080 1085
Trp Leu Pro Gly Phe Ala Phe Asn Asp Gin Phe He Gin He Ser Thr
1090 1095 1100
Arg Leu Pro Ser Glu Tyr He Tyr Gly Phe Gly Glu Val Glu His Thr 105 1110 1115 1120
Ala Phe Lys Arg Asp Leu Asn Trp Asn Thr Trp Gly Met Phe Thr Arg
1125 1130 1135
Asp Gin Pro Pro Gly Tyr Lys Leu Asn Ser Tyr Gly Phe His Pro Tyr
1140 1145 1150
Tyr Met Ala Leu Glu Glu Glu Gly Asn Ala His Gly Val Phe Leu Leu
1155 1160 1165
Asn Ser Asn Ala Met Asp Val Thr Phe Gin Pro Thr Pro Ala Leu Thr
1170 1175 1180 Tyr Arg Thr Val Gly Gly He Leu Asp Phe Tyr Met Phe Leu Gly Pro
185 1190 1195 1200
Thr Pro Gin Val Ala Thr Lys Gin Tyr His Glu Val He Gly His Pro
1205 1210 1215
Val Met Pro Ala Tyr Trp Ala Leu Gly Phe Gin Leu Cys Arg Tyr Gly
1220 1225 1230
Tyr Ala Asn Thr Ser Glu Val Arg Glu Leu Tyr Asp Ala Met Val Ala
1235 1240 1245
Ala Asn He Pro Tyr Asp Val Gin Tyr Thr Asp He Asp Tyr Met Glu 1250 1255 1260
Arg Gin Leu Asp Phe Thr He Gly Glu Ala Phe Gin Asp Leu Pro Gin 265 1270 1275 1280
Phe Val Asp Lys He Arg Gly Glu Gly Met Arg Tyr He He He Leu
1285 1290 1295
Asp Pro Ala He Ser Gly Asn Glu Thr Lys Thr Tyr Pro Ala Phe Glu
1300 1305 1310
Arg Gly Gin Gin Asn Asp Val Phe Val Lys Trp Pro Asn Thr Asn Asp 1315 1320 1325 He Cys Trp Ala Lys Val Trp Pro Asp Leu Pro Asn He Thr He Asp 1330 1335 1340
Lys Thr Leu Thr Glu Asp Glu Ala Val Asn Ala Ser Arg Ala His Val 345 1350 1355 1360
Ala Phe Pro Asp Phe Phe Arg Thr Ser Thr Ala Glu Trp Trp Ala Arg
1365 1370 1375
Glu He Val Asp Phe Tyr Asn Glu Lys Met Lys Phe Asp Gly Leu Trp
1380 1385 1390
He Asp Met Asn Glu Pro Ser Ser Phe Val Asn Gly Thr Thr Thr Asn
1395 1400 1405
Gin Cys Arg Asn Asp Glu Leu Asn Tyr Pro Pro Tyr Phe Pro Glu Leu
1410 1415 1420 Thr Lys Arg Thr Asp Gly Leu His Phe Arg Thr He Cys Met Glu Ala
425 1430 1435 1440
Glu Gin He Leu Ser Asp Gly Thr Ser Val Leu His Tyr Asp Val His
1445 1450 1455
Asn Leu Tyr Gly Trp Ser Gin Met Lys Pro Thr His Asp Ala Leu Gin
1460 1465 1470
Lys Thr Thr Gly Lys Arg Gly He Val He Ser Arg Ser Thr Tyr Pro
1475 1480 1485
Thr Ser Gly Arg Trp Gly Gly His Trp Leu Gly Asp Asn Tyr Ala Arg 1490 1495 1500
Trp Asp Asn Met Asp Lys Ser He He Gly Met Met Glu Phe Ser Leu 505 1510 1515 1520
Phe Gly He Ser Tyr Thr Gly Ala Asp He Cys Gly Phe Phe Asn Asn
1525 1530 1535
Ser Glu Tyr His Leu Cys Thr Arg Trp Met Gin Leu Gly Ala Phe Tyr
1540 1545 1550
Pro Tyr Ser Arg Asn His Asn He Ala Asn Thr Arg Arg Gin Asp Pro 1555 1560 1565 Ala Ser Trp Asn Glu Thr Phe Ala Glu Met Ser Arg Asn He Leu Asn 1570 1575 1580
He Arg Tyr Thr Leu Leu Pro Tyr Phe Tyr Thr Gin Met His Glu He 585 1590 1595 1600
His Ala Asn Gly Gly Thr Val He Arg Pro Leu Leu His Glu Phe Phe
1605 1610 1615
Asp Glu Lys Pro Thr Trp Asp He Phe Lys Gin Phe Leu Trp Gly Pro
1620 1625 1630
Ala Phe Met Val Thr Pro Val Leu Glu Pro Tyr Val Gin Thr Val Asn 1635 1640 1645
Ala Tyr Val Pro Asn Ala Arg Trp Phe Asp Tyr His Thr Gly Lys Asp
1650 1655 1660
He Gly Val Arg Gly Gin Phe Gin Thr Phe Asn Ala Ser Tyr Asp Thr 665 1670 1675 1680
He Asn Leu His Val Arg Gly Gly His He Leu Pro Cys Gin Glu Pro
1685 1690 1695
Ala Gin Asn Thr Phe Tyr Ser Arg Gin Lys His Met Lys Leu He Val 1700 1705 1710 Ala Ala Asp Asp Asn Gin Met Ala Gin Gly Ser Leu Phe Trp Asp Asp 1715 1720 1725
Gly Glu Ser He Asp Thr Tyr Glu Arg Asp Leu Tyr Leu Ser Val Gin
1730 1735 1740
Phe Asn Leu Asn Gin Thr Thr Leu Thr Ser Thr He Leu Lys Arg Gly 745 1750 1755 1760
Tyr He Asn Lys Ser Glu Thr Arg Leu Gly Ser Leu His Val Trp Gly
1765 1770 1775
Lys Gly Thr Thr Pro Val Asn Ala Val Thr Leu Thr Tyr Asn Gly Asn 1780 1785 1790
Lys Asn Ser Leu Pro Phe Asn Glu Asp Thr Thr Asn Met He Leu Arg
1795 1800 1805
He Asp Leu Thr Thr His Asn Val Thr Leu Glu Glu Pro He Glu He
1810 1815 1820
Asn Trp Ser 825
(2) INFORMATION FOR SEQ ID NO: 180:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 2284 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (ix) FEATURE:
(A) NAME/KEY: Coding Sequence
(B) LOCATION: 45...2099 (D) OTHER INFORMATION:
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 180:
GCCTTACTGC AGGAAGGCAC TCCGAAGACA TAAGTCGGTG AGAC ATG GCT GAA GAT 56
Met Ala Glu Asp
1 AAA AGC AAG AGA GAC TCC ATC GAG ATG AGT ATG AAG GGA TGC CAG ACA 104 Lys Ser Lys Arg Asp Ser He Glu Met Ser Met Lys Gly Cys Gin Thr 5 10 15 20
AAC AAC GGG TTT GTC CAT AAT GAA GAC ATT CTG GAG CAG ACC CCG GAT 152 Asn Asn Gly Phe Val His Asn Glu Asp He Leu Glu Gin Thr Pro Asp 25 30 35
CCA GGC AGC TCA ACA GAC AAC CTG AAG CAC AGC ACC AGG GGC ATC CTT 200 Pro Gly Ser Ser Thr Asp Asn Leu Lys His Ser Thr Arg Gly He Leu 40 45 50
GGC TCC CAG GAG CCC GAC TTC AAG GGC GTC CAG CCC TAT GCG GGG ATG 248 Gly Ser Gin Glu Pro Asp Phe Lys Gly Val Gin Pro Tyr Ala Gly Met 55 60 65
CCC AAG GAG GTG CTG TTC CAG TTC TCT GGC CAG GCC CGC TAC CGC ATA 296 Pro Lys Glu Val Leu Phe Gin Phe Ser Gly Gin Ala Arg Tyr Arg He 70 75 80
CCT CGG GAG ATC CTC TTC TGG CTC ACA GTG GCT TCT GTG CTG GTG CTC 344 Pro Arg Glu He Leu Phe Trp Leu Thr Val Ala Ser Val Leu Val Leu 85 90 95 100
ATC GCG GCC ACC ATA GCC ATC ATT GCC CTC TCT CCA AAG TGC CTA GAC 392 He Ala Ala Thr He Ala He He Ala Leu Ser Pro Lys Cys Leu Asp 105 110 115
TGG TGG CAG GAG GGG CCC ATG TAC CAG ATC TAC CCA AGG TCT TTC AAG 440
Trp Trp Gin Glu Gly Pro Met Tyr Gin He Tyr Pro Arg Ser Phe Lys 120 125 130
GAC AGT AAC AAG GAT GGG AAC GGA GAT CTG AAA GGT ATT CAA GAT AAA 488 Asp Ser Asn Lys Asp Gly Asn Gly Asp Leu Lys Gly He Gin Asp Lys 135 140 145 CTG GAC TAC ATC ACA GCT TTA AAT ATA AAA ACT GTT TGG ATT ACT TCA 536 Leu Asp Tyr He Thr Ala Leu Asn He Lys Thr Val Trp He Thr Ser 150 155 160
TTT TAT AAA TCG TCC CTT AAA GAT TTC AGA TAT GGT GTT GAA GAT TTC 584 Phe Tyr Lys Ser Ser Leu Lys Asp Phe Arg Tyr Gly Val Glu Asp Phe 165 170 175 180
CGG GAA GTT GAT CCC ATT TTT GGA ACG ATG GAA GAT TTT GAG AAT CTG 632 Arg Glu Val Asp Pro He Phe Gly Thr Met Glu Asp Phe Glu Asn Leu
185 190 195
GTT GCA GCC ATA CAT GAT AAA GGT TTA AAA TTA ATC ATC GAT TTC ATA 680 Val Ala Ala He His Asp Lys Gly Leu Lys Leu He He Asp Phe He
200 205 210
CCA AAC CAC ACG AGT GAT AAA CAT ATT TGG TTT CAA TTG AGT CGG ACA 728 Pro Asn His Thr Ser Asp Lys His He Trp Phe Gin Leu Ser Arg Thr 215 220 225
CGG ACA GGA AAA TAT ACT GAT TAT TAT ATC TGG CAT GAC TGT ACC CAT 776 Arg Thr Gly Lys Tyr Thr Asp Tyr Tyr He Trp His Asp Cys Thr His 230 235 240
GAA AAT GGC AAA ACC ATT CCA CCC AAC AAC TGG TTA AGT GTG TAT GGA 824 Glu Asn Gly Lys Thr He Pro Pro Asn Asn Trp Leu Ser Val Tyr Gly 245 250 255 260
AAC TCC AGT TGG CAC TTT GAC GAA GTG CGA AAC CAA TGT TAT TTT CAT 872 Asn Ser Ser Trp His Phe Asp Glu Val Arg Asn Gin Cys Tyr Phe His 265 270 275
CAG TTT ATG AAA GAG CAA CCT GAT TTA AAT TTC CGC AAT CCT GAT GTT 920 Gin Phe Met Lys Glu Gin Pro Asp Leu Asn Phe Arg Asn Pro Asp Val 280 285 290
CAA GAA GAA ATA AAA GAA ATT TTA CGG TTC TGG CTC ACA AAG GGT GTT 968 Gin Glu Glu He Lys Glu He Leu Arg Phe Trp Leu Thr Lys Gly Val 295 300 305
GAT GGT TTT AGT TTG GAT GCT GTT AAA TTC CTC CTA GAA GCA AAG CAC 1016 Asp Gly Phe Ser Leu Asp Ala Val Lys Phe Leu Leu Glu Ala Lys His 310 315 320
CTG AGA GAT GAG ATC CAA GTA AAT AAG ACC CAA ATC CCG GAC ACG GTC 1064 Leu Arg Asp Glu He Gin Val Asn Lys Thr Gin He Pro Asp Thr Val 325 330 335 340 ACA CAA TAC TCG GAG CTG TAC CAT GAC TTC ACC ACC ACG CAG GTG GGA 1112 Thr Gin Tyr Ser Glu Leu Tyr His Asp Phe Thr Thr Thr Gin Val Gly 345 350 355
ATG CAC GAC ATT GTC CGC AGC TTC CGG CAG ACC ATG GAC CAA TAC AGC 1160 Met His Asp He Val Arg Ser Phe Arg Gin Thr Met Asp Gin Tyr Ser 360 365 370
ACG GAG CCC GGC AGA TAC AGG TTC ATG GGG ACT GAA GCC TAT GCA GAG 1208 Thr Glu Pro Gly Arg Tyr Arg Phe Met Gly Thr Glu Ala Tyr Ala Glu 375 380 385
AGT ATT GAC AGG ACC GTG ATG TAC TAT GGA TTG CCA TTT ATC CAA GAA 1256 Ser He Asp Arg Thr Val Met Tyr Tyr Gly Leu Pro Phe He Gin Glu 390 395 400
GCT GAT TTT CCC TTC AAC AAT TAC CTC AGC ATG CTA GAC ACT GTT TCT 1304 Ala Asp Phe Pro Phe Asn Asn Tyr Leu Ser Met Leu Asp Thr Val Ser 405 410 415 420
GGG AAC AGC GTG TAT GAG GTT ATC ACA TCC TGG ATG GAA AAC ATG CCA 1352 Gly Asn Ser Val Tyr Glu Val He Thr Ser Trp Met Glu Asn Met Pro 425 430 435
GAA GGA AAA TGG CCT AAC TGG ATG ATT GGT GGA CCA GAC AGT TCA CGG 1400 Glu Gly Lys Trp Pro Asn Trp Met He Gly Gly Pro Asp Ser Ser Arg 440 445 450
CTG ACT TCG CGT TTG GGG AAT CAG TAT GTC AAC GTG ATG AAC ATG CTT 1448 Leu Thr Ser Arg Leu Gly Asn Gin Tyr Val Asn Val Met Asn Met Leu 455 460 465
CTT TTC ACA CTC CCT GGA ACT CCT ATA ACT TAC TAT GGA GAA GAA ATT 1496 Leu Phe Thr Leu Pro Gly Thr Pro He Thr Tyr Tyr Gly Glu Glu He 470 475 480
GGA ATG GGA AAT ATT GTA GCC GCA AAT CTC AAT GAA AGC TAT GAT ATT 1544 Gly Met Gly Asn He Val Ala Ala Asn Leu Asn Glu Ser Tyr Asp He 485 490 495 500
AAT ACC CTT CGC TCA AAG TCA CCA ATG CAG TGG GAC AAT AGT TCA AAT 1592
Asn Thr Leu Arg Ser Lys Ser Pro Met Gin Trp Asp Asn Ser Ser Asn 505 510 515
GCT GGT TTT TCT GAA GCT AGT AAC ACC TGG TTA CCT ACC AAT TCA GAT 1640 Ala Gly Phe Ser Glu Ala Ser Asn Thr Trp Leu Pro Thr Asn Ser Asp 520 525 530 TAC CAC ACT GTG AAT GTT GAT GTC CAA AAG ACT CAG CCC AGA TCG GCT 1688 Tyr His Thr Val Asn Val Asp Val Gin Lys Thr Gin Pro Arg Ser Ala 535 540 545
TTG AAG TTA TAT CAA GAT TTA AGT CTA CTT CAT GCC AAT GAG CTA CTC 1736 Leu Lys Leu Tyr Gin Asp Leu Ser Leu Leu His Ala Asn Glu Leu Leu 550 555 560
CTC AAC AGG GGC TGG TTT TGC CAT TTG AGG AAT GAC AGC CAC TAT GTT 1784 Leu Asn Arg Gly Trp Phe Cys His Leu Arg Asn Asp Ser His Tyr Val 565 570 575 580
GTG TAC ACA AGA GAG CTG GAT GGC ATC GAC AGA ATC TTT ATC GTG GTT 1832 Val Tyr Thr Arg Glu Leu Asp Gly He Asp Arg He Phe He Val Val 585 590 595
CTG AAT TTT GGA GAA TCA ACA CTG TTA AAT CTA CAT AAT ATG ATT TCG 1880 Leu Asn Phe Gly Glu Ser Thr Leu Leu Asn Leu His Asn Met He Ser 600 605 610
GGC CTT CCC GCT AAA ATA AGA ATA AGG TTA AGT ACC AAT TCT GCC GAC 1928 Gly Leu Pro Ala Lys He Arg He Arg Leu Ser Thr Asn Ser Ala Asp 615 620 625
AAA GGC AGT AAA GTT GAT ACA AGT GGC ATT TTT CTG GAC AAG GGA GAG 1976 Lys Gly Ser Lys Val Asp Thr Ser Gly He Phe Leu Asp Lys Gly Glu 630 635 640
GGA CTC ATC TTT GAA CAC AAC ACG AAG AAT CTC CTT CAT CGC CAA ACA 2024 Gly Leu He Phe Glu His Asn Thr Lys Asn Leu Leu His Arg Gin Thr 645 650 655 660
GCT TTC AGA GAT AGA TGC TTT GTT TCC AAT CGA GCA TGC TAT TCC AGT 2072 Ala Phe Arg Asp Arg Cys Phe Val Ser Asn Arg Ala Cys Tyr Ser Ser 665 670 675 GTA CTG AAC ATA CTG TAT ACC TCG TGT TAGGCACCTT TATGAAGAGA TGAAGAC 2126 Val Leu Asn He Leu Tyr Thr Ser Cys 680 685
ACTGGCATTT CAGTGGGATT GTAAGCATTT GTAATAGCTT CATGTACAGC ATGCTGCTTG 2186 GTGAACAATC ATTAATTCTT CGATATTTCT GTAGCTTGAA TGTAACCGCT TTAAGAAAGG 2246 TTCTCAAATG TTTTGAAAAA AATAAAATGT TTAAAAGT 2284
(2) INFORMATION FOR SEQ ID NO: 181:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 685 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 181:
Met Ala Glu Asp Lys Ser Lys Arg Asp Ser He Glu Met Ser Met Lys 1 5 10 15
Gly Cys Gin Thr Asn Asn Gly Phe Val His Asn Glu Asp He Leu Glu
20 25 30
Gin Thr Pro Asp Pro Gly Ser Ser Thr Asp Asn Leu Lys His Ser Thr
35 40 45
Arg Gly He Leu Gly Ser Gin Glu Pro Asp Phe Lys Gly Val Gin Pro
50 55 60
Tyr Ala Gly Met Pro Lys Glu Val Leu Phe Gin Phe Ser Gly Gin Ala 65 70 75 80 Arg Tyr Arg He Pro Arg Glu He Leu Phe Trp Leu Thr Val Ala Ser
85 90 95
Val Leu Val Leu He Ala Ala Thr He Ala He He Ala Leu Ser Pro
100 105 110
Lys Cys Leu Asp Trp Trp Gin Glu Gly Pro Met Tyr Gin He Tyr Pro
115 120 125
Arg Ser Phe Lys Asp Ser Asn Lys Asp Gly Asn Gly Asp Leu Lys Gly
130 135 140
He Gin Asp Lys Leu Asp Tyr He Thr Ala Leu Asn He Lys Thr Val 145 150 155 160
Trp He Thr Ser Phe Tyr Lys Ser Ser Leu Lys Asp Phe Arg Tyr Gly
165 170 175
Val Glu Asp Phe Arg Glu Val Asp Pro He Phe Gly Thr Met Glu Asp
180 185 190
Phe Glu Asn Leu Val Ala Ala He His Asp Lys Gly Leu Lys Leu He
195 200 205
He Asp Phe He Pro Asn His Thr Ser Asp Lys His He Trp Phe Gin
210 215 220 Leu Ser Arg Thr Arg Thr Gly Lys Tyr Thr Asp Tyr Tyr He Trp His
225 230 235 240
Asp Cys Thr His Glu Asn Gly Lys Thr He Pro Pro Asn Asn Trp Leu
245 250 255
Ser Val Tyr Gly Asn Ser Ser Trp His Phe Asp Glu Val Arg Asn Gin
260 265 270
Cys Tyr Phe His Gin Phe Met Lys Glu Gin Pro Asp Leu Asn Phe Arg
275 280 285
Asn Pro Asp Val Gin Glu Glu He Lys Glu He Leu Arg Phe Trp Leu 290 295 300
Thr Lys Gly Val Asp Gly Phe Ser Leu Asp Ala Val Lys Phe Leu Leu 305 310 315 320
Glu Ala Lys His Leu Arg Asp Glu He Gin Val Asn Lys Thr Gin He
325 330 335
Pro Asp Thr Val Thr Gin Tyr Ser Glu Leu Tyr His Asp Phe Thr Thr
340 345 350
Thr Gin Val Gly Met His Asp He Val Arg Ser Phe Arg Gin Thr Met 355 360 365 Asp Gin Tyr Ser Thr Glu Pro Gly Arg Tyr Arg Phe Met Gly Thr Glu 370 375 380
Ala Tyr Ala Glu Ser He Asp Arg Thr Val Met Tyr Tyr Gly Leu Pro 385 390 395 400
Phe He Gin Glu Ala Asp Phe Pro Phe Asn Asn Tyr Leu Ser Met Leu
405 410 415
Asp Thr Val Ser Gly Asn Ser Val Tyr Glu Val He Thr Ser Trp Met
420 425 430
Glu Asn Met Pro Glu Gly Lys Trp Pro Asn Trp Met He Gly Gly Pro 435 440 445
Asp Ser Ser Arg Leu Thr Ser Arg Leu Gly Asn Gin Tyr Val Asn Val
450 455 460
Met Asn Met Leu Leu Phe Thr Leu Pro Gly Thr Pro He Thr Tyr Tyr 465 470 475 480
Gly Glu Glu He Gly Met Gly Asn He Val Ala Ala Asn Leu Asn Glu
485 490 495
Ser Tyr Asp He Asn Thr Leu Arg Ser Lys Ser Pro Met Gin Trp Asp
500 505 510
Asn Ser Ser Asn Ala Gly Phe Ser Glu Ala Ser Asn Thr Trp Leu Pro
515 520 525
Thr Asn Ser Asp Tyr His Thr Val Asn Val Asp Val Gin Lys Thr Gin 530 535 540
Pro Arg Ser Ala Leu Lys Leu Tyr Gin Asp Leu Ser Leu Leu His Ala 545 550 555 560
Asn Glu Leu Leu Leu Asn Arg Gly Trp Phe Cys His Leu Arg Asn Asp
565 570 575
Ser His Tyr Val Val Tyr Thr Arg Glu Leu Asp Gly He Asp Arg He
580 585 590
Phe He Val Val Leu Asn Phe Gly Glu Ser Thr Leu Leu Asn Leu His 595 600 605 Asn Met He Ser Gly Leu Pro Ala Lys He Arg He Arg Leu Ser Thr 610 615 620
Asn Ser Ala Asp Lys Gly Ser Lys Val Asp Thr Ser Gly He Phe Leu 625 630 635 640
Asp Lys Gly Glu Gly Leu He Phe Glu His Asn Thr Lys Asn Leu Leu
645 650 655
His Arg Gin Thr Ala Phe Arg Asp Arg Cys Phe Val Ser Asn Arg Ala
660 665 670
Cys Tyr Ser Ser Val Leu Asn He Leu Tyr Thr Ser Cys 675 680 685
(2) INFORMATION FOR SEQ ID NO: 182:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 54 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 182:
Leu Val Pro Arg Gly Ser Pro Gly He Pro Gly Ser Arg Val Gly Gin
1 5 10 15
Cys Thr Asp Ser Asp Val Arg Arg Pro Trp Ala Arg Ser Cys Ala His 20 25 30 Gin Gly Cys Gly Ala Gly Thr Arg Asn Ser His Gly Cys He Thr Arg 35 40 45
Pro Leu Arg Gin Ala Ser 50
(2) INFORMATION FOR SEQ ID NO: 183:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 19 amino acids (B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 183:
Ser Ala Arg Asp Ser Gly Pro Ala Glu Asp Gly Ser Arg Ala Val Arg 1 5 10 15
Leu Asn Gly
(2) INFORMATION FOR SEQ ID NO: 184:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:184:
Asp Gly Ser Arg Ala Val Arg Leu Asn Gly Val Glu Asn Ala Asn Thr
1 5 10 15
Arg Lys Ser Ser Arg 20
(2) INFORMATION FOR SEQ ID NO: 185:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 185:
Glu Asn Ala Asn Thr Arg Lys Ser Ser Arg Ser Asn Pro Arg Gly Arg
1 5 10 15
Arg His Pro
(2) INFORMATION FOR SEQ ID NO: 186: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 11 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:186:
Thr Arg Lys Ser Ser Arg Ser Asn Pro Arg Gly
1 5 10
(2) INFORMATION FOR SEQ ID NO: 187:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 187:
Ser Arg Pro Tyr Ser Val Asp Ser Asp Ser Asp Thr Asn Ala Lys His 1 5 10 15 Ser Ser His Asn Arg 20
(2) INFORMATION FOR SEQ ID NO: 188:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 188:
Thr Asn Ala Lys His Ser Ser His Asn Arg Arg Leu Arg Thr Arg Ser
1 5 10 15
Arg Pro Asn
(2) INFORMATION FOR SEQ ID NO: 189:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 24 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 189:
Arg Tyr Lys His Asp He Gly Cys Asp Ala Gly Val Asp Lys Lys Ser
1 5 10 15
Ser Ser Val Arg Gly Gly Cys Gly 20
(2) INFORMATION FOR SEQ ID NO:190: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 26 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 190:
Gly Cys Asp Ala Gly Val Asp Lys Lys Ser Ser Ser Val Arg Gly Gly
1 5 10 15
Cys Gly Ala His Ser Ser Pro Pro Arg Ala 20 25
(2) INFORMATION FOR SEQ ID NO: 191:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 21 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 191: Gly Ala His Ser Ser Pro Pro Arg Ala Gly Arg Gly Pro Arg Gly Thr 1 5 10 15
Met Val Ser Arg Leu 20
(2) INFORMATION FOR SEQ ID NO:192:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 192:
Glu Asn Ala Asn Thr Arg Lys Ser Ser Arg 1 5 10
(2) INFORMATION FOR SEQ ID NO:193:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 39 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 193:
Lys Lys Arg He Ala Gly Leu Pro Trp Tyr Arg Cys Arg Thr Val Ala
1 5 10 15
Phe Glu Thr Gly Met Gin Asn Thr Gin Leu Cys Ser Thr He Val Gin
20 25 30
Leu Ser Phe Thr Pro Glu Glu 35 (2) INFORMATION FOR SEQ ID NO: 194:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 194:
Arg Lys Ser Ser Arg Ser Asn Pro Arg Gly 1 5 10
(2) INFORMATION FOR SEQ ID NO: 195:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 9 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 195; Ser Asn Pro Arg Gly Arg Arg His Pro 1 5
(2) INFORMATION FOR SEQ ID NO:196:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 9 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 196:
Thr Asn Ala Lys His Ser Ser His Asn 1 5
(2) INFORMATION FOR SEQ ID NO: 197:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 10 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 197: Ser Ser His Asn Arg Arg Leu Arg Thr Arg 1 5 10
(2) INFORMATION FOR SEQ ID NO: 198:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (°) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 198:
Arg Arg Leu Arg Thr Arg Ser Arg Pro Asn 1 5 10 (2) INFORMATION FOR SEQ ID NO: 199:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS :
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 199:
Arg Val Gly Gin Cys Thr Asp Ser Asp Val Arg Arg Pro Trp Ala Arg
1 5 10 15
Ser Cys Ala
(2) INFORMATION FOR SEQ ID NO: 200:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY : unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 200:
Val Arg Arg Pro Trp Ala Arg Ser Cys Ala His Gin Gly Cys Gly Ala 1 5 10 15
Gly Thr Arg Asn Ser 20
(2) INFORMATION FOR SEQ ID NO: 201:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 19 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 201:
Gly Thr Arg Asn Ser His Gly Cys He Thr Arg Pro Leu Arg Gin Ala
1 5 10 15
Ser Gin His
(2) INFORMATION FOR SEQ ID NO:202:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 40 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 202:
Ser Thr Pro Pro Ser Arg Glu Ala Tyr Ser Arg Pro Tyr Ser Val Asp
1 5 10 15
Ser Asp Ser Asp Thr Met Ala Lys His Ser Ser His Asn Arg Arg Leu 20 25 30
Arg Thr Arg Ser Arg Pro Asn Gly 35 40
(2) INFORMATION FOR SEQ ID NO:203:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:203:
Tyr Ser Lys Val 1
(2) INFORMATION FOR SEQ ID NO: 204;
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 204:
1
(2) INFORMATION FOR SEQ ID NO: 205:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 205:
Tyr Arg Gly Val
1 , (2). INFORMATION FOR SEQ ID NO:206:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:206:
Tyr Gin Thr He
1
(2) INFORMATION FOR SEQ ID NO: 207: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 207:
Thr Glu Gin Phe
1
(2) INFORMATION FOR SEQ ID NO: 208:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 208:
Thr Glu Val Met 1
(2) INFORMATION FOR SEQ ID NO: 209:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 209:
Thr Ser Ala Phe
1
(2) INFORMATION FOR SEQ ID NO: 210:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 210:
Tyr Thr Arg Phe
1
(2) INFORMATION FOR SEQ ID NO: 211:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 717 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear (ii) MOLECULE TYPE: DNA
(ix) FEATURE:
(A) NAME/KEY: Coding Sequence
(B) LOCATION: 1...714 (D) OTHER INFORMATION:
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 211:
ATG TCC CCT ATA CTA GGT TAT TGG AAA ATT AAG GGC CTT GTG CAA CCC 48
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15
ACT CGA CTT CTT TTG GAA TAT CTT GAA GAA AAA TAT GAA GAG CAT TTG 96 Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30 TAT GAG CGC GAT GAA GGT GAT AAA TGG CGA AAC AAA AAG TTT GAA TTG 144 Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
GGT TTG GAG TTT CCC AAT CTT CCT TAT TAT ATT GAT GGT GAT GTT AAA 192
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60
TTA ACA CAG TCT ATG GCC ATC ATA CGT TAT ATA GCT GAC AAG CAC AAC 240 Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80 ATG TTG GGT GGT TGT CCA AAA GAG CGT GCA GAG ATT TCA ATG CTT GAA 288 Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95
GGA GCG GTT TTG GAT ATT AGA TAC GGT GTT TCG AGA ATT GCA TAT AGT 336 Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110
AAA GAC TTT GAA ACT CTC AAA GTT GAT TTT CTT AGC AAG CTA CCT GAA 384 Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
ATG CTG AAA ATG TTC GAA GAT CGT TTA TGT CAT AAA ACA TAT TTA AAT 432 Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn 130 135 140
GGT GAT CAT GTA ACC CAT CCT GAC TTC ATG TTG TAT GAC GCT CTT GAT 480 Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
GTT GTT TTA TAC ATG GAC CCA ATG TGC CTG GAT GCG TTC CCA AAA TTA 528 Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu 165 170 175
GTT TGT TTT AAA AAA CGT ATT GAA GCT ATC CCA CAA ATT GAT AAG TAC 576 Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190
TTG AAA TCC AGC AAG TAT ATA GCA TGG CCT TTG CAG GGC TGG CAA GCC 624
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205
ACG TTT GGT GGT GGC GAC CAT CCT CCA AAA TCG GAT CTG GTT CCG CGT 672 Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220 GGA TCC CCA GGA ATT CCC GGG TCG ACT CGA GCG GCC GCA TCG TGA 717
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser 225 230 235
(2) INFORMATION FOR SEQ ID NO: 212:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 238 amino acids (B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:212:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro l 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu 165 170 175 Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser 225 230 235 (2) INFORMATION FOR SEQ ID NO:213:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 282 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 213:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45 Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190 Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Gin
225 230 235 240
Gly Ser Lys Gin Cys Met Gin Tyr Arg Thr Gly Arg Leu Thr Val Gly
245 250 255
Ser Glu Tyr Gly Cys Gly Met Asn Pro Ala Arg His Ala Thr Pro Ala
260 265 270
Tyr Pro Ala Arg Leu Leu Pro Arg Tyr Arg 275 280
(,2,) INFORMATION FOR SEQ ID NO:214:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 282 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:214:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110 Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Asp 225 230 235 240
His Ala Leu Gly Thr Asn Leu Arg Ser Asp Asn Ala Lys Glu Pro Gly 245 250 255 Asp Tyr Asn Cys Cys Gly Asn Gly Asn Ser Thr Gly Arg Lys Val Phe 260 265 270
Asn Arg Arg Arg Pro Ser Ala He Pro Thr 275 280
(2) INFORMATION FOR SEQ ID NO: 215:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 279 amino acids (B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 215:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110 Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Pro 225 230 235 240
Cys Gly Gly Ser Trp Gly Arg Phe Met Gin Gly Gly Leu Phe Gly Gly 245 250 255 Arg Thr Asp Gly Cys Gly Ala His Arg Asn Arg Thr Ser Ala Ser Leu 260 265 270
Glu Pro Pro Ser Ser Asp Tyr 275
(2) INFORMATION FOR SEQ ID NO: 216:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 277 amino acids (B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:216:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80 Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Arg Gly 225 230 235 240
Ser Thr Gly Thr Ala Gly Gly Glu Arg Ser Gly Val Leu Asn Leu His
245 250 255
Thr Arg Asp Asn Ala Ser Gly Ser Gly Phe Lys Pro Trp Tyr Pro Ser
260 265 270
Asn Arg Gly His Lys 275
(2) INFORMATION FOR SEQ ID NO: 217:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 277 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:217: Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140 Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp
145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser His 225 230 235 240
Ser Gly Gly Met Asn Arg Ala Tyr Gly Asp Val Phe Arg Glu Leu Arg 245 250 255
Asp Arg Trp Asn Ala Thr Ser His His Thr Arg Pro Thr Pro Gin Leu
260 265 270
Pro Arg Gly Pro Asn 275
(2) INFORMATION FOR SEQ ID NO:218:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 248 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 218:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60 Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn 130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205 Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser His 225 230 235 240
Ser Gly Gly Met Asn Arg Ala Tyr 245
(2) INFORMATION FOR SEQ ID NO:219: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 248 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 219:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160 Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Gly Asp 225 230 235 240
Val Phe Arg Glu Leu Arg Asp Arg 245
(2) INFORMATION FOR SEQ ID NO: 220:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 248 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 220:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15 Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160 Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Trp Asn 225 230 235 240
Ala Thr Ser His His Thr Arg Pro 245
(2) INFORMATION FOR SEQ ID NO: 221:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 247 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 221:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110 Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Thr Pro 225 230 235 240
Gin Leu Pro Arg Gly Pro Asn 245
(2) INFORMATION FOR SEQ ID NO: 222:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 258 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:222: Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205 Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Gly Asp 225 230 235 240
Val Phe Arg Glu Leu Arg Asp Arg Trp Asn Ala Thr Ser His His Thr
245 250 255
Arg Pro
(2) INFORMATION FOR SEQ ID NO: 223:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 257 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 223
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45 Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Trp Asn 225 230 235 240
Ala Thr Ser His His Thr Arg Pro Thr Pro Gin Leu Pro Arg Gly Pro
245 250 255
Asn
(2) INFORMATION FOR SEQ ID NO: 224:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 267 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 224:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125 Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn 130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Gly Asp 225 230 235 240
Val Phe Arg Glu Leu Arg Asp Arg Trp Asn Ala Thr Ser His His Thr
245 250 255
Arg Pro Thr Pro Gin Leu Pro Arg Gly Pro Asn 260 265
(2) INFORMATION FOR SEQ ID NO: 225:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 277 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 225:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60 Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205 Tnr Pne Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser His 225 230 235 240
Ser Gly Gly Met Asn Arg Ala Tyr Gly Asp Val Phe Arg Glu Leu Arg
245 250 255
Asp Arg Trp Asn Ala Thr Ser Ala Ala Thr Arg Pro Thr Pro Gin Leu
260 265 270
Pro Arg Gly Pro Asn 275
(2) INFORMATION FOR SEQ ID NO:226:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 277 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 226:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30 Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn
65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Ala 225 230 235 240
Arg Asp Ser Gly Pro Ala Glu Asp Gly Ser Arg Ala Val Arg Leu Asn
245 250 255
Gly Val Glu Asn Ala Asn Thr Arg Lys Ser Ser Arg Ser Asn Pro Arg 260 265 270 Gly Arg Arg His Pro 275
(2) INFORMATION FOR SEQ ID NO: 227:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 257 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 227:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15 Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160 Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Ala 225 230 235 240
Arg Asp Ser Gly Pro Ala Glu Asp Gly Ser Arg Ala Val Arg Leu Asn 245 250 255 Gly
(2) INFORMATION FOR SEQ ID NO: 228:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 259 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 228:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95 Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu 165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Asp Gly 225 230 235 240 Ser Arg Ala Val Arg Leu Asn Gly Val Glu Asn Ala Asn Thr Arg Lys
245 250 255
Ser Ser Arg
(2) INFORMATION FOR SEQ ID NO: 229:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 257 amino acids (B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 229:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110 Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Glu Asn 225 230 235 240
Ala Asn Thr Arg Lys Ser Ser Arg Ser Asn Pro Arg Gly Arg Arg His 245 250 255 Pro
(2) INFORMATION FOR SEQ ID NO: 230:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 248 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 230:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95 Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190 Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Glu Asn 225 230 235 240
Ala Asn Thr Arg Lys Ser Ser Arg 245 (2) INFORMATION FOR SEQ ID NO: 231:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 248 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 231:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45 Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190 Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Arg Lys 225 230 235 240
Ser Ser Arg Ser Asn Pro Arg Gly 245 (2) INFORMATION FOR SEQ ID NO: 232:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 247 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 232:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140 Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp
145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Asn 225 230 235 240
Pro Arg Gly Arg Arg His Pro 245
(2) INFORMATION FOR SEQ ID NO: 233:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 249 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 233: Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu 165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Thr Arg 225 230 235 240 Lys Ser Ser Arg Ser Asn Pro Arg Gly
245
(2) INFORMATION FOR SEQ ID NO:234:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 277 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:234:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95 Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu 165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Thr 225 230 235 240 Pro Pro Ser Arg Glu Ala Tyr Ser Arg Pro Tyr Ser Val Asp Ser Asp
245 250 255
Ser Asp Thr Asn Ala Lys His Ser Ser His Asn Arg Arg Leu Arg Thr
260 265 270
Arg Ser Arg Pro Asn
275 (2) INFORMATION FOR SEQ ID NO: 235:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 258 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:235:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15 Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160 Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Thr 225 230 235 240
Pro Pro Ser Arg Glu Ala Tyr Ser Arg Pro Tyr Ser Val Asp Ser Asp
245 250 255
Ser Asp
(2) INFORMATION FOR SEQ ID NO: 236:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 259 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:236: Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95 Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu 165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Arg 225 230 235 240 Pro Tyr Ser Val Asp Ser Asp Ser Asp Thr Asn Ala Lys His Ser Ser
245 250 255
His Asn Arg
(2) INFORMATION FOR SEQ ID NO: 237:
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 257 amino acids (B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:237:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80 Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Thr Asn 225 230 235 240
Ala Lys His Ser Ser His Asn Arg Arg Leu Arg Thr Arg Ser Arg Pro
245 250 255
Asn
(2) INFORMATION FOR SEQ ID NO: 238:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 247 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 238:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro 1 5 10 15 Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu 20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu 85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160 Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Thr Asn 225 230 235 240
Ala Lys His Ser Ser His Asn 245
(2) INFORMATION FOR SEQ ID NO: 239:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 248 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 239:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110 Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Ser Ser 225 230 235 240
His Asn Arg Arg Leu Arg Thr Arg 245
(2) INFORMATION FOR SEQ ID NO: 240:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 248 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 240:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110 Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp
145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205 Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Arg Arg 225 230 235 240
Leu Arg Thr Arg Ser Arg Pro Asn 245
(2) INFORMATION FOR SEQ ID NO: 241: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 282 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:241:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60 Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn
65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn 130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205 Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Arg Val 225 230 235 240
Gly Gin Cys Thr Asp Ser Asp Val Arg Arg Pro Trp Ala Arg Ser Cys
245 250 255
Ala His Gin Gly Cys Gly Ala Gly Thr Arg Asn Ser His Gly Cys He
260 265 270
Thr Arg Pro Leu Arg Gin Ala Ser Ala His 275 280
(2) INFORMATION FOR SEQ ID NO: 242:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 257 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 242:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125 Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn 130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Arg Val 225 230 235 240
Gly Gin Cys Thr Asp Ser Asp Val Arg Arg Pro Trp Ala Arg Ser Cys
245 250 255
Ala
(2) INFORMATION FOR SEQ ID NO:243:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 259 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS :
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 243:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu
115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn 130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205 Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Val Arg 225 230 235 240
Arg Pro Trp Ala Arg Ser Cys Ala His Gin Gly Cys Gly Ala Gly Thr
245 250 255
Arg Asn Ser
(2) INFORMATION FOR SEQ ID NO: 244:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 257 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 244:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45 Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190 Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Gly Thr
225 230 235 240
Arg Asn Ser His Gly Cys He Thr Arg Pro Leu Arg Gin Ala Ser Gin
245 250 255
His
(2) INFORMATION FOR SEQ ID NO: 245:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 282 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (xi) SEQUENCE DESCRIPTION: SEQ ID NO:245:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125 Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn 130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Arg Tyr 225 230 235 240
Lys His Asp He Gly Cys Asp Ala Gly Val Asp Lys Lys Ser Ser Ser
245 250 255
Val Arg Gly Gly Cys Gly Ala His Ser Ser Pro Pro Arg Ala Gly Arg 260 265 270 Gly Pro Arg Gly Thr Met Val Ser Arg Leu 275 280
(2) INFORMATION FOR SEQ ID NO:246:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 262 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: (D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:246:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu
35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125 Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn 130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala
195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Arg Tyr 225 230 235 240
Lys His Asp He Gly Cys Asp Ala Gly Val Asp Lys Lys Ser Ser Ser
245 250 255
Val Arg Gly Gly Cys Gly 260
(2) INFORMATION FOR SEQ ID NO: 247:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 264 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 247:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45
Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys
50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser 100 105 110 Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp
145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr
180 185 190
Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205 Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg 210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Gly Cys 225 230 235 240
Asp Ala Gly Val Asp Lys Lys Ser Ser Ser Val Arg Gly Gly Cys Gly
245 250 255
Ala His Ser Ser Pro Pro Arg Ala 260 (2) INFORMATION FOR SEQ ID NO: 248:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 259 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO-.248:
Met Ser Pro He Leu Gly Tyr Trp Lys He Lys Gly Leu Val Gin Pro
1 5 10 15
Thr Arg Leu Leu Leu Glu Tyr Leu Glu Glu Lys Tyr Glu Glu His Leu
20 25 30
Tyr Glu Arg Asp Glu Gly Asp Lys Trp Arg Asn Lys Lys Phe Glu Leu 35 40 45 Gly Leu Glu Phe Pro Asn Leu Pro Tyr Tyr He Asp Gly Asp Val Lys 50 55 60
Leu Thr Gin Ser Met Ala He He Arg Tyr He Ala Asp Lys His Asn 65 70 75 80
Met Leu Gly Gly Cys Pro Lys Glu Arg Ala Glu He Ser Met Leu Glu
85 90 95
Gly Ala Val Leu Asp He Arg Tyr Gly Val Ser Arg He Ala Tyr Ser
100 105 110
Lys Asp Phe Glu Thr Leu Lys Val Asp Phe Leu Ser Lys Leu Pro Glu 115 120 125
Met Leu Lys Met Phe Glu Asp Arg Leu Cys His Lys Thr Tyr Leu Asn
130 135 140
Gly Asp His Val Thr His Pro Asp Phe Met Leu Tyr Asp Ala Leu Asp 145 150 155 160
Val Val Leu Tyr Met Asp Pro Met Cys Leu Asp Ala Phe Pro Lys Leu
165 170 175
Val Cys Phe Lys Lys Arg He Glu Ala He Pro Gin He Asp Lys Tyr 180 185 190 Leu Lys Ser Ser Lys Tyr He Ala Trp Pro Leu Gin Gly Trp Gin Ala 195 200 205
Thr Phe Gly Gly Gly Asp His Pro Pro Lys Ser Asp Leu Val Pro Arg
210 215 220
Gly Ser Pro Gly He Pro Gly Ser Thr Arg Ala Ala Ala Ser Gly Ala 225 230 235 240
His Ser Ser Pro Pro Arg Ala Gly Arg Gly Pro Arg Gly Thr Met Val
245 250 255
Ser Arg Leu
(2) INFORMATION FOR SEQ ID NO: 249: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 44 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (xi) SEQUENCE DESCRIPTION: SEQ ID NO: 249:
Ser Gly Ser Pro Pro Cys Cys Cys Ser Trp Gly Arg Phe Met Gin Gly
1 5 10 15
Gly Leu Phe Gly Gly Arg Thr Asp Gly Cys Gly Ala His Arg Asn Arg
20 25 30
Thr Ser Ala Ser Leu Glu Pro Pro Ser Ser Asp Tyr 35 40 (2) INFORMATION FOR SEQ ID NO: 250:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 40 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 250:
Ser His Ser Gly Gly Met Asn Arg Ala Tyr Gly Asp Val Phe Arg Glu
1 5 10 15
Leu Arg Asp Arg Trp Asn Ala Thr Ser His His Thr Arg Pro Thr Pro
20 25 30
Gin Leu Pro Arg Gly Pro Asn Ser 35 40
(2) INFORMATION FOR SEQ ID NO: 251:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 21 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 251:
Asp Thr Asn Ala Lys His Ser Ser His Asn Arg Arg Leu Arg Thr Arg
1 5 10 15
Ser Arg Pro Asn Gly 20 (2) INFORMATION FOR SEQ ID NO: 252:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 23 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:252:
Cys Gly Ala Gly Thr Arg Asn Ser His Gly Cys He Thr Arg Pro Leu 1 5 10 15
Arg Gin Ala Ser Ala His Gly 20
(2) INFORMATION FOR SEQ ID N0:253:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 11 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (ix) FEATURE:
(A NAME/KEY: Modified Site (B LOCATION : 1 (D OTHER INFORMATION: "Xaa=Ser or Thr"
(A NAME/KEY: Modified Site (B LOCATION : 3 (D OTHER INFORMATION: "Xaa=Arg or Lys"
(A NAME/KEY: Modified Site
(B : LOCATION: 4 (D: OTHER INFORMATION: "Xaa=Lys or Arg"
(A NAME/KEY: Modified Site (B LOCATION : 6 (D OTHER INFORMATION: "Xaa=Ser or Leu"
(A NAME/KEY: Modified Site (B LOCATION: 7 (D OTHER INFORMATION: "Xaa=Arg, He, Val or Ser"
(A NAME/KEY: Modified Site (B LOCATION: 8 (D OTHER INFORMATION: "Xaa=Ser, Tyr, Phe or His"
(A NAME/KEY: Modified Site (B LOCATION: 10 (D OTHER INFORMATION: "Xaa=Phe, His or Arg"
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 253:
Xaa Thr Xaa Xaa Ser Xaa Xaa Xaa Asn Xaa Arg 1 5 10
(2) INFORMATION FOR SEQ ID NO: 254:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (ix) FEATURE:
(A) NAME/KEY: Modified Site
(B) LOCATION: 2
(D) OTHER INFORMATION: "Xaa=Ser, Ala or Gly"
(A) NAME/KEY: Modified Site
(B) LOCATION: 4
(D) OTHER INFORMATION: "Xaa=Val or Gin"
(A) NAME/KEY: Modified Site
(B) LOCATION: 7
(D) OTHER INFORMATION: "Xaa=Pro, Gly or Ser"
(A) NAME/KEY: Modified Site
(B) LOCATION: 8
(D) OTHER INFORMATION: "Xaa=Trp or Tyr"
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 254:
Asp Xaa Asp Xaa Arg Arg Xaa Xaa 1 5
(2) INFORMATION FOR SEQ ID NO: 255: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide (ix) FEATURE: (A) NAME/KEY: Modified Site
(B) LOCATION: 7 (D) OTHER INFORMATION: "Xaa=Ala or Phe"
(A) NAME/KEY: Modified Site
(B) LOCATION: 8
(D) OTHER INFORMATION: "Xaa=Arg or His"
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 255:
Val Arg Ser Gly Cys Gly Xaa Xaa Ser Ser 1 5 10
(2) INFORMATION FOR SEQ ID NO: 256:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 11 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO-.256:
Asn Thr Arg Lys Ser Ser Arg Ser Asn Pro Arg 1 5 10
(2) INFORMATION FOR SEQ ID NO: 257:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 11 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 257: Ser Thr Lys Arg Ser Leu He Tyr Asn His Arg
1 5 10
(2) INFORMATION FOR SEQ ID NO: 258:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 10 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:258:
Ser Thr Gly Arg Lys Val Phe Asn Arg Arg 1 5 10
(2) INFORMATION FOR SEQ ID NO: 259:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 11 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:259:
Thr Asn Ala Lys His Ser Ser His Asn Arg Arg 1 5 10
(2) INFORMATION FOR SEQ ID NO: 260: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:260:
Asp Ser Asp Val Arg Arg Pro Trp
1 5
(2) INFORMATION FOR SEQ ID NO: 261:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 8 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 261:
Ala Ala Asp Gin Arg Arg Gly Trp 1 5
(2) INFORMATION FOR SEQ ID NO:262
(i) SEQUENCE CHARACTERISTICS: (A) LENGTH: 8 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:262:
Asp Gly Arg Gly Gly Arg Ser Tyr
1 5
(2) INFORMATION FOR SEQ ID NO: 263:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 4 amino acids
(B) TYPE: amino acid (C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 263:
Arg Val Arg Ser
1
(2) INFORMATION FOR SEQ ID NO:264:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown (ϋ) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:264:
Ser Val Arg Ser Gly Cys Gly Phe Arg Gly Ser Ser 1 5 10
(2) INFORMATION FOR SEQ ID NO: 265: (i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 11 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS:
(D) TOPOLOGY: unknown
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:265:
Ser Val Arg Gly Gly Cys Gly Ala His Ser Ser 1 5 10