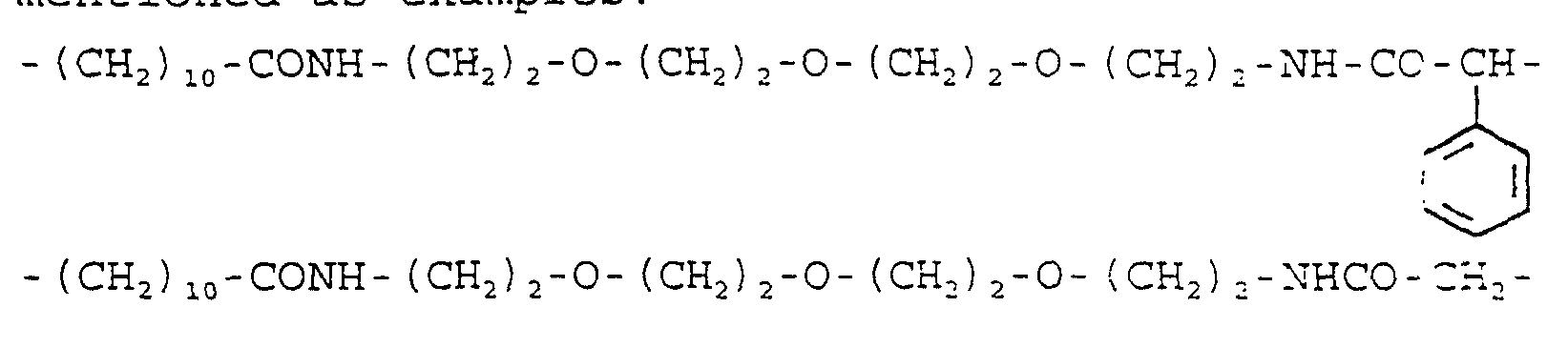WO1996040273A2 - Conjugates of ferrites and oligonucleotides, which bond specifically to certain target structures - Google Patents
Conjugates of ferrites and oligonucleotides, which bond specifically to certain target structures Download PDFInfo
- Publication number
- WO1996040273A2 WO1996040273A2 PCT/EP1996/002442 EP9602442W WO9640273A2 WO 1996040273 A2 WO1996040273 A2 WO 1996040273A2 EP 9602442 W EP9602442 W EP 9602442W WO 9640273 A2 WO9640273 A2 WO 9640273A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- oligonucleotide
- group
- oligonucleotides
- optionally
- target
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K49/00—Preparations for testing in vivo
- A61K49/06—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations
- A61K49/18—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by a special physical form, e.g. emulsions, microcapsules, liposomes
- A61K49/1818—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by a special physical form, e.g. emulsions, microcapsules, liposomes particles, e.g. uncoated or non-functionalised microparticles or nanoparticles
- A61K49/1821—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by a special physical form, e.g. emulsions, microcapsules, liposomes particles, e.g. uncoated or non-functionalised microparticles or nanoparticles coated or functionalised microparticles or nanoparticles
- A61K49/1824—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by a special physical form, e.g. emulsions, microcapsules, liposomes particles, e.g. uncoated or non-functionalised microparticles or nanoparticles coated or functionalised microparticles or nanoparticles coated or functionalised nanoparticles
- A61K49/1827—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by a special physical form, e.g. emulsions, microcapsules, liposomes particles, e.g. uncoated or non-functionalised microparticles or nanoparticles coated or functionalised microparticles or nanoparticles coated or functionalised nanoparticles having a (super)(para)magnetic core, being a solid MRI-active material, e.g. magnetite, or composed of a plurality of MRI-active, organic agents, e.g. Gd-chelates, or nuclei, e.g. Eu3+, encapsulated or entrapped in the core of the coated or functionalised nanoparticle
- A61K49/1851—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by a special physical form, e.g. emulsions, microcapsules, liposomes particles, e.g. uncoated or non-functionalised microparticles or nanoparticles coated or functionalised microparticles or nanoparticles coated or functionalised nanoparticles having a (super)(para)magnetic core, being a solid MRI-active material, e.g. magnetite, or composed of a plurality of MRI-active, organic agents, e.g. Gd-chelates, or nuclei, e.g. Eu3+, encapsulated or entrapped in the core of the coated or functionalised nanoparticle having a (super)(para)magnetic core coated or functionalised with an organic macromolecular compound, i.e. oligomeric, polymeric, dendrimeric organic molecule
- A61K49/1857—Nuclear magnetic resonance [NMR] contrast preparations; Magnetic resonance imaging [MRI] contrast preparations characterised by a special physical form, e.g. emulsions, microcapsules, liposomes particles, e.g. uncoated or non-functionalised microparticles or nanoparticles coated or functionalised microparticles or nanoparticles coated or functionalised nanoparticles having a (super)(para)magnetic core, being a solid MRI-active material, e.g. magnetite, or composed of a plurality of MRI-active, organic agents, e.g. Gd-chelates, or nuclei, e.g. Eu3+, encapsulated or entrapped in the core of the coated or functionalised nanoparticle having a (super)(para)magnetic core coated or functionalised with an organic macromolecular compound, i.e. oligomeric, polymeric, dendrimeric organic molecule the organic macromolecular compound being obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. PLGA
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K51/00—Preparations containing radioactive substances for use in therapy or testing in vivo
- A61K51/12—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by a special physical form, e.g. emulsion, microcapsules, liposomes, characterized by a special physical form, e.g. emulsions, dispersions, microcapsules
- A61K51/1241—Preparations containing radioactive substances for use in therapy or testing in vivo characterised by a special physical form, e.g. emulsion, microcapsules, liposomes, characterized by a special physical form, e.g. emulsions, dispersions, microcapsules particles, powders, lyophilizates, adsorbates, e.g. polymers or resins for adsorption or ion-exchange resins
Definitions
- This invention relates to the object characterized in the claims, i.e., oligonucleotide conjugates, which contain a metal oxide sheathed with a coating agent.
- Imaging diagnosis has achieved great progress in past decades and continues to develop. It is now possible to make visible the blood vessel system, most organs and many tissues in the living body without major intervention. In many cases, diseases are diagnosed, since they lead to clear changes of shape, size and position of anatomical structures in the body. Such anatomical data from the inside of the body can be obtained by x-ray technology, ultrasound diagnosis and magnetic resonance tomography.
- Each of the mentioned technologies can be improved in efficiency by the use of pharmaceutical agents for enhancing the natural contrasts of the tissue and body fluids in the resulting image.
- the pharmaceutical agents in question are introduced into body cavities or injected into blood vessels with the purpose of changing in contrast the cavities or vessels. In addition, they are dispersed by the blood stream into the organism and can change organs and tissues in visibility.
- superparamagnetic compounds are ferrite particles, which for stabilization in most cases are sheathed with a coating agent, for example a polysaccharide, a protein or a silane (see US 4,827,945, Groman et al.). Based on their pronounced relaxation effects, superparamagnetic contrast media, i.a., have the advantage that they can be dosed very low.
- nuclear diagnosis is based on substances which themselves can be made visible.
- radioactive isotopes which emit far-reaching radiation, are introduced in the body.
- the dispersing of these substances in the organism can be tracked by means of suitable detectors.
- An advantage of the nuclear medicine process is the high effectiveness at low dosage of the signal-transmitting radioactive substances designated as radiopharmaceutical agents.
- radiopharmaceutical agents can also be used for therapeutic purposes, e.g., for destruction of tumors.
- the same goal can also be achieved by the fact that harmless isotopes or substances are introduced into the body and are converted to a therapeutically effective form there by, e.g., neutron or x-ray radiation, ultrasound or radio waves.
- a general problem is the diagnosis and localization of pathological changes at a time at which no marked changes of shape, structure and circulation of the organs and tissues in question are present.
- Such a diagnosis and follow-up are of decisive importance, e.g., in the case of tumor diseases including the search for metasta ses, the evaluation of an inferior supply of tissues with oxygen and in the case of certain infections as well as metabolic diseases.
- contrast media in the trade are quite predominantly so-called unspecific preparations. They are spread passively in those spaces into which they are introduced, e.g., by injection.
- US Patent No. 4,707,352 is concerned with a special process to label complexing molecules with radioactive isotopes, but no well-suited complexing agents for the bonding of metal ions are described.
- EP-A-0 285 057 describes nucleotide-complexing agent conjugates, which are not suitable, i.a., because of the in vivo stability of the nucleotides used for the application as in vivo diagnostic agents or therapeutic agents and also meet hardly any of the other requirements of compatibility and pharmacokinetics.
- a multiplicity of US patents such as, for example, US Patent No. 4,707,4440, is concerned with modified polymers, which contain a detectable chemical group.
- the polymers can be polynucleotides and oligonucleotides, but the latter are stabilized neither against degradation by naturally occurring nucleases, nor selected by a special process, so that they bond specifically with high bonding affinity to target structures.
- Special embodiments of these detectable molecules are mentioned in US Patents No. 4,843,122 and 4,943,523.
- An individual nucleotide modified in this way is claimed in US Patent No.
- the object of this invention is to make available specifically bonding agents for the detection of target structures, by which, for example, the visualization of organs, tissues and their pathological changes in vitro and in vivo is made possible.
- conjugates consisting of oligonucleotides bonding specifically with high affinity to target structures, containing modifications significantly limiting the degradation by naturally occurring nucleases, reduced by one hydroxyl group in 3'-position or in 5'-position or by one hydroxymethyl group in
- N means an oligonucleotide bonding specifically with high affinity to target structures, containing modifications significantly limiting the degradation by naturally occurring nucleases, reduced by one OH group in the terminal 3'-position or in the terminal 5'-position,
- B means a bonding component X-Y-Z, in which X means a direct bond or a group -NH or -NR with R meaning a C1 to C4 alkyl chain,
- Y means a direct bond or a spacer and Z means a direct bond or a sulfur atom
- L means a linker
- A means a group bound on the terminal 3'- or 5'- position
- M means a chemically modified, optionally radioac- tively-labeled ferrite coated with a polysaccharide, and m means a number between 1 and 100,
- Oligonucleotide N consists of 5 to 200 nucleotides, preferably 15 to 100 nucleotides. Its structure is characterized in that
- phosphodiesters independently of one another, being used as internucleotide bond, are replaced by phosphorothioates, phosphorodithioates or methyl phosphonates,
- the terminal radicals in 3'- and 5'-positions optionally contain modified internucleotide bonds as described in b), which connect up to 5 thymidines.
- Oligonucleotide PN is characterized in that it bonds specifically with high bonding affinity to other target structures, and in that it can be obtained in that a mixture of oligonucleotides, containing random sequences, is brought together with the target structure, and certain oligonucleotides exhibit an increased affinity to the target structure relative to the mixture of oligonucleotides, the latter are separated from the remainder of the oligonucleotide mixture, then the oligonucleotides with increased affinity to the target structure are amplified to obtain a mixture of oligonucleotides, which exhibits an increased portion of oligonucleotides, which bond on the target structures.
- oligonucleotide PN is characterized in that it bonds specifically with high bonding affinity to other target structures and in that it can be obtained in that
- a) first a DNA strand is produced by chemical synthesis, so that this DNA strand exhibits a defined sequence on the 3'-end, which is complementary to a promoter for an RNA polymerase and at the same time complementary to a primer of the polymerase chain reaction (PCR) and in that this DNA strand exhibits a defined DNA sequence on the 5'-end, which is complementary to a primer sequence for the polymerase chain reaction, and the sequence contains a random sequence between the defined sequences, and in that
- this DNA strand is transferred with the help of an RNA polymerase in a complimentary RNA strand, and the nucleotides that are modified in 2'-position of the ribose unit are offered to the polymerase, and in that c) the RNA oligonucleotides, produced in this way, with the target structure on which the oligonucleotide is to bond specifically, are brought together and in that d) those oligonucleotides that have bonded on the target structure are separated first together with the target structure from the nonbonding oligonucleotides and then the bound oligonucleotides are again separated from the target structure and in that
- these target-structure-specific RNA oligonucleotides are transferred with the help of reverse transcriptase to a complementary DNA strand and in that
- the DNA oligonucleotides amplified in this way are then transferred again with the help of the RNA polymerase and with modified nucleotides to RNA oligonucleotide and in that
- oligonucleotides which are characterized by a high bonding affinity to the target structure, are sufficiently selected and then the sequences of the thus obtained oligonucleotides optionally can be determined.
- the target structure is selected from among macromolecules, tissue structures of higher organisms such as animals or humans, organs or parts of organs of an animal or human, cells, tumor cells or tumors.
- oligonucleotides that can be used according to the invention are stabilized against degradation by in vivo occurring nucleases.
- Unmodified oligonucleotides or polynucleotides are cleaved in vivo by endonucleases and exonucleases.
- the degradation reaction in the RNA series begins with an activation of the 2'-hydroxy group.
- Other catabolic enzymes are, e.g., ribozymes, which cleave the phosphodiester bond of RNA (see Science 261, 709 (1993)).
- the in vivo stability of RNA derivatives can be increased by partial or complete exchange of the 2'-hydroxyl group for other substituents.
- substituents are, e.g., alkoxy groups, especially the methoxy group (see, e.g., Chem. Pharm. Bull.
- the stabilization can be achieved in that the
- hydroxyl groups in 2'-position of the ribose units are modified.
- Such a modification can be achieved by a replacement of this hydroxyl group by an OR 2 group, a halogen atom, especially a fluorine atom, a hydrogen atom or an amine radical, especially by an amino group.
- Radical R 2 of the alkoxy group stands, in this case, for a straight-chain or branched alkyl radical with 1 to 20 C atoms, such as methyl, ethyl, propyl, isopropyl, butyl, tert-butyl, pentyl or hexyl or a cyclic unsubstituted or substituted alkyl radical with 4 to 20 C atoms, such as cyclopentyl or cyclohexyl, which optionally contains 1-2 hydroxy groups and is optionally interrupted by 1-5 oxygen atoms.
- the stabilization is also increased in that existing hydroxyl groups in 3-' and 5'-positions are etherified with the radical R 2 .
- phosphodiesters being used as internucleotide bond independently of one another, are replaced by phosphorothioates, phosphorodithioates or alkylphos phonates, with a C1 to C6 alkyl -- especially a methyl group.
- These internucleotide bonds can also be linked to the terminal radicals in 3'- and 5'-positions or else also connect the 3'-3'- or 5'-5'-positions.
- the phosphodiester bond further makes possible linkages with
- hydroxyalkyl radicals which are present on nitrogen or carbon atoms of the nucleobases.
- two thymidines can be linked with the hydroxyalkyl chains present in 3-position or two purine bases with the radical present in 8-position.
- the linkage can also take place in hydroxyl groups in 2'- or 3'- or 5'-position.
- the modified internucleotide bonds can optionally occur preferably at the end of the polynucleotide, and they are especially preferably bound on the thymidine.
- N used are not limited to certain oligonucleotide
- oligonucleotides are preferred that bond specifically with high bonding affinity to target structures.
- the SELEX method involves selection from a mixture of candidate oligonucleotides and step-wise iterations of binding, partitioning and amplification, using the same general selection scheme, to achieve virtually any desired criterion of binding affinity and selectivity.
- the SELEX method includes steps of contacting the mixture with the target under conditions favorable for binding, partitioning unbound nucleic acids from those nucleic acids which have bound specifically to target molecules, dissociating the nucleic acid-target complexes, amplifying the nucleic acids dissociated from the nucleic acid-target complexes to yield a ligand-enriched mixture of nucleic acids, then reiterating the steps of binding, partitioning, dissociating and amplifying through as many cycles as desired to yield highly specific, high affinity nucleic acid ligands to the target molecule.
- the SELEX method encompasses the identification of high-affinity nucleic acid ligands containing modified nucleotides conferring improved characteristics on the ligand, such as improved in vivo stability or improved delivery characteristics. Examples of such modifications include chemical substitutions at the ribose and/or phosphate and/or base substitutions.
- SELEX-identified nucleic acid ligands containing modified nucleotides are described in U.S. patent application Ser. No. 08/117,991, filed September 8, 1993, that describes oligonucleotides containing nucleotide derivatives chemically modified at the 5- and 2'-positions of pyrimidines.
- the SELEX method encompasses combining selected oligonucleotides with other selected oligonucleotides and non-oligonucleotide functional units as described in U.S. patent applications Ser. No. 08/284,063, filed August 2, 1994, and Ser. No. 08/234,997, filed April 28, 1994, respectively. These applications allow the combination of the broad array of shapes and other properties, and the efficient amplification and replication properties, of oligonucleotides with the desirable properties of other molecules.
- the SELEX process may be defined by the following series of steps :
- a candidate mixture of nucleic acids of differing sequence is prepared.
- the candidate mixture generally includes regions of fixed sequences (i.e., each of the members of the candidate mixture contains the same sequences in the same location) and regions of randomized sequences.
- the fixed sequence regions are selected either: (a) to assist in the amplification steps
- the randomized sequences can be totally randomized (i.e., the probability of finding a base at any position being one in four) or only partially randomized (e.g., the probability of finding a base at any location can be selected at any level between 0 and
- the candidate mixture is contacted with the selected target under conditions favorable for binding between the target and members of the candidate mixture. Under these circumstances, the interaction between the target and the nucleic acids of the candidate mixture can be considered as forming nucleic acid-target pairs between the target and those nucleic acids having the strongest affinity for the target.
- nucleic acids with the highest affinity for the target are partitioned from those nucleic acids with lesser affinity to the target. Because only an extremely small number of sequences (and possibly only one molecule of nucleic acid) corresponding to the highest affinity nucleic acids exist in the candidate mixture, it is generally desirable to set the partitioning criteria so that a significant amount of the nucleic acids in the candidate mixture (approximately 5-50%) are retained during partitioning.
- nucleic acids selected during partitioning as having the relatively higher affinity to the target are then amplified to create a new candidate mixture that is enriched in nucleic acids having a relatively higher affinity for the target.
- the newly formed candidate mixture contains fewer and fewer unique sequences, and the average degree of affinity of the nucleic acids to the target will generally increase.
- the SELEX process will yield a candidate mixture containing one or a small number of unique nucleic acids representing those nucleic acids from the original candidate mixture having the highest affinity to the target molecule.
- the SELEX patents and applications describe and elaborate on this process in great detail. Included are targets that can be used in the process; methods for partitioning nucleic acids within a candidate mixture; and methods for amplifying partitioned nucleic acids to generate enriched candidate mixture.
- the SELEX patents and applications also describe ligands obtained to a number of target species, including both protein targets where the protein is and is not a nucleic acid binding protein. Therefore, the SELEX process can be used to provide high affinity ligands of a target molecule.
- Target molecules are preferably proteins, but can also include among others carbohydrates, peptidoglycans and a variety of small molecules.
- nucleic acid antibodies oligonucleotide ligands
- Oligonucleotide ligands can be employed to target biological structures, such as cell surfaces or viruses, through specific interaction with a molecule that is an integral part of that biological structure. Oligonucleotide ligands are advantageous in that they are not limited by self tolerance, as are conventional antibodies. Also nucleic acid antibodies do not require animals or cell cultures for synthesis or production, since SELEX is a wholly in vitro process.
- nucleic acids can bind to complementary nucleic acid sequences.
- nucleic acids This property of nucleic acids has been extensively utilized for the detection, quantitation and isolation of nucleic acid molecules.
- the methods of the present invention are not intended to encompass these well-known binding capabilities between nucleic acids.
- the methods of the present invention related to the use of nucleic acid antibodies are not intended to encompass known binding affinities between nucleic acid molecules.
- a number of proteins are known to function via binding to nucleic sequences, such as regulatory proteins which bind to nucleic acid operator sequences. The known ability of certain nucleic acid binding proteins to bind to their natural sites, for example, has been employed in the detection, quantitation, isolation and purification of such proteins.
- oligonucleotide ligands are not intended to encompass the known binding affinity between nucleic acid binding proteins and nucleic acid sequences to which they are known to bind.
- novel, non- naturally-occurring sequences which bind to the same nucleic acid binding proteins can be developed using SELEX.
- the oligonucleotide ligands of the present invention bind to such target molecules which comprise a three dimensional chemical structure, other than a polynucleotide that binds to said oligonucleotide ligand through a mechanism which predominantly depends on Watson/Crick base pairing or triple helix binding, wherein said oligonucleotide ligand is not a nucleic acid having the known physiological function of being bound by the target molecule.
- SELEX allows very rapid determination of nucleic acid sequences that will bind to a protein and, thus, can be readily employed to determine the structure of unknown operator and binding site sequences which sequences can then be .employed for applications as described herein.
- SELEX is thus a general method for use of nucleic acid molecules for the detection, quantitation, isolation and purification of proteins which are not known to bind nucleic acids.
- certain nucleic acid antibodies isolatable by SELEX can also be employed to affect the function, for example inhibit, enhance or activate the function, of specific target molecules or structures.
- nucleic acid antibodies can be employed to inhibit, enhance or activate the function of proteins.
- oligonucleotides used in the conjugates according to the invention are obtained in a preferred embodiment according to the process described below.
- Suitable oligonucleotides thus can be obtained in that a mixture of oligonucleotides, containing random sequences, is brought together with the target structure, certain oligonucleotides exhibiting an increased affinity to the target structure relative to the mixture of oligonucleotides, the latter being separated from the radical of the oligonucleotide mixture, then the oligonucleotides with increased affinity to the target structure being amplified to obtain a mixture of oligonucleotides, which exhibits an increased portion of oligonucleotides, which bond on the target structures.
- a DNA strand is first produced in a preferred way by chemical synthesis.
- This DNA strand has on the 3'-end a known sequence, which is used as a promoter for an RNA polymerase and at the same time is complementary to a primer sequence for the polymerase chain reaction (PCR).
- PCR polymerase chain reaction
- the promoter for the T7 RNA polymerase is involved.
- a random sequence is synthesized on the promoter.
- the random sequence can be obtained in that the four suitable bases are input in the same ratio in the synthesis machine. Completely random DNA sequences thus result.
- the length of the random sequence is about 15 to 100 nucleotides in the preferred embodiment.
- another DNA sequence is synthesized, which can be used for the polymerase chain reaction (PCR).
- RNA polymerase RNA polymerase
- those nucleotides that are modified are supplied to the RNA polymerase.
- the ribose is modified in 2'-position. In this case, a substitution of the hydrogen atom or of the hydroxyl group by an alkoxy group, preferably a methoxy group, an amino group or a fluorine atom can be involved.
- the RNA oligonucleotides produced in this way are then introduced into the selection process.
- Target structure is defined as a structure on which the oligonucleotide is to bond specifically and with high affinity.
- Such structures are, e.g., macromolecules, tissue structures of higher organisms such as animals or human, organs or parts of organs, cells, especially tumor cells or tumors.
- the target structure does not absolutely have to be present in pure form, it can also be present in a naturally occurring organ or on a cell surface. Stringency may applied to the selection process by the addition of polyamino (tRNA, heparin), plasma or whole blood to the SELEX reaction.
- polyamino tRNA, heparin
- an isolated protein is involved in this case, the latter can be bound on a solid phase, for example a filter.
- a solid phase for example a filter.
- an excess of target structure relative to the RNA mixture is used.
- the specific oligonucleotide molecules bond on the target structures, while the nonbound oligonucleotides are separated from the mixture, for example by washing.
- the oligonucleotide molecules are separated from the target molecules or removed by washing with suitable buffers or solvents.
- RNA oligonucleotide found is then transferred to the complementary DNA strand.
- RNA oligonucleotides amplified in this way are then transferred with the help of the RNA polymerase again to RNA oligonucleotides and the thus obtained RNA oligonucleotides can be used in another selection step (as described above).
- RNA oligonucleotides obtained in the second selection step, from the target molecules
- the latter are again transferred in DNA with the help of reverse transcriptase
- the thus obtained complementary DNA oligonucleotides are amplified with the help of the polymerase chain reaction and then transcribed with the help of the RNA polymerase again to RNA oligonucleotides, which are available for an additional selection step.
- the desired high specificities and high bonding affinities can be obtained if the selection steps are repeated several times. Rarely is the desired oligonucleotide sequence to be obtained already after one or two selection steps. As soon as the desired specificity and bonding affinity between target structure and oligonucleotide is obtained, the oligonucleotide(s) can be sequenced, by which the sequence of the specifically bonding oligonucleotides can be determined.
- the above-mentioned selection process can also be performed on purified target structures. But it is essential especially for the in vivo diagnosis that specificity of the oligonucleotide is given for the target structure in the living environment. Therefore, the selection processes can be performed also on cells or cell cultures, on tissues or tissue sections, on perfused organs and even on living organisms.
- the modified oligonucleotides can withstand the degradation by the almost omnipresent RNAs.
- the desired oligonucleotide sequences are themselves concentrated in the selection processes on living organisms, since appropriate naturally occurring oligonucleotides are catabolized by the RNAs.
- Ferrite M consists of a signal -transmitting metal oxide nucleus, which for stabilization is sheathed with a polysaccharide, preferably a dextran (see, e.g., EP 0 186 616, Gries et al.).
- a polysaccharide preferably a dextran (see, e.g., EP 0 186 616, Gries et al.).
- the polysaccharide is
- ferrites in principle all microcrystalline metal oxides stabilized by coating agents, proposed for NMR diagnosis, are suitable, especially superparamagnetic materials, such as, for example, a magnetite AMI-25 designated as Endorem (R) (Guerbet Company, Paris), a magnetite designated as AMI-227 (Advanced Magnetics
- Ferrite particles, which are stabilized with glycosaminoglycans as coating agent are also suitable. Dextran-magnetites are especially preferred.
- the ferrites or the ferrite conjugates according to the invention have the value of nanoparticles, in which the metal oxide nucleus exhibits a diameter less than 30 nm, preferably less than 15 nm.
- the ferrites optionally can also be radioactive.
- isotopes there can be mentioned, for example: Fe-57, Ga-67, Tc-99m, In-111, 1-123, Ti-201 and Yb-169 for radiodiagnosis and Y-90 for radiotherapy.
- B means a bonding component X-Y-Z, in which X is a direct bond or a group -NH or -NR with R meaning a C1 to C4 alkyl chain, Y is a direct bond or a spacer and Z is a direct bond or a sulfur atom, L means a linker and
- A means a carbonyl group bound on the terminal 3 - ' or 5 ' -position
- the linkage of group A with oligonucleotide N takes place on an oligonucleotide radical reduced on the terminal 3'-end or 5'-end by a hydroxyl group or on an oligonucleotide radical reduced in 4'-position by a hydroxy- methyl group, the linkage of group X with ferrite M takes place on a ferrite derivative carrying functional groups.
- functional groups the formyl group, the carboxyl group, the glycidyl group and the amino group can be mentioned as examples.
- Y has the meaning of a spacer, it stands for a straight-chain or branched, saturated or unsaturated C1 to C200, preferably C1 to C50 alkylene chain, which optionally contains 1 to 10 imino, preferably 1 to 5 imino, 1 to 3 phenylene, 1 to 3 phenylenoxy, 1 to 10 amido, 1 to 2 hydrazido, 1 to 10 carbonyl, 1 succinimido, 1 6-ethylpyridin-2-yl groups, and which optionally is interrupted by 1 to 60 oxygen atoms and/or 1 to 5 sulfur atoms and which optionally is substituted by 1 to 5 hydroxy, 1 to 10 oxo, 1 to 3 carboxy, 1 to 5 carboxy-C1 to C4 alkyl, 1 to 3 hydroxy-C1 to C4 alkyl, 1 to 3 C1 to C7 alkoxy and/or 1 to 2 phenyl groups, which optionally are substituted by a carboxy, a cyano
- spacers Y As spacers Y, the following structures can be mentioned as examples:
- Linker L stands for a straight-chain or branched, saturated or unsaturated C1 to C20 alkylene chain, which optionally contains 1 to 3 imino, 1 to 3 oxo, a phenylene or a phenylenoxy group and optionally is interrupted by 1 to 6 oxygen atoms and/or 1 to 3 sulfur acorns.
- the invention relates to a process for the production of the conjugates according to the invention.
- PN means an oligonucleotide bonding specifically with high affinity to target structures, containing modifications significantly limiting the degradation by naturally occurring nucleases, reduced by one hydroxyl group in the terminal
- B means a bonding component X-Y-Z, in which X means a direct bond or a group -NH or -NR with R meaning a C1 to C4 alkylene chain,
- Y means a direct bond or a spacer and Z is a direct bond or a sulfur atom
- L means a linker
- A means a group bound on the terminal 3'- or 5' -position
- M means an optionally radioactively-labeled ferrite coated with a polysaccharide and chemically modified
- n means a number between 1 and 100
- E 1 means a leaving group such as F, Br, Cl, OTs or OMes and
- Y' has the same meaning as Y or
- E 2 means an acceptor group, such as
- Y' means spacer Y reduced by E 2 -H
- R 1 stands for hydrogen, C1 to C10 alkyl or for phenyl optionally substituted with -OCH 3 , -CN,
- E stands for a functional group E 3 means a formyl, carboxyl, carboxylalkyl or glycidyl group
- reaction cited under a) takes place in aqueous medium at an alkaline pH, preferably at 8 to 9, at room temperature under protective gas, preferably under argon.
- X stands for -NH or -NR with R in the above-mentioned meaning and Y and E have the above-mentioned meanings,
- G represents a protective group familiar to one skilled in the art, such as, for example, a tert- butyl-oxycarbonyl, an Fmoc or a trifluoroacetyl group
- E 1 and R 1 have the above-mentioned meaning and Y 1 -NR-CO-CHR 1 stands for Y, or
- E 2 has the above-mentioned meaning and Y 1 -NR-CO- Y 2 stands for Y.
- the reactions take place in organic solvents, such as, for example, dichloromethane, dimethylformamide, tetrahydrofuran, dioxane or dichloromethane at temperatures between -5°C and 50°C, preferably at room temperature.
- organic solvents such as, for example, dichloromethane, dimethylformamide, tetrahydrofuran, dioxane or dichloromethane at temperatures between -5°C and 50°C, preferably at room temperature.
- the acid group is activated in a way known to one skilled in the art, for example, by boiling with thionyl chloride as acid chloride; also, mixed anhydrides are suitable [Krejcarek and Tucker, Biochem. Biophys.
- organic amines such as triethylamine, pyridine, N-ethylmorpholine, are used.
- ⁇ -halogen acids of formula VIIa are obtained by reacting the acids with elementary bromine according to the process of C.
- the partially protected amines of general formula VI can be obtained from the commercially available compounds of general formula X
- X and Y 1 have the above-mentioned meaning, e.g., by reaction with di-t-butyl pyrocarbonate [HoppeSeyler's Z. Physiol. Chem., 357, 1651 (1976)].
- the compounds of general formula III are obtained according to standard methods of nucleotide chemistry (Oligonucleotides and Analogues, A Practical Approach, Ed. F. Eckstein, Oxford University Press, Oxford, New York, Tokyo, 1991) from the partially protected polynucleotide, which has a free 3'- or 5'-position, by reaction with suitable linker precursors.
- the 5'-[6- mercaptohexylphosphoric acid ester] of the oligonucleotide is obtained by a condensation with ⁇ -cyanoethyl-N,N- diisopropylamino-S-trityl-6-mercapto-phosphoramidite, subsequent oxidation of the formed phosphite with iodine to the phosphotriester and downstream hydrolysis with ammonia solution to the S-trityl derivative of the target compound.
- the free SH compound is obtained by hydrolysis of the trityl compound with silver nitrate solution.
- 2-mercaptopyridyl group and the mercaptomethyl group are suitable as sulfur protective groups, which can be cleaved reductively (see T. W. Greene, loc. cit.).
- X stands for a group -NH or -NR with R in the above-mentioned meaning
- L, A and N have the above-mentioned meaning
- a and PN have the above-mentioned meaning, and L' means radical L reduced by a methylene group, and the reaction preferably is performed in aqueous medium at neutral pH, at room temperature and under protective gas.
- Suitable additives are, for example, physiologically harmless buffers (such as, e.g., sodium citrate) or electrolytes, such as, e.g., sodium chloride or antioxidants, such as, e.g., ascorbic acid or mannitol (or other osmotically active substances) or stabilizers, such as, e.g., sodium lactate.
- physiologically harmless buffers such as, e.g., sodium citrate
- electrolytes such as, e.g., sodium chloride or antioxidants, such as, e.g., ascorbic acid or mannitol (or other osmotically active substances)
- stabilizers such as, e.g., sodium lactate.
- the pharmaceutical agents according to the invention contain preferably 0.1 ⁇ mol/1 to 0.1 mmol/1 of the oligonucleotide conjugates according to the invention and are generally administered in amounts of 0.01 nmol/kg to 60 ⁇ mol/kg, preferably 5 to 20 ⁇ mol of metal/kg. They are intended for enteral and parenteral administration.
- nanoparticles In the preparations of nanoparticles, low-viscosity aqueous colloidal solutions or suspensions of metal oxides containing stabilized particles in the nanometer range are involved.
- the solutions of nanoparticles do not contain any substantial aggregates, so that requirements of international pharmacopoeia on parenteralia with respect to the particle size are met .
- the solutions or suspensions are colored reddishbrown to black, which is attributable to the intensive colors of crystals containing iron.
- the pronounced inherent colors can be used for visual detection, e.g., as a marker substance in surgical medicine.
- the nanoparticles are superparamagnetic or contain superparamagnetic portions. The particles physically show very high saturated magnetizations, which are achieved even in the case of low applied field strengths and, after turning off an external magnet, exhibit no more residual magnetization, they show no remanence.
- the nanoparticles are formulated as solutions (suspensions) and can be administered without further preparation. Since the solutions of the nanoparticles are compatible with usual medicinal solvents, such as physiological sodium chloride solution, electrolyte solutions or sugar solutions, the particles can be" diluted at will and are also infused, e.g., for special applications.
- This invention further relates to a process for detecting target structures. In this case, one or more of the above-described compounds are brought together with the samples to be examined in vivo or in vitro. In this case, the oligonucleotide bonds specifically and with high bonding affinity to the target structure to be detected.
- the target structure If the target structure is present in the sample, it can be detected there based on the signal.
- the process is especially suitable for a noninvasive diagnosis of diseases.
- one or more of the above- described compounds, preferably labeled with radioisotopes, are administered in vivo. Based on the signal, it can be detected whether the target structure, on which the oligonucleotide bonds specifically and with high affinity, is present in the organism to be examined.
- the conjugates and agents according to the invention meet the varied requirements, which are to be imposed on a diagnostic agent. They are distinguished especially by a high specificity or affinity relative to the target structures in question. Relative to known oligonucleotide conjugates, the conjugates according to the invention exhibit an especially high in vivo stability. This was achieved by a substitution of the 2'-hydroxy group. Surprisingly, the specificity of the oligonucleotide is significantly impaired neither by this modification, nor by the coupling with the ferrite. Other advantages are the controllable pharmacokinetics as well as the low dosage that is advantageous with respect to the compatibility.
- oligonucleotide components Depending on the properties of the oligonucleotide components, many areas of use arise for special indications such as the MR-lymphography after intravenous or local interstitial administration, the tumor visualization, the visualization of functions or disturbed functions, the plaque visualization (atherosclerotic imaging), the visualization of clots and vessel occlusions, MR angiography, perfusion tests, the visualization of infarctions, the visualization of endothelial impairments, receptor imaging, the visualization of the integrity of the blood-brain barrier, etc. and for the differential diagnosis, especially for distinguishing tumors/metastases and hyperplastic tissue.
- the MR-lymphography after intravenous or local interstitial administration
- the tumor visualization the visualization of functions or disturbed functions
- the plaque visualization the visualization of clots and vessel occlusions
- MR angiography perfusion tests
- the visualization of infarctions the visualization of endothelial impairments
- receptor imaging the visualization of the integrity of the
- 29.24 g (100 mmol) of the amine produced under la) is dissolved in 300 ml of dichloromethane. It is mixed with 10.12 g (100 mmol) of triethylamine and then, 23.35 g (100 mmol) of 2-bromo-2-phenyl-acetyl chloride, dissolved in 30 ml of dichloromethane, is instilled in it with stirring and cooling with ice water. After stirring overnight, it is poured into ice water, the organic solution is separated, it is washed quickly with cold 2 N hydrochloric acid, then with saturated sodium bicarbonate solution, it is dried on sodium sulfate and evaporated to dryness in a vacuum. The title compound is obtained as oil.
- the oxidation of the formed phosphite to the completely protected phosphotriester takes place with iodine in tetrahydrofuran. Then, the column is washed in succession with methanol and water. To remove the modified oligonucleotide from the solid support, the content of the column is transferred to a multivial, mixed with 5 ml of 30% ammonia solution, the vessel is sealed and shaken overnight at 55°C. It is then cooled to 0°C, centrifuged, the support is washed with 5 ml of water and the combined aqueous phases are subjected to a freeze- drying.
- the solid material is taken up in 2 ml of water, mixed with 2 ml of 0.5 M ammonium acetate solution, then with 10 ml of ethanol. It is allowed to stand overnight at -20°C, centrifuged, the residue is washed with 1 ml of ethanol (-20°C) and finally dried in a vacuum at room temperature. 9 mg of the S-tritylated title compound is obtained.
- the product is dissolved in 0.5 ml of water, mixed with 0.1 ml of 1 M silver nitrate solution and stirred for 1 hour at room temperature. Then, it is mixed with 0.1 ml of 1 M dithiothreitol solution. After 15 minutes, it is centrifuged and the supernatant solution is extracted several times with ethyl acetate.
- the column is reacted with an acetonitrile solution of 50 ⁇ mol of ⁇ -cyanoethyl- N,N-diisopropylamino-6-(trifluoroacetamido)-1-hexyl-phosphoramidite (produced according to Nucl. Acids. Res. 16, 2659-2669 (1988)) in the presence of tetrazole.
- the oxidation of the formed phosphite to the completely protected phosphotriester takes place with iodine in tetrahydrofuran.
- the column is washed in succession with methanol and water.
- the content of the column is transferred to a multivial, mixed with 5 ml of 30% ammonia solution, the vessel is sealed and shaken overnight at 55°C. It is then cooled to 0°C, centrifuged, the support is washed with 5 ml of water and the combined aqueous phases are subjected to a freeze-drying.
- the solid material is taken up in 2 ml of water, mixed with 2 ml of 0.5 M ammonium acetate solution and mixed with 10 ml of ethanol, it is allowed to standovernight at -20°C, centrifuged, the residue is washed with 1 ml of ethanol (-20°C) and finally dried in a vacuum at room temperature. 8 mg of the title compound is obtained as colorless powder.
- WO 94/03501 108 mg of iron; 108 mg of carboxydextran
- Amicon Diaflo stirring cell with a membrane filter (cutoff 30 kDa) and ultrafil- tered.
- the retentate is again filled up with bidistilled water and the ultrafiltration is continued.
- the retentate is obtained and diluted to 20.0 ml with bidistilled water.
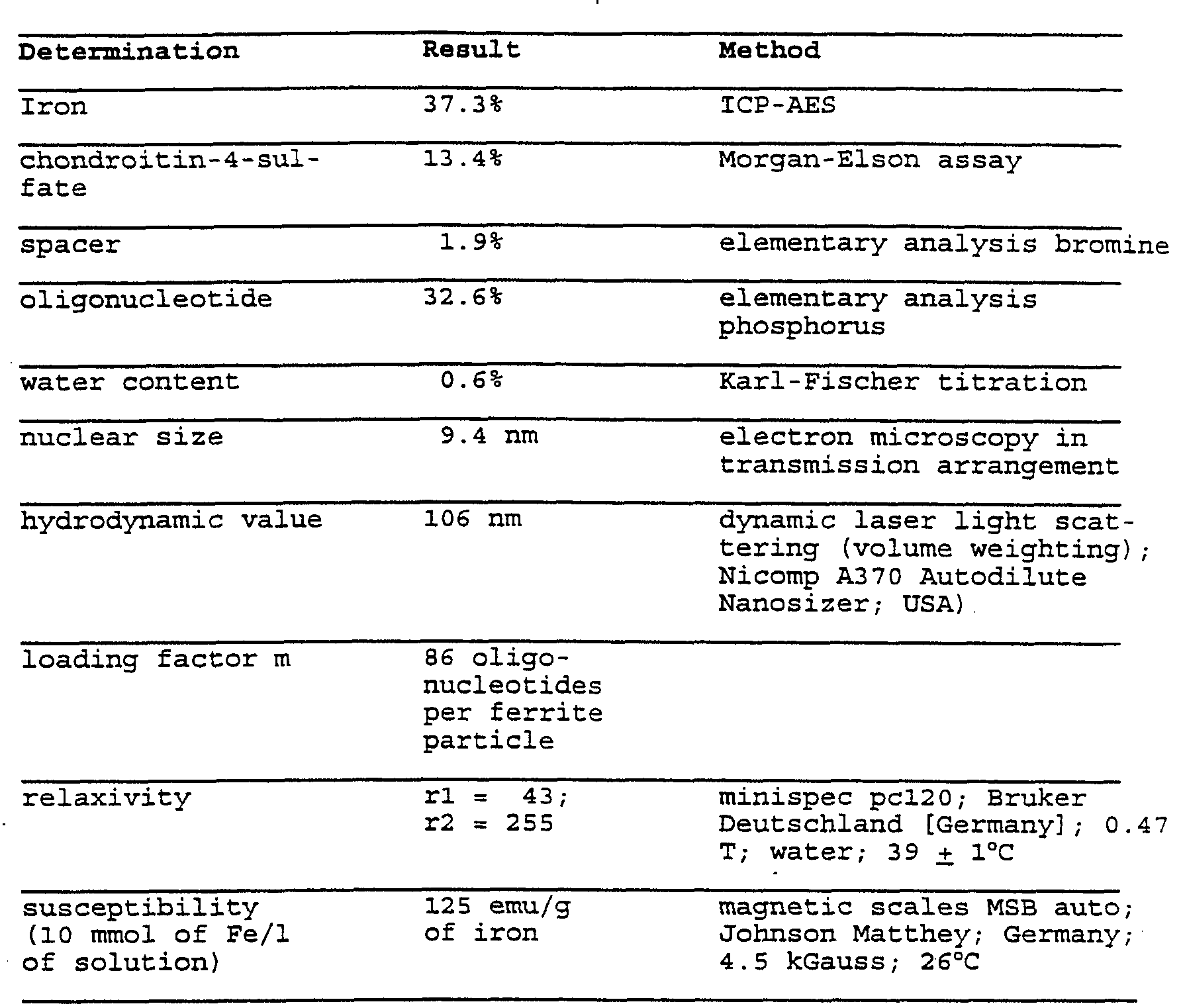
- the iron determination with ICP-AES inductively coupled plasma atomic emission spectroscopy produces an iron content of 5.4 mg/ml and the carboxydextran concent was determined as 1.6 mg/ml (photometric determination with anthrone, Dische Z, in Whistler and Wolfrom, Mechods in Carbohydrate Chemistry I, Academic Press, New York, London, 490-491, 1962).
- ICP-AES inductively coupled plasma atomic emission spectroscopy
- Diaflo stirring cell until iodate can no longer be determined in the filtrate (detection with aqueous silver nitrate solution, Jander, G. and Blasius, E., Lehrbuch der analytician und praparativen anorganischen Chemie [Textbook of Analytical and Preparative Inorganic Chemistry], S. Hirzel Verlag Stuttgart, 161, 1979).
- the generated aldehydes were determined with hydroxylamine
- the activated ferrite is mixed with a 5-fold excess
- Example A (69.2 mg) and stirred for 30 minutes.
- the purification of nonbound spacer takes place by an Amicon
- Diaflo stirring cell with a cutoff 30 kDa membrane Diaflo stirring cell with a cutoff 30 kDa membrane.
- the retentate is again filled up right through the middle with water and the purification is continued as long as the conductivity in the filtrate is less than 10 ⁇ S/cm.
- the filtrates are combined and the nonbound spacers are determined by the determination of the bromine content (elementary analysis).
- the portion of bound spacer is produced by the difference in the amount used and was determined as 13.8 mg.
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under protective gas argon mixed with a 2-fold excess (relative to the spacer content) of the oligonucleotide according to Example C (711 mg).
- the reaction solution is stirred for 3 days and nonbound oligonucleotide is then separated by filtration on Amicon Diaflo stirring cells with a cutoff 30 kDa filter and recovered.
- the purification is completed when the extinction in the filtrate (260 nm; reference bidistilled water) can no longer be measured (OD ⁇ 0.002 AU).
- the determination of the bound oligonucleotide takes place destructively afcer conversion of the phosphoric acid ester to inorganic phosphate (heating in sulfuric acid) and yields a bound portion of a total of 355.6 mg of oligonucleotide.
- the oligonucleotide-ferrite solution is then filtered by a membrane filter with 0.22 ⁇ m pore size and freeza-dried.
- Example 1 After the reaction and purification as in Example1), a CHO content of 0.014 mmol is produced.
- the activated ferrite is mixed with a 5-fold excess (relative to the aldehyde groups) of spacer according to Example B (40.5 mg) and treated as in Example 1.
- the portion of bound spacer arises by the difference in the amount used and was determined as 8.1.
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2-fold excess (relative to the spacer content) of the oligonucleotide according to Example C) (283 mg) and reacted with the oligonucleotide analogously to Example 1).
- the bound portion of the oligonucleotide was determined as 141.7 mg.
- the oligonucleotide- ferrite solution is then filtered by membrane filter with 0.22 ⁇ m pore size and freeze-dried.
- the activated ferrite is mixed with a 5-fold excess (relative to the aldehyde groups) of spacer according to Example A (30 mg) and treated as in Example 1.
- the portion of bound spacer is produced by the difference in the amount used and was determined as 6 mg.
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2-fold excess (relative to the spacer content) of the oligonucleotide according to Example C (308.5 mg) and reacted with the oligonucleotide analogously to Example 1.
- the bound portion of oligonucleotide was determined as 154 mg.
- the oligonucleotide ferrite solution is then filtered via membrane filter with 0.22 ⁇ m pore size and is freeze-dried. Yield : 331 mg of brownish-black powder with an uncharacteristic decomposition point
- the activated ferrite is mixed with a 5-fold excess (relative to the aldehyde groups) of spacer according to Example A (28.5 mg) and treated as in Example 1.
- the portion of bound spacer arises by the difference in the amount used and was determined as 5.7 mg .
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2 -fold excess (relative to the spacer content) of the oligonucleotide according to Example C (293.3 mg) and reacted with the oligonucleotide analogously to Example 1.
- the bound portion of the oligonucleotide was determined as 146.7 mg.
- the oligonucleotide-ferrite solution is then filtered via membrane filter with 0.22 ⁇ m pore size and freeze-dried.
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2-fold excess (relative to the spacer content) of the oligonucleotide according to Example C (46.8 mg) and reacted with the oligonucleotide analogously to Example 1.
- the bound portion of the oligonucleotide was determined as 23.4 mg.
- the oligonucleotide-ferrite solution is then filtered by membrane filter with 0.22 ⁇ m pore size and freeze-dried.
- the activated ferrite is mixed with a 5-fold excess (relative to the aldehyde groups) of spacer according to Example A (10.3 mg) and treated as in Example 1.
- the portion of bound spacer arises by the difference in the amount used and was determined as 2.0 mg.
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2- fold excess (relative to the spacer content) of the oligonucleotide according to Example C (105.5 mg) and reacted with the oligonucleotide analogously to Example 1.
- the bound portion of the oligonucleotide was determined as 53 mg.
- the oligonucleotide-ferrite solution is then fil tered via membrane filter with 0 . 22 ⁇ m pore size and freeee-dried .
- Example 1 1.0 ml of chondroitin-4-sulfate-ferrite according to EP 0,516,252, Example 1 (56 mg of iron, 24 mg of chondroitin-4-sulfate) is diluted with bidistilled water 1:10 and adjusted to pH 4.75 with 0.1 N HCl.
- the ferrite solution is mixed with 1.0 ml of a freshly-prepared solution of 9.2 mg of EDC [1-ethyl-3-(3-dimethylaminopropyl)carbodiimide HCl] in 10 ml of water and the pH is kept constant at 4.75 for two hours (titration system TPC 2000; Schott, Germany).
- the reaction of the EDC can be tracked by the acid consumption in the pH-STAT titration and is calculated as 2.4 ⁇ mol of activated acid.
- the separation of low-molecular reactants takes place by an Amicon Diaflo ultrafiltration with a cutoff of 30 kDa.
- the iron determination in the retentate with ICP-AES provides an iron content of 5 mg/ml and the chondroitin content was determined as 2.1 mg/ml (photometric determination with the hexosamine method according to Morgan-Elson, Morgan, W. T., and Elson, L. A., J. Biochem. 51, 1824, 1933).
- the activated ferrite is mixed with a 5-fold excess (relative to the activated acid groups) of spacer according to Example B (13.7 mg) and treated as in Example 1.
- the portion of bound spacer arises by the difference in the amount used and was determined as 2.7 mg.
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2-fold excess (relative to the spacer content) of the oligonucleotide according to Example C (96 mg) and reacted with the oligonucleotide analogously to Example 1.
- the bound portion of the oligonucleotide was determined as 46 mg.
- the oligonucleotide-ferrite solution is then filtered via membrane filter with 0.22 ⁇ m pore size and freeze-dried.
- Example 13 1.0 ml of chondroitin-4-sulfate-ferrite according to EP 0,516,252, Example 13 (22.4 mg of iron, 19.6 mg of chondroitin-4-sulfate) is diluted with bidistilled water 1:200 and diafiltered from bidistilled water in an Amicon ultrafiltration unit with an RS2000 tank and a hollow fiber membrane with a cutoff of 100 kDA to separate free coating polymer. Then, the solution is concentrated up to about 8 ml and then filled up to 10.0 ml with bidistilled water. For activation, the ferrite solution is adjusted to pH 4.75 with 0.1 N HCl.
- the ferrite solution is then mixed with 1.0 ml of a freshly-prepared solution of 4.2 mg of EDC [1-ethyl-3-(3-dimethylaminopropyl)carbodiimide HCl] in 10 ml of water and the pH is kept constant at 4.75 for two hours (titration system TPC 2000; Schott, Germany).
- the reaction of the EDC can be tracked by the acid consumption in the pH-STAT titration and is calculated at 2.2 ⁇ mol of activated acid.
- the separation of low-molecular reactants takes place by an Amicon Diaflo ultrafiltration with a cutoff of 30 kDa.
- the iron determination in the retentate with ICP-AES provides an iron content of 1.8 mg/ml and the chondroitin content was determined as 0.8 mg/ml (photometric determination of the hexosamine method according to Morgan-Elson).
- the activated ferrite is mixed with a 5-fold excess (relative to the aldehyde groups) of spacer according to Example A (4.3 mg) and treated as in Example 1.
- the portion of bound spacer arises by the difference in the amount used and was determined as 0.86 mg.
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2-fold excess (relative to the spacer content) of the oligonucleotide according to Example C (44 mg) and reacted with the oligonucleotide analogously to Example 1.
- the bound portion of the oligonucleotide was determined as 22 mg.
- the olig ⁇ nucleotide-ferrite solution is then filtered by membrane filter with 0.22 ⁇ m pore size and freeze-dried.
- the acid-substituted ferrite is mixed with the double volume of ethanol and the precipitate is centrifuged off (10 minutes, 1000 g). Then, the precipitate is resuspended again in 5 ml of bidistilled water, and pressed through a 0.22 ⁇ m cellulose acetate filter and then filled up with bidis tilled water at 5.0 ml.
- the degree of substitution of the carboxyldextran with the hexanoic acid the reaction was performed under the same conditions on pure stabilizer polymer (carboxydextran) and the content of acid groups was determined by potentiometric titration as 19.9 ⁇ 1.2%.
- ferrite solution 54 mg of iron, 48 mg of carboxydextran
- 1.0 ml of ferrite solution is diluted to 5 ml with bidistilled water and mixed with 103.8 mg of spacer according to Example A, and the mixture is then adjusted to pH 4.75 with 0.1 N HCl.
- the solution is mixed with 10.2 mg of EDC-HCl/1 ml of bidistilled water (freshly prepared), and the pH is kept constant at pH 4.75 for 2 hours by an automatic titration system (TPC 2000, Schott, Germany).
- TPC 2000, Schott, Germany automatic titration system
- the purification of low-molecular reactants and free, nonbound or adsorbed carboxydextran stabilizers takes place by dialysis (Visking dialyzer tube, Serva, Germany).
- the portion of bound spacer is produced by the difference in the amount used and was .determined as 20.5 mg.
- the ferrite solution is adjusted to pH 8.5 with 0.1
- the oligonucleotide-ferrite solution is then filtered via membrane filter with 0.22 ⁇ m pore size and freeze-dried.
- the precipitate is redispersed in 5 ml of water and pressed through a 0.22 ⁇ m cellulose acetate filter and ther. filled up to 5.0 ml with bidistilled water.
- the reaction was performed under the same conditions on pure stabilizer polymer (carboxydextran) and the content of acid groups was determined by potentiometric titration. One carboxymethyl group per glucose unit is produced on the average.
- Example A Example A, and the mixture is then adjusted to pH 4.75 with 0.1 N HCl.
- the solution is mixed with 10.2 mg of EDC-HCl/1 ml of bidistilled water (freshly prepared), and the pH is kept constant at pH 4.75 for 2 hours by an automatic titration system (TPC 2000, Schott, Germany).
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2-fold excess (relative to the spacer content) of the oligonucleotide according to Example C (1088.9 mg) and reacted with the oligonucleotide analogously to Example 1.
- the bound portion of the oligonucleotide was
- Example 1 After the reaction and purification as in Example 1, a CHO content of 0.011 mmol is produced.
- the activated ferrite is mixed with a 5- fold excess (relative to the aldehyde groups) of spacer according to Example A (20.5 mg) and treated as in Example 1.
- the portion of bound spacer is produced by the difference in the amount used and was determined as 4.1 mg.
- the ferrite solution is adjusted to pH 8.5 with 0.1 N NaOH and under argon as protective gas mixed with a 2-fold excess (relative to the spacer content) of the oligonucleotide according to Example D (211.1 mg) and reacted with the oligonucleotide analogously to Example 1.
- the bound portion of the oligonucleotide was determined as 105.6 mg.
- the oligonucleotide-ferrite solution is then filtered by membrane filter with 0.22 ⁇ m pore size anc freeze-dried.
- the yield is 94 mg of brownish-black powder with an uncharacteristic decomposition point.
- the solution After 30 minutes, it is mixed with 62 mg of sodium cyanoborohydride and the solution is purified after another 30 minutes by ultrafiltration. It is then adjusted to pH 8.5 by adding 0.1 N sodium hydroxide solution and reacted for 2 hours at room temperature under argon protective gassing with 3.5 mg of the 35-mer oligonucleotide obtained according to Example C. After another ultrafiltration, the solution is adjusted to a concentration of 20 mmol/1 of iron and then sterilized by filtration.
Abstract
Description
Claims
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| AU62224/96A AU6222496A (en) | 1995-06-07 | 1996-06-05 | Conjugates of ferrites and oligonucleotides, which bond specifically to certain target structures |
| JP9500149A JPH11507027A (en) | 1995-06-07 | 1996-06-05 | Ferrite and oligonucleotide conjugates that specifically bind to specific target structures |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US47611695A | 1995-06-07 | 1995-06-07 | |
| US08/476,116 | 1995-06-07 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO1996040273A2 true WO1996040273A2 (en) | 1996-12-19 |
| WO1996040273A3 WO1996040273A3 (en) | 1997-03-27 |
Family
ID=23890573
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/EP1996/002442 WO1996040273A2 (en) | 1995-06-07 | 1996-06-05 | Conjugates of ferrites and oligonucleotides, which bond specifically to certain target structures |
Country Status (4)
| Country | Link |
|---|---|
| JP (1) | JPH11507027A (en) |
| AU (1) | AU6222496A (en) |
| WO (1) | WO1996040273A2 (en) |
| ZA (1) | ZA964871B (en) |
Cited By (3)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP2054894A2 (en) * | 2006-08-11 | 2009-05-06 | MDS (Canada) Inc. | Composition apparatus and method for use in imaging |
| US8906345B2 (en) | 2006-09-20 | 2014-12-09 | Isis Innovation Limited | Multimeric particles |
| US9028845B2 (en) | 2001-06-21 | 2015-05-12 | Dynavax Technologies Corporation | Chimeric immunomodulatory compounds and methods of using the same-IV |
Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1986005815A1 (en) * | 1985-03-25 | 1986-10-09 | Genetics International Inc. | Nucleic acid sequences attached to materials sensitive to magnetic fields, and methods of assay and apparatus using such attached sequences. |
| EP0295965A2 (en) * | 1987-06-18 | 1988-12-21 | Amoco Corporation | Oscillator-based methods of detecting a member of a specific binding pair |
| US5160725A (en) * | 1987-03-24 | 1992-11-03 | Silica Gel Gesellschaft Mbh Adsorptions-Technik, Apparatebau | Magnetic liquid compositions |
| WO1993023570A1 (en) * | 1992-05-11 | 1993-11-25 | Pharmagenics, Inc. | Oligonucleotides having conjugates attached at the 2'-position of the sugar moiety |
| US5270163A (en) * | 1990-06-11 | 1993-12-14 | University Research Corporation | Methods for identifying nucleic acid ligands |
| WO1994001448A1 (en) * | 1992-07-06 | 1994-01-20 | Pharmagenics, Inc. | Oligonucleotides modified with conjugate groups |
| EP0640350A2 (en) * | 1991-09-16 | 1995-03-01 | Syngenix Limited | Ceramic particles and their preparation |
| DE4424922A1 (en) * | 1994-07-14 | 1996-01-18 | Schering Ag | Nuclease-resistant oligo:nucleotide conjugates |
| WO1996002669A1 (en) * | 1994-07-14 | 1996-02-01 | Schering Aktiengesellschaft | Conjugates of metal complexes and oligonucleotides, which specifically bond to specific target structures, agents containing these conjugates, their use in nmr diagnosis as well as process for their production |
| US5516670A (en) * | 1991-09-30 | 1996-05-14 | Kuehnle; Adelheid R. | Magnetophoretic particle delivery method and apparatus for the treatment of cells |
-
1996
- 1996-06-05 AU AU62224/96A patent/AU6222496A/en not_active Abandoned
- 1996-06-05 WO PCT/EP1996/002442 patent/WO1996040273A2/en active Application Filing
- 1996-06-05 JP JP9500149A patent/JPH11507027A/en active Pending
- 1996-06-07 ZA ZA9604871A patent/ZA964871B/en unknown
Patent Citations (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO1986005815A1 (en) * | 1985-03-25 | 1986-10-09 | Genetics International Inc. | Nucleic acid sequences attached to materials sensitive to magnetic fields, and methods of assay and apparatus using such attached sequences. |
| US5160725A (en) * | 1987-03-24 | 1992-11-03 | Silica Gel Gesellschaft Mbh Adsorptions-Technik, Apparatebau | Magnetic liquid compositions |
| EP0295965A2 (en) * | 1987-06-18 | 1988-12-21 | Amoco Corporation | Oscillator-based methods of detecting a member of a specific binding pair |
| US5270163A (en) * | 1990-06-11 | 1993-12-14 | University Research Corporation | Methods for identifying nucleic acid ligands |
| EP0640350A2 (en) * | 1991-09-16 | 1995-03-01 | Syngenix Limited | Ceramic particles and their preparation |
| US5516670A (en) * | 1991-09-30 | 1996-05-14 | Kuehnle; Adelheid R. | Magnetophoretic particle delivery method and apparatus for the treatment of cells |
| WO1993023570A1 (en) * | 1992-05-11 | 1993-11-25 | Pharmagenics, Inc. | Oligonucleotides having conjugates attached at the 2'-position of the sugar moiety |
| WO1994001448A1 (en) * | 1992-07-06 | 1994-01-20 | Pharmagenics, Inc. | Oligonucleotides modified with conjugate groups |
| DE4424922A1 (en) * | 1994-07-14 | 1996-01-18 | Schering Ag | Nuclease-resistant oligo:nucleotide conjugates |
| WO1996002669A1 (en) * | 1994-07-14 | 1996-02-01 | Schering Aktiengesellschaft | Conjugates of metal complexes and oligonucleotides, which specifically bond to specific target structures, agents containing these conjugates, their use in nmr diagnosis as well as process for their production |
Non-Patent Citations (6)
| Title |
|---|
| ANTICANCER DRUG DES, SEP 1996, VOL. 11, NO. 6, PAGE(S) 439-49, XP000615940 KAIREMO KJ ET AL: "Oligoradionuclidetherapy using radiolabelled antisense oligodeoxynucleotide phosphorothioates." * |
| ANTISENSE RES DEV, FALL 1992, VOL. 2, NO. 3, PAGE(S) 223-33, XP000615946 IVERSEN PL ET AL: "Binding of antisense phosphorothioate oligonucleotides to murine lymphocytes is lineage specific and inducible." * |
| ANTISENSE RES DEV, WINTER 1994, VOL. 4, NO. 4, PAGE(S) 285-9, XP002024861 PIRRUCCELLO SJ ET AL: "HIV-1 rev antisense phosphorothioate oligonucleotide binding to human mononuclear cells is cell type specific and inducible." * |
| BIOCONJUG CHEM, JUL-AUG 1991, VOL. 2, NO. 4, PAGE(S) 195-200, XP002024862 DEWANJEE MK ET AL: "Development of sensitive radioiodinated anti-sense oligonucleotide probes by conjugation technique." * |
| J NUCL MED, APR 1996, VOL. 37, NO. 4 SUPPL, PAGE(S) 3S-6S, XP002024863 O'DONOGHUE JA: "Strategies for selective targeting of Auger electron emitters to tumor cells." * |
| JPN. J. CANCER CHEMOTHER., 1994, VOL. 21, NO. 3, PAGE(S) 320-324, XP000615980 TAKAKURA Y. ET AL: "Control of in vivo characteristics of gene and antisense DNA disposition" * |
Cited By (5)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9028845B2 (en) | 2001-06-21 | 2015-05-12 | Dynavax Technologies Corporation | Chimeric immunomodulatory compounds and methods of using the same-IV |
| EP2054894A2 (en) * | 2006-08-11 | 2009-05-06 | MDS (Canada) Inc. | Composition apparatus and method for use in imaging |
| EP2054894A4 (en) * | 2006-08-11 | 2012-11-21 | Nordion Canada Inc | Composition apparatus and method for use in imaging |
| EP3355314A1 (en) * | 2006-08-11 | 2018-08-01 | Biocompatibles Uk Ltd. | Composition apparatus and method for use in pet imaging |
| US8906345B2 (en) | 2006-09-20 | 2014-12-09 | Isis Innovation Limited | Multimeric particles |
Also Published As
| Publication number | Publication date |
|---|---|
| ZA964871B (en) | 1997-02-06 |
| AU6222496A (en) | 1996-12-30 |
| JPH11507027A (en) | 1999-06-22 |
| WO1996040273A3 (en) | 1997-03-27 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| RU2081881C1 (en) | Macromolecular paramagnetic compound, method of synthesis of macromolecular paramagnetic compound, diagnostic contrasting agent, method of diagnostic contrasting agent preparing | |
| KR100605072B1 (en) | Tenascin-C Nucleic Acid Ligands | |
| JPH11506455A (en) | Magnetic resonance imaging agents for the detection of physiological substances | |
| IE76315B1 (en) | Contrast media synthesised from polyaldehydes | |
| JPS61155338A (en) | Diagnotic agent | |
| JP2003505111A5 (en) | ||
| CN101641366A (en) | Diagnostic and radiotherapeutic contrast agents and process for their preparation | |
| JP2009197024A (en) | Conjugate made of metal complex and oligonucleotide, medicine containing the conjugate, and their use in radiodiagnosis as well as method for their production | |
| WO1996040273A2 (en) | Conjugates of ferrites and oligonucleotides, which bond specifically to certain target structures | |
| EP1897562A1 (en) | Aptamers labelled with Gallium-68 | |
| US20020077306A1 (en) | Conjugates made of metal complexes and oligonucleotides, agents containing the conjugates, their use in radiodiagnosis as well as process for their production | |
| WO2009033876A1 (en) | Aptamers labeled with 18f | |
| US7368099B2 (en) | MRI contrast agents | |
| WO1996002669A1 (en) | Conjugates of metal complexes and oligonucleotides, which specifically bond to specific target structures, agents containing these conjugates, their use in nmr diagnosis as well as process for their production | |
| AU721330B2 (en) | Conjugates made of metal complexes and oligonucleotides | |
| US7005260B1 (en) | Tenascin-C nucleic acid ligands |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Kind code of ref document: A2 Designated state(s): AU BY CA CN CZ HU IL JP KR MX NO NZ PL RU SK UA VN |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A2 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LU MC NL PT SE |
|
| DFPE | Request for preliminary examination filed prior to expiration of 19th month from priority date (pct application filed before 20040101) | ||
| AK | Designated states |
Kind code of ref document: A3 Designated state(s): AU BY CA CN CZ HU IL JP KR MX NO NZ PL RU SK UA VN |
|
| AL | Designated countries for regional patents |
Kind code of ref document: A3 Designated state(s): AT BE CH DE DK ES FI FR GB GR IE IT LU MC NL PT SE |
|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application | ||
| WWE | Wipo information: entry into national phase |
Ref document number: 1996920793 Country of ref document: EP |
|
| ENP | Entry into the national phase |
Ref country code: JP Ref document number: 1997 500149 Kind code of ref document: A Format of ref document f/p: F |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1996920793 Country of ref document: EP |
|
| NENP | Non-entry into the national phase |
Ref country code: CA |
|
| 122 | Ep: pct application non-entry in european phase |