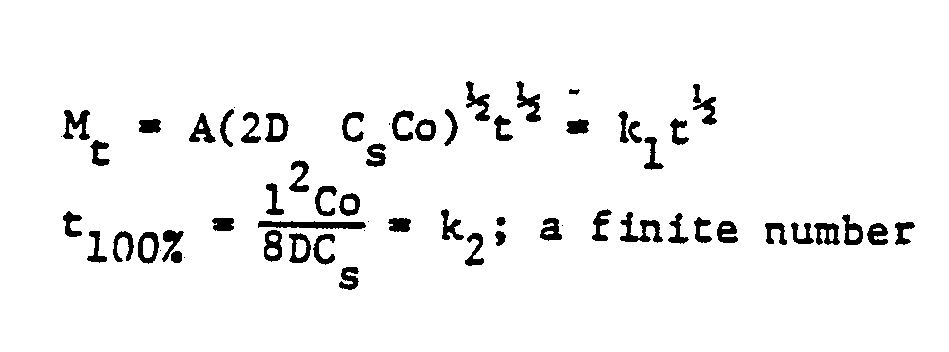WO1984004674A1 - Dry direct compression compositions for controlled release dosage forms - Google Patents
Dry direct compression compositions for controlled release dosage forms Download PDFInfo
- Publication number
- WO1984004674A1 WO1984004674A1 PCT/US1984/000807 US8400807W WO8404674A1 WO 1984004674 A1 WO1984004674 A1 WO 1984004674A1 US 8400807 W US8400807 W US 8400807W WO 8404674 A1 WO8404674 A1 WO 8404674A1
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- weight
- tablets
- parts
- tablet
- hydrophobic
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2013—Organic compounds, e.g. phospholipids, fats
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/205—Polysaccharides, e.g. alginate, gums; Cyclodextrin
- A61K9/2054—Cellulose; Cellulose derivatives, e.g. hydroxypropyl methylcellulose
Definitions
- This invention relates to compositions and processes for making suitable controlled release dosage compressed forms of biologically active agents such as drugs, nutrients, pesticides, other biocides and fragrances. More particularly, it relates to a dry direct compression binder from hydrophobic carbohydrate polymers and to a mixture of a hydrophobic carbohydrate polymer and at least one water insoluble or sparingly soluble component composition as a dry, directly compressible matrix for controlled release of agents, particularly drugs and nutritional supplements, into the gastrointestinal tract after oral ingestion of said composition or after implantation. DESCRIPTION OF THE PRIOR ART
- compositions in which a biologically active agent is held on an ion-exchange resin are provided.
- the use of a solvent is undesirable since the formulation can be easily tainted by a solvent residue which is not safe for human or animal ingestion, environmental pollution may attend the solvent removal step, occupational hazard may attend handling of the solvent and the biologically active agent may also be soluble in the solvent resulting in an undesirable dissolved system of release rather than the preferred dispersed controlled release system.
- composition adapted for controlling release of biologically active agents comprising and admixture of a fatty acid material or a neutral lipid, and preferably both, a wax and at least a binding amount of a hydrophobic cellulose derivative hereinafter defined as a hydrophobic carbohydrate polymer whereby said composition can be directly compressed in a dry state into tablet form having a hardness of 6 to 25 kg as measured on a Pfizer hardness tester.
- a controlled release formulation comprising from 0.01 to 95 wt. % of biologically active agent and from 5 to 99.9 wt. % of a controlled and continuous release binder admixture, said wt. % being based on the total weight of formulation and said admixture containing from 2 to 97 parts by weight of a fatty acid material or a neutral lipid and preferably both from 2 to 97 parts by weight of a wax preferably having a melting point between 60°C and 90oC and from 1 to 96 parts by weight of a hydrophobic cellulose derivative, i.e. a hydrophobic carbohydrate polymer.
- a fatty acid material particularly those of the class consisting of fatty acids having from 12 to 28 carbons, fatty monoalcohols having 12 to 28 carbons, fatty amines and amides having 12 to 28 carbons and mixtures thereof (e.g. an optimum mixture of 85 wt. % stearic acid and 15 wt.
- % palmitic acid or a neutral lipid, particularly of the class consisting of stearin, palmitin, castorwax, phospholipids, glycolipids, glycerides such as glyceryl monostearate and glyceryl distearate, hydrogenated cottonseed oil, hydrogenated tallow and metal salts of C 12 to C 28 fatty acids, optimally hydrogenated cottonseed oil, or a mixture of said fatty acid material and said neutral lipid; (b) a wax preferably having a melting point between 60oC and 90oC, particularly of the class consisting of carnauba wax, spermaceti, beeswax, candelilla wax, esparto and paraffins, optimally carnauba wax; and (c) a hydrophobic carbohydrate polymer, preferably of the class consisting of ethyl cellulose, propyl cellulose, cellulose acetate, cellulose acetate-butyrate, and cellulose acetate
- the percentage of the active and the percentage of the combination in said formulation are readily varied to modify the controlled release rate of the active agent from a few hours to several days.
- the combination can be used for controlled release solid dosage forms of any particulate agent, preferably particles of less than 20 mesh in size.
- hydrophobic carbohydrate polymer synergistically cooperates with the digestivedifficulty soluble controlled release component, particularly with the fatty acid, to surprisingly strengthen the tableted combination containing the particulate biologically active material by imparting increased vertical strength and enhanced resistance to delamination from an external force.
- the teachings herein now provide a controlled release dosage form that can be produced by a dry, direct compression process that overcomes the serious disadvantages of the prior art including the adverse and deleterious impact of solvents and/or heat which teachings result in compressed structures of surprisingly superior physical integrity and resistance to delamination.
- DETAILED DESCRIPTION OF THE INVENTION From the foregoing it must be evident that the invention herein relates to dry compressed products obtained from a directly compressible hydrophobic carbohydrate polymer and more preferably from a combination or an admixture of a hydrophobic carbohydrate polymer and various water insoluble ingredients which provide for controlled continuous release of the biologically active agent in the body when taken by mouth in tablet form or by implantation.
- the unique property provided by this admixture is one of dry compressibility so that useful tablets or implants can be readily produced in an inexpensive, facile, hazard free and environmentally safe manner. BIOLOGICALLY ACTIVE AGENTS
- the biologically active agents which can be admixed with the excipients to provide the controlled release tablets according to this invention include all substances which when introduced into the body of a human, animal, plants, soil and water is biologically active, usually in a therapeutic sense, nutritional purpose or biocidal effects.
- Such biologically active agents are: a. vitamins, minerals and other nutritional supplements including all of the water soluble vitamins including Vitamin C, B vitamins and choline, inositol, bioflavinoids, iron, selenium, para amino benzoic acid, iodine, zinc, 1-lysine, 1-glutamine, 1-cysteine, calcium , magnesium, potassium, etc. b. ANALGESIC drugs such as acetaminophen, aspirin, codeine, salicylamide propoxyphene, pentazocine HC1, malbuphine HC1, ibuprofen, indomethacin, meperidine HC1, morphine and oxaprozin.
- vitamins, minerals and other nutritional supplements including all of the water soluble vitamins including Vitamin C, B vitamins and choline, inositol, bioflavinoids, iron, selenium, para amino benzoic acid, iodine, zinc, 1-lysine, 1-glutamine, 1-cysteine, calcium
- ANOREXIC drugs such as amphetamines, phentermine, phenylpropanolamine and phenmetrazine.
- ANTHELMINTIC drugs such as peperazine citrate, pyrantel pamoate, thiabendazole, mebendazole, levamisole and their derivatives.
- ANTIASTHMA drugs such as terbutaline sulfate, isoetharine, theophylline and sodium glycinate.
- ANTIBACTERIAL drugs such as trimethoprim and sulfamethoxazole.
- ANTIBIOTIC and ANTIMICROBIAL drugs such as metronidazole, amoxicillin, erythromycin, ampicillin, penicillin, tetracycline, aminosalicylate, rifampin, cycloserine, amikacin, cefazolin, cephradine, cefaclor, cephalor idine, chloramphenicol, clindamycin, demeclocycline, kanamycin, cephaloridine, cefamandole nafate, cyclacillin, carbenicillin, vancomycin, cephradine, fluphenazine, hetacillin, streptomycin, ethambutol, methenamine, gentamicin and toburamicin.
- metronidazole amoxicillin, erythromycin, ampicillin, penicillin, tetracycline, aminosalicylate, rifampin, cycloserine, amikacin, cefazolin,
- ANTICEPTIC drugs such as nitrofurantoin and sulfonamides.
- ANTICOAGULANT drugs such as warfarin.
- ANTICONVULSANT drugs such as clonazepam, nalproic acid, phenytoin, diazepam and primidone.
- ANTIDEPRESSANT drugs such as trimipramine maleate, imipramine HC1 and imipramine pamoate.
- ANTIDIABETIC drugs such as chlorpropamide, acetohexamide, tolbytamide and tolazamide.
- ANTI-GOUT drugs such as probenecid, sul finpy razone and allopurinol.
- ANTIFUNGAL drugs such as griseofulvin, flucytosine, nystatin, clotrimazole and miconazole.
- ANTIHISTAMINE drugs such as triprolidine HC1, diphenhydramine HC1, chlorpheniramine maleate, brompheniramine maleate and hydroxyzine HC1.
- ANTI-INFLAMMATORY drugs such as phenylbutazones, steroids, sulfonamides and salicylates.
- ANTIMALARIAL drugs such as chloroquine phosphate, hydroxychloroquine sulfate and pyrimethamine.
- ANTIMIGRAINE drugs such as ergotamine tartrate, propranolol HC1, isometheptene and mucate.
- ANTIMOTION SICKNESS drugs such as dimenhydrinate.
- ANTINAUSEANT drugs such as hydroxyzine HCl, buclizine
- ANTINEOPLASTIC drugs such as tamoxifen citrate, mitotane, megestrol acetate, tetolacone, flurouracil, busulfar, chlorambucil, melphalan, amsacrine, streptozocin, anthracyline agents, azacitidine, bleomycide, vinca allkaloids, cytrarabine, hexamethylmelamine, methotrexate, hydroxyaren, chlorotriansene, cisplatin, cyclophosphamide, dacarbazine, dactinomycine, mithramycine, mitomycino, procarbazine, azathioprine, mercaptopurine, thioguanine and nitrosoureas.
- tamoxifen citrate such as tamoxifen citrate, mitotane, megestrol acetate, tetolacone, flurouracil
- COUGH & COLD PREPARATION drugs such as guaifenesin, promethazine HCl, benzonatate, noscapine and dextromethorphan HBr.
- DECONGESTANT drugs such as b rompheniramine maleate and phenylephrine HCl.
- DIURETIC drugs such as thiazides, acetazolamide, furosemide and triamterene.
- MUSCLE RELAXANT drugs such as dantrolene sodium, cyclobenzaprine, chlorzoxazone and quinine sulfate.
- PARASYMPATHOLYTIC drugs such as oxyphenomium bromide, atropine, hyoscyamine sulfate, glycopy rrolate and propantheline bromide.
- SEDATIVE drugs such as barbiturates, meprobamate, promethazine HCl and methaqualone.
- TRANQUILIZER drugs such as diazepam, chlorazepate monopotassium, prazepam, ch loridiazepoxide HCl and chloralhydrate.
- AGRICHEMICALS including herbicides such as 2,4-D and its derivatives, class of nitrobenzen amines, prometone, atrazine, simazine, trifluralin, picloram, lindane, batoxyethanolesters, dimethylamine, diquat silvex, tok, machete, lasso, avenge, prowl and their derivatives.
- ALGICIDES such as chlorine compounds, e.g. calcium hypochlorite, and their derivatives.
- ANTIFOULING AGENTS such as organotin compounds and ⁇ rganolead compounds.
- FUNGICIDES such as kitazin and their derivatives.
- INSECTICIDES such as DDUP, class of phosphoro thioate compounds, aldicarb, hexamethyl phosphoric triamide, malathion, parathion, pyrenium, sumithion, elsan, aldrin and their derivatives.
- MOLLUSCICIDE such as copper bis (tri-n-butyltin) oxide, niclosamide and N-tritylmorpholine.
- PHERMONES such as methyleugenol and grandlure.
- PLANT GROWTH REGULATOR such as gibberelline and auxin
- RODENTICIDES such as decarboximide and their derivatives.
- OTHER BIOCIDES m. FERTILIZERS such as urea and other mineral nutrients n. FLAVORS, FRAGRANCES, AND PERFUMES
- the biologically active particulate solids which preferably should be smaller than 10 mesh (U.S. sieve grade), optimally pass through a 20 mesh screen, ranges broadly in an amount of 0.01 to 95, preferably 0.1 to 90, wt. % of the total formulation compressed into the controlled continuous release tablets.
- the dry directly compressible hydrophobic carbohydrate polymer binder or the admixture which makes possible the products of the invention provides a matrix for the biologically active particulate agent which ranges in an amount of 5 to 99.99, preferably from 10 to 99.9 wt. % based upon the total weight of said tablets derived from said compressed formulation.
- the dry directly compressible hydrophobic carbohydrate polymer or the admixture can also include as desired: flow aid materials in an amount ranging from 0.5 to 2 wt . %, said flow materials being represented by finely divided silica and talc; and from 0.5 to 2 wt. % of a lubricating material to facilitate tablet ejection e.g. a metal salt of a fatty acid, preferably magnesium stearate.
- flow aid materials in an amount ranging from 0.5 to 2 wt . %, said flow materials being represented by finely divided silica and talc; and from 0.5 to 2 wt. % of a lubricating material to facilitate tablet ejection e.g. a metal salt of a fatty acid, preferably magnesium stearate.
- the combination of the invention which provides said matrix is either a dry directly compressible hydrophobic carbohydrate polymer or an admixture containing a hydrophobic carbohydrate polymer and a one to three digestive-difficulty soluble components readily produced by dry blending of powders smaller than 20 mesh, preferably 30 mesh, from the fatty acid material and/or the neutral lipid and/or said wax and the aforesaid hydrophobic carbohydrate polymer.
- the physiologically active particulate agent and additional excipients as desired are readily dispersed into the blended powders providing the matrix whereby the resultant formulation can be dry and direct compressed on a press under a pressure of 1.5 to 20, preferably 3 to 9, tons/square inch to produce the tablets.
- Tablets obtained from this teaching of the invention have a useful hardness, i.e. 3 to 25 kg as measured on a Pfizer hardness tester, to provide a commercially marketable product.
- the hydrophobic carbohydrate polymer constitutes from 1 to 100, preferably 3 to 50, optimally 5 to 30, wt. % of said matrix composition and provides said matrix composition with the integrity necessary to realize the binding of the controlled release tablets according to this invention.
- Preferred hydrophobic carbohydrate polymers are those of the class of hydrophobic cellulose derivatives in which the R-moiety of the cellulose-R or cellulose-ROH or other R derivative is either an aliphatic acyl group of 2 to 22 carbons or aliphatic alkyl of from 1 to 8 carbons and chitin.
- the most preferred hydrophobic carbohydrate polymers are ethyl cellulose, propyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate-butyrate, cellulose acetate propionate.
- the optimum hydrophobic carbohydrate polymer is ethyl cellulose having an ethoxy content of from 43-50%.
- hydrophobic carbohydrate polymer can be either singly used or in combination with a difficulty soluble component, i.e. any one or all of the components which are difficulty soluble in the digestive tract, i.e. wax, fatty acid and neutral lipid, which for purposes of this disclosure have been collectively designated the digestive-difficulty soluble component.
- a difficulty soluble component i.e. any one or all of the components which are difficulty soluble in the digestive tract, i.e. wax, fatty acid and neutral lipid, which for purposes of this disclosure have been collectively designated the digestive-difficulty soluble component.
- Each has the property of slowly dissolving or disintegrating in the digestive tract.
- the useful waxes are those which are obtained from plant and animal sources or as a petroleum product.
- the wax in this invention increases the hardness and compactness of the matrix, forming a cohesive hard tablet under the compressive forces of the process of this invention.
- the useful waxes have a melting point ranging from 50°C to 100°C and constitute from 0 to 99, preferably
- waxes are carnauba wax, spermaceti, beeswax, paraffin wax as well as synthetic waxes e.g. polyethylene.
- the optimum wax is carnauba wax.
- the fatty acid materials preferably along with the neutral lipid constitutes from 0 to 99, preferably 5 to 80, optimally 10 to 70, wt. % of the matrix composition of the invention which provides for controlled release of the active agent.
- Fatty acid materials assist in the control or regulation of the rate at which release of the active agent occurs and are generally characterized by having a melting point above 43°C.
- the fatty acid materials preferably are of the class consisting of: fatty acids having 12 to 28 carbons, e.g. stearic acid, palmitic acid, lauric acid, eleostearic acid, etc.; fatty alcohols having from 16 to 44 carbons, e.g.
- stearyl alcohol, palmitol, etc. a fatty amine having 13 to 45 carbons; and, a fatty amide having 11 to 45 carbons.
- a highly useful commercially available fatty acid material is Hystrene sold by Humko Sheffield (a division of Witco Chemical Co.) of Memphis, Tennessee which is a mixture of 85 wt. % stearic acid and 15 wt. % palmitic acid. 3.
- Humko Sheffield a division of Witco Chemical Co.
- the neutral lipid which can be used as an alternative to a fatty acid material, but preferably in combination therewith constitutes from 0 to 99, preferably 5 to 80, optimally 10 to 70, wt. % of said matrix admixture of this invention.
- the neutral lipid cooperates with the fatty acid material in the control of the rate at which release of the agent occurs and is characterized by having a melting point greater than 43°C.
- the neutral lipids are preferably of the class consisting of monoglyceride, diglyceride, triglyceride, phosphatides, glycolipids, steroids and neutral metal and organic salts of fatty acids having from 12 to 29 carbons.
- Representative examples of the preferred neutral lipids include stearin, palmitin, castor wax, lecithin, hydrogenated cottonseed oil, hydrogenated tallow, magnesium stearate and calcium and aluminum salts of palmitic and other fatty acids.
- a highly useful commercially available neutral lipid is hydrogenated cottonseed o i l wh ich h as b een ob t a in ed f r om : Humko Shef field as Neustrene; Capital City Products as Ditrex or Sterotex; and Durkee as Lubritab.
- the dry, direct compressed tablets and implants can be achieved by a composition adapted for controlling release of biologically active agents comprising an admixture of from 2 to 97 parts by weight of a fatty acid material or a neutral lipid and preferably both, from 2 to 97 parts by weight of a wax preferably having a melting point between 50°C and 100°C and from 1 to 96 parts by weight of a hydrophobic carbohydrate polymer.
- a controlled release tablet containing ascorbic acid as the biologically active agent was prepared as follows: 67.8 parts by weight of ascorbic acid having a particle size that passed through a 20 mesh screen was blended with 30.8 parts by weight of a mixture of 45.0 weight percent Hystrene, 24.4 weight percent Neustrene (a commercial product of hydrogenated cottonseed oil), 17.8 weight percent carnauba wax, 12.2 wt. % ethyl cellulose and 1.4 wt. % tableting excipients.
- the resulting blend was passed through a 20 mesh screen and compression molded at a pressure of 3 to 9 tons per square inch into oval tablets containing 1000 mg of ascorbic acid and having a total weight of about 1530 mg, a thickness of about 7 mm and a hardness of about 14 Kg (measures on Pfizer hardness tester).
- Table I The controlled continuous release of tableted formulations obtained in this example is shown by the data of Table I.
- a number of tablets were subjected to a test in which a single tablet was placed in a beaker containing 100 ml of water maintained at 37°C. After a period of time, the tablet was taken from the water, the porous outer matrix of the test tablet was rubbed off until a solid core was left for drying and then air dried on a filter paper overnight, the solid core was then weighed.
- Table I The consolidated results are hereinafter set forth in Table I.
- a controlled release tablet containing multi vitamins and minerals as biologicallya ctive agents was prepared as follows:
- the resulting blend was passed through a 20 mesh screen and compression molded at a pressure of 3 to 9 tons per square inch into oval tablets having a weight of 1550 mg, a thickness about 6 mm and hardness of 15 kg (measured on Pfizer hardness tester).
- the data of the several examples show that the disintegration hence the release of these nutrients are continuous upon time.
- the release pattern is parabolic i.e. the rate, faster in the beginning and slower at the later times, which is often desirable for quickly achieving the desired blood level with subsequent prophylactic level for a prolonged period.
- compositions containing ascorbic acid as the biologically active agent were prepared as follows: Ascorbic acid having a particle size that passed through a 20 mesh screen was blended with three different formulas as set forth below.
- the blends in parts by weight are as follows:
- Hystrene is a fatty acid mixture of 85 wt . % estearic acid and 15 wt. % palmitic acid sold by Humko Sheffield (a division of Witco Chemicals)
- Ethocel is ethoxylated cellulose commercially available from Dow Chemical Company of Midland, Michigan.
- Aerosil 200 is a fumed silica sold by DeGussa, Inc. of Teterboro, New Jersey.
- Example 12 and 13 i.e. the blends of invention showed excellent hardness and was clearly superior in enhanced resistance to delamination forces.
- the enhanced resistance to delamination makes possible ejection of the tablet from the tableting die cavity with reduced breaking of tablets arising from the delamination forces imposed on the tablet during ejection from the uneven walls of the cavity.
- composition of the invention provides useful controlled release of dosage forms of biologically active particulates.
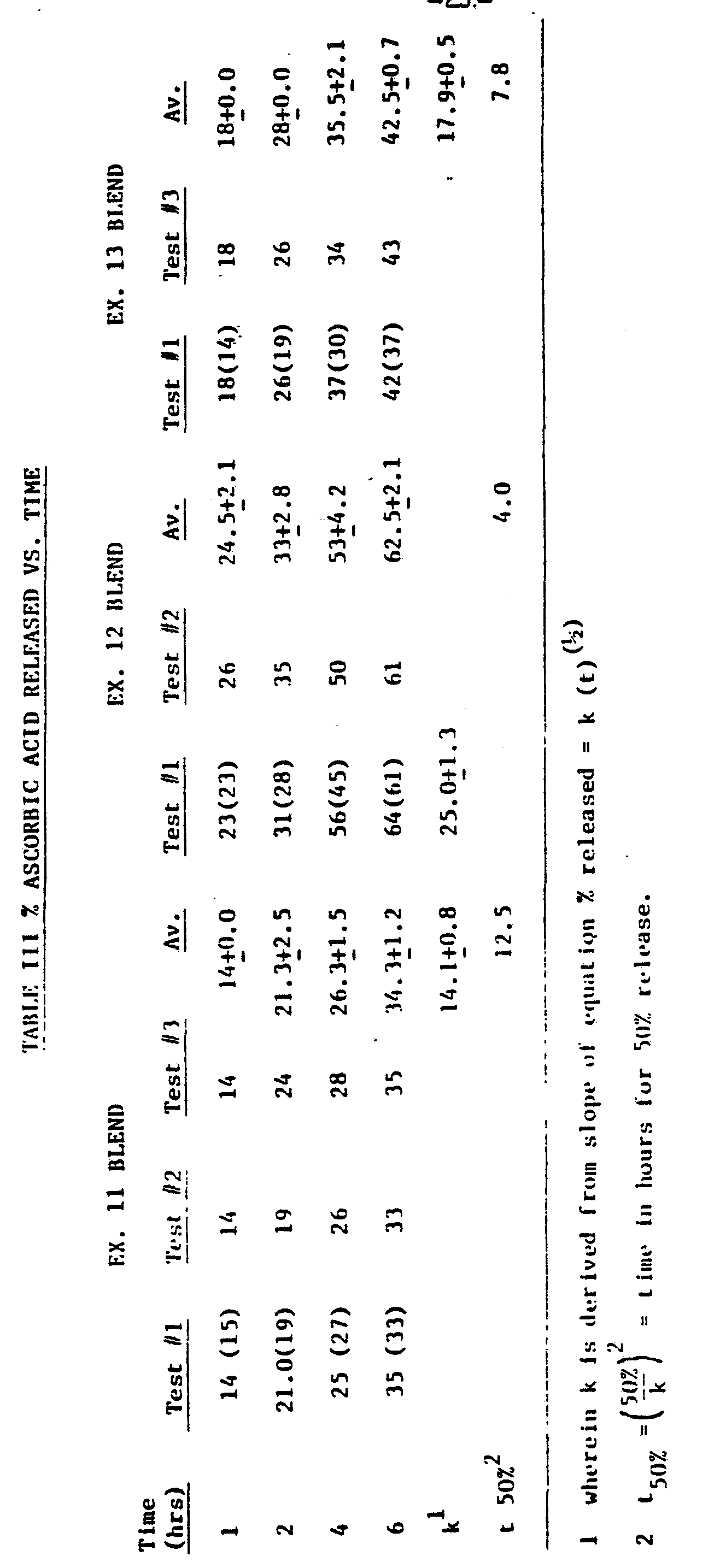
- the percent of ascorbic acid released was calculated based upon the total quantity of ascorbic acid per tablet. Duplicate or triplicate release tests were performed and the average values were calculated. Also the porous outer matrix of the tablets was rubbed off until a solid core was left for drying and then air dried on a filter paper overnight. The solid core was then weighed. The consolidated results are hereinafter set forth in Table III as percent released.
- % released k (t) 1/2 where k is a constant and t represents time in hours.
- fatty acids are the prolonged release rate controlling substance and the hydrophobic carbohydrate polymer, e.g. ethylcellulose, is poor for that purpose.
- ethy lcellulose is an excellent dry direct compression tableting binder for controlled slow release tablets and as shown in hardness data and breaking pattern (Example 14), the presence of ethyl cellulose in dry-direct compression compositions does improve markedly the Tablet integrity by reducing delamination during the tablet compression, henceforth, the commercial controlled release tablet production.
- the data of Example 15 shows that the slow dissolution, hence the release of biologically active particulates are, continuous upon time for a release admixture according to this invention while providing tablets of excellent strength and enhanced resistance to delamination.
- one or more of the matrix blend components as a coating on the biologically active material and/or an excipient in the practice of this invention, e.g. introduce the ethyl cellulose as a coating on either or both into the dry mix blending step.
- hydrophobic carbohydrate polymer as described herein is exemplified in preferred form by cellulose polymers wherein the hydroxyl or charged groups of the molecule are modified, i.e. derivatized, into hydrophobic groups by alkylation, acylation or similar processes.
- Other suitable (for the purposes of this invention) carbohydrate polymeric substances which can be similarly derivatized to provide the essential hydrophobic property are starch, dextran, gums, inulin, mucopolysaccharides and chitin.
- the latter carbohydrate polymeric substances are themselves sufficiently hydrophobic to be used without further derivat ization, e.g. chitin in its natural state possesses acetamido moieties which usefully modifies its cellulosic properties for use in matrix blends according to this invention.
- hydrophobic fumed silicas are well known commercially available materials, e.g. Aerosil ⁇ R-972 sold by Degussa, Inc. of Teterboro, N.3., Cabosil ⁇ N70-TS sold by Cabot Corp..of Tuscola, Illinois, Tullanox ⁇ 500 sold by Tulco, Inc., (all of which are preferred for use herein), of the general class of amorphous precipitated silicas but of the pyrogenic (fumed) type which provides according to Kirk-Othmer's Encyclopedia of Chemical Technology (Third Edition) Vol.20 at pages 768 and 778-779 an ultimate particle size, nm of 1-100 and an aggregate particle size, urn of 2-3.
- hydrophob icity of the fumed silica is achieved by replacement of the hydroxyl groups of the surface with OR 5 or OOR 5 wherein the R 5 moiety is 1 to 5 carbon aliphatic alkyl or aliphatic acyl groups.
- the most preferred hydrophobic silica is the methylated fumed silica. Hydrophobic silicas are discussed in Kirk-Othmer's Encyclopedia of Chemical Technology (Third Edition) Vol. 7 at pages 440-441.
- Cab-O-sil ⁇ N70TS is a hydrophobic fumed silica sold by Cabot Corporation, Tuscola, Illinois.
- Thickness (ins.) 0.284+0.002 0.281+0.002 0.272+0.001 0.274+0.003
- the release test data of the following Table V were obtained by analysis of the medium for ascorbic acid content. At each indicated time interval the release medium was replaced by fresh release medium and subjected to the analysis for Vitamin C content. Each of the release mediums were held at a conatant 37oC while exposed to the immersed tablet. Ascorbic acid release was analyzed by the USP XX methods. Release rates of tablets (av. of 4 to 6 tests) obtained from each of the blends of Exs. 16 through Ex. 19 are shown in Table V.
- hydrophobic fumed silica can be an effective dry controlled release agent when used in conjunction with other hydrophobic materials as taught herein.
- hydrophobic fumed silica is applicable to other known controlled release processes such as those approaches earlier discussed in this application and the dry direct compression process using a micropulverized lipid, e.g. as described in U.S. Patent 3,279,998 to produce tablets since its presence in said tablet in the amounts described herein will reduce the tendency of the tablet to desintegrate in water environments markedly reducing the dissolution rate.
- the 0.05 to 3 weight percent of hydrophobic fumed silica dispersed throughout the tablet as by blending the silica into the formulation prior to tableting provides lipid excipient controlled release tablets having better control of release, slower release if desired and improved physical integrity in aqueous environments such as found in the digestive tract.
- the present dry direct compression process and compositions are applied to pharmaceuticals to make compressed controlled release dosage forms.
- the formula is shown in the following Table VI. These blends of dry blend compositions are directly compressed in standard rotary tablet press as before.
- the resultant directly compressed controlled release pharmaceutical tablets are subjected to release for the three hours in water having a pH of 3.0 and thereafter the pH was adjusted to 7.0 with the water temperature held constant at 37oC.
- the amounds of the drug released were measured by Perkin Elmer Lamda 3A doublebeam UV Spectrophotometer.
- Table VII Table VII. k and t100% (time for 100% release) were calculated according to the equation set forth in Ex. 15.
- calcium hypochlorite tablets were prepared according to the present invention as follows. wt. %
- Tablets formed by the new dry direct compression process are very different from the tablets formed by known processes using solvents, heat or plastic polymers.
- the tablets of the invention can be distinguished from the products of known processes by the following criteria:
- the amounts of impurities in the tablets of the present process is only limited to the amounts of impurities present in raw material according to the supplier's specificat ions often by the certificates of analysis after dilution factors. For example, if ascorbic acid has 0.01% impurity and if the ascorbic acid content is 505 of the tablet weight then total percent of impurity for the tablet becomes 0.005%.
- the amounts of impurities in raw material drugs and food additives are limited by USP or Food Chemicals Codex specifications.
- Ranges of purity by assay are 99.05-100.05 (some examples: acetaminophen, 100.4%, procainamide HCl, 99.3%, ascorbic acid, 99.5%, lobeline sulfate, 100.3%, l-glutamine, 99.9%, pyridoxine HCl, 99.8%, etc.).
- Heat fusion and solvent solution of lipid or hydrophobic polymeric material both result in a more homogeneous liquid state. Cooling or solvent removal forms mass interlinked agglomerated skeletal structure with crystal or solid state structures changed from the original dry particle structures before the treatment.
- the thin cross sections of the tablets provided by the dry direct process of the invention shows clear cut boundaries between particles and crystals of composition components with the size and shapes the same or closely similar (allowing compression shrinkage of hydrophobic carbohydrate polymers) to the sizes and shapes of the precompression state.
- Those products using heat/solvent give fused together or glued together structures markedly different from the pretreatment state. More amorphous and less crystalline states are also detected by a polarizing microscope with far less clear boundary between excipients.
- the particle size after treatment usually depends on sieve sizes of the granulation processes used for prior art processes.
- Co-melting by heat and co-solubilization by solvents of the active and the controlled release material also changes crystal structure of the controlled release material by formation of a solid solution or a eutectic mixture.
- X-ray diffraction, electron microscope, polarizing microscope and thermal analysis techniques easily detect such changes.
- the present dry direct process preserves the physical characteristics of the active and controlled release material intact and native to precompression state when observed by these techniques.
- Electron and polarizing microscopic examinations of the tablet from the present dry direct process show even distribution of the active and lipid and hydrophobic carbohydrate polymer components in the matrix structure. Tablets of the prior art process using heat and solvent show that greater concentration of the controlled release material around the active component to be coated.
- Patents #3,965,256 and #3,317,394 will show that the lipid material coats the active component; and, U.S. Patents #4,167,558, #3,062,720, #3,362,880, #3,344,029 and #3,147,187 show that hydrophobic polymer coated the active agent forming completed or partially encapsulated forms.
- Such coating by lipid or hydrophobic polymers is not present in the tablet of the present process.
- the prior art process has two steps of first encapsulating the active and then dry mixing with other filler or lipid material, then by using differential solvents which dissolve only the second step material, one can isolate the active-encapsulating material ccibination and examine it by microscope or IR and thermal analysis technique.
- the tablets of the present process do not have such partially or completed encapsulated structure and with the above differential dissolving method the active agents and the hydrophobic material become physically separated.
- the tablets of the present dry direct process are a true matrix system where the active component particles are evenly and truly dispersed between dry direct composition components without solubiiizing into each other.
- tablets of the prior art processes due to heat fusion/solvent solubilization between the active and the controlled release material contain solid solutions and eutectic mixtures as explained before.
- the release profiles of the biologically active components in biological fluid medium, i.e. water or gastric juice are different since the products of the invention provide a dispersed system whereas the products of the prior art products provide a partially or completely dissolved system as illustrated by R. W. Baker and H. K. Lonsdale in p. 40.1 Controlled Release Symposium 1974 published by the University of Akron, Akron, Ohio.
- the release pro fi le can be presented as
- the release profile can be presented as
- U.S. Patents #3,317,394 and #3,432,592 use a single thermoplastic or injection moldable polymeric material.
- Many prior art processes such as in U.S. Patents #3,147,187, #3,976,256 and #3,302,880 use water swellable hydroxycellulose or hydrocolloid polymers as binders.
- Patent #4,167,558 uses a hydrocolloid as buoyant material. All these water swellable polymers can be identified by a standard analytical method.
- U.S. Patent #3,402,240 uses glucose, a simple sugar as a binder. The present process does not use such polymers or glucose as binder or buoyant material.
- U.S. Patent #3,279,998 uses dry direct compression process with only micropulverized lipid material without any hydrophobic carbohydrate polymer.
- the directly compressed tablets of U.S. Patent #3,577,514 using an enteric substance are easily distinguished from the tablets of the present process.
- the former does not release the biologically active component in the acid pH and release only in the medium of alkaline pH.
- the present process tablet releases the active continuously regardless of pH conditions. The former therefore can not be used as controlled release dosage forms.
- the present new process of direct compression with dry blend controlled release compositions without heat and solvent procedures produces very different controlled release tablets from those obtained from published processes.
- the product of the present process should be able to be easily distinguished in the market places from those of the prior art processes.
- Analytical tools and methods for differentiation are UV, IR, Fourier Transform IR, NMR, Solid State NMR Spectroscopics, X-ray diffraction, light, electron and polarized microscopy, mass spectroscopy, thermal analysis, gaa chromatography, high pressure liquid chromatography, other residue analysis methods and release test methods.
Abstract
The selection of: a hydrophobic carbohydrate polymer, e.g. ethyl cellulose; and, generally at least one digestive-difficulty soluble component, i.e., a wax, e.g. carnauba wax, fatty acid material or neutral lipid provides upon dry direct compression a controlled and continuous release matrix for tablets or implants of biologically active agents. Preferred for producing dry direct compressed products is the combination of: a hydrophobic cellulose derivative; a wax, and, a fatty acid material and/or a neutral lipid since it provides upon dry direct compression a controlled and continuous release tablet or implant of improved structurally integrity against externally imposed forces.
Description
DRY DIRECT COMPRESSION COMPOSITIONS FOR CONTROLLED RELEASE
DOSAGE FORMS
BACKGROUND OF THE INVENTION
This invention relates to compositions and processes for making suitable controlled release dosage compressed forms of biologically active agents such as drugs, nutrients, pesticides, other biocides and fragrances. More particularly, it relates to a dry direct compression binder from hydrophobic carbohydrate polymers and to a mixture of a hydrophobic carbohydrate polymer and at least one water insoluble or sparingly soluble component composition as a dry, directly compressible matrix for controlled release of agents, particularly drugs and nutritional supplements, into the gastrointestinal tract after oral ingestion of said composition or after implantation. DESCRIPTION OF THE PRIOR ART
There is a recognized need for controlled release dosage forms of biologically active agents for humans and animals having the property of being released within the gastro-intestinal tract over a defined period of time and at a pre-determined rate of release after introduction of the agent into the body, as by oral ingestion of a tablet containing said agent or by implantation.
Various types of controlled release compositions have been developed and/or commercialized and can be classified according to at least the following three categories: a. compositions in which granules or tablets containing a biologically active agent are coated with a water insoluble material, such as a wax or a synthetic resin (see U.S. 3,062,720); b. compositions in which a biologically active agent is dispersed within a melt of water insoluble material
(see U.S. 3,147,187) or is mixed with said water insoluble material and a water soluble material (see
U.S. 3,577,514);
c. compositions in which a biologically active agent is held on an ion-exchange resin.
There are many disadvantages however in the preparation of such controlled release compositions. The disadvantages are related to the fact that virtually all prior art compositions utilize a hydrophobic material to retard dissolution, disintegration or release of the biologically active agent. These hydrophobic materials are oftentimes hydrocarbons and their derivatives such as lipids, waxes, paraffins and hydrophobic polymers.
To retard the release of the biologically active agent by means of the hydrophobic material, virtually all these prior art compositions use heat or solvent to melt or dissolve the hydrophobic material and thereby achieve effective binding and desired release of the agent. Unfortunately the requisite use of heat or solvent results in related shortcomings, inconveniences and/or excessive costs of production. Attendant with the use of heat is: the risk that the biologically active agent may be harmed since many are unstable to heat; the fact that hot melting equipment is expensive and/or hazardous to operate; and, costly procedures of several additional processing steps.
Similarly, the use of a solvent is undesirable since the formulation can be easily tainted by a solvent residue which is not safe for human or animal ingestion, environmental pollution may attend the solvent removal step, occupational hazard may attend handling of the solvent and the biologically active agent may also be soluble in the solvent resulting in an undesirable dissolved system of release rather than the preferred dispersed controlled release system.
An approach to overcome the problems attendant with the heat or solvent processes would be to use a dry, direct compression process such as is taught in U.S. patent 3,279,998 wherein a blend of the biologically active agent and a micro-pulverized lipid, e.g. the fat, fatty acid, wax, etc., is compressed into tablets or in U.S. 3,577,514 wherein the biologically active agent is
blended with a mixture of wax, enteric substance (acid-insoluble release agent) and water-soluble or dispersible binder. The requisite micropulverization of the lipid results in compression/adhesion difficulties for the former dry process whereas the latter process does not provide a system for controlled continuous release but rather a delayed release until an alkalin pH is reached.
Another approach to controlled release formulations has been to utilize physiologically tolerable synthetic resins having the property of flowing under high pressures to encapsulate the medicament (see U.S. patent
3,096,248) or to provide a skeletal matrix for tablet integrity during medicament release (see U.S. 3,317,394) or to provide a coating for active agents whereby said coated agents can be injection molded into medicaments in solid form (see U.S. 3,432,592). Unfortunately, this use of synthetic resins requires excessive (medicament injurious) temperatures and/or solvents so that these processes suffer the common disadvantages of the earlier discussed prior art approaches.
It is an object of this invention to provide a composition for the controlled release dosage forms of biologically active agents which overcomes the disadvantages of the prior art. More particularly, it is an object of this invention to provide a dry compression/adhesion-dry compressible binder and more preferably a combination of this dry binder and excipients that collectively cooperate to form a dry, compressible matrix which as the property of the controlled release dosage forms of biologically active solid particles dispersed throughout said matrix and a method of using said combination to obtain a controlled release tablet of useful integrity and desired release rates. SUMMARY OF INVENTION in my U.S. Patent Application Serial No. 34,580, filed April 30, 1979 it is taught that the combination of a hydrophobic cellulose derivative, a fatty acid material or a neutral lipid and a wax provides a matrix for particulate biologically active agent which can be dry
compressed to provide a useful tablet having the property of controlled continuous release of said agent in the gastro-intestinal tract. It provides a composition adapted for controlling release of biologically active agents comprising and admixture of a fatty acid material or a neutral lipid, and preferably both, a wax and at least a binding amount of a hydrophobic cellulose derivative hereinafter defined as a hydrophobic carbohydrate polymer whereby said composition can be directly compressed in a dry state into tablet form having a hardness of 6 to 25 kg as measured on a Pfizer hardness tester.
More specifically, the aforesaid application teaches a controlled release formulation comprising from 0.01 to 95 wt. % of biologically active agent and from 5 to 99.9 wt. % of a controlled and continuous release binder admixture, said wt. % being based on the total weight of formulation and said admixture containing from 2 to 97 parts by weight of a fatty acid material or a neutral lipid and preferably both from 2 to 97 parts by weight of a wax preferably having a melting point between 60°C and 90ºC and from 1 to 96 parts by weight of a hydrophobic cellulose derivative, i.e. a hydrophobic carbohydrate polymer. More particularly, the aforesaid application teaches that the described combinations: (a) a fatty acid material, particularly those of the class consisting of fatty acids having from 12 to 28 carbons, fatty monoalcohols having 12 to 28 carbons, fatty amines and amides having 12 to 28 carbons and mixtures thereof (e.g. an optimum mixture of 85 wt. % stearic acid and 15 wt. % palmitic acid) or a neutral lipid, particularly of the class consisting of stearin, palmitin, castorwax, phospholipids, glycolipids, glycerides such as glyceryl monostearate and glyceryl distearate, hydrogenated cottonseed oil, hydrogenated tallow and metal salts of C12 to C28 fatty acids, optimally hydrogenated cottonseed oil, or a mixture of said fatty acid material and said neutral lipid; (b) a wax preferably having a melting point between 60ºC and 90ºC, particularly of the class consisting of
carnauba wax, spermaceti, beeswax, candelilla wax, esparto and paraffins, optimally carnauba wax; and (c) a hydrophobic carbohydrate polymer, preferably of the class consisting of ethyl cellulose, propyl cellulose, cellulose acetate, cellulose acetate-butyrate, and cellulose acetate-propionate optimally ethyl cellulose; can be used to make controlled continuous release solid dosage forms of biologically active particulate agents by simple dry blending or slugging/granulation and compression (without a need of micropulverization, heat or solvent). The percentage of the active and the percentage of the combination in said formulation are readily varied to modify the controlled release rate of the active agent from a few hours to several days. The combination can be used for controlled release solid dosage forms of any particulate agent, preferably particles of less than 20 mesh in size.
In my U.S. Patent Application Serial No. 54,856 filed July 6, 1979 the discovery was reported that the combination of the hydrophobic cellulose derivative (herein a hydrophobic carbohydrate polymer) in admixture with one of the digestive-difficulty soluble controlled release components, i.e. a fatty acid material, neutral lipid or wax, can be dry compressed with a particulate biologically active agent to provide a useful tablet having a hardness of 6 to 25 kg as measured on a Pfizer hardness tester.
It appears that the hydrophobic carbohydrate polymer synergistically cooperates with the digestivedifficulty soluble controlled release component, particularly with the fatty acid, to surprisingly strengthen the tableted combination containing the particulate biologically active material by imparting increased vertical strength and enhanced resistance to delamination from an external force.
The teachings herein now provide a controlled release dosage form that can be produced by a dry, direct compression process that overcomes the serious disadvantages of the prior art including the adverse and
deleterious impact of solvents and/or heat which teachings result in compressed structures of surprisingly superior physical integrity and resistance to delamination. DETAILED DESCRIPTION OF THE INVENTION From the foregoing it must be evident that the invention herein relates to dry compressed products obtained from a directly compressible hydrophobic carbohydrate polymer and more preferably from a combination or an admixture of a hydrophobic carbohydrate polymer and various water insoluble ingredients which provide for controlled continuous release of the biologically active agent in the body when taken by mouth in tablet form or by implantation. The unique property provided by this admixture is one of dry compressibility so that useful tablets or implants can be readily produced in an inexpensive, facile, hazard free and environmentally safe manner. BIOLOGICALLY ACTIVE AGENTS
The biologically active agents which can be admixed with the excipients to provide the controlled release tablets according to this invention include all substances which when introduced into the body of a human, animal, plants, soil and water is biologically active, usually in a therapeutic sense, nutritional purpose or biocidal effects.
Representative of such biologically active agents are: a. vitamins, minerals and other nutritional supplements including all of the water soluble vitamins including Vitamin C, B vitamins and choline, inositol, bioflavinoids, iron, selenium, para amino benzoic acid, iodine, zinc, 1-lysine, 1-glutamine, 1-cysteine, calcium , magnesium, potassium, etc. b. ANALGESIC drugs such as acetaminophen, aspirin, codeine, salicylamide propoxyphene, pentazocine HC1, malbuphine HC1, ibuprofen, indomethacin, meperidine HC1, morphine and oxaprozin.
ANOREXIC drugs such as amphetamines, phentermine, phenylpropanolamine and phenmetrazine.
ANTHELMINTIC drugs such as peperazine citrate, pyrantel pamoate, thiabendazole, mebendazole, levamisole and their derivatives.
ANTIASTHMA drugs such as terbutaline sulfate, isoetharine, theophylline and sodium glycinate.
ANTIBACTERIAL drugs such as trimethoprim and sulfamethoxazole.
ANTIBIOTIC and ANTIMICROBIAL drugs such as metronidazole, amoxicillin, erythromycin, ampicillin, penicillin, tetracycline, aminosalicylate, rifampin, cycloserine, amikacin, cefazolin, cephradine, cefaclor, cephalor idine, chloramphenicol, clindamycin, demeclocycline, kanamycin, cephaloridine, cefamandole nafate, cyclacillin, carbenicillin, vancomycin, cephradine, fluphenazine, hetacillin, streptomycin, ethambutol, methenamine, gentamicin and toburamicin.
ANTICEPTIC drugs such as nitrofurantoin and sulfonamides. ANTICOAGULANT drugs such as warfarin.
ANTICONVULSANT drugs such as clonazepam, nalproic acid, phenytoin, diazepam and primidone. ANTIDEPRESSANT drugs such as trimipramine maleate, imipramine HC1 and imipramine pamoate. ANTIDIABETIC drugs such as chlorpropamide, acetohexamide, tolbytamide and tolazamide.
ANTI-GOUT drugs such as probenecid, sul finpy razone and allopurinol.
ANTIFUNGAL drugs such as griseofulvin, flucytosine, nystatin, clotrimazole and miconazole.
ANTIHISTAMINE drugs such as triprolidine HC1, diphenhydramine HC1, chlorpheniramine maleate, brompheniramine maleate and hydroxyzine HC1.
ANTI-INFLAMMATORY drugs such as phenylbutazones, steroids, sulfonamides and salicylates.
ANTIMALARIAL drugs such as chloroquine phosphate, hydroxychloroquine sulfate and pyrimethamine. ANTIMIGRAINE drugs such as ergotamine tartrate, propranolol HC1, isometheptene and mucate.
ANTIMOTION SICKNESS drugs such as dimenhydrinate.
ANTINAUSEANT drugs such as hydroxyzine HCl, buclizine
HCl, prochlorperazine and promethazine HCl.
ANTINEOPLASTIC drugs such as tamoxifen citrate, mitotane, megestrol acetate, tetolacone, flurouracil, busulfar, chlorambucil, melphalan, amsacrine, streptozocin, anthracyline agents, azacitidine, bleomycide, vinca allkaloids, cytrarabine, hexamethylmelamine, methotrexate, hydroxyaren, chlorotriansene, cisplatin, cyclophosphamide, dacarbazine, dactinomycine, mithramycine, mitomycino, procarbazine, azathioprine, mercaptopurine, thioguanine and nitrosoureas.
COUGH & COLD PREPARATION drugs such as guaifenesin, promethazine HCl, benzonatate, noscapine and dextromethorphan HBr.
DECONGESTANT drugs such as b rompheniramine maleate and phenylephrine HCl.
DIURETIC drugs such as thiazides, acetazolamide, furosemide and triamterene.
MUSCLE RELAXANT drugs such as dantrolene sodium, cyclobenzaprine, chlorzoxazone and quinine sulfate.
PARASYMPATHOLYTIC drugs such as oxyphenomium bromide, atropine, hyoscyamine sulfate, glycopy rrolate and propantheline bromide.
SEDATIVE drugs such as barbiturates, meprobamate, promethazine HCl and methaqualone.
TRANQUILIZER drugs such as diazepam, chlorazepate monopotassium, prazepam, ch loridiazepoxide HCl and chloralhydrate.
AGRICHEMICALS including herbicides such as 2,4-D and its derivatives, class of nitrobenzen amines, prometone, atrazine, simazine, trifluralin, picloram, lindane, batoxyethanolesters, dimethylamine, diquat silvex, tok, machete, lasso, avenge, prowl and their derivatives.
ALGICIDES such as chlorine compounds, e.g. calcium hypochlorite, and their derivatives.
e. ANTIFOULING AGENTS such as organotin compounds and σrganolead compounds. f. FUNGICIDES such as kitazin and their derivatives. g. INSECTICIDES such as DDUP, class of phosphoro thioate compounds, aldicarb, hexamethyl phosphoric triamide, malathion, parathion, pyrenium, sumithion, elsan, aldrin and their derivatives. h. MOLLUSCICIDE such as copper bis (tri-n-butyltin) oxide, niclosamide and N-tritylmorpholine. i. PHERMONES such as methyleugenol and grandlure. j. PLANT GROWTH REGULATOR such as gibberelline and auxin, k. RODENTICIDES such as decarboximide and their derivatives. 1. OTHER BIOCIDES m. FERTILIZERS such as urea and other mineral nutrients n. FLAVORS, FRAGRANCES, AND PERFUMES
The kinds of biological agents for this invention are not limited to the names listed herein. Numerous other compounds can be incorporated into the excipients to make controlled release dosage forms according to the teachings herein.
The biologically active particulate solids which preferably should be smaller than 10 mesh (U.S. sieve grade), optimally pass through a 20 mesh screen, ranges broadly in an amount of 0.01 to 95, preferably 0.1 to 90, wt. % of the total formulation compressed into the controlled continuous release tablets. The dry directly compressible hydrophobic carbohydrate polymer binder or the admixture which makes possible the products of the invention provides a matrix for the biologically active particulate agent which ranges in an amount of 5 to 99.99, preferably from 10 to 99.9 wt. % based upon the total weight of said tablets derived from said compressed formulation. The dry directly compressible hydrophobic carbohydrate polymer or the admixture can also include as desired: flow aid materials in an amount ranging from 0.5 to 2 wt . %, said flow materials being represented by finely divided silica and talc; and from 0.5 to 2 wt. % of
a lubricating material to facilitate tablet ejection e.g. a metal salt of a fatty acid, preferably magnesium stearate.
The combination of the invention which provides said matrix is either a dry directly compressible hydrophobic carbohydrate polymer or an admixture containing a hydrophobic carbohydrate polymer and a one to three digestive-difficulty soluble components readily produced by dry blending of powders smaller than 20 mesh, preferably 30 mesh, from the fatty acid material and/or the neutral lipid and/or said wax and the aforesaid hydrophobic carbohydrate polymer. The physiologically active particulate agent and additional excipients as desired are readily dispersed into the blended powders providing the matrix whereby the resultant formulation can be dry and direct compressed on a press under a pressure of 1.5 to 20, preferably 3 to 9, tons/square inch to produce the tablets. Under some circumstances it is useful to granulate the resulting tablets and recompress the granules with or without additional excipients to obtain the desired release rate. Tablets obtained from this teaching of the invention have a useful hardness, i.e. 3 to 25 kg as measured on a Pfizer hardness tester, to provide a commercially marketable product. HYDROPHOBIC CARBOHYDRATE POLYMER
The hydrophobic carbohydrate polymer constitutes from 1 to 100, preferably 3 to 50, optimally 5 to 30, wt. % of said matrix composition and provides said matrix composition with the integrity necessary to realize the binding of the controlled release tablets according to this invention.
Preferred hydrophobic carbohydrate polymers are those of the class of hydrophobic cellulose derivatives in which the R-moiety of the cellulose-R or cellulose-ROH or other R derivative is either an aliphatic acyl group of 2 to 22 carbons or aliphatic alkyl of from 1 to 8 carbons and chitin.
The most preferred hydrophobic carbohydrate polymers are ethyl cellulose, propyl cellulose, cellulose acetate, cellulose propionate, cellulose acetate-butyrate, cellulose acetate propionate. The optimum hydrophobic carbohydrate polymer is ethyl cellulose having an ethoxy content of from 43-50%. DIGESTIVE-DIFFICULTY SOLUBLE COMPONENTS
As indicated above this invention has now taught that the hydrophobic carbohydrate polymer can be either singly used or in combination with a difficulty soluble component, i.e. any one or all of the components which are difficulty soluble in the digestive tract, i.e. wax, fatty acid and neutral lipid, which for purposes of this disclosure have been collectively designated the digestive-difficulty soluble component. Each has the property of slowly dissolving or disintegrating in the digestive tract.
1. WAX
The useful waxes are those which are obtained from plant and animal sources or as a petroleum product.
In addition to its uses as a hydrophobic matrix material, the wax in this invention increases the hardness and compactness of the matrix, forming a cohesive hard tablet under the compressive forces of the process of this invention. The useful waxes have a melting point ranging from 50°C to 100°C and constitute from 0 to 99, preferably
5 to 70, optimally 10 to 40 wt. % of said matrix composition. Illustrative of the preferred waxes are carnauba wax, spermaceti, beeswax, paraffin wax as well as synthetic waxes e.g. polyethylene. The optimum wax is carnauba wax.
2. FATTY ACID MATERIALS
The fatty acid materials preferably along with the neutral lipid constitutes from 0 to 99, preferably 5 to 80, optimally 10 to 70, wt. % of the matrix composition of the invention which provides for controlled release of the active agent.
Fatty acid materials assist in the control or regulation of the rate at which release of the active agent occurs and are generally characterized by having a melting point above 43°C. The fatty acid materials preferably are of the class consisting of: fatty acids having 12 to 28 carbons, e.g. stearic acid, palmitic acid, lauric acid, eleostearic acid, etc.; fatty alcohols having from 16 to 44 carbons, e.g. stearyl alcohol, palmitol, etc.; a fatty amine having 13 to 45 carbons; and, a fatty amide having 11 to 45 carbons. A highly useful commercially available fatty acid material is Hystrene sold by Humko Sheffield (a division of Witco Chemical Co.) of Memphis, Tennessee which is a mixture of 85 wt. % stearic acid and 15 wt. % palmitic acid. 3. NEUTRAL LIPID
The neutral lipid which can be used as an alternative to a fatty acid material, but preferably in combination therewith constitutes from 0 to 99, preferably 5 to 80, optimally 10 to 70, wt. % of said matrix admixture of this invention. The neutral lipid cooperates with the fatty acid material in the control of the rate at which release of the agent occurs and is characterized by having a melting point greater than 43°C.
The neutral lipids are preferably of the class consisting of monoglyceride, diglyceride, triglyceride, phosphatides, glycolipids, steroids and neutral metal and organic salts of fatty acids having from 12 to 29 carbons. Representative examples of the preferred neutral lipids include stearin, palmitin, castor wax, lecithin, hydrogenated cottonseed oil, hydrogenated tallow, magnesium stearate and calcium and aluminum salts of palmitic and other fatty acids. A highly useful commercially available neutral lipid is hydrogenated cottonseed o i l wh ich h as b een ob t a in ed f r om : Humko Shef field as Neustrene; Capital City Products as Ditrex or Sterotex; and Durkee as Lubritab.
As earlier noted it is taught herein that an optimum approach to admixtures which provide for dry and direct compression of controlled release dosage tablets
and implants is for the admixture to comprise the combination of a hydrophobic carbohydrate polymer, a fatty acid material and/or a neutral lipid and a wax to serve as a matrix for the particulate biologically active agent. Thus, the dry, direct compressed tablets and implants can be achieved by a composition adapted for controlling release of biologically active agents comprising an admixture of from 2 to 97 parts by weight of a fatty acid material or a neutral lipid and preferably both, from 2 to 97 parts by weight of a wax preferably having a melting point between 50°C and 100°C and from 1 to 96 parts by weight of a hydrophobic carbohydrate polymer.
The following examples demonstrate the practice and utility of this invention. EXAMPLE 1
A controlled release tablet containing ascorbic acid as the biologically active agent was prepared as follows: 67.8 parts by weight of ascorbic acid having a particle size that passed through a 20 mesh screen was blended with 30.8 parts by weight of a mixture of 45.0 weight percent Hystrene, 24.4 weight percent Neustrene (a commercial product of hydrogenated cottonseed oil), 17.8 weight percent carnauba wax, 12.2 wt. % ethyl cellulose and 1.4 wt. % tableting excipients.
The resulting blend was passed through a 20 mesh screen and compression molded at a pressure of 3 to 9 tons per square inch into oval tablets containing 1000 mg of ascorbic acid and having a total weight of about 1530 mg, a thickness of about 7 mm and a hardness of about 14 Kg (measures on Pfizer hardness tester).
The controlled continuous release of tableted formulations obtained in this example is shown by the data of Table I. A number of tablets were subjected to a test in which a single tablet was placed in a beaker containing 100 ml of water maintained at 37°C. After a period of time, the tablet was taken from the water, the porous outer matrix of the test tablet was rubbed off until a
solid core was left for drying and then air dried on a filter paper overnight, the solid core was then weighed. The consolidated results are hereinafter set forth in Table I. A
EXAMPLE 2
A controlled release tablet containing multi vitamins and minerals as biologicallya ctive agents was prepared as follows:
57.5 parts by weight of the blend of high potency multi vitamins and minerals having a size that passed through a 20 mesh screen was blended with 40 parts by weight of a dry direct compression mixture of 62.9 weight percent Hystrene, 17.4 weight percent Neustrene, 12.6 weight percent carnauba wax and 7.1 weight percent ethyl cellulose.
The resulting blend was passed through a 20 mesh screen and compression molded at a pressure of 3 to 9 tons per square inch into oval tablets having a weight of 1550 mg, a thickness about 6 mm and hardness of 15 kg (measured on Pfizer hardness tester).
90 weight percent of the tablet is disintegrated after 16.5 hours.
EXAMPLES 3 -in
In each example, all the identified ingredients are sieved on a 20 mesh screen and blended together into a formulation. The formulation was then compressed on a tablet press to provide the controlled release tablets with the desired release times. The several exmaples with the respective formulations and corresponding time for disinte gration of 90 weight percent of the tablet in 100 ml of H2O held at a constant 37ºC are set forth in Table II hereinafter set forth.
The data of the several examples show that the disintegration hence the release of these nutrients are continuous upon time. The release pattern is parabolic i.e. the rate, faster in the beginning and slower at the later times, which is often desirable for quickly achieving the desired blood level with subsequent prophylactic level for a prolonged period.
EXAMPLES 11 - 13
Three controlled release tablet compositions containing ascorbic acid as the biologically active agent were prepared as follows: Ascorbic acid having a particle size that passed through a 20 mesh screen was blended with three different formulas as set forth below.
Each of the resulting blends were passed through a 20 mesh screen and compression molded at a pressure of 3.1 tons per square inch into round convex tablets containing 350 mg of ascorbic acid and having a total weight of about 700 mg.
The blends in parts by weight are as follows:
Ex. 11 Ex. 12 Ex. 13
Blend Blend Blend ascorbic acid 49.26 49.26 49.26
Hystrene1 49.26 - 34.48
Ethocel2 - 49.26 14.78
Magnesium stearate 0.98 0.98 0.98
Aerosil 2003 0.49 0.49 0.49
1. Hystrene is a fatty acid mixture of 85 wt . % estearic acid and 15 wt. % palmitic acid sold by Humko Sheffield (a division of Witco Chemicals)
2. Ethocel is ethoxylated cellulose commercially available from Dow Chemical Company of Midland, Michigan.
3. Aerosil 200 is a fumed silica sold by DeGussa, Inc. of Teterboro, New Jersey.
EXAMPLE 14
The tablets resulting from each blend of Examples 11, 12 and 13 had the following properties:
I
The blends of Example 12 and 13, i.e. the blends of invention showed excellent hardness and was clearly superior in enhanced resistance to delamination forces.
The enhanced resistance to delamination makes possible ejection of the tablet from the tableting die cavity with reduced breaking of tablets arising from the delamination forces imposed on the tablet during ejection from the uneven walls of the cavity.
As will be seen from the Table III of Example 15, the composition of the invention provides useful controlled release of dosage forms of biologically active particulates. EXAMPLE 15
The controlled continuous release of tableted formulations obtained from the blends of Examples 11-13 are shown by the data of Table III. Four tablets of each blend were subjected to a test in which a single tablet was placed in a breaker containing 100 ml of distilled water maintained at 37°C. After a period of time, the release medium was analyzed for the amount of ascorbic acid release from the tablets by the USP methods as published in the United States Pharmacopeia (20th Revision) Official from July 1, 1980 on page 55 of USP.
The percent of ascorbic acid released was calculated based upon the total quantity of ascorbic acid per tablet. Duplicate or triplicate release tests were performed and the average values were calculated. Also the porous outer matrix of the tablets was rubbed off until a solid core was left for drying and then air dried on a filter paper overnight. The solid core was then weighed. The consolidated results are hereinafter set forth in Table III as percent released.
The core method somewhat underestimating the amount ascorbic acid released is probably due to incomplete rubbing (figures in parenthesis).
Since the matrix controlled release can be expressed according to the authors of a well-known textbook (Controlled Release of Biologically active
Agents, Edited by A.C. Tanquary & R. E. Lacey, Plenum Press, New York 1974) by the following equation:
% released = k (t)1/2 where k is a constant and t represents time in hours.
When the % released is plotted against the square root of the time, a straight line is obtained. Its slope is a good measure of release rates and experimental reproducibility of release rates. The time for 50% release (t50%) is also shown in the Table III by t50% =
From Table III, fatty acids are the prolonged release rate controlling substance and the hydrophobic carbohydrate polymer, e.g. ethylcellulose, is poor for that purpose. However, ethy lcellulose is an excellent dry direct compression tableting binder for controlled slow release tablets and as shown in hardness data and breaking pattern (Example 14), the presence of ethyl cellulose in dry-direct compression compositions does improve markedly the Tablet integrity by reducing delamination during the tablet compression, henceforth, the commercial controlled release tablet production. The data of Example 15 shows that the slow dissolution, hence the release of biologically active particulates are, continuous upon time for a release admixture according to this invention while providing tablets of excellent strength and enhanced resistance to delamination.
Two blends comparable to the blends of Examples 11 and 13 were formulated except that carnauba wax was substituted for the Hystrene with the result that the modified blend of Example 13 according to this invention exhibited enhanced resistance to delamination from an external force. This was evidenced by tablets of the modified blend of Example 13 exhibiting an average
breaking force of 11.3 kilograms (kg) whereas the tablets of the modified blend of Example 11 exhibited an average breaking force of 9.7 kg.
In the foregoing the components providing the matrix blend have been admixed in the dry blending stage.
However, it is possible to provide one or more of the matrix blend components as a coating on the biologically active material and/or an excipient in the practice of this invention, e.g. introduce the ethyl cellulose as a coating on either or both into the dry mix blending step.
The hydrophobic carbohydrate polymer as described herein is exemplified in preferred form by cellulose polymers wherein the hydroxyl or charged groups of the molecule are modified, i.e. derivatized, into hydrophobic groups by alkylation, acylation or similar processes. Other suitable (for the purposes of this invention) carbohydrate polymeric substances which can be similarly derivatized to provide the essential hydrophobic property are starch, dextran, gums, inulin, mucopolysaccharides and chitin. In some applications the latter carbohydrate polymeric substances are themselves sufficiently hydrophobic to be used without further derivat ization, e.g. chitin in its natural state possesses acetamido moieties which usefully modifies its cellulosic properties for use in matrix blends according to this invention.
During the investigation of excipients which can be introduced into the formulation of the invention, it has been discovered that then presence of 0.01 to 10, preferably 0.01 to 5, optimally 0.05 to 3, wt. % of hydrophobic fumed silica (a silica falling within the class of finely divided silica), said wt. % based on the total tablet weight, can surprisingly prolong the release rates by 20 to 40 percent as measured by t 100% (time to release 100% of the biologically active agents). Such prolongation can effectively reduced the amount of admixture necessary for dry direct compressible matrix to obtain desired release rates thus reducing the volume (size) of tablet required for a given dosage of
biologically active material and/or increasing the amount of biologically active material per unit volume of the tablet.
HYDROPHOBIC FUMED SILICA The hydrophobic fumed silicas are well known commercially available materials, e.g. Aerosil● R-972 sold by Degussa, Inc. of Teterboro, N.3., Cabosil● N70-TS sold by Cabot Corp..of Tuscola, Illinois, Tullanox● 500 sold by Tulco, Inc., (all of which are preferred for use herein), of the general class of amorphous precipitated silicas but of the pyrogenic (fumed) type which provides according to Kirk-Othmer's Encyclopedia of Chemical Technology (Third Edition) Vol.20 at pages 768 and 778-779 an ultimate particle size, nm of 1-100 and an aggregate particle size, urn of 2-3. The hydrophob icity of the fumed silica is achieved by replacement of the hydroxyl groups of the surface with OR5 or OOR5 wherein the R5 moiety is 1 to 5 carbon aliphatic alkyl or aliphatic acyl groups. The most preferred hydrophobic silica is the methylated fumed silica. Hydrophobic silicas are discussed in Kirk-Othmer's Encyclopedia of Chemical Technology (Third Edition) Vol. 7 at pages 440-441.
Examples of blends containing hydrophobic fumed silica according to this invention are set forth in Examples 16 through 19 which follow.
wt.% Bl % . 1 2 O
- -
2. As described for Exs. 11, 12, 13
3. As described for Exs. 11, 12, 13
Example 20
The tablets of Exs. 16 through 19 blends were made according to the procedures of Examples 11 through 13 end thereafter measured for weight, thickness and hardness prior to evaluation of the release rates. The properties are shown in Table IV.
Tables IV Physical Data of Tablets from blends of Exs. 16 through 19. Property Ex.16 Ex.17 Ex.18 Ex.19
(av.of 20 tabs.) Blend Blend Blend Blend
Weight (gr.) 0.703+0.008 0.702+0.009 0.635+0.009 0.704+0.008
Thickness (ins.) 0.284+0.002 0.281+0.002 0.272+0.001 0.274+0.003
Hardness (kg.) 4.3 +0.8 6.3 +1.7 3.7 +0.6 4.8 +0.5
The release test data of the following Table V were obtained by analysis of the medium for ascorbic acid content. At each indicated time interval the release medium was replaced by fresh release medium and subjected to the analysis for Vitamin C content. Each of the release mediums were held at a conatant 37ºC while exposed to the immersed tablet. Ascorbic acid release was analyzed by the USP XX methods. Release rates of tablets (av. of 4 to 6 tests) obtained from each of the blends of Exs. 16 through Ex. 19 are shown in Table V.
2
The data of Table V shows that the presence of only 1 % of the Cab-o-sil● N70TS hydrophobic fumed silica increased the tiQQg by 27% (the 6.8 hr. increase in the tablet of Ex. 17 compared with the tablet of the blend of Ex. 16 and similarly the 2.8 hr. increase in Ex. 19 over Ex. 18) for the tablets containing both 50 and 60 weight percent of ascorbic acid when it is added to the dry directly compressible mixture of this invention.
This dramatic increase in slowing release rates by a small amount is very important in perfecting controlled release tablets especially the tablet size.
In accordance with this invention, the presence of a small amount of hydrophobic fumed silica can be an effective dry controlled release agent when used in conjunction with other hydrophobic materials as taught herein.
The discovery of the remarkable utility of the presence of the hydrophobic fumed silica is applicable to other known controlled release processes such as those approaches earlier discussed in this application and the dry direct compression process using a micropulverized lipid, e.g. as described in U.S. Patent 3,279,998 to produce tablets since its presence in said tablet in the amounts described herein will reduce the tendency of the tablet to desintegrate in water environments markedly reducing the dissolution rate. The 0.05 to 3 weight percent of hydrophobic fumed silica dispersed throughout the tablet as by blending the silica into the formulation prior to tableting provides lipid excipient controlled release tablets having better control of release, slower release if desired and improved physical integrity in aqueous environments such as found in the digestive tract. EXAMPLE 21
The present dry direct compression process and compositions are applied to pharmaceuticals to make compressed controlled release dosage forms. The formula is shown in the following Table VI. These blends of dry
blend compositions are directly compressed in standard rotary tablet press as before. The resultant directly compressed controlled release pharmaceutical tablets are subjected to release for the three hours in water having a pH of 3.0 and thereafter the pH was adjusted to 7.0 with the water temperature held constant at 37ºC. The amounds of the drug released were measured by Perkin Elmer Lamda 3A doublebeam UV Spectrophotometer. The results are shown in the following Table VII. k and t100% (time for 100% release) were calculated according to the equation set forth in Ex. 15.
EXAMPLE 22
As an example for an algicide controlled release tablet form, calcium hypochlorite tablets were prepared according to the present invention as follows. wt. %
Calcium Hypochlorite 74.3
Dritex 9.3
Hystrene 4.7
Carnauba Wax 6.8 Ethylcellulose 3.7
Aerosil 200 0.1
Magnesium Stearate 1.0
100% Release rates were measured by immersing the tablet into room temperature water for 10 minutes and dried for 20 minutes and repeated this process 10 times as if the tablet was soaked with water only during the swimming pool filtering periods. The result shows an average 1% of the active component release each time. This rate will permit 100 times soaking of the tablet for each 10 minute period or 100 days for 10 minutes soaking each day. Products of the Invention
Tablets formed by the new dry direct compression process are very different from the tablets formed by known processes using solvents, heat or plastic polymers.
The tablets of the invention can be distinguished from the products of known processes by the following criteria:
1. Detectability of chemical compounds not supposed to be present in the finished product, such as heat degraded compounds or solvent residues;
2. Physical structure differences;
3. Release rate profile differences;
4. Identification of components. The known processes set forth in U.S. Patents
#3,317,394, #3,432,592, #3,344,029, #4,167,558, #3,402,240, #3,062,720, #3,577,514, #3,147,187, #3,965,256
and #3,362,880 result in products which are fully distinguishable by the above criteria from the tablets of the invention.
Many commercial defects and shortcomings as controlled release tablets are very apparent in the tablets resultant from the very complicated prior art processes. The tablets of the inventive dry direct compression process overcome those defects and shortcomings with a simple, easy and extremely low cost process. The aforesaid defects and shortcomings are amplified hereafter.
1. Detectability of hazardous chemical compounds. Previous processes using heat to make controlled release tablets deactivate the biologically active components resulting in reduced potency of the active and cause chemical reactions, oftentimes of a totally unknown nature. Many biologically active compounds are highly sensitive to the exposure of the heat required to melt or glassify hydrophobic lipids and polymeric material used as controlled release medium (see U.S. Patents #3,147,187, #3,432,592, #3,317,394, #3,344,029 and #3,965,256).
Not only is the instability of the biologically active compounds a shortcoming but also the degraded or deactivated or chemically newly formed compounds is potentially hazardous to human and animal health, to plant life and the environment. The present dry direct compression process hardly has any chance to form such potentially hazardous compounds and does not produce tablets of reduced potency.
The amounts of impurities in the tablets of the present process is only limited to the amounts of impurities present in raw material according to the supplier's specificat ions often by the certificates of analysis after dilution factors. For example, if ascorbic acid has 0.01% impurity and if the ascorbic acid content is 505 of the tablet weight then total percent of impurity for the tablet becomes 0.005%. The amounts of impurities in raw material drugs and food additives are limited by
USP or Food Chemicals Codex specifications. Ranges of purity by assay are 99.05-100.05 (some examples: acetaminophen, 100.4%, procainamide HCl, 99.3%, ascorbic acid, 99.5%, lobeline sulfate, 100.3%, l-glutamine, 99.9%, pyridoxine HCl, 99.8%, etc.).
The reduced potency and the presence of the newly formed compounds are easily detected by common analytical procedures using UV, IR, GLC, GC, HPLC, NMR and other residue analysis methods. Heat degradation of the biologically active components can occur during the solvent removal step for the processes using solvents (see U.S. Patents #3,362,880, #3,147,187, #4,167,558 and #3,344,029).
Especially the degradation of the biologically active components is more pronounced in the presence of water at elevated temperatures (see U.S. Patents #3,362,880, #3,965,256, #3,062,720, #3,402,240). The presence of moisture in the tablet from processes using water accelerates the degradation of many active components in finished product.
Thus a comparison of stability data will also distinguish the product of present process.
Another undesirable chemical component present in the tablets of the prior processes are solvent residues. The product of the present dry direct process is free of solvent residues.
Again the amounts of solvent residues are only limited to those of raw material before compression after volume/weight dilution factors. Most raw material certificates of analysis state loss after drying as the amount of volatile compounds (organic solvents or water)(see Table VIII for representative compounds).
Products from the known processes using solvents have solvent residues no matter how effective the solvent removal process. The solvent residues can be easily detected by standard analytical techniques.
When the solvent contents and types are analyzed for the finished tablets, the dry directly compressed tablets show only amounts of those inherited from raw material as shown in Table VIII.
On the other hand, tablets of prior arts using solvents show higher amounts of those solvent residues in addition to the types and amounts inherited from raw material. Those prior teachings using solvents include
U.S. Patents #3,344,029, #3,062,720, #3,147,187, #3,965,256 and #3,362,880. There are potential hazards to health and environment of the residual solvent. Many solvents used in the prior art such as dichlorethy lene
(U.S. Patent #3,147,187), benzene (U.S. Patent 3,317,394), carbon tetrachloride (U.S. Patent 3,344,029), and methylene chloride are known to cause cancer in animals and humans. U.S. Patent #3, 344 , 029 u se s me t hy l a l c o h o l which is high ly tox ic .
2. Physical Structure Difference. Because the heat or solvent process involve physicochemically liquid states, products resulting from the prior process using such have physical structures of tablets very different from the product made by the present dry direct process which only involves solid states. These physical structure differences can be examined mainly under three different aspects.
Heat fusion and solvent solution of lipid or hydrophobic polymeric material both result in a more homogeneous liquid state. Cooling or solvent removal forms mass interlinked agglomerated skeletal structure with crystal or solid state structures changed from the original dry particle structures before the treatment.
First, interlinked skeletal structures are evident after the active components are leached out. The empty honeycomb of fused or glued binders and lipid or polymer material have much stronger structures than those of the present dry direct process with identical wt. % compositions. The product of the present process easily crumbles to the particle size o f the precompression state . This is particularl y the case for hydrophobic carbohydrate polymers. Change in crystal structures or morphology after heat/solvent process are easily detected by x-ray diffraction and electron microscopy. Especially the extent of crystallinity in polymers are reduced due to the presence of solvent residues with their plasticizing effect (see U.S. patents #3,362,880 and #3,344,029).
The easier crumbling and disintegration of the excipients are more desirable for the environment. Tablets which are unbreakable or difficult to crumble and retain the structure after leaching as by passage through the gut will give doubt to consumers of the incomplete release of the biologically active agents when viewed in the stool by the user. Such are the products of U.S. Patents #3,432,592, #3,317,394, #3,147,187, #3,344,029 and #3,062,720. This physical structure difference is also easily examined by a polarizing microscopic technique. The thin cross sections of the tablets provided by the dry direct process of the invention shows clear cut boundaries between particles and crystals of composition components with the size and shapes the same or closely similar (allowing compression shrinkage of hydrophobic carbohydrate polymers) to the sizes and shapes of the precompression state. Those products using heat/solvent give fused together or glued together structures markedly different from the pretreatment state.
More amorphous and less crystalline states are also detected by a polarizing microscope with far less clear boundary between excipients. The particle size after treatment usually depends on sieve sizes of the granulation processes used for prior art processes. A less clear boundary between the active and controlled release materials and change of particle size, crystal forms of the biologically active component are also observed due to melting and solubilizatioπ of the active by heat or solvent common to solubilize both the active and controlled release material. Ethanol and water are typically such solvents which are used to dissolve both active and controlled release materials. For example aspirin has solubility of 1g/100cc in H2O and 1g/5cc in ethanol. The prior art process using ethanol to dissolve ethylcellulose will dissolve also aspirin and after drying alter the crystal structures. Shape and size of the aspirin become different from those of pretreatment state. X-ray diffraction, electron and polarizing microscopes detect the changes resulting from the solvent process (see U.S. Patents #4,167,558, #3,402,240, #3,965,256, #3,062,7820, #3,317,394, #3,432,592, #3,362,880, #3,344,029 and #3,147,187).
Co-melting by heat and co-solubilization by solvents of the active and the controlled release material also changes crystal structure of the controlled release material by formation of a solid solution or a eutectic mixture. X-ray diffraction, electron microscope, polarizing microscope and thermal analysis techniques easily detect such changes. On the contrary the present dry direct process preserves the physical characteristics of the active and controlled release material intact and native to precompression state when observed by these techniques. Electron and polarizing microscopic examinations of the tablet from the present dry direct process show even distribution of the active and lipid and hydrophobic carbohydrate polymer components in the matrix structure. Tablets of the prior art process using heat and solvent
show that greater concentration of the controlled release material around the active component to be coated. An examination of the products of: U.S. Patents #3,965,256 and #3,317,394 will show that the lipid material coats the active component; and, U.S. Patents #4,167,558, #3,062,720, #3,362,880, #3,344,029 and #3,147,187 show that hydrophobic polymer coated the active agent forming completed or partially encapsulated forms. Such coating by lipid or hydrophobic polymers is not present in the tablet of the present process. Where the prior art process has two steps of first encapsulating the active and then dry mixing with other filler or lipid material, then by using differential solvents which dissolve only the second step material, one can isolate the active-encapsulating material ccibination and examine it by microscope or IR and thermal analysis technique. The tablets of the present process do not have such partially or completed encapsulated structure and with the above differential dissolving method the active agents and the hydrophobic material become physically separated.
3) Release Rate Profile Difference. The tablets of the present dry direct process are a true matrix system where the active component particles are evenly and truly dispersed between dry direct composition components without solubiiizing into each other. On the other hand, tablets of the prior art processes due to heat fusion/solvent solubilization between the active and the controlled release material contain solid solutions and eutectic mixtures as explained before. The release profiles of the biologically active components in biological fluid medium, i.e. water or gastric juice, are different since the products of the invention provide a dispersed system whereas the products of the prior art products provide a partially or completely dissolved system as illustrated by R. W. Baker and H. K. Lonsdale in p. 40.1 Controlled Release Symposium 1974 published by the University of Akron, Akron, Ohio.
For d i s pe r sed system the release pro fi le can be presented as
For a dissolved system the release profile can be presented as
-(3)
Mt = amount release at time t A = Surface area
Cs = Solubility in the dispersed medium C0 = The initial concentration in the device I = thickness of the device
D = diffusion constant t100% = Time for 100% release or exhaustion time M100% = 100% content of the active By examining the release profiles, tablets using above mentioned prior art processes give extremely long t100% at a verv low ineffective level as Mt follows an exponential rate (see equations 3 & 4)(dragging on) whereas the tablets of the present process give clear cut end points (see equations 1 & 2). Illustrative diagrams of these two systems for release profile are included in the above Baker and Lonsdale's publication. Those products of the prior art which form solid solutions are described in U.S. Patents #3,317,394, #3,432,592, #3,344,029, #4,167,558, #3,402,240, #3,062,720, #3,147,187, #3,965,256 and #3,362,880.
4. Identification of Controlled Release Components. Tablets of prior art processes are also distinguished from the tablet of present process and compositions by identification of controlled release material. U.S. Patents #3,317,394 and #3,432,592 use a single thermoplastic or injection moldable polymeric material. Many prior art processes such as in U.S. Patents #3,147,187, #3,976,256 and #3,302,880 use water swellable hydroxycellulose or hydrocolloid polymers as binders. U.S. Patent #4,167,558 uses a hydrocolloid as buoyant material. All these water swellable polymers can be identified by a standard analytical method. U.S. Patent #3,402,240 uses glucose, a simple sugar as a binder. The present process does not use such polymers or glucose as binder or buoyant material. U.S. Patent #3,279,998 uses dry direct compression process with only micropulverized lipid material without any hydrophobic carbohydrate polymer.
The directly compressed tablets of U.S. Patent #3,577,514 using an enteric substance are easily distinguished from the tablets of the present process. The former does not release the biologically active component in the acid pH and release only in the medium of alkaline pH. The present process tablet releases the active continuously regardless of pH conditions. The former therefore can not be used as controlled release dosage forms. The present new process of direct compression with dry blend controlled release compositions without heat and solvent procedures produces very different controlled release tablets from those obtained from published processes. The product of the present process should be able to be easily distinguished in the market places from those of the prior art processes. Analytical tools and methods for differentiation are UV, IR, Fourier Transform IR, NMR, Solid State NMR Spectroscopics, X-ray diffraction, light, electron and polarized microscopy, mass spectroscopy, thermal analysis, gaa chromatography, high pressure liquid chromatography, other residue analysis methods and release test methods.
Claims
WHAT IS CLAIMED IS:
1. A dry, direct compressed product containing controlled release dosage forms of therapeutically active particulate agents produced without heat or solvents by the steps comprising: (a) dry blending blend particles, all of which have particle sizes smaller than 20 mesh, consisting essentially of from 0.61 to 95 weight parts of biologically active particulate solids with from 5 to 99.99 weight parts of a matrix blend combination consisting essentially of from 1 to 96 parts by weight of a hydrophobic carbohydrate polymer of the class of ethyl cellulose, propyl cellulose, cellulose acetate, cellulose propionate, cellulose acetatebutryate and cellulose acetate-propionate and from 4 to 99 parts by weight of a digestive-difficulty soluble component of the class consisting of a wax of the class consisting of carnauba wax, spermceti, beeswax, candelilla wax, esparto and paraffins; a fatty acid material of the class consisting of fatty acids having from 12 to 28 carbon atoms, fatty monoalchols having 12 to 28 carbon atoms, fatty amines and amides having from 12 to 28 carbon atoms; a neutral lipid of the class consisting of stearin, palmitin, castorwax, phospholipids, glycolipids, glycerides, hydrogenated cottonseed oil, hydrogenated cottonseed oil, hydrogenated tallow and metal and organic salts of C11-C28 fatt acids; and, mixtures thereof; (b) compressing said blended solids and blend under a pressure of 1.5 to 20 tons per square inch; and (c) thereafter, recovering said compressed product having a hardness of 4 to 25 kg and excellent resistance to delamination when subjected to an external longitudinal force.
2. A dry, direct compressed product according to claim 1 wherein said hydrophobic polymer is ethyl cellulose present in from 5 to 30 weight parts and the balance of said combination being selected from carnauba wax, hydrogenated cottonseed oil, a fatty acid having from 12 to 28 carbons or mixtures thereof.
3. A matrix composition according to claim 1 wherein: said hydrophobic cellulose polymer is ethyl cellulose present in from 3 to 50 parts by weight; said wax is carnauba wax present in from 5 to 70 parts by weight; said fatty acid material is a mixture of stearic acid and palmitic acid; said neutral lipid is hydrogenated cottonseed oil; and, said fatty acid material or said neutral lipid or both is present in from 5 to 80 parts by weight.
4. A product according to claim 1 wherein said compressing is followed by the further steps (c) of granulating said tablets and blending them with additional excipients and (d) finally, compressing said blend into tablets with desired release rates.
5. A product according to claim 1 wherein said mixture of said digestive-difficulty soluble component comprises from 2 to 97 parts by weight of said wax and from 2 to 97 parts by weight of said fatty acid material, of said neutral lipid and of mixtures thereof.
6. A product according to claim 1 wherein: said polymer is present in from 5 to 30 parts by weight; said wax is present in from 10 to 40 parts by weight; said fatty acid material is present in from 10 to 70 parts by weight; and, said neutral lipid is present in from 10 to 70 parts by weight.
7. A product according to claim 1 wherein said blended particles of step (a) additionally contains from 0.01 to 10 weight parts of hydrophobic fumed silica whereby the dissolution release rate is reduced.
8. A product according to claim 7 wherein said silica amounts to from 0.05 to 3 weight parts.
9. A method for reducing the controlled release rate of tablets containing both a biologically active agent and a lipid controlled release excipient comprising the step of dispersing from 0.05 to 3 weight percent of hydrophobic fumed silica throughout said tablet.
10. A dry, direct compression tablet comprising at least one particulate biologically active agent evenly dispersed within a controlled release excipient binder matrix characterized in that the tablet has a hardness of 3 to 25 kilograms and the absence of: a derivative of said agent resulting from its degradation by heat and/or solvent; solvent residue; interlinked excipient skeletal structures; and, solid solutions and eutectic mixtures of the tablet components.
11. The tablet of claim 10 wherein said binder matrix contains as an essential ingredient a hydrophobic carbohydrate polymer.
12. The tablet of claim 11 wherein said agent is from 0.01 to 95 wt. % and said matrix is from 99.99 to 5 wt. % of said tablet and said matrix contains from 1 to 100 wt. % of said polymer and up to 99 wt. % of a difficulty soluble component.
14. A method of preparing a controlled release tablet comprising the steps of: (a) dry blending a desired level of biologically active particulate agent into an excipient binder having as an essential ingredient a hydrophobic carbohydrate polymer, said blend having a particle size smaller than 10 mesh; and (b) compressing said blend into tablets of from 3 to 25 kg hardness as measured on a Pfizer hardness tester.
15. A method according to Claim 14 wherein said compressing is followed by the further steps (c) of granulating said tablets and blending them with additional excipients and (d) finally, compressing said blend into tablets with desired release rates.
16. The method according to Claim 14 wherein said desired level ranged from 0.01 to 95 wt. 5, said binder comprising at least one difficulty soluble component and said polymer is ethyl cellulose and present in about 10 to 30 wt. %, said wt. 5 based on the total weight of said blend.
Priority Applications (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP59502292A JPH0759502B2 (en) | 1983-05-31 | 1984-05-29 | Dry direct compression composition for modified release dosage forms |
| BR8406921A BR8406921A (en) | 1983-05-31 | 1984-05-29 | DRY COMPRESSION COMPOSITES, DIRECT FOR CONTROLLED RELEASE DOSAGE FORMS |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US49922183A | 1983-05-31 | 1983-05-31 | |
| US60047284A | 1984-04-16 | 1984-04-16 |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| WO1984004674A1 true WO1984004674A1 (en) | 1984-12-06 |
Family
ID=27053077
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/US1984/000807 WO1984004674A1 (en) | 1983-05-31 | 1984-05-29 | Dry direct compression compositions for controlled release dosage forms |
Country Status (5)
| Country | Link |
|---|---|
| EP (1) | EP0147437A4 (en) |
| JP (1) | JPH0759502B2 (en) |
| AU (2) | AU581177B2 (en) |
| BR (1) | BR8406921A (en) |
| WO (1) | WO1984004674A1 (en) |
Cited By (15)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0208144A1 (en) | 1985-07-04 | 1987-01-14 | HEUMANN PHARMA GMBH & CO | Pharmaceutical granulate containing ambroxol or bromohexine |
| EP0224146A2 (en) * | 1985-11-18 | 1987-06-03 | F. Hoffmann-La Roche Ag | Pharmaceutical preparation for the controlled release of active ingredients |
| WO1991005547A1 (en) * | 1989-10-18 | 1991-05-02 | Eastman Kodak Company | Direct compression carrier composition |
| US5385887A (en) * | 1993-09-10 | 1995-01-31 | Genetics Institute, Inc. | Formulations for delivery of osteogenic proteins |
| US5458888A (en) * | 1994-03-02 | 1995-10-17 | Andrx Pharmaceuticals, Inc. | Controlled release tablet formulation |
| WO2002100389A2 (en) * | 2001-06-12 | 2002-12-19 | Rhodia, Inc. | Compressible guaifenesin compositions and method for producing them |
| US7658938B2 (en) * | 1999-02-22 | 2010-02-09 | Merrion Reasearch III Limited | Solid oral dosage form containing an enhancer |
| US7704977B2 (en) | 2006-04-07 | 2010-04-27 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US7820722B2 (en) | 2001-05-11 | 2010-10-26 | Merrion Research Iii Limited | Permeation enhancers |
| US20130259937A1 (en) * | 2011-12-02 | 2013-10-03 | Pegasus Laboratories, Inc. | Amphipathic lipid-based sustained release compositions |
| US8802114B2 (en) | 2011-01-07 | 2014-08-12 | Merrion Research Iii Limited | Pharmaceutical compositions of iron for oral administration |
| US8999383B2 (en) | 2008-05-07 | 2015-04-07 | Merrion Research Iii Limited | Compositions of GnRH related compounds and processes of preparation |
| US9089484B2 (en) | 2010-03-26 | 2015-07-28 | Merrion Research Iii Limited | Pharmaceutical compositions of selective factor Xa inhibitors for oral administration |
| US20170114189A1 (en) * | 2015-10-27 | 2017-04-27 | Tenstech Inc. | Cellulosic microsheets |
| US10265384B2 (en) | 2015-01-29 | 2019-04-23 | Novo Nordisk A/S | Tablets comprising GLP-1 agonist and enteric coating |
Families Citing this family (1)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| SE455836B (en) * | 1985-10-11 | 1988-08-15 | Haessle Ab | PREPARATION WITH CONTROLLED RELEASE CONTAINING A SALT OF METOPROLOL AND METHOD FOR PREPARING THIS PREPARATION |
Citations (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3062720A (en) * | 1959-05-20 | 1962-11-06 | Philips Roxane | Sustained release pharmaceutical tablet |
| US3096248A (en) * | 1959-04-06 | 1963-07-02 | Rexall Drug & Chemical Company | Method of making an encapsulated tablet |
| US3115441A (en) * | 1962-06-06 | 1963-12-24 | Victor M Hermelin | Timed release pharmaceutical preparations and method of making the same |
| US3147187A (en) * | 1962-09-10 | 1964-09-01 | Don Hall Lab | Sustained release pharmaceutical |
| US3279998A (en) * | 1962-06-22 | 1966-10-18 | Smith Kline French Lab | Method of preparing sustained release tablets |
| US3317394A (en) * | 1955-12-22 | 1967-05-02 | Haessle Ab | Medicinal tablet and a method for its preparation |
| US3336200A (en) * | 1963-05-28 | 1967-08-15 | Warner Lambert Pharmaceutical | Tablet structure |
| US3344029A (en) * | 1963-06-03 | 1967-09-26 | U S Ethicals Inc | Sustained release composition |
| US3362880A (en) * | 1963-09-20 | 1968-01-09 | Dow Chemical Co | Compressed drug tablets of ethyl cellulose, glyceryl monostearate, karaya gum, tragacanth, talc, and magnesium stearate |
| US3400197A (en) * | 1965-01-26 | 1968-09-03 | Robins Co Inc A H | Compressible sustained release pharmaceutical tablet lipid-colloidal silica gel matrix fragment granules |
| US3402240A (en) * | 1957-06-25 | 1968-09-17 | Pfizer & Co C | Medicinal tablet and process of making same |
| US3432592A (en) * | 1962-08-31 | 1969-03-11 | Ciba Geigy Corp | Injection-moulded oral medicament in solid form |
| US3577514A (en) * | 1968-06-10 | 1971-05-04 | Pfizer | Sustained release pharmaceutical tablets |
| US3950508A (en) * | 1972-05-10 | 1976-04-13 | Laboratoires Servier | Process for obtaining pharmaceutical sustained releases |
| US3965256A (en) * | 1972-05-16 | 1976-06-22 | Synergistics | Slow release pharmaceutical compositions |
| US4167558A (en) * | 1976-02-13 | 1979-09-11 | Hoffmann-La Roche Inc. | Novel sustained release tablet formulations |
Family Cites Families (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3108046A (en) * | 1960-11-25 | 1963-10-22 | Smith Kline French Lab | Method of preparing high dosage sustained release tablet and product of this method |
| DE2050701C3 (en) * | 1969-10-17 | 1979-12-20 | Shinetsu Chemical Co., Tokio | Use of a pulverulent cellulose ether as an adjuvant for the production of easily disintegratable solid pharmaceutical forms |
| ZA836627B (en) * | 1982-10-08 | 1984-05-30 | Verex Lab | Constant release rate solid oral dosage formulation of pharmaceutical compounds having a high degree of water solubility |
| US4797288A (en) * | 1984-10-05 | 1989-01-10 | Warner-Lambert Company | Novel drug delivery system |
-
1984
- 1984-05-29 BR BR8406921A patent/BR8406921A/en unknown
- 1984-05-29 EP EP19840902301 patent/EP0147437A4/en not_active Withdrawn
- 1984-05-29 AU AU29676/84A patent/AU581177B2/en not_active Ceased
- 1984-05-29 WO PCT/US1984/000807 patent/WO1984004674A1/en not_active Application Discontinuation
- 1984-05-29 JP JP59502292A patent/JPH0759502B2/en not_active Expired - Lifetime
-
1989
- 1989-05-15 AU AU34751/89A patent/AU3475189A/en not_active Abandoned
Patent Citations (16)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US3317394A (en) * | 1955-12-22 | 1967-05-02 | Haessle Ab | Medicinal tablet and a method for its preparation |
| US3402240A (en) * | 1957-06-25 | 1968-09-17 | Pfizer & Co C | Medicinal tablet and process of making same |
| US3096248A (en) * | 1959-04-06 | 1963-07-02 | Rexall Drug & Chemical Company | Method of making an encapsulated tablet |
| US3062720A (en) * | 1959-05-20 | 1962-11-06 | Philips Roxane | Sustained release pharmaceutical tablet |
| US3115441A (en) * | 1962-06-06 | 1963-12-24 | Victor M Hermelin | Timed release pharmaceutical preparations and method of making the same |
| US3279998A (en) * | 1962-06-22 | 1966-10-18 | Smith Kline French Lab | Method of preparing sustained release tablets |
| US3432592A (en) * | 1962-08-31 | 1969-03-11 | Ciba Geigy Corp | Injection-moulded oral medicament in solid form |
| US3147187A (en) * | 1962-09-10 | 1964-09-01 | Don Hall Lab | Sustained release pharmaceutical |
| US3336200A (en) * | 1963-05-28 | 1967-08-15 | Warner Lambert Pharmaceutical | Tablet structure |
| US3344029A (en) * | 1963-06-03 | 1967-09-26 | U S Ethicals Inc | Sustained release composition |
| US3362880A (en) * | 1963-09-20 | 1968-01-09 | Dow Chemical Co | Compressed drug tablets of ethyl cellulose, glyceryl monostearate, karaya gum, tragacanth, talc, and magnesium stearate |
| US3400197A (en) * | 1965-01-26 | 1968-09-03 | Robins Co Inc A H | Compressible sustained release pharmaceutical tablet lipid-colloidal silica gel matrix fragment granules |
| US3577514A (en) * | 1968-06-10 | 1971-05-04 | Pfizer | Sustained release pharmaceutical tablets |
| US3950508A (en) * | 1972-05-10 | 1976-04-13 | Laboratoires Servier | Process for obtaining pharmaceutical sustained releases |
| US3965256A (en) * | 1972-05-16 | 1976-06-22 | Synergistics | Slow release pharmaceutical compositions |
| US4167558A (en) * | 1976-02-13 | 1979-09-11 | Hoffmann-La Roche Inc. | Novel sustained release tablet formulations |
Non-Patent Citations (1)
| Title |
|---|
| See also references of EP0147437A4 * |
Cited By (26)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| EP0208144A1 (en) | 1985-07-04 | 1987-01-14 | HEUMANN PHARMA GMBH & CO | Pharmaceutical granulate containing ambroxol or bromohexine |
| EP0224146A2 (en) * | 1985-11-18 | 1987-06-03 | F. Hoffmann-La Roche Ag | Pharmaceutical preparation for the controlled release of active ingredients |
| EP0224146A3 (en) * | 1985-11-18 | 1987-09-23 | F. Hoffmann-La Roche & Co. Aktiengesellschaft | Pharmaceutical preparation for the controlled release of active ingredients |
| US4892742A (en) * | 1985-11-18 | 1990-01-09 | Hoffmann-La Roche Inc. | Controlled release compositions with zero order release |
| WO1991005547A1 (en) * | 1989-10-18 | 1991-05-02 | Eastman Kodak Company | Direct compression carrier composition |
| US5385887A (en) * | 1993-09-10 | 1995-01-31 | Genetics Institute, Inc. | Formulations for delivery of osteogenic proteins |
| US5458888A (en) * | 1994-03-02 | 1995-10-17 | Andrx Pharmaceuticals, Inc. | Controlled release tablet formulation |
| US8828431B2 (en) | 1999-02-22 | 2014-09-09 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US7658938B2 (en) * | 1999-02-22 | 2010-02-09 | Merrion Reasearch III Limited | Solid oral dosage form containing an enhancer |
| US8053429B2 (en) | 1999-02-22 | 2011-11-08 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8323690B2 (en) | 1999-02-22 | 2012-12-04 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8323689B2 (en) | 1999-02-22 | 2012-12-04 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US7820722B2 (en) | 2001-05-11 | 2010-10-26 | Merrion Research Iii Limited | Permeation enhancers |
| US8039444B2 (en) | 2001-05-11 | 2011-10-18 | Isis Pharmaceuticals, Inc. | Antisense permeation enhancers |
| WO2002100389A2 (en) * | 2001-06-12 | 2002-12-19 | Rhodia, Inc. | Compressible guaifenesin compositions and method for producing them |
| WO2002100389A3 (en) * | 2001-06-12 | 2003-10-30 | Rhodia | Compressible guaifenesin compositions and method for producing them |
| US8883201B2 (en) | 2006-04-07 | 2014-11-11 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8883203B2 (en) | 2006-04-07 | 2014-11-11 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US7704977B2 (en) | 2006-04-07 | 2010-04-27 | Merrion Research Iii Limited | Solid oral dosage form containing an enhancer |
| US8999383B2 (en) | 2008-05-07 | 2015-04-07 | Merrion Research Iii Limited | Compositions of GnRH related compounds and processes of preparation |
| US9089484B2 (en) | 2010-03-26 | 2015-07-28 | Merrion Research Iii Limited | Pharmaceutical compositions of selective factor Xa inhibitors for oral administration |
| US8802114B2 (en) | 2011-01-07 | 2014-08-12 | Merrion Research Iii Limited | Pharmaceutical compositions of iron for oral administration |
| US20130259937A1 (en) * | 2011-12-02 | 2013-10-03 | Pegasus Laboratories, Inc. | Amphipathic lipid-based sustained release compositions |
| US10722458B2 (en) * | 2011-12-02 | 2020-07-28 | Pegasus Laboratories, Inc. | Amphipathic lipid-based sustained release compositions |
| US10265384B2 (en) | 2015-01-29 | 2019-04-23 | Novo Nordisk A/S | Tablets comprising GLP-1 agonist and enteric coating |
| US20170114189A1 (en) * | 2015-10-27 | 2017-04-27 | Tenstech Inc. | Cellulosic microsheets |
Also Published As
| Publication number | Publication date |
|---|---|
| EP0147437A1 (en) | 1985-07-10 |
| JPS60501459A (en) | 1985-09-05 |
| AU2967684A (en) | 1984-12-18 |
| EP0147437A4 (en) | 1987-03-05 |
| BR8406921A (en) | 1985-06-04 |
| AU3475189A (en) | 1989-09-07 |
| JPH0759502B2 (en) | 1995-06-28 |
| AU581177B2 (en) | 1989-02-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US4882167A (en) | Dry direct compression compositions for controlled release dosage forms | |
| US4590062A (en) | Dry direct compression compositions for controlled release dosage forms | |
| AU581177B2 (en) | Dry direct compression compositions for controlled release dosage forms | |
| US5486364A (en) | Readily available konjac glucomannan as a sustained release excipient | |
| DE69901938T3 (en) | FAST CRUMPING TABLET | |
| US5830576A (en) | Solid dosage forms | |
| DE69915718T2 (en) | Flowable, directly compressible starch as a binder, disintegrant and filler for pressed tablets and hard gelatin capsules | |
| DE69434640T2 (en) | Heterodisperse hydrogel systems for the sustained release of insoluble drugs | |
| DE69627934T2 (en) | PHARMACEUTICAL AUXILIARY SUBSTANCE WITH IMPROVED COMPRESSIBILITY | |
| US4708874A (en) | Devices for the controlled release of active substances, as well as process for the preparation thereof | |
| DE60036205T2 (en) | Granules consisting of starch and lactose | |
| DE69802543T3 (en) | IMPROVED MULTIPARTICLE TABLET WITH FAST RESOLUTION | |
| AT398165B (en) | METHOD FOR PRODUCING A PHARMACEUTICAL CAPSULE FOR ORAL ADMINISTRATION WITH DELAYED ACTIVE SUBSTANCE RELEASE OR. COMBINED DELAYED / RAPID RELEASE OF ACTIVE SUBSTANCE | |
| AU657114B2 (en) | Carrier for active agents, and solid dosage forms prepared therewith | |
| KR100236326B1 (en) | Directly compressible high load acetaminophen formulations | |
| US3147187A (en) | Sustained release pharmaceutical | |
| DE69821553T2 (en) | Granule preparation containing simethicone and granulated anhydrous calcium phosphate | |
| US4880830A (en) | Slow release formulation | |
| AT392901B (en) | METHOD FOR PRODUCING A GRANULAR SHAPE WITH DELAYED RELEASE OF PHARMACEUTICALLY ACTIVE SUBSTANCES | |
| DE69838300T2 (en) | PHARMACEUTICAL SUSPENSION TABLET COMPOSITIONS | |
| FI96095C (en) | A method of preparing a sustained release medicament | |
| EP0108218A2 (en) | Constant release rate solid oral dosage formulation of pharmaceutical compounds having a high degree of water solubility | |
| JPS61502759A (en) | Erodible matrix for use in pre-release biologically active compositions | |
| CH646331A5 (en) | DOSING UNIT IN THE FORM OF A CAPSULE CONTAINING PHARMACEUTICAL ACTIVE MATERIAL. | |
| DE60019550T2 (en) | SHOCK ABSORBERS FOR THE MANUFACTURE OF SOLID FORMIC OBJECTS |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AK | Designated states |
Designated state(s): AU BR DK FI HU JP NO RO SU US |
|
| AL | Designated countries for regional patents |
Designated state(s): AT BE CF CG CH CM DE FR GA GB LU MR NL SE SN TD TG |
|
| WWP | Wipo information: published in national office |
Ref document number: 1984902301 Country of ref document: EP |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 1984902301 Country of ref document: EP |
|
| WWW | Wipo information: withdrawn in national office |
Ref document number: 1984902301 Country of ref document: EP |