USRE37599E1 - Poly(arylene ether) compositions and the method for their manufacture - Google Patents
Poly(arylene ether) compositions and the method for their manufacture Download PDFInfo
- Publication number
- USRE37599E1 USRE37599E1 US09/659,380 US65938000A USRE37599E US RE37599 E1 USRE37599 E1 US RE37599E1 US 65938000 A US65938000 A US 65938000A US RE37599 E USRE37599 E US RE37599E
- Authority
- US
- United States
- Prior art keywords
- formula
- composition
- integer
- compound
- comprised
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Expired - Fee Related
Links
- 0 CO*OC1=C(F)C(F)=C(C2=C(F)C(F)=C(C)C(F)=C2F)C(F)=C1F Chemical compound CO*OC1=C(F)C(F)=C(C2=C(F)C(F)=C(C)C(F)=C2F)C(F)=C1F 0.000 description 17
- VVDKCESGQNRCFY-UHFFFAOYSA-N C.C.C.C.C.C1=CC2=C3C(=C1)/C=C\C1=C3C(=CC=C1)C=C2.C1=CC=C2C(=C1)C=CC1=C2C=CC=C1.CC.CC.CC.CC.CC.CC1=C2C=CC=CC2=CC2=C1C=CC=C2.CC1=C2C=CC=CC2=CC=C1.CC1=C2C=CC=CC2=NC2=C1C=CC=C2.CC1=CC(C)=C(C)C(C)=C1.CC1=CC=CC2=NC=CN=C12.CC1=CC=CC2=NC=CN=C12 Chemical compound C.C.C.C.C.C1=CC2=C3C(=C1)/C=C\C1=C3C(=CC=C1)C=C2.C1=CC=C2C(=C1)C=CC1=C2C=CC=C1.CC.CC.CC.CC.CC.CC1=C2C=CC=CC2=CC2=C1C=CC=C2.CC1=C2C=CC=CC2=CC=C1.CC1=C2C=CC=CC2=NC2=C1C=CC=C2.CC1=CC(C)=C(C)C(C)=C1.CC1=CC=CC2=NC=CN=C12.CC1=CC=CC2=NC=CN=C12 VVDKCESGQNRCFY-UHFFFAOYSA-N 0.000 description 5
- ONUFSRWQCKNVSL-UHFFFAOYSA-N FC1=C(F)C(F)=C(C2=C(F)C(F)=C(F)C(F)=C2F)C(F)=C1F Chemical compound FC1=C(F)C(F)=C(C2=C(F)C(F)=C(F)C(F)=C2F)C(F)=C1F ONUFSRWQCKNVSL-UHFFFAOYSA-N 0.000 description 4
- WNXPHLIWYUTFAZ-UHFFFAOYSA-N [H]C1=C([H])C(C2=CC(F)=C(F)C(F)=C2)=C([H])C([H])=C1C1=CC(F)=C(C)C(F)=C1 Chemical compound [H]C1=C([H])C(C2=CC(F)=C(F)C(F)=C2)=C([H])C([H])=C1C1=CC(F)=C(C)C(F)=C1 WNXPHLIWYUTFAZ-UHFFFAOYSA-N 0.000 description 3
- FNJDHOHLVCAGQY-UHFFFAOYSA-N C.C/C1=C2\C=C/C=C3/C4=CC=CC5=C4/C4=C6/C(=CC=C/C6=C/5C)C5=C(C1=CC=C5)C4=C32.CC1=C(C2=CC=CC=C2)C=C(C2=CC(C3=CC=CC=C3)=C(C)C(C3=CC=CC=C3)=C2)C=C1C1=CC=CC=C1.CC1=CC(F)=C(C2=C(F)C=C(C)CC2F)C(F)=C1.CC1=CC2=C3C(=C1)/C=C\C1=C3C(=CC(C)=C1)C=C2.CC1=CC=C(C2(C3=CC=CC=C3)C3=C(C=CC=C3)C3=C\C=C/C=C\32)C=C1.CC1=CC=C(C2=C(C3=CC=CC=C3)C(C3=CC=CC=C3)=C(C3=CC=C(C)C=C3)C(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1.CC1=CC=C(C2=CC=C(C)C=C2OC(F)(F)F)C(OC(F)(F)F)=C1.CC1=CC=C(C2=CC=C(C)CC2C(F)(F)F)C(C(F)(F)F)=C1 Chemical compound C.C/C1=C2\C=C/C=C3/C4=CC=CC5=C4/C4=C6/C(=CC=C/C6=C/5C)C5=C(C1=CC=C5)C4=C32.CC1=C(C2=CC=CC=C2)C=C(C2=CC(C3=CC=CC=C3)=C(C)C(C3=CC=CC=C3)=C2)C=C1C1=CC=CC=C1.CC1=CC(F)=C(C2=C(F)C=C(C)CC2F)C(F)=C1.CC1=CC2=C3C(=C1)/C=C\C1=C3C(=CC(C)=C1)C=C2.CC1=CC=C(C2(C3=CC=CC=C3)C3=C(C=CC=C3)C3=C\C=C/C=C\32)C=C1.CC1=CC=C(C2=C(C3=CC=CC=C3)C(C3=CC=CC=C3)=C(C3=CC=C(C)C=C3)C(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1.CC1=CC=C(C2=CC=C(C)C=C2OC(F)(F)F)C(OC(F)(F)F)=C1.CC1=CC=C(C2=CC=C(C)CC2C(F)(F)F)C(C(F)(F)F)=C1 FNJDHOHLVCAGQY-UHFFFAOYSA-N 0.000 description 2
- ZIOZFPYIGFIILJ-UHFFFAOYSA-N C1=CC=C2N=C3C(=NC2=C1)C1=CC=CC=C1C1=C3C=CC(C2=C/C3=C(\C=C/2)C2=NC4=CC=CC=C4N=C2C2=CC=CC=C23)=C1.CC.CC.CC.CC1=C/C2=C3/C4=C(/C=C(C)/C=C4\C4=C(C=CC=C4)\C3=C/1)C1=CC=CC=C12.CC1=C2/C=C\C3=C4C2=C2C(=C1)/C=C\C1=C2C2=C/4C(=C/C(C)=C\2C=C1)/C=C\3.CC1=CC=C(C2=NC3=CC=CC=C3N=C2C2=CC=CC=C2)C=C1.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)C1=NC3=CC=CC=C3N=C21.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)C1=NC3=CC=CC=C3N=C21 Chemical compound C1=CC=C2N=C3C(=NC2=C1)C1=CC=CC=C1C1=C3C=CC(C2=C/C3=C(\C=C/2)C2=NC4=CC=CC=C4N=C2C2=CC=CC=C23)=C1.CC.CC.CC.CC1=C/C2=C3/C4=C(/C=C(C)/C=C4\C4=C(C=CC=C4)\C3=C/1)C1=CC=CC=C12.CC1=C2/C=C\C3=C4C2=C2C(=C1)/C=C\C1=C2C2=C/4C(=C/C(C)=C\2C=C1)/C=C\3.CC1=CC=C(C2=NC3=CC=CC=C3N=C2C2=CC=CC=C2)C=C1.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)C1=NC3=CC=CC=C3N=C21.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)C1=NC3=CC=CC=C3N=C21 ZIOZFPYIGFIILJ-UHFFFAOYSA-N 0.000 description 2
- YFCKNEOMNTZLJQ-UHFFFAOYSA-N CC1=C2C=CC=CC2=C(C2=C3C=CC=CC3=C(C)C=C2)C=C1.CC1=CC=C(C#CC2=CC=CC=C2)C(C2=C(C#CC3=CC=CC=C3)C=CC(C)=C2)=C1.CC1=CC=C(C2=C(C#CC3=CC=CC=C3)C=C(C)C=C2)C(C#CC2=CC=CC=C2)=C1.CC1=CC=C2C(=C1)OC1=CC(C)=CC=C1C21C2=CC=CC=C2C2=C1C=CC=C2 Chemical compound CC1=C2C=CC=CC2=C(C2=C3C=CC=CC3=C(C)C=C2)C=C1.CC1=CC=C(C#CC2=CC=CC=C2)C(C2=C(C#CC3=CC=CC=C3)C=CC(C)=C2)=C1.CC1=CC=C(C2=C(C#CC3=CC=CC=C3)C=C(C)C=C2)C(C#CC2=CC=CC=C2)=C1.CC1=CC=C2C(=C1)OC1=CC(C)=CC=C1C21C2=CC=CC=C2C2=C1C=CC=C2 YFCKNEOMNTZLJQ-UHFFFAOYSA-N 0.000 description 2
- ZTVXQGZDIOAWKV-UHFFFAOYSA-N OC1=CC=C(C#CC2=CC=CC=C2)C(C2=C(C#CC3=CC=CC=C3)C=CC(O)=C2)=C1.OC1=CC=C(C2=C(C#CC3=CC=CC=C3)C=C(O)C=C2)C(C#CC2=CC=CC=C2)=C1 Chemical compound OC1=CC=C(C#CC2=CC=CC=C2)C(C2=C(C#CC3=CC=CC=C3)C=CC(O)=C2)=C1.OC1=CC=C(C2=C(C#CC3=CC=CC=C3)C=C(O)C=C2)C(C#CC2=CC=CC=C2)=C1 ZTVXQGZDIOAWKV-UHFFFAOYSA-N 0.000 description 2
- MRCDXYXNQUIHJT-UHFFFAOYSA-N C.C.C.C.C.C.C1=CC2=C3C(=C1)/C=C\C1=C3C(=CC=C1)C=C2.C1=CC=C2C(=C1)C=CC1=C2C=CC=C1.CC.CC.CC.CC.CC.CC1=C2C=CC=CC2=CC2=C1C=CC=C2.CC1=C2C=CC=CC2=CC=C1.CC1=C2C=CC=CC2=NC2=C1C=CC=C2.CC1=CC(C)=C(C)C(C)=C1.CC1=CC=CC2=NC=CN=C12.CC1=CC=CC2=NC=CN=C12 Chemical compound C.C.C.C.C.C.C1=CC2=C3C(=C1)/C=C\C1=C3C(=CC=C1)C=C2.C1=CC=C2C(=C1)C=CC1=C2C=CC=C1.CC.CC.CC.CC.CC.CC1=C2C=CC=CC2=CC2=C1C=CC=C2.CC1=C2C=CC=CC2=CC=C1.CC1=C2C=CC=CC2=NC2=C1C=CC=C2.CC1=CC(C)=C(C)C(C)=C1.CC1=CC=CC2=NC=CN=C12.CC1=CC=CC2=NC=CN=C12 MRCDXYXNQUIHJT-UHFFFAOYSA-N 0.000 description 1
- IKRZLKMKKZACAK-UHFFFAOYSA-N C.C.C1=CC2=C3C(=C1)/C=C\C1=C3C(=CC=C1)C=C2.C1=CC=C2C(=C1)C=CC1=C2C=CC=C1.CC.CC.CC.CC.CC.CC1=C2C=CC=CC2=CC2=C1C=CC=C2.CC1=C2C=CC=CC2=CC=C1.CC1=C2C=CC=CC2=NC2=C1C=CC=C2.CC1=CC(C)=C(C)C(C)=C1.CC1=CC=CC2=NC=CN=C12.CC1=CC=CC2=NC=CN=C12 Chemical compound C.C.C1=CC2=C3C(=C1)/C=C\C1=C3C(=CC=C1)C=C2.C1=CC=C2C(=C1)C=CC1=C2C=CC=C1.CC.CC.CC.CC.CC.CC1=C2C=CC=CC2=CC2=C1C=CC=C2.CC1=C2C=CC=CC2=CC=C1.CC1=C2C=CC=CC2=NC2=C1C=CC=C2.CC1=CC(C)=C(C)C(C)=C1.CC1=CC=CC2=NC=CN=C12.CC1=CC=CC2=NC=CN=C12 IKRZLKMKKZACAK-UHFFFAOYSA-N 0.000 description 1
- WJAOGKAMBRNTPI-UHFFFAOYSA-N C/C1=C2\C=C/C=C3/C4=CC=CC5=C4/C4=C6/C(=CC=C/C6=C/5C)C5=C(C1=CC=C5)C4=C32.CC1=C(C2=CC=CC=C2)C=C(C2=CC(C3=CC=CC=C3)=C(C)C(C3=CC=CC=C3)=C2)C=C1C1=CC=CC=C1.CC1=C2/C=C\C3=C4C2=C2C(=C1)/C=C\C1=C2C2=C/4C(=C/C(C)=C\2C=C1)/C=C\3.CC1=CC2=C3C(=C1)/C=C\C1=C3C(=CC(C)=C1)C=C2.CC1=CC=C(C2(C3=CC=CC=C3)C3=C(C=CC=C3)C3=C\C=C/C=C\32)C=C1.CC1=CC=C(C2=C(C3=CC=CC=C3)C(C3=CC=CC=C3)=C(C3=CC=C(C)C=C3)C(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1.CC1=CC=C(C2=CC=C(C)C=C2OC(F)(F)F)C(OC(F)(F)F)=C1 Chemical compound C/C1=C2\C=C/C=C3/C4=CC=CC5=C4/C4=C6/C(=CC=C/C6=C/5C)C5=C(C1=CC=C5)C4=C32.CC1=C(C2=CC=CC=C2)C=C(C2=CC(C3=CC=CC=C3)=C(C)C(C3=CC=CC=C3)=C2)C=C1C1=CC=CC=C1.CC1=C2/C=C\C3=C4C2=C2C(=C1)/C=C\C1=C2C2=C/4C(=C/C(C)=C\2C=C1)/C=C\3.CC1=CC2=C3C(=C1)/C=C\C1=C3C(=CC(C)=C1)C=C2.CC1=CC=C(C2(C3=CC=CC=C3)C3=C(C=CC=C3)C3=C\C=C/C=C\32)C=C1.CC1=CC=C(C2=C(C3=CC=CC=C3)C(C3=CC=CC=C3)=C(C3=CC=C(C)C=C3)C(C3=CC=CC=C3)=C2C2=CC=CC=C2)C=C1.CC1=CC=C(C2=CC=C(C)C=C2OC(F)(F)F)C(OC(F)(F)F)=C1 WJAOGKAMBRNTPI-UHFFFAOYSA-N 0.000 description 1
- BYYNOWPDRMMLCN-UHFFFAOYSA-N C1=CC=C2N=C3C(=NC2=C1)C1=CC=CC=C1C1=C3C=CC(C2=C/C3=C(\C=C/2)C2=NC4=CC=CC=C4N=C2C2=CC=CC=C23)=C1.CC.CC.CC.CC1=C/C2=C3/C4=C(/C=C(C)/C=C4\C4=C(C=CC=C4)\C3=C/1)C1=CC=CC=C12.CC1=CC2=C(C=C1)C1=NC3=CC=CC=C3N=C1C1=CC=CC=C12.CC1=CC=C(C#CC2=CC=CC=C2)C(C2=C(C#CC3=CC=CC=C3)C=CC(C)=C2)=C1.CC1=CC=C(C2=NC3=CC=CC=C3N=C2C2=CC=CC=C2)C=C1.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)C1=NC3=CC=CC=C3N=C21.CC1=CC=C2C(=C1)OC1=CC(C)=CC=C1C21C2=CC=CC=C2C2=C1C=CC=C2 Chemical compound C1=CC=C2N=C3C(=NC2=C1)C1=CC=CC=C1C1=C3C=CC(C2=C/C3=C(\C=C/2)C2=NC4=CC=CC=C4N=C2C2=CC=CC=C23)=C1.CC.CC.CC.CC1=C/C2=C3/C4=C(/C=C(C)/C=C4\C4=C(C=CC=C4)\C3=C/1)C1=CC=CC=C12.CC1=CC2=C(C=C1)C1=NC3=CC=CC=C3N=C1C1=CC=CC=C12.CC1=CC=C(C#CC2=CC=CC=C2)C(C2=C(C#CC3=CC=CC=C3)C=CC(C)=C2)=C1.CC1=CC=C(C2=NC3=CC=CC=C3N=C2C2=CC=CC=C2)C=C1.CC1=CC=C2C(=C1)C1=C(C=CC(C)=C1)C1=NC3=CC=CC=C3N=C21.CC1=CC=C2C(=C1)OC1=CC(C)=CC=C1C21C2=CC=CC=C2C2=C1C=CC=C2 BYYNOWPDRMMLCN-UHFFFAOYSA-N 0.000 description 1
- ZPVNKPIRINBMLL-UHFFFAOYSA-N CC#CC1=C(C2=C(C#CC)C=CC=C2)C=CC=C1.CO.CO Chemical compound CC#CC1=C(C2=C(C#CC)C=CC=C2)C=CC=C1.CO.CO ZPVNKPIRINBMLL-UHFFFAOYSA-N 0.000 description 1
- LVBMUQYNGJMBOM-UHFFFAOYSA-N CC1=CC(C#CC2=CC=CC=C2)=C(C2=CC=C(O)C=C2C#CC2=CC=CC=C2)C=C1.CC1=CC(C2=CC(O)=CC=C2C#CC2=CC=CC=C2)=C(C#CC2=CC=CC=C2)C=C1 Chemical compound CC1=CC(C#CC2=CC=CC=C2)=C(C2=CC=C(O)C=C2C#CC2=CC=CC=C2)C=C1.CC1=CC(C2=CC(O)=CC=C2C#CC2=CC=CC=C2)=C(C#CC2=CC=CC=C2)C=C1 LVBMUQYNGJMBOM-UHFFFAOYSA-N 0.000 description 1
- FDZOBCVCTRQMIL-UHFFFAOYSA-N CC1=CC=C(C2=C(C#CC3=CC=CC=C3)C=C(C)C=C2)C(C#CC2=CC=CC=C2)=C1 Chemical compound CC1=CC=C(C2=C(C#CC3=CC=CC=C3)C=C(C)C=C2)C(C#CC2=CC=CC=C2)=C1 FDZOBCVCTRQMIL-UHFFFAOYSA-N 0.000 description 1
Images
Classifications
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G65/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G65/34—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from hydroxy compounds or their metallic derivatives
- C08G65/38—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from hydroxy compounds or their metallic derivatives derived from phenols
- C08G65/40—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from hydroxy compounds or their metallic derivatives derived from phenols from phenols (I) and other compounds (II), e.g. OH-Ar-OH + X-Ar-X, where X is halogen atom, i.e. leaving group
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/52—Arrangements for conducting electric current within the device in operation from one component to another, i.e. interconnections, e.g. wires, lead frames
- H01L23/522—Arrangements for conducting electric current within the device in operation from one component to another, i.e. interconnections, e.g. wires, lead frames including external interconnections consisting of a multilayer structure of conductive and insulating layers inseparably formed on the semiconductor body
- H01L23/532—Arrangements for conducting electric current within the device in operation from one component to another, i.e. interconnections, e.g. wires, lead frames including external interconnections consisting of a multilayer structure of conductive and insulating layers inseparably formed on the semiconductor body characterised by the materials
- H01L23/5329—Insulating materials
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G65/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G65/34—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from hydroxy compounds or their metallic derivatives
- C08G65/38—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from hydroxy compounds or their metallic derivatives derived from phenols
- C08G65/40—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule from hydroxy compounds or their metallic derivatives derived from phenols from phenols (I) and other compounds (II), e.g. OH-Ar-OH + X-Ar-X, where X is halogen atom, i.e. leaving group
- C08G65/4006—(I) or (II) containing elements other than carbon, oxygen, hydrogen or halogen as leaving group (X)
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/28—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection
- H01L23/29—Encapsulations, e.g. encapsulating layers, coatings, e.g. for protection characterised by the material, e.g. carbon
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L23/00—Details of semiconductor or other solid state devices
- H01L23/48—Arrangements for conducting electric current to or from the solid state body in operation, e.g. leads, terminal arrangements ; Selection of materials therefor
- H01L23/488—Arrangements for conducting electric current to or from the solid state body in operation, e.g. leads, terminal arrangements ; Selection of materials therefor consisting of soldered or bonded constructions
- H01L23/498—Leads, i.e. metallisations or lead-frames on insulating substrates, e.g. chip carriers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2650/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G2650/28—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule characterised by the polymer type
- C08G2650/46—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule characterised by the polymer type containing halogen
- C08G2650/48—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule characterised by the polymer type containing halogen containing fluorine, e.g. perfluropolyethers
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G2650/00—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule
- C08G2650/28—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule characterised by the polymer type
- C08G2650/60—Macromolecular compounds obtained by reactions forming an ether link in the main chain of the macromolecule characterised by the polymer type containing acetylenic group
-
- H—ELECTRICITY
- H01—ELECTRIC ELEMENTS
- H01L—SEMICONDUCTOR DEVICES NOT COVERED BY CLASS H10
- H01L2924/00—Indexing scheme for arrangements or methods for connecting or disconnecting semiconductor or solid-state bodies as covered by H01L24/00
- H01L2924/0001—Technical content checked by a classifier
- H01L2924/0002—Not covered by any one of groups H01L24/00, H01L24/00 and H01L2224/00
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10S—TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10S428/00—Stock material or miscellaneous articles
- Y10S428/901—Printed circuit
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/24—Structurally defined web or sheet [e.g., overall dimension, etc.]
- Y10T428/24802—Discontinuous or differential coating, impregnation or bond [e.g., artwork, printing, retouched photograph, etc.]
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/24—Structurally defined web or sheet [e.g., overall dimension, etc.]
- Y10T428/24802—Discontinuous or differential coating, impregnation or bond [e.g., artwork, printing, retouched photograph, etc.]
- Y10T428/24917—Discontinuous or differential coating, impregnation or bond [e.g., artwork, printing, retouched photograph, etc.] including metal layer
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/31504—Composite [nonstructural laminate]
- Y10T428/3154—Of fluorinated addition polymer from unsaturated monomers
Definitions
- the present invention relates to fluorinated poly(arylene ether) compositions for use in coating microelectronic structures, such as integrated circuits, and the method for their manufacture. More particularly, this invention relates to the use of novel bisphenol reactants in the method of manufacturing novel poly(arylene ether) compositions which possess improved properties such as higher glass transition temperature and lower dielectric constant.
- Fluorinated poly(arylene ethers) based upon decafluorobiphenyl have been used extensively as replacements for polyimides for use in spin-coating substrates, such as multichip modules, printed circuit boards, integrated circuits and other microelectronic devices in intermetal dielectric production.
- fluorinated poly(arylene ethers) which are commercially available from AlliedSignal Inc. under the tradename “FLARETM”, not only exhibit a thermal stability comparable to that of polyimides but also possess about ten to forty times lower moisture absorption rate, a dielectric constant in the range of about 2.35 to about 2.65, and good retention of storage modulus above their glass transition temperature (“Tg”). These properties are relevant not only to enhanced circuit performance, i.e.
- R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition
- n is an integer of from about 0 to about 50;
- n is an integer of from about 1 to about 200.
- r is an integer of from about 0 to about 50
- q is an integer of from about 0 to about 49
- R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition under conditions sufficient to produce the composition.
- This invention is also directed to a process for producing a fluorinated poly(arylene ether) mixture suitable for spin-coating onto a substrate comprising:
- R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition
- n is an integer of from about 0 to about 50;
- n is an integer of from about 1 to about 200
- This invention is also directed to a process for producing a cured, coated substrate comprising:
- R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition
- n is an integer of from about 0 to about 50;
- n is an integer of from about 1 to about 200;
- step b applying the solution of step b onto a substrate to form a film thereon;
- step c heating the film of step c under conditions sufficient to cure the film.
- Another embodiment of the invention is directed to a process for making ethynylated, hydroxy-substituted biphenyl compounds comprising:
- R′ is comprised of an alkyl group having from about 1 to about 20 carbon atoms, an alkenyl group having from about 1 to about 20 carbon atoms, an aryl group, a heteroaryl group, and derivatives or mixtures thereof to form 2,2′-bis(C ⁇ CR′)-5,5′-bis(tetrahydropyranoxy)biphenyl; and
- poly(arylene ether) compositions of this invention not only possess a Tg in excess of about 350° C. without having to highly cross-link the compound or include cross-linkable pendent groups thereto as well as without sacrificing any of its other properties such as solubility, spin-coatability, high thermal stability, low moisture absorptivity, low dielectric constant, and planarizability.
- FIG. 1 illustrates a method for the synthesis of 2,2′-Diiodo-5,5′-dimethoxybiphenyl.
- FIG. 2 illustrates a method for synthesizing 2,2′-Bis(phenylethynyl)-5,5′-dihydroxybiphenyl.
- FIG. 3 illustrates a method for synthesizing poly(arylene ether) compositions according to the process of the present invention.
- FIG. 4a illustrates the thermal behavior of a poly(arylene ether) composition containing 2,2′-bis(phenylethynyl) biphenyl-5,5′-dioxy linkages during two thermal cycles under conditions of differential scanning calorimetry (“DSC”).
- FIG. 4b illustrates an enlarged view of the second scan of FIG. 4 a.
- FIG. 5 illustrates the thermal behavior of a poly(arylene ether) composition containing 2,2′-bis(phenylethynyl) biphenyl-5,5′-dioxy linkages under conditions of dynamic mechanical analysis.
- ethynylated aromatic compound starting materials which are preferably ethynylated hydroxy-substituted biphenyl (“bisphenol”) compounds corresponding to Formula B
- R is an aromatic nucleus that imparts a Tg of greater than about 260° C., and preferably from about 350° C. to about 450° C., to the compounds which contain them, are either commercially available and/or may readily be synthesized by one skilled in the art without undue experimentation based upon a conventional boron tribromide demethylation reaction of the R groups with methoxy substituents. Details of such reactions are well-known in the art and described at, for example, McOmie, J. F. W., et al., 24 Tetrahedron 2289-2292 (1972) [hereinafter “McOmie”].
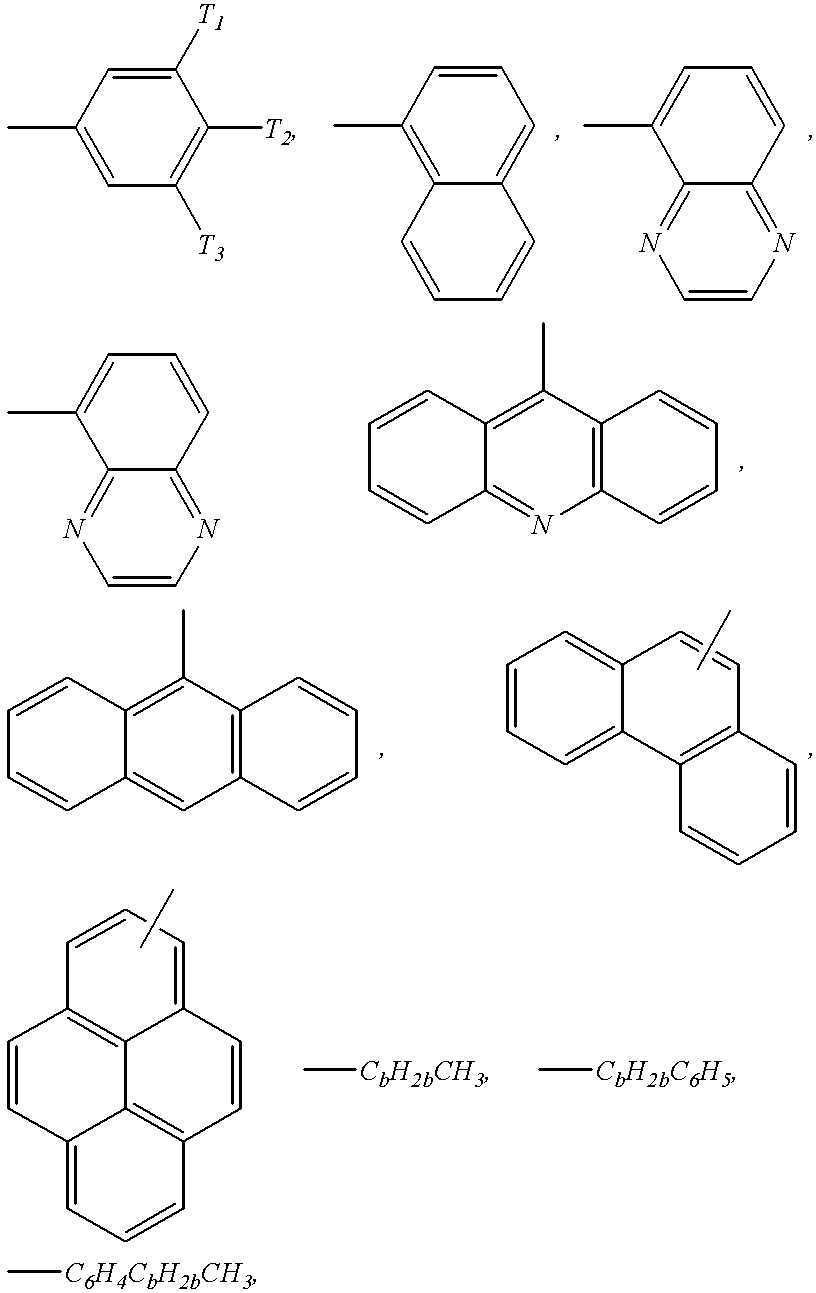
- R groups examples include
- Preferred bisphenol compounds include
- o-dianisidine 3,3′-dimethoxy-4,4′-diaminobiphenyl
- o-dianisidine 3,3′-dimethoxy-4,4′-diaminobiphenyl
- the 3,3′-dimethoxybiphenyl is converted into 2,2′-dihalo-5,5′-dimethyoxybiphenyl, and preferably 2,2′-diiodo-5,5′-dimethyoxy-biphenyl, via an initial reaction with a halogen such as chlorine or bromine, and preferably bromine, miscible in glacial acid such as carboxylic acids and anhydrides thereof including but not limited to propionic acid, butyric acid, and acetic acid under a temperature of from about 60° C. to about 150° C., and preferably from about 100° C. to about 120° C.
- a halogen such as chlorine or bromine, and preferably bromine
- miscible in glacial acid such as carboxylic acids and anhydrides thereof including but not limited to propionic acid, butyric acid, and acetic acid under a temperature of from about 60° C. to about 150° C., and preferably from about 100° C. to about 120
- Acetic acid is preferred.
- the period of reaction may range from about 1 hour to about 10 hours, and preferably from about 2 hours to about 4 hours.
- the concentration of halogen in the acid-containing mixture is from about 5 to about 50%, and preferably from about 10 to about 20%, based upon the total weight of the halogen-acid mixture.
- the molar ratio of 3,3′-dimethoxybiphenyl to the halogen desired is about 1 to 2, preferably about 1 to 3, and most preferably about 1 to 2.2.
- the product of the initial halogen reaction i.e., 2,2′-dibromo-5,5′dimethoxybiphenyl
- 2,2′-dibromo-5,5′dimethoxybiphenyl is then halogenated into a second 2,2′-dihalo-5,5′-dimethoxybiphenyl, i.e.
- 2,2′-diiodo-5,5′-dimethoxybiphenyl via a lithiation exchange reaction in the presence of an organolithium reagent, such as n-butyllithium, to form a dilithiated intermediate which is then reacted with the desired halogen, or via a Grignard reaction with magnesium to form a bis-Grignard intermediate, which is then reacted with the desired halogen.
- the lithiation-halogenation reactions occur at a temperature of from about ⁇ 78° C. to about ⁇ 20° C., and preferably from about ⁇ 78° C. to about ⁇ 50° C. and ambient pressure, while the Grignard-halogenation reactions occur under conditions of ambient temperature and pressure.
- Iodine is the preferred halogen.
- the molar ratio of the second 2,2′-dihalo-5,5′dimethoxybiphenyl to halogen is about 1:2, preferably about 1:3, and most preferably about 1:2.2.
- the molar ratio of the second 2,2′-dihalo-5,5′dimethoxybiphenyl to the organolithium reagent or magnesium is about 1:2, preferably about 1:3, and most preferably about 1:2.5.
- the period of these halogenation reactions may depend upon the specific chemical structure, but will generally be from about 0.5 hours to about 4 hours, and preferably from about 1 hour to about 2 hours.
- the second 2,2′-dihalo-5,5′-dimethoxybiphenyl and preferably 2,2′-diiodo-5,5′-dimethoxybiphenyl, is then demethylated under temperatures of about ⁇ 78° C. to about ⁇ 20° C., and preferably from about ⁇ 78° C.
- a bromide-containing compound such as hydrogen bromide, boron tribromide, or mixtures thereof in a chlorinated solvent such as a chlorohydrocarbon including but not limited to dichloromethane to yield 2,2′-dihalo-5,5′-dihydroxybiphenyl, and preferably 2,2′-diiodo-5,5′-dihydroxybiphenyl.
- a chlorinated solvent such as a chlorohydrocarbon including but not limited to dichloromethane to yield 2,2′-dihalo-5,5′-dihydroxybiphenyl, and preferably 2,2′-diiodo-5,5′-dihydroxybiphenyl.
- Boron tribromide is the preferred reactant.
- the concentration of the bromide-containing compound in the chlorinated solvent may range from 0.05M to about 1M, and preferably from about 0.1M to about 0.5M.
- the period of the demethylation reaction may depend upon the chemical structure, but will generally be from about 5 minutes to about 2 hours, and preferably from about 15 minutes to about 1 hour.
- the molar ratio of 2,2′-diiodo-5,5′dimethoxybiphenyl to the bromide-containing compound is about 1:3 to 1:10, and preferably about 1:2.5 to 1:5.
- the product of the demethylation reaction i.e. 2,2′-diiodo-5,5′-dihydroxybiphenyl
- a compound containing a hydroxyl protective group preferably dihydropyran
- a protection catalyst under temperature conditions ranging between 0° C. to about 30° C., and preferably from about 20° C. to about 25° C., and ambient pressures to form 2,2′-diiodo-5,5′-bis(tetrahydropyranoxy)biphenyl.
- Suitable protection catalysts have a purity of at least about 99% and include arylsulfonic acids such as p-toluenesulfonic acid, benzenesulfonic acid, and p-bromophenylsulfonic acid, with p-toluenesulfonic acid being preferred.
- the molar ratio of the hydroxyl-containing compound to 2,2′-diiodo-5,5′-dihydroxybiphenyl is from about 100:1 to 500:1, more preferably from about 200:1 to 250:1, and most preferably from 100:1 to 150:1.
- the period of the hydroxy reaction may depend upon the reactivity, but will generally be from about 1 hour to about 48 hours, and preferably from about 12 hours to about 24 hours.
- the 2,2′-diiodo-5,5′-bis(tetrahydropyranoxy)biphenyl, and preferably 2,2′-diiodo-5,5′-bis(tetrahydropyranoxy)biphenyl, is then ethynylated with a solution of a copper acetylide compound in a solvent having a boiling point of from about 100° C. to about 120° C.
- R′ is a moiety comprising an alkyl having from about 1 to about 20, and preferably from about 1 to about 6 carbon atoms; an alkenyl having from about 1 to about 20, and preferably from about 2 to about 10 carbon atoms; an aryl such as those having a substituted phenyl ring as illustrated by tolyl or anisyl, or those having a fused aromatic nucleus such as naphthalene, anthracene, phenanthrene, and pyrene; a heteroaryl such as furan, pyrrole, thiophene, pyridine, quinoline, quinoxaline, and derivatives and mixtures thereof, and preferably is a phenyl, a pentafluorophenyl, or a biphenylyl group, and most preferably a phenyl group.
- R 1 , R 2 , and R 3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl comprising phenyl, or biphenylyl, or terphenylyl, or mixtures thereof; and n is an integer of from about 0 to about 20, and preferably about 1 to about 5.
- the temperature of the ethynylation reaction may range from about 80° C. to about 1 50° C., and preferably from about 90° C. to about 110° C .
- the pressure is ambient.
- the molar ratio of 2,2′-dihalo-5,5′-bis(tetrahydropyranoxy) biphenyl, i.e. 2,2′-dihalo-5,5′-bis(tetrahydropyranoxy)biphenyl, to copper acetylide compound is about 1 to 10, preferably about 1 to 5 and most preferably about 1 to 2.5.
- the period of the ethynylation reaction will depend upon reactivity of the haloaromatic, i.e. 2,2′-diiodo-5,5′-bis(tetrahydropyranoxy)biphenyl, but will generally be from about 30 minutes to about 48 hours, and preferably from about 4 hours to about 30 hours.
- the copper[I] acetylide compound is of the formula
- R′ is as described above.
- the preferred copper[I] acetylide compound is copper[I] phenylacetylide. Further details of the phenylethynylation of haloaromatics by copper[I] phenylacetylide is described in, for example, Stephens, R. D., et al, 28 J. Org. Chem. 3313-3315 (1963) and Castro, C. E., et al., 31 Journal of Organic Chemistry 4071-4078 (1966).
- suitable solvents for the ethynylation reaction include pyridine and pyridines substituted with moieties such as alkyl having from about I to about 10, and preferably from about 1 to about 5 carbon atoms. Pyridine is preferred.
- the solvents have a purity of at least about 95% and a copper[I] acetylide concentration of from about 10% to about 40%, and preferably from about 10% to about 20%.
- tetrahydropyranyl groups are removed from 2,2′-bis(C ⁇ CR′)-5,5′-bis(tetrahydropyranoxy)biphenyl by acid hydrolysis under ambient conditions to form ethynylated aromatic compounds of the formula 2,2′-bis(C ⁇ CR′)-5,5′-dihydroxybiphenyl, wherein the substituent R′ is as above-described.
- Suitable acid solutions contain, based upon the total weight of the solution, from about greater than 0% to about 20%, and preferably from about 5% to about 10% acid. Any acid may be used, but hydrochloric acid is preferred.
- suitable solvents include any aprotic solvent such as toluene.
- the molar ratio of the acid solution to 2,2′-bis(C ⁇ CR′)-5,5′-bis(tetrahydropyranoxy)biphenyl is from about 80:20 to 50:50, and preferably from about 80:20 to 75:25.
- the period of the hydrolysis reaction generally will be from about 1 hour to about 10 hours, and preferably from about 1 hour to about 3 hours.
- ethnylated aromatic compounds produced according to the process of the present invention include those 2,2-bis(substituted ethynyl)-5,5′-bis(tetrahydropryanoxy)biphenyl compounds of the formula:
- the preferred ethynylated aromatic compound is 2,2′-bis(phenylethynyl)-5,5′-dihydroxybiphenyl.
- FIG. 2 illustrates the preferred synthetic sequence for the synthesis of the preferred monomer, 2,2′-bis(phenylethynyl)-5,5′-dihydroxybiphenyl.
- the products of each above-described reaction step are recovered from any products, solvent and catalyst that may be present prior to commencement of the next reaction step.
- the products should be about 85-100% , and more preferably about 95-99% pure.
- the products may be recovered by any means well known in the art including but not limited to distillation, reprecipitation, extraction, and recrystallization.
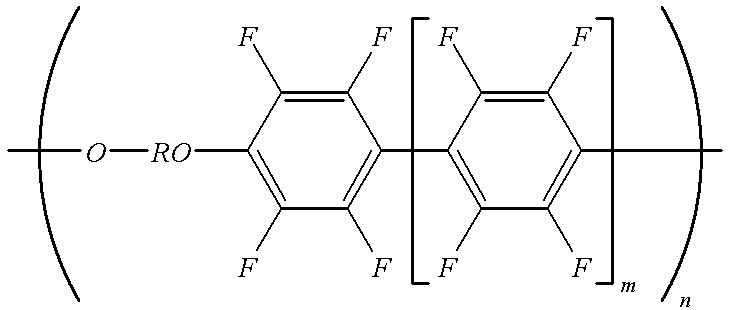
- the other starting material for producing the poly(arylene ethers) of the present invention includes the perfluorinated phenylenes of Formula A:
- r is an integer of from about 0 to about 50, preferably from about 0 to 10, and more preferably from about 0 to about 3;
- q is an integer of from about 0 to about 49, preferably from about 0 to about 9, and more preferably from about 0 to about 3.
- the fluorinated poly(arylene ethers) of Formula C may be synthesized by reacting the starting materials, i.e. the bisphenols of Formula B and the fluorinated phenylene compounds of Formulas A or D, under conditions sufficient to affect the polymerization reaction.
- the bisphenol moieties of Formula A or D are replaced with, for example, bis(phenylethynyl) biphenyl moieties such as the 2,2′-bis(phenylethynyl)-4,4′-dihydroxybiphenyl or 2,2′-bis(phenylethynyl)-5,5′-dihydroxybiphenyl of Formula B, to form a polymer corresponding to Formula C having a glass transition temperature greater than about 350° C.:
- R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition
- n is an integer of from about 0 to about 50, and preferably from about I to about 25, and more preferably from about 1 to about 10;
- n is an integer of from about 1 to about 200, and preferably from about 1 to about 100 and more preferably from about 25 to about 100.
- R groups examples include
- Preferred bisphenol compounds include
- the temperature at which the polymerization reaction is conducted and the reaction time will depend on the molecular weight build-up desired in the compound having Formula C.
- One of ordinary skill in the art can readily optimize the conditions of the reaction to get the claimed results, but the temperature will generally be in the range of from about 100° C. to about 175° C., and preferably from about 110° C. to about 130° C., for a period of, for example, from about 12 to about 48 hours, and preferably from about 24 to about 30 hours. Pressure is not critical.
- the molar ratio of compounds having Formula A or D to those having Formula B is about 1 to 1, preferably about 1 to 0.90, and most preferably from about 1 to 0.95.
- Compounds corresponding to Formula C have a number-average molecular weight (“Mn”) of from about 8,000 g/Mol to about 30,000 g/Mol, and preferably from about 10,000 g/Mol to about 20,000 g/Mol, and are produced in a yield of at least from about 50% to about 100%, and preferably from about at least 75% to about 90%, based upon the weight of monomers corresponding to Formula A, D, or B consumed.
- Mn number-average molecular weight
- the compounds corresponding to Formula C are preferably recovered from any inorganic by-products produced in the synthesis reaction via conventional isolation methods known in the art such as precipitation, extraction, washing with a non-solvent and drying, and combinations thereof.
- Z is any of the above-described aromatic nuclei in the compounds having Formula B or 1,5-naphthalenediyl, and preferably is 1,5-napthalenediyl;
- Y is independently
- T 1 , T 2 , and T 3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl comprising phenyl, or biphenylyl, or terphenylyl, or mixtures thereof; and preferably is phenyl,
- a is independently an integer of from about 0 to about 200, preferably from about 10 to about 100, and more preferably from about 10 to about 50;
- b is an integer of from about 0 to about 20, and preferably about 1 to about 5,
- a preferred compound of Formula C′′ is the copolymer corresponding to Formula C′:
- c is independently an integer from about I to about 200, preferably from about 10 to about 100, and more preferably from about 10 to about 50;
- the Y group of the compound having Formula C′′ is a phenyl.
- Z is any of the above-described aromatic nuclei in the compounds having Formula B or 1,5-naphthalenediyl, or mixtures thereof, and preferably is 1,5-napthalenediyl;
- Y is independently
- T 1 , T 2 , and T 3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl comprising phenyl, or biphenylyl, or terphenylyl, or mixtures thereof; and preferably is phenyl,
- d is independently an integer of from about 1 to about 200, preferably from about 10 to about 100, and more preferably from about 10 to about 50;
- b is an integer of from about 0 to about 20, and preferably about 1 to about 5, and
- q is independently an integer of from about 0 to about 50, preferably from about 0 to 20, and more preferably from about 0 to about 3
- the preferred inorganic base is potassium carbonate.
- the fluorinated poly(arylene ether) composition of the present invention may be formed into a solution suitable for application onto the substrate by any conventional means, such as spin-coating.
- the solution is centrally applied to the substrate, which is then spun at speeds ranging between about 500 and about 6000 rpm, preferably between about 1500 and about 4000 rpm, and most preferably at 2900 rpm to 3,100 rpm, for about 2 to about 60 seconds, and preferably from about 5 to about 30 seconds, in order to spread the solution evenly across the substrate surface.
- Solvents suitable for use in the solutions of the present invention include aprotic solvents such as cyclopentanone; cyclic ketones such as cyclohexanone, cycloheptanone, and cyclooctanone; cyclic amides such as N-alkylpyrrolidinone wherein the alkyl has form about 1 to about 4 carbons atoms and N-cyclohexylpyrrolidinone and mixtures thereof. Cyclopentanone is preferred.
- the solution is formed by combining the solvent and poly(arylene ether) composition under ambient conditions in any conventional apparatus having, a non-metallic lining, and preferably in a glass-lined apparatus to produce a solution comprised of, based upon the total weight of the solution, from about 1 to about 50%, and preferably from about 3% to about 20% of the fluorinated poly(arylene ether) composition and from about 50% to about 99%, and preferably from about 80% to about 97% of solvent.
- the resulting mixed solution is then filtered under ambient conditions via filtration means well-known in the art including, but not limited to commercially available filtration cartridges having the desired porosity.
- these filtration cartridges consist of a non-stick, scratch resistant material such as Teflon® available from DuPont.
- Teflon® available from DuPont.
- the mixed solution is filtered through a variety of filtration means having different pore sizes. More preferably, the solution is filtered sequentially through about 4 filtration means having a pore size of about 1.0 ⁇ m, about 0.5 ⁇ m, about 0.2 ⁇ m, and about 0.1 ⁇ m, respectively.
- the solutions of this invention are applied by standard spin-coating techniques well-known in the art onto wafer substrates, such as silicon or metal wafers having an aluminum wiring circuit pattern on their surface, to be processed into integrated circuits and/or other microelectronic devices.
- the coated substrate is then heated by any conventional means known in the art.
- the combination is heated by placing it on top of a hot plate.
- a hot plate typically this is done commercially via a conventional integrated spin-coater/hot plate system. This type of heating not only rapidly and directly expels the solvent from the solution and causes the film to flow, but is also easily adaptable for continuous processing operations.
- the coated substrate is typically heated for about 30 seconds to about 5.0 minutes at temperatures ranging between about 50° C. and about 250° C., and more preferably about 100C and about 200° C.
- Multiple hot plates i.e. between about 2 and about 5, and preferably from about 2 to about 3, can be used to process the coated substrates under similar conditions.
- the coated substrate is baked for up to about 2 minutes, and preferably from about 50 to about 70 seconds, on a hot plate set at a temperature of about 70° C. to about 150° C., and preferably from about 100° C. to about 150° C., then cured with thermal or electron beam energy in a conventional curing means such as an oven chamber or an autoclave, within a temperature range of about 300° C. to about 450° C., and preferably from about 375° C. to about 425° C., for about 0.5 to about 4 hours, and preferably from about 1.0 to about 2.0 hours.
- the cure pressure may range between about 1 torr to about 2 torr.
- the length of exposure will generally range from about 2 to about 45 minutes, and preferably from about 5 to about 25 minutes for a dose of electron beam energy ranging from about 1000 to about 50,000, and preferably from about 2500 to about 10,000 ⁇ C/cm 2 .
- the accelerating voltage of the electron beam may vary from about I to about 25 KeV. Further details regarding such curing methods may be found in U.S. Serial No. 60,000,239 filed on Jun. 15, 1995 (pending) and U.S. Pat. No. 5,003,178 to Livesay, et al, which is incorporated by reference.
- the baked, uncured coated substrate may be then baked a second time or third time for up to about 2 minutes/baking, and preferably from about 50 to about 70 seconds/baking, on a hot plate set at a temperature of about 90° C. to about 200° C., and preferably from about 130° C. to about 150° C., prior to being cured with thermal or electron beam energy under the time and temperature conditions set forth above.
- the thickness of the resulting thin-film ranges from about 0.5 ⁇ m to about 40 ⁇ m, preferably from about 0.8 ⁇ m to about 2.0 ⁇ m.
- the films produced by this invention generally exhibit a thickness standard deviation less than 2%, and preferably less than 1%, of the average film thickness.
- the films produced according to the present invention possess several beneficial properties such as a Tg of greater than about 350° C. and a dielectric constant below about 2.8.
- a Tg of greater than about 350° C.
- a dielectric constant below about 2.8.
- the high Tg is a result of the intramolecular cyclization (“IMC”) reaction of the two proximal phenylethynyl pendent groups in bisphenol compounds such as 2,2′-bis (phenylethynyl)-5,5′-dihydroxybiphenyl.
- the film is uniform and can be produced with a high degree of consistency.
- the film is resistant to organic solvents, and has minimal loss in mechanical strength and modulus above its Tg to less than 1.5 orders of magnitude.
- thermomechanical and electrical properties The following nonlimiting measurements illustrate the properties of the poly(arylene ether) solutions and thin films of the present invention. It can be seen that the film possesses superior thermomechanical and electrical properties:
- a coated wafer is placed inside the furnace chamber of a FlexusTM model 2410 Film Stress Measurement System available from Tencor Instruments. After inputting the value of a films' thickness into the System, the film's stress is computed therefrom.
- GPC gel phase chromatography system
- PS-2 polystyrene standards available from Polymer Labs under the tradename “PS-2”. These standards have a MW ranging from 580 g/Mol to 400,000 g/Mol. Further details of this procedure are set forth in Rosen, S., “Fundamental Principles of Polymeric Materials 53-81 (2nd Ed. 1993).
- the dielectric constant of a poly(arylene ether) film is determined using the capacitance-voltage (“CV”) measurement technique.
- Metal-oxide semiconductor (“MOS”) capacitors are constructed by depositing the polymer film onto a thermally grown silicon dioxide layer on a low-resistivity n-type silicon wafer. The silicon wafer acts as the bottom electrode of the capacitor structure.
- the top electrode (gate electrode) is formed by sputter deposition through a shadow mask of 1 ⁇ m of Al onto the polymer film. Owing to the fact that the radius of the Al dot is much greater than the combined polymer film and thermal oxide thicknesses, the parallel plate approximation is used to determine the dielectric constant of the polymer film.
- C is the measured total capacitance of the film
- k O is the permittivity of the free space (8.854 ⁇ 10 ⁇ 14 F/cm)
- A is the measured area of the deposited Al dot (electrode)
- d is the measured thickness of the polymer film
- d ox is the known thickness of the thermal oxide layer
- C the total measured capacitance
- the capacitance is measured using a Hewlett-Packard Model 4061A semiconductor measurement system consisting primarily of a sensitive multifrequency (10 KHz-10 MHz) Induction Capacitance and Resistance (“LCR”) meter, current and voltage sources, ramp generator, and picoammeter. All measurements are made at a frequency of 1 Mhz. Prior to a set of measurements, the stray capacitance due to the coaxial cable and probe unit is zeroed out.
- LCR Induction Capacitance and Resistance
- the test structure incorporating the capping layer is comprised of a thin multilayer metal liner applied directly over the aluminum underlayer, before the deposition of the poly(arylene ether).
- the capping liner which is comprised of two outer layers of chromium with a copper layer therebetween, has a total thickness of about 200 ⁇ to about 300 ⁇ . Details of this test procedure are set forth in Lau, K. S. Y., et al, “Characterization and Thin-Film Properties of FLARETM, A Low Dielectric Constant, High Tg Fluorinated Poly(arylene ether), as an Intermetal Dielectric for Interconnect Applications,” Proceedings of Low Dielectric Constant Materials and Interconnects Workshop (California Apr. 30- May 1, 1996).
- TMA Thermal Mechanical Analysis
- Tg Film disks having a 10 mm diameter and a 30 ⁇ m to 40 ⁇ m thickness are placed into a Perkin-Elmer Thermal Mechanical Analyzer. As the temperature therein is increased at a rate of 10° C./min from ⁇ 50° C. to 300° C., a continuous force of about 2 mN to about 3 mN is applied to the film in extension.
- CTE coefficient of thermal expansion
- the CTE was also derived via the “bending beam” procedure, details of which are set forth in “McKerrow”.
- the resulting 6 inch diameter wafer with a cured film layer of 1 micron thickness thereon was cleaved via conventional or focused ion beam milling procedures.
- the cross-sectional view of the coated wafer was examined under a JEOL Model JSM6300F scanning electron microscope (“SEM”) in order to determine what gap sizes and trench depths on the wafer were completely filled. The reported values represent the smallest gap sizes which could be completely filled without defects.
- the wafer was heated to a temperature of 400° C. or 450° C. over a time period of 60 min in the System simultaneously with the recording of a stress measurement of the film at minute intervals.
- the temperature of the System was then decreased to 25° C. over a time period of 60 minutes simultaneously with the recording of a stress measurement of the film at minute intervals.
- the temperature in the System was then held constant at 25° C. for 6 hours simultaneously with the recording of a stress measurement of the film at 6 minute intervals. This cycle was repeated 4 additional times.
- the reported Residual Stress values are recorded after the completion of five cycles.
- the Tg was determined from the temperature at which the film exhibited zero stress.
- thermomechanical and electrical properties of the film were measured as illustrated below in Table 1.
- TMA Residual Stress
- Stress hysteresis 31-37 MPa Residual Stress
- Bending Beam 45 MPa CTE (ppm/° C.)
- TMA 43-52 CTE (ppm/° C.)
- Bending Beam 40 Thermal Stability 1) 1-2% (400° C.) 1) ITGA weight loss (% in 2 hr) 3-5% (425° C.) 2) thickness change (%) 5-6% (450° C.) 2) zero change Gapfill Ability (type of substrate 0.30 ⁇ m (SiO 2 ) surface) 0.25 ⁇ m (Al)
- FIG. 6 illustrates the DMA results.
- the MIM dielectric constant for DOM structures was higher than that for the capped structures due to the interaction between the polymer and the metal during the testing procedure. Such interactions were eliminated by the use of the capping layer in the capped structures, the results for which thus more accurately represent the dielectric constant property of the polymer film.
- Example 1 Fifty mL of the solution of Example 1 was concentrated to 25% solids content and then poured onto the surface of an optically polished quartz plate having a diameter of 10 cm to cover an area of about 1 cm by 6 cm. The solution was then leveled across the plate's surface with a doctor's blade set at a nominal setting, typically in the range of 35 ⁇ m to 80 ⁇ m, then baked on a hot plate. The coated plate was exposed to a variant temperature increasing at a rate of 5° C./min until the hot plate reached a temperature of 150° C., at which point it was baked at a constant temperature for about 2 hours.
- the baked coated plate was then heated, in the presence of nitrogen, inside the furnace chamber of a Tencor Flexus Model 2410 stress gauge at a variant temperature increasing at a rate of 5° C./min until the furnace reached a temperature of 425° C., then heated for 1 hour at 425° C., and then cooled at a variant temperature decreasing at a rate of 2°/min until the temperature in the furnace was 25° C.
- the coated plate was then placed in water set at a temperature of 90° C. for 1 to 3 hours after which time the film was removed from the plate.
- the resulting film possesses a thickness of 30 to 60 ⁇ m, as desired.
- a 1.132-kb quantity (5.290 Mol) of 3,3′-dimethoxybiphenyl and 12 L of acetonitrile were charged into a reaction vessel set at a temperature of 25° C. under ambient pressure with stirring. The temperature of a surrounding external cooling bath was then lowered such that the internal temperature of the reaction mixture was reduced to approximately 15° C.
- N-bromosuccinimide (“NBS”) were added over a four-hour period while maintaining the reaction mixture temperature at 15° C. After removing the cooling bath, the reaction mixture was then stirred at ambient temperature for approximately 15 hours.
- the crystallized product was isolated from the mother liquor by suction filtration in a Büchner filter funnel using approximately 200 torr of pressure, washed with approximately 2 L of acetonitrile, then dried under ambient conditions while still on the Büchner filter for about 1 hour and then in an oven under 10 torr of pressure and set at a temperature of about 60° C. for about 16 hours.
- the yield of the product was 1.338 kg (68%; theoretical yield was 1.968 kg).
- the melting range matched the literature value of 134-136° C.
- the purity of the product which was determined by gas chromatography, using a Hewlett-Packard Model 5890 gas chromatograph equipped with a standard 2-meter glass capillary column (“GC”), was 98.4%.
- This example illustrates an improved method for producing 2,2′-Dibromo-5,5′-dimethoxybiphenyl which results in yields that are significantly higher than those of the prior art processes.
- THF tetrahydrofuran
- a reaction vessel equipped with a mechanical stirrer was charged with 110 grams (4.58 Mol) of magnesium tumings (Aldrich 20,909-5).
- a 500-mL portion of a solution containing 700 grams (1.88 Mol) of the 2,2′-dibromo-5,5′-dimethoxybiphenyl produced in Example 4 in 3,000 mL of dry THF was added to the vessel.
- the reaction mixture was heated for about 10 minutes with a heating mantle to a temperature of 60° C. while 2 grams of an iodine reaction initiator was added thereto.
- a 1,050-gram (4.134 Mol) quantity of iodine and 3,000 ml of anhydrous THF were charged into a 22-L reaction flask equipped with mechanical stirrer which was maintained at a temperature of 0° C. via an external cooling bath.
- the above Grignard reagent was added thereto via a double tipped syringe over a period of 3 hours.
- the reaction mixture was then stirred for an additional three hours.
- TLC thin-layer chromatography
- Example 7 illustrates a reliable method of producing 2,2′-Diiodo-5,5′-bis(tetrahydropyranyl)biphenyl at a yield higher than that reported in the prior art, i.e. “Lindley”.
- the polymer was rinsed with 500 mL of methanol and dried under vacuum at 70° C. for 15 hours, it was then dissolved in cyclopentanone at the desired solids content. The solution was filtered through the series of 4 Teflon cartridges with 1.0, 0.5, 0.2 and 0.1 ⁇ m porosities.
Landscapes
- Chemical & Material Sciences (AREA)
- Power Engineering (AREA)
- Condensed Matter Physics & Semiconductors (AREA)
- Engineering & Computer Science (AREA)
- Computer Hardware Design (AREA)
- Microelectronics & Electronic Packaging (AREA)
- Physics & Mathematics (AREA)
- General Physics & Mathematics (AREA)
- Health & Medical Sciences (AREA)
- Chemical Kinetics & Catalysis (AREA)
- Medicinal Chemistry (AREA)
- Polymers & Plastics (AREA)
- Organic Chemistry (AREA)
- Polyethers (AREA)
- Organic Low-Molecular-Weight Compounds And Preparation Thereof (AREA)
Abstract
A method is disclosed for making hydroxy-substituted ethynylated biphenyl compounds and for reacting such compounds with a family of noncross-linking thermosetting poly(arylene ethers) to produce novel poly(arylene ether) compositions which, when cured at glass transition temperatures greater than about 350° C. to form thin films, possess properties such as low dielectric constant, low moisture absorption, and high thermal stability. These films are suitable for use as intermetal dielectrics for multilevel interconnection.
Description
This application claims the benefit of provisional application number 60/000,514 filed on Jun. 26, 1995, which is incorporated herein by reference.
1. Field of the Invention
The present invention relates to fluorinated poly(arylene ether) compositions for use in coating microelectronic structures, such as integrated circuits, and the method for their manufacture. More particularly, this invention relates to the use of novel bisphenol reactants in the method of manufacturing novel poly(arylene ether) compositions which possess improved properties such as higher glass transition temperature and lower dielectric constant.
2. Background of the Invention
Fluorinated poly(arylene ethers) based upon decafluorobiphenyl have been used extensively as replacements for polyimides for use in spin-coating substrates, such as multichip modules, printed circuit boards, integrated circuits and other microelectronic devices in intermetal dielectric production. These fluorinated poly(arylene ethers), which are commercially available from AlliedSignal Inc. under the tradename “FLARE™”, not only exhibit a thermal stability comparable to that of polyimides but also possess about ten to forty times lower moisture absorption rate, a dielectric constant in the range of about 2.35 to about 2.65, and good retention of storage modulus above their glass transition temperature (“Tg”). These properties are relevant not only to enhanced circuit performance, i.e. higher speed, lower power consumption, and reduced signal-to-noise ratios, but also to lower processing costs. By highly cross-linking these compounds with cross-linking agents or cross-linkable pendent groups, their performance may be enhanced under process integration conditions, i.e. a Tg increase to greater than about 300° C. See Lau, A. N. K., et al., Am. Chem. Soc., Polymer Preprints, 33(1), 996-997 (1992).
Also well-known in the art are methods for synthesizing ethynylated biphenyl monomers such as 2,2′-bis(phenylethynyl)-5,5′-diaminobiphenyl by first producing 2,2′-diiodo-5,5′dimethoxybiphenyl from 3,3′-dimethoxybiphenyl. See Lindley, P. M., et al., in J. Polym. Sci., Part A: Polym. Chem., 1061-1071 (1991) and U.S. Pat. No. 4,683,340 to Lindley, et al. [together hereinafter referred to as “Lindley”]. However, the yield of ethylnylated aromatic compounds produced therefrom is very low, i.e. 4% to 14%.
It is further well known in the art to incorporate such ethynylated biphenyl monomers such as 2,2′-bis(phenylethynyl)-5,5′-diaminobiphenyl and 2,2′-bis(phenylethynyl)-5,5′-diaminobenzidine in the synthesis of high-temperature resistant, high-performance polyphenylquinoxalines and polybenzimidazoles. However, such methods are also laborious, costly, and low-yielding. See Lau, K. S. Y., “Chemistry, Characterization, and Processing of IMC Curing Polymers,” Final Report, Air Force Contract F33615-79-C-5101, for period of September, 1979 to June, 1983, Hughes Aircraft Company (1983).
It would be desirable to provide an improved fluorinated poly(arylene ether) composition which would possess a Tg in excess of about 300° C. without having to highly cross-link the compound or include cross-linkable pendent groups as well as without sacrificing any of the other properties associated with known fluorinated poly(arylene ether) compounds. It would also be desirable to provide an economical, high-yielding process for producing such compositions.
wherein
R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition;
m is an integer of from about 0 to about 50; and
n is an integer of from about 1 to about 200.
In accordance with another aspect of this invention, there is provided a process for manufacturing the above composition comprising:
wherein
r is an integer of from about 0 to about 50,
wherein
q is an integer of from about 0 to about 49,
with a compound of Formula B
wherein
R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition under conditions sufficient to produce the composition.
This invention is also directed to a process for producing a fluorinated poly(arylene ether) mixture suitable for spin-coating onto a substrate comprising:
wherein
R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition;
m is an integer of from about 0 to about 50; and
n is an integer of from about 1 to about 200
and an aprotic solvent; and
b) filtering the solution through at least one filter having a pore size of about 0.1 micrometers.
This invention is also directed to a process for producing a cured, coated substrate comprising:
wherein
R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition;
m is an integer of from about 0 to about 50; and
n is an integer of from about 1 to about 200;
and an aprotic solvent;
b) filtering the solution;
c) applying the solution of step b onto a substrate to form a film thereon; and
d) heating the film of step c under conditions sufficient to cure the film.
Another embodiment of the invention is directed to a process for making ethynylated, hydroxy-substituted biphenyl compounds comprising:
a) halogenating 3,3′-dimethoxybiphenyl under conditions sufficient to form 2,2′-dihalo-5,5′-dimethoxybiphenyl;
b) reacting said 2,2′dihalo-5,5′-dimethoxybiphenyl with a bromide containing compound in a chlorinated solvent under conditions sufficient to form 2,2′-dihalo-5,5′-dihydroxybiphenyl;
c) reacting said 2,2′-dihalo-5,5′-dihydroxybiphenyl with a hydroxyl-containing compound in the presence of a protection catalyst under conditions sufficient to form 2,2′-dihalo-5,5′-bis(tetrahydropyranoxy)biphenyl;
d) ethynylating said 2,2′-dihalo-5,5′-bis(tetrahydropyranoxy)biphenyl with a compound of the formula
wherein R′ is comprised of an alkyl group having from about 1 to about 20 carbon atoms, an alkenyl group having from about 1 to about 20 carbon atoms, an aryl group, a heteroaryl group, and derivatives or mixtures thereof to form 2,2′-bis(C≡CR′)-5,5′-bis(tetrahydropyranoxy)biphenyl; and
e) hydrolyzing said 2,2′-bis(C≡CR′)-5,5′-bis(tetrahydropyranoxy)biphenyl under conditions sufficient to form 2,2′-bis(C≡CR′)-5,5′-bis(dihydroxy)biphenyl, wherein R′ is as described in step d.
The poly(arylene ether) compositions of this invention not only possess a Tg in excess of about 350° C. without having to highly cross-link the compound or include cross-linkable pendent groups thereto as well as without sacrificing any of its other properties such as solubility, spin-coatability, high thermal stability, low moisture absorptivity, low dielectric constant, and planarizability.
The invention will be more fully understood and further advantages will become apparent when reference is made to the following detailed description of the preferred embodiments of the invention and the accompanying drawings, in which:
FIG. 1 illustrates a method for the synthesis of 2,2′-Diiodo-5,5′-dimethoxybiphenyl.
FIG. 2 illustrates a method for synthesizing 2,2′-Bis(phenylethynyl)-5,5′-dihydroxybiphenyl.
FIG. 3 illustrates a method for synthesizing poly(arylene ether) compositions according to the process of the present invention.
FIG. 4a illustrates the thermal behavior of a poly(arylene ether) composition containing 2,2′-bis(phenylethynyl) biphenyl-5,5′-dioxy linkages during two thermal cycles under conditions of differential scanning calorimetry (“DSC”). FIG. 4b illustrates an enlarged view of the second scan of FIG. 4a.
FIG. 5 illustrates the thermal behavior of a poly(arylene ether) composition containing 2,2′-bis(phenylethynyl) biphenyl-5,5′-dioxy linkages under conditions of dynamic mechanical analysis.
Unless indicated otherwise, all references herein are specified in weight. As used herein, “glacial” means the absence of water or undiluted. Although the below-described reactions may be conducted in any type of conventional round-bottom reaction vessels, it is preferable to use a nonmetallic-lined vessels.
The ethynylated aromatic compound starting materials, which are preferably ethynylated hydroxy-substituted biphenyl (“bisphenol”) compounds corresponding to Formula B
wherein
R is an aromatic nucleus that imparts a Tg of greater than about 260° C., and preferably from about 350° C. to about 450° C., to the compounds which contain them, are either commercially available and/or may readily be synthesized by one skilled in the art without undue experimentation based upon a conventional boron tribromide demethylation reaction of the R groups with methoxy substituents. Details of such reactions are well-known in the art and described at, for example, McOmie, J. F. W., et al., 24 Tetrahedron 2289-2292 (1972) [hereinafter “McOmie”].
with 2,2′-bis(phenylethynyl)-5,5′- dihydroxybiphenyl being most preferred.
In a preferred embodiment for producing ethnylated aromatic compounds, commercially available 3,3′-dimethoxy-4,4′-diaminobiphenyl (“o-dianisidine”), is diazotized under conditions sufficient to form 3,3′-dimethoxybiphenyl. Details of this reaction are well known in the art and are described in, for example, U.S. Pat. No. 3,320,320 to Kamlet et al., which is incorporated herein by reference; Organic Synthesis 30-55 (N.Y. 1941); and Organic Synthesis Collective Volume III, 295-299 (1955).
The 3,3′-dimethoxybiphenyl is converted into 2,2′-dihalo-5,5′-dimethyoxybiphenyl, and preferably 2,2′-diiodo-5,5′-dimethyoxy-biphenyl, via an initial reaction with a halogen such as chlorine or bromine, and preferably bromine, miscible in glacial acid such as carboxylic acids and anhydrides thereof including but not limited to propionic acid, butyric acid, and acetic acid under a temperature of from about 60° C. to about 150° C., and preferably from about 100° C. to about 120° C. and ambient pressure to form 2,2′-dihalo-5,5′dimethoxybiphenyl, and preferably 2,2′-dibromo-5,5′dimethoxybiphenyl. Acetic acid is preferred. The period of reaction may range from about 1 hour to about 10 hours, and preferably from about 2 hours to about 4 hours. The concentration of halogen in the acid-containing mixture is from about 5 to about 50%, and preferably from about 10 to about 20%, based upon the total weight of the halogen-acid mixture. The molar ratio of 3,3′-dimethoxybiphenyl to the halogen desired is about 1 to 2, preferably about 1 to 3, and most preferably about 1 to 2.2.
As illustrated in FIG. 1, the product of the initial halogen reaction, i.e., 2,2′-dibromo-5,5′dimethoxybiphenyl, is then halogenated into a second 2,2′-dihalo-5,5′-dimethoxybiphenyl, i.e. 2,2′-diiodo-5,5′-dimethoxybiphenyl, via a lithiation exchange reaction in the presence of an organolithium reagent, such as n-butyllithium, to form a dilithiated intermediate which is then reacted with the desired halogen, or via a Grignard reaction with magnesium to form a bis-Grignard intermediate, which is then reacted with the desired halogen. The lithiation-halogenation reactions occur at a temperature of from about −78° C. to about −20° C., and preferably from about −78° C. to about −50° C. and ambient pressure, while the Grignard-halogenation reactions occur under conditions of ambient temperature and pressure. Iodine is the preferred halogen. The molar ratio of the second 2,2′-dihalo-5,5′dimethoxybiphenyl to halogen is about 1:2, preferably about 1:3, and most preferably about 1:2.2. The molar ratio of the second 2,2′-dihalo-5,5′dimethoxybiphenyl to the organolithium reagent or magnesium is about 1:2, preferably about 1:3, and most preferably about 1:2.5. The period of these halogenation reactions may depend upon the specific chemical structure, but will generally be from about 0.5 hours to about 4 hours, and preferably from about 1 hour to about 2 hours.
The second 2,2′-dihalo-5,5′-dimethoxybiphenyl , and preferably 2,2′-diiodo-5,5′-dimethoxybiphenyl, is then demethylated under temperatures of about −78° C. to about −20° C., and preferably from about −78° C. to about −50° C., and ambient pressure, with a bromide-containing compound such as hydrogen bromide, boron tribromide, or mixtures thereof in a chlorinated solvent such as a chlorohydrocarbon including but not limited to dichloromethane to yield 2,2′-dihalo-5,5′-dihydroxybiphenyl, and preferably 2,2′-diiodo-5,5′-dihydroxybiphenyl. Boron tribromide is the preferred reactant. The concentration of the bromide-containing compound in the chlorinated solvent may range from 0.05M to about 1M, and preferably from about 0.1M to about 0.5M. The period of the demethylation reaction may depend upon the chemical structure, but will generally be from about 5 minutes to about 2 hours, and preferably from about 15 minutes to about 1 hour. The molar ratio of 2,2′-diiodo-5,5′dimethoxybiphenyl to the bromide-containing compound is about 1:3 to 1:10, and preferably about 1:2.5 to 1:5.
The product of the demethylation reaction, i.e. 2,2′-diiodo-5,5′-dihydroxybiphenyl, is then reacted with a compound containing a hydroxyl protective group, preferably dihydropyran, in the presence of a protection catalyst under temperature conditions ranging between 0° C. to about 30° C., and preferably from about 20° C. to about 25° C., and ambient pressures to form 2,2′-diiodo-5,5′-bis(tetrahydropyranoxy)biphenyl. Details of this reaction are well-known in the art and are described in, for example, Lindley which is incorporated herein by reference, with the exception that we have found that it is preferable to use from about 100% to about 500%, and more preferably from about 200% to about 250% by weight catalyst relative to 2,2′-dihalo-5,5′-dihydroxybiphenyl. Suitable protection catalysts have a purity of at least about 99% and include arylsulfonic acids such as p-toluenesulfonic acid, benzenesulfonic acid, and p-bromophenylsulfonic acid, with p-toluenesulfonic acid being preferred. The molar ratio of the hydroxyl-containing compound to 2,2′-diiodo-5,5′-dihydroxybiphenyl is from about 100:1 to 500:1, more preferably from about 200:1 to 250:1, and most preferably from 100:1 to 150:1. The period of the hydroxy reaction may depend upon the reactivity, but will generally be from about 1 hour to about 48 hours, and preferably from about 12 hours to about 24 hours.
The 2,2′-diiodo-5,5′-bis(tetrahydropyranoxy)biphenyl, and preferably 2,2′-diiodo-5,5′-bis(tetrahydropyranoxy)biphenyl, is then ethynylated with a solution of a copper acetylide compound in a solvent having a boiling point of from about 100° C. to about 120° C. to form 2,2′-bis(C≡CR′)-5,5′-bis(tetrahydro-pyranoxy)biphenyl compounds, wherein R′ is a moiety comprising an alkyl having from about 1 to about 20, and preferably from about 1 to about 6 carbon atoms; an alkenyl having from about 1 to about 20, and preferably from about 2 to about 10 carbon atoms; an aryl such as those having a substituted phenyl ring as illustrated by tolyl or anisyl, or those having a fused aromatic nucleus such as naphthalene, anthracene, phenanthrene, and pyrene; a heteroaryl such as furan, pyrrole, thiophene, pyridine, quinoline, quinoxaline, and derivatives and mixtures thereof, and preferably is a phenyl, a pentafluorophenyl, or a biphenylyl group, and most preferably a phenyl group. Suitable R′ groups include, but are not limited to
or mixtures thereof;
R1, R2, and R3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl comprising phenyl, or biphenylyl, or terphenylyl, or mixtures thereof; and n is an integer of from about 0 to about 20, and preferably about 1 to about 5.
The temperature of the ethynylation reaction may range from about 80° C. to about 1 50° C., and preferably from about 90° C. to about 110° C . The pressure is ambient. The molar ratio of 2,2′-dihalo-5,5′-bis(tetrahydropyranoxy) biphenyl, i.e. 2,2′-dihalo-5,5′-bis(tetrahydropyranoxy)biphenyl, to copper acetylide compound is about 1 to 10, preferably about 1 to 5 and most preferably about 1 to 2.5. The period of the ethynylation reaction will depend upon reactivity of the haloaromatic, i.e. 2,2′-diiodo-5,5′-bis(tetrahydropyranoxy)biphenyl, but will generally be from about 30 minutes to about 48 hours, and preferably from about 4 hours to about 30 hours.
The copper[I] acetylide compound is of the formula
wherein R′ is as described above.
The preferred copper[I] acetylide compound is copper[I] phenylacetylide. Further details of the phenylethynylation of haloaromatics by copper[I] phenylacetylide is described in, for example, Stephens, R. D., et al, 28 J. Org. Chem. 3313-3315 (1963) and Castro, C. E., et al., 31 Journal of Organic Chemistry 4071-4078 (1966).
Examples of suitable solvents for the ethynylation reaction include pyridine and pyridines substituted with moieties such as alkyl having from about I to about 10, and preferably from about 1 to about 5 carbon atoms. Pyridine is preferred. The solvents have a purity of at least about 95% and a copper[I] acetylide concentration of from about 10% to about 40%, and preferably from about 10% to about 20%.
The tetrahydropyranyl groups are removed from 2,2′-bis(C≡CR′)-5,5′-bis(tetrahydropyranoxy)biphenyl by acid hydrolysis under ambient conditions to form ethynylated aromatic compounds of the formula 2,2′-bis(C≡CR′)-5,5′-dihydroxybiphenyl, wherein the substituent R′ is as above-described. Suitable acid solutions contain, based upon the total weight of the solution, from about greater than 0% to about 20%, and preferably from about 5% to about 10% acid. Any acid may be used, but hydrochloric acid is preferred. Examples of suitable solvents include any aprotic solvent such as toluene. The molar ratio of the acid solution to 2,2′-bis(C≡CR′)-5,5′-bis(tetrahydropyranoxy)biphenyl is from about 80:20 to 50:50, and preferably from about 80:20 to 75:25. The period of the hydrolysis reaction generally will be from about 1 hour to about 10 hours, and preferably from about 1 hour to about 3 hours.
Examples of ethnylated aromatic compounds produced according to the process of the present invention include those 2,2-bis(substituted ethynyl)-5,5′-bis(tetrahydropryanoxy)biphenyl compounds of the formula:
wherein R′ is as above described. The preferred ethynylated aromatic compound is 2,2′-bis(phenylethynyl)-5,5′-dihydroxybiphenyl. FIG. 2 illustrates the preferred synthetic sequence for the synthesis of the preferred monomer, 2,2′-bis(phenylethynyl)-5,5′-dihydroxybiphenyl.
Preferably, the products of each above-described reaction step are recovered from any products, solvent and catalyst that may be present prior to commencement of the next reaction step. Preferably the products should be about 85-100% , and more preferably about 95-99% pure. The products may be recovered by any means well known in the art including but not limited to distillation, reprecipitation, extraction, and recrystallization.
The other starting material for producing the poly(arylene ethers) of the present invention includes the perfluorinated phenylenes of Formula A:
wherein
r is an integer of from about 0 to about 50, preferably from about 0 to 10, and more preferably from about 0 to about 3;
wherein
q is an integer of from about 0 to about 49, preferably from about 0 to about 9, and more preferably from about 0 to about 3.
Compounds corresponding to both Formulas A and D can be readily synthesized by one of ordinary skill in the art without undue experimentation.
Examples of suitable perfluorinated phenylenes include hexafluorobenzene (m=0); decafluorobiphenyl (m=1); the three terphenyl structures (m=2), i.e., tetradecafluoro-para-terphenyl, tetradecafluoro-meta-terphenyl, and tetradecafluoro-ortho-terphenyl; and tetraphenyl structures (m=3) such as octadecafluoro-para, para, para-tetraphenyl and octadecafluoro-para, para, meta-tetraphenyl, octadecafluoro-para, para, ortho-tetraphenyl, and all isomers thereof due to ring catenation, and mixtures and copolymers thereof. Decafluorobiphenyl is preferred.
In another embodiment of the present invention, the fluorinated poly(arylene ethers) of Formula C may be synthesized by reacting the starting materials, i.e. the bisphenols of Formula B and the fluorinated phenylene compounds of Formulas A or D, under conditions sufficient to affect the polymerization reaction. More specifically, the bisphenol moieties of Formula A or D are replaced with, for example, bis(phenylethynyl) biphenyl moieties such as the 2,2′-bis(phenylethynyl)-4,4′-dihydroxybiphenyl or 2,2′-bis(phenylethynyl)-5,5′-dihydroxybiphenyl of Formula B, to form a polymer corresponding to Formula C having a glass transition temperature greater than about 350° C.:
wherein
R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition;
m is an integer of from about 0 to about 50, and preferably from about I to about 25, and more preferably from about 1 to about 10; and
n is an integer of from about 1 to about 200, and preferably from about 1 to about 100 and more preferably from about 25 to about 100.
or mixtures thereof.
with 2,2′-bis(phenylethynyl)-5,5′- dihydroxybiphenyl being most preferred.
The temperature at which the polymerization reaction is conducted and the reaction time will depend on the molecular weight build-up desired in the compound having Formula C. One of ordinary skill in the art can readily optimize the conditions of the reaction to get the claimed results, but the temperature will generally be in the range of from about 100° C. to about 175° C., and preferably from about 110° C. to about 130° C., for a period of, for example, from about 12 to about 48 hours, and preferably from about 24 to about 30 hours. Pressure is not critical.
The molar ratio of compounds having Formula A or D to those having Formula B is about 1 to 1, preferably about 1 to 0.90, and most preferably from about 1 to 0.95.
Compounds corresponding to Formula C have a number-average molecular weight (“Mn”) of from about 8,000 g/Mol to about 30,000 g/Mol, and preferably from about 10,000 g/Mol to about 20,000 g/Mol, and are produced in a yield of at least from about 50% to about 100%, and preferably from about at least 75% to about 90%, based upon the weight of monomers corresponding to Formula A, D, or B consumed.
The compounds corresponding to Formula C are preferably recovered from any inorganic by-products produced in the synthesis reaction via conventional isolation methods known in the art such as precipitation, extraction, washing with a non-solvent and drying, and combinations thereof.
wherein
Z is any of the above-described aromatic nuclei in the compounds having Formula B or 1,5-naphthalenediyl, and preferably is 1,5-napthalenediyl;
T1, T2, and T3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl comprising phenyl, or biphenylyl, or terphenylyl, or mixtures thereof; and preferably is phenyl,
a is independently an integer of from about 0 to about 200, preferably from about 10 to about 100, and more preferably from about 10 to about 50; and
b is an integer of from about 0 to about 20, and preferably about 1 to about 5,
may be produced by reacting compounds corresponding to Formula A with a compound corresponding to Formula B, an optional but preferred inorganic base such as potassium carbonate, sodium carbonate, potassium hydroxide, sodium hydroxide or mixtures thereof, and another bisphenol-containing coreactant corresponding to Formula Z under conditions set forth in the above polymerization reaction. The preferred inorganic base is potassium carbonate. The molar ratio of compounds containing Formula B to those coreactant compounds having Formula Z is about 20:1, and preferably about 5:1, and more preferably from about 2:1. A preferred compound of Formula C″ is the copolymer corresponding to Formula C′:
wherein
c is independently an integer from about I to about 200, preferably from about 10 to about 100, and more preferably from about 10 to about 50;
Z is defined above for Formula C″;
and the Y group of the compound having Formula C″ is a phenyl.
wherein
Z is any of the above-described aromatic nuclei in the compounds having Formula B or 1,5-naphthalenediyl, or mixtures thereof, and preferably is 1,5-napthalenediyl;
and preferably is phenyl,
T1, T2, and T3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl comprising phenyl, or biphenylyl, or terphenylyl, or mixtures thereof; and preferably is phenyl,
d is independently an integer of from about 1 to about 200, preferably from about 10 to about 100, and more preferably from about 10 to about 50;
b is an integer of from about 0 to about 20, and preferably about 1 to about 5, and
q is independently an integer of from about 0 to about 50, preferably from about 0 to 20, and more preferably from about 0 to about 3
may be produced by reacting compounds corresponding to Formula D with a compound corresponding to Formula B, an optional but preferred inorganic base as described above, and another bisphenol-containing coreactant corresponding to Formula Z under conditions set forth in the above polymerization reaction for compounds having formula C. The preferred inorganic base is potassium carbonate.
The fluorinated poly(arylene ether) composition of the present invention may be formed into a solution suitable for application onto the substrate by any conventional means, such as spin-coating. Preferably, the solution is centrally applied to the substrate, which is then spun at speeds ranging between about 500 and about 6000 rpm, preferably between about 1500 and about 4000 rpm, and most preferably at 2900 rpm to 3,100 rpm, for about 2 to about 60 seconds, and preferably from about 5 to about 30 seconds, in order to spread the solution evenly across the substrate surface.
Solvents suitable for use in the solutions of the present invention include aprotic solvents such as cyclopentanone; cyclic ketones such as cyclohexanone, cycloheptanone, and cyclooctanone; cyclic amides such as N-alkylpyrrolidinone wherein the alkyl has form about 1 to about 4 carbons atoms and N-cyclohexylpyrrolidinone and mixtures thereof. Cyclopentanone is preferred.
The solution is formed by combining the solvent and poly(arylene ether) composition under ambient conditions in any conventional apparatus having, a non-metallic lining, and preferably in a glass-lined apparatus to produce a solution comprised of, based upon the total weight of the solution, from about 1 to about 50%, and preferably from about 3% to about 20% of the fluorinated poly(arylene ether) composition and from about 50% to about 99%, and preferably from about 80% to about 97% of solvent.
The resulting mixed solution is then filtered under ambient conditions via filtration means well-known in the art including, but not limited to commercially available filtration cartridges having the desired porosity. Preferably, these filtration cartridges consist of a non-stick, scratch resistant material such as Teflon® available from DuPont. Although one skilled in the art can readily determine without undue experimentation to the ultimate pore size of the filtration means based upon the desired application, it is generally preferable to use a means having a pore size less than 1.0 μm, and preferably less than 0.1 μm.
In a preferred embodiment, the mixed solution is filtered through a variety of filtration means having different pore sizes. More preferably, the solution is filtered sequentially through about 4 filtration means having a pore size of about 1.0 μm, about 0.5 μm, about 0.2 μm, and about 0.1 μm, respectively.
Typically the solutions of this invention are applied by standard spin-coating techniques well-known in the art onto wafer substrates, such as silicon or metal wafers having an aluminum wiring circuit pattern on their surface, to be processed into integrated circuits and/or other microelectronic devices.
The coated substrate is then heated by any conventional means known in the art. Preferably, the combination is heated by placing it on top of a hot plate. Typically this is done commercially via a conventional integrated spin-coater/hot plate system. This type of heating not only rapidly and directly expels the solvent from the solution and causes the film to flow, but is also easily adaptable for continuous processing operations. The coated substrate is typically heated for about 30 seconds to about 5.0 minutes at temperatures ranging between about 50° C. and about 250° C., and more preferably about 100C and about 200° C. Multiple hot plates, i.e. between about 2 and about 5, and preferably from about 2 to about 3, can be used to process the coated substrates under similar conditions.
In a preferred embodiment, the coated substrate is baked for up to about 2 minutes, and preferably from about 50 to about 70 seconds, on a hot plate set at a temperature of about 70° C. to about 150° C., and preferably from about 100° C. to about 150° C., then cured with thermal or electron beam energy in a conventional curing means such as an oven chamber or an autoclave, within a temperature range of about 300° C. to about 450° C., and preferably from about 375° C. to about 425° C., for about 0.5 to about 4 hours, and preferably from about 1.0 to about 2.0 hours. The cure pressure may range between about 1 torr to about 2 torr. The length of exposure will generally range from about 2 to about 45 minutes, and preferably from about 5 to about 25 minutes for a dose of electron beam energy ranging from about 1000 to about 50,000, and preferably from about 2500 to about 10,000 μC/cm2. The accelerating voltage of the electron beam may vary from about I to about 25 KeV. Further details regarding such curing methods may be found in U.S. Serial No. 60,000,239 filed on Jun. 15, 1995 (pending) and U.S. Pat. No. 5,003,178 to Livesay, et al, which is incorporated by reference. Depending on the processing characteristics of the polymer, the baked, uncured coated substrate may be then baked a second time or third time for up to about 2 minutes/baking, and preferably from about 50 to about 70 seconds/baking, on a hot plate set at a temperature of about 90° C. to about 200° C., and preferably from about 130° C. to about 150° C., prior to being cured with thermal or electron beam energy under the time and temperature conditions set forth above.
The thickness of the resulting thin-film ranges from about 0.5 μm to about 40 μm, preferably from about 0.8 μm to about 2.0 μm. The films produced by this invention generally exhibit a thickness standard deviation less than 2%, and preferably less than 1%, of the average film thickness.
The films produced according to the present invention possess several beneficial properties such as a Tg of greater than about 350° C. and a dielectric constant below about 2.8. Although not wishing to be bound by any theory, we believe that the high Tg is a result of the intramolecular cyclization (“IMC”) reaction of the two proximal phenylethynyl pendent groups in bisphenol compounds such as 2,2′-bis (phenylethynyl)-5,5′-dihydroxybiphenyl. The film is uniform and can be produced with a high degree of consistency. The film is resistant to organic solvents, and has minimal loss in mechanical strength and modulus above its Tg to less than 1.5 orders of magnitude.
The following nonlimiting measurements illustrate the properties of the poly(arylene ether) solutions and thin films of the present invention. It can be seen that the film possesses superior thermomechanical and electrical properties:
1) Film Thickness (Å)
Using a calibrated Nanospec® AFT-Y CTS-102 model 010-180 reflectometer Film Thickness Measurement System available from Nanometrics, Co., light of wavelengths from about 480 nm to 790 nm locally illuminates a coated sample. The reflected light is detected and converted to a film thickness in Angstroms (Å) via its internal numerical algorithm. Measurements are acquired for five different locations on the wafer. The average of these five values is recorded.
2) Film Stress (MPa)
A coated wafer is placed inside the furnace chamber of a Flexus™ model 2410 Film Stress Measurement System available from Tencor Instruments. After inputting the value of a films' thickness into the System, the film's stress is computed therefrom.
3) Molecular Weight (“MW”)
About 50 μl of a 0.5% solution of the subject poly(arylene ether) in N-methylpyrrolieinone (“NMP”) is injected into a gel phase chromatography system (“GPC”) from Waters. This GPC system has B columns containing gels with an average cavity size of 10μ, which are available from Polymer Labs. A continuous flow of 0.0125 M solution of LiBr in NMP is maintained. The GPC is calibrated using polystyrene standards available from Polymer Labs under the tradename “PS-2”. These standards have a MW ranging from 580 g/Mol to 400,000 g/Mol. Further details of this procedure are set forth in Rosen, S., “Fundamental Principles of Polymeric Materials 53-81 (2nd Ed. 1993).
4) Dielectric Constant
The dielectric constant of a poly(arylene ether) film is determined using the capacitance-voltage (“CV”) measurement technique. Metal-oxide semiconductor (“MOS”) capacitors are constructed by depositing the polymer film onto a thermally grown silicon dioxide layer on a low-resistivity n-type silicon wafer. The silicon wafer acts as the bottom electrode of the capacitor structure. The top electrode (gate electrode) is formed by sputter deposition through a shadow mask of 1 μm of Al onto the polymer film. Owing to the fact that the radius of the Al dot is much greater than the combined polymer film and thermal oxide thicknesses, the parallel plate approximation is used to determine the dielectric constant of the polymer film. The following formula is valid for the parallel plate geometry used:
where
C is the measured total capacitance of the film Cox is the capacitance due to the oxide film (Cox=kO.kox.A/dox)
kO is the permittivity of the free space (8.854×10−14 F/cm)
kox is known as 4.0
kF is the dielectric constant
A is the measured area of the deposited Al dot (electrode)
d is the measured thickness of the polymer film
dox is the known thickness of the thermal oxide layer
The value of C, the total measured capacitance, is determined with the MOS capacitor biased into the accumulation mode, which occurs when a positive gate potential is applied. The capacitance is measured using a Hewlett-Packard Model 4061A semiconductor measurement system consisting primarily of a sensitive multifrequency (10 KHz-10 MHz) Induction Capacitance and Resistance (“LCR”) meter, current and voltage sources, ramp generator, and picoammeter. All measurements are made at a frequency of 1 Mhz. Prior to a set of measurements, the stray capacitance due to the coaxial cable and probe unit is zeroed out.
Further details of this procedure are set forth in “SOG Dielectric Constant Theory”, report by AlliedSignal Inc., Advanced Microelectronic Materials Division (Jan. 3, 1995). Additional details for dielectric constant measurements are set forth in Solymar, L. et. al., “Lectures on the Electrical Properties of Materials,” (2nd Ed. 1979).
The metal-insulator-metal (“MIM”) structure used in the electrical measurements can be a direct-on-metal (“DOM”) structure (i.e., Al/polymer/Al) or with a capping layer (liner) in the total MIM structure (i.e., Al/Polymer/Cr/Cu/Cr/Al). The DOM structure comprises an underlayer of aluminum onto which a layer of poly(arylene ether) film has been deposited by standard spin-coating technique and cured at 425° C. for 1 hour. A layer of Al dots is then deposited by standard plasma vapor deposition (“PVD”) through a shadow mask. The thickness of each layer ranges from about 0.5 to about 1 μm. The test structure incorporating the capping layer is comprised of a thin multilayer metal liner applied directly over the aluminum underlayer, before the deposition of the poly(arylene ether). The capping liner, which is comprised of two outer layers of chromium with a copper layer therebetween, has a total thickness of about 200 Å to about 300 Å. Details of this test procedure are set forth in Lau, K. S. Y., et al, “Characterization and Thin-Film Properties of FLARE™, A Low Dielectric Constant, High Tg Fluorinated Poly(arylene ether), as an Intermetal Dielectric for Interconnect Applications,” Proceedings of Low Dielectric Constant Materials and Interconnects Workshop (California Apr. 30-May 1, 1996).
Alternatively, from measurements of the out-of-plane refractive index, nTM, the out-of-plane dielectric constant was also calculated from the Maxwell identity, K=nTM 2. Similarly, from measurements of the in-plane refractive index, nTE, the in-plane dielectric constant was calculated from the Maxwell identity, K=nTE 2. The Maxwell identity expressed in this form assumes that the film is nonmagnetic, which is the case. Details of this study are set forth in McKerrow, A. J., et al. “Characterization of Low k Dielectrics for ILD Applications,” Proceedings of Low Dielectric Constant Materials and Interconnects Workshop (California Apr. 30-May 1, 1996) [hereinafter “McKerrow”].
5) Differential Scanning Calorimetry (“DSC”)
About 5 mg of poly(arylene ether) powder was heated at a rate of 10° C./min in a purged nitrogen environment in a Perkin-Elmer Model DSC 7 system from a temperature of 25° C. to 450° C. The film was maintained therein at a temperature of 450° C. for 5 minutes before cooling at a rate of 10° C./min to 25° C. The Tg was calculated from a deflection in the temperature (°C.) versus heat flow (Watt/g) curve as known in the art.
6) Dynamic Mechanical Analysis (“DMA”)
Measures storage modulus (MPa) and loss modulus (MPa); 1 cm×5 cm×1 mm samples are placed into a Dynamic Mechanical Spectrometer 110 available from Seiko Instruments Inc., U.S.A. set in the Flexural Mode (3 point bending mode) with a frequency of 1 Hz; the samples are heated at a rate of 3° C./minute from −20° C. to 300° C. The Tg is determined by the position of the tan δ peak (=storage modulus/loss modulus).
7) Thermal Mechanical Analysis (“TMA”)
Measures Tg; Film disks having a 10 mm diameter and a 30 μm to 40 μm thickness are placed into a Perkin-Elmer Thermal Mechanical Analyzer. As the temperature therein is increased at a rate of 10° C./min from −50° C. to 300° C., a continuous force of about 2 mN to about 3 mN is applied to the film in extension.
The coefficient of thermal expansion (“CTE”) is derived from the slope of the graph of the film dimension increase (ppm) versus temperature (°C.) as recorded by the Analyzer. The Tg is obtained from the deflection in the curve of the graph as is known in the art.
The CTE was also derived via the “bending beam” procedure, details of which are set forth in “McKerrow”.
8) Gapfill Ability (μm)
After coating a patterned wafer with the subject poly (arylene ether) and curing, the resulting 6 inch diameter wafer with a cured film layer of 1 micron thickness thereon was cleaved via conventional or focused ion beam milling procedures. The cross-sectional view of the coated wafer was examined under a JEOL Model JSM6300F scanning electron microscope (“SEM”) in order to determine what gap sizes and trench depths on the wafer were completely filled. The reported values represent the smallest gap sizes which could be completely filled without defects.
9) Solution Viscosity (cP)
Approximately 20 ml of a poly(arylene ether) solution were placed in a Brookfield Synchro-lectric viscometer, Model LVT 62238 to measure the solution viscosity, typically at ambient temperature.
10) Film Thickness Uniformity (%)
The thickness as determined by a DekTek profilometer or by ellipsometry of the film at 20 various locations was averaged. The reported value is the percent of nonuniformity of the coated wafer.
11) Refractive Index—Out of Plane and In Plane, (“nTE”) and (“nTM”), Respectively
Details of this test procedure are set forth in “McKerrow.”.
12) Residual Stress (MPa)
After measuring the residual stress of a coated, cured poly(arylene ether) film-coated wafer in the above-described Tencor System, the wafer was heated to a temperature of 400° C. or 450° C. over a time period of 60 min in the System simultaneously with the recording of a stress measurement of the film at minute intervals. The temperature of the System was then decreased to 25° C. over a time period of 60 minutes simultaneously with the recording of a stress measurement of the film at minute intervals. The temperature in the System was then held constant at 25° C. for 6 hours simultaneously with the recording of a stress measurement of the film at 6 minute intervals. This cycle was repeated 4 additional times.
The reported Residual Stress values are recorded after the completion of five cycles. The Tg was determined from the temperature at which the film exhibited zero stress.
The Residual Stress of a similar poly(arylene ether) thin film was also measured via the “bending beam” procedure as set forth in “McKerrow.”.
13) Thermal Stability
Both the isothermal thermal graphometric analysis (“ITGA”)(microgram loss/time) and thickness change (%) were determined according to the procedure set forth in Lau, K S. Y., et al, “Characterization and Thin-Film Properties of FLARE™, A Low Dielectric Constant, High Tg Fluorinated Poly(arylene ether), as an Intermetal Dielectric for Interconnect Applications,” Proceedings, Thirteenth International VLSI Multilevel Interconnection (VMIC) Conference & Poster Session (California Jun. 18-20, 1996) [hereafter “Layu”].
After preparing a 12% solution of a poly(arylene ether) corresponding to formula C′ wherein Z is 1,5-naphthalenediyl and n is about 10, available from Allied-Signal under the tradename “FLARE™ 1.51”, by dissolving the appropriate quantity of the solid polymer in cyclopentanone under ambient conditions in a glass lined reactor, the solution was filtered through a series of four Teflon filtration cartridges at different pore sizes, i.e. 1.0, 0.5, 0.2 and 0.1 micrometers.
About 3 ml to about 7 ml of the solution prepared in Example I was processed onto the surface of a silicon wafer having a diameter of 6 inches, using a spin coater manufactured by Silicon Valley Group, Inc. (SVG), Model No. 8826, and a multiple hot plate oven track manufactured by SVG, Model 8840, with the initial spin conditions set at 72° F., 20-30 mmHg, and a spin cup humidity of 40%. The spin-bake-cure recipe was in accordance with the conditions set forth in “Lau”.
Various thermomechanical and electrical properties of the film were measured as illustrated below in Table 1.
| TABLE 1 |
| PROPERTIES OF FLARE ™ 1.51 POLYARYLENE ETHER FILM |
| Poly(arylene ether) | |||
| PROPERTY | FLARE ™ 1.51 | ||
| Cured film thickness | Nominally 1 micron | ||
| Film Thickness Uniformity | <2% | ||
| Refractive Index (out-of-plane, | 1.62 | ||
| DTM) | |||
| Refractive Index (in-plane, DTE) | 1.63 | ||
| Dielectric Constant (out-of-plane) | 2.62 | ||
| calculated from Maxwell identity | |||
| k = DTM 2: | |||
| Dielectric Constant (in-plane) | 2.63 | ||
| calculated from Maxwell identity | |||
| k = DTE 2: | |||
| Dielectric Constant (out-of-plane) | |||
| MIM (caped plate) and 1 MHz | 2.52 | ||
| MIM (DOM) and 1 MHz | 2.84 | ||
| Glass Transition Temperature | >350° C. (DMA) | ||
| 378° C. (TMA) | |||
| Residual Stress (stress hysteresis) | 31-37 | ||
| MPa | |||
| Residual Stress (Bending Beam) | 45 | ||
| MPa | |||
| CTE (ppm/° C.) (TMA) | 43-52 | ||
| CTE (ppm/° C.) (Bending Beam) | 40 | ||
| Thermal Stability | 1) | 1-2% (400° C.) | |
| 1) ITGA weight loss (% in 2 hr) | 3-5% (425° C.) | ||
| 2) thickness change (%) | 5-6% (450° C.) | ||
| 2) | zero change |
| Gapfill Ability (type of substrate | 0.30 μm (SiO2) | ||
| surface) | |||
| 0.25 μm (Al) | |||
As illustrated in FIG. 4, analysis of the first DSC scan on the film recorded a distinct exotherm having a maximum temperature (“Tmax”) at 226° C., with an onset coinciding with a shallow endotherm (softening point) at a temperature of 133° C. Although not wishing to be bound by any theory, we believe that the exotherm at 226° C. (ΔH=304.6 J/g) is likely due to the IMC reaction that rigidizes the bis (phenylethynyl)biphenyl units.
Upon subjecting the samples to a second DSC scan, neither of these exotherms and endotherms were apparent, and no new features assignable to a glass transition were observed between 200° and 300° C. A mild calorimetric change was seen in the 350°-400° C. range, but it was ambiguous. At temperatures of 350°-400° C., a possible position for a Tg at a temperature of about 360° C. and an exotherm (which was ˜10% of the magnitude of the exotherm recorded at 226° C.) at 393° C. We also believe that this mild exotherm likely corresponds to the cross-linking reaction of residual phenylethynyl groups left over from the IMC reaction. We further believe that the combination of these IMC reactions contributes to the high Tg of the composition of the present invention.
The results of DMA and TMA analysis confirmed that the film possesses a high Tg, i.e. greater than 350° C. FIG. 6 illustrates the DMA results.
The MIM dielectric constant for DOM structures was higher than that for the capped structures due to the interaction between the polymer and the metal during the testing procedure. Such interactions were eliminated by the use of the capping layer in the capped structures, the results for which thus more accurately represent the dielectric constant property of the polymer film.
Fifty mL of the solution of Example 1 was concentrated to 25% solids content and then poured onto the surface of an optically polished quartz plate having a diameter of 10 cm to cover an area of about 1 cm by 6 cm. The solution was then leveled across the plate's surface with a doctor's blade set at a nominal setting, typically in the range of 35 μm to 80 μm, then baked on a hot plate. The coated plate was exposed to a variant temperature increasing at a rate of 5° C./min until the hot plate reached a temperature of 150° C., at which point it was baked at a constant temperature for about 2 hours.
In order to cure the film, the baked coated plate was then heated, in the presence of nitrogen, inside the furnace chamber of a Tencor Flexus Model 2410 stress gauge at a variant temperature increasing at a rate of 5° C./min until the furnace reached a temperature of 425° C., then heated for 1 hour at 425° C., and then cooled at a variant temperature decreasing at a rate of 2°/min until the temperature in the furnace was 25° C.
The coated plate was then placed in water set at a temperature of 90° C. for 1 to 3 hours after which time the film was removed from the plate. The resulting film possesses a thickness of 30 to 60 μm, as desired.
It is evident from this Example that free standing film samples of poly(arylene ether) FLARE™ 1.51 can be routinely prepared to a thickness ranging from about 30 μm to about 60 μm.
A 1.132-kb quantity (5.290 Mol) of 3,3′-dimethoxybiphenyl and 12 L of acetonitrile were charged into a reaction vessel set at a temperature of 25° C. under ambient pressure with stirring. The temperature of a surrounding external cooling bath was then lowered such that the internal temperature of the reaction mixture was reduced to approximately 15° C.
To this solution under cooling, 1.883 kg (10.58 Mol) of solid N-bromosuccinimide (“NBS”) were added over a four-hour period while maintaining the reaction mixture temperature at 15° C. After removing the cooling bath, the reaction mixture was then stirred at ambient temperature for approximately 15 hours.
The crystallized product was isolated from the mother liquor by suction filtration in a Büchner filter funnel using approximately 200 torr of pressure, washed with approximately 2 L of acetonitrile, then dried under ambient conditions while still on the Büchner filter for about 1 hour and then in an oven under 10 torr of pressure and set at a temperature of about 60° C. for about 16 hours. The yield of the product was 1.338 kg (68%; theoretical yield was 1.968 kg). The melting range matched the literature value of 134-136° C. The purity of the product, which was determined by gas chromatography, using a Hewlett-Packard Model 5890 gas chromatograph equipped with a standard 2-meter glass capillary column (“GC”), was 98.4%.
After concentrating the mother liquor on a Büchi rotary evaporator, the precipitated solids therefrom were partitioned between heptane and water phases at reflux. The succinimide-containing water layer was then discarded, and the wet heptane solution layer was dried by simple azeotropic distillation at a pot temperature of about 80° C., while the distillate mixture was collected in the Dean-Stark trap until the heptane phase appeared clear and virtually no more water droplets separated in the cooled distillate. After treating the anhydrous heptane with about 20 grams of activated charcoal such as “Darco G-60” from Aldrich Chemical Co., the solution was filtered through a bed of a “Celite” filtering aid, also available from Aldrich, and placed inside a Büchner filter funnel. The heptane filtrate was then cooled to allow crystallization of a tan-colored solid (360 g, 18% yield), which was then distilled on a Kugelrohr distillation device at 2-5 torr pressure, to give 300 g (15% yield) of a colorless solid. GC analysis indicated a >98% purity, suitable for the Grignard reaction.
The heptane mother liquor was concentrated to yield 197 g of a solid residue. GC analysis of the solid revealed the presence of a mixture of mono and dibromo derivatives.
This example illustrates an improved method for producing 2,2′-Dibromo-5,5′-dimethoxybiphenyl which results in yields that are significantly higher than those of the prior art processes.
Preparation of Grignard Reagent
Approximately 5 L of tetrahydrofuran (THF) (Aldrich 14,7222-2) were mixed with 0.5 L of a 65% solution of sodium bis(2-methoxyethoxy)aluminium hydride in toluene, a reagent available from Aldrich Chemical Co. under the tradename “Red-AI®” (19,610-3), under ambient conditions. The mixture was heated to reflux and distilled under nitrogen to produce anhydrous THF.
A reaction vessel equipped with a mechanical stirrer was charged with 110 grams (4.58 Mol) of magnesium tumings (Aldrich 20,909-5). A 500-mL portion of a solution containing 700 grams (1.88 Mol) of the 2,2′-dibromo-5,5′-dimethoxybiphenyl produced in Example 4 in 3,000 mL of dry THF was added to the vessel. The reaction mixture was heated for about 10 minutes with a heating mantle to a temperature of 60° C. while 2 grams of an iodine reaction initiator was added thereto. Then, the rest of the THF-2,2′-dibromo-5,5′-dimethoxybiphenyl solution was added dropwise to the reaction mixture at a rate sufficient to maintain reflux. After heating the mixture at reflux (60° C.) for one hour, 1,500 mL of dry THF were added thereto while maintaining the temperature of the reaction mixture at about 60° C.
Iodination
A 1,050-gram (4.134 Mol) quantity of iodine and 3,000 ml of anhydrous THF were charged into a 22-L reaction flask equipped with mechanical stirrer which was maintained at a temperature of 0° C. via an external cooling bath. The above Grignard reagent was added thereto via a double tipped syringe over a period of 3 hours. The reaction mixture was then stirred for an additional three hours.
After slowly adding 10 L of a 20% aqueous sodium bisulfite solution to the reaction mixture, the resulting mixture was stirred for 16 hours. The solid product was collected therefrom via the suction filtration procedure set forth in Example 4. The filtered cake was washed four times with 1,000 mL of water, then dried in the vacuum oven described in Example 4 for 24 hours at 60° C. In general, 800-824 g (92-94% yield; theoretical yield is 876 g) of 2,2′-Diiodo-5,5′-dimethoxybiphenyl (93% purity as determined by GC) was produced.
A 250-mL (230.5 grams; 2.83 Mol) quantity of dihydropyran (Aldrich 310.620-8) was charged into a reaction flask equipped with a mechanical stirrer at 25° C. and ambient pressure. Over a minimum of three hours, 200 g 2,2′-diiodo-5,5′-dihydroxybiphenyl was added in the absence of catalyst.
Samples of the reaction mixture were analyzed by thin-layer chromatography (“TLC”) using a hexane-ethyl acetate eluant in a 5:1 molar ratio. When the TLC results showed complete disappearance of starting materials, 450 mL of heptane were charged into the reaction vessel, and the resulting mixture was stirred for 16 hours. The solid product was collected via the suction filtration procedure set forth in Example 4, washed twice with 200 mL of heptane, and then dried in the vacuum oven used in Example 4 at a temperature of 50° C. for 24 hours. A 220-g quantity (80% yield) of 2,2′-Diiodo-5,5′-bis(tetrahydropyranyl)biphenyl was produced having a melting point of 116.5-117.4° C. (literature melting point of 112° C. to 113° C. as set forth in Lindley).
Example 7 illustrates a reliable method of producing 2,2′-Diiodo-5,5′-bis(tetrahydropyranyl)biphenyl at a yield higher than that reported in the prior art, i.e. “Lindley”.
1.0000 Mol of decafluorobiphenyl followed by 0.6633 Mol of 2,2′-bis(phenylethynyl)-5,5′-dihydroxy-biphenyl were added to a nitrogen purged reaction vessel containing 600 mL of N,N-dimethylacetamide (“DMAC”). The mixture was heated to a temperature of 110° C. using an internal Teflon®-coated thermocouple. Potassium carbonate (2.48 Mol) was then added to the stirred reaction mixture while maintaining its internal temperature at 110° C.
After four hours of stirring, 0.3317 Mol of 1,5-dihydroxynaphthalene were added thereto with stirring. After about 17 hours of additional stirring, the mixture was cooled to ambient temperature then poured into 2 L of water. The resulting white polymer precipitate was filtered via the suction filtration procedure set forth in Example 4, washed once with 200 mL of water, placed inside an extraction thimble in the Soxhlet tower, and continuously extracted with a recycling stream of 15% ethanol/water sustained by volatilizing the solvent up to the water-cooled condenser, condensing the solvent to drop into the thimble inside the Soxhlet tower, and siphoning back to the heating pot. Extraction was continued for 2 to 3 days. After the polymer was rinsed with 500 mL of methanol and dried under vacuum at 70° C. for 15 hours, it was then dissolved in cyclopentanone at the desired solids content. The solution was filtered through the series of 4 Teflon cartridges with 1.0, 0.5, 0.2 and 0.1 μm porosities.
Claims (41)
1. A process for producing a poly(arylene ether) composition comprising:
where r is an integer of from about 0 to about 50,
wherein q is an integer of from about 0 to about 49 with a compound of formula B
wherein R is an aromatic nucleus which imparts a glass transition temperature of greater than 300° C. to the composition, under conditions sufficient to produce the composition.
2. The process of claim 1 wherein r is from about 1 to about 4.
3. The process of claim 2 wherein said compound of the formula A is comprised of hexafluorobenzene, decafluoro-biphenyl having ortho-, meta-, and para-catenation between its aromatic rings, perfluoro-para-terphenyl, perfluoro-meta-terphenyl, perfluoro-ortho-terphenyl, tetradecafluoro-para-terphenyl, tetradecafluoro-meta-terphenyl, tetradecafluoro-ortho-terphenyl, octadecafluoro-para,para-terphenyl or all positional isomers thereof due to ring catenation, perfluoro-para,para-tetraphenyl and all positional isomers thereof due to ring catenation, or mixtures thereof.
4. The process of claim 1 wherein q is from about 0 to about 3.
5. The process of claim 1 wherein the temperature of said reaction is from about 100° C. to about 175° C.
6. The process of claim 1 wherein the molar ratio of compounds having formula A or formula D to compounds having Formula B is about 1:1 to about 1:0.95.
wherein m is an integer of from about 0 to about 50; and n is an integer of from about 1 to about 200; comprising:
wherein r is an integer of from about 0 to about 50 with a compound of formula B:
or mixtures thereof, under conditions sufficient to produce the composition.
9. The process of claim 7 wherein said R group is comprised of 2,2′-bis(phenylethynyl)-4,4′biphenylenediyl or 2,2′-bis(phenylethynyl)-5,5′-biphenylenediyl.
10. The process of claim 7 wherein m is from about 0 to about 4.
11. The process of claim 7 wherein n is from about 1 to about 200.
12. The process of claim 1 further comprising recovering the poly(arylene ether) composition.
13. The composition produced according to the process of claim 1 .
14. The composition produced according to the process of claim 12 .
15. The process of claim 1 wherein the compound having formula A and a first compound having formula B are further reacted with a compound containing formula Z under conditions sufficient to produce a product corresponding to formula C″
wherein
Z is comprised of 1,5-naphthalenediyl, an aromatic nucleus in a second compound having formula B which is independent from said first compound having formula B, or mixtures thereof;
or mixtures thereof:
T1, T2, and T3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl, or mixtures thereof; a is independently an integer from about 1 to about 200; and b is an integer of from about 0 to about 20.
wherein c is independently an integer from about 1 to about 200; and Z is comprised of 1,5-naphthalenediyl, an aromatic nucleus in a second compound having formula B which is independent from said first compound having formula B, or mixtures thereof.
17. The process of claim 15 wherein an inorganic base is optionally reacted with said compound having formula A, said first compound having formula B, and said compound containing formula Z.
18. The process of claim 17 wherein said inorganic base is potassium carbonate.
19. The process of claim 17 wherein the molar ratio of said compound having formula Z to said first compound having formula B is about 20 to about 1.
20. The process of claim 17 wherein Z is 1,5-naphthalenediyl.
21. The process of claim 1 wherein the compound having formula D and a first compound having formula B are further reacted with a compound containing formula Z under conditions sufficient to produce a product corresponding to formula D″
wherein
Z is comprised of 1,5-naphthalenediyl, an aromatic nucleus in a second compound having formula B which is independent from said first compound having formula B, or mixtures thereof;
T1, T2, and T3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl, or mixtures thereof; d is independently an integer from about 1 to about 200; b is an integer of from about 0 to about 20; and q is independently an integer of from about 0 to about 20.
22. A film comprised of the composition of claim 13 .
23. A substrate coated with the composition of claim 13 .
24. A film comprised of the composition of claim 14 .
25. A microelectronic device containing the film of claim 22 .
26. A microelectronic device containing the film of claim 24 .
wherein
Z is comprised of 1,5-naphthalenediyl, an R group wherein R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition, or mixtures thereof;
T1, T2, and T3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl, or mixtures thereof;
d is independently an integer from about 1 to about 200;
b is an integer of from about 0 to about 20; and
q is an integer of from about 0 to about 20.
wherein
Z is comprised of 1,5-naphthalenediyl, an R group wherein R is an aromatic nucleus which imparts a glass transition temperature of greater than 260° C. to the composition, or mixtures thereof;
and mixtures thereof;
T1, T2, and T3 are independently comprised of an alkyl having from about 1 to about 10 carbons, an aryl, or mixtures thereof;
a is independently an integer from about 1 to about 200; and
b is an integer of from about 0 to about 20.
30. A process for producing a fluorinated poly(arylene ether) mixture for spin-coating onto a substrate comprising:
wherein
R is an aromatic nucleus which imparts a glass transition temperature of greater than 300° C. to the composition;
m is an integer of from about 0 to about 50; and
n is an integer of from about 1 to about 200 and an aprotic solvent; and
b) filtering the solution through at least one filter having a pore size of about 0.1 micrometers.
31. The process of claim 30 wherein said aprotic solvent is comprised of cyclic ketones, cyclic amides, and mixtures thereof.
32. The process of claim 30 wherein said aprotic solvent is comprised of cyclopentanone, cyclohexanone, cycloheptanone, cyclooctanone, N-methylpyrrolidinone, N-cyclohexyl-pyrrolidinone, or mixtures thereof.
33. The process of claim 32 wherein said solvent is cyclopentanone.
34. The process of claim 30 wherein said solution is comprised of, based upon the total weight of said solution, from about 50% to about 99% of said solvent and from about 1 to about 50% of said fluorinated poly(arylene ether) composition.
35. The process of claim 34 wherein said solution is comprised of, based upon the total weight of said solution, from about 80% to about 97% of said solvent and from about 3 to about 20% of said fluorinated poly(arylene ether) composition.
36. The process of claim 30 wherein said solution is filtered sequentially through at least two filters, each of said filters having a different pore size.
37. A process for producing a cured, coated substrate comprising:
wherein
R is an aromatic nucleus which imparts a glass transition temperature of greater than 300° C. to the composition;
m is an integer of from about 0 to about 50; and
n is an integer of from about 1 to about 200 and an aprotic solvent;
b) filtering the solution;
c) applying the solution of step b via spin-coating onto a substrate to form a film thereon; and
d) heating the film of step c under conditions sufficient to cure the film.
39. A film comprised of the composition of claim 27 .
40. A substrate coated with the film of claim 39 .
41. A microelectronic device containing the film of claim 39 .
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/659,380 USRE37599E1 (en) | 1995-06-26 | 2000-09-08 | Poly(arylene ether) compositions and the method for their manufacture |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US51495P | 1995-06-26 | 1995-06-26 | |
| US08/665,132 US5986045A (en) | 1995-06-26 | 1996-06-14 | Poly(arylene ether) compositions and the method for their manufacture |
| US09/659,380 USRE37599E1 (en) | 1995-06-26 | 2000-09-08 | Poly(arylene ether) compositions and the method for their manufacture |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/665,132 Reissue US5986045A (en) | 1995-06-26 | 1996-06-14 | Poly(arylene ether) compositions and the method for their manufacture |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| USRE37599E1 true USRE37599E1 (en) | 2002-03-19 |
Family
ID=26667757
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/665,132 Ceased US5986045A (en) | 1995-06-26 | 1996-06-14 | Poly(arylene ether) compositions and the method for their manufacture |
| US09/659,380 Expired - Fee Related USRE37599E1 (en) | 1995-06-26 | 2000-09-08 | Poly(arylene ether) compositions and the method for their manufacture |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US08/665,132 Ceased US5986045A (en) | 1995-06-26 | 1996-06-14 | Poly(arylene ether) compositions and the method for their manufacture |
Country Status (8)
| Country | Link |
|---|---|
| US (2) | US5986045A (en) |
| EP (1) | EP0835275B1 (en) |
| JP (1) | JP3751978B2 (en) |
| KR (1) | KR19990028450A (en) |
| DE (1) | DE69620405T2 (en) |
| HK (1) | HK1010203A1 (en) |
| TW (1) | TW428005B (en) |
| WO (1) | WO1997001593A1 (en) |
Cited By (4)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6715200B2 (en) | 1999-02-12 | 2004-04-06 | General Electric Company | Methods for making data storage media |
| US7179551B2 (en) * | 1999-02-12 | 2007-02-20 | General Electric Company | Poly(arylene ether) data storage media |
| US20070106050A1 (en) * | 2005-11-08 | 2007-05-10 | Sokolowski Alex D | Crosslinked poly(arylene ether) composition, method, and article |
| US20080132669A1 (en) * | 2005-06-24 | 2008-06-05 | Asahi Glass Company, Limited | Crosslinkable fluorinated aromatic prepolymer and its uses |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| KR19990024596A (en) * | 1997-09-04 | 1999-04-06 | 윤종용 | Polyarylene ether for optical communication |
| US6235353B1 (en) * | 1998-02-24 | 2001-05-22 | Alliedsignal Inc. | Low dielectric constant films with high glass transition temperatures made by electron beam curing |
| US6660875B1 (en) | 1998-06-09 | 2003-12-09 | Ppt Technologies, Llc | Ion exchange purification of dielectric condensate precursor fluids and silicate esters such as tetraethylorthosilicate (TEOS) |
| US6333141B1 (en) | 1998-07-08 | 2001-12-25 | International Business Machines Corporation | Process for manufacture of integrated circuit device using inorganic/organic matrix comprising polymers of three dimensional architecture |
| US6093636A (en) * | 1998-07-08 | 2000-07-25 | International Business Machines Corporation | Process for manufacture of integrated circuit device using a matrix comprising porous high temperature thermosets |
| WO2000031183A1 (en) | 1998-11-24 | 2000-06-02 | The Dow Chemical Company | A composition containing a cross-linkable matrix precursor and a poragen, and a porous matrix prepared therefrom |
| US6399666B1 (en) | 1999-01-27 | 2002-06-04 | International Business Machines Corporation | Insulative matrix material |
| JP2001181577A (en) | 1999-12-27 | 2001-07-03 | Sumitomo Chem Co Ltd | Coating liquid for forming porous organic film and method for forming porous organic film |
| KR100795714B1 (en) | 2000-08-21 | 2008-01-21 | 다우 글로벌 테크놀로지스 인크. | Organosilicate resins as hardmasks for organic polymer dielectrics in fabrication of microelectronic devices |
| US6593155B2 (en) | 2000-12-28 | 2003-07-15 | Dow Global Technologies Inc. | Method for determination of cure and oxidation of spin-on dielectric polymers |
| JP3509760B2 (en) * | 2001-02-08 | 2004-03-22 | 株式会社半導体先端テクノロジーズ | Method for manufacturing semiconductor device |
| US6465267B1 (en) * | 2001-04-02 | 2002-10-15 | Advanced Micro Devices, Inc. | Method of measuring gate capacitance to determine the electrical thickness of gate dielectrics |
| CN1643030A (en) * | 2001-04-06 | 2005-07-20 | 霍尼韦尔国际公司 | Low dielectric constant materials and methods of preparation thereof |
| US20040247896A1 (en) * | 2001-12-31 | 2004-12-09 | Paul Apen | Organic compositions |
| US6716955B2 (en) * | 2002-01-14 | 2004-04-06 | Air Products And Chemicals, Inc. | Poly(arylene ether) polymer with low temperature crosslinking grafts and adhesive comprising the same |
| US7030209B2 (en) | 2002-02-04 | 2006-04-18 | E. I. Du Pont De Nemours And Company | Halogenated optical polymer composition |
| US6787633B2 (en) * | 2002-09-13 | 2004-09-07 | General Electric Company | Method and apparatus for preparing a poly(arylene ether) |
| WO2004066360A2 (en) | 2003-01-22 | 2004-08-05 | Honeywell International Inc | Apparatus and methods for ionized deposition of a film or thin layer |
| TW200505966A (en) | 2003-04-02 | 2005-02-16 | Dow Global Technologies Inc | Organosilicate resin formulation for use in microelectronic devices |
| JP4501391B2 (en) | 2003-09-30 | 2010-07-14 | 旭硝子株式会社 | Crosslinkable fluorine-containing aromatic prepolymer and use thereof |
| CA2511112A1 (en) * | 2004-06-30 | 2005-12-30 | Her Majesty The Queen In Right Of Canada, As Represented By The Minister Of National Research Council Of Canada | Synthesis of poly(arylene)s copolymers containing pendant sulfonic acid groups bonded to naphthalene as proton exchange membrane meterials |
| US20080173541A1 (en) * | 2007-01-22 | 2008-07-24 | Eal Lee | Target designs and related methods for reduced eddy currents, increased resistance and resistivity, and enhanced cooling |
| US8702919B2 (en) | 2007-08-13 | 2014-04-22 | Honeywell International Inc. | Target designs and related methods for coupled target assemblies, methods of production and uses thereof |
| KR101637267B1 (en) * | 2010-12-02 | 2016-07-08 | 현대자동차 주식회사 | Poly(arylene ether) copolymer having cation-exchange group, process of manufacturing the same, and use thereof |
| JP2013035959A (en) * | 2011-08-09 | 2013-02-21 | Nippon Shokubai Co Ltd | Fluorine-containing aromatic polymer |
Citations (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4252937A (en) | 1979-06-08 | 1981-02-24 | The United States Of America As Represented By The Secretary Of The Air Force | Polyaromatic ether-keto-sulfones and their synthesis |
| US4683340A (en) | 1986-10-08 | 1987-07-28 | The United States Of America As Represented By The Secretary Of The Air Force | Bis(benzilyloxy) compounds |
| EP0373784A2 (en) | 1988-12-12 | 1990-06-20 | Imperial Chemical Industries Plc | Aromatic polymer |
| WO1991009071A1 (en) | 1989-12-08 | 1991-06-27 | Raychem Corporation | Electronic articles containing a fluorinated poly(arylene ether) dielectric |
| WO1991009070A1 (en) | 1989-12-08 | 1991-06-27 | Raychem Corporation | Fluorinated poly(naphthyl ethers) |
| WO1991016370A1 (en) | 1990-04-17 | 1991-10-31 | Raychem Corporation | Crosslinkable fluorinated aromatic ether compositions |
| US5114780A (en) | 1990-04-17 | 1992-05-19 | Raychem Corporation | Electronic articles containing a fluorinated poly(arylene ether) dielectric |
| US5145936A (en) | 1989-12-08 | 1992-09-08 | Raychem Corporation | Fluorinated poly (naphthyl ether) |
-
1996
- 1996-06-14 US US08/665,132 patent/US5986045A/en not_active Ceased
- 1996-06-25 EP EP19960922563 patent/EP0835275B1/en not_active Expired - Lifetime
- 1996-06-25 KR KR1019970709766A patent/KR19990028450A/en not_active Application Discontinuation
- 1996-06-25 JP JP50450997A patent/JP3751978B2/en not_active Expired - Fee Related
- 1996-06-25 WO PCT/US1996/010811 patent/WO1997001593A1/en not_active Application Discontinuation
- 1996-06-25 DE DE1996620405 patent/DE69620405T2/en not_active Expired - Fee Related
- 1996-07-26 TW TW85109119A patent/TW428005B/en not_active IP Right Cessation
-
1998
- 1998-10-13 HK HK98111230A patent/HK1010203A1/en not_active IP Right Cessation
-
2000
- 2000-09-08 US US09/659,380 patent/USRE37599E1/en not_active Expired - Fee Related
Patent Citations (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4252937A (en) | 1979-06-08 | 1981-02-24 | The United States Of America As Represented By The Secretary Of The Air Force | Polyaromatic ether-keto-sulfones and their synthesis |
| US4683340A (en) | 1986-10-08 | 1987-07-28 | The United States Of America As Represented By The Secretary Of The Air Force | Bis(benzilyloxy) compounds |
| EP0373784A2 (en) | 1988-12-12 | 1990-06-20 | Imperial Chemical Industries Plc | Aromatic polymer |
| WO1991009071A1 (en) | 1989-12-08 | 1991-06-27 | Raychem Corporation | Electronic articles containing a fluorinated poly(arylene ether) dielectric |
| WO1991009070A1 (en) | 1989-12-08 | 1991-06-27 | Raychem Corporation | Fluorinated poly(naphthyl ethers) |
| US5108840A (en) | 1989-12-08 | 1992-04-28 | Raychem Corporation | Multilayer electronic circuit article having a poly(naphthyl ether) dielectric |
| US5145936A (en) | 1989-12-08 | 1992-09-08 | Raychem Corporation | Fluorinated poly (naphthyl ether) |
| WO1991016370A1 (en) | 1990-04-17 | 1991-10-31 | Raychem Corporation | Crosslinkable fluorinated aromatic ether compositions |
| US5114780A (en) | 1990-04-17 | 1992-05-19 | Raychem Corporation | Electronic articles containing a fluorinated poly(arylene ether) dielectric |
Non-Patent Citations (2)
| Title |
|---|
| Journal Polym. Sci. : Part A : Polymer Chemistry, vol. 29, 1991,pp. 1061-1071, Lindley et al., "Intramolecular Cyclization of Pendant Phenylethynyl Groups". |
| Polymeric Materials Sci. & Eng., vol. 66, 1992, pp. 198-199, Mercer et al.., "Low Dielectric Constant Fluorinated Aryl Ethers". |
Cited By (8)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6715200B2 (en) | 1999-02-12 | 2004-04-06 | General Electric Company | Methods for making data storage media |
| US6752952B2 (en) | 1999-02-12 | 2004-06-22 | General Electric Company | Embossing methods |
| US20040143958A1 (en) * | 1999-02-12 | 2004-07-29 | Feist Thomas P. | Methods for making data storage media and the resultant media |
| US20050233151A1 (en) * | 1999-02-12 | 2005-10-20 | Feist Thomas P | Data storage media |
| US7179551B2 (en) * | 1999-02-12 | 2007-02-20 | General Electric Company | Poly(arylene ether) data storage media |
| US20080132669A1 (en) * | 2005-06-24 | 2008-06-05 | Asahi Glass Company, Limited | Crosslinkable fluorinated aromatic prepolymer and its uses |
| US7538177B2 (en) | 2005-06-24 | 2009-05-26 | Asahi Glass Company, Limited | Crosslinkable fluorinated aromatic prepolymer and its uses |
| US20070106050A1 (en) * | 2005-11-08 | 2007-05-10 | Sokolowski Alex D | Crosslinked poly(arylene ether) composition, method, and article |
Also Published As
| Publication number | Publication date |
|---|---|
| JP3751978B2 (en) | 2006-03-08 |
| EP0835275B1 (en) | 2002-04-03 |
| KR19990028450A (en) | 1999-04-15 |
| JPH10510580A (en) | 1998-10-13 |
| HK1010203A1 (en) | 1999-06-17 |
| DE69620405D1 (en) | 2002-05-08 |
| WO1997001593A1 (en) | 1997-01-16 |
| DE69620405T2 (en) | 2002-10-02 |
| EP0835275A1 (en) | 1998-04-15 |
| TW428005B (en) | 2001-04-01 |
| US5986045A (en) | 1999-11-16 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| USRE37599E1 (en) | Poly(arylene ether) compositions and the method for their manufacture | |
| US6124421A (en) | Poly(arylene ether) compositions and methods of manufacture thereof | |
| US5959157A (en) | Process for making hydroxy-substituted ethynylated biphenyl compounds | |
| US5874516A (en) | Nonfunctionalized poly(arylene ethers) | |
| EP0758664A1 (en) | Nonhalogenated poly(arylene ether) dielectrics | |
| US6303733B1 (en) | Poly(arylene ether) homopolymer compositions and methods of manufacture thereof | |
| US7166250B2 (en) | Poly(arylene ether) dielectrics | |
| US7772344B2 (en) | Insulating film-forming composition | |
| JPWO2005114724A1 (en) | Laminated body and semiconductor device | |
| KR102543300B1 (en) | Novel polymer with a good heat resistance and Storage Stability, underlayer film composition containing the polymer and process for forming underlayer film using the composition | |
| JP3969779B2 (en) | Manufacturing method of organic insulating film material | |
| JP3195247B2 (en) | Organic insulating film material and method of manufacturing the same | |
| JP4045621B2 (en) | Electrical insulating material containing fluorine-containing polyimide resin and electronic component using the same | |
| JP3195248B2 (en) | Organic insulating film material and method of manufacturing the same | |
| JP3462941B2 (en) | Composition for interlayer insulating film and / or surface protective film of semiconductor substrate and semiconductor device | |
| JP3769929B2 (en) | Phenylene group-containing polymer composition, thin film and electronic component | |
| JPH06271772A (en) | Silsesquioxane polymer composition | |
| JP2000186140A (en) | Thermosetting polymer, solution containing the same and film using the solution | |
| JP2010215819A (en) | Composition for film forming, insulator film and semiconductor device | |
| JPH10189567A (en) | Interlayer insulating film and/or surface protective composition of semiconductor substrate as well as semiconductor device | |
| JPH11323249A (en) | Polyarylene ether solution and its cross-linked film |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| FPAY | Fee payment |
Year of fee payment: 4 |
|
| FEPP | Fee payment procedure |
Free format text: PAYER NUMBER DE-ASSIGNED (ORIGINAL EVENT CODE: RMPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY Free format text: PAYOR NUMBER ASSIGNED (ORIGINAL EVENT CODE: ASPN); ENTITY STATUS OF PATENT OWNER: LARGE ENTITY |
|
| FPAY | Fee payment |
Year of fee payment: 8 |
|
| REMI | Maintenance fee reminder mailed | ||
| LAPS | Lapse for failure to pay maintenance fees |