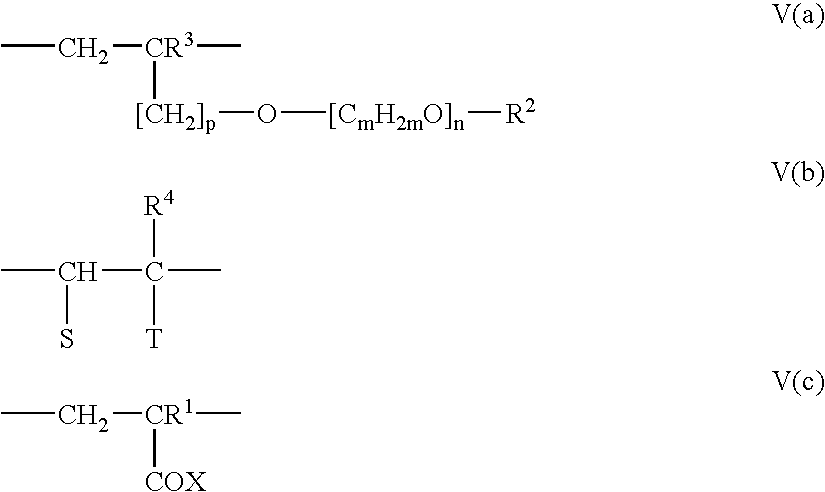US20060280898A1 - Modifiers for gypsum slurries and method of using them - Google Patents
Modifiers for gypsum slurries and method of using them Download PDFInfo
- Publication number
- US20060280898A1 US20060280898A1 US11/152,317 US15231705A US2006280898A1 US 20060280898 A1 US20060280898 A1 US 20060280898A1 US 15231705 A US15231705 A US 15231705A US 2006280898 A1 US2006280898 A1 US 2006280898A1
- Authority
- US
- United States
- Prior art keywords
- gypsum
- dispersant
- slurry
- modifier
- water
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [4*]OCCOCC([5*])(C)C([6*])C Chemical compound [4*]OCCOCC([5*])(C)C([6*])C 0.000 description 3
- KISSZFZZJXHEHH-UHFFFAOYSA-M C.C.C.C.[H]OCCOC(C)CC(C(=O)OCCOC)C(C)C(=O)[O-][Na+] Chemical compound C.C.C.C.[H]OCCOC(C)CC(C(=O)OCCOC)C(C)C(=O)[O-][Na+] KISSZFZZJXHEHH-UHFFFAOYSA-M 0.000 description 1
- PFEOZHBOMNWTJB-UHFFFAOYSA-N CCC(C)CC Chemical compound CCC(C)CC PFEOZHBOMNWTJB-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- C—CHEMISTRY; METALLURGY
- C04—CEMENTS; CONCRETE; ARTIFICIAL STONE; CERAMICS; REFRACTORIES
- C04B—LIME, MAGNESIA; SLAG; CEMENTS; COMPOSITIONS THEREOF, e.g. MORTARS, CONCRETE OR LIKE BUILDING MATERIALS; ARTIFICIAL STONE; CERAMICS; REFRACTORIES; TREATMENT OF NATURAL STONE
- C04B28/00—Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder, e.g. polycarboxylate cements
- C04B28/14—Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder, e.g. polycarboxylate cements containing calcium sulfate cements
-
- Y—GENERAL TAGGING OF NEW TECHNOLOGICAL DEVELOPMENTS; GENERAL TAGGING OF CROSS-SECTIONAL TECHNOLOGIES SPANNING OVER SEVERAL SECTIONS OF THE IPC; TECHNICAL SUBJECTS COVERED BY FORMER USPC CROSS-REFERENCE ART COLLECTIONS [XRACs] AND DIGESTS
- Y10—TECHNICAL SUBJECTS COVERED BY FORMER USPC
- Y10T—TECHNICAL SUBJECTS COVERED BY FORMER US CLASSIFICATION
- Y10T428/00—Stock material or miscellaneous articles
- Y10T428/23—Sheet including cover or casing
- Y10T428/232—Encased layer derived from inorganic settable ingredient
Definitions
- This invention relates to improved gypsum products. More specifically, it relates to an improved gypsum slurry that is flowable at low water concentrations, with less expense and less retardive effects of using carboxylate dispersants alone.
- Gypsum products are commonly used as building materials for many reasons, such as wallboard.
- Wallboard sheets are easily joined together to make continuous walls of any size and shape. They are easily patched and have fire and sound proofing properties.
- Decorative finishes, such as wallpaper or paint readily adhere to plaster or wallboard surfaces to allow for a large variety of decorating options.
- the strength of gypsum products made from full density slurries is inversely proportional to the amount of water used in their manufacture.
- Some of the water that is added to the gypsum slurry is used to hydrate the calcined gypsum, also known as calcium sulfate hemihydrate, to form an interlocking matrix of calcium sulfate dihydrate crystals.
- Excess water evaporates or is driven off in a kiln, leaving voids in the matrix once occupied by the water. Where large amounts of water were used to fluidize the gypsum slurry, more and larger voids remain in the product when it is completely dry. These voids decrease the product density and strength in the finished product.
- polycarboxylate superplasticizers are very effective in allowing water reduction and the resultant increase in product strength, however, there are disadvantages known to be associated with use of large doses of polycarboxylate dispersants. These materials are relatively expensive. When used in large doses, polycarboxylate dispersants can be the one of the single, most expensive additives in making gypsum products. The high price of this component can overcome the narrow margins afforded these products in a highly competitive marketplace.
- Gypsum board is made on high speed production lines where the slurry is mixed, poured, shaped and dried in a matter of minutes. The board must be able to hold its shape to be moved from one conveyor line to another to put the board into the kiln. Damage can occur if the boards have not attained a minimum green strength by the time they are stacked and wrapped for shipping. If the board line has to be slowed down because the board is not sufficiently set to move on to the next step in the process, production costs are driven up, resulting in an economically uncompetitive product.
- U.S. Pat. No. 5,718,759 teaches the addition of silicates to mixtures of beta-calcined gypsum and cement.
- lignosulfates or naphthalene sulfonates are used as water-reducing agents.
- pozzolanic materials, including silicates is credited with reducing expansion due to the formation of ettringite.
- the composition is suggested for use in building materials, such as backer boards, floor underlayments, road patching materials fire-stopping materials and fiberboard.
- Luongo in U.S. Pat. No. 6,391,958, teaches a novel wallboard composition combining gypsum with sodium silicates and a synthetic, cross-linking binder. Vinyl acetate polymers were the preferred cross-linking binder.
- the addition of sodium silicates reduces the amount of calcined gypsum that is needed to make a given number of panels. The weight of the building panel is reduced, making it easier for workers to move the panels before and during installations.
- a number of polycarboxylate dispersants are disclosed in U.S. Pat. No. 6,005,040.
- a water soluble polymer is disclosed having one repeating unit of the formula:
- the polymer also includes a water-soluble repeating unit selected from a group containing acrylic acid and methacrylic acid, among others.
- the polymer is used in an unfired, ceramic precursor material.
- Other polymers are disclosed in the application, including some for use in the core forming process of gypsum wallboard or the preparation of gypsum slurries.
- this invention includes the addition of an efficacy modifier to a gypsum slurry that increases the fluidity of slurries made with polycarboxylate dispersant.
- an efficacy modifier to a gypsum slurry that increases the fluidity of slurries made with polycarboxylate dispersant.
- the invention relates to an improved gypsum slurry, that includes water, calcium sulfate hemihydrate, a polycarboxylate dispersant and a modifier.
- the modifier is chemically configured to improve the efficacy of the polycarboxylate dispersant.
- Preferred modifiers include cement, lime, slaked lime, soda ash, carbonates, silicates, phosphonates and phosphates.
- a gypsum panel is made from at least one facing sheet and a core made from the improved gypsum slurry.
- Yet another aspect of this invention is a method of making the gypsum slurry that includes selecting a modifier, mixing the modifier with a polycarboxylate dispersant and adding the calcium sulfate hemihydrate.
- modifiers herein described improves the efficacy of the dispersant in fluidizing the gypsum slurry. This allows less of the dispersant to be used and still obtain high flowability in low water slurries for strength.
- Polycarboxylate dispersants are often one of the most expensive components in products that use them. Using lower dosages of the dispersant reduces the cost so that a competitively priced gypsum product can be made.
- the improved efficacy of the dispersant can also be used to reduce the amount of water used to make the gypsum slurry.
- the manufacturing process can be made more fuel efficient, conserving fossil fuels and realizing the cost savings. Fuel savings can be based on either reduced kiln temperatures or shorter residence time in the kiln.
- the gypsum slurry of this invention is made using water, calcined gypsum, a polycarboxylate dispersant and a modifier. Although the benefits of this invention are most clear when used in a slurry for a high strength product, it can be used with any slurry using a polycarboxylate, even those that already utilize low doses of polycarboxylate dispersant.
- any calcined gypsum or stucco is useful in this slurry. It has unexpectedly been discovered that some gypsum sources are more responsive to the dispersant and the modifier than other sources. Laboratory tests indicate that gypsums from different geographical areas contain different salts and impurities. While not wishing to be bound by theory, it is believed that the salts present in the gypsum influence to the fluidity in the slurry.
- Reduction in the amount of water used to make the slurry is achieved by the addition of a polycarboxylate dispersant.
- the dispersant attaches itself to the calcium sulfate, then charged groups on the backbone and the side chains on the branches of the polymer repel each other, causing the gypsum particles to spread out and flow easily.
- the amount of water can be reduced and still obtain a flowable fluid. In general, reduction in water results in increased product strength and lower drying costs.
- any polycarboxylate dispersant that is useful for improving fluidity in gypsum may be used in the slurry of this invention.
- a number of polycarboxylate dispersants, particularly polycarboxylic ethers, are preferred types of dispersants.
- One of the preferred class of dispersants used in the slurry includes two repeating units. It is described further in co-pending U.S. Ser. No. 11/_______ (Attorney Ref. No 2033.72740), entitled “Gypsum Products Utilizing a Two-Repeating Unit System and Process for Making Them”, previously incorporated by reference.
- These dispersants are products of Degussa Construction Polymers, GmbH (Trostberg Germany) and are supplied by Degussa Corp. (Kennesaw, Ga.) (hereafter “Degussa”) and are hereafter referenced as the “PCE211-Type Dispersants”.
- the first repeating unit is an olefinic unsaturated mono-carboxylic acid repeating unit, an ester or salt thereof, or an olefinic unsaturated sulphuric acid repeating unit or a salt thereof.
- Preferred first repeating units include acrylic acid or methacrylic acid.
- Mono- or divalent salts are suitable in place of the hydrogen of the acid group.
- the hydrogen can also be replaced by hydrocarbon group to form the ester.
- the second repeating unit satisfies Formula I, R 1 —O—R 2 I
- R 1 is an alkenyl group of Formula II
- R 2 is derived from an unsaturated (poly)alkylene glycol ether group according to Formula III. —(C m H 2m O) x —(C n H 2n O) y —R 4 III
- the alkenyl repeating unit optionally includes a C 1 to C 3 alkyl group between the polymer backbone and the ether linkage.
- the value of p is an integer from 0-3, inclusive.
- p is either 0 or 1.
- R 3 is either a hydrogen atom or an aliphatic C 1 to C 5 hydrocarbon group, which may be linear, branched, saturated or unsaturated. Examples of preferred repeating units include acrylic acid and methacrylic acid.
- the polyether group of Formula III contains multiple C 2 -C 4 alkyl groups, including at least two different alkyl groups, connected by oxygen atoms.
- M and n are integers from 2 to 4, inclusive. Preferably, at least one of m and n is 2.
- X and y are integers from 55 to 350, inclusive.
- R 4 is hydrogen or an aliphatic C 1 to C 20 hydrocarbon group, a cycloaliphatic C 5 to C 8 hydrocarbon group, a substituted C 6 to C 14 aryl group or a group conforming at least one of Formula IV(a), IV(b) and VI(c).
- R 5 and R 7 independently of each other, represent an alkyl, aryl, aralkyl or alkylaryl group.
- R 6 is a bivalent alkyl, aryl, aralkyl or alkylaryl group.
- PCE211 A particularly useful dispersant of the PCE211-Type Dispersants is designated PCE211 (hereafter “211”).
- Other polymers in this series known to be useful in wallboard include PCE111.
- PCE211-Type dispersants are described more fully in U.S. Ser. No. 11/_______ filed concurrently herewith, by Degussa Construction Polymers (Attorney Ref. DCP3) entitled “Polyether-Containing Copolymer”, herein incorporated by reference.
- the molecular weight of the PCE211 Type dispersant is preferably from about 20,000 to about 60,000 Daltons. Surprisingly, it has been found that the lower molecular weight dispersants cause less retardation of set time than dispersants having a molecular weight greater than 60,000 Daltons. Generally longer side chain length, which results in an increase in overall molecular weight, provides better dispensability. However, tests with gypsum indicate that efficacy of the dispersant is reduced at molecular weights above 50,000 Daltons.
- R 1 preferably makes up from about 300 to about 99 mole % of the total repeating units, more preferably from about 40 to about 80%. From about 1 to about 70 mole % of the repeating units are R 2 , more preferably from about 10 to about 60 mole %.
- the dispersant includes at least three repeating units shown in Formula V(a), V(b) and V(c).
- R 4 is hydrogen or a methyl group, depending on whether the structural units are acrylic or methacrylic.
- P can be from 0 to 3.
- M is an integer from 2 to 4, inclusive, and n is an integer from 0 to 200, inclusive.
- PCE211-Type and 2641-Type dispersants are manufactured by Degussa Construction Polymers, GmbH (Tröstberg, Germany) and marketed in the United States by Degussa Corp. (Kennesaw, Ga.).
- Preferred 2641-Type Dispersants are sold by Degussa as MELFLUX 2641F, MELFLUX 2651F and MELFLUX 2500L dispersants.
- 2641-Type dispersants (MELFLUX is a registered trademark of Degussa Construction Polymers, Gm bH) are described for use in wallboard and gypsum slurries in U.S. Ser. No. 11/_______ (Attorney Ref. No. 2033.72380), entitled “Fast Drying Wallboard”, previously incorporated by reference.
- 1641-Type Dispersants Yet another preferred dispersant family is sold by Degussa and referenced as “1641-Type Dispersants”. This dispersant is more fully described in U.S. Pat. No. 5,798,425, herein incorporated by reference.
- a particularly preferred 1641-Type Dispersant is shown in Formula VI and marketed as MELFLUX 1641F dispersant by Degussa. This dispersant is made primarily of two repeating units, one a vinyl ether and the other a vinyl ester. In Formula VI, m and n are the mole ratios of the component repeating units, which can be randomly positioned along the polymer chain.
- dispersants are particularly well-suited for use with gypsum. While not wishing to be bound by theory, it is believed that the acid repeating units bind to the hemihydrate crystals while the long polyether chains of the second repeating unit perform the dispersing function. Since it is less retardive than other dispersants, it is less disruptive to the manufacturing process of gypsum products such as wallboard.
- the dispersant is used in any effective amount. To a large extent, the amount of dispersant selected is dependent on the desired fluidity of the slurry. As the amount of water decreases, more dispersant is required to maintain a constant slurry fluidity.
- polycarboxylate dispersants are relatively expensive components, it is preferred to use a small dose, preferably less than 2% or more preferably less than 1% by weight based on the weight of the dry stucco.
- the dispersant is used in amounts of about 0.05% to about 0.5% based on the dry weight of the stucco. More preferably, the dispersant is used in amounts of about 0.01% to about 0.2% on the same basis. In measuring a liquid dispersant, only the polymer solids are considered in calculating the dosage of the dispersant, and the water from the dispersant is considered when a water/stucco ratio is calculated.
- the ratio of the acid-containing repeating units to the polyether-containing repeating unit is directly related to the charge density.
- the charge density of the co-polymer is in the range of about 300 to about 3000 ⁇ equiv. charges/g co-polymer. It has been found that the most effective dispersant tested for water reduction in this class of dispersants, MELFLUX 2651F, has the highest charge density.
- the increase in charge density further results in an increase in the retardive effect of the dispersant.
- Dispersants with a low charge density such as MELFLUX 2500L, retard the set times less than the MELFLUX 2651F dispersant that has a high charge density. Since retardation in set times increases with the increase in efficacy obtained with dispersants of high charge density, making a slurry with low water, good flowability and reasonable set times requires keeping of the charge density in a mid-range. More preferably, the charge density of the co-polymer is in the range of about 600 to about 2000 ⁇ equiv. charges/g co-polymer.
- the modifier can be any substance, liquid or solid, which when combined with a polycarboxylate dispersant in a gypsum slurry, leads to an improvement the efficacy of the dispersant.
- Modifiers are not intended to be dispersants in themselves, but serve to allow the dispersant to be more effective. For example, at constant concentrations of dispersant, better fluidity is obtained when the modifier is used compared to the same slurry without the modifier.
- Preferred modifiers include cement, lime, also known as quicklime or calcium oxide, slaked lime, also known as calcium hydroxide, soda ash, also known as sodium carbonate, potassium carbonate, also known as potash, and other carbonates, silicates, hydroxides, phosphonates and phosphates.
- Preferred carbonates include sodium and potassium carbonate.
- Sodium silicate is a preferred silicate.
- lime or slaked lime When lime or slaked lime is used as the modifier, it is used in concentrations of about 0.15% to about 1.0% based on the weight of the dry calcium sulfate hemihydrate.
- lime In the presence of water, lime is quickly converted to calcium hydroxide, or slaked lime, and the pH of the slurry becomes alkaline. The sharp rise in pH can cause a number of changes in the slurry chemistry.
- Certain additives, including trimetaphosphate break down as the pH increases. There can also be problems with hydration and, where the slurry is used to make wallboard or gypsum panels, there are problems with paper bond at high pH.
- modifiers include carbonates, phosphonates, phosphates and silicates.
- the modifiers are used in amounts less than 0.25% based on the weight of the dry calcium sulfate hemihydrate. Above these concentrations, increases in the amount of modifier causes a decrease in the dispersant efficacy. These modifiers are preferably used in amounts of from about 0.05 to about 0.2 weight %.
- the charge density of the dispersant has also been found to affect the ability of the modifier to interact with the dispersant. Given a family of dispersants with the same repeating units, the modifier causes a greater increase in efficacy in the dispersant having the higher charge density. It is important to note that although the general trend is to obtain a higher efficacy boost with higher charge density, when comparing the effectiveness of dispersants having different repeating units, the effectiveness of the dispersants may be considerably different at the same charge density. Thus, adjustment of the charge density may not be able to overcome poor fluidity with a particular family of dispersants for that application.
- Modifiers appear to be less effective if the calcium sulfate hemihydrate is wetted with the dispersant before the modifier is added to the mixture. It is, therefore, preferred that the dispersant and the modifier be combined prior to mixture with the stucco. If either the modifier or the dispersant is in a liquid form, the liquid is preferably added to the process water. The other of the modifier or the dispersant is then added to the water prior to addition of the calcium sulfate hemihydrate. Only a few seconds of mixing is needed to blend the modifier and the dispersant together. If both the modifier and the dispersant are in dry form, they can be mixed together and added simultaneously with the stucco.
- the reaction of the polycarboxylate dispersants and the modifiers react differently when used in different gypsum media. While not wishing to be bound by theory, the impurities present in gypsum are believed to contribute to the efficacy of both the dispersant and the modifier.
- the impurities present in stucco are salts that vary by geographical location. Many salts are known to be set accelerators or set retarders. These same salts may also change the efficacy of the polycarboxylate dispersant by affecting the degree of fluidity that can be achieved.
- Some preferred polycarboxylates, including the PCE211-Type Dispersants are best utilized with a low salt stucco. Other dispersants, such as the 2641-Type Dispersants are suitable for use with high-salt stuccos.
- the amount of water used to fluidize the slurry can be reduced compared to slurries made without these additives.
- the stucco source, the calcining technique, the dispersant family, the charge density and the modifier all work together to produce a slurry of a given fluidity. In the laboratory, it is possible to reduce the water level close to that theoretically required to fully hydrate the calcium sulfate hemihydrate. When used in a commercial setting, process considerations may not allow water reduction to this degree.
- any amount of water may be used to make the slurry of this invention as long as the slurry has sufficient fluidity for the application being considered.
- the amount of water varies greatly, depending on the source of the stucco, how it is calcined, the additives and the product being made.
- WSR water to stucco ratio
- Pourable flooring utilizes a WSR of from about 0.17 to about 0.45, preferably from 0.17 to about 0.34. Flooring compositions using this dispersant are revealed in U.S. Ser. No. 11/________ (Attorney Ref.
- the slurry is used to make gypsum panels or wallboard having increased strength.
- the slurry is poured onto at least one sheet of facing material. Facing materials are well known to an artisan of gypsum panels. Multi-ply paper is the preferred facing material, however, single-ply paper, cardboard, plastic sheeting and other facing materials may be used.
- CSA is a set accelerator comprising 95% calcium sulfate dihydrate co-ground with 5% sugar and heated to 250° F. (121° C.) to caramelize the sugar.
- CSA is available from USG Corporation, Southard, Okla. plant, and is made according to U.S. Pat. No. 3,573,947, herein incorporated by reference.
- Potassium sulfate is another preferred accelerator.
- HRA is calcium sulfate dihydrate freshly ground with sugar at a ratio of about 5 to 25 pounds of sugar per 100 pounds of calcium sulfate dihydrate. It is further described in U.S. Pat. No. 2,078,199, herein incorporated by reference. Both of these are preferred accelerators.
- wet gypsum accelerator WGA
- WGA wet gypsum accelerator
- a description of the use of and a method for making wet gypsum accelerator are disclosed in U.S. Pat. No. 6,409,825, herein incorporated by reference.
- This accelerator includes at least one additive selected from the group consisting of an organic phosphonic compound, a phosphate-containing compound or mixtures thereof.
- This particular accelerator exhibits substantial longevity and maintains its effectiveness over time such that the wet gypsum accelerator can be made, stored, and even transported over long distances prior to use.
- the wet gypsum accelerator is used in amounts ranging from about 5 to about 80 pounds per thousand square feet (24.3 to 390 g/m 2 ) of board product.
- additives are included in the gypsum slurry to modify one or more properties of the final product.
- Additives are used in the manner and amounts as are known in the art. Concentrations are reported in amounts per 1000 square feet of finished board panels (“MSF”). Starches are used in amounts from about 3 to about 20 lbs./MSF (14.6 to 97.6 g/m 2 ) to increase the density and strengthen the product. Glass fibers are optionally added to the slurry in amounts of at least 11 lb./MSF (54 g/m 2 ). Up to 15 lb./MSF (73.2 g/m 2 ) of paper fibers are also added to the slurry. Wax emulsions are added to the gypsum slurry in amounts up to 90 lbs./MSF (0.4 kg/m 2 ) to improve the water-resistency of the finished gypsum board panel.
- any of the conventional foaming agents known to be useful in preparing foamed set gypsum products can be employed.
- Many such foaming agents are well known and readily available commercially, e.g. from GEO Specialty Chemicals, Ambler, Pa.
- Foams and a preferred method for preparing foamed gypsum products are disclosed in U.S. Pat. No. 5,683,635, herein incorporated by reference. If foam is added to the product, the polycarboxylate dispersant can be divided between the process water and the foam water prior to its addition to the calcium sulfate hemihydrate.
- trimetaphosphate with lime or other modifiers that raise the alkalinity of the slurry. Above a pH of about 9.5, the trimetaphosphate loses its ability to strengthen the product and the slurry becomes severely retardive.
- biocides to reduce growth of mold, mildew or fungi are biocides to reduce growth of mold, mildew or fungi.
- the biocide can be added to the covering, the gypsum core or both.
- examples of biocides include boric acid, pyrithione salts and copper salts.
- Biocides can be added to either the facing or the gypsum core. When used, biocides are used in the facings in amounts of less than about 500 ppm.
- the gypsum composition optionally can include a starch, such as a pregelatinized starch and/or an acid-modified starch.
- a starch such as a pregelatinized starch and/or an acid-modified starch.
- the inclusion of the pregelatinized starch increases the strength of the set and dried gypsum cast and minimizes or avoids the risk of paper delamination under conditions of increased moisture (e.g., with regard to elevated ratios of water to calcined gypsum).
- pregelatinizing raw starch such as, for example, cooking raw starch in water at temperatures of at least about 185° F. (85° C.) or other methods.
- pregelatinized starch examples include, but are not limited to, PCF 1000 starch, commercially available from Lauhoff Grain Company and AMERIKOR 818 and HQM PREGEL starches, both commercially available from Archer Daniels Midland Company. If included, the pregelatinized starch is present in any suitable amount. For example, if included, the pregelatinized starch can be added to the mixture used to form the set gypsum composition such that it is present in an amount of from about 0.5% to about 10% percent by weight of the set gypsum composition. Starches such as USG95 (United States Gypsum Company, Chicago, Ill.) are also optionally added for core strength.
- PCF 1000 starch commercially available from Lauhoff Grain Company and AMERIKOR 818 and HQM PREGEL starches, both commercially available from Archer Daniels Midland Company.
- the pregelatinized starch is present in any suitable amount.
- the pregelatinized starch can be added to the mixture used to form the set gy
- gypsum board is made in a continuous process formed into a long panel and cut into panels of desired lengths.
- the formed facing material is obtained and put into place to receive the gypsum slurry.
- the facing material is of a width to form a continuous length of panel that requires no more than two cuts to make a panel with the desired finished dimensions.
- Any known facing material is useful in making the wallboard panels, including paper, glass mat and plastic sheeting. Facing material is continuously fed to the board line.
- the slurry is formed by mixing the dry components and the wet components together in any order.
- liquid additives are added to the water, and the mixer is activated for a short time to blend them. Water is measured directly into the mixer. If modifiers are used, preferably the modifiers and dispersants are predissolved in the mixer water prior to introduction of the stucco. Dry components of the slurry, the calcined gypsum and any dry additives, are preferablyblended together prior to entering the mixer. The dry components are added to the liquid in the mixer, and blended until the dry components are moistened.
- the slurry is then mixed to achieve a homogeneous slurry.
- an aqueous foam is mixed into the slurry to control the density of the resultant core material.
- Such an aqueous foam is usually generated by high shear mixing of an appropriate foaming agent, water and air to prior to the introduction of the resultant foam into the slurry.
- the foam can be inserted into the slurry in the mixer, or preferably, into the slurry as it exits the mixer in a discharge conduit. See, for example, U.S. Pat. No. 5,683,635, herein incorporated by reference.
- frequently solids and liquids are continuously added to a mixer, while the resultant slurry is continuously discharged from the mixer, and has an average residence time in the mixer of less than 30 seconds.
- the slurry is continuously dispensed through one or more outlets from the mixer through a discharge conduit and deposited onto a moving conveyor carrying the facing material and formed into a panel.
- Another paper cover sheet is optionally placed on top of the slurry, so that the slurry is sandwiched between two moving cover sheets which become the facings of the resultant gypsum panel.
- the thickness of the resultant board is controlled by a forming plate, and the edges of the board are formed by appropriate mechanical devices which continuously score, fold and glue the overlapping edges of the paper. Additional guides maintain thickness and width as the setting slurry travels on a moving belt. While the shape is maintained, the calcined gypsum is kept under conditions sufficient (i.e. temperature of less than about 120° F.) to react with a portion of the water to set and form an interlocking matrix of gypsum crystals. The board panels are then cut, trimmed and passed to dryers to dry the set but still somewhat wet boards.
- a two-stage drying process is employed.
- the panels are first subjected to a high temperature kiln to rapidly heat up the board and begin to drive off excess water.
- the temperature of the kiln and the residence time of the board vary with the thickness of the panel.
- a 1 ⁇ 2-inch board (12.7 mm) is preferably dried at temperatures in excess of 300° F. (149° C.) for approximately 20 to 50 minutes.
- a second-stage oven has temperatures less than 300° F. (149° C.) to limit calcination of the board.
- the amount of water needed to make a patty of standard diameter decreases as the amount of the modifier increases.
- the water demand again rises.
- the set time consistently drops as the amount of soda ash increases.
- Hydroxides act as modifiers, allowing less water to be used to obtain a standard fluidity which produces a standard patty size. Although the efficacy is good, hydroxides are not suitable for some products, such as wallboard, because the slurry becomes very alkaline, causing loss of efficacy of some preferred additives, including trimetaphosphate. Even at 0.05% CaMg(OH) 4 , the pH was above 10. For products where pH of the product is not a problem, hydroxides can be used effectively as modifiers.
- the preferred 211 dispersant was tested with a variety of modifiers to determine the improvement in efficacy.
- Reagent grade tetra sodium phosphate (“TSP”), tetra sodium pyrophosphate (“TSPP”) and sodium carbonate (soda ash) were tested.
- Dequest 2006 Solutia, Inc. St. Louis, Mo.
- a penta sodium salt of aminotri methylene phosphonic acid
- DEQUEST 2006 yields a much smaller patty for about the same set time and TSPP has a smaller patty size but has a higher set time.
Abstract
Description
- This application is related to co-pending U.S. Ser. No. 11/______ (Attorney Ref. No. 2033.72380), entitled “Fast Drying Wallboard”, U.S. Ser. No. 11/______ (Attorney Ref. 2033.72699), entitled “High Strength Flooring Compositions”, U.S. Ser. No. 11/______ (Attorney Ref. No. 2033.72740), entitled “Gypsum Products Utilizing a Two-Repeating Unit System and Process for Making Them”; U.S. Ser. No. 11/______ (Attorney Ref. No. 2033.73064), entitled “Method of Making a Gypsum Slurry with Modifiers and Dispersants” and U.S. Ser. No. 11/______ (Attorney Ref. No. 2033.73130), entitled, “Effective Use of Dispersants in Wallboard Containing Foam”, all filed concurrently herewith and all hereby incorporated by reference.
- This invention relates to improved gypsum products. More specifically, it relates to an improved gypsum slurry that is flowable at low water concentrations, with less expense and less retardive effects of using carboxylate dispersants alone.
- Gypsum products are commonly used as building materials for many reasons, such as wallboard. Wallboard sheets are easily joined together to make continuous walls of any size and shape. They are easily patched and have fire and sound proofing properties. Decorative finishes, such as wallpaper or paint readily adhere to plaster or wallboard surfaces to allow for a large variety of decorating options.
- The strength of gypsum products made from full density slurries is inversely proportional to the amount of water used in their manufacture. Some of the water that is added to the gypsum slurry is used to hydrate the calcined gypsum, also known as calcium sulfate hemihydrate, to form an interlocking matrix of calcium sulfate dihydrate crystals. Excess water evaporates or is driven off in a kiln, leaving voids in the matrix once occupied by the water. Where large amounts of water were used to fluidize the gypsum slurry, more and larger voids remain in the product when it is completely dry. These voids decrease the product density and strength in the finished product.
- Attempts have been made to reduce the amount of water used to make a fluid slurry using dispersants. Polycarboxylate superplasticizers are very effective in allowing water reduction and the resultant increase in product strength, however, there are disadvantages known to be associated with use of large doses of polycarboxylate dispersants. These materials are relatively expensive. When used in large doses, polycarboxylate dispersants can be the one of the single, most expensive additives in making gypsum products. The high price of this component can overcome the narrow margins afforded these products in a highly competitive marketplace.
- Another disadvantage associated with polycarboxylate dispersants is the retardation of the setting reaction. Gypsum board is made on high speed production lines where the slurry is mixed, poured, shaped and dried in a matter of minutes. The board must be able to hold its shape to be moved from one conveyor line to another to put the board into the kiln. Damage can occur if the boards have not attained a minimum green strength by the time they are stacked and wrapped for shipping. If the board line has to be slowed down because the board is not sufficiently set to move on to the next step in the process, production costs are driven up, resulting in an economically uncompetitive product.
- Lime has been used in plaster to improve its workability. It gives the plaster a good “feel”, imparting a smoothness and plasticity that makes it easy to trowel. Since it is alkaline, lime acts to make some retarders more efficient, increasing the open time of the plaster. Finally, the lime present in the plaster oxidizes over time to form calcium carbonate which gives the surface a hardness beyond that obtainable with plaster alone.
- U.S. Pat. No. 5,718,759 teaches the addition of silicates to mixtures of beta-calcined gypsum and cement. In the examples, lignosulfates or naphthalene sulfonates are used as water-reducing agents. The addition of pozzolanic materials, including silicates, is credited with reducing expansion due to the formation of ettringite. The composition is suggested for use in building materials, such as backer boards, floor underlayments, road patching materials fire-stopping materials and fiberboard.
- Luongo, in U.S. Pat. No. 6,391,958, teaches a novel wallboard composition combining gypsum with sodium silicates and a synthetic, cross-linking binder. Vinyl acetate polymers were the preferred cross-linking binder. The addition of sodium silicates reduces the amount of calcined gypsum that is needed to make a given number of panels. The weight of the building panel is reduced, making it easier for workers to move the panels before and during installations.
-
- where P is an integer from 1-10 and R2, R3, R4, R5 and R6 are not all hydrogens, but any one of them can be a hydrogen. The polymer also includes a water-soluble repeating unit selected from a group containing acrylic acid and methacrylic acid, among others. The polymer is used in an unfired, ceramic precursor material. Other polymers are disclosed in the application, including some for use in the core forming process of gypsum wallboard or the preparation of gypsum slurries.
- The prior art has failed to adequately address the problem of improving the efficacy of a given polycarboxylate dispersant. Improving the efficacy of a dispersant would reduce the cost of the dispersant and maintaining the reasonable price of gypsum products.
- Thus, there is a need in the art to reduce the dosage of dispersants used in a gypsum slurry while maintaining flowability of the slurry. Reduction in dispersant use would result in saving of costs spent on the dispersant and would reduce adverse side effects, such as set retardation.
- These and other problems are improved by this invention which includes the addition of an efficacy modifier to a gypsum slurry that increases the fluidity of slurries made with polycarboxylate dispersant. When one or more of the modifiers is used, less dispersant is required to achieve a given fluidity resulting as lower dispersant cost and generally less retardation.
- More specifically, the invention relates to an improved gypsum slurry, that includes water, calcium sulfate hemihydrate, a polycarboxylate dispersant and a modifier. The modifier is chemically configured to improve the efficacy of the polycarboxylate dispersant. Preferred modifiers include cement, lime, slaked lime, soda ash, carbonates, silicates, phosphonates and phosphates.
- In another embodiment of this invention, a gypsum panel is made from at least one facing sheet and a core made from the improved gypsum slurry. Yet another aspect of this invention is a method of making the gypsum slurry that includes selecting a modifier, mixing the modifier with a polycarboxylate dispersant and adding the calcium sulfate hemihydrate.
- Use of the modifiers herein described improves the efficacy of the dispersant in fluidizing the gypsum slurry. This allows less of the dispersant to be used and still obtain high flowability in low water slurries for strength. Polycarboxylate dispersants are often one of the most expensive components in products that use them. Using lower dosages of the dispersant reduces the cost so that a competitively priced gypsum product can be made.
- Lowering the concentration of the dispersant also minimizes the disadvantageous effects of the polycarboxylate dispersant. At a lower dose, there is less retardation of the setting reactions. Less set accelerator would be needed in the product to overcome the effects of set retardation, reducing the price paid for the accelerator.
- Instead of or in addition to reducing the dispersant dosage, the improved efficacy of the dispersant can also be used to reduce the amount of water used to make the gypsum slurry. The manufacturing process can be made more fuel efficient, conserving fossil fuels and realizing the cost savings. Fuel savings can be based on either reduced kiln temperatures or shorter residence time in the kiln.
- The gypsum slurry of this invention is made using water, calcined gypsum, a polycarboxylate dispersant and a modifier. Although the benefits of this invention are most clear when used in a slurry for a high strength product, it can be used with any slurry using a polycarboxylate, even those that already utilize low doses of polycarboxylate dispersant.
- Any calcined gypsum or stucco is useful in this slurry. It has unexpectedly been discovered that some gypsum sources are more responsive to the dispersant and the modifier than other sources. Laboratory tests indicate that gypsums from different geographical areas contain different salts and impurities. While not wishing to be bound by theory, it is believed that the salts present in the gypsum influence to the fluidity in the slurry.
- Reduction in the amount of water used to make the slurry is achieved by the addition of a polycarboxylate dispersant. The dispersant attaches itself to the calcium sulfate, then charged groups on the backbone and the side chains on the branches of the polymer repel each other, causing the gypsum particles to spread out and flow easily. When the slurry flows more easily, the amount of water can be reduced and still obtain a flowable fluid. In general, reduction in water results in increased product strength and lower drying costs.
- Any polycarboxylate dispersant that is useful for improving fluidity in gypsum may be used in the slurry of this invention. A number of polycarboxylate dispersants, particularly polycarboxylic ethers, are preferred types of dispersants. One of the preferred class of dispersants used in the slurry includes two repeating units. It is described further in co-pending U.S. Ser. No. 11/______ (Attorney Ref. No 2033.72740), entitled “Gypsum Products Utilizing a Two-Repeating Unit System and Process for Making Them”, previously incorporated by reference. These dispersants are products of Degussa Construction Polymers, GmbH (Trostberg Germany) and are supplied by Degussa Corp. (Kennesaw, Ga.) (hereafter “Degussa”) and are hereafter referenced as the “PCE211-Type Dispersants”.
- The first repeating unit is an olefinic unsaturated mono-carboxylic acid repeating unit, an ester or salt thereof, or an olefinic unsaturated sulphuric acid repeating unit or a salt thereof. Preferred first repeating units include acrylic acid or methacrylic acid. Mono- or divalent salts are suitable in place of the hydrogen of the acid group. The hydrogen can also be replaced by hydrocarbon group to form the ester.
- The second repeating unit satisfies Formula I,
R1—O—R2 I -
- and R2 is derived from an unsaturated (poly)alkylene glycol ether group according to Formula III.
—(CmH2mO)x—(CnH2nO)y—R4 III - Referring to Formula II, the alkenyl repeating unit optionally includes a C1 to C3 alkyl group between the polymer backbone and the ether linkage. The value of p is an integer from 0-3, inclusive. Preferably, p is either 0 or 1. R3 is either a hydrogen atom or an aliphatic C1 to C5 hydrocarbon group, which may be linear, branched, saturated or unsaturated. Examples of preferred repeating units include acrylic acid and methacrylic acid.
- The polyether group of Formula III contains multiple C2-C4 alkyl groups, including at least two different alkyl groups, connected by oxygen atoms. M and n are integers from 2 to 4, inclusive. Preferably, at least one of m and n is 2. X and y are integers from 55 to 350, inclusive. R4 is hydrogen or an aliphatic C1 to C20 hydrocarbon group, a cycloaliphatic C5 to C8 hydrocarbon group, a substituted C6 to C14 aryl group or a group conforming at least one of Formula IV(a), IV(b) and VI(c).
- In the above formulas, R5 and R7, independently of each other, represent an alkyl, aryl, aralkyl or alkylaryl group. R6 is a bivalent alkyl, aryl, aralkyl or alkylaryl group.
- A particularly useful dispersant of the PCE211-Type Dispersants is designated PCE211 (hereafter “211”). Other polymers in this series known to be useful in wallboard include PCE111. PCE211-Type dispersants are described more fully in U.S. Ser. No. 11/______ filed concurrently herewith, by Degussa Construction Polymers (Attorney Ref. DCP3) entitled “Polyether-Containing Copolymer”, herein incorporated by reference.
- The molecular weight of the PCE211 Type dispersant is preferably from about 20,000 to about 60,000 Daltons. Surprisingly, it has been found that the lower molecular weight dispersants cause less retardation of set time than dispersants having a molecular weight greater than 60,000 Daltons. Generally longer side chain length, which results in an increase in overall molecular weight, provides better dispensability. However, tests with gypsum indicate that efficacy of the dispersant is reduced at molecular weights above 50,000 Daltons.
- R1 preferably makes up from about 300 to about 99 mole % of the total repeating units, more preferably from about 40 to about 80%. From about 1 to about 70 mole % of the repeating units are R2, more preferably from about 10 to about 60 mole %.
- Another class of polycarboxylate compounds that are useful in this invention is disclosed in U.S. Pat. No. 6,777,517, herein incorporated by reference and hereafter referenced as the “2641-Type Dispersant”. Preferably, the dispersant includes at least three repeating units shown in Formula V(a), V(b) and V(c).
- In this case, both acrylic and maleic acid repeating units are present, yielding a higher ratio of acid groups to vinyl ether groups. R1 represents a hydrogen atom or an aliphatic hydrocarbon radical having from 1 to 20 carbon atoms. X represents OM, where M is a hydrogen atom, a monovalent metal cation, an ammonium ion or an organic amine radical. R2 can be hydrogen, an aliphatic hydrocarbon radical having from 1 to 20 carbon atoms, a cycloaliphatic hydrocarbon radical having from 6 to 14 carbon atoms, which may be substituted. R3 is hydrogen or an aliphatic hydrocarbon radical having from 1 to 5 carbon atoms, which are optionally linear or branched, saturated or unsaturated. R4 is hydrogen or a methyl group, depending on whether the structural units are acrylic or methacrylic. P can be from 0 to 3. M is an integer from 2 to 4, inclusive, and n is an integer from 0 to 200, inclusive. PCE211-Type and 2641-Type dispersants are manufactured by Degussa Construction Polymers, GmbH (Tröstberg, Germany) and marketed in the United States by Degussa Corp. (Kennesaw, Ga.). Preferred 2641-Type Dispersants are sold by Degussa as MELFLUX 2641F, MELFLUX 2651F and MELFLUX 2500L dispersants. 2641-Type dispersants (MELFLUX is a registered trademark of Degussa Construction Polymers, Gm bH) are described for use in wallboard and gypsum slurries in U.S. Ser. No. 11/______ (Attorney Ref. No. 2033.72380), entitled “Fast Drying Wallboard”, previously incorporated by reference.
- Yet another preferred dispersant family is sold by Degussa and referenced as “1641-Type Dispersants”. This dispersant is more fully described in U.S. Pat. No. 5,798,425, herein incorporated by reference. A particularly preferred 1641-Type Dispersant is shown in Formula VI and marketed as MELFLUX 1641F dispersant by Degussa. This dispersant is made primarily of two repeating units, one a vinyl ether and the other a vinyl ester. In Formula VI, m and n are the mole ratios of the component repeating units, which can be randomly positioned along the polymer chain.
- These dispersants are particularly well-suited for use with gypsum. While not wishing to be bound by theory, it is believed that the acid repeating units bind to the hemihydrate crystals while the long polyether chains of the second repeating unit perform the dispersing function. Since it is less retardive than other dispersants, it is less disruptive to the manufacturing process of gypsum products such as wallboard. The dispersant is used in any effective amount. To a large extent, the amount of dispersant selected is dependent on the desired fluidity of the slurry. As the amount of water decreases, more dispersant is required to maintain a constant slurry fluidity. Since polycarboxylate dispersants are relatively expensive components, it is preferred to use a small dose, preferably less than 2% or more preferably less than 1% by weight based on the weight of the dry stucco. Preferably, the dispersant is used in amounts of about 0.05% to about 0.5% based on the dry weight of the stucco. More preferably, the dispersant is used in amounts of about 0.01% to about 0.2% on the same basis. In measuring a liquid dispersant, only the polymer solids are considered in calculating the dosage of the dispersant, and the water from the dispersant is considered when a water/stucco ratio is calculated.
- Many polymers can be made with the same repeating units using different distributions of them. The ratio of the acid-containing repeating units to the polyether-containing repeating unit is directly related to the charge density. Preferably, the charge density of the co-polymer is in the range of about 300 to about 3000 μequiv. charges/g co-polymer. It has been found that the most effective dispersant tested for water reduction in this class of dispersants, MELFLUX 2651F, has the highest charge density.
- However, it has also been discovered that the increase in charge density further results in an increase in the retardive effect of the dispersant. Dispersants with a low charge density, such as MELFLUX 2500L, retard the set times less than the MELFLUX 2651F dispersant that has a high charge density. Since retardation in set times increases with the increase in efficacy obtained with dispersants of high charge density, making a slurry with low water, good flowability and reasonable set times requires keeping of the charge density in a mid-range. More preferably, the charge density of the co-polymer is in the range of about 600 to about 2000 μequiv. charges/g co-polymer.
- The modifier can be any substance, liquid or solid, which when combined with a polycarboxylate dispersant in a gypsum slurry, leads to an improvement the efficacy of the dispersant. Modifiers are not intended to be dispersants in themselves, but serve to allow the dispersant to be more effective. For example, at constant concentrations of dispersant, better fluidity is obtained when the modifier is used compared to the same slurry without the modifier.
- Although the exact chemistry involved in the use of modifiers is not fully understood, at least two different mechanisms are responsible for the increase in dispersant efficacy. Lime, for example, reacts with the polycarboxylate in the aqueous solution to uncoil the dispersant molecule. In contrast, soda ash reacts on the gypsum surface to help improve the dispersant effect. Any mechanism can be used by the modifier to improve the efficacy of the dispersant for the purposes of this invention. Theoretically, if the two mechanisms work independently, combinations of modifiers can be found that utilize the full effect of both mechanisms and result in even better dispersant efficacy.
- Preferred modifiers include cement, lime, also known as quicklime or calcium oxide, slaked lime, also known as calcium hydroxide, soda ash, also known as sodium carbonate, potassium carbonate, also known as potash, and other carbonates, silicates, hydroxides, phosphonates and phosphates. Preferred carbonates include sodium and potassium carbonate. Sodium silicate is a preferred silicate.
- When lime or slaked lime is used as the modifier, it is used in concentrations of about 0.15% to about 1.0% based on the weight of the dry calcium sulfate hemihydrate. In the presence of water, lime is quickly converted to calcium hydroxide, or slaked lime, and the pH of the slurry becomes alkaline. The sharp rise in pH can cause a number of changes in the slurry chemistry. Certain additives, including trimetaphosphate, break down as the pH increases. There can also be problems with hydration and, where the slurry is used to make wallboard or gypsum panels, there are problems with paper bond at high pH. For workers who come in contact with the slurry, strongly alkaline compositions can be irritating to the skin and contact should be avoided. Above pH of about 11.5, lime no longer causes an increase in fluidity. Therefore, it is preferred in some applications to hold the pH below about nine for maximum performance from this modifier. In other applications, such as flooring, a high pH has the benefit of minimizing mold and mildew. Alkali metal hydroxides, especially sodium and potassium hydroxides are preferred for use in flooring.
- Other preferred modifiers include carbonates, phosphonates, phosphates and silicates. Preferably, the modifiers are used in amounts less than 0.25% based on the weight of the dry calcium sulfate hemihydrate. Above these concentrations, increases in the amount of modifier causes a decrease in the dispersant efficacy. These modifiers are preferably used in amounts of from about 0.05 to about 0.2 weight %.
- The charge density of the dispersant has also been found to affect the ability of the modifier to interact with the dispersant. Given a family of dispersants with the same repeating units, the modifier causes a greater increase in efficacy in the dispersant having the higher charge density. It is important to note that although the general trend is to obtain a higher efficacy boost with higher charge density, when comparing the effectiveness of dispersants having different repeating units, the effectiveness of the dispersants may be considerably different at the same charge density. Thus, adjustment of the charge density may not be able to overcome poor fluidity with a particular family of dispersants for that application.
- Modifiers appear to be less effective if the calcium sulfate hemihydrate is wetted with the dispersant before the modifier is added to the mixture. It is, therefore, preferred that the dispersant and the modifier be combined prior to mixture with the stucco. If either the modifier or the dispersant is in a liquid form, the liquid is preferably added to the process water. The other of the modifier or the dispersant is then added to the water prior to addition of the calcium sulfate hemihydrate. Only a few seconds of mixing is needed to blend the modifier and the dispersant together. If both the modifier and the dispersant are in dry form, they can be mixed together and added simultaneously with the stucco. The preferred method of combining water, dispersant, modifier and stucco is further described in U.S. Ser. No. 11/______ (Attorney Ref. No. 2033.73064), entitled “Method of Making a Gypsum Slurry with Modifiers and Dispersants”, previously incorporated by reference.
- It has also been noted that the reaction of the polycarboxylate dispersants and the modifiers react differently when used in different gypsum media. While not wishing to be bound by theory, the impurities present in gypsum are believed to contribute to the efficacy of both the dispersant and the modifier. Among the impurities present in stucco are salts that vary by geographical location. Many salts are known to be set accelerators or set retarders. These same salts may also change the efficacy of the polycarboxylate dispersant by affecting the degree of fluidity that can be achieved. Some preferred polycarboxylates, including the PCE211-Type Dispersants, are best utilized with a low salt stucco. Other dispersants, such as the 2641-Type Dispersants are suitable for use with high-salt stuccos.
- As a result of the use of fluidity enhancing dispersants and modifiers to boost their performance, the amount of water used to fluidize the slurry can be reduced compared to slurries made without these additives. It must be understood that the stucco source, the calcining technique, the dispersant family, the charge density and the modifier all work together to produce a slurry of a given fluidity. In the laboratory, it is possible to reduce the water level close to that theoretically required to fully hydrate the calcium sulfate hemihydrate. When used in a commercial setting, process considerations may not allow water reduction to this degree.
- Any amount of water may be used to make the slurry of this invention as long as the slurry has sufficient fluidity for the application being considered. The amount of water varies greatly, depending on the source of the stucco, how it is calcined, the additives and the product being made. For wallboard applications, a water to stucco ratio (“WSR”) of 0.18 to about 0.8 is used, preferably from about 0.2 to about 0.5. Pourable flooring utilizes a WSR of from about 0.17 to about 0.45, preferably from 0.17 to about 0.34. Flooring compositions using this dispersant are revealed in U.S. Ser. No. 11/______ (Attorney Ref. 2033.72699), entitled “High Strength Flooring Compositions”, previously incorporated by reference, that utilize water to stucco ratios less than 0.3. Castable products utilize a WSR from about 0.1 to about 0.3, preferably from about 0.16 to about 0.25. In the laboratory, water to stucco ratios of less than 0.1 are attainable, however, commercially, the water to stucco ratio is typically from 0.5 to 0.7. Generally, water to stucco ratios of about 0.2 to about 0.6 are preferred. The amount of water can be diminished compared to other slurries, resulting in fuel savings and higher product strength.
- In a second aspect of this invention, the slurry is used to make gypsum panels or wallboard having increased strength. To form gypsum panels, the slurry is poured onto at least one sheet of facing material. Facing materials are well known to an artisan of gypsum panels. Multi-ply paper is the preferred facing material, however, single-ply paper, cardboard, plastic sheeting and other facing materials may be used.
- Other additives are also added to the slurry as are typical for the particular application to which the gypsum slurry will be put. Set retarders (up to about 2 lb./MSF (9.8g/m2)) or dry accelerators (up to about 35 lb./MSF (170 g/m2)) are added to modify the rate at which the hydration reactions take place. “CSA” is a set accelerator comprising 95% calcium sulfate dihydrate co-ground with 5% sugar and heated to 250° F. (121° C.) to caramelize the sugar. CSA is available from USG Corporation, Southard, Okla. plant, and is made according to U.S. Pat. No. 3,573,947, herein incorporated by reference. Potassium sulfate is another preferred accelerator. HRA is calcium sulfate dihydrate freshly ground with sugar at a ratio of about 5 to 25 pounds of sugar per 100 pounds of calcium sulfate dihydrate. It is further described in U.S. Pat. No. 2,078,199, herein incorporated by reference. Both of these are preferred accelerators.
- Another accelerator, known as wet gypsum accelerator or WGA, is also a preferred accelerator. A description of the use of and a method for making wet gypsum accelerator are disclosed in U.S. Pat. No. 6,409,825, herein incorporated by reference. This accelerator includes at least one additive selected from the group consisting of an organic phosphonic compound, a phosphate-containing compound or mixtures thereof. This particular accelerator exhibits substantial longevity and maintains its effectiveness over time such that the wet gypsum accelerator can be made, stored, and even transported over long distances prior to use. The wet gypsum accelerator is used in amounts ranging from about 5 to about 80 pounds per thousand square feet (24.3 to 390 g/m2) of board product.
- In some embodiments of the invention, additives are included in the gypsum slurry to modify one or more properties of the final product. Additives are used in the manner and amounts as are known in the art. Concentrations are reported in amounts per 1000 square feet of finished board panels (“MSF”). Starches are used in amounts from about 3 to about 20 lbs./MSF (14.6 to 97.6 g/m2) to increase the density and strengthen the product. Glass fibers are optionally added to the slurry in amounts of at least 11 lb./MSF (54 g/m2). Up to 15 lb./MSF (73.2 g/m2) of paper fibers are also added to the slurry. Wax emulsions are added to the gypsum slurry in amounts up to 90 lbs./MSF (0.4 kg/m2) to improve the water-resistency of the finished gypsum board panel.
- In embodiments of the invention that employ a foaming agent to yield voids in the set gypsum-containing product to provide lighter weight, any of the conventional foaming agents known to be useful in preparing foamed set gypsum products can be employed. Many such foaming agents are well known and readily available commercially, e.g. from GEO Specialty Chemicals, Ambler, Pa. Foams and a preferred method for preparing foamed gypsum products are disclosed in U.S. Pat. No. 5,683,635, herein incorporated by reference. If foam is added to the product, the polycarboxylate dispersant can be divided between the process water and the foam water prior to its addition to the calcium sulfate hemihydrate. A preferred method of incorporating one or more dispersants into the mixer water and the foam water is disclosed in U.S. Ser. No. 11/______ (Attorney Ref. No. 2033.73130), entitled, “Effective Use of Dispersants in Wallboard Containing Foam”, previously incorporated by reference.
- A trimetphosphate compound is added to the gypsum slurry in some embodiments to enhance the strength of the product and to reduce sag resistance of the set gypsum. Preferably the concentration of the trimetaphosphate compound is from about 0.07% to about 2.0% based on the weight of the calcined gypsum. Gypsum compositions including trimetaphosphate compounds are disclosed in U.S. Pat. Nos. 6,342,284 and 6,632,550, both herein incorporated by reference. Exemplary trimetaphosphate salts include sodium, potassium or lithium salts of trimetaphosphate, such as those available from Astaris, LLC., St. Louis, Mo. Care must be exercised when using trimetaphosphate with lime or other modifiers that raise the alkalinity of the slurry. Above a pH of about 9.5, the trimetaphosphate loses its ability to strengthen the product and the slurry becomes severely retardive.
- Other potential additives to the wallboard are biocides to reduce growth of mold, mildew or fungi. Depending on-the biocide selected and the intended use for the wallboard, the biocide can be added to the covering, the gypsum core or both. Examples of biocides include boric acid, pyrithione salts and copper salts. Biocides can be added to either the facing or the gypsum core. When used, biocides are used in the facings in amounts of less than about 500 ppm.
- In addition, the gypsum composition optionally can include a starch, such as a pregelatinized starch and/or an acid-modified starch. The inclusion of the pregelatinized starch increases the strength of the set and dried gypsum cast and minimizes or avoids the risk of paper delamination under conditions of increased moisture (e.g., with regard to elevated ratios of water to calcined gypsum). One of ordinary skill in the art will appreciate methods of pregelatinizing raw starch, such as, for example, cooking raw starch in water at temperatures of at least about 185° F. (85° C.) or other methods. Suitable examples of pregelatinized starch include, but are not limited to, PCF 1000 starch, commercially available from Lauhoff Grain Company and AMERIKOR 818 and HQM PREGEL starches, both commercially available from Archer Daniels Midland Company. If included, the pregelatinized starch is present in any suitable amount. For example, if included, the pregelatinized starch can be added to the mixture used to form the set gypsum composition such that it is present in an amount of from about 0.5% to about 10% percent by weight of the set gypsum composition. Starches such as USG95 (United States Gypsum Company, Chicago, Ill.) are also optionally added for core strength.
- Other known additives may be used as needed to modify specific properties of the product. Sugars, such as dextrose, are used to improve the paper bond at the ends of the boards. Wax emulsions or polysiloxanes are used for water resistance. If stiffness is needed, boric acid is commonly added. Fire retardancy can be improved by the addition of vermiculite. These and other known additives are useful in the present slurry and wallboard formulations.
- While individual gypsum panels can be made in a batch process, in a preferred process, gypsum board is made in a continuous process formed into a long panel and cut into panels of desired lengths. The formed facing material is obtained and put into place to receive the gypsum slurry. Preferably, the facing material is of a width to form a continuous length of panel that requires no more than two cuts to make a panel with the desired finished dimensions. Any known facing material is useful in making the wallboard panels, including paper, glass mat and plastic sheeting. Facing material is continuously fed to the board line.
- The slurry is formed by mixing the dry components and the wet components together in any order. Typically, liquid additives are added to the water, and the mixer is activated for a short time to blend them. Water is measured directly into the mixer. If modifiers are used, preferably the modifiers and dispersants are predissolved in the mixer water prior to introduction of the stucco. Dry components of the slurry, the calcined gypsum and any dry additives, are preferablyblended together prior to entering the mixer. The dry components are added to the liquid in the mixer, and blended until the dry components are moistened.
- The slurry is then mixed to achieve a homogeneous slurry. Usually, an aqueous foam is mixed into the slurry to control the density of the resultant core material. Such an aqueous foam is usually generated by high shear mixing of an appropriate foaming agent, water and air to prior to the introduction of the resultant foam into the slurry. The foam can be inserted into the slurry in the mixer, or preferably, into the slurry as it exits the mixer in a discharge conduit. See, for example, U.S. Pat. No. 5,683,635, herein incorporated by reference. In a gypsum board plant, frequently solids and liquids are continuously added to a mixer, while the resultant slurry is continuously discharged from the mixer, and has an average residence time in the mixer of less than 30 seconds.
- The slurry is continuously dispensed through one or more outlets from the mixer through a discharge conduit and deposited onto a moving conveyor carrying the facing material and formed into a panel. Another paper cover sheet is optionally placed on top of the slurry, so that the slurry is sandwiched between two moving cover sheets which become the facings of the resultant gypsum panel. The thickness of the resultant board is controlled by a forming plate, and the edges of the board are formed by appropriate mechanical devices which continuously score, fold and glue the overlapping edges of the paper. Additional guides maintain thickness and width as the setting slurry travels on a moving belt. While the shape is maintained, the calcined gypsum is kept under conditions sufficient (i.e. temperature of less than about 120° F.) to react with a portion of the water to set and form an interlocking matrix of gypsum crystals. The board panels are then cut, trimmed and passed to dryers to dry the set but still somewhat wet boards.
- Preferably, a two-stage drying process is employed. The panels are first subjected to a high temperature kiln to rapidly heat up the board and begin to drive off excess water. The temperature of the kiln and the residence time of the board vary with the thickness of the panel. By way of example, a ½-inch board (12.7 mm) is preferably dried at temperatures in excess of 300° F. (149° C.) for approximately 20 to 50 minutes. As water at the surface evaporates, it is drawn by capillary action from the interior of the panel to replace the surface water. The relatively rapid water movement assists migration of the starch and the pyrithione salt into the paper. A second-stage oven has temperatures less than 300° F. (149° C.) to limit calcination of the board.
- Tests were conducted to determine the effect of the addition of potassium carbonate on two different dispersants. In each of the following samples, a gypsum slurry was made from 400 grams of stucco from Southard, Okla., 180 grams of water and 0.2% dispersant based on the dry weight of the stucco. The dispersant type and amount of potassium carbonate are shown in Table I below, together with the results of the patty size and the stiffening rate tests.
TABLE I Potassium Stiffening Dispersant Carbonate, g Patty Size, cm Time 211 0.6 30.3 6:00 211 0.0 19.8 2:05 Melflux 2500L 0.6 26.0 10:30 Melflux 2500L 0.0 15.5 2:35 - As is seen in the data in Table I above, the addition of potassium carbonate increases the slurry fluidity as evidenced by the increase patty size. The modifier addition also retarded the stiffening time compared to the samples where no potassium carbonate was used.
- Tests were run to determine the effect of lime on dispersant MELFLUX 2500L with two different stuccos.
TABLE II Water to Stucco Ratio at Given Dispersant Dose Stucco Lime 0.0% 0.05% 0.11% 0.22% Shoals 0 0.65 0.64 0.58 0.50 Shoals 0.25% 0.68 0.61 0.52 0.41 Galena 0 0.60 0.53 0.45 0.39 Galena 0.25% 0.64 0.50 0.39 0.30 - Tests presented above show that lime is an effective modifer with stuccos from Shoals and Galena Park.
- Soda ash was tested in the laboratory for suitability as a modifier. The amount of water listed in Table III, the water demand, was added to 50 cc of board stucco, a beta-calcined hemihydrate. This amount of water was selected to produce a standard 3-¾″ patty when the stucco, dispersant, modifier and water were combined. The dispersant was added at the rate of 1.5#/MSF on a solids basis as if it were being added to the slurry for a ½″ wallboard. The dispersant was a PCE211, two-repeating unit dispersant, identified as PCE49.
- Sodium carbonate was added at the concentrations shown in Table III, ranging from none to 0.6% by weight based on the weight of the dry stucco. The water demand and the set time are shown in Table III.
TABLE III Na2CO3 cc Water Added Set Time 0.0% 52 17 0.05% 47 17 0.10% 45 17 0.20% 45 15 0.40% 47 11 0.60% 50 11 - Up to and including, 0.2%, the amount of water needed to make a patty of standard diameter decreases as the amount of the modifier increases. At 0.40% and 0.60% levels, the water demand again rises. The set time consistently drops as the amount of soda ash increases.
- A similar study was conducted, except that CaMg(OH)4 was used as the modifier. Water was added in the amounts reported in Table IV. The same stucco and dispersant were used in the same amounts.
TABLE IV CaMg(OH)4 cc Water Added Set Time 0.0% 52 17 0.05% 48 13 0.10% 47 11 0.20% 45 11 0.40% 44.5 11 0.60% 45 9 - Hydroxides act as modifiers, allowing less water to be used to obtain a standard fluidity which produces a standard patty size. Although the efficacy is good, hydroxides are not suitable for some products, such as wallboard, because the slurry becomes very alkaline, causing loss of efficacy of some preferred additives, including trimetaphosphate. Even at 0.05% CaMg(OH)4, the pH was above 10. For products where pH of the product is not a problem, hydroxides can be used effectively as modifiers.
- The preferred 211 dispersant was tested with a variety of modifiers to determine the improvement in efficacy. Reagent grade tetra sodium phosphate (“TSP”), tetra sodium pyrophosphate (“TSPP”) and sodium carbonate (soda ash) were tested. Dequest 2006 (Solutia, Inc. St. Louis, Mo.), a penta sodium salt of aminotri (methylene phosphonic acid), was also tested.
- For all testing samples, the water to stucco ratio was 0.5. Wet gypsum accelerator (WGA) was based on the dry weight of the stucco was added. The control sample had only 0.5% by weight WGA. The amount of each modifier added is shown in Table V, along with the set time and patty size produced by each sample.
- The modifier and dispersant were added to the water, followed by addition of the stucco and WGA. The slurry was stirred until it was consistent.
TABLE V DEQUEST Soda Modifier Control 2006 TSP TSPP Ash Amount 0 0.05% 0.05% 0.05% 0.15% Patty Size 20 cm 23.7 cm 21.5 cm 25.5 cm 27.5 cm Stiffening 2:15 2:35 2:15 2:55 2:30 Time - Even though more soda ash was used to obtain these results, it is considered to be effective because it costs one third the price of the other modifiers. Further, it increases the patty size by 37% while increasing the set time only 11%. DEQUEST 2006 yields a much smaller patty for about the same set time and TSPP has a smaller patty size but has a higher set time.
- While a particular embodiment of the modifiers for gypsum products, it will be appreciated by those skilled in the art that changes and modifications may be made thereto without departing from the invention in its broader aspects and as set forth in the following claims.
Claims (18)
Priority Applications (21)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/152,317 US20060280898A1 (en) | 2005-06-14 | 2005-06-14 | Modifiers for gypsum slurries and method of using them |
| PCT/US2006/018415 WO2006138002A2 (en) | 2005-06-14 | 2006-05-11 | Modifiers for gypsum slurries and method of using them |
| US11/450,068 US7608347B2 (en) | 2005-06-14 | 2006-06-09 | Modifiers for gypsum slurries and method of using them |
| AU2006259585A AU2006259585B2 (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them |
| KR1020087001090A KR20080016960A (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them |
| CNA2006800202774A CN101312925A (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them |
| NZ562969A NZ562969A (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them |
| UAA200800408A UA95077C2 (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method for their use |
| EP06773002A EP1896376A4 (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them |
| JP2008517006A JP2008546619A (en) | 2005-06-14 | 2006-06-13 | Gypsum slurry regulators and methods for their use |
| PCT/US2006/022936 WO2006138277A2 (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them |
| TW095121005A TW200704618A (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them |
| BRPI0612042-3A BRPI0612042A2 (en) | 2005-06-14 | 2006-06-13 | gypsum paste, gypsum panel and method of manufacturing a gypsum paste comprising a polycarboxylate dispersant |
| MX2007015965A MX2007015965A (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them. |
| RU2008101423/03A RU2416581C2 (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum suspensions and method of their application |
| CA002607973A CA2607973A1 (en) | 2005-06-14 | 2006-06-13 | Modifiers for gypsum slurries and method of using them |
| ARP060102521A AR053628A1 (en) | 2005-06-14 | 2006-06-14 | A PLASTER MILK, PLASTER PANEL AND METHOD TO MAKE SUCH MILK. |
| IL187021A IL187021A0 (en) | 2005-06-14 | 2007-10-30 | Modifiers for gypsum slurries and method of using them |
| ZA200709448A ZA200709448B (en) | 2005-06-14 | 2007-11-01 | Modifiers for gypsum slurries and method of using them |
| NO20075725A NO20075725L (en) | 2005-06-14 | 2007-11-09 | Modifiers for gypsum slurries and methods for using them |
| US12/512,612 US7932308B2 (en) | 2005-06-14 | 2009-07-30 | Modifiers for gypsum slurries and method of using them |
Applications Claiming Priority (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US11/152,317 US20060280898A1 (en) | 2005-06-14 | 2005-06-14 | Modifiers for gypsum slurries and method of using them |
Related Child Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/450,068 Continuation-In-Part US7608347B2 (en) | 2005-06-14 | 2006-06-09 | Modifiers for gypsum slurries and method of using them |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20060280898A1 true US20060280898A1 (en) | 2006-12-14 |
Family
ID=37522959
Family Applications (3)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/152,317 Abandoned US20060280898A1 (en) | 2005-06-14 | 2005-06-14 | Modifiers for gypsum slurries and method of using them |
| US11/450,068 Active 2026-01-05 US7608347B2 (en) | 2005-06-14 | 2006-06-09 | Modifiers for gypsum slurries and method of using them |
| US12/512,612 Expired - Fee Related US7932308B2 (en) | 2005-06-14 | 2009-07-30 | Modifiers for gypsum slurries and method of using them |
Family Applications After (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US11/450,068 Active 2026-01-05 US7608347B2 (en) | 2005-06-14 | 2006-06-09 | Modifiers for gypsum slurries and method of using them |
| US12/512,612 Expired - Fee Related US7932308B2 (en) | 2005-06-14 | 2009-07-30 | Modifiers for gypsum slurries and method of using them |
Country Status (12)
| Country | Link |
|---|---|
| US (3) | US20060280898A1 (en) |
| JP (1) | JP2008546619A (en) |
| CN (1) | CN101312925A (en) |
| BR (1) | BRPI0612042A2 (en) |
| IL (1) | IL187021A0 (en) |
| MX (1) | MX2007015965A (en) |
| NZ (1) | NZ562969A (en) |
| RU (1) | RU2416581C2 (en) |
| TW (1) | TW200704618A (en) |
| UA (1) | UA95077C2 (en) |
| WO (1) | WO2006138002A2 (en) |
| ZA (1) | ZA200709448B (en) |
Cited By (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20080148997A1 (en) * | 2006-12-22 | 2008-06-26 | Blackburn David R | Gypsum compositions with naphthalene sulfonate and modifiers |
| WO2009086390A1 (en) * | 2007-12-28 | 2009-07-09 | United States Gypsum Company | Decreased evaporation with retarder for a high water to stucco ratio lightweight board |
| US7670427B2 (en) | 2007-06-06 | 2010-03-02 | United States Gypsum Company | Very fast setting cementitious composition with high early-age compressive strength |
| US20110054081A1 (en) * | 2009-09-02 | 2011-03-03 | Frank Dierschke | Formulation and its use |
| WO2011029711A1 (en) | 2009-09-02 | 2011-03-17 | Basf Construction Polymers Gmbh | Formulation and its use |
| WO2011078708A1 (en) * | 2009-12-24 | 2011-06-30 | Carbon Credit Corporation New Zealand Limited | Carbon plaster board |
| WO2012049077A2 (en) | 2010-10-11 | 2012-04-19 | Basf Construction Polymers Gmbh | Dispersant containing gypsum slurry |
| US8197952B2 (en) | 2005-06-09 | 2012-06-12 | United States Gypsum Company | High starch light weight gypsum wallboard |
| US8210310B1 (en) * | 2010-12-29 | 2012-07-03 | United States Gypsum Company | Tunable acoustical plaster system and method of making it |
| USRE44070E1 (en) | 2005-06-09 | 2013-03-12 | United States Gypsum Company | Composite light weight gypsum wallboard |
| WO2015085129A1 (en) * | 2013-12-06 | 2015-06-11 | Georgia-Pacific Gypsum Llc | Gypsum composites containing cementitious materials and methods |
| US9802866B2 (en) | 2005-06-09 | 2017-10-31 | United States Gypsum Company | Light weight gypsum board |
| WO2017201270A1 (en) | 2016-05-20 | 2017-11-23 | United States Gypsum Company | Gypsum slurries with linear polycarboxylate dispersants |
| US9840066B2 (en) | 2005-06-09 | 2017-12-12 | United States Gypsum Company | Light weight gypsum board |
| WO2018022819A1 (en) | 2016-07-28 | 2018-02-01 | United States Gypsum Company | Methods for making gypsum boards with polymer coating and gypsum boards made by the method |
| WO2018048633A1 (en) * | 2016-09-06 | 2018-03-15 | Lhoist North America, Inc. | High quality hydroxide slurries |
| WO2018057456A1 (en) | 2016-09-22 | 2018-03-29 | United States Gypsum Company | Gypsum boards with polymer coating and methods for making same |
| WO2018071351A1 (en) | 2016-10-12 | 2018-04-19 | United States Gypsum Company | Methods for making a lightweight gypsum composition with internally generated foam and products made from same |
| US10207475B2 (en) | 2016-05-13 | 2019-02-19 | United States Gypsum Company | Mat-faced board |
| US10221100B2 (en) | 2016-06-07 | 2019-03-05 | United States Gypsum Company | Pourable compositions |
| US10309771B2 (en) | 2015-06-11 | 2019-06-04 | United States Gypsum Company | System and method for determining facer surface smoothness |
| WO2019199741A1 (en) | 2018-04-11 | 2019-10-17 | United States Gypsum Company | A method for making a lightweight gypsum composition with internally generated foam and products made from same |
| US11306028B2 (en) | 2005-06-09 | 2022-04-19 | United States Gypsum Company | Light weight gypsum board |
| US11338548B2 (en) | 2005-06-09 | 2022-05-24 | United States Gypsum Company | Light weight gypsum board |
Families Citing this family (14)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20060280899A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Method of making a gypsum slurry with modifiers and dispersants |
| FR2897863B1 (en) * | 2006-02-28 | 2008-07-11 | Bpb Plc | METHOD OF HYDROFUGATING A GYPSUM-BASED PRODUCT FROM A PLASTER-BASED COMPOSITION |
| US8323785B2 (en) | 2011-02-25 | 2012-12-04 | United States Gypsum Company | Lightweight, reduced density fire rated gypsum panels |
| KR102006667B1 (en) * | 2012-03-14 | 2019-08-02 | 요시노 셋고 가부시키가이샤 | Gypsum dispersant |
| EP2826760B1 (en) * | 2012-03-14 | 2022-07-20 | Yoshino Gypsum Co., Ltd. | Gypsum dispersant |
| UA110269C2 (en) * | 2012-12-03 | 2015-12-10 | Saint Gobain Placo | Chemical additives for gypsum products |
| CN106470813B (en) * | 2014-06-05 | 2019-12-27 | 可耐福石膏两合公司 | Method for producing gypsum plasterboards and gypsum plasterboard obtained thereby |
| CN106193496B (en) * | 2015-04-30 | 2018-09-11 | 宁波高新区起兴机电有限公司 | A kind of production method of soft decorative panel for building |
| JP2015205820A (en) * | 2015-08-03 | 2015-11-19 | 日東石膏ボード株式会社 | Putty material and joint treatment method |
| CN109665739A (en) * | 2018-11-07 | 2019-04-23 | 宁夏宝塔化工中心实验室(有限公司) | A kind of efficient gypsum reinforcing agent |
| KR102183145B1 (en) * | 2019-04-08 | 2020-11-25 | 주식회사 실크로드시앤티 | Admixture for gypsum board, composition for forming gypsum board, and gypsum board using the same |
| KR102332242B1 (en) * | 2020-06-11 | 2021-11-30 | 주식회사 실크로드시앤티 | An admixture for a gypsum board |
| KR102551585B1 (en) * | 2020-10-06 | 2023-07-05 | 주식회사 실크로드시앤티 | A method for producing an admixture for a gypsum board and an admixture thereof, composition for forming a gypsum board |
| CN115259819A (en) * | 2022-06-24 | 2022-11-01 | 汕头市固迪诗新材料有限公司 | Gypsum-based composite slurry and application method thereof |
Citations (56)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4028125A (en) * | 1976-03-25 | 1977-06-07 | The Dow Chemical Company | Cement composition |
| US4202857A (en) * | 1977-07-19 | 1980-05-13 | Pitun-Unicrete Limited | Casting of articles from compositions containing calcined gypsum and Portland cement |
| US4238239A (en) * | 1978-10-25 | 1980-12-09 | Weston Research, Corporation | Dry wall joint and finishing compounds |
| US4341560A (en) * | 1979-10-02 | 1982-07-27 | Kurashiki Boseki Kabushiki Kaisha | Waterproof gypsum molded product |
| US4561986A (en) * | 1984-07-19 | 1985-12-31 | Villa Jose L | Combined dispersant fluid loss control additives |
| US4666971A (en) * | 1983-11-03 | 1987-05-19 | General Electric Company | Thermal-sensitive insulating composition comprising cured acrylonitrile butadiene carboxylic acid rubbers containing filler materials |
| US4814014A (en) * | 1986-12-09 | 1989-03-21 | W. R. Grace & Co. | Hydraulic cement additives and hydraulic cement compositions containing same |
| US4927463A (en) * | 1988-04-08 | 1990-05-22 | Biochemie Ladenburg Gmbh | Aqueous dispersion of gypsum and its use as a filler and coating pigment in the production of paper and cardboard |
| US4960465A (en) * | 1986-12-09 | 1990-10-02 | W. R. Grace & Co. | Hydraulic cement additives and hydraulic cement compositions containing same |
| US5109030A (en) * | 1989-11-22 | 1992-04-28 | Rohm And Haas Company | Foamed hydraulic compositions containing copolymeric foam stabilizers |
| US5118751A (en) * | 1990-09-27 | 1992-06-02 | Wacker Chemie Gmbh | Redispersible powder composition |
| US5223036A (en) * | 1990-12-12 | 1993-06-29 | W. R. Grace & Co.-Conn. | Additive composition for cement admixture |
| US5362323A (en) * | 1992-02-14 | 1994-11-08 | W. R. Grace & Co. Conn. | Cement admixture composition |
| US5369198A (en) * | 1993-02-01 | 1994-11-29 | Chemie Linz Gesellschaft M.B.H | Copolymers based on maleic acid derivatives and vinyl monomers, their production and application |
| US5387626A (en) * | 1991-09-03 | 1995-02-07 | Hoechst Aktiengesellschaft | Additive combination for improving the processing properties of water-containing mixtures of building materials |
| US5393343A (en) * | 1993-09-29 | 1995-02-28 | W. R. Grace & Co.-Conn. | Cement and cement composition having improved rheological properties |
| US5401798A (en) * | 1991-06-14 | 1995-03-28 | Bayer Aktiengesellschaft | Gypsum-based materials, process for their preparation and their use |
| US5424099A (en) * | 1993-03-12 | 1995-06-13 | W.R. Grace & Co.-Conn. | High strength pourable gypsum floor underlayments and methods of providing same |
| US5556460A (en) * | 1995-09-18 | 1996-09-17 | W.R. Grace & Co.-Conn. | Drying shrinkage cement admixture |
| US5614017A (en) * | 1996-03-26 | 1997-03-25 | Arco Chemical Technology, L.P. | Cement additives |
| US5643978A (en) * | 1993-09-29 | 1997-07-01 | W. R. Grace & Co.-Conn. | Cement admixture product having improved rheological properties and process of forming same |
| US5661206A (en) * | 1993-06-11 | 1997-08-26 | Mbt Holding Ag | Fluidity control of cementitious compositions |
| US5670578A (en) * | 1996-12-10 | 1997-09-23 | Arco Chemical Technology, L.P. | Cement additives |
| US5685903A (en) * | 1994-06-03 | 1997-11-11 | National Gypsum Company | Cementitious gypsum-containing compositions and materials made therefrom |
| US5703174A (en) * | 1995-06-21 | 1997-12-30 | W. R. Grace & Co.-Conn. | Air controlling superplasticizers |
| US5725656A (en) * | 1996-05-29 | 1998-03-10 | The Trustees Of Colombia University In The City Of New York | Gypsum composition |
| US5725657A (en) * | 1995-07-24 | 1998-03-10 | Darwin; David Charles | Cement admixture product |
| US5739212A (en) * | 1992-12-08 | 1998-04-14 | Skw Trostberg Aktiengesellschaft | Water-soluble graft polymers |
| US5779786A (en) * | 1996-04-26 | 1998-07-14 | National Gypsum Company | Ready mixed setting-type joint compound, and method of making same |
| US5798425A (en) * | 1995-04-07 | 1998-08-25 | Skw Trostberg Aktiengesellschaft | Co-polymers based on oxyalkyleneglycol alkenyl ethers and unsaturated dicarboxylic acid derivatives |
| US5834576A (en) * | 1995-02-28 | 1998-11-10 | Nippon Shokubai Co., Ltd. | Acrylic acid derivatives, method for preparing the acrylic acid derivatives, and acrylic acid polymers |
| US5858083A (en) * | 1994-06-03 | 1999-01-12 | National Gypsum Company | Cementitious gypsum-containing binders and compositions and materials made therefrom |
| US5925984A (en) * | 1995-12-22 | 1999-07-20 | Patent-Treuhand-Gesellschaft Fuer Elektrische Gluehlampen Mbh | Circuit arrangement having LC parallel tuned drive circuitry |
| US5985989A (en) * | 1997-07-09 | 1999-11-16 | Arco Chemical Technology, Lp | Method of making a water reducing additive for cement |
| US6034208A (en) * | 1997-08-25 | 2000-03-07 | Arco Chemical Technology, L.P. | Copolymers useful as cement additives and a process for their preparation |
| US6043329A (en) * | 1997-04-07 | 2000-03-28 | Cement Intellectual Property Ltd. (Cip) | Acrylic copolymers |
| US6150437A (en) * | 1994-06-21 | 2000-11-21 | Skw Trostberg Aktiengesellschaft | Flow improving agents for binder suspensions containing cement |
| US6166112A (en) * | 1997-03-10 | 2000-12-26 | Nippon Shokubai Co., Ltd. | Cement admixture and cement composition |
| US6187887B1 (en) * | 1998-02-17 | 2001-02-13 | Skw Bauchemie Gmbh | Water-soluble or water-swellable copolymers containing sulfonic groups and methods of preparation |
| US6221317B1 (en) * | 1999-04-30 | 2001-04-24 | Ccs Packard, Inc. | Universal pipette tip box |
| US6264739B1 (en) * | 1995-02-20 | 2001-07-24 | Kao Corporation | Dispersant for plaster |
| US6281307B1 (en) * | 1995-01-31 | 2001-08-28 | Ciba Specialty Chemicals Corporation | Polymerizable composition, process for producing cross linked polymers, and cross-linkable polymers |
| US6294015B1 (en) * | 1998-01-22 | 2001-09-25 | Nippon Shokubai Co., Ltd. | Cement admixture and cement composition |
| US6376581B1 (en) * | 1995-07-13 | 2002-04-23 | Mbt Holding Ag | Cement dispersant, method for production thereof, and cement composition using the dispersant |
| US20030019401A1 (en) * | 2001-04-11 | 2003-01-30 | Arco Chemical Technology L.P. | Use of comb-branched copolymers in gypsum compositions |
| US20030127026A1 (en) * | 2001-11-05 | 2003-07-10 | James Edward Anderson | High early-strength cementitious composition |
| US20030167973A1 (en) * | 2002-01-08 | 2003-09-11 | Boral Material Technologies, Inc. | Admixture for producing cementitious compositions having good fluidity and high early compressive strength |
| US6620879B1 (en) * | 1999-02-10 | 2003-09-16 | Degussa Construction Chemicals Gmbh | Powdery polyether carboxylate-based polymeric compositions |
| US20040045481A1 (en) * | 2002-09-11 | 2004-03-11 | National Gypsum Properties, Llc | Lightweight wallboard compositions containing excess starch |
| US20040072939A1 (en) * | 2002-10-11 | 2004-04-15 | Cornman Charles R. | Viscosity modifying agents and water reducers |
| US20040149172A1 (en) * | 2003-01-30 | 2004-08-05 | Jardine Leslie A. | High solids pumpable cement additives |
| US20040149174A1 (en) * | 2003-02-05 | 2004-08-05 | Mbt Holding Ag | Accelerating admixture for concrete |
| US20040170873A1 (en) * | 2002-12-13 | 2004-09-02 | G-P Gypsum Corporation | Gypsum panel having UV-cured moisture resistant coating and method for making the same |
| US20040198873A1 (en) * | 2003-02-26 | 2004-10-07 | Construction Research And Technology Gmbh | Strength improvement admixture |
| US20040211342A1 (en) * | 2003-04-25 | 2004-10-28 | Mbt Holding Ag | Rheology stabilizer for cementitious compositions |
| US6852159B2 (en) * | 2002-12-16 | 2005-02-08 | Takemoto Yushi Kabushiki Kaisha | Gypsum slurry compositions |
Family Cites Families (24)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JPS5830257B2 (en) | 1979-09-17 | 1983-06-28 | 宇部興産株式会社 | Water-resistant gypsum composition |
| JPS5925876A (en) | 1982-08-04 | 1984-02-09 | Nitto Chem Ind Co Ltd | Grouting method |
| DE3344291A1 (en) * | 1983-12-07 | 1985-06-13 | Skw Trostberg Ag, 8223 Trostberg | DISPERSING AGENT FOR SALTY SYSTEMS |
| JPS6140861A (en) | 1984-07-31 | 1986-02-27 | 菊水化学工業株式会社 | Mortar composition |
| US5286412A (en) * | 1991-10-16 | 1994-02-15 | Georgia-Pacific Corporation | Modified lignosulfonate dispersant for gypsum |
| GB9319205D0 (en) | 1993-09-16 | 1993-11-03 | Brown Jonathon L | Cement products and a method of manufacture thereof |
| AU7949494A (en) | 1993-10-21 | 1995-05-08 | Chichibu Onoda Cement Corporation | Self-leveling water-base composition |
| IL113587A (en) | 1994-06-03 | 1999-05-09 | Nat Gypsum Co | Cementitious gypsum-containing compositions and materials made therefrom |
| TW419447B (en) | 1996-02-22 | 2001-01-21 | Nippon Catalytic Chem Ind | Cement composition |
| JPH10158051A (en) * | 1996-11-26 | 1998-06-16 | Kao Corp | Aqueous slurry of gypsum as by-product and its production |
| DE19834173A1 (en) | 1997-08-01 | 1999-02-04 | Sueddeutsche Kalkstickstoff | Copolymer based on unsaturated di:carboxylic acid derivatives and oxyalkylene glycol-alkenyl ether(s) |
| US6437027B1 (en) * | 1998-11-30 | 2002-08-20 | Taiheiyo Cement Corporation | Process for producing dispersant for powdery hydraulic composition |
| US6409824B1 (en) | 2000-04-25 | 2002-06-25 | United States Gypsum Company | Gypsum compositions with enhanced resistance to permanent deformation |
| US7105587B2 (en) * | 2001-03-07 | 2006-09-12 | Innovative Construction And Building Materials | Method and composition for polymer-reinforced composite cementitious construction material |
| CA2478323C (en) | 2002-03-27 | 2011-01-04 | United States Gypsum Company | High strength flooring compositions |
| US6774146B2 (en) * | 2002-08-07 | 2004-08-10 | Geo Specialty Chemicals, Inc. | Dispersant and foaming agent combination |
| CN100542989C (en) * | 2003-03-05 | 2009-09-23 | 株式会社日本触媒 | The method of cement additive, cement composition and laying method thereof and production hardening of cement product |
| BRPI0408504A (en) | 2003-03-19 | 2006-03-07 | United States Gypsum Co | acoustic panel comprising seated plaster interlacing matrix and method for doing the same |
| US6869988B2 (en) | 2003-04-16 | 2005-03-22 | Arco Chemical Technology, L.P. | Solid supported comb-branched copolymers as an additive for gypsum compositions |
| US7700017B2 (en) * | 2003-08-25 | 2010-04-20 | Icestone Llc | Method for producing materials from recycled glass and cement compositions |
| US7875114B2 (en) * | 2005-06-14 | 2011-01-25 | United States Gypsum Company | Foamed slurry and building panel made therefrom |
| US20060280899A1 (en) * | 2005-06-14 | 2006-12-14 | United States Gypsum Company | Method of making a gypsum slurry with modifiers and dispersants |
| US7070648B1 (en) | 2005-06-16 | 2006-07-04 | Lyondell Chemical Technology, L.P. | Preparation of gypsum compositions |
| US20080148997A1 (en) * | 2006-12-22 | 2008-06-26 | Blackburn David R | Gypsum compositions with naphthalene sulfonate and modifiers |
-
2005
- 2005-06-14 US US11/152,317 patent/US20060280898A1/en not_active Abandoned
-
2006
- 2006-05-11 WO PCT/US2006/018415 patent/WO2006138002A2/en active Application Filing
- 2006-06-09 US US11/450,068 patent/US7608347B2/en active Active
- 2006-06-13 NZ NZ562969A patent/NZ562969A/en unknown
- 2006-06-13 JP JP2008517006A patent/JP2008546619A/en active Pending
- 2006-06-13 TW TW095121005A patent/TW200704618A/en unknown
- 2006-06-13 RU RU2008101423/03A patent/RU2416581C2/en not_active IP Right Cessation
- 2006-06-13 CN CNA2006800202774A patent/CN101312925A/en active Pending
- 2006-06-13 MX MX2007015965A patent/MX2007015965A/en active IP Right Grant
- 2006-06-13 BR BRPI0612042-3A patent/BRPI0612042A2/en not_active IP Right Cessation
- 2006-06-13 UA UAA200800408A patent/UA95077C2/en unknown
-
2007
- 2007-10-30 IL IL187021A patent/IL187021A0/en unknown
- 2007-11-01 ZA ZA200709448A patent/ZA200709448B/en unknown
-
2009
- 2009-07-30 US US12/512,612 patent/US7932308B2/en not_active Expired - Fee Related
Patent Citations (58)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4028125A (en) * | 1976-03-25 | 1977-06-07 | The Dow Chemical Company | Cement composition |
| US4202857A (en) * | 1977-07-19 | 1980-05-13 | Pitun-Unicrete Limited | Casting of articles from compositions containing calcined gypsum and Portland cement |
| US4238239A (en) * | 1978-10-25 | 1980-12-09 | Weston Research, Corporation | Dry wall joint and finishing compounds |
| US4341560A (en) * | 1979-10-02 | 1982-07-27 | Kurashiki Boseki Kabushiki Kaisha | Waterproof gypsum molded product |
| US4666971A (en) * | 1983-11-03 | 1987-05-19 | General Electric Company | Thermal-sensitive insulating composition comprising cured acrylonitrile butadiene carboxylic acid rubbers containing filler materials |
| US4561986A (en) * | 1984-07-19 | 1985-12-31 | Villa Jose L | Combined dispersant fluid loss control additives |
| US4814014A (en) * | 1986-12-09 | 1989-03-21 | W. R. Grace & Co. | Hydraulic cement additives and hydraulic cement compositions containing same |
| US4960465A (en) * | 1986-12-09 | 1990-10-02 | W. R. Grace & Co. | Hydraulic cement additives and hydraulic cement compositions containing same |
| US4927463A (en) * | 1988-04-08 | 1990-05-22 | Biochemie Ladenburg Gmbh | Aqueous dispersion of gypsum and its use as a filler and coating pigment in the production of paper and cardboard |
| US5109030A (en) * | 1989-11-22 | 1992-04-28 | Rohm And Haas Company | Foamed hydraulic compositions containing copolymeric foam stabilizers |
| US5118751A (en) * | 1990-09-27 | 1992-06-02 | Wacker Chemie Gmbh | Redispersible powder composition |
| US5223036A (en) * | 1990-12-12 | 1993-06-29 | W. R. Grace & Co.-Conn. | Additive composition for cement admixture |
| US5401798A (en) * | 1991-06-14 | 1995-03-28 | Bayer Aktiengesellschaft | Gypsum-based materials, process for their preparation and their use |
| US5387626A (en) * | 1991-09-03 | 1995-02-07 | Hoechst Aktiengesellschaft | Additive combination for improving the processing properties of water-containing mixtures of building materials |
| US5362323A (en) * | 1992-02-14 | 1994-11-08 | W. R. Grace & Co. Conn. | Cement admixture composition |
| US5739212A (en) * | 1992-12-08 | 1998-04-14 | Skw Trostberg Aktiengesellschaft | Water-soluble graft polymers |
| US5369198A (en) * | 1993-02-01 | 1994-11-29 | Chemie Linz Gesellschaft M.B.H | Copolymers based on maleic acid derivatives and vinyl monomers, their production and application |
| US5424099A (en) * | 1993-03-12 | 1995-06-13 | W.R. Grace & Co.-Conn. | High strength pourable gypsum floor underlayments and methods of providing same |
| US5661206A (en) * | 1993-06-11 | 1997-08-26 | Mbt Holding Ag | Fluidity control of cementitious compositions |
| US5393343A (en) * | 1993-09-29 | 1995-02-28 | W. R. Grace & Co.-Conn. | Cement and cement composition having improved rheological properties |
| US5643978A (en) * | 1993-09-29 | 1997-07-01 | W. R. Grace & Co.-Conn. | Cement admixture product having improved rheological properties and process of forming same |
| US5685903A (en) * | 1994-06-03 | 1997-11-11 | National Gypsum Company | Cementitious gypsum-containing compositions and materials made therefrom |
| US5858083A (en) * | 1994-06-03 | 1999-01-12 | National Gypsum Company | Cementitious gypsum-containing binders and compositions and materials made therefrom |
| US6150437A (en) * | 1994-06-21 | 2000-11-21 | Skw Trostberg Aktiengesellschaft | Flow improving agents for binder suspensions containing cement |
| US6281307B1 (en) * | 1995-01-31 | 2001-08-28 | Ciba Specialty Chemicals Corporation | Polymerizable composition, process for producing cross linked polymers, and cross-linkable polymers |
| US6264739B1 (en) * | 1995-02-20 | 2001-07-24 | Kao Corporation | Dispersant for plaster |
| US5834576A (en) * | 1995-02-28 | 1998-11-10 | Nippon Shokubai Co., Ltd. | Acrylic acid derivatives, method for preparing the acrylic acid derivatives, and acrylic acid polymers |
| US5798425A (en) * | 1995-04-07 | 1998-08-25 | Skw Trostberg Aktiengesellschaft | Co-polymers based on oxyalkyleneglycol alkenyl ethers and unsaturated dicarboxylic acid derivatives |
| US5703174A (en) * | 1995-06-21 | 1997-12-30 | W. R. Grace & Co.-Conn. | Air controlling superplasticizers |
| US6376581B1 (en) * | 1995-07-13 | 2002-04-23 | Mbt Holding Ag | Cement dispersant, method for production thereof, and cement composition using the dispersant |
| US5725657A (en) * | 1995-07-24 | 1998-03-10 | Darwin; David Charles | Cement admixture product |
| US5556460A (en) * | 1995-09-18 | 1996-09-17 | W.R. Grace & Co.-Conn. | Drying shrinkage cement admixture |
| US5925984A (en) * | 1995-12-22 | 1999-07-20 | Patent-Treuhand-Gesellschaft Fuer Elektrische Gluehlampen Mbh | Circuit arrangement having LC parallel tuned drive circuitry |
| US5614017A (en) * | 1996-03-26 | 1997-03-25 | Arco Chemical Technology, L.P. | Cement additives |
| US5779786A (en) * | 1996-04-26 | 1998-07-14 | National Gypsum Company | Ready mixed setting-type joint compound, and method of making same |
| US5725656A (en) * | 1996-05-29 | 1998-03-10 | The Trustees Of Colombia University In The City Of New York | Gypsum composition |
| US5670578A (en) * | 1996-12-10 | 1997-09-23 | Arco Chemical Technology, L.P. | Cement additives |
| US6166112A (en) * | 1997-03-10 | 2000-12-26 | Nippon Shokubai Co., Ltd. | Cement admixture and cement composition |
| US6043329A (en) * | 1997-04-07 | 2000-03-28 | Cement Intellectual Property Ltd. (Cip) | Acrylic copolymers |
| US5985989A (en) * | 1997-07-09 | 1999-11-16 | Arco Chemical Technology, Lp | Method of making a water reducing additive for cement |
| US6034208A (en) * | 1997-08-25 | 2000-03-07 | Arco Chemical Technology, L.P. | Copolymers useful as cement additives and a process for their preparation |
| US6294015B1 (en) * | 1998-01-22 | 2001-09-25 | Nippon Shokubai Co., Ltd. | Cement admixture and cement composition |
| US6187887B1 (en) * | 1998-02-17 | 2001-02-13 | Skw Bauchemie Gmbh | Water-soluble or water-swellable copolymers containing sulfonic groups and methods of preparation |
| US6620879B1 (en) * | 1999-02-10 | 2003-09-16 | Degussa Construction Chemicals Gmbh | Powdery polyether carboxylate-based polymeric compositions |
| US6221317B1 (en) * | 1999-04-30 | 2001-04-24 | Ccs Packard, Inc. | Universal pipette tip box |
| US20030019401A1 (en) * | 2001-04-11 | 2003-01-30 | Arco Chemical Technology L.P. | Use of comb-branched copolymers in gypsum compositions |
| US6527850B2 (en) * | 2001-04-11 | 2003-03-04 | Arco Chemical Technology L.P. | Use of comb-branched copolymers in gypsum compositions |
| US20030127026A1 (en) * | 2001-11-05 | 2003-07-10 | James Edward Anderson | High early-strength cementitious composition |
| US20030167973A1 (en) * | 2002-01-08 | 2003-09-11 | Boral Material Technologies, Inc. | Admixture for producing cementitious compositions having good fluidity and high early compressive strength |
| US20040045481A1 (en) * | 2002-09-11 | 2004-03-11 | National Gypsum Properties, Llc | Lightweight wallboard compositions containing excess starch |
| US20040072939A1 (en) * | 2002-10-11 | 2004-04-15 | Cornman Charles R. | Viscosity modifying agents and water reducers |
| US20040170873A1 (en) * | 2002-12-13 | 2004-09-02 | G-P Gypsum Corporation | Gypsum panel having UV-cured moisture resistant coating and method for making the same |
| US6852159B2 (en) * | 2002-12-16 | 2005-02-08 | Takemoto Yushi Kabushiki Kaisha | Gypsum slurry compositions |
| US20040149172A1 (en) * | 2003-01-30 | 2004-08-05 | Jardine Leslie A. | High solids pumpable cement additives |
| US6800129B2 (en) * | 2003-01-30 | 2004-10-05 | W. R. Grace & Co.-Conn. | High solids pumpable cement additives |
| US20040149174A1 (en) * | 2003-02-05 | 2004-08-05 | Mbt Holding Ag | Accelerating admixture for concrete |
| US20040198873A1 (en) * | 2003-02-26 | 2004-10-07 | Construction Research And Technology Gmbh | Strength improvement admixture |
| US20040211342A1 (en) * | 2003-04-25 | 2004-10-28 | Mbt Holding Ag | Rheology stabilizer for cementitious compositions |
Cited By (35)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9840066B2 (en) | 2005-06-09 | 2017-12-12 | United States Gypsum Company | Light weight gypsum board |
| US9802866B2 (en) | 2005-06-09 | 2017-10-31 | United States Gypsum Company | Light weight gypsum board |
| US11884040B2 (en) | 2005-06-09 | 2024-01-30 | United States Gypsum Company | Light weight gypsum board |
| US11338548B2 (en) | 2005-06-09 | 2022-05-24 | United States Gypsum Company | Light weight gypsum board |
| US11306028B2 (en) | 2005-06-09 | 2022-04-19 | United States Gypsum Company | Light weight gypsum board |
| US10406779B2 (en) | 2005-06-09 | 2019-09-10 | United States Gypsum Company | Light weight gypsum board |
| US8197952B2 (en) | 2005-06-09 | 2012-06-12 | United States Gypsum Company | High starch light weight gypsum wallboard |
| US10407345B2 (en) | 2005-06-09 | 2019-09-10 | United States Gypsum Company | Light weight gypsum board |
| US8257489B2 (en) | 2005-06-09 | 2012-09-04 | United States Gypsum Company | Slurries and methods of making light weight gypsum board |
| USRE44070E1 (en) | 2005-06-09 | 2013-03-12 | United States Gypsum Company | Composite light weight gypsum wallboard |
| US8470461B2 (en) | 2005-06-09 | 2013-06-25 | United States Gypsum Company | Light weight gypsum board |
| US20080148997A1 (en) * | 2006-12-22 | 2008-06-26 | Blackburn David R | Gypsum compositions with naphthalene sulfonate and modifiers |
| US7670427B2 (en) | 2007-06-06 | 2010-03-02 | United States Gypsum Company | Very fast setting cementitious composition with high early-age compressive strength |
| US20100291305A1 (en) * | 2007-12-28 | 2010-11-18 | United States Gypsum Company | Decreased evaporation with retarder for a high water to stucco radio lightweight board |
| WO2009086390A1 (en) * | 2007-12-28 | 2009-07-09 | United States Gypsum Company | Decreased evaporation with retarder for a high water to stucco ratio lightweight board |
| WO2011029711A1 (en) | 2009-09-02 | 2011-03-17 | Basf Construction Polymers Gmbh | Formulation and its use |
| US20110054081A1 (en) * | 2009-09-02 | 2011-03-03 | Frank Dierschke | Formulation and its use |
| WO2011078708A1 (en) * | 2009-12-24 | 2011-06-30 | Carbon Credit Corporation New Zealand Limited | Carbon plaster board |
| WO2012049077A2 (en) | 2010-10-11 | 2012-04-19 | Basf Construction Polymers Gmbh | Dispersant containing gypsum slurry |
| US8210310B1 (en) * | 2010-12-29 | 2012-07-03 | United States Gypsum Company | Tunable acoustical plaster system and method of making it |
| WO2015085129A1 (en) * | 2013-12-06 | 2015-06-11 | Georgia-Pacific Gypsum Llc | Gypsum composites containing cementitious materials and methods |
| US10309771B2 (en) | 2015-06-11 | 2019-06-04 | United States Gypsum Company | System and method for determining facer surface smoothness |
| US10207475B2 (en) | 2016-05-13 | 2019-02-19 | United States Gypsum Company | Mat-faced board |
| US10968138B2 (en) | 2016-05-20 | 2021-04-06 | United States Gypsum Company | Gypsum slurries with linear polycarboxylate dispersants |
| US10442732B2 (en) | 2016-05-20 | 2019-10-15 | United States Gypsum Company | Gypsum slurries with linear polycarboxylate dispersants |
| WO2017201270A1 (en) | 2016-05-20 | 2017-11-23 | United States Gypsum Company | Gypsum slurries with linear polycarboxylate dispersants |
| US10221100B2 (en) | 2016-06-07 | 2019-03-05 | United States Gypsum Company | Pourable compositions |
| US9945119B2 (en) | 2016-07-28 | 2018-04-17 | United States Gypsum Company | Methods for making gypsum boards with polymer coating and gypsum boards made by the method |
| WO2018022819A1 (en) | 2016-07-28 | 2018-02-01 | United States Gypsum Company | Methods for making gypsum boards with polymer coating and gypsum boards made by the method |
| WO2018048633A1 (en) * | 2016-09-06 | 2018-03-15 | Lhoist North America, Inc. | High quality hydroxide slurries |
| WO2018057456A1 (en) | 2016-09-22 | 2018-03-29 | United States Gypsum Company | Gypsum boards with polymer coating and methods for making same |
| US10737981B2 (en) | 2016-10-12 | 2020-08-11 | United States Gypsum Company | Method for making a lightweight gypsum composition with internally generated foam and products made from same |
| WO2018071351A1 (en) | 2016-10-12 | 2018-04-19 | United States Gypsum Company | Methods for making a lightweight gypsum composition with internally generated foam and products made from same |
| WO2019199741A1 (en) | 2018-04-11 | 2019-10-17 | United States Gypsum Company | A method for making a lightweight gypsum composition with internally generated foam and products made from same |
| US11414352B2 (en) | 2018-04-11 | 2022-08-16 | United States Gypsum Company | Method for making a lightweight gypsum composition with internally generated foam and products made from same |
Also Published As
| Publication number | Publication date |
|---|---|
| JP2008546619A (en) | 2008-12-25 |
| US7932308B2 (en) | 2011-04-26 |
| US20090292045A1 (en) | 2009-11-26 |
| US7608347B2 (en) | 2009-10-27 |
| MX2007015965A (en) | 2008-03-06 |
| BRPI0612042A2 (en) | 2010-10-13 |
| RU2008101423A (en) | 2009-07-20 |
| ZA200709448B (en) | 2008-11-26 |
| CN101312925A (en) | 2008-11-26 |
| IL187021A0 (en) | 2008-02-09 |
| UA95077C2 (en) | 2011-07-11 |
| US20060278134A1 (en) | 2006-12-14 |
| WO2006138002A3 (en) | 2007-10-11 |
| TW200704618A (en) | 2007-02-01 |
| NZ562969A (en) | 2011-02-25 |
| WO2006138002A2 (en) | 2006-12-28 |
| RU2416581C2 (en) | 2011-04-20 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US7608347B2 (en) | Modifiers for gypsum slurries and method of using them | |
| AU2006259585B2 (en) | Modifiers for gypsum slurries and method of using them | |
| US7572328B2 (en) | Fast drying gypsum products | |
| US7572329B2 (en) | Method of making a gypsum slurry with modifiers and dispersants | |
| US7767019B2 (en) | Gypsum products utilizing a two-repeating unit dispersant and a method for making them | |
| US7544242B2 (en) | Effective use of dispersants in wallboard containing foam | |
| US20060278128A1 (en) | Effective use of dispersants in wallboard containing foam | |
| US20080148997A1 (en) | Gypsum compositions with naphthalene sulfonate and modifiers | |
| US7601214B2 (en) | Modified landplaster as a wallboard filler | |
| US7819971B2 (en) | Method of using landplaster as a wallboard filler | |
| US9328025B2 (en) | Effective use of melamine sulfonate condensate dispersants in wallboard containing foam |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: UNITES STATES GYPSUM COMPANY, ILLINOIS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:LETTKEMAN, DENNIS M.;SHAKE, MICHAEL P.;LIU, QINGXIA;AND OTHERS;REEL/FRAME:016832/0506;SIGNING DATES FROM 20050705 TO 20050726 |
|
| AS | Assignment |
Owner name: UNITED STATES GYPSUM COMPANY, ILLINOIS Free format text: CORRECTIVE ASSIGNMENT TO CORRECT THE DOC DATE PREVIOUSLY RECORDED ON REEL 016832 FRAME 0506;ASSIGNORS:LETTKEMAN, DENNIS M.;SHAKE, MICHAEL P.;LIU, QINGXIA;AND OTHERS;REEL/FRAME:017197/0190;SIGNING DATES FROM 20050705 TO 20050726 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |