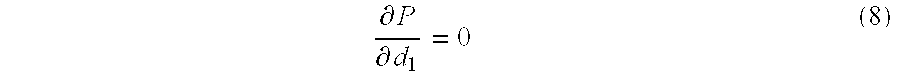US20040208751A1 - Microchip integrated multi-channel electroosmotic pumping system - Google Patents
Microchip integrated multi-channel electroosmotic pumping system Download PDFInfo
- Publication number
- US20040208751A1 US20040208751A1 US10/478,612 US47861203A US2004208751A1 US 20040208751 A1 US20040208751 A1 US 20040208751A1 US 47861203 A US47861203 A US 47861203A US 2004208751 A1 US2004208751 A1 US 2004208751A1
- Authority
- US
- United States
- Prior art keywords
- microchannels
- microchannel
- microfluidic
- electroosmotic
- pump
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 238000005086 pumping Methods 0.000 title abstract description 65
- 239000012530 fluid Substances 0.000 claims abstract description 32
- 239000000758 substrate Substances 0.000 claims description 29
- 238000004458 analytical method Methods 0.000 claims description 14
- 239000000463 material Substances 0.000 claims description 14
- 238000000034 method Methods 0.000 claims description 10
- 238000004891 communication Methods 0.000 claims description 6
- 239000011521 glass Substances 0.000 claims description 5
- 239000005373 porous glass Substances 0.000 claims description 5
- 238000001514 detection method Methods 0.000 claims description 3
- OKTJSMMVPCPJKN-UHFFFAOYSA-N Carbon Chemical compound [C] OKTJSMMVPCPJKN-UHFFFAOYSA-N 0.000 claims description 2
- 239000000919 ceramic Substances 0.000 claims description 2
- 229910002804 graphite Inorganic materials 0.000 claims description 2
- 239000010439 graphite Substances 0.000 claims description 2
- 229910021420 polycrystalline silicon Inorganic materials 0.000 claims description 2
- 229920005591 polysilicon Polymers 0.000 claims description 2
- 239000010453 quartz Substances 0.000 claims description 2
- 229910052710 silicon Inorganic materials 0.000 claims description 2
- 239000010703 silicon Substances 0.000 claims description 2
- VYPSYNLAJGMNEJ-UHFFFAOYSA-N silicon dioxide Inorganic materials O=[Si]=O VYPSYNLAJGMNEJ-UHFFFAOYSA-N 0.000 claims description 2
- 229910010272 inorganic material Inorganic materials 0.000 claims 1
- 239000011147 inorganic material Substances 0.000 claims 1
- 239000011368 organic material Substances 0.000 claims 1
- 239000007788 liquid Substances 0.000 abstract description 5
- 238000005370 electroosmosis Methods 0.000 description 34
- 239000002904 solvent Substances 0.000 description 11
- 238000000926 separation method Methods 0.000 description 10
- 230000001419 dependent effect Effects 0.000 description 9
- 238000010586 diagram Methods 0.000 description 8
- 239000003480 eluent Substances 0.000 description 8
- 239000002699 waste material Substances 0.000 description 8
- 238000013461 design Methods 0.000 description 7
- 238000002347 injection Methods 0.000 description 7
- 239000007924 injection Substances 0.000 description 7
- 238000004519 manufacturing process Methods 0.000 description 7
- 238000005516 engineering process Methods 0.000 description 6
- 230000007246 mechanism Effects 0.000 description 6
- 230000008901 benefit Effects 0.000 description 5
- 239000003153 chemical reaction reagent Substances 0.000 description 5
- 150000002500 ions Chemical class 0.000 description 5
- 238000004811 liquid chromatography Methods 0.000 description 5
- 239000000872 buffer Substances 0.000 description 4
- 238000002330 electrospray ionisation mass spectrometry Methods 0.000 description 4
- 230000010354 integration Effects 0.000 description 4
- 239000011148 porous material Substances 0.000 description 4
- 230000008021 deposition Effects 0.000 description 3
- 238000009826 distribution Methods 0.000 description 3
- 230000000694 effects Effects 0.000 description 3
- 230000005684 electric field Effects 0.000 description 3
- 230000009471 action Effects 0.000 description 2
- 238000004401 flow injection analysis Methods 0.000 description 2
- 125000000524 functional group Chemical group 0.000 description 2
- 238000011068 loading method Methods 0.000 description 2
- 238000001906 matrix-assisted laser desorption--ionisation mass spectrometry Methods 0.000 description 2
- 239000012528 membrane Substances 0.000 description 2
- 238000012856 packing Methods 0.000 description 2
- 239000002245 particle Substances 0.000 description 2
- 238000000206 photolithography Methods 0.000 description 2
- 238000004094 preconcentration Methods 0.000 description 2
- 108090000765 processed proteins & peptides Proteins 0.000 description 2
- 238000012546 transfer Methods 0.000 description 2
- 238000003631 wet chemical etching Methods 0.000 description 2
- 230000005526 G1 to G0 transition Effects 0.000 description 1
- 241001465754 Metazoa Species 0.000 description 1
- 239000004696 Poly ether ether ketone Substances 0.000 description 1
- 238000001042 affinity chromatography Methods 0.000 description 1
- 230000004075 alteration Effects 0.000 description 1
- 238000013459 approach Methods 0.000 description 1
- 230000009286 beneficial effect Effects 0.000 description 1
- JUPQTSLXMOCDHR-UHFFFAOYSA-N benzene-1,4-diol;bis(4-fluorophenyl)methanone Chemical compound OC1=CC=C(O)C=C1.C1=CC(F)=CC=C1C(=O)C1=CC=C(F)C=C1 JUPQTSLXMOCDHR-UHFFFAOYSA-N 0.000 description 1
- 239000007853 buffer solution Substances 0.000 description 1
- 238000005251 capillar electrophoresis Methods 0.000 description 1
- 238000002045 capillary electrochromatography Methods 0.000 description 1
- 239000011248 coating agent Substances 0.000 description 1
- 230000001010 compromised effect Effects 0.000 description 1
- 229920001940 conductive polymer Polymers 0.000 description 1
- 238000001816 cooling Methods 0.000 description 1
- 230000007423 decrease Effects 0.000 description 1
- 238000011161 development Methods 0.000 description 1
- 238000001312 dry etching Methods 0.000 description 1
- 238000007787 electrohydrodynamic spraying Methods 0.000 description 1
- 238000004049 embossing Methods 0.000 description 1
- 238000005530 etching Methods 0.000 description 1
- 238000011049 filling Methods 0.000 description 1
- 238000001914 filtration Methods 0.000 description 1
- 230000036541 health Effects 0.000 description 1
- 238000010438 heat treatment Methods 0.000 description 1
- 238000012203 high throughput assay Methods 0.000 description 1
- 238000013537 high throughput screening Methods 0.000 description 1
- 238000003018 immunoassay Methods 0.000 description 1
- 238000010348 incorporation Methods 0.000 description 1
- 238000001802 infusion Methods 0.000 description 1
- 238000001746 injection moulding Methods 0.000 description 1
- -1 inter alia Substances 0.000 description 1
- 238000011835 investigation Methods 0.000 description 1
- 238000001155 isoelectric focusing Methods 0.000 description 1
- 238000000608 laser ablation Methods 0.000 description 1
- 238000002156 mixing Methods 0.000 description 1
- 238000012544 monitoring process Methods 0.000 description 1
- 230000003287 optical effect Effects 0.000 description 1
- 230000035699 permeability Effects 0.000 description 1
- 238000005191 phase separation Methods 0.000 description 1
- 229920002530 polyetherether ketone Polymers 0.000 description 1
- 230000008569 process Effects 0.000 description 1
- 230000009467 reduction Effects 0.000 description 1
- 238000011160 research Methods 0.000 description 1
- 230000004044 response Effects 0.000 description 1
- 239000000243 solution Substances 0.000 description 1
- 239000011877 solvent mixture Substances 0.000 description 1
- 238000001179 sorption measurement Methods 0.000 description 1
- 239000000126 substance Substances 0.000 description 1
- 230000001502 supplementing effect Effects 0.000 description 1
Images
Classifications
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/416—Systems
- G01N27/447—Systems using electrophoresis
- G01N27/44756—Apparatus specially adapted therefor
- G01N27/44791—Microapparatus
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J19/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J19/0093—Microreactors, e.g. miniaturised or microfabricated reactors
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
- B01L3/50273—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip characterised by the means or forces applied to move the fluids
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L3/00—Containers or dishes for laboratory use, e.g. laboratory glassware; Droppers
- B01L3/50—Containers for the purpose of retaining a material to be analysed, e.g. test tubes
- B01L3/502—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures
- B01L3/5027—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip
- B01L3/502738—Containers for the purpose of retaining a material to be analysed, e.g. test tubes with fluid transport, e.g. in multi-compartment structures by integrated microfluidic structures, i.e. dimensions of channels and chambers are such that surface tension forces are important, e.g. lab-on-a-chip characterised by integrated valves
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F04—POSITIVE - DISPLACEMENT MACHINES FOR LIQUIDS; PUMPS FOR LIQUIDS OR ELASTIC FLUIDS
- F04B—POSITIVE-DISPLACEMENT MACHINES FOR LIQUIDS; PUMPS
- F04B17/00—Pumps characterised by combination with, or adaptation to, specific driving engines or motors
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F04—POSITIVE - DISPLACEMENT MACHINES FOR LIQUIDS; PUMPS FOR LIQUIDS OR ELASTIC FLUIDS
- F04B—POSITIVE-DISPLACEMENT MACHINES FOR LIQUIDS; PUMPS
- F04B19/00—Machines or pumps having pertinent characteristics not provided for in, or of interest apart from, groups F04B1/00 - F04B17/00
- F04B19/006—Micropumps
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16K—VALVES; TAPS; COCKS; ACTUATING-FLOATS; DEVICES FOR VENTING OR AERATING
- F16K99/00—Subject matter not provided for in other groups of this subclass
- F16K99/0001—Microvalves
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16K—VALVES; TAPS; COCKS; ACTUATING-FLOATS; DEVICES FOR VENTING OR AERATING
- F16K99/00—Subject matter not provided for in other groups of this subclass
- F16K99/0001—Microvalves
- F16K99/0003—Constructional types of microvalves; Details of the cutting-off member
- F16K99/0017—Capillary or surface tension valves, e.g. using electro-wetting or electro-capillarity effects
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16K—VALVES; TAPS; COCKS; ACTUATING-FLOATS; DEVICES FOR VENTING OR AERATING
- F16K99/00—Subject matter not provided for in other groups of this subclass
- F16K99/0001—Microvalves
- F16K99/0003—Constructional types of microvalves; Details of the cutting-off member
- F16K99/0025—Valves using microporous membranes
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16K—VALVES; TAPS; COCKS; ACTUATING-FLOATS; DEVICES FOR VENTING OR AERATING
- F16K99/00—Subject matter not provided for in other groups of this subclass
- F16K99/0001—Microvalves
- F16K99/0034—Operating means specially adapted for microvalves
- F16K99/0042—Electric operating means therefor
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16K—VALVES; TAPS; COCKS; ACTUATING-FLOATS; DEVICES FOR VENTING OR AERATING
- F16K99/00—Subject matter not provided for in other groups of this subclass
- F16K99/0001—Microvalves
- F16K99/0034—Operating means specially adapted for microvalves
- F16K99/0042—Electric operating means therefor
- F16K99/0049—Electric operating means therefor using an electroactive polymer [EAP]
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N27/00—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means
- G01N27/26—Investigating or analysing materials by the use of electric, electrochemical, or magnetic means by investigating electrochemical variables; by using electrolysis or electrophoresis
- G01N27/416—Systems
- G01N27/447—Systems using electrophoresis
- G01N27/44704—Details; Accessories
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00781—Aspects relating to microreactors
- B01J2219/00783—Laminate assemblies, i.e. the reactor comprising a stack of plates
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00781—Aspects relating to microreactors
- B01J2219/00851—Additional features
- B01J2219/00853—Employing electrode arrangements
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00781—Aspects relating to microreactors
- B01J2219/00851—Additional features
- B01J2219/00858—Aspects relating to the size of the reactor
- B01J2219/0086—Dimensions of the flow channels
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01J—CHEMICAL OR PHYSICAL PROCESSES, e.g. CATALYSIS OR COLLOID CHEMISTRY; THEIR RELEVANT APPARATUS
- B01J2219/00—Chemical, physical or physico-chemical processes in general; Their relevant apparatus
- B01J2219/00781—Aspects relating to microreactors
- B01J2219/00891—Feeding or evacuation
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0809—Geometry, shape and general structure rectangular shaped
- B01L2300/0816—Cards, e.g. flat sample carriers usually with flow in two horizontal directions
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0861—Configuration of multiple channels and/or chambers in a single devices
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0861—Configuration of multiple channels and/or chambers in a single devices
- B01L2300/0864—Configuration of multiple channels and/or chambers in a single devices comprising only one inlet and multiple receiving wells, e.g. for separation, splitting
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2300/00—Additional constructional details
- B01L2300/08—Geometry, shape and general structure
- B01L2300/0861—Configuration of multiple channels and/or chambers in a single devices
- B01L2300/0867—Multiple inlets and one sample wells, e.g. mixing, dilution
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/04—Moving fluids with specific forces or mechanical means
- B01L2400/0403—Moving fluids with specific forces or mechanical means specific forces
- B01L2400/0415—Moving fluids with specific forces or mechanical means specific forces electrical forces, e.g. electrokinetic
-
- B—PERFORMING OPERATIONS; TRANSPORTING
- B01—PHYSICAL OR CHEMICAL PROCESSES OR APPARATUS IN GENERAL
- B01L—CHEMICAL OR PHYSICAL LABORATORY APPARATUS FOR GENERAL USE
- B01L2400/00—Moving or stopping fluids
- B01L2400/04—Moving fluids with specific forces or mechanical means
- B01L2400/0403—Moving fluids with specific forces or mechanical means specific forces
- B01L2400/0415—Moving fluids with specific forces or mechanical means specific forces electrical forces, e.g. electrokinetic
- B01L2400/0418—Moving fluids with specific forces or mechanical means specific forces electrical forces, e.g. electrokinetic electro-osmotic flow [EOF]
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16K—VALVES; TAPS; COCKS; ACTUATING-FLOATS; DEVICES FOR VENTING OR AERATING
- F16K99/00—Subject matter not provided for in other groups of this subclass
- F16K2099/0073—Fabrication methods specifically adapted for microvalves
- F16K2099/0074—Fabrication methods specifically adapted for microvalves using photolithography, e.g. etching
-
- F—MECHANICAL ENGINEERING; LIGHTING; HEATING; WEAPONS; BLASTING
- F16—ENGINEERING ELEMENTS AND UNITS; GENERAL MEASURES FOR PRODUCING AND MAINTAINING EFFECTIVE FUNCTIONING OF MACHINES OR INSTALLATIONS; THERMAL INSULATION IN GENERAL
- F16K—VALVES; TAPS; COCKS; ACTUATING-FLOATS; DEVICES FOR VENTING OR AERATING
- F16K99/00—Subject matter not provided for in other groups of this subclass
- F16K2099/0082—Microvalves adapted for a particular use
- F16K2099/0094—Micropumps
-
- G—PHYSICS
- G01—MEASURING; TESTING
- G01N—INVESTIGATING OR ANALYSING MATERIALS BY DETERMINING THEIR CHEMICAL OR PHYSICAL PROPERTIES
- G01N1/00—Sampling; Preparing specimens for investigation
- G01N1/02—Devices for withdrawing samples
- G01N1/10—Devices for withdrawing samples in the liquid or fluent state
- G01N1/14—Suction devices, e.g. pumps; Ejector devices
Definitions
- Microfluidics and instrument miniaturization 1 have experienced significant growth in activity in recent years in response to the significant increase in use of microchips as bioanalytical tools.
- a major aspect of microfluidics refers to the manipulation of fluid flows in the microchip channels.
- 2-5 flow streams are typically generated using electrical forces (electroosmotic flow, or EOF).
- EOF electroosmotic flow
- EOF is dependent on the surface charge of the channel walls and consequently is sensitive to the physicochemical properties of the sample (pH, ionic strength, organic content). For example, the EOF can be easily reduced or even suppressed if the channel surface is altered by contact with specific sample types.
- micro-liquid chromatography ⁇ LC
- FOA flow injection analysis
- samples such as those containing cells that require zero electric field conditions for their manipulation
- fluid flows on microchips may, or must, be generated by differential pressure. 6-8
- this is accomplished by connecting external devices to the microchip, e.g., vacuum, gas pressure generators or syringe pumps.
- external devices e.g., vacuum, gas pressure generators or syringe pumps.
- the connection of external devices to microchips while effective, increases the complexity of the system, and integration and multiplexing capabilities may be compromised.
- the invention is directed to a microchip integrated microfabricated, microfluidic, multichannel, preferably electroosmotic pump and pumping system.
- the electroosmotic pump of the invention comprises a plurality of microchannels, which begin and end in common compartments, completed into an array.
- the microchannels within the pump have substantially identical, optimal dimensions of cross-section and length such that sufficient pressure for optimal flow of fluid (e.g., liquid or gas) and pressure is generated by the pump and flow rates are. stable and reproducible.
- the microchannels of the electroosmotic pump of the invention are open channels, i.e., not containing packed or porous materials.
- an electroosmotic pump of the invention is coupled to a single channel of a larger cross-section.
- a similar structure is also used in an electroosmotic valve of the invention, where samples are introduced into an analytical device.
- the microfluidic electroosmotic pumping system of the invention generates sufficient flow and pressures by optimizing the dimensional parameters of cross-section and length of the microchannels.
- FIG. 1 shows a schematic diagram of the microfabricated electroosmotic pumping system in accordance with the invention
- FIG. 2 shows a schematic diagram of a different embodiment of the microfabricated electroosmotic pumping system in accordance with the invention
- FIG. 3A shows representative flow, diameter and length in a schematic representation of a micropump with one pumping channel
- FIG. 3B shows a graph of the flow distribution, or the F/F eof coefficients calculated for a micropump with one microchannel at various d 1 /d 2 ratios;
- FIG. 4A shows a schematic representation of a micropump with n pumping channels
- FIG. 7A shows a schematic diagram of an injection of a well delimited sample plug moving through the electroosmotic valve of the invention
- FIG. 7B shows a schematic diagram of a sample plug being transported down the channel in the electroosmotic valve of the invention
- FIG. 8A shows a schematic diagram of an electroosmotic pumping system functioning as an electroosmotic valve
- FIG. 8B shows a schematic diagram of an electroosmotic pumping system functioning as an electroosmotic valve
- FIG. 9 shows an exemplary multiplexed pumping system in accordance with the invention.
- FIG. 10A and FIG. 10B show a cross-sectional view and a plan view, respectively, of a micropump according to the invention having filtering and gating elements.
- the microfabricated microfluidic pumping system of the invention comprises one or more miniaturized pumping units, which are capable of stable fluid delivery at flow rates and backpressures compatible with common analytical applications carried out on a microchip and sufficiently small to enable multiplexing of individual pumps.
- a multiple channel micropump based on electroosmotic pumping principles in accordance with the invention comprises a plurality of parallel, small cross-section microchannels (e.g., narrow or shallow) that deliver fluid to a microchannel of larger cross-section.
- This design allows the simultaneous generation of both flow and pressure and can be implemented in both packed channel and open-channel configuration.
- an open-channel means that no packed particles or porous materials are added to or contained in the microchannels.
- the pumping system of the invention offers numerous advantages. First, it can easily be integrated on microfluidic platforms and utilized for fluid propulsion on a microchip. Secondly, its fabrication using standard photolithographic and wet chemical etching technologies (or other microelectromechanical systems (MEMS) technologies) ensures high manufacturing and operating reproducibility. Thirdly, the simplicity of the design ensures robustness, reliability and trouble free operation.
- MEMS microelectromechanical systems
- the micropump is designed to fit into an integrated microfluidic analysis scheme for delivery of, e.g., peptide samples for, e.g., electrospray ionization-mass spectrometry (ESI-MS) analysis.
- ESI-MS electrospray ionization-mass spectrometry
- ⁇ -TAS micro-total analysis systems
- the principle of ⁇ -TAS is based on integrating all necessary parts and methods for analysis in miniaturized devices.
- the benefits of miniaturizing an analysis system include, but are not limited to, generation of multiple units at low cost; reduction in sample and reagent consumption and waste production; high throughput assays; small compact design; simple and reliable operation; and integration of several units or with existing systems.
- the micropump of the invention can be used to deliver fluid flows in electric field free regions and perform sample transfer, gradient generation, or fraction collection/deposition.
- a large variety of applications can be envisioned for a micropump according to the invention, including, inter alia, eluent flow and/or pressure generation for condensed phase separation techniques (e.g., micro liquid chromatography in open, packed, monolithic or microfabricated channels, pressure assisted capillary electrochromatography or capillary electrophoresis, isoelectric focusing, affinity chromatography, immunoassays); single, parallel or sequential sample delivery for electrospray ionization-mass spectrometry (ESI-MS), matrix assisted laser desorption ionization (MALDI)-MS, optical detectors, or other detection systems; single, parallel or sequential sample delivery systems to microreactors, mixers, preconcentrators, filters, or other functional elements integrated on the microchip; single, parallel or sequential eluent or sample delivery systems to off-chip applications; solvent gradient generation
- FIG. 1 illustrates a simplified schematic drawing of an exemplary microfabricated electroosmotic pumping system of the invention.
- a pumping unit 10 can be incorporated on one microchip device. However, multiple pumping units can also be incorporated, e.g., in parallel as shown in FIG. 2 and FIG. 9.
- the microchip device may be about a few square millimeters, e.g., 5-50 mm 2 .
- a multiplexed device (see, e.g., FIG. 9) that may contain, for example, eight, sixteen, or even 96 individual pumps may require a larger size chip.
- Pumping channel system 11 of pumping unit 10 comprises a plurality of first shallow microchannels 12 (e.g., 2-10,000 microchannels).
- All pumping channels 12 have first and second ends, which are in fluid communication with a common inlet 14 and a common outlet compartment or reservoir 16 , respectively. Electrodes inserted in reservoirs 14 and 16 are connected to high voltage power supply 30 and are used to apply a voltage drop across the pump.
- the pumping channels are exposed only to buffer solutions and do not come in contact with any sample that may be added to the microchip.
- the pump size is quite variable and depends on the application (e.g., 1 ⁇ 10, 2 ⁇ 10, 5 ⁇ 50, 1 ⁇ 50 mm).
- the length of each microchannel may range from, e.g., about 5-50 mm.
- the channel depth, width, equivalent diameter or cross-section may range from about less than 1 ⁇ m to a few micrometers (e.g., 0.5-10 ⁇ m).
- the number of microchannels in one pump may range from two to more than a thousand (e.g., 2-10,000 channels).
- the spacing between the microchannels is in the low micrometer range (e.g., 1-50 ⁇ m).
- the pump unit of the invention comprises 4 microchannels, 10 microchannels, 25 microchannels, 100 microchannels or 1000 microchannels.
- the pumping (or valve) system of the invention is activated by introducing into the channels a buffer or solvent, appropriate for preserving or increasing the surface charge on the pumping channel walls.
- the microchannels 12 can be filled by capillary action or by action of a pressure differential. Through electrodes placed in the reservoirs 14 and 16 , a potential differential is applied across the microchannels 12 . Depending on the surface properties of the channel (whether negatively or positively charged), the larger voltage must be applied to the appropriate reservoir, such that the electroosmotic flow will have the desired direction. For example, for a glass micropump having uncoated pumping channel walls, by applying a 2000 V on reservoir 14 and connecting reservoir 16 to ground, electroosmotic flow will be generated from reservoir 14 in the direction of reservoir 16 .
- Electroosmotic flow is created due to the following mechanism.
- the functional groups on the pumping microchannel walls ionize in the presence of the solvent/fluid that is filling the microchannels and attract a layer of oppositely charged ions.
- this layer of oppositely charged ions start to move in the direction of the appropriate electrode (negative electrode for positively charged ions), dragging along bulk fluid flow.
- this electroosmotic flow is restricted from evolving freely by the structure of the system or by the rest of the microfluidic channel network on the chip, pressure will be created inside the microfluidic structure, and the electroosmotic flow will be distributed through all the channels of the device through a pressure controlled mechanism.
- the direction of pumping will be reversed, and the pump will draw fluid out from the rest of the channels.
- the pumping system delivers fluid via a common outlet compartment, connected to reservoir 16 , to larger diameter second microchannel 13 , which may be connected to a network of channels or to other devices.
- second microchannel 13 is connected to network channel 20 for, e.g., sample infusion or separation.
- Channel 20 comprises electroosmotic valve 17 of the invention, which is used, e.g., for sample introduction.
- the electroosmotic valve is open to electroosmotic driven flows and essentially closed to pressure driven flows.
- an electrospray emitter 32 for example, or a detection or a sample collection device using any of the systems described above, can be integrated for analysis of a sample.
- Sample injection in a microfabricated analysis systems is accomplished electrokinetically 2,3 or by pressure gradients, 44 and sample manipulations are performed with electrokinetic forces. If sample manipulations are to be performed using pressure driven flows, a fluid valving system, such as described here, is necessary to prevent leakage of the fluid flow into the sample or sample waste reservoirs.
- electroosmotic valving is accomplished using very narrow channels for the sample injection and waste lines, in a configuration similar to that of the electooosmotic pump. In a pressurized analysis system, narrow injection channels 45 can act as efficient valving components. As shown in FIG.
- a sample in a valve according to the invention, can be infused electroosmotically from reservoir 22 to reservoir 24 through the sample and sample waste pluralities off channels, 26 and 28 , respectively, and a double-T injector 18 .
- the double-T injector can be an open or packed channel, e.g., as part of a preconcentration or ⁇ LC system. After completion of the injection, the pump can be started. For sample introduction and waste channels with similar dimensions to the pumping microchannels, additional pressure driven fluid loss through these channels could be negligible.
- valve 17 and pump 10 are first filled with an appropriate eluent.
- Sample is injected into the valve inlet reservoir 22 , and a voltage drop is established between reservoirs 22 and 24 to allow for a sample plug to be loaded into microchannel 20 .
- the sample is electroosmotically carried from reservoir 22 in the direction of reservoir 24 by the application of a potential difference, e.g., 2000 V, between reservoir 22 and reservoir 24 .
- a very small voltage drop (e.g., 100 V) between pump reservoir 14 and pump reservoir 16 may be needed on the pump unit 12 as well to help focus the injected plug in the microchannel 20 .
- a large voltage drop e.g., 2000 V
- the electrodes in reservoirs 22 and 24 are maintained at the same value as the voltage on reservoir 16 , or they are removed from these reservoirs, in order to suppress the electroosmotic flow in the direction of the sample and sample waste reservoirs.
- the voltage drop across the pump will generate electroosmotic flow in the pump and pressurized flow in the rest of the system.
- the large hydraulic resistance in the sample inlet and outlet channels will prevent back pressure leakage, or will allow for only a small flow leakage through these channels. Consequently, the sample plug will be transported only down the microchannel 20 .
- an electroosmotic pumping system composed of two parallel pumps can function, alternatively, as an electroosmotic valve, as well.
- One of the pumps, e.g., pump 10 is used for loading the sample, while the other pump, e.g., pump 100 , is used for loading the eluent.
- Small, well delimited sample plugs cannot be injected with this configuration.
- FIG. 8B for injecting the plug for analysis, a large voltage drop is applied across pump 100 , for example, 2000 V between reservoir 104 and reservoir 106 .
- the voltage on reservoirs 14 and 16 is maintained at the same value as the voltage on reservoir 106 , or the electrodes are removed from these reservoirs.
- the voltage drop across the pump 100 will generate electroosmotic flow in this pump, and pressurized flow in the rest of the system. For properly designed pumping channel diameters, the pressurized leakage flow through pump 12 will be minimal.
- the potential differential for electroosmotic flow (EOF) generation is applied between the inlet and outlet reservoirs and may be provided by a power supply.
- EEF electroosmotic flow
- the necessary voltage drop for the operation of the electroosmotic micropump may vary from a few tens to thousands of volts (e.g., 50-2000 V/cm).
- outlet compartment or reservoir 16 can be common for systems with multiple pumps.
- a semi-permeable gate 34 that allows for an exchange of ions but not of the bulk eluent flow (liquid or gas), is created at the bottom of the outlet reservoir.
- the gate prevents EOF leakage in the direction of the exit electrode 35 ′ placed in the outlet reservoir.
- a porous glass disc e.g., 5 mm in diameter, 0.8-1 mm in width, and 40-50 ⁇ pore size, prepared by Chang Associates (Worcester, Mass.)
- other materials may be used, e.g., graphite, polymeric organic or inorganic membrane materials.
- reservoir 16 is fabricated from a PEEK external nut.
- the porous glass disc is secured (e.g., between two gasket elements) at the bottom of reservoir 16 with a corresponding internal nut.
- This arrangement provides for robustness and exchangeability of the porous glass disc.
- the pumping microchannels act as a filter, clogging of some of the channels can occur.
- a short filter element 36 e.g., 100 ⁇ m in length
- Filter elements 36 are spaced from the ends of the pumping channels by intermediate compartments 38 and terminate at end compartments 14 ′ and 16 ′, respectively.
- end compartments 14 ′, 16 ′ are connected to orifaces 40 in the substrate, which make connection with reservoirs 14 and 16 on the exterior surface of the substrate.
- the pump can be fabricated in a variety of substrate materials such as, inter alia, glass, quartz, silicon, polysilicon, polymeric materials (organic or inorganic), ceramic, or other materials.
- the micropumps can be made using microfabrication techniques, for example, photolithography and wet chemical etching, or other microelectromechanical systems (MEMS) technologies (e.g., dry etching, laser ablation, injection molding, embossing, stamping).
- MEMS microelectromechanical systems
- Microfabricated devices that contain an electroosmotic micropump of the invention can be fabricated from one or two substrates made of any of the materials described above.
- the microfluidic channels can be made using one of the substrates described above by any of the microfabrication techniques. For example, micropump and sample handling channels defined on a photomask by 2 and 20 ⁇ m wide lines (other dimensions are possible) are transferred to the substrate using photolithography. After exposure, substrate etching is conducted to achieve channel depths of 0.5-10 ⁇ m for the micropump and 20-100 ⁇ m for sample handling. This substrate is placed into contact with the second (or top) substrate such that the surface of the second substrate is covering the channels of the first substrate. The two substrates are then bonded together to seal the enclosed channels.
- the micropump channels may be fabricated in the bottom substrate, while the rest of the microfluidic network of channels and chambers may be fabricated in the top substrate, made of the same or a different material as the bottom substrate. They come in contact with each other by proper alignment prior to bonding.
- the micropump may be fabricated in a substrate that is sandwiched between other two substrates.
- the walls of the pumping channels may be the bare, unmodified surface or the chemically/physically altered surface of the substrate material that is used for the fabrication of the device.
- chemical treatment with, or physical adsorption of an appropriate surface coating agent on the pumping channel walls may be necessary in order to provide for sufficient and adequate charged functional groups on these walls.
- the holes or the apertures necessary to access the pumping channel and the other channels of the microfabricated device are fabricated prior to bonding in one or both of the substrates. These holes (typically of 1-2 mm in diameter) are used for the introduction of fluids and reagents into the chip, and for the placement of electrodes that ensure electrical contact with the network of microfluidic channels. These holes can also function as reservoirs for the solvent and reagents that are to be manipulated on the chip. To increase the volume of solvent or reagents that may be handled by the chip, additional reservoir structures may be attached to the access holes. Cooling or heating devices may be attached to the pump for specific applications.
- the straight microchannels can be replace by a tortuous arrangement, or even a more complicated microfabricated monolithic structure.
- the electrodes may be embedded in the microfabricated device at appropriate positions. Embedded electrodes are fabricated using MEMS technologies prior to bonding of the two substrates.
- the inlet and outlet reservoirs may be placed not directly on the pump inlet or outlet, but at the terminus of some larger channels, with small electrical resistance, that come in direct contact with the inlet and outlet of the pumping channels.
- a structure with similar properties to the semi-permeable glass disc that is inserted in the outlet reservoir, can be created by MEMS technologies directly in the microchip body, at appropriate positions.
- This alternate gate structure can be used as fabricated, or it may be filled before utilization with an electrically conductive polymer that is replaceable. It will have the same function, to allow the exchange of ions but prevent transport of bulk flow.
- the walls of the pumping microchannels of one or both pumps may be chemically or physically altered such that their surface is positively charged in the case of one of the pumps and negatively charged in the case of the second pump.
- both pumps will generate electroosmotic flow in the same direction, but one will function under a positive voltage gradient, while the other will function under a negative voltage gradient.
- This configuration eliminates the need for the outlet reservoir 16 and the semi-permeable gate.
- the electrospraying or sample deposition capillary 32 may be replaced by a structure fabricated by MEMS technologies.
- microchannels are filled with solvent or buffer.
- the solvent or buffer (SA) in reservoir 104 is replaced with a different solvent or buffer (SB).
- a porous glass disc 34 is placed and secured in reservoir 16 and 106 .
- An identical voltage is applied to both reservoir 16 and 106 .
- Voltages are then applied to reservoir 14 and 104 in a ratio that will provide the desired solvent mixture.
- the ratio of the two voltages is modulated (in steps or continuously) to generate a desired solvent gradient that is delivered to the system.
- the generated flow or pressure is monitored or measured.
- a pump with a large number of narrow or shallow open microchannels is designed to produce EOF.
- the multiple microchannels ensure the generation of sufficient flow rate, while the small dimensions of the microchannels result in the necessary hydraulic resistance to pressurized back flow leakage.
- a single, large diameter open-channel electroosmotic micropump could generate flow only if the backpressure is small, and the flow would be highly dependent on the backpressure.
- multiple channel pump configurations with small diameters according to the invention overcome this shortcoming, being capable of generating sufficient flow that is independent of backpressure. The pumping principles are further explained below.
- Electroosmotic flow generation in single channels To illustrate the principle of the open-channel electroosmotic micropump, an ideal system composed of cylindrical capillaries is considered and the conditions that must be met for proper pumping are examined. For this discussion, the contributions of local hydraulic resistances to the total pressure drop will be neglected.
- the F eof will distribute into a forward flow through the large channel (F) and a back flow through the narrow channel (F b ).
- Pressurized fluid flow will be proportional to d 1 4 /d 2 4 regardless of the method used to produce it.
- the flow will be distributed in a ratio F/F b of 1:10,000, i.e., essentially all the flow will be directed through the large channel.
- F/F eof ratio seems to be dependent on channel dimensions, but independent of pressure or the actual EOF in the system.
- F/F eof could be defined as an efficiency coefficient for a given pump, since it will determine what fraction of the originally produced EOF is actually pumped forward in the system.
- F/F eof values can be calculated from equation (5) and represented as a function of d 1 /d 2 and L 1 /L 2 (FIG. 3B).
- small diameter and long pumping channels i.e., small d 1 /d 2 and large L 1 /L 2 ratios
- Electroosmotic flow generation in multiple channels For very small d, values, the actual EOF in a channel and consequently the overall flow in the system will be quite low.
- L 1 /L 2 0.001
- the F/F eof ratio drops from 0.9 to 0.09 when n is increased from 1 to 100.
- microchip implementation of fully integrated micro-LC separations performed on polymeric monolithic stationary phases that are commonly performed under 100 psi 39 thus becomes feasible with the integration of such pumping systems.
- pressure generation and not flow were the main purpose of these micropumps, and if microchip material and connecting elements would be capable of withstanding the high pressures, hunrdreds of bars could be produced with sub-micrometer sized channel EOF pumps.
- a stand-alone microfabricated device with an integrated multichannel EOF pump according to the invention has been constructed for producing controllable pressure driven flows within microfluidic channels.
- the pump can be constructed as a stand-alone module and can be employed to deliver fluid flow for on-chip or off-chip applications, as well.
- Micropumps with 4-100 pumping channels were constructed that produced flow rates in the range of 10-400 nL/min and developed pressures up to 80 psi. The flow rate and eluent gradients were adjusted by varying the potential drop over the pump. The experimental results followed closely the trends predicted by the theoretical calculations.
- the electroosmotic pump according to the invention may be used with any other valving component, and the electroosmotic valve of the invention may be used with any other pump or pressure generating element (in particular, an electroosmotic/electrokinetic pump.
- the output of a pump and/or valve of the invention may be monitored via connection to a flow or pressure monitoring/controlling sensor and/or controller.
- a cluster of pumps may be attached to an individual network channel for, e.g., gradient generation or the introduction of a sequence of solvents into the system.
- a microfluidic device with a multiple channel structure constructed according to the invention allows for material transport driven by any mechanism (electric, magnetic, etc.) other than a pressure driven mechanism.
- a microfluidic pump according to the invention can serve as an actuator for closing/opening of another valving component.
- the above microfluidic devices and pumps operate with aqueous and/or organic solutions, or other fluids (e.g., any liquid or gas).
Abstract
A microchip integrated microfabricated, microfluidic, multichannel, preferably electroosmotic pump and pumping system is disclosed. The electroosmotic pump of the invention comprises a plurality of microchannels, which begin and end in common compartments, complexed into an array. The microchannels within the pump have substantially identical, optimal dimensions of cross-section and length such that sufficient pressure for optimal flow of fluid (e.g., liquid or gas) and pressure is generated by the pump and flow rates are stable and reproducible. To effectuate efficient flow of fluid without the hindrance of backpressure, an electroosmotic pump of the invention is coupled to a single channel of a larger cross-section. A similar structure is also used in an electroosmotic valve of the invention, where samples are introduced into an analytical device. The microfluidic electroosmotic pumping system of the invention generates sufficient flow and pressures by optimizing the dimensional parameters of cross-section and length to the microchannels.
Description
- This application claims the benefit under 35 U.S.C. §119(e) of U.S. Provisional Patent Application No. 60/292,780, filed May 22, 2001, entitled MICROCHIP INTEGRATED OPEN-CHANNEL ELECTROOSMOTIC PUMPING SYSTEM, the whole of which is hereby incorporated by reference herein.
- [0002] Part of the work leading to this invention was carried out with United States Government support provided under a grant from the National Institutes of Health, Grant No. GM15847. Therefore, the U.S. Government has certain rights in this invention.
- Microfluidics and instrument miniaturization 1 have experienced significant growth in activity in recent years in response to the significant increase in use of microchips as bioanalytical tools. A major aspect of microfluidics refers to the manipulation of fluid flows in the microchip channels. For analytical processes, such as fluid valving,2,3 mixing,4 or electrically driven separations,2-5 flow streams are typically generated using electrical forces (electroosmotic flow, or EOF). However, EOF is dependent on the surface charge of the channel walls and consequently is sensitive to the physicochemical properties of the sample (pH, ionic strength, organic content). For example, the EOF can be easily reduced or even suppressed if the channel surface is altered by contact with specific sample types. Alternatively, for some techniques such as micro-liquid chromatography (μLC) or flow injection analysis (FIA), or for some samples, such as those containing cells that require zero electric field conditions for their manipulation, fluid flows on microchips may, or must, be generated by differential pressure.6-8 Typically, this is accomplished by connecting external devices to the microchip, e.g., vacuum, gas pressure generators or syringe pumps. However, the connection of external devices to microchips, while effective, increases the complexity of the system, and integration and multiplexing capabilities may be compromised.
- A number of micropumps have been described in the art. Mechanical pumps that use a membrane actuated by various forces (e.g., piezoelectric, electromagnetic, pneumatic) 9-15 are capable of pumping fluids of various physicochemical properties; however, the flow is pulsed, and the fabrication of the micropump is relatively complex. Non-mechanical pumps, with no moving parts, that operate on the basis of a large variety of principles (e.g., electrokinetic, centrifugal, electrohydrodynamic, etc.)16-29 have been implemented. The flow produced by these pumps is generally pulse free and varies from a few nL/min to hundreds of μL/min. However, the generated pressures are low, up to only a few psi. Fabrication procedures are often complex, as well.
- The use of electroosmosis for pressurized pumping was advanced almost forty years ago 30,31 and demonstrated later in open32 and packed16,17 capillary columns, and for open-channel microchip platforms.18-22 While packed electroosmotic pumps were shown to be capable of generating both flow and pressure, having packed particles or porous materials within the pump structure may, inter alia, impose limitations on the pumping channel size, may necessitate additional steps in pump fabrication and may affect reproducibility from one pump to another. The open-channel configurations were capable of producing flow but not sufficient pressure for the intended applications. Accordingly, it would be useful to have a microchip-integrated pumping device that simultaneously generates both sufficient flow and pressure for most analytical applications on a microchip and that is easy to fabricate, implement and use. Furthermore, an optimum pump design would ensure that a generated EOF is delivered to the analysis system and is not leaked out of the pump itself.
- The invention is directed to a microchip integrated microfabricated, microfluidic, multichannel, preferably electroosmotic pump and pumping system. The electroosmotic pump of the invention comprises a plurality of microchannels, which begin and end in common compartments, completed into an array. The microchannels within the pump have substantially identical, optimal dimensions of cross-section and length such that sufficient pressure for optimal flow of fluid (e.g., liquid or gas) and pressure is generated by the pump and flow rates are. stable and reproducible. In one aspect, the microchannels of the electroosmotic pump of the invention are open channels, i.e., not containing packed or porous materials. To effectuate efficient flow of fluid without the hindrance of backpressure, an electroosmotic pump of the invention is coupled to a single channel of a larger cross-section. A similar structure is also used in an electroosmotic valve of the invention, where samples are introduced into an analytical device. The microfluidic electroosmotic pumping system of the invention generates sufficient flow and pressures by optimizing the dimensional parameters of cross-section and length of the microchannels.
- Other features and advantages of the invention will be apparent from the following description of the preferred embodiments thereof and from the claims, taken in conjunction with the accompanying drawings, in which:
- FIG. 1 shows a schematic diagram of the microfabricated electroosmotic pumping system in accordance with the invention;
- FIG. 2 shows a schematic diagram of a different embodiment of the microfabricated electroosmotic pumping system in accordance with the invention;
- FIG. 3A shows representative flow, diameter and length in a schematic representation of a micropump with one pumping channel;
- FIG. 3B shows a graph of the flow distribution, or the F/F eof coefficients calculated for a micropump with one microchannel at various d1/d2 ratios;
- FIG. 4A shows a schematic representation of a micropump with n pumping channels;
- FIG. 4B shows a graph of the flow distribution, or the F/F eof coefficients calculated for a micropump with n microchannels at d1/d2=0.1;
- FIG. 5A shows a graph of a total forward flow generated in a pumping system with d 1/d2=0.1 and L1/L2=0.1 with varying number of pumping channels;
- FIG. 5B shows a graph of a total forward flow generated in a pumping system with d 1/d2=0.1 and L1/L2=0.001 with varying number of pumping channels;
- FIG. 6A shows a graph of achievable pressure as a function of the micropump channel diameter, for a restriction with d 2=30 μm diameter (L1=0.03 m, L2=30 m, U=3000 V);
- FIG. 6B shows a graph of achievable pressure as a function of the micropump channel diameter, for a restriction with d 2=50 μm diameter (L1=0.03 m, L2=30 m, U=3000 V);
- FIG. 7A shows a schematic diagram of an injection of a well delimited sample plug moving through the electroosmotic valve of the invention;
- FIG. 7B shows a schematic diagram of a sample plug being transported down the channel in the electroosmotic valve of the invention;
- FIG. 8A shows a schematic diagram of an electroosmotic pumping system functioning as an electroosmotic valve;
- FIG. 8B shows a schematic diagram of an electroosmotic pumping system functioning as an electroosmotic valve;
- FIG. 9 shows an exemplary multiplexed pumping system in accordance with the invention; and
- FIG. 10A and FIG. 10B show a cross-sectional view and a plan view, respectively, of a micropump according to the invention having filtering and gating elements.
- The microfabricated microfluidic pumping system of the invention comprises one or more miniaturized pumping units, which are capable of stable fluid delivery at flow rates and backpressures compatible with common analytical applications carried out on a microchip and sufficiently small to enable multiplexing of individual pumps. A multiple channel micropump based on electroosmotic pumping principles in accordance with the invention comprises a plurality of parallel, small cross-section microchannels (e.g., narrow or shallow) that deliver fluid to a microchannel of larger cross-section. This design allows the simultaneous generation of both flow and pressure and can be implemented in both packed channel and open-channel configuration. In the context of this invention, an open-channel means that no packed particles or porous materials are added to or contained in the microchannels. The pumping system of the invention offers numerous advantages. First, it can easily be integrated on microfluidic platforms and utilized for fluid propulsion on a microchip. Secondly, its fabrication using standard photolithographic and wet chemical etching technologies (or other microelectromechanical systems (MEMS) technologies) ensures high manufacturing and operating reproducibility. Thirdly, the simplicity of the design ensures robustness, reliability and trouble free operation.
- To illustrate the utility of an electroosmotic pumping system according to the invention, the micropump is designed to fit into an integrated microfluidic analysis scheme for delivery of, e.g., peptide samples for, e.g., electrospray ionization-mass spectrometry (ESI-MS) analysis. In addition, the ability to reproducibly control very low flow rates, at low and high pressure drops, where conventional systems do not perform well, enables the utilization of the micropump for many other micro-total analysis systems (μ-TAS) applications. The principle of μ-TAS is based on integrating all necessary parts and methods for analysis in miniaturized devices. The benefits of miniaturizing an analysis system include, but are not limited to, generation of multiple units at low cost; reduction in sample and reagent consumption and waste production; high throughput assays; small compact design; simple and reliable operation; and integration of several units or with existing systems.
- Generally, the micropump of the invention can be used to deliver fluid flows in electric field free regions and perform sample transfer, gradient generation, or fraction collection/deposition. A large variety of applications can be envisioned for a micropump according to the invention, including, inter alia, eluent flow and/or pressure generation for condensed phase separation techniques (e.g., micro liquid chromatography in open, packed, monolithic or microfabricated channels, pressure assisted capillary electrochromatography or capillary electrophoresis, isoelectric focusing, affinity chromatography, immunoassays); single, parallel or sequential sample delivery for electrospray ionization-mass spectrometry (ESI-MS), matrix assisted laser desorption ionization (MALDI)-MS, optical detectors, or other detection systems; single, parallel or sequential sample delivery systems to microreactors, mixers, preconcentrators, filters, or other functional elements integrated on the microchip; single, parallel or sequential eluent or sample delivery systems to off-chip applications; solvent gradient generation for on or off-chip applications; solvent and reagent delivery for flow injection analysis; sample or sample fraction generation/dispensing/collection from microfluidic systems; sample or sample fraction deposition on MALDI-MS targets; sample transfer/delivery to, or from, combinatorial libraries; sample delivery for medical applications (external or animal implanted devices); generation of microreactors or sample alteration devices by chemically or physically modifying the surface of the micropuming channel walls, while simultaneously maintaining pumping capabilities; generation of multiplexed devices for high throughput screening by integration of a series of pumps on one single microchip platform; and generation of pressure or vacuum in a liquid or gas phase within a microfluidic device, or an attached additional device.
- FIG. 1 illustrates a simplified schematic drawing of an exemplary microfabricated electroosmotic pumping system of the invention. A
pumping unit 10 can be incorporated on one microchip device. However, multiple pumping units can also be incorporated, e.g., in parallel as shown in FIG. 2 and FIG. 9. The microchip device may be about a few square millimeters, e.g., 5-50 mm2. A multiplexed device (see, e.g., FIG. 9) that may contain, for example, eight, sixteen, or even 96 individual pumps may require a larger size chip. Pumping channel system 11 of pumpingunit 10 comprises a plurality of first shallow microchannels 12 (e.g., 2-10,000 microchannels). All pumpingchannels 12 have first and second ends, which are in fluid communication with acommon inlet 14 and a common outlet compartment orreservoir 16, respectively. Electrodes inserted inreservoirs voltage power supply 30 and are used to apply a voltage drop across the pump. The pumping channels are exposed only to buffer solutions and do not come in contact with any sample that may be added to the microchip. The pump size is quite variable and depends on the application (e.g., 1×10, 2×10, 5×50, 1×50 mm). The length of each microchannel may range from, e.g., about 5-50 mm. The channel depth, width, equivalent diameter or cross-section may range from about less than 1 μm to a few micrometers (e.g., 0.5-10 μm). The number of microchannels in one pump may range from two to more than a thousand (e.g., 2-10,000 channels). The spacing between the microchannels is in the low micrometer range (e.g., 1-50 μm). In one aspect, the pump unit of the invention comprises 4 microchannels, 10 microchannels, 25 microchannels, 100 microchannels or 1000 microchannels. - The pumping (or valve) system of the invention is activated by introducing into the channels a buffer or solvent, appropriate for preserving or increasing the surface charge on the pumping channel walls. The
microchannels 12 can be filled by capillary action or by action of a pressure differential. Through electrodes placed in thereservoirs microchannels 12. Depending on the surface properties of the channel (whether negatively or positively charged), the larger voltage must be applied to the appropriate reservoir, such that the electroosmotic flow will have the desired direction. For example, for a glass micropump having uncoated pumping channel walls, by applying a 2000 V onreservoir 14 and connectingreservoir 16 to ground, electroosmotic flow will be generated fromreservoir 14 in the direction ofreservoir 16. Electroosmotic flow is created due to the following mechanism. The functional groups on the pumping microchannel walls ionize in the presence of the solvent/fluid that is filling the microchannels and attract a layer of oppositely charged ions. When the potential differential is applied, this layer of oppositely charged ions start to move in the direction of the appropriate electrode (negative electrode for positively charged ions), dragging along bulk fluid flow. If this electroosmotic flow is restricted from evolving freely by the structure of the system or by the rest of the microfluidic channel network on the chip, pressure will be created inside the microfluidic structure, and the electroosmotic flow will be distributed through all the channels of the device through a pressure controlled mechanism. By reversing the polarity on the electrodes inreservoir - The pumping system delivers fluid via a common outlet compartment, connected to
reservoir 16, to larger diametersecond microchannel 13, which may be connected to a network of channels or to other devices. As shown in FIGS. 1, 7 and 8,second microchannel 13 is connected to networkchannel 20 for, e.g., sample infusion or separation.Channel 20 compriseselectroosmotic valve 17 of the invention, which is used, e.g., for sample introduction. The electroosmotic valve is open to electroosmotic driven flows and essentially closed to pressure driven flows. At the other end of thenetwork channel 20, anelectrospray emitter 32, for example, or a detection or a sample collection device using any of the systems described above, can be integrated for analysis of a sample. - Sample injection in a microfabricated analysis systems is accomplished electrokinetically 2,3 or by pressure gradients,44 and sample manipulations are performed with electrokinetic forces. If sample manipulations are to be performed using pressure driven flows, a fluid valving system, such as described here, is necessary to prevent leakage of the fluid flow into the sample or sample waste reservoirs. According to the invention, electroosmotic valving is accomplished using very narrow channels for the sample injection and waste lines, in a configuration similar to that of the electooosmotic pump. In a pressurized analysis system, narrow injection channels45 can act as efficient valving components. As shown in FIG. 1, 7 and 8, in a valve according to the invention, a sample can be infused electroosmotically from
reservoir 22 toreservoir 24 through the sample and sample waste pluralities off channels, 26 and 28, respectively, and a double-T injector 18.46 The double-T injector can be an open or packed channel, e.g., as part of a preconcentration or μLC system. After completion of the injection, the pump can be started. For sample introduction and waste channels with similar dimensions to the pumping microchannels, additional pressure driven fluid loss through these channels could be negligible. Theoretically, if the number of injection and waste channels equals the number of pumping channels, the coefficient F/Feof would be unaffected by the injection/waste channels when d1/d2=0.01, and would drop to only 50% of its original value when d1/d2=0.1 (L1:L2=1:1000 in both cases). - In operation of the electroosmotic valve (as shown in FIG. 7A), all the appropriate channels and reservoirs of
valve 17 and pump 10 are first filled with an appropriate eluent. Sample is injected into thevalve inlet reservoir 22, and a voltage drop is established betweenreservoirs microchannel 20. The sample is electroosmotically carried fromreservoir 22 in the direction ofreservoir 24 by the application of a potential difference, e.g., 2000 V, betweenreservoir 22 andreservoir 24. A very small voltage drop (e.g., 100 V) betweenpump reservoir 14 andpump reservoir 16 may be needed on thepump unit 12 as well to help focus the injected plug in themicrochannel 20. - As depicted in FIG. 7B, for injecting the sample plug down the
microchannel 20 for analysis, a large voltage drop, e.g., 2000 V, betweenreservoir 14 andreservoir 16 is applied to the pumping unit 11. The electrodes inreservoirs reservoir 16, or they are removed from these reservoirs, in order to suppress the electroosmotic flow in the direction of the sample and sample waste reservoirs. The voltage drop across the pump will generate electroosmotic flow in the pump and pressurized flow in the rest of the system. The large hydraulic resistance in the sample inlet and outlet channels will prevent back pressure leakage, or will allow for only a small flow leakage through these channels. Consequently, the sample plug will be transported only down themicrochannel 20. - As shown in FIG. 8A, an electroosmotic pumping system composed of two parallel pumps can function, alternatively, as an electroosmotic valve, as well. One of the pumps, e.g., pump 10, is used for loading the sample, while the other pump, e.g., pump 100, is used for loading the eluent. Small, well delimited sample plugs, however, cannot be injected with this configuration.
- In FIG. 8B, for injecting the plug for analysis, a large voltage drop is applied across
pump 100, for example, 2000 V betweenreservoir 104 andreservoir 106. The voltage onreservoirs reservoir 106, or the electrodes are removed from these reservoirs. The voltage drop across thepump 100 will generate electroosmotic flow in this pump, and pressurized flow in the rest of the system. For properly designed pumping channel diameters, the pressurized leakage flow throughpump 12 will be minimal. - The potential differential for electroosmotic flow (EOF) generation is applied between the inlet and outlet reservoirs and may be provided by a power supply. Depending on the length of the pumping channels and the desired flow rate and pressure, the necessary voltage drop for the operation of the electroosmotic micropump may vary from a few tens to thousands of volts (e.g., 50-2000 V/cm).
- In some configurations, outlet compartment or
reservoir 16 can be common for systems with multiple pumps. Referring now to FIG. 10, a semi-permeable gate 34, that allows for an exchange of ions but not of the bulk eluent flow (liquid or gas), is created at the bottom of the outlet reservoir. The gate prevents EOF leakage in the direction of theexit electrode 35′ placed in the outlet reservoir. For example, a porous glass disc (e.g., 5 mm in diameter, 0.8-1 mm in width, and 40-50 Å pore size, prepared by Chang Associates (Worcester, Mass.)) can be used as the gate, but other materials may be used, e.g., graphite, polymeric organic or inorganic membrane materials. While most reservoirs on a microchip are made of glass,reservoir 16 is fabricated from a PEEK external nut. The porous glass disc is secured (e.g., between two gasket elements) at the bottom ofreservoir 16 with a corresponding internal nut. This arrangement provides for robustness and exchangeability of the porous glass disc. Since the pumping microchannels act as a filter, clogging of some of the channels can occur. To reduce this effect, a short filter element 36 (e.g., 100 μm in length), of the same size and density as the collection of pumping channels, can be introduced, preferably at each end of the pump.Filter elements 36 are spaced from the ends of the pumping channels byintermediate compartments 38 and terminate at end compartments 14′ and 16′, respectively. In the embodiment shown, end compartments 14′, 16′ are connected to orifaces 40 in the substrate, which make connection withreservoirs - The pump can be fabricated in a variety of substrate materials such as, inter alia, glass, quartz, silicon, polysilicon, polymeric materials (organic or inorganic), ceramic, or other materials. The micropumps can be made using microfabrication techniques, for example, photolithography and wet chemical etching, or other microelectromechanical systems (MEMS) technologies (e.g., dry etching, laser ablation, injection molding, embossing, stamping).
- Microfabricated devices that contain an electroosmotic micropump of the invention can be fabricated from one or two substrates made of any of the materials described above. The microfluidic channels can be made using one of the substrates described above by any of the microfabrication techniques. For example, micropump and sample handling channels defined on a photomask by 2 and 20 μm wide lines (other dimensions are possible) are transferred to the substrate using photolithography. After exposure, substrate etching is conducted to achieve channel depths of 0.5-10 μm for the micropump and 20-100 μm for sample handling. This substrate is placed into contact with the second (or top) substrate such that the surface of the second substrate is covering the channels of the first substrate. The two substrates are then bonded together to seal the enclosed channels. Alternatively, the micropump channels may be fabricated in the bottom substrate, while the rest of the microfluidic network of channels and chambers may be fabricated in the top substrate, made of the same or a different material as the bottom substrate. They come in contact with each other by proper alignment prior to bonding. Alternatively, the micropump may be fabricated in a substrate that is sandwiched between other two substrates.
- The walls of the pumping channels may be the bare, unmodified surface or the chemically/physically altered surface of the substrate material that is used for the fabrication of the device. In the case of certain substrate materials, chemical treatment with, or physical adsorption of an appropriate surface coating agent on the pumping channel walls, may be necessary in order to provide for sufficient and adequate charged functional groups on these walls.
- The holes or the apertures necessary to access the pumping channel and the other channels of the microfabricated device are fabricated prior to bonding in one or both of the substrates. These holes (typically of 1-2 mm in diameter) are used for the introduction of fluids and reagents into the chip, and for the placement of electrodes that ensure electrical contact with the network of microfluidic channels. These holes can also function as reservoirs for the solvent and reagents that are to be manipulated on the chip. To increase the volume of solvent or reagents that may be handled by the chip, additional reservoir structures may be attached to the access holes. Cooling or heating devices may be attached to the pump for specific applications.
- Alternatively, in order to increase the resistance to back flow leakage, the straight microchannels can be replace by a tortuous arrangement, or even a more complicated microfabricated monolithic structure.
- Alternatively, the electrodes may be embedded in the microfabricated device at appropriate positions. Embedded electrodes are fabricated using MEMS technologies prior to bonding of the two substrates.
- Alternatively, the inlet and outlet reservoirs may be placed not directly on the pump inlet or outlet, but at the terminus of some larger channels, with small electrical resistance, that come in direct contact with the inlet and outlet of the pumping channels.
- Alternatively, a structure (porous or multiple channel structure, etc.) with similar properties to the semi-permeable glass disc that is inserted in the outlet reservoir, can be created by MEMS technologies directly in the microchip body, at appropriate positions. This alternate gate structure can be used as fabricated, or it may be filled before utilization with an electrically conductive polymer that is replaceable. It will have the same function, to allow the exchange of ions but prevent transport of bulk flow.
- Alternatively, in the case of configurations with two pumping units, the walls of the pumping microchannels of one or both pumps may be chemically or physically altered such that their surface is positively charged in the case of one of the pumps and negatively charged in the case of the second pump. Thus, by applying a potential differential between the two inlet reservoirs, 14 and 104, both pumps will generate electroosmotic flow in the same direction, but one will function under a positive voltage gradient, while the other will function under a negative voltage gradient. This configuration eliminates the need for the
outlet reservoir 16 and the semi-permeable gate. - In another embodiment, the electrospraying or
sample deposition capillary 32 may be replaced by a structure fabricated by MEMS technologies. - As shown in FIG. 2, to apply an eluent gradient with multiple pumps, e.g., two
pumps reservoir 104 is replaced with a different solvent or buffer (SB). A porous glass disc 34 is placed and secured inreservoir reservoir reservoir - In the present invention, a pump with a large number of narrow or shallow open microchannels is designed to produce EOF. Advantageously, the multiple microchannels ensure the generation of sufficient flow rate, while the small dimensions of the microchannels result in the necessary hydraulic resistance to pressurized back flow leakage. In contrast, a single, large diameter open-channel electroosmotic micropump could generate flow only if the backpressure is small, and the flow would be highly dependent on the backpressure. 37 Properly designed, multiple channel pump configurations with small diameters according to the invention overcome this shortcoming, being capable of generating sufficient flow that is independent of backpressure. The pumping principles are further explained below.
- Electroosmotic flow generation in single channels. To illustrate the principle of the open-channel electroosmotic micropump, an ideal system composed of cylindrical capillaries is considered and the conditions that must be met for proper pumping are examined. For this discussion, the contributions of local hydraulic resistances to the total pressure drop will be neglected. The standard equations that describe fluid flow and velocity in pressure driven (F Δp and vΔp) and electroosmotic driven (Feof and Veof) open capillary systems are as follows:38
- where Δp =the pressure drop across a capillary of diameter d and length L, ρ=the viscosity of the fluid, ε o=the electrical permitivity of the vacuum, εr=the relative permitivity of the medium (or dielectric constant), ζ=the zeta potential at the capillary wall, and U=the voltage applied across the capillary of length L. From equations (1) and (3), it can be seen that FΔp (Poisseuille flow) and Feof (electroosmotic flow) are dependent on d4 and on d2, respectively, due to the fact that vΔp is dependent on d2, while veof is independent of d. Therefore, if fluid flow generation and distribution in a microfluidic structure occurs by both Δp and EOF, a net fluid flow to be induced in preferential directions is expected.
- Consider a system comprised of a single narrow channel (I) with dimensions d 1 and L1 connected to a large channel (II) with dimensions d2 and L2 (FIG. 3A). If a potential drop is applied only to the narrow channel to generate flow through an electroosmotic mechanism (i.e., ˜d1 2), the flow in both channels can be redistributed through a pressure-controlled mechanism (i.e., ˜d1 4 and d2 4). Pressure generation is due to the fact that the field free capillary (II) acts as a restrictor for the Feof that is produced in capillary (I) under the influence of the electric field. The Feof will distribute into a forward flow through the large channel (F) and a back flow through the narrow channel (Fb). Pressurized fluid flow will be proportional to d1 4/d2 4 regardless of the method used to produce it. Practically, for a ratio of d1/d2 of only 1:10 (L1 being equal to L2), the flow will be distributed in a ratio F/Fb of 1:10,000, i.e., essentially all the flow will be directed through the large channel.
- When both pressure and a potential gradient are applied to a capillary, the resulting flow is a sum of the Poiseuille and electroosmotic flows. 30 By balancing the electroosmotic input flow (Feof) given by equation (3) with the pressurized output flows (F and Fb) given by equation (1), it can be shown that the pressurized forward flow (F) in the large channel is dependent on the EOF in the narrow channel (Feof), according to the following equation:
- The F/F eof ratio seems to be dependent on channel dimensions, but independent of pressure or the actual EOF in the system. F/Feof could be defined as an efficiency coefficient for a given pump, since it will determine what fraction of the originally produced EOF is actually pumped forward in the system. F/Feof values can be calculated from equation (5) and represented as a function of d1/d2 and L1/L2 (FIG. 3B). As seen, in this figure, small diameter and long pumping channels (i.e., small d1/d2 and large L1/L2 ratios), result in large F/Feof values and a more efficient electroosmotic pump. For example, for d1/d2=0.1, a relatively effective pump is created, since the F/Feof coefficient decreases from 1 to only 0.9 even when the large channel is very long and imposes a considerable backpressure (L1/L2=0.001).
- Electroosmotic flow generation in multiple channels. For very small d, values, the actual EOF in a channel and consequently the overall flow in the system will be quite low. For a channel diameter of 1 μm, the EOF will be 0.17 nL/min [calculated from equation (3) for a capillary with L 1=0.03 m and U=3000 V, and for parameters given in reference 45: εo=8.85×10−12 C2 N−1 m−2, εr=80, ζ=50×10−3 V, and n=0.001 N s m−2]. In order to compensate for this low EOF, multiple small diameter channels (n) can be connected to one large channel (FIG. 4A). However, in this case, by balancing again the flows, F will be defined by equation (6), and F/Feof will be dependent on n:
- The larger n, the greater the total F eof, but F/Feof becomes smaller. F/Feof f values are represented as a function of n and L1/L2 for d1/d2=0.1 in FIG. 4B. For example, for d1/d2=0.1 and L1/L2=0.001, the F/Feof ratio drops from 0.9 to 0.09 when n is increased from 1 to 100.
- The optimum number (n) and dimensions (d 1 and L1) of the micropump channels that can generate the desired flow in the main channel can be calculated. If F/Feof is determined from equation (6) and multiply this value with the total EOF value calculated according to equation (3), diagrams that show the total pressure driven flow for given system characteristics (in this case d1/d2=0.1) can be constructed (FIG. 5). As expected, the total flow rate increases with the increase of d, and n. However, it is important to observe that when the backpressure increases significantly (i.e., L2 becomes much larger than L1), supplementing the pump with additional microchannels does not bring any additional benefit in increasing the flow rate (compare FIG. 5B and FIG. 5A). At high backpressures, the flow cannot be pumped, but rather will leak backwards in proportion to the number of channels n. The beneficial effect of increasing the number of microchannels requires properly chosen dimensions. At progressively smaller d1/d2 ratios, the back flow leakage becomes negligible. For example, as shown in Table 1, the F/Feof coefficients maintain a high and relatively constant value for a large range of pumping microchannels when d1/d2 drops to a value of 0.01. Based on the above considerations, and depending on the specific applications, micropumps with 100 to 1000 pumping channels of 1-3 μm in depth would perform as effective pumping systems on microfluidic platforms.
TABLE 1 Variation of F/Feof coefficient with d1/d2 and n (L1/L2 = 0.001) d1/d2 n = 1 n = 10 n = 100 n = 1000 n = 10000 1 0.001 1E−04 1E−5 1E−6 1E−7 0.1 0.909 0.5 0.091 0.009 0.001 0.01 0.999 0.999 0.999 0.990 0.910 -
- A plot of this pressure as a function of pumping channel diameter, for given system characteristics, is shown in FIG. 6. It is worth noting that the pressure depends on the number and dimensions of the pumping microchannels, the voltage applied to the pump, the zeta potential, and the relative permitivity of the medium. The pressure is, however, not dependent on the fluid viscosity. In a system with enhanced restriction, i.e., d 2=30 μm (FIG. 6A), the pressure will be higher than in a system with a lower restriction, i.e., d2=50 μm (FIG. 6B). The condition of maximum pressure:
-
- The absolute maximum pressure that can be generated with microchannels of a given dimension can be inferred from conditions of zero flow out from the system [.e., the second term in the denominator of equation (7) is set to zero], and is dependent only on the channel diameter, applied voltage and channel surface properties:
- For a pump that would be used for a LC separation, once the parameters of the separation column (d 2, L2) and the optimum eluent flow rate are determined, the pressure necessary to generate this flow can be calculated, and the appropriate pump configuration (n, d1, and L1) can be chosen from diagrams such as shown in FIGS. 5 or 6. For a packed column, d2 and L2 are the dimensions of an equivalent open tubular LC column that produces the same pressure drop. Alternatively, the d2 4/L2 ratio in the denominator of equation (7) could be replaced by a term that takes into consideration the porosity and the permeability of the column. The microchip implementation of fully integrated micro-LC separations performed on polymeric monolithic stationary phases that are commonly performed under 100 psi39 thus becomes feasible with the integration of such pumping systems. Theoretically, (see equation 10), if pressure generation and not flow were the main purpose of these micropumps, and if microchip material and connecting elements would be capable of withstanding the high pressures, hunrdreds of bars could be produced with sub-micrometer sized channel EOF pumps.
- Use
- A stand-alone microfabricated device with an integrated multichannel EOF pump according to the invention has been constructed for producing controllable pressure driven flows within microfluidic channels. If desired, the pump can be constructed as a stand-alone module and can be employed to deliver fluid flow for on-chip or off-chip applications, as well. Micropumps with 4-100 pumping channels were constructed that produced flow rates in the range of 10-400 nL/min and developed pressures up to 80 psi. The flow rate and eluent gradients were adjusted by varying the potential drop over the pump. The experimental results followed closely the trends predicted by the theoretical calculations.
- The new approach for microfluidic valving according to the invention, e.g., “electroosmotic valving,” in which sample plugs can be injected in pressurized systems, was tested for the investigation of peptide samples by MS analysis. The incorporation of an adsorbing medium for sample preconcentration in the double-T injector could prove useful in avoiding the electrophoretic bias effect, inherently associated with the use of electrokinetic forces for sample mobilization.
- Two examples of system design to accomplish specific results follow:
- Consider conducting a liquid chromatography separation in an open tubular capillary where the optimum separation parameters have been determined to be: d 2=20 μm,. L2=0.5 m, and F=100 nL/min. The required pump design that can generate this flow rate in a liquid chromatography column can be determined from plots such as shown in FIG. 4A, which was constructed for d1/d2=0.1 and L1/L2=0.1. A pump with d1=2 μm, L1=0.05 m, and n=200, would generate about 110 nL/min.
- Consider conducting a liquid chromatography separation in a column filled with monolith packing. The optimum separation parameters are: d 2=150 μm, L2=0.05 m, F=700 nL/min, and the necessary pressure to generate this flow rate through the monolithic packing is about 3.4 bar. The equivalent column length that would generate a 3.4 bar pressure drop, at 700 nL/min and d2=150 μm, is L2=362 m. The appropriate pump design can be chosen, again from plots such as shown in FIG. 4A, or can be directly calculated. For instance, a pump with d1=2 μm, L1=0.05 m, and n=2000, would generate a total Feof=1320 nL/min. For the selected parameters, one can determine d1/d2=0.013, L1/L2=1.38, and F/Feof =0.70. Consequently, F can be calculated. A flow of F=910 nL/min will be produced, which is enough to drive the separation. Alternatively, a more efficient pump (d1=1-1.5 μm) that would guarantee a larger F/Feof coefficient could be chosen, as well.
- In other embodiments, the electroosmotic pump according to the invention may be used with any other valving component, and the electroosmotic valve of the invention may be used with any other pump or pressure generating element (in particular, an electroosmotic/electrokinetic pump. The output of a pump and/or valve of the invention may be monitored via connection to a flow or pressure monitoring/controlling sensor and/or controller. A cluster of pumps may be attached to an individual network channel for, e.g., gradient generation or the introduction of a sequence of solvents into the system. A microfluidic device with a multiple channel structure constructed according to the invention allows for material transport driven by any mechanism (electric, magnetic, etc.) other than a pressure driven mechanism. Additionally, a microfluidic pump according to the invention can serve as an actuator for closing/opening of another valving component. The above microfluidic devices and pumps operate with aqueous and/or organic solutions, or other fluids (e.g., any liquid or gas).
- 1. Manz, A.; Graber, N.; Widmer, H. M. Sens. Actuators, 1990, B1, 244-248.
- 2. Harrison D. J.; Manz, A.; Fan, Z.; Lüdi, H.; Widmer, H. M. Anal. Chem. 1992, 64, 1926-1932.
- 3. Jacobson, S. C.; Hergenröder, R.; Koutny, L. B.; Warmack, R. J.; Ramsey, J. M. Anal. Chem. 1994, 66, 1107-1113.
- 4. Jacobson, S. C.; McKnight, T. E.; Ramsey, J. M. Anal. Chem. 1999, 71, 4455-4459.
- 5. Jacobson, S. C.; Hergenroder, R.; Koutny, L. B.; Ramsey, J. M. Anal. Chem. 1994, 66, 2369-2373.
- 6. McEnery, M.; Tan, A.; Alderman, J.; Patterson, J.; O'Mathuna, S. C.; Glennon, J. D. Analyst 2000, 125, 25-27.
- 7. Huang, Y.; Rubinsky, B. Biomedical Microdevices 1999, 2(2), 145-150.
- 8. Liu, H.; Felten, C.; Xue, Q; Zhang, B.; Jedrzjewski, P.;
- Karger, B. L.; Foret, F. Anal. Chem. 2000, 72, 3303-3310.
- 9. Francais, O.; Dufour, I. J. Micromech. Microeng. 2000, 10, 282-286.
- 10. Gong, Q.; Zhou, Z.; Yang, Y.; Wang, X. Sens. Actuators A 2000, 83, 200-207:
- 11. Accoto, D.; Carozza, M. C.; Dario, P. J. Micromech. Microeng. 2000, 10, 277-281.
- 12. Laurell, T.; Waliman, L.; Nilsson, J. J. Micromech. Microeng. 1999, 9, 369-376.
- 13. Kar, S.; McWhorter, S.; Ford, S. M.; Soper, S. A. Analyst 1998, 123, 1435-1441.
- 14. Jeong, O. C.; Yang, S. S. Sens. Actuators A 2000, 83, 249-255.
- 15. Handique, K.; Burke, D. T.; Mastrangelo, C. H.; Burns, M. A. Anal. Chem. 2001, 73 (8), 1831-1838.
- 16. Paul, P. H.; Arnold, D. W.; Rakestraw, D. J. Proceedings of the Micro Total Analysis Systems '98 Workshop, p. 49-52. Banff, Canada, 13-16 Oct., 1998.
- 17. Paul, P. H.; Arnold, D. W.; Neyer, D. W.; Smith, K. B. Proceedings of the Micro Total Analysis Systems 2000 Symposium, p. 583-590. Enschede, Netherlands, 14-18 May, 2000.
- 18. Lazar, I. M.; Ramsey, R. S.; Jacobson, S. C.; Foote, R. S.; Ramsey, J. M. J. Chromatogr. A 2000, 892, 195-201.
- 19. McKnight, T. E.; Culbertson, C. T.; Jacobson, S. C.; Ramsey, J. M. Anal. Chem. 2001, 73(16), 4045-4049.
- 20. Zeng, S.; Chen, C.-H.; Mikkelsen, J. C., Jr.; Santiago, J. C. Sensors and Actuators B, 2001, 79, 107-114.
- 21. Morf, W. E.; Guenat, O. T.; de Rooij, N. F. Sensors and Actuators B, 2001, 72, 266-272.
- 22. Guenat, O. T.; Ghiglione, D.; Morf, W. E.; de Rooij, N. F. Sensors and Actuators B, 2001, 72, 273-282.
- 23. McBride, S. E.; Moroney, R. M.; Chiang, W. Proceedings of the Micro Total Analysis Systems '98 Workshop, p. 45-48. Banff, Canada, 13-16 Oct., 1998.
- 24. Jang, J.; Lee, S. S. Sensors and
Actuators A 2000, 80, 84-89. - 25. Lemoff, A. V.; Lee, A. P. Sensors and Actuators B 2000, 63, 178-185.
- 26. Sammarco, T. S.; Burns, M. A. J. Micromech. Microeng. 2000, 10, 42-55.
- 27. Prins, M. W. J.; Welters, W. J. J.; Weekamp, J. W. Science, 2001, 291(12), 277-280.
- 28. Rife, J. C.; Bell, M. I.; Horwitz, J. S.; Kabler, M. N.; Auyeung, R. C. Y.; Kim, W. J. Sens. Actuators A 2000, 86, 135-140.
- 29. Duffy, D. C.; Gillis, H. L.; Lin, J.; Sheppard, N. F., Jr.;
- Kellogg, G. J. Anal. Chem. 1999, 71(20), 4669-4678.
- 30. Rice, C. L.; Whitehead, R. J. Phys. Chem. 1965, 69(11), 4017-4024.
- 31. Pretorius, V.; Hopkins, B. J.; Schieke, J. D. J. Chromatogr. 1974, 99, 23-30.
- 32. Dasgupta, P. K.; Liu, S. Anal. Chem. 1994, 66, 1792-1798.
- 33. Wallingford, R. A.; Ewing, A. G. Anal. Chem. 1987, 59, 1762-1766.
- 34. O'Shea, T. J.; Greenhagen, R. D.; Lunte, S. M.; Lunte, C. E.; Smyth, M. R.; Radzik, D. M.; Watanabe, N. J. Chromatogr. 1992, 593, 305-312.
- 35. Hu, S.; Wang, Z.-L.; Li, P.-B.; Cheng, J.-K. Anal. Chem. 1997, 69, 264-267.
- 3 6. Lazar, I. M.; Ramsey, R. S., Ramsey, J. M. Anal. Chem. 2001, 73, 1733-1739.
- 37. Parce, J. W., U.S. Pat. No. 6012902, 2000.
- 38. Knox, J. H.; Grant, I. H. Chromatographia, 1987, 24, 135-143.
- 39. Moore, R. E.; Licklider L.; Schumann, D.; Lee, T. D. Anal. Chem. 1998, 70, 4879-4884.
- 40. Stevens, T. S.; Cortes, H. J. Anal. Chem. 1983, 55, 1365-1370.
- 41. Kutter, J. P.; Jacobson, S. C.; Ramsey, J. M. Anal. Chem. 1997, 69, 5165-5171.
- 42. Jakeway, S. C.; de Mello, A. J.; Russell, E. L. Fresenius J. Anal. Chem. 2000, 366, 525-539.
- 43. He, B.; Burke, B. J.; Zhang, X.; Zhang, R.; Regnier, F. E. Anal. Chem. 2001, 73, 1942-1947.
- 44. Zhang, B.; Foret, F.; Karger, B. L. Anal. Chem. 2001, 73, 2675-2681.
- 45. Zhang, C.-X.; Manz, A. Anal. Chem. 2001, 73, 2656-2662.
- 46. Effenhauser, C. S.; Manz, A.; Widmer, H. M. Anal. Chem. 1993, 65, 2637-2642.
- While the present invention has been described in conjunction with a preferred embodiment, one of ordinary skill, after reading the foregoing specification, will be able to effect various changes, substitutions of equivalents, and other alterations to the compositions and methods set forth herein. It is therefore intended that the protection granted by Letters Patent hereon be limited only by the definitions contained in the appended claims and equivalents thereof.
Claims (20)
1. A microfluidic pump or valve device comprising:
a substrate, said substrate comprising:
a plurality of first microchannels, each of said first microchannels having a first and a second end, wherein said first ends of said first microchannels originate at a common inlet compartment and wherein said second ends of said first microchannels terminate at a common outlet compartment.
2. The microfluidic device of claim 1 , wherein said first microchannels are open-channeled.
3. The microfluidic device of claim 1 , wherein said plurality of first microchannels comprises at least 5 microchannels.
4. The microfluidic device of claim 1 , wherein said plurality of first microchannels comprises at least 50 microchannels.
5. The microfluidic device of claim 1 , wherein said plurality of first microchannels comprises at least 100 microchannels.
6. The microfluidic device of claim 1 , wherein said plurality of first microchannels comprises at least 1,000 microchannels.
7. The microfluidic device of claim 1 , wherein said plurality of first microchannels comprises at least 10,000 microchannels.
8. The microfluidic device of claim 1 , wherein said plurality of first microchannels are in a substantially parallel configuration.
9. The microfluidic device of claim 1 , wherein said plurality of first microchannels are in a tortuous configuration.
10. The microfluidic device of claim 1 , said device further comprising an electrode embedded in said common outlet compartment.
11. The microfluidic device of claim 1 , said device further comprising an electrode embedded in said common inlet compartment.
12. The microfluidic device of claim 1 , said device further comprising an oriface connecting said inlet compartment to a surface of said substrate.
13. The microfluidic device of claim 1 , said device further comprising an oriface connecting said outlet compartment to a surface of said substrate.
14. The microfluidic device of claim 1 , said device further comprising a second microchannel coupled to said outlet compartment, wherein said second microchannel is larger in cross-section than an individual said first microchannel.
15. A microfluidic system configured in a substrate, said system comprising:
(a) an electroosmotic pump comprising:
(i) a plurality of first microchannels fabricated in said substrate, each of said first microchannels having a first and a second end, wherein said first ends of said first microchannels originate at a common inlet compartment and wherein said second ends of said first microchannels terminate at a common outlet compartment; and
(ii) a voltage source coupled to said inlet and outlet compartments for said first microchannels;
(b) a second microchannel, said second microchannel having a larger cross-section than each of said plurality of first microchannels, said second microchannel having first and second ends, wherein said common outlet compartment of said first microchannels is in fluid communication with said first end of said second microchannel; and
(c) an electroosmotic valve comprising:
(i) two sets of a plurality of third microchannels fabricated on said substrate, said third microchannels each having a smaller cross-section than said said second channel, each of said third microchannels having a first and a second end, wherein said first ends of said first set of a plurality of third microchannels originate at a common inlet compartment, wherein said second ends of said first set of a plurality of third microchannels is in fluid communication with said second microchannel, wherein said second ends of said second set of a plurality of third microchannels is also in fluid communication with said second microchannel and wherein said first ends of said second set of a plurality of third microchannels terminate at a common outlet compartment; and
(vi) a voltage source coupled to said inlet and outlet compartments.
16. The microfluidic system system of claim 15 , wherein the ratio of the diameter of a said first microchannel to the diameter of said second microchannel is 1:1.25-1:1000.
17. The microfluidic system system of claim 15 , wherein said substrate material is selected from the group consisting of glass, quartz, silicon, polysilicon, polymeric materials (organic or inorganic) and ceramic.
18. The microfluidic system system of claim 15 , wherein said outlet compartment associated with said plurality of firsts microchannels comprises a semi-permeable gate.
19. The microfluidic system system of claim 15 , wherein said semi-permeable gate is selected from the group consisting of a porous glass, graphite, and polymeric organic or inorganic material.
20. A method of transporting a material for analysis through a microchannel in a microchip, said method comprising the steps of:
providing in said microchip a plurality of first microchannels and a second microchannel, wherein said second microchannel has a first and a second end, wherein the cross-section of each of said first microchannels is smaller than the cross-section of said second microchannel, wherein said first end of said second microchannel is in fluid communication with said plurality of first microchannels at one end of each and wherein said second end of said second microchannel is in communication with a detection system for analysis of a sample;
introducing a sample into said second microchannel; and
applying a potential difference across a length of said plurality of microchannels to electroosmotically move said sample in said second microchannel.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/478,612 US20040208751A1 (en) | 2001-05-22 | 2002-05-22 | Microchip integrated multi-channel electroosmotic pumping system |
Applications Claiming Priority (3)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US29278001P | 2001-05-22 | 2001-05-22 | |
| US10/478,612 US20040208751A1 (en) | 2001-05-22 | 2002-05-22 | Microchip integrated multi-channel electroosmotic pumping system |
| PCT/US2002/016207 WO2002094440A2 (en) | 2001-05-22 | 2002-05-22 | Microchip integrated multichannel electroosmotic pumping system |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040208751A1 true US20040208751A1 (en) | 2004-10-21 |
Family
ID=23126156
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US10/478,612 Abandoned US20040208751A1 (en) | 2001-05-22 | 2002-05-22 | Microchip integrated multi-channel electroosmotic pumping system |
Country Status (2)
| Country | Link |
|---|---|
| US (1) | US20040208751A1 (en) |
| WO (1) | WO2002094440A2 (en) |
Cited By (34)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004002878A2 (en) * | 2002-06-26 | 2004-01-08 | California Institute Of Technology | Microfluidic devices and methods with electrochemically actuated sample processing |
| US20040241004A1 (en) * | 2003-05-30 | 2004-12-02 | Goodson Kenneth E. | Electroosmotic micropump with planar features |
| US20050220681A1 (en) * | 2004-03-19 | 2005-10-06 | State of Oregon acting by and through the State Board of Higher Education on behalf of | Microchemical nanofactories |
| US20060193748A1 (en) * | 2002-06-26 | 2006-08-31 | Yu-Chong Tai | Integrated LC-ESI on a chip |
| US20060254913A1 (en) * | 2005-05-10 | 2006-11-16 | Myers Alan M | Orientation independent electroosmotic pump |
| WO2007034267A1 (en) * | 2005-07-07 | 2007-03-29 | Obshchestvo S Ogranichennoj Otvetstvennostyu 'institut Rentgenovskoj Optiki' | Electrokinetic micropump |
| US20070184576A1 (en) * | 2005-11-29 | 2007-08-09 | Oregon State University | Solution deposition of inorganic materials and electronic devices made comprising the inorganic materials |
| WO2008033021A1 (en) * | 2006-09-15 | 2008-03-20 | Stichting Voor De Technische Wetenschappen | Moving field capillary electrophoresis with sample plug dispersion compensation |
| US20080073506A1 (en) * | 2006-08-24 | 2008-03-27 | Lazar Iuliana M | Microfluidic devices and methods facilitating high-throughput, on-chip detection and separation techniques |
| US20080102119A1 (en) * | 2006-11-01 | 2008-05-01 | Medtronic, Inc. | Osmotic pump apparatus and associated methods |
| US20080108122A1 (en) * | 2006-09-01 | 2008-05-08 | State of Oregon acting by and through the State Board of Higher Education on behalf of Oregon | Microchemical nanofactories |
| US20080152509A1 (en) * | 2006-02-24 | 2008-06-26 | Hsueh-Chia Chang | Integrated micro-pump and electro-spray |
| US20100120260A1 (en) * | 2008-11-12 | 2010-05-13 | Jiou-Kang Lee | Multi-Step Process for Forming High-Aspect-Ratio Holes for MEMS Devices |
| US20100168457A1 (en) * | 2006-11-07 | 2010-07-01 | Waters Technologies Corporation | Monolithic electrokinetic pump fabrication |
| US20110005605A1 (en) * | 2008-04-09 | 2011-01-13 | Arkray, Inc. | Liquid delivery control method and liquid delivery control system |
| US20110023728A1 (en) * | 2009-07-29 | 2011-02-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Pasteurization system and method |
| US8137554B2 (en) | 2004-10-06 | 2012-03-20 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Microfluidic devices, particularly filtration devices comprising polymeric membranes, and method for their manufacture and use |
| US8236599B2 (en) | 2009-04-09 | 2012-08-07 | State of Oregon acting by and through the State Board of Higher Education | Solution-based process for making inorganic materials |
| CN103041879A (en) * | 2012-12-31 | 2013-04-17 | 苏州汶颢芯片科技有限公司 | Micro-fluidic chip for micro/nano liter quota-sampling and preparation method thereof |
| CN103071554A (en) * | 2012-12-31 | 2013-05-01 | 苏州汶颢芯片科技有限公司 | Microfluidic chip for circularly driving solution and preparation method thereof |
| US8501009B2 (en) | 2010-06-07 | 2013-08-06 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Fluid purification system |
| US8580161B2 (en) | 2010-05-04 | 2013-11-12 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Fluidic devices comprising photocontrollable units |
| US8603834B2 (en) | 2011-12-15 | 2013-12-10 | General Electric Company | Actuation of valves using electroosmotic pump |
| US8801922B2 (en) | 2009-06-24 | 2014-08-12 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Dialysis system |
| US9328969B2 (en) | 2011-10-07 | 2016-05-03 | Outset Medical, Inc. | Heat exchange fluid purification for dialysis system |
| US9402945B2 (en) | 2014-04-29 | 2016-08-02 | Outset Medical, Inc. | Dialysis system and methods |
| WO2016053936A3 (en) * | 2014-09-29 | 2016-09-22 | The University Of Florida Research Foundation, Inc. | Electro-fluid transducers |
| US9545469B2 (en) | 2009-12-05 | 2017-01-17 | Outset Medical, Inc. | Dialysis system with ultrafiltration control |
| US9599407B2 (en) | 2009-07-29 | 2017-03-21 | Tokitae Llc | System and structure for heating or sterilizing a liquid stream |
| US10046322B1 (en) | 2018-03-22 | 2018-08-14 | Talis Biomedical Corporation | Reaction well for assay device |
| US10820847B1 (en) | 2019-08-15 | 2020-11-03 | Talis Biomedical Corporation | Diagnostic system |
| US11045767B2 (en) * | 2012-03-29 | 2021-06-29 | Hoffmann-La Roche Inc. | Micro flow filtration system and integrated microfluidic element |
| US11534537B2 (en) | 2016-08-19 | 2022-12-27 | Outset Medical, Inc. | Peritoneal dialysis system and methods |
| US11951241B2 (en) | 2022-11-28 | 2024-04-09 | Outset Medical, Inc. | Peritoneal dialysis system and methods |
Families Citing this family (9)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| GB2382538C (en) * | 2001-09-20 | 2008-12-12 | Univ Hull | Device having a liquid flowpath |
| DE60312990T2 (en) * | 2002-08-02 | 2007-12-13 | GE Healthcare (SV) Corp., Sunnyvale | Integrated microchip design |
| NL1023404C2 (en) * | 2003-05-13 | 2004-11-16 | Berkin Bv | Device for controlling the mass flow rate of a liquid flow in a liquid channel. |
| FR2857099B1 (en) * | 2003-07-04 | 2005-10-07 | Jean Marie Billiotte | METHOD AND DEVICE FOR CHEMICAL OR BIOLOGICAL ANALYSIS BY MONOLITHIC CHAMBER SENSOR IN MULTI-MICRO-TUBULAR GERB AND LATERAL TRANSDUCER OF INTEGRAL MEASUREMENT |
| US7722817B2 (en) | 2003-08-28 | 2010-05-25 | Epocal Inc. | Lateral flow diagnostic devices with instrument controlled fluidics |
| US7084495B2 (en) | 2003-10-16 | 2006-08-01 | Intel Corporation | Electroosmotic pumps using porous frits for cooling integrated circuit stacks |
| US6992381B2 (en) | 2003-10-31 | 2006-01-31 | Intel Corporation | Using external radiators with electroosmotic pumps for cooling integrated circuits |
| JP4704351B2 (en) | 2003-11-06 | 2011-06-15 | ライフスキャン・インコーポレイテッド | Drug introduction pen with event notification means |
| CN102946988B (en) * | 2010-06-09 | 2015-04-15 | 英派尔科技开发有限公司 | Adjustable pressure microreactor |
Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4908112A (en) * | 1988-06-16 | 1990-03-13 | E. I. Du Pont De Nemours & Co. | Silicon semiconductor wafer for analyzing micronic biological samples |
| US5296375A (en) * | 1992-05-01 | 1994-03-22 | Trustees Of The University Of Pennsylvania | Mesoscale sperm handling devices |
| US5632876A (en) * | 1995-06-06 | 1997-05-27 | David Sarnoff Research Center, Inc. | Apparatus and methods for controlling fluid flow in microchannels |
| US6062261A (en) * | 1998-12-16 | 2000-05-16 | Lockheed Martin Energy Research Corporation | MicrofluIdic circuit designs for performing electrokinetic manipulations that reduce the number of voltage sources and fluid reservoirs |
| US6319469B1 (en) * | 1995-12-18 | 2001-11-20 | Silicon Valley Bank | Devices and methods for using centripetal acceleration to drive fluid movement in a microfluidics system |
| US6440645B1 (en) * | 1997-07-18 | 2002-08-27 | Cambridge Sensors Limited | Production of microstructures for use in assays |
| US6766817B2 (en) * | 2001-07-25 | 2004-07-27 | Tubarc Technologies, Llc | Fluid conduction utilizing a reversible unsaturated siphon with tubarc porosity action |
-
2002
- 2002-05-22 US US10/478,612 patent/US20040208751A1/en not_active Abandoned
- 2002-05-22 WO PCT/US2002/016207 patent/WO2002094440A2/en not_active Application Discontinuation
Patent Citations (7)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4908112A (en) * | 1988-06-16 | 1990-03-13 | E. I. Du Pont De Nemours & Co. | Silicon semiconductor wafer for analyzing micronic biological samples |
| US5296375A (en) * | 1992-05-01 | 1994-03-22 | Trustees Of The University Of Pennsylvania | Mesoscale sperm handling devices |
| US5632876A (en) * | 1995-06-06 | 1997-05-27 | David Sarnoff Research Center, Inc. | Apparatus and methods for controlling fluid flow in microchannels |
| US6319469B1 (en) * | 1995-12-18 | 2001-11-20 | Silicon Valley Bank | Devices and methods for using centripetal acceleration to drive fluid movement in a microfluidics system |
| US6440645B1 (en) * | 1997-07-18 | 2002-08-27 | Cambridge Sensors Limited | Production of microstructures for use in assays |
| US6062261A (en) * | 1998-12-16 | 2000-05-16 | Lockheed Martin Energy Research Corporation | MicrofluIdic circuit designs for performing electrokinetic manipulations that reduce the number of voltage sources and fluid reservoirs |
| US6766817B2 (en) * | 2001-07-25 | 2004-07-27 | Tubarc Technologies, Llc | Fluid conduction utilizing a reversible unsaturated siphon with tubarc porosity action |
Cited By (62)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| WO2004002878A3 (en) * | 2002-06-26 | 2007-04-26 | California Inst Of Techn | Microfluidic devices and methods with electrochemically actuated sample processing |
| US20040124085A1 (en) * | 2002-06-26 | 2004-07-01 | California Institute Of Technology | Microfluidic devices and methods with electrochemically actuated sample processing |
| US7976779B2 (en) * | 2002-06-26 | 2011-07-12 | California Institute Of Technology | Integrated LC-ESI on a chip |
| US20060193748A1 (en) * | 2002-06-26 | 2006-08-31 | Yu-Chong Tai | Integrated LC-ESI on a chip |
| WO2004002878A2 (en) * | 2002-06-26 | 2004-01-08 | California Institute Of Technology | Microfluidic devices and methods with electrochemically actuated sample processing |
| US20040241004A1 (en) * | 2003-05-30 | 2004-12-02 | Goodson Kenneth E. | Electroosmotic micropump with planar features |
| US7316543B2 (en) * | 2003-05-30 | 2008-01-08 | The Board Of Trustees Of The Leland Stanford Junior University | Electroosmotic micropump with planar features |
| US20050220681A1 (en) * | 2004-03-19 | 2005-10-06 | State of Oregon acting by and through the State Board of Higher Education on behalf of | Microchemical nanofactories |
| US7507380B2 (en) * | 2004-03-19 | 2009-03-24 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Microchemical nanofactories |
| US8273245B2 (en) | 2004-10-06 | 2012-09-25 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Microfluidic devices, particularly filtration devices comprising polymeric membranes, and methods for their manufacture and use |
| US8137554B2 (en) | 2004-10-06 | 2012-03-20 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Microfluidic devices, particularly filtration devices comprising polymeric membranes, and method for their manufacture and use |
| US20060254913A1 (en) * | 2005-05-10 | 2006-11-16 | Myers Alan M | Orientation independent electroosmotic pump |
| US7645368B2 (en) | 2005-05-10 | 2010-01-12 | Intel Corporation | Orientation independent electroosmotic pump |
| WO2007034267A1 (en) * | 2005-07-07 | 2007-03-29 | Obshchestvo S Ogranichennoj Otvetstvennostyu 'institut Rentgenovskoj Optiki' | Electrokinetic micropump |
| US20070184576A1 (en) * | 2005-11-29 | 2007-08-09 | Oregon State University | Solution deposition of inorganic materials and electronic devices made comprising the inorganic materials |
| US8679587B2 (en) | 2005-11-29 | 2014-03-25 | State of Oregon acting by and through the State Board of Higher Education action on Behalf of Oregon State University | Solution deposition of inorganic materials and electronic devices made comprising the inorganic materials |
| US20080152509A1 (en) * | 2006-02-24 | 2008-06-26 | Hsueh-Chia Chang | Integrated micro-pump and electro-spray |
| US20080073506A1 (en) * | 2006-08-24 | 2008-03-27 | Lazar Iuliana M | Microfluidic devices and methods facilitating high-throughput, on-chip detection and separation techniques |
| US7744762B2 (en) * | 2006-08-24 | 2010-06-29 | Virginia Tech Intellectual Properties, Inc. | Microfluidic devices and methods facilitating high-throughput, on-chip detection and separation techniques |
| US20080108122A1 (en) * | 2006-09-01 | 2008-05-08 | State of Oregon acting by and through the State Board of Higher Education on behalf of Oregon | Microchemical nanofactories |
| WO2008033021A1 (en) * | 2006-09-15 | 2008-03-20 | Stichting Voor De Technische Wetenschappen | Moving field capillary electrophoresis with sample plug dispersion compensation |
| US20080102119A1 (en) * | 2006-11-01 | 2008-05-01 | Medtronic, Inc. | Osmotic pump apparatus and associated methods |
| US20110184389A1 (en) * | 2006-11-01 | 2011-07-28 | Medtronic, Inc. | Osmotic pump apparatus and associated methods |
| US8680311B2 (en) | 2006-11-07 | 2014-03-25 | Waters Technologies Corporation | Monolithic electrokinetic pump fabrication |
| US20100168457A1 (en) * | 2006-11-07 | 2010-07-01 | Waters Technologies Corporation | Monolithic electrokinetic pump fabrication |
| US20110005605A1 (en) * | 2008-04-09 | 2011-01-13 | Arkray, Inc. | Liquid delivery control method and liquid delivery control system |
| US7923379B2 (en) | 2008-11-12 | 2011-04-12 | Taiwan Semiconductor Manufacturing Company, Ltd. | Multi-step process for forming high-aspect-ratio holes for MEMS devices |
| US20100120260A1 (en) * | 2008-11-12 | 2010-05-13 | Jiou-Kang Lee | Multi-Step Process for Forming High-Aspect-Ratio Holes for MEMS Devices |
| US8236599B2 (en) | 2009-04-09 | 2012-08-07 | State of Oregon acting by and through the State Board of Higher Education | Solution-based process for making inorganic materials |
| US8801922B2 (en) | 2009-06-24 | 2014-08-12 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Dialysis system |
| US9930898B2 (en) * | 2009-07-29 | 2018-04-03 | Tokitae Llc | Pasteurization system and method |
| US9599407B2 (en) | 2009-07-29 | 2017-03-21 | Tokitae Llc | System and structure for heating or sterilizing a liquid stream |
| US20110023728A1 (en) * | 2009-07-29 | 2011-02-03 | Searete Llc, A Limited Liability Corporation Of The State Of Delaware | Pasteurization system and method |
| US9545469B2 (en) | 2009-12-05 | 2017-01-17 | Outset Medical, Inc. | Dialysis system with ultrafiltration control |
| US8580161B2 (en) | 2010-05-04 | 2013-11-12 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Fluidic devices comprising photocontrollable units |
| US8524086B2 (en) | 2010-06-07 | 2013-09-03 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Fluid purification system |
| US11724013B2 (en) | 2010-06-07 | 2023-08-15 | Outset Medical, Inc. | Fluid purification system |
| US9138687B2 (en) | 2010-06-07 | 2015-09-22 | Oregon State University | Fluid purification system |
| US10668201B2 (en) | 2010-06-07 | 2020-06-02 | Oregon State University | Dialysis system |
| US10105476B2 (en) | 2010-06-07 | 2018-10-23 | Oregon State University | Fluid purification system |
| US9895480B2 (en) | 2010-06-07 | 2018-02-20 | Oregon State University | Dialysis system |
| US8501009B2 (en) | 2010-06-07 | 2013-08-06 | State Of Oregon Acting By And Through The State Board Of Higher Education On Behalf Of Oregon State University | Fluid purification system |
| US9328969B2 (en) | 2011-10-07 | 2016-05-03 | Outset Medical, Inc. | Heat exchange fluid purification for dialysis system |
| US8603834B2 (en) | 2011-12-15 | 2013-12-10 | General Electric Company | Actuation of valves using electroosmotic pump |
| US9188113B2 (en) | 2011-12-15 | 2015-11-17 | General Electric Company | Actuation of valves using electroosmotic pump |
| US11045767B2 (en) * | 2012-03-29 | 2021-06-29 | Hoffmann-La Roche Inc. | Micro flow filtration system and integrated microfluidic element |
| CN103041879A (en) * | 2012-12-31 | 2013-04-17 | 苏州汶颢芯片科技有限公司 | Micro-fluidic chip for micro/nano liter quota-sampling and preparation method thereof |
| CN103071554A (en) * | 2012-12-31 | 2013-05-01 | 苏州汶颢芯片科技有限公司 | Microfluidic chip for circularly driving solution and preparation method thereof |
| US9402945B2 (en) | 2014-04-29 | 2016-08-02 | Outset Medical, Inc. | Dialysis system and methods |
| US9579440B2 (en) | 2014-04-29 | 2017-02-28 | Outset Medical, Inc. | Dialysis system and methods |
| US9504777B2 (en) | 2014-04-29 | 2016-11-29 | Outset Medical, Inc. | Dialysis system and methods |
| US11305040B2 (en) | 2014-04-29 | 2022-04-19 | Outset Medical, Inc. | Dialysis system and methods |
| US10378565B2 (en) | 2014-09-29 | 2019-08-13 | University Of Florida Research Foundation, Inc. | Electro-fluid transducers |
| WO2016053936A3 (en) * | 2014-09-29 | 2016-09-22 | The University Of Florida Research Foundation, Inc. | Electro-fluid transducers |
| US10788062B2 (en) | 2014-09-29 | 2020-09-29 | University Of Florida Research Foundation, Inc. | Electro-fluid transducers |
| US11534537B2 (en) | 2016-08-19 | 2022-12-27 | Outset Medical, Inc. | Peritoneal dialysis system and methods |
| US10046322B1 (en) | 2018-03-22 | 2018-08-14 | Talis Biomedical Corporation | Reaction well for assay device |
| US11633736B2 (en) | 2018-03-22 | 2023-04-25 | Talis Biomedical Corporation | Optical reaction well for assay device |
| US10618047B2 (en) | 2018-03-22 | 2020-04-14 | Talis Biomedical Corporation | Reaction well for assay device |
| US11008627B2 (en) | 2019-08-15 | 2021-05-18 | Talis Biomedical Corporation | Diagnostic system |
| US10820847B1 (en) | 2019-08-15 | 2020-11-03 | Talis Biomedical Corporation | Diagnostic system |
| US11951241B2 (en) | 2022-11-28 | 2024-04-09 | Outset Medical, Inc. | Peritoneal dialysis system and methods |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2002094440A2 (en) | 2002-11-28 |
| WO2002094440A3 (en) | 2003-03-13 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US20040208751A1 (en) | Microchip integrated multi-channel electroosmotic pumping system | |
| JP5124054B2 (en) | Microfluidic devices and systems incorporating protective layers | |
| US20020166592A1 (en) | Apparatus and method for small-volume fluid manipulation and transportation | |
| Lazar et al. | Multiple open-channel electroosmotic pumping system for microfluidic sample handling | |
| US6488897B2 (en) | Microfluidic devices and systems incorporating cover layers | |
| US20040124085A1 (en) | Microfluidic devices and methods with electrochemically actuated sample processing | |
| US5644395A (en) | Miniaturized flow injection analysis system | |
| US7497994B2 (en) | Microfluidic devices and systems incorporating cover layers | |
| US6756019B1 (en) | Microfluidic devices and systems incorporating cover layers | |
| US6752922B2 (en) | Microfluidic chromatography | |
| US20050189225A1 (en) | Apparatus and method for small-volume fluid manipulation and transportation | |
| US20060147344A1 (en) | Fully packed capillary electrophoretic separation microchips with self-assembled silica colloidal particles in microchannels and their preparation methods | |
| JP2005537923A (en) | Mounting of microfluidic components in a microfluidic system | |
| US20050224351A1 (en) | Microfluidic devices for introducing and dispensing fluids from microfluidic systems | |
| US20100322825A1 (en) | Microfluidic molecular-flow fractionator and bioreactor with integrated active/passive diffusion barrier | |
| EP1997772A2 (en) | Method of fabrication of a microfluidic device | |
| US20010035351A1 (en) | Cross channel device for serial sample injection | |
| US20030057092A1 (en) | Microfluidic methods, devices and systems for in situ material concentration | |
| KR100865634B1 (en) | Thermopneumatic capillary micropump and manufacturing method thereof | |
| US20050011761A1 (en) | Microfluidic methods, devices and systems for in situ material concentration | |
| US20090041590A1 (en) | Apparatus, system, and method for electrochemical pump-based chromatography separations in microfabricated devices | |
| US11717830B2 (en) | Open microfluidic system and various functional arrangements therefore | |
| Willis et al. | Microchip capillary electrophoresis for in situ planetary exploration | |
| Sweedler | DOE-FG02-00ER62797 Final Report |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: NORTHEASTERN UNIVERSITY, MASSACHUSETTS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNORS:LAZAR, IULIANA;KARGER, BARRY L.;REEL/FRAME:016297/0657;SIGNING DATES FROM 20040113 TO 20040115 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |
|
| AS | Assignment |
Owner name: NATIONAL INSTITUTES OF HEALTH (NIH), U.S. DEPT. OF Free format text: CONFIRMATORY LICENSE;ASSIGNOR:NORTHEASTERN UNIVERSITY;REEL/FRAME:026833/0723 Effective date: 20110818 |