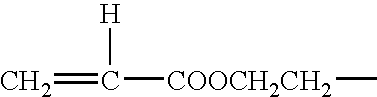US20040054106A1 - Ocular lens material and process for producing the same - Google Patents
Ocular lens material and process for producing the same Download PDFInfo
- Publication number
- US20040054106A1 US20040054106A1 US10/644,776 US64477603A US2004054106A1 US 20040054106 A1 US20040054106 A1 US 20040054106A1 US 64477603 A US64477603 A US 64477603A US 2004054106 A1 US2004054106 A1 US 2004054106A1
- Authority
- US
- United States
- Prior art keywords
- group
- ocular lens
- lens material
- siloxane
- carbon atoms
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 [3*][Si]([4*])([2*]O[1*]C)O[Si]([5*])([6*])O[Si]([7*])([8*])[2*]O[1*]C Chemical compound [3*][Si]([4*])([2*]O[1*]C)O[Si]([5*])([6*])O[Si]([7*])([8*])[2*]O[1*]C 0.000 description 13
- ZRTPILQCURMQTA-UHFFFAOYSA-N [H]N(CC1(C)CC(N([H])C(=O)OC2COC(=O)C2([H])=C)CC(C)(C)C1)C(=O)OC Chemical compound [H]N(CC1(C)CC(N([H])C(=O)OC2COC(=O)C2([H])=C)CC(C)(C)C1)C(=O)OC ZRTPILQCURMQTA-UHFFFAOYSA-N 0.000 description 3
- WRSMCUOUYLOXNO-UHFFFAOYSA-N C.C.C.C.C.C.CCCOCCC[Si](C)(C)[Si](C)(C)(=O)O1O2(C3(CCCOCC3)[Si]2(C)C)[Si]1(C)CCC(F)(F)F Chemical compound C.C.C.C.C.C.CCCOCCC[Si](C)(C)[Si](C)(C)(=O)O1O2(C3(CCCOCC3)[Si]2(C)C)[Si]1(C)CCC(F)(F)F WRSMCUOUYLOXNO-UHFFFAOYSA-N 0.000 description 1
- YYMMGIFHTQETMF-UHFFFAOYSA-N C=CC1=CC=CC=C1.CC.CC Chemical compound C=CC1=CC=CC=C1.CC.CC YYMMGIFHTQETMF-UHFFFAOYSA-N 0.000 description 1
- AGINNBJBZIUVDC-UHFFFAOYSA-N CC1=CC2=NN(C3=CC(C)=CC(C)=C3O)N=C2C=C1 Chemical compound CC1=CC2=NN(C3=CC(C)=CC(C)=C3O)N=C2C=C1 AGINNBJBZIUVDC-UHFFFAOYSA-N 0.000 description 1
- PNXMTCDJUBJHQJ-UHFFFAOYSA-N [H]C(=C)C(=O)OCCC Chemical compound [H]C(=C)C(=O)OCCC PNXMTCDJUBJHQJ-UHFFFAOYSA-N 0.000 description 1
- NYXHSRNBKJIQQG-UHFFFAOYSA-N [H]N(C)C(=O)OC Chemical compound [H]N(C)C(=O)OC NYXHSRNBKJIQQG-UHFFFAOYSA-N 0.000 description 1
Classifications
-
- G—PHYSICS
- G02—OPTICS
- G02C—SPECTACLES; SUNGLASSES OR GOGGLES INSOFAR AS THEY HAVE THE SAME FEATURES AS SPECTACLES; CONTACT LENSES
- G02C7/00—Optical parts
- G02C7/02—Lenses; Lens systems ; Methods of designing lenses
- G02C7/04—Contact lenses for the eyes
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/18—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F218/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid or of a haloformic acid
- C08F218/02—Esters of monocarboxylic acids
- C08F218/04—Vinyl esters
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F283/00—Macromolecular compounds obtained by polymerising monomers on to polymers provided for in subclass C08G
- C08F283/12—Macromolecular compounds obtained by polymerising monomers on to polymers provided for in subclass C08G on to polysiloxanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F290/00—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups
- C08F290/02—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups on to polymers modified by introduction of unsaturated end groups
- C08F290/06—Polymers provided for in subclass C08G
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F290/00—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups
- C08F290/02—Macromolecular compounds obtained by polymerising monomers on to polymers modified by introduction of aliphatic unsaturated end or side groups on to polymers modified by introduction of unsaturated end groups
- C08F290/06—Polymers provided for in subclass C08G
- C08F290/068—Polysiloxanes
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F8/00—Chemical modification by after-treatment
- C08F8/12—Hydrolysis
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L51/00—Compositions of graft polymers in which the grafted component is obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers
- C08L51/08—Compositions of graft polymers in which the grafted component is obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers grafted on to macromolecular compounds obtained otherwise than by reactions only involving unsaturated carbon-to-carbon bonds
- C08L51/085—Compositions of graft polymers in which the grafted component is obtained by reactions only involving carbon-to-carbon unsaturated bonds; Compositions of derivatives of such polymers grafted on to macromolecular compounds obtained otherwise than by reactions only involving unsaturated carbon-to-carbon bonds on to polysiloxanes
-
- G—PHYSICS
- G02—OPTICS
- G02B—OPTICAL ELEMENTS, SYSTEMS OR APPARATUS
- G02B1/00—Optical elements characterised by the material of which they are made; Optical coatings for optical elements
- G02B1/04—Optical elements characterised by the material of which they are made; Optical coatings for optical elements made of organic materials, e.g. plastics
- G02B1/041—Lenses
- G02B1/043—Contact lenses
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F218/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and at least one being terminated by an acyloxy radical of a saturated carboxylic acid, of carbonic acid or of a haloformic acid
- C08F218/02—Esters of monocarboxylic acids
- C08F218/04—Vinyl esters
- C08F218/08—Vinyl acetate
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08F—MACROMOLECULAR COMPOUNDS OBTAINED BY REACTIONS ONLY INVOLVING CARBON-TO-CARBON UNSATURATED BONDS
- C08F230/00—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and containing phosphorus, selenium, tellurium or a metal
- C08F230/04—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and containing phosphorus, selenium, tellurium or a metal containing a metal
- C08F230/08—Copolymers of compounds having one or more unsaturated aliphatic radicals, each having only one carbon-to-carbon double bond, and containing phosphorus, selenium, tellurium or a metal containing a metal containing silicon
Definitions
- the present invention relates to an ocular lens material and a process for producing the same. More particularly, the present invention relates to an ocular lens material suitably used for, for example, soft contact lens and soft intraocular lens, which is excellent not only in lipid-stain resistance, wettability and oxygen permeability, but also in flexibility, particularly, shape recovery at the same time, and a process for producing the ocular lens material.
- a contact lens comprising a copolymer obtained by polymerizing siloxanyl (meth)acrylate and vinyl ester is disclosed in Japanese Unexamined Patent Publication No. 163811/1988
- a contact lens comprising a copolymer obtained by polymerizing siloxanyl (meth)acrylate, a vinyl ester, and vinyl (meth)acrylate and/or allyl (meth)acrylate is disclosed in Japanese Unexamined Patent Publication No. 301919/1988.
- a water-containing contact lens obtained by saponifying a polymer comprising a (meth)acrylate polymer, which has at least one polymerizable group on average, a vinyl monomer, a vinyl ester of fatty acid and a crosslinkable monomer is disclosed in Japanese Unexamined Patent Publication No. 102471/1994, and a silicone-containing hydrogel material formed from a polymer prepared by using a monomer mixture comprising a polysiloxane pre-polymer, a bulk polysiloxanylalkyl (meth)acrylate monomer and a hydrophilic monomer is disclosed in Japanese Unexamined Patent Publication No. 508063/1995. Also, in International Publication No.
- an ocular lens material obtained by hydrating, by using a water-soluble organic solvent, a polymer prepared by solution polymerization of a polymerizable silicon-containing compound and/or a polymerizable fluorine-containing compound, hydroxyalkyl (meth)acrylate, and a cross-linked compound is disclosed.
- contact lenses and materials have properties such as high oxygen permeability, lipid-stain resistance and wettability, they only have one or a plurality of such properties, and do not show flexibility, particularly shape recovery, which is one of the important properties required for an ocular lens material, in particular, a soft ocular lens material.
- An object of the present invention is to provide an ocular lens material which has not only high oxygen permeability, excellent wettability and superior lipid-stain resistance, but also flexibility, particularly shape recovery at the same time, and an easy process for producing the ocular lens material.
- the present invention relates to
- an ocular lens material comprising a siloxane-containing polymer obtained by polymerizing a monomer mixture containing
- an ocular lens material comprising a polymer prepared by saponifying a siloxane-containing polymer obtained by polymerizing a monomer mixture containing
- the ocular lens material of the present invention comprises a siloxane-containing polymer obtained by polymerizing a monomer mixture containing (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and (B) a vinyl ester of lower fatty acid as essential components.
- the siloxane macromonomer (A) is a component which mainly imparts flexibility as typified by shape recovery and mechanical strength to the ocular lens material I.
- the active unsaturated group in the siloxane macromonomer (A) is an active unsaturated group which can be subjected to radical polymerization.
- the active unsaturated group are (meth)acryloyl group, vinyl group, allyl group, (meth)acryloyloxy group, vinyl carbamate group and the like.
- acryloyloxy group and vinyl group are preferable from the viewpoint that they can impart excellent flexibility to the ocular lens material I and copolymerizability with other monomers is excellent.
- (meth)acryl - - - means “acryl - - - and/or methacryl - - - ”.
- a number average molecular weight of the siloxane macromonomer (A) is at least 2,000, preferably at least 2,500 and more preferably at least 3,000 in order to impart excellent flexibility to the ocular lens material I without increasing hardness extremely. Also, it is desired that a number average molecular weight of the siloxane macromonomer (A) is at most 100,000, preferably at most 50,000, more preferably at most 10,000 not to make shape recovery inferior though the ocular lens material I becomes softened.
- siloxane macromonomer (A) for example, the macromonomer of dialkyl siloxane having an active unsaturated group described in U.S. Pat. No. 4,189,546 specification can be used to obtain the aimed ocular lens material I.
- siloxane macromonomer (A) used in the present invention a siloxane macromonomer having a urethane group represented by the formula:
- the siloxane macromonomer (A) contains the above urethane group, suitable mechanical strength and excellent wettability can be imparted to the ocular lens material I. It is desired that the number of the urethane group in the siloxane macromonomer (A) is at least 2, preferably at least 4 on average in order to impart sufficient mechanical strength and wettability. On the other hand, when too many urethane groups are introduced, flexibility of the ocular lens material I decreases. Therefore, it is desired that the average number of the urethane group in the siloxane macromonomer (A) is at most 20, preferably at most 14.
- the macromonomer to which hydrophilic parts are introduced at the both ends of the siloxane structure described in U.S. Pat. No. 4,495,361 specification or U.S. Pat. No. 5,807,944 specification can also be used as the siloxane macromonomer (A) in order to obtain the ocular lens material I whose flexibility, particularly shape recovery, is improved.
- siloxane macromonomer (A) a macromonomer represented by the formula (I-1) is preferably used:
- each of A 1 and A 2 is independently an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms;
- U 1 is a diurethane type group which contains urethane bonds formed with adjacent A 1 and adjacent S 1 on both sides or which contains urethane bonds with adjacent two S 1 on both sides;
- U 2 is a diurethane type group which contains urethane bonds formed with adjacent A 1 and adjacent S 2 on both sides or which contains urethane bonds with adjacent S 1 and adjacent S 2 on both sides;
- U 3 is a diurethane type group which contains urethane bonds formed with adjacent S 2 and adjacent A 2 on both side;
- each of S 1 and S 2 is independently a group represented by the formula:
- each of R 1 and R 2 is independently an alkylene group having 1 to 20 carbon atoms
- each of R 3 , R 4 , R 5 , R 6 , R 7 and R 8 is independently a linear, branched or cyclic alkyl group having 1 to 20 carbon atoms, which may be substituted with a fluorine atom, or a group represented by the formula:
- a 3 is an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms
- U 4 is a urethane type group which contains urethane bonds formed with adjacent A 3 and adjacent R 1 on both sides, and each of R 1 and R 2 is the same as above, x is an integer of 1 to 1500, y is 0 or an integer of 1 to 1499, and x+y is an integer of 1 to 1500;
- n is 0 or an integer of 1 to 10;
- B 1 is an active unsaturated group having urethane bond
- S 3 is a group represented by the formula:
- each of R 1 and R 2 is independently an alkylene group having 1 to 20 carbon atoms
- each of R 3 , R 4 , R 5 , R 6 , R 7 and R 8 is independently a linear, branched or cyclic alkyl group having 1 to 20 carbon atoms, which may be substituted with fluorine atom, or a group represented by the formula:
- a 3 is an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms
- U 4 is a urethane type group which contains urethane bonds formed with adjacent A 3 and adjacent R 1 on both sides, and each of R 1 and R 2 is the same as above
- x is an integer of 1 to 1500
- y is 0 or an integer of 1 to 1499
- x+y is an integer of 1 to 1500.
- examples of the active unsaturated group represented by A 1 and A 2 are (meth)acryloyl group, vinyl group, allyl group, (meth)acryloyloxy group, vinyl carbamate group and the like as mentioned above.
- acryloyloxy group and vinyl group are preferable and acryloyloxy group is particularly preferable, since more excellent flexibility can be imparted to the ocular lens material I and copolymerizability with other monomers is excellent.
- the above active unsaturated group has an alkylene group or an alkylene glycol group
- the number of carbon atoms of the alkylene group or the alkylene glycol group is 1 to 20, preferably 1 to 10.
- each of R 1 , R 2 , R 3 , R 4 , R 5 , R 6 , R 7 , R 8 , x and y is the same as above, each of R 1 and R 2 is preferably an alkylene group having 1 to 5 carbon atoms, and each of R 3 , R 4 , R 5 , R 6 , R 7 and R 8 is preferably an alkyl group having 1 to 5 carbon atoms.
- the active unsaturated group has an alkylene group or an alkylene glycol group
- the number of carbon atoms of the alkylene group or the alkylene glycol group is 1 to 20, preferably 1 to 10 carbon atoms.
- x is an integer of 1 to 500
- y is 0 or an integer of 1 to 499
- x+y is an integer of 1 to 500.
- n is 0 or an integer of 1 to 5.
- examples of the active unsaturated group having urethane bond, represented by B 1 are (meth)acryloylisocyanate group, (meth)acryloyloxyisocyanate group, allylisocyanate group, vinylbenzylisocyanate group and the like.
- the group represented by S 3 in the formula (I-2) is the same as the group represented by S 1 or S 2 in the above formula (I-1).
- each of A 1 , A 2 , U 1 , U 2 , U 3 , S 1 and S 2 is the same as above, and n′ is an integer of 1 to 4, are preferable.
- A is a group represented by the formula:
- a is an integer of 20 to 50, is preferable.
- the above vinyl ester of lower fatty acid (B) is a component which mainly imparts shape recovery to the ocular lens material I and hydrophilic property to the ocular lens material II according to the saponification mentioned below.
- Typical examples of the vinyl ester of lower fatty acid (B) is a compound represented by the formula (II):
- R is hydrogen atom or an alkyl group having 1 to 15 carbon atoms, which may be substituted with a halogen atom.
- the compound are vinyl formate, vinyl acetate, vinyl propionate, vinyl butyrate, vinyl pivalate, vinyl versatate, vinyl laurate, vinyl stearate, vinyl monochloroacetate, vinyl monofluoroacetate, vinyl trichloroacetate, vinyl trifluoroacetate and the like. These can be used alone or in admixture thereof.
- vinyl esters of lower fatty acid (B) because shape recovery and hydrophilic property can be greatly imparted to the lens material, vinyl acetate, vinyl propionate and vinyl pivalate are preferable. In particular, vinyl acetate is preferable.
- the siloxane-containing polymer which constitutes the ocular lens material I of the present invention is prepared by polymerizing the monomer mixture containing the siloxane macromonomer (A) and the vinyl ester of lower fatty acid (B) as essential components.
- the monomer mixture can also contain, for instance, a silicon-containing monomer (C).
- the above silicon-containing monomer (C) is preferably used since it is a component which mainly imparts oxygen permeability and further, flexibility, particularly shape recovery, to the ocular lens material I.
- Typical examples of the silicon-containing monomer (C) are a silicon-containing (meth)acrylate such as pentamethyldisiloxanylmethyl (meth)acrylate, trimethylsiloxydimethylsilylpropyl (meth)acrylate, methylbis(trimethylsiloxy) silylpropyl (meth)acrylate, tris(trimethylsiloxy)silylpropyl (meth)acrylate, mono[methylbis(tri methylsiloxy) siloxy]bis(trimethylsiloxy) silylpropyl (meth)acrylate, tris[methylbis(trimethylsiloxy) siloxy]silylpropyl (meth) acrylate, trimethylsilylmethyl (meth)acrylate, trimethylsilylpropyl (meth)acrylate, methylbis(trimethylsiloxy)silylethyltetramethyldisiloxanylmethyl (meth) acrylate, tetramethyltriisopropy
- p is an integer of 1 to 15
- q is 0 or 1
- r is an integer of 1 to 15, such as tris(trimethylsiloxy)silylstyrene, methylbis(trimethylsiloxy)silylstyrene, dimethylsilylstyrene, trimethylsilylstyrene, tris(trimethylsiloxy) siloxanyldimethylsilylstyrene, [methylbis(trimethylsiloxy) siloxanyl]dimethylsilylstyrene, pentamethyldisiloxanylstyrene, heptamethyltrisiloxanylstyrene, nonamethyltetrasiloxanylstyrene, pentadecamethylheptasiloxanylstyrene, heneicosamethyldecasiloxanylstyrene, heptacosamethyltridecasiloxanylstyrene,
- the silicon-containing (meth)acrylate is preferable.
- tris(trimethylsiloxy)silylpropyl acrylate is preferable.
- the monomer mixture can also contain, for instance, a fluorine-containing monomer (D), in addition to the siloxane macromonomer (A), the vinyl ester of lower fatty acid (B) and the silicon-containing monomer (C).
- a fluorine-containing monomer D
- the siloxane macromonomer A
- the vinyl ester of lower fatty acid B
- the silicon-containing monomer C
- the fluorine-containing monomer (D) is a component which mainly imparts oxygen permeability and flexibility to the ocular lens material I, improving lipid-stain resistance at the same time.
- Typical examples of the fluorine-containing monomer (D) are 2,2, 2-trifluoroethyl (meth)acrylate, 2,2,3,3-tetrafluoropropyl (meth)acrylate, 2,2,3,3-tetrafluoro-t-pentyl (meth)acrylate, 2,2,3,4,4,4-hexafluorobutyl (meth)acrylate, 2,2,3,4,4,4-hexafluoro-t-hexyl (meth)acrylate, 2,3,4,5,5,5-hexafluoro-2,4-bis(trifluoromethyl)pentyl (meth)acrylate, 2,2,3,3,4,4-hexafluorobutyl (meth)acrylate, 2,2,2,2′,2′,2′-hexafluoroisopropyl (meth)acrylate, 2,2,3,3,4,4,4-heptafluorobutyl (meth)acrylate, 2,2,3,3,4,4,5, 5-o
- R 1 is a fluoroalkyl group having 3 to 15 carbon atoms and R 2 is hydrogen atom or a methyl group, such as 3-perfluorobutyl-2-hydroxypropyl (meth)acrylate, 3-perfluorohexyl-2-hydroxypropyl (meth)acrylate, 3-perfluorooctyl-2-hydroxypropyl (meth)acrylate, 3-(perfluoro-3-methylbutyl)-2-hydroxypropyl (meth)acrylate, 3-(perfluoro-5-methylhexyl)-2-hydroxypropyl (meth)acrylate or 3-(perfluoro-7-methyloctyl)-2-hydroxypropyl (meth)acrylate; and the like.
- fluoroalkyl acrylate is preferable since flexibility can be greatly imparted to the ocular lens material I particularly out of oxygen permeability, flexibility and lipid-stain resistance.
- fluorine-containing compounds can impart oxygen permeability and lipid-stain resistance to a material, but are bad in wettability.
- a fluoroalkyl acrylate having a hydroxyl group represented by the formula:
- R′ 1 is a perfluoroalkyl group having 3 to 15, preferably 3 to 8, more preferably 4 to 6 carbon atoms is particularly preferable from the viewpoint that flexibility and wettability can be imparted to the ocular lens material I without lacking oxygen permeability and lipid-stain resistance.
- the proportion of the siloxane macromonomer (A), the vinyl ester of lower fatty acid (B) and the silicon-containing monomer (C) used as occasion demands is preferably defined as mentioned below. That is, in order to impart sufficient flexibility, mechanical strength and oxygen permeability to the ocular lens material I, it is desired that the ratio of the total weight of the siloxane macromonomer (A) and the silicon-containing monomer (C) to the weight of the vinyl ester of lower fatty acid (B), the total weight of (A) and (C)/the weight of (B), that is, when the silicon-containing monomer (C) is not used, the weight of (A)/the weight of (B), is at least 30/70, preferably at least 50/50. In order to impart sufficient shape recovery and hydrophilic property to the ocular lens material I, it is desired that the above ratio is at most 90/10, preferably at most 80/20.
- the ratio of the weight of the siloxane macromonomer (A) to the weight of the silicon-containing monomer (C), the weight of (A)/the weight of (C), is preferably defined as mentioned below. That is, in order to impart sufficient mechanical strength to the ocular lens material I without lowering shape recovery, it is desired that the weight of (A)/the weight of (C) is at least 20/80, preferably at least 25/75. In order to impart sufficient oxygen permeability to the ocular lens material I, it is desired that the above ratio is at most 90/10, preferably at most 80/20.
- the proportion of the siloxane macromonomer (A) and the vinyl ester of lower fatty acid (B), and the silicon-containing monomer (C) and the fluorine-containing monomer (D) which are used as occasion demands is preferably defined as mentioned below.
- the ratio of the total weight of siloxane macromonomer (A), the vinyl ester of lower fatty acid (B) and the silicon-containing monomer (C) to the weight of the fluorine-containing monomer (D), the total weight of (A), (B) and (C)/the weight of (D), is at least 20/80, preferably at least 40/60.
- the above ratio is at most 90/10, preferably at most 85/15.
- the monomer mixture can also contain a crosslinkable compound (E) having at least two polymerizable groups.
- the above crosslinkable compound (E) is a component which mainly imparts optical properties such as transparency to the ocular lens material I and further improves mechanical strength thereof so that the ocular lens material I can be used as a lens material.
- Typical examples of the crosslinkable compound (E) are ethylene glycol di(meth)acrylate, diethylene glycol di(meth)acrylate, triethylene glycol di(meth)acrylate, propylene glycol di(meth)acrylate, dipropylene glycol di(meth)acrylate, allyl (meth)acrylate, vinyl (meth) acrylate; trimethylolpropane tri(meth) acrylate, methacryloyloxydiethyl acrylate, divinylbenzene, diallyl phthalate, diallyl adipate, diethylene glycol diallyl ether, triallylisocyanurate, ⁇ -methylene-N-vinylpyrrolidone, 4-vinylbenzyl (meth)acrylate, 3-vinylbenzyl (meth)acrylate, 2,2-bis(p-(meth)acryloyloxyphenyl) hexafluoropropane, 2,2-bis(m-(meth)(me
- ethylene glycol di(meth)acrylate, diethylene glycol di(meth)acrylate, diallyl adipate and diethylene glycol diallyl ether are preferable.
- ethylene glycol di(meth)acrylate and diethylene glycol diallyl ether are preferable.
- the amount of the crosslinkable compound (E) in the monomer mixture is at least 0.01% by weight, preferably at least 0.05% by weight.
- the amount of the crosslinkable compound (E) in the monomer mixture is at most 15% by weight, preferably at most 10% by weight.
- the above monomer mixture may further contain an ultraviolet-ray absorbing agent in order to improve ultraviolet-ray absorbability of the ocular lens material I.
- Examples of the ultraviolet-ray absorbing agent are a benzotriazole ultraviolet-ray absorbing agent such as 2-(2′-hydroxy-5′-methacryloxyethyleneoxy-t-butylphenyl)-5-methylbenzotriazole or a compound represented by the formula (III):
- Z 1 is hydrogen atom, a halogen atom such as chlorine atom, bromine atom or iodine atom, an alkyl group having 1 to 6 carbon atoms or an alkoxyl group having 1 to 6 carbon atoms
- each of Z 2 and Z 3 is independently hydrogen atom or an alkyl group having 1 to 6 carbon atoms, such as 2-(2′-hydroxy-5′-methylphenyl)benzotriazole, 5-chloro-2-(3′-t-butyl-2′-hydroxy-5′-methylphenyl)benzotriazole or 2-(5-chloro-2H-benzotriazole-2-yl)-6-(1,1-dimethyl)-4-methylphenol; a benzophenone ultraviolet-ray absorbing agent such as 2-hydroxy-4-methoxybenzophenone or 2-hydroxy-4-octoxybenzophenone; a salicylic acid derivative ultraviolet-ray absorbing agent; a hydroxyacetophenone derivative ultraviolet-ray absorbing agent;
- the benzotriazole ultraviolet-ray absorbing agent is preferable from the viewpoint of ultraviolet-ray absorbability.
- the compound represented by the formula (III) such as 2-(5-chloro-2H-benzotriazole-2-yl)-6-(1,1-dimethyl)-4-methylphenol is preferable.
- the above ultraviolet-ray absorbing agent may or may not have a polymerizable group, and is not particularly limited.
- the amount of the above ultraviolet-ray absorbing agent in the monomer mixture is at least 0.01% by weight, preferably at least 0.05% by weight in order to impart sufficient ultraviolet-ray absorbability to the ocular lens material I. Also, it is desired that the amount is at most 5% by weight, more preferably at most 3% by weight in order to prevent relative decrease in other properties and induction of polymerization inhibition caused by excessive addition.
- a siloxane-containing polymer can be prepared by polymerizing the above monomer mixture.
- a radical polymerization initiator, a photo sensitizer and the like are usually added first to the monomer mixture obtained by adjusting kind and amount of monomers. They are added in accordance with a radical polymerization method such as a thermal polymerization method or a photo polymerization method mentioned below, wherein radical generated in an active unsaturated group is subjected to polymerization reaction.
- Typical examples of the radical polymerization initiator are a thermal polymerization initiator such as azobisisobutyronitrile, azobisdimethylvaleronitrile, benzoyl peroxide, t-butyl hydroperoxide, cumene peroxide; a benzoin photo polymerization initiator such as methylorthobenzoyl benzoate, methylbenzoyl formate, benzoin methyl ether, benzoin ethyl ether, benzoin isopropyl ether, benzoin isobutyl ether or benzoin-n-butyl ether; a phenone photo polymerization initiator such as 2-hydroxy-2-methyl-1-phenylpropane-1-one, p-isopropyl- ⁇ -hydroxyisobutylphenone, p-t-butyltrichloroacetophenone, 2,2-dimethoxy-2-phenylacetophenone, ⁇ , ⁇ -dichloro-4
- the photo sensitizer is not activated independently by irradiation of ultraviolet-ray. However, when used together with a photo initiator, the photo sensitizer effects as a promotor and show more excellent effects than the photo initiator alone.
- the photo sensitizer are 1,2-benzoanthraquinone; an amine compound such as n-butylamine, di-n-butylamine or triethylamine; tri-n-butylphosphine; allylthiourea; s-benzylisothiuronium-p-toluenesulfinate; diethylaminoethyl methacrylate; and the like.
- the radical polymerization initiator and the photo sensitizer can be suitably selected and used alone or in admixture thereof. It is desired that the amount thereof is about 0.002 to 2 parts by weight (hereinafter referred to as “part(s)”), preferably about 0.01 to 1 part, based on 100 parts of the total amount of the monomer mixture.
- radical polymerization method only a radical polymerization initiator and a photo sensitizer may be used for polymerization of the monomer mixture.
- a diluent can be also used, for instance, to improve compatibility of each monomer.
- Typical examples of the diluent are an alcohol such as methanol, ethanol, propanol, butanol, pentanol or hexanol; a ketone such as acetone or methyl ethyl ketone; an ether such as diethyl ether or tetrahydrofuran; and the like. These can be used alone or in admixture thereof.
- diluents an alcohol having 1 to 6 carbon atoms is preferable since the monomer mixture gains excellent solubility.
- n-propanol, n-butanol and n-pentanol are preferable.
- the monomer mixture is admixed with the diluent according to the following proportion. That is, in order to dissolve the monomer mixture in the diluent sufficiently, it is desired that the weight ratio of the monomer mixture to the diluent, the monomer mixture/the diluent, is at most 90/10, preferably at most 80/20. In order to remove fear that the aimed polymer becomes cloud in white to lower optical property and the polymer lacks mechanical strength, it is desired that the above weight ratio is at least 30/70, preferably at least 50/50.
- the ocular lens material I can be prepared by means of every conventional method. However, from the viewpoint of achieving maximum benefit from the properties of the ocular lens material I, it is particularly preferable to produce a lens by preparing a siloxane-containing polymer according to polymerization reaction after injecting the above monomer mixture and, if necessary, the above diluent into a mold composed of two parts, one part corresponding to the front shape of a lens, such as, a contact lens or an intraocular lens, and the other part corresponding to the back shape of the lens, and sealing the mold.
- a mold having a shape corresponding to a one-piece intraocular lens in which an optic and haptics are united with each other may be used. Also, a mold having a shape corresponding to the optic and a mold having a shape corresponding to the haptics may be used.
- the methods for the polymerization are, for instance, a thermal polymerization method, wherein a mixture of a monomer mixture containing the above radical polymerization initiator and, if necessary, a diluent, is heated at about 30° C. to 60° C. for several hours to several 10 hours to polymerize the mixture, and then the temperature of reaction system is gradually increased to about 120° C. to 140° C.
- the above mixture can be heated in a thermostat container or a thermostat room, and can be irradiated with electromagnetic wave such as microwave.
- the temperature of the mixture may be increased stepwise.
- the photo sensitizer may be further added to the mixture.
- the above ocular lens material I can be subjected to mechanical process such as cutting process or polishing process as occasion demands.
- the above ocular lens material I has excellent properties sufficient and suitable for an ocular lens material.
- the above siloxane-containing polymer is subjected to saponification to obtain a material whose wettability is further improved.
- the ocular lens material of the present invention comprises a polymer prepared by saponifying a siloxane-containing polymer obtained by polymerizing a monomer mixture containing (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and (B) a vinyl ester of lower fatty acid as essential components.
- the process of the present invention is characterized by the above saponification.
- the saponification in the present invention is to form vinyl alcohol as mentioned below by subjecting units derived from the vinyl ester of lower fatty acid (B) in the polymer, which can decompose by saponification, to alkali treatment with an alkaline compound or to acid treatment with, for instance, sulfuric acid according to the conventionally known saponification method of polyvinyl ester.
- saponification by the acid treatment there are defects that saponification speed is late, uniform saponification is difficult and that side reaction occurs. For these reasons, saponification by the alkali treatment is preferred.
- hydrophilic property in other words surface wettability, can be imparted to the ocular lens material II without much increase of water content.
- Examples of the alkaline compound used for the alkali treatment are ammonia, an alkaline metal hydroxide, an alkaline earth metal hydroxide and the like. Concrete examples of the alkaline compound are ammonium hydroxide, sodium hydroxide, potassium hydroxide, calcium hydroxide and the like. Because most alkaline compounds are solids, it is preferred that these alkaline compounds are dissolved in water, an alcohol, an ether or the like to give an alkaline solution and the alkaline solution is used for saponification.
- Examples of the alcohol are methanol, ethanol, propanol, butanol and the like.
- Examples of the ether are diethyl ether, tetrahydrofuran and the like.
- alkaline solutions of alkaline compound used for saponification those obtained from an alcohol are preferable.
- a methanol aqueous solution of sodium hydroxide having a concentration of 0.01 to 1 mol/L is more preferable.
- An ocular lens material can be subjected to dyeing in case where saponification is carried out by alkali treatment in the present invention.
- a siloxane-containing polymer is prepared as mentioned above, then immersed and swollen in a mixture obtained by dispersing, or dissolving a dye in an organic solvent such as methanol, ethanol or 2-propanol, and non-reacted residual monomers in the siloxane-containing polymer are eluted while the dye is dispersed in the polymer at the same time.
- the dye can be fixed to the siloxane-containing polymer in subsequent saponification by alkali treatment.
- the above dye there is no particular limitation for the above dye.
- reactive dyes such as C. I. Reactive Black 5, C. I.: standing for “Color Index”, hereinafter referred to as the same, C. I. Reactive Blue 21, C. I. Reactive Orange 78, C. I. Reactive Yellow 15, C. I. Reactive Blue 4, C. I. Reactive Red 11, C. I. Reactive Yellow 86, C. I. Reactive Blue 163, C. I. Reactive Red 180, and the like.
- the amount of the dye is not particularly limited as long as it is used in such an amount that sufficient dyeing of the ocular lens material II can be achieved.
- the siloxane-containing polymer is subjected to saponification by immersing the polymer in the above alkaline solution or the above solution of acidic compound.
- the temperature in saponification is not particularly limited. In general, it is desired that the temperature is about 0° C. to about 100° C., preferably about 10° C. to about 70° C.
- the treatment time for saponification which is not determined as a whole, can be changed depending on kind of alkaline compound and acidic compound, concentration of alkaline compound and acidic compound, or temperature of saponification.
- the treatment time is at least 0.1 hour, preferably at least 0.5 hour in order to improve hydrophilic property of the ocular lens material II effectively.
- the treatment time is at most 30 hours, preferably at most 15 hours, in order to remove fears that an inappropriate material for an ocular lens is produced because the material becomes cloud in white to lower transparency, or because mechanical strength thereof is lowered, and that working efficiency is lowered due to extended treatment time.
- the saponified polymer can be boiled in physiological saline (0.9% sodium chloride aqueous solution) for several hours.
- the ocular lens material II obtained by the saponification of alkali treatment after photo-irradiation to the siloxane-containing polymer has further increased surface wettability.
- light to be irradiated is ultraviolet ray having a wavelength of preferably at most 380 nm, more preferably at most 300 nm from the viewpoint of efficient improvement of wettability. It is particularly preferable to irradiate ultraviolet ray having a wavelength near 185 nm and ultraviolet ray having a wavelength near 250 nm simultaneously.
- the photo-irradiation time is 0.1 to 600 minutes, preferably 1 to 60 minutes from the viewpoint of efficient improvement of wettability without affecting productivity to produce a desired ocular lens from the ocular lens material II.
- Either of the above steps may precede when both of the steps are employed together, one step being such that a siloxane-containing polymer is immersed and swollen in a mixture obtained by dispersing, or dissolving, a dye in an organic solvent to elute residual monomers in the siloxane-containing polymer which did not react while the dye is dispersed in the polymer at the same time, and the other step being such that the siloxane-containing polymer is irradiated with photo.
- the photo-irradiation step is carried out after the elution step, light may be irradiated after a sample is dried for increased efficiency of photo-irradiation.
- the ocular lens material I of the present invention is most suitable for a soft ocular lens material in particular. This is because the ocular lens material I has excellent flexibility, particularly shape recovery, in addition to advantages of conventional soft ocular lens materials, such as high oxygen permeability, superior lipid-stain resistance and excellent wettability. The reason why a material excellent in shape recovery is suitable for a soft ocular lens material can be seen when a material inferior in shape recovery is taken into account. When used as a contact lens, a material inferior in shape recovery disturbs stable eyesight immediately after wearing lenses, causes uncomfortable feeling to patients during wearing lenses, and further, induces troubles in eyeball.
- the ocular lens material I of the present invention is very suitable for an ocular lens material for the reason that it has excellent flexibility, particularly shape recovery, in addition to high oxygen permeability, superior lipid-stain resistance and excellent wettability.
- the ocular lens material II of the present invention has not only high oxygen permeability and excellent flexibility as typified by shape recovery, but also excellent hydrophilic property, surface wettability in other words, and lipid stain resistance at the same time due to saponification, showing most suitable properties for an ocular lens material all together.
- the mold was transferred into a constant temperature condition and photo polymerization was carried out by irradiating ultraviolet ray having a wavelength of 360 nm in about 1 mW/cm 2 to the content in the mold for an hour by using a mercury lamp to give a siloxane-containing polymer having shape of a contact lens.
- a polymer having shape of a contact lens was obtained by directly saponifying the obtained siloxane-containing polymer, and a polymer having shape of a contact lens was obtained by irradiating ultraviolet ray having wavelength of 185 nm and ultraviolet ray having a wavelength of 254 nm for 10 minutes and then saponifying the obtained siloxane-containing polymer.
- These polymers to which ultraviolet ray was irradiated were used only for measuring dynamic contact angle mentioned below.
- Oxygen permeability, shape recovery, flexibility in other words, surface wettability and lipid-stain resistance of polymers having shape of a contact lens in Examples 1 to 11 and Comparative Examples 1 to 6 were examined in accordance with the following method. The results are shown in Tables 3 to 5.
- shape recovery coefficient (%) was calculated on the basis of the following equation.
- shape recovery coefficient is at least 18%
- shape recovery coefficient is inappropriate for an ocular lens, especially for a soft contact lens or a soft intraocular lens.
- soft contact lens such material is not suitable because feeling for wearing becomes poor and unstable eyesight may be caused at wearing the lens immediately after the lens is handled by fingers.
- soft intraocular lens such material is not suitable because trouble is caused for folding in operation and mark is left on the lens after insertion.
- Shape recovery coefficient (%) ⁇ ( T 1 ⁇ T 2 )/ T 1 ⁇ 100
- ⁇ Wettability for water is not good. Repelling and cloud is partly observed, or the material is clouded on the whole.
- x Wettability for water is poor. Repelling and cloud are observed.
- Example 6 and 10 as well as Comparative Examples 1 and 4, the obtained polymer was cut into strip test pieces having a size of 5 mm wide, about 12 mm long and about 0.6 mm thick (which were prepared by using a punching knife to have the same width, obtained by changing an immersing solution from saline to distilled water; and whose thickness is measured for each piece).
- Dynamic contact angle was measured by using a dynamic contact angle measuring device DCA-322 made by CAHN Co., Ltd., equipped with cover glass, wherein distilled water for injection is used as a test solution. The measuring process is as follows.
- the cover glass was used as a test piece to measure surface tension T of the test solution.
- dynamic contact angle of each test piece was measured in accordance with measuring principle of Wilhelmy method under the following conditions.
- Rate of immersion 90 ⁇ m/s
- Test weight 500.0 mg
- Test solution distilled water for injection
- Dynamic contact angle is an index for wettability of a polymer.
- a polymer whose dynamic contact angle is at least 120° has poor wettability and is inappropriate for an ocular lens material.
- the obtained polymer was put in a glass bottle containing 2 ml of an artificial tear lipid solution, a buffer solution of pH 7, which comprises 0.3 g of oleic acid, 0.3 g of linoleic acid, 4.0 g of tripalmitic acid, 1.0 g of cetyl alcohol, 0.3 g of palmitic acid, 4.0 g of spermaceti, 0.4 g of cholesterol, 0.4 g of cholesterol palmitate and 14.0 g of yolk lecithin.
- the glass bottle was shaken at 37° C. for 5 hours.
- the polymer has poor lipid-stain resistance, and is not suitable for an ocular lens such as a contact lens or an intraocular lens.
- SiMa1 a macromonomer represented by the formula:
- A is a group represented by the formula:
- SiMa2 a macromonomer represented by the formula:
- A′ is a group represented by the formula:
- a′ is an integer of 10 to 20 and a′′ is an integer of 20 to 40;
- SiMa3 a macromonomer represented by the formula:
- A′′ is a group represented by the formula:
- a′′′ is an integer of 5 to 25 and b is an integer of 1 to 5;
- V1 vinyl acetate
- V2 vinyl propionate
- V3 vinyl pivalate
- SiMo1 tris(trimethylsiloxy)silylpropyl acrylate
- SiMo2 tris(trimethylsiloxy)silylpropyl methacrylate
- F2 2-hydroxy-3-perfluorooctylpropyl acrylate
- C2 diethylene glycol diallyl ether
- NVP N-vinylpyrrolidone
- C.I. Reactive Blue 4 available from Mitsui BASF Dye Co., Ltd. was dissolved in 20 ml of distilled water in an amount of 0.0511 g to obtain a solution.
- 2-propanol was added 1 ml of the above solution at 25° C.
- a siloxane-containing polymer obtained in the same manner as in Example 2 (Example 12), and a siloxane-containing polymer obtained in the same manner as in Example 10 without ultraviolet-ray irradiation before saponification Example 13 were immersed in the mixture for 16 hours, respectively.
- these polymers were immersed in the same methanol aqueous solution containing sodium hydroxide as used in Example 2 for 6 hours at 25° C., and then each polymer was taken out therefrom, transferred to distilled water, and taken out therefrom after 120 minutes.
- the ocular lens material of the present invention is excellent not only in lipid-stain resistance, wettability and oxygen permeability, but also in flexibility, particularly, shape recovery at the same time, and can be suitably used for ocular lenses such as soft contact lens and soft intraocular lens. According to the process of the present invention, the above ocular lens material is easily produced.
Abstract
An ocular lens material comprising a siloxane-containing polymer obtained by polymerizing a monomer mixture containing (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and (B) a vinyl ester of lower fatty acid as essential components. An ocular lens material comprising a polymer obtained by saponification of the siloxane-containing polymer. Processes for producing the ocular lens materials. The ocular lens materials are excellent not only in lipid-stain resistance, wettability and oxygen permeability, but also in flexibility, particularly, shape recovery at the same time.
Description
- The present invention relates to an ocular lens material and a process for producing the same. More particularly, the present invention relates to an ocular lens material suitably used for, for example, soft contact lens and soft intraocular lens, which is excellent not only in lipid-stain resistance, wettability and oxygen permeability, but also in flexibility, particularly, shape recovery at the same time, and a process for producing the ocular lens material.
- Recently, consumer's demands for contact lenses, for example, are directed to a soft contact lens with high oxygen permeability, and for intraocular lenses, for example, a foldable intraocular lens. For these reasons, various materials having properties such as high oxygen permeability and excellent flexibility in particular are suggested.
- For instance, a contact lens comprising a copolymer obtained by polymerizing siloxanyl (meth)acrylate and vinyl ester is disclosed in Japanese Unexamined Patent Publication No. 163811/1988, and a contact lens comprising a copolymer obtained by polymerizing siloxanyl (meth)acrylate, a vinyl ester, and vinyl (meth)acrylate and/or allyl (meth)acrylate is disclosed in Japanese Unexamined Patent Publication No. 301919/1988.
- However, it cannot be said that these contact lenses are excellent in flexibility taking the monomer mixture into account though all of them have high oxygen permeability and excellent stain resistance.
- In addition to the above, a water-containing contact lens obtained by saponifying a polymer comprising a (meth)acrylate polymer, which has at least one polymerizable group on average, a vinyl monomer, a vinyl ester of fatty acid and a crosslinkable monomer, is disclosed in Japanese Unexamined Patent Publication No. 102471/1994, and a silicone-containing hydrogel material formed from a polymer prepared by using a monomer mixture comprising a polysiloxane pre-polymer, a bulk polysiloxanylalkyl (meth)acrylate monomer and a hydrophilic monomer is disclosed in Japanese Unexamined Patent Publication No. 508063/1995. Also, in International Publication No. WO 97/09169, an ocular lens material obtained by hydrating, by using a water-soluble organic solvent, a polymer prepared by solution polymerization of a polymerizable silicon-containing compound and/or a polymerizable fluorine-containing compound, hydroxyalkyl (meth)acrylate, and a cross-linked compound is disclosed.
- However, though these contact lenses and materials have properties such as high oxygen permeability, lipid-stain resistance and wettability, they only have one or a plurality of such properties, and do not show flexibility, particularly shape recovery, which is one of the important properties required for an ocular lens material, in particular, a soft ocular lens material.
- As mentioned above, ocular lens materials having not only high oxygen permeability, excellent lipid-stain resistance and superior wettability but also shape recovery at the same time have not been provided yet. Accordingly, development of such materials as the above has been expected.
- The present invention has been carried out from the viewpoint of the above prior arts. An object of the present invention is to provide an ocular lens material which has not only high oxygen permeability, excellent wettability and superior lipid-stain resistance, but also flexibility, particularly shape recovery at the same time, and an easy process for producing the ocular lens material.
- The present invention relates to
- (1) an ocular lens material comprising a siloxane-containing polymer obtained by polymerizing a monomer mixture containing
- (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and
- (B) a vinyl ester of lower fatty acid as essential components;
- (2) an ocular lens material comprising a polymer prepared by saponifying a siloxane-containing polymer obtained by polymerizing a monomer mixture containing
- (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and
- (B) a vinyl ester of lower fatty acid as essential components; and
- (3) a process for producing the above ocular lens material, characterized by preparing a siloxane-containing polymer by polymerization of a monomer mixture containing
- (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and
- (B) a vinyl ester of lower fatty acid as essential components; and then subjecting said siloxane-containing polymer to saponification.
- The ocular lens material of the present invention, hereinafter referred to as an ocular lens material I, comprises a siloxane-containing polymer obtained by polymerizing a monomer mixture containing (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and (B) a vinyl ester of lower fatty acid as essential components.
- The siloxane macromonomer (A) is a component which mainly imparts flexibility as typified by shape recovery and mechanical strength to the ocular lens material I.
- The active unsaturated group in the siloxane macromonomer (A) is an active unsaturated group which can be subjected to radical polymerization. Examples of the active unsaturated group are (meth)acryloyl group, vinyl group, allyl group, (meth)acryloyloxy group, vinyl carbamate group and the like. Among these, acryloyloxy group and vinyl group are preferable from the viewpoint that they can impart excellent flexibility to the ocular lens material I and copolymerizability with other monomers is excellent.
- In the instant specification, “(meth)acryl - - -” means “acryl - - - and/or methacryl - - - ”.
- It is desired that a number average molecular weight of the siloxane macromonomer (A) is at least 2,000, preferably at least 2,500 and more preferably at least 3,000 in order to impart excellent flexibility to the ocular lens material I without increasing hardness extremely. Also, it is desired that a number average molecular weight of the siloxane macromonomer (A) is at most 100,000, preferably at most 50,000, more preferably at most 10,000 not to make shape recovery inferior though the ocular lens material I becomes softened.
- As the siloxane macromonomer (A), for example, the macromonomer of dialkyl siloxane having an active unsaturated group described in U.S. Pat. No. 4,189,546 specification can be used to obtain the aimed ocular lens material I.
- Usually, many siloxane macromonomers are bad in wettability and relatively lack mechanical strength when each of the macromonomers is homopolymerized. Accordingly, as the siloxane macromonomer (A) used in the present invention, a siloxane macromonomer having a urethane group represented by the formula:
- in the macromonomer structure is preferable in order to improve wettability.
- When the siloxane macromonomer (A) contains the above urethane group, suitable mechanical strength and excellent wettability can be imparted to the ocular lens material I. It is desired that the number of the urethane group in the siloxane macromonomer (A) is at least 2, preferably at least 4 on average in order to impart sufficient mechanical strength and wettability. On the other hand, when too many urethane groups are introduced, flexibility of the ocular lens material I decreases. Therefore, it is desired that the average number of the urethane group in the siloxane macromonomer (A) is at most 20, preferably at most 14.
- In the present invention, the macromonomer to which hydrophilic parts are introduced at the both ends of the siloxane structure described in U.S. Pat. No. 4,495,361 specification or U.S. Pat. No. 5,807,944 specification can also be used as the siloxane macromonomer (A) in order to obtain the ocular lens material I whose flexibility, particularly shape recovery, is improved.
- In the present invention, as the siloxane macromonomer (A), a macromonomer represented by the formula (I-1) is preferably used:
- A1-(-U1—S1—)n—U2—S2—U3-A2 (I-1)
- wherein each of A 1 and A2 is independently an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms;
- U 1 is a diurethane type group which contains urethane bonds formed with adjacent A1 and adjacent S1 on both sides or which contains urethane bonds with adjacent two S1 on both sides;
- U 2 is a diurethane type group which contains urethane bonds formed with adjacent A1 and adjacent S2 on both sides or which contains urethane bonds with adjacent S1 and adjacent S2 on both sides;
- U 3 is a diurethane type group which contains urethane bonds formed with adjacent S2 and adjacent A2 on both side;
-
- wherein each of R 1 and R2 is independently an alkylene group having 1 to 20 carbon atoms, each of R3, R4, R5, R6, R7 and R8 is independently a linear, branched or cyclic alkyl group having 1 to 20 carbon atoms, which may be substituted with a fluorine atom, or a group represented by the formula:
- A3-U4—R1—O—R2-
- in which A 3 is an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms, U4 is a urethane type group which contains urethane bonds formed with adjacent A3 and adjacent R1 on both sides, and each of R1 and R2 is the same as above, x is an integer of 1 to 1500, y is 0 or an integer of 1 to 1499, and x+y is an integer of 1 to 1500;
- n is 0 or an integer of 1 to 10;
- or a macromonomer represented by the following formula (I-2) is preferably used:
- B1—S3—B1 (I-2)
- wherein B 1 is an active unsaturated group having urethane bond; and
-
- wherein each of R 1 and R2 is independently an alkylene group having 1 to 20 carbon atoms, each of R3, R4, R5, R6, R7 and R8 is independently a linear, branched or cyclic alkyl group having 1 to 20 carbon atoms, which may be substituted with fluorine atom, or a group represented by the formula:
- A3-U4—R1—O—R2-
- in which A 3 is an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms, U4 is a urethane type group which contains urethane bonds formed with adjacent A3 and adjacent R1 on both sides, and each of R1 and R2 is the same as above, x is an integer of 1 to 1500, y is 0 or an integer of 1 to 1499, and x+y is an integer of 1 to 1500.
- In the formula (I-1), examples of the active unsaturated group represented by A 1 and A2 are (meth)acryloyl group, vinyl group, allyl group, (meth)acryloyloxy group, vinyl carbamate group and the like as mentioned above. Among them, acryloyloxy group and vinyl group are preferable and acryloyloxy group is particularly preferable, since more excellent flexibility can be imparted to the ocular lens material I and copolymerizability with other monomers is excellent.
- When the above active unsaturated group has an alkylene group or an alkylene glycol group, it is desired that the number of carbon atoms of the alkylene group or the alkylene glycol group is 1 to 20, preferably 1 to 10.
-
- wherein each of R 1, R2, R3, R4, R5, R6, R7, R8, x and y is the same as above, each of R1 and R2 is preferably an alkylene group having 1 to 5 carbon atoms, and each of R3, R4, R5, R6, R7 and R8 is preferably an alkyl group having 1 to 5 carbon atoms. A3 in the formula: A3-U4—R1—O—R2-, which represents R3, R4, R5, R6, R7 or R8, is the same active unsaturated group as the above-exemplified one. When the active unsaturated group has an alkylene group or an alkylene glycol group, it is desired that the number of carbon atoms of the alkylene group or the alkylene glycol group is 1 to 20, preferably 1 to 10 carbon atoms. In addition, it is desired that x is an integer of 1 to 500, y is 0 or an integer of 1 to 499, and x+y is an integer of 1 to 500.
- In the formula (I-1), it is desired that n is 0 or an integer of 1 to 5.
- In the formula (I-2), examples of the active unsaturated group having urethane bond, represented by B 1 are (meth)acryloylisocyanate group, (meth)acryloyloxyisocyanate group, allylisocyanate group, vinylbenzylisocyanate group and the like. The group represented by S3 in the formula (I-2) is the same as the group represented by S1 or S2 in the above formula (I-1).
- Among the above macromonomers, because flexibility such as shape recovery and mechanical strength can be greatly imparted to the lens material, a macromonomer represented by the formula:
- A1-U2—S2—U3-A2
- wherein each of A 1, A2, U2, U3 and S2 is the same as above; and a macromonomer represented by the formula:
- A1-(-U1—S1—)n.—U2—S2—U3-A2
-
-
- and a is an integer of 20 to 50, is preferable.
- The above vinyl ester of lower fatty acid (B) is a component which mainly imparts shape recovery to the ocular lens material I and hydrophilic property to the ocular lens material II according to the saponification mentioned below.
-
- wherein R is hydrogen atom or an alkyl group having 1 to 15 carbon atoms, which may be substituted with a halogen atom. Concrete examples of the compound are vinyl formate, vinyl acetate, vinyl propionate, vinyl butyrate, vinyl pivalate, vinyl versatate, vinyl laurate, vinyl stearate, vinyl monochloroacetate, vinyl monofluoroacetate, vinyl trichloroacetate, vinyl trifluoroacetate and the like. These can be used alone or in admixture thereof.
- Among the above vinyl esters of lower fatty acid (B), because shape recovery and hydrophilic property can be greatly imparted to the lens material, vinyl acetate, vinyl propionate and vinyl pivalate are preferable. In particular, vinyl acetate is preferable.
- The siloxane-containing polymer which constitutes the ocular lens material I of the present invention is prepared by polymerizing the monomer mixture containing the siloxane macromonomer (A) and the vinyl ester of lower fatty acid (B) as essential components. The monomer mixture can also contain, for instance, a silicon-containing monomer (C).
- The above silicon-containing monomer (C) is preferably used since it is a component which mainly imparts oxygen permeability and further, flexibility, particularly shape recovery, to the ocular lens material I.
- Typical examples of the silicon-containing monomer (C) are a silicon-containing (meth)acrylate such as pentamethyldisiloxanylmethyl (meth)acrylate, trimethylsiloxydimethylsilylpropyl (meth)acrylate, methylbis(trimethylsiloxy) silylpropyl (meth)acrylate, tris(trimethylsiloxy)silylpropyl (meth)acrylate, mono[methylbis(tri methylsiloxy) siloxy]bis(trimethylsiloxy) silylpropyl (meth)acrylate, tris[methylbis(trimethylsiloxy) siloxy]silylpropyl (meth) acrylate, trimethylsilylmethyl (meth)acrylate, trimethylsilylpropyl (meth)acrylate, methylbis(trimethylsiloxy)silylethyltetramethyldisiloxanylmethyl (meth) acrylate, tetramethyltriisopropylcyclotetrasiloxanylpropyl (meth)acrylate, tetramethyltriisopropylcyclotetrasiloxybis(trimethylsiloxy) silylpropyl (meth)acrylate or trimethylsiloxydimethylsilylpropyl (meth)acrylate; a silicon-containing styrene derivative represented by the formula:
- wherein p is an integer of 1 to 15, q is 0 or 1 and r is an integer of 1 to 15, such as tris(trimethylsiloxy)silylstyrene, methylbis(trimethylsiloxy)silylstyrene, dimethylsilylstyrene, trimethylsilylstyrene, tris(trimethylsiloxy) siloxanyldimethylsilylstyrene, [methylbis(trimethylsiloxy) siloxanyl]dimethylsilylstyrene, pentamethyldisiloxanylstyrene, heptamethyltrisiloxanylstyrene, nonamethyltetrasiloxanylstyrene, pentadecamethylheptasiloxanylstyrene, heneicosamethyldecasiloxanylstyrene, heptacosamethyltridecasiloxanylstyrene, hentriacontamethylpentadecasiloxanylstyrene, trimethylsiloxypentamethyldisiloxymethylsilylstyrene, tris(pentamethyldisiloxy) silylstyrene, [tris(trimethylsiloxy)siloxanyl]bis(trimethylsiloxy)silylstyrene, methylbis (heptamethyltrisiloxy) silylstyrene, tris[methylbis(trimethylsiloxy) siloxy]silylstyrene, trimethylsiloxybis[tris(trimethylsiloxy) siloxy]silylstylene, heptakis(trimethylsiloxy) trisiloxanylstyrene, tris[tris(trimethylsiloxy) siloxy] silylstyrene, [tris(trimethylsiloxy)hexamethyltetrasiloxy] [tris(trimethylsiloxy)-siloxy]trimethylsiloxysilylstyrene, nonakis(trimethylsiloxy)tetrasiloxanylstyrene, methylbis(tridecamethylhexasiloxy) silylstyrene, heptamethylcyclotetrasiloxanylstyrene, heptamethylcyclotetrasiloxybis(trimethylsiloxy)silylstyrene or tripropyltetramethylcyclotetrasiloxanylstyrene; and the like. These can be used alone or in admixture thereof.
- Among the above silicon-containing monomers (C), because high oxygen permeability and flexibility, in particular, shape recovery can be greatly imparted to the ocular lens material I at the same time, the silicon-containing (meth)acrylate is preferable. In particular, tris(trimethylsiloxy)silylpropyl acrylate is preferable.
- Furthermore, the monomer mixture can also contain, for instance, a fluorine-containing monomer (D), in addition to the siloxane macromonomer (A), the vinyl ester of lower fatty acid (B) and the silicon-containing monomer (C).
- The fluorine-containing monomer (D) is a component which mainly imparts oxygen permeability and flexibility to the ocular lens material I, improving lipid-stain resistance at the same time.
- Typical examples of the fluorine-containing monomer (D) are 2,2, 2-trifluoroethyl (meth)acrylate, 2,2,3,3-tetrafluoropropyl (meth)acrylate, 2,2,3,3-tetrafluoro-t-pentyl (meth)acrylate, 2,2,3,4,4,4-hexafluorobutyl (meth)acrylate, 2,2,3,4,4,4-hexafluoro-t-hexyl (meth)acrylate, 2,3,4,5,5,5-hexafluoro-2,4-bis(trifluoromethyl)pentyl (meth)acrylate, 2,2,3,3,4,4-hexafluorobutyl (meth)acrylate, 2,2,2,2′,2′,2′-hexafluoroisopropyl (meth)acrylate, 2,2,3,3,4,4,4-heptafluorobutyl (meth)acrylate, 2,2,3,3,4,4,5, 5-octafluoropentyl (meth)acrylate; a fluoroalkyl (meth)acrylate represented by the formula:
- wherein R 1 is a fluoroalkyl group having 3 to 15 carbon atoms and R2 is hydrogen atom or a methyl group, such as 3-perfluorobutyl-2-hydroxypropyl (meth)acrylate, 3-perfluorohexyl-2-hydroxypropyl (meth)acrylate, 3-perfluorooctyl-2-hydroxypropyl (meth)acrylate, 3-(perfluoro-3-methylbutyl)-2-hydroxypropyl (meth)acrylate, 3-(perfluoro-5-methylhexyl)-2-hydroxypropyl (meth)acrylate or 3-(perfluoro-7-methyloctyl)-2-hydroxypropyl (meth)acrylate; and the like.
- Among them, fluoroalkyl acrylate is preferable since flexibility can be greatly imparted to the ocular lens material I particularly out of oxygen permeability, flexibility and lipid-stain resistance.
-
- wherein R′ 1 is a perfluoroalkyl group having 3 to 15, preferably 3 to 8, more preferably 4 to 6 carbon atoms is particularly preferable from the viewpoint that flexibility and wettability can be imparted to the ocular lens material I without lacking oxygen permeability and lipid-stain resistance.
- The proportion of the siloxane macromonomer (A), the vinyl ester of lower fatty acid (B) and the silicon-containing monomer (C) used as occasion demands is preferably defined as mentioned below. That is, in order to impart sufficient flexibility, mechanical strength and oxygen permeability to the ocular lens material I, it is desired that the ratio of the total weight of the siloxane macromonomer (A) and the silicon-containing monomer (C) to the weight of the vinyl ester of lower fatty acid (B), the total weight of (A) and (C)/the weight of (B), that is, when the silicon-containing monomer (C) is not used, the weight of (A)/the weight of (B), is at least 30/70, preferably at least 50/50. In order to impart sufficient shape recovery and hydrophilic property to the ocular lens material I, it is desired that the above ratio is at most 90/10, preferably at most 80/20.
- When the silicon-containing monomer (C) is used, the ratio of the weight of the siloxane macromonomer (A) to the weight of the silicon-containing monomer (C), the weight of (A)/the weight of (C), is preferably defined as mentioned below. That is, in order to impart sufficient mechanical strength to the ocular lens material I without lowering shape recovery, it is desired that the weight of (A)/the weight of (C) is at least 20/80, preferably at least 25/75. In order to impart sufficient oxygen permeability to the ocular lens material I, it is desired that the above ratio is at most 90/10, preferably at most 80/20.
- The proportion of the siloxane macromonomer (A) and the vinyl ester of lower fatty acid (B), and the silicon-containing monomer (C) and the fluorine-containing monomer (D) which are used as occasion demands is preferably defined as mentioned below. That is, in order to impart effects from the siloxane macromonomer (A), the vinyl ester of lower fatty acid (B) and the silicon-containing monomer (C) sufficiently to the ocular lens material 1, it is desired that the ratio of the total weight of siloxane macromonomer (A), the vinyl ester of lower fatty acid (B) and the silicon-containing monomer (C) to the weight of the fluorine-containing monomer (D), the total weight of (A), (B) and (C)/the weight of (D), is at least 20/80, preferably at least 40/60. In order to impart sufficient lipid-stain resistance in particular to the ocular lens material I, it is desired that the above ratio is at most 90/10, preferably at most 85/15.
- As occasion demands, the monomer mixture can also contain a crosslinkable compound (E) having at least two polymerizable groups.
- The above crosslinkable compound (E) is a component which mainly imparts optical properties such as transparency to the ocular lens material I and further improves mechanical strength thereof so that the ocular lens material I can be used as a lens material.
- Typical examples of the crosslinkable compound (E) are ethylene glycol di(meth)acrylate, diethylene glycol di(meth)acrylate, triethylene glycol di(meth)acrylate, propylene glycol di(meth)acrylate, dipropylene glycol di(meth)acrylate, allyl (meth)acrylate, vinyl (meth) acrylate; trimethylolpropane tri(meth) acrylate, methacryloyloxydiethyl acrylate, divinylbenzene, diallyl phthalate, diallyl adipate, diethylene glycol diallyl ether, triallylisocyanurate, α-methylene-N-vinylpyrrolidone, 4-vinylbenzyl (meth)acrylate, 3-vinylbenzyl (meth)acrylate, 2,2-bis(p-(meth)acryloyloxyphenyl) hexafluoropropane, 2,2-bis(m-(meth)acryloyloxyphenyl)hexafluoropropane, 2,2-bis(o-(meth) acryloyloxyphenyl)hexafluoropropane, 1,4-bis(2-(meth)acryloyloxyhexafluoroisopropyl)benzene, 1,3-bis(2-(meth)acryloyloxyhexafluoroisopropyl)benzene, 1,2-bis (2-(meth) acryloyloxyhexafluoroisopropyl) benzene, 1,4-bis(2-(meth)acryloyloxyisopropyl)benzene, 1,3-bis(2-(meth)acryloyloxyisopropyl)benzene, 1,2-bis(2-(meth)acryloyloxyisopropyl)benzene and the like. These can be used alone or in admixture thereof.
- Among the above crosslinkable compounds (E), because optical property and mechanical strength can be greatly imparted to the ocular lens material I and handling is easy, ethylene glycol di(meth)acrylate, diethylene glycol di(meth)acrylate, diallyl adipate and diethylene glycol diallyl ether are preferable. In particular, ethylene glycol di(meth)acrylate and diethylene glycol diallyl ether are preferable.
- In order to impart sufficient optical property and mechanical strength to the ocular lens material I, it is desired that the amount of the crosslinkable compound (E) in the monomer mixture is at least 0.01% by weight, preferably at least 0.05% by weight. In order to remove fear that flexibility of the ocular lens material I is lowered although mechanical strength is imparted to the ocular lens material I, it is desired that the amount of the crosslinkable compound (E) in the monomer mixture is at most 15% by weight, preferably at most 10% by weight.
- The above monomer mixture may further contain an ultraviolet-ray absorbing agent in order to improve ultraviolet-ray absorbability of the ocular lens material I.
-
- wherein Z 1 is hydrogen atom, a halogen atom such as chlorine atom, bromine atom or iodine atom, an alkyl group having 1 to 6 carbon atoms or an alkoxyl group having 1 to 6 carbon atoms, and each of Z2 and Z3 is independently hydrogen atom or an alkyl group having 1 to 6 carbon atoms, such as 2-(2′-hydroxy-5′-methylphenyl)benzotriazole, 5-chloro-2-(3′-t-butyl-2′-hydroxy-5′-methylphenyl)benzotriazole or 2-(5-chloro-2H-benzotriazole-2-yl)-6-(1,1-dimethyl)-4-methylphenol; a benzophenone ultraviolet-ray absorbing agent such as 2-hydroxy-4-methoxybenzophenone or 2-hydroxy-4-octoxybenzophenone; a salicylic acid derivative ultraviolet-ray absorbing agent; a hydroxyacetophenone derivative ultraviolet-ray absorbing agent; and the like. Among them, the benzotriazole ultraviolet-ray absorbing agent is preferable from the viewpoint of ultraviolet-ray absorbability. In particular, the compound represented by the formula (III), such as 2-(5-chloro-2H-benzotriazole-2-yl)-6-(1,1-dimethyl)-4-methylphenol is preferable.
- The above ultraviolet-ray absorbing agent may or may not have a polymerizable group, and is not particularly limited.
- It is desired that the amount of the above ultraviolet-ray absorbing agent in the monomer mixture is at least 0.01% by weight, preferably at least 0.05% by weight in order to impart sufficient ultraviolet-ray absorbability to the ocular lens material I. Also, it is desired that the amount is at most 5% by weight, more preferably at most 3% by weight in order to prevent relative decrease in other properties and induction of polymerization inhibition caused by excessive addition.
- In order to produce the ocular lens material I of the present invention, a siloxane-containing polymer can be prepared by polymerizing the above monomer mixture.
- In preparing the above siloxane-containing polymer, a radical polymerization initiator, a photo sensitizer and the like are usually added first to the monomer mixture obtained by adjusting kind and amount of monomers. They are added in accordance with a radical polymerization method such as a thermal polymerization method or a photo polymerization method mentioned below, wherein radical generated in an active unsaturated group is subjected to polymerization reaction.
- Typical examples of the radical polymerization initiator are a thermal polymerization initiator such as azobisisobutyronitrile, azobisdimethylvaleronitrile, benzoyl peroxide, t-butyl hydroperoxide, cumene peroxide; a benzoin photo polymerization initiator such as methylorthobenzoyl benzoate, methylbenzoyl formate, benzoin methyl ether, benzoin ethyl ether, benzoin isopropyl ether, benzoin isobutyl ether or benzoin-n-butyl ether; a phenone photo polymerization initiator such as 2-hydroxy-2-methyl-1-phenylpropane-1-one, p-isopropyl-α-hydroxyisobutylphenone, p-t-butyltrichloroacetophenone, 2,2-dimethoxy-2-phenylacetophenone, α,α-dichloro-4-phenoxyacetophenone or N,N-tetraethyl-4,4-diaminobenzophenone; 1-hydroxycyclohexyl phenyl ketone; 1-phenyl-1,2-propanedione-2-(o -ethoxycarbonyl)oxime; a thioxanthone photo polymerization initiator such as 2-chlorothioxanthone or 2-methylthioxanthone; dibenzosvarron; 2-ethylanthraquinone; benzophenone acrylate; benzophenone; benzyl; and the like.
- The photo sensitizer is not activated independently by irradiation of ultraviolet-ray. However, when used together with a photo initiator, the photo sensitizer effects as a promotor and show more excellent effects than the photo initiator alone. Examples of the photo sensitizer are 1,2-benzoanthraquinone; an amine compound such as n-butylamine, di-n-butylamine or triethylamine; tri-n-butylphosphine; allylthiourea; s-benzylisothiuronium-p-toluenesulfinate; diethylaminoethyl methacrylate; and the like.
- The radical polymerization initiator and the photo sensitizer can be suitably selected and used alone or in admixture thereof. It is desired that the amount thereof is about 0.002 to 2 parts by weight (hereinafter referred to as “part(s)”), preferably about 0.01 to 1 part, based on 100 parts of the total amount of the monomer mixture.
- In the radical polymerization method, only a radical polymerization initiator and a photo sensitizer may be used for polymerization of the monomer mixture. However, a diluent can be also used, for instance, to improve compatibility of each monomer.
- Typical examples of the diluent are an alcohol such as methanol, ethanol, propanol, butanol, pentanol or hexanol; a ketone such as acetone or methyl ethyl ketone; an ether such as diethyl ether or tetrahydrofuran; and the like. These can be used alone or in admixture thereof.
- Among the diluents, an alcohol having 1 to 6 carbon atoms is preferable since the monomer mixture gains excellent solubility. In particular, n-propanol, n-butanol and n-pentanol are preferable.
- The monomer mixture is admixed with the diluent according to the following proportion. That is, in order to dissolve the monomer mixture in the diluent sufficiently, it is desired that the weight ratio of the monomer mixture to the diluent, the monomer mixture/the diluent, is at most 90/10, preferably at most 80/20. In order to remove fear that the aimed polymer becomes cloud in white to lower optical property and the polymer lacks mechanical strength, it is desired that the above weight ratio is at least 30/70, preferably at least 50/50.
- The ocular lens material I can be prepared by means of every conventional method. However, from the viewpoint of achieving maximum benefit from the properties of the ocular lens material I, it is particularly preferable to produce a lens by preparing a siloxane-containing polymer according to polymerization reaction after injecting the above monomer mixture and, if necessary, the above diluent into a mold composed of two parts, one part corresponding to the front shape of a lens, such as, a contact lens or an intraocular lens, and the other part corresponding to the back shape of the lens, and sealing the mold. In case of producing an intraocular lens, a mold having a shape corresponding to a one-piece intraocular lens in which an optic and haptics are united with each other may be used. Also, a mold having a shape corresponding to the optic and a mold having a shape corresponding to the haptics may be used.
- After a monomer mixture and, if necessary, a diluent are injected into the mold, polymerization reaction is carried out to give a siloxane-containing polymer. Any usual method can be employed for the polymerization without particular limitation.
- The methods for the polymerization are, for instance, a thermal polymerization method, wherein a mixture of a monomer mixture containing the above radical polymerization initiator and, if necessary, a diluent, is heated at about 30° C. to 60° C. for several hours to several 10 hours to polymerize the mixture, and then the temperature of reaction system is gradually increased to about 120° C. to 140° C. over several hours to ten and several hours to complete the polymerization; a photo polymerization method, wherein the above mixture is irradiated with light ray such as ultraviolet-ray, which has wavelength corresponding to absorption band of activation of the radical polymerization initiator and then, the monomer mixture is polymerized; and a combination method of thermal polymerization and photo polymerization.
- When the thermal polymerization method is carried out, the above mixture can be heated in a thermostat container or a thermostat room, and can be irradiated with electromagnetic wave such as microwave. The temperature of the mixture may be increased stepwise. When the photo polymerization method is carried out, the photo sensitizer may be further added to the mixture.
- The thus-obtained siloxane-containing polymer is put out from the mold to give the ocular lens material I of the present invention.
- The above ocular lens material I can be subjected to mechanical process such as cutting process or polishing process as occasion demands.
- The above ocular lens material I has excellent properties sufficient and suitable for an ocular lens material. However, the above siloxane-containing polymer is subjected to saponification to obtain a material whose wettability is further improved.
- That is, the ocular lens material of the present invention, hereinafter referred to as ocular lens material II, comprises a polymer prepared by saponifying a siloxane-containing polymer obtained by polymerizing a monomer mixture containing (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and (B) a vinyl ester of lower fatty acid as essential components. The process of the present invention is characterized by the above saponification.
- The saponification in the present invention is to form vinyl alcohol as mentioned below by subjecting units derived from the vinyl ester of lower fatty acid (B) in the polymer, which can decompose by saponification, to alkali treatment with an alkaline compound or to acid treatment with, for instance, sulfuric acid according to the conventionally known saponification method of polyvinyl ester. In saponification by the acid treatment, however, there are defects that saponification speed is late, uniform saponification is difficult and that side reaction occurs. For these reasons, saponification by the alkali treatment is preferred. By saponification, hydrophilic property, in other words surface wettability, can be imparted to the ocular lens material II without much increase of water content.
- wherein R is the same as the above.
- Examples of the alkaline compound used for the alkali treatment are ammonia, an alkaline metal hydroxide, an alkaline earth metal hydroxide and the like. Concrete examples of the alkaline compound are ammonium hydroxide, sodium hydroxide, potassium hydroxide, calcium hydroxide and the like. Because most alkaline compounds are solids, it is preferred that these alkaline compounds are dissolved in water, an alcohol, an ether or the like to give an alkaline solution and the alkaline solution is used for saponification.
- Examples of the alcohol are methanol, ethanol, propanol, butanol and the like. Examples of the ether are diethyl ether, tetrahydrofuran and the like.
- Among the alkaline solutions of alkaline compound used for saponification, those obtained from an alcohol are preferable. In order to carry out saponification efficiently, a methanol aqueous solution of sodium hydroxide having a concentration of 0.01 to 1 mol/L is more preferable. In particular, a methanol aqueous solution of sodium hydroxide, in which the volume ratio of methanol to water, methanol/water, is 30/70 to 90/10, is preferable.
- An ocular lens material can be subjected to dyeing in case where saponification is carried out by alkali treatment in the present invention.
- Specifically, a siloxane-containing polymer is prepared as mentioned above, then immersed and swollen in a mixture obtained by dispersing, or dissolving a dye in an organic solvent such as methanol, ethanol or 2-propanol, and non-reacted residual monomers in the siloxane-containing polymer are eluted while the dye is dispersed in the polymer at the same time. The dye can be fixed to the siloxane-containing polymer in subsequent saponification by alkali treatment.
- There is no particular limitation for the above dye. Preferable examples thereof are, for example, reactive dyes such as C. I. Reactive Black 5, C. I.: standing for “Color Index”, hereinafter referred to as the same, C. I. Reactive Blue 21, C. I. Reactive Orange 78, C. I. Reactive Yellow 15, C. I. Reactive Blue 4, C. I. Reactive Red 11, C. I. Reactive Yellow 86, C. I. Reactive Blue 163, C. I. Reactive Red 180, and the like. The amount of the dye is not particularly limited as long as it is used in such an amount that sufficient dyeing of the ocular lens material II can be achieved.
- The siloxane-containing polymer is subjected to saponification by immersing the polymer in the above alkaline solution or the above solution of acidic compound.
- The temperature in saponification is not particularly limited. In general, it is desired that the temperature is about 0° C. to about 100° C., preferably about 10° C. to about 70° C.
- The treatment time for saponification, which is not determined as a whole, can be changed depending on kind of alkaline compound and acidic compound, concentration of alkaline compound and acidic compound, or temperature of saponification. However, it is desired that the treatment time is at least 0.1 hour, preferably at least 0.5 hour in order to improve hydrophilic property of the ocular lens material II effectively. Also, it is desired that the treatment time is at most 30 hours, preferably at most 15 hours, in order to remove fears that an inappropriate material for an ocular lens is produced because the material becomes cloud in white to lower transparency, or because mechanical strength thereof is lowered, and that working efficiency is lowered due to extended treatment time.
- The saponified polymer can be boiled in physiological saline (0.9% sodium chloride aqueous solution) for several hours.
- Further, it is also possible to carry out the above saponification after photo-irradiation to the prepared siloxane-containing polymer in the present invention.
- The ocular lens material II obtained by the saponification of alkali treatment after photo-irradiation to the siloxane-containing polymer has further increased surface wettability.
- It is preferable to carry out photo-irradiation before the saponification to a siloxane-containing polymer as mentioned above, but there is no particular limitation for the condition of the siloxane-containing polymer as long as the polymer is dried or swollen by an extraction solution employed for removing residual monomers during extraction process.
- It is desired that light to be irradiated is ultraviolet ray having a wavelength of preferably at most 380 nm, more preferably at most 300 nm from the viewpoint of efficient improvement of wettability. It is particularly preferable to irradiate ultraviolet ray having a wavelength near 185 nm and ultraviolet ray having a wavelength near 250 nm simultaneously.
- It is desired that the photo-irradiation time is 0.1 to 600 minutes, preferably 1 to 60 minutes from the viewpoint of efficient improvement of wettability without affecting productivity to produce a desired ocular lens from the ocular lens material II.
- Either of the above steps may precede when both of the steps are employed together, one step being such that a siloxane-containing polymer is immersed and swollen in a mixture obtained by dispersing, or dissolving, a dye in an organic solvent to elute residual monomers in the siloxane-containing polymer which did not react while the dye is dispersed in the polymer at the same time, and the other step being such that the siloxane-containing polymer is irradiated with photo. When the photo-irradiation step is carried out after the elution step, light may be irradiated after a sample is dried for increased efficiency of photo-irradiation.
- The ocular lens material I of the present invention is most suitable for a soft ocular lens material in particular. This is because the ocular lens material I has excellent flexibility, particularly shape recovery, in addition to advantages of conventional soft ocular lens materials, such as high oxygen permeability, superior lipid-stain resistance and excellent wettability. The reason why a material excellent in shape recovery is suitable for a soft ocular lens material can be seen when a material inferior in shape recovery is taken into account. When used as a contact lens, a material inferior in shape recovery disturbs stable eyesight immediately after wearing lenses, causes uncomfortable feeling to patients during wearing lenses, and further, induces troubles in eyeball. Meanwhile, when an ocular lens material is used as a soft intraocular lens, the best advantage of the soft intraocular lens resides in the fact that incision of eyeball can be extremely small since the lens is foldable when inserted in the eye. However, when an ocular lens material is inferior in shape recovery, wide incision is therefore necessary because the material is difficult to fold. In addition, when shape recovery is inferior, it is difficult for a lens to recover its original shape after insertion in the eye and function as a lens is not achieved, resulting in lowering of eyesight. Accordingly, the ocular lens material I of the present invention is very suitable for an ocular lens material for the reason that it has excellent flexibility, particularly shape recovery, in addition to high oxygen permeability, superior lipid-stain resistance and excellent wettability.
- Furthermore, in recent growing development of soft ocular lens materials with high oxygen permeability, use of a silicon-containing component is becoming essentially important for high oxygen permeability. However, though high oxygen permeability is imparted to the material, the material becomes extremely vulnerable to stain by lipid and inferior in hydrophilic property when used for an ocular lens because of high affinity of the silicon-containing component with a lipid component. Such characteristics are also inappropriate for an ocular lens material. On the contrary, the ocular lens material II of the present invention has not only high oxygen permeability and excellent flexibility as typified by shape recovery, but also excellent hydrophilic property, surface wettability in other words, and lipid stain resistance at the same time due to saponification, showing most suitable properties for an ocular lens material all together.
- Next, the ocular lens material and the process for producing the same of the present invention are explained in detail, but the present invention is not limited thereto.
- Each of monomer mixtures shown in Tables 1 and 2 was mixed with 2-hydroxy-2-methyl-1-phenylpropane-1-one, as a polymerization initiator, in an amount of 0.2 part based on the monomer mixture. Then, the obtained mixture was injected into a mold having shape of a contact lens (made of polypropylene, corresponding to a contact lens having a diameter of 13.8 mm and a thickness of 0.2 mm).
- Next, the mold was transferred into a constant temperature condition and photo polymerization was carried out by irradiating ultraviolet ray having a wavelength of 360 nm in about 1 mW/cm 2 to the content in the mold for an hour by using a mercury lamp to give a siloxane-containing polymer having shape of a contact lens.
- The saponification of siloxane-containing polymers shown in Tables 1 and 2 was carried out by immersing the polymer in a 60% methanol aqueous solution containing 0.25 mol/L of sodium hydroxide whose temperature was 25° C. for six hours.
- In Examples 6 and 10, a polymer having shape of a contact lens was obtained by directly saponifying the obtained siloxane-containing polymer, and a polymer having shape of a contact lens was obtained by irradiating ultraviolet ray having wavelength of 185 nm and ultraviolet ray having a wavelength of 254 nm for 10 minutes and then saponifying the obtained siloxane-containing polymer. These polymers to which ultraviolet ray was irradiated were used only for measuring dynamic contact angle mentioned below.
- Oxygen permeability, shape recovery, flexibility in other words, surface wettability and lipid-stain resistance of polymers having shape of a contact lens in Examples 1 to 11 and Comparative Examples 1 to 6 were examined in accordance with the following method. The results are shown in Tables 3 to 5.
- (a) Oxygen Permeability
- Dk values defined by ISO which stands for “International Organization for Standardization” were measured according to Fatt method.
- (b) Shape Recovery (Flexibility)
- The periphery of a polymer was fixed, while the center thereof was fixed to an apparatus for loading by using a spherical tool whose tip diameter was about 3 mm.
- Stress was given to the fixed polymer up to about 20 g. This stress was removed and loss of repulsive force from the polymer was measured. The value T 1, the maximum load on the polymer, and then the value T2, repulsive force from the polymer after 30 seconds were measured.
- Using the measured values T 1 and T2, shape recovery coefficient (%) was calculated on the basis of the following equation.
- The larger the shape recovery coefficient is, the more inferior the shape recovery is. Particularly, when shape recovery coefficient is at least 18%, such material is inappropriate for an ocular lens, especially for a soft contact lens or a soft intraocular lens. In case of the soft contact lens, such material is not suitable because feeling for wearing becomes poor and unstable eyesight may be caused at wearing the lens immediately after the lens is handled by fingers. In case of the soft intraocular lens, such material is not suitable because trouble is caused for folding in operation and mark is left on the lens after insertion.
- Shape recovery coefficient (%)={(T 1 −T 2)/T 1}×100
- (a) Surface wettability
- After immersing the obtained polymer in saline for about 16 hours, the polymer was taken out and the surface thereof was observed with naked eyes. Evaluation was made according to the following criteria.
- (Criteria for Evaluation)
- ⊚: Wettability for water is excellent on the whole. There is no repelling and cloud at all.
- ◯: Repelling is partly observed, whereas there is no cloud and wettability is good.
- Δ: Wettability for water is not good. Repelling and cloud is partly observed, or the material is clouded on the whole.
- x: Wettability for water is poor. Repelling and cloud are observed.
- In Examples 6 and 10 as well as Comparative Examples 1 and 4, the obtained polymer was cut into strip test pieces having a size of 5 mm wide, about 12 mm long and about 0.6 mm thick (which were prepared by using a punching knife to have the same width, obtained by changing an immersing solution from saline to distilled water; and whose thickness is measured for each piece). Dynamic contact angle was measured by using a dynamic contact angle measuring device DCA-322 made by CAHN Co., Ltd., equipped with cover glass, wherein distilled water for injection is used as a test solution. The measuring process is as follows.
- First, the cover glass was used as a test piece to measure surface tension T of the test solution. Second, dynamic contact angle of each test piece was measured in accordance with measuring principle of Wilhelmy method under the following conditions.
- Rate of immersion: 90 μm/s
- Depth of immersion: 10 mm
- Test weight: 500.0 mg
- Test solution: distilled water for injection
- Surface tension of test solution: the above measured value T
- Cycle: 3 cycles
- As a result of the above measurement, the value at the second cycle was employed as dynamic contact angle (°)
- Dynamic contact angle is an index for wettability of a polymer. A polymer whose dynamic contact angle is at least 120° has poor wettability and is inappropriate for an ocular lens material.
- (b) Lipid-Stain Resistance
- The obtained polymer was put in a glass bottle containing 2 ml of an artificial tear lipid solution, a buffer solution of pH 7, which comprises 0.3 g of oleic acid, 0.3 g of linoleic acid, 4.0 g of tripalmitic acid, 1.0 g of cetyl alcohol, 0.3 g of palmitic acid, 4.0 g of spermaceti, 0.4 g of cholesterol, 0.4 g of cholesterol palmitate and 14.0 g of yolk lecithin. The glass bottle was shaken at 37° C. for 5 hours.
- After five hours, the polymer was picked up from the artificial tear lipid solution and then, lipid components which adhered to the polymer were extracted by immersing the polymer in 1 ml of a mixed solution of ethanol and ether (ethanol:ether=3:1 (volume ratio)).
- To 500 μl of the obtained lipid extracted solution was added 1 ml of concentrated sulfuric acid and then, 3 mg of vanillin and 2 ml of phosphoric acid were added thereto. Adhering lipid amount (mg/cm 2) of the polymer was quantitated.
- When adhering lipid amount is more than 0.3 mg/cm 2, lipid easily adheres to the polymer. Thus, the polymer has poor lipid-stain resistance, and is not suitable for an ocular lens such as a contact lens or an intraocular lens.
- Abbreviations in Tables 1 and 2 indicate the following compounds.
-
-
- in which a is an integer of 20 to 50;
- whose number average molecular weight is 6,000; and having four urethane groups on average.
-
-
- in which a′ is an integer of 10 to 20 and a″ is an integer of 20 to 40;
- whose number average molecular weight is 7,500; and having four urethane groups on average.
-
-
- in which a′″ is an integer of 5 to 25 and b is an integer of 1 to 5;
- whose number average molecular weight is 9,000; and having eight urethane groups on average.
- V1: vinyl acetate
- V2: vinyl propionate
- V3: vinyl pivalate
- SiMo1: tris(trimethylsiloxy)silylpropyl acrylate
- SiMo2: tris(trimethylsiloxy)silylpropyl methacrylate
- F1: 2-hydroxy-3-perfluorohexylpropyl acrylate
- F2: 2-hydroxy-3-perfluorooctylpropyl acrylate
- C 1: ethylene glycol dimethacrylate
- C2: diethylene glycol diallyl ether
- 2HEMA: 2-hydroxyethyl methacrylate
- 2HEA: 2-hydroxyethyl acrylate
- DMAA: N,N-dimethylacrylamide
- NVP: N-vinylpyrrolidone
- Number average molecular weights of the above SiMa 1, SiMa2 and SiMa3 were converted to polystylene basis according to size exclusion chromatography.
TABLE 1 Monomer mixture (part) (E) Ex. (A) (B) (C) (D) C1/C2 = 1/2 No. SiMa1 SiMa2 SiMa3 V1 V2 V3 SiMo1 SiMo2 F1 F2 (weight ratio) Saponification 1 40 — — 30 — — 30 — — — 1 not saponified 2 40 — — 30 — — 30 — — — 1 saponified 3 20 — — 40 — — 40 — — — 1 not saponified 4 50 — — 35 — — 15 — — — 1 saponified 5 — 50 — 35 — — 15 — — — 1 saponified 6 — — 40 30 — — 30 — — — 1 saponified 7 50 — — 35 — — — 15 — — 1 saponified 8 50 — — — 35 — 15 — — — 1 saponified 9 50 — — — — 35 15 — — — 1 saponified 10 40 — — 28 — — 12 — 20 — 1 saponified 11 40 — — 28 — — 12 — — 20 1 saponified -
TABLE 2 Monomer mixture (part) (E) (A) (C) C1/C2 = 1/2 Others Com. Ex. No. SiMa1 SiMa3 SiMo1 (weight ratio) 2HEMA 2HEA DMAA NVP Saponification 1 40 — 30 1 30 — — — not saponified 2 40 — 30 1 30 — — — saponified 3 — 40 30 1 30 — — — not saponified 4 40 — 30 1 — 30 — — not saponified 5 40 — 30 1 — — 30 — not saponified 6 40 — 30 1 — — — 30 not saponified -
TABLE 3 Properties of polymer (ocular lens material) Lipid-stain resistance Oxygen permeability Shape recovery Surface wettability (Adhering lipid amount Ex. No. (DK value) (Shape recovery coefficient (%)) for water (mg/cm2)) 1 115 5 ◯ 0.2≧ 2 110 5 ⊚ 0.2≧ 3 105 11 ◯ 0.2≧ 4 130 9 ⊚ 0.2≧ 5 130 11 ⊚ 0.2≧ 6 110 10 ⊚ 0.2≧ 7 130 11 ⊚ 0.2≧ 8 130 10 ⊚ 0.2≧ 9 130 10 ⊚ 0.2≧ 10 130 10 ⊚ 0.1≧ 11 130 10 ⊚ 0.1≧ -
TABLE 4 Surface wettability for water of polymer (ocular lens material) Ultraviolet-ray (dynamic contact angle (°)) irradiation Ex. No. 6 98 not irradiated 60 irradiated 10 95 not irradiated 60 irradiated Com. Ex. No. 1 130 not irradiated 4 140 not irradiated -
TABLE 5 Properties of polymer (ocular lens material) Com. Lipid stain resistance Ex. Oxygen permeability Shape recovery Surface wettability (Adhering lipid amount No. (DK value) (Shape recovery coefficient (%)) for water (mg/cm2)) 1 110 25 Δ 0.5 2 80 25 ◯ 0.5 3 110 25 Δ 0.5 4 110 9 Δ 0.6 5 110 9 Δ 0.6 6 110 6 Δ 0.5 - The results in Table 3 prove that a polymer which has high oxygen permeability, superior lipid-stain resistance, excellent surface wettability, and excellent shape recovery at the same time can be obtained when a siloxane macromonomer (A) and a vinyl ester of lower fatty acid (B) are used together as in Examples 1 to 11. The results also show that a polymer which has further improved surface wettability can be obtained when saponification is carried out as in Examples 2 and 4 to 11. It is also proved that a polymer which has particularly excellent lipid-stain resistance in addition to the above advantages can be obtained when a fluorine-containing monomer (D) is used as in Examples 10 and 11. The results in Table 4 indicate that when short wavelength ultraviolet ray is irradiated before saponification as in Examples 6 and 10, a polymer has smaller dynamic contact angle and more excellent surface wettability than those in Comparative Examples 1 and 4 and cases in Examples 6 and 10 where ultraviolet ray was not irradiated.
- On the contrary, the results in Table 5 shows that when a hydrophilic monomer of 2-hydroxyethyl (meth)acrylate, N,N-dimethylacrylamide or N-vinylpyrrolidone is used instead of the vinyl ester of lower fatty acid (B) as in Comparative Examples 1 to 6, surface wettability is inferior as in Comparative Examples 1 and 3 to 6, shape recovery is poor as in Comparative Examples 1 to 3, and lipid-stain resistance is particularly low.
- C.I. Reactive Blue 4 available from Mitsui BASF Dye Co., Ltd. was dissolved in 20 ml of distilled water in an amount of 0.0511 g to obtain a solution. To 20 ml of 2-propanol was added 1 ml of the above solution at 25° C. A siloxane-containing polymer obtained in the same manner as in Example 2 (Example 12), and a siloxane-containing polymer obtained in the same manner as in Example 10 without ultraviolet-ray irradiation before saponification (Example 13) were immersed in the mixture for 16 hours, respectively. Next, these polymers were immersed in the same methanol aqueous solution containing sodium hydroxide as used in Example 2 for 6 hours at 25° C., and then each polymer was taken out therefrom, transferred to distilled water, and taken out therefrom after 120 minutes.
- All polymers assumed uniform and transparent blue color, and was equal to polymers obtained in Examples 2 or 10 in high oxygen permeability, and excellent lipid-stain resistance, surface wettability and shape recovery.
- The ocular lens material of the present invention is excellent not only in lipid-stain resistance, wettability and oxygen permeability, but also in flexibility, particularly, shape recovery at the same time, and can be suitably used for ocular lenses such as soft contact lens and soft intraocular lens. According to the process of the present invention, the above ocular lens material is easily produced.
Claims (22)
1. An ocular lens material comprising a siloxane-containing polymer obtained by polymerizing a monomer mixture containing (A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and (B) a vinyl ester of lower fatty acid as essential components.
2. The ocular lens material of claim 1 , wherein said monomer mixture further contains (C) a silicon-containing monomer.
3. The ocular lens material of claim 1 , wherein said monomer mixture further contains (D) a fluorine-containing monomer.
4. The ocular lens material of claim 2 , wherein the weight ratio of the total of said siloxane macromonomer (A) and said silicon-containing monomer (C) to said vinyl ester of lower fatty acid (B), the total weight of (A) and (C)/the weight of (B), is 30/70 to 90/10.
5. The ocular lens material of claim 3 , wherein the weight ratio of the total of said siloxane macromonomer (A), said vinyl ester of lower fatty acid (B) and said silicon-containing monomer (C) to said fluorine-containing monomer (D), the total weight of (A), (B) and (C)/the weight of (D), is at least 20/80.
6. The ocular lens material of claim 2 , wherein the weight ratio of said siloxane macromonomer (A) to said silicon-containing monomer (C), the weight of (A)/the weight of (C), is at least 20/80.
7. The ocular lens material of claim 1 , wherein said siloxane macromonomer (A) is a macromonomer represented by the formula (I-1):
A1-(-U1—S1—)n—U2—S2—U3-A2 (I-1)
wherein each of A1 and A2 is independently an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms;
U1 is a diurethane type group which contains urethane bonds formed with adjacent A1 and adjacent S1 on both sides or which contains urethane bonds with adjacent two S1 on both sides;
U2 is a diurethane type group which contains urethane bonds formed with adjacent A1 and adjacent S2 on both sides or which contains urethane bonds with adjacent S1 and adjacent S2 on both sides;
U3 is a diurethane type group which contains urethane bonds formed with adjacent S2 and adjacent A2 on both sides;
each of S1 and S2 is independently a group represented by the formula:
in which each of R1 and R2 is independently an alkylene group having 1 to 20 carbon atoms, each of R3, R4, R5, R6, R7 and R8 is independently a linear, branched or cyclic alkyl group having 1 to 20 carbon atoms which may be substituted with a fluorine atom, or a group represented by the formula:
A3-U4—R—O—R2-
in which A3 is an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms, U4 is a urethane type group which contains urethane bonds formed with adjacent A3 and adjacent R1 on both sides, each of R1 and R2 is the same as the above, x is an integer of 1 to 1500, y is 0 or an integer of 1 to 1499, and x+y is an integer of 1 to 1500; and
n is 0 or an integer of 1 to 10; or
a macromonomer represented by the formula (I-2):
B1—S3—B1 (I-2)
wherein B1 is an active unsaturated group having urethane bond, and S3 is a group represented by the formula:
in which each of R1 and R2 is independently an alkylene group having 1 to 20 carbon atoms, each of R3, R4, R5, R6, R7 and R8 is independently a linear, branched or cyclic alkyl group having 1 to 20 carbon atoms which may be substituted with a fluorine atom, or a group represented by the formula:
A3—U4—R1 —O—R2-
in which A3 is an active unsaturated group, an active unsaturated group having an alkylene group having 1 to 20 carbon atoms or an active unsaturated group having an alkylene glycol group having 1 to 20 carbon atoms, U4 is a urethane type group which contains urethane bonds formed with adjacent A3 and adjacent R1 on both sides, each of R1 and R2 is the same as the above, x is an integer of 1 to 1500, y is 0 or an integer of 1 to 1499, and x+y is an integer of 1 to 1500.
9. The ocular lens material of claim 1 , wherein said vinyl ester of lower fatty acid (B) is vinyl acetate, vinyl propionate or vinyl pivalate.
10. The ocular lens material of claim 2 , wherein said silicon-containing monomer (C) is a silicon-containing (meth)acrylate.
11. The ocular lens material of claim 2 , wherein said silicon-containing monomer (C) is tris(trimethylsiloxy)silylpropyl acrylate.
12. The ocular lens material of claim 3 , wherein said fluorine-containing monomer (D) is a fluoroalkyl (meth)acrylate.
13. An ocular lens material comprising a polymer prepared by saponifying a siloxane-containing polymer obtained by polymerizing a monomer mixture containing
(A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and
(B) a vinyl ester of lower fatty acid as essential components.
14. A process for producing an ocular lens material, characterized by preparing a siloxane-containing polymer by polymerization of a monomer mixture containing
(A) a siloxane macromonomer having at least two active unsaturated groups and a number average molecular weight of 2,000 to 100,000; and
(B) a vinyl ester of lower fatty acid as essential components; and then subjecting said siloxane-containing polymer to saponification.
15. The process of claim 14 , wherein said saponification is carried out by alkali treatment.
16. The process of claim 15 , wherein a methanol aqueous solution containing sodium hydroxide in a concentration of 0.01 to 1 mol/L is used for said alkali treatment as a treatment solution.
17. The process of claim 16 , wherein the volume ratio of methanol to water in said methanol aqueous solution, methanol/water, is 30/70 to 90/10.
18. The process of claim 15 , wherein after preparing said siloxane-containing polymer, a dye is dispersed in the siloxane-containing polymer and then, said dye is fixed to the siloxane-containing polymer during said saponification by said alkali treatment.
19. The process of claim 14 , wherein after preparing said siloxane-containing polymer, photo-irradiation is conducted and saponification is carried out subsequently.
20. The process of claim 19 , wherein said photo-irradiation is conducted by using ultraviolet ray whose wavelength is at most 380 nm.
21. The process of claim 19 , wherein said photo-irradiation is conducted out by using ultraviolet ray whose wavelength is at most 300 nm.
22. The process of claim 19 , wherein said photo-irradiation is conducted out for 0.1 to 600 minutes.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/644,776 US20040054106A1 (en) | 1999-05-12 | 2003-08-21 | Ocular lens material and process for producing the same |
Applications Claiming Priority (4)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| JP13167899 | 1999-05-12 | ||
| JP131678/1999 | 1999-05-12 | ||
| US09/926,459 US6727336B1 (en) | 1999-05-12 | 2000-05-09 | Ocular lens materials and process for producing the same |
| US10/644,776 US20040054106A1 (en) | 1999-05-12 | 2003-08-21 | Ocular lens material and process for producing the same |
Related Parent Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/926,459 Continuation US6727336B1 (en) | 1999-05-12 | 2000-05-09 | Ocular lens materials and process for producing the same |
| PCT/JP2000/002939 Continuation WO2000070388A1 (en) | 1999-05-12 | 2000-05-09 | Ocular lens materials and process for producing the same |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20040054106A1 true US20040054106A1 (en) | 2004-03-18 |
Family
ID=15063674
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/926,459 Expired - Lifetime US6727336B1 (en) | 1999-05-12 | 2000-05-09 | Ocular lens materials and process for producing the same |
| US10/644,776 Abandoned US20040054106A1 (en) | 1999-05-12 | 2003-08-21 | Ocular lens material and process for producing the same |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/926,459 Expired - Lifetime US6727336B1 (en) | 1999-05-12 | 2000-05-09 | Ocular lens materials and process for producing the same |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US6727336B1 (en) |
| EP (1) | EP1176454B1 (en) |
| JP (1) | JP3939927B2 (en) |
| KR (1) | KR20020040661A (en) |
| AT (1) | ATE246222T1 (en) |
| AU (1) | AU4318800A (en) |
| CA (1) | CA2372930A1 (en) |
| DE (1) | DE60004200T2 (en) |
| WO (1) | WO2000070388A1 (en) |
Cited By (10)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050154080A1 (en) * | 2001-09-10 | 2005-07-14 | Mccabe Kevin P. | Biomedical devices containing internal wetting agents |
| US20070138692A1 (en) * | 2002-09-06 | 2007-06-21 | Ford James D | Process for forming clear, wettable silicone hydrogel articles |
| US20070149699A1 (en) * | 2003-12-19 | 2007-06-28 | Masihiro Matsumoto | Contact lens material |
| US20070229757A1 (en) * | 2001-09-10 | 2007-10-04 | Mccabe Kevin P | Biomedical devices containing internal wetting agents |
| US20080094570A1 (en) * | 2004-09-30 | 2008-04-24 | Takehisa Kamiya | Highly Oxygen-Permeable Hydrated Ocular Lens |
| US20140171544A1 (en) * | 2006-11-22 | 2014-06-19 | Sauflon Cl Limited | Contact lens |
| US10591749B2 (en) * | 2011-08-17 | 2020-03-17 | Toray Industries, Inc. | Medical device, combination of coating solutions, and method for producing medical device |
| US20210163650A1 (en) * | 2019-02-26 | 2021-06-03 | Menicon Co., Ltd. | Polymer material |
| US11119338B2 (en) * | 2016-04-04 | 2021-09-14 | Seed Co., Ltd. | Soft contact lens and method for suppressing attachment of soft contact lens onto cornea |
| US11226553B2 (en) * | 2016-05-11 | 2022-01-18 | Dic Corporation | Photo-imprinting curable composition and pattern transferring method using the same |
Families Citing this family (32)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE10215962A1 (en) * | 2002-04-11 | 2003-10-30 | Wacker Polymer Systems Gmbh | Organopolymers of silicone and their saponification products |
| AU2002950469A0 (en) * | 2002-07-30 | 2002-09-12 | Commonwealth Scientific And Industrial Research Organisation | Improved biomedical compositions |
| WO2004073566A1 (en) * | 2003-02-21 | 2004-09-02 | Menicon Co., Ltd. | Coating material for medical use |
| US7628810B2 (en) | 2003-05-28 | 2009-12-08 | Acufocus, Inc. | Mask configured to maintain nutrient transport without producing visible diffraction patterns |
| DE10338479A1 (en) * | 2003-08-21 | 2005-04-14 | Wacker Polymer Systems Gmbh & Co. Kg | Silicone-containing polyvinyl acetals |
| HUE044130T2 (en) * | 2004-08-27 | 2019-09-30 | Coopervision Int Holding Co Lp | Silicone hydrogel contact lenses |
| US9322958B2 (en) | 2004-08-27 | 2016-04-26 | Coopervision International Holding Company, Lp | Silicone hydrogel contact lenses |
| AU2007247850B2 (en) | 2006-05-03 | 2013-05-02 | Vision Crc Limited | Eye treatment |
| US7828432B2 (en) * | 2007-05-25 | 2010-11-09 | Synergeyes, Inc. | Hybrid contact lenses prepared with expansion controlled polymeric materials |
| CA2770735C (en) | 2009-08-13 | 2017-07-18 | Acufocus, Inc. | Masked intraocular implants and lenses |
| US10004593B2 (en) | 2009-08-13 | 2018-06-26 | Acufocus, Inc. | Intraocular lens with elastic mask |
| CA2773223C (en) * | 2009-10-01 | 2015-11-24 | Coopervision International Holding Company, Lp | Silicone hydrogel contact lenses and methods of making silicone hydrogel contact lenses |
| WO2012118674A2 (en) | 2011-02-28 | 2012-09-07 | Coopervision International Holding Company, Lp | Silicone hydrogel contact lenses |
| MY151110A (en) | 2011-02-28 | 2014-04-15 | Coopervision Int Holding Co Lp | Dimensionally stable silicone hydrogel contact lenses |
| CN103890044B (en) | 2011-02-28 | 2015-11-25 | 库柏维景国际控股公司 | Silicone hydrogel contact lenses |
| TWI497151B (en) | 2011-02-28 | 2015-08-21 | Coopervision Int Holding Co Lp | Phosphine-containing hydrogel contact lenses |
| MX2013009217A (en) | 2011-02-28 | 2014-09-08 | Coopervision Int Holding Co Lp | Silicone hydrogel contact lenses having acceptable levels of energy loss. |
| WO2012118675A2 (en) | 2011-02-28 | 2012-09-07 | Coopervision International Holding Company, Lp | Wettable silicone hydrogel contact lenses |
| CN103764724B (en) | 2011-02-28 | 2016-04-06 | 库柏维景国际控股公司 | Silicone hydrogel contact lenses and compositions related and method |
| CA2857306C (en) | 2011-12-02 | 2017-07-25 | Acufocus, Inc. | Ocular mask having selective spectral transmission |
| US9427922B2 (en) | 2013-03-14 | 2016-08-30 | Acufocus, Inc. | Process for manufacturing an intraocular lens with an embedded mask |
| JP6356635B2 (en) * | 2014-06-12 | 2018-07-11 | 信越化学工業株式会社 | Monomers for ophthalmic device manufacturing |
| EP3220859B8 (en) | 2014-11-19 | 2020-06-10 | AcuFocus, Inc. | Fracturable mask for treating presbyopia |
| EP3359987B1 (en) | 2015-10-05 | 2024-02-28 | AcuFocus, Inc. | Methods of molding intraocular lenses |
| CA3005891C (en) | 2015-11-24 | 2023-12-12 | Acufocus, Inc. | Toric small aperture intraocular lens with extended depth of focus |
| EP3323387B1 (en) * | 2016-11-22 | 2019-10-02 | REPER Sàrl | A biocompatible copolymer material and an ophthalmic implant consisting thereof |
| KR102554567B1 (en) * | 2017-01-18 | 2023-07-11 | 동우 화인켐 주식회사 | Photocurable composition and photocurable layer formed from the same |
| KR102595791B1 (en) | 2017-06-07 | 2023-10-31 | 알콘 인코포레이티드 | silicone hydrogel contact lenses |
| HUE055667T2 (en) | 2017-06-07 | 2021-12-28 | Alcon Inc | Method for producing silicone hydrogel contact lenses |
| CN110709731B (en) | 2017-06-07 | 2022-05-24 | 爱尔康公司 | Silicone hydrogel contact lenses |
| WO2019217471A1 (en) | 2018-05-09 | 2019-11-14 | Acufocus, Inc. | Intraocular implant with removable optic |
| WO2020261502A1 (en) | 2019-06-27 | 2020-12-30 | 株式会社メニコン | Ophthalmic medical instrument including photochromic polymer and production method for ophthalmic medical instrument |
Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4136250A (en) * | 1977-07-20 | 1979-01-23 | Ciba-Geigy Corporation | Polysiloxane hydrogels |
| US4410674A (en) * | 1981-11-17 | 1983-10-18 | Ivani Edward J | Silicone-vinyl acetate composition for contact lenses |
| US4500695A (en) * | 1981-11-17 | 1985-02-19 | Ivani Edward J | Silicone-vinyl acetate composition for contact lenses |
| US5244470A (en) * | 1989-09-20 | 1993-09-14 | Menicon Co., Ltd. | Method for marking a water-absorptive contact lens: dyeing dry lens with a mark using reduced vat dye and then making the dye insoluble |
| US5413863A (en) * | 1993-11-04 | 1995-05-09 | E. I. Du Pont De Nemours And Company | Recording medium with inproved adhesion to glass |
| US6440571B1 (en) * | 1999-05-20 | 2002-08-27 | Bausch & Lomb Incorporated | Surface treatment of silicone medical devices with reactive hydrophilic polymers |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| JP2554483B2 (en) | 1986-12-26 | 1996-11-13 | セイコーエプソン株式会社 | contact lens |
| JP3195662B2 (en) | 1992-08-24 | 2001-08-06 | 株式会社メニコン | Ophthalmic lens materials |
| JP3088857B2 (en) | 1992-09-22 | 2000-09-18 | 株式会社メニコン | Hydrous contact lens |
| JPH0749470A (en) | 1993-08-04 | 1995-02-21 | Menicon Co Ltd | Water-containing contact lens and its manufacture |
| JPH08245652A (en) | 1995-03-08 | 1996-09-24 | Asahi Chem Ind Co Ltd | Polyfunctional organic siloxane macromer and soft material for ophthalmic medical use using the same |
| JPH11287971A (en) | 1998-04-02 | 1999-10-19 | Kuraray Co Ltd | Lens material for eye |
-
2000
- 2000-05-09 EP EP00922989A patent/EP1176454B1/en not_active Expired - Lifetime
- 2000-05-09 KR KR1020017013439A patent/KR20020040661A/en not_active Application Discontinuation
- 2000-05-09 AT AT00922989T patent/ATE246222T1/en not_active IP Right Cessation
- 2000-05-09 CA CA002372930A patent/CA2372930A1/en not_active Abandoned
- 2000-05-09 JP JP2000618770A patent/JP3939927B2/en not_active Expired - Fee Related
- 2000-05-09 AU AU43188/00A patent/AU4318800A/en not_active Abandoned
- 2000-05-09 US US09/926,459 patent/US6727336B1/en not_active Expired - Lifetime
- 2000-05-09 DE DE60004200T patent/DE60004200T2/en not_active Expired - Lifetime
- 2000-05-09 WO PCT/JP2000/002939 patent/WO2000070388A1/en not_active Application Discontinuation
-
2003
- 2003-08-21 US US10/644,776 patent/US20040054106A1/en not_active Abandoned
Patent Citations (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4136250A (en) * | 1977-07-20 | 1979-01-23 | Ciba-Geigy Corporation | Polysiloxane hydrogels |
| US4410674A (en) * | 1981-11-17 | 1983-10-18 | Ivani Edward J | Silicone-vinyl acetate composition for contact lenses |
| US4500695A (en) * | 1981-11-17 | 1985-02-19 | Ivani Edward J | Silicone-vinyl acetate composition for contact lenses |
| US5244470A (en) * | 1989-09-20 | 1993-09-14 | Menicon Co., Ltd. | Method for marking a water-absorptive contact lens: dyeing dry lens with a mark using reduced vat dye and then making the dye insoluble |
| US5413863A (en) * | 1993-11-04 | 1995-05-09 | E. I. Du Pont De Nemours And Company | Recording medium with inproved adhesion to glass |
| US6440571B1 (en) * | 1999-05-20 | 2002-08-27 | Bausch & Lomb Incorporated | Surface treatment of silicone medical devices with reactive hydrophilic polymers |
Cited By (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US9097914B2 (en) | 2001-09-10 | 2015-08-04 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US8796353B2 (en) | 2001-09-10 | 2014-08-05 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US11360241B2 (en) | 2001-09-10 | 2022-06-14 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US20070229757A1 (en) * | 2001-09-10 | 2007-10-04 | Mccabe Kevin P | Biomedical devices containing internal wetting agents |
| US20080015282A1 (en) * | 2001-09-10 | 2008-01-17 | Mccabe Kevin P | Biomedical devices containing internal wetting agents |
| US8168720B2 (en) | 2001-09-10 | 2012-05-01 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US7666921B2 (en) | 2001-09-10 | 2010-02-23 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US8431669B2 (en) | 2001-09-10 | 2013-04-30 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US10641926B2 (en) | 2001-09-10 | 2020-05-05 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US10254443B2 (en) | 2001-09-10 | 2019-04-09 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US7691916B2 (en) | 2001-09-10 | 2010-04-06 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US8450387B2 (en) * | 2001-09-10 | 2013-05-28 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US20050154080A1 (en) * | 2001-09-10 | 2005-07-14 | Mccabe Kevin P. | Biomedical devices containing internal wetting agents |
| US9958577B2 (en) | 2001-09-10 | 2018-05-01 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US8895687B2 (en) | 2001-09-10 | 2014-11-25 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US10935696B2 (en) | 2001-09-10 | 2021-03-02 | Johnson & Johnson Vision Care, Inc. | Biomedical devices containing internal wetting agents |
| US20070138692A1 (en) * | 2002-09-06 | 2007-06-21 | Ford James D | Process for forming clear, wettable silicone hydrogel articles |
| US20070149699A1 (en) * | 2003-12-19 | 2007-06-28 | Masihiro Matsumoto | Contact lens material |
| US20080094570A1 (en) * | 2004-09-30 | 2008-04-24 | Takehisa Kamiya | Highly Oxygen-Permeable Hydrated Ocular Lens |
| US7781536B2 (en) * | 2004-09-30 | 2010-08-24 | Seed Co., Ltd. | Highly oxygen-permeable hydrated ocular lens |
| US20140171544A1 (en) * | 2006-11-22 | 2014-06-19 | Sauflon Cl Limited | Contact lens |
| US10591749B2 (en) * | 2011-08-17 | 2020-03-17 | Toray Industries, Inc. | Medical device, combination of coating solutions, and method for producing medical device |
| US11119338B2 (en) * | 2016-04-04 | 2021-09-14 | Seed Co., Ltd. | Soft contact lens and method for suppressing attachment of soft contact lens onto cornea |
| US11226553B2 (en) * | 2016-05-11 | 2022-01-18 | Dic Corporation | Photo-imprinting curable composition and pattern transferring method using the same |
| US20210163650A1 (en) * | 2019-02-26 | 2021-06-03 | Menicon Co., Ltd. | Polymer material |
Also Published As
| Publication number | Publication date |
|---|---|
| EP1176454A4 (en) | 2002-06-19 |
| JP3939927B2 (en) | 2007-07-04 |
| EP1176454A1 (en) | 2002-01-30 |
| ATE246222T1 (en) | 2003-08-15 |
| WO2000070388A1 (en) | 2000-11-23 |
| DE60004200T2 (en) | 2004-02-26 |
| KR20020040661A (en) | 2002-05-30 |
| AU4318800A (en) | 2000-12-05 |
| EP1176454B1 (en) | 2003-07-30 |
| US6727336B1 (en) | 2004-04-27 |
| CA2372930A1 (en) | 2000-11-23 |
| DE60004200D1 (en) | 2003-09-04 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6727336B1 (en) | Ocular lens materials and process for producing the same | |
| EP0726474B1 (en) | Colored contact lens and method for producing the same | |
| US8703875B2 (en) | Polymers | |
| EP1197782B1 (en) | Material for ocular lens | |
| EP0611781B1 (en) | Ophthalmic lens polymer incorporating acyclic monomer | |
| EP0940693B1 (en) | Soft contact lenses | |
| JP2006126797A (en) | Highly oxygen-permeable hydrated ocular lens | |
| EP0882996A2 (en) | Process for producing controlled drug-release contact lens, and controlled drug-release contact lens thereby produced | |
| CA2307634C (en) | Methods and compositions for manufacturing tinted ophthalmic lenses | |
| EP1080381B1 (en) | High refractive index ophthalmic device materials prepared using a post-polymerization cross-linking method | |
| US20070231293A1 (en) | Methods and systems for releasing silicone hydrogel ophthalmic lenses using surfactants | |
| JP6857784B2 (en) | Polymer material | |
| US20010031318A1 (en) | Process for preparing ultraviolet-ray-absorbable and low water-containing soft contact lens | |
| JPH031647B2 (en) | ||
| US11873361B2 (en) | Ophthalmic devices | |
| JP2591154B2 (en) | contact lens | |
| JP2803232B2 (en) | contact lens | |
| JP2803241B2 (en) | contact lens | |
| JP2803152B2 (en) | contact lens | |
| US20240010777A1 (en) | Surface treatment agent for ophthalmic device | |
| CN116490532A (en) | Modifying agent for ophthalmic device | |
| JP2004075948A (en) | Soft ocular lens material and its preparation process | |
| JPH05277173A (en) | Hydrous lens |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |