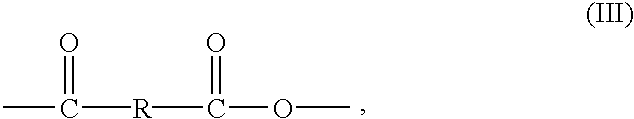US20030035787A1 - Polyanhydrides with biologically active degradation products - Google Patents
Polyanhydrides with biologically active degradation products Download PDFInfo
- Publication number
- US20030035787A1 US20030035787A1 US10/254,191 US25419102A US2003035787A1 US 20030035787 A1 US20030035787 A1 US 20030035787A1 US 25419102 A US25419102 A US 25419102A US 2003035787 A1 US2003035787 A1 US 2003035787A1
- Authority
- US
- United States
- Prior art keywords
- composition
- host
- administered
- polyanhydrides
- skin
- Prior art date
- Legal status (The legal status is an assumption and is not a legal conclusion. Google has not performed a legal analysis and makes no representation as to the accuracy of the status listed.)
- Abandoned
Links
- 0 COC(=O)[Ar]*[Ar]C(C)=O Chemical compound COC(=O)[Ar]*[Ar]C(C)=O 0.000 description 9
- CRSOQBOWXPBRES-UHFFFAOYSA-N CC(C)(C)C Chemical compound CC(C)(C)C CRSOQBOWXPBRES-UHFFFAOYSA-N 0.000 description 3
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/20—Pills, tablets, discs, rods
- A61K9/2004—Excipients; Inactive ingredients
- A61K9/2022—Organic macromolecular compounds
- A61K9/2031—Organic macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds, e.g. polyethylene glycol, polyethylene oxide, poloxamers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/60—Salicylic acid; Derivatives thereof
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/74—Synthetic polymeric materials
- A61K31/765—Polymers containing oxygen
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/30—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds
- A61K8/33—Cosmetics or similar toiletry preparations characterised by the composition containing organic compounds containing oxygen
- A61K8/36—Carboxylic acids; Salts or anhydrides thereof
- A61K8/368—Carboxylic acids; Salts or anhydrides thereof with carboxyl groups directly bound to carbon atoms of aromatic rings
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K8/00—Cosmetics or similar toiletry preparations
- A61K8/18—Cosmetics or similar toiletry preparations characterised by the composition
- A61K8/72—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds
- A61K8/84—Cosmetics or similar toiletry preparations characterised by the composition containing organic macromolecular compounds obtained by reactions otherwise than those involving only carbon-carbon unsaturated bonds
- A61K8/85—Polyesters
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61L—METHODS OR APPARATUS FOR STERILISING MATERIALS OR OBJECTS IN GENERAL; DISINFECTION, STERILISATION OR DEODORISATION OF AIR; CHEMICAL ASPECTS OF BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES; MATERIALS FOR BANDAGES, DRESSINGS, ABSORBENT PADS OR SURGICAL ARTICLES
- A61L27/00—Materials for grafts or prostheses or for coating grafts or prostheses
- A61L27/14—Macromolecular materials
- A61L27/18—Macromolecular materials obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P17/00—Drugs for dermatological disorders
- A61P17/06—Antipsoriatics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P27/00—Drugs for disorders of the senses
- A61P27/02—Ophthalmic agents
- A61P27/12—Ophthalmic agents for cataracts
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P31/00—Antiinfectives, i.e. antibiotics, antiseptics, chemotherapeutics
- A61P31/12—Antivirals
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P35/00—Antineoplastic agents
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P7/00—Drugs for disorders of the blood or the extracellular fluid
- A61P7/02—Antithrombotic agents; Anticoagulants; Platelet aggregation inhibitors
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61P—SPECIFIC THERAPEUTIC ACTIVITY OF CHEMICAL COMPOUNDS OR MEDICINAL PREPARATIONS
- A61P9/00—Drugs for disorders of the cardiovascular system
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08G—MACROMOLECULAR COMPOUNDS OBTAINED OTHERWISE THAN BY REACTIONS ONLY INVOLVING UNSATURATED CARBON-TO-CARBON BONDS
- C08G67/00—Macromolecular compounds obtained by reactions forming in the main chain of the macromolecule a linkage containing oxygen or oxygen and carbon, not provided for in groups C08G2/00 - C08G65/00
- C08G67/04—Polyanhydrides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L73/00—Compositions of macromolecular compounds obtained by reactions forming a linkage containing oxygen or oxygen and carbon in the main chain, not provided for in groups C08L59/00 - C08L71/00; Compositions of derivatives of such polymers
- C08L73/02—Polyanhydrides
-
- C—CHEMISTRY; METALLURGY
- C08—ORGANIC MACROMOLECULAR COMPOUNDS; THEIR PREPARATION OR CHEMICAL WORKING-UP; COMPOSITIONS BASED THEREON
- C08L—COMPOSITIONS OF MACROMOLECULAR COMPOUNDS
- C08L77/00—Compositions of polyamides obtained by reactions forming a carboxylic amide link in the main chain; Compositions of derivatives of such polymers
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q17/00—Barrier preparations; Preparations brought into direct contact with the skin for affording protection against external influences, e.g. sunlight, X-rays or other harmful rays, corrosive materials, bacteria or insect stings
- A61Q17/005—Antimicrobial preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q19/00—Preparations for care of the skin
- A61Q19/08—Anti-ageing preparations
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61Q—SPECIFIC USE OF COSMETICS OR SIMILAR TOILETRY PREPARATIONS
- A61Q5/00—Preparations for care of the hair
- A61Q5/006—Antidandruff preparations
Definitions
- aromatic polyanhydrides are produced from ortho-substituted bis-aromatic carboxylic acid anhydrides having the structure of Formula II:
Abstract
Polyanhydrides which degrade into biologically active salicylates and alpha-hydroxy acids and methods of using these polyanhydrides to deliver the salicylates and alpha-hydroxy acids to a host are provided.
Description
- This application is a continuation-in-part of PCT Application No. PCT/US98/18816, filed Sep. 10, 1998, which claims the benefit of provisional Application Serial No. 60/058,328, filed Sep. 10, 1997.
- Biocompatible polyanhydrides having improved degradation properties and processability with useful degradation products have now been developed. In one embodiment, the polyanhydrides are ortho-substituted aromatic polyanhydrides produced from ortho-substituted bis-aromatic carboxylic acid anhydrides which degrade into biologically active materials such as salicylates. In another embodiment, the polyanhydrides are aliphatic in structure and degrade into alpha-hydroxy acids. Salicylates are used routinely as anti-inflammatory, antipyretic, analgesic, and anti-oxidant agents, while alpha-hydroxy acids are incorporated into many skin moisturizers, cleansers, lotions, creams shampoos, tanning products and lipsticks to promote smoother, clearer skin with fewer wrinkles. Thus, the biocompatible polyanhydrides of the present invention can be administered to a host via a variety of routes including, but not limited to orally, subcutaneously, intramuscularly, intradermally and topically, depending upon the degradation product of the polyanhydride and the selected use for the degradation product.
- Polymers comprising aromatic or aliphatic anhydrides have been studied extensively over the years for a variety of uses. For example, in the 1930s fibers comprising aliphatic polyanhydrides were prepared for use in the textile industry. In the mid 1950s, aromatic polyanhydrides were prepared with improved film and fiber forming properties. More recently, attempts have been made to synthesize polyanhydrides with greater thermal and hydrolytic stability and sustained drug release properties.
- U.S. Pat. Nos. 4,757,128 and 4,997,904 disclose the preparation of polyanhydrides with improved sustained drug release properties from pure, isolated prepolymers of diacids and acetic acid. However, these biocompatible and biodegradable aromatic polyanhydrides have radical or aliphatic bonds resulting in compounds with slow degradation times as well as relatively insoluble degradation products unless incorporated into a copolymer containing a more hydrophilic monomer, such as sebacic acid. The aromatic polyanhydrides disclosed in the '128 Patent and the '904 Patent are also insoluble in most organic solvents. A bioerodible controlled release device produced as a homogenous polymeric matrix from polyanhydrides with aliphatic bonds having weight average molecular weights greater than 20,000 and an intrinsic velocity greater than 0.3 dL/g and a biologically active substance is also described in U.S. Pat. No. 4,888,176. Another bioerodible matrix material for controlled delivery of bioactive compounds comprising polyanhydride polymers with a uniform distribution of aliphatic and aromatic residues is disclosed in U.S. Pat. No. 4,857,311.
- Biocompatible and biodegradable aromatic polyanhydrides prepared from para-substituted bis-aromatic dicarboxylic acids for use in wound closure devices are disclosed in U.S. Pat. No. 5,264,540. However, these compounds exhibit high melt and glass transition temperatures and decreased solubility, thus making them difficult to process. The disclosed polyanhydrides also comprise radical or aliphatic bonds which can not be hydrolyzed by water.
- Polyanhydride polymeric matrices have also been described for use in orthopedic and dental applications. For example, U.S. Pat. No. 4,886,870 discloses a bioerodible article useful for prosthesis and implantation which comprises a biocompatible, hydrophobic polyanhydride matrix. U.S. Pat. No. 5,902,599 also discloses biodegradable polymer networks for use in a variety of dental and orthopedic applications which are formed by polymerizing anhydride prepolymers.
- Biocompatible and biodegradable polyanhydrides have now been developed with improved degradation, processing and solubility properties, as well as utilities based upon their degradation products.
- An object of the present invention is to provide biocompatible and biodegradable polyanhydrides which degrade into biologically active products. In one embodiment, aromatic polyanhydrides which degrade into biologically active salicylates are prepared from ortho-substituted bis-aromatic carboxylic acid anhydrides. Ortho substitution disrupts the crystallinity of the resulting polymer, enhancing solubility and processability, as well as degradation properties. The use of hydrolyzable bonds such as esters, amides, urethanes, carbamates and carbonates as opposed to radical or aliphatic bonds in these compounds further enhances these properties. In this embodiment, the polyanhydride comprises a repeating unit within the structure of Formula I:
- wherein Ar is a substituted or unsubstituted aromatic ring and R is a difunctional organic moiety substituted on each Ar ortho to the anhydride group. Ar and R are preferably selected so that the hydrolysis products of the polyanhydrides have a chemical structure resembling biologically active materials, particularly salicylates such as aspirin, non-steroidal anti-inflammatory naphthyl or phenyl propionates such as ibuprofen, ketoprofen, naproxen, and the like, or other aromatic anti-inflammatory compounds such as indomethacin, indoprofen, and the like. Ar is preferably a phenyl group and R is preferably —Z 1—R1—Z1— in which R1, is a difunctional moiety and both Z1s are independently either an ester, amide, anhydride, carbonate, urethane or sulfide groups. R1 is preferably an alkylene group containing from 1 to 20 carbon atoms, or a group with 2-20 carbon atoms having a structure selected from (—CH2—CH2—O—)m, (CH2—CH2—CH2—O—)m and (—CH2—CHCH3—O—)m.
-
- wherein Ar and R, and the preferred species thereof, are the same as described above with respect to Formula I and R is substituted on each Ar ortho to the anhydride group.
-
-
- wherein x is from 1 to 20 and Z 1 and Z2 are OH so that the R group contains from 1 to 40 hydroxyl groups.
- The present invention relates to compositions and methods of using compositions comprising polyanhydrides of Formula (I) or (III) in applications wherein delivery of a salicylate or an alpha-hydroxy acid to a host is desired. By “host” it is meant to include both animals and plants.
- A more complete appreciation of the invention and other intended advantages can be readily obtained by reference to the following detailed description of the preferred embodiments and claims, which disclose the principles of the invention and the best modes which are presently contemplated for carrying them out.
- Polyanhydrides which degrade into useful biologically active products such as salicylates and alpha-hydroxy acids have now been developed. Compounds comprising these polyanhydrides are useful in a variety of applications wherein delivery of a salicylate or alpha-hydroxy acid is desired.
-
- wherein Ar is a substituted or unsubstituted aromatic ring and R is a difunctional organic moiety substituted on each Ar ortho to the anhydride group. In this embodiment, Ar and R are preferably selected so that the hydrolysis products of the polyanhydrides have a chemical structure resembling biologically active materials, particularly salicylates such as aspirin, nonsteroidal anti-inflammatory naphthyl or phenyl propionates such as ibuprofen, ketoprofen, naproxen, and the like, or other aromatic anti-inflammatory compounds such as indomethacin, indoprofen, and the like. Examples of the biologically active salicylates include, but are not limited to, thymotic acid, 4,4-sulfinyldinailine, 4-sulfanilamidosalicylic acid, sulfanilic acid, sulfanilylbenzylamine, sulfaloxic acid, succisulfone, salicylsulfuric acid, salsallate, salicylic alcohol, orthocaine, mesalamine, gentisic acid, enfenamic acid, cresotic acid, aminosalicylic acid, aminophenylacetic acid, acetylsalicylic acid, and the like. The identification of Ar and R moieties that provide aromatic polyanhydrides that hydrolyze to form such biologically active salicylates can be readily determined by those of ordinary skill in the art without undue experimentation. In particular, Ar is preferably a phenyl group and R is preferably —Z 1—R1—Z1— in which R1, is a difunctional moiety and both Z1s are independently either an ester, amide, anhydride, carbonate, urethane or sulfide groups. R1 is preferably an alkylene group containing from 1 to 20 carbon atoms, or a group with 2-20 carbon atoms having a structure selected from (—CH2—CH2—O—)m, (CH2—CH2—CH2—O—)m and (—CH2—CHCH3—O—)m, or R1 may have the structure —R2—Z2—R3-1 wherein R2 and R3 are independently alkylene groups containing from 1 to 19 carbon atoms or groups having from 2 to 18 carbon atoms having a structure selected from (—CH2—CH2—O—)m, (—CH2—CH2—CH2—O—)m, and (—CH2—CHCH3—O—)m, and Z2 is selected from the difunctional moieties described above with respect to Z1.
- Ar may be an alkylaryl group, in which a difunctional organic moiety is positioned between each anhydride carbonyl group and the corresponding aromatic ring. Preferably, however, each carbonyl group is directly substituted on the corresponding aromatic ring.
- Preferred polymers of this embodiment have repeating units with the structure of Formula I in which Ar is a phenyl ring and R is selected from —Z 1—(—CH2—)n—Z1—, —Z1(—CH2—CH2—0—)m—Z1—, —Z1(—CH2—CH —2CH —02—) —Z —ml,1 and —Z (—CH —CHCH 2—0—) —3Z—, wherein Z1 is an ester or amide group and n is from 1 to 20 inclusive, and preferably is 6, and m is selected so that R has from 2 to 20, and preferably 6, carbon atoms.
- The aromatic polyanhydrides of this embodiment of the present invention may be prepared by the method described in Conix, Macromol. Synth., 2, 95-99 (1996), in which dicarboxylic acids are acetylated in an excess of acetic anhydride at reflux temperatures followed by melt condensation of the resulting carboxylic acid anhydride at 180° C. for 2-3 hours. The resulting polymers are isolated by precipitation into diethylether from methylene chloride. The described process is essentially the conventional method for polymerizing bisaromatic dicarboxylic acid anhydrides into aromatic polyanhydrides.
- Aromatic polyanhydrides in accordance with this embodiment of the present invention have average molecular weights of at least about 1500 daltons, up to about 100,000 daltons, calculated by Gel Permeation Chromatography (GPC) relative to narrow molecular weight polystyrene standards.
-
- in which Ar, R and the preferred species thereof are the same as described above with respect to Formula I. As noted above, ortho-substituted bis-aromatic carboxylic acid anhydrides are prepared by acetylation of the corresponding ortho-substituted bis-aromatic carboxylic acids in an excess of acetic anhydride at reflux temperatures. The dicarboxylic acids have the structure of Formula IV,
- wherein Ar, R and the preferred species thereof are the same as described above with respect to Formula I.
- The dicarboxylic acids are prepared by reacting a stoichiometric ratio of aromatic carboxylic acid having the structure Z 3—Ar—COOH and a compound having a structure Z4—R—Z4 wherein Ar is a substituted or unsubstituted aromatic ring on which Z3 is substituted ortho to the carboxylic acid group, R is a difunctional organic moiety and Z3 and Z4 are functional groups selected to provide the linkage desired between the difunctional organic moiety and the two aromatic rings.
- Suitable Z 3 and Z4 functional groups, and the manner in which they may be reacted to produce the bis-aromatic dicarboxylic acids of the present invention, may be readily determined by those of ordinary skill in the art without undue experimentation. For example, for aromatic polyanhydrides having the structure of Formula I in which Ar is a phenyl group and R is —O—(CH2—)6—O—, the ortho-substituted bisaromatic dicarboxylic acid starting material may be prepared by reacting o-salicylic acid with 1,6-dibromohexane.
-
-
- wherein x is from 1 to 20 and Z 1 and Z2 are OH so that the R group contains from 1 to 40 hydroxyl groups. Examples of biologically active alpha-hydroxy acids include, but are not limited to, citric acid and malic acid. These polyanhydrides are prepared in the same fashion as described for aromatic polyanhydrides.
- Polyanhydrides used in the present invention can be isolated by known methods commonly employed in the field of synthetic polymers to produce a variety of useful products with valuable physical and chemical properties. The new polymers can be readily processed into pastes or solvent cast to yield films, coatings, microspheres and fibers with different geometric shapes for design of various medical implants, and may also be processed by compression molding and extrusion. Medical implant applications include the use of aromatic polyanhydrides to form shaped articles such as vascular grafts and stents, bone plates, sutures, implantable sensors, implantable drug delivery devices, stents for tissue regeneration, and other articles that decompose harmlessly within a known time period. Polyanhydrides of the present invention can also be incorporated into oral formulations and into products such as skin moisturizers, cleansers, pads, plasters, lotions, creams, gels, ointments, solutions, shampoos, tanning products and lipsticks for topical application.
- The quantity of aromatic polyanhydride that hydrolyzes to form an amount of biologically active salicylate or alpha-hydroxy acid effective for the selected use can be readily determined by those of ordinary skill in the art without undue experimentation. The quantity essentially corresponds stoichiometrically to the amount of salicylate or alpha-hydroxy acid known to produce an effective treatment for the selected use.
- The present invention relates to methods of using compositions comprising these polyanhydrides in any application wherein delivery of a salicylate or alpha-hydroxy acid is desired. For example, salicylates such as salicylic acid are used routinely to treat many skin disorders including, but not limited to, acne, dandruff, psoriasis, seborrheic dermatitis of the skin and scalp, calluses, corns, common warts and plantar warts. Salicylic acid is also topically applied as an antiseptic for wounds, ulcers, and skin abscesses as it is known to exert powerful static effects against Gram-negative and Gram-positive bacteria, yeasts, dermatophytes, molds and other microbes. These antifungal properties also render salicylic acid useful in the treatment of athlete's foot. Accordingly, topical application of a composition comprising an aromatic polyanhydride of the present invention which degrades to a biologically active salicylate is expected to be useful in the treatment of all of these conditions and/or injuries.
- The anti-bacterial activity of salicylic acid also renders these polyanhydrides useful in agricultural applications. Solutions comprising a polyanhydride of Formula (I) can be applied topically to plants to establish microbial resistance against a wide range of pathogens. Salicylic acid treatment has also been shown to induce thermotolerance in mustard seedlings. Accordingly, topical application of polyanhydrides of Formula (I) is also expected to induce thermotolerance in plants.
- Salicylic acid has also been shown to have anti-cataract activity in patients suffering from galactosemic cataracts. Accordingly, a solution comprising an aromatic polyanhydride of Formula (I) can also be topically applied to the eye to inhibit cataract formation.
- Salicylic acid is also a powerful anti-oxidant, neutralizing highly reactive, cell damaging molecules called free radicals. In fact, salicylic acid is often the standard by which the effectiveness of other anti-oxidants is measured. Anti-oxidants are administered orally and/or topically as antiviral agents. Anti-oxidants also inhibit UV-induced signal transduction and can be used as chemopreventative agents for skin cancer. In addition, the anti-oxidant properties of salicylates have been associated with anti-aging properties, protection against ischemia and reperfusion injury, and lowering of cholesterol levels and inhibition of clotting of blood. It is believed that compositions comprising an aromatic polyanhydride of Formula (I) will also exhibit these antioxidant properties. Thus, compositions comprising an aromatic polyanhydride of Formula (I) can also be used as antiviral agents, chemopreventative agents for skin cancer, anti-aging agents, and anti-clotting agents, and to provide protection against ischemia and reperfusion injury.
- Compositions of the present invention comprising a polyanhydride of Formula (III) which degrades to an alpha-hydroxy acid can be incorporated into various topical formulations and applied to the skin to promote smoother, clearer skin with less wrinkles. It is generally accepted that regular use of alpha-hydroxy acids improves the appearance of the skin by minimizing fine lines, softening dry, rough skin patches and fading age spots. Alpha-hydroxy acids are effective exfoliators which dissolve the links that bind surface skin cells together causing dead cells to slough off. This process reveals the more youthful looking skin underneath which has more even skin tone, retains moisture and is less likely to form wrinkles. Topical application of a composition comprising a polyanhydride of Formula (III) provides an effective means for delivering alpha-hydroxy acids to the skin to promote smoother, clearer skin with less wrinkles.
- The following non-limiting examples set forth hereinbelow illustrate certain aspects of the invention. All parts and percentages are by weight unless otherwise noted and all temperatures are in degrees Celsius. Except for acetic anhydride and ethyl ether (Fisher Scientific), all solvents and reagents were obtained from Aldrich Chemical. All solvents were HPLC grade. All other reagents were of analytical grade and were purified by distillation or recrystallization.
- All compounds were characterized by a proton nuclear magnetic resonance (NMR) spectroscopy, infrared (IR) spectroscopy, gel permeation chromatography (GPC), high performance liquid chromatography (HPLC), differential scanning calorimetry (DSC), and thermal gravimetric analysis (TGA). Infrared spectroscopy was performed on an ATI Mattson Genesis (M100) FTIR Spectrophotometer. Samples were prepared by solvent casting on NaCl plates. 1H and 13C NMR spectroscopy was obtained on a Varian 200 MHZ or Varian 400 MHZ spectrometer in solutions of CDCl3 or DMSO-d6 with solvent as the internal reference.
- GPC was performed on a Perkin-Elmer Advanced LC Sample Processor (ISS 200) with PE Series 200 LC Pump and a PE Series LC Refractive Index Detector to determine molecular weight and polydispersity. The data analysis was carried out using Turbochrom 4 software on a DEC Celebris 466 computer. Samples were dissolved in tetrahydrofuran and eluted through a mixed bed column (PE PL gel, 5 μm mixed bed) at a flow rate of 0.5 mL/minute. Samples (about 5 mg/mL) were dissolved into the tetrahydrofuran and filtered using 0.5 μm PTFE syringe filters prior to column injection. Molecular weights were determined relative to narrow molecular weight polystyrene standards (Polysciences, Inc.).
- Thermal analysis was performed on a Perkin-Elmer system consisting of a TGA 7 thermal gravimetric analyzer equipped with PE AD-4 autobalance and Pyris 1 DSC analyzer. Pyris software was used to carry out data analysis on a DEC Venturis 5100 computer. For DSC, an average sample weight of 5-10 mg was heated at 10° C./minute at a 30 psi flow of N 2. For TGA, an average sample weight of 10 mg was heated at 20° C./minute under a 8 psi flow of N2. Sessile drop contact angle measurements were obtained with an NRL Goniometer (Rame-hart) using distilled water. Solutions of polymer in methylene chloride (10% wt/volume) were spun-coated onto glass slips, at 5,000 rpm for 30 seconds.
- Preparation of 1,6-Bis(o-Carboxyphenoxy) Hexane Dicarboxylic Acid
- To a mixture of salicylic acid (77.12 g, 0.5580 mole) and distilled water (84 mL) sodium hydroxide (44.71 g, 1.120 mole) was added. The reaction was brought to reflux temperature before 1,6-dibromohexane (45.21 g, 0.2790 mole) was added drop-wise. Reflux was continued for 23 hours after which additional sodium hydroxide (11.17 g, 0.2790 mole) was added. The mixture was refluxed for 16 more hours, cooled, filtered, and washed with methanol. The yield was 48.8%.
- Preparation of 1,6-Bis(o-Carboxyphenoxy) Hexane Monomer (o-CPH)
- The dicarboxylic acid of Example 1 was acetylated in an excess of acidic anhydride at reflux temperature. The resulting monomer was precipitated with methylene chloride into an excess of diethyl ether. The yield was 66.8%.
- Preparation of Poly(1,6-Bis(o-Carboxyphenoxy) Hexane) (Poly(o-CPH))
- The monomer of Example 2 was polymerized in a melt condensation performed at 180° C. for 3 hours under vacuum in a reaction vessel with a side arm. The polymerization vessel was flushed with nitrogen at frequent intervals. The polymer was isolated by precipitation into diethyl ether from methylene chloride. The yield was quantitative.
- All compounds were characterized by nuclear magnetic resonance spectroscopy, GPC, differential scanning calorimetry (DSC), thermal gravimetric analysis, contact angle measurements, UV spectroscopy, mass spectroscopy, elemental analysis and high pressure liquid chromatography (HPLC).
- The o-CPH monomer was polymerized by melt polycondensation for 60 minutes at temperatures ranging from 100° C. to 300° C. Analysis of the resulting polymers by GPC indicated that the highest molecular weight, coupled with the lowest polydispersity index occurred at 260° C.
- The poly(o-CPH) was generally soluble in methylene chloride and chloroform, while the poly(p-CPH) was not. The poly(o-CPH) was slightly soluble in tetrahydrofuran, acetone and ethyl acetate.
- Disks of poly(o-CPH), poly(p-CPH) and, as a reference, poly(lactic acid glycolic acid) were prepared and placed in 0.1 phosphate buffer solution at 37° C. for 4 weeks. The degradation media was replaced periodically. The degradation profile was linear up to three weeks time. In prior art polyanhydride systems, the aromatic groups are para-substituted. This substitution pattern results in higher melt and glass transition temperatures and decreased solubility, thus ultimately making these parasubstituted polymers difficult to process.
- Poly(o-CPH), unlike poly(p-CPH), has both a lower melting point (65° C. vs. 143° C.) and glass transition temperature (35° C. vs. 47° C.). It is also possible to solution cast poly(o-CPH) using low-boiling solvents whereas poly(p-CPH) is relatively insoluble in most organic and aqueous solvents. This structural modification gives a polymer whose hydrolysis products are chemically similar to aspirin. Aspirin is an anti-inflammatory agent derived from salicylic acid, which is one of the reagents used to synthesize the inventive polyanhydrides. Therefore, the degradation products of this polymer actually aid in patient recovery. Because of pliability and ease of processing, the aromatic polyanhydrides of the present invention have great potential as polymer scaffolds for wound healing.
- Preparation of 1,3-bis(o-carboxyphenoxy)propane dicarboxylic acid
- 1,3-dibromopropane (14.7 mL, 0.145 mole) was added to a mixture of salicylic acid (40.0 g, 0.290 mole), distilled water (44 mL) and sodium hydroxide (23.2 g, 0.580 mole) using the method described in Example 1. After 4 hours, additional sodium hydroxide (5.79 g, 0.145 mole) was added to the reaction mixture. Reflux was continued for another 4 hours, after which the mixture was cooled, filtered and washed using the methods described in Example 1. The yield was 37.7%
- Preparation of poly(1,3-bis(o-carboxyphenoxy) propane)
- The dicarboxylic acid of Example 4 was acetylated using the methods of Example 2. The acetylated dicarboxylic acid was then polymerized using the methods described in Example 3. The resulting polymer had a M w of 8,500 daltons and a polydispersity of 2.3.
- Contact angle measurements on solvent-cast films demonstrated that the hexyl chain of the polymer of Example 3 increased the surface hydrophobicity relative to the shorter propyl chain of the polymer of Example 5. A comparison of thermal characteristics emphasized the effects of lengthening the alkyl chain. In particular, the polymer of Example 3 has a T g of 34° C. and a Td of 410° C., while the polymer of Example 5 had a Tg of 50° C. and a Td of 344° C. Thus, the hexyl chain decreased the glass transition temperature (Tg) relative to the propyl chain, reflecting the increased flexibility of the polymer chain. The opposite trend was observed for decomposition temperatures (Td), with the longer alkyl chain increasing the Td.
- Optimum polycondensation conditions were determined for the polymer of Example 3. Optimum conditions were defined as those that yielded a crude polymer with the highest molecular weight and highest T g. Higher reaction temperatures decreased the Mw values (measured by GPC) with a concurrent increase in polydispersity. As expected for a condensation polymerization, longer reaction times yielded polymers with higher molecular weights. However, over longer reaction times, there appeared a subsequent decrease in Tg. Based on these results, the optimum conditions were defined as temperatures of 220° C. for 150 minutes under a vacuum.
- Preparation of 1,8-bis[o-(benzylcarboxy)carboxy phenyl] octane dicarboxylic acid ester
- The initial synthesis of poly(anhydride-ester) dicarboxylic acid monomers was attempted using the same methodology used for the poly(anhydride-ether) dicarboxylic monomers of Example 3. It was found, however, that the reactivity of the phenol was enhanced by benzylation of the carboxylic acid group. In addition, the solubility of benzyl salicylate in organic media increased the ability of the reaction to move forward.
- Thus, benzyl salicylate (1.530 g, 6.720 mmole) and distilled tetrahydrofuran were combined under an inert atmosphere in a reaction flask. An ice-salt bath was placed under the reaction flask and the addition of 60% sodium hydride (0.4840 g, 12.10 mmole) followed. After one hour, sebacoyl chloride (0.7850 g, 3.280 mmole) was added drop-wise to the 0° C. reaction mixture. After 30 minutes, the reaction mixture was vacuum filtered, the filtrate collected and the solvent removed to yield the free carboxylate as a white solid residue. Purification was performed using a chromatron with ethyl acetate/methylene chloride (20/80) as the solvent system. The yield was 43%.
- Polymerization of Poly(1,8-bis(o-dicarboxyphenyl) octane)
- To remove the benzyl protecting groups, the 1,8-bis[(benzylcarboxy)carboxyphenyl]octane dicarboxylic acid ester of Example 6 (0.06000 g, 0.9620 mmole) was dissolved in methylene chloride in a reaction flask (60.00 mL). The catalyst Pd-C (10%, 1.200 g) was added to the reaction flask. After 30 minutes, the reaction was complete. The reaction mixture was filtered and the solvent removed to yield the free dicarboxylic acid as a white solid residue which was recrystallized using petroleum ether and methylene chloride. The yield was 45%.
- The dicarboxylic acid was acetylated using the methods described in Example 2 and the acetylated dicarboxylic acid was then polymerized using the methods described in Example 3. The resulting polymer had a M w of 3,000 daltons and a polydispersity of 1.40.
- Subsequent polymerizations yielded polymers with M w's ranging from 2,000 to 5,000 daltons with corresponding polydispersities of approximately 1.40.
- The poly(anhydride esters) of Example 7 were compression molded into circular discs and placed in phosphate buffered saline solution under acidic, neutral and basic conditions. Over the course of a three-week degradation study, the polymers in the acidic and neutral solutions showed no observable changes, whereas the polymer in the basic media showed significant morphological changes over time.
- Preparation of Poly[(1,8-bis(o-dicarboxyphenyl) octane)-(1,6-bis(p-carboxyphenoxy)hexane]copolymers
- The 1,8-bis(o-dicarboxyphenyl) octane of Example 2 was copolymerized with 1,6-bis(p-carboxyphenoxy) hexane using the methods described in Example 3. In an in vivo mouse study, each mouse was implanted with 2 polymers, the copolymer of Example 8 and poly(1,6-bis(p-carboxyphenoxy)hexane). Each polymer was compression molded for 1 to 5 minutes at 1 to 20 K psi depending on the thickness of polymer needed. The polymer was placed under the palatal gingival mucosa adjacent to the first maxillary molars.
Claims (15)
1. A composition comprising a polyanhydride which degrades to a biologically active salicylate or alpha-hydroxy acid, said polyanhydride comprising a repeating unit having the structure of Formula (I):
wherein Ar is a substituted or unsubstituted aromatic ring and R is —Z1—R1—Z1— substituted on each Ar ortho to the anhydride group, wherein R1 is a difunctional organic moiety and Z1 is a difunctional moiety selected from the group consisting of esters, amides, urethanes, carbamates and carbonates; or the structure of Formula (III):
wherein R is an alkylene group containing from 1 to 20 carbon atoms, —(CH2)x— wherein x is from 1 to 20, or
wherein x is from 1 to 20 and Z1 and Z2 are OH so that the R group contains from 1 to 40 hydroxyl groups.
2. A method of delivering a salicylate to a host comprising administering to a host a composition of claim 1 wherein the polyanhydride comprises Formula (I).
3. The method of claim 2 wherein the composition is administered topically.
4. The method of claim 3 wherein the composition is topically administered to the host to treat a skin disorder selected from the group consisting of acne, dandruff, psoriasis, seborrheic dermatitis of the skin and scalp, calluses, corns, common warts and plantar warts.
5. The method of claim 3 wherein the composition is topically administered to a host to prevent microbial infection.
6. The method of claim 5 wherein the host is an animal and the composition is topically applied as an antiseptic to a wound, ulcer or skin abscess.
7. The method of claim 5 wherein the host is a plant.
8. The method of claim 2 wherein the composition is administered to the host so that the salicylate can act as an anti-oxidant thereby neutralizing free radicals.
9. The method of claim 8 wherein the composition is administered to treat viral infections.
10. The method of claim 8 wherein the composition is administered to inhibit UV-induced signal transduction and development of skin cancer.
11. The method of claim 8 wherein the composition is administered to prevent blood clotting.
12. The method of claim 8 wherein the composition is administered to prevent tissue injury caused by ischemia and reperfusion.
13. The method of claim 2 wherein the composition is administered topically to the eye to inhibit cataract formation.
14. A method of delivering an alpha-hydroxy acid to a host comprising administering to a host a composition of claim 1 wherein the polyanhydride comprises Formula (III).
15. The method of claim 14 wherein the composition is administered topically to promote smoother, clearer skin with less wrinkles.
Priority Applications (1)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US10/254,191 US20030035787A1 (en) | 1999-10-21 | 2002-09-24 | Polyanhydrides with biologically active degradation products |
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| US09/422,294 US6468519B1 (en) | 1997-09-10 | 1999-10-21 | Polyanhydrides with biologically active degradation products |
| US10/254,191 US20030035787A1 (en) | 1999-10-21 | 2002-09-24 | Polyanhydrides with biologically active degradation products |
Related Parent Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/422,294 Continuation US6468519B1 (en) | 1997-09-10 | 1999-10-21 | Polyanhydrides with biologically active degradation products |
Publications (1)
| Publication Number | Publication Date |
|---|---|
| US20030035787A1 true US20030035787A1 (en) | 2003-02-20 |
Family
ID=23674230
Family Applications (2)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/422,294 Expired - Fee Related US6468519B1 (en) | 1997-09-10 | 1999-10-21 | Polyanhydrides with biologically active degradation products |
| US10/254,191 Abandoned US20030035787A1 (en) | 1999-10-21 | 2002-09-24 | Polyanhydrides with biologically active degradation products |
Family Applications Before (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| US09/422,294 Expired - Fee Related US6468519B1 (en) | 1997-09-10 | 1999-10-21 | Polyanhydrides with biologically active degradation products |
Country Status (9)
| Country | Link |
|---|---|
| US (2) | US6468519B1 (en) |
| EP (2) | EP1246627B1 (en) |
| JP (1) | JP2003511550A (en) |
| AT (1) | ATE314078T1 (en) |
| AU (1) | AU7877100A (en) |
| CA (1) | CA2387558A1 (en) |
| DE (1) | DE60025272D1 (en) |
| MX (1) | MXPA02003890A (en) |
| WO (1) | WO2001028492A2 (en) |
Cited By (22)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20040038948A1 (en) * | 1999-12-07 | 2004-02-26 | Uhrich Kathryn E. | Therapeutic compositions and methods |
| US20040096476A1 (en) * | 2002-07-17 | 2004-05-20 | Uhrich Kathryn E. | Therapeutic devices for patterned cell growth |
| US20050053577A1 (en) * | 2000-07-27 | 2005-03-10 | Rutgers, The State University Of New Jersey | Therapeutic polyanhydride compounds for drug delivery |
| US20050255079A1 (en) * | 2004-05-14 | 2005-11-17 | Santerre Paul J | Polymeric coupling agents and pharmaceutically-active polymers made therefrom |
| US20060188546A1 (en) * | 1997-09-10 | 2006-08-24 | Polymerix Corporation | Medical devices employing novel polymers |
| US7122615B1 (en) | 1998-09-10 | 2006-10-17 | Rutgers, The State University Of New Jersey | Polyanhydrides with therapeutically useful degradation products |
| JP2007537168A (en) * | 2004-05-14 | 2007-12-20 | インターフェース バイオロジクス インコーポレーティッド | Polymer coupling agents and pharmaceutically active polymers made therefrom |
| WO2008128193A1 (en) | 2007-04-12 | 2008-10-23 | Rutgers, The State University Of New Jersey | Biodegradable polyanhydrides with natural bioactive molecules |
| US20090253806A1 (en) * | 2006-03-23 | 2009-10-08 | Varshney Sunil K | Polyanhydride polymers and their uses in biomedical devices |
| WO2010148104A2 (en) * | 2009-06-16 | 2010-12-23 | L'oreal S.A. | Topical compositions containing a polymer for releasing at least one salicylic acid compound |
| WO2010148101A2 (en) * | 2009-06-16 | 2010-12-23 | L'oreal S.A. | Topical compositions containing a polymer for releasing at least one salicylic acid compound |
| US20110045049A1 (en) * | 2006-11-06 | 2011-02-24 | Ifat Rubin-Bejerano | Immunomodulating compositions and methods of use |
| US20110223232A1 (en) * | 2006-10-23 | 2011-09-15 | Olexander Hnojewyj | drug-release composition having a therapeutic carrier |
| US9144579B2 (en) | 2012-08-17 | 2015-09-29 | Rutgers, The State University Of New Jersey | Polyesters and methods of use thereof |
| US9387250B2 (en) | 2013-03-15 | 2016-07-12 | Rutgers, The State University Of New Jersey | Therapeutic compositions for bone repair |
| US9457047B2 (en) | 2006-11-06 | 2016-10-04 | Whitehead Institute | Immunomodulating compositions and methods of use thereof |
| US9782432B2 (en) | 2012-10-25 | 2017-10-10 | Rutgers, The State University Of New Jersey | Polymers and methods thereof for wound healing |
| US9862672B2 (en) | 2013-05-29 | 2018-01-09 | Rutgers, The State University Of New Jersey | Antioxidant-based poly(anhydride-esters) |
| US10023521B2 (en) | 2014-06-13 | 2018-07-17 | Rutgers, The State University Of New Jersey | Process and intermediates for preparing poly(anhydride-esters) |
| US10543162B2 (en) | 2015-04-10 | 2020-01-28 | Rutgers, The State University Of New Jersey | Kojic acid polymers |
| US10588862B2 (en) | 2018-02-02 | 2020-03-17 | Ripple Therapeutics Corporation | Dexamethasone prodrug compositions and uses thereof |
| US11279729B2 (en) | 2020-05-01 | 2022-03-22 | Ripple Therapeutics Corporation | Heterodimer compositions and methods for the treatment of ocular disorders |
Families Citing this family (25)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US6468519B1 (en) * | 1997-09-10 | 2002-10-22 | Rutgers, The State University Of New Jersey | Polyanhydrides with biologically active degradation products |
| ATE444086T1 (en) * | 1999-12-07 | 2009-10-15 | Univ Rutgers | THERAPEUTIC COMPOSITIONS AND METHODS FOR TREATING PERIODONTITIS WITH ANTI-INFLAMMATORY AGENTS |
| US6527801B1 (en) | 2000-04-13 | 2003-03-04 | Advanced Cardiovascular Systems, Inc. | Biodegradable drug delivery material for stent |
| JP5244279B2 (en) * | 2000-07-27 | 2013-07-24 | ラトガーズ,ザ ステイト ユニバーシティ | Therapeutic polyesters and polyamides |
| AU2001279064A1 (en) | 2000-07-27 | 2002-02-13 | Rutgers, The State University | Therapeutic azo-compounds for drug delivery |
| CN1684582A (en) * | 2001-11-23 | 2005-10-19 | 拉特格斯州立大学 | Improved synthesis of polyanhydrides |
| US6932930B2 (en) * | 2003-03-10 | 2005-08-23 | Synecor, Llc | Intraluminal prostheses having polymeric material with selectively modified crystallinity and methods of making same |
| US9445901B2 (en) * | 2003-03-12 | 2016-09-20 | Deger C. Tunc | Prosthesis with sustained release analgesic |
| CA2525631C (en) * | 2003-05-13 | 2012-12-18 | Medtronic, Inc. | Moisture curable materials for delivery of agents, methods, and medical devices |
| WO2005039489A2 (en) * | 2003-09-24 | 2005-05-06 | Polymerix Corporation | Compositions and methods for the inhibition of bone growth and resorption |
| US8352712B2 (en) * | 2004-05-06 | 2013-01-08 | International Business Machines Corporation | Method and system for specualtively sending processor-issued store operations to a store queue with full signal asserted |
| US7858077B2 (en) * | 2005-01-28 | 2010-12-28 | Bezwada Biomedical Llc | Functionalized phenolic esters and amides and polymers therefrom |
| US7691364B2 (en) * | 2005-01-28 | 2010-04-06 | Bezwada Biomedical, Llc | Functionalized drugs and polymers derived therefrom |
| US20060193891A1 (en) * | 2005-02-25 | 2006-08-31 | Robert Richard | Medical devices and therapeutic delivery devices composed of bioabsorbable polymers produced at room temperature, method of making the devices, and a system for making the devices |
| EP1898832A4 (en) * | 2005-05-23 | 2013-01-02 | Univ Rutgers | Fast degrading polymers |
| WO2007053794A2 (en) * | 2005-10-21 | 2007-05-10 | Bezwada Biomedical Llc | Functionalized phenolic compounds and absorbable therefrom |
| US8007526B2 (en) | 2005-12-01 | 2011-08-30 | Bezwada Biomedical, Llc | Difunctionalized aromatic compounds and polymers therefrom |
| US7935843B2 (en) | 2005-12-09 | 2011-05-03 | Bezwada Biomedical, Llc | Functionalized diphenolics and absorbable polymers therefrom |
| US9603941B2 (en) * | 2006-01-24 | 2017-03-28 | Minghui Chai | Method of preparing dendritic drugs |
| KR20090024242A (en) * | 2006-06-06 | 2009-03-06 | 루트거스, 더 스테이트 유니버시티 오브 뉴 저지 | Iodinated polymers |
| EP2102144A4 (en) | 2006-09-13 | 2011-03-23 | Univ Rutgers | Active agents and their oligomers and polymers |
| US8217134B2 (en) | 2007-08-30 | 2012-07-10 | Bezwada Biomedical, Llc | Controlled release of biologically active compounds |
| US8026285B2 (en) * | 2007-09-04 | 2011-09-27 | Bezwada Biomedical, Llc | Control release of biologically active compounds from multi-armed oligomers |
| US8048980B2 (en) | 2007-09-17 | 2011-11-01 | Bezwada Biomedical, Llc | Hydrolysable linkers and cross-linkers for absorbable polymers |
| US8741317B2 (en) | 2010-08-19 | 2014-06-03 | Rutgers, The State University Of New Jersey | Slow-degrading polymers comprising salicylic acid for undelayed and sustained drug delivery |
Citations (47)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4062855A (en) * | 1971-09-27 | 1977-12-13 | University Of Washington | Synthetic polymers furnishing controlled release of a biologically active component during degradation |
| US4287174A (en) * | 1978-03-31 | 1981-09-01 | The Proctor & Gamble Company | Anti-ulcer composition |
| US4298595A (en) * | 1978-12-20 | 1981-11-03 | Dynapol | Pharmaceutical preparations containing a polymeric agent for releasing 5-aminosalicylic acid or its salts into the gastrointestinal tract |
| US4363815A (en) * | 1975-07-23 | 1982-12-14 | Yu Ruey J | Alpha hydroxyacids, alpha ketoacids and their use in treating skin conditions |
| US4483854A (en) * | 1983-04-12 | 1984-11-20 | Key Pharmaceuticals, Inc. | Systemic treatment of psoriasis using certain salicylates |
| US4612302A (en) * | 1983-11-14 | 1986-09-16 | Brigham And Women's Hospital | Clinical use of somatostatin analogues |
| US4665063A (en) * | 1983-06-13 | 1987-05-12 | Rafa Laboratories Ltd. | Method of treating acne |
| US4684620A (en) * | 1984-09-04 | 1987-08-04 | Gibson-Stephens Neuropharmaceuticals, Inc. | Cyclic polypeptides having mu-receptor specificity |
| US4757128A (en) * | 1986-08-01 | 1988-07-12 | Massachusetts Institute Of Technology | High molecular weight polyanhydride and preparation thereof |
| US4857311A (en) * | 1987-07-31 | 1989-08-15 | Massachusetts Institute Of Technology | Polyanhydrides with improved hydrolytic degradation properties |
| US4868274A (en) * | 1988-05-23 | 1989-09-19 | Hoechst Celanese Corp. | Polyanhydride from carboxy aryloxy alkanoic acid |
| US4886870A (en) * | 1984-05-21 | 1989-12-12 | Massachusetts Institute Of Technology | Bioerodible articles useful as implants and prostheses having predictable degradation rates |
| US4888176A (en) * | 1984-05-21 | 1989-12-19 | Massachusetts Institute Of Technology | Controlled drug delivery high molecular weight polyanhydrides |
| US4891225A (en) * | 1984-05-21 | 1990-01-02 | Massachusetts Institute Of Technology | Bioerodible polyanhydrides for controlled drug delivery |
| US4906474A (en) * | 1983-03-22 | 1990-03-06 | Massachusetts Institute Of Technology | Bioerodible polyanhydrides for controlled drug delivery |
| US4916204A (en) * | 1987-07-31 | 1990-04-10 | Massachusetts Institute Of Technology | Pure polyanhydride from dicarboxylic acid and coupling agent |
| US4938949A (en) * | 1988-09-12 | 1990-07-03 | University Of New York | Treatment of damaged bone marrow and dosage units therefor |
| US4997904A (en) * | 1989-08-25 | 1991-03-05 | Nova Pharmaceutical Corporation | Aromatic polyanhydride compositions |
| US4999417A (en) * | 1989-03-30 | 1991-03-12 | Nova Pharmaceutical Corporation | Biodegradable polymer compositions |
| US5055524A (en) * | 1987-07-16 | 1991-10-08 | Ppg Industries, Inc. | Polyol-modified polyanhydride curing agent for polyepoxide powder coatings |
| US5082925A (en) * | 1990-08-16 | 1992-01-21 | Ethicon, Inc. | Homopolymers and copolymers of salicylate lactones |
| US5151415A (en) * | 1991-05-23 | 1992-09-29 | Dallas Sirany | Method of treating a papova-type viral infection |
| US5175235A (en) * | 1990-06-04 | 1992-12-29 | Nova Pharmaceutical Corporation | Branched polyanhydrides |
| US5259968A (en) * | 1988-02-29 | 1993-11-09 | Exxon Chemical Patents Inc. | Dispersant additive comprising the reaction product of a polyanhydride and a mannich condensation product |
| US5264540A (en) * | 1992-07-20 | 1993-11-23 | Ethicon, Inc. | Aromatic polyanhydrides |
| US5422352A (en) * | 1989-07-07 | 1995-06-06 | Nycomed Dak A/S | Slimming pharmaceutical composition |
| US5498729A (en) * | 1989-12-26 | 1996-03-12 | Domb; Abraham J. | Prodrug compositions |
| US5514764A (en) * | 1990-11-19 | 1996-05-07 | Cornell Research Foundation, Inc. | Hyperbranched polyesters and polyamides |
| US5545409A (en) * | 1989-02-22 | 1996-08-13 | Massachusetts Institute Of Technology | Delivery system for controlled release of bioactive factors |
| US5660851A (en) * | 1989-12-26 | 1997-08-26 | Yissum Research Development Company Of The Hebrew Univ. Of Jerusalem | Ocular inserts |
| US5703122A (en) * | 1993-04-26 | 1997-12-30 | Avon Products, Inc. | Ascorbic acid compositions for reducing irritation of topically applied active ingredients |
| US5798115A (en) * | 1996-02-15 | 1998-08-25 | Santerre; Paul J. | Bioresponsive pharmacologically-active polymers and articles made therefrom |
| US5837278A (en) * | 1994-01-06 | 1998-11-17 | Ed Geistlich Sohne Ag Fur Chemische Industrie | Resorbable collagen membrane for use in guided tissue regeneration |
| US5869069A (en) * | 1994-07-22 | 1999-02-09 | Coletica | Lipophilic hydroxylated acid, its use in cosmetics and pharmacy, and its process of preparation |
| US5882665A (en) * | 1997-11-18 | 1999-03-16 | Elizabeth Arden Co., Division Of Conopco, Inc. | Phytosphingosine salicylates in cosmetic compositions |
| US5902110A (en) * | 1995-12-18 | 1999-05-11 | The Block Drug Company | Bone regeneration |
| US5942252A (en) * | 1986-10-24 | 1999-08-24 | Southern Research Institute | Method for delivering bioactive agents into and through the mucosally-associated lymphoid tissues and controlling their release |
| US6025331A (en) * | 1996-02-16 | 2000-02-15 | Children's Medical Center Corporation | Pharmaceutical compositions comprising troponin subunits, fragments and analogs thereof and methods of their use to inhibit angiogenesis |
| US6071530A (en) * | 1989-07-24 | 2000-06-06 | Atrix Laboratories, Inc. | Method and composition for treating a bone tissue defect |
| US6153212A (en) * | 1998-10-02 | 2000-11-28 | Guilford Pharmaceuticals Inc. | Biodegradable terephthalate polyester-poly (phosphonate) compositions, articles, and methods of using the same |
| US6468519B1 (en) * | 1997-09-10 | 2002-10-22 | Rutgers, The State University Of New Jersey | Polyanhydrides with biologically active degradation products |
| US6486214B1 (en) * | 1997-09-10 | 2002-11-26 | Rutgers, The State University Of New Jersey | Polyanhydride linkers for production of drug polymers and drug polymer compositions produced thereby |
| US6602915B2 (en) * | 2000-07-27 | 2003-08-05 | Rutgers, The State University Of New Jersey | Therapeutic azo-compounds for drug delivery |
| US6685928B2 (en) * | 1999-12-07 | 2004-02-03 | Rutgers, The State University Of New Jersey | Therapeutic compositions and methods |
| US6689350B2 (en) * | 2000-07-27 | 2004-02-10 | Rutgers, The State University Of New Jersey | Therapeutic polyesters and polyamides |
| US20040038948A1 (en) * | 1999-12-07 | 2004-02-26 | Uhrich Kathryn E. | Therapeutic compositions and methods |
| US20040096476A1 (en) * | 2002-07-17 | 2004-05-20 | Uhrich Kathryn E. | Therapeutic devices for patterned cell growth |
Family Cites Families (17)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| DE288311C (en) | 1915-10-27 | |||
| DE288387C (en) | ||||
| CH666185A5 (en) * | 1985-09-16 | 1988-07-15 | Dieter Dr Russmann | Salicylic acid or sodium salt - used for treating inflammatory intestinal disorders |
| CH671402A5 (en) | 1985-10-02 | 1989-08-31 | Sandoz Ag | |
| DE3670206D1 (en) | 1986-05-20 | 1990-05-17 | Massachusetts Inst Technology | BIOERODABLE PRODUCTS FOR USE AS IMPLANTS OR PROSTHESIS WITH A PREDICTABLE LEVEL OF RESORBATION. |
| WO1990009779A1 (en) | 1989-02-28 | 1990-09-07 | Benhuri Marc N | Method and composition for treatment of periodontal disease |
| US5317079A (en) * | 1990-01-19 | 1994-05-31 | Nova Pharmaceutical Corporation | Fatty acid terminated polyanhydride |
| NL9000237A (en) | 1990-01-31 | 1991-08-16 | Re Novative Drugs For Dermatol | Topical medicaments contg. 5-amino-salicylic acid - for treating inflammatory, erosive or ulcerative disorders of oral cavity or vagina |
| US5198572A (en) * | 1991-02-04 | 1993-03-30 | General Electric Company | Copolymers of dicarboxylic acids and salicylic acids |
| US5902599A (en) | 1996-02-20 | 1999-05-11 | Massachusetts Institute Of Technology | Biodegradable polymer networks for use in orthopedic and dental applications |
| IE960308A1 (en) | 1996-04-23 | 1997-11-05 | Kinerton Ltd | Sustained release ionic conjugate |
| US5955096A (en) * | 1996-06-25 | 1999-09-21 | Brown University Research Foundation | Methods and compositions for enhancing the bioadhesive properties of polymers using organic excipients |
| MXPA99007662A (en) | 1997-02-18 | 2002-07-22 | Univ Rutgers | Monomers derived from hydroxy acids and polymers prepared therefrom. |
| JP2001513503A (en) * | 1997-08-06 | 2001-09-04 | スミスクライン・ビーチャム・コーポレイション | Macrophage scavenger receptor antagonists used in the treatment of cardiovascular diseases |
| WO1999012990A1 (en) | 1997-09-10 | 1999-03-18 | Rutgers, The State University | Polyanhydrides with therapeutically useful degradation products |
| DE19754063A1 (en) | 1997-12-05 | 1999-06-10 | Bayer Ag | Degradation of biodegradable polymers |
| US6060212A (en) * | 1998-06-11 | 2000-05-09 | Clariant Finance (Bvi) Limited | 193 nm positive-working photoresist composition |
-
1999
- 1999-10-21 US US09/422,294 patent/US6468519B1/en not_active Expired - Fee Related
-
2000
- 2000-10-11 JP JP2001531088A patent/JP2003511550A/en active Pending
- 2000-10-11 CA CA002387558A patent/CA2387558A1/en not_active Abandoned
- 2000-10-11 MX MXPA02003890A patent/MXPA02003890A/en active IP Right Grant
- 2000-10-11 DE DE60025272T patent/DE60025272D1/en not_active Expired - Lifetime
- 2000-10-11 AT AT00968928T patent/ATE314078T1/en not_active IP Right Cessation
- 2000-10-11 EP EP00968928A patent/EP1246627B1/en not_active Expired - Lifetime
- 2000-10-11 WO PCT/US2000/027962 patent/WO2001028492A2/en active IP Right Grant
- 2000-10-11 AU AU78771/00A patent/AU7877100A/en not_active Abandoned
- 2000-10-11 EP EP04007101A patent/EP1464673A3/en not_active Withdrawn
-
2002
- 2002-09-24 US US10/254,191 patent/US20030035787A1/en not_active Abandoned
Patent Citations (53)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4126445A (en) * | 1971-09-27 | 1978-11-21 | University Of Washington | Synthetic polymers furnishing controlled release of a biologically active component during degradation |
| US4062855A (en) * | 1971-09-27 | 1977-12-13 | University Of Washington | Synthetic polymers furnishing controlled release of a biologically active component during degradation |
| US4363815A (en) * | 1975-07-23 | 1982-12-14 | Yu Ruey J | Alpha hydroxyacids, alpha ketoacids and their use in treating skin conditions |
| US4287174A (en) * | 1978-03-31 | 1981-09-01 | The Proctor & Gamble Company | Anti-ulcer composition |
| US4298595A (en) * | 1978-12-20 | 1981-11-03 | Dynapol | Pharmaceutical preparations containing a polymeric agent for releasing 5-aminosalicylic acid or its salts into the gastrointestinal tract |
| US4906474A (en) * | 1983-03-22 | 1990-03-06 | Massachusetts Institute Of Technology | Bioerodible polyanhydrides for controlled drug delivery |
| US4483854A (en) * | 1983-04-12 | 1984-11-20 | Key Pharmaceuticals, Inc. | Systemic treatment of psoriasis using certain salicylates |
| US4665063A (en) * | 1983-06-13 | 1987-05-12 | Rafa Laboratories Ltd. | Method of treating acne |
| US4612302A (en) * | 1983-11-14 | 1986-09-16 | Brigham And Women's Hospital | Clinical use of somatostatin analogues |
| US4891225A (en) * | 1984-05-21 | 1990-01-02 | Massachusetts Institute Of Technology | Bioerodible polyanhydrides for controlled drug delivery |
| US4886870A (en) * | 1984-05-21 | 1989-12-12 | Massachusetts Institute Of Technology | Bioerodible articles useful as implants and prostheses having predictable degradation rates |
| US4888176A (en) * | 1984-05-21 | 1989-12-19 | Massachusetts Institute Of Technology | Controlled drug delivery high molecular weight polyanhydrides |
| US4684620A (en) * | 1984-09-04 | 1987-08-04 | Gibson-Stephens Neuropharmaceuticals, Inc. | Cyclic polypeptides having mu-receptor specificity |
| US4757128A (en) * | 1986-08-01 | 1988-07-12 | Massachusetts Institute Of Technology | High molecular weight polyanhydride and preparation thereof |
| US5942252A (en) * | 1986-10-24 | 1999-08-24 | Southern Research Institute | Method for delivering bioactive agents into and through the mucosally-associated lymphoid tissues and controlling their release |
| US5055524A (en) * | 1987-07-16 | 1991-10-08 | Ppg Industries, Inc. | Polyol-modified polyanhydride curing agent for polyepoxide powder coatings |
| US4916204A (en) * | 1987-07-31 | 1990-04-10 | Massachusetts Institute Of Technology | Pure polyanhydride from dicarboxylic acid and coupling agent |
| US4857311A (en) * | 1987-07-31 | 1989-08-15 | Massachusetts Institute Of Technology | Polyanhydrides with improved hydrolytic degradation properties |
| US5259968A (en) * | 1988-02-29 | 1993-11-09 | Exxon Chemical Patents Inc. | Dispersant additive comprising the reaction product of a polyanhydride and a mannich condensation product |
| US4868274A (en) * | 1988-05-23 | 1989-09-19 | Hoechst Celanese Corp. | Polyanhydride from carboxy aryloxy alkanoic acid |
| US4938949A (en) * | 1988-09-12 | 1990-07-03 | University Of New York | Treatment of damaged bone marrow and dosage units therefor |
| US5629009A (en) * | 1989-02-22 | 1997-05-13 | Massachusetts Institute Of Technology | Delivery system for controlled release of bioactive factors |
| US5545409A (en) * | 1989-02-22 | 1996-08-13 | Massachusetts Institute Of Technology | Delivery system for controlled release of bioactive factors |
| US4999417A (en) * | 1989-03-30 | 1991-03-12 | Nova Pharmaceutical Corporation | Biodegradable polymer compositions |
| US5422352A (en) * | 1989-07-07 | 1995-06-06 | Nycomed Dak A/S | Slimming pharmaceutical composition |
| US6071530A (en) * | 1989-07-24 | 2000-06-06 | Atrix Laboratories, Inc. | Method and composition for treating a bone tissue defect |
| US4997904A (en) * | 1989-08-25 | 1991-03-05 | Nova Pharmaceutical Corporation | Aromatic polyanhydride compositions |
| US5498729A (en) * | 1989-12-26 | 1996-03-12 | Domb; Abraham J. | Prodrug compositions |
| US5660851A (en) * | 1989-12-26 | 1997-08-26 | Yissum Research Development Company Of The Hebrew Univ. Of Jerusalem | Ocular inserts |
| US5175235A (en) * | 1990-06-04 | 1992-12-29 | Nova Pharmaceutical Corporation | Branched polyanhydrides |
| US5082925A (en) * | 1990-08-16 | 1992-01-21 | Ethicon, Inc. | Homopolymers and copolymers of salicylate lactones |
| US5514764A (en) * | 1990-11-19 | 1996-05-07 | Cornell Research Foundation, Inc. | Hyperbranched polyesters and polyamides |
| US5151415A (en) * | 1991-05-23 | 1992-09-29 | Dallas Sirany | Method of treating a papova-type viral infection |
| US5264540A (en) * | 1992-07-20 | 1993-11-23 | Ethicon, Inc. | Aromatic polyanhydrides |
| US5703122A (en) * | 1993-04-26 | 1997-12-30 | Avon Products, Inc. | Ascorbic acid compositions for reducing irritation of topically applied active ingredients |
| US5837278A (en) * | 1994-01-06 | 1998-11-17 | Ed Geistlich Sohne Ag Fur Chemische Industrie | Resorbable collagen membrane for use in guided tissue regeneration |
| US5869069A (en) * | 1994-07-22 | 1999-02-09 | Coletica | Lipophilic hydroxylated acid, its use in cosmetics and pharmacy, and its process of preparation |
| US5902110A (en) * | 1995-12-18 | 1999-05-11 | The Block Drug Company | Bone regeneration |
| US5798115A (en) * | 1996-02-15 | 1998-08-25 | Santerre; Paul J. | Bioresponsive pharmacologically-active polymers and articles made therefrom |
| US6025331A (en) * | 1996-02-16 | 2000-02-15 | Children's Medical Center Corporation | Pharmaceutical compositions comprising troponin subunits, fragments and analogs thereof and methods of their use to inhibit angiogenesis |
| US6486214B1 (en) * | 1997-09-10 | 2002-11-26 | Rutgers, The State University Of New Jersey | Polyanhydride linkers for production of drug polymers and drug polymer compositions produced thereby |
| US6468519B1 (en) * | 1997-09-10 | 2002-10-22 | Rutgers, The State University Of New Jersey | Polyanhydrides with biologically active degradation products |
| US5882665A (en) * | 1997-11-18 | 1999-03-16 | Elizabeth Arden Co., Division Of Conopco, Inc. | Phytosphingosine salicylates in cosmetic compositions |
| US6153212A (en) * | 1998-10-02 | 2000-11-28 | Guilford Pharmaceuticals Inc. | Biodegradable terephthalate polyester-poly (phosphonate) compositions, articles, and methods of using the same |
| US6685928B2 (en) * | 1999-12-07 | 2004-02-03 | Rutgers, The State University Of New Jersey | Therapeutic compositions and methods |
| US20040038948A1 (en) * | 1999-12-07 | 2004-02-26 | Uhrich Kathryn E. | Therapeutic compositions and methods |
| US6602915B2 (en) * | 2000-07-27 | 2003-08-05 | Rutgers, The State University Of New Jersey | Therapeutic azo-compounds for drug delivery |
| US6613807B2 (en) * | 2000-07-27 | 2003-09-02 | Rutgers, The State University Of New Jersey | Therapeutic polyanhydride compounds for drug delivery |
| US6689350B2 (en) * | 2000-07-27 | 2004-02-10 | Rutgers, The State University Of New Jersey | Therapeutic polyesters and polyamides |
| US20040044125A1 (en) * | 2000-07-27 | 2004-03-04 | Rutgers, The State University Of New Jersey | Therapeutic AZO-compounds for drug delivery |
| US20050031577A1 (en) * | 2000-07-27 | 2005-02-10 | Rutgers, The State University Of New Jersey | Therapeutic polyesters and polyamides |
| US20050053577A1 (en) * | 2000-07-27 | 2005-03-10 | Rutgers, The State University Of New Jersey | Therapeutic polyanhydride compounds for drug delivery |
| US20040096476A1 (en) * | 2002-07-17 | 2004-05-20 | Uhrich Kathryn E. | Therapeutic devices for patterned cell growth |
Cited By (38)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20100074937A1 (en) * | 1997-09-10 | 2010-03-25 | Uhrich Kathryn E | Polyanhydrides with therapeutically useful degradation products |
| US20060188546A1 (en) * | 1997-09-10 | 2006-08-24 | Polymerix Corporation | Medical devices employing novel polymers |
| US20070213500A1 (en) * | 1997-09-10 | 2007-09-13 | Rutgers, The State University Of New Jersey | Polyanhydrides with therapeutically useful degradation products |
| US8017714B2 (en) | 1997-09-10 | 2011-09-13 | Rutgers, The State University Of New Jersey | Polyanhydrides with therapeutically useful degradation products |
| US7985415B2 (en) | 1997-09-10 | 2011-07-26 | Rutgers, The State University Of New Jersey | Medical devices employing novel polymers |
| US7534852B2 (en) | 1997-09-10 | 2009-05-19 | Rutgers, The State University Of New Jersey | Polyanhydrides with therapeutically useful degradation products |
| US7122615B1 (en) | 1998-09-10 | 2006-10-17 | Rutgers, The State University Of New Jersey | Polyanhydrides with therapeutically useful degradation products |
| US8088405B2 (en) | 1999-12-07 | 2012-01-03 | Rutgers, The State University of New Jersery | Therapeutic compositions and methods |
| US20040038948A1 (en) * | 1999-12-07 | 2004-02-26 | Uhrich Kathryn E. | Therapeutic compositions and methods |
| US20050053577A1 (en) * | 2000-07-27 | 2005-03-10 | Rutgers, The State University Of New Jersey | Therapeutic polyanhydride compounds for drug delivery |
| US20040096476A1 (en) * | 2002-07-17 | 2004-05-20 | Uhrich Kathryn E. | Therapeutic devices for patterned cell growth |
| US20110112259A1 (en) * | 2004-05-14 | 2011-05-12 | Interface Biologics, Inc | Polymeric coupling agents and pharmaceutically-active polymers made therefrom |
| US8349309B2 (en) | 2004-05-14 | 2013-01-08 | Interface Biologics Inc. | Polymeric coupling agents and pharmaceutically-active polymers made therefrom |
| US20050255079A1 (en) * | 2004-05-14 | 2005-11-17 | Santerre Paul J | Polymeric coupling agents and pharmaceutically-active polymers made therefrom |
| JP2007537168A (en) * | 2004-05-14 | 2007-12-20 | インターフェース バイオロジクス インコーポレーティッド | Polymer coupling agents and pharmaceutically active polymers made therefrom |
| US20090253806A1 (en) * | 2006-03-23 | 2009-10-08 | Varshney Sunil K | Polyanhydride polymers and their uses in biomedical devices |
| US7674285B2 (en) | 2006-03-23 | 2010-03-09 | Bioabsorbable Therapeutics, Inc. | Polyanhydride polymers and their uses in biomedical devices |
| US20110223232A1 (en) * | 2006-10-23 | 2011-09-15 | Olexander Hnojewyj | drug-release composition having a therapeutic carrier |
| US8580253B2 (en) | 2006-11-06 | 2013-11-12 | Whitehead Institute | Immunomodulating compositions and methods of use |
| US20110045049A1 (en) * | 2006-11-06 | 2011-02-24 | Ifat Rubin-Bejerano | Immunomodulating compositions and methods of use |
| US9457047B2 (en) | 2006-11-06 | 2016-10-04 | Whitehead Institute | Immunomodulating compositions and methods of use thereof |
| WO2008128193A1 (en) | 2007-04-12 | 2008-10-23 | Rutgers, The State University Of New Jersey | Biodegradable polyanhydrides with natural bioactive molecules |
| WO2010148101A2 (en) * | 2009-06-16 | 2010-12-23 | L'oreal S.A. | Topical compositions containing a polymer for releasing at least one salicylic acid compound |
| WO2010148104A3 (en) * | 2009-06-16 | 2011-05-05 | L'oreal S.A. | Topical compositions containing a polymer for releasing at least one salicylic acid compound |
| WO2010148104A2 (en) * | 2009-06-16 | 2010-12-23 | L'oreal S.A. | Topical compositions containing a polymer for releasing at least one salicylic acid compound |
| WO2010148101A3 (en) * | 2009-06-16 | 2011-05-05 | L'oreal S.A. | Topical compositions containing a polymer for releasing at least one salicylic acid compound |
| US9144579B2 (en) | 2012-08-17 | 2015-09-29 | Rutgers, The State University Of New Jersey | Polyesters and methods of use thereof |
| US9782432B2 (en) | 2012-10-25 | 2017-10-10 | Rutgers, The State University Of New Jersey | Polymers and methods thereof for wound healing |
| US9387250B2 (en) | 2013-03-15 | 2016-07-12 | Rutgers, The State University Of New Jersey | Therapeutic compositions for bone repair |
| US9862672B2 (en) | 2013-05-29 | 2018-01-09 | Rutgers, The State University Of New Jersey | Antioxidant-based poly(anhydride-esters) |
| US10023521B2 (en) | 2014-06-13 | 2018-07-17 | Rutgers, The State University Of New Jersey | Process and intermediates for preparing poly(anhydride-esters) |
| US10543162B2 (en) | 2015-04-10 | 2020-01-28 | Rutgers, The State University Of New Jersey | Kojic acid polymers |
| US10588862B2 (en) | 2018-02-02 | 2020-03-17 | Ripple Therapeutics Corporation | Dexamethasone prodrug compositions and uses thereof |
| US10632075B2 (en) | 2018-02-02 | 2020-04-28 | Ripple Therapeutics Corporation | Glass formulations and uses thereof |
| US10945958B2 (en) | 2018-02-02 | 2021-03-16 | Ripple Therapeutics Corporation | Dexamethasone prodrug compositions and uses thereof |
| US10959954B2 (en) | 2018-02-02 | 2021-03-30 | Ripple Therapeutics Corporation | Dexamethasone prodrug compositions and uses thereof |
| US11612567B2 (en) | 2018-02-02 | 2023-03-28 | Ripple Therapeutics Corporation | Ocular inserts comprising a covalently linked steroid dimer |
| US11279729B2 (en) | 2020-05-01 | 2022-03-22 | Ripple Therapeutics Corporation | Heterodimer compositions and methods for the treatment of ocular disorders |
Also Published As
| Publication number | Publication date |
|---|---|
| AU7877100A (en) | 2001-04-30 |
| WO2001028492A2 (en) | 2001-04-26 |
| DE60025272D1 (en) | 2006-02-02 |
| EP1246627B1 (en) | 2005-12-28 |
| JP2003511550A (en) | 2003-03-25 |
| EP1464673A2 (en) | 2004-10-06 |
| EP1246627A2 (en) | 2002-10-09 |
| US6468519B1 (en) | 2002-10-22 |
| MXPA02003890A (en) | 2002-12-13 |
| WO2001028492A3 (en) | 2001-09-13 |
| EP1464673A3 (en) | 2007-12-19 |
| ATE314078T1 (en) | 2006-01-15 |
| EP1246627A4 (en) | 2003-04-02 |
| CA2387558A1 (en) | 2001-04-26 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| US6468519B1 (en) | Polyanhydrides with biologically active degradation products | |
| EP1032605B1 (en) | Polyanhydrides with therapeutically useful degradation products | |
| US8017714B2 (en) | Polyanhydrides with therapeutically useful degradation products | |
| US7666398B2 (en) | Polyanhydride linkers for production of drug polymers and drug polymer compositions produced thereby | |
| EP1261347B1 (en) | Therapeutic compositions and methods for treating periodontitis with antiinflamatory compounds | |
| US20080234235A1 (en) | Synthesis of Polyanhydrides | |
| AU2005248970A1 (en) | Polyanhydrides with biologically active degradation products | |
| AU2002301261B2 (en) | Polyanhydrides with therapeutically useful degradation products | |
| AU2006202416A1 (en) | Polyanhydrides with therapeutically useful degradation products | |
| MXPA00002494A (en) | Polyanhydrides with therapeutically useful degradation products |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| AS | Assignment |
Owner name: AMT CAPITAL, LTD., TEXAS Free format text: ASSIGNMENT OF ASSIGNORS INTEREST;ASSIGNOR:POLYMERIX CORPORATION;REEL/FRAME:014250/0921 Effective date: 20030519 |
|
| AS | Assignment |
Owner name: POLYMERIX CORPORATION, NEW JERSEY Free format text: RELEASE OF SECURITY INTEREST;ASSIGNOR:AMT CAPITAL, LTD.;REEL/FRAME:014683/0487 Effective date: 20031023 |
|
| STCB | Information on status: application discontinuation |
Free format text: ABANDONED -- FAILURE TO RESPOND TO AN OFFICE ACTION |